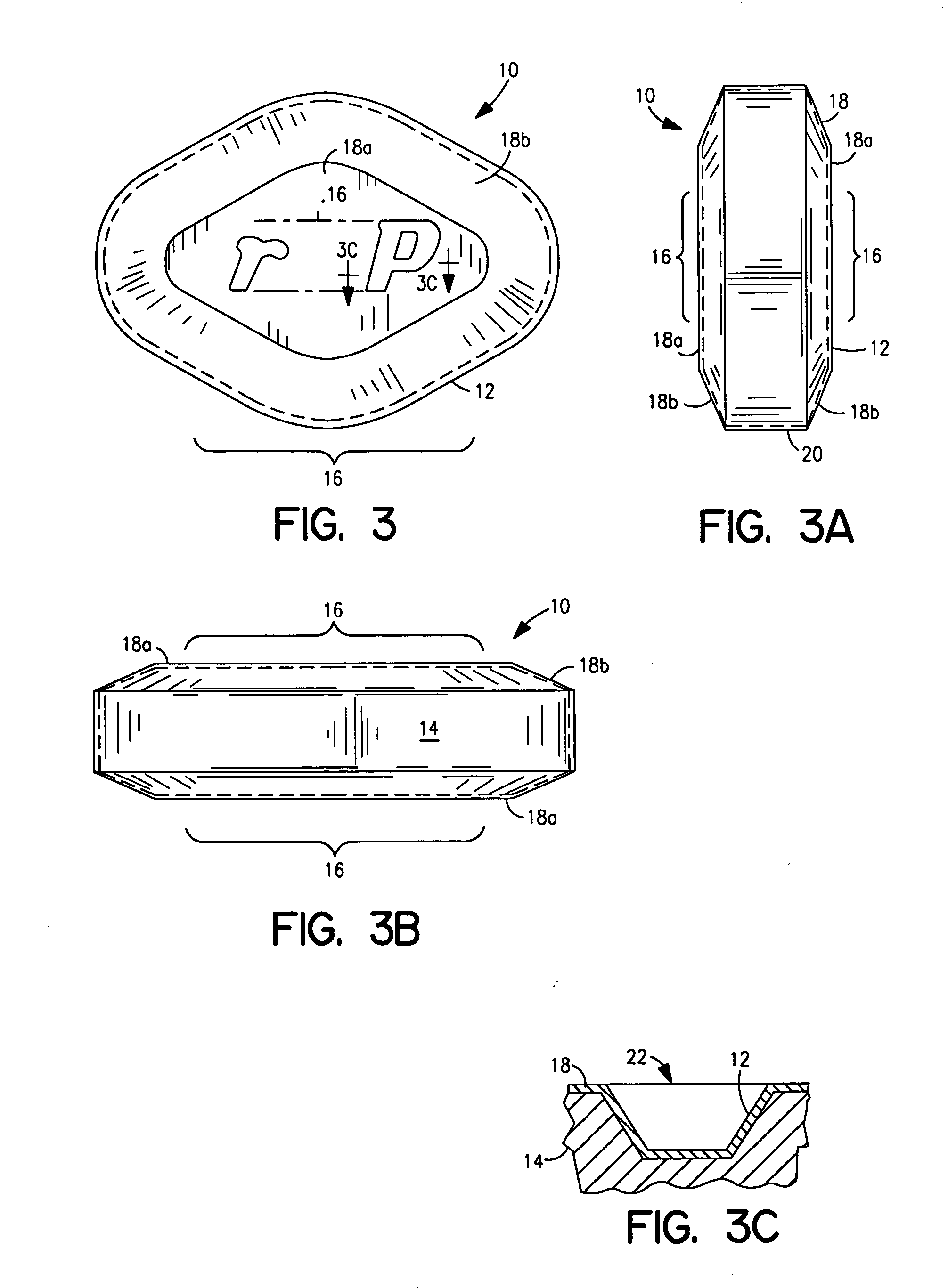
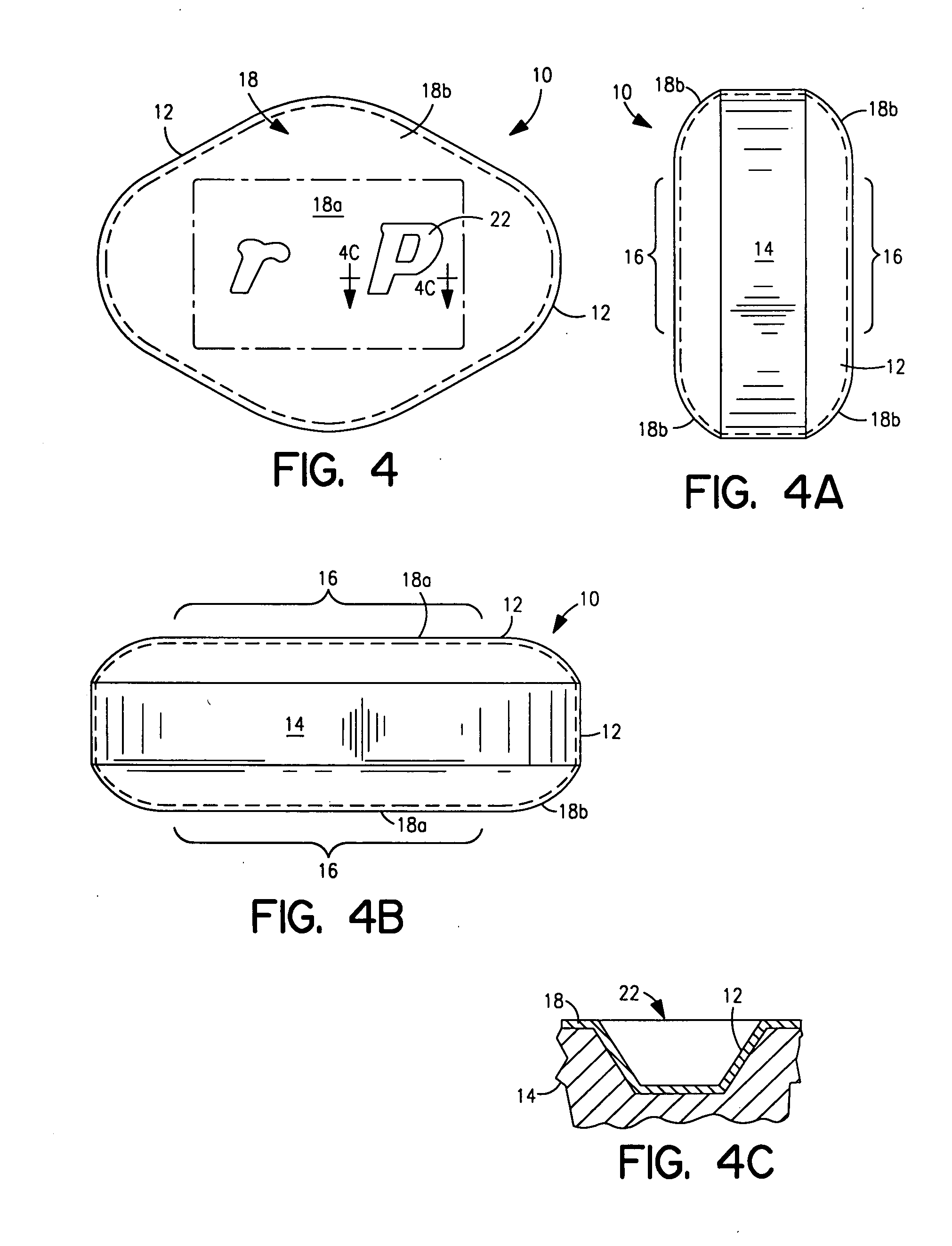
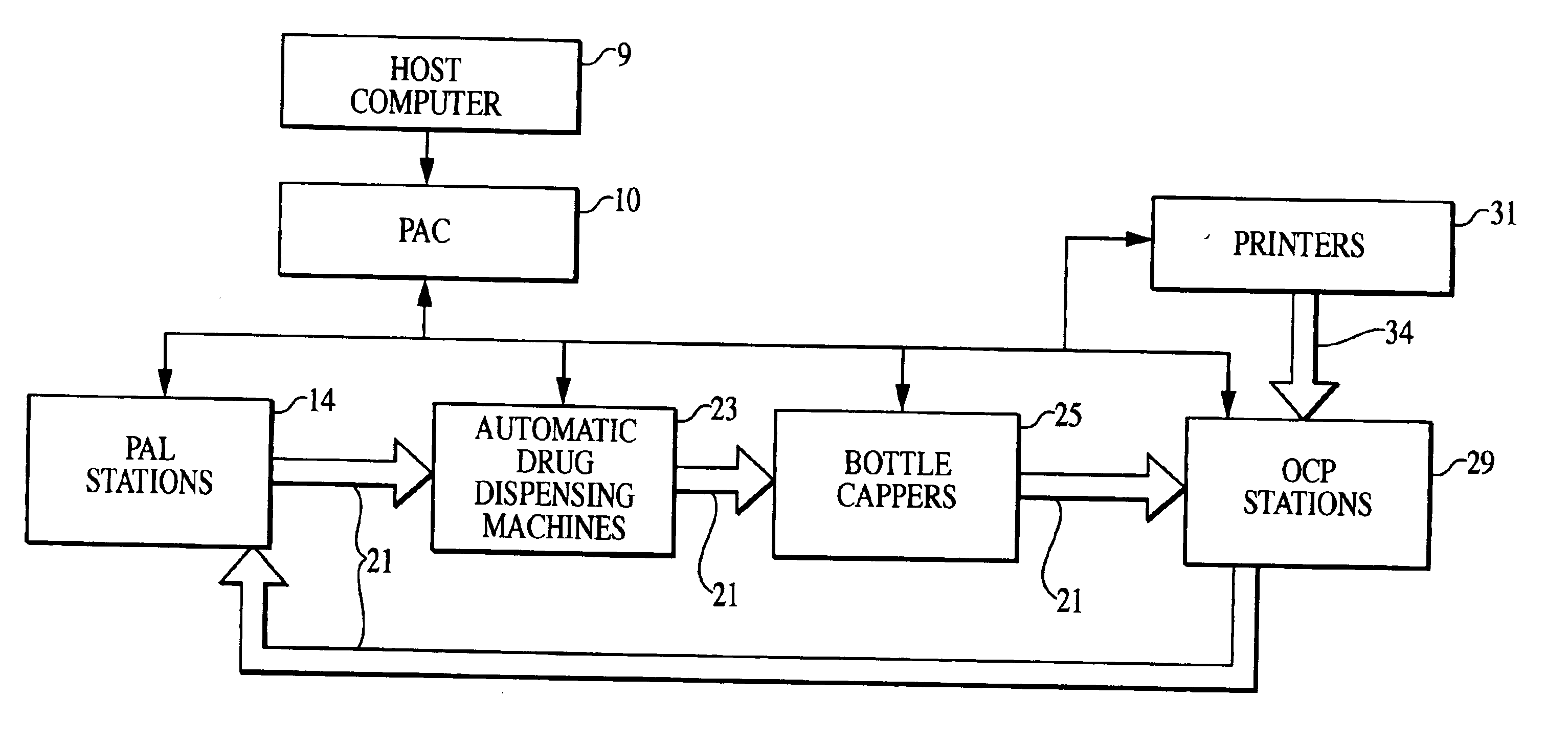
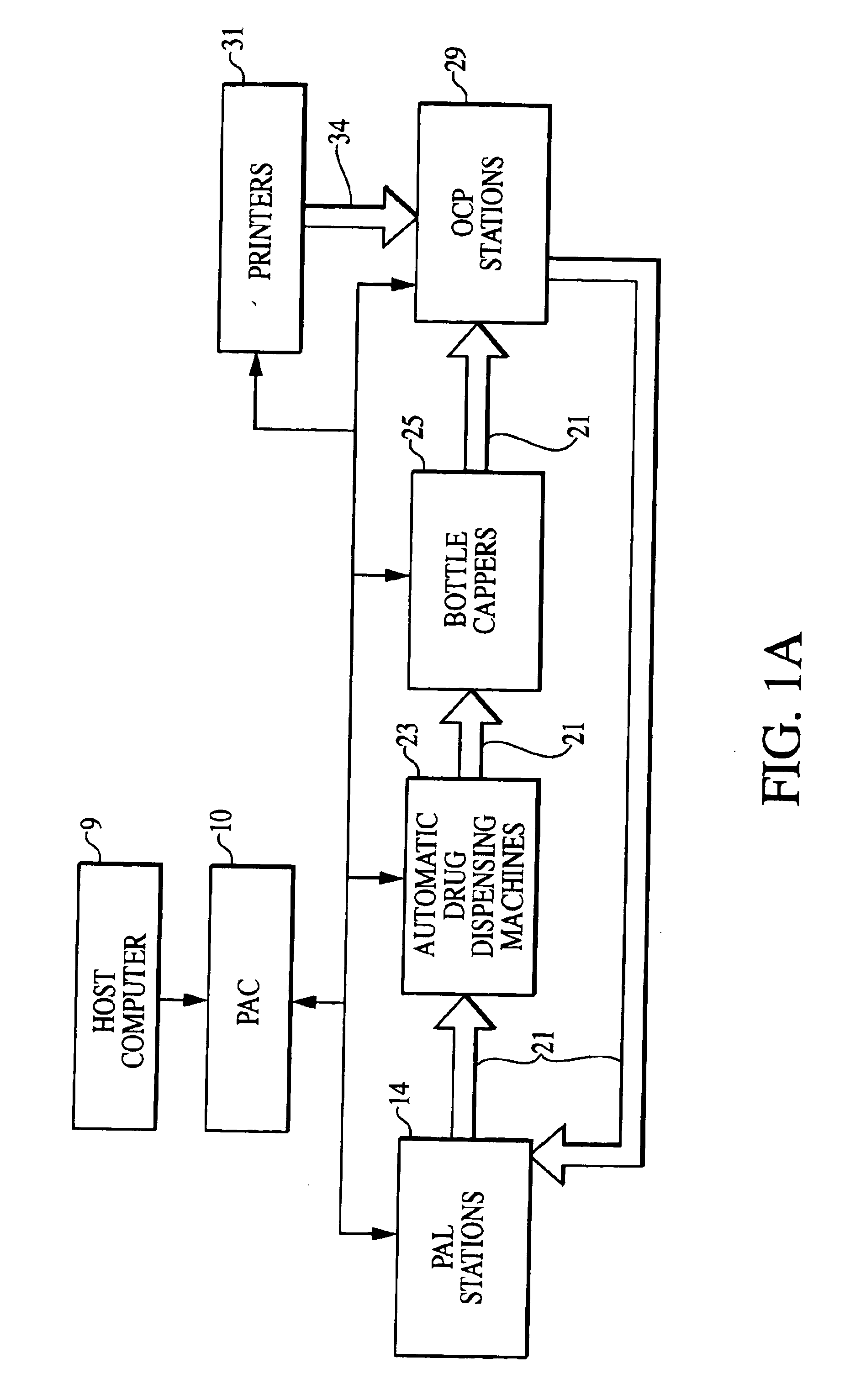
Patents
Literature
8637 results about "Drug product" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug product means “a finished dosage form, for example, tablet, capsule, or solution that contains a drug substance, generally, but not necessarily, in association with one or more other ingredients.” [21 CFR 314.3; Title 21-Food And Drugs; Chapter I-Food And Drug Administration,...
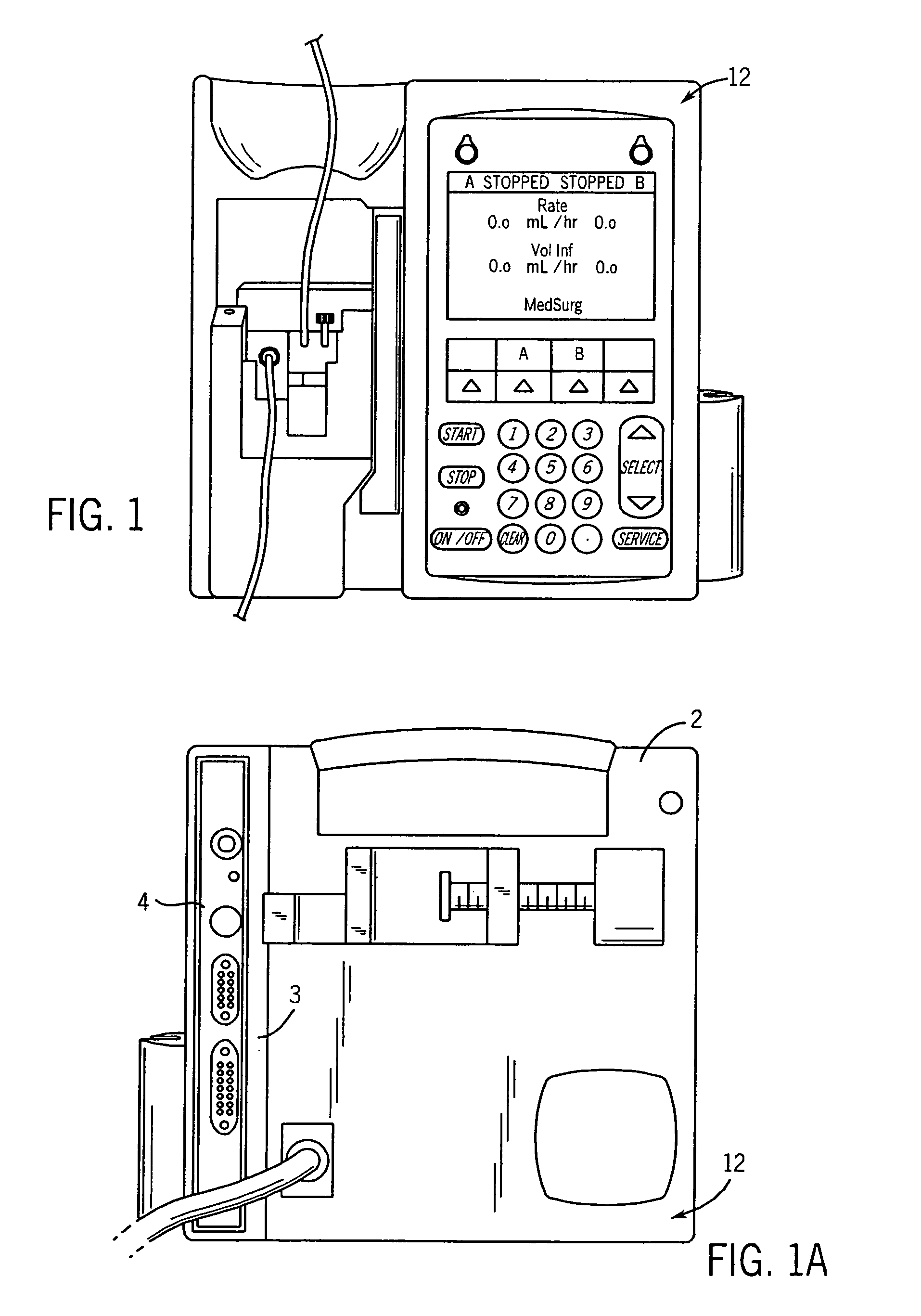
Medication administration system
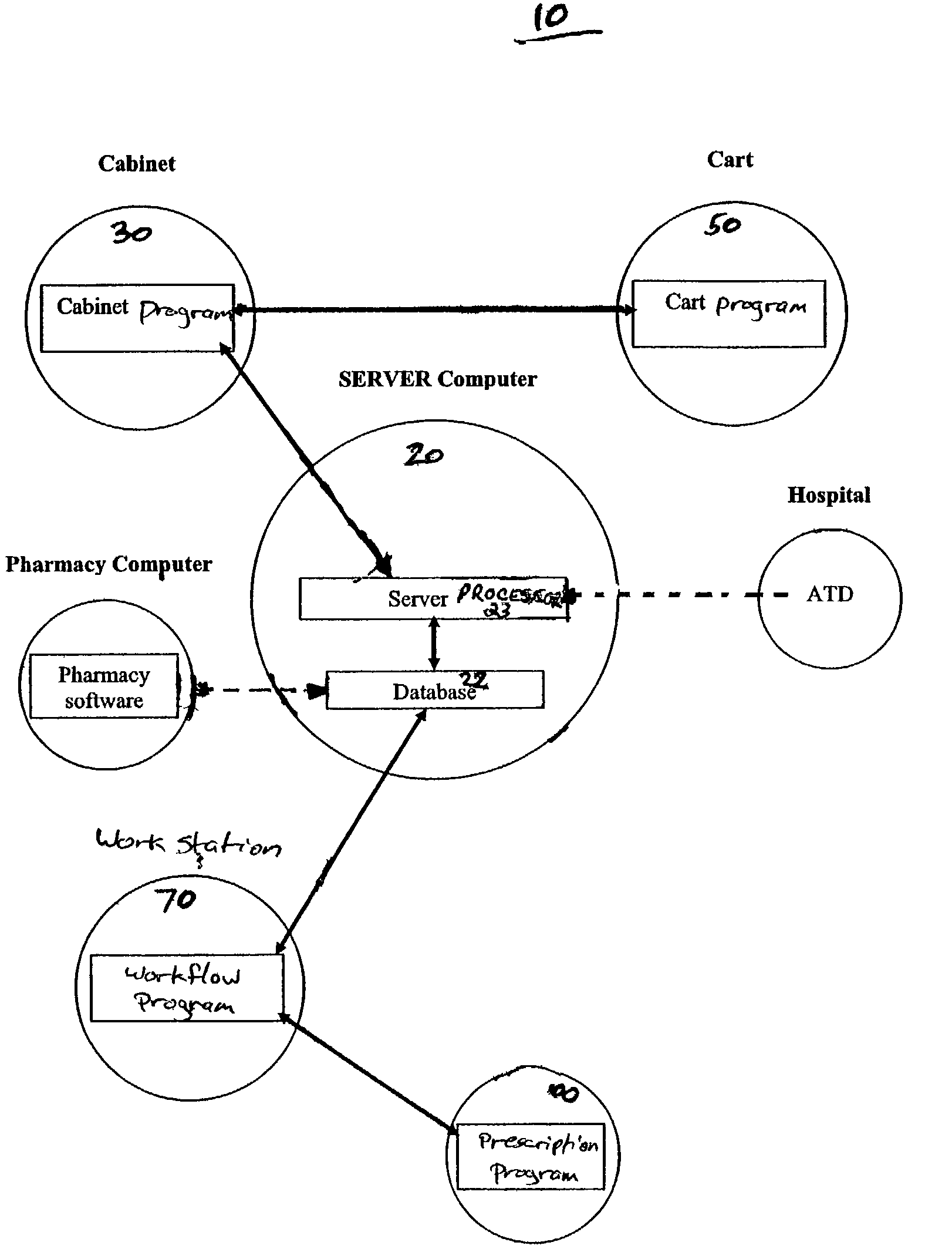
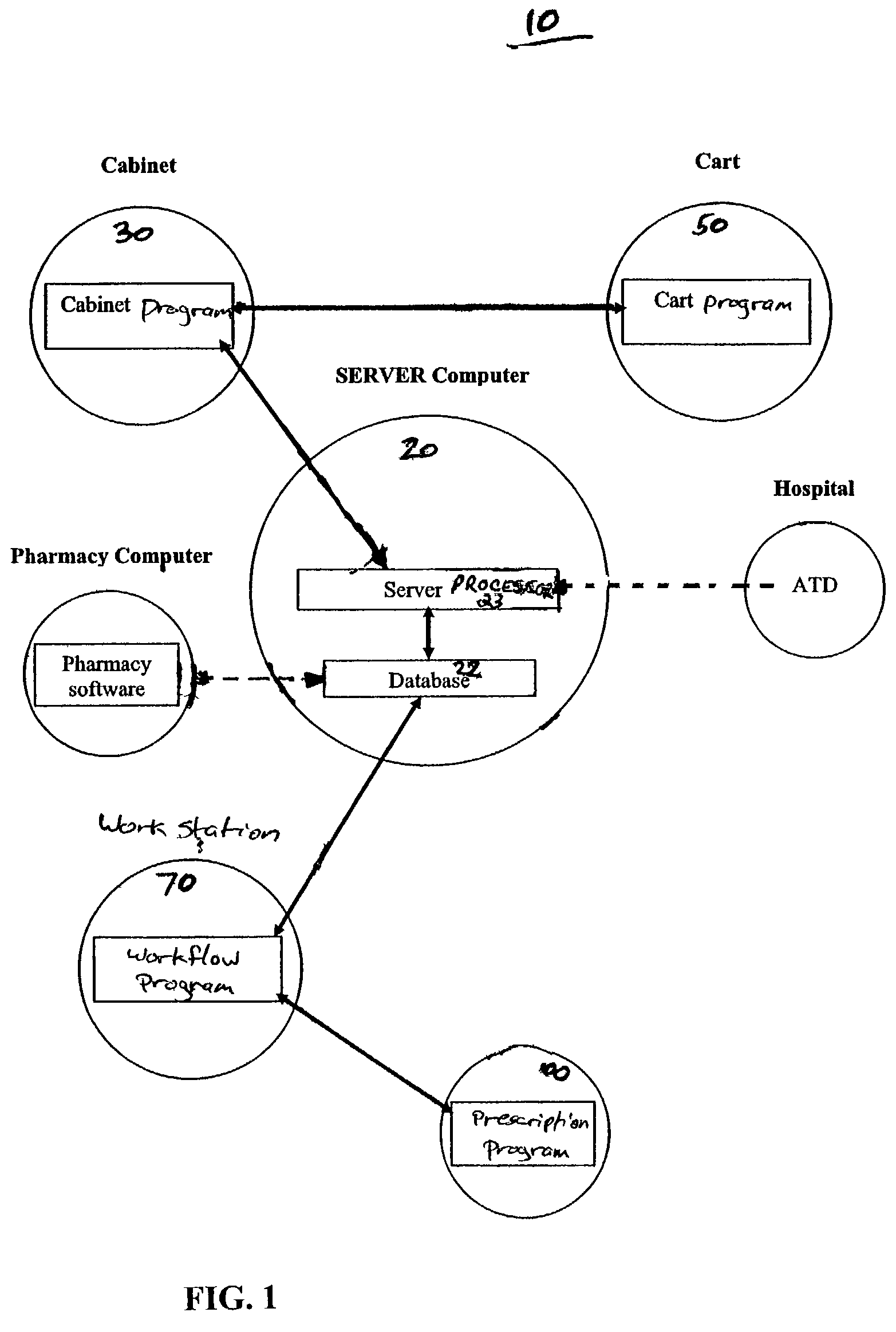
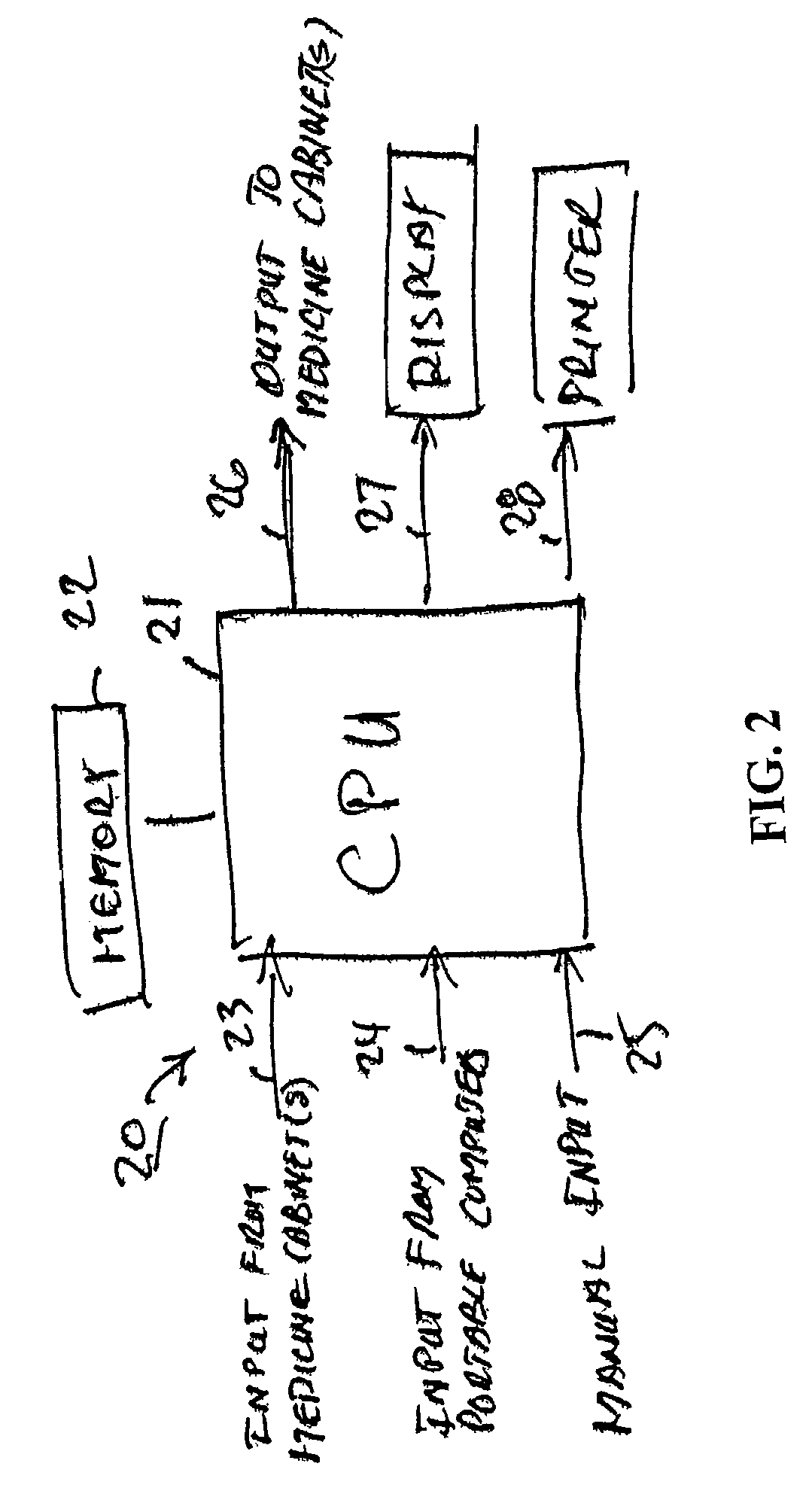
The present invention comprises a system and method for administering medications to a plurality of patients in a medication institution. A preferred system comprises a workflow program for generating a scheduler, wherein the scheduler coordinates the administration of medications to the patients, a medicine cabinet, responsive to said scheduler, for storing medications and dispensing the medications to an authorized user for administration to the patients, the workflow program providing the cabinet with patient specific information relating to said dispensation of the medications including a physician order for each patient, and a medicine cart, coupled to the medicine cabinet, for instructing said authorized user in the administration of said medication to each of said patients. The cart comprises a plurality of patient specific cart drawers for storing the medication to be administered to each patient, wherein each cart drawer remains unidentified as patient specific until the medication cart receives said patient specific information, and a cart processor, wherein the cart drawers are filled with medicine from the medicine cabinet for each patient associated with each patient specific cart drawer in accordance with the respective physician order for each patient.
