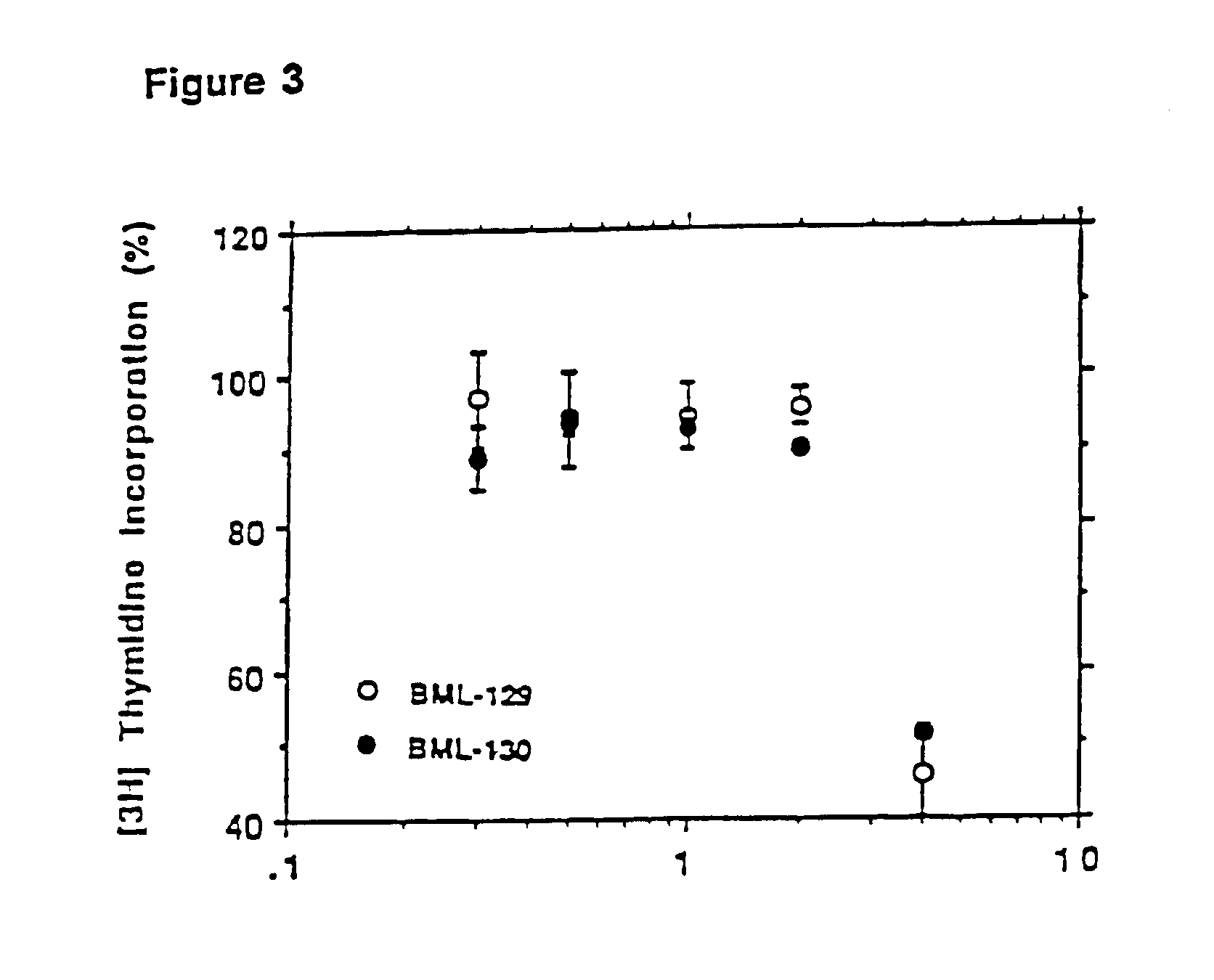
