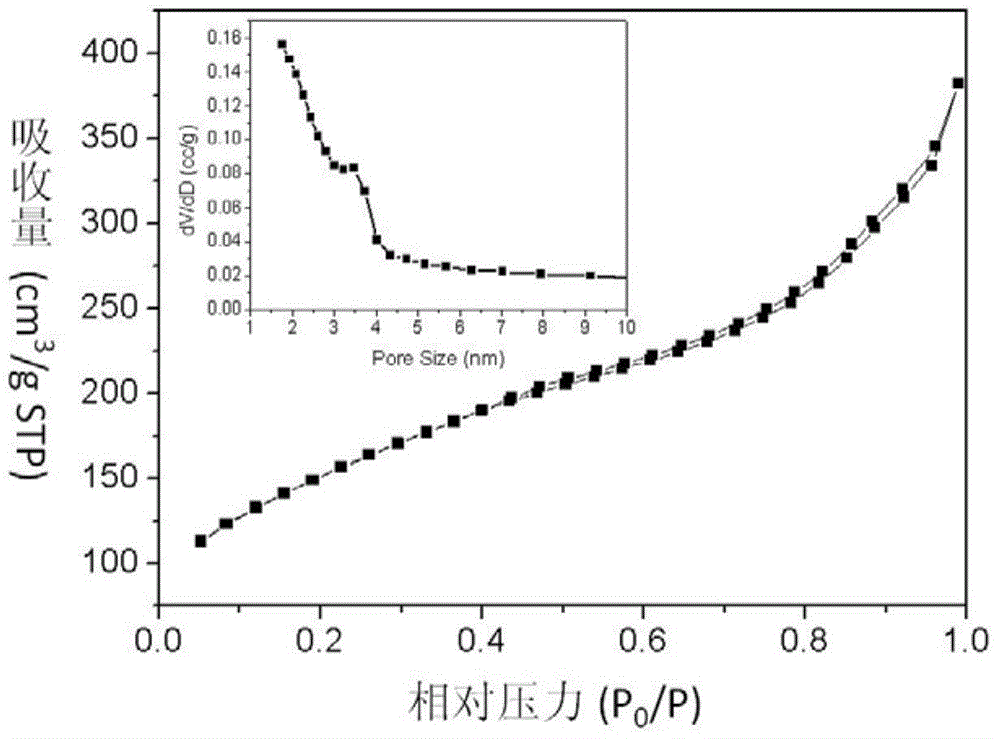
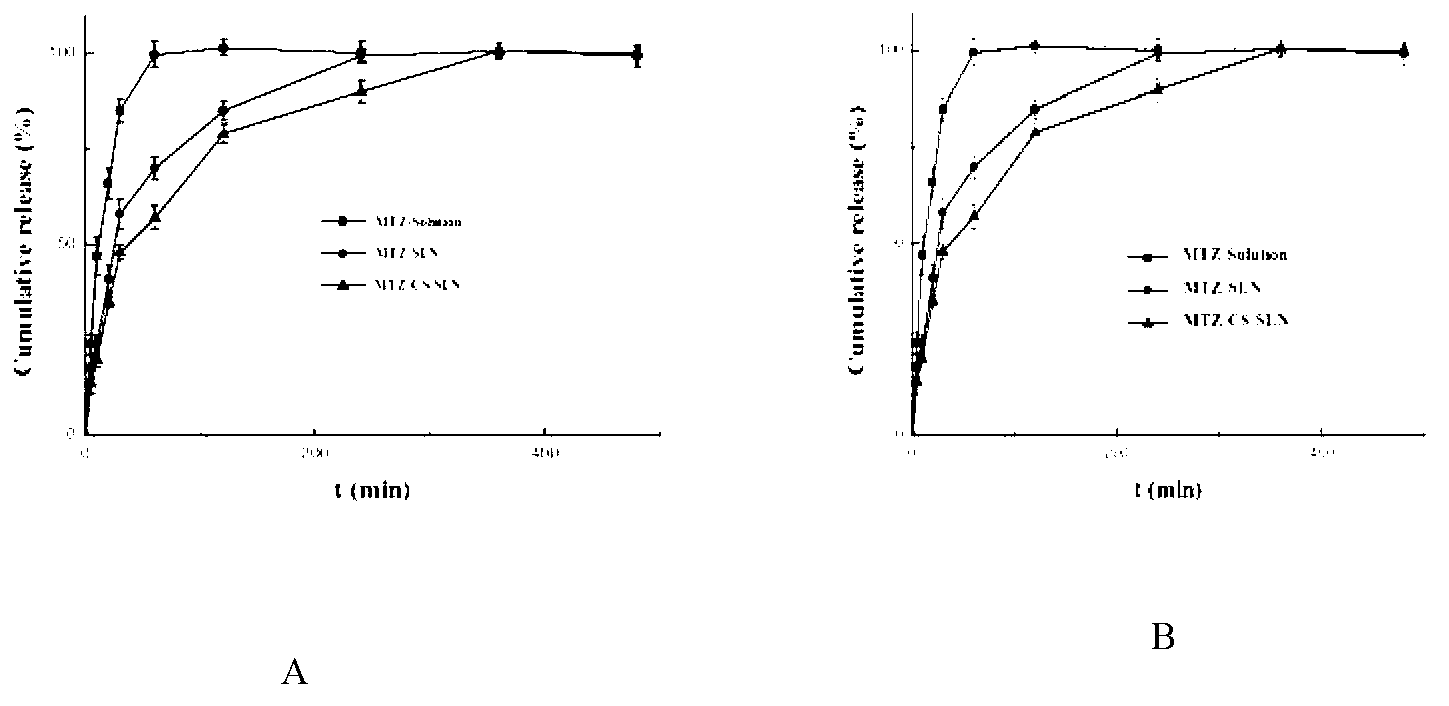
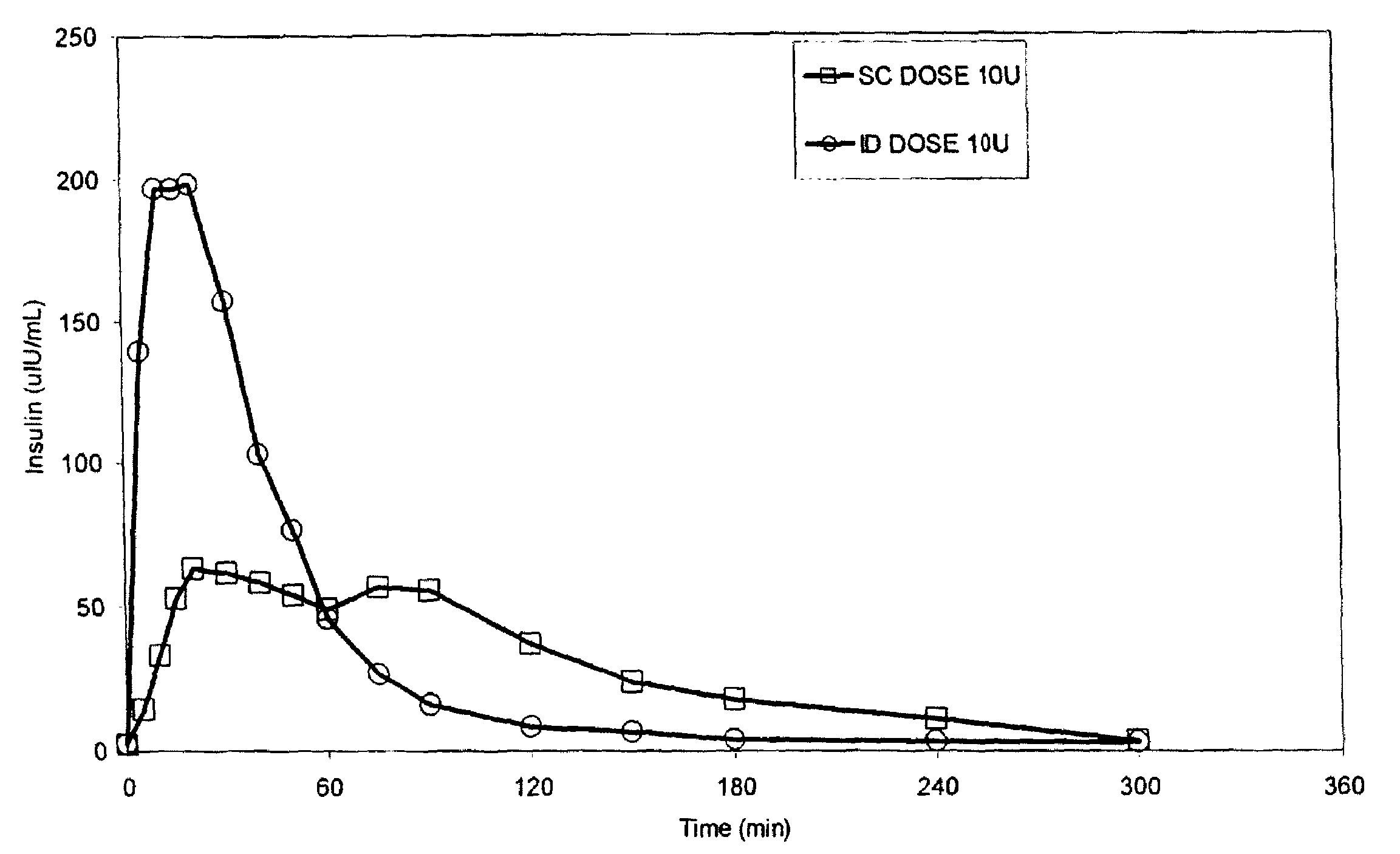
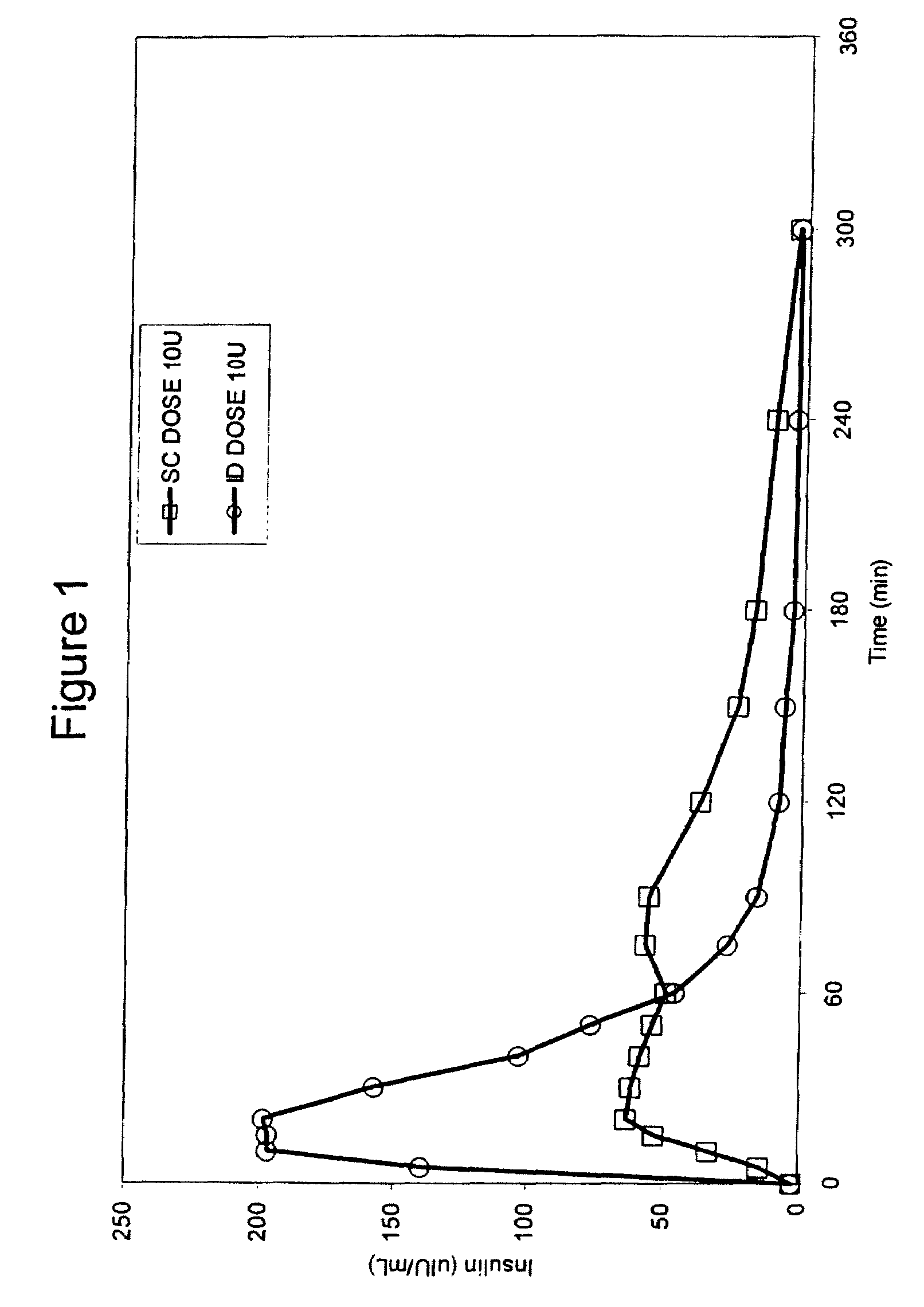
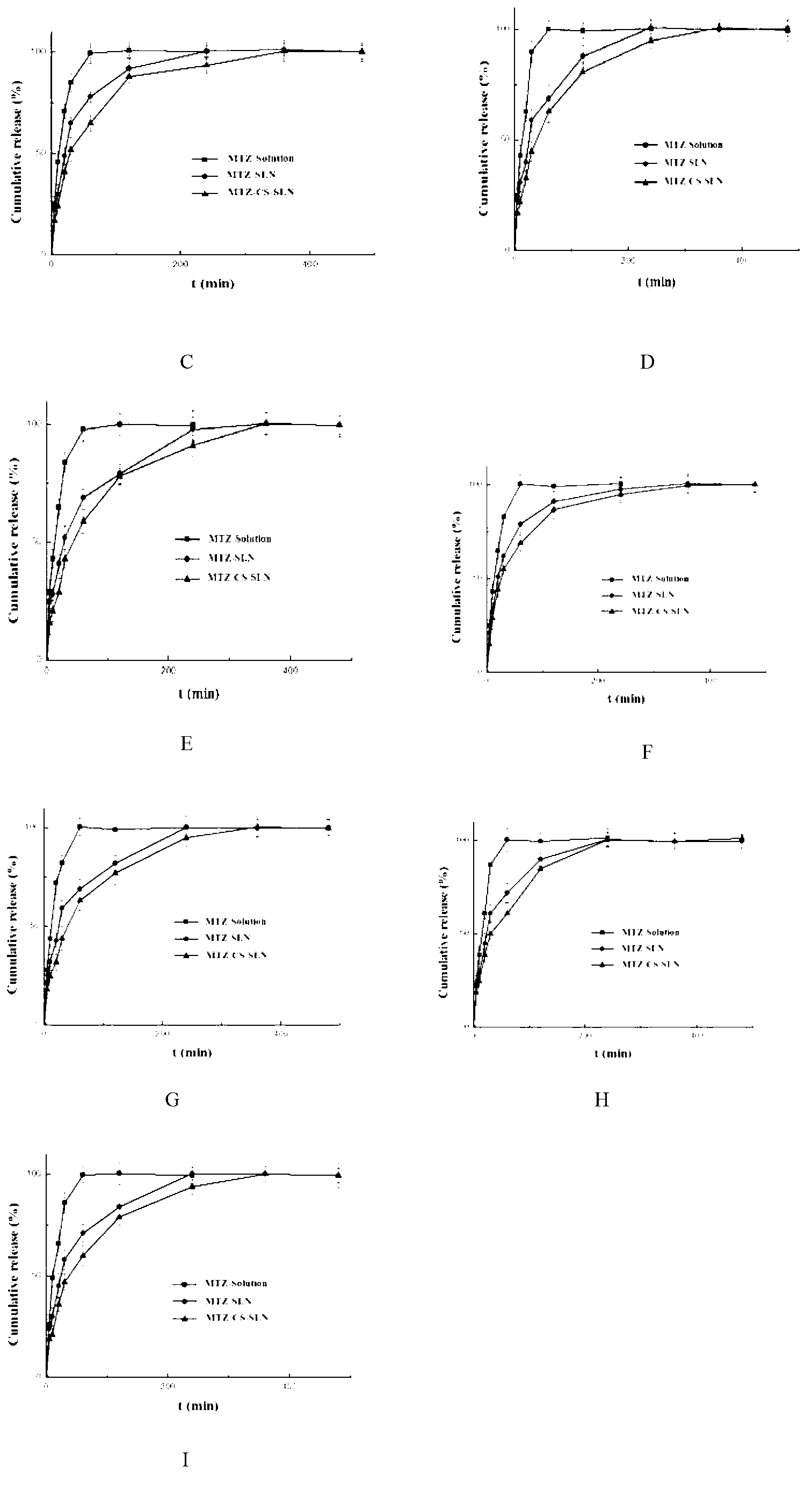Patents
Literature
197results about How to "Increase uptake" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
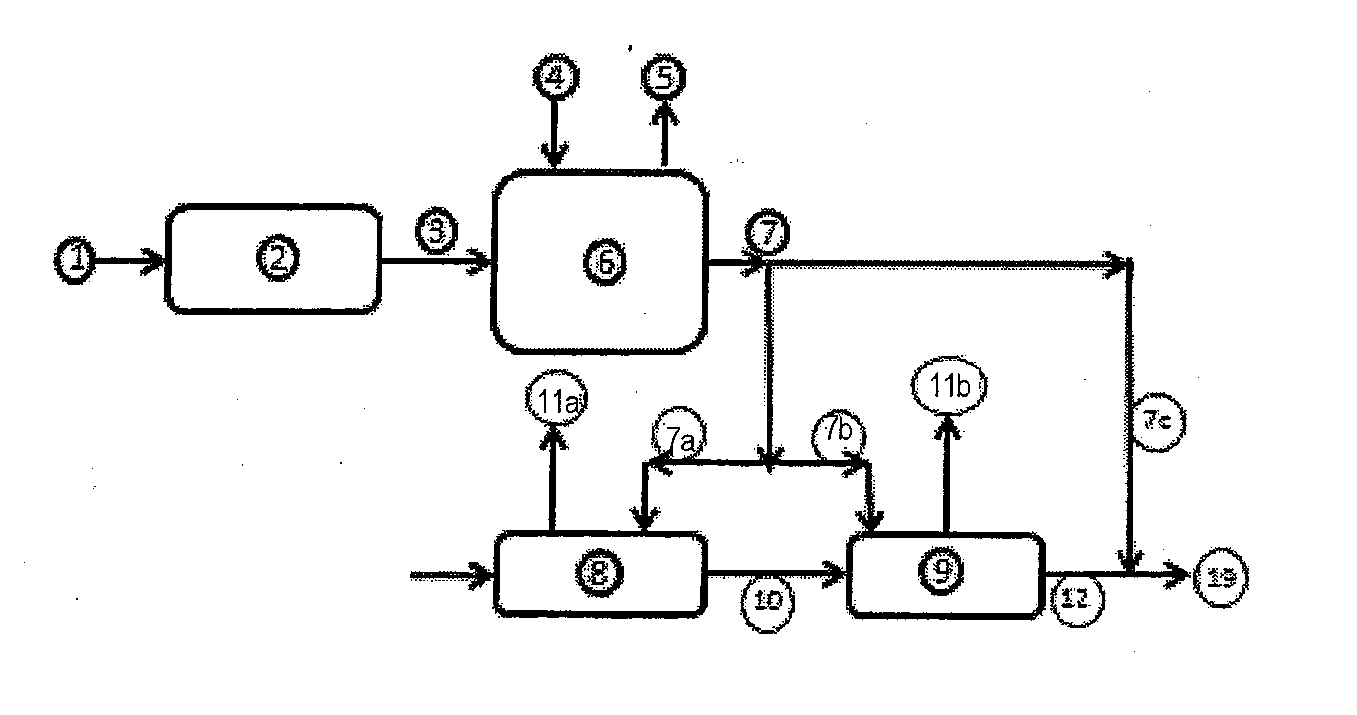
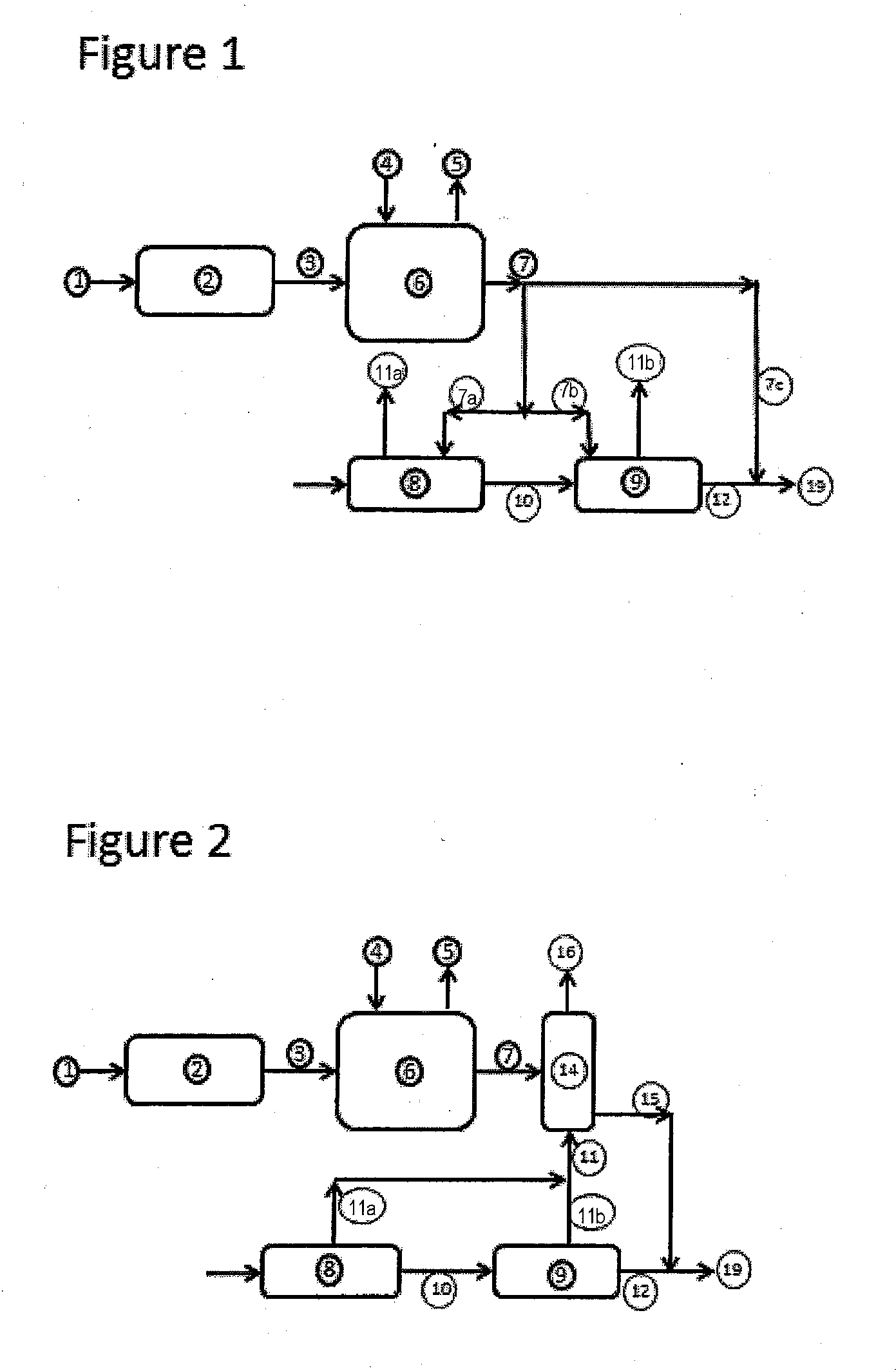
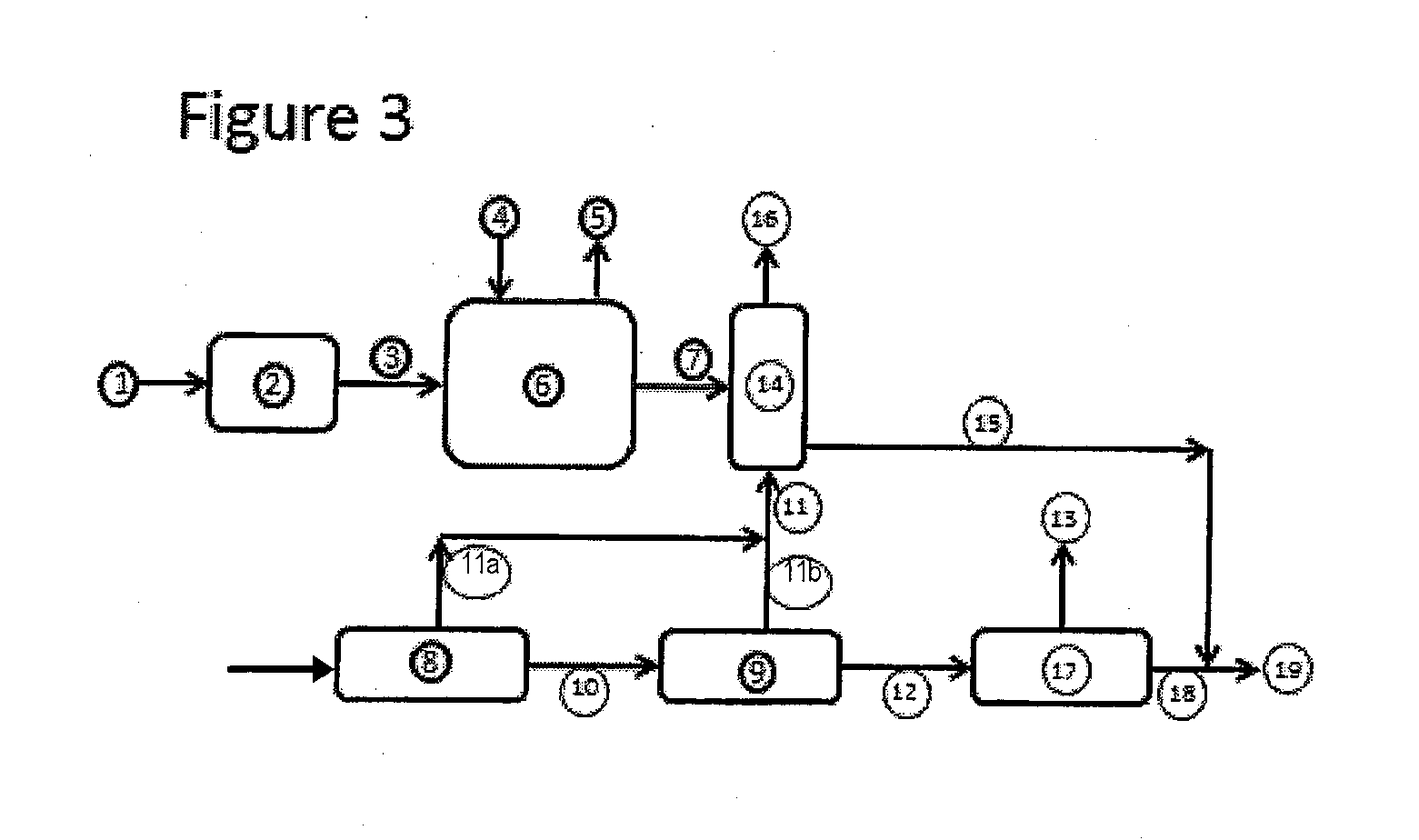
Method and composition for the treatment of diabetes
InactiveUS6153632AIncrease uptakeImprove utilizationBiocidePeptide/protein ingredientsIGT - Impaired glucose toleranceGlycosidase inhibitor
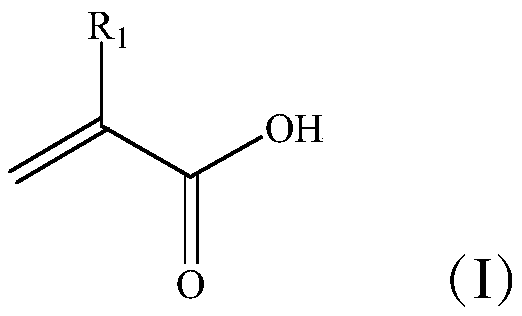
This invention is directed to a novel method and composition for the treatment of diabetes mellitus (Type I, Impaired Glucose Tolerance ["IGT"]and Type II). More specifically, this invention pertains to a novel method of treating diabetes mellitus by incorporating a therapeutic amount of one or more insulin sensitizers along with one or more of an orally ingested insulin, an injected insulin, a sulfonylurea, a biguanide or an alpha-glucosidase inhibitor for the treatment of diabetes mellitus.
