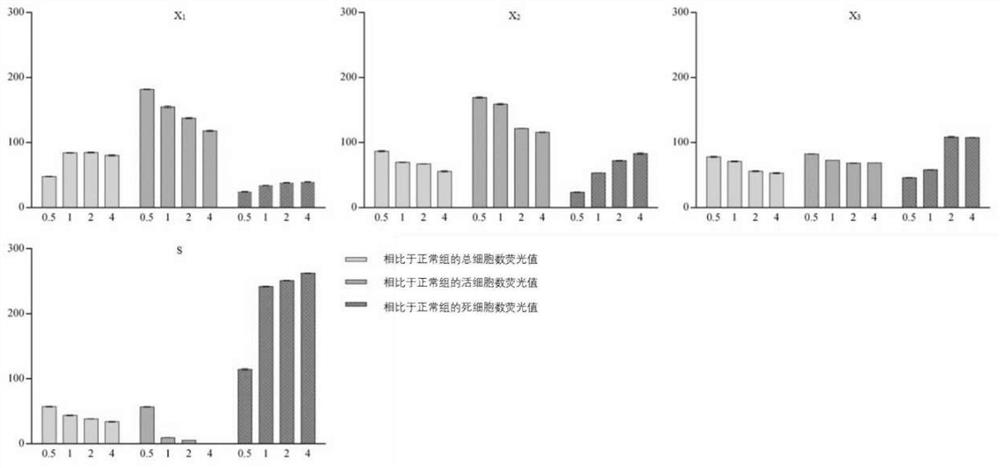
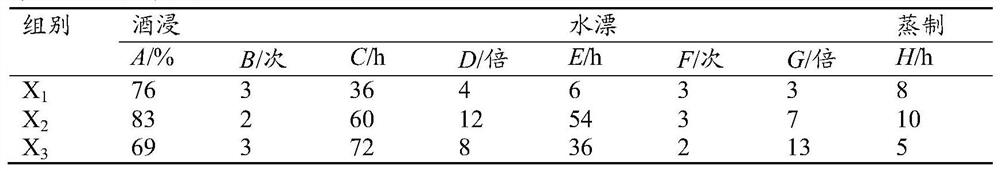
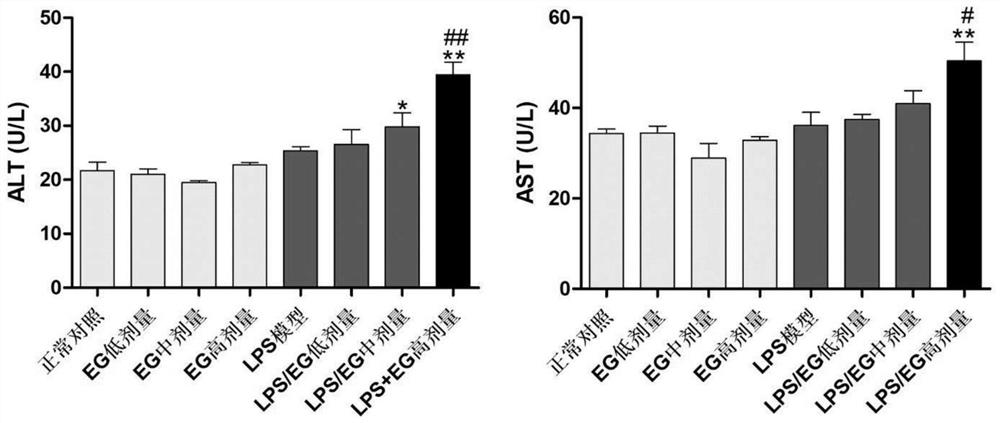
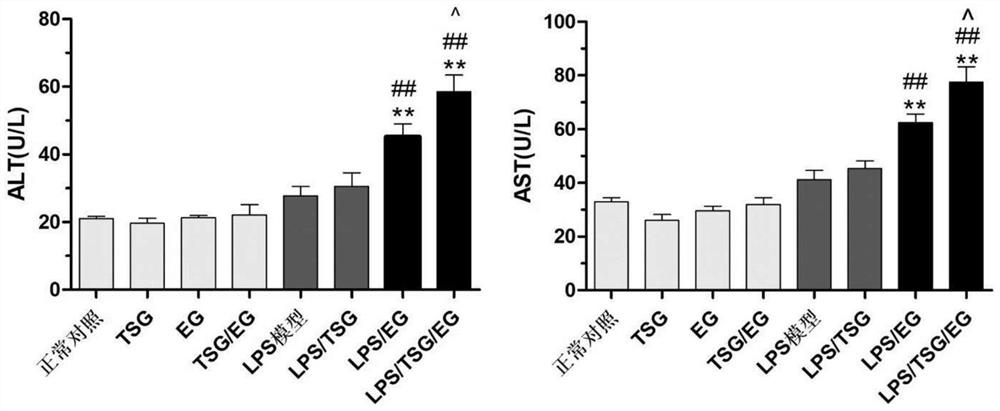
Patents
Literature
82 results about "Hepatic toxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
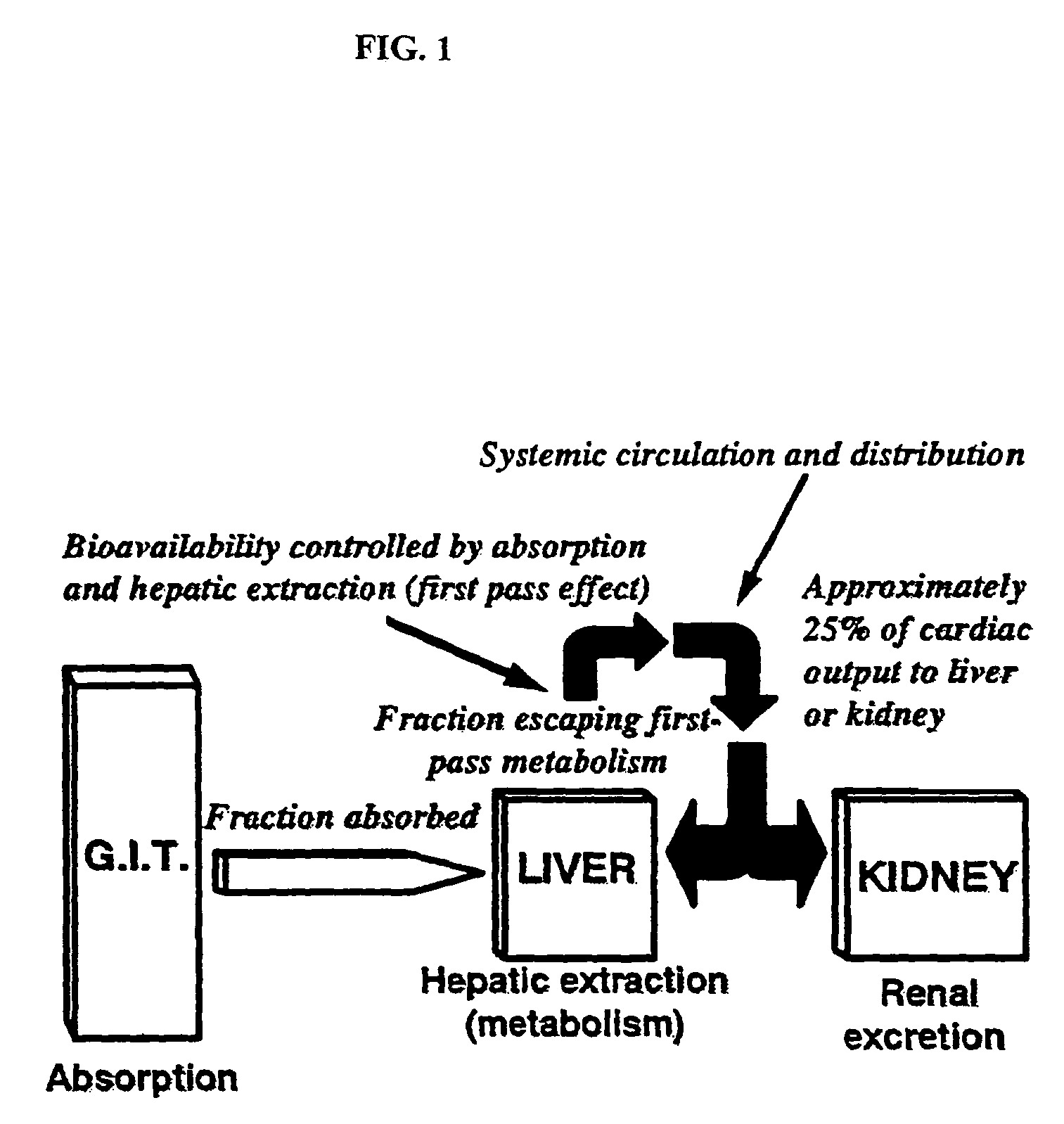
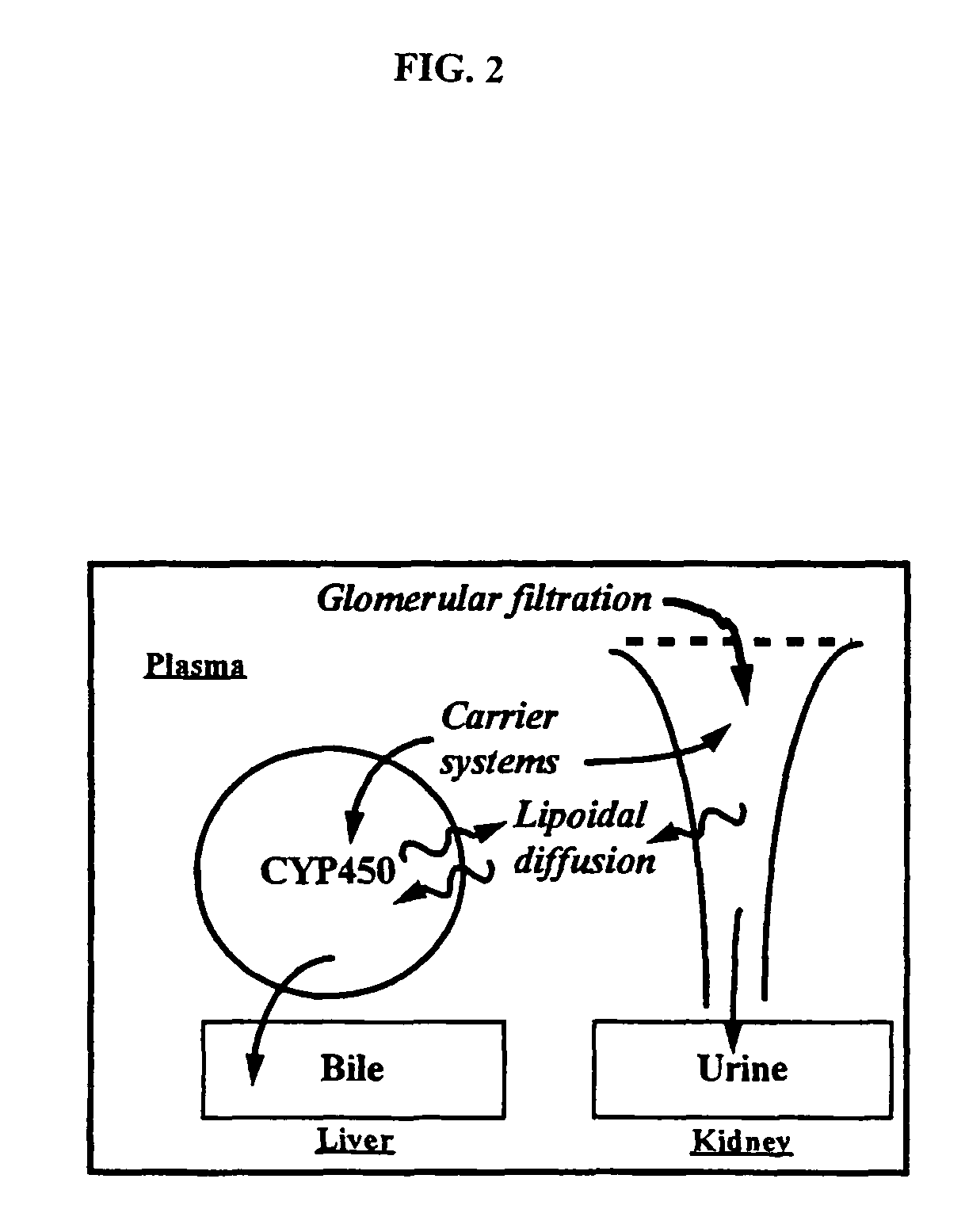
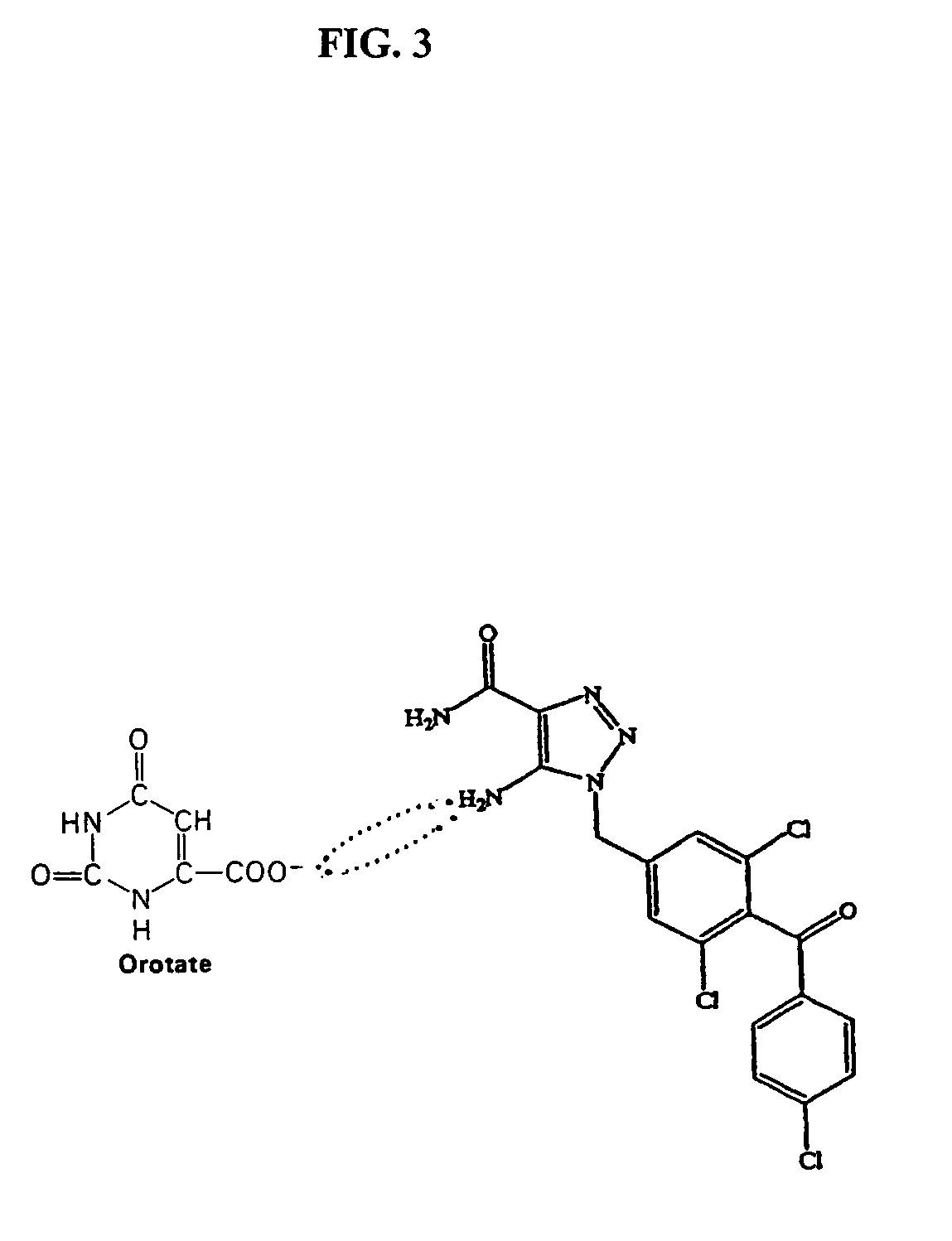
Method of increasing drug oral bioavailability and compositions of less toxic orotate salts
ActiveUS20060189640A1Improve bioavailabilityImprove drug bioavailabilitySalicyclic acid active ingredientsBiocideSide effectDrug interaction
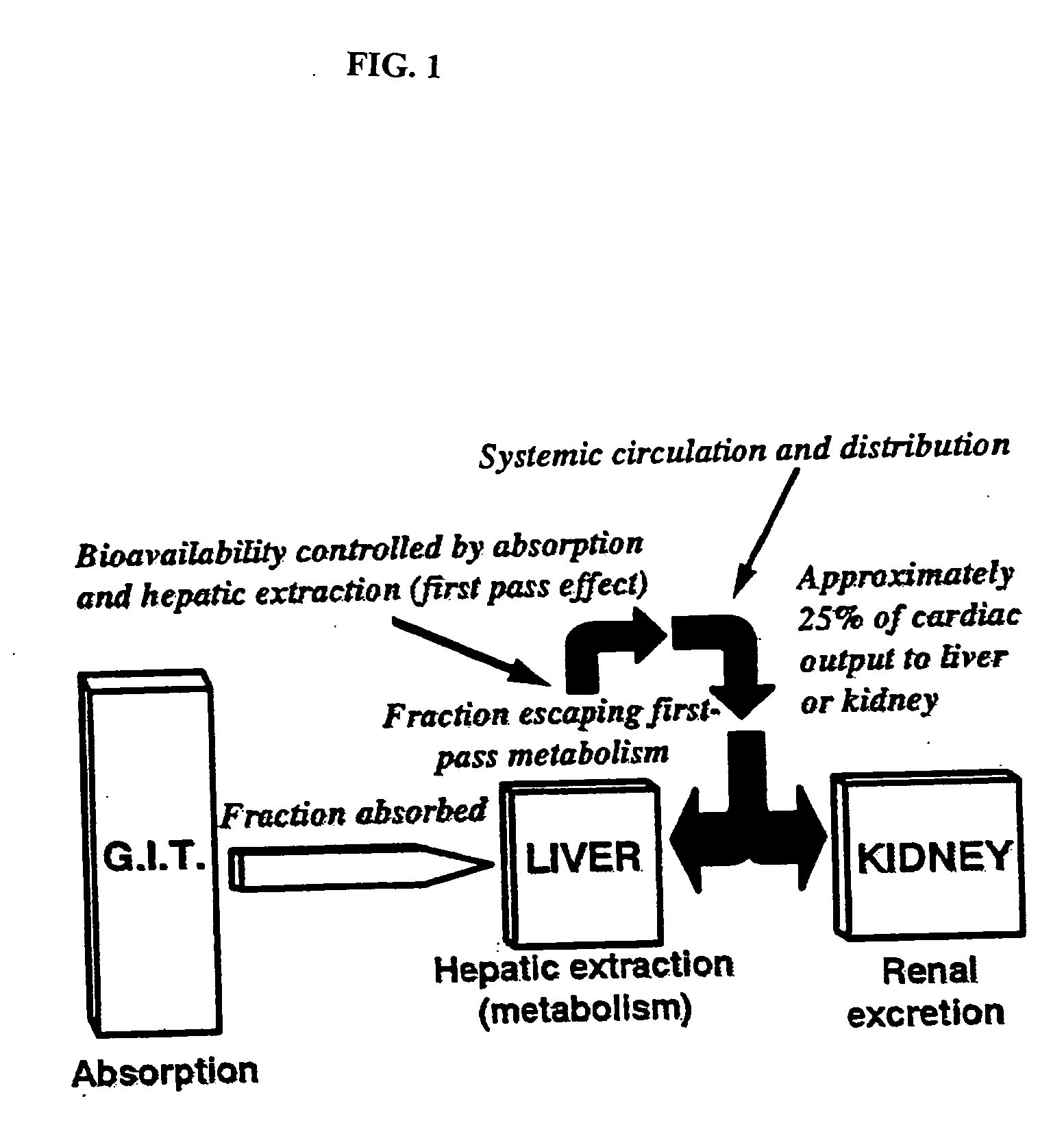
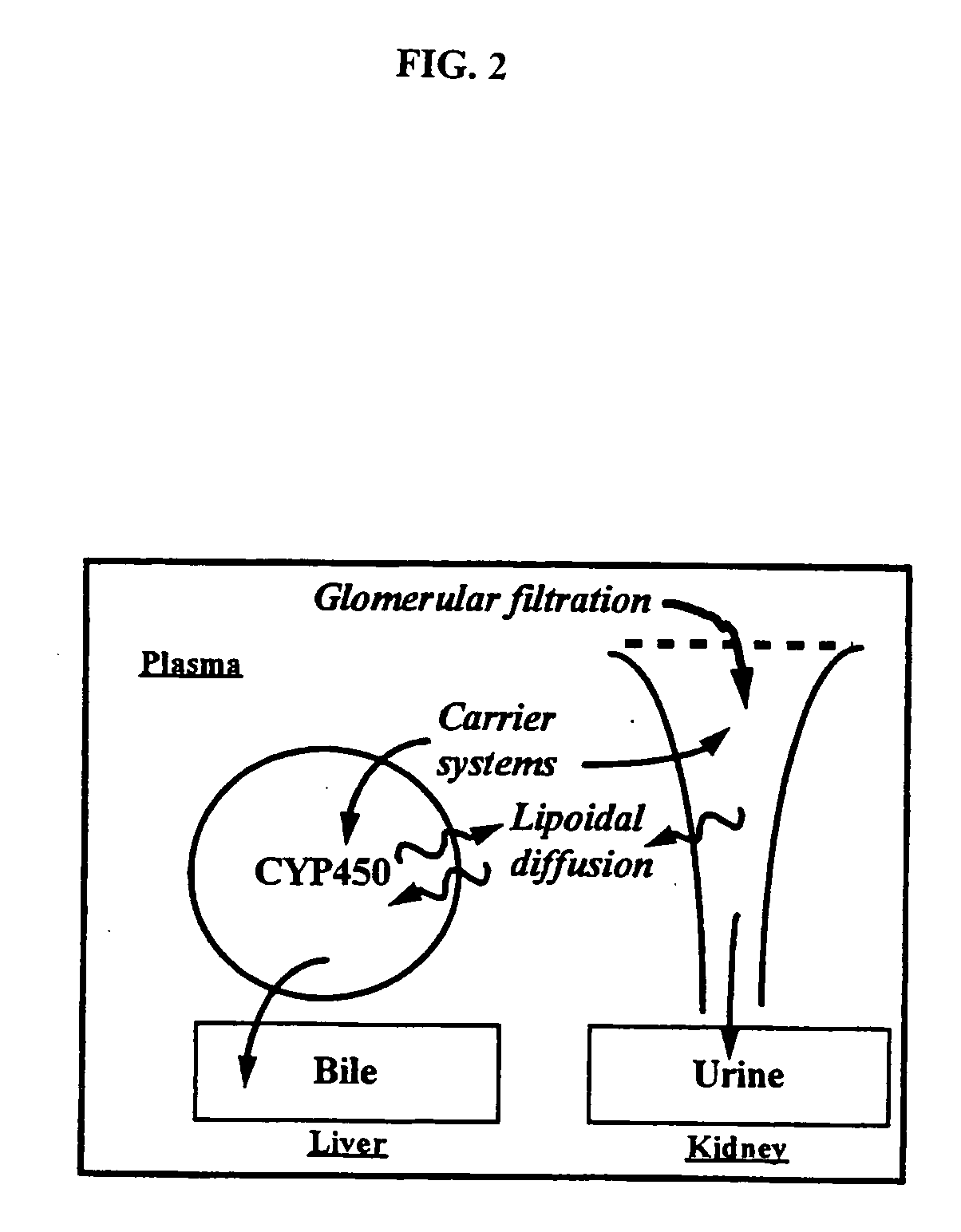
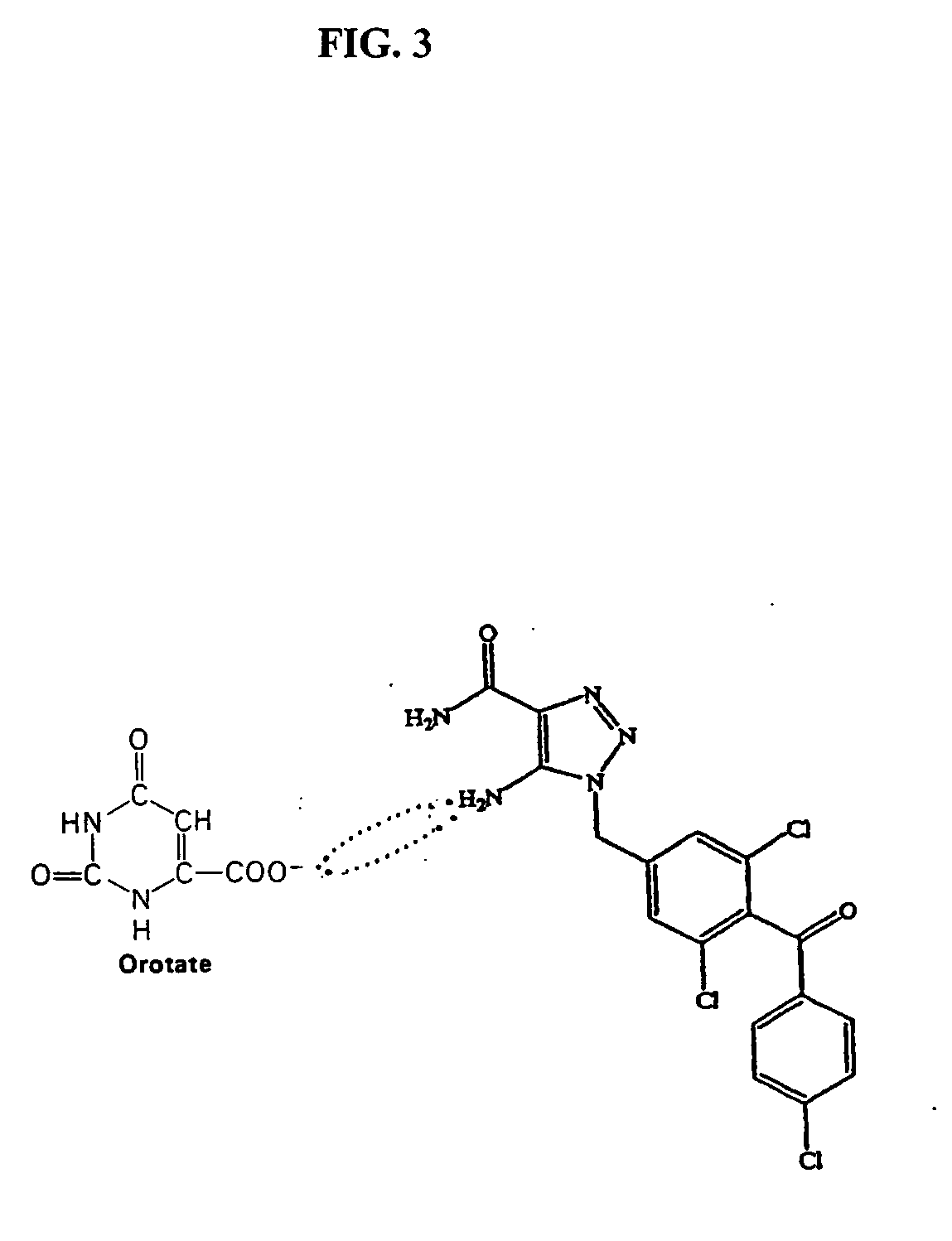
The present invention relates generally to the method of increasing the oral bioavailability, reducing chemotherapy induced toxicity and side effects, and improving the effectiveness of pharmaceutical agents that are poorly absorbed from the gastrointestinal tract. Specifically, the invention relates to poorly absorbed pharmaceutical drugs and converting them to orotate salts. The orotate salts of the drugs can be dosed at lower doses to provide the efficacy benefits of a higher dose, while reducing the drugs' toxic effects at lower doses. Additionally, the orotate salts of pharmaceutical agents have better clearance and reduce the potential for drug-induced hepatic toxicity. Therefore, an especially useful formulation of the orotate salt of the pharmaceutical agent can provide rapid and consistent action using a lower dose while reducing drug interactions and side-effects.
Owner:SAVVIPHARM INC
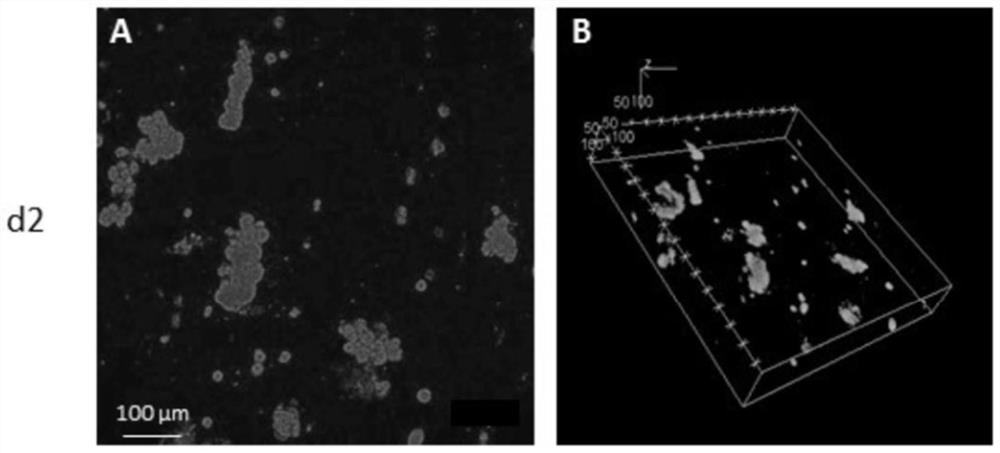
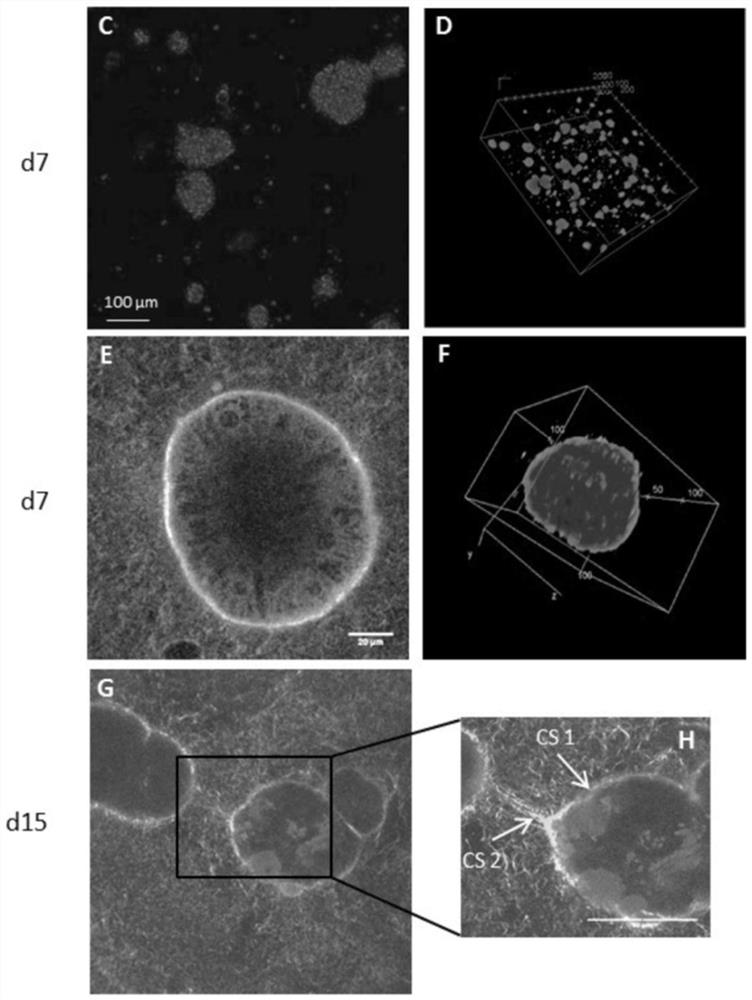
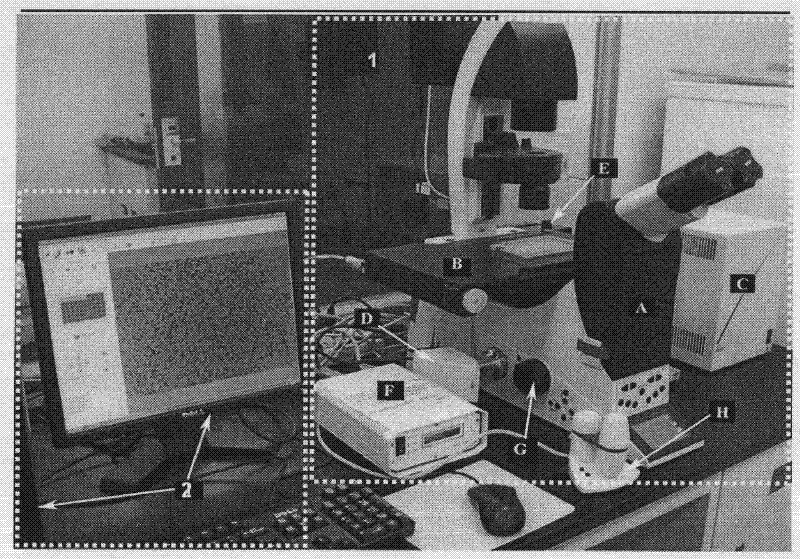
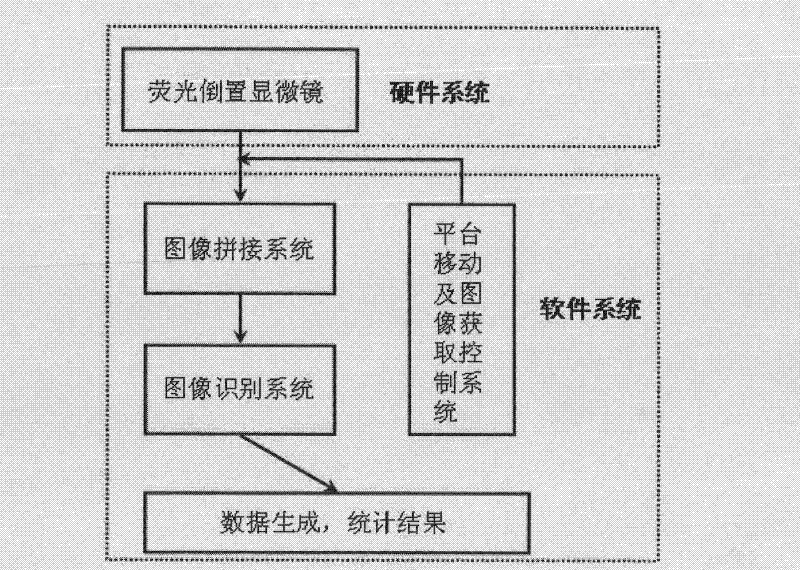
