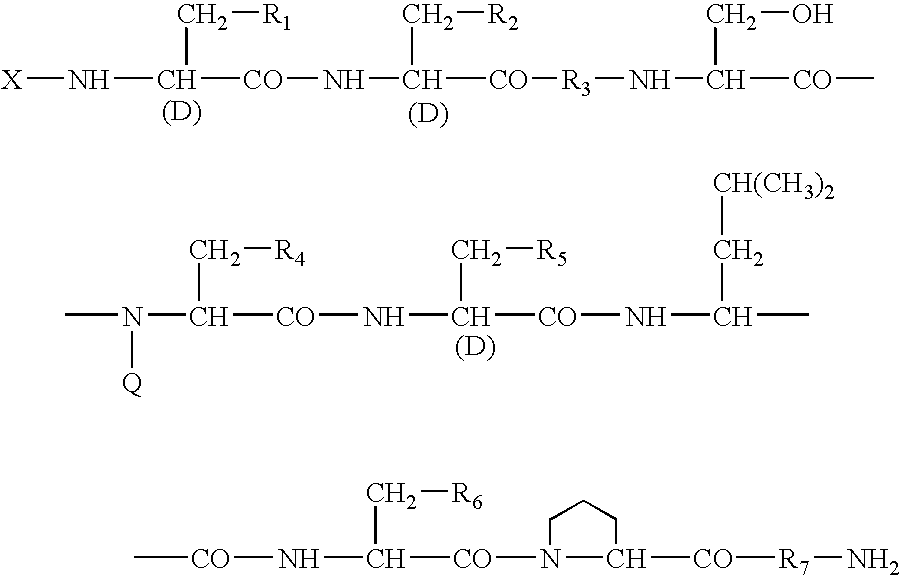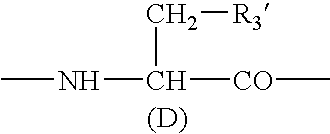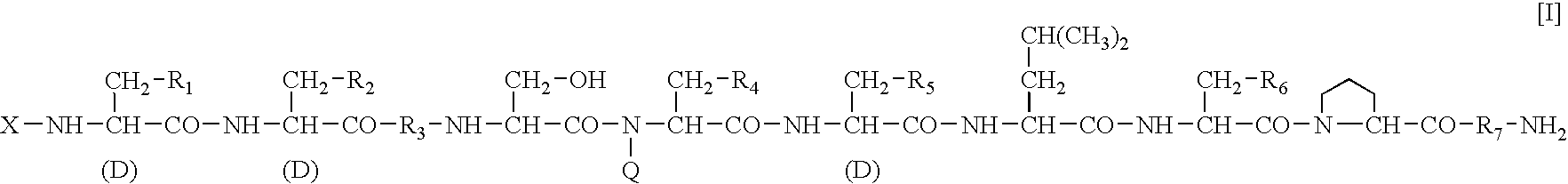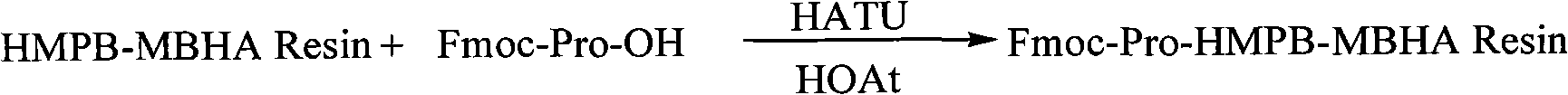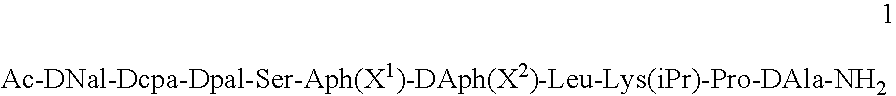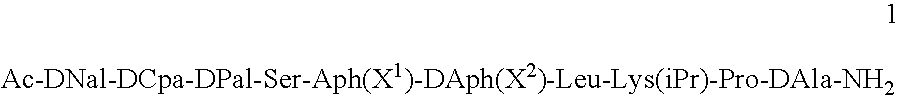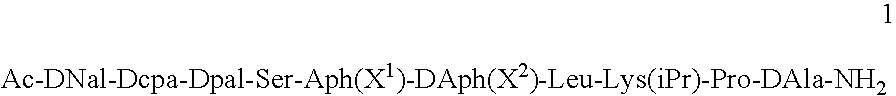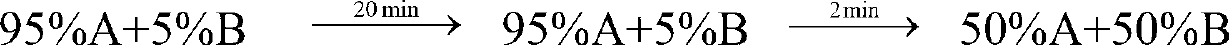Patents
Literature
322results about "Luteinising hormone-releasing hormone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
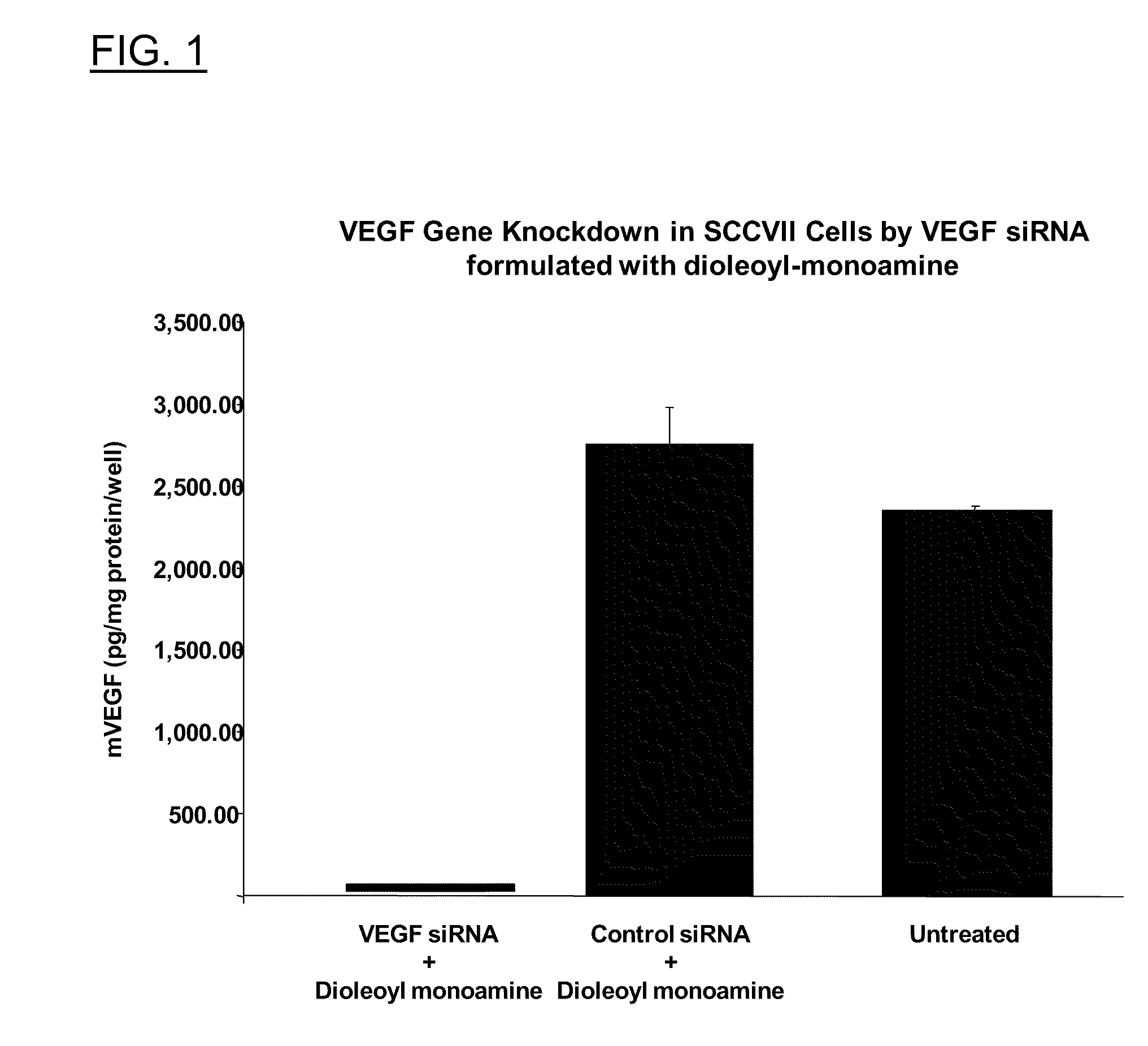
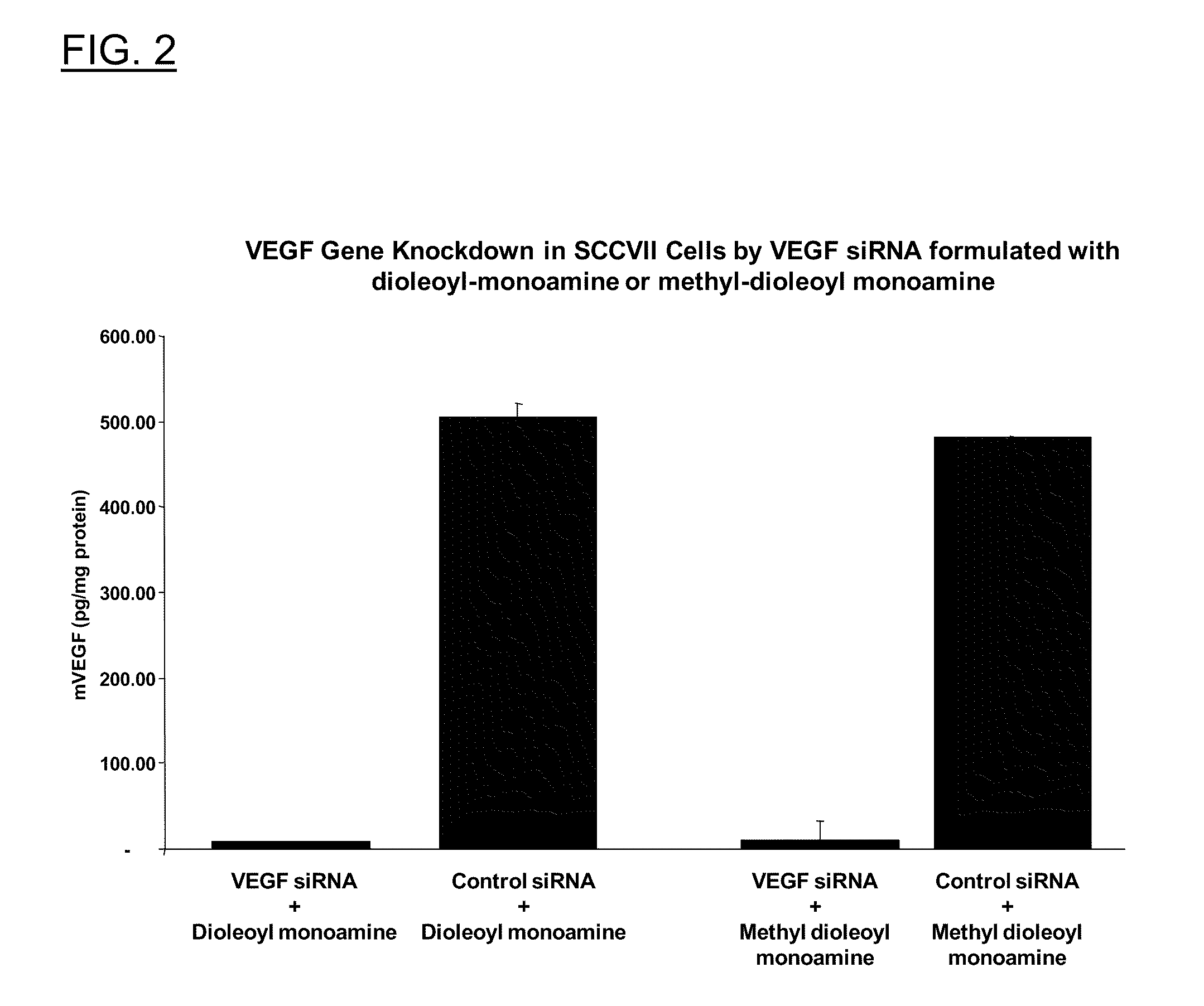
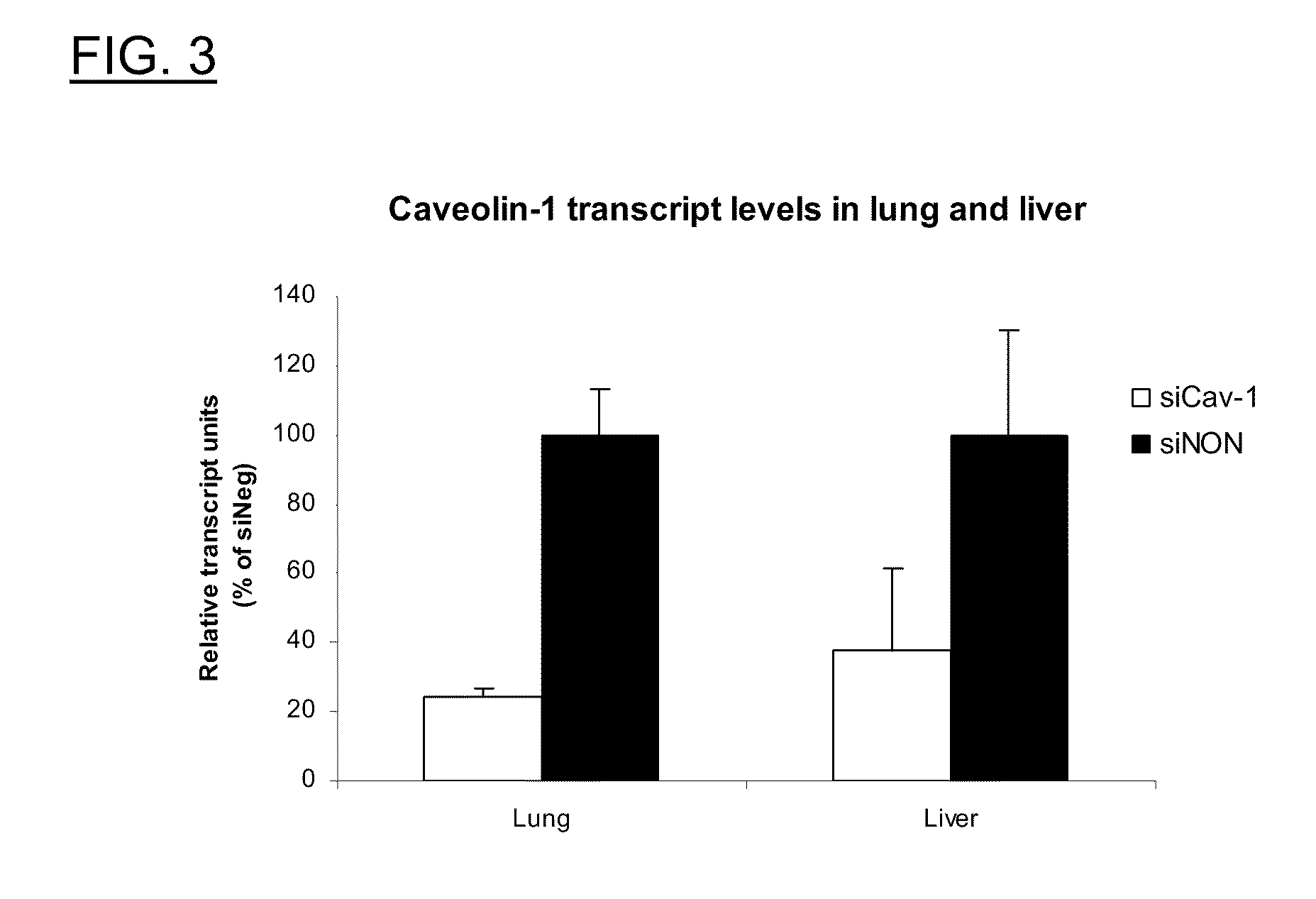
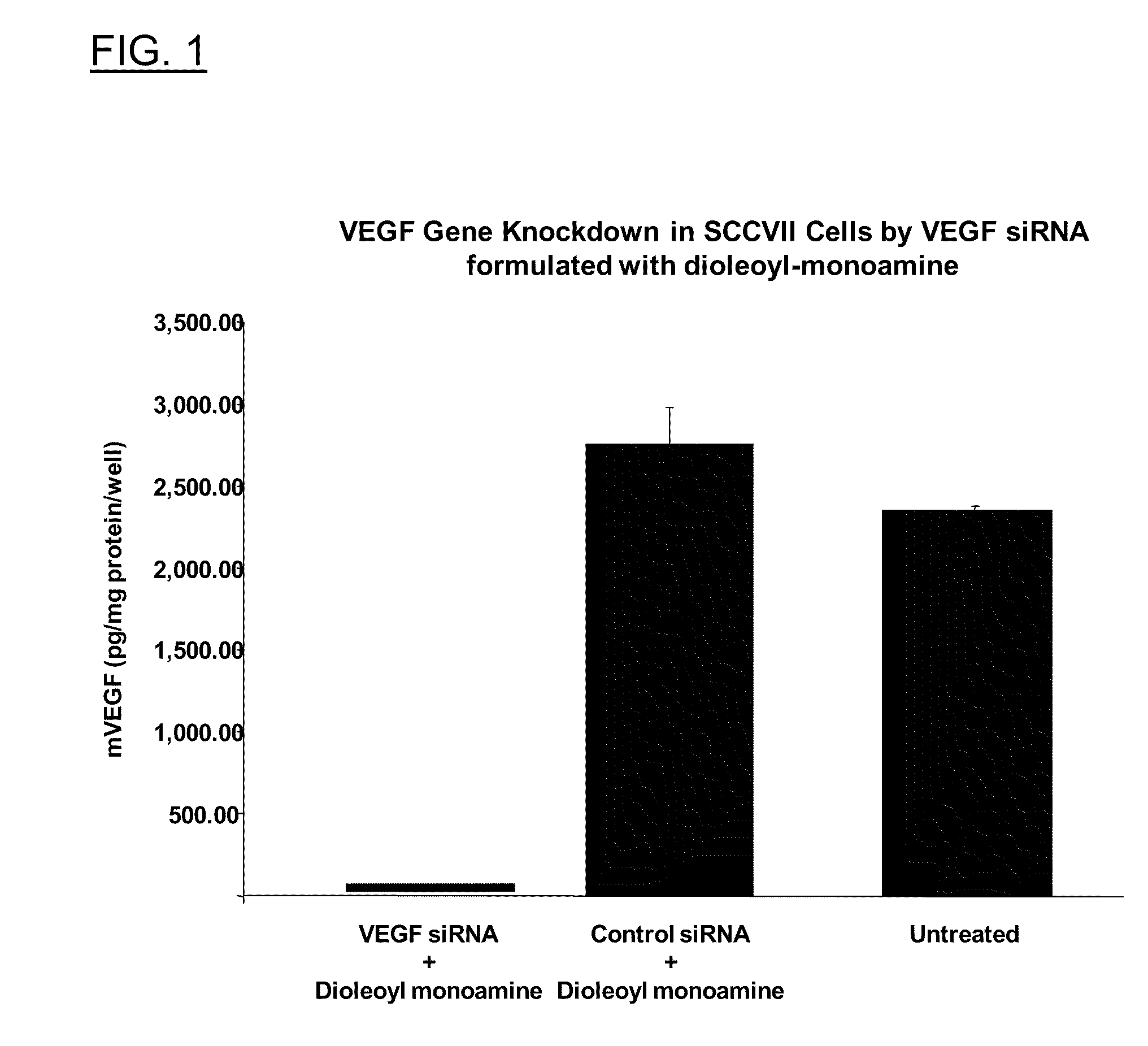
Polyamine Derivatives
ActiveUS20100260817A1Improve efficiencyImproving of local deliveryOrganic active ingredientsSenses disorderWhole bodyMedicinal chemistry
Owner:CLSN LAB
Polyamine derivatives
ActiveUS8460696B2Reduce deliveryImprove efficiencyOrganic active ingredientsSenses disorderMedicinal chemistryPolyamine
Owner:CLSN LAB
Ligand/lytic peptide compositions and methods of use
InactiveUS6635740B1Prevents sexual maturationInhibition of maturationHormone peptidesPeptide/protein ingredientsLytic peptideAbnormal tissue growth
Amphipathic lytic peptides are ideally suited to use in a ligand / cytotoxin combination to specifically inhibit cells that are driven by or are dependent upon a specific ligand interaction; for example, to induce sterility or long-term contraception, or to attack tumor cells, or to selectively lyse virally-infected cells, or to attack lymphocytes responsible for autoimmune diseases. The peptides act directly on cell membranes, and need not be internalized. Administering a combination of gonadotropin-releasing hormone (GnRH) (or a GnRH agonist) and a membrane-active lytic peptide produces long-term contraception or sterilization in animals in vivo. Administering in vivo a combination of a ligand and a membrane-active lytic peptide kills cells with a receptor for the ligand. The compounds are relatively small, and are not antigenic. Lysis of gonadotropes has been