Owner:RIEVELEY CHERYL ANNE
Delivery of highly lipophilic agents via medical devices
InactiveUS20060240070A1Easy to transportIncrease drug retentionBiocideFibrinogenMedicineMedical device
An apparatus and system for delivering a lipophilic agent associated with a medical device including: a medical device, a first lipophilic agent capable of penetrating a body lumen, wherein the transfer coefficients of the first lipophilic agent is by an amount that is statistically significant of at least approximately 5,000, wherein the first lipophilic agent is associated with the medical device, wherein the first lipophilic agent / medical device is placed adjacent to said body lumen, and wherein a therapeutically effective amount of the first lipophilic agent is delivered to a desired area within a subject. Furthermore, the invention relates to a method for improving patency in a subject involving placement of a medical device in a body lumen for treating and / or preventing adjacent diseases or maintaining patency of the body lumen.
Owner:ABBOTT LAB INC
Bioabsorbable polymeric composition for a medical device
ActiveUS20080118546A1Less inflammatoryLower potential for traumaStentsSurgeryMedical deviceCopolymer
Owner:ORBUSNEICH MEDICAL PTE LTD
Intradermal delivery of substances
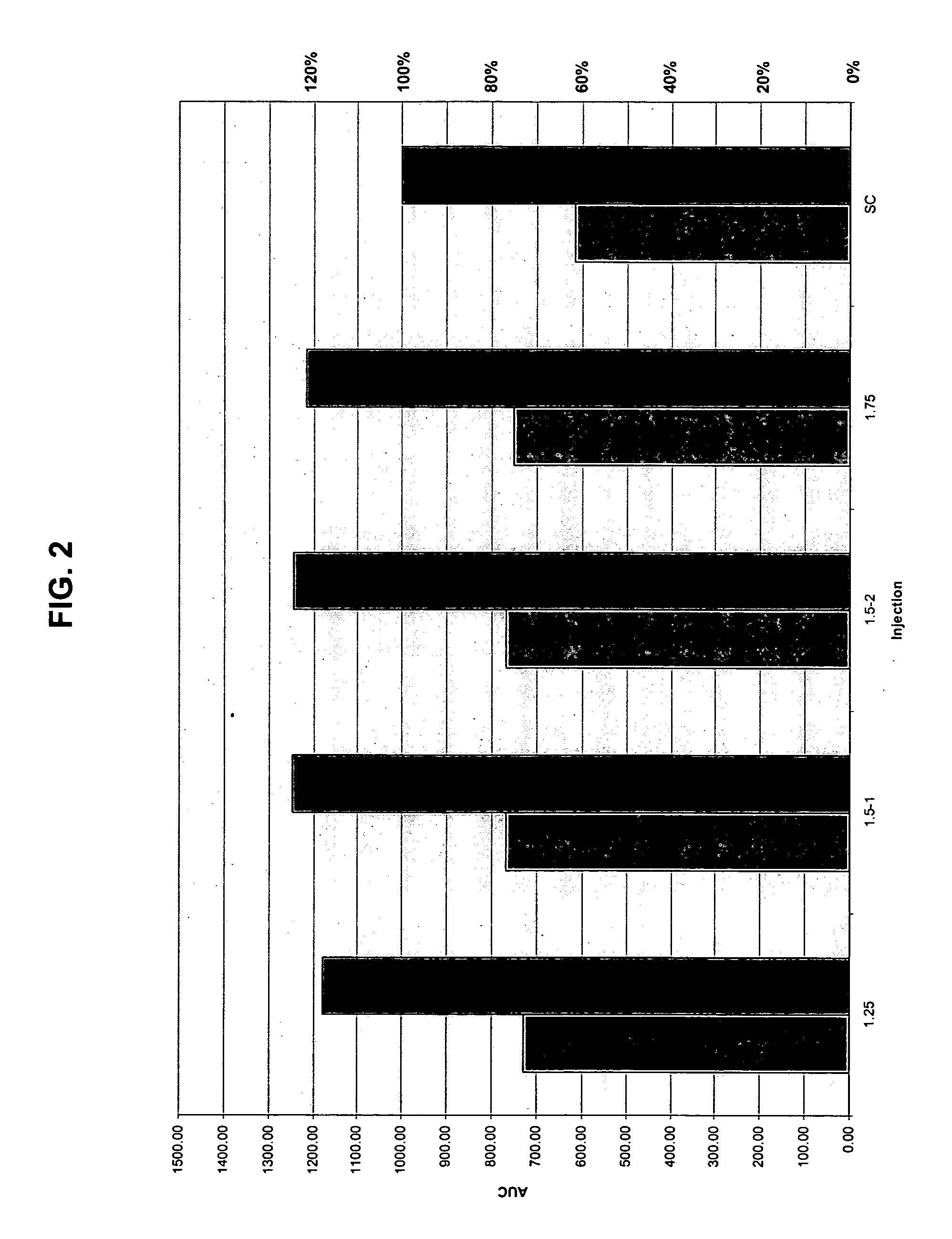
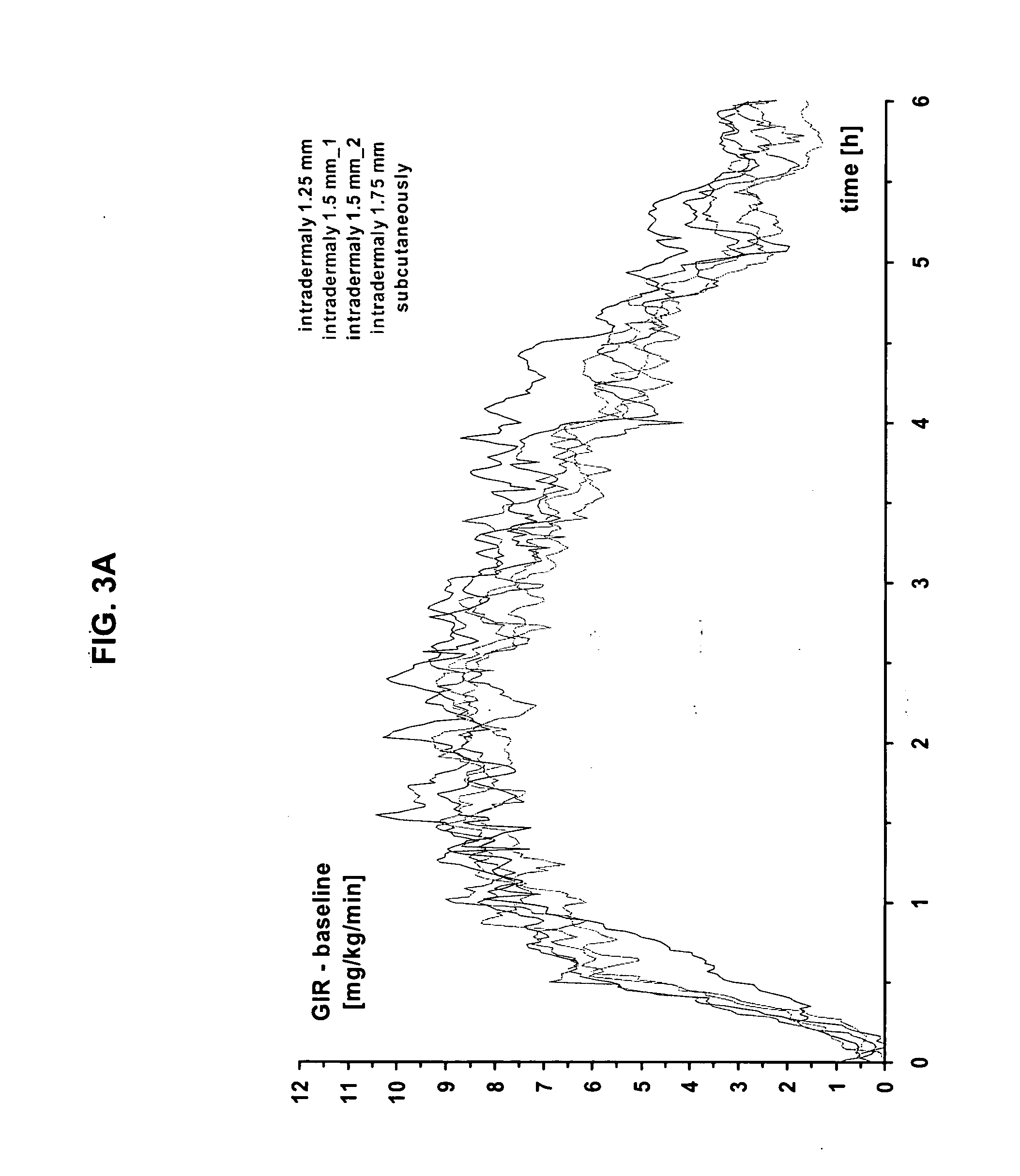
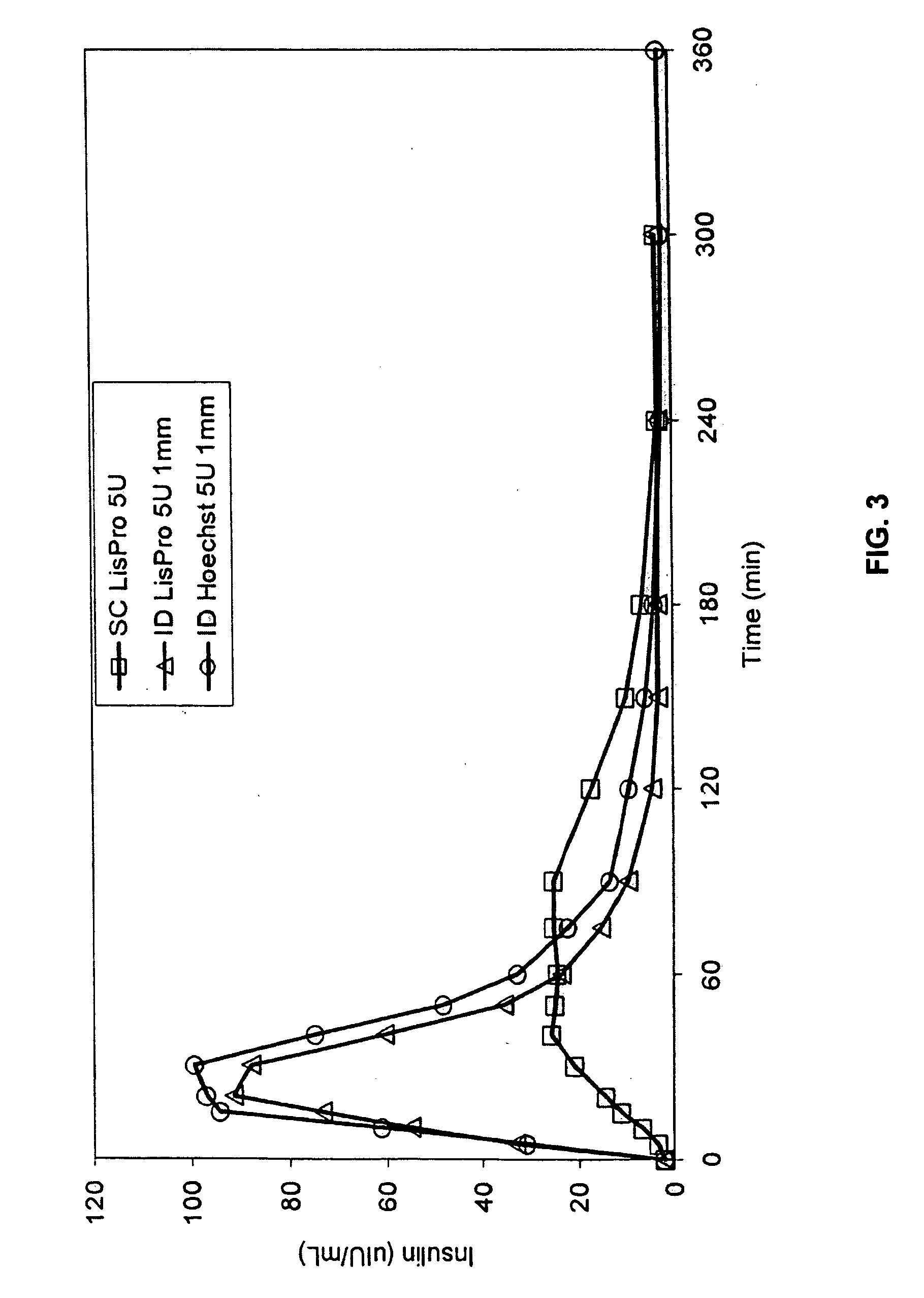
InactiveUS20040073160A1Increase uptakeRapid uptake rateJet injection syringesPeptide/protein ingredientsWhole bodyGrowth hormone
A method for administration of a substance into the dermis of a mammal is disclosed. The method involves administration into the dermis by injection which results in improved systemic absorption relative to that obtained upon subcutaneous administration of the substance. The substance administered may be a growth hormone, a low molecular weight heparin or a dopamine receptor agonist.
Owner:PHARMACIA CORP
Unagglomerated core/shell nanocomposite particles
ActiveUS20050281884A1Improve stabilityPromote absorptionPowder deliveryMaterial nanotechnologyAlcoholMicroemulsion
The present invention provides a method for the synthesis of unagglomerated, highly dispersed, stable core / shell nanocomposite particles comprised of preparing a reverse micelle microemulsion that contains nanocomposite particles, treating the microemulsion with a silane coupling agent, breaking the microemulsion to form a suspension of the nanocomposite particles by adding an acid / alcohol solution to the microemulsion that maintains the suspension of nanocomposite particles at a pH of between about 6 and 7, and simultaneously washing and dispersing the suspension of nanocomposite particles, preferably with a size exclusion HPLC system modified to ensure unagglomeration of the nanocomposite particles. The primary particle size of the nanocomposite particles can range in diameter from between about 1 to 100 nm, preferably from between about 10 to 50 nm, more preferably about 10 to 20 nm, and most preferably about 20 nm.
Owner:PENN STATE RES FOUND
Methods and devices for administration of substances into the intradermal layer of skin for systemic absorption
InactiveUS20030100885A1Increase uptakeRapid onsetOrganic active ingredientsAmpoule syringesWhole bodySystemic absorption
Methods and devices for administration of substances into the intradermal layer of skin for systemic absorption.
Owner:BECTON DICKINSON & CO
Enhanced pharmacokinetic profile of intradermally delivered substances
InactiveUS20030073609A1Increase uptakeEnhanced iontophoresisPowder deliveryOrganic active ingredientsWhole bodyGrowth hormone
A method for administration of a substance into the dermis of a mammal is disclosed. The method involves administration into the dermis by injection which results in improved systemic absorption relative to that obtained upon subcutaneous administration of the substance. The substance administered may be a growth hormone, a low molecular weight heparin or a dopamine receptor agonist.
Owner:PHARMACIA CORP
Method and device for reducing therapeutic dosage
InactiveUS20020156453A1Increase uptakeRapid uptake rateAmpoule syringesJet injection syringesBiomedical engineeringBioavailability
Methods and devices for administration of substances into the intradermal layer of skin with improved bioavailability.
