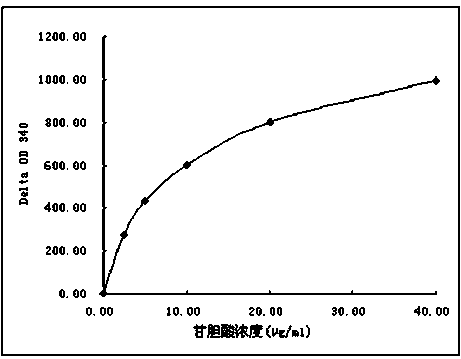
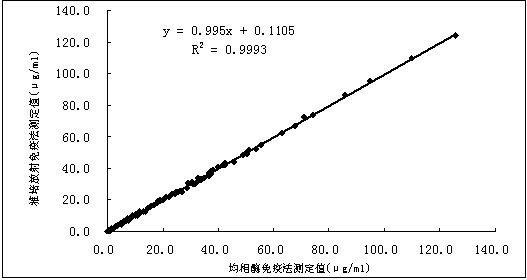
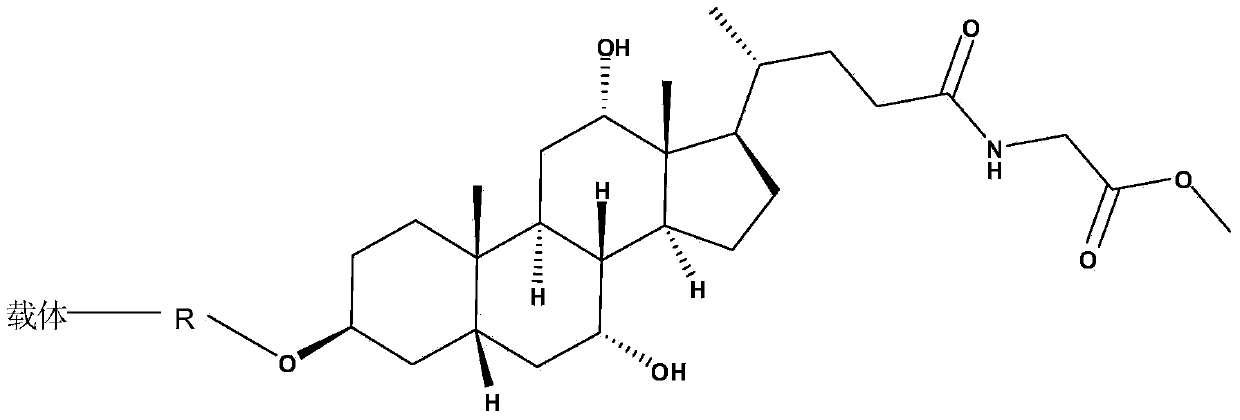
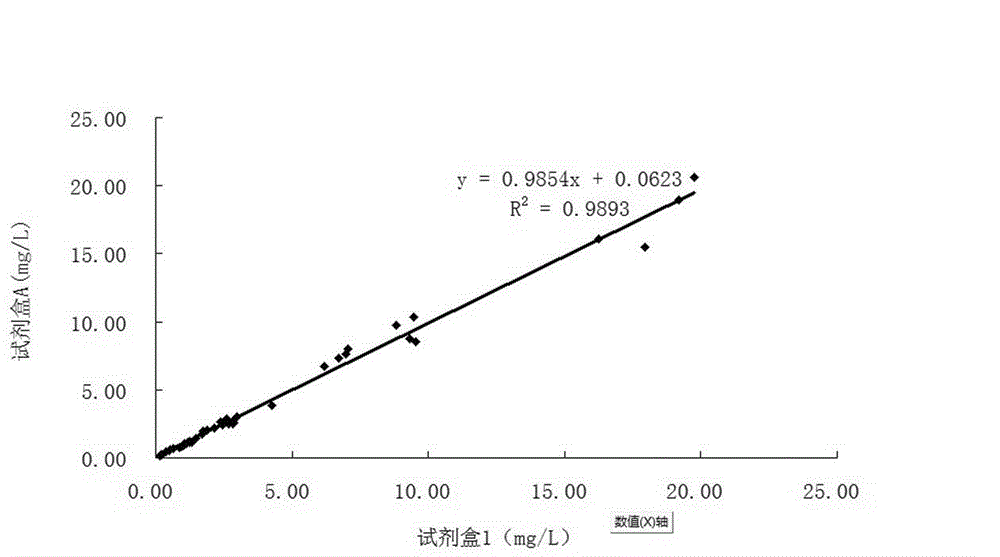
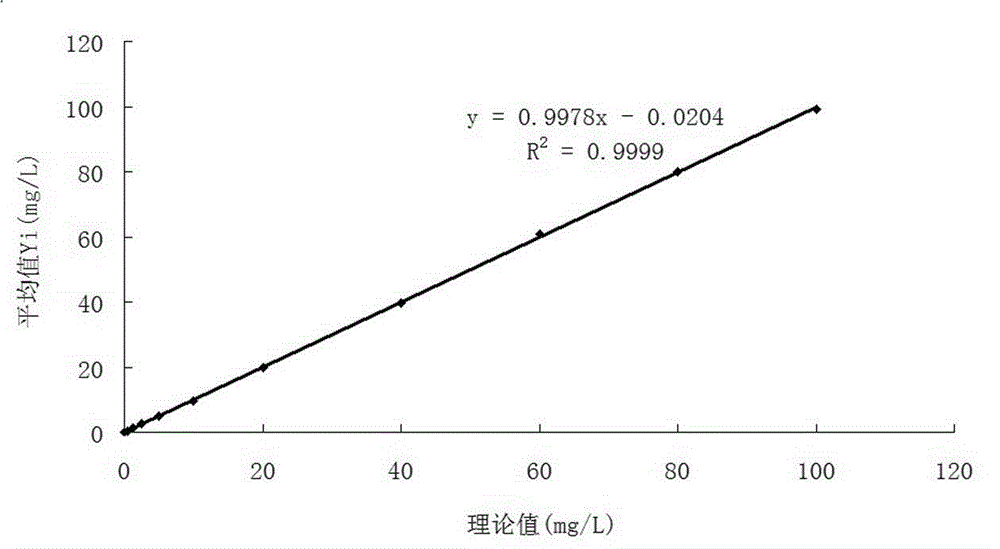
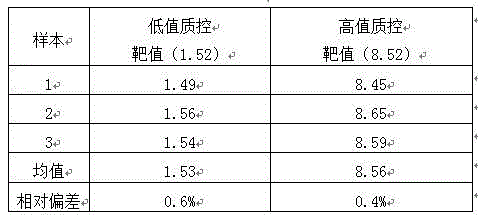
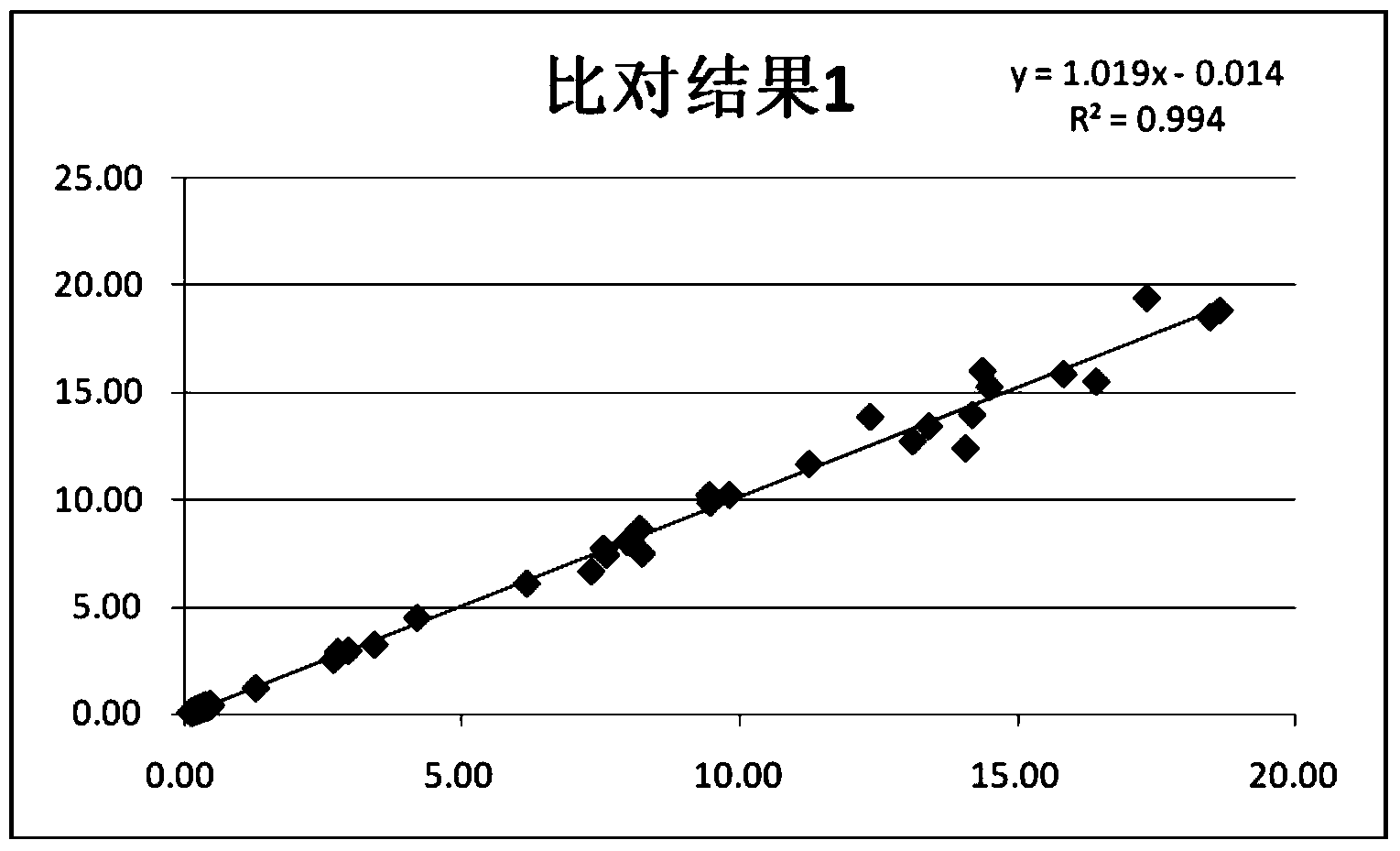
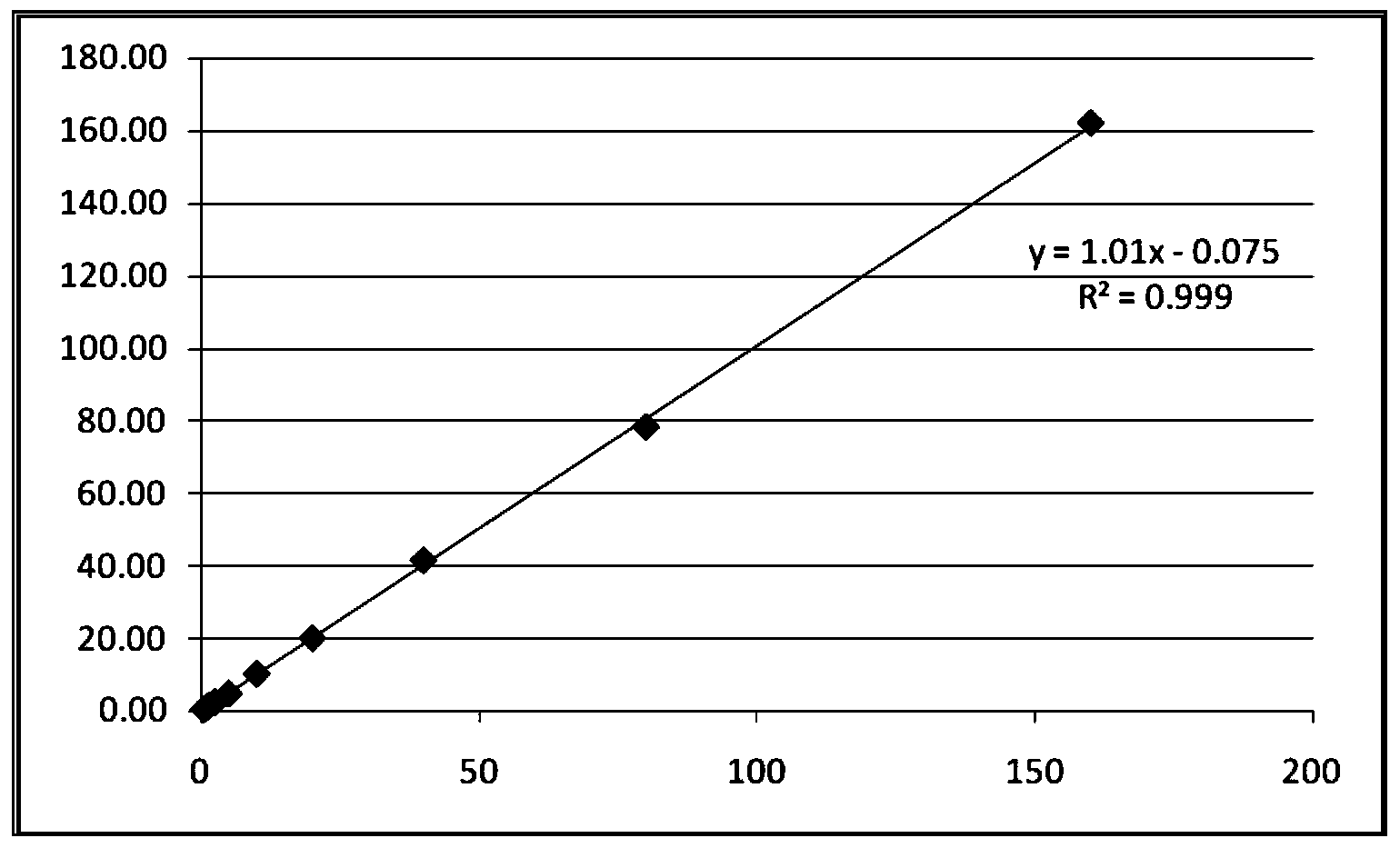
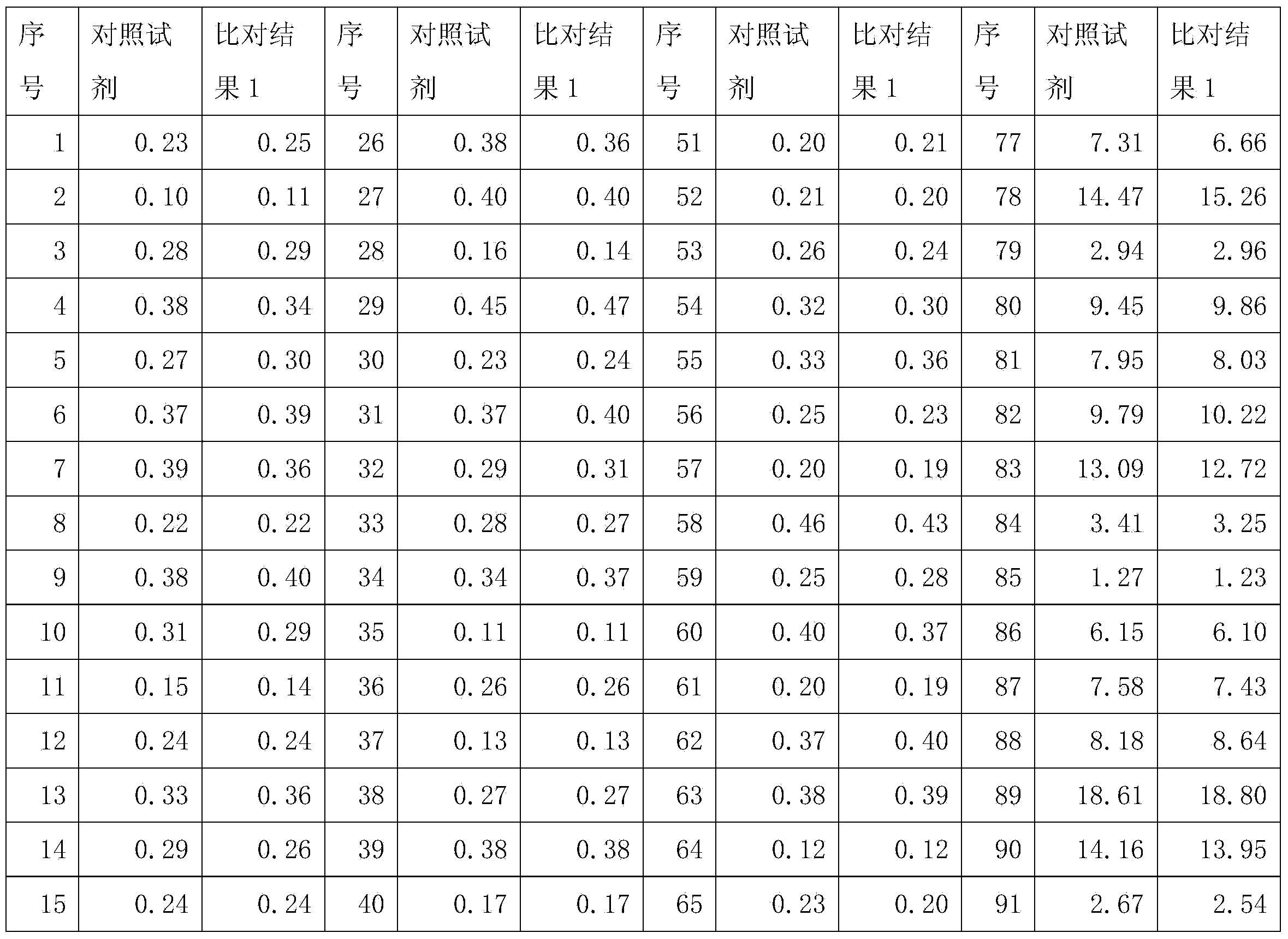
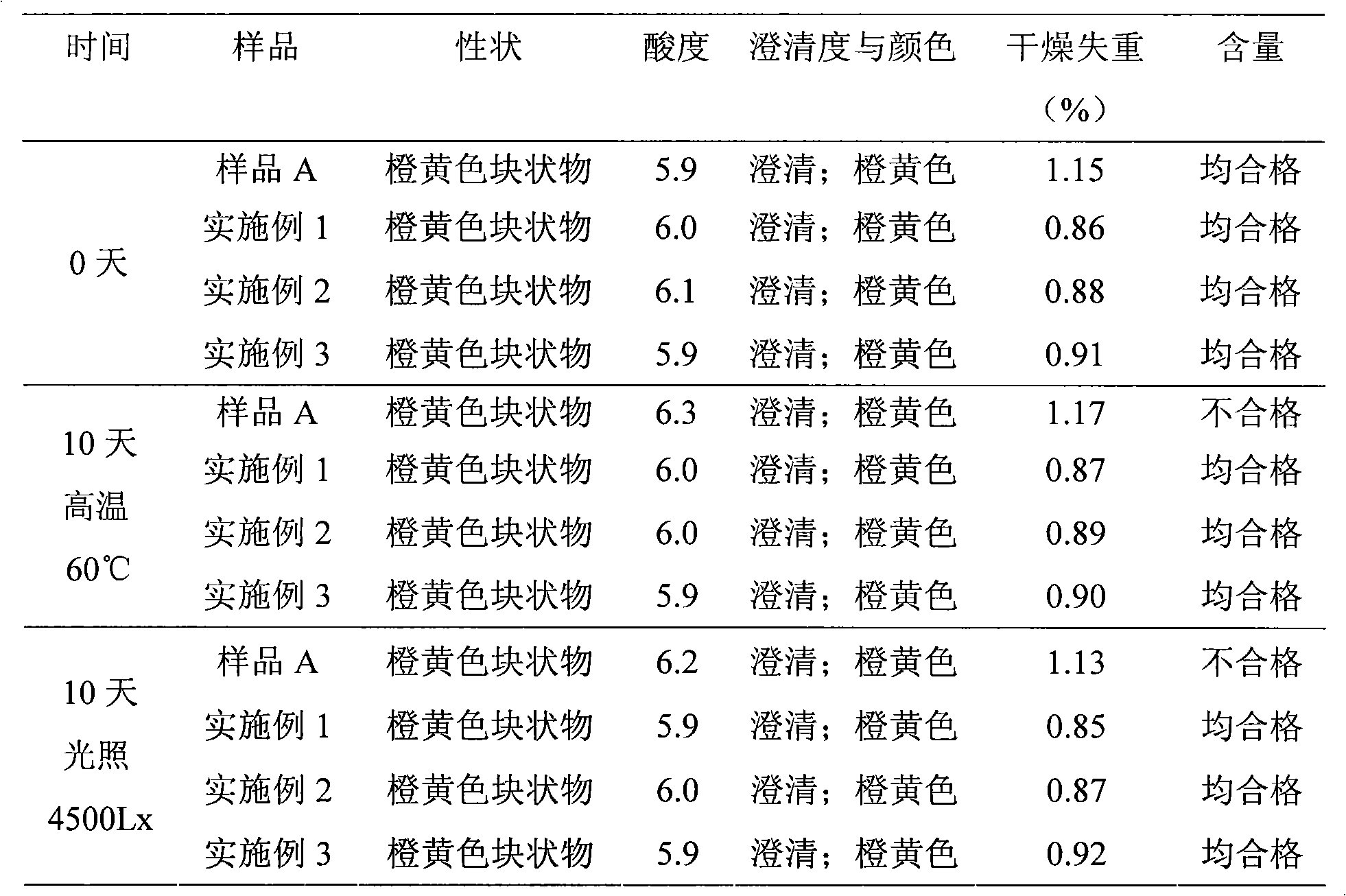
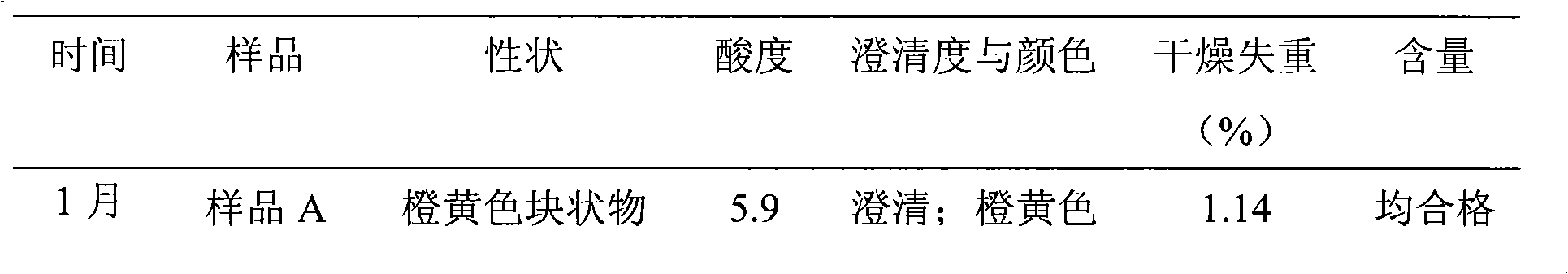
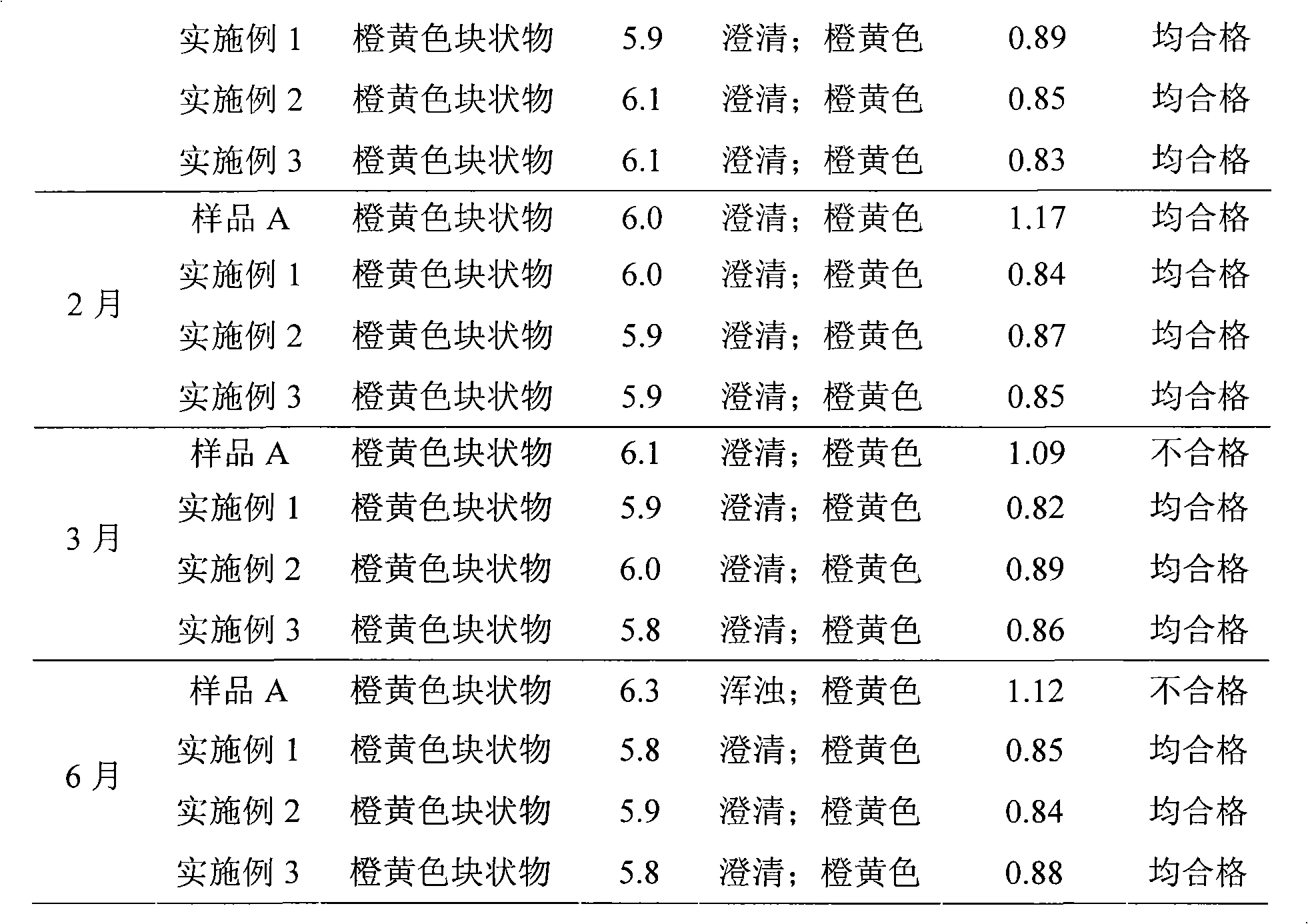
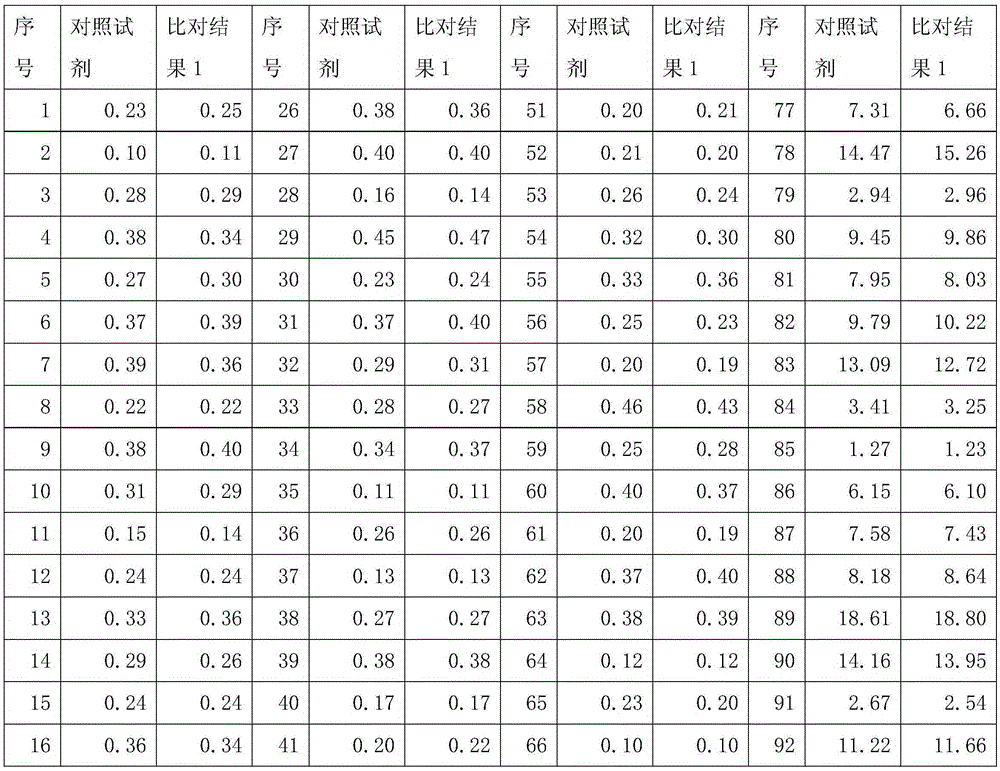
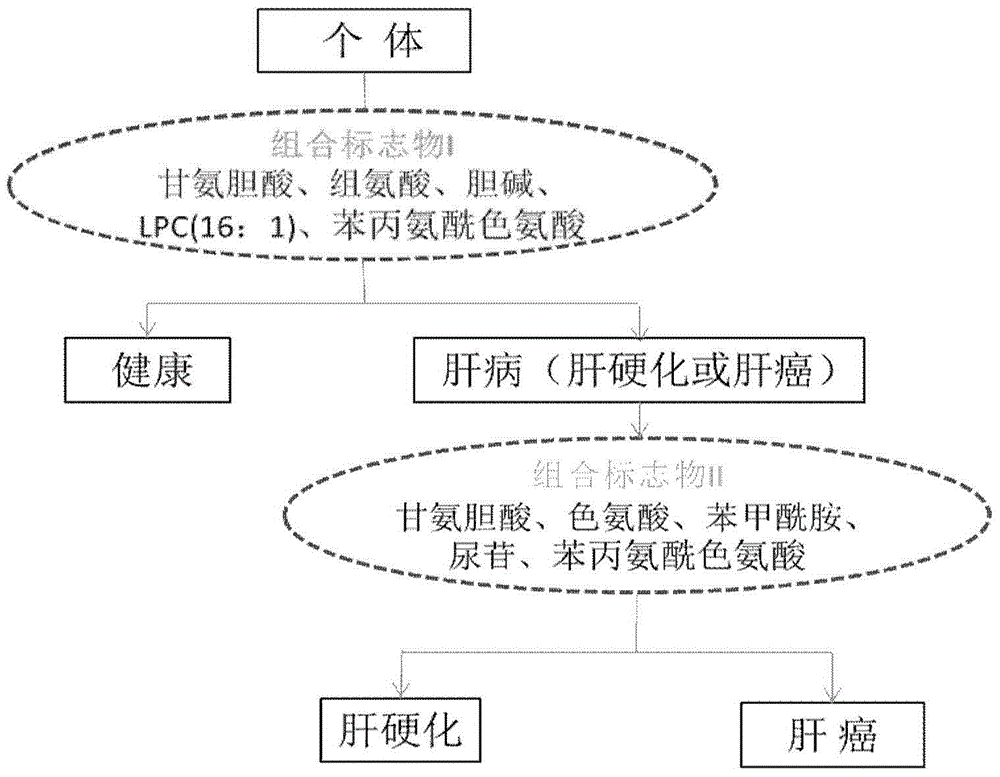
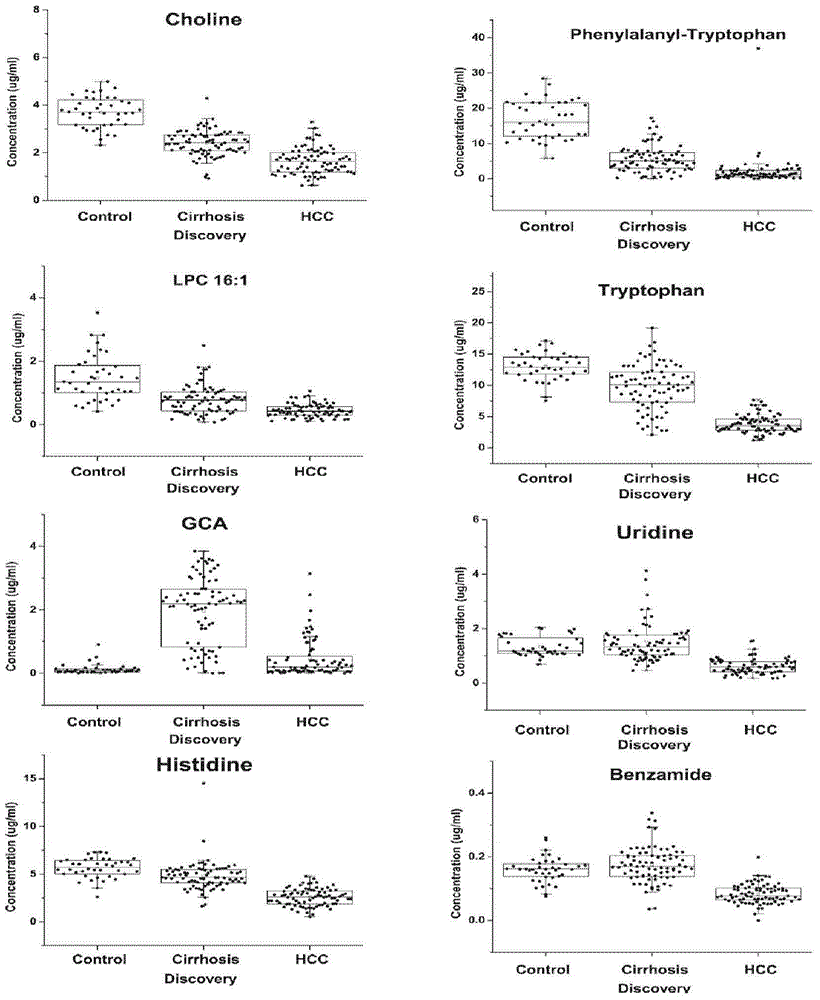
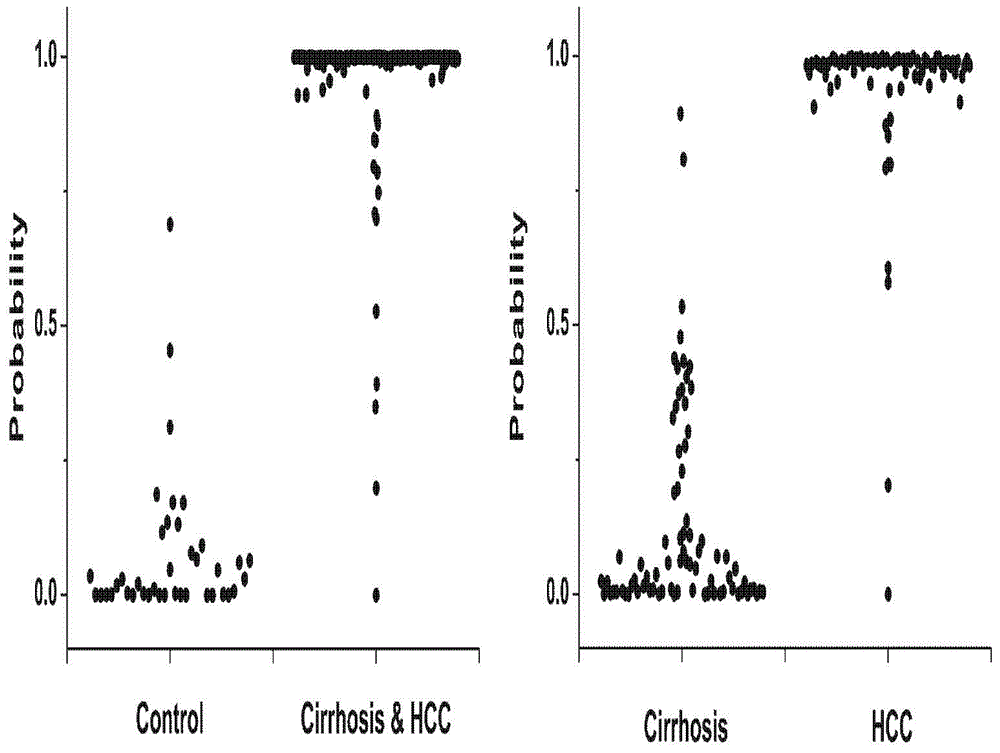
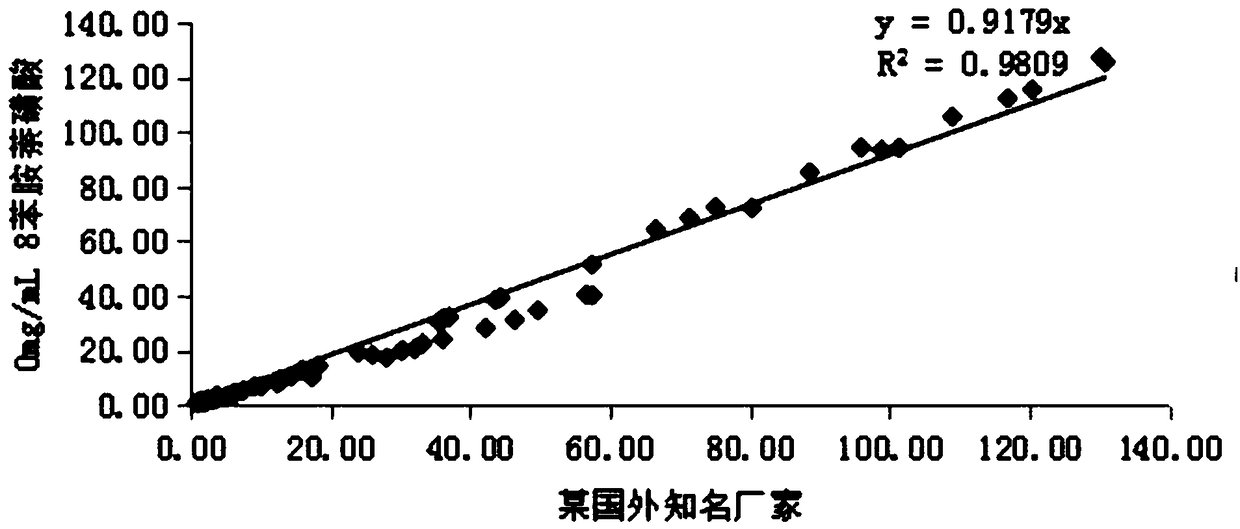
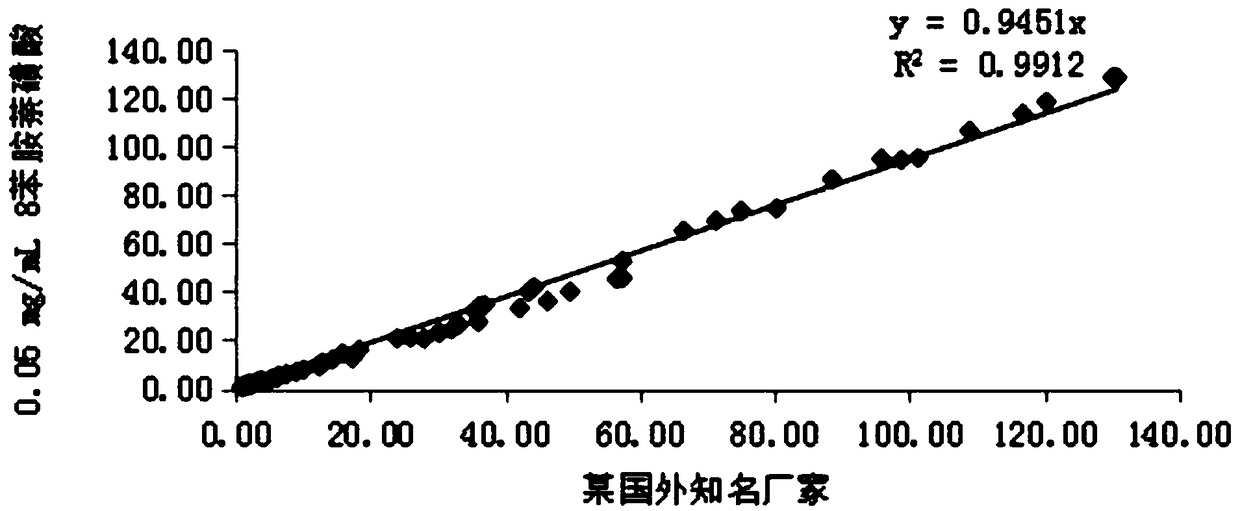
