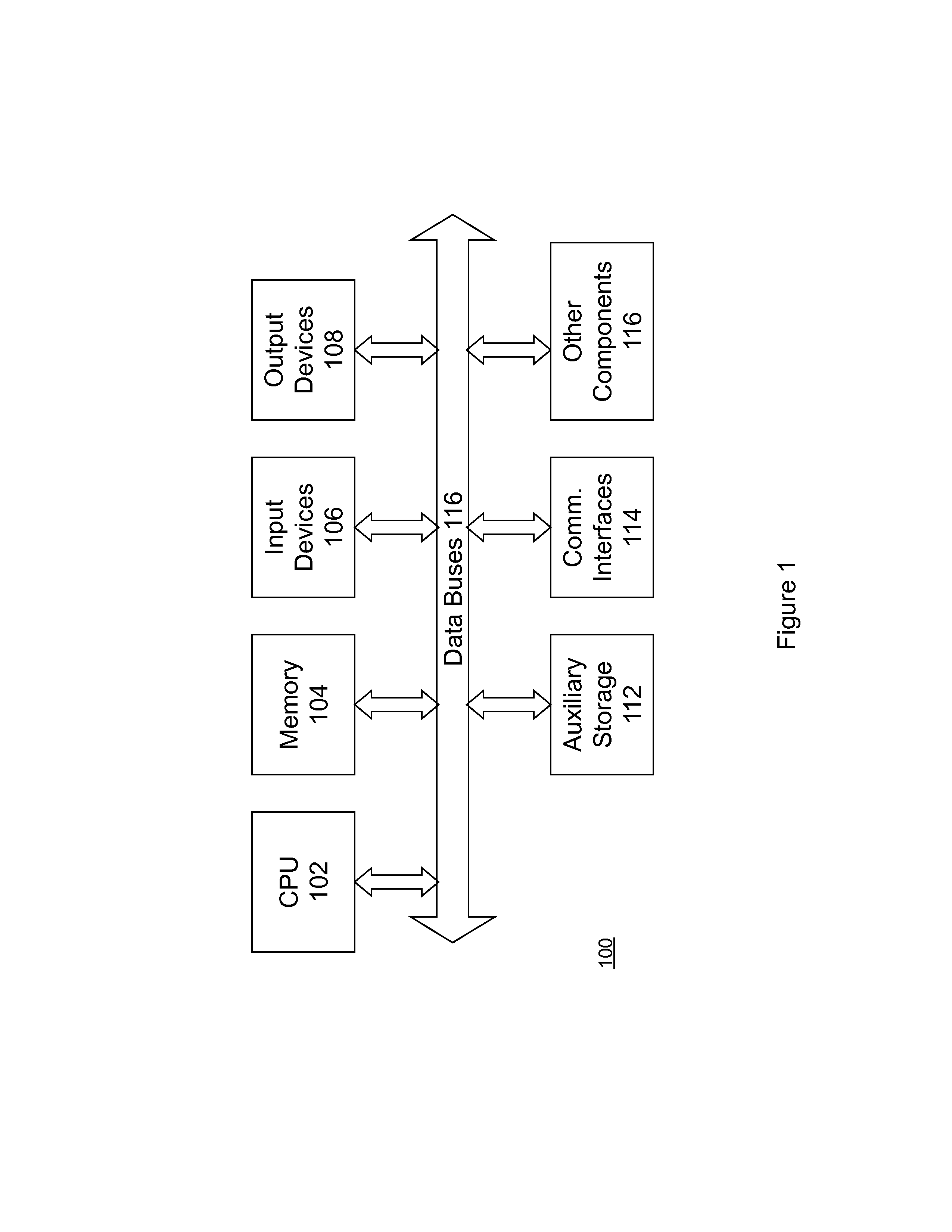
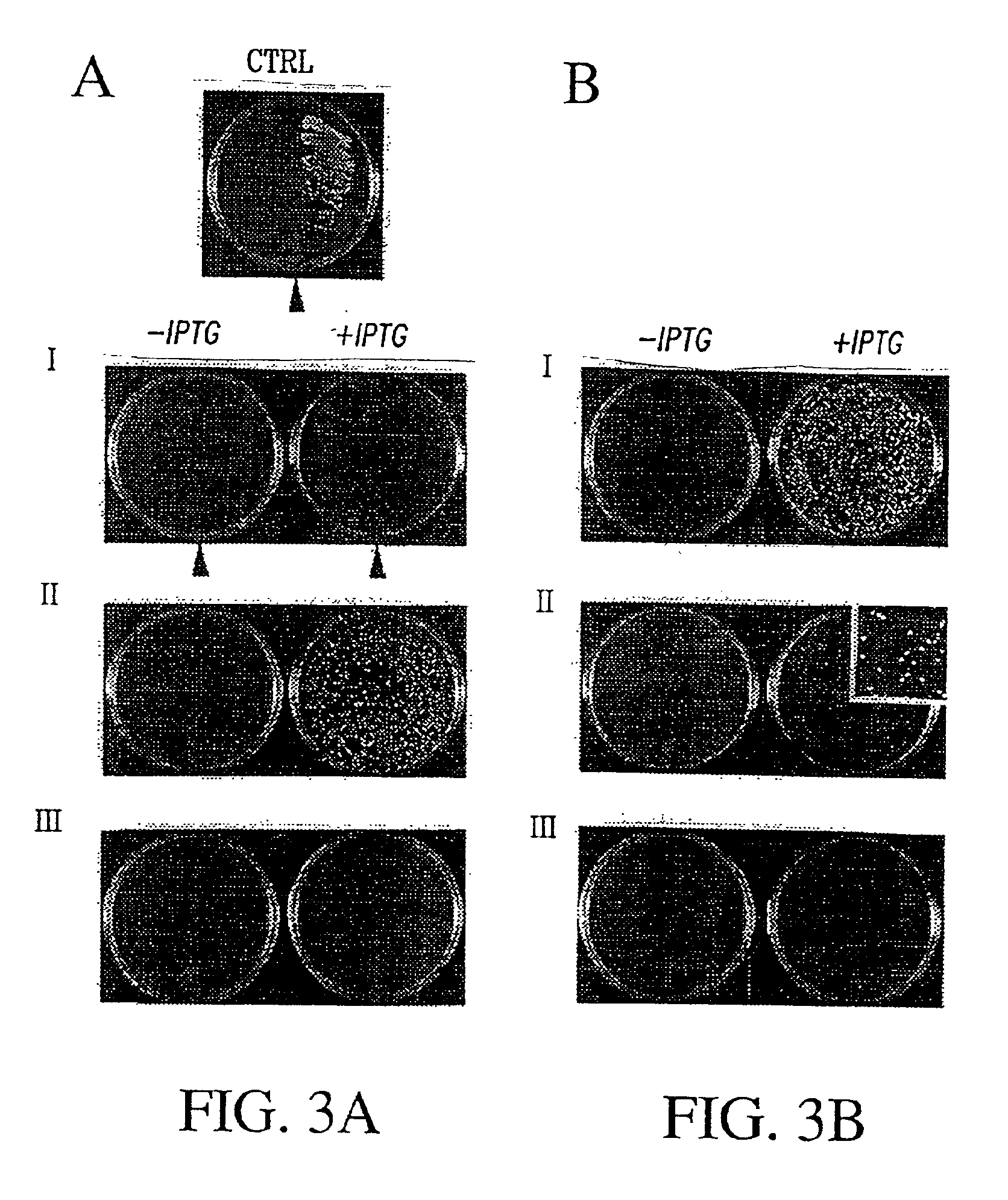
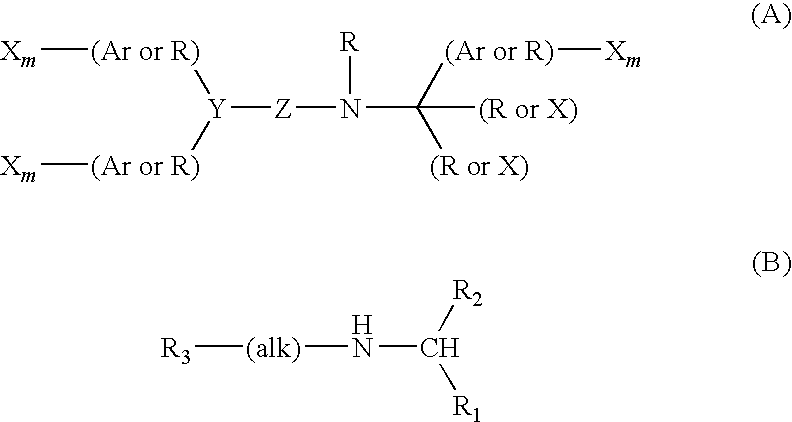
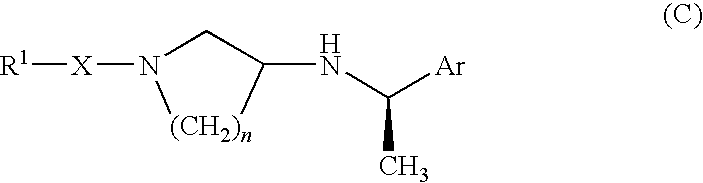
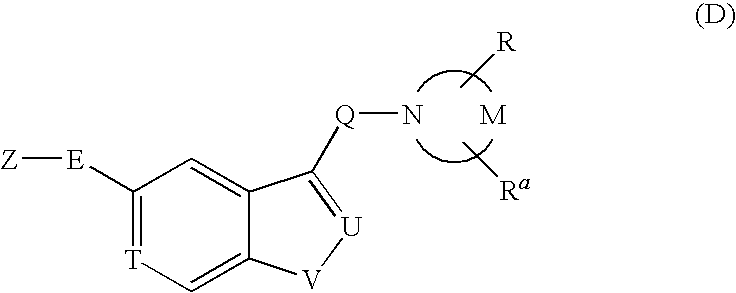
Patents
Literature
194 results about "Drug interaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
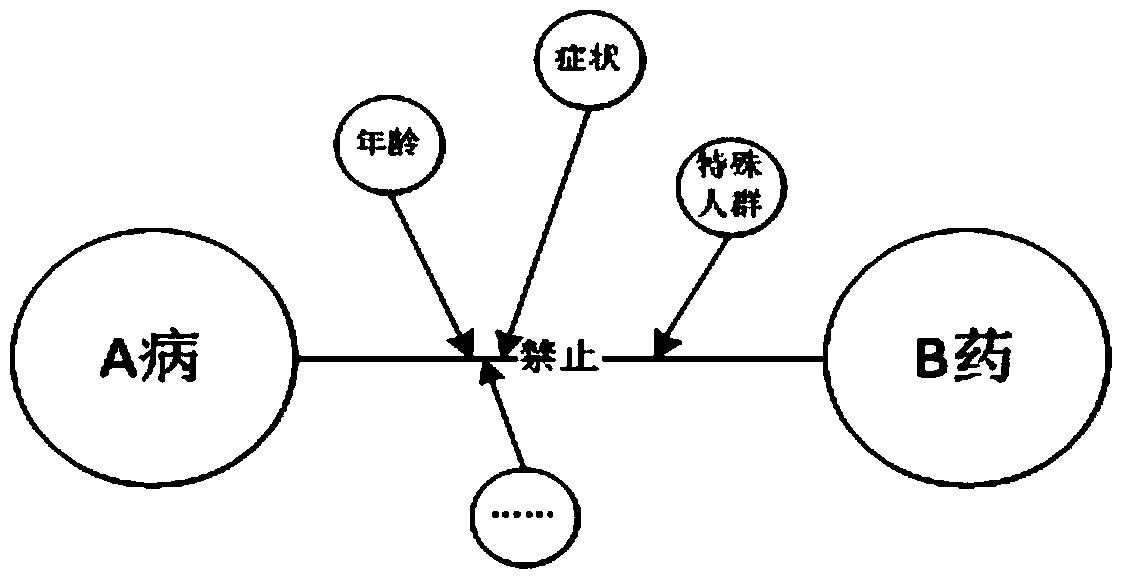
A drug interaction is a change in the action or side effects of a drug caused by concomitant administration with a food, beverage, supplement, or another drug. There are many causes of drug interactions. For example, one drug may alter the pharmacokinetics of another. Alternatively, drug interactions may result from competition for a single receptor or signaling pathway.
Integrated pharmaceutical accounts management system and method
InactiveUS20020002495A1Facilitate real-time adjudicationEasy to processHand manipulated computer devicesDrug and medicationsData warehouseDrug interaction
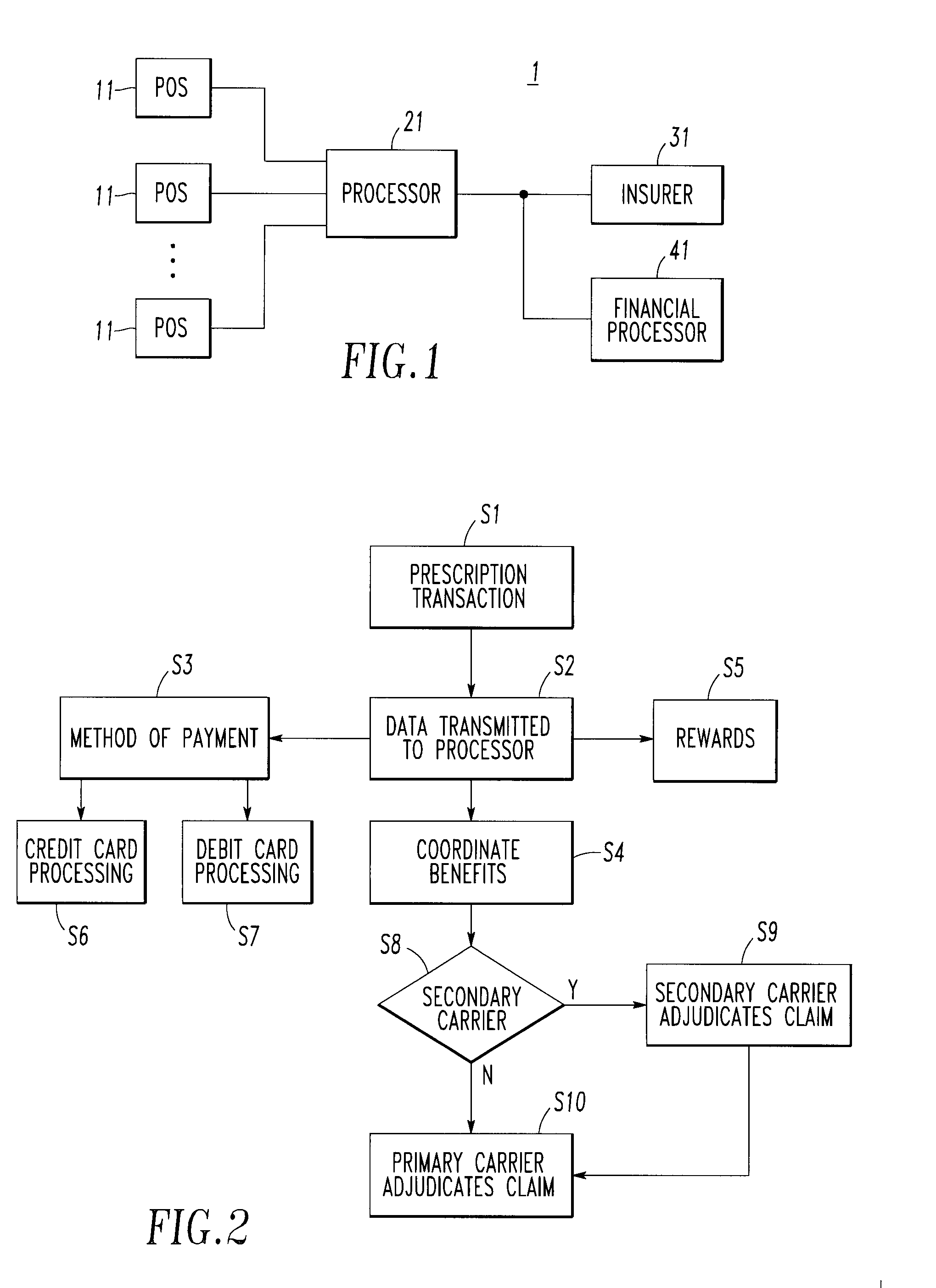
<heading lvl="0">Abstract of Disclosure< / heading> An integrated suite of services for consumers, service providers and manufacturers in the pharmaceutical industry is disclosed. The present invention utilizes one or more of the NCPDP standard formats and adopts the switch for an integrated system of, for example, instant adjudication of prescriptions, consumer data warehousing and / or incentive rewards for the consumer. A participating consumer with one card, can instantly purchase pharmaceuticals and charge the transaction to a credit card and earn and apply savings dollars redeemable for pharmaceutical purchases. For a participating service provider, instant adjudication and instant validation of consumer eligibility can be performed. Moreover, a service provider may receive messages related to the patient's medications. Significantly, data is recorded for consumers even when consumers make the pharmaceutical purchase with cash. The system includes a unique card issued to participating consumers. The card is adapted to encode conventional credit or debit card information specific to the participating consumer so that the consumer can consummate a transaction for the purchase of pharmaceuticals without possession of an additional credit card. The system further includes a host processor coupled to the point of sale at the service provider through a leased line or public switch network or the like. When a customer performs a pharmaceutical transaction at the point of sale of the service provider, the host processor coordinates any benefits and data with other prescription benefit management systems through messages transmitted and received from any primary or secondary carrier systems. The host processor further is adapted to facilitate real-time adjudication of claims and checks for any dangerous drug-to-drug interactions. The host processor additionally facilitates any financial processing including the accumulation and redemption of any bonus dollars earned by the consumer. Furthermore, since the card used by the consumer can be encoded with credit or debit card information, the host processor determines the desired payment method and performs the actual financial transaction. Even if the transaction at the point of sale is a cash purchase, the consumer may desire to use his unique card for the accrual of bonus dollars. Therefore, data concerning the transaction (i.e., pharmacy number, prescription number, etc.) can be recorded even for transactions conducted with cash.
Owner:NPAX
Method and system for administering anticoagulation therapy
InactiveUS20090234674A1Efficient managementOptimizationDrug and medicationsOffice automationWarfarinTime schedule
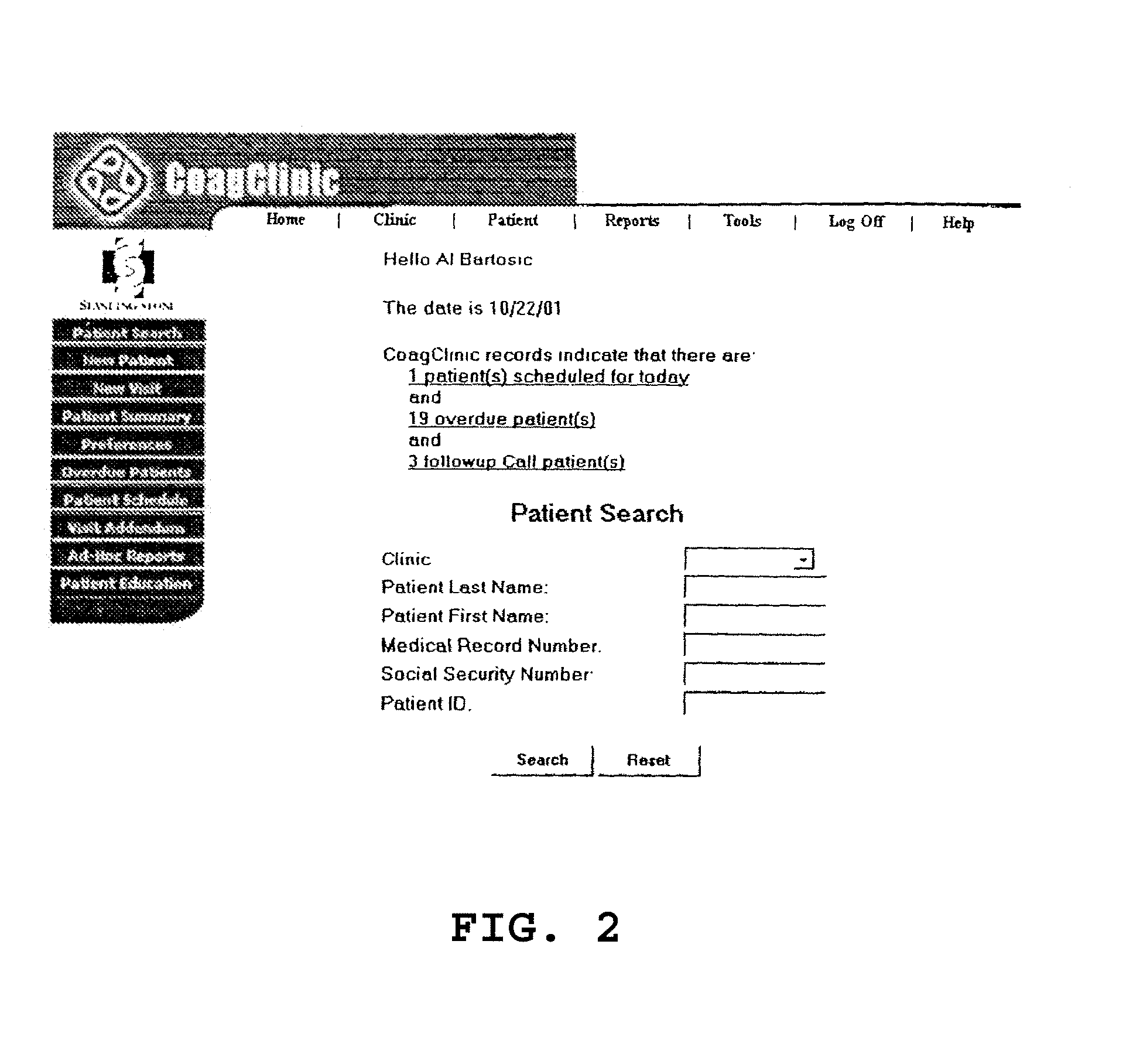
A method and system for effectively administering anticoagulation therapy, including providing a warfarin dose weekly schedule and converting a total weekly requirement into daily dosages based on the number of milligrams in the pills selected for treatment. A default pill size can be selected as well as other customizable features. Medications can be recorded simultaneously and potential drug interactions are highlighted. Dates on which the patient should return to the clinic are automatically calculated for review. The patient is provided with a hardcopy of the visit as well as the recommended warfarin dose schedule. The user is provided with several customizable options for recording pertinent visit data.