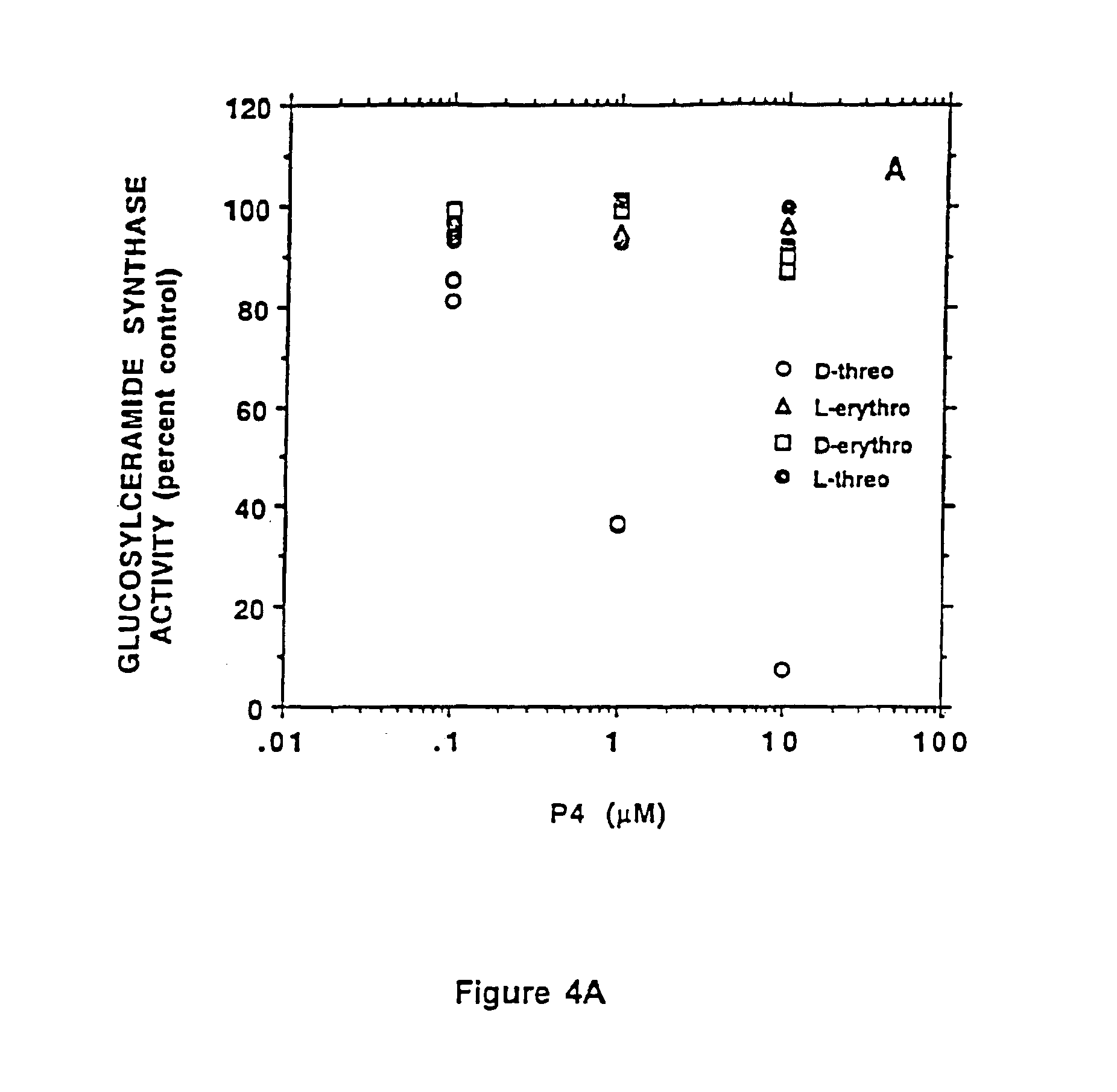
