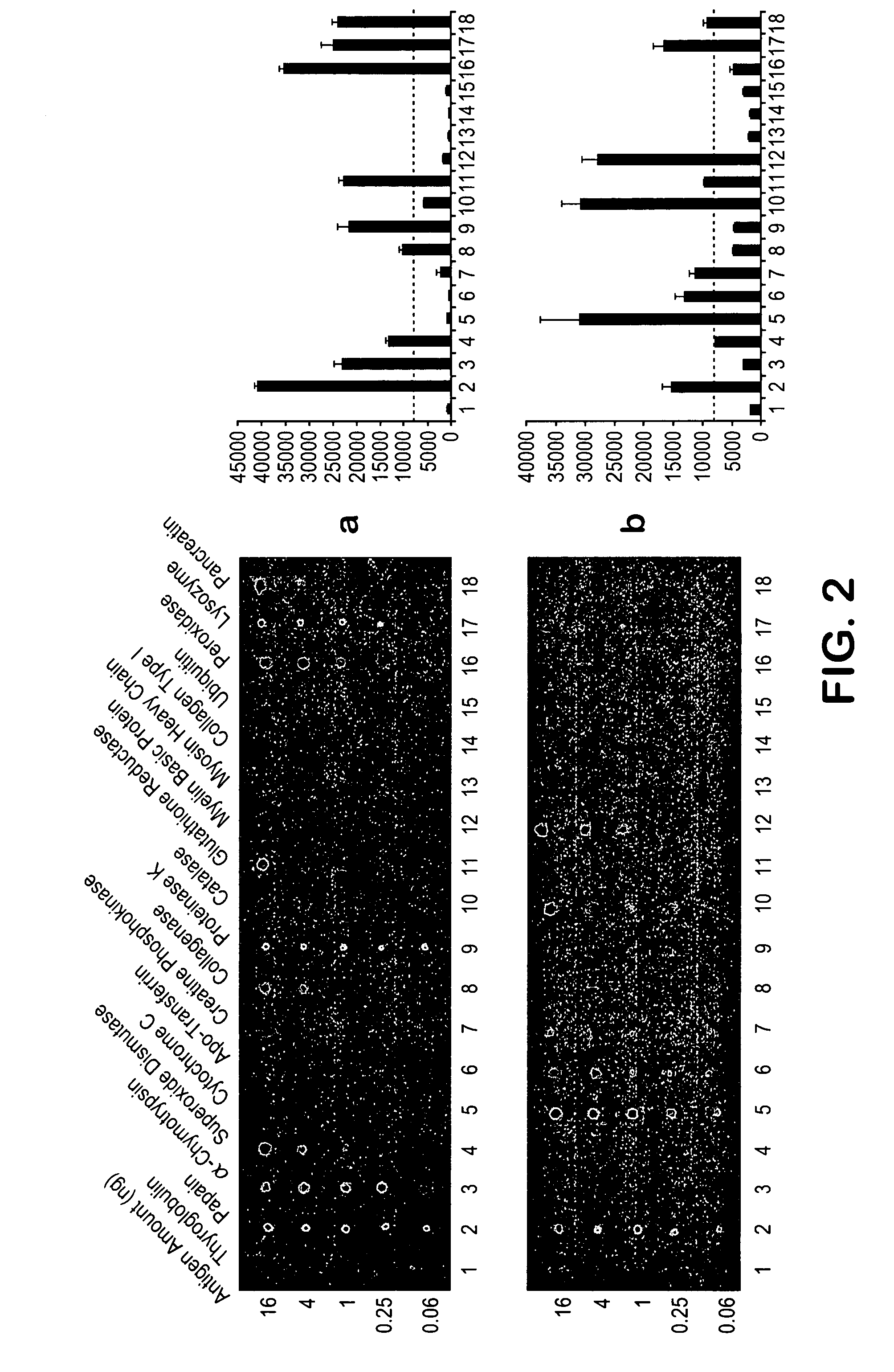
Patents
Literature
118 results about "Antibody formation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
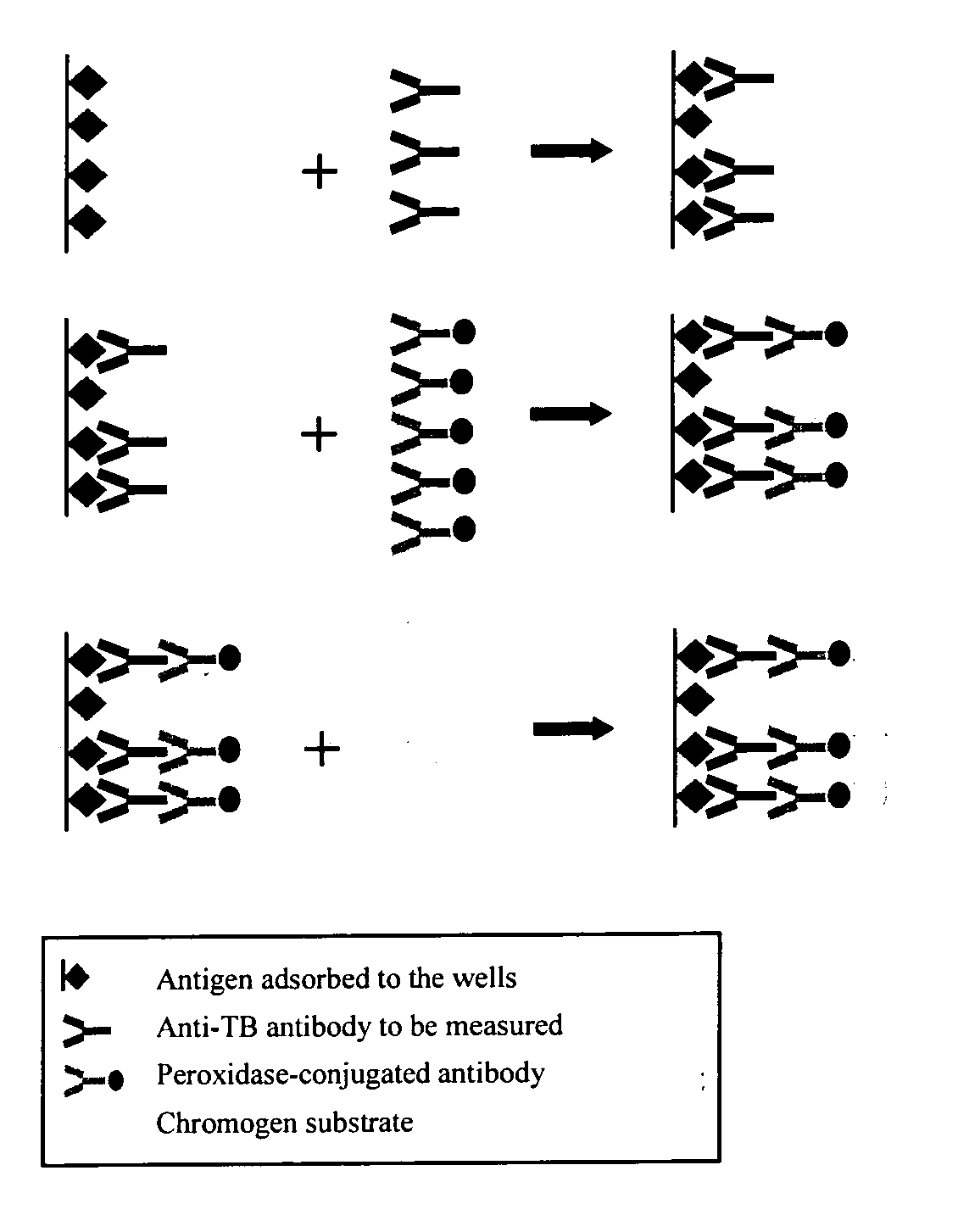
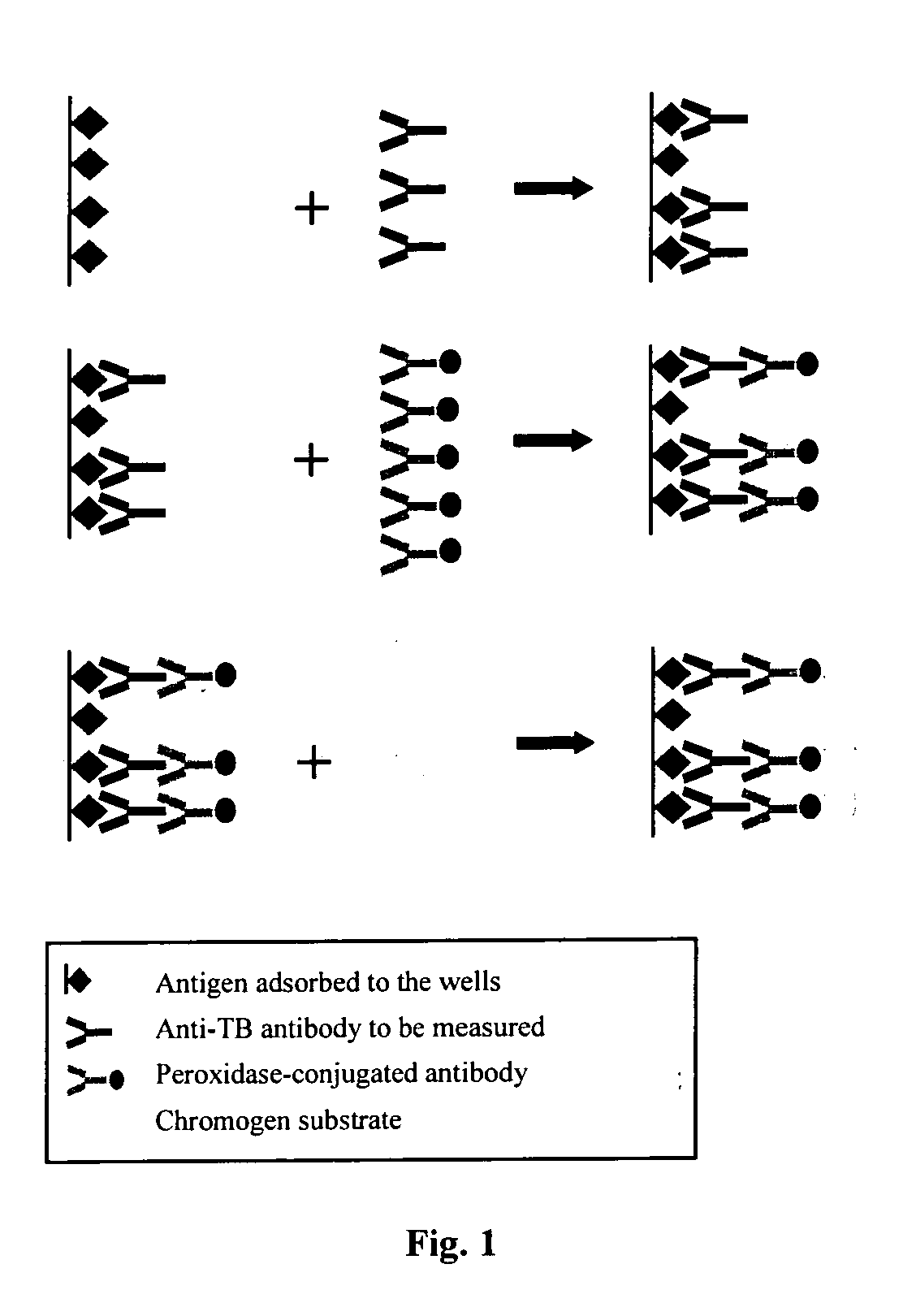
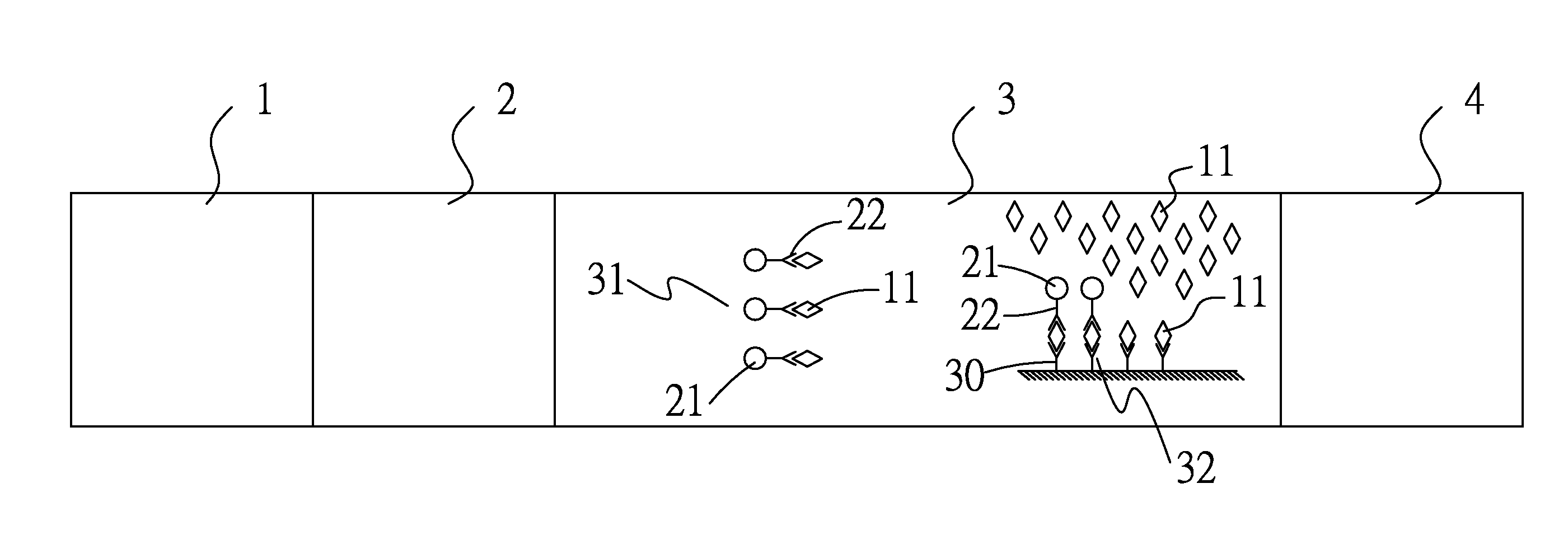
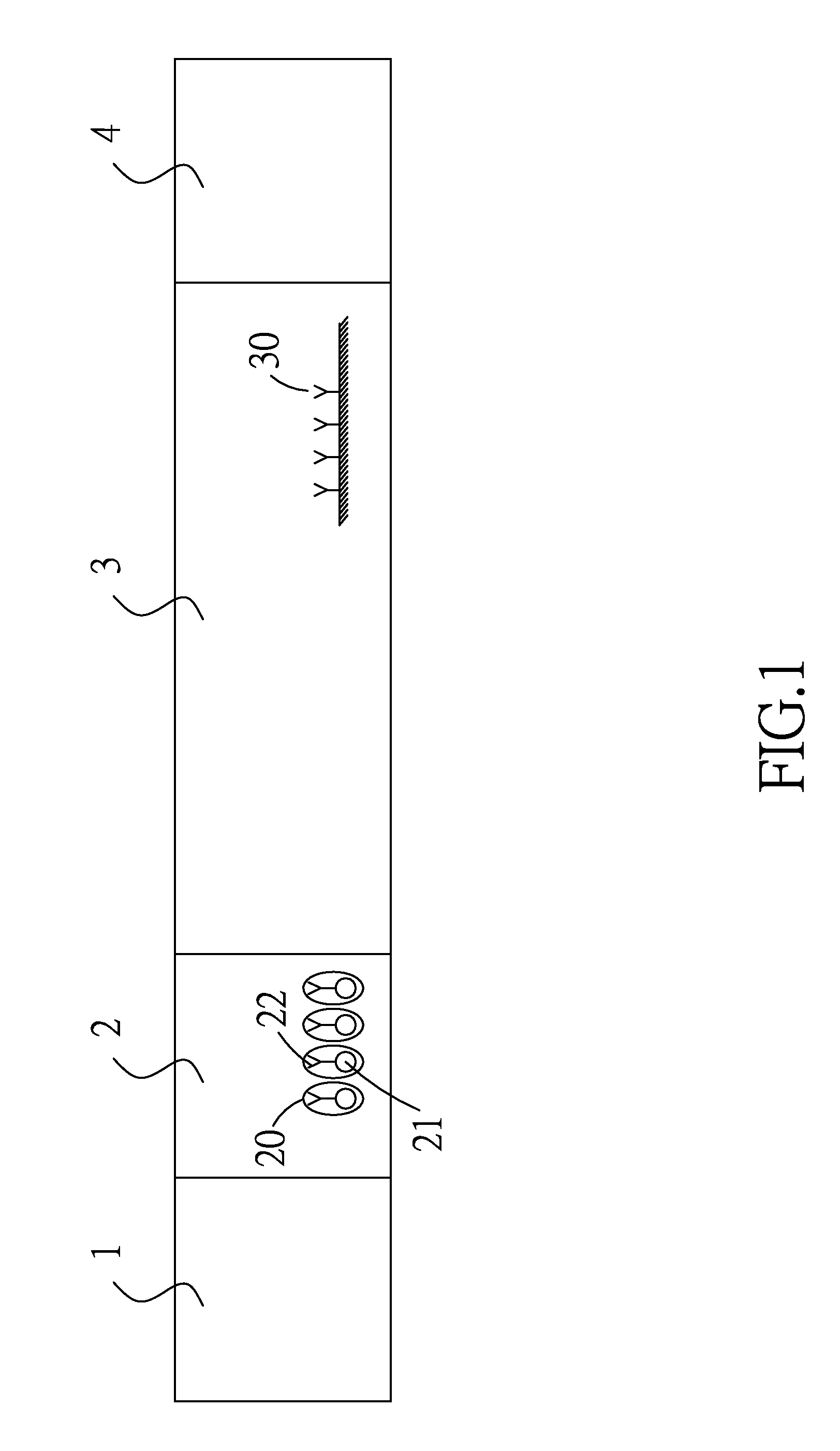
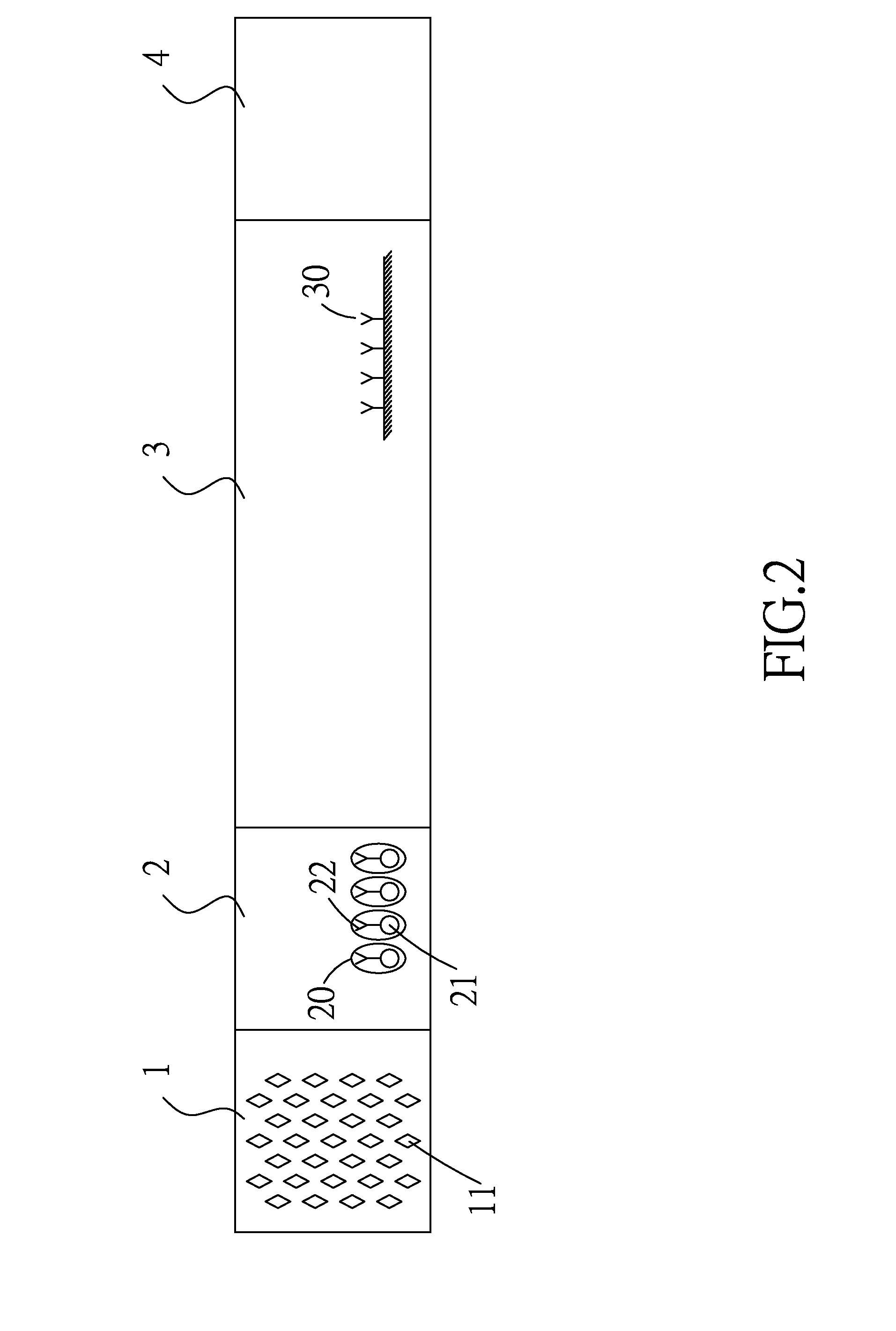
Formation of Antibodies. Antibodies are formed as a result of the entry of micro-organisms that produce antigens(protein macromolecules) which in turn cause the lymphocytes(white blood cells) to produce corresponding antibodies.
