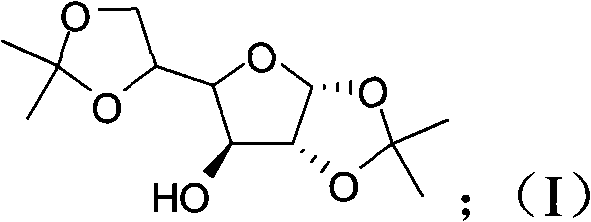
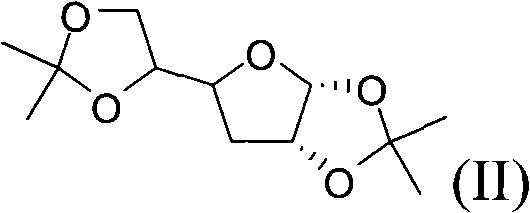
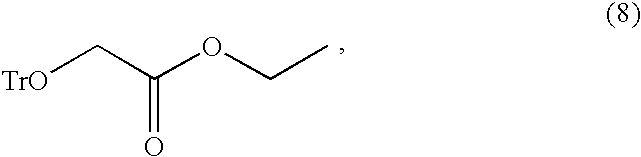Patents
Literature
38 results about "Trityl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
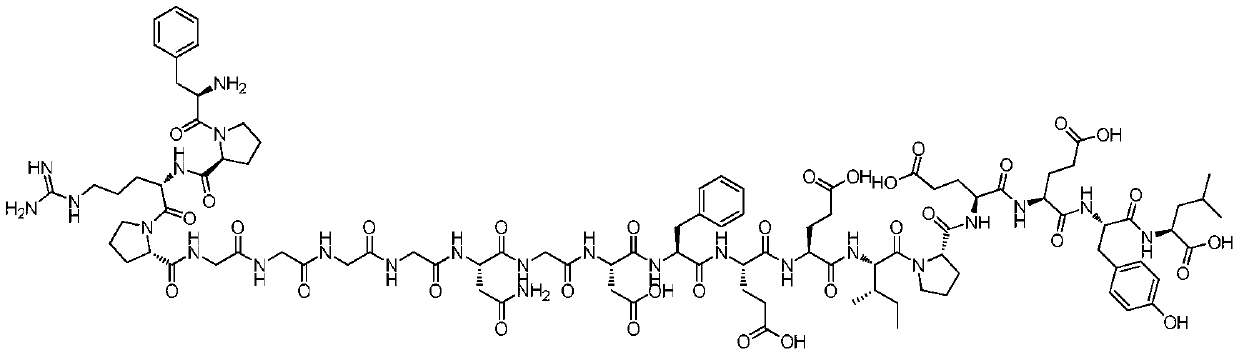
Triphenylmethyl chloride or trityl chloride (TrCl) is a white solid with the chemical formula C 19 H 15 Cl. It is an alkyl halide, sometimes used to introduce the trityl protecting group
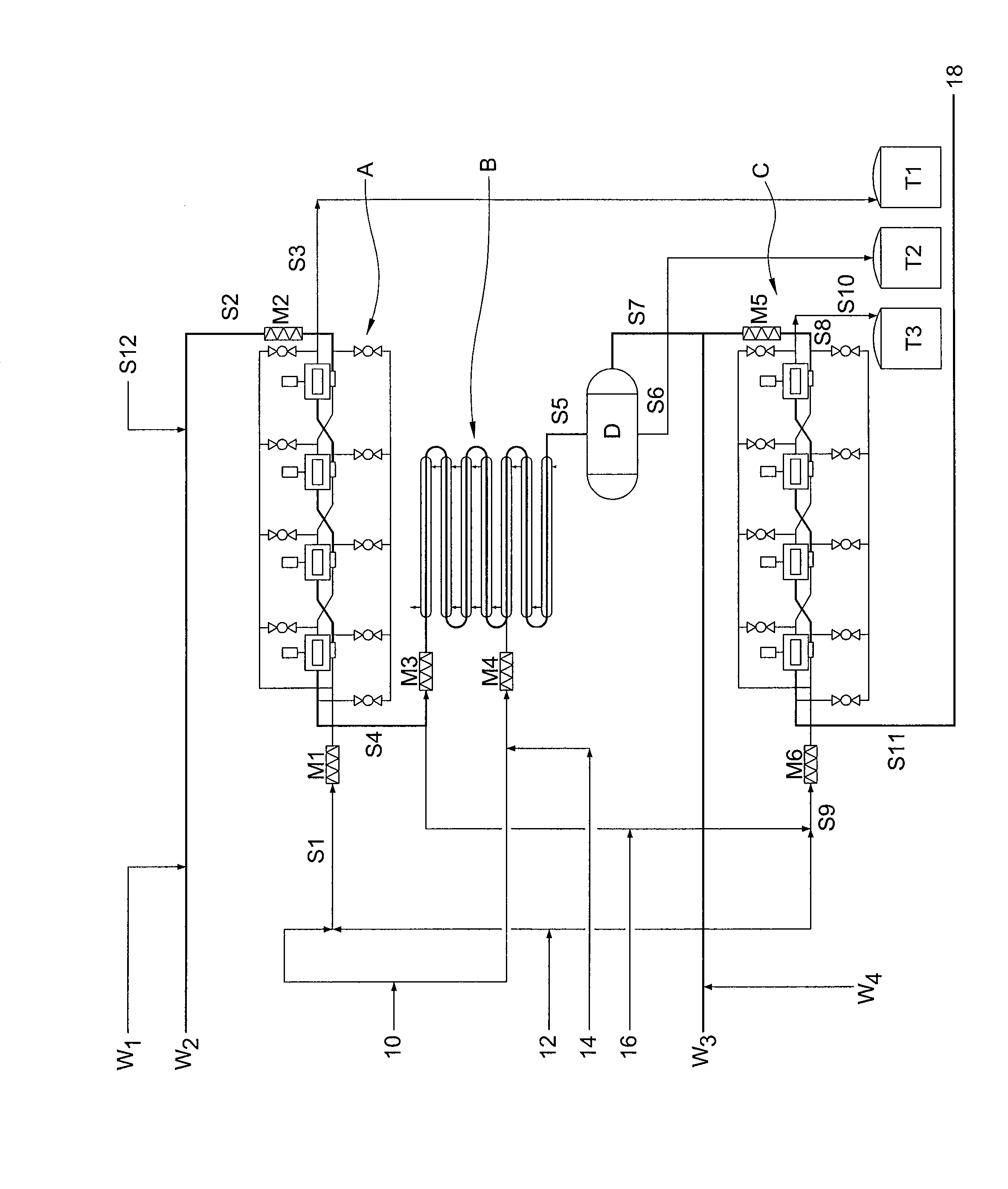
Method of preparing bivalirudin
InactiveUS20080051558A1Improve availabilityQuality improvementPeptide-nucleic acidsPeptide/protein ingredientsSide chainHigh pressure
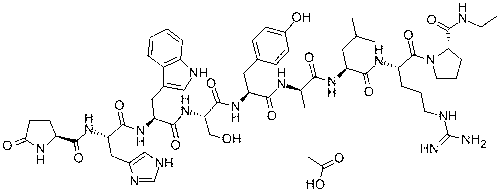
The present invention relates to a novel solid phase peptide synthesis method for Bivalirudin. This method contains following steps: serving Trityl Chloride Resin, 4-Methyltrityl Chloride Resin, 4-Methoxytrityl Chloride Resin, or 2-Cl Trityl Chloride Resin, or attaching of Wang Resin as a start raw material); according to general solid phase peptide synthesis rules, coupling protected amino acids after deprotection of Fmoc-protection group and then deprotecting side chain protection group; cleaving peptides from resin; and then obtaining crude Bivalirudin product. C18 high pressure liquid chromatography (HPLC) column is applied to purify the product of Bivalirudin. This method is suitable and effective for mass production, in addition to its features of high quality, low production cost, high synthetic yield, avoidance of usage of fatal toxic chemical such as HF, and less environmental pollution. The high yield rate of 99% is achieved for each synthetic step and total yield rate is 14%.
Owner:ZHOU YIMING +1
Solid-liquid phase synthesis method for alarelin acetate
ActiveCN102702327ALuteinising hormone-releasing hormonePeptide preparation methodsFluid phaseSide chain
The invention relates to a solid-liquid phase synthesis method for alarelin acetate, and mainly solves the technical problems of low yield, high cost, severe reaction conditions and serious pollution existing in the conventional synthesis method. The solid-liquid phase synthesis method mainly comprises the synthesis steps of: (1) coupling each protective amino acid one by one by using a fluorenylmethoxycarbonyl solid phase synthesis method by taking proline-dichloro-trityl-chlorine resin as initial resin and synthesizing to obtain side chain fully protected peptide chain resin; (2) cutting the side chain fully protected peptide chain resin to obtain a fully protected peptide chain segment pGluP-9; (3) performing C-terminal ethylamination on the fully protected peptide chain segment pGluP-9 to obtain a fully protected segment of the alarelin acetate; and (4) cutting the fully protected segment of the alarelin acetate to remove a side chain protective group to obtain alarelin acetate rough product peptide. The solid-liquid phase synthesis method has the advantages of high large-scale production capacity, easy operation, stable process, low production cost and total yield of exceeding 40 percent.
Owner:GL BIOCHEM SHANGHAI +2
Synthesis and preparation process of RGD cyclopeptide
ActiveCN103588863AReasonable workmanshipEasy to operatePeptide preparation methodsCyclic peptidesSide chainTrityl chloride
The invention discloses a synthesis and preparation process of an RGD cyclopeptide in the field of solid-phase polypeptide synthesis. A new method comprises the steps as follows: a 2-chlorine trityl chloride resin is selected and taken as a carrier; D aspartic acid amino acid with a special protection group of a first side-chain carboxyl is grafted firstly; then a linear peptide of an RGD sequence peptide is grafted on the resin; after the last amino acid is grafted, the protection group FMOC of the amino group is not required to be removed by using piperidine, a special catalyst is added, and the side-chain carboxyl protection group of the first D aspartic acid is removed from the resin directly; then piperidine is added, and the amino protection group FMOC of the terminal amino acid is removed; then a condensing agent is directly added to the resin, the carboxyl and the amino group which are exposed at the head end and the tail end of the linear peptide are subjected to dehydration synthesis, so that the cyclopeptide is formed in an amido bond manner; and finally, the cyclopeptide is cut down from the resin directly by using a cutting liquid. The synthesis and preparation process of the RGD cyclopeptide has the advantages as follows: the process is advanced, the operation is simple, the product yield is high, the synthetic efficiency is improved, and the like.
Owner:苏州强耀生物科技有限公司
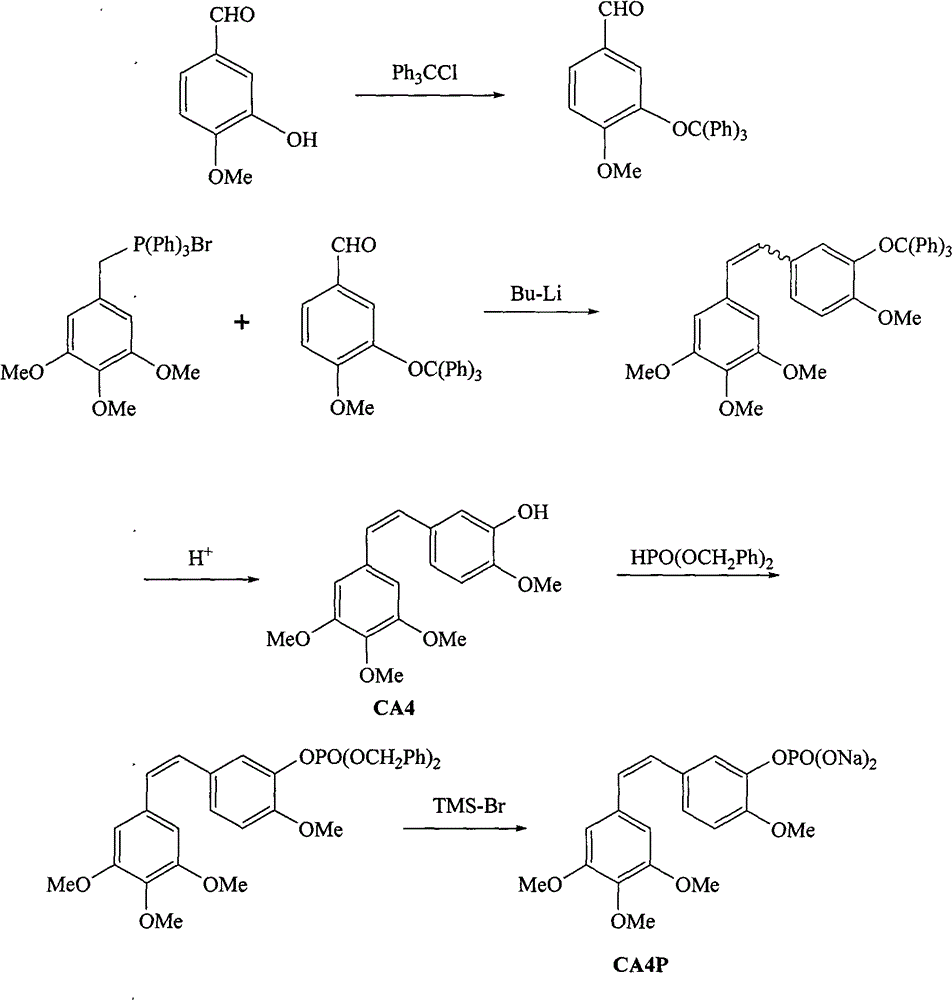
Method for synthesizing CA4P
ActiveCN101885738ARaw materials are cheap and easy to getMild reaction conditionsMethine/polymethine dyesGroup 5/15 element organic compoundsChemical synthesisPhosphate
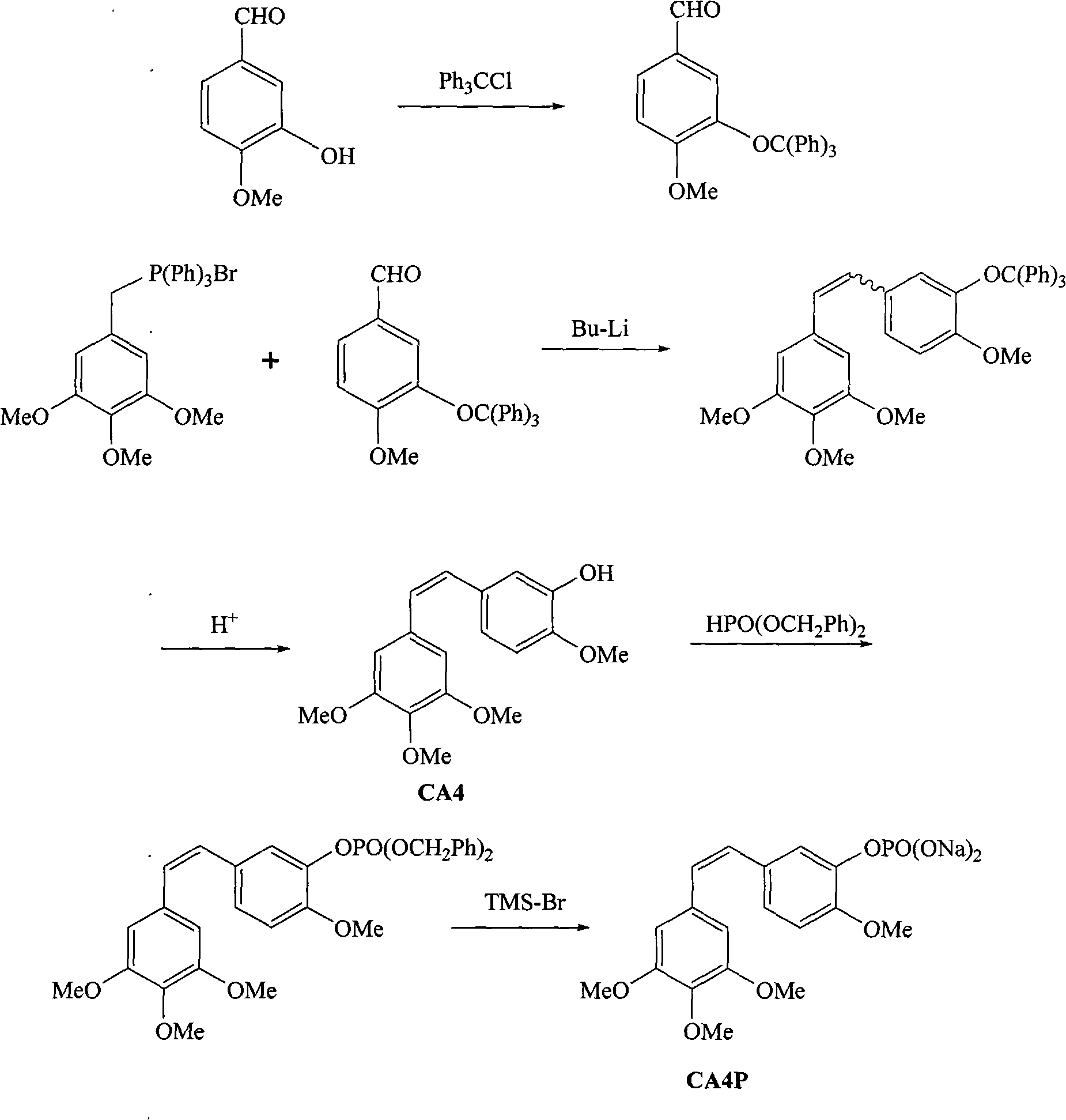
The invention belongs to the field of chemical synthesis and relates to a method for preparing CA4P, in particular to a method for synthesizing CA4P by the following steps that: isovanillin and trityl chloride, which serve as raw materials, are used to form 3- triphenylmethoxy-4-methoxybenzaldehyde which is an intermediate isovanillin protector; the 3- triphenylmethoxy-4-methoxybenzaldehyde and 3,4,5-trimethoxy-triphenyl benzylidene bromide phosphine salt undergo a Wittig reaction , and the protective group is removed by hydrolysis to obtain CA4; and the CA4 and phosphonic acid bis(phenylmethyl)ester react to form benzyl phosphate, and the benzyl group is removed to form a sodium salt to obtain the target compound, namely CA4P.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD
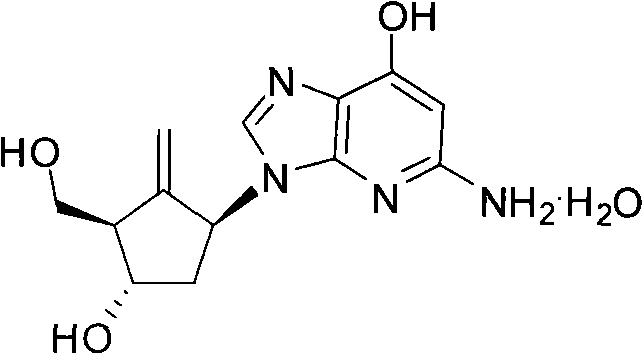
Preparation method of antiviral drug Entecavir
ActiveCN102417506AWide variety of sourcesLow costOrganic chemistryBulk chemical productionPurification methodsEntecavir
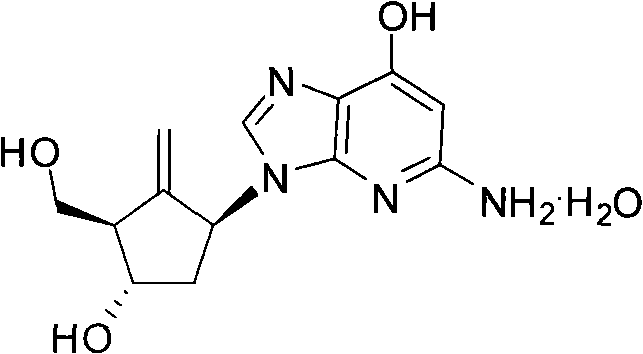
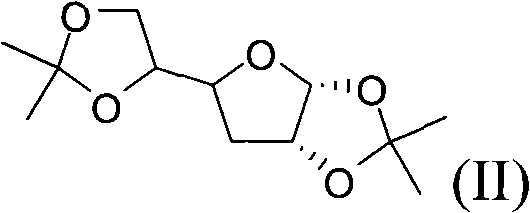
The invention relates to a preparation method of an antiviral drug Entecavir. Taking D-glucose and acetone as starting materials, the method has advantages of easy operation, high yield, easy separation and purification, wide source of raw materials and low cost. The selectivity and stereospecificity are controlled at the beginning of a reaction to effectively inhibit the production of chiral isomer. The purification method of product is simple with high yield. A key intermediate of the reaction is 4- methylol-5- methylene cyclopentane-1,3-diol, of which 4- methylol is selectively protected by trityl chloride, and the intermediate combines with 2-amino-6-chloropurine by mitsunobu. Because of the steric hinderance effect, the protecting group can effectively protect target group and the operation is easy to carry out while removing the protecting group.