Owner:STANDING STONE
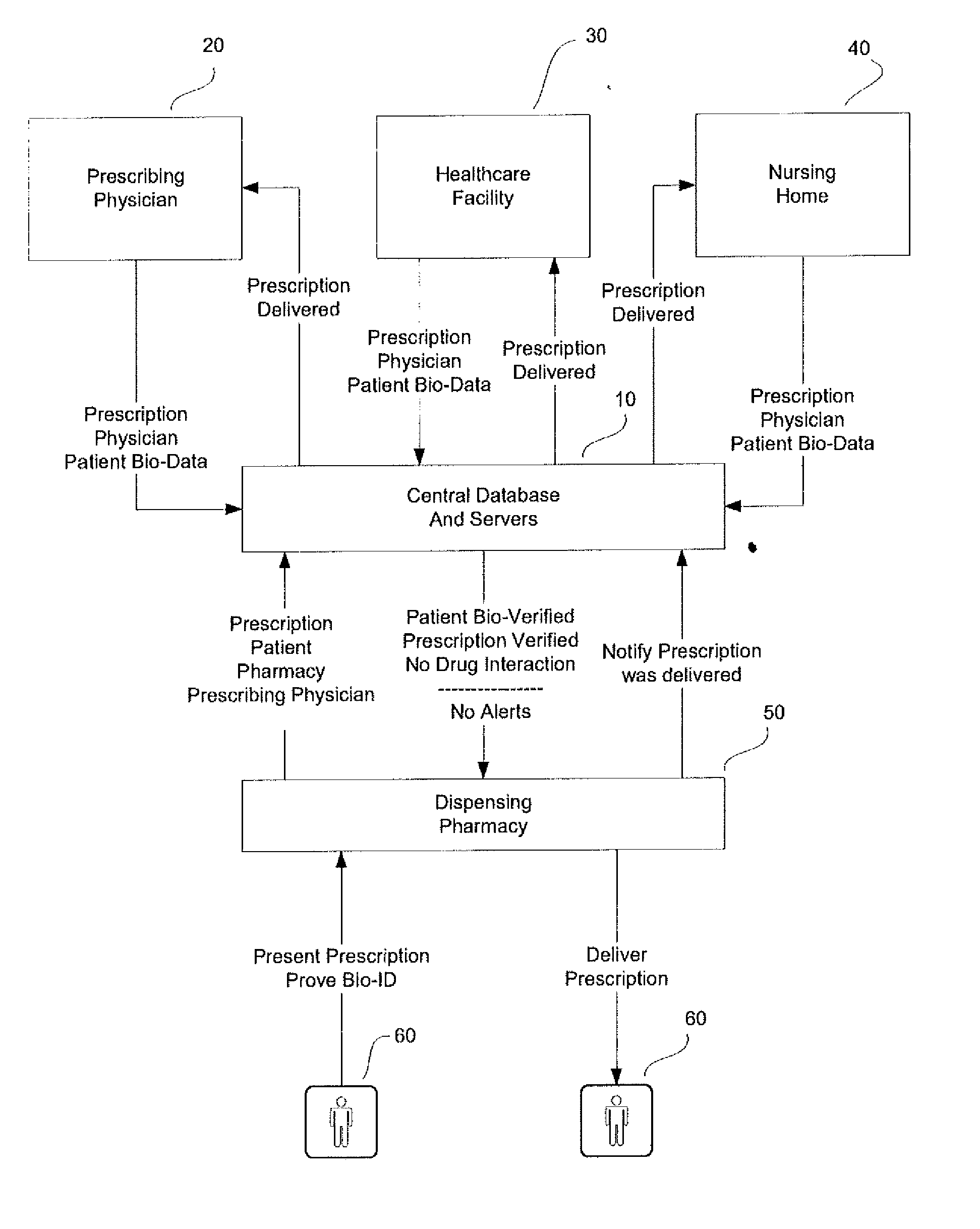
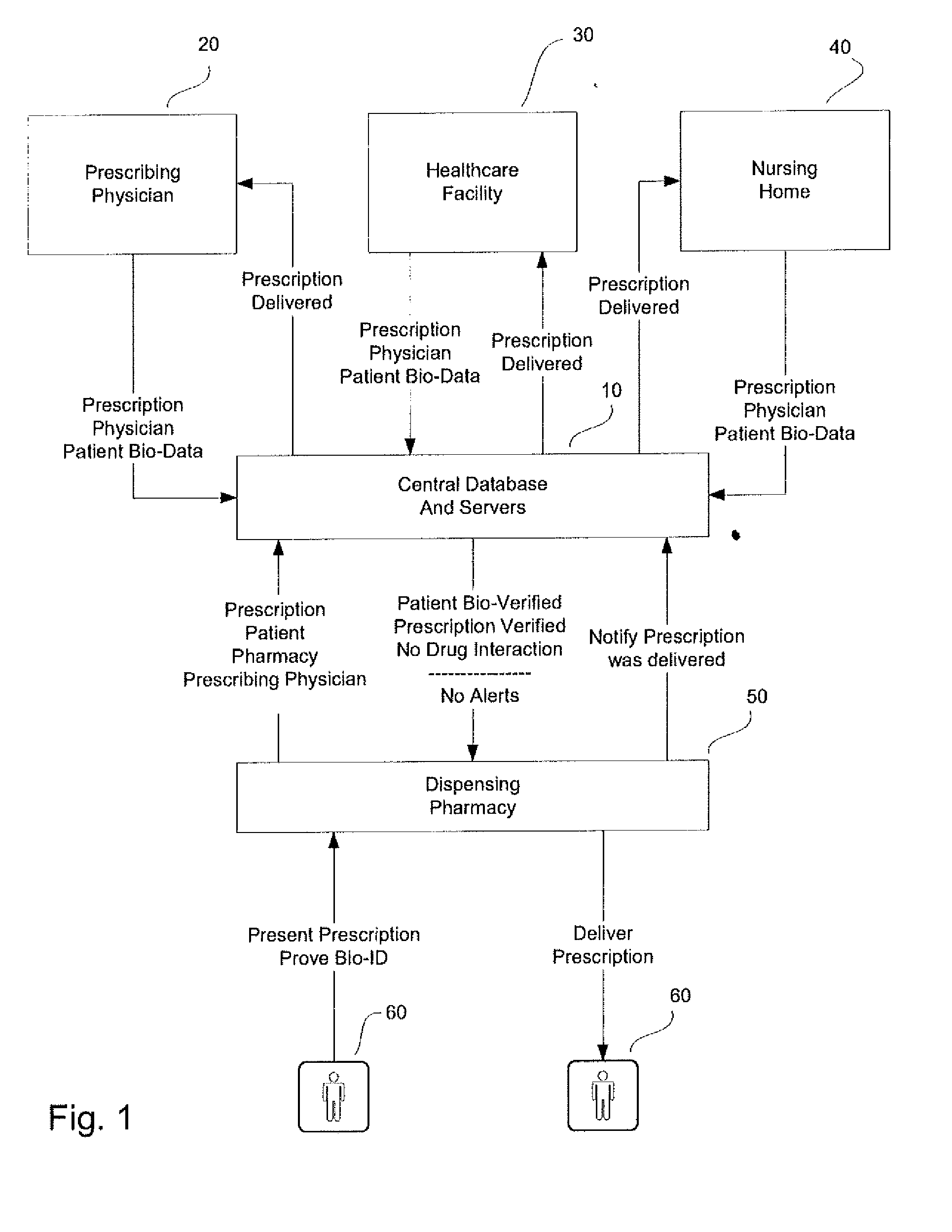
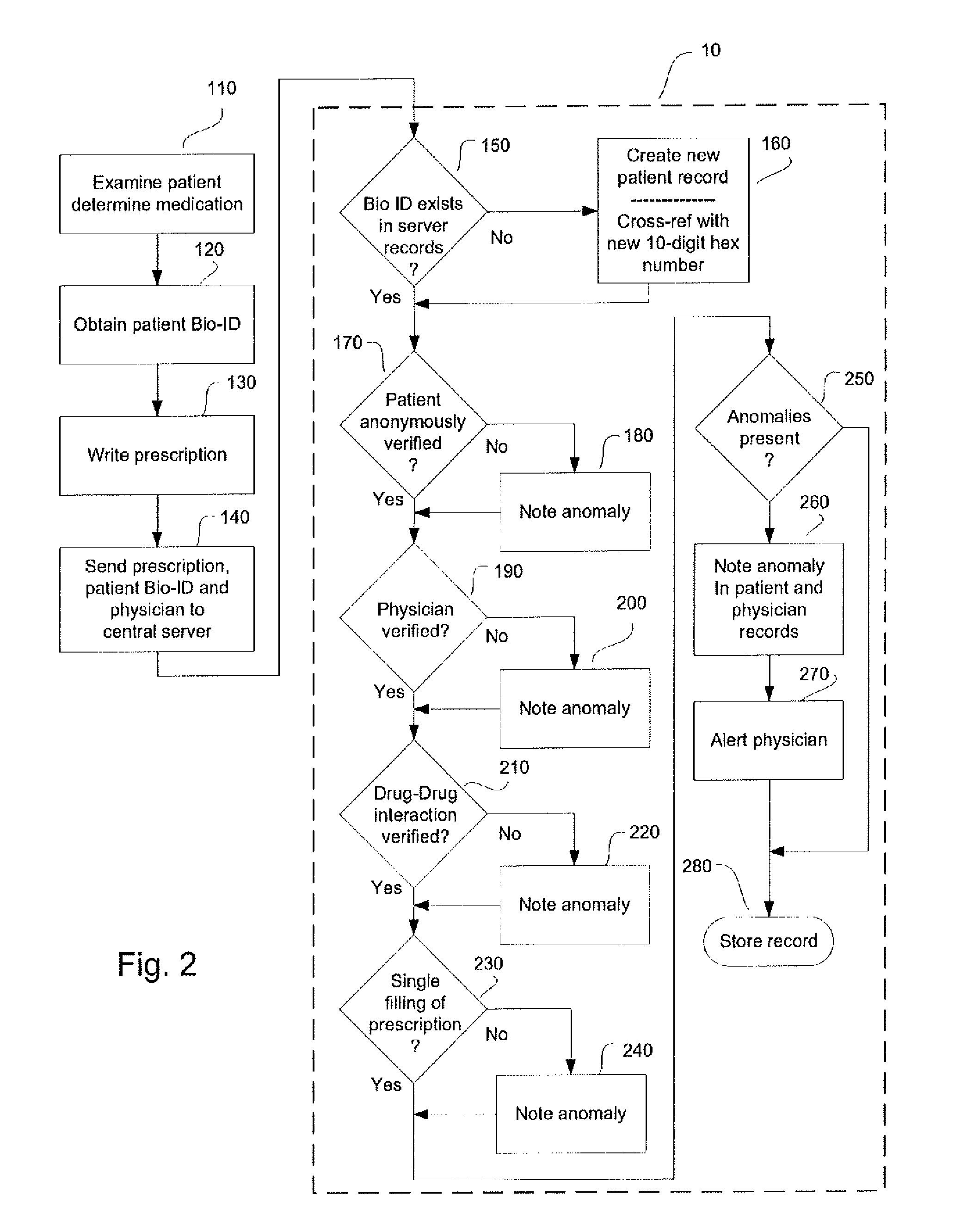
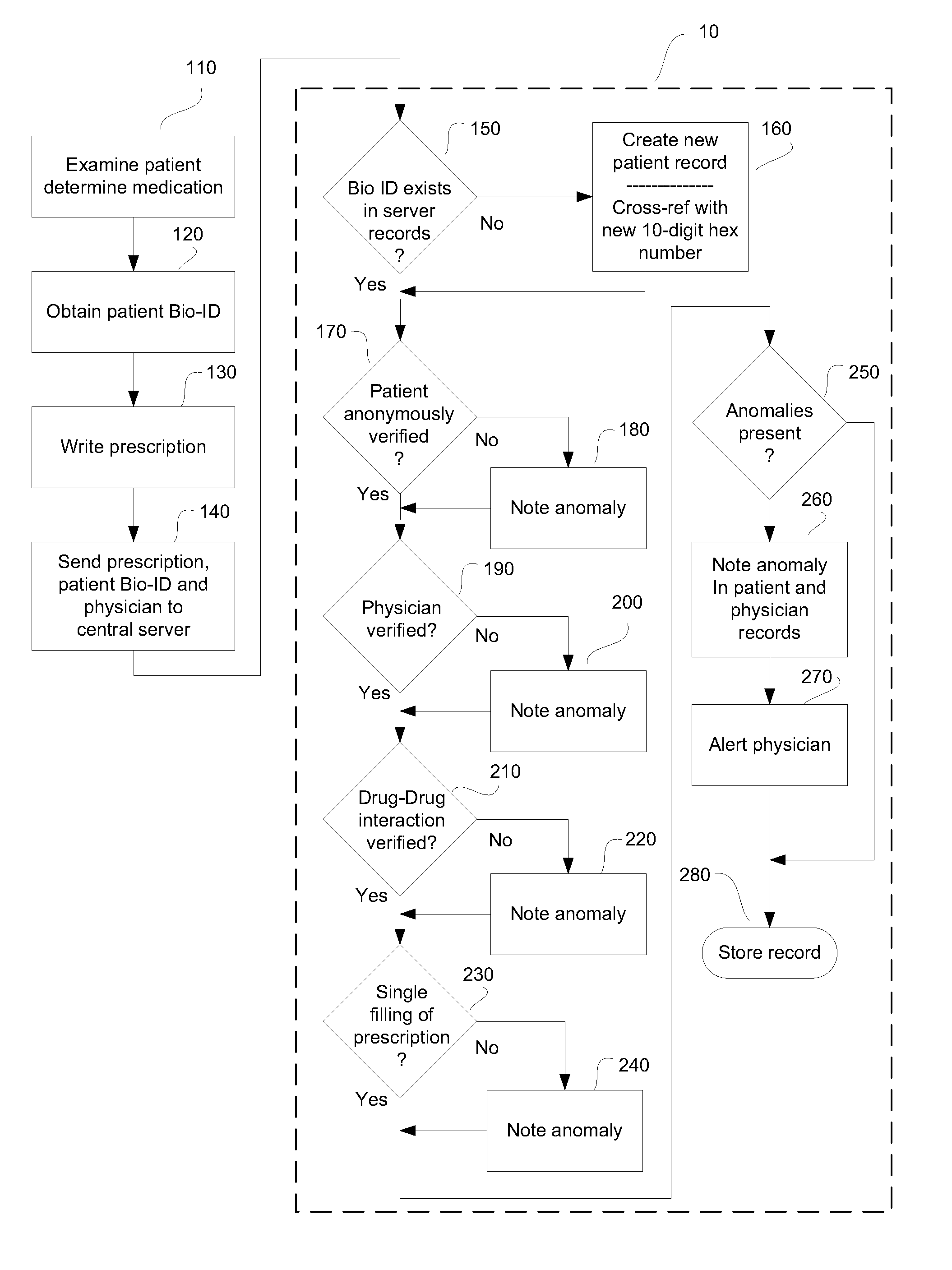
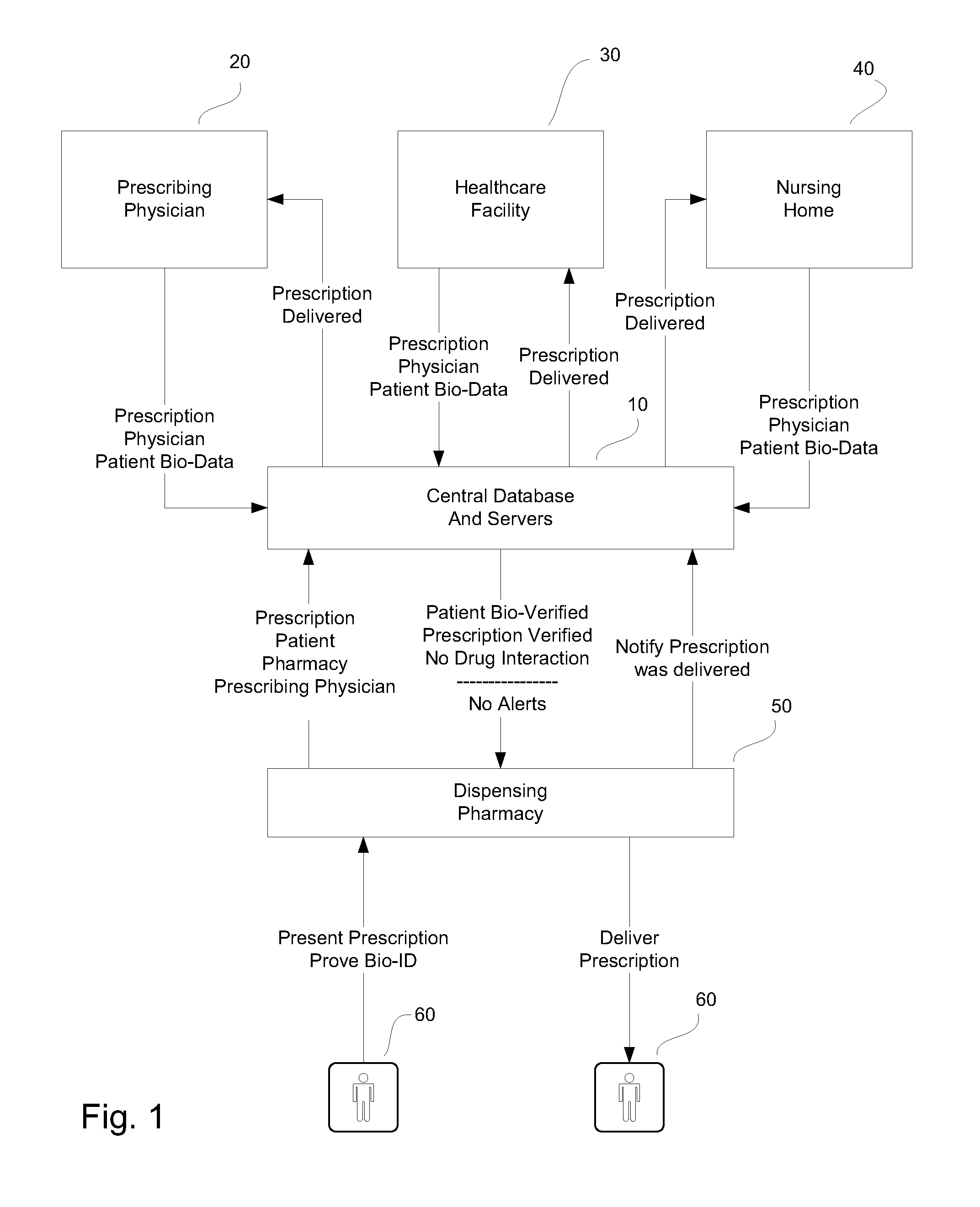
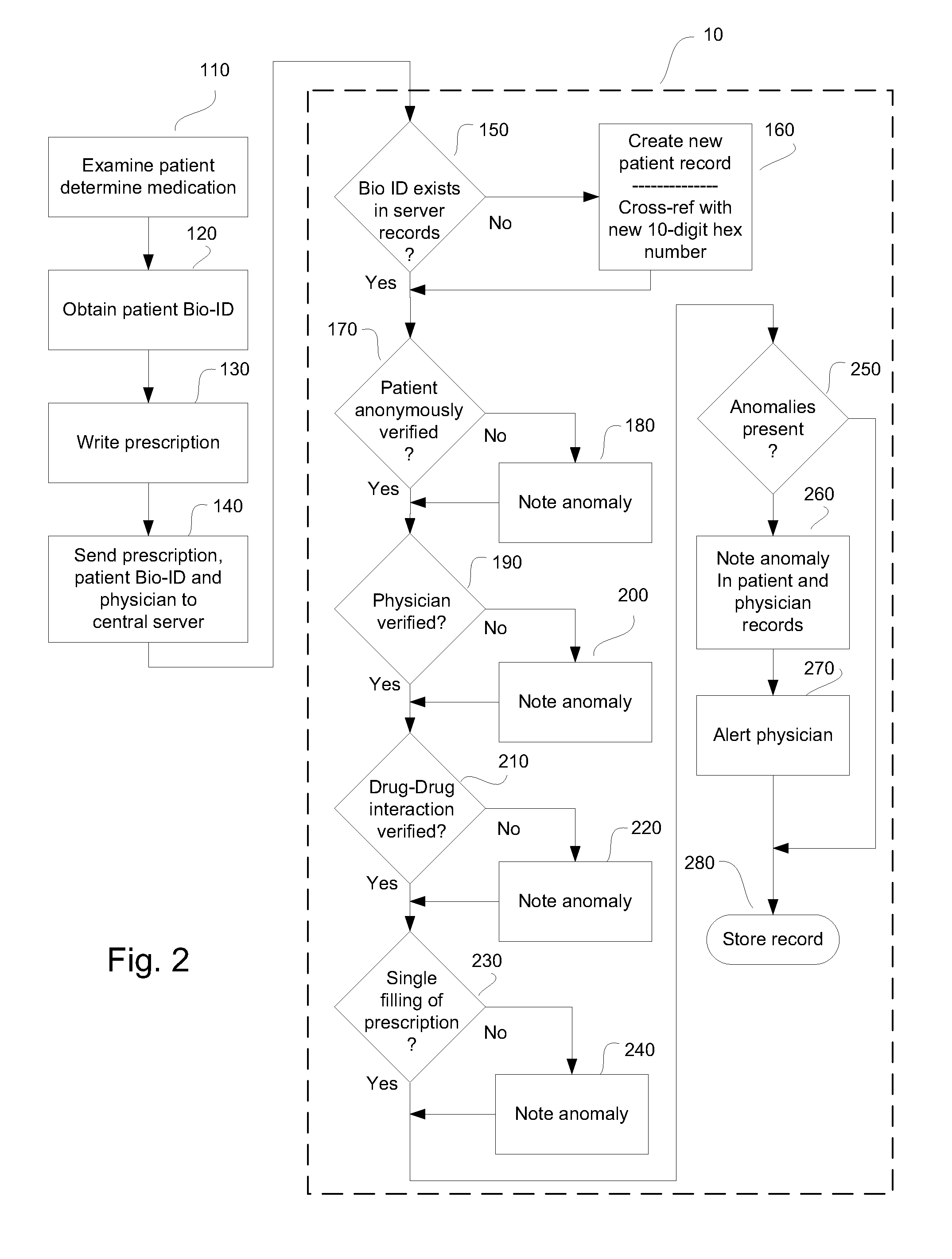
System and method for monitoring medication prescriptions using biometric identification and verification
InactiveUS20100299158A1Quick measurementEasily verifiable biometricDrug and medicationsResourcesBiometric dataDrug interaction
This invention is a system and method for a matching system that cross-references patients anonymously with doctors and pharmacies. The invention uses patient-unique biometrics, such as fingerprinting, retinal scanning, or another such unique identifier, for patient identification and verification without revealing a patient's name or other personal information. The system tracks patient prescriptions and produces alerts to indicate potential problems, such as drug interactions or possible fraudulent behavior. The system utilizes the biometric data of the prescribing physician as authorization of the prescription. The system can produce a traceable paper trail, for investigation or prosecution, and protects the medical industry, patients, and the public from the consequences of doctor shopping.
Owner:BIO TECH MEDICAL SOFTWARE
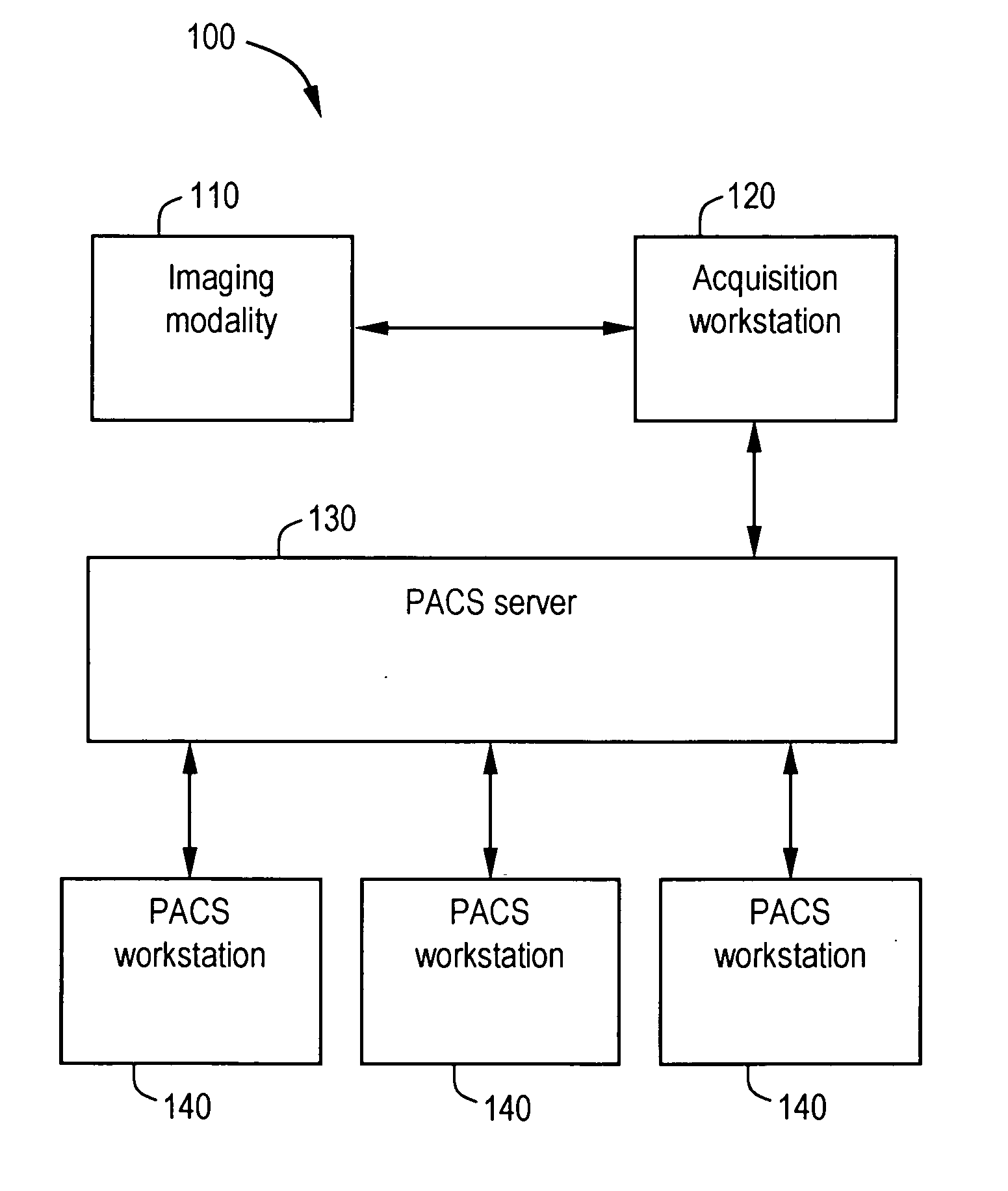
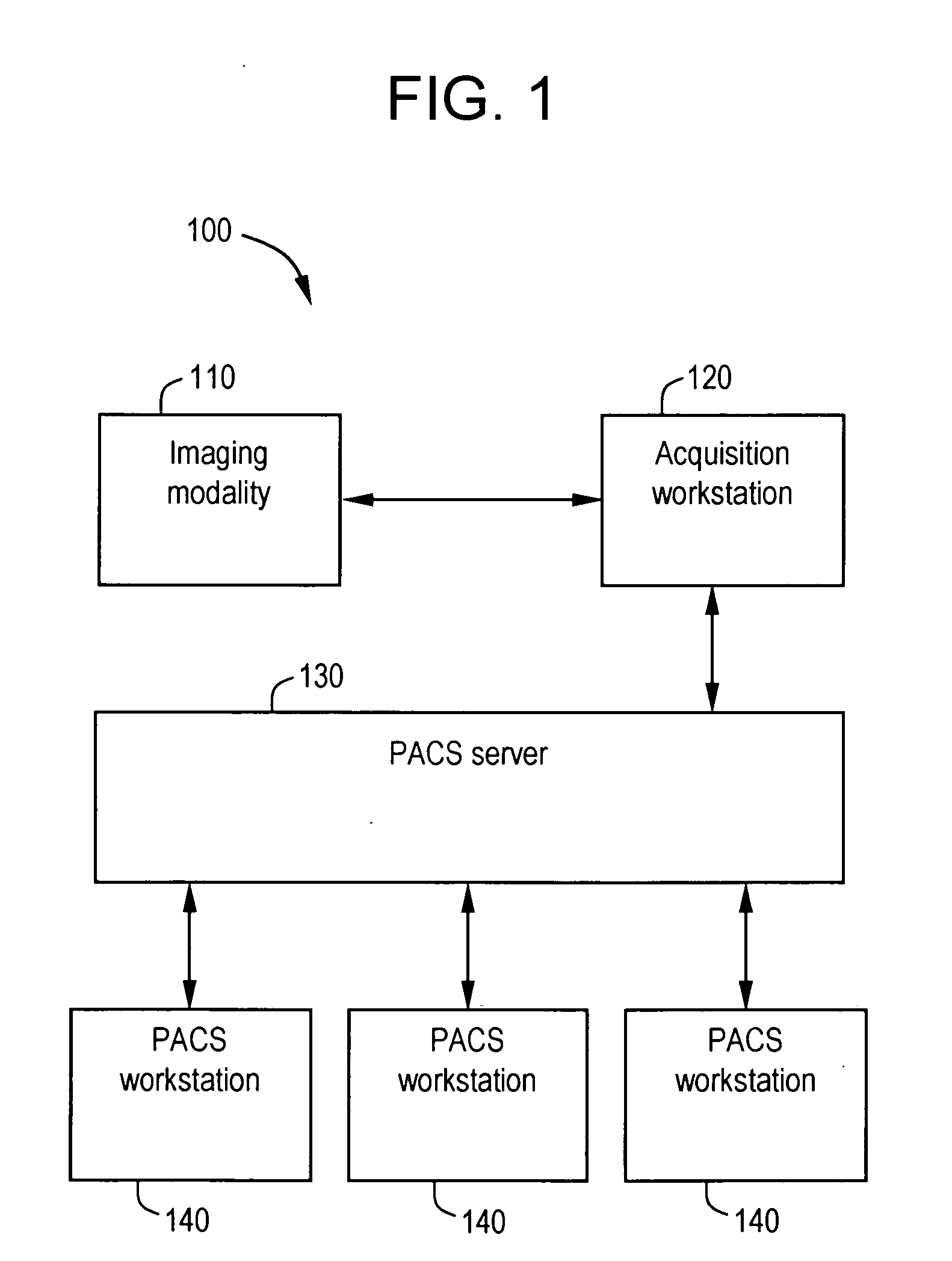
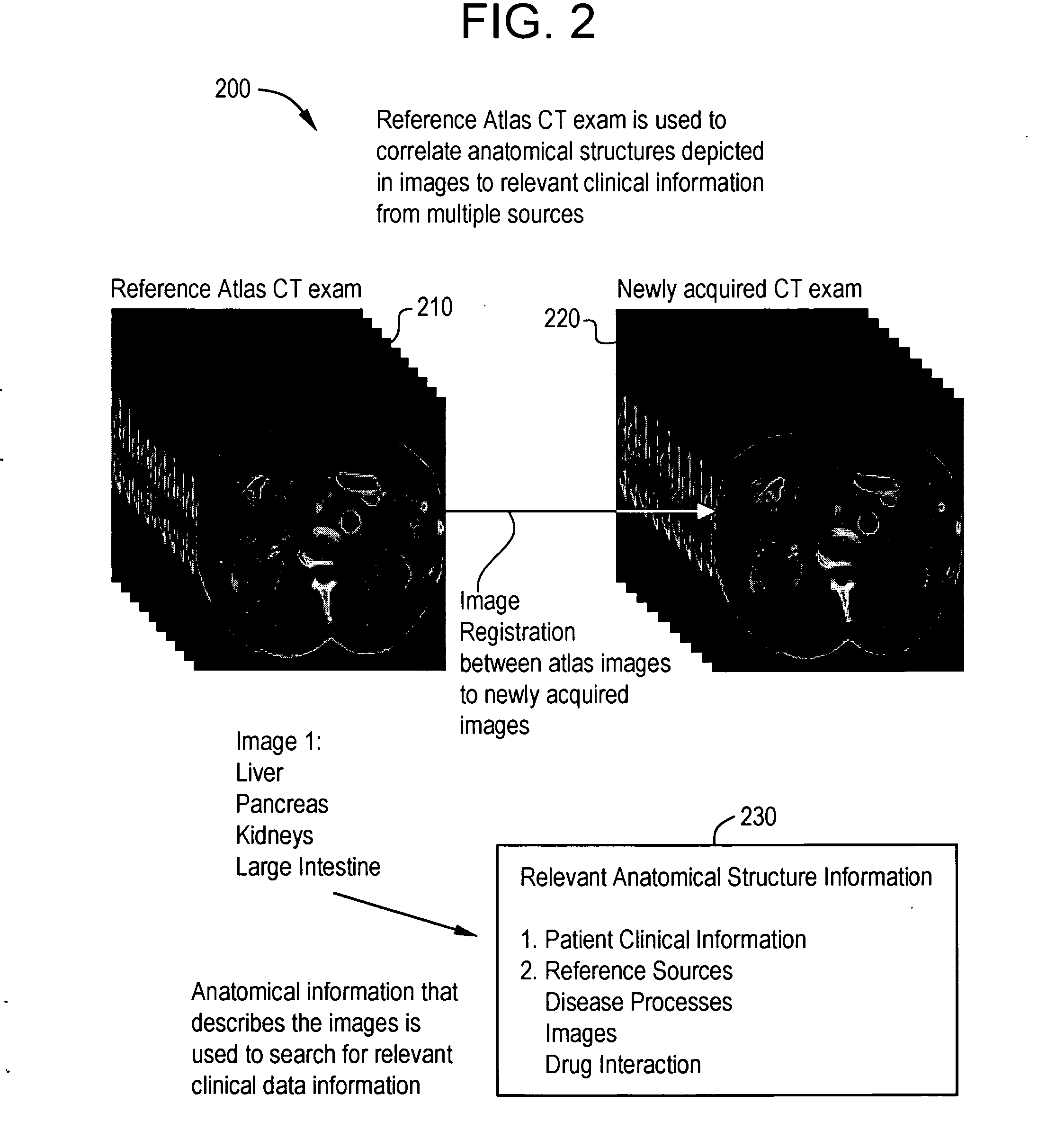
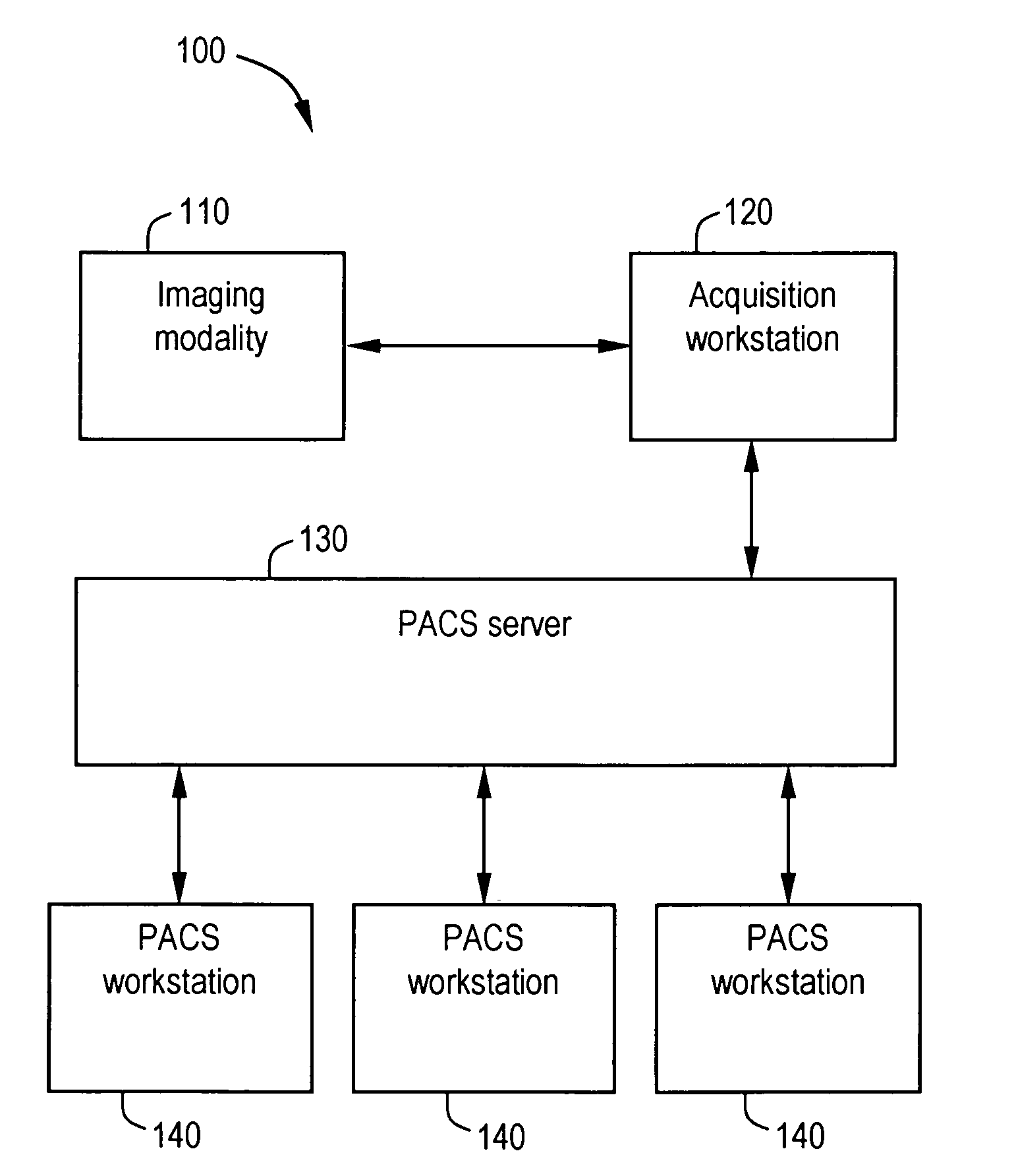
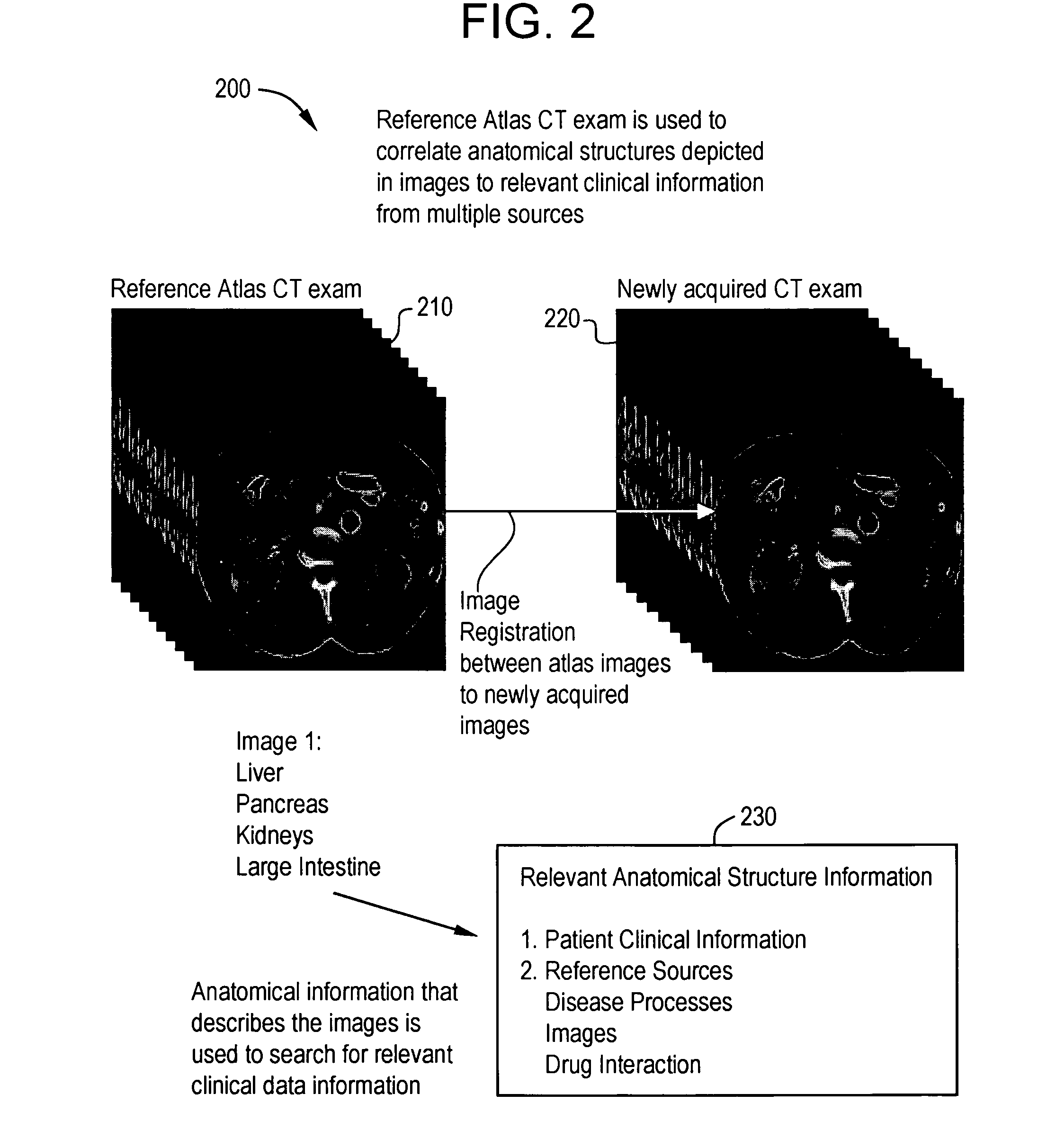
System and method for anatomy labeling on a PACS
ActiveUS20070127790A1Narrow selectionImage enhancementImage analysisClinical informationDrug interaction
Certain embodiments of the present invention provide a system and method for image registration and display of relevant information. The method includes identifying one or more anatomical parts in an acquired image, mapping the acquired image to a reference image based on the one or more anatomical parts, storing anatomy information in relation to the acquired image, and displaying the acquired image based on the anatomy information. The method may also include controlling the displaying of the acquired image based on a voice command related to the anatomy information. Anatomy information may be displayed with the acquired image. Anatomy information may include clinical information, reference information, disease process information, a related image, and / or drug interaction information, for example. The acquired image may be displayed according to a display setting, such as a window level setting and / or other display setting, based on the anatomy information.
