Patents
Literature
448 results about "Affitin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Affitins (commercial name Nanofitins) are artificial proteins with the ability to selectively bind antigens. They are structurally derived from the DNA binding protein Sac7d, found in Sulfolobus acidocaldarius, a microorganism belonging to the archaeal domain. By randomizing the amino acids on the binding surface of Sac7d and subjecting the resulting protein library to rounds of ribosome display, the affinity can be directed towards various targets, such as peptides, proteins, viruses, and bacteria. Affitins are antibody mimetics and are being developed as an alternative to antibodies as tools in biotechnology. They have also been used as specific inhibitors for various enzymes. Affitins can be utilized in biochemical purification techniques, specifically in Affinity chromatography. The ability of Affitins to effectively and selectively bind antigens is used to target specific proteins. Scientists have been able purify human immunoglobulin G (hIgG), bacterial PulD protein, and chicken egg lysozyme using Affitin columns with a high degree of purity.
Liquid phase chip for joint detection of multiple tumor markers and preparation method thereof
The invention relates to a liquid phase chip for joint detection of multiple tumor markers and a preparation method thereof. The liquid phase chip comprises micro-balloons of a coupled antibody (at least two of the followings: AFP, CEA, CA125, CA153, CA19-9, CA242, CA72-4, PSA, HGH, Beta-HCG), corresponding biotin-labeled detection antibodies, streptavidin phycoerythrin and vegetable hydrosol or polysaccharide hydrosol which has a solute content of 1-10 wt per thousand and does not contain protein. The preparation of the liquid phase chip comprises refining and purification of hydrosol, coupling between captured antibodies and micro-balloons, preparation of biotin-labeled antibodies, dispersing the coupled micro-balloons into the vegetable hydrosol or the polysaccharide hydrosol, and the like. The liquid phase chip has the advantages of stable performance, good micro-balloon dispersivity, long preservation time, fast and convenient use and operation, small amount of samples in use, high detection sensitivity, wide linear scope and low detection cost, can detect ten tumor markers at most at one time, and requires a cost which is a quarter of the total fee of conventional methods.
Owner:HENAN YUKANG BIOTECH
Magnetic microparticle separating chemiluminescence immune analysis determination reagent kit for detecting related sign object and preparing method thereof
ActiveCN101377490AEnsure sensitivityEnsure effectivenessChemiluminescene/bioluminescenceBiological testingDiseaseMicroparticle
The invention relates to the immunoassay medical field, particularly provides a magnetic particle separation chemiluminescence immunoassay assay kit and a preparation method thereof used for detecting disease related markers. The kit of the invention comprises: 1) a calibrator; 2) magnetic particles which are coated with streptavidin; 3) disease related marker antibodies of enzyme markers and biotin markers; and 4) a chemiluminescence substrate. Further, the method for preparing the kit according to the invention includes the following steps: 1) pure raw materials are used to prepare the calibrator; 2) the streptavidin is used to coat the magnetic particles; 3) the mixed liquid of the enzyme and the biotin markers are prepared; 4) the calibrator, the chemiluminescence substrate as well as the mixed liquid of the enzyme and the biotin markers are packaged in a separated way; and 5) a finished product is packaged. The kit has the advantages of convenience, rapidness, sensitivity, stability, and the like.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Anti-growth factor receptor avidin fusion proteins as universal vectors for drug delivery
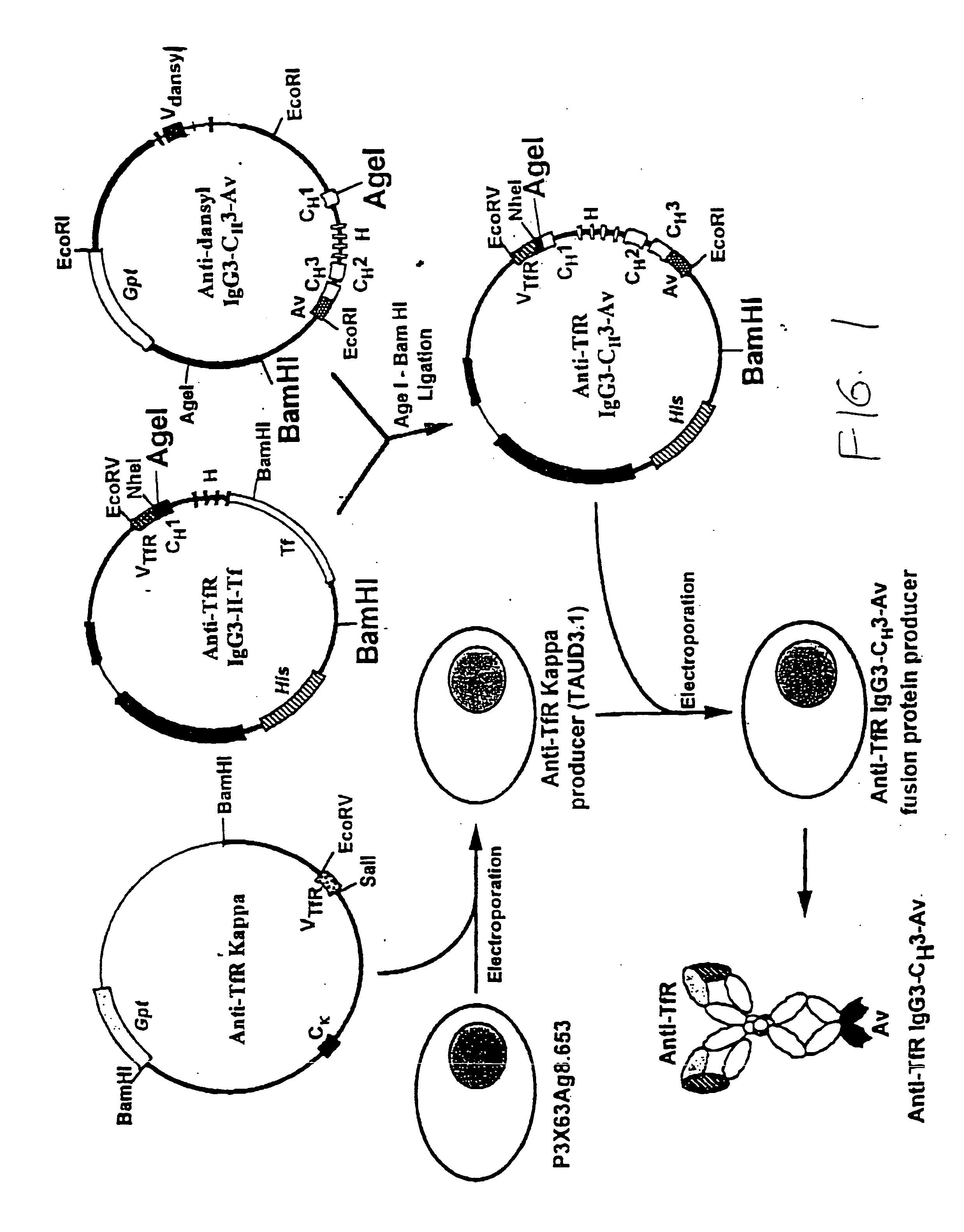
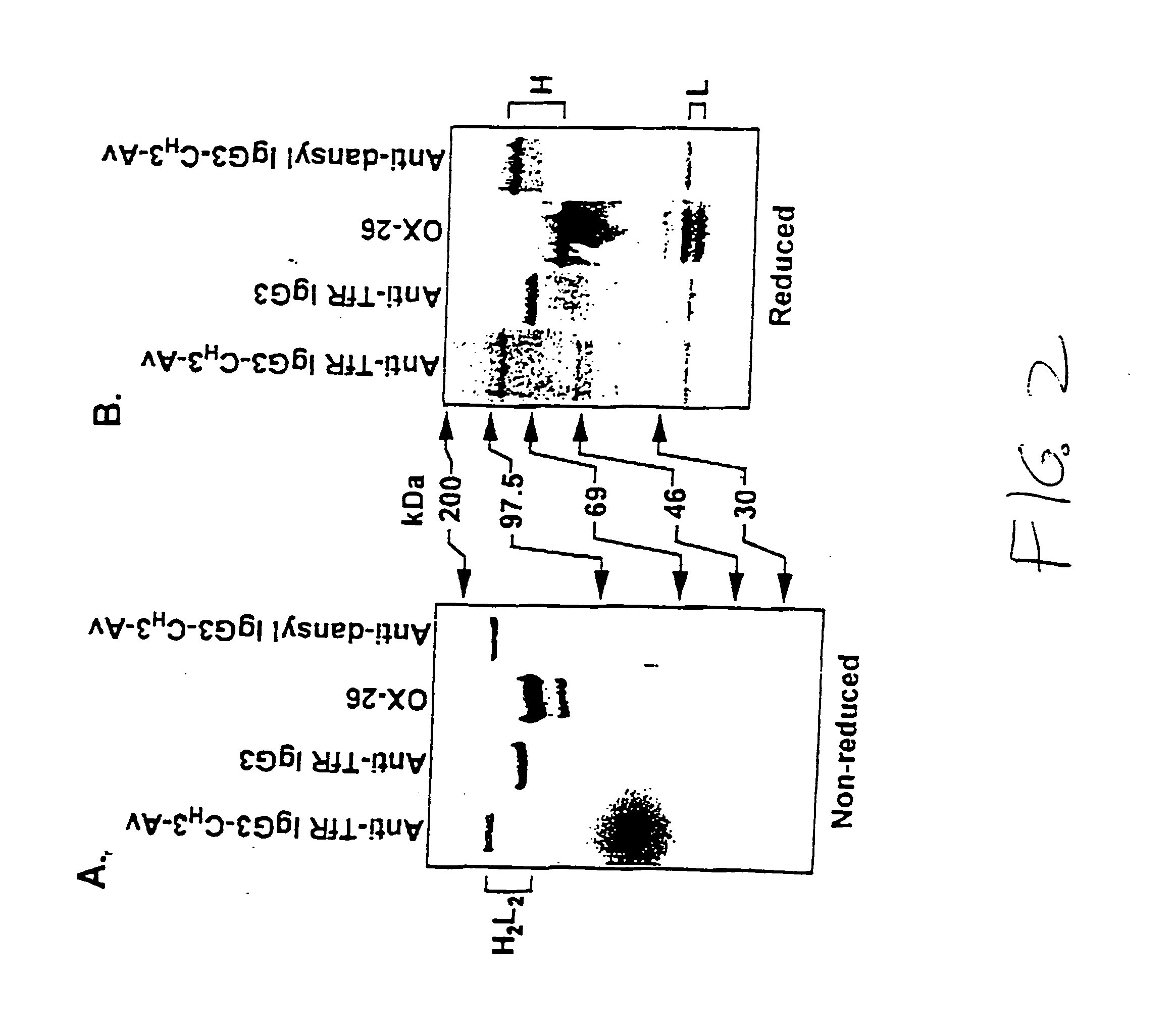
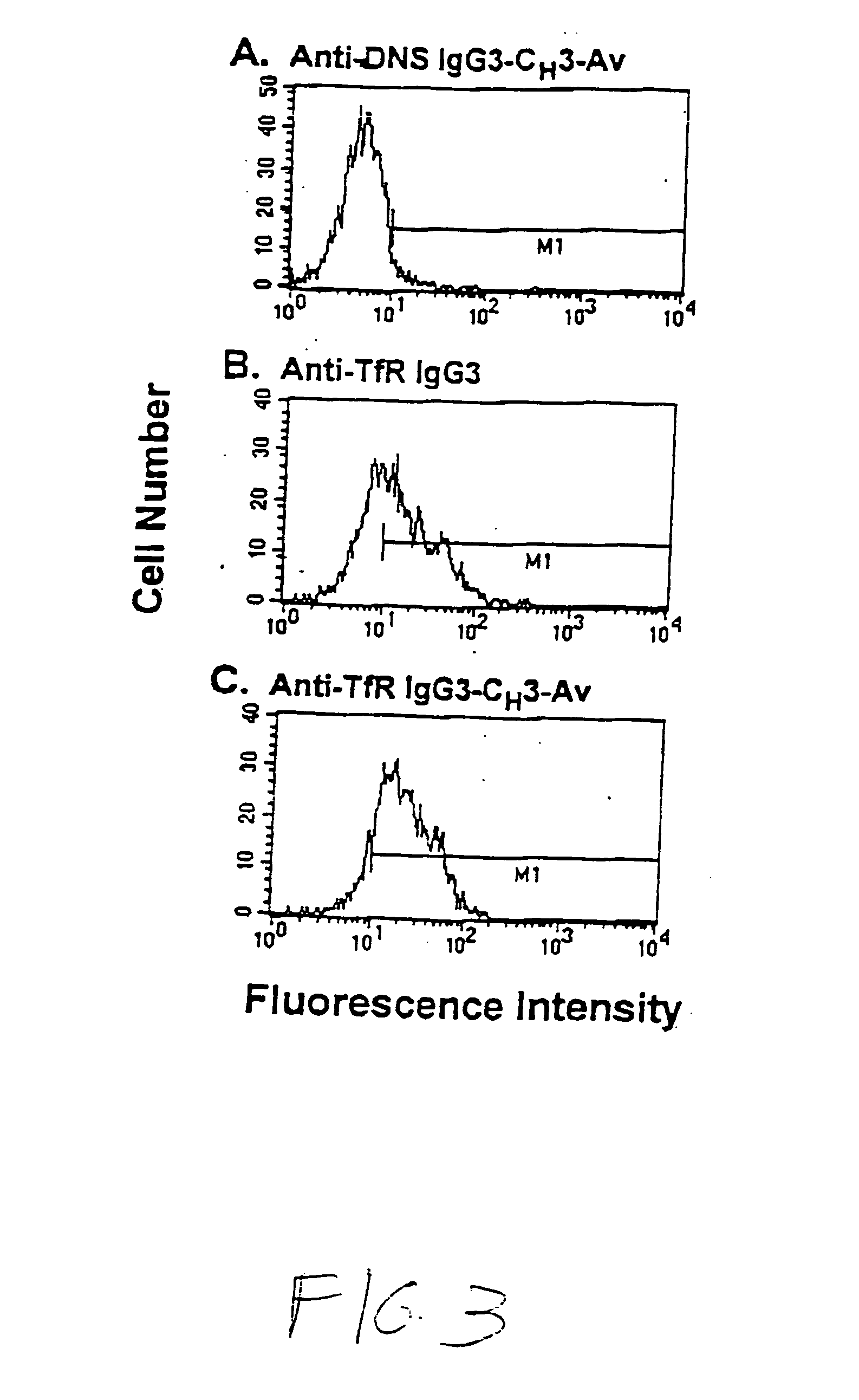
A fusion protein for delivery of a wide variety of agents to a cell via antibody-receptor-mediated endocytosis comprises a first segment and a second segment: the first segment comprising a variable region of an antibody that recognizes an antigen on the surface of a cell that after binding to the variable region of the antibody undergoes antibody-receptor-mediated endocytosis, and, optionally, further comprises at least one domain of a constant region of an antibody; and the second segment comprising a protein domain selected from the group consisting of avidin, an avidin mutein, a chemically modified avidin derivative, streptavidin, a streptavidin mutein, and a chemically modified streptavidin derivative. Typically, the antigen is a protein. Typically, the protein antigen on the surface of the cell is a receptor such as a transferrin receptor-or an insulin receptor. The invention also includes an antibody construct incorporating the fusion protein that is either a heavy chain or a light chain together with a complementary light chain or heavy chain to form an intact antibody molecule. The invention further includes targeting methods and screening methods.