Owner:BECTON DICKINSON & CO
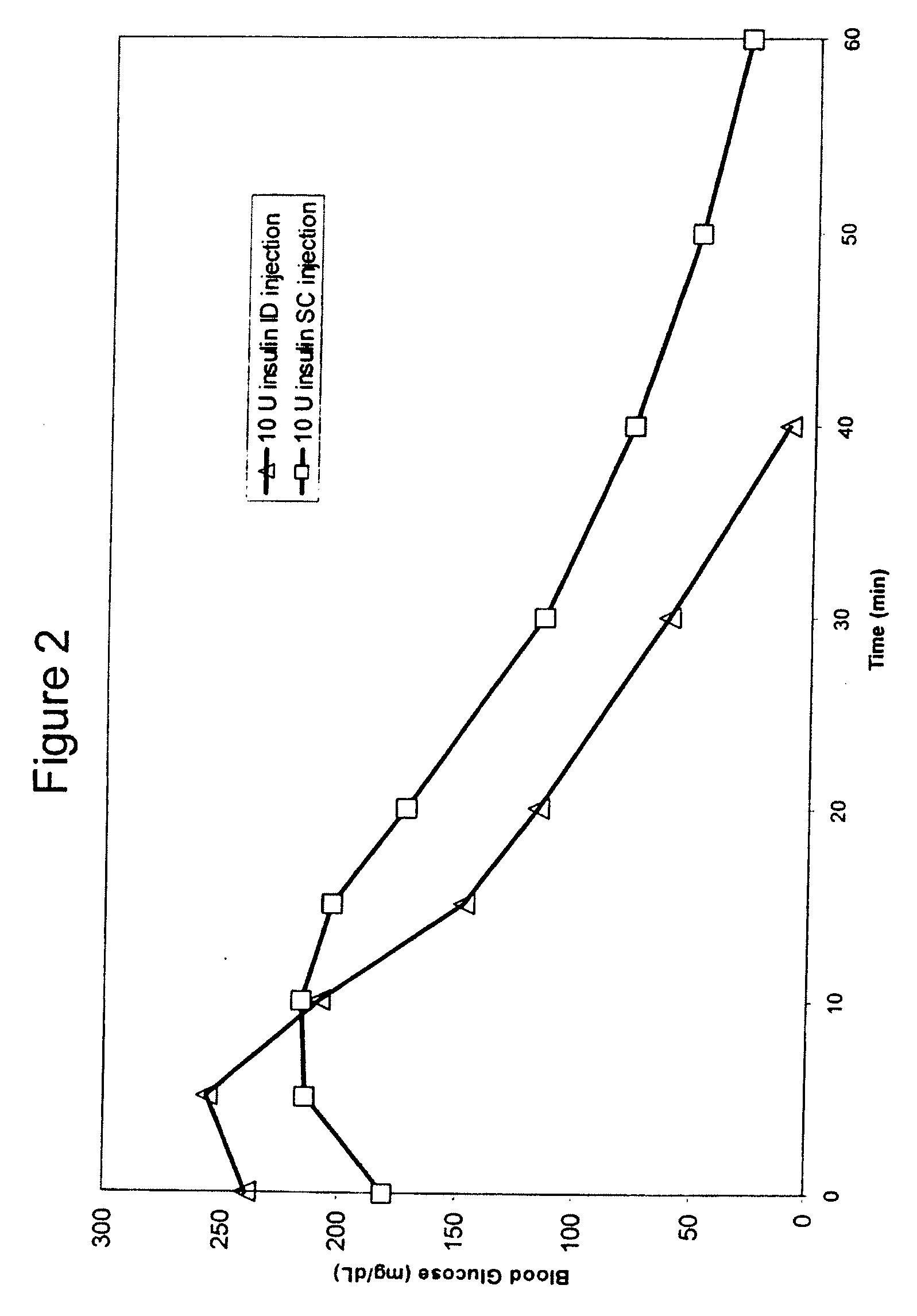
Method for altering insulin pharmacokinetics
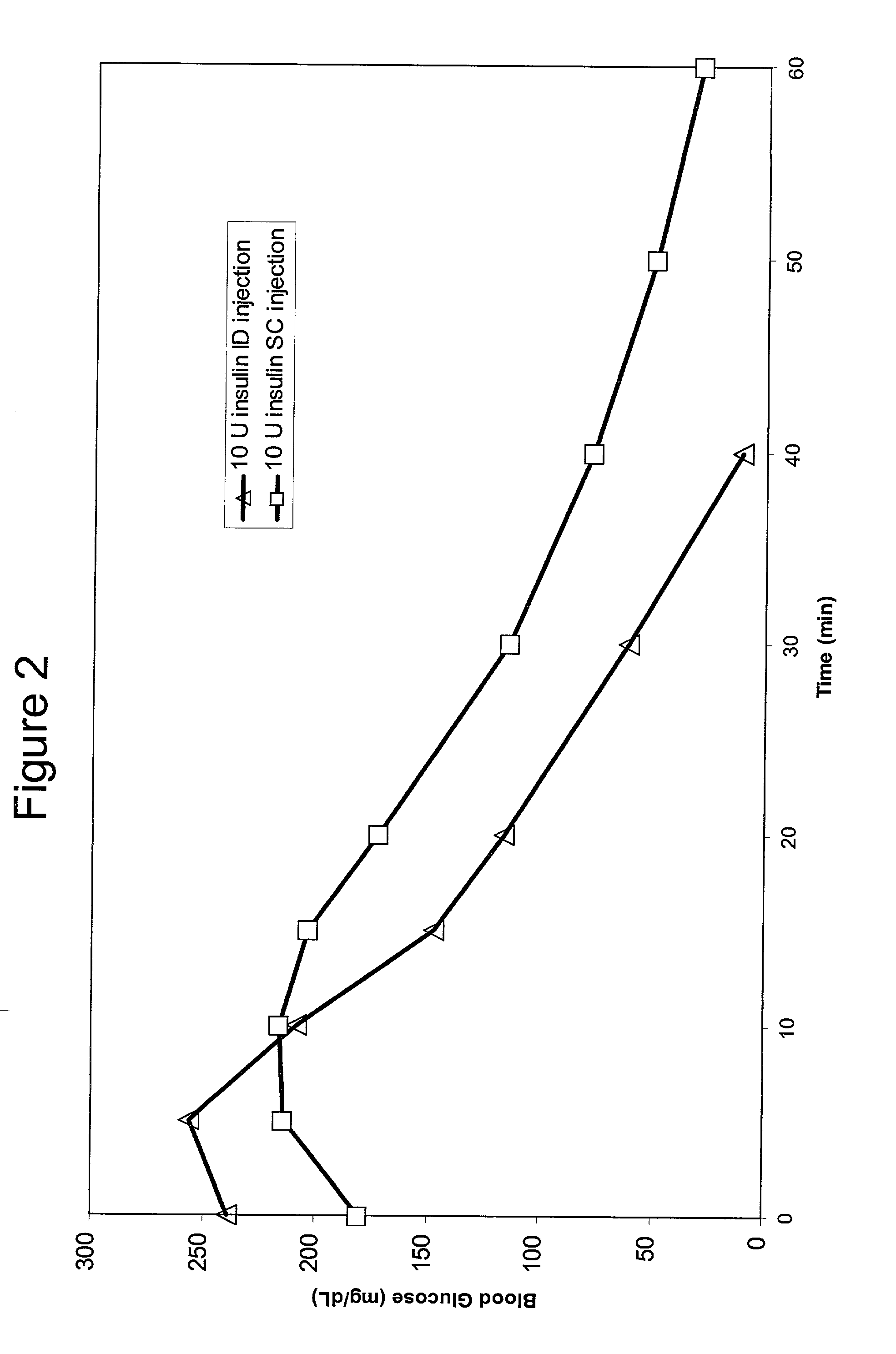
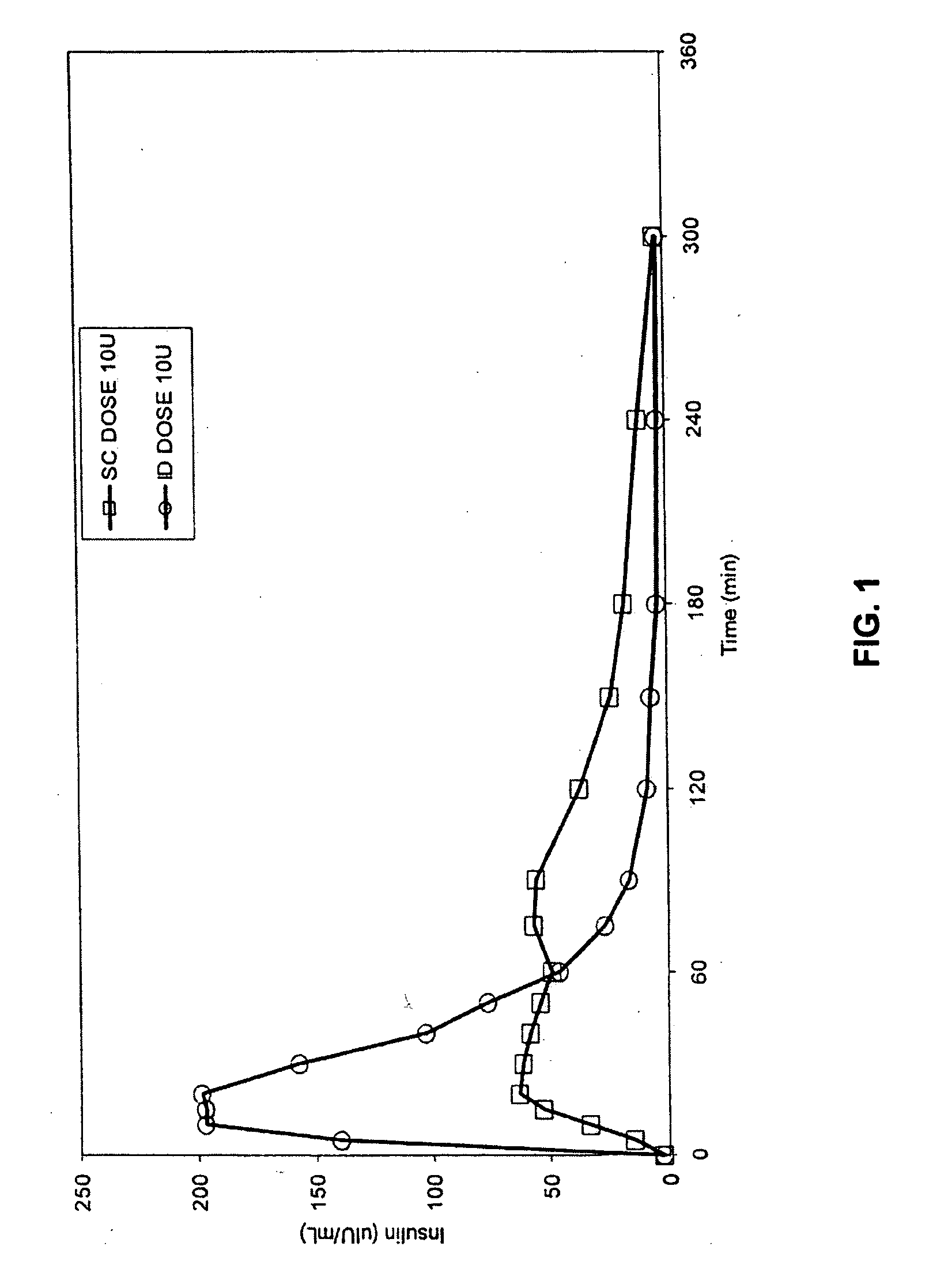
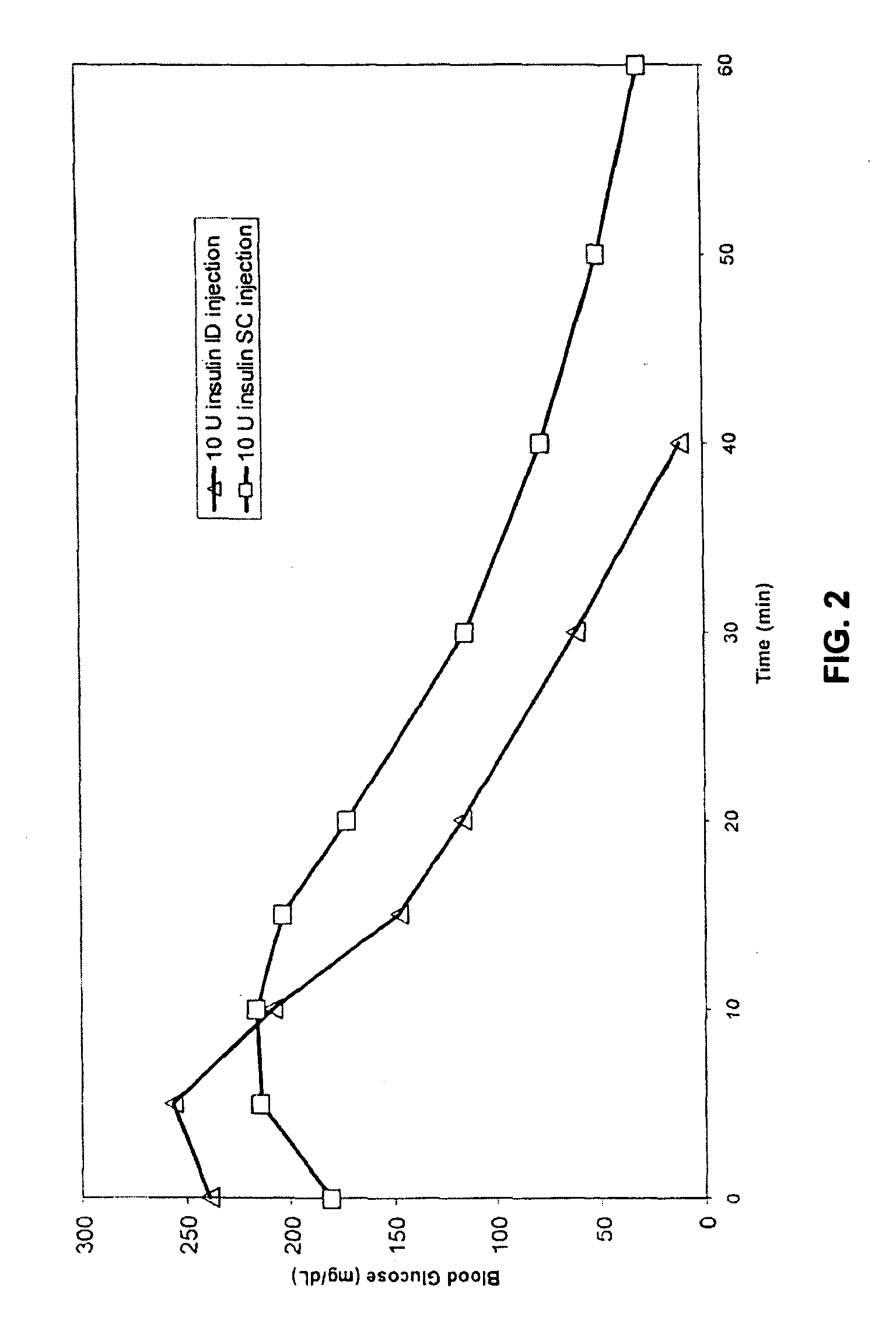
InactiveUS20050055010A1Enhance bioavailabilityHigh bioavailabilityPeptide/protein ingredientsMetabolism disorderNon fastingClinical efficacy
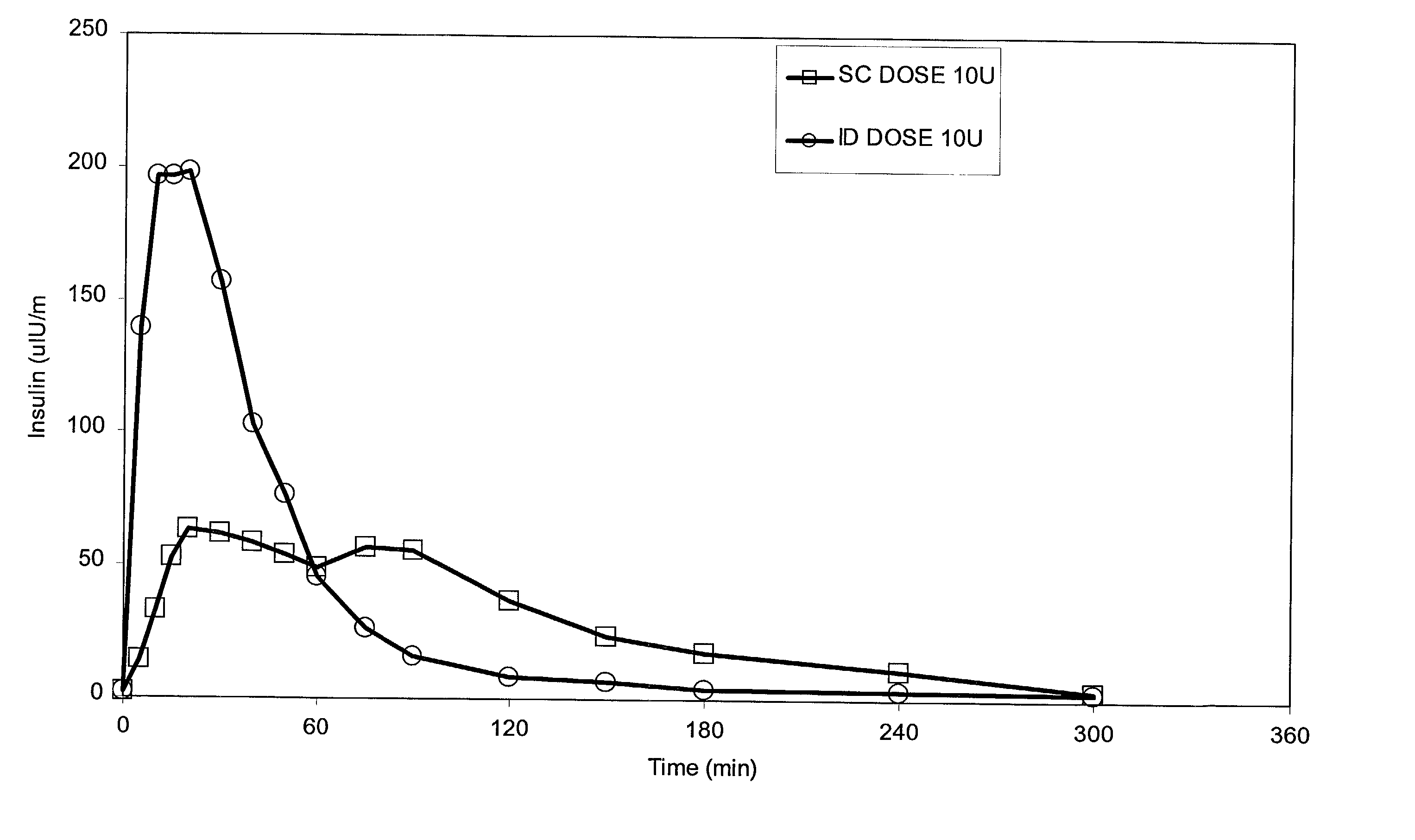
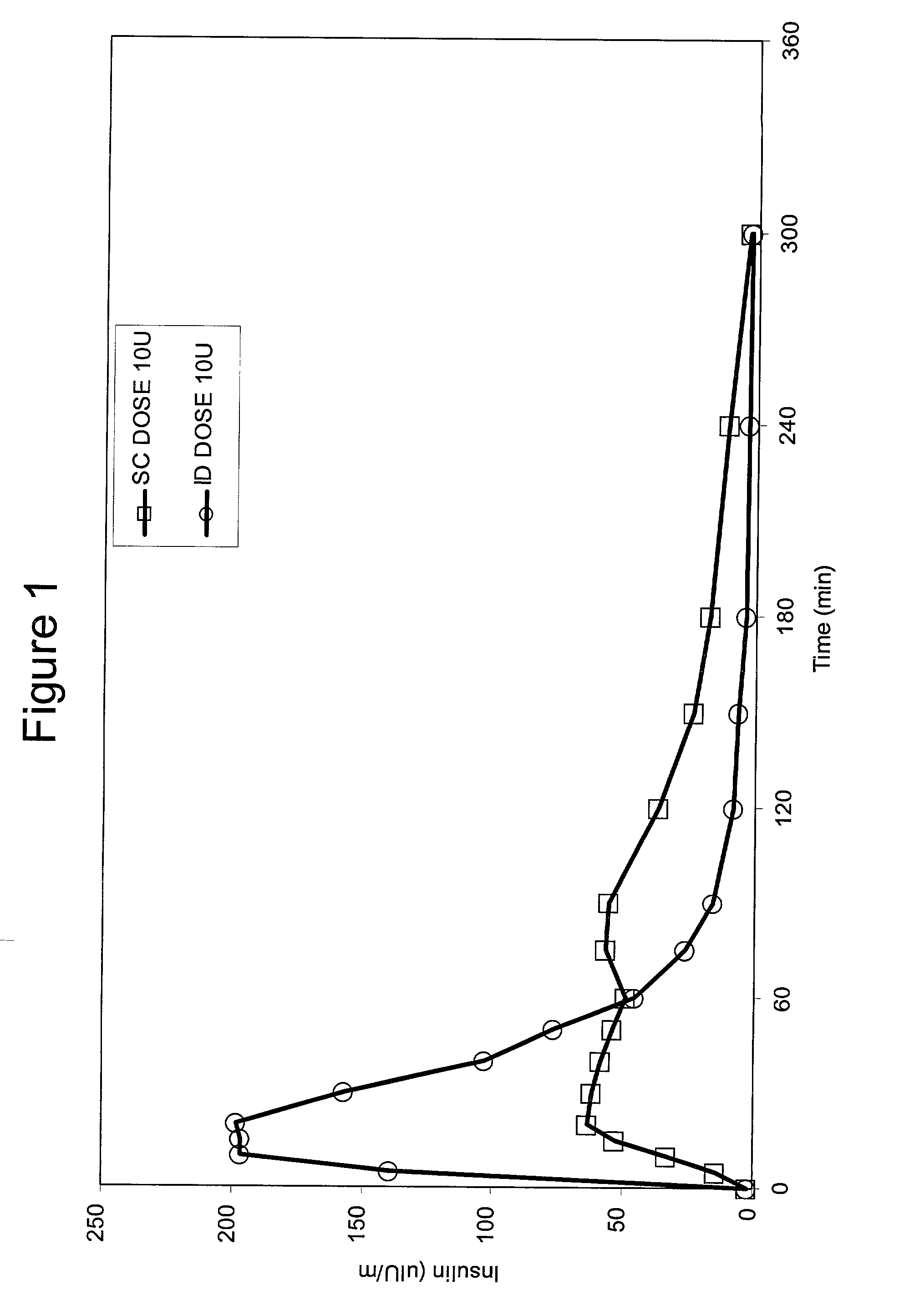
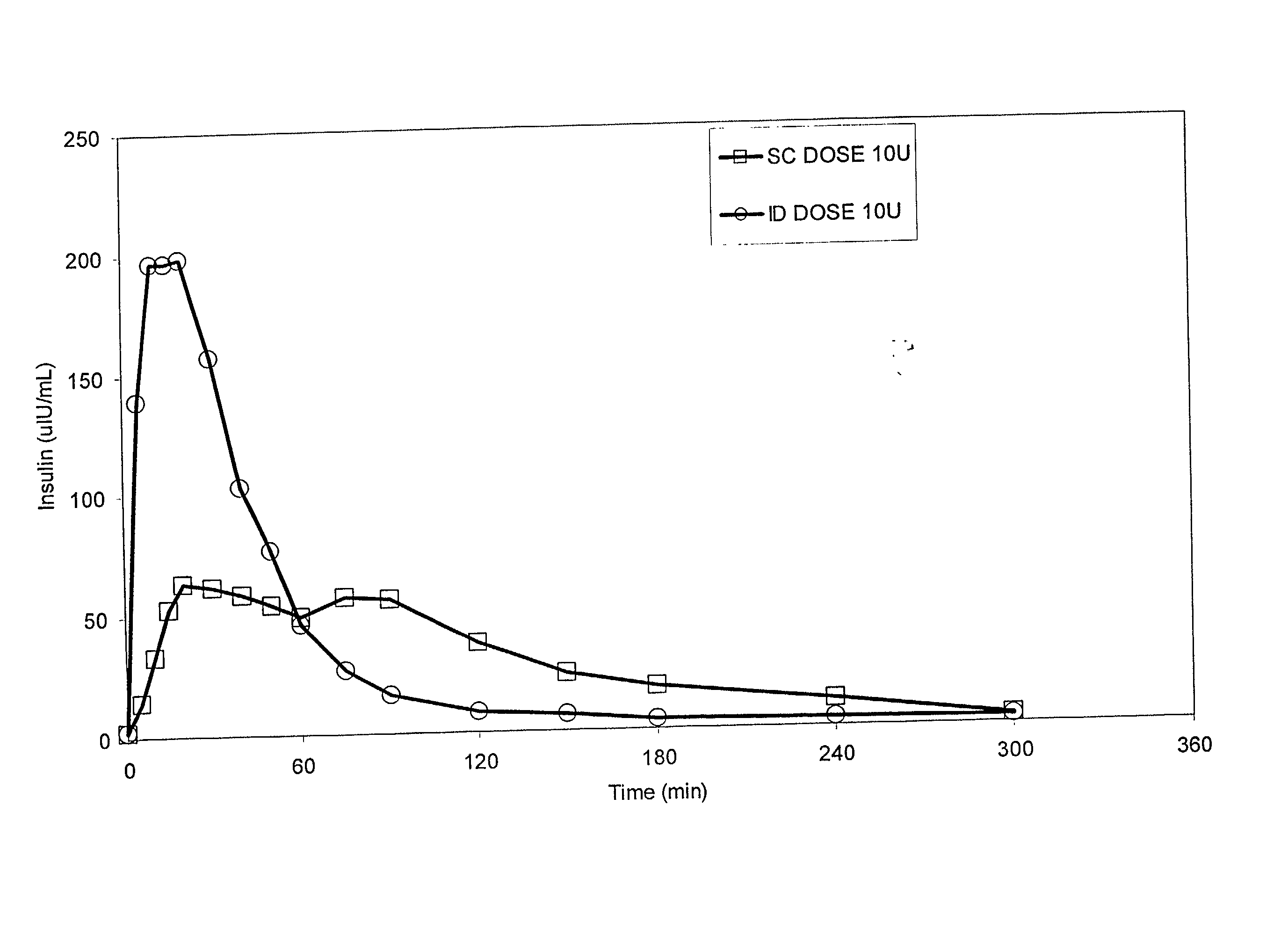
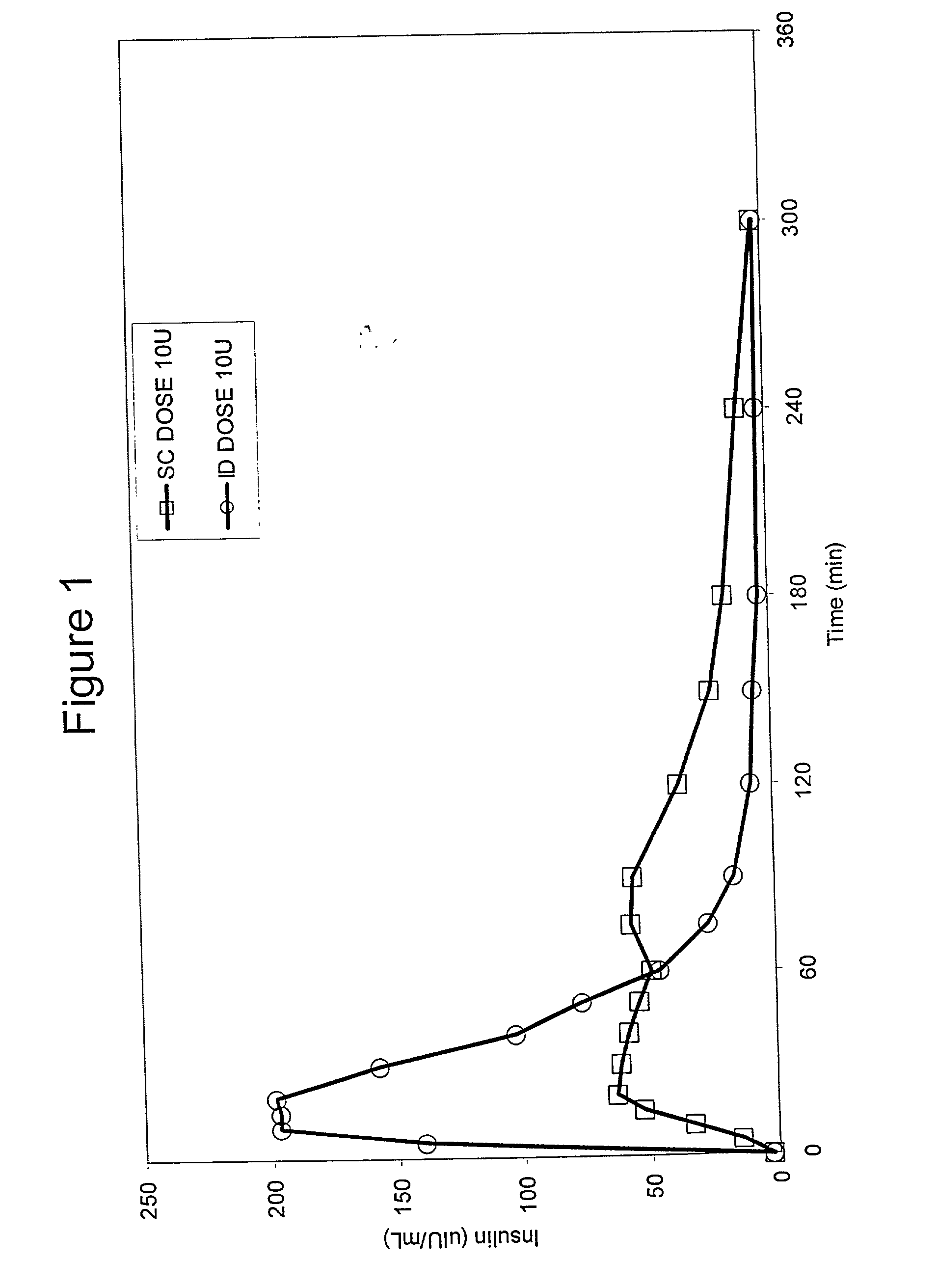
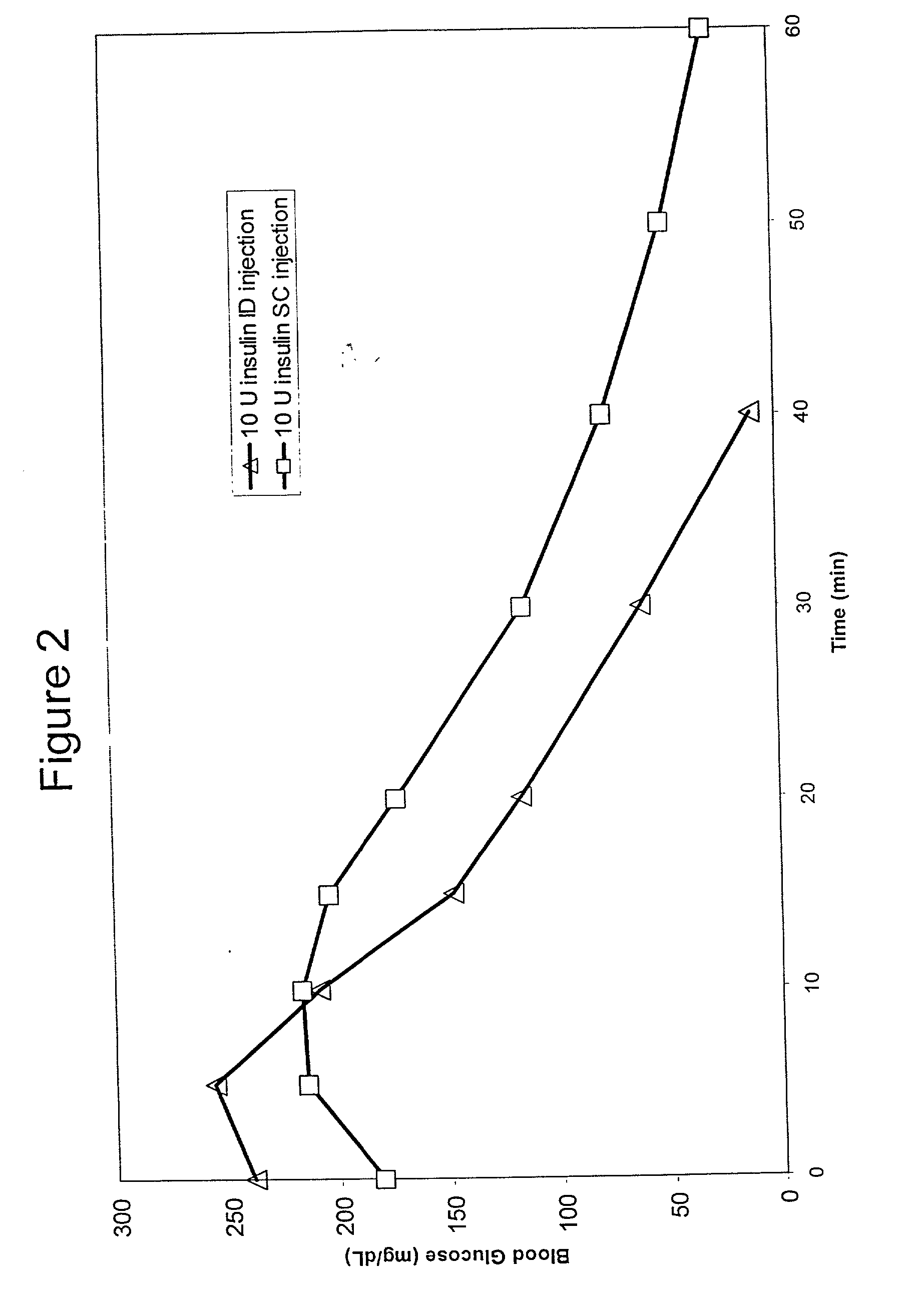
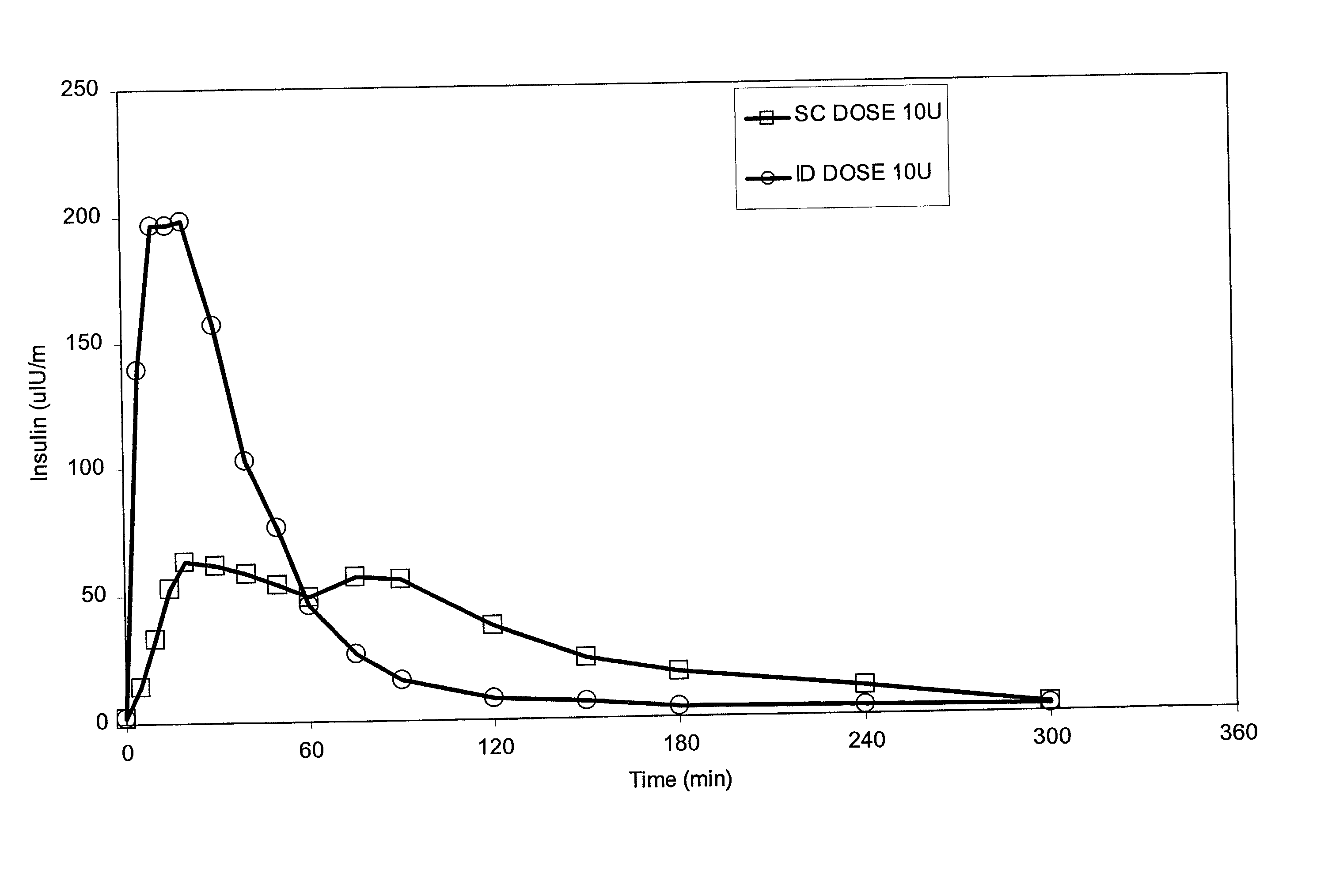
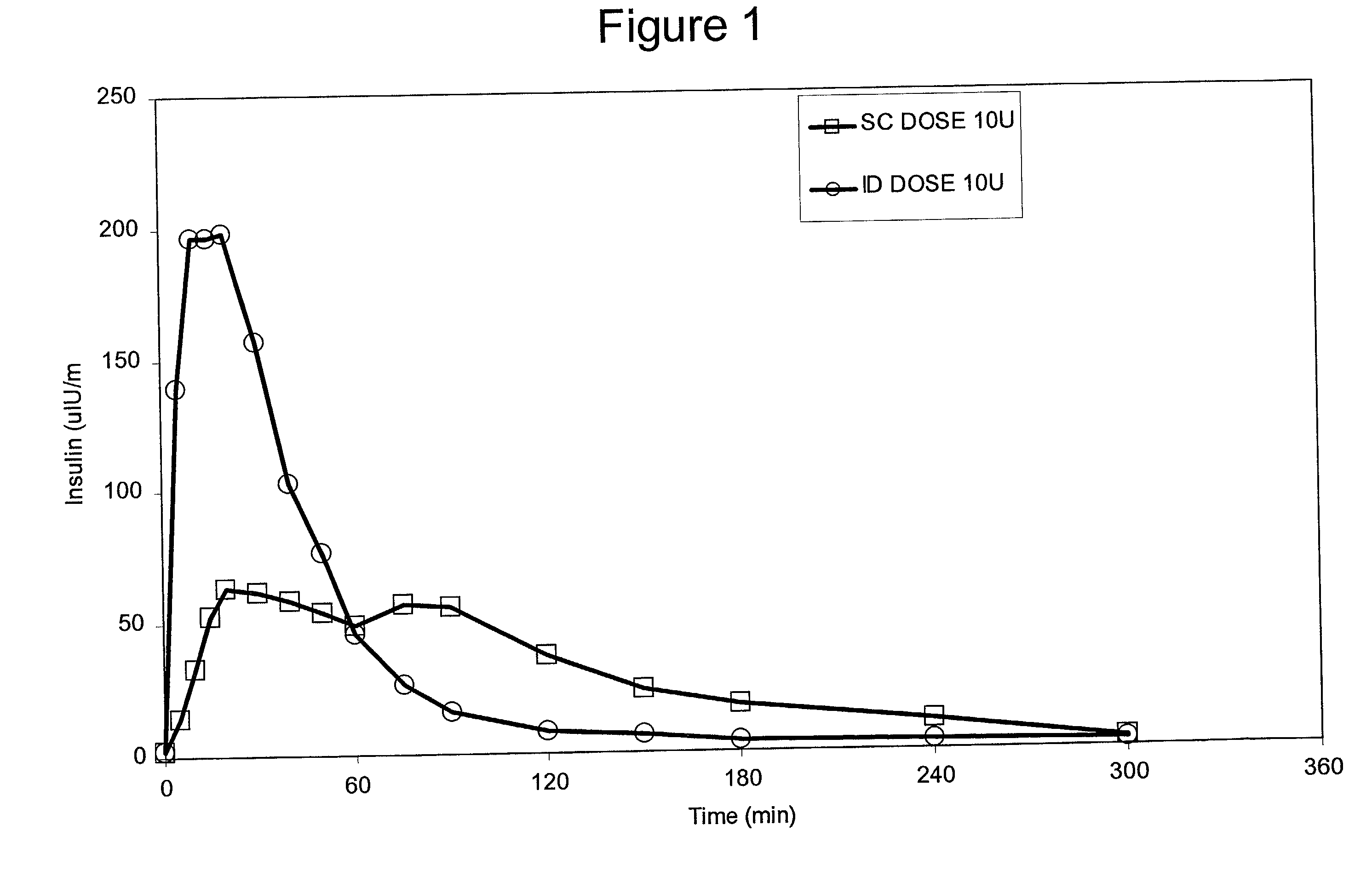
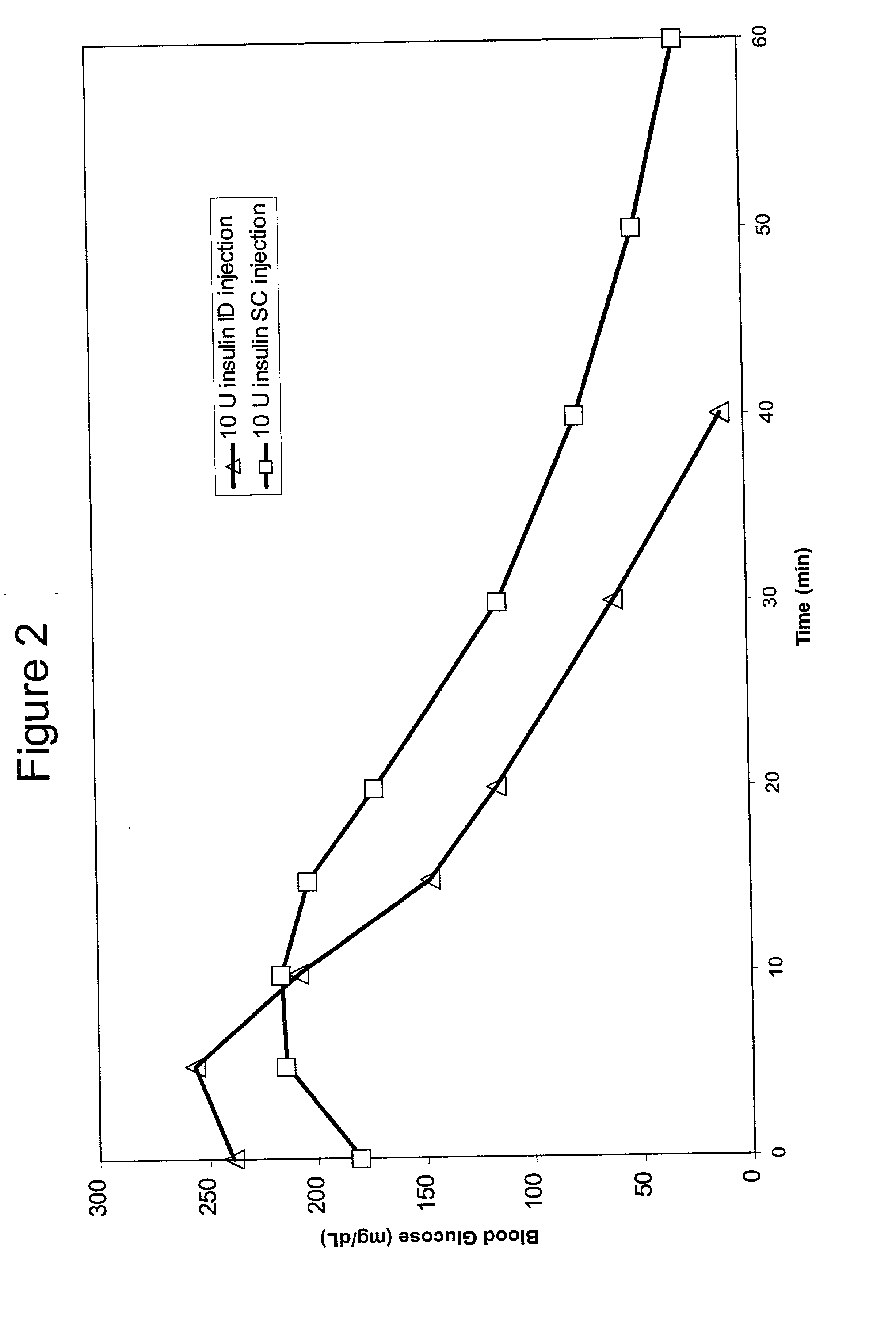
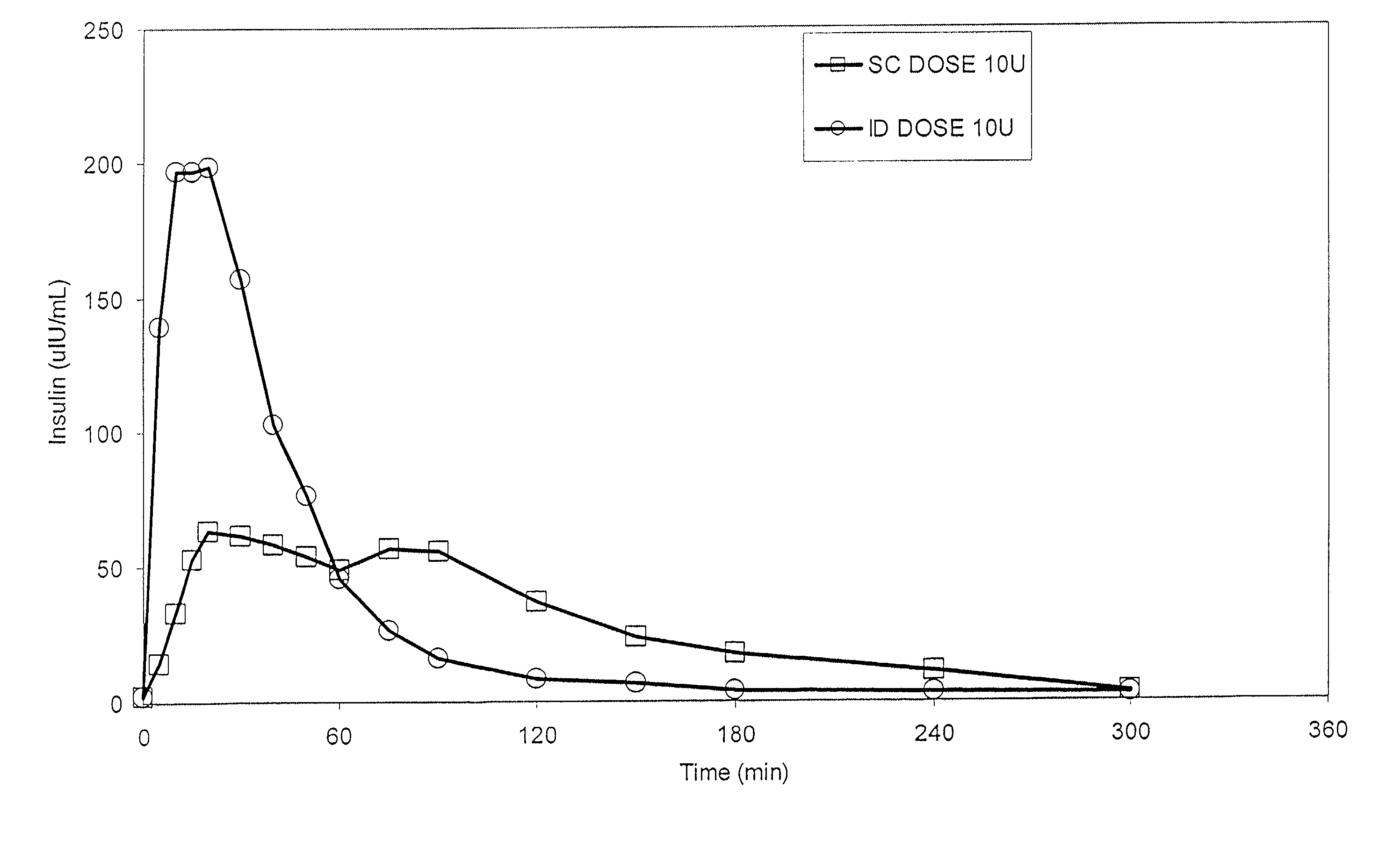
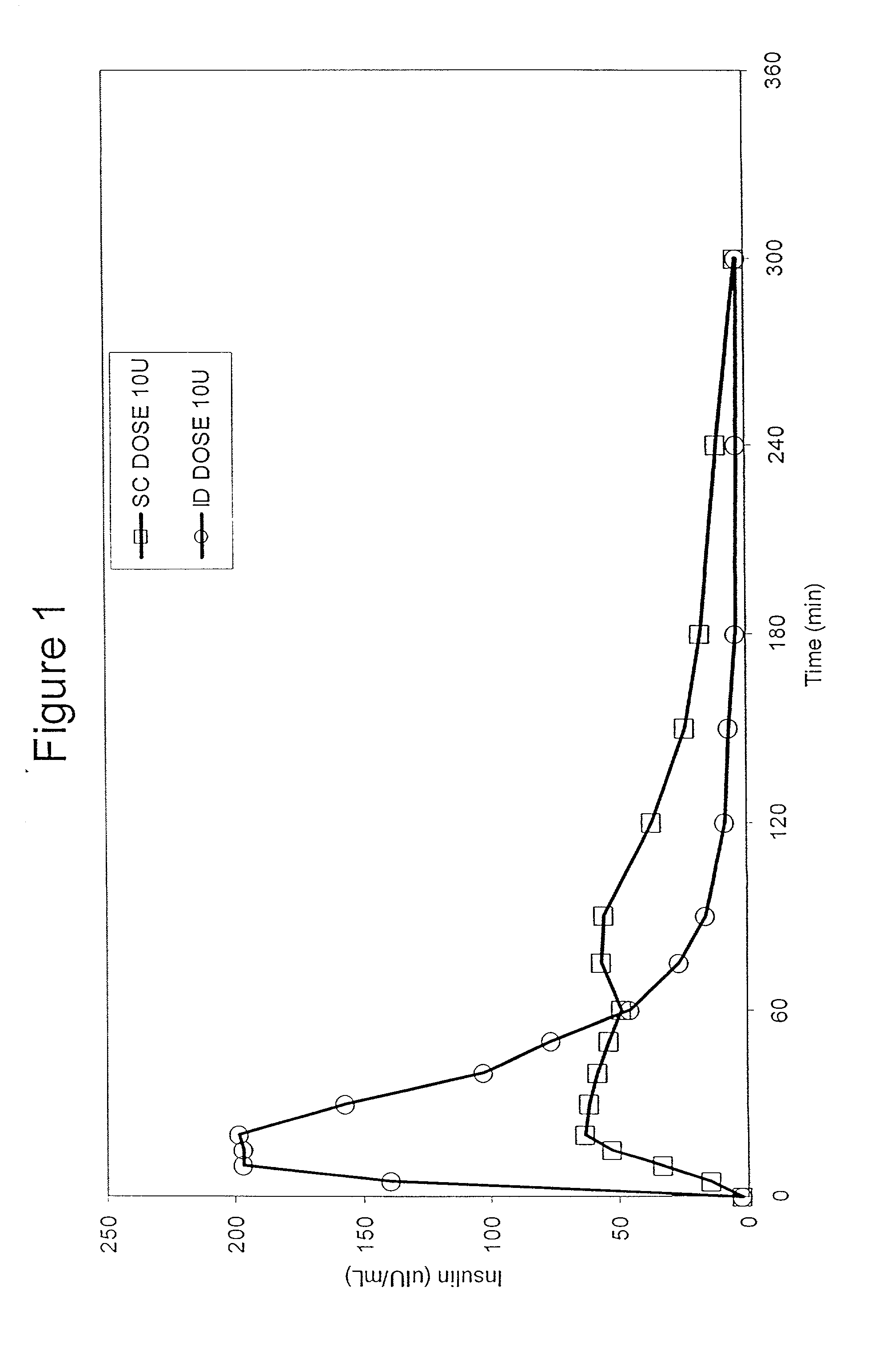
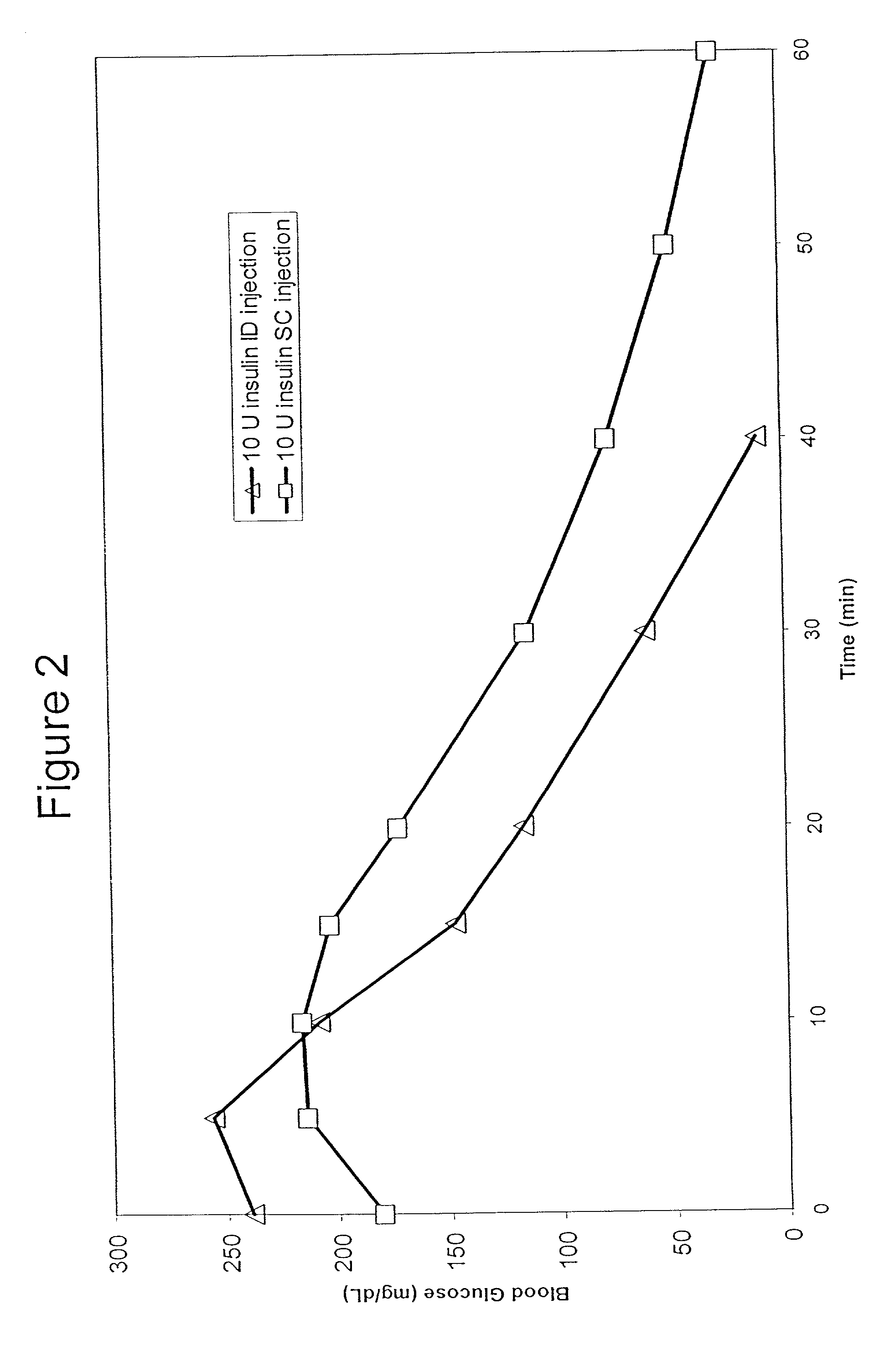
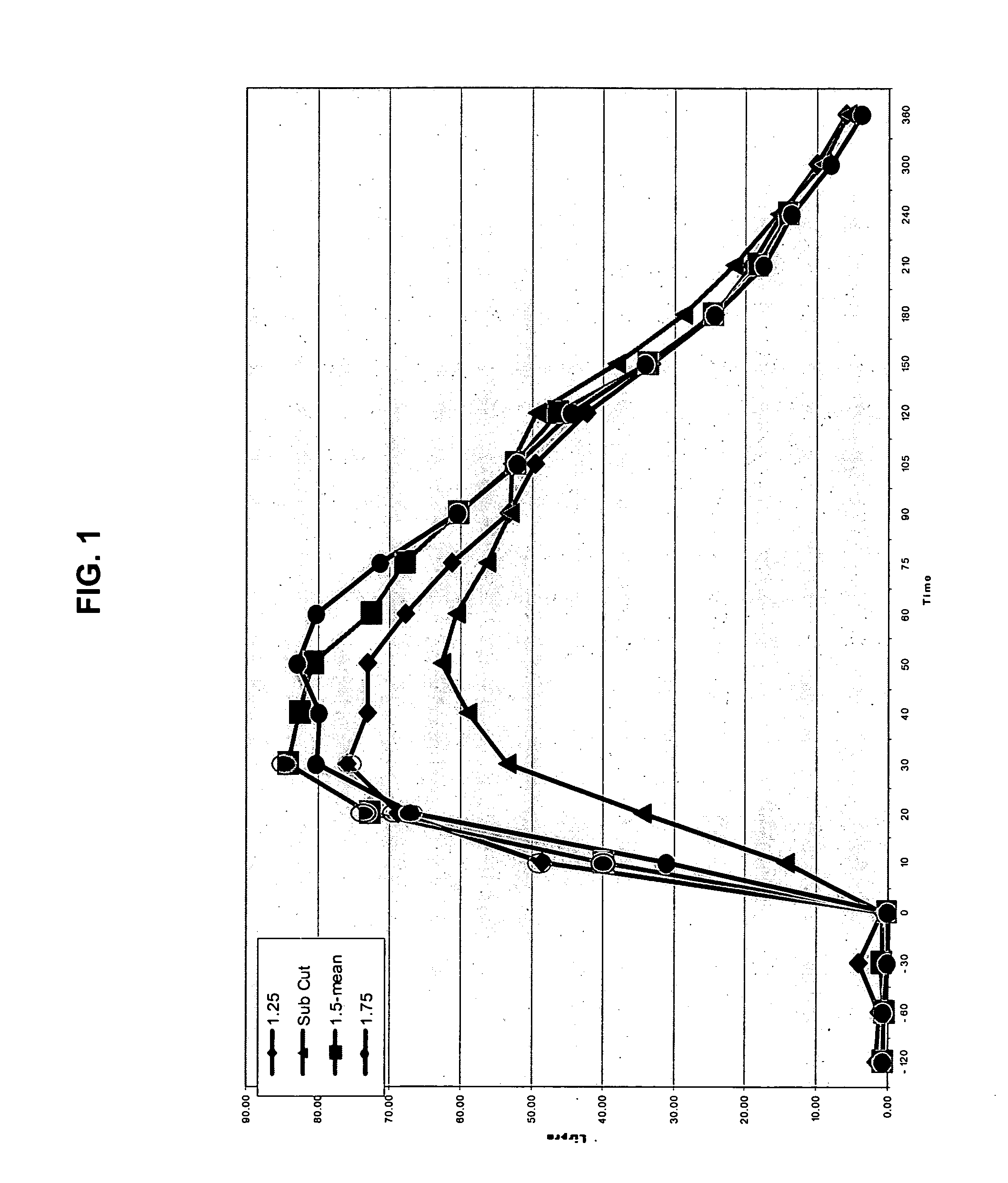
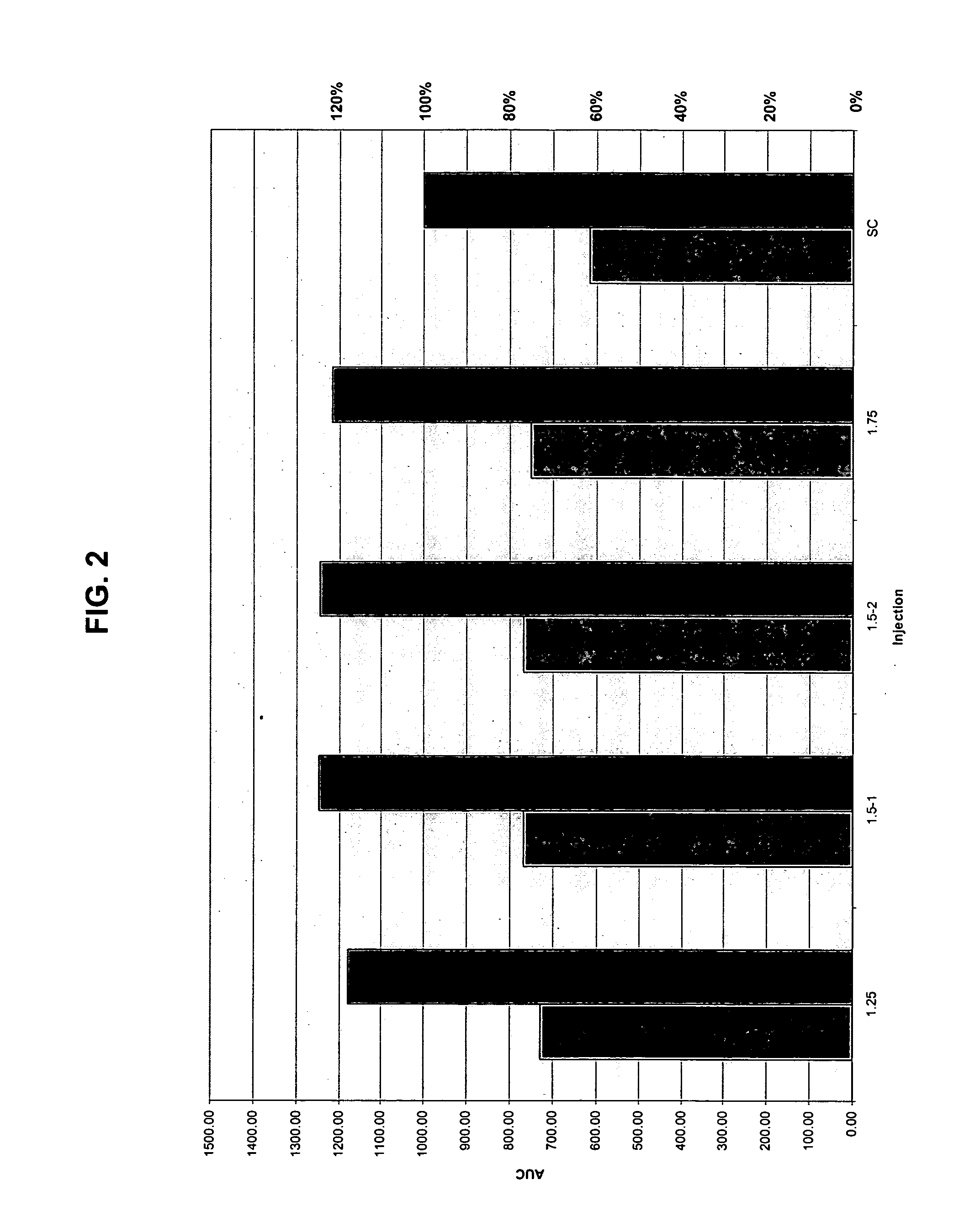
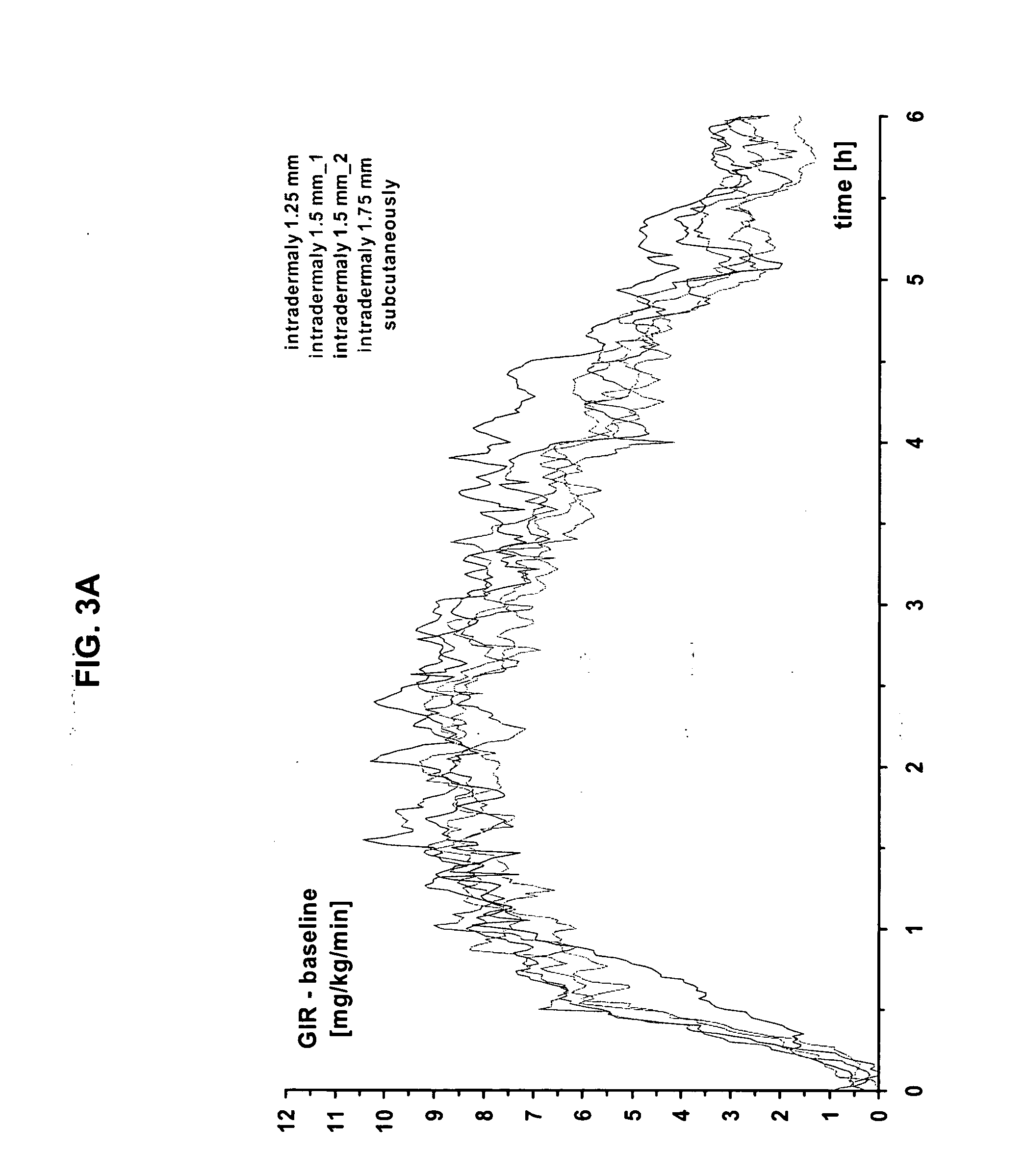
The present invention relates to methods for administration of insulin into the intradermal compartment of subject's skin, preferably to the dermal vasculature of the intradermal compartment. The methods of the present invention enhance the pharmacokinetic and pharmacodynamic parameters of insulin delivery and effectively result in a superior clinical efficacy in the treatment and / or prevention of diabetes mellitus. The methods of the instant invention provide an improved glycemic control of both non-fasting (i.e., post-prandial) and fasting blood glucose levels and thus have an enhanced therapeutic efficacy in treatment, prevention and / or management of diabetes relative to traditional methods of insulin delivery, including subcutaneous insulin delivery.
