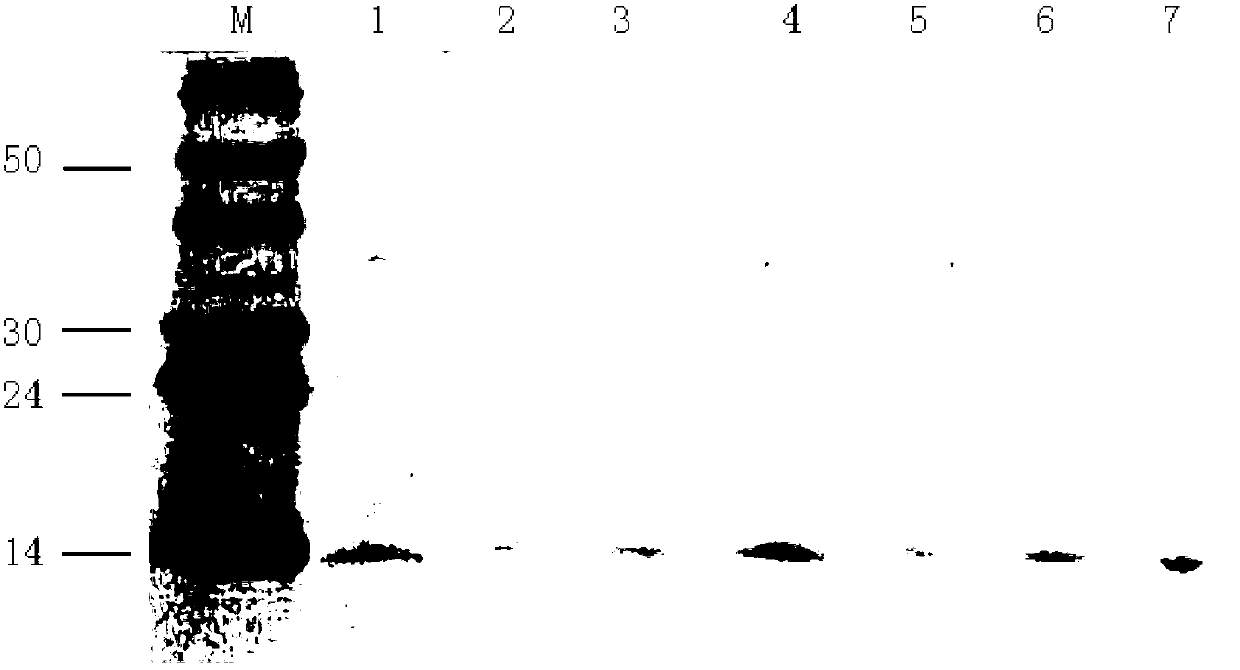
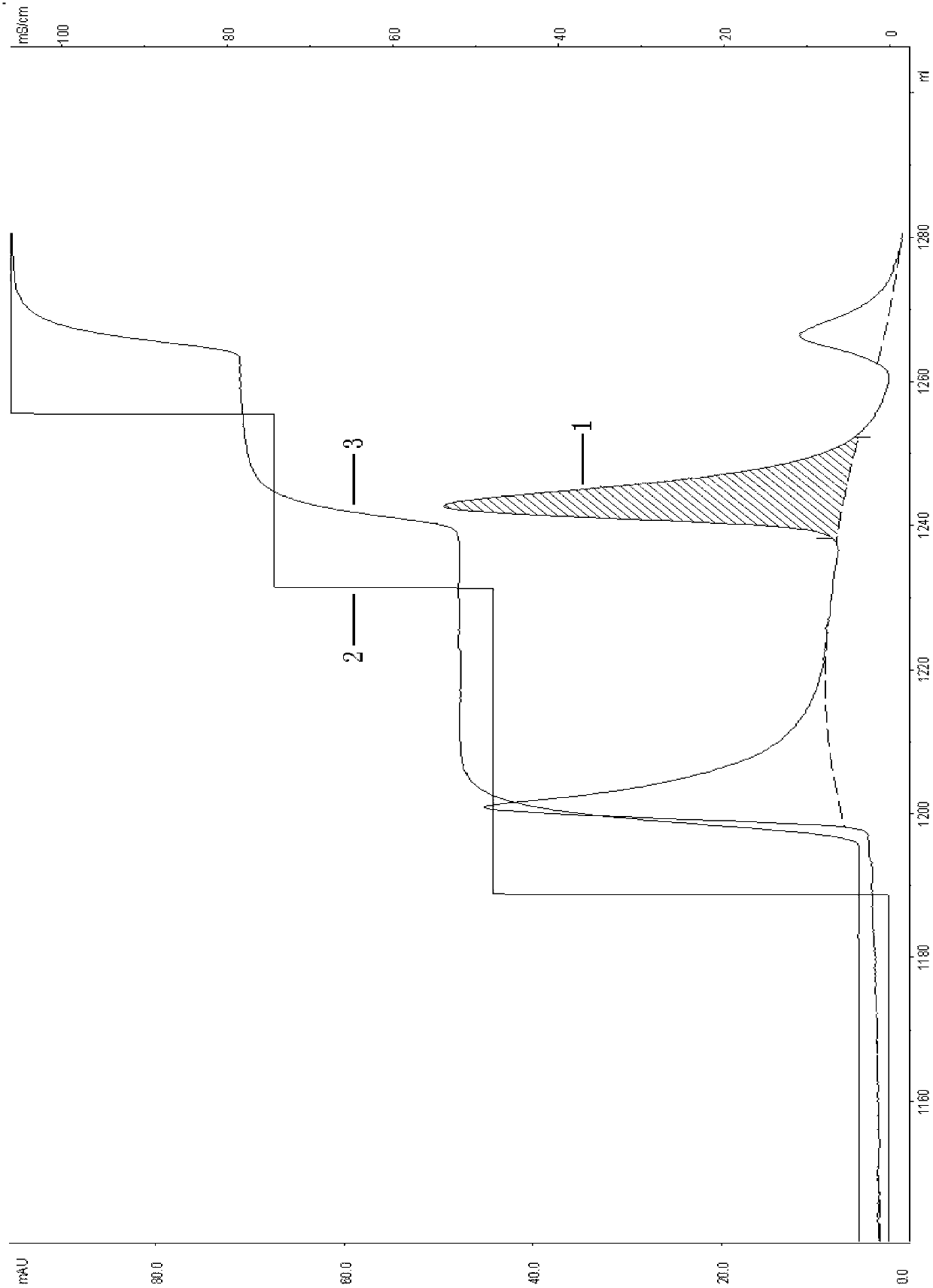
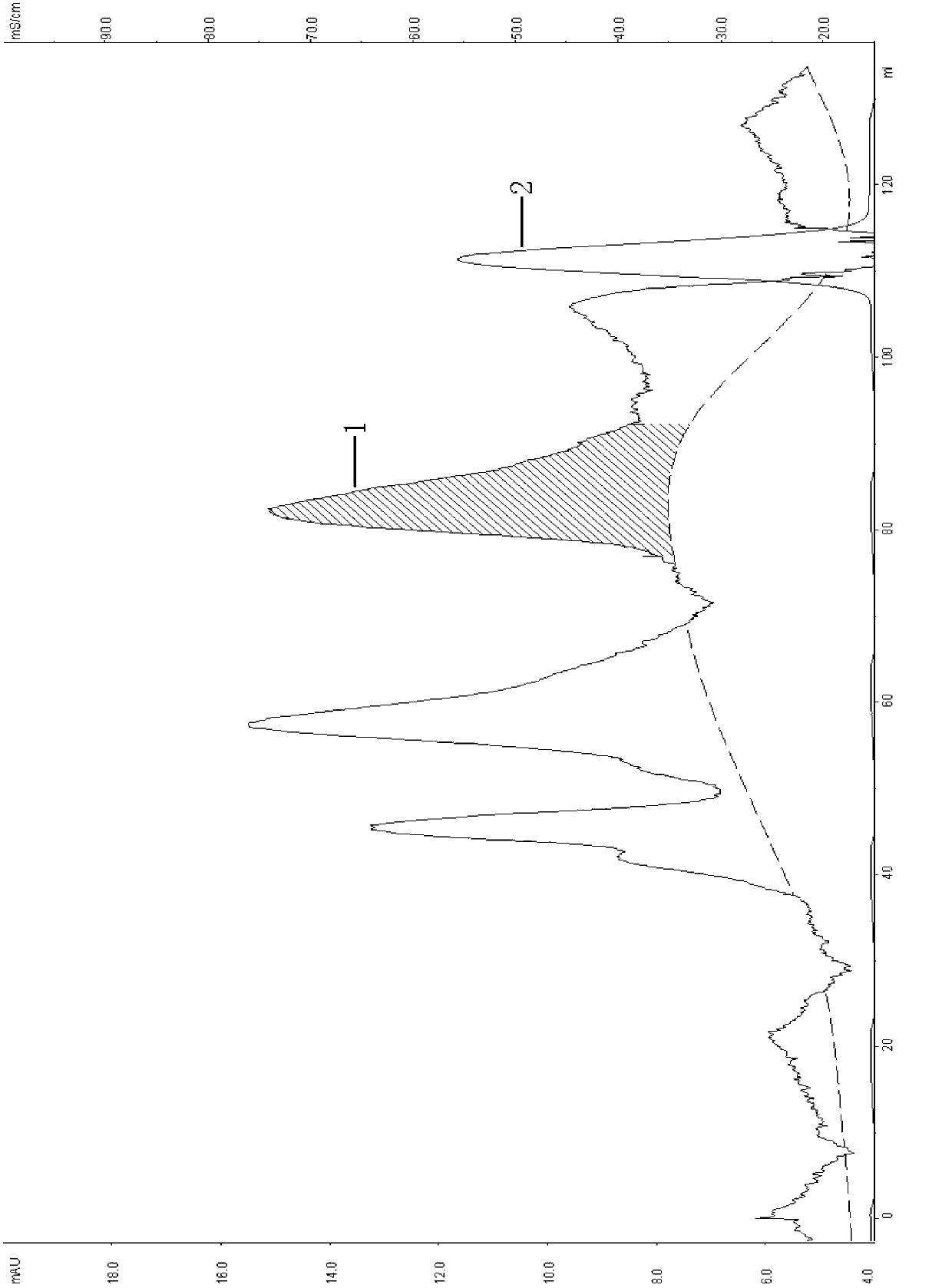
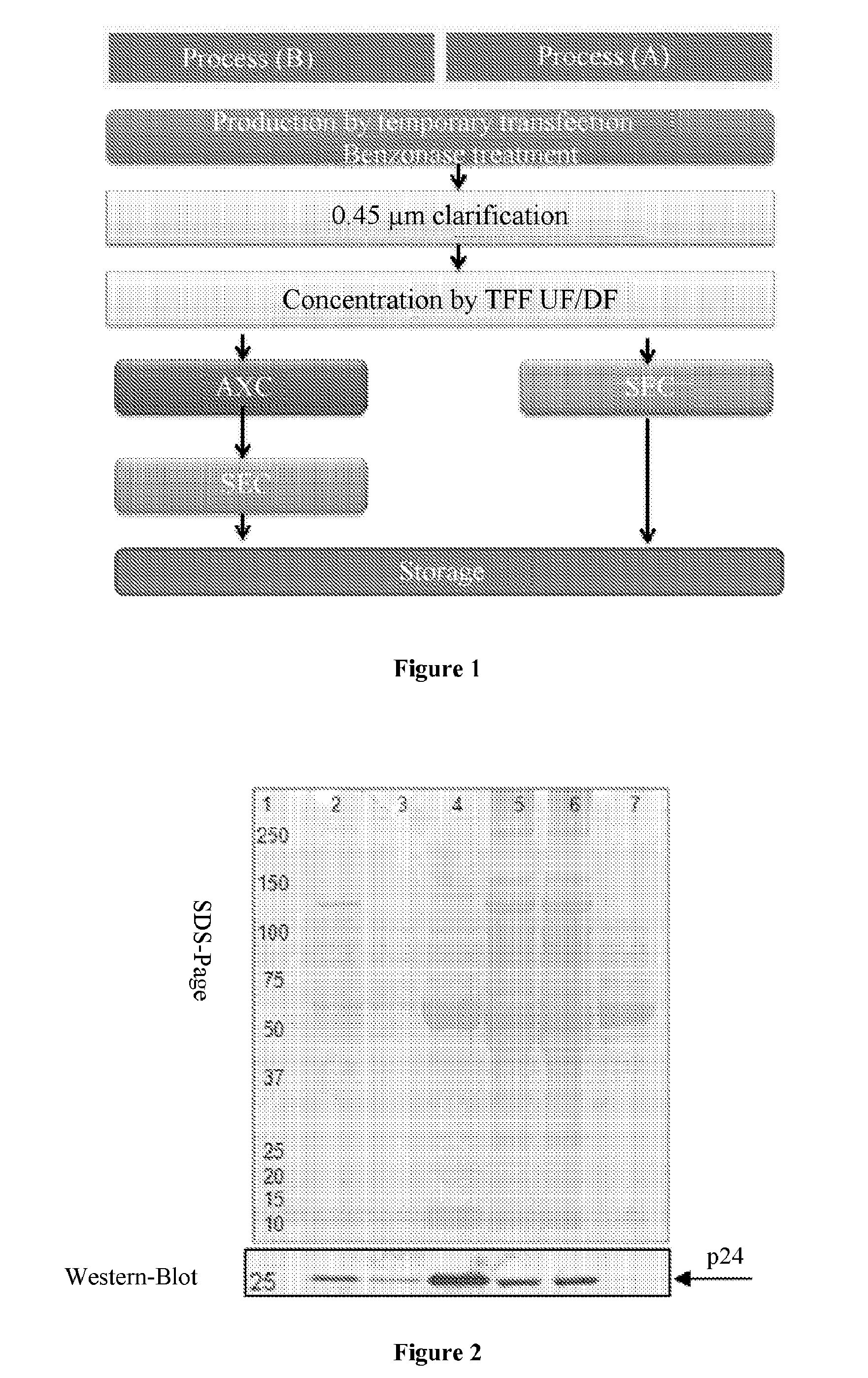
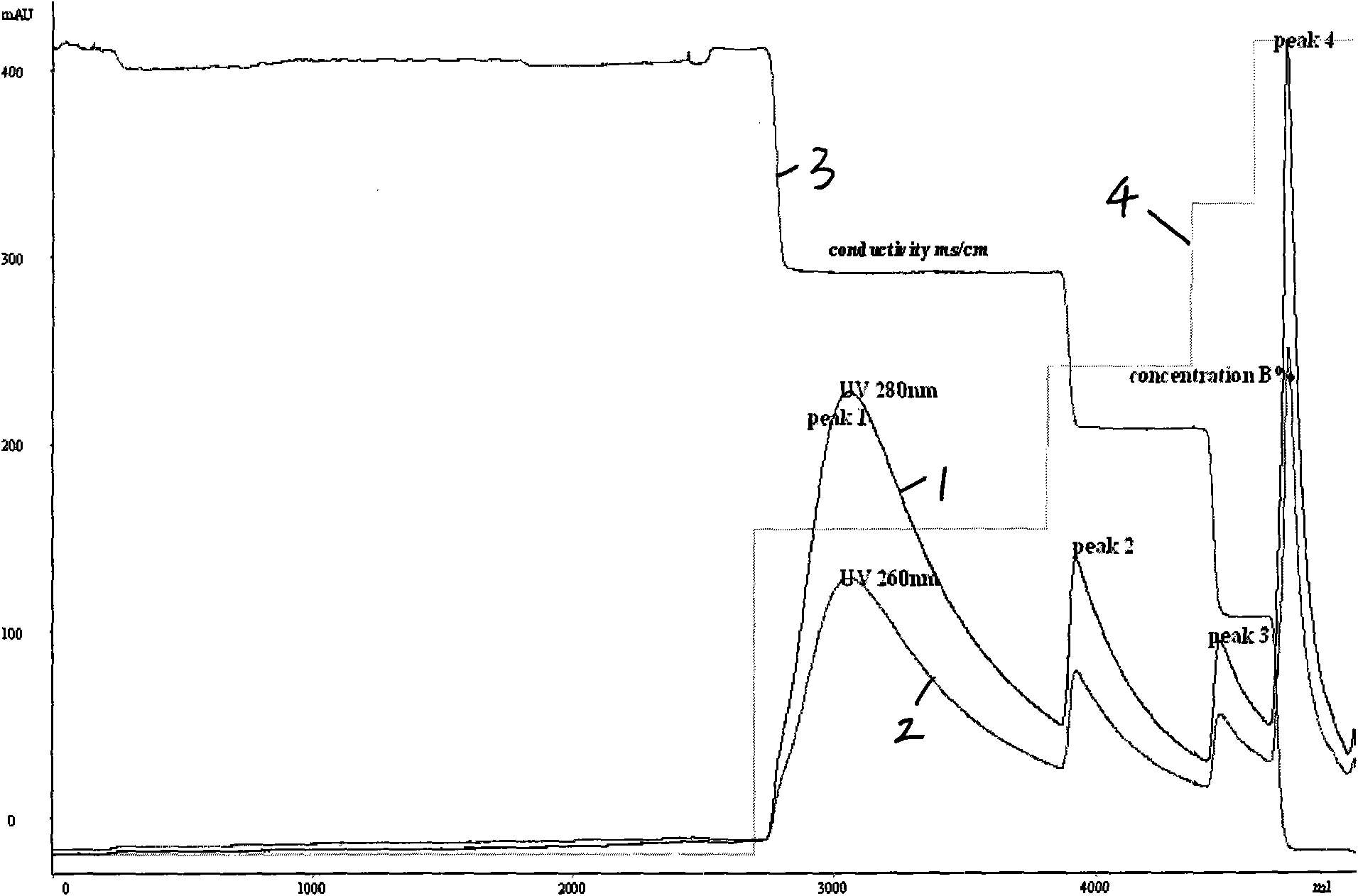
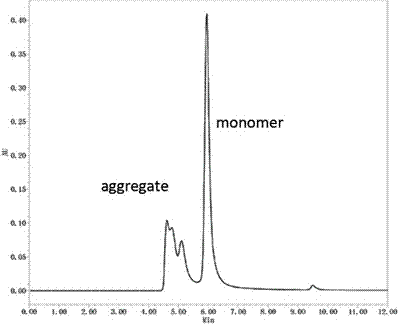
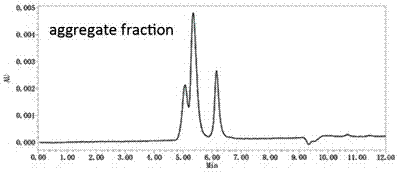
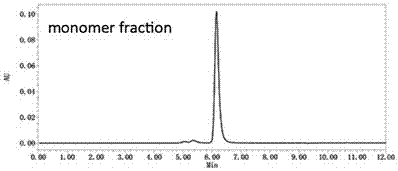
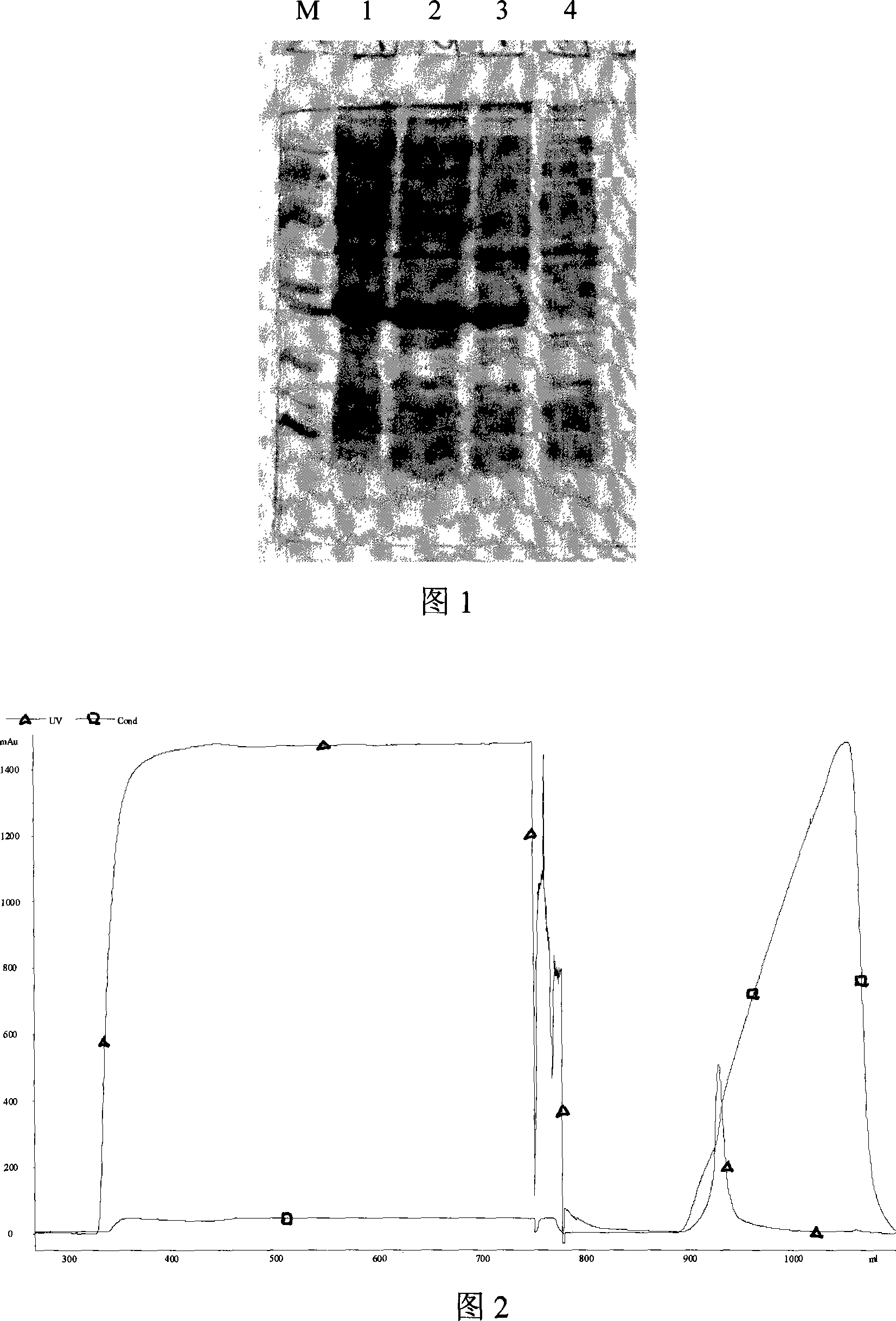
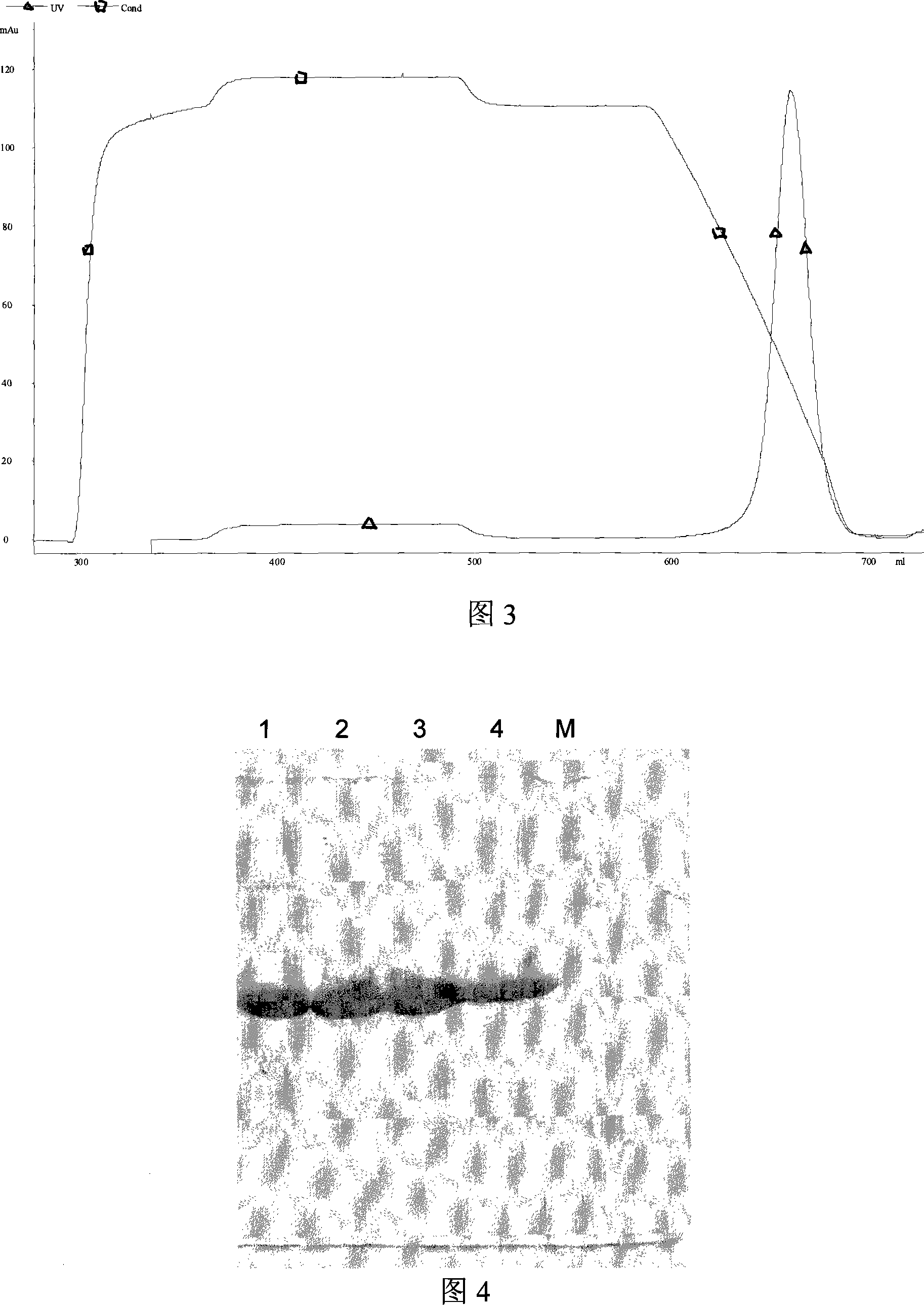
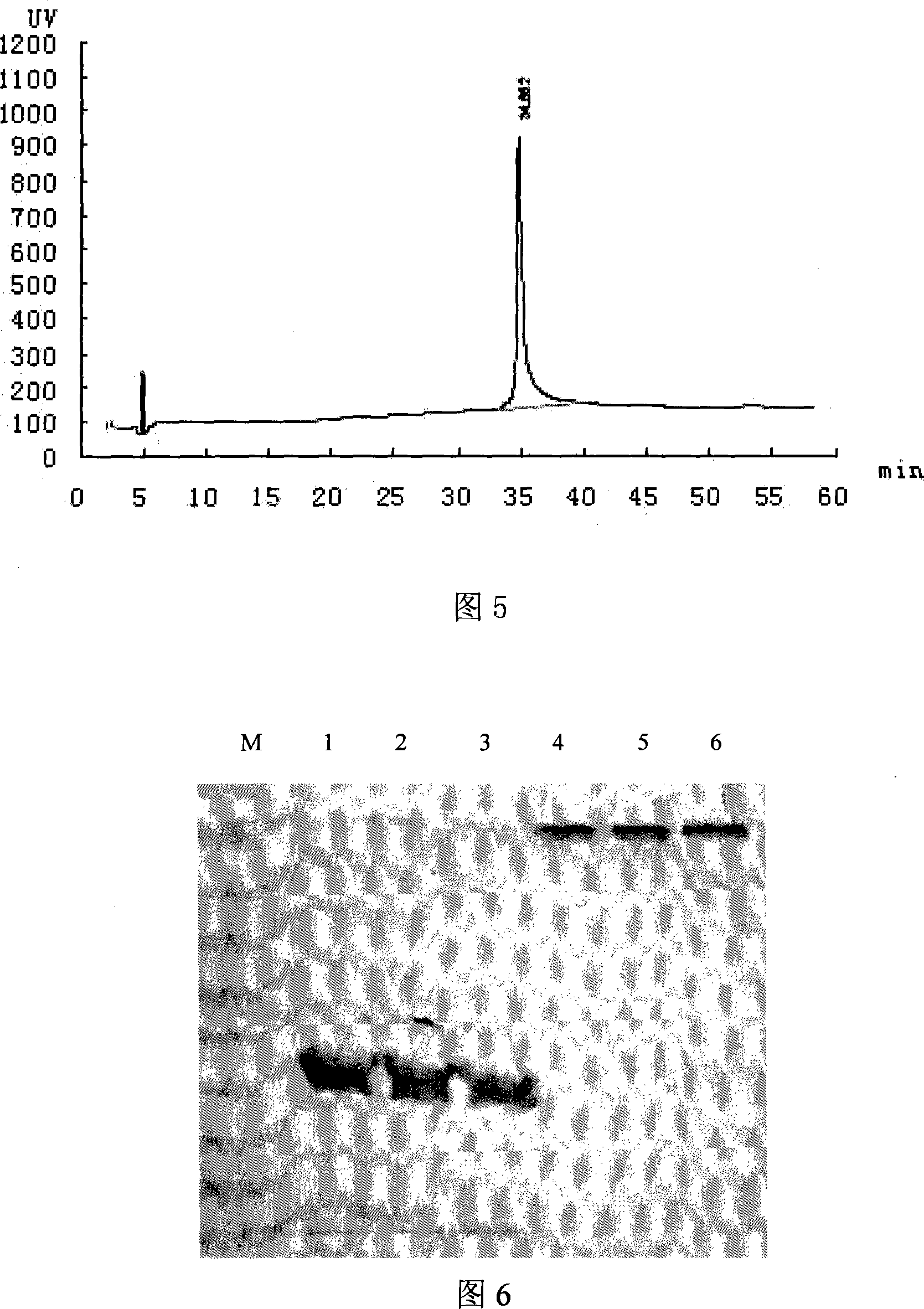
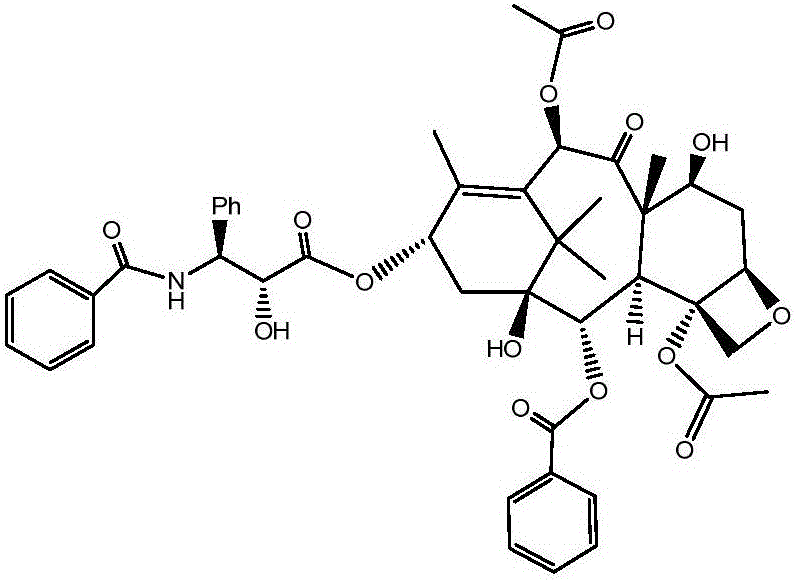
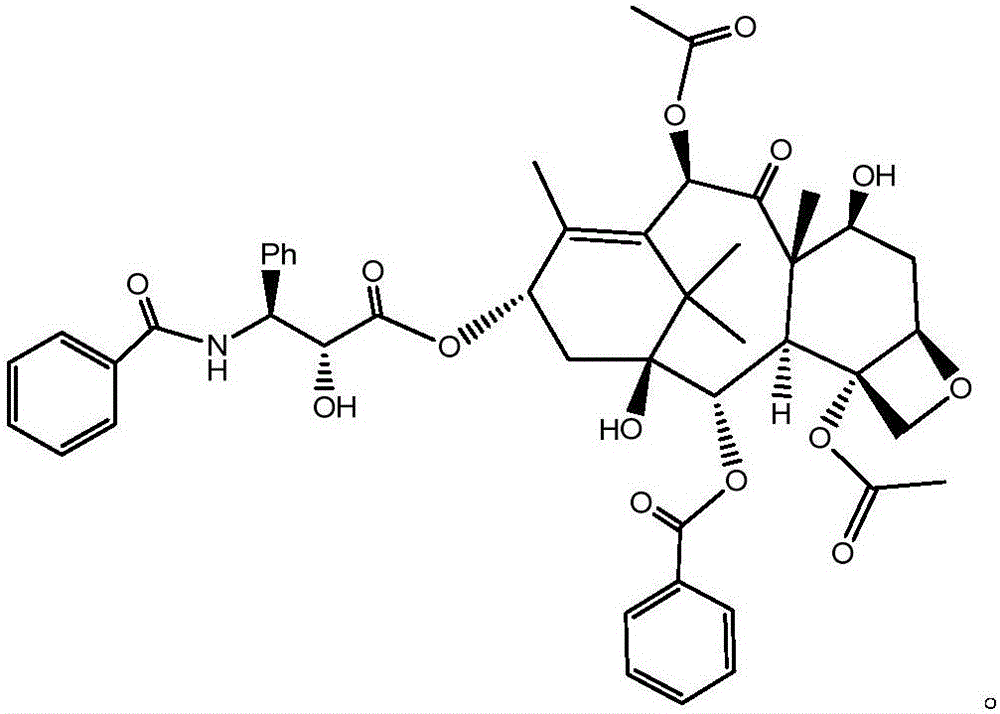
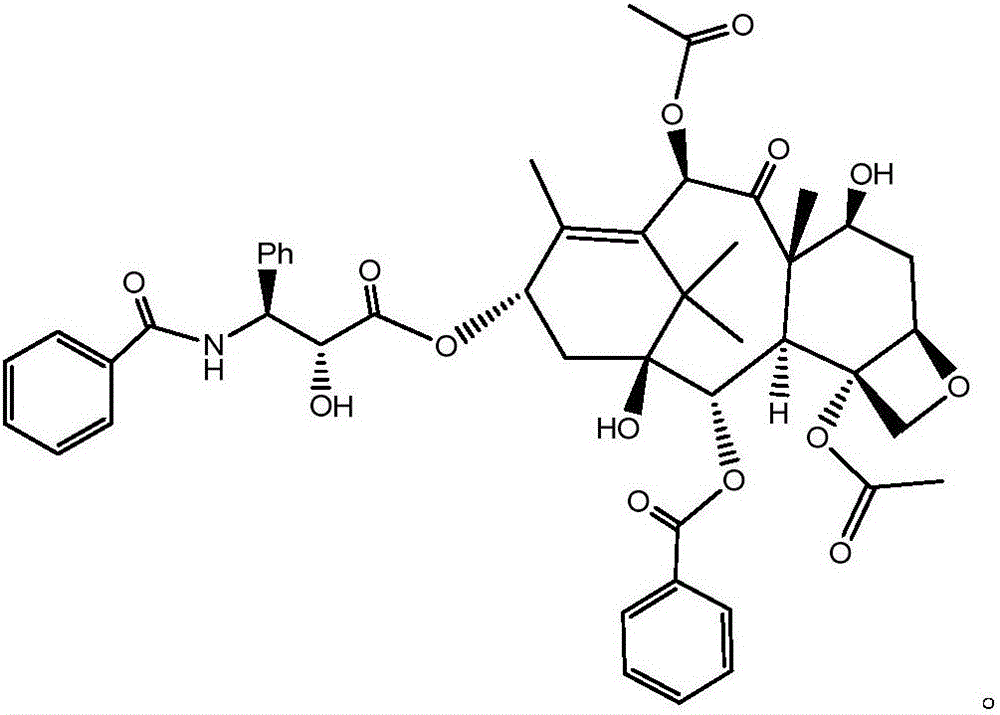
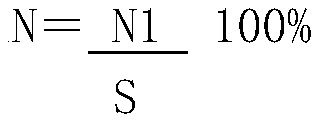Patents
Literature
138results about How to "High purification yield" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
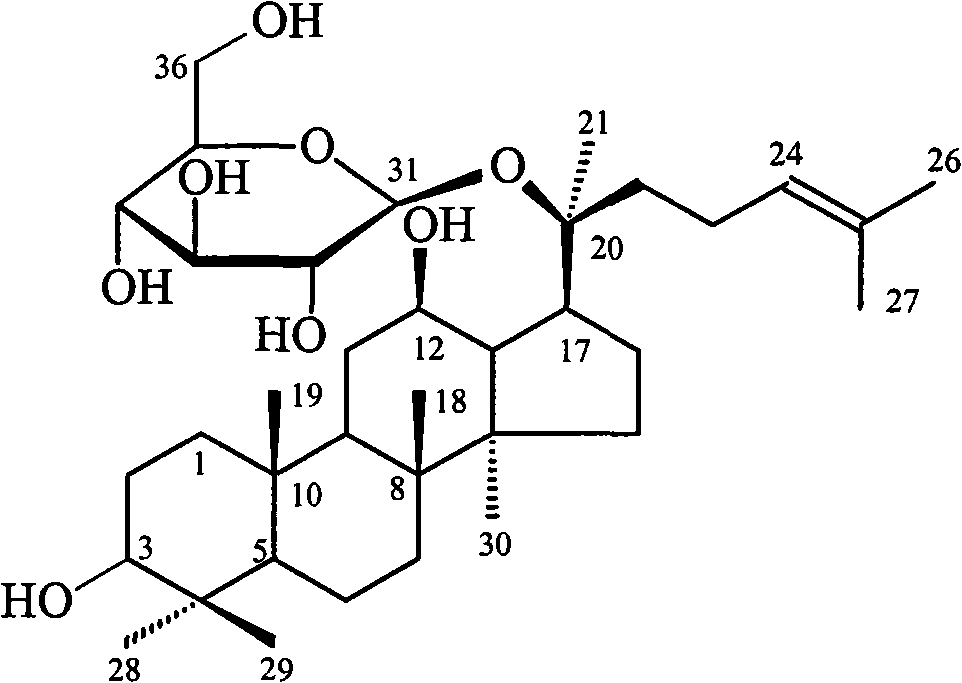
Method for purifying panaxoside compound-K by applying macroporous resin
ActiveCN101921304AReduce usageEasy to operateIon-exchange process apparatusIon-exchanger regenerationHigh concentrationMicroorganism
The invention relates to a method for separating and purifying a panaxoside compound-K from microorganism zymocyte by applying macroporous resin for preparing a raw medicine. The purifying yield