observed to be very rapid (on the order of ten minutes.) Lysis of tumor cells is rapid. The two components-the ligand and the lytic peptide-may optionally be administered as a fusion peptide, or they may be administered separately, with the ligand administered slightly before the lytic peptide, to activate cells with receptors for the ligand, and thereby make those cells susceptible to lysis by the lytic peptide. The compounds may be used in gene therapy to treat malignant or non-malignant tumors, and other diseases caused by clones or populations of "normal" host cells bearing specific receptors (such as lymphocytes), because genes encoding a lytic peptide or encoding a lytic peptide / peptide hormone fusion may readily be inserted into hematopoietic stem cells or myeloid precursor cells.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Methods and pharmaceutical compositions for gnrh-II and gnrh-II modulation of t-cell activity, adhesion, migration and extravasation
Methods and compositions comprising GnRH-I and GnRH-II, GnRH-I and GnRH-II antibodies, anti-receptor antibodies, polynucleotide constructs and GnRH-I and GnRH-II analogs for immune enhancement and suppression, prevention and treatment of diseases and conditions characterized by abnormal T-cell activity, treatment of viral and prion-related diseases, and treatment of T-cell related neoplastic diseases are disclosed.
Owner:KOCH YITZHAK PROF +1
Gonadotropin releasing hormone antagonists in gel-forming concentrations
InactiveUS20050245455A1Peptide/protein ingredientsLuteinising hormone-releasing hormoneActive agentGnRH Antagonist
Pharmaceutical compositions are provided for the treatment of steroid-dependent and other diseases. The compositions are solutions for subcutaneous or intramuscular injection, and the active agent is a GnRH antagonist peptide according to general formula (1): Ac-DNal-DCpa-DPal-Ser-Aph(X1)-DAph(X2)-Leu-Lys(iPr)-Pro-DAla-NH2 present at a concentration sufficient to from a gel following administration.
