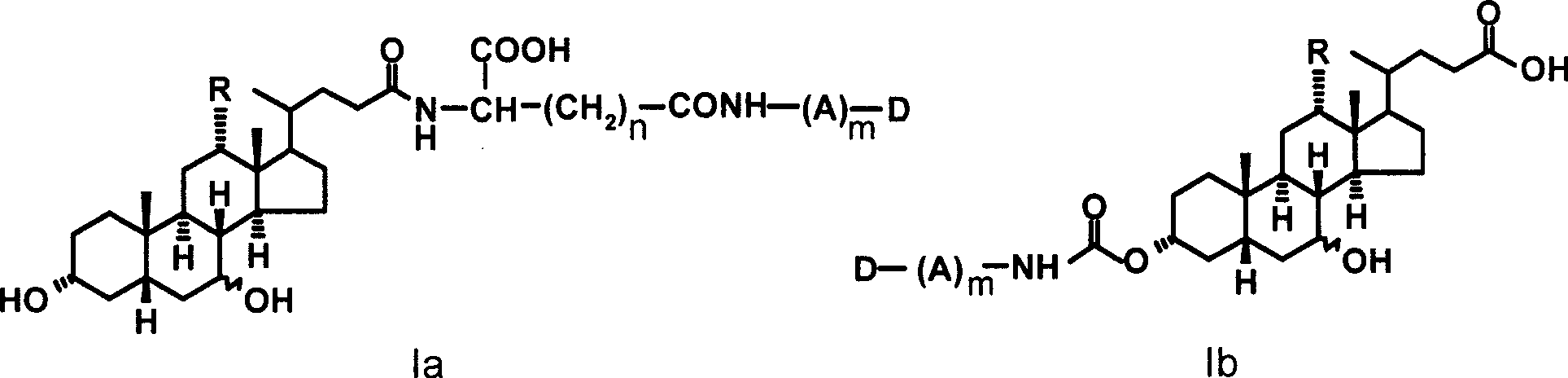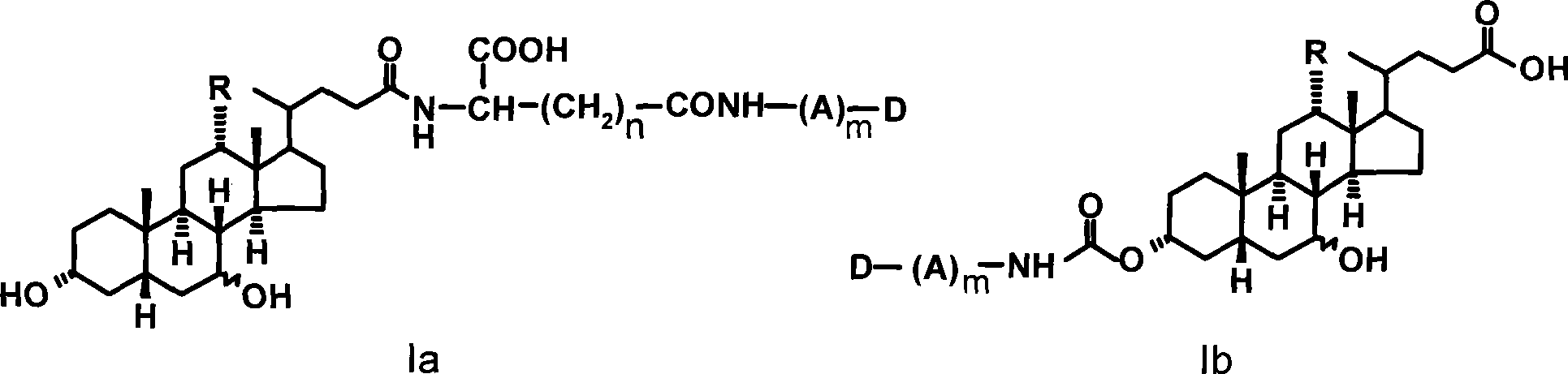Patents
Literature
37 results about "Hyocholic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
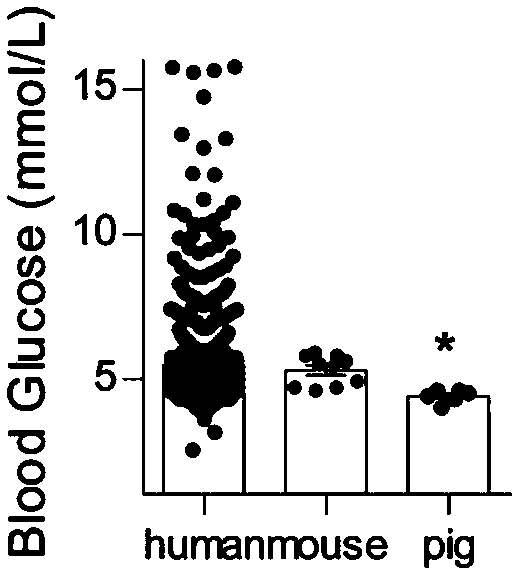
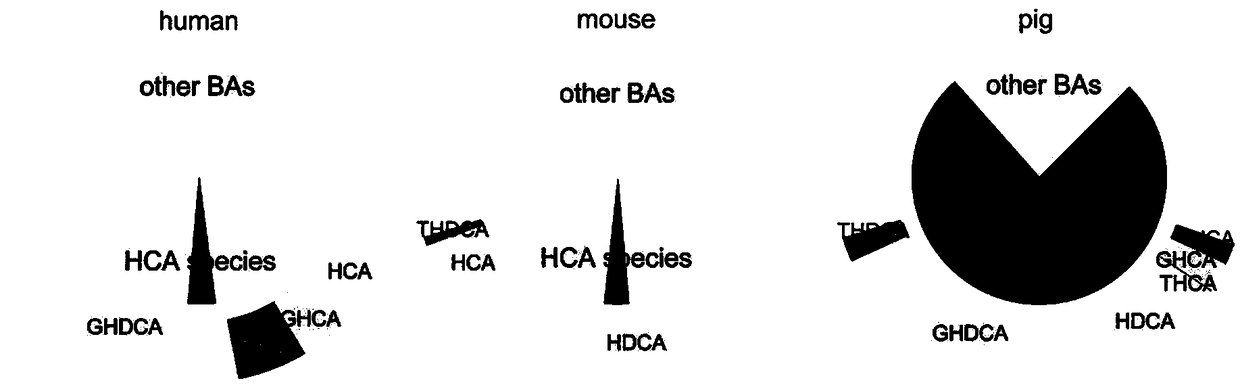
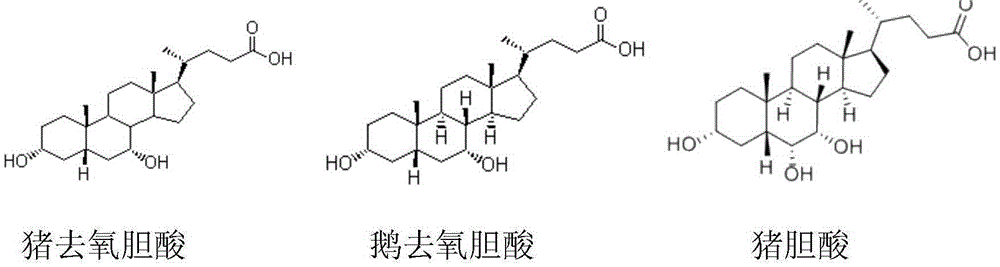
Hyocholic acid or 3α,6α,7α-trihydroxy-5β-cholan-24-oic acid is a bile acid found as one of the main forms in pig, and at low concentrations in other species including humans.
High efficiency technique for extracting bilirubin and bile acid by using animal bile as raw material
The present invention relates to process of extracting cholic acid, deoxycholic acid and bilirubin from bile of pig, ox and sheep. Bile of pig, ox and sheep consists of water in about 97 %, bile acid in about 2.5 % and bilirubin in about 0.4 %; and contains also phospholipid, cholesterol, Na, K, Ca, phosphate, carbonate, small amount of protein, and other components. Fresh bile is treated through cooling, filtering to defat, basic hydrolysis, acidification and organic solvent extracting to obtain bilirubin; the rest solution is further treated through deep saponification, acidification and organic solvent precipitation to obtain the mixture of cholic acid and deoxycholic acid; and the mixture is re-crystallization separated to obtain high purity cholic acid and deoxycholic acid.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method for removing hyocholic acid from chenodeoxycholic acid
The invention discloses a method for removing hyocholic acid from chenodeoxycholic acid. The method comprises the steps of dissolving, residue removing, complexing, crystallizing, centrifugal separating, emulsifying, acidifying, drying, mother liquor treating and the like; high-boiling-point butyl acetate is utilized as solvent, volatilization of the solvent is reduced, a good separation effect is achieved by utilizing the unique dissolution property of butyl acetate to impurities, and the solvent belongs to a three-level low-toxicity dangerous chemical; the selective complexing characteristic of sec-butylamine to bile acid is utilized, and the hyocholic acid and other impurities can be separated to a higher degree. According to the separation and purification method, impurity removing is qualified in one time, the loss rate of the chenodeoxycholic acid is 5% or below and is greatly lower than the effective ingredient loss of 30% in a conventional crystallization method, the solvent can be recycled, three wastes are not generated, and the method is an environment-friendly, low-cost and efficient separation method.