Owner:3AM IP
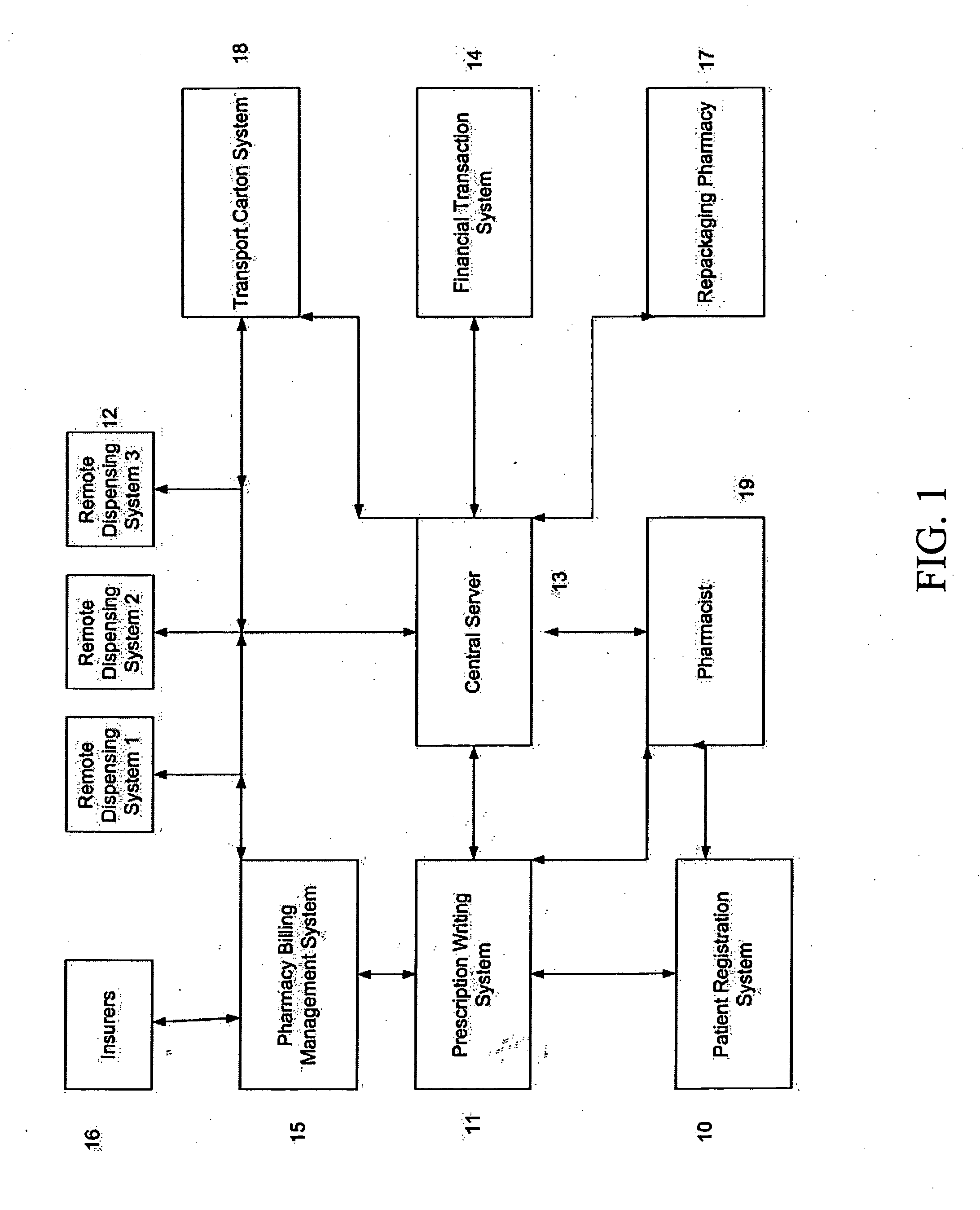
Integrated pharmaceutical accounts management system and method
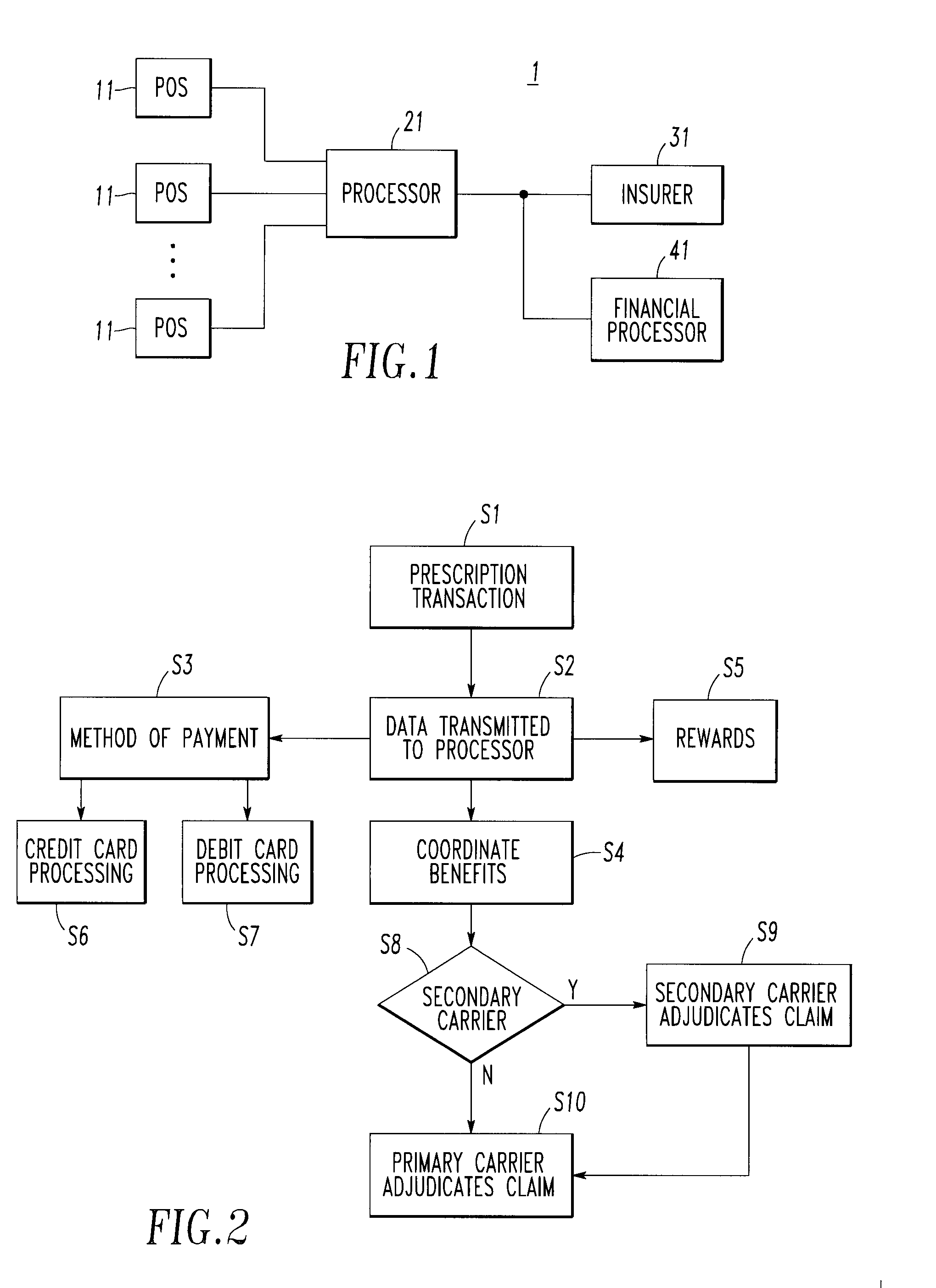
InactiveUS20020002495A1Facilitate real-time adjudicationEasy to processHand manipulated computer devicesDrug and medicationsData warehouseDrug interaction
<heading lvl="0">Abstract of Disclosure< / heading> An integrated suite of services for consumers, service providers and manufacturers in the pharmaceutical industry is disclosed. The present invention utilizes one or more of the NCPDP standard formats and adopts the switch for an integrated system of, for example, instant adjudication of prescriptions, consumer data warehousing and / or incentive rewards for the consumer. A participating consumer with one card, can instantly purchase pharmaceuticals and charge the transaction to a credit card and earn and apply savings dollars redeemable for pharmaceutical purchases. For a participating service provider, instant adjudication and instant validation of consumer eligibility can be performed. Moreover, a service provider may receive messages related to the patient's medications. Significantly, data is recorded for consumers even when consumers make the pharmaceutical purchase with cash. The system includes a unique card issued to participating consumers. The card is adapted to encode conventional credit or debit card information specific to the participating consumer so that the consumer can consummate a transaction for the purchase of pharmaceuticals without possession of an additional credit card. The system further includes a host processor coupled to the point of sale at the service provider through a leased line or public switch network or the like. When a customer performs a pharmaceutical transaction at the point of sale of the service provider, the host processor coordinates any benefits and data with other prescription benefit management systems through messages transmitted and received from any primary or secondary carrier systems. The host processor further is adapted to facilitate real-time adjudication of claims and checks for any dangerous drug-to-drug interactions. The host processor additionally facilitates any financial processing including the accumulation and redemption of any bonus dollars earned by the consumer. Furthermore, since the card used by the consumer can be encoded with credit or debit card information, the host processor determines the desired payment method and performs the actual financial transaction. Even if the transaction at the point of sale is a cash purchase, the consumer may desire to use his unique card for the accrual of bonus dollars. Therefore, data concerning the transaction (i.e., pharmacy number, prescription number, etc.) can be recorded even for transactions conducted with cash.
Owner:NPAX
Anti-infection compound preparation and its preparation method
InactiveCN1380098AImprove immunityEffective excretionAntibody ingredientsUnknown materialsSide effectSuppository
The anti-infective medicine, including powder, mixture, aerosol, capsule, injection, suppository, ointment and microcapsule, is characterized by that on the theroretical basis of combining traditional Chinese medicine and modern immunology the immunoglobulin, effective components of Chinese medicinal materials which are extracted according to the compound prescription and auxiliary preparation are combined together organically, and undergone the fine preparation process to obtain a high-efficiency, safe, stable, environment-protecting type anti-infection medicine having no toxic side effect and having no drug resistance.
Owner:张勇飞 +2
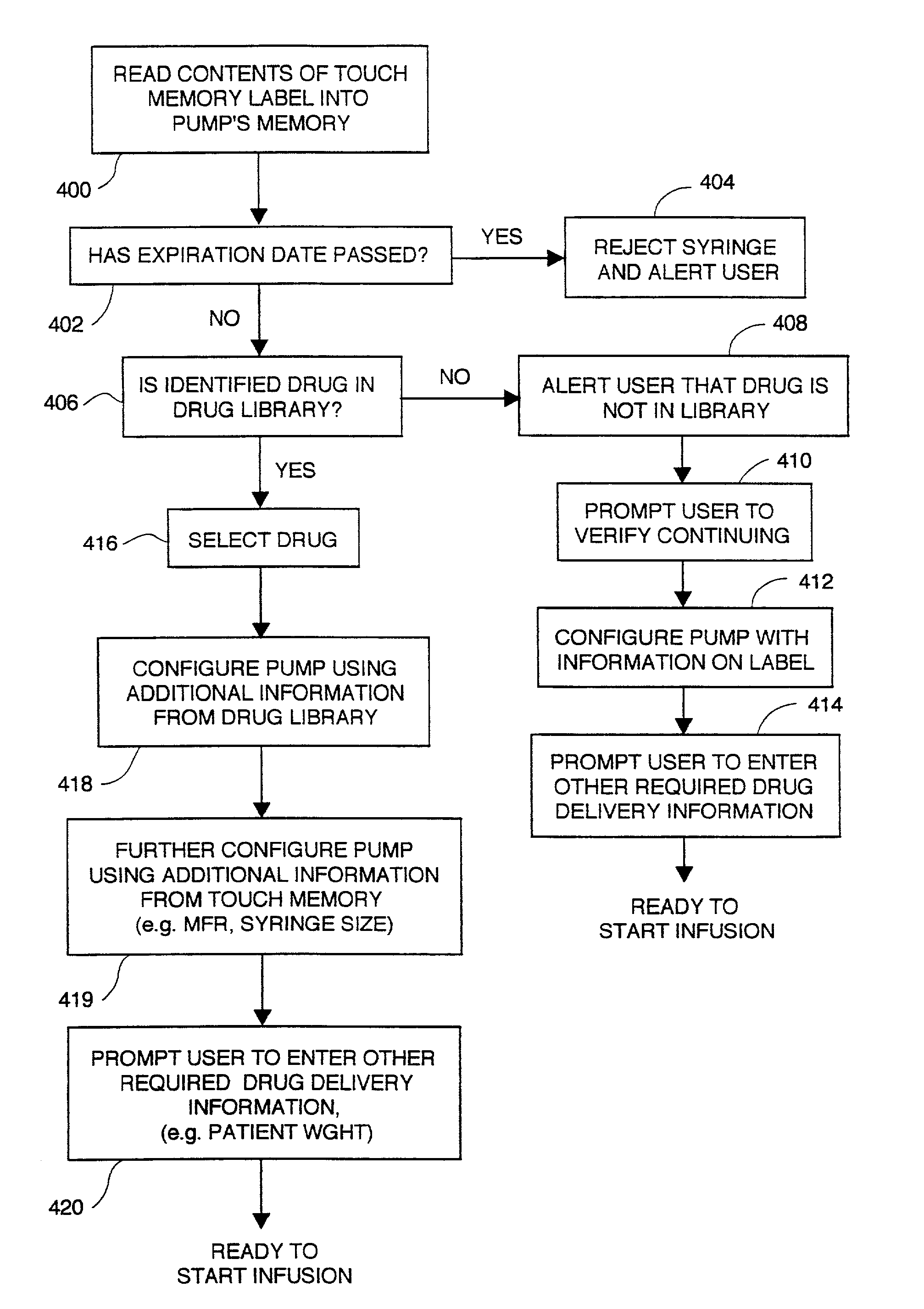
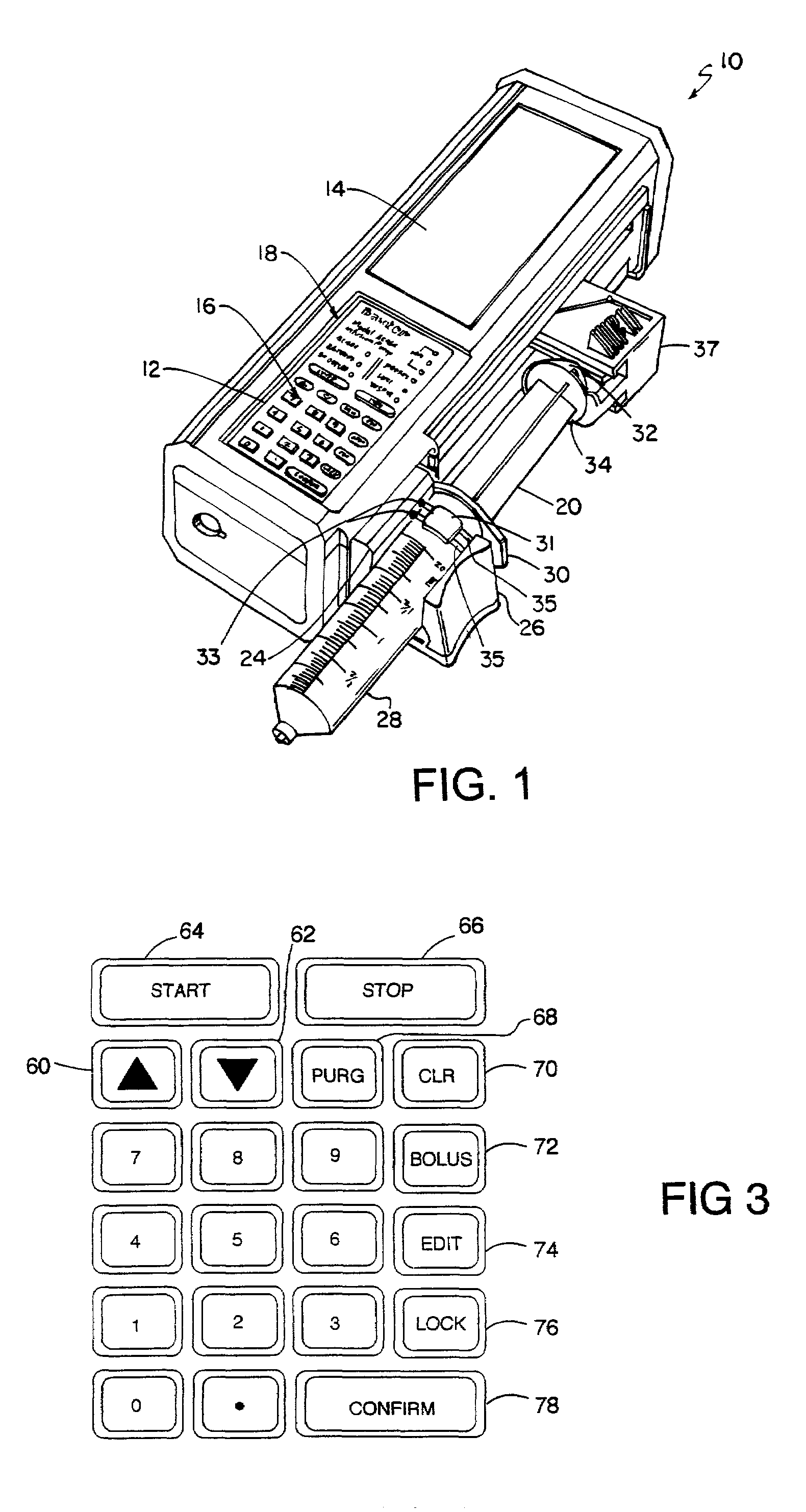
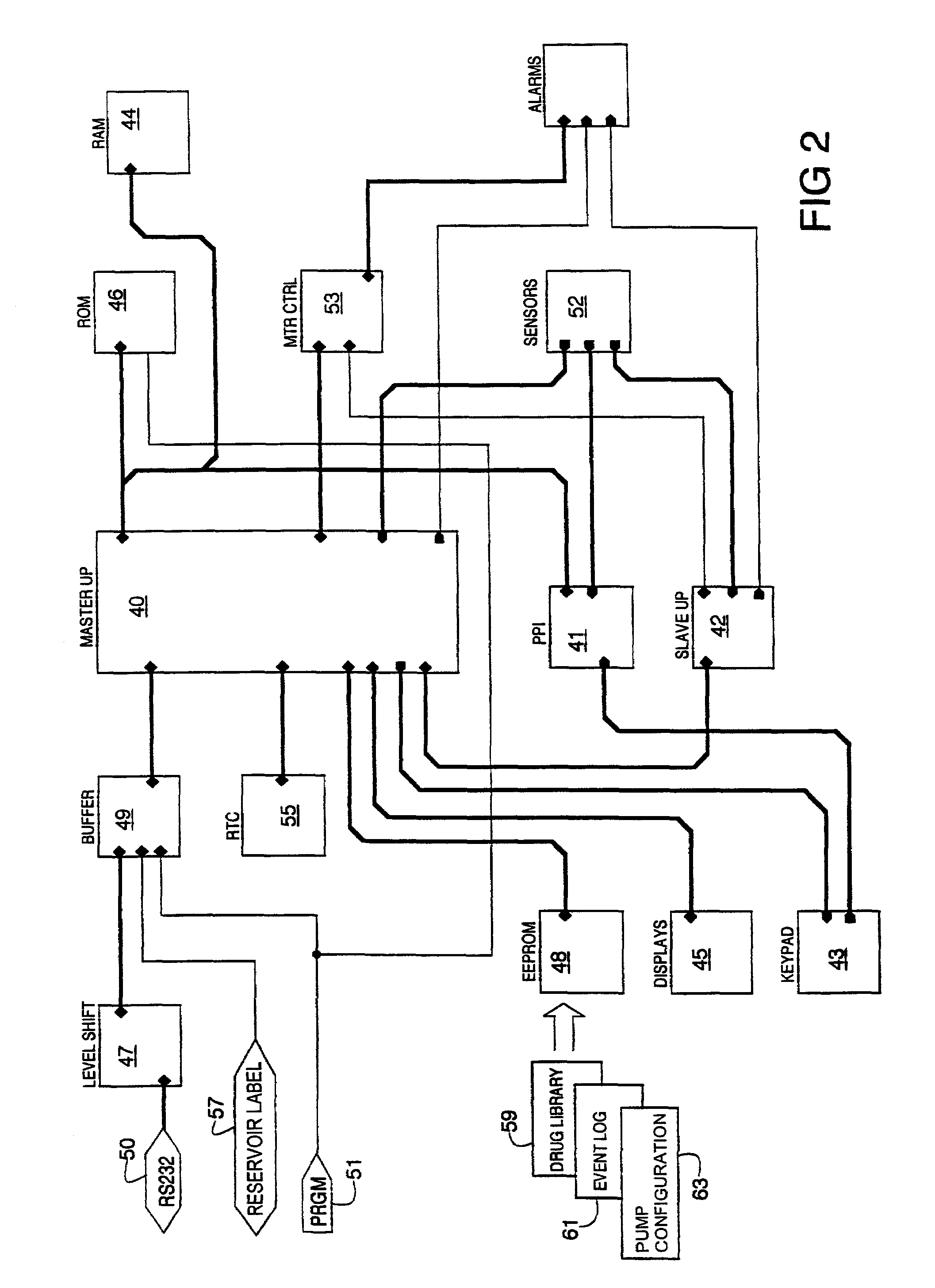
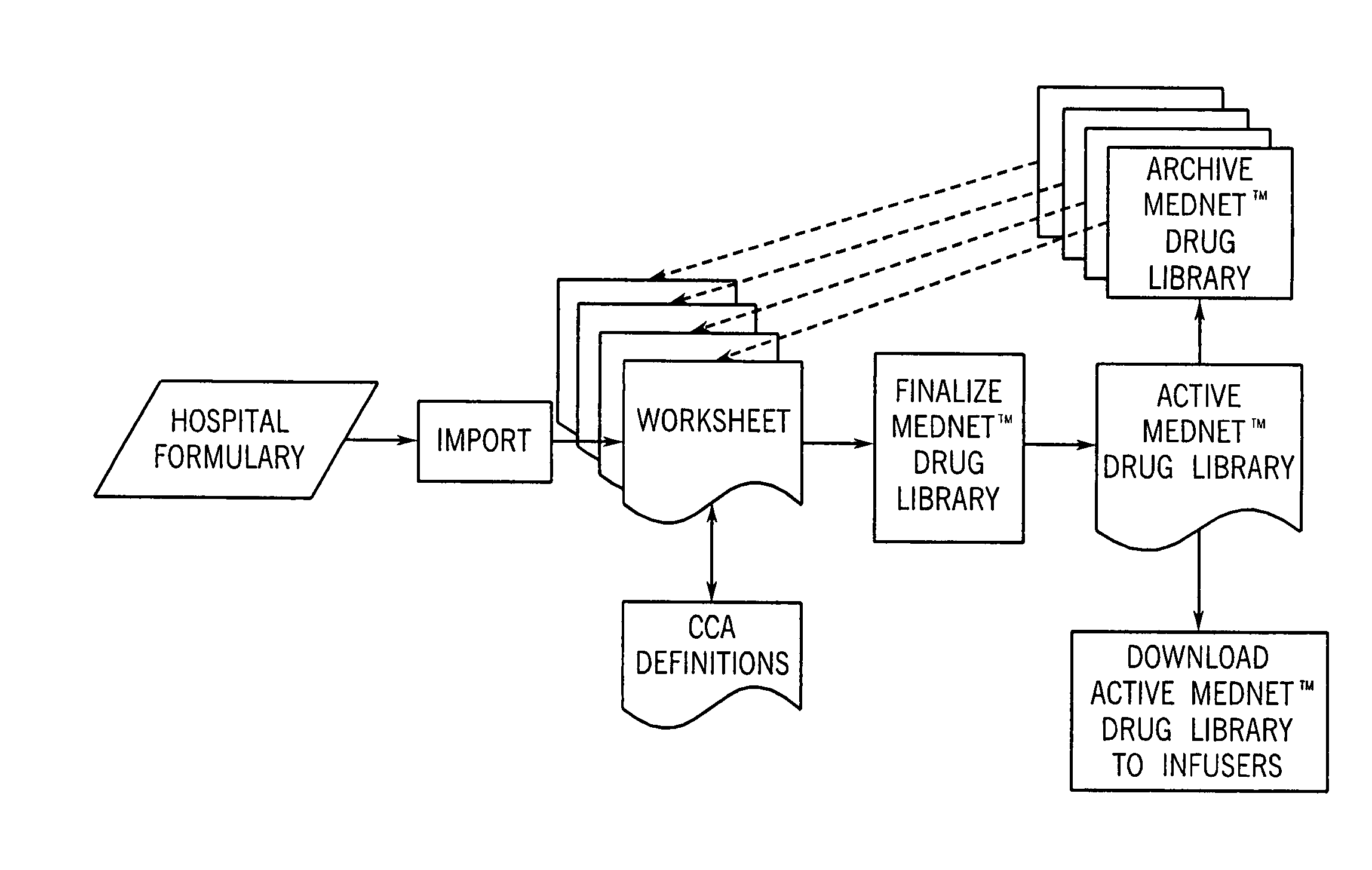
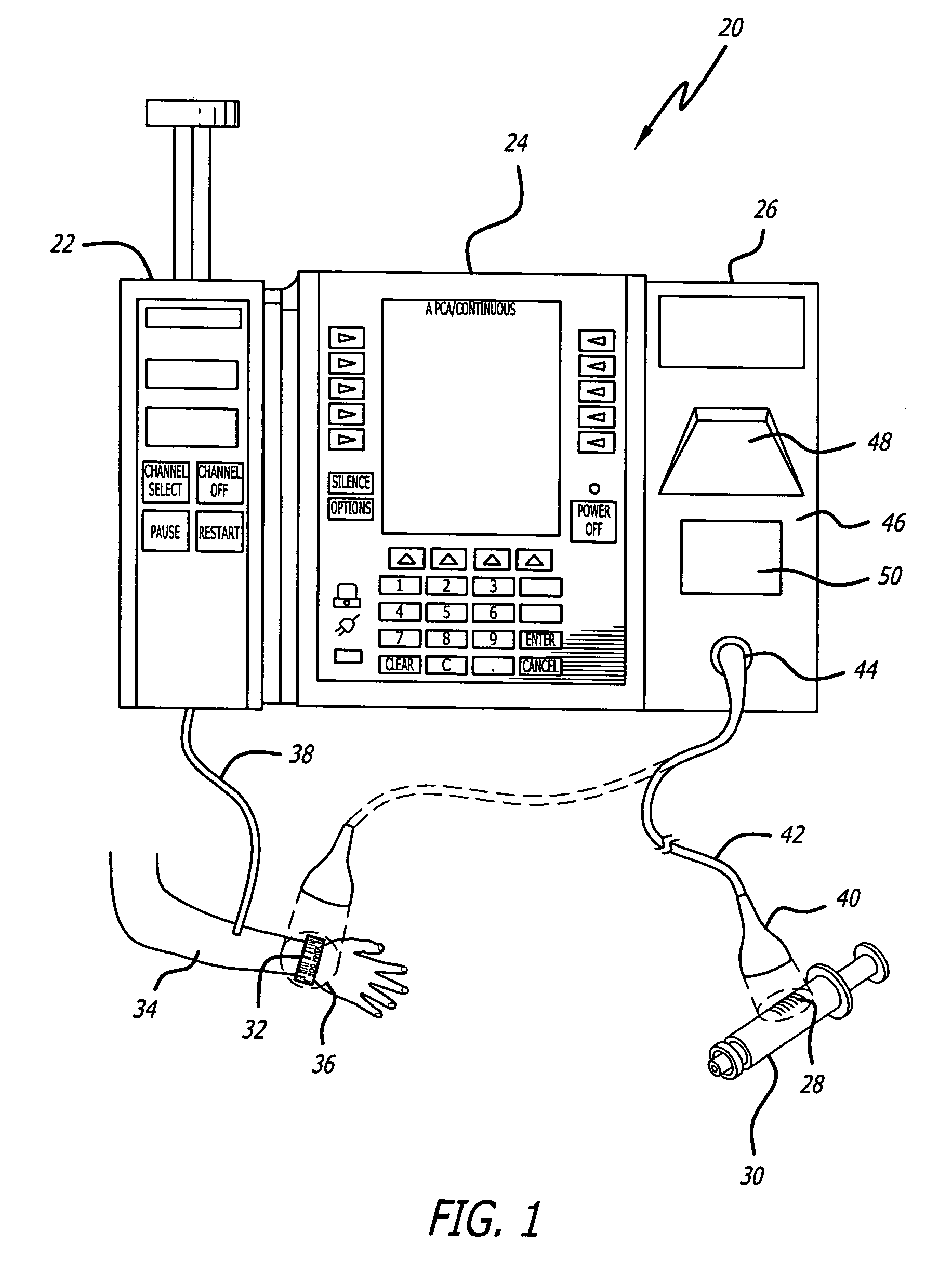
Infusion pump with an electronically loadable drug library and label reader
InactiveUS7471994B2Easy to customizeEasy injectionDrug and medicationsMedical devicesMedicineDrug product
A system for creating a customized drug library for an electronically loadable drug infusion pump, the system including a drug library containing a plurality of drug entries, there being associated with each drug entry a set of associated drug delivery parameters and / or drug delivery protocols for configuring the drug infusion pump; a tool for selecting a set of drug entries from among the plurality of drug entries in said drug library; a tool for adding the selected drug entries along with the sets of drug delivery information associated therewith to a customized library; and a loading tool for causing the system to electronically load the customized library into the drug infusion pump.