Owner:GENERAL ELECTRIC CO
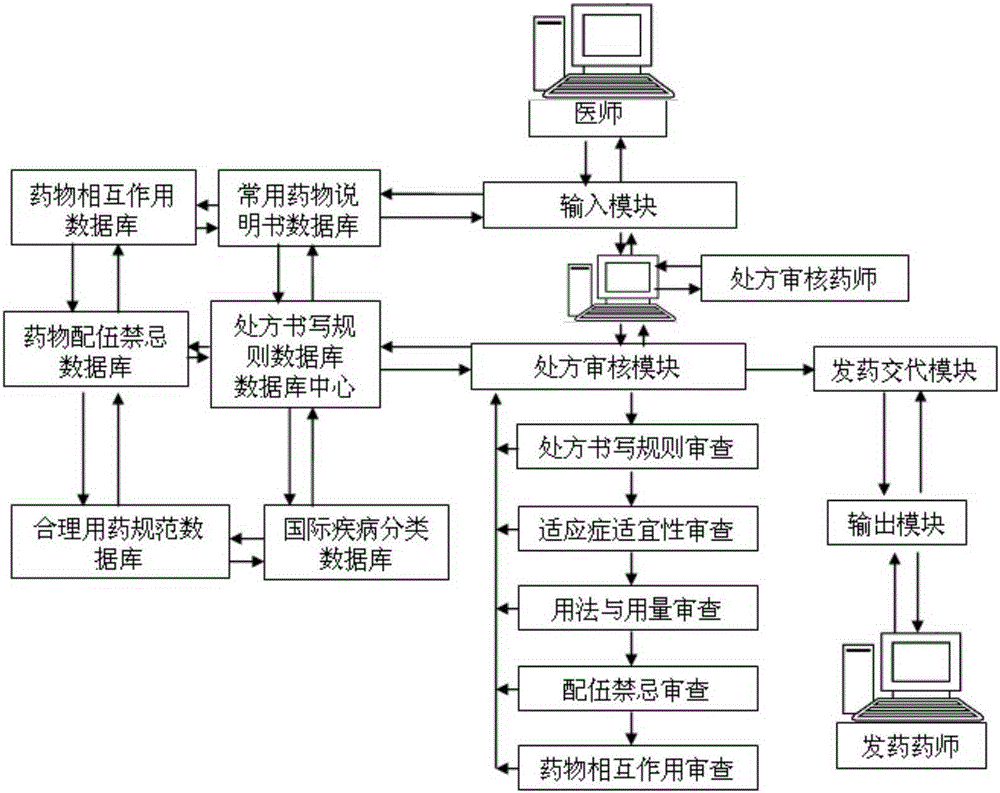
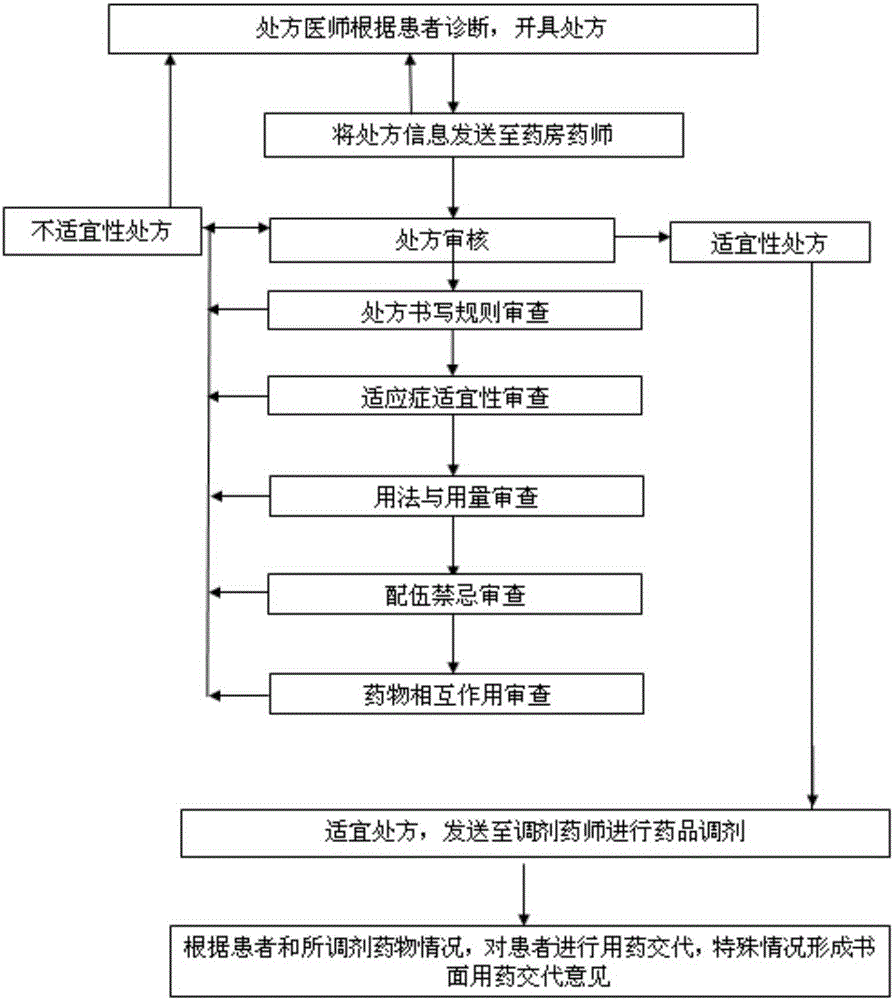
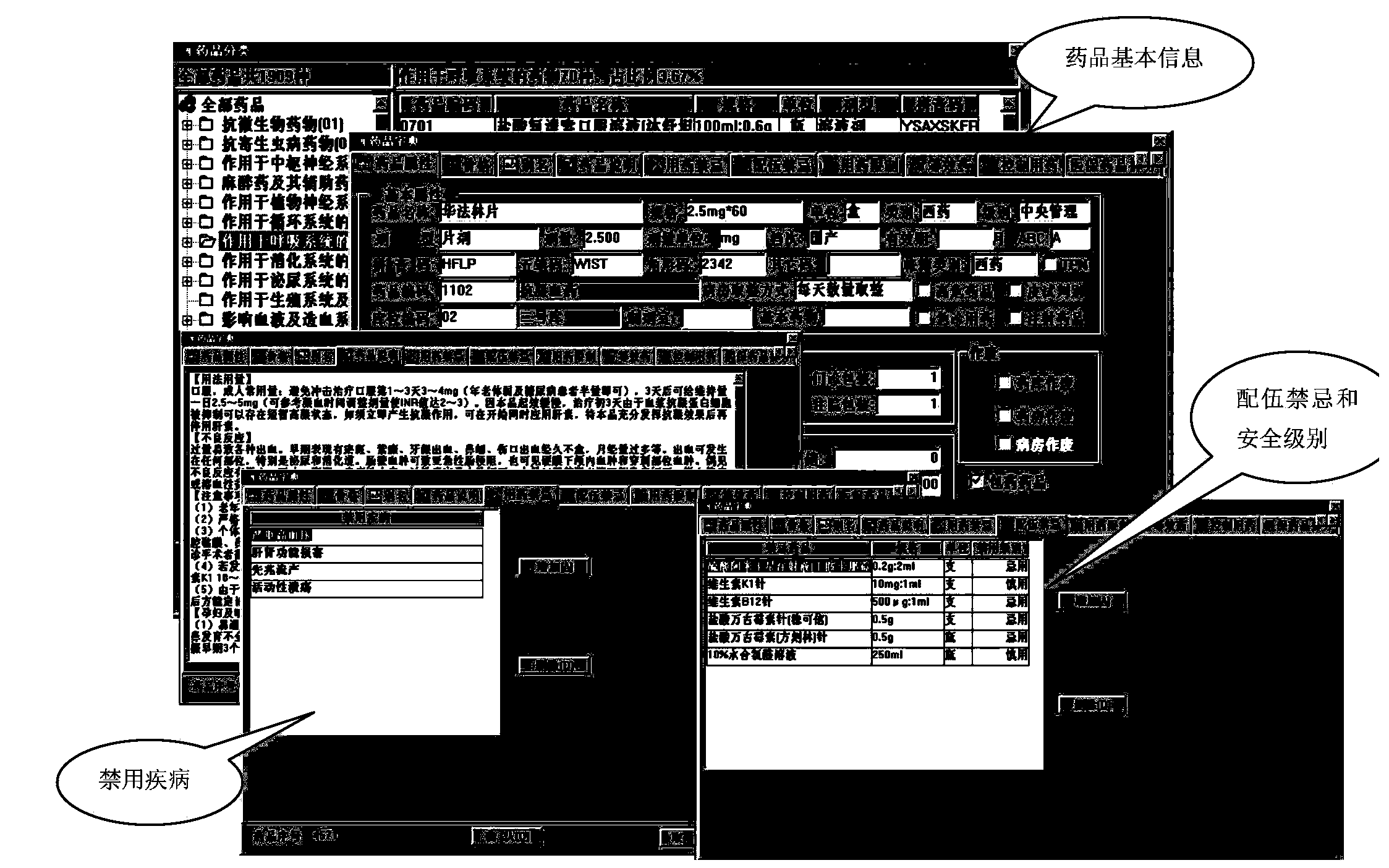
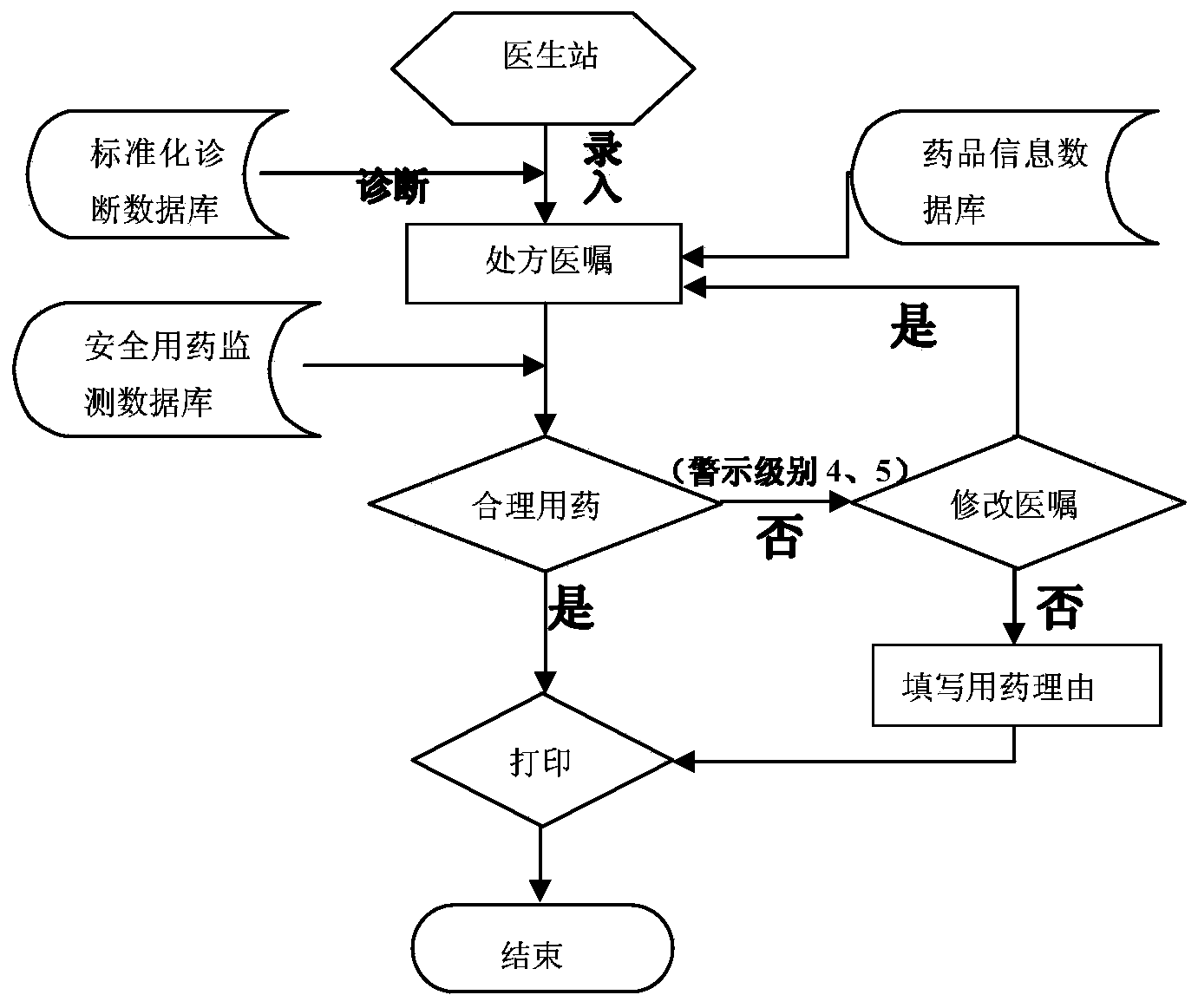
Intelligent prescription auditing system and method
InactiveCN105224794ARegulate Prescribing BehaviorNormative behaviorSpecial data processing applicationsDrug interactionControl system
The invention provides an intelligent prescription auditing system and an intelligent prescription auditing method. The system is provided with an input module, various databases, a prescription auditing module, a dispensing explaining module, and an output module. The method is implemented as follows: a doctor refines a prescription through the input module and then sends the prescription to the prescription auditing module; after receiving the prescription, the pharmacist reviews writing rules, indications, dosage, incompatibility and drug interactions of the prescription according to various databases; the doctor returns checking comments of the disqualified prescription to the prescription input module so as to modify or re-issue the prescription or give suggestions, and sends the qualified prescription to the dispensing explaining module; after receiving the prescription, an allocation doctor allocates medicines; after receiving the prescription, a dispensing doctor generates prescription information, a medication explaining matter and the like of dispensing explaining, which are printed by the output module and then explained to a patient. The invention provides a medicine dispensing control system and a medicine dispensing control method which are capable of effectively avoiding great prescription, irregular prescription, unsuitable medication for the prescription and unconventional prescription.
Owner:石庆平
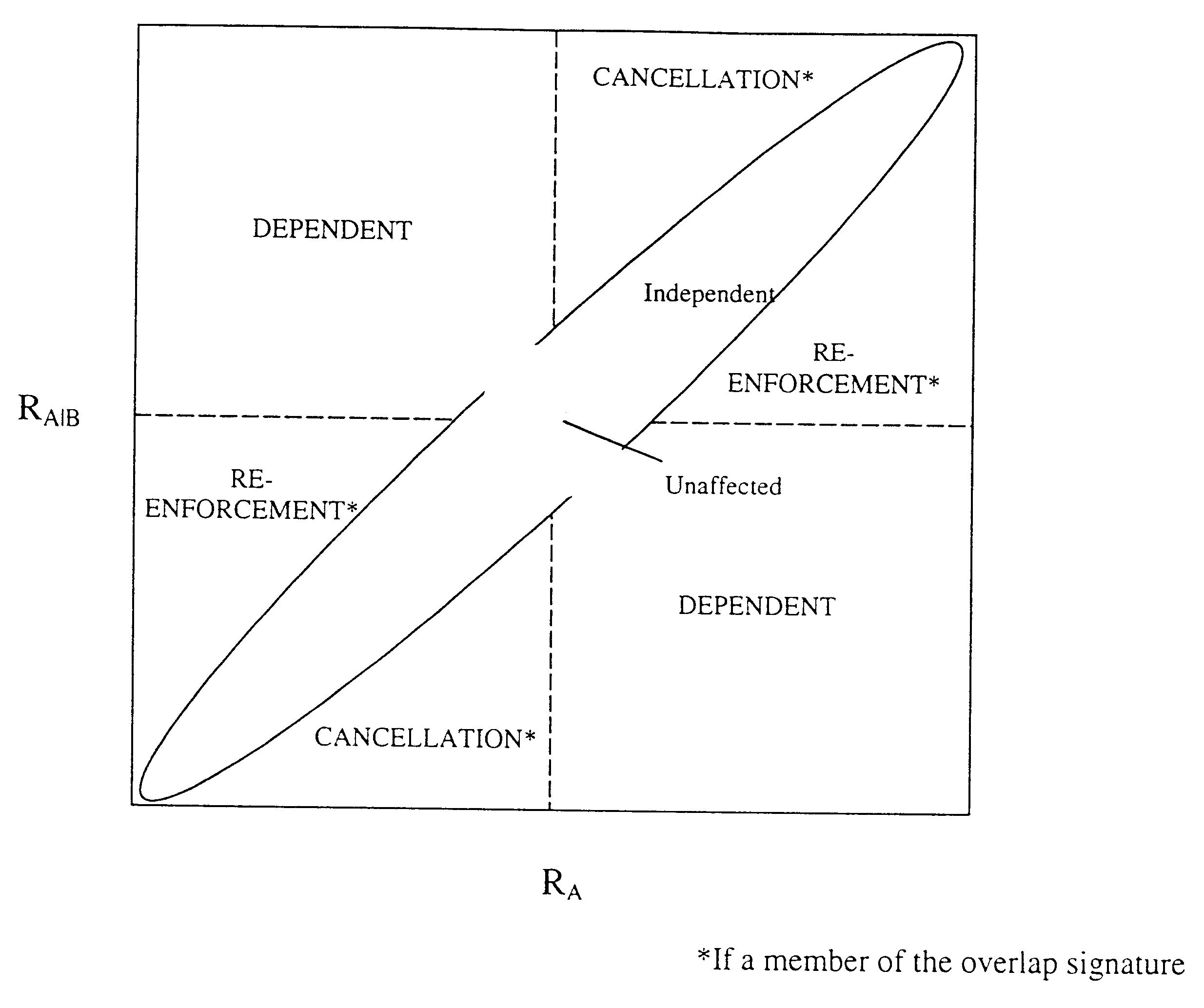
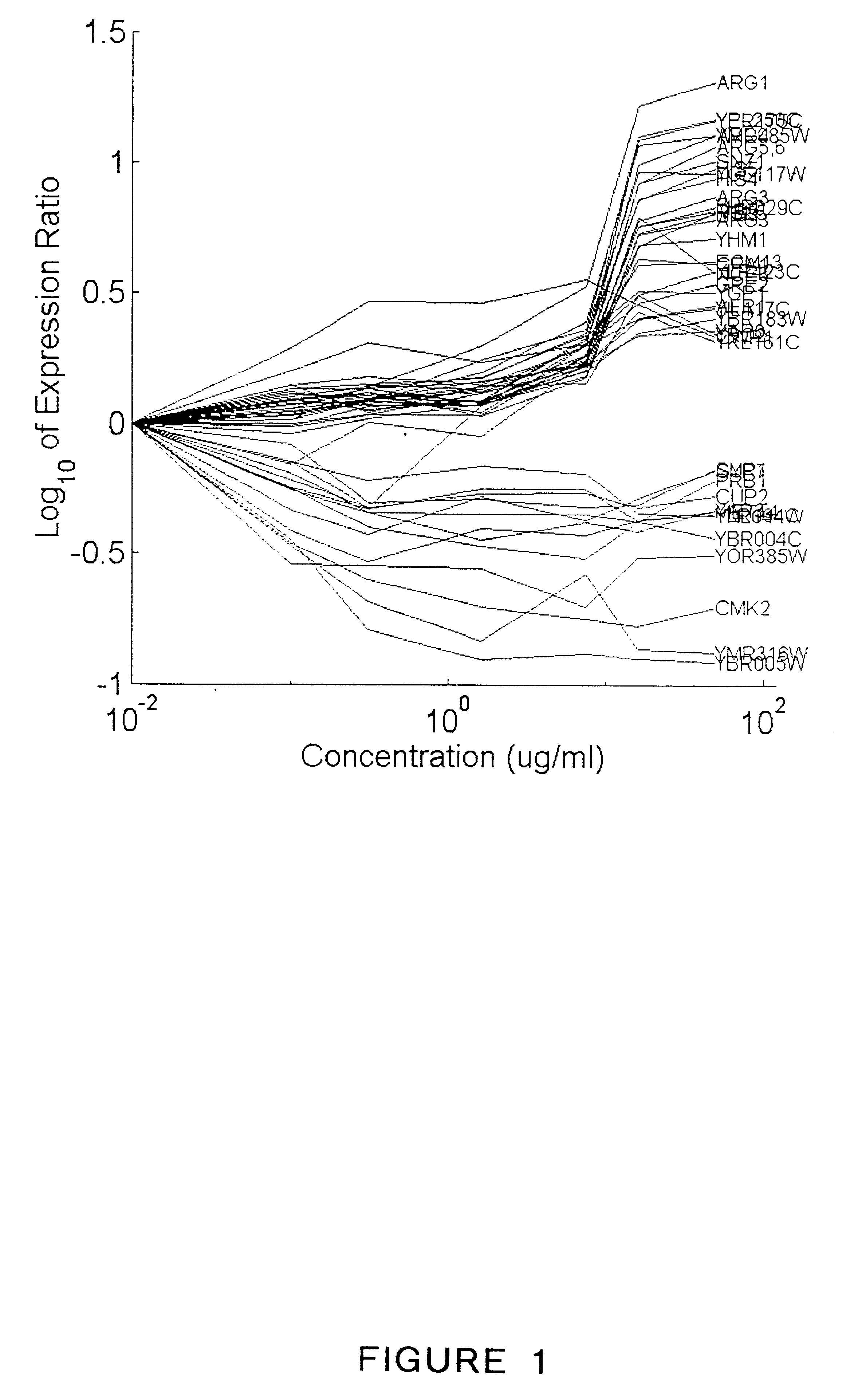
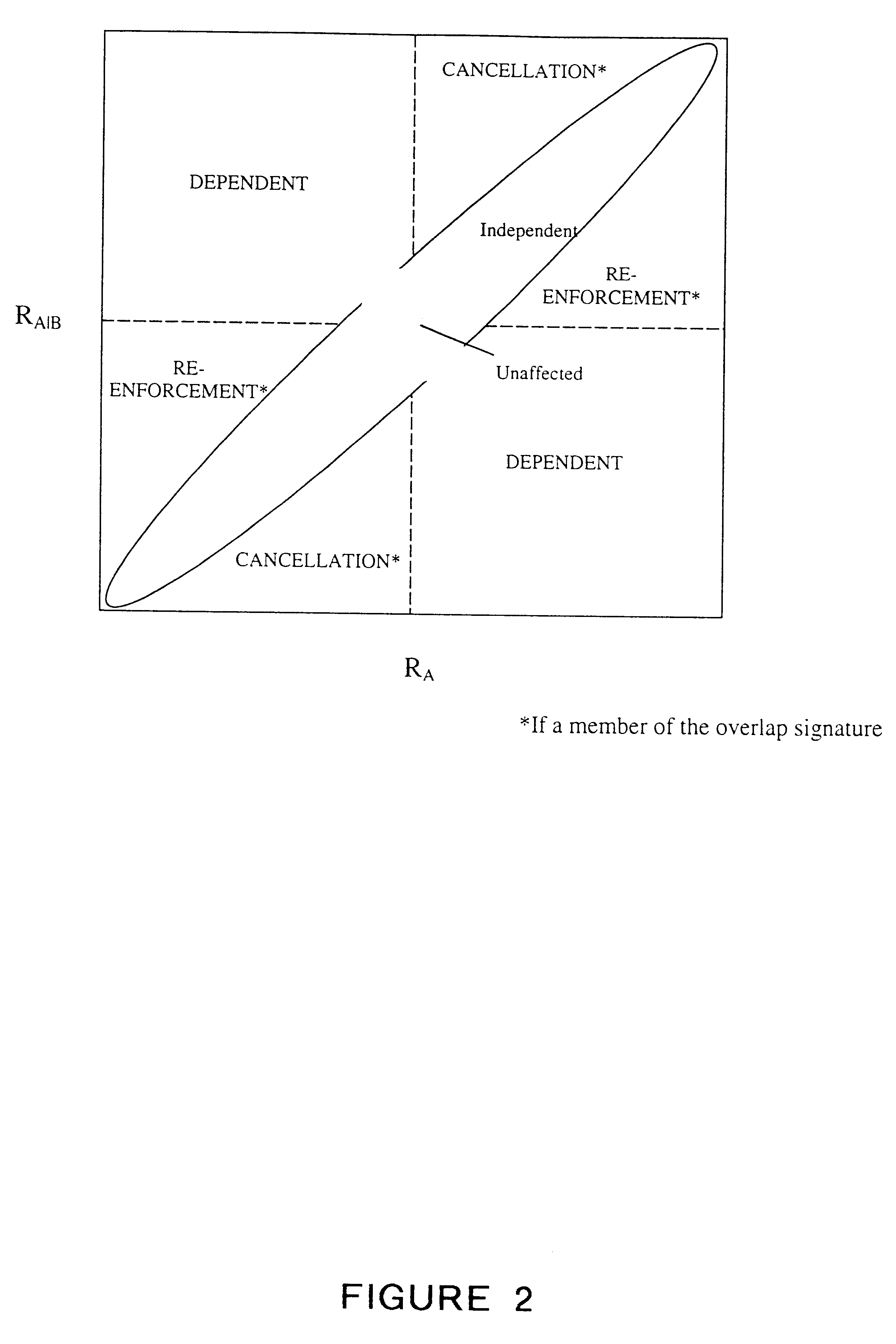
Methods for drug interaction prediction using biological response profiles
This invention provides methods for detecting and predicting drug interaction. In one embodiment of the invention, a plurality of cellular constituents in a biological sample is monitored as the sample is subject to various drug treatments. The response of the cellular constituents are analyzed to detect interactions. This method is particularly useful for predicting drug interaction using a model organism. It is also useful for analyzing interaction between any perturbations to a biological system.