reaches more than 72 percent, and the content of the panaxoside compound-K in a product is more than 96 percent. The method mainly comprises the following steps of: adding ethanol into zymocyte for extracting and concentrating; decoloring by active carbon and filtering; after adding water into filter liquor for diluting, leading the filter liquor to pass through a macroporous resin chromatographic column; washing by a low-concentration organic solvent aqueous solution for removing pigment and high-polarity saponin; eluting by a high-concentration organic solvent aqueous solution to obtain a panaxoside compound-K component; and finally crystallizing to obtain the panaxoside compound-K raw medicine with the content of more than 96 percent. The invention can provide a great amount of qualified raw medicine samples for the pharmaceutical development of the panaxoside compound-K.
Owner:ZHEJIANG HISUN PHARMA CO LTD
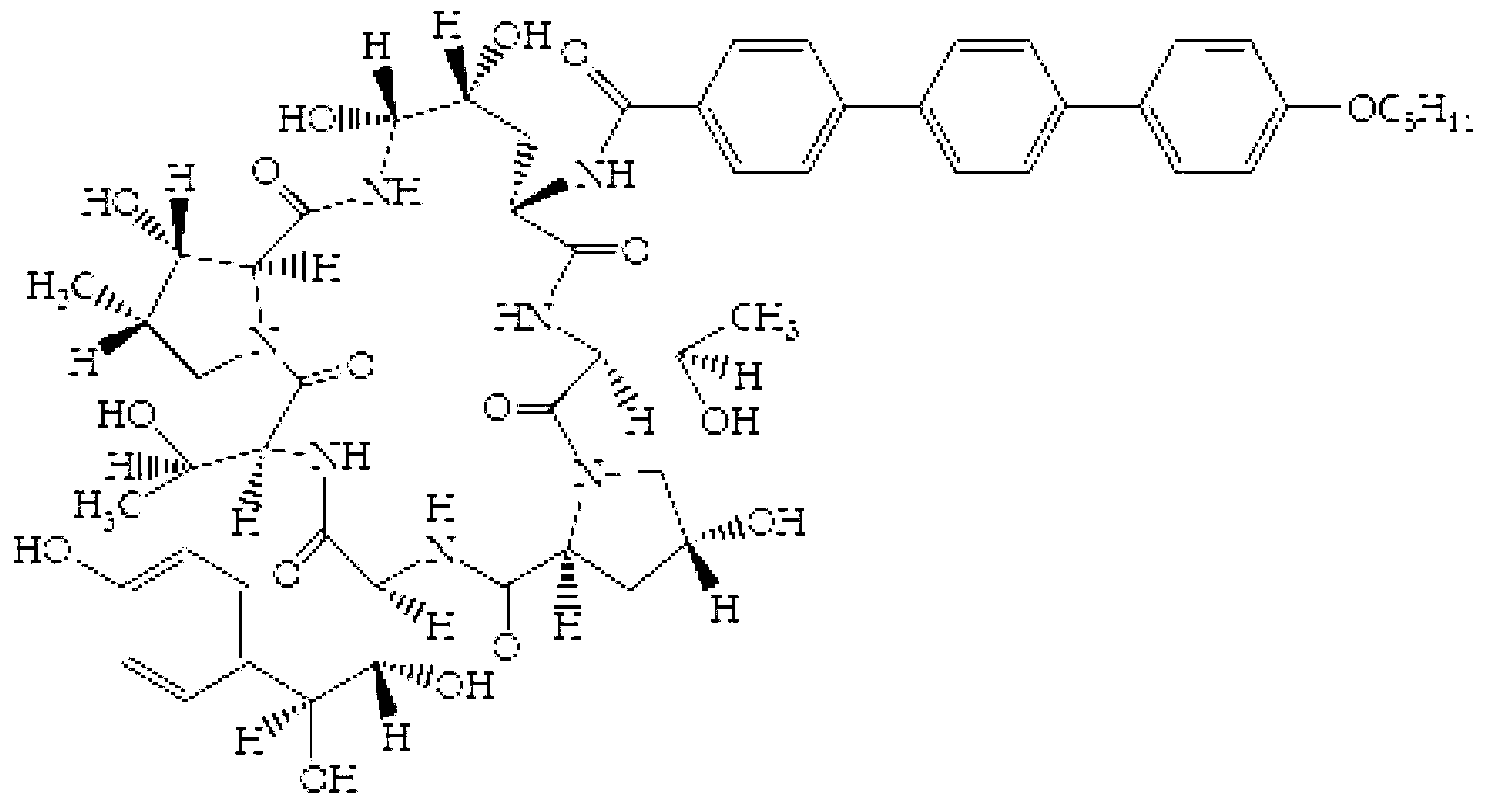
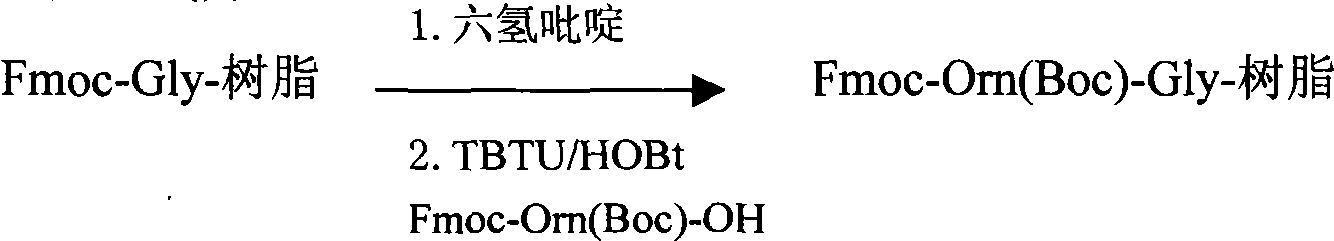
Preparation method of teduglutide
InactiveCN104418949AEase of mass productionAvoid side effects such as degradationPeptide preparation methodsGlucagonsCombinatorial chemistrySide reaction
The invention belongs to the field of medicinal chemistry, and in particular relates to a preparation method of teduglutide. The preparation method of the invention is as below: respectively synthesizing a teduglutide sequence site No.4-33 peptide resin fragment and a teduglutide sequence No.1-3 peptide resin fragment through solid phase synthesis; then coupling the teduglutide sequence site No.4-33 peptide resin fragment and the teduglutide sequence No.1-3 peptide resin fragment by an appropriate resin vector to obtain a teduglutide resin; and finally cracking to obtain crude teduglutide, and purifying to obtain the pure teduglutide. The preparation method provided by the invention can effectively avoid side reaction such as degradation caused by an Asp-Gly structure of the No. 3-4 site, also can reduce the His1 racemization, thus effectively improving the purity of crude peptide and improve the purification yield. Compared with the prior art, the preparation method of the invention has the advanategs of high purity of the prepared teduglutide product, less by-product and high product yield, and is beneficial for mass production of teduglutide.