Owner:THE GENERAL HOSPITAL CORP +1
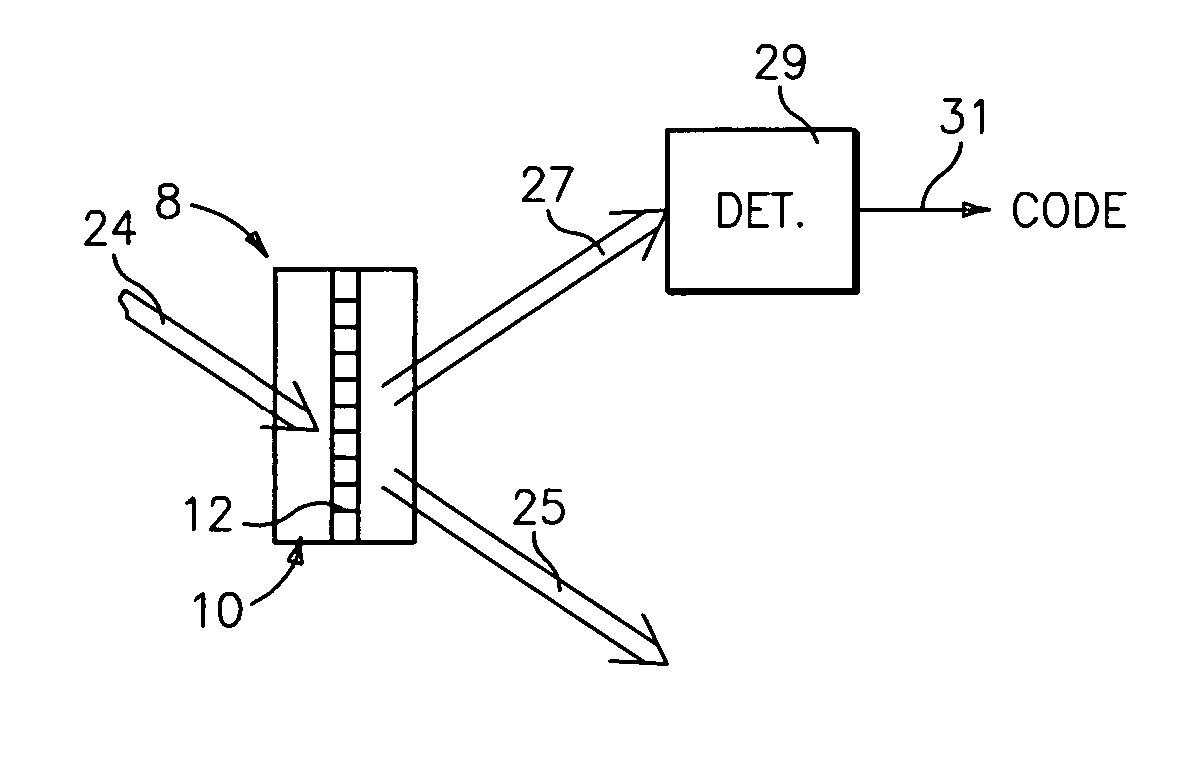
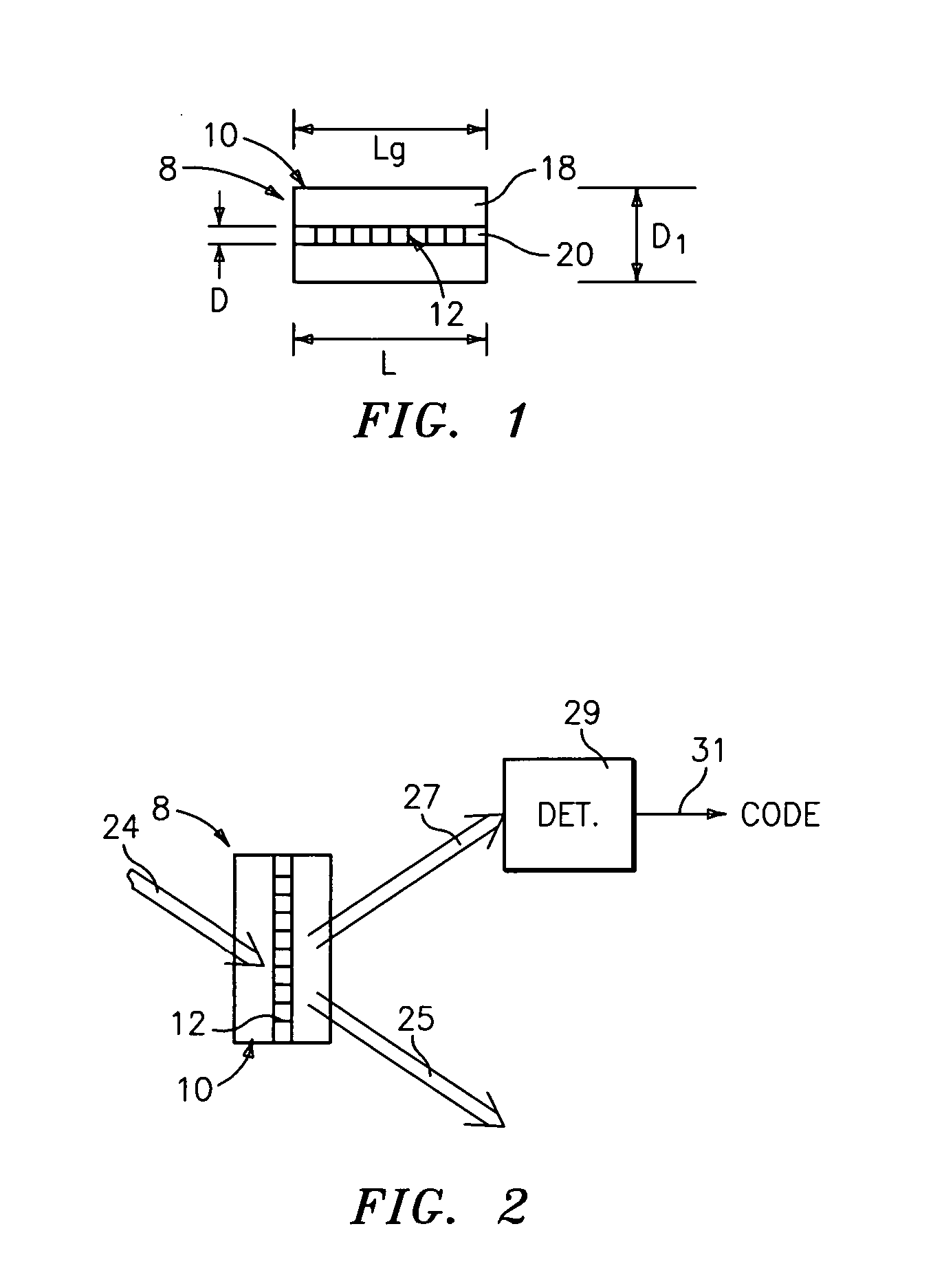
Method and apparatus for drug product tracking using encoded optical identification elements
ActiveUS20060028727A1Made smallMade flexiblePaper-money testing devicesPharmaceutical delivery mechanismAuthenticationPhysics
A method and apparatus for drug product tracking (or other pharmaceutical, health care or cosmetics products, and / or the packages or containers they are supplied with) using diffraction grating-based encoded optical identification elements 8 includes an optical substrate 10 having at least one diffraction grating 12 disposed therein. The grating 12 has one or more colocated pitches Λ which represent a unique identification digital code that is detected when illuminated by incident light 24. The incident light 24 may be directed transversely from the side of the substrate 10 (or from an end) with a narrow band (single wavelength) or multiple wavelength source, and the code is represented by a spatial distribution of light or a wavelength spectrum, respectively, or a combination thereof. The encoded element 8 may be used to label any desired item, such as drugs or medicines, or other pharmaceutical or health care products or cosmetics. The label may be used for many different purposes, such as for sorting, tracking, identification, verification, authentication, anti-theft / anti-counterfeit, security / anti-terrorism, or for other purposes. In a manufacturing environment, the elements 8 may be used to track inventory for production information or sales of goods / products. Such labeling provides product identification at the pill or liquid medicine level, which provides traceability of these products to their manufacturer, thereby reducing counterfeit products in the marketplace. Also, the elements 8 may be incorporated into a film, liquid, coating or adhesive tape at attached to the product package.
Owner:ILLUMINA INC
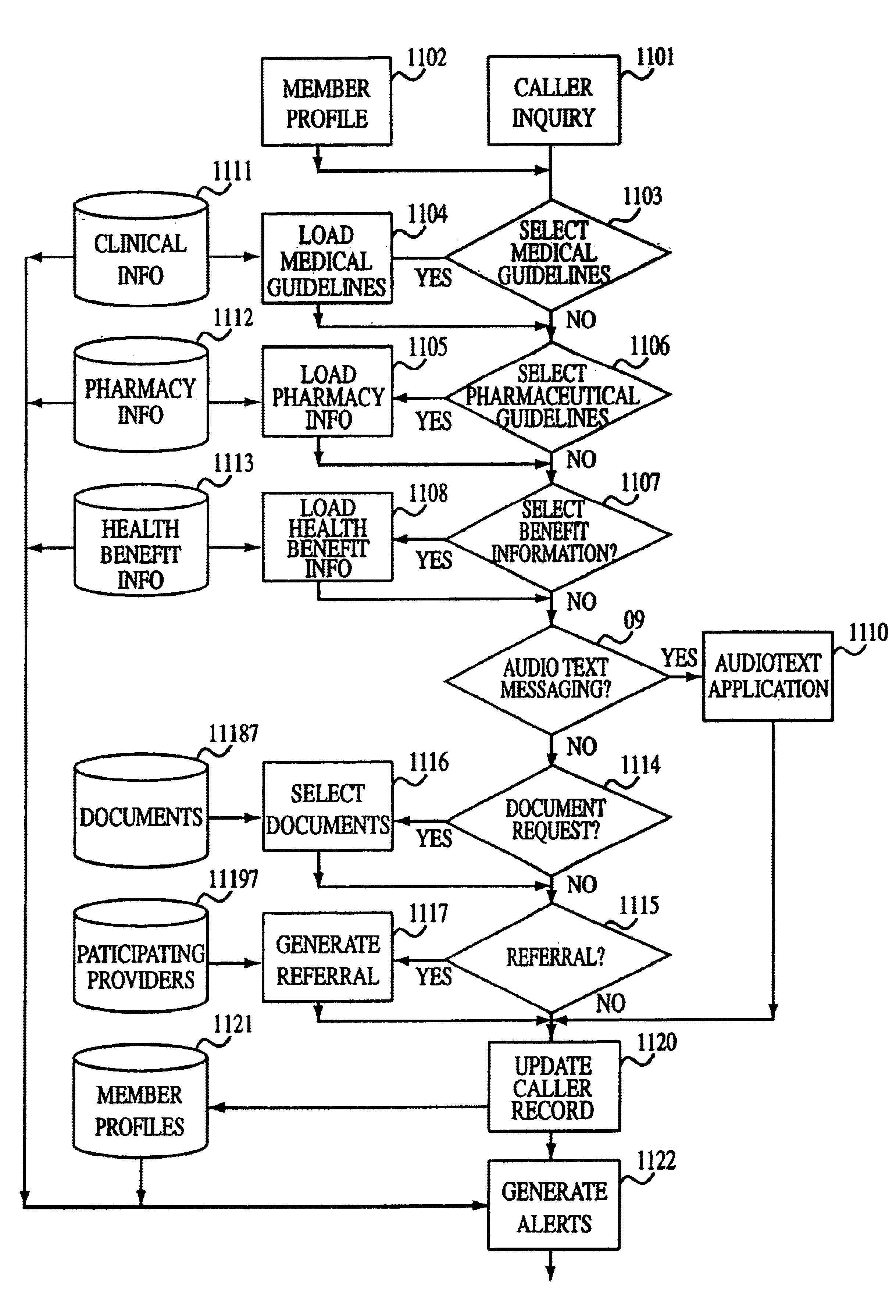
Computer implemented medical integrated decision support system
InactiveUS6697783B1Easy constructionOffice automationSpecial data processing applicationsMedication informationGuideline
A software-based, integrated member decision support system provides a method for corporations, insurance carriers, health maintenance organizations, physicians, physician groups, or other clients to efficiently provide medical, pharmaceutical, and health benefit advice and information for an enrolled population. The system contains one or more databases which include member profiles, clinical information and guidelines, pharmaceutical information and guidelines, health benefit information, and optional additional information. A caller establishes communication with the system, which directs the caller to an operator who provides the caller with medical, pharmaceutical, and / or health benefit advice based on an inquiry from the caller and the information stored on the system. The system may automatically alert the caller or the operator of important medical or pharmaceutical information. At the conclusion of the call, the system or the system with the operator's input, may update the caller's member profile, request written materials, generate referrals, order prescriptions, or generate reports.
Owner:EXPRESS SCRIPTS STRATEGIC DEV INC
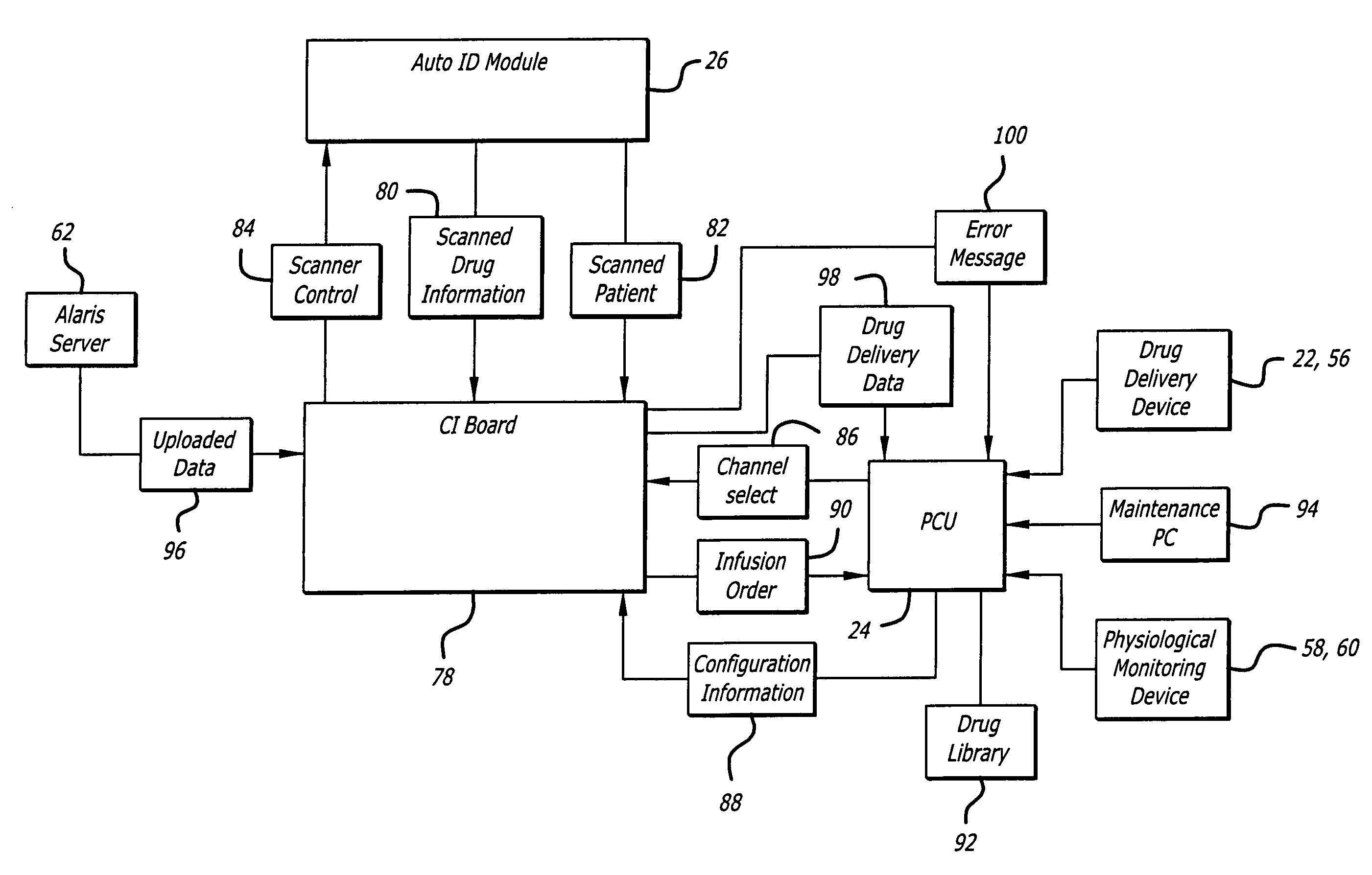
Identification system and method for medication management
An identification system and method for medication management comprises a controller, a drug library accessible by the controller, the drug library containing drug library profiles including a data set of drug information, and an identification module configured to read patient identification information from machine-readable identification devices worn by patients and to read drug information from machine-readable identification devices affixed to drugs or containers of drugs. The controller compares the read information to each other and to the drug library profile and provides alerts or error signals in the event of an inconsistency. In one case, identification devices are read by an optical reader fixedly mounted to the identification module. In another case, identification devices are read by an optical reader that is hand-held and mobile and can be moved to the location of the identification device. The hand-held reader communicates with the identification module by wired or wireless means. In another case, a third reader that is non-optical and wireless is fixedly mounted to the identification module. The controller is also configured to provide alerts or error messages in the event that there is an inconsistency between the drug information on the drug or drug container when compared to the drug library profile associated with the controller.
Owner:CAREFUSION 303 INC
Medicinal product order processing system
InactiveUS20060136266A1Improve monitoring qualityProcess is repeatedDrug and medicationsPatient personal data managementRepeated prescriptionRegimen
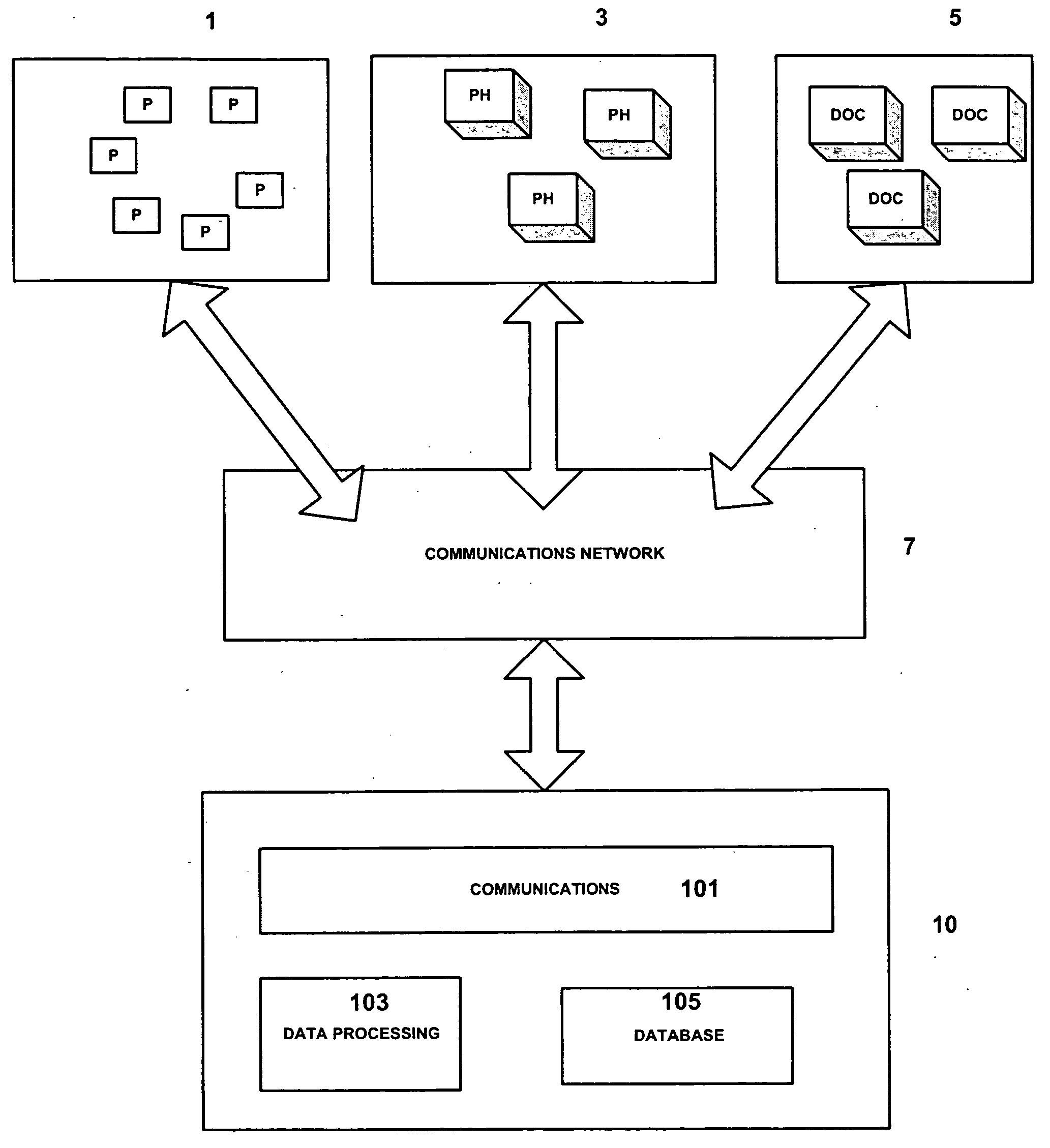
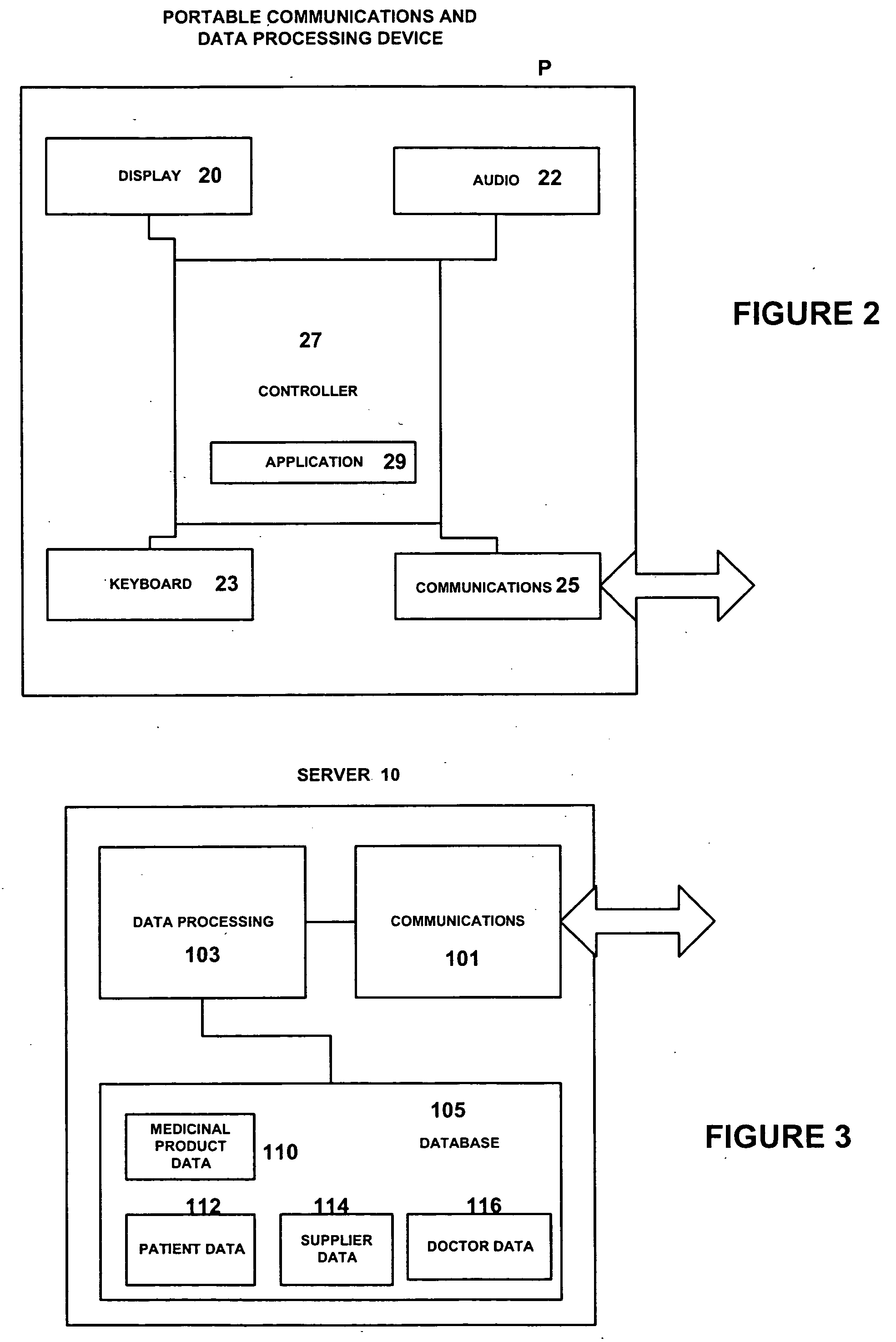
A repeat prescription ordering system for allowing patients requiring resupply of medication or medical products to access a server using a portable communications and data processing device such as a smart phone or personal digital assistant. The server supplies to the patient a list of medication and medical products which they are authorized to order. The patient can select the products required, and the order is logged by the server and allocated to a supplier for completion of the order. The server maintains an estimate of the amount of medication or medical product held by the patient, this being based on the prescribed dosage regimen and information entered by the patient on their usage and, optionally, on checks on their own health. The patient may be alerted when the estimate indicates that their supplies are running low. The estimate is allowed to go below zero, this implying a possible departure from the prescribed medication regimen.