Owner:BECTON DICKINSON & CO
Method and composition for the treatment of diabetes
InactiveUS6291495B1Increase uptakeImprove utilizationBiocideHeavy metal active ingredientsIGT - Impaired glucose toleranceGlycosidase inhibitor
This invention is directed to a novel method and composition for the treatment of diabetes mellitus (Type I, Impaired Glucose Tolerance ["IGT"] and Type II). More specifically, this invention pertains to a novel method of treating diabetes mellitus by incorporating a therapeutic amount of one or more insulin sensitizers along with one or more of an orally ingested insulin, an injected insulin, a sulfonylurea, a biguanide or an alpha-glucosidase inhibitor for the treatment of diabetes mellitus.
Owner:RIEVELEY ROBERT B
Lipids and lipid assemblies comprising transfection enhancer elements
ActiveUS20080306153A1Promote absorptionImprove fusion effectAntibacterial agentsBiocideLipid formationLiposome
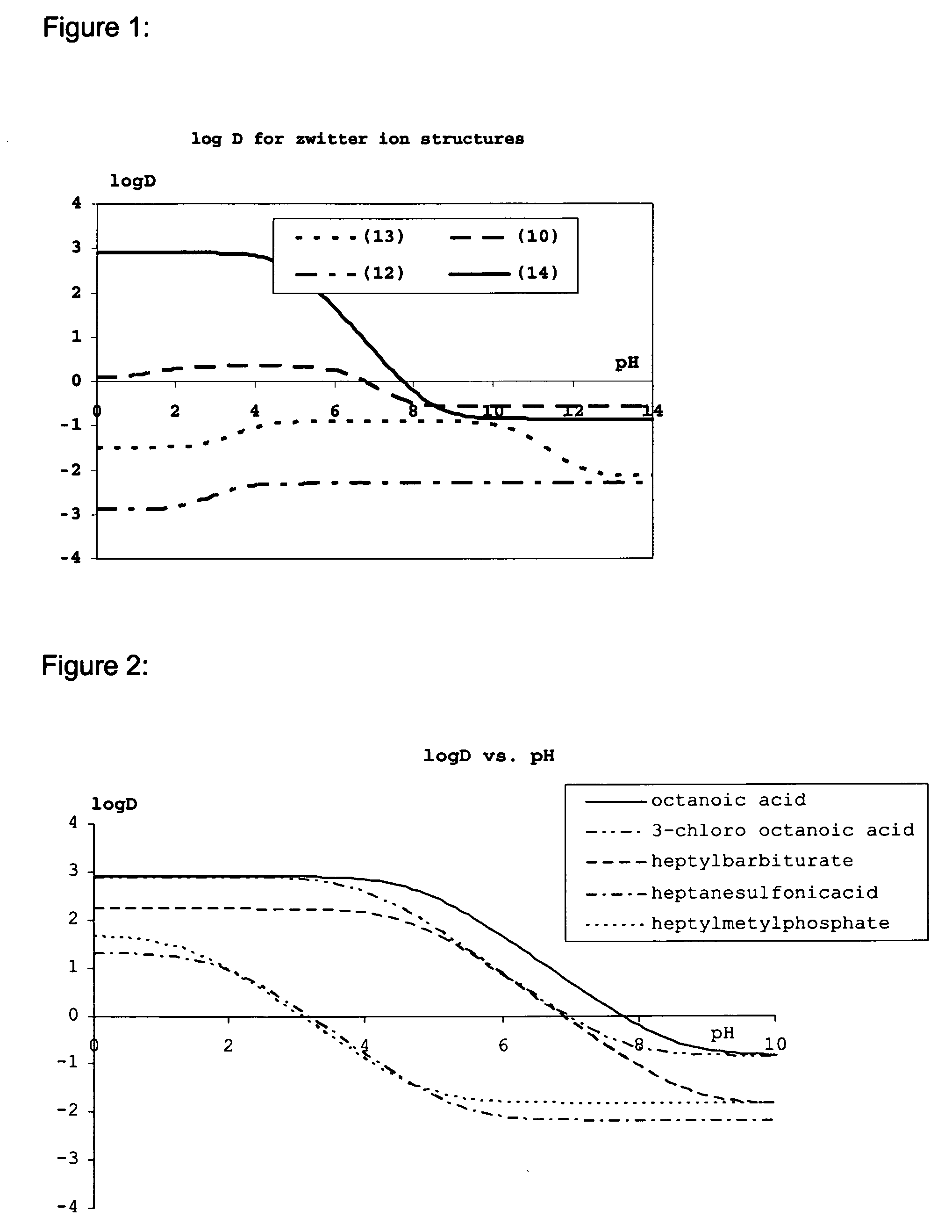
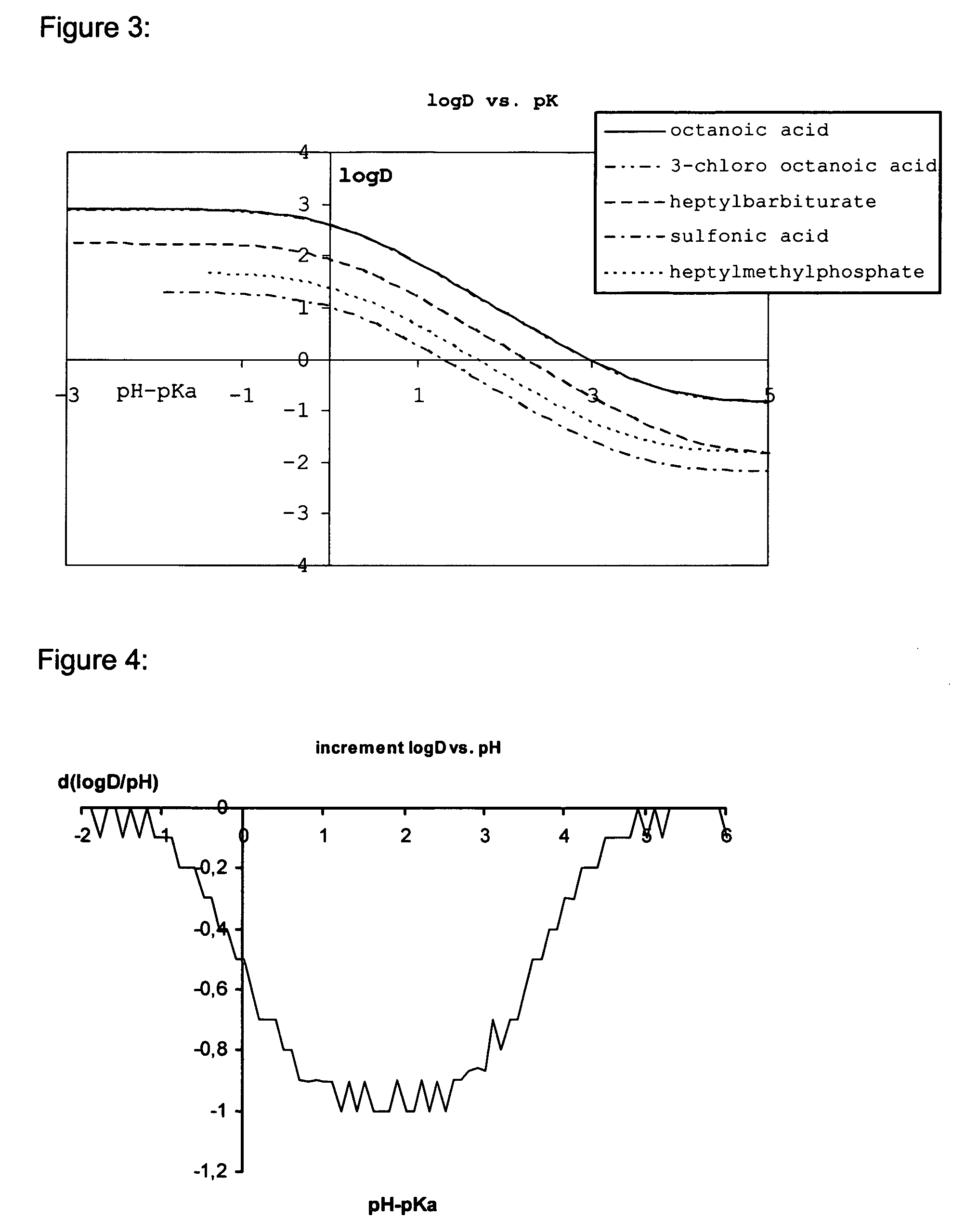
Lipid assemblies, such as liposomes, comprising transfection enhancer elements (TEE's), which are complexed with the lipid assemblies by means of ionic interactions, or lipids incorporating such TEE's are disclosed for enhancing the fusogenicity of the lipid assemblies. The TEE's have the formula:hydrophobic moiety-pH sensitive hydrophilic moiety (II)The pH sensitive hydrophilic moiety of each TEE is a weak acid having a pka of between 2 and 6 or a zwitterionic structure comprising a combination of acidic groups with weak bases having a pKa of between 3 and 8. Lipids incorporating one or more such TEEs have the formula (I):Lipid moiety-[Hydrophobic moiety-pH sensitive hydrophilic moiety] (I)