Owner:MICROSOFT TECH LICENSING LLC
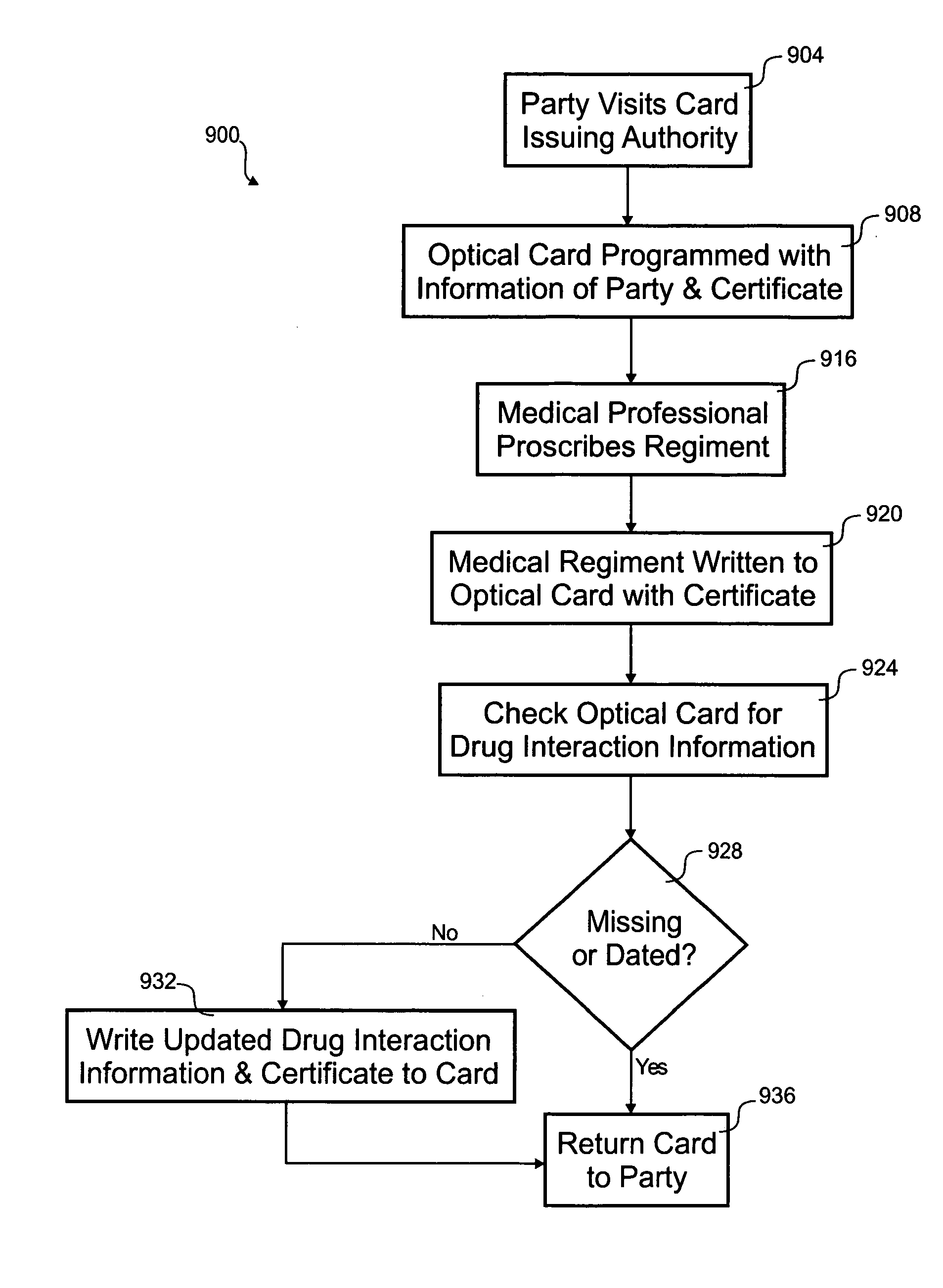
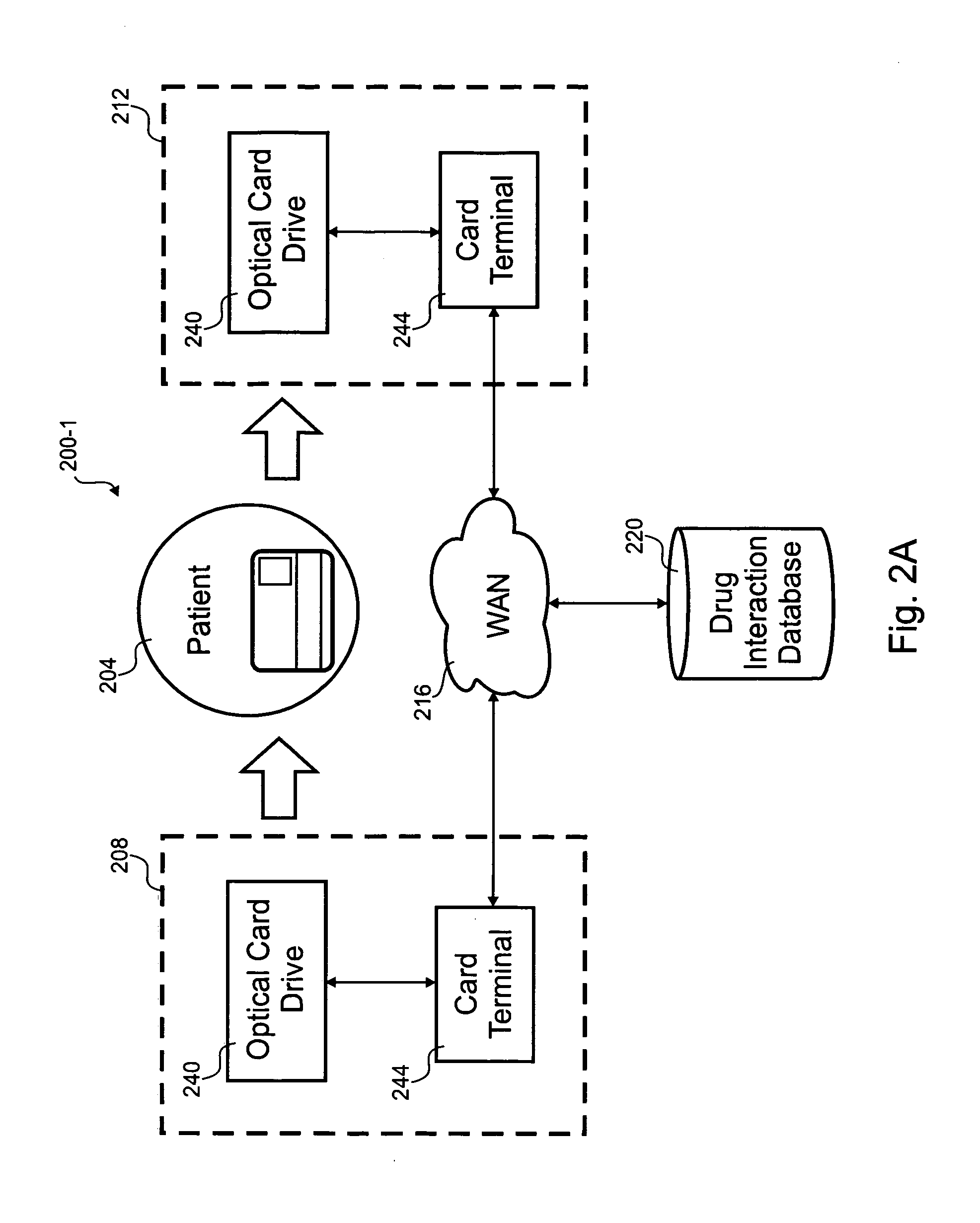
Optical prescription card
According to the invention, a method for distributing drug interaction information for pharmacological compounds is disclosed. In one step, a request is received for a pharmacological compound for administration to a party. A list is received that includes a plurality of other pharmacological compounds associated with the party. A listing of possible drug interactions related to at least one of the pharmacological compound or the plurality of other pharmacological compounds is read from an optical card. It is determined if the pharmacological compound is contraindicated from the listing of possible drug interactions. A subset of the listing of possible drug interactions relevant to use of the pharmacological compound with the plurality of other pharmacological compounds is printed.
Owner:BSI2000
Interactive prescription processing and managing system
InactiveUS20090106313A1Drug and medicationsPatient personal data managementDrug interactionComputer science
An electronic prescription processing system. The system allows users to process prescriptions for multiple patients, access individual patient and medication data over a computer network and to use that data to perform all tasks necessary to fill and refill prescriptions including, checking for drug interactions, alerting the user of alternative medications, and numerous other tasks useful for managing one or more prescriptions for one or multiple patients.
Owner:BOLDYGA RANDY
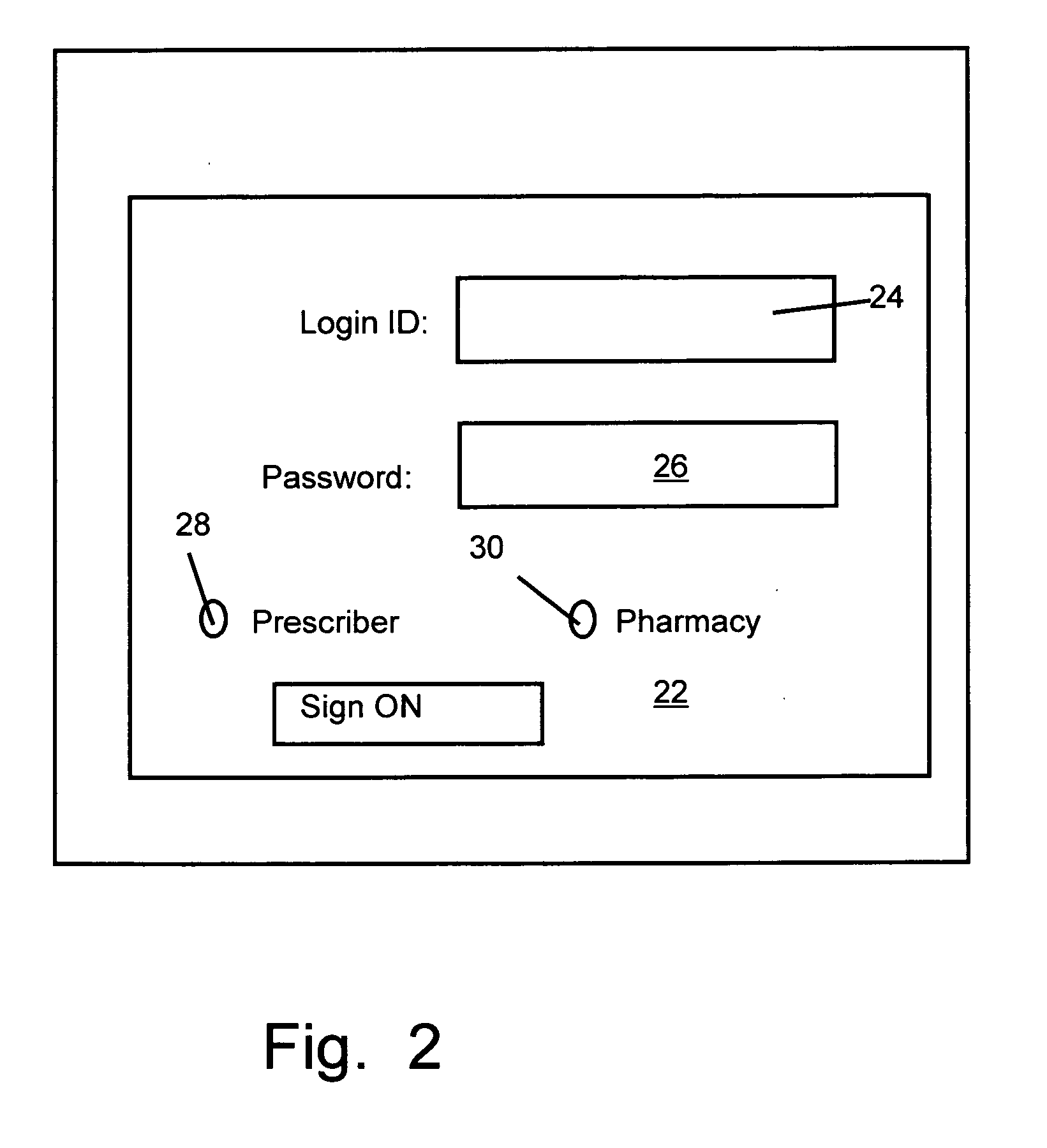
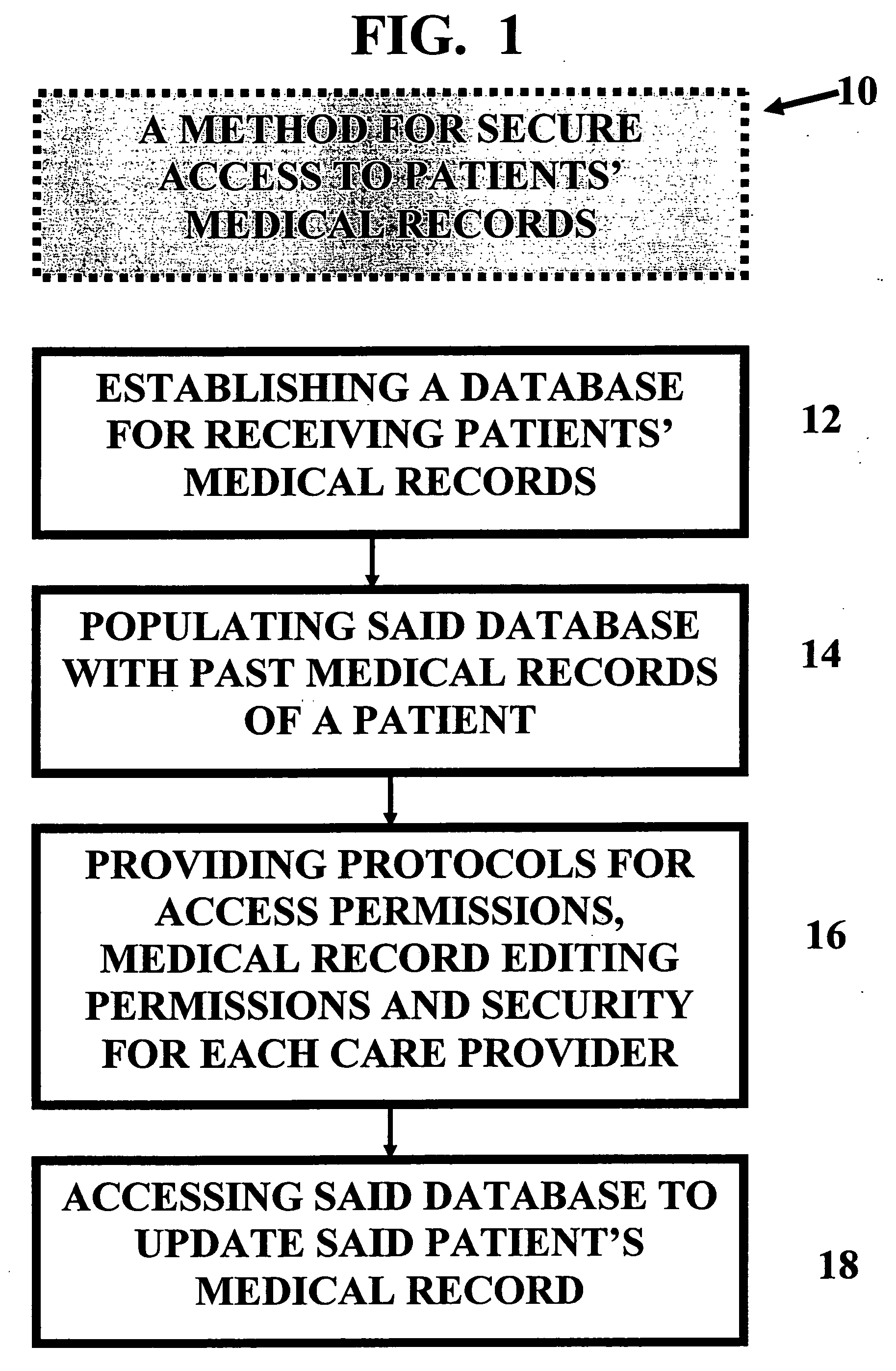
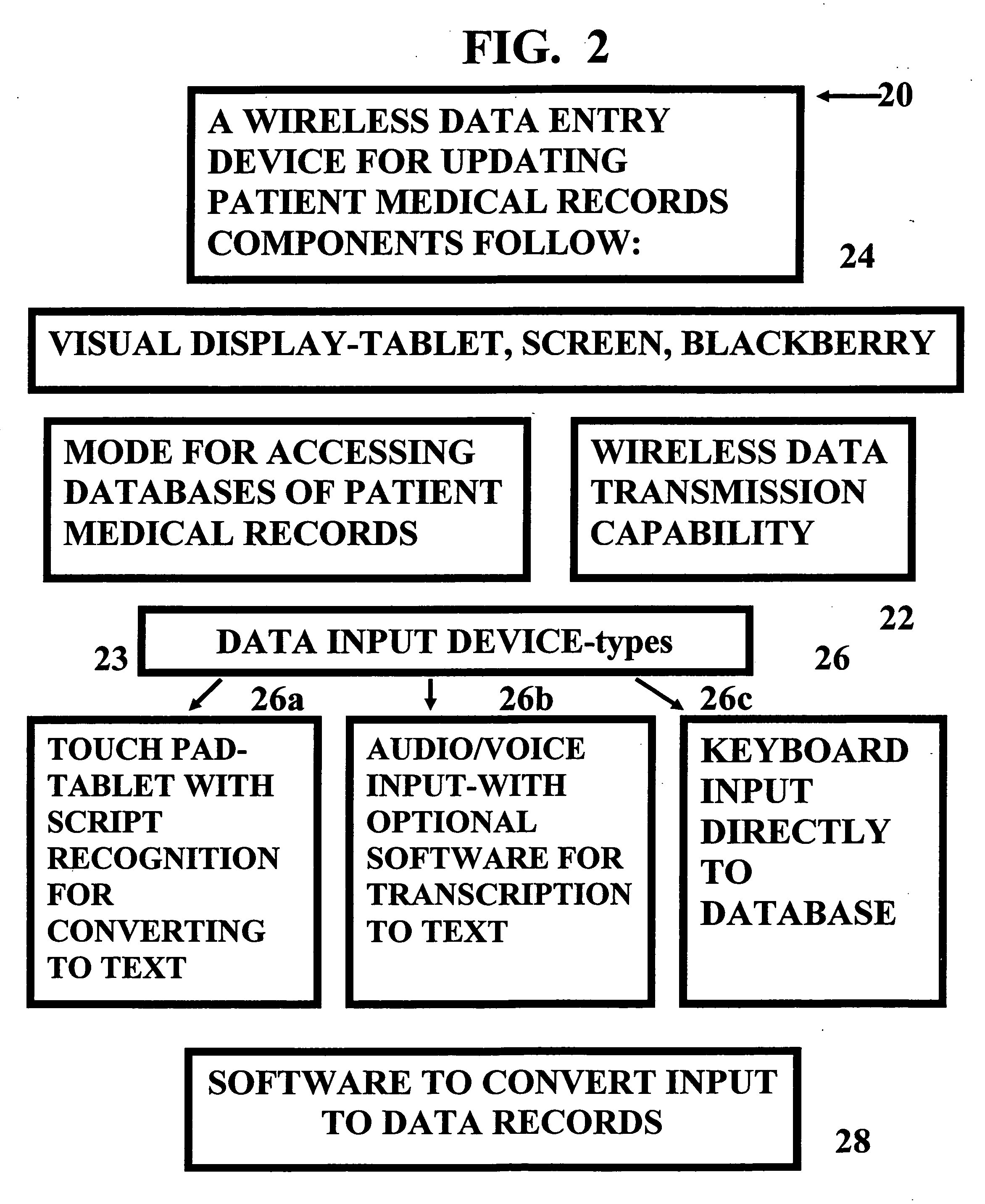
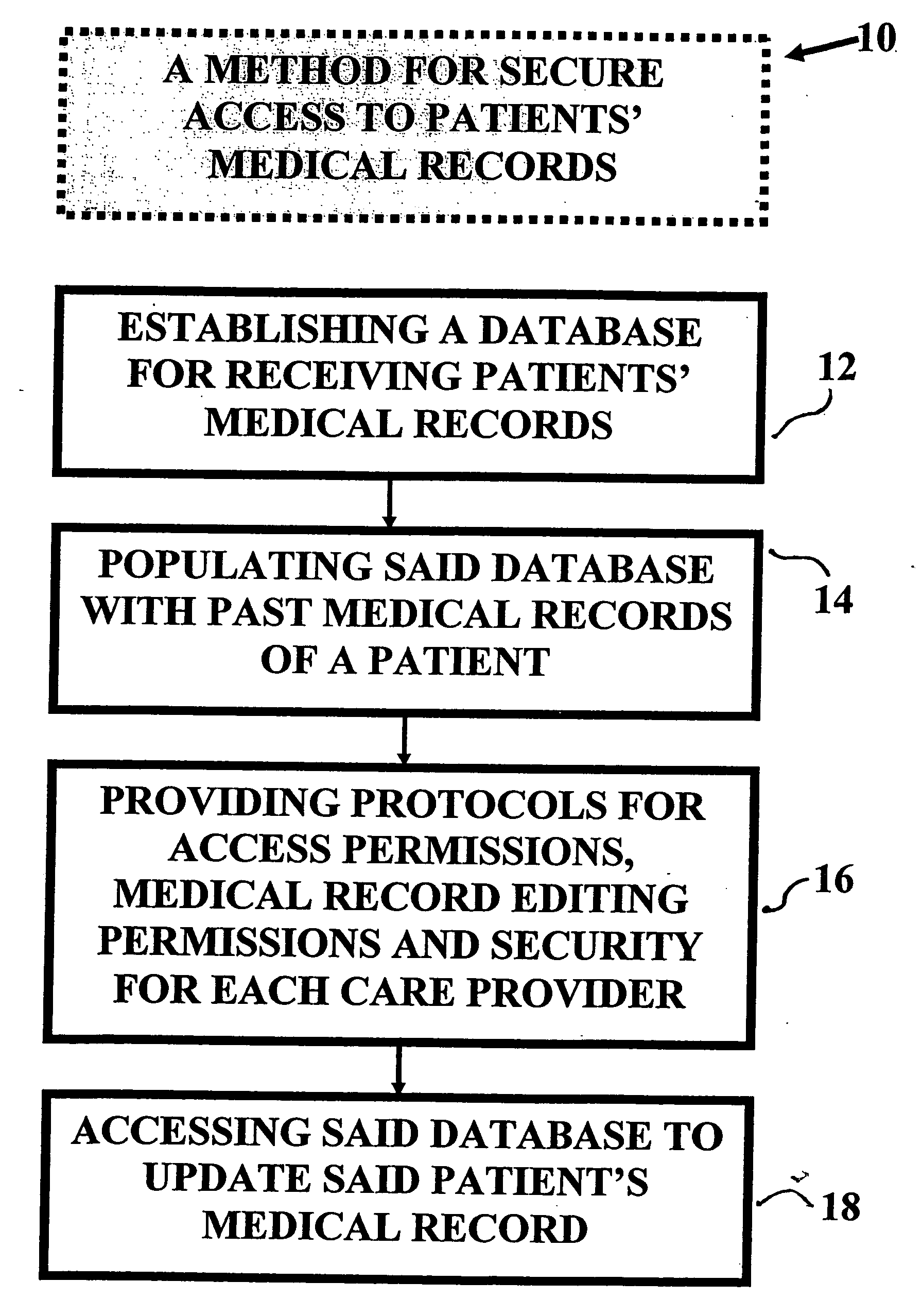
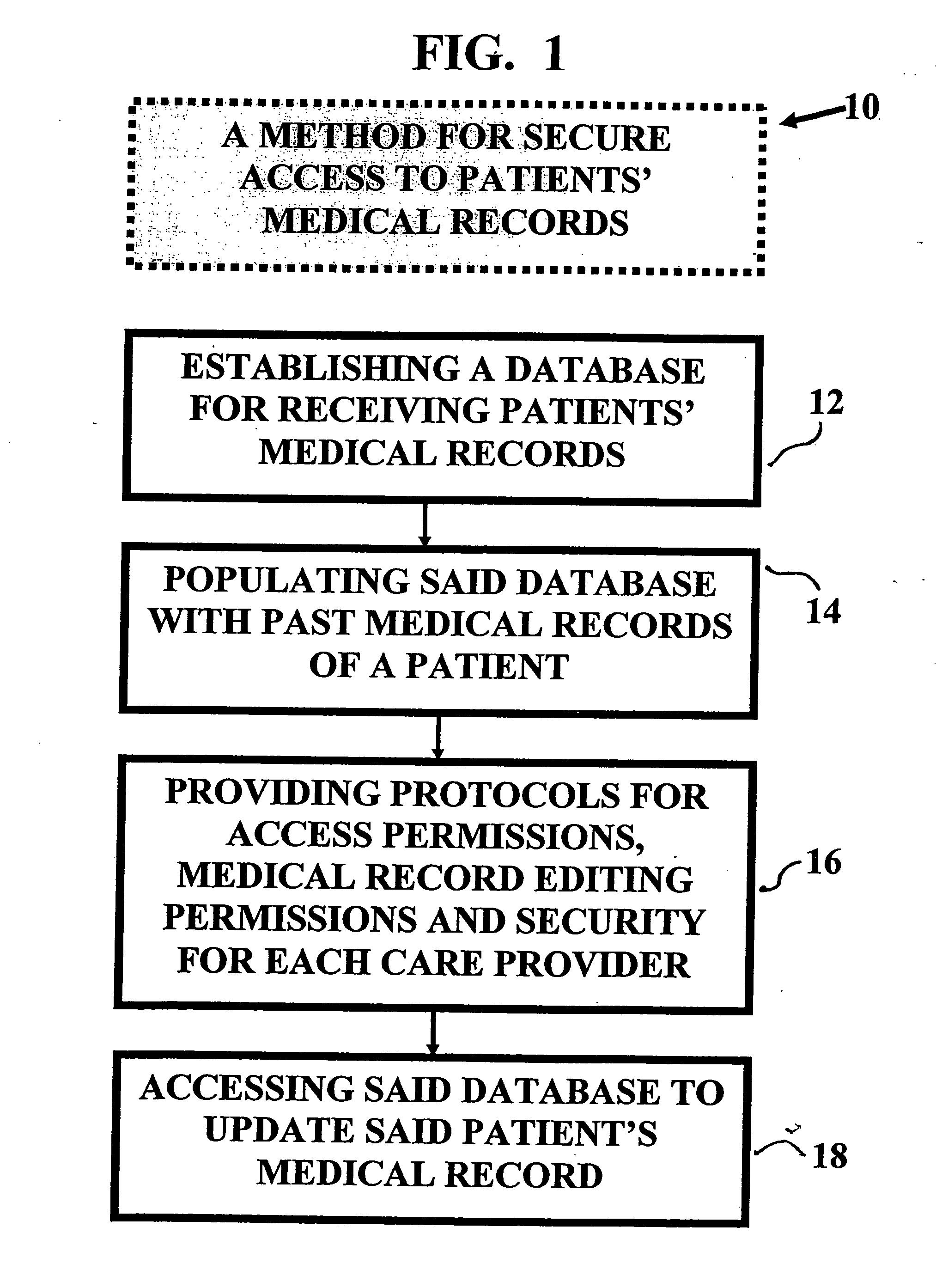
Method and device for maintaining and providing access to electronic clinical records
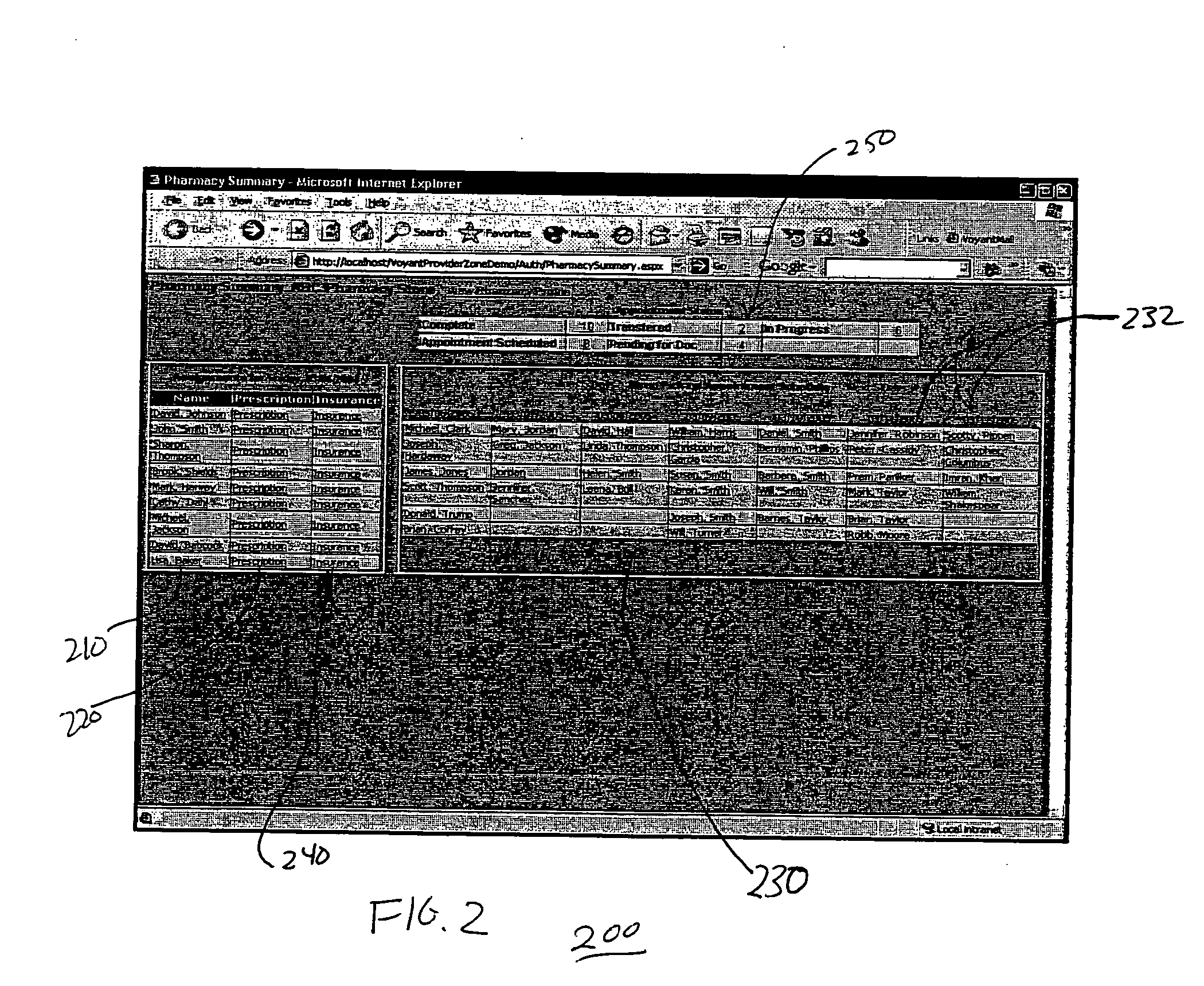
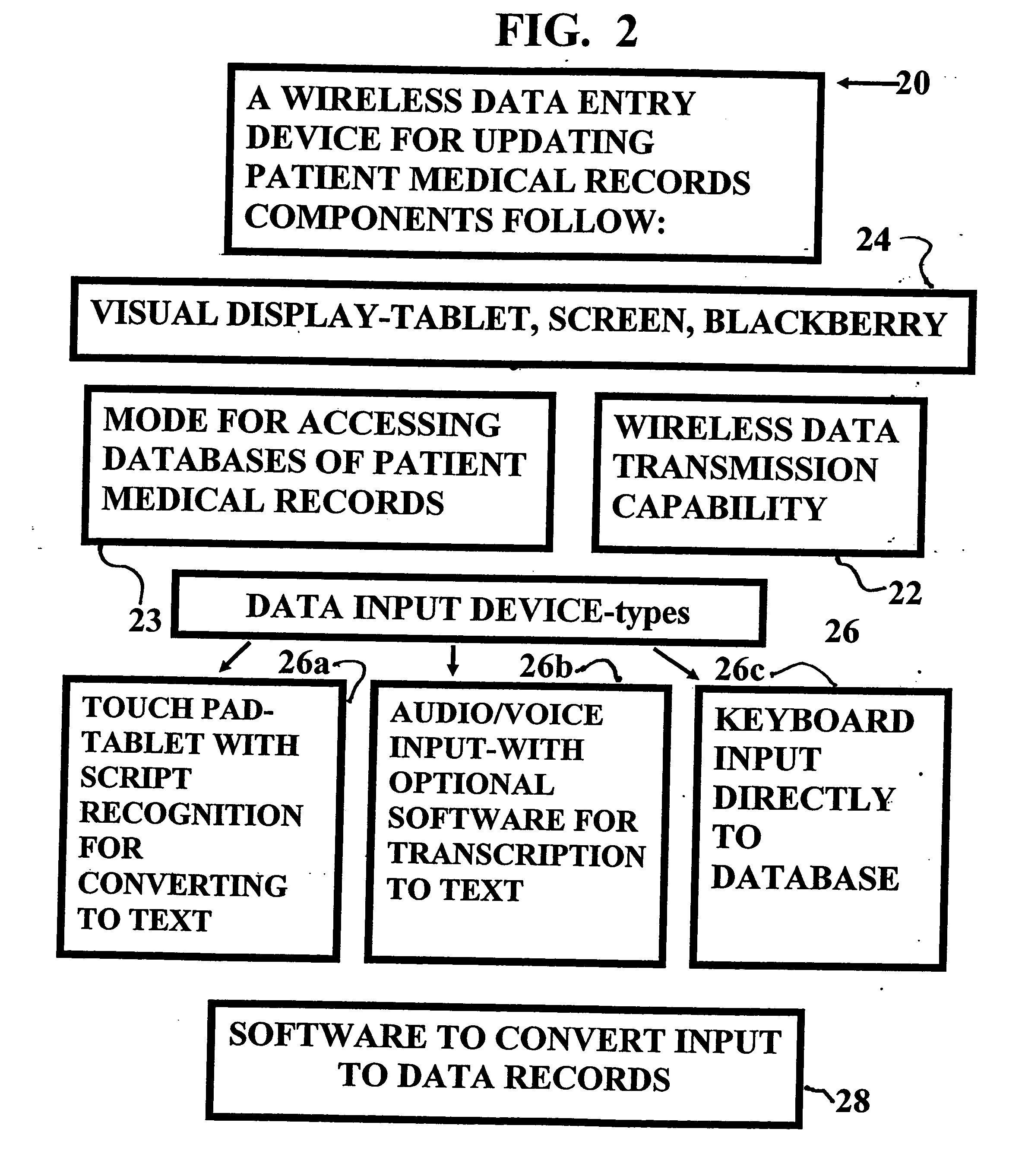
InactiveUS20070005397A1Easy to understandUser friendly to manipulateData processing applicationsDrug and medicationsMedical recordTablet computer
A method for maintaining and providing access to electronic medical records preferably utilizing multifunctional handheld tablet computers for real-time access to patients location and their records and rapidly updating those records. Using the tablets, tests can be ordered, results accessed and prescriptions sent electronically to pharmacists after being checked for allergic or drug interaction complications. Many medical provider's' records on the same patient reside on a central database giving a full view of the patient's complete medical history. Privacy of patient records is assured using permission and security protocols to secure the records from unauthorized access.
