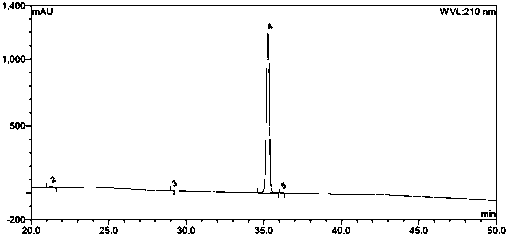
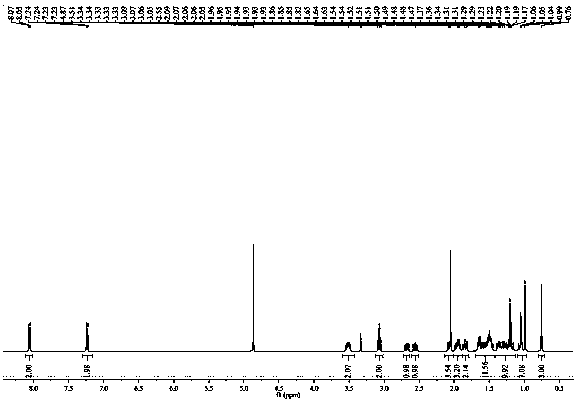
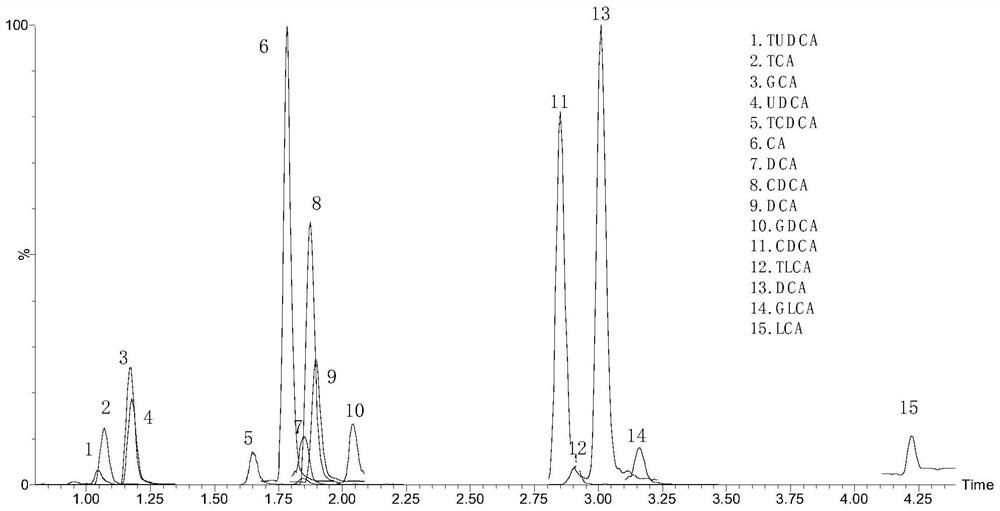
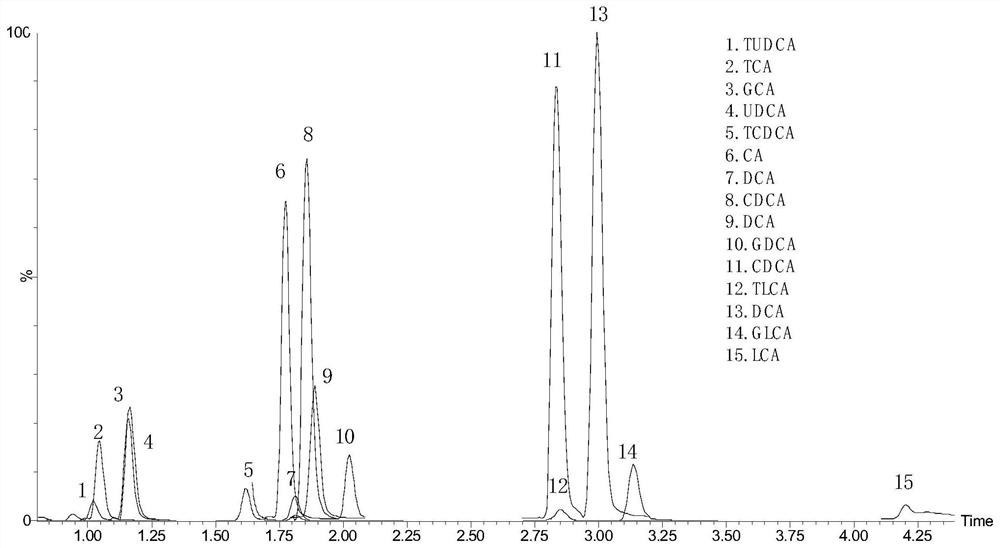
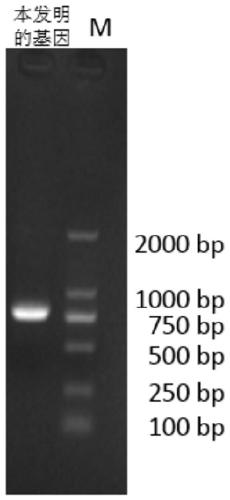
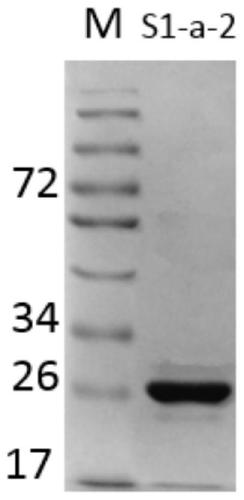
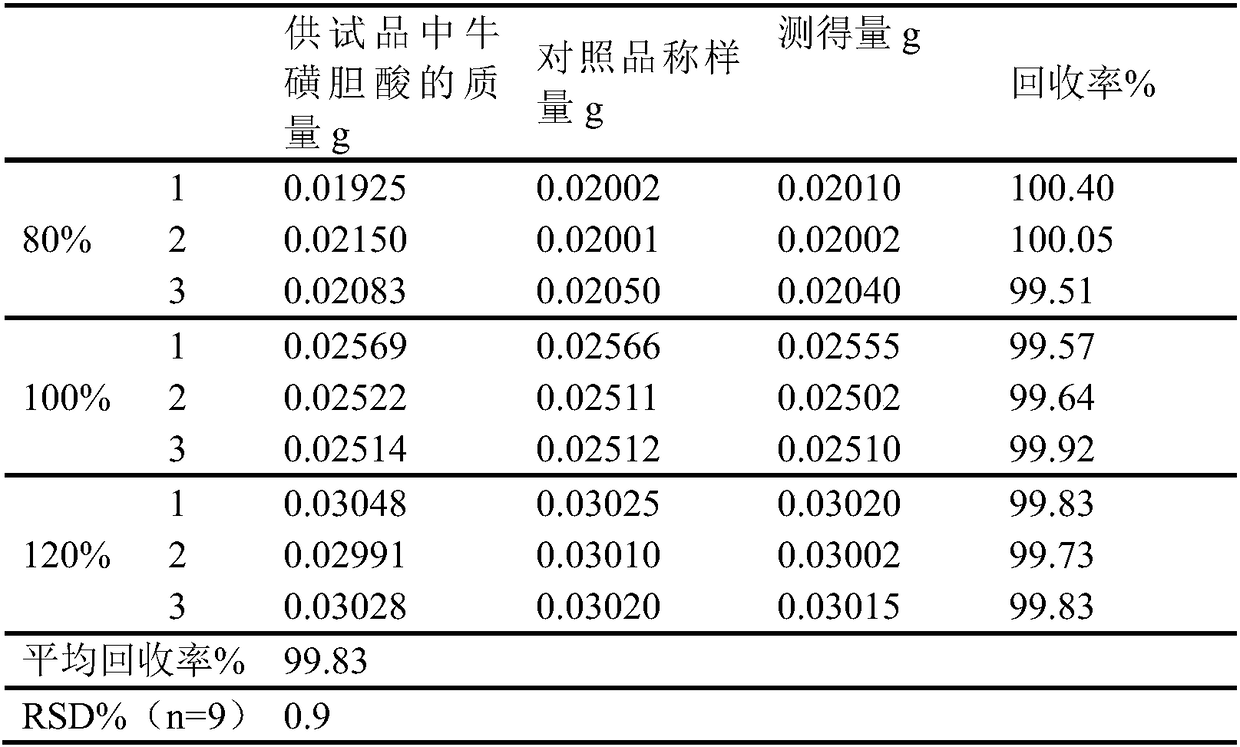
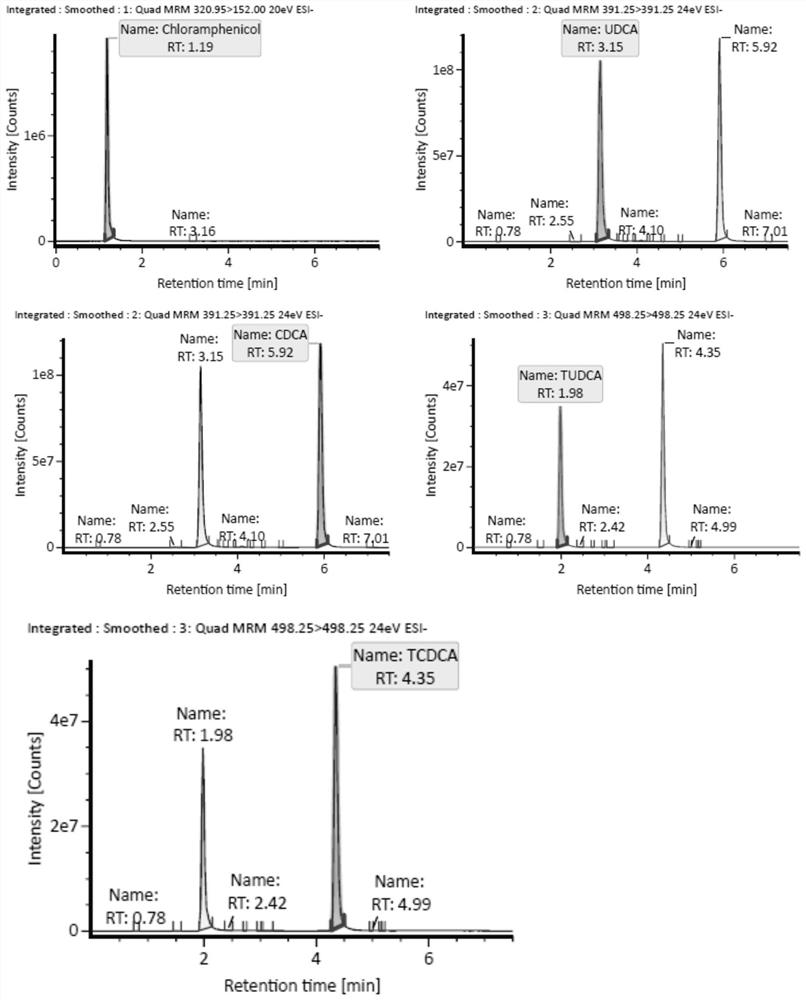
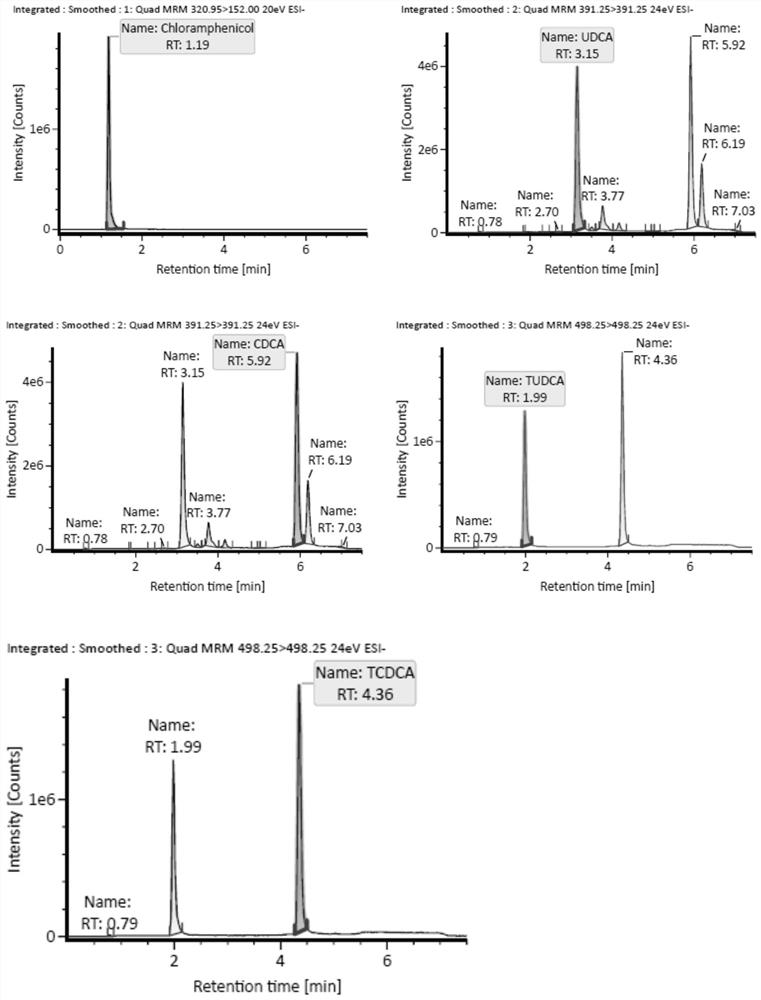
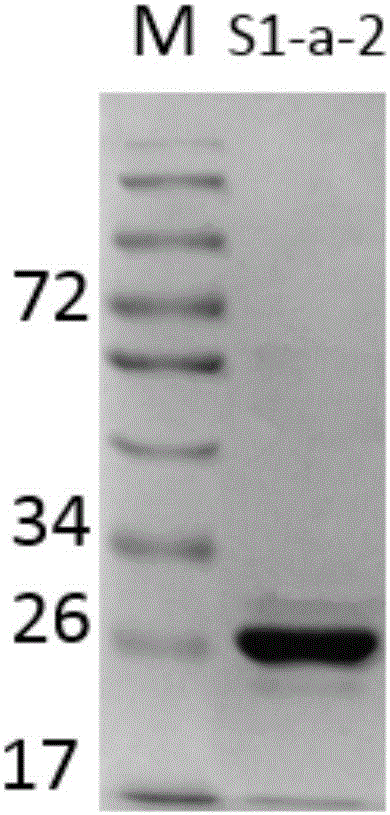
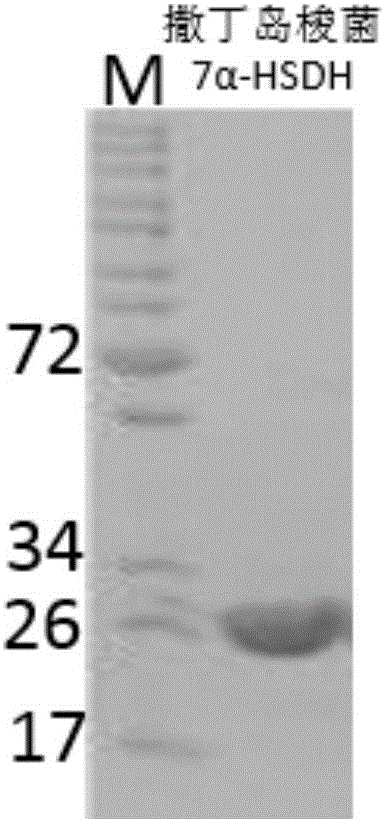
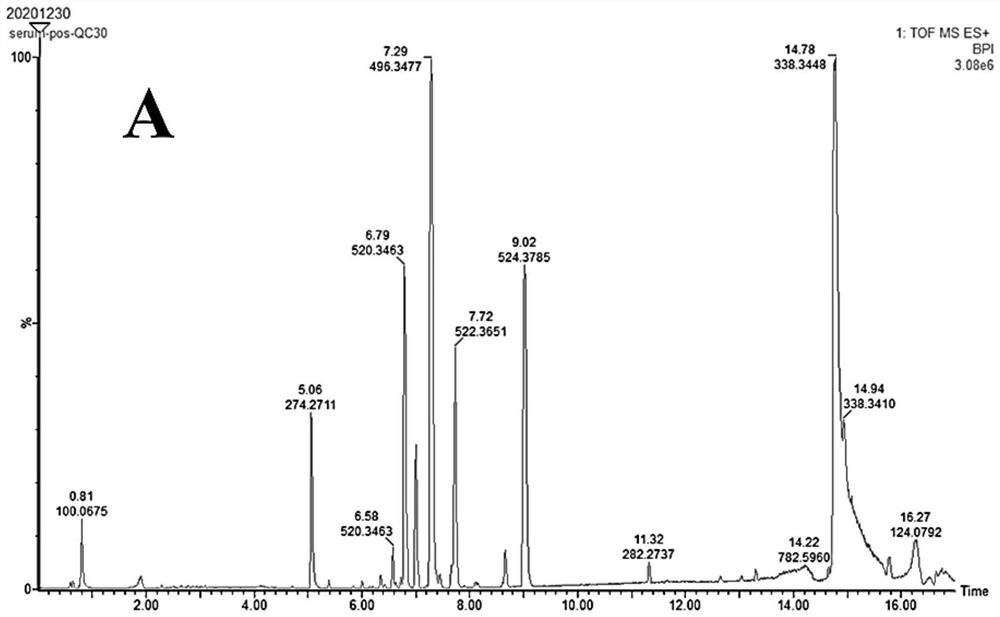
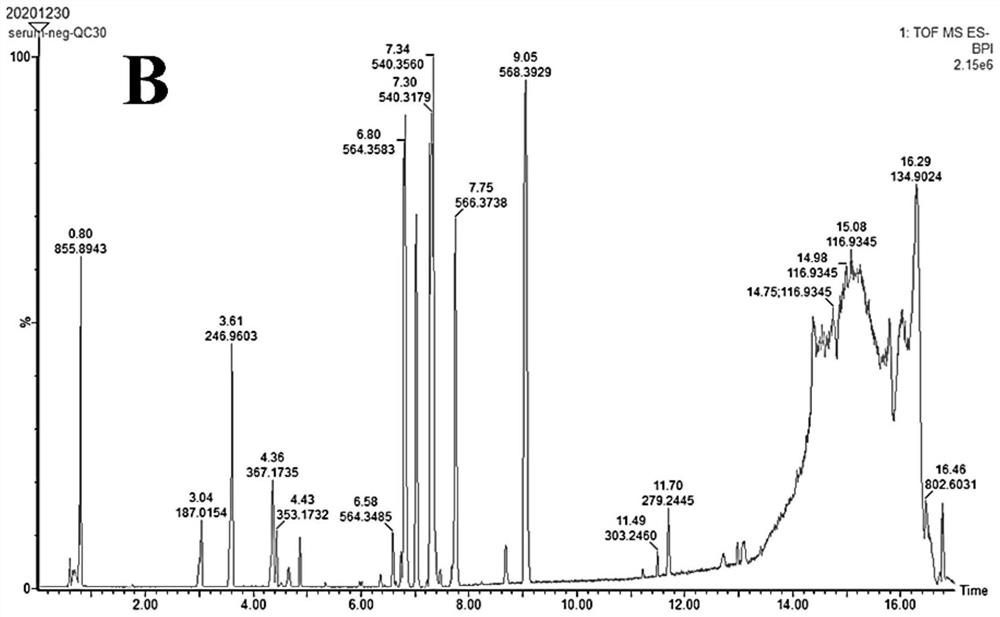
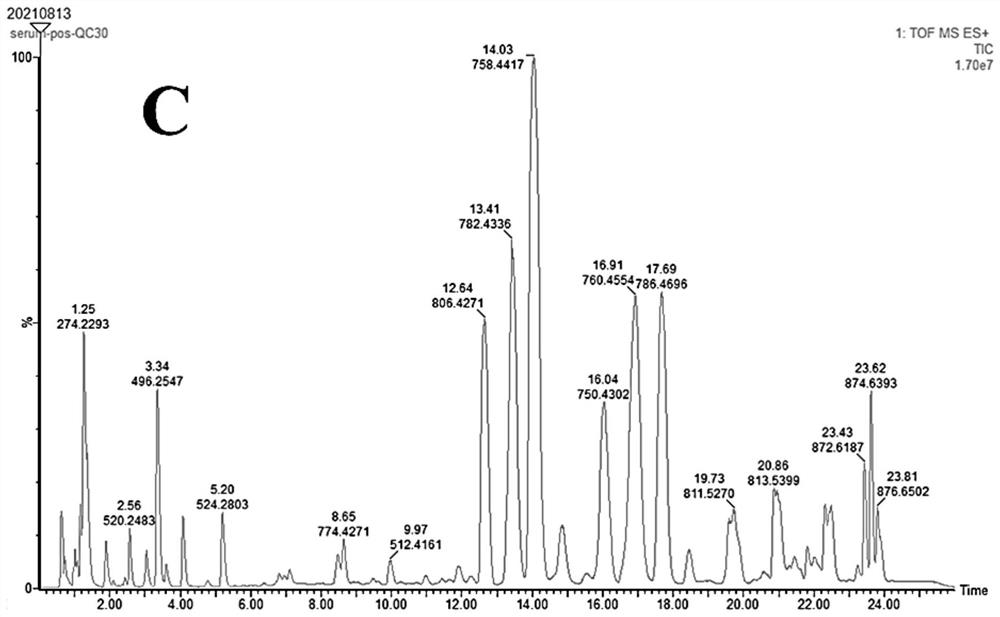
