Novel controlled release capsule and preparation method thereof
A capsule and capsule technology, applied in the field of medicine, can solve the problems of being unsuitable for the preparation of osmotic pump tablets, many restrictive factors of drugs, complicated preparation process, etc., and achieve the effects of fast onset of drug effect, simplified selection of excipients, and simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
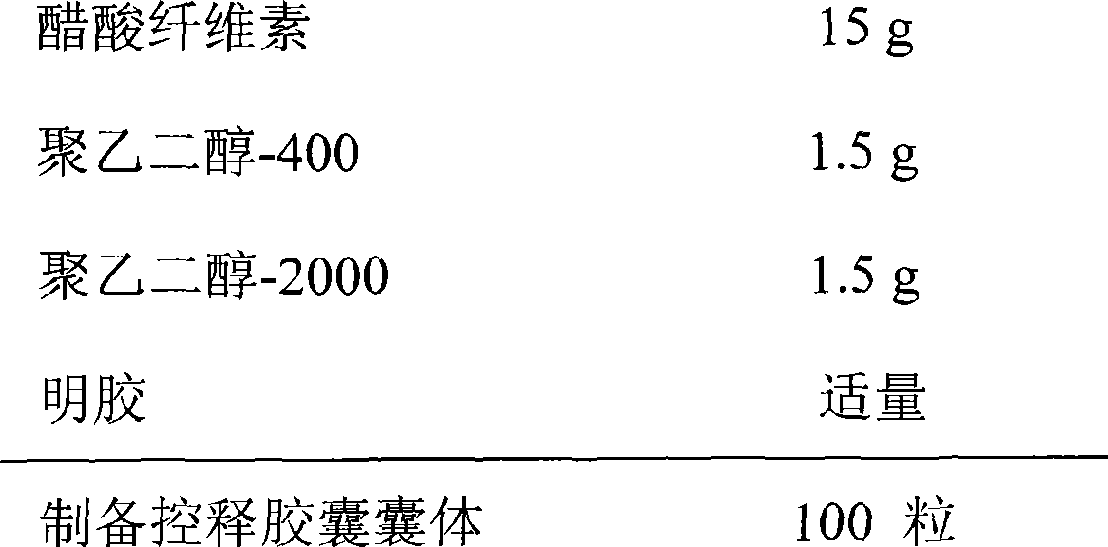
[0029] Controlled release capsule capsule body composition:
[0030]
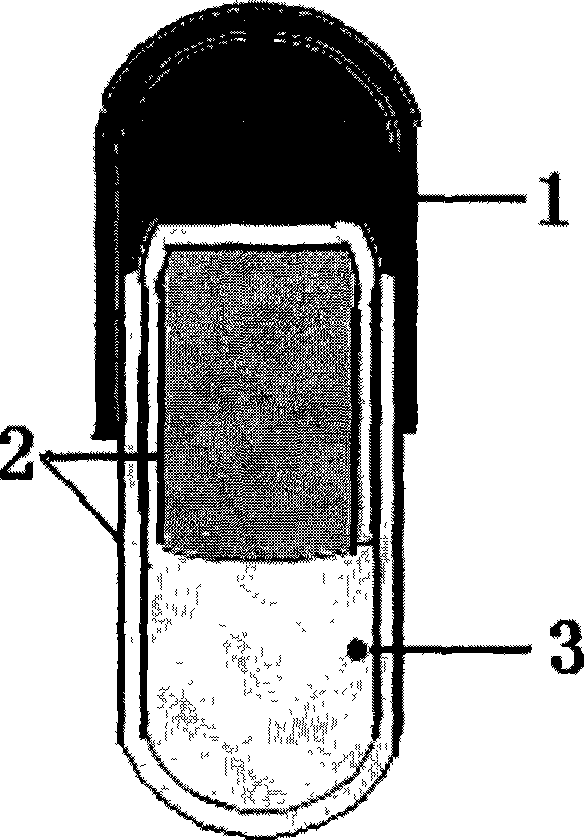
[0031] Preparation process: Preparation of controlled-release capsule capsules: first dissolve cellulose acetate in a solvent of acetone: absolute ethanol = 4:1, add polyethylene glycol-400 and polyethylene glycol-2000 as plasticizers and The porogen is made by dipping in glue, dried, and a drug release hole with a diameter of 1.0mm is prepared on the capsule by mechanical drilling, sealed with gelatin solution and dried, pulled out, cut, and sorted to obtain controlled release. The cap and body of the capsule body.
