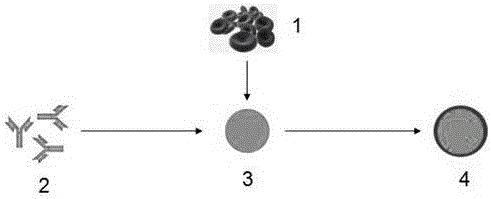
Antibody nanoparticles coated by red cell membranes for antibody drug delivery and preparation method
A technology of nanoparticles and red blood cell membranes, which is applied in the direction of antibodies, drug combinations, anti-tumor drugs, etc., can solve the problems of the difficulty of excreting macromolecular PEG, high selectivity of modified molecules, and insufficient purity of products, etc., and prolong the time of circulation in the body , low clearance rate, high cancer cell uptake effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of Anti-hTERT Monoclonal Antibody Nanoparticles
[0028] Put the anti-hTERT monoclonal antibody raw material into a 10kD dialysis bag, dialyze in ultrapure water, change the water after 2h and 4h, and dialyze overnight. The antibodies in the dialysis bag were collected, freeze-dried, and stored at -20°C. Take 3mg of lyophilized antibody, dissolve it in 0.5mL of 0.01N HCl containing 0.2% Tween80, add 0.01NNaOH dropwise under magnetic stirring, until the smoke-like precipitation that appears reaches the maximum amount. Centrifuge at 6500rpm for 5 minutes, carefully remove the supernatant to obtain anti-hTERT monoclonal antibody nanoparticles.
Embodiment 2
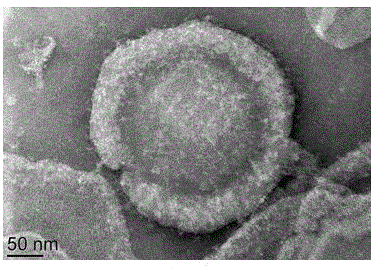
[0030] Preparation of Anti-hTERT Monoclonal Antibody Nanoparticles Wrapped in Erythrocyte Membrane
[0031] After the SD rats were fully anesthetized with 2% pentobarbital sodium solution, the xiphoid process and the above parts were cut open, and after the heart was exposed, a needle was inserted from the left ventricle, and 1 mL of blood was collected from a syringe washed with 0.5% heparin sodium. The blood was transferred to a 2m EP tube containing sodium heparin. Put the whole blood in a 4°C centrifuge at 900g for 20min, discard the supernatant, leave a dark red precipitate, take 3 times the volume of the precipitate in 1*PBS, blow it evenly, and put it in a 4°C centrifuge for centrifugation 2500g, 15min, this is the first washing. Put it into a centrifuge and centrifuge at 2500g for 15min, which is the second washing. Repeat the above steps again, this is the third wash. Discard the supernatant, leave the precipitate, take the same amount of 1*PBS as the precipitate a...
Embodiment 3
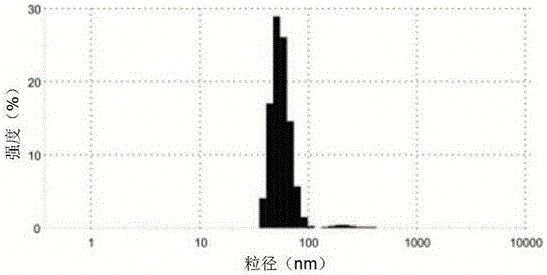
[0033] The particle size distribution of embodiment 3 nanoparticles
[0034] Using the prepared anti-hTERT monoclonal antibody nanoparticles wrapped in the red blood cell membrane, the particle size distribution was detected by DLS, and the results can be found in figure 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com