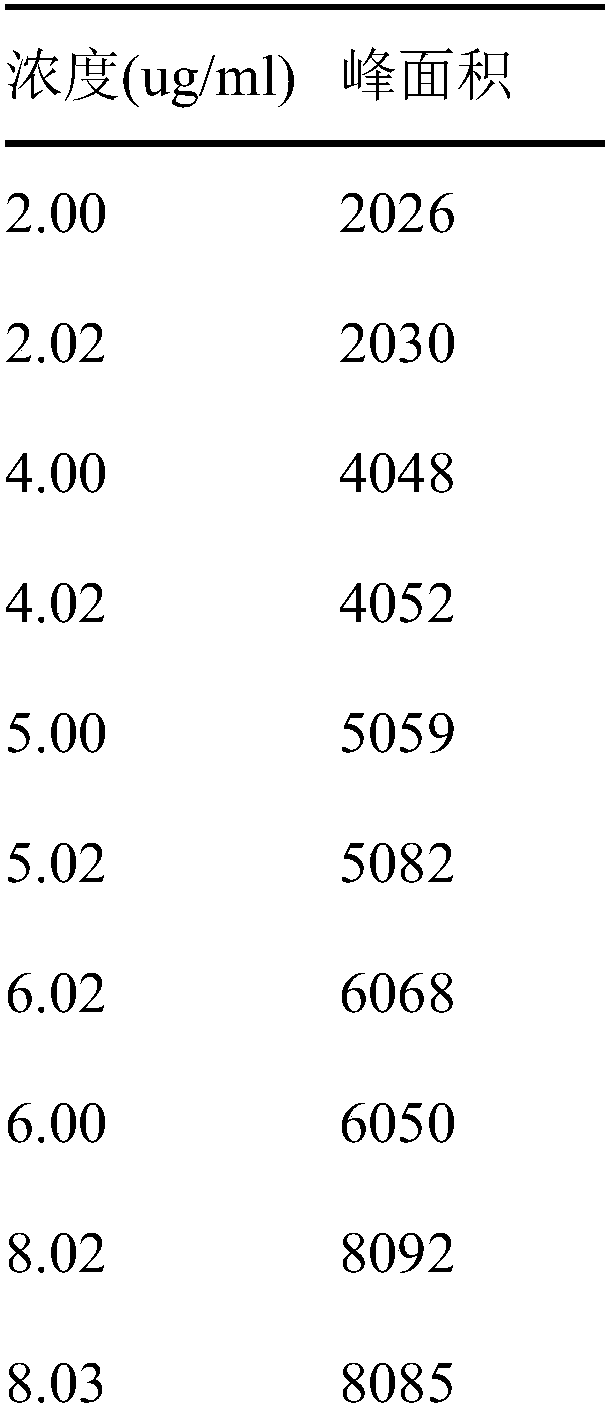Patents
Literature
49 results about "Taurochenodeoxycholic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Taurochenodeoxycholic acid is a bile acid formed in the liver of most species, including humans, by conjugation of chenodeoxycholic acid with taurine. It is secreted into bile and then into intestine. It is usually ionized at physiologic pH, although it can be crystallized as the sodium salt. It acts as detergent to solubilize fats in the small intestine and is itself absorbed by active transport in the terminal ileum. It is used as a cholagogue and choleretic.