Owner:BIONTECH DELIVERY TECH GMBH
Bioabsorbable Polymeric Composition for a Medical Device
ActiveUS20090281249A1Less inflammatoryLower potential for traumaStentsSurgeryMedical deviceCopolymer
Owner:ORBUSNEICH MEDICAL PTE LTD
Liquorice vegetable drink for improving gastrointestinal function and enhancing immunity and preparation method of liquorice vegetable drink
The invention discloses a liquorice vegetable drink for improving a gastrointestinal function and enhancing the immunity and a preparation method of the liquorice vegetable drink. Herb of liquorice is used as a main raw material; liquorice concentrated juice, an edible mushroom extract, wolfberry fruit concentrated juice, modified dietary fibers, a corn bee pollen extract, pectin hydrolyzate and the like are scientifically compounded; the raw materials are treated by adopting a modern low-temperature and biological extraction technology, so that the content of effective components of the raw materials is greatly increased; various functional components are organically mixed, so that the optimal synergistic effect is achieved; meanwhile, by adoption of methods for thermal hybrid concentration of unstable concentrated liquid, jointed removal of cold and heat, sterile filtration, and the like, the flavor of the liquorice vegetable drink is greatly enhanced, and the expiration date of a product is prolonged; the liquorice vegetable drink with the effects of improving the gastrointestinal function and enhancing the whole immunity of a human body is prepared.