Owner:HYBIO PHARMA
Method of preparing veralkanol from Chinese medicinal herb plant polygonum cuspidatum
InactiveCN1724495AHigh purification yieldRich in plant resourcesOrganic chemistryOrganic compound preparationCelluloseFreeze-drying
The invention provides a method for preparing veralkanol giant knotweed rhizome a Chinese medicinal herbal plant, which consists of collecting giant knotweed rhizome, drying and preserving, using ethanol-water mixed solvent as extracting agent, heating and extracting through return flow, concentrating the mixed solvent extract into grease form substance through vacuum rotary concentration, thus obtaining giant knotweed rhizome crude extract, dissolving the grease form substance with distilled water, extracting the solution with equal-volume of acetic ester, drying the acetic ester through vacuum rotary concentration, dissolving with methanol, isolating and purifying with column chromatography, using cellulose as the fixed phase in column chromatography, carrying out column chromatography elution with methanol, collecting the eluent, concentrating the collected veralkanol, and freeze drying. The yield of the veralkanol is 0.4%.
Owner:SHANGHAI JIAO TONG UNIV
Purification method of echinocandins antifungal drug anidulafungin
ActiveCN103193868ASimple stepsEasy to recyclePeptide preparation methodsEchinocandinPurification methods
The invention discloses a purification method of echinocandins antifungal drug anidulafungi. The method comprises the following steps of: (1) preparing a dry sample: adding organic solvent in a crude product of the anidulafungin to dissolve the crude product, adding silica gel after the crude product of the anidulafungin is adequately dissolved, and uniformly mixing and drying the mixture to obtain the anidulafungin dry sample; (2) pressurizing and eluting: uniformly filling the anidulafungin dry samples in the top end of a chromatographic column with silica gel, pressurizing and eluting the chromatographic column with the anidulafungin dry samples by adding elution solvent, utilizing a high-effective liquid phase chromatography to monitor, and collecting the elution solution with the anidulafungin content being greater than 98 percent; and (3) concentrating: concentrating the elution solution with the anidulafungin content being greater than 98 percent until dryness to obtain a pure product with the anidulafungin content being greater than 98 percent. By adopting the column chromatography, simplicity in operation is realized, and the equipment cost is low; the organic solvent with low toxicity and low boiling point is adopted as the elution solution, so that the subsequent recycling treatment is simple, and the environmental pressure can be greatly reduced; and the purification separation time is short, the purification effect is good, the purification yield is high, and the purification method is applicable to industrialized mass production.
Owner:NCPC NEW DRUG RES & DEV
Method for synthesizing atosiban acetate from solid phase polypeptide
ActiveCN101357937AConvenient sourceHigh peptide yieldPeptide preparation methodsBulk chemical productionPreparative hplcSide chain
The invention discloses a preparation method of solid phase peptide synthesis atosiban which includes the following steps: taking Rink Amide resins, Rink Amide MBHA resins or Rink Amide AM resins as starting materials and taking Fmoc amino acids as monomers, amino acids are grafted one by one and mercaptopropionic acids (Map(SX)) are protected by the last peptide chain; after protected nonapeptide resins are obtained, the acellular side-chain protective groups and cutting peptides are synchronized; then cutting peptides is carried out, and reduced crude atosiban is collected; the pH value is adjusted to 7.5 to 10.0, and oxidized crude atosiban is collected; target products are obtained by the separation and purification by preparative HPLC(C18 or C8 column). The preparation method is convenient in material source, simplifies technology and reduces cost; and the preparation method is low in the pollution of three wastes and is high in yield, and the preparation method is convenient for being industrialized and has good industrialization prospect.
Owner:SHANGHAI SOHO YIMING PHARMA
Method for removing cupric salt catalyst from product of ATRP
InactiveCN101519466AReduce pollutionSimple processPurification methodsAtom-transfer radical-polymerization
The invention belongs to a method for purifying the product of atom transfer radical polymerization (ATRP) and particularly relates to a method for removing a cupric salt catalyst from the product of ATRP. The process of the method comprises the following steps of: dissolving a product of the ATRP in a solvent I; extracting the product with an extract solution for three times; concentrating the product under reduced pressure; adding a solvent II into the concentrated product to precipitate the product; washing the product till the solution is colorless and neutral; collecting the product and drying the product under vacuum till the weight of the product is constant; and obtaining the purified ATRP polymerization product. The method has the advantages that: the purification process is simple, environmental pollution is little, the product yield is more than 80 percent, and the residual Cu content after purification is less than 2ppm.
Owner:NANJING UNIV OF TECH
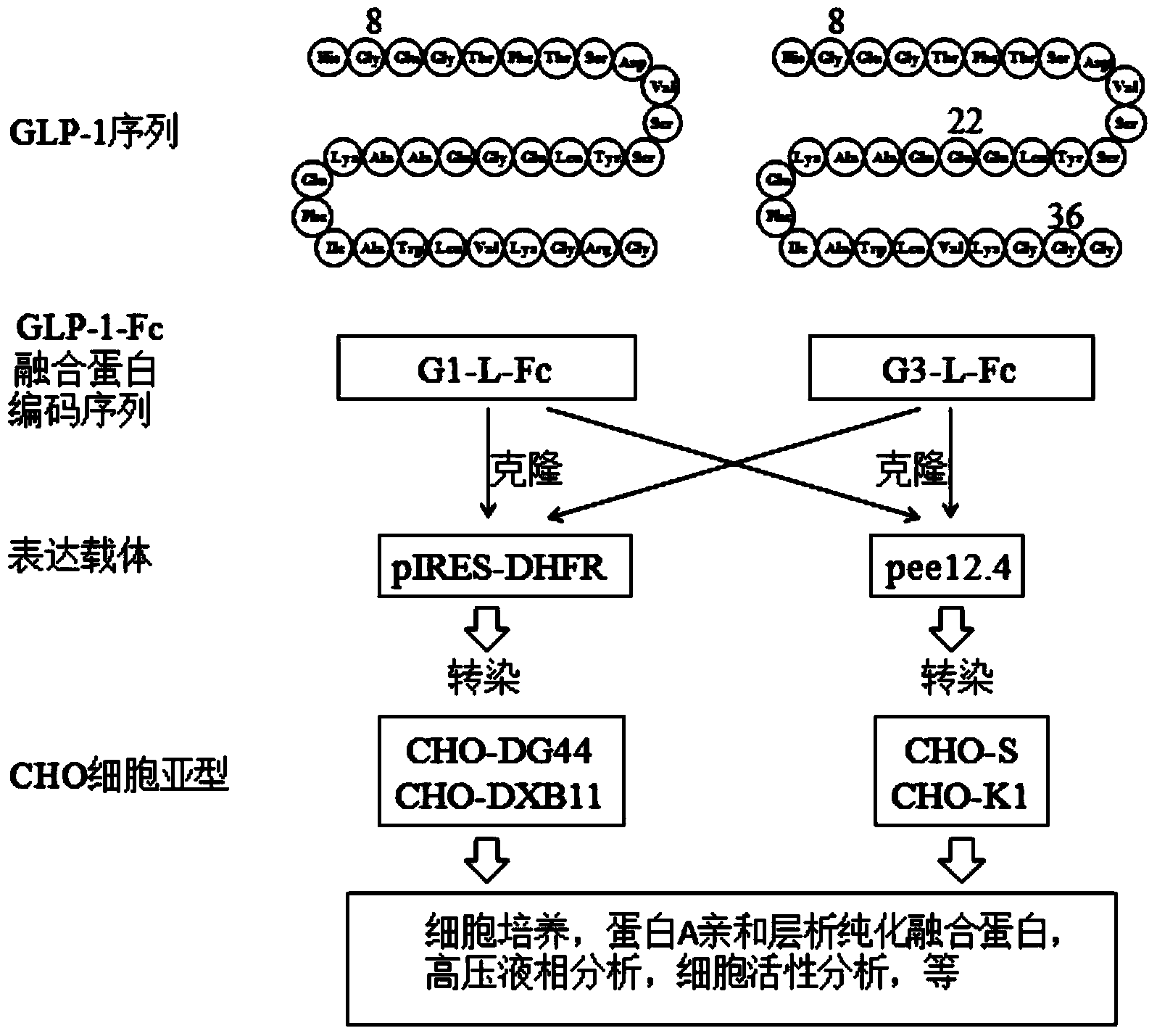
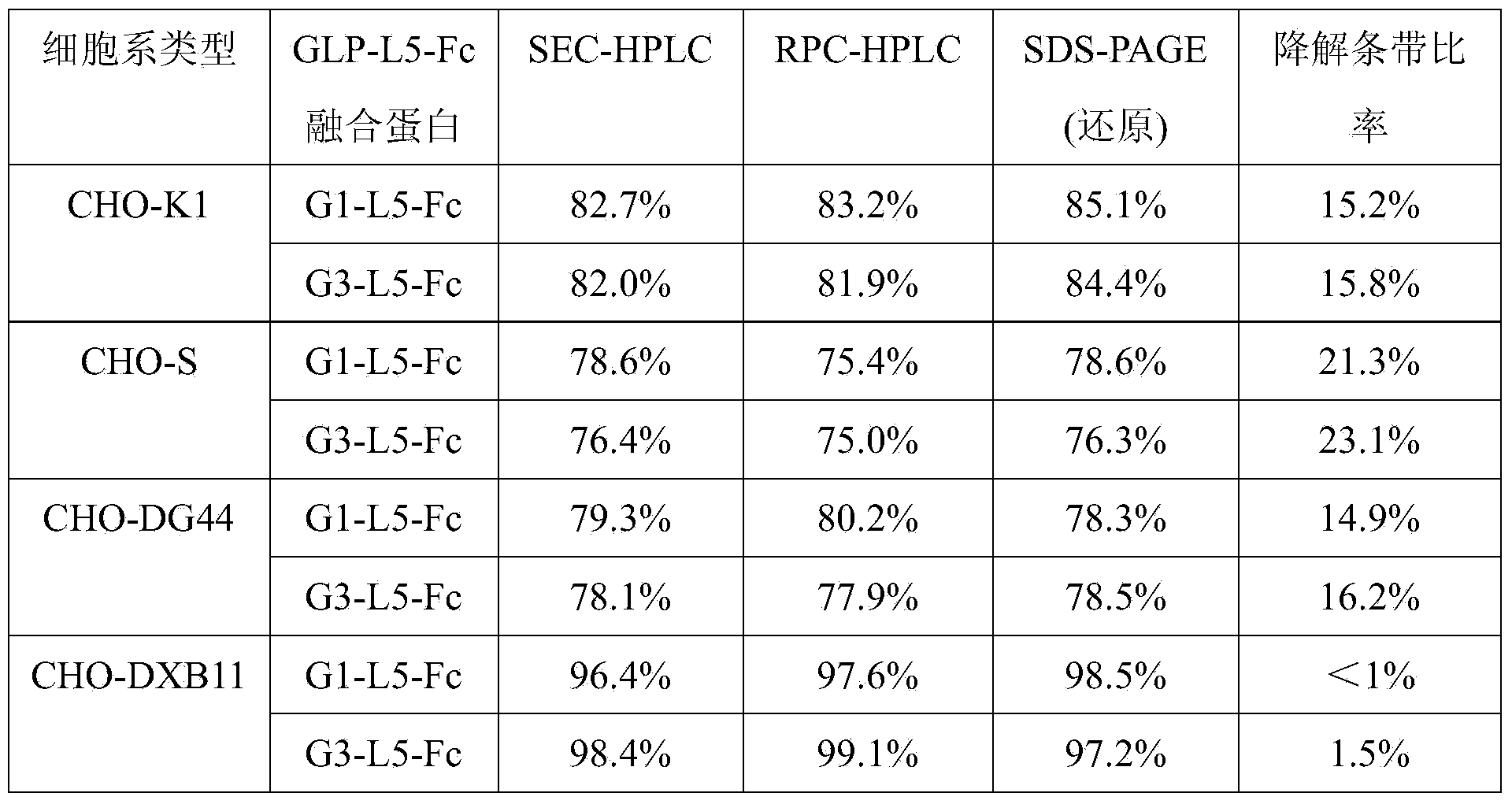
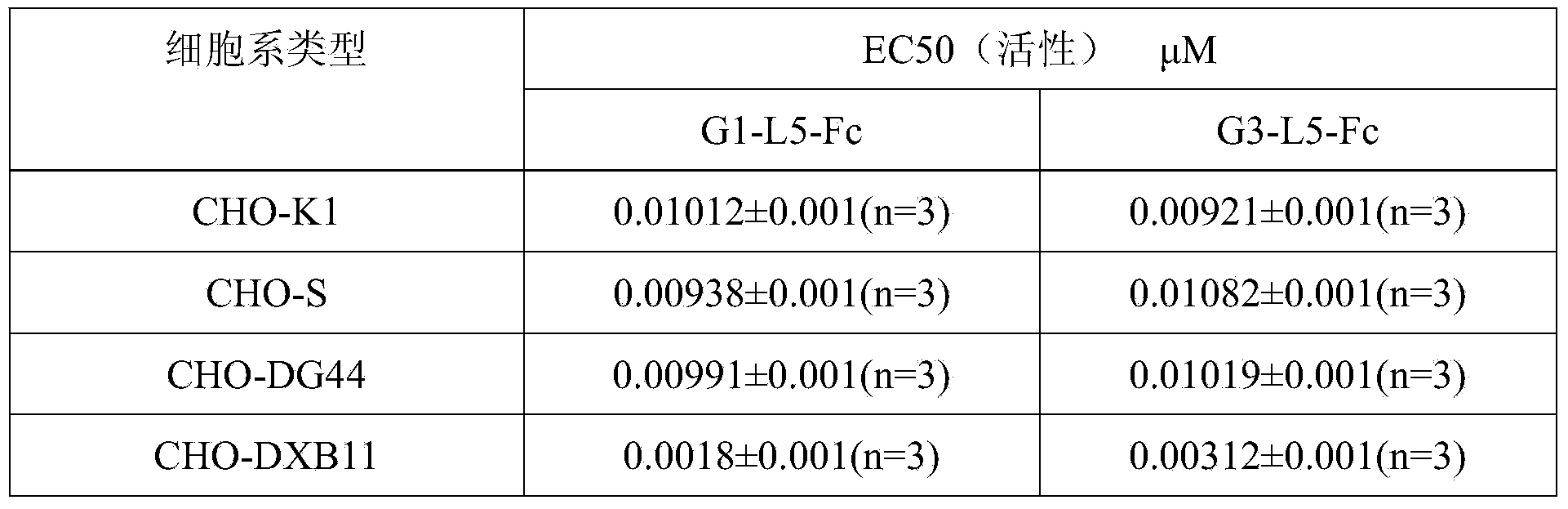
Preparation method of GLP (Glucagon-Like Peptide) -1 or analogue thereof and antibody Fc fragment fusion protein
ActiveCN104293834AReduce quality problemsIncrease the difficulty of purificationPeptide/protein ingredientsMetabolism disorderCell strainDrug biological activity
The invention relates to the technical field of biology and particularly relates to a preparation method of a GLP (Glucagon-Like Peptide) -1 or analogues thereof and an antibody Fc fragment fusion protein. The preparation method of the GLP-1 or the analogues thereof and the antibody Fc fragment fusion protein comprises the following steps: cloning sequences for encoding the GLP-1 or the analogues thereof and the antibody Fc fragment fusion protein into an expression vector; transfecting the expression vector with CHO-DXB11 cells, culturing and screening a positive cell strain; expressing the obtained cell strain and purifying to obtain the fusion protein. By virtue of the preparation method provided by the invention, when the GLP-1-Fc molecule is expressed in DXB11 of the CHO cell line, the problem of degradation cannot be caused, the subsequent purification yield is greatly improved, the biological activity is not decreased and thus the preparation method is very suitable for the requirement of industrial production.