Owner:CHANGDE YUNGANG BIOTECHNOLOGY CO LTD
Method for preparing chenodeoxycholic acid and ursodesoxycholic acid by directly extracting and synthetizing from porcine bile paste or leftovers
The invention provides a method for preparing chenodeoxycholic acid and ursodesoxycholic acid by directly extracting and synthetizing from porcine bile paste or leftovers, comprising the following steps: using lower content (15-35%) porcine bile paste or leftovers and pre-processing it to remove off non-cholic acids impurities; removing off cholic acid impurities below the chenodeoxycholic acid in the thin layer chromatography by bromine oxidation or chromium oxidation; removing off cholic acid impurities above the chenodeoxycholic acid or the ursodesoxycholic acid in the thin layer chromatography by conventional reduction method; directly preparing the chenodeoxycholic acid or the ursodesoxycholic acid.
Owner:郑州药凰中医药研究有限公司
Quality control method of Danle capsule
The invention relates to a quality detection and control method of a compound preparation of Danle capsule which is made from traditional Chinese medicinal materials of 75g of hyodeoxycholic acid, 75g of dried tangerine peel, 240g of turmeric root tuber and 600g of longtube ground ivy herb; the method comprises a content measure item which adopts an HPLC-ELSD method to measure the content of hyodeoxycholic acid C24H40O4 in the Danle capsule; and the method is also can be used for measuring the content of the hyodeoxycholic acid C24H40O4 in the hyocholic acid. The method ensures the accuracy and the advanced nature of the quality detection standard of the Danle capsule and is capable of effectively controlling the quality of the Danle capsule, thus the method can be considered as the index of the quality control and the technological stability observation of the Danle capsule.
Owner:ZHEJIANG YONGNING PHARMA
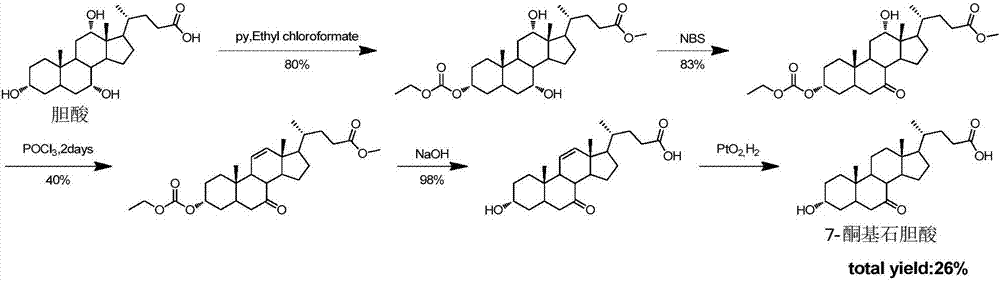
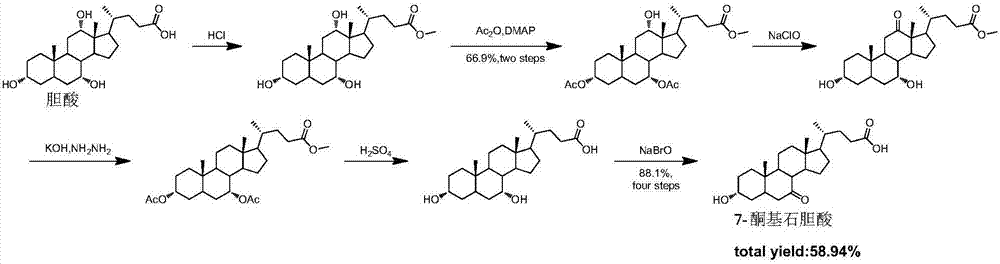
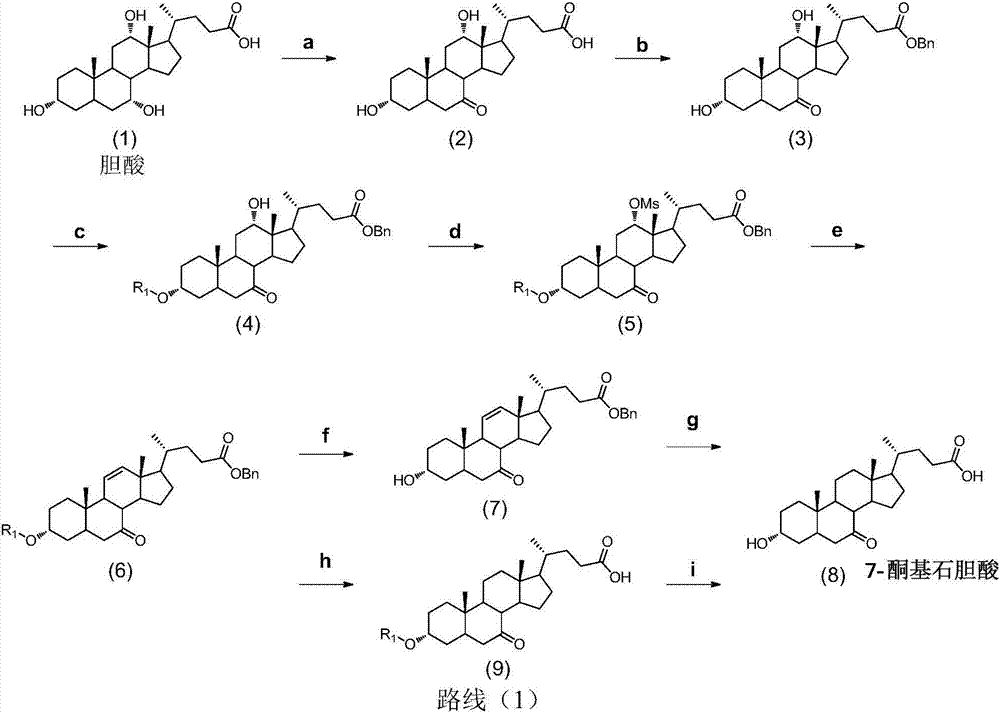
Synthesis method of intermediate 7-ketolithocholic acid of ursodeoxycholic acid
The invention discloses a chemical synthesis method of intermediate 7-ketolithocholic acid (3alpha-hydroxy-7-one-5beta-cholestan-2-4-acid) of ursodeoxycholic acid, and belongs to the field of organic chemical synthesis. According to the method, cholic acid is adopted as a material, and is subjected to reactions including selective oxidation of 7alpha-hydroxy, benzyl esterification of a side chain carboxyl group, esterification of 3alpha-hydroxy, methanesulfonic acid esterification of 12alpha-hydroxy, elimination, hydrogenation and hydrolysis to synthesize the intermediate 7-ketolithocholic acid of the ursodeoxycholic acid; the cheap cholic acid is adopted as the material, the synthesis method has the advantages of being novel, low in cost, high in yield and environment-friendly and industrial production is facilitated.
Owner:EAST CHINA NORMAL UNIV
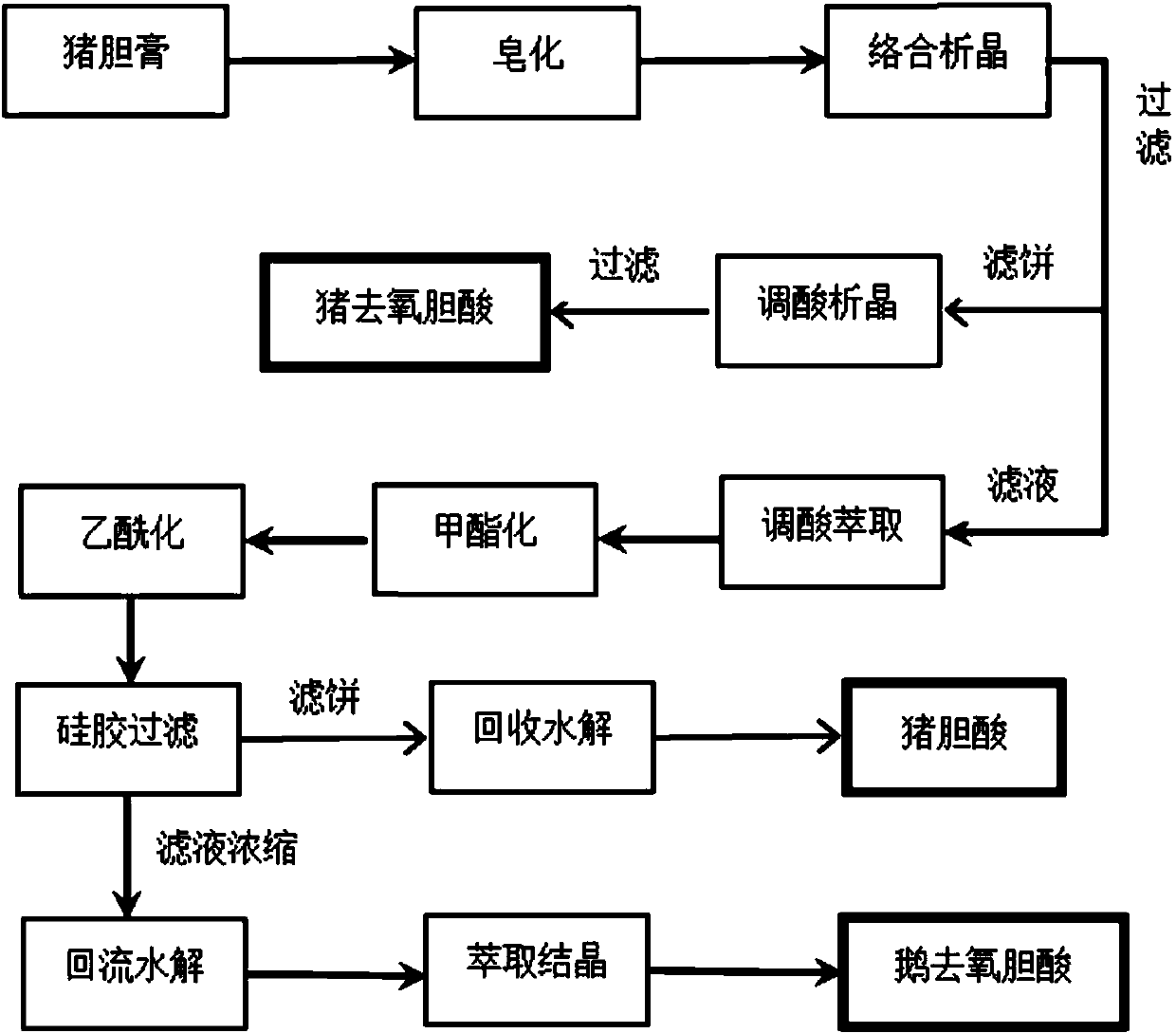
Method for extracting chenodeoxycholic acid from pig gall paste
The invention discloses a method for extracting chenodeoxycholic acid from pig gall paste. The method comprises the steps such as saponification, complexation crystallization, extraction esterification and acetylation, and the purpose that hyodeoxycholic acid, hyocholic acid and the chenodeoxycholic acid are separate from the pig gall paste in one time is achieved. The purity of the obtained threetypes of products is high, reagents are all cheap and easy to obtain, most solvents can be recycled and reused, and produced waste water is less.