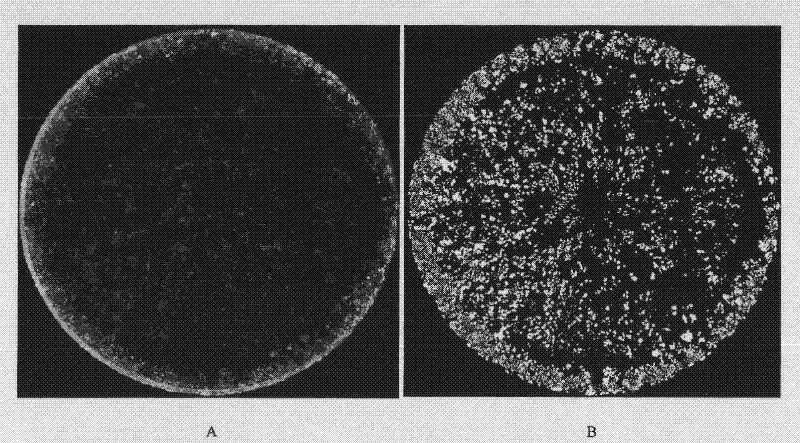
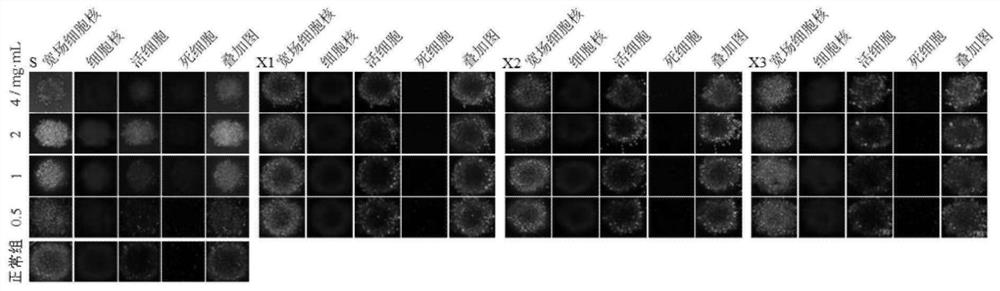
Hepatoxic substance sieving and evaluating method based on fluorescence labeling
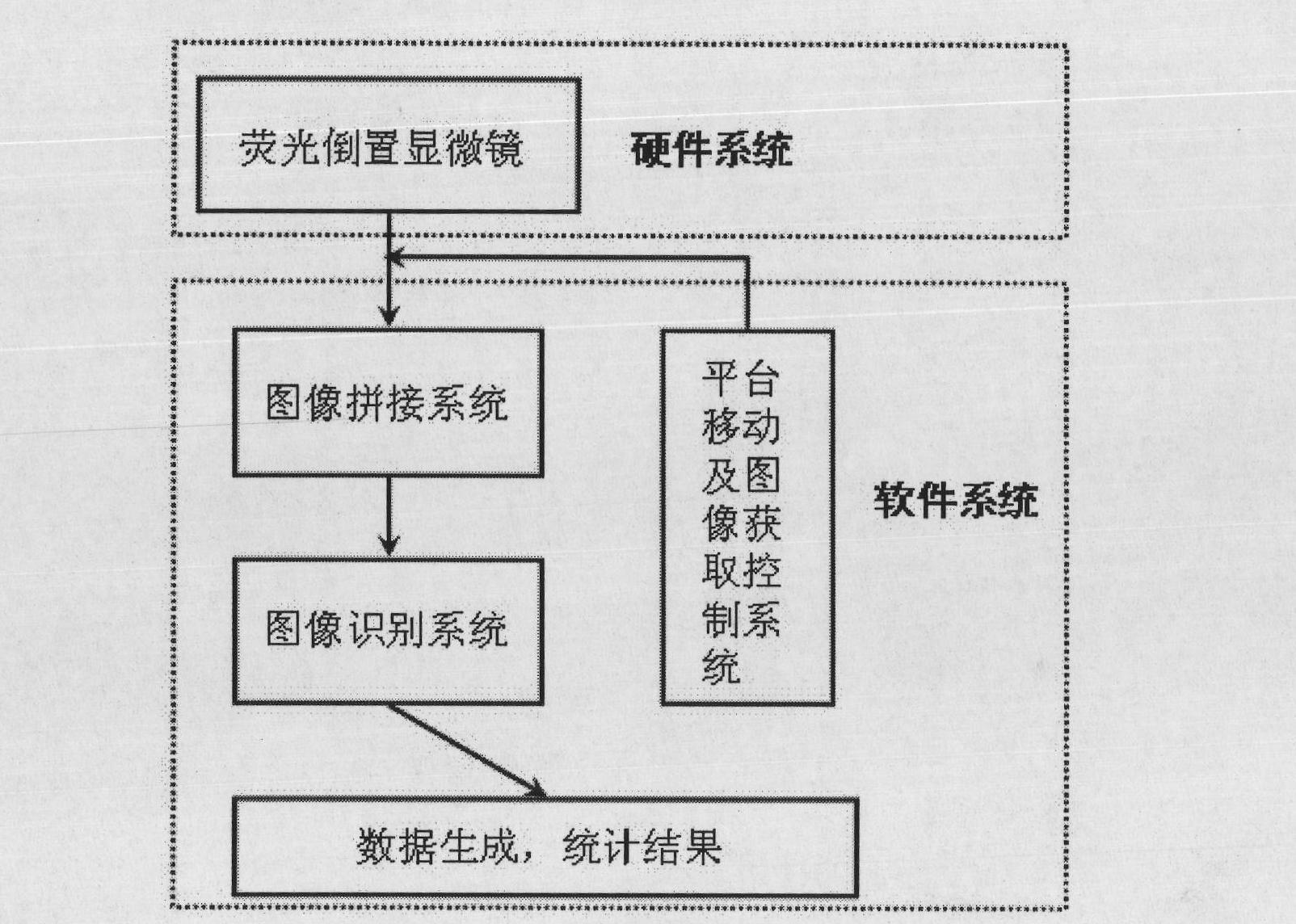
InactiveCN101788480AReliable resultsRealize screeningMicrobiological testing/measurementFluorescence/phosphorescenceOn cellsChemistry
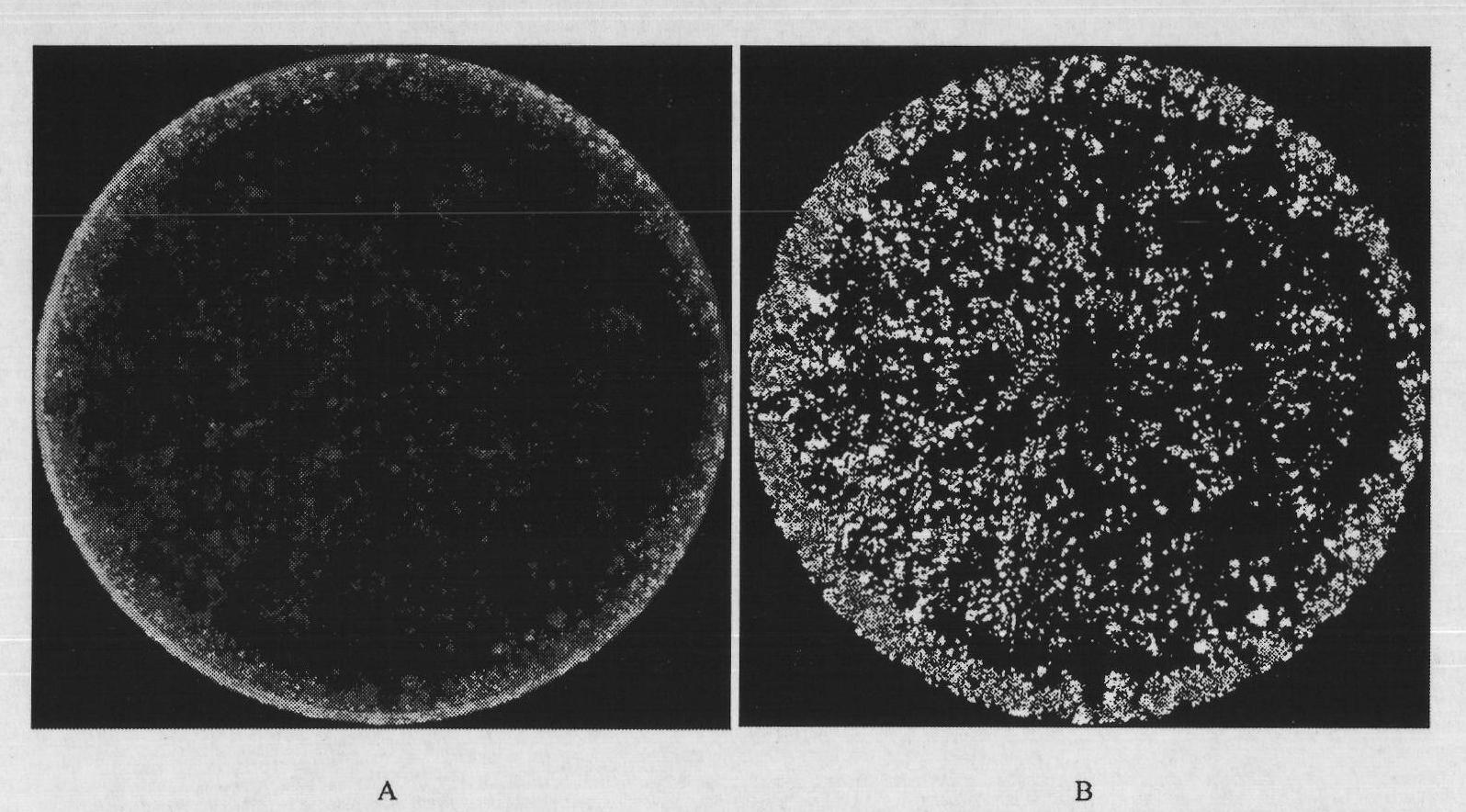
The invention provides a hepatoxic substance sieving and evaluating method based on cell states labeled by fluorescence, which is a sieving and evaluating method combining a hardware / software system. The images are acquired by a fluorescence inverted microscope and a computer in real time, the biological information is processed and visualized, the liner dynamic range width of the living cell is measured by combining FDA dyeing, and then the relevant parameters for evaluating the hepatoxic substances are provided. The method is quick, sensitive and economical, and is used for quickly detecting the influence of the sieved sample on cell quantity, forms, migration, apoptasis and the like under the condition of maintaining cell structure and function integrity, accurately carrying out quantitative analysis on the activity of the cell labeled by the fluorescence specialty and detecting the activity change of the cell under the function of the hepatoxic substances. The method has reasonable design. The provided sieving and evaluating system has complete structure, and can be used for sieving and evaluating the hepatoxic substances.
Owner:ZHEJIANG UNIV
Combination of MTP inhibitors or apoB-secretion inhibitors with fibrates for use as pharmaceuticals
The invention relates to the use of fibrates for lowering the liver toxicity of MTP inhibitors as well as pharmaceutical compositions containing an MTP inhibitor and a fibrate.
