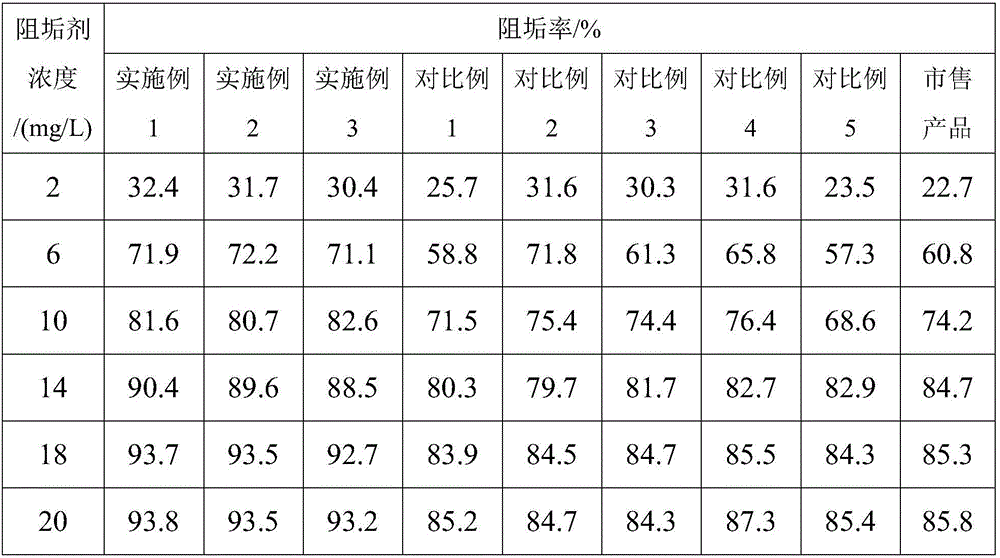
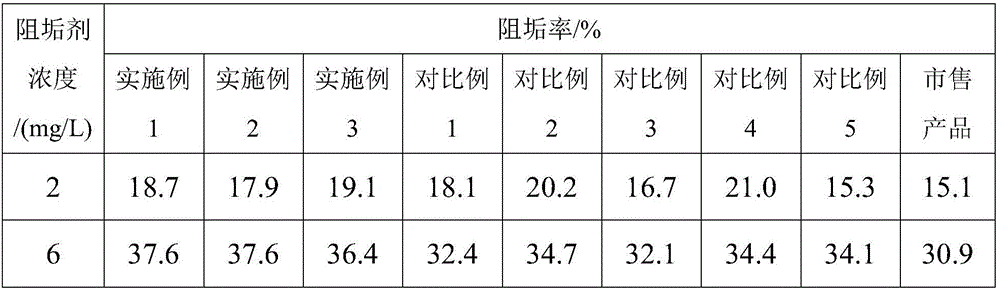
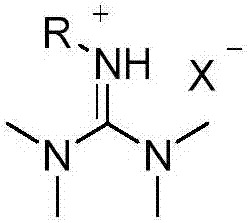
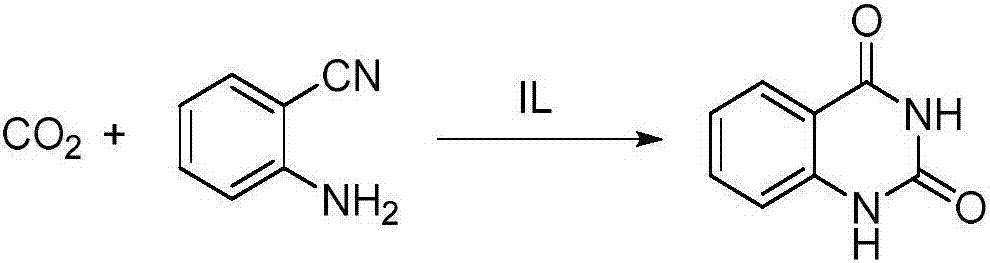Patents
Literature
65 results about "Methylguanidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A product of putrefaction. Poisonous.
Ectoparasite control compositions
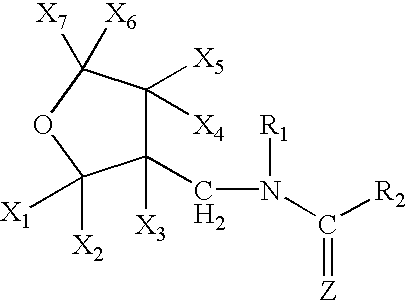
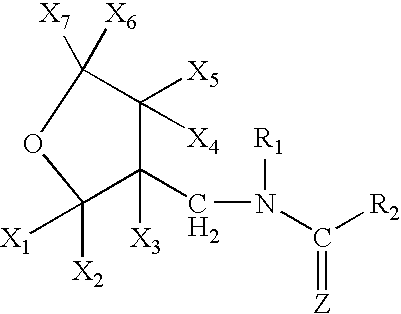
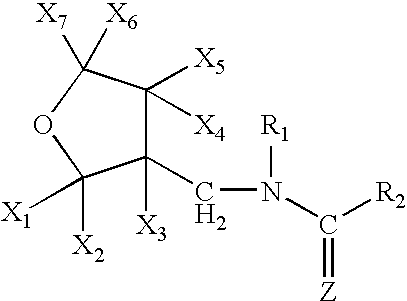
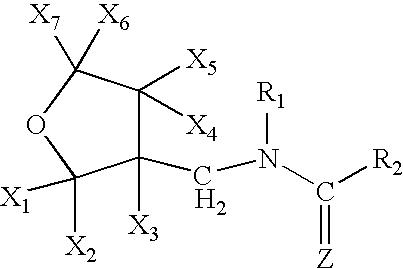
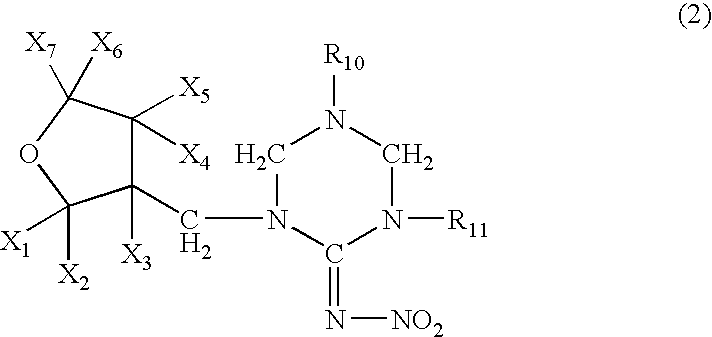
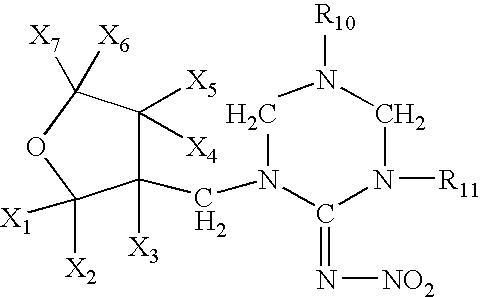
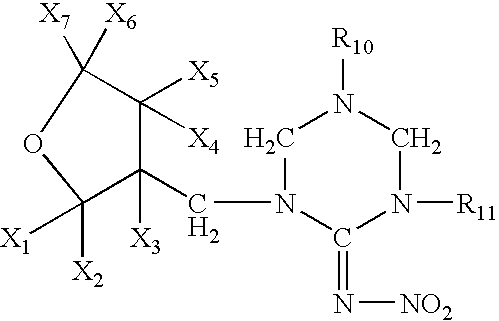
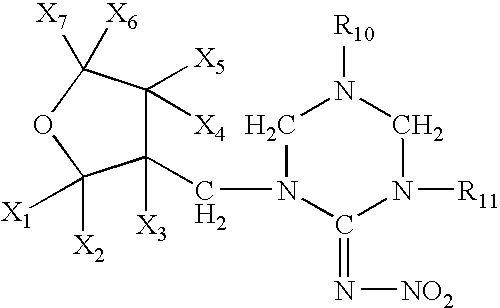
Provided are ectoparasite control compositions, a method of controlling an ectoparasite and uses of a composition for controlling an ectoparasite. The ectoparasite control compositions comprise a solvent and 1-methyl-2-nitro-3-[(3-tetrahydrofuryl)methyl]guanidine, wherein said solvent contains mainly N-methyl-2-pyrrolidone. The methods of controlling an ectoparasite, comprise applying to a host animal, an ectoparasite control composition which comprises a solvent and 1-methyl-2-nitro-3-[(3-tetrahydrofuryl)methyl]guanidine, wherein said solvent contains mainly N-methyl-2-pyrrolidone.
Owner:SUMITOMO CHEM CO LTD
High concentration topical insecticide
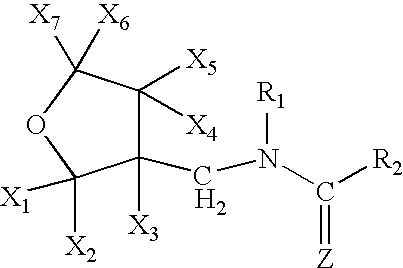
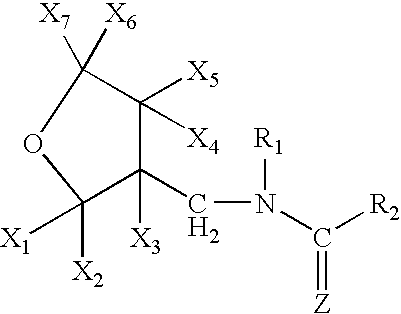
A topical insecticide is provided which can be safe to use and avoids many common deleterious side effects of conventional topical insecticides. In one preferred embodiment of the invention, the active ingredient of the insecticide formulation is an amine derivative, having a nitro-methylene group, a nitroamino group or a cyanoamino group, which can be formulated to have low toxicity and excellent insecticidal activity. One particularly suitable insecticide is 1-{(tetrahydro-3-furanyl)methyl}-2-nitro-3-methylguanidine (dinotefuran), an aldulticide that will kill adult fleas, dissolved in phenyl methanol or ethanol and ethyl lactate.
Owner:CEVA ANIMAL HEALTH
High concentration topical insecticide containing pyriproxyfen
InactiveUS6867223B2Reduce the amount requiredReduce efficacyBiocideDead animal preservationHigh concentrationSide effect
A topical insecticide is provided which can be safe to use and avoids many common deleterious side effects of conventional topical insecticides. In one preferred embodiment of the invention, the active ingredient of the insecticide formulation is an amine derivative, having a nitro-methylene group, a nitroamino group or a cyanoamino group, which can be formulated to have low toxicity and excellent insecticidal activity. One particularly suitable insecticide is 1-{(tetrahydro-3-furanyl)methyl}-2-nitro-3-methylguanidine (dinotefuran), an aldulticide that will kill adult fleas combined with pyriproxyfen.
Owner:CEVA ANIMAL HEALTH
Topical insecticide
A topical insecticide is provided which can be safe to use and avoids many common deleterious side effects of conventional topical insecticides. In one preferred embodiment of the invention, the active ingredient of the insecticide formulation is an amine derivative, having a nitro-methylene group, a nitroamino group or a cyanoamino group, which can be formulated to have low toxicity and excellent insecticidal activity. One particularly suitable insecticide is 1-{(tetrahydro-3-furanyl)methyl}-2-nitro-3-methylguanidine (dinotefuran), an aldulticide that will kill adult fleas, dissolved in a solvent such as ethanol and / or DPM.