Owner:RGT UNIV OF CALIFORNIA
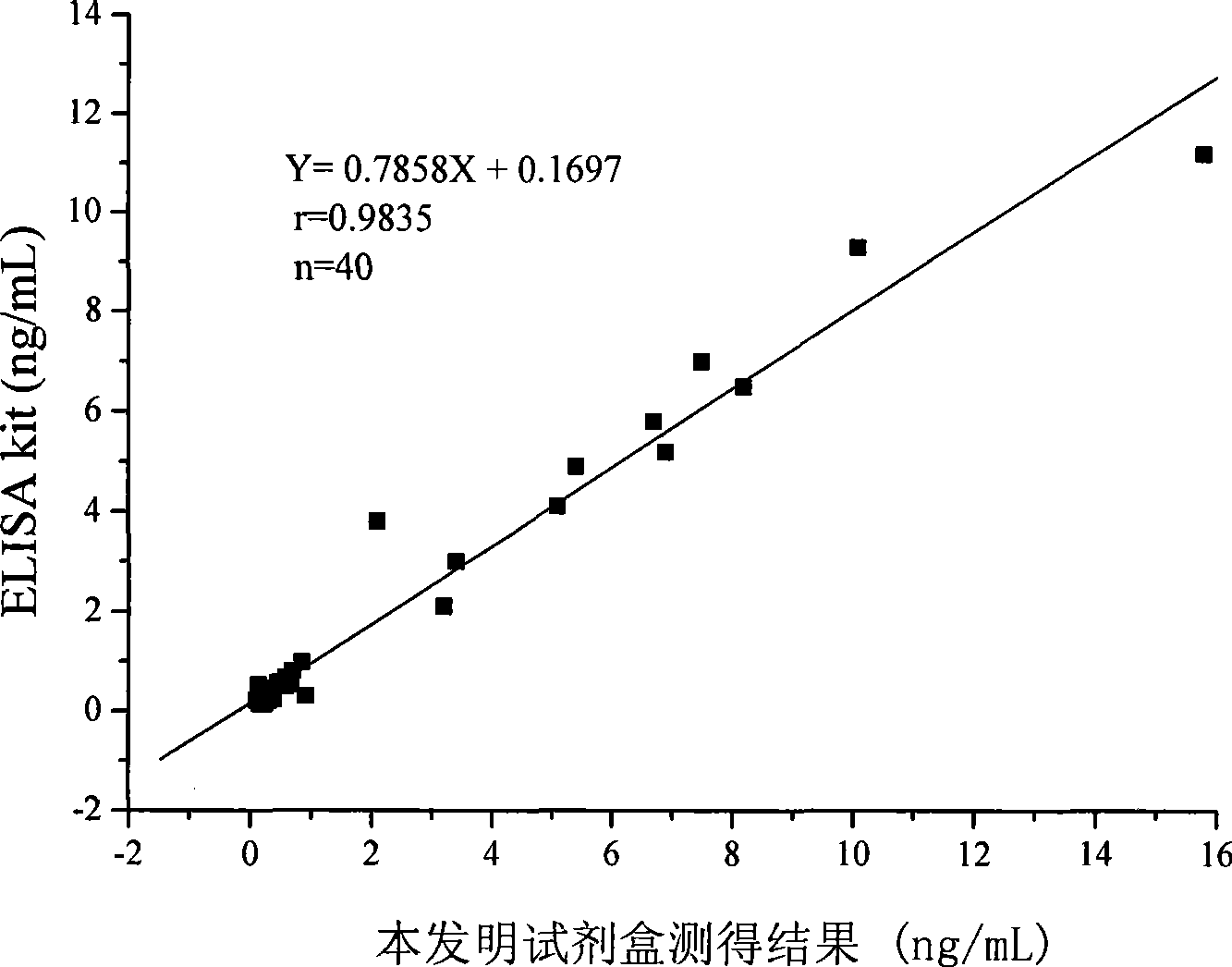
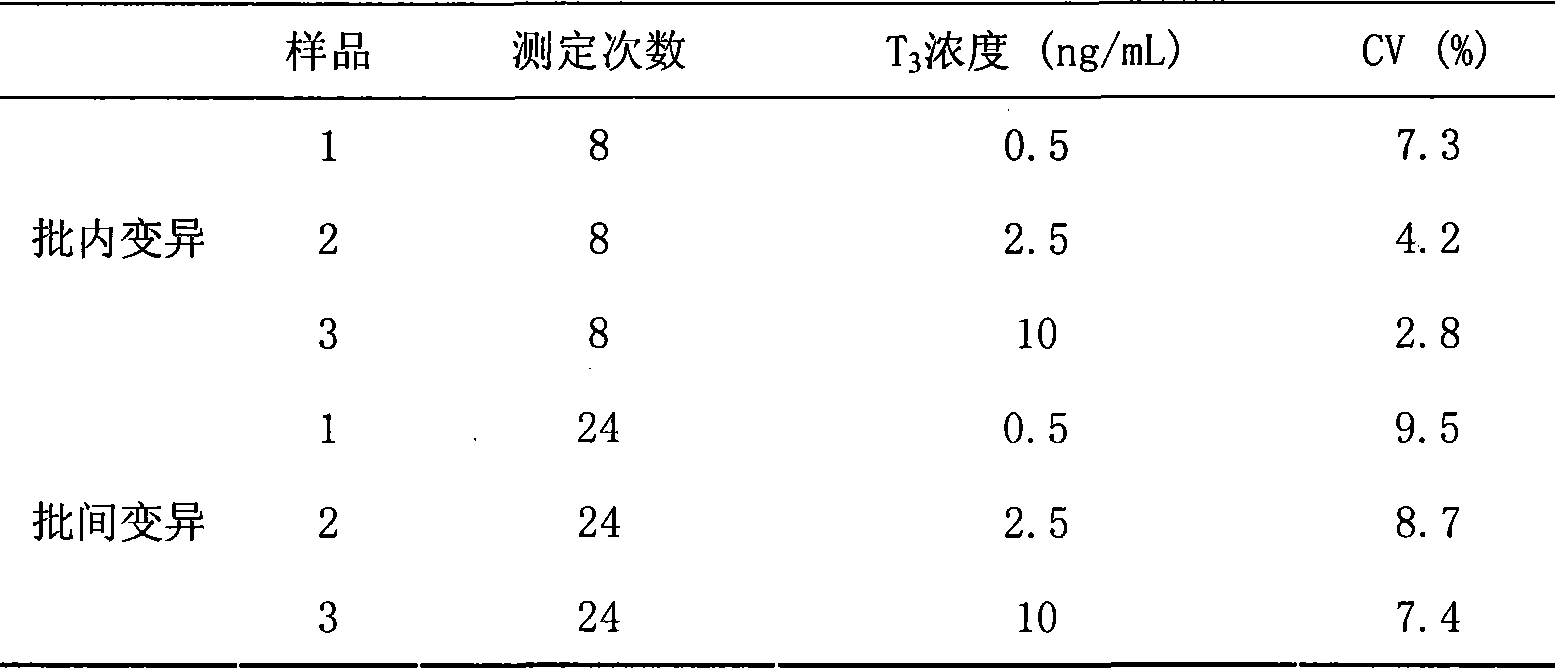
Chemoluminescence immunoassay measuring kit and preparation method thereof for triiodothyronine magnetic particles
InactiveCN101545913AEasy to measureStability determinationChemiluminescene/bioluminescenceBiological testingBiotin-streptavidin complexAntigen
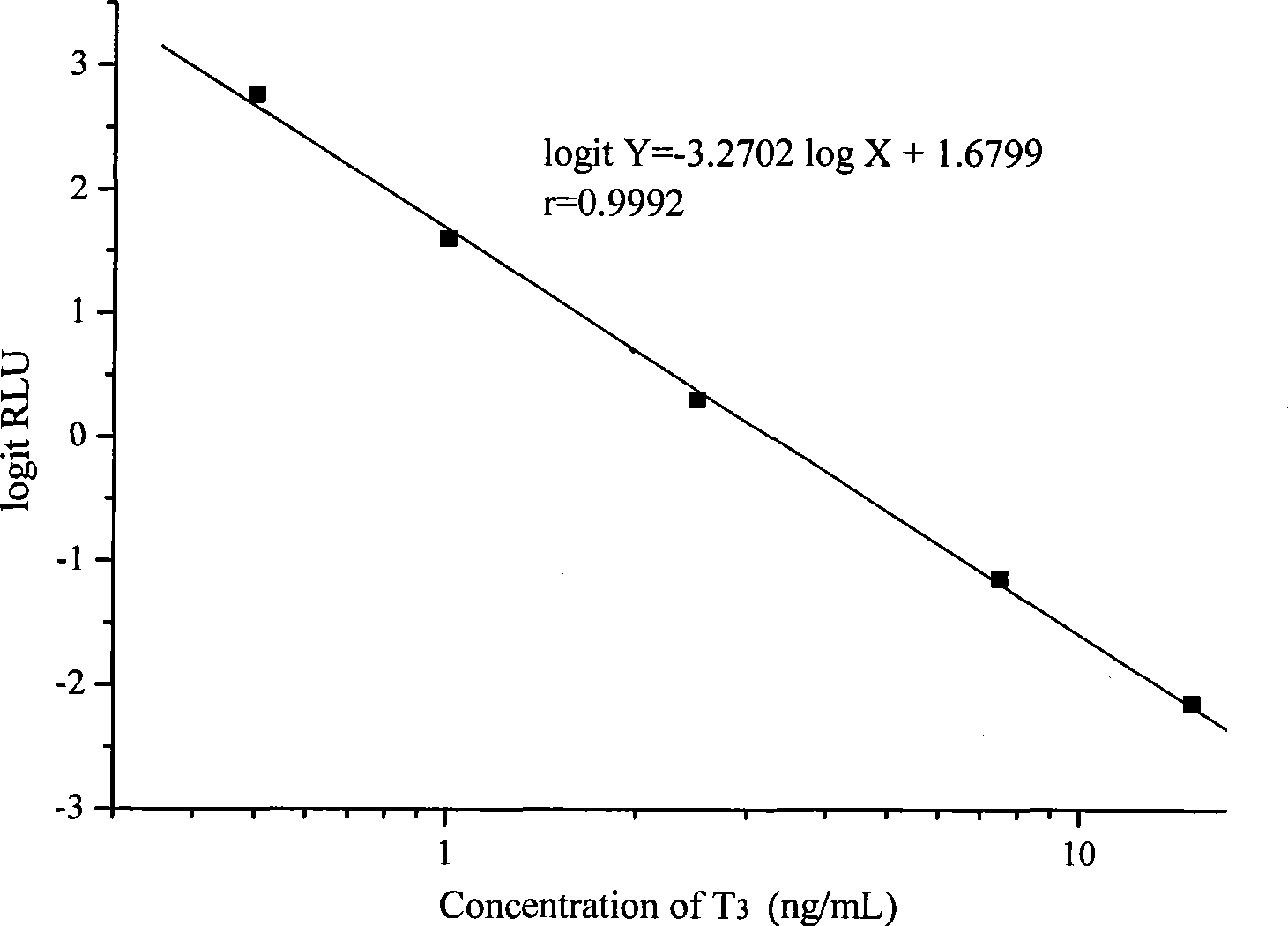
The invention provides a chemoluminescence immunoassay measuring kit and a preparation method thereof for quantificationally detect triiodothyronine (T3) magnetic particles. The kit mainly comprises a triiodothyronine serial calibration sample, magnetic particle solution coated by an anti-fluorescein isothiocyanate (FITC) monoclonal antibody, a T3 antigen marked by biotin, T3 monoclonal antibody marked by FITC, streptavidin marked by alkaline phosphatase, chemoluminescence substrate solution and 20-time concentrated washing solution. The invention adopts a competitive-method reaction mode, effectively utilizes the chemoluminescence technology combined with magnetic particles and biotin-avidin immunity magnifying technology principle to quantificationally detect the content of T3 in blood serum and blood plasma samples of human bodies and ensure the sensitivity of the detection. The kit is simple, convenient, fast, sensitive and stable to use, and provides a very valuable detection method for clinic diagnosis and scientific research works.
Owner:北京科美东雅生物技术有限公司
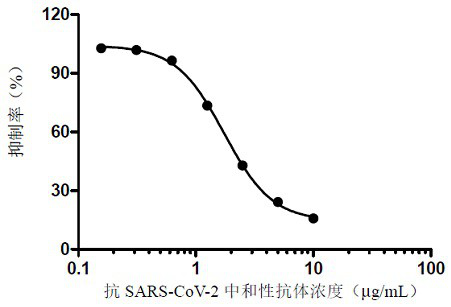
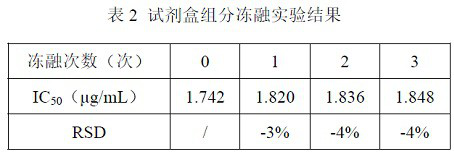
ELISA kit for detecting titer of novel coronavirus neutralizing antibody
ActiveCN111781354AThe pre-processing process is simpleShort timeImmunoassaysElisa kitHorseradish peroxidase
Owner:ACROBIOSYSTEMS INC
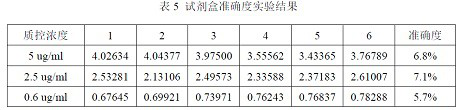
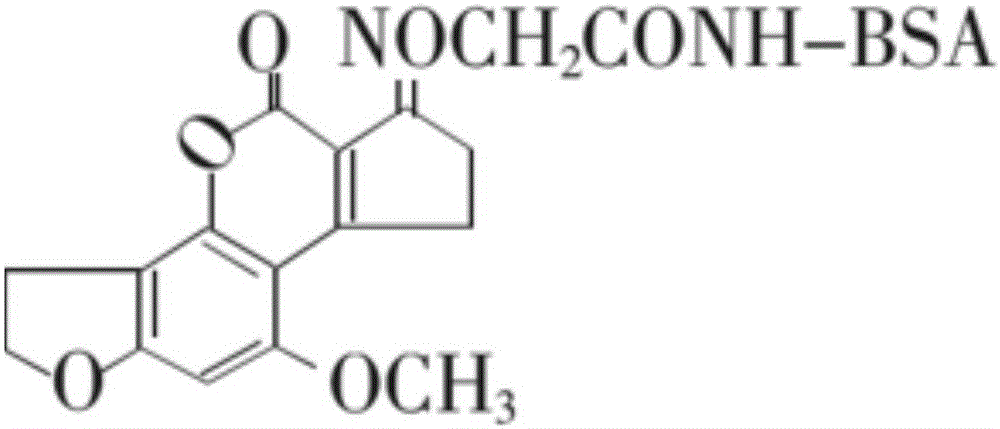
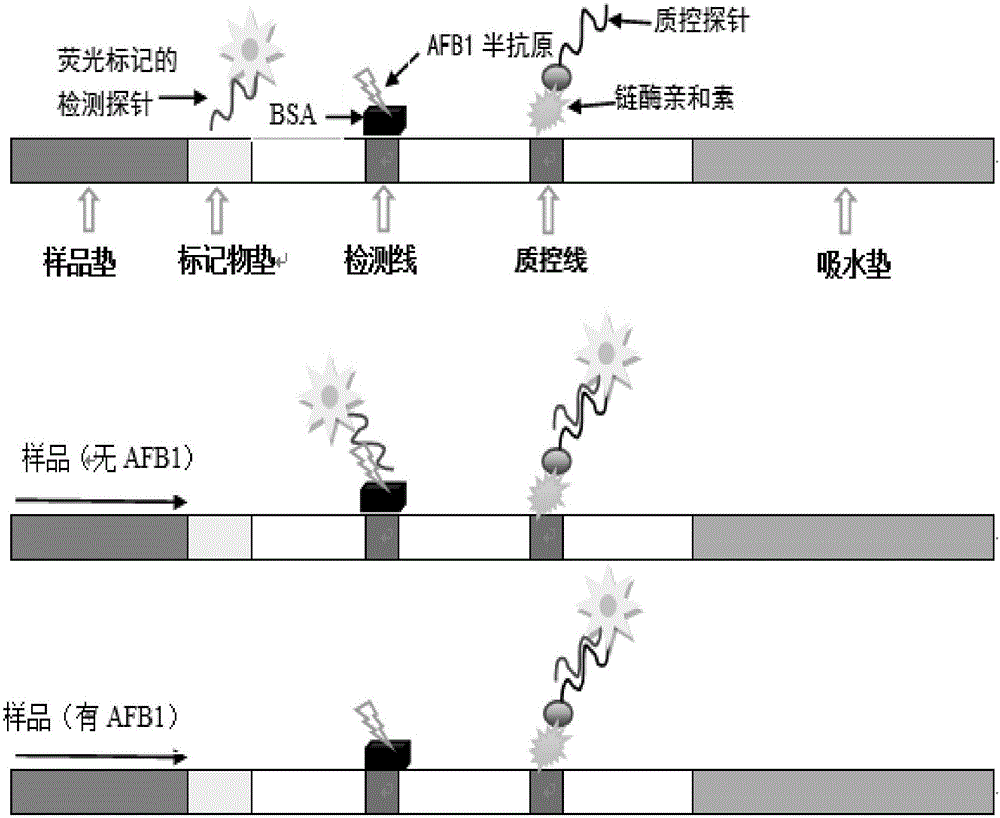
Test strip for detecting aflatoxin B1 or M1 by utilizing aptamer
The invention discloses a test strip for detecting aflatoxin B1 or M1 by utilizing an aptamer. The test strip for detecting the aflatoxin B1 or M1 by utilizing the aptamer, provided by the invention, comprises a sample absorption pad, a marker pad, a reaction film and a water absorption pad, wherein the marker pad is coated with a detection probe; the detection probe is an aflatoxin B1 aptamer marked by fluorescein; the nucleotide sequence of the aflatoxin B1 aptamer is sequence 1; the reaction film comprises a detection region and a quality control region; the detection region is coated with a conjugate formed by an aflatoxin B1 hapten and a carrier protein; and the quality control region is coated with a quality control probe, and the quality control probe is a conjugate formed by avidin conjugation probe marked aflatoxin B1 complementary single strand DNA (Deoxyribose Nucleic Acid)) molecules. The test strip for detecting the aflatoxin B1 or M1, provided by the invention, has the advantages of high sensitivity, accuracy in quantifying, strong specificity, simplicity and convenience, and short detection time.
