A novel coronavirus antibody detection kit based on magnetic particle chemiluminescence
A coronavirus and antibody detection technology, applied in chemiluminescence/bioluminescence, analysis through chemical reaction of materials, measurement devices, etc., can solve the problems of prone to false negatives, low sensitivity, and prone to false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
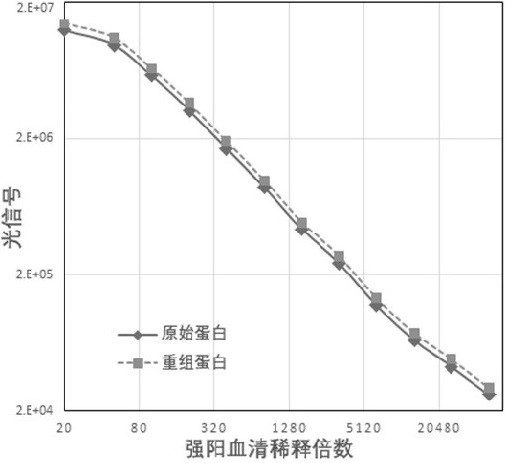
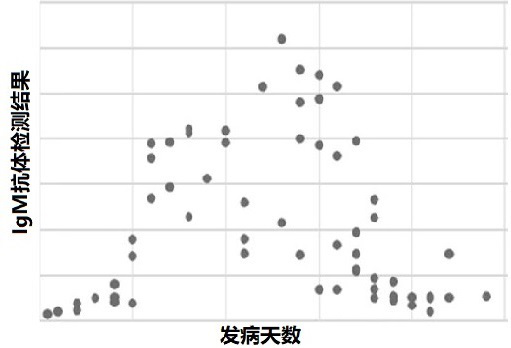
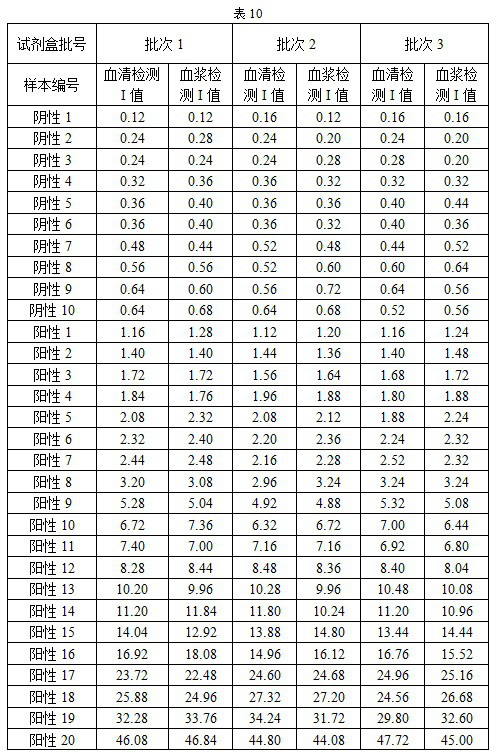
Image
Examples
Embodiment 1
[0078] This embodiment provides a novel coronavirus IgM antibody detection kit based on magnetic particle chemiluminescence, specifically including:
[0079] 0.3 mg / mL streptavidin magnetic particles, biotin-labeled novel coronavirus antigen, acridine sulfonamide-labeled goat anti-human IgM antibody, sample diluent, positive quality control, negative quality control, sample diluent and washing liquid.
[0080] Among them, (1) The preparation method of the biotin-labeled novel coronavirus antigen is:
[0081] Dilute 0.3 mg of the recombinant nucleocapsid protein of the new coronavirus and 0.2 mg of the recombinant spike protein S1 with 0.02 MPBS (pH7.2) buffer, and the final concentration of the mixed antigen is 1 mg / mL;
[0082] Wherein, the sequence of the recombinant nucleocapsid protein is SEQ ID NO.1; the sequence of the recombinant spike protein S1 is SEQ ID NO.3:
[0083] Take 12 μL of 10 mg / mL activated biotin and add it to the above antigen buffer, mix well, and keep...
Embodiment 2
[0090] This embodiment provides a new coronavirus IgM antibody detection kit based on magnetic particle chemiluminescence. The difference from Example 1 is that the mass ratio of recombinant nucleocapsid protein and recombinant spike protein S1 is set to 5:0, 4:0, respectively. 1, 2:3, 1:4 and 0:5;
[0091] Detection discrimination, the results are shown in Table 1:
[0092]
[0093] Among them, the reference samples are standard samples, which are the samples to be tested during the development of the kit, and are numbered N01~N15 (negative samples), P01~05 (positive samples) and L1~L3 (quality control samples); from the above table It can be seen that when the ratio of nucleocapsid protein and spike protein S1 is 5:0, 4:1, 1:4, and 0:5, the negative coincidence rate (that is, the discrimination degree is less than 1) is 14 / 15;
[0094] Specifically: when the ratio is 5:0, the discrimination of N10 samples is 2.08; when the ratio is 4:1, the discrimination of N10 samples ...
Embodiment 3
[0097] This example provides a new coronavirus IgM antibody detection kit based on magnetic particle chemiluminescence. The difference from Example 1 is that the addition amount of Sulfo-NHS-LC-Biotin solution is set to 3, 6 and 24 μL respectively.
[0098] The specific results are shown in Table 2 below;
[0099]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com