Owner:BOEHRINGER INGELHEIM PHARM KG
Methods for improving the therapeutic index for a chemotherapeutic drug
PendingUS20190038761A1Reduce riskLow toxicityPowder deliveryOrganic active ingredientsChemotherapeutic drugsLiver toxicity
This disclosure relates to methods for improving the therapeutic index of a chemotherapeutic drug in the treatment of patients afflicted with cancer, including, for example, reducing chemotherapy-related toxicity (e.g., liver toxicity).
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Non-invasive apparatus system for monitoring drug hepatoxicity and uses thereof
The invention relates to a non-invasive apparatus system for monitoring drug hepatotoxicity, and its uses in monitoring drug-induced hepatotoxicity and abnormal liver function.
Owner:IND TECH RES INST
Method for stabilizing aminotransferase activity in a biological fluid
InactiveUS6465202B1Easy to collectHigh activityMicrobiological testing/measurementBiological material analysisAminotransferase activityLiver toxicity
Methods and compositions for the stabilization of aminotransferase activity of plasma or serum are provided. Such methods will be useful in the accurate determination of tests associated with liver toxicity.
Owner:BIOSAFE LAB
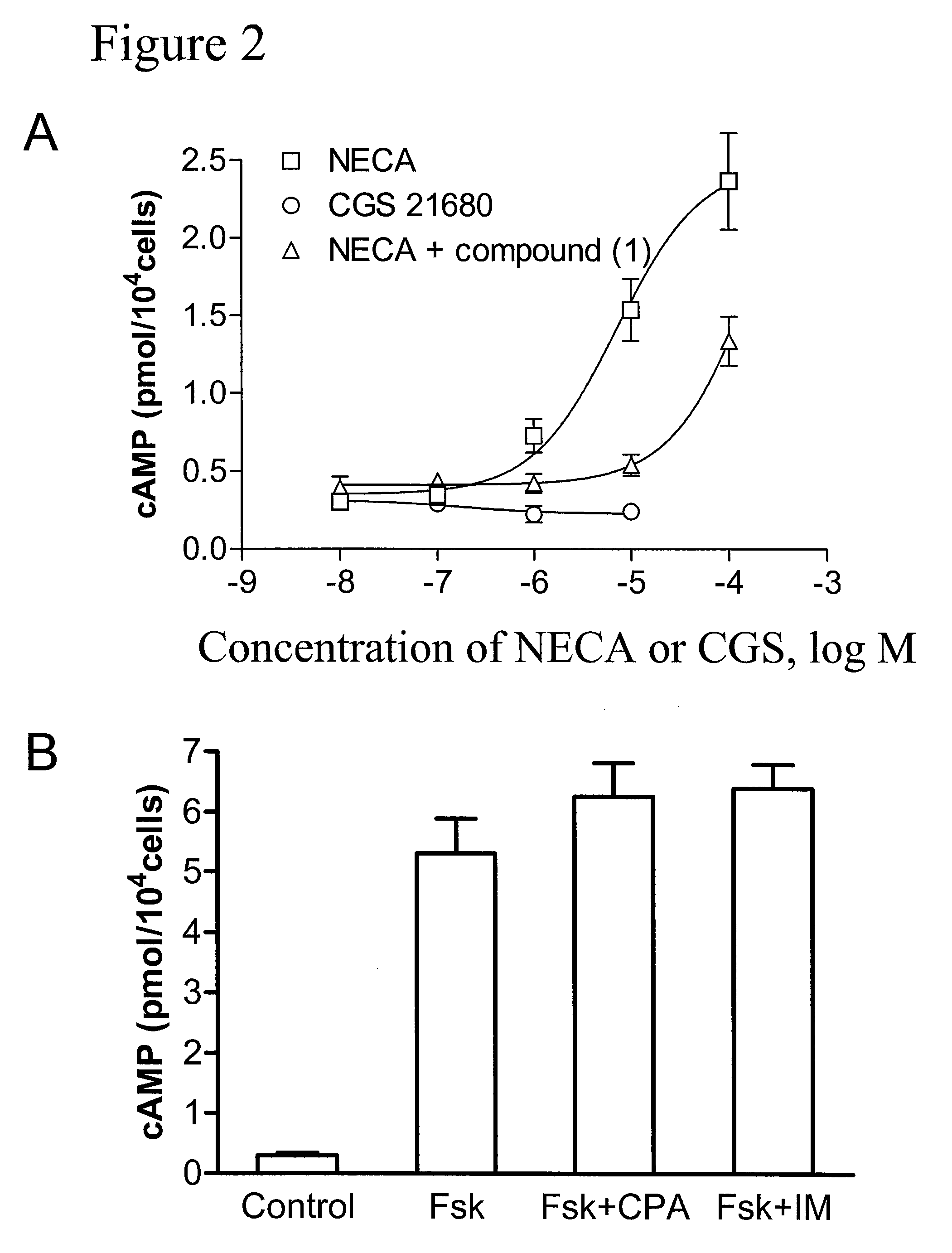
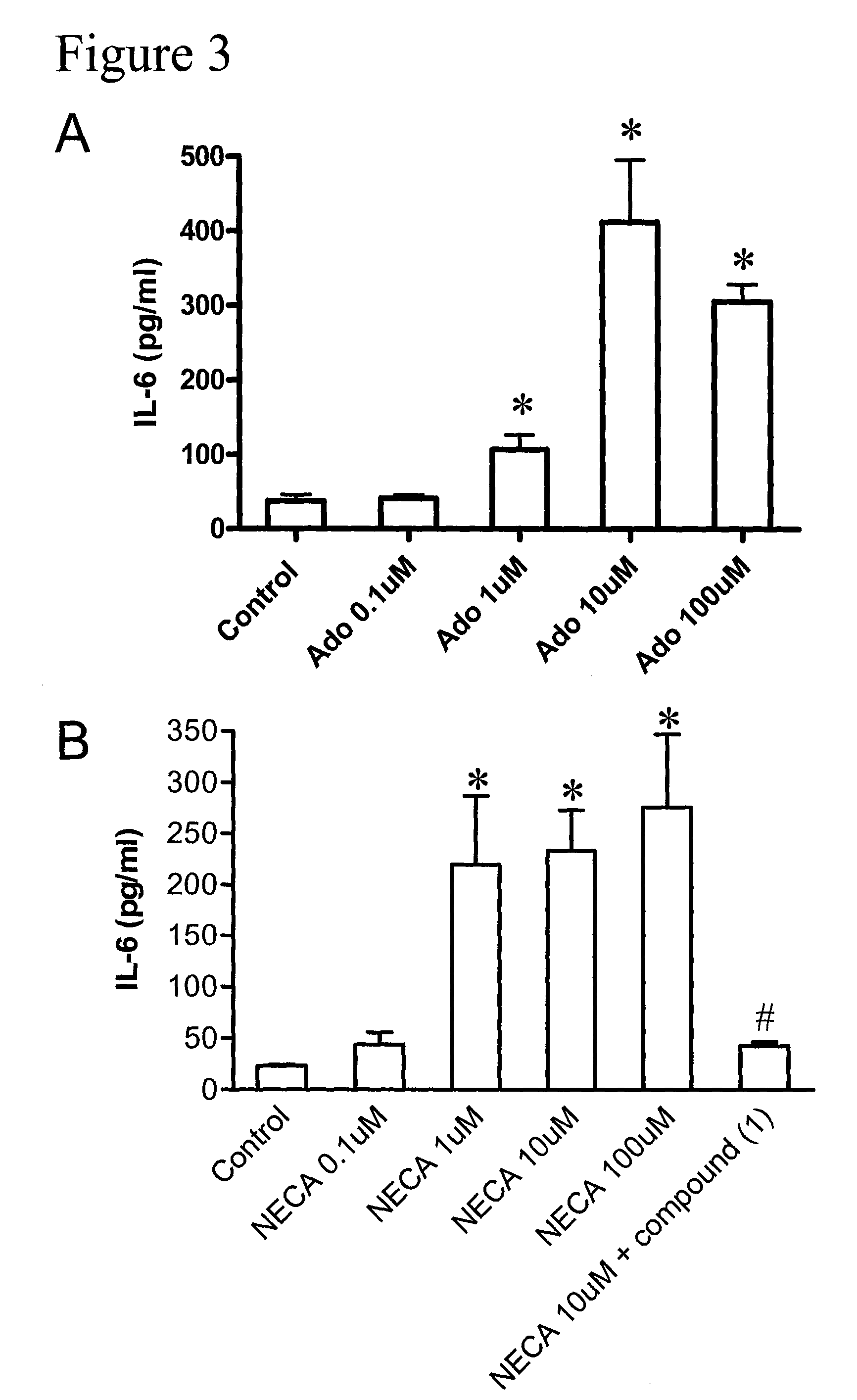
Method of treating hepatic disease using A2B adenosine receptor antagonists
ActiveUS7795268B2Decreasing hepatotoxic side effectBiocideOrganic chemistryAlcohol abuseHepatic inflammation
The invention is related to methods of treating hepatic fibrosis using A2B adenosine receptor antagonists and utility in the treatment of liver damage caused by alcohol abuse, surgical intervention, viral hepatitis, the ingestion of hepatotoxic drugs, or other hepatic diseases.