Owner:HAINAN PULIN PHARMA +1
Method for synthesizing tetrapeptide isomers by using solid phase peptide synthesis method and applications of tetrapeptide isomers
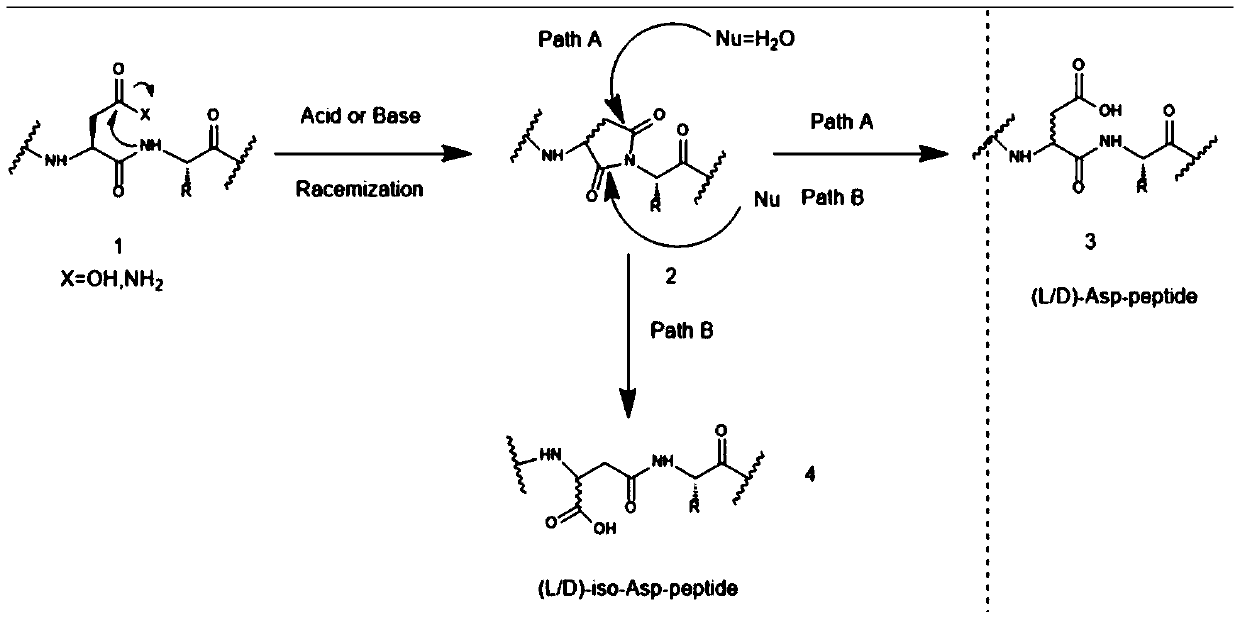
The invention discloses a method for synthesizing tetrapeptide isomers by using solid phase peptide. The method includes using any of trityl chloride-type resins as a starting raw material, sequentially linking fmoc-protected amino acids according to the solid phase synthesis method, obtaining tetrapeptide isomer resins, meanwhile sequentially removing fmoc protecting groups, using a condensing agent to perform a peptide synthesis reaction, simultaneously removing side chain protecting groups and cutting peptides after obtaining protected tetrapeptide isomer resins, obtaining crude tetrapeptide isomers, separating and purifying the crude tetrapeptide isomers through C18 or C8 chromatographic column, and then obtaining the tetrapeptide isomers. According to the protected tetrapeptide isomer synthesis method required by the method for synthesizing the tetrapeptide isomers, raw and auxiliary materials are convenient to collect, the process is stable, the production cycle is short, the production cost is low, the yield is high, the purity is fine, the quality is stable, large-scale production can be achieved, and the market competition ability is high.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
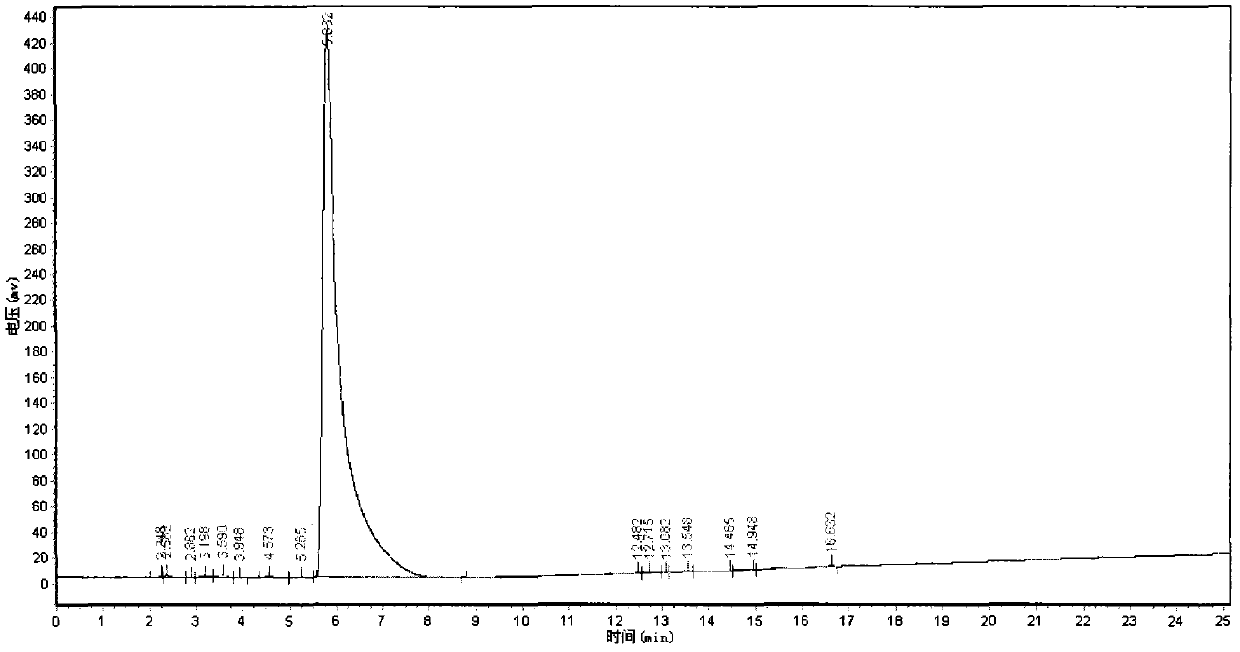
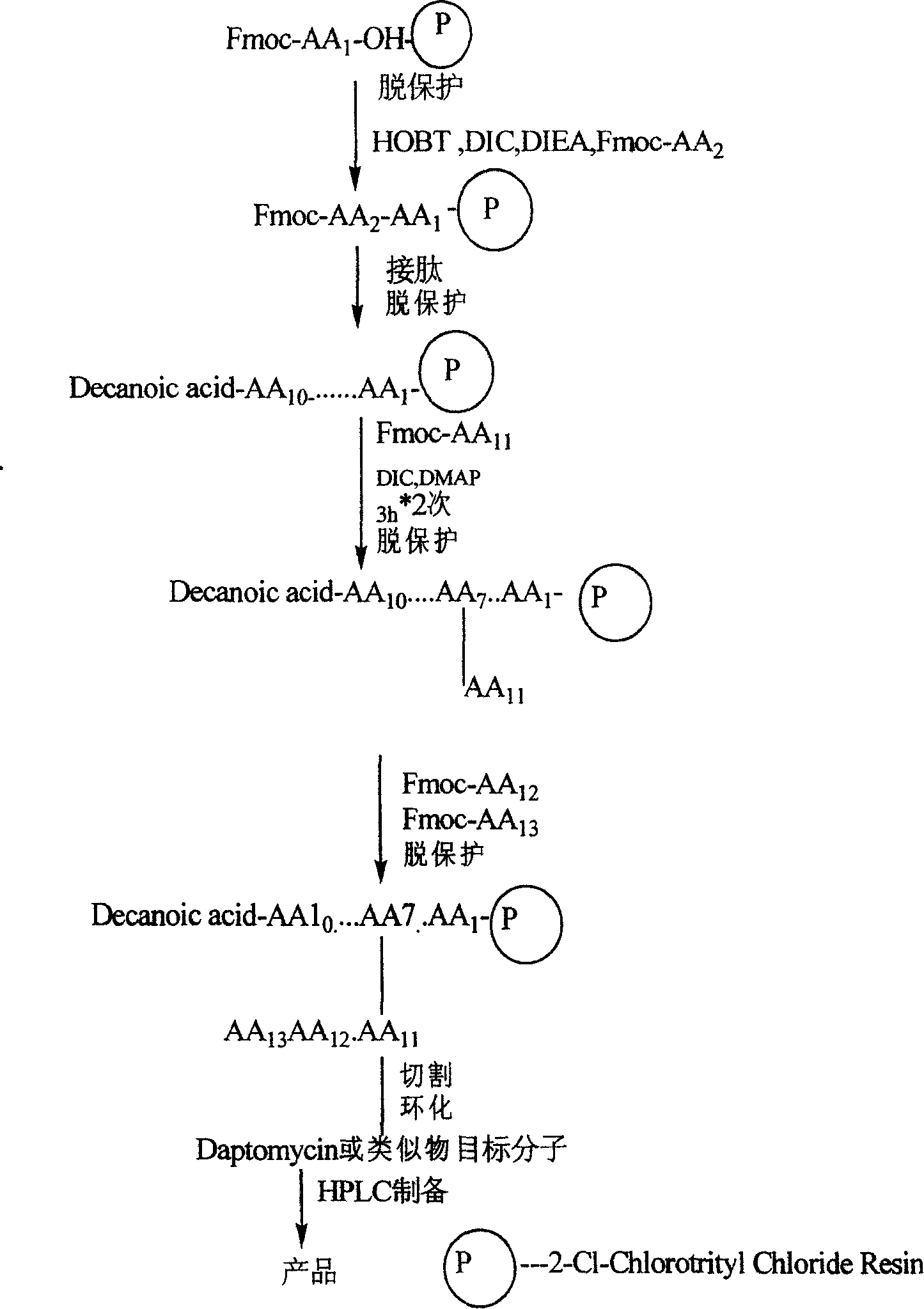
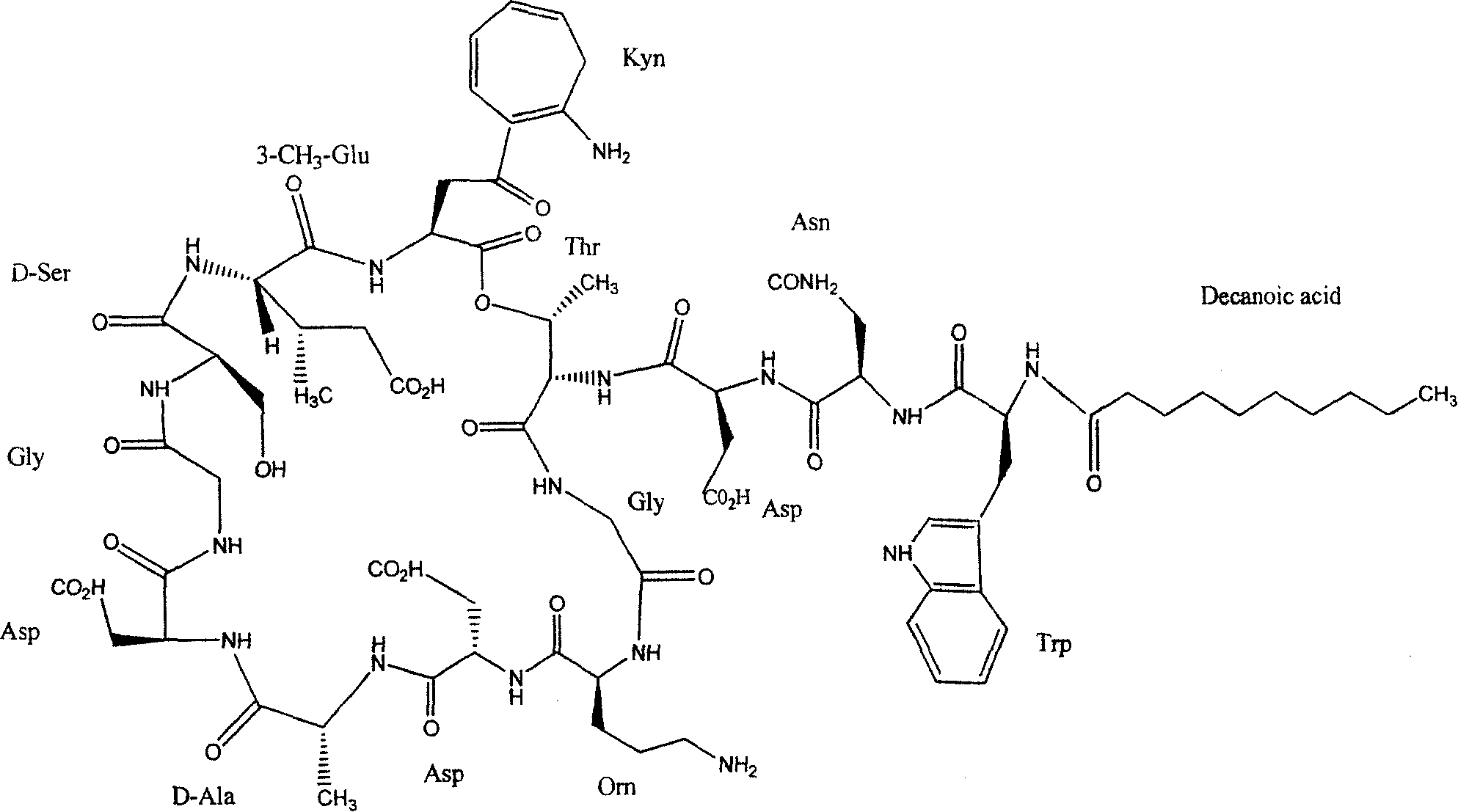
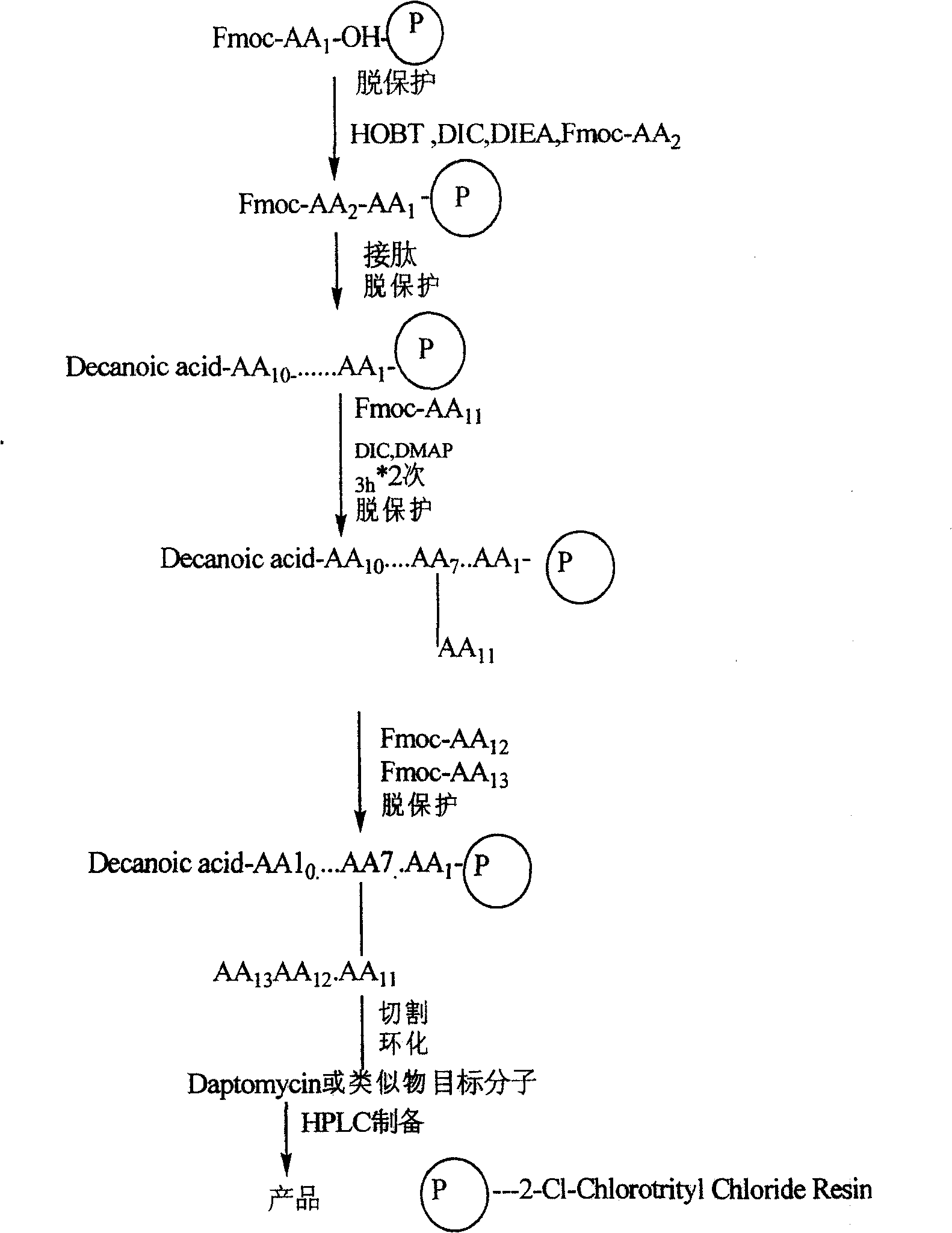
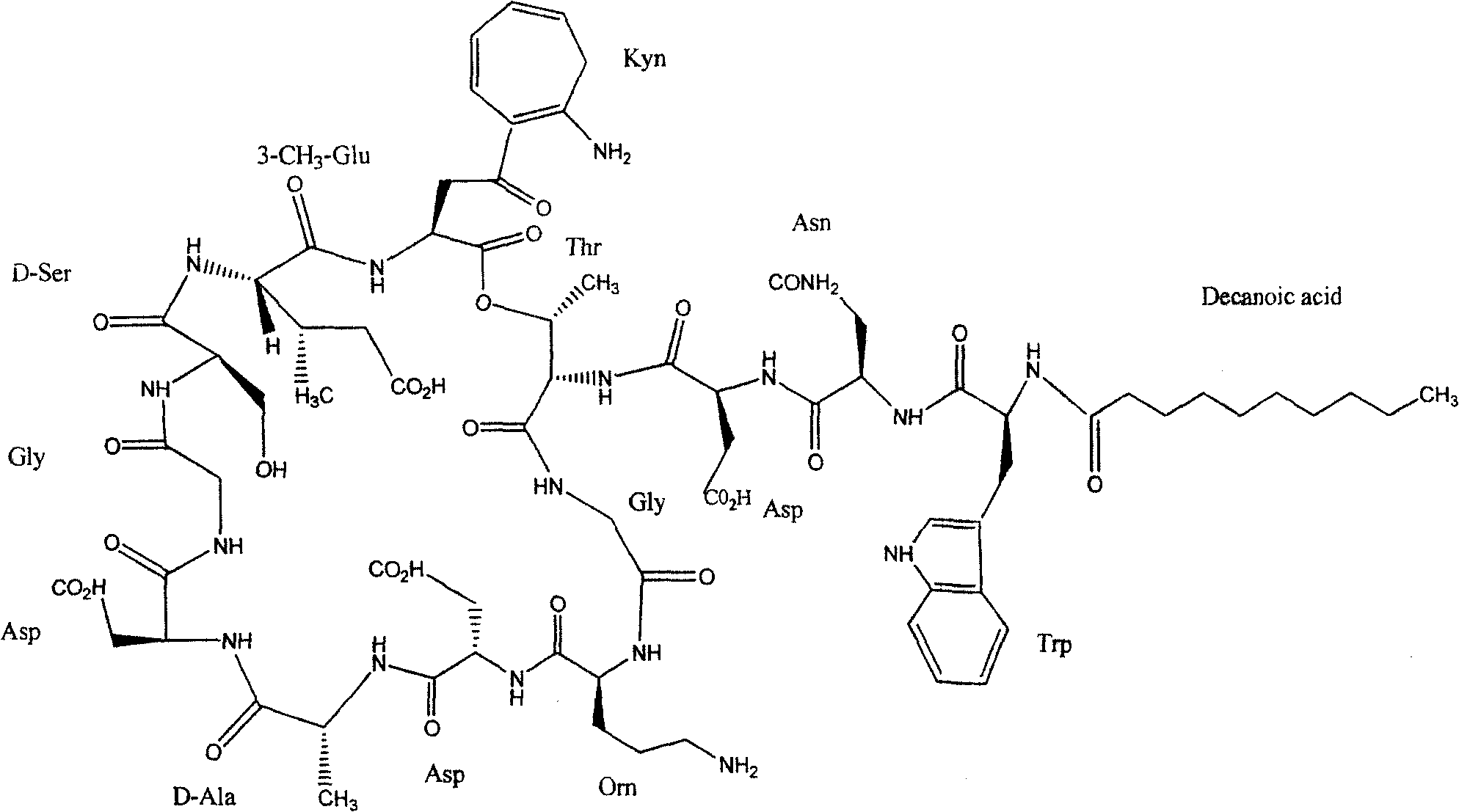
Synthesis method of daptomycin
ActiveCN101235080AAvoid it happening againGood effectPeptide preparation methodsBulk chemical productionSynthesis methodsSolid-phase synthesis
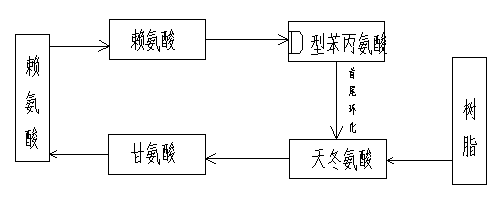
The invention relates to a daptomycin synthesis method for resolving the technical problem of prior art which uses rose spore streptomycete as raw material to cost high synthesis cost. The synthesis method comprises a, using 2-chlorine trityl chloride resin as carrier, via solid synthesis method to connect the amino acids with protective groups, to obtain protective decapeptide resin while the Fmoc-protective groups are removed in turn, B, connecting decanoic acid via same method, connecting next amino acid via esterification, removing Fmoc-protective groups, and connecting left two amino acids via normal solid method, removing