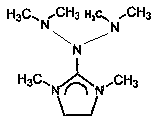
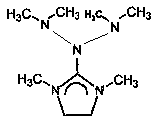
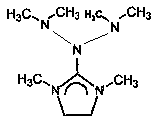
Preparation method and application of N-bis (dimethylamino)-1, 3-dimethylimidazoline
A dimethylimidazoline and dimethylamine group technology, which is applied in the field of compound preparation, can solve the problems of hidden dangers in production and transportation, phosgene and diphosgene are highly toxic, and cannot be used for catalytic fluorination, and can achieve high temperature heat. Good stability, lower production costs, and reduced polymerization effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] A preparation method of N-bis(dimethylamino)-1,3-dimethylimidazoline, which is characterized in that it comprises the following steps:
[0023] S1: Take 1,3-dimethyl-2-imidazoline and bis(trichloromethyl) carbonate as raw materials, carry out chlorination reaction to synthesize chloro 1,3-dimethyl-2-chloroimidazoline; this It not only solves the safety and environmental protection problems of other chlorinated reagents, but also greatly improves the product yield (over 87%).
[0024] S2: Add tetramethylguanidine dropwise to the chloride salt generated above to perform condensation reaction;
[0025] S3: Neutralize the above products to obtain N-bis(dimethylamino)methylene-1,3-dimethyl-2-chloroimidazoline ammonium chloride.
[0026] A use of N-bis(dimethylamino)-1,3-dimethylimidazoline, which is characterized in that it is used as a catalyst.
[0027] Preferably, the said is suitable for use as a catalyst in the synthesis of aromatic fluorine-containing compounds which are diffic...
Embodiment 1
[0029] Chlorination reaction: pump 500 kg of toluene into the upper tank, dissolve 200 kg of bis(trichloromethyl) carbonate, pump 200 kg of DMI into the reactor, add dropwise at 10-20 degrees, keep warm for 1 hour after dropping, and warm up To 40 degrees, keep for 5 hours, distill to recover toluene, and cool down. 284 kg of white crystalline product chloro 1,3-dimethyl-2-chloroimidazoline was obtained, the HPLC purity was 99.70%, and the yield was 96%.
[0030] Condensation reaction: 480 kg of dichloroethane is sucked into the high tank and dropped into the chlorination reactor in the previous step, and then 370 kg of tetramethylguanidine is added dropwise. The temperature in the kettle is controlled at 40 degrees. After the dripping is completed, the temperature is kept at 40 degrees for 1 hour , Heating up and recovering dichloroethane, then adding lye dropwise until the reaction solution is neutral, centrifuging to obtain the product N-bis(dimethylamino)methylene-1,3-dimethy...
Embodiment 2
[0032] Chlorination reaction: pump 500 kg of toluene into the upper tank, dissolve 200 kg of bis(trichloromethyl) carbonate, pump 200 kg of DMI into the reactor, add dropwise at 10-20 degrees, keep warm for 1 hour after dropping, and warm up To 40 degrees, keep for 5 hours, distill to recover toluene, and cool down. 287 kg of white crystalline product chloro 1,3-dimethyl-2-chloroimidazoline was obtained, with HPLC purity of 99.72% and yield of 97%.
[0033] Condensation reaction: 480 kg of dichloroethane is sucked into the high tank and dropped into the chlorination reactor in the previous step, and then 370 kg of tetramethylguanidine is added dropwise. The temperature in the kettle is controlled at 40 degrees. After the dripping is completed, the temperature is kept at 40 degrees for 1 hour , Heating up and recovering dichloroethane, then adding lye dropwise until the reaction solution is neutral, centrifuging to obtain the product N-bis(dimethylamino)methylene-1,3-dimethyl-2-ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com