Hepatic carcinoma targeted photo-thermal therapeutic agent as well as preparation method and application thereof
A photothermal therapy agent, a technology for liver cancer, applied in the field of biomedical engineering, can solve the problems of RGO cannot be independently imaged and has no active targeting ability, and achieve the effect of excellent clinical application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
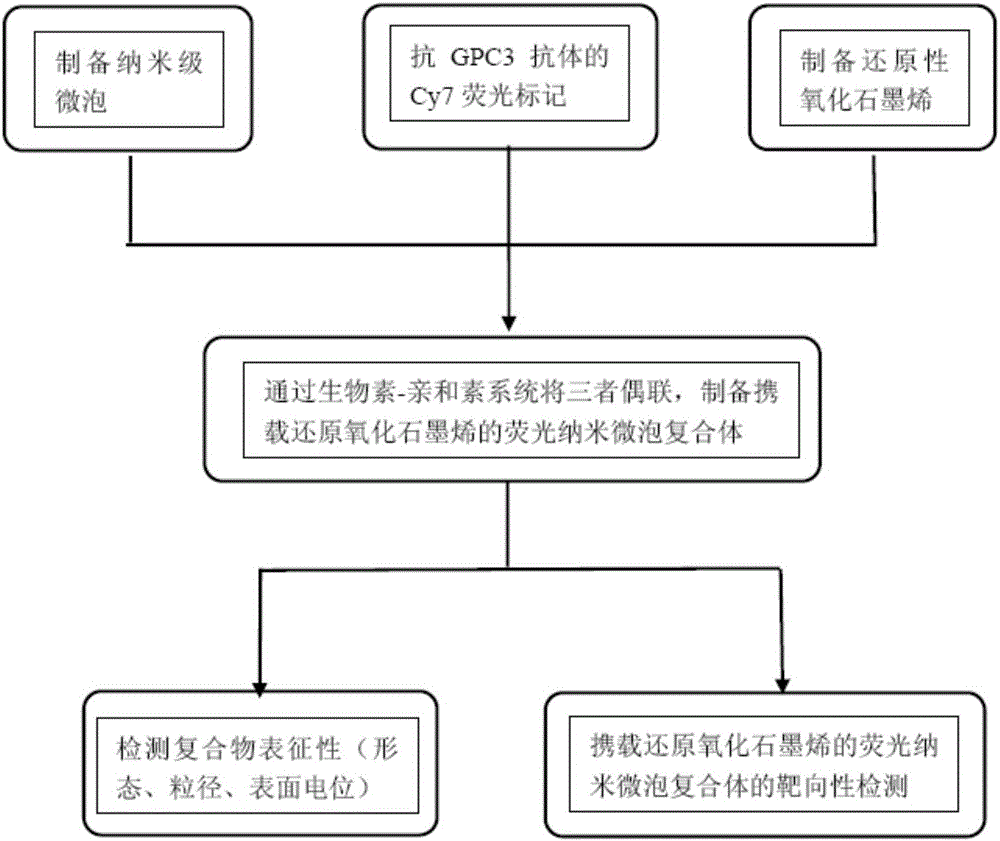
[0042] Example 1 Preparation of Biotinylated Nanobubbles
[0043] ① Select DSPC, DSPE-PEG2000, and DSPE-PEG2000-biotin with a mass ratio of 18:1:1 as raw materials, put 10 mg of phospholipid mixture in a 20 ml round bottom flask, and add a mixed solution of chloroform and methanol drop by drop (volume ratio 2: 1) until the lipid mixture is completely dissolved. Place the flask in a rotary evaporator and evaporate to dryness at 40°C under vacuum;
[0044] ②After evaporating to dryness, add 5ml of PBS buffer solution (pH=7.4, 0.15mol / l) to the flask to redissolve, transfer the liquid into a centrifuge tube, seal it, and replace the internal air with C 3 f 8 ;
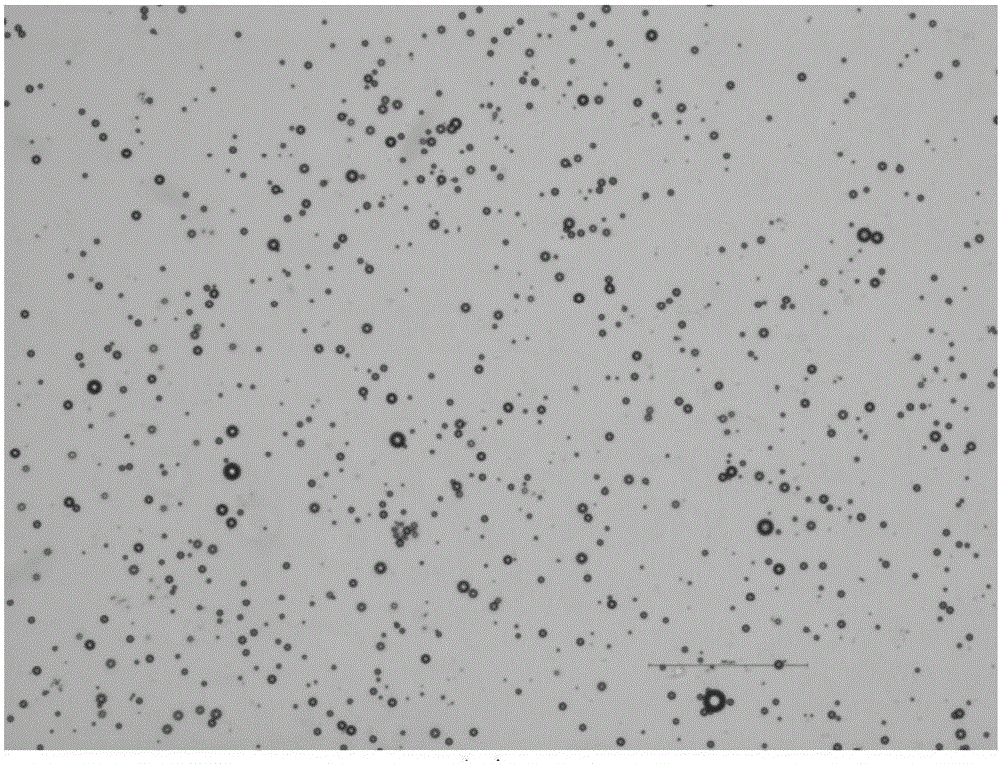
[0045] ③ Vibrate with an amalgam oscillator for 40 seconds, then place the solution in a water bath at a temperature of 55° C., and process it with an ultrasonic homogenizer (working frequency 20 kHz) for 20 seconds. After the treatment, incubate at the same temperature for 60 minutes, and then continue the ultrasonic...