Fmoc-protective groups, c, using trifluoroacetic acid or carrene solution to cut off total protective peptide from resin, drying and completing end-to-end liquid cyclisation in organic solvent, d, using the mixture of trifluoroacetic acid, water and benzene methyl sulfide to cut off peptide from resin to obtain crude product. The invention can synthesize daptomycin.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
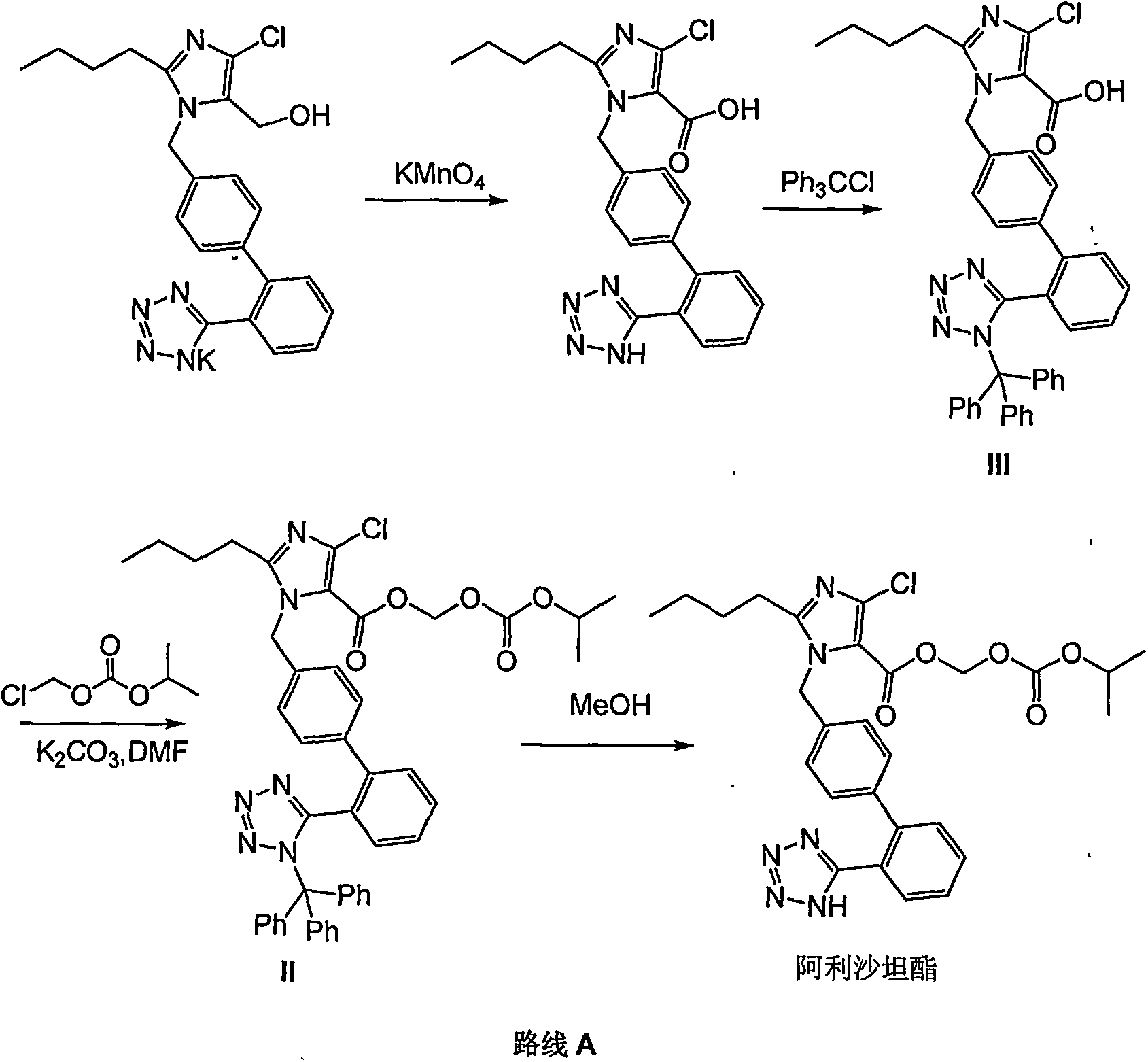
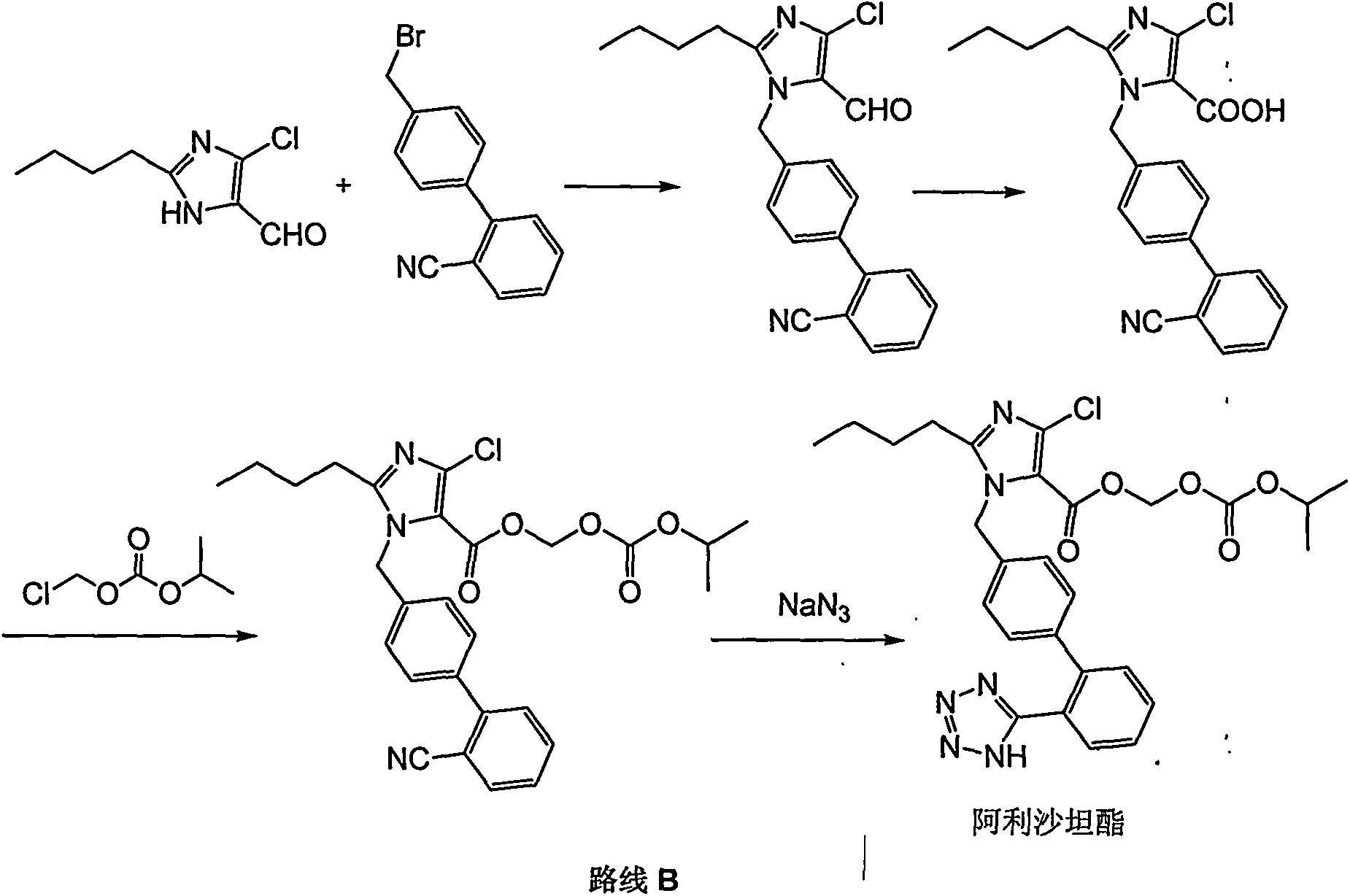
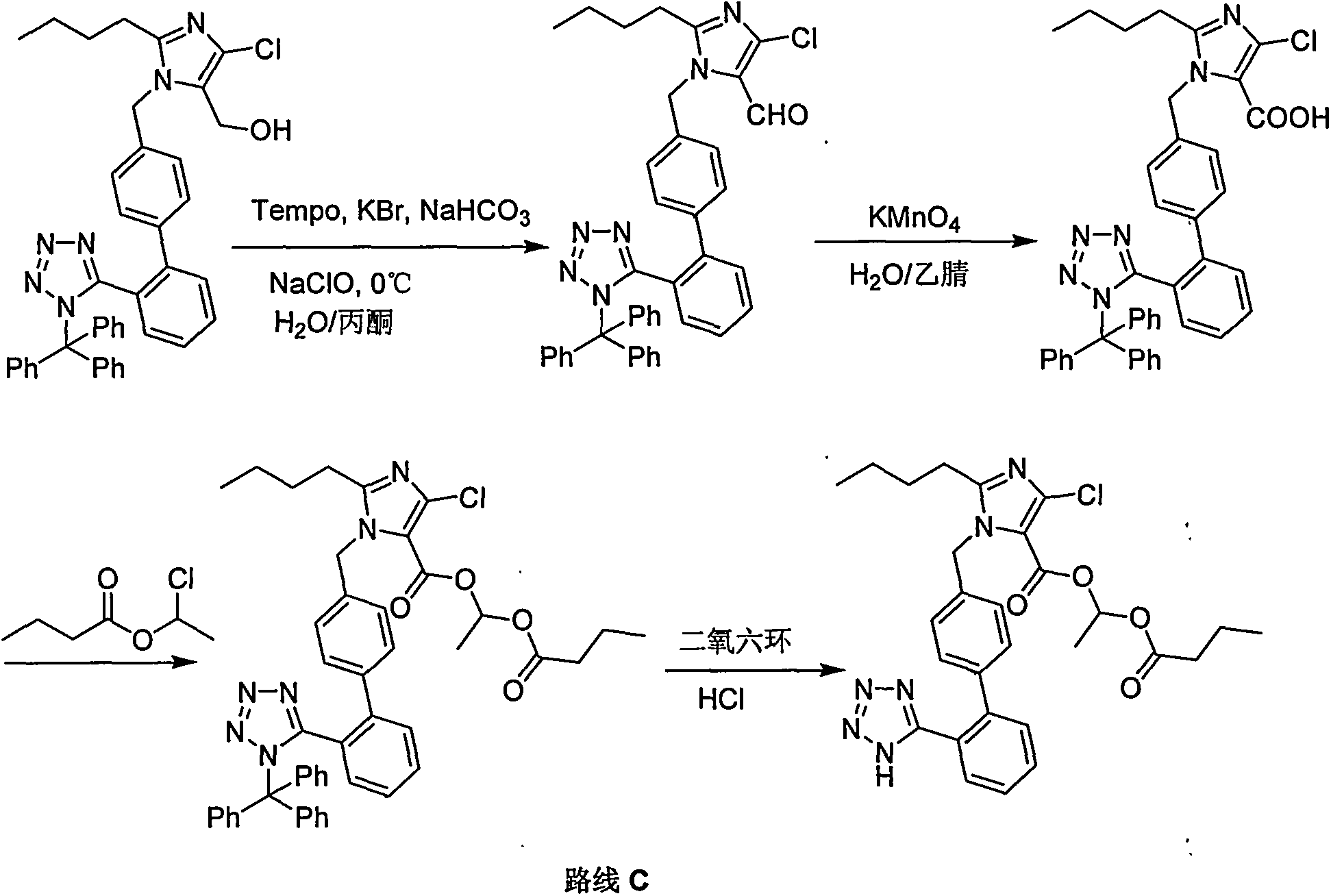
Preparation method for Allisartan Isoproxil
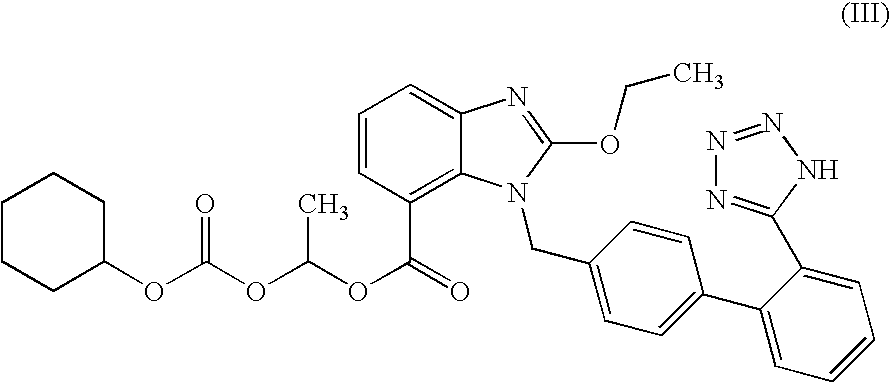
The invention relates to a preparation and refining method for Allisartan Isoproxil. The preparation method comprises the following steps: trityl chloride losartan is oxidized in a polarity organic solvent at the presence of a catalyst to obtain a compound expressed in formula IV; the compound expressed in the formula IV is further oxidized by oxidation reagent hydrogen peroxide / sodium chlorite, Resorcino / sodium chlorite, sulfamic acid / sodium chlorite or 2-methyl-2-butene / sodium chlorite to obtain Allisartan Isoproxil intermediate expressed in formula III; then the Allisartan Isoproxil intermediate is subjected to ester formation and deprotection to obtain an Allisartan Isoproxil crude product; finally, the Allisartan Isoproxil crude product is refined by ethanol / normal heptane to obtain purified Allisartan Isoproxil. According to the preparation method for Allisartan Isoproxil, the yield and efficiency are high, the by-product is few, the preparation method is environmental-friendly and suitable for industrialized production, the operation is simple and safe and the cost is low.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Preparation method of 2-chloro trityl chloride resin
The invention discloses a preparation method of 2-chloro trityl chloride resin in the fields of medicine, biological chemistry and chemical engineering, which comprises the following steps that: 2-chlorobenzophenone is adopted as the raw material to synthesize 1-chlorine-2- dichloro-diphenyl benzene, and the 1-chlorine-2- dichloro-diphenyl benzene is chemically coupled with low cross-linked polystyrene (PS) white balls to obtain the 2-chloro trityl chloride (2-CTC) resin. The method overcomes the defects of a traditional 2- chloro trityl chloride resin preparation method that the steps are complicated and organic