Owner:CEVA ANIMAL HEALTH
High concentration dinotefuran formulations containing methoprene
InactiveUS6889632B2Reduce the amount requiredReduce efficacyBiocideDead animal preservationMethopreneHigh concentration
Owner:CEVA ANIMAL HEALTH
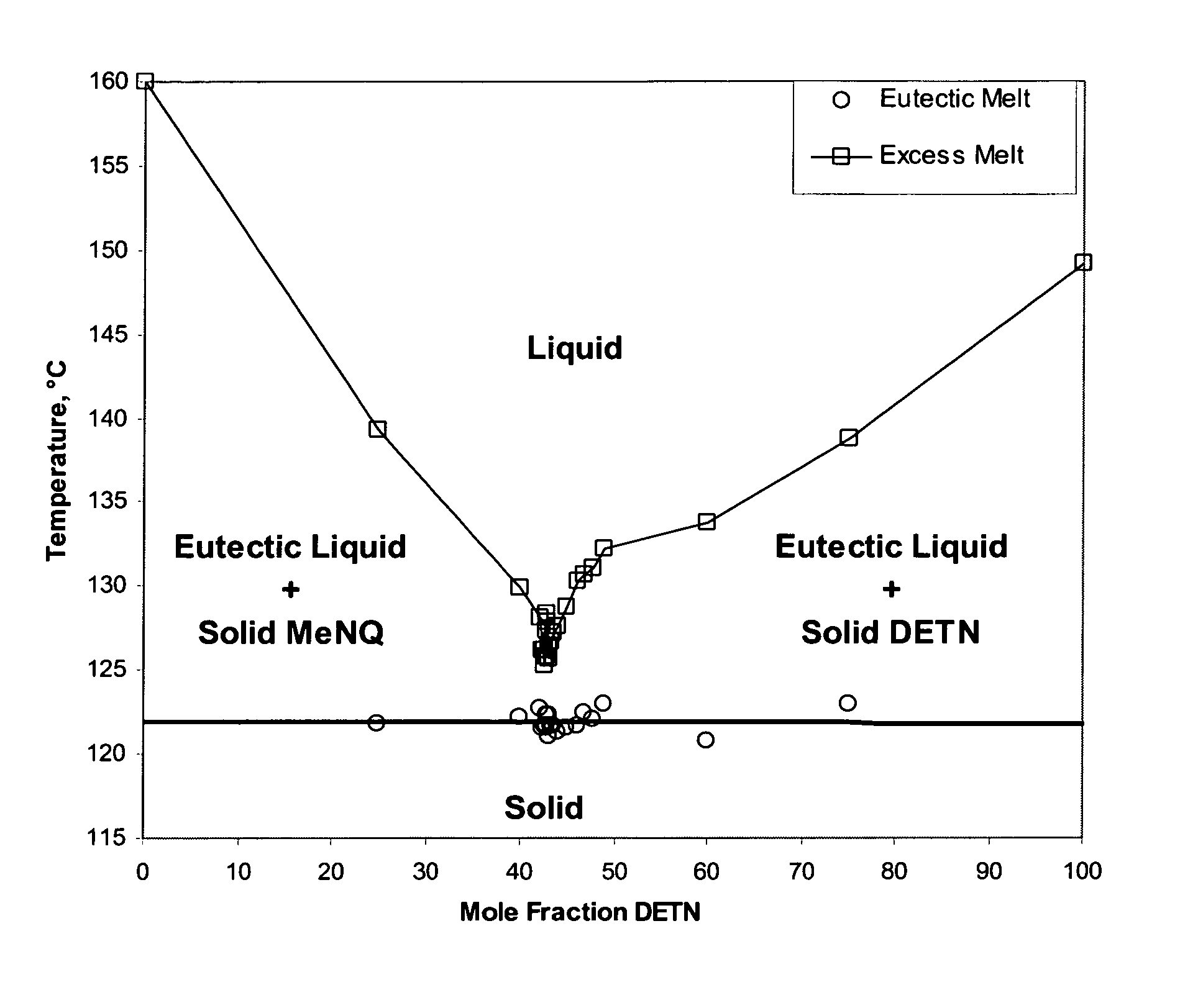
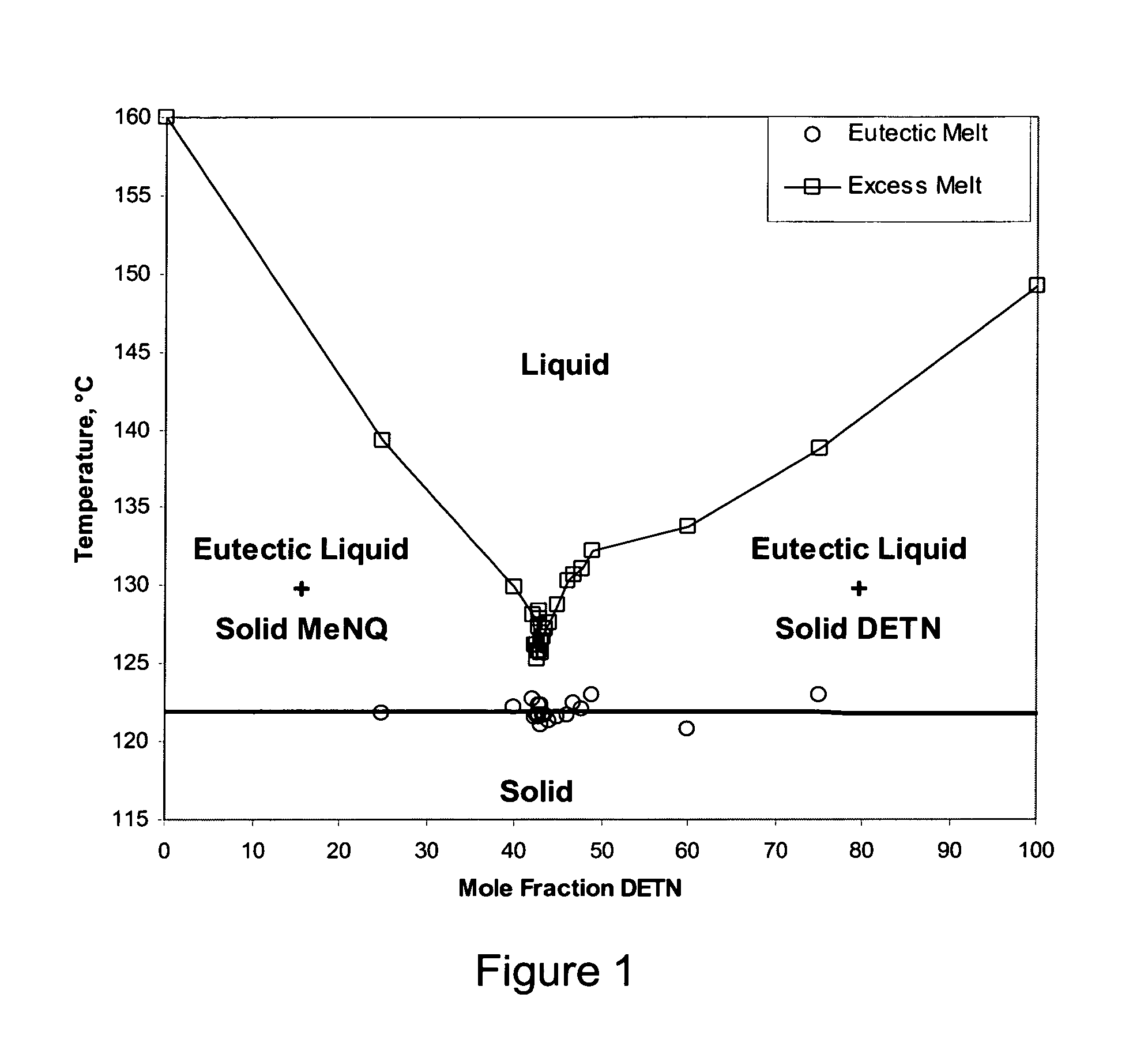
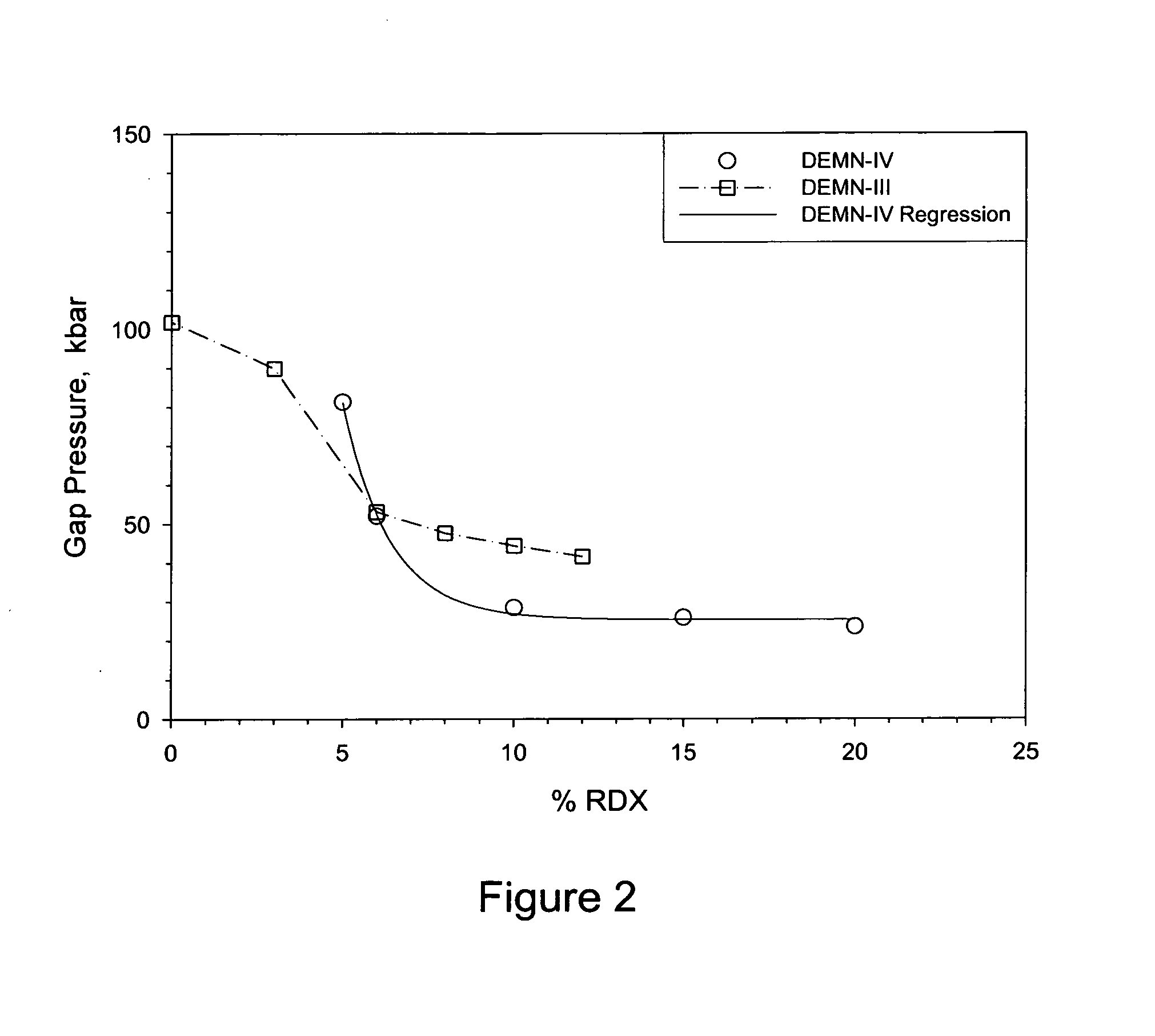
Melt cast insensitive eutectic explosive
InactiveUS8663406B1Nitrated acyclic/alicyclic/heterocyclic amine explosive compositionsExplosive mixture shapingDiethylenetriamineMelting temperature
A insensitive explosive comprises:34.9 wt % diethylenetriamine trinitrate (DETN),33.4 wt % ethylenediamine dinitrate (EDD),25.4 wt % methyl-guanidine (MeNQ), and6.3 wt % guanidine (NQ).This quaternary eutectic is used in combination with a sensitive explosive. A low melting temperature facilitates melt casting to fill 155 mm artillery shells.
