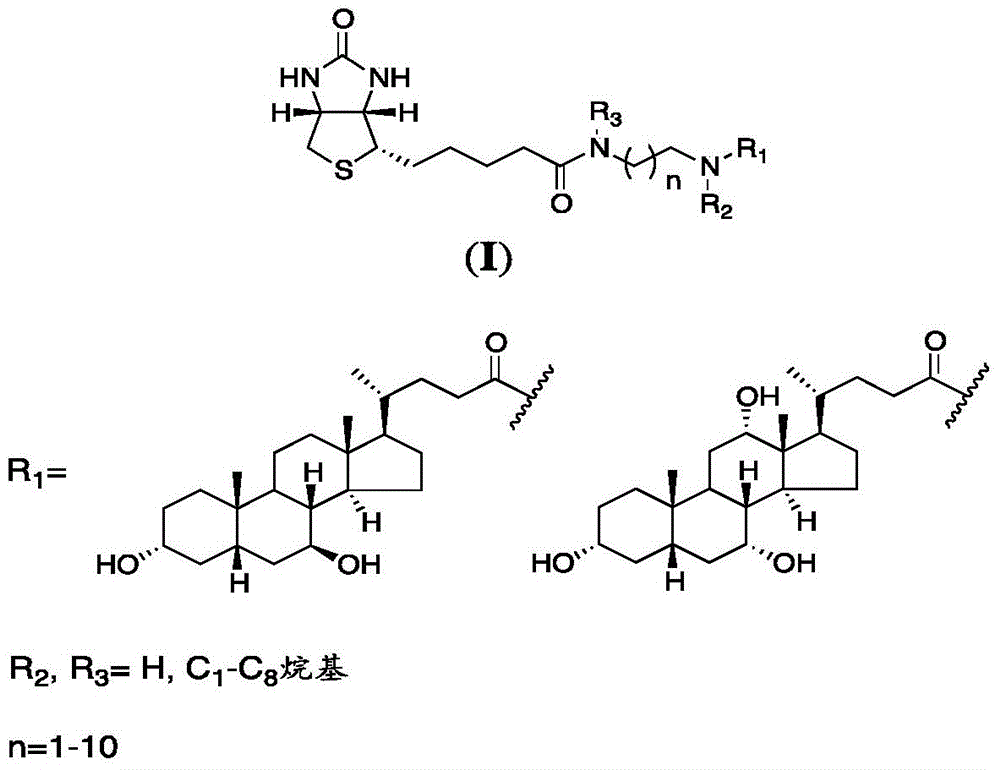
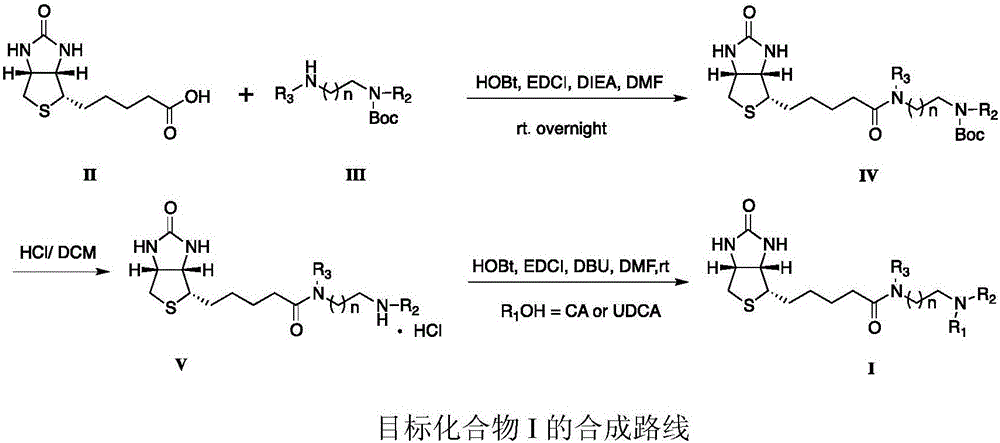
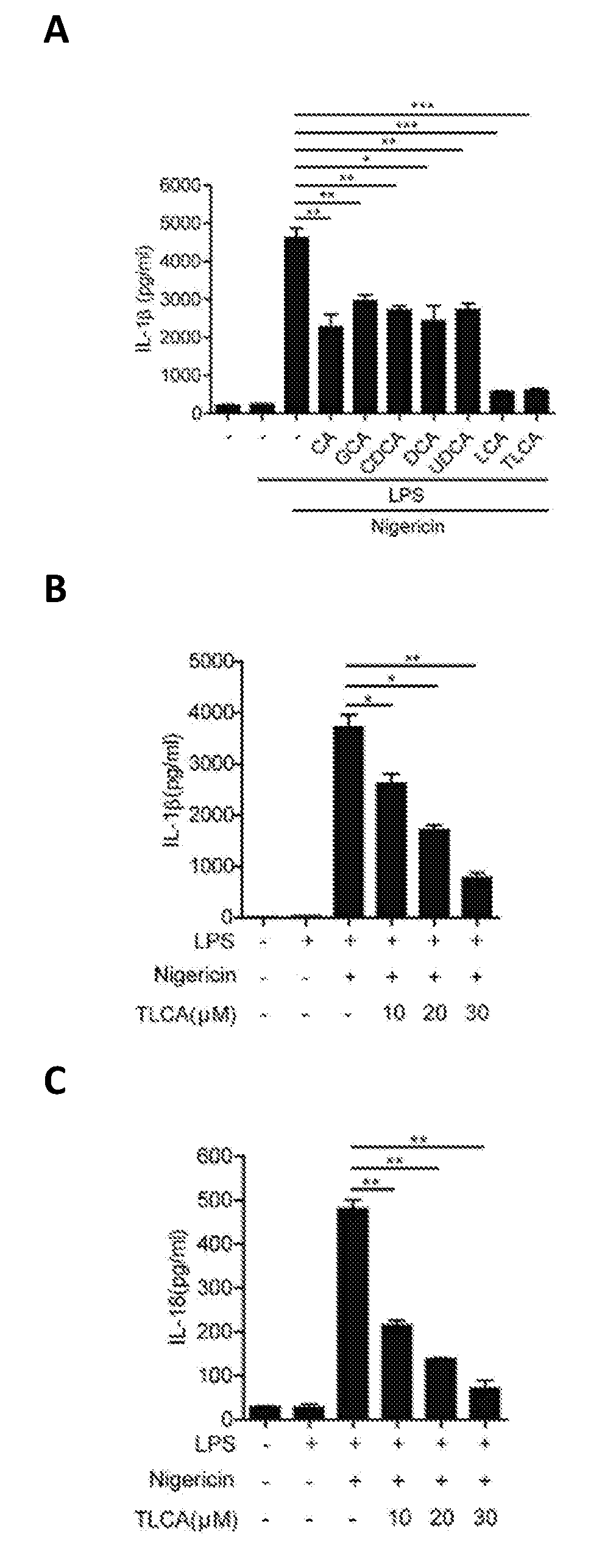
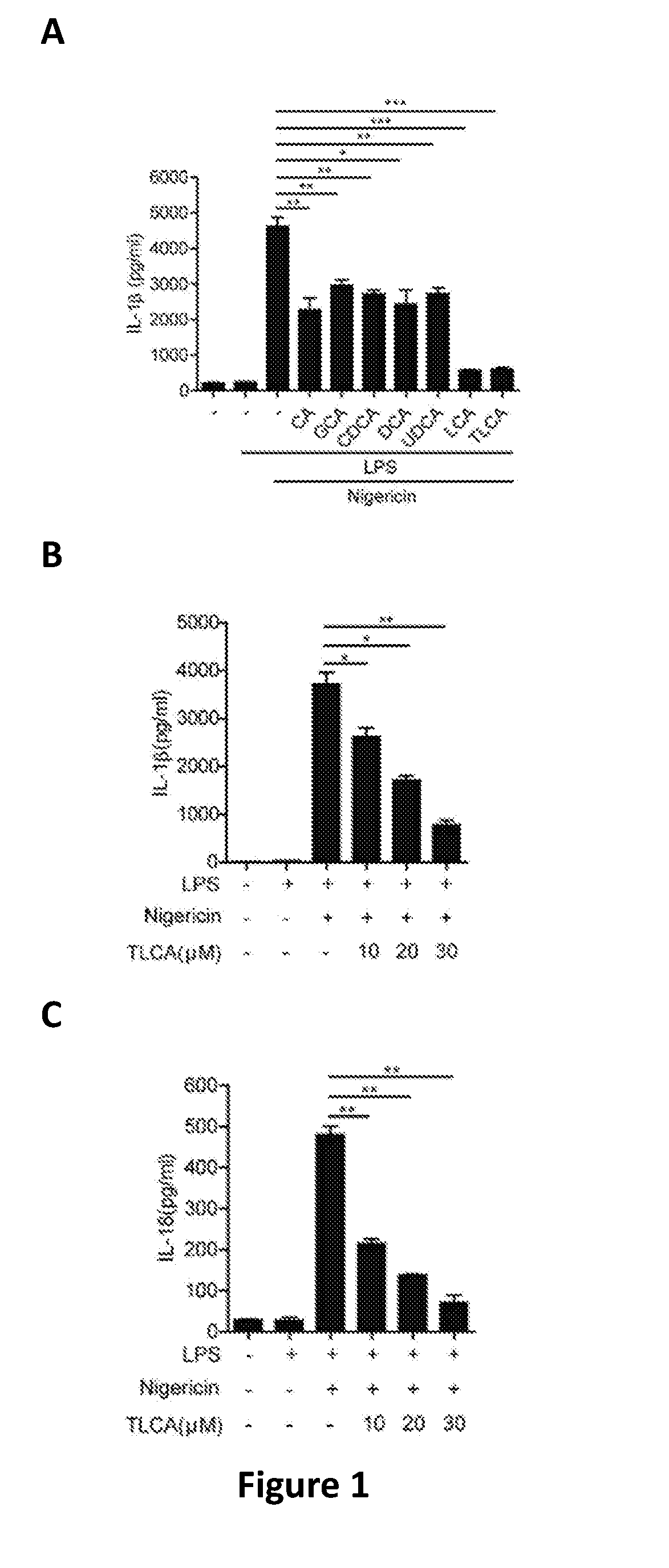
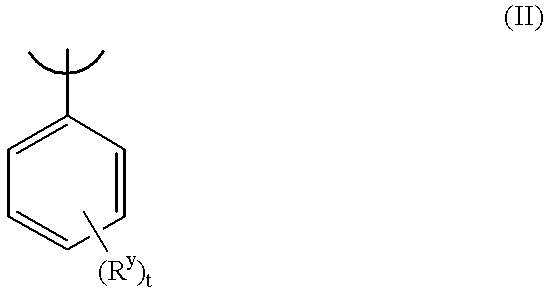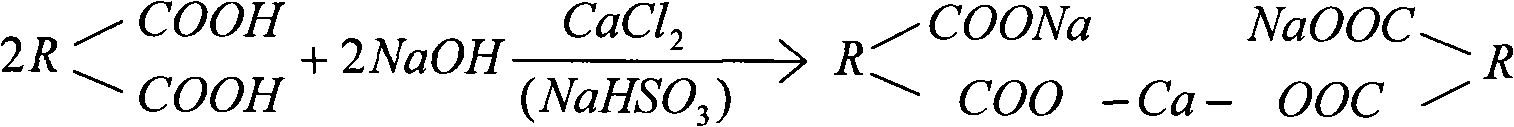Patents
Literature
41 results about "Taurocholic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
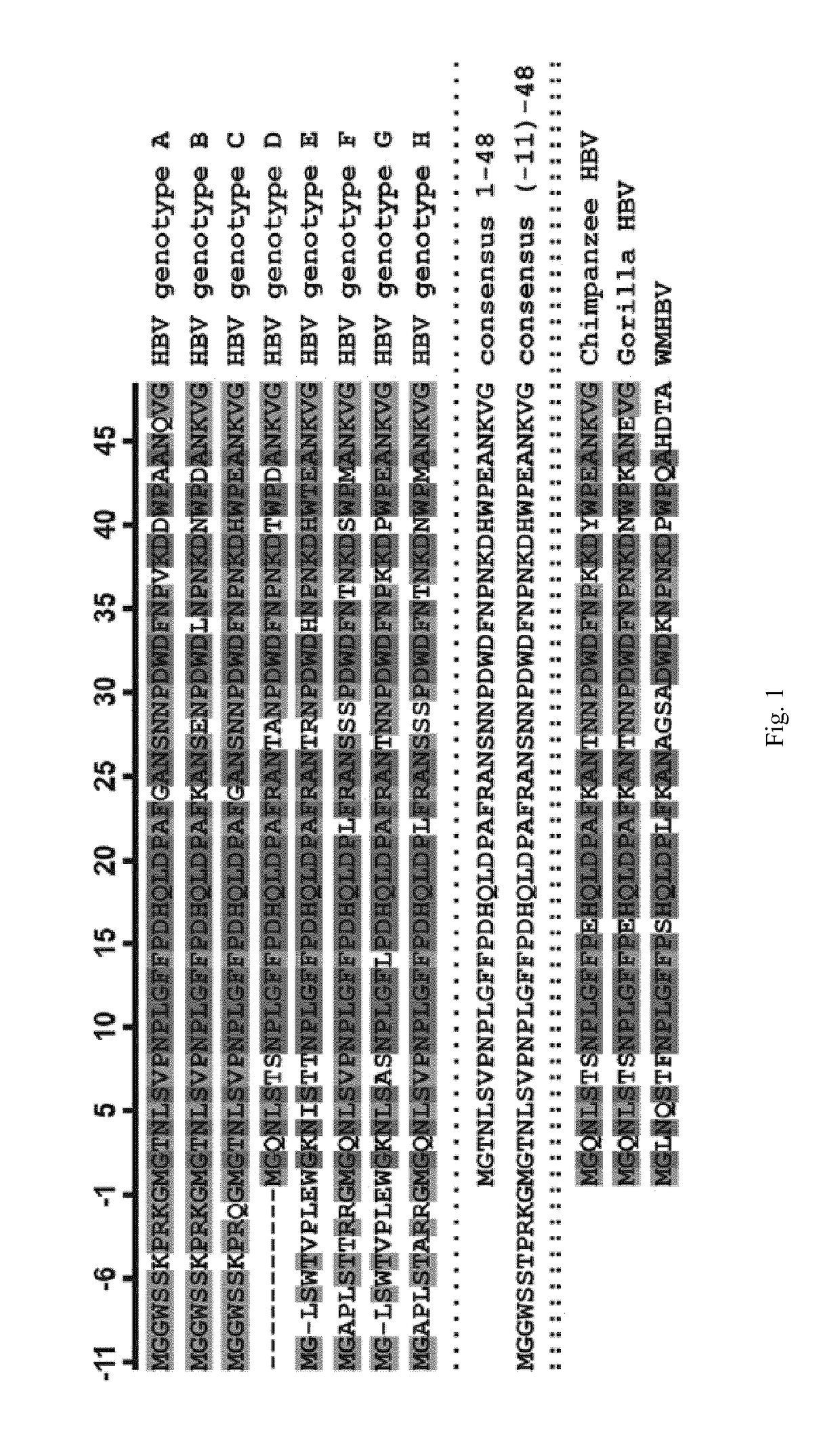
Taurocholic acid, known also as cholaic acid, cholyltaurine, or acidum cholatauricum, is a deliquescent yellowish crystalline bile acid involved in the emulsification of fats. It occurs as a sodium salt in the bile of mammals. It is a conjugate of cholic acid with taurine. In medical use, it is administered as a cholagogue and choleretic.