Owner:GILEAD SCI INC
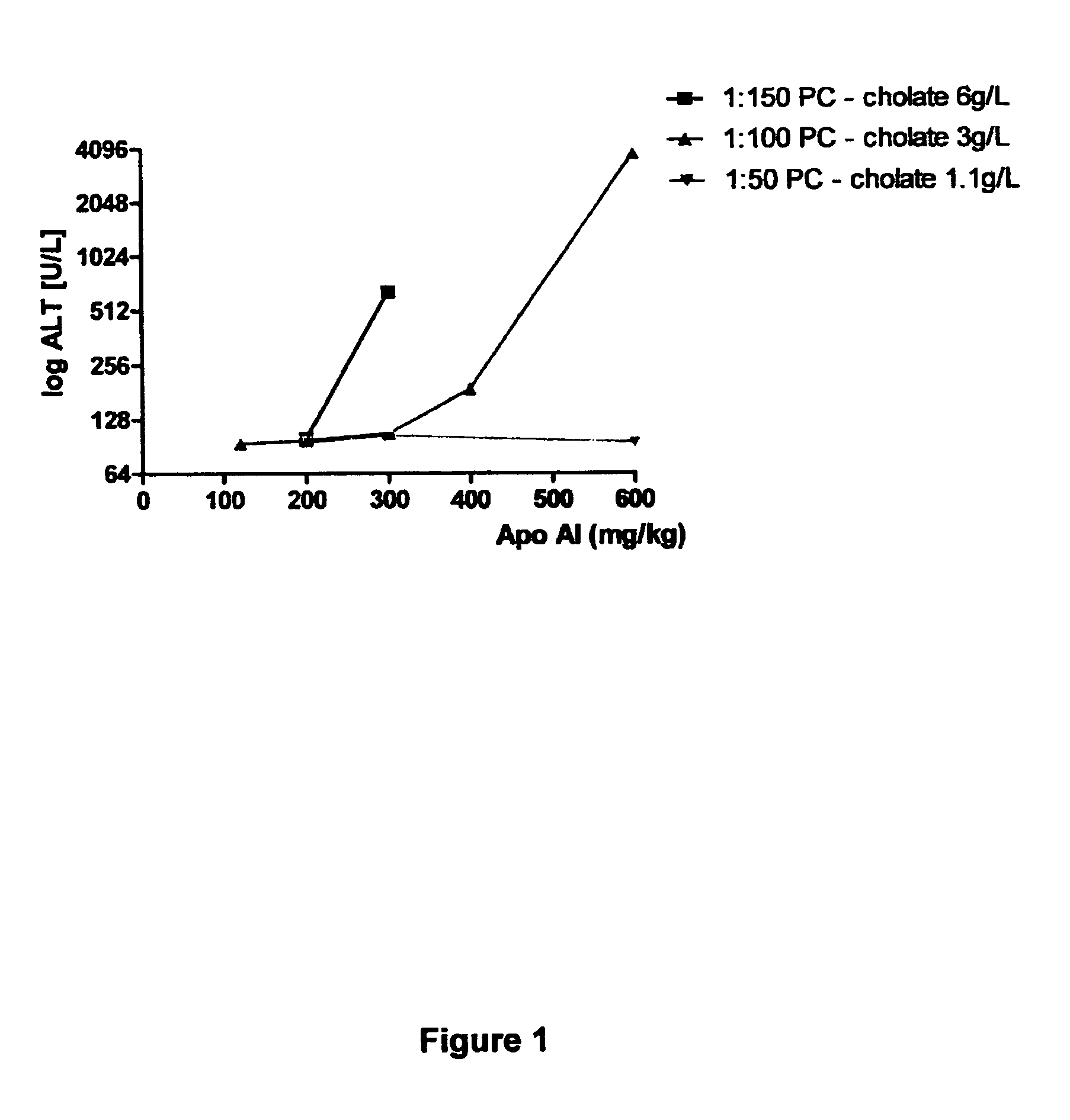
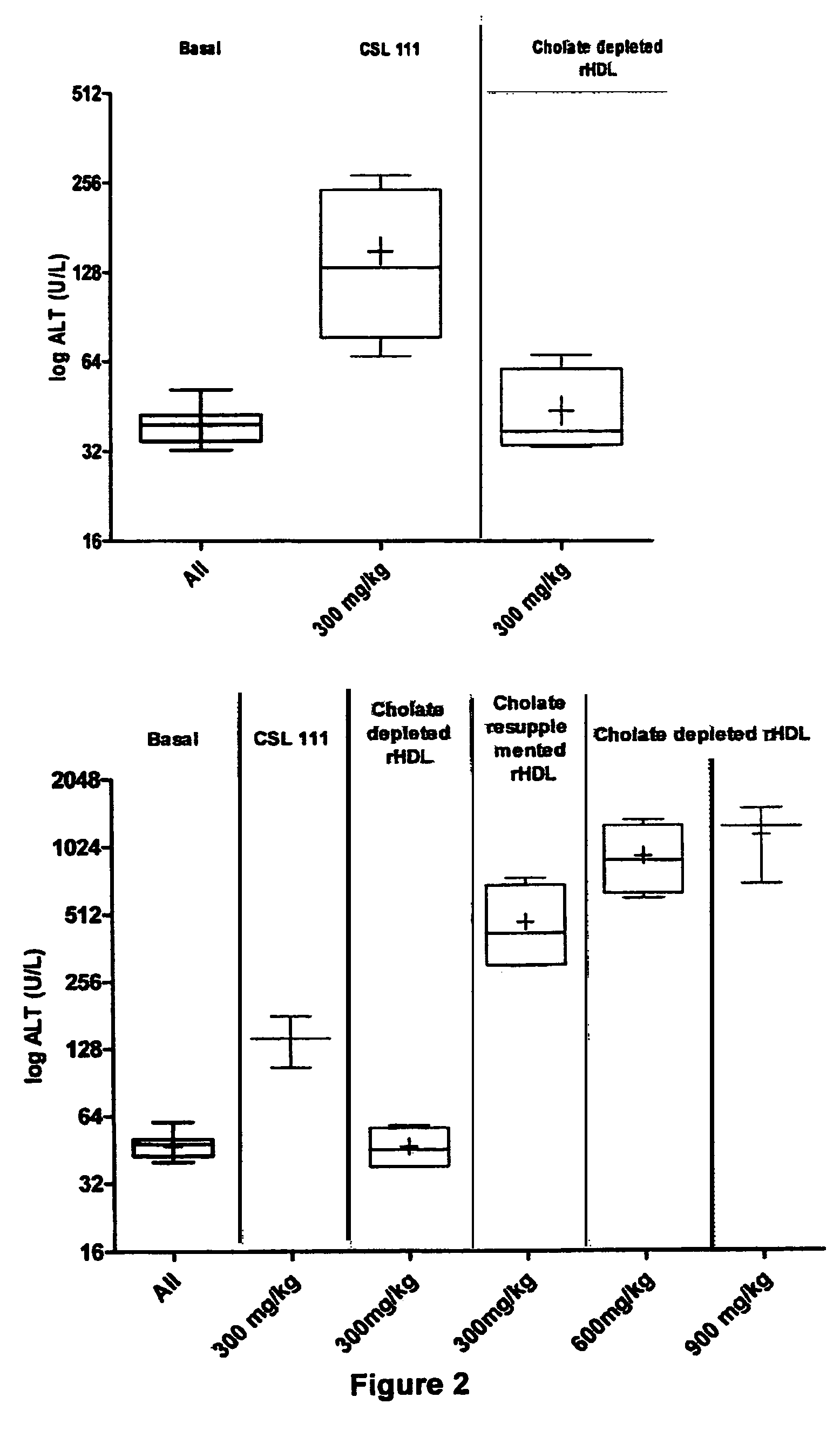
Reconstituted high density lipoprotein formulation and production method thereof
ActiveUS8999920B2Reduced and minimal toxicityOrganic active ingredientsPeptide/protein ingredientsLipid formationApolipoproteins E
A reconstituted high density lipoprotein formulation having relatively low toxicity comprises an apolipoprotein such as ApoAI or fragment thereof, a lipid and a detergent at a level which is about 5-50% of that which would normally cause liver toxicity upon administration to a human. The lipid is optimally phosphatidylcholine at about 30-50 g / L and the molar ratio of apolipoprotein:lipid is optimally in the range 1:40 to 1:75. The formulation is useful for treating diseases or conditions such as cardiovascular disease, hypercholesterolaemia and hypocholesterolaemia inclusive of acute coronary syndrome (ACS), atherosclerosis and myocardial infarction.
Owner:CSL LTD
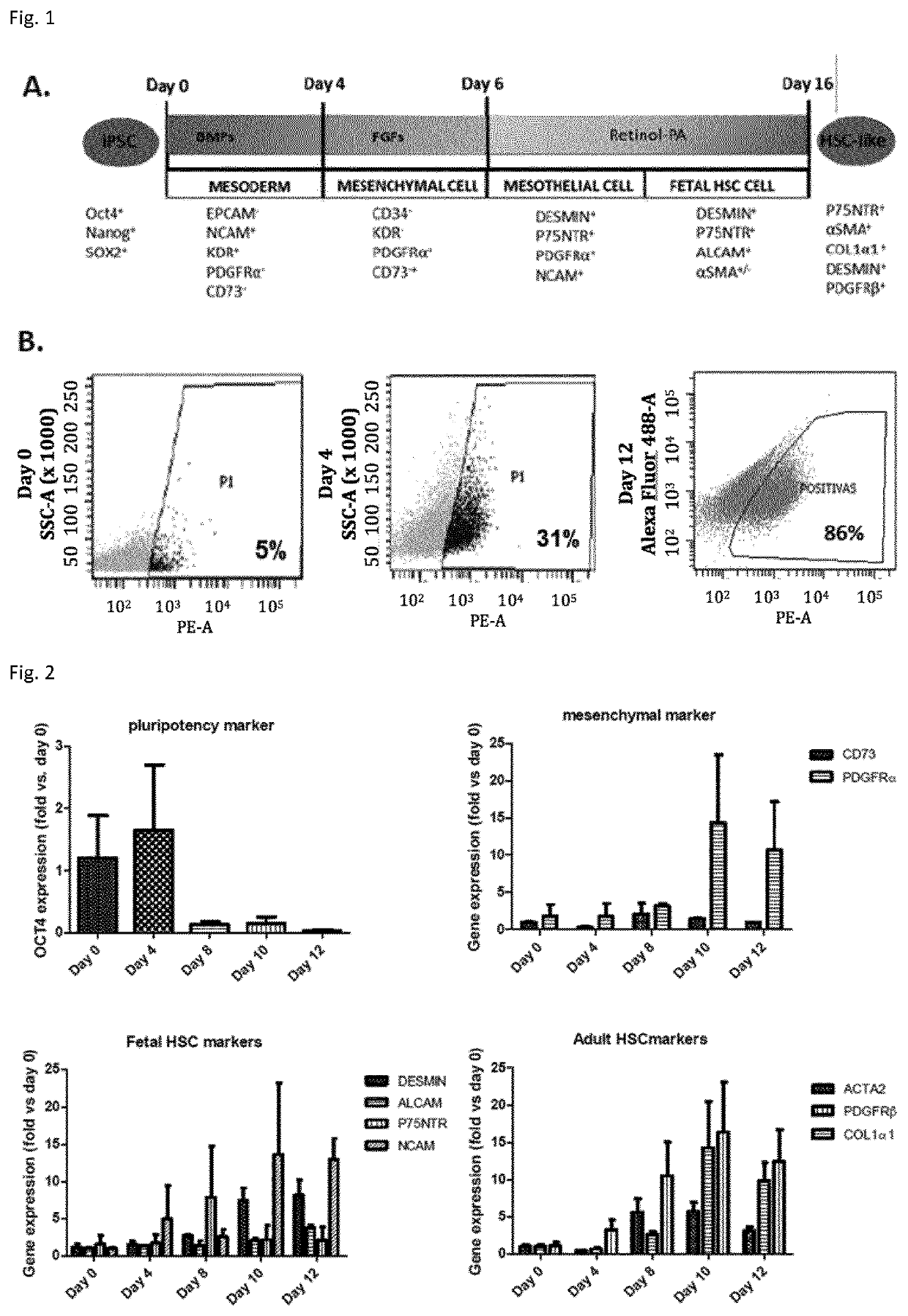
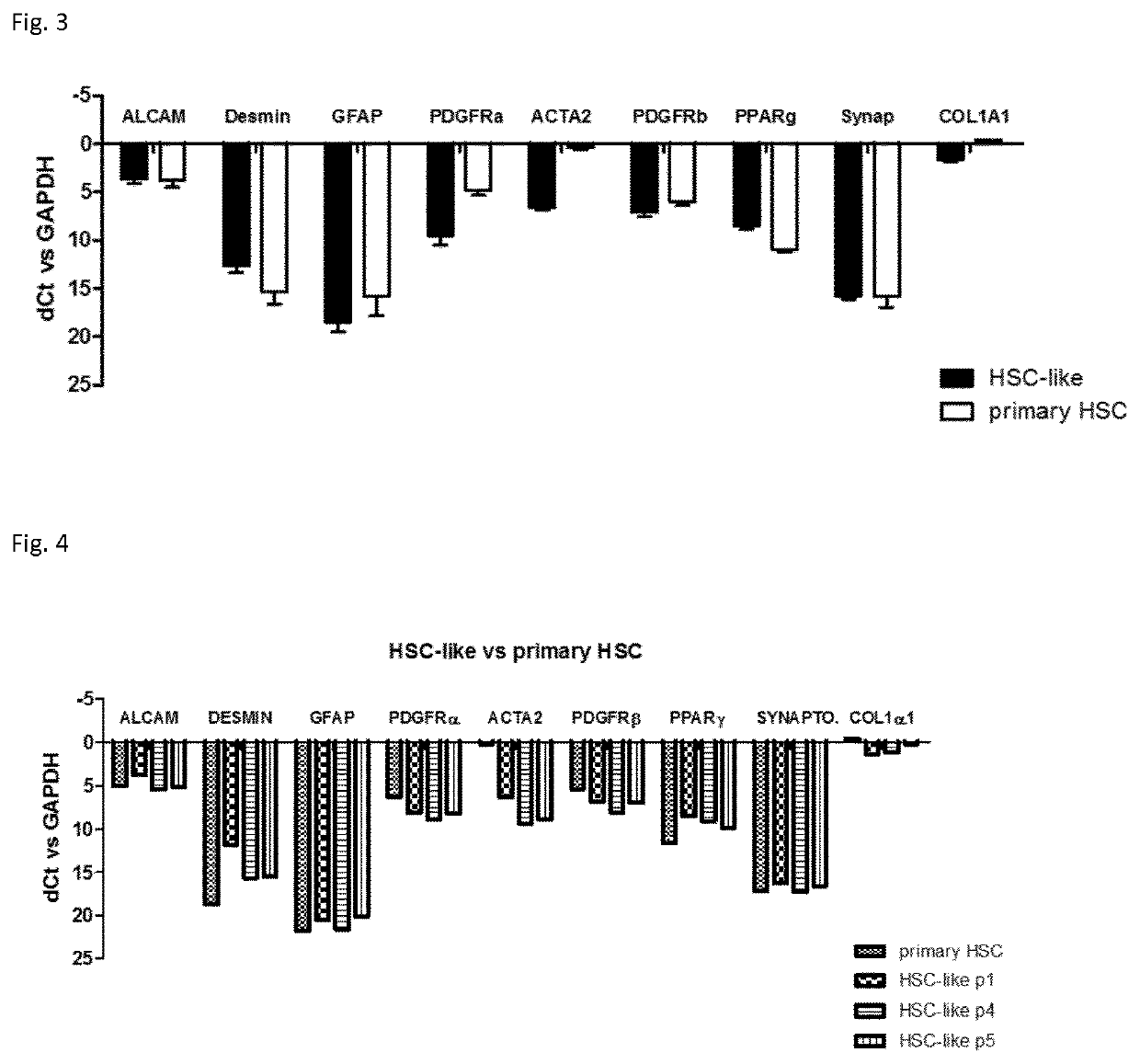
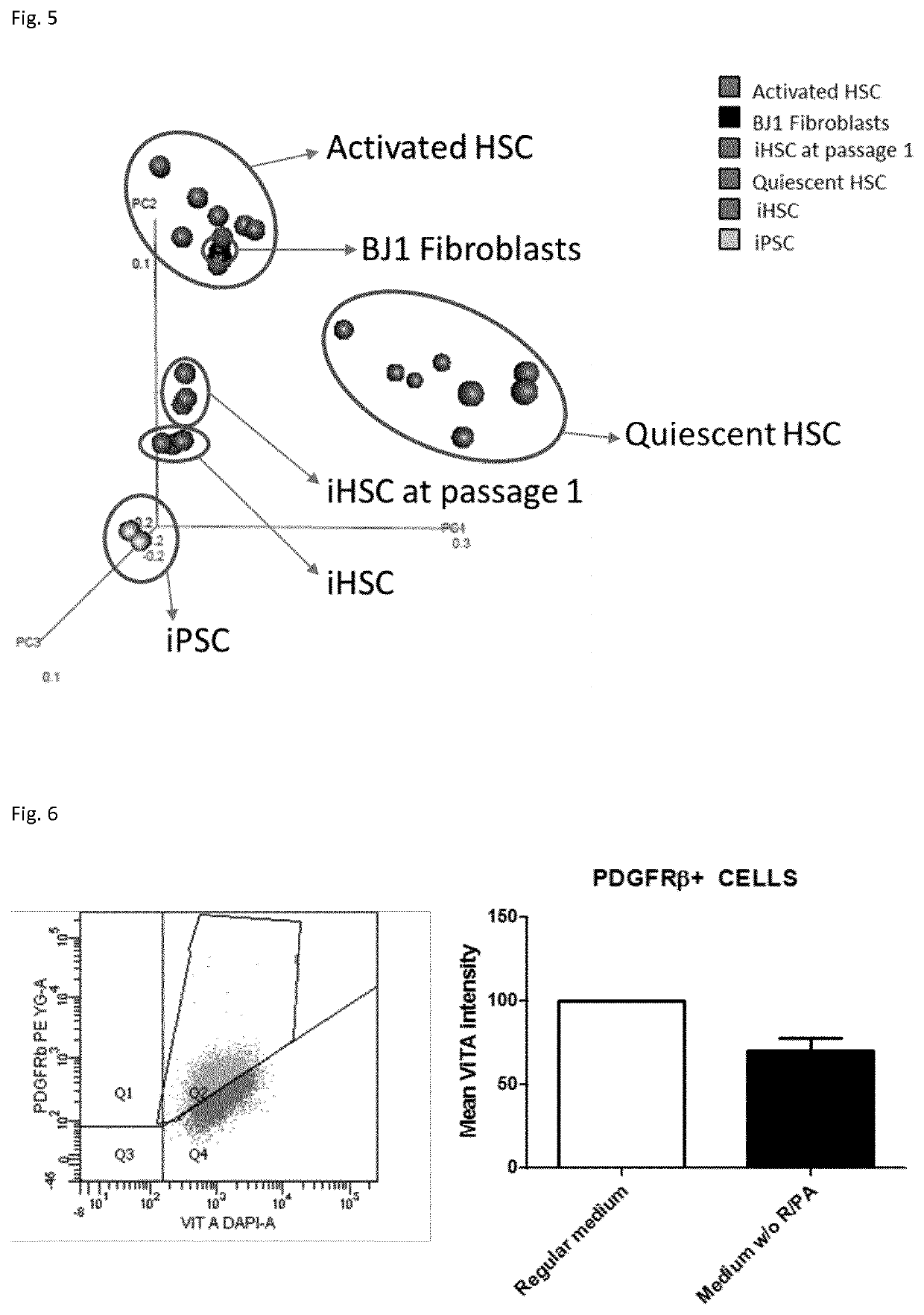
Methods for Differentiating Cells into Hepatic Stellate Cells and Hepatic Sinusoidal Endothelial Cells, Cells Produced by the Methods, and Methods for Using the Cells
ActiveUS20120009672A1Artificial cell constructsArtificially induced pluripotent cellsCulture cellLiver metabolism
The invention is directed to methods for culturing cells so that the cells are induced to differentiate into cells that express a hepatic stellate phenotype and cells that express a hepatic sinusoidal endothelial phenotype. The invention is also directed to cells produced by the methods of the invention. The cells are useful, among other things, for treatment of liver deficiency, liver metabolism studies, and liver toxicity studies.
Owner:KATHOLIEKE UNIV LEUVEN
Method of increasing drug oral bioavailability and compositions of less toxic orotate salts
ActiveUS8034823B2Improve bioavailabilityImprove drug bioavailabilityBiocideSalicyclic acid active ingredientsSide effectDrug induced hepatotoxicity
The present invention relates generally to the method of increasing the oral bioavailability, reducing chemotherapy induced toxicity and side effects, and improving the effectiveness of pharmaceutical agents that are poorly absorbed from the gastrointestinal tract. Specifically, the invention relates to poorly absorbed pharmaceutical drugs and converting them to orotate salts. The orotate salts of the drugs can be dosed at lower doses to provide the efficacy benefits of a higher dose, while reducing the drugs' toxic effects at lower doses. Additionally, the orotate salts of pharmaceutical agents have better clearance and reduce the potential for drug-induced hepatic toxicity. Therefore, an especially useful formulation of the orotate salt of the pharmaceutical agent can provide rapid and consistent action using a lower dose while reducing drug interactions and side-effects.
Owner:SAVVIPHARM INC
Culturing method for improving in-vitro differentiation phenotype and function of hepatic cells
InactiveCN105695392ABiocompatibleSupport growthHepatocytesArtificial cell constructsCell-Extracellular MatrixECM Protein
The invention discloses a culturing method for improving the in-vitro differentiation phenotype and function of hepatic cells. The culturing method is characterized by comprising the following steps that the hepatic cells are adopted as seed cells, a porous silk protein scaffold is taken as an in-vitro culturing carrier of the hepatic cells, the hepatic cells, hepatogenic interstitial cells and an extracellular matrix are inoculated to the porous silk protein scaffold according to a certain proportion, and the porous silk protein scaffold is put in a simulated hepatic cell in-vitro culturing environment to be cultured. A three-dimensional hepatic cell co-culturing system built through the method not only can be applied to tissue-engineered hepatic cell function units, but also can be applied to in-vitro evaluation of an influence of drugs on a hepatic metabolism enzyme and hepatic toxicity detection, and therefore a novel screening and evaluating tool is supplied to new drug research and development.