Owner:SYNDEGEN SHANGHAI BIOTECH
Recombinant human nerve growth factor purifying method based on CHO cell expression system
ActiveCN102702341AImprove bindingHigh purification yieldPeptide preparation methodsAnimals/human peptidesProtein targetChromatography column
The invention relates to a recombinant human nerve growth factor purifying method based on a CHO cell expression system. The recombinant human nerve growth factor purifying method based on the CHO cell expression system comprises the following steps: 1), centrifugating supernatant, successively removing living cells and cell fragments, and removing impurities and bacteria by filtering; 2), applying a supernatant sample to an EMD SO3-(M) chromatography column, rinsing unbound protein and impure protein, eluting and collecting a target protein peak; 3), concentrating the protein with ultrafiltration equipment with a molecular weight cutoff of 3K; and 4), applying the sample to Superdex 75-containing chromatography column and collecting the target protein peak, namely a recombinant human nerve growth factor stoste. According to the CHO cell expression system and the recombinant human nerve growth factor purifying method based on the CHO cell expression system, the recombinant human nerve growth factor with the purity greater than 98% and the specific activity higher than the 5* 10 to the power of 5 AU / mg can be prepared; compared with other methods, the recombinant human nerve growth factor purifying method based on the CHO cell expression system has the advantages that the steps are reduced, the production cycle is greatly shortened, and the processing capacity and the yield are improved; therefore, the recombinant human nerve growth factor purifying method based on the CHO cell expression system is application for large-scale production.
Owner:北京福睿君安科技有限公司
Method for double-solvent lactide recrystallization
InactiveCN101157680AHigh purification yieldGood recrystallization effectOrganic chemistryAcetic acidLactide
The invention provides a method of using two solvents for recrystallization to get the lactide with the high yield and high purity at the same time. That is also the aim of the invention. The invention is characterized in that the invention uses two solvents successively for recrystallization to get the lactide according to the features that the ethanol is conductive to the improvement of purification yield of lactide, but the molecular weight of the obtained polymer is not high, while the ethyl acetate is conductive to the improvement of the purity of the lactide and can get the polylactic acid with the high molecular weight by polymerization, but the yield is not high. Compared with the public recrystallization methods for the purification of lactide, the invention is characterized by higher yield of recrystallization product and easier obtainment of polylactic acid with high molecular weight by polymerization.
Owner:NANJING UNIV OF TECH
Method for synthesizing glucagon-like peptide (GLP)-1 analogue in solid-phase mode
InactiveCN102977204AReduce generationReduce the difficulty of purificationHormone peptidesPeptide preparation methodsSolid-phase synthesisGLP-1 Analogue
The invention relates to the field of polypeptide solid-phase synthesis and provides a method for synthesizing glucagon-like peptide (GLP)-1 analogue in a solid-phase mode. The method for synthesizing the GLP-1 analogue in the solid-phase mode solves the problems that in the process of synthesizing the GLP-1 analogue in the solid-phase mode, peptide deficiency products and by-products are generated because of difficult or incomplete amino acid sequence connection, and the by-products are resulted to be difficulty to separate in subsequent purification. The peptide deficiency products and by-products are quite same in properties. According to the method for synthesizing the GLP-1 analogue in the solid-phase mode, a segment convergent synthesis method and a substituent-introduced method are adopted in difficult-connection points and polypeptide synthesis yield coefficient is improved.
Owner:JILIN AOTENG BIOTECH
Cetrorelix purification and separation method
InactiveCN107312073AEfficient separationHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsFreeze-dryingEvaporation
The invention discloses a cetrorelix purification and separation method. The method includes steps: dissolving a crude product of cetrorelix in acetonitrile water solution, and filtering through a filter membrane to obtain crude solution for standby application; adopting a mobile phase A for balancing a reversed phase column, loading the crude solution into the reversed phase column, performing gradient eluting for separation and purification, wherein the mobile phase A refers to sodium dihydrogen phosphate aqueous solution, and a mobile phase B refers to acetonitrile; subjecting target peptide solution with purity higher than 99.5% to vacuum rotary evaporation and concentration at a water temperature not higher than 38 DEG C; adopting acetic acid aqueous solution for balancing the reversed phase column, loading a sample of concentrated liquid into the reversed phase column, and adopting an acetic acid aqueous solution / acetonitrile system for salt conversion; subjecting the converted acetate and the target peptide solution with purity higher than 99.5% to vacuum rotary evaporation and concentration at a water temperature not higher than 38 DEG C, and performing freeze drying to obtain powdery cetrorelix. The obtained cetrorelix is high in purity and yield, meets industrial production requirements and has a high economic value and a promising application prospect.
Owner:ZHEJIANG PEPTITES BIOTECH CO LTD
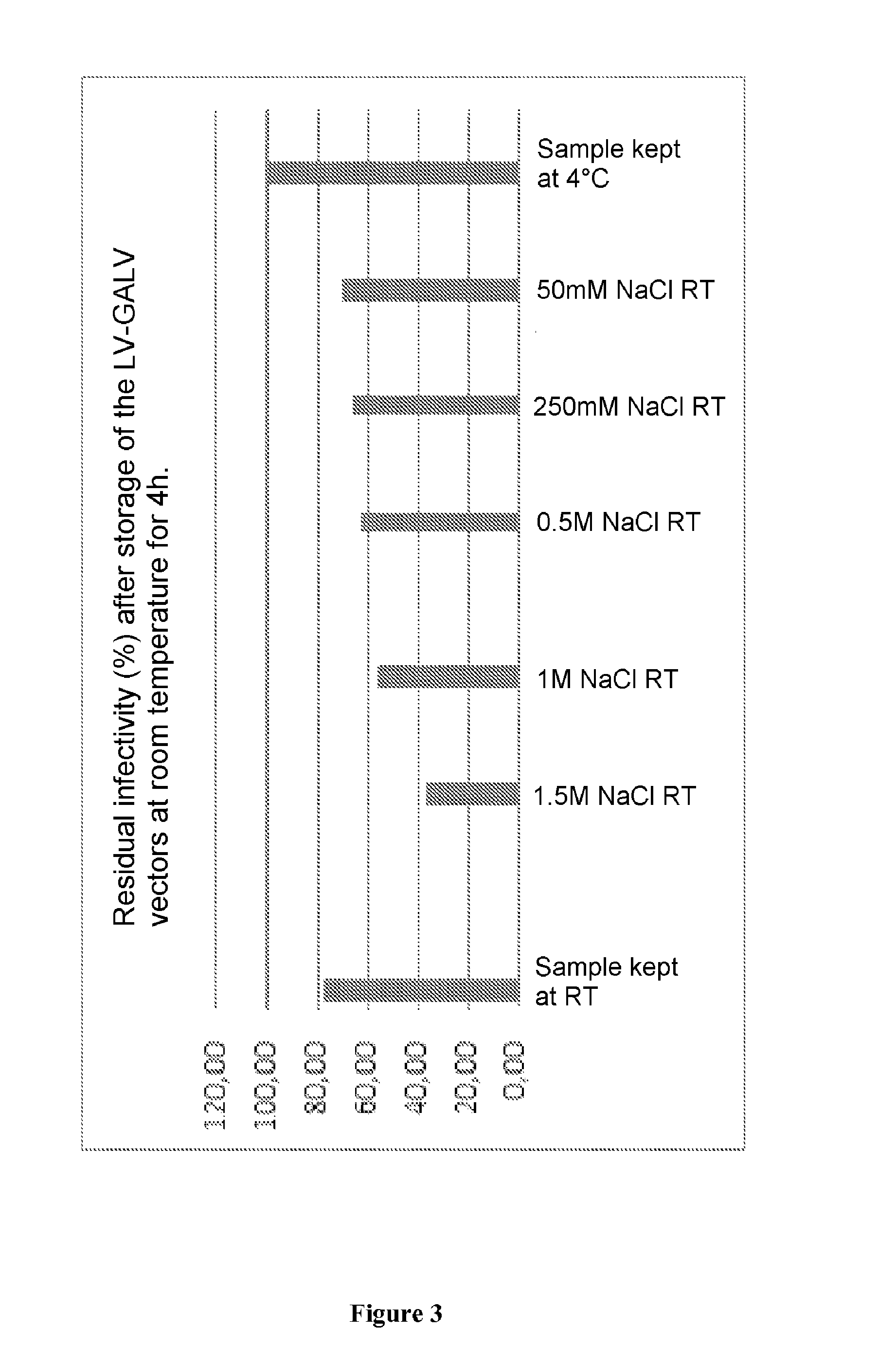
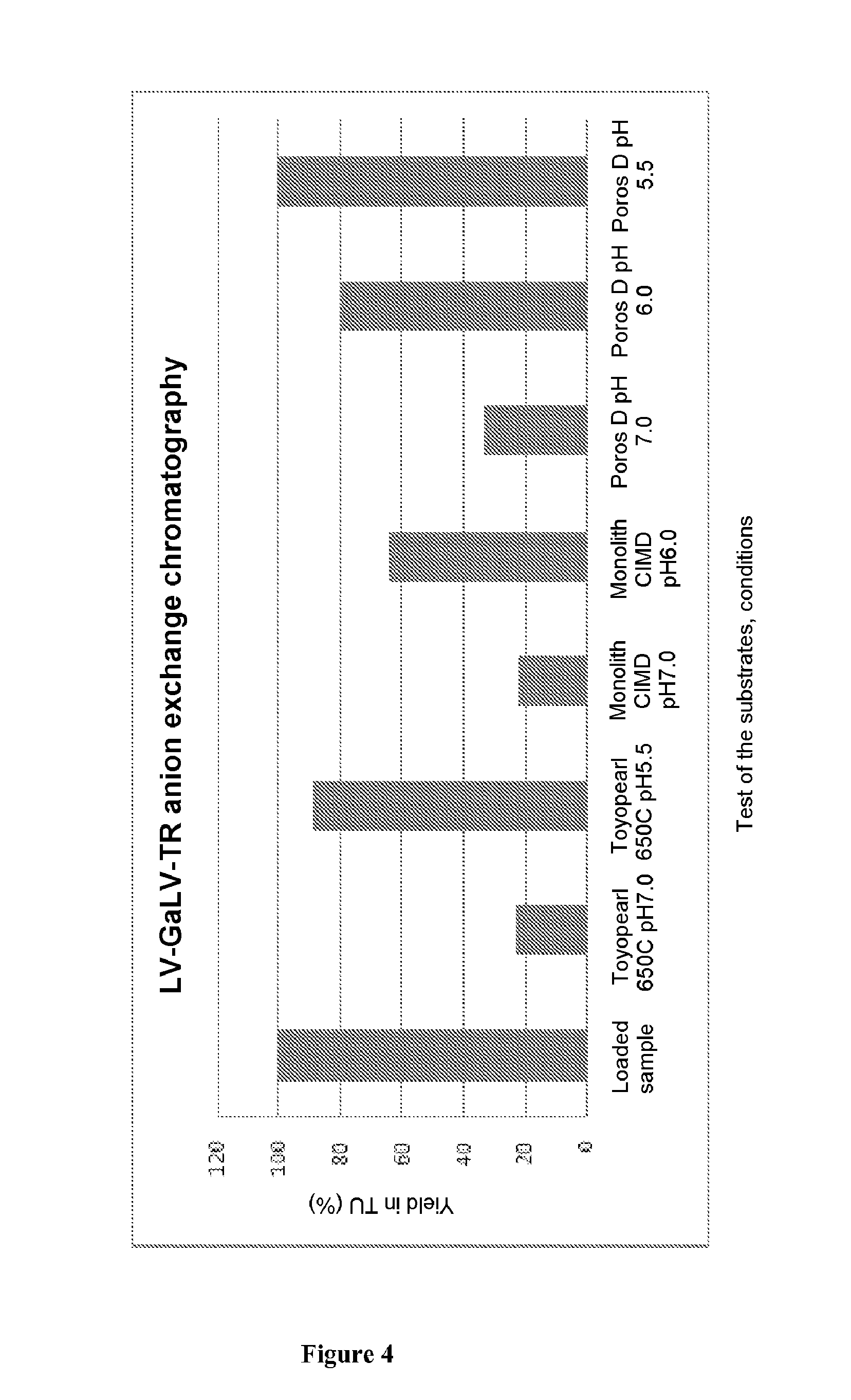
Method for purifying enveloped viruses or viral vectors
ActiveUS20170002332A1High purification yieldIncrease productionVector-based foreign material introductionReverse transcribing RNA virusesClinical gradeViral vector
The invention relates to a process for purifying enveloped viruses. The process of the invention is useful for recovering at a large scale enveloped viruses under conditions complying with good manufacturing practices and allowing viruses of a clinical grade to be obtained.