Immunomodulatory compositions containing an immunostimulatory sequence linked to antigen and methods of use thereof
InactiveUS7223398B1Reduced antibody productionReduce productionGenetic material ingredientsAntiviralsNucleotideImmuno modulation
The invention provides classes of immunomodulatory compositions which comprise an average of one or more immunostimulatory sequence (ISS) containing polynucleotide conjugated, or attached, to antigen. The extent of conjugation affects immunomodulatory properties, such as extent of antigen-specific antibody formation, including Th1-associated antibody formation, and thus these various conjugate classes are useful for modulating the type and extent of immune response. The invention also includes methods of modulating an immune response using these compositions.
Owner:DYNAVAX TECH CORP
Prediction and assessment of immunogenicity
InactiveUS20060148009A1Low immunogenicityBiological material analysisVaccine efficacyImmunocompatibility
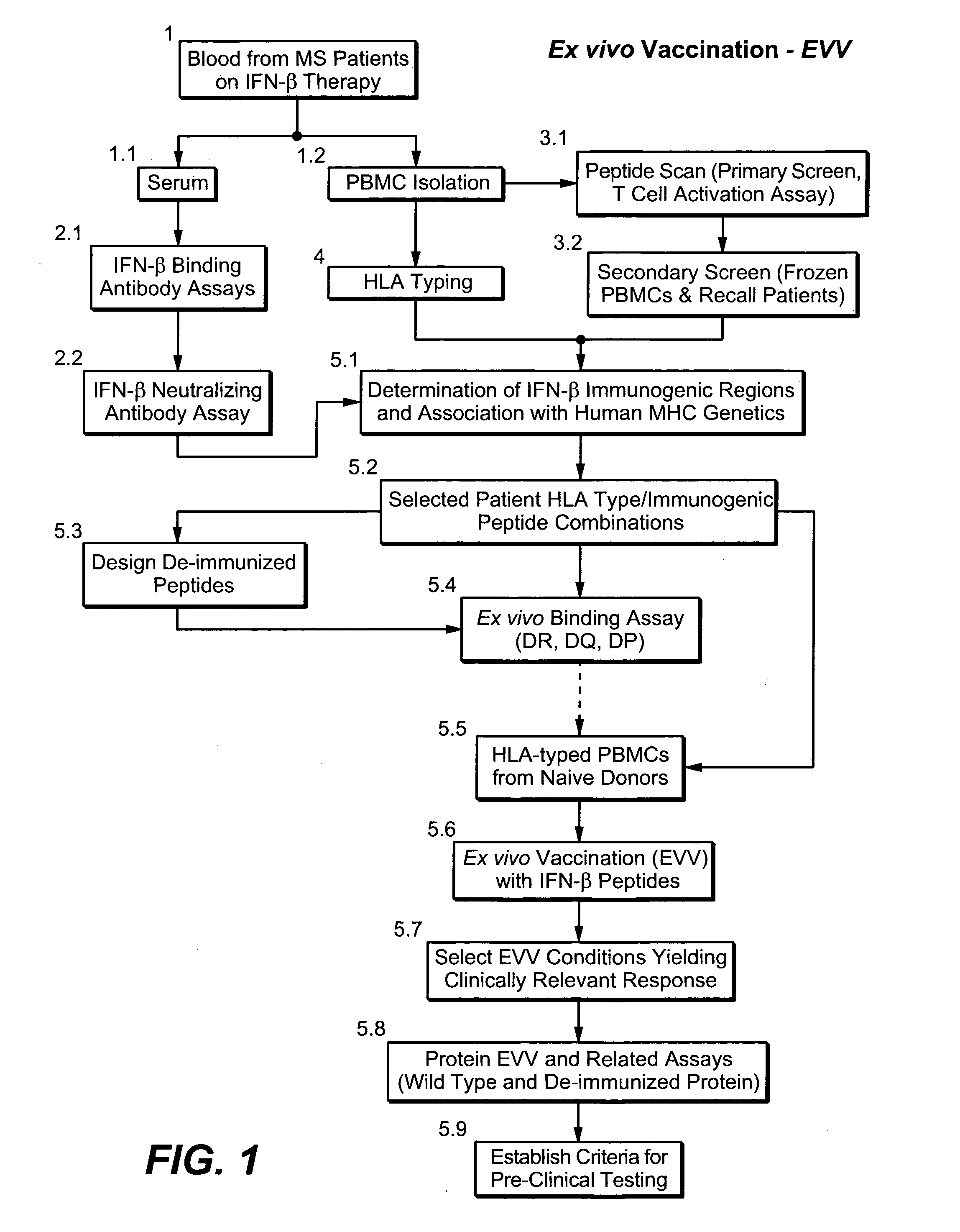
A system and method to predict and assess immunogenicity, especially prior to on-set of immunogenic conditions. Also disclosed are methods to identify relevant peptides associated with the formation of antibodies in patients treated with a given protein therapeutic. In various aspects, the present application is directed to methods of determining the immunological compatibility of a subject with a therapeutic agent such as a proteinaceous therapeutic agent, methods of determining vaccine efficacy by determining the immunological compatibility of a subject with a therapeutic agent, and selecting a therapeutic agent for a subject in need of treatment. Methods of designing a therapeutic agent with reduced immunogenicity for a subject and methods for designing vaccines with enhanced immunogenicity for a subject are also contemplated.
Owner:XENCOR
Real-time quantitative detection reagent and method of time-resolved fluorescence immune chromatography
ActiveCN102879559AImprove stabilityImprove signal-to-noise ratioBiological testingEmulsionFluorescence
The invention provides a real-time quantitative detection reagent and a method of time-resolved fluorescence immune chromatography. The invention provides emulsion particles containing lanthanide elements coated by a gel, an antibody compound formed by the emulsion particles and an antibody coupled to the emulsion particles, a detection strip containing the antibody compound and a kit containing the detection strip, and applications thereof. The emulsion particles, the antibody compound and the kit can greatly eliminate non-specificity combination, and enables signal-to-noise ratio of detection signals to be increased significantly. The stability can be improved by coating the emulsion particles with the gel. The detection method has the advantages of high speed, high sensitivity, high specificity, etc.
Owner:上海执诚生物科技有限公司
Compositions and methods for determining successful immunization by one or more vaccines
ActiveUS8956859B1Bioreactor/fermenter combinationsAnalysis using chemical indicatorsTernary complexIgM binding
A host antigen-specific antibody testing system and method. The a ternary complex of the antigen, a ligand-bound anti-host IgM, and a non-host anti-antigen IgG detector conjugate selectively form a quaternary complex with host antibodies, wherein the host antibodies and IgG compete for the antigen, and the IgM binds the host antibodies. The quaternary complex is retained by an immobilized IgM ligand binding agent, and any residual ternary is retained by a later encountered immobilized anti-non-host IgG. If sufficient host antibodies have a high affinity for the antigen, the complex is detected at the quaternary complex detection region based on the presence of the detector, and if there are insufficient high affinity host antibodies, the ternary complex migrates past the quaternary complex detection region and is retained and detected at a control region.
Owner:AVIEX TECH
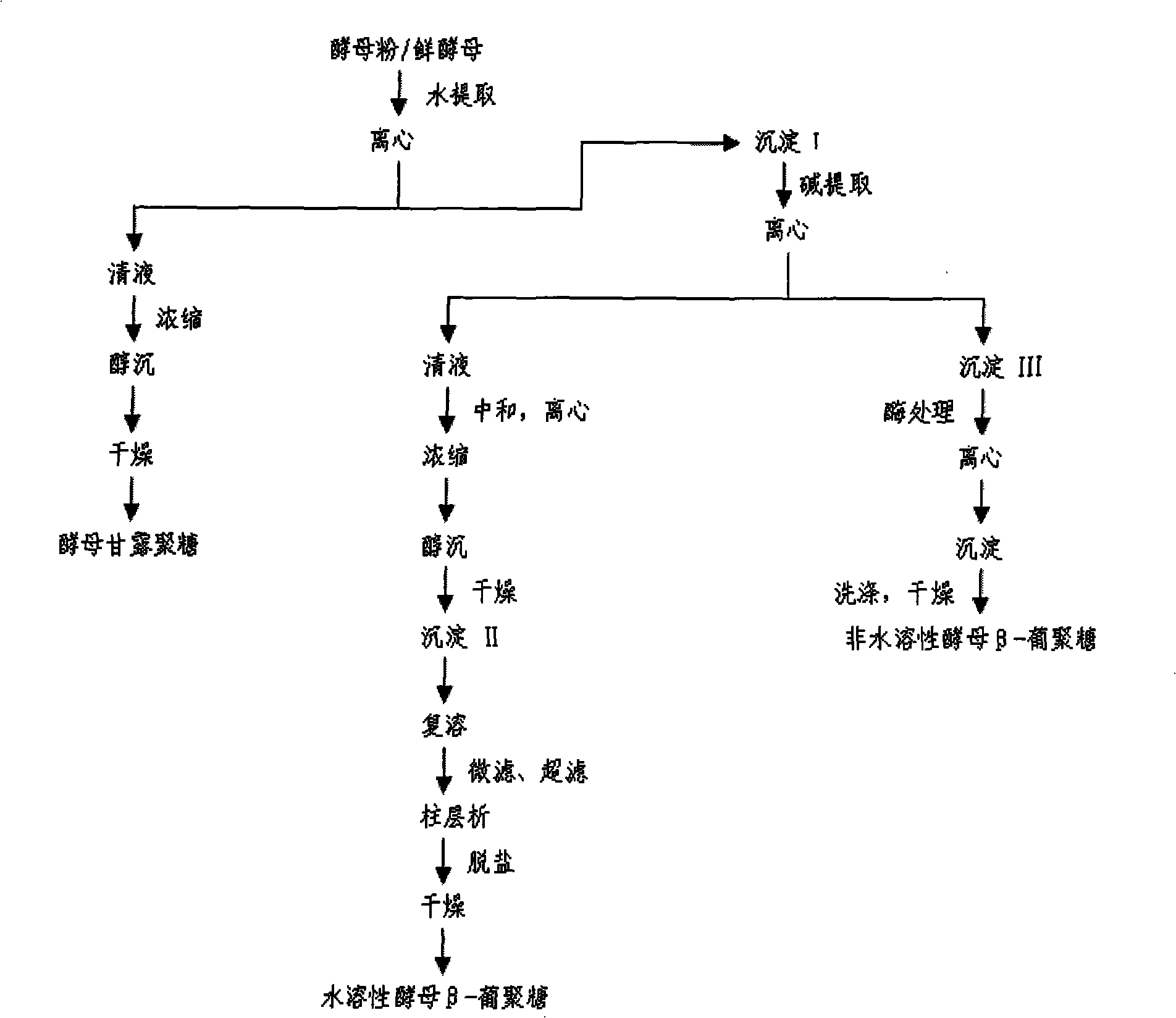
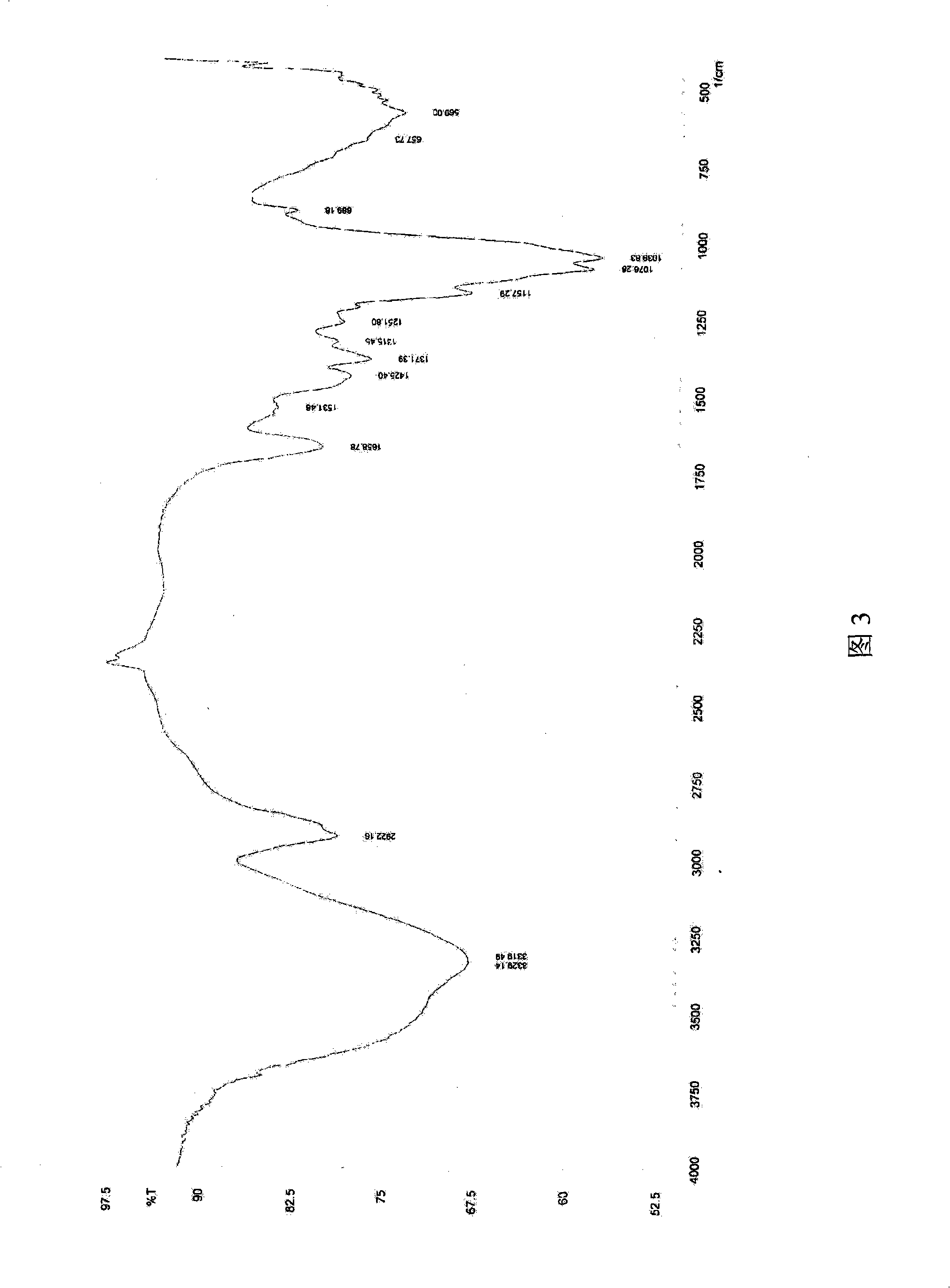
Water-soluble yeast beta-dextran and preparation thereof
ActiveCN101353383AAchieve maximum utilizationSimple processMilk preparationImmunological disordersYeastDairy foods
The invention relates to a water-soluble barm Beta-dextran and a preparation method thereof. In the water-soluble barm Beta-dextran chain, Beta-1, 3-dextran is taken as a main chain, Beta-1, 6-dextran is taken as a branched chain, the molecular weight is 0.08 to 0.2 million Daltun. The preparation method of the water-soluble barm Beta-dextran includes the following steps of: water extraction, alkali extraction and impurity processing. The preparation technique of the invention is simple; the technique parameters are easy to be operated and controlled; both the yield and the purity of the product water-soluble barm Beta-dextran are higher. The obtained water-soluble barm Beta-dextran has remarkable immune activation effect and especially can improve the phagocytic function and the antibody formation capacity of a macrophage to a large extent, thereby improving the body immunity and resistance. The water-soluble barm Beta-dextran can be widely applied in the fields such as food, dairy food and drinks, etc.
