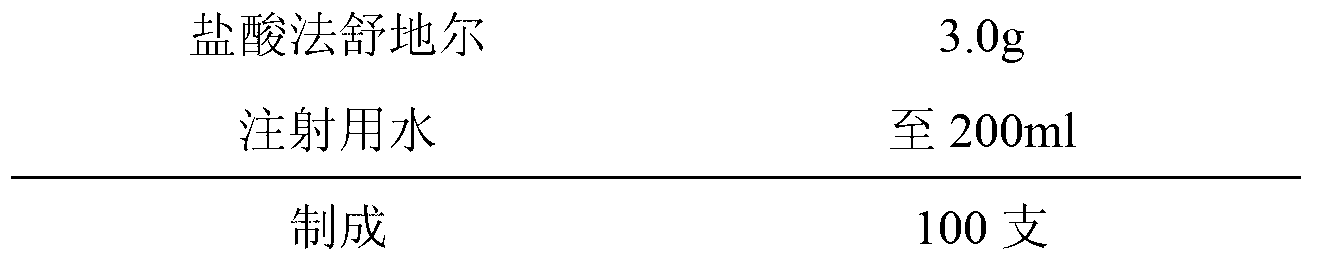Patents
Literature
413results about How to "Simple prescription" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
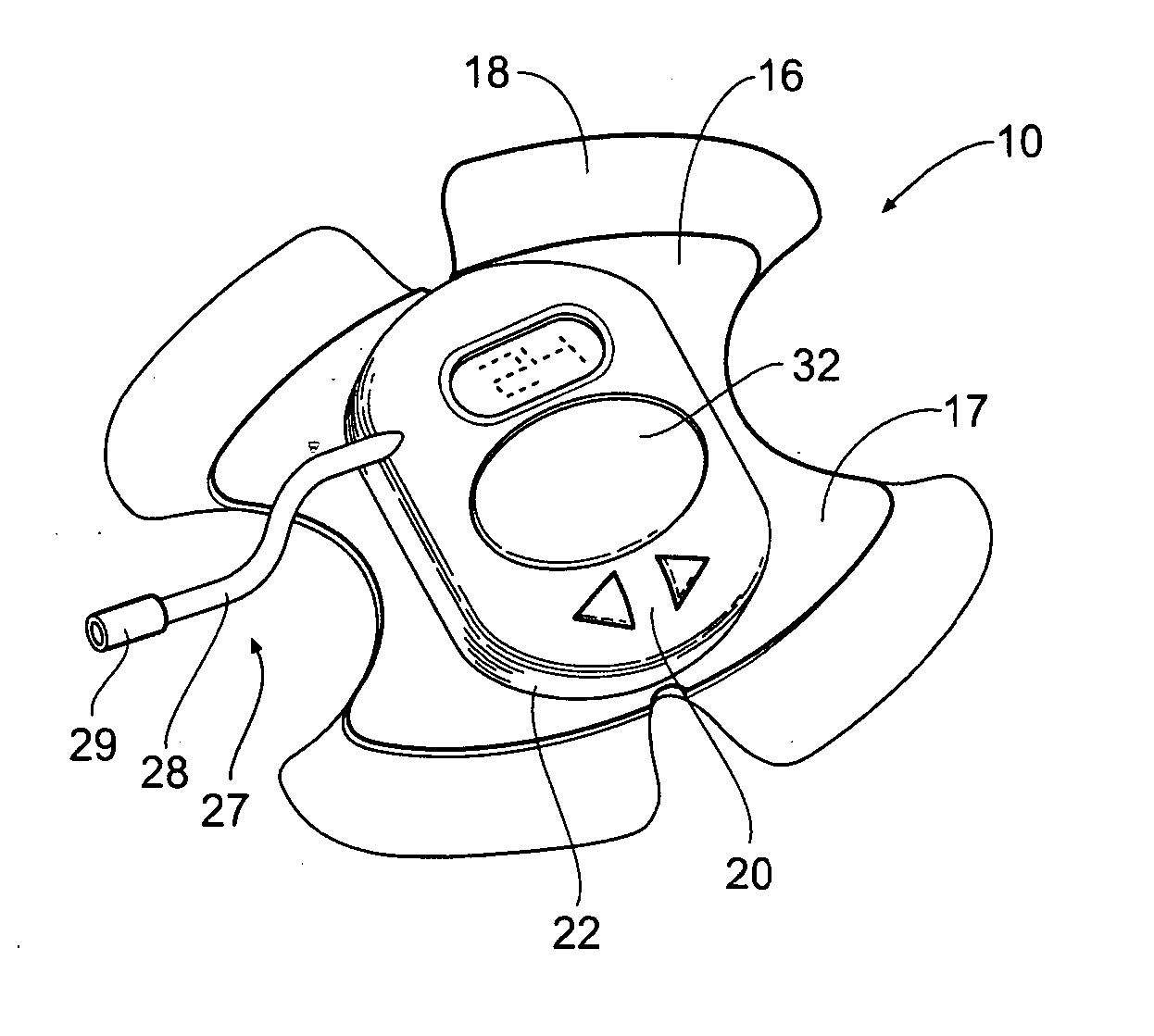
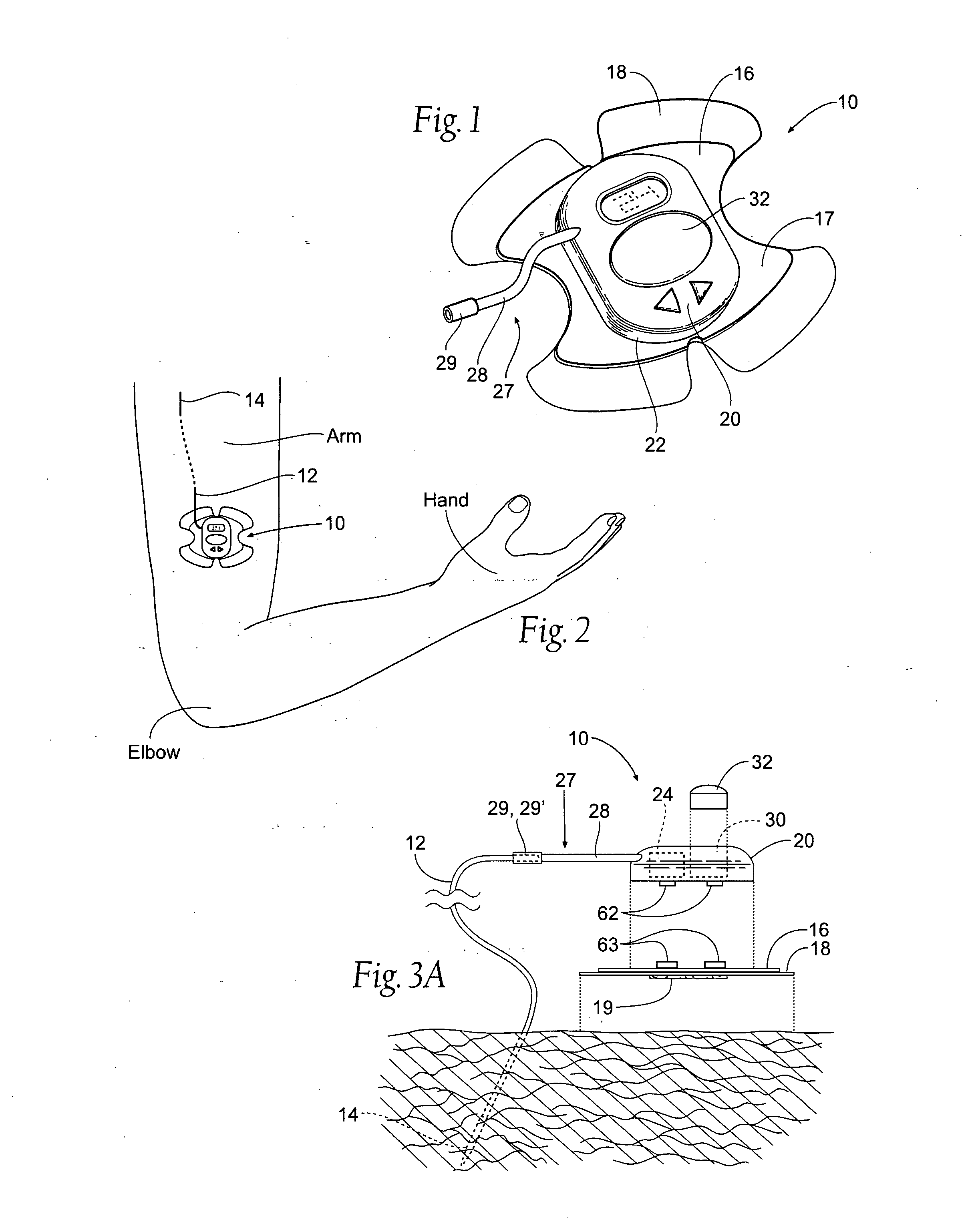
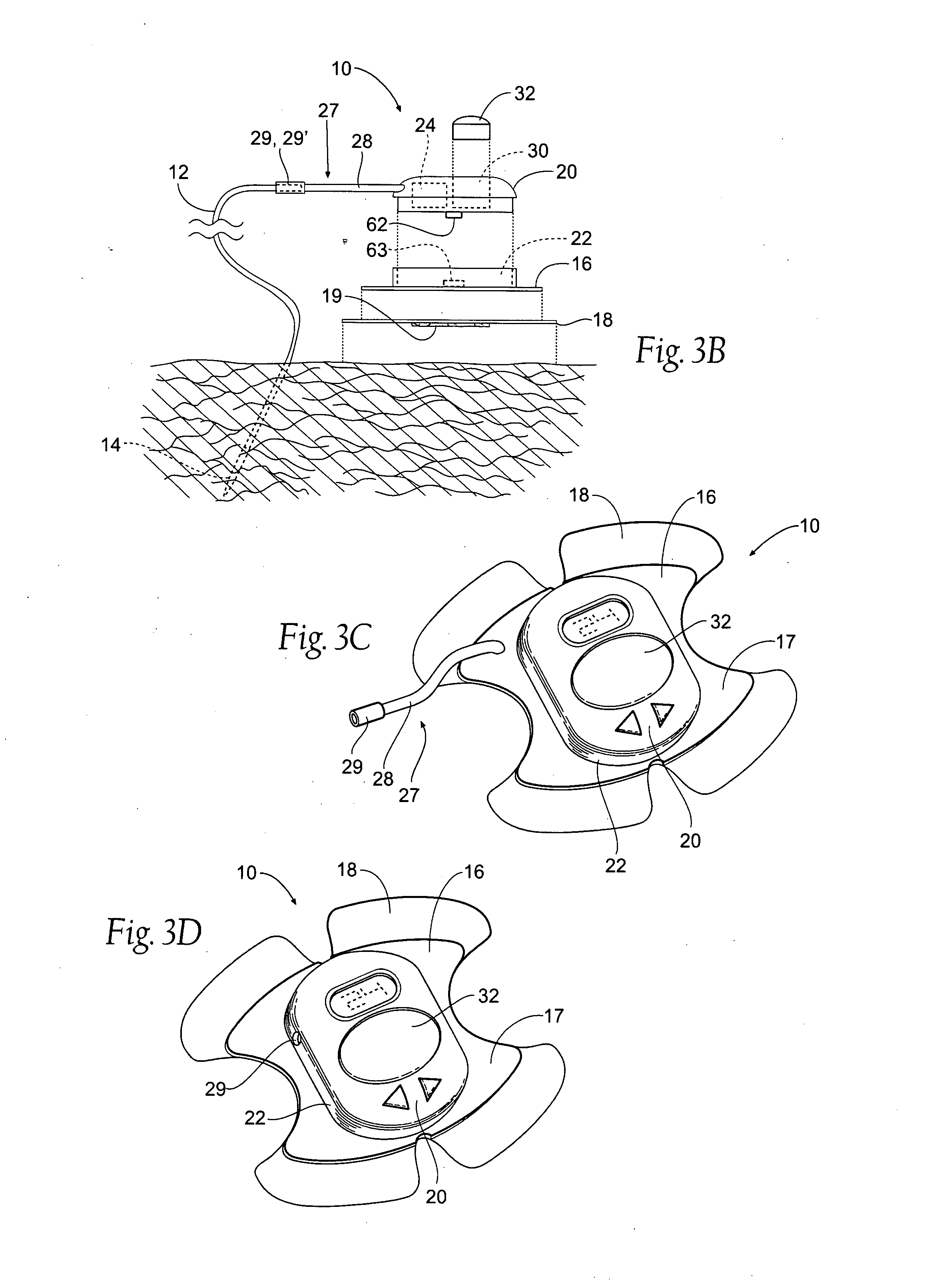
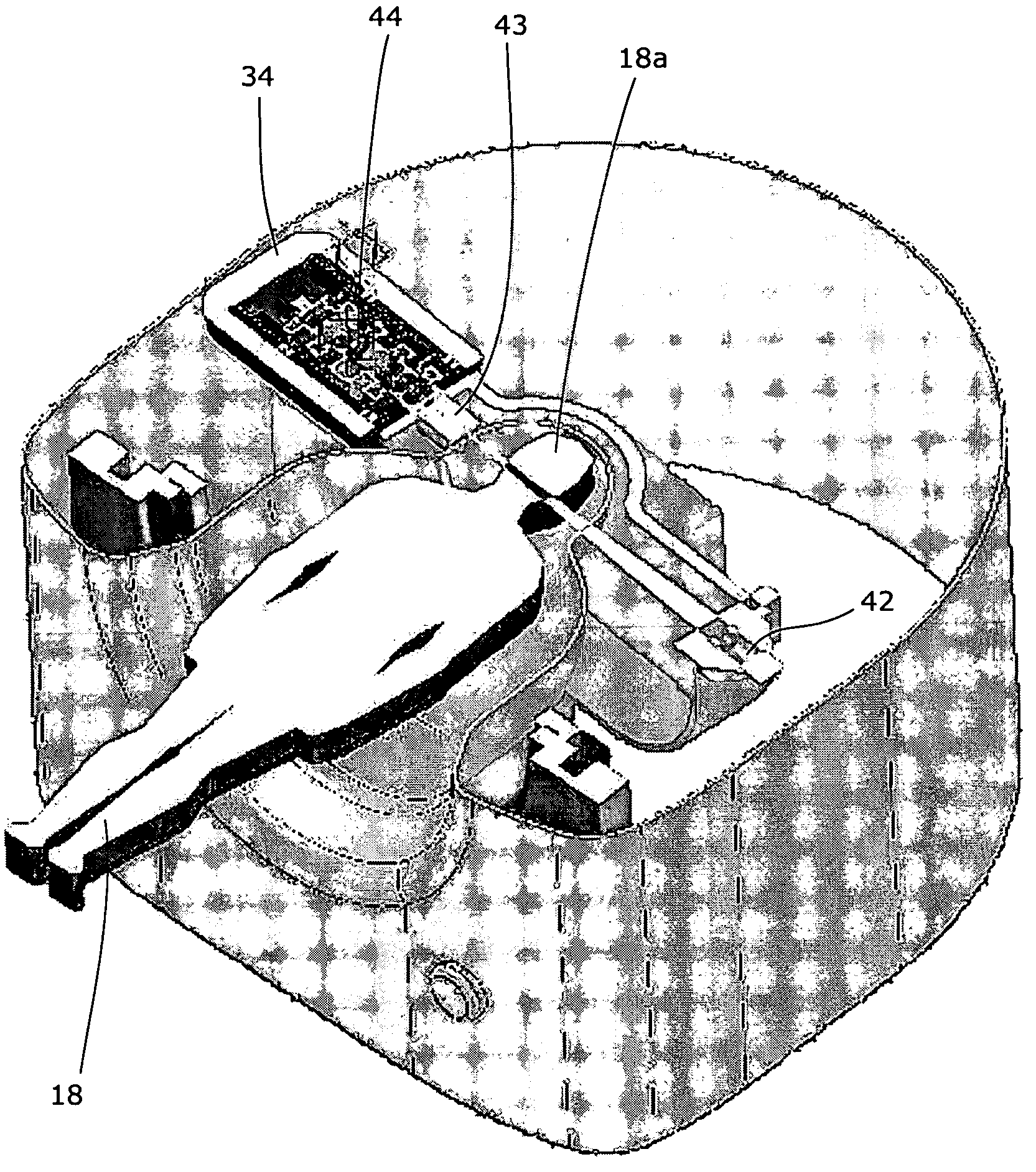
Portable assemblies, systems, and methods for providing functional or therapeutic neurostimulation
InactiveUS20080065182A1Easy to carryAllows and mobilitySpinal electrodesHead electrodesElectricityMedicine
Neurostimulation assemblies, systems, kits, and methods make possible the providing of short-term therapy or diagnostic testing by providing electrical connections between muscles or nerves inside the body and stimulus generators or recording instruments mounted on the surface of the skin or carried outside the body. Neurostimulation assemblies, systems, and methods may include a carrier and a removable electronics pod, the electronics pod including stimulation generation circuitry, a power input bay to hold a disposable power source, and user interface components. The assemblies, systems, and methods are adapted to provide coordinated neurostimulation to multiple regions of the body.
