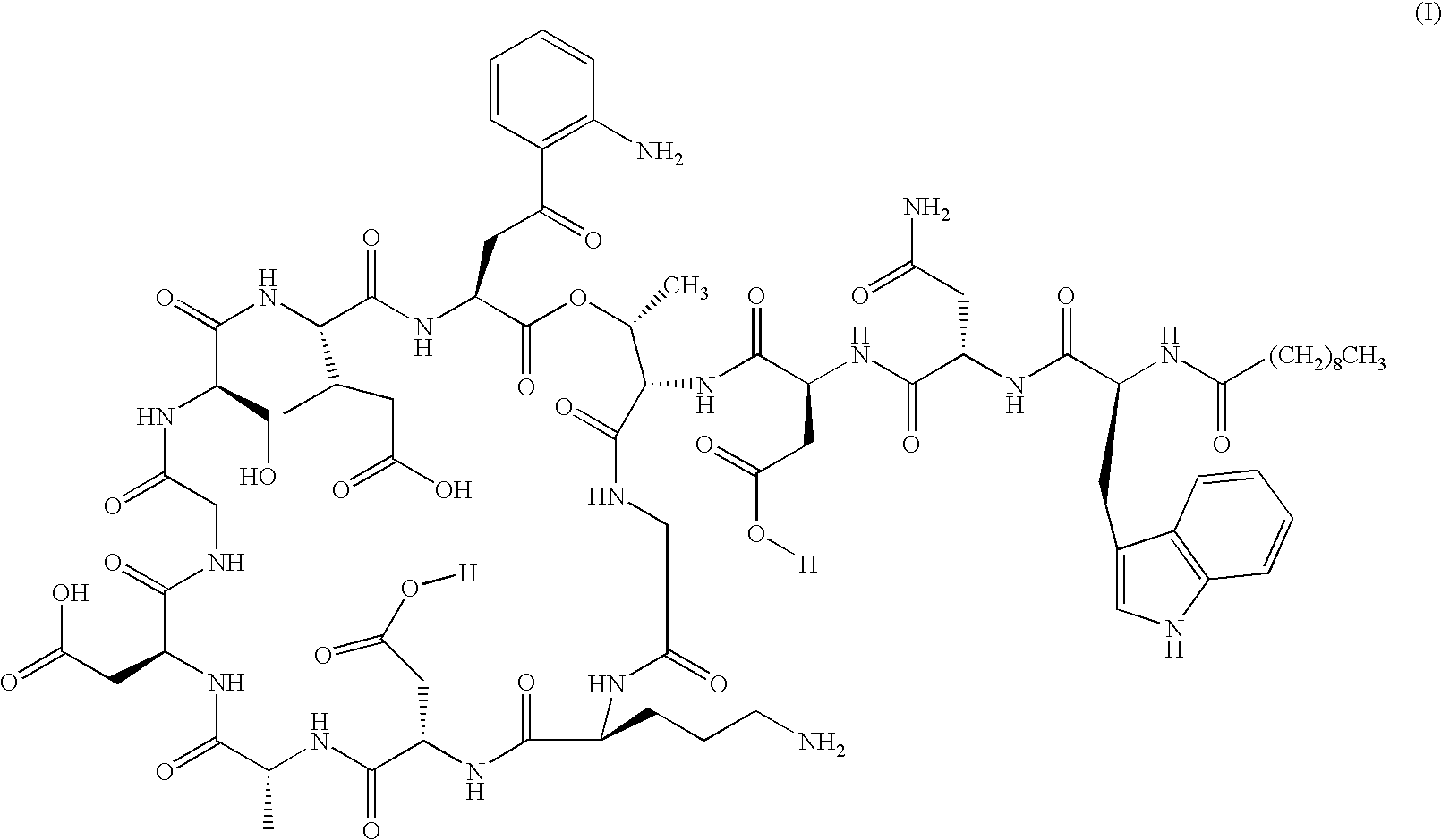
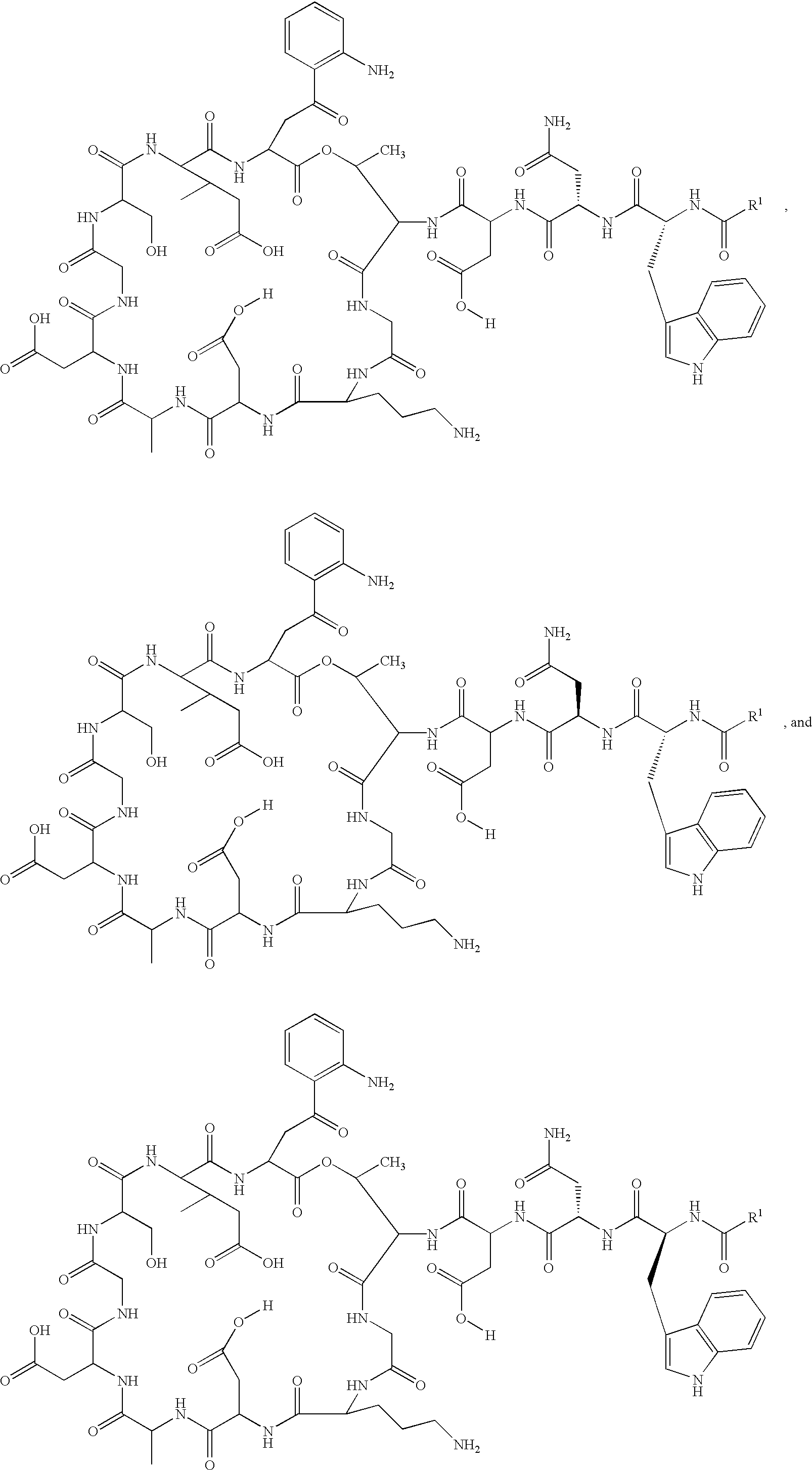
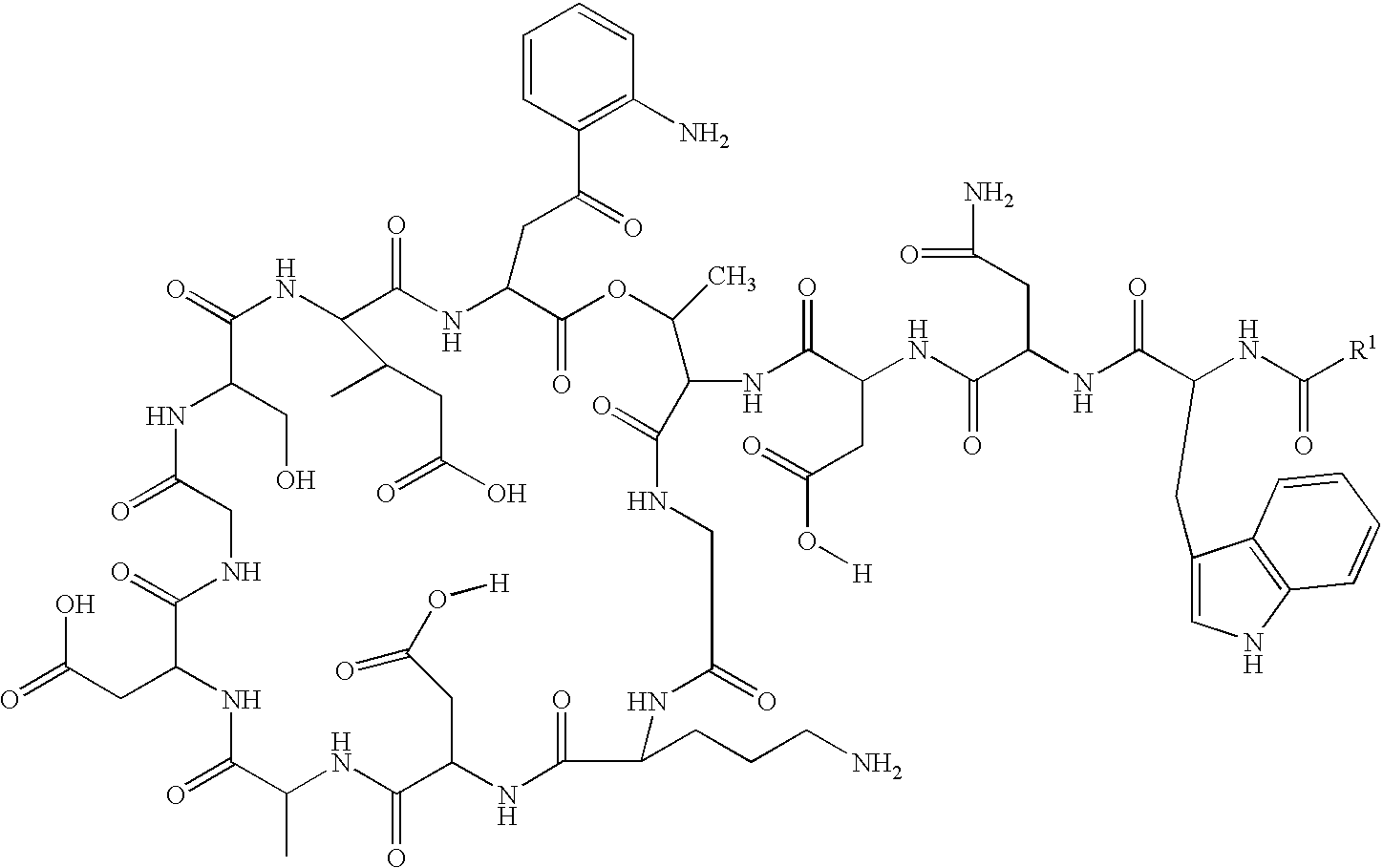
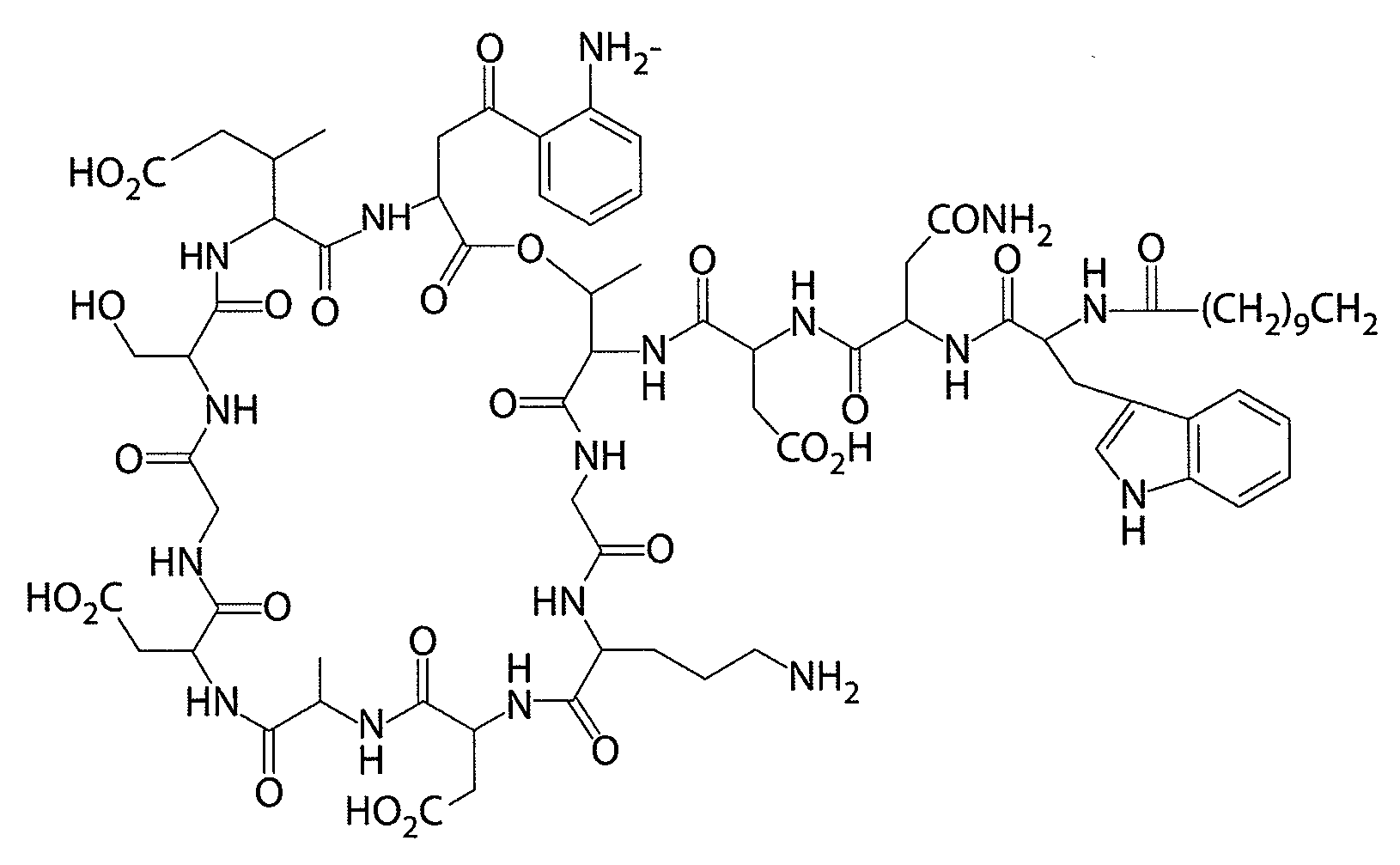
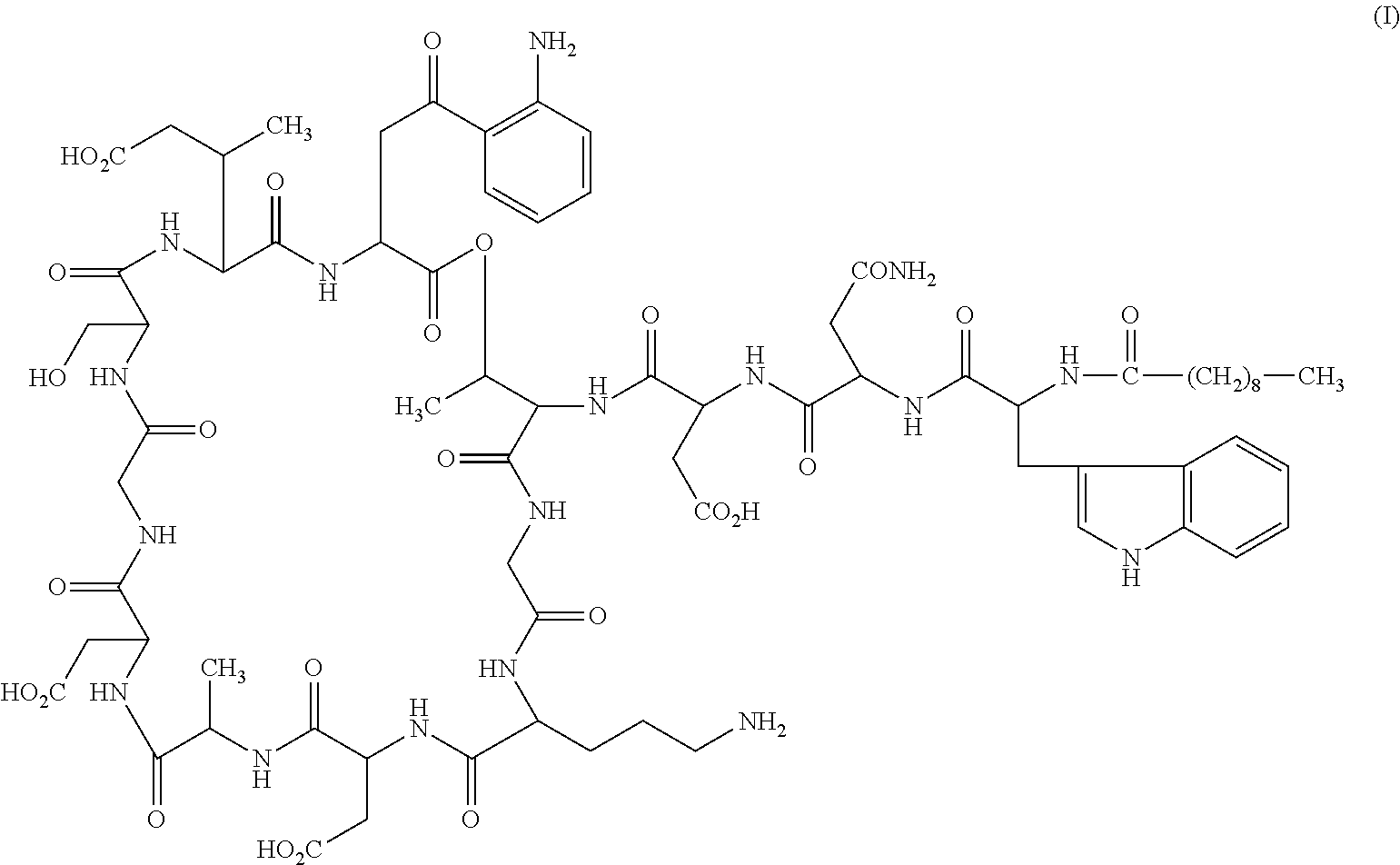
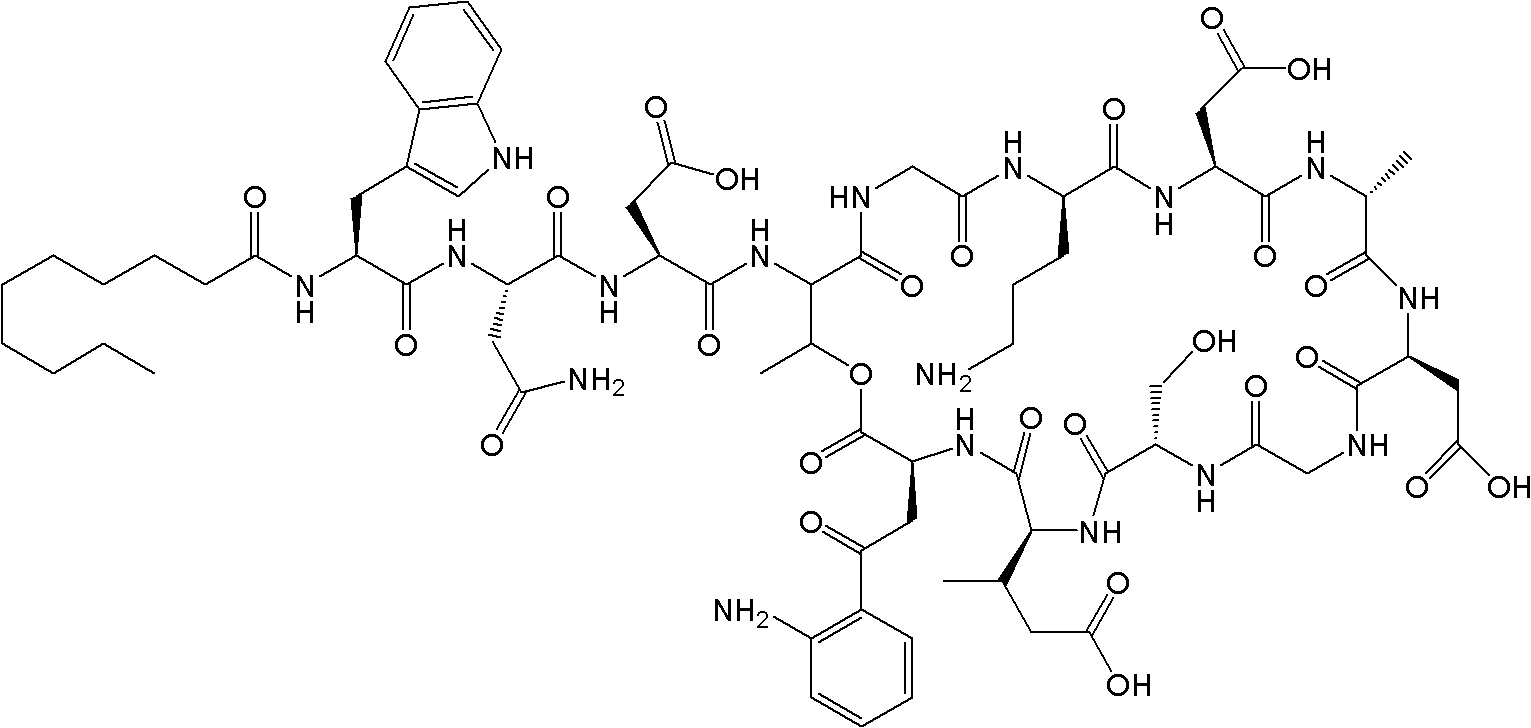
Patents
Literature
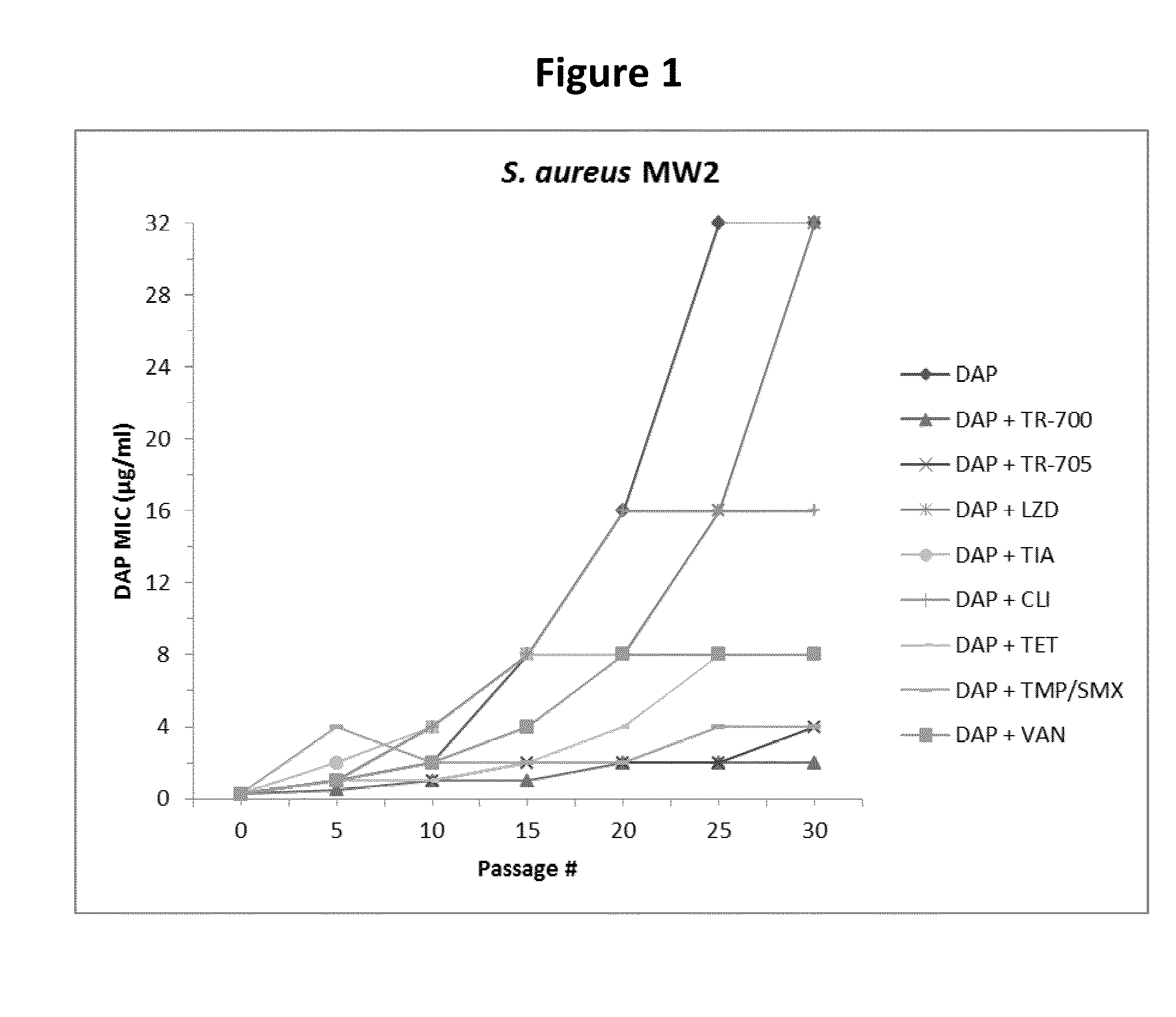
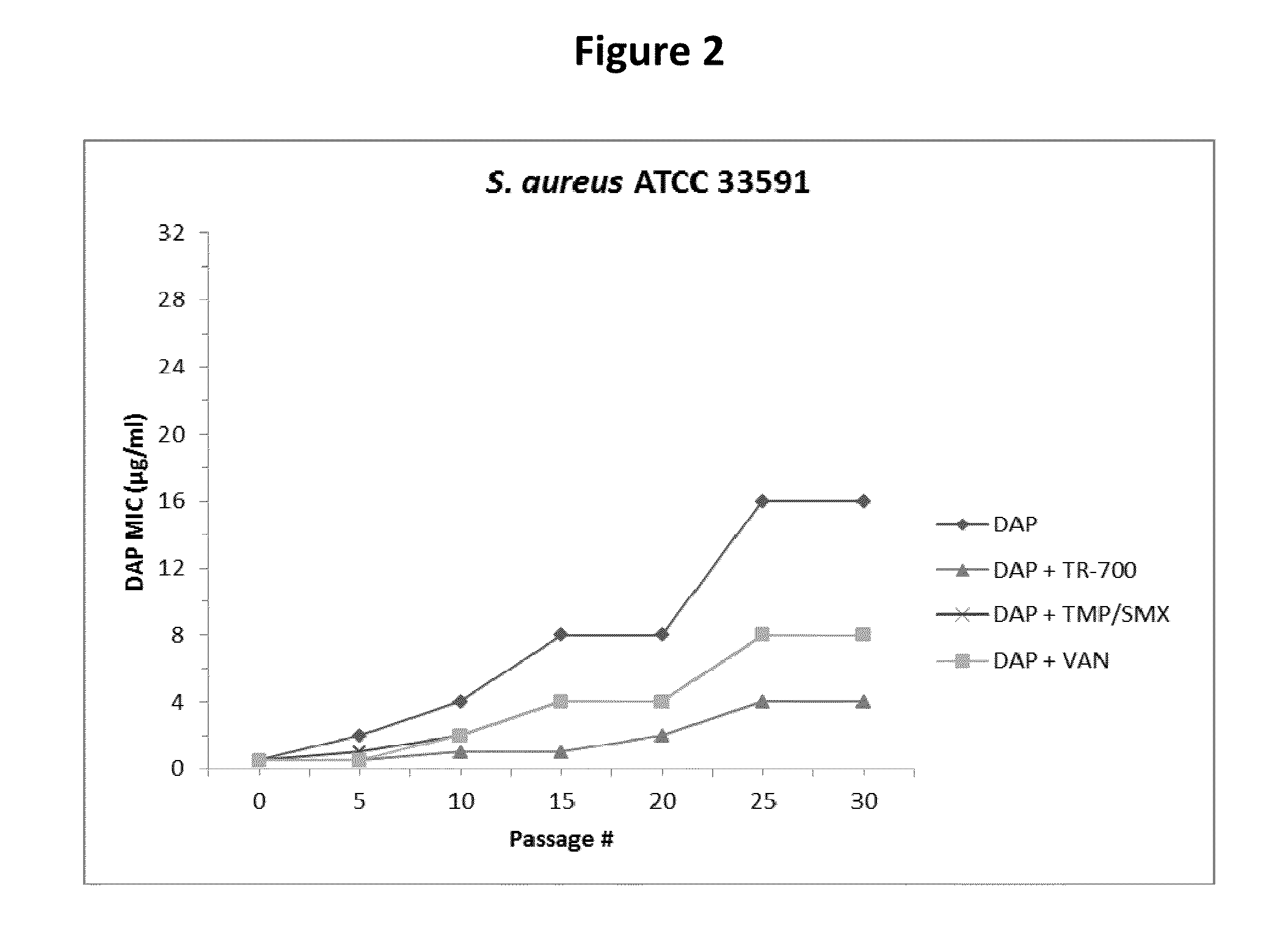
140 results about "Daptomycin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
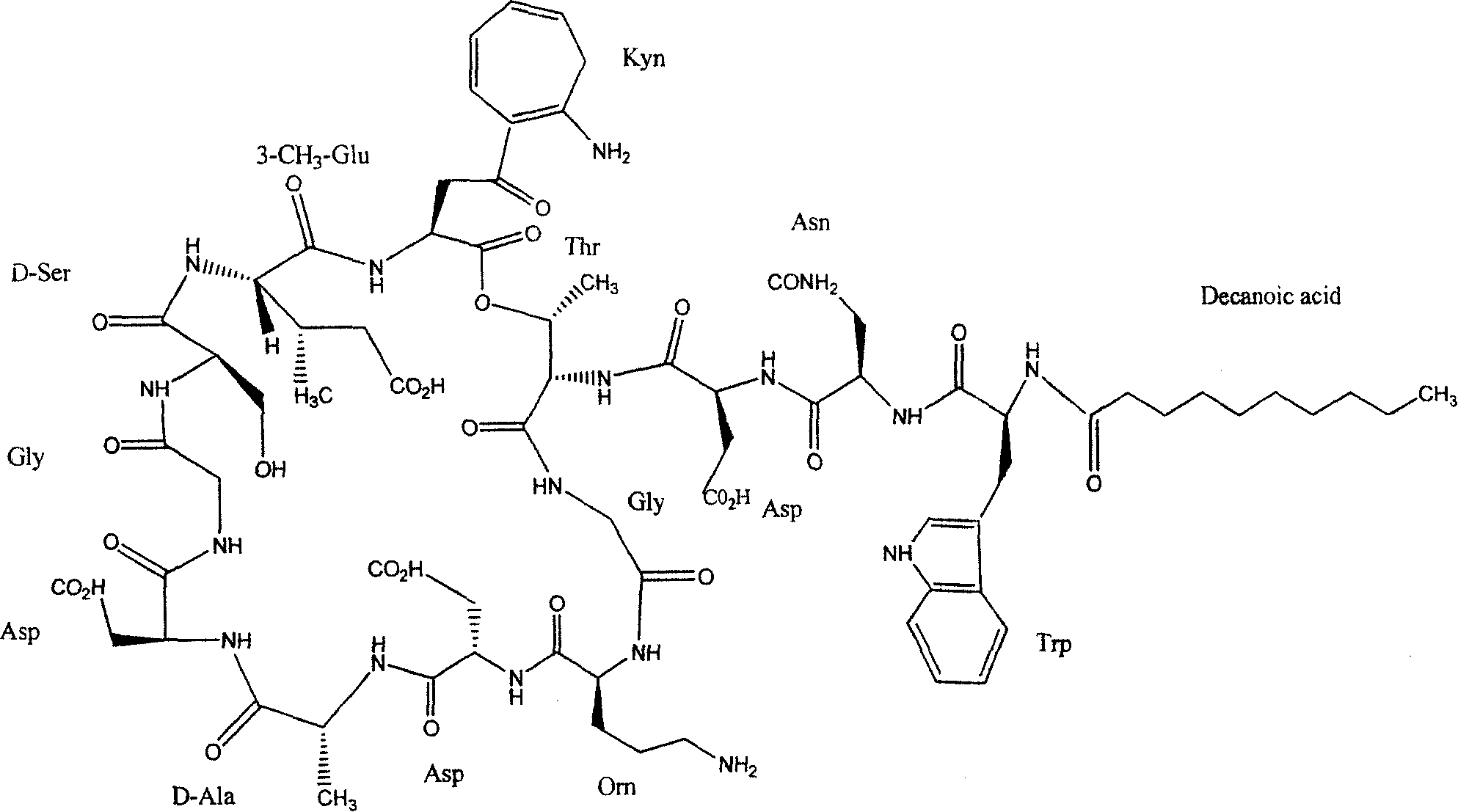
This medication is an antibiotic used to treat serious bacterial infections.
Daptomycin freeze-dried preparation for injection and preparing method
InactiveCN1616083AImprove solubilityEasy to storeAntibacterial agentsPowder deliveryFreeze-dryingAntibiotic Y
Active medicine daptomycin is mixed with certain amount of sodium hydroxide and pH regulator, the mixture is compounded with injection water to form solution in certain concentration, and the solution is filled into antibiotic bottle and freeze dried for 30-60 hr to prepare loose and water soluble freeze dried preparation. The freeze dried daptomycin preparation for injection has excellent medicine stability and convenience in carrying and clinical application. The preparation is used specially for intravenous injection to treat skin infection with resistance to other antibiotics.