Owner:GENETHON
Preparation process of lactide
The invention discloses a preparing method of lactide in the macromolecular material preparing domain, which comprises the following steps: a. adopting L-lactic acid as raw material to obtain the low-molecular polymer of lactic acid; b. placing the low-molecular polymer of lactic acid in the reactor to react; controlling the reacting condition under normal pressure; heating to 150-250 deg.c; c. aerating inert gas into reactor at 200-500 deg.c; carrying the reacting product out of reactor; cooling to 80-150 deg.c; collecting; purifying extracted compound to obtain the product.
Owner:RUGAO FIBERGLASS FACTORY
Trelagliptin purification method
The invention relates to a trelagliptin purification method. The method comprises heating and dissolving trelagliptin in a mixed solvent, carrying out crystallization and separating solids, wherein the mixed solvent comprises isopropanol and one of methyl acetate, acetonitrile and ethanol. The trelagliptin purification method realizes a high trelagliptin yield and high trelagliptin purity.
Owner:CHONGQING PHARMA RES INST
Acidic proteinase and preparation method thereof
InactiveCN101948820AIncrease profitHigh extraction and purification yieldHydrolasesMicroorganism based processesLeather industryAspergillus niger
The invention relates to an acidic proteinase and a preparation method thereof and the acidic proteinase belongs to the microbial acidic proteinase. The invention is characterized in that 1) the enzymatic characteristics of the acidic proteinase are as follows: the optimum pH value is 2.5-3.5, the stable pH value is 2.5-6.0; the optimum temperature is 40-50 DEG C, the temperature stability range is 30-50 DEG C; and 2) the preparation method uses Aspergillus Niger which is stored in the China Center of Industrial Culture Collection (CICC) and has a preservation number of 2238, as the enzyme-producing strain and uses wheat bran as the raw material; the solid fermentation technology is adopted for preparation; the fermentation and enzyme-producing capability is not less than 47000u / g (dry yeast), the liquid enzyme yield is not less than 85% and the soid enzyme yield is not less than 80%, which all achieve the food grade sanitation standard. The invention provides the acidic proteinase and the preparation method thereof, wherein the utilization rate of the fermentation equipment is high, the byproduct of crop processing is used as the main raw material, the enzyme activity for fermentation is high, the extracting and purifying rate of enzyme is high and the production cost is low. The acidic proteinase of the invention is suitable to be used as the raw material or product additive of the products in the leather industry, the pharmaceutical industry, the brewing industry and the feed industry.
Owner:SHANDONG LONGKETE ENZYME PREPARATION
Affinity particle and affinity separation method
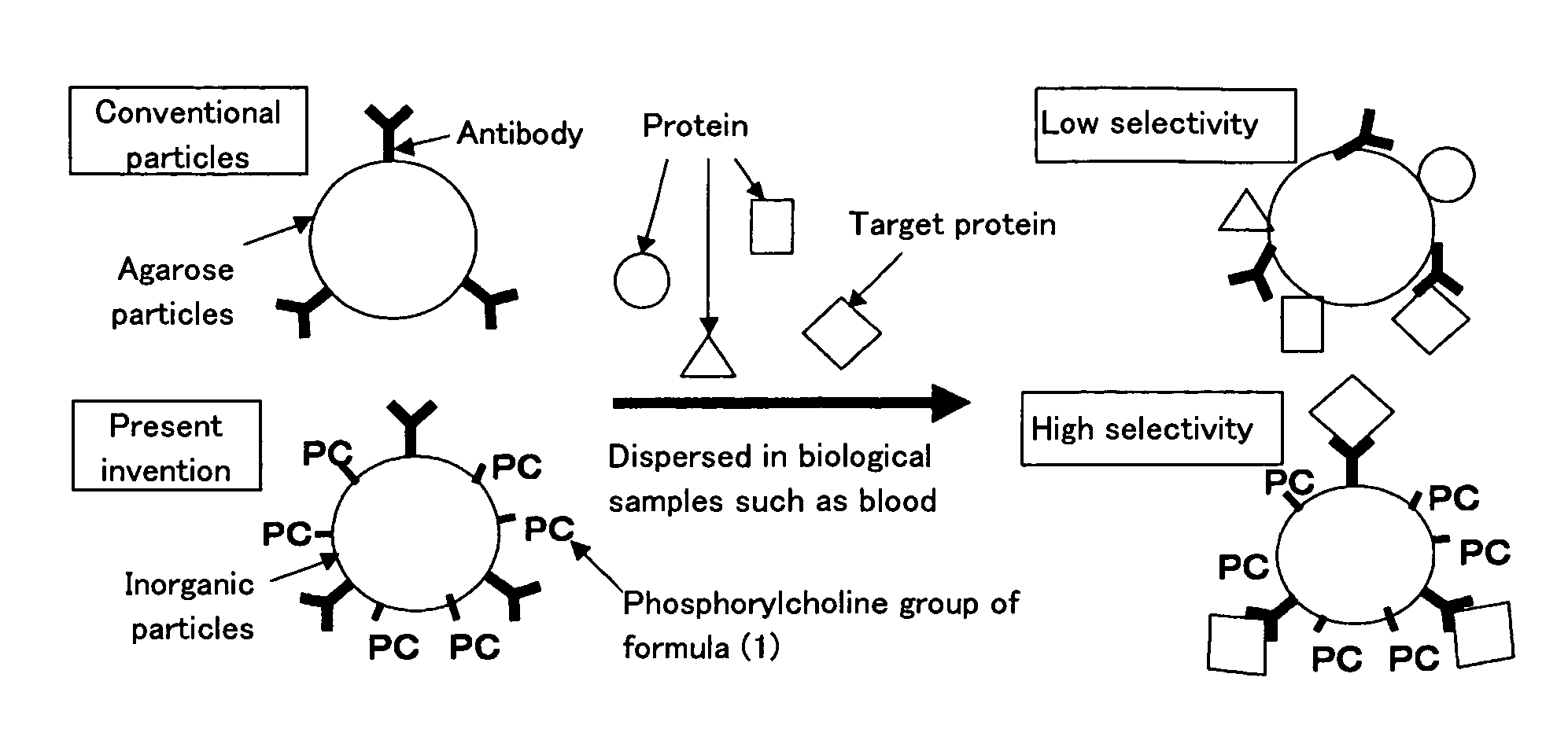
InactiveUS20070181503A1Inhibition of adsorptionHigh separation selectivityIon-exchanger regenerationGlass/slag layered productsInorganic particlePhosphorylcholine
The present invention is affinity particles that are characterized by having phosphorylcholine groups represented by the following formula (1) covalently bonded onto the surface of inorganic powder and also by having ligands having specific affinity with a certain target substance covalently bonded or adsorbed onto the surface of inorganic powder. The object of the present invention is to provide an affinity separation method that uses affinity particles utilizing inexpensive inorganic particles and is capable of separating the target substance easily and with high accuracy.
Owner:SHISEIDO CO LTD
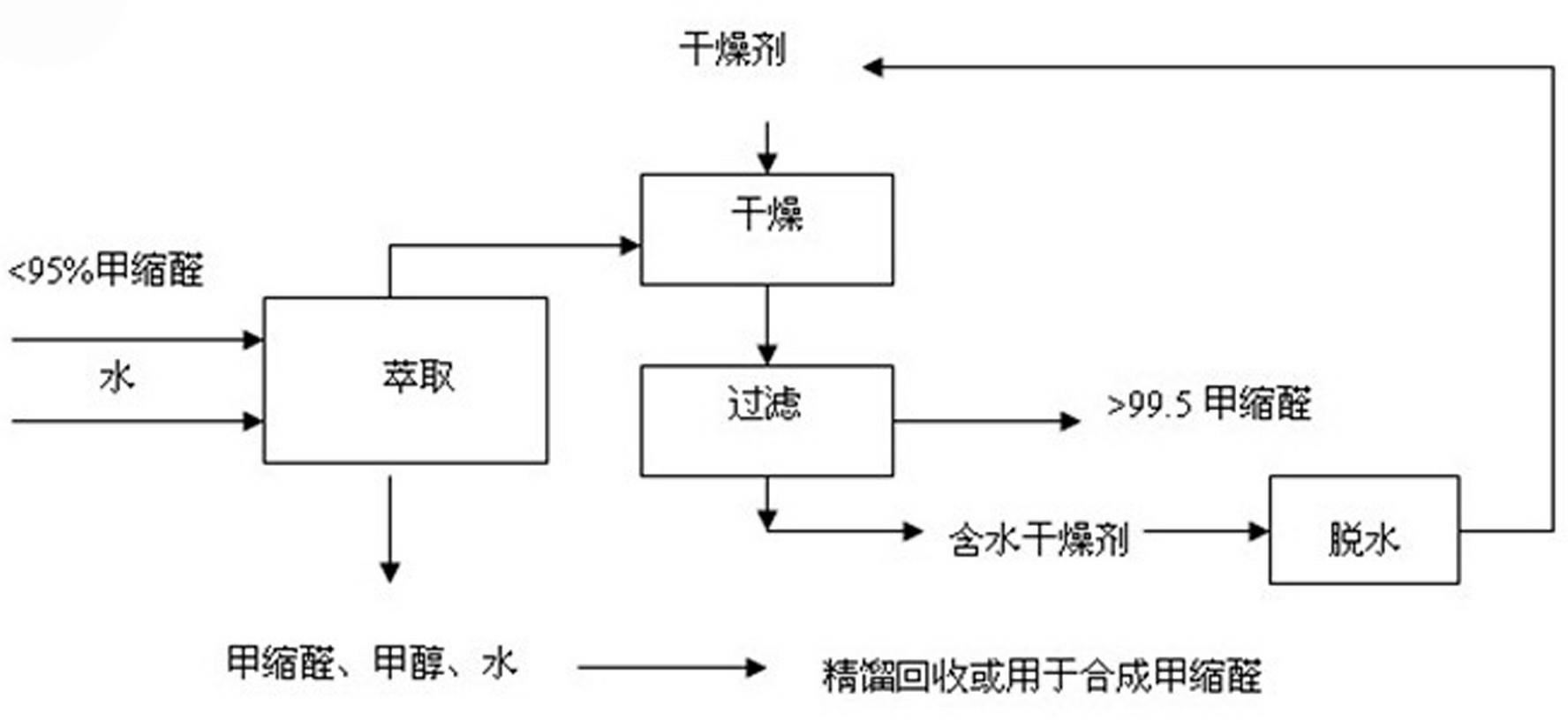
Method for purifying methylal
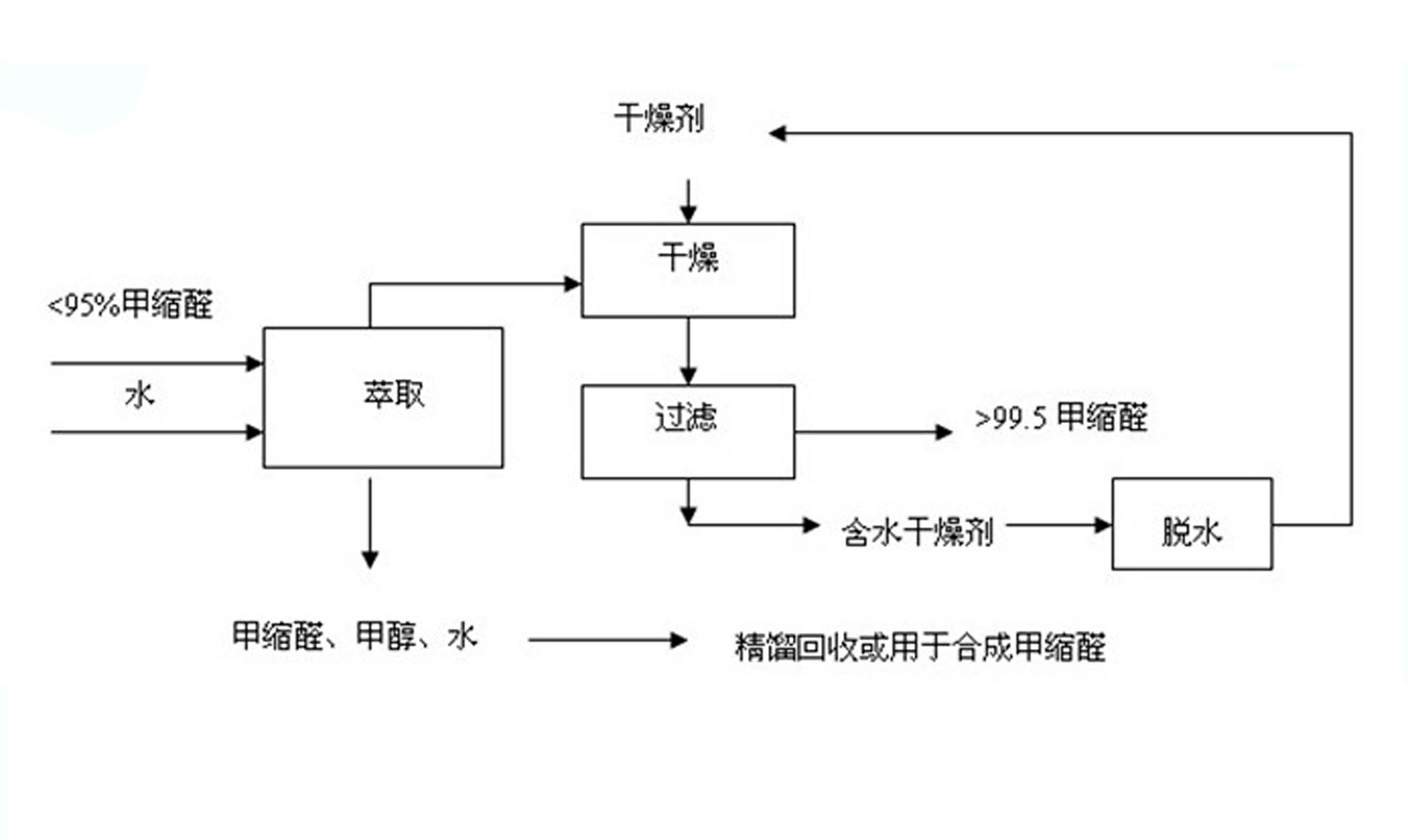
InactiveCN102320940AWide range of usesLower quality requirementsOrganic compound preparationHydroxy compound preparationDesiccantHazardous substance
The invention relates to a method for purifying methylal. The method comprises the following steps of: adding water into methylal of which the mass concentration is less than 95 percent and uniformly stirring, wherein the added water is 0.1-3 times the weight of the methylal; standing for 20-40 minutes for layering; adding a drying agent into an upper layer for drying, and stirring for 5-15 minutes, wherein the water sucking weight of the added drying agent is 1-12 times the wet weight of the methylal in the upper layer; and drying, and filtering the drying agent out to obtain the methylal of which the mass concentration is more than 99.5 percent, wherein the water-containing drying agent can be recycled by dehydrating. The method has the advantages: water is added into the methylal for extracting, and water is taken as an extracting agent, so that the cost is low, the purifying process is simple, the condition is mild, the purifying amount is large, industrial continuous production is facilitated, the mass concentration is stable and reliable, and other impurities are eliminated. The methylal is purified in a way of rectifying with a high-pressure rectifying system, so that the requirement on raw material quality is low, the purifying yield is high, the energy demand is low, industrial continuous production is facilitated, harmful substances are not discharged, and environmental protection is facilitated.
