Sardinia clostridium 7alpha-hydroxy steroid dehydrogenase mutant K179M
A hydroxysteroid, K179M technology, applied in the direction of enzymes, genetic engineering, oxidoreductase, etc., can solve the problems of difficult recovery, expensive auxiliary reagents, low stereoselectivity, etc., and achieve the effect of huge application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1. Preparation of CA 7α-HSDH mutants
[0056] 1. Mutant Gene Synthesis
[0057] Original sequence: codon-optimized wild-type CA 7α-HSDH gene sequence (see patent publication CN102827848A), the nucleotide sequence of which is shown in SEQ ID NO:4.
[0058] By comparing the similarities and differences between wild-type CA 7α-HSDH and homologous enzyme proteins from the primary structure to the high-order structure, the site that affects the enzymatic properties is the 179th amino acid of wild-type CA 7α-HSDH , the amino acid is lysine, and the corresponding nucleotide sequence is the 535th-537th codon.
[0059] The 535th-537th codon of the wild-type CA 7α-HSDH gene sequence was changed from AAA to ATG, and the original lysine was replaced with methionine to obtain a CA 7α-HSDH mutant, named CA 7α-HSDH The nucleotide sequence of the K179M mutant is shown in SEQ ID NO: 3, and the amino acid sequence is shown in SEQ ID NO: 2.
[0060] 2. Construction of vectors ...
Embodiment 2K179
[0121] Example 2. K179M mutant enzyme activity detection
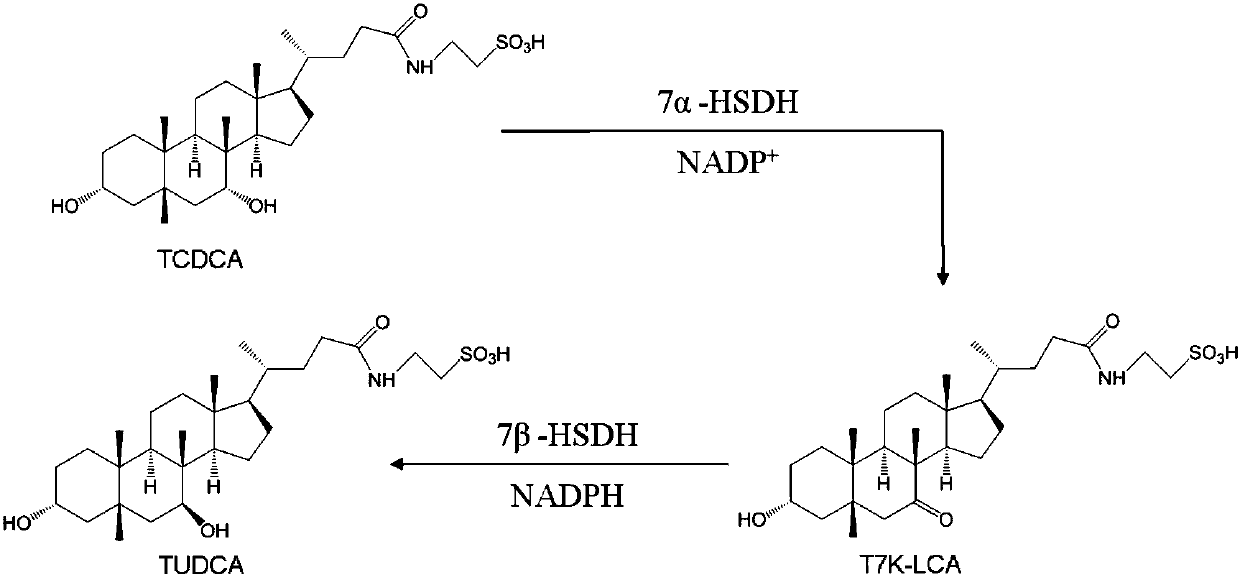
[0123] At room temperature, add 1958uL 50mM Tris-HCl (PH=8.0) solution, 20uL 50mM NADP to the cuvette + solution, and then add 2uL of the K179M enzyme protein solution obtained in Example 1, mix well and adjust to zero, and finally add 20uL of 50mM TCDCA solution, mix well, and start timing. The change in absorbance at 340 nm is read every minute.
[0124] At the same time, the enzymatic activity of CA7α-HSDH was measured in the same manner as above.
[0125] The results show that the mutant K179M of the present invention catalyzes the epimerization of the 7-hydroxyl group of TCDCA to generate TUDCA intermediate taurine 7-ketolithocholic acid (T7K-LCA). The specific activities of K179M enzyme protein of the present invention and Clostridium sardinia 7α-HSDH enzyme protein to TCDCA are shown in Table 1.
[0126] Specific activity (U / mg) Definition: Under the above reaction conditions,...
Embodiment 5K179
[0130] Embodiment 5. K179M mutant thermostability research
[0131] The enzyme sample (without glycerol) was placed in a metal bath at 50° C. for 20 minutes, and the residual enzyme activity was detected according to the method for measuring enzyme activity in Example 2. Each sample was repeated no less than 3 times.
[0132] The results are shown in Table 2. After heat treatment for 20 minutes, the enzyme activity of wild-type CA 7α-HSDH decreased rapidly, and only 32.33% of the enzyme activity remained, while K179M still retained 45.09% of the enzyme activity. It shows that compared with the wild-type CA 7α-HSDHK179M, it has better thermal stability and is more suitable for industrial applications.
[0133] Table 2. Comparison of thermal stability between wild-type CA 7α-HSDH and 7α-HSDHJ-1-1
[0134]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com