Owner:邵素英
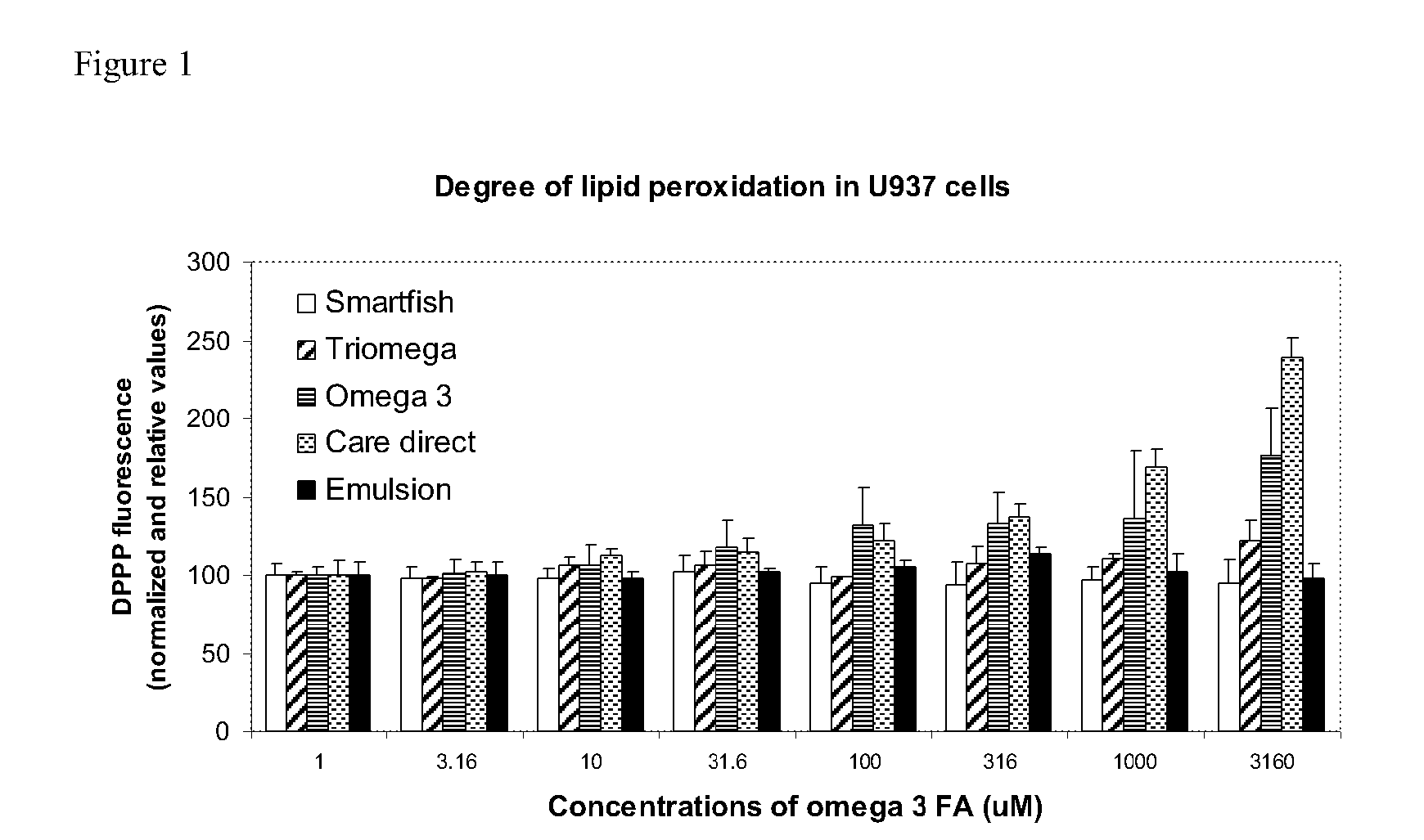
Drink formula comprising fresh marine omega-3 oil and antioxidants
InactiveUS20110135745A1Improve promotion effectReduce deliveryNervous disorderHydrocarbon active ingredientsEmulsionAntioxidant
Owner:SMARTFISH AS
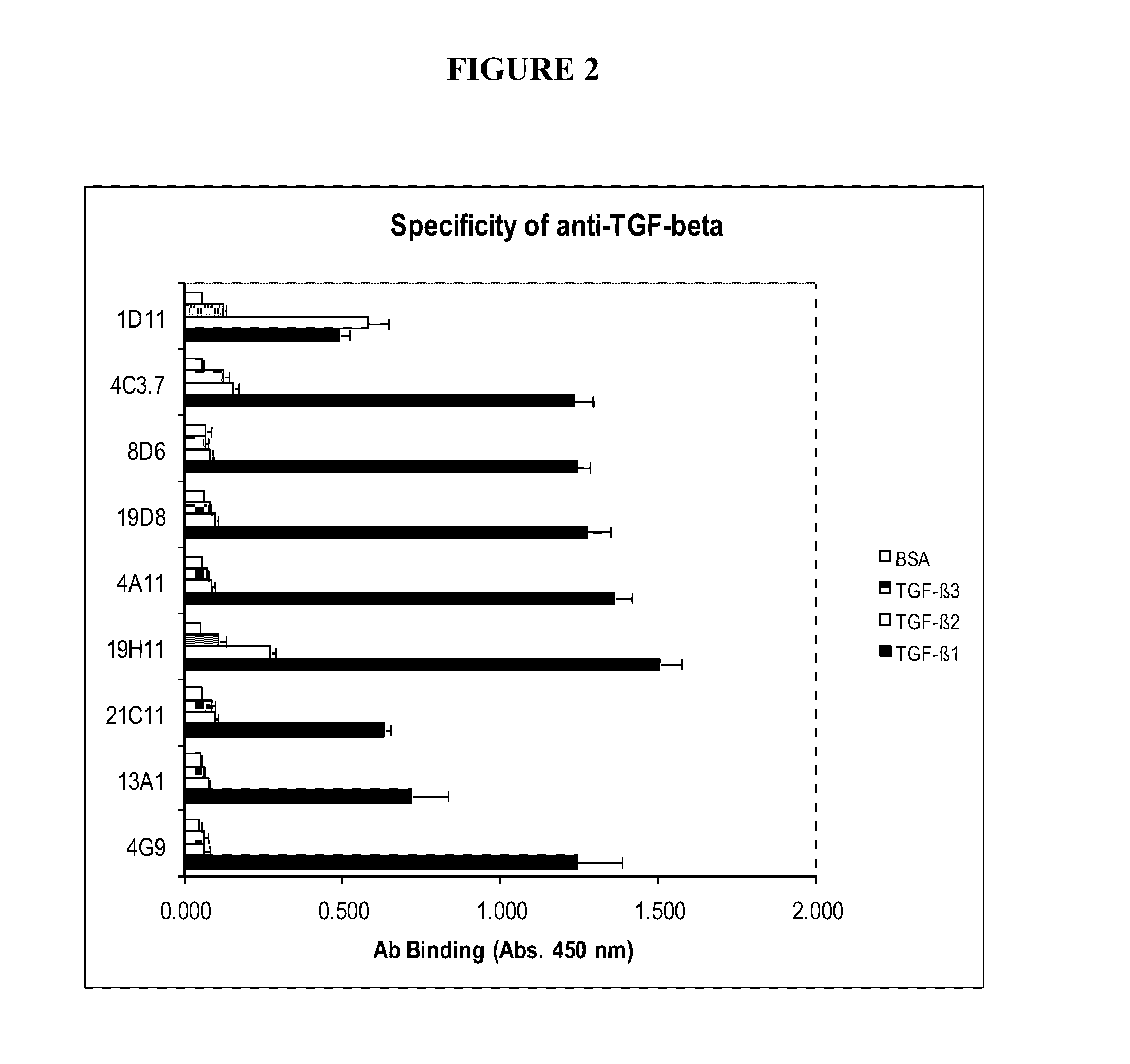
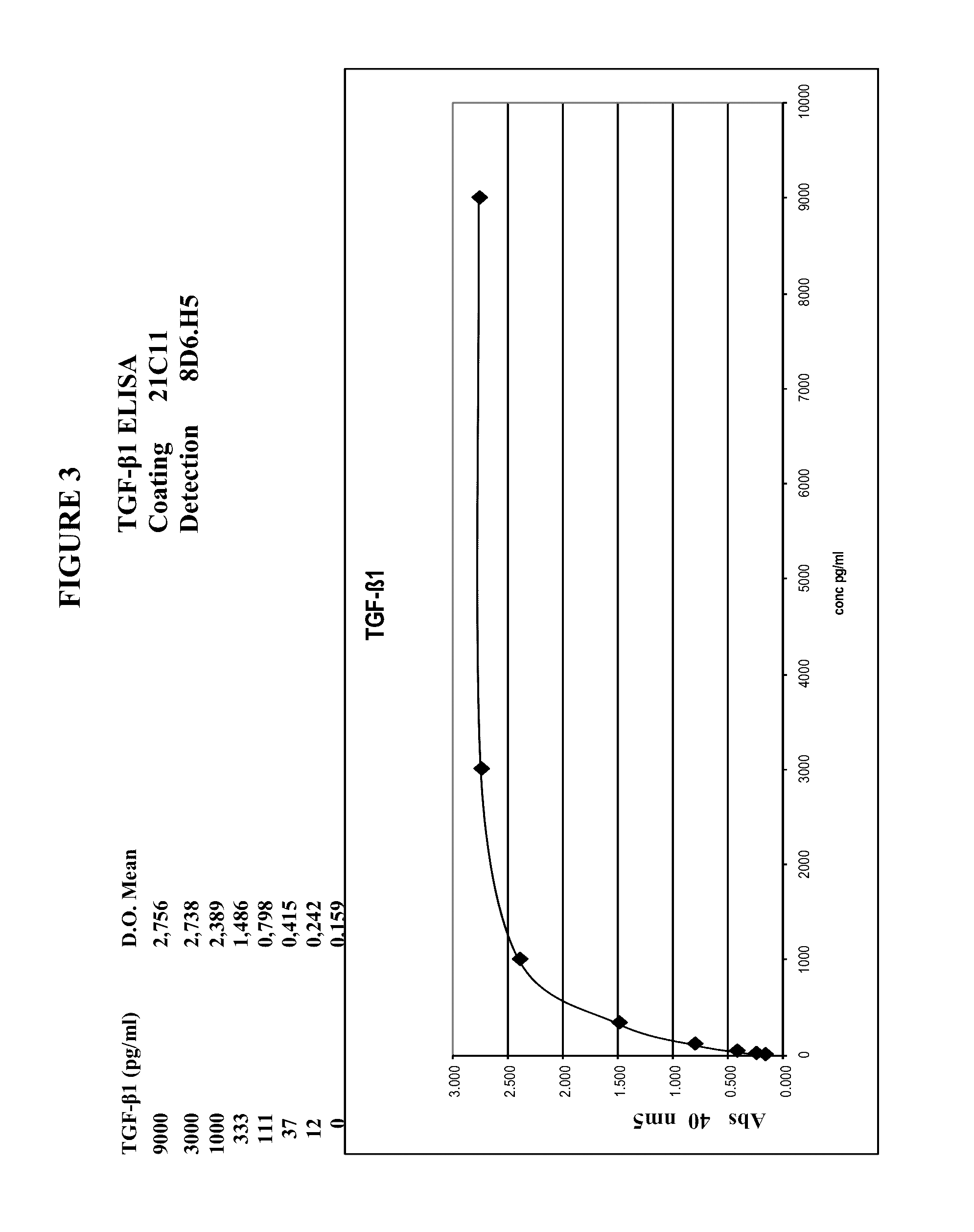
TGF-β1 specific antibodies and methods and uses thereof
ActiveUS9518112B2Less responseLess toxicityImmunoglobulins against growth factorsAntibody ingredientsDiseaseAnticarcinogen
Specific binding members, particularly antibodies and fragments thereof, which bind to transforming growth factor beta 1 (TGF-β1) are provided, particularly recognizing human and mouse TGF-β1 and not recognizing or binding TGF-β2 or TGF-β3. Particular antibodies are provided which specifically recognize and neutralize TGF-β1. These antibodies are useful in the diagnosis and treatment of conditions associated with activated or elevated TGF-β1, including cancer, and for modulating immune cells and immune response, including immune response to cancer or cancer antigens. The anti-TGF-β1 antibodies, variable regions or CDR domain sequences thereof, and fragments thereof may also be used in therapy in combination with chemotherapeutics, immune modulators, or anti-cancer agents and / or with other antibodies or fragments thereof. Antibodies of this type are exemplified by the novel antibodies hereof, including antibody 13A1, whose sequences are provided herein.
Owner:LUDWIG INST FOR CANCER RES LTD
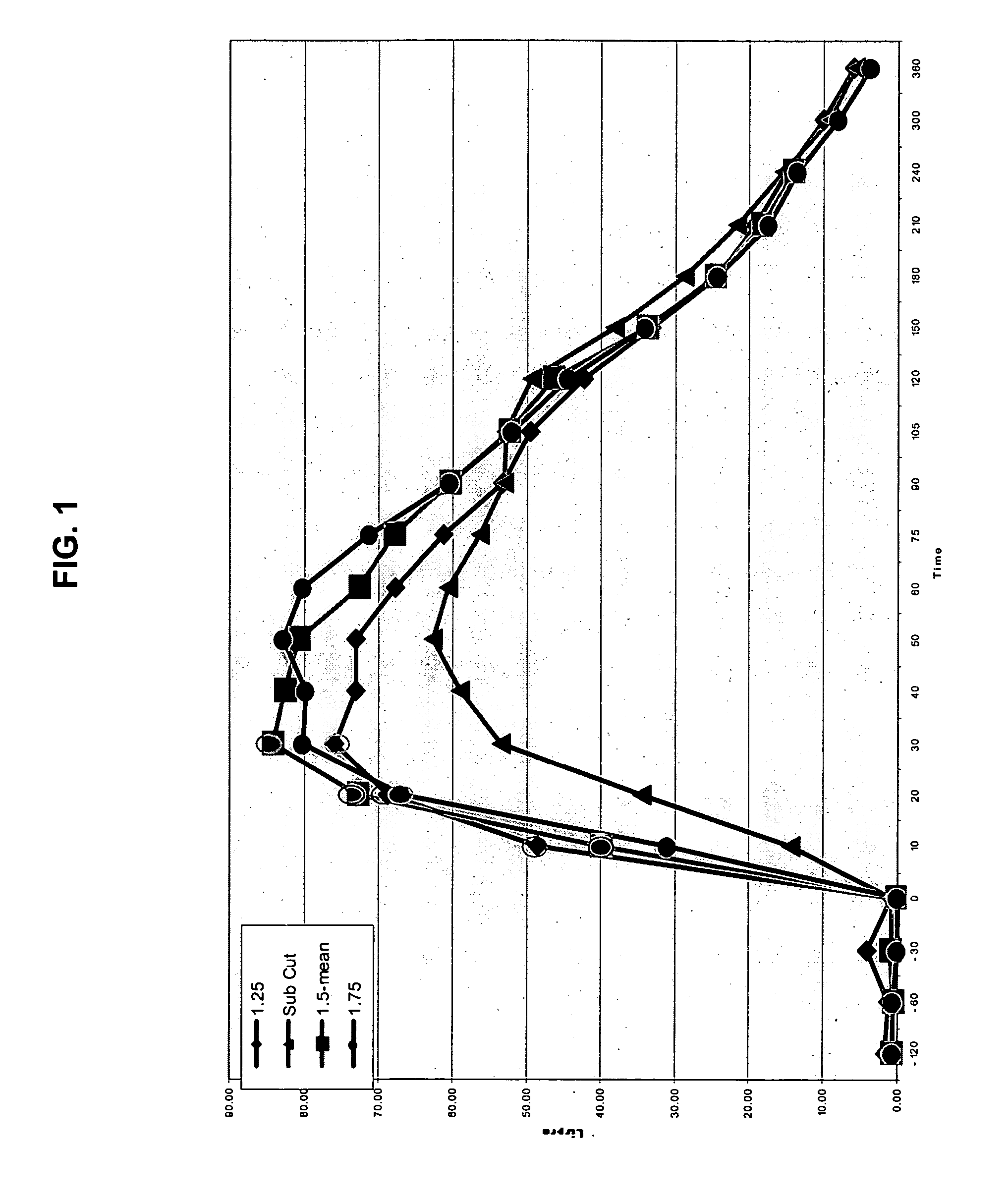
Method for altering insulin pharmacokinetics
InactiveUS20060264886A9Increase in hypoglycemic eventImprove blood sugar controlPeptide/protein ingredientsMetabolism disorderClinical efficacySubcutaneous insulin
The present invention relates to methods for administration of insulin into the intradermal compartment of subject's skin, preferably to the dermal vasculature of the intradermal compartment. The methods of the present invention enhance the pharmacokinetic and pharmacodynamic parameters of insulin delivery and effectively result in a superior clinical efficacy in the treatment and / or prevention of diabetes mellitus. The methods of the instant invention provide an improved glycemic control of both non-fasting (i.e., post-prandial) and fasting blood glucose levels and thus have an enhanced therapeutic efficacy in treatment, prevention and / or management of diabetes relative to traditional methods of insulin delivery, including subcutaneous insulin delivery.
Owner:BECTON DICKINSON & CO
Method for delivering therapeutic proteins to the intradermal compartment
InactiveUS20050196380A1Increase uptakeEnhanced iontophoresisPeptide/protein ingredientsPharmaceutical delivery mechanismTherapeutic proteinBioavailability
The present invention relates to methods and devices for intradermal delivery of substances, preferably therapeutic substances by targeting the substance to the intradermal compartment of a subject's skin. Substances delivered in accordance with the methods of the invention have an improved clinical utility and therapeutic efficacy relative to other drug delivery methods including intramuscular, and subcutaneous delivery. The present invention provides benefits and improvements over conventional drug delivery methods including but not limited to, improved pharmacokinetics and bioavailability.
Owner:BECTON DICKINSON & CO
Edible grain, bean and potato coarse cereal rice composition and preparing method thereof
InactiveCN104855830AHigh economic valueImprove immunityNatural extract food ingredientsFood ingredient functionsHydrolysateEdible Grain
The invention discloses an edible grain, bean and potato coarse cereal rice composition and a preparing method thereof and belongs to the technical field of grain deep processing. According to the edible grain, bean and potato coarse cereal rice composition, broken rice is used as a main raw material, after ultrasonic cleaning, microwave curing, drying and sterilizing of the broken rice, the broken rice, grain powder with a special flavor, enzymatic hydrolysate and other functional raw materials are pelletized, and then grain arrangement and processing through a microwave drying device are carried out, so that the edible grain, bean and potato coarse cereal rice composition is obtained. The edible grain, bean and potato coarse cereal rice composition has a tissue structure, granularity, hardness and viscosity which are similar to those of rice, can be boiled and cooked with rice, has the taste and chewiness, which are similar to those of cooked rice, is unique in flavor, soft in fragrance, high in appetite attractiveness, easy to digest and comprehensive in nutrition, and can improve the immunity and disease resistance of the human bodies and improve the gastrointestinal function. The guarantee period is three times of that of commercial rice. More importantly, a shortcut is developed for the efficient value-added comprehensive utilization of the broken rice, the road for the deep processing of the broken rice is broadened, and the economical value of the broken rice is increased. Better social benefits and economic benefits are realized and a sustainable development road for the deep processing of agricultural byproducts and the effective solving of Three-Rural problems is explored.