Owner:INST OF QUALITY STANDARD & TESTING TECH FOR AGRO PROD OF CAAS
Preparation method of electrochemical sensor for detection of heavy metal lead contaminants
ActiveCN107621493AImprove catalytic performanceHigh sensitivityMaterial analysis by electric/magnetic meansElectrochemical gas sensorBimetallic nanoparticle
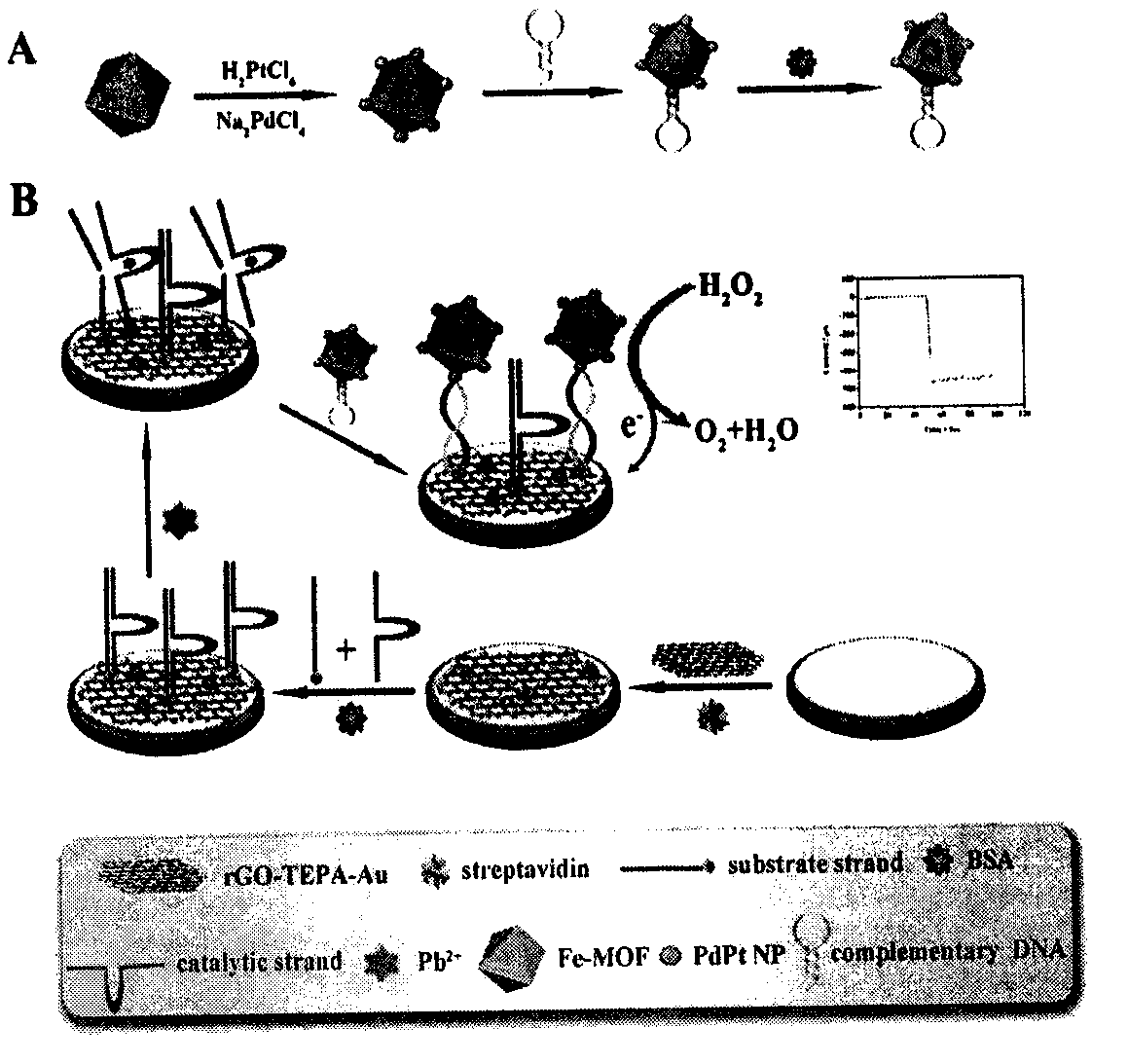
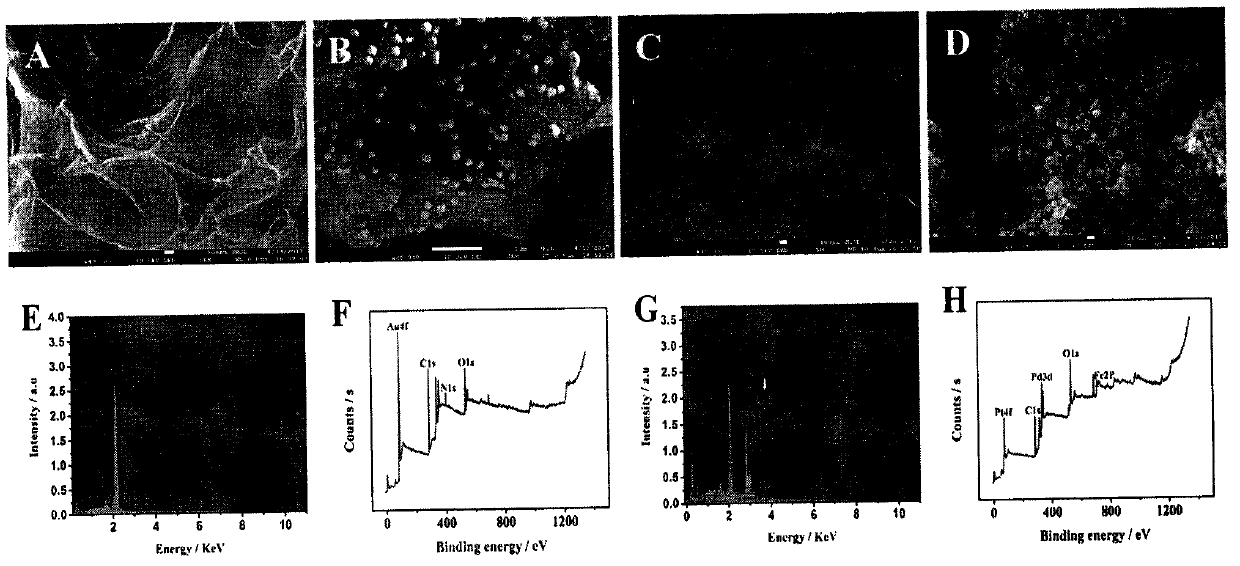
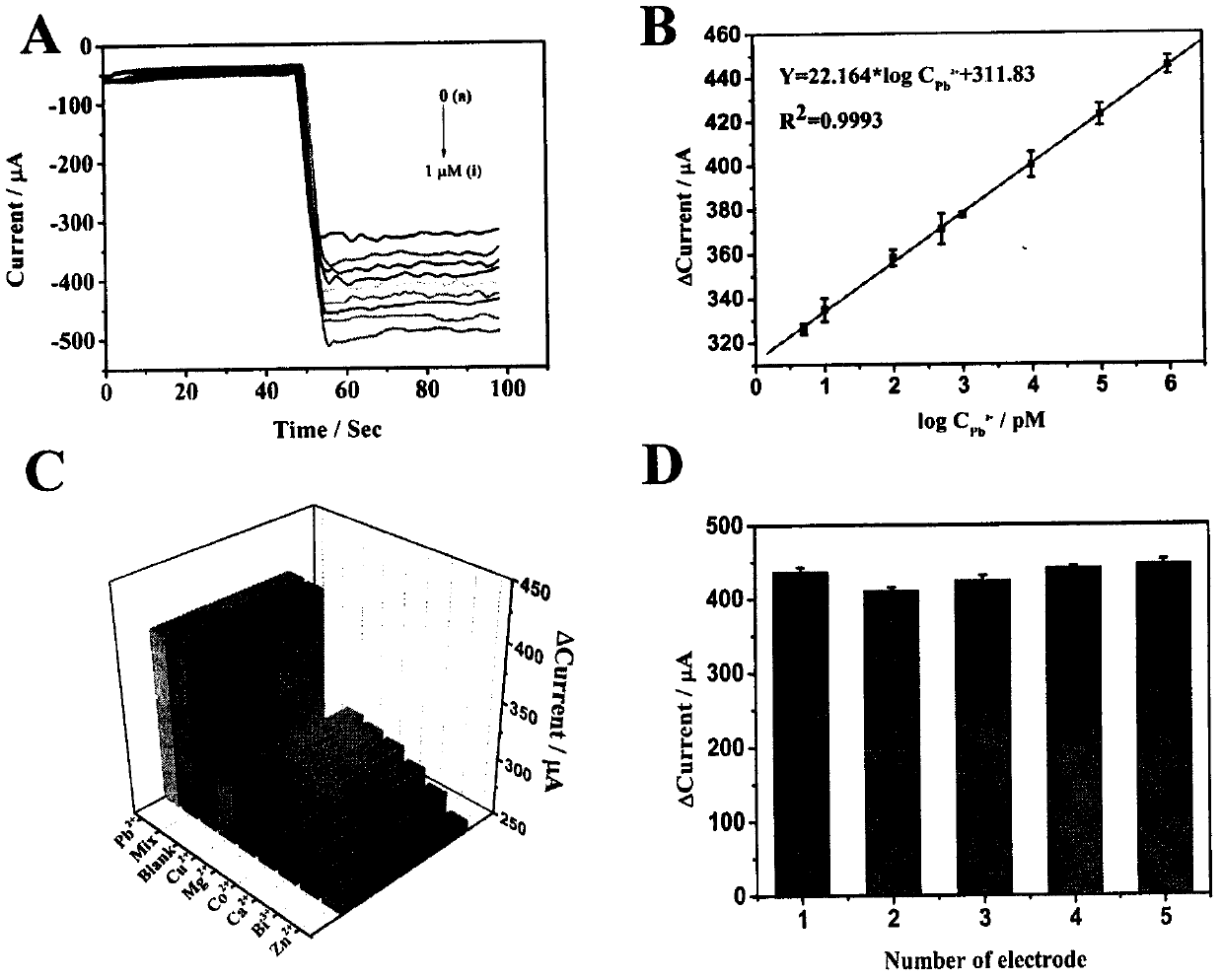
The invention relates to a preparation method and application of an electrochemical sensor for detection of heavy metal lead contaminants and belongs to the technical field of electrochemical detection. The preparation method is characterized by including; performing synthesis to obtain a Fe-MOFs nano material, reducing palladium-platinum bimetallic nanoparticles onto the Fe-MOFs nano material, and mixing a hairpin type DNA signal probe with the composite material to obtain a biological signal probe; performing layer-by-layer self-assembly through rGO-TEPA and avidin for fixing of '8-17'DNAzyme to obtain the electrochemical sensor for detection of the heavy metal lead contaminants. The sensor is successfully applied to detection of lead contaminants in the environment. The preparation method and application has the advantages of high flexibility, high specificity and rapid and convenient detection; experimental evidence is provided for research of lead contamination detection technology, and new concepts and new technology platforms are provided for monitoring the lead contaminants in the environment are provided.
Owner:CHONGQING MEDICAL UNIVERSITY
Method for qualitatively and quantitatively detecting target substance to be detected in blood serum by utilizing light initiated chemiluminescence immune assay
InactiveCN102944672AEliminate the influence of Hooks effectIncreased Quantitative Detection RangeChemiluminescene/bioluminescenceBiological testingBiotin-streptavidin complexMicrosphere
The invention discloses a method for qualitatively and quantitatively detecting a target substance to be detected in blood serum by utilizing light initiated chemiluminescence immune assay, and the method comprises the following steps of placing luminous microspheres coating a substance antibody, biotin-coated target substance antibody and optical sensitization microspheres wrapped by streptavidin (SA) into a specimen to be detected to perform a primary immunity reaction and detection, and the method also comprises the step of placing anti-He agent into the reaction system to facilitate the target substance immune superimposition reaction, and carrying out the secondary optical excitation chemicalluminescence immune detection. A purpose for comprehensively correcting a hooks effect can be realized by comprehensively analyzing detection results of twice light initiated chemiluminescence assay (LiCA), and the analysis process comprises the following steps of classifying the specimen to be detected into five concentration intervals such as cathode, low anode, middle anode, high anode and ultrahigh anode according to the signal characteristics of the primary LiCA detection and the secondary LiCA detection; and quantitatively analyzing the specimen in the low anode concentration interval and the ultrahigh anode concentration interval according to the primary LiCA detection. The method has the characteristics of accuracy in result, simplicity and convenience in operation, wide application range and the like.
Owner:李方和
Liquid phase chip reagent kit detecting various anti EB viral antigen antibody
ActiveCN101063683AHigh throughputSmall amount of sampleMaterial analysisViral antigensNasopharyngeal carcinoma
This invention discloses one liquid phase chip test agent case and its process method for multiple kinds of anti-EB virus antibody, which comprises the following parts: coupling affinity element multi-color microball covering on polypeptide; at least one kind of coupling hydroxyl group multi-color microball covering on natural or gene antigen, wherein the antigen is EB virus crack object or shell antigen VCA; gene antigen is of one amino acid in list of No. 33-No. 37; the said integration polypeptide is one from No. 1-No. 32.