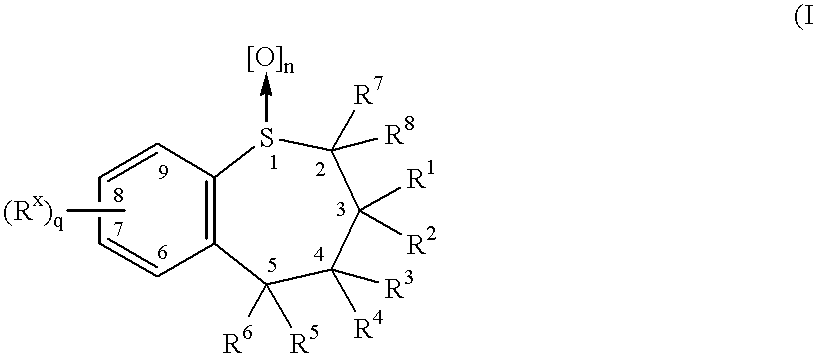
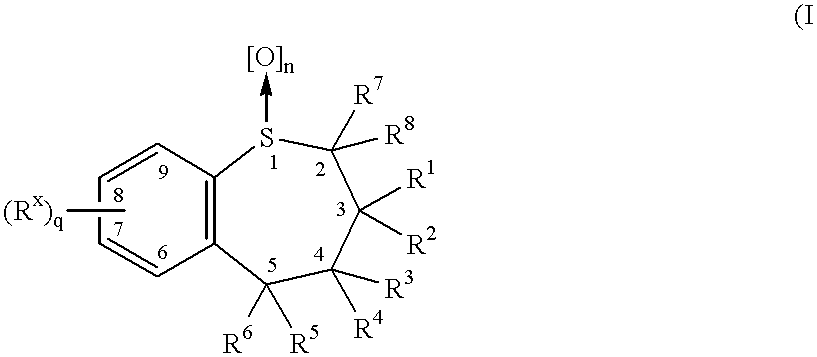
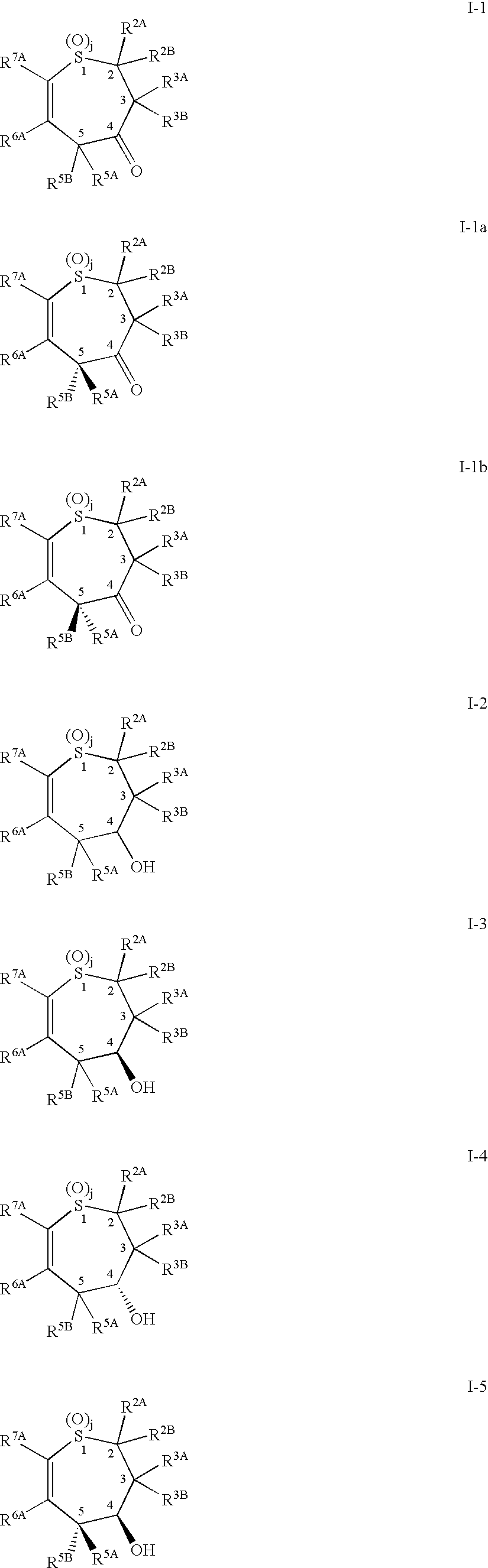
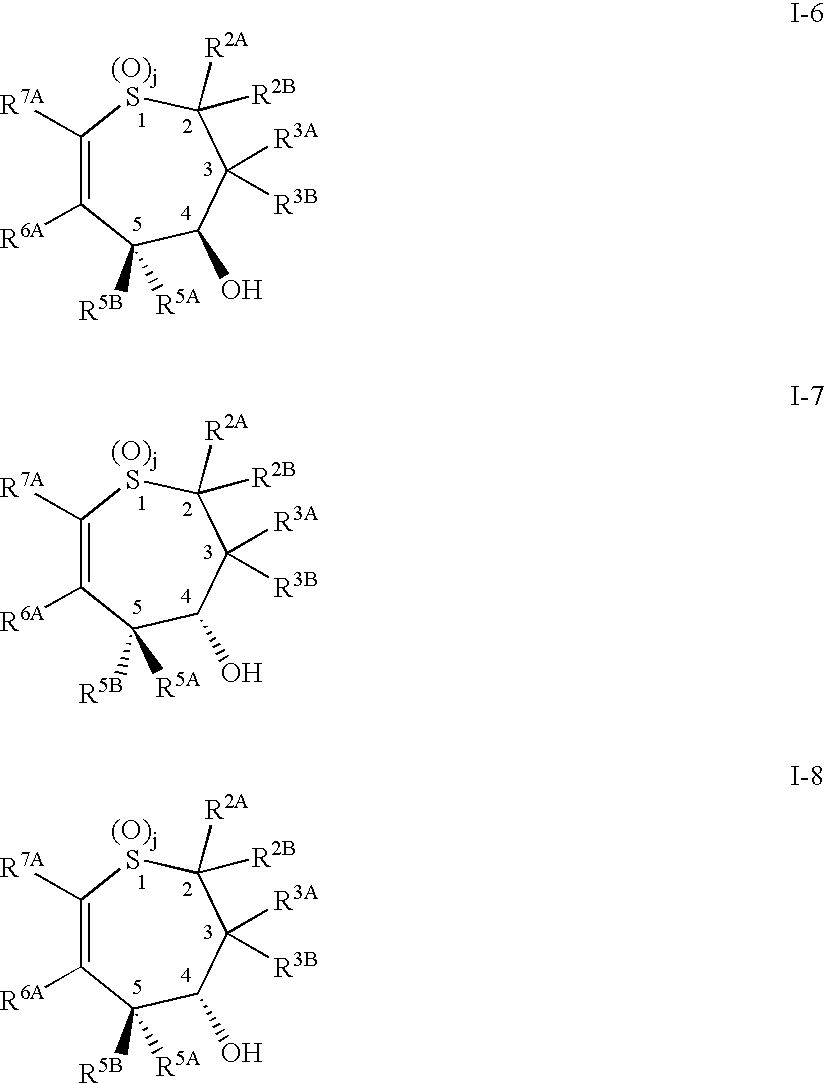
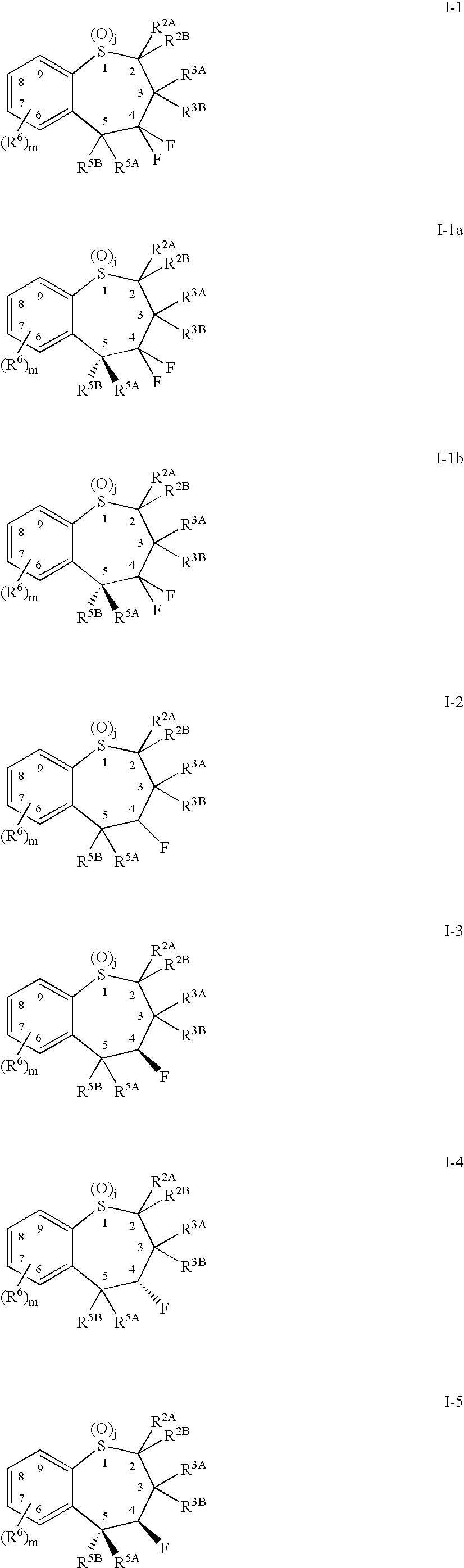
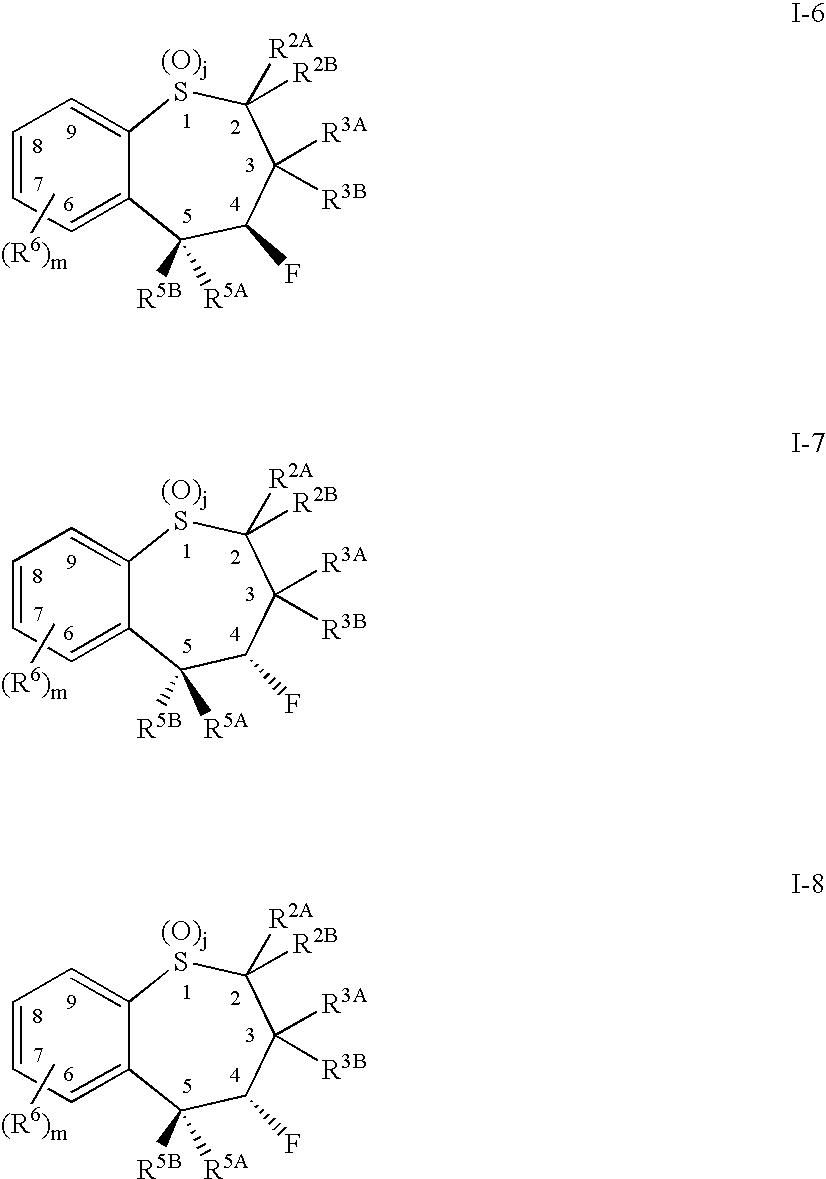
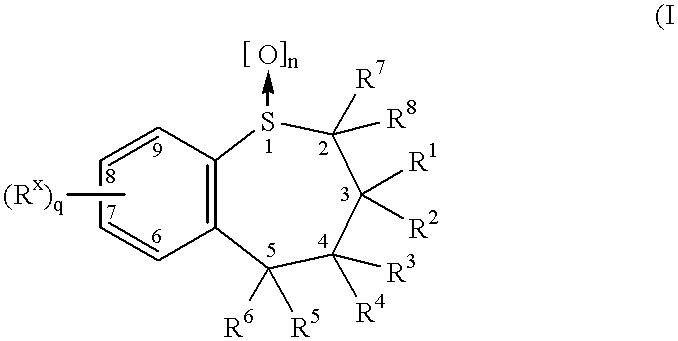
Benzothiepines having activity as inhibitors of ileal bile acid transport and taurocholate uptake
Provided are novel benzothiepines, derivatives, and analogs thereof; pharmaceutical compositions containing them; and methods of using these compounds and compositions in medicine, particularly in the prophylaxis and treatment of hyperlipidemic conditions such as those associated with atherosclerosis or hypercholesterolemia, in mammals.
Owner:GD SEARLE & CO
Substituted 5-aryl-benzothiepines having activity as inhibitors of ileal bile acid transport and taurocholate uptake
Provided are novel benzothiepines, derivatives, and analogs thereof; pharmaceutical compositions containing them; and methods of using these compounds and compositions in medicine, particularly in the prophylaxis and treatment of hyperlipidemic conditions such as those associated with atherosclerosis or hypercholesterolemia, in mammals.
Owner:GD SEARLE & CO
Alkyl/aryl hydroxy or keto thiepine compounds as inhibitors of apical sodium co-dependent bile acid transport (ASBT) and taurocholate uptake
Owner:PHARMACIA CORP
Mono- and di-fluorinated benzothiepine compounds as inhibitors of apical sodium co-dependent bile acid transport (ASBT) and taurocholate uptake
Owner:GD SEARLE & CO
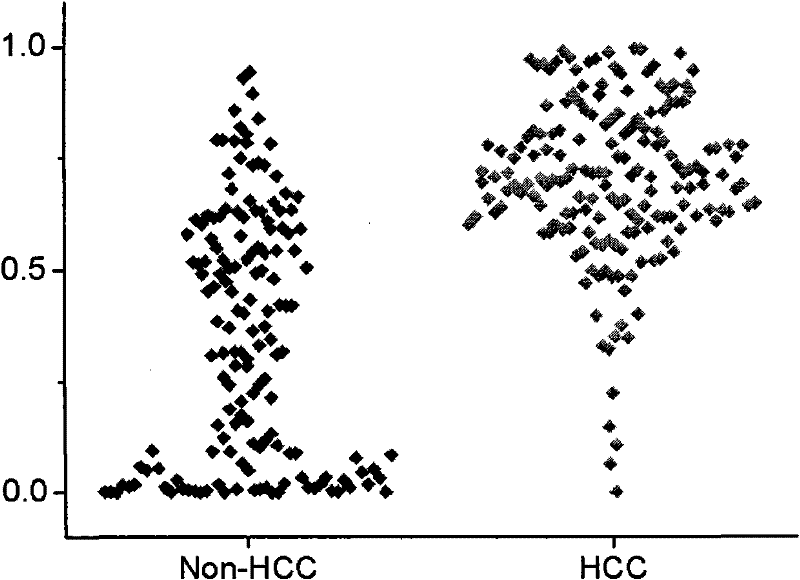
Small molecule metabolite map for identifying liver cancer, hepatitis or liver cirrhosis and manufacturing method thereof
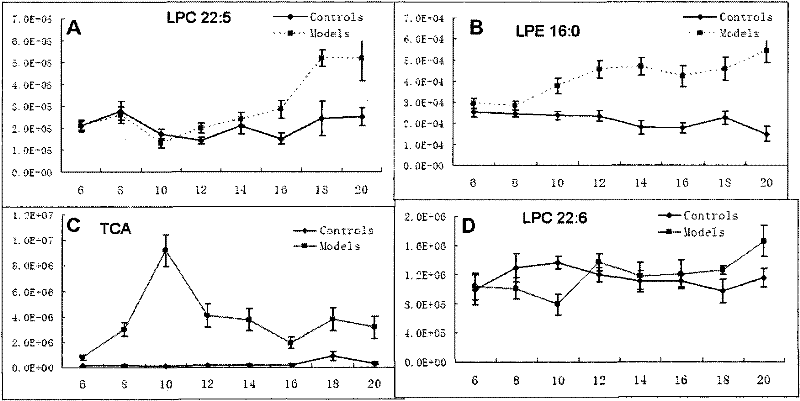
The invention relates to the technical field of medical diagnostics, in particular to a small molecule metabolite map for identifying liver cancer, hepatitis or liver cirrhosis and a manufacturing method thereof. The small molecule metabolites are four blood metabolites markers, i.e. palmitoyl hemolysis phosphoric acid ethanolamine glyceride, docosapentaenoic acyl hemolysis phosphatidyl choline, docosahexaenoic acyl hemolysis phosphatidyl choline and taurocholic acid, which respectively have the molecular weight of 453.2855, 569.3481, 567.3319 and 516.2916, and the corresponding ions of 454.2928, 570.3547, 568.3391 and 480.2776 detected through mass spectrum. Through experimentation on animals, the accuracy for determination of the liver cancer phase of a rat is 85 percent, and the accuracy for determination of the hepatitis or the liver cirrhosis is 94 percent. Clinical experiment results show that the accuracy for determination of the liver cancer is 85 percent, and the accuracy for determination of non-liver cancer is 71.9 percent. The method provided by the invention has the advantages of high sensitivity and high throughput, is superior to the existing liver cancer single diagnosis marker, and is applicable to screening and assisted diagnosis of the liver cancer.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY +1
Novel alkyl/aryl hydroxy or keto thiepine compounds as inhibitors of apical sodium co-dependent bile acid transport (ASBT) and taurocholate uptake
Owner:PHARMACIA CORP
Intermediates and processes for the preparation of benzothiepines having activity as inhibitors of ileal bile acid transport and taurocholate uptake
Provided are novel benzothiepines, derivatives, and analogs thereof; pharmaceutical compositions containing them; and methods of using these compounds and compositions in medicine, particularly in the prophylaxis and treatment of hyperlipidemic conditions such as those associated with atherosclerosis or hypercholesterolemia, in mammals.