Owner:魏雪纹 +2
Methods for administration of antibiotics
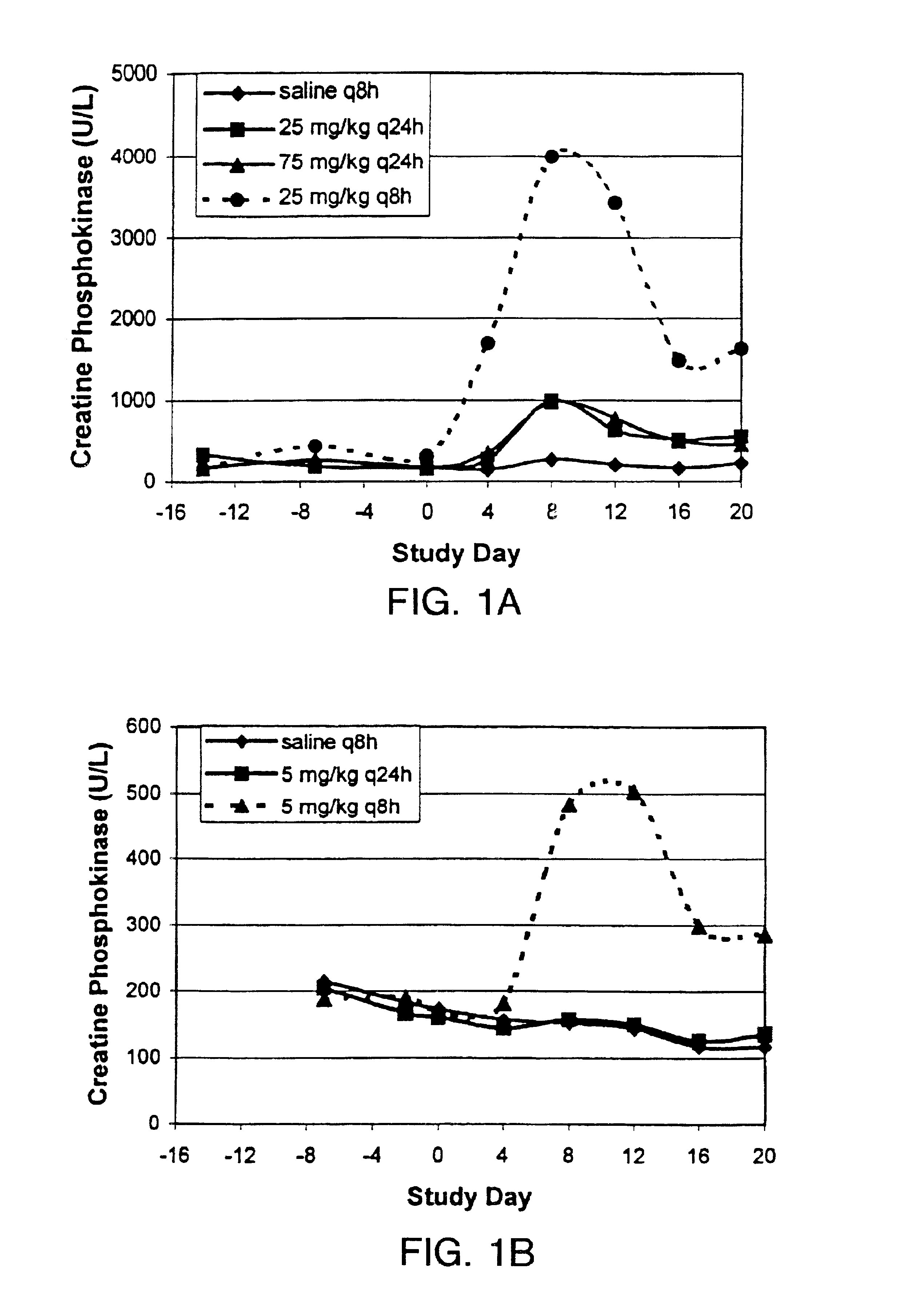
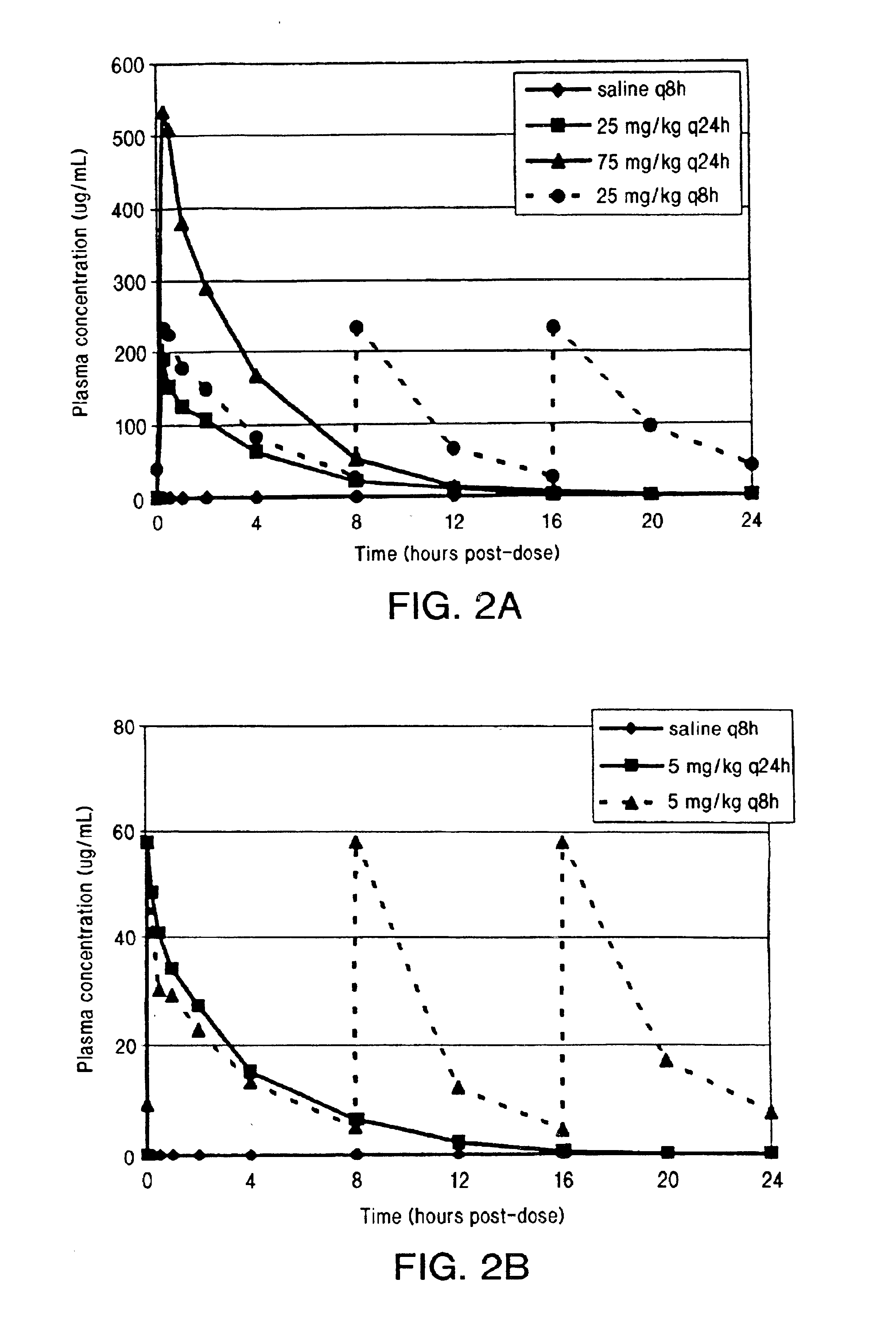
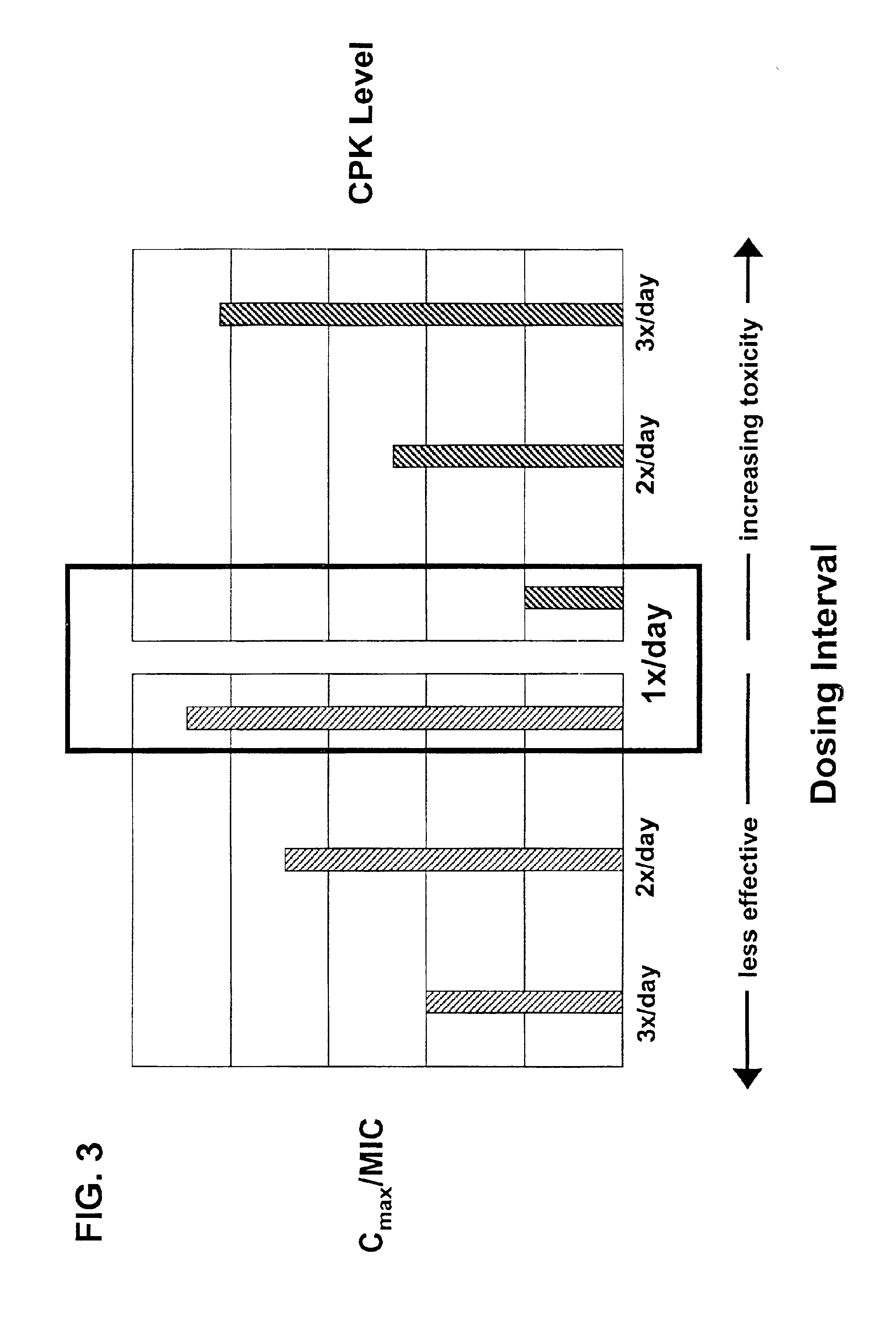
InactiveUS6852689B2Minimizes skeletal muscle toxicitySufficient efficacy levelAntibacterial agentsBiocideAntibiotic YSkeletal muscle
The invention provides methods for administering a therapeutically effective amount of daptomycin while minimizing skeletal muscle toxicity. The methods provide daptomycin administration at a dosing interval of 24 hours or greater. This long dosing interval minimizes skeletal muscle toxicity and allows for higher peak concentrations of daptomycin, which is related to daptomycin's efficacy. The invention also provides methods of administering lipopeptide antibiotics other than daptomycin while minimizing skeletal muscle toxicity by administering a therapeutically effective amount of the lipopeptide antibiotic at a dosage interval that does not result in muscle toxicity. The invention also provides methods of administering quinupristin / dalfopristin while minimizing skeletal muscle toxicity by administering a therapeutically effective amount of quinupristin / dalfopristin at a dosage interval that dos not result in muscle toxicity.
Owner:CUBIST PHARMA INC
Purification and preparation method of high-purity Daptomycin
ActiveCN101830970ASuitable for large-scale productionAntibacterial agentsPeptide preparation methodsCompound aElution
The invention discloses a purification and preparation method of high-purity Daptomycin, which comprises the following steps of compounding a buffer solution and coarse Daptomycin into a sample solution, absorbing by using composite YT-01reverse phase silica gel columns and carrying out gradient elution or constant elution with the water solution of a strong polar solvent, which is used as a resolution agent. The chromatographic purity is higher than 98%.
Owner:CHENGDU YATU BIOLOGICAL TECH
Methods for preparing purified lipopeptides
The present invention relates to crystalline and crystal-like forms of lipopeptides, including daptomycin, a lipopeptide antibiotic with potent bactericidal activity against gram-positive bacteria, including strains that are resistant to conventional antibiotics. The present invention relates to methods of purifying lipopeptides, including daptomycin, a lipopeptide antibiotic with potent bactericidal activity against gram-positive bacteria, including strains that are resistant to conventional antibiotics. The present invention also relates to pharmaceutical compositions comprising the purified form of the lipopeptide and methods of using these compositions.
Owner:CUBIST PHARMA INC
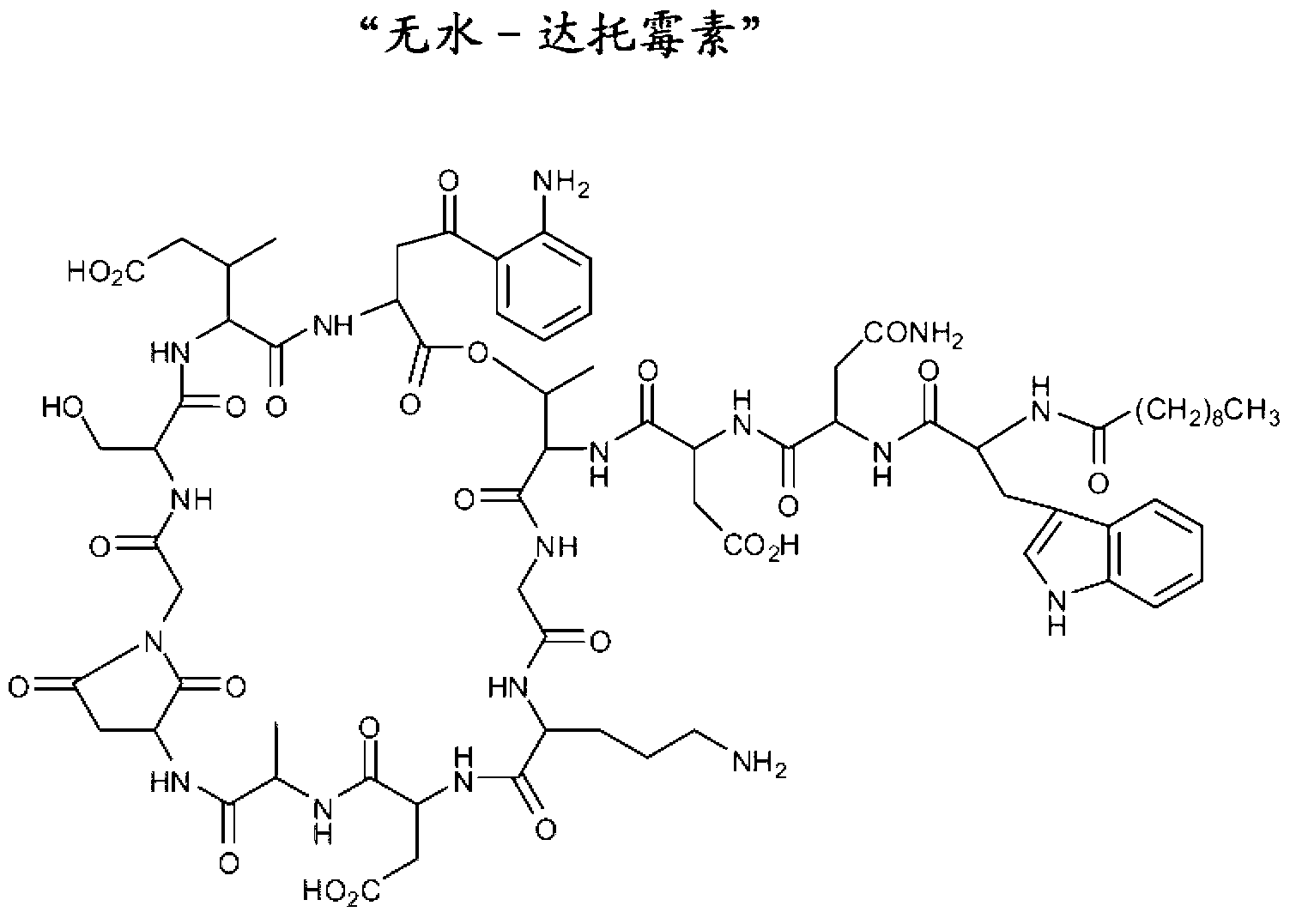
Formulations of daptomycin
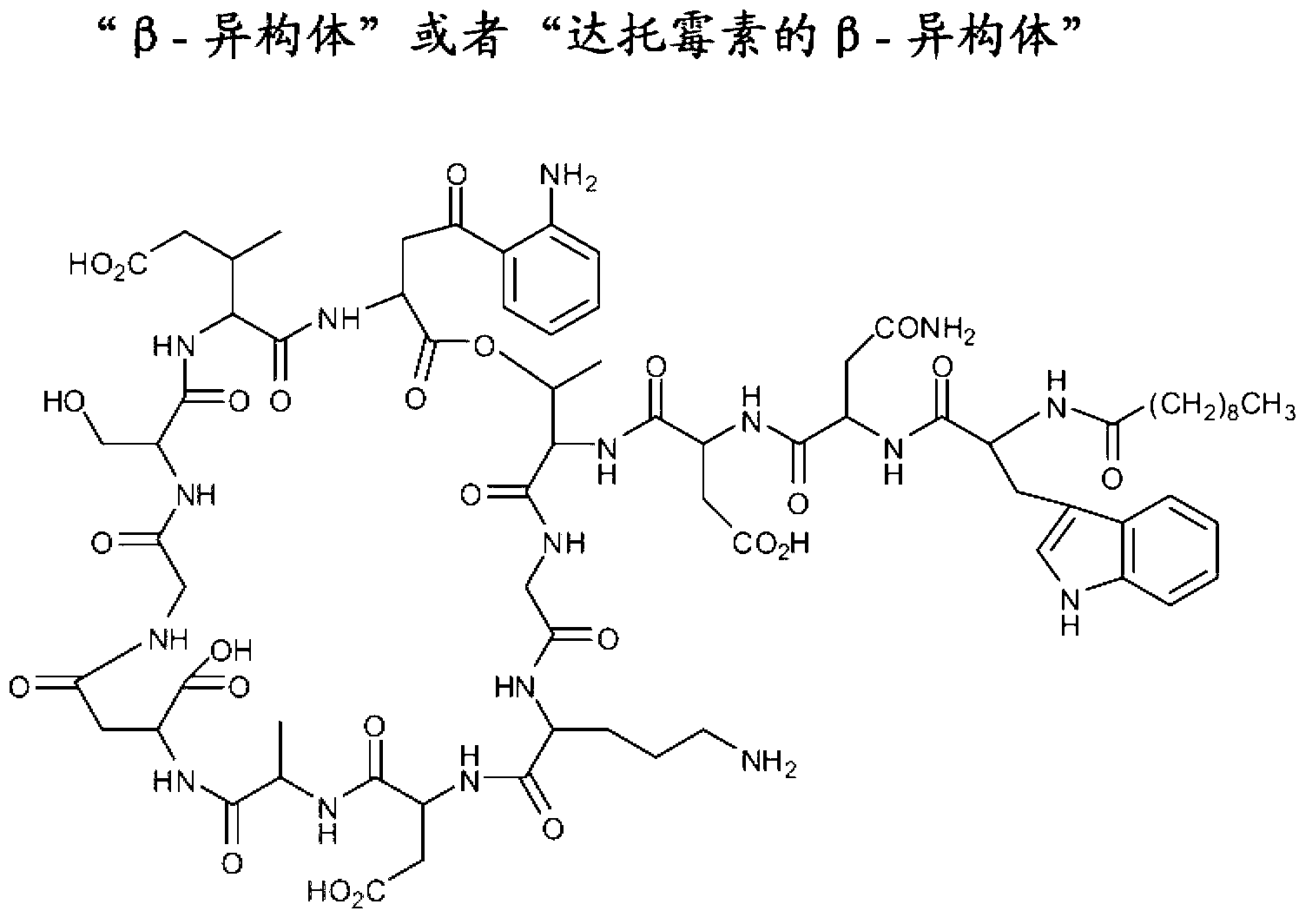
Long term storage stable daptomycin-containing compositions are disclosed. The compositions include a pharmacologically acceptable fluid including daptomycin or a pharmaceutically acceptable salt thereof at a concentration of less than or equal to about 25 mg / mL, and a calcium source. The formulations are surprisingly free of degradation products such as the hydrolysis product of daptomycin, the β-isomer of daptomycin and anhydro daptomycin after storage periods of at least about 18 months under refrigerated conditions.