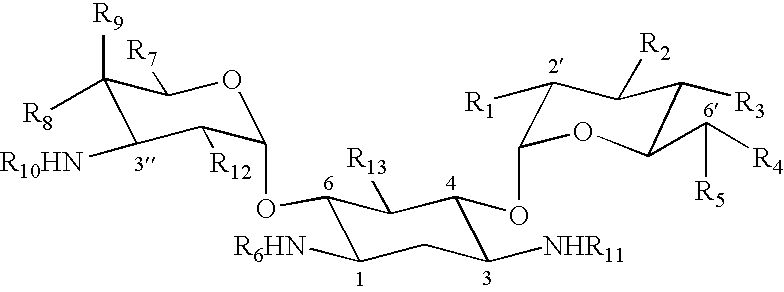
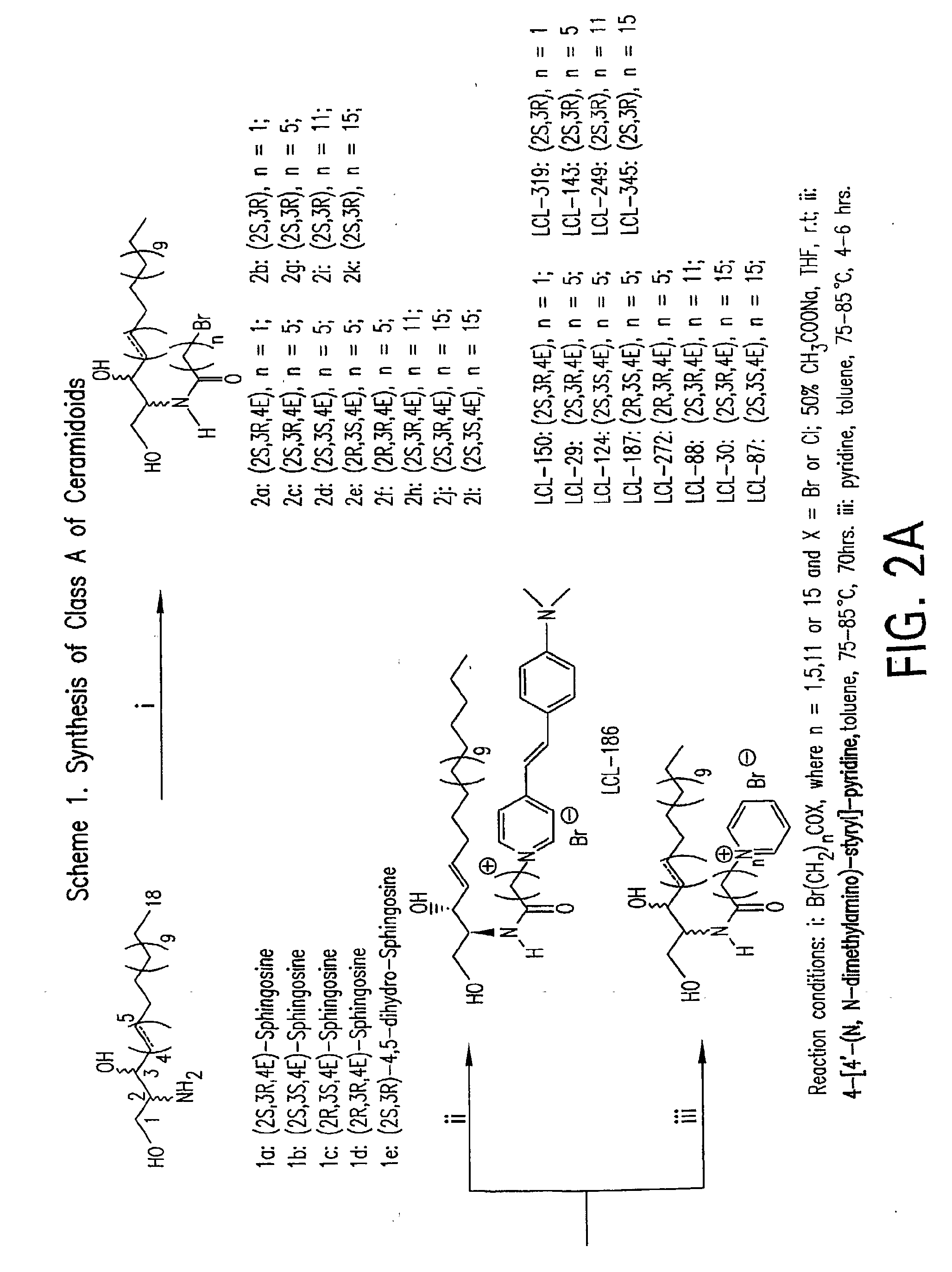