Owner:GD SEARLE & CO
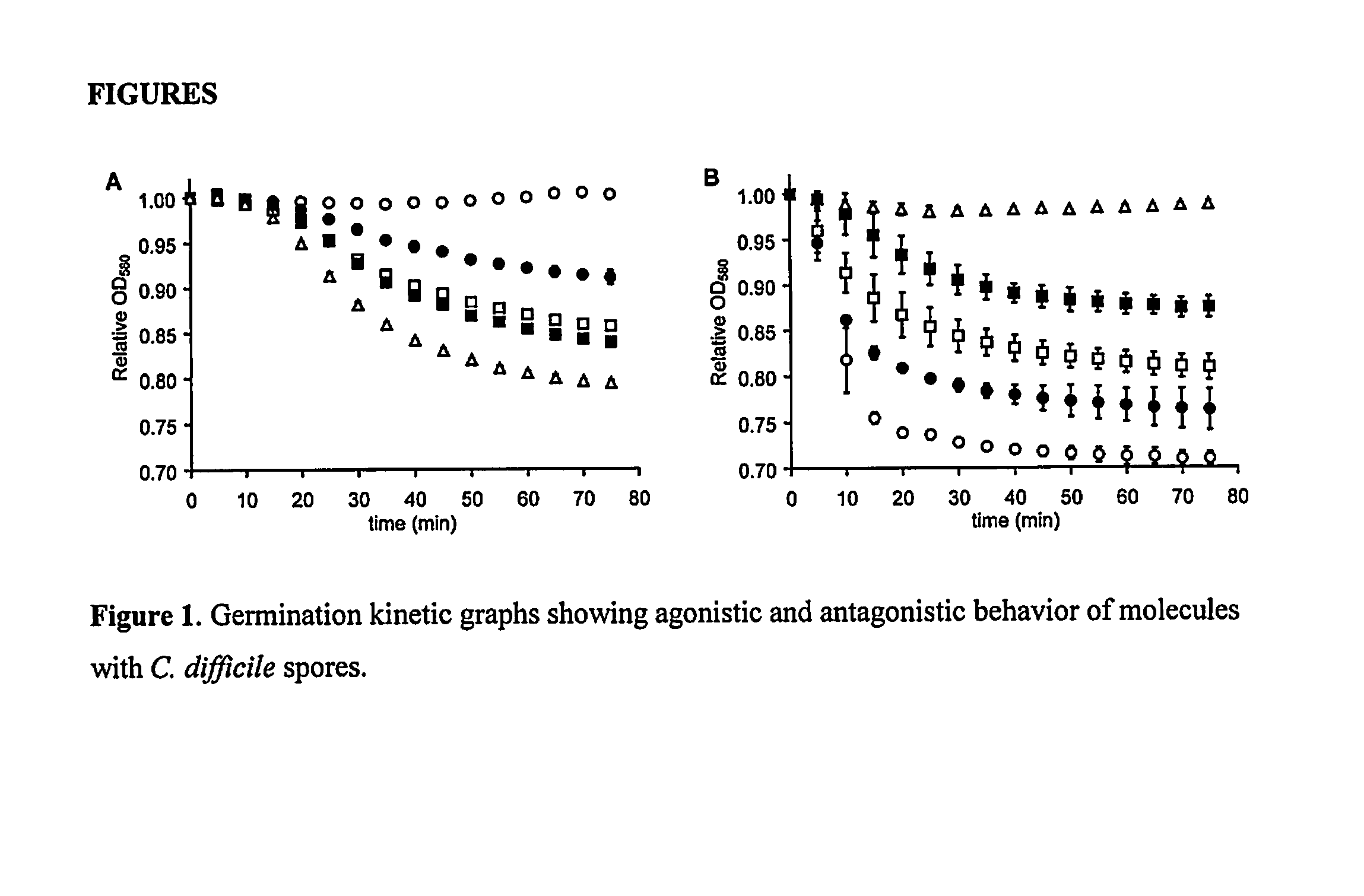
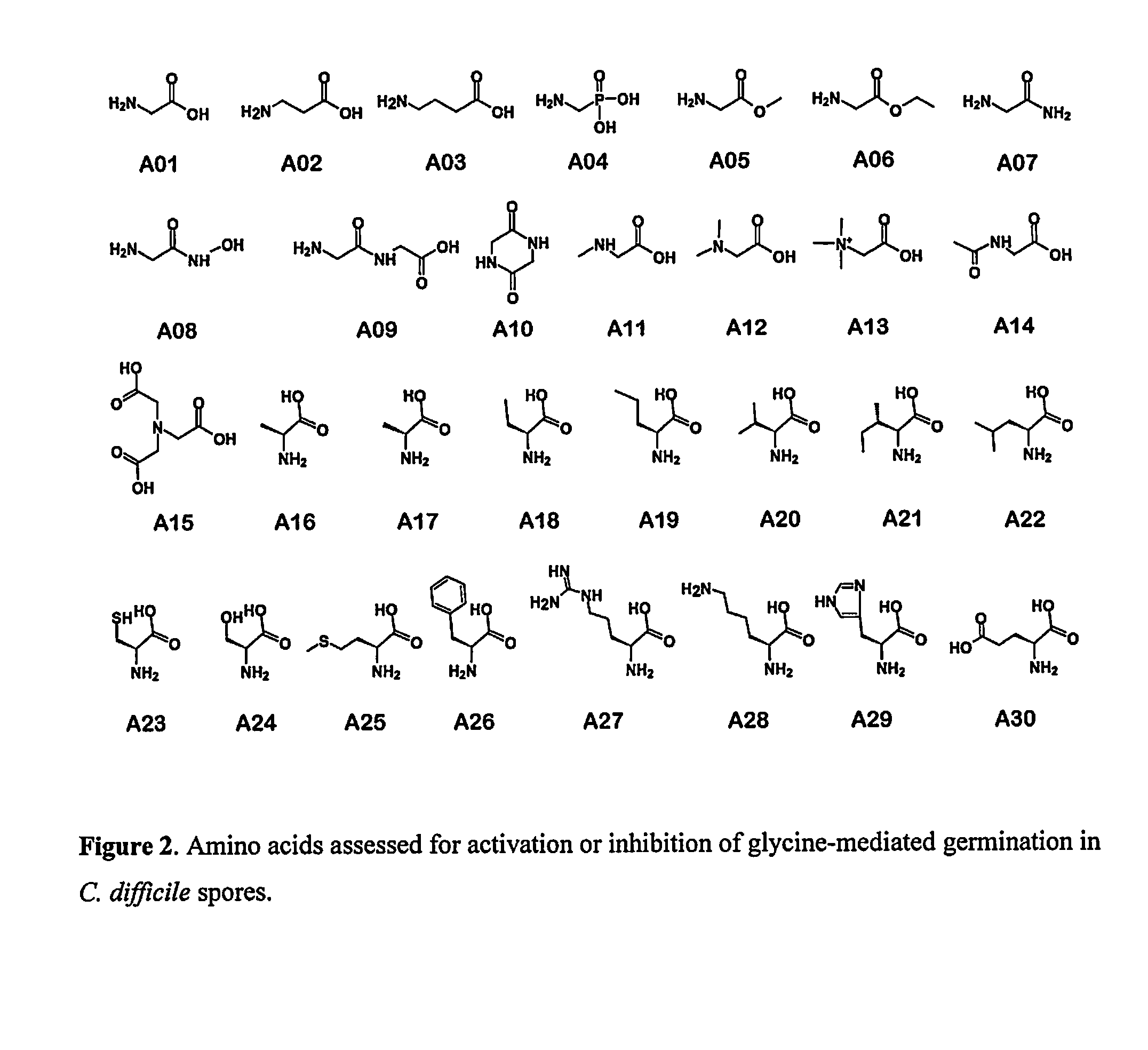
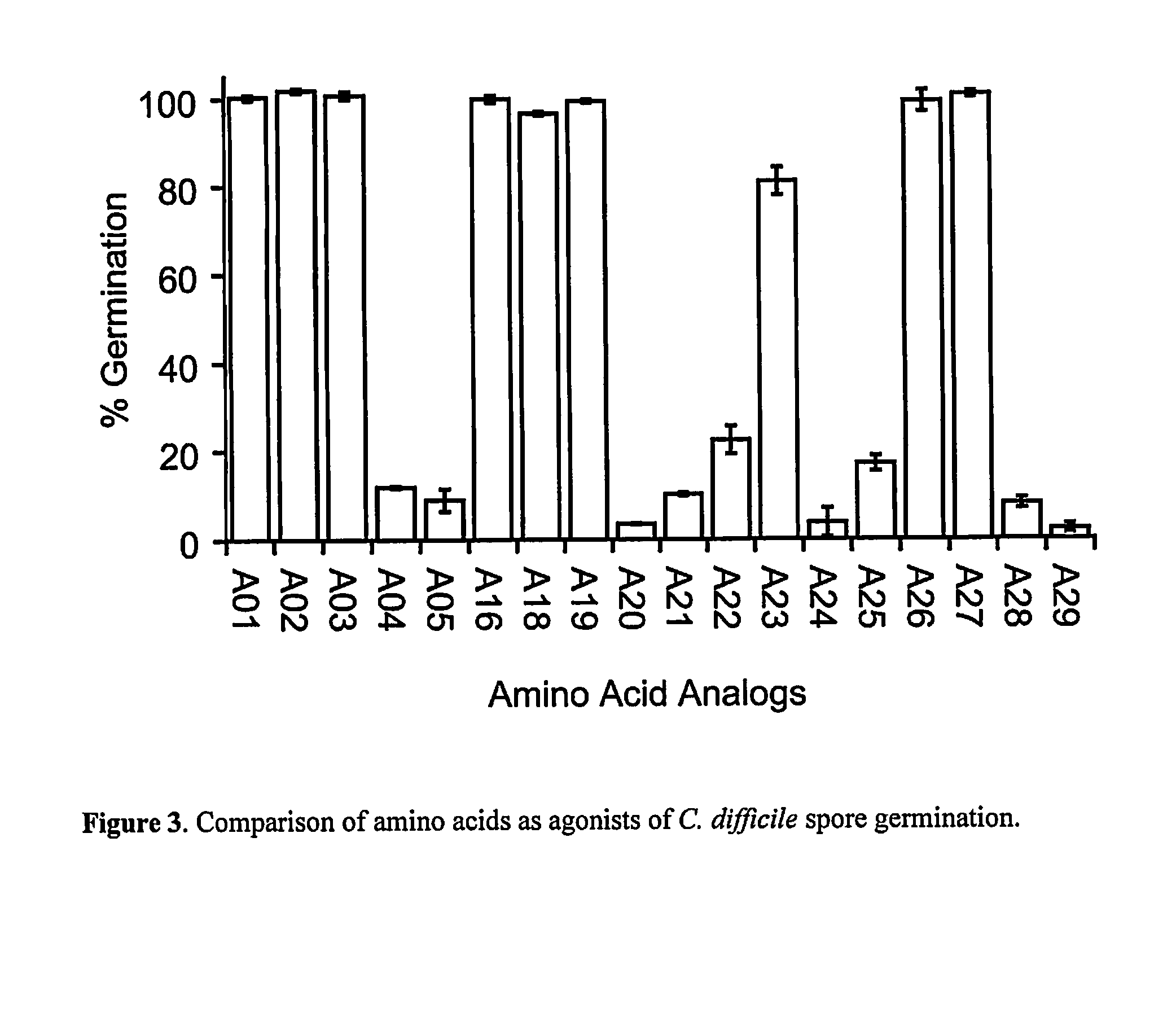
Reducing Risk of Contracting Clostridium-Difficile Associated Disease
ActiveUS20140045808A1Reduce development riskOrganic active ingredientsBiocideClostridium difficileCvd risk
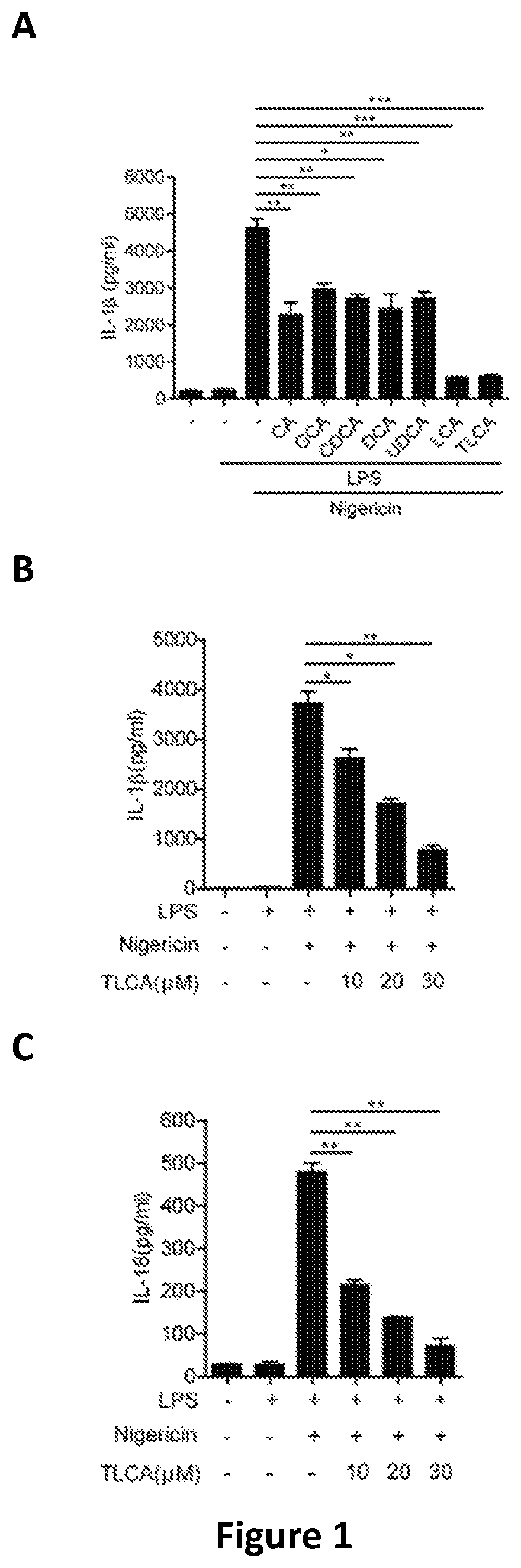
A method of treating a patient to reduce risk of developing Clostridium difficile-associated disease or reducing existing Clostridium difficile-associated disease in a mammalian subject involves administering to a mammalian subject an effective amount of a germination-inhibiting compound derived from taurocholate. Novel compounds of this class are also provided.