Owner:SCIDOSE
Streptomyces parvus and application thereof for preparing daptomycin
The invention discloses a streptomyces parvus and application thereof for preparing daptomycin. The strain is reserved in the CCTCC and has the reservation number of CCTCC NO: M2010136, and the reservation data is June 4, 2010. The method for preparing the daptomycin is as follows: a, taking streptomyces parvus CCTCC NO: M2010136 as a fermentation strain; b, strain cultivation: inoculating slope lawn into a shake flask seeding tank to cultivate to obtain shake flask seeding liquid; inoculating the shake flask seeding liquid into the seeding tank to be cultivated; inoculating the seeding tank culture solution into fermentation tank culture medium to be cultivated, and collecting fermentation liquor; carrying out feed supplement in the fermentation and cultivation process; and c, extracting a fermentation product. The fermentation technology provided by the invention has stable production capability, high fermentation unit and few fermentation byproducts by the pilot plant test and the experiment in a 10-ton fermentation tank, greatly lowers post-extracting difficulty and is suitable for industrial production.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
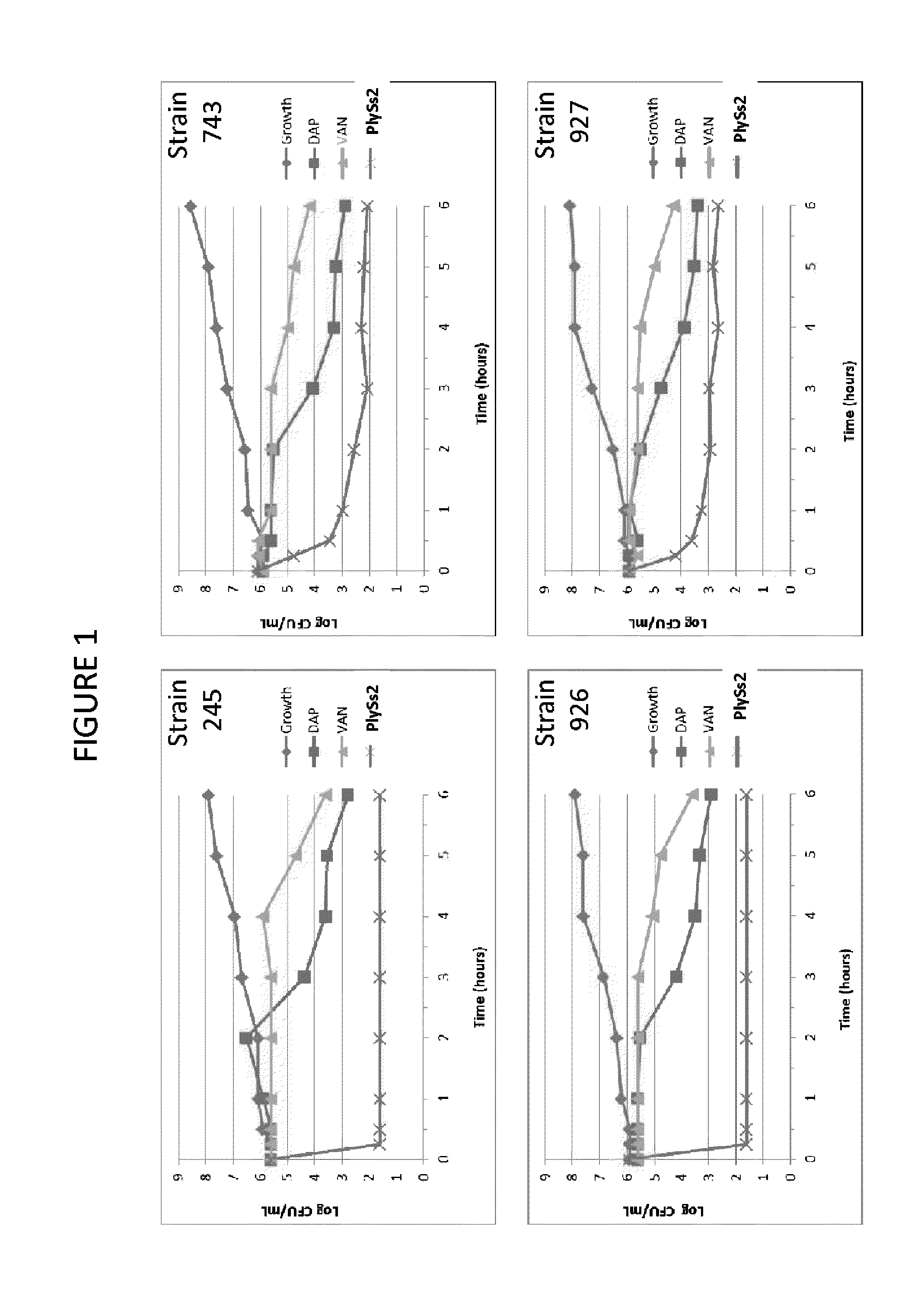
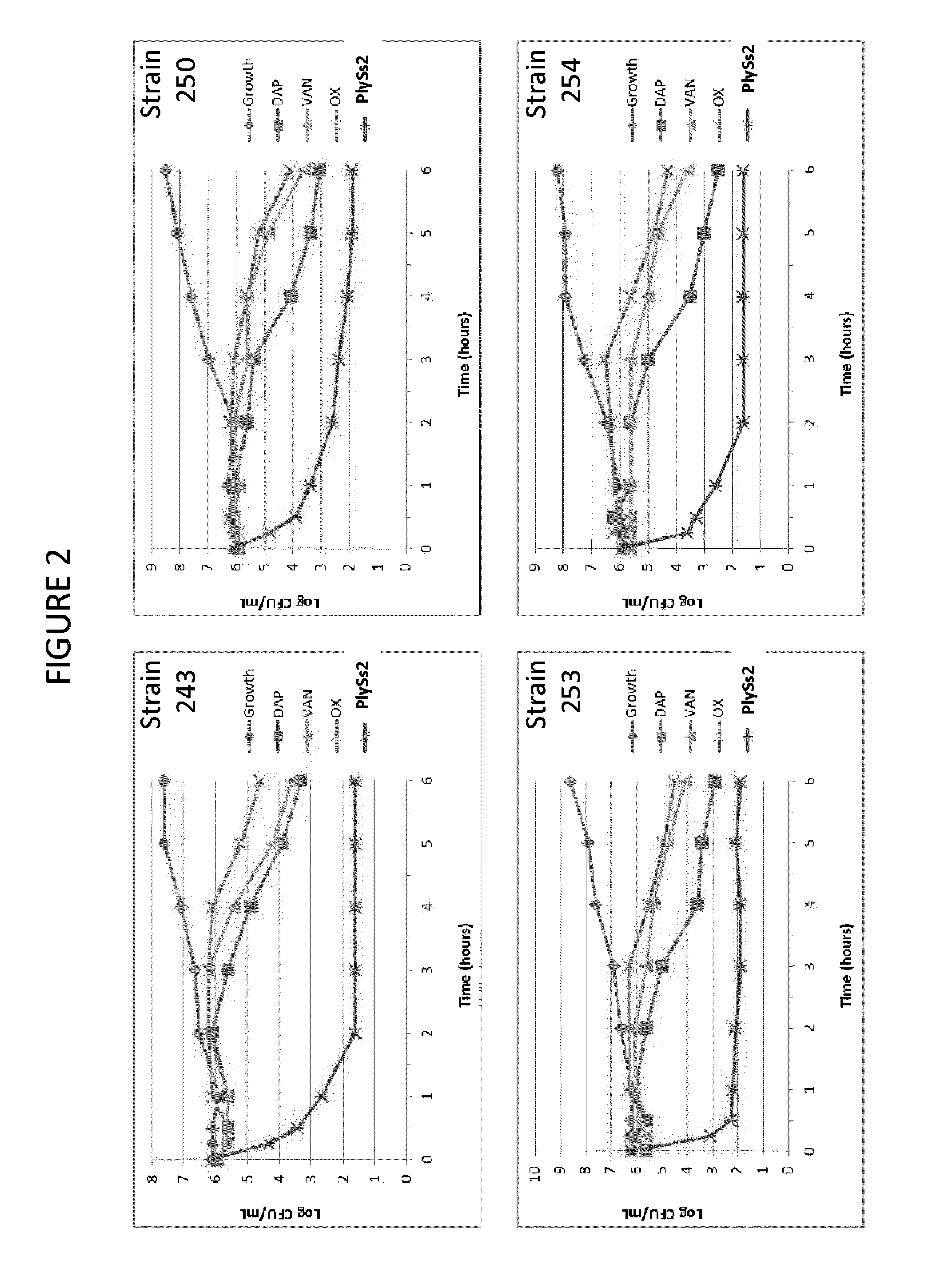
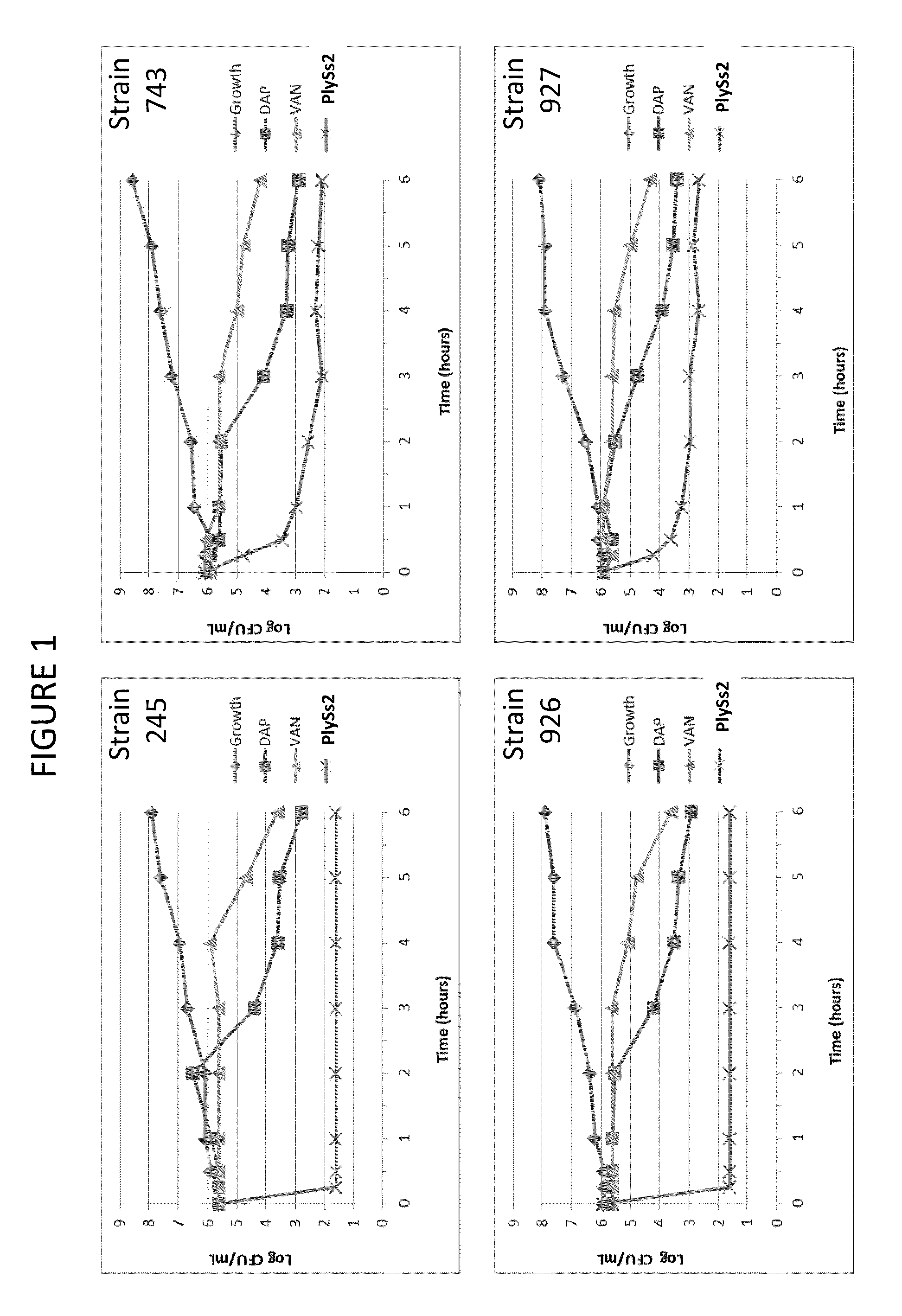
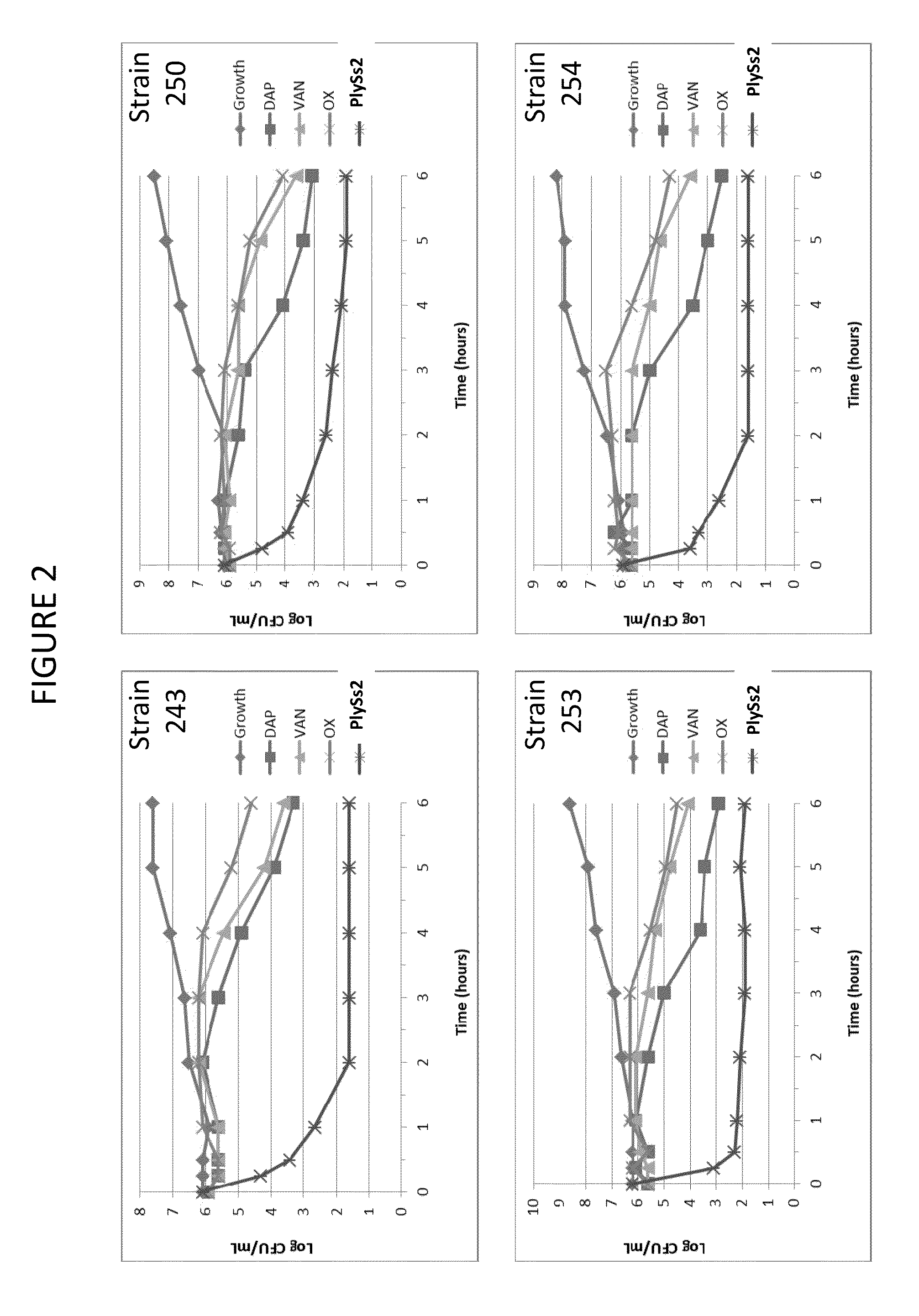
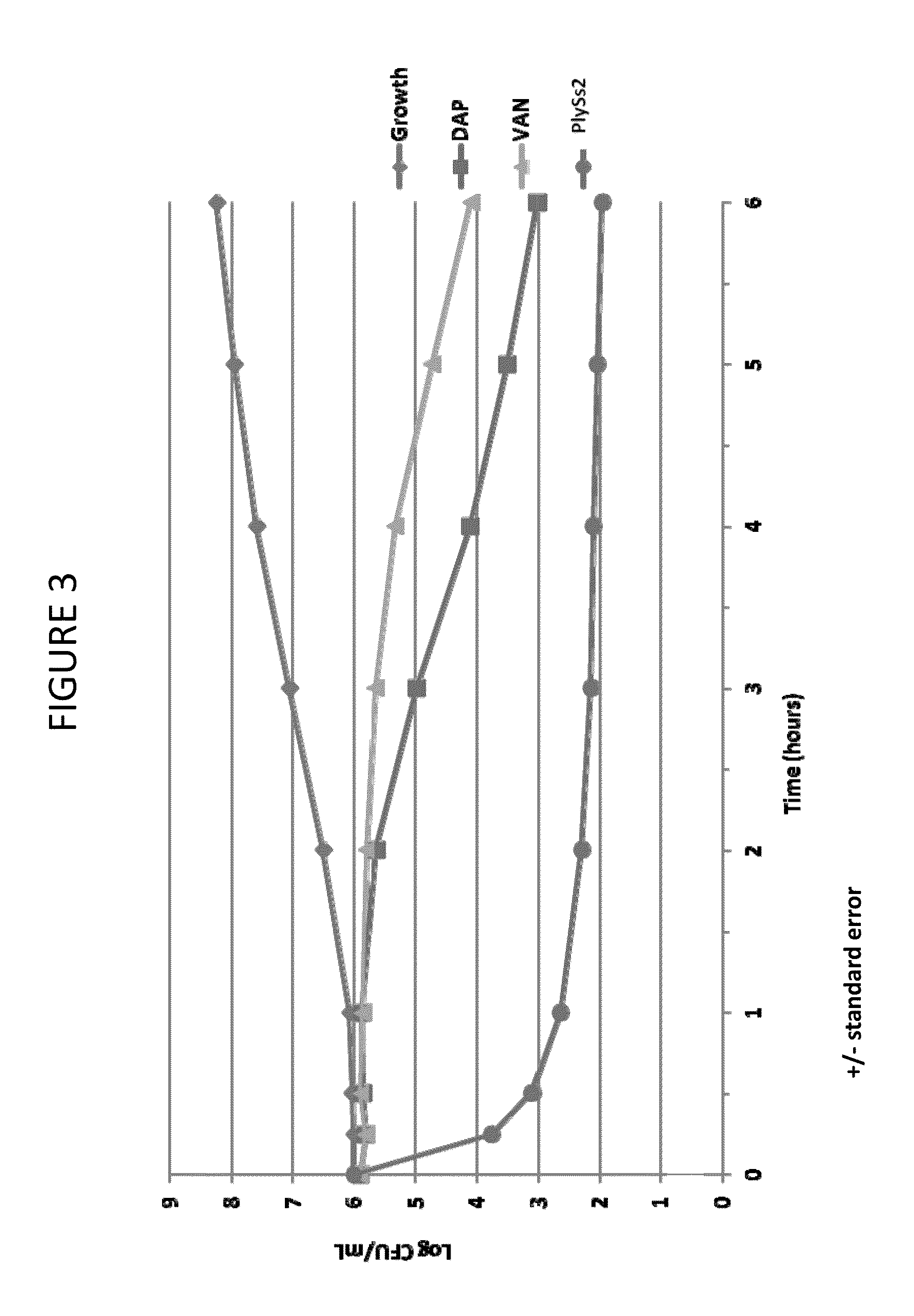
Bacteriophage lysin and antibiotic combinations against gram positive bacteria
ActiveUS20130302306A1Quick killBroad killing activityAntibacterial agentsOrganic active ingredientsLysinLinezolid
The present invention provides compositions and methods for prevention, amelioration and treatment of gram positive bacteria, particularly Staphylococcal bacteria, with combinations of lysin, particularly Streptococcal lysin, particularly the lysin PlySs2, and one or more antibiotic, including daptomycin, vancomycin, oxacillin, linezolid, or related antibiotic(s).
Owner:CONTRAFECT CORP
Method for separating and purifying daptomycin
ActiveCN102718839ALittle side effectsRaw materials are simplePeptide preparation methodsChemistryDaptomycin
The invention provides a method for separating and purifying daptomycin. The method overcomes the defects of the exiting extraction technology of the daptomycin, the purity of the daptomycin is improved, and the final purity of the daptomycin reaches higher than 99.2, so a method capable of industrially purifying the daptomycin is provided.
Owner:鲁南新时代生物技术有限公司
Preparation method of high-purity Daptomycin
The invention provides a preparation method of high-purity Daptomycin, which comprises the steps of: first removing mycelium, soluble protein, culture medium, partial pigments and the like from fermented liquid by adopting primary membrane filtration; then concentrating by a secondary membrane filtration system to remove some micromolecular impurities like monosaccharide and a large number of inorganic salts so as to result in clarified Daptomycin concentrated solution; and then causing the clarified Daptomycin concentrated solution to be subject to chromatography of cation exchange resin and neutralization of anion exchange resin as well as nanofiltration membrane concentration and normal reduced pressure concentration in order to obtain high-purity Daptomycin by means of crystallization. Based on the comprehensive usage of membrane separation technology and resin chromatography technology, the invention provides a production-feasible and operationally-easy method for preparing high-purity Daptomycin.
Owner:ANHUI BBCA FERMENTATION TECH ENG RES
Purification method of daptomycin
ActiveCN102276696ARaw materials are easy to getSave raw materialsPeptide preparation methodsPurification methodsRaw material
Owner:SHANGHAI LAIYI BIOMEDICAL RES & DEV CENT +1
Lipopeptide stereoisomers, methods for preparing same, and useful intermediates
The present invention provides daptomycin stereoisomeric compounds, methods and intermediates for preparing daptomycin and daptomycin stereoisomoeric compounds, as well as pharmaceutical compositions of these compounds and methods of using these compositions as antibacterial agents.