metal reagent must be used, is simple and feasible, does not need to use the organic metal reagent, the uploading level of the first amino acid, swelling property and peptide solid-phase synthesis efficiency of the prepared 2-CTC resin are better than those of similar commodities 2-CTC resin, and the mass quality is stable, the repeatability is good and the industrial production is easy.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
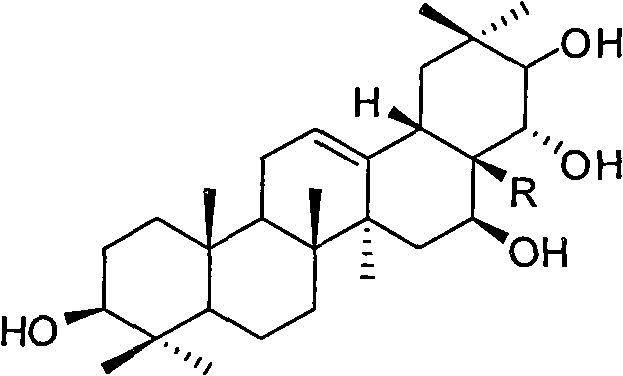
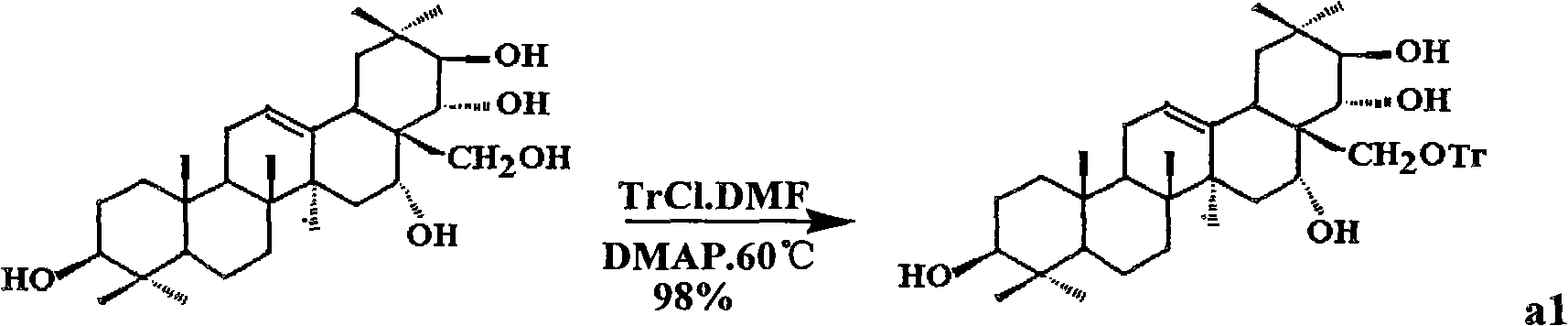
Theasapogenol derivative with anti-HIV (Human Immunodeficiency Virus) activity, preparation method and application thereof
InactiveCN102030807AHigh anti-HIV activitySimple preparation processOrganic active ingredientsAntiviralsAcetic anhydridePyridinium
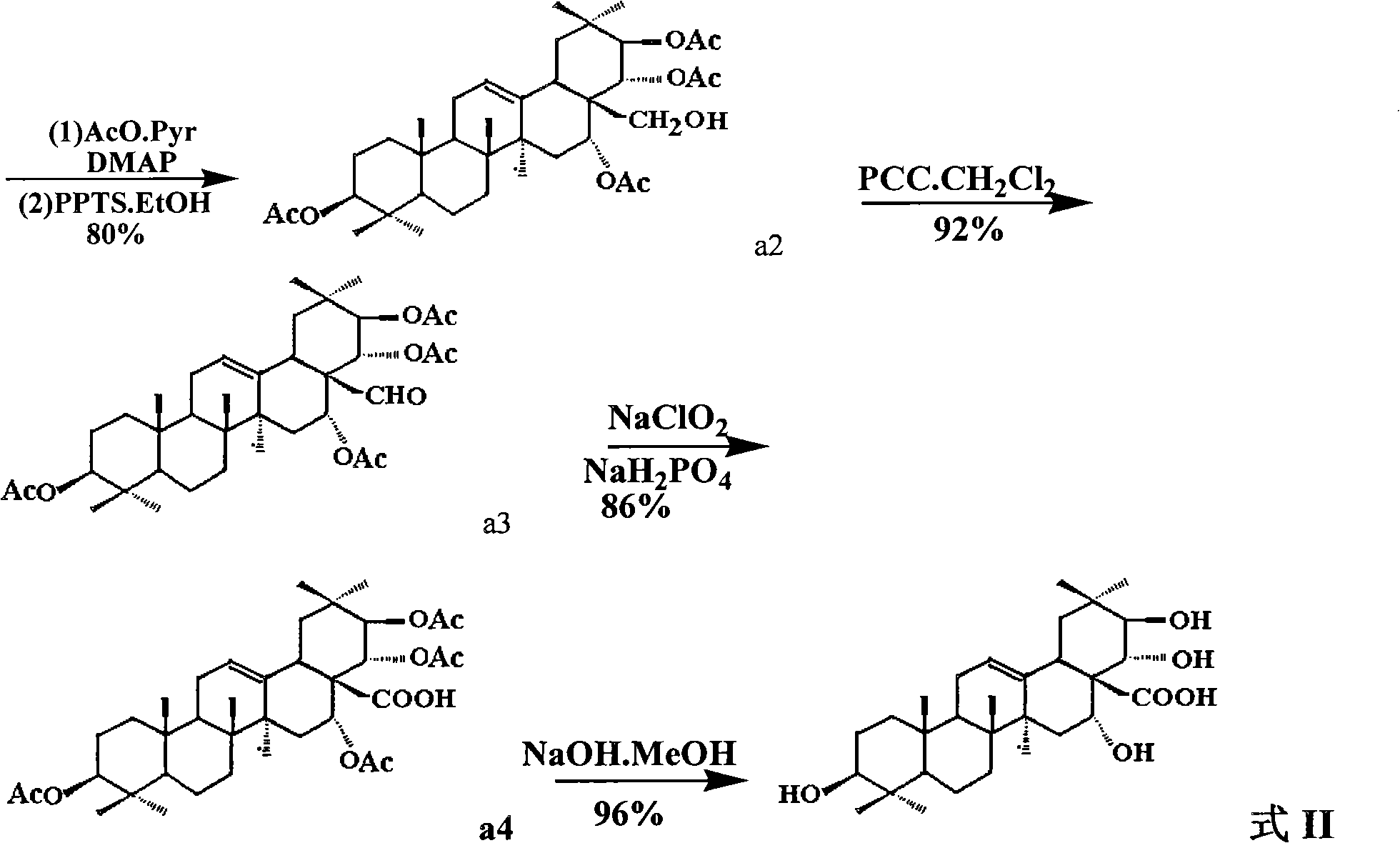
The invention discloses a theasapogenol derivative with anti-HIV (Human Immunodeficiency Virus) activity, a preparation method and application thereof. The theasapogenol derivative with anti-HIV activity has a structure disclosed as a formula I. The preparation method comprises the following steps of: (1) reacting the 4-dimethylaminopyridine with trityl chloride to obtain a compound a1, wherein theasapogenol as a raw material, and 4-dimethylaminopyridine is used as a catalyst in a pyridine solvent; (2) reacting the a1 with acetic anhydride by using the dimethylaminopyridine as the catalyst inan anhydrous pyridine solvent and adding sulfanilic acid pyridinium into a solution for reaction to obtain a compound a2; (3) oxidizing the a2 by using pyridinum chlorochromate salt to obtain a compound a3; (4) oxidizing the a3 by using NaClO2 and NaH2PO4 to obtain a4; and (5) hydrolyzing the a4 by using an aqueous solution of methanol and sodium hydroxide to obtain the theasapogenol derivative with anti-HIV activity disclosed as the formula I. The theasapogenol derivative has the advantages of high anti-HIV activity, simple preparation technology, high product purity, easy control of reaction conditions and industrial production.
Owner:SOUTH CHINA UNIV OF TECH
Process for the preparation of candesartan cilexetil
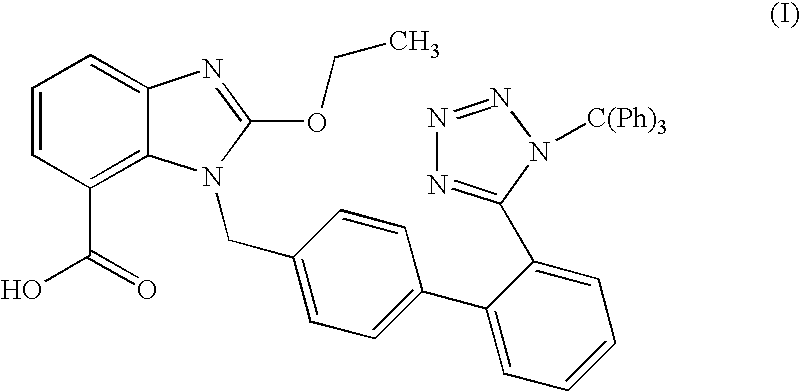
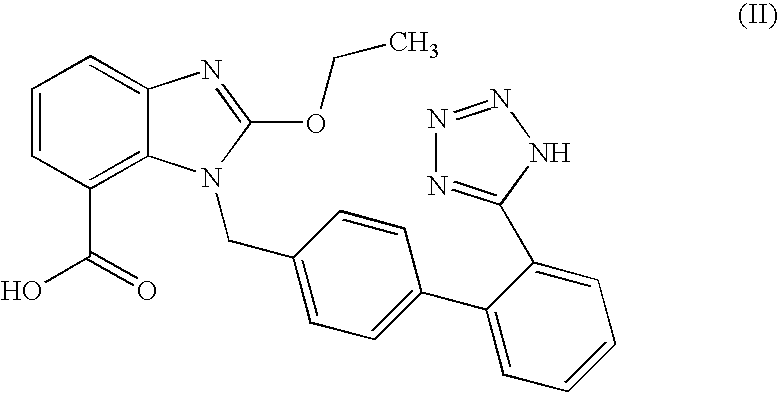
The present invention relates to an improved process for the preparation of tritylated candesartan acid of formula (I)comprising a step of, reacting candesartan acid of formula (II)with trityl chloride in the presence of a base in a ketonic solvent.