Owner:NDI MEDICAL LLC - CHARTER NO 1766209
Method and apparatus for treatment by ionizing radiation
ActiveUS20050089141A1Improve accuracyEasy to operateMaterial analysis using wave/particle radiationRadiation/particle handlingBeam directionLight beam
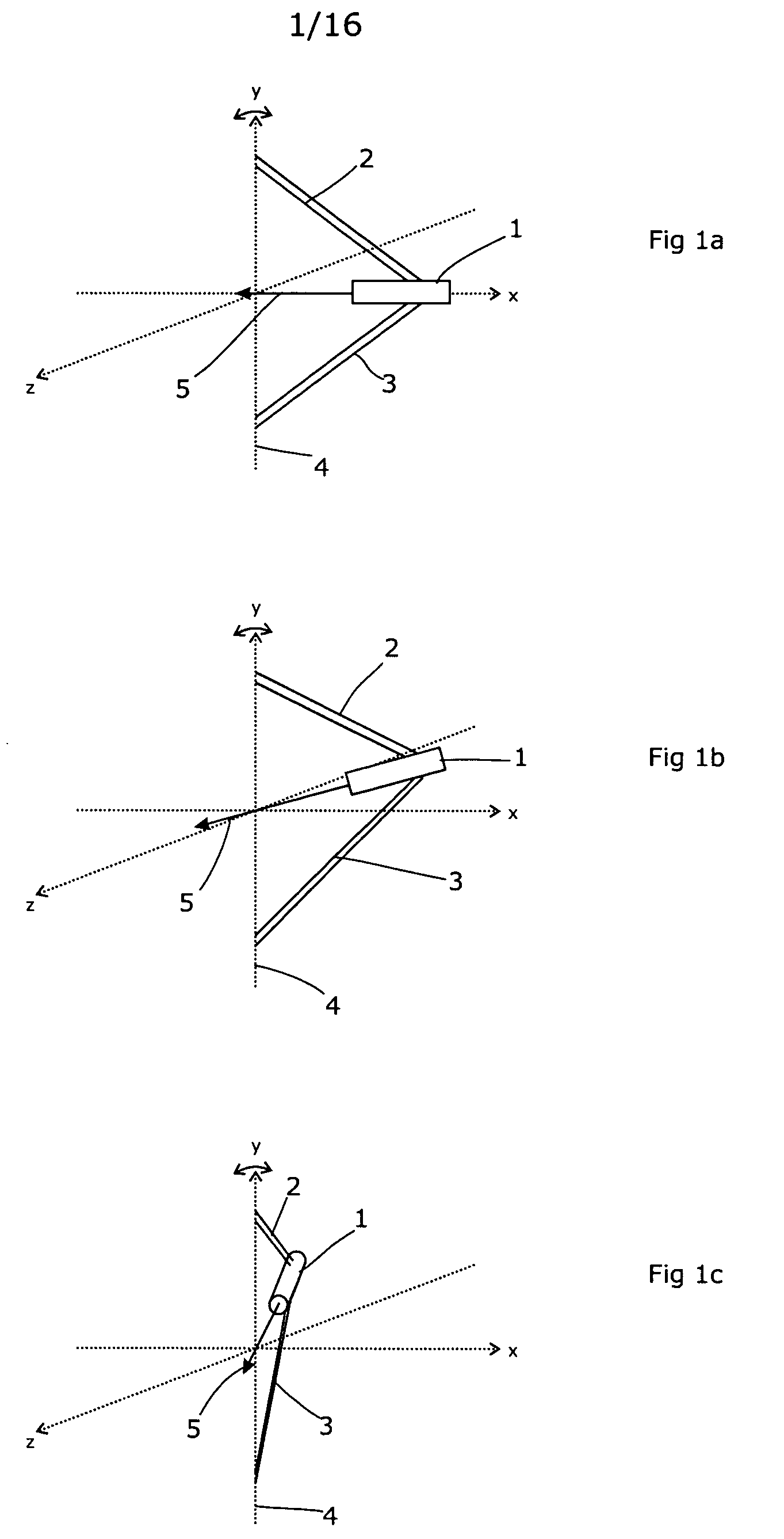
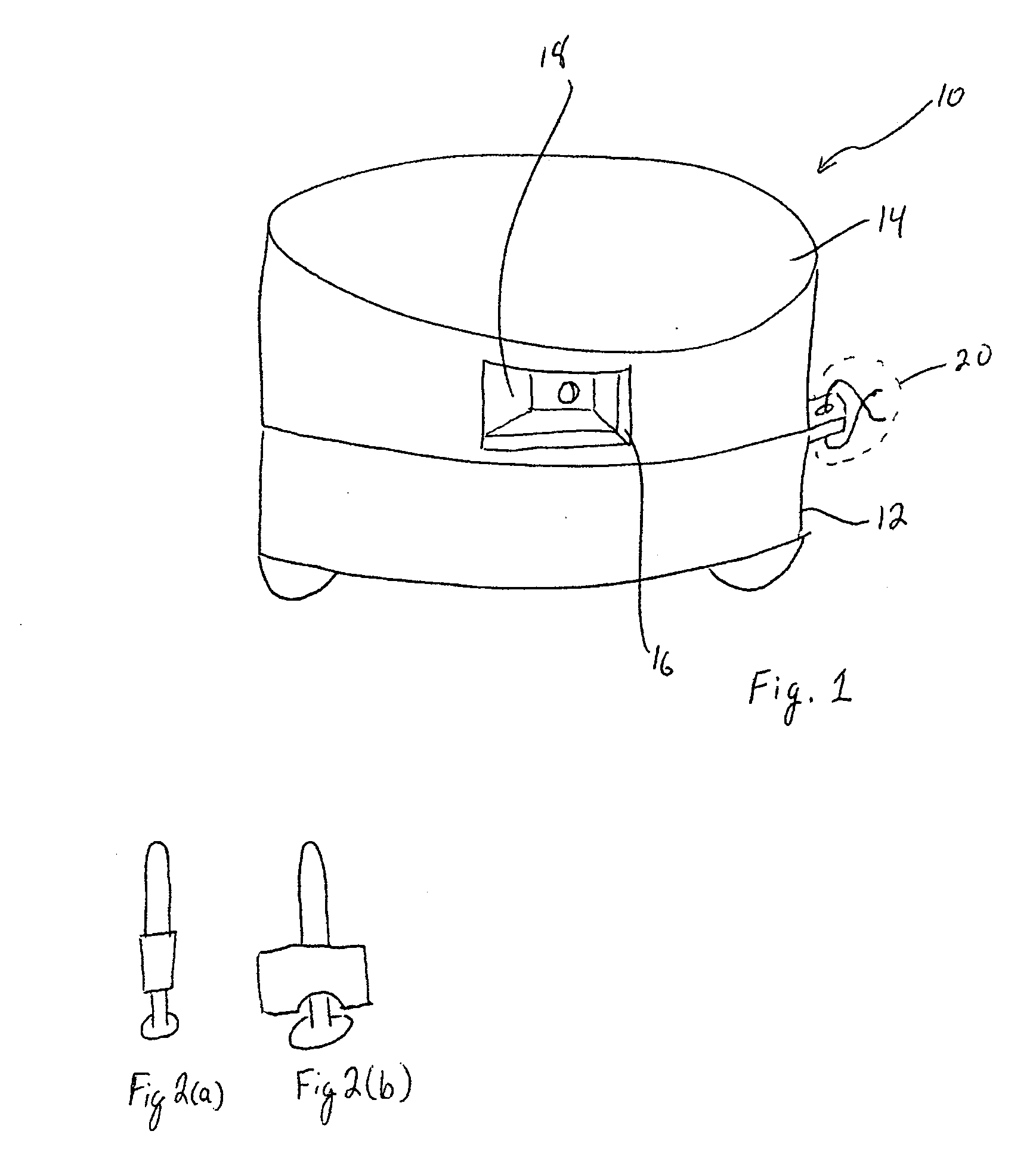
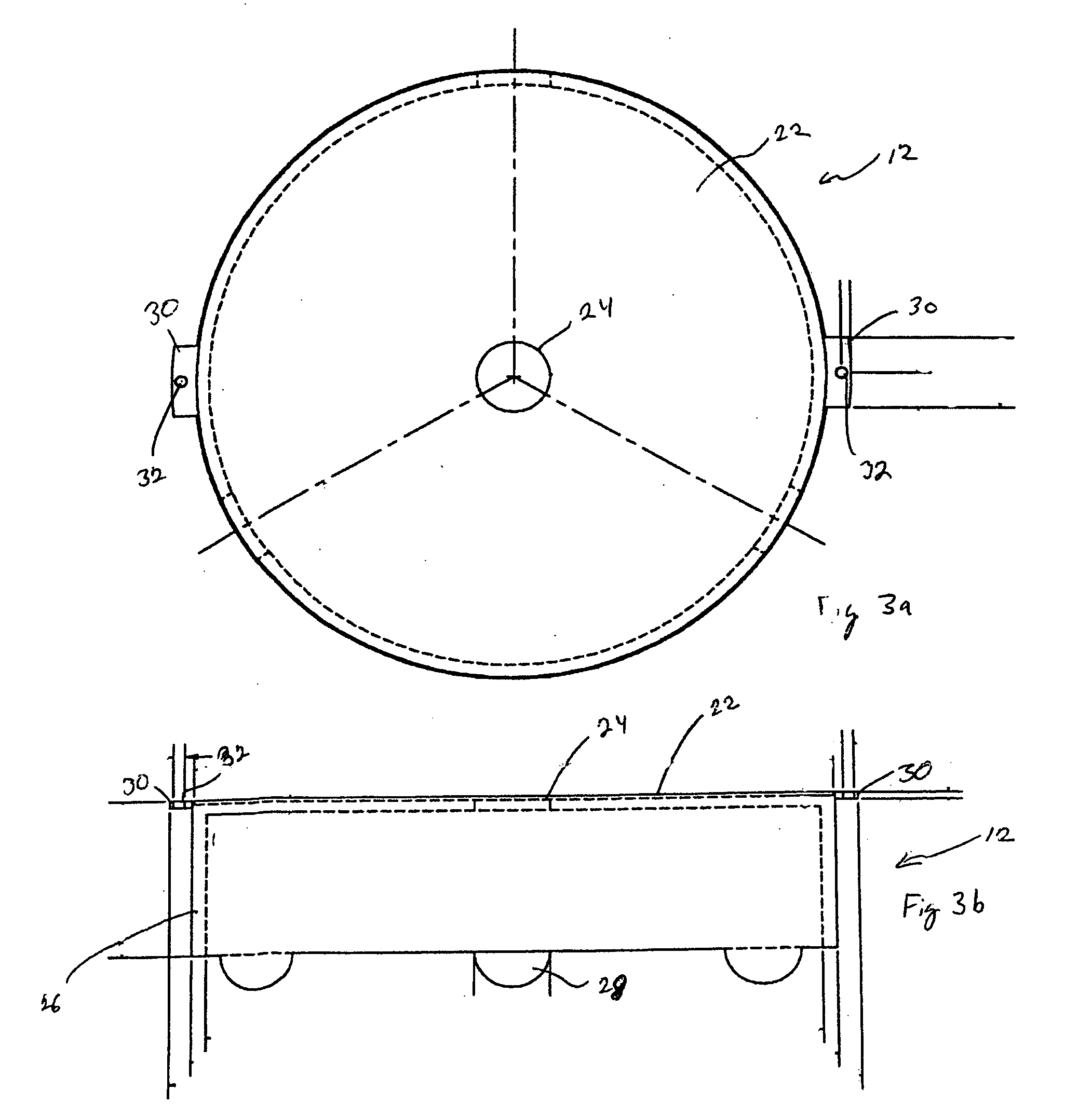
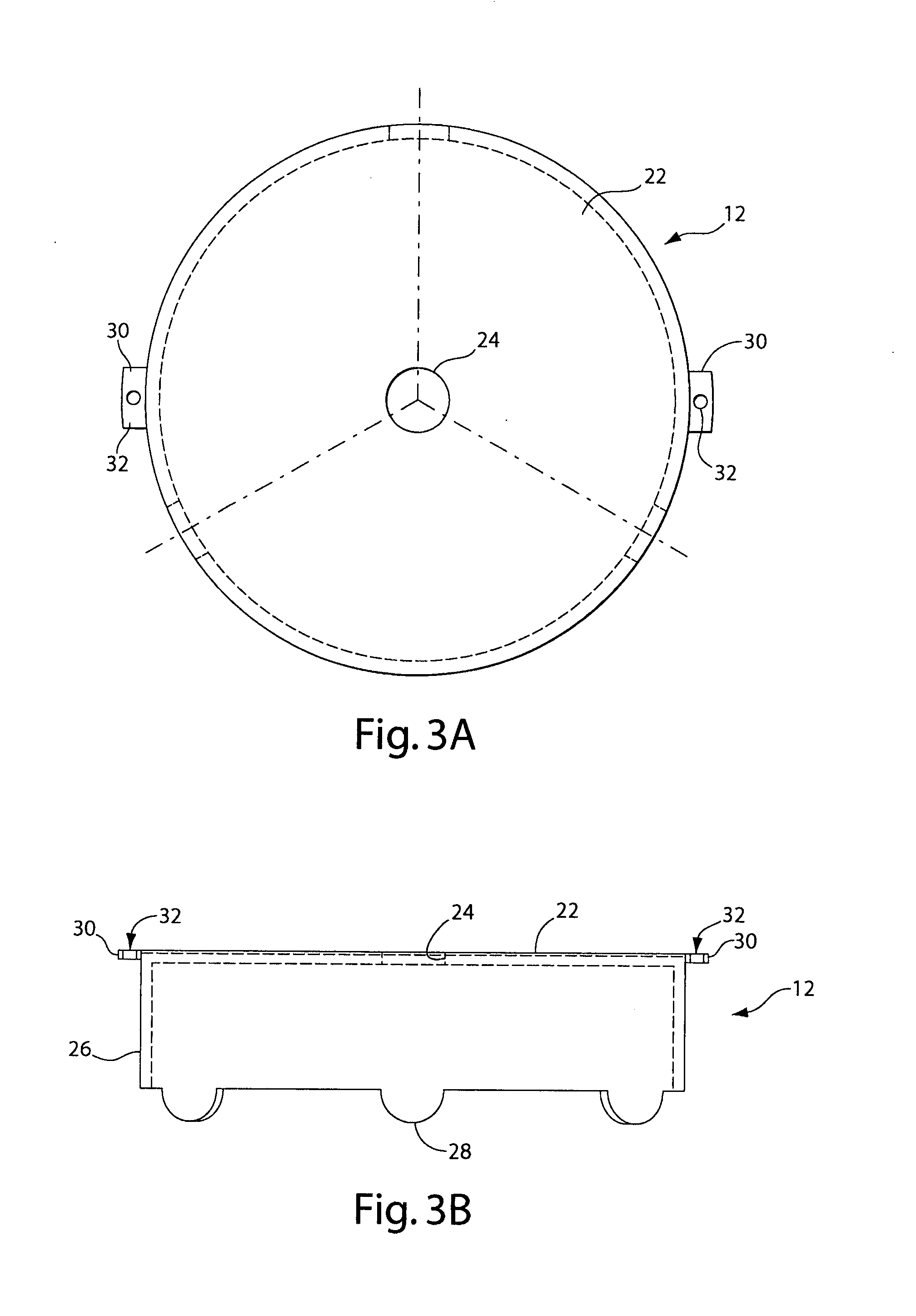
A radiation therapy / surgery device optimised to meet the needs of the Neurosurgeon is provided, i.e. one for the treatment of tumours in the brain. It combines the qualities of a good penumbra and accuracy, simple prescription and operation, together with high reliability and minimal technical support. The device comprises a rotateable support, on which is provided a mount extending from the support out of the plane of the circle, and a radiation source attached to the mount via a pivot, the pivot having an axis which passes through the axis of rotation of the support, the radiation source being aligned so as to produce a beam which passes through the co-incidence of the rotation axis and the pivot. It will generally be easier to engineer the apparatus if the rotateable support is planar, and more convenient if the rotateable support is disposed in an upright position. The rotation of the rotateable support will be eased if this part of the apparatus is circular. A particularly preferred orientation is one in which the radiation source is spaced from the rotateable support, to allow it to pivot without fouling the latter. It is thus preferred that the mount extends transverse to the support. In this way, the pivot axis is spaced from the rotateable support providing free space in which the radiation source can pivot. Another way of expressing this preference is to state that the pivot axis is located out of the plane of the rotateable support. To simplify the geometry of the device and the associated arithmetic, it is preferred both that the pivot axis is substantially perpendicular to the rotation axis, and that the beam direction is perpendicular to the pivot axis. It is preferred that the radiation source is a linear accelerator. The output of the radiation source is preferably collimated to conform to the shape of the area to be treated.
Owner:ELEKTA AB
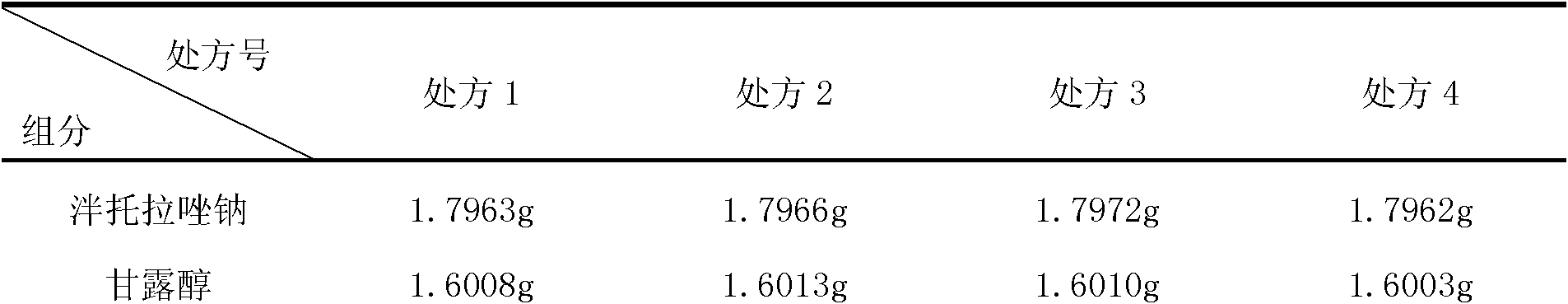
Freeze-dried powder injection of pantoprazole sodium and its preparation
ActiveCN1679563ALittle side effectsImprove stabilityOrganic active ingredientsPowder deliveryDisodium EdetateFreeze-drying
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
Pemetrexed disodium freeze-dried powder injection and preparation method thereof
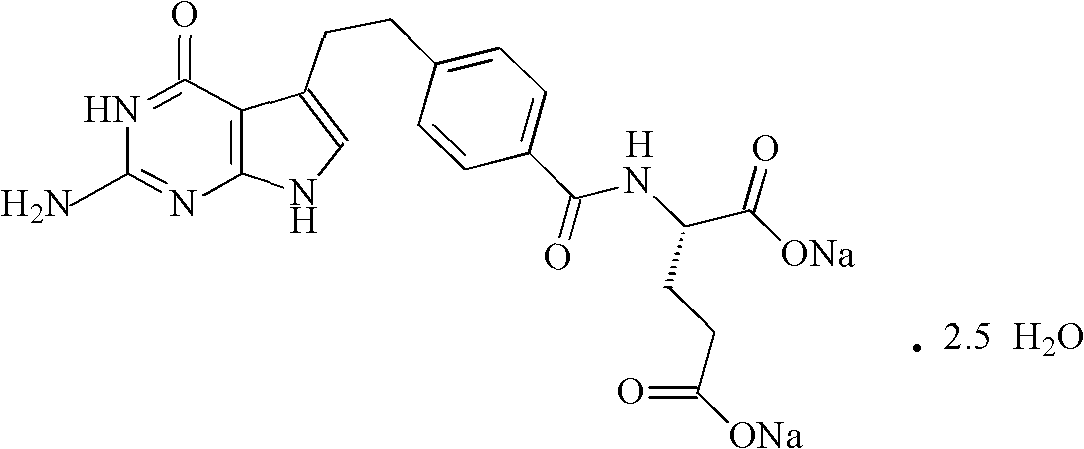
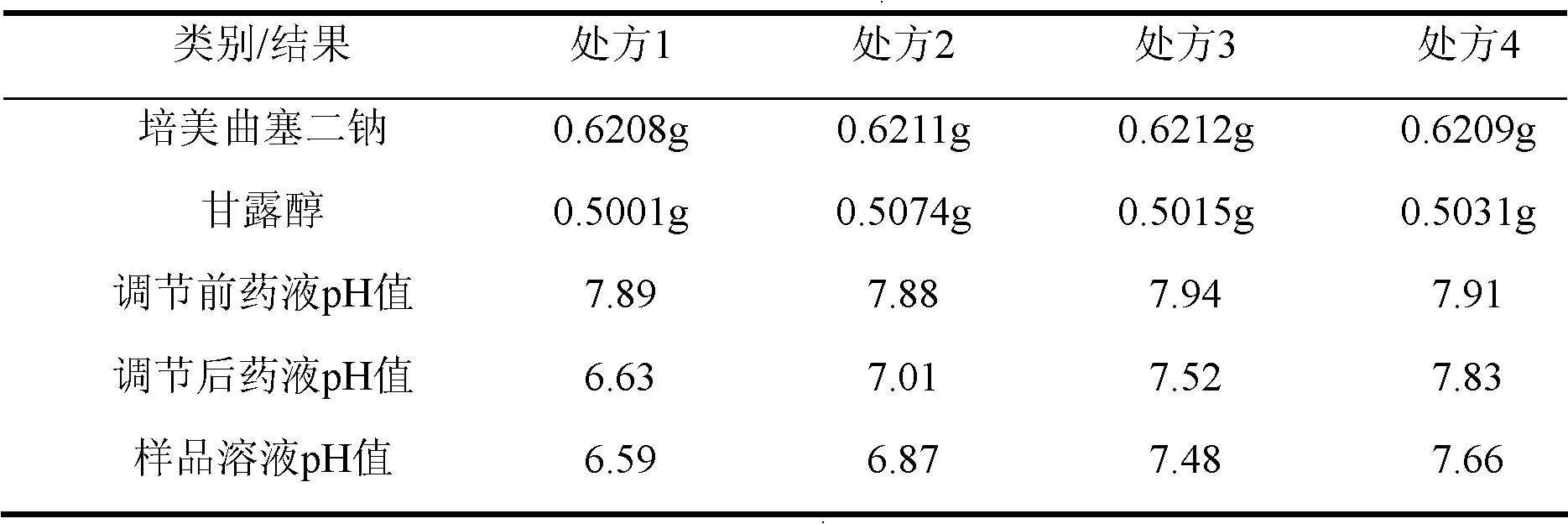
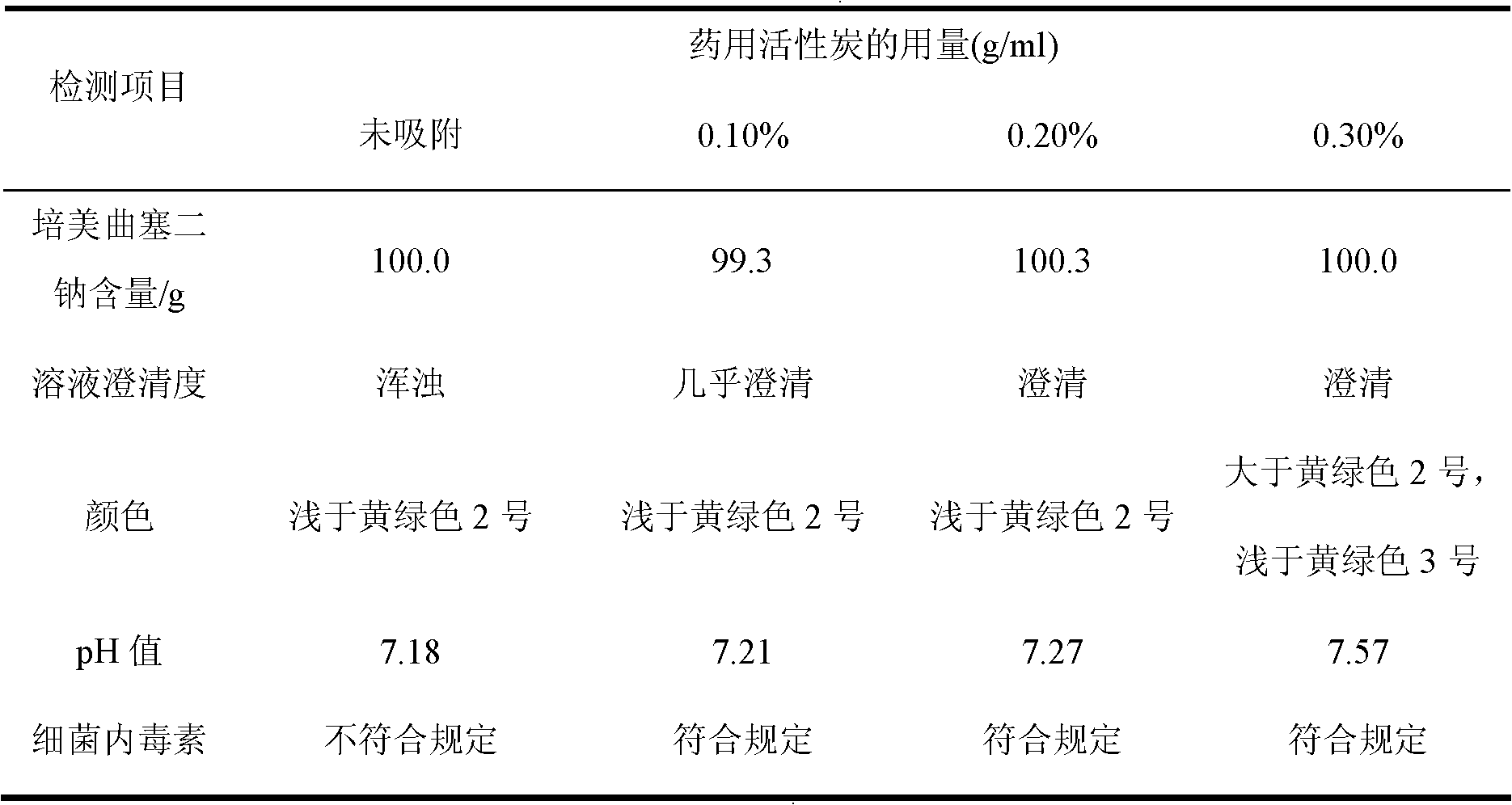