Owner:敖汉旗北方粮仓农产品批发市场有限公司
Preparation method of carboxyl and polypeptide modified AIE polymer nanoparticles
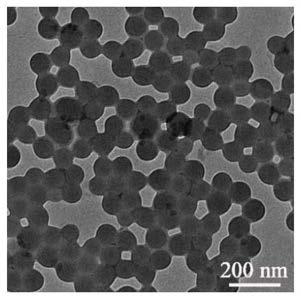
ActiveCN110092858AHigh fluorescence intensityIncrease uptakeLuminescent compositionsFunctional monomerOil phase
The invention relates to a preparation method of carboxyl and polypeptide modified AIE polymer nanoparticles, the method comprises the following steps: 1) preparing an emulsifier aqueous solution; 2)dissolving AIE molecules and an Austrian ripening effect inhibitor in a mixed solution of carboxyl functional monomers and hydrophobic monomers to obtain an oil phase solution; 3) adding the emulsifier aqueous solution into the oil phase solution, stirring and pre-emulsifying to obtain crude emulsion, and performing ultrasonic treatment to obtain monomer miniemulsion; introducing nitrogen to remove oxygen, adding a water-soluble initiator to react; 4) dissolving a carbodiimide condensing agent in an acidic pH buffer solution, and adding the carbodiimide condensing agent into the emulsion prepared by the step 3) for activation; 5) dissolving omega-aminomaleimide in an alkaline pH buffer solution to obtain an omega-aminomaleimide solution; 6) adding the omega-aminomaleimide solution to the emulsion prepared in the step 4) for reaction; and (7) adding a polypeptide aqueous solution containing cysteine sequence units at the tail end to the emulsion prepared in the step (6) for reaction toprepare the carboxyl and polypeptide modified AIE polymer nanoparticles.
Owner:ZHEJIANG SCI-TECH UNIV
Axially substitutive silicon phthalocyanine complex and doxorubicin conjugate thereof
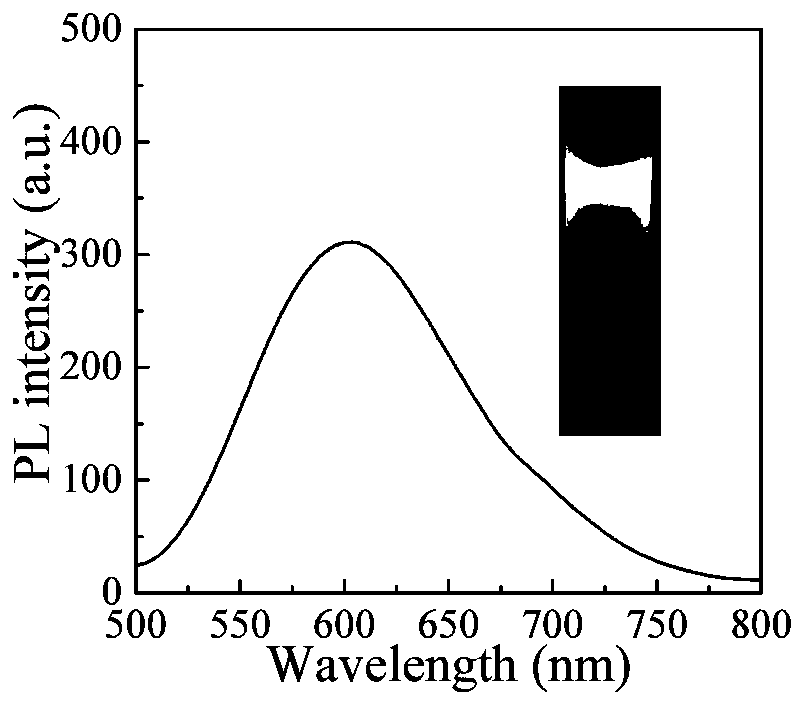
ActiveCN105669735AGood amphipathyHigh uptake by cancer cellsSilicon organic compoundsOrganic active ingredientsAnti cancer drugsDoxorubicin
The invention discloses an axially substitutive silicon phthalocyanine complex and a doxorubicin conjugate thereof and a preparation method and application of the axially substitutive silicon phthalocyanine complex and the doxorubicin conjugate thereof, and belongs to the technical field of photosensitizer and drug preparation.The complex can be prepared into a novel efficient photodynamic drug or photosensitizer, and the complex and doxorubicin can be prepared into the silicon phthalocyanine-doxorubicin conjugate.The coupling agent has both a photodynamic therapy effect and a chemotherapy effect and can be prepared into a novel anti-cancer drug.
Owner:FUZHOU UNIVERSITY
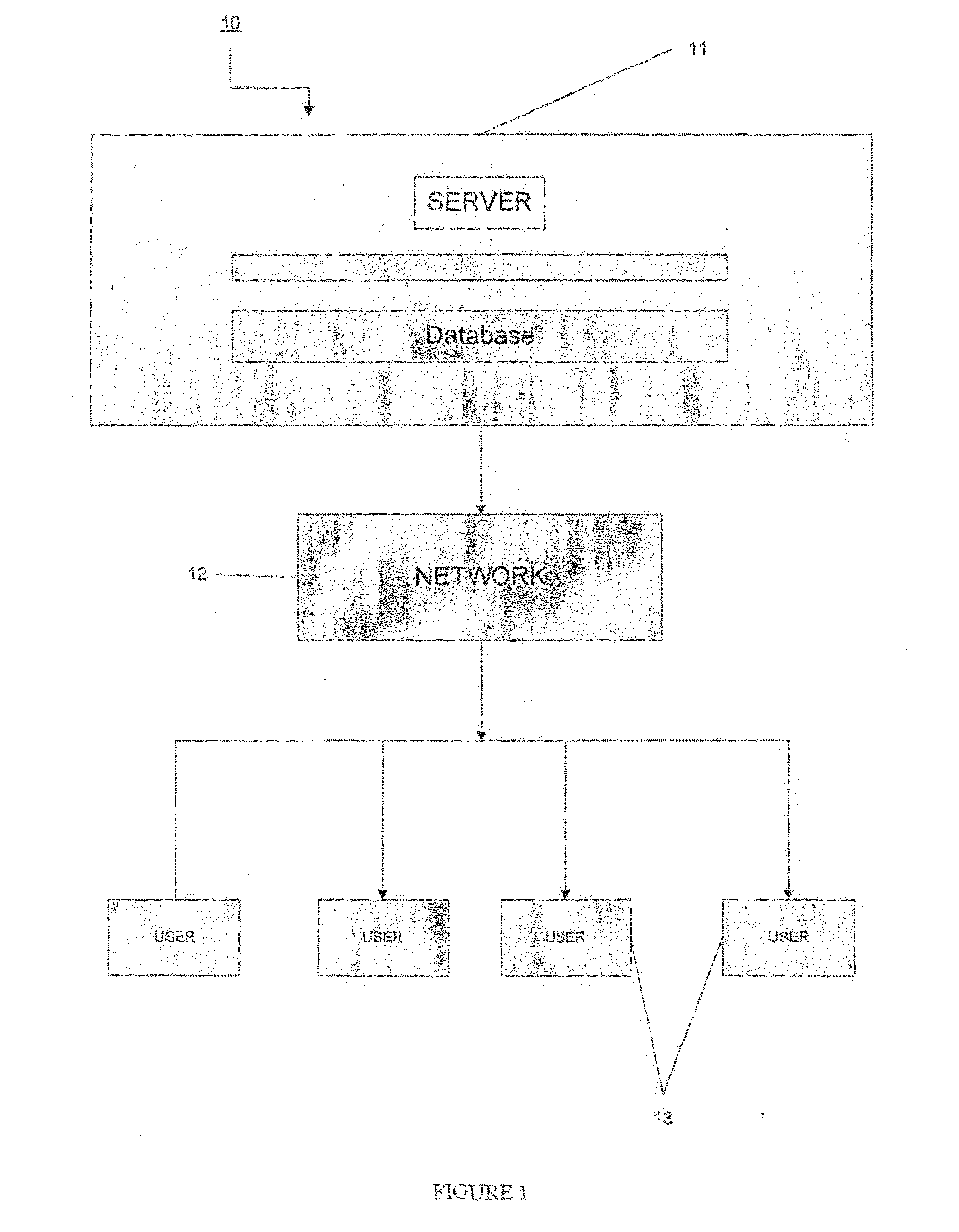
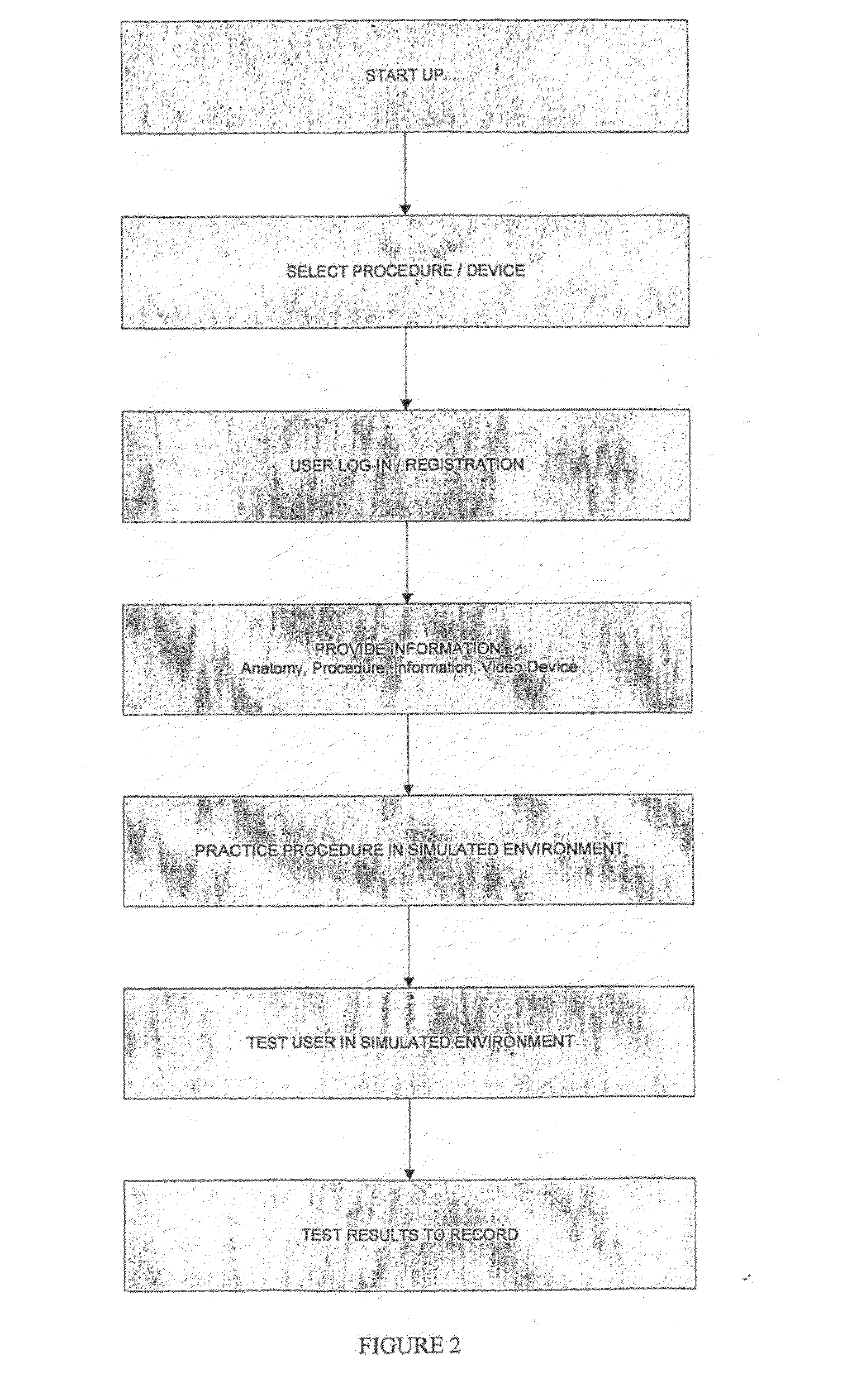
Medical instruction system
InactiveUS20090317781A1Increase uptakeIncrease exposureEducational modelsElectrical appliancesComputer programMedical treatment
The invention relates to a method, system, method of doing business, and computer program product for providing instruction and device training. More particularly, the invention provides information and training around medical, veterinary and anatomical procedures. The procedures are presented as a multimedia interactive platform to provide improved instruction and training.
Owner:GO VIRTUAL MEDICAL
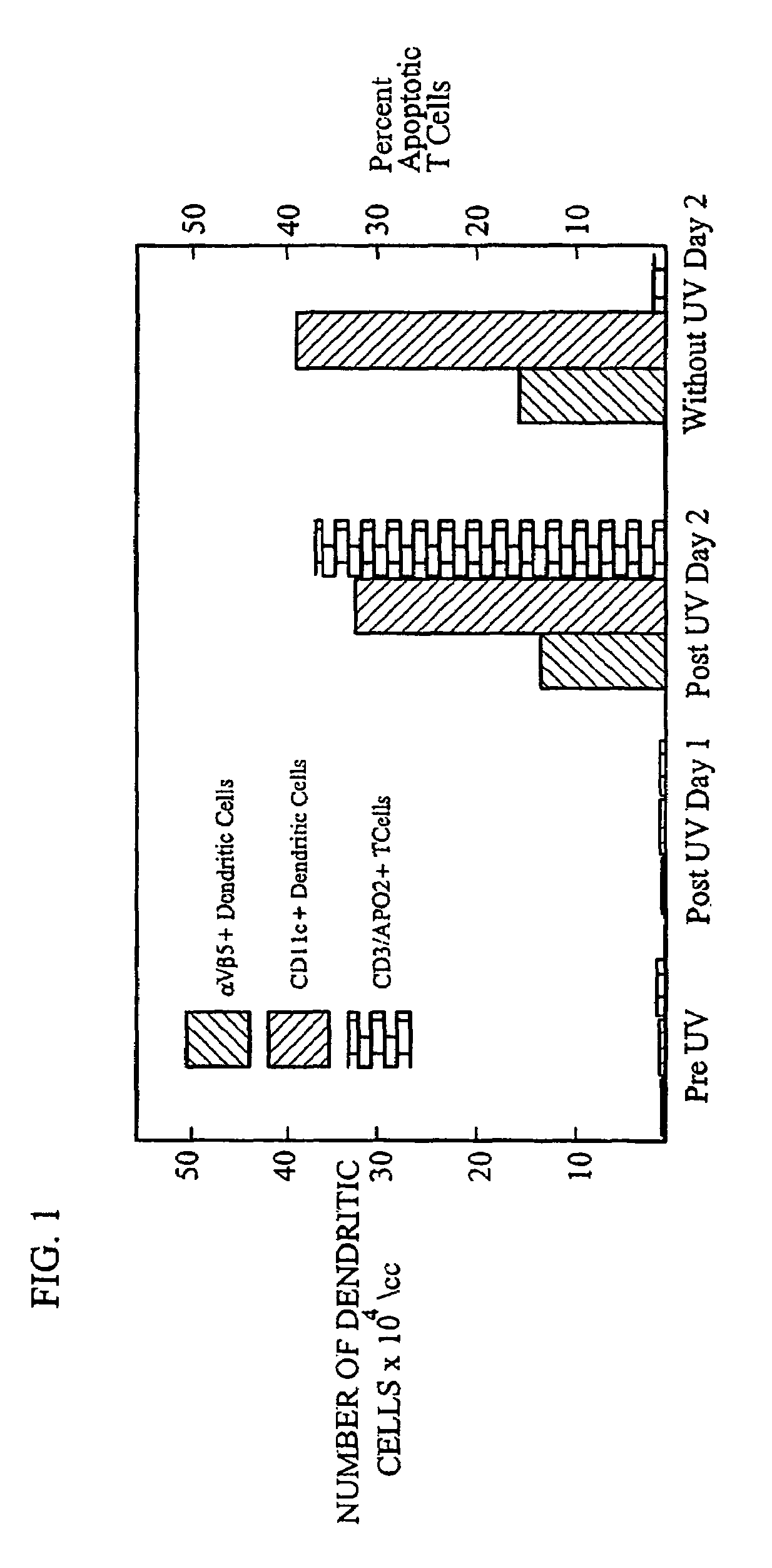
Methods for inducing the differentiation of monocytes into functional dendritic cells and immunotherapeutic compositions including such dendritic cells
A method for inducing differentiation of monocytes contained in an extracorporeal quantity of a subject's blood into functional dendritic antigen presenting cells is provided. The monocytes are induced to differentiate into dendritic cells by activation forces resulting from flow of the monocytes through a treatment apparatus having plastic channels. The interior surface of the plastic channel may be modified to increase the available surface area for interaction with blood monocytes. Platelets and serum protein may be removed from the blood prior to treatment to reduce or eliminate contamination of the plastic channel by these blood components. Functional dendritic cells generated from induced monocytes are incubated together with apoptotic or inactivated disease effector agents to enhance the presentation of at least one disease-causing antigen expressed by the disease effector agents. Compositions including dendritic cells derived from induced monocytes and compositions including such dendritic cells incubated with disease effector agents are also provided for use in immunotherapeutic treatment.
Owner:YALE UNIV
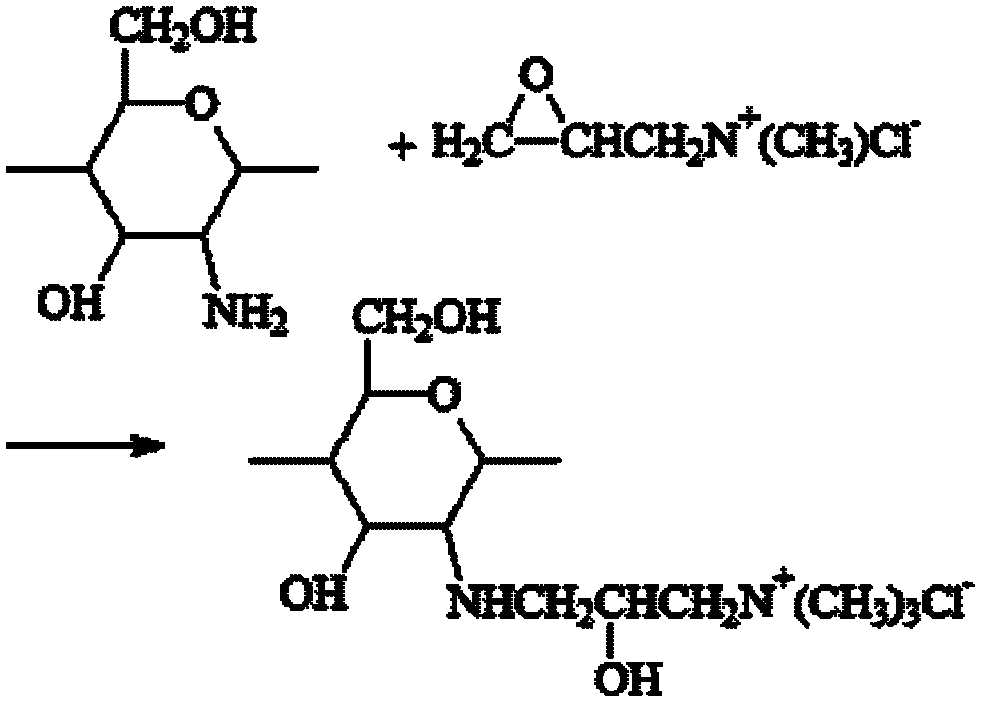
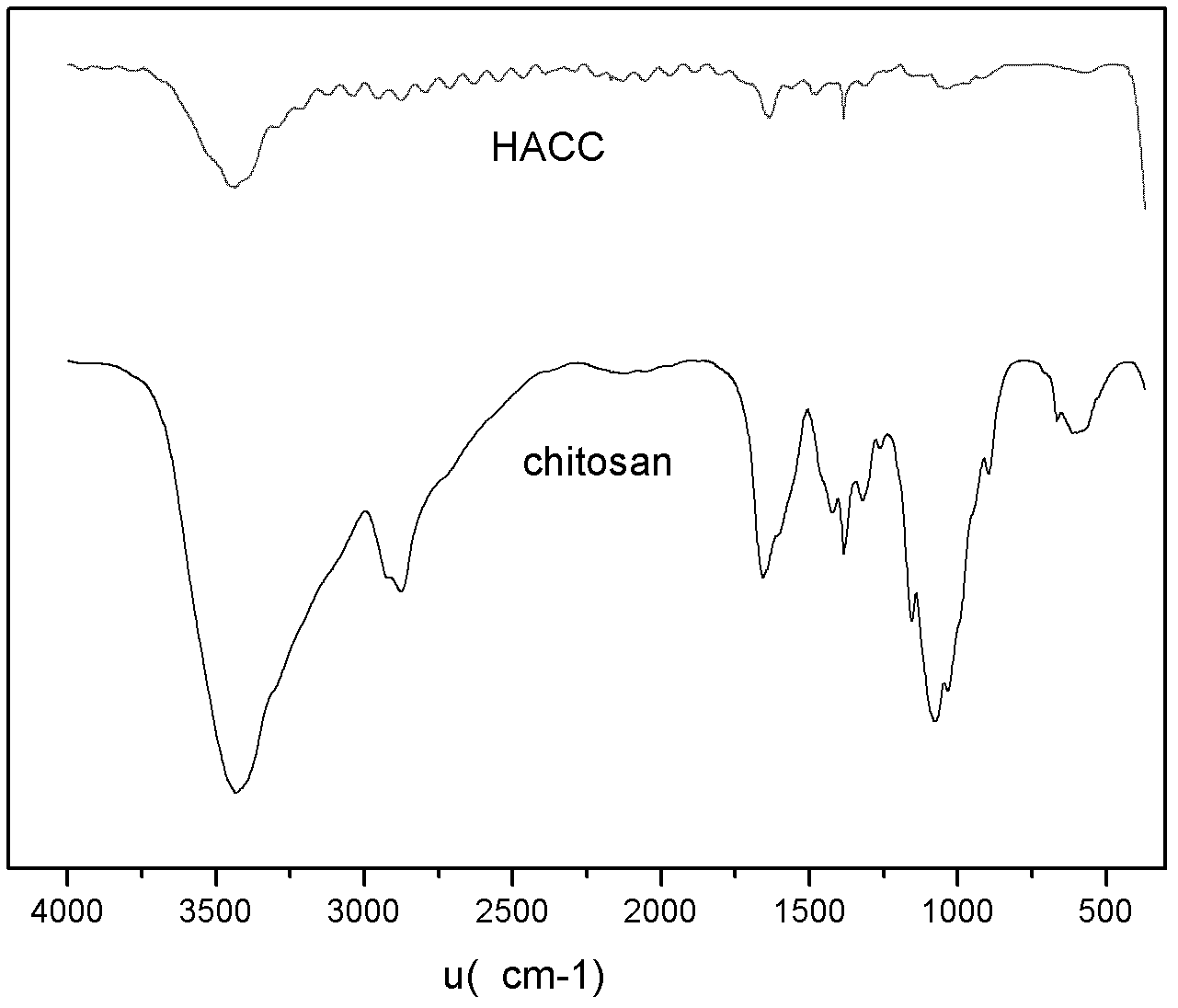
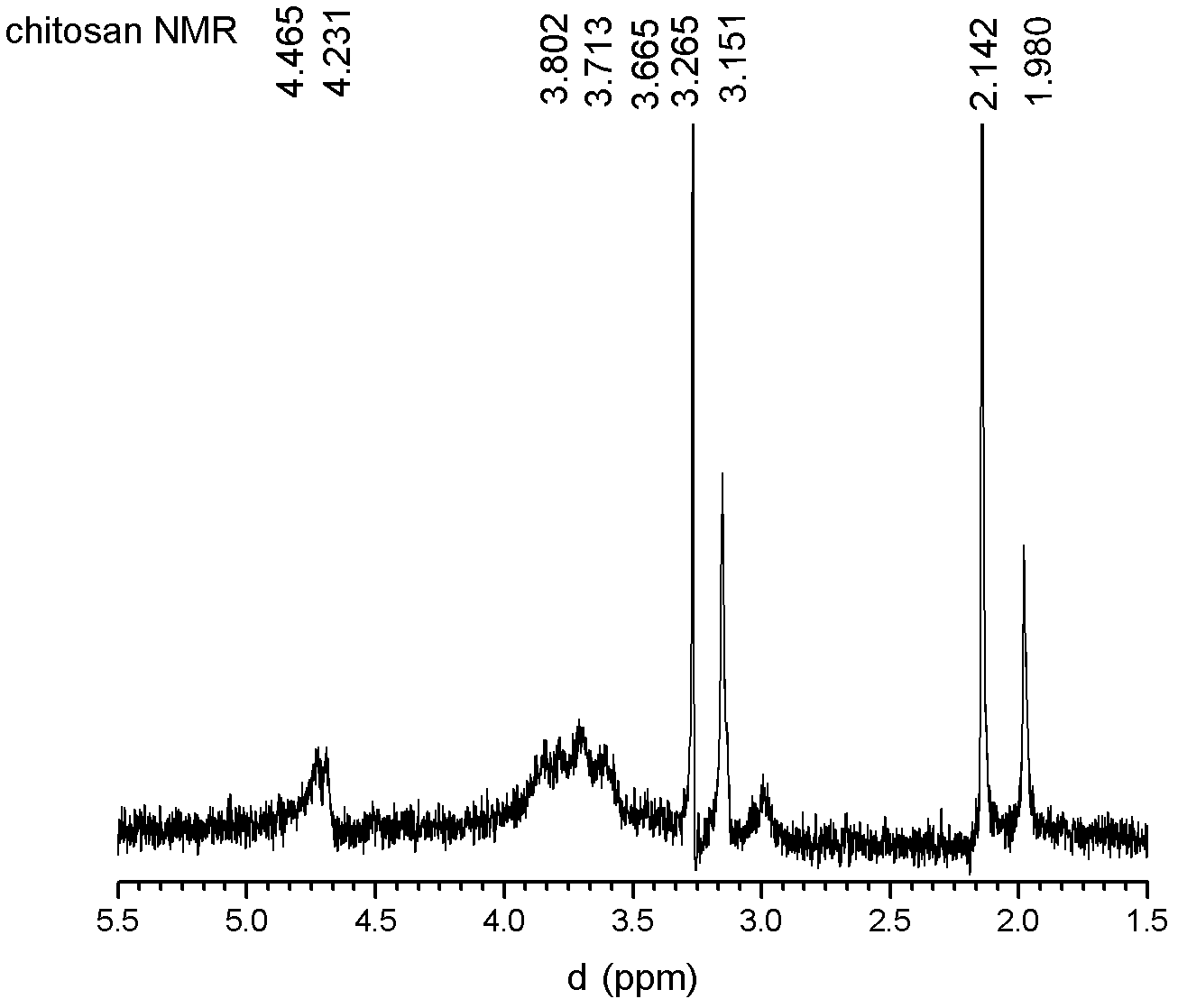
Synthetic method of N-2-hydroxypropyl trimethyl ammonium chloride chitosan and preparation method of Newcastle disease attenuated live vaccine-loaded nanoparticles of N-2-hydroxypropyl trimethyl ammonium chloride chitosan
ActiveCN102432695ARaw materials are easy to getEasy to operatePowder deliveryViral antigen ingredientsSide effectFreeze-drying
The invention provides a synthetic method of N-2-hydroxypropyl trimethyl ammonium chloride chitosan and a preparation method of Newcastle disease attenuated live vaccine-loaded nanoparticles of the N-2-hydroxypropyl trimethyl ammonium chloride chitosan, relating to a synthetic method of chitosan and a preparation method of vaccine-loaded nanoparticles of the chitosan. The synthetic method comprises the following steps of: deacelation of the chitosan; dip-treatment of the chitosan; crude preparation of the N-2-hydroxypropyl trimethyl ammonium chloride chitosan; and refined preparation of the N-2-hydroxypropyl trimethyl ammonium chloride chitosan. The preparation method comprises the following steps of: adding a Newcastle disease virus solution into an N-2-hydroxypropyl trimethyl ammonium chloride chitosan solution to obtain a solution A; adding sodium tripolyphosphate, PBS (phosphate buffer solution) and span-80 into the solution A to obtain a solution B; and centrifuging the solution B to obtain a deposit, adding PBS for suspension, adding mycose skimmed milk, and performing freeze drying to finish the preparation. The nanoparticles prepared by using the method has the advantages of easiness in control of particle size, small particle size of drug-loaded nanoparticles, high entrapment efficiency, large drug-loading quantity, mild preparation conditions, low drug toxic or side effect, long slow release time, simple preparation process, lower production cost and easiness in large-scale production.
