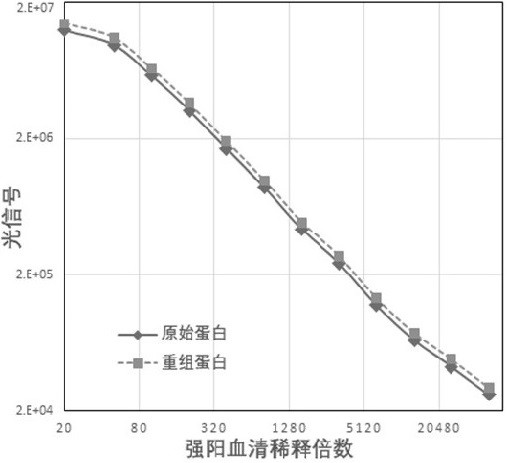
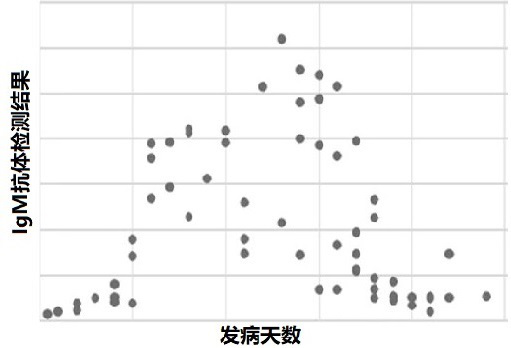
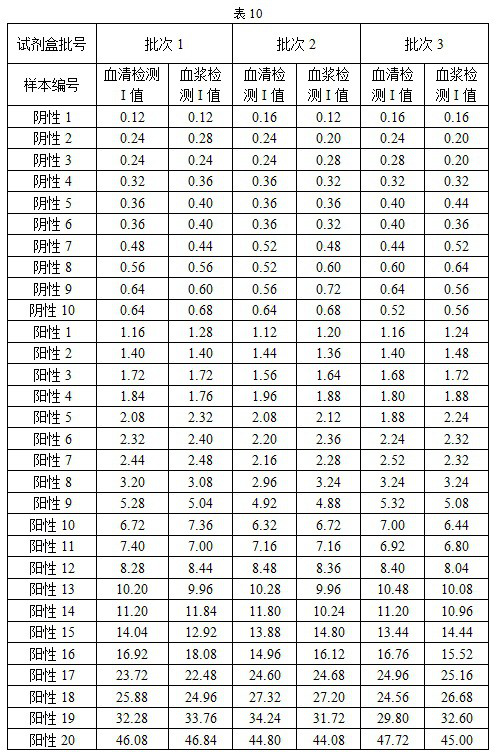
Novel coronavirus antibody detection kit based on magnetic particle chemiluminescence
A coronavirus and antibody detection technology, applied in chemiluminescence/bioluminescence, analysis through chemical reactions of materials, measurement devices, etc., can solve problems such as false negatives, false positives, and biosafety risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] This embodiment provides a novel coronavirus IgM antibody detection kit based on magnetic particle chemiluminescence, specifically including:
[0079] 0.3 mg / mL streptavidin magnetic particles, biotin-labeled novel coronavirus antigen, acridine sulfonamide-labeled goat anti-human IgM antibody, sample diluent, positive quality control, negative quality control, sample diluent and washing liquid.
[0080] Among them, (1) The preparation method of the biotin-labeled novel coronavirus antigen is:
[0081] Dilute 0.3 mg of the recombinant nucleocapsid protein of the new coronavirus and 0.2 mg of the recombinant spike protein S1 with 0.02 M PBS (pH7.2) buffer, and the final concentration of the mixed antigen is 1 mg / mL;
[0082] Wherein, the sequence of the recombinant nucleocapsid protein is SEQ ID NO.1; the sequence of the recombinant spike protein S1 is SEQ ID NO.3:
[0083] Take 12 μL of 10 mg / mL activated biotin and add it to the above antigen buffer, mix well, and kee...
Embodiment 2
[0090] This embodiment provides a new coronavirus IgM antibody detection kit based on magnetic particle chemiluminescence. The difference from Example 1 is that the mass ratio of recombinant nucleocapsid protein and recombinant spike protein S1 is set to 5:0, 4:0, respectively. 1, 2:3, 1:4 and 0:5;
[0091] Detection discrimination, the results are shown in Table 1:
[0092]
[0093] Among them, the reference samples are standard samples, which are the samples to be tested during the development of the kit, and are numbered N01~N15 (negative samples), P01~05 (positive samples) and L1~L3 (quality control samples); from the above table It can be seen that when the ratio of nucleocapsid protein and spike protein S1 is 5:0, 4:1, 1:4, and 0:5, the negative coincidence rate (that is, the discrimination degree is less than 1) is 14 / 15;
[0094] Specifically: when the ratio is 5:0, the discrimination of N10 samples is 2.08; when the ratio is 4:1, the discrimination of N10 samples ...
Embodiment 3
[0097] This example provides a new coronavirus IgM antibody detection kit based on magnetic particle chemiluminescence. The difference from Example 1 is that the addition amount of Sulfo-NHS-LC-Biotin solution is set to 3, 6 and 24 μL respectively.
[0098] The specific results are shown in Table 2 below;
[0099]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com