ActiveCN102106833AReduce adverse effectsSimple prescriptionOrganic active ingredientsPowder deliveryActivated carbonFreeze-drying
The invention belongs to the technical field of medication, and in particular relates to a pemetrexed disodium freeze-dried powder injection and a preparation method thereof. The pemetrexed disodium freeze-dried powder injection consists of pemetrexed disodium and mannitol, wherein the mass ratio of the mannitol to the pemetrexed disodium is (0.6-2.0):1. The preparation method comprises the following steps: adding injecting water into a liquid preparation tank; adding the pemetrexed disodium weighted according to the formula; stirring until the pemetrexed disodium completely dissolved; adding the mannitol; regulating the pH by utilizing a hydrochloric acid solution or a sodium hydroxide solution; adding activated carbon for decoloration; filtering to remove the carbon; finely filtering with a filter membrane; subpackaging; and freezing and drying. The pemetrexed disodium freeze-dried powder injection has excellent moldability; the appearance of the solution before freezing is clear; the frozen and dry product has good re-dissolubility; and the re-dissolved product has the advantages of good clarity, low impurity content, low moisture content, good stability and controllable quality.
Owner:HAINAN JINRUI PHARMA
Method and apparatus for treatment by ionizing radiation
ActiveUS7295648B2Improve accuracyEasy to operateMaterial analysis using wave/particle radiationRadiation/particle handlingBeam directionLight beam
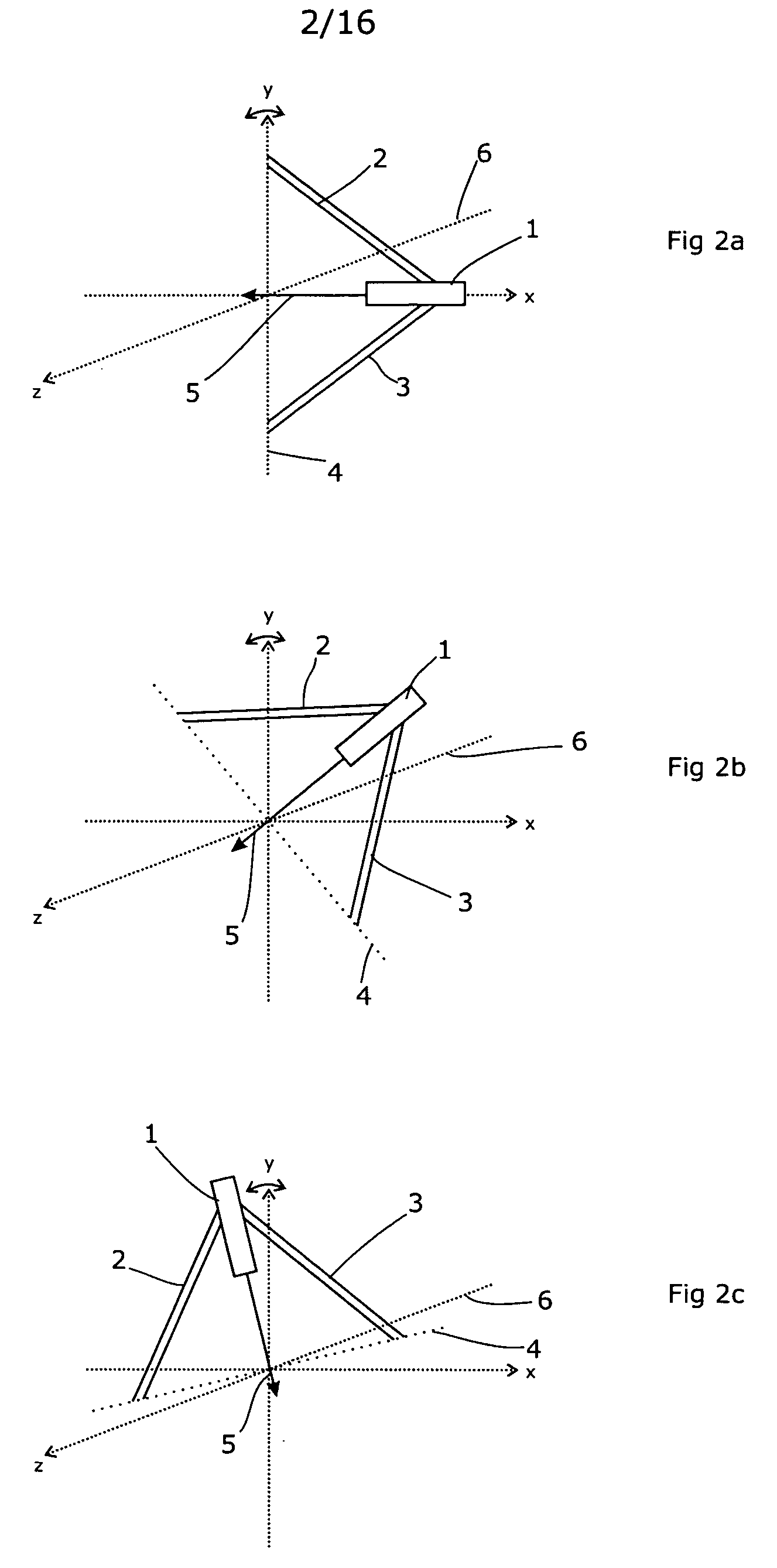
A radiation therapy / surgery device optimised to meet the needs of the Neurosurgeon is provided, i.e. one for the treatment of tumours in the brain. It combines the qualities of a good penumbra and accuracy, simple prescription and operation, together with high reliability and minimal technical support. The device comprises a rotateable support, on which is provided a mount extending from the support out of the plane of the circle, and a radiation source attached to the mount via a pivot, the pivot having an axis which passes through the axis of rotation of the support, the radiation source being aligned so as to produce a beam which passes through the co-incidence of the rotation axis and the pivot. It will generally be easier to engineer the apparatus if the rotateable support is planar, and more convenient if the rotateable support is disposed in an upright position. The rotation of the rotateable support will be eased if this part of the apparatus is circular. A particularly preferred orientation is one in which the radiation source is spaced from the rotateable support, to allow it to pivot without fouling the latter. It is thus preferred that the mount extends transverse to the support. In this way, the pivot axis is spaced from