Owner:DALIAN MEDICAL UNIVERSITY
Combinations of a squalene synthase inhibitor and a HMG-CoA reductase inhibitor for treating hyperlipidemia
InactiveCN101189005AImprove serum lipidsEliminate side effectsMetabolism disorderDigestive systemHMG-CoA reductaseSecondary hyperlipidemia
A pharmaceutical composition useful for the prevention and / or treatment of hyperlipidemia, which comprises combining an effective amount of a squalene synthase inhibitor and a HMG-CoA reductase inhibitor is provided. Furthermore, a method for preventing and / or treating hepatic toxicity caused by the administration of a HM6-C0A veductase inhibitor, which comprisis adminisvering an effective amount of a sgualence synthase inhibitor, is provided .
Owner:TAKEDA PHARMA CO LTD
Diagnostic kit containing anti-dog hemoglobin monoclonal antibody and application thereof
ActiveCN102226171AHigh sensitivityStrong specificityImmunoglobulins against animals/humansMicroorganism based processesGastrointestinal toxicityMicroorganism
A diagnostic kit containing an anti-dog hemoglobin monoclonal antibody and an application thereof. The invention relates to a monoclonal antibody, and particularly relates to an anti-dog hemoglobin monoclonal antibody. The invention provides a mouse hybridoma cell strain, which is a strain preserved in China general microbiological culture collection center with a preservation number of CGMCC No. 4619. The invention also provides a monoclonal antibody generated by the mouse hybridoma cell strain with a preservation number of CGMCC No. 4619, and a kit containing the monoclonal antibody. The kit provided by the invention has high sensitivity, strong specificity, good accuracy and precision, and good stability, can detect dog hemoglobin in a sample to be detected specifically and sensitively, can exactly evaluate the gastrointestinal toxicity, the hepatic toxicity, the hematopoietic system toxicity, and the like of a medicament, and provides more scientific and accurate evaluation of the nonclinical safety of a medicament.
Owner:成都华西海圻医药科技有限公司
Application of schisandra chinensis in the preparation of medicine for preventing and reducing toxic and side effect of antitumor agent
InactiveCN1919258ASmall toxicityImprove heart functionAntineoplastic agentsCardiovascular disorderSide effectKidney Toxicity
The invention relates to the use of schisandra fruit in preparing medicament for prevention and abatement of toxic and side effects of antineoplastic drugs including cardiovascular toxicity, liver toxicity, kidney toxicity, or bone marrow depression, or immunological suppression or trichomadesis.
Owner:胡汛 +1
Method for detecting metabolite relative to psoralea corylifolia hepatotoxicity and application thereof
ActiveCN104251890AEasy for security monitoringThe detection method is simpleComponent separationMetaboliteMass spectrometric
The invention provides a method for detecting metabolite relative to psoralea corylifolia hepatotoxicity. The metabolite is psoralen and / or a dihydrodiol metabolite of isopsoralen; the method comprises acquisition of psoralen and / or the dihydrodiol metabolite of isopsoralen, a tandem mass spectrum is used, and a multiple reaction monitoring method is used for detecting the metabolite. The provided method in the application for monitoring hepatotoxicity of psoralen, isopsoralen, a composition containing psoralen and / or isopsoralen provides basis for safe medication and early warning of medicine toxicity.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Asarin lipid nanospheres and method for preparing same
ActiveCN102085183AGood physical and chemical stabilityReduce degradationNervous disorderEther/acetal active ingredientsLipid formationGlycerol