Owner:SUN YAT SEN UNIV CANCER CENT
Immunosensor based on hybrid chain reaction and single molecule counting and application of immunosensor
InactiveCN103940989AHigh detection sensitivityReduce usageBiological material analysisFluorescence/phosphorescenceSmall sampleAnti tnf alpha
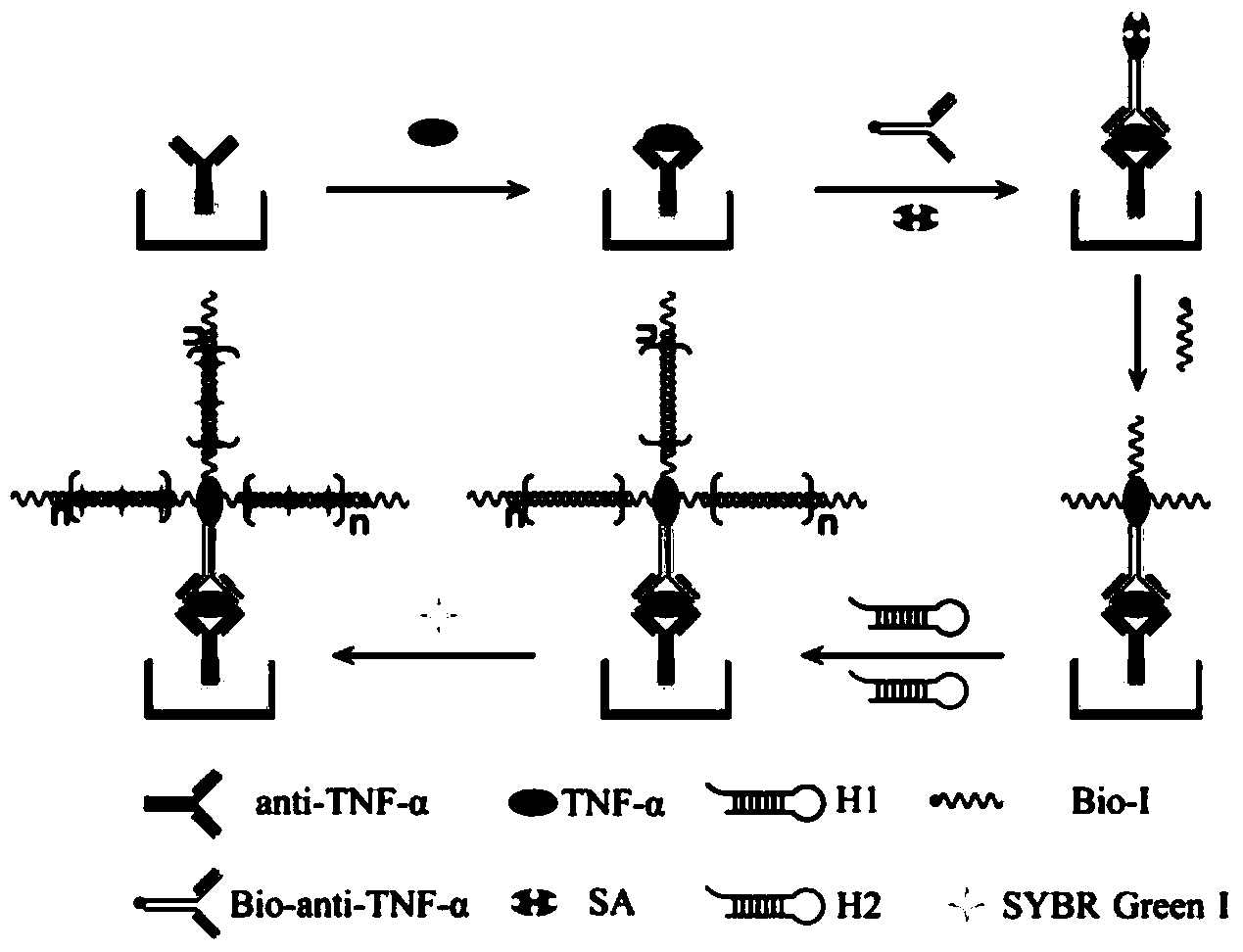
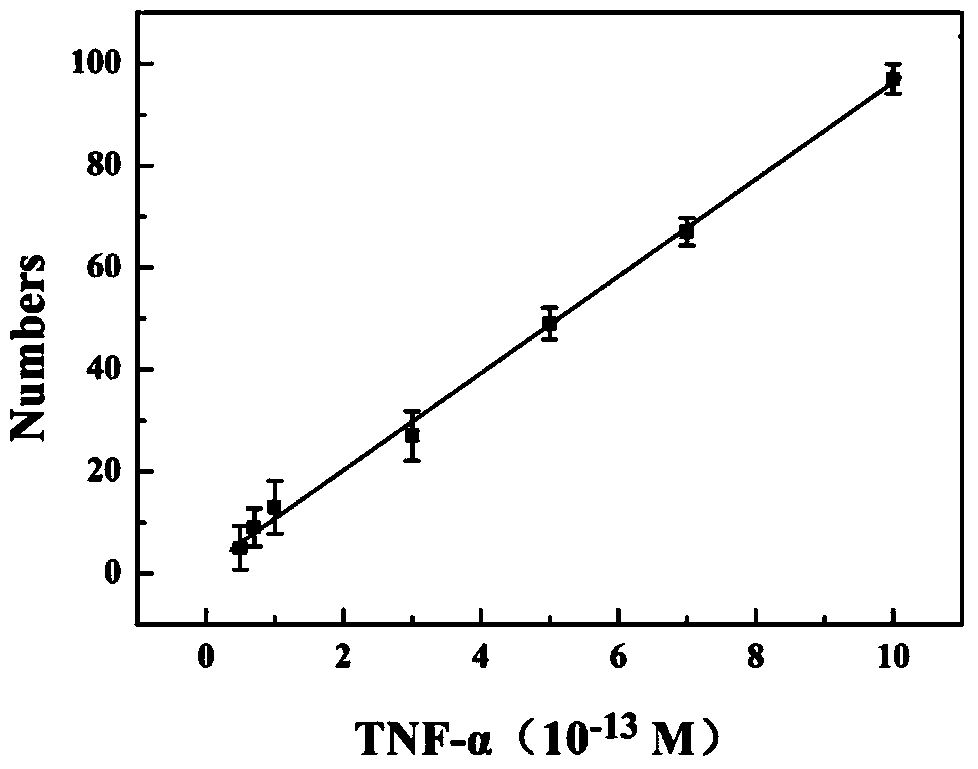
The invention discloses an immunosensor based on hybrid chain reaction and single molecule counting. The immunosensor consists of a capturing antibody, a detection antibody, streptavidin, a biotinylated primer and a hairpin probe, wherein the capturing antibody is anti-TNF-alpha; the detection antibody is Bio-anti-TNF-alpha; a base sequence of the biotinylated primer (Bio-I) is 5'-AGT CTA GGA TTC GGC GTG GGT TAA TTT TTT TTT-biotin-3'; the hairpin probe consists of a hairpin probe H1 and a hairpin probe H2; a base sequence of the hairpin probe H1 is 5'-TTA ACC CAC GCC GAA TCC TAG ACT CAA AGT AGT CTA GGA TTC GGC GTG-3'; a base sequence of the hairpin probe H2 is 5'-AGT CTA GGA TTC GGC GTG GGT TAA CAC GCC GAA TCC TAG ACT ACT TTG-3'. The invention also provides a preparation method of the immunosensor and application of the immunosensor in detection of the TNF-alpha. The immunosensor disclosed by the invention has the advantages of high detection sensitivity, small sample use amount, no need of enzyme amplification and the like, and quantitative detection on low-concentration TNF-alpha can be realized.
Owner:SHANDONG UNIV
Monophosphoinositide proteoglycans-3 chemiluminescence immune analysis determination reagent kit and preparing method thereof
InactiveCN101377506AIncrease the effective amountReliable clinical reference valueChemiluminescene/bioluminescenceTreatment effectChemiluminescent immunoassay
The invention relates to the medical field of immunoassay, more specially, the invention provides a chemiluminescent immunoassay detection kit for phosphatidylinositol proteoglycan-3(GPC-3) and a preparation method thereof, and realizes the simultaneous serological detection of GPC-3 N terminal and C-terminal protein with the chemiluminescent immunoassay method. The kit has the advantages of simple sampling, convenient detection and accurate and specific technical method. The invention adopts a biotin-strapavidin system to coat antibodies and improve the efficiency of antibody coating and the linear range of detection as well as sensitivity, and can be conveniently used for the tracing observation of early diagnosis or treatment effect for primary carcinoma of liver.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Kit and method for detecting dermatophagoides pteronyssinus allergen specificity IgE antibody
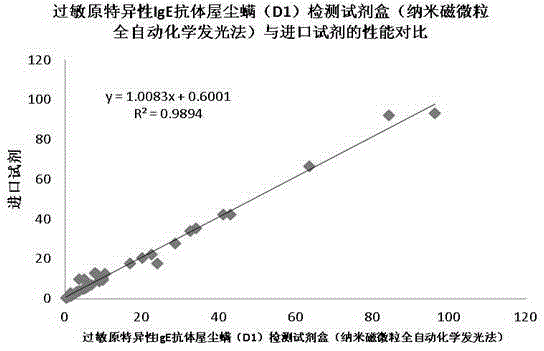
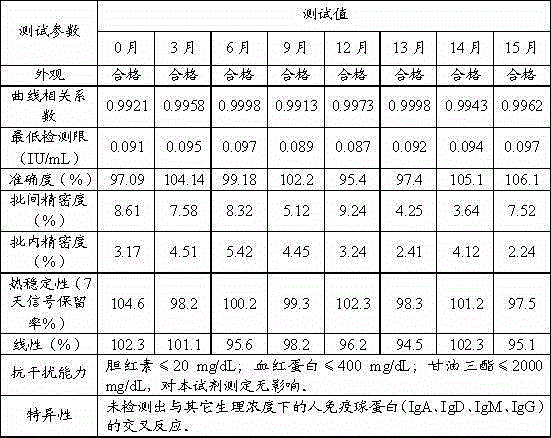
The invention discloses a kit and a method for detecting a dermatophagoides pteronyssinus allergen specificity IgE antibody. The kit comprises a calibration material, a quality control material, a biotin-marked dermatophagoides pteronyssinus allergen solution, an alkaline phosphatase-marked mouse-antihuman IgE second antibody solution and a streptavidin-marked nanometer magnetic particle suspension liquid. The kit is utilized to detect the dermatophagoides pteronyssinus allergen specificity IgE antibody. In such a mode, all reagent components in the kit are excellent in stability; the period of validity can reach over one year; the detecting sensitivity is high, the peculiar performance is excellent and the variation is small; a perfect uniform technique is acquired through technical optimization in a large number of experiments; the production is performed in strict accordance with standard production operation specification and quality control regulation; a user can acquire a reliable result by only performing standard operation according to the operation specification; in the clinical research, the conforming relevance with a foreign imported reagent reaches up to over 90% and the cost of the kit is only 1 / 2 of the cost of the foreign imported reagent.
Owner:SUZHOU HAOOUBO BIOPHARML
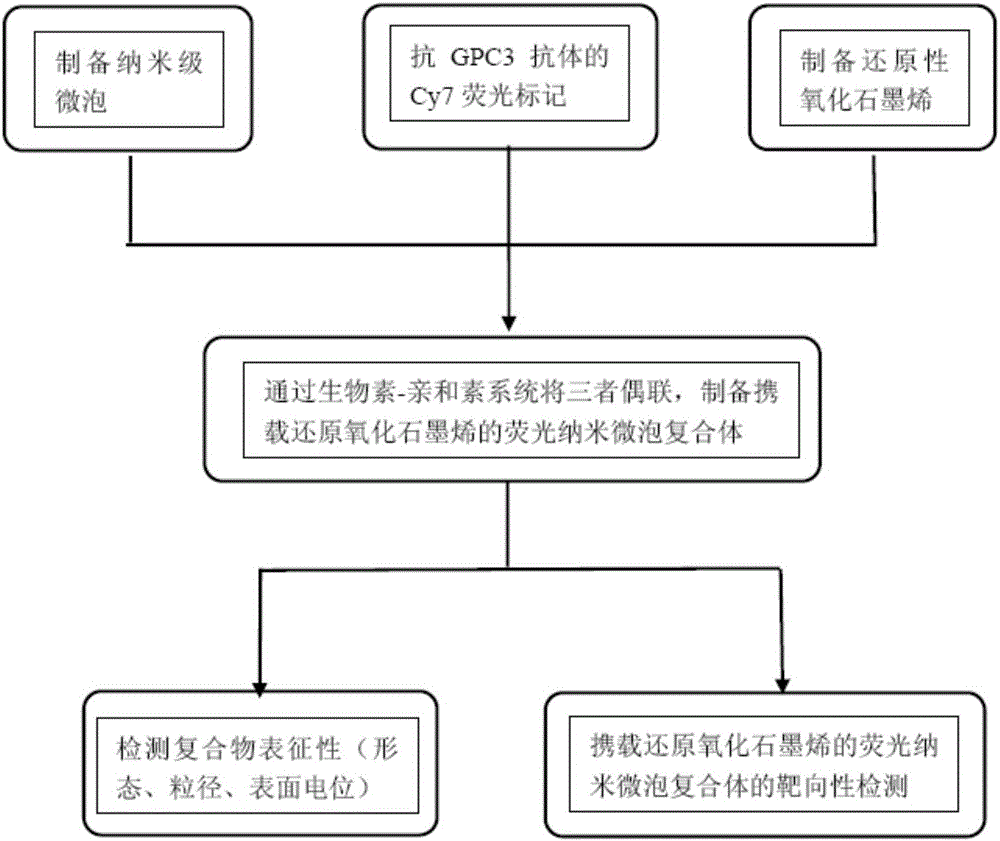
Hepatic carcinoma targeted photo-thermal therapeutic agent as well as preparation method and application thereof
ActiveCN106075440AGood separation and purification effectWith ultrasoundEnergy modified materialsDigestive systemFluorescenceHepatic carcinoma
The invention provides a hepatic carcinoma targeted photo-thermal therapeutic agent as well as a preparation method and an application thereof and belongs to the field of biomedical engineering. The hepatic carcinoma targeted photo-thermal therapeutic agent is prepared with the method comprising the following steps: (1) preparing biotinylation nanometer microbubbles; (2) preparing a Cy7 fluorescently-labeled biotinylation anti-GPC3 (glypican3) antibody; (3) preparing biotinylation RGO (reduced graphene oxide); (4) coupling the products prepared in the step (1), step (2) and step (3) through a biotin-avidin system to obtain the hepatic carcinoma targeted photo-thermal therapeutic agent. The hepatic carcinoma targeted photo-thermal therapeutic agent has ultrasonic and fluorescence imaging functions, mediates RGO targeted delivery with an ultrasound-targeted microbubble destruction technology, monitors a photo-thermal therapeutic process in real time, can be used for preparing drugs for hepatic carcinoma therapy and has excellent clinical application value.