Owner:ALEMBIC LTD
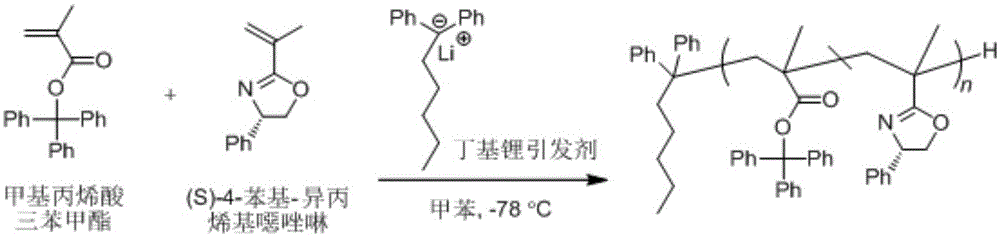
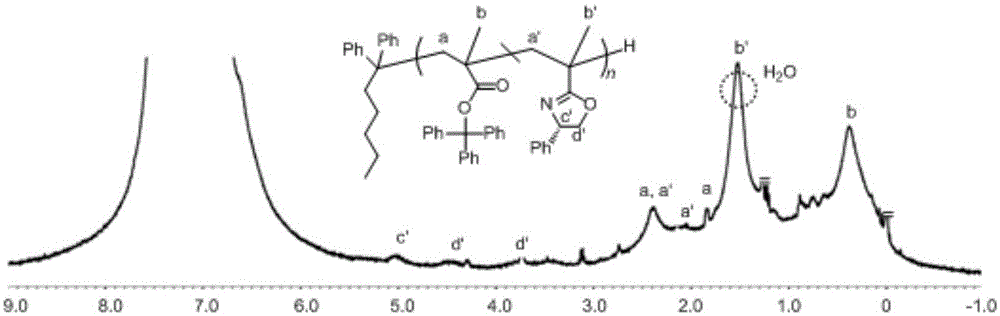
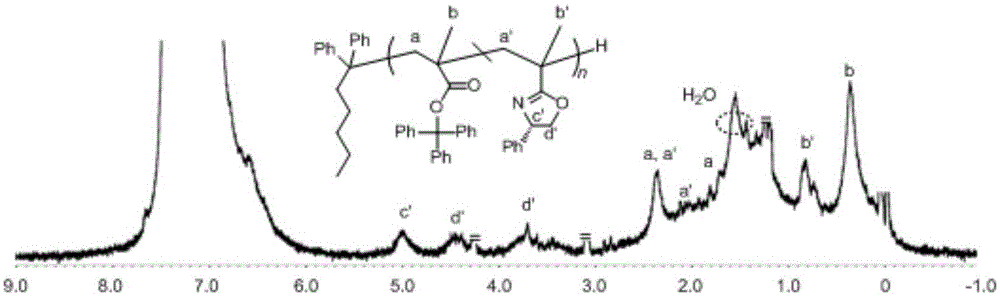
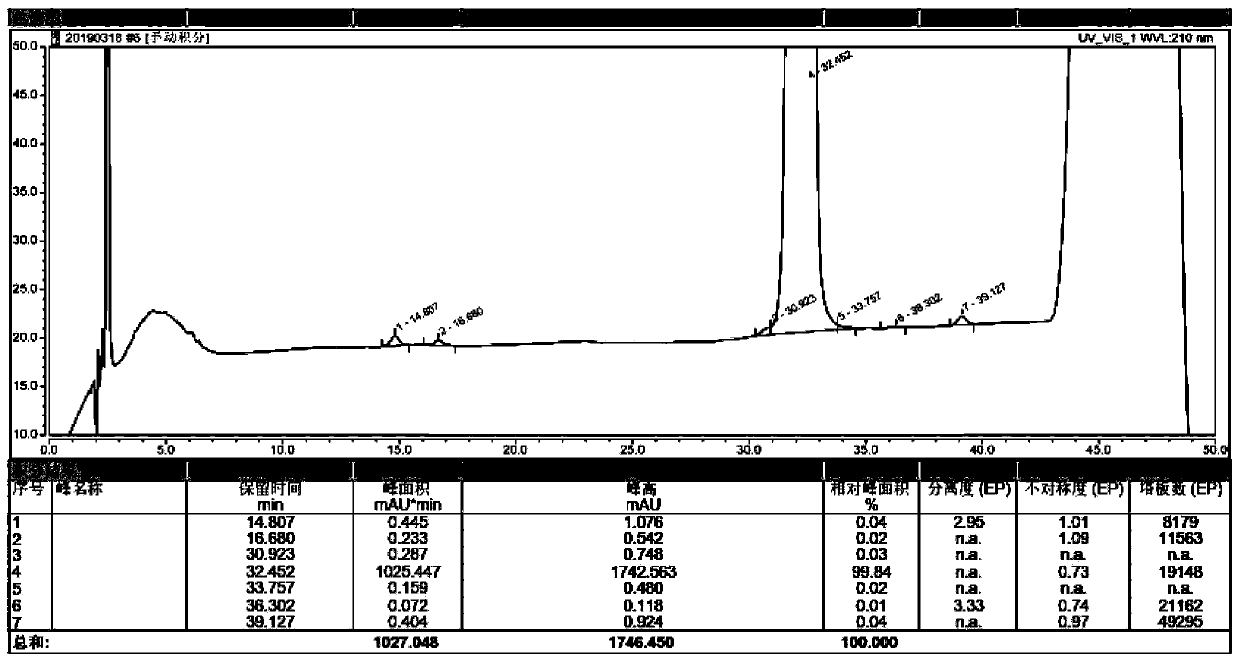
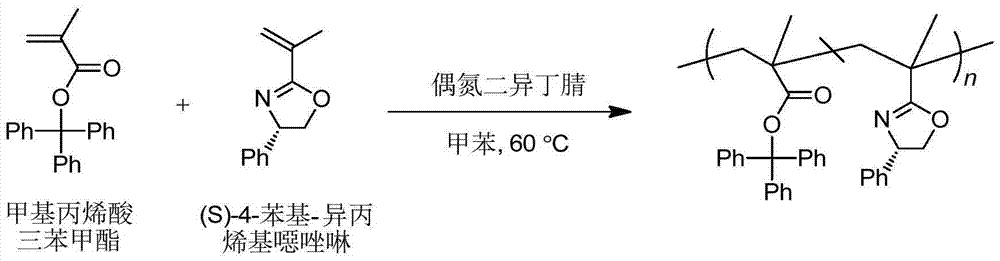
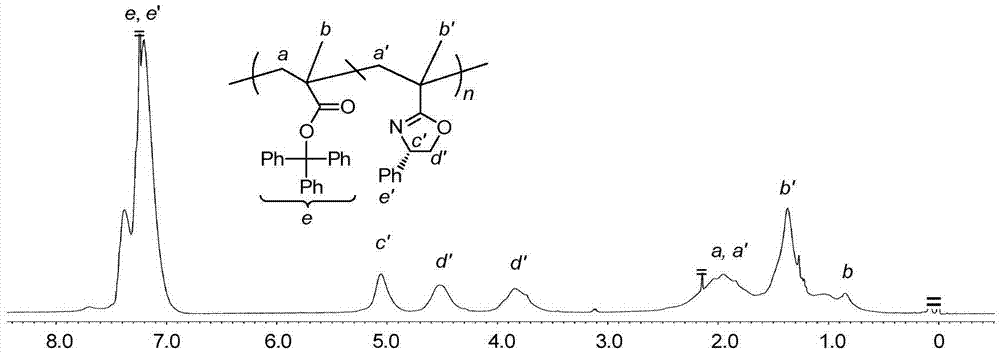
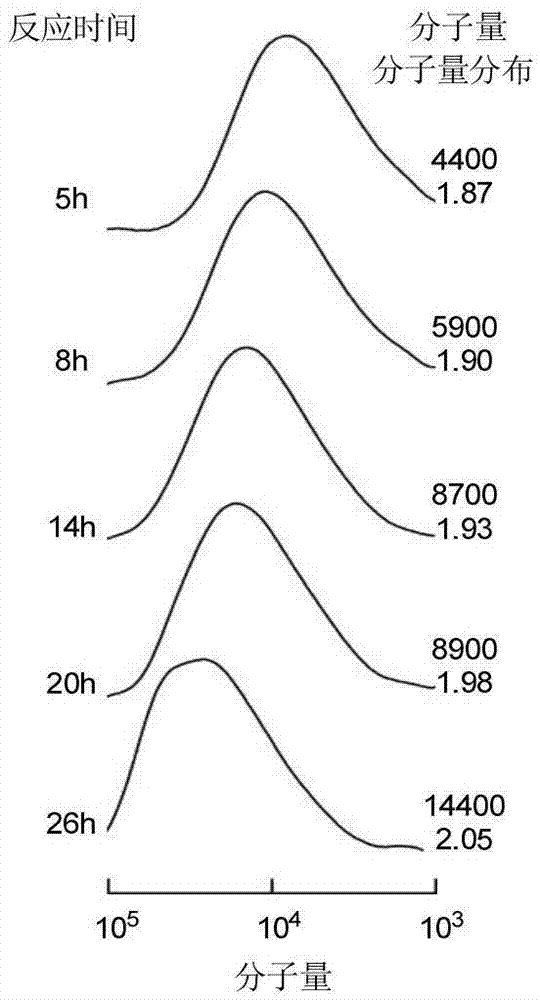
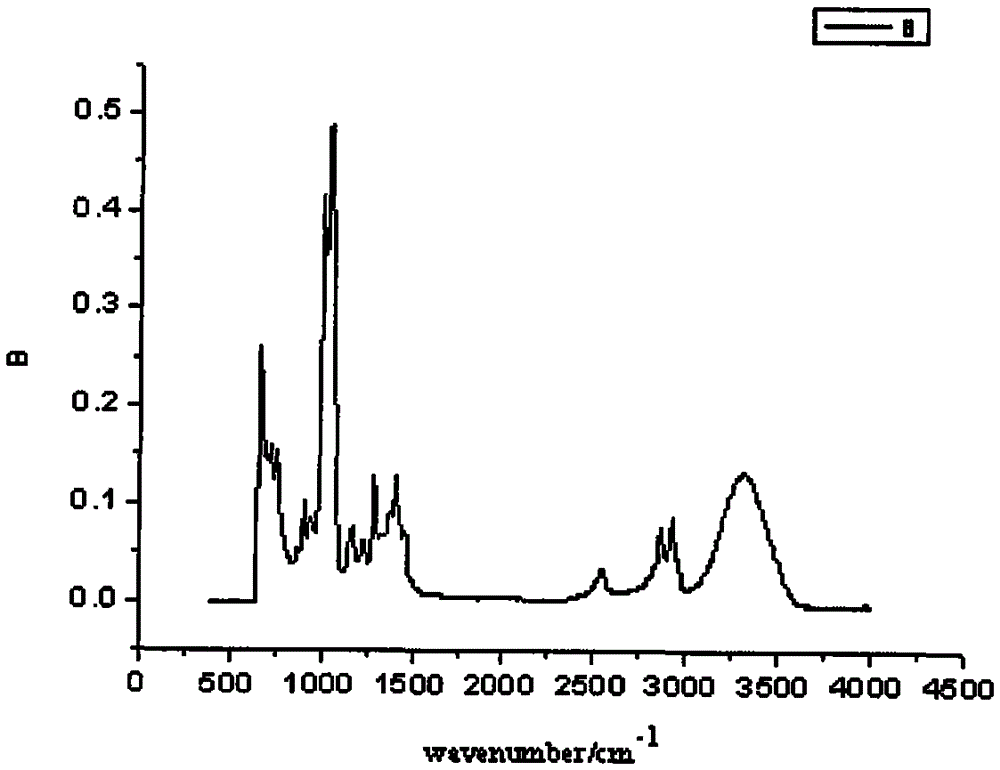
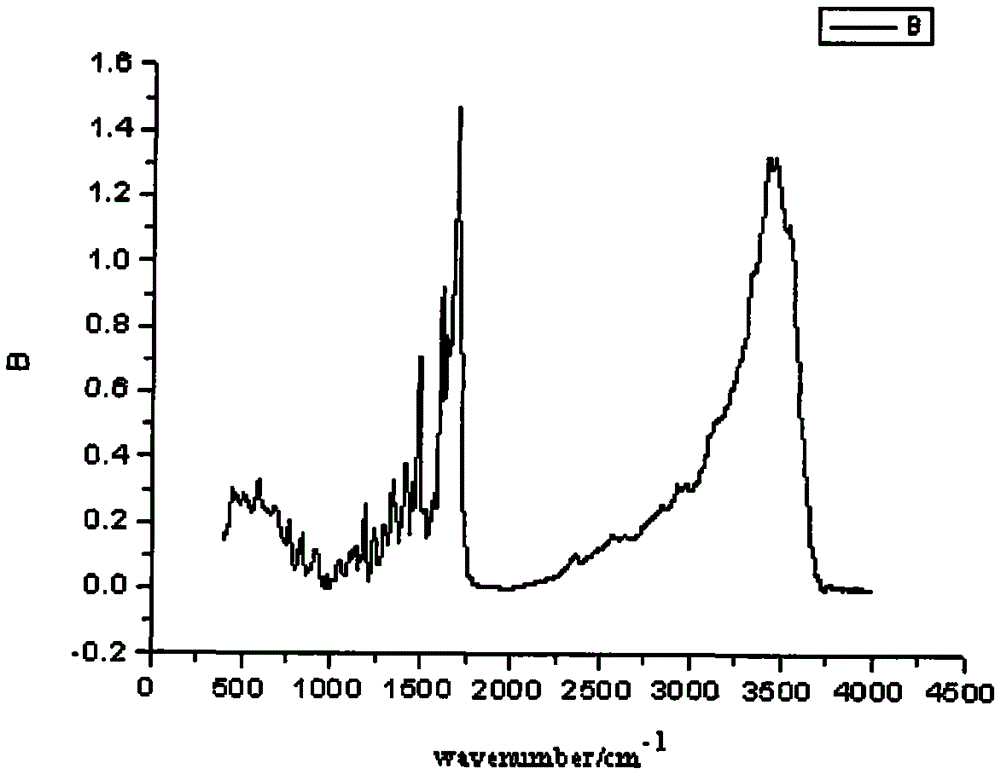
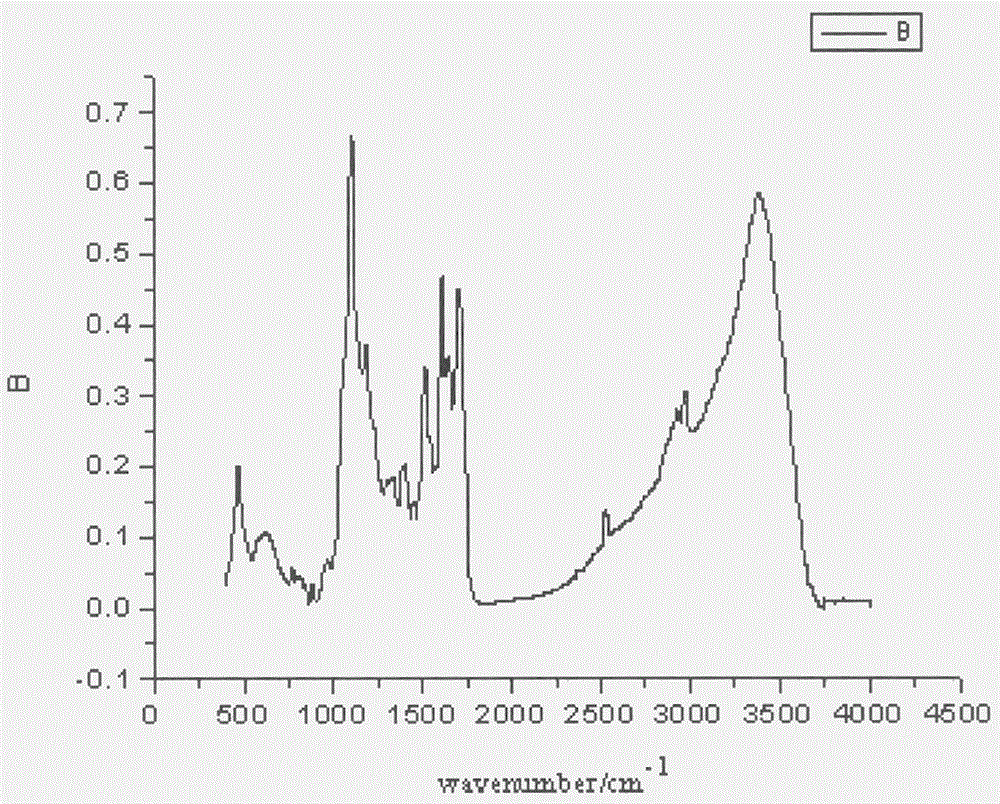
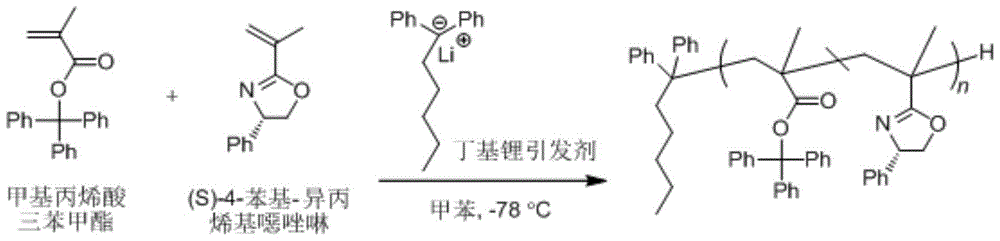
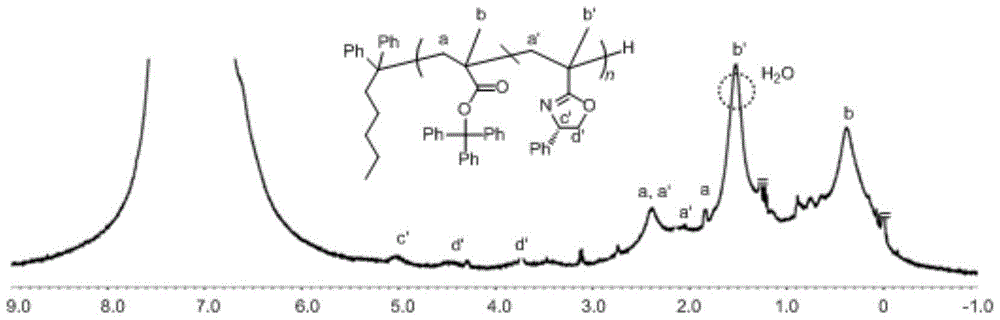
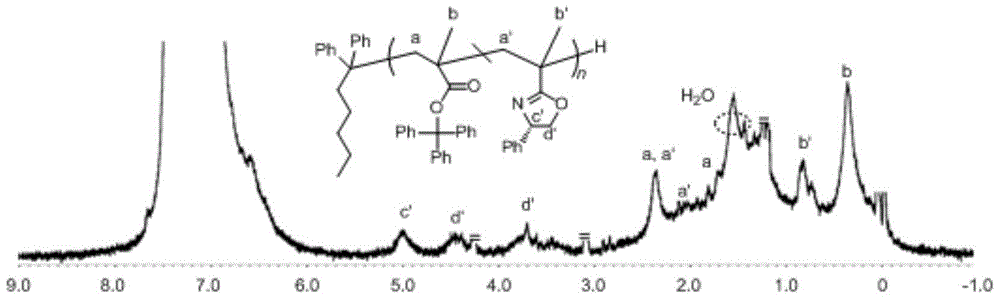
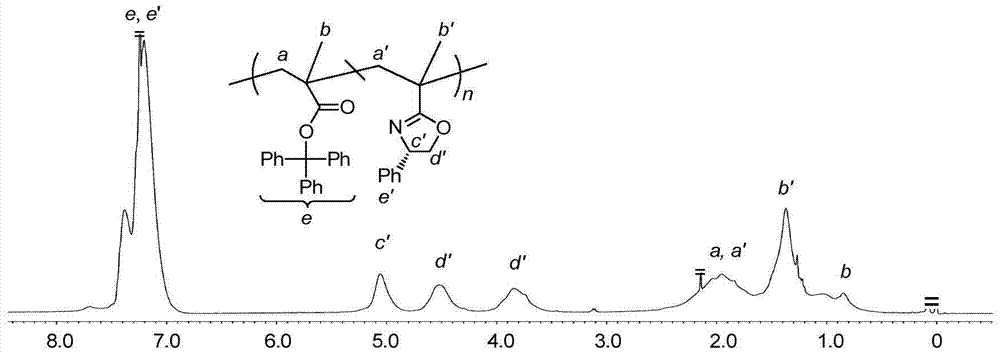
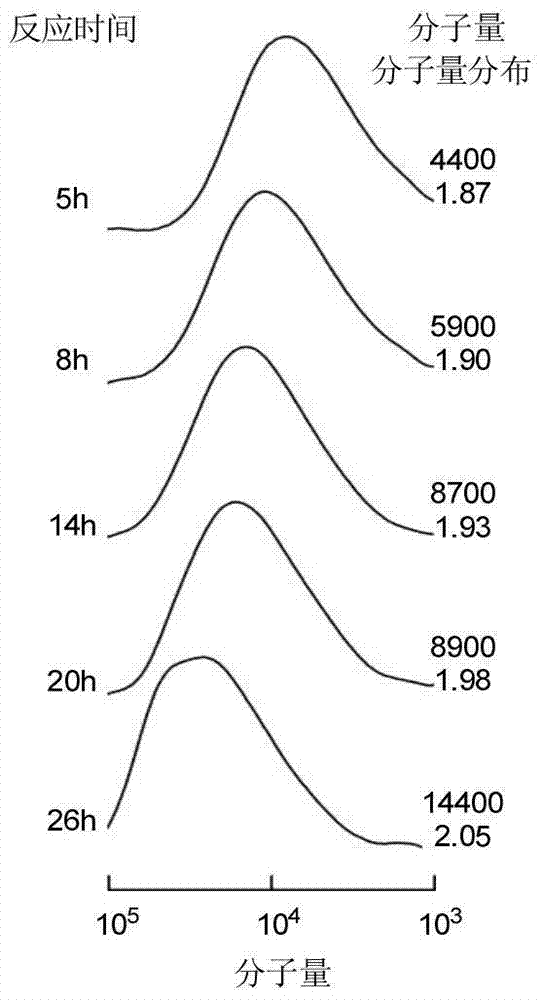
Asymmetric anionic copolymerization method of methacrylate chiral polymer
The invention provides an asymmetric anionic copolymerization method of a methacrylate chiral polymer. The method comprises the following steps: reacting methacrylic acid with trityl chloride to generate a large-volume triphenylmethyl acrylate monomer, carrying out an asymmetric anionic copolymerization reaction on the large-volume triphenylmethyl acrylate monomer and a self-made chiral function monomer under specific anion conditions to obtain a large-volume methacrylate chiral polymer; and purifying the polymer to finally obtain a target product. The structural characterization and analysis of the obtained polymer are carried out by using nuclear magnetic resonance hydrogen spectrum (<1>H-NMR) and an element analysis technology to determine that the molecular structure of the polymer and proportions of all components of the polymer accord with design requirements. Performances of the synthesized copolymer are deeply analyzed through using a gel permeation chromatograph and a polarimeter to obtain the molecular weight of the novel chiral copolymer and the distribution thereof, and the optical active characteristics of the copolymer. The method has the advantages of clear and feasible synthesis route, manure process, simple operation, easy realization, and large-scale batch production.
Owner:HARBIN ENG UNIV
Solid-phase fragment method for bivalirudin synthesis
PendingCN110204611ASolve the technical problems of low purity and low total product yieldSolve technical problems of low total yieldPeptide preparation methodsBulk chemical productionTrityl chloridePeptide sequence
The invention discloses a solid-phase fragment method for bivalirudin synthesis. The method includes: taking trityl chloride resin as starting resin, performing solid-phase synthesis of 1-9 full-protection peptide resin, cutting full-protection nonapeptide off resin, performing inoculation of 10-20 peptide resin in a solid phase, and cracking to obtain a bivalirudin crude product. The method has advantages that by replacement of a step-by-step method with the solid-phase fragment method for bivalirudin synthesis, deprotection times can be reduced, five-membered ring rearrangement side reactionof a 10Gly-9Asn peptide sequence structure under alkaline conditions is avoided, and the content of impurities including Asp9 and beta-Asp9 is effectively reduced. The method is simple in process operation and high in crude product purity, prepared product purity reaches 99.8%, the single impurity content is smaller than 0.1%, and a promising industrial production prospect is achieved.
Owner:HAINAN ZHONGHE PHARM CO LTD
Trityl chloride recovery
Methods of recovering a triarylmethyl halide from a sucrose derivatization process include the steps of(a) forming a mixture including1) a triarylmethylated sucrose derivative including at least one triarylmethyl substituent and at least one acyl substituent on the sucralose,2) triarylmethylated sucrose ester byproducts, and3) an amine;(b) separating from the output of step (a)i) the triarylmethylated sucrose derivative, andii) a mixture including the triarylmethylated sucrose ester byproducts and the amine;(c) removing the amine from the mixture of step (b) ii);(d) contacting the product of step (c) with hydrogen halide to cleave triarylmethyl groups and thereby form a crude triarylmethyl halide component;(e) contacting the crude triarylmethyl halide component with hydrogen halide to form a purified triarylmethyl halide component; and(f) recovering the triarylmethyl halide from the output of step (e).
Owner:TATE & LYLE TECH LTD
Method for copolymerizing asymmetrical free-radicals of methacrylate chiral polymer