the rotateable support providing free space in which the radiation source can pivot. Another way of expressing this preference is to state that the pivot axis is located out of the plane of the rotateable support. To simplify the geometry of the device and the associated arithmetic, it is preferred both that the pivot axis is substantially perpendicular to the rotation axis, and that the beam direction is perpendicular to the pivot axis. It is preferred that the radiation source is a linear accelerator. The output of the radiation source is preferably collimated to conform to the shape of the area to be treated.
Owner:ELEKTA AB
Improved cataplasm ground-mass and use thereof
InactiveCN101416955AImprove adhesionGood molding effectPharmaceutical non-active ingredientsSheet deliveryCross-linkAnalysis method
The invention discloses an improved substrate of a cataplasma and the application thereof to loxoprofen sodium cataplasma. The substrate of the cataplasma adopts the combined application of two or more cross-linking agents and achieves the purposes of increasing the viscosity of a paste and improving the ductibility and moldability of the paste. The application of the substrate can reduce the variety and usage of thickening accessories or can increase the viscosity of the cataplasma to the extent required by a viscous force even without adding special thickening agents. The substrate has advantages in ensuring the good ductibility and moldability of the cataplasma and quickly establishing analytical methods, and the like, thus being significant for the further promotion and application ofthe cataplasma.
Owner:CHONGQING PHARMA RES INST
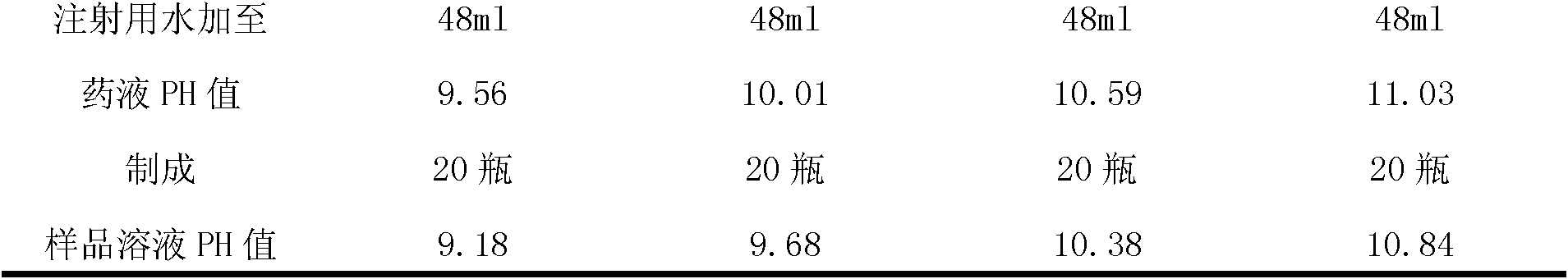
Pantoprazole sodium freeze-dried powder injection and preparation method thereof
ActiveCN102085190AShorten the secondary drying timeGood lookingOrganic active ingredientsPowder deliveryCLARITYFreeze-drying
The invention relates to a pantoprazole sodium freeze-dried powder injection and a preparation method thereof. The powder injection is prepared from pantoprazole sodium and mannitol, wherein the consumption ratio of the pantoprazole sodium to the mannitol is (1:0.8)-(1:1.6), and the PH value is 10.5-11.0. In the invention, by lowering the pre-freezing temperature, properly lowering the freezing temperature, maintaining the lowered freezing temperature for a proper time, properly shortening two-stage drying time and carrying out other adjustment processes, good appearance and quality of the product can be kept under the condition that the content of the mannitol is low, the processes are reliable and feasible, and the effect is obvious. The prepared product has low content of related substances and has controllable quality, and the freeze-dried product has good clarity and formability after being redissolved.