Use of taurochenodeoxycholic acid in prevention and treatment of osteoporosis
The invention relates to new use of taurochenodeoxycholic acid, in particular to the new use of the taurochenodeoxycholic acid in prevention and treatment of osteoporosis. Through the study on taurochenodeoxycholic acid, the inventor found that the taurochenodeoxycholic acid can promote bone cells to secrete osteocalcin and improve lowered bone density. Thus, the taurochenodeoxycholic acid can beused in prevention or treatment of osteoporosis. After being administrated directly or being formed into various formulations, the taurochenodeoxycholic acid can be used for preventing or treating osteoporosis and other related diseases or states of osteoporosis.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
High-purity tauro ursodesoxy cholic acid and preparation method thereof
ActiveCN102477059AHigh purityImprove securityOrganic active ingredientsDigestive systemEthyl chloroformateCholic acid
The invention relates to high-purity tauro ursodesoxy cholic acid and a preparation method thereof. The content of taurochenodeoxycholic acid in the tauro ursodesoxy cholic acid is less than 0.7%. The tauro ursodesoxy cholic acid is safe and effective and does not have toxic and side effects in clinical application. The invention further provides a mixed anhydride reaction of ursodesoxycholic acid and ethyl chloroformate by taking acetone as a solvent. By means of control of a reaction condition and a reaction solvent, the tauro ursodesoxy cholic acid has the advantages of simple process, low cost, environmental friendlessness and industrial production; furthermore, the high-purity tauro ursodesoxy cholic acid can be obtained.
Owner:CHENGDU GUOHONG PHARMA
Method of preparing tauroursodeoxycholic acid by biotransformation and application of method
ActiveCN109402212AImprove conversion rateAvoid affinity purificationAntibody mimetics/scaffoldsNucleic acid vectorDrug biotransformationSubstrate concentration
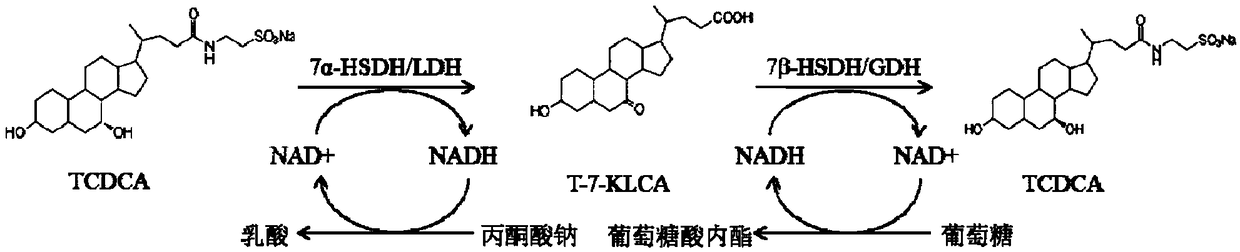
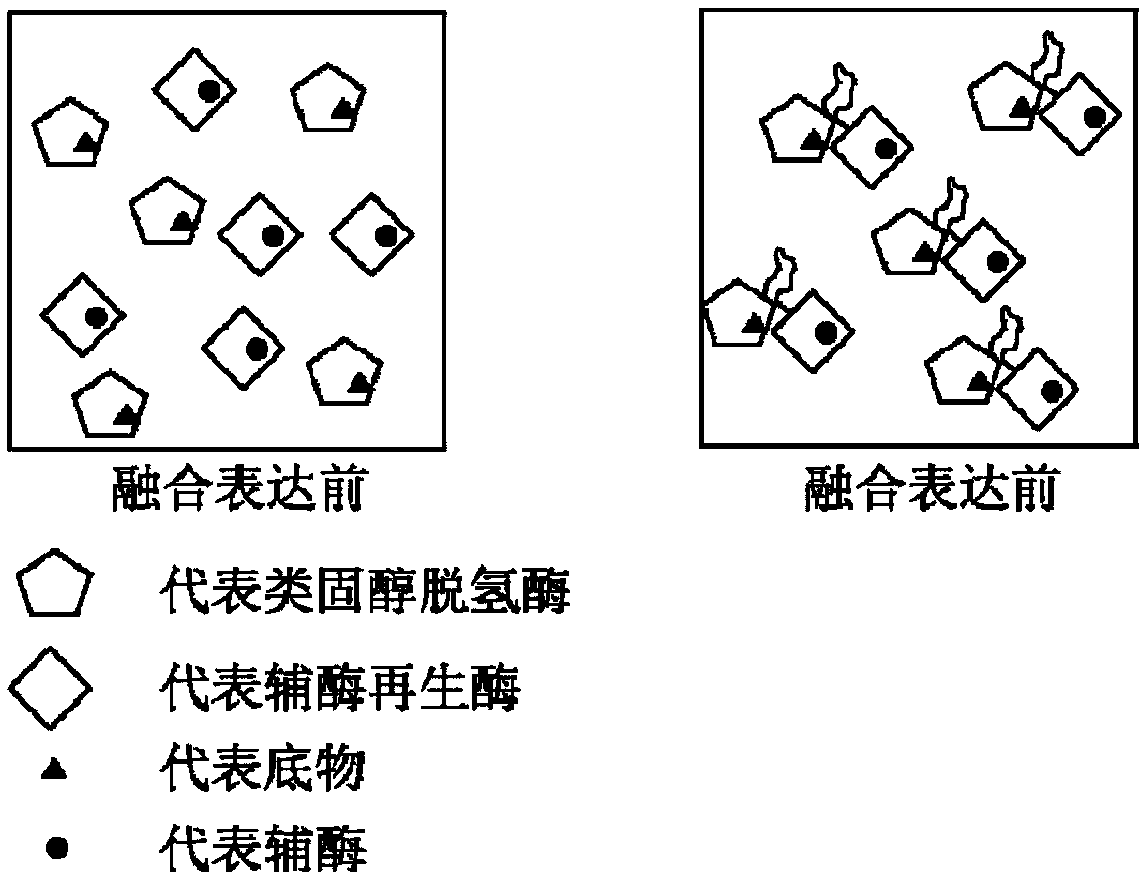
The invention discloses a method of preparing tauroursodeoxycholic acid by biotransformation and application of the method. Biotransformation includes genetic codon optimization, engineered bacteria construction, engineered bacteria cultivation, substrate transformation and product preparation. Tauroursodeoxycholic acid is prepared by transforming a substrate through direct fermentation of engineered bacteria; the substrate is taurochenodeoxycholic acid. The substrate may reach 250 g / L in concentration; the reaction time is short; substrate transformation rate reaches 98% and above; the obtained product reaches 99% and above in purity; cyclic regeneration of NAD+ (nicotinamide adenine dinucleotide +) in the reaction system helps greatly reduce the usage of the coenzyme NAD+; the cost of enzymic catalytic reaction is reduced; industrial amplification is benefited. hydroxysteroid dehydrogenase and the regenerated coenzyme are connected via a flexible polypeptide sequence to form a protein fusion polymer; binding distances to the substrate and coenzyme are shorter; transformation progress is more facilitated; the number of fermenting times in industrial production is decreased; the process is simplified; time cost and material cost are saved.
Owner:JIANGSU BANGZE BIOLOGICAL MEDICINE TECH CO LTD
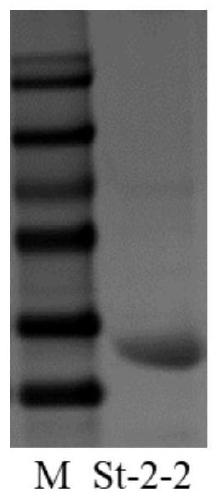
7alpha-hydroxysteroid dehydrogenase (St-2-2) mutants
ActiveCN111254126AHigh catalytic efficiencyBacteriaMicroorganism based processesHydroxysteroid DehydrogenasesHomosteroids
The invention relates to hydroxysteroid dehydrogenase, and in particular to 7alpha-hydroxysteroid dehydrogenase (St-2-2) mutants. The amino acid sequences of the mutants are shown as SEQ ID NO:2, 3, 4, 5, 6, 7, 8, 9 or 10, and are obtained by changing the 255th amino acid of the 7alpha-hydroxysteroid dehydrogenase with an amino acid sequence of SEQ ID NO:1 from Ile to Tyr, Gln, Leu, Thr, Gly, Asn,Ser, Ala or Phe. In the presence of same substrates TCDCA and NADP<+>, enzyme activity of the mutants is respectively 1.49, 1.78, 1.79, 1.79, 1.93, 2.44, 2.58, 2.97 and 3.34 times of enzyme activityof a wild type hydroxysteroid dehydrogenase, and the mutant has a great application potential in a process of obtaining tauroursodeoxycholic acid (TUDCA) through biotransformation of taurochenodeoxycholic acid (TCDCA).
Owner:CHONGQING UNIV
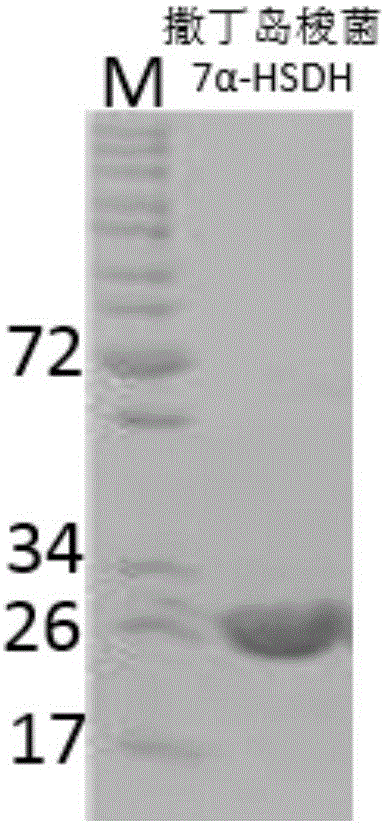
Sardinia clostridium 7alpha-hydroxy steroid dehydrogenase mutant K179M
ActiveCN107841489ACatalytic asymmetric reduction reactionOxidoreductasesGenetic engineeringCholic acidWild type
The invention belongs to the technical field of biologics and in particular relates to a Sardinia clostridium 7alpha-hydroxy steroid dehydrogenase mutant K179M of which the amino acid sequence is as shown in SEQ ID NO:2, and is generated by converting a 179-site amino acid of 7alpha-hydroxy steroid dehydrogenase of which the nucleotide sequence is as shown in SEQ ID NO:1 into methionine. The catalysis ratio activity of the mutant to TCDCA (Taurochenodeoxycholic Acid) is 2.9 times of that of a wild type. The K179M mutant provided by the invention has great application potential in the industrial production process in biological conversion of TCDCA into TUDCA (Tauro Ursodesoxy Cholic Acid).