Owner:HEILONGJIANG UNIV
Nano-medicinal carrier with magnetocaloric effect as well as preparation method and application thereof
InactiveCN104083765AStrong specificityGood delivery efficiencyInorganic non-active ingredientsNanomedicineAnti cancer drugsNanometre
The invention provides a nano-medicinal carrier with magnetocaloric effect. The nano-medicinal carrier with grain diameter of 50-200 nanometers comprises mesoporous silica particles and Fe3O4 nano-particles embedded inside the mesoporous silica particles, wherein the mesoporous diameter of the mesoporous silica particles is 2-10 nanometers, and the mesoporous channel is in a radial shape from the inner core to outside; the diameter of the Fe3O4 particles is 15-20 nanometers. The invention further provides a preparation method of the nano-medicinal carrier and application of the nano-medicinal carrier in loading anti-cancer medicaments. The nano-medicinal carrier is high in drug storage capacity, and can be used for remarkably improving the anti-cancer drug high-performance transmission efficiency, remarkably improving the curative effect of tumor treatment, and realizing cancer therapy by virtue of medical chemotherapy and magnetocaloric therapy.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Antibody nanoparticles coated by red cell membranes for antibody drug delivery and preparation method
ActiveCN105769818AImprove stabilityIncrease uptakeAntibody ingredientsPharmaceutical non-active ingredientsImmune clearanceClearance rate
The invention discloses antibody nanoparticles coated by natural red cell membranes for antibody drug delivery and a preparation method of the antibody nanoparticles. The red cell membranes in the invention are extracted from experimental rats, most of the contents and unrelated proteins are removed after the red cell membranes are treated, and protein nanoparticles formed by antibodies are coated with the obtained red cell membranes. The method is suitable for coating nanoparticles formed by different antibodies through the red cell membranes, can improve the stabilities and the in-vivo circulation times of free antibodies, and further reduces the body immunity clearance rate.
Owner:EAST CHINA NORMAL UNIV
Processes and plants for reducing ammonia loss and odor from organic material or waste to the atmosphere
ActiveUS20150299056A1Reducing ammonia lossReduce odorBio-organic fraction processingMethane captureNitritePlasma generator
The invention relates to processes for reducing ammonia loss and odor from organic material or waste to the atmosphere. A plasma generator is applied to upgrade organic waste and manure with a mixture of acidic nitrates and nitrites. The present invention also relates to an acidic nitrate solution, suitable for reducing ammonia loss and odor from organic material or waste to the atmosphere, and a process for producing such an acidic nitrate solution. The invention further comprises plants for reducing ammonia loss and odor from organic material or waste to the atmosphere.
Owner:N2 APPLIED
Chitosan-modified methazolamide solid lipid nanoparticles and preparation method thereof
InactiveCN102793672AEasy to stayIncrease uptakeOrganic active ingredientsPowder deliveryLipid formationCorneal permeability
The invention provides chitosan-modified methazolamide solid lipid nanoparticles and a preparation method thereof. The chitosan-modified methazolamide solid lipid nanoparticles can be used as eye drops. The preparation method is suitable for industrial production. The chitosan-modified methazolamide solid lipid nanoparticles are characterized in that based on 30ml of a nanoparticle-containing solution, the chitosan-modified methazolamide solid lipid nanoparticles comprise 5mg of methazolamide, 25 to 150mg of at least one lipid material, 25 to 150mg of phospholipid, 5 to 100mg of chitosan, 50 to 250mg of at least one non-phospholipid surfactant and 50 to 250mg of at least one assistant surfactant. The chitosan-modified methazolamide solid lipid nanoparticles have small particle sizes and high drug entrapment efficiency. Compared with solid lipid nanoparticles which are not modified by chitosan, the chitosan-modified methazolamide solid lipid nanoparticles have higher stability and better corneal permeability because of positive charges on the surfaces of the chitosan-modified methazolamide solid lipid nanoparticles so that drug bioavailability is improved. Therefore, the chitosan-modified methazolamide solid lipid nanoparticles have a large clinical application potential in glaucoma treatment.
Owner:NANJING MEDICAL UNIV
Methods and devices for administration of substances into the intradermal layer of skin for systemic absorption
InactiveUS20080118465A1Increase uptakeShorter TmaxAmpoule syringesJet injection syringesWhole bodySystemic absorption
Methods and devices for administration of substances into the intradermal layer of skin for systemic absorption.
Owner:PETTIS RONALD J +2
Synthetic method of LDH@SiO2 shell-nuclear nano composite material
ActiveCN104310408AGood immune effectImprove immunityMaterial nanotechnologySilicaAnhydrous ethanolAmmonia
The invention relates to a synthetic method of an LDH@SiO2 shell-nuclear nano composite material. The method comprises the following steps: I, magnetically stirring and uniformly mixing anhydrous ethanol, deionized water and ammonia water, then, adding ethyl orthosilicate, continuously stirring, standing, centrifuging, collecting precipitates, washing to neutral and drying; II, dissolving NaOH and NaOH3 in deionized water with CO2 removed and dispersing SiO2 nano particles therein to obtain liquid A; III, dissolving 15-20g of Mg(NO3)3.6H2O and 10-12g of Al(NO3)3.9H2O in deionized water without CO2 to obtain liquid B; and IV, dropwise adding the liquid B into the liquid A while stirring, stirring at constant temperature, washing the precipitates by using the deionized water without CO2, and drying in vacuum to obtain the material. The material provided by the invention is uniform in particle size distribution and easy to control, simple in preparation process, relatively low in production cost and easy for scaled production.
Owner:HEILONGJIANG UNIV
1-3 generation arylene ether dendritic phthalocyanine complex and polymer nano-particle thereof
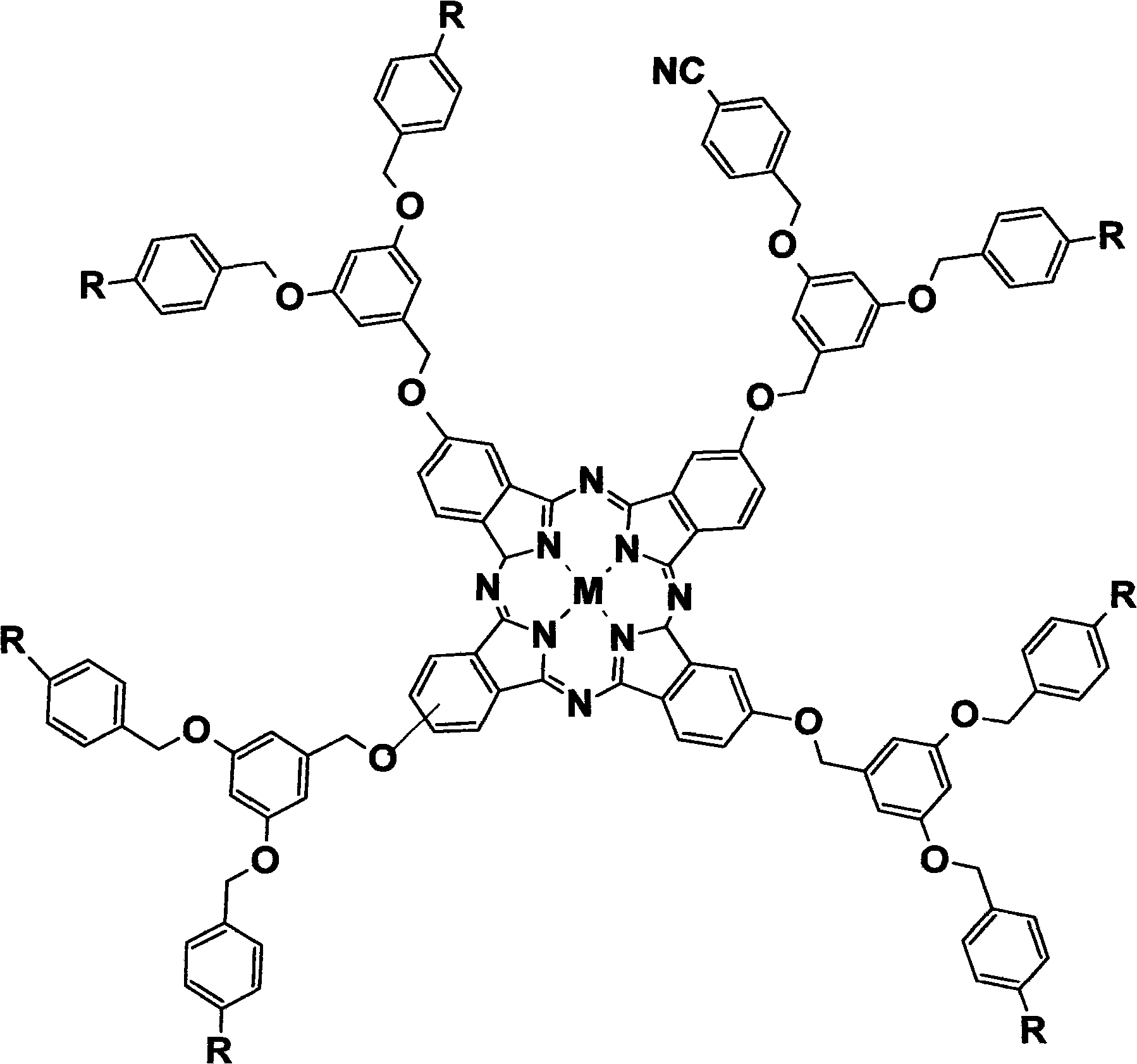
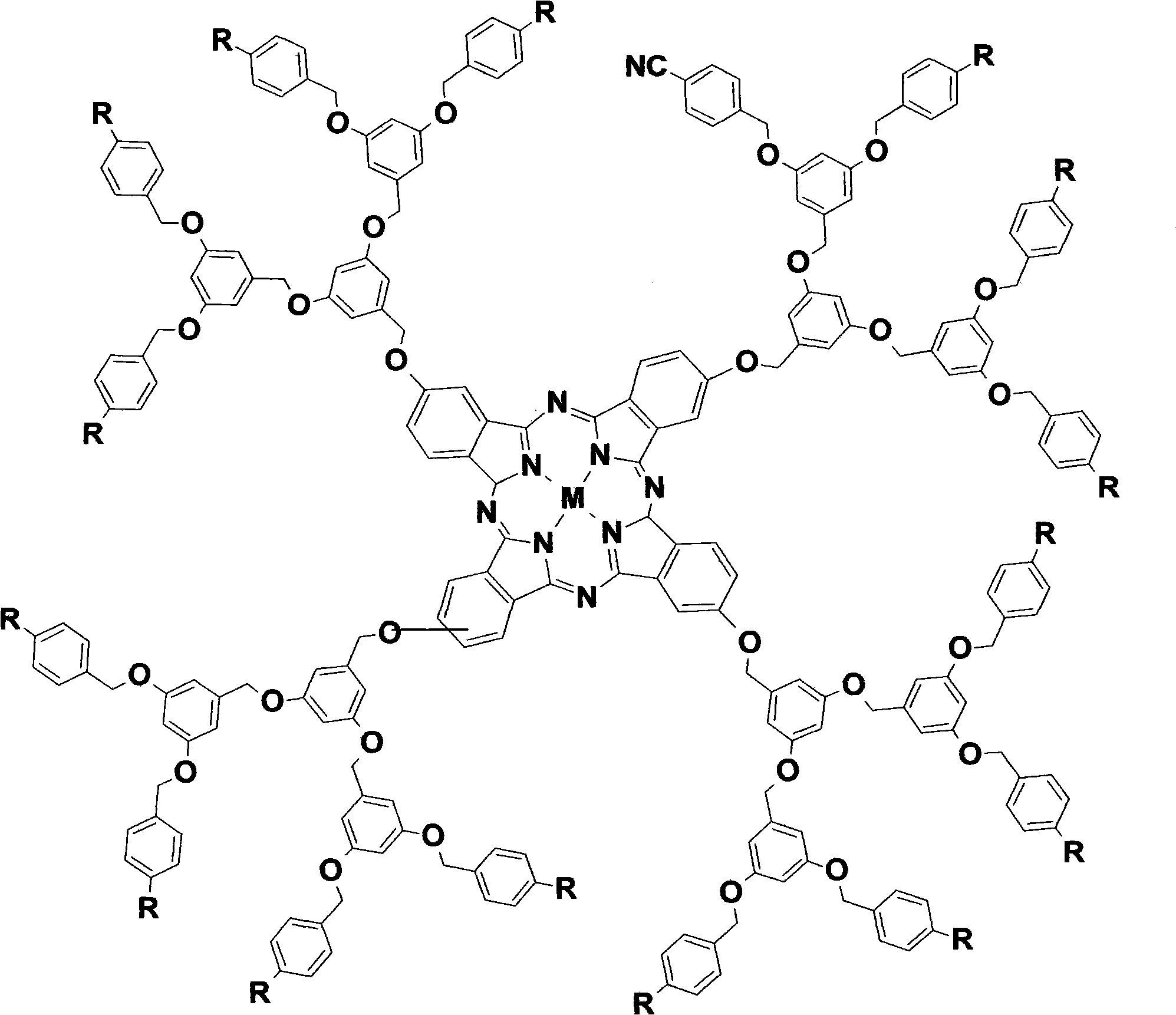
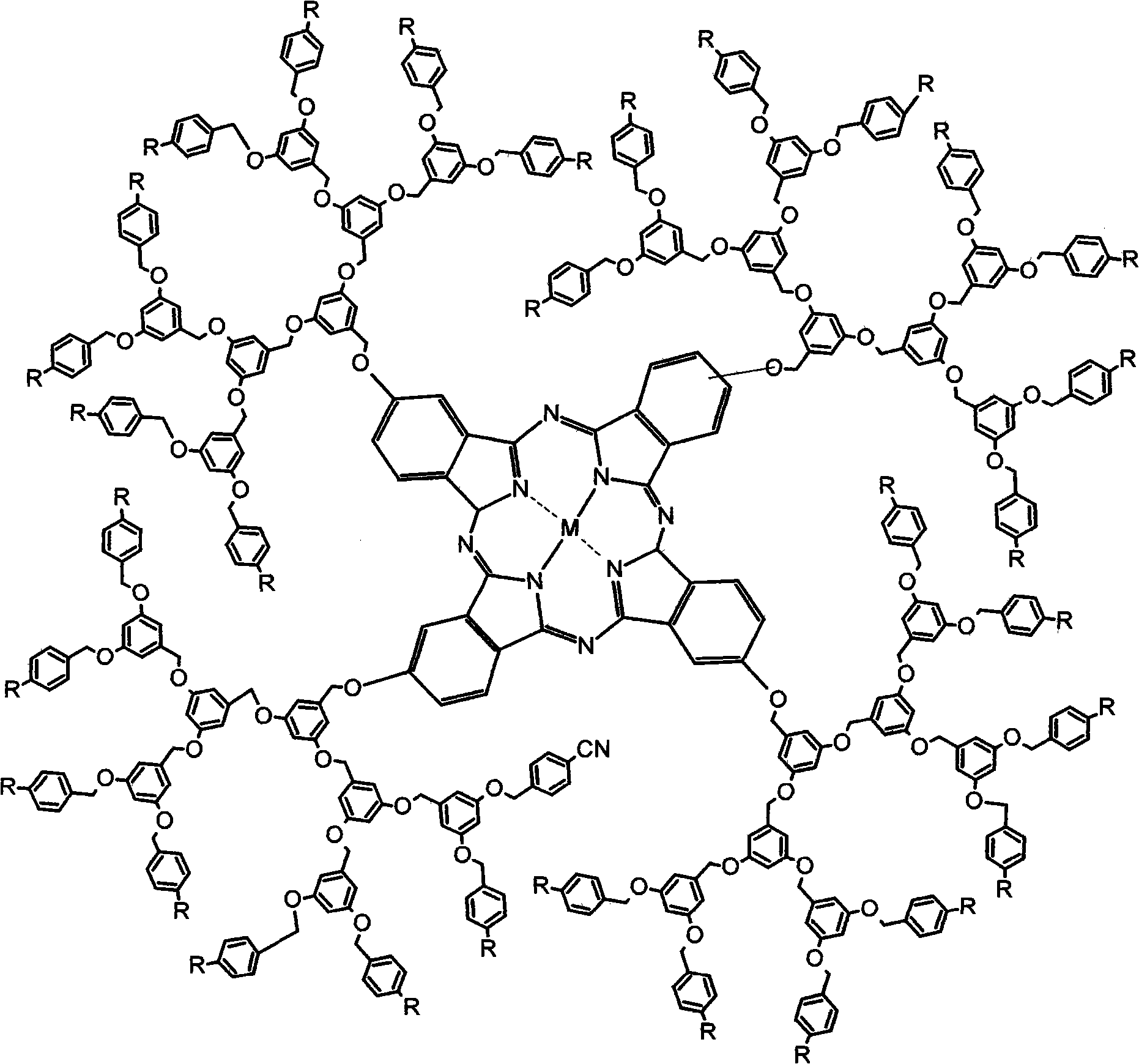
InactiveCN101580506AGood water solubilityHigh photosensitivityOrganic active ingredientsOrganic chemistryDendrimerEnd-group
The invention discloses a 1-3 generation arylene ether-substituted dendritic phthalocyanine zinc complex, a preparation method and an application thereof. The method comprises the following steps of adopting a Frechet synthesis method to synthesize the first to third generation alcohol molecules which take the 1-3 generation cyan as an end group; leading the first to third generation alcohols to react with 4-nitrophthalonitrile respectively, thus synthesizing corresponding phthalocyanine precursor of dendrimer taking the 1-3 generation cyan as the end group; subsequently, leading the phthalocyanine precursor ring of dendrimer taking the 1-3 generation cyan as the end group to synthesize corresponding arylene ether dendritic phthalocyanine taking the first to third generation cyan as the end group and the arylene ether dendritic phthalocyanine taking the first to third generation carboxyl as the end group generated by hydrolysis. The 1-3 generation arylene ether dendritic phthalocyanine complex and the amphiphilic block copolymer are automatically dissolved to form the polymer nano-particle loading the 1-3 generation arylene ether dendritic phthalocyanine complex. The 1-3 generation diaryl ether dendritic phthalocyanine zinc complex and the loaded polymer nano-particle thereof are used as photosencitizers for photodynamic therapy.
Owner:FUJIAN NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com