Owner:CHAMBROAD CHEM IND RES INST CO LTD
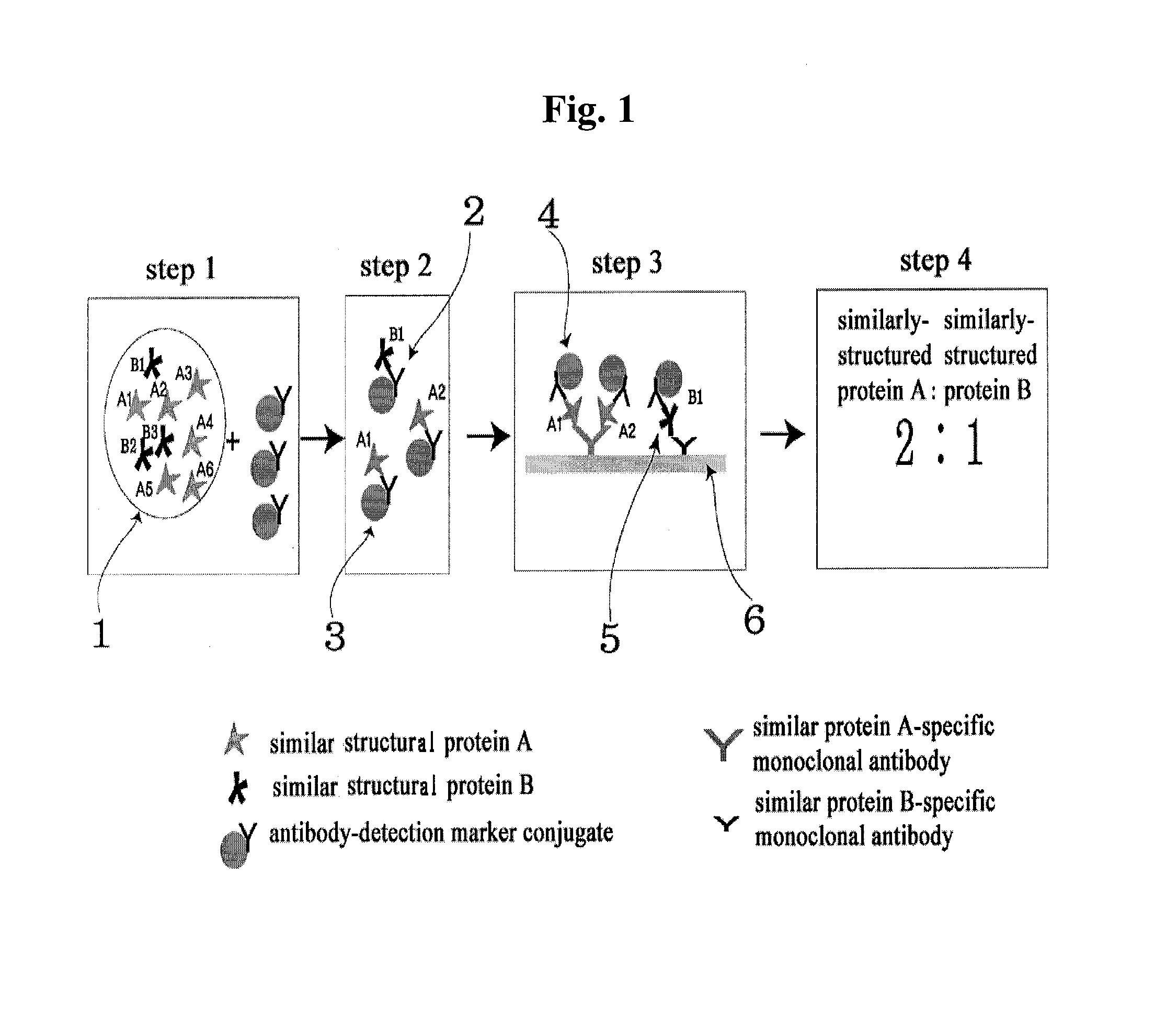
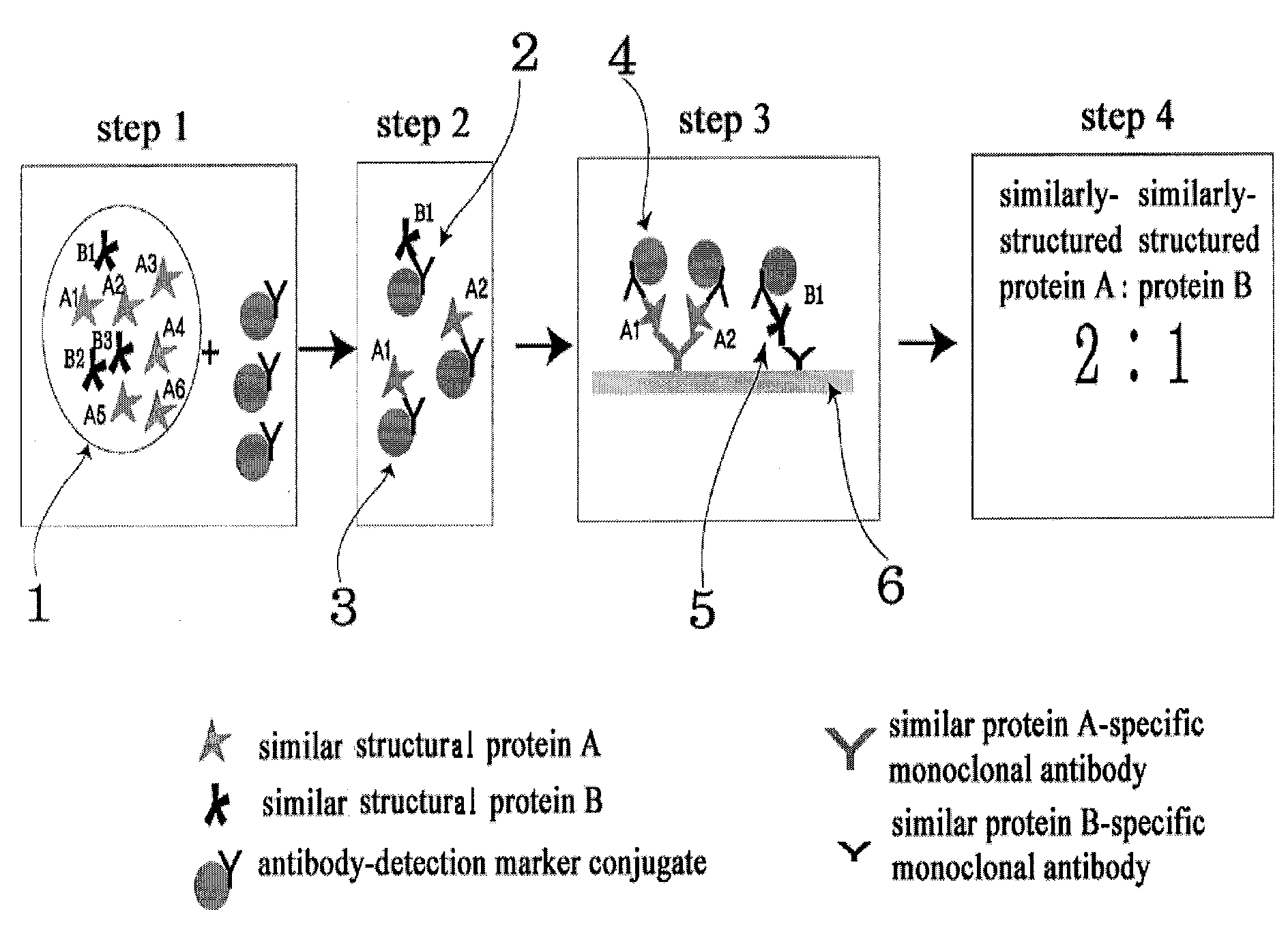
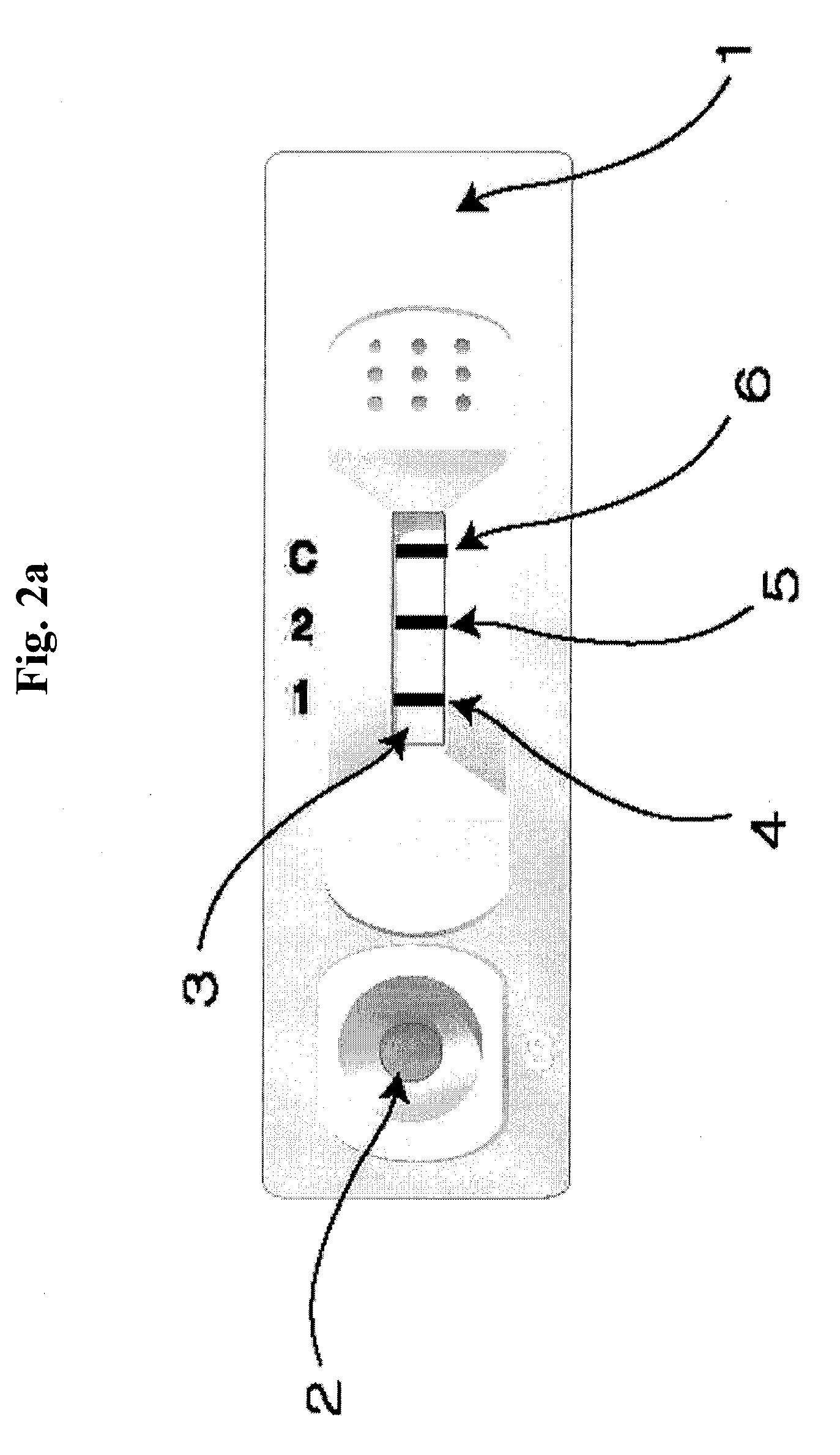
Diagnose device for measuring the ratio of proteins with similar structure
ActiveUS8101369B2Advantage of simplicityEasy to testSamplingPeptide/protein ingredientsAntiendomysial antibodiesObstetrics
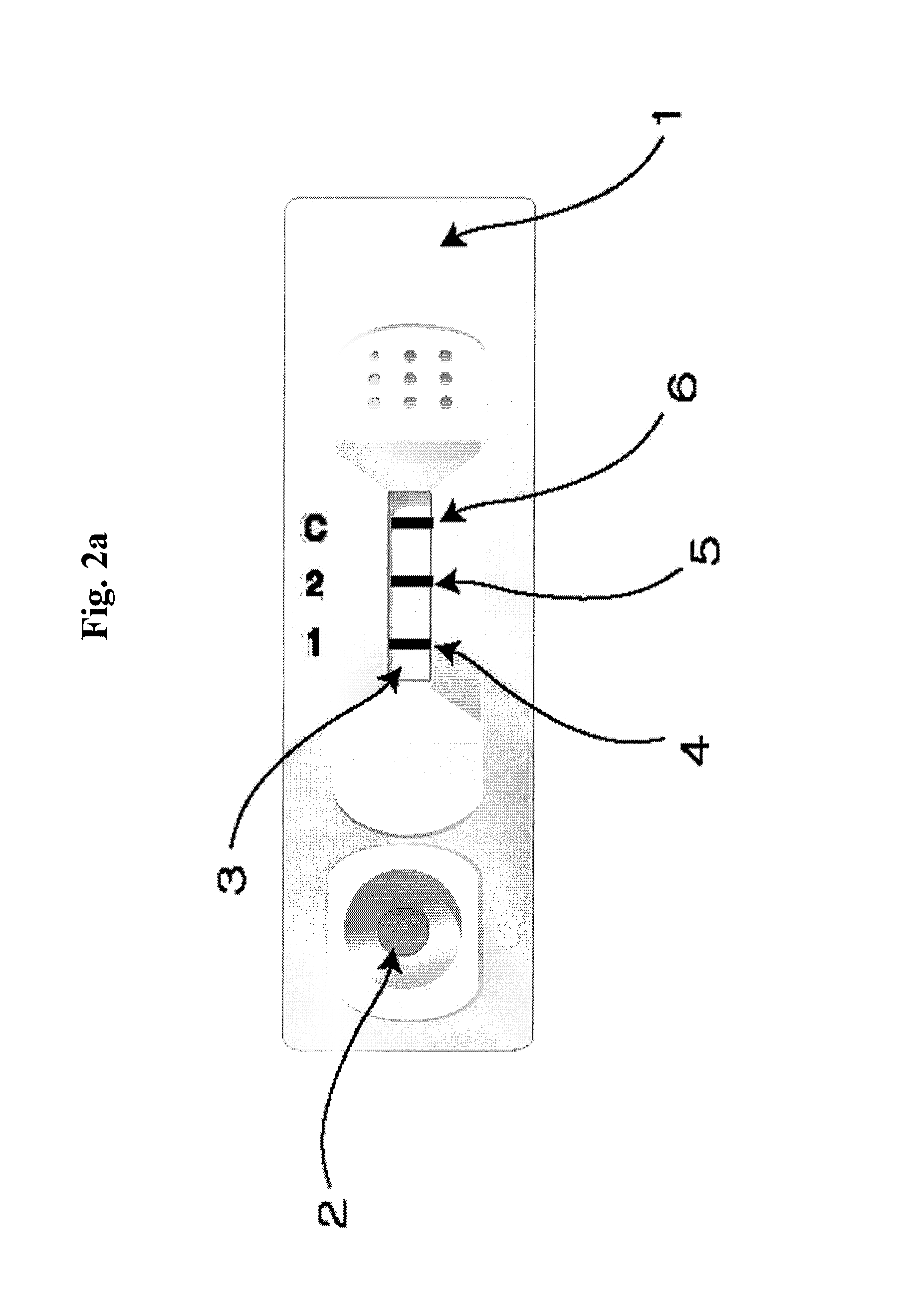
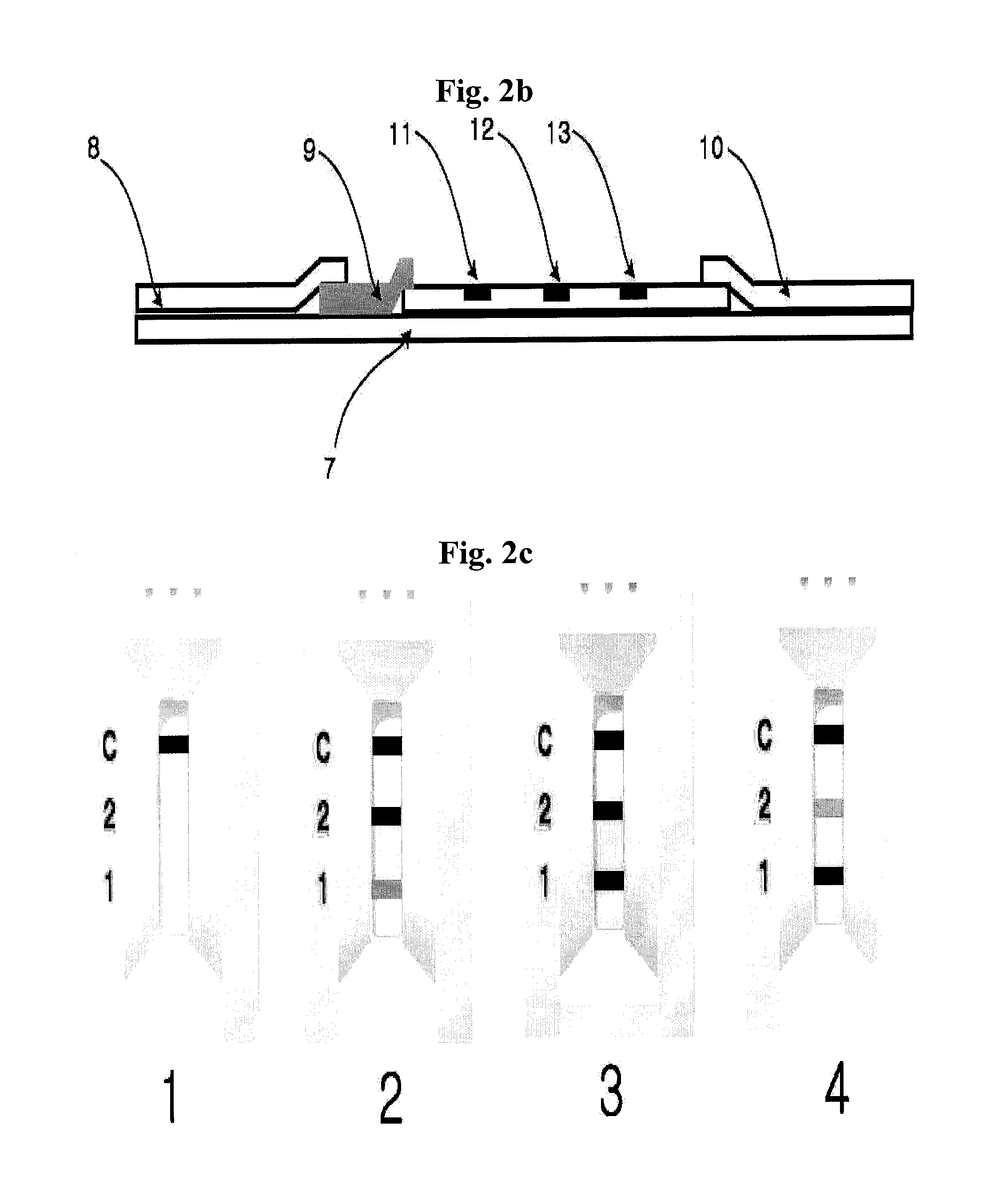
The present invention relates to a diagnostic device for measuring the ratio of similar structural proteins among the proteins secreted in a liquid test sample taken from diagnosis subject. In further detail, the test device according to the present invention comprises detection marker-antibody conjugate recognizing the same site on two or more similar structural proteins and a detection zone in which antibody specifically recognizes each of said proteins via formation of sandwich type complex, wherein said antibodies form a set, and the present Invention relates to a diagnostic device for early diagnosis of polycystic ovary syndrome, abnormal pregnancy, prostatic carcinoma etc. based on determination of the ratio of follicle stimulating hormone and luteinizing hormone in case of polycystic ovary syndrome, the ratio between hCG isomers in case of abnormal pregnancy, and the ratio of prostate-specific antigens (PSA) in case of prostatic carcinoma.