Owner:FERRING BV
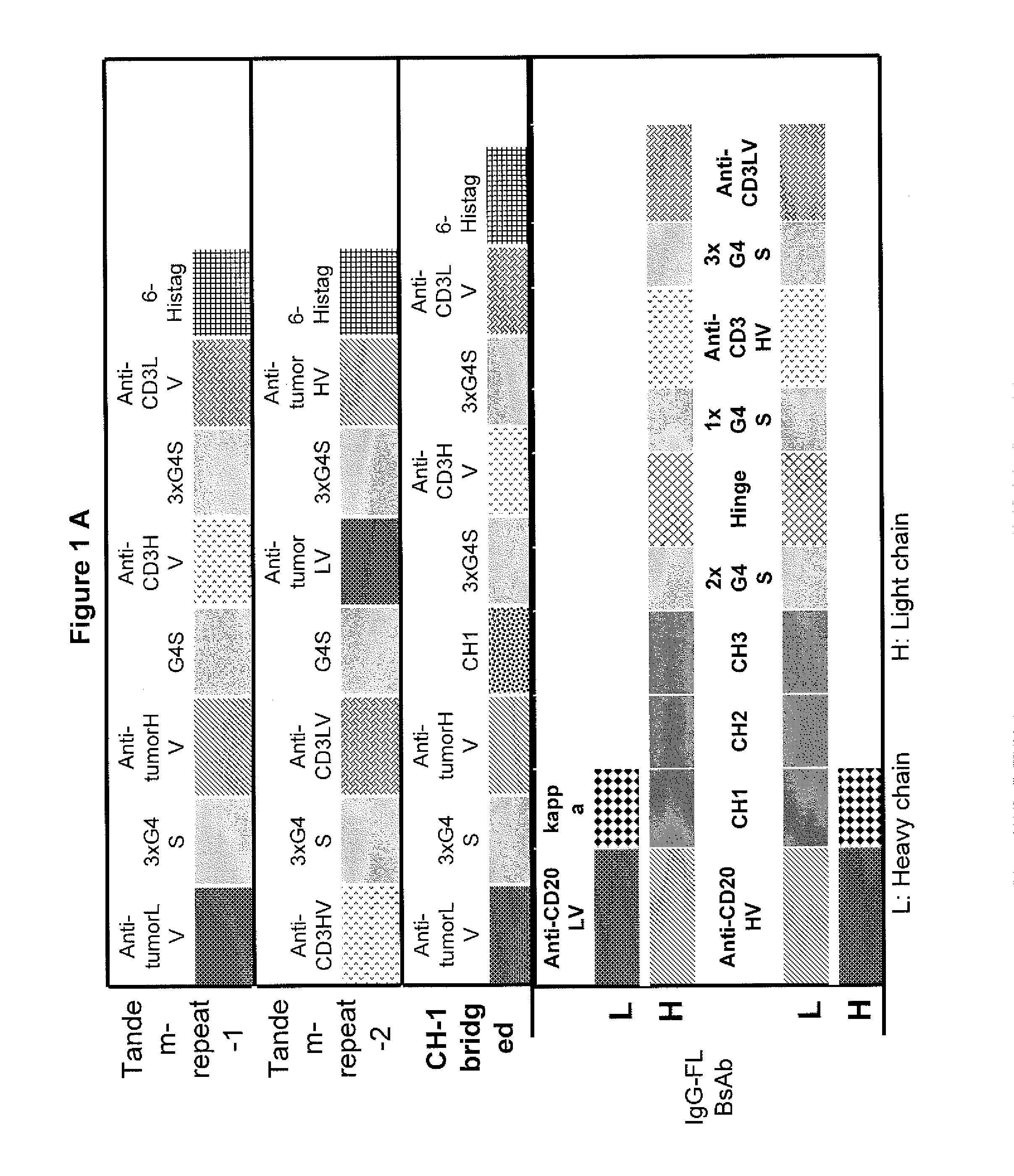
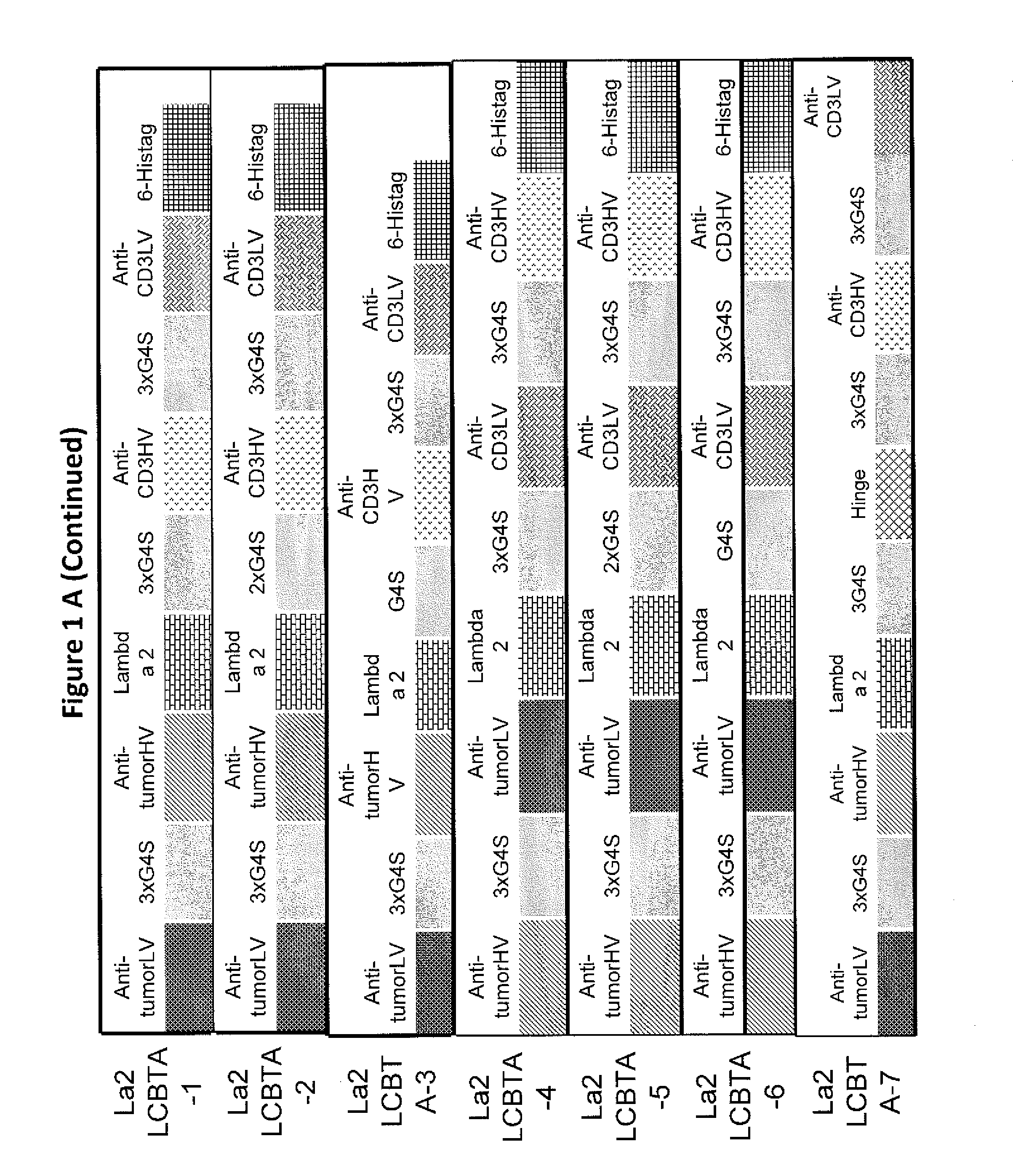
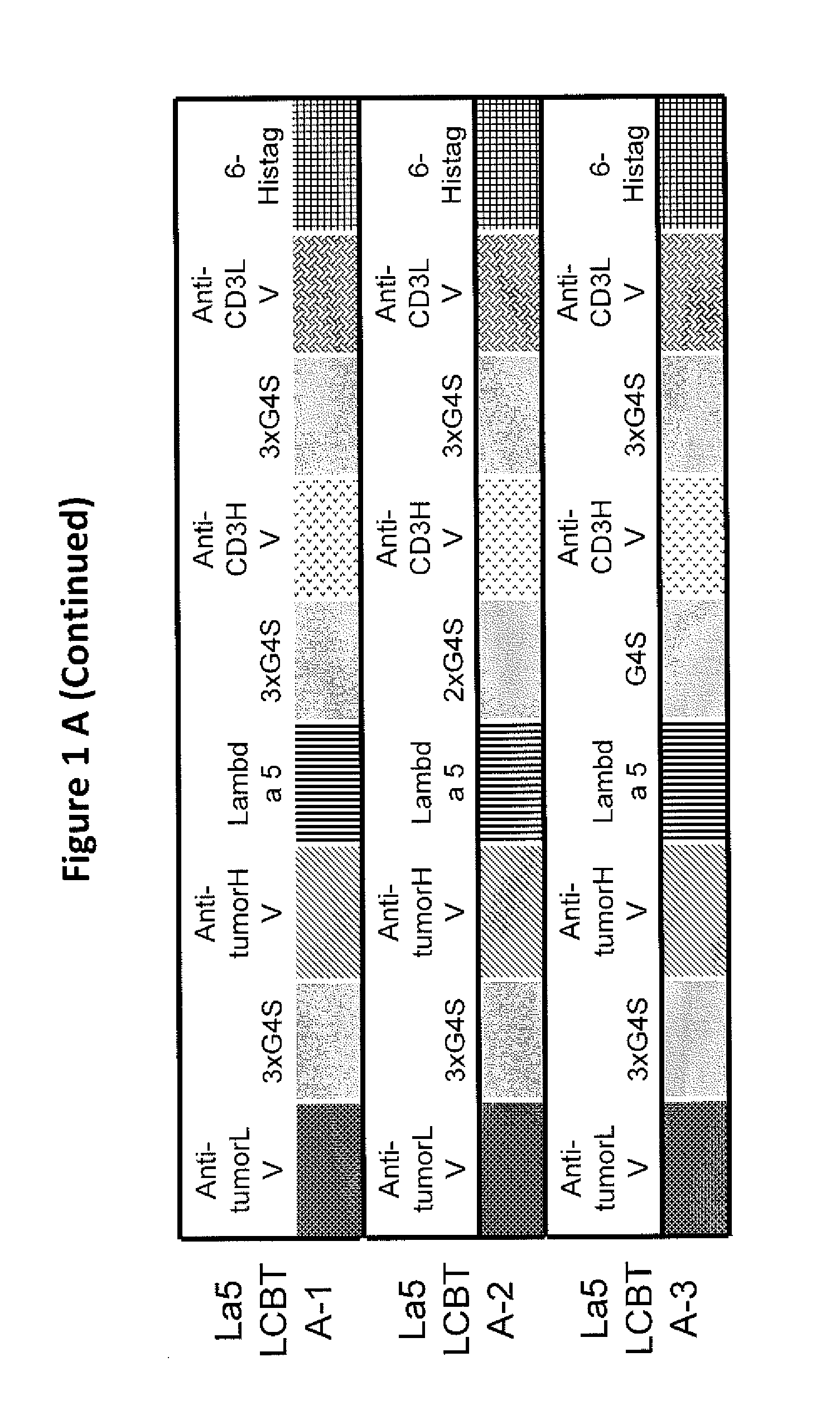
Light chain-bridged bispecific antibody
InactiveUS20130165638A1Hybrid immunoglobulinsAntibody mimetics/scaffoldsBispecific antibodyClinical therapy
This invention describes a novel format of monomeric bispecific fusion protein with immune activating property for clinical therapies. A bispecific fusion protein includes a first targeting domain with a specificity for a first target of interest; a bridging domain derived from a constant region of a light chain or heavy chain of an immunoglobulin, which may be a human immunoglobulin; and a second targeting domain with a specificity for a second target of interest. The bispecific fusion protein may further include a linker fused to the N-terminus or the C-terminus of the bridging domain. The first targeting domain is fused to the bridging domain and the second targeting domain is fused to the bridging domain or the linker. The linker may include a GGGGS sequence. The first target of interest may be CD20, Her2 / neu, or EpCAM, and the second targeting domain is a T-lymphocyte activating domain.