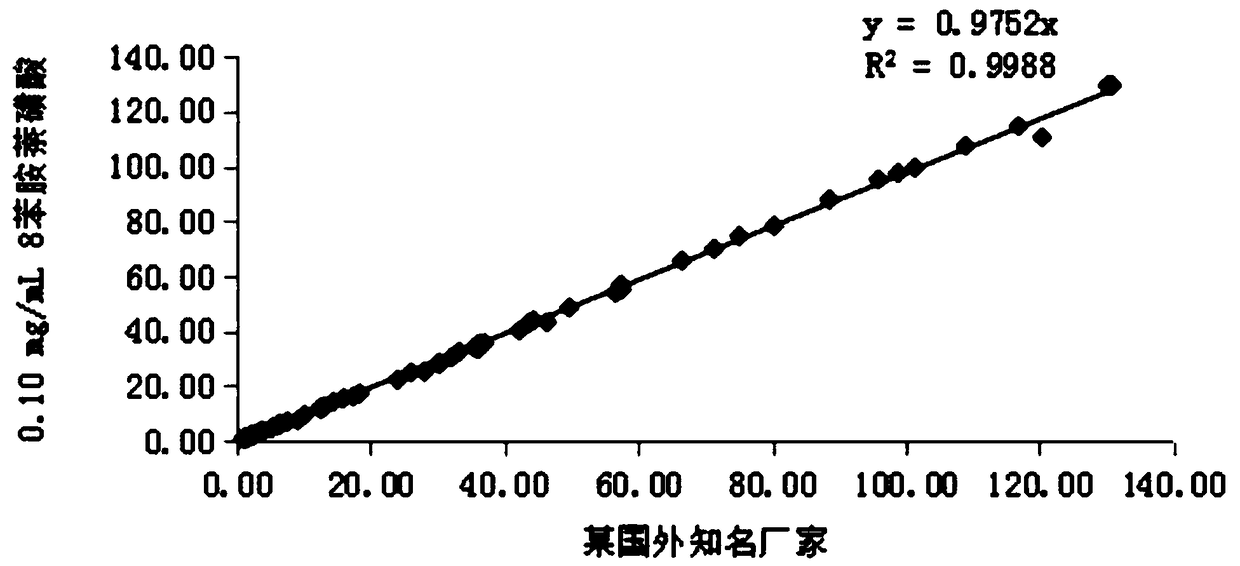
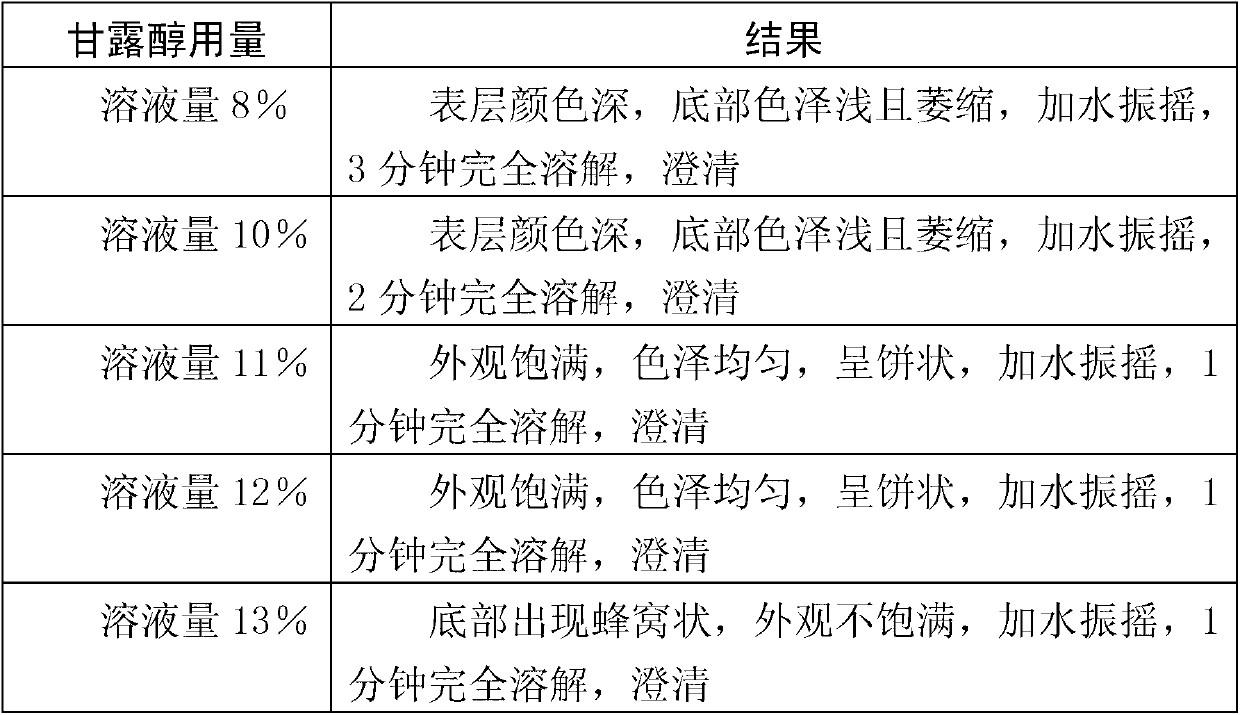
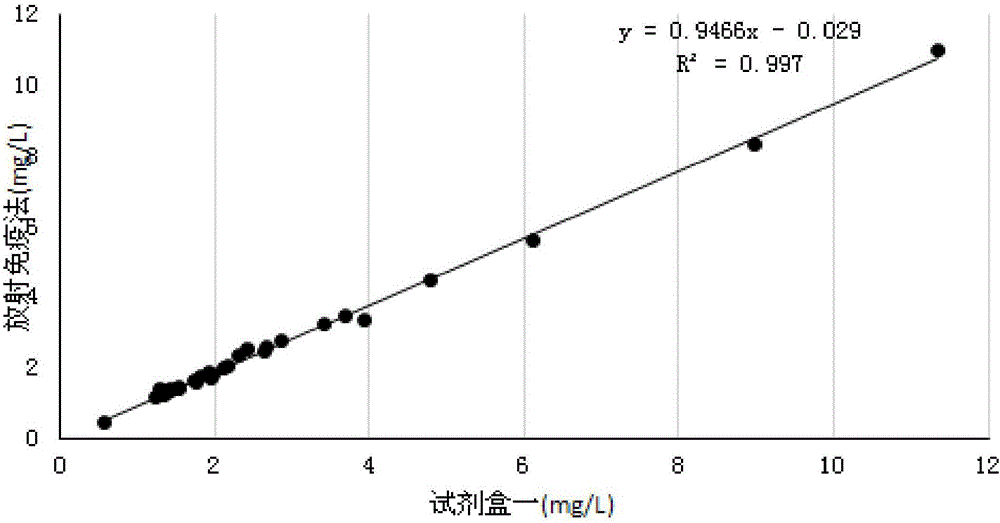
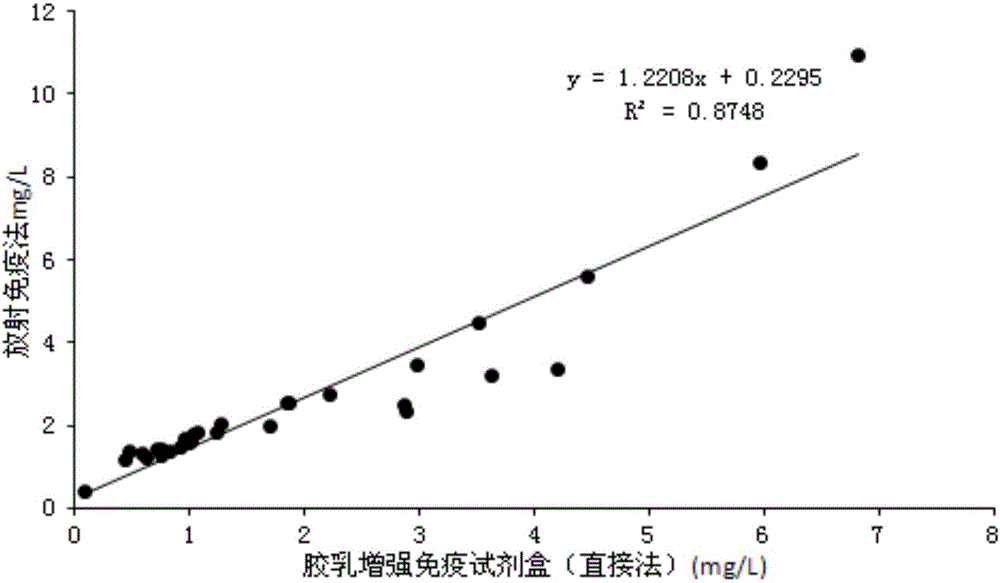
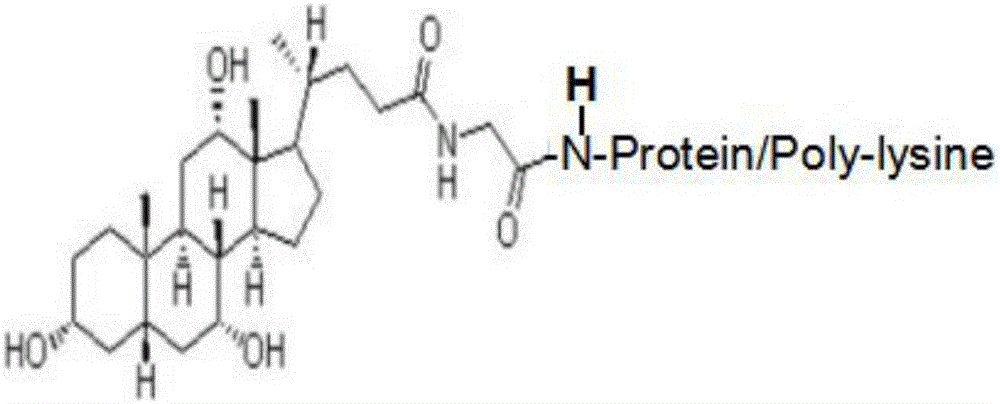
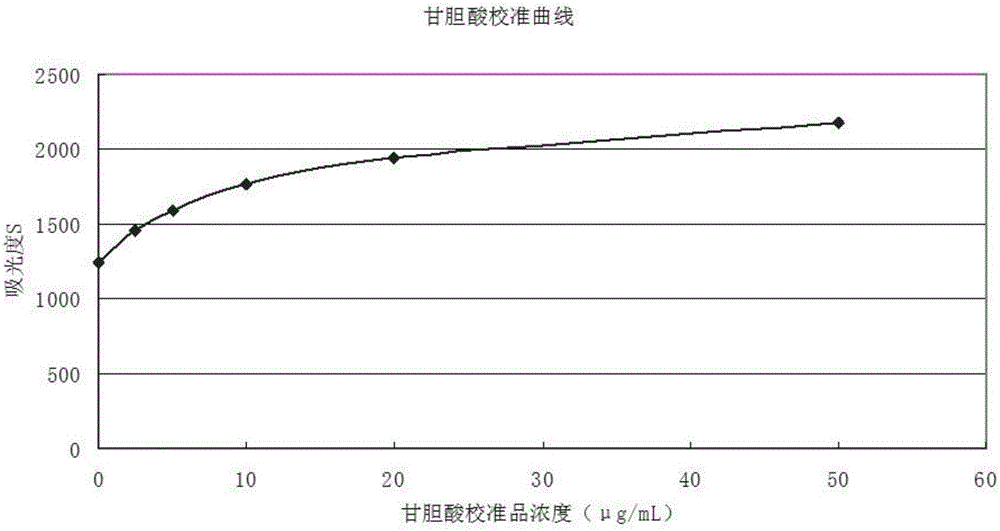
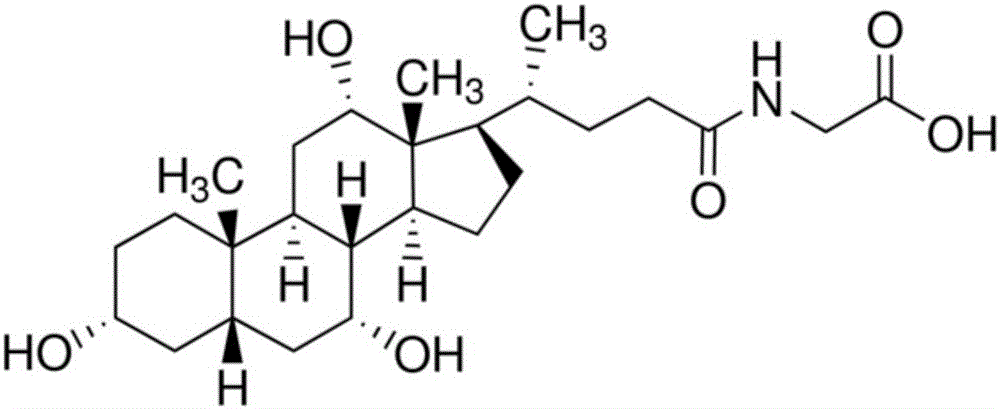
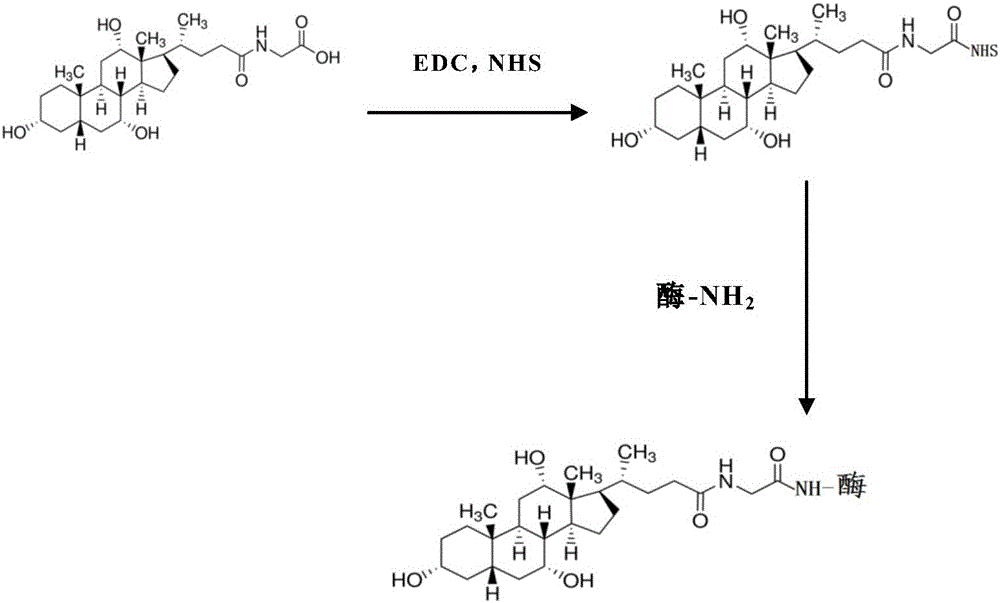
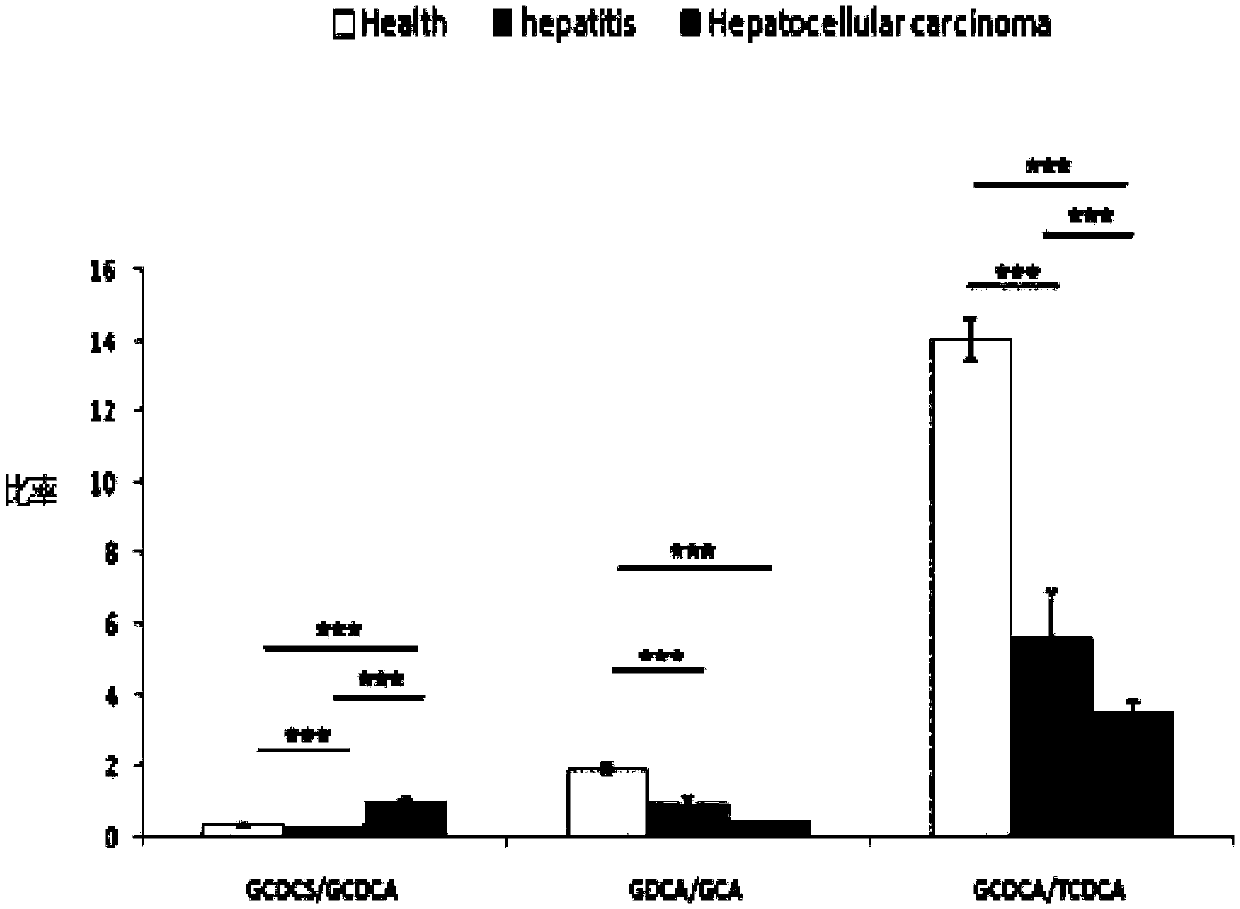
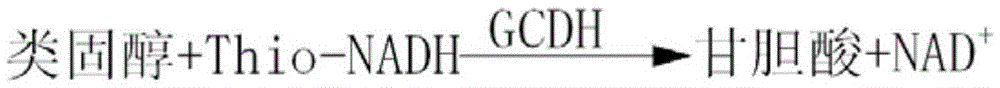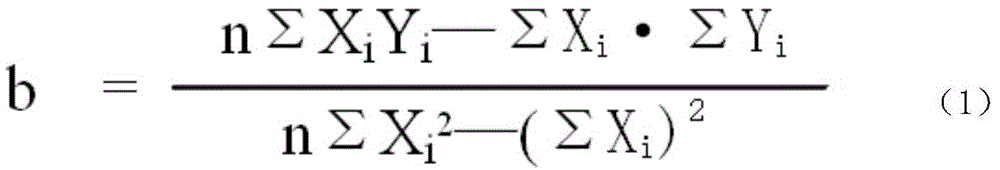Patents
Literature
114 results about "Glycocholic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
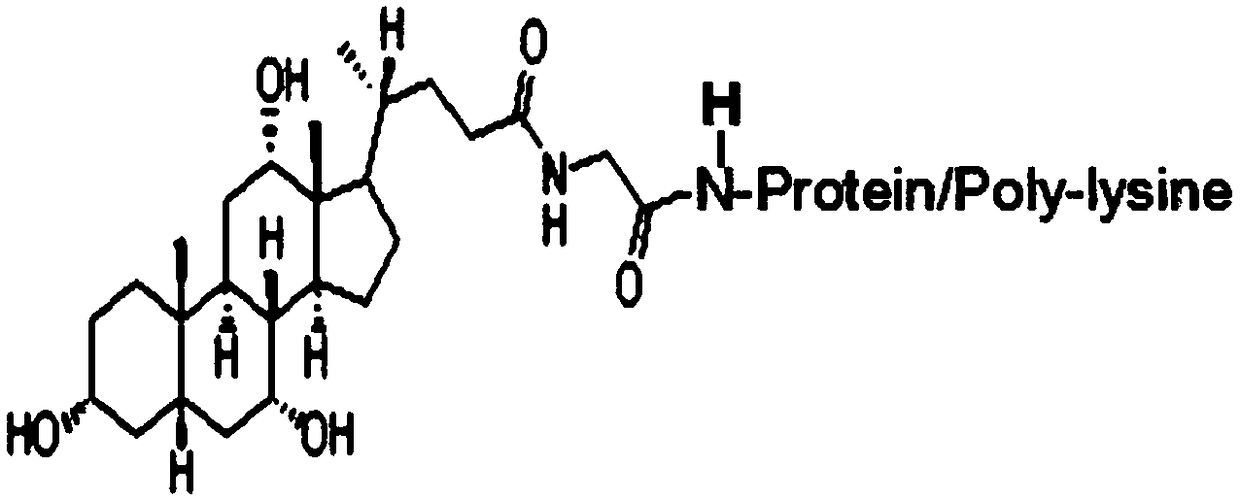
Glycocholic acid, or cholylglycine, is a crystalline bile acid involved in the emulsification of fats. It occurs as a sodium salt in the bile of mammals. It is a conjugate of cholic acid with glycine. Its anion is called glycocholate.