Owner:HUMASIS
Method for Detecting Anti-Transglutaminase Antibodies
InactiveUS20080038760A1Easy to detectEnhanced signalBiological material analysisBiological testingSaliva sampleGlutaminase
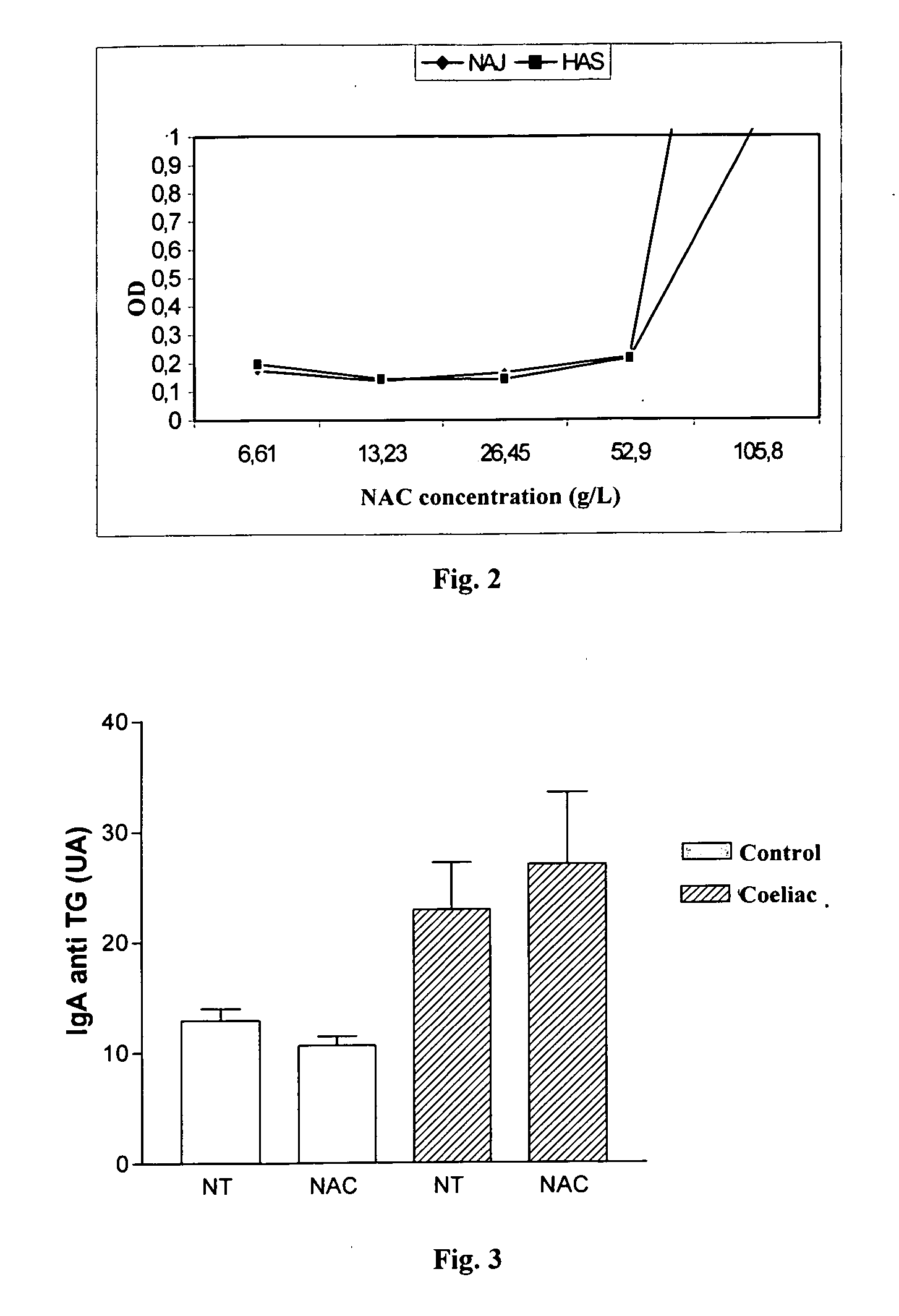
Methods for detecting anti-transglutaminase antibodies in a saliva sample containing the antibodies are disclosed. Saliva samples are pre-treated. The antibodies are subsequently detected in the pre-treated saliva by an immune reaction with a transglutaminase under conditions suitable for forming immuno-complexes with the antibodies. The method is useful for diagnosing and / or therapeutically controlling celiac disease.
Owner:UNIV LIBRE DE BRUXELIES
Immunoassays for citrullinated proteins
ActiveUS20110244492A1Reduce in quantityEnhanced radiationDisease diagnosisBiological testingRadiation injuryImmuno assay
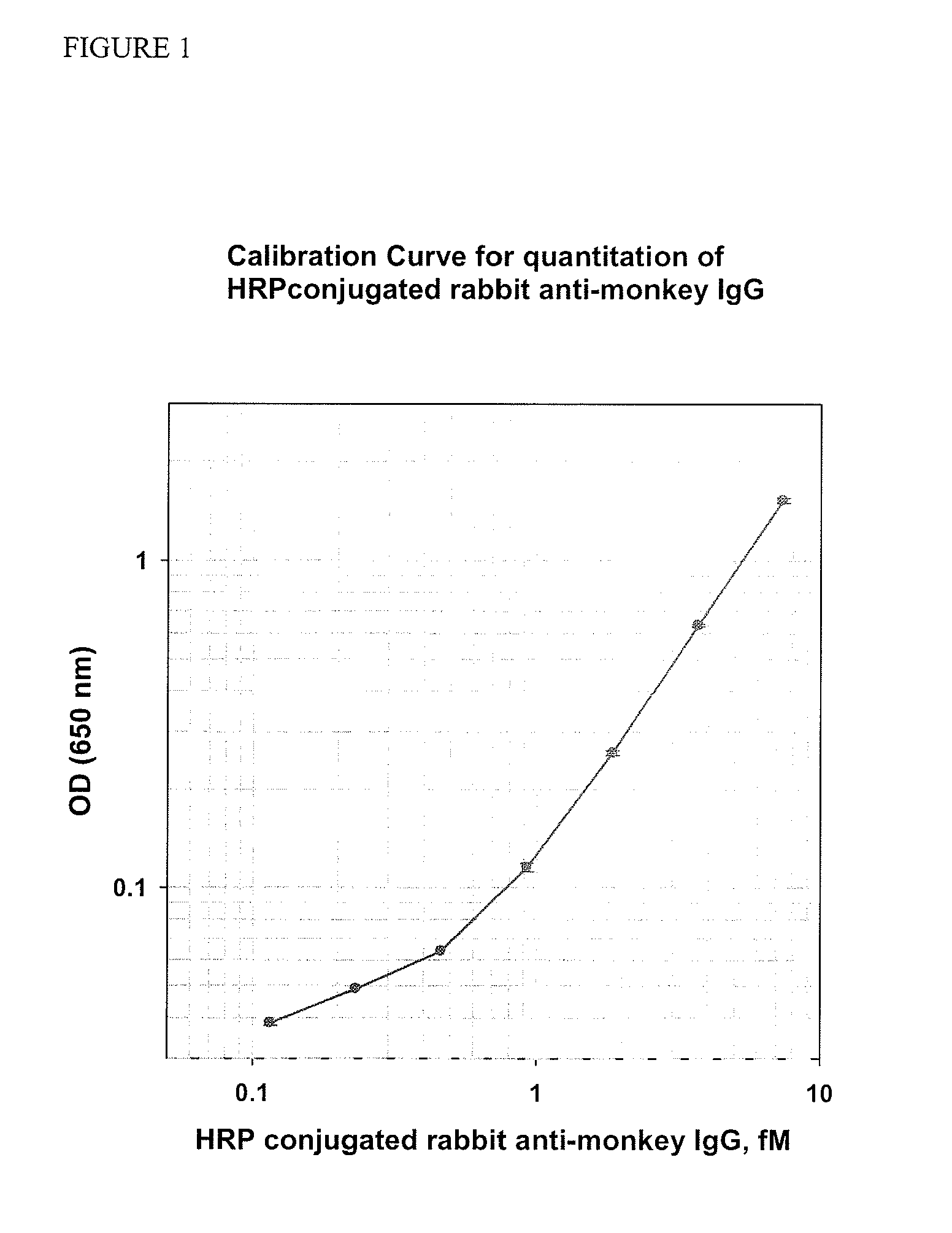
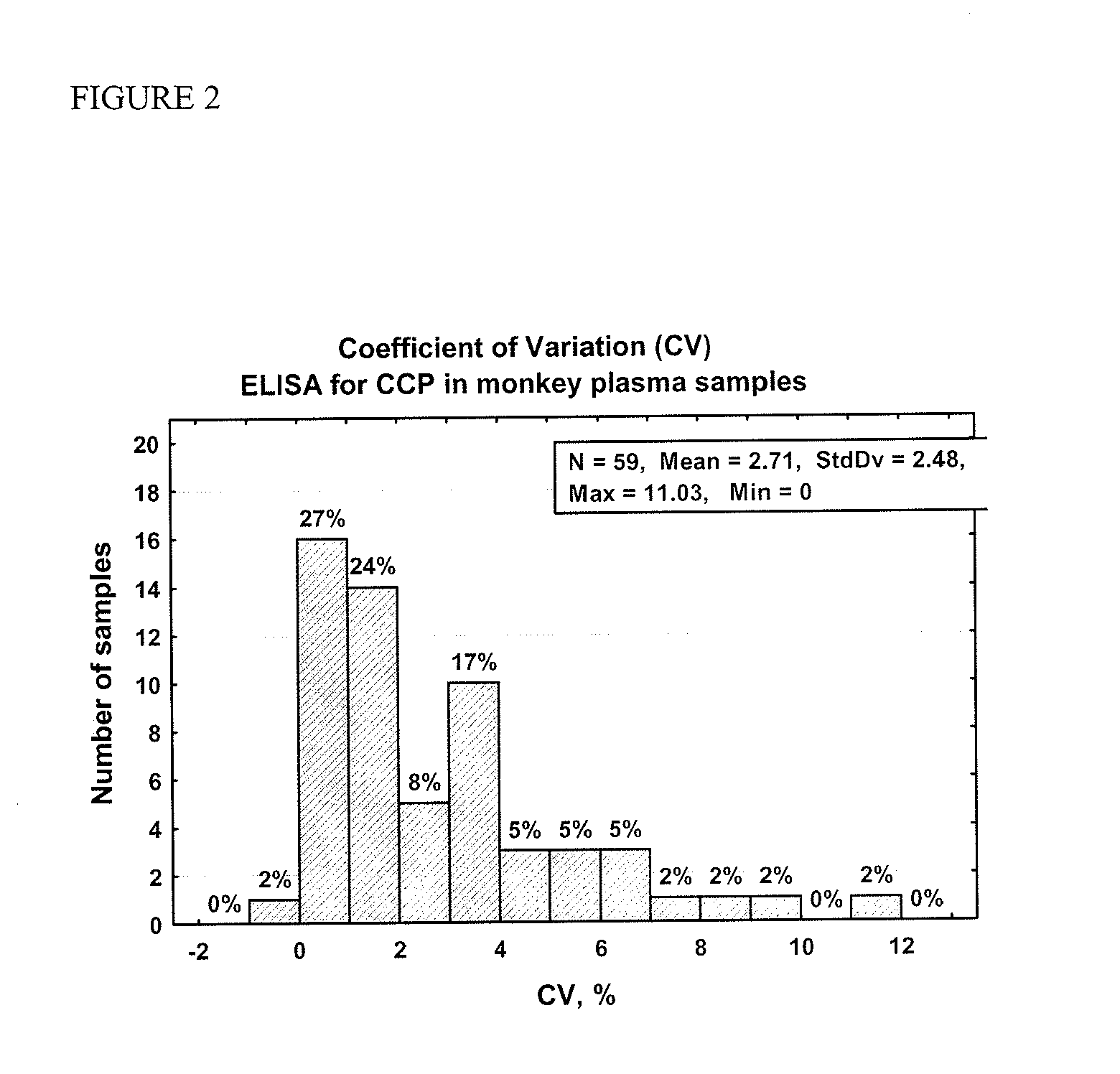
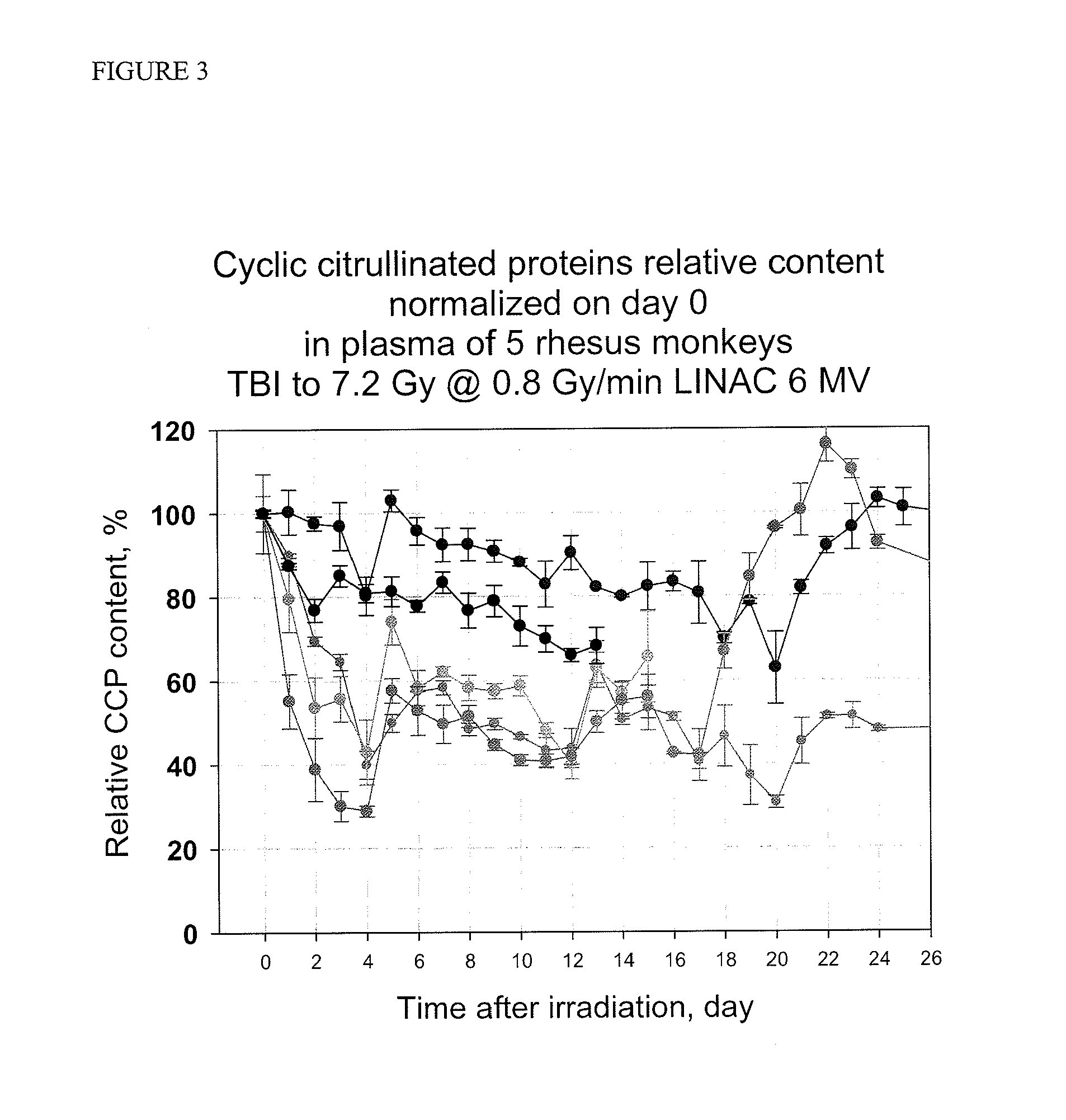
Methods and kits are provided for assessing radiation injury and exposure in a mammal. The methods comprise the steps of: obtaining one or more test samples from the mammal, contacting the test samples with an antibody immunoreactive with a citrullinated protein to form an immunocomplex; and detecting the immunocomplex with an ELISA; wherein a decrease in the quantity of the immunocomplex in the test samples, as compared to the quantity of immunocomplexes formed under identical conditions with the same antibody and a control sample from one or more mammals known to have a lower degree of radiation injury or exposure, indicates a higher degree of radiation injury and exposure to the mammal. The information obtained from such methods can be used by a clinician to accurately assess the extent of radiation injury / exposure in the mammal, and thus will provide a valuable tool for determining treatment protocols on a subject by subject basis.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Device for detecting hepatitis b virus marker in mouth cavity liquid
The present invention provides a detecting device for the hepatitis B virus marker in oral cavity liquid, which relates to a device which can quickly detect the hepatitis B virus marker in the oral cavity liquid. The present invention consists of an external lid which is a oral cavity liquid gathering part, a shell upper layers of test paper which are overlapped together, a piece of test paper and the lower layer of the test paper. The present invention comprises the oral cavity liquid sample gathering part which can complete by directly gathering the oral cavity liquid through putting the paper into mouth or by using a gatherer, and reaction and result observation part which comprises antigen, antibody reaction and observation part. If the gathered oral cavity liquid has corresponding antigen, a composite is formed by the antigen, golden dot antibody, and over-membrane, a red line appears positive (sandwich method). The corresponding antibody can be detected in the same way. Or the antigen and the antibody can be detected by a competition method. And the detecting sensitivity of the sample is obviously increased by combining biotin and affinity element amplification system. The present invention can detect the hepatitis b virus marker in the oral cavity liquid quickly and conveniently and is provided with high sensitivity and specificity.