Owner:印海平
Acid protease and preparation method thereof
InactiveCN101638647AHigh enzyme productionImprove performanceHydrolasesPeptide/protein ingredientsMicroorganismYeast
The invention provides acid protease which is derived from microorganism sources and a preparation method thereof. The acid protease is characterized in that a. enzymology characteristics: the optimumpH is 2.5-3.5, the stable pH is 2.5-6.0, the optimum temperature is 40-50 DEG C, and the temperature stability ranges from 30 DEG C to 50 DEG C; b. with A. niger as an enzyme producing strain and wheat bran as main materials, the acid protease is prepared by solid fermentation process; the fermenting and enzyme producing capabilities are more than or equal to 47000u / g (based on dry yeast), the yield of liquid enzyme is more than or equal to 85% and that of solid enzyme is more than or equal to 80%, which all reach the food grade hygienic standards. The acid protease takes grain processing by-products as main raw materials and has high fermentation enzyme activity, high enzyme extraction purification yield and low production cost. The acid protease is suitable for serving as the preparation raw material or the additive of the products in such industries as tanning, medicine, brewing and feed.
Owner:SHANDONG LONGKETE ENZYME PREPARATION
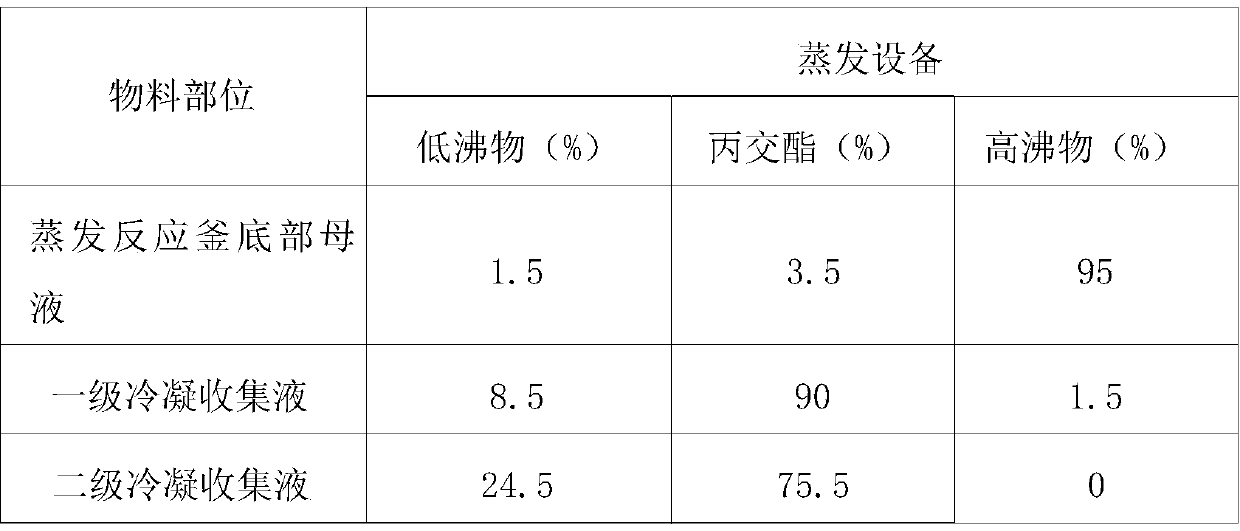
Method for industrial continuous preparation of high-purity lactide
The invention discloses a method for industrial continuous preparation of high-purity lactide. The method includes the following steps that of crude lactide evaporation and melt crystallization. Crudelactide evaporation includes the following steps that crude lactide is conveyed into evaporation equipment with a secondary condenser, heating and evaporation are carried out under vacuum conditions,and a lactide intermediate product with most high-boiling-point components removed is collected from a primary condenser. Melt crystallization includes the following steps that the lactide intermediate product is collected from the primary condenser and conveyed into a melt crystallizer, the melt crystallizer is used for cooling and crystallization, and mother liquor is discharged; crystals leftin the melt crystallizer are subjected to crystal sweating, and the high-purity lactide is collected. The new technology is short in process, convenient to operate, low in energy consumption and highin product yield and quality, the optical purity and chemical purity of lactide can both reach 99.6% or above, and the purification yield is 90% or above.
Owner:北京朗净汇明生物科技有限公司 +1
Preparation method of high-content kasugamycin aqueous solution
ActiveCN106818752AReduce bring inReduce salt contentBiocideSugar derivativesHigh concentrationAqueous solution
The invention relates to a preparation method of a high-content kasugamycin aqueous solution. By the method, high-concentration kasugamycin aqueous solution with the content being 5 to 8 percent can be prepared, the salt concentration of a product is extremely low, the use effect is good, use convenience is achieved, the use requirement of plane prevention with high requirement on concentration can be met, and the storage and transportation cost is low.
Owner:陕西绿盾生物药业创新中心有限公司
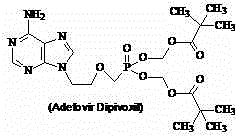
Adefovir dipivoxil monohydrate and preparation method thereof
ActiveCN104387421AImprove stabilityGood water solubilityGroup 5/15 element organic compoundsFreeze-dryingSolvent
The invention relates to adefovir dipivoxil monohydrate and a preparation method thereof. According to the preparation method, water-containing acetone is taken as a solvent and is subjected to freeze drying so as to generate freeze-dried powder of adefovir dipivoxil monohydrate. The preparation method is easy and convenient to operate, monohydrate can be selectively prepared, the yield of adefovir dipivoxil monohydrate is high, and the purity of adefovir dipivoxil monohydrate is good; the preparation method is very applicable to large-scale production.
Owner:SUZHOU ERYE PHARMA CO LTD
Technique for purifying recombined human interferon alpha 1b
ActiveCN1727361AShort processing cycleHigh purification yieldPeptide preparation methodsInterferonsChemistryInterferon Alfa-1b
A process for purifying recombinant human interferon alpha 16 includes such steps as breaking the engineered bacteria able to express human interferon alpha 16 in buffering Tris-HCl solution, adsorbing on expanding bed by use of anionic gel, buffering Tris-HCl solution and the eluting liquid containing NaCl and Tris-HCl, affinity chromatography by use of the gel coupled by its monoclonal antibody, PBS as balancing liquid and washing liquid, Gly-HCl as eluting liquid, Tris-HCl as regeneration liquid and NaAc-Hac as buffering liquid, and gel filtering by use of Sephacryl gel and PBS as buffering system.
Owner:SHENZHEN KEXING PHARMA CO LTD
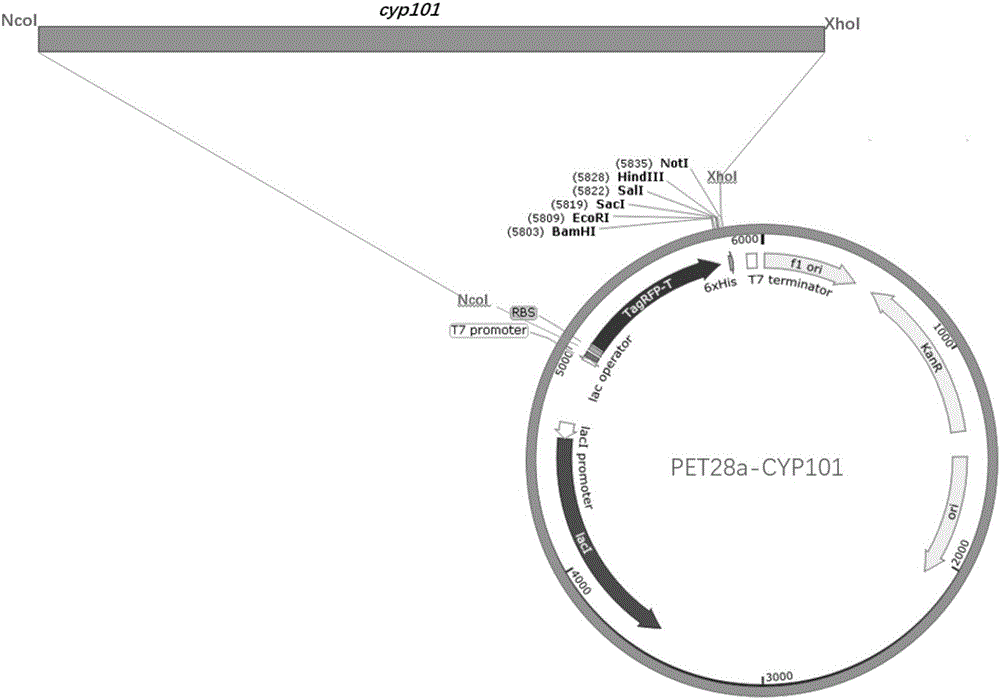
CYP101 enzyme recombinant vector, construction method thereof and CYP101 enzyme high-efficiency expression and purification method
InactiveCN106497956ASmall protein structureImprove expression levelBacteriaOxidoreductasesIon exchangeCulture mediums
The invention relates to a CYP101 enzyme recombinant vector, a construction method thereof and a CYP101 enzyme high-efficiency expression and purification method, and belongs to the field of biotechnology. A PET28a-CYP101 high-efficiency expression vector is constructed and transformed into BL21 plysS strain, and high-purity enzyme with purity higher than 95% is obtained by a simple two-step purification method including affinity chromatography and ion exchange chromatography after inducible expression. Compared with the prior art, CYP101 is simply and efficiently produced, the cost is low, purification is facilitated, and 23mg of target protein with the purity higher than 95% can be obtained by per liter of culture media. The CYP101 enzyme high-efficiency expression and purification method can be used for expressing and purifying CYP101 enzyme, the production efficiency of a laboratory is greatly improved, and experimental cost is greatly reduced. The CYP101 enzyme high-efficiency expression and purification method has an excellent application prospect for large-scale CYP101 enzyme production and purification.
Owner:SHANGHAI JIAO TONG UNIV
Method for producing recombinant human bone morphogenetic protein-2 mature peptide
ActiveCN102336829ARealize industrialized mass productionEasy to separatePeptide preparation methodsAnimals/human peptidesIon exchangeIon
The invention relates to a method for producing recombinant human bone morphogenetic protein-2 mature peptide. The method comprises the following steps: sampling a recombinant human bone morphogenetic protein-2 mature peptide solution with good renaturation into a balanced hydrophobic chromatography column, then performing a stepped-gradient elution which gradually reduces the salinity by an elution buffer solution, and collecting target peaks. In order to further enhance the protein purity of the target peaks, the purification method can be a multi-step hydrophobic interaction chromatography or be used by combining with the ion exchange chromatography. The method has the advantages of simple operation, lower cost, higher purification yield (more than 20%), higher purity (SDS-PAGE, HPLC and HPCE are greater than 97%) and the like, and is suitable for producing the recombinant human bone morphogenetic protein family.