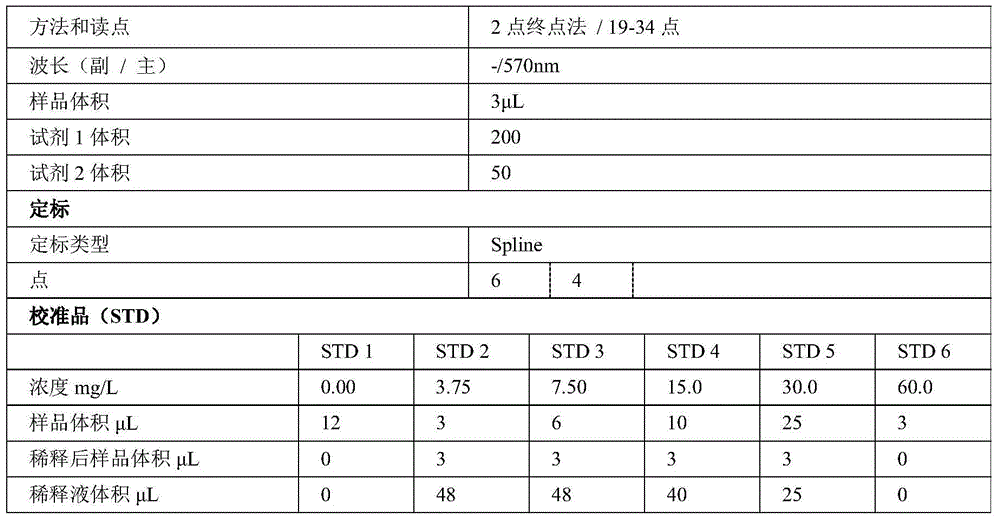
Kit for determining glycocholic acid in human blood
ActiveCN102955033ARapid determinationEasy to operateMaterial analysisAntiendomysial antibodiesActive agent
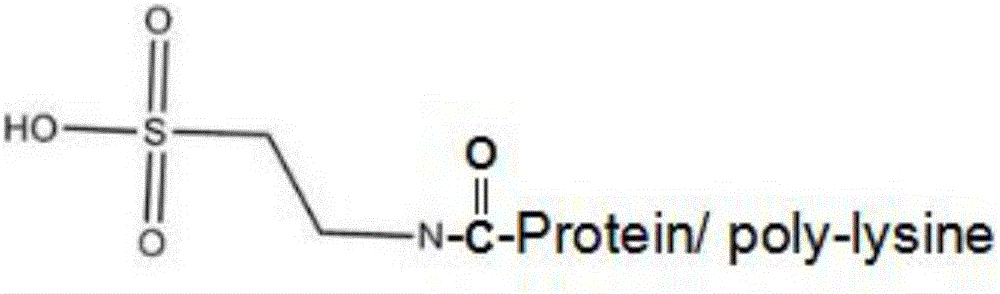
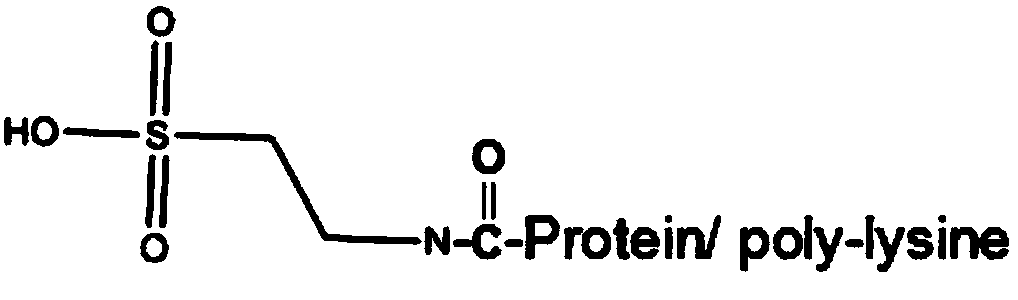
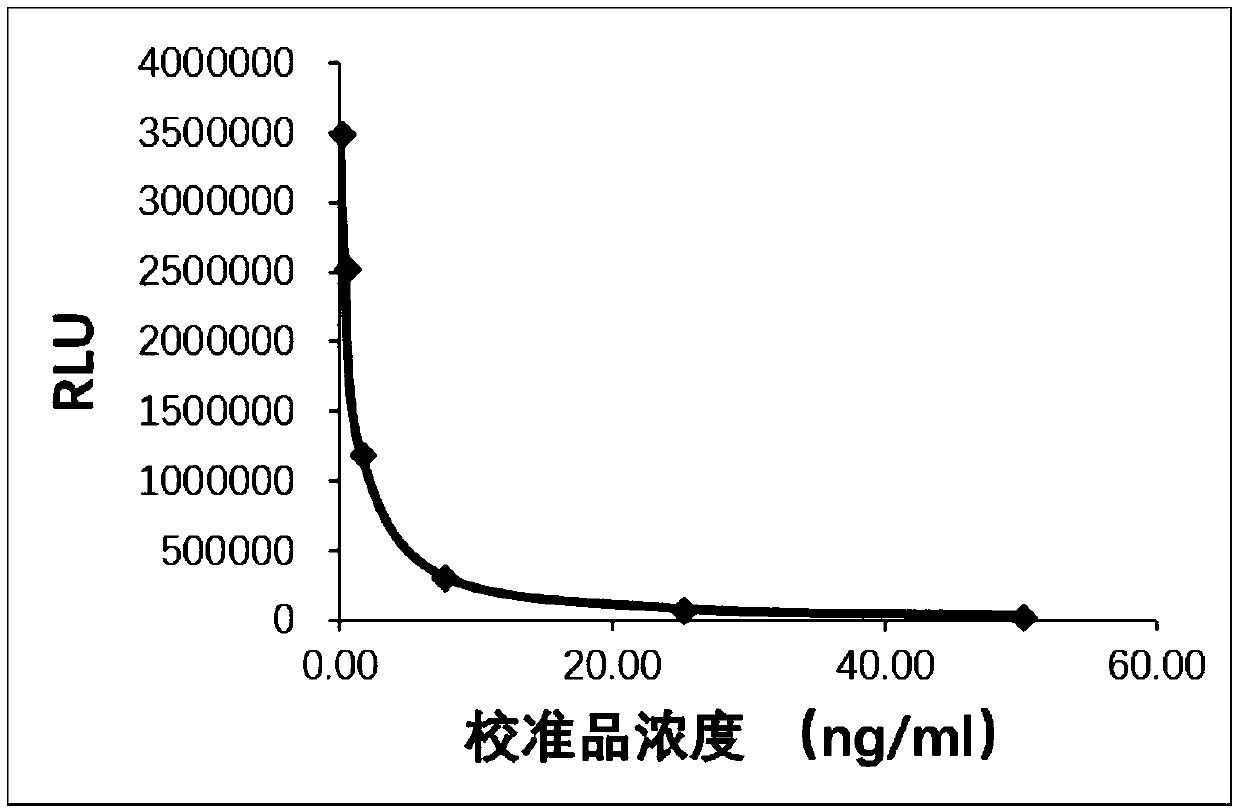
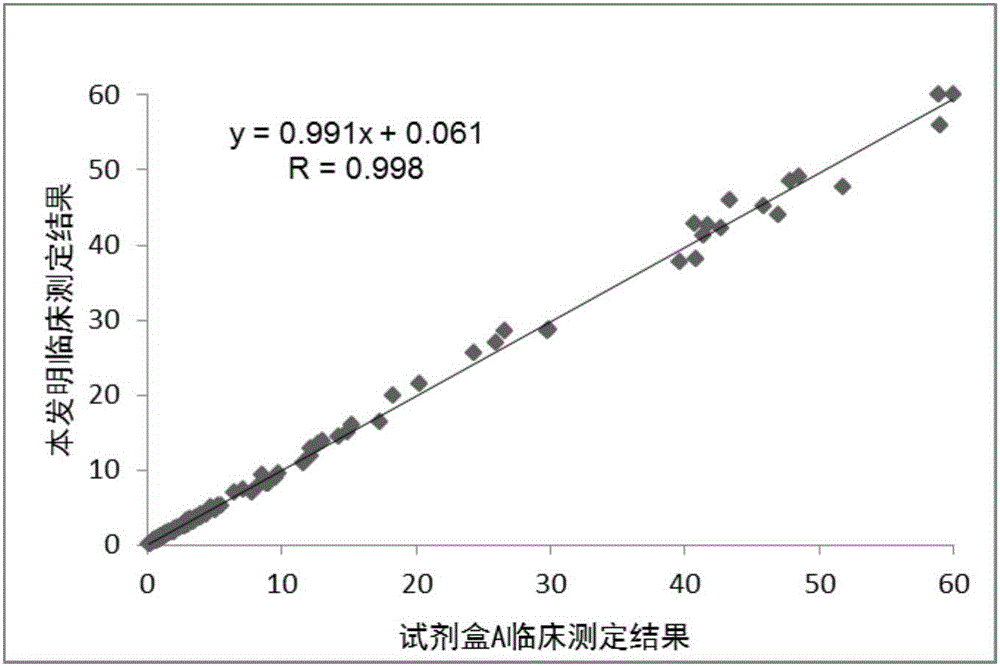
The invention discloses a kit for determining glycocholic acid in human blood. The kit comprises a reagent A, a reagent B and a calibrator for glycocholic acid with known concentration, wherein the mass ratio of the reagent A to the reagent B is (1-5):1; the reagent A comprises a microorganism inhibiting agent, EDTA (Ethylene Diamine Tetraacetic Acid), glycerol, albumin bovine serum, a surface active agent, a reactive reinforcing agent, sodium chloride and a Good's buffer solution with the pH of 6-8; and the reagent B comprises the microorganism inhibiting agent, the EDTA (Ethylene Diamine Tetraacetic Acid), the glycerol, the albumin bovine serum, the surface active agent, the reactive reinforcing agent, sodium chloride, the Good's buffer solution with the pH of 6-8 and nanoparticles crosslinked with a glycocholic acid monoclonal antibody. The kit is simple, convenient and fast in detection and operation, can be used for simultaneously and quickly determining a large amount of samples and has good detection accuracy and sensitivity.