Owner:CHONGQING UNIV
Applications of diagnostic markers in biliary atresia of newborns
InactiveCN102818866AOptimal operation timeImprove the curative effect of surgeryComponent separationCholic acidChenodeoxycholic acid
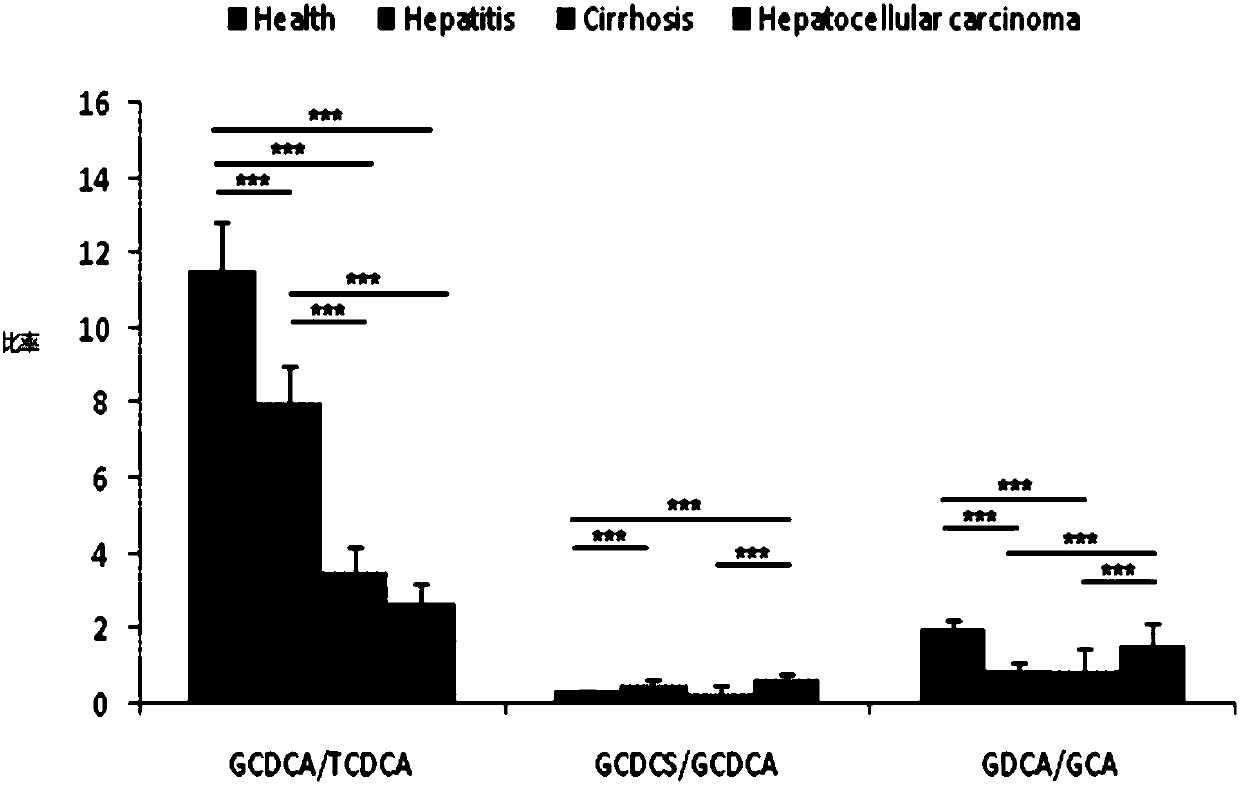
The invention relates to applications of serum taurochenodeoxycholic acid and chenodeocycholic acid as diagnostic markers in the preparation of medical instruments for diagnosing biliary atresia of newborns. The diagnostic markers of the medical instruments are content ratio of the taurochenodeoxycholic acid and the chenodeocycholic axid in serum. The invention also provides the application of chenodeocycholic acid. The application of diagnostic markers in biliary atresia of the newborns has the advantages that the application of the taurochenodeoxycholic acid and the chenodeocycholic acid as diagnostic markers of biliary atresia is proposed for the firstly time, so that the biliary atresia can be distinguished from other cholestasis diseases of newborns, the best operation time of children can be strived for, and therefore, the operation effect can be improved.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
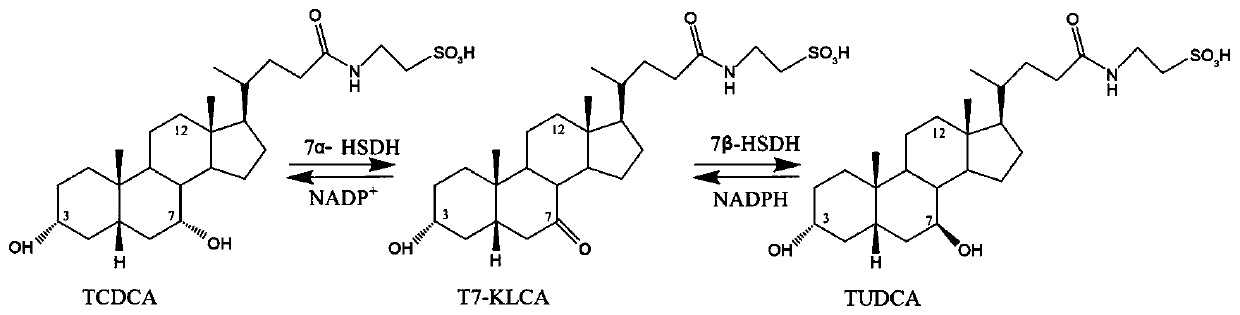
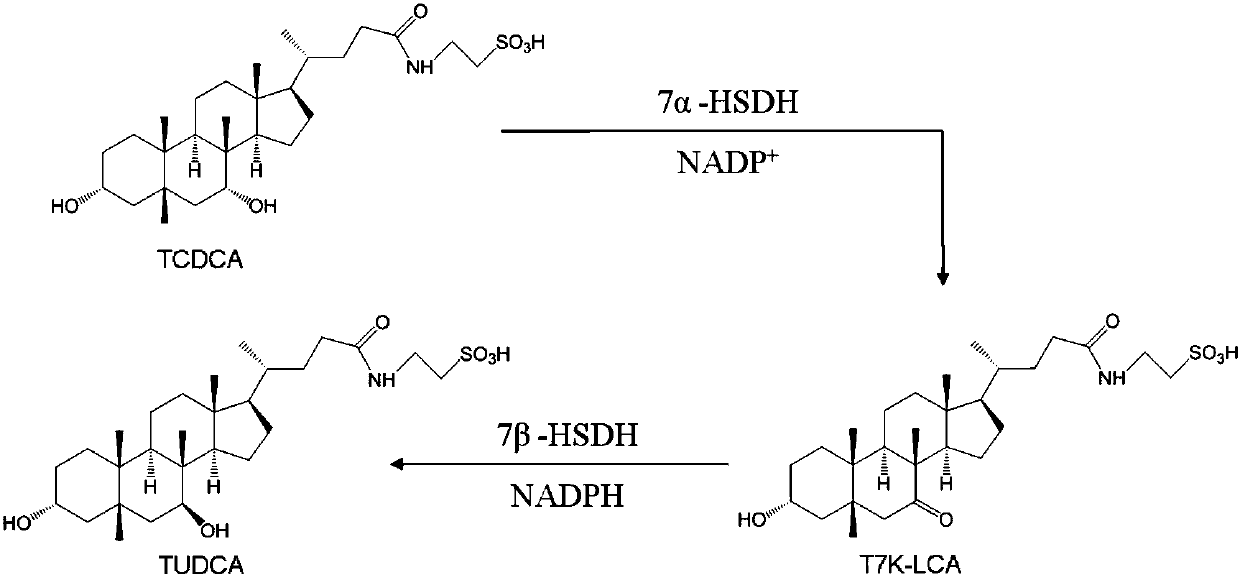
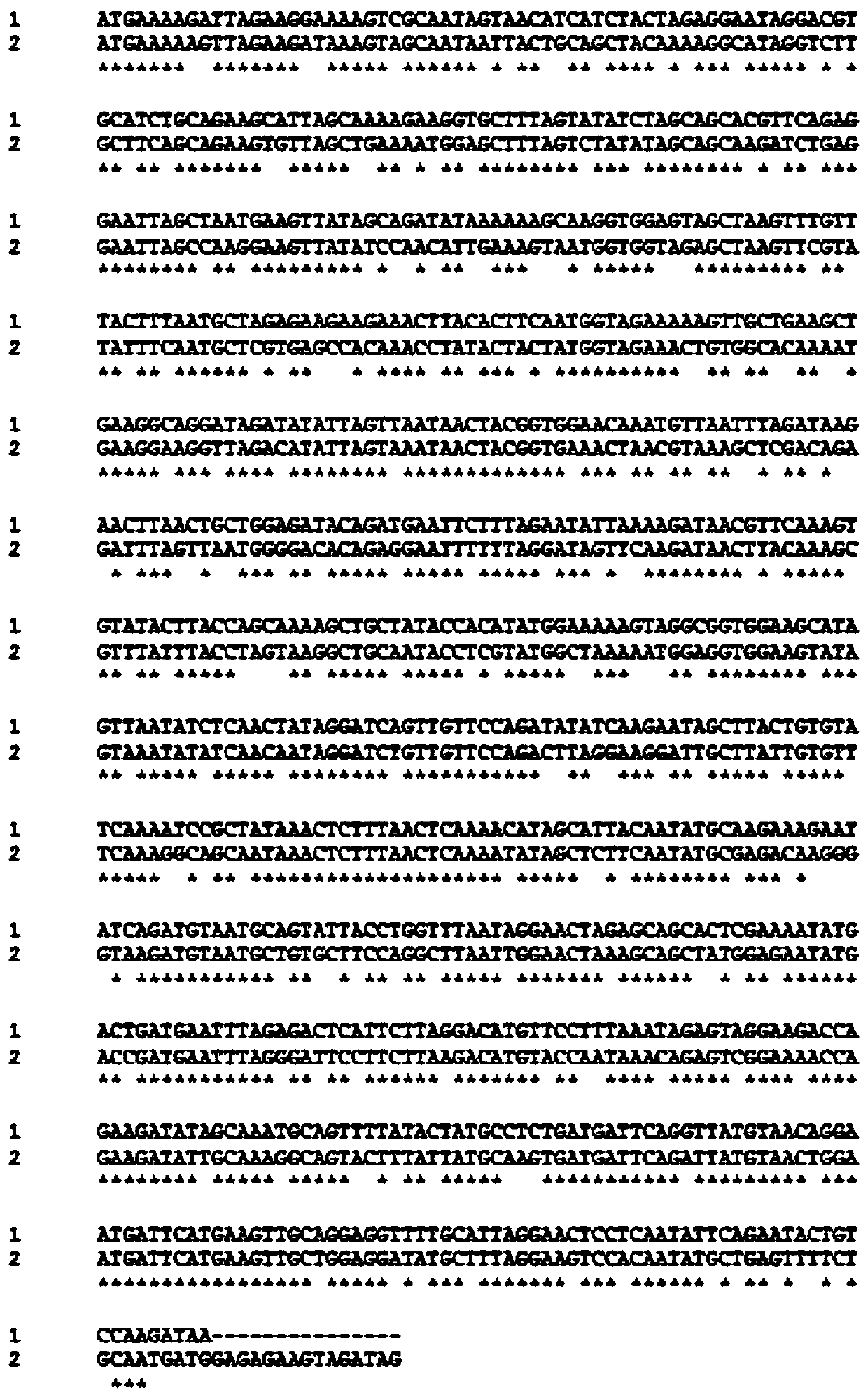
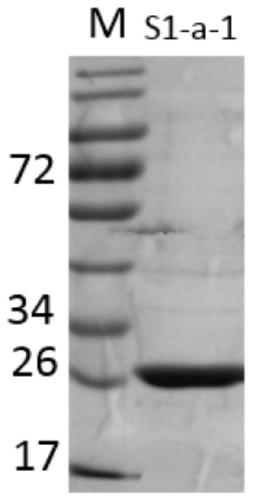
Gene S1-a-1 of novel 7 alpha-HSDH (hydroxysteroid dehydrogenase)
ActiveCN106701707ACatalytic carbonyl asymmetric reduction reactionBacteriaOxidoreductasesChenodeoxycholic acidNucleotide
The invention relates to HSDH (hydroxysteroid dehydrogenase), in particular to a gene S1-a-1 of novel 7 alpha-HSDH. A nucleotide sequence of the gene is shown as SEQ ID NO.2, the novel 7 alpha-HSDH is encoded, a nucleotide sequence of the novel 7 alpha-HSDH is shown as SEQ ID NO.1, the novel 7 alpha-HSDH can be used for catalyzing CDCA (chenodeoxycholic acid) and TCDCA (taurochenodeoxycholic acid) to generate 7K-LCA (7-ketone lithocholic acid) and T7K-LCA (taurine-7-ketone lithocholic acid), wherein the catalytic activity of the novel 7 alpha-HSDH for CDCA is about 5 times of that of 7 alpha-HSDH of Sardinia clostridium, and the catalytic activity of the novel 7 alpha-HSDH for TCDCA is about over 2.5 times of that of 7 alpha-HSDH of Sardinia clostridium, therefore, the novel 7 alpha-HSDH has a great industrial application value.