Owner:马北峰
Recombinant fusion protein and polynucleotide construct for immunotoxin production
The present invention relates to a polynucleotide construct encoding a fusion protein consisting of a domain which binds the immunoglobulin Fc region, genetically fused to a truncated form of Pseudomonas exotoxin A (PE). In particular, the invention discloses the fusion protein, ZZ-PE38, and further provides immunotoxins, formed from complexes of the fusion protein with antibodies for targeted cell killing.
Owner:RAMOT AT TEL AVIV UNIV LTD
Method for detecting high antigen concentration and device therefor
InactiveUS20080311680A1Rapid lateral flowIncrease concentrationBioreactor/fermenter combinationsBiological substance pretreatmentsAntibody antigenSolid phases
A method for detecting high antigen concentration is disclosed. The method enables the mobile-phase antibody, in the presence of excessive amount of antigen, to form the antibody-antigen-antibody sandwich with the immobilized solid-phase antibody effectively in a rapid lateral flow chromatographic immunoassay. The mobile-phase and / or immobilized solid-phase antibody are treated with soluble coatings to generate a delaying mechanism, so that antigen-antibody binding occurs only when both phases of antibodies and antigen are in very close proximity. A user friendly immunoassay device with a sample over-flow mechanism also facilitates such antigen-antibody binding.
Owner:CHIU JOHN
Antibody nanoparticles coated by red cell membranes for antibody drug delivery and preparation method
ActiveCN105769818AImprove stabilityIncrease uptakeAntibody ingredientsPharmaceutical non-active ingredientsImmune clearanceClearance rate
The invention discloses antibody nanoparticles coated by natural red cell membranes for antibody drug delivery and a preparation method of the antibody nanoparticles. The red cell membranes in the invention are extracted from experimental rats, most of the contents and unrelated proteins are removed after the red cell membranes are treated, and protein nanoparticles formed by antibodies are coated with the obtained red cell membranes. The method is suitable for coating nanoparticles formed by different antibodies through the red cell membranes, can improve the stabilities and the in-vivo circulation times of free antibodies, and further reduces the body immunity clearance rate.
Owner:EAST CHINA NORMAL UNIV
Novel coronavirus antibody detection kit based on magnetic particle chemiluminescence
ActiveCN112229994AReduce False Positive FactorsStrong specificityChemiluminescene/bioluminescenceImmunoassaysAcridineCoronavirus antibody
The invention provides a novel coronavirus antibody detection kit based on magnetic particle chemiluminescence. The detection kit comprises streptavidin magnetic particles, a biotin-labeled novel coronavirus antigen, an acridine sulfonamide-labeled secondary antibody, a sample diluent and a quality control material, wherein the biotin-labeled novel coronavirus antigen comprises a recombinant nucleocapsid protein and a recombinant spinous process protein S1. A to-be-detected sample, the biotin-labeled antigen and the streptavidin magnetic particles are mixed, incubated and washed, then the acridine sulfonamide-labeled antibody is added, a magnetic particle-streptavidin-biotin-antigen-novel coronavirus antibody-secondary antibody compound is formed, and then the luminous intensity is detected to achieve qualitative analysis of the to-be-detected sample.
Owner:DYNAMIKER BIOTECH TIANJIN
Immunomodulatory compositions containing an immunostimulatory sequence linked to antigen and methods of use thereof
InactiveUS20070190073A1Reduce productionGenetic material ingredientsAntiviralsImmunomodulationsPolynucleotide
The invention provides classes of immunomodulatory compositions which comprise an average of one or more immunostimulatory sequence (ISS) containing polynucleotide conjugated, or attached, to antigen. The extent of conjugation affects immunomodulatory properties, such as extent of antigen-specific antibody form ration, including Th1-associated antibody formation, and thus these various conjugate classes are useful for modulating the type and extent of immune response. The invention also includes methods of modulating an immune response using these compositions.
Owner:DYNAVAX TECH CORP
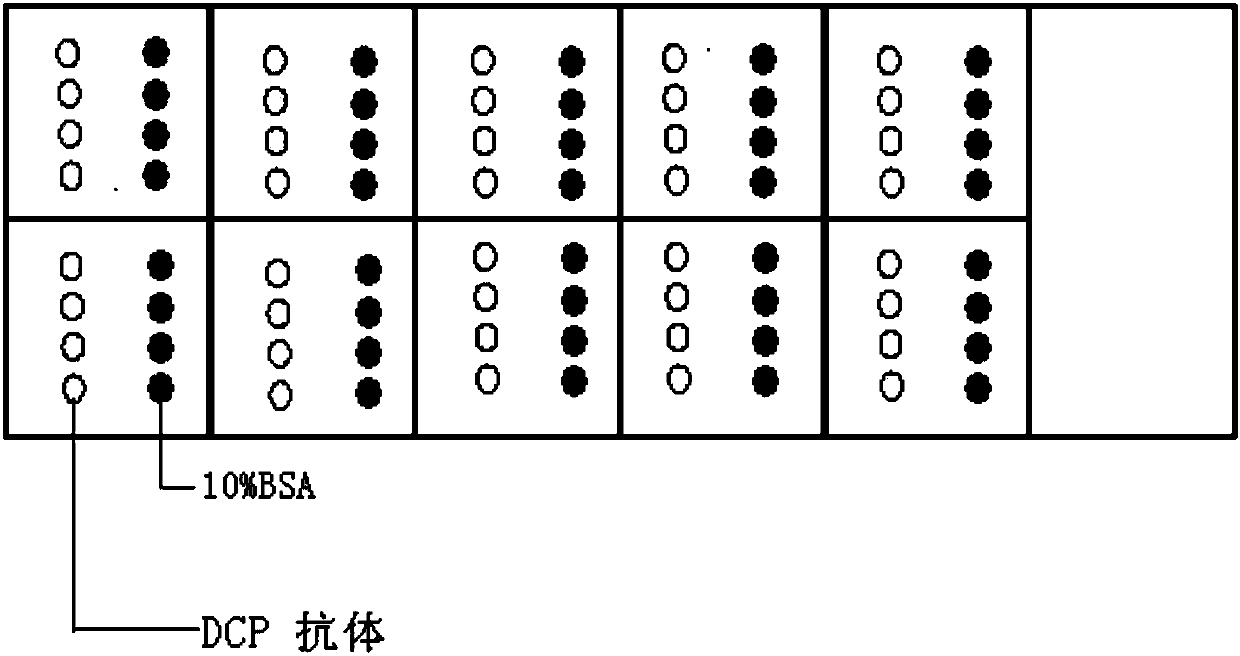
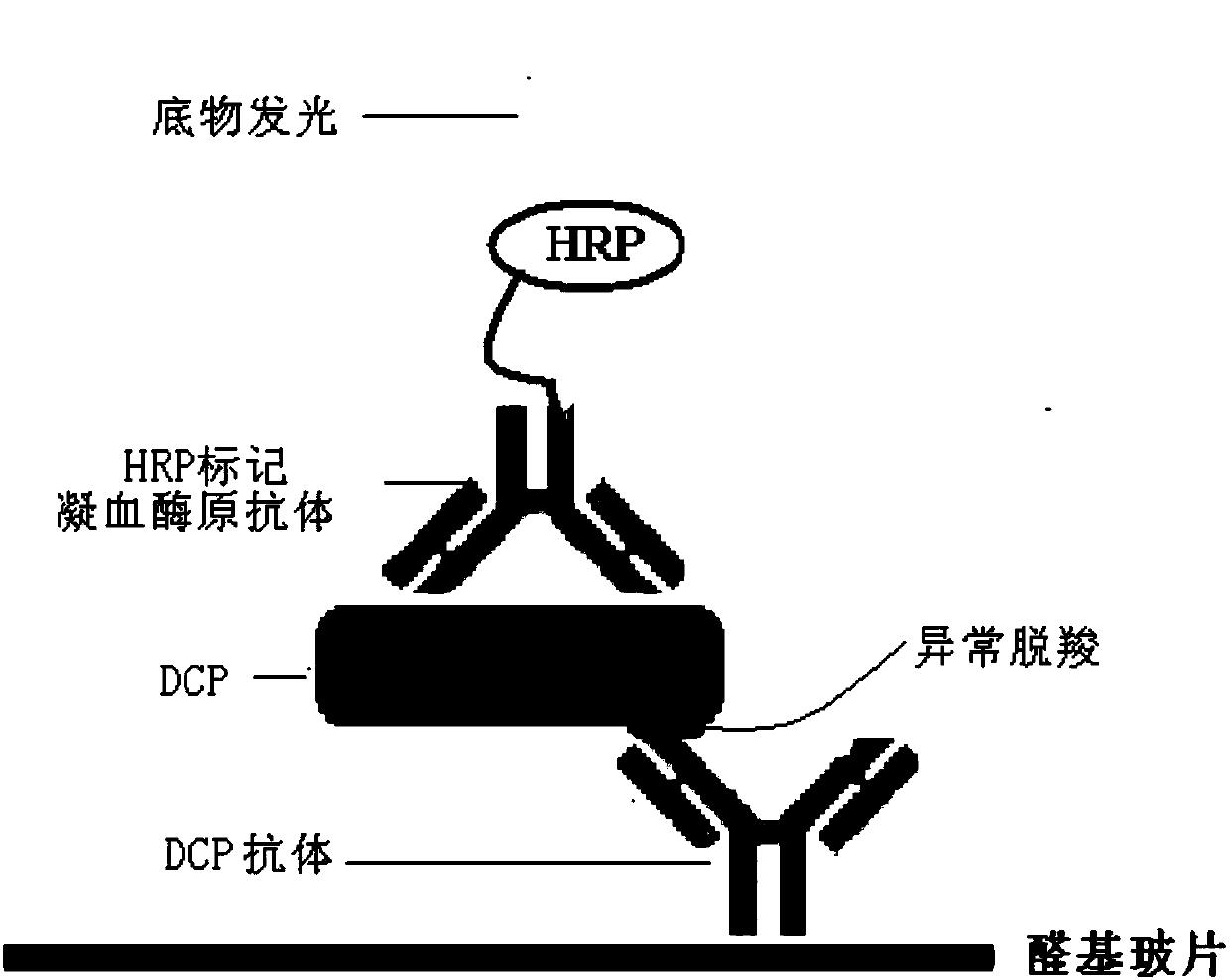
Protein chip and kit for detecting abnormal DCP (Des--Carboxy-Prothrombin) in serum and preparation method thereof
InactiveCN107727864AEnable high-throughput detectionReduce testing costsMicrobiological testing/measurementBiological material analysisSerum igeProtein detection
The invention relates to a protein chip and a kit for detecting abnormal DCP (Des--Carboxy-Prothrombin) in serum and a preparation method thereof, and belongs to the technology of protein detection. The protein chip for detecting the abnormal DCP in the serum is characterized in that substrate slide glass of the protein chip at least comprises a detecting subarea, and one detecting subarea detectsone serum sample; a detecting spot area and a control spot area are formed in the detecting subarea; the detecting spot area has detecting spots formed by fixing a DCP specific antibody; the controlspot area has control spots formed by fixing bovine serum albumin; the concentrations of the substances on all detecting spots in the same detecting spot area are the same; the total amount of the DCPspecific antibody fixed on each of the detecting spot is 3nl; each detecting spot is formed by spraying 10 times, and 300pl is spotted each time. The protein chip and the kit disclosed by the invention can accurately detect the abnormal DCP; in clinical use, the protein chip and the kit have the advantages of high sensitivity, time conservation, convenience and quickness, economy and the like.