Owner:浙江强盛生物科技有限公司
Glycocholic acid immunodetection reagent and preparing method and detecting method thereof
ActiveCN103760348AStrong immunogen specificityStrong specificityDisease diagnosisAntiendomysial antibodiesPharmaceutical drug
The invention relates to a glycocholic acid detecting reagent and a preparing method and a detecting method thereof, in particular to a glycocholic acid immunodetection reagent and a preparing method and a detecting method thereof. The glycocholic acid immunodetection reagent comprises a glycocholic acid specificity resisting antibody and an indicating reagent, wherein the indicating reagent is used for detecting the glycocholic acid specificity resisting antibody and a glycocholic acid composite; the glycocholic acid specificity resisting antibody is obtained from glycocholic acid immunogen immune animals. The glycocholic acid immunodetection reagent has the benefits that the glycocholic acid immunogen specificity is strong, the immunogenicity is high, and the prepared glycocholic acid specificity resisting antibody has strong specificity and high valence and does not have any cross reaction with 45 common drugs; a homogeneous enzyme immunodetection reagent containing the glycocholic acid specificity resisting antibody can conveniently, rapidly and accurately determine the content of glycocholic acid in a sample and can simultaneously test multiple samples on a fully-automatic biochemical analysis instrument; the high-throughout rapid measurement of the glycocholic acid is realized, the accuracy is high, the specificity is strong, and both the precision and the detection efficiency are greatly improved.
Owner:苏州博源医疗科技有限公司
Kit (Latex-enhanced immunoturbidimetry) for detecting content of glycocholic acid in blood serum
ActiveCN102944673AHigh sensitivityAccurate automated detectionMaterial analysis by observing effect on chemical indicatorLatex particleSurface-active agents
The invention relates to a kit of latex-enhanced immunoturbidimetry for detecting content of glycocholic acid in blood serum. Specifically, the provided glycocholic acid detection kit comprises a reagent R1, a reagent R2 and a calibrator, wherein the reagent R1 comprises a reaction promotion agent, a preservative, a surface active agent, a stabilizer, an electrolyte and a buffer solution; the reagent R2 comprises latex particles combined with a glycocholic acid antibody, a preservative, a surface active agent, a stabilizer, an electrolyte and a buffer solution; and the calibrator comprises a preservative, an electrolyte, a stabilizer, glycocholic acid pure products and a buffer solution. The kit for detecting the content of the glycocholic acid in the blood serum, disclosed by the invention, ensures the high sensitivity and wide linear range of a kit by utilizing a method of coating the latex particles by utilizing polyclonal antibodies, also has the advantages of high accuracy, good repeatability, strong specificity, simplicity in operation and the like, and can be applied to a clinical general full automatic biochemical analyzer.
Owner:CO HEALTH BEIJING LAB
Detection kit for glycocholic acid for eliminating chyle interference
InactiveCN106124439AEliminate distractionsPrevention of missing elimination agentColor/spectral properties measurementsPreservativeMonoclonal antibody
The invention discloses a detection kit for glycocholic acid for eliminating chyle interference. The detection kit comprises a reagent R1, a reagent R2 and a calibrator. The reagent R1 contains buffer solution a, chyle elimination agent, stabilizer a, surfactant a, reaction promoter a and preservative a; the reagent R2 contains buffer solution b, latex particles of antihuman glycocholic acid monoclonal antibody, stabilizer b, surfactant b, preservative b and reaction promoter b; the calibrator contains buffer solution c, glycocholic acid standard, stabilizer c, surfactant c, reaction promoter c and preservative c. The detection kit is short in detection time, strong in anti-interference capacity, good in stability and repeatability and high in accuracy and detection sensitivity.
Owner:山东康华生物医疗科技股份有限公司
Kit for determining glycocholic acid content in human body and preparation method
ActiveCN103940816AIncrease signal strengthStrong turbidity responseMaterial analysis by observing effect on chemical indicatorInorganic saltsPreservative
The invention discloses a kit for determining glycocholic acid content in human body. The kit is characterized by comprising a glycocholic acid R1 reagent, a glycocholic acid R2 reagent and a glycocholic acid calibrator, wherein the R2 reagent is prepared by coating glycocholic acid coupled on protein by latex particles and suspending in a special buffer solution. The glycocholic acid is firstly coupled on macro-molecular protein through a chemical bond to form a plurality of sites, while protein relatively high in molecular weight can be better bonded with the latex particles, and protein coupled with the glycocholic acid further coats the surfaces of the latex particles through a chemical cross-linking method. According to the kit disclosed by the invention, the R1 reagent is prepared by adding a glycocholic acid-protein conjugate to the special buffer solution, wherein inorganic salt, a coagulation accelerator, protein and a preservative are added into the special buffer solution. Through the reagent disclosed by the invention, signal strength of immune reaction is greatly improved so that low-content substances can also generate relatively strong turbidity reaction in immune binding for detection.
Owner:ANHUI DAQIAN BIO ENG LIMITED
Polyene phosphatidyl choline injection and method for preparing the same
The invention discloses a polyene phosphatidyl choline injection and the preparation method. The components and the ratio (portion) of the polyene phosphatidyl choline injection of the invention is: 465 portion of polyene phosphatidyl choline injection, 88 portion of benzyl zlcohol, 50 to 800 portion of glycocholic acid, cholic acid or tween-80, 50 to 80 portion of alcohol, propanediol or glycerin, 15 to 200 portion of sodium hydroxide or sodium carbonate, 0.5 to 5 portion of 2,6-D-itert-butyl-p-cresol, 0.8 to 8 portion of Tertiary butyl-4-hydroxyl anisole, 3 to 25 portion of Vitamin E by weight. The polyene phosphatidyl choline injection of the invention has good clarity, high stability, simple preparation process and easy operation.
Owner:SICHUAN HAISCO PHARMA CO LTD +1
Composite liposome for injection containing 12 vitamins and preparation method thereof
InactiveCN101491499ASafety proofMetabolism disorderAmide active ingredientsAlpha-TocopherolSodium phosphates
The invention relates to a liposome lyophilized preparation prepared from twelve compound vitamins. The lyophilized preparation is characterized in that the lyophilized preparation is prepared from a liposome which consists of soybean lecithin and glycocholic acid and is encapsulated with twelve vitamins of retinyl palmitate, cocarboxylase tetrahydrate, riboflavin sodium phosphate, pyridoxine hydrochloride, cyanocobalamin, cholecalciferol, ascorbic acid, racemization-alpha tocopherol, D-biotin, niacinamide, folacin and dexpanthenol.
Owner:灵康药业集团股份有限公司
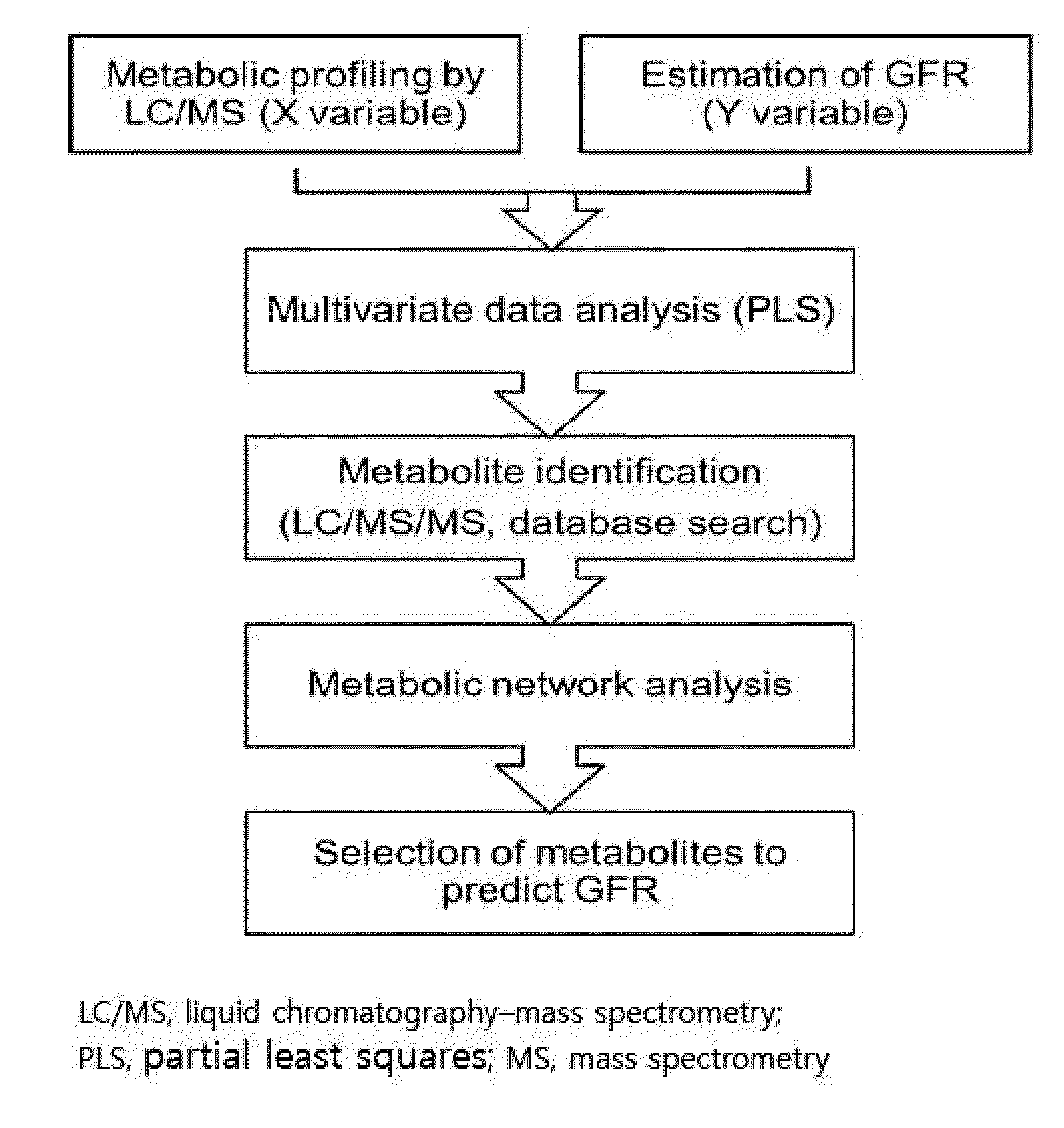
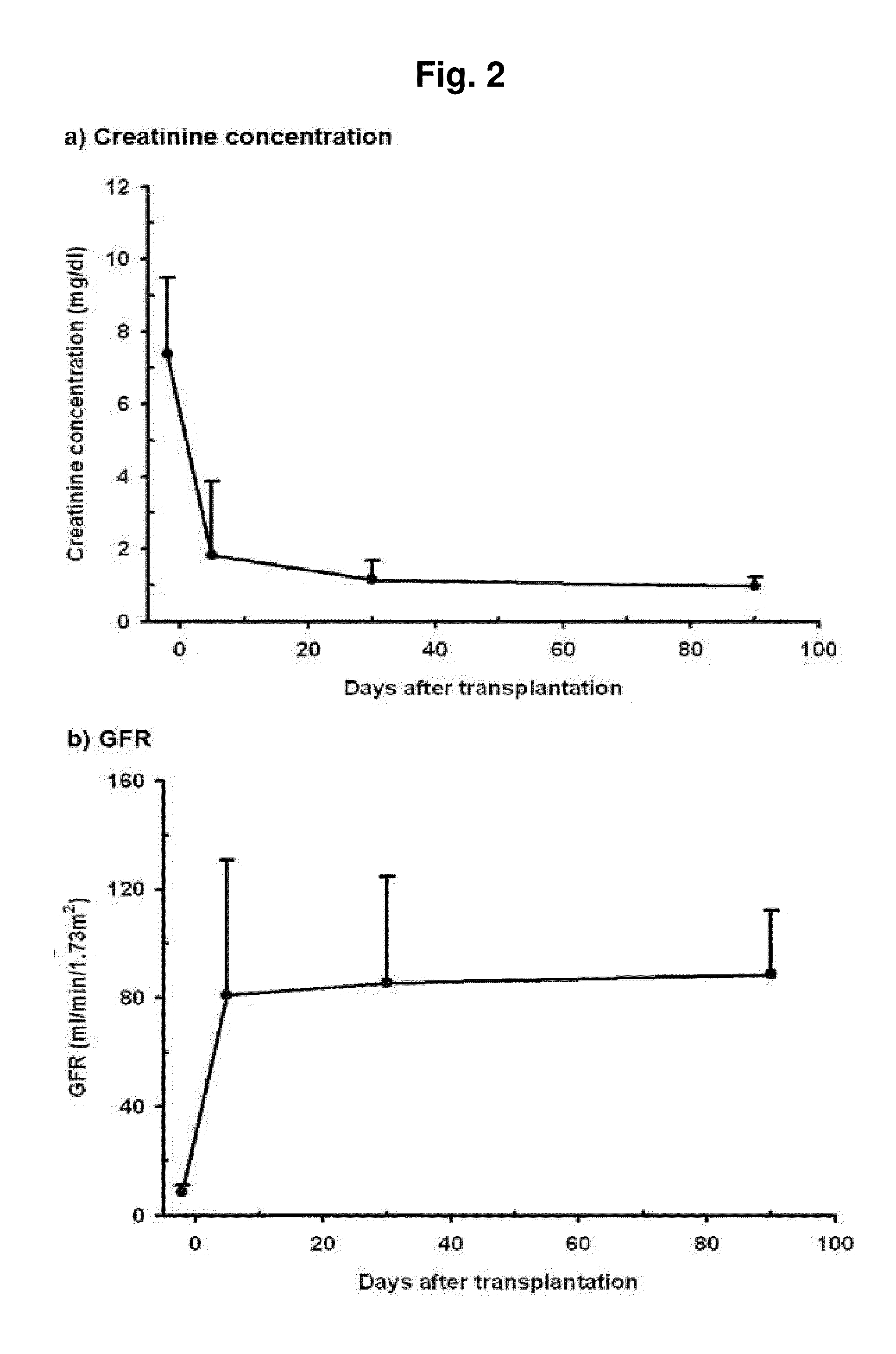
Prediction method of glomerular filtration rate from urine samples after kidney transplantation
Disclosed is a prediction method of glomerular filtration rate (GFR) from urine samples after kidney transplantation to provide an information needed for predict renal function after the transplantation, more particularly to a prediction method of glomerular filtration rate (GFR) from urine samples after kidney transplantation, which comprises detecting metabolic profiles of five biomarkers, 5a-androst-3-en-17-one (AS), glycocholic acid (GC), sphingosine (SG), tryptophan (TR) and histidine (HT), from urine samples of patients. Glomerular filtration rate (GFR) after kidney transplantation can be predicted more rapidly and precisely to provide an information needed for predict renal function after the transplantation by using five metabolites as biomarkers. The method provides more specific, sensitive, and reliable biomarkers that monitor clinical outcomes and adverse renal events after kidney transplantation, such as rejection, drug toxicity, delayed graft function, and infection.