Owner:GORVERNMENT OF THE UNITED STATES
High concentration dinotefuran formulations
InactiveUS20070254951A1Amount of liquid can be minimizedQuantity minimizationBiocideDead animal preservationHigh concentrationSide effect
A topical insecticide is provided which can be safe to use and avoids many common deleterious side effects of conventional topical insecticides. In one preferred embodiment of the invention, the active ingredient of the insecticide formulation is an amine derivative, having a nitro-methylene group, a nitroamino group or a cyanoamino group, which can be formulated to have low toxicity and excellent insecticidal activity. One particularly suitable insecticide is 1-{(tetrahydro-3-furanyl)methyl}-2-nitro-3-methylguanidine (dinotefuran), an aldulticide that will kill adult fleas dissolved in ethyl lactate.
Owner:SUMMIT VETPHARM
High concentration dinotefuran formulations containing methoprene
A topical insecticide is provided which can be safe to use and avoids many common deleterious side effects of conventional topical insecticides. In one preferred embodiment of the invention, the active ingredient of the insecticide formulation is an amine derivative, having a nitro-methylene group, a nitroamino group or a cyanoamino group, which can be formulated to have low toxicity and excellent insecticidal activity. One particularly suitable insecticide is 1-{(tetrahydro-3-furanyl)methyl}-2-nitro-3-methylguanidine (dinotefuran), an aldulticide that will kill adult fleas combined with methoprene.
Owner:CEVA ANIMAL HEALTH
High concentration topical insecticide containing pyriproxyfen
A topical insecticide is provided which can be safe to use and avoids many common deleterious side effects of conventional topical insecticides. In one preferred embodiment of the invention, the active ingredient of the insecticide formulation is an amine derivative, having a nitro-methylene group, a nitroamino group or a cyanoamino group, which can be formulated to have low toxicity and excellent insecticidal activity. One particularly suitable insecticide is 1-{(tetrahydro-3-furanyl)methyl}-2-nitro-3-methylguanidine (dinotefuran), an aldulticide that will kill adult fleas combined with pyriproxyfen.
Owner:CEVA ANIMAL HEALTH
Preparation method of rosuvastatin
ActiveCN102311457AReduce pollutionFew reaction stepsGroup 5/15 element organic compoundsAcyl groupPhenyl group
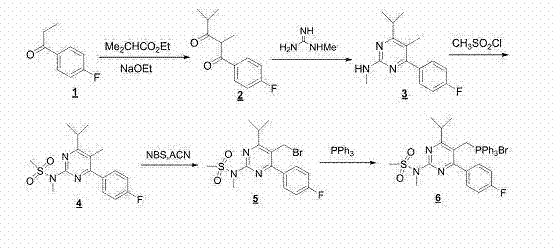
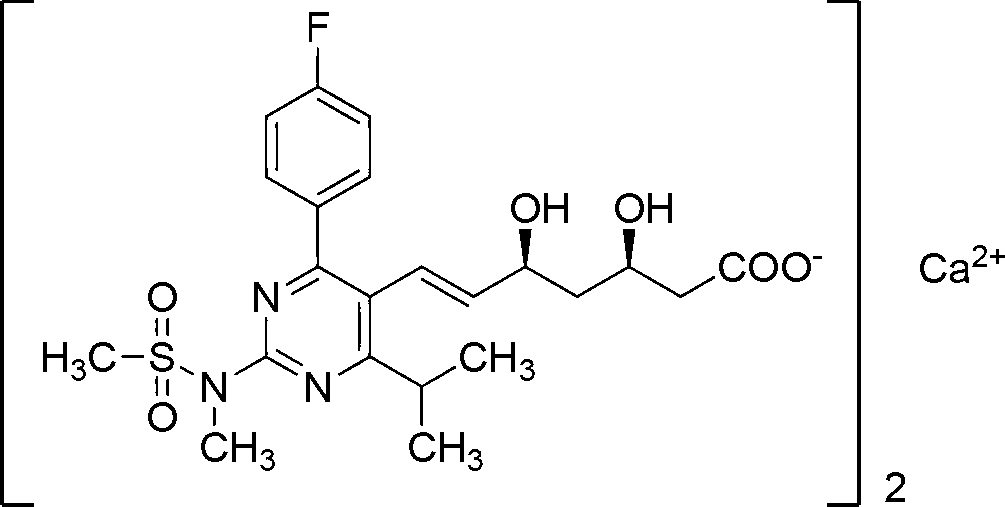
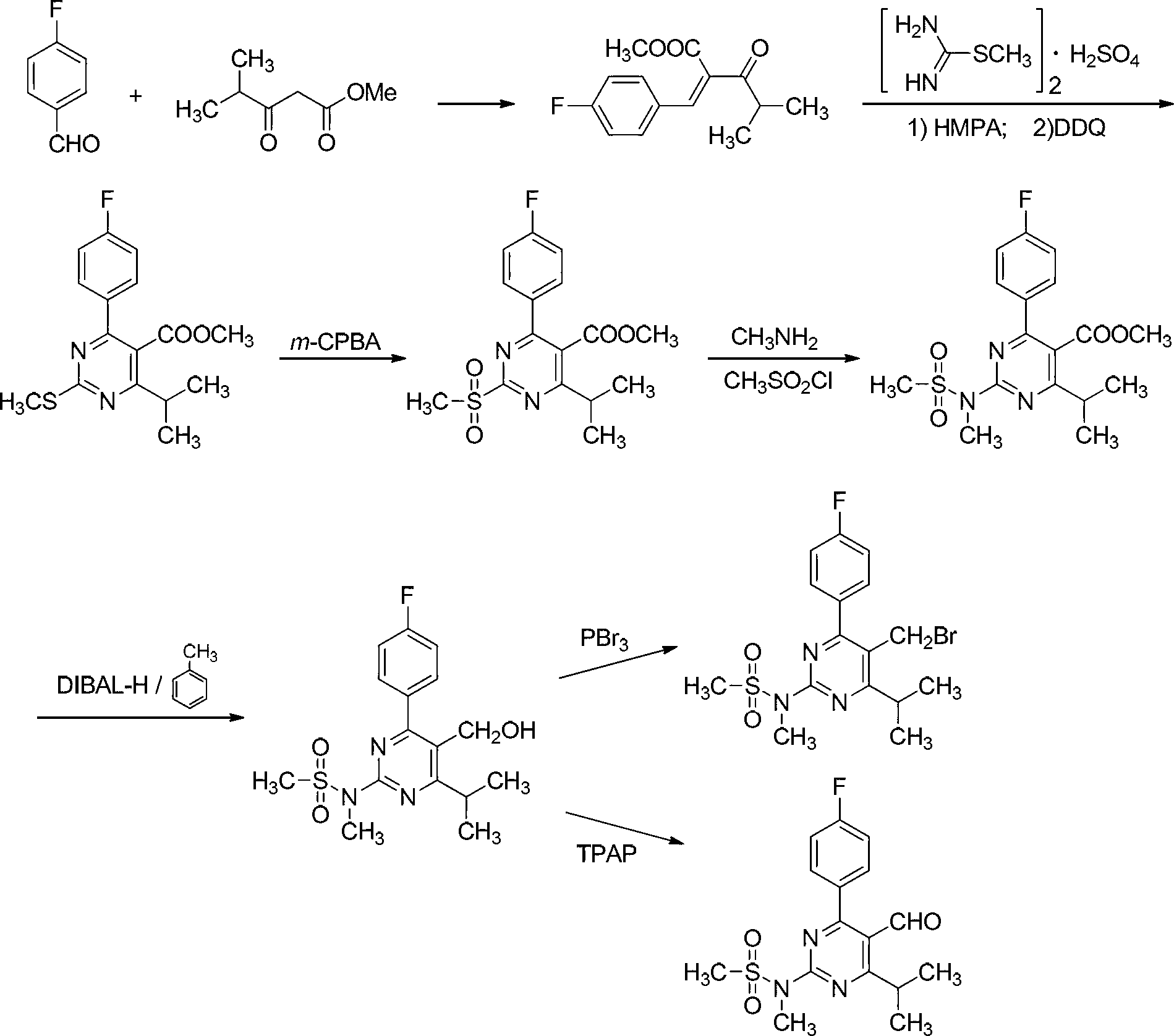
The invention discloses a preparation method of rosuvastatin, comprising the following steps of: using 4-fluoropropiophenone as a raw material, followed by a condensation reaction with ethyl isobutyrate to obtain 1-(4-fluorophenyl)-2,4-dimethylpentane-1,3-dione, performing a cyclization reaction with 1-methylguanidine to obtain 4-(4-fluorophenyl)-6-isopropyl-N,5-dimethyl pyrimidine-2-amine, followed by mesyl substituent and bromination to obtain N-(5-(bromomethyl)-4-(4-fluorophenyl)-6-isopropyl pyrimidine-2-yl)-N-methyl methanesulfonamide, and reacting with triphenyl phosphine to form a wittig reagent so as to prepare rosuvastatin provided by the invention. By the adoption of the preparation method provided by the invention, reaction steps are shortened from nine step reactions in original technology to fine step reactions; in addition, two oxidation reactions and an ultralow temperature reduction reaction are avoided, the production efficiency is effectively increased, the product quality is raised, the environmental pollution is minimized, and the production cost is reduced.
Owner:苏州莱克施德药业有限公司
Topical insecticide
A topical insecticide is provided which can be safe to use and avoids many common deleterious side effects of conventional topical insecticides. In one preferred embodiment of the invention, the active ingredient of the insecticide formulation is an amine derivative, having a nitro-methylene group, a nitroamino group or a cyanoamino group, which can be formulated to have low toxicity and excellent insecticidal activity. One particularly suitable insecticide is 1-{(tetrahydro-3-furanyl)methyl}-2-nitro-3-methylguanidine(dinotefuran), an aldulticide that will kill adult fleas, dissolved in a solvent such as ethanol and / or DPM.
Owner:CEVA ANIMAL HEALTH
Ceftriaxone sodium tetrahydrate compound
The invention discloses a ceftriaxone sodium tetrahydrate compound and a preparation method thereof. Each mole of ceftriaxone sodium contains four mole of water. According to the compound, sulfochlorides and dimethylformamide are adopted to be reacted to generate an activator, the activator is directly reacted with 7-aminoceftriazine tetramethylguanidine salt to obtain the ceftriaxone sodium tetrahydrate compound. The operation is simple, the obtaining of a reactant is easy, the reaction condition is milder, and the yield is high. The ceftriaxone sodium tetrahydrate compound has the advantagesof low hygroscopicity, low impurity content, good fluidity, good thermodynamic stability and more extensive application prospects.
Owner:陕西顿斯制药有限公司 +2
High concentration dinotefuran formulations
InactiveUS20080038214A1Amount of liquid can be minimizedQuantity minimizationBiocideCosmetic preparationsHigh concentrationSide effect
A topical insecticide is provided which can be safe to use and avoids many common deleterious side effects of conventional topical insecticides. In one preferred embodiment of the invention, the active ingredient of the insecticide formulation is an amine derivative, having a nitro-methylene group, a nitroamino group or a cyanoamino group, which can be formulated to have low toxicity and excellent insecticidal activity. One particularly suitable insecticide is 1-{(tetrahydro-3-furanyl)methyl}-2-nitro-3-methylguanidine (dinotefuran), an aldulticide that will kill adult fleas dissolved in ethyl lactate.