Owner:BEIJING YOUAN HOSPITAL CAPITAL MEDICAL UNIV
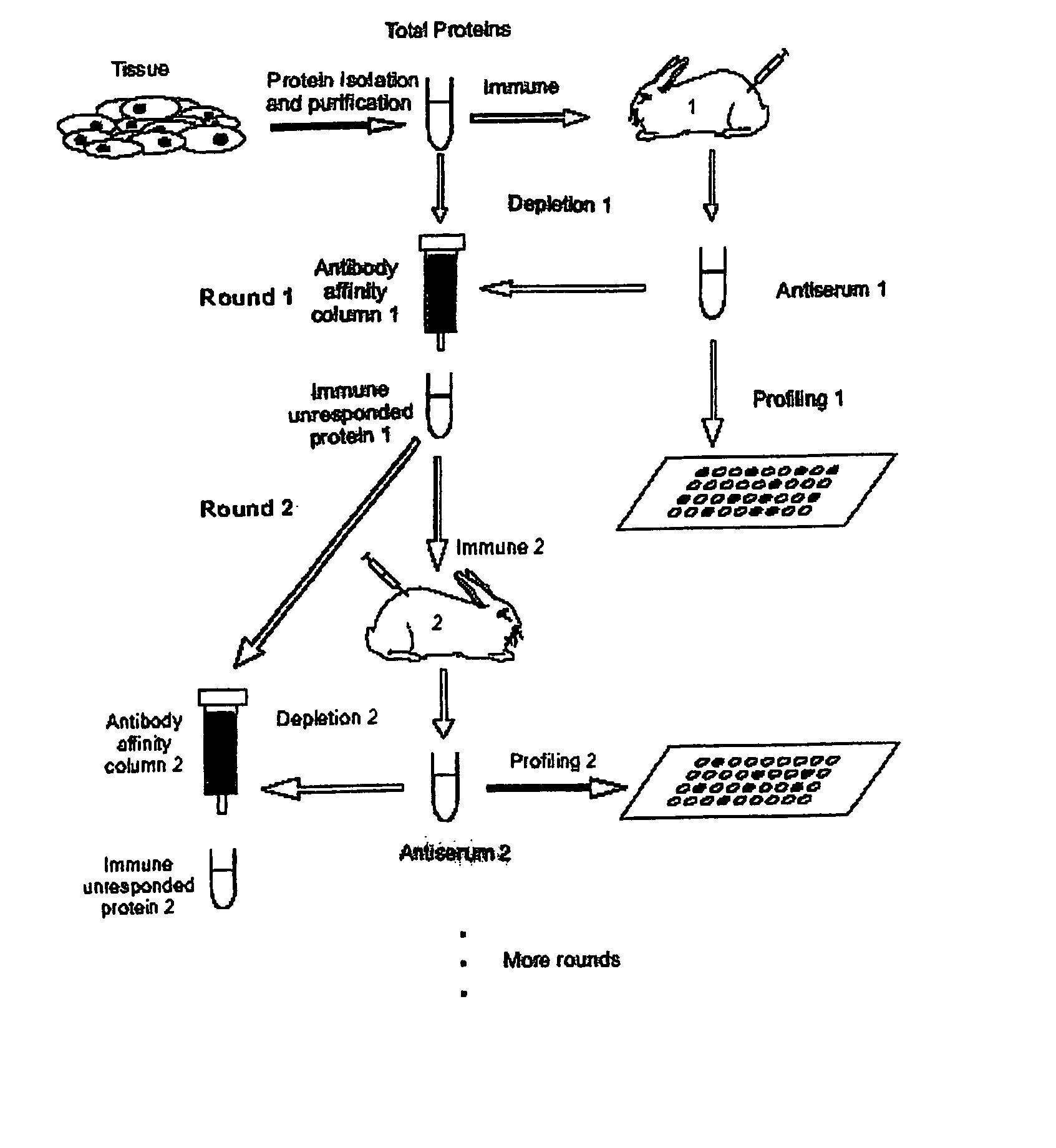
Method for proteomic analysis utilizing immune recognition and cumulative subtraction
InactiveUS20080008699A1Promote accumulationSimple and noninvasive collectionSnake antigen ingredientsImmunoglobulins against animals/humansBiological bodyImmune recognition
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
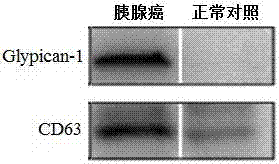
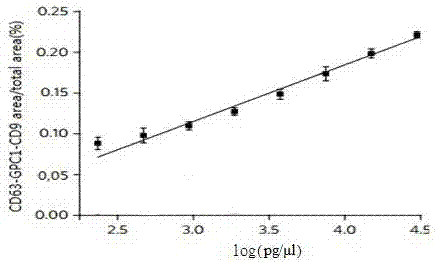
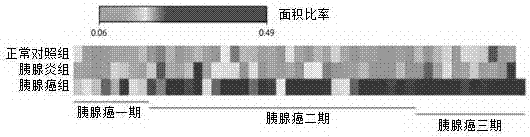
Application of Glypican-1 protein in diagnosis of pancreatic cancer, detection method of positive exosome concentration, and use of detection method
InactiveCN106950374AServe as a diagnosisPlay the role of staging judgment, etc.Raman scatteringBlood plasmaBiology
The invention discloses an application of a Glypican-1 protein in the diagnosis of pancreatic cancer, a detection method of a Glypican-1 positive exosome concentration, and a use of the detection method. A nano-plasma enhanced scattering (nPES) detection technology can be used to quantify exosomes in serum, an exosome extracting process is omitted, the characteristic antigen of the exosomes is used as a target, and the antigen and a corresponding antibody carrying gold nanoparticles form an antigen-antibody complex in order to obtain exosomes, and the amount of the exosomes is reflected by using the light radiation principle of the gold nanoparticles. The Glypican-1 is a pancreatic cancer-derived exosome biomarker, and the content of Glypican-1 positive exosomes in blood plasma has specific specificity even in early pancreatic cancer, so the exosome content index can be used to diagnose the pancreatic cancer, and the exosome level is closely related to the staging and progression of pancreatic cancer patients. Experiments prove that the method using the nPES detection technology to detect the content of the Glypican-1 exosomes in the serum is concise and accurate, and has better sensitivity and specificity than CA19-9 in the diagnosis of the pancreatic cancer.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
Calibration product stabilizer, detection kit for determining C peptide and detection method
ActiveCN109917134ALow costImprove responseChemiluminescene/bioluminescenceBiological testingC-peptideReaction curve
The invention belongs to the technical field of biomedical examination, and particularly relates to a calibration product stabilizer, a C peptide determination detection kit and a detection method. The kit comprises a calibration product, a reagent 1, a reagent 2, a reagent R3 and a reagent R4. According to the kit, an acridinium ester-labeled anti-C peptide antibody, an antigen and a horse radishperoxidase-labeled anti-C peptide antibody are utilized to form an antibody-antigen-antibody compound. A triggering agent is added into the compound without a washing process, the compound is continuously detected for a period of time, the peak area is calculated every 0.02-0.05 S, and a dosage-reaction curve is made by using C peptide with known concentration and the calculated peak area; and the content of the C peptide in the sample to be detected is calculated according to the curve. The kit for detecting the C peptide by adopting the spatial proximity chemiluminiscence method provided bythe invention has the advantages of strong calibration product stability, strong reagent anti-interference capability, high accuracy, good specificity and wide linear range, and is suitable for beingused by medical and research institutions at all levels in combination with instrument measurement.
Owner:GUANGZHOU JINDE BIOTECH
SPA-antibody tripolymer, cell treating kit containing tripolymer, preparation method and application thereof
InactiveCN101799473ASimple methodLow costBlood/immune system cellsBiological testingProgenitorRed blood cell
The invention relates to an SPA-antibody tripolymer, a cell treating kit containing the tripolymer, a preparation method and application thereof. The tripolymer comprises an anti erythrocyte antibody, an SPA and an antibody of a certain anti leukocyte antigen or other antigens. The tripolymer is combined with an erythrocyte through the anti erythrocyte antibody per se, the other antibody is combined with a corresponding leukocyte antigen (or other antigens), and then leukocyte (other cells, factors) and the erythrocyte are deposited through an erythrocyte sedimentation process or other methods of density gradient centrifugation and the like, thereby achieving the purpose of eliminating ingredients of corresponding cells and the like, connecting the erythrocyte with a corresponding antigen by the SPA, and being used as a mean for detecting a certain antigen. The invention has convenience and simpleness, and can be directly used for clinically separating and extracting stem cells / progenitor cells; and the collected and extracted stem cells / progenitor cells have no external markers. Meanwhile, the kit based on the tripolymer can be industrially produced so as to realize the popularization of the separation and purification technology of hematopoietic stem cells.
Owner:王信
Method for portably detecting vascular endothelial growth factor by using hybrid chain reaction, and nucleic acid sequence used by method
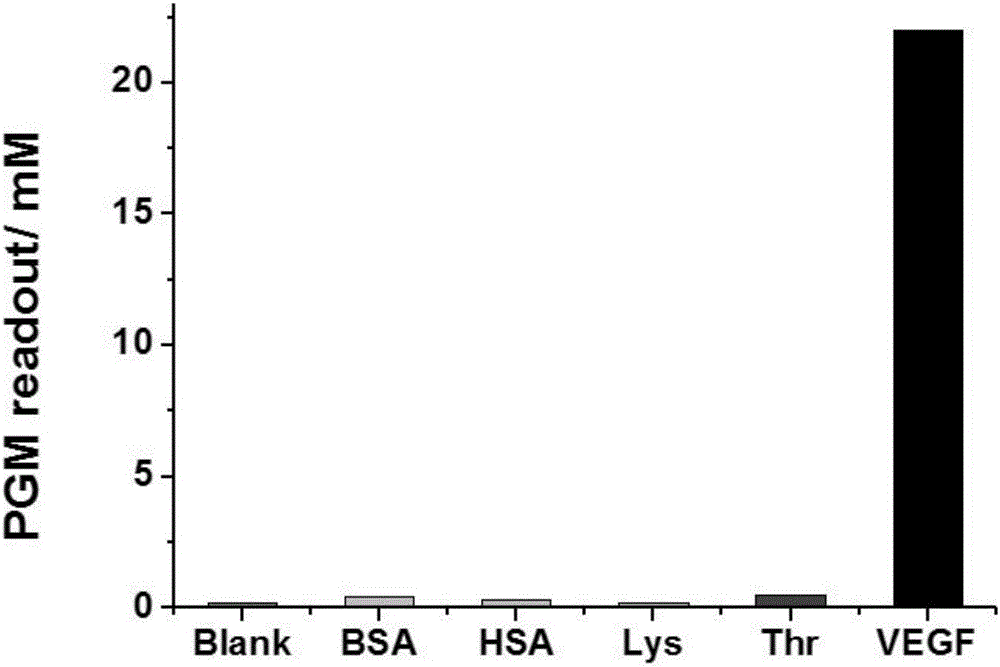
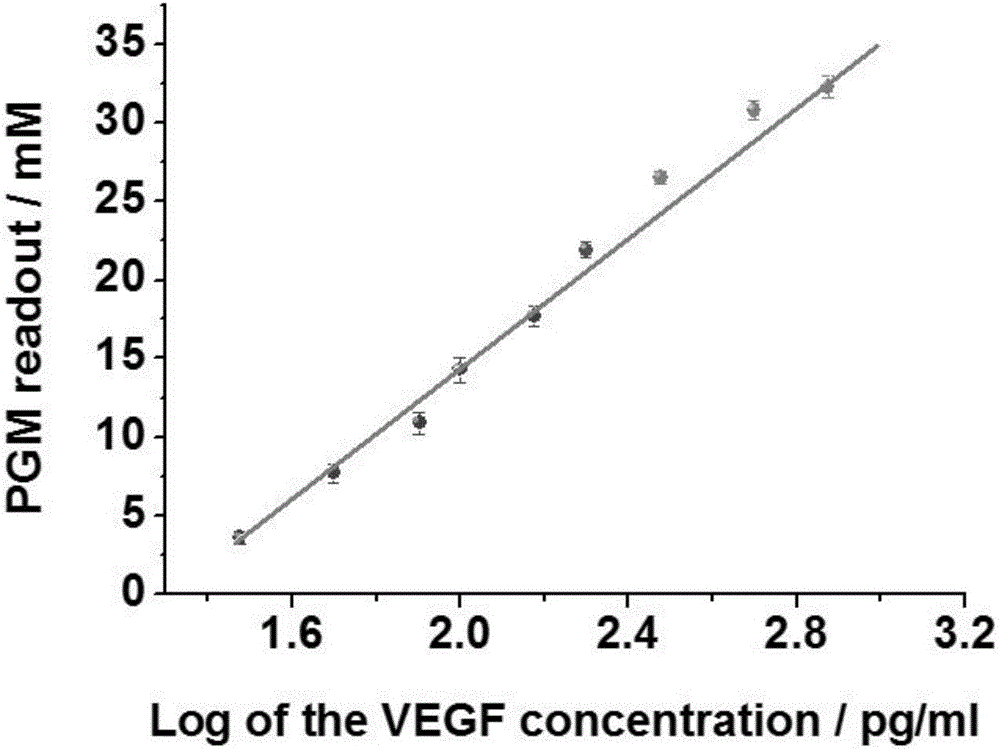
InactiveCN106093438AImprove defects with low detection sensitivityEfficient detectionBiological testingDNA/RNA fragmentationSucroseBlood vessel
The invention relates to a method for portably detecting a vascular endothelial growth factor by using a hybrid chain reaction, and a nucleic acid sequence used by the method. The method for detecting the vascular endothelial growth factor comprises the following steps: VEGF, an aptamer and an antibody form an antibody-protein-aptamer sandwich structure on polyethylene micro-pores, the tail end of the aptamer includes a 18-base extended sequence, two fragments of synthesized sucrose transferase labeled auxiliary probes initial a chain hybrid amplification reaction with the 18-base extended sequence as a template in order to immobilize a large amount of sucrose transferase in the micro-pores, the sucrose transferase catalyzes conversion of sucrose into glucose, and the concentration of the generated glucose is indicated by a glucometer. The detection method has the advantages of portability, simplicity, convenience in operation, high sensitivity, high selectivity, and realization of effective detection of the content of the vascular endothelial growth factor in a target to be analyzed.
Owner:FUJIAN AGRI & FORESTRY UNIV
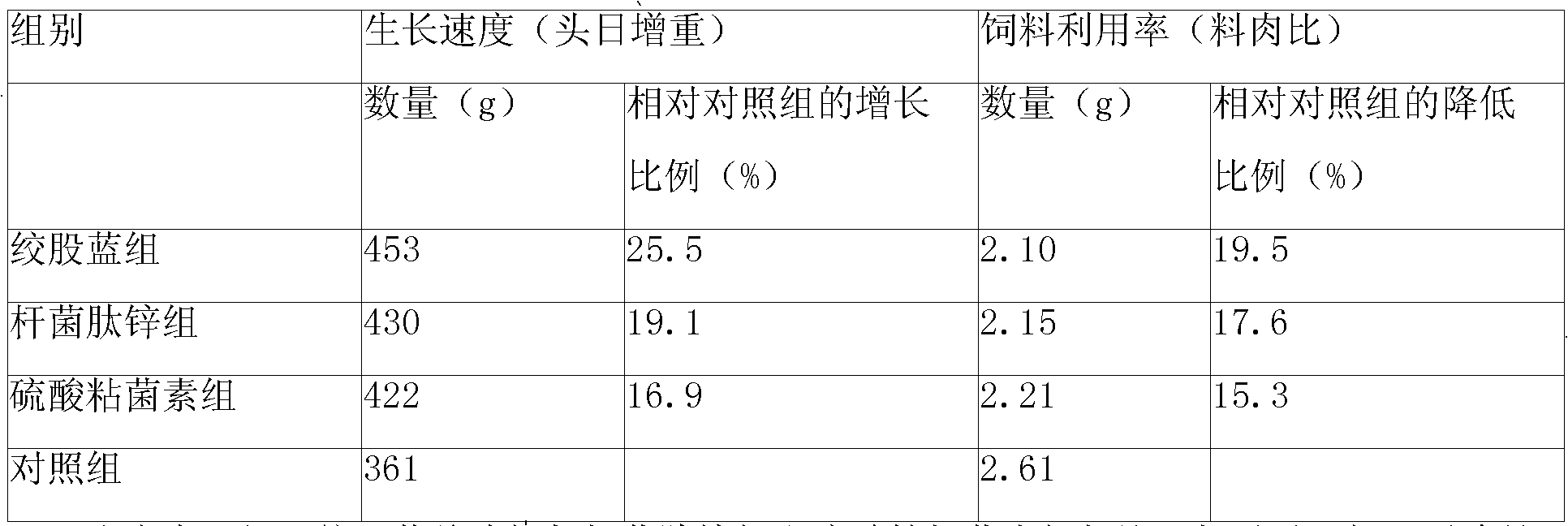
Gynostemma pentaphylla total glycoside composite feed additive and feed comprising same
ActiveCN101653201ACan regulate immunityPromote growth and developmentFood processingAnimal feeding stuffBiotechnologyDisease
A gynostemma pentaphylla total glycoside composite feed additive and a feed comprising the same. The additive is composed of main drugs including gynostemma pentaphylla total glycoside, Radix Astragali polysaccharide, hesperidin, licorice extract or glycyrrhizic acid and auxiliary materials. The additive comprises the Chinese herbal medicament active ingredients, could substitute for present antibiotic additive, has the functions of invigorating the spleen, benefiting the stomach and enhancing the digestion-absorption function, promotes the growth and development of pig, chicken and rabbit, induces the organism to generate interferon to adjust animal organism immunity, promotes to form antibody for preventing bacteria, virus and disease.
Owner:湖南康诺华生物科技有限公司
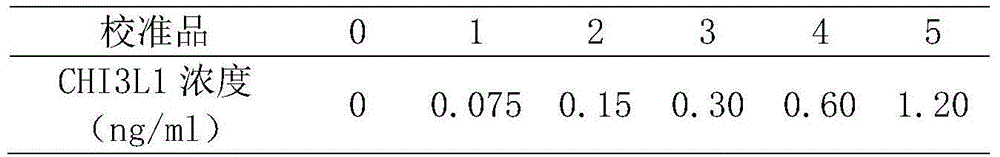
Liver cirrhosis detection kit and detection method thereof
InactiveCN104865385AEasy to operateThe test result is accurateDisease diagnosisBiological testingSolid phasesChemistry
This invention provides a liver cirrhosis detection kit and detection method, and belongs to the biotechnology field. According to the detection method, anti-human chitinase 3 protein 1 (CHI3L1) antibody coats a microporous plate for preparation of a solid phase antibody, when in use, a to-be-tested sample is added into the microporous plate coated with the antibody, if the to-be-tested sample contains a tested matter, the to-be-tested sample can be combined on the solid phase antibody to form an antigen-antibody complex; then a biotin-labeled anti-human CHI3L1 antibody is added to form an antibody-antigen-biotin-labeled antibody complex; horse radish peroxidase (HRP)-labeled avidin is added to form an antibody-antigen-biotin-labeled antibody-enzyme labeled avidin complex; and finally the complex is added into 3, 3 ', 5, 5 '-tetramethyl benzidine substrate system for chromogenic reaction. After the chromogenic reaction is completed, an enzyme standard instrument is used or testing. The liver cirrhosis detection kit has the advantages of fast, simple and accurate determination of test results.
Owner:TIANYIKANG TIANJIN HOSPITAL MANAGEMENT CO LTD
Protein peptide powder for enhancing immunity and preparation method and application thereof
InactiveCN106937748AImprove immunityImprove proliferative abilityFood ingredient functionsProtein food ingredientsLymphocyte proliferationIsomaltooligosaccharide
The invention relates to protein peptide powder for enhancing immunity and a preparation method and application thereof. The protein peptide powder is prepared from the following raw materials by weight: 30%-45% of marine fish skin collagen oligopeptide powder, 30%-50% of soybean oligopeptide powder, 10%-20% of Isomaltooligosaccharide, 8%-13% of polydextrose and 0%-0.5% of a sweetener. The protein peptide powder can increase macrophage phagocytic rate and phagocytic index and serum hemolysin content, enhances lymphocyte proliferation and NK cell activity, promotes antibody formation cell number increase, improves the body's immunity, and enhances body immunity.