The present invention provides a method for asymmetrical free-radicals of methacrylate chiral polymer. The method comprises: firstly performing a reaction on methacrylic acid with trityl chloride to generate a methacrylic acid / trityl chloride monomer in a large volume; then performing asymmetrical free-radical copolymerization on the monomer and a chiral functional monomer under specific free-radical polymerization conditions; and obtaining a methacrylate chiral copolymer in a large volume; and performing purification on the obtained polymer to finally obtain a target product. NMR (1H-NMR) and elemental analytical techonlogies are are used for structural characterization and analysis of the obtained polymer to determine that the molecular structure and the component ratio meet design requirements. Gel permeation chromatography and a polarimeter are used for performing in-depth analysis on performances of the synthesized copolymer to obtain molecular weight and distribution of the new chiral copolymer and features of optical activity. The synthesis route is clear and feasible, the process is sophisticated, the operations are simple, and the method is easy to implement; and the method can be used for large-scale batch production.
Owner:HARBIN ENG UNIV
Trityl chloride recovery
Methods of recovering a triarylmethyl halide from a sucrose derivatization process include the steps of(a) forming a mixture including1) a triarylmethylated sucrose derivative including at least one triarylmethyl substituent and at least one acyl substituent on the sucrose,2) triarylmethylated sucrose ester byproducts, and3) an amine;(b) separating from the output of step (a)i) the triarylmethylated sucrose derivative, andii) a mixture including the triarylmethylated sucrose ester byproducts and the amine;(c) removing the amine from the mixture of step (b) ii);(d) contacting the product of step (c) with hydrogen halide to cleave triarylmethyl groups and thereby form a crude triarylmethyl halide component;(e) contacting the crude triarylmethyl halide component with hydrogen halide to form a purified triarylmethyl halide component; and(f) recovering the triarylmethyl halide from the output of step (e).
Owner:TATE & LYLE TECH LTD
Preparation method of folic acid sulfhydrylation derivative
ActiveCN103980277AHigh yieldImprove separation efficiencyOrganic chemistryPharmaceutical non-active ingredientsThiolAcid derivative
The invention discloses a preparation method of a folic acid sulfhydrylation derivative. The preparation method comprises the following steps that -SH in thiol is protected to obtain a derivative of thiol, so that sulfydryl having high reaction activity is protected; then, in the presence of a catalyst and a dehydrating agent, esterification reaction is carried out between hydroxyl on the protected thiol derivative and folic acid, so that a modified folic acid derivative is obtained; a carbon sulphur bond in the folic acid derivative is broken through reduction reaction; sulfydryl is formed again in a product, and therefore, the target product, namely sulfhydrylation folic acid, is obtained. According to the preparation method disclosed by the invention, trityl chloride is replaced by triphenylcarbinol; the disadvantages of difficulty in separation of products and lower product yield are overcome; the product yield is increased; in the esterification reaction, a solid catalyst (macroporous cation exchange resin) is adopted, so that the separation efficiency of products is greatly improved; micromolecules, such as 4-dimethylamino-pyridine (DMAP), are adopted in normal biological preparation; the micromolecules are easily dissolved in a solvent and difficult to separate.
Owner:QINGDAO UNIV
Prepn of Nim-tribenzyl histidine
The present invention is the preparation process of Nim-tribenzyl histidine in medicine chemical technology field. The preparation process includes dropping or solution of dichloro dimethyl silane into mixture of histidine and organic solvent, dropping triethylamine to neutralize HCl produced in the forgoing step, reflux reaction and lowering temperature, adding organic solution of trityl chloride while dropping triethylamine to neutralize HCl produced, stirring to react to obtain protected silane compound, adding diluent ethyl acetate, adding water to eliminate protection to obtain coarse Nim-tribenzyl histidine product containing silane with less polarity and triphenyl methanol as impurity, extracting with ethyl acetate to eliminate impurity, washing with deionized water before recrystallization, collecting solid and drying to obtain Nim-tribenzyl histidine product. The process may be used in large-scale production with medium yield of 60-65%.