Owner:E SAN LTD
Porous drug matrices and methods of manufacture thereof
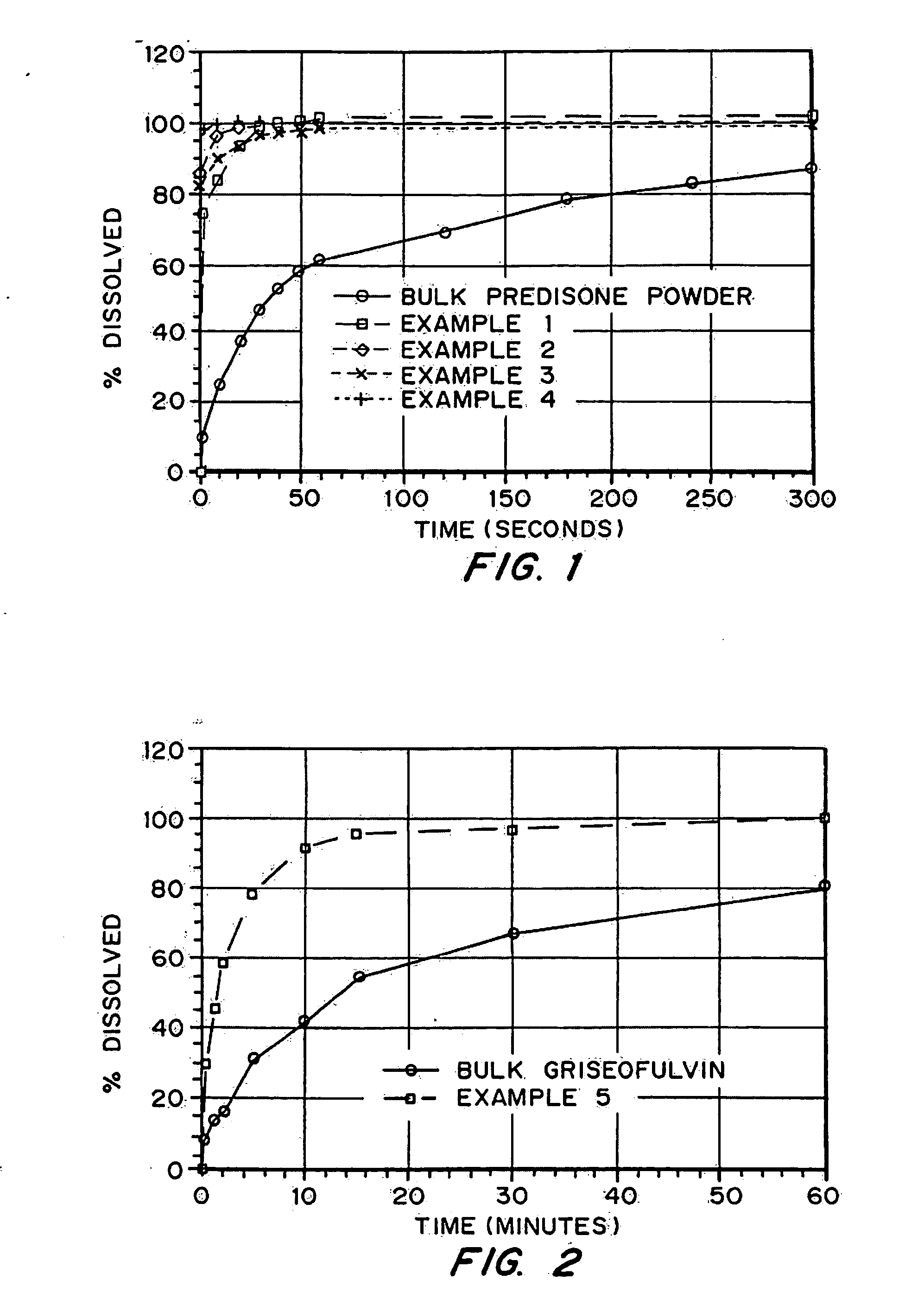
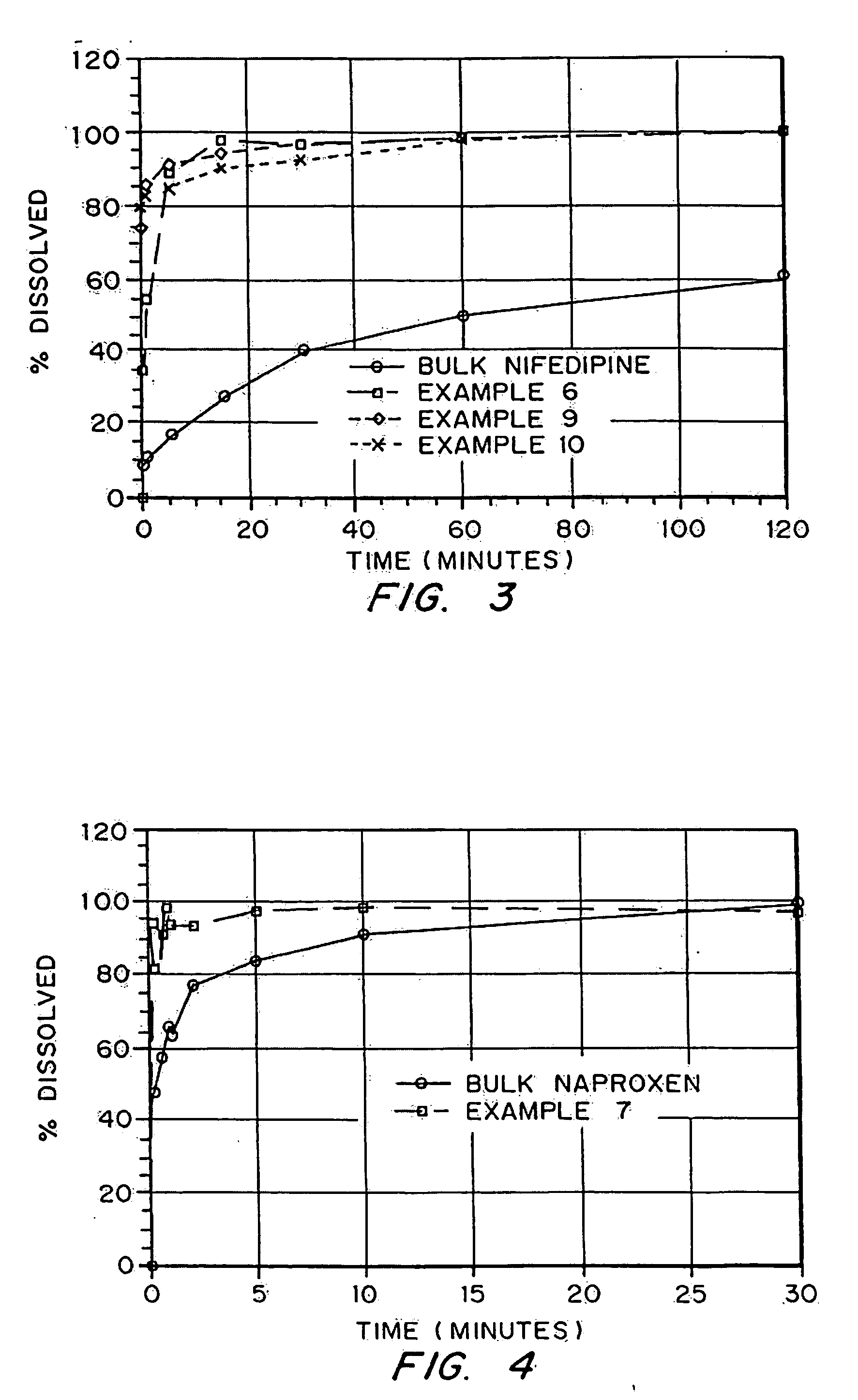
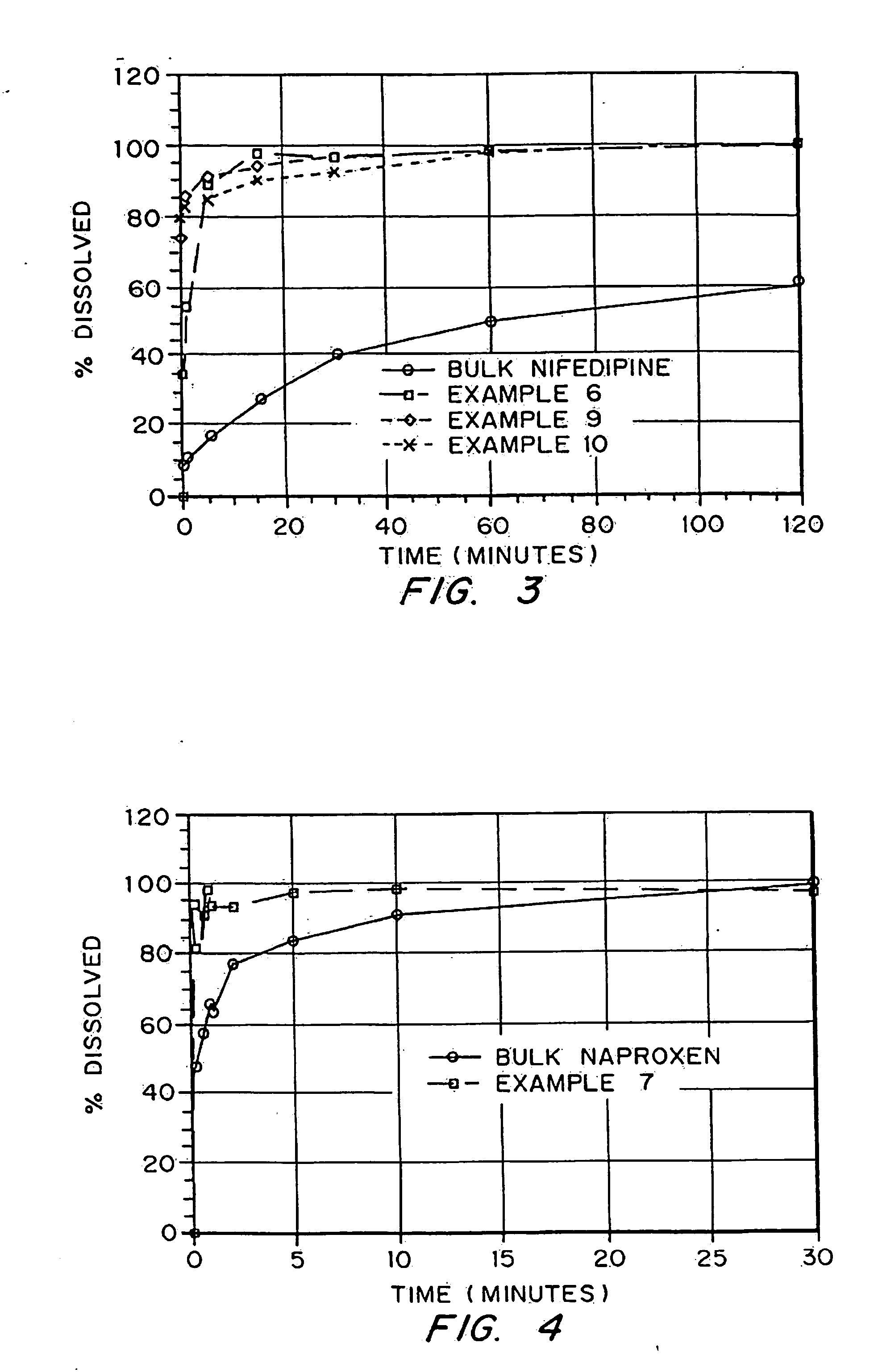
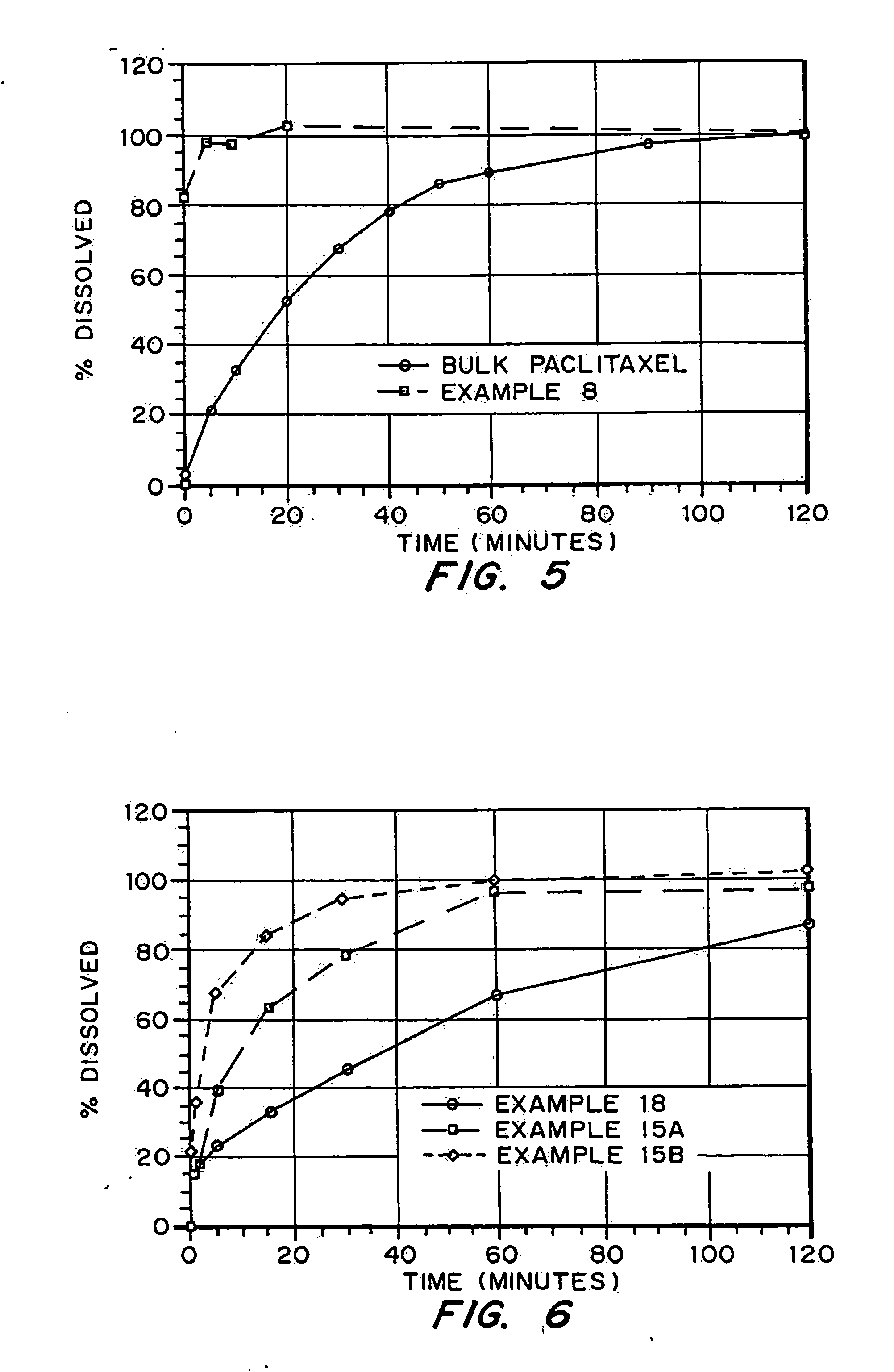
InactiveUS20050048116A1Fast dissolutionHigh dissolution ratePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid-compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
System for maintaining drug information and communicating with medication delivery devices
ActiveUS8065161B2Data processing applicationsDrug and medicationsSoftware designBiomedical technology
Owner:ICU MEDICAL INC
Identification system and method for medication management
An identification system and method for medication management comprises a controller, a drug library accessible by the controller, the drug library containing drug library profiles including a data set of drug information, and an identification module configured to read patient identification information from machine-readable identification devices worn by patients and to read drug information from machine-readable identification devices affixed to drugs or containers of drugs. The controller compares the read information to each other and to the drug library profile and provides alerts or error signals in the event of an inconsistency. In one case, identification devices are read by an optical reader fixedly mounted to the identification module. In another case, identification devices are read by an optical reader that is hand-held and mobile and can be moved to the location of the identification device. The hand-held reader communicates with the identification module by wired or wireless means. In another case, a third reader that is non-optical and wireless is fixedly mounted to the identification module. The controller is also configured to provide alerts or error messages in the event that there is an inconsistency between the drug information on the drug or drug container when compared to the drug library profile associated with the controller.
Owner:CAREFUSION 303 INC
High alcohol content gel-like and foaming compositions
ActiveUS20070027055A1Little dryingEasy to manufactureBiocideCosmetic preparationsAdditive ingredientLiquid composition
This invention relates to a “high lower alcohol content” (>40% v / v of a C1-4 alcohol) liquid composition able to be either dispensed as a stable foam with the use of non-propellant foam dispensing devices from non-pressurized containers or as an alcohol gel composition which does not use thickener and gelling agents that leave undesirable deposits or a sticky after-feel and that has a final viscosity less than 4,000 cps. The liquid compositions comprise an alcohol, C1-4 (>40% v / v), a fluorosurfactant of at least 0.001% by weight to prepare a foamable composition or from 0-2.0% to prepare a gel-like composition of a final viscosity less than 4,000 cps, 0-10% w / w of additional minor components added to obtain the desired performance (a foamable composition or a gel-like composition with a viscosity less than 4,000 cps), and the balance being purified water. The compositions may include emulsifier-emollients and mosturizers, secondary surfactants, foam stabilizers, fragrances, antimicrobial agents, other type of medicinal ingredients, and the like ingredients or additives or combinations thereof commonly added to alcohol gels or foams, aerosol compositions or to toiletries, cosmetics, pharmaceuticals and the like.
Owner:DEB IP
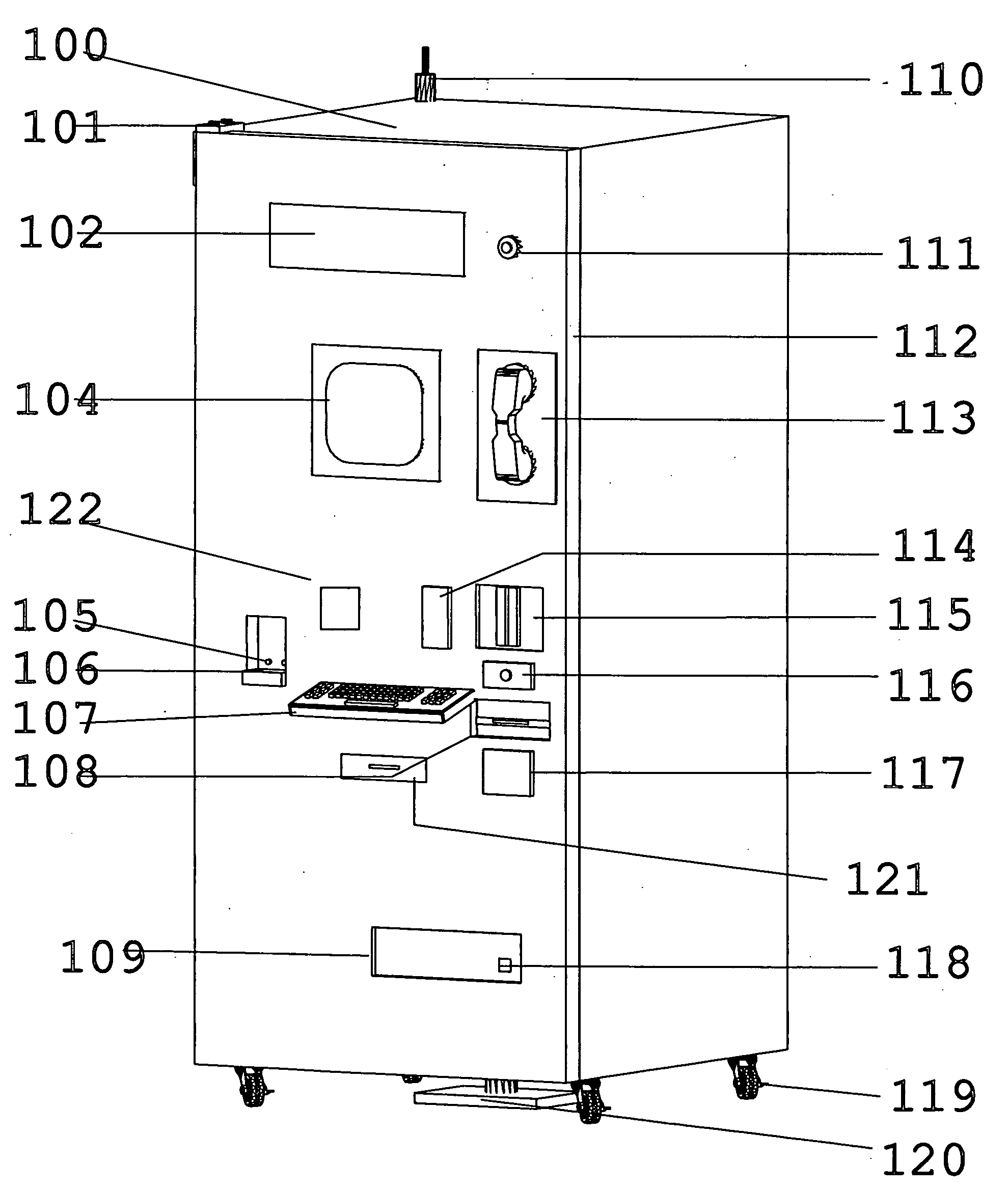
Inventory control and prescription dispensing system
ActiveUS20070043469A1Ensure integrityEnsure safetyDrug and medicationsCoin-freed apparatus detailsEngineeringPatient registration
An inventory control and prescription management and dispensing system including a dispensing vault for storing and dispensing prescriptions, the dispensing vault in communication with a central computer system that, in turn, communicates with prescription providers, insurance companies, and other third parties; the dispensing vault including robotic means for randomly accessing pre-filled prescriptions within the vault, with the vault further including RFID, bar code, and other means for verifying the content and internal location of pre-filled prescriptions; a customer interface that uses customer biometrics to ID a customer to ensure that prescriptions are only dispensed to the correct person; a patient registration system in communication with the central computer system for collecting insurance, doctor, biometric, and other information to facilitate transactions; a labeling system for labeling pre-filled prescriptions with customer specific information upon dispensing; transport container that integrate into the dispensing vault and provide secure transportation from a pharmaceutical manufacturer or repackager to the dispensing vault, security provided through RFID tags which communicate with the central computer to verify that the transport container contains the correct formulary and that the integrity of the container (temperature range, time in transit, tampering) has not been compromised; a payment system integral to the dispensing vault that is in communication with third party banks and pharmacy billing management systems, credit agencies and the central computer; a verification system for ensuring that pre-filled bottles received from the manufacturer (before they are placed into the storage locations inside the vault) have not had their integrity compromised, with this system evaluating each container's weight, size, moisture content, shape, velocity change, color, pattern, and physical integrity (all comparisons made against standards stored in the central computer).
Owner:DRAPER LONNIE
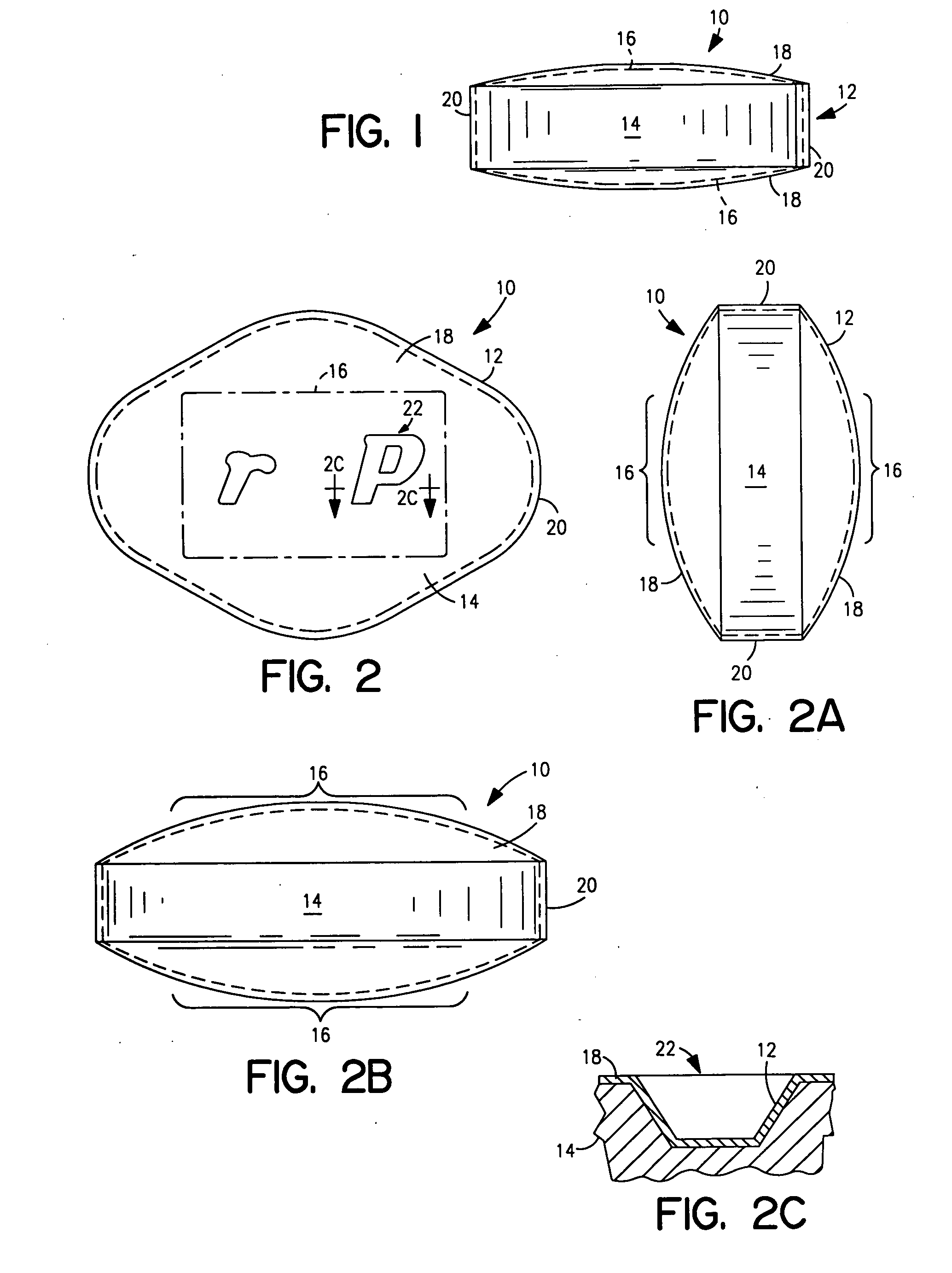
Edible holographic products, particularly pharmaceuticals and methods and apparatus for producing same
InactiveUS20060068006A1Preserve product integrityCheap productionPretreated surfacesDiffraction gratingsCoated tabletsPlasticizer
An edible product such as a unit dosage form of a pharmaceutically active substance includes a layer of a material that can receive and retain a high resolution microrelief that can convey information. The microrelief is themo-formable, preferably formed from an aqueous solution of HPMC and / or HPC plus a plasticizer and colorant. Other additives such as strengtheners, surfactants and adherents may be used depending on the application. The materials are selected and proportioned to control the fading or change in color of the visual image or effect produced by the relief to indicate exposure to an unacceptable degree of heat or humidity. The dosage form can be the relief-containing layer itself with the pharmaceutical carried therein. In a preferred form, the layer is an outer coating over a core containing the pharmaceutically active substance. Coated tablets are configured to resist twinning. To produce such dosage forms, the coated core is transported in unison with a flexible mold or transfer plate that can heat-replicate the microrelief on the outer layer of the dosage form, followed by a cooling and release of the transfer plate from the coating.
Owner:EDWARDS ANGELL PALMER & DODGE
Automated prescription filling system/method with automated labeling and packaging system/method automated order consolidation system/method
Computer assisted systems, methods and mediums for filling one or more orders. One embodiment of the present invention is a system that includes an order consolidation station configured to receive at least one bottle containing pills individually counted and / or at least one package containing pharmaceutical products without having been designated for any of the orders when the package was created and / or at least one literature pack optionally including patient specific information. The order consolidation station is further configured to combine automatically the received bottle and / or package and / or literature pack into a container to be sent to a recipient including, for example, mail order pharmacies, wholesalers and / or central fill dealers for subsequent distribution or sale including retailer distribution or sale. The bottle is specifically designated for the order, and the order generally includes at least one prescription for the package.