Owner:CUBIST PHARMA INC
Methods for preparing purified lipotides
The present invention relates to crystalline and crystal-like forms of lipopeptides, including daptomycin, a lipopeptide antibiotic with potent bactericidal activity against gram-positive bacteria, including strains that are resistant to conventional antibiotics. The present invention relates to methods of purifying lipopeptides, including daptomycin, a lipopeptide antibiotic with potent bactericidal activity against gram-positive bacteria, including strains that are resistant to conventional antibiotics. The present invention also relates to pharmaceutical compositions comprising the purified form of the lipopeptide and methods of using these compositions.
Owner:CUBIST PHARMA INC
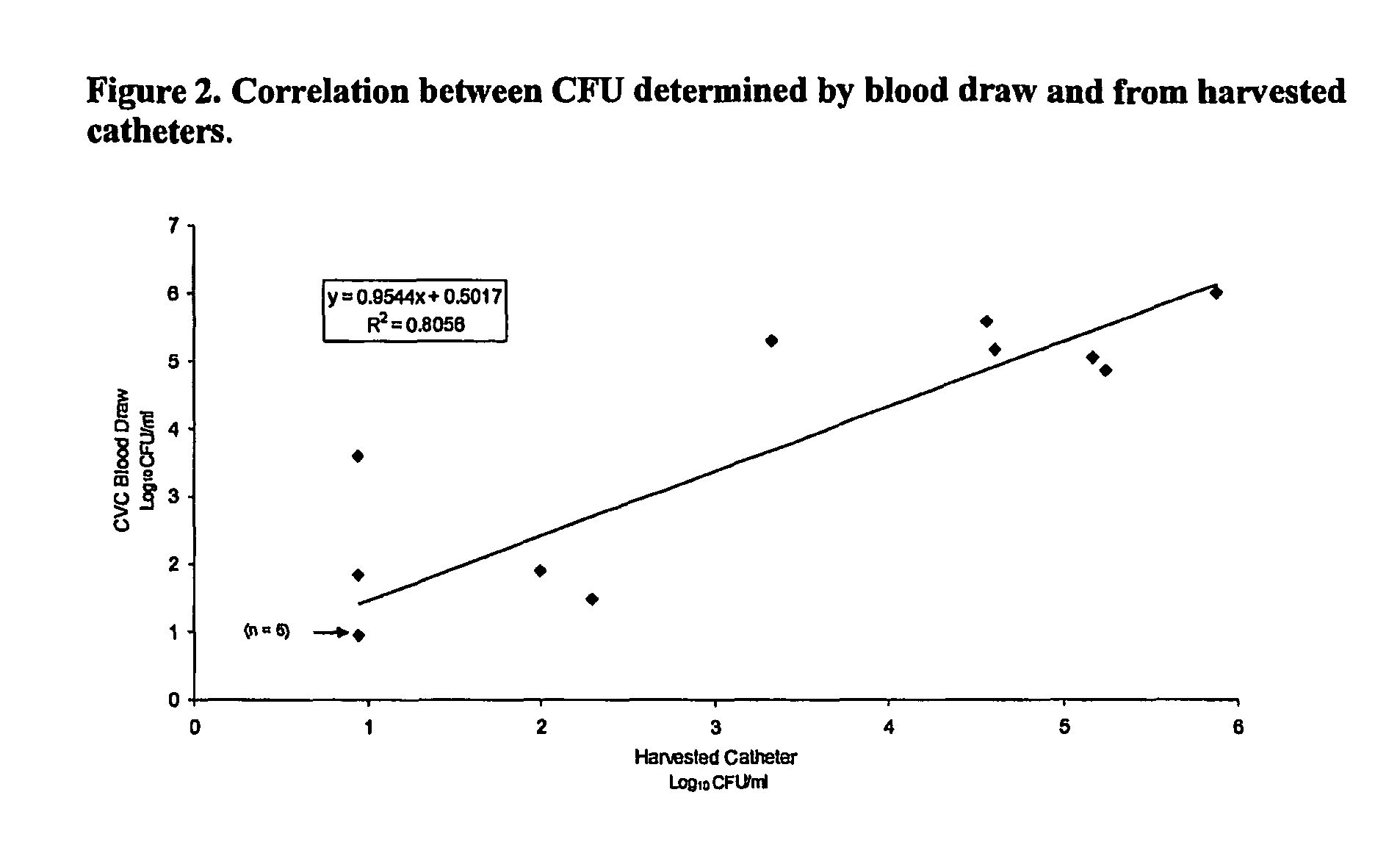
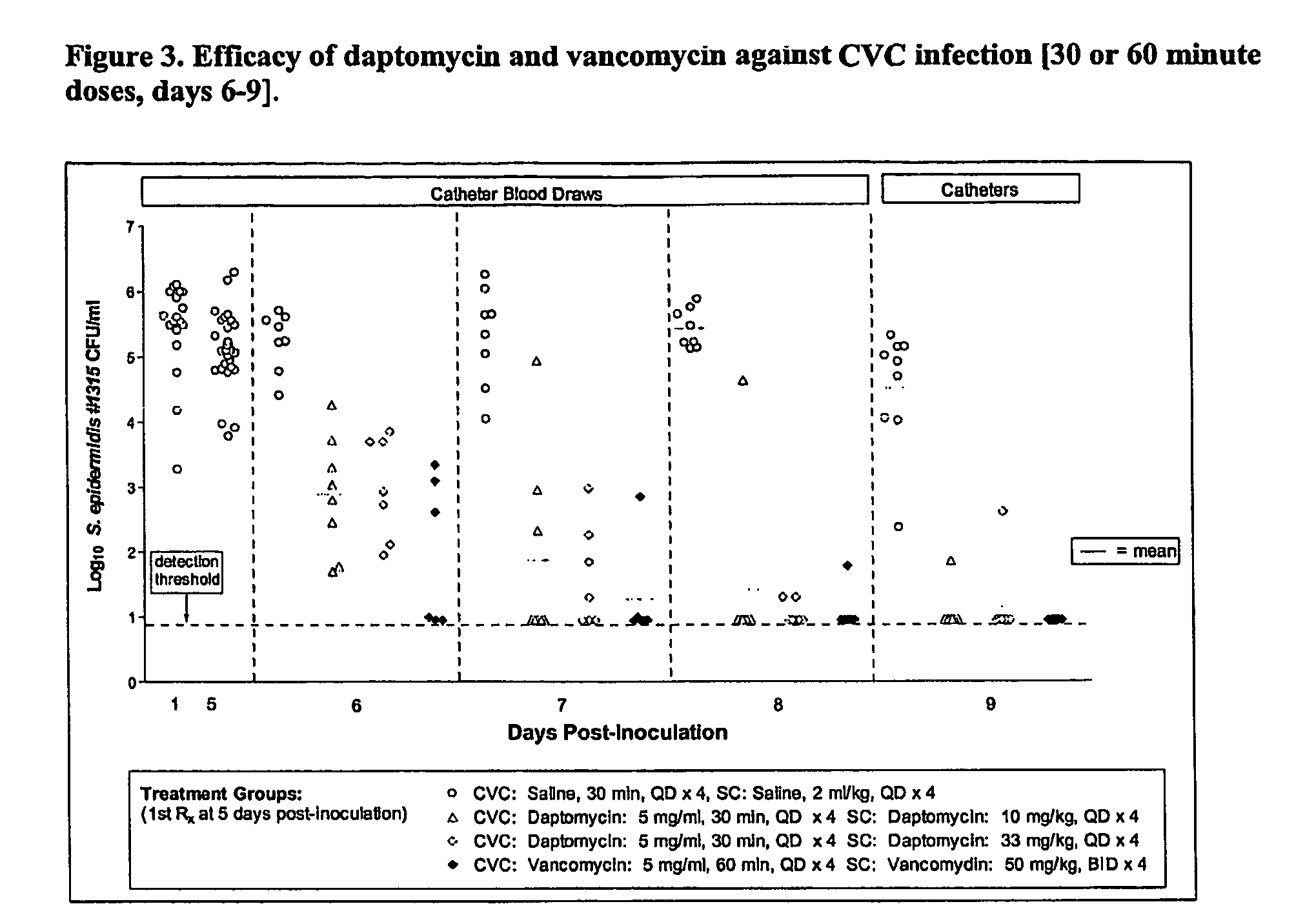
Daptomycin for the treatment of biofilm and catheter salvage
Daptomycin can be used for biofilm treatment (particularly central venous catheter salvage for S. epidermidis infected catheters). Catheter salvage with daptomycin shows rapid cidality, activity against stationary phase bacteria, and penetration and activity in biofilms. The present inventions provide formulations, methods, and articles of manufacture useful for biofilm treatment or catheter salvage involving daptomycin. Particular formulations include daptomycin in lactated Ringer's solution having a rapid kill curve against the bacteria of the biofilm.
Owner:CUBIST PHARMA INC
Formulations of daptomycin
ActiveUS8431539B2Cyclic peptide ingredientsPharmaceutical non-active ingredientsHydrolysateChemistry
Long term storage stable daptomycin-containing compositions are disclosed. The compositions include daptomycin or a pharmaceutically acceptable salt thereof at a concentration of less than or equal to about 25 mg / mL, a buffer having an acidic functional group and have a pH of from about 6.0 to about 7. The formulations are surprisingly free of degradation products such as the hydrolysis product of daptomycin and the β-isomer of daptomycin after storage periods of at least about 18 months.
Owner:EAGLE PHARMACEUTICALS INC
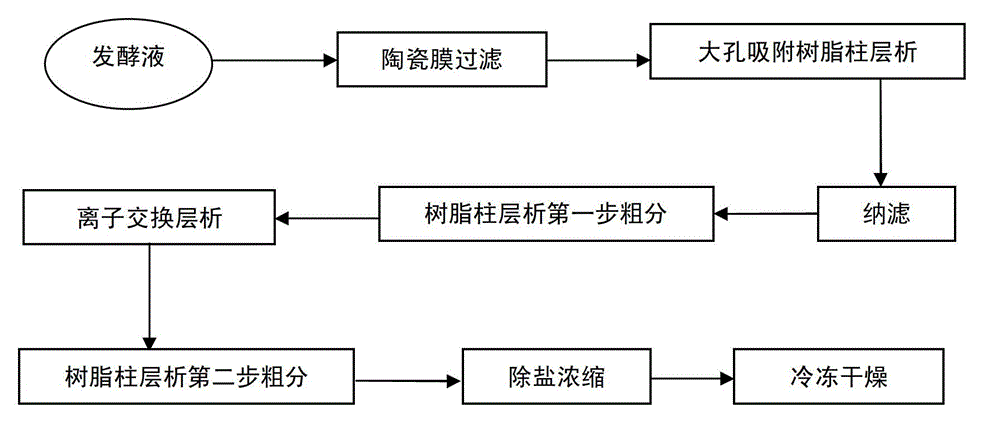
Extraction method for daptomycin
The invention discloses an extraction method for daptomycin. The method comprises the following steps of: forming micelles from daptomycin in fermentation solution and filtering the micelles via a ceramic membrane system at first; then performing separation and purification by virtue of a multi-section macroporous adsorption resin separation system and a weak-base anion-exchange resin separation system; and finally concentrating and freeze-drying to obtain a daptomycin solid having a chromatographic purity of greater than 96%. The method disclosed by the invention is simple and practicable, low in cost, and suitable for industrialized production.
Owner:NEW FOUNDER HLDG DEV LLC +2
Daptomycin extraction method
ActiveCN105481950AReduce healthy stressLittle side effectsPeptide preparation methodsFiltrationGradient elution
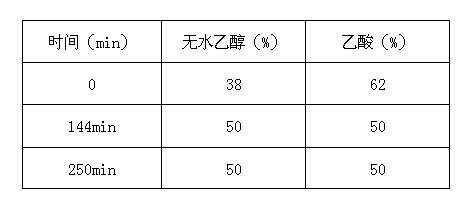
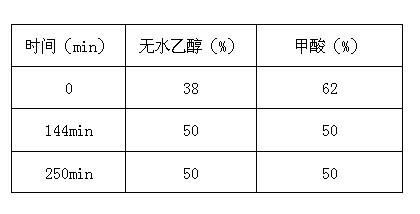
The invention relates to the technical field of antibiotic production, in particular to a daptomycin extraction method. The method includes the following steps of firstly, filtering daptomycin fermentation liquor through a ceramic film; secondly, making filtered liquor pass through large-pore adsorption resin so that eluant can be collected; thirdly, mixing NM-Q anion exchange resin with the obtained eluant, and collecting eluant; fourthly, making the eluant obtained in the third step pass through a reverse phase silica gel MNsil-C18 column, conducting gradient elution on the column through an ammonium acetate ethanol solution, and collecting eluant; fifthly, concentrating the daptomycin eluant obtained in the fourth step, adding calcium acetate and isopropyl alcohol, and conducting standing, crystallization, suction filtration and drying to obtain daptomycin crystallization powder. The method has the advantages that the virulent acetonitrile solution is not used as the eluant, pressure on the environment and health of production personnel is reduced, the use of acid-base regulator is reduced, and the production cost is reduced.
Owner:LIVZON GROUP FUZHOU FUXING PHARMACEUTICAL CO LTD
Extraction and purification method of daptomycin
ActiveCN102675426AReduce manufacturing costThe process steps are simplePeptide preparation methodsBiotechnologyEngineering
The invention discloses an extraction and purification method of daptomycin.. The method comprises the following steps: enriching daptomycin in a fermentation broth; and performing crude extraction and refining on the enriched daptomycin; and finally lyophilizing to obtain daptomycin, wherein acidic column chromatography and neutral column chromatography are sequentially adopted in the crude extraction; and neutral column chromatography and acidic column chromatography are sequentially adopted in refining. By virtue of improvement of the extraction and purification method, massive pigment generated in the fermenting process, homologous compounds, isomers and other side products with similar structure to the daptomycin can be well removed. The final product which is subjected to extraction and purification has high purity; in addition, the process is simple and is suitable for industrial production.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Formulations of daptomycin
ActiveUS20110172167A1Cyclic peptide ingredientsPharmaceutical non-active ingredientsHydrolysateChemistry
Long term storage stable daptomycin-containing compositions are disclosed. The compositions include daptomycin or a pharmaceutically acceptable salt thereof at a concentration of less than or equal to about 25 mg / mL, a buffer having an acidic functional group and have a pH of from about 6.0 to about 7. The formulations are surprisingly free of degradation products such as the hydrolysis product of daptomycin and the β-isomer of daptomycin after storage periods of at least about 18 months.
Owner:EAGLE PHARMACEUTICALS INC
Method for separating and purifying daptomycin
The invention discloses a method for separating and purifying daptomycin. The method comprises the steps of allowing daptomycin to form micelles, filtering by a ceramic membrane system, performing separation and purification and discoloring treatment by using a weak-base anion exchange resin separating system and a macroporous resin separating system, crystallizing, and thus a daptomycin solid with a chromatographic purity higher than 98% is obtained. The method is simple and practicable, is low in cost, and is suitable for industrialized production.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Daptomycin purifying method
ActiveCN106589065AHigh purityEasy to operatePeptide preparation methodsPurification methodsDesalination
The invention provides a daptomycin purifying method. Specifically, the method comprises the steps that firstly, fermentation liquor is clarified and then is separated by macro-porous adsorption resin and anion exchange resin, then nanofiltration concentration is adopted to remove endotoxin, and desalination, concentration, filtration and the like are performed to obtain high-purity daptomycin, wherein macro-porous adsorption resin separation and anion exchange resin separation can be exchanged in step sequence. The product obtained by the adoption of the daptomycin purifying method is high in purity and contains few impurities, and operation is simple.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Method for extracting daptomycin from fermentation broth
InactiveCN102492024AReduce acidolysisGood medical market prospectPeptide preparation methodsSodium acetateIon exchange
A method for extracting daptomycin from fermentation broth relates to fermentation broth. Supernatant liquor is obtained after the fermentation broth is centrifuged, the daptomycin enters an organic phase after the supernatant liquor is extracted by an organic solvent, sodium acetate-acetic acid buffer solution is used to perform re-extraction to reach a water phase so as to obtain strip-extraction liquid, the strip-extraction liquid is purified through hydrophobic interaction chromatography, isopropanol-sodium acetate-acetic acid buffer solution with constant intensity is used for elution to obtain hydrophobic interaction chromatography eluent, the eluent is separated and purified through ion-exchange column chromatography to obtain ion-exchange eluent, the sodium acetate-acetic acid buffer solution is used as basic liquid, and neutral salt is added for elution so as to complete extraction of the daptomycin from the fermentation broth. Organic solvent extraction and resin chromatography technology is applied comprehensively, and the daptomycin with purity more than 85% can be separated and purified from the fermentation broth by using the method. Compared with other separating and purifying technology, the method is moderate in operating condition, low in cost and capable of effectively reducing acidolysis of the daptomycin. The daptomycin serving as novel lipopeptide antibiotics has good medical market prospect.
Owner:XIAMEN UNIV
Therapeutic combination of daptomycin and protein synthesis inhibitor antibiotic, and methods of use
A therapeutic combination comprises an antibacterially effective amount of daptomycin, and an amount of protein synthesis inhibitor antibiotic effective to prevent the development of daptomycin non-susceptibility in bacteria. Related combination therapies and methods are also included.
Owner:MERCK SHARP & DOHME LLC
Bacteriophage lysin and antibiotic combinations against gram positive bacteria
ActiveUS20150290299A1Quick killBroad killing activityAntibacterial agentsOrganic active ingredientsLysinLinezolid
The present invention provides compositions and methods for prevention, amelioration and treatment of gram positive bacteria, particularly Staphylococcal bacteria, with combinations of lysin, particularly Streptococcal lysin, particularly the lysin PlySs2, and one or more antibiotic, including daptomycin, vancomycin, oxacillin, linezolid, or related antibiotic(s).
Owner:CONTRAFECT CORP
Lipopeptide compositions and related methods
The present disclosure provides novel powder daptomycin formulations which have improved chemical stability and faster reconstitution times when in the solid state. Some examples of the compositions comprise daptomycin and sucrose.