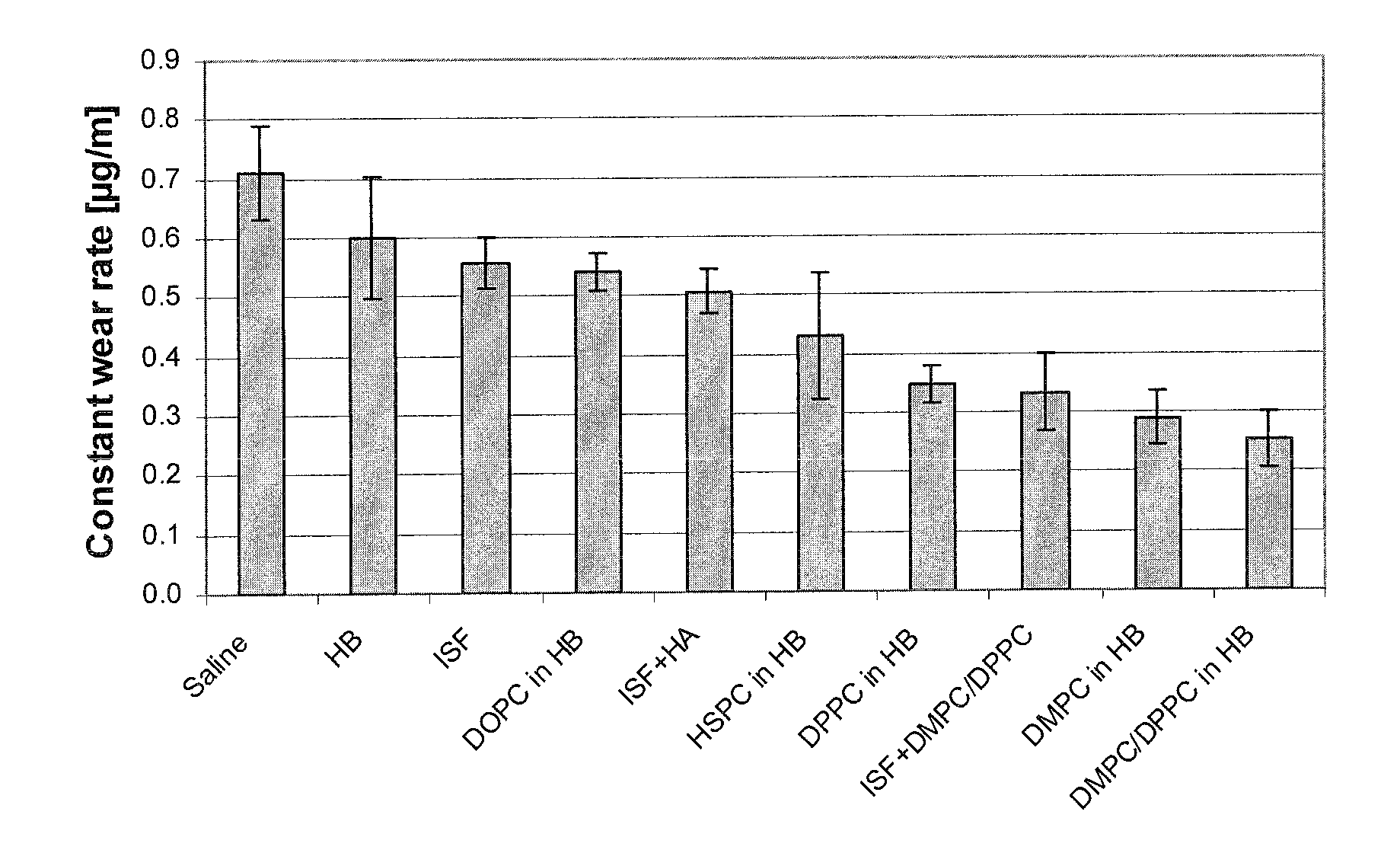