Owner:HUNAN JIUDIAN PHARMA +1
Method for extracting cholic acid of ducks and chenodeoxycholic acid (CDCA) from bile of ducks
The invention discloses a method for extracting cholic acid of ducks and chenodeoxycholic acid (CDCA) from bile of ducks. The method is used for directly extracting CDCA from the bile of ducks and is also used for extracting cholic acid of ducks. The preparation method is simple, is easy to operate and is suitable for industrial application. The preparation method can be used for preparing CDCA and cholic acid of ducks at the same time. The purity of the prepared CDCA and cholic acid of ducks is higher and is more than or equal to 95% and more than or equal to 90% respectively.
Owner:四川新功生物科技集团有限公司
Preparation method of alpha-murine cholic acid
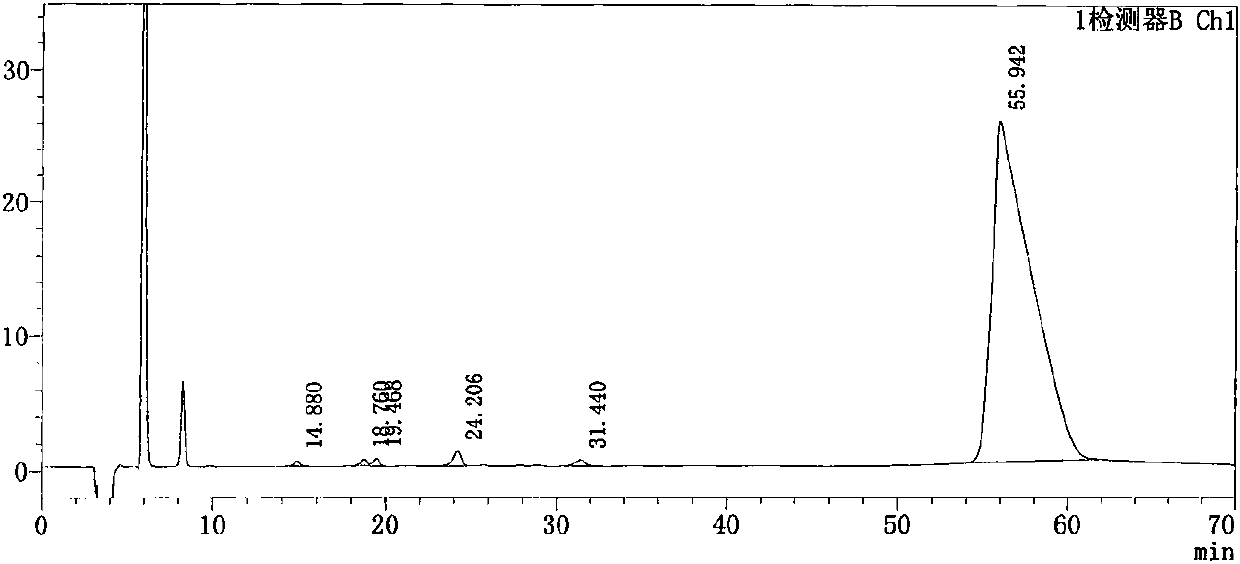
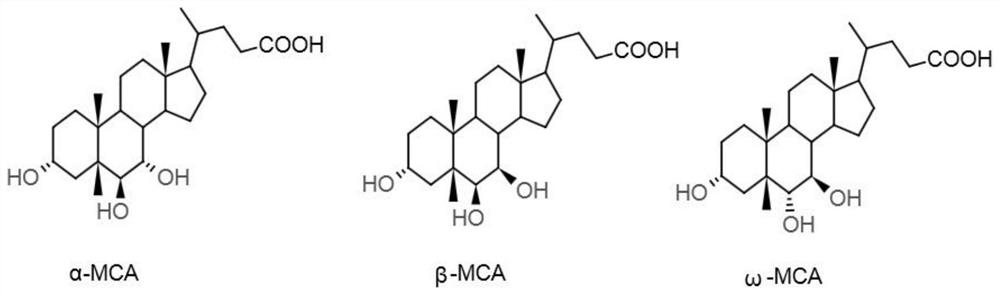
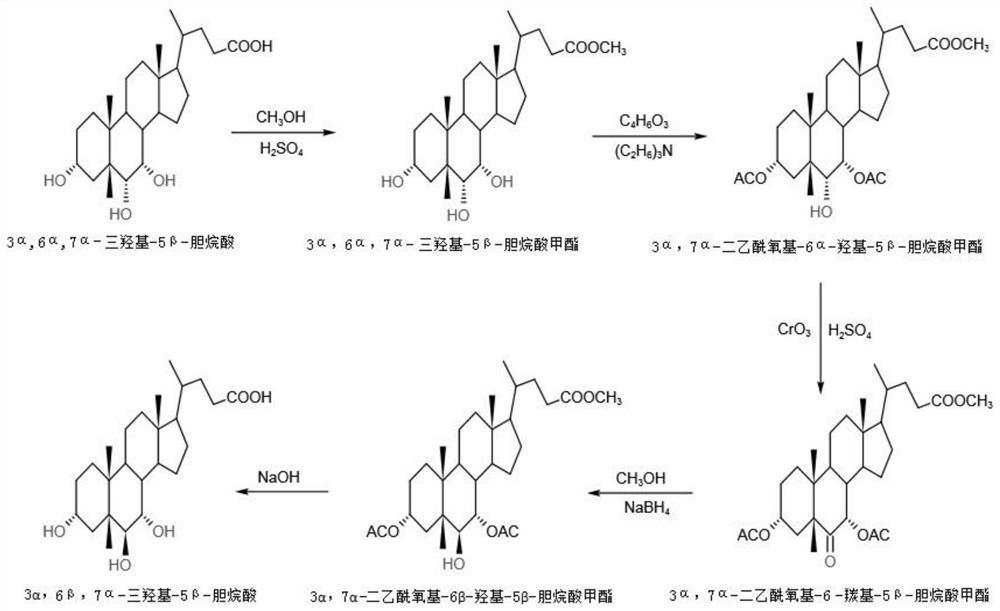
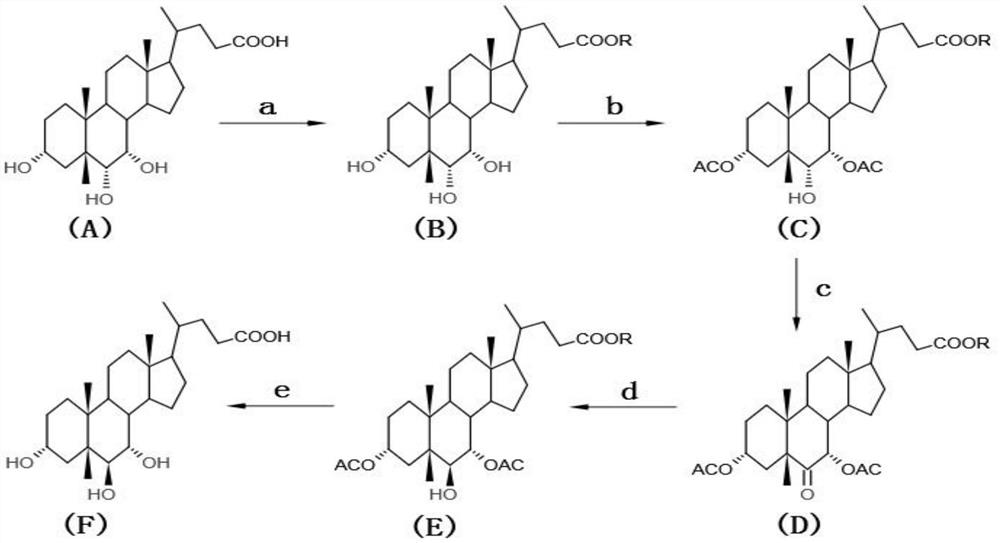
The invention discloses a preparation method of alpha-murine cholic acid. The method takes 3alpha, 6alpha, 7alpha-trihydroxy-5beta-cholanic acid (hyocholic acid) as an initial raw material, and comprises the following steps: carboxyl esterification, acetylation of 3-site and 7-site hydroxyl, oxidation of 6-site hydroxyl, reduction of 6-site carbonyl, hydrolysis and recrystallization to obtain high-purity 3alpha, 6beta, 7alpha-trihydroxy-5beta-cholanic acid, namely alpha-murine cholic acid. The alpha-murine cholic acid synthesized and prepared by the route is wide in raw material source; and the method has the advantages of high yield, high purity and few side reactions, and is suitable for large-scale preparation.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Method for extracting ursodesoxycholic acid
Owner:ANHUI PRINTING MECHANICAL & ELECTRICAL
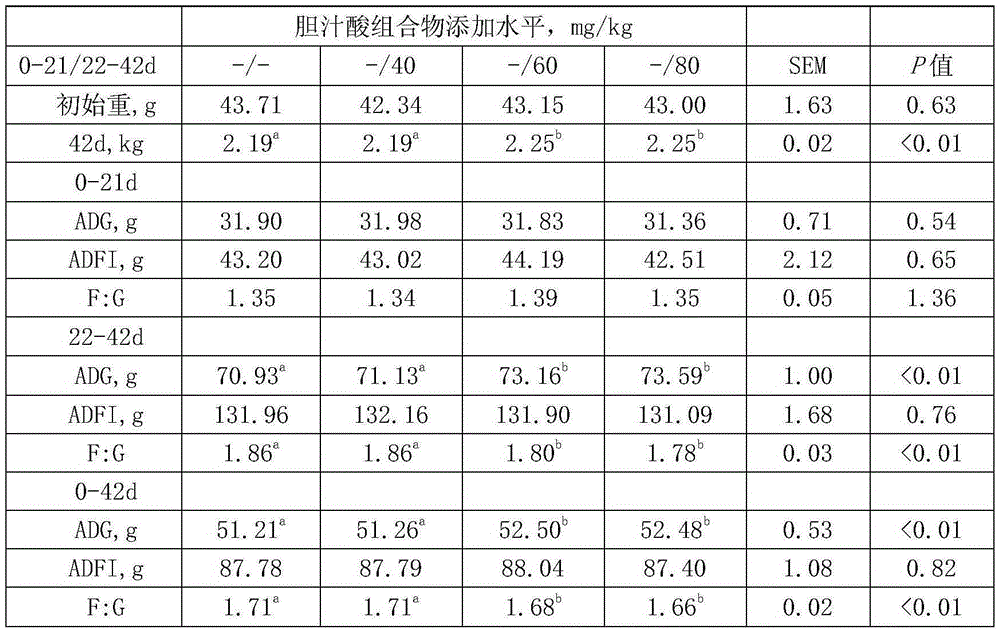
Composition for predicting and curing treating type 2 diabetes mellitus, evaluation method and preparation of composition for predicting and treating type 2 diabetes mellitus
PendingCN109364269ABuy time for treatmentLower blood sugarCompounds screening/testingOrganic active ingredientsTreatment effectCvd risk
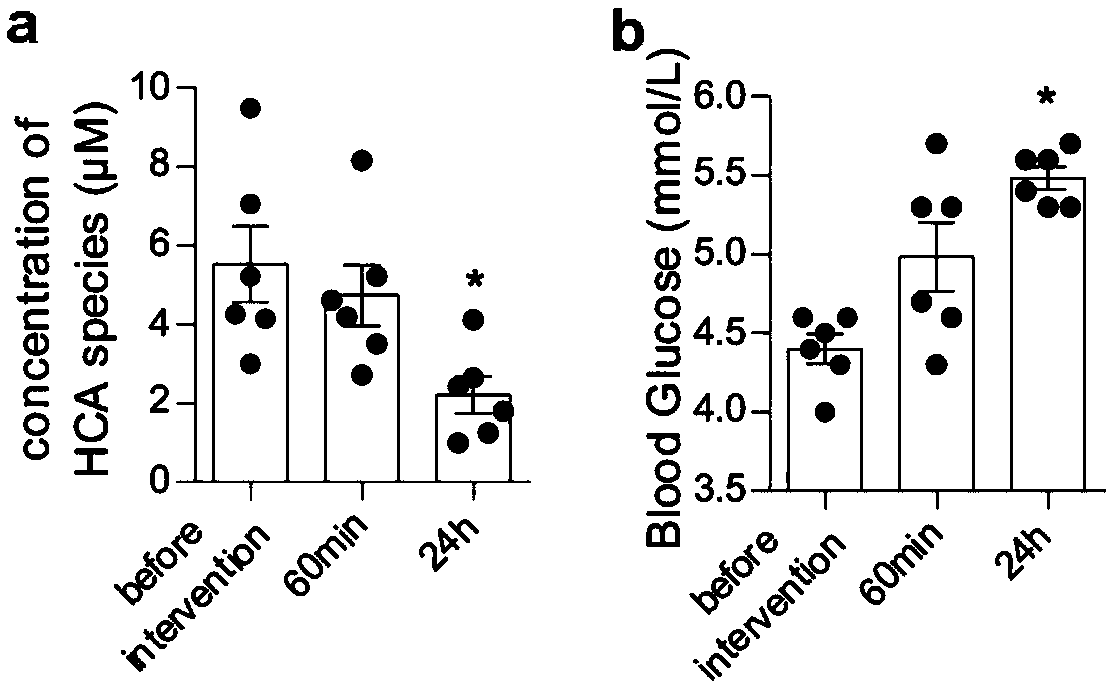