Owner:CUBIST PHARMA INC
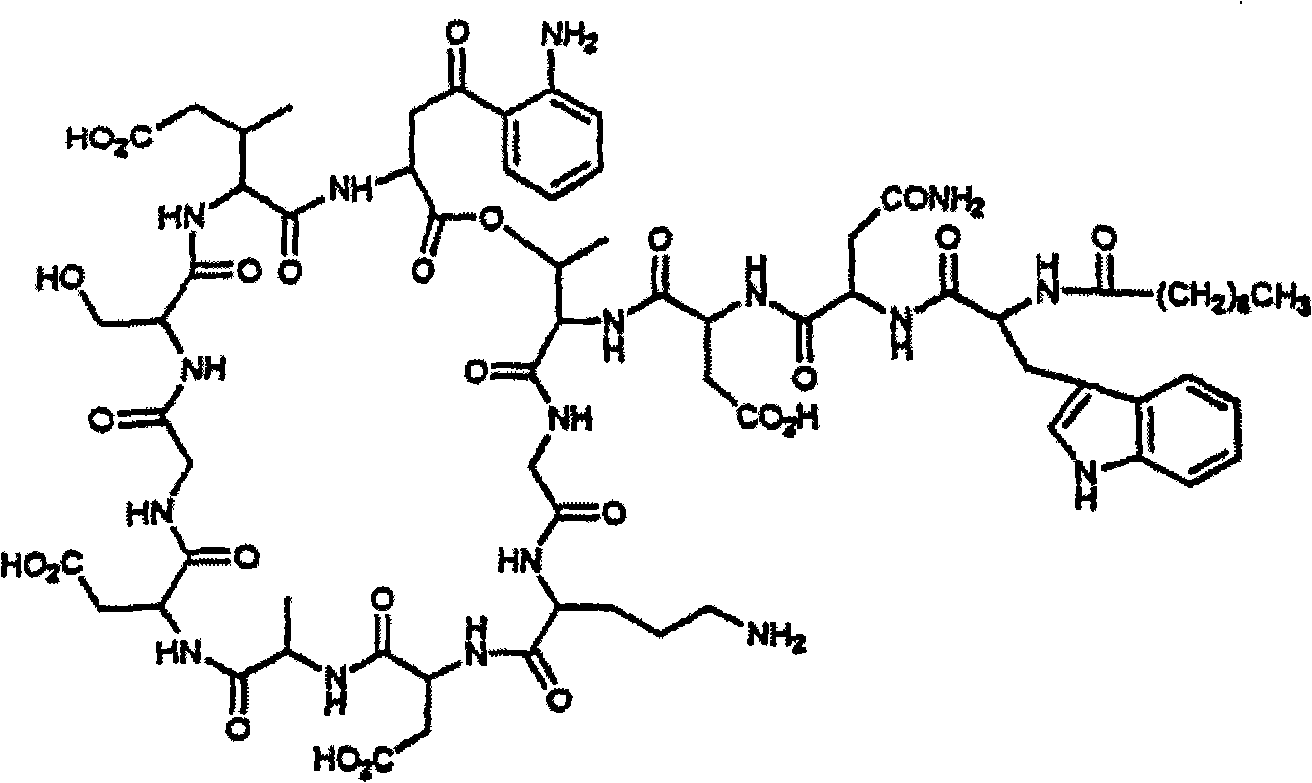
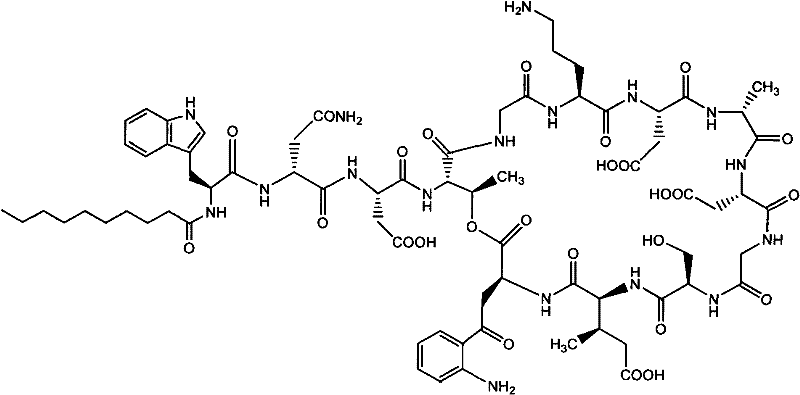
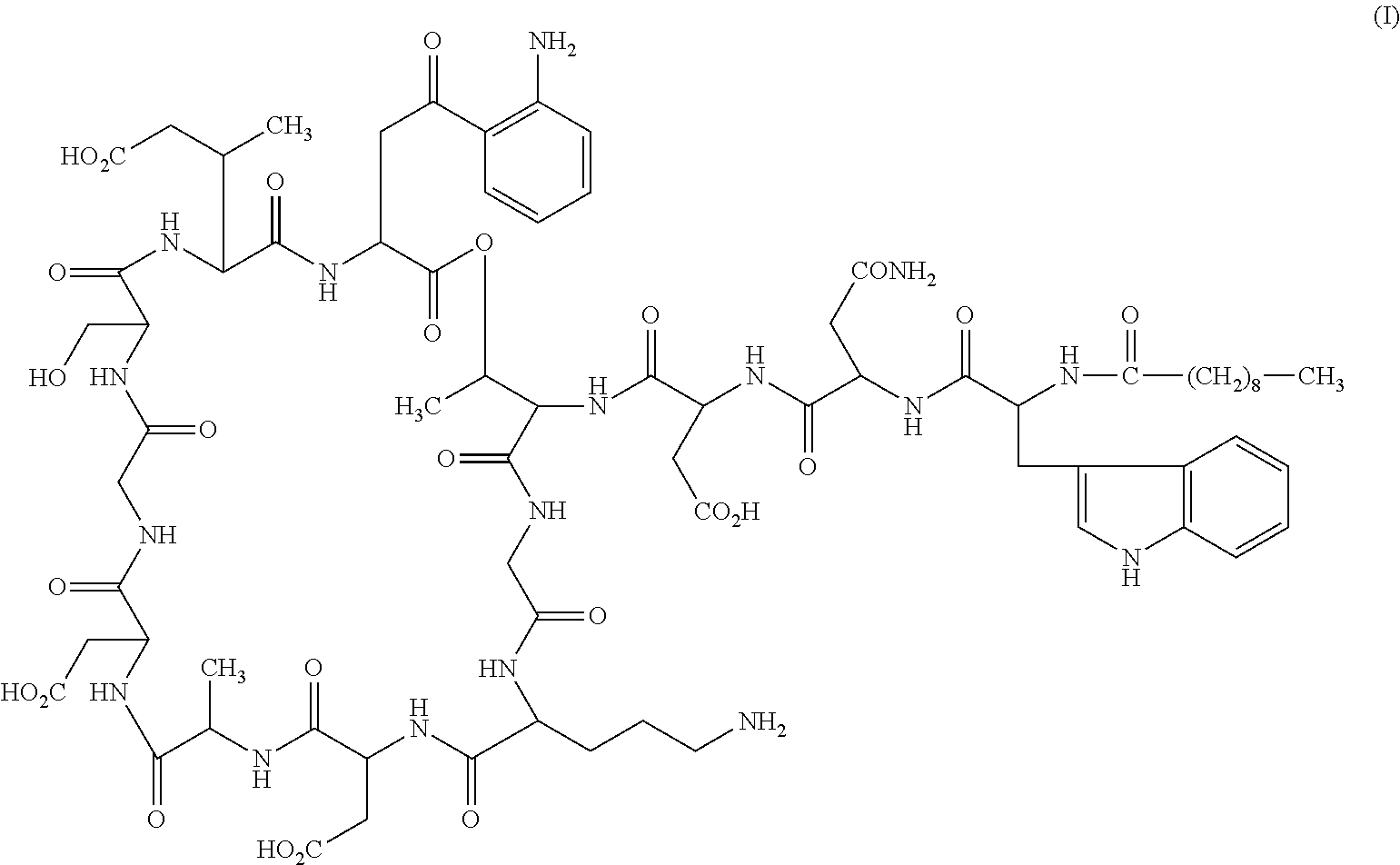
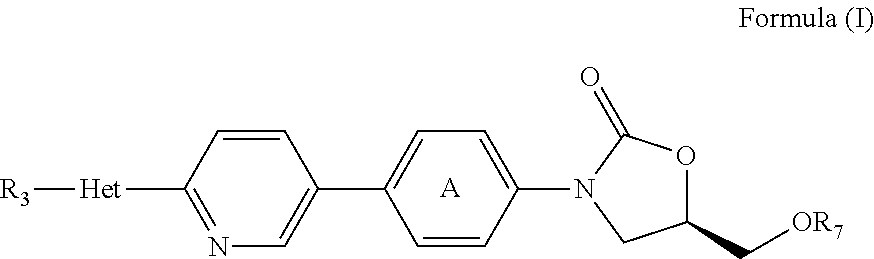
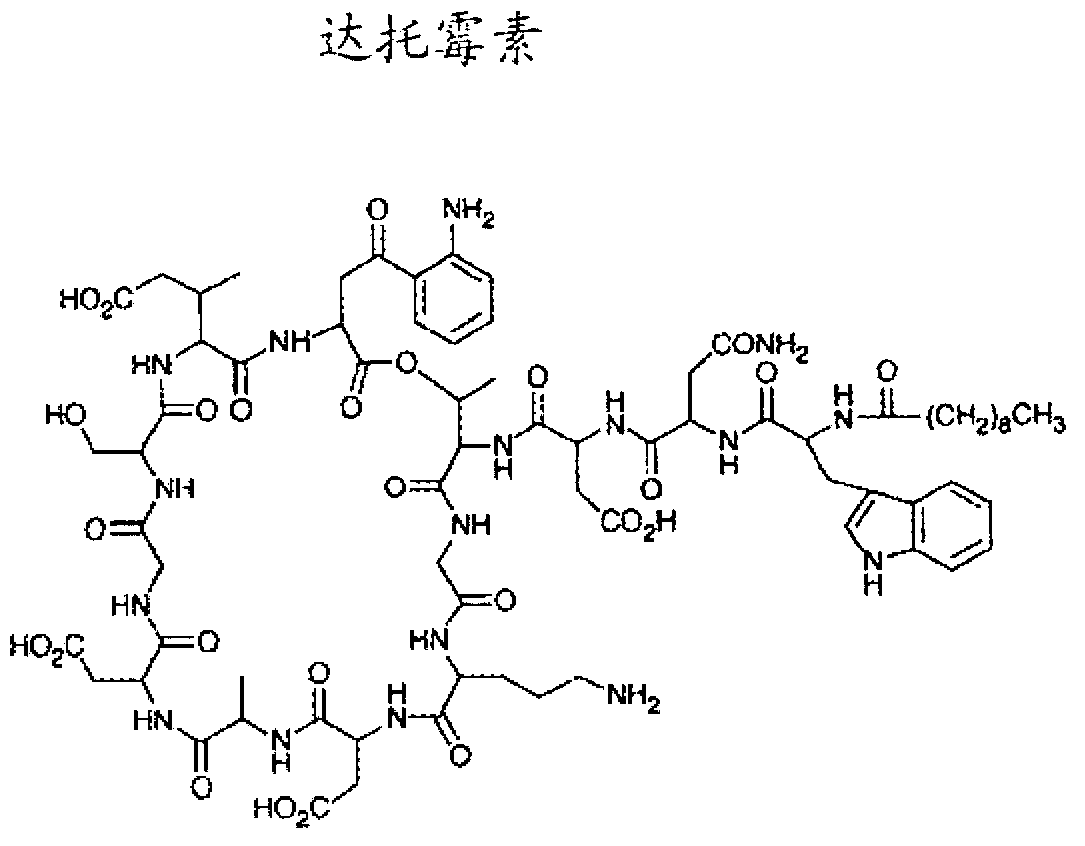
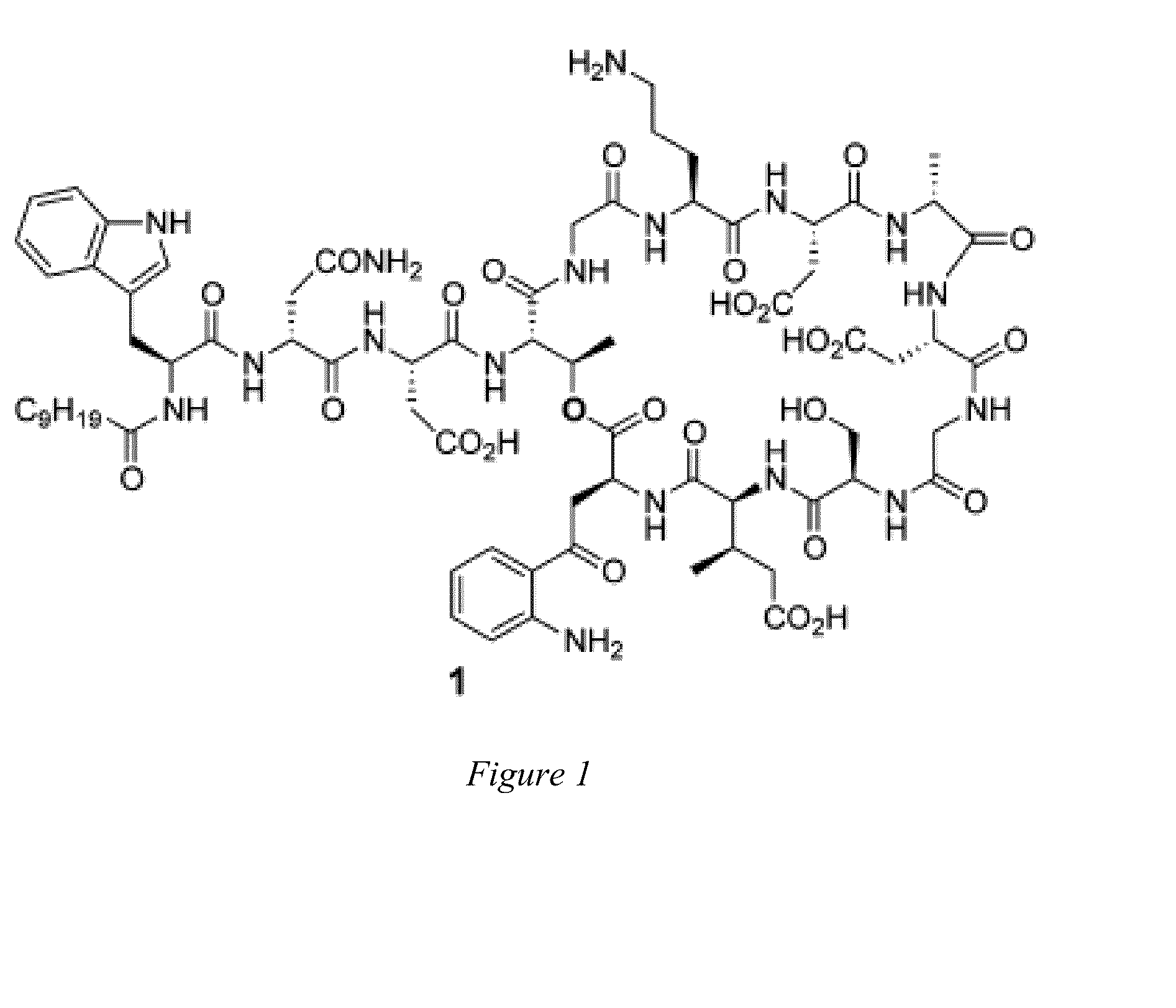
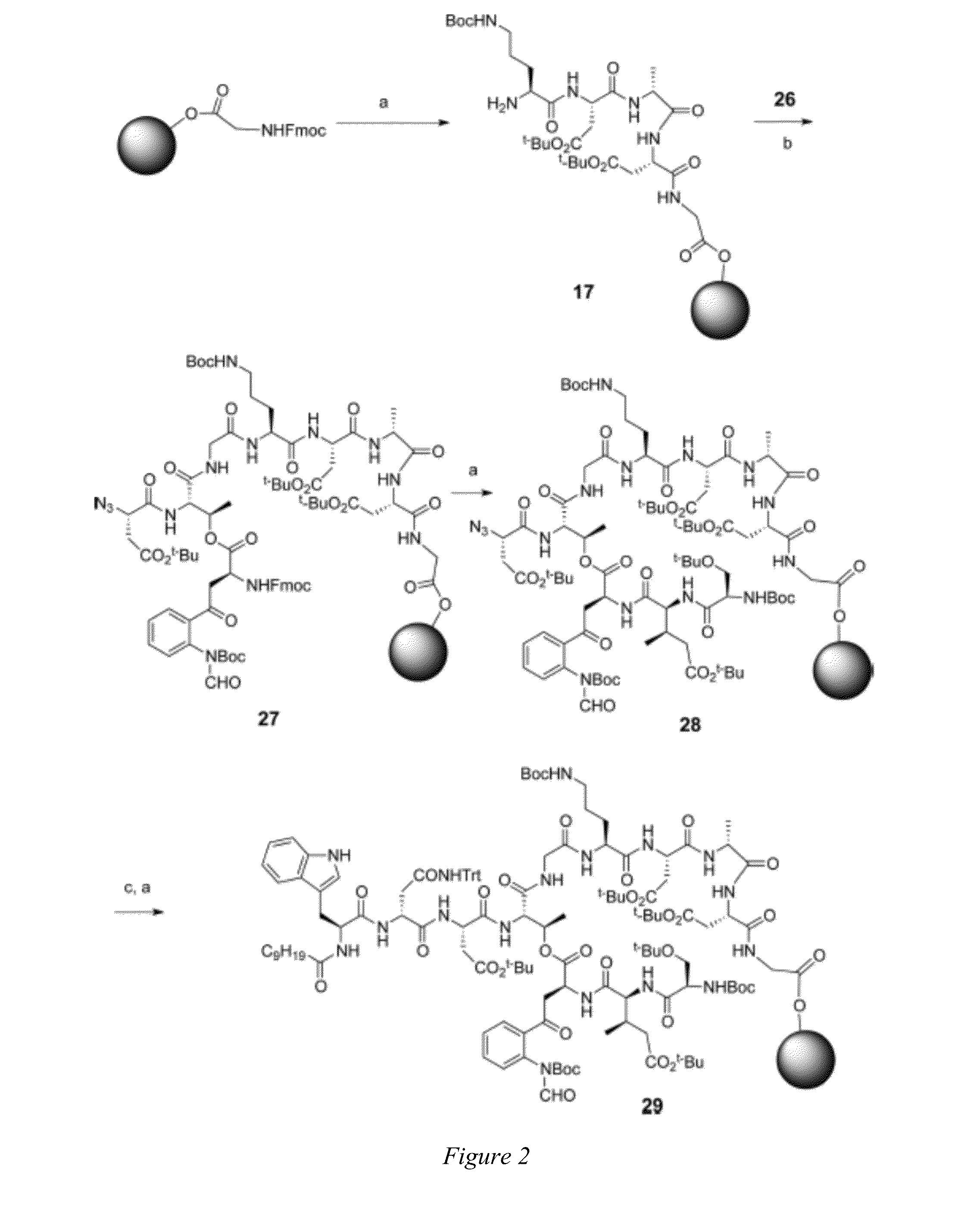
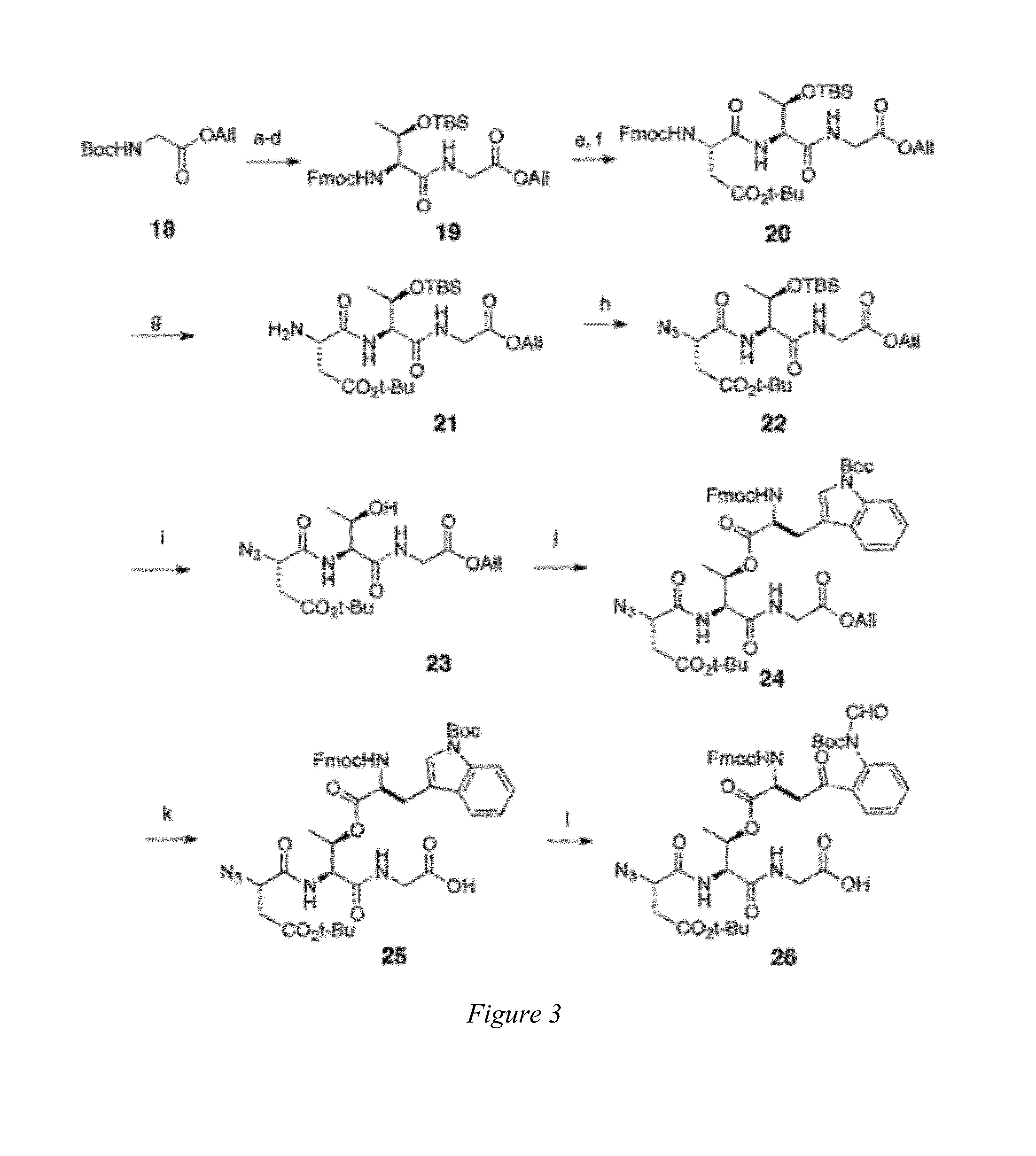
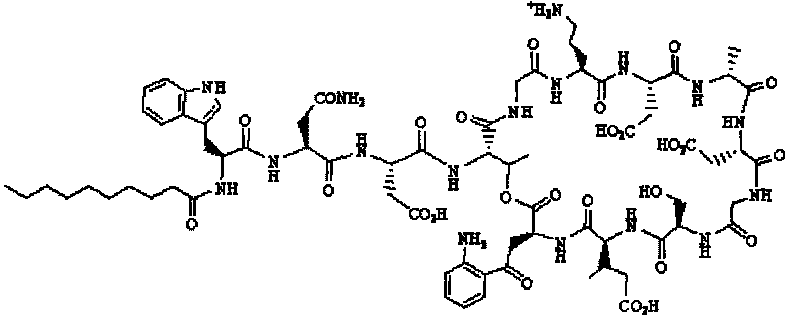
Daptomycin analogues and a method for the preparation of daptomycin or a daptomycin analogue
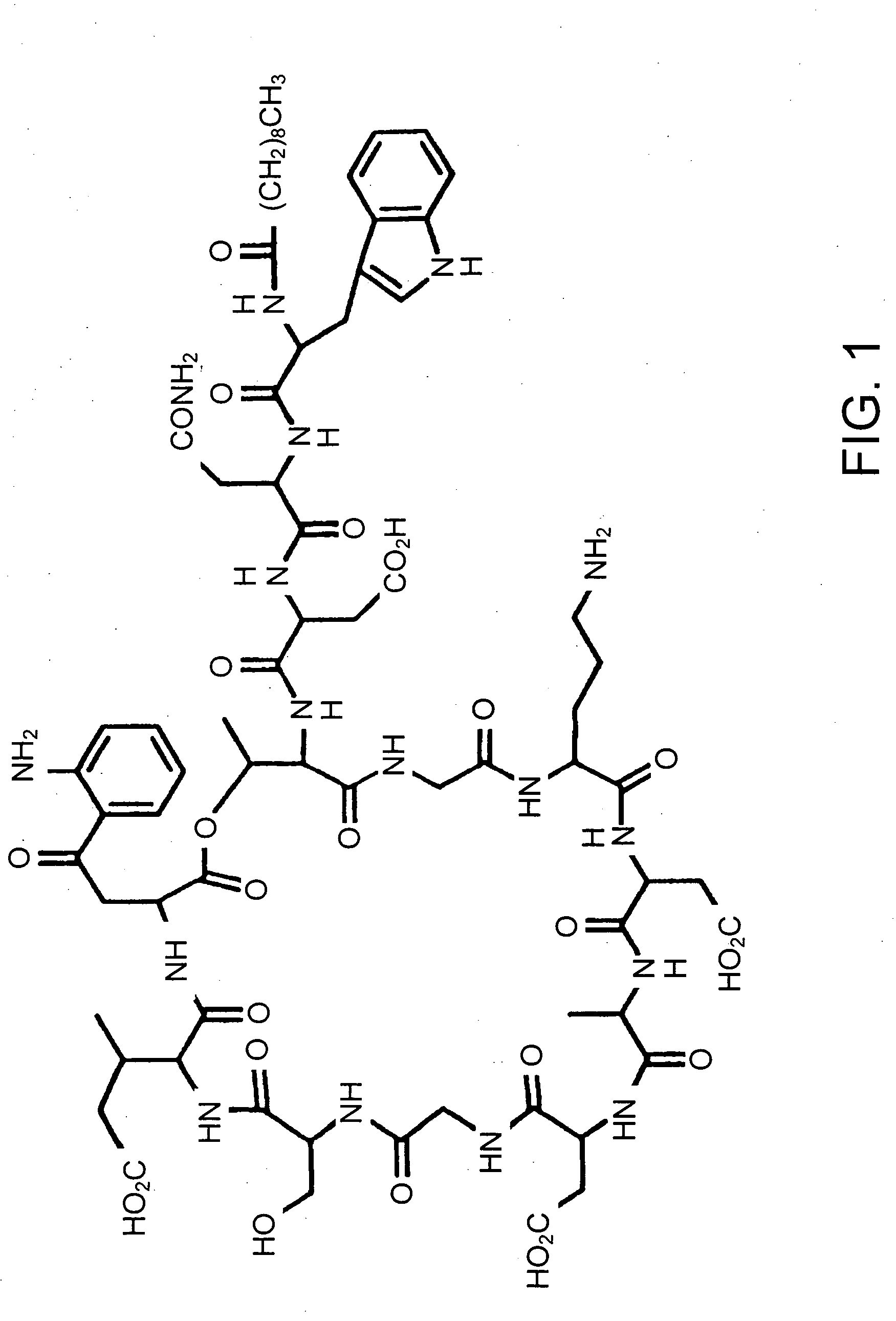
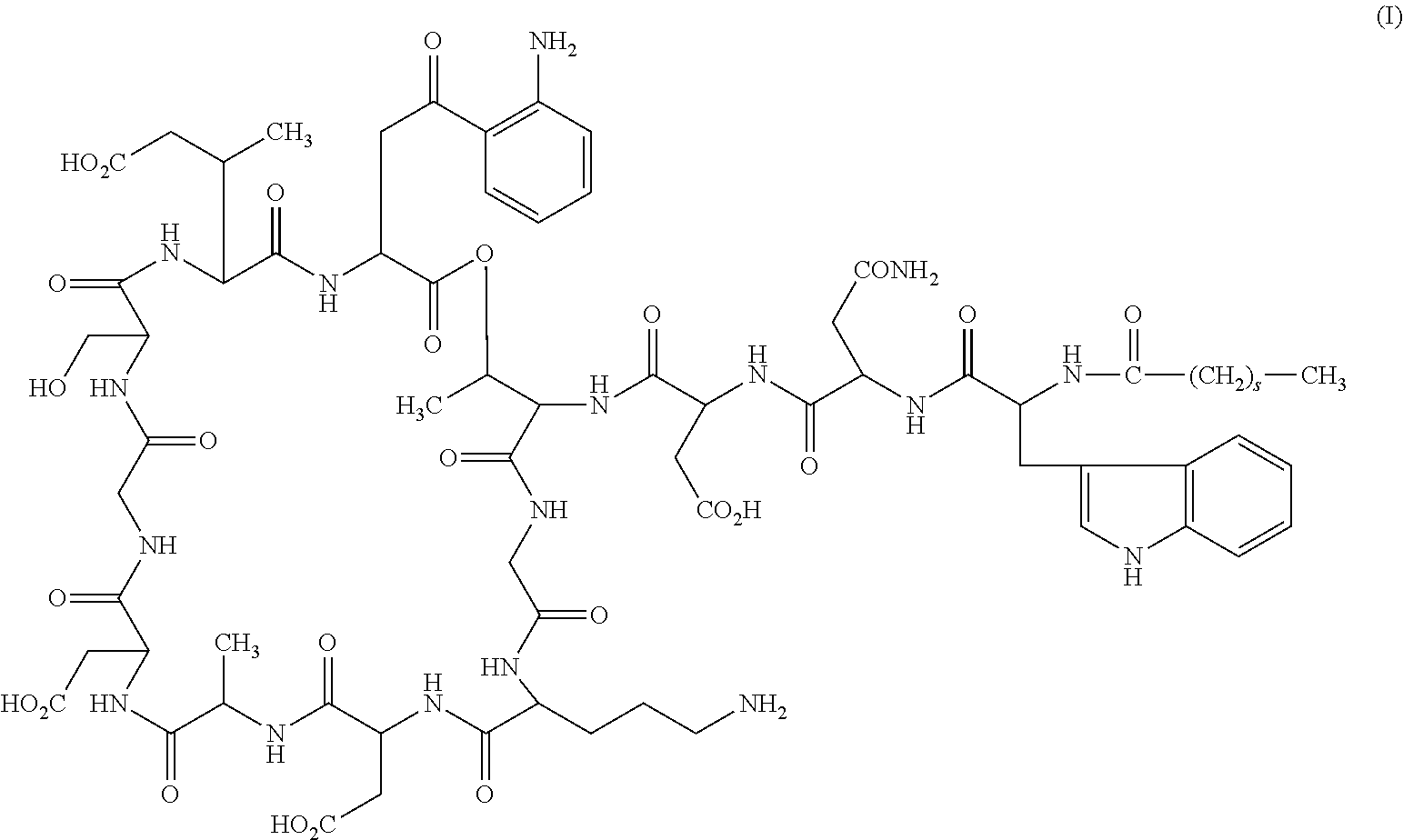
