Synthesis method of abiraterone acetate highly finished product
A kind of technology of abiraterone acetate and synthesis method, applied in the direction of steroids, organic chemistry, etc., can solve the problems of long synthesis steps, purification method and product yield, serious corrosion of equipment, etc., to achieve improved results efficiency, shorten the synthesis steps, and improve the effect of purification yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0079] In order to make the object, technical solution and advantages of the present invention more clear, the present invention will be further described in detail below in conjunction with the examples. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
[0080] Such as figure 1 As shown, the synthetic method of abiraterone acetate refined product provided by the embodiments of the present invention comprises the following steps:
[0081] The reaction scheme of the synthetic side of the refined product of abiraterone acetate that the embodiment of the present invention provides is:
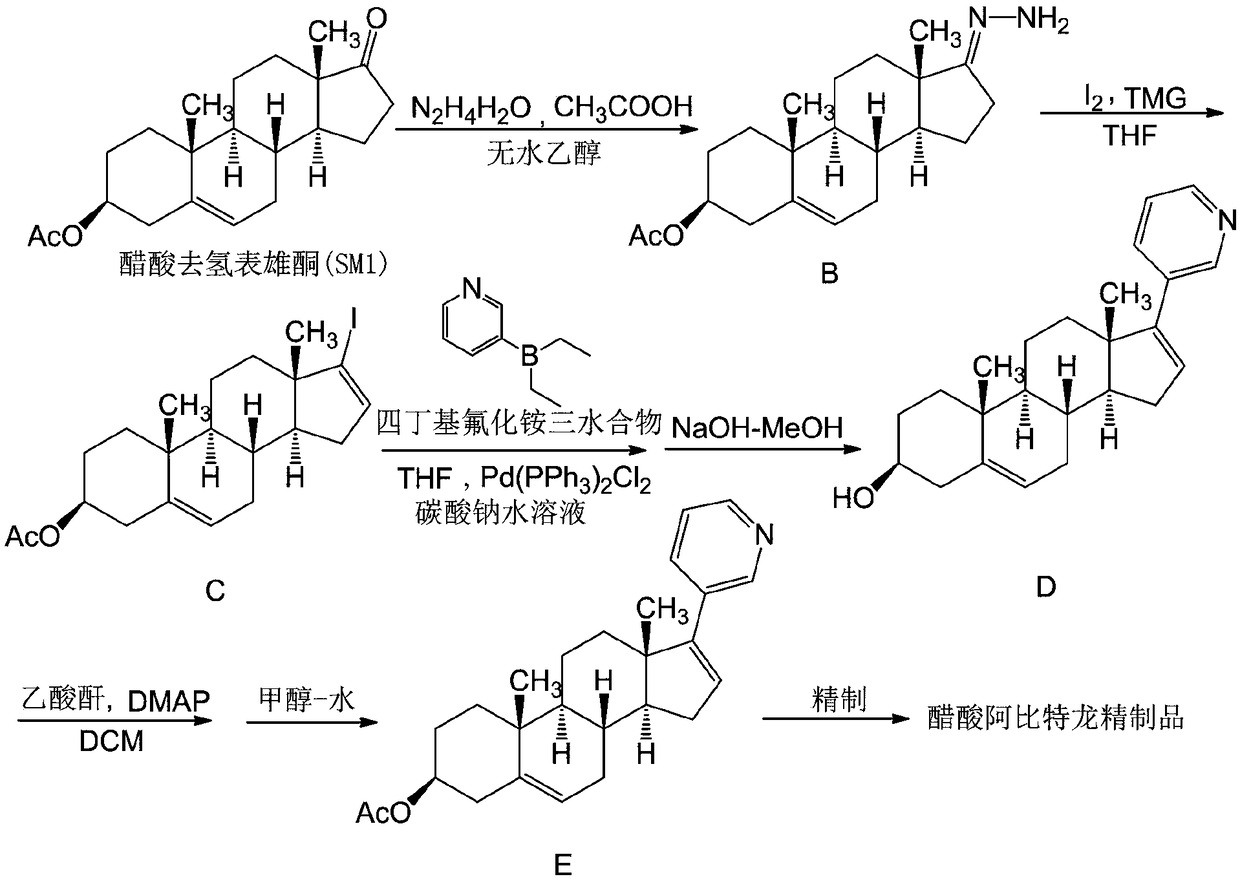
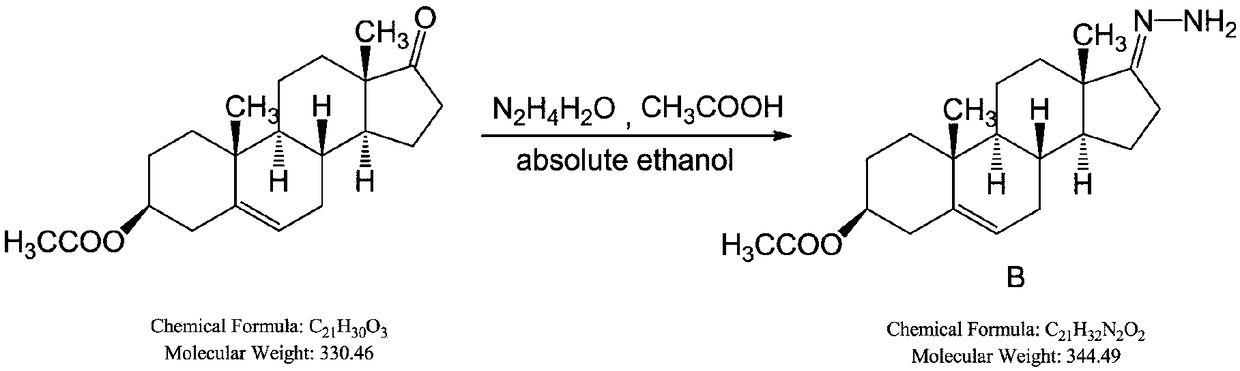
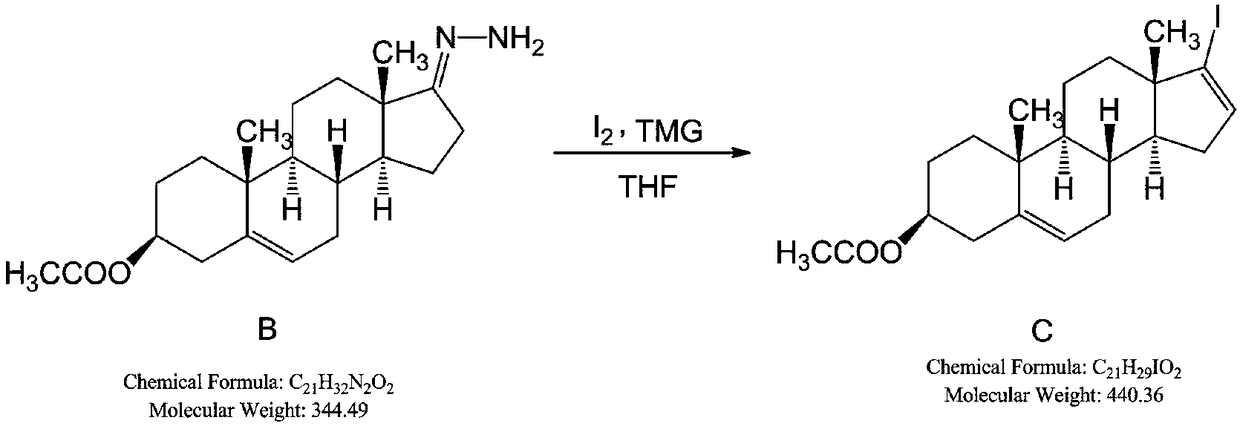
[0082] Add dehydroepiandrosterone acetate (SM1), sequentially add absolute ethanol, hydrazine hydrate and glacial acetic acid at 20-30°C to prepare reactant B; add tetrahydrofuran and iodine grains, -5-5°C, slowly add tetramethyl Guanidine, control the temperature below 10°C, add the tetrahyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com