Owner:BOARD OF RGT NEVADA SYST OF HIGHER EDUCATION ON BEHALF OF THE UNIV OF NEVADA RENO
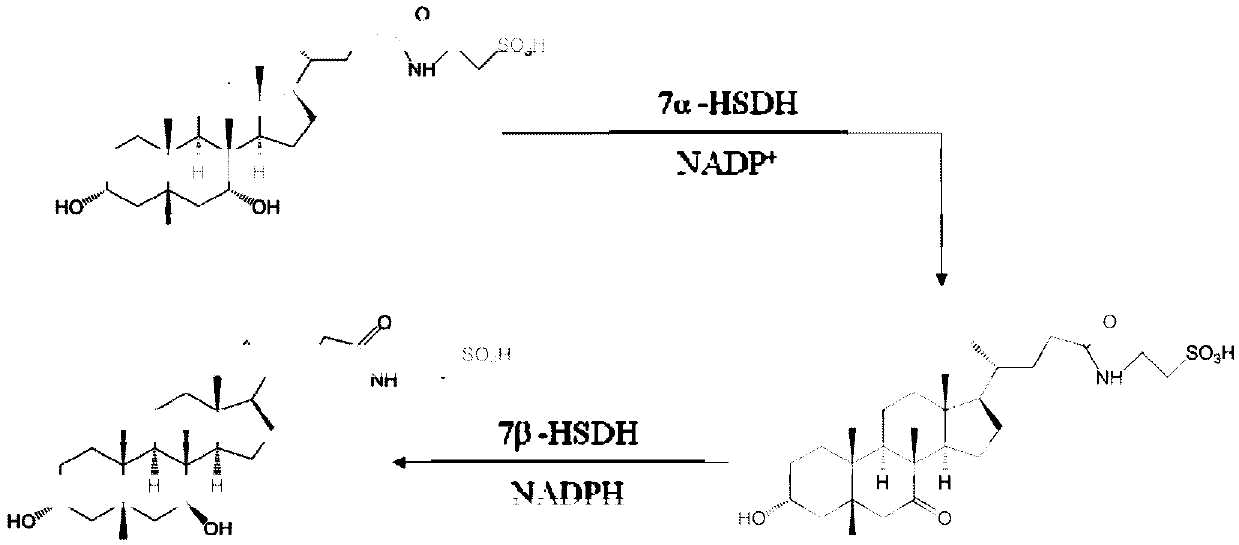
7 alpha-hydroxysteroid dehydrogenase as well as coding gene and application thereof
ActiveCN108034643ACatalytic asymmetric reduction reactionOxidoreductasesFermentationNucleotideKetone
The invention discloses 7 alpha-hydroxysteroid dehydrogenase as well as a coding gene and application thereof. An amino acid sequence of the 7 alpha-hydroxysteroid dehydrogenase provided by the invention is shown as SEQ ID NO. 1, and a nucleotide sequence of the 7 alpha-hydroxysteroid dehydrogenase gene is disclosed and shown as SEQ ID NO. 2. The 7 alpha-hydroxysteroid dehydrogenase can catalyze asubstrate taurocholic acid (TCA) to generate taurine 7-ketone cholic acid (T7K-CA), catalyze a substrate glycocholic acid (GCA) to generate glycine 7-ketone cholic acid (G7K-CA), catalyze a substratetaurochenodeoxycholic acid (TCDCA) to generate taurine 7-ketone lithocholic acid (T7K-LCA), catalyze a substrate glycochenodeoxycholic acid (GCDCA) to generate glycine 7-ketone lithocholic acid (G7K-LCA) and catalyze a substrate ethyl benzoylformate (EB) to generate ethyl 2-hydroxy-2-phenylacetate, and has better catalytic activity, has a catalytic activity to TCDCA, GCDCA and EB 10, 5, and 3 times that of sardinia clostridium 7 alpha-HSDH, correspondingly, and has great industrial application value.
Owner:CHONGQING UNIV
Kit, preparation method of kit and method for detecting peripheral blood glycocholic acid through kit
ActiveCN106226512ALow specificityHigh detection sensitivityColor/spectral properties measurementsTaurocholic acidTaurine
The invention discloses a kit. The kit comprises a reagent R1 and a reagent R2, the reagent R1 contains a trihydroxymethyl aminomethane (Tris-HCL) buffer solution, an anti-taurine antibody, an anti-glycocholic acid antibody and glucose-6-phosphate (G6P), and the reagent R2 contains the trihydroxymethyl aminomethane (Tris-HCL) buffer solution, a glycocholic acid-G-6-PD conjugate, NADP and BSA. The invention further discloses a preparation method of the kit, particularly discloses a preparation method of the anti-taurine antibody the anti-glycocholic acid antibody and further discloses a method for detecting peripheral blood glycocholic acid through the kit. Accordingly, interference of taurocholic acid can be avoided, the detection sensitivity reaches up to 0.1 microgram per milliliter, and the advantages of high specificity and detection repeatability, good stability and the like are achieved.
Owner:杭州利安生物科技有限公司 +1
A kit, a preparation method of the kit, and a detection method of glycocholic acid in peripheral blood realized by using the kit
ActiveCN106226512BLow specificityClosed interferenceColor/spectral properties measurementsTaurocholic acidTaurine
The invention discloses a kit. The kit comprises a reagent R1 and a reagent R2, the reagent R1 contains a trihydroxymethyl aminomethane (Tris-HCL) buffer solution, an anti-taurine antibody, an anti-glycocholic acid antibody and glucose-6-phosphate (G6P), and the reagent R2 contains the trihydroxymethyl aminomethane (Tris-HCL) buffer solution, a glycocholic acid-G-6-PD conjugate, NADP and BSA. The invention further discloses a preparation method of the kit, particularly discloses a preparation method of the anti-taurine antibody the anti-glycocholic acid antibody and further discloses a method for detecting peripheral blood glycocholic acid through the kit. Accordingly, interference of taurocholic acid can be avoided, the detection sensitivity reaches up to 0.1 microgram per milliliter, and the advantages of high specificity and detection repeatability, good stability and the like are achieved.
Owner:杭州利安生物科技有限公司 +1
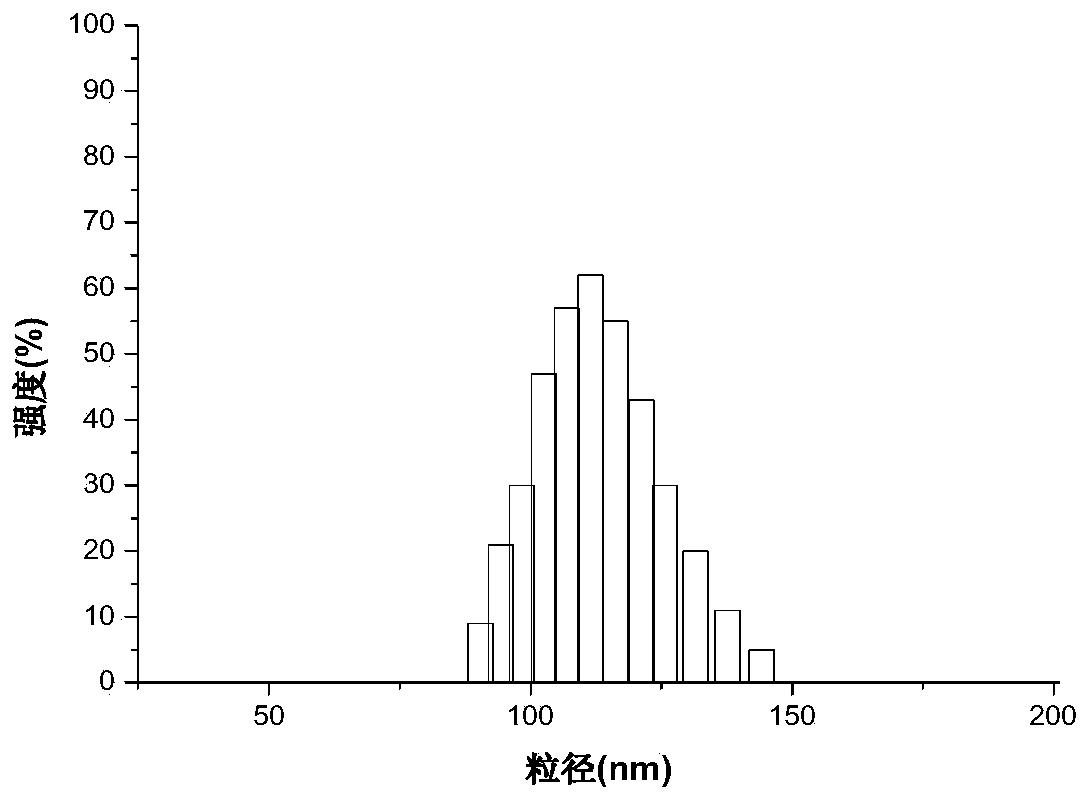
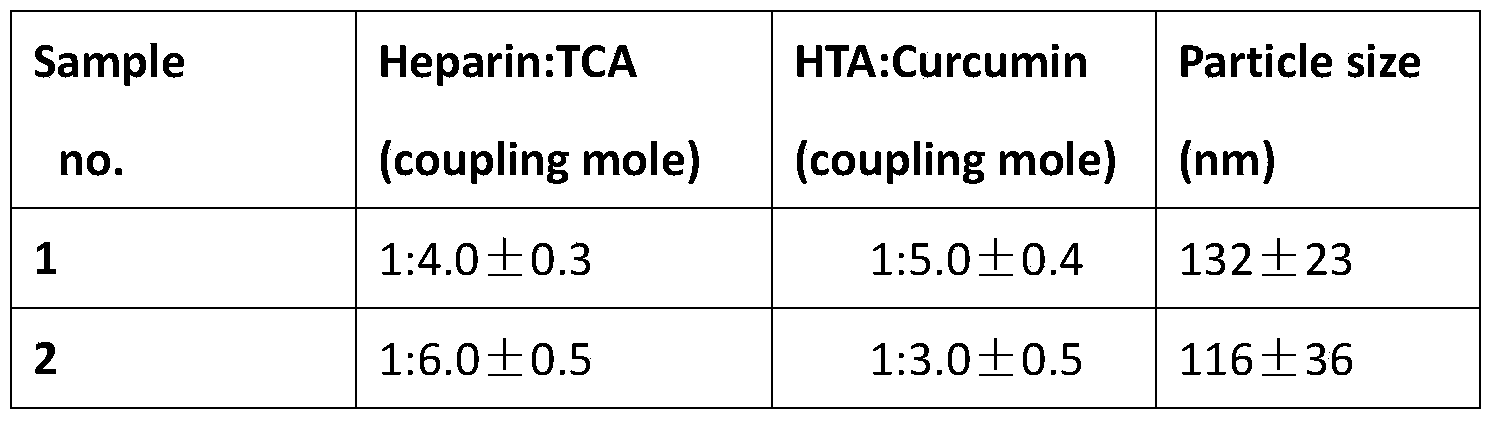
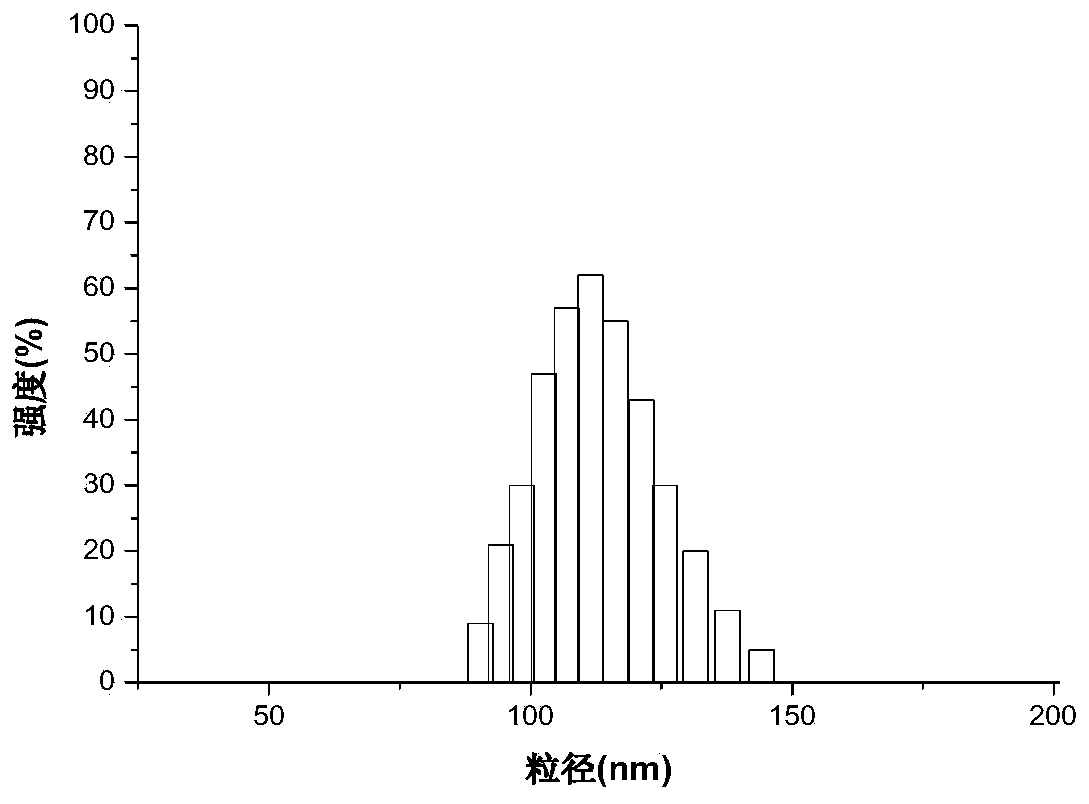
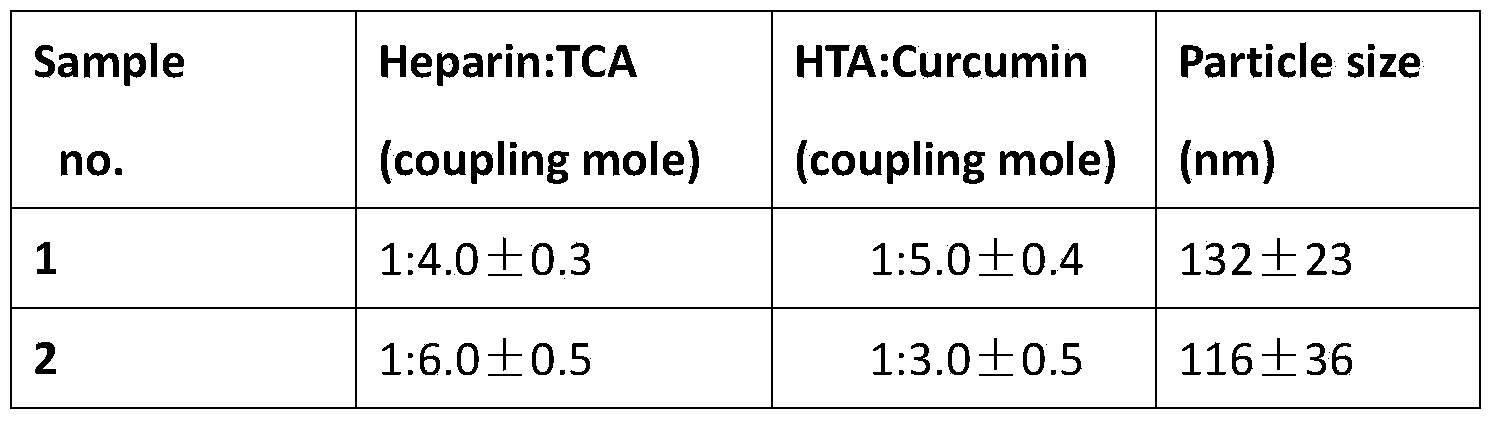
Oral curcumin nano-granule and preparation method thereof
InactiveCN103446057BGood water solubilityOvercome the disadvantage of low bioavailabilityPowder deliveryNervous disorderSolubilityThrombus
Owner:THE FIRST PEOPLES HOSPITAL OF CHANGZHOU +1
Method for extracting taurocholic acid in sheep bile through column chromatography isolation method
The invention discloses a method for extracting taurocholic acid in sheep bile through a column chromatography isolation method. The method comprises the following steps: filtering, performing protein precipitation treatment, performing depigmentation treatment, salting out, extracting, dewatering concentration and performing column chromatography isolation. According to the method, the sheep bile serves as an extracting raw material, the extraction cost of the taurocholic acid is reduced, the extraction process is simple, and the income is higher. The taurocholic acid is extracted through the column chromatography isolation, the column chromatography isolation is good in purification effect, the extracted taurocholic acid does not have a fishy smell and is high in purity, the product is high in yield, the extraction process is simple, and industrial production is promoted.