Owner:COTTRELL IAN W +3
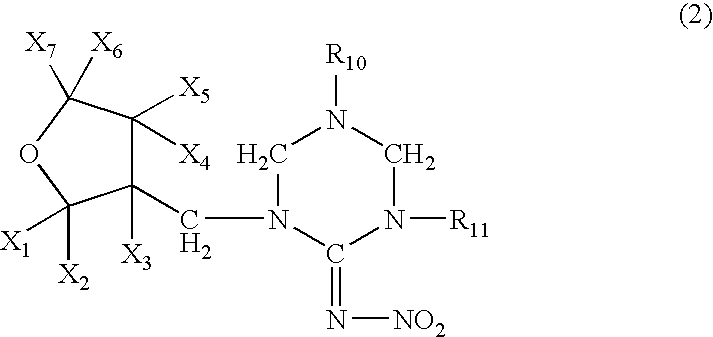
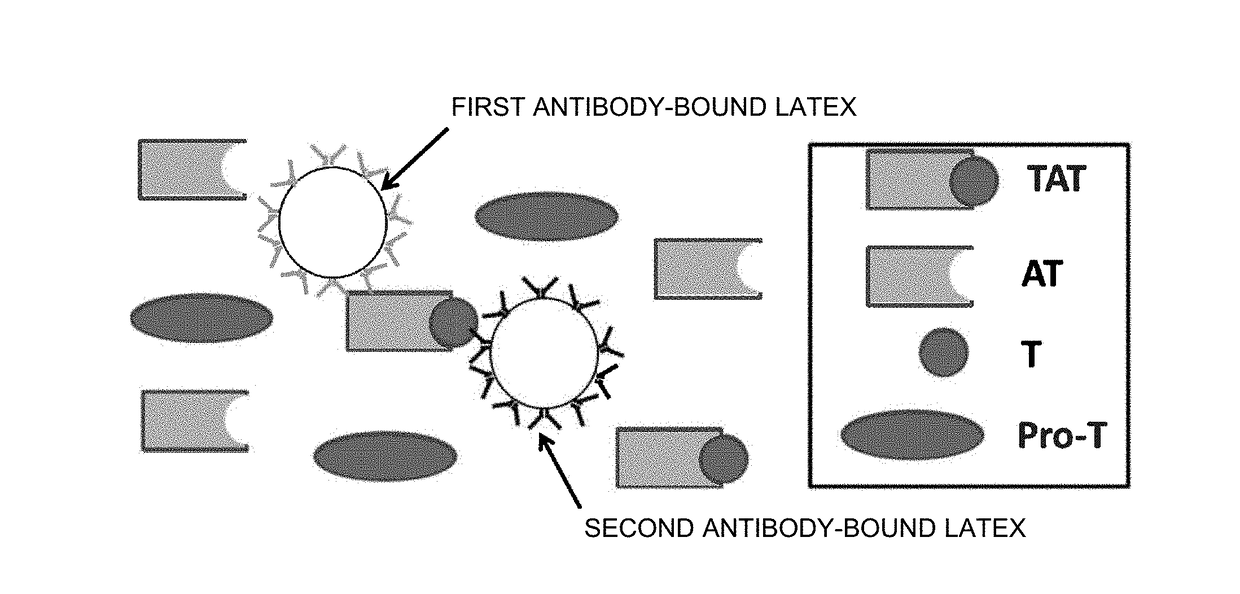
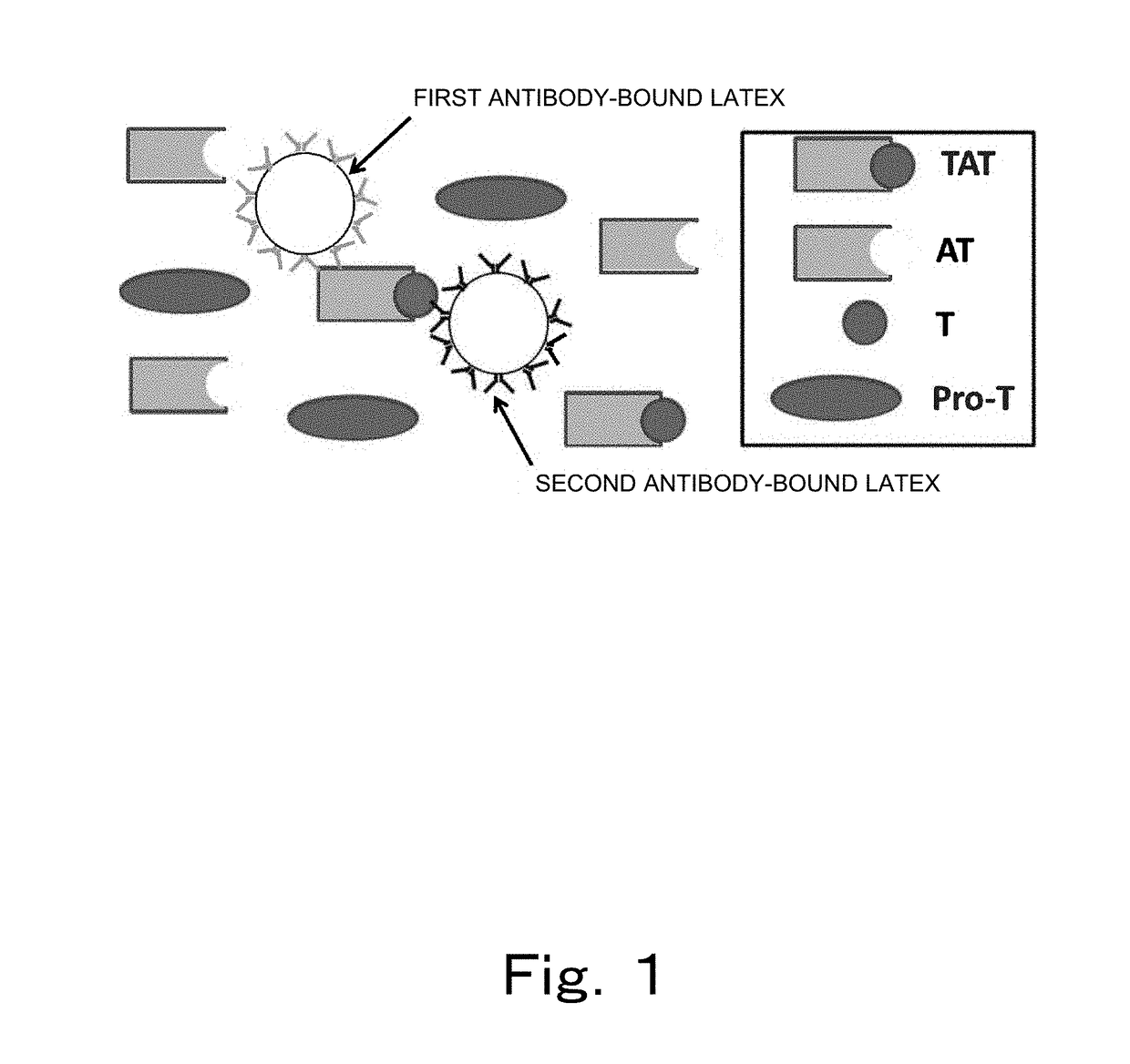
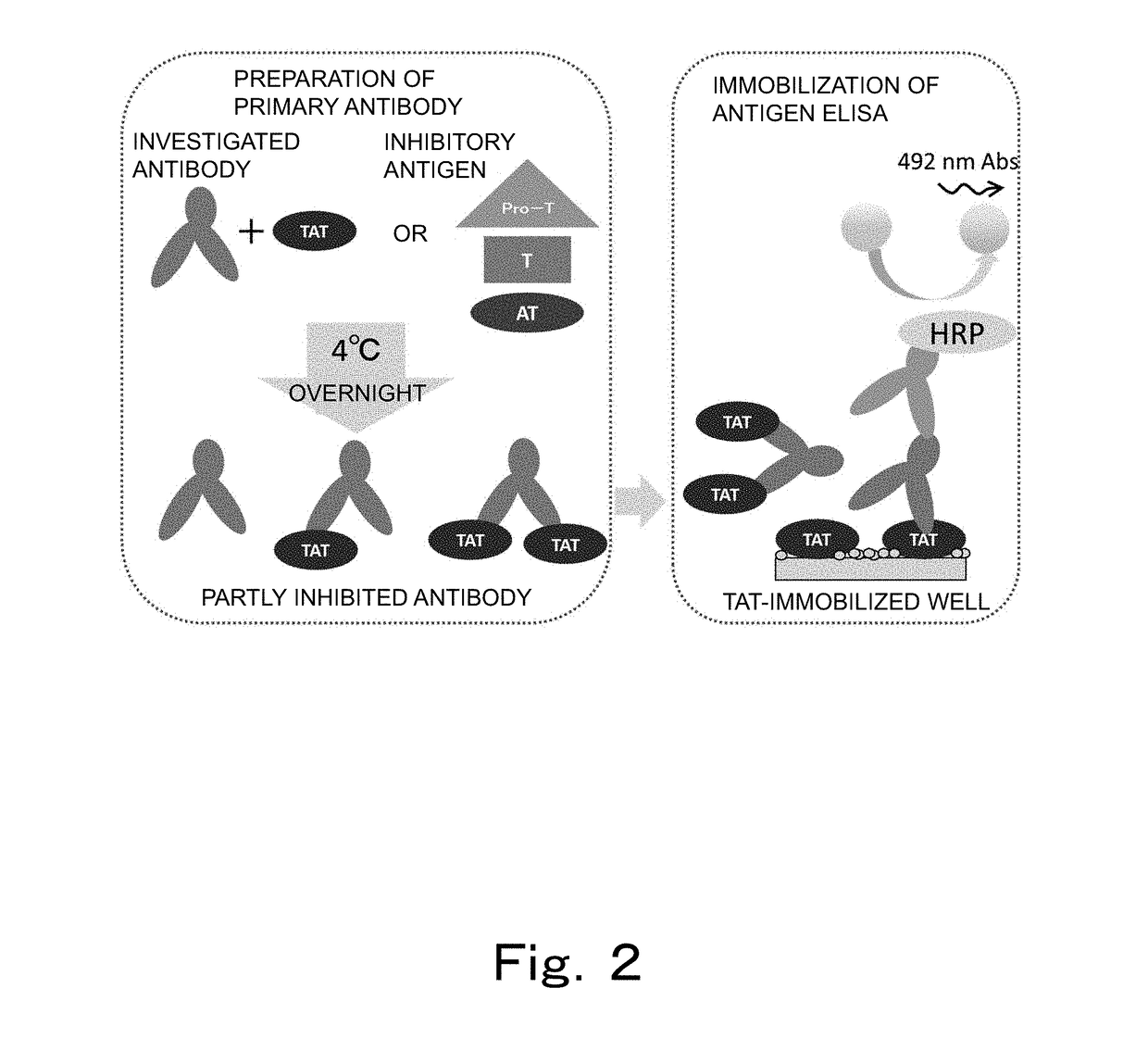
Reagent and method for measuring thrombin-antithrombin complex
ActiveUS20180238871A1Small amountAccurate measurementDisease diagnosisBiological testingDextranAmmonium chloride mixture
Owner:MITSUBISHI CHEM MEDIENCE
Synthetic method of kreatine
InactiveCN102584636AShort reaction timeSimple operation processOrganic chemistryOrganic compound preparationMethylguanidineEthanol
The invention discloses a synthetic method of kreatine. The synthetic method of kreatine comprises the following steps: adding water in a stainless steel reaction kettle with a stirring, heating and refluxing device, then adding N-methylguanidine ethanol, adding manganese dioxide in stirring, next heating to be in-kettle temperature and reacting continuously, finishing the reaction, filtering immediately to obtain a kreatine solution, cooling to be 20 DEG C, filtering, and drying to obtain kreatine. Through the way, the invention can shorten the synthetic time, and the operation in the reaction process is simple.