Owner:CHONGQING KINBEAR BIOTECHNOLOGY CO LTD
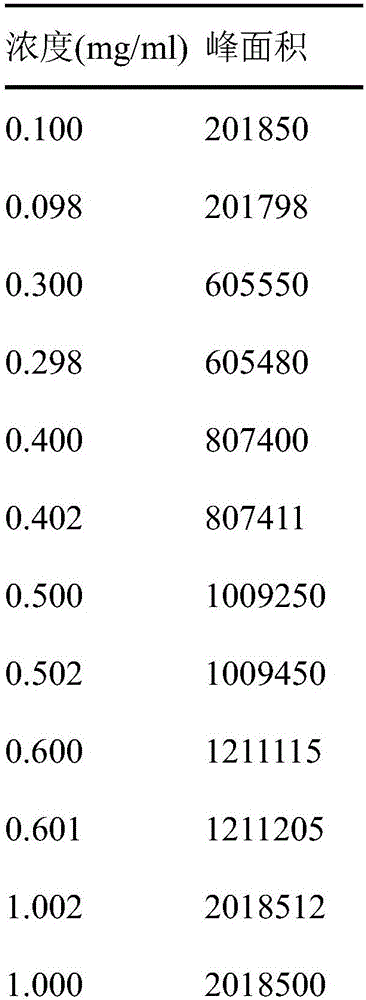
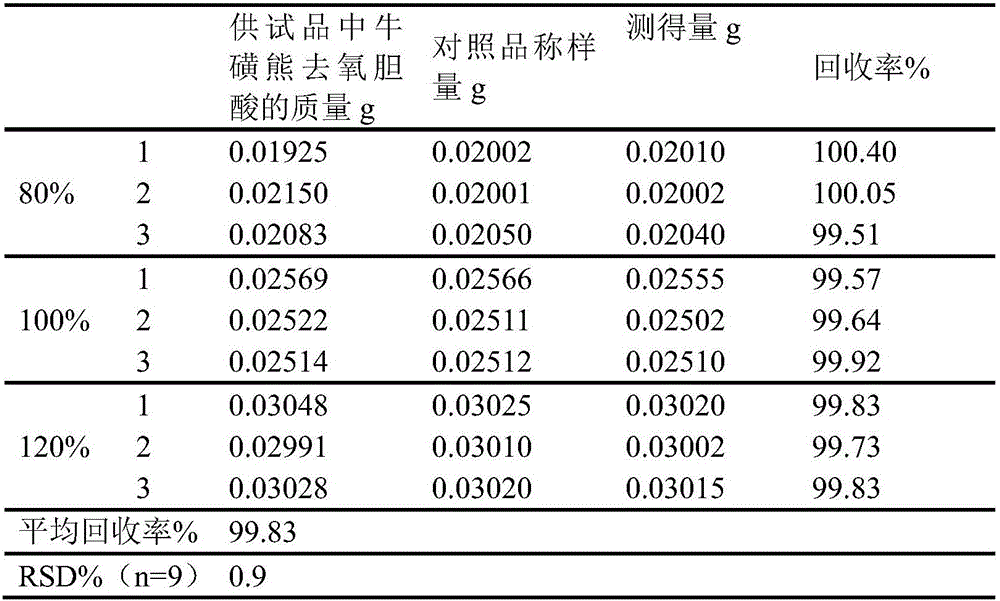
Novel detection method of tauroursodeoxycholic acid content and relevant substances
ActiveCN105891351AAccurate measurementReduce testing costsComponent separationCholic acidTest article
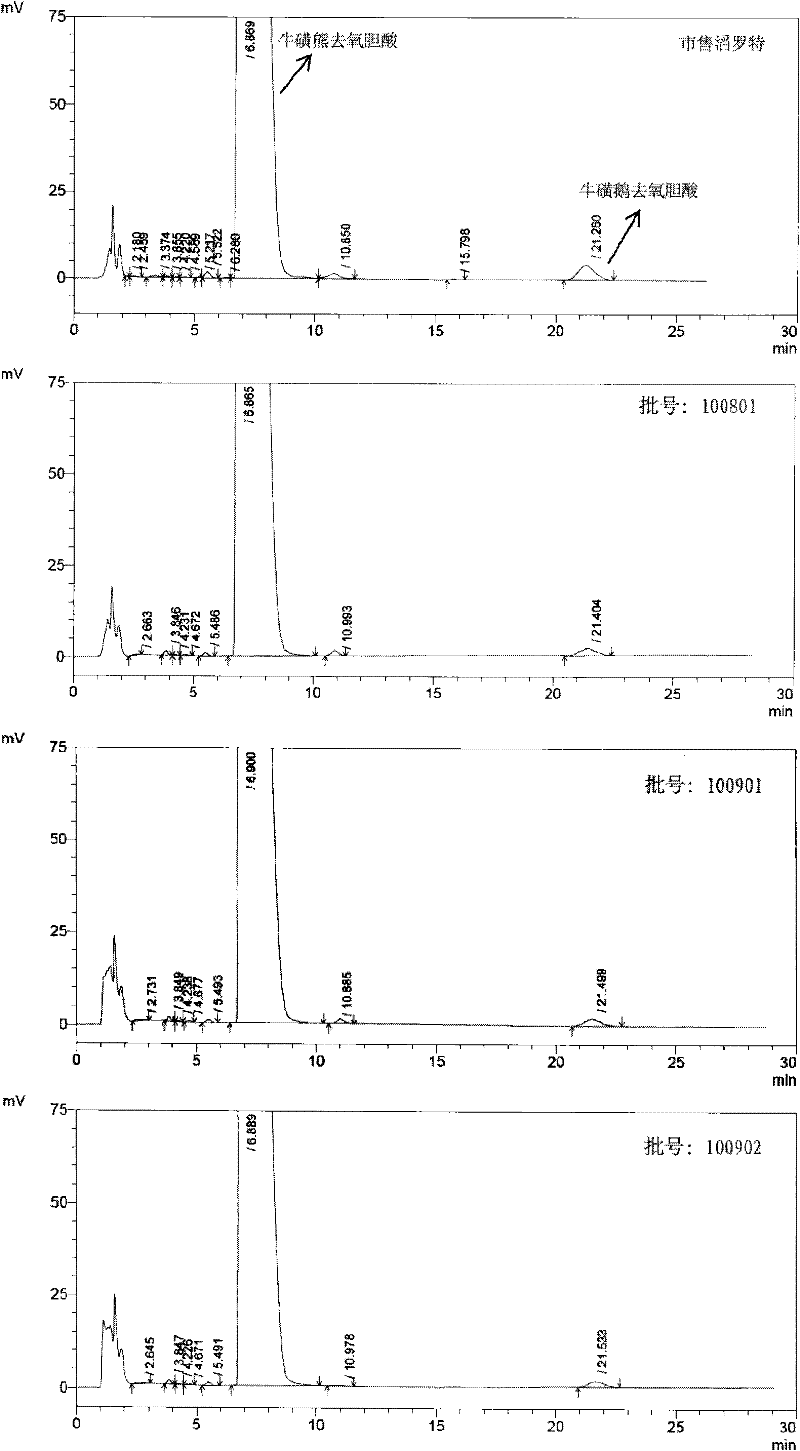
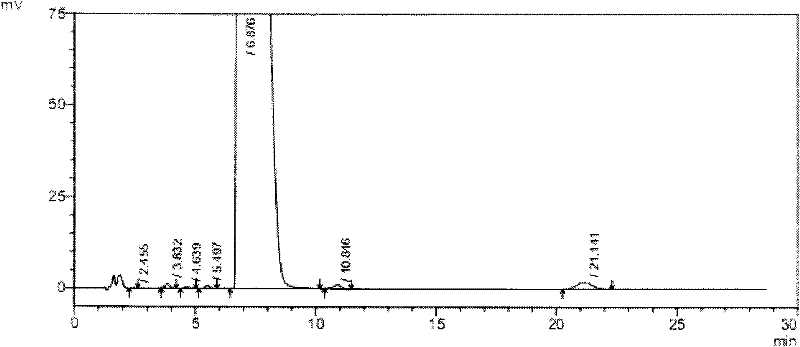
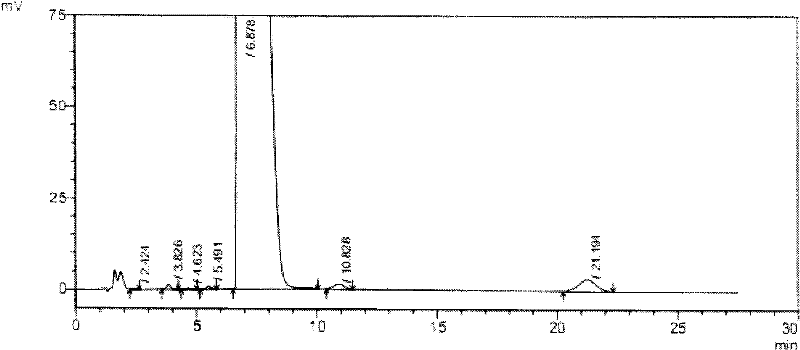
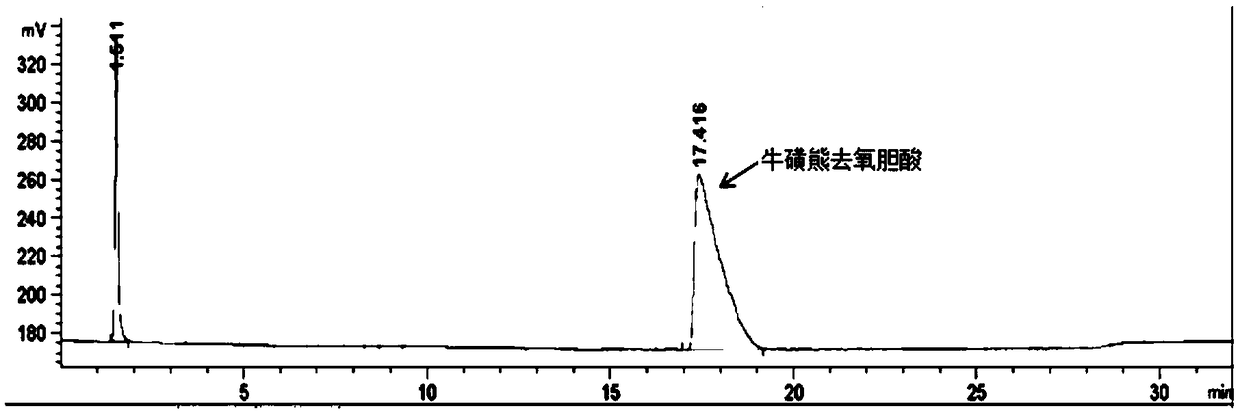
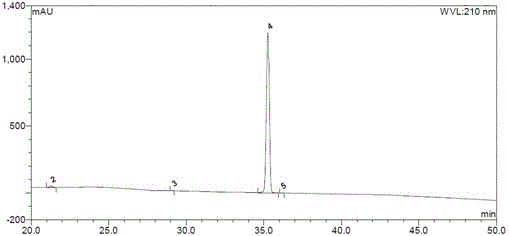
The invention discloses a novel detection method of the tauroursodeoxycholic acid content and relevant substances. The relevant substances are taurochenodeoxycholic acid and ursodesoxycholic acid. The method includes the steps of preparing a reference substance solution and a reference substance test article solution, injecting the solutions into a liquid phase chromatographic instrument, recording the peak area of tauroursodeoxycholic acid in a chromatograph, calculating the HPLC content through anhydrous tauroursodeoxycholic acid according to an external standard method, preparing an impurity reference substance solution and a sample solution, injecting the solutions into the liquid phase chromatographic instrument, recording the peak areas of taurochenodeoxycholic acid and ursodesoxycholic acid in the chromatogram, and calculating the amounts of the relevant substances in the tauroursodeoxycholic acid sample according to the external standard method. By means of the method, an RID is applied through the efficient liquid phase chromatography. Under the same liquid phase spectrum conditions, two cholic acid substances, namely tauroursodeoxycholic acid and ursodesoxycholic acid which are large in polarity difference, can be effectively separated and accurately and quantitatively detected.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Preparation process of artificial bear gall powder
PendingCN111407780AKeep aliveImprove biotransformation efficiencyPowder deliveryUnknown materialsBiotechnologyCholic acid
The invention relates to the field of biological medicine, relates to a preparation process of bear gall powder, and in particular relates to a preparation process of artificial bear gall powder. According to the scheme, a conversion solution containing cell active substances is used, so that the technical problems that the purification step of enzyme is complex, the cost is high and the enzyme activity is not easy to maintain are solved. According to the preparation process, after bioconversion is performed on livestock gall powder, the ratio of tauroursodeoxycholic acid to taurochenodeoxycholic acid of the obtained artificial bear gall powder is similar to that of natural bear gall powder. The artificial bear gall powder in the scheme can be used as a substitute of the natural bear gallpowder for deep processing and further application. The artificial bear gall powder product produced by adopting the preparation process is small in batch difference, the production process can be standardized, the conversion efficiency is improved, and industrial large-scale production can be achieved.
Owner:CHONGQING KINBEAR BIOTECHNOLOGY CO LTD
Chicken gallbladder extraction composition and extraction method thereof
InactiveCN103893210AImprove stabilityIncrease contentDigestive systemUnknown materialsCholic acidEvaporation
The present invention relates to a chicken gallbladder extraction composition, which comprises, by mass, 45-55% of taurochenodeoxycholic acid, 10-15% of other cholic acids, 5-15% of amino acids, 23-28% of proteins, and 0.5-2% of fat, wherein the chicken gallbladder extraction composition has the high taurochenodeoxycholic acid content, contains plural kinds of the amino acids, has the high amino acid content, has significant efficacy, and has high stability. The present invention further relates to an extraction method for the chicken gallbladder extraction composition. The extraction method comprises: freezing, chopping, melting and pressing chicken gallbladder, filtering to obtain the bile, adding ethanol to extract, standing, carrying out protein precipitation, filtering to obtain the supernatant, adding petroleum ether to extract, collecting the ethanol layer, and carrying out concentration evaporation dryness to obtain the chicken gallbladder extraction composition. The method has characteristics of simple process, easy operation, low cost and short purification period.