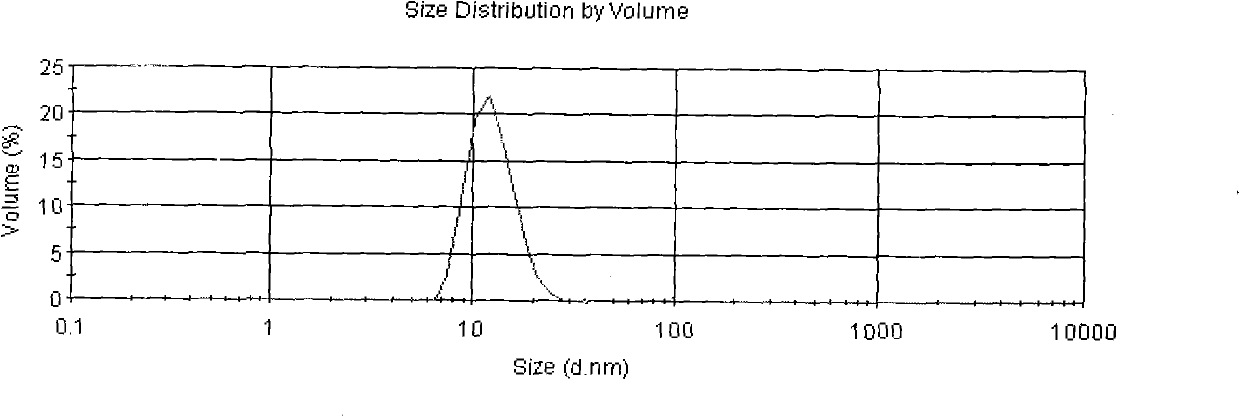
The invention discloses asarin lipid nanospheres and a method for preparing the same, and belongs to the technical field of medicaments. The asarin lipid nanospheres are prepared by the homogenization of the following raw materials in part by weight: 0.2 to 1.0 part of asarin, 5 to 15 parts of medium chain oil, soybean oil or the mixture of the medium chain oil and the soybean oil, 0.5 to 5 parts of lecithin, 1 to 30 parts of polyethylene glycol-12-methyl hydroxystearate, 1 to 4 parts of glycerol and 40 to 100 parts of water for injection. The preparation method comprises the following steps of: adding the oil phase into the aqueous phase, stirring the mixed solution to obtain the coarse product, adjusting the pH value, and repeatedly homogenizing the coarse product by a homogenizer to obtain the finished product. The average grain size of the lipid nanospheres is less than 100nm; compared with asarin injections and big-grain size emulsions which are sold on markets, the asarin lipid nanospheres obviously improve the targeting property in the lung, obviously reduce the distribution in the hepatic tissue, lower the potential hepatic toxicity and improve the drug efficacy.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES
Anti-tumor agent
InactiveUS20100216755A1Increased toxicitySafety zone can be expandedBiocideHydroxy compound active ingredientsAnti-inflammatoryHepatic toxicity
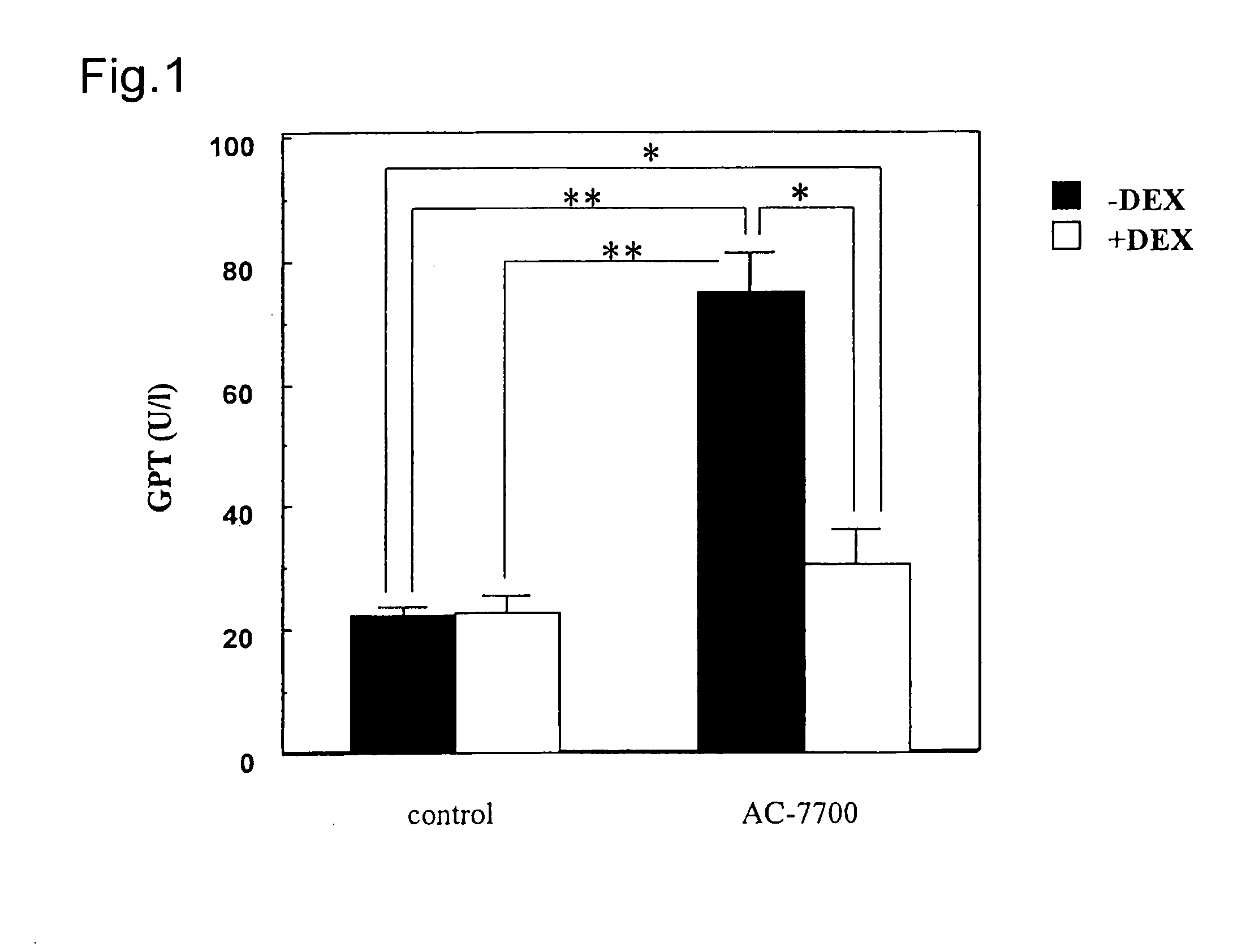
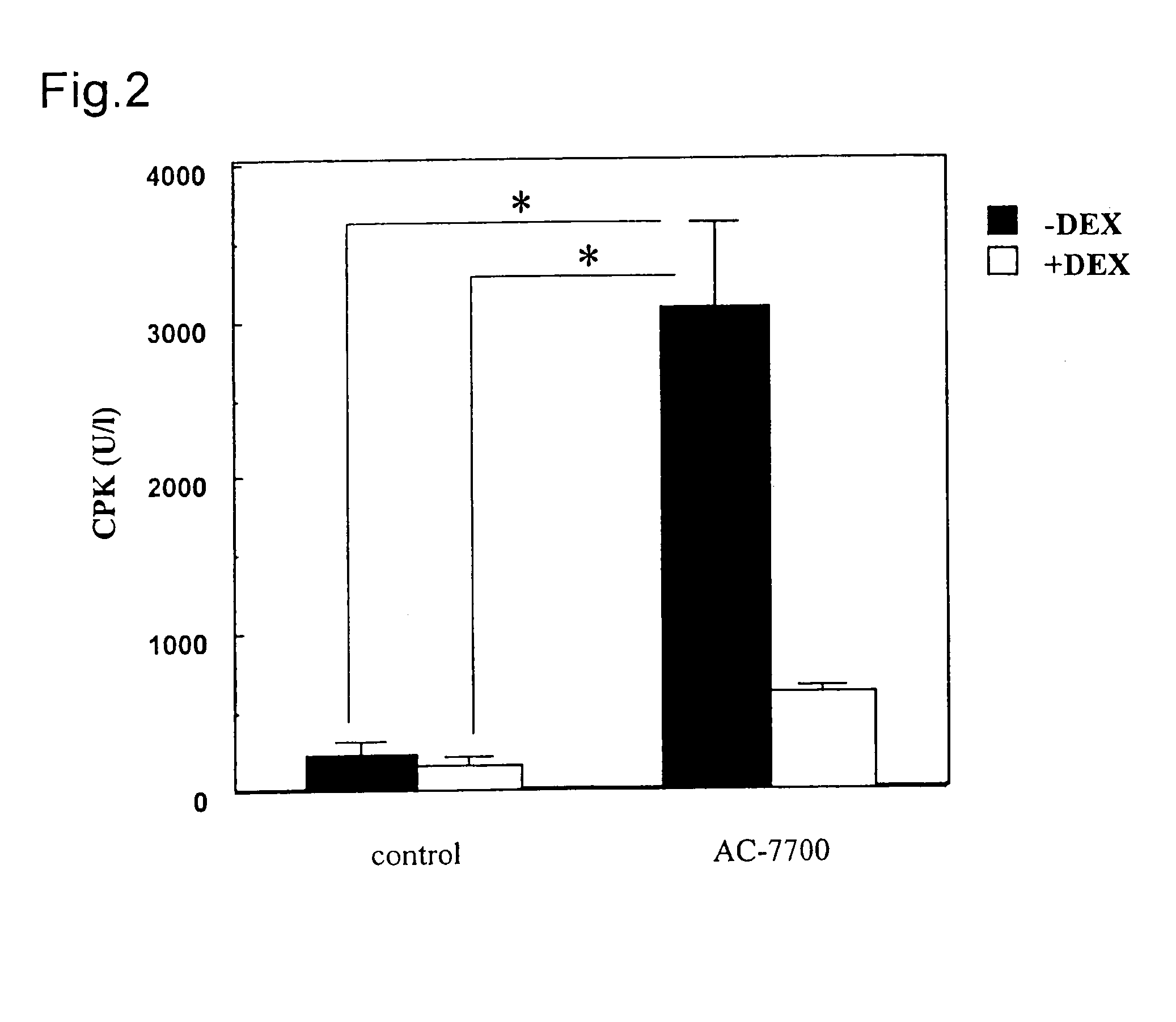
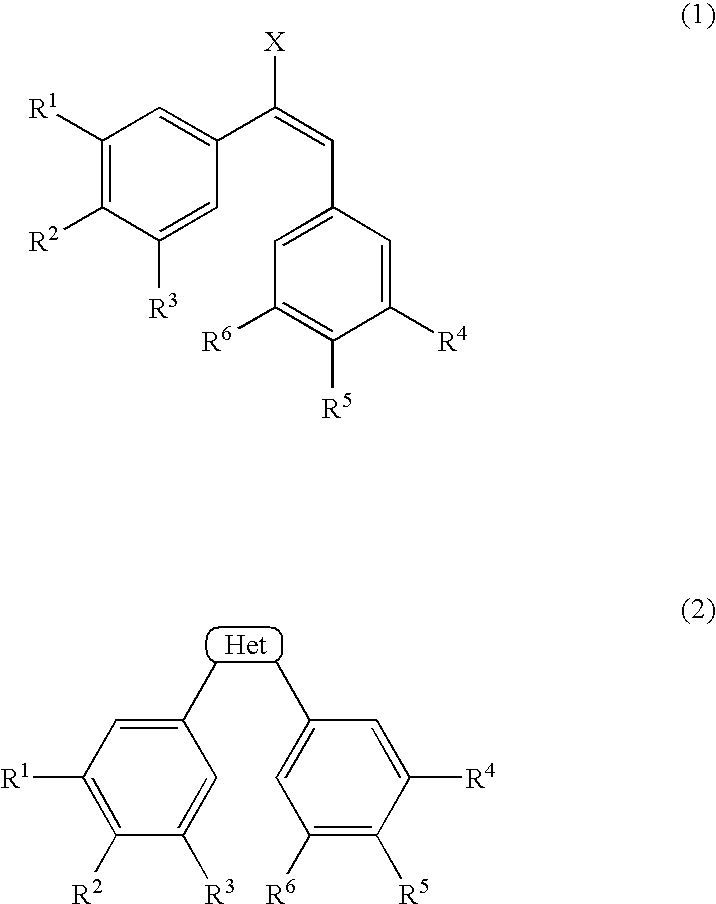
The present application provides a method for the treatment of tumors where the lethal dose of (Z)—N-[2-methoxy-5-[2-(3,4,5-trimethoxyphenyl)vinyl]phenyl]-L-serinamide is increased to twice or more, the toxicity at the pharmaceutically effective dosage of (Z)—N-[2-methoxy-5-[2-(3,4,5-trimethoxyphenyl)vinyl]phenyl]-L-serinamide is reduced, gastrointestinal toxicity at the pharmaceutically effective dosage of (Z)—N-[2-methoxy-5-[2-(3,4,5-trimethoxyphenyl)vinyl]phenyl]-L-serinamide is reduced, hepatic toxicity at the pharmaceutically effective dosage of (Z)—N-[2-methoxy-5-[2-(3,4,5-trimethoxyphenyl)vinyl]phenyl]-L-serinamide is reduced, and / or cardiovascular toxicity at the pharmaceutically effective dosage of (Z)—N-[2-methoxy-5-[2-(3,4,5-trimethoxyphenyl)vinyl]phenyl]-L-serinamide is reduced, by administering to a subject in need thereof a composition containing:(a) an effective amount of an anti-inflammatory active substance, wherein the anti-inflammatory active substance is a Dexamethasone selected from the group consisting Dexamethasone, an ester of Dexamethasone, and a salt of Dexamethasone; and(b) (Z)—N-[2-methoxy-5-[2-(3,4,5-trimethoxyphenyl)vinyl]phenyl]-L-serinamide or a salt thereof.
Owner:AJINOMOTO CO INC
Hepatotoxic component of airpotato yam and determination method thereof
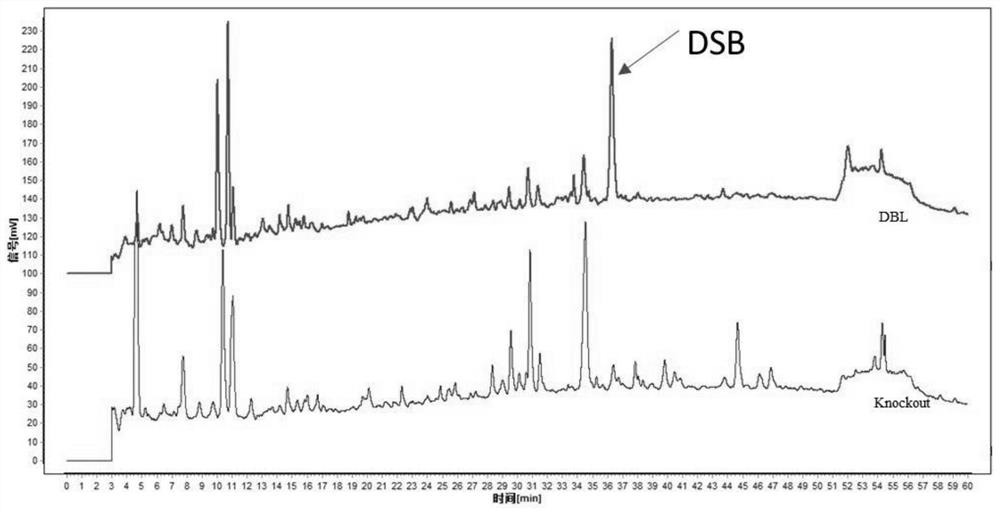
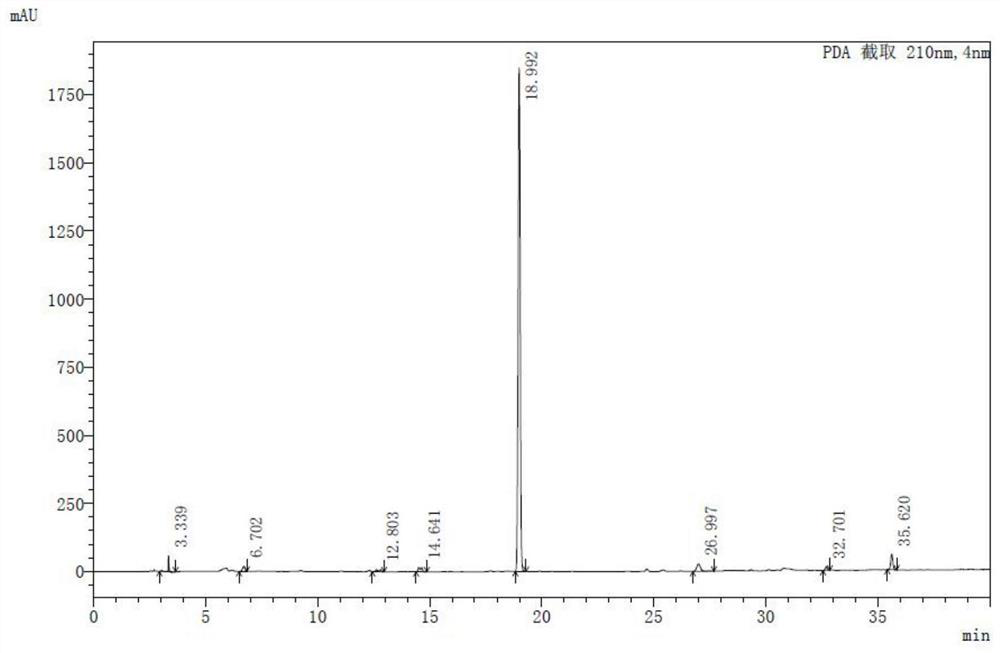
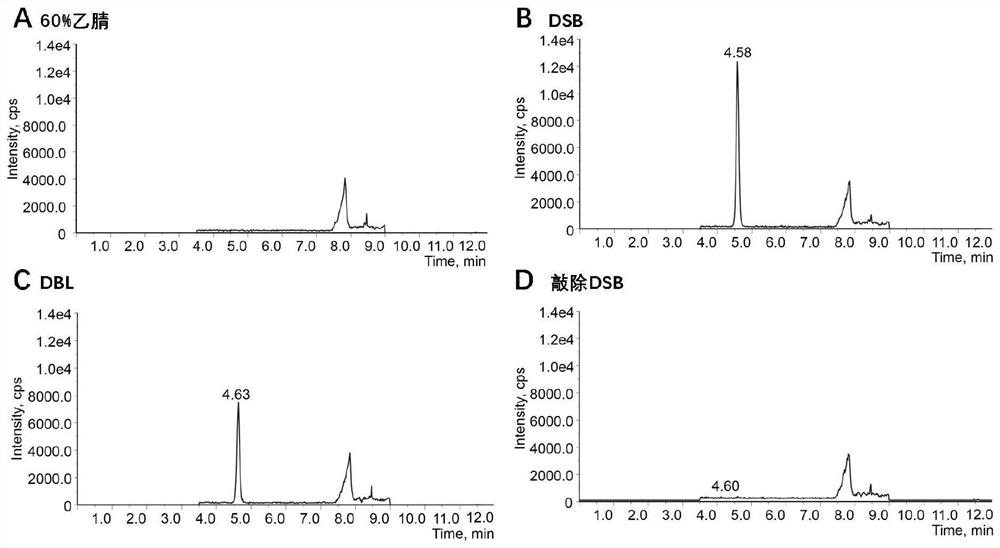
PendingCN113230338ANot hepatotoxicLow hepatotoxicityCompounds screening/testingPlant ingredientsBiotechnologyMicrobiology
The invention discloses a determination method of hepatotoxic component of airpotato yam. The method comprises the following steps: knocking out xanthotoxin B from all-component extract of airpotato yam, respectively representing the hepatotoxicity of the xanthotoxin B and the hepatotoxicity of the airpotato yam extract from which the xanthotoxin B is knocked out, and determining that the hepatotoxic component of the airpotato yam is the xanthotoxin B and is only the xanthotoxin B. The airpotato yam rhizome extract without the flavanotoxin B has no hepatotoxicity or is relatively weak in hepatotoxicity. The method eliminates the prejudice or misunderstanding of common technicians in the field, including researchers in the field, on the basis of the liver toxic substances of the airpotato yam.