Owner:HAINAN JINRUI PHARMA CO LTD
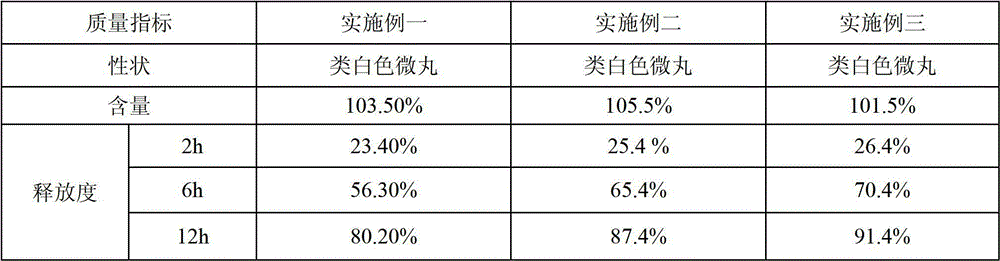
Vitamin C sustained-release pellets and method for preparing same
ActiveCN102908319AHigh drug loadingLarge particle sizeOrganic active ingredientsMetabolism disorderSustained release pelletsVitamin C
A vitamin C sustained-release pellet applied to the vitamin C sustained-release preparation field and a method for preparing the same are disclosed. The vitamin C sustained-release pellet is composed of a vitamin C sustained-release pill and a sustained-release coating, wherein the vitamin C sustained-release pill is composed of a mother nucleus and a lamination layer, or composed of a vitamin C and vitamin C pill accessory, or composed of vitamin C; the sustained-release coating is composed of a sustained-release coating material and a sustained-release coating accessory, or composed of the sustained-release coating material; the vitamin C sustained-release pill accessory is one or two selected from a filler and a binder; the sustained-release coating accessory is one or two selected from a plasticizer and an antisticking agent; the weight percentage content of the vitamin C in the mother nucleus is the same as that in the lamination layer; and the filler is one or several selected from microcrystalline cellulose, powdered sugar, starch, dextrin and lactose. The vitamin C sustained-release pellet disclosed by the invention is simple in prescription, free of metal-chelator or antioxidant, great in unit volume drug loading capacity, good in stability and capable of keeping sustained release for a long time; and the preparation method of the vitamin C sustained-release pellet is short in operation time and low in cost.
Owner:SHENYANG NO 1 PHARMA FACTORY DONGBEI PHARMA GRP
Trimetazidine dihydrochloride sustained-release tablet and preparation method thereof
InactiveCN102885795AThe solution is not easy to cleanSimple preparation stepsOrganic active ingredientsPharmaceutical non-active ingredientsTrimetazidine DihydrochlorideSustained Release Tablet
The invention discloses a trimetazidine dihydrochloride sustained-release tablet and a preparation method thereof. The trimetazidine dihydrochloride sustained-release tablet comprises the following constituents in percentage by mass: 5-60% of trimetazidine dihydrochloride, 10-25% of sustained-release framework material, 1-8% of adhesive, 20-80% of filler, 0.1-5% of glidant and 0.2-3% of lubricant. According to the trimetazidine dihydrochloride sustained-release tablet, medicine can be slowly and uniformly released by adding the sustained-release framework material, so as to achieve regulation and control for a release speed, reduce the peak-valley ratio of the medicine, improve the efficacy, reduce the toxic and side effects of the medicine, reduce daily medicine-taking times and enhance the compliance of the patient on the medicine. The preparation method of the trimetazidine dihydrochloride sustained-release tablet disclosed by the invention is simple in process, does not need specially process production equipment, and is low in cost and good for batch amplification and industrialized production for products.
Owner:AC PHARMA CO LTD
Omeprazole sodium freeze-dried powder injection and preparation method thereof
ActiveCN101703483AReduce the risk of adverse reactionsComply with the requirements of human intravenous injectionPowder deliveryOrganic active ingredientsOmeprazole SodiumFiltration
The invention discloses an omeprazole sodium freeze-dried powder injection and a preparation method thereof. The omeprazole sodium freeze-dried powder injection contains an active ingredient, namely, omeprazole sodium monohydrate, and auxiliary materials, namely, calcium disodium edetate and sodium hydroxide. The preparation method of the omeprazole sodium freeze-dried powder injection is characterized by comprising the following steps: weighing the calcium disodium edetate of prescription amount and dissolving the calcium disodium edetate in water for injection, stirring, dissolving, and regulating pH value to 10.0-12.0 by using 10% of sodium hydroxide solution; weighing omeprazole sodium of the prescription amount and adding the omeprazole sodium in the mixture, stirring at room temperature for dissolution, supplementing and adding the water for injection to full amount; adding active carbon, stirring at room temperature for decoloration and endotoxin removal, conducting rough filtration to remove carbon firstly, and then conducting refining filtration by using a filter membrane of 0.22 Mum; taking refining filtrate to test intermediate, conducting encapsulation after meeting requirements; and freeze-drying and unboxing, thus obtaining the omeprazole sodium freeze-dried powder injection. The freeze-drying technology of the omeprazole sodium freeze-dried powder injection takes temperature below minus 40 DEG C as pre-freezing temperature; after pre-freezing for at least two hours, sublimation is started, wherein the sublimation temperature is 5-12 DEG C, the sublimation time is over 14 hours; and then drying is conducted for over 2 hours at the temperature of 20-35 DEG C. Unboxing is carried out after a stopper is added and a cover is put in place, thus obtaining the finished product of the omeprazole sodium freeze-dried powder injection.
Owner:HAINAN LEVTEC PHARMA
Immunomodulator slow-release preparation and preparation method thereof
ActiveCN103610658AProlong the action timeUniform and constant action timeOrganic active ingredientsPill deliveryBlood concentrationProlonged-release tablet
The invention discloses an immunomodulator slow-release preparation and a preparation method thereof. A lenalidomide slow-release tablet is composed of a slow-release layer and an optional quick-release layer, wherein the slow-release layer contains active ingredients of lenalidomide and a slow-release framework material simultaneously; the quick-release layer does not contain the slow-release framework material. The lenalidomide slow-release tablet disclosed by the invention is capable of slowly and uniformly releasing medicines by virtue of the slow-release framework material, so as to reduce the release speed, delay the time to peak, prolong the action time of lenalidomide, and provide a uniform and constant blood concentration. Moreover, The lenalidomide slow-release tablet disclosed by the invention is simple in prescription and excellent in quality stability; the preparation process is simple to operate, free from special treatment and production equipment, low in production cost, and beneficial to batch-enlarged industrial production for the product; the preparation method is high in yield, the granulation and crushing procedures are simple and practicable to operate, the intermediate material is good in stability, flowability, compressibility and content uniformity, and completely meets the requirements of tabletting, and the surface of the prepared tablet is smooth and beautiful.
Owner:AC PHARMA CO LTD
Composition for repairing skin barriers
ActiveCN105748315APromote absorptionEasy to prepareCosmetic preparationsToilet preparationsMedicineMedical prescription
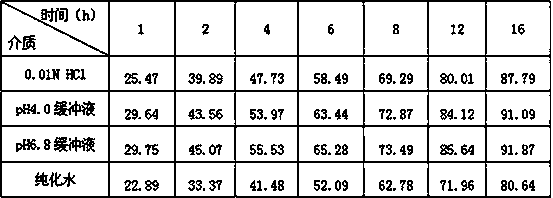
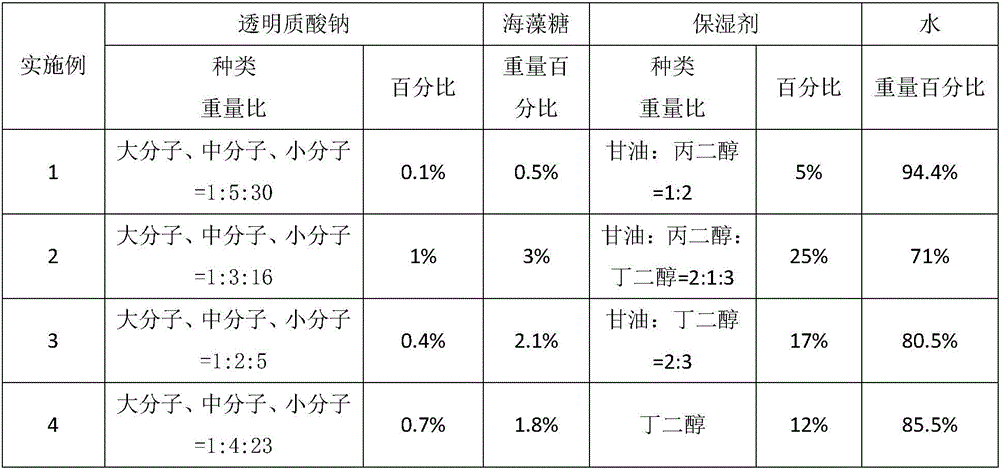
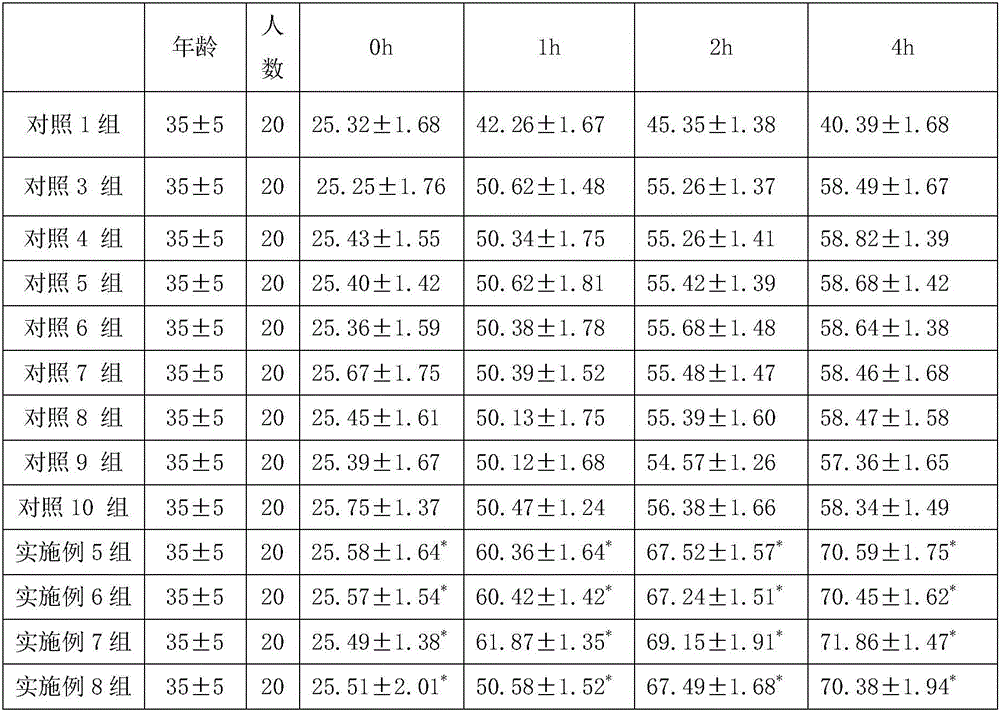
The invention discloses a composition for repairing skin barriers. The composition is prepared from the following raw materials in percentage by weight: 0.1-1% of sodium hyaluronate, 0.5-3% of trehalose, 5-25% of a humectants and 71-94.4% of water. Experiments prove that the composition for repairing skin barriers, disclosed by the invention, can effectively relieve and treat the symptoms of redness and swelling, hot pain, skin shedding, pruritus and the like of skin caused by beautifying means of laser beautification, tartaric acid skin change and the like, and is good in absorption effect, simple in preparation method, short in production cycle, compendious in prescription, and low in cost.