Owner:EXPRESS SCRIPTS STRATEGIC DEV INC
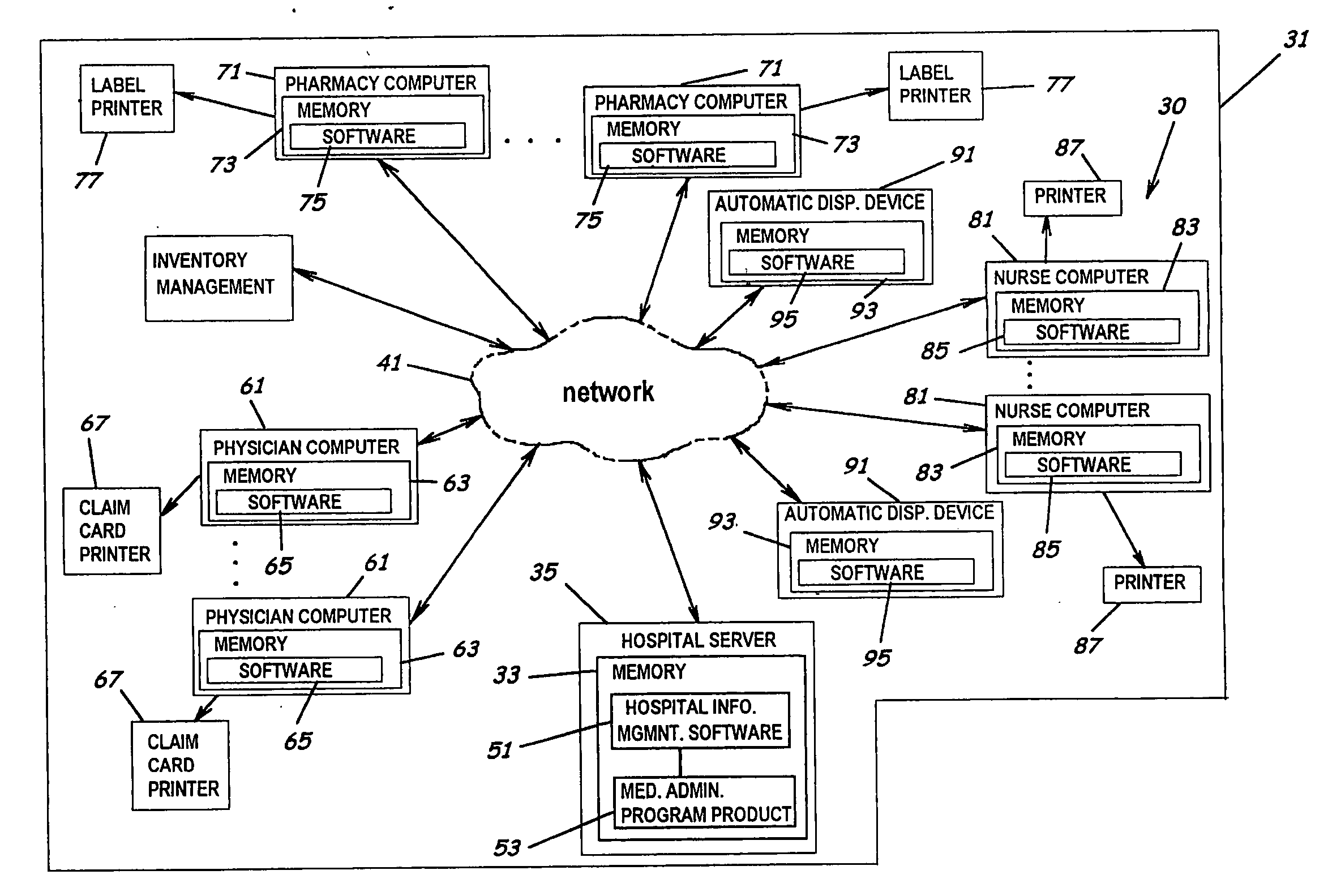
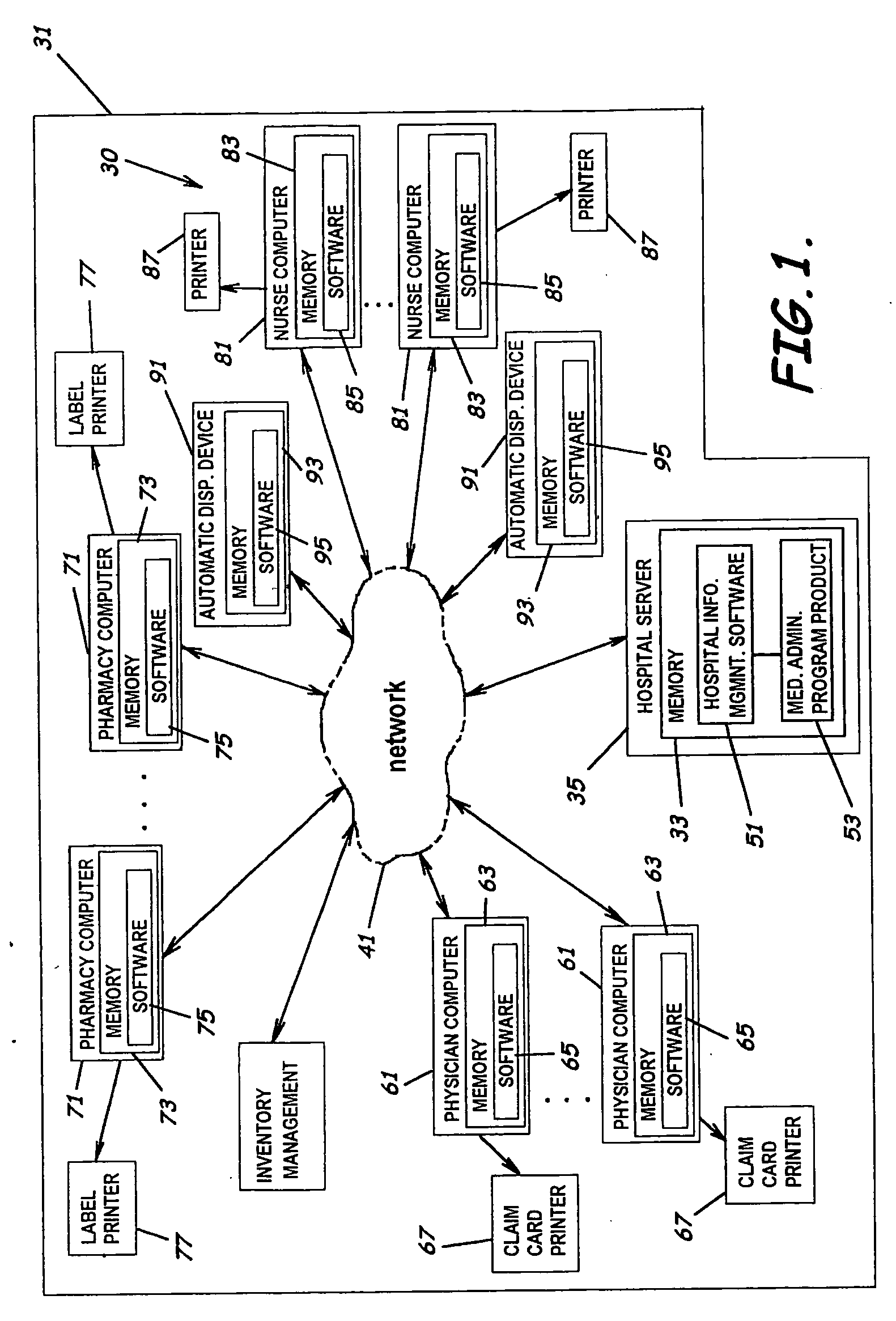
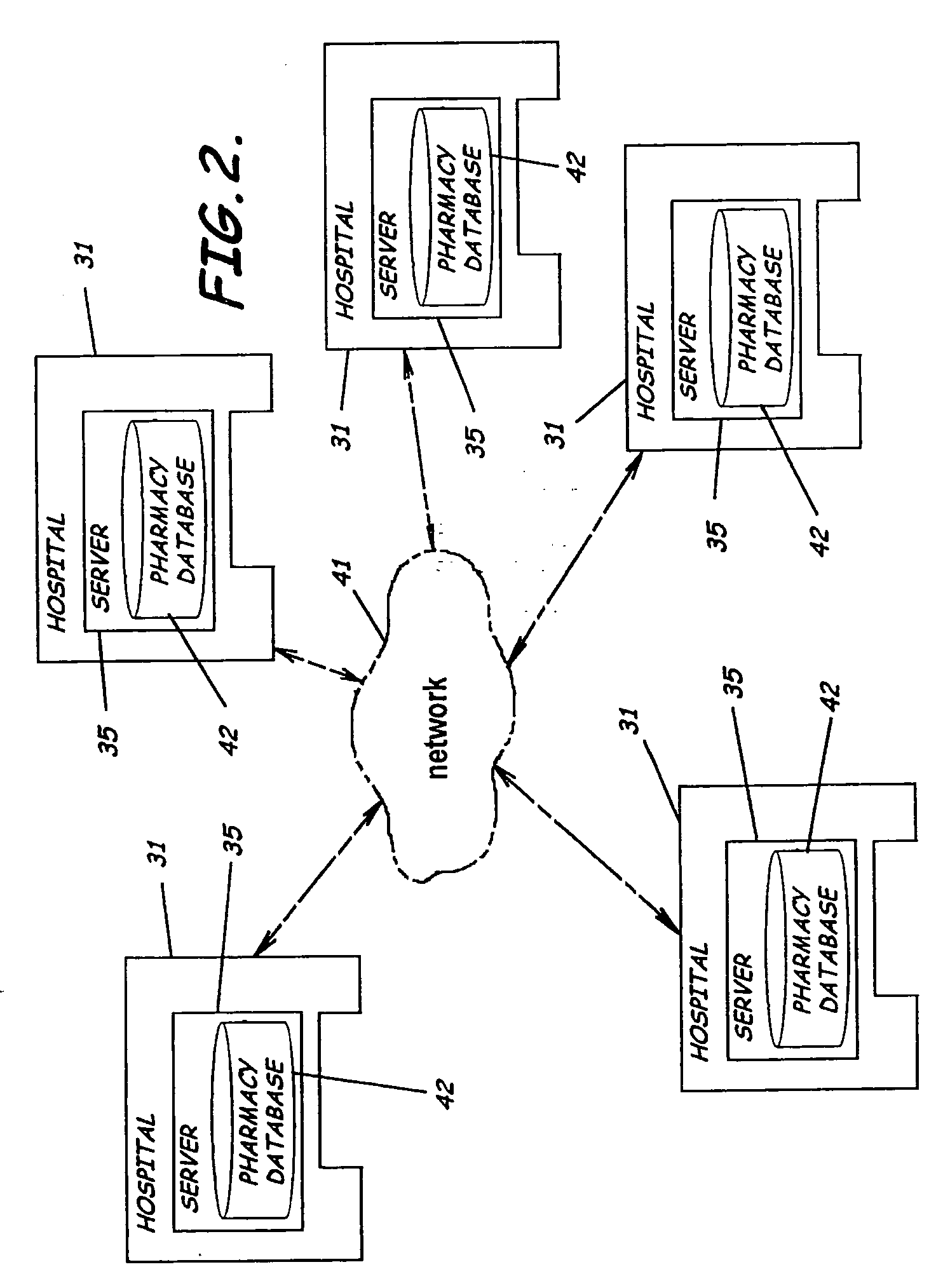
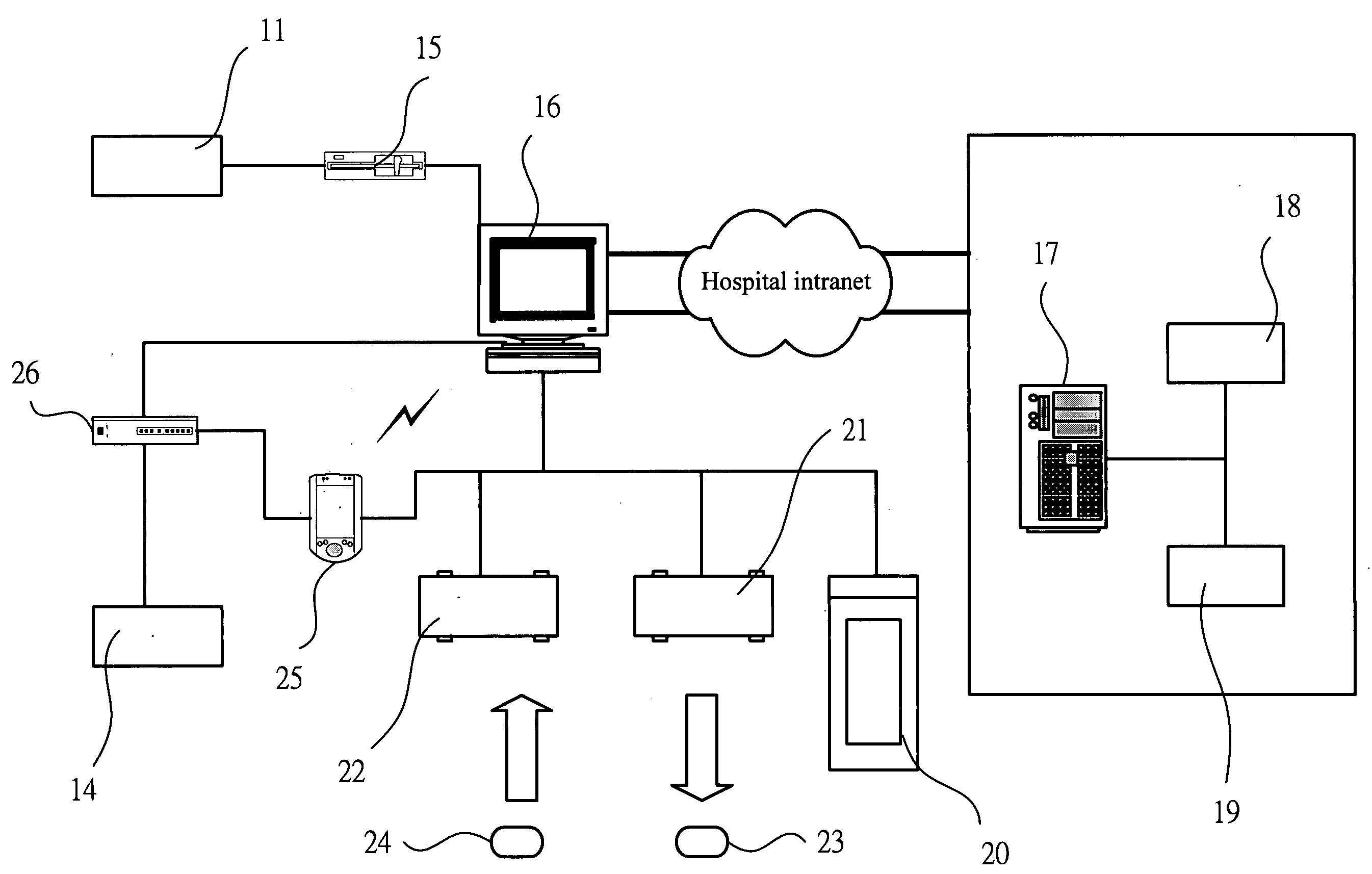
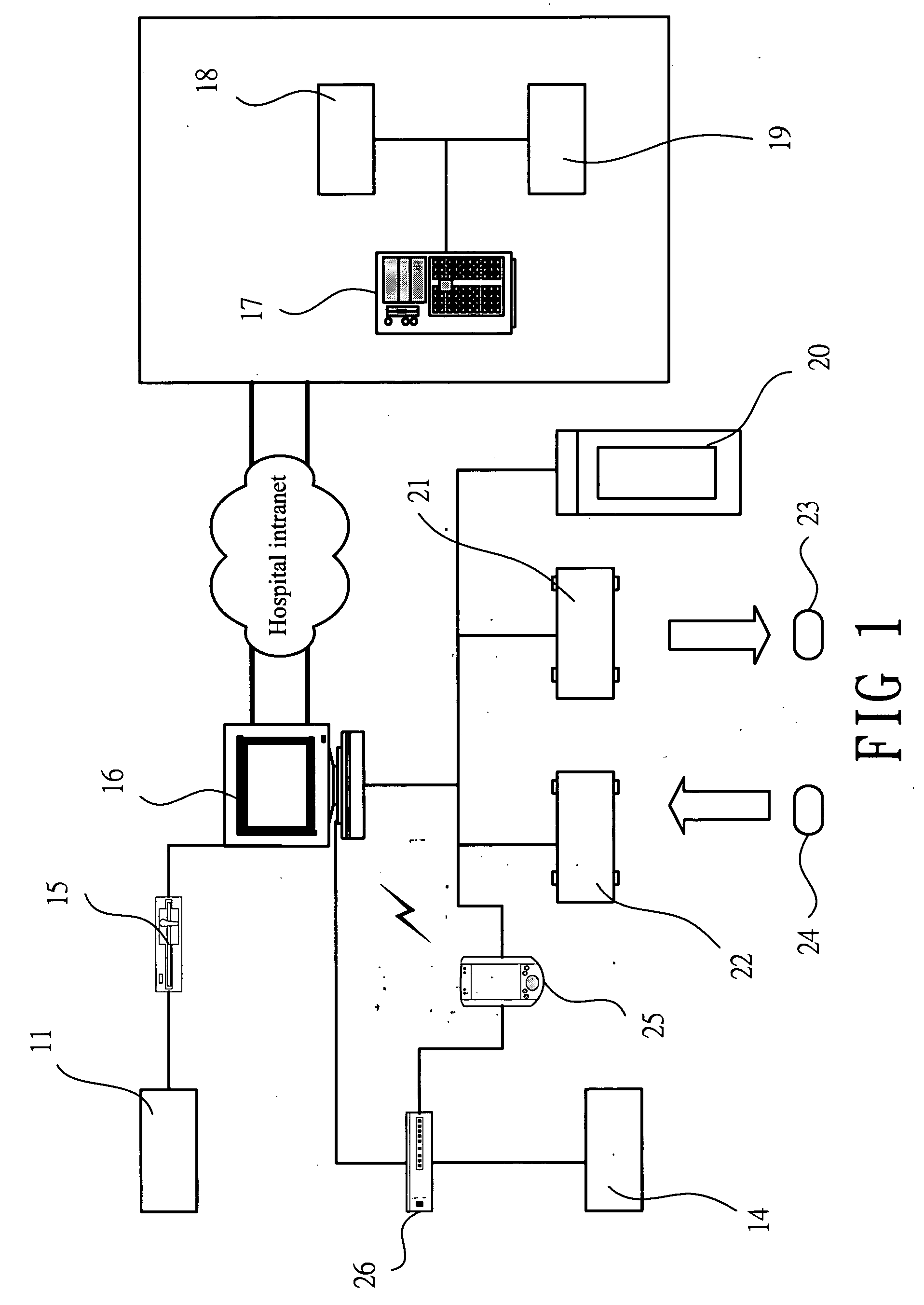
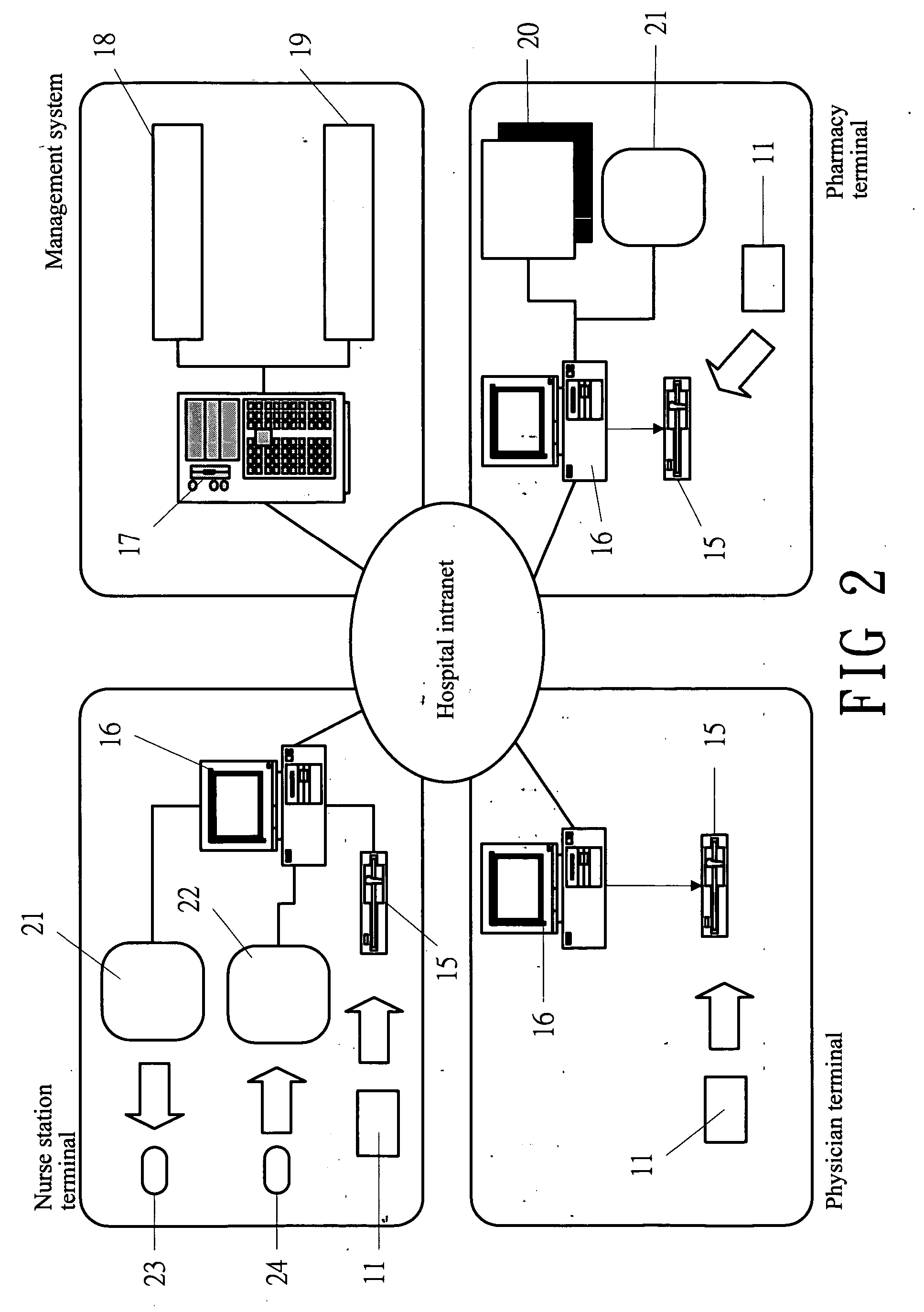
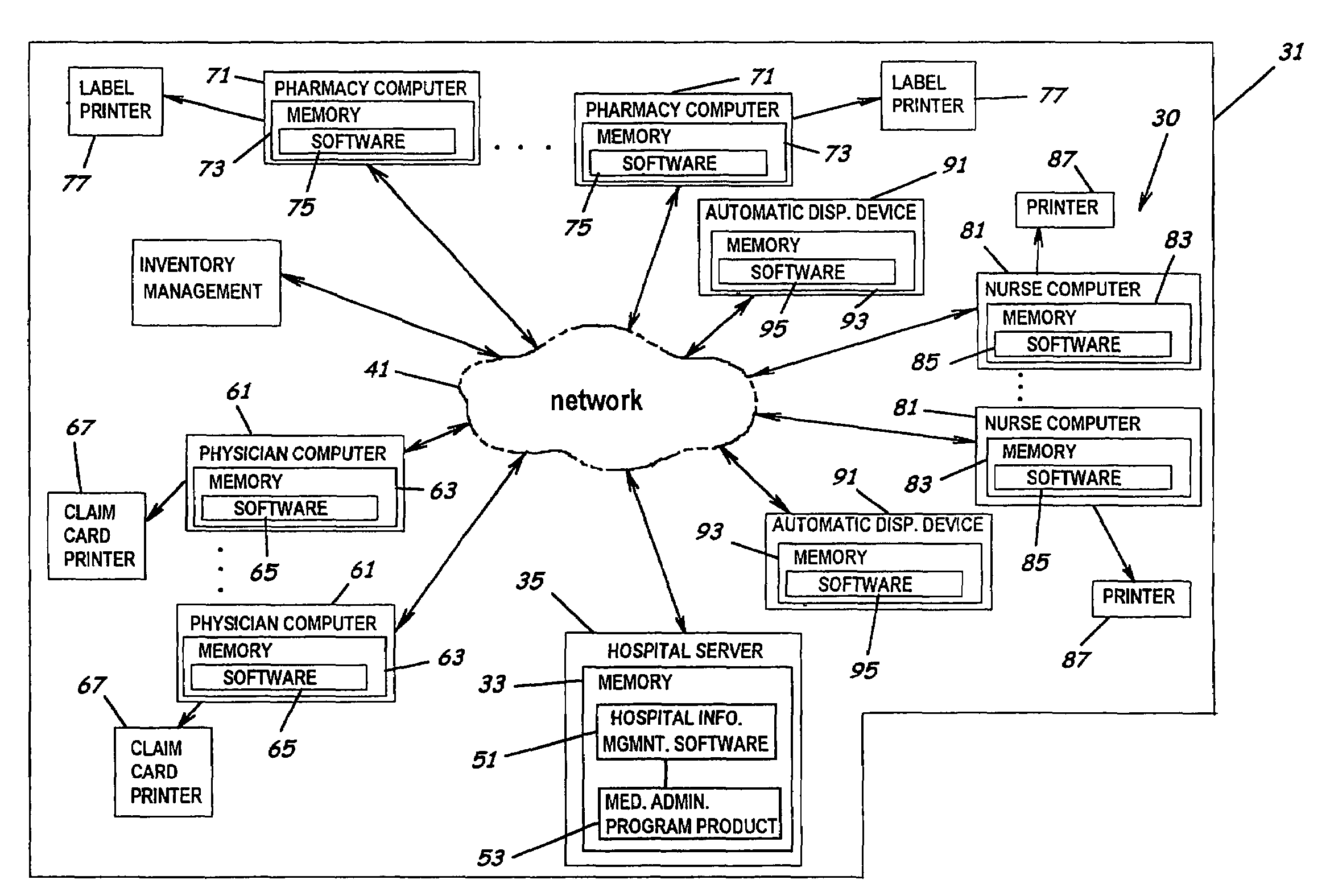
System and software of enhanced pharmacy services and related methods
InactiveUS20060149416A1Service interruptionIncrease heightDrug and medicationsOffice automationMedication informationPharmacist
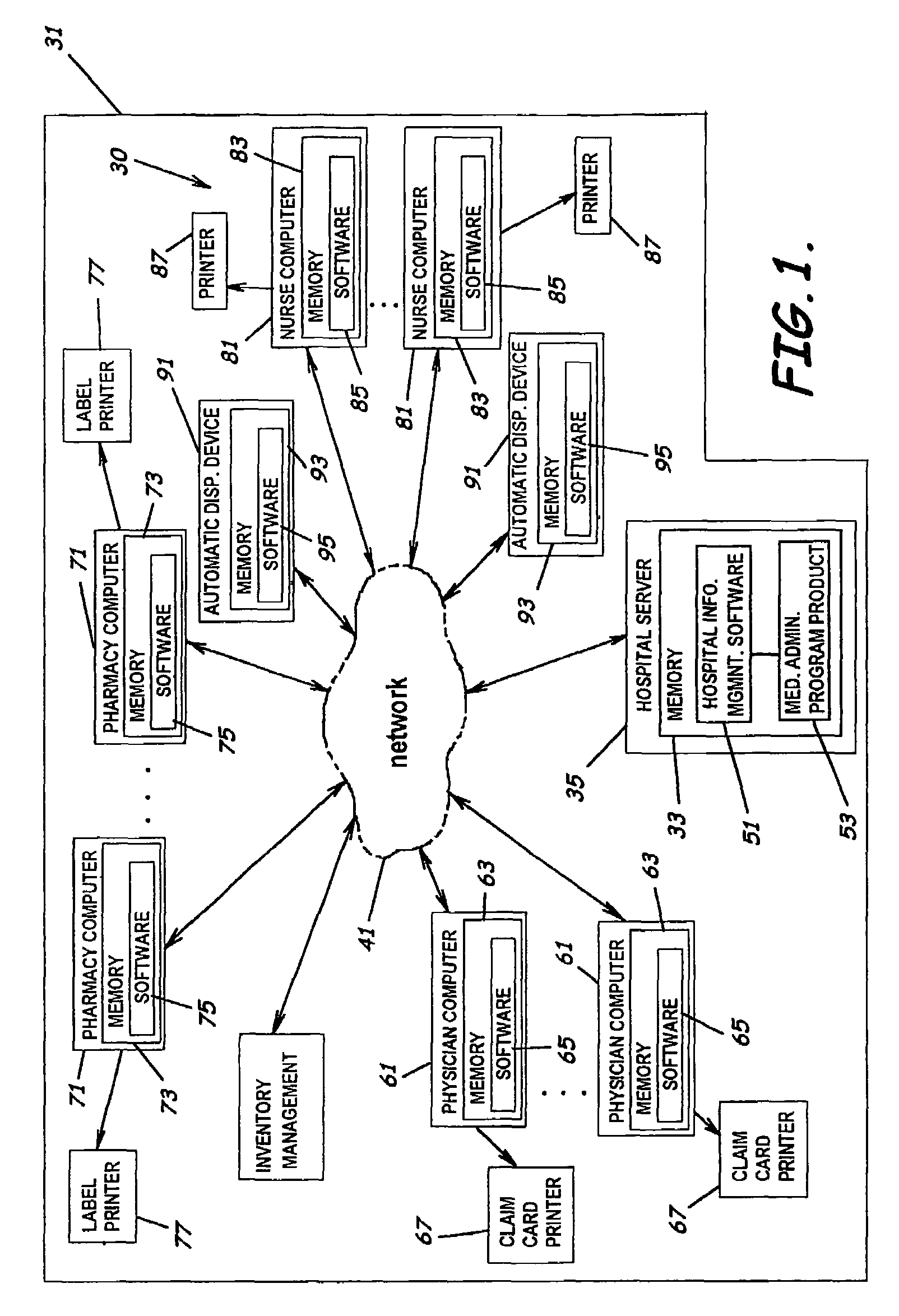
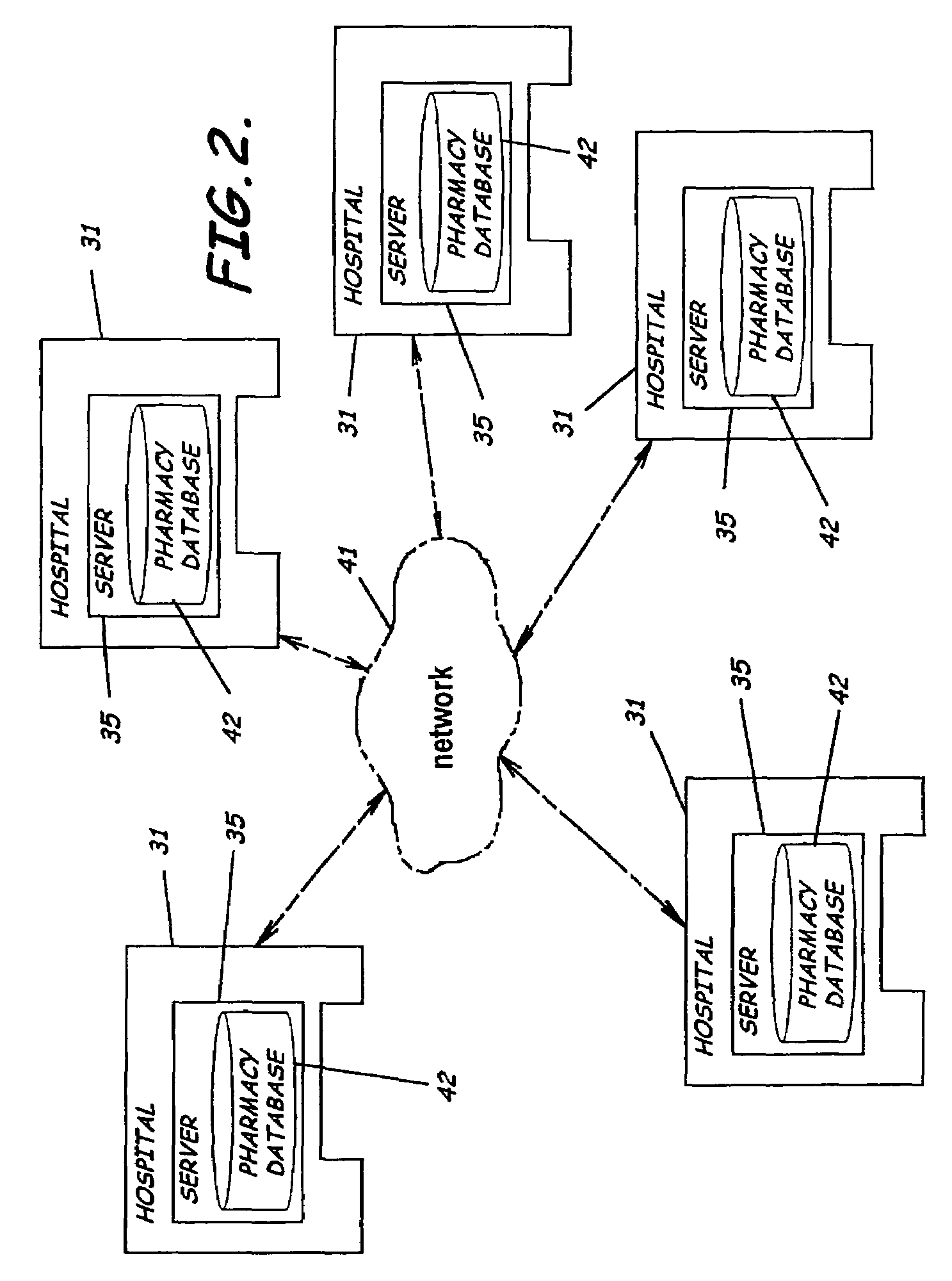
A system, software, and methods related to enhanced pharmaceutical order entry and administration by medical personnel, and enhanced pharmaceutical inventory control within a medical institution are provided. An embodiment of the system includes a pharmaceutical information management server having memory and a medication administration program product including a set of instructions stored in the memory of the pharmaceutical information management server to enhance provision of pharmacy services. The system also includes medical institution physician computers to provide for computerized physician medication order entry, pharmacy computers to provide for review and verification by a pharmacist of electronic medication orders placed through the physician computers, and medical institution nursing unit computers, to provide for review of and input to electronic medication administration records.
Owner:JOHNS HOPKINS ARAMCO HEALTHCARE
Medicine dispensing machine for patient and medicine dispensing method of medicine dispensing machine
ActiveCN104207943AReduce volumeSolve the cumbersomeness of taking medicine and dispensing medicineOral administration deviceSpecial data processing applicationsLiquid-crystal displayDrive shaft
The invention provides a medicine dispensing machine for a patient. The medicine dispensing machine is composed of a plurality of medicine bottles and a medicine dispensing control system device, wherein a core rotary shaft of each medicine bottle is mounted on a driving shaft of a stepping motor and an infrared counter is arranged on one side under a medicine outlet hole of each medicine bottle; the infrared counters and the stepping motors are mounted on a base plate; the medicine dispensing control system device comprises a single board computer, a liquid crystal display module and an SD (Secure Digital) card reader; the single board computer is further connected with the plurality of stepping motors with a driver and the infrared counter under each medicine bottle. A medicine dispensing method comprises the following steps: inputting medicine taking information, namely medicine taking time, medicine taking names and medicine taking quantity into the SD card; inserting the SD card after mounting the medicine bottles according to the requirements; switching on a power supply of the medicine dispensing machine; and discharging medicines by the medicine dispensing machine on time according to the varieties and the quantity of the medicines. By adopting the medicine dispensing machine, the problems that manual medicine taking and medicine dispensing of the patient are tedious and the varieties, the time and the quantity of the medicines are wrong and forgotten, caused by the manual medicine taking and medicine dispensing are solved. The medicine dispensing machine is small in size so that the machine can be taken along and the power consumption is low so that the machine can be powered by a battery.