Embodiment 2
[0047] Example 2 Cy7 fluorescent labeling of biotinylated anti-GPC3 antibody
[0048] ①Biotinylated anti-GPC3 antibody was purchased from ABCAM Company, UK;
[0049] ②Configuration of cross-linking reaction solution: Accurate NaHCO 3 Powder 0.378g, Na 2 CO 3 Put 0.053g of powder and 0.368g of NaCl powder in a 50ml volumetric flask, add sterile double distilled water to make up to 50ml;
[0050] ③Cy7 powder is dissolved in DMSO, the concentration is 1mg / ml, and the whole process is protected from light;
[0051] ④ Dissolve the antibody in the cross-linking reaction solution at a concentration of 2mg / ml, and react according to the ratio of antibody to Cy7 mass ratio of 1mg to 150ug, slowly add Cy7 fluorescent dye to the antibody cross-linking reaction solution, shake gently to mix, Protect from light and react at 4°C for 8 hours;
[0052] ⑤ Add 5mol / L NH dropwise 4 Stop with 25 μL of Cl solution, and continue to react for 8 hours at 4°C in the dark;
[0053] ⑥ The obtaine...
Embodiment 3
[0054] Embodiment 3 Preparation of biotinylated reduced graphene oxide
[0055] ① Weigh 1g of graphite powder, add about 40g of NaCl powder and mix well, and grind the mixture thoroughly with a ceramic mortar until no obvious graphite particles are visible. Add water to dissolve and filter to wash off the NaCl powder in the mixture, and place the finely ground graphite in an oven to dry. Add the dried graphite powder into a 250mL round-bottomed flask, carefully add about 23mL of concentrated sulfuric acid while stirring, and stir at room temperature for 8 hours until the concentrated sulfuric acid and graphite are fully mixed. Place the reaction vessel in an ice-water bath. After the temperature of the mixture in the flask drops below 15°C, slowly add a total of 3 g of potassium permanganate solids one by one. Stir continuously during the addition to ensure that the temperature in the flask remains uniform and stable during the process. After adding the solid potassium perman...
PUM
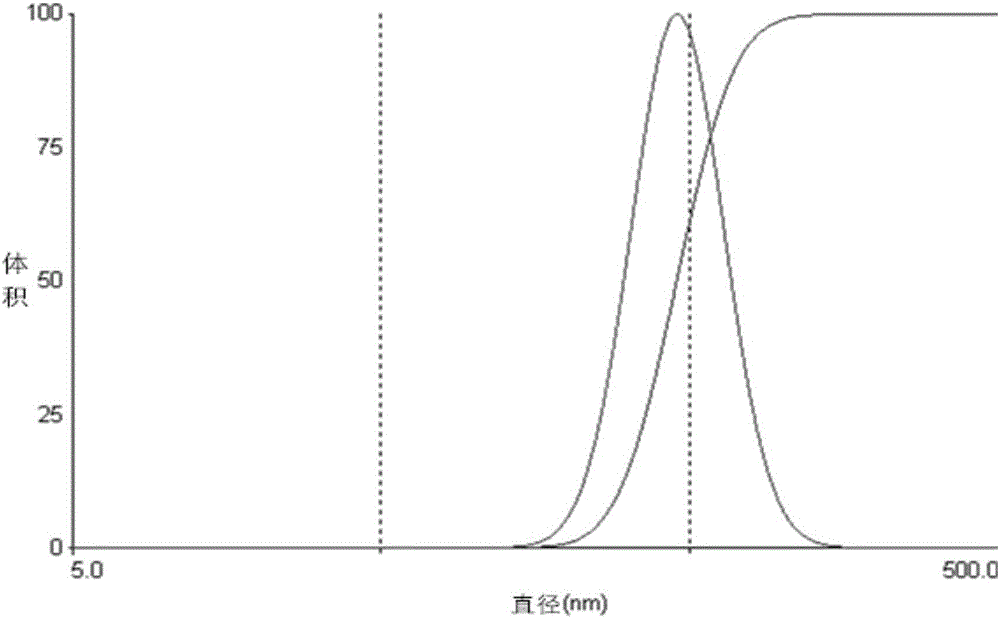
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com