Owner:辽宁省药物研究院
Penetration promoting and eyesight improving eye drop
InactiveCN101862311ASpeed up entryGood treatment effectSenses disorderHydroxy compound active ingredientsUveitisChenodeoxycholic acid
The invention relates to an eye drop, in particular to a penetration promoting and eyesight improving eye drop. The invention aims to solve the problem of treatment period prolongation of an eye disease due to less effective components of the traditional eye drop, which really enter the inner side of an eyeball, by the isolation of a blood-eye barrier, a cornea barrier and an eye surface barrier. A penetration promoting and eyesight improving medicament in the first scheme is any combination of one or more of borneol, menthol, taurochenodeoxycholic acid or tauroursodeoxycholic acid; and a penetration promoting and eyesight improving medicament in the second technical scheme is any combination of one or more of borneol, menthol, chenodeoxycholic acid or ursodesoxycholic acid. The eye drop is used for a patient with conjunctivitis, keratitis and uveitis and used as a penetration promoting agent of other eye drops or an ophthalmic pharmaceutical partner for increasing the amount of the eye drops dropping in eyes. The eye drop can penetrate through the blood-eye barrier, the cornea barrier and the eye surface barrier; and compared with the prior art, the invention can improve the effective component entering the inner side of the eyeballs by 10-200 percent.
Owner:崔浩
Use of combined serum metabolic marker in preparation of kit for diagnosing progress of hepatopathy, kit, and method using kit to screen serum metabolic markers
InactiveCN107656007AAlleviate the problem of difficult development monitoringRapid diagnosisComponent separationSerum igeMetabolite
The invention provides a use of a combined serum metabolic marker in the preparation of a kit for diagnosing the progress of hepatopathy, the kit, and a method using the kit to screen serum metabolicmarkers, and relates to the technical field of serum metabolic markers. The combined serum metabolic marker is mainly composed of sodium glycochenodeoxycholate sulfate, glycochenodeoxycholic acid, glycodesoxycholic acid, glycocholic acid and taurochenodeoxycholic acid. The kit executes combined screening detection on the content of above serum metabolites in serum in order to realize assisted diagnosis of the progress of the hepatopathy of hepatopathy patients, so the difficult monitoring problem of the occurrence and development of the hepatopathy in existing clinic therapy of the hepatopathyis effectively alleviated.
Owner:HANGZHOU HEALTH BANK MEDICAL LAB CO LTD
Method for synthesizing tauroursodeoxycholic acid
The invention discloses a method for synthesizing tauroursodeoxycholic acid. The method comprises the steps that chenodeoxycholic acid is adopted as an initial raw material, reacting is carried out under the action of alkyl chloroformate to obtain mixed anhydride, then mixed anhydride and taurine react to obtain taurochenodeoxycholic acid, and taurochenodeoxycholic acid is sequentially subjected to an oxidation reaction and a hydrogenation reaction to obtain tauroursodeoxycholic acid. The method is low in cost, easy to operate, environmentally friendly and suitable for industrial production. Tauroursodeoxycholic acid prepared through the method is high in purity and yield.
Owner:四川新功生物科技集团有限公司
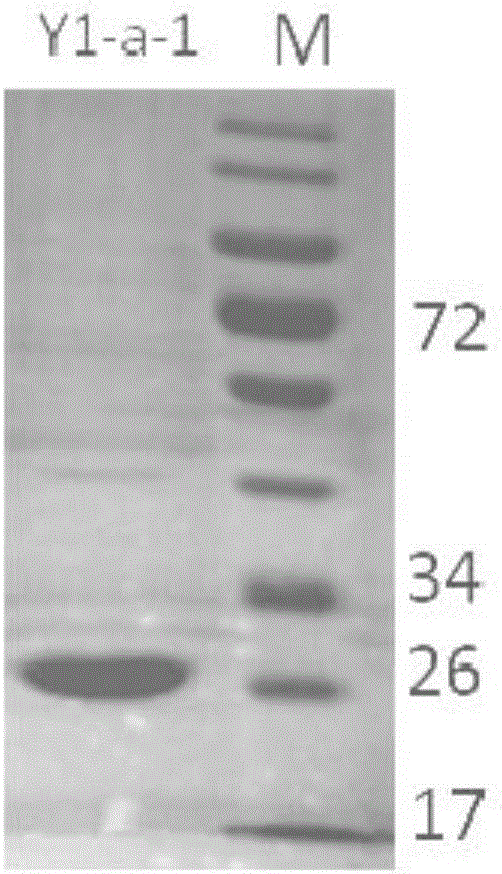
New 7alpha-hydroxysteroid dehydrogenase gene Y1-a-1
ActiveCN106701708AHigh industrial application valueBetter than activityBacteriaOxidoreductasesChenodeoxycholic acidNucleotide
The invention relates to hydroxysteroid dehydrogenase, in particular to a new 7alpha-hydroxysteroid dehydrogenase gene Y1-a-1. The nucleotide sequence of the gene is as shown in SEQ ID NO. 2. New 7alpha-hydroxysteroid dehydrogenase is coded, and the amino acid sequence of new 7alpha-hydroxysteroid dehydrogenase is as shown in SEQ ID NO.1; new 7alpha-hydroxysteroid dehydrogenase can catalyze chenodeoxycholic acid (CDCA) and taurochenodeoxycholic acid (TCDCA) to generate 7-ketone lithocholic acid (7K-LCA) and tauro 7-ketone lithocholic acid (T7K-LCA); the catalysis activity for CDCA or TCDCA is more than two times that of existing Sardinia clostridium 7alpha-HSDH, and the new 7alpha-hydroxysteroid dehydrogenase gene Y1-a-1 has an extremely large industrial application value.
Owner:CHONGQING UNIV
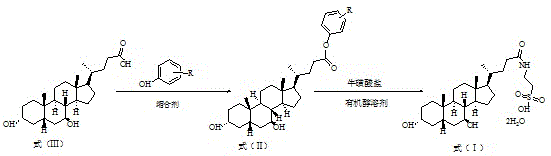
Method for preparing tauro ursodesoxy cholic acid
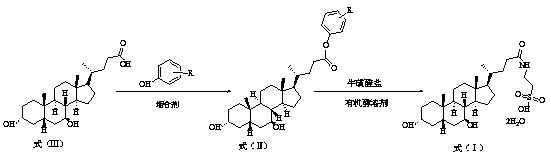
The invention discloses a method for preparing tauro ursodesoxy cholic acid. The synthetic method comprises the following steps: a step (1) of adding ursodeoxycholic acid and phenolic compounds into chloralkane organic solvent at room temperature, performing cooling to 10-20 DEG C, adding a condensing agent; after the condensing agent is added dropwise, reacting completely at 5-45 DEG C, performing filtration and then concentrating filtrate to obtain a crude product, performing recrystallization and then obtaining high-finished product ursodesoxycholic acid phenolic ester as shown in a formula (II); a step (2) of adding the high-finished product ursodesoxycholic acid phenolic ester and taurine salt into an organic alcohol solvent to undergo the reaction completely, lowering the temperature to room temperature and then performing filtration, dissolving filter cake in water, using acid to adjust the pH to 1.0-3.0, and performing stirring and crystallization to obtain tauro ursodesoxy cholic acid as shown in a formula (I). The synthetic method is low cost, simple to operate, controllable and high in product yield and purity, an isomer impurity taurochenodeoxycholic acid of tauro ursodesoxy cholic acid can be controlled well, and the method is suitable for industrial production.
Owner:HANGZHOU HEZE PHARMA TECH
Tauroursodeoxycholic acid synthesis method
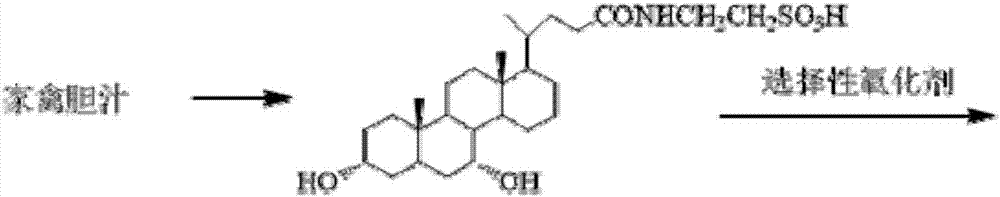
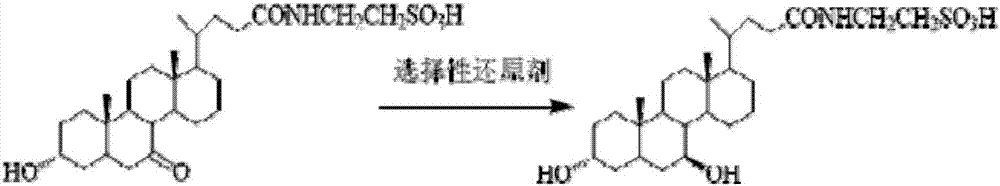
The invention discloses a tauroursodeoxycholic acid synthesis method. The method includes: taking poultry bile as a raw material, and performing process steps of dissolving, decontaminating, degreasing, deproteinizing, acidifying, treatment through ion-exchange resin and the like to obtain high-purity taurochenodeoxycholic acid; performing 7-hydroxyl selective oxidation of the taurochenodeoxycholic acid to obtain 3alpha-hydroxyl-7-keto-cholan-24-oyl-N-taurine; performing 7-keto selective reduction of 3alpha-hydroxyl-7-keto-cholan-24-oyl-N-taurine to obtain N-(3alpha,7beta-dihydroxy-5-beta-cholan-24-oyl)-taurine; finally performing re-crystallization and purification to obtain high-purity tauroursodeoxycholic acid. Cheap raw materials are adopted for acquisition of high-additional-value tauroursodeoxycholic acid through two-step reaction, short chemical synthesis route, high yield, high target product purity, simple and controllable process, few impurities, reduction of acid and alkaline emission and environmental friendliness are realized, industrialization can be realized beneficially, and the method has a promising application prospect in the pharmaceutical field.
Owner:GUANGZHOU YINGYU PHARMA TECH CO LTD
7α-hydroxysteroid dehydrogenase gene s1-a-1
ActiveCN106701707BCatalytic carbonyl asymmetric reduction reactionBacteriaOxidoreductasesCholic acidChenodeoxycholic acid
The invention relates to HSDH (hydroxysteroid dehydrogenase), in particular to a gene S1-a-1 of novel 7 alpha-HSDH. A nucleotide sequence of the gene is shown as SEQ ID NO.2, the novel 7 alpha-HSDH is encoded, a nucleotide sequence of the novel 7 alpha-HSDH is shown as SEQ ID NO.1, the novel 7 alpha-HSDH can be used for catalyzing CDCA (chenodeoxycholic acid) and TCDCA (taurochenodeoxycholic acid) to generate 7K-LCA (7-ketone lithocholic acid) and T7K-LCA (taurine-7-ketone lithocholic acid), wherein the catalytic activity of the novel 7 alpha-HSDH for CDCA is about 5 times of that of 7 alpha-HSDH of Sardinia clostridium, and the catalytic activity of the novel 7 alpha-HSDH for TCDCA is about over 2.5 times of that of 7 alpha-HSDH of Sardinia clostridium, therefore, the novel 7 alpha-HSDH has a great industrial application value.