The invention provides a composition for predicting and curing treating type 2 diabetes mellitus, and an evaluation method and a preparation of the composition for predicting and treating type 2 diabetes mellitus. The composition is prepared from hyocholic acid, hyodeoxycholic acid, glycohyocholic acid, glycohyodeoxycholic acid, taurohyocholic acid, taurohyodeoxycholic acid, and a steroidal amidehyocholic acid derivative prepared fromformed byprepared from the hyocholic acid or the hyodeoxycholic acid and amino acids except for glycine and taurine. One of targets for a drug prepared from thecomposition is intestinal epithelial endocrine cells, through drug stimulation, a bile acid membrane receptor TGR5 can be excited, meanwhile, a bile acid nucleus receptor FXR is antagonized, the effects of stimulating the intestinal endocrine cells to secrete GLP1 is achieved jointly, and thus blood sugar is reduced; meanwhile, the content of the composition of a human body is detected, the futurediabetes mellitus risk can be predicted, diabetes mellitus can be screened or early detected, and the curing time is savedprolonged for patients; and the curing effect can also be evaluated by testing the content of the composition after curing.
Owner:SHENZHEN YUNHE PHARM TECH PARTNERSHIP LTD
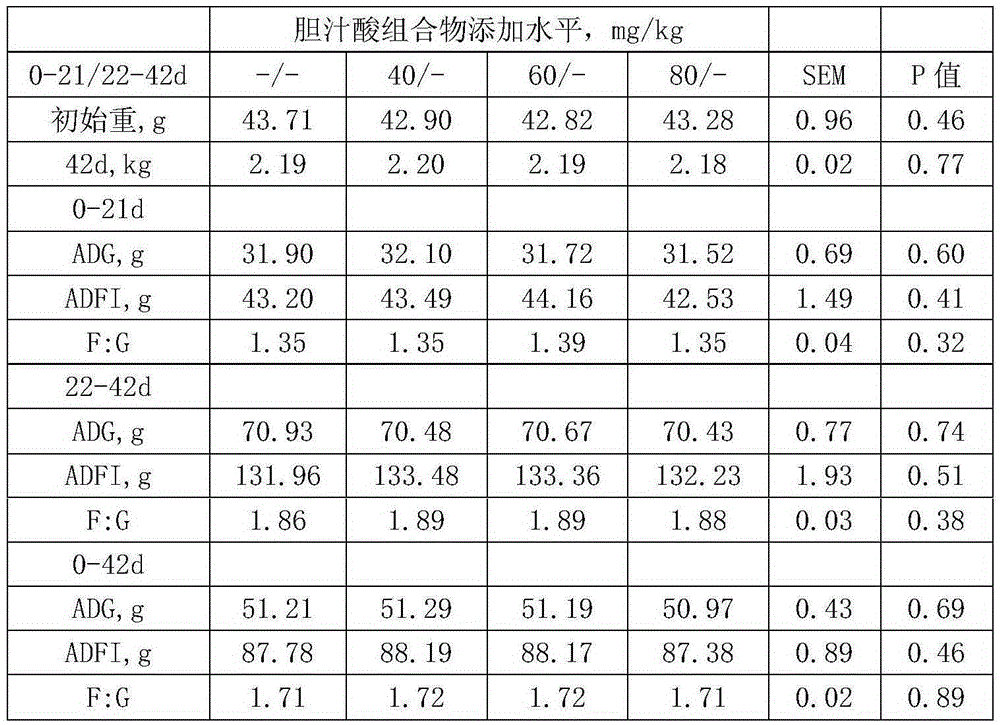
Application of bile acid in the preparation of additives for improving the growth performance of weaned piglets
InactiveCN108783015AImprove growth performanceImprove digestibilityAccessory food factorsAnimal scienceLowering plants
The invention provides application of bile acid in the preparation of additives for improving the growth performance of weaned piglets. The bile acid includes the following components: hyocholic acid,hyodeoxycholic acid and chenodeoxycholic acid. The bile acid is proved by experiments that adding the bile acid to daily rations of weaned piglets can improve the growth performance, improve the digestibility of nutrients (especially fat) and reduce the anti-nutritional factors of plant proteins, thereby comprehensively improving the growth performance of piglets, improving the efficiency of feedutilization and the evenness of final weight, reducing the cost of meat production and improving the economic benefits and returns.