Owner:四川三高生化股份有限公司
Modified producing method for trichlorosaccharose
InactiveCN101125869AHigh recovery rateReduced responseSugar derivativesSugar derivatives preparationSucroseTrityl chloride
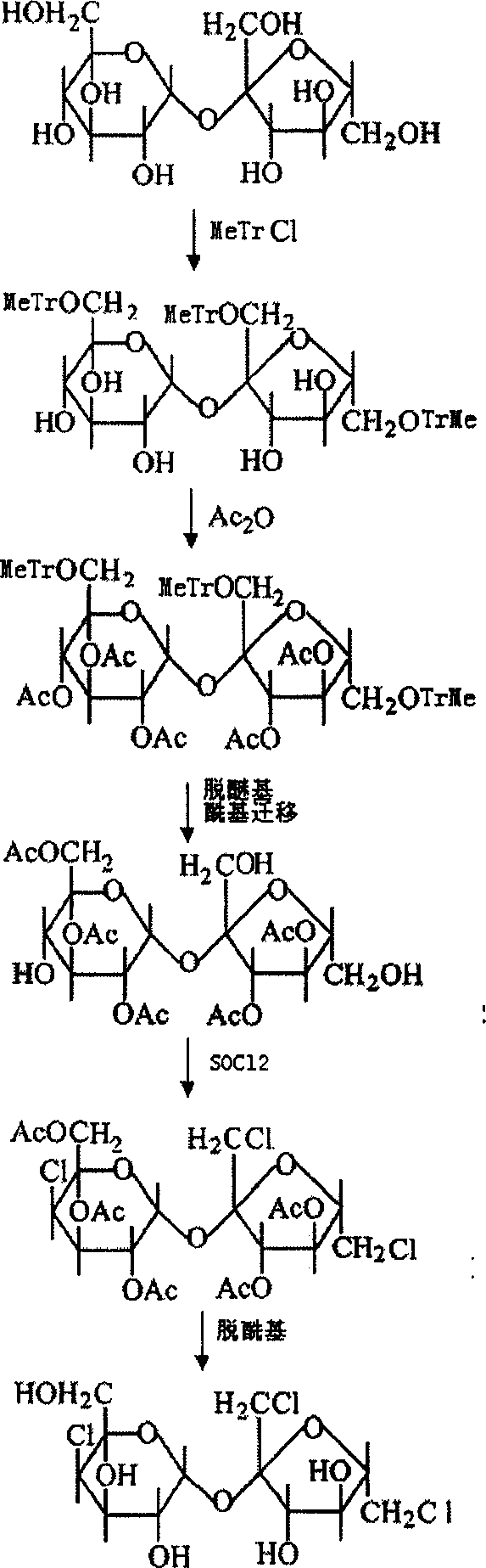
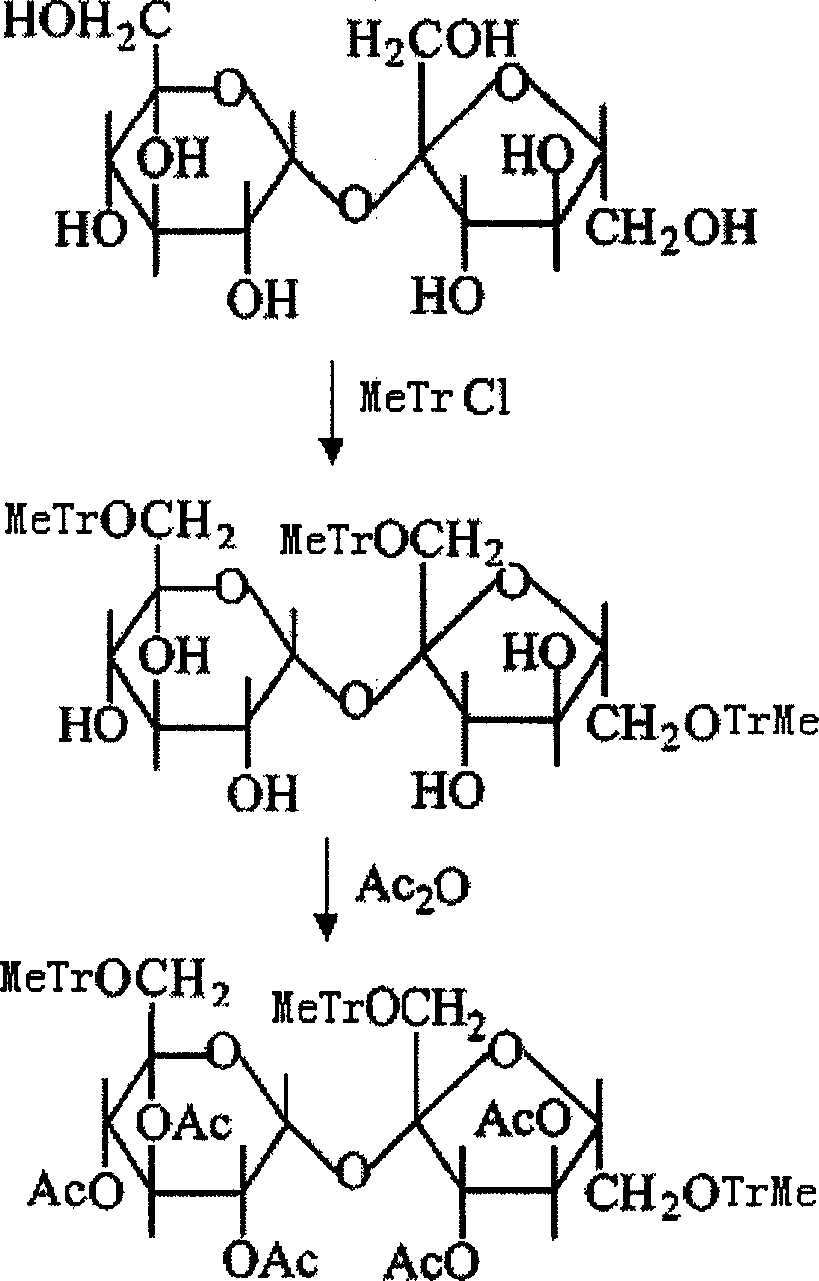
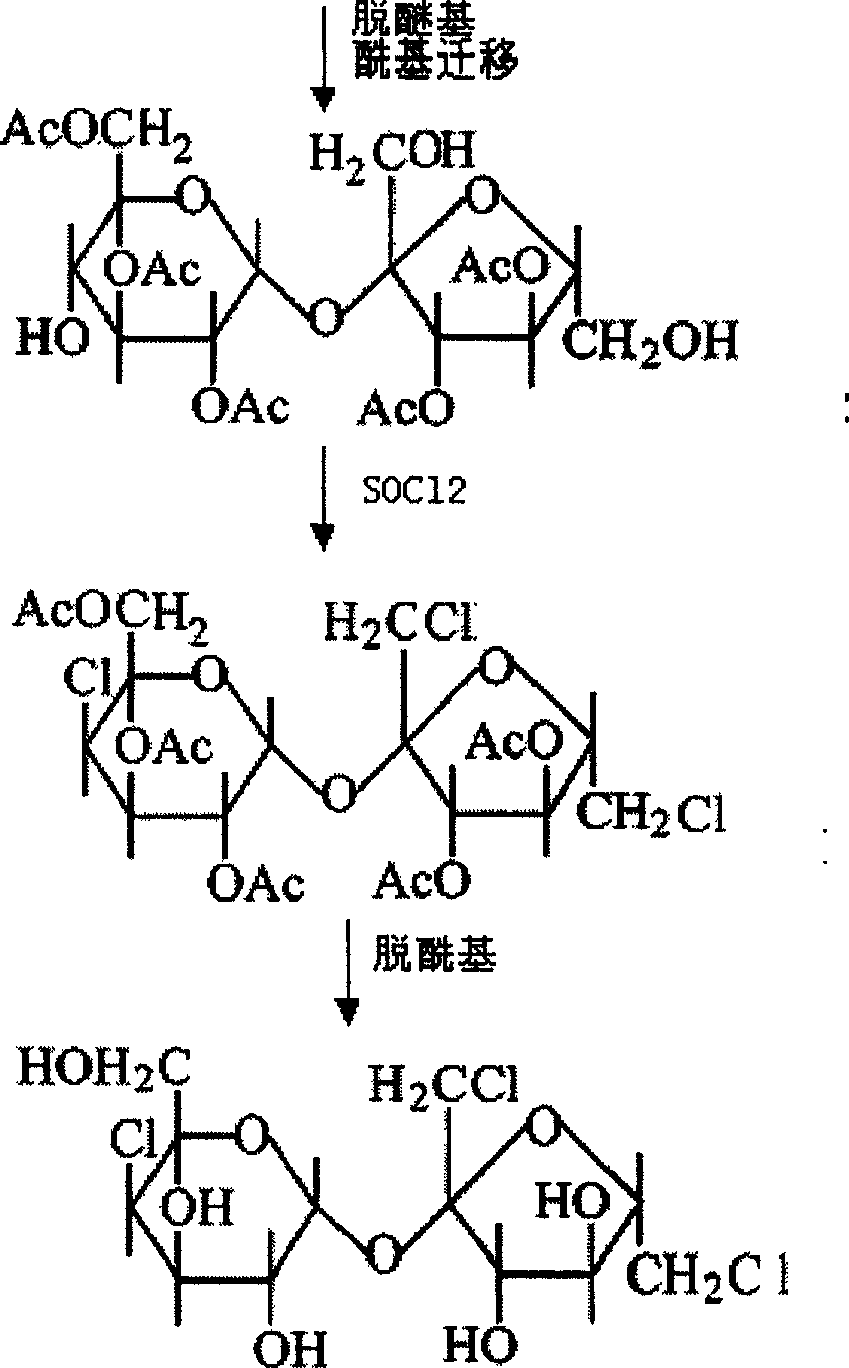
An improved production method of sucralose is characterized by comprising the steps: (1) methoxy on benzene ring is used for replacing the three ortho-hydroxide radical of 6, 1' and 6' in trityl chloride etherified sucrose molecules and acylation reaction is carried out to prepare 6, 1', 6'-trimethoxy benzyl-penta acetic sucrose through etherealization and fine purification; (2) etherealization and acyl removal reactions are carried out to the 6, 1', 6'-trimethoxy benzyl-penta acetic sucrose in water-bearing weak acid at the same time, product 6-PAS generated from the reaction is refined and enters into the next chlorination reaction step; (3) 4, 1', 6'- trichloro-4, 1', 6'-trideoxidation galacto-sucrose (namely the sucralose) is prepared through the chlorination and acyl reaction of the 6-PAS. The invention combines the advantages of a full protective line and a single protective line, spurns the defects of the existing production methods and changes the original 'five steps' of the full protective line into 'four steps', thereby promoting the recovery rate of the full protective line greatly with less reaction steps and consumption, thus resulting in a more economic and environment protective production technology compared with the traditional production technologies of the full protective line or the single protective line.
Owner:清远天基谷醣实业有限公司
Synthesis method of daptomycin
ActiveCN101235080BGood effectSimple methodPeptidesBulk chemical productionSynthesis methods2-chlorotrityl chloride
The invention relates to a daptomycin synthesis method for resolving the technical problem of prior art which uses rose spore streptomycete as raw material to cost high synthesis cost. The synthesis method comprises a, using 2-chlorine trityl chloride resin as carrier, via solid synthesis method to connect the amino acids with protective groups, to obtain protective decapeptide resin while the Fmoc-protective groups are removed in turn, B, connecting decanoic acid via same method, connecting next amino acid via esterification, removing Fmoc-protective groups, and connecting left two amino acids via normal solid method, removing Fmoc-protective groups, c, using trifluoroacetic acid or carrene solution to cut off total protective peptide from resin, drying and completing end-to-end liquid cyclisation in organic solvent, d, using the mixture of trifluoroacetic acid, water and benzene methyl sulfide to cut off peptide from resin to obtain crude product. The invention can synthesize daptomycin.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
Method for synthesizing CA4P
ActiveCN101885738BRaw materials are cheap and easy to getMild reaction conditionsMethine/polymethine dyesGroup 5/15 element organic compoundsChemical synthesisPhosphate
The invention belongs to the field of chemical synthesis and relates to a method for preparing CA4P, in particular to a method for synthesizing CA4P by the following steps that: isovanillin and trityl chloride, which serve as raw materials, are used to form 3- triphenylmethoxy-4-methoxybenzaldehyde which is an intermediate isovanillin protector; the 3- triphenylmethoxy-4-methoxybenzaldehyde and 3,4,5-trimethoxy-triphenyl benzylidene bromide phosphine salt undergo a Wittig reaction , and the protective group is removed by hydrolysis to obtain CA4; and the CA4 and phosphonic acid bis(phenylmethyl)ester react to form benzyl phosphate, and the benzyl group is removed to form a sodium salt to obtain the target compound, namely CA4P.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD
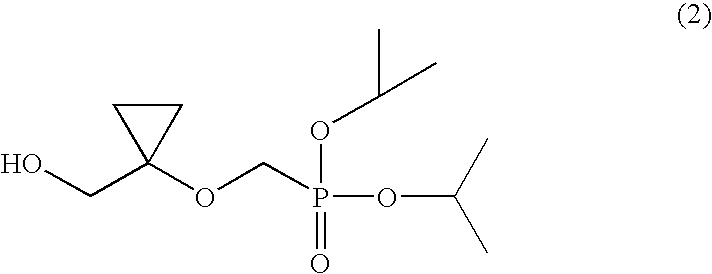
Process for preparing diisopropyl((1-(hydroxymethyl)-cyclopropyl)oxy)methylphosphonate
InactiveUS7795463B2High purityHigh yieldOrganic compound preparationPhosphorus organic compoundsAcetic acidTrityl chloride
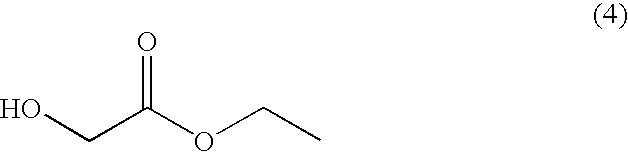
Disclosed is a process for preparing a compound of the following formula (2):including the steps of reacting a compound of the following formula (4):with trityl chloride to prepare trityloxy-acetic acid ethyl ester of the following formula (8):reacting the compound of formula (8) with ethyl magnesium halide to prepare 1-trityloxymethyl-cyclopropanol of the following formula (9):combining the 1-trityloxymethyl-cyclopropanol of formula (9) with diisopropylbromo-methylphosphonate in a solvent in the presence of a base to prepare (1-trityloxymethyl-cyclopropoxymethyl)-phosphonic acid diisopropyl ester of the following formula (10):as a solid form, and converting the trityl group of the compound of formula (10) into a hydroxyl group.
Owner:LG LIFE SCI LTD
Method for synthesizing sucralose
InactiveCN101654467AEase of large-scale industrial productionIncrease profitSugar derivativesSugar derivatives preparationSucroseTrityl chloride
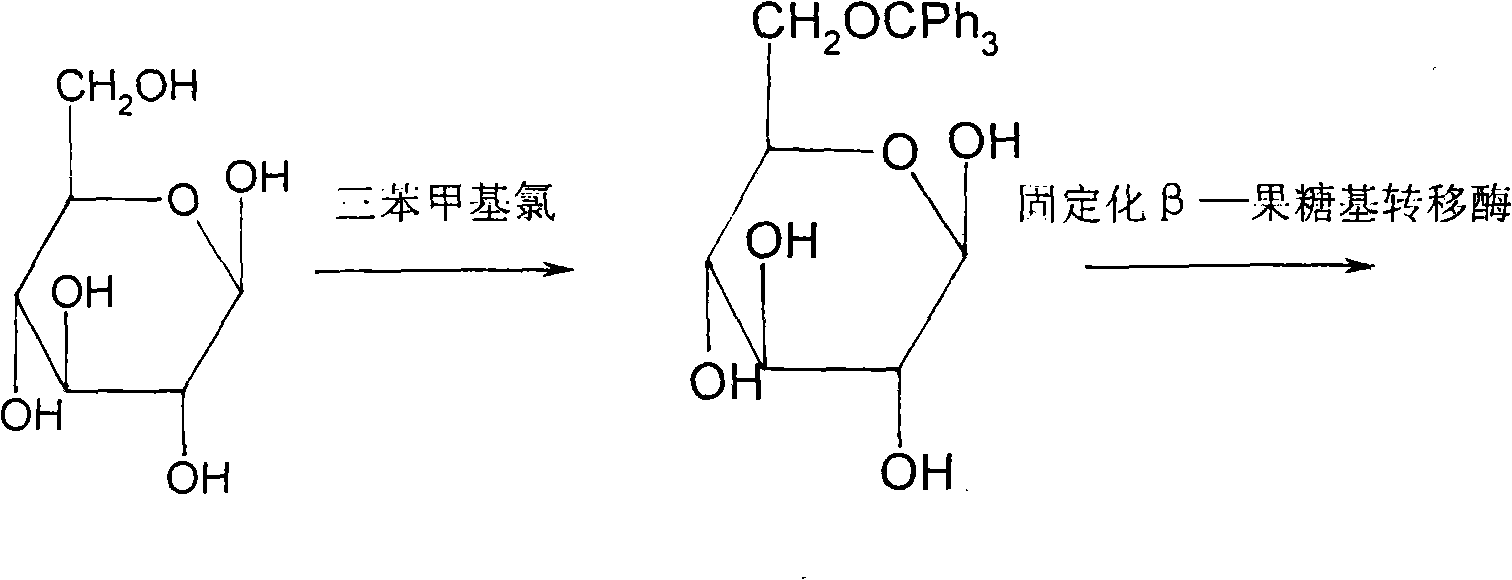
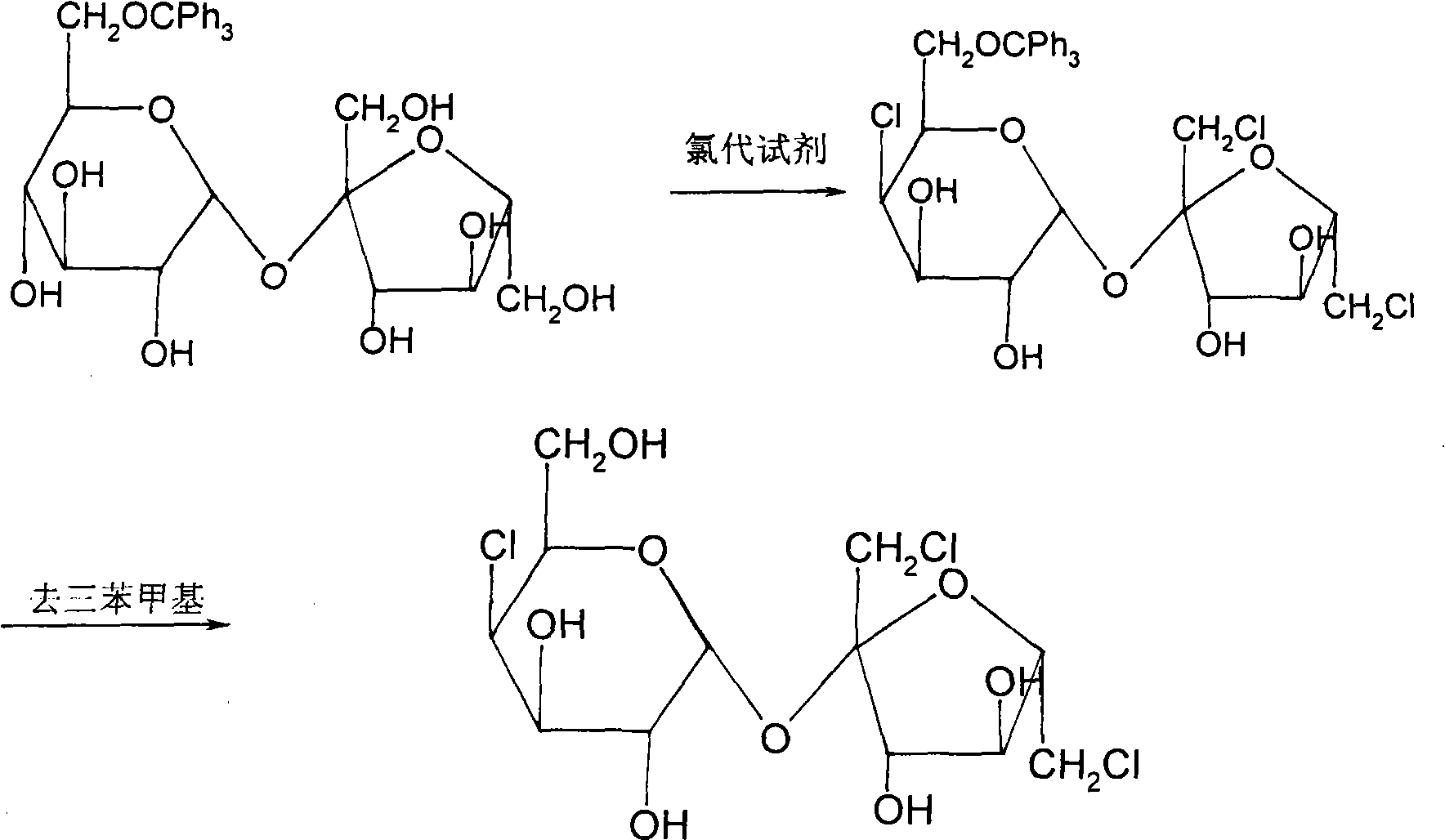
The invention discloses a method for synthesizing sucralose, which is characterized by comprising the following steps that: glucose reacts with trityl chloride in a solvent DMF to form 6-O-triphenyl methyl glucose; the 6-O-triphenyl methyl glucose reacts with sucrose to produce 6-O-triphenyl methyl sucrose in the presence of an immobilized beta-fructosyltransferase; the 6-O-triphenyl methyl sucrose is chloridized; and the triphenyl methyl is removed from a chlorination product obtained to form sucralose. The method for synthesizing sucralose has the advantages of high sucralose yield, a few purification steps, compact and simple process, few side reactions and easy realization of scale industrial production.