Owner:TIANJIN JIASHITANG SCI & TECH
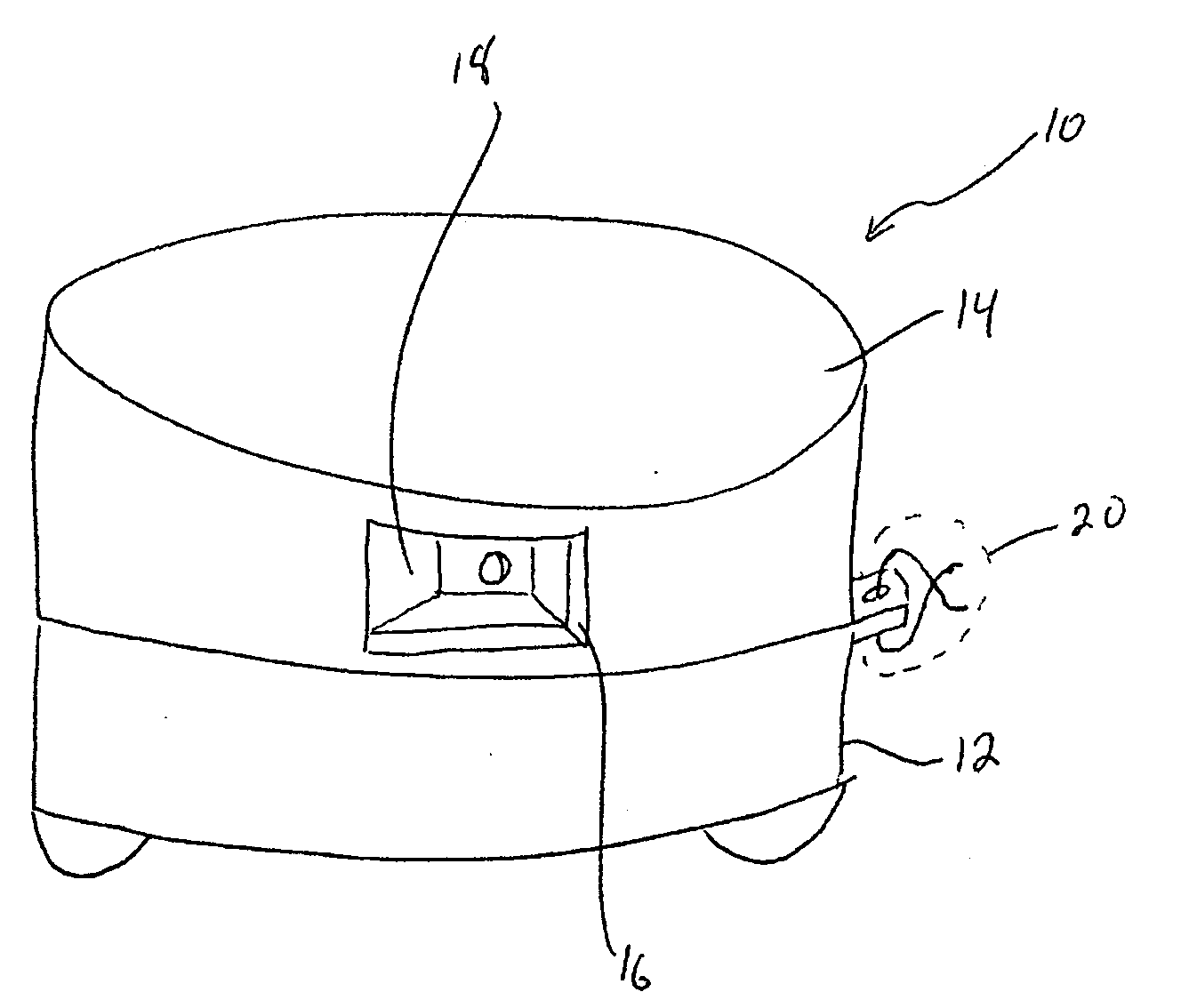
Dispensing device
InactiveUS20080017658A1Simple prescriptionSimple equipmentOral administration devicePackagingDrugBiomedical engineering
Owner:JAVELIN PHARMA INC
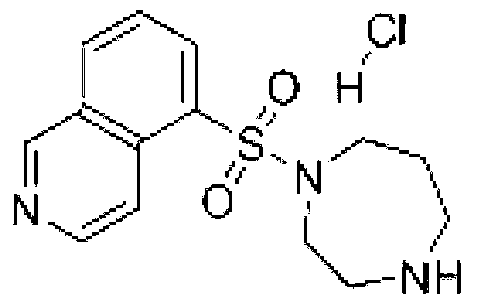
Fasudil hydrochloride injection composition and its preparation method
ActiveCN103222953AReduce contentSimple prescriptionOrganic active ingredientsPharmaceutical delivery mechanismSodium calcium edetateMedical prescription
The invention provides a novel Fasudil hydrochloride injection composition and its preparation method. A prescription and a preparation technology are simple. As a metal ion chelating agent, sodium calcium edetate in the prescription can be used to reduce degradation of the product under a high-light condition. In addition, the preparation technology provided by the invention is easy to operate. The quality of the prepared product is controllable, and the product has good stability and meets regulations of SFDA (State Food and Drug Administration).
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Drug composition containing hydroxyfasudil compound
ActiveCN103040738ASimple prescriptionReduce symptoms of hypotensionPowder deliveryOrganic active ingredientsMethionine biosynthesisMedical prescription
The invention provides a drug composition containing a hydroxyfasudil compound, which comprises hydroxyfasudil, sodium dihydrogen phosphate, dextran 40, methionine and water for injection. A preparation method of the drug composition comprises the following steps of stirring and dissolving sodium dihydrogen phosphate, methionine, hydroxyfasudil and dextran with the water for the injection, adding active carbon for decoloration, filtering removing carbon, and then conducting filter sterilization and autoclaved sterilization. The drug composition containing hydroxyfasudil is simple in prescription and reduces the possibility of hypotension, the stability is improved by reasonable configuration, and application and popularization of a hydroxyfasudil injection is facilitated.
Owner:罗诚
Irinotecan hydrochloride liquor type injection and preparation method thereof
ActiveCN101953781AReduce drug riskImprove product qualityOrganic active ingredientsPharmaceutical delivery mechanismStabilizing AgentsChemistry
The invention discloses an irinotecan hydrochloride liquor type injection and a preparation method thereof, which is characterized in that the injection contains buffer salt, no stabilizing agent, i.e. sorbierite. In addition, the invention also discloses a preparation method of the irinotecan hydrochloride injection. The irinotecan hydrochloride injection obtained by utilizing the method has stable quality, pharmacy safety and low production cost.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
Novel controlled release capsule and preparation method thereof
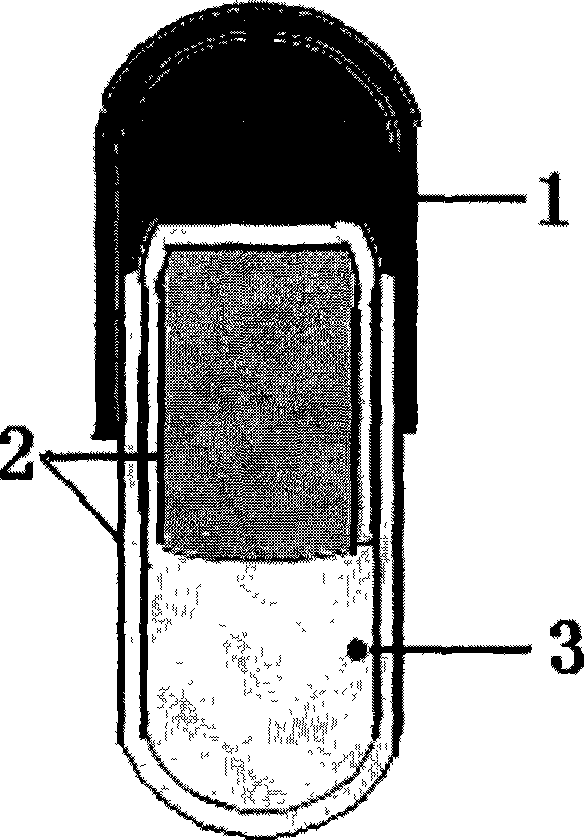
InactiveCN101485644AQuick effectBroaden your optionsPharmaceutical delivery mechanismOsmotic pumpControlled Release Capsule
The invention relates to a novel controlled-release capsule and a method for preparing the same. The capsule has the advantages of simultaneously having a quick release part and a controlled-release part, along with simple preparation process and wide drug selection range. The novel controlled-release capsule consists of a capsule cap and a controlled-release capsule body, wherein the capsule cap is a common capsule shell sold in markets, the controlled-release capsule body is an osmotic pump controlled-release capsule which can be used to encapsulate chemicals, traditional Chinese medicines, biological product medicines and the like, and the medicines are packaged in the capsule cap and the controlled-release capsule body respectively. Tests show that the capsule preparation can quickly release the medicines, and the release rate of the remained medicines is constant.
Owner:WENZHOU MEDICAL UNIV
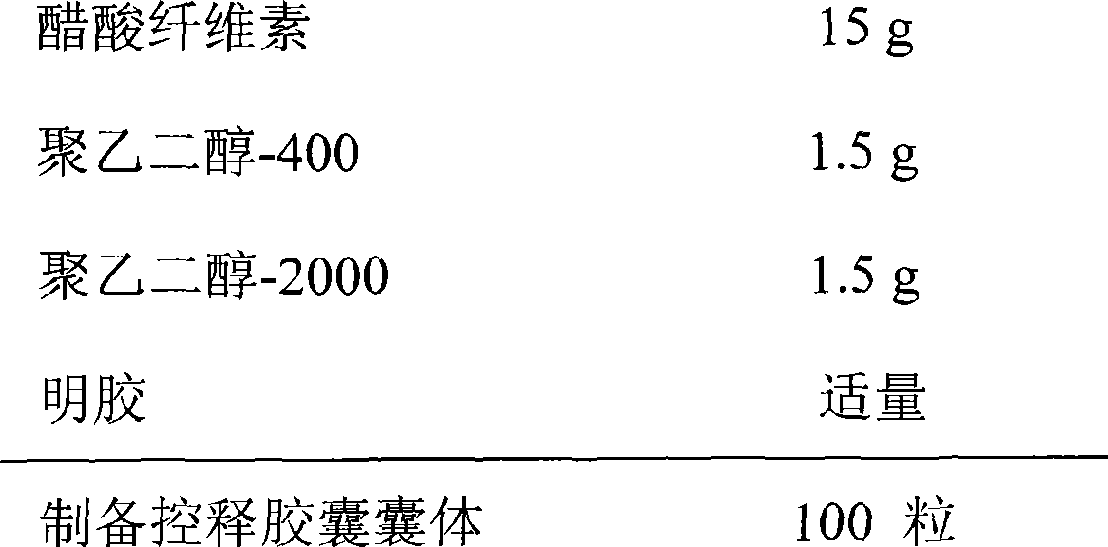
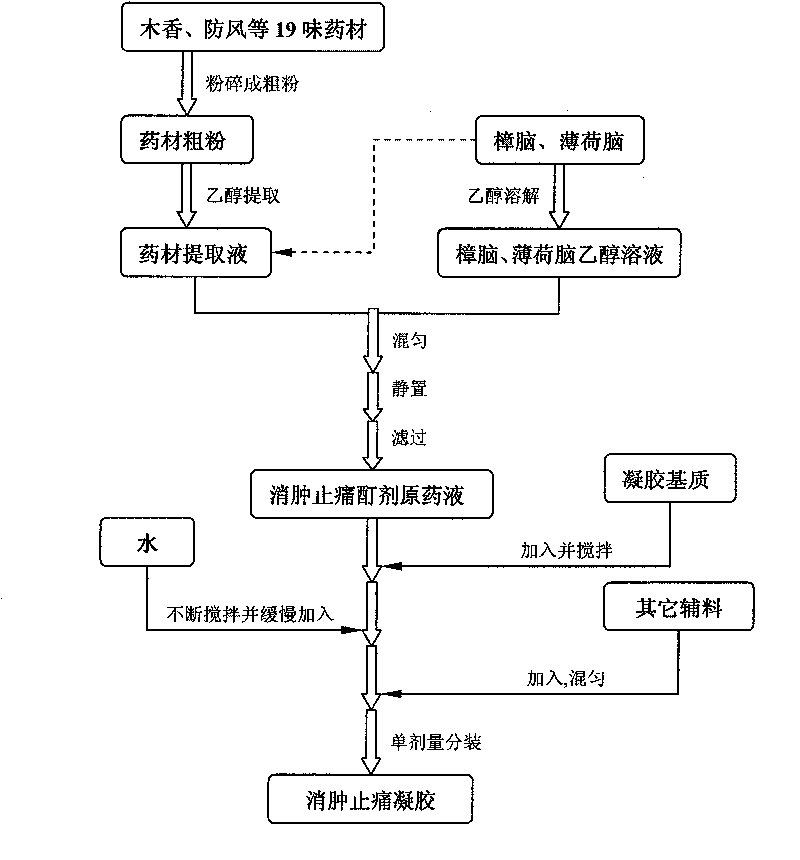
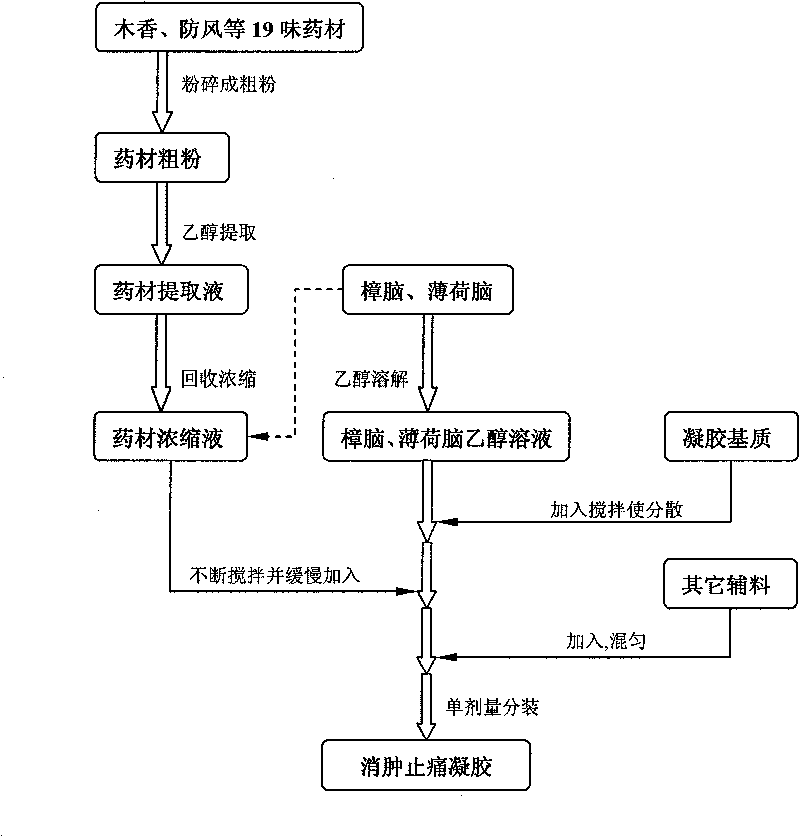
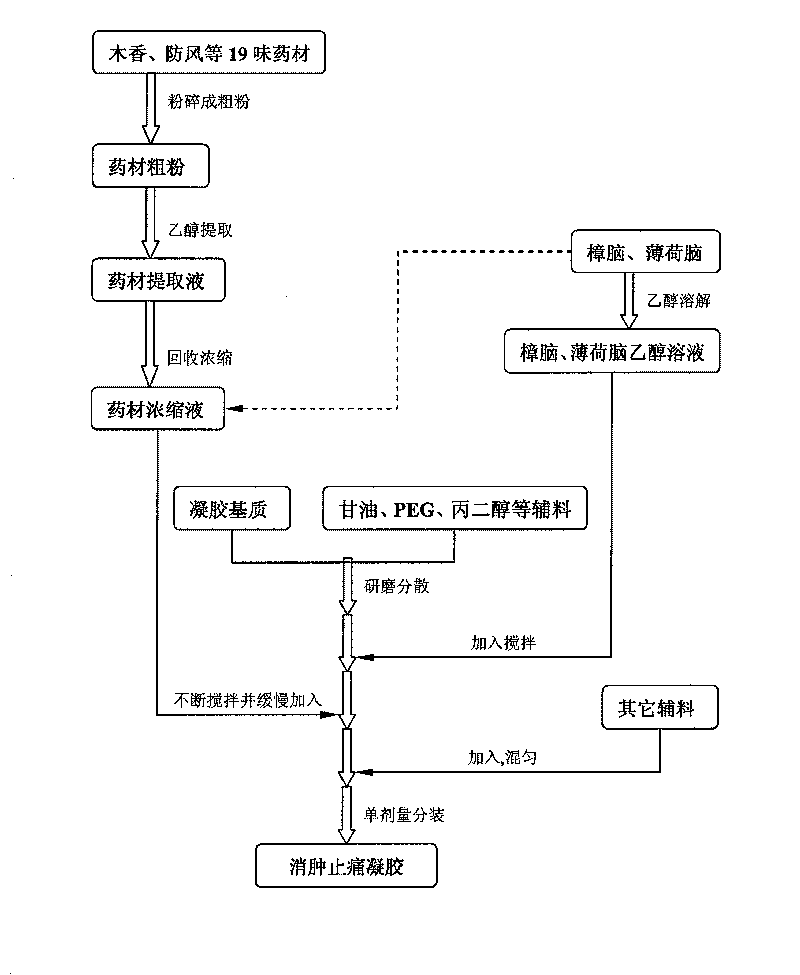
Swelling reducing and pain easing gel and preparation method thereof
InactiveCN101757522AReduce manufacturing costReduce dosageOrganic active ingredientsAntipyreticRheumatismPharmaceutical Adjuvants
The invention relates to swelling reducing and pain easing gel and preparation method thereof. The gel mainly contains swelling reducing and pain easing tincture liquid medicine, gel substrate and other pharmaceutical adjuvant and has the characteristics of small dosage of the adjuvant and good stability. The gel acts locally, has the functions of stimulating blood circulation and causing the muscles and joints to relax, reducing swelling and easing pain, and is used for curing injuries from falls, rheumatism, unknown swelling and toxin, and parotitis swelling. The gel has the advantages of long dwell time, durable effect, simple formulation process, low production cost and strong operability in industrial production.