Owner:DEV CENT FOR BIOTECHNOLOGY +1
Application of initial doses of LHRH analogues and maintenance doses of LHRH antagonists for the treatment of hormone-dependent cancers and corresponding pharmaceutical kits
ActiveUS20080032935A1Negative hormone withdrawal symptomsPeptide/protein ingredientsLuteinising hormone-releasing hormoneGynecologyInitial dose
LHRH analogues and LHRH antagonists for use in the treatment or prophylaxis of hormone-dependent cancers, in particular prostate cancer, prostate carcinoma and / or advanced prostate carcinoma, by administering an initial dose of an LHRH analogue over a first period sufficient to effect hormonal castration, then administering a maintenance dose of an LHRH antagonist over a second period, the dose being insufficient to achieve and / or maintain hormonal castration.
Owner:AETERNA ZENTARIS GMBH
Sustained-release preparation
A sustained-release preparation which comprises a physiologically active peptide of general formulawherein X represents an acyl group;R1, R2 and R4 each represents an aromatic cyclic group;R3 represents a D-amino acid residue or a group of the formulawherein R3′ is a heterocyclic group;R5 represents a group of the formula —(CH2)n—R5′ wherein n is 2 or 3, and R5′ is an amino group which may optionally be substituted, an aromatic cyclic group or an O-glycosyl group;R6 represents a group of the formula —(CH2)n—R6′ wherein n is 2 or 3, and R6′ is an amino group which may optionally be substituted;R7 represents a D-amino acid residue or an azaglycyl residue; andQ represents hydrogen or a lower alkyl group, or a salt thereof and a biodegradable polymer having a terminal carboxyl group.The sustained-release preparation shows a constant release of the peptide over a long time and is substantially free from an initial burst.
Owner:TAKEDA PHARMA CO LTD
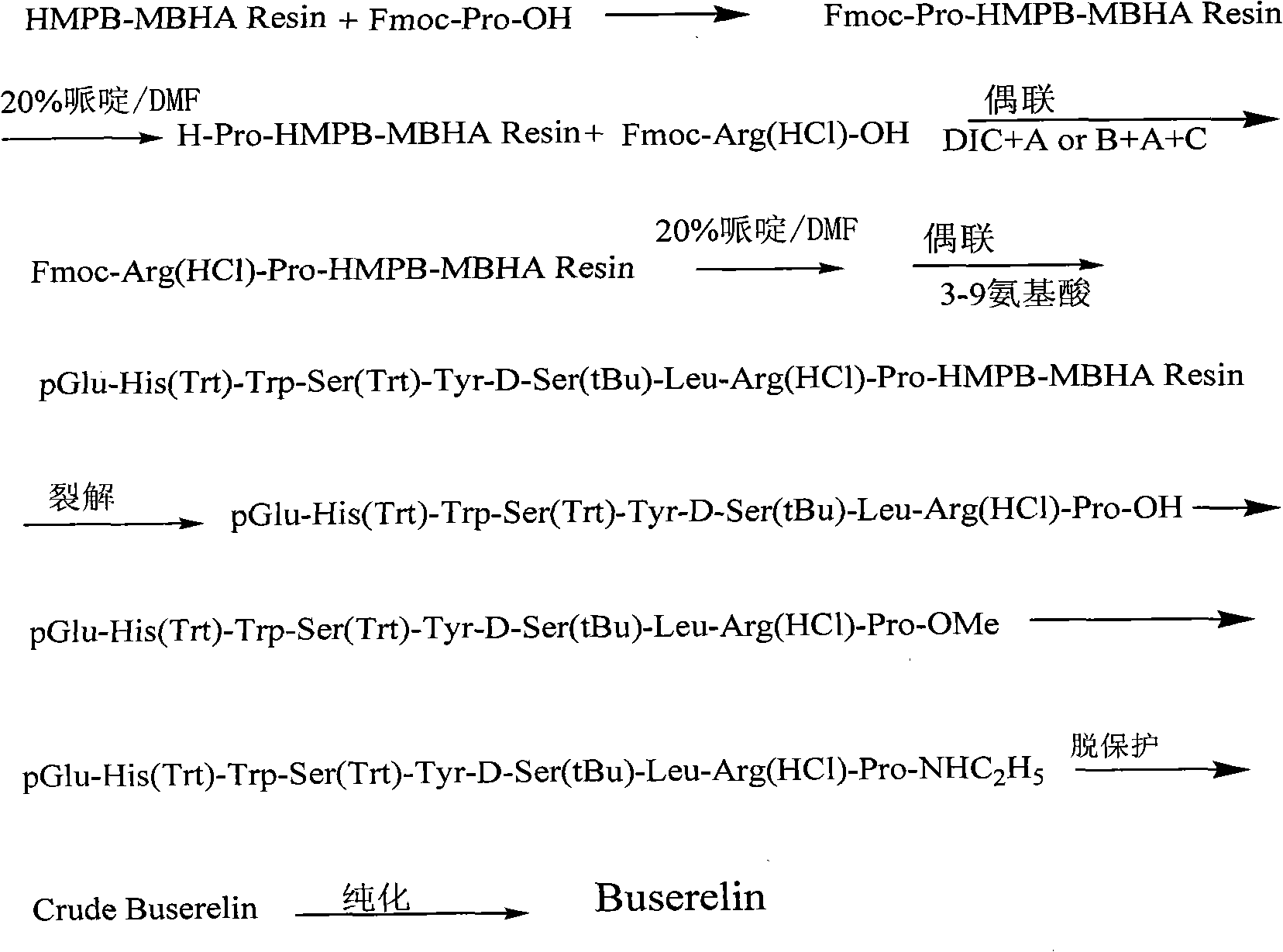
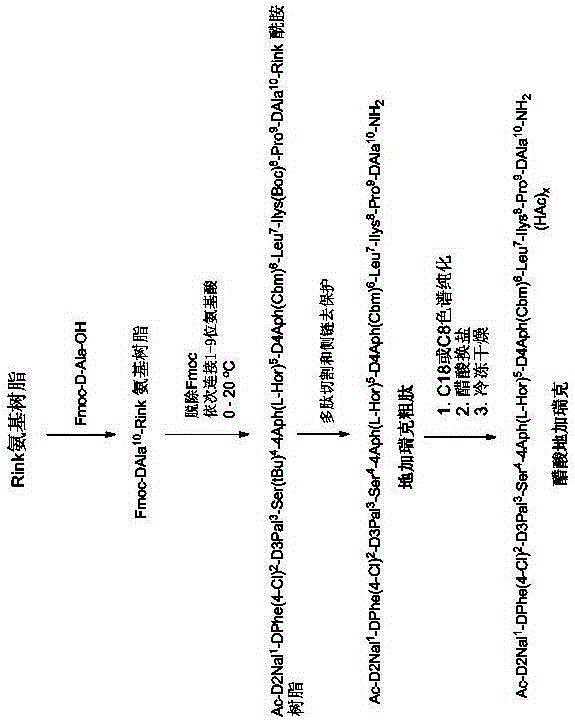
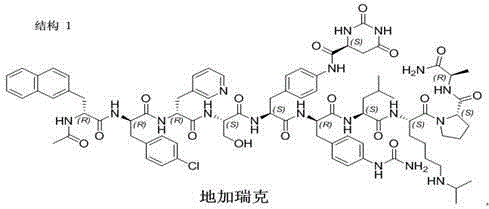
Solid-phase preparation method for buserelin
InactiveCN101935339AHigh yieldLow costLuteinising hormone-releasing hormonePeptide preparation methodsPeptide sequenceCombinatorial chemistry
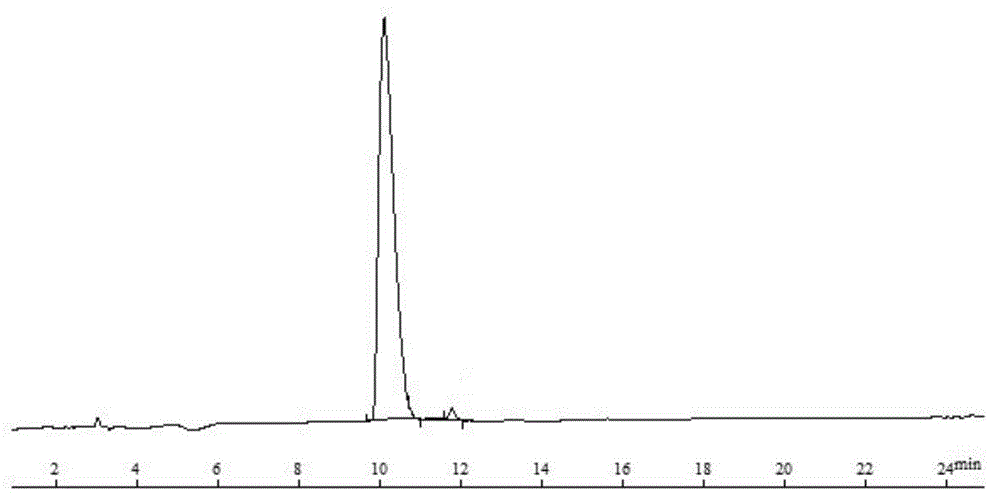
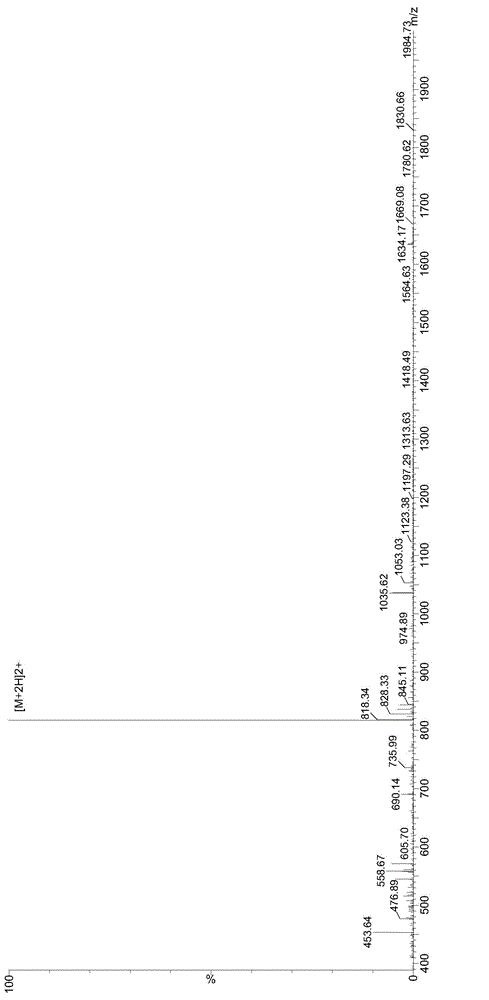
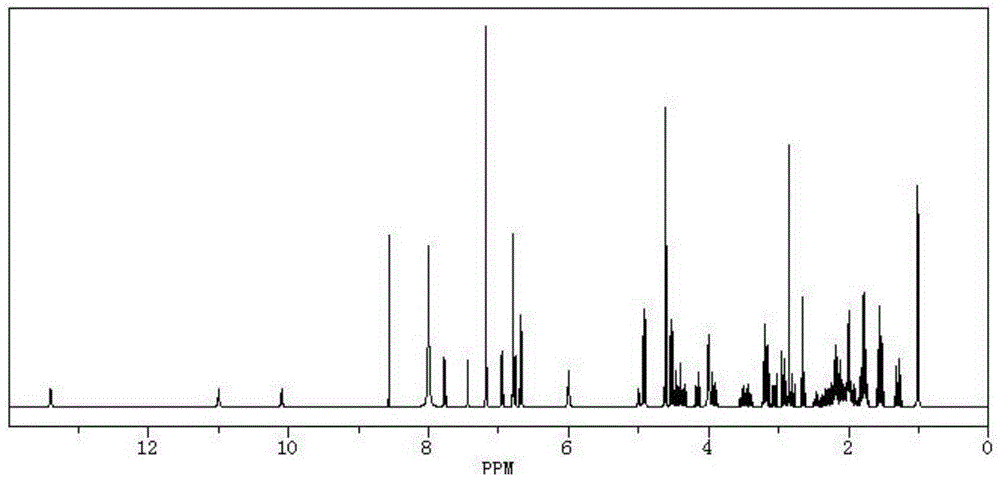
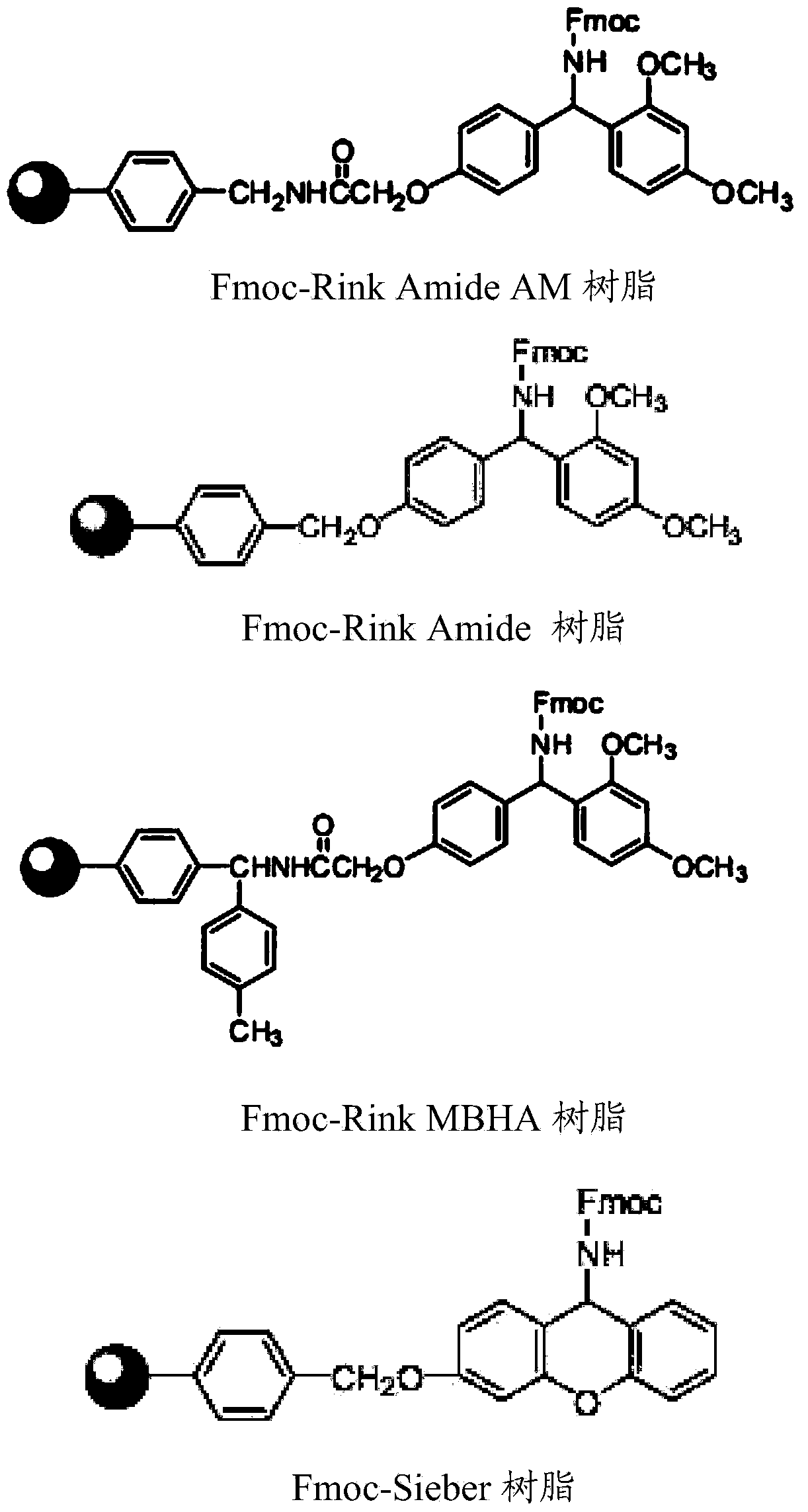
The invention provides a solid-phase preparation method for buserelin. The method comprises the following steps of: 1) preparing a Fmoc-Pro-HMPB-MBHA resin with degree of substitution of between 0.15 and 0.80mmol / g from Fmoc-Pro-OH and a HMPB-MBHA resin with degree of substitution of between 0.2 and 0.9mmol / g by a solid-phase synthesis method; 2) gradually coupling remaining protected amino acid of the Fmoc-Pro-HMPB-MBHA resin according to a peptide sequence to obtain buserelin-HMPB-MBHA resin; 3) cracking the buserelin-HMPB-MBHA resin to obtain fully-protected peptide; and 4) performing ethyl amination, deprotection and purification on the obtained fully-protected peptide to obtain buserelin. The invention aims to provide a buserelin solid-phase synthesis method which has the advantages of high yield, low cost, mild reaction conditions, small environmental pollution and contribution to realizing industrialization.
Owner:HYBIO PHARMA
Use of a GnRH antagonist peptide in the treatment of sex hormone-dependent diseases
InactiveUS20090018085A1Peptide/protein ingredientsLuteinising hormone-releasing hormonePrecocious pubertyPhysiology
Owner:FERRING BV
Method for improving stability of polypeptide active pharmaceutical ingredients
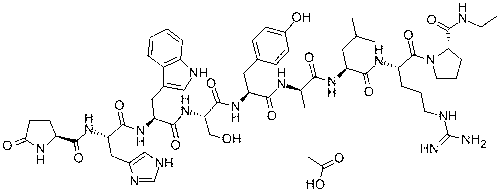
ActiveCN106188218AChange particle shapeChange moistureOxytocins/vasopressinsThymosin peptidesOxytocinBivalirudin
The invention discloses a method for improving the stability of polypeptide active pharmaceutical ingredients. The polypeptide active pharmaceutical ingredients comprise but are not limited to bivalirudin, octreotide acetate, lanreotide acetate, eptifibatide or cetrorelix acetate, ganirelix acetate, degarelix, liraglutide, oxytocin, thymosin alpha1, leuprolide acetate, goserelin acetate, terlipressin or linaclotide. The method comprises the steps that after polypeptide drugs are salified, a polypeptide solution containing compensation ions is obtained, and a target polypeptide product is prepared through an ultralow temperature vacuum freeze-drying method. According to the method for improving the stability of the polypeptide active pharmaceutical ingredients, the problem that the polypeptide active pharmaceutical ingredients are prone to degradation after being placed for a long time is solved, the uniformity of the product is improved, and the drug risk is reduced.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Solid-phase synthesis method of degarelix