Owner:SHANDONG LONGCHANG ANIMAL HEALTH PROD
Preparation method of Huodan dropping pills
ActiveCN104069170AAvoid potential damageShort heating timeOrganic active ingredientsPharmaceutical product form changeMelting tankHeating time
The invention relates to a preparation method of Huodan dropping pills. The method comprises the following steps: respectively putting a raw material hyocholic acid sieved by a 60-mesh sieve and an auxiliary material polyethylene glycol 6000 according to a prescription quantity into a mixer for fully mixing, throwing the mixed material into a melting tank and melting in a heating manner so as to obtain an evenly-dispersed undertint medicine liquid while the medium is molten; adding patchouli oil in a specified prescription quantity in a stirring state; evenly stirring the medicine liquid for forming dropping pills; then, dropping the medicine liquid into cooling oil array pipes with certain temperature gradients in a speed control manner under a certain heat preservation condition; and collecting the formed dropping pills, centrifugally removing the cooling oil and collecting the dropping pills. According to the method, the dropping temperature cannot exceed 85 DEG C, so that the volatilization loss of the patchouli oil taken as an effective medicine is obviously reduced. Compared with a powdery feeding process, according to the method provided by the invention, the heating time of the materials is shorted by half.
Owner:SHANGHAI LEIYUNSHANG PHARMA
Novel method for preparing calculus bovis factitius and raw materials thereof
The invention discloses a novel method for preparing calculus bovis factitius and raw materials thereof. The method comprises the following steps: weighing the following components in percentage by weight: 20-30 percent of calcium bilirubinate, 10-20 percent of sodium bilirubinate, 3-8 percent of taurine, 20-30 percent of deoxycholic acid, 2-5 percent of cholic acid or hyocholic acid, 2-5 percent of binding cholesterol or free cholesterol, 2-5 percent of zinc sulfate and 2-5 percent of magnesium sulfate; stirring for 20-60 minutes, adding the balance of ox gallbladder powder, and keeping on stirring for 20-60 minutes to obtain the calculus bovis factitius. According to the method for preparing calcium bilirubinate and sodium bilirubinate serving as effective components in the calculus bovis factitius, high-quality calculus bovis factitius is prepared according to a specific proportion, and the requirement of market for performance of calculus bovis factitius can be met.
Owner:张隶军
A method for extracting chenodeoxycholic acid
The invention discloses a method for extracting chenodeoxycholic acid, and belongs to the technical field of bioengineering. The method comprises the following steps of (1) preparing bile acid sodium salt; (2) preparing bile acid amine salt; (3) extracting and separating a bile acid amine salt solution; (4) salting out and carrying out alkaline hydrolysis. According to the method, when the chenodeoxycholic acid is extracted, chenodeoxycholic acid amine salt and cholic acid amine salt regulated through organic acid in a butyl ester solution have a remarkable solubility difference, so that the chenodeoxycholic acid amine salt and the cholic acid amine salt are crude-separated, and the content of cholic acid is reduced to below 3 percent through crude separation; the chenodeoxycholic acid amine salt and the cholic acid amine salt have a larger solubility difference in a high-concentration butyl ester solvent and a dichloromethane water solution system, and the cholic acid is cleaned and treated again, so that the chenodeoxycholic acid is refined and purified, the content of the cholic acid is reduced to be within 0.2 percent, and the extraction ratio of the chenodeoxycholic acid is greatly improved.
Owner:CHANGDE YUNGANG BIOTECHNOLOGY CO LTD
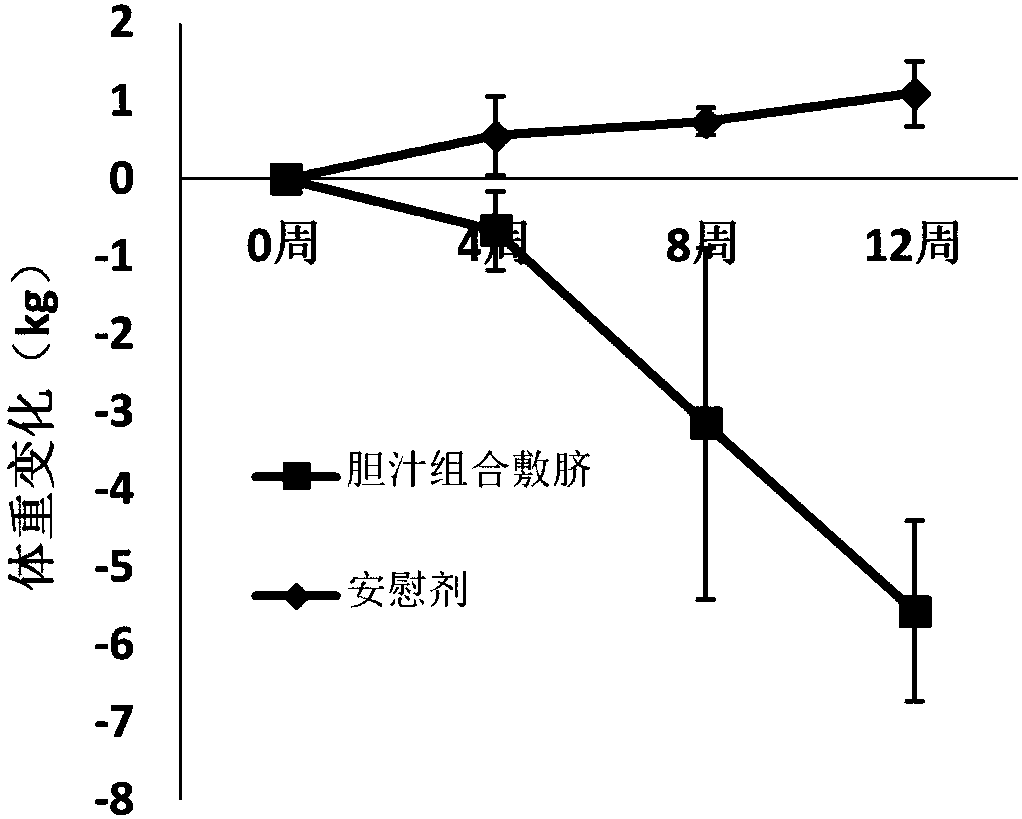
Composition applied to navel for preventing and treating obesity as well as preparation method and application method
InactiveCN108042785AHigh expressionDelayed gastric emptyingMetabolism disorderUnknown materialsSide effectNavel
The invention provides a composition applied on a navel for preventing and treating obesity, belonging to the technical field of obesity treatment. The composition is prepared from cattle bitter gallpowder, pig bitter gall powder, radix et rhizoma rhei and ginger juice; the weight ratio of the cattle bitter gall powder to the pig bitter gall powder to the radix et rhizoma rhei is (10-20) to (10-20) to (1-2), the ratio of the mass of the cattle bitter gall powder to the volume of the ginger juice is (10-20)g to (2-5)ml; the cattle bitter gall powder is prepared from taurocholic acid, calculusbovis deoxycholic acid, glycocholic acid and glycodeoxycholic acid hydrate; the pig bitter gall powder is prepared from hyocholic acid and hyodeoxycholic acid. The composition for preventing and treating obesity can be used for reducing weight, and any side effects are not seen.