Owner:安徽九凤生物科技有限公司
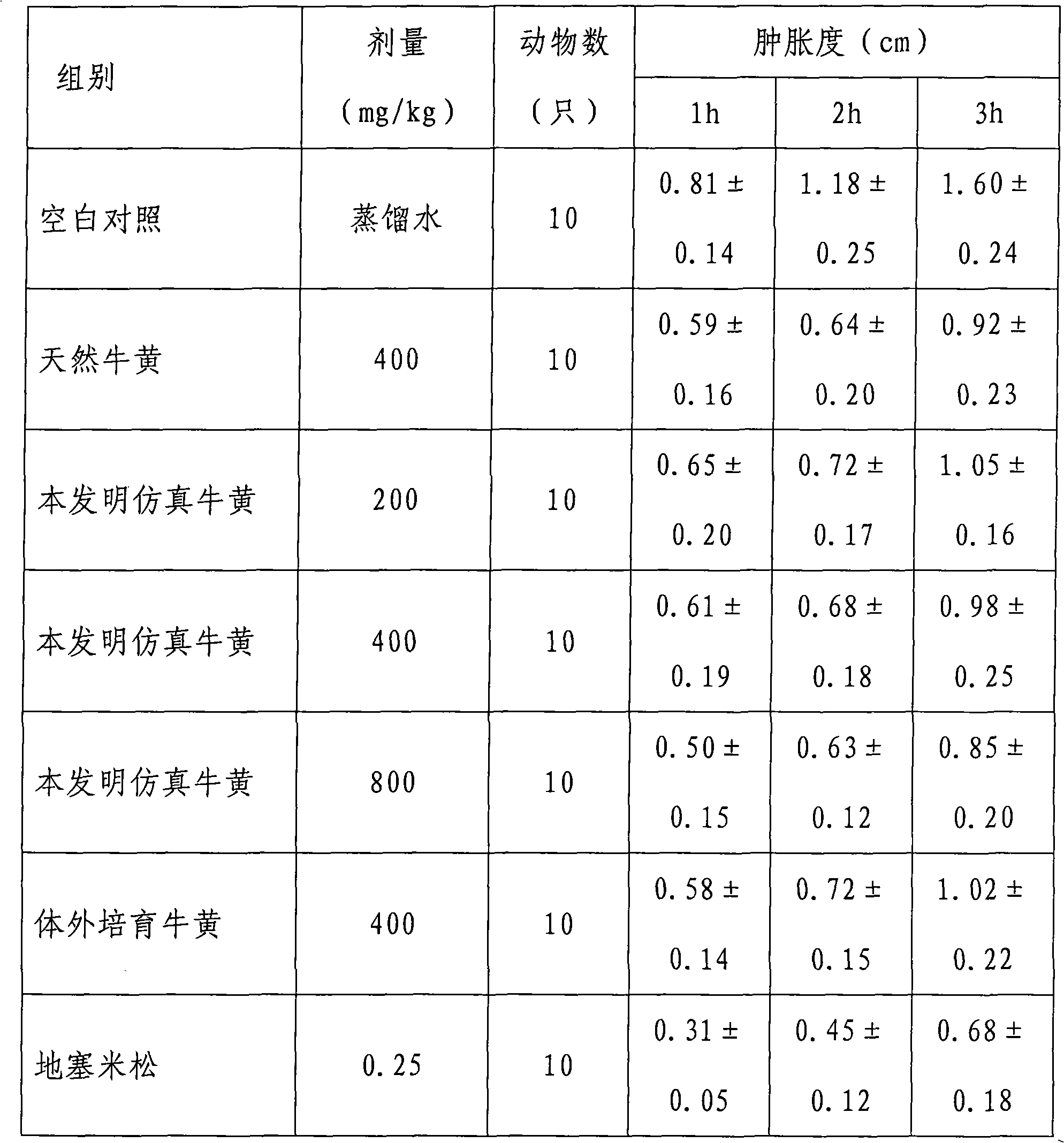
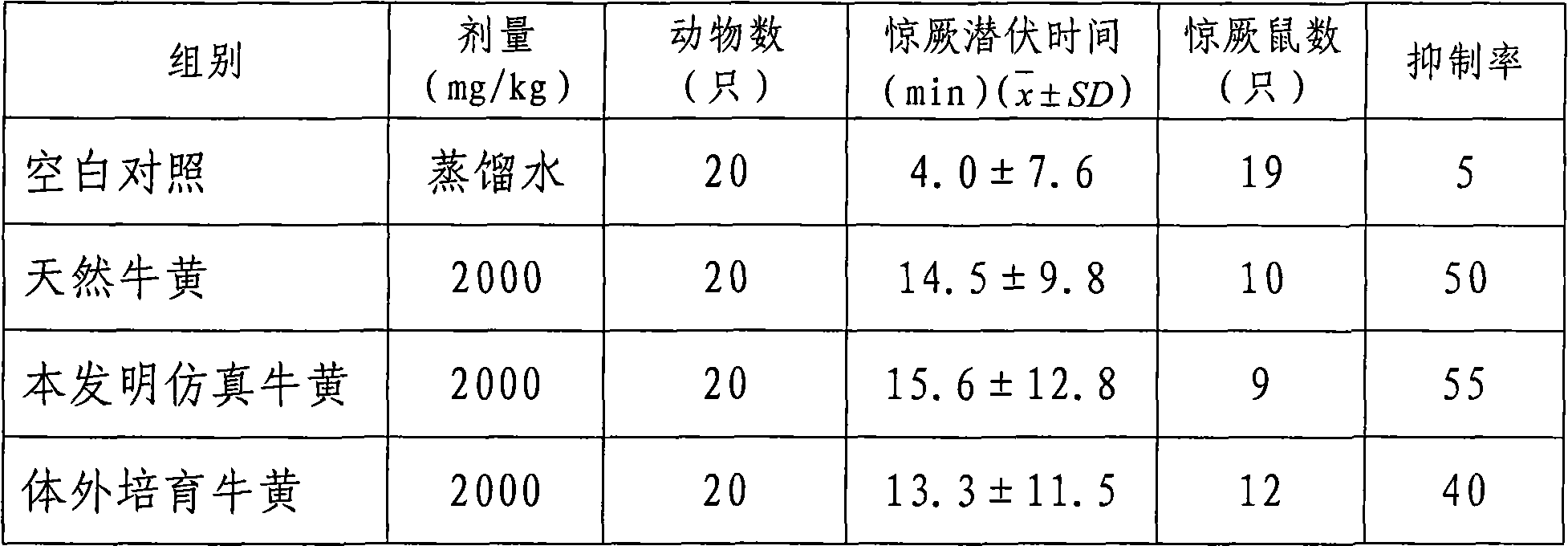
Medicinal simulation calculus bovis and manufacturing method thereof
The invention provides a medicinal simulation calculus bovis; compared with the existing other artificial calculus bovis, the medicinal simulation calculus bovis is characterized in that the medicinal simulation calculus bovis uses calcium bilirubinate as main component, a relatively large amount of conjugated bile acid (glycocholic acid, taurocholic acid, and the like) is added, proteins (comprising mucoprotein, polypeptide, and the like) and lecithin are used as component substances, the hyodeoxycholic acid in the traditional formula of artificial calculus bovis is removed so as to reduce the ratio of free cholesteric acid, the technology of preparing calcium bilirubinate with a bacterial fermentation method is eliminated and starch is removed as accessory, and the compound ratio and existing manner in the formula is almost completely the same as natural calculus bovis. The medicinal simulation calculus bovis has better drug effect than the existing other artificial calculus bovis.
Owner:石朝周
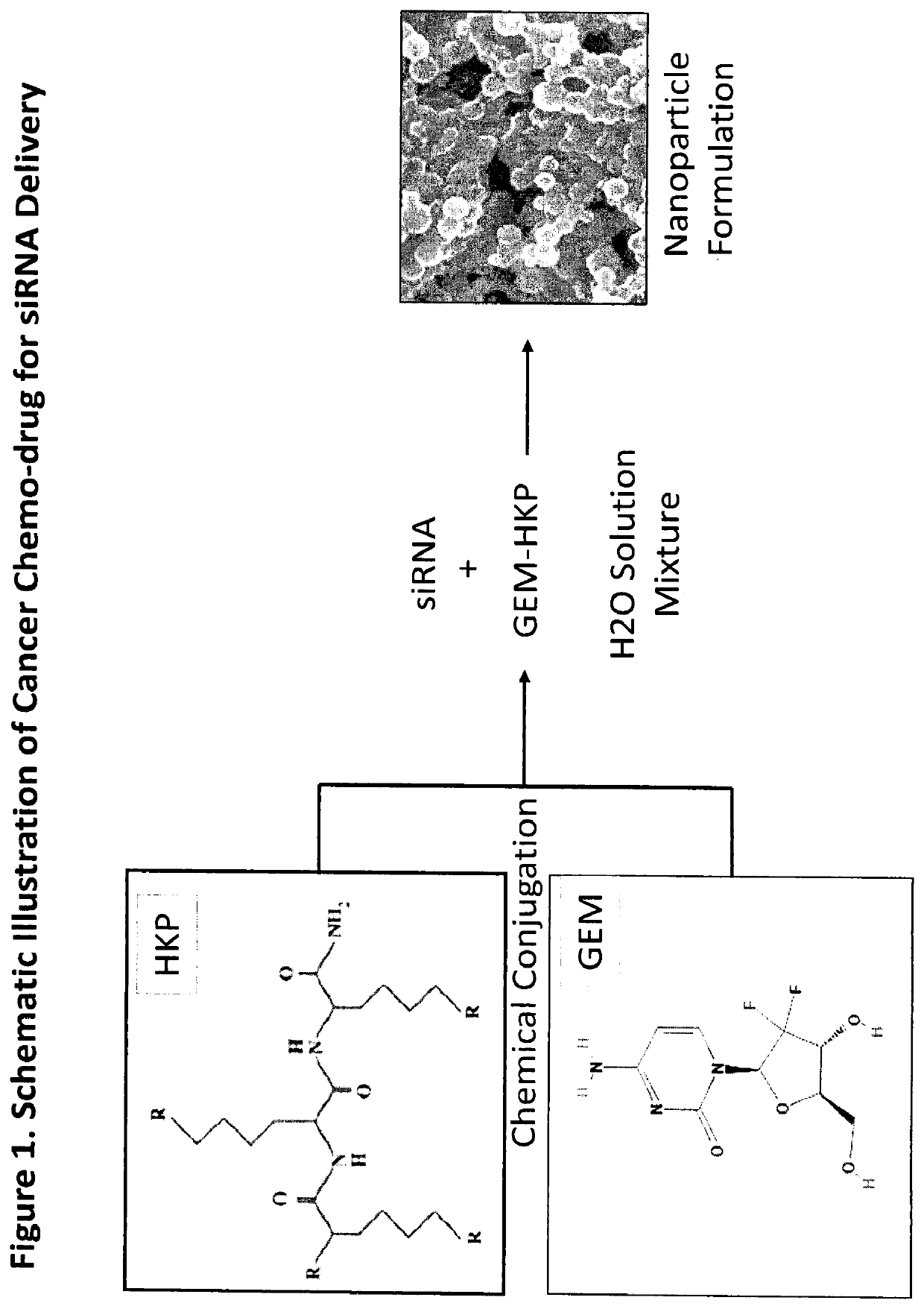
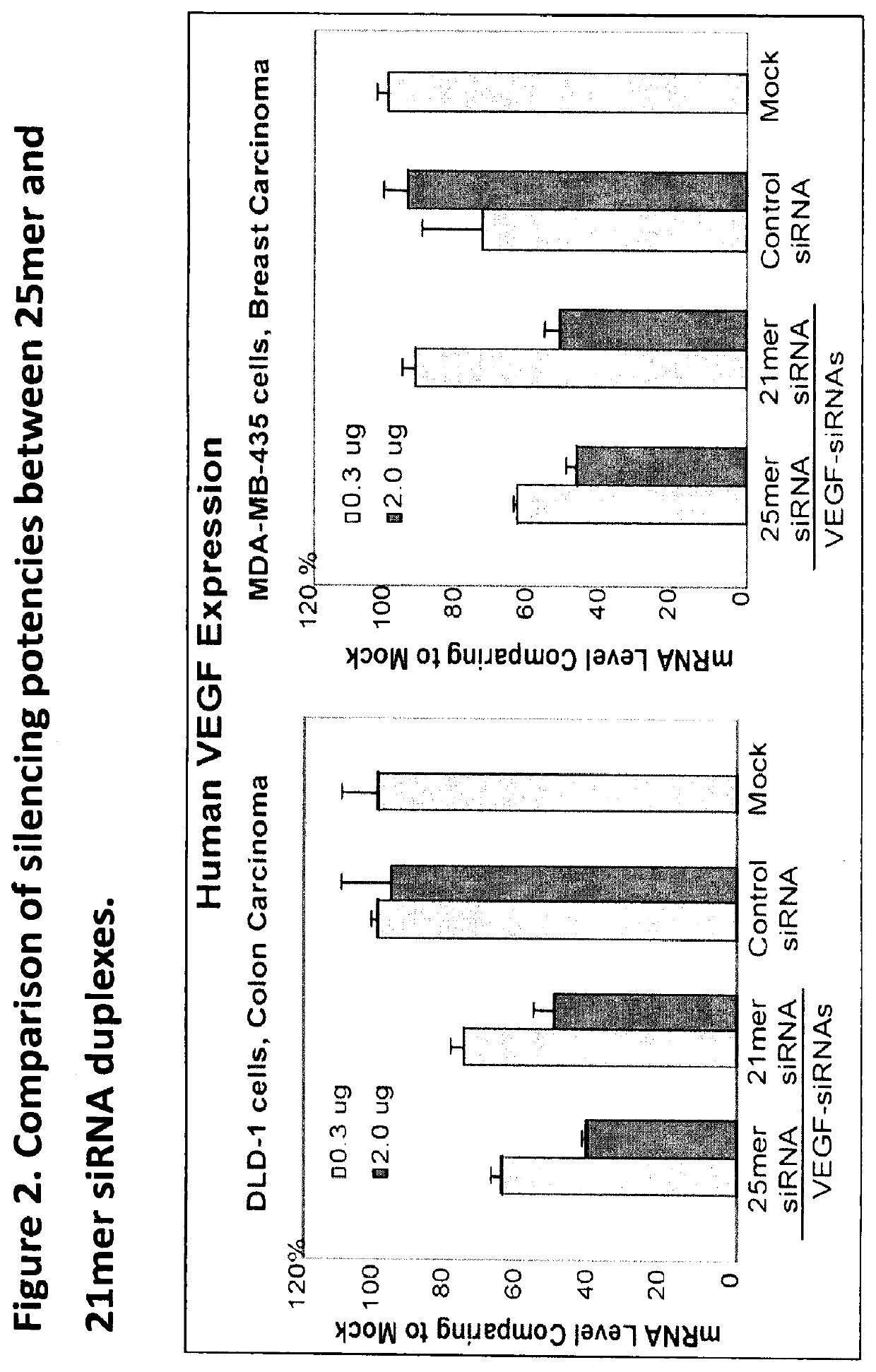
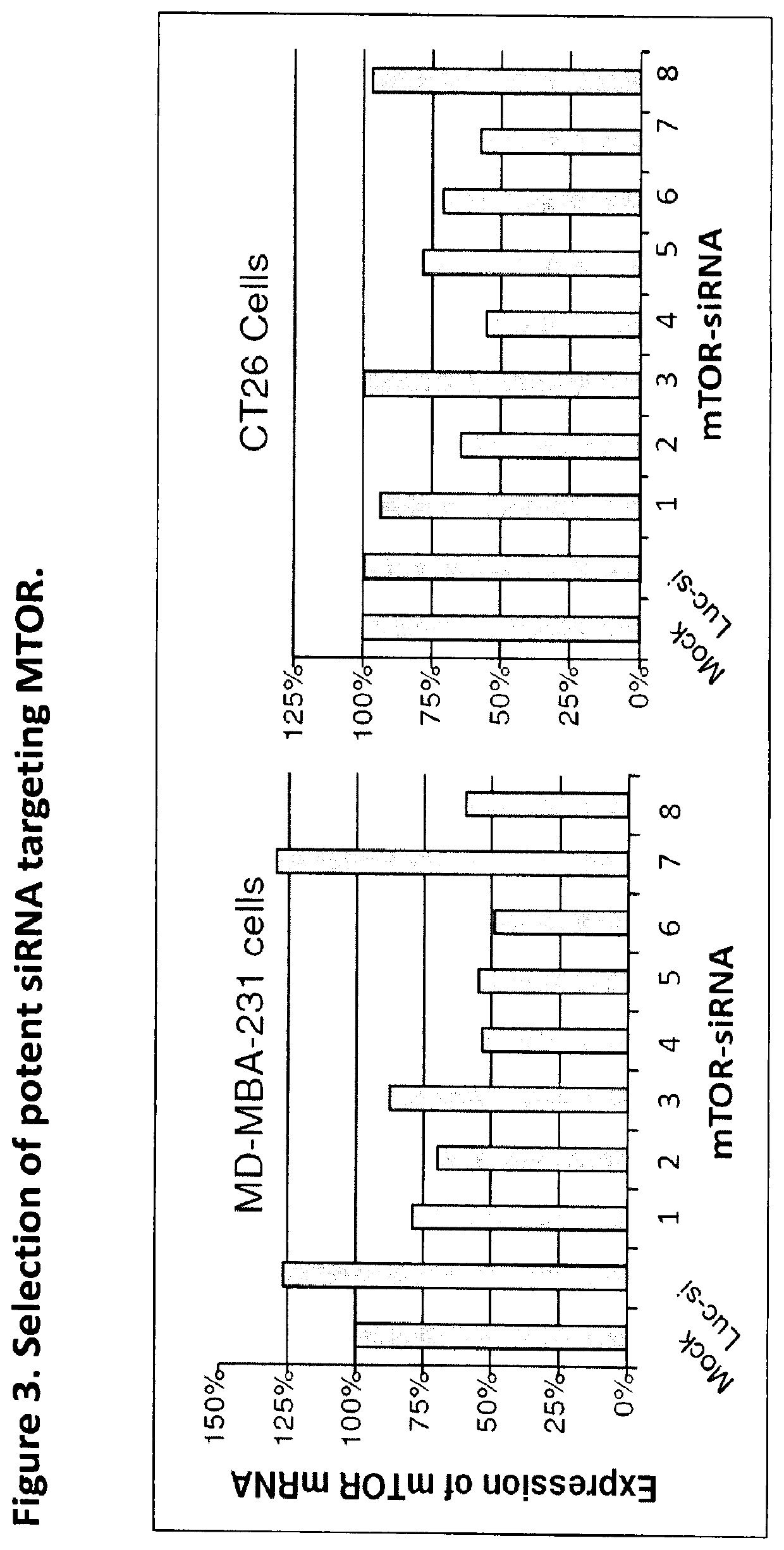
Gemcitabine Derivatives for Cancer Therapy
The present invention provides pharmaceutical compositions comprising the chemotherapy drug gemcitabine (GEM) and certain derivatives, a taurocholic acid (TCA) formulation, and a Histidine-Lysine Polymer (HKP) conjugate, for enhancement of RNAi cancer therapeutics.