Owner:陈莹胤
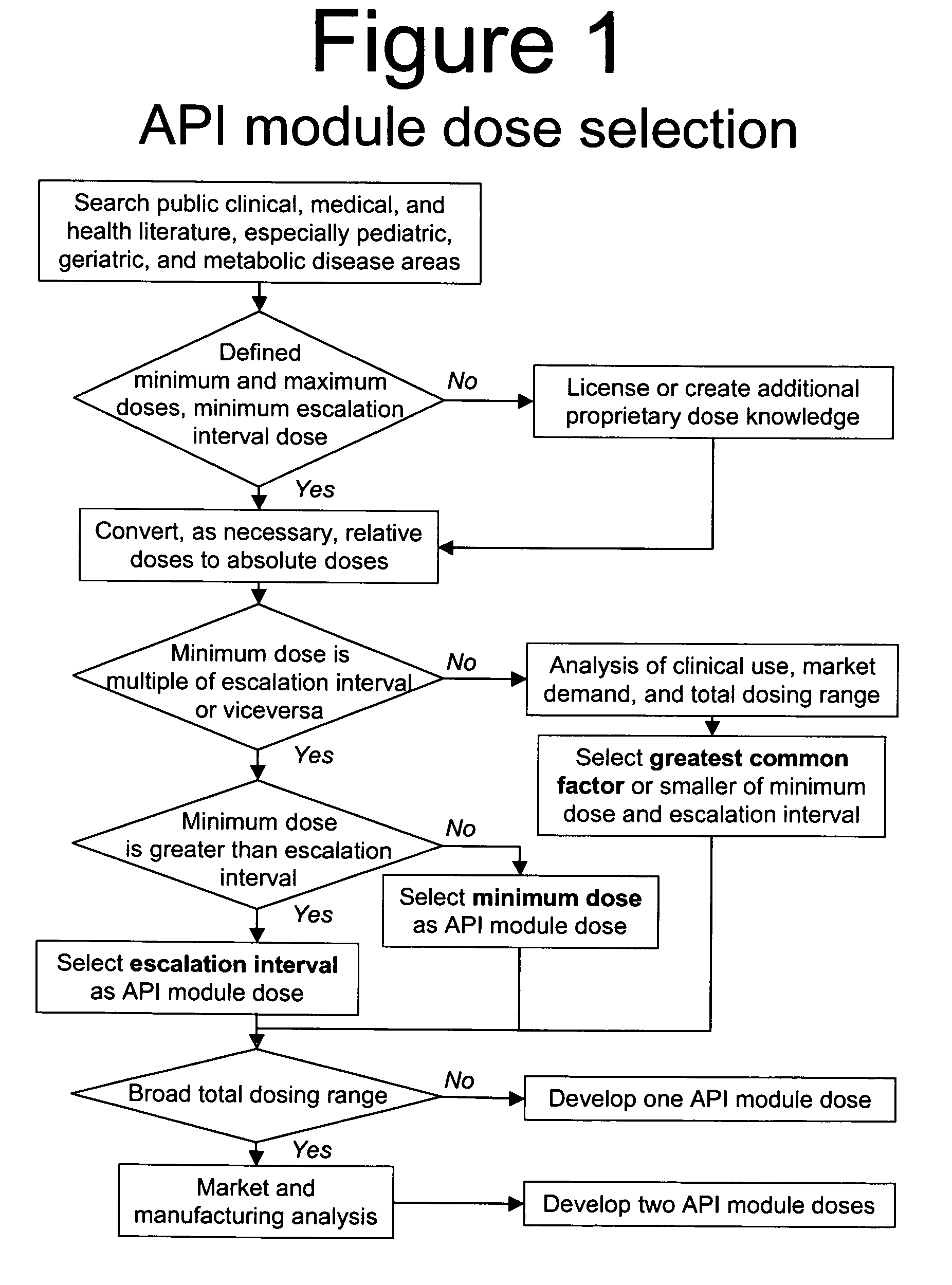
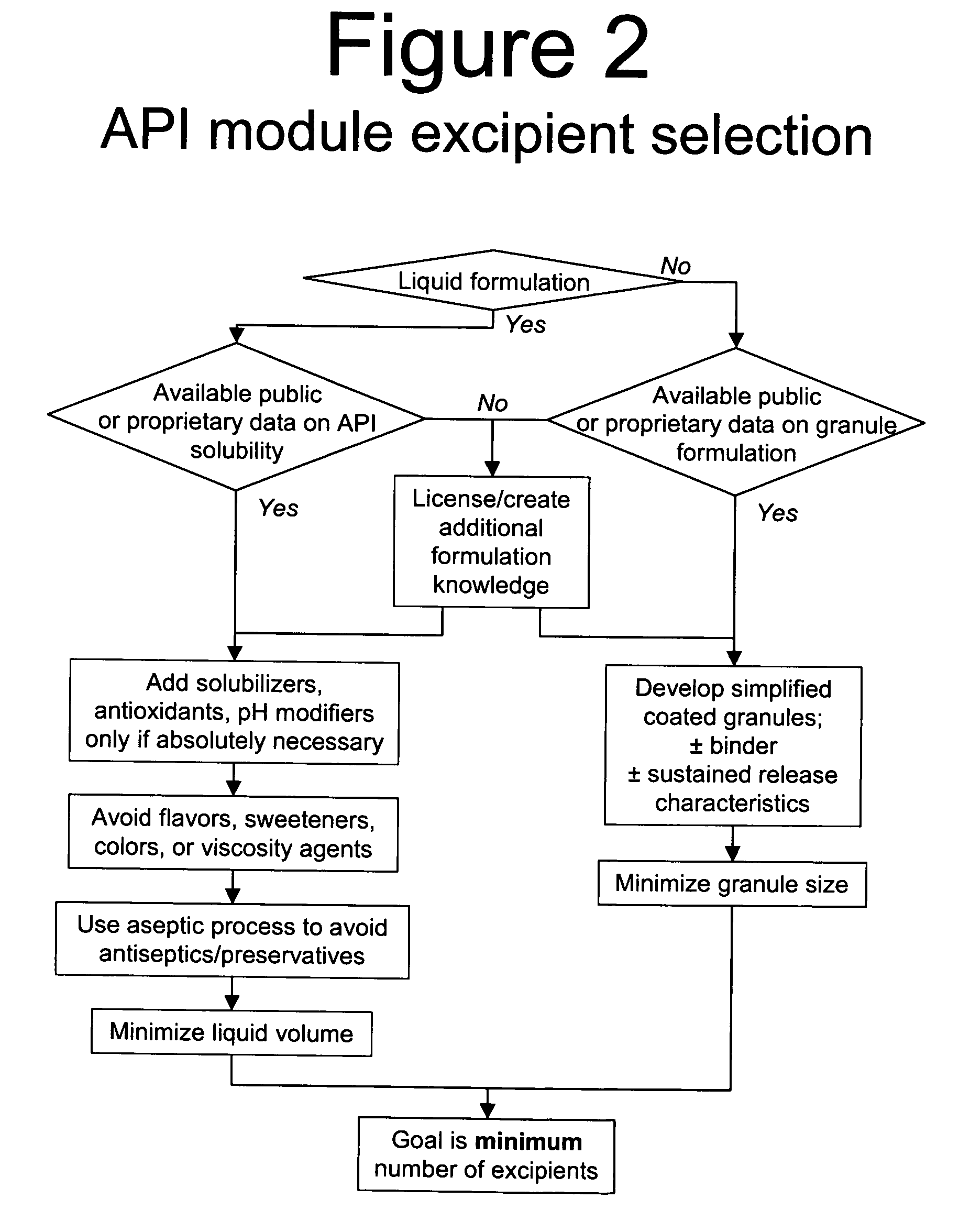
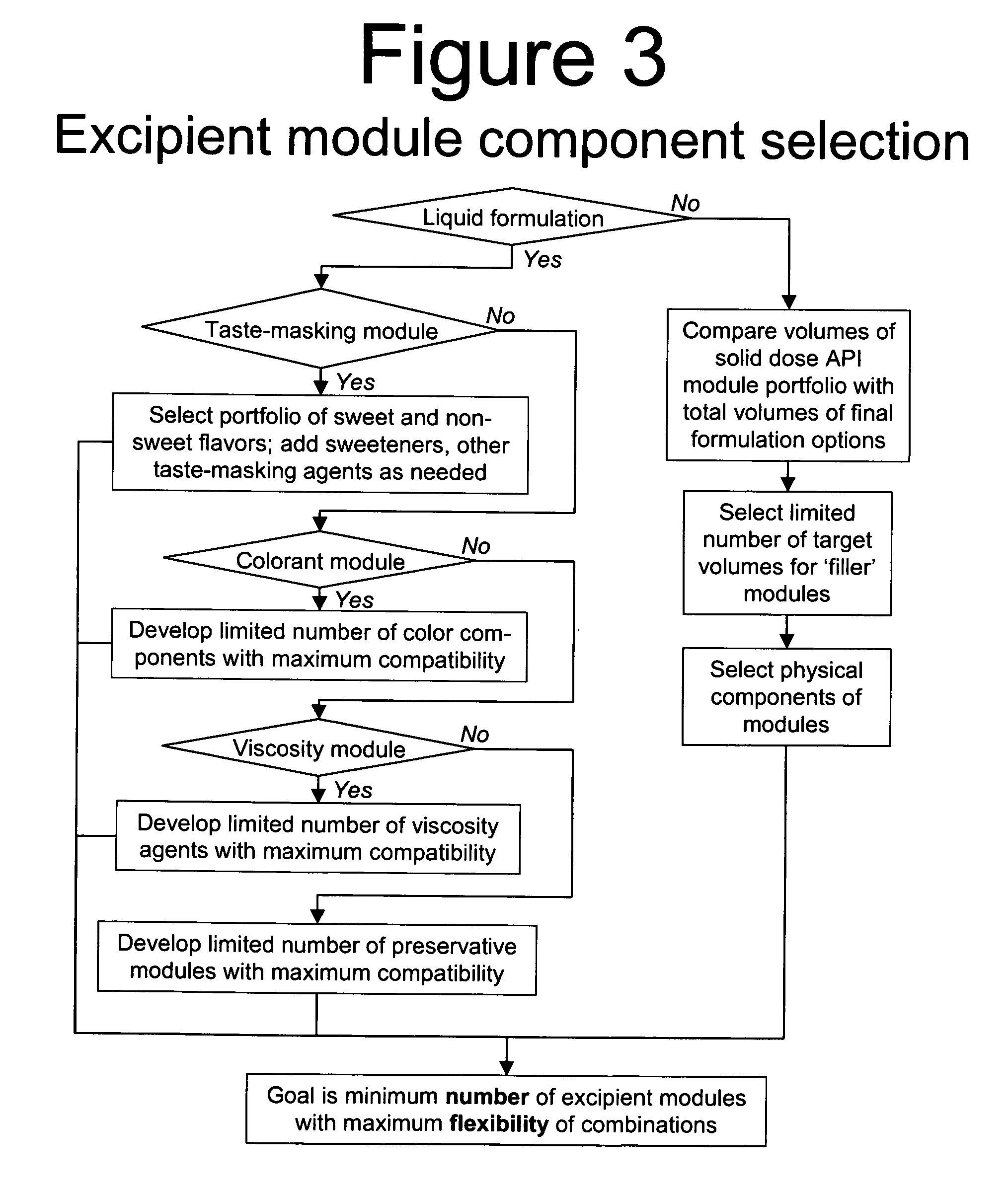
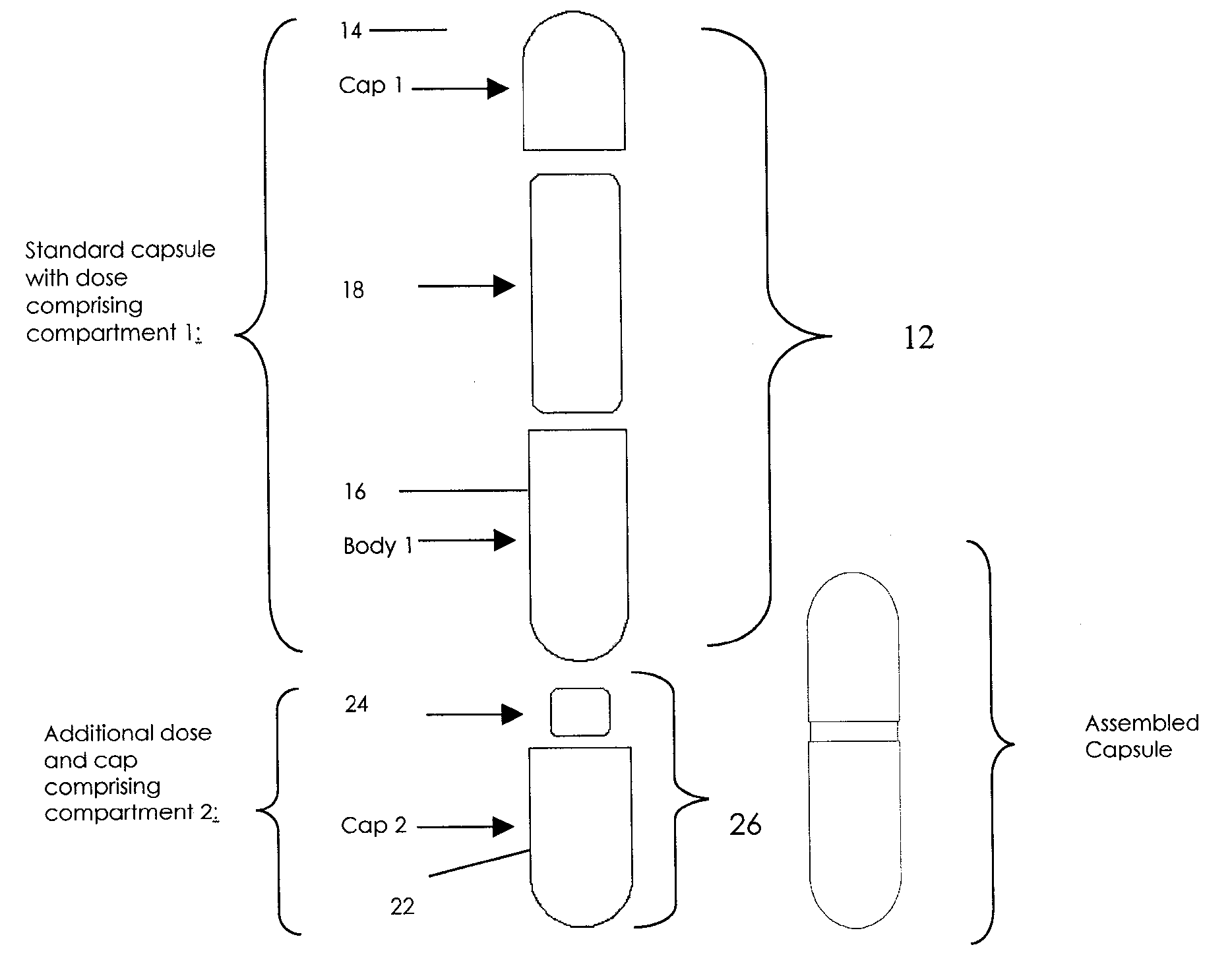
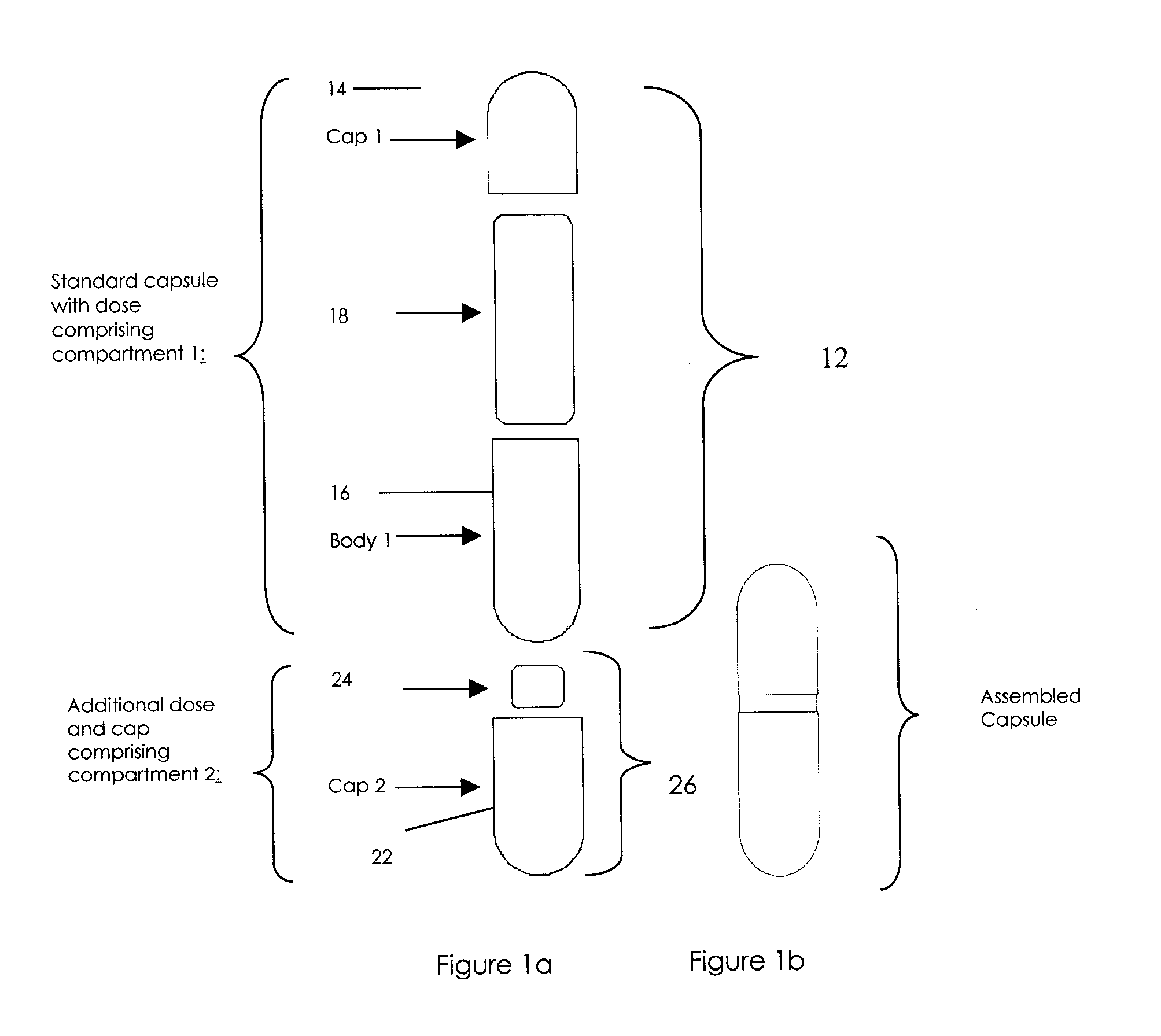
Methods of Making Pharmaceutical Components for Customized Drug Products
InactiveUS20100015184A1Maximize desired pharmaceutical characteristicLow costDrug and medicationsPharmaceutical product form changeFlavorAdditive ingredient
Methods and systems for developing and manufacturing componentized drug product precursor modules that can be simply assembled to create customized drug products are disclosed. Each module contains components of a final drug product (e.g., active pharmaceutical ingredients, nutritional ingredients, and / or excipients) in a fixed mixture selected to maximize desired pharmaceutical characteristics (e.g., stability, manufacturing efficiency) and minimize cost. The modules can be extensively tested for quality and assembled immediately, or at a later time, in multiple combinations to customize the final drug product characteristics (e.g., multiple active ingredients, doses, flavor, viscosity, etc.) to meet individual patient / consumer needs and / or preferences while assuring high quality. Permitted combinations may be maintained in a database to enable networked drug product selection, prescribing, and ordering. Each resulting customized drug product dose can be labeled to facilitate compliance and reduce the number of drug products administered per day.
Owner:TUEL STEPHEN M
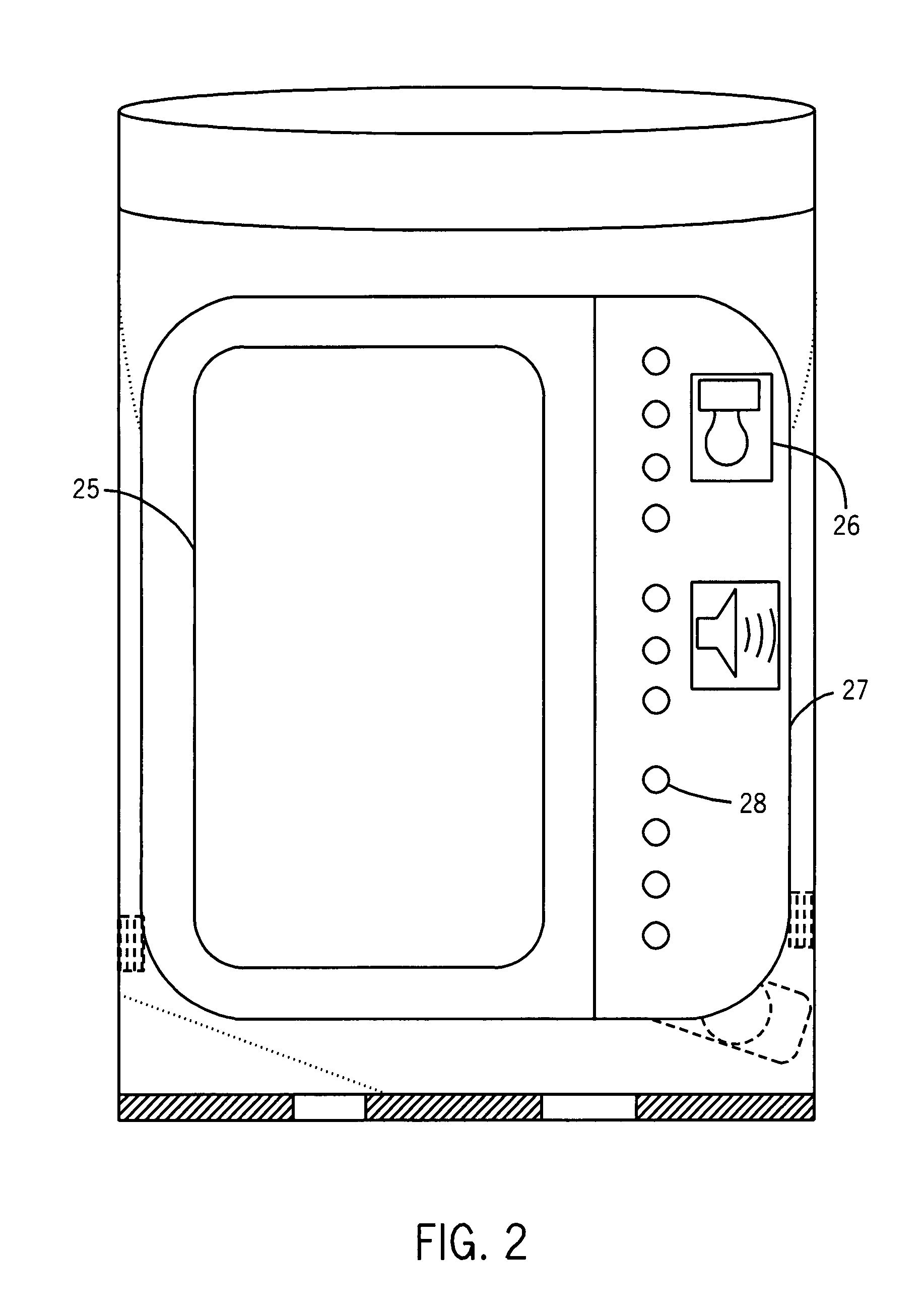
Smart medicine container
InactiveUS7269476B2Prevent misuse of prescription medicationsAssembly is accurate and reliableDrug and medicationsCoin-freed apparatus detailsDiseaseGlucose meter device
Owner:RATNAKAR NITESH
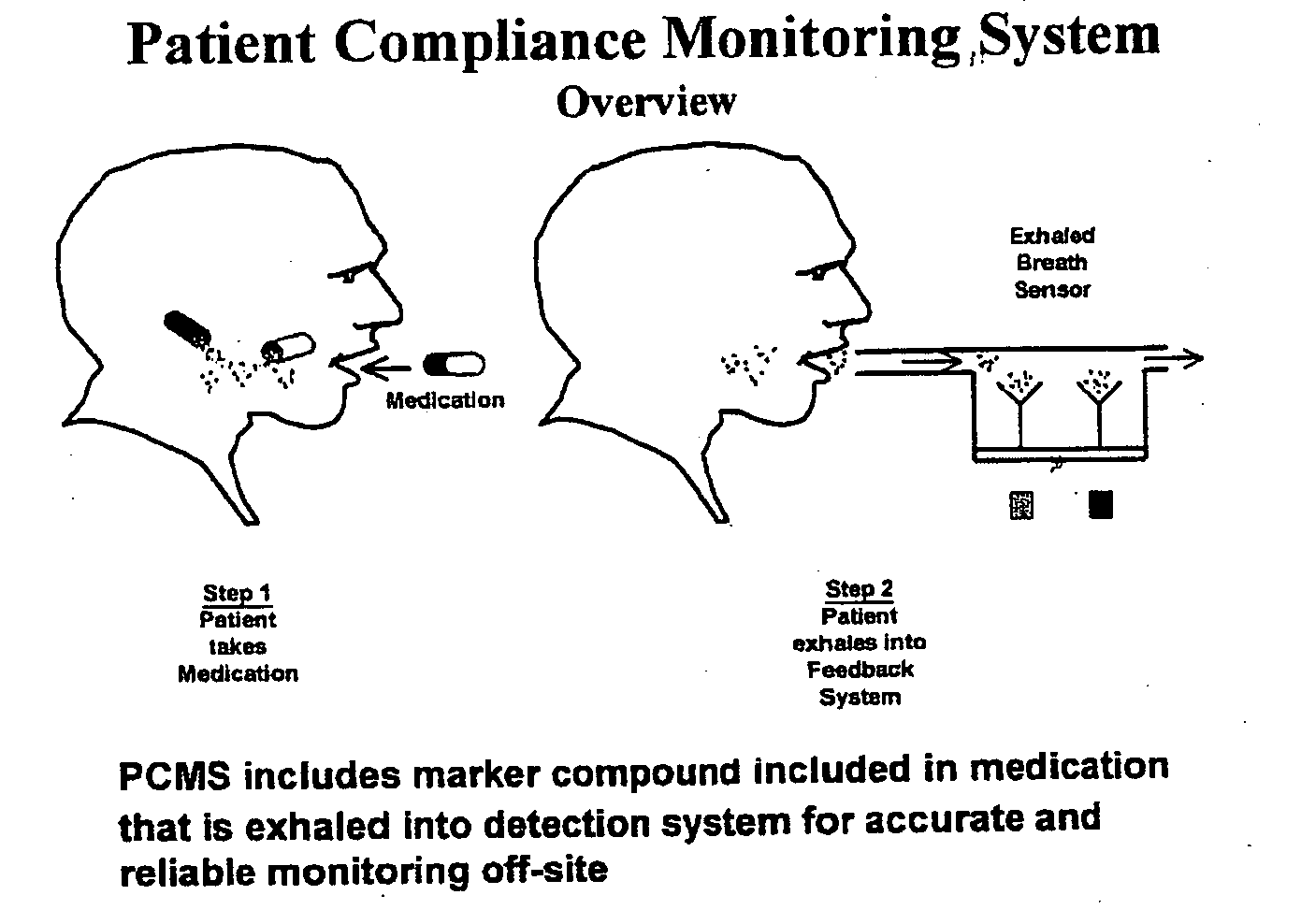
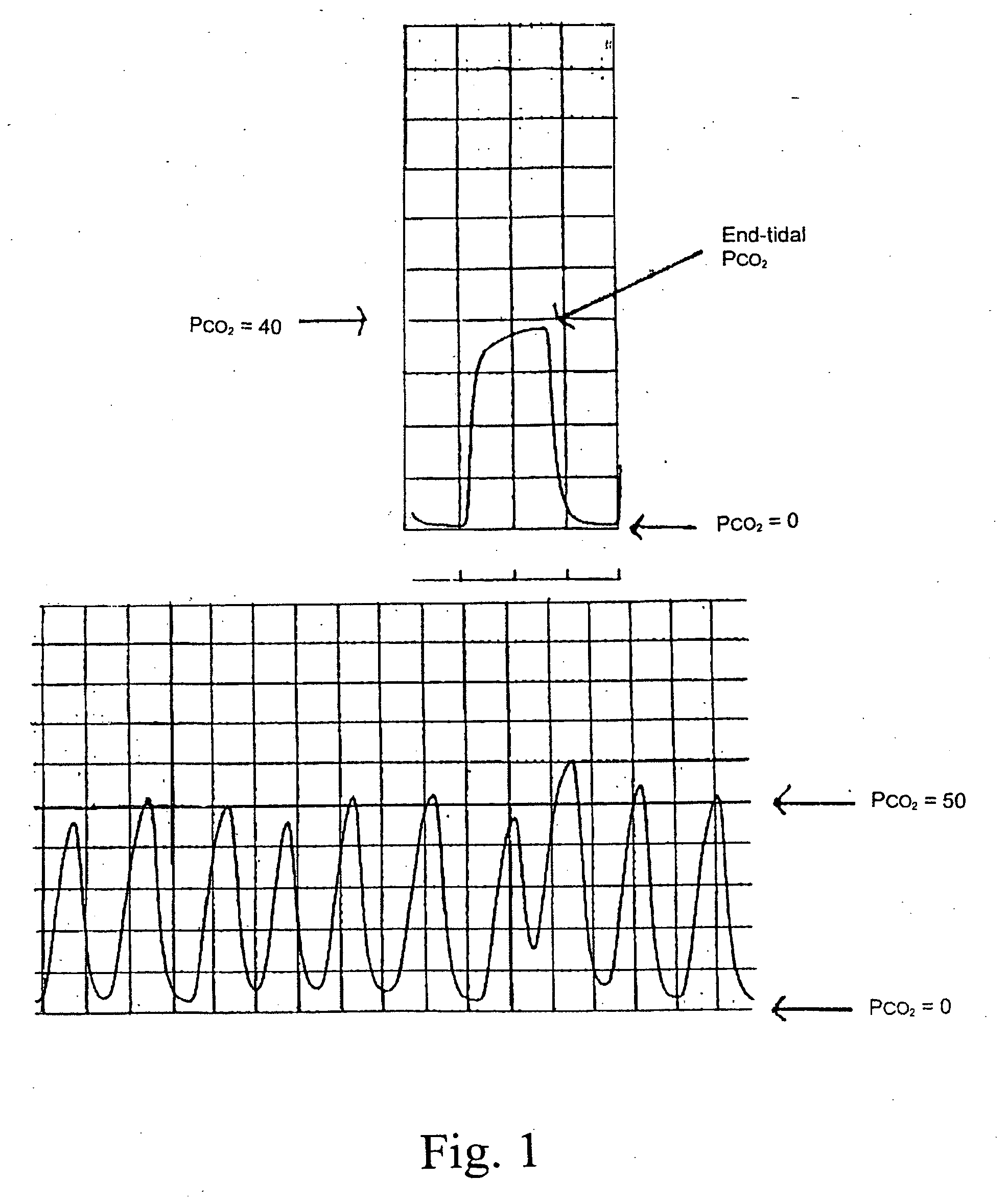
Marker detection method and apparatus to monitor drug compliance
InactiveUS20050233459A1Accurate assessmentPatient complianceDiagnostic recording/measuringSensorsNoseEnvironmental health
The present invention includes systems and methods for monitoring therapeutic drug concentration in blood by detecting markers, such as odors, upon exhalation by a patient after the drug is taken, wherein such markers result either directly from the drug itself or from an additive combined with the drug. In the case of olfactory markers, the invention preferably utilizes electronic sensor technology, such as the commercial devices referred to as “artificial” or “electronic” noses or tongues, to non-invasively monitor drug levels in blood. The invention further includes a reporting system capable of tracking drug concentrations in blood (remote or proximate locations) and providing the necessary alerts with regarding to ineffective or toxic drug dosages in a patient.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Application of levo-oxiracetam in preparation of medicine for treating memory and intelligence disturbance