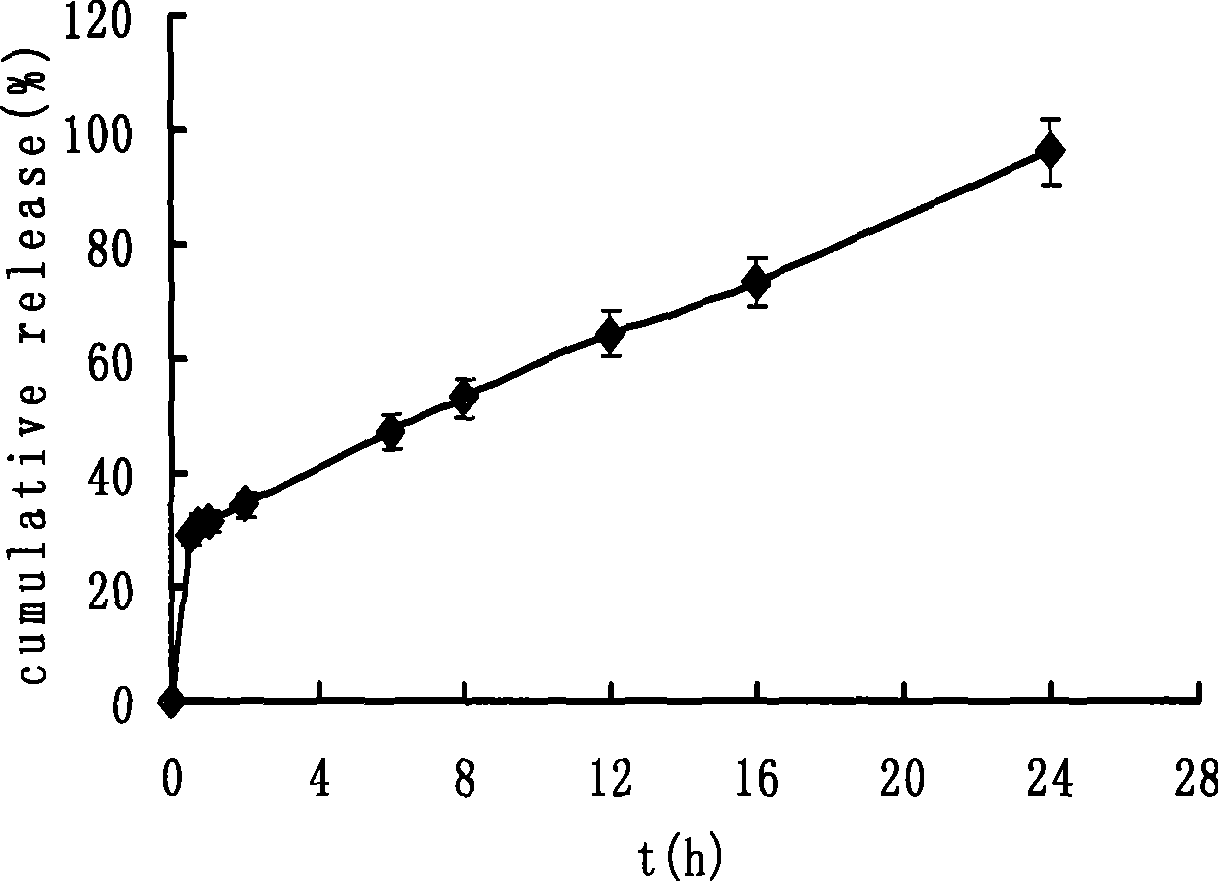
[0032] Taking paracetamol as the model drug, 30mg of paracetamol raw material is filled into the capsule cap of ordinary capsules in powder form; another 70mg of paracetamol raw material and 35mg of sodium chloride are mixed, and filled into the controlled-release capsule body in powder form, and the controlled-release capsule The body is sealed with the same glue as the capsule material; ...
Embodiment 2
[0035] Controlled release capsule capsule body composition:
[0036]
[0037] Preparation process: Preparation of controlled-release capsules: first dissolve cellulose acetate in acetone, add polyethylene glycol-200, PVP-K30, and polyethylene glycol-4000 as plasticizers and porogens, and the preparation process Same as Example 1, prepare a drug release hole with a diameter of 0.8mm on the capsule body by mechanical drilling method, seal the gelatin solution and dry, pull out the shell, cut, arrange, and obtain the capsule shell of the osmotic pump.
[0038] Taking paracetamol as the model drug, 30mg of paracetamol raw material is filled into the capsule cap of ordinary capsules in powder form; in addition, 70mg of paracetamol raw material and 70mg of sucrose are mixed, and filled into the controlled-release capsule body in powder form, which is used for the controlled-release capsule body Seal with the same glue as the capsule material; then nest the capsule cap of the ordi...
Embodiment 3
[0040] Controlled release capsule capsule body composition:
[0041]
[0042] Preparation process: Preparation of controlled-release capsules: first dissolve ethyl cellulose in 95% ethanol, add polyethylene glycol-400 and sodium carboxymethyl cellulose as plasticizers and porogens, add titanium dioxide and stir Evenly, the preparation process is the same as in Example 1. A drug release hole with a diameter of 1.0 mm is prepared on the capsule body with a mechanical punching method, and the gelatin solution is sealed.
[0043] Taking sagrelate hydrochloride as the model drug, 30mg of sargrelate hydrochloride raw material is filled into the capsule cap of ordinary capsules in powder form; another 70mg of sarcogrelate hydrochloride raw material and 60mg of lactose are mixed, and filled into controlled-release capsules in powder form In the body, the capsule body of the controlled-release capsule is sealed with the same glue as the capsule material; then the capsule cap of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com