ActiveCN103992392AThe process steps are simpleMild conditionsLuteinising hormone-releasing hormonePeptide preparation methodsAcetic anhydrideDiethyl ether
The invention discloses a solid-phase synthesis method of degarelix. The solid-phase synthesis method of degarelix comprises the following steps: on the basis of taking Fmoc-amino resin as a solid-phase carrier, replacing orotic acid fragments connected with an alanine benzene ring in the fifth site and an amino in the fourth site with ivdde, and performing sequential condensation reaction from the end C to the end N so as to connect 10 protected amino acids, thereby obtaining a full-protective peptide resin; then removing the Fmoc protecting group of end N D-Nal, acetylating by using acetic anhydride and pyridine, replacing ivDde with Hor, cutting the peptide resin by using a splitting agent, and precipitating by using frozen diethyl ether to obtain crude peptide. Besides, the invention also relates to a method for synthesizing a raw material Fmoc-Aph(ivDde)-OH by use of ivDde-OH and Fmoc-Aph-OH. The solid-phase synthesis method of degarelix has the advantages that the ivDde is introduced to synthesize decapeptide firstly and then the Hor is used as a substitute to avoid a rearrangement side reaction; the solid-phase synthesis method is simple in process steps, easy to control, low in influence on a human body and the environment, high in yield, and suitable for large-scale production.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Controlled release composition and method of producing the same
InactiveUS20030134800A1Suppressing initial excess releaseStable releasing speed for a long period of timeNervous disorderTetrapeptide ingredientsControlled release3-hydroxy-2-naphthoic acid
A controlled release composition containing a physiologically active substance in high content, suppressing the initial excess release, and achieving a stable release speed over a long period of time is provided. A controlled release composition comprising (1) a physiologically active substance or salt thereof in an amount of about 14% (w / w) to about 24% (w / w) based on the total composition weight, (2) hydroxynaphthoic acid selected from the group consisting of 3-hydroxy-2-naphthoic acid and 1-hydroxy-2-naphthoic acid or salt thereof, and (3) a lactic acid polymer or salt thereof having a weight-average molecular weight of 15000 to 50000 in which the content of polymers having molecular weights of 5000 or less is about 5% by weight or less, wherein the molar ratio of said hydroxynaphthoic acid or salt thereof to said physiologically active substance or salt thereof is from 3:4 to 4:3.
Owner:TAKEDA PHARMA CO LTD
Compositions and methods for contraception in or sterilization of mammals
InactiveUS6680058B1Prevents sexual maturationInhibition of maturationHormone peptidesPeptide/protein ingredientsLytic peptideMammal
Amphipathic lytic peptides are ideally suited to use in a ligand / cytotoxin combination to induce sterility or long-term contraception in mammals. The peptides act directly on cell membranes, and need not be internalized. Administering a combination of gonadotropin-releasing hormone (GnRH) (or a GnRH agonist) and a membrane-active lytic peptide produces long-term contraception or sterilization in mammals in vivo. The compounds are relatively small, and are not antigenic. Lysis of gonadotropes has been observed to be very rapid (on the order of ten minutes.) The two components-the ligand and the lytic peptide-may optionally be administered as a fusion peptide, or they may be administered separately, with the ligand administered slightly before the lytic peptide, to activate cells with receptors for the ligand, and thereby make those cells susceptible to lysis by the lytic peptide.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Method For The Manufacture Of Degarelix
ActiveCN102428097APeptide/protein ingredientsLuteinising hormone-releasing hormoneOrganic solventOrganic base
In a step-wise synthesis of degarelix comprising 0.3 % by weight or less of 4-([2-(5-hydantoyl)]acetylamino)-phenylalanine analog on (solid support)-NH2 a step comprises providing a solution of an amino acid or peptide of which the a-amino group is protected by Fmoc; contacting the support with the solution in the presence of reagent for forming a peptide bond between a carboxyl group of the amino acid or peptide and (solid support)-NH2; removing Fmoc by contacting the support with an organic base, in particular piperidine, in an organic solvent. Also disclosed is degarelix of high purity prepared by the method of the invention and the use of Fmoc in the synthesis of degarelix.