Owner:KYUNGPOOK NAT UNIV IND ACADEMIC COOP FOUND
Preparation method of kit for measuring glycocholic acid content in human body
ActiveCN105301255AIncrease signal strengthStrong turbidity responseMaterial analysis by observing effect on chemical indicatorSerum igeSerum samples
The invention discloses a preparation method of a kit for measuring glycocholic acid content in the human body. The kit comprises a glycocholic acid R1 reagent, a glycocholic acid R2 reagent and a glycocholic acid calibrator. The glycocholic acid R1 reagent is obtained by sufficiently mixing glycocholic acid-protein conjugate with buffer liquid, the glycocholic acid R2 reagent is obtained by mixing latex particles wrapped by a glycocholic acid-protein antibody with suspension buffer liquid, and the glycocholic acid calibrator is composed of human-derived glycocholic acid and buffer liquid. The preparation method comprises the steps that human plasma containing glycocholic acid, a serum sample and the glycocholic acid-protein conjugate are competitively bound with the glycocholic acid-protein antibody latex particles, the glycocholic acid-protein antibody latex particles bound with the glycocholic acid do not produce turbidity, while, the glycocholic acid-protein antibody latex particles bound with the glycocholic acid-protein conjugate will produce turbidity changes, and quantitative analysis on the glycocholic acid is carried out by measuring the lowering degree of the system turbidity after the reaction. By means of the preparation method, the immunoreaction signal intensity is greatly improved, and even low-content matter can produce strong turbidity reaction during immune binding for detection.
Owner:ANHUI DAQIAN BIO ENG LIMITED
Novel serum metabolite composition and application thereof as liver cancer diagnosis marker
ActiveCN105738626AMaintain stabilityHas the value of clinical development and applicationMaterial analysisTryptophanUridine Nucleotides
The invention discloses a novel serum metabolite composition and an application thereof as a marker for preparing an early-stage liver cancer diagnosis kit. The serum metabolite composition includes: glycocholic acid, tryptophan, histidine, uridine, choline, benzamide, lysophosphatidyl choline 16:1 and phenylalanyl tryptophan. The serum metabolite composition can be used for early-stage diagnosis of liver cancer, is low in detection cost, is high in repeatability and sensitivity, and has excellent complementarity with a conventional clinical diagnosis marker serum alpha fetoprotein (AFP).
Owner:HANGZHOU HEALTH BANK MEDICAL LAB CO LTD
Preparation method of quality control serum for quality control of centrifugal microfluidic chips
ActiveCN108152519ASufficient sourceEasy to getBiological testingFreeze-dryingMonopotassium phosphate
The invention discloses a preparation method of quality control serum for quality control of centrifugal microfluidic chips. The method comprises the following steps: adding 0.6 to 1.0 percent of cholesteryl sodium sulfate into bovine plasma, and adding 0.02 to 0.06 percent of monopotassium phosphate; sequentially adding 0.04 to 0. 08 percent of ammonium ferric sulfate dodecahydrate, 0.5 to 0.9 percent of calcium chloride, 0.2 to 0.6 percent of bitter salt, 0.6 to 1 percent of urea, 9 to 13 percent of sodium chloride, 0.01 to 0.03 percent of zinc vitriol, 0.01 to 0.03 percent of chalcanthite,0.06 to 0.2 percent of glycocholic acid, 2 to 4 percent of glucose, 0.03 to 0.06 percent of creatinine, 0.08 to 0.2 percent of uric acid, and 0.07 to 0.11 percent of triolein into water; uniformly mixing obtained solution; adding glycol, saccharose and triton X-100, and uniformly mixing; sequentially adding albumin bovine serum, sodium azide, alanine aminotransferase, aspartic transaminase, alkaline phosphatase, lipase and creatine kinase, uniformly mixing, and performing freeze drying. The method has the advantages that the source of the raw materials is sufficient, the raw materials are easyto get, possible matrix effect is furthest avoided, and precipitation of the raw materials is prevented.
Owner:NINGBO MEIKANG BAOSHENG BIOMEDICAL ENG
Reagent kit and method for measuring concentration of glycocholic acid
ActiveCN105988000AExclude non-specific cross-reactivityImprove accuracyMaterial analysisLatex particleMonoclonal antibody
The invention discloses a reagent kit and method for measuring the concentration of glycocholic acid. The reagent kit comprises a reagent (1) prepared from latex particles coated with a glycocholic acid resisting monoclonal antibody, and a reagent (2) prepared from latex particles coated with glycocholic acid-inert carrier conjugates. When used for detecting glycocholic acid, the reagent kit can easily enhance immunity turbidity and amplify detection signals, has the advantages of being free of special instruments, easy and convenient to operate, high in sensitivity and low in cost, is capable of quickly measuring large-flow samples clinically and has extremely high economic value, and it becomes possible that glycocholic acid becomes a clinical conventional detection item.
Owner:上海微鸿企业管理有限公司
Glycocholic-acid immunodetection reagent based on anti-glycocholic acid specific antibody and preparation method thereof
InactiveCN105481977AStrong specificityHigh potencySerum albuminImmunoglobulinsReactive siteImmunochromatographic test
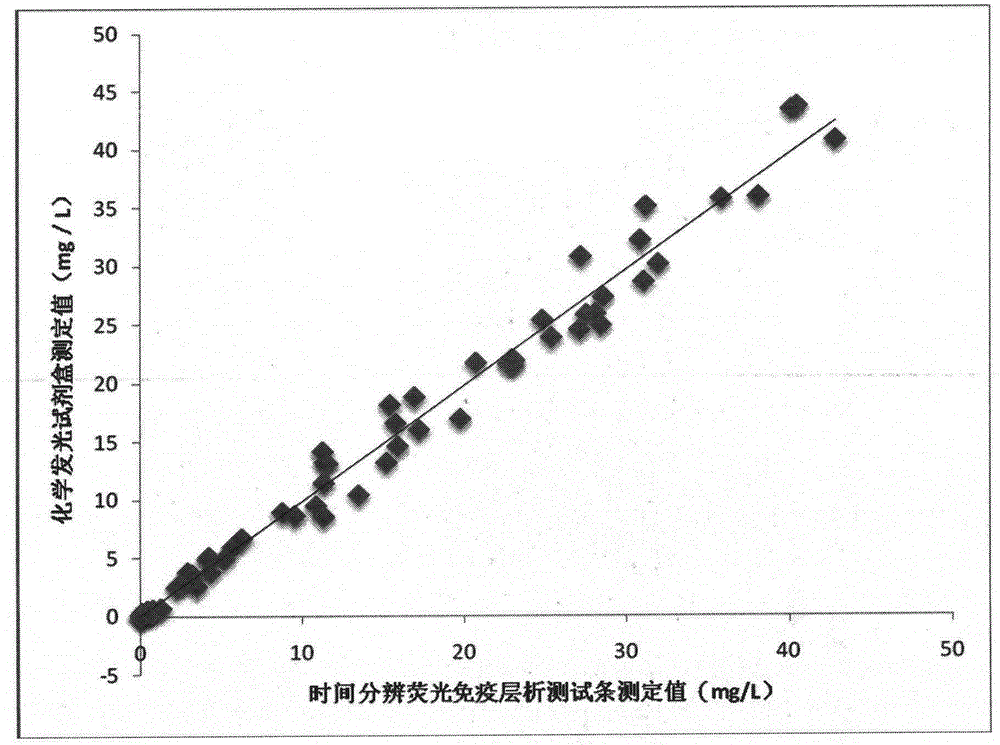
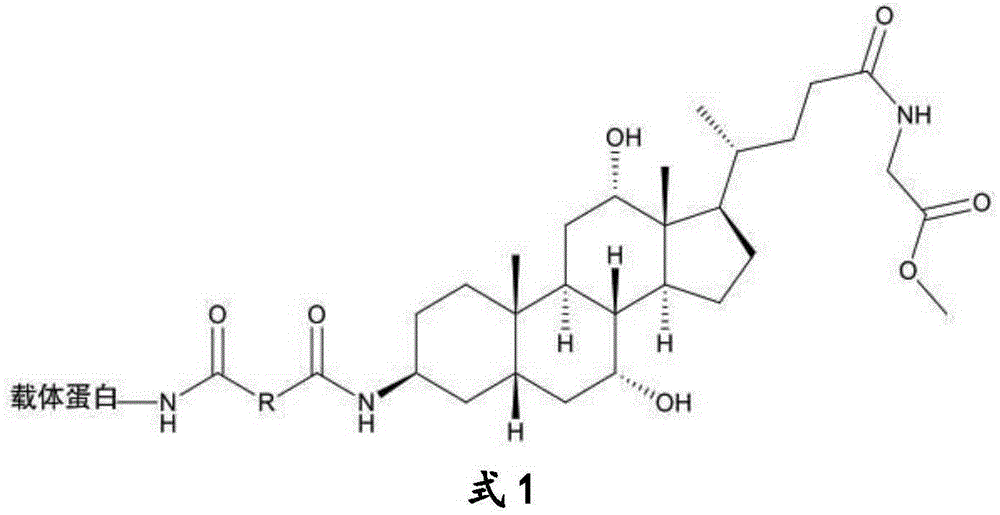
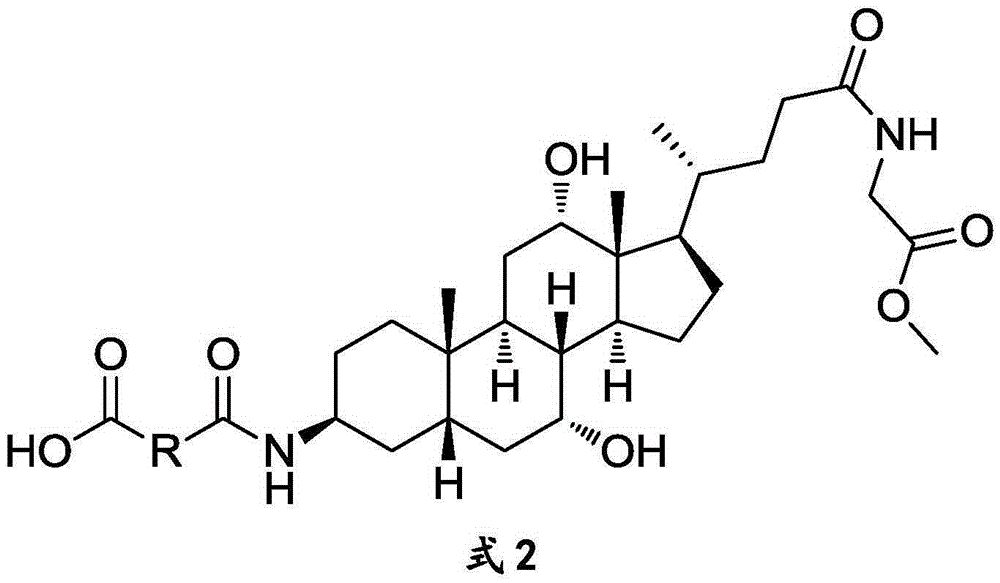
The invention discloses a glycocholic acid specific semiantigen, a preparation method for a glycocholic acid specific monoclonal antibody based on the semiantigen, and an immunity chromatography detection method for detecting glycocholic acid. The above specific monoclonal antibody is obtained from a glycocholic-acid-specific-semiantigen-coupled protein immune animal. The immunogen prepared through coupling of the glycocholic acid specific semiantigen and protein keeps a specific active site of glycocholic acid, and the cross reaction rates of the obtained monoclonal antibody with other 8 kinds of bile acids are all smaller than 10%. A time-resolved immunochromatographic test strip prepared by using the above glycocholic acid specific monoclonal antibody is capable of rapidly determining the content of glycocholic acid in a sample, and specificity and accuracy of current glycocholic acid clinic diagnosis are improved.
Owner:BEIJING HUAXINYUAN BIOTECH CO LTD
A lyophilized powder injection of polyene phosphatidylcholine and preparation method thereof
The invention relates to a phosphatidyl choline freeze powder injection and relative preparation, wherein said formula is formed by phosphatidyl choline, solubilizer, support agent and other additive components; the phosphatidyl choline content is 10-60%; the solubilizer can colalin, glycocholic acid, cholaic acid, deoxycholic acid, anthrodsoxycholic acid, or taurodeoxycholic acid and relative salt, loxapine and tween; the support agent can be freeze protector. The invention has high stability, which can be stored for 1 year at normal temperature, without allergic and excitation.
Owner:四川思达康药业有限公司
Glycocholic acid detection kit
PendingCN109444399ASimple preparation stepsLow costDisease diagnosisColor/spectral properties measurementsLatex particleAniline
The invention provides a glycocholic acid detection kit and a preparation method of a glycocholic acid-protein conjugate and glycocholic acid antibody coated rubber latex particles in the glycocholicacid detection kit. According to the glycocholic acid detection kit, glycocholic acid is detected by a competition method, 8-aniline-1-naphthalene sulfonic acid is added into the kit, so that the analysis sensitivity and precision of the kit are obviously improved, the linear range of detection is widened, preparation steps of specific substances in the kit are simple, and the kit is low in cost and more applicable to industrial production and clinic application.
Owner:ZYBIO INC
Pharmaceutical composition of 12 complex vitamins for injection and preparation method thereof
InactiveCN103006683AHydroxy compound active ingredientsMetabolism disorderThiamine pyrophosphateAlpha-Tocopherol
The invention provides a pharmaceutical composition of 12 complex vitamins for injection and a preparation method thereof. The pharmaceutical composition of 12 complex vitamins for injection provided by the invention comprises the active ingredients of vitamin A palmitate, cholecalciferol, racemic alpha-tocopherol, ascorbic acid, nicotinamide, dexpanthenol, pyridoxine hydrochloride, riboflavin sodium phosphate, tetrahydrate thiamine pyrophosphate, folic acid, D-biotin and cyanocobalamin, and auxiliary materials namely polysorbate 80 and mannitol. The prescription provided by the invention does not contain auxiliary material glycocholic acid, and can be clinically used for people with over-high glycocholic acid.
Owner:SHANXI PUDE PHARMA CO LTD
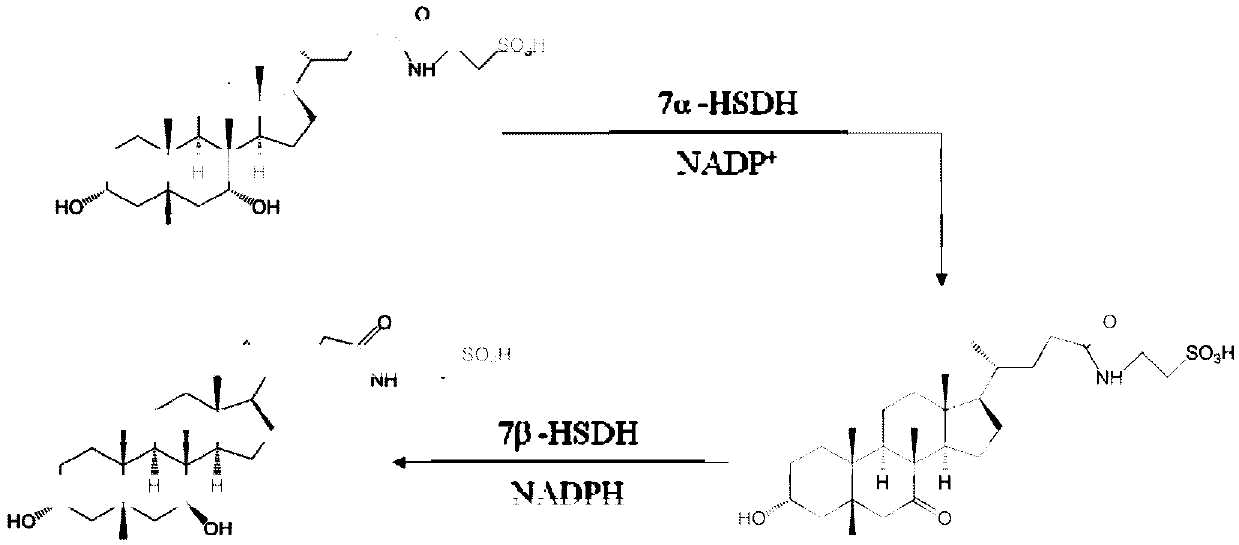
7 alpha-hydroxysteroid dehydrogenase as well as coding gene and application thereof
ActiveCN108034643ACatalytic asymmetric reduction reactionOxidoreductasesFermentationNucleotideKetone
The invention discloses 7 alpha-hydroxysteroid dehydrogenase as well as a coding gene and application thereof. An amino acid sequence of the 7 alpha-hydroxysteroid dehydrogenase provided by the invention is shown as SEQ ID NO. 1, and a nucleotide sequence of the 7 alpha-hydroxysteroid dehydrogenase gene is disclosed and shown as SEQ ID NO. 2. The 7 alpha-hydroxysteroid dehydrogenase can catalyze asubstrate taurocholic acid (TCA) to generate taurine 7-ketone cholic acid (T7K-CA), catalyze a substrate glycocholic acid (GCA) to generate glycine 7-ketone cholic acid (G7K-CA), catalyze a substratetaurochenodeoxycholic acid (TCDCA) to generate taurine 7-ketone lithocholic acid (T7K-LCA), catalyze a substrate glycochenodeoxycholic acid (GCDCA) to generate glycine 7-ketone lithocholic acid (G7K-LCA) and catalyze a substrate ethyl benzoylformate (EB) to generate ethyl 2-hydroxy-2-phenylacetate, and has better catalytic activity, has a catalytic activity to TCDCA, GCDCA and EB 10, 5, and 3 times that of sardinia clostridium 7 alpha-HSDH, correspondingly, and has great industrial application value.
