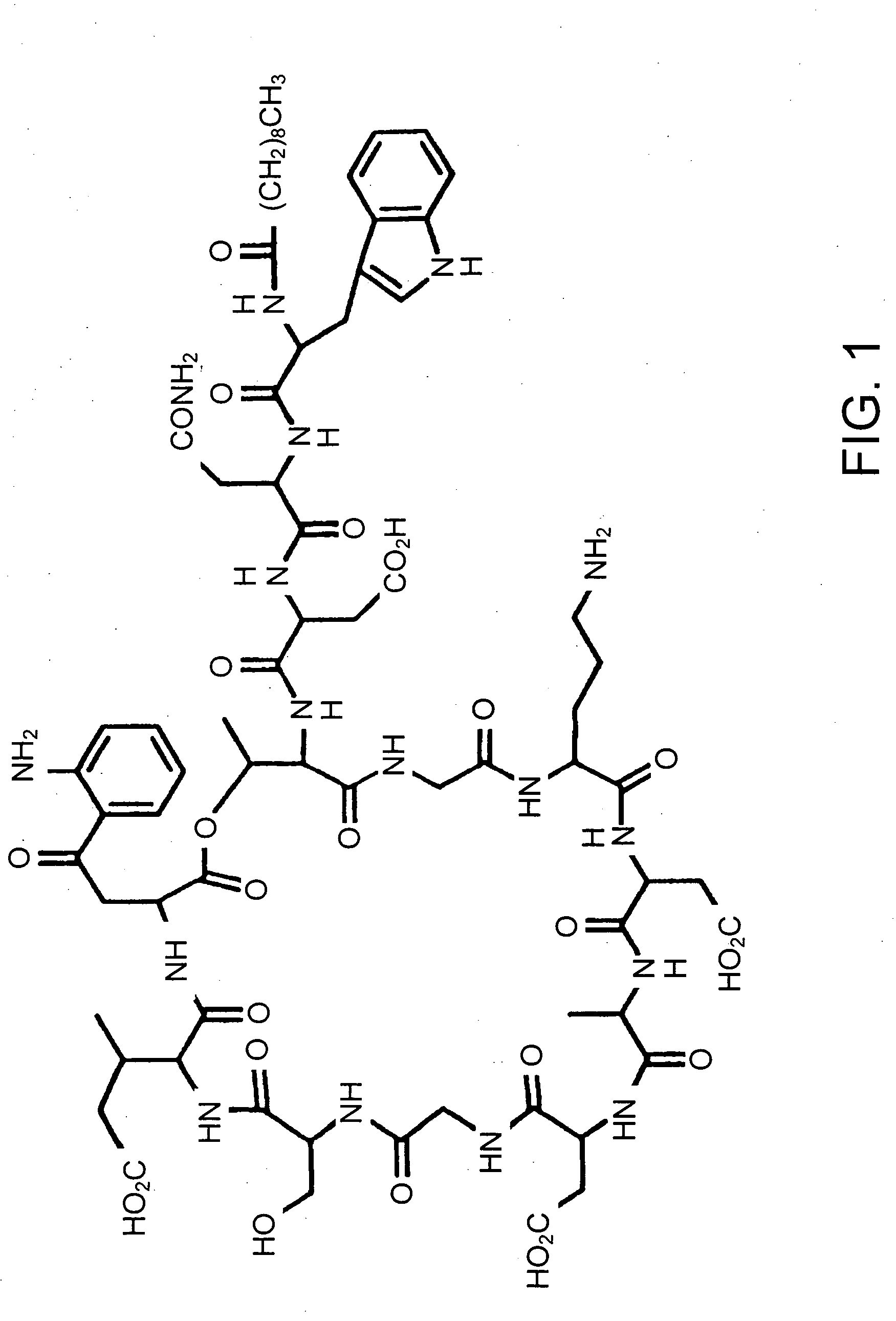
Methods for preparing purified lipopeptides
a technology of lipopeptides and purified water, which is applied in the direction of peptide/protein ingredients, drug compositions, antibacterial agents, etc., can solve the problems of no simple and robust method, no method disclosed in these u.s. patents, and no effective method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0135] Daptomycin was prepared by conventional techniques. The daptomycin preparation was a pale yellow amorphous powder, with a solubility at 25° C. of greater than 1 g / mL in water and a solubility of 2.8 mg / mL in ethanol. The amorphous daptomycin preparation was hygroscopic and decomposed at 215° C.