Owner:POLYPEPTIDE LAB AS
Transient and/or permanent modification of sexual behavior and/or fertility using recombinant chimeric GnRH
InactiveUS20050239701A1Improve sensory propertiesDecreased interest in and no desireBiocidePeptide/protein ingredientsFertilityImmunogenicity
The invention provides an immunogenic composition comprising a GnRH multimer and an antigenic carrier, an immunogenic composition comprising a recombinant vector containing a nucleic acid molecule encoding a GnRH multimer and optionally an antigenic carrier, antibodies elicited by the immunogenic compositions, and methods of using the immunogenic compositions and antibodies for modifying sexual physiology and behavior, improving the organoleptic properties of meat, and treating androgen-dependent prostate tumors and GnRH-sensitive ovarian tumors.
Owner:BAKER HENRY +2
GnRH polypeptide-methotrexate conjugate, and preparation method and application thereof
ActiveCN104371009AOrganic active ingredientsLuteinising hormone-releasing hormoneTumor targetTumor targeting
The invention discloses a GnRH polypeptide-methotrexate conjugate shown as a formula in the specification, and the GnRH polypeptide-methotrexate conjugate can be prepared from GnRH polypeptide or analogs thereof and methotrexate or derivatives thereof. The invention also provides a preparation method of the GnRH polypeptide-methotrexate conjugate and application of the GnRH polypeptide-methotrexate conjugate to prepare antitumor medicines. The GnRH polypeptide-methotrexate conjugate is simple in preparation technology, high in yield and easy to purify, has good tumor targeting property on the aspect of resisting tumors, and is capable of centralizing a medicine at the tumor position.
Owner:上海市生物医药技术研究院
Method for synthesizing degarelix
ActiveCN104177478AHigh purityHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsD alanineBiological activation
The invention relates to the field of synthesis of medicines and discloses a method for synthesizing degarelix. The method for synthesizing the degarelix comprises the following steps: enabling D-alanine with a protective group coupled with an N end to carry out esterification reaction with amino resin with a protective group coupled with an amino in the presence of a condensation reagent and an activation reagent to obtain peptide resin 1; extending and coupling other protective amino acids one by one by starting from the peptide resin 1 according to a sequence from C end to N end of the amino acid sequence of the degarelix in the presence of the condensation reagent and the activation reagent, to obtain corresponding peptide resins after extending and coupling each time and finally obtain degarelix resin, then carrying out acidolysis to obtain a degarelix crude product, and purifying the degarelix crude product to obtain a degarelix pure product. By virtue of the method for synthesizing the degarelix, a proper synthesizing scheme is selected; the adaptive amino resin and an acidolysis solution are selected; the overall synthesis process is optimized; the purity of the degarelix is obviously improved; the degarelix has a relatively high total yield and is free of pollution to any environment.
Owner:CHENGDU SHENGNUO BIOPHARM
Preparation method of cetrorelix
InactiveCN104610433AReduce pollutionReduce harmLuteinising hormone-releasing hormonePeptide preparation methodsChromatographic columnSide chain
The invention discloses a preparation method of cetrorelix. According to the preparation method, a Fmoc-Linker-amino resin is taken as the starting raw material, nine amino acids with Fmoc-protecting groups are orderly connected from the C terminal to the N terminal, and after the Fmoc-protecting groups are removed, Ac-D-2-Nal-OH is further connected to obtain an N-terminal acetylated peptide resin, namely an Ac-full-protected decapeptide resin. The peptide resin is cut by use of a cracking reagent, and the cutting fluid is precipitated by use of ice ether to obtain a cetrorelix crude peptide, and then the cetrorelix crude peptide is separated and purified by use of an HPLC preparative chromatographic column, and freeze-dried to obtain a cetrorelix trifluoroacetate or a cetrorelix acetate. According to the preparation method of the cetrorelix, the minimal protection principle is adopted, and the N-terminal acetylated amino acid Ac-D-2-Nal-OH is used for replacing Fmoc-D-2-Nal-OH in advance to avoid the side reaction of the acetic anhydride in an acetylation reagent to the D-Cit side chain carbamido; and as a result, the product purity is improved, the yield of the coarse peptide is high and large-scale industrial production can be realized favorably.
Owner:泰州启瑞医药科技有限公司
Goserelin acetate solid-phase synthesis method
ActiveCN104910257ATo solve such a drawback that cannot be monitoredLow costLuteinising hormone-releasing hormonePeptide preparation methodsSide chainEthylic acid
The invention relates to a goserelin acetate solid-phase synthesis method, which comprises the following steps: HBTU / DIPEA is employed as a condensation system, Fmoc-Ser-OH, Fmoc-Trp-OH are successively coupled; a whole-protection lysate with corresponding voluminal amount according to 10 times of resin weight is added, a carrier 2-CTC Resin in an intermediate is removed, all side chain protective groups are reserved; the whole-protection lysate is adjusted to slight alkaline by using DIPEA(N,N-diisopropylethylaine), semicarbazide hydrochloride and PyBop(1H-benzotriazole-1-oxygen tripyrrole alkyl hexafluorophosphate) (used for a coupling agent of peptide) are added in the whole-protection lysate for reaction coupling, a goserelin peptide solution with the side chain protective group is obtained; the lysate with 20% of TFA / DCM is added in a freezing ether for settling to obtain the white solid crude peptide; the white solid crude peptide is dried under vacuum for solving by methyl alcohol, ammonium formate and Pa / c are added for a hydrogenation reaction to remove the side chain protective group in a peptide sequence. According to the invention, side reaction phenomena can be avoided, target peptide purity is increased, yield is high, operation is convenient and feasible, the intermediate can be tracked and controlled, and the whole process is benefit for enlarged production.
Owner:苏州天马医药集团天吉生物制药有限公司
Lhrh-ii peptide analogs
The invention is directed to analogs of LHRH-II and, more generally, to analogs of the LHRH family in which modifications have been made that confer enhanced binding affinity for LHRH receptors and / or improved metabolic stability.The invention is further directed to methods of targeted therapy and targeted imaging in patients with sex-hormone-related cancers or other LHRH-mediated diseases.