Owner:南京仕浪药业有限公司
A kind of preparation method of folic acid thiolated derivative
ActiveCN103980277BHigh yieldImprove separation efficiencyOrganic chemistryPharmaceutical non-active ingredientsSolventIon-exchange resin
The invention discloses a preparation method of a folic acid sulfhydrylation derivative. The preparation method comprises the following steps that -SH in thiol is protected to obtain a derivative of thiol, so that sulfydryl having high reaction activity is protected; then, in the presence of a catalyst and a dehydrating agent, esterification reaction is carried out between hydroxyl on the protected thiol derivative and folic acid, so that a modified folic acid derivative is obtained; a carbon sulphur bond in the folic acid derivative is broken through reduction reaction; sulfydryl is formed again in a product, and therefore, the target product, namely sulfhydrylation folic acid, is obtained. According to the preparation method disclosed by the invention, trityl chloride is replaced by triphenylcarbinol; the disadvantages of difficulty in separation of products and lower product yield are overcome; the product yield is increased; in the esterification reaction, a solid catalyst (macroporous cation exchange resin) is adopted, so that the separation efficiency of products is greatly improved; micromolecules, such as 4-dimethylamino-pyridine (DMAP), are adopted in normal biological preparation; the micromolecules are easily dissolved in a solvent and difficult to separate.
Owner:QINGDAO UNIV
Asymmetric anionic copolymerization method of methacrylate chiral polymer
The invention provides an asymmetric anionic copolymerization method of a methacrylate chiral polymer. The method comprises the following steps: reacting methacrylic acid with trityl chloride to generate a large-volume triphenylmethyl acrylate monomer, carrying out an asymmetric anionic copolymerization reaction on the large-volume triphenylmethyl acrylate monomer and a self-made chiral function monomer under specific anion conditions to obtain a large-volume methacrylate chiral polymer; and purifying the polymer to finally obtain a target product. The structural characterization and analysis of the obtained polymer are carried out by using nuclear magnetic resonance hydrogen spectrum (<1>H-NMR) and an element analysis technology to determine that the molecular structure of the polymer and proportions of all components of the polymer accord with design requirements. Performances of the synthesized copolymer are deeply analyzed through using a gel permeation chromatograph and a polarimeter to obtain the molecular weight of the novel chiral copolymer and the distribution thereof, and the optical active characteristics of the copolymer. The method has the advantages of clear and feasible synthesis route, manure process, simple operation, easy realization, and large-scale batch production.
Owner:HARBIN ENG UNIV
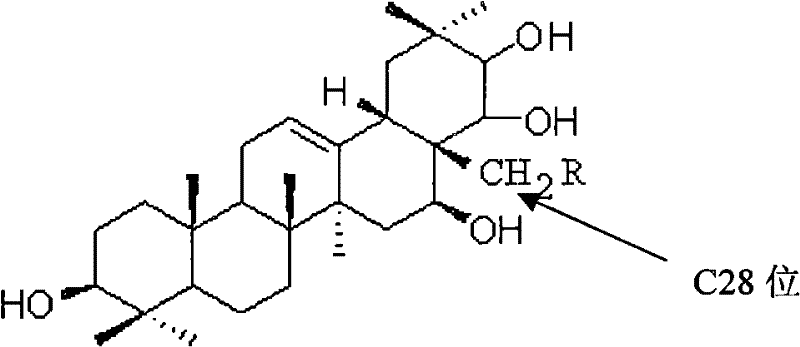
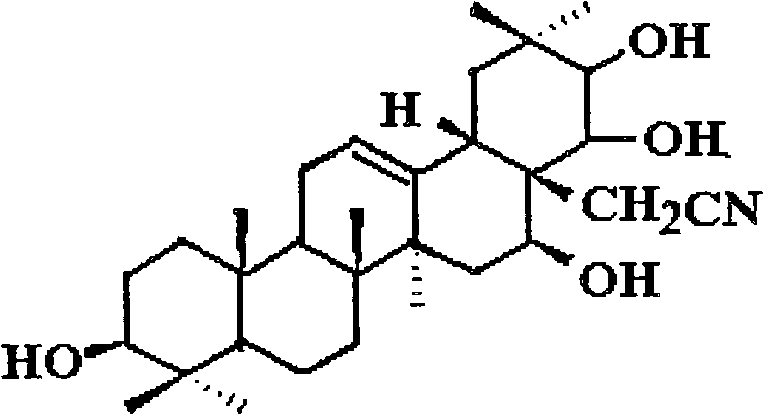
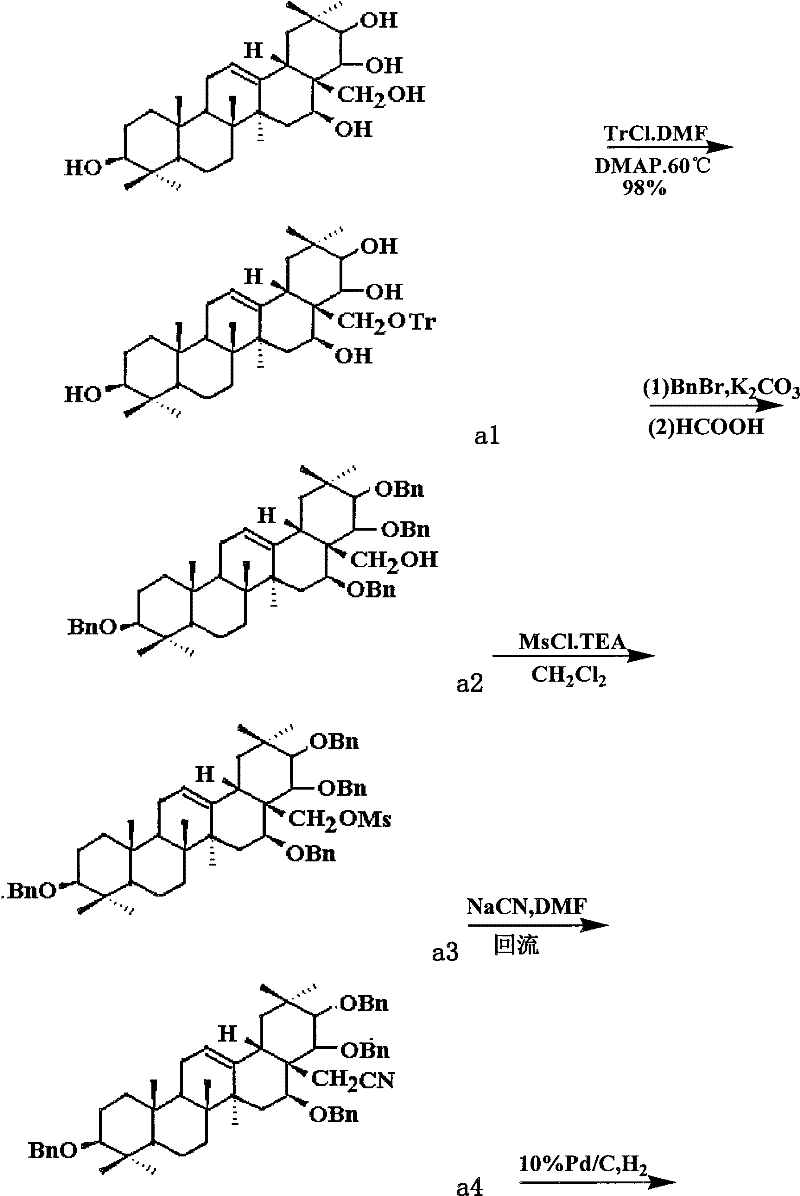
Theasapogenol derivative with anti-tumour activity and preparing method and application thereof
InactiveCN102030805BImprove anti-tumor activityTo achieve directed connectionOrganic active ingredientsSteroids preparationBenzoyl bromideSolvent
The present invention discloses a theasapogenol derivative with anti-tumour activity and preparing method and application thereof. The theasapogenol derivative with anti-tumour activity has a structure shown as formula I; and the preparing method comprises the following steps of: (1) reacting theasapogenol, which is the raw material, with trityl chloride in a pyridine solvent by using 4-dimethylamino pyridine as a catalyst, reacting the reaction product with benzyl bromide, and reacting the product thereof with formic acid to obtain alcohol terpenoid compound; (2) introducing in methylsulfonyl at C-28 bit based on the alcohol terpenoid compound; introducing in cyano at the C-28 bit with NaCN as a cyaniding reagent; and finally removing benzyl at palladium / carbon under hydrogen reduction condition, so as to obtain the theasapogenol derivative with anti-tumour activity shown as formula I. The theasapogenol derivative of the present invention has high anti-tumour activity; the preparing method and process are simple; and the theasapogenol derivative is convenient for industrial production.
Owner:SOUTH CHINA UNIV OF TECH
Synthesizing nucleoside analog for antivirus
InactiveCN1247579CHigh yieldIncreased site selectivityOrganic chemistryAntiviralsAnti virusBenzoic acid
Compound 3 is dissolved in dry pyridine, reacting with 4,4-dimethoxy trityl chloride to obtain compound 9. Said compound 9 is then removed its hydrogen chloride by reacting with 5-butanol potassium to obtain compound 10, then reacting with m-chloro peroxy benzoic acid to obtain compound 11. Compound 11 and compound 12 have obvious different of Rf values on silca-gel plate, so they are separated by silica column. Compound 11 and compound 12 have obvious difference of Rf values on silia-gel plate, so they are separated by silica column. Compound 11 and compound 12 react with alkali base respectively to obtain compound 13 or compound 15, then being reacted with acetic acid to remove its 4,4-dimethoxy triphenmethyl to obtain final invented products: compound 1, compound 2, compound 14 or compound 16. advantages are: simple operation, single raw material more kinds of final products, with spectrum anti-virus activity.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
A kind of nucleoside bisphosphoramidite and preparation method thereof
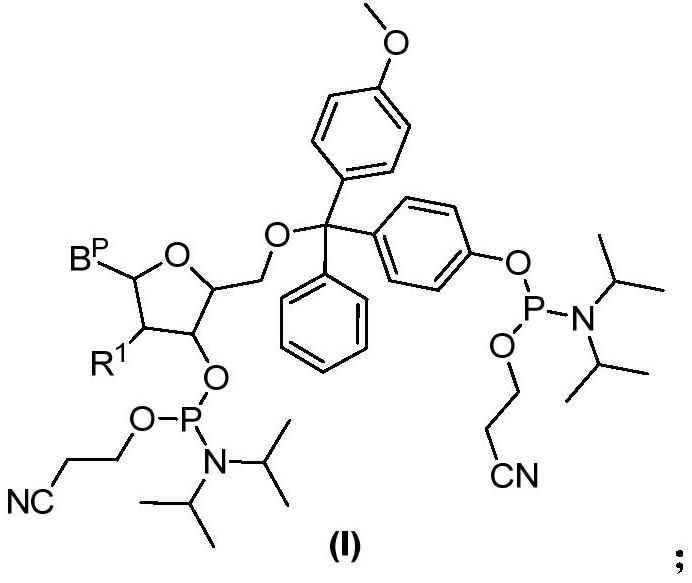
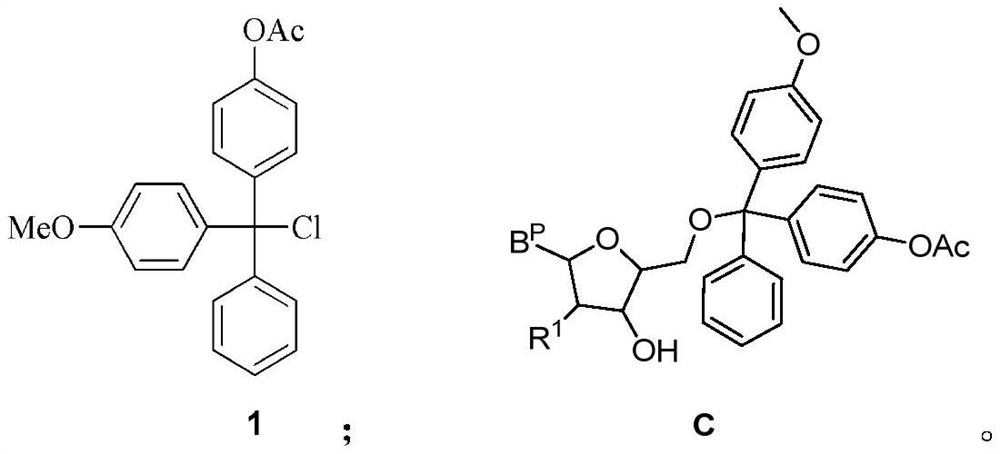
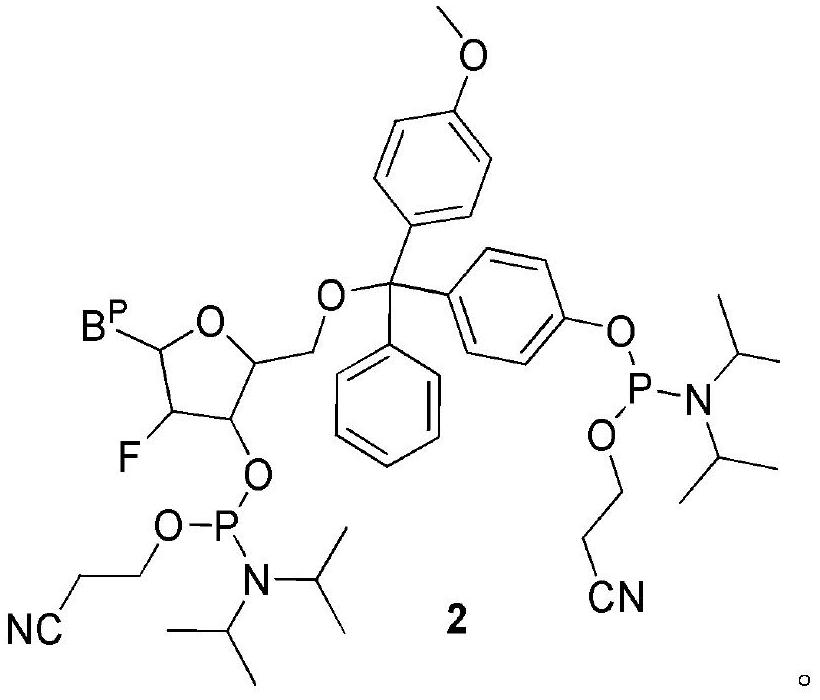
The invention relates to a nucleoside bisphosphoramidite and a preparation method thereof. The method of the present invention is based on the preparation method of the nucleoside bisphosphoramidite starting from 4-methoxy-4'-acetoxytrityl chloride. The nucleoside bisphosphoramidite designed and prepared by the invention can be used for impurity calibration and analysis in oligonucleotide synthesis, thereby facilitating large-scale synthesis, purification and quality control of oligonucleotide drugs.
Owner:SHANGHAI ZHAOWEI TECH DEV
Asymmetric Free Radical Copolymerization of Methacrylate Chiral Polymers
The present invention provides a method for asymmetrical free-radicals of methacrylate chiral polymer. The method comprises: firstly performing a reaction on methacrylic acid with trityl chloride to generate a methacrylic acid / trityl chloride monomer in a large volume; then performing asymmetrical free-radical copolymerization on the monomer and a chiral functional monomer under specific free-radical polymerization conditions; and obtaining a methacrylate chiral copolymer in a large volume; and performing purification on the obtained polymer to finally obtain a target product. NMR (1H-NMR) and elemental analytical techonlogies are are used for structural characterization and analysis of the obtained polymer to determine that the molecular structure and the component ratio meet design requirements. Gel permeation chromatography and a polarimeter are used for performing in-depth analysis on performances of the synthesized copolymer to obtain molecular weight and distribution of the new chiral copolymer and features of optical activity. The synthesis route is clear and feasible, the process is sophisticated, the operations are simple, and the method is easy to implement; and the method can be used for large-scale batch production.
Owner:HARBIN ENG UNIV
A kind of preparation method of antiviral drug entecavir
ActiveCN102417506BWide variety of sourcesLow costOrganic chemistryBulk chemical productionPurification methodsEntecavir
The invention relates to a preparation method of an antiviral drug Entecavir. Taking D-glucose and acetone as starting materials, the method has advantages of easy operation, high yield, easy separation and purification, wide source of raw materials and low cost. The selectivity and stereospecificity are controlled at the beginning of a reaction to effectively inhibit the production of chiral isomer. The purification method of product is simple with high yield. A key intermediate of the reaction is 4- methylol-5- methylene cyclopentane-1,3-diol, of which 4- methylol is selectively protected by trityl chloride, and the intermediate combines with 2-amino-6-chloropurine by mitsunobu. Because of the steric hinderance effect, the protecting group can effectively protect target group and the operation is easy to carry out while removing the protecting group.
Owner:HAINAN PULIN PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com