Owner:张利生
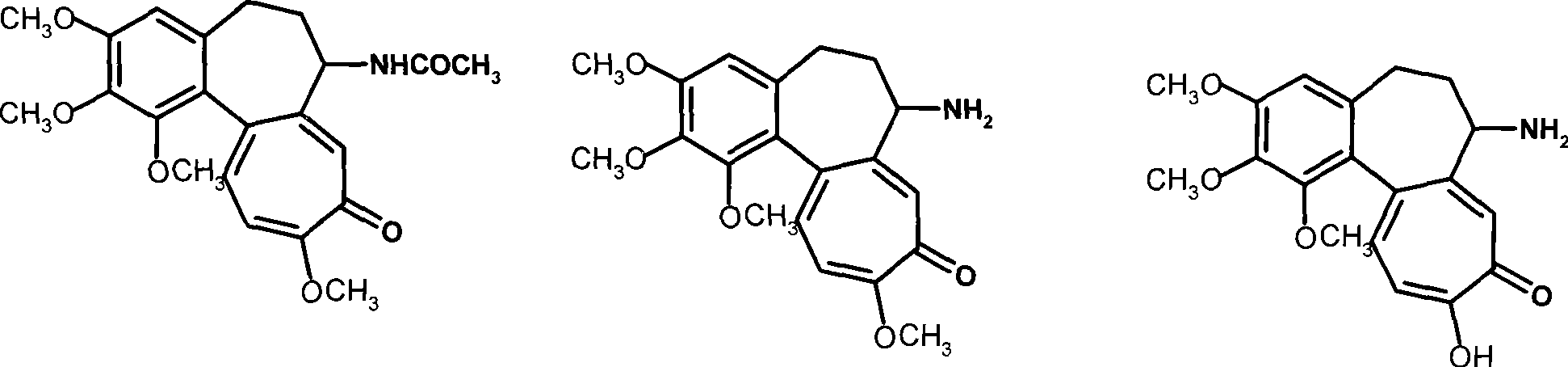
Colchicine derivative-bile acid coupling compounds and medical use thereof
The invention provides a coupling compound of cholic acid or bear deoxycholic acid and colchicine derivative which are as shown in formulas (Ia and Ib), salt thereof which is acceptable in non-toxicity pharmacy, a method for preparing the coupling compound and the salt and compositions and application of drugs containing the compounds. In a structural formula, R represents OH (cholic acid) or H (bear deoxycholic acid), A represents amino acid, n is an integer between 1 and 3, m is an integer between 0 and 2, the structural type of the amino acid is D type or L type, and D represents acetyl colchicine or colchicine and other active metabolites of the colchicine.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
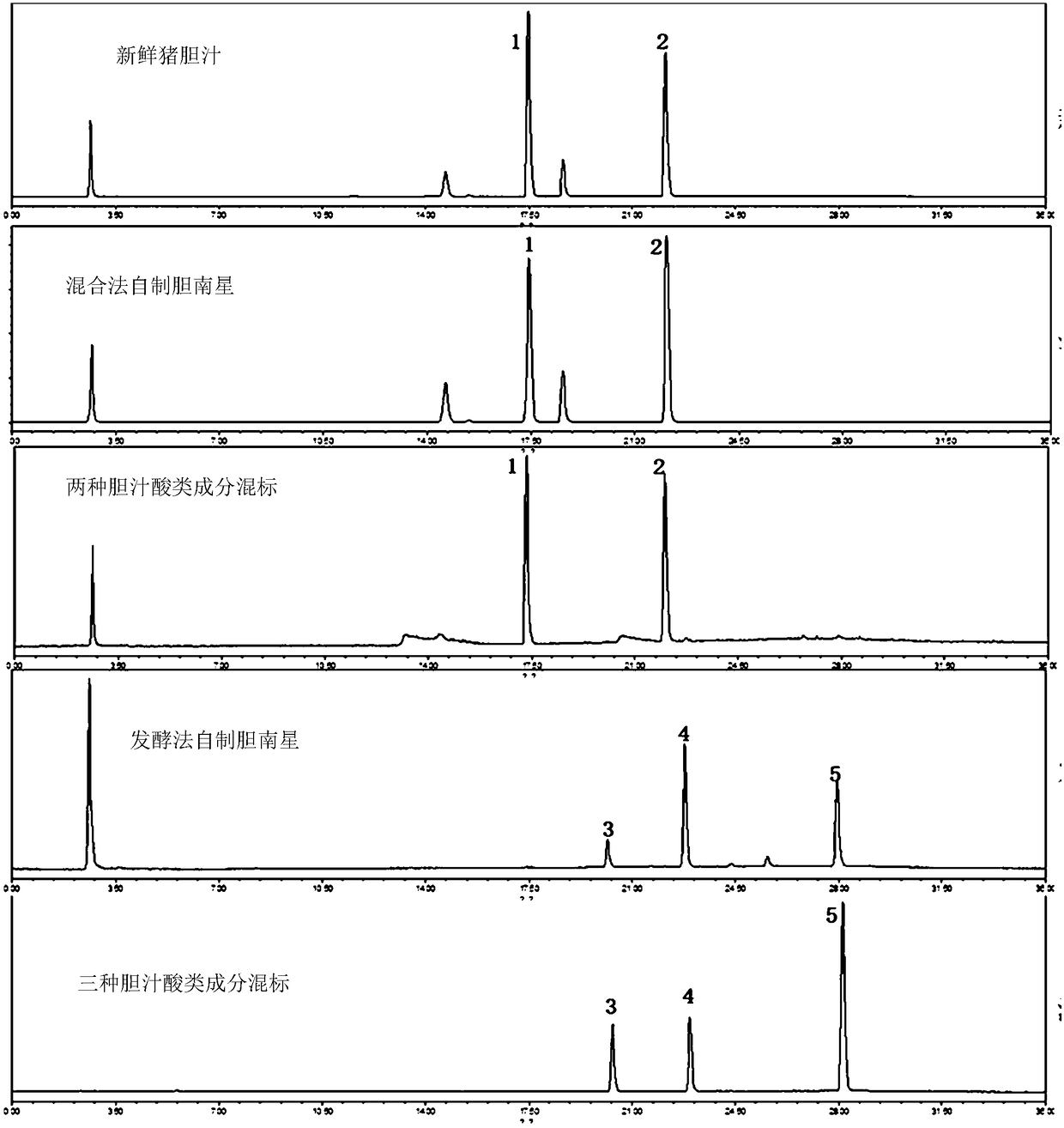
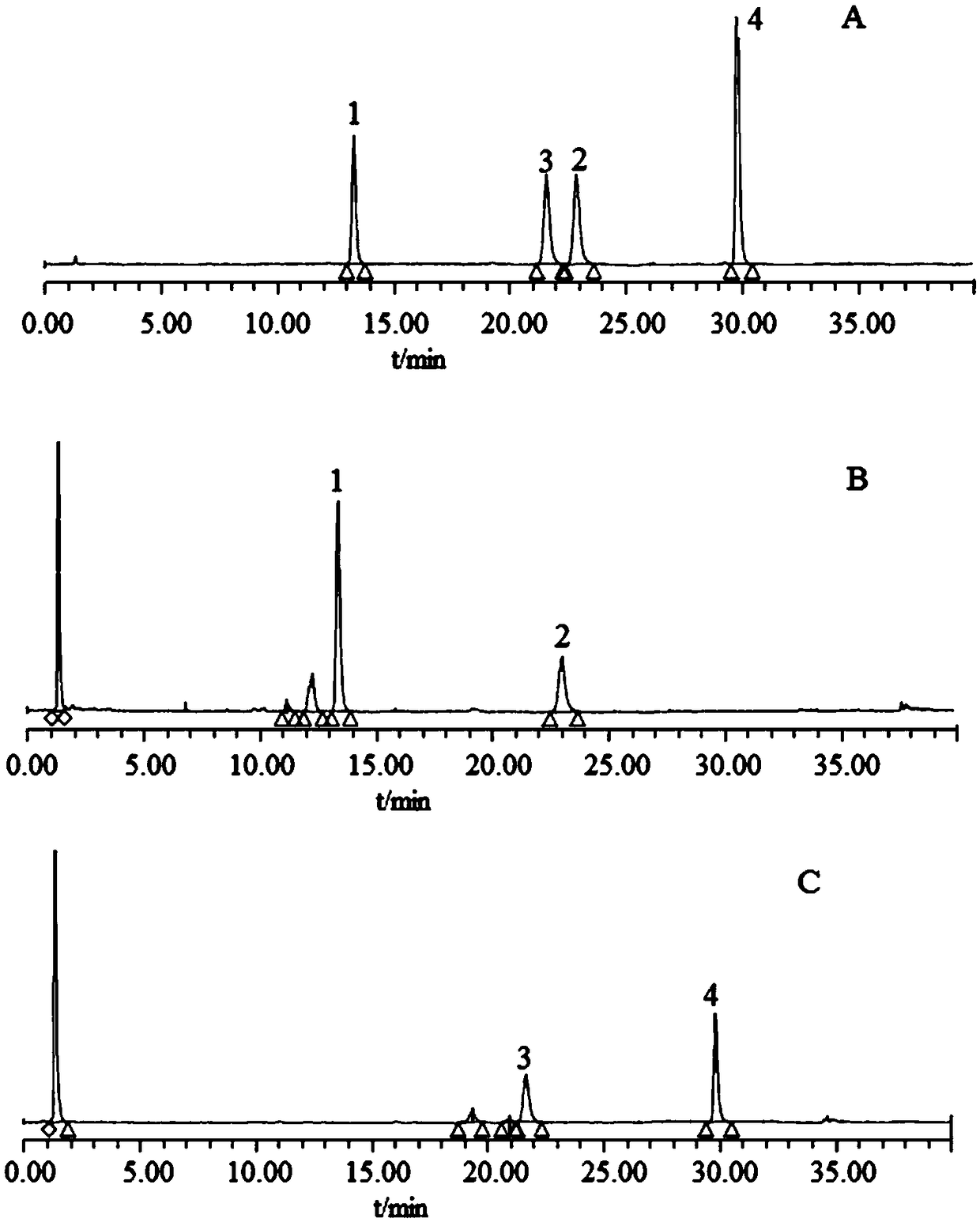
Identification method for fermented and mixed-steamed bile arisaema processed from porcine bile
ActiveCN108717096AQuality improvementEasy to identifyComponent separationAdditive ingredientBile fluid
Disclosed is an identification method for fermented and mixed-steamed bile arisaema processed from porcine bile. The identification method includes: taking hyocholic acid (HCA), hyodeoxycholic acid (HDCA) and chenodeoxycholic acid (CDCA) as a group of markers, taking glycohyodeoxycholic acid (GHDCA) and glycochenodeoxycholic acid (GCDCA) as another group of markers, and applying the groups of markers in identifications of the fermented and mixed-steamed bile arisaema processed from the porcine bile. According to the identification method, thin-layer chromatography and high-performance liquid chromatography are provided to identify bile acid ingredients in the bile arisaema processed from the porcine bile, so that different products of the bile arisaema processed from the porcine bile can be identified simply and quickly, and quality of the bile arisaema can be controlled better. The identification method has the advantages of high specificity and good reproducibility.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE +1
Method for preparing chenodeoxycholic acid by extraction complexing process
The invention relates to a method for preparing chenodeoxycholic acid by an extraction complexing process. The method comprises the following steps: 1, extracting; 2, concentrating; 3, complexing; 4,removing magnesium; 5, crystallizing; 6, recrystallizing for obtaining the chenodeoxycholic acid finished product. According to the method, impurities such as sterols and proteins in chenodeoxycholicacid are extracted with petroleum ether, then ethyl ester is washed with weak alkaline water to remove hyocholic acid impurities in chenodeoxycholic acid, chenodeoxycholic acid is easy to complex under high-temperature catalysis of ethyl acetate, and complete separation of chenodeoxycholic acid is achieved. The method has the beneficial effects that the yield is high and is 20-25%, the melting point is high and is 160-170 DEG C, and the content of high-purity products is 98% or above through HPLC detection. The method has the characteristics of simplicity in operation, short process flow, reduction of organic reagent consumption, cost saving, safety, environmental protection and the like.