Owner:GUANGZHOU BAIYUSN GUANGHUA PHARMA
Method of modulating neutralizing antibodies formation in mammals, and uses thereof in gene therapy, animal trangenesis and in functional inactivation of endogenous proteins
InactiveUS20080220015A1Long-term expressionLong-term therapyVirusesPeptide/protein ingredientsMammalTransgene
Disclosed is a method of modulating neutralizing antibodies formation against a heterologous protein. The method may be used to induce tolerance of the immune system towards the protein, such tolerance being useful to allow long-term gene therapy or transgene expression. The method may also be used to provide an animal with a reproducible functional inactivation phenotype of an endogenous protein of the animal.
Owner:NOKAD
Immunological assay reagents and assay method
An immunological analyzing reagent comprising a composition containing a biotin-conjugated antigen or antibody against a substance to be analyzed, and a composition containing an avidin-conjugated microparticle, wherein the biotin-conjugated antigen or antibody contains an amount of biotin which does not substantially agglutinate with the avidin-conjugated microparticle in the absence of the substance to be analyzed, is disclosed. Further, an immunological analyzing method comprising the steps of: bringing into contact a sample, a biotin-conjugated antigen or antibody against a substance to be analyzed, and an avidin-conjugated microparticle, the biotin-conjugated antigen or antibody containing an amount of biotin which does not agglutinate with the avidin-conjugated microparticle in the absence of the substance to be analyzed; and detecting the degree of aggregation between the avidin-conjugated microparticle, and an immunocomplex formed from the substance to be analyzed and the biotin-conjugated antigen or antibody, is disclosed. According to the immunological analyzing reagent and analyzing method, a procedure in which an antibody or antigen is carried on a latex particle is not necessary; self-aggregation of latex particles does not occur; a precise measurement of the substance to be analyzed may be carried out; and an excellent reactivity the same as or superior to that obtained by the conventional sensitized latex method may be obtained.
Owner:MITSUBISHI KAGAKA IATRON INC
Mode identification method based on immune antibody network
InactiveCN101655911AImprove the correct recognition rateEasy to calculateBiological modelsCharacter and pattern recognitionApplication areasContinuous training
The invention relates to a mode identification method based on an immune antibody network, which is used for mode identification. The technical scheme of the invention comprises the following steps: generating an original antibody in the immune antibody network by extracting all classes of training samples with a certain number stochastically and completing the initialization of the immune antibody network; taking all the training samples as an input antigen, training the immune antibody network according to an antibody formation algorithm and extracting the mode characteristics of all classesof training samples effectively; and training the immune antibody network repeatedly, stopping training when the continuous training results of two times are accordant, preserving the obtained immuneantibody network and carrying out the mode identification. The invention has simple and convenient calculation and very high correct identification rate without manually setting parameters and thresholds and is suitable for the computer application fields of character identification, fault diagnosis and the like.
Owner:NORTH CHINA ELECTRIC POWER UNIV (BAODING)
Homogeneous chemiluminescence immune assay method based on adjacent position striking effect
InactiveCN104165999AEasy to operateLow costMicrobiological testing/measurementChemiluminescene/bioluminescenceMolecular beaconImmune complex deposition
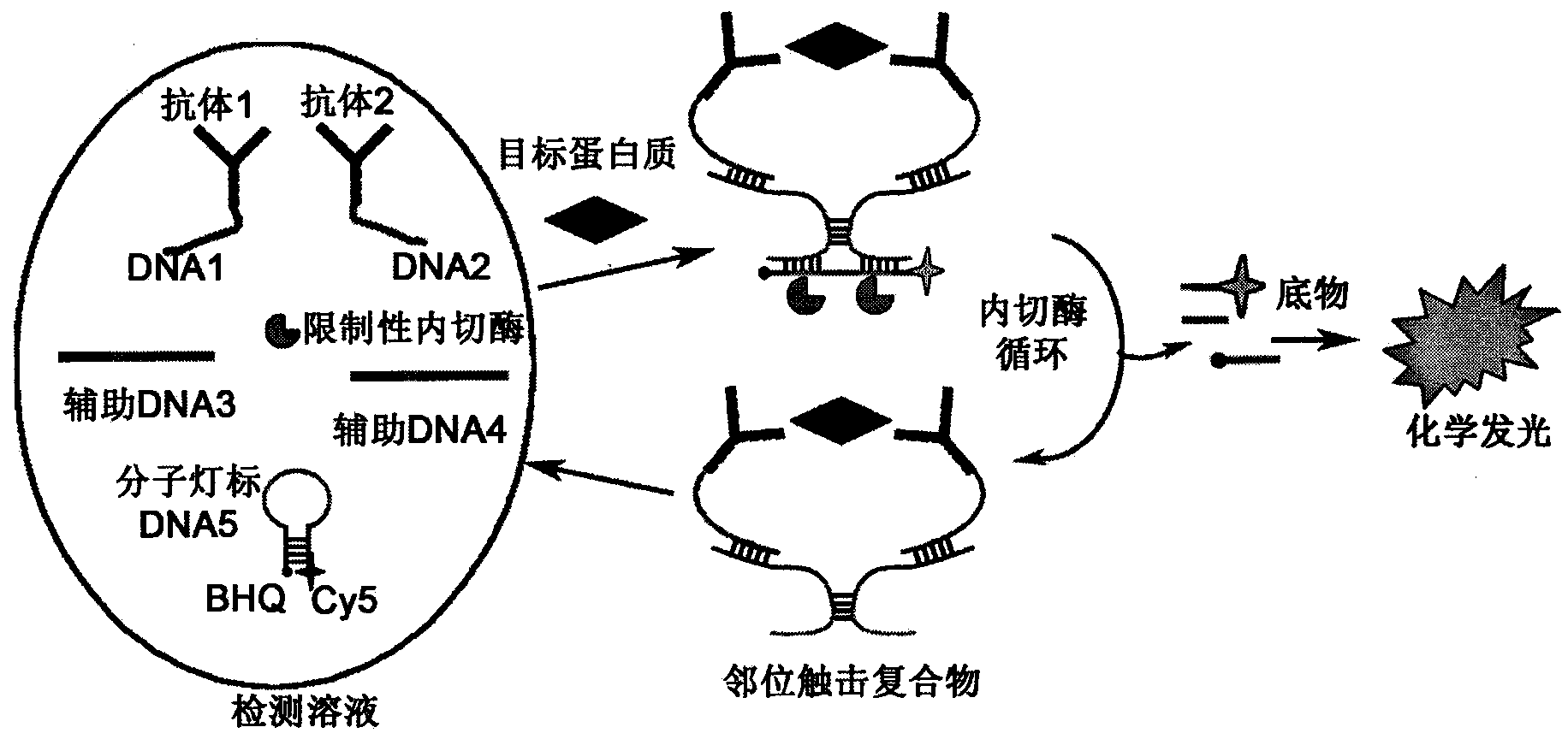
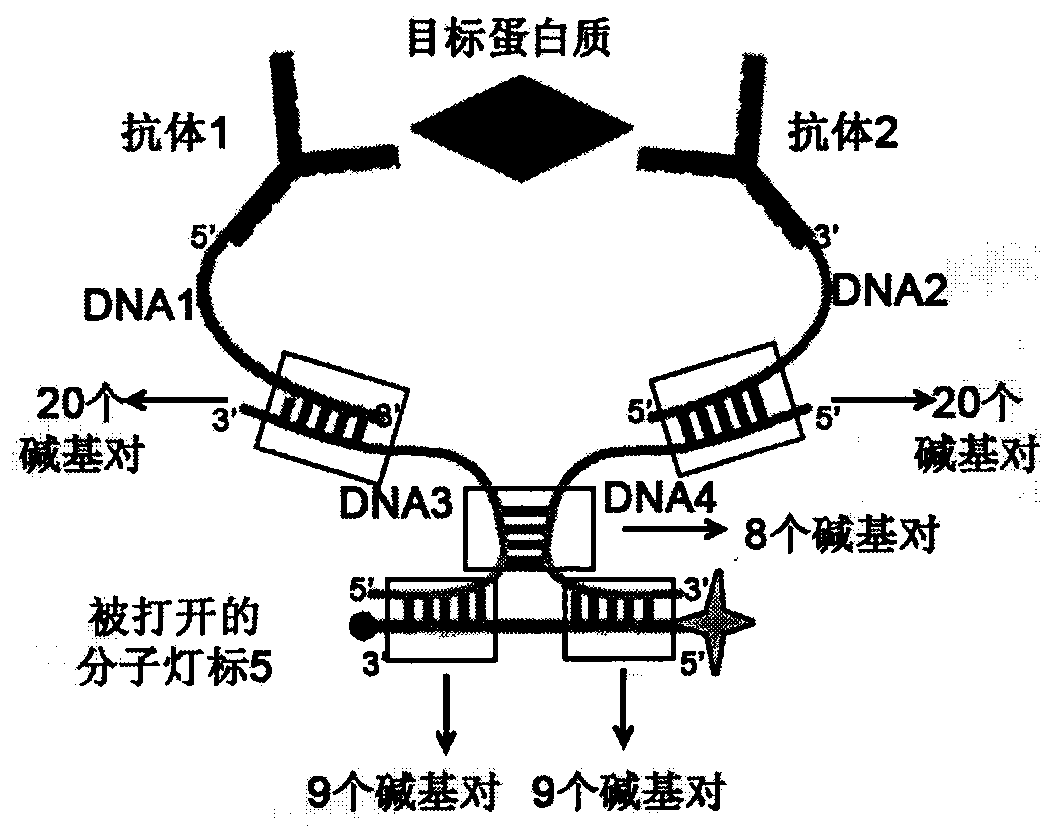
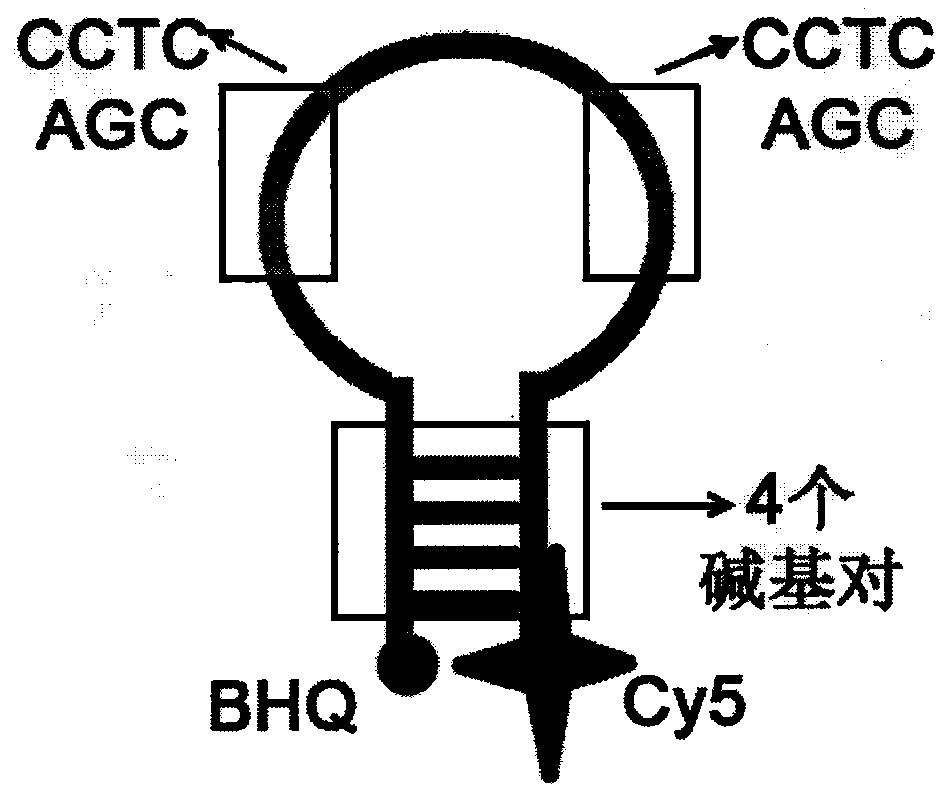
The invention relates to a homogeneous chemiluminescence immune assay method based on an adjacent position striking effect. A detection solution of the method includes a DNA1-antibody 1 conjugate, a DNA2-antibody 2 conjugate, auxiliary DNA3, auxiliary DNA4, molecular beacon DNA5 and restrictive endonuclease. When a target protein exists, a DNA1-antibody 1 and a DNA2-antibody 2 form a sandwich immune complex to make DNA3 and DNA4 respectively hybridized with DNA1 and DNA2 close to each other in order to form an adjacent position strike complex, the adjacent position strike complex can be hybridized with the DNA5 to open its hairpin structure, and a dye Cy5 goes away from a quencher to generate chemiluminiscence. A double chain formed by the adjacent position strike complex and the DNA5 can be identified by endonuclease, new DNA5 is opened after the DNA5 is cut, and the cycle of above steps can realize onsite amplified luminescence in order to realize the highly-sensitive quantitative analysis of the target protein. The method has the advantages of realization of fast one-step protein detection, simple operation and high universality.