Owner:CHONGQING PHARMA RES INST
Budesonide nano crystallizing preparation and preparation method thereof
InactiveCN101961320AImprove stabilityPreparation process is easy to scale upOrganic active ingredientsPowder deliverySolubilityOral medication
The invention relates to application of a new medicament delivery system in budesonide delivery, in particular to a preparation method for a budesonide nano crystallizing mixed suspension and application thereof. Budesonide has poor water solubility and low bioavailability by oral administration, seriously influences the application in clinic and increases the economical burden of patients. Accordingly, the invention provides the preparation method of the budesonide nano crystallizing mixed suspension for more favorably solving the problem of poor water solubility. The budesonide nano crystallizing mixed suspension can rapidly dissolve effective components, shorten the action time, reduce adverse reaction and is safe and convenient for use. The invention provides the budesonide nano crystallizing mixed suspension which overcomes the defects in current clinic practice and has a plurality of other advantages.
Owner:SHANDONG XINBO PHARMA R&D
Oral solution containing ambroxol hydrochloride and salbutamol sulfate
InactiveCN104622855ASimple prescriptionGuaranteed stabilityOrganic active ingredientsPharmaceutical delivery mechanismDiseaseMedicine
The invention provides an oral solution containing ambroxol hydrochloride and salbutamol sulfate, and belongs to the technical field of medicines. An auxiliary material of the oral solution mainly comprises preservatives and corrigents. The oral solution is used for treating respiratory system diseases such as acute and chronic bronchitis, asthmatoid bronchitis and bronchial asthma, has a simple prescription, a good medication effect and a good taste, and is quick in response.
Owner:CP PHARMA QINGDAO CO LTD
Chinese medicine composition for reducing blood fat
ActiveCN102058761AGood blood fat-lowering effectSimple prescriptionMetabolism disorderPlant ingredientsMedicinal herbsMedical prescription
The invention provides a Chinese medicine composition for reducing blood fat, which comprises the following Chinese medicinal herbs in parts by weight: 12-25 parts of root of common peony, 3-15 parts of fruit of trifoliate orange and 3-15 parts of allium macrostemon. The composition has better blood fat reducing efficacy and has the advantages of simple prescription and low cost. According to the extraction, separation and preparation technology of the modern Chinese medicine, the Chinese medicine composition can be prepared into a reasonable preparation.
Owner:ZHEJIANG UNIV +1
Dispensing device
InactiveUS20080054008A1Simple prescriptionSimple equipmentOral administration devicePackagingTime controlBiomedical engineering
Owner:JAVELIN PHARMA INC
Drug for treatment of migraine
InactiveCN103417945ASimple drug prescriptionLow priceHeavy metal active ingredientsNervous disorderSoybean sproutStalactite
The invention relates to drug for the treatment of migraine. The drug is made with, by weight, capillary wormwood herb, tree peony bark, leaf of paniculate microcos, rhizome of broadleaf common valeriana, Lapis Chloriti, common coltsfoot flower, pummelo peel, Chinese thorowax root, Divaricate Saposhnikovia root, sea-ear shell, Turpinia leaf, rhizome of oriental water plantain, Chinese starjasmine stem, eucommia bark, Zaocys dhumnades, Szechwan chinaberry fruit, gordon euryale seed, chingma abutilon seed, rhizome of paniculate Bolbostemma, small centipeda herb, indigoplant leaf, Philippine violet herb, Szechuan lovage rhizome, pine nodular branch, scorpion, Japanese ampelopsis root, stalactite, fruit of Polygonum Orientale, dried black soybean sprout, wild buckwheat rhizome, Chinese honey locust, Chinese honeylocust spine, Chinese Torreya seed, common lophatherum herb, tabasheer, long pepper, Psammosilene Tunicoides root, dried longan pulp, common Jasminorange leaf and twig, fruit of Rangoon creeper, humifuse euphorbia herb, Chinese wax gourd peel, manyinflorescenced sweetvetch root, longtube ground ivy herb, ophicslcite, Entadae stem, cockscomb flower, dried ginger, lotus leaf and common dayflower herb. A prescription of the drug is simple; the drug is cheap, has specific curative effect, highly suitable for patients, and applicable to large-scale clinical popularization.
Owner:潘德利
Agent for disinfection of operating room
The invention relates to an agent for disinfection of an operating room. The agent is prepared by the following raw materials in parts by weight: 4-8 parts of rhizoma zingiberis, 6-10 parts of semen brassicae, 1-5 parts of liquorice, 1-3 parts of euphorbia kansui, 1-5 parts of euchresta japonica, 3-6 parts of medulla stachyuri, 1-5 parts of acanthopanax, 10-15 parts of saposhnikovia divaricata, 3-7 parts of cortex albiziae, 10-20 parts of notopterygium root, 3-6 parts of radix stemonae, 10-15 parts of cinnamon, 4-8 parts of endothelium corneum gigeriae galli, 1-5 parts of tangerine peel, 1-3 parts of radix ophiopogonis, and 3-7 parts of cocklebur fruit. The agent for the disinfection of the operating room is simple in prescription, low in price, and definite in curative effect, and is suitable for large-scale popularization in the operating room.
Owner:周静
Parecoxib sodium pharmaceutical composition for injection and preparation method thereof
The invention provides a parecoxib sodium pharmaceutical composition for injection and a preparation method thereof. The parecoxib sodium pharmaceutical composition for injection has the advantages that prescription of the parecoxib sodium pharmaceutical composition for injection is simple, dosage of disodium hydrogen phosphate is decreased greatly as compared with that of the prior art, and requirement for injection medicine safety is met well; since parecoxib sodium is in the shape of crystal, time for dissolution is shortened and time for dosing is then shortened, quality stability is guaranteed, and production efficiency is improved; by a rapid freezing process during preparation, free-dried products maintain good pore structure with quite uniform granularity which is favorable for complete sublimation of moisture; since a nitrogen charge operation is omitted, production procedure is simplified, cost is reduced, and socialized mass production is facilitated.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Dragon blood nano medicament crystallized preparation and preparation method thereof
InactiveCN102579737AImprove utilizationGood treatment effectPowder deliveryAntipyreticFreeze-dryingSuspending Agents
The invention discloses a dragon blood nano medicament crystallized preparation and a preparation method thereof, and relates to the field of medicinal preparation, and contains a dragon blood, a stabilizing agent and a mixed solvent, wherein the stabilizing agent contains a surfactant and a suspending agent, and the weight ratio of each component is as follows: the dragon blood 0.01-5.0g; the surfactant 0.01-2.0 g; the suspending agent 0.002-1.0 g; the mixed solvent 0.01-5.0g. The preparation technology of the dragon blood nano medicament crystallized preparation has the advantages of easy enlargement, simple prescription, improved medicament stability after freeze drying and reduced administration volume; the medication is prepared into an oral nanometer crystallized suspension for improving bioavailability, reducing administration dosage and saving resource effectively.