Owner:E WEB
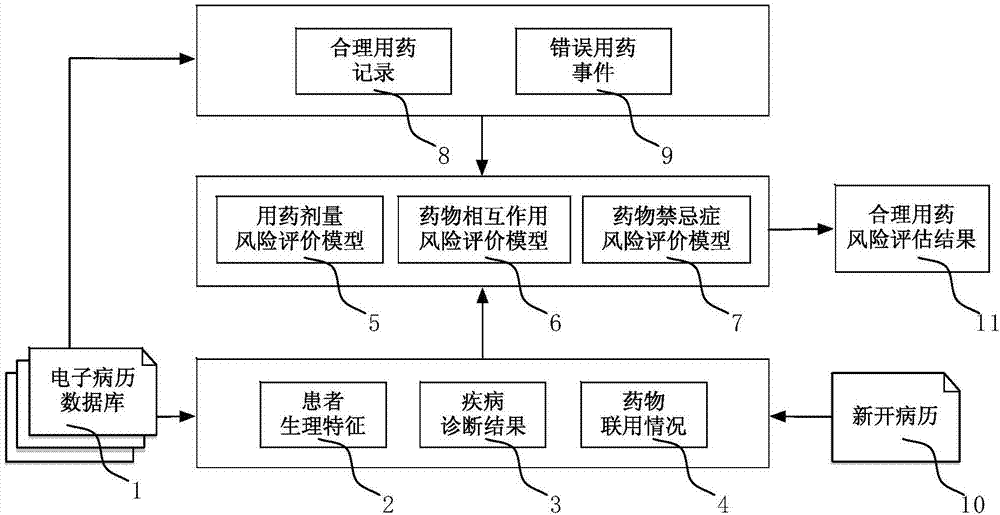
Electronic medical record big data-based clinical reasonable drug use risk assessment method
InactiveCN107341345AEnsure medication safetyRational drug use risk individualizationResourcesSpecial data processing applicationsMedical recordRational use
The invention discloses a clinical rational drug use risk assessment method based on big data of electronic medical records, which comprises the following steps: S1 extracting rational drug use records and wrong drug use events from electronic medical record data, according to the patient's physiological characteristics, disease diagnosis results, drug combination Based on factors such as drug use, automatically establish a risk assessment model for rational drug use such as drug dosage, drug interactions, and drug contraindications; S2 extracts corresponding patient physiological characteristics, disease diagnosis results, drug combination conditions, etc. from the new electronic medical records Factors, using the risk assessment model for rational drug use, to obtain individual drug risk assessment, to provide decision-making basis for clinical drug affairs management. The clinical rational drug use risk evaluation method of the present invention can automatically evaluate the clinical drug use risk, and the result is accurate and effective, and can provide decision-making basis for the practice of clinical rational drug use.
Owner:XIAMEN UNIV
Systems and methods for managing electronic prescriptions
InactiveUS20060261145A1Drug and medicationsPatient personal data managementDrug interactionApplication server
A method for managing prescriptions includes receiving a patient assessment from a physician at an application server, receiving a prescription based on the patient assessment, determining drug interactions based on the received prescription, sending the prescription to a pharmacy based on the drug interaction determination and validating the prescription. Validating the prescription comprises a three-way match of prescription information stored on a centralized database with the filled medicine container and the bulk container. Prescriptions can be prescribed and patient records reviewed using remote devices linked to the prescription management application server and centralized database.
Owner:ROBERTSON SCOTT +3
Method and device for maintaining and providing access to electronic clinical records
InactiveUS20070005396A1Easy to understandUser friendly to manipulateData processing applicationsDrug and medicationsMedical recordTablet computer
A method and device for maintaining and providing access to electronic medical records preferably utilizing multifunctional handheld tablet computers for real-time access to patients location and their records and rapidly updating those records. Using the tablets, tests can be ordered, results accessed and prescriptions sent electronically to pharmacists after being checked for allergic or drug interaction complications. Many medical providers' records on the same patient reside on a central database giving a full view of the patient's complete medical history. Privacy of patient records is assured using permission and security protocols to secure the records from unauthorized access.
Owner:E WEB
System and method for monitoring medication prescriptions using biometric identification and verification
InactiveUS20090216560A1Eliminate fraudQuick measurementDrug and medicationsComputer-assisted medicine prescription/deliveryPharmacyDrug interaction
This invention is a system and method for a matching system that cross-references patients anonymously with doctors and pharmacies. The invention uses patient-unique biometrics, such as fingerprinting, retinal scanning, or another such unique identifier, for patient identification and verification without revealing a patient's name or other personal information. The system tracks patient prescriptions and produces alerts to indicate potential problems, such as drug interactions or possible fraudulent behavior. The system can produce a traceable paper trail, for investigation or prosecution, and protects the medical industry, patients, and the public from the consequences of doctor shopping.
Owner:BIO TECH MEDICAL SOFTWARE
Hospital medication management system with medication safety monitoring and intervening functions
The invention relates to a hospital medication management system with the medication safety monitoring and intervening functions. The hospital medication management system with the medication safety monitoring and intervening functions is composed of a medication safety monitoring system and an early informatization intervening system. The computer database technology and other technologies are adopted for the medication safety monitoring system. According to the professional examination principle in the medicine science and the pharmaceutical science, with the specialized knowledge in the medicine science and the pharmaceutical science as standards, relevant medicine data information can be provided when doctor's advice is entered and examination of drug allergy histories, drug interactions, contraindications, side effects, external injection medicine compatibility and the like is performed on the doctor's advice to assist a doctor in correctly screening medicines and determining the doctor's advice, a prompt and an alarm can be given timely when problems are found, and thus the possibility of errors is reduced.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
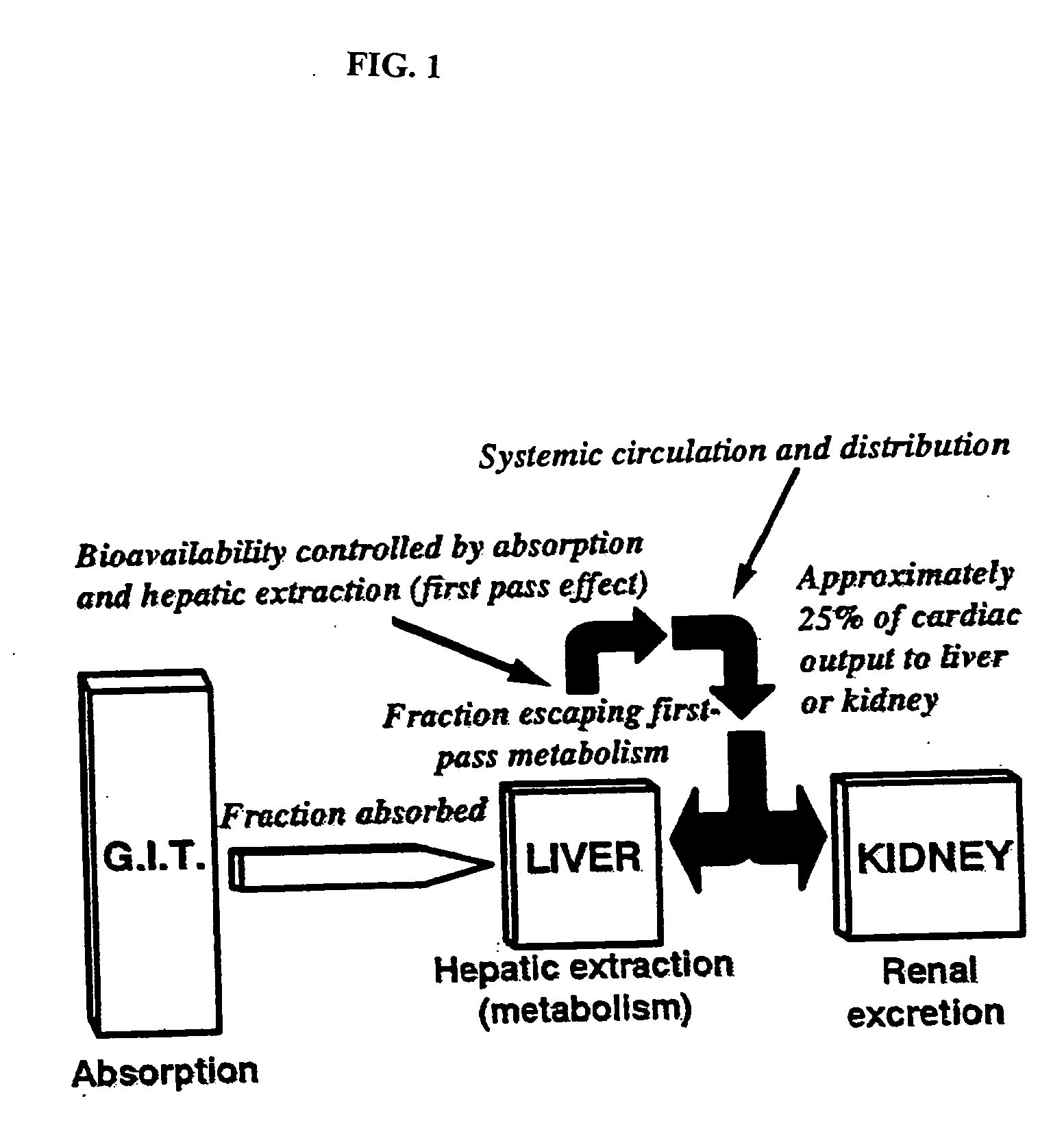
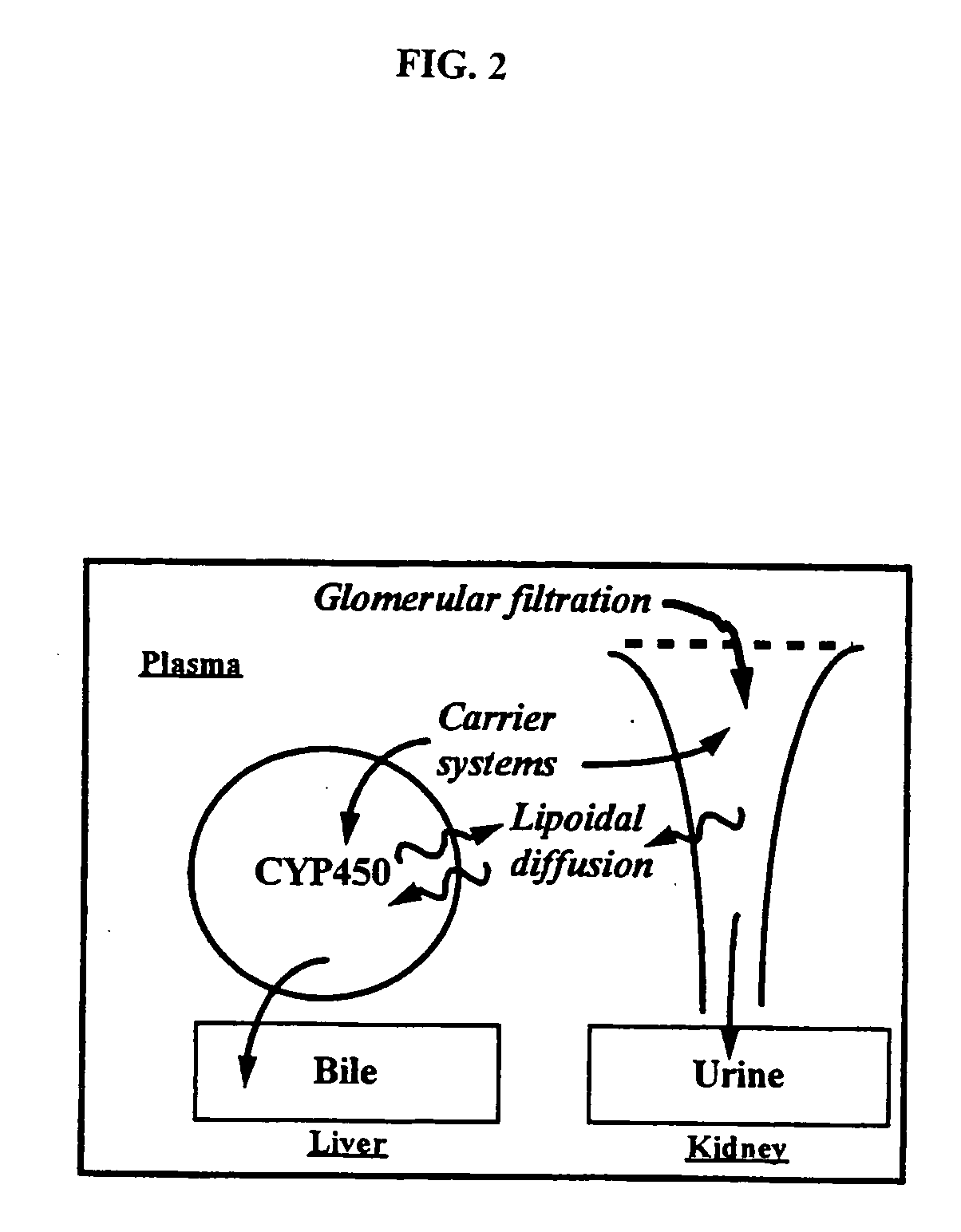
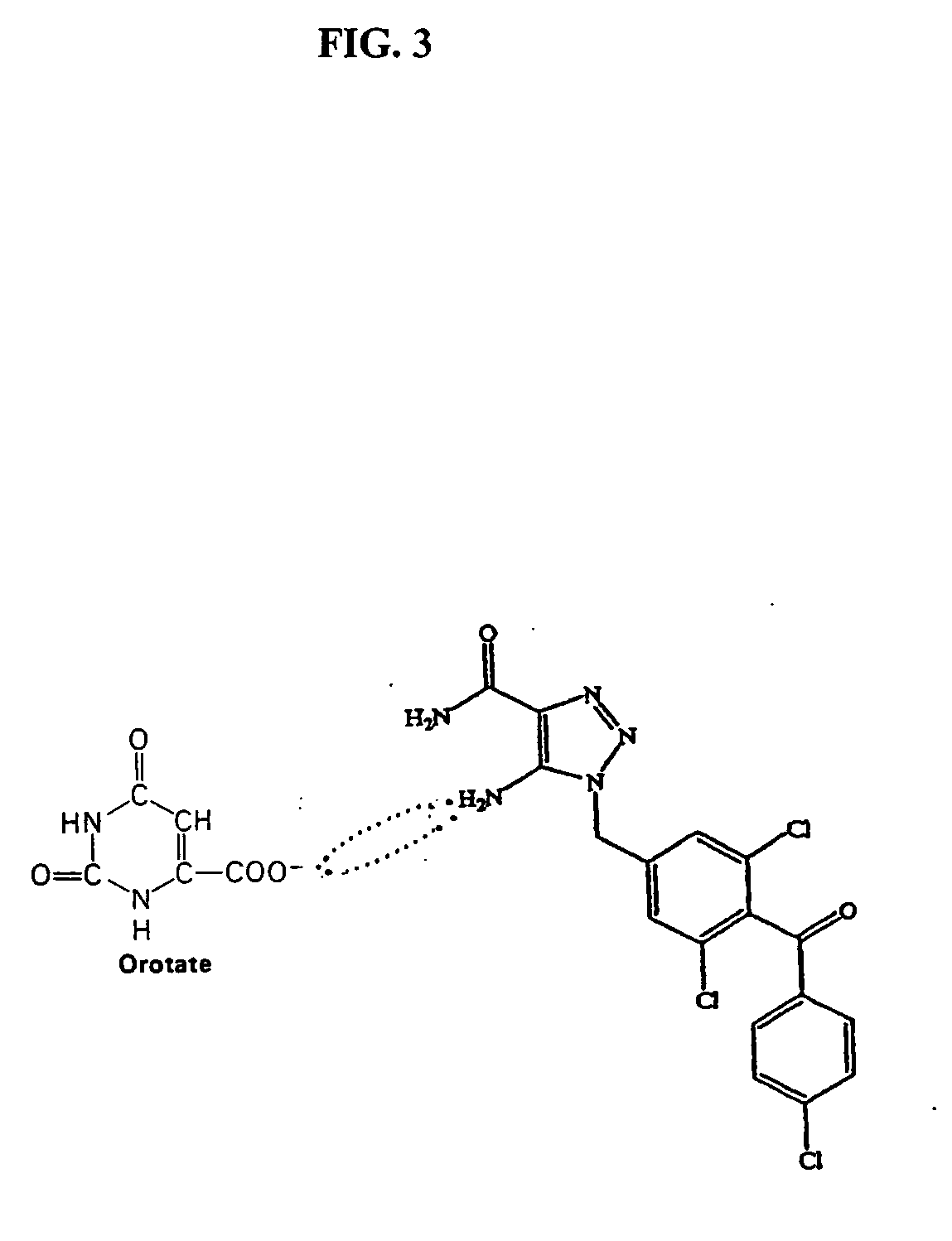
Method of increasing drug oral bioavailability and compositions of less toxic orotate salts
ActiveUS20060189640A1Improve bioavailabilityImprove drug bioavailabilitySalicyclic acid active ingredientsBiocideSide effectDrug interaction
The present invention relates generally to the method of increasing the oral bioavailability, reducing chemotherapy induced toxicity and side effects, and improving the effectiveness of pharmaceutical agents that are poorly absorbed from the gastrointestinal tract. Specifically, the invention relates to poorly absorbed pharmaceutical drugs and converting them to orotate salts. The orotate salts of the drugs can be dosed at lower doses to provide the efficacy benefits of a higher dose, while reducing the drugs' toxic effects at lower doses. Additionally, the orotate salts of pharmaceutical agents have better clearance and reduce the potential for drug-induced hepatic toxicity. Therefore, an especially useful formulation of the orotate salt of the pharmaceutical agent can provide rapid and consistent action using a lower dose while reducing drug interactions and side-effects.