Owner:BRACCO IMAGINIG SPA
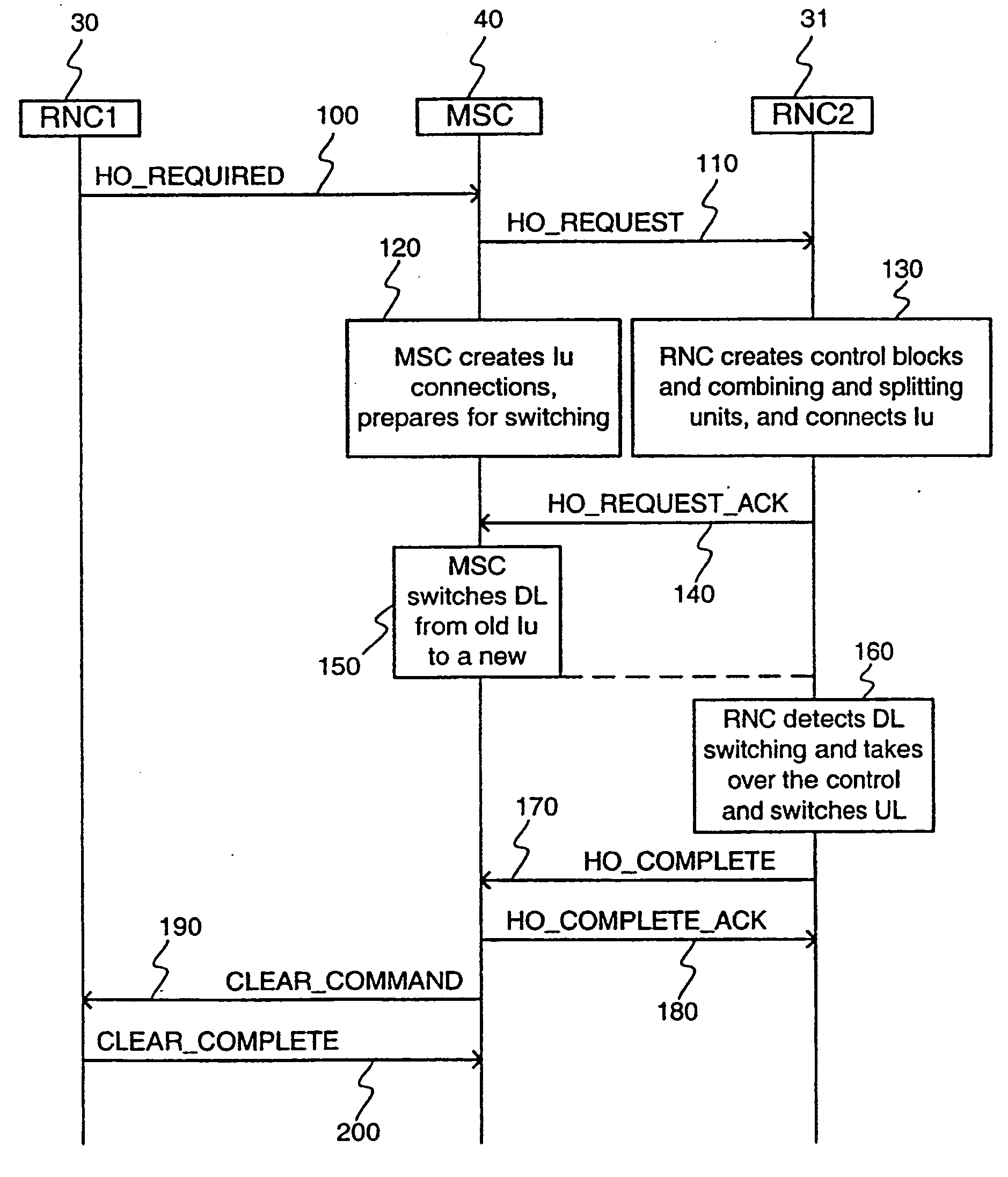
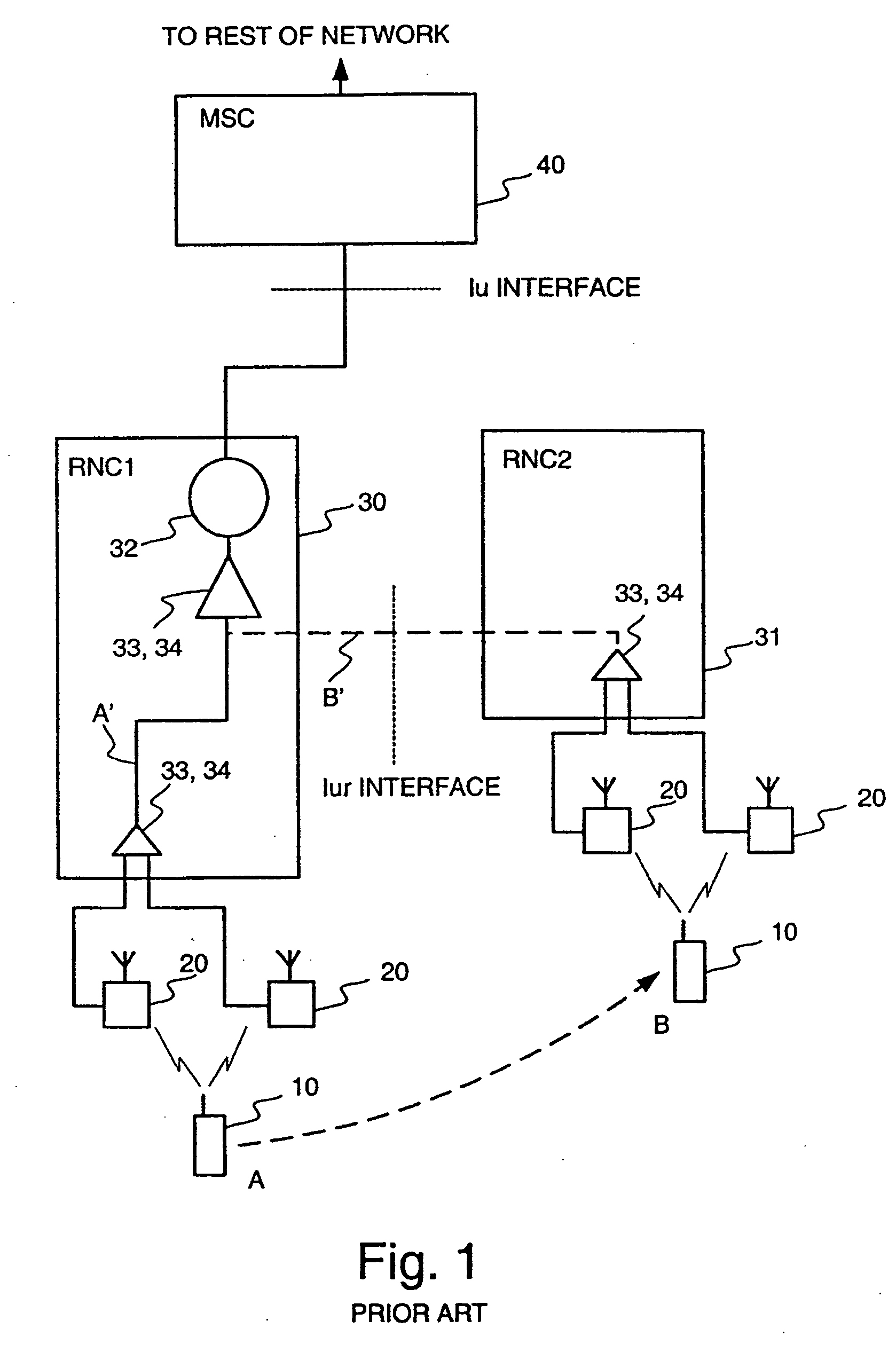
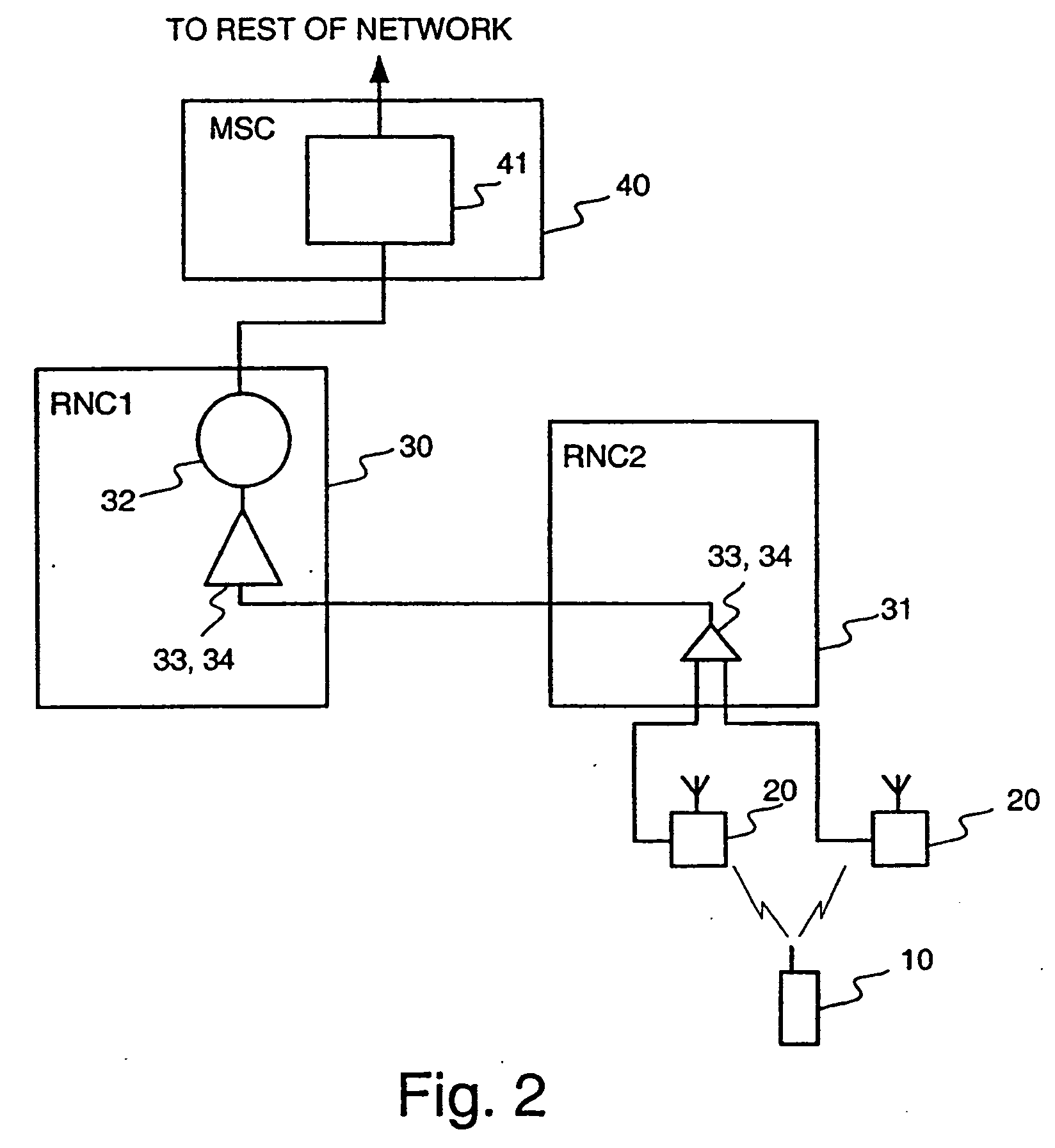
Method for controlling connections to a mobile station
InactiveUS20040252660A1Reduce network loadPeptide/protein ingredientsLuteinising hormone-releasing hormoneTelecommunications networkStart time
When a new connection is set up between a mobile station and a cellular telecommunications network, the starting frame of the new connection is selected using a simple rule so that the starting times of the interleaving periods of all the presently active connections between the mobile station and the cellular network are periodically aligned, thereby substantially simplifying the handover process. In addition, the length of the interleaving period can be set such that the periodic alignment is more frequent.
Owner:QUALCOMM INC
Preparation method for degarelix
InactiveCN105085634ALuteinising hormone-releasing hormonePeptide preparation methodsFreeze-dryingEthylic acid
The invention discloses a safe, high-efficiency degarelix synthesis method, particularly a preparation method for a degarelix. With amino resin as an initial resin carrier, the method disclosed by the invention sequentially connects corresponding Fmoc-amino acids in a degarelix sequence by solid-phase synthesis, wherein Ac-D-2Nal-OH is adopted as the last amino acid, and by condensation reaction and deprotection reaction, fully protected degarelix peptide resin is obtained; acidolysis segmentation and ether sedimentation are then carried out, so that crude degarelix peptide is obtained; by chromatographic purification, acetic acid salinization and freeze drying, the acetic acid degarelix is obtained. The preparation method chooses Oxyma as safe, high-efficiency coupling reagent in order to increase the safety of the process and reduce the byproducts of the process. The process can adopt not only solid-phase synthesis but also liquid-phase synthesis, the byproducts of the process are little, and separation and purification are easy. The purity of the obtained final acetic acid degarelix product is up to 99 percent, the yield is up to 61 percent, and the final acetic acid degarelix product has considerable economically applicable value and a broad application prospect.
Owner:CHINESE PEPTIDE CO
Purification method of ganirelix acetate
ActiveCN102993274AGood removal effectHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsGanirelixPurification methods
The invention provides a purification method of ganirelix acetate. The purification method comprises the steps of (1) purification of ganirelix crude peptide, wherein octadecylsilane bonded silica is adopted as a fixed phase, perchlorate / phosphoric acid solution with certain concentration is taken as an A phase and acetonitrile is taken as a B phase, the ganirelix crude peptide is purified by a gradient-elution high performance liquid chromatography (HPLC) method; (2) salt conversion and purification, wherein the alkylsilane bonded silica is taken as the fixed phase, glacial acetic acid solution with a certain concentration is taken as the A phase and the acetonitrile is taken as the B phase, salt conversion and purification are carried out by adopting the gradient elution HPLC method, and the solution collected and subjected to freeze-drying to obtain the ganirelix acetate. The invention aims at providing the purification method of the ganirelix acetate with stable and controllable process, high yield, high purity, and wide practical value and application prospect.
Owner:HYBIO PHARMA
Methods and Compositions Related to Improving Properties of Pharmacological Agents Targeting Nervous System
InactiveUS20090281031A1Improve blood-brain barrier permeabilityIncreased lipophilic characterNervous disorderPeptide/protein ingredientsNervous systemMedicine
Disclosed are compositions and methods related to improving pharmacological properties of bioactive compounds targeting nervous system.
Owner:UNIV OF UTAH RES FOUND
Preparation method of cetrorelix
InactiveCN104892732ADoes not affect yield issuesReduce pollutionLuteinising hormone-releasing hormonePeptide preparation methodsFluid phaseSide chain
The invention discloses a solid-phase synthesis method of cetrorelix. The method completely avoids toxic [D-Cit(Ac)]-cetrorelix impurities of D-citrulline side chain acetylation. According to the method, amino resin is used as an initial resin carrier, corresponding Fmoc-amino acids in a cetrorelix sequence are sequentially connected with the solid-phase synthesis method, and all-protected cetrorelix peptide resin is obtained through a condensation reaction and a deprotection reaction; then acidolysis cutting and sedimentation by the aid of diethyl ether are performed, and crude cetrorelix peptides are obtained; cetrorelix acetate is obtained through chromatographic purification, acetate conversion and freeze-drying. According to the method, Ac-D-2Nal-OH is selected as connection of the last amino acid, so that acetylation termination is avoided. Both a solid-phase synthesis technology and a liquid-phase synthesis technology can be adopted, few technological byproducts are produced, and separation and purification are easy. The purity of the finally obtained cetrorelix acetate product is up to 99%, the yield is up to 40%, and the method has the considerable economical application value and the broad application prospect.
Owner:CHINESE PEPTIDE CO
Method for synthesizing Degarelix
ActiveCN105524143AHigh purityThe synthesis process is simpleFollicle-stimulating hormoneLuteinising hormone-releasing hormoneCombinatorial chemistryProtecting group
The invention relates to the field of medicine synthesizing and discloses a method for synthesizing Degarelix. The method is characterized in that the synthesizing of the whole Degarelix is separated into two parts from the fifth amino acid position to the sixth amino acid position, appropriate protecting groups are used for parts of protecting amino acid, and the whole synthesizing process is completed by using specific acidulate agents. The method has the advantages that an appropriate synthesizing scheme is selected, the suitable protecting groups and acidulate agents are selected, the whole synthesizing process is optimized, the purity of the Degarelix is increased evidently, high total yield is achieved, and generation of toxic hydantoin degradation products is avoided.