Owner:SIRNAOMICS INC
Oral curcumin nano-granule and preparation method thereof
InactiveCN103446057AGood water solubilityOvercome the disadvantage of low bioavailabilityPowder deliveryNervous disorderSolubilityBile acid transport
The invention belongs to the technical field of pharmaceutical preparation and particularly relates to an oral curcumin nano-granule and a preparation method thereof. The nano-granule is prepared by self-assembling a ternary conjugated compound which is obtained by connecting taurocholic acid, heparin and curcumin through amido bonds, the curcumin is a core of the nano-granule, and the hydrophilic taurocholic acid is on the surface of the nano-granule. The oral curcumin nano-granule overcomes the poor water solubility of curcumin, the absorption of the nano-granule can be enhanced by utilizing the interaction of taurocholic acid and bile acid transporter of small intestine, the defect that the bioavailability of the oral curcumin is low can be overcome, and the heparin also has anti-thrombosis and anti-angiogenesis effect.
Owner:THE FIRST PEOPLES HOSPITAL OF CHANGZHOU +1
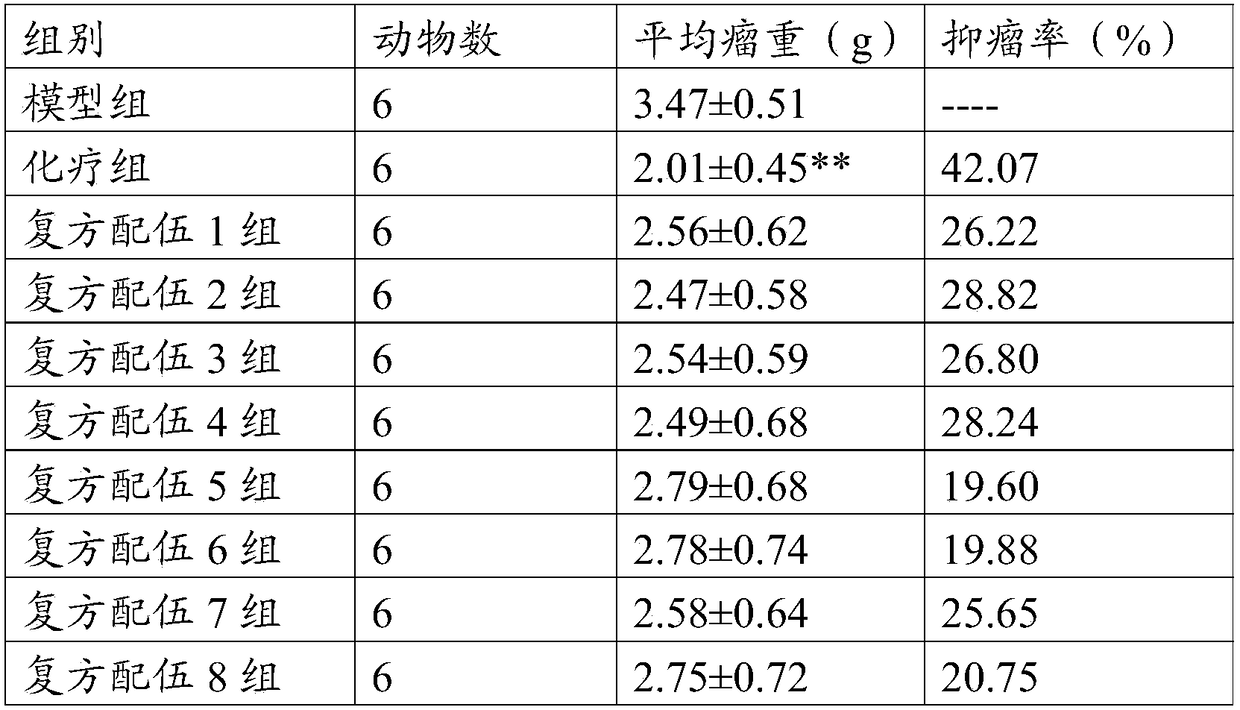
Antitumor traditional Chinese medicinal composition and antitumor active component composition, and use and preparation thereof
InactiveCN108553495AReduce tumor weightImprove the quality of lifeOrganic active ingredientsMammal material medical ingredientsCholic acidPANAX NOTOGINSENG ROOT
The invention relates to a traditional Chinese medicinal composition, and concretely relates to an antitumor traditional Chinese medicinal composition and an antitumor active component composition, and a use and a preparation thereof. The antitumor traditional Chinese medicinal composition comprises, by weight, 30-60 parts of Panax Notoginseng, 5-15 parts of artificial bezoar, 1-8 parts of artificial musk and 1-10 parts of snake galls. The antitumor active component composition comprises, by weight, 10-30 parts of Panax notoginseng saponins, 1-8 parts of cholic acid, 0.1-1 part of musk ketoneand 0.1-1 part of taurocholic acid. The traditional Chinese medicinal composition or the active component composition has the advantages of low price, clear components, clear mechanism, accurate dosage, safety and effectiveness, and is used for preventing or treating various malignant tumors.
Owner:FUJIAN UNIV OF TRADITIONAL CHINESE MEDICINE
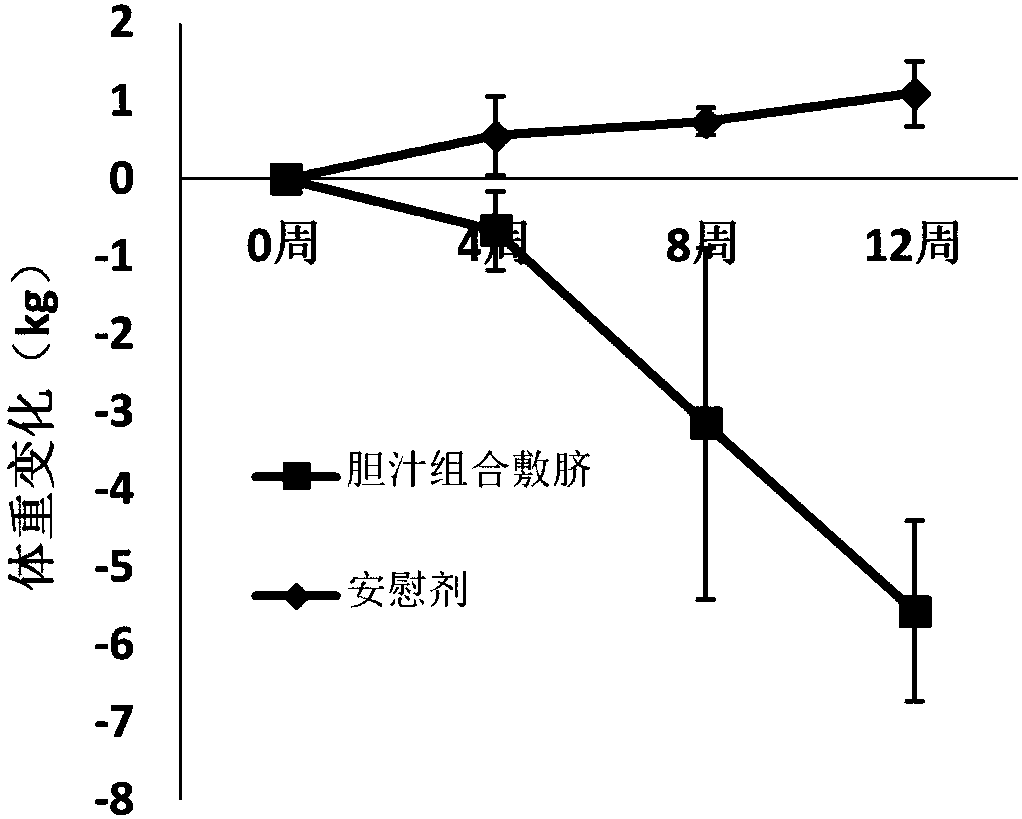
Composition applied to navel for preventing and treating obesity as well as preparation method and application method
InactiveCN108042785AHigh expressionDelayed gastric emptyingMetabolism disorderUnknown materialsSide effectNavel
The invention provides a composition applied on a navel for preventing and treating obesity, belonging to the technical field of obesity treatment. The composition is prepared from cattle bitter gallpowder, pig bitter gall powder, radix et rhizoma rhei and ginger juice; the weight ratio of the cattle bitter gall powder to the pig bitter gall powder to the radix et rhizoma rhei is (10-20) to (10-20) to (1-2), the ratio of the mass of the cattle bitter gall powder to the volume of the ginger juice is (10-20)g to (2-5)ml; the cattle bitter gall powder is prepared from taurocholic acid, calculusbovis deoxycholic acid, glycocholic acid and glycodeoxycholic acid hydrate; the pig bitter gall powder is prepared from hyocholic acid and hyodeoxycholic acid. The composition for preventing and treating obesity can be used for reducing weight, and any side effects are not seen.
Owner:张利生
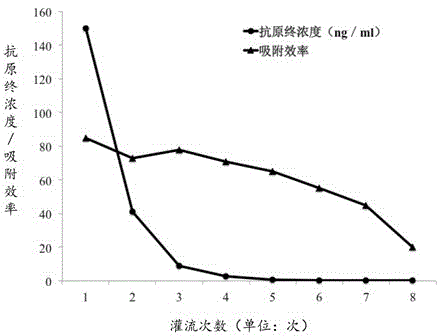
Adsorbing material of hepatitis B antigen protein and preparation method of material