Owner:SAVVIPHARM INC
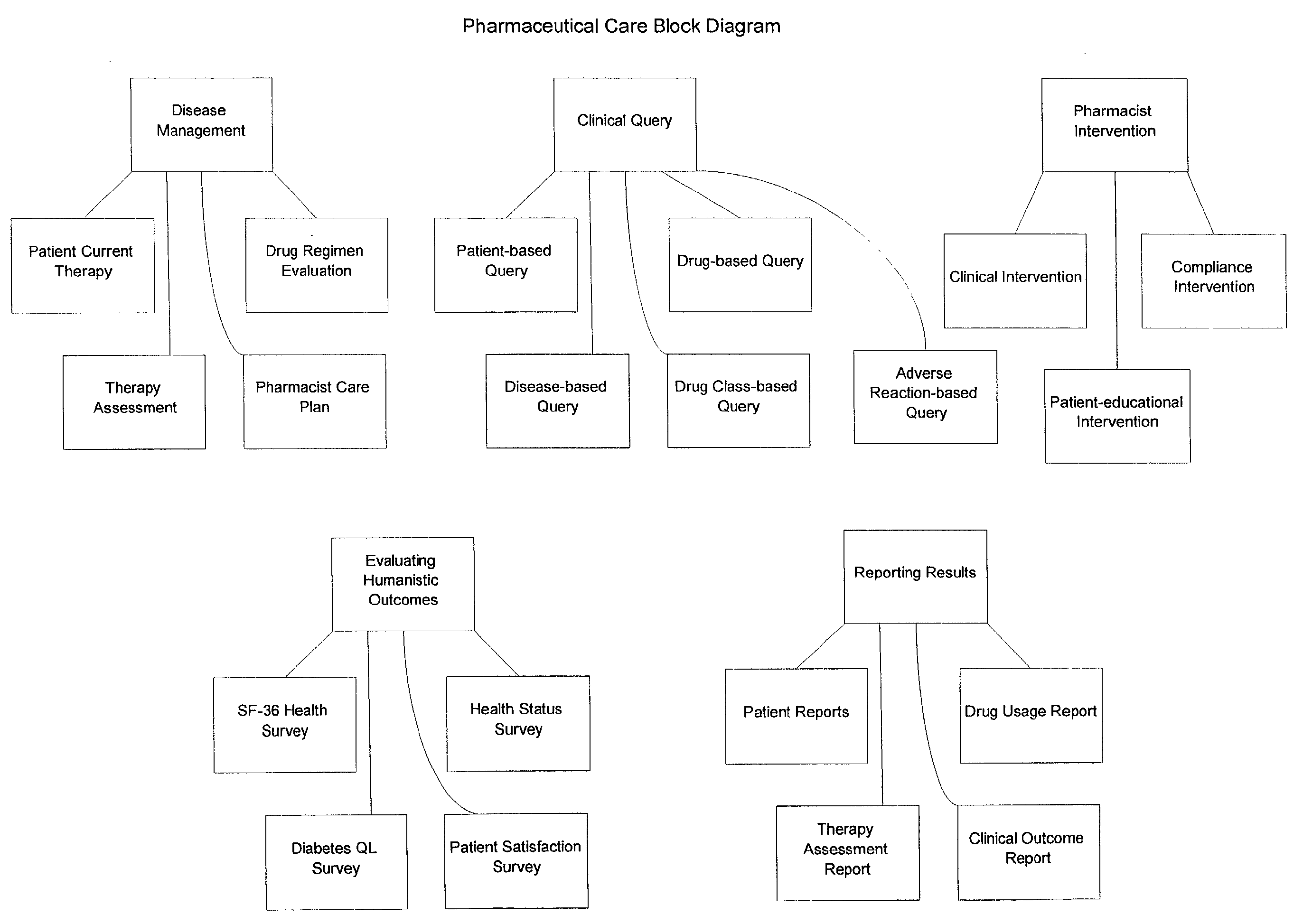
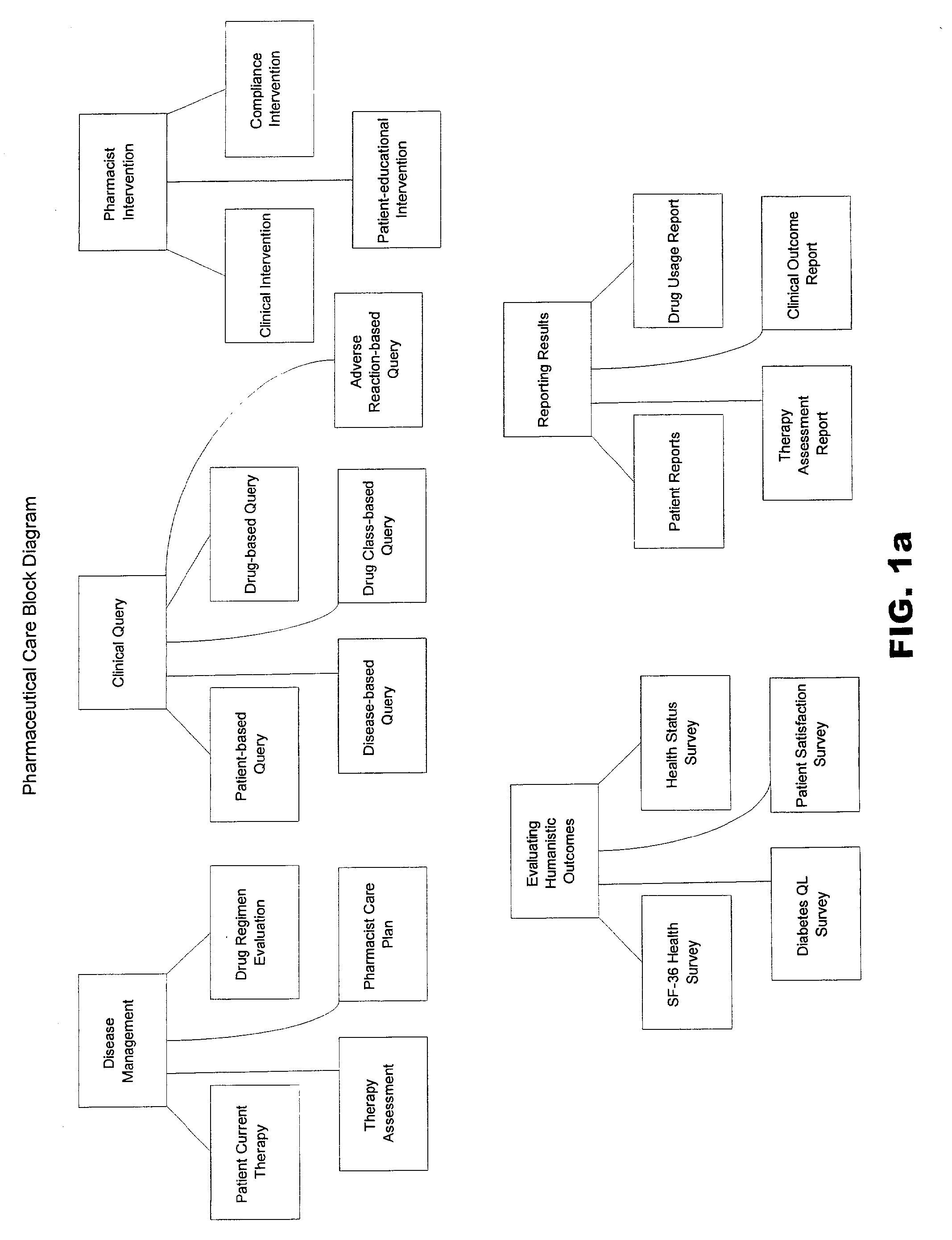
Systems and methods for managing patient pharmaceutical care
Systems and methods are described for allowing a pharmacist to practice pharmaceutical care in an accurate and efficient manner. The present invention provides the systems for gathering, organizing, and maintaining the necessary clinical and patient data, and providing pharmacists access thereto, through integrated computer software. The clinical data classifies drugs into therapeutic classes, and for each class there is associated therewith known indications, contra-indications, recommended dosages, known adverse reactions, and drug interactions. A clinical database and a patient database are used. In the clinical database, each drug is assigned a unique identification code including a therapeutic cross reference (TXR). The TXR allows access to information associated with the drug's adverse reactions, and dosage recommendations, and also to disease indications and contra-indications via a link to the ICD-9s (International Classification of Diseases) associated with the diseases. The patient data includes patient diagnosis profiles and allergy profiles.
Owner:CERNER INNOVATION
System and method for anatomy labeling on a PACS
ActiveUS7590440B2Narrow selectionImage enhancementImage analysisDrug interactionRelevant information
Owner:GENERAL ELECTRIC CO
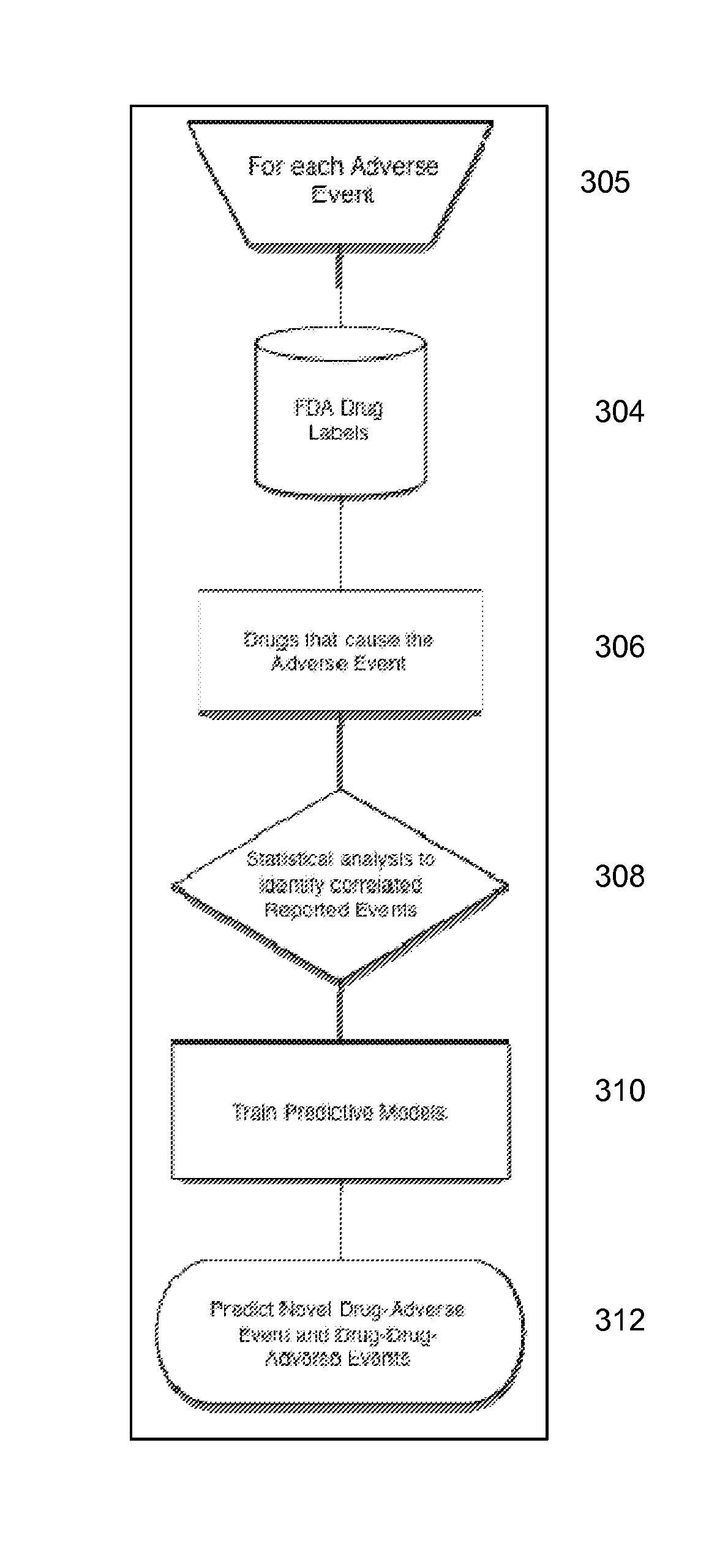
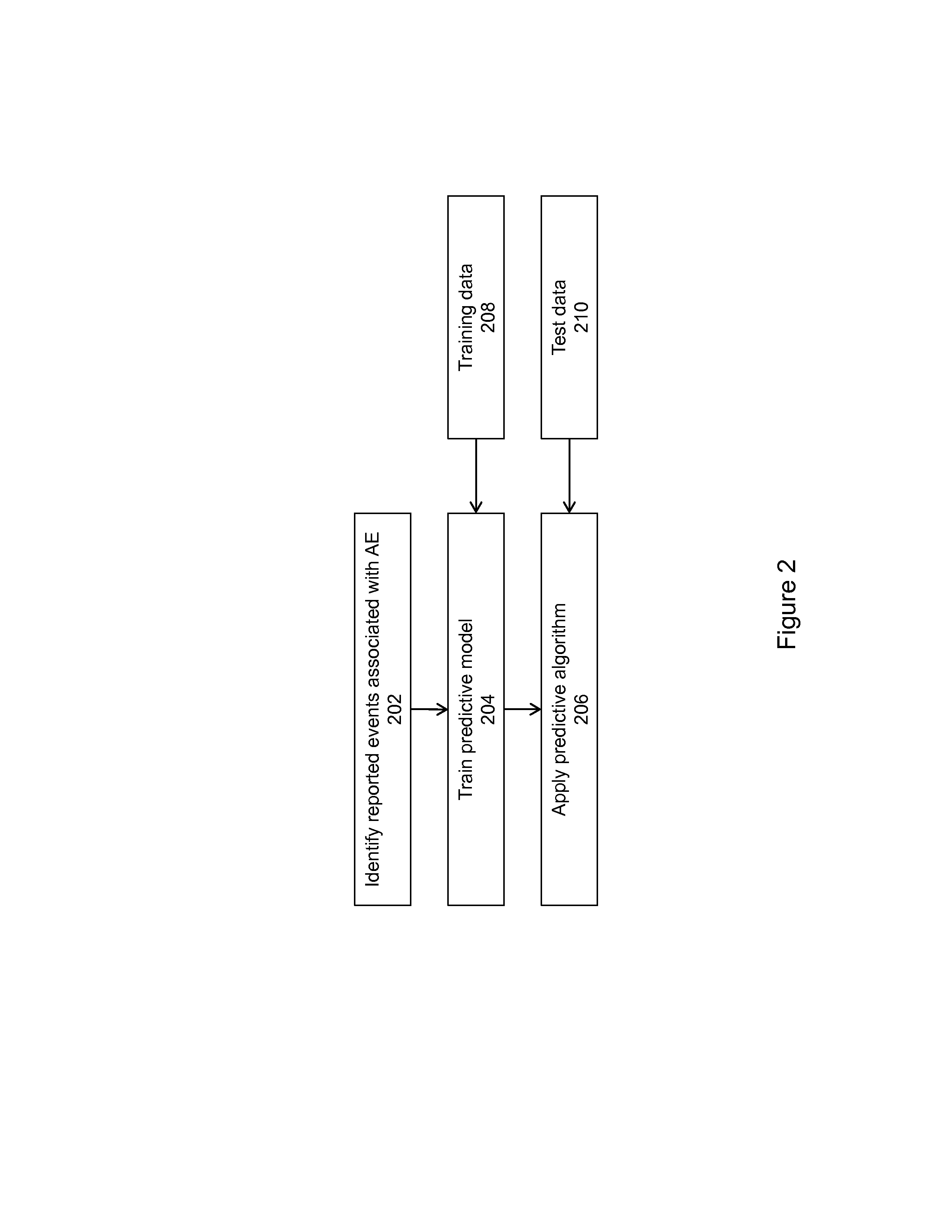
Signal Detection Algorithms to Identify Drug Effects and Drug Interactions
ActiveUS20130179375A1Improve performanceMedical simulationDrug and medicationsObservational databaseDrug interaction
An algorithm according to an embodiment of the present invention provides for latent signal detection of adverse events. Embodiments infer the presence of adverse drug events from large observational databases housed by the FDA, WHO, and other governmental organizations. The disclosed algorithms do not require the adverse event to be reported explicitly. Instead, the algorithms infer the presence of adverse events through more common secondary effects. In an embodiment, machine learning techniques are used for this purpose.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
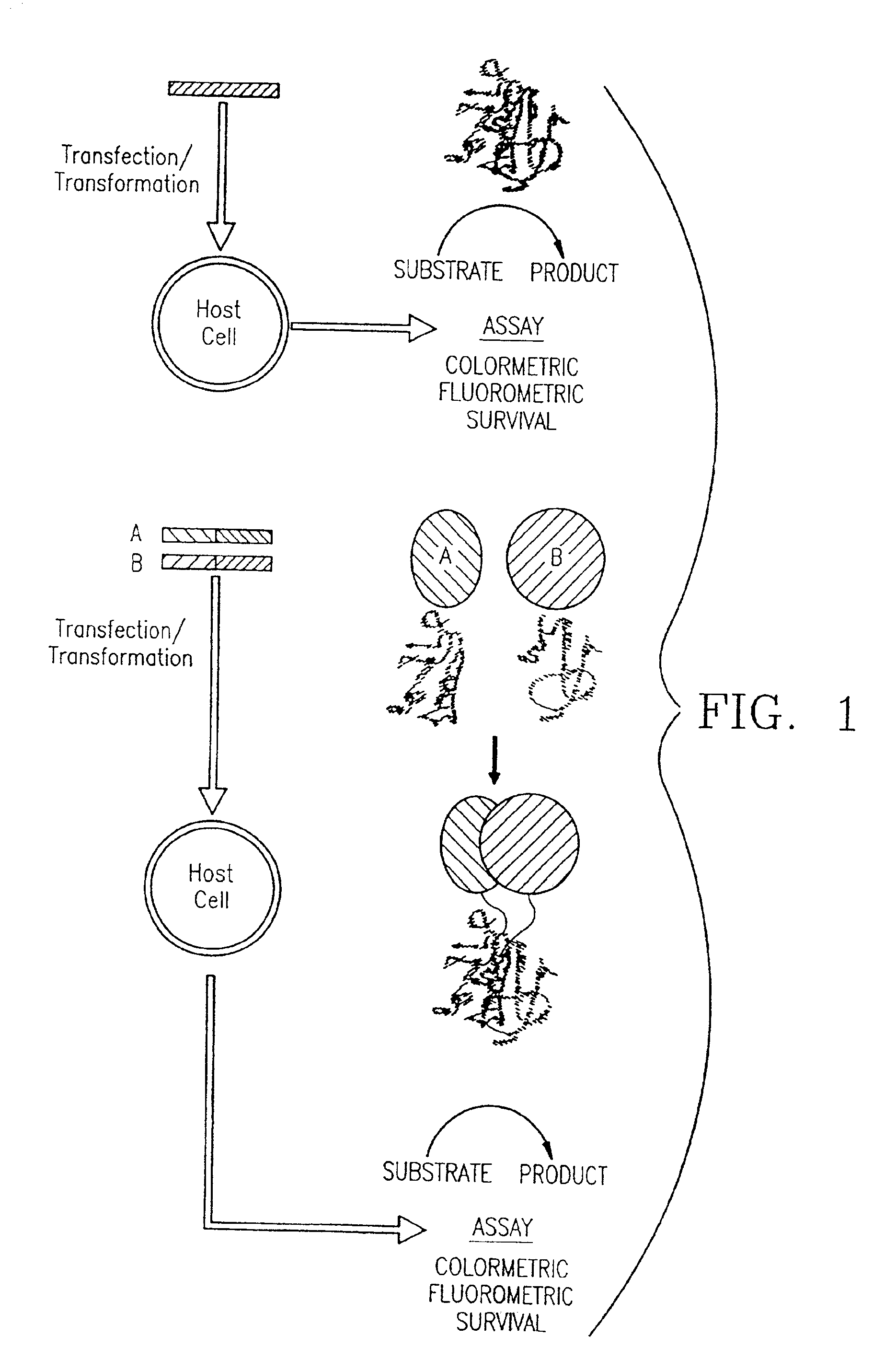
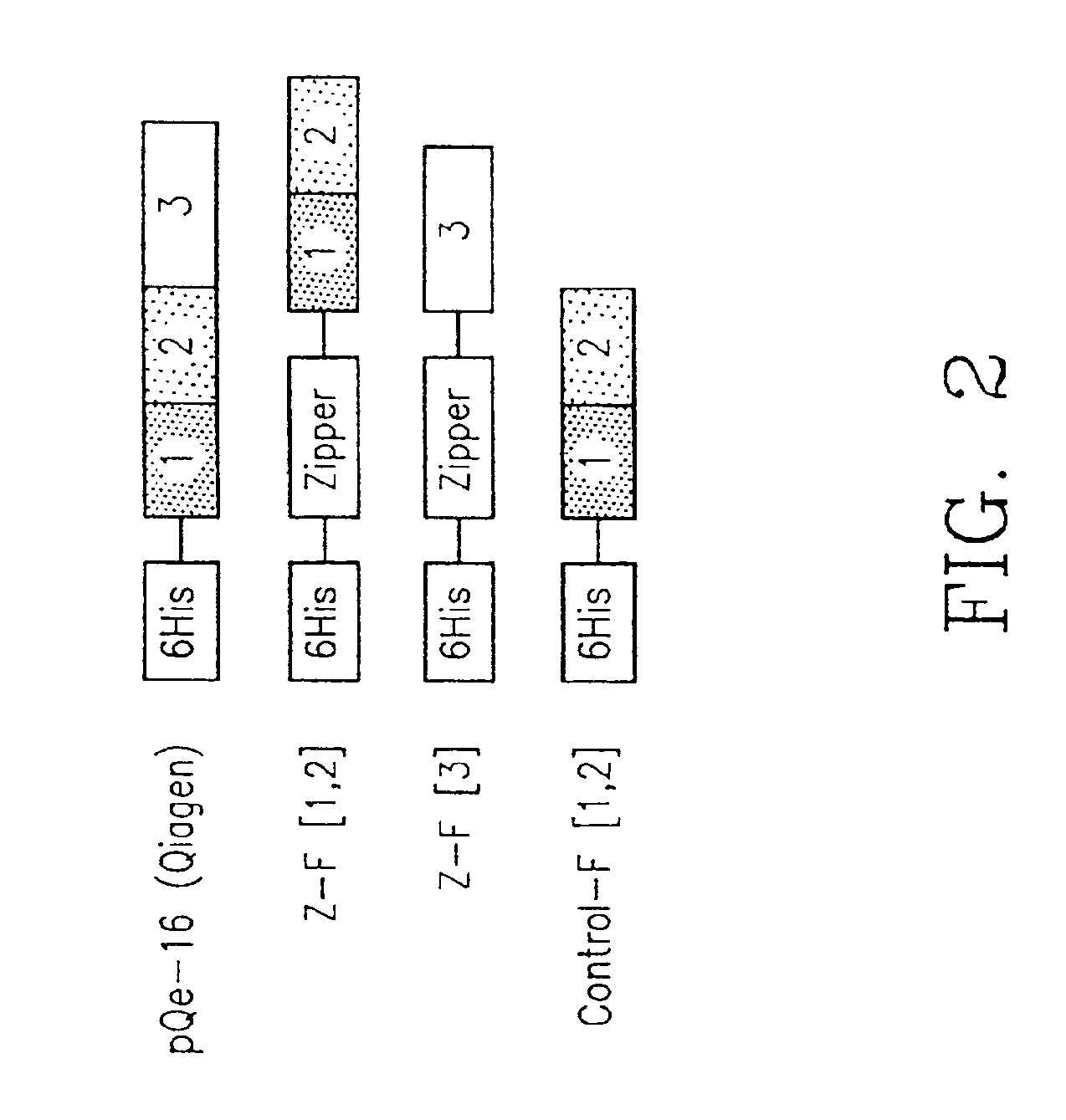
Protein fragment complementation assays for the detection of biological or drug interactions
InactiveUS6929916B2Level of simplicitySimple versatilityBacteriaAntibody mimetics/scaffoldsDrug interactionProtein Fragment
The present invention describes a method for detecting biomolecular interactions said method comprising: (a) selecting an appropriate reporter molecule selected from the group consisting of a protein, a fluorescent protein, a luminescent protein and a phosphorescent protein; (b) effecting fragmentation of said reporter molecule such that said fragmentation results in reversible loss of reporter function; (c) fusing or attaching fragments of said reporter molecule separately to other molecules; followed by (d) reassociation of said reporter fragments through interactions of the molecules that are fused to said fragments; and (e) detecting said biomolecular interactions by reconstitution of activity of the reporter molecule with the proviso that said protein is not ubiquitin.
Owner:ODYSSEY THERA INC
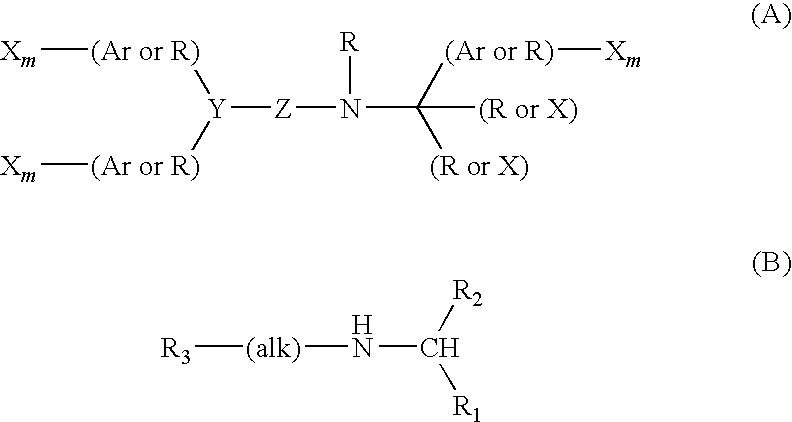
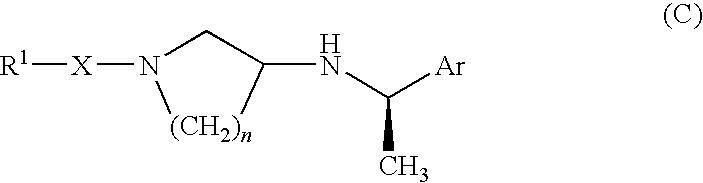
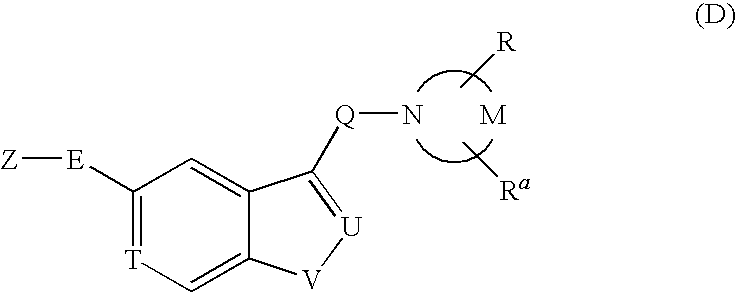
Pyrrolidine derivative or salt thereof
InactiveUS20090062366A1Excellent CaSR agonistic activityHigh activityBiocideOrganic chemistryDiseaseRenal osteodystrophy
[Problem] To provide a compound which may be used in treating diseases in which a calcium sensing receptor (CaSR) is concerned, particularly hyperparathyroidism.[Means for Resolution] It was found that novel pyrrolidine derivatives which are characterized by the possession of aminomethyl group substituted with arylalkyl group or the like, or salts thereof, have excellent CaSR agonistic regulatory activity and also have excellent selectivity with CYP2D6 inhibitory activity having a possibility of causing drug interaction. Based on the above, these novel pyrrolidine derivatives are useful as therapeutic agents for treating diseases in which CaSR is concerned (hyperparathyroidism, renal osteodystrophy, hypercalcemia and the like).
Owner:ASTELLAS PHARMA INC
Method for the quantification of methylated DNA
InactiveUS20050287553A1Reliable valueMicrobiological testing/measurementFermentationDiseaseProbe type
Particular aspects of the present invention provide a method for quantification of two different variations of a DNA sequence. Particularly, the invention relates to a quantification of methylated DNA, and for this purpose, the test DNA is converted so that cytosine is converted to uracil, while 5-methylcytosine remains unchanged. The converted DNA is amplified by means of a real-time PCR, wherein two labeled real-time probe types are utilized: one specific for methylated DNA; and one for unmethylated DNA. Preferably, the degree of methylation of the test DNA is calculated from the ratio of the signal intensities of the probes or from the Ct values. The inventive methods have substantial utility for diagnosis and prognosis of cancer and other disorders associated with altered or characteristic DNA methylation status, as well as having substantial utility for analysis of SNPs, allelic expression, and prediction of drug response, drug interactions, among other uses.