Owner:HANGZHOU JIUYUAN GENE ENG
Method for purifying antibody and buffer solution used therein
ActiveCN107446044AHigh purification yieldReduce the ratioAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsMANNITOL/SORBITOLBuffer solution
The invention provides a method for purifying an antibody and a buffer solution used therein, belonging to the field of biomedicine. The method and the buffer solution are used for reducing aggregate formed in the low-pH value treatment process of antibody molecules. According to a technical scheme in the invention, the method comprises a step of adding mannitol with a certain concentration into the low-pH value buffer solution. The method can reduce the proportion of aggregate formed by IgG4 subtype antibodies (including a fusion protein containing an IgG4 subtype Fc segment) at a lower pH value to 2% from original 30%, so the purification yield of the antibody is improved, and the activity and safety of antibody drugs can be enhanced.
Owner:越海百奥药业(绍兴)有限公司
Method for preparing enzyme of dissolving staphylococcal bacteria, its derivative, and method for preparing the derivative
ActiveCN101092618AExpression scheme is simpleSimple purification processBacteriaRecombinant DNA-technologyEscherichia coliProtein target
This invention relates to a method for preparing lysostaphin and its derivatives. The method comprises: designing and synthesizing lysostaphin gene, inserting lysostaphin gene into the digestion site of expression vector (plasmid pET), transforming E. coli, screening positive clones, culturing, inducing the expression of target protein, collecting E. coli, lysing, centrifuging, collecting the supernatant, and purifying the supernatant through chromatography. The method has such advantages as simple process, high expression level, simple purification, high yield, soluble product, and no requirement for renaturation, and is suitable for mass production. The lysostaphin derivatives are obtained by modifying lysostaphin with polyethylene glycol, and have such advantages as low immunogenicity and long half value period. The lysostaphin and its derivatives are suitable for treating Staphylococcus infection in clinic.
Owner:HANGZHOU LION BIOTECH
Crystallization purification method for paclitaxel
InactiveCN106243066AMeet the requirementsImprove the purification effectOrganic chemistry methodsAlkaneImpurity
The invention discloses a method for preparing a crude paclitaxel product prepared from a specific three-component system consisting of dichloromethane, acetone and alkane through crystallization purification and having a structure as shown in the following formula which is described in the specification. The method comprises the following steps: 1) dissolving the crude paclitaxel product in a mixed solution of dichloromethane and acetone; 2) adding alkane into the mixed solution drop by drop so as to allow paclitaxel to be precipitated; and 3) carrying out filtering and drying so as to obtain a high-purity paclitaxel product. The method provided by the invention has excellent purifying effect and can prepare paclitaxel with HPLC purity of 99% or above; the method has a wide application scope and exerts substantial purifying effect on almost all the impurities including specific impurities hard to purify, such as 7-epitaxol; the method is simple to operate, only needs simple reaction tanks and is suitable for large-scale production; and the method is high in purification yield, wherein crystallization yield is 92% or above.
Owner:SHANGHAI BIOMAN PHARMA
Method for purifying L-valine from L-valine complex
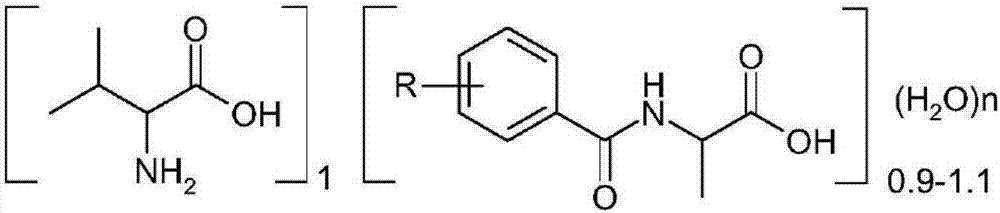
ActiveCN107488129AHigh purityEfficient use ofOrganic compound preparationAmino-carboxyl compound preparationPurification methodsOrganic solvent
The present invention relates to a method for purifying L-valine from a L-valine complex. The method comprises: A, complexing racemic DL-valine and a resolving agent N-benzoyl-L-alanine to obtain an L-valine complex, adding 0.5-5 times the organic solvent to the L-valine complex, and carrying out heating reflux for 1-3 h in a reaction kettle; B, filtering the mixture obtained in the step A while hot, returning the solid to the kettle, adding 0.5-5 times the organic solvent, carrying out stirring reflux for 1-3 h, repeatedly performing three times, and filtering to obtain an L-valine crude product; C, adding 10-20 times the water to the crude product obtained in the step B, carrying out heating dissolving, decolorizing with active carbon, and filtering to obtain a decolorizing liquid; and D, carrying out pressure reducing concentration crystallization on the decolorizing liquid obtained in the step C in a crystallization kettle, filtering, and drying the wet product to obtain the L-valine finished product. According to the present invention, the hetero amino acids are not generated, the purification yield is obviously improved, the finished product quality is improved, the decolorizing liquid separation with the adsorption column in the traditional purification method is eliminated, and the operation is simple.
Owner:HUANGGANG WELLMAN BIOSCI
Human apolipoprotein AI genetic engineering preparation method and expression vector and engineering bacteria thereof
InactiveCN101921793AIncrease productionOvercome the problem of being easily degradedApolipeptidesBacteriaEscherichia coliProtein target
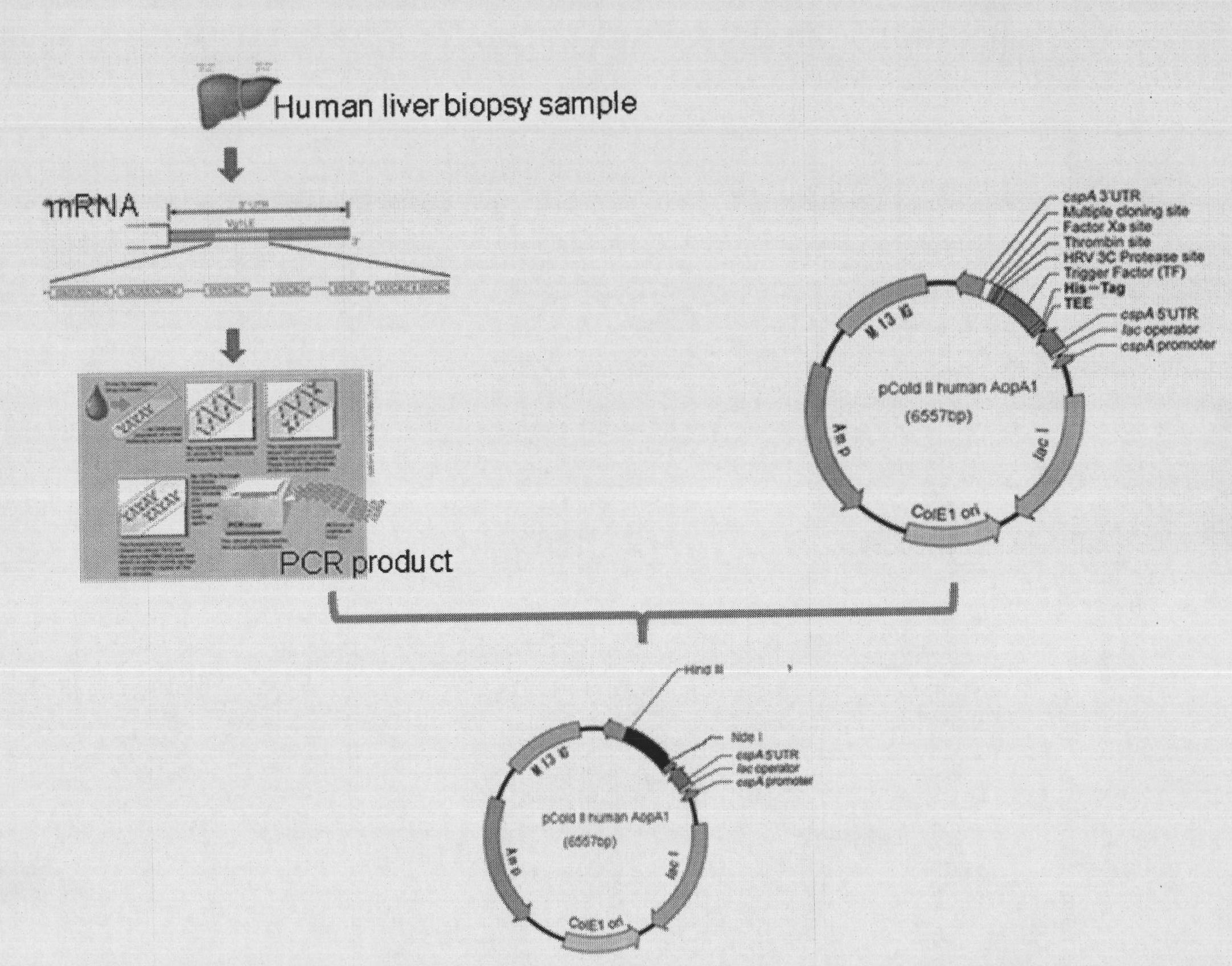
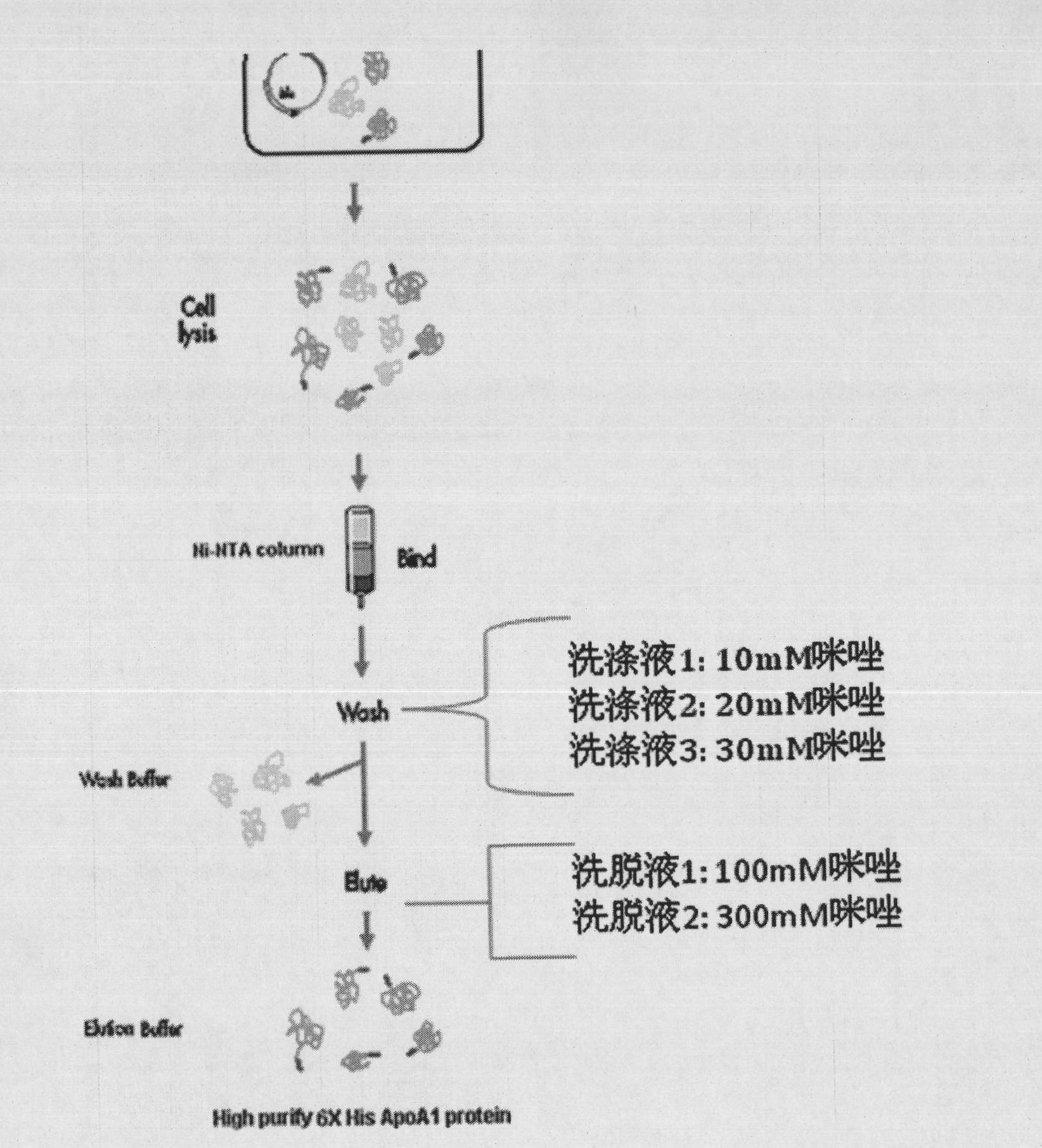
The invention provides a high efficiency soluble human apolipoprotein AI genetic engineering preparation method and expression vector and engineering bacteria thereof. Plasmid pCold containing recombinant human apolipoprotein AI gene sequence is selected as fusion expression vector, ApoA1 coding sequence is arranged between Nde I and Hind III endonuclease site on pCold vector. The engineering bacteria is escherichia coli, and the escherichia coli is converted into pCold-ApoA1 / Rosetta-gami by virtue of the expression vector plasmid pCold of the invention, and low temperature culture is carried out at 10-20 DEG C, thus inducing expression of soluble target protein. The invention adopts nickel crosslinking affinity column one-step method, operation is simple, yield is high, time is short, and the purity of the obtained target protein is higher than 90%, thus being applicable to industrialized mass production.
Owner:DIASYS DIAGNOSTIC SYST SHANGHAI
Medicine for curing diarrhea and its preparing process
InactiveCN101100690AHigh activityRealize industrial productionPeptide/protein ingredientsDigestive systemPharmaceutical drugBiochemistry
Process is concerned with high active human enteric trilobite factor protein and product concerned. Also, its usage in preparation of pharmaceutics is disclosed herewith. Meanwhile, ITF protein with high ratio of double bodies is obtained with low cost.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com