Owner:CHENGDU SHENGNUO BIOPHARM
Method for preparing cetrorelix
InactiveCN104086632ADoes not affect yieldLow synthetic purityLuteinising hormone-releasing hormonePeptide preparation methodsPolymer scienceBackbone chain
The invention discloses a preparation method of cetrorelix. The method has the specific steps that: (A) AM resin is adopted as initial resin; according to cetrorelix main-chain peptide sequence, amino acids with N-terminal Fmoc protection and side-chain protection are coupled sequentially, wherein peptide sequence 6-site amino acid coupling adopts Fmoc-D-Orn(Dde)-OH; (B) when coupling is finished, Fmoc protecting group is removed, and an acetylation reaction is carried out on the N terminal; (C) a mixed solution with hydrazine hydrate and DMF with a volume ratio of 3:97 is used for removing D-ornithine side-chain Dde protecting group; tert-butyl isocyanate is added, such that D-ornithine side-chain in decapeptide resin is subjected to a modification reaction, and fully protected cetrorelix peptide resin is obtained; and (D) peptide resin is subjected to cracking, purification, desalination, and lyophilization, such that cetrorelix can be obtained. The content of a process impurity [D-Cit(Ac)<6>]-cetrorelix is less than 0.1%. The preparation process provided by the invention has the advantages of high product purity and low cost. The preparation method is suitable for large-scale cetrorelix production. With the process, the content of the impurity [D-Cit(Ac)<6>]-cetrorelix can be effectively controlled without influencing the yield of cetrorelix.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Solid-liquid phase synthesis method for alarelin acetate
ActiveCN102702327ALuteinising hormone-releasing hormonePeptide preparation methodsFluid phaseSide chain
The invention relates to a solid-liquid phase synthesis method for alarelin acetate, and mainly solves the technical problems of low yield, high cost, severe reaction conditions and serious pollution existing in the conventional synthesis method. The solid-liquid phase synthesis method mainly comprises the synthesis steps of: (1) coupling each protective amino acid one by one by using a fluorenylmethoxycarbonyl solid phase synthesis method by taking proline-dichloro-trityl-chlorine resin as initial resin and synthesizing to obtain side chain fully protected peptide chain resin; (2) cutting the side chain fully protected peptide chain resin to obtain a fully protected peptide chain segment pGluP-9; (3) performing C-terminal ethylamination on the fully protected peptide chain segment pGluP-9 to obtain a fully protected segment of the alarelin acetate; and (4) cutting the fully protected segment of the alarelin acetate to remove a side chain protective group to obtain alarelin acetate rough product peptide. The solid-liquid phase synthesis method has the advantages of high large-scale production capacity, easy operation, stable process, low production cost and total yield of exceeding 40 percent.
Owner:GL BIOCHEM SHANGHAI +2
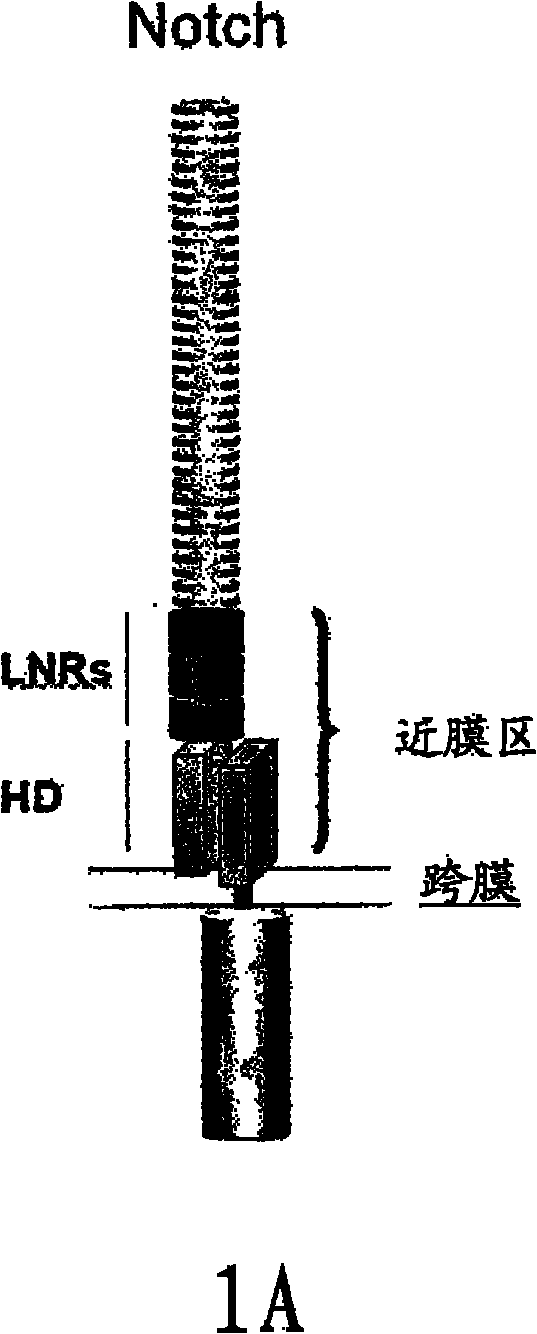
NOTCH1 receptor binding agents and methods of use thereof
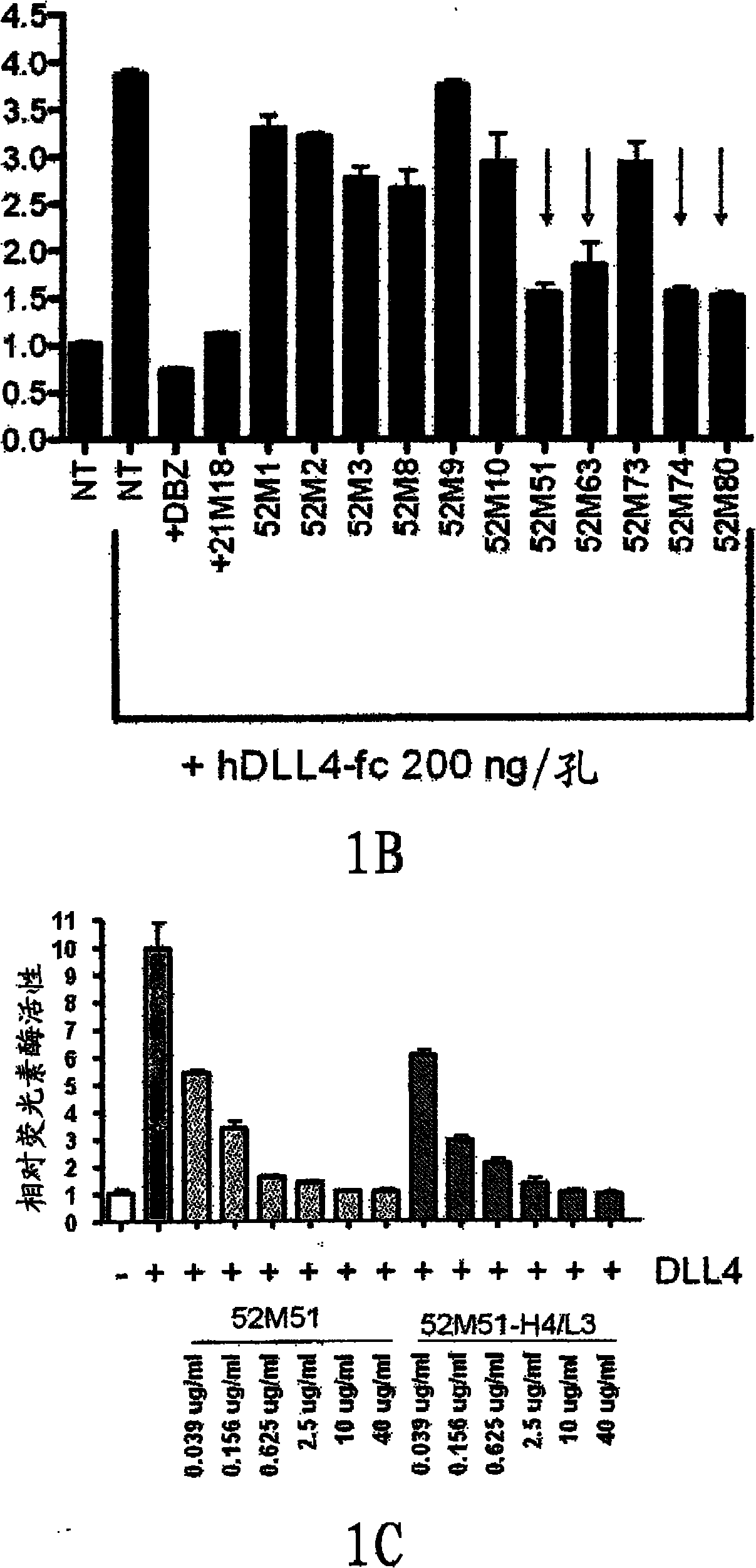
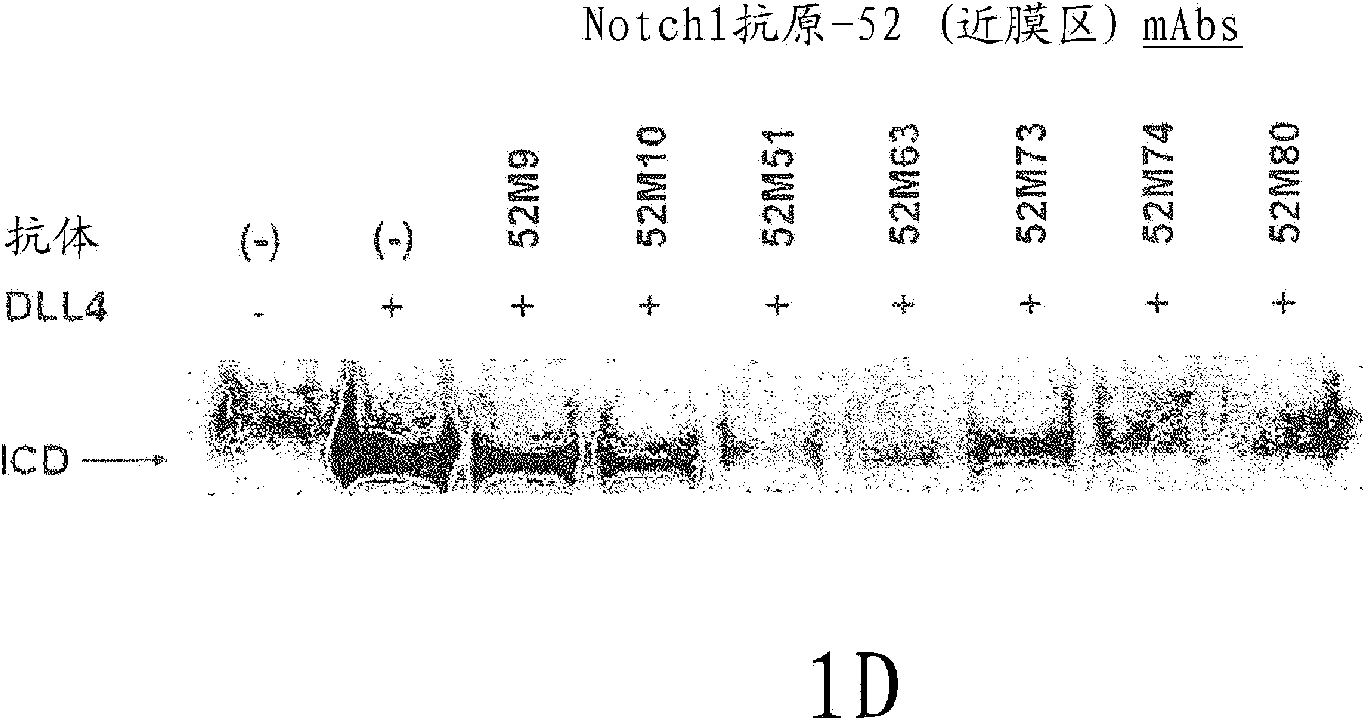
InactiveCN102112490AOrganic active ingredientsImmunoglobulins against growth factorsWilms' tumorCancer research
The present invention discloses means and methods for the diagnosis, characterization, prognosis and treatment of cancer and specifically targeting cancer stem cells. The present invention provides an antibody that specifically binds to a non-hgand binding membrane proximal region of the extracellular domain of a human Notch receptor and inhibits tumor growth, and a method of treating cancer, wherein the method comprises administering the antibody for study subjects.
Owner:ONCOMED PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com