Owner:JIANGSU YUANYANG PHARMA
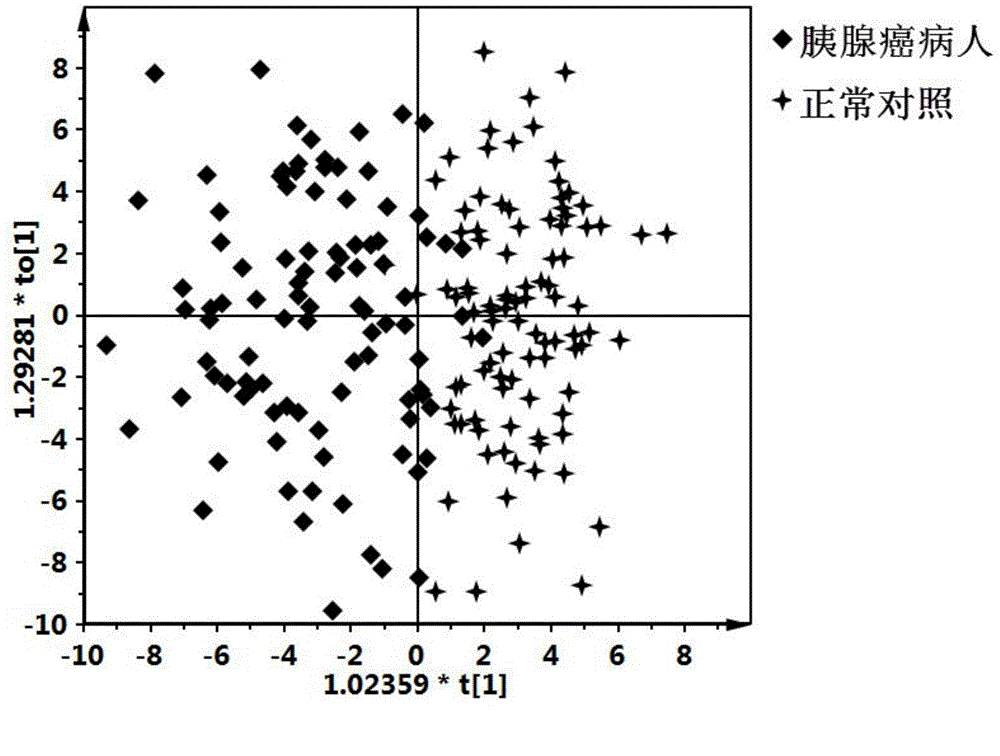
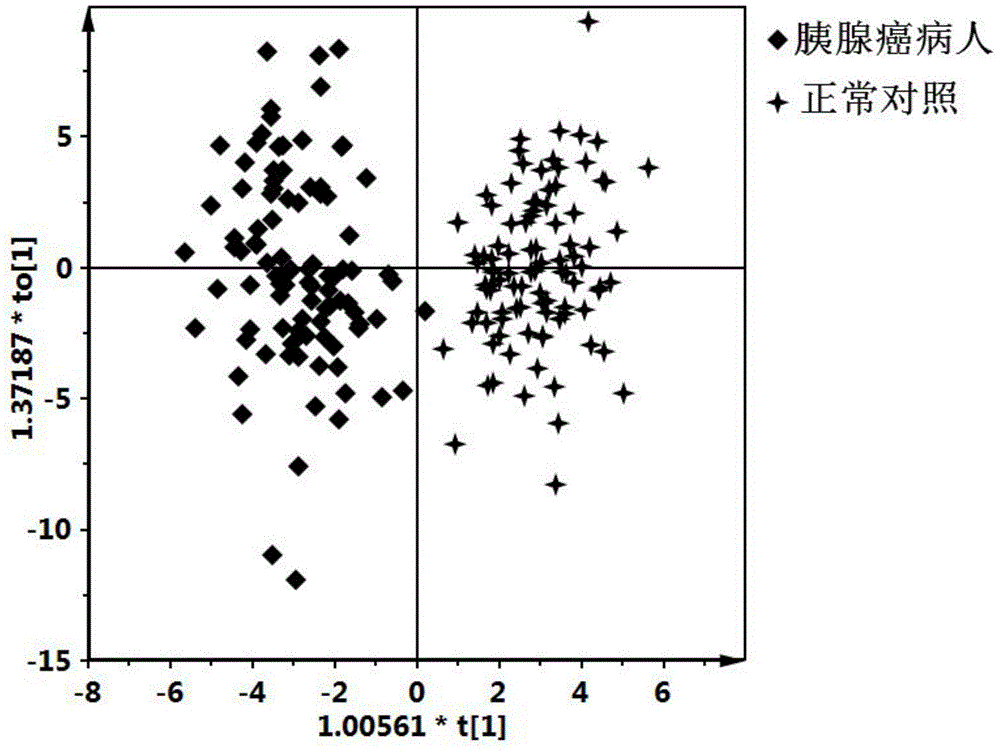
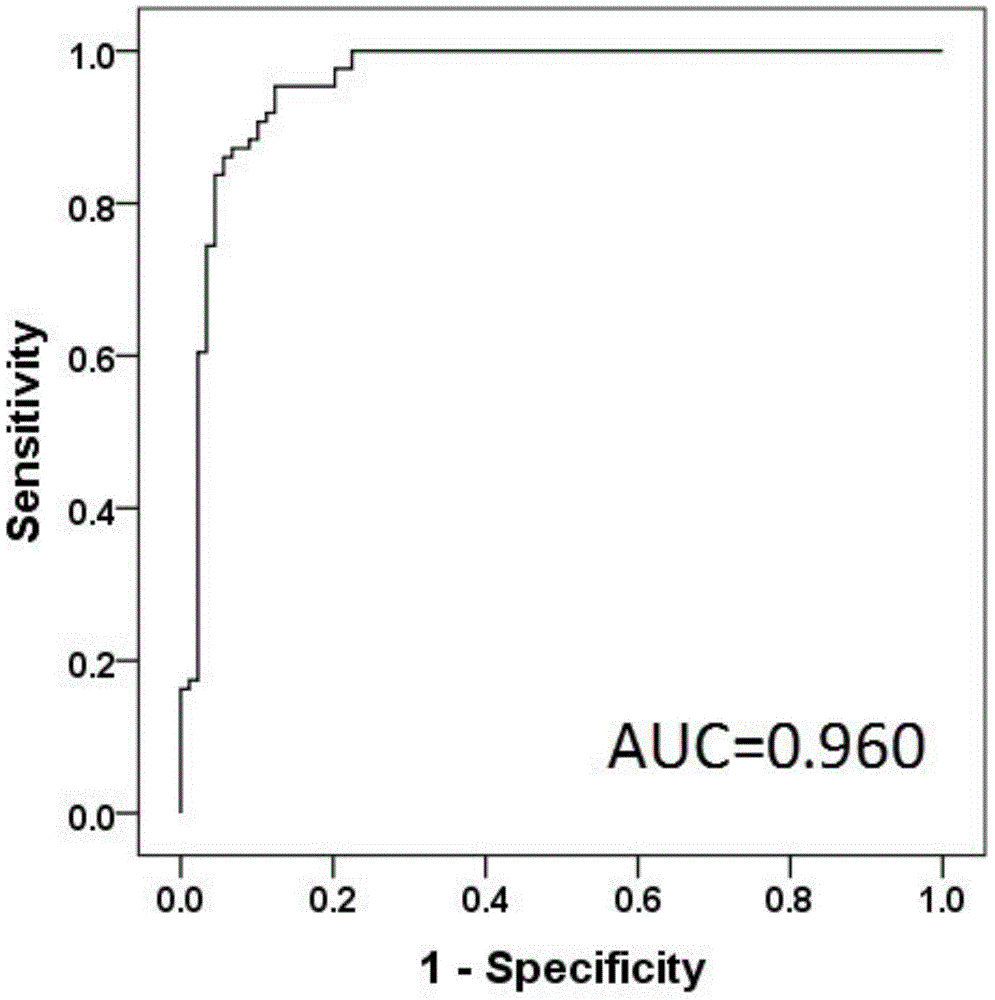
Pancreatic cancer diagnostic marker combination as well as application and determination method thereof
ActiveCN105572276AImprove clinical treatment effectGood clinical effectComponent separationBetaineMetabolite
The invention discloses a pancreatic cancer diagnostic marker combination as well as application and a determination method thereof. The pancreatic cancer diagnostic marker combination comprises 15 differentiated metabolites (2,5-dihydroxybenzoic acid, talopyranose, proline, glutamate, choline, 1,5-anhydro-D-glucitol, tryptophan, glutamine, betaine, 2-oxoglutaric acid, methylguanidine, adenine, glycocholic acid, valine and 2-aminobutyric acid). The invention further provides a combination of the 15 differentiated metabolites to serve as a marker for early discovery and diagnosis of pancreatic cancer, application and a diagnostic marker determination method, and the method is a liquid-phase / gas-phase chromatography-mass spectrometry combined metabonomics analysis method based on plasma / serum of patients with pancreatic cancer. The pancreatic cancer diagnostic marker combination provided by the technical scheme of the invention has the characteristics of being high in sensitiveness and specificity, has relatively high sensitiveness and specificity on early pancreatic cancer diagnosis, can be used for early discovery of pancreatic cancer, gains time for the patients to receive treatment as soon as possible, and improves the clinical treatment effect.
Owner:麦特绘谱生物科技(上海)有限公司
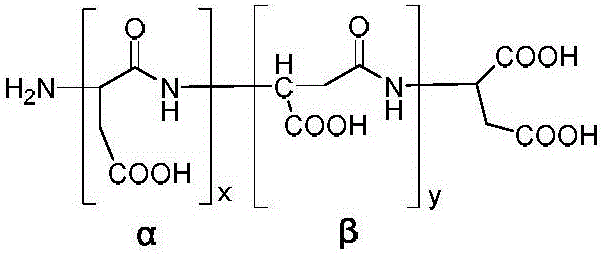
Polyaspartic acid for water treatment scale inhibitor, as well as preparation method and application of polyaspartic acid
ActiveCN106007015AGood anti-scaling effectFast biodegradabilitySpecific water treatment objectivesScale removal and water softeningAcetic acidPolyaspartic acid
The invention discloses polyaspartic acid for a water treatment scale inhibitor. The structural formula of the polyaspartic acid is shown in formula I of the description, where x / (x+y) is more than or equal to 0.50 and less than or equal to 0.8. The preparation method for the polyaspartic acid comprises the following steps: performing contact reaction on polysuccinimide and a mixed base in the presence of triethanolamine and zinc acetate, and regulating the pH of a solution to 6 to 7 by virtue of hydrochloric acid, wherein the mixed base is prepared from potassium hydroxide and tetramethyl guanidine of which the mass ratio is (1 to 3):1. The polyaspartic acid is higher in scale inhibition performance and biological degradation performance and more environmentally-friendly.
Owner:山东玉泉食品有限公司
High concentration dinotefuran formulations
InactiveUS20050209318A1Amount of liquid can be minimizedQuantity minimizationBiocideDead animal preservationHigh concentrationSide effect
A topical insecticide is provided which can be safe to use and avoids many common deleterious side effects of conventional topical insecticides. In one preferred embodiment of the invention, the active ingredient of the insecticide formulation is an amine derivative, having a nitro-methylene group, a nitroamino group or a cyanoamino group, which can be formulated to have low toxicity and excellent insecticidal activity. One particularly suitable insecticide is 1-{(tetrahydro-3-furanyl)methyl}-2-nitro-3-methylguanidine (dinotefuran), an aldulticide that will kill adult fleas dissolved in ethyl lactate.
Owner:CEVA ANIMAL HEALTH
Pesticidal aerosol compositions and pesticidal methods
The present invention provides pesticidal aerosol compositions and pesticidal methods. The pesticidal aerosol compositions comprise a propellant, 1-methyl-2-nitro-3-[(3-tetrahydrofuryl)methyl]guanidine and N-methyl-2-pyrrolidone, wherein a weight to weight ratio of said 1-methyl-2-nitro-3-[(3-tetrahydrofuryl)methyl]guanidine to said N-methyl-2-pyrrolidone is at a pesticidally synergistic weight to weight ratio. The pesticidal methods comprise applying to a pest or to a habitat of a pest, at least one the pesticidal aerosol compositions.