[0136] The remaining examples describe crystallizing or precipitating lipopeptides in the presence or absence of an organic precipitant (e.g., PEG).
example 2
[0137] In a microbatch crystallization, 25 μL of a daptomycin stock (20 mg / mL in methanol) was sequentially mixed with 15 μL of reagent stock (200 mM calcium acetate, 0.1 M cacodylate (pH 6.5), 18% [w / v] PEG 8000 and 15 μL ethylene glycol) to give a solution that was 27.5% aqueous component, 45% methanol and 27.5% ethylene glycol. Urchin-like crystals were formed at a yield of 50% with a purity of 98% as measured by HPLC.
example 3
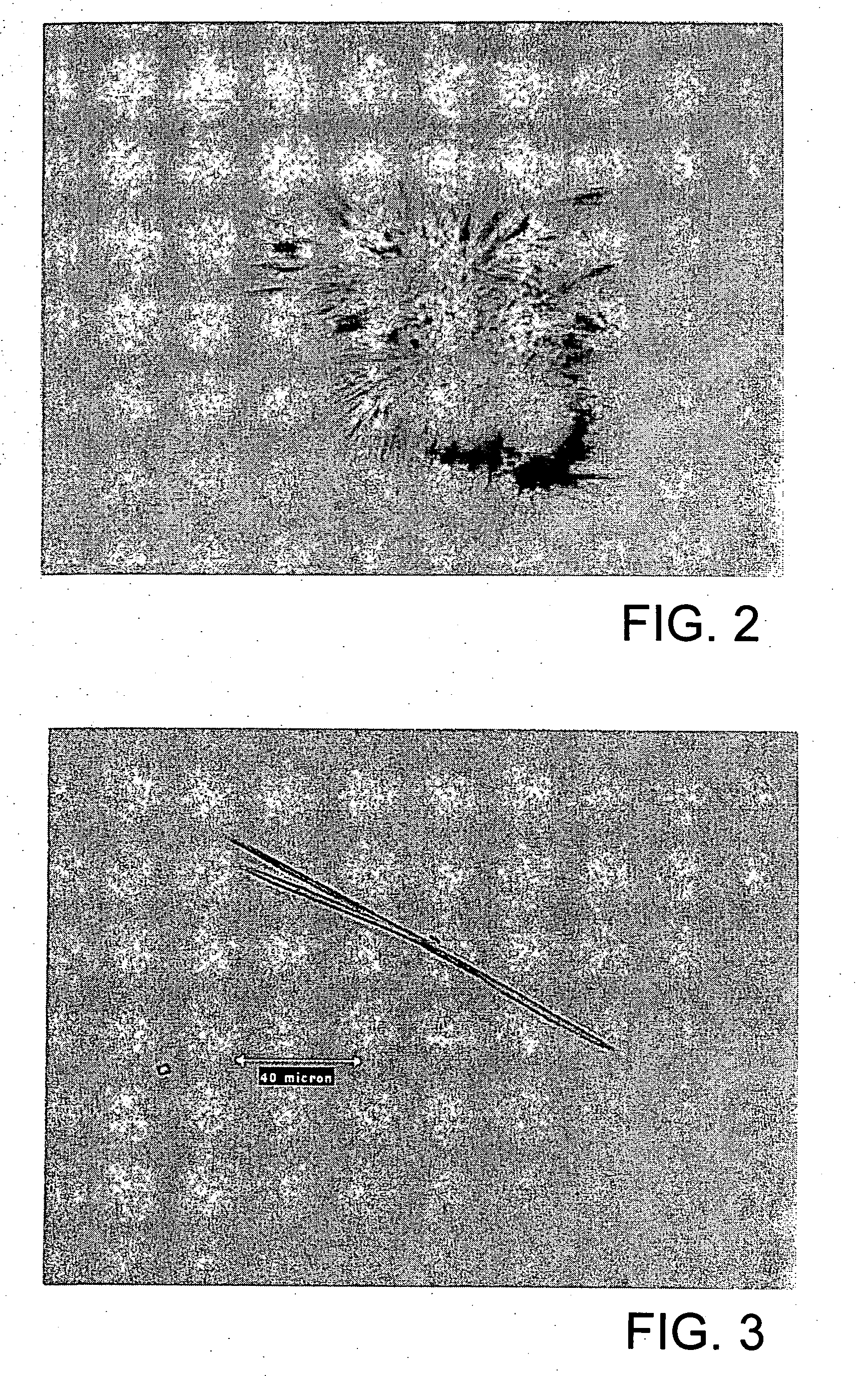
[0138] A daptomycin stock was prepared by dissolving 440 mg daptomycin in 1 mL of a buffer containing 25 mM sodium acetate (pH 5.0) and 5 mM CaCl2. Crystallization was done by the vapor diffusion (hanging drop) method, in which 5 μL of the daptomycin stock was added to 5 μL of 0.1 M tri-sodium citrate dihydrate (pH 5.6), and 35% [v / v] tert-butanol in water to form a drop. The drop was suspended over a reservoir solution (0.1 M tri-sodium citrate dihydrate (pH5.6), and 35% [v / v] tert-butanol in water) in an=air-tight-environment until crystallization occurred. This method yielded urchin-like daptomycin crystals. See, e.g., FIG. 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com