Owner:TAISHAN MEDICAL UNIV
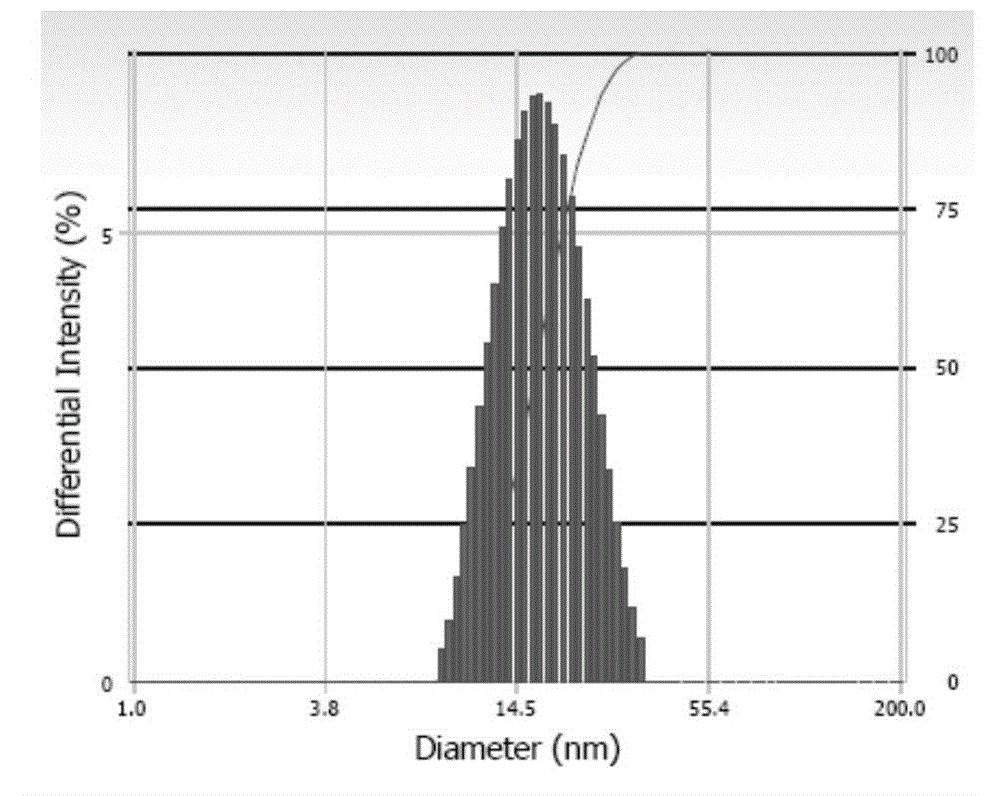
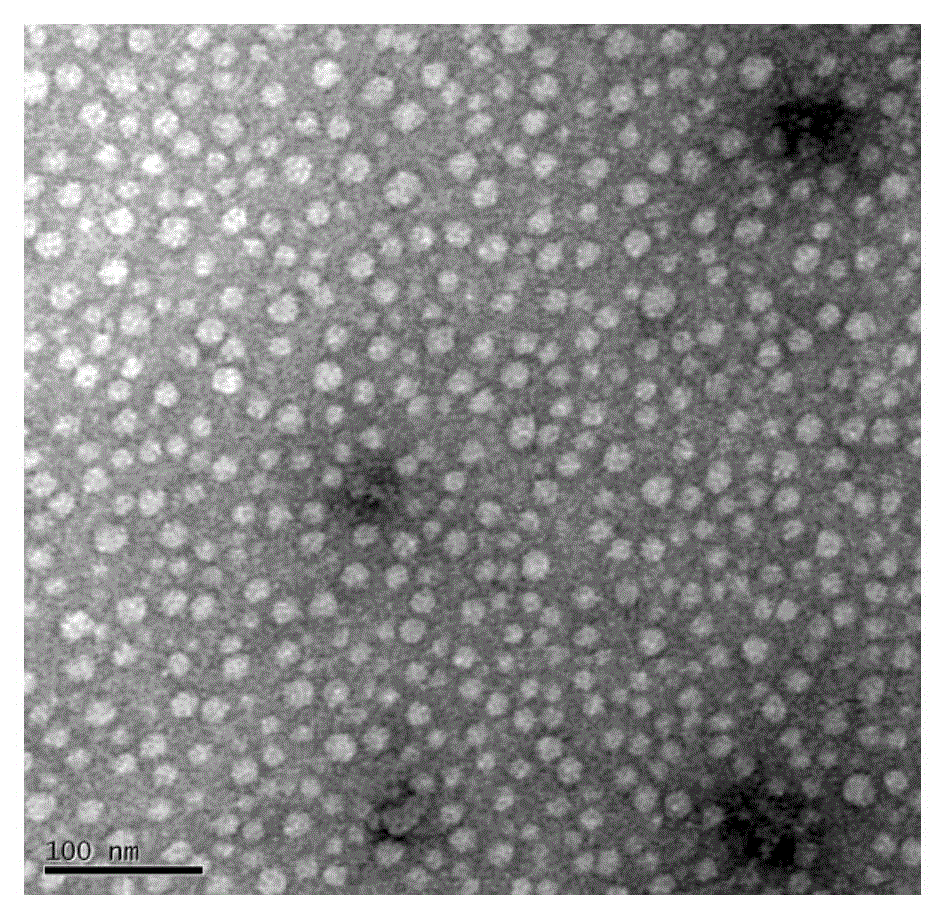
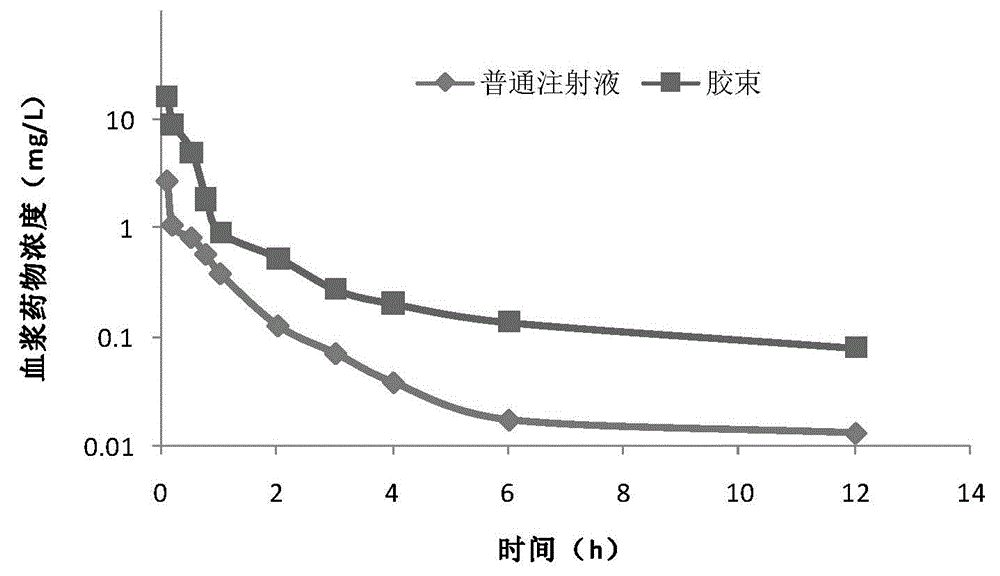
Andrographolide polymer micelle, preparation method and medicinal application thereof
InactiveCN104415029ASolve the problem that the drug cannot be injected directlyImprove bioavailabilityPowder deliveryOrganic active ingredientsTert butylMicelle
The invention relates to a novel micelle drug delivery system formed by an amphipathic segmented copolymer and andrographolide. The amphipathic segmented copolymer includes a hydrophilic chain segment and a hydrophobic chain segment, wherein the hydrophobic chain segment is terminated by a hydrophobic group being one of an acetyl group, a tert-butyryl group, a tert-butyl acetyl group, a benzoyl group, an amino acid residual group or an amino acid derivative residual group. Not only is compatibility between a medicine molecule and the hydrophobic chain segment in the segmented copolymer improved and an acting force therebetween enhanced, but also a larger space is provided for accommodating the molecular molecule. The micelle can limit the medicine molecule within a core of the micelle more effectively so that the medicine molecule is not liable to dissolve out, thereby obtaining the drug delivery micelle being high in stability.
Owner:CHANGZHOU TARGET MEDICINE TECH CO LTD
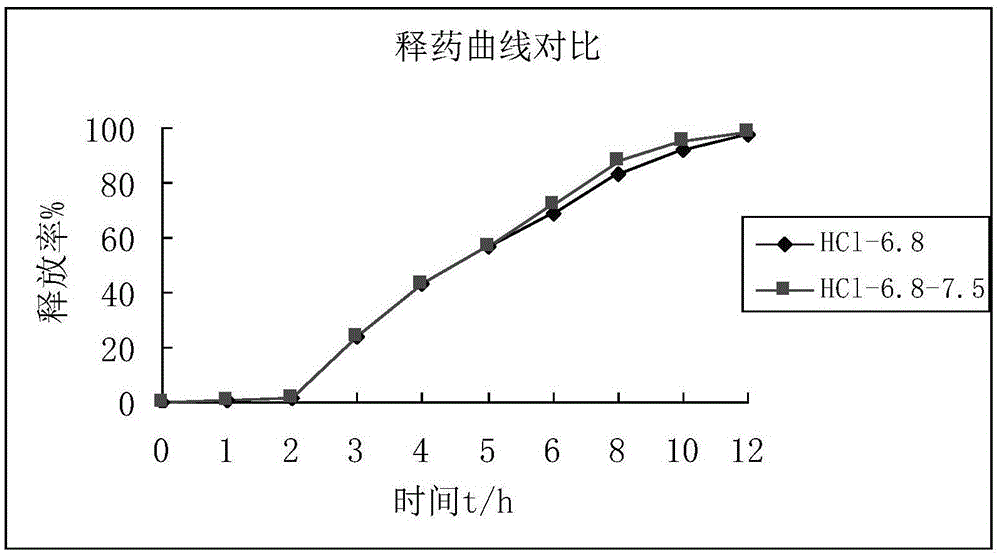
Mesalazine sustained-release pellets, preparation method thereof and mesalazine sustained-release capsule
ActiveCN105456223ASimple prescriptionHigh drug loadingOrganic active ingredientsDigestive systemSustained release pelletsProcedure Agents
The invention provides mesalazine sustained-release pellets, a preparation method thereof and a mesalazine sustained-release capsule. The preparation method comprises the steps that 5-aminosalicylic acid, microcrystalline cellulose and a binding agent are mixed, and cores with pills are obtained through an extrusion rolling technology; materials comprising ethyecellulose are adopted, and cores with the pills are coated with sustained-release coating layers; the sustained-release coating layers are coated with enteric coating layers by the adoption of enteric materials and a processing aid, and mesalazine sustained-release pellets are obtained; the enteric materials are methacrylic acid and ethyl acrylate copolymer. PH dependent form and time dependent form drug release mechanisms are combined to achieve an ideal drug release curve, and the drug release position accuracy is improved. The mesalazine sustained-release capsule comprises the mesalazine sustained-release pellets, can be better released in the gastrointestinal tract and is beneficial to treatment on ulcerative colitis and Crohn's disease.
Owner:XINAN PHARMA
Fludarabine phosphate freeze-dried powder injection and preparation method thereof
ActiveCN102091046AThe appearance is plump and does not shrinkUniform colorOrganic active ingredientsPowder deliveryFreeze-dryingCLARITY
The invention belongs to the technical field of medicines, and relates to a fludarabine phosphate freeze-dried powder injection and a preparation method thereof. The fludarabine phosphate freeze-dried powder injection consists of fludarabine phosphate and mannitol in a mass ratio of 1:(0.6-1.2). The method for preparing the fludarabine phosphate freeze-dried powder injection comprises the following steps of: adding water for injection into a liquid preparing tank, adding the fludarabine phosphate in a prescription amount, adding alkali, stirring until the mixture is dissolved completely; and adding the mannitol, regulating the pH value, adding active carbon for decolorizing, filtering for decolorizing, performing fine filtering by using a filter membrane, subpackaging, and performing freeze drying. The fludarabine phosphate freeze-dried powder injection has excellent formability, clarified appearance of solution to be frozen, high redissolution performance of freeze-dried products, high clarity after redissolution, low impurity content and moisture content, high stability and controllable quality.
Owner:HAINAN JINRUI PHARMA
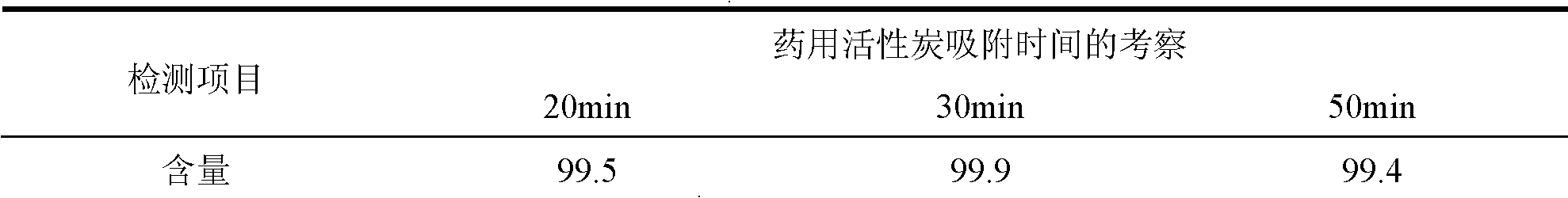
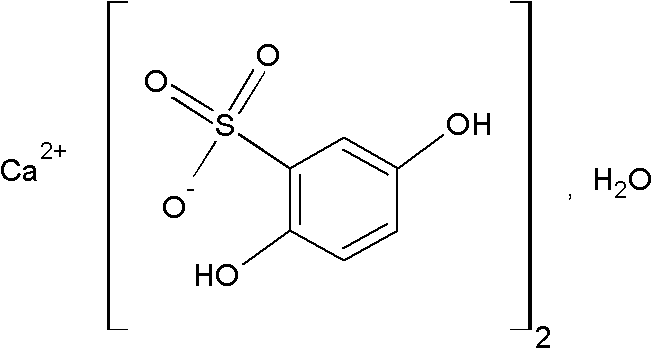
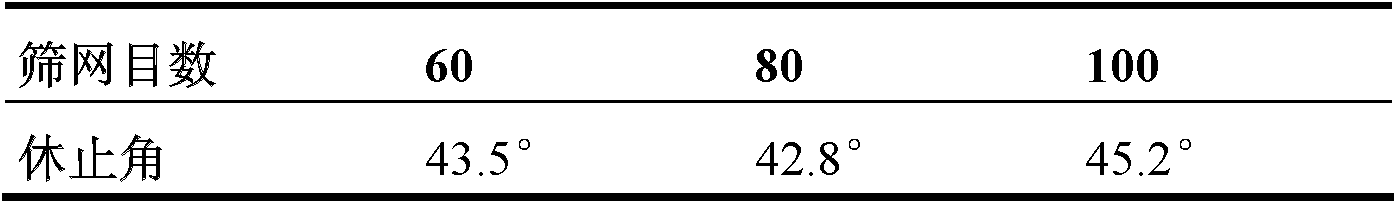
Calcium dobesilate capsule and preparation method thereof
InactiveCN102091055AThe effect is fully verifiedAvoid loading differencesSenses disorderMetabolism disorderDissolutionCroscarmellose sodium
The invention discloses a calcium dobesilate capsule and a preparation method thereof. The calcium dobesilate capsule is prepared from calcium dobesilate, croscarmellose sodium, magnesium stearate and polyvinyl pyrrolidone, wherein every 1000 calcium dobesilate capsules contain 500g of calcium dobesilate, 20-50g of croscarmellose sodium and 2-6g of magnesium stearate. In addition, a traditional preparation method is improved in the invention, and the improved method comprises the following steps: on the basis of taking the croscarmellose sodium as a disintegrating agent and taking the magnesium stearate as a lubricating agent, adding a proper amount of the polyvinyl pyrrolidone to prepare a bonding agent; preparing the bonding agent and the calcium dobesilate as well as the croscarmellosesodium into granules by a multi-step granulation method; and spraying the ethanol solution of the magnesium stearate onto the surfaces of the granules to obtain calcium dobesilate capsules with stable quality, high dissolution rate and small content uniformity.
Owner:HAINAN JINRUI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com