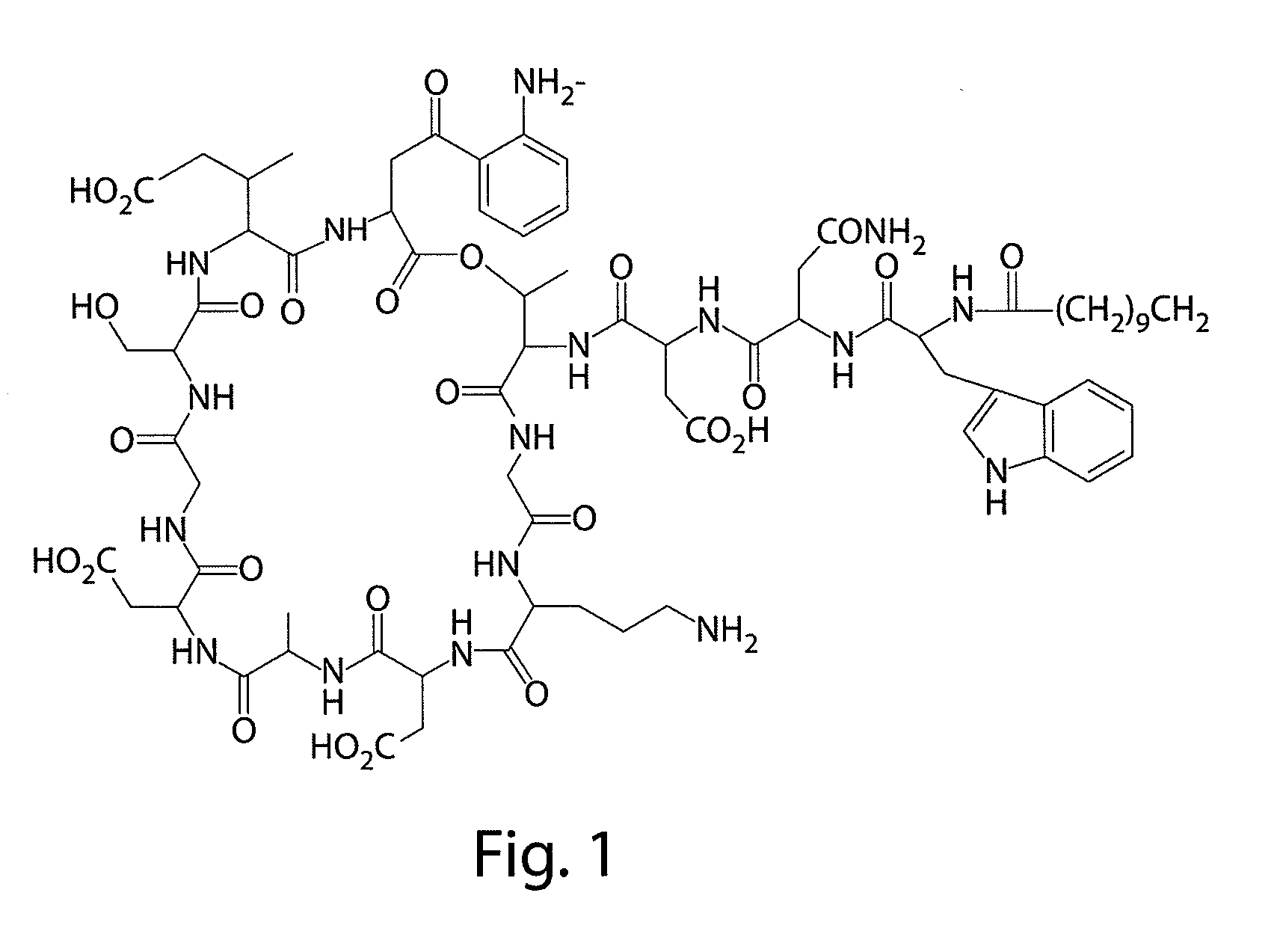
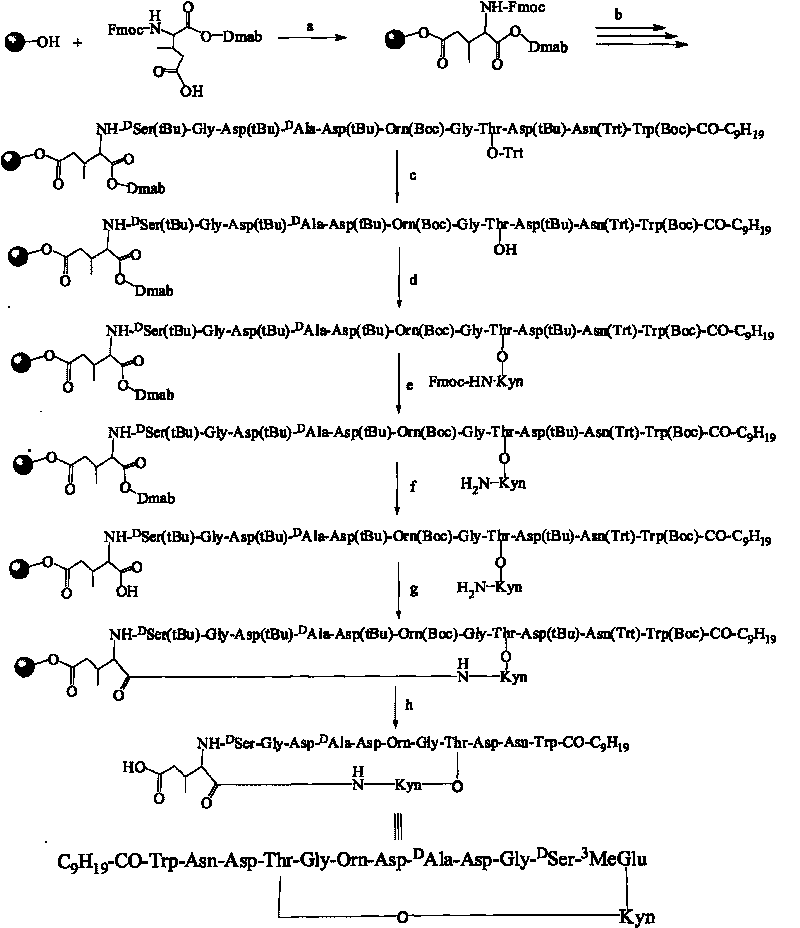
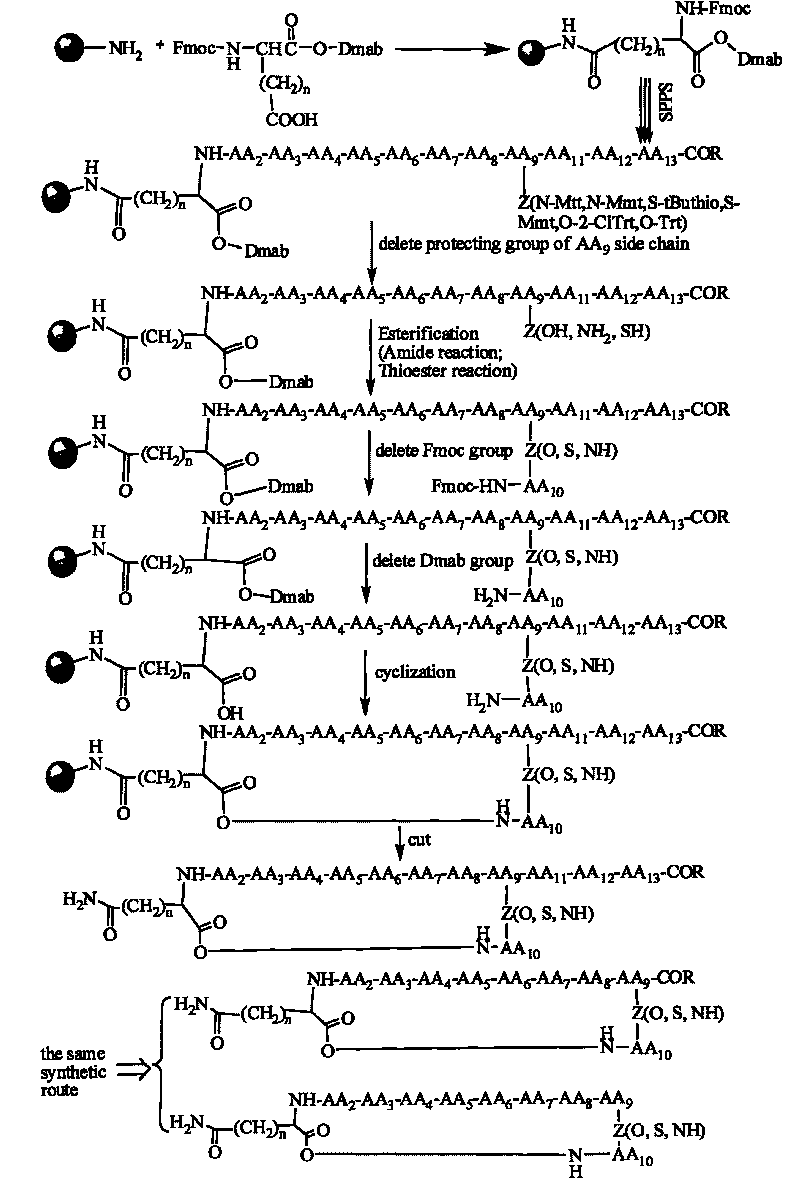
A method for the synthesis of daptomycin or a daptomycin analogue is carried out on a resin to form a linear precursor followed by a serine ligation macrocyclization in solution. Daptomycin analogues can differ from daptomycin by substitution of amino acids residues and / or deletion or addition of amino acid residues. Daptomycin analogues can include a different fatty acid in the side arm of the daptomycin analogue.