Owner:CHONGQING KINBEAR BIOTECHNOLOGY CO LTD
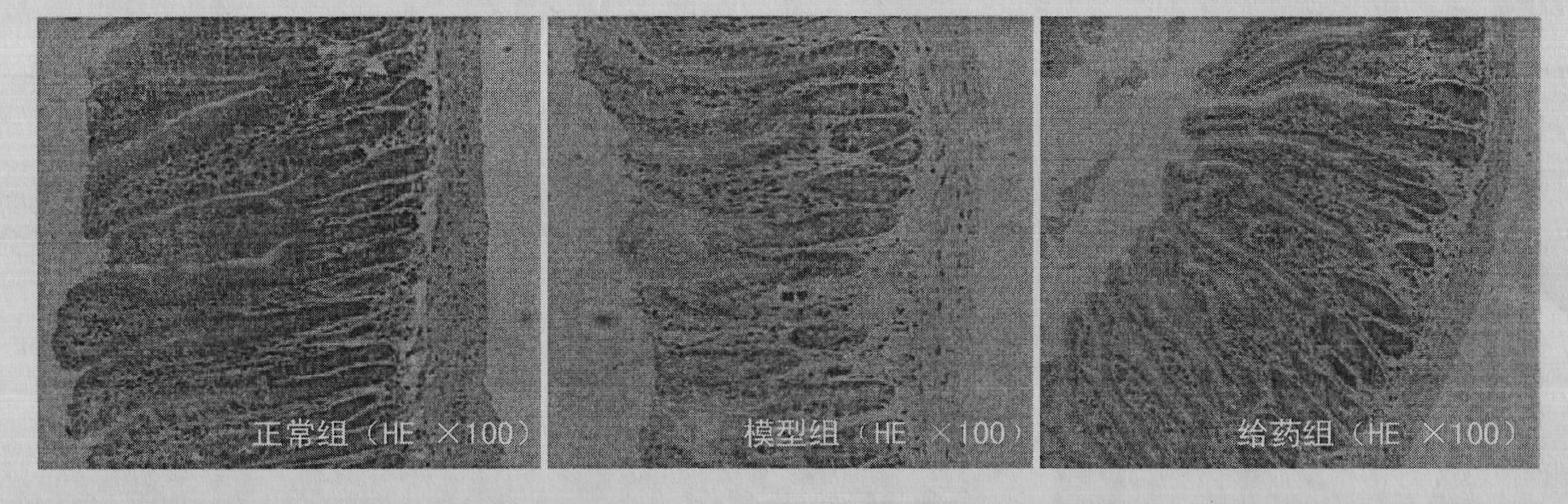
Application of taurochenodeoxycholic acid to protection of gastrointestinal tract function
The invention relates to new application of taurochenodeoxycholic acid, in particular to new application of taurochenodeoxycholic acid to protection of a gastrointestinal tract function. The inventor researches the taurochenodeoxycholic acid to find that the taurochenodeoxycholic acid can enhance the immune function of mucosa, can promote proliferation of enteral stem cells and can alleviate alimentary tract tissue structure injury, so that the taurochenodeoxycholic acid can be applied to protecting the gastrointestinal tract function, can be administrated directly, and can also be prepared into various formulations of medicaments for enhancing mucosal immunization and preventing and treating gastrointestinal diseases.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
7alpha-oxhydryl sterol dehydrogenase and encoding gene and application thereof
The invention relates to 7alpha-oxhydryl sterol dehydrogenase and an encoding gene and application thereof, and belongs to the technical field of biology. The 7alpha-oxhydryl sterol dehydrogenase is protein shown in SEQ ID NO.1 or protein with the same functions, obtained through substitution and / or deletion and / or addition of one or more amino acid residues of an amino acid sequence shown in theSEQ ID NO.1. The 7alpha-oxhydryl sterol dehydrogenase can catalyze an asymmetric reduction reaction of carbanyl groups of C7alpha-oxhydryl groups of taurocholic acid, glycocholic acid, taurochenodeoxycholic acid and glycochenodeoxycholic acid and catalyze an asymmetric reduction reaction of carbanyl groups and oxhydryl groups in benzoyl groups of ethyl benzoylformate. Compared with 7alpha-oxhydrylsterol dehydrogenase in clostridium sardiniense, the 7alpha-oxhydryl sterol dehydrogenase has higher catalytic activity and thermostability, and has very high industrial application value.
Owner:CHONGQING UNIV
Application of taurochenodeoxycholic acid in preventing and treating adverse reactions of glucocorticoid medicaments
InactiveCN102178684APromote proliferationPromote secretionOrganic active ingredientsDigestive systemOsteoblastAdrenal cell
The invention relates to novel application of taurochenodeoxycholic, and particularly relates to novel application of the taurochenodeoxycholic acid in treating adverse reactions of glucocorticoid medicaments. Through studying on the taurochenodeoxycholic acid, the inventor discovers that the taurochenodeoxycholic acid can resist apoptosis of suprarenal gland cells and promote multiplication of adrenal cortex stem cells so as to recover the hypothalamic-pituitary-adrenal axis inhibition caused by the glucocorticoid medicaments, promotes osteoblast to secrete osteocalcin and improve the reduced bone mineral density so as to recover osteoporosis caused by the glucocorticoid medicaments, and can reinforce the mucosal immunity function and promote multiplication of small intestine stem cells; therefore, the taurochenodeoxycholic acid can be directly administrated or made into various preparations or mixtures for preventing and treating adverse reactions caused by the glucocorticoid medicaments.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Preparation method of tauroursodeoxycholic acid
The invention discloses a preparation method of tauroursodeoxycholic acid. A synthesis method comprises the following steps of (1) adding the ursodesoxycholic acid and a phenolic compound into a chloroalkane organic solvent at a room temperature, cooling to 10-20 DEG C, adding a condensing agent, reacting at 5-45 DEG C until the dropwise addition is completed, filtering, concentrating the filtrate to obtain a crude product, and recrystallizing to obtain a refined ursodesoxycholic acid phenolic ester product shown in a formula (II); and (2) at 50-80 DEG C, adding the ursodeoxycholic acid phenolic ester refined product and the taurine salt into an organic alcohol solvent for complete reaction, cooling to the room temperature, filtering, dissolving a filter cake into water, adjusting the pHvalue to 1.0-3.0 with acid, stirring and crystallizing to obtain the tauroursodeoxycholic acid shown as a formula (I). The synthesis method is low in cost, simple and controllable in operation, high in product yield and purity, is capable of well controlling the isomer impurity of taurochenodeoxycholic acid, and is suitable for industrial production.
Owner:HANGZHOU HEZE PHARMA TECH
Kit for detecting 15 bile acids in serum and application of kit
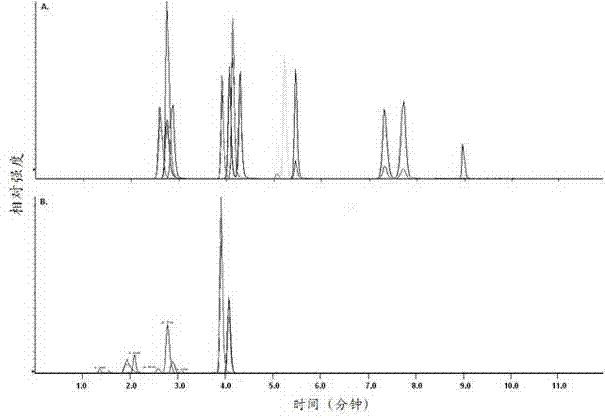
The invention discloses a kit for detecting 15 bile acids in serum and an application of the kit, and belongs to the technical field of blood detection. The 15 bile acids comprise a cholic acid, a glycocholic acid, a taurocholic acid, a lithocholic acid, a glycolithocholic acid, a taurolithocholic acid, a deoxycholic acid, a glycodeoxycholic acid, a taurodeoxycholic acid, a chenodeoxycholic acid,a glycochenodeoxycholic acid, a taurochenodeoxycholic acid, an ursodeoxycholic acid, a glycoursodeoxycholic acid and a tauroursodeoxycholic acid. The kit comprises an eluent, a calibration product solution, a mixed internal standard working solution, a protein precipitant and a quality control product. When the kit is used for detecting the bile acids in the serum, a to-be-detected sample does notneed derivatization treatment, pretreatment is simple, a sample dosage is small, sensitivity is high, specificity is strong, more types can be detected, the 15 types of bile acid can be simultaneously detected within 6.5 minutes, and the kit can be used for clinical diagnosis and health assessment of serum bile acids.
Owner:NANJING PINSHENG MEDICAL TECH CO LTD
Engineering saccharomyces cerevisiae and method for preparing artificial bear bile powder
PendingCN112779175ASimple and fast operationIncrease enzyme activityFungiMicroorganism based processesCholic acidHomosteroids
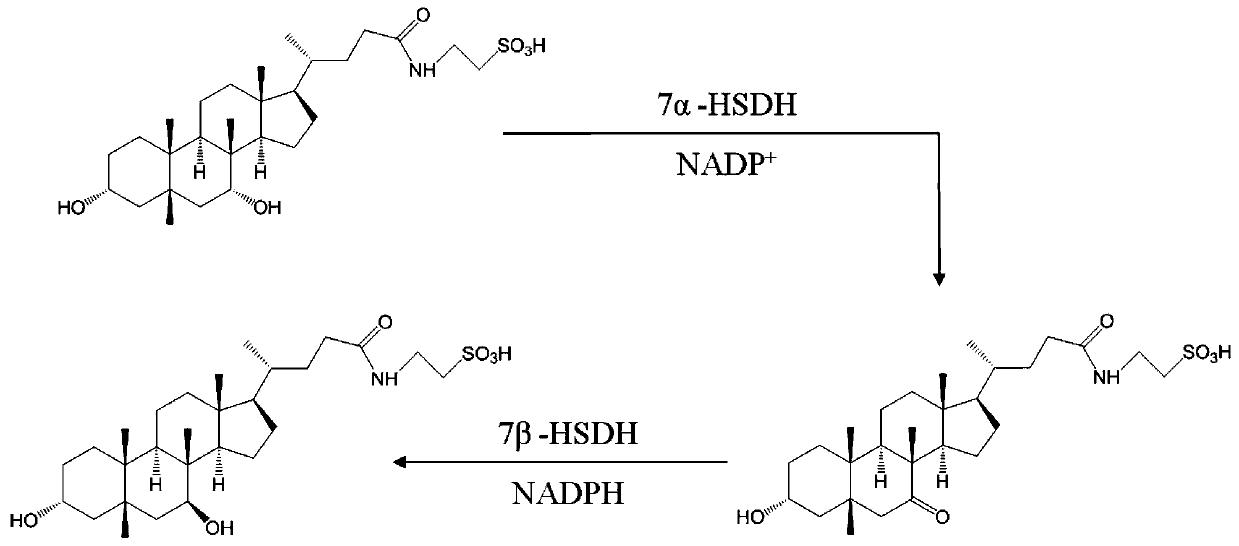
The invention relates to the field of biotechnology and medicine, in particular to a method for preparing artificial bear bile powder by biocatalysis of active substances expressed by engineering saccharomyces cerevisiae. The method comprises the following steps: performing liquid culture on saccharomyces cerevisiae which simultaneously expresses 7alpha-hydroxysteroid dehydrogenase (7alpha-HSDH) and 7beta-hydroxysteroid dehydrogenase (7beta-HSDH) genes to prepare a crude enzyme extract; directly converting taurochenodeoxycholic acid in poultry bile products such as chicken gall powder into tauroursodeoxycholic acid with a certain proportion. According to the method, in-vitro double-enzyme catalysis is used, NADP+ is added as an auxiliary factor to promote the reaction, the crude enzyme preparation process is simple, the catalytic reaction condition is mild, the result is stable, the whole process is green, safe, rapid, environment-friendly, pollution-free and controllable, and great significance is achieved by utilizing the biotechnology to replace resources with bear bile powder.
Owner:SHANGHAI UNIV OF T C M
7α-hydroxysteroid dehydrogenase gene s1-a-2
ActiveCN106676079BCatalytic carbonyl asymmetric reduction reactionBacteriaOxidoreductasesCholic acidChenodeoxycholic acid
The invention relates to hydroxysteroid dehydrogenase, in particular to a novel 7alpha-hydroxysteroid dehydrogenase gene S1-a-2. A nucleotide sequence of the gene is shown as SEQ ID NO.2. A novel 7alpha-hydroxysteroid dehydrogenase with an amino acid sequence shown as SEQ ID NO.1 is coded and is capable of catalyzing CDCA (chenodeoxycholic acid) and TCDCA (taurochenodeoxycholic acid) to generate 7K-LCA (7-ketone lithocholic acid) and T7K-LCA (tauro-7-keto lithocholic acid), catalytic activity of the novel 7alpha-hydroxysteroid dehydrogenase for the CDCA or TCDCA is about twice of that of Clostridium sardiniense 7alpha-HSDH for the same, and accordingly the novel 7alpha-hydroxysteroid dehydrogenase is high in industrial application value.