Owner:SUMITOMO CHEM CO LTD
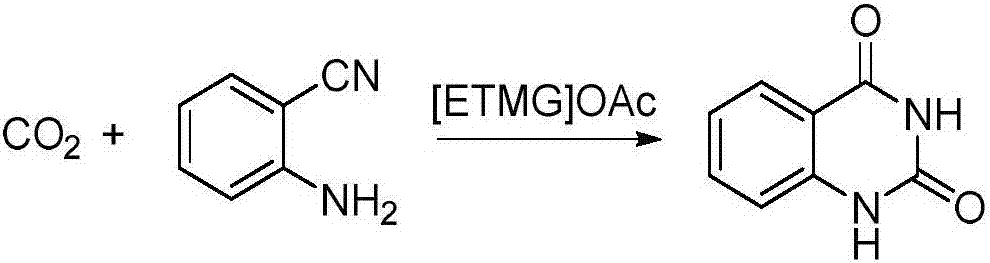
5-alkyl guanidine ionic liquid, and preparation and application thereof
InactiveCN106883151AHigh catalytic activityThe synthesis process is simpleOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIon exchangeSolvent
The invention discloses 5-alkyl guanidine ionic liquid, and preparation and application thereof. After tetramethyl guanidine and alkyl halide are adopted to be ionized, ion exchange with metal salt is performed to prepare 5-alkyl guanidine acid radical ionic liquid, and the ionic liquid is used as a catalyst to synthesize quinazoline-2,4(1H,3H)-ketone and derivatives thereof in the carboxylation and cyclization reaction of carbon dioxide and anthranilonitrile. Compared with the prior art, the 5-alkyl guanidine ionic liquid is simple in synthetic process, low in costs and has excellent performance in a homogeneous catalysis carbon dioxide reaction process; particularly, when the 5-alkyl guanidine ionic liquid is used in the carboxylation and cyclization reaction synthesis of the carbon dioxide and the anthranilonitrile, quinazoline-2,4(1H,3H)-diketone and derivatives thereof can be obtained in a relatively mild condition; the catalytic performance is good; the reaction condition is mild; and the postprocessing is easy. Meanwhile, the 5-alkyl guanidine ionic liquid can be used as a solvent and a catalyst; the use of a large quantity of organic solvent is avoided; and the 5-alkyl guanidine ionic liquid has important significance on researching medicinal chemistry and compounds of medical intermediates.
Owner:EAST CHINA NORMAL UNIVERSITY
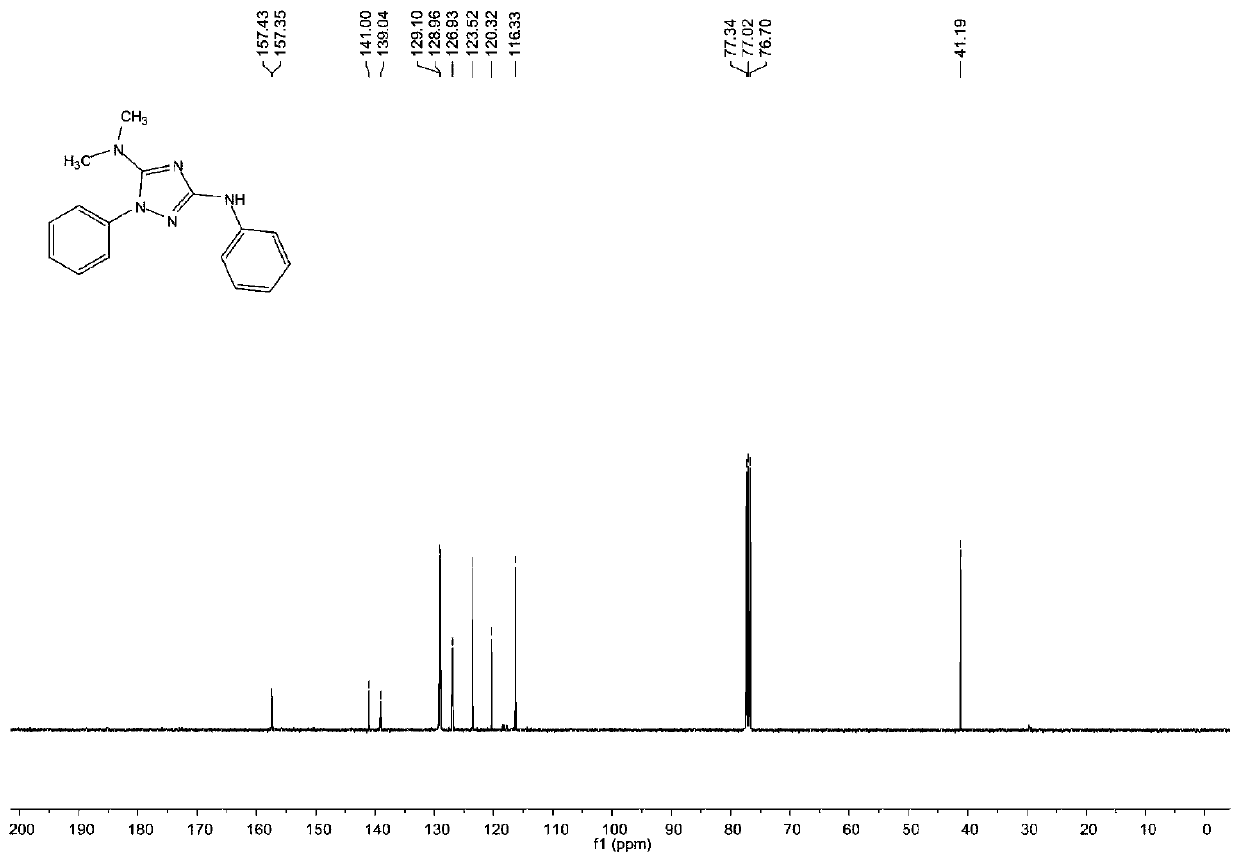
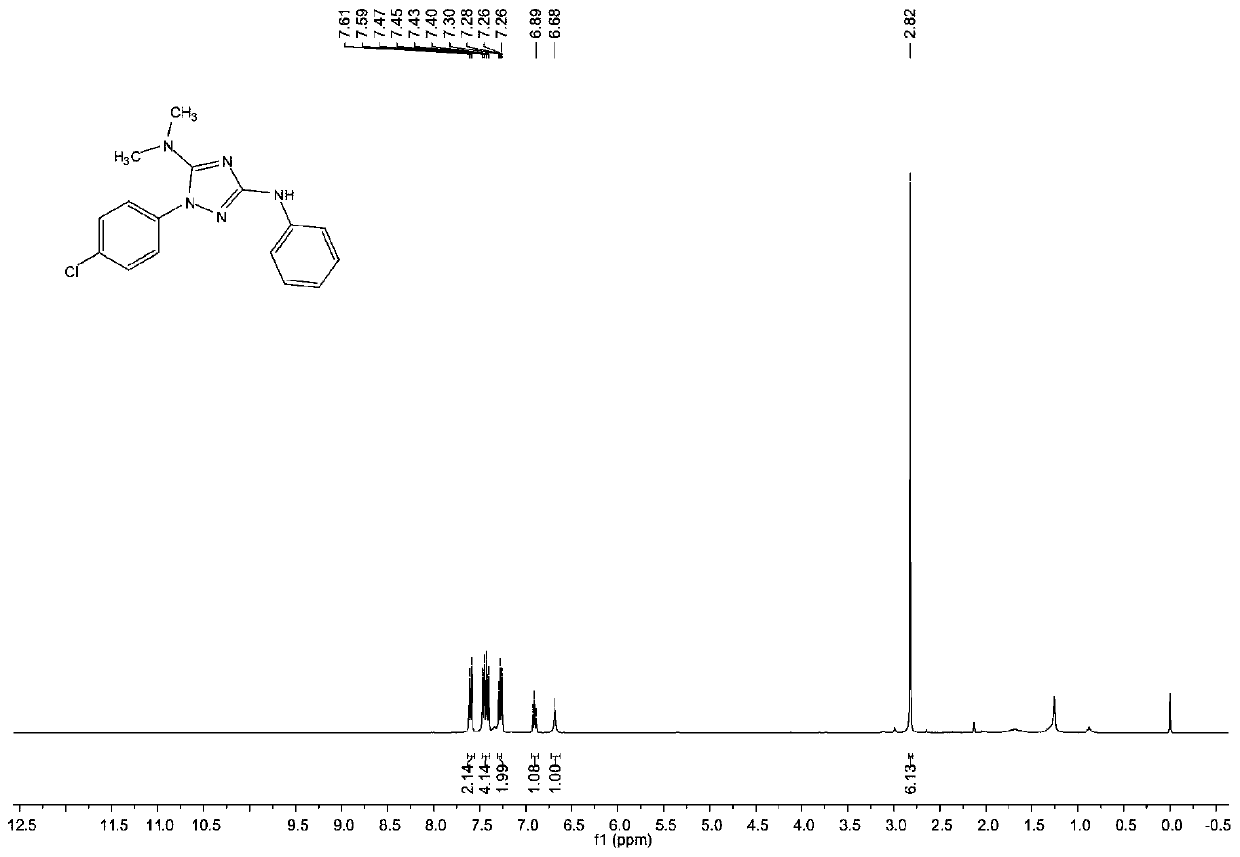
1, 2, 4-triazole compound and preparation method thereof
The invention provides a 1, 2, 4-triazole compound and a preparation method thereof, and relates to the technical field of organic intermediates. The 1, 2, 4-triazole compound provided by the invention has a structure as shown in a formula I, is a 1, 2, 4-triazole compound with a novel structure, expands the variety of the 1, 2, 4-triazole compound, and can be applied to pharmaceutical chemistry.The invention also provides a preparation method of the 1, 2, 4-triazole compound, which comprises the following steps: mixing a hydrazine compound, an isothiocyanate compound, tetramethylguanidine, aphotocatalyst and a polar organic solvent, and carrying out cyclization reaction on an obtained mixed solution under the conditions of illumination and temperature of 50-80 DEG C to obtain the 1, 2,4-triazole compound. The 1, 2, 4-triazole compound can be obtained at the temperature of 50-80 DEG C by providing energy required by reaction through illumination, the reaction process is safe and stable, conditions are mild, operation is convenient, and control is easy.
