Tauroursodeoxycholic acid synthesis method
A technology for tauroursodeoxycholic acid and tauroursodeoxycholic acid is applied in the field of tauroursodeoxycholic acid synthesis, which can solve the problems of unstable amplification process, complex related substances and high production cost, and achieves chemical The effect of short synthesis route, high purity and few impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
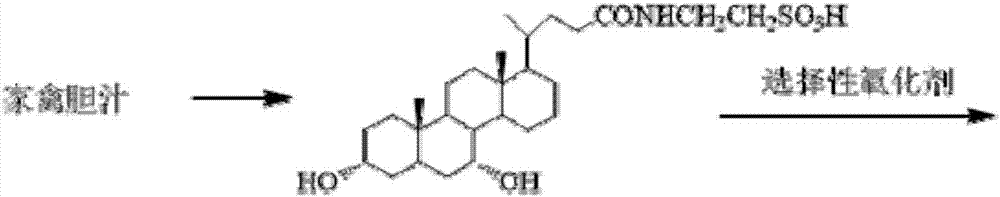
[0038] (1) Take chicken gall paste 100g, add 500mL water, stir to dissolve, centrifuge, filter, wash the filtrate three times with ethyl acetate (200mL*3), collect the lower layer solution; add 2 to 3 times the amount of ethanol to the filtrate, centrifuge, filter , the filtrate is concentrated to dryness, then 200ml of ethanol is added, centrifuged, filtered, the filtrate is adjusted to pH 2-5 with hydrochloric acid, the filtrate is evaporated to dryness, the concentrate is added to pure water to dissolve completely, super acidic ion exchange resin, the collection purity is greater than 95% The taurochenodeoxycholic acid component is concentrated to obtain about 10.3 g of taurochenodeoxycholic acid.
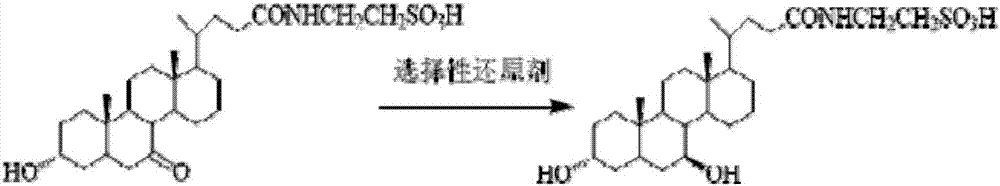
[0039] (2) Dissolve taurochenodeoxycholic acid (10.0 g, 20.0 mmol) in 50 mL of acetone aqueous solution (acetone (V) / water (V) = 4 / 1), and slowly add N in batches after the dissolution is complete. -Bromosuccinimide (5.6g, 30mmol), stirred at room temperature for 4h; sampled on ...
Embodiment 2
[0043](1) Take 100g of chicken gall paste, add 500mL of 0.1M sodium hydroxide aqueous solution, stir to dissolve, centrifuge, filter, wash the filtrate three times with ethyl acetate (200mL*3), collect the lower layer solution; the amount of solution added to the filtrate is 2-3 Double the amount of ethanol, centrifuge, filter, concentrate the filtrate to dryness, then add 200ml of ethanol, centrifuge, filter, adjust the pH value of the filtrate to 2-5 with hydrochloric acid, evaporate the filtrate, add pure water to dissolve the concentrate completely, super strong acid ion The resin was exchanged to collect taurochenodeoxycholic acid components with a purity greater than 95%, and concentrated to obtain about 10.2 g of taurochenodeoxycholic acid.
[0044] (2) step is the same as (2) step of embodiment 1.
[0045] (3) step is the same as (3) step of embodiment 1.
Embodiment 3
[0047] (1) Take 100g of chicken gall paste, add 500mL of water, stir to dissolve, centrifuge, filter, wash the filtrate with petroleum ether three times (200mL*3), collect the lower layer; add ethanol 2 to 3 times the amount of the solution to the filtrate, centrifuge, filter, Concentrate the filtrate to dryness, then add 200ml of ethanol, centrifuge, filter, adjust the pH value of the filtrate to 2-5 with hydrochloric acid, evaporate the filtrate to dryness, add pure water to the concentrate and dissolve it completely, and use a super-acidic ion exchange resin to collect the purity greater than 95%. The taurochenodeoxycholic acid component is concentrated to obtain about 9.8 g of taurochenodeoxycholic acid.
[0048] (2) step is the same as (2) step of embodiment 1.
[0049] (3) step is the same as (3) step of embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com