Owner:ANHUI KEBAO BIOLOGICAL ENG CO LTD
A kind of bile acid composition and preparation method thereof
ActiveCN104041678BHigh yieldHigh purityAccessory food factorsSteroidsOrganic solventAdditive ingredient
The invention especially discloses a bile acid composition and a preparation method thereof. The bile acid composition has effective ingredients including hyocholic acid, hyodeoxycholic acid and chenodeoxycholic acid, wherein the sum of the weight percentages of hyocholic acid and hyodeoxycholic acid is not less than 78.0%, the weight percentage of chenodeoxycholic acid is not less than 18.0%, and the balance is water and ash. The bile acid composition and the preparation method thereof have the beneficial effects that: the preparation method has the advantages of simple operation and mild conditions, does not adopt harmful and toxic organic solvents in the preparation process, and can be easily applied in industrialized production; the prepared bile acid is high in yield and high in purity, is added into a feed for feeding broiler chickens, can significantly improve the growth performance and slaughter performance of the broiler chickens, is beneficial for the broiler chickens to utilize fat, reduces the use of broiler chicken own fat, thereby improving the growth performance and slaughter performance of the broiler chickens, solving the problems of fat digestion and absorption in the feed, and improving the broiler chicken carcass quality.
Owner:SHANDONG LONGCHANG ANIMAL HEALTH PROD
7-ketolithocholic acid intermediate and preparation process and application thereof
ActiveCN110423261AThe reaction route is simpleThe reaction conditions are mild and not harshSteroidsBulk chemical productionLeaving groupSilanes
The invention discloses a 7-ketolithocholic acid intermediate and a preparation process and an application thereof. Hyocholic acid is utilized as a starting material, an esterification reaction is carried out first, then a silane protection group protects a 3-hydroxyl group with high selectivity, then a specific spatial structure of 6,7-hydroxyl group of the hyocholic acid is utilized, a conventional protection method can be selectively connected to a strong leaving group at 6-position to obtain the 7-ketolithocholic acid intermediate, and the 7-ketolithocholic acid intermediate is subjected to oxidation, removal, reduction, and hydrolysis to remove a protection group to obtain a target product 7-ketolithocholic acid. The 7-ketolithocholic acid prepared by the process is high in degree ofpurity, is also simple in process step, enables a synthetize route to be greatly simplified, and enables the industrialization cost to be saved.
Owner:成都百途医药科技有限公司 +1
A method for removing hyocholic acid from chenodeoxycholic acid
Owner:CHANGDE YUNGANG BIOTECHNOLOGY CO LTD
Biomarker detection kit for cholestasis indication prognosis
ActiveCN113504324AProven reliabilityGood prediction accuracyComponent separationDisease diagnosisCholic acidBile Juice
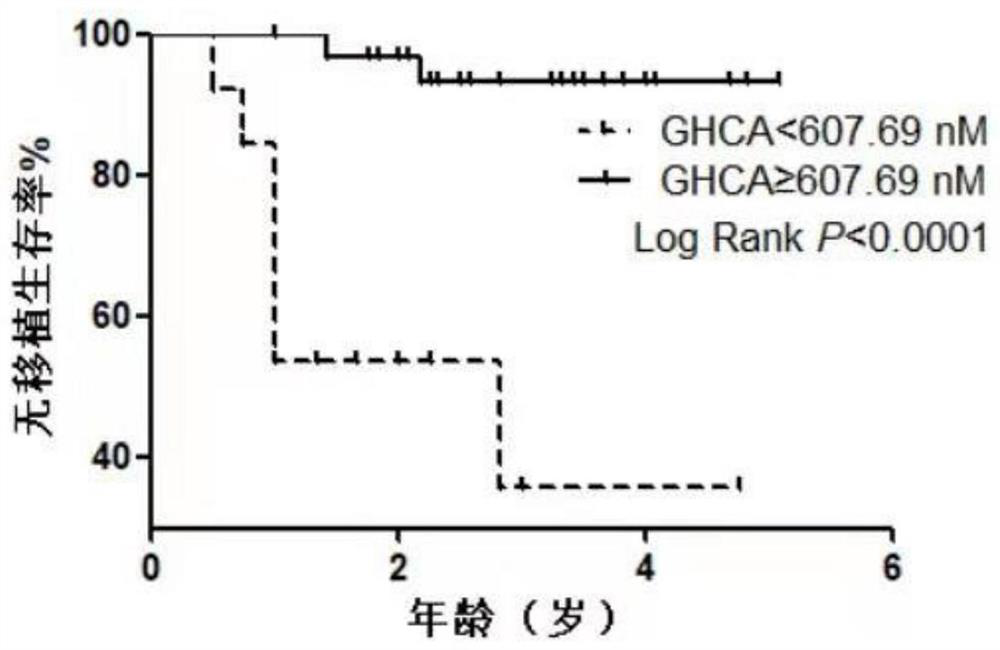
The invention relates to a biomarker for cholestasis indication prognosis, a marker detection method and a corresponding kit. The prognosis indication biomarker is selected from one or more of glycyl acylated hyocholic acid (Glyco-hyoCA or GHCA), taurosylated-2 [beta], 3 [alpha], 7 [alpha], 12 [alpha]-tetrahydroxycholic acid (Tauro-2 [beta], 3 [alpha], 7 [alpha], 12 [alpha]-Tetraphydroxy bileacid or Tauro-2 [beta], 3 [alpha], 7 [alpha], 12 [alpha]-THBA),taurosylated hyocholic acid (Tauro-hyoCA or THCA), Tauro-3 [alpha], 6 [beta], 7 [alpha], 12 [alpha]-THBA, Tauro-3 [alpha], 6 [alpha], 7 [alpha], 12 [alpha]-THBA, and total Tauro-THBAs. The detection finds that bile acid spectrums in serum / plasma samples of subjects are concentrated, different bile acids are screened out to serve as candidate indication prognosis markers, meanwhile, corresponding cutoff values are formulated, and the cutoff values are applied to a verification set to verify the indication prognosis efficiency of the verification set. The invention provides the method and the kit for prognosis judgment of cholestasis for the first time, the detection efficiency is high, the result is accurate, the method and the kit are suitable for early prognosis effect judgment of patients with cholestasis, and guidance is provided for individual treatment and medication schemes.
Owner:CHILDRENS HOSPITAL OF FUDAN UNIV +1
7-ketone lithocholic acid intermediate, preparation method and applications thereof
ActiveCN110437296AThe reaction conditions are mild and not harshImprove securitySteroidsBulk chemical productionCholic acidLeaving group
The invention discloses a 7-ketone lithocholic acid intermediate, a preparation method and applications thereof. The preparation method comprises: carrying out an esterification reaction on hyocholicacid as a starting raw material; adding 2,2-dimethylpropane to carry out a reaction with the unique 6,7 synclastic hydroxyl structure to form cross protection; selectively protecting the 3-site hydroxyl group; selectively linking the leaving group to the 6-site to form a 7-ketone lithocholic acid intermediate by using the special spatial structure of hyocholic acid; selectively oxidizing the unique 7-site exposed hydroxyl group of the 7-ketone lithocholic acid intermediate to form an alpha hydroxyketone intermediate with a leaving group linked to the 6-site; simultaneously and directly removing the 6-site hydroxyl group and the leaving group by using the hydroxyketone to form an intermediate 7; and removing the protection group through simple hydrolysis of the intermediate 7 to obtain the7-ketone lithocholic acid. According to the present invention, the 7-ketone lithocholic acid prepared by the method has high purity, and the method has characteristics of simple process and no specialpurification method, and is suitable for industrial production.
Owner:成都百途医药科技有限公司
Preparation method of directly extracting and synthesizing chenodeoxycholic acid and ursodeoxycholic acid from pig gall paste or leftovers
Owner:郑州药凰中医药研究有限公司
7-ketolithocholic acid intermediate and its preparation method and application
ActiveCN110437296BHigh yieldSuitable for industrial scale-upSteroidsBulk chemical productionCholic acidKetone
The invention discloses a 7-ketolithocholic acid intermediate and its preparation method and application. The invention uses hyocholic acid as a starting material, first performs esterification reaction, and then adds 2,2-dimethylpropane and hyocholic acid The unique 6,7 hydroxyl structure reacts to form parallel fork protection, and then selectively protects the 3-position hydroxyl group, and then uses the special space structure of hyocholic acid to selectively connect the leaving group to the 6-position to form 7-ketone Keystone cholic acid intermediate, 7-ketolithocholic acid intermediate unique 7-position exposed hydroxyl group, selective oxidation of 7-position hydroxyl group to form α-hydroxy ketone intermediate 6 with 6-position connecting leaving group, this type of hydroxyketone can be directly The hydroxyl group at the 6th position and the leaving group are removed simultaneously to form intermediate 7, and intermediate 7 can be deprotected by simple hydrolysis to obtain the target product 7-ketolithocholic acid. The 7-ketolithocholic acid prepared by the method of the present invention not only has high purity, but also has a simple process flow and no special purification method in the preparation process, which is suitable for industrial production.