The invention relates to new use of levo-oxiracetam in pharmaceutical field, and in particular relates to application of levo-oxiracetam in preparation of a medicine for treating memory and intelligence disturbance. The experiment result shows that the levo-oxiracetam is a main active ingredient for playing efficacy in oxiracetam, the clinical dosage can be greatly reduced by singly using the levo-oxiracetam, and the potential toxic and side effect is reduced. According to the invention, the levo-oxiracetam is a single active ingredient, the raw material purity is greater than 99.5% so as to effectively avoid the toxicity risk caused by other impurities in the medicine, the pharmacy is safer, the medicine quality is more controllable, and the curative effect is more precise.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +2
Porous drug matrices and methods of manufacture thereof
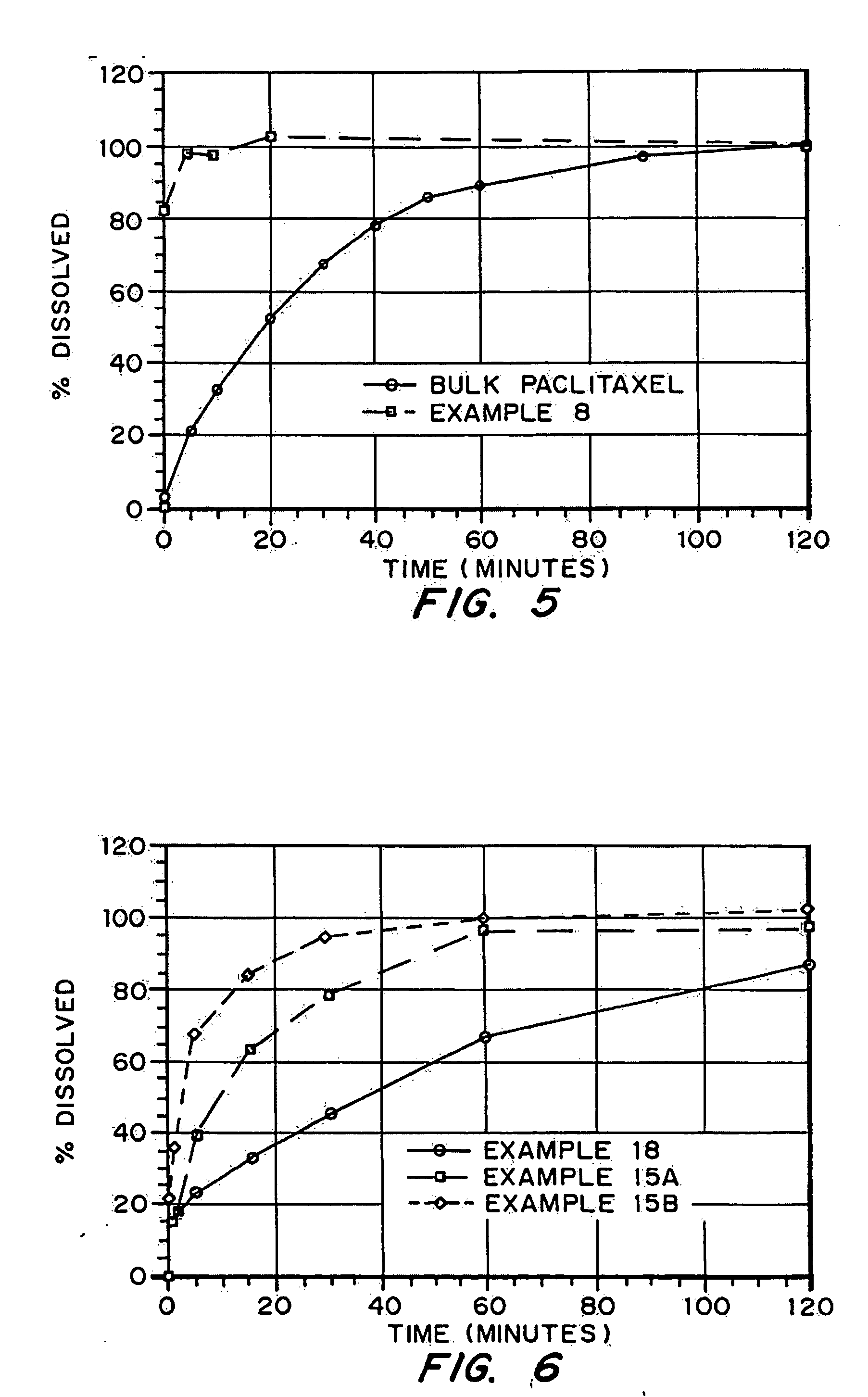
InactiveUS20050058710A1Fast dissolutionExtended half-lifePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Oral dosage combination pharmaceutical packaging
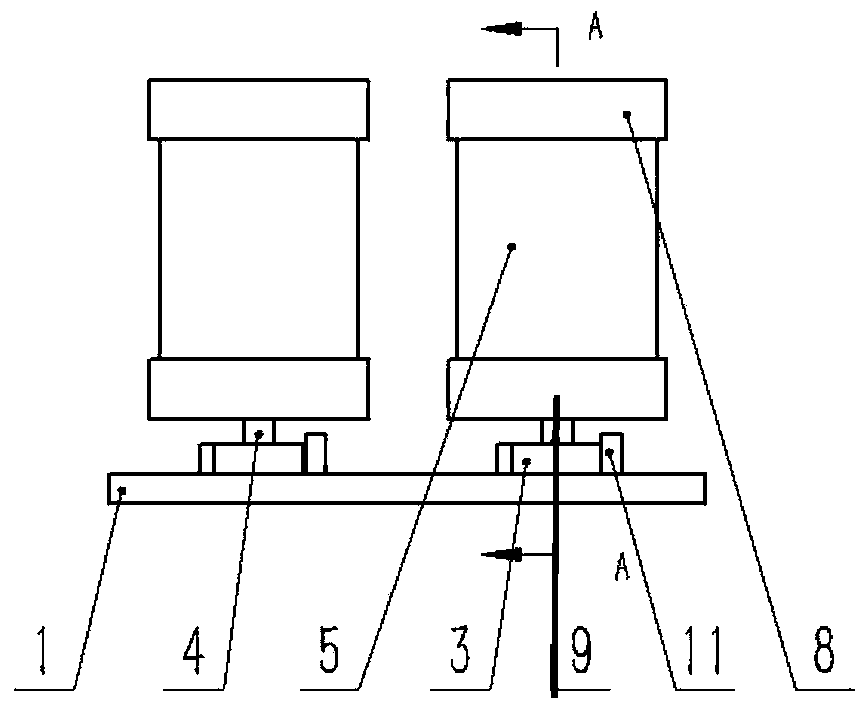
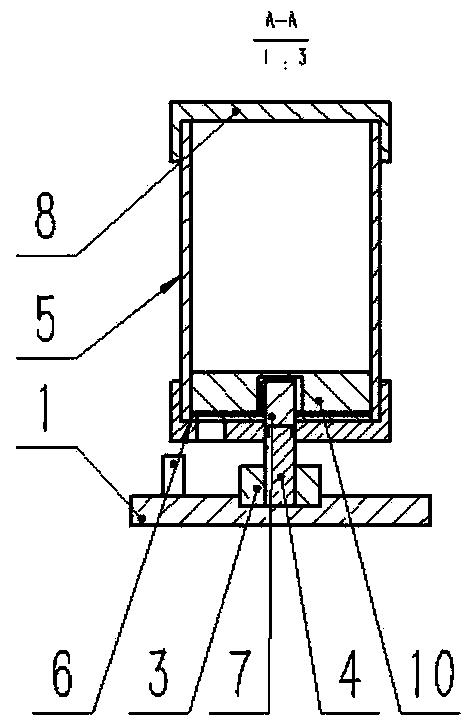
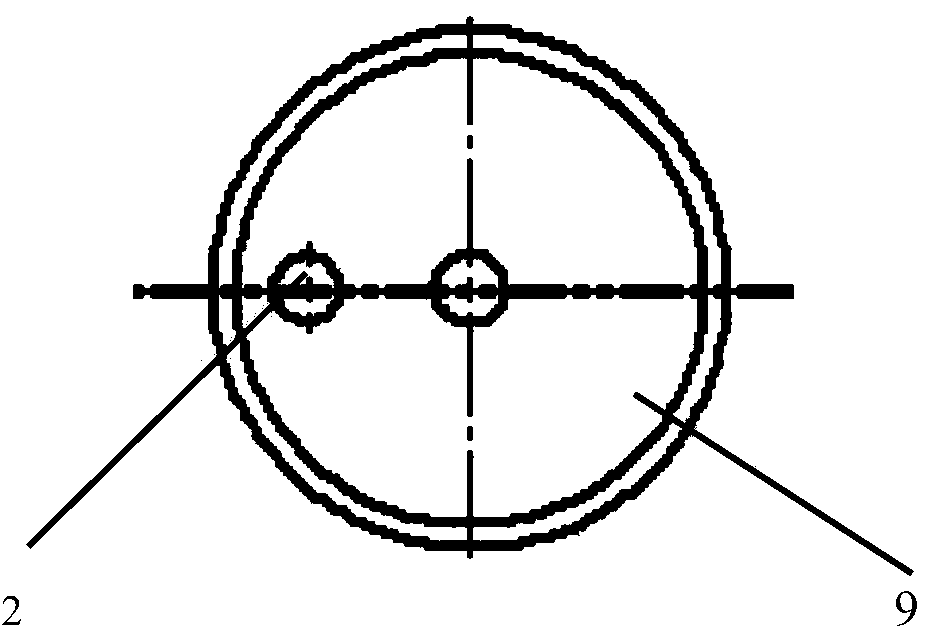
InactiveUS20090087483A1Overall design flexibilityIncreased riskAntibacterial agentsBiocideMedicinePharmaceutical packaging
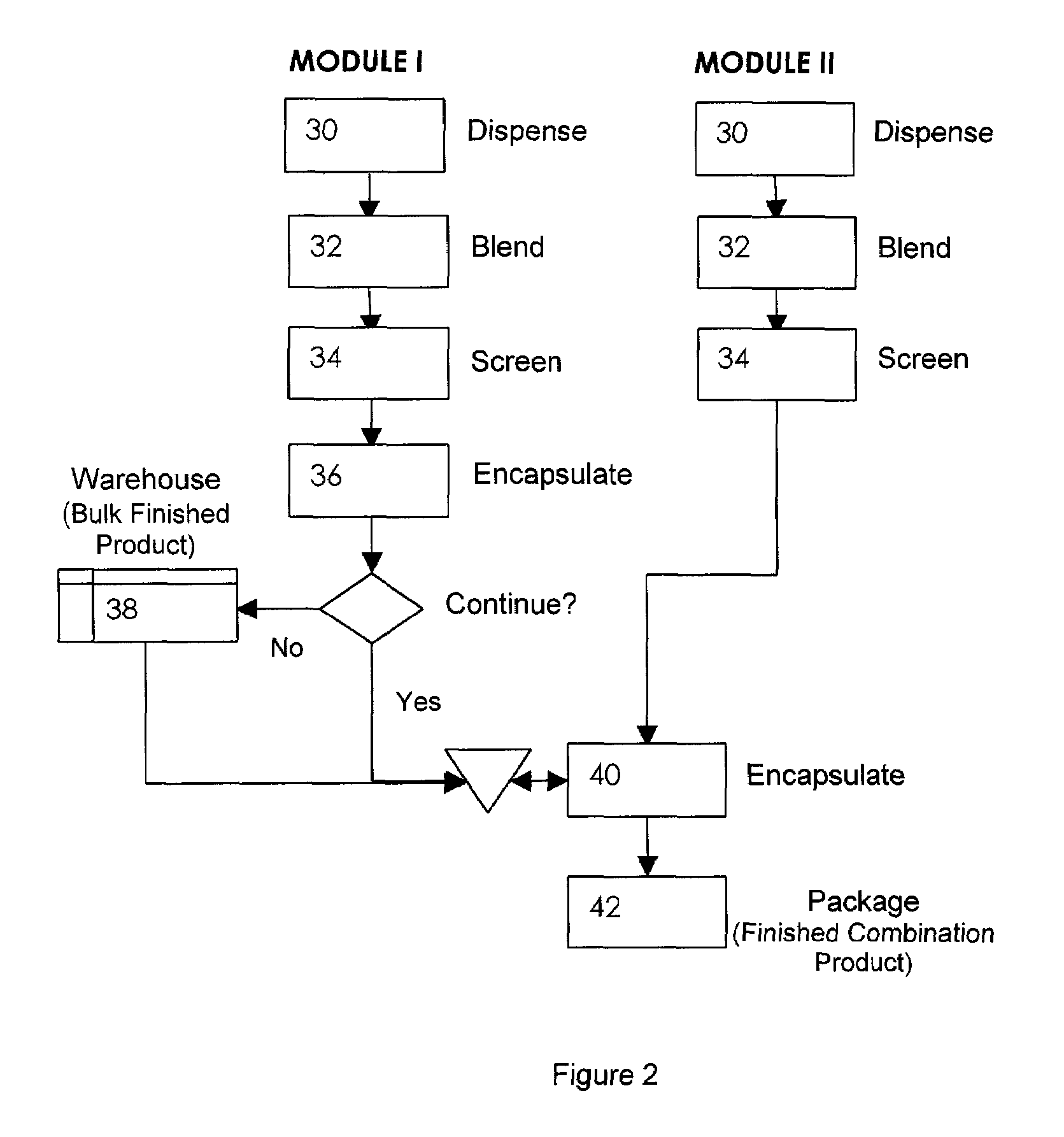
Pharmaceutical fixed dose combination products are formed by merging a fixed dose of a first pharmaceutical formulation from primary module, with a fixed dose of a second pharmaceutical formulation from a secondary module. In a preferred embodiment the first and second pharmaceutical formulations are separated from one another in a three piece capsule, a capsule-in-a-capsule or a tablet-in-a-capsule, and the primary and secondary modules are interchangeable.
Owner:MICRODOSE THERAPEUTX INC
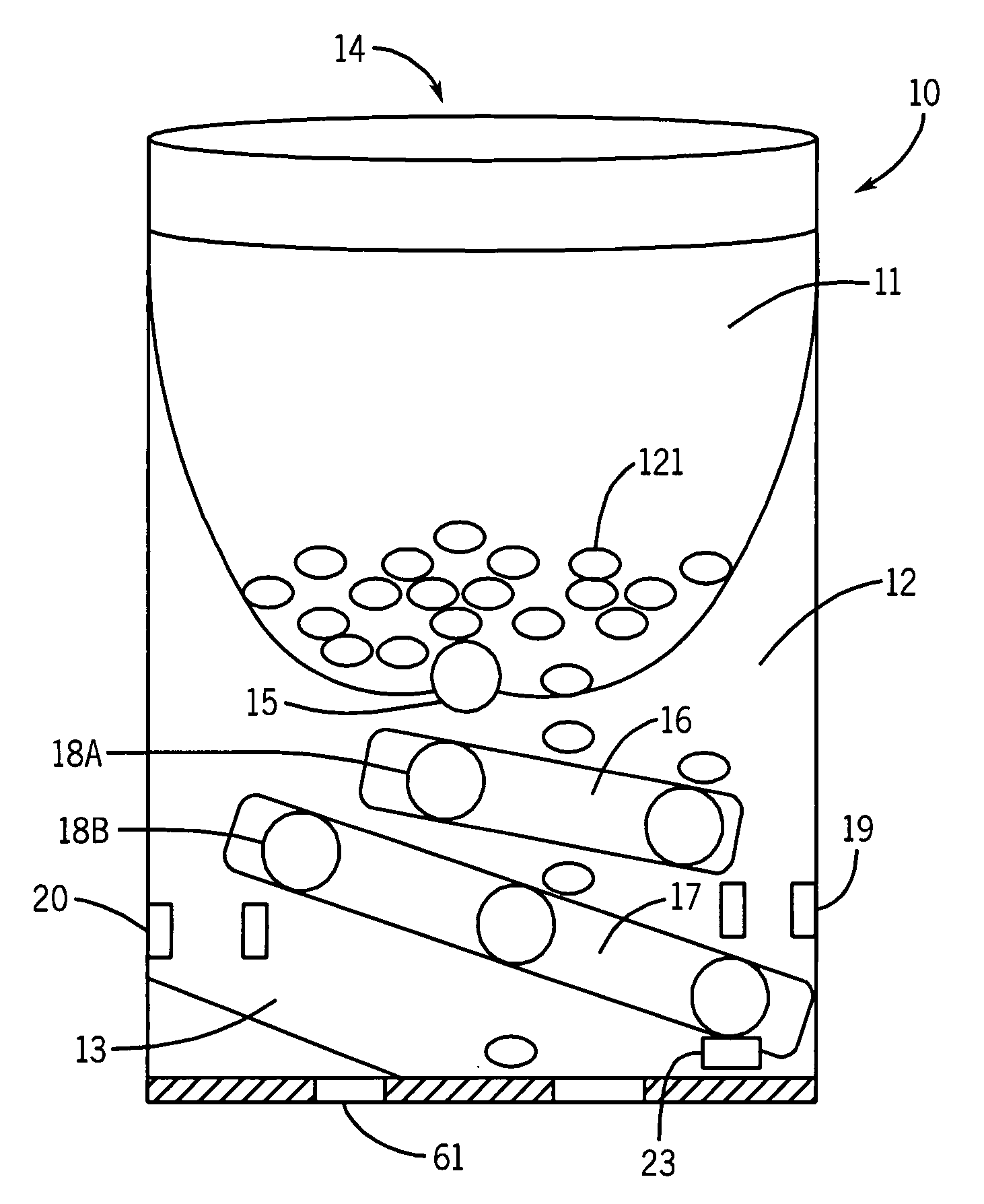
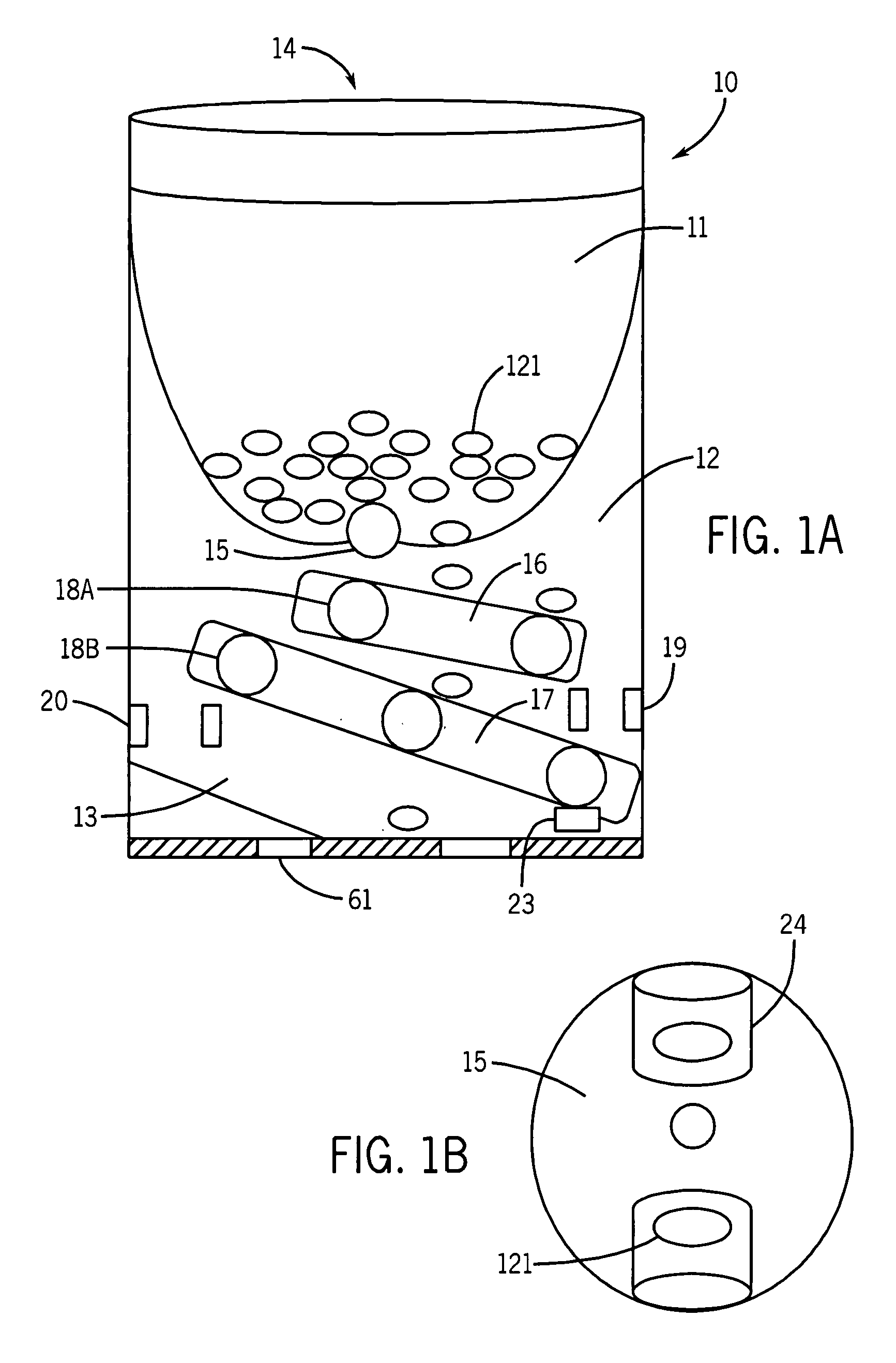
Automatic dispensation device and medicine
InactiveUS20070150092A1Efficient and automated drug dispensingDrug and medicationsCoin-freed apparatus detailsMedication DispenserMedicine
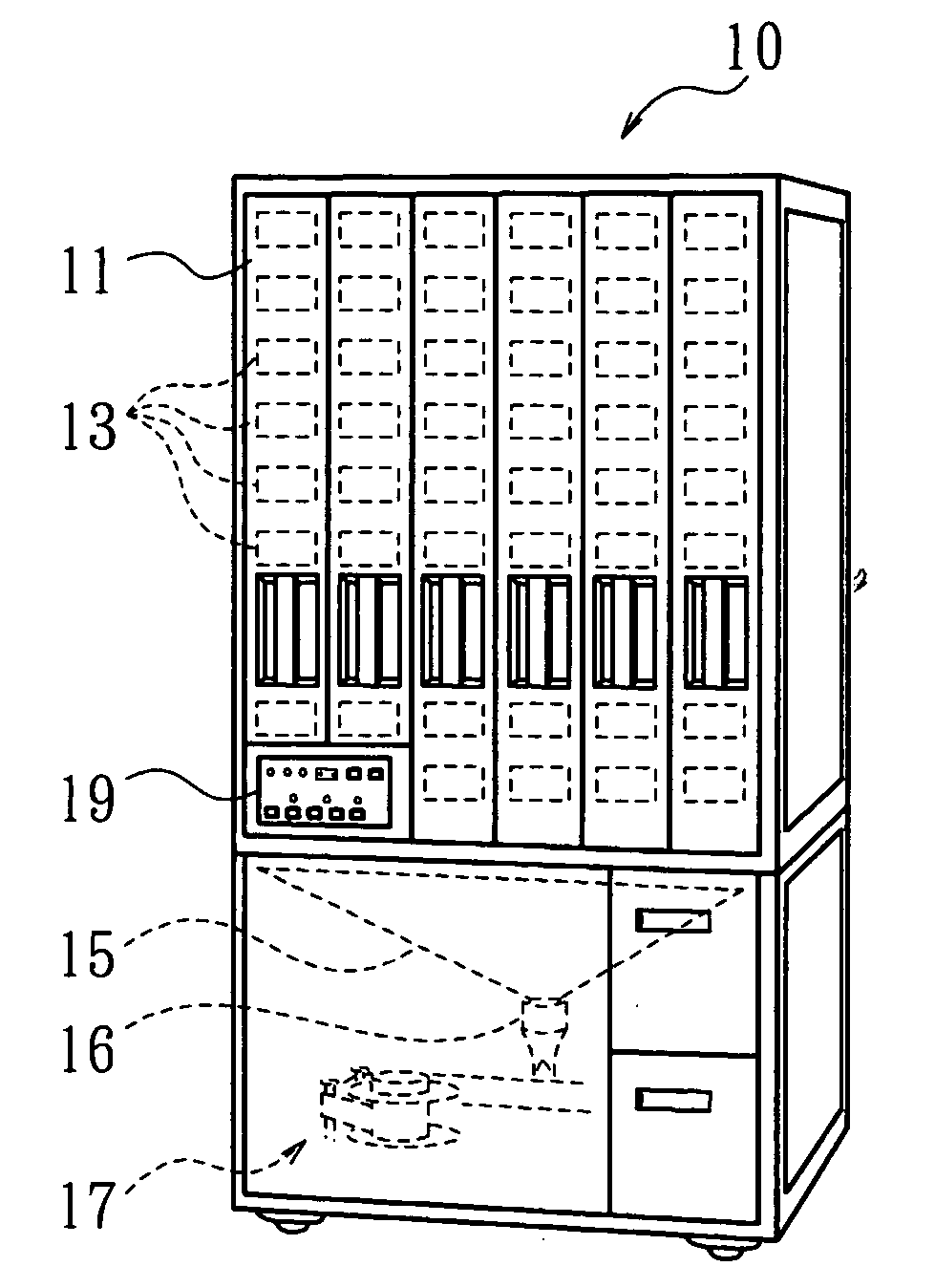
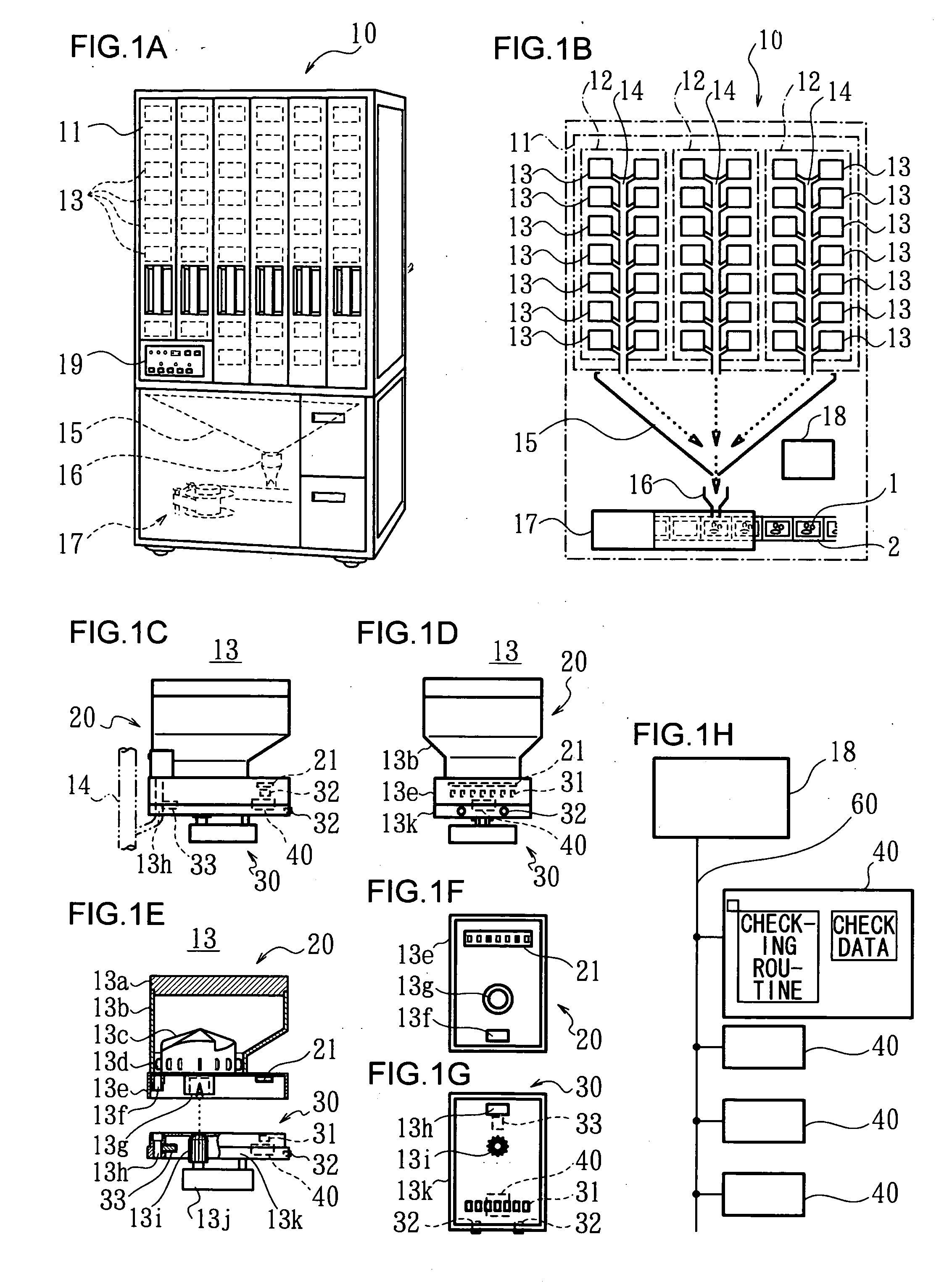
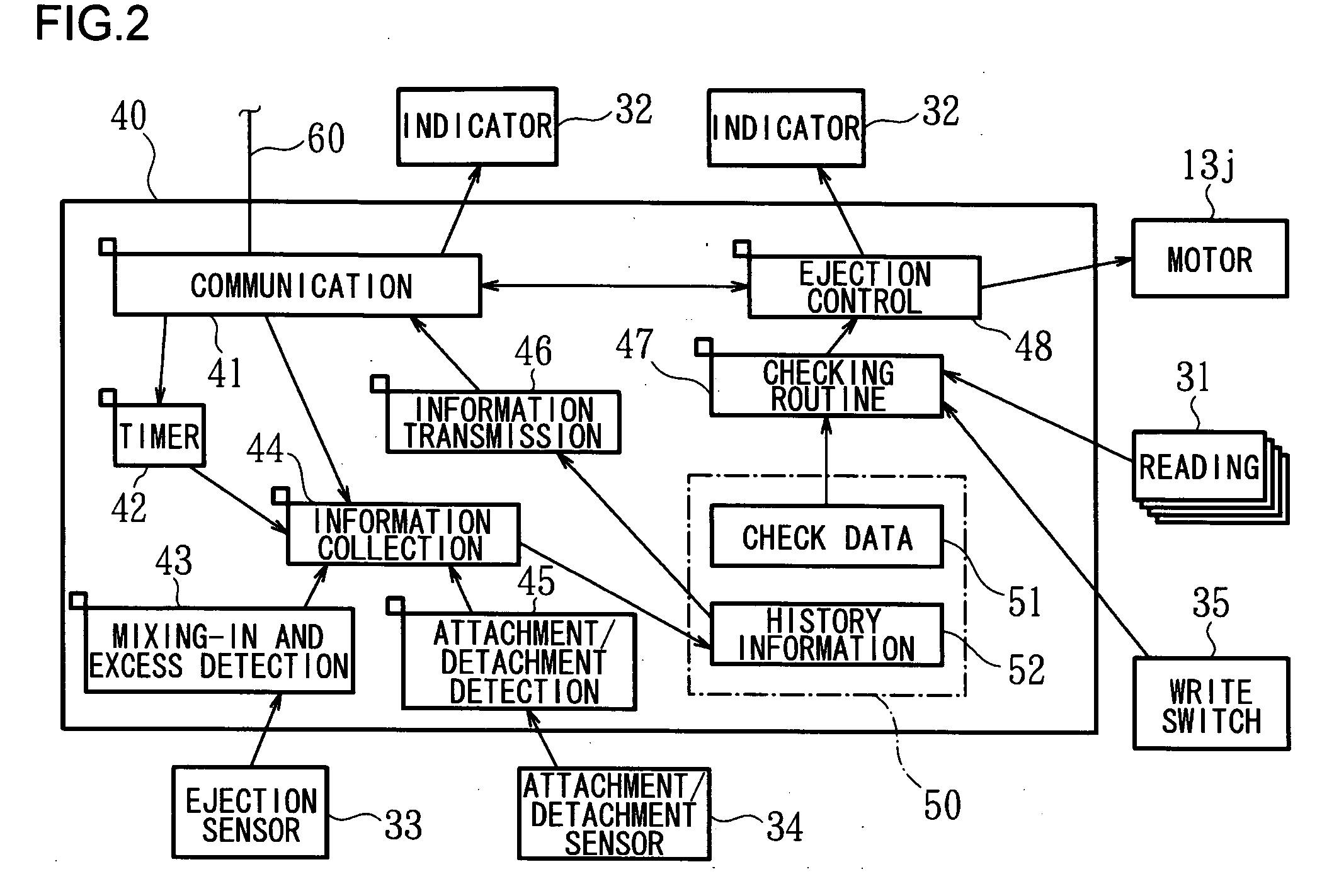
An automatic drug dispenser includes: a drug cassette which ejectably accommodates drugs; a base unit which detachably supports the drug cassette and drives a motor to eject drugs; a drug feeder storage which is designed to store a large number of base units; a reading device which is provided in each of the base units and reads identification information assigned to the drug cassette; and a checking means which compares a result of reading with pre-stored check data, wherein a microprocessor is mounted in each of the base units, and the checking means, the check data and history information related to the cassette are built in each microprocessor in a distributed manner.