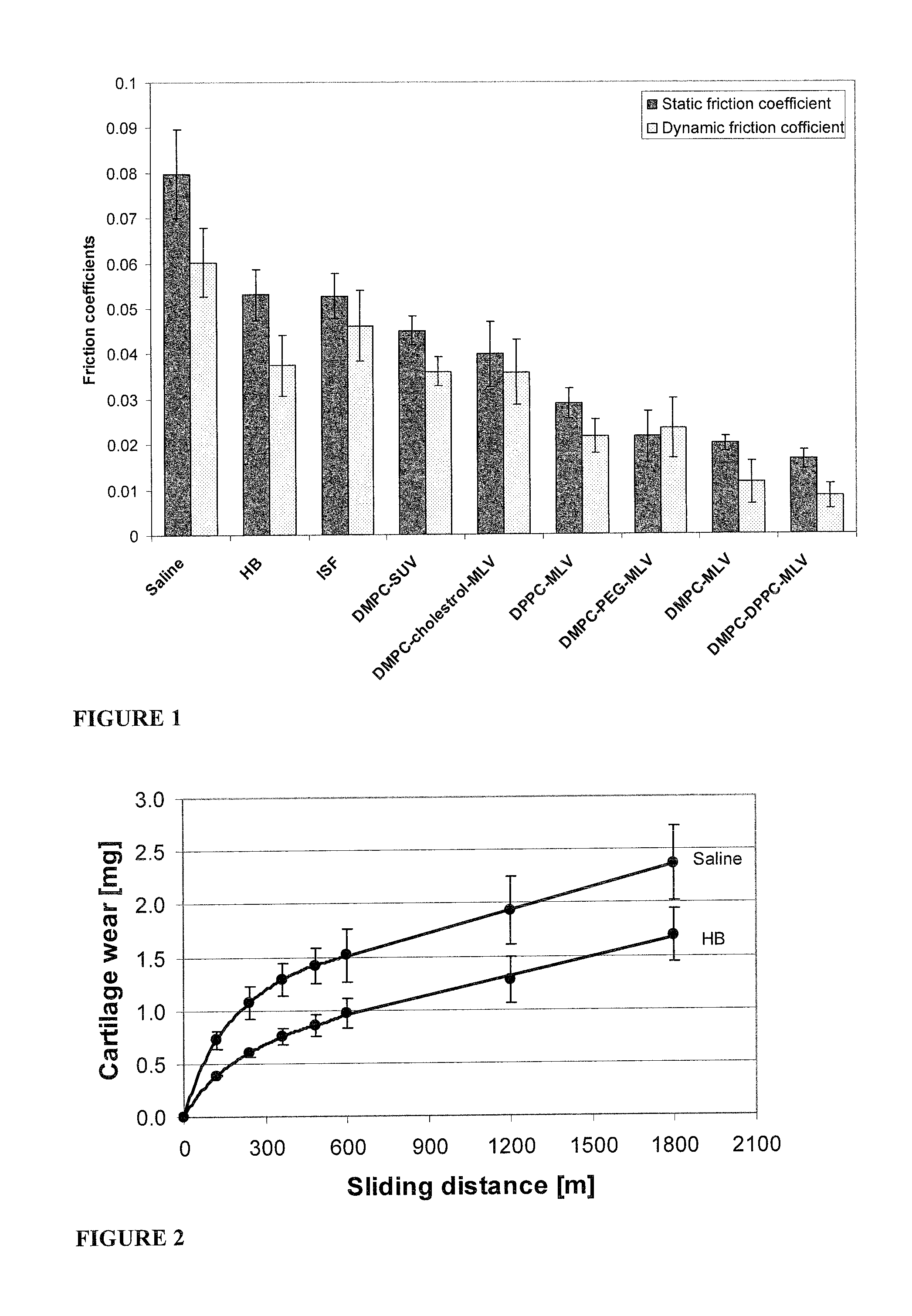