Owner:GANNAN NORMAL UNIV
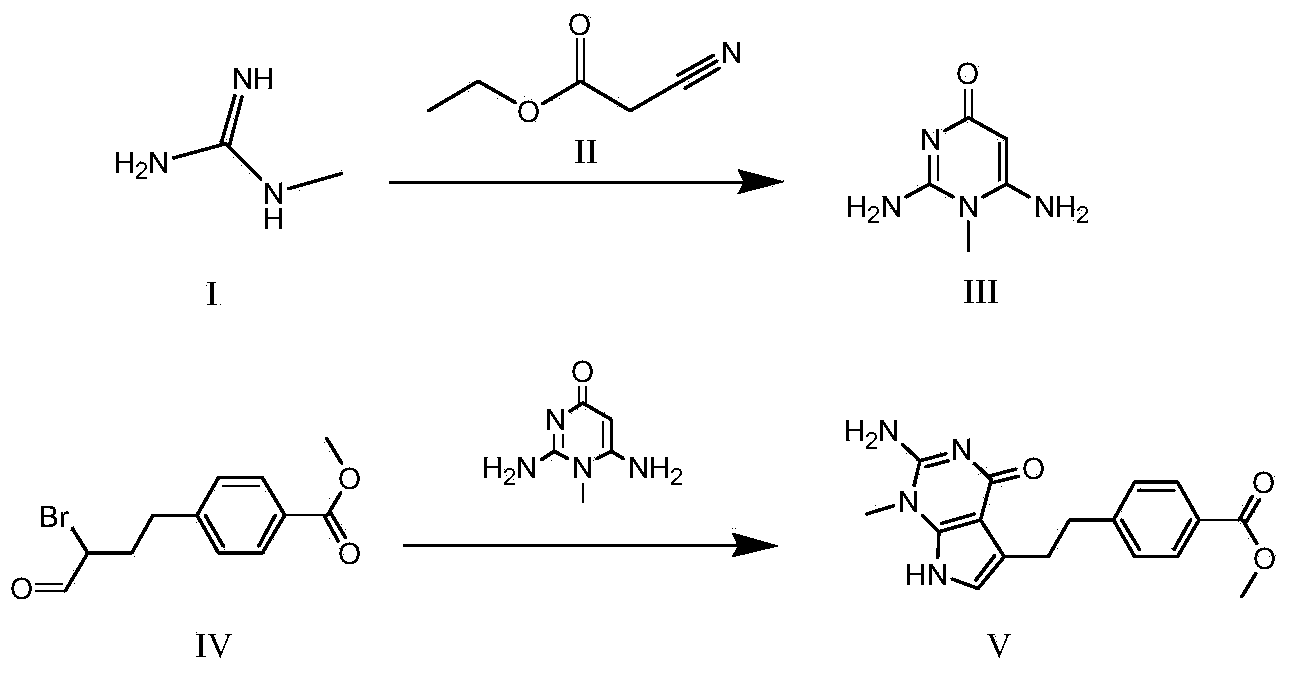
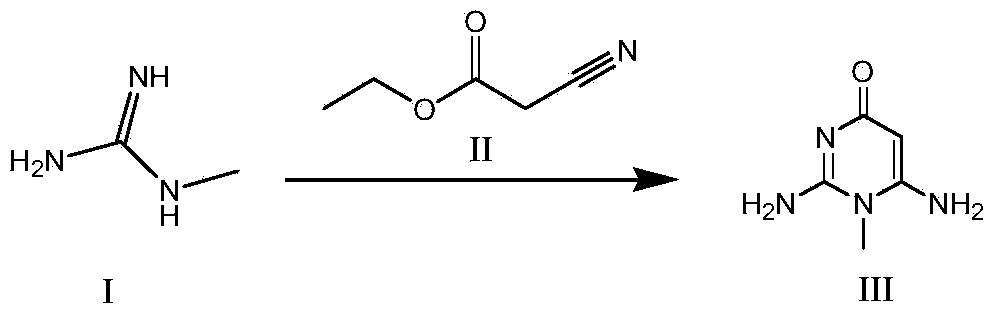
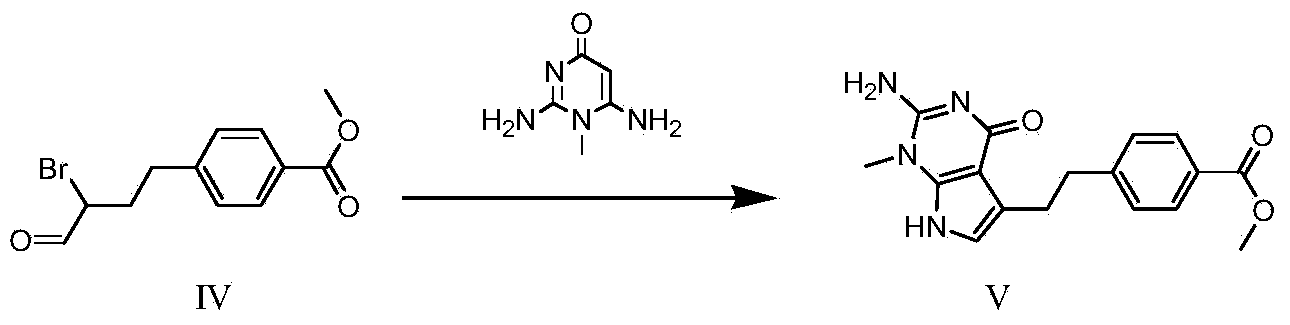
Synthesis method for intermediate of impurity A of pemetrexed disodium
The invention discloses a synthesis method for an intermediate of an impurity A of pemetrexed disodium and belongs to the field of drug synthesis. (4-(2-(2-amino-1-methyl-4,7-dihydro-4-oxo-1H-pyrrolo(2,3-d)pyrimidine-5-yl)ethyl)benzoyl)-L-glutamic acid is an impurity A of pemetrexed disodium, and 4-(2-(2-amino-1-methyl-4,7-dihydro-4-oxo-1H-pyrrolo(2,3-d)pyrimidine-5-yl)ethyl)methyl benzoate is an important intermediate required for synthesis of the impurity A. The synthesis method of the intermediate comprises the following steps: carrying out a reaction on a compound I 1-methylguanidine and a compound II ethyl cyanoacetate to obtain a compound III 2,6-diamino-4-carbonyl-1-methylpyrimidine, and carrying out a reaction on a compound IV 4-(4-carbonyl-3 brombutyl)methyl benzoate and the compound III to obtain a compound V 4-(2-(2-amino-1-methyl-4,7-dihydro-4-oxo-1H-pyrrolo(2,3-d)pyrimidine-5-yl)ethyl)methyl benzoate.
Owner:SHANDONG BOYUAN PHARM CO LTD
Activating agent of gas oil sweetening catalyst
InactiveCN102614924AImprove conversion rateGood miscibilityOrganic-compounds/hydrides/coordination-complexes catalystsRefining with oxygen compoundsAlkali freeGasoline
The invention relates to an activating agent of a gas oil sweetening catalyst, which belongs to the field of chemical engineering, aims at overcoming the shortcomings of the gas oil alkali-free fixed bed sweetening process and is high in efficiency. The activating agent is composed of the following raw materials by weight: 95% ethanol, sodium hydroxide, chlorinated octadecyl dimethyl benzyl ammonium, tetramethylguanidine and N-methyldiethanolamine. The activating agent has the advantages of being good in intersolubility with gas oil and high in mercaptan conversion rate, having good washing effects on colloid and aromatic hydrocarbon substances which are adsorbed on the surface of a bed catalyst, being capable of maintain high activity state accordingly, prolonging service, having no bad effects on performance of oil if retained in the oil, being an environment-friendly product for cleaning and being simple in manufacture process, less in dosage of chlorinated octadecyl dimethyl benzyl ammonium in raw material components and low in preparation cost.
Owner:长春惠鹏石油化工科技有限公司
Preparation method and application of N-bis (dimethylamino)-1, 3-dimethylimidazoline
InactiveCN110845414AHigh activityLow reaction temperatureOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsCatalytic effectMethyl palmoxirate
The invention relates to the field of preparation methods and application of compounds, in particular to a preparation method and application of N-bis (dimethylamino)-1, 3-dimethylimidazoline, whereinthe chemical molecular formula is C9H23N5. The preparation method comprises the following steps: S1, taking 1, 3-dimethyl-2-imidazoline and bis (trichloromethyl) carbonate as raw materials, and carrying out chlorination reaction to synthesize chlorinated 1, 3-dimethyl-2-chloroimidazoline; therefore, the problems of safety and environmental protection of other chlorinated reagents are solved, andthe product yield (87% or above) is greatly improved. S2, dropwise adding tetramethylguanidine into the generated chlorine salt, and carrying out condensation reaction; S3, carrying out neutralizationreaction on an obtained product to obtain N-bis (dimethylamino) methylene-1, 3-dimethyl-2-chloroimidazoline ammonium chloride salt. The process is high in operability, mild in reaction condition, safe, green, environmentally friendly, good in product catalytic effect and wide in application range, and almost no other impurities are generated except that high-content potassium chloride is generated as a byproduct.
Owner:JINING KANGSHENG RAINBOW BIOTECHNOLOGY CO LTD
Adjuvant Compositions Comprising a Tetramethylguanidine and a 4-Isothiazolin-3-One
PendingUS20210235699A1High bactericidal activityLess pollutionBiocideAnimal repellantsAdjuvantMethyl palmoxirate
The present invention relates to biocidal compositions comprising an isothiazolone biocidal active compound in combination with a guanidine adjuvant. These compositions are highly effective in preventing deterioration and decay caused by microbial contamination in very low concentrations so that the environmental or toxicological burden is low.
Owner:ARXADA LLC
Preparation method of saturated urushiol-based functional coating
ActiveCN109575770ANo lacquer allergyImprove drynessPolyurea/polyurethane coatings4-methylmorpholineNitrogen
The invention provides a preparation method of saturated urushiol-based functional coating, which includes: dissolving saturated urushiol in a solvent, using tetramethyl guanidine or 1,8-diazabicycloundec-7-ene as a catalyst, adding a crosslinker to catalyze crosslinking reaction, adding a capping agent to allow capping reaction, and subjecting the saturated urushiol to matrix modification to obtain the saturated urushiol-based functional coating. With saturated urushiol used as a raw material, tetramethyl guanidine, 1,8-diazabicycloundec-7-ene or 4-methylmorpholine as a catalyst, isophorone diisocyanate or toluene 2,4-diisocyanate as a crosslinker, and acetamide or epsilon-caprolactam as a capping agent, the saturated urushiol-based functional coating is synthesized. The saturated urushiol-based functional coating is free of allergy of raw lacquer and good in dryness and has good construction convenience.
Owner:中华全国供销合作总社西安生漆涂料研究所
Alkyl-substituted ethyl acetate-based guanidine ionic liquid as well as preparation and application thereof
ActiveCN111393332AHigh catalytic activityThe synthesis process is simpleOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMethylanilineDimethylaniline N-oxide
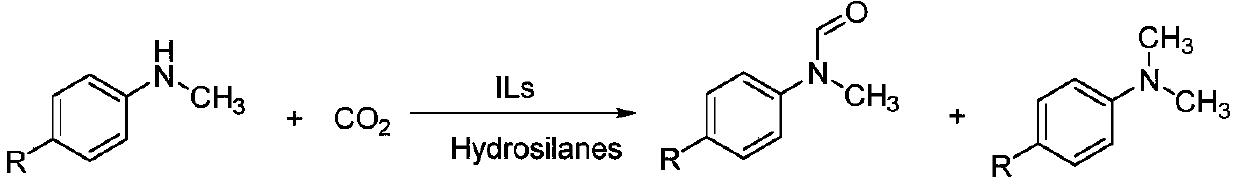
The invention discloses alkyl-substituted ethyl acetate-based guanidine ionic liquid as well as preparation and application thereof, which are characterized in that tetramethylguanidine and 2-bromo ester are ionized to obtain alkyl-substituted ethyl acetate-based guanidine ionic liquid, and the alkyl-substituted ethyl acetate-based guanidine ionic liquid is applied as a catalyst to formylation and methylation reactions of carbon dioxide, N-methylaniline and derivatives of the N-methylaniline to selectively generate N-methylformylaniline or N, N-dimethylaniline and derivatives thereof. Compared with the prior art, the alkyl-substituted ethyl acetate-based guanidine ionic liquid has the advantages of good catalytic performance, mild reaction conditions, simple post-treatment, simple synthesis, low cost, greenness and high efficiency, avoids the use of a large amount of organic solvents when being used as a solvent and a catalyst at the same time, and has important meanings in the research of medicinal chemistry and medical intermediate compounds.
Owner:EAST CHINA NORMAL UNIV
Process for synthesizing pregabalin
InactiveCN105130832AFew reaction stepsMild process conditionsOrganic compound preparationAmino-carboxyl compound preparationPtru catalystNitromethane
The invention discloses a process for synthesizing pregabalin. According to a synthesis route in the process, isovaleraldehyde and propane diacid are condensed for esterification; under the catalytic action of tetramethyl guanidine, a Michael addition is performed with nitromethane; a palladium-carbon catalyst of which the mass fraction is 10% is adopted in glacial acetic acid for reduction and hydrogenation; a raceme (+ / -)-1 is obtained by directly performing hydrolysis in a hydrochloric acid solution without separation, and can be split to obtain (S)-1. The process provided by the invention is available, a few of reaction steps are taken, process conditions are mild, and the total yield is greatly improved on the basis of the prior art.