Owner:CHONGQING UNIV
Determination reagent for glycocholic acid and preparation method of determination reagent
The invention relates to a determination reagent for glycocholic acid and a preparation method of the determination reagent, and belongs to the technical field of biochemical means testing. The determination reagent comprises an R1 reagent, an R2 reagent and a glycocholic acid standard sample; a solution formed by dissolving a sulfo-oxidation type coenzyme into purified water added with a buffer solution is adopted as the R1 reagent; a solution formed by dissolving a reduction type coenzyme into purified water added with a buffer solution, glycocholic acid dehydrogenase and sodium azide is adopted as the R2 reagent; a solution formed by dissolving a sodium glycocholate hydrate, a buffer solution and sodium azide into purified water is adopted as the glycocholic acid standard sample. The determination reagent is applied to glycocholic acid determination and has the advantages of being quick, sensitive, good in accuracy, high in specificity, good in stability and the like.
Owner:浙江达美生物技术有限公司
Reagent kit for measuring glycocholic acid in human serum and method for applying reagent kit
The invention discloses a reagent kit for measuring glycocholic acid in human serum. The reagent kit comprises glycocholic acid reagents R1, glycocholic acid reagents R2 and glycocholic acid standard substances. The reagent kit has the advantages that competition processes are used in detection procedures, accordingly, variation of the turbidity of test solution can be controlled in wide ranges, the glycocholic acid can be detected by the aid of common analytical instruments, and the reagent kit is favorable for guaranteeing the accuracy of the instruments.
Owner:北京美德美康生物技术有限公司
Kit, preparation method of kit and method for detecting peripheral blood glycocholic acid through kit
ActiveCN106226512ALow specificityHigh detection sensitivityColor/spectral properties measurementsTaurocholic acidTaurine
The invention discloses a kit. The kit comprises a reagent R1 and a reagent R2, the reagent R1 contains a trihydroxymethyl aminomethane (Tris-HCL) buffer solution, an anti-taurine antibody, an anti-glycocholic acid antibody and glucose-6-phosphate (G6P), and the reagent R2 contains the trihydroxymethyl aminomethane (Tris-HCL) buffer solution, a glycocholic acid-G-6-PD conjugate, NADP and BSA. The invention further discloses a preparation method of the kit, particularly discloses a preparation method of the anti-taurine antibody the anti-glycocholic acid antibody and further discloses a method for detecting peripheral blood glycocholic acid through the kit. Accordingly, interference of taurocholic acid can be avoided, the detection sensitivity reaches up to 0.1 microgram per milliliter, and the advantages of high specificity and detection repeatability, good stability and the like are achieved.
Owner:杭州利安生物科技有限公司 +1
A kit, a preparation method of the kit, and a detection method of glycocholic acid in peripheral blood realized by using the kit
ActiveCN106226512BLow specificityClosed interferenceColor/spectral properties measurementsTaurocholic acidTaurine
The invention discloses a kit. The kit comprises a reagent R1 and a reagent R2, the reagent R1 contains a trihydroxymethyl aminomethane (Tris-HCL) buffer solution, an anti-taurine antibody, an anti-glycocholic acid antibody and glucose-6-phosphate (G6P), and the reagent R2 contains the trihydroxymethyl aminomethane (Tris-HCL) buffer solution, a glycocholic acid-G-6-PD conjugate, NADP and BSA. The invention further discloses a preparation method of the kit, particularly discloses a preparation method of the anti-taurine antibody the anti-glycocholic acid antibody and further discloses a method for detecting peripheral blood glycocholic acid through the kit. Accordingly, interference of taurocholic acid can be avoided, the detection sensitivity reaches up to 0.1 microgram per milliliter, and the advantages of high specificity and detection repeatability, good stability and the like are achieved.
Owner:杭州利安生物科技有限公司 +1
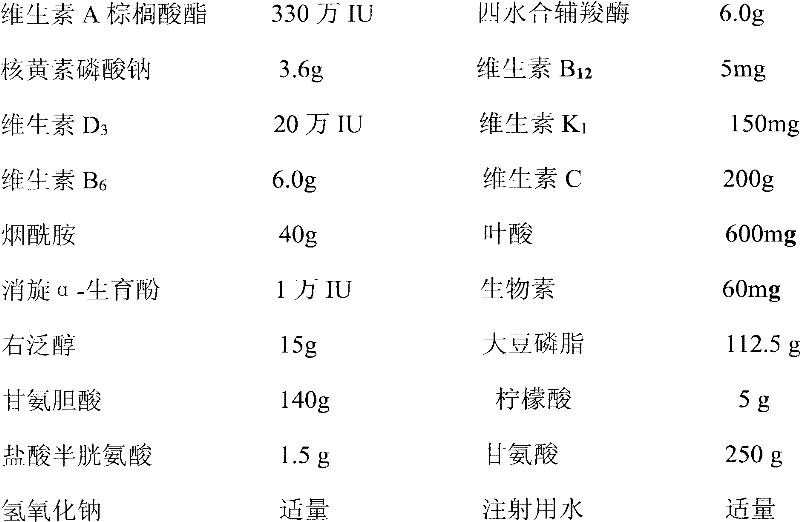
Pharmaceutical composition containing 13 kinds of vitamins
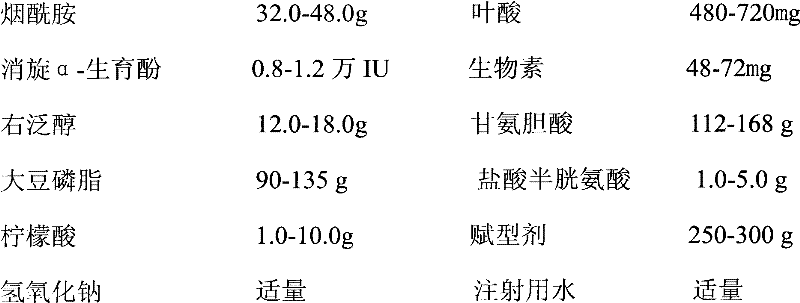
The invention discloses a pharmaceutical composition containing 13 kinds of vitamins, which is characterized in that the pharmaceutical composition is an injection preparation formed by vitamin A palmitate, tetrahydrate cocarboxylase, lactoflavin sodium phosphate, vitamin B12, vitamin D3, vitamin K1, vitamin B6, vitamin C, niacinamide, folacin, racemic alpha-tocopheryl, biotin, dexpanthenol, soybean phospholipid, glycocholic acid, citric acid, cysteine hydrochloride and excipients. According to the pharmaceutical composition, the deficiencies in the prior art are overcome; and indicated by experimental results, the formed injection preparation has the advantages of long-term stability in quality and more safety in clinical use.
Owner:郑飞雄
Magnetic particle chemiluminiscence detection kit for determining content of glycocholic acid in human body
InactiveCN109521005ALow pre-processing requirementsGuaranteed SensitivityChemiluminescene/bioluminescenceAntigenFluorescein isothiocyanate
The invention discloses a preparation method of a magnetic particle chemiluminiscence detection kit for determining the content of glycocholic acid in a human body. The kit contains a glycocholic acidR1 reagent, a glycocholic acid R2 reagent, a magnetic separation reagent, a calibration product liquid series and a chemiluminiscence substrate liquid, wherein the glycocholic acid R1 reagent is fluorescein isothiocyanate labeled anti-glycocholic acid-BSA mouse monoclonal antibody diluent; the glycocholic acid R2 reagent is an alkaline phosphatase labeled glycocholic acid antigen diluent; the magnetic separation reagent is an anti-fluorescein isothiocyanate mouse monoclonal antibody coated magnetic particle diluent; glycocholic acid calibration product liquid contains synthetic glycocholic acid and a buffer solution; and the chemiluminiscence substrate liquid is an alkaline phosphatase catalyzed Tris-HCl buffer solution containing dioxane. According to the kit, the signal strength and sensitivity of immunoreactions are greatly improved, a low-content substance can generate very strong chemiluminiscence signals during immune binding, and the relatively accurate, precise, convenient andrapid method is provided for the detection of glycocholic acid.
Owner:BEIJING LEADMAN BIOCHEM
Kit for detecting glycocholic acid content in serum and preparation method of kit
The invention discloses a kit for detecting the glycocholic acid content in serum. The kit comprises a resistant reagent A, a resistant reagent B, a magnetic particle reagent, a calibration product, a quality control product, luminous substrate liquid and concentrated washing liquid; the resistant reagent A is a glycocholic acid derivative resistant reagent A marked with alkaline phosphatase; the resistant reagent B is a glycocholic acid antibody marked with FITC; the magnetic particle reagent is carboxyl magnetic particles coated with FITC antibodies. The invention further discloses a preparation method of the kit. According to the kit, a reaction system close to a homogeneous phase is provided by combining a chemiluminiscence technology with the immune magnetic particles; in addition, a one-step reaction mode is adopted, so that the detection properties (such as the flexibility, the precision and the detection range) are greatly improved, the reaction time is greatly shortened, and the time from sample adding to detection result obtaining is shorter than 35 min and obviously shorter than that of a kit of the same kind; the coupling efficiency is high, bonding is firm, the process is stable, and the product cost is greatly reduced while the product performance is improved.
Owner:TAIZHOU ZECEN BIOTECH CO LTD
Metabolic marker for diagnosing and distinguishing unstable angina pectoris and acute myocardial infarction
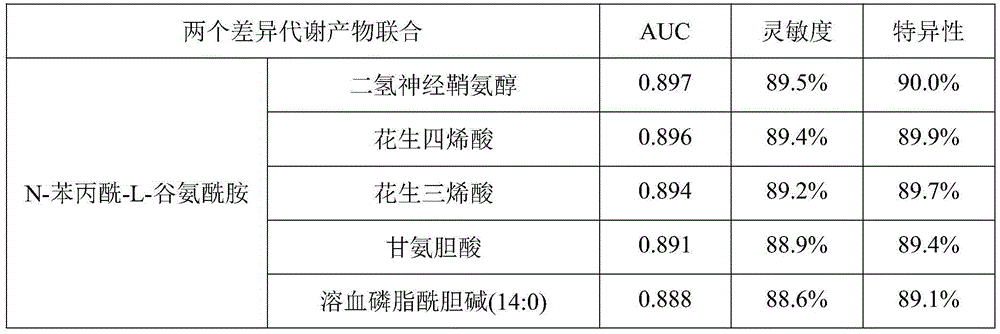
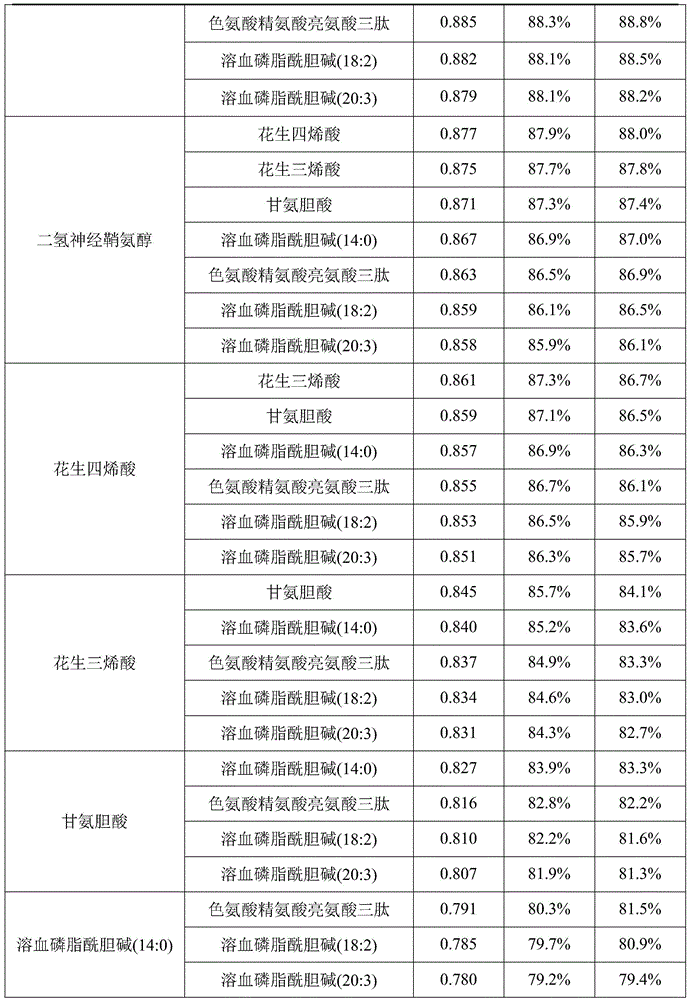
The invention discloses a metabolic marker for diagnosing and distinguishing unstable angina pectoris and acute myocardial infarction, comprising one or more of N-phenylalanyl-L-glutamine, sphinganine, arachidonic acid, eicosatrienoic acid, glycocholic acid, lysophosphatidylcholine (14:0), tryptophan-arginine-leucine tripeptide, lysophosphatidylcholine (18:2), and lysophosphatidylcholine (20:3). In the single use of diagnosing and distinguishing patients with acute myocardial infarction and patients with unstable angina pectoris, each ROC (receiver operating characteristic) AUC (area under the curve) is greater than 0.7, and clinical diagnostic significance is provided; in the joint use for diagnosis, AUC further increases as a joint quantity increases, a highest AUC up to 0.991 is obtained in a case of nine members jointed, and sensitivity and specificity are respectively 99.2% and 98.9% under an optimal cutoff value. The metabolic marker can accurately diagnose and distinguish unstable angina pectoris and acute myocardial infarction, with high accuracy and high sensitivity and specificity.
Owner:齐炼文
Preparation method for homogeneous enzyme immunodiagnosis reagent used for glycocholic acid
InactiveCN106405069AEasy to operateReliable responseColor/spectral properties measurementsBiological activationGlucose phosphate
The invention relates to a preparation method for a homogeneous enzyme immunodiagnosis reagent used for glycocholic acid, belonging to the technical field of biological medicine. The preparation method comprises the following steps: preparation of a glycocholic acid antibody solution; preparation of a glycocholic acid-enzyme conjugate solution; and preparation of a glycocholic acid calibrating substance. The glycocholic acid-enzyme conjugate solution is prepared by adding glycocholic acid into a MES buffer solution, adding a carboxyl activator for carboxyl activation, then adding 6-phosphogluconate dehydrogenase for a condensation reaction so as to obtain a crude glycocholic acid-enzyme conjugate product, carrying out dialysis and purification, adding the treated crude glycocholic acid-enzyme conjugate product into a Tris-HCl buffer solution, adding an auxiliary reagent and carrying out uniform mixing so as to obtain the glycocholic acid-enzyme conjugate solution. The homogeneous enzyme immunodiagnosis reagent for glycocholic acid prepared by using the method is safe, rapid, highly efficient and sensitive and can accurately detect the content of glycocholic acid in a to-be-detected sample.
Owner:李松羊
Kit for quickly determining content of glycocholic acid (CG) in serum or blood plasma
The invention relates to the field of biological detection and in particular relates to a detection kit used for quantitatively detecting glycocholic acid (CG) as well as a preparation method and application of the detection kit. The detection kit used for quantitatively detecting CG comprises a test paper card, wherein the test paper card comprises a base plate as well as a sample pad, a gold mark pad, a nitrocellulose membrane and a water absorption pad which are sequentially arranged from a sampling end positioned on the surface of the base plate, the gold mark pad contains a CG antibody, the nitrocellulose membrane is coated with a detection line and a quality control line, and the CG antibody on the gold mark pad is marked by adopting a fluorescent microsphere. The detection kit provided by the invention can be used for detecting CG by adopting a fluorescent microsphere immunochromatographic technology initially, has sensitivity and specificity and has the advantages of quickness and simplicity in operation, accurate result, economic applicability and the like.