Owner:THE UNIVERSITY OF HONG KONG
Daptomycin analog and full solid phase synthesis preparation method thereof
InactiveCN101696235AImprove biological activitySimple structurePeptide preparation methodsBulk chemical productionAmino acid side chainCombinatorial chemistry
The invention relates to a daptomycin analog and a full solid phase synthesis preparation method thereof. The full solid phase synthesis preparation method is technically characterized by comprising the steps of: linking a first Fmoc amino acid (Alpha-carboxyl thereof is protected by a selected group) to a solid phase resin carrier, wherein the side chain of the first Fmoc amino acid is provided with carboxyl; continuing to sequentially link 7 Fmoc amino acids, and sequentially removing Fmoc; linking a ninth Fmoc amino acid and removing Fmoc, wherein the side chain of the ninth Fmoc amino acid is provided with carboxyl (sulfydryl or amino) and the ninth Fmoc amino acid is protected by a selective group; continuing to sequentially link the subsequent 3 Fmoc amino acids and removing Fmoc; after linking fatty acid, selectively removing the protective group on the carboxyl (sulfydryl or amino) of the side chain of the ninth amino acid, then reacting with the carboxyl of the last Fmoc amino acid and removing Fmoc; after removing the protective group of the Alpha-carboxyl of the first amino acid, carrying out the cyclization on solid phase resin directly; and at last, cutting the product from the resin, and obtaining the product through the precipitation of cold ethyl ether.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
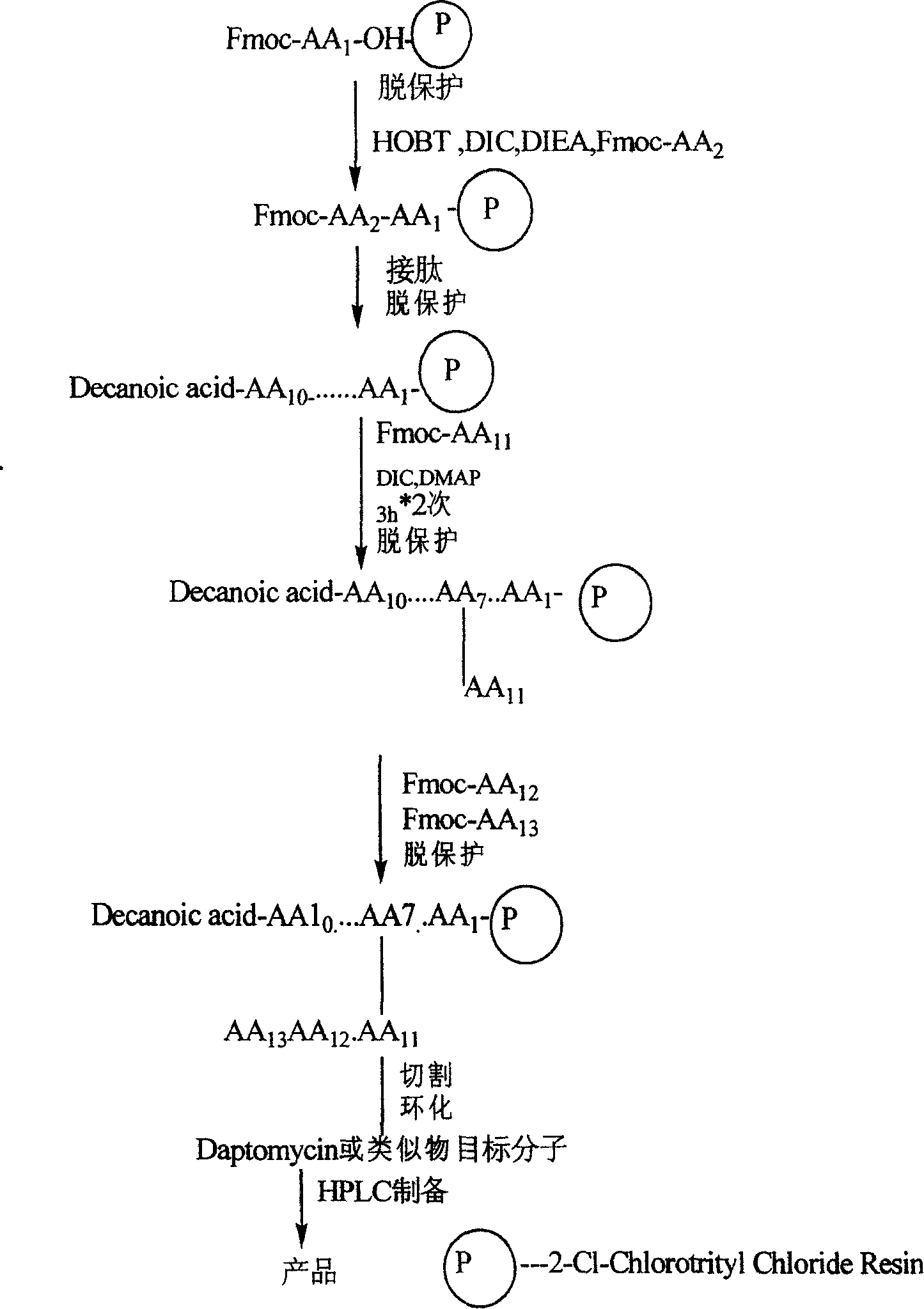
Synthesis method of daptomycin
ActiveCN101235080AAvoid it happening againGood effectPeptide preparation methodsBulk chemical productionSynthesis methodsSolid-phase synthesis
The invention relates to a daptomycin synthesis method for resolving the technical problem of prior art which uses rose spore streptomycete as raw material to cost high synthesis cost. The synthesis method comprises a, using 2-chlorine trityl chloride resin as carrier, via solid synthesis method to connect the amino acids with protective groups, to obtain protective decapeptide resin while the Fmoc-protective groups are removed in turn, B, connecting decanoic acid via same method, connecting next amino acid via esterification, removing Fmoc-protective groups, and connecting left two amino acids via normal solid method, removing Fmoc-protective groups, c, using trifluoroacetic acid or carrene solution to cut off total protective peptide from resin, drying and completing end-to-end liquid cyclisation in organic solvent, d, using the mixture of trifluoroacetic acid, water and benzene methyl sulfide to cut off peptide from resin to obtain crude product. The invention can synthesize daptomycin.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
Method for fermenting daptomycin by adding caprate
ActiveCN101880703AExtended pathLow toxicityMicroorganism based processesFermentationDecanoatesPotassium
The invention relates to a method for fermenting daptomycin by adding caprate. In the fermentation tank culturing process, the daptomycin is produced by adding 5.0 percent potassium decanoate solution, 10.0 percent sodium decanoate solution or 8.0 percent ammonium decanoate solution, namely the decanoates are used as precursors for fermenting the deptomycin. The method has the advantages that: while the daptomycin fermenting path is enlarged, the adding effect can be ensured because the precursors have small toxicity to cells, can exist in a liquid form at normal temperature and cannot easily crystallize due to low temperature.
Owner:FUJIAN INST OF MICROBIOLOGY
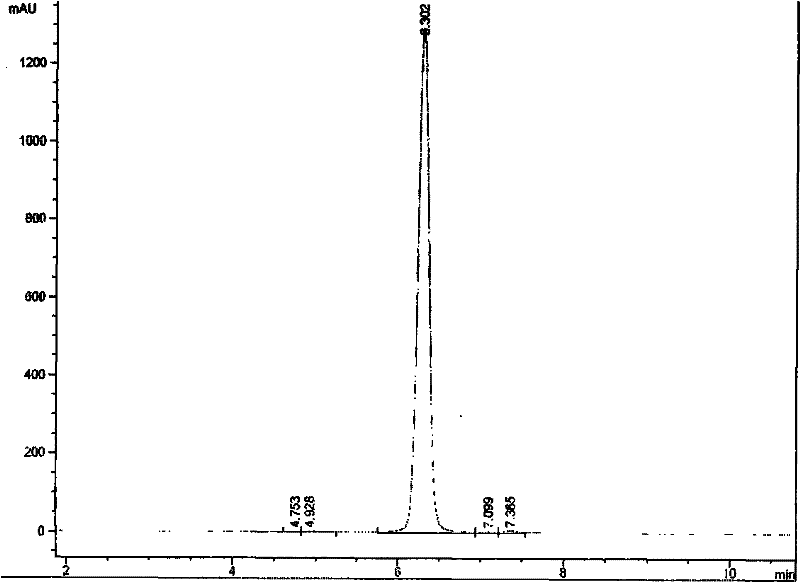
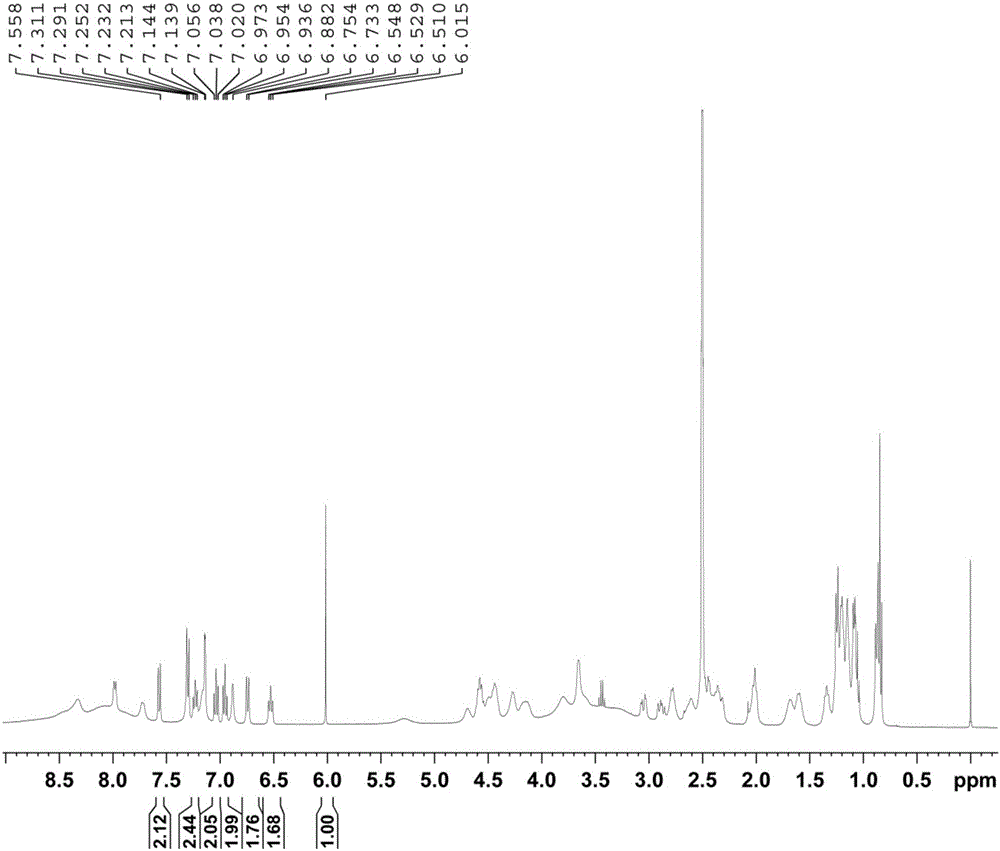
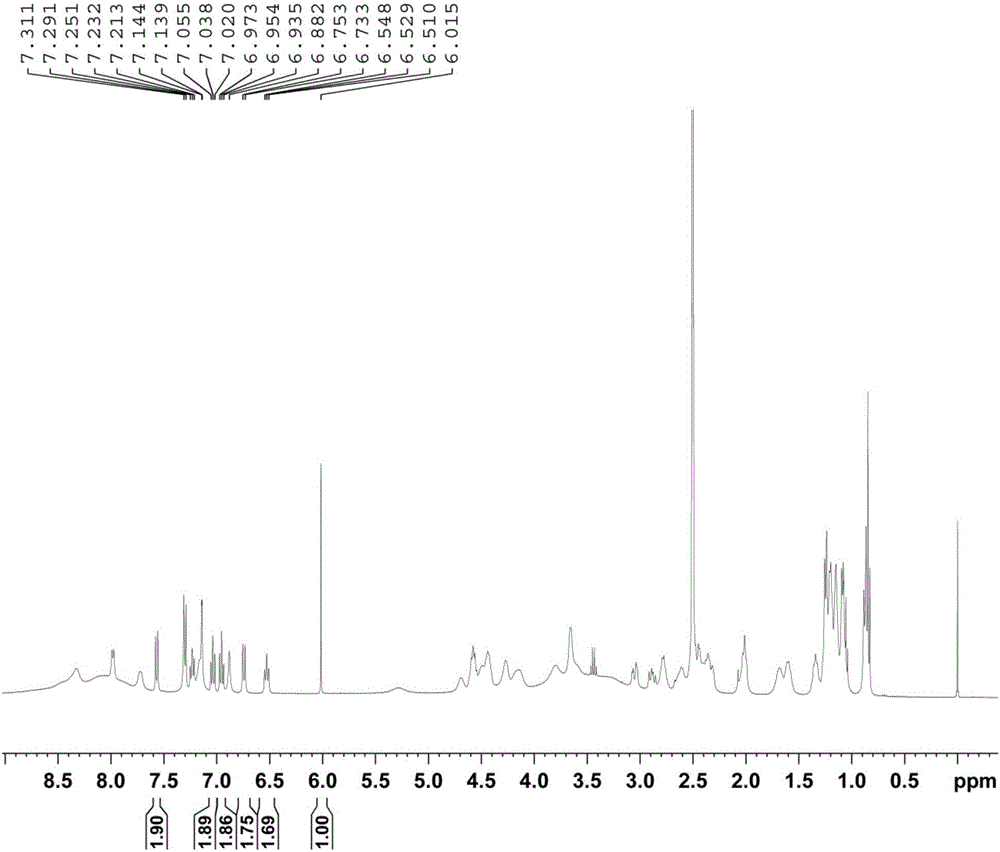
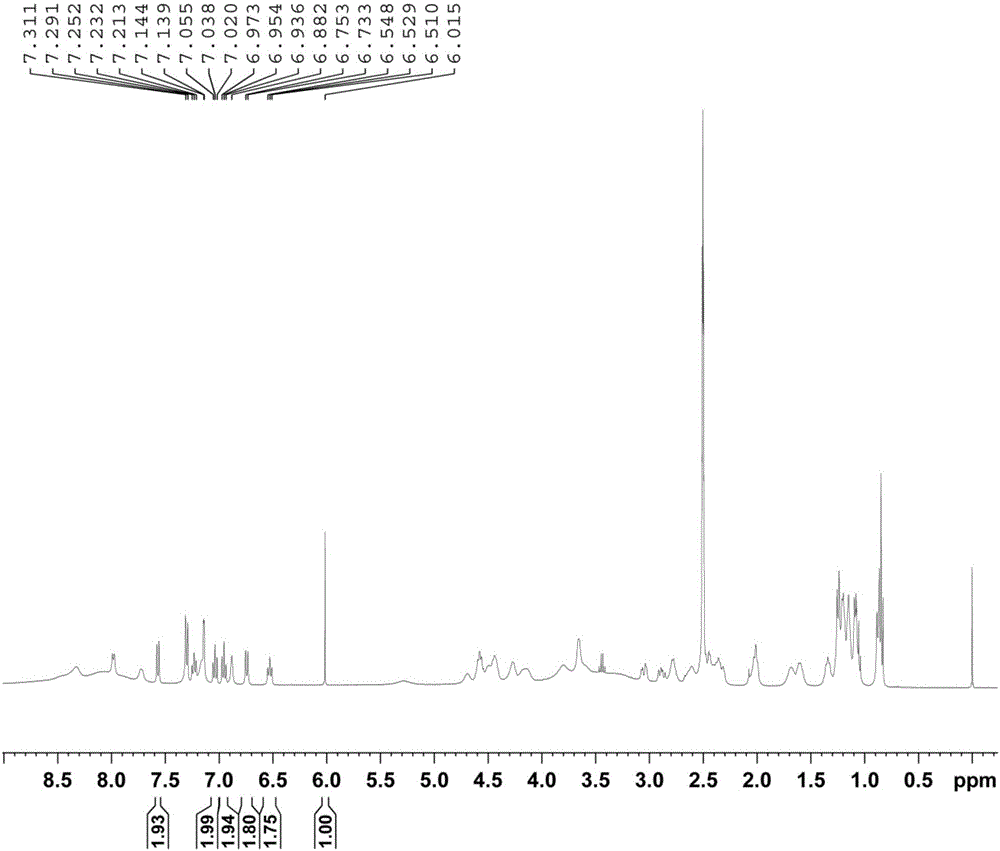
Method for determining purity of daptomycin on basis of hydrogen nuclear magnetic resonance
InactiveCN106018456AAccurately get the absolute contentReduce analysis costsAnalysis using nuclear magnetic resonanceDelayed timeProton NMR
The invention discloses a method for determining the purity of daptomycin on the basis of hydrogen nuclear magnetic resonance. The method comprises the following steps that 1, longitudinal relaxation time (T<1>) of quantitative target peaks of daptomycin and an internal standard substance in a deuterated reagent is determined; 2, a flip angle of a nuclear magnetic resonance wave spectrometer and relaxation delay time (d<1>) are set according to the determined T<1> value; 3, integral areas of all quantitative target peaks of a daptomycin sample and the internal standard substance are determined according to the parameters set in the second step, and the molar mass ratio of daptomycin to the internal standard substance is obtained; 4, the purity of daptomycin is calculated according to the mass of the internal standard substance, and the purity of daptomycin is calculated according to the following formula of W%=(m<IS> *H<IS>*A<S>*M<S>*100%) / (M<IS>*A<IS>*m<S>*H<S>). The method has the advantages of being good in repeatability and easy and convenient to operate, can rapidly and accurately detect the purity of daptomycin and provides a novel method for strictly controlling the purity of daptomycin.
Owner:SHANDONG ANALYSIS & TEST CENT
Method for purifying lipopetide compound
ActiveCN102241732AAchieve the effect of separating and purifying lipopeptide compoundsSuitable for commercial productionPeptide preparation methodsEnvironmental resistancePurification methods
The invention relates to a method for purifying a lipopetide compound, in particular to a method for precipitating daptomycin. The method comprises the following steps of: concentrating a daptomycin solution and then controlling concentration of the solution, regulating pH, adding a precipitation promoter and a filter aid, stirring and precipitating. The precipitate obtained by the method is redissolved and can be then directly refined by adopting methods such as reversed-phase chromatography, ion exchange and the like. The method provided by the invention has the advantages of economy, environment friendliness, suitability for commercialization and the like.
Owner:HISUN PHARMACEUTICAL (HANGZHOU) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com