ActiveCN103111270BLow chance of rejectionReduce the risk of immune rejectionOther chemical processesSolid sorbent liquid separationSynthesis methodsSepharose
The invention relates to a specific adsorbing material for removing hepatitis B virus protein in blood plasma, and a preparation method of the adsorbing material, and discloses a high polymer material which is prepared by coupling sepharose gel with gene-modified sodion taurocholic acid cotransporting polypeptide. The preparation method of the material comprises the steps of reacting the sepharose gel, which is taken as a carrier matrix, with a bi-glycidyl ether coupling reagent to obtain an active carrier, and subsequently, conducting coupling reaction on the active carrier with the sodion taurocholic acid cotransporting polypeptide which is fused with polypeptide tags. The synthesis method of the material is simple and convenient, the process route is short and the preparation is safe; and the material has the characteristics of strong specificity, high adsorption rate to the hepatitis B virus protein, good regenerability, harmlessness and no heat source reaction, and can be applied to clinical adsorption treatment to eliminate the hepatitis B virus protein in blood plasma in a specificity mode.
Owner:WUHAN RUIFA MEDICAL DEVICES CO LTD
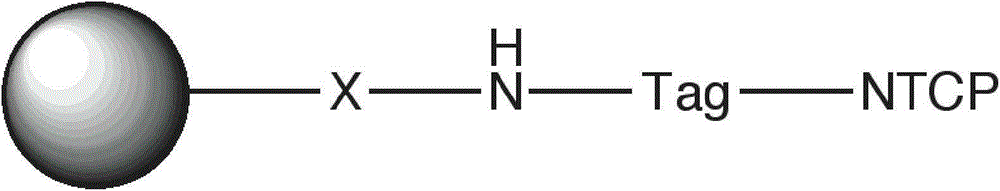
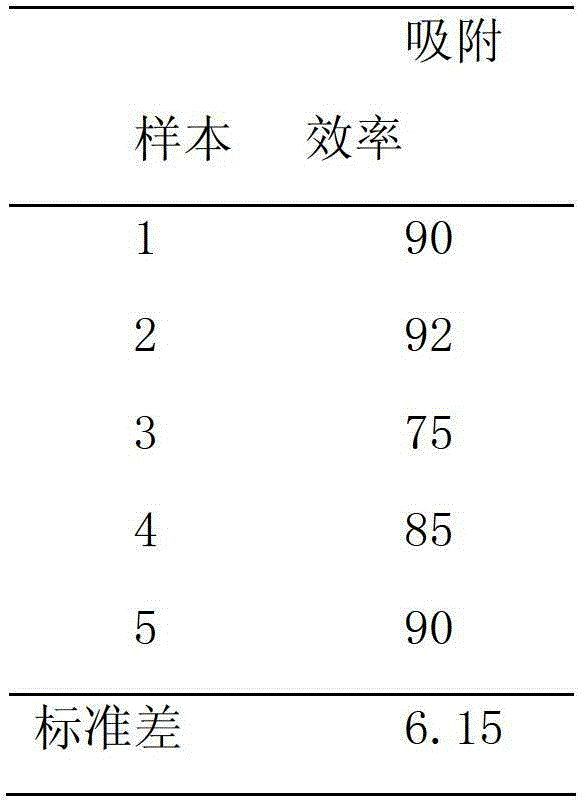
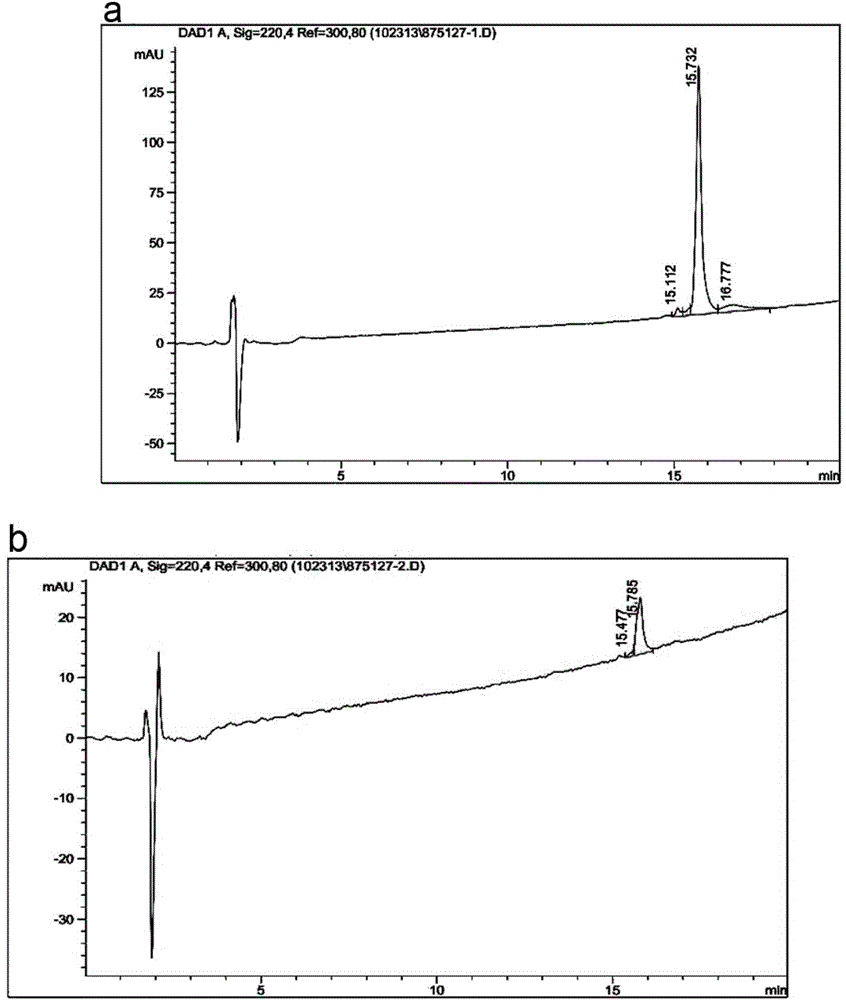
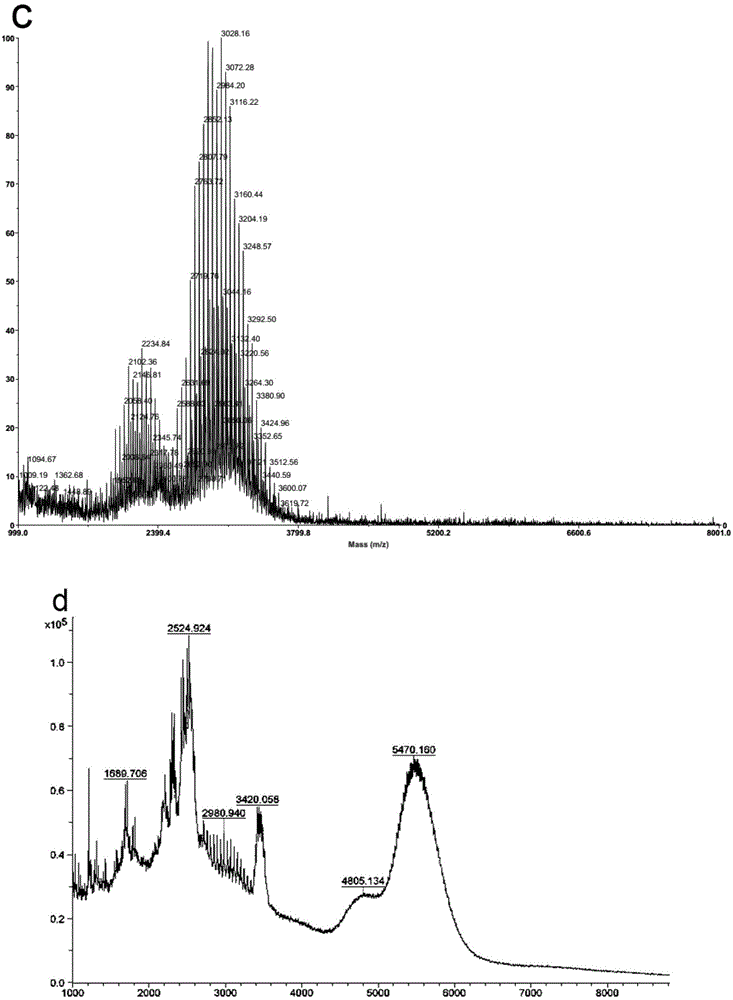
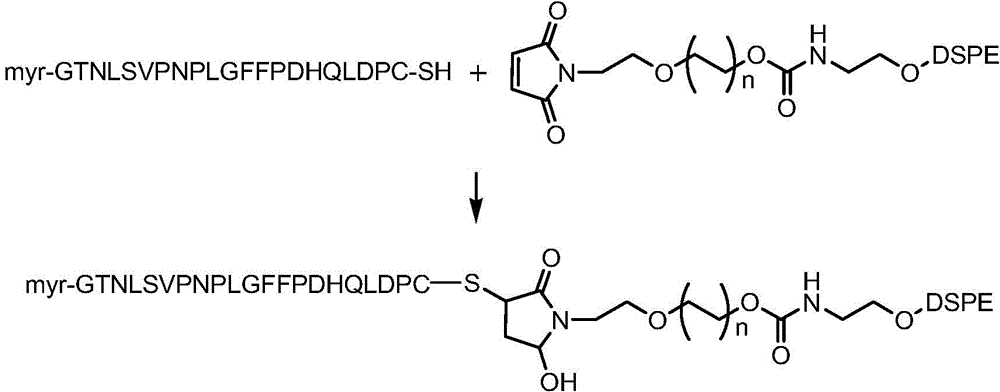
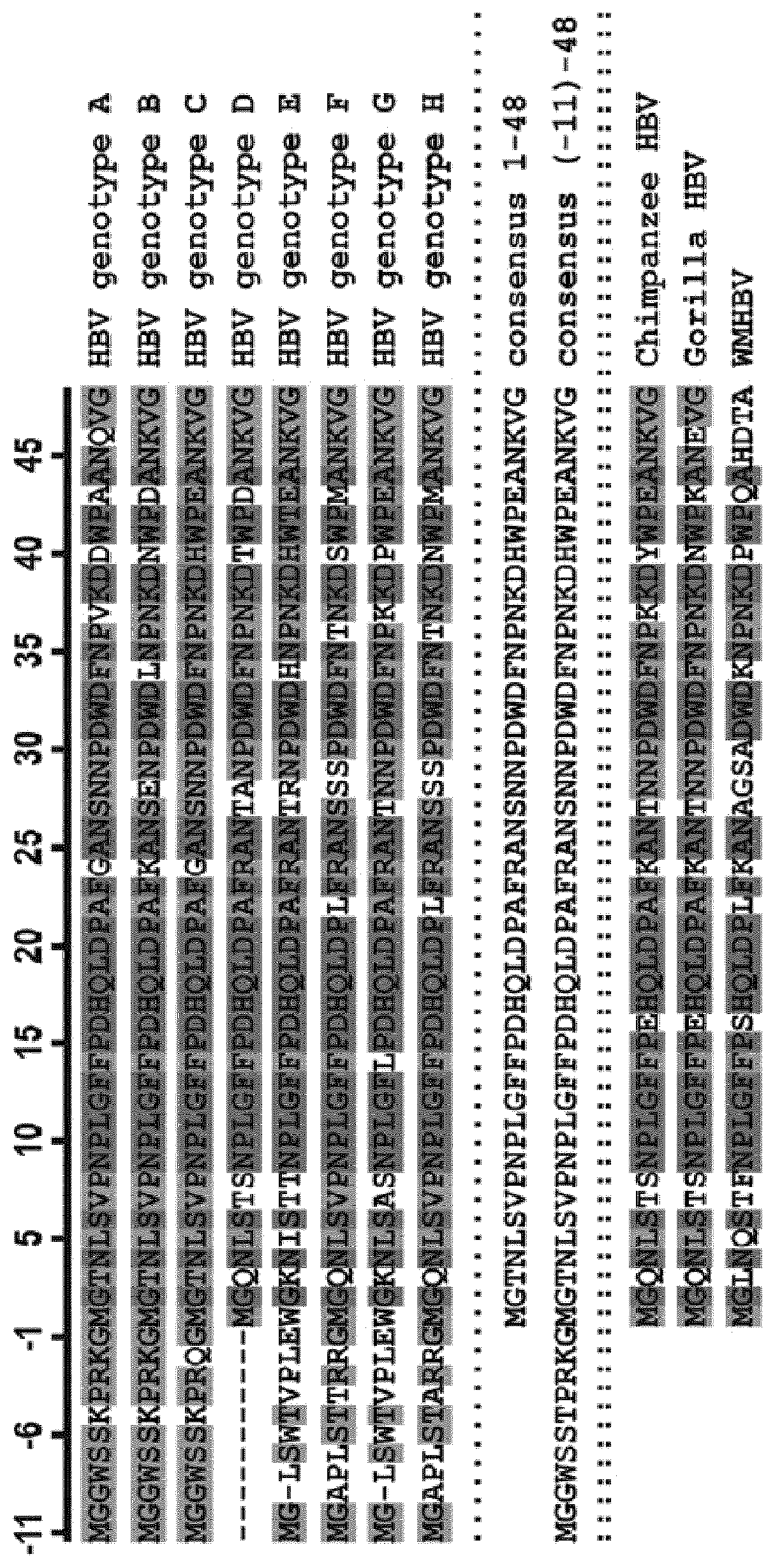
Preparation of targeted liposome and application thereof
The present invention relates to preparation of target liposom and uses thereof, particularly to the target hepatocellular compound of formula 1, the composition and pharmaceutical composition containing the compound for delivering drugs. In formula 1, X is a polypeptide derived from hepatitis B virus adventitial protein PreS1 zone, specifically binding with natrium ion-taurocholic acid co-transferred polypeptide (NTCP); B is a maleimide-polyethyleneglycol-stearoyl phosphatidyl ethanolamine polymer represented by formula 2; in formula 2, n is an integer of 2-100, DSPE is stearoyl phosphatidyl ethanolamine; X is linked with B by the maleimide portion of formula 2.
Owner:SHANGHAI JIBEI PHARMA TECH
Novel sodium taurocholate cotransporting polypeptide (NTCP) inhibitor
The invention relates to synthesis of a novel sodium taurocholate cotransporting polypeptide (NTCP) inhibitor and inhibiting effects of the inhibitor on the NTCP. The experimental research indicates that the compound has high inhibition activity for NTCP and indicates directions for antivirus, anti-bile siltation and metabolic regulation effects of anti-bile acid transporting drugs. The compound or composition thereof is hopeful to be developed into a novel NTCP inhibitor with great potential.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI +1
Combination therapy of hbv and hdv infection
InactiveUS20180228804A1SsRNA viruses negative-senseCell receptors/surface-antigens/surface-determinantsChronic hepatitisInterferon alpha
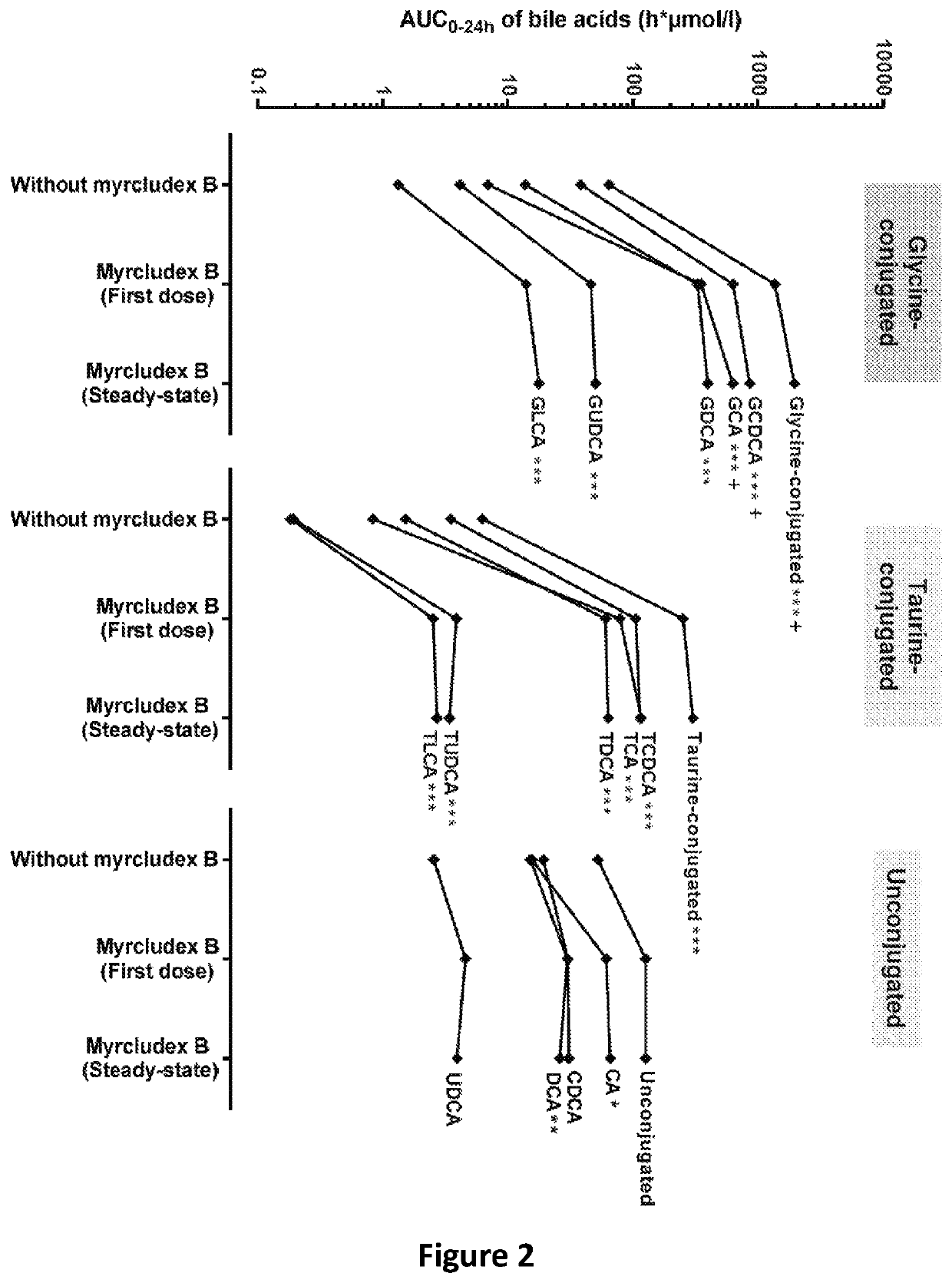
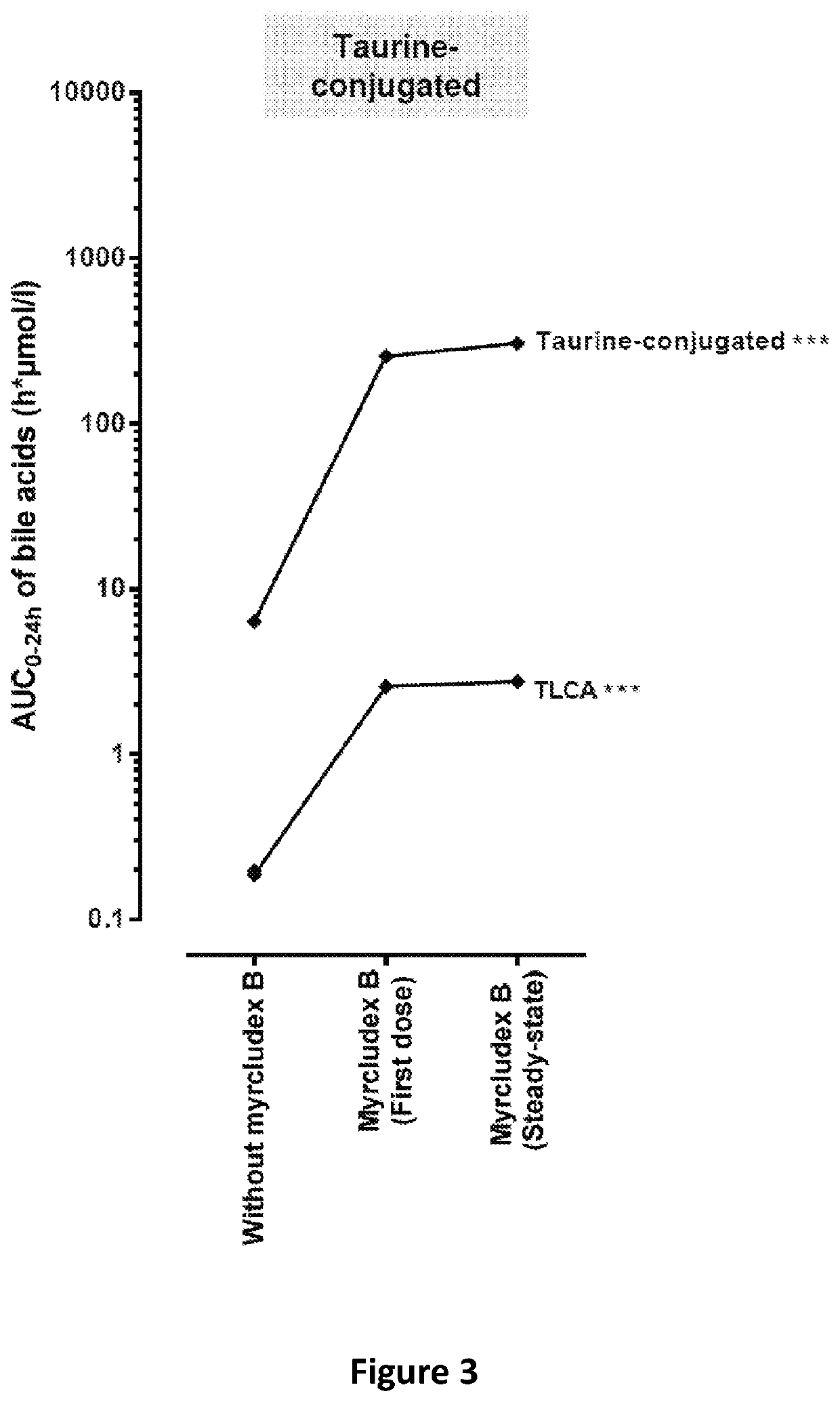
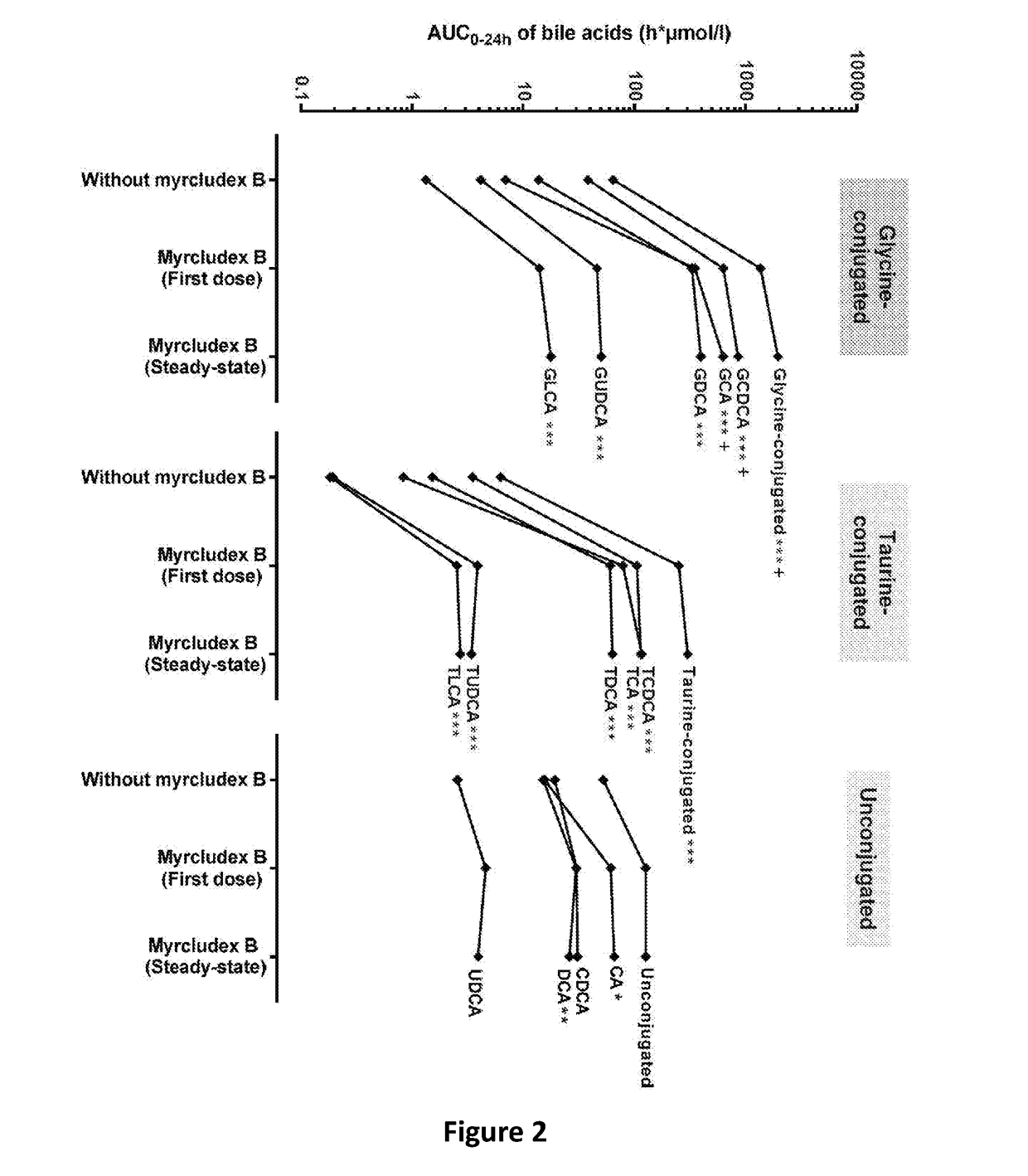
The invention provides a composition comprising an inhibitor of Na+-taurocholate cotransporting polypeptide (NTCP) and a active ingredient selected from the group consisting of a nucleoside analogue such as lamivudine, telbivudine, or entecavir, a nucleotide analogue such as tenofovir, adefovir and an immunomodulator such as interferon alpha. The NTCP inhibitor inhibits HBV / HDV entry into a cell and is preferably derived from an HBV pre-S1 peptide. Also provided are methods of treating HBV and HDV infection, hepatitis B and D, or chronic hepatitis B and D.