Owner:EPIGENOMICS AG
System and Method for Providing Pharmacy Services
InactiveUS20150261934A1Improves pharmaceutical healthcare serviceReducing medicalData processing applicationsDrug and medicationsDrug interactionDrug dispensing
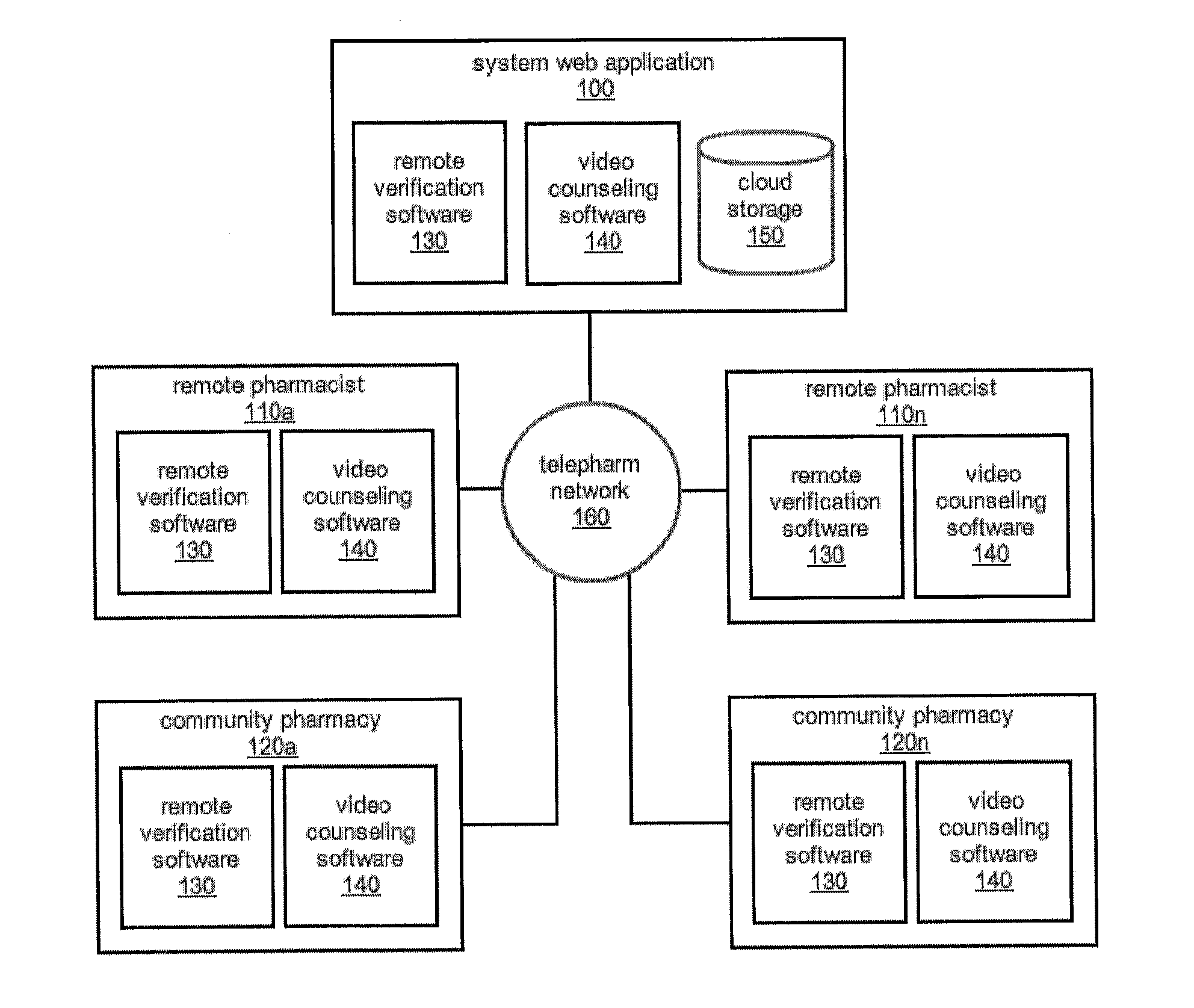
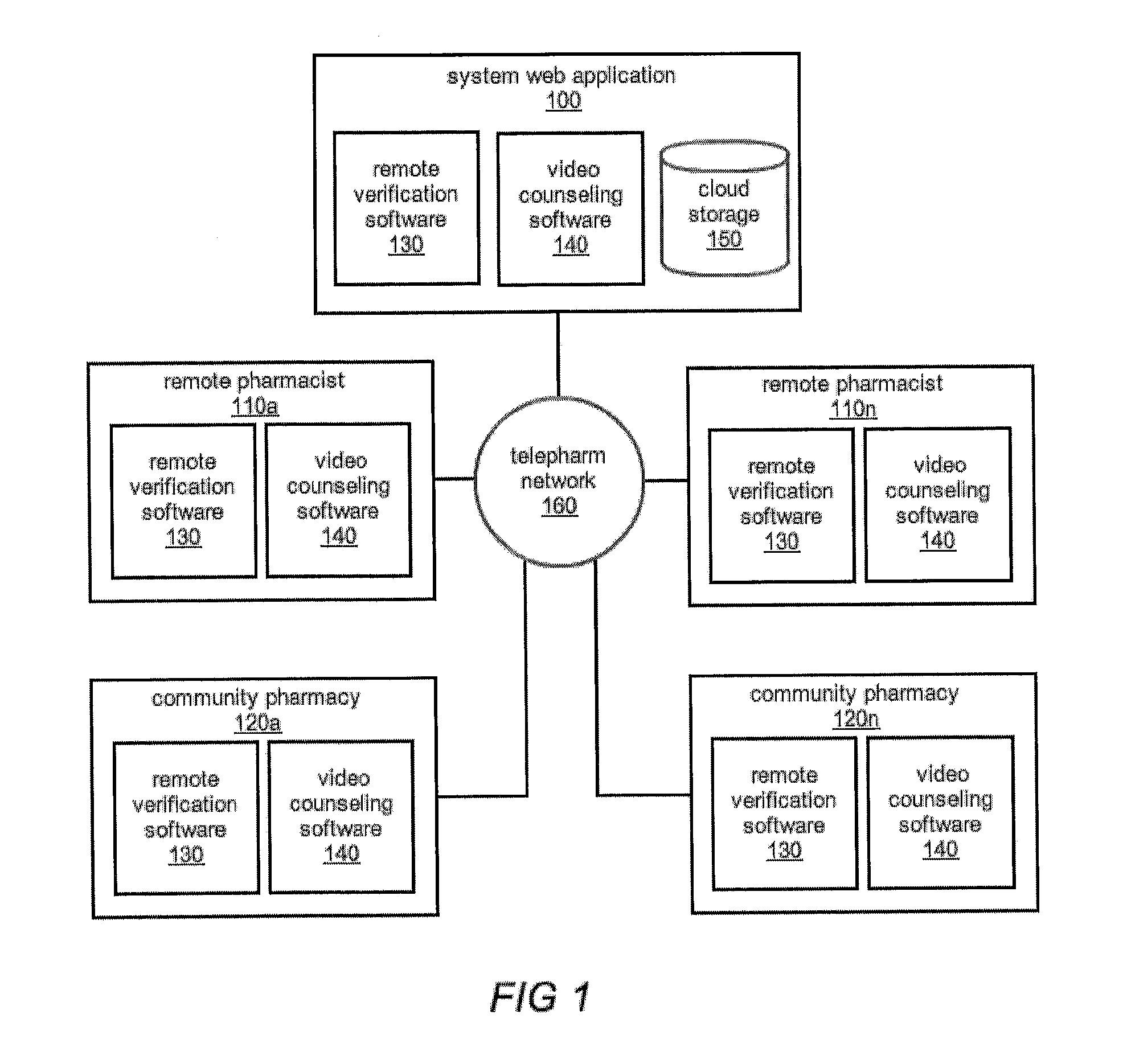
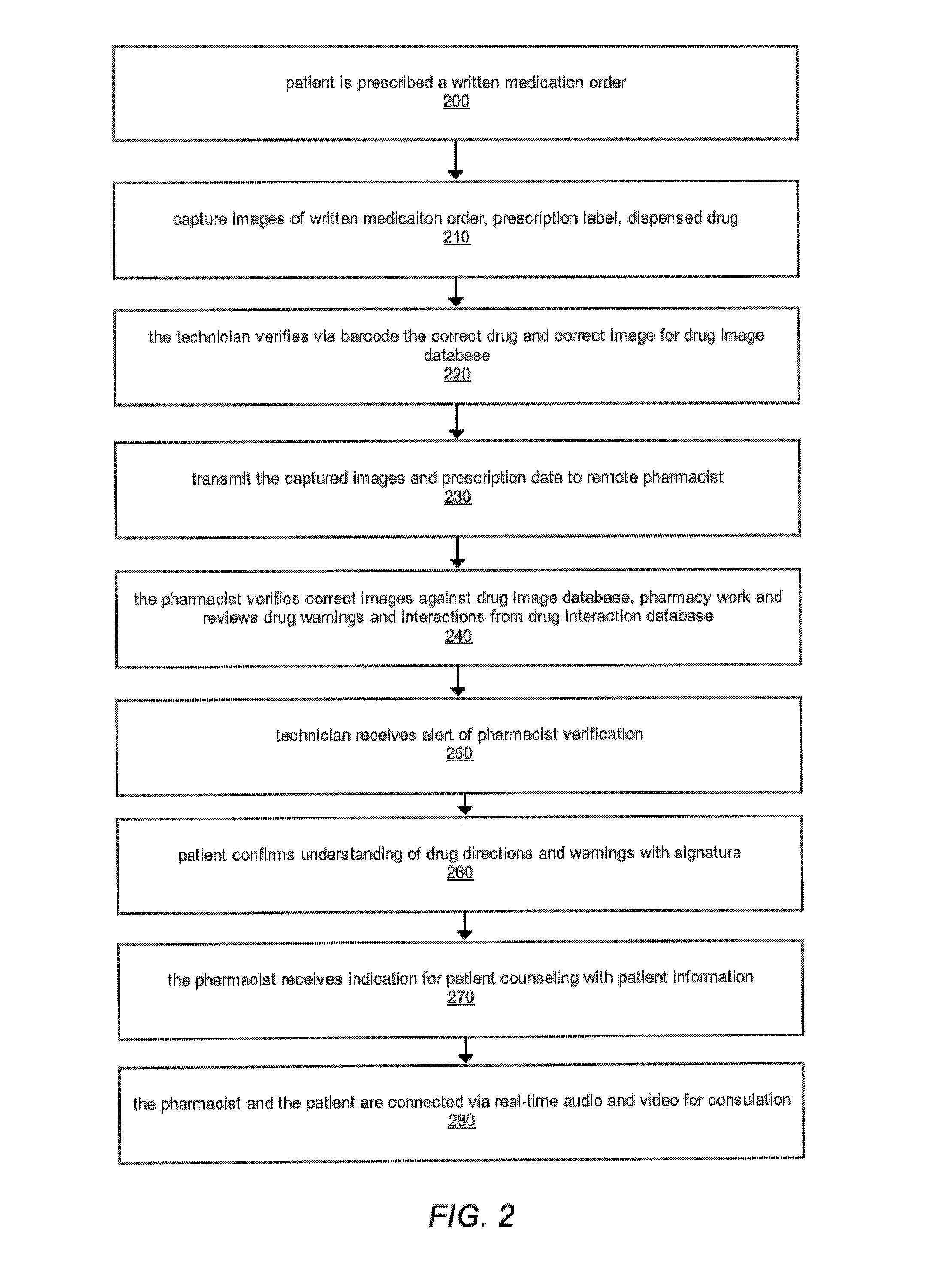
The comprehensive and controlled web-based system facilitates provision of pharmaceutical care and dispensing of medications to patients. The system includes multiple retail or pharmacy outlets whose prescription filling activities are supervised and verified by pharmacists at remote sites. The supervision includes visual confirmation by images for the prescription and the drug dispensed and systematic provision of information pertaining to drug interactions and instructions with affirmative patient confirmation. The system ensures patient safety and education, prescription accuracy, and reduction of expense while assisting rural or economically challenged areas to retain local access to pharmacy services.
Owner:TELEPHARM
Pharmacogenetic drug interaction management system
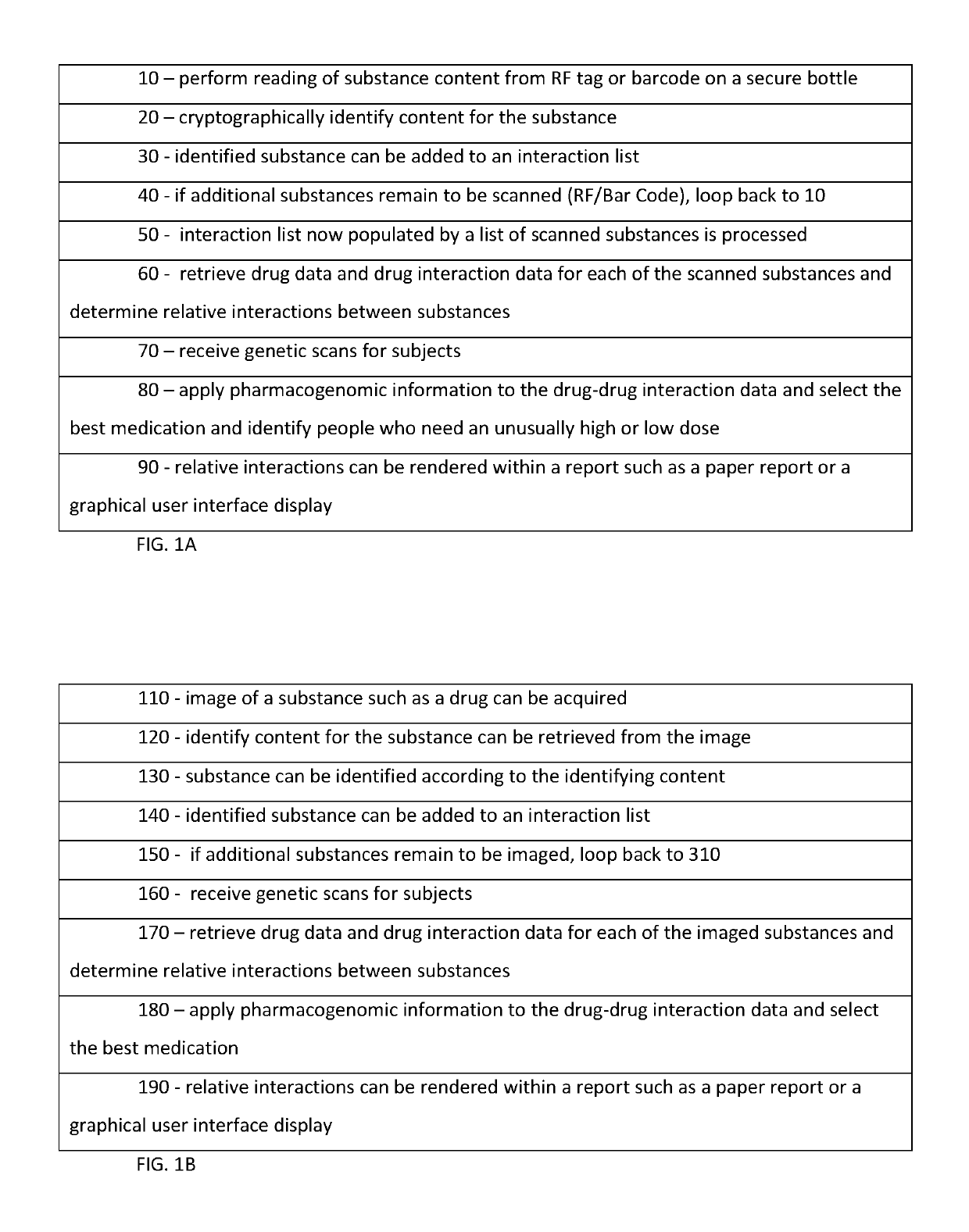
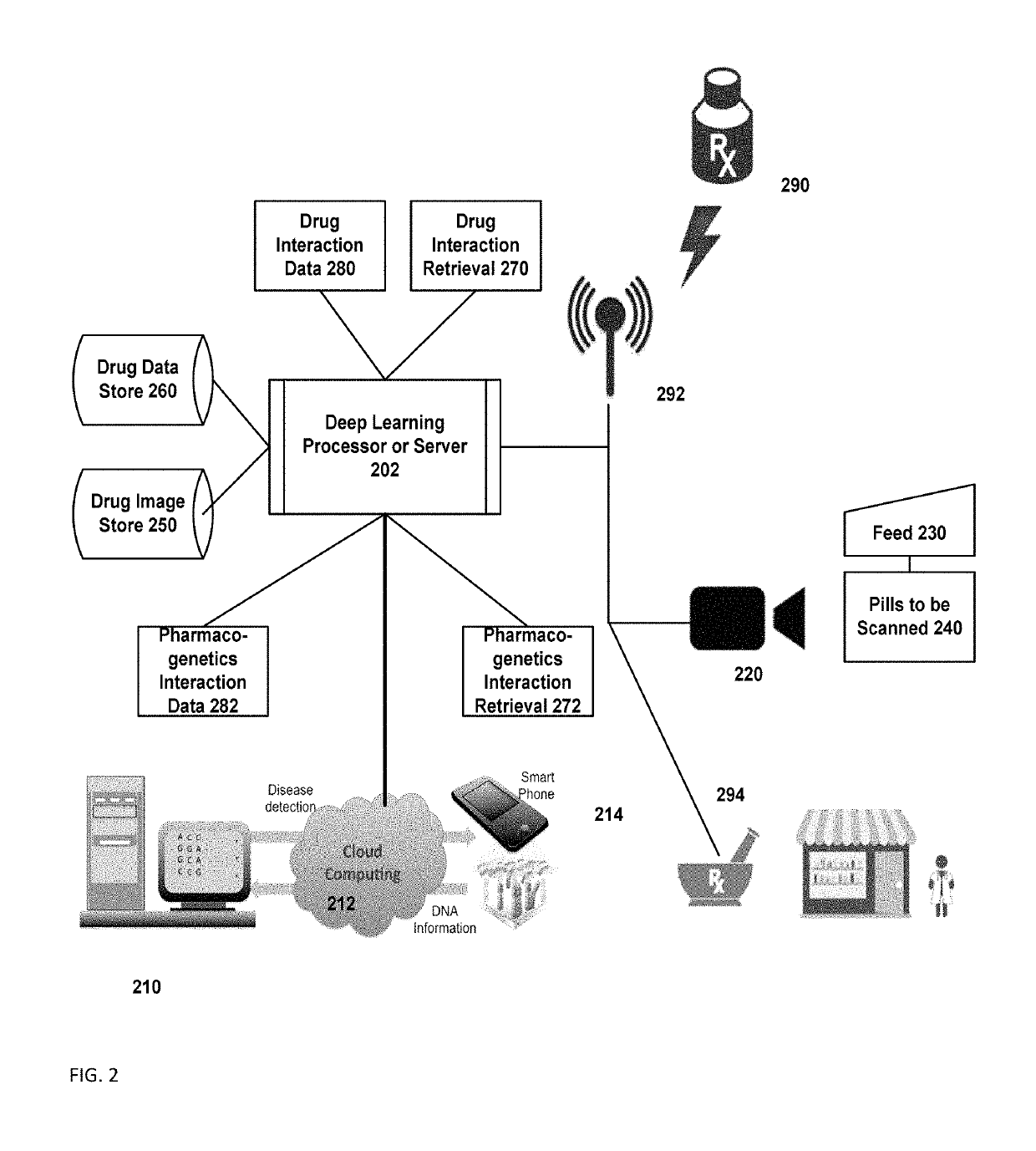
InactiveUS10262107B1Medical trials more efficientLow costMathematical modelsHealth-index calculationDrug interactionGenomic Biomarker
A system includes a substance to be consumed by a subject and one or more indicia labeling the substance with: genomic biomarkers; drug exposure and clinical response variability; risk for adverse events; genotype-specific dosing; polymorphic drug target and disposition genes; and treatment based on the biomarker.
Owner:TRAN BAO
Systems and methods for managing electronic prescriptions
InactiveUS7438228B2Drug and medicationsPatient personal data managementDrug interactionApplication server
A method for managing prescriptions includes receiving a patient assessment from a physician at an application server, receiving a prescription based on the patient assessment, determining drug interactions based on the received prescription, sending the prescription to a pharmacy based on the drug interaction determination and validating the prescription. Validating the prescription comprises a three-way match of prescription information stored on a centralized database with the filled medicine container and the bulk container. Prescriptions can be prescribed and patient records reviewed using remote devices linked to the prescription management application server and centralized database.
Owner:ROBERTSON SCOTT +3
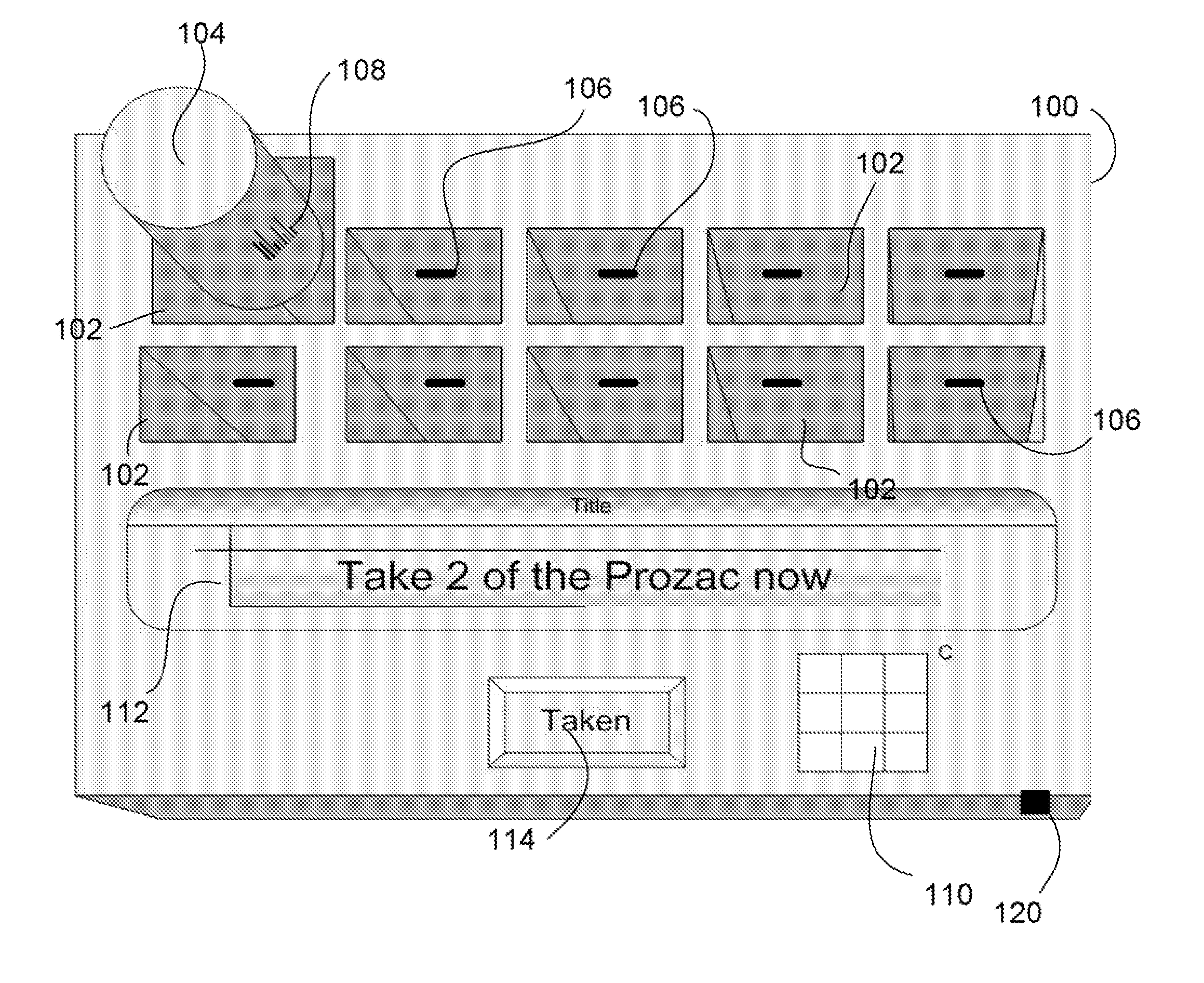
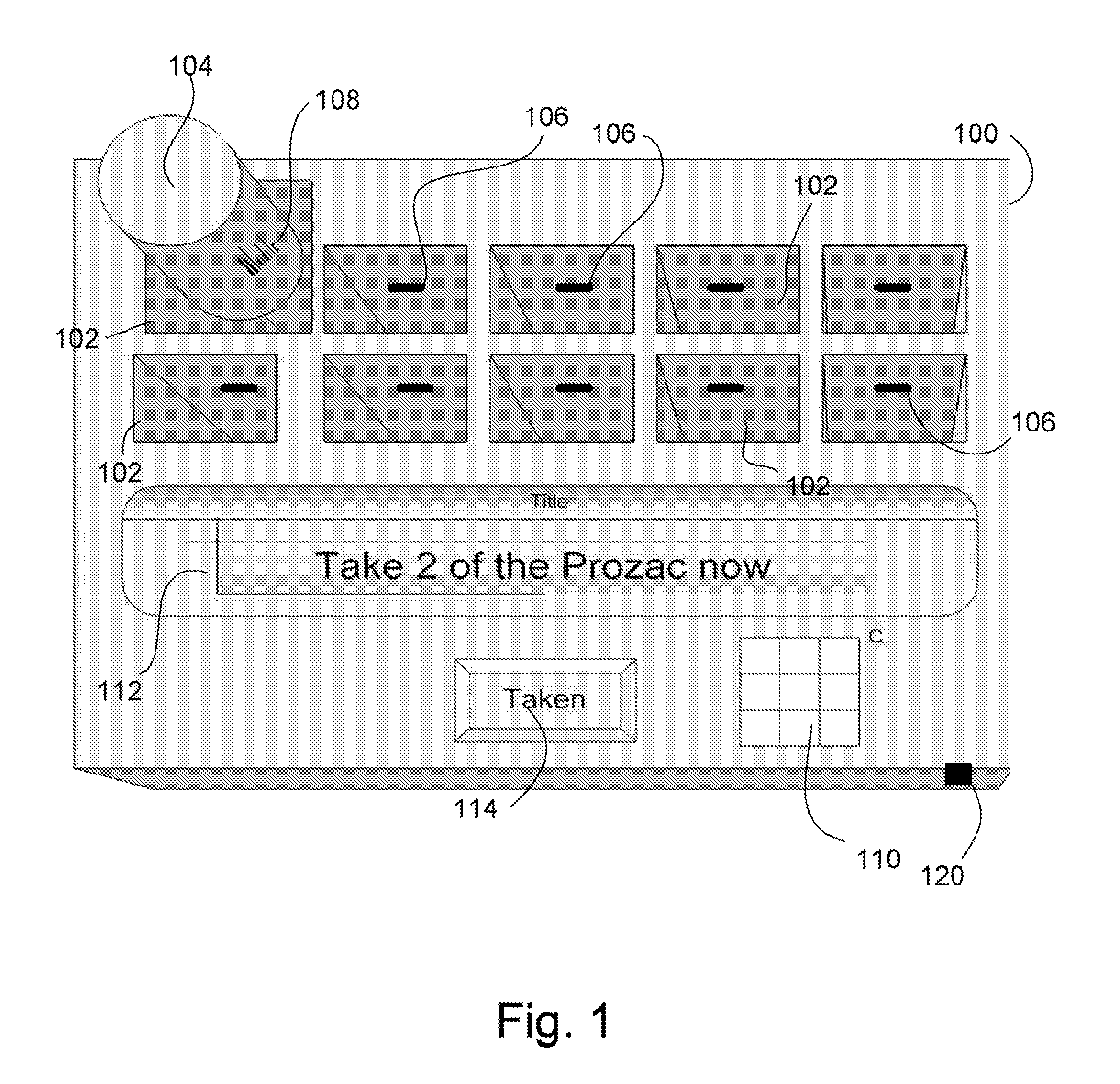
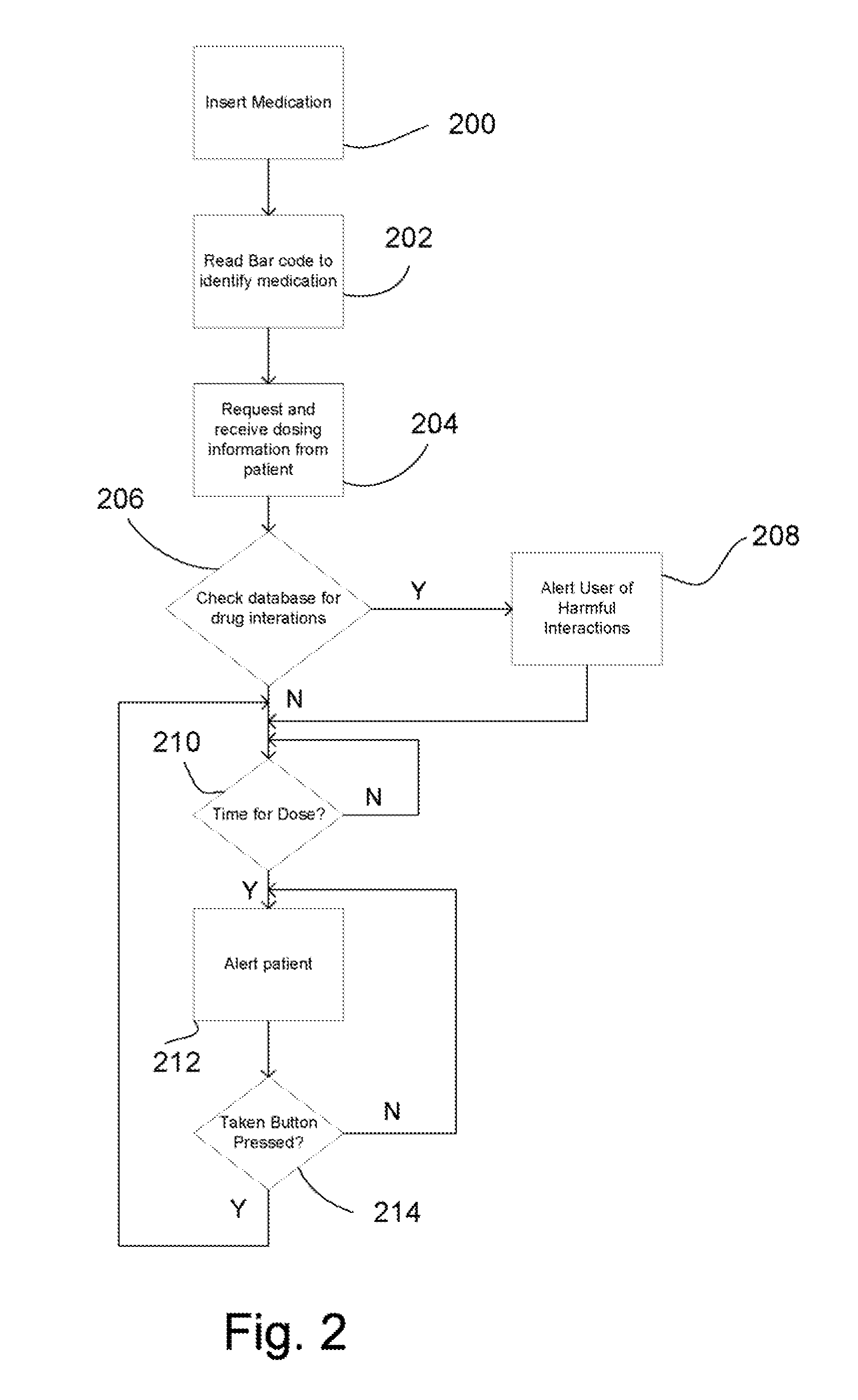
Intelligent medication tracker
InactiveUS20090001093A1Managing their medications regimenImprove patient safety in the hospital settingDrug and medicationsCoin-freed apparatus detailsPharmacyMedication Dispenser
A medication dispenser comprises slots that may be sized to accommodate medication containers of various sizes. Each slot has an associated barcode reader. A user inserts the pharmacy-provided pill or medication container into one slot. The barcode on the container is read. From the barcode the medication in the container is identified. The device contains a simple user interface that allows the user to enter the dosing frequency prescribed, how many pills or cc's are in the container, how many refills and a starting time. The dispenser alerts users to possible drug interactions with medications in other slots as well as alerts them to dosing times and other pertinent information.
Owner:INTEL GE CARE INNOVATIONS
Induced Hepatic Stem Cell And Process For Production Thereof, And Applications Of The Cell
InactiveCN102858958AEfficient developmentGenetically modified cellsMicrobiological testing/measurementDrug interactionDrug target
Disclosed are: an induced hepatic stem cell useful for safety tests, toxicity tests, metabolism tests, drug interaction tests, anti-viral activity tests, screening tests for medicinal agents such as hyperlipemia therapeutic agents, hypertension therapeutic agents, pharmaceutical low-molecular-weight compound agents and pharmaceutical antibody agents, the screening for potential drug targets, the production of animal models, the production of hepatocyte-produced proteins, and regenerative medicine; a process for producing the cell; and applications of the cell. The induced hepatic stem cell is characterized by fulfilling at least the following requirements (1) to (3): (1) at least 15 genes selected from specific genes that are marker genes for embryonic stem cells are expressed in the cell; (2) the cell has properties of hepatocytes; and (3) the cell can be proliferatively cultured or sub-cultured for at least three days.