Owner:GUIZHOU MEDICAL UNIV +1
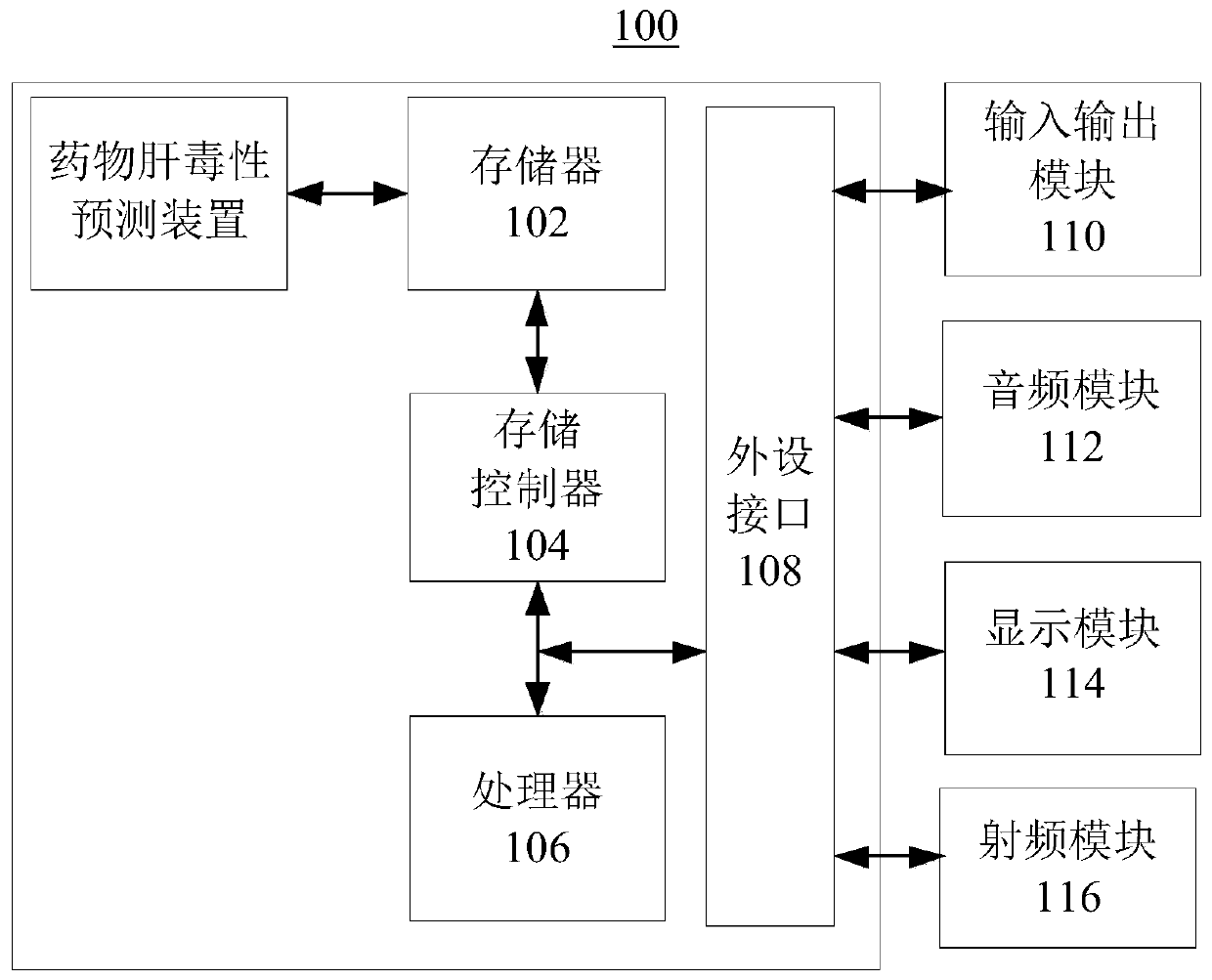
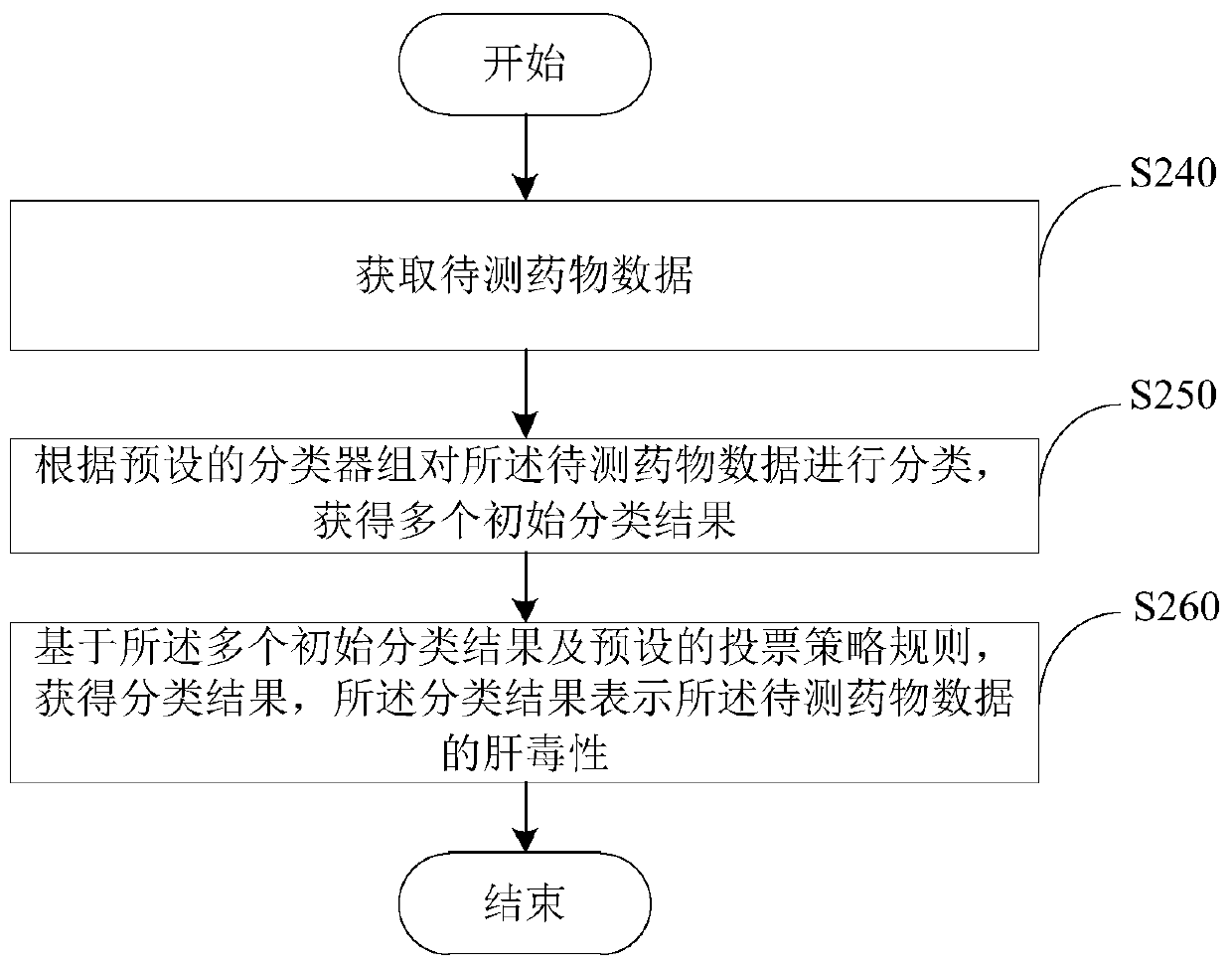
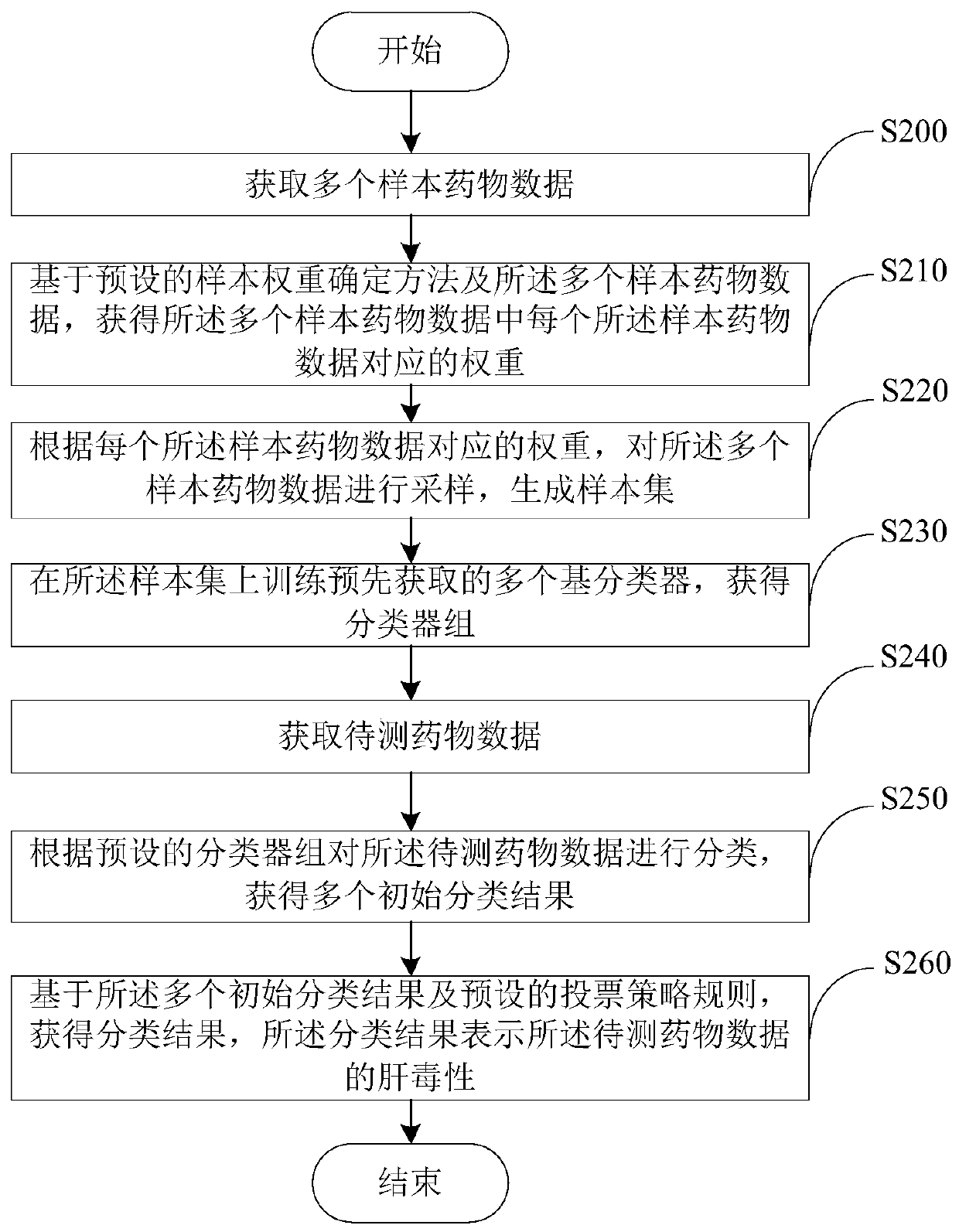
Drug hepatotoxicity prediction method and device
InactiveCN108021941BAdapt to future development needsImprove forecasting efficiencyCharacter and pattern recognitionLaboratory analysis dataPharmacyMedicine
Embodiments of the present invention provide a drug hepatotoxicity prediction method and device, which relate to the technical field of drug prediction. The method comprises: after acquiring the data of the drug to be tested, classifying the data of the drug to be tested according to a preset classifier group to obtain a plurality of initial classification results; and then based on the plurality of initial classification results and the preset voting strategy According to the rule, a classification result is obtained, and the classification result represents the hepatotoxicity of the drug data to be tested. By classifying the drug data through the preset classifier group and voting strategy, the hepatotoxicity of the drug data can be obtained, the prediction efficiency and accuracy can be improved, and the future development needs of the pharmaceutical industry can be adapted. More effective control of development costs.
Owner:SICHUAN UNIV
Application of schisandra chinensis monomer compound in preparation of drugs for prevention and treatment of hepatotoxicity caused by acetaminophen
ActiveCN103751174ACurb consumptionImprove necrosisDigestive systemEther/acetal active ingredientsLiver necrosisAlanine aminotransferase
The invention discloses an application of a schisandra chinensis monomer compound in preparation of drugs for prevention and treatment of hepatotoxicity caused by acetaminophen (APAP), wherein the schisandra chinensis monomer compound is deoxyschizandrin, schisandrin C, schisandrin or schisantherin a. Experiment results show that: with the schisandra chinensis monomer compound, the ALT content and the AST content in the APAP-induced liver injury animal model can be significantly reduced, consumption of glutathione in liver cells can be inhibited, the liver cell necrosis degree can be effectively improved, and the schisandra chinensis monomer compound provides significant treatment and protection effects for hepatotoxicity symptoms caused by APAP.
Owner:SUN YAT SEN UNIV
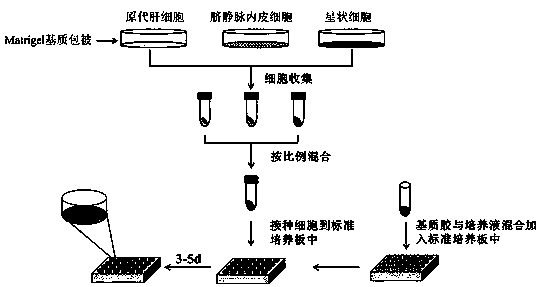
Preparation method of tissue-engineered hepatic model
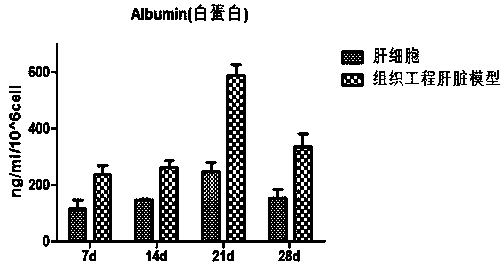
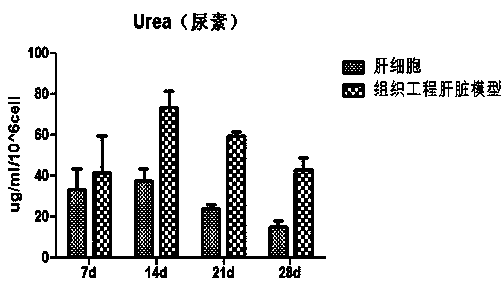
InactiveCN110684712AAchieve exposureHave albumin secretionHepatocytesCulture processEfficacyCytochrome P450 activity
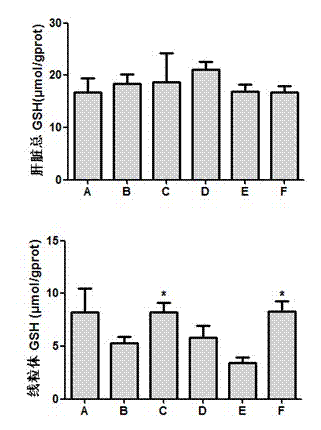
The invention relates to a preparation method of a tissue-engineered hepatic model. The preparation method of the tissue-engineered hepatic model comprises the following steps that step one, seed cells are cultivated and amplified; step two, liquid and matrix for model building are prepared; step three, cells are collected; and step four, the model is built. The tissue-engineered hepatic model iscomposed of primary hepatocytes, endothelial cells and hepatic stellate cells, a bile duct sample structure similar to natural hepatic tissue is formed, functions of albumin secretion and bile acid transport are achieved, cytochrome P450 activity is high, and the mitochondrial function is high. Compared with individually cultured hepatic cells, the albumin and urea expression level of the built hepatic tissue model is obviously improved, the tissue activity can be maintained in vitro for 28 days, long-term drug exposure can be realized, and a more sensitive and accurate test model is providedfor hepatotoxicity and efficacy evaluation based on vitro cells.
Owner:GUANGDONG BOXI BIO TECH CO LTD
Methods and formulations for treatment of and/or protection against acute liver failure and other hepatotoxic conditions
ActiveUS20190015424A1Loss of functionOrganic active ingredientsPowder deliveryAcute hepatic failureActive agent
Methods, formulations and kits for treating and / or protecting against acute liver failure and other hepatotoxicities in an individual employ a combination of a first active agent which replenishes, or decreases a loss of, functional glutathione in the individual, and a second active agent comprising a manganese complex selected from the group consisting of (i) a calcium manganese mixed metal complex of N,N′-bis-(pyridoxal-5-phosphate)-ethylenediamine-N,N′-diacetic acid (DPDP) having a molar ratio of calcium to manganese in a range of from 1 to 10, or a pharmaceutically acceptable salt thereof, (ii) a mixture of manganese DPDP (MnDPDP), or a pharmaceutically acceptable salt thereof, and a non-manganese-containing DPDP compound, or (iii) a mixture of manganese pyridoxyl ethylenediamine (MnPLED), or a pharmaceutically acceptable salt thereof, and a non-manganese-containing pyridoxyl ethylenediamine (PLED) compound.
Owner:EGETIS THERAPEUTICS AB
Method for processing rhizoma alismatis
The invention discloses a method for processing rhizoma alismatis. The method includes manufacturing steps of cleaning, slicing, drying, ultraviolet lamp irradiating, braising, microwave drying, packaging and the like. The method has the advantages that the problems of certain hepatic toxicity and renal toxicity of existing rhizoma alismatis in the traditional technological procedures for processing the existing rhizoma alismatis can be solved by the aid of the method; destruction of toxic substances in the rhizoma alismatis can be accelerated by means of ultraviolet lamp irradiation, and accordingly hepatic and renal toxicity of the rhizoma alismatis can be reduced; technologies for processing the rhizoma alismatis are optimized, accordingly, alisol A and alisol B in processed rhizoma alismatis medicinal slices can be easily decocted to seep out, and effects of inducing diuresis, excreting dampness and clearing kidney fire can be enhanced.