Owner:HARBIN MEDICAL UNIVERSITY
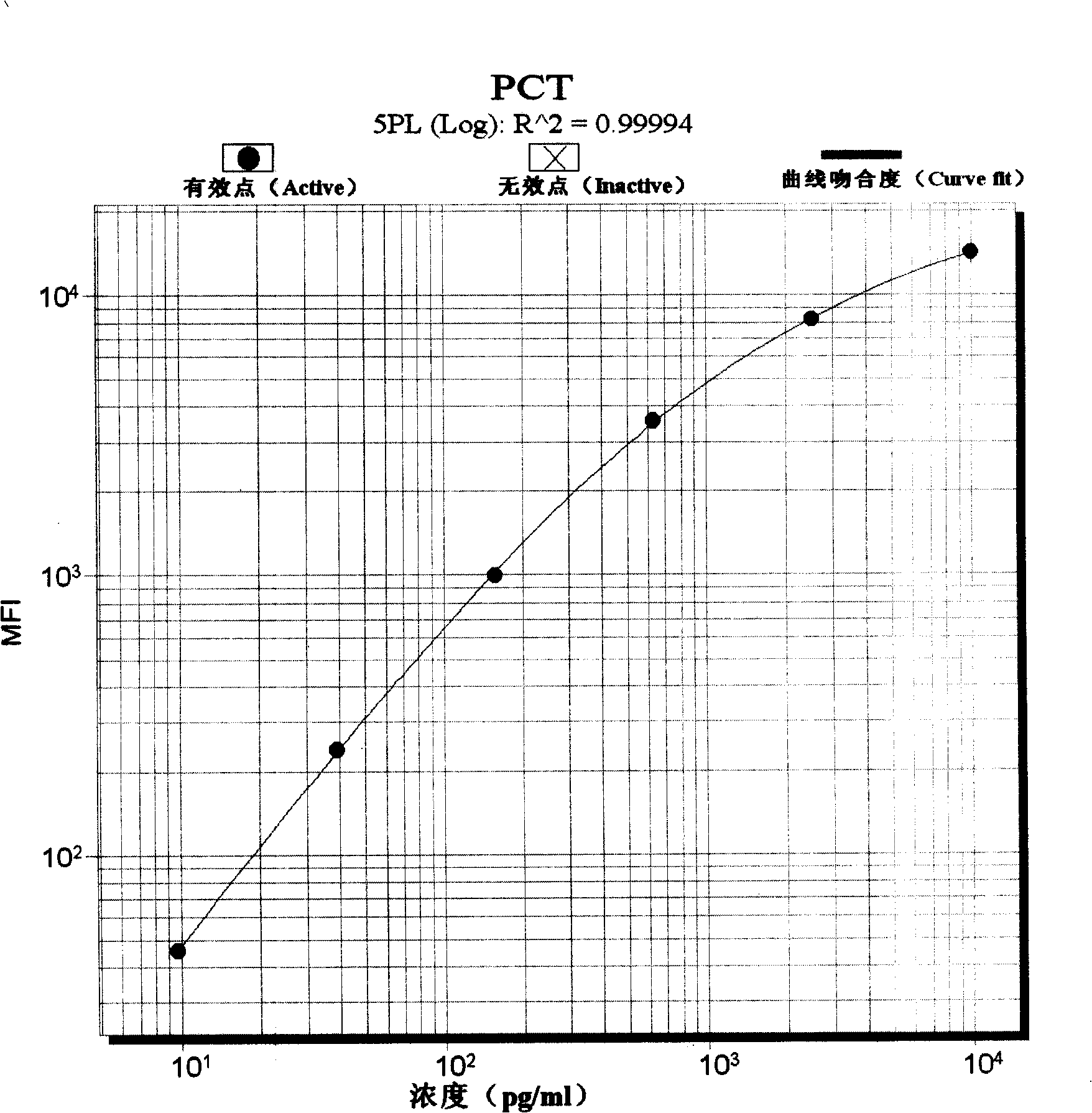
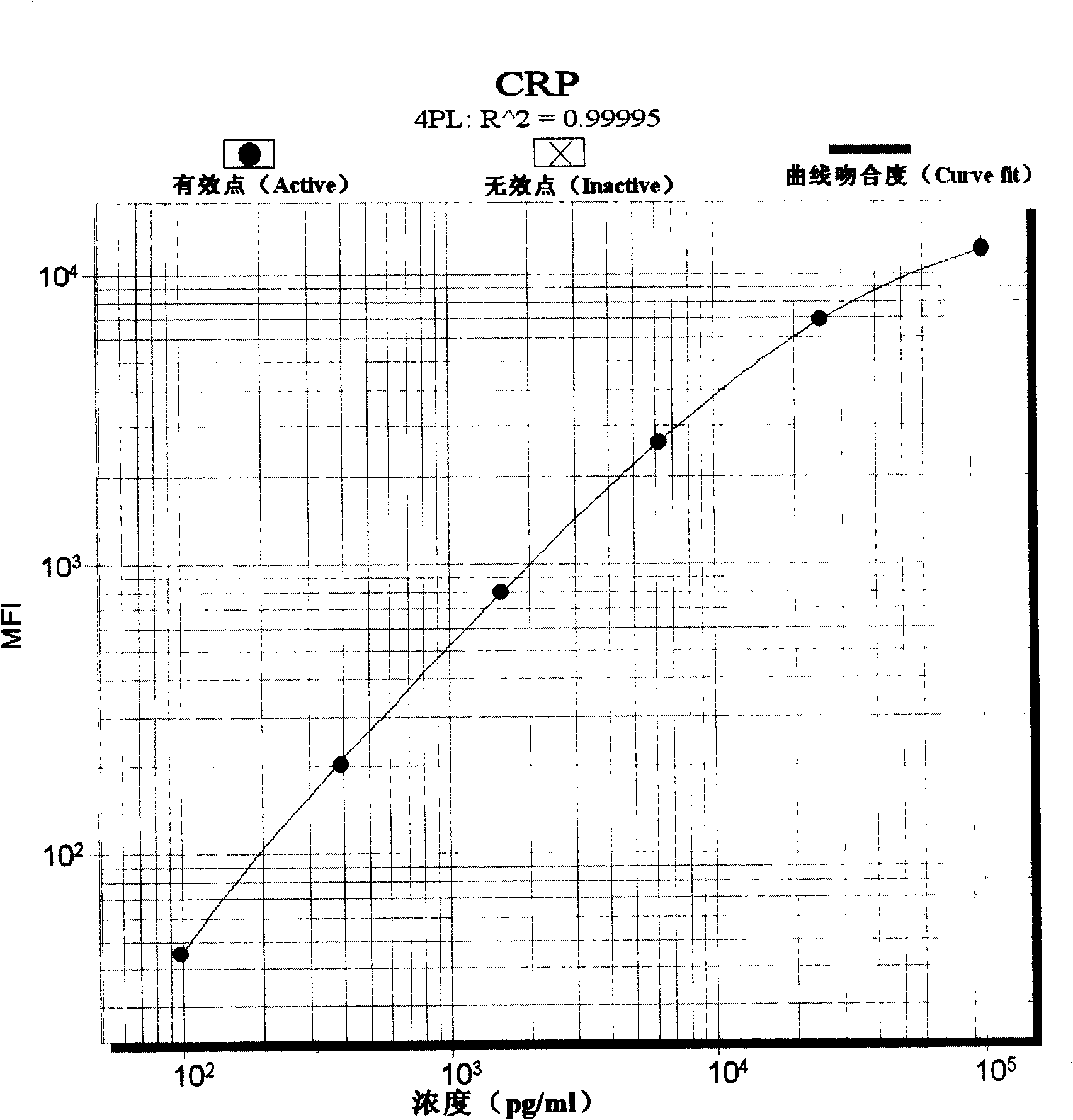
Pyemia early diagnosis liquid phase chip and method for producing the same
InactiveCN101246163AImprove detection efficiencySmall sample sizeMaterial analysisMicrospherePhycoerythrin
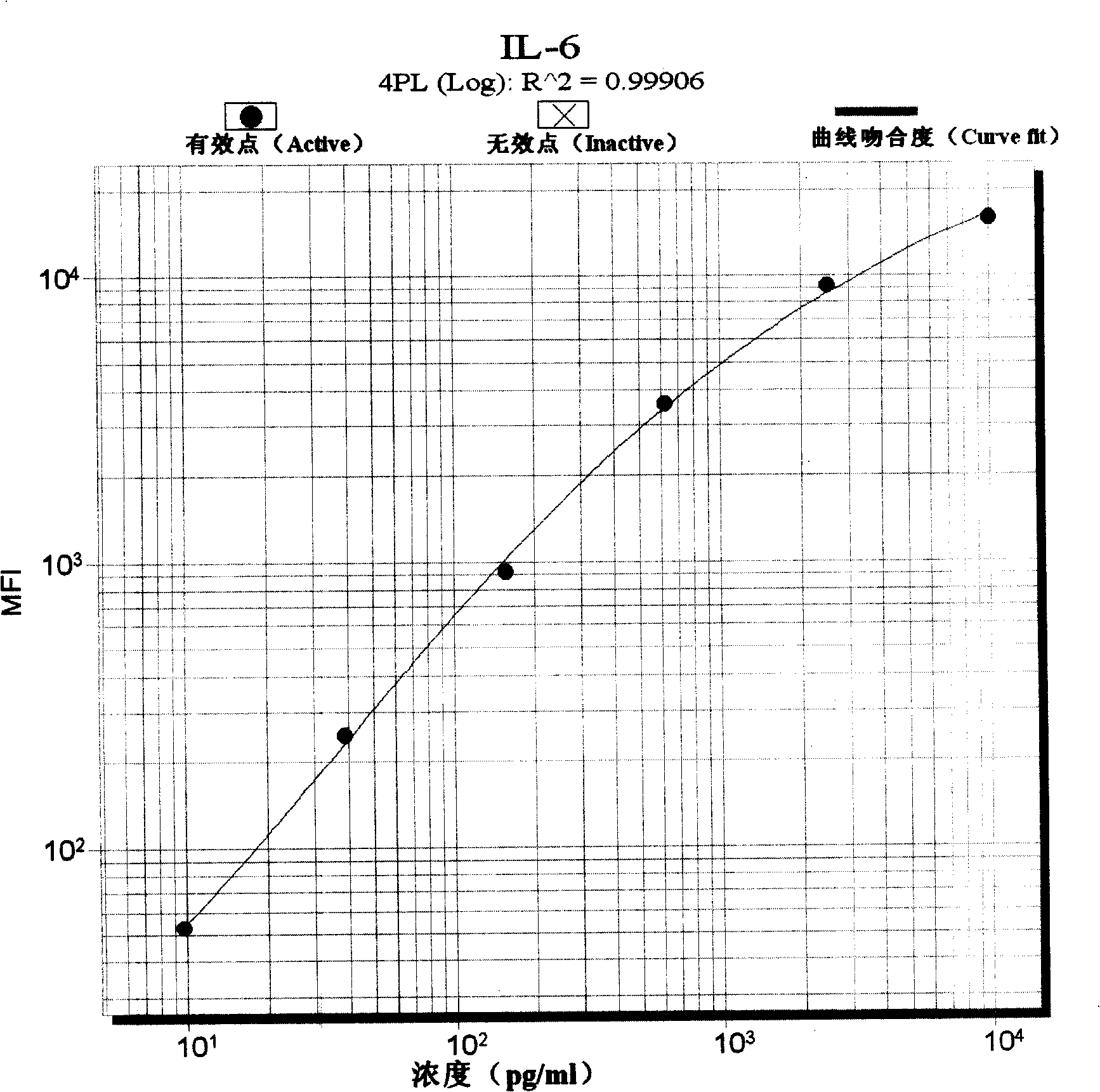
The invention discloses a sepsis early diagnosis liquid chip which mainly includes the following components: microsphere coated with PCT capturing antibody, microsphere coated with CRP capturing antibody, microsphere coated with IL-6 capturing antibody, microsphere coated with Neopterin capturing antibody, these microspheres have codes with different colors; biotin-labeled test antibody; avidin-linked phycoerythrin. The invention sepsis early diagnosis liquid chip has advantages of high detection efficiency, a small amount of sample, high specificity and high sensitivity. And at the same time, the invention can test four kinds of specific markers of sepsis simultaneously, improve sensitivity and specificity of sepsis early diagnosis, distinguish sepsis caused by bacterial and viral, judge severity and poor prognosis of sepsis and monitor continuously for judging reflection of patient to certain kind of treatment.
Owner:SUREXAM BIO TECH
Double-antibody biotin-Avidin ELISA (enzyme-linked immuno sorbent assay) detection kit for cattle viral diarrhea virus and application method thereof
InactiveCN102023217AAvoid missing detectionStrong specificityMaterial analysisBovine virus diarrhea virus AntigenAssay
The invention relates to a double-antibody biotin-Avidin ELISA (enzyme-linked immuno sorbent assay) detection kit for cattle viral diarrhea virus antigen and an application method thereof. The kit comprises washing liquid for ELISA detection, developing liquid, stopping liquid, Streptavidin-HRP, positive control and negative control and is characterized by further comprising an ELISA plate coating cattle viral diarrhea virus single antibody 3D8 and a cattle viral diarrhea virus single antibody 3F9 marked by biotin. The detection without cross reaction is performed by using the kit, thereby being capable of preventing leak detection caused by low titer in the antibody and having high specificity; a biotin-affine sensitizer is used for amplification, thereby increasing the flexibility, improving the detection rate, and simultaneously reducing the amount of second antibody; and the kit is suitable for being used widely.
Owner:YANGZHOU UNIV
Fluorescent latex granular immune chromatography by time resolution
InactiveCN1818653AFast flowResolving directly coated antibodiesFluorescence/phosphorescenceAntigenFluorescence
A microparticle immune chromatography of time-resolution fluorescence emulsion includes preparing biotinylation antibody and preparing immune time-resolution fluorescence microparticles, using Fusion 5 film to prepare avidin solid phase film detection line and quality control line by utilizing avidin to envelop said film, finally using double-antibody sandwich method to detect antigen quickly.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Tumor-associated antigen 125 magnetic microparticle chemiluminescence immune assay determination kit and method for making same
InactiveCN101398431ASimple structureLow priceChemiluminescene/bioluminescenceBiotin-streptavidin complexMonoclonal antibody
The invention relates to the medical field of immunoassay, particularly provides a measuring kit of the chemiluminescence immunoassay of a CA125 magnetic particle. The kit of the invention comprises: 1) a CA125 working calibrator; 2) the mixed solution of the magnetic particle coated with a CA125 monoclonal antibody and the CA125 monoclonal antibody marked by biotin; 3) streptavidin marked by horse radish peroxidase; 4) a chemiluminescence substrate; and 5) a reaction tube. Further, the method for preparing the kit according to the invention comprises the following steps: 1) the working calibrator is prepared by CA125 pure products; 2) the mixed solution of the magnetic particle coated with the monoclonal antibody and the monoclonal antibody marked by the biotin is prepared; 3) the horse radish peroxidase is used for marking the streptavidin; 4) the chemiluminescence substrate is prepared; 5) the working calibrator, the mixed solution, the enzyme markers, the chemiluminescence substrate and the reaction tube are sub-assembled; and 6) assembly is carried out to obtain a finished product. The kit of the invention has the advantages of simpleness and convenience, fastness, sensitivity and stability and the like.
Owner:北京科美东雅生物技术有限公司
Novel coronavirus rapid detection test paper and detection method
PendingCN111337669ASuitable for on-site screeningReduce the risk of infectionImmunoassaysReceptorHorse radish peroxidase
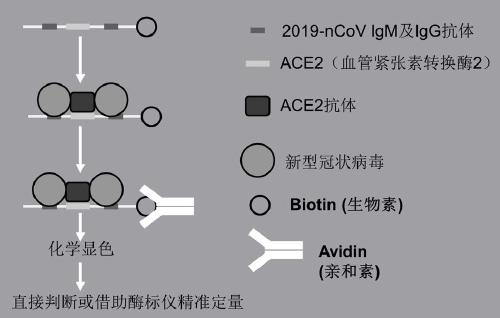
The invention discloses novel coronavirus rapid detection test paper and a detection method. The novel coronavirus rapid detection test paper is composed of a sample pad, a colloidal gold pad, a reaction color development area, a water absorption area and a supporting plate; the sample pad and the water absorption pad are arranged on the supporting plate and located on the two opposite sides of the supporting plate respectively, and the sample pad is suction filter paper or a glass fiber membrane and used for bearing an object to be detected; a nitrocellulose membrane is fixed on the supporting plate, and the absorbent pad is absorbent paper; through double binding of a novel coronavirus antibody and a receptor ACE2 protein of a virus, a coronavirus protein antigen is specifically capturedand bound by adopting double enzyme-linked immunosorbent assay; the ACE2 antibody in the test paper reaction color development area is combined with biotin, avidin is connected with horse radish peroxidase, and through a biotin-avidin system, rapid judgment can be achieved through chemical color development, and the virus content can also be accurately detected through a microplate reader.
