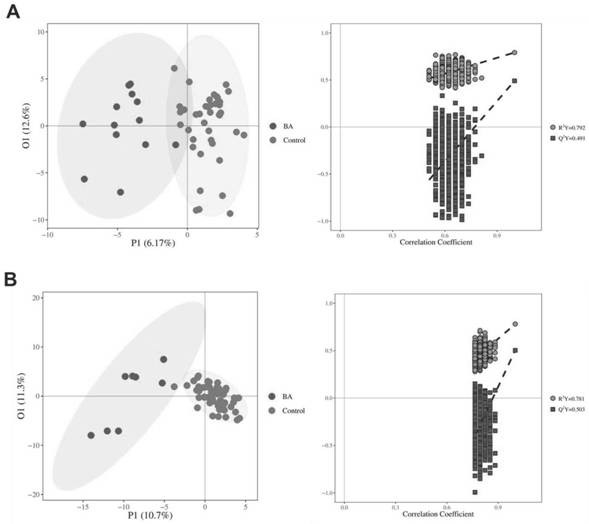
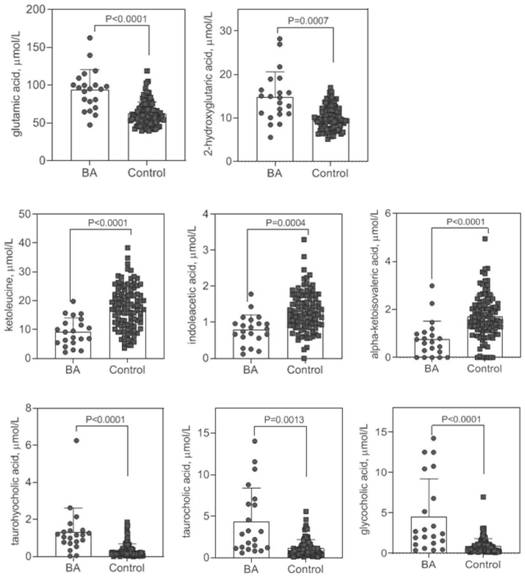
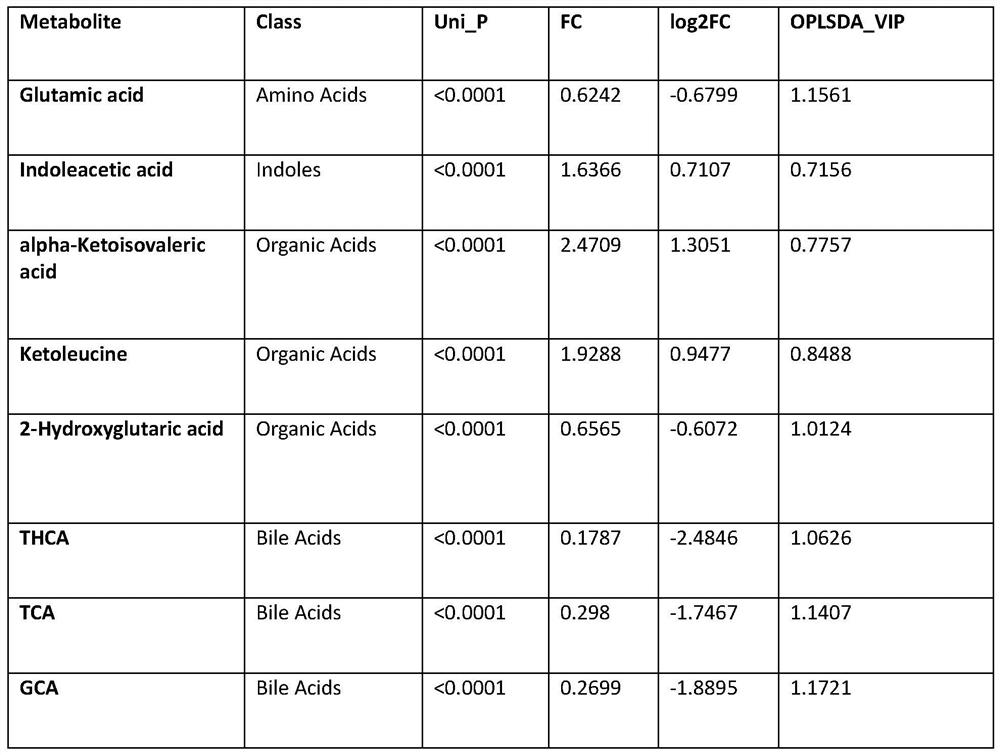
Early screening marker for biliary atresia based on neonatal blood spot metabolite and application of early screening marker
A biomarker, biliary atresia technology, applied in the field of early screening markers for biliary atresia, can solve the problems of early screening of newborn babies, poor feasibility of blood drawing and high risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0070] 1. Preparation and Collection of Neonatal Blood Spots
[0071] 1) Prepare blood spot samples within 3-4 days after birth. The specific process is as follows:
[0072] S1. Gently massage and wipe the plantar blood collection site with 75% ethanol or iodophor cotton swab.
[0073] S2. Use a disposable sterilized blood collection needle to pierce the blood collection site for 2-3 mm, and immediately withdraw the needle.
[0074] S3. After the blood flows out naturally, suck the blood with a disposable micropipette or drop the blood on the filter paper, and then press the wound with a sterile dry cotton ball to stop the bleeding.
[0075] S4. Identify the collected samples. Dry in a cool place away from light for 4 hours, put in an aluminum foil bag, and store at -80°C.
[0076] 2) Blood spot samples for this project were collected from the Neonatal Metabolic Disease Detection Platform of Shanghai Institute of Pediatric Medicine. A total of 121 cases of neonatal blood ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com