Owner:成都百途医药科技有限公司
A kind of 7-ketolithocholic acid intermediate and its preparation process and application
ActiveCN110423261BLow cost of industrializationHigh yieldSteroidsBulk chemical productionCholic acidLeaving group
Owner:成都百途医药科技有限公司 +1
A method for distinguishing fermented dannanxing processed from pig bile and mixed steamed dannanxing
Disclosed is an identification method for fermented and mixed-steamed bile arisaema processed from porcine bile. The identification method includes: taking hyocholic acid (HCA), hyodeoxycholic acid (HDCA) and chenodeoxycholic acid (CDCA) as a group of markers, taking glycohyodeoxycholic acid (GHDCA) and glycochenodeoxycholic acid (GCDCA) as another group of markers, and applying the groups of markers in identifications of the fermented and mixed-steamed bile arisaema processed from the porcine bile. According to the identification method, thin-layer chromatography and high-performance liquid chromatography are provided to identify bile acid ingredients in the bile arisaema processed from the porcine bile, so that different products of the bile arisaema processed from the porcine bile can be identified simply and quickly, and quality of the bile arisaema can be controlled better. The identification method has the advantages of high specificity and good reproducibility.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE +1
A method for extracting chenodeoxycholic acid and allocholic acid from duck bile
ActiveCN106749473BRealize refining and purificationHigh extraction rateSteroidsAlkaneChenodeoxycholic acid
The invention discloses a method for extracting chenodeoxycholic acid and allocholic acid from duck bile, and belongs to the technical field of biological engineering. The method comprises the following steps of (1) preparing of bile acid extracting liquid: obtaining duck bile, saponifying, cooling, adjusting a pH (potential of hydrogen) value to 7 to 8, adding a mixed solvent of ester and alkane, adjusting the pH value to 2 to 4, extracting, and discoloring, so as to obtain the bile acid extracting liquid; (2) preparing of bile acid magnesium salt: adjusting the pH value of the bile acid extracting liquid in step (1) to 4.5 to 5.5, dissolving the magnesium salt, adding into the bile acid extracting liquid, refluxing, cooling, and separating, so as to respectively obtain the magnesium allocholate and a chenodeoxycholic acid solution; refining and purifying the chenodeoxycholic acid solution, so as to obtain a finished product of henodeoxycholic acid; (3) extracting of the allocholic acid: adding water and carbonate into the prepared magnesium allocholate in step (2), heating and dissolving, separating solid from liquid, adjusting the pH value of a solution to 1 to 2, and separating solid from liquid, so as to obtain a finished product of the allocholic acid. The method has the advantages that the purities and extracting rates of the chenodeoxycholic acid and the allocholic acid are greatly improved, the operation is safe, and the method is suitable for industrialization.
Owner:CHANGDE YUNGANG BIOTECHNOLOGY CO LTD
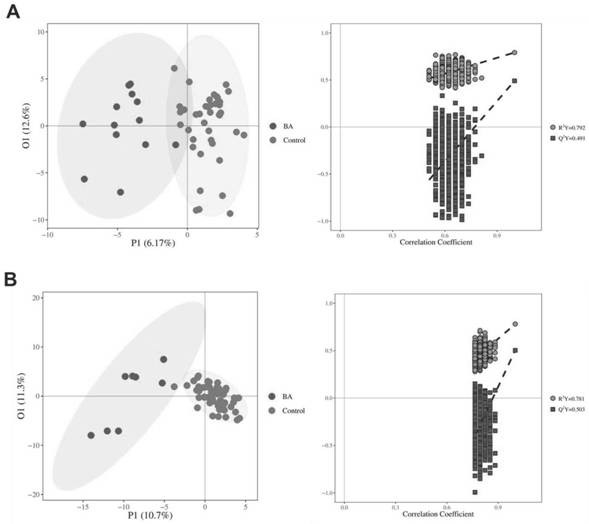
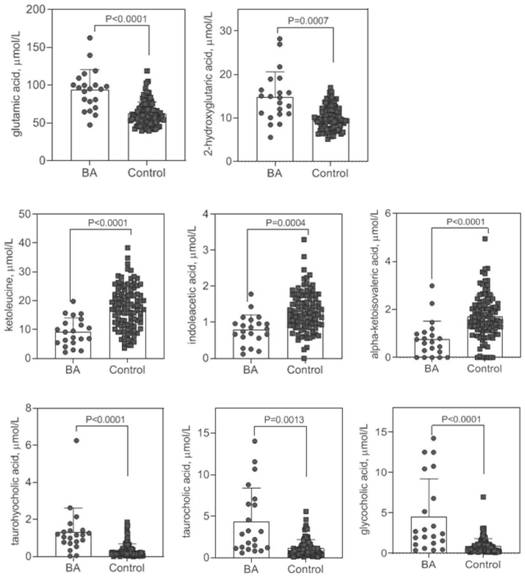
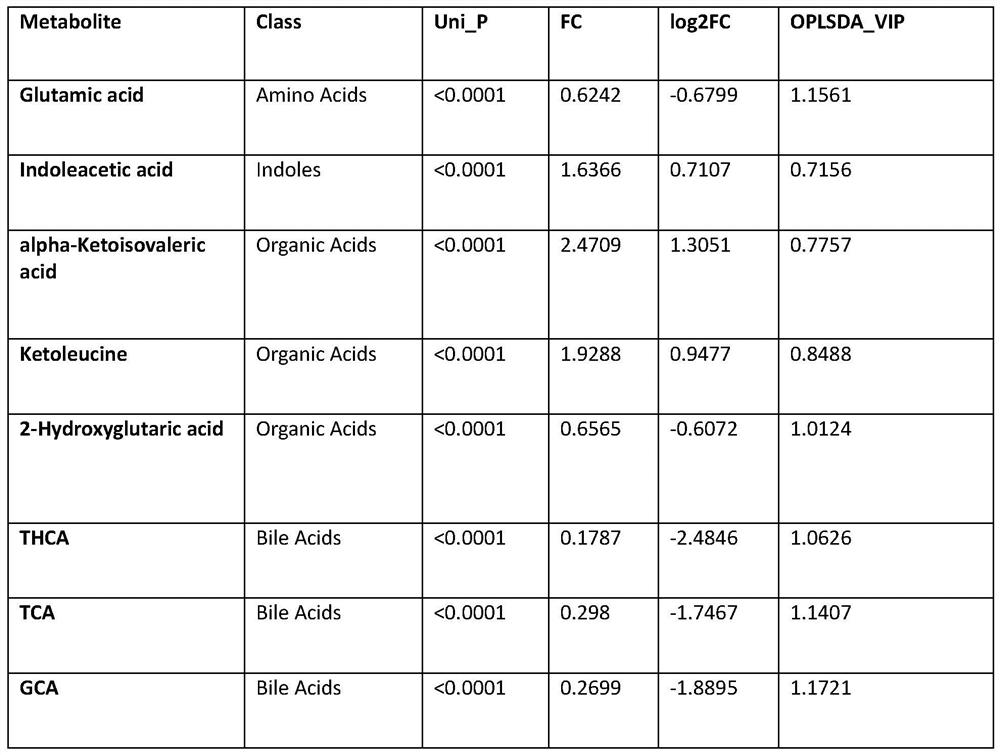
Early screening marker for biliary atresia based on neonatal blood spot metabolite and application of early screening marker
The invention provides application of a biomarker. The biomarker is characterized in that the biomarker is selected from one or more of the following specific biomarkers: A. Glutamic acid; B, indoleacetic acid (Indoleaceticacid); C, [alpha]-ketoisovalerate (alpha-ketoisovalerate), and C, [alpha]-ketoisovalerate ([alpha]-ketoisovalerate); D, ketoleucine (Ketoleucine) is used as a raw material; E. 2-hydroxyglutaric acid (2-hydroxyglutaric acid), and E. 2-hydroxyglutaric acid (2-hydroxyglutaric acid); F, taurohyocholic acid (THCA); G, taurocholic acid (TCA); and H, glycocholic acid (GCA). The application is used for preparing a screening kit for detecting early risk of neonatal biliary atresia.
Owner:SHANGHAI INST OF PEDIATRIC RES
Method for synthesizing ursodesoxycholic acid by using blanking material after extraction of bilirubin from pig gall
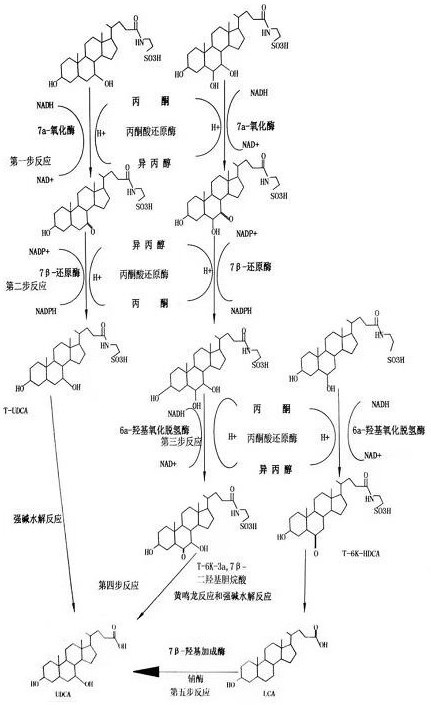
ActiveCN114317663ASolve the problem of insufficient sourcesDifficult to form large-scale productionSteroidsFermentationReaction intermediateOxygen
According to the method, chenodeoxycholic acid, hyocholic acid and hyodeoxycholic acid do not need to be separated and purified, but total bile acid in the pig gall subjected to bilirubin extraction is directly catalyzed by a compound enzyme, and then the ursodeoxycholic acid is synthesized by the ursodeoxycholic acid and the pig gall subjected to bilirubin extraction. Compared with the prior art, the method has the advantages that the conversion yield of the ursodesoxycholic acid can be increased by more than one time, meanwhile, the problem that three bile acids and reaction intermediate products thereof are impurities in the prior art is solved, the problem that purification needs to be performed firstly in the traditional conversion method is solved, the use of organic solvents in the reaction process is reduced, and the production cost is reduced. And after the reaction is finished, recycling can be realized, and environmental pollution is reduced.
Owner:CHANGDE YUNGANG BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com