Owner:NANTONG UNIVERSITY +1
Magnetic molecular targeted ultrasound contrast agent microsphere and preparation method thereof
InactiveCN101780284AReduce investmentEasy to operateEchographic/ultrasound-imaging preparationsLipid formationUltrasound contrast media
The invention relates to a novel magnetic molecular targeted ultrasound contrast agent, in particular to a gas-wrapped magnetic liposome microsphere suspension with the mean diameter of 1-4 micrometers and a preparation method thereof. The preparation method of the magnetic molecular targeted ultrasound contrast agent microsphere comprises a condition that a lipid layer of the microsphere and / or the surface of the lipid layer contains (or connects) magnetic response materials. A contrast agent microsphere wall material contains phospholipids, polyethylene glycol (PEG), PEG phospholipid polymers, biotinylated and / or polypeptide modificatory PEG phospholipid polymer, poloxamer, ligands (monoclonal antibodies or polypeptide, and the like) and the magnetic response materials and / or avidin bridging materials, wherein the ligand (monoclonal antibodies or polypeptide, and the like) has specific affinity to target molecules, the wrapped gas is a fluorine carbon gas, and a solvent is an aqueous medium (distilled water). The invention also provides the preparation method of the magnetic molecular targeted ultrasound contrast agent microsphere, which comprises a condition that the specific ligand is connected with the microsphere in a covalence and avidin bridging way. The novel magnetic molecular targeted ultrasound contrast agent has good targeted developing effect, can be used for evaluating the change of vessel endothelial molecules of an arterious and venous system of an organic tissue and has good application prospect in treatment.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
Kit and method for detection of thyroid peroxidase antibody
InactiveCN105467122AImprove accuracyHigh precisionChemiluminescene/bioluminescenceAntigenBiotin-streptavidin complex
The present invention discloses a kit and method for detection of thyroid peroxidase antibody. The kit includes thyroid peroxidase antibody series standards, a magnetic separation reagent of a magnetic particle suspension conjugated with streptavidin and pigment, a first reagent of an antigen solution containing biotin N-hydroxysuccinimide ester labeled thyroid peroxidase antigen, a second reagent of a solution containing alkaline phosphatase labeled mouse-anti-human IgG monoclonal antibody. The kit is used for detection of thyroid peroxidase antibody. By the above-described manner, the invention uses a biotin-streptavidin signal amplification system and alkaline phosphatase labeling to solve the traditional problem of low ELISA sensitivity; the nanometer magnetic particle separation system achieves high sensitivity for radioimmunoassay (RIA), but has no radioactive contamination, and has greatly improved period of validity, security and environmental performance.
Owner:SUZHOU HAOOUBO BIOPHARML
Magnetic particle and single base extension based SNP automatic detection method
InactiveCN101445834AImprove accuracyImprove throughputMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceEnzyme
The invention discloses a novel single nucleotide polymorphism detection method based on magnetic particles and single base extension technology. The detection method is characterized in that through extension primer of a detected locus marked with biotin, specific ddNTP marked with fluorescence is extended to the 3' end of the primer under the action of Taq enzyme, so as to amplify the genotype signal of the template through thermal cycle reaction including denaturation, annealing and extension. Then the extension primer is fixed to the surface of magnetic particles modified with avidin to detect the subtype signal of the specimen. The detection method has the advantages of simplicity, accuracy, low cost, high flux and high sensitivity, can realize automatic operation and has higher practicability than the traditional method.
Owner:SOUTHEAST UNIV
Preparation and application of chemiluminescent immunoprobe based on dendrimer double-amplification labeling
InactiveCN108344864AImprove performanceIncrease the amount of probe molecule bindingChemiluminescene/bioluminescenceDendrimerStreptavidin
The invention relates to a preparation and application of a chemiluminescent immunoprobe based on dendrimer double-amplification labeling, and belongs to the field of chemiluminescent labeling in chemiluminescence in-vitro diagnosis. The preparation method of the amplified chemiluminescence immunodetection probe comprises the following steps: 1, preparation of a dendrimer-streptavidin-chemiluminescent signal substance complex; 2, biotinylation labeling of an antibody; and 3, preparation of a dual-amplification chemiluminescent immunolabeling detection probe complex. According to the dual-amplifying chemiluminescence immunodetection probe, the mode of enhancing the fluorescent intensity and the mode of increasing the detecting molecule combining weight of fluorescent microspheres are effectively combined, one-time amplification is achieved through the combination effect between biotin and avidin, second-time amplification is achieved by use of a signal-substance-labeled dendrimer, and the signal intensity of chemiluminescence immunoassay is greatly improved through the dual amplifying mode.
Owner:NANJING TZONE BIOLOGICAL SCI & TECH
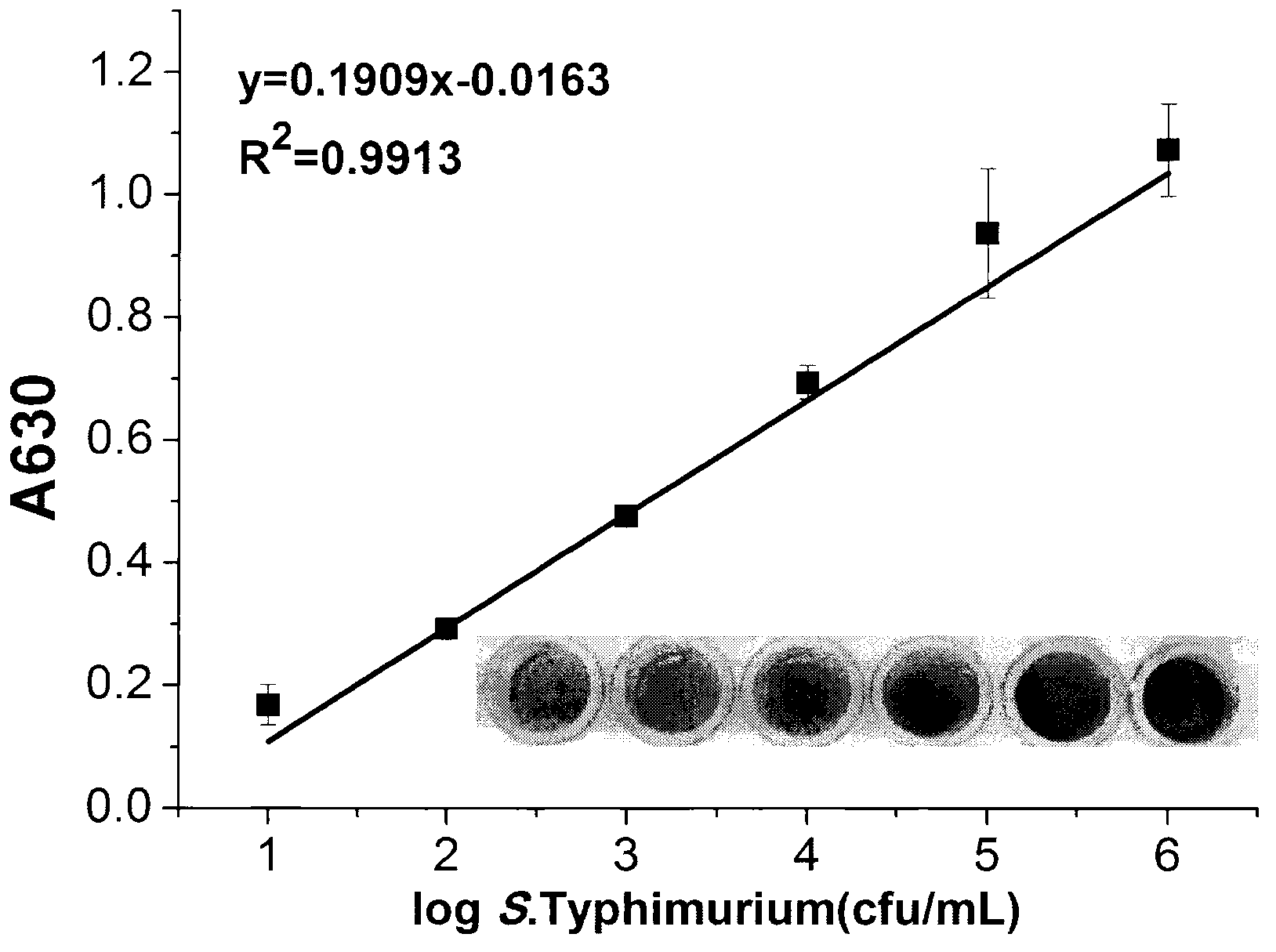
Visual detection method for mouse typhus salmonella based on aptamer recognition
InactiveCN103014163AStrong specificityEasy to operateMicrobiological testing/measurementAgainst vector-borne diseasesAptamerSalmonella frintrop
The invention provides a visual detection method for mouse typhus salmonella based on aptamer recognition. The basic principle of the method is that the surface of a microwell plate is coated with avidin, sulfhydrylation salmonella aptamer which is marked by biotinylation salmonella aptamer and nanogold is adopted to recognize targeted salmonella, and on the basis, a silver enhancement technique is utilized to further amplify a detection signal, so that the visual detection is achieved. The visual detection method for the mouse typhus salmonella based on the aptamer recognition has the advantages of high specificity, strong sensitivity, simplification and rapidness, thereby providing the possibility for rapidly detecting the mouse typhus salmonella in an environment.
Owner:JIANGNAN UNIV +1
Immunochromatographic test paper for detecting novel coronavirus
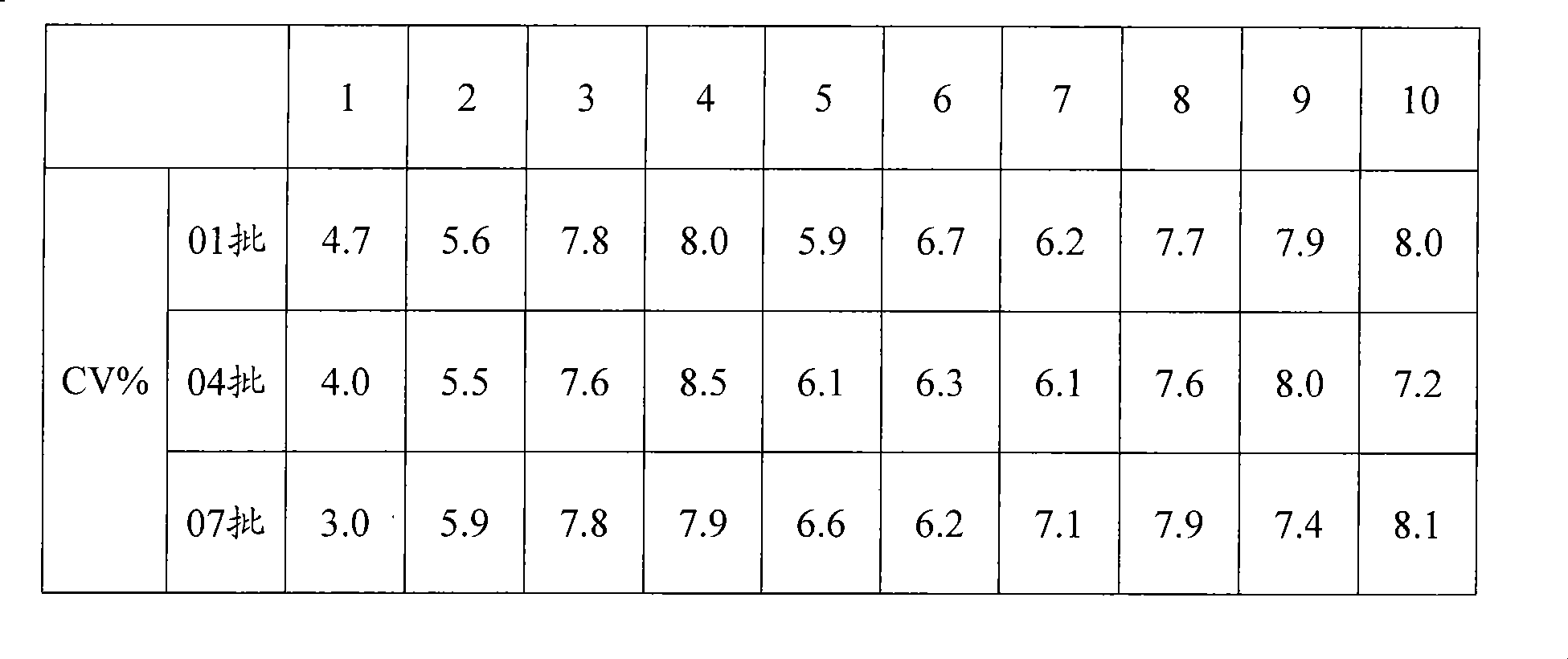
ActiveCN111879933AAccurate identificationAvoid missing detectionMaterial analysisAgainst vector-borne diseasesBlood vesselAffitin
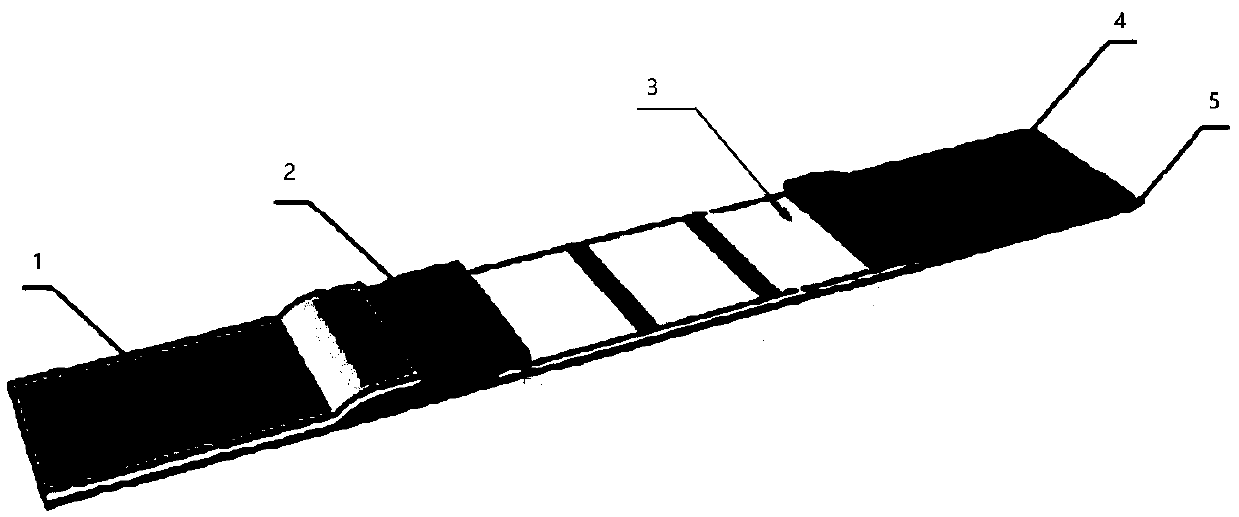
The invention relates to immunochromatographic test paper for detecting novel coronavirus SARS-CoV-2. The test paper comprises a substrate, and a sample pad, a combination pad, a nitrocellulose membrane and a water absorption pad which are arranged on the substrate and are sequentially and fixedly adhered to the substrate along the flowing direction of a to-be-detected liquid sample, the substrateis a PVC plate. The sample pad is coated with a biotin labeled anti-SARS-CoV-2N protein monoclonal antibody and biotin labeled angiotensin converting enzyme 2 (ACE2), the combination pad is coated with avidin crosslinked colloidal gold, the nitrocellulose membrane is provided with a detection line and a quality control line, the detection line is coated with an anti-SARS-CoV-2M protein monoclonalantibody, and the quality control line is coated with goat anti-mouse IgG. The immunochromatographic test paper provided by the invention achieves high-sensitivity, high-specificity, high-speed and convenient-to-operate detection, and can be applied to large-scale rapid screening of people in primary hospitals and communities.