Owner:江西古方原中药饮片有限公司
Sphingolipids in treatment and prevention of steatosis and of steatosis or of hepatotoxicity and its sequelae
InactiveUS7906488B2Avoid developmentReduce severityBiocideMetabolism disorderTG - TriglycerideLiver steatosis
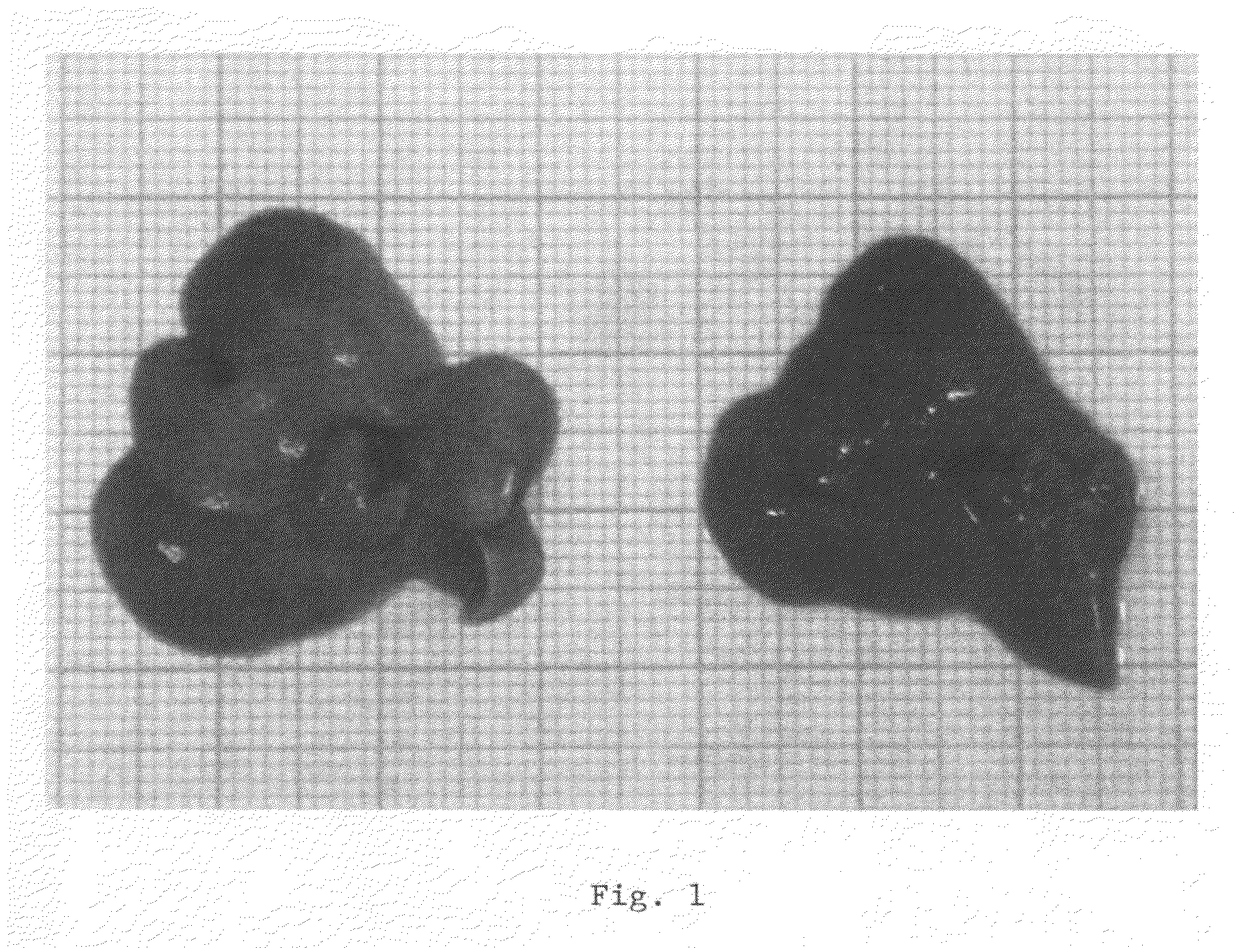
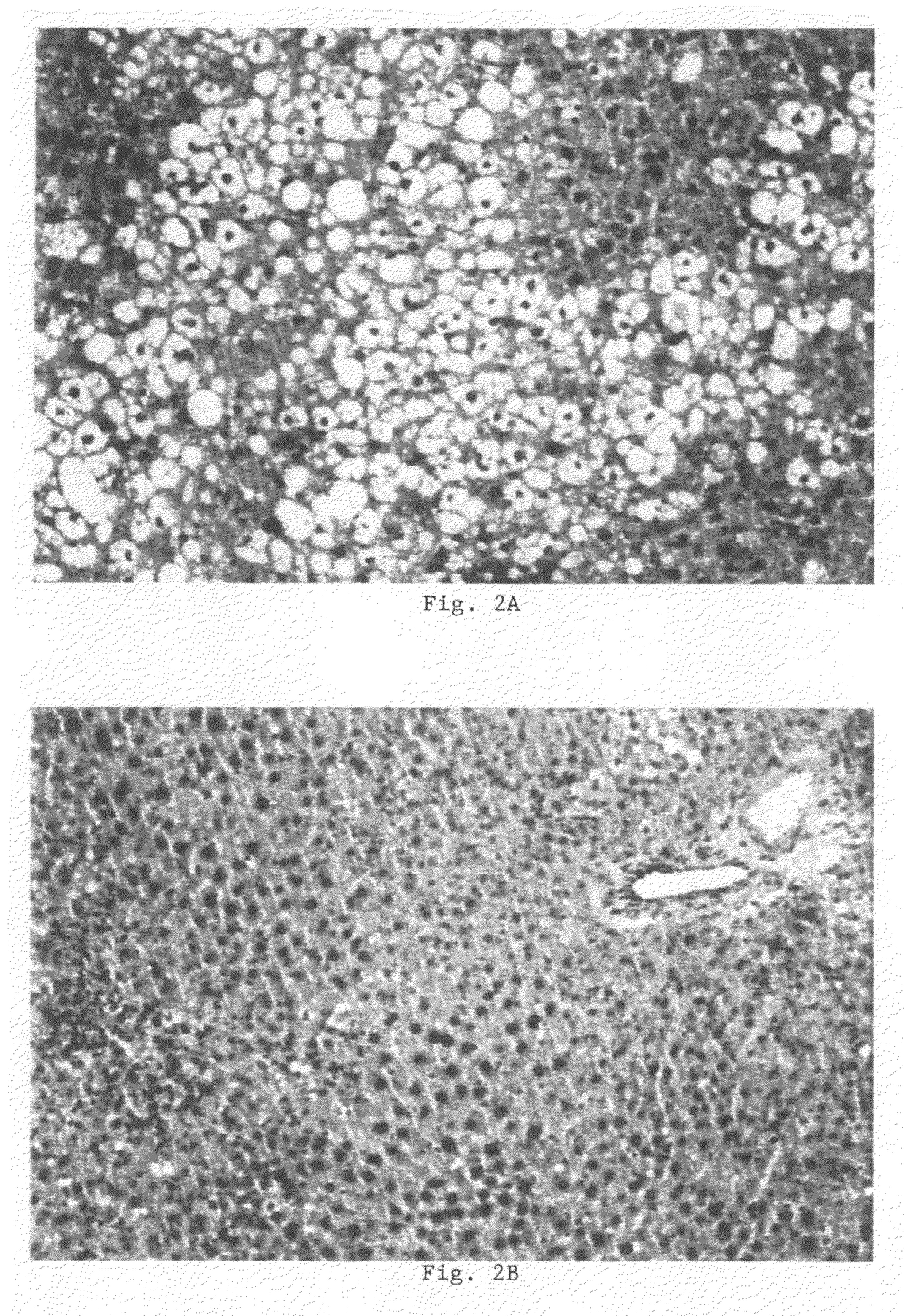
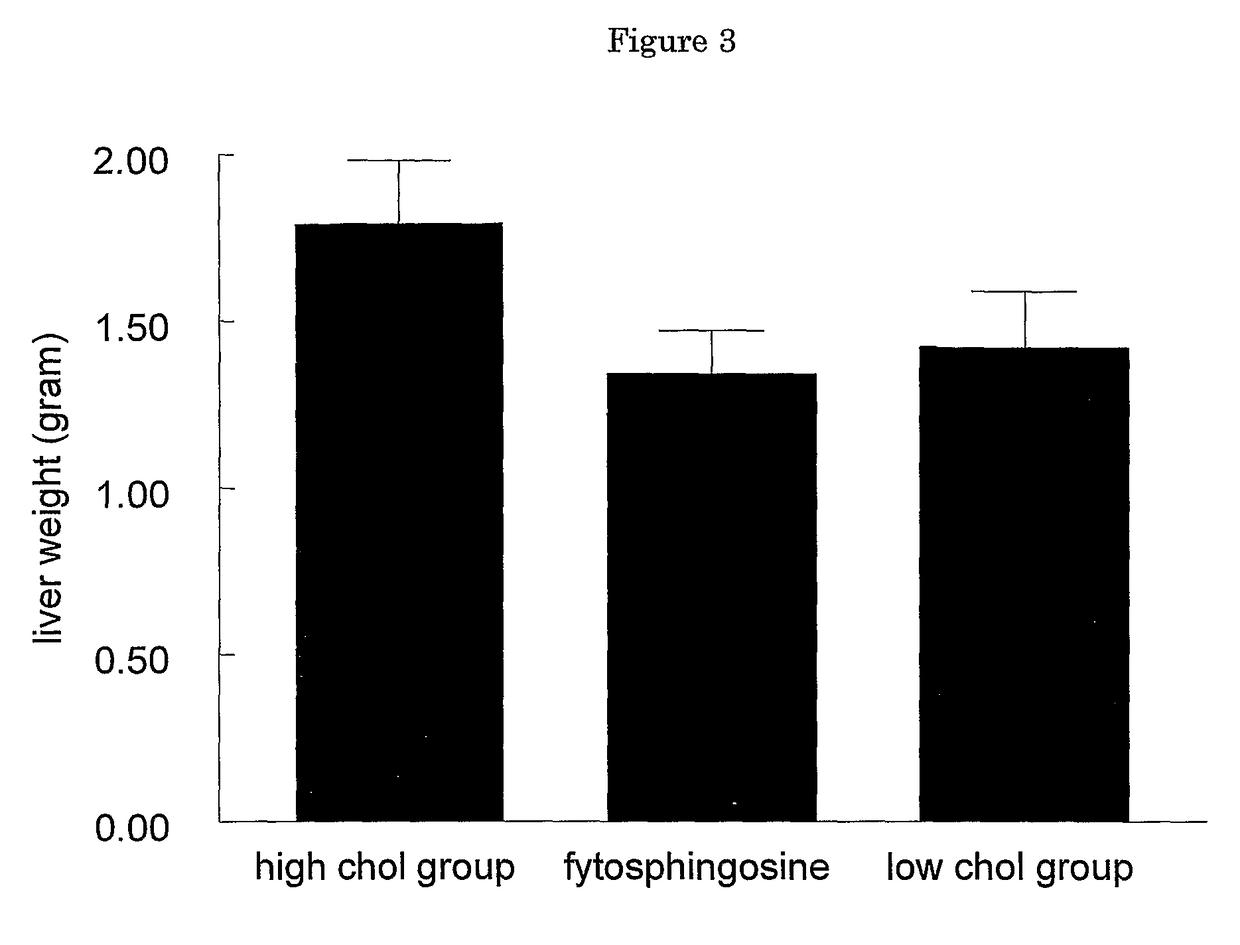
The present invention relates to the use of sphingolipids for the preparation of a food item, a food supplement and / or a medicament for the prevention and / or treatment of elevated blood levels of cholesterol and triglycerides related to steatosis and hepatotoxic effects of medicaments and viral infections. In particular, the invention relates to the use of a sphingolipid such as phytosphingosine, sphingosine, sphinganine, ceramide, glycosylceramide and / or sphingomyelin, or a precursor or a derivative of a sphingolipid, or a pharmaceutically acceptable salt thereof, for the prevention and / or treatment of hepatotoxic effects such as hepatic steatosis, fibrosis and / or cirrhosis.
Owner:NEDERLANDSE ORG VOOR TOEGEPAST NATUURWETENSCHAPPELIJK ONDERZOEK TNO
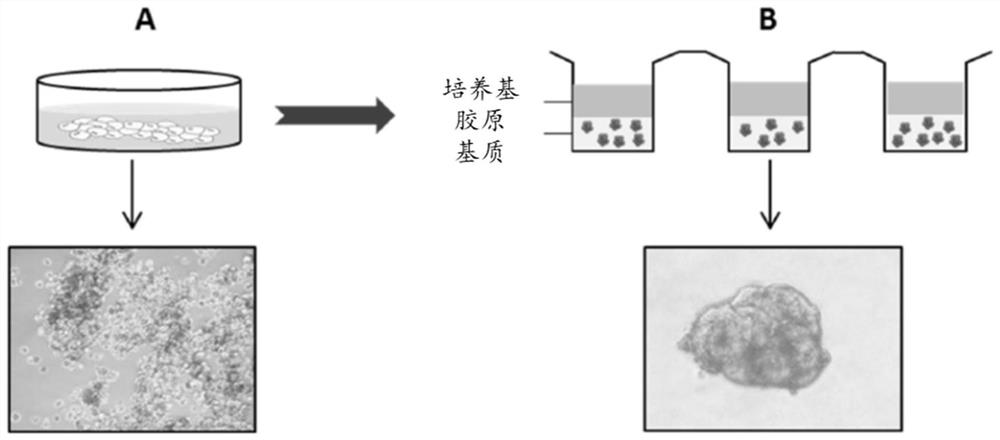
Method of culturing proliferative hepatocytes
The present invention relates to a method of culturing animal cells, preferably primary hepatocytes, comprising a first step of culturing the animal cells in non-adherent culture vessel, preferably alow or ultra-low attachment culture vessel, a second step of embedding the animal cells in a collagen matrix or in a gelatin matrix, and a third step of culturing the animal cells embedded in the collagen matrix or in the gelatin matrix, thereby obtaining 3D animal cell structures comprising proliferative animal cells, preferably spheroids comprising proliferative primary hepatocytes. The invention also relates to a spheroid comprising proliferative primary hepatocytes and the uses thereof for engineering an artificial liver model or an artificial liver organ, and for assessing in vitro the liver toxicity, genotoxicity and / or the effects of a drug or a compound.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Hepatoxic substance sieving and evaluating method based on fluorescence labeling
InactiveCN101788480BRealize screeningRealize evaluationMicrobiological testing/measurementFluorescence/phosphorescenceStainingSoftware system
The invention provides a hepatoxic substance sieving and evaluating method based on cell states labeled by fluorescence, which is a sieving and evaluating method combining a hardware / software system. The images are acquired by a fluorescence inverted microscope and a computer in real time, the biological information is processed and visualized, the liner dynamic range width of the living cell ismeasured by combining FDA dyeing, and then the relevant parameters for evaluating the hepatoxic substances are provided. The method is quick, sensitive and economical, and is used for quickly detecting the influence of the sieved sample on cell quantity, forms, migration, apoptasis and the like under the condition of maintaining cell structure and function integrity, accurately carrying out quantitative analysis on the activity of the cell labeled by the fluorescence specialty and detecting the activity change of the cell under the function of the hepatoxic substances. The method has reasonable design. The provided sieving and evaluating system has complete structure, and can be used for sieving and evaluating the hepatoxic substances.
Owner:ZHEJIANG UNIV
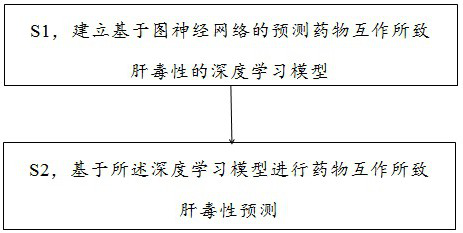
Method for predicting hepatotoxicity caused by drug interaction based on graph neural network model
ActiveCN114792574AReduce investmentRelieve painHealth-index calculationDrug referencesDrug interactionPreclinical toxicity
The invention discloses a method for predicting hepatotoxicity caused by drug interaction based on a graph neural network model, which comprises the following steps: establishing a deep learning model for predicting hepatotoxicity caused by drug interaction based on a graph neural network, and predicting hepatotoxicity caused by drug interaction based on the deep learning model, the method comprises the following steps: encoding two drug molecules by using a drug molecule encoder constructed based on a graph neural network, predicting a hepatotoxicity score caused by interaction of the two drugs through a full-connection neural network, and judging whether the combination of the two drugs can cause hepatotoxicity according to the predicted hepatotoxicity score. The method not only reduces the fund and time investment of drug combination preclinical toxicity research and development to a certain extent, but also can accurately predict the drug interaction hepatotoxicity during drug combination, and avoids the injury to the liver of a patient due to combination of a plurality of drugs. Unnecessary medicine combination clinical experiments during development of a new treatment scheme are reduced, and the success rate of the clinical experiments is increased.
Owner:PRECISION SCI BIOMEDICINE (SUZHOU) CO LTD +2
Method for reducing hepatotoxicity of fructus psoraleae
The invention discloses a method for reducing hepatotoxicity of fructus psoraleae. The method comprises the following steps that the fructus psoraleae is pre-treated, the fructus psoraleae is sequentially soaked with an ethanol water solution and rinsed with water, and then the fructus psoraleae rinsed with water is steamed and dried. The fructus psoraleae prepared by the method can effectively reduce the hepatotoxicity of the fructus psoraleae and does not affect the efficacy of the fructus psoraleae.
Owner:北京圆融科技有限公司
Method for controlling polygonum multiflorum extra-heterogeneous hepatotoxicity
PendingCN112305211AMicrobiological testing/measurementMaterial analysis by optical meansStatistical analysisPharmaceutical drug
The invention discloses a method for controlling polygonum multiflorum extra-heterogeneous hepatotoxicity. The method comprises the following steps: determining dosage of emodin-8-O-glucoside; preparing an emodin-8-O-glucoside solution; giving the emodin-8-O-glucoside solution to a rat through intragastric administration, and collecting plasma and liver specimens; performing index detection; usingSPSS software for statistical analysis. The invention further provides application of the emodin-8-O-glucoside in preparation of a medicine which does not cause the special liver injury and application of the emodin-8-O-glucoside and the trans-stilbene glucoside in evaluation of the special liver injury. The invention also provides a safe content limit of emodin-8-O-glucoside so as to control theliver injury risk of polygonum multiflorum.
Owner:北京圆融科技有限公司
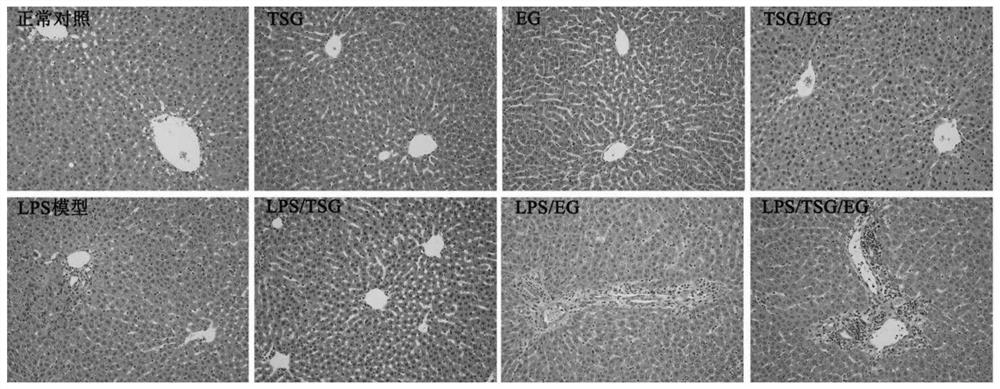
Methods for differentiating cells into hepatic stellate cells
The invention is directed to methods for culturing cells so that the cells are induced to differentiate into cells that express a hepatic stellate phenotype. The invention is also directed to cells produced by the methods of the invention. The cells are useful, among other applications, for treatment of liver deficiencies, liver metabolism studies, and liver toxicity studies, fibrogenic studies, or to support hepatocyte function in co-culture setting.
Owner:INST DINVESTIGACIONS BIOMEDIQUES AUGUST PI I SUNYER (IDIBAPS) +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com