Owner:ZYBIO INC
Use of combined serum metabolic marker in preparation of kit for diagnosing progress of hepatopathy, kit, and method using kit to screen serum metabolic markers
InactiveCN107656007AAlleviate the problem of difficult development monitoringRapid diagnosisComponent separationSerum igeMetabolite
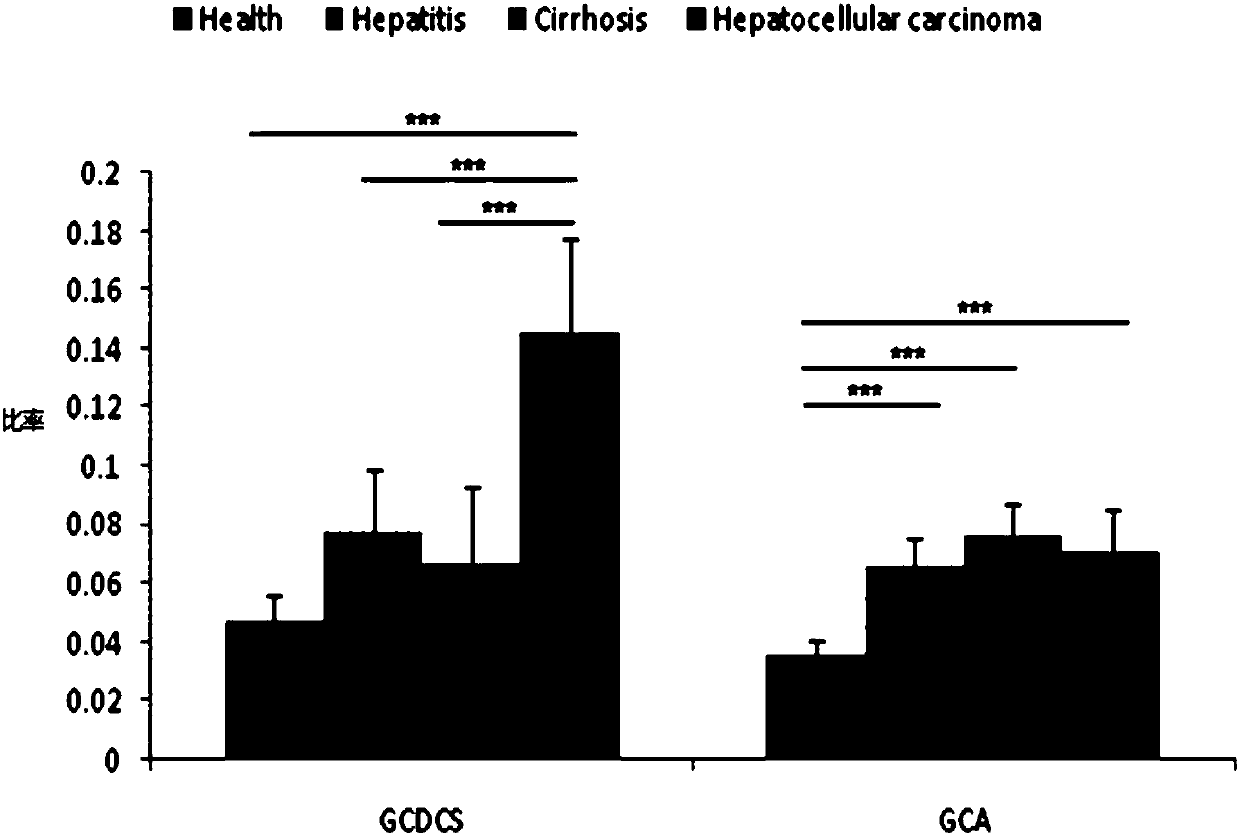
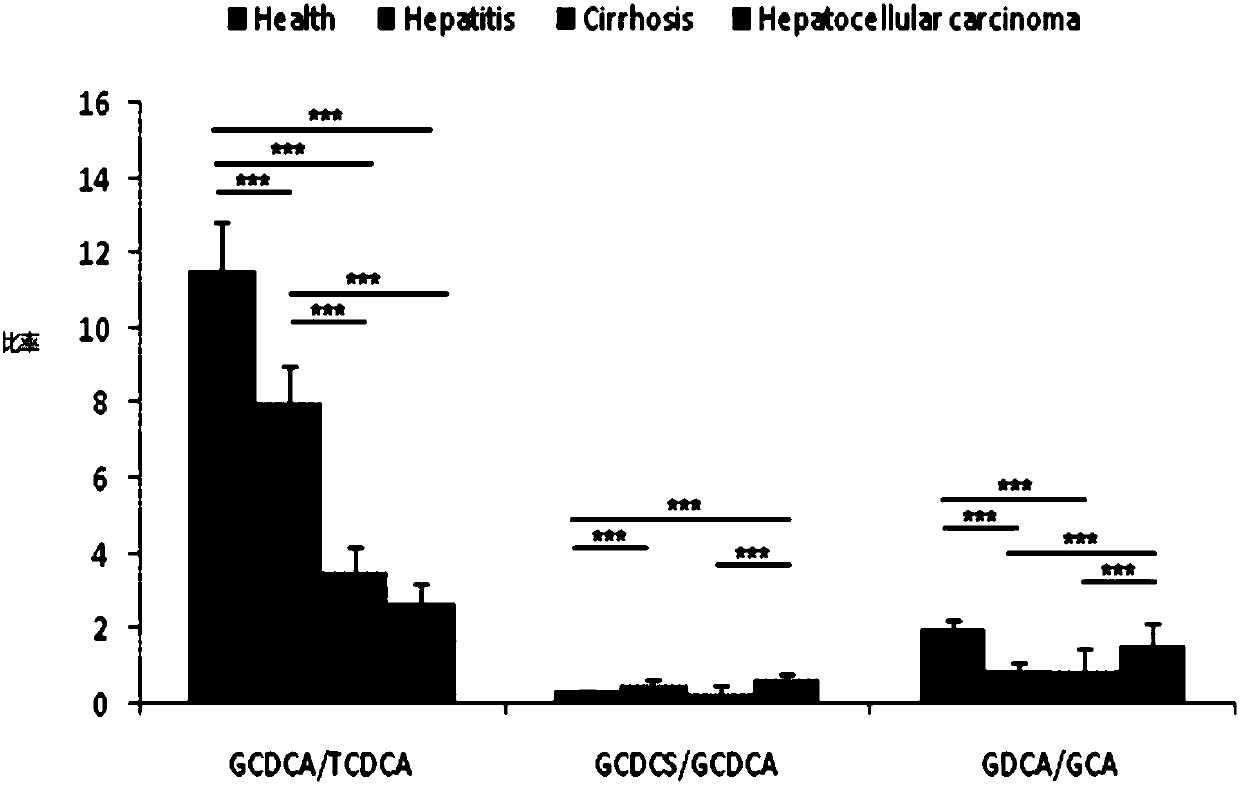
The invention provides a use of a combined serum metabolic marker in the preparation of a kit for diagnosing the progress of hepatopathy, the kit, and a method using the kit to screen serum metabolicmarkers, and relates to the technical field of serum metabolic markers. The combined serum metabolic marker is mainly composed of sodium glycochenodeoxycholate sulfate, glycochenodeoxycholic acid, glycodesoxycholic acid, glycocholic acid and taurochenodeoxycholic acid. The kit executes combined screening detection on the content of above serum metabolites in serum in order to realize assisted diagnosis of the progress of the hepatopathy of hepatopathy patients, so the difficult monitoring problem of the occurrence and development of the hepatopathy in existing clinic therapy of the hepatopathyis effectively alleviated.
Owner:HANGZHOU HEALTH BANK MEDICAL LAB CO LTD
Pancreatic cancer diagnostic marker combination as well as application and determination method thereof
ActiveCN105572276AImprove clinical treatment effectGood clinical effectComponent separationBetaineMetabolite
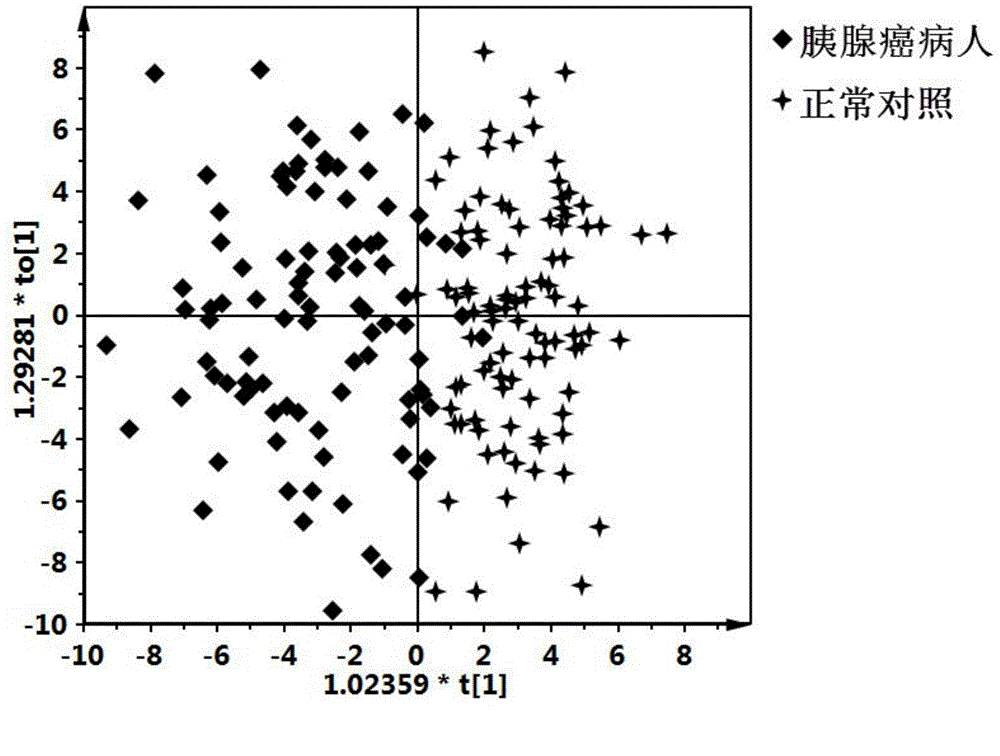
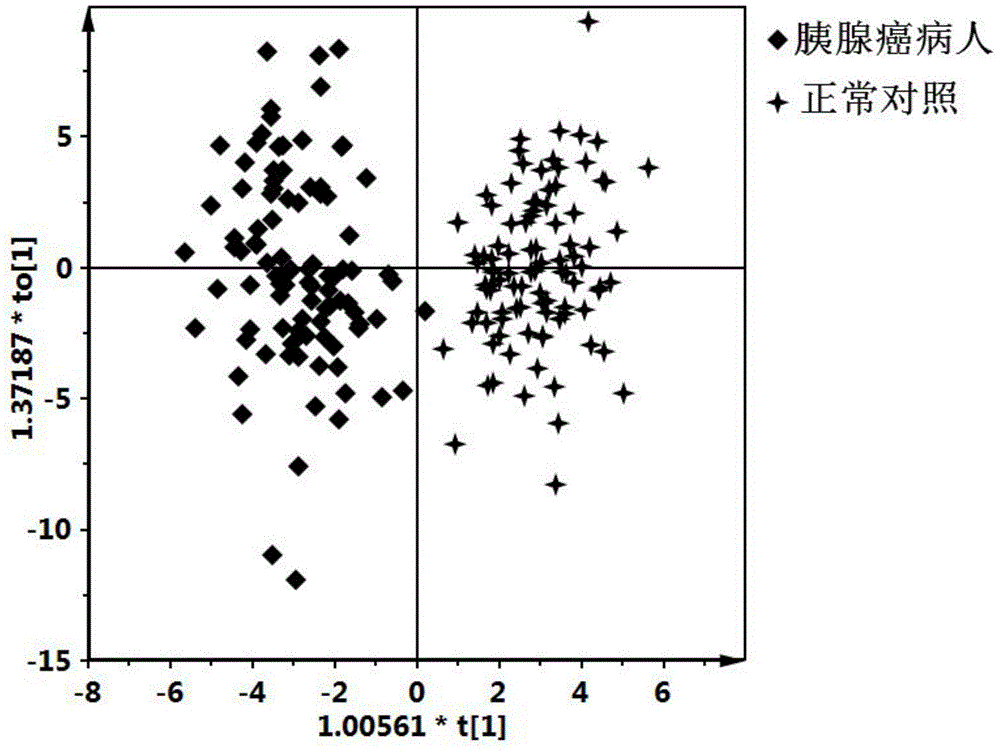
The invention discloses a pancreatic cancer diagnostic marker combination as well as application and a determination method thereof. The pancreatic cancer diagnostic marker combination comprises 15 differentiated metabolites (2,5-dihydroxybenzoic acid, talopyranose, proline, glutamate, choline, 1,5-anhydro-D-glucitol, tryptophan, glutamine, betaine, 2-oxoglutaric acid, methylguanidine, adenine, glycocholic acid, valine and 2-aminobutyric acid). The invention further provides a combination of the 15 differentiated metabolites to serve as a marker for early discovery and diagnosis of pancreatic cancer, application and a diagnostic marker determination method, and the method is a liquid-phase / gas-phase chromatography-mass spectrometry combined metabonomics analysis method based on plasma / serum of patients with pancreatic cancer. The pancreatic cancer diagnostic marker combination provided by the technical scheme of the invention has the characteristics of being high in sensitiveness and specificity, has relatively high sensitiveness and specificity on early pancreatic cancer diagnosis, can be used for early discovery of pancreatic cancer, gains time for the patients to receive treatment as soon as possible, and improves the clinical treatment effect.
Owner:麦特绘谱生物科技(上海)有限公司
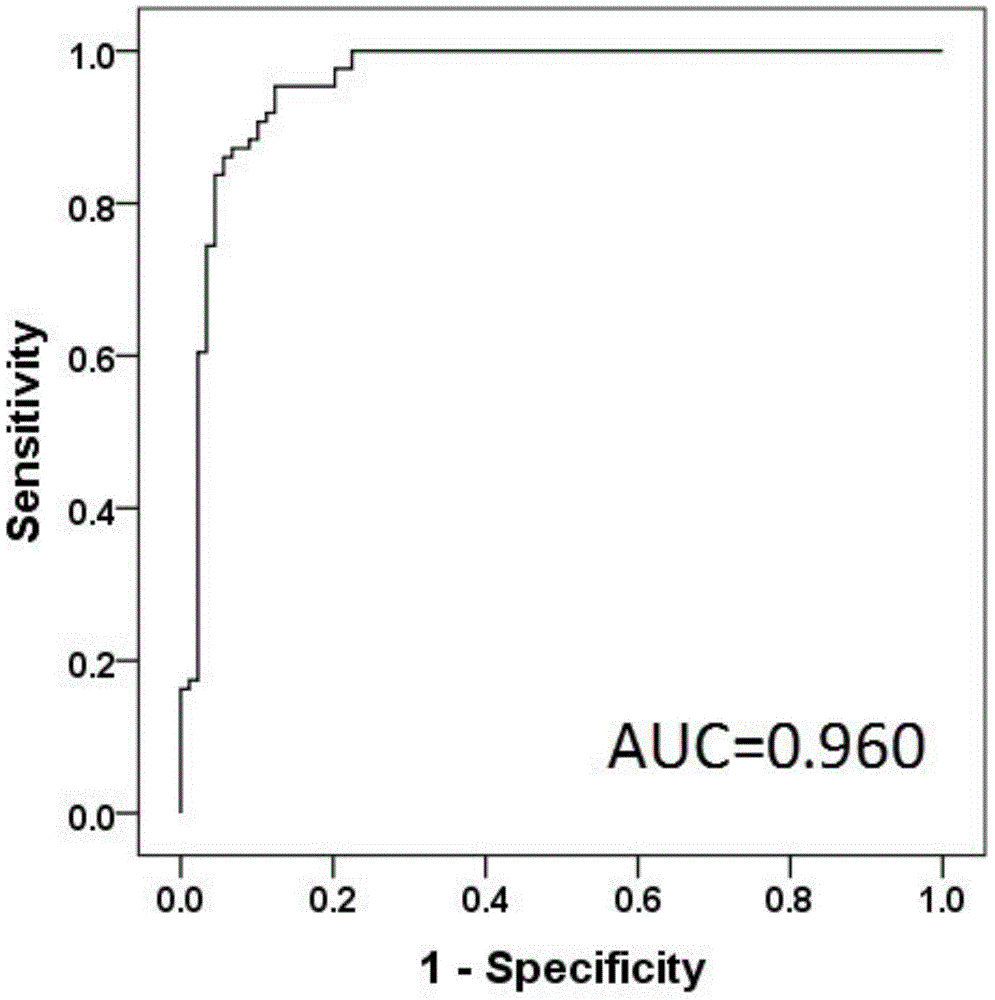
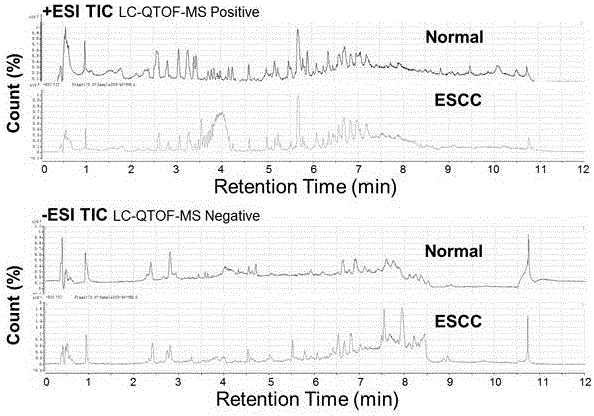
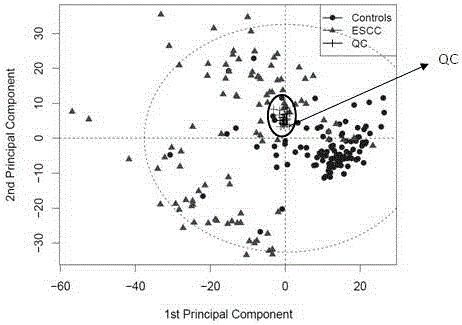
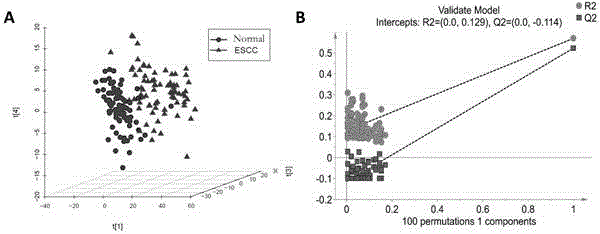
Diagnosis marker suitable for early-stage esophageal squamous cell cancer diagnosis
The invention discloses a diagnosis marker suitable for an early-stage esophageal squamous cell cancer diagnosis. The diagnosis marker is a composition of two or more than two kinds of materials from the following five kinds of serum metabolic markers including L-tyrosine, L-tryptophan, glycocholic acid, taurocholate and cortisol. When the diagnosis marker provided by the invention is adopted, a diagnosis model can be built. The diagnosis model has the advantages of good effect, high sensitivity and good specificity, is suitable for late-stage and early-stage esophagus cancer diagnoses, and has good clinic use and popularization value.
Owner:SHANDONG RES INST OF TUMOUR PREVENTION TREATMENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com