Owner:广州德成生物科技有限公司
Immune detection reagent for detecting respiratory syncytial virus
ActiveCN103048459AHigh sensitivityImprove featuresMaterial analysisTrue positive rateMonoclonal antibody
The invention provides an immune detection reagent for detecting respiratory syncytial virus and a corresponding reagent box. The immune detection reagent comprises anti-respiratory syncytial virus monoclonal antibody 1 as peridium antibody, anti-respiratory syncytial virus monoclonal antibody 2 as biotin labeling antibody and avidin-HRP (horseradish peroxidase). The invention also provides a method for detecting the respiratory syncytial virus by applying the immune detection reagent or the reagent box. The method has relatively high sensitivity and specificity and is easy to clinically use.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
MicroRNA (ribonucleic acid) detection probe and method for detecting microRNA
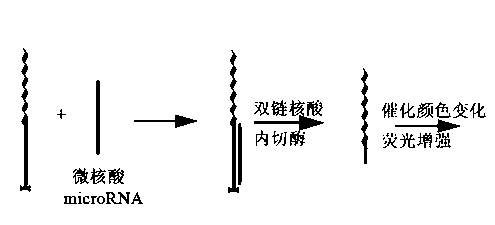
InactiveCN103160611AStrong specificityEnhanced signalMicrobiological testing/measurementDNA/RNA fragmentationSingle strandA-DNA
The invention relates to a microRNA (ribonucleic acid) detection probe and a method for detecting microRNA. The detection probe comprises three parts, an intermediate region is of single-stranded DNA (deoxyribonucleic acid) which is complementary to the microRNA, one end of the single-stranded DNA is connected with a DNA enzyme as a signal output part, and labeled biotin is arranged at the other end of the single-stranded DNA. The probe is added in a system to be detected, the single-stranded DNA of the probe and the target microRNA are complementary and paired, DSN (duplex-specific nuclease) selectively digests a DNA-RNA hybrid DNA chain, and the RNA can be recycled. Streptavidin magnetic beads are added, and the magnetic beads and the probe labeled by the non-reacted biotin are removed from a water solution through a ferromagnetic body. The part of the solution is still used for further signal output reaction. The detection method disclosed by the invention adopts DSN amplification and ribozyme amplification, and becomes a method for detecting the microRNA, which has the advantages of low background, high sensitivity and strong specificity.
Owner:WUHAN UNIV
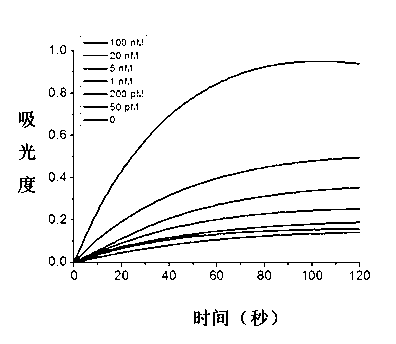
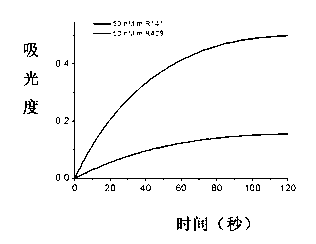
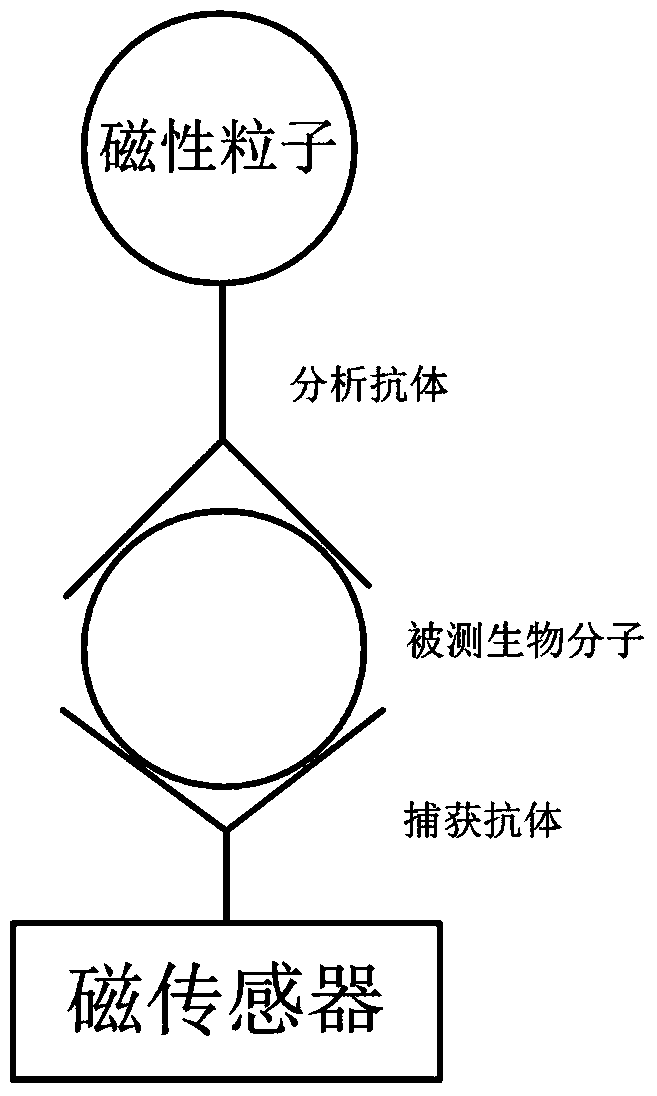
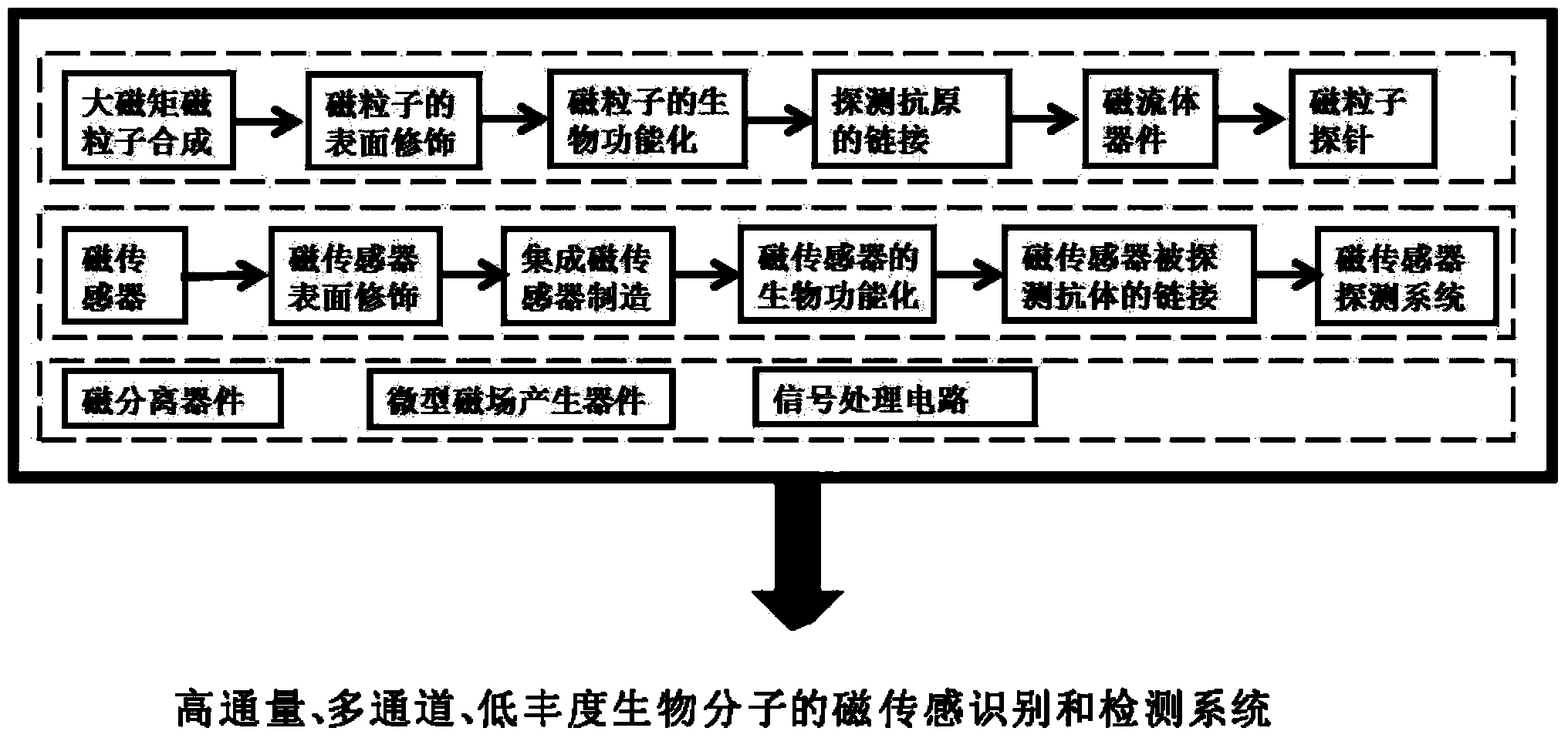
Magnetic sensing identification method for high-flux multi-channel low-abundance biomolecules
ActiveCN104034881AHigh sensitivityImprove throughputBiological testingMaterial magnetic variablesAntigenSomatic cell
The invention relates to a magnetic sensing identification method for high-flux multi-channel low-abundance biomolecules, and belongs to the technical field of biomolecule identification. The invention mainly aims at an immunolabelling biomolecule detection, monitoring and identification method based on systems such as antigens-antibodies, cell factors-cell factor acceptors, bioactive peptides-acceptors, biotin-avidin and chiral molecules. A biomolecule probe and detection system consists of superparamagnetic particles and high-performance magnetic sensors. The magnetic sensing identification method can be applied to the application fields of biology, medicines, food safety and the like, and can be used for biomolecule identification, detection and monitoring, clinical disease diagnose, food safety detection and virus and germ detection.
Owner:NANJING YIDEGUAN ELECTRONICS TECH
Chemiluminescence immune analytic reagent kit for detecting thyroid peroxidase autoantibody
InactiveCN101377496AGuaranteed SensitivityHigh sensitivityChemiluminescene/bioluminescenceAntigenChemiluminescent immunoassay
