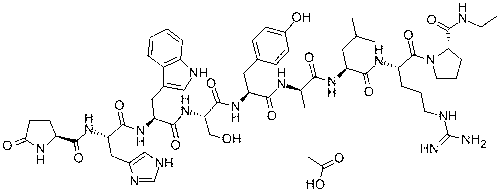
Solid-liquid phase synthesis method for alarelin acetate
A synthesis method, solid-liquid phase technology, applied in the field of solid-liquid phase synthesis method of alarelin, can solve the problems of high cost, large pollution, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] 1) Preparation of Fmoc-Arg(pbf)-Pro-2Cl-Trt-Cl resin
[0063] Weigh 50 grams of H-Pro-2Cl-Trt-Cl resin (Loading 0.6mmol / g, 30mmol), soak it with 800ml DCM for 30 minutes, fully swell the resin, drain it, add Fmoc-Arg(pbf)-OH (MW : 648.8, 60mmol) 38.9g, TBTU (MW: 321.1, 60mmol) 19.3g, HOBT (MW: 135.1, 60mmol) 8.1g, DIEA (MW: 129.24, 120mmol) 20ml, DMF500ml, reacted for 1.5 hours, ninhydrin detection The resin was colorless and transparent, drained, washed 3 times with DMF, and dried to obtain Fmoc-Arg(pbf)-Pro-2Cl-Trt-Cl resin.
[0064] 2) Preparation of pGlu-His(Trt)-Trp(Boc)-Ser(tbu)-Tyr(tbu)-D-Ala-Leu-Arg(pbf)-Pro-2Cl-Trt-Cl resin
[0065] In the Fmoc-Arg(pbf)-Pro-2Cl-Trt-Cl resin in step (1), add 800ml decapping reagent (20% PIP / DMF(v / v) solution), react for 30 minutes, drain, and use Wash with DMF 6 times, drain, use DMF as solvent, add amino acid with Fmoc protection, DIEA, TBTU / HOBT, react for 1.5 hours, detect the reaction end point, drain, wash with DMF 3-5 ti...
Embodiment 2
[0074] 1) Preparation of Fmoc-Arg(pbf)-Pro-2Cl-Trt-Cl resin
[0075] Weigh 67 grams of H-Pro-2Cl-Trt-Cl resin (Loading 0.45mmol / g, 30mmol), soak it with 800ml DCM for 30 minutes, fully swell the resin, drain it, add Fmoc-Arg(pbf)-OH (MW : 648.8, 90mmol) 58.4g, HBTU (MW: 379.2, 90mmol) 34.1g, HOBT (MW: 135.1, 90mmol) 12.2g, NMM (MW: 102.1, 180mmol) 20ml, DMF500ml, reaction for 1 hour, ninhydrin detection The resin was colorless and transparent, drained, washed 3 times with DMF, and dried to obtain Fmoc-Arg(pbf)-Pro-2Cl-Trt-Cl resin.
[0076] 2) Preparation of pGlu-His(Trt)-Trp(Boc)-Ser(tbu)-Tyr(tbu)-D-Ala-Leu-Arg(pbf)-Pro-2Cl-Trt-Cl resin
[0077] In the Fmoc-Arg(pbf)-Pro-2Cl-Trt-Cl resin in step (1), add 800ml decapping reagent (20% PIP / DMF(v / v) solution), react for 30 minutes, drain, and use Wash with DMF 6 times, drain, use DMF as solvent, add amino acid with Fmoc protection, NMM, HBTU / HOBT, react for 1 hour, detect the reaction end point, drain, wash with DMF 3-5 times, d...
Embodiment 3
[0086]1) Preparation of Fmoc-Arg(pbf)-Pro-2Cl-Trt-Cl resin
[0087] Weigh 42 grams of H-Pro-2Cl-Trt-Cl resin (Loading 0.72mmol / g, 30mmol), soak it with 800ml DCM for 30 minutes, fully swell the resin, drain it, add Fmoc-Arg(pbf)-OH (MW :648.8, 60mmol) 38.9g, HATU (MW: 380.23, 60mmol) 22.8g, HOAT (MW: 136.1, 60mmol) 8.2g, NMM (MW: 102.1, 120mmol) 14ml, DMF500ml, reacted for 0.5 hours, ninhydrin detection The resin was colorless and transparent, drained, washed 3 times with DMF, and dried to obtain Fmoc-Arg(pbf)-Pro-2Cl-Trt-Cl resin.
[0088] 2) Preparation of pGlu-His(Trt)-Trp(Boc)-Ser(tbu)-Tyr(tbu)-D-Ala-Leu-Arg(pbf)-Pro-2Cl-Trt-Cl resin
[0089] In the Fmoc-Arg(pbf)-Pro-2Cl-Trt-Cl resin in step (1), add 800ml decapping reagent (20% PIP / DMF(v / v) solution), react for 30 minutes, drain, and use Wash with DMF 6 times, drain, use DMF as solvent, add amino acid with Fmoc protection, NMM, HATU / HOAT, react for 0.5 hours, detect the reaction end point, drain, wash with DMF 3-5 times...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com