Owner:MYR
Therapy of atherosclerosis, primary biliary cirrhosis and NRLP3 inflammasome-associated disease by HTCP inhibitors
An inhibitor of Na+-taurocholate cotransporting polypeptide (NTCP) for use in a method of treatment of primary biliary cirrhosis, atherosclerosis, or an NRLP3 inflammasome-associated disease in a subject.
Owner:MYR
Compound oral solution with pholcodine
PendingCN106310219AImprove autoimmunityDefinite curative effectOrganic active ingredientsPharmaceutical delivery mechanismBlueberry extractSide effect
The invention discloses a compound oral solution containing pholcodine, prepared from, by weight, 4-6 arts of pholcodine, 6-8 parts of triprolidine, 2-4 parts of glutathione, 15-20 parts of radix isatidis, 5-12 parts of stevioside, 8-12 parts of artemisia apiacea, 4-10 parts of fresh ginger, 4-8 parts of andrographis paniculata, 4-8 parts of semen ziziphi spinosae, 2-6 parts of liquorice root, 1-2 parts of taurocholic acid, 2-4 parts of honey, 0.2-0.4 parts of Pu-erh tea extract, 0.2-0.4 parts of celery leaf extract, and 0.2-0.4 parts of blueberry extract; Compared with the prior technology, the compound oral solution has synergistic effect between the raw material components, the treatment effect is well with quick effect, the drug resistance is small, the medicinal safety is high, the side effect is small, the stability is good, the shelf life is long , which is easy to carry, the preparing process is simple, suitable for large-scale production and so on.
Owner:南昌立健药业有限公司
Production process of Niuchan granules with good palatability
InactiveCN107929328AReduces unpleasant taste effectsReduce taste impactAmphibian material medical ingredientsHydroxy compound active ingredientsBetaineFiltration
The invention discloses a production process of Niuchan granules with good palatability. According to the present invention, at the clear paste preparation stage during Niuchan granule production, a clear paste and a beta-cyclodextrin saturated aqueous solution are uniformly mixed and stirred, cold preservation, filtration and drying are performed to obtain a beta-cyclodextrin inclusion compound,the beta-cyclodextrin inclusion compound, betaine, fishy attractant and sodium glutamate are uniformly mixed according to a mass ratio of 97:1.5:1:0.5, and a required auxiliary is added to prepare theNiuchan granules; by using the molecular coating technology, taurocholic acid affecting the palatability, venenum bufonis and borneol are coated with beta-cyclodextrin while the flavoring agent is added, such that the poor taste of the Niuchan granules is significantly reduced; with the application of the molecular coating technology to prepare the Niuchan granules, the stability of the drug is increased, and the adverse effects of the oxidation effect, the temperature, the pH value and other factors on the effective components are avoided; and the production process is suitable for the veterinary dosage forms of known Niuchan granules with different treatment purposes and different use methods.
Owner:广西普大动物保健品有限公司
A kind of synthetic method of taurocholic acid
ActiveCN103755764BThe synthesis method is simpleSimple processSteroidsCholic acidEthyl chloroformate
The invention discloses a synthetic method of taurocholic acid. The method comprises the following steps: A, mixing and stirring cholic acid with acetone and cooling to -10 to 30 DEG C, adding organic alkali, dripping ethyl chloroformate below 30 DEG C after evenly stirring, reacting for 1-3 hours after dripping is finished, and filtering to obtain filtrate; B, adding the filtrate in the step A to a solution formed by taurine and water, adjusting the pH to 7-11 by using organic alkali, then reacting at 20-30 DEG C for 3-5 hours, and filtering to obtain filtrate after reaction is finished; C, adjusting the pH of the filtrate in the step B to below 1 by using 10-30% of hydrochloric acid, concentrating in vacuum at 40-65 DEG C to dry, adding methanol, heating for dissolving, adding acetone to evenly stir, and cooling to -5 to 0 DEG C for crystallizing; filtering after crystallization is finished; washing a filter cake by acetone, draining and drying in vacuum. The invention aims at providing the synthetic method of taurocholic acid, which is simple in technology, relatively low in production cost, high in yield of taurocholic acid, and convenient for industrial production.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Immunomodulator for treating colorectal cancer lung metastasis and composition for predicting and detecting immunomodulator
PendingCN114588265AThe detection method is simpleShorten the timeMicrobiological testing/measurementMicroorganism based processesCholic acidBacteroides
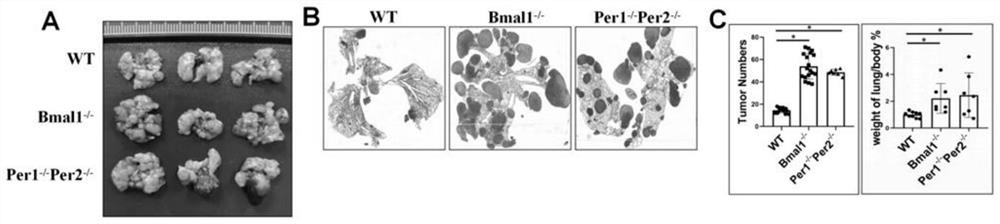
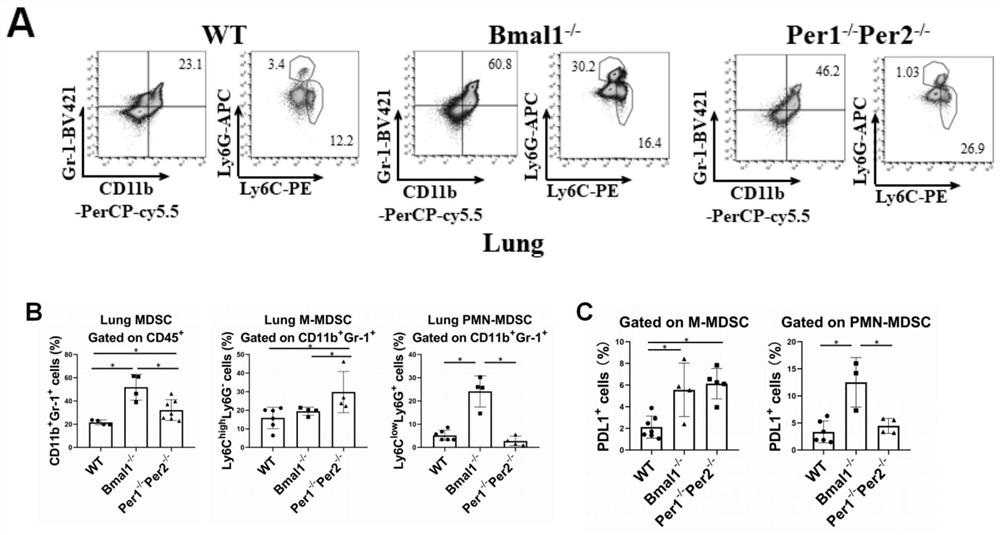
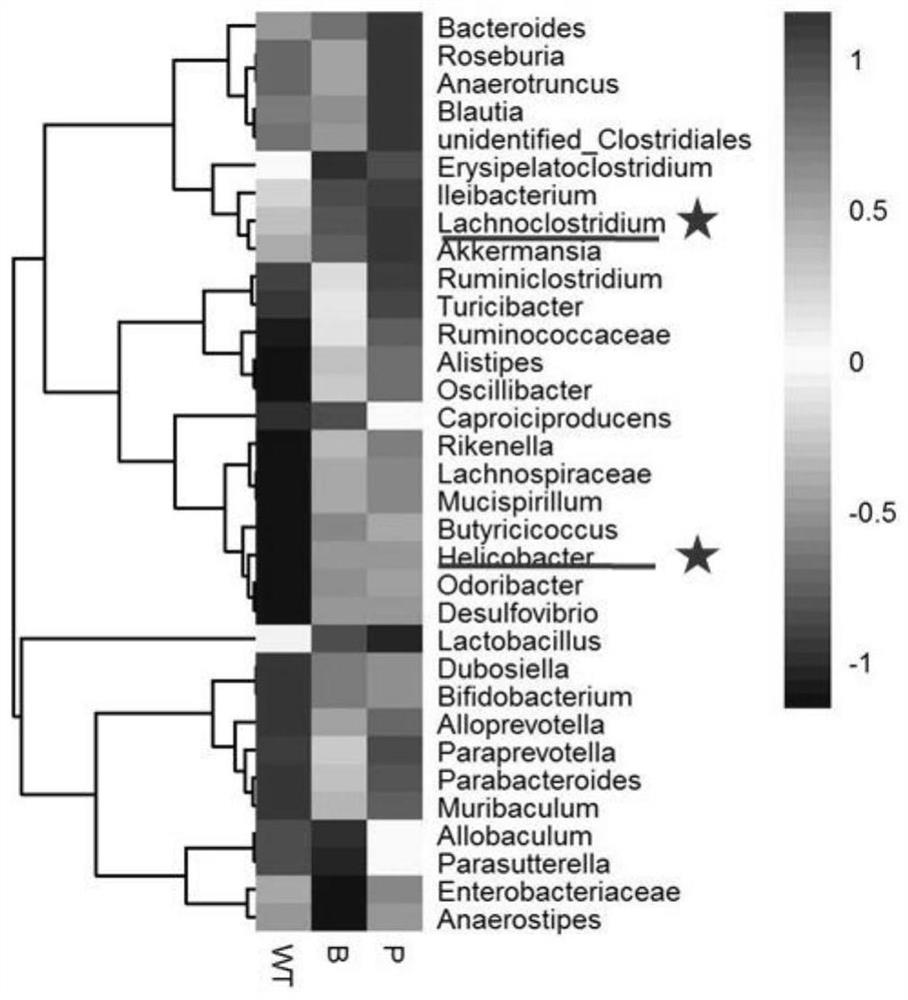
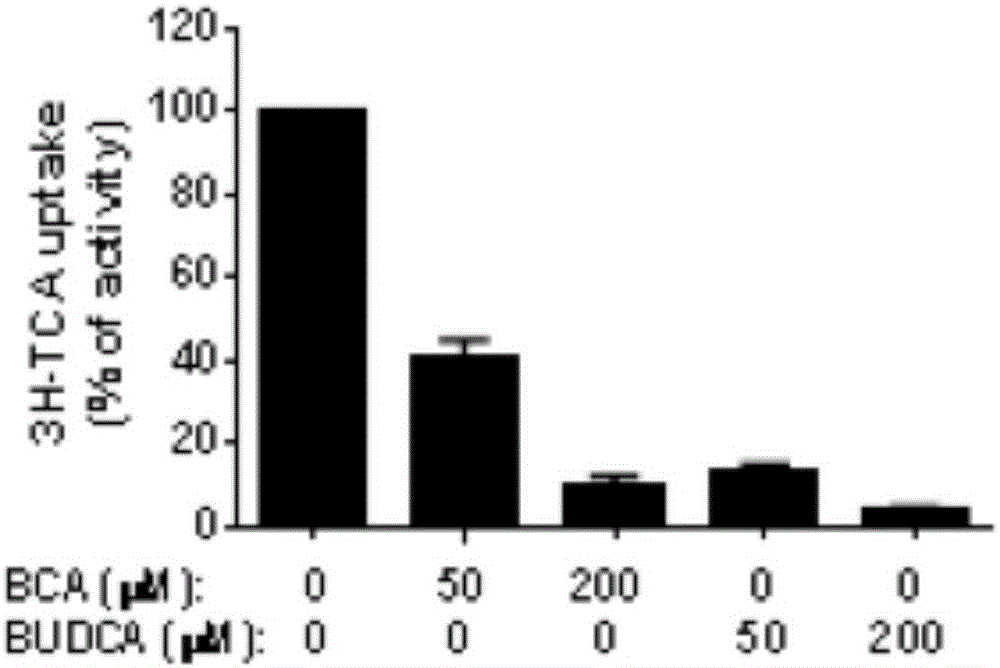
The invention relates to an immunomodulator for treating lung metastasis of colorectal cancer and a composition for prediction and detection of the immunomodulator, the immunomodulator comprises a substance for inhibiting deletion of a Bmal1 gene and / or a substance for inhibiting deletion of a Per1 gene and a Per2 gene, and the composition for predicting or detecting lung metastasis of colorectal cancer comprises a reagent for detecting Helicobacter helicobacter and Lacnoctustridium bacteroides, a reagent for detecting Helicobacter helicobacter and Lacnoctustridium bacteroides, and a reagent for predicting or detecting lung metastasis of colorectal cancer. And / or a reagent for detecting bile acid taurocholic acid. The development process of colorectal cancer lung metastasis is influenced by adjusting the tumor immune microenvironment, the risk of colorectal cancer lung metastasis is predicted by detecting the abundance of intestinal bacteria and metabolites thereof, the detection mode is simple and convenient, and accurate detection is convenient to achieve.
Owner:SUZHOU UNIV
Sodium ion taurocholic acid co-transporter peptide inhibitor
The invention relates to synthesis of a novel sodium taurocholate cotransporting polypeptide (NTCP) inhibitor and inhibiting effects of the inhibitor on the NTCP. The experimental research indicates that the compound has high inhibition activity for NTCP and indicates directions for antivirus, anti-bile siltation and metabolic regulation effects of anti-bile acid transporting drugs. The compound or composition thereof is hopeful to be developed into a novel NTCP inhibitor with great potential.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI +1
Therapy of atherosclerosis, primary biliary cirrhosis and nrlp3 inflammasome-associated disease by htcp inhibitors
ActiveUS20180296634A1Easy to optimizeReduce inflammationSsRNA viruses negative-senseNervous disorderCholic acidDisease
An inhibitor of Na+-taurocholate cotransporting polypeptide (NTCP) for use in a method of treatment of primary biliary cirrhosis, atherosclerosis, or an NRLP3 inflammasome-associated disease in a subject.
Owner:MYR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com