The invention provides a chemiluminescent immunoassay kit for thyroid peroxidase autoantibodies and a preparation method thereof. The invention has the advantages that the reaction pattern of the double antigen sandwich method is adopted, the chemiluminescent technology and the biotin-avidin immune amplifying technological principle are effectively utilized, horse radish peroxidase is adopted to be as the marker enzyme, the content of the thyroid peroxidase autoantibodies in human serum samples is quantitatively detected, and the detection sensitivity can be ensured. The kit of the invention has the advantages of high sensitivity, good repeatability, good stability and high detection efficiency, and can provide a timely and reliable experimental basis for the clinical diagnosis and the treatment of thyroid disorders.
Owner:北京科美东雅生物技术有限公司
High sensitive and jettisonable multicomponent chemiluminescent imaging immunosensor
InactiveCN103575896AAchieving Simultaneous High Sensitivity Image ImmunoassaysEasy to makeMaterial analysis by optical meansBiological testingSensor arrayPeroxidase
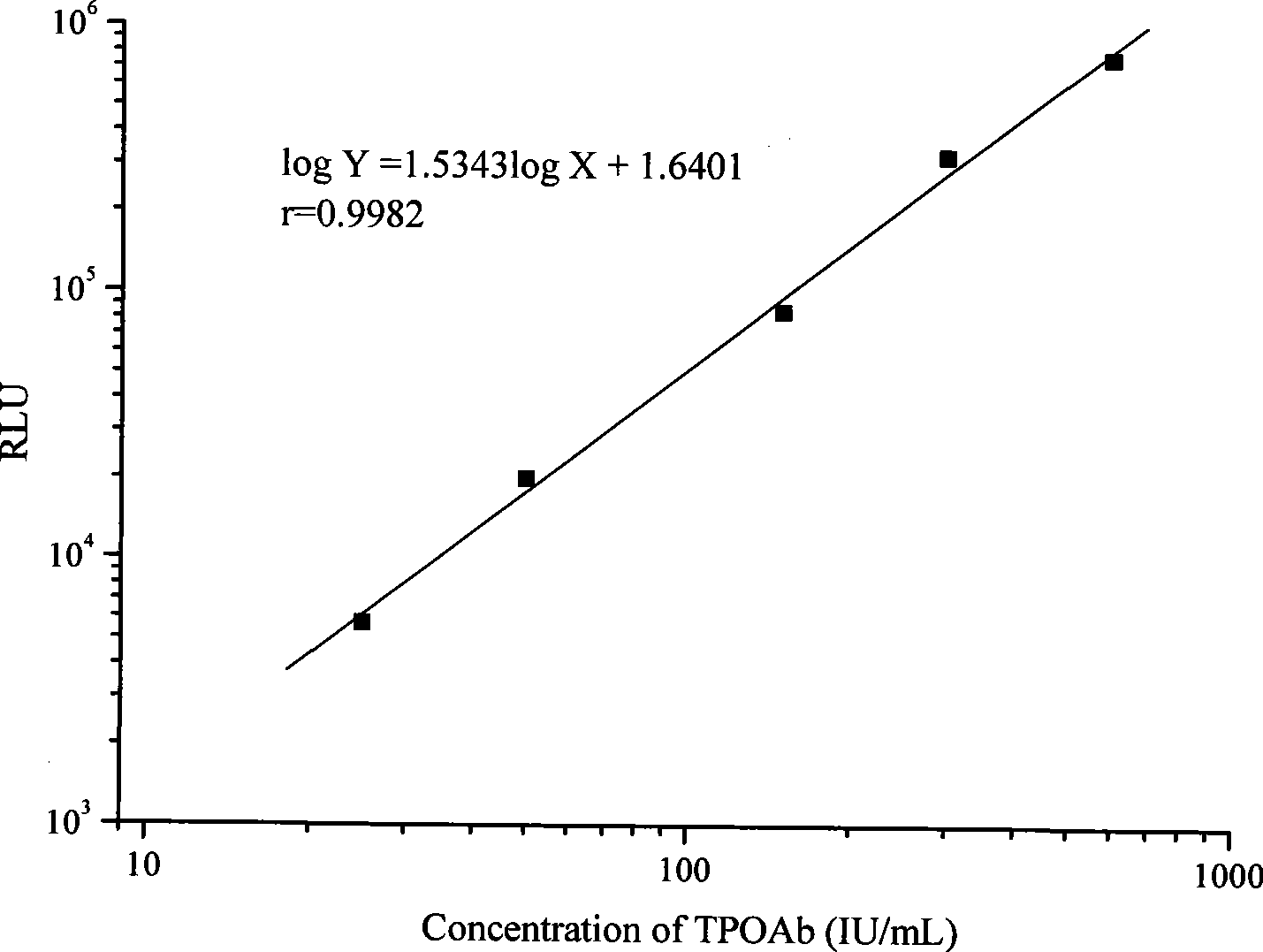
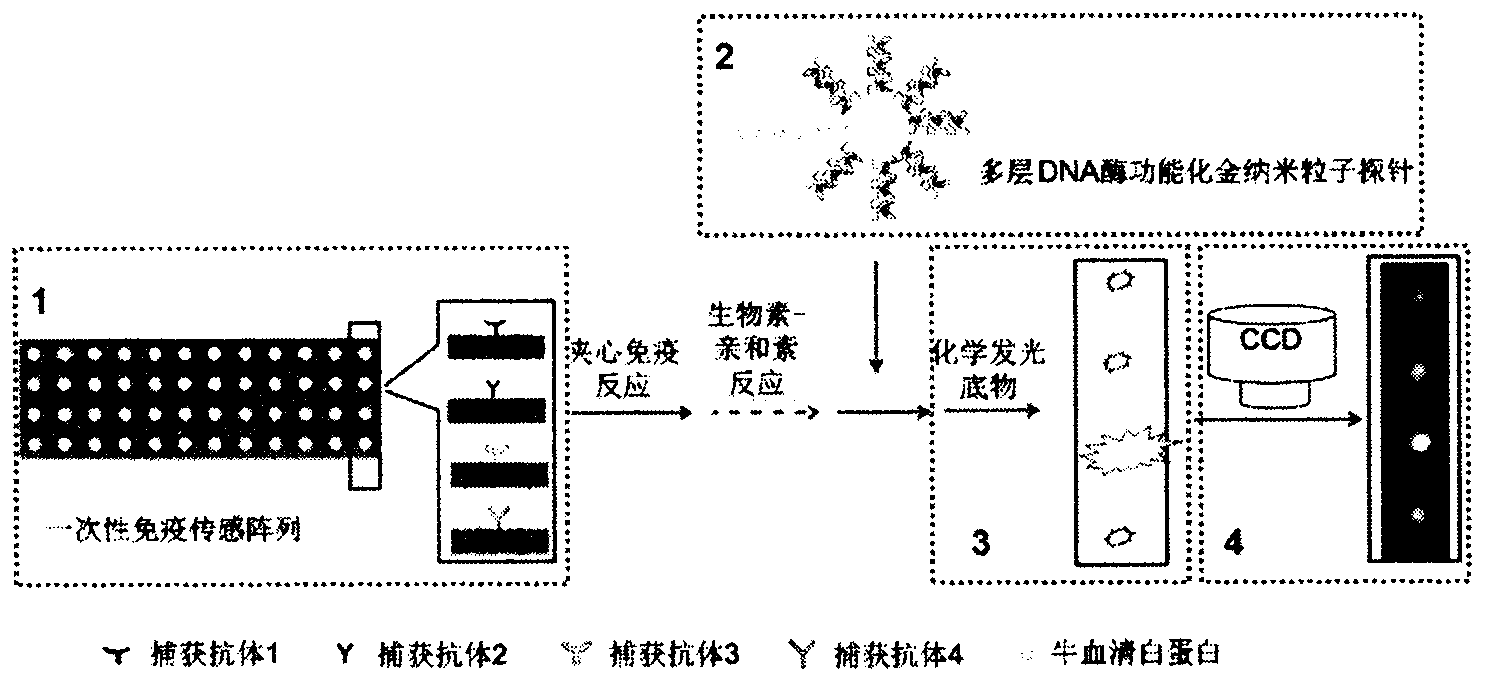
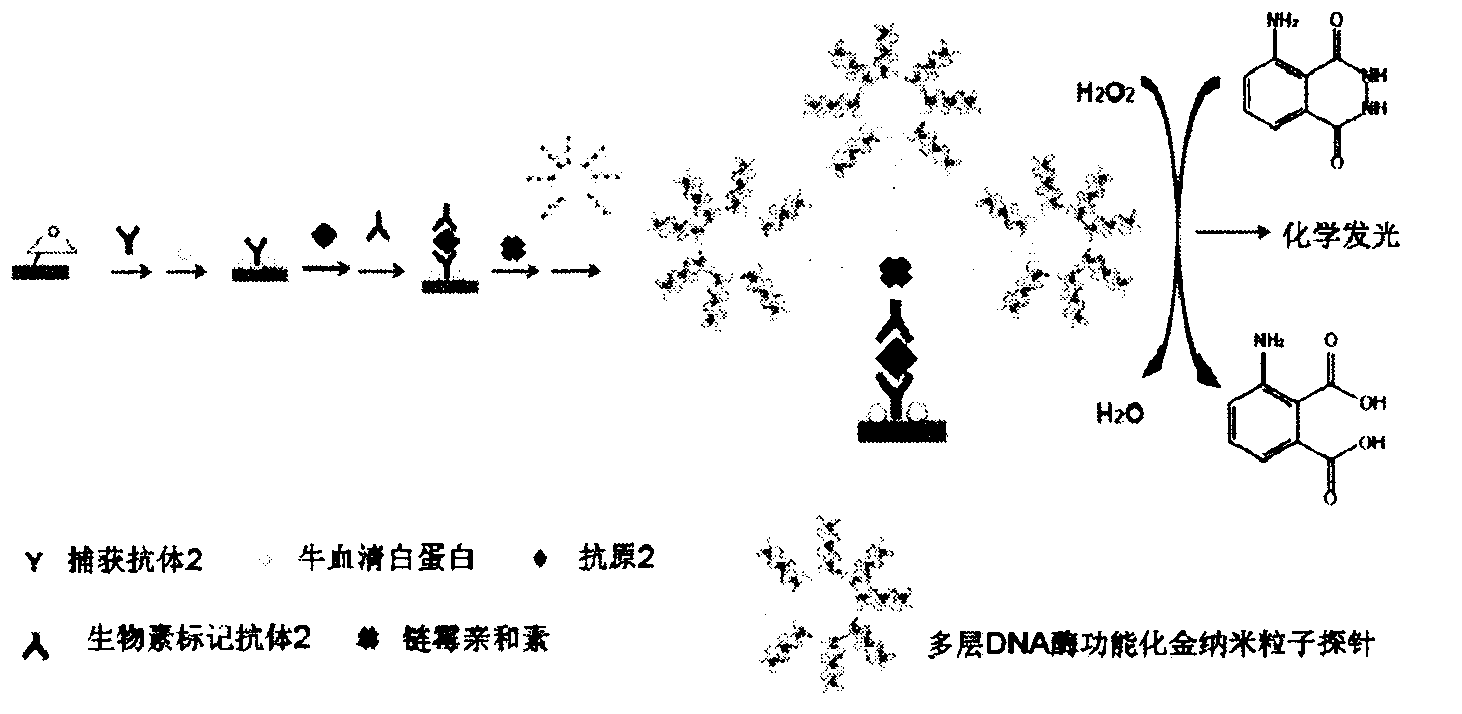
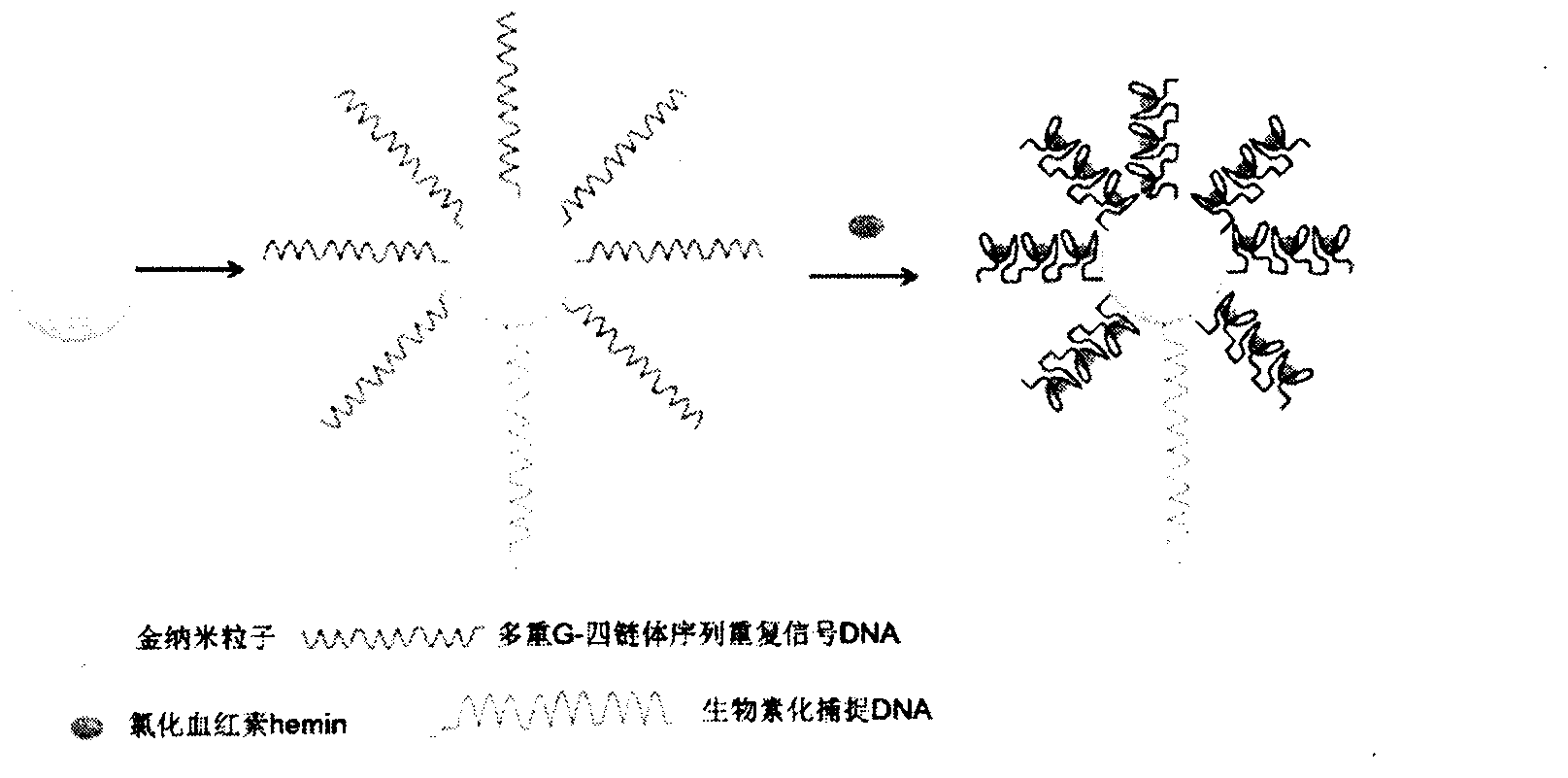
The invention relates to a high sensitive and jettisonable multicomponent chemiluminescent imaging immunosensor. The immunosensor is prepared by: constructing a 4*12 array on a silanized glass slide by a screen printing technique, and coating the array points with different capture antibodies to construct a jettisonable multicomponent immune sensor array; immobilizing biotinylated capture DNA and multiple G-quadruplex sequence repeat signal DNA on gold nanoparticle surfaces simultaneously, combining G-quadruplex signal DNA with heme to form DNA enzyme so as to prepare a multilayer DNA enzyme functionalized gold nanoparticle probe; and based on sandwich immunoassay, forming a sandwich immune complex on the sensor array, carrying out biotin-avidin reaction, labeling different immune complexes with the multilayer DNA enzyme functionalized gold nanoparticle probe; and making use of the peroxidase characteristics of DNA enzyme to catalyze reaction between chemiluminescent substrate H2O2-luminol to obtain a sensitive chemiluminescent signal, thus realizing high sensitive image immunoassay of a variety of protein. The immunosensor has the advantages of simple design, low cost, high sensitivity, high throughput and good repeatability, etc., and has certain clinical application value.
Owner:JIANGSU CANCER HOSPITAL +1
Preparation and application method of pharmaceutical albumin nanoparticle
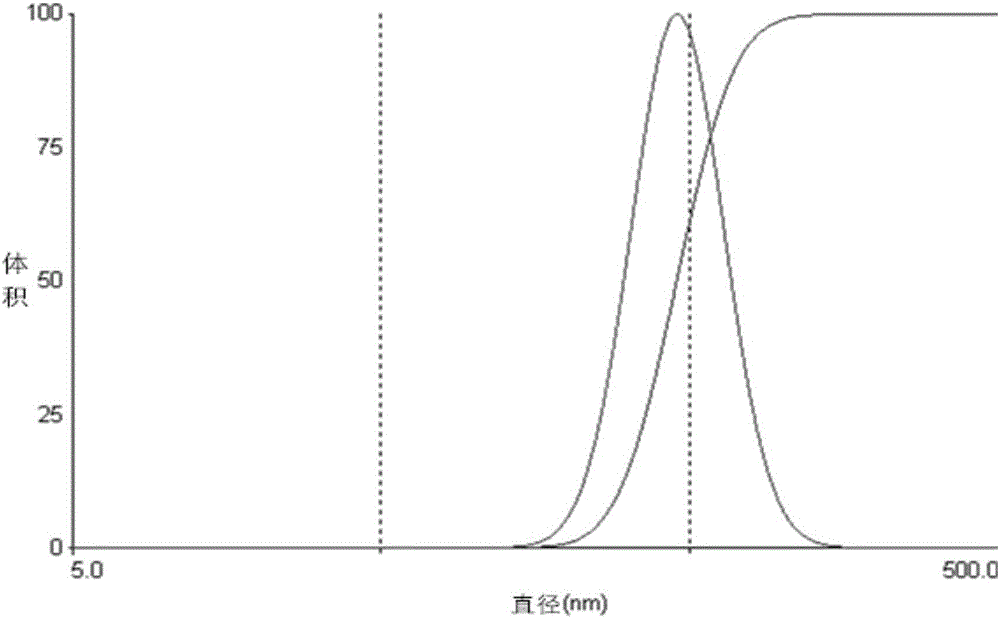
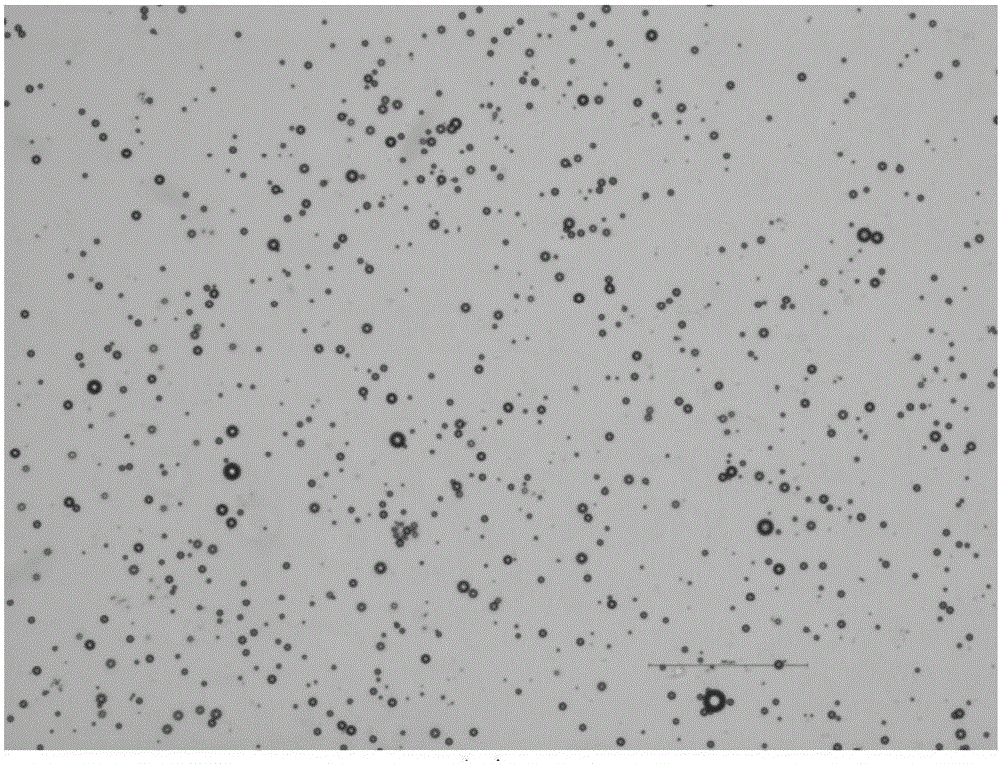
InactiveCN105816885AUniform particle size distributionGood effectPowder deliveryKetone active ingredientsTumor targetingMedicine
The invention belongs to the field of pharmaceutical preparations, and provides a pharmaceutical albumin nanoparticle, a preparation method and application of the nanoparticle to tumor targeting therapy. The nanoparticle is made from two types of proteins, namely avidin and albumin, encapsulating slightly-soluble antitumor drugs. The electrostatic interaction exists between avidin and albumin, and avidin in the nanoparticle can be combined with biotin. Avidin can target the nanoparticle encapsulating the drugs on a biotin enrichment part. Before the application of the nanoparticle, a biotinylated antibody is used for pre-targeting on a tumor part, and can improve the distribution of a nanoparticle preparation in the tumor part. The biotinylated antibody and the nanoparticle preparation are combined for therapy, thereby playing better antitumor effect than the effect played by the combination of a monoclonal antibody and an albumin nanoparticle without avidin.
Owner:PEKING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com