Owner:CHONGQING UNIV
Novel method for detecting taurocholic acid content and related substances
ActiveCN108254461AAccurate measurementReduce testing costsComponent separationCholic acidChenodeoxycholic acid
The invention discloses a novel method for detecting a taurocholic acid content and related substances. The method comprises the following steps: taking a taurocholic acid-TRC reference substance as aknown reference substance, dissolving the reference substance by a diluent to form a reference substance solution, and dissolving a taurocholic acid sample by a diluent to form a reference substancesample solution; injecting 20ul of each solution into a liquid chromatograph respectively, recording a peak area, and carrying out calculation according to an external standard method on the basis ofanhydrous taurocholic acid; taking cholic acid as a reference substance, dissolving the reference substance by a mobile phase to form a concentration impurity reference substance solution, and dissolving the taurocholic acid sample by a diluent to form a concentration sample solution; injecting 20ul of each solution into the liquid chromatograph respectively, recording the peak areas of sodium taurocholate and cholic acid in a chromatogram, and calculating the sizes of related substances in the taurocholic acid sample according to the external standard method. Effective separation and accuratequantitative detection of taurochenodeoxycholic acid and chenodeoxycholic acid which are two cholic acid substances with large difference in polarities are realized under the same liquid chromatography condition.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Applications of diagnostic markers in biliary atresia of newborns
InactiveCN102818866BOptimal operation timeImprove the curative effect of surgeryComponent separationCholic acidChenodeoxycholic acid
The invention relates to applications of serum taurochenodeoxycholic acid and chenodeocycholic acid as diagnostic markers in the preparation of medical instruments for diagnosing biliary atresia of newborns. The diagnostic markers of the medical instruments are content ratio of the taurochenodeoxycholic acid and the chenodeocycholic axid in serum. The invention also provides the application of chenodeocycholic acid. The application of diagnostic markers in biliary atresia of the newborns has the advantages that the application of the taurochenodeoxycholic acid and the chenodeocycholic acid as diagnostic markers of biliary atresia is proposed for the firstly time, so that the biliary atresia can be distinguished from other cholestasis diseases of newborns, the best operation time of children can be strived for, and therefore, the operation effect can be improved.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
LC-MS/MS method for rapidly and quantitatively detecting four cholic acid components in blood plasma
PendingCN111830152AThe result is accurateHigh degree of automationComponent separationCholic acidChenodeoxycholic acid
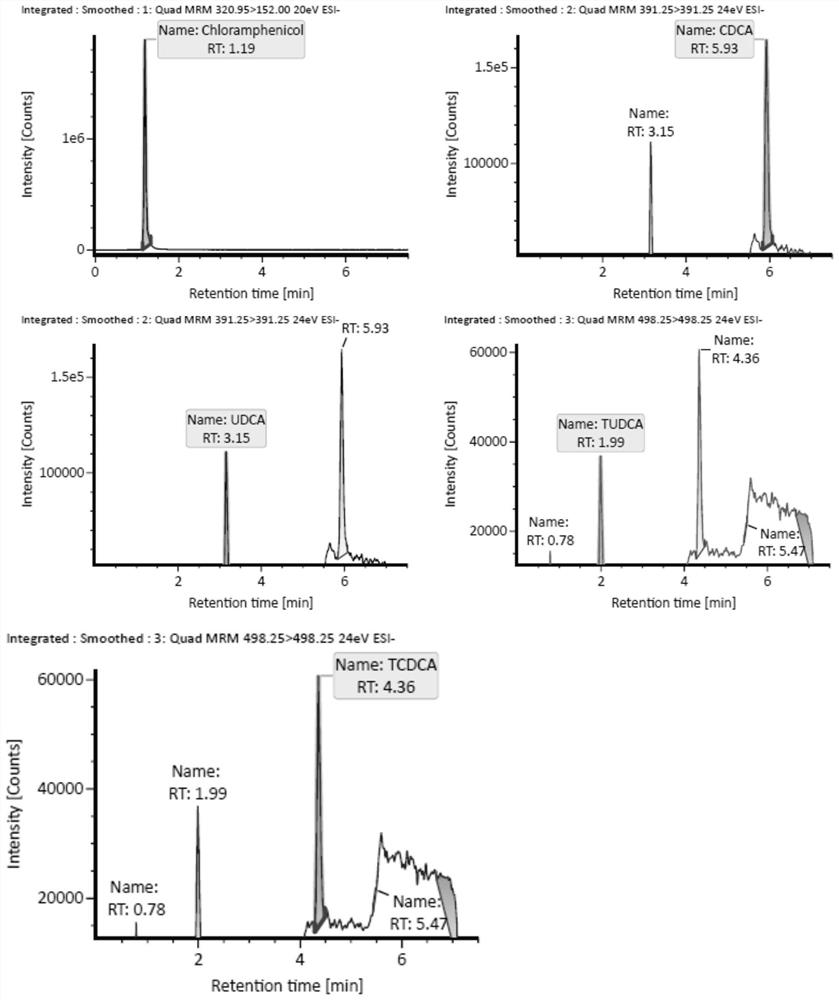
The invention discloses an LC-MS / MS method for rapidly and quantitatively detecting four cholic acid components in blood plasma. The method comprises the following steps of: (1) establishing a cholicacid component standard curve: a, preparing a reference substance solution, b, preparing a series of concentration matrix mixed reference substance solutions, and c, respectively sucking the series ofconcentration matrix mixed reference substance solutions, and injecting the solutions into an LC-MS / MS instrument, and determining the peak area to obtain the cholic acid component standard curve; (2) determining the content of cholic acid components in a to-be-determined sample: d, preparing a test solution, and e, determining the test solution. According to the LC-MS / MS method for rapidly detecting the four cholic acid components in the blood plasma, free ursodeoxycholic acid, chenodeoxycholic acid, tauroursodeoxycholic acid and / or taurochenodeoxycholic acid in the blood can be detected atthe same time, the result is accurate, the automation degree is high, the speed is high, the sensitivity is high, and the method has very important significance.
Owner:成都华西海圻医药科技有限公司
Novel 7alpha-hydroxysteroid dehydrogenase gene S1-a-2
ActiveCN106676079AHigh industrial application valueBetter than activityBacteriaOxidoreductasesChenodeoxycholic acidNucleotide
The invention relates to hydroxysteroid dehydrogenase, in particular to a novel 7alpha-hydroxysteroid dehydrogenase gene S1-a-2. A nucleotide sequence of the gene is shown as SEQ ID NO.2. A novel 7alpha-hydroxysteroid dehydrogenase with an amino acid sequence shown as SEQ ID NO.1 is coded and is capable of catalyzing CDCA (chenodeoxycholic acid) and TCDCA (taurochenodeoxycholic acid) to generate 7K-LCA (7-ketone lithocholic acid) and T7K-LCA (tauro-7-keto lithocholic acid), catalytic activity of the novel 7alpha-hydroxysteroid dehydrogenase for the CDCA or TCDCA is about twice of that of Clostridium sardiniense 7alpha-HSDH for the same, and accordingly the novel 7alpha-hydroxysteroid dehydrogenase is high in industrial application value.
Owner:CHONGQING UNIV
Cerebral infarction early diagnosis marker, screening method and application, and construction method and application of cerebral infarction early diagnosis model
ActiveCN114137226AImproved prognosisImprove survival rateComponent separationBiostatisticsCholic acidLipidome
The invention discloses a cerebral infarction early diagnosis marker, a screening method and application, and a construction method and application of a cerebral infarction early diagnosis model. The diagnostic marker is composed of 4-dimethylallyltryptophan, taurochenodeoxycholic acid-3-sulfate, tri-hexose ceramide (d18: 1 / 18: 0), lysophosphatidylcholine (18: 0), arginine-alanine, asparaginic acid-tryptophan, methionine-arginine, sphingomyelin d37: 5, phosphatidyl glycerol (12: 0 / 21: 0) and glucosylceramide (d18: 0 / 18: 0). The diagnostic marker is screened out by simultaneously carrying out metabonomics analysis and lipidomics analysis on serum by utilizing a UPLC-MS (Ultra Performance Liquid Chromatography-Mass Spectrometry) technology; the sensitivity, the specificity, the accuracy and the AUC of the method for constructing the diagnosis model by utilizing the diagnosis marker are all greater than 0.9, and the method can be used for accurately distinguishing cerebral infarction patients from healthy people and can be used for early diagnosis of cerebral infarction.
Owner:CAPITAL NORMAL UNIVERSITY
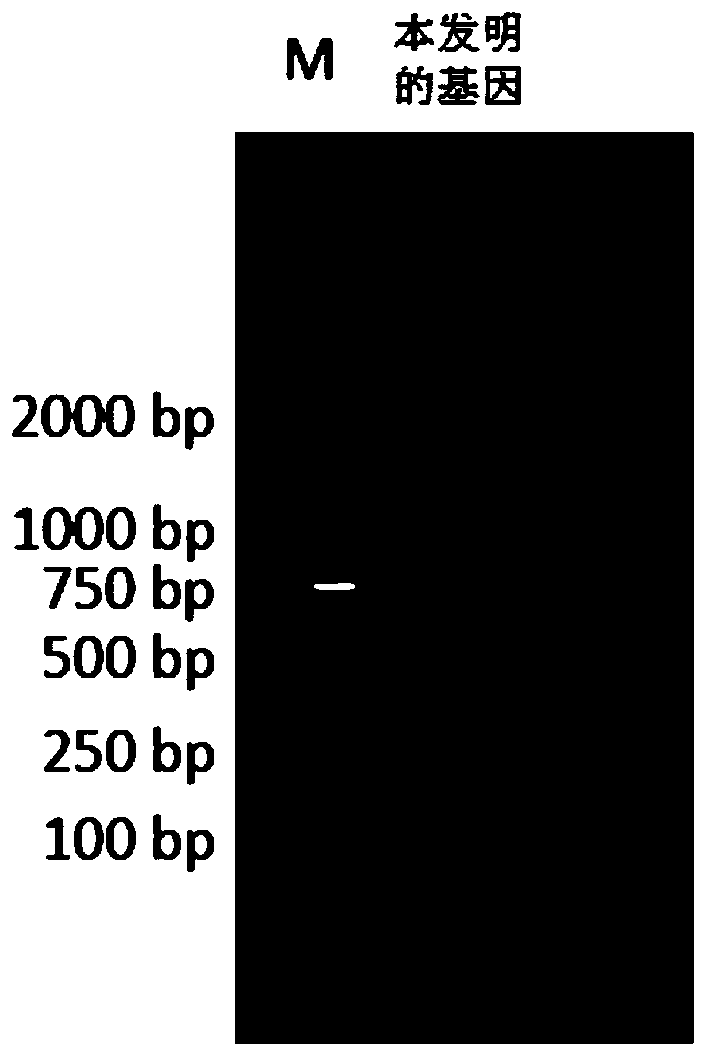
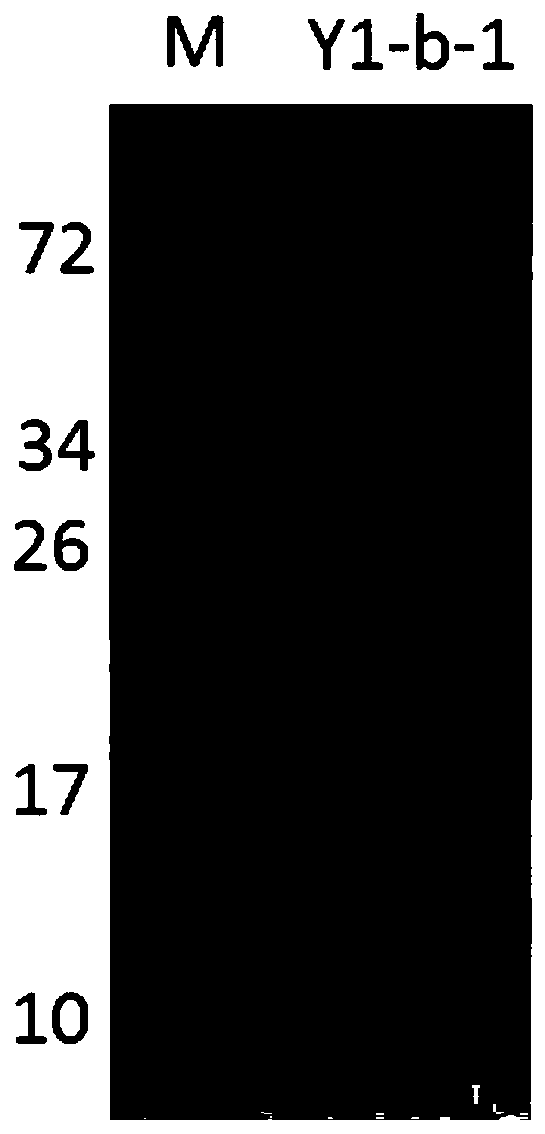
7β-hydroxysteroid dehydrogenase gene y1-b-1
ActiveCN107058250BCatalytic carbonyl asymmetric reduction reactionBacteriaOxidoreductasesCholic acidChenodeoxycholic acid
The invention relates to a hydroxysteroid dehydrogenase and particularly relates to a novel 7beta-hydroxysteroid dehydrogenase gene Y1-b-1. A nucleotide sequence of the gene is as shown in SEQ ID NO.2; a novel 7beta-hydroxysteroid dehydrogenase is encoded and an amino acid sequence thereof is as shown in SEQ ID NO.1. Epimerization of 7-hydroxy of ursodesoxycholic acid (UDCA) and tauroursodeoxycholic acid (TUDCA) can be catalyzed to generate intermediates 7-ketone lithocholic acid (7K-LCA) and taurine 7-ketone lithocholic acid (T7K-LCA) of chenodeoxycholic acid (CDCA) and taurochenodeoxycholic acid (TCDCA). The specific activity of the novel 7beta-hydroxysteroid dehydrogenase gene Y1-b-1 on the UDCA and the TUDCA is equivalent to that of existing clostridium sardiniense 7beta-HSDH, but the novel 7beta-hydroxysteroid dehydrogenase gene Y1-b-1 has better heat stability and a great industrial application prospect.
Owner:CHONGQING KINBEAR BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com