Owner:NANJING UNIV +1
Method of quickly detecting and/or assaying antigen by fluorescence correlation spectrometry
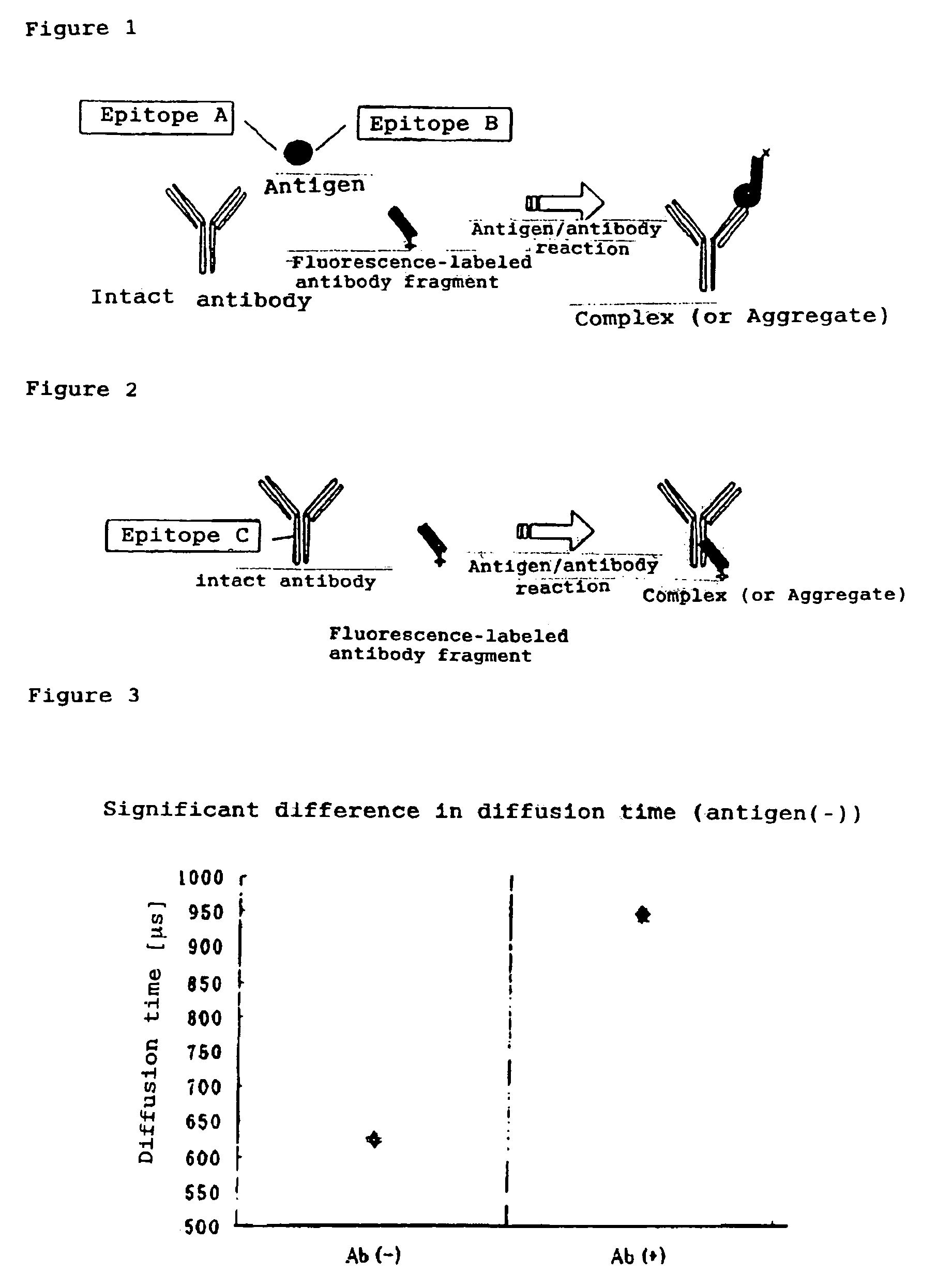
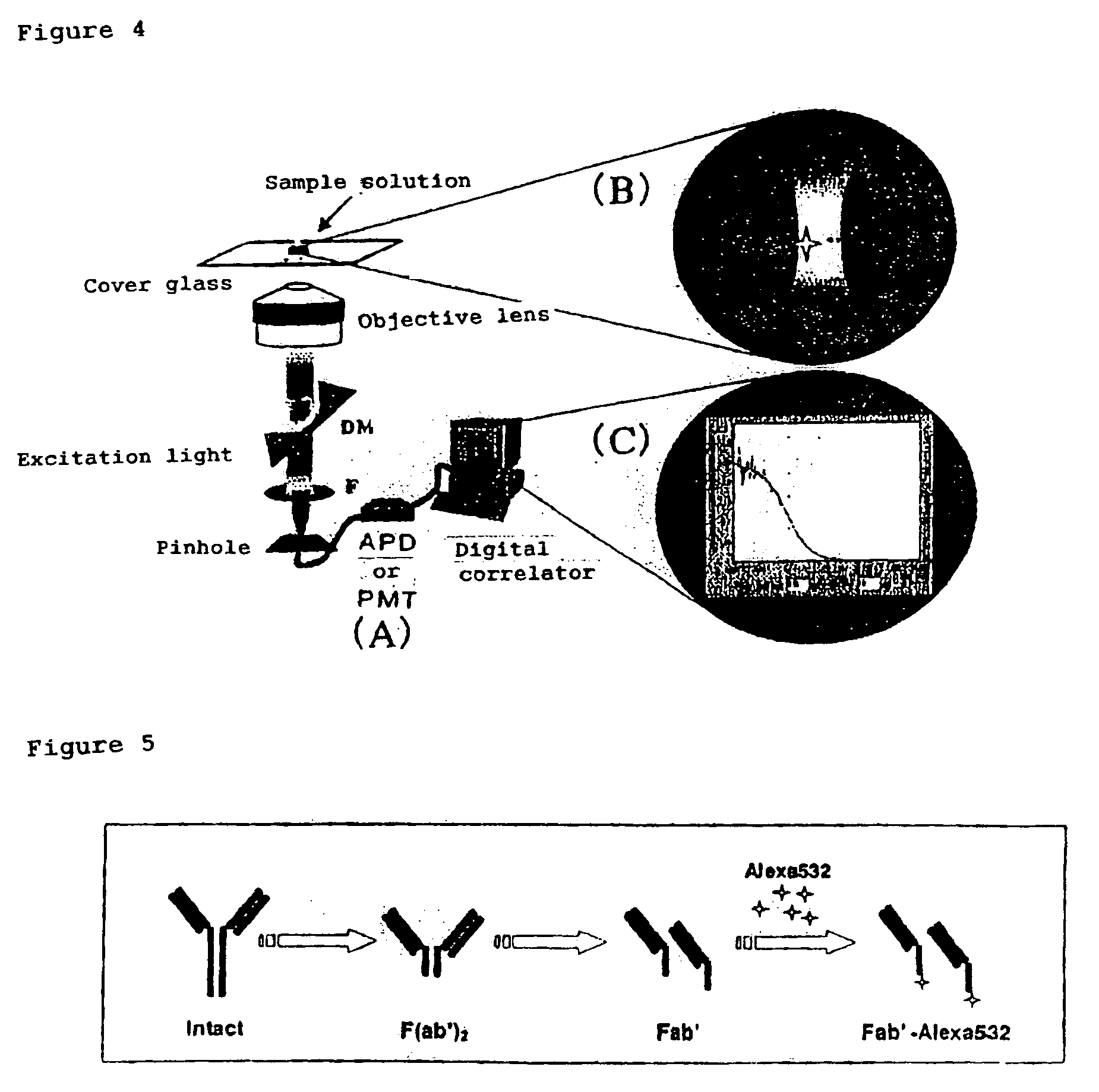
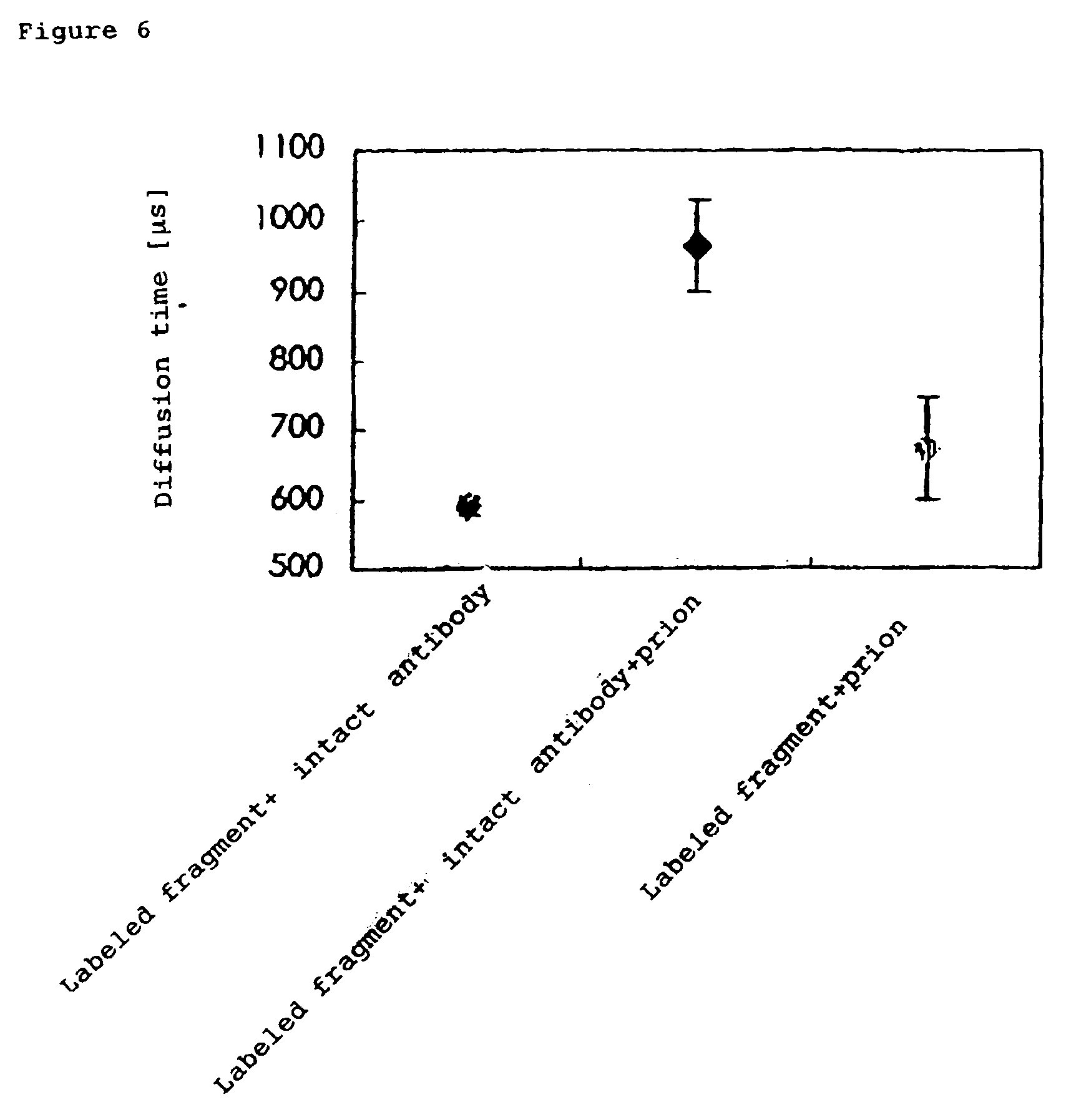
InactiveUS7476545B2Quick and convenient methodAvoid narrow scopeMaterial analysis by observing effect on chemical indicatorChemiluminescene/bioluminescenceAntibody fragmentsAntigen binding
The present invention provides a method of quickly and accurately detecting and / or assaying an antigen using fluorescence correlation spectroscopy (FCS), which involves a fluorescence-labeled antibody fragment and a non-fluorescence-labeled intact antibody that form a complex with the antigen. There is a significant difference in diffusion rate between the fluorescence-labeled antibody fragment not bonded to the antigen and the complex formed by the the fluorescence-labeled antibody fragment, the antigen, and the non-fluorescence-labeled intact antibody, and this diffusion rate can be determined using FCS. The antigen can be an antigenic protein, such as an abnormal prion or a harmful protein contained in a food material. According to this method, antigens over a wide scope can be assayed regardless of the shape or molecular weight.
Owner:JAPAN SCI & TECH CORP
Rapid classification of biological components
InactiveUS20050191692A1Bioreactor/fermenter combinationsBiological substance pretreatmentsImmune complex depositionUnknown Source
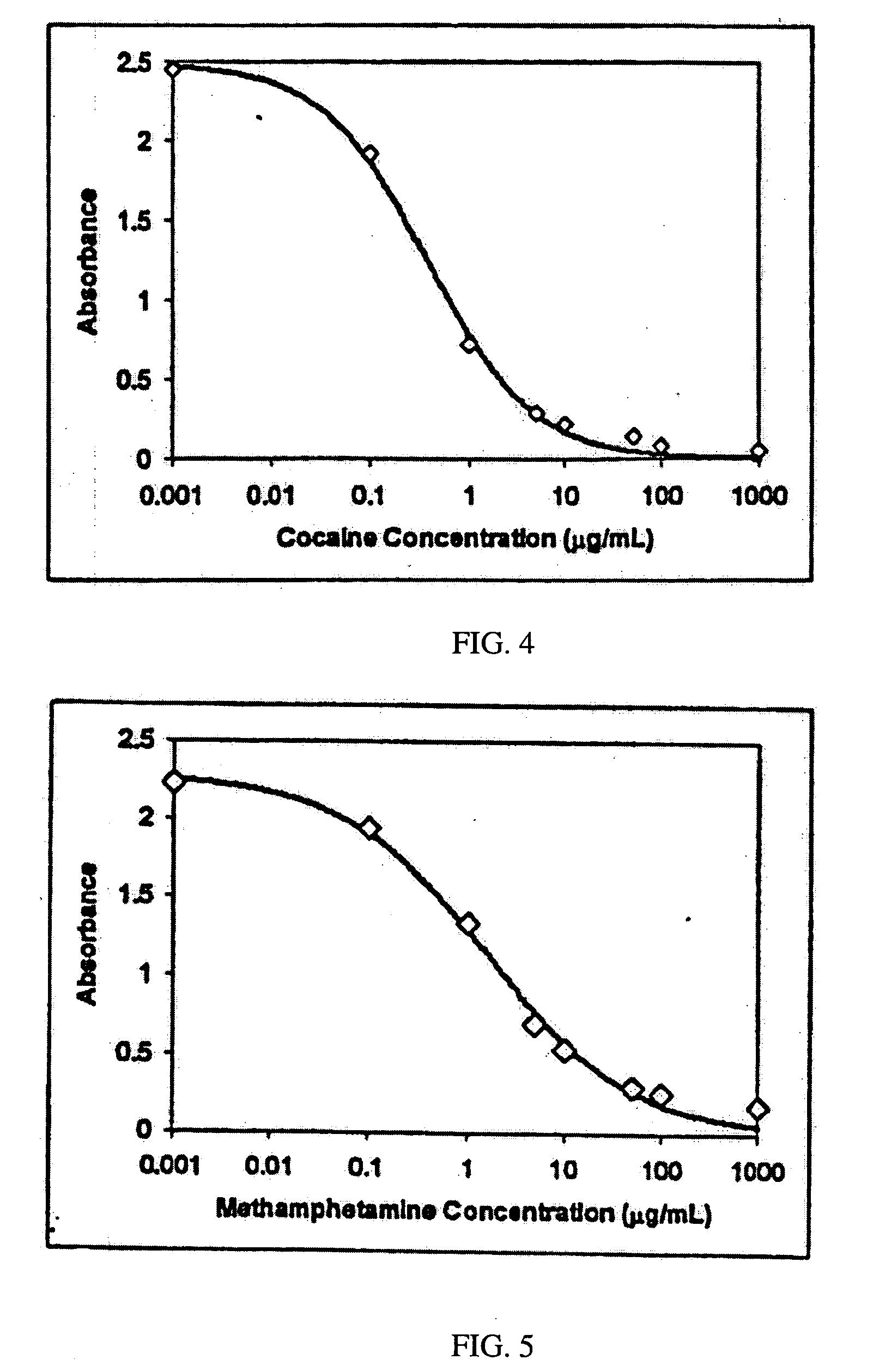
A method is disclosed for analyzing a biological sample by antibody profiling for identifying forensic samples or for detecting the presence of an analyte. In an illustrative embodiment of the invention, the analyte is a drug, such as marijuana, cocaine, methamphetamine, methyltestosterone, or mesterolone. The method involves attaching antigens to the surface of a solid support in a preselected pattern to form an array wherein the locations of the antigens are known; contacting the array with the biological sample such that a portion of antibodies in the sample reacts with and binds to antigens in the array, thereby forming immune complexes; washing away antibodies that do form immune complexes; and detecting the immune complexes, thereby forming an antibody profile. Forensic samples are identified by comparing a sample from an unknown source with a sample from a known source. Further, an assay, such as a test for illegal drug use, can be coupled to a test for identity such that the results of the assay can be positively correlated to the subject's identity.
Owner:BATTELLE ENERGY ALLIANCE LLC
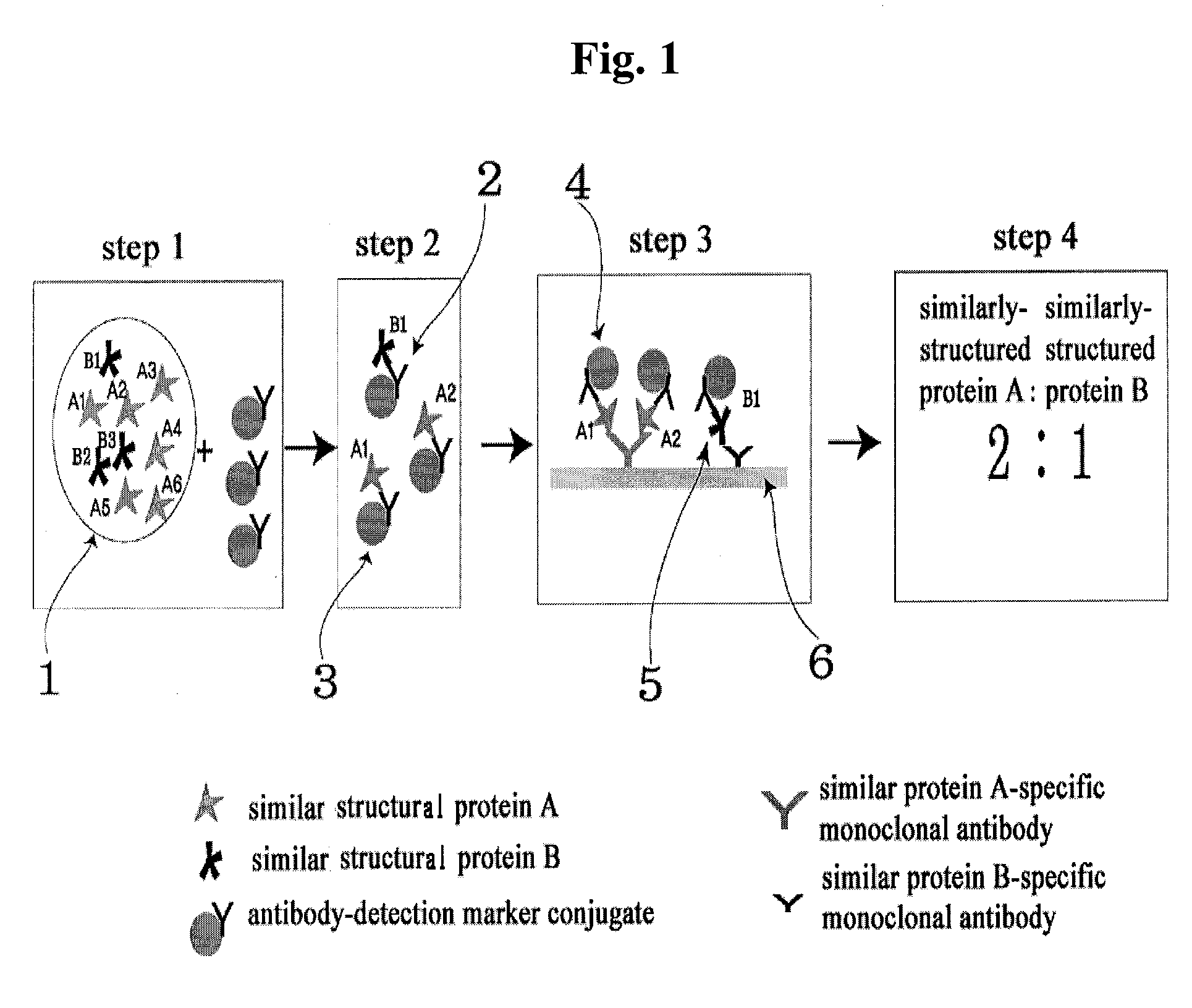
Diagnose device for measuring the ratio of proteins with similar structure
ActiveUS20090208983A1Advantage of simplicityEasy to testBioreactor/fermenter combinationsBiological substance pretreatmentsAntibody conjugateStructural protein
The present invention relates to a diagnostic device for measuring the ratio of similar structural proteins among the proteins secreted in a liquid test sample taken from diagnosis subject. In further detail, the test device according to the present invention comprises detection marker-antibody conjugate recognizing the same site on two or more similar structural proteins and a detection zone in which antibody specifically recognizes each of said proteins via formation of sandwich type complex, wherein said antibodies form a set, and the present Invention relates to a diagnostic device for early diagnosis of polycystic ovary syndrome, abnormal pregnancy, prostatic carcinoma etc. based on determination of the ratio of follicle stimulating hormone and luteinizing hormone in case of polycystic ovary syndrome, the ratio between hCG isomers in case of abnormal pregnancy, and the ratio of prostate-specific antigens (PSA) in case of prostatic carcinoma.
Owner:HUMASIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com