Owner:TOUSHIYOU KK
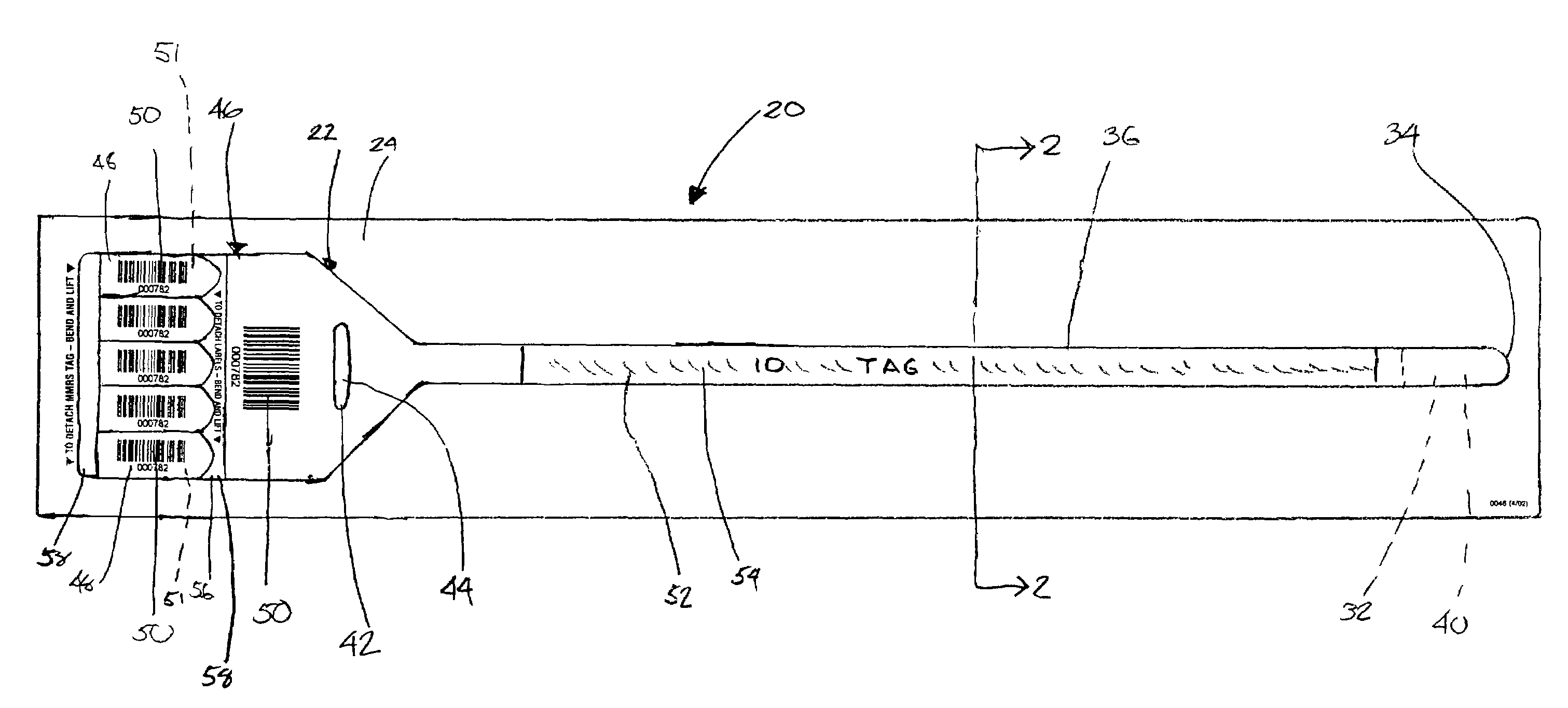
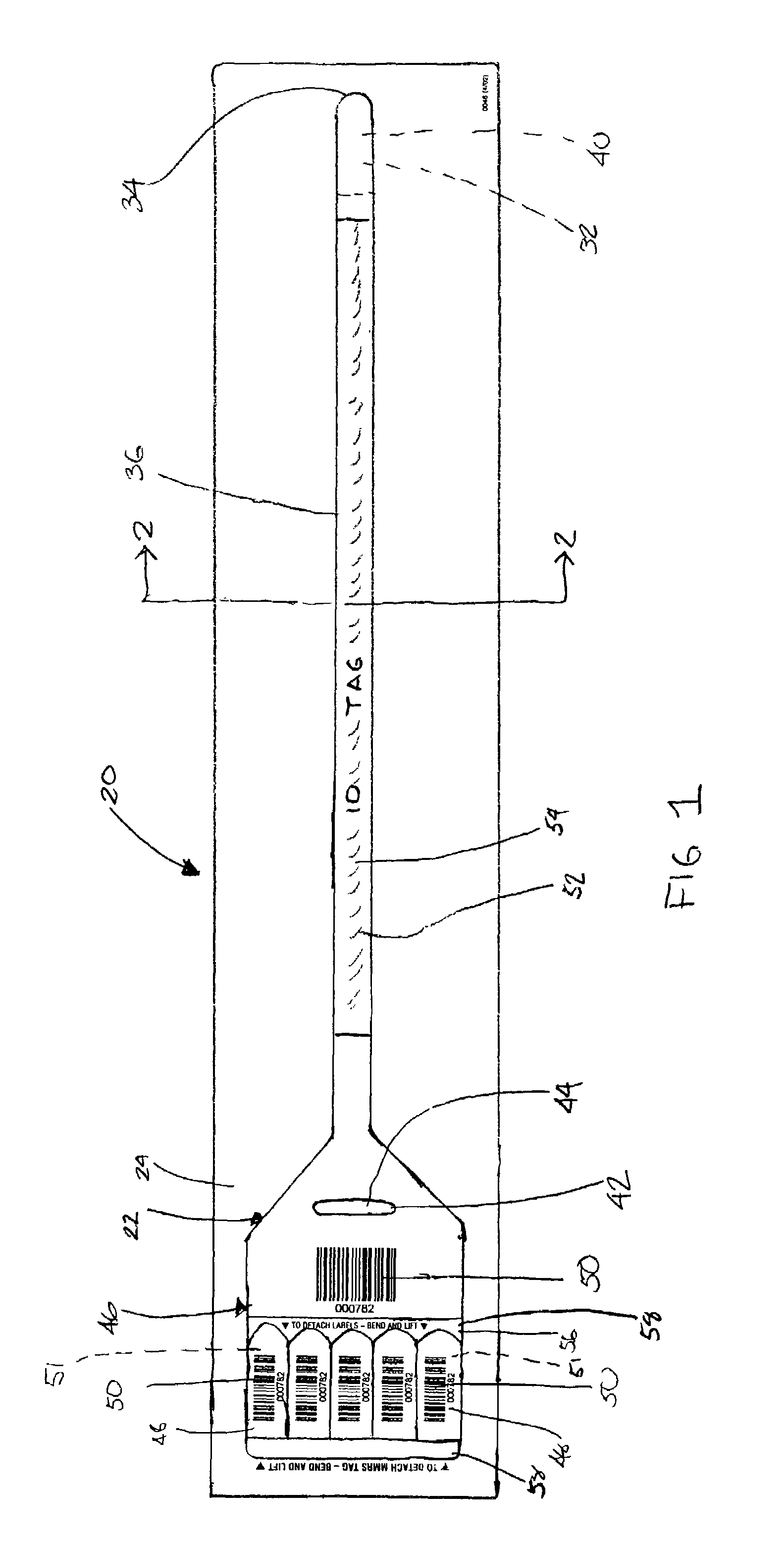
Wristband/label assembly business form and method
A business form particularly adapted for use during medical emergencies includes in a first embodiment a wristband / label assembly that is readily separable from a carrier, with the wristband including a single end for looping around a victim's appendage through a cinch and a tab carrying a plurality of labels with the wristband and each label having an identifying indicia such as a bar code printed thereon. The wristband bar code thus becomes associated with the victim and the labels are used to identify items associated with the patient such as his possessions, medical charts, medicines, etc. The wristband may be color coded so that as the medical personnel triage victims they are categorized by color as to their need for medical care, with the color coding thus being readily ascertainable by others as multiple victims are processed. A second embodiment includes a pre-printed form having a tab portion with the bar code labels as in the first embodiment and also a series of tear off tabs for indicating the medical condition of the patient. Additionally, the medical condition tabs may also be bar coded so that the patient's ID and medical condition may both be “swiped” into a data base using bar code information. Once the data is collected, it is conveniently input into a computer with the computer then transmitting the information to a server for display at a web site. The server and related software is fully capable of handling input from multiple computers in real time so that victim information is made available over the internet almost immediately as the victims are processed.
Owner:ZEBRA TECH CORP
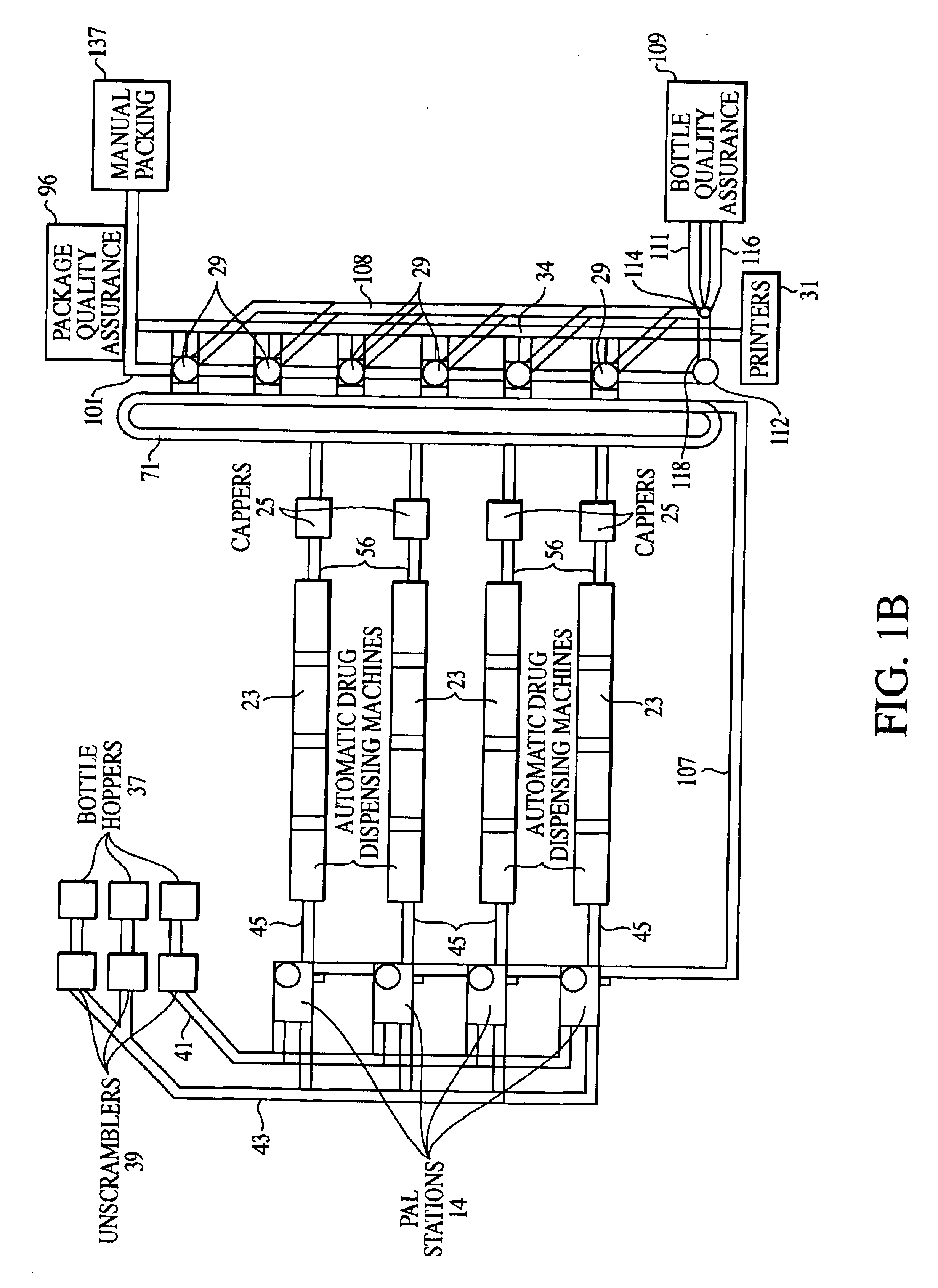
Packaging and delivery of pharmaceuticals and drugs
Owner:MICRODOSE THERAPEUTX INC
Method for sorting discarded and spent pharmaceutical items
ActiveUS7318529B2Easy to classifyEncourages and facilitates complianceSustainable waste treatmentDispensing apparatusMedical wasteWorkstation
Owner:CAREFUSION 303 INC
Pharmaceutical administrative system for ordering and receiving prescribed medication
InactiveUS7636718B1Drug and medicationsDigital data processing detailsPharmacyDispensing medications
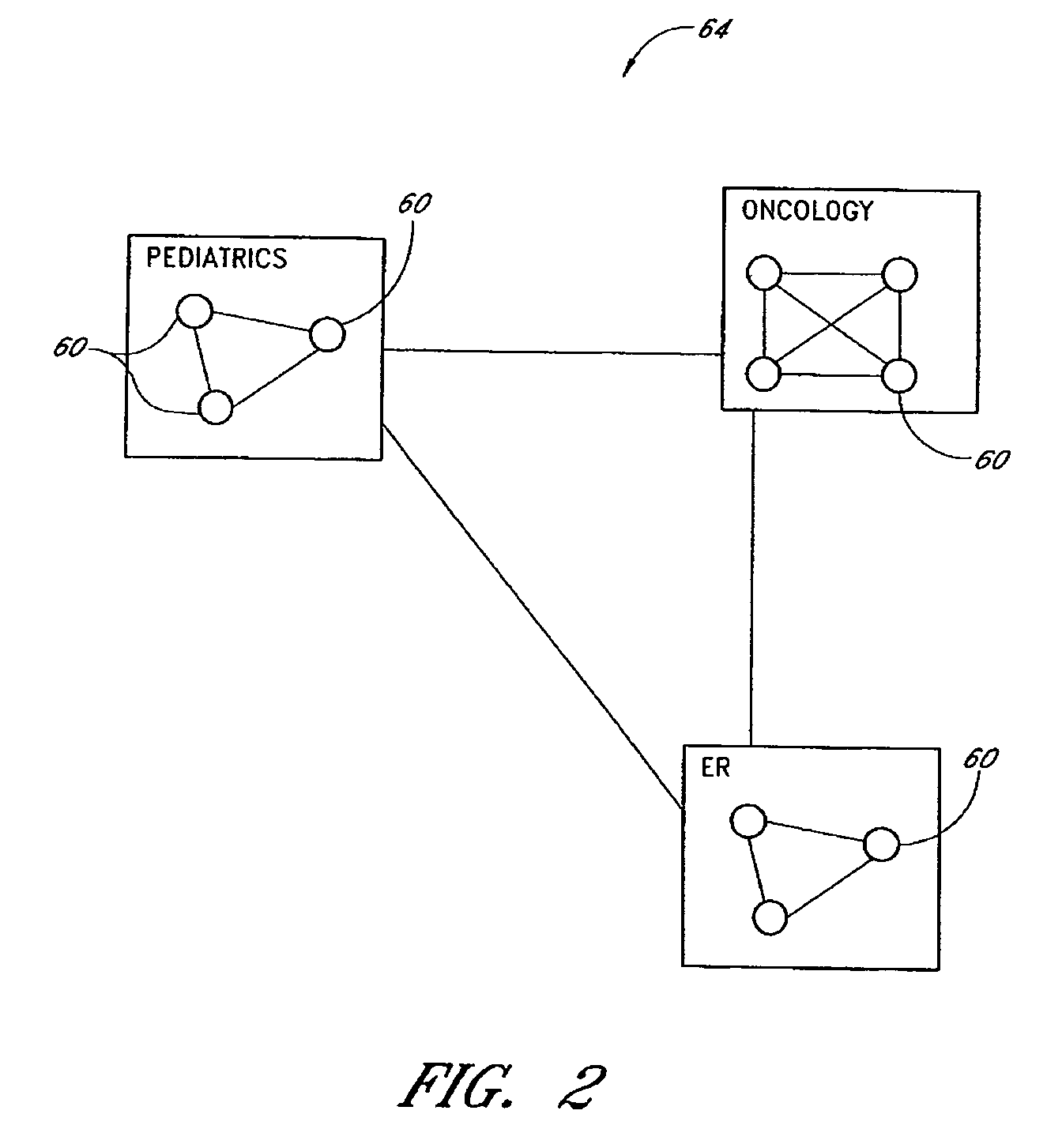
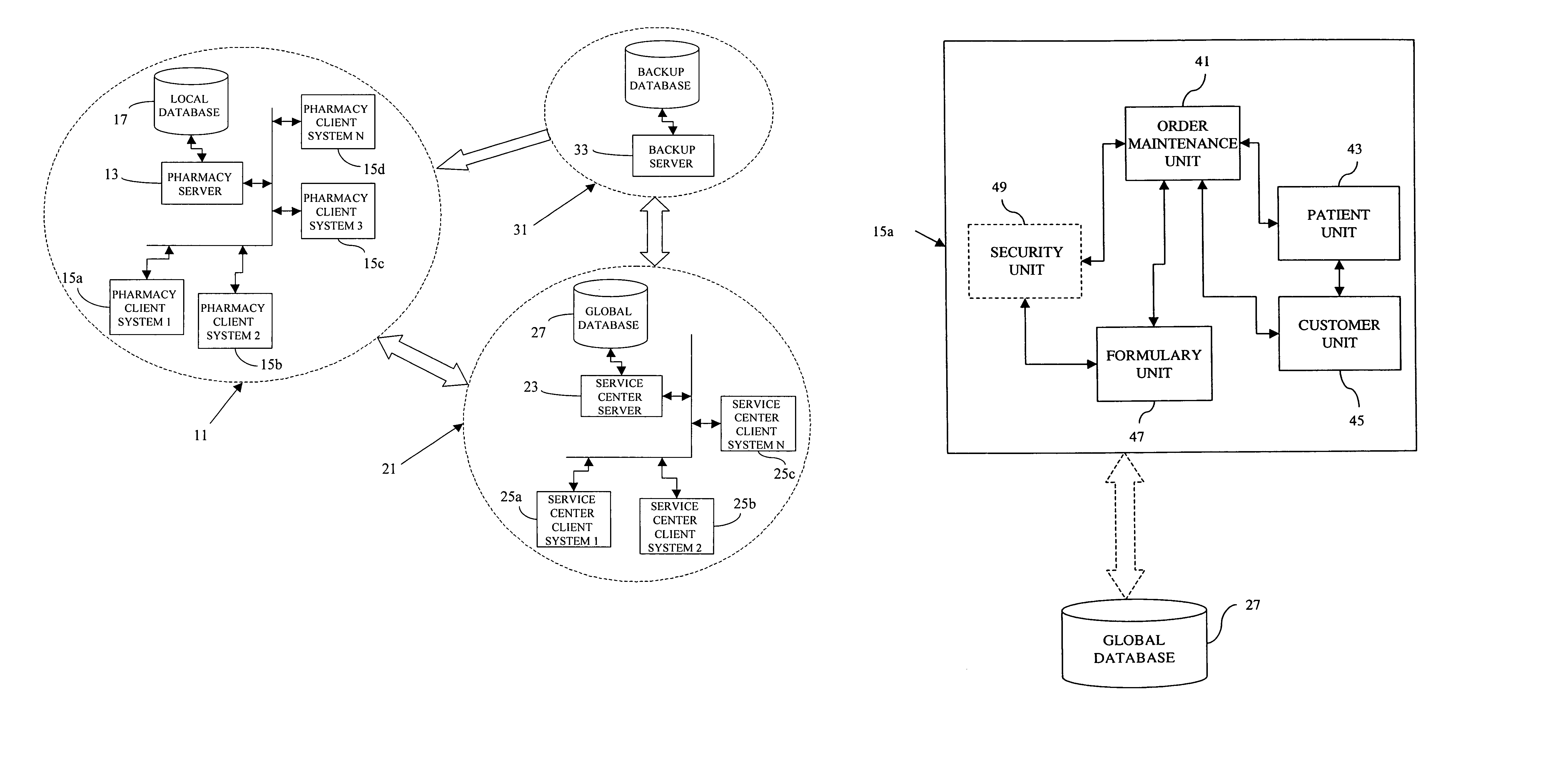
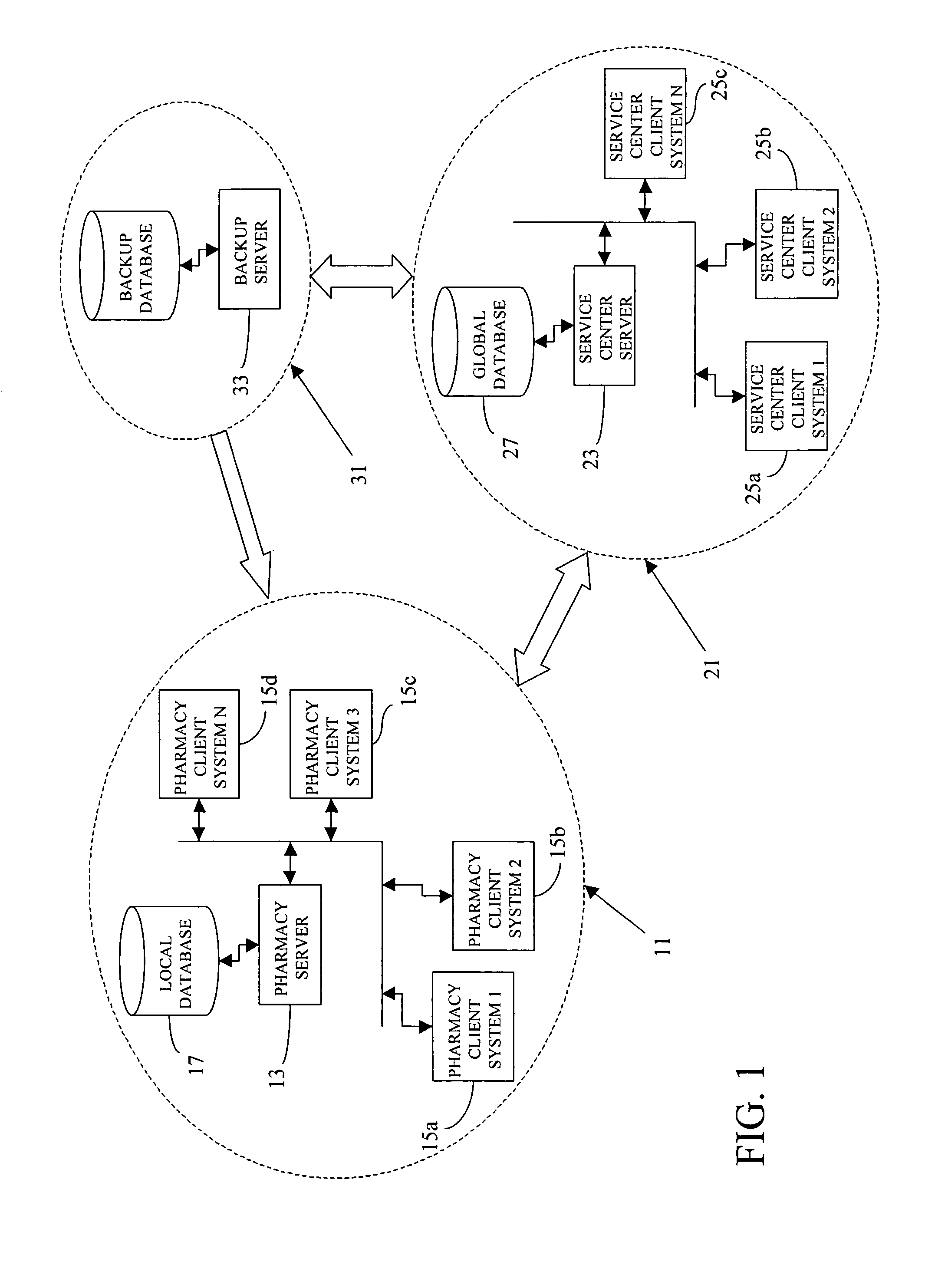
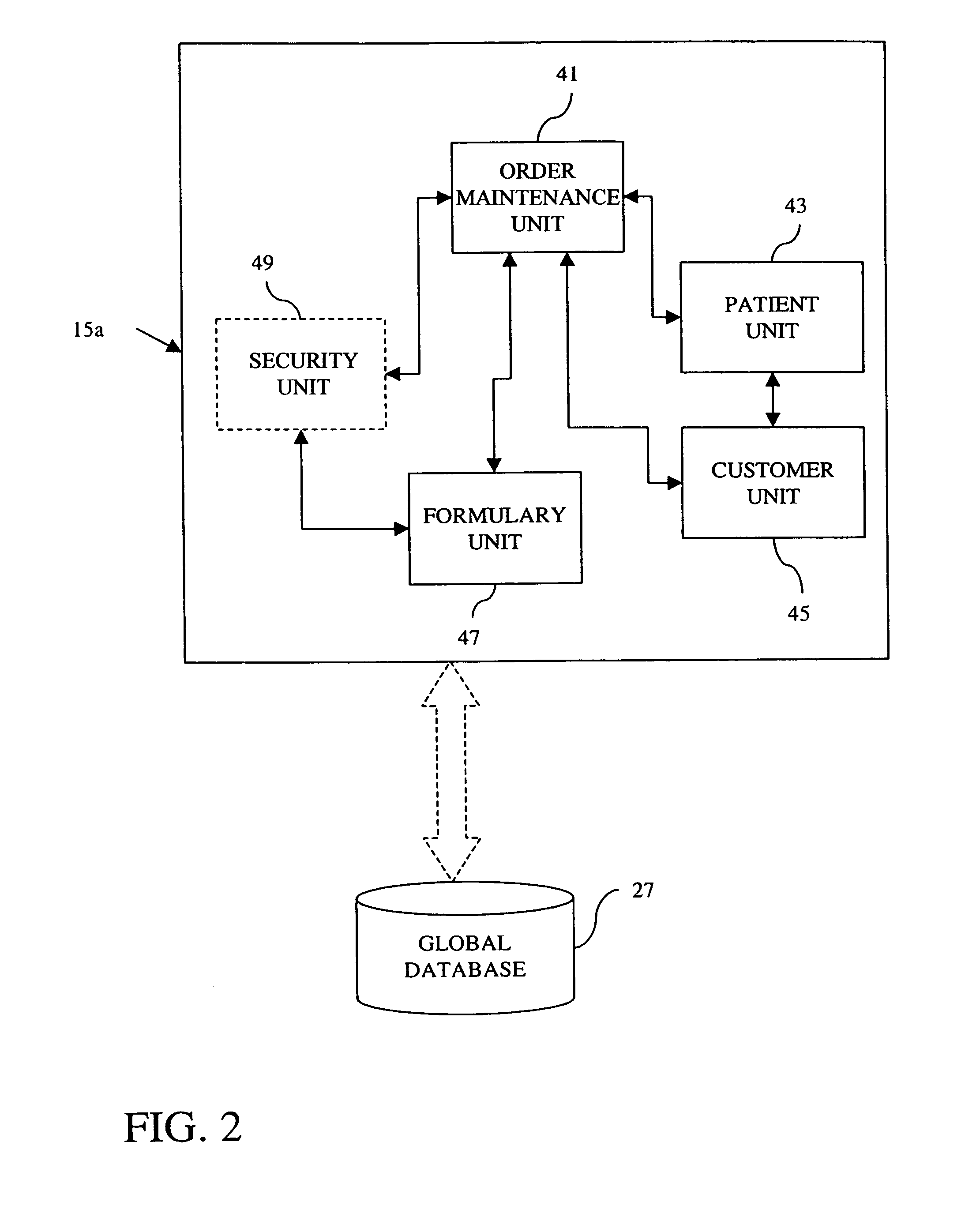
A pharmaceutical administrative system with a pharmacy and a service center network for preparing and dispensing medication. The pharmacy network prepares orders for medication from various customers for various patients. By retrieving information from a global database in the service center network, the pharmacy network conveys patient, customer and formulary information to users of the pharmacy network. Also, the pharmacy network prepares medication specific labels to identify and verify the contents of the medication. Furthermore, the pharmacy network provides additional safeguards and information, including balancing orders and displaying and / or generating hardcopies of solubility curves, to a health care provider using the pharmacy network with the additional ability to customize the medication.
Owner:B BRAUN MEDICAL
Means and method of applying RFID and PKI technologies for patient safety
InactiveUS20060089858A1Preventing improper useInhibition releaseData processing applicationsDrug and medicationsMedical recordMedicine
A system and a method for applying RFID (Radio Frequency Identification) and PKI (Public Key Infrastructure) technologies for patient health safety to install RFID tag and RFID reader on the medicine-storing appliance. By sensing the RFID tag on medicine, the RFID reader stores the medical records such as medicine usage and movement, and transmit to central database. In this way, the circulation of medicine could be controlled, the improper use and distribution of medicines could be avoided, the medical resource management could be improved, and it could ensure that the correct medicine to enhance the process of five rights (right time, right route, right dose, right patient and right drug) and improve the quality of patient safety.
Owner:LINGANG COMM CORP
System and software of enhanced pharmacy services and related methods
InactiveUS7706915B2Improvement in administrationImproved and timely accessDrug and medicationsOffice automationMedication informationPharmacist
Owner:JOHNS HOPKINS ARAMCO HEALTHCARE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com