Owner:NAT CANCER CENT
Pyrrolidine derivative or salt thereof
[Problem] To provide a compound which may be used in treating diseases in which a calcium sensing receptor (CaSR) is concerned, particularly hyperparathyroidism. [Means for Resolution] It was found that novel pyrrolidine derivatives which are characterized by the possession of aminomethyl group substituted with arylalkyl group or the like, or salts thereof, have excellent CaSR agonistic regulatory activity and also have excellent selectivity with CYP2D6 inhibitory activity having a possibility of causing drug interaction. Based on the above, these novel pyrrolidine derivatives are useful as therapeutic agents for treating diseases in which CaSR is concerned (hyperparathyroidism, renal osteodystrophy, hypercalcemia and the like).
Owner:ASTELLAS PHARMA INC
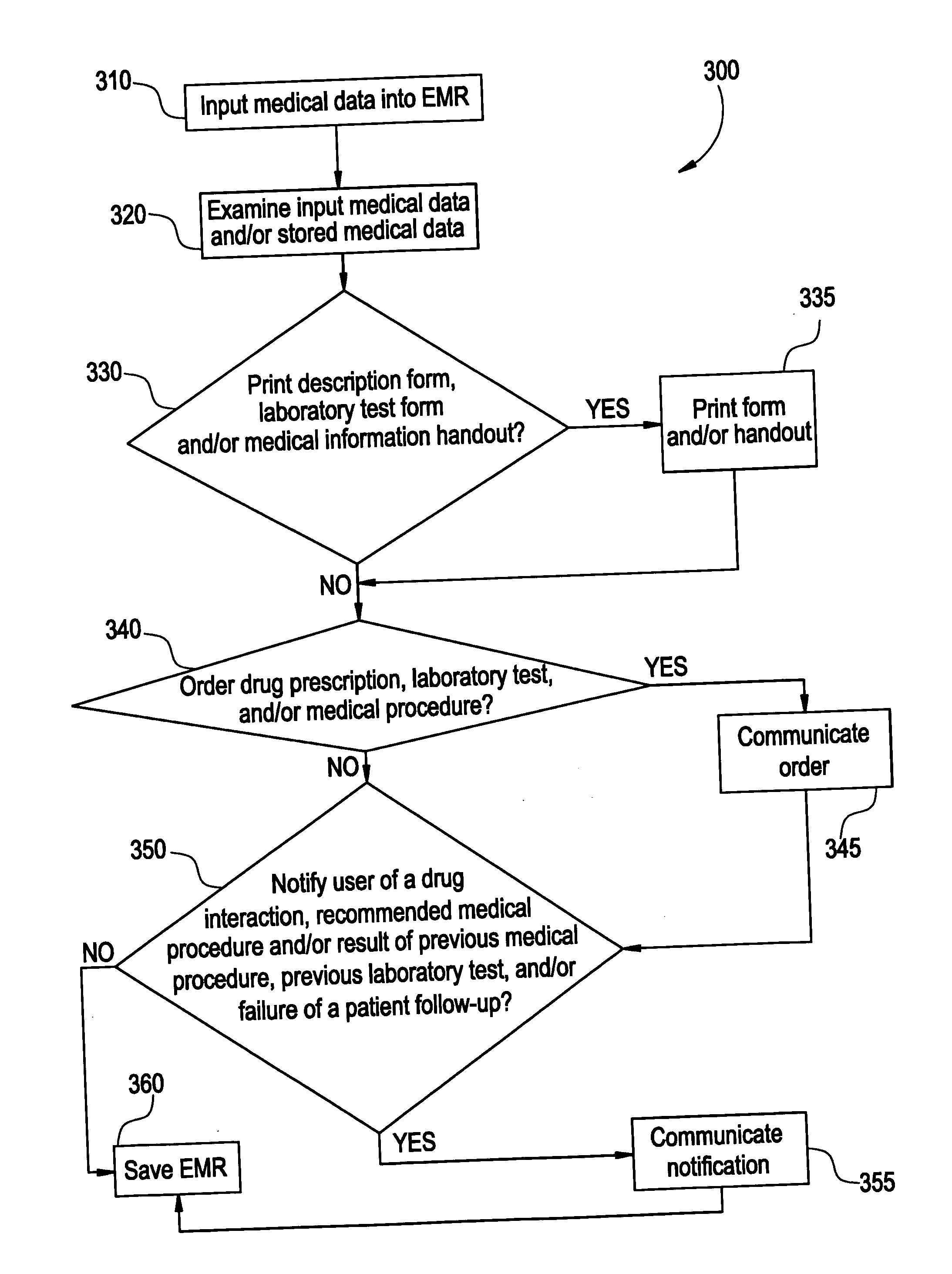
System and method for macro-enhanced clinical workflow
InactiveUS20070136090A1Data processing applicationsDrug and medicationsMedical recordDrug interaction
The present invention provides a method and system for macro-enhanced clinical workflow. The method and system enables printing of a prescription form, a laboratory test form, and a medical information handout based on at least user input to an electronic medical record (“EMR”). The method and system also enables the ordering one or more of a drug prescription, a laboratory test, and a medical procedure based on at least user input to the EMR. The method and system additionally provides for notifying a user of one or more of a drug interaction, a recommended medical procedure, and a result of at least one of a previous medical procedure, previous laboratory test, and a failure of the patient to follow-up to a physician request based on at least user input to the EMR.
Owner:GENERAL ELECTRIC CO
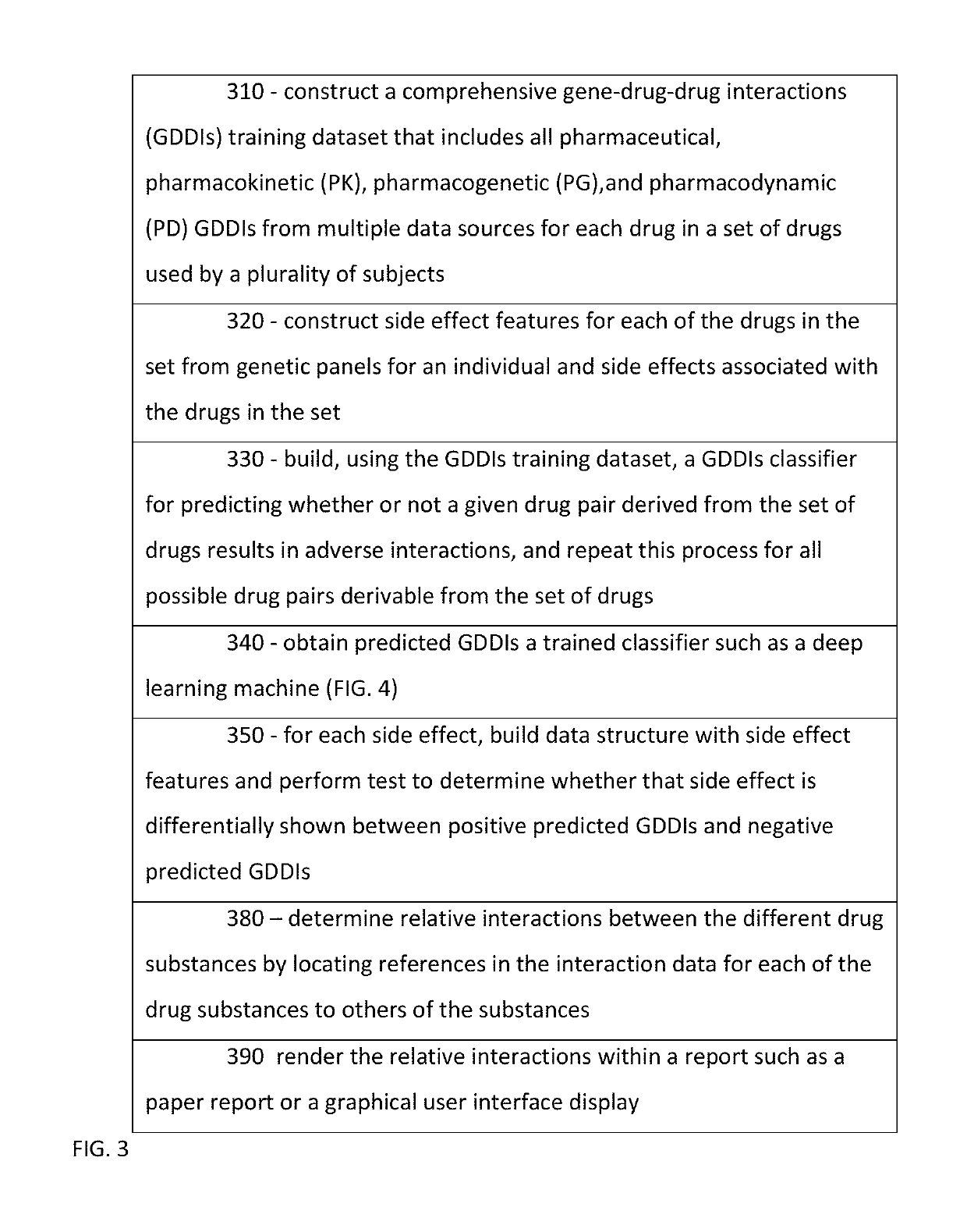
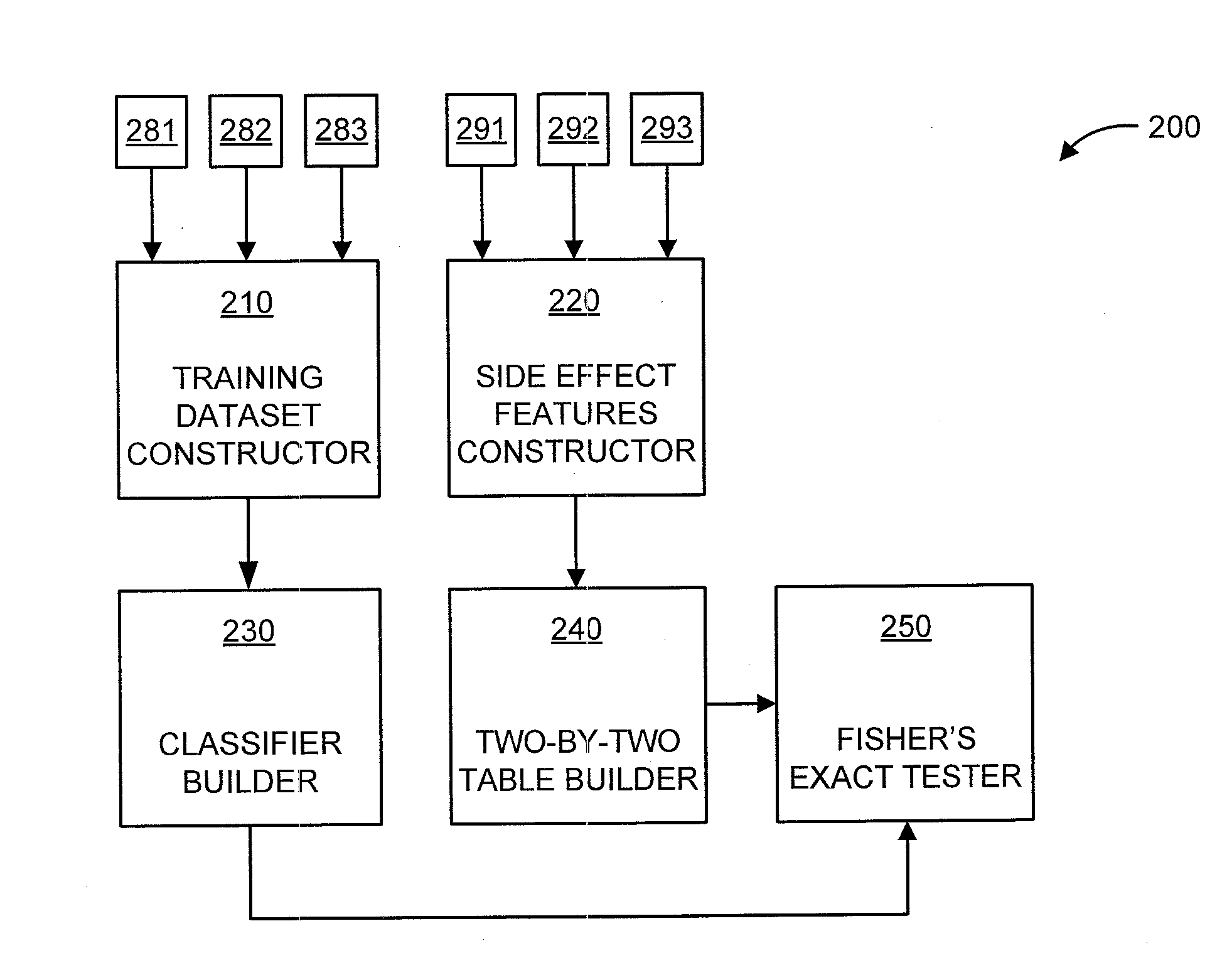
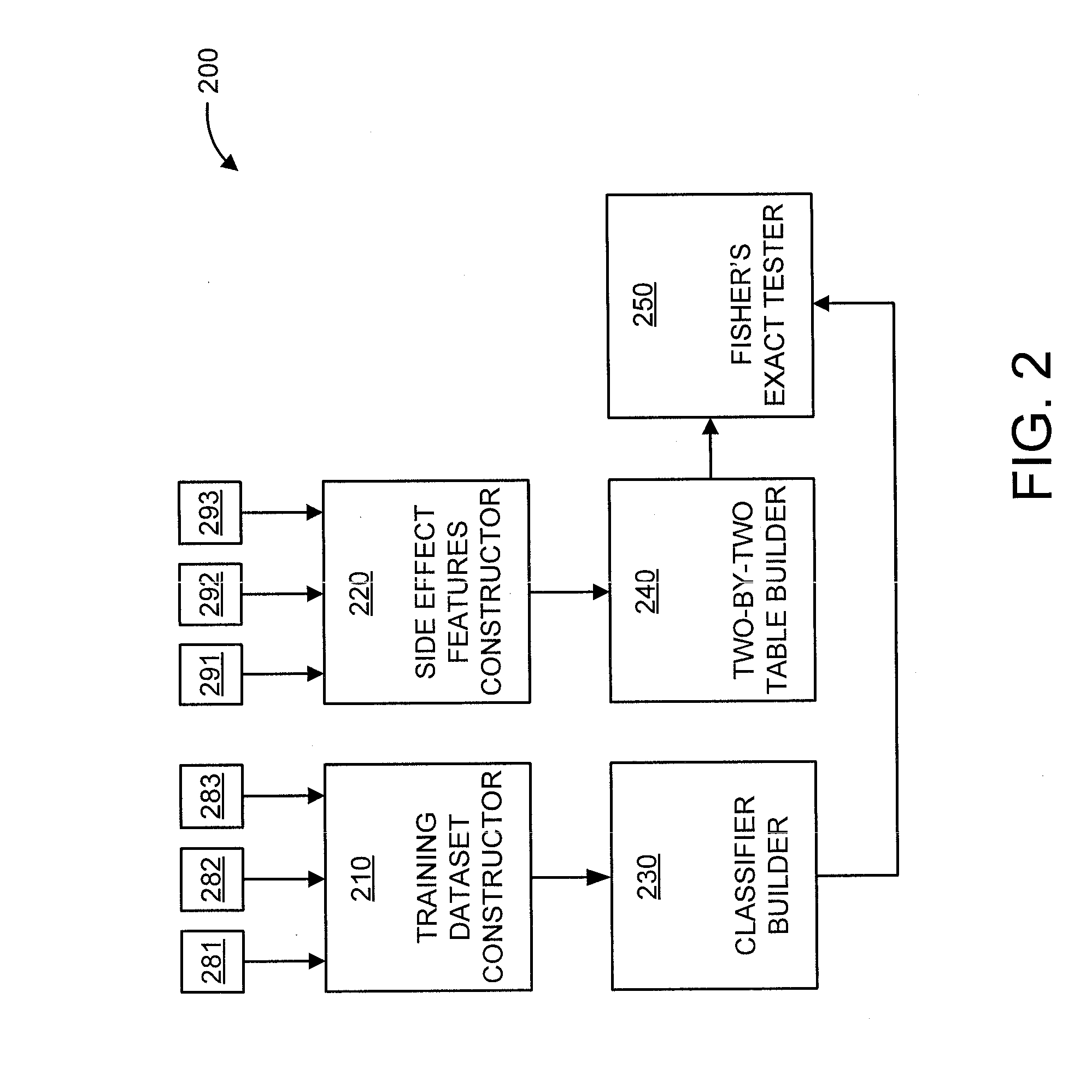
Predicting drug-drug interactions based on clinical side effects
A processor-implemented method, computer program product and system are provided for predicting drug-drug interactions based on clinical side effects. The method includes constructing a drug-drug interactions training dataset that includes pharmaceutical, pharmacokinetic or pharmacodynamics drug-drug interactions from multiple data sources for each of a plurality of drugs. The method also includes constructing side effect features for each of the drugs from side effects associated with the drugs. The method further includes building, using the drug-drug interactions training dataset, a drug-drug interactions classifier that predicts adverse drug-drug interactions for drug pairs derivable from the drugs. The method additionally includes for each of the side effects, building a two-by-two table using the side effect features, and performing a Fisher's exact test using the two-by-two table to determine whether a given one of side effects is differentially shown between positive predicted drug-drug interactions and negative predicted drug-drug interactions.
Owner:IBM CORP
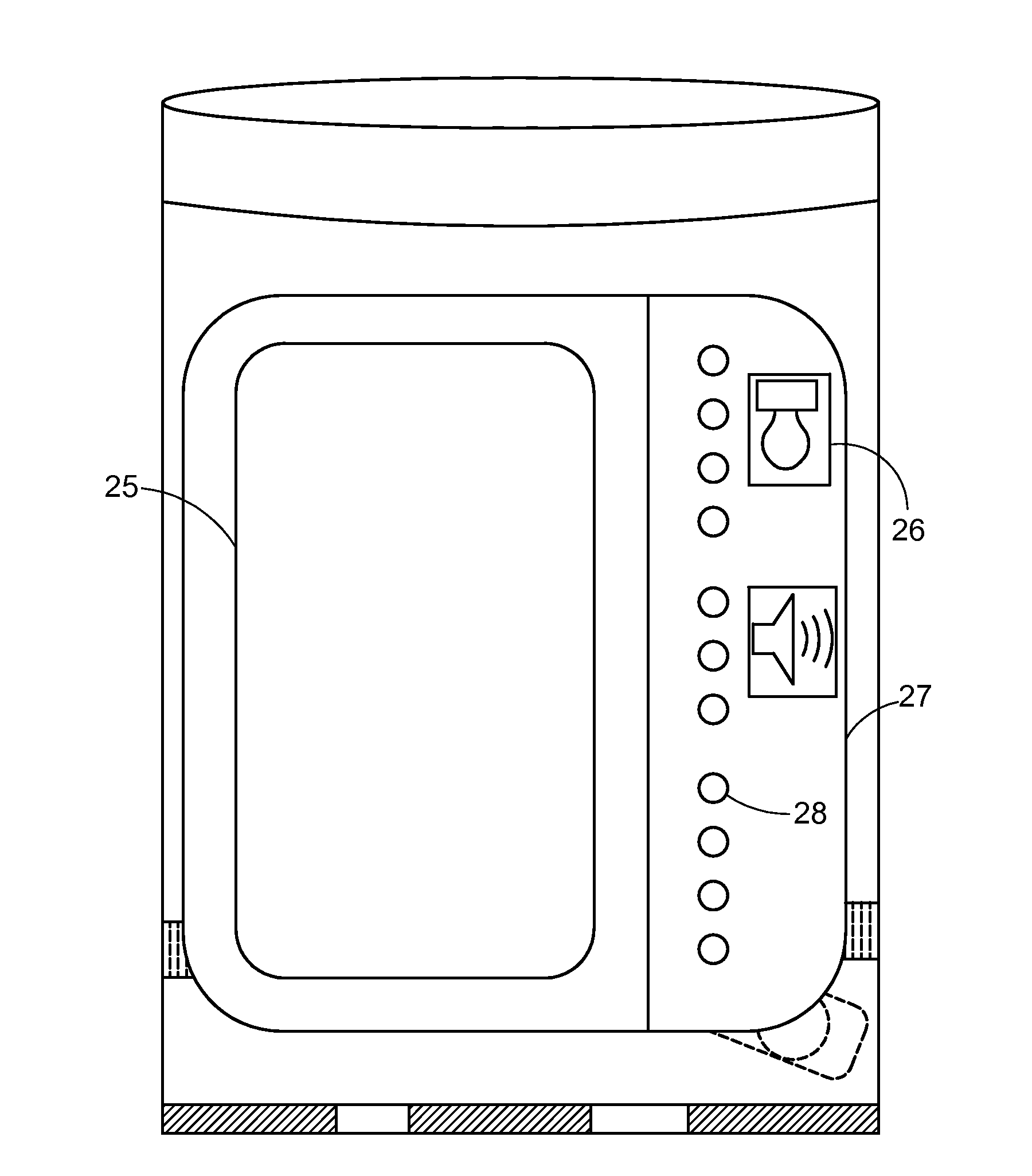
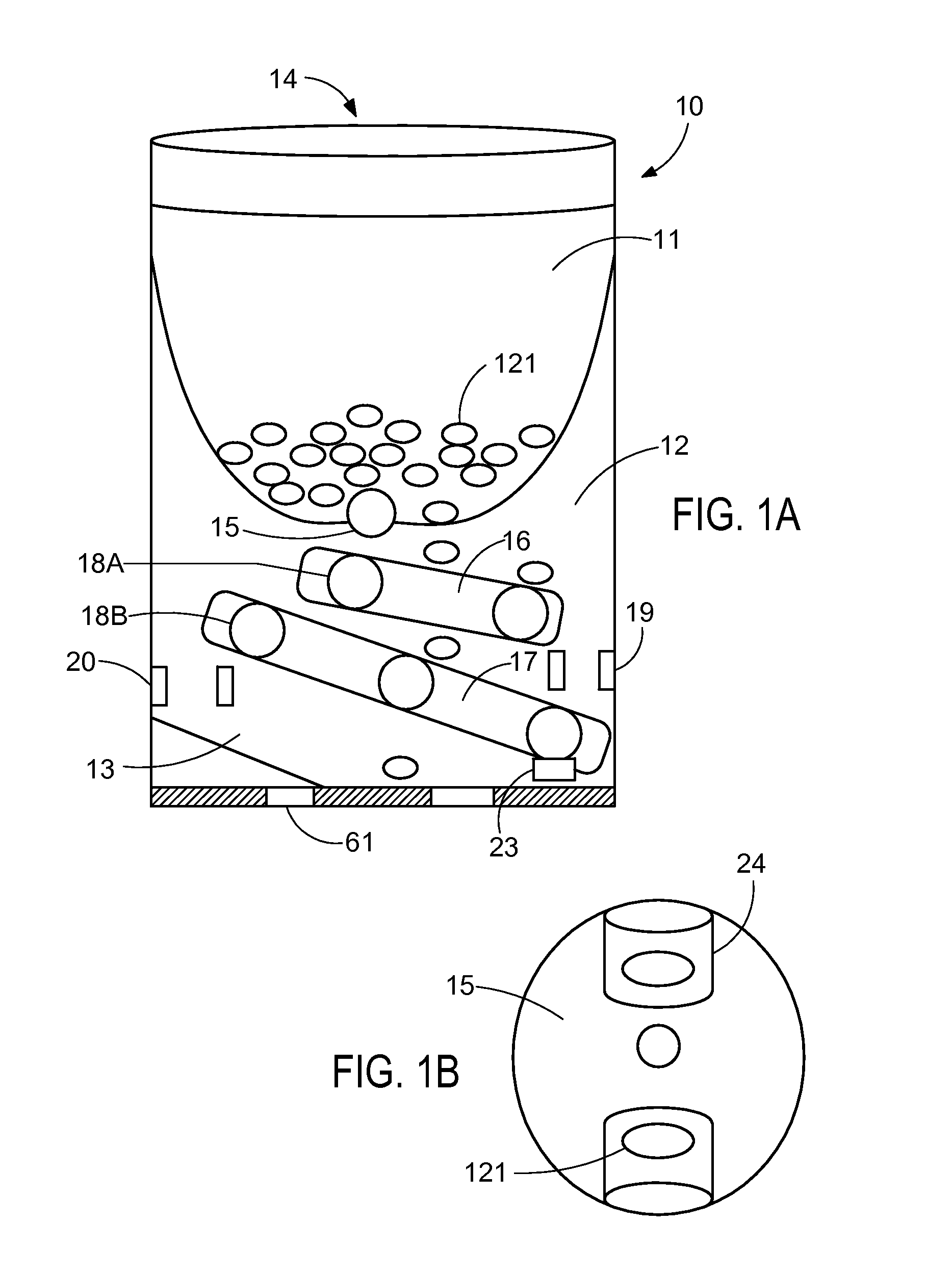
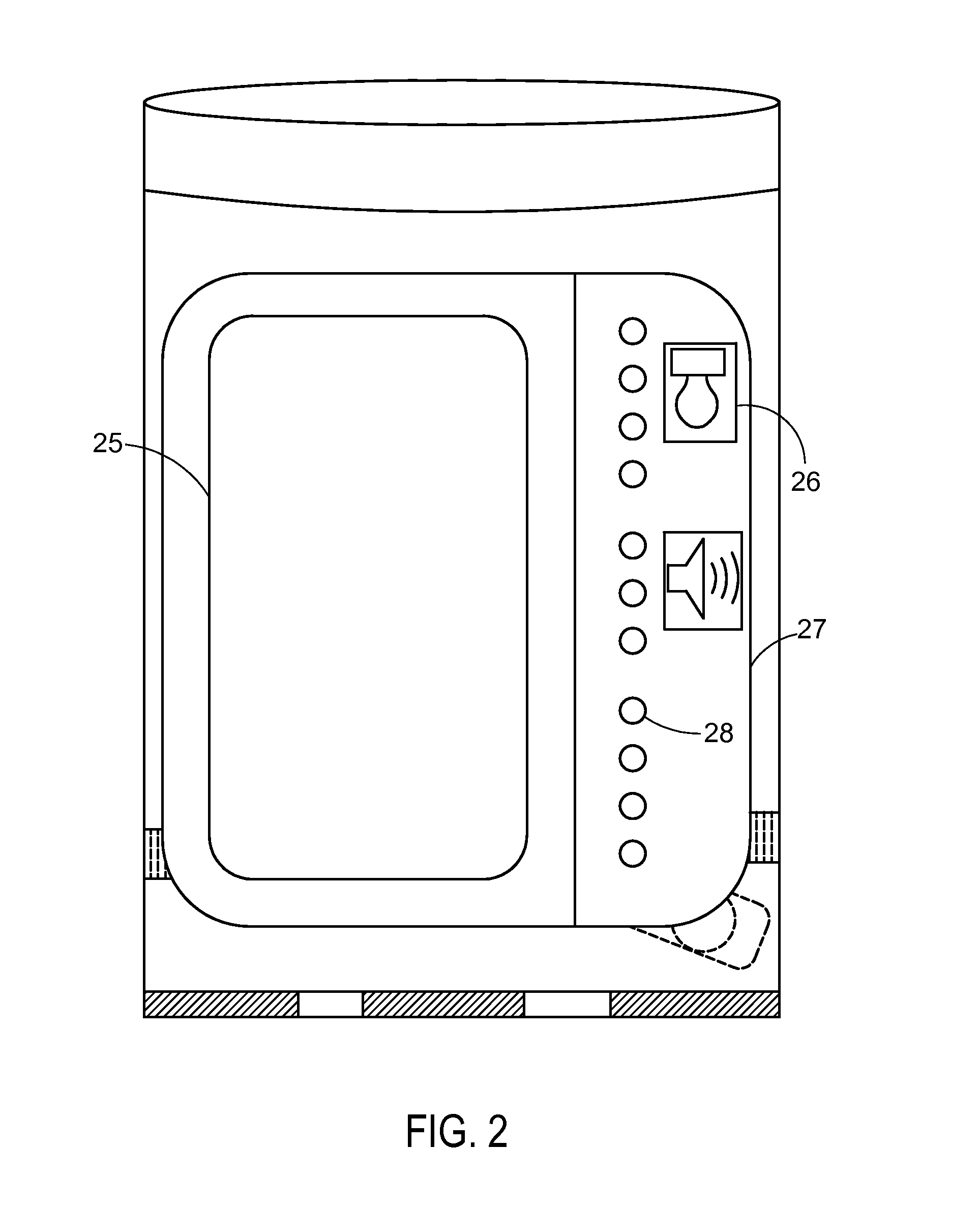
System and apparatus for displaying drug interactions on drug storage containers
The present disclosure relates to a system and apparatus for displaying drug interaction and side effects on a drug storage container. The system includes a server and a plurality of medicine dispensing devices communicatively connectable to the server. Each of the dispensing devices includes a medicine storage portion having a discharge port, a dispensing assembly configured to selectively receive the pills from the discharge port, an electronic display screen, and a control system. The control system is configured to associate a medicine stored in the medicine storage portion of the medicine dispensing device, generate a list of potential drug interactions associated with the medicine and a second medicine associated with a user, receive, from the server, the list of potential drug interactions associated with the medicine, and display the list of potential drug interactions on the electronic display screen of the first medicine dispensing device.
Owner:RATNAKAR NITESH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com