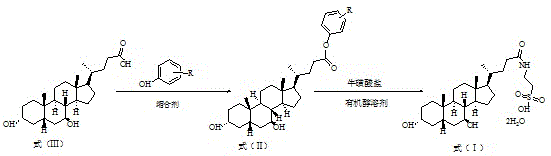
Method for preparing tauro ursodesoxy cholic acid
A technology of tauroursodeoxycholic acid and ursodeoxycholic acid, which is applied in the field of preparation of tauroursodeoxycholic acid, can solve the problems of many side reactions, low purity of the crude product, strong reactivity, etc., and achieve the product High yield and purity, simple operation and post-treatment, suitable for large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
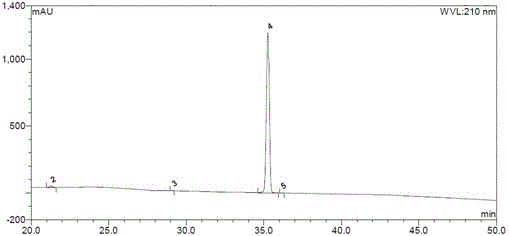
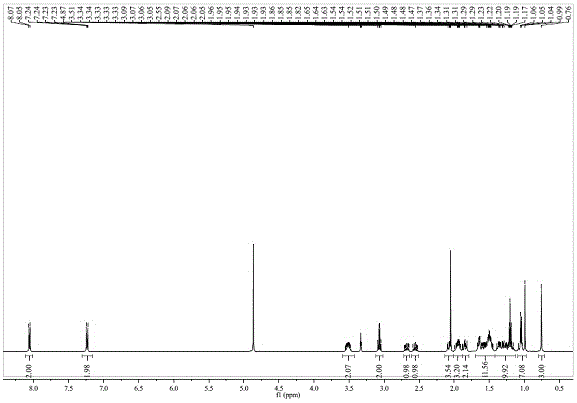
Image
Examples
Embodiment 1
[0037] Preparation of Tauroursodeoxycholic Acid
[0038] At room temperature, add 20.0 g (50.9 mmol) of ursodeoxycholic acid and 4.79 g (50.9 mmol) of phenol into 100 mL of dichloromethane, stir and cool down to 10-20°C. Slowly add the dichloromethane solution of N,N'-dicyclohexylcarbodiimide and 4-dimethylaminopyridine dropwise, after the dropwise addition, react at 30-45°C for 2 hours, filter to remove insoluble matter, and concentrate the filtrate Obtained 22.68 g of light yellow oily substance, yield 95.0%.
[0039] The above oil was recrystallized from acetonitrile to obtain a white sticky substance, which was directly used in the next reaction.
[0040] At room temperature, 12.55 g (76.4 mmol) of potassium taurate and the above oil were added to n-butanol and stirred. After heating to 80°C and keeping it warm for 6 hours, TLC (ethyl acetate:petroleum ether=1:1) monitored that the reaction was complete. The reaction system was cooled to room temperature, filtered and d...
Embodiment 2
[0042] Preparation of Tauroursodeoxycholic Acid
[0043] At room temperature, 10.0 g (25.4 mmol) of ursodeoxycholic acid and 3.90 g (28.0 mmol) of p-nitrophenol were added to 80 mL of dichloromethane, stirred and cooled to 15°C.
[0044] At 10-20°C, slowly add the dichloromethane solution of N,N'-dicyclohexylcarbodiimide and 4-dimethylaminopyridine dropwise, after the addition is complete, react at 5-20°C for 2 hours, then filter. The insoluble matter was removed, and the filtrate was concentrated to obtain 12.03 g of a light yellow oil, with a yield of 92.0%.
[0045] The above oil was recrystallized from acetonitrile to obtain a white sticky substance, which was directly used in the next reaction.
[0046] At room temperature, 6.28 g (38.2 mmol) of potassium taurate and the above-mentioned oil were added to n-butanol, and stirred. After heating to 80°C and keeping the temperature for 6 hours,
[0047] The reaction system was cooled to room temperature, filtered and dried to...
Embodiment 3
[0049] Preparation of Tauroursodeoxycholic Acid
[0050] At room temperature, 50.0 g (127.4 mmol) of ursodeoxycholic acid and 21.04 g (140.1 mmol) of 4-hydroxypropiophenone were added to 200 mL of dichloromethane, stirred and cooled to 15°C.
[0051] At 10-20°C, slowly add the dichloromethane solution of N,N'-dicyclohexylcarbodiimide and 4-dimethylaminopyridine dropwise, after the addition is complete, react at 30-45°C for 2 hours, then filter. Insoluble matter was removed, and the filtrate was concentrated to obtain 65.63 g of light yellow oil.
[0052] The above oil was added into acetonitrile for recrystallization to obtain 65.03 g of white solid tauroursodeoxycholic acid 4-propionylphenol ester, with a yield of 97.3% and a purity of 99.6%.
[0053] At room temperature, 30.53 g (0.19 mol) of potassium taurate and the above-mentioned white solid were added to n-butanol and stirred. After heating to 80°C and keeping it warm for 6 hours, the reaction system was cooled to roo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com