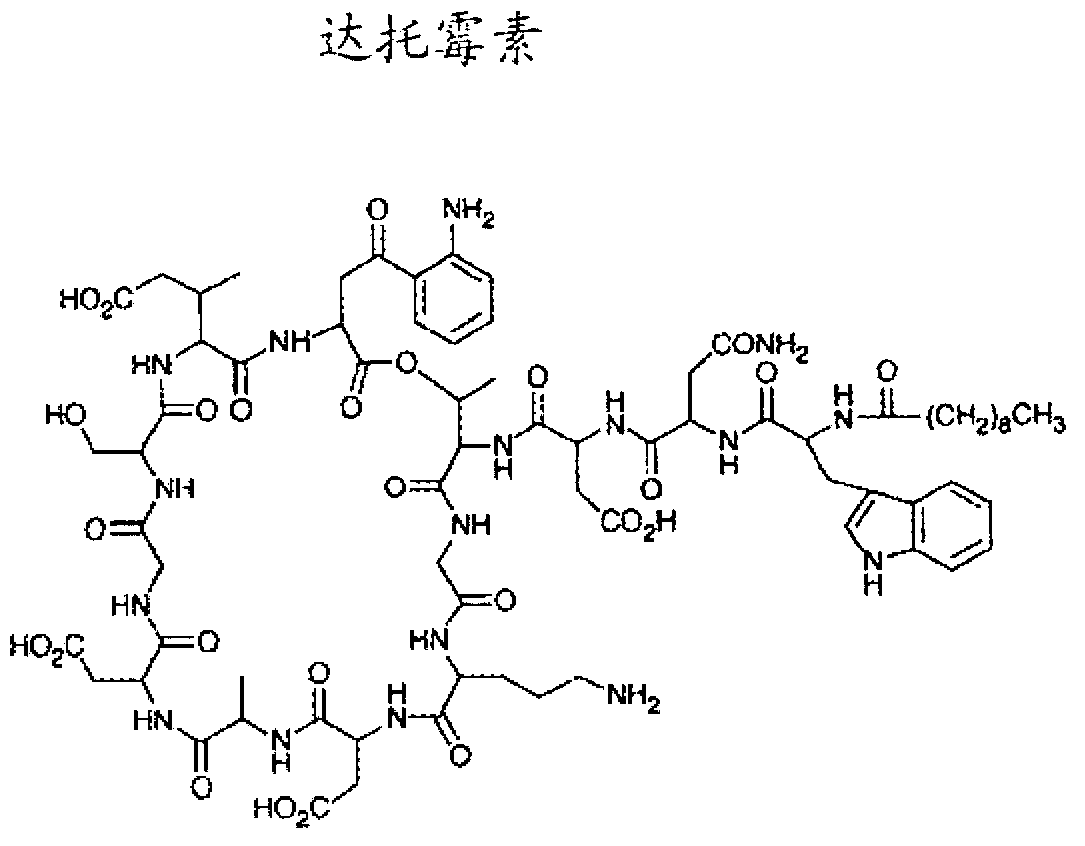
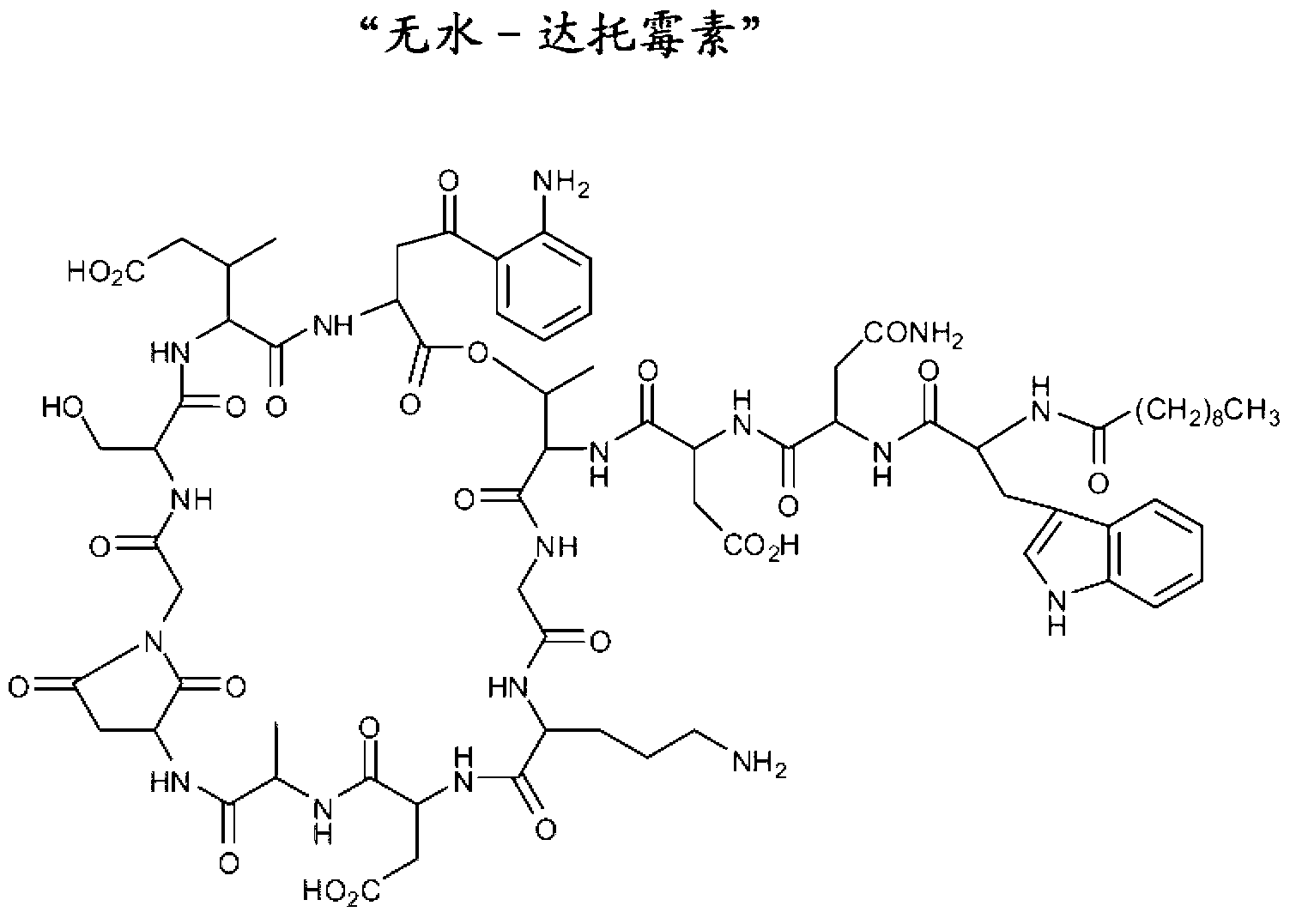
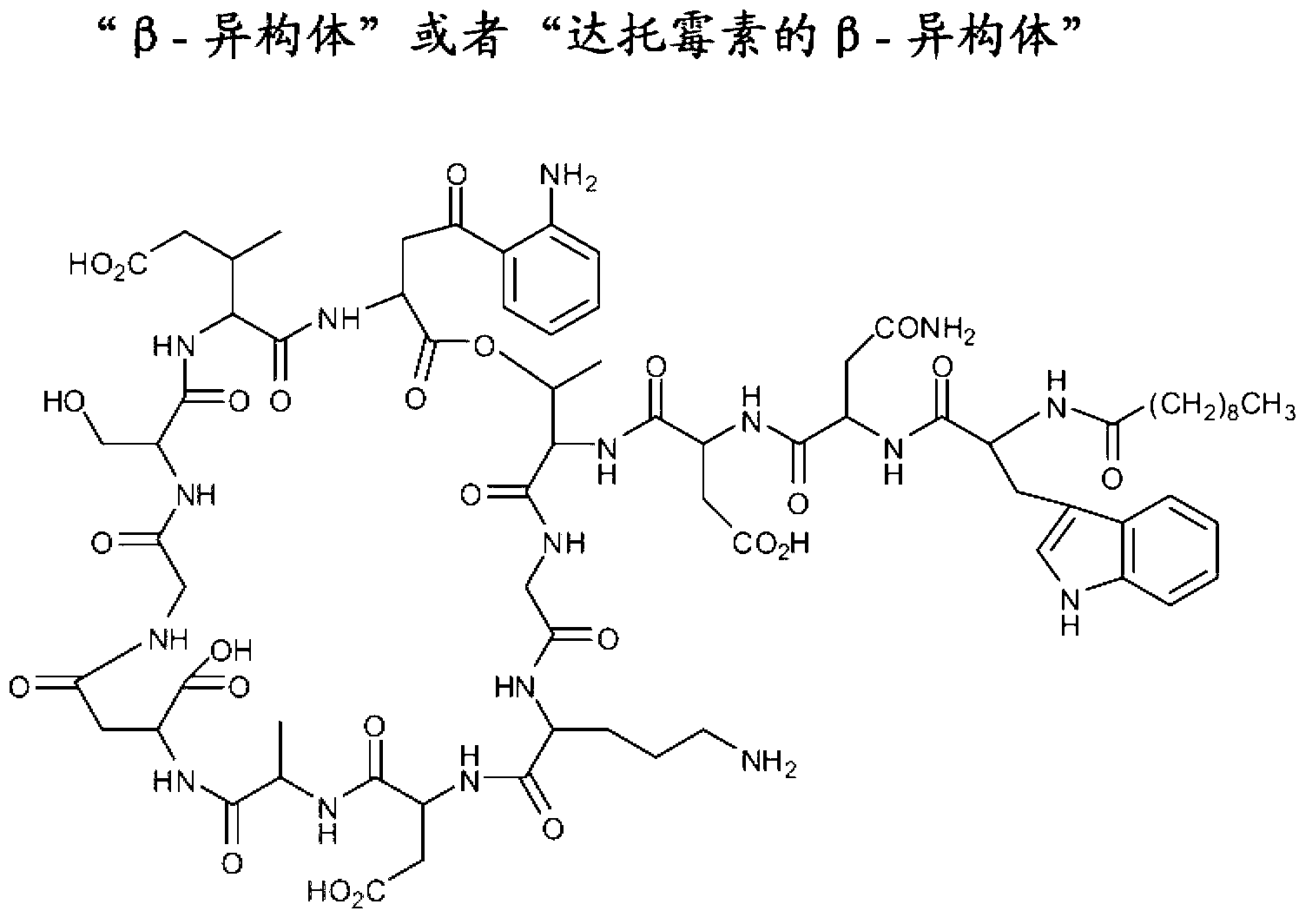
Lipopeptide compositions and related methods
A technology for drugs and substances, applied in chemical instruments and methods, drug combinations, peptides, etc., can solve the problems of inability to stabilize daptomycin, inability to prevent daptomycin isomerization, and inability to prevent degradation of daptomycin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0096] Example 1A: Comparative Preparation Method A (lyophilized daptomycin without sugar or amino acid at pH 4.7)
[0097] Complexation of comparative daptomycin formulations without sugar or glycine was performed under refrigerated (2-10°C) conditions. Daptomycin active pharmaceutical ingredient (API) is supplied as a frozen liquid at pH 3.0 in the concentration range of 125-130 mg / mL. Complexation was initiated by obtaining liquid daptomycin API (eg, thawing frozen daptomycin API provided at a pH of about 3.0), followed by adjusting the pH to a target pH of about 4.7 using 3N NaOH. The bulk solution was further diluted with