Owner:CHENGDU AIBIKE BIOTECH
Method for preparing rosuvastatin calcium intermediate containing brooethyl, hydroxymethyl or formyl
InactiveCN102936225AMild reaction conditionsHigh yieldOrganic chemistryRosuvastatin CalciumHydroxymethyl
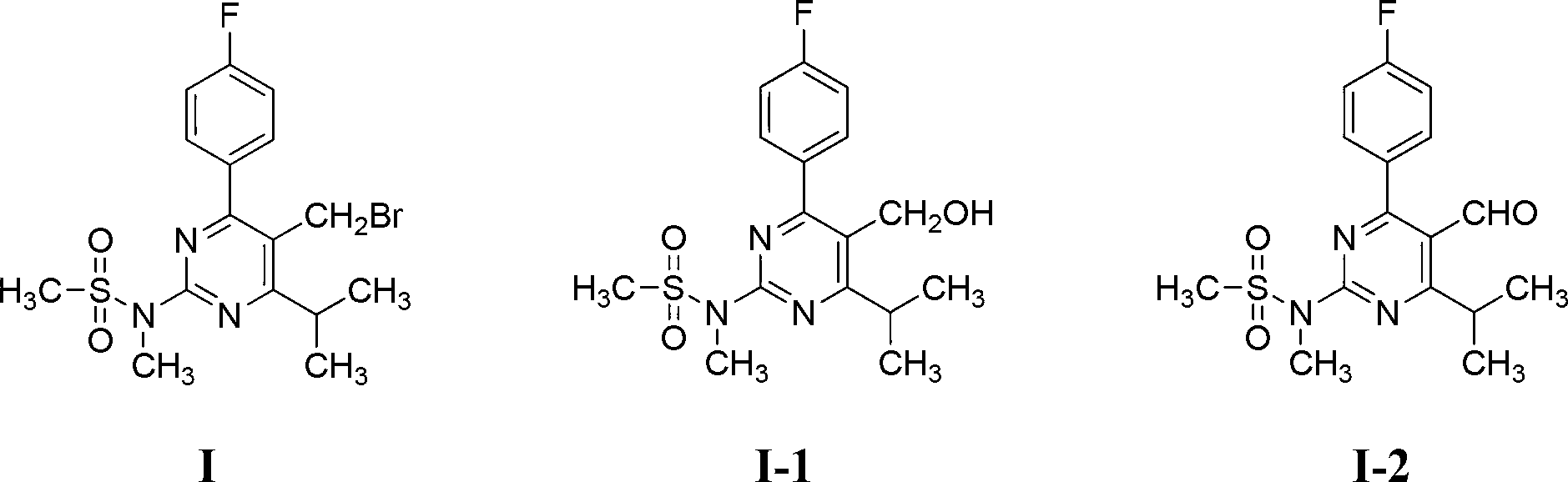
The invention discloses a method for preparing a rosuvastatin calcium intermediate containing brooethyl, hydroxymethyl or formyl. The method includes enabling fluoro-acetophenone to be reacted with ethyl iso-butyrate to prepare a compound V, enabling the compound V to be reacted with methyl iodide to synthesize a compound as shown in a formula IV, then enabling the compound as shown in the formula IV to be reacted with methylguanidine hydrochloride and cesium carbonate to obtain a compound as shown in a formula III, enabling the compound as shown in the formula III to be reacted with triethylamine and methylsulfonyl chloride to prepare a compound as shown in a formula II, and finally enabling the compound as shown in the formula II to be reacted with NBS (N-bromosuccinimide) to obtain a compound as shown in a formula I; and then preparing a compound as shown in a formula I-1 and a compound in a formula I-2. Compared with an existing method, the method has the advantages of mild reaction conditions, cheap utilized reagents and high yield.
Owner:JIANGSU ALPHA PHARM CO LTD
Synthesis method of abiraterone acetate highly finished product
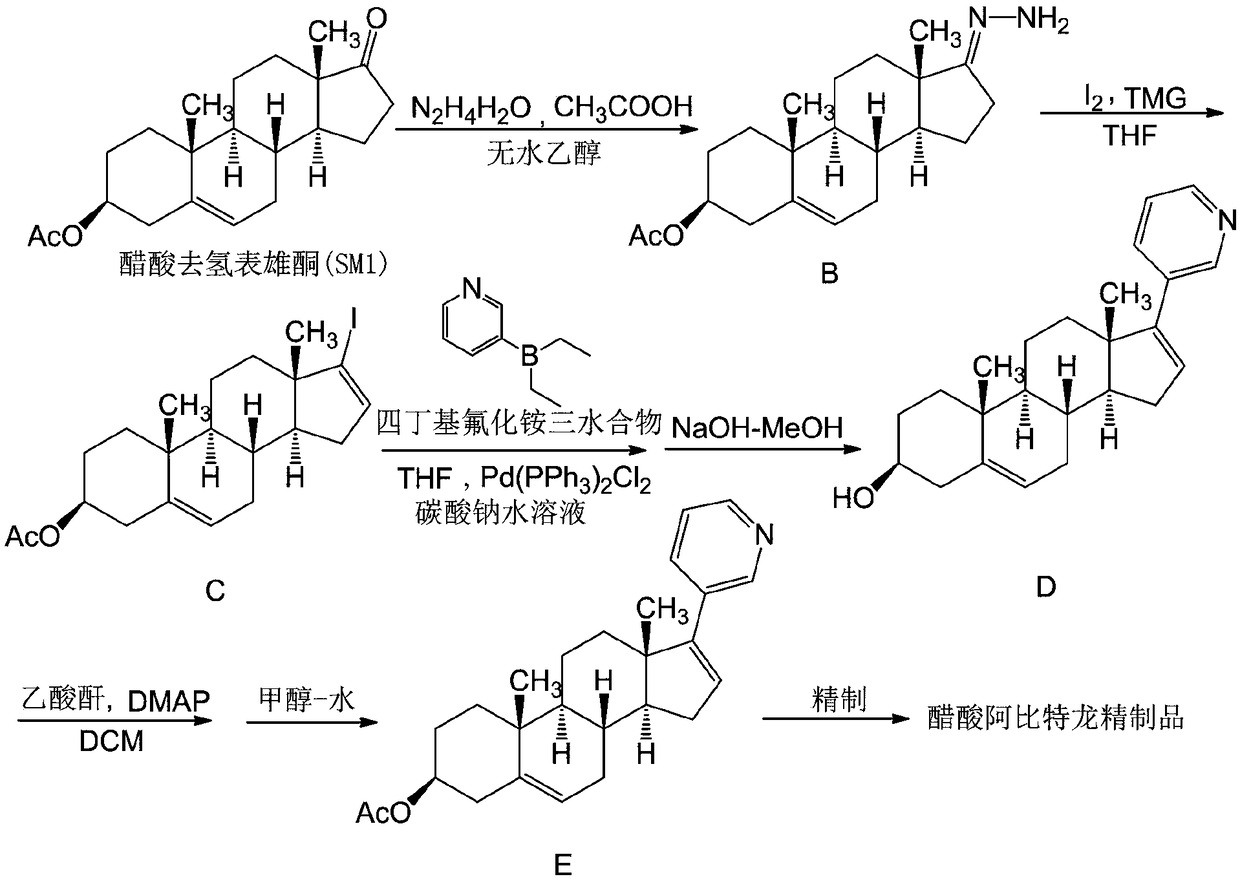
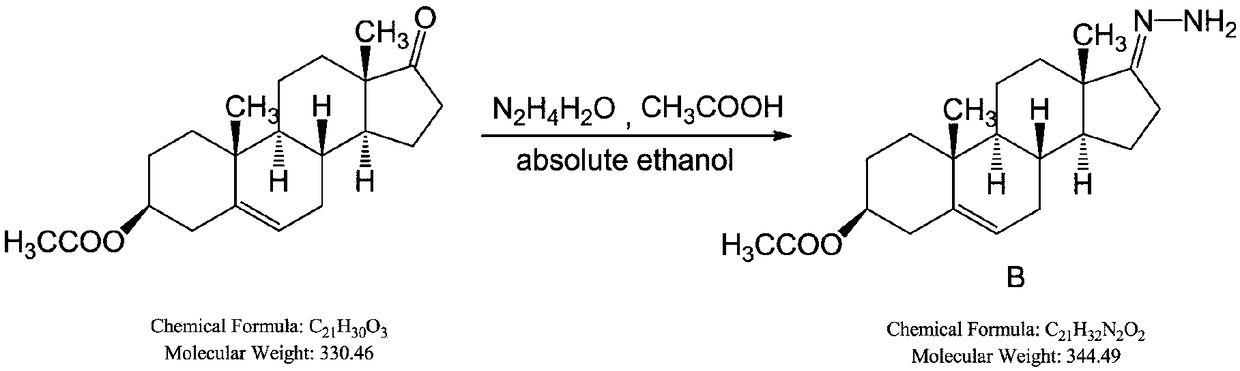
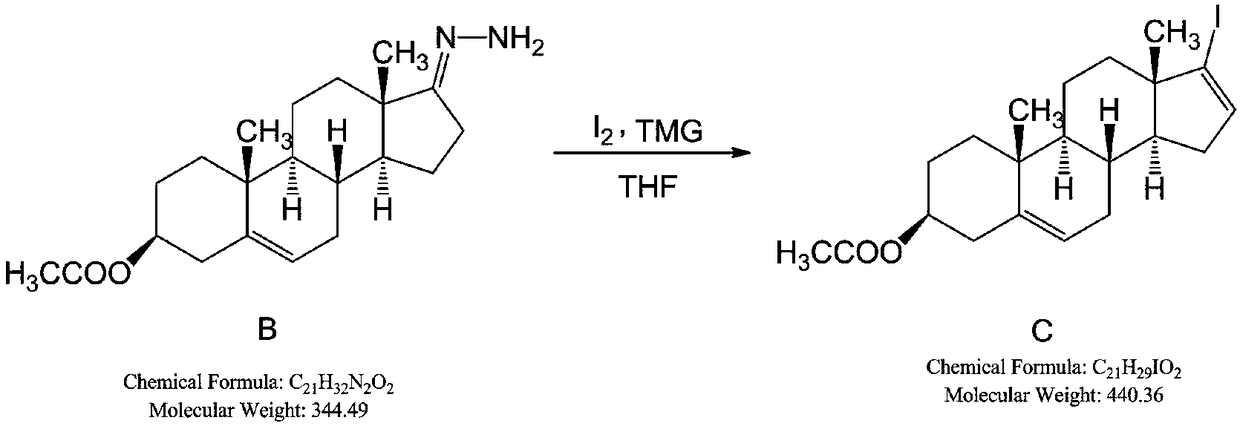
The invention belongs to the technical field of organic chemistry, and discloses a synthesis method of an abiraterone acetate highly finished product. The synthesis method comprises the following steps: sequentially adding absolute ethyl alcohol, hydrazine hydrate and glacial acetic acid in dehydroisoandrosterone 3-acetate (SM1), and preparing a reactant B at the temperature of 20-30 DEG C; addingtetrahydrofuran and iodine, slowly adding tetramethyl guanidine at the temperature of -5 to 5 DEG C, controlling the temperature to be 10 DEG C or below, and dropwise adding a tetrahydrofuran solution of the compound B at the temperature of -5 to 5 DEG C for 6 h to obtain a compound C; sequentially adding tetrahydrofuran, diethyl-(3-pyridine)borane, trans-dichlorobis(triphenyl-phosphine)palladiumand a tetrabutylammonium fluoride trihydrate in the compound C to obtain a compound D; and adding dichloromethane and 4-dimethylaminopyridine in the compound D, slowly adding acetic anhydride at thetemperature of 15-35 DEG C, and adding activated carbon and methanol-water after finishing the reaction to obtain a compound E, and highly finishing the compound E to prepare the abiraterone acetate highly finished product.
Owner:XUZHOU COLLEGE OF INDAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com