sWFI to a target concentration of 105 mg / mL and mixed to ensure a homogeneous solution (again at 2-10°C). The bulk product solution was 0.2 μm filtered and filled into 10 mL vials, followed by lyophilization according to the current lyophilization cycle set forth in Example 3. The drug product formulation was capped and sealed under nitrogen.
Embodiment 1B
[0098] Example 1B: Comparative Preparation Method B (lyophilized daptomycin without sugar or amino acid at pH 7.0)
[0099] Compounding of the overall formulation was performed under refrigerated (2-10°C) conditions. Daptomycin API is supplied as a frozen liquid at pH 3.0 in the concentration range of 125-130 mg / mL. The population was complexed by thawing the API and then adjusting the pH to a target pH of approximately 7.0 using 3N NaOH under frozen (2-10°C) conditions, followed by dilution with sWFI to a target concentration of 105 mg / mL and mixing to ensure a homogeneous solution. preparation. The formulated drug product was 0.2 μm filtered and filled into 10 mL vials, followed by lyophilization according to the modified lyophilization cycle set forth in Example 3. The drug product formulation was capped and sealed under nitrogen.
Embodiment 2A
[0100] Example 2A: Preparation Method A (lyophilized at pH 4.7)
[0101] Compounding of the improved daptomycin formulation was performed under refrigerated (2-10°C) conditions. Daptomycin active pharmaceutical ingredient (API) is supplied as a frozen liquid at pH 3.0 in the concentration range of 125-130 mg / mL. By obtaining liquid daptomycin API (e.g., thawing frozen daptomycin API provided at a pH of about 3.0), then adjusting the pH to a target pH of about 4.7 using 3N NaOH, followed by adding one or more sugars ( Such as sucrose) to start the complex. The bulk solution was further diluted with sWFI to a target concentration of 105 mg / mL and mixed to ensure a homogeneous solution (again at 2-10°C). The bulk product solution was 0.2 μm filtered and filled into 10 mL vials, followed by lyophilization according to the current lyophilization cycle set forth in Example 3. The drug product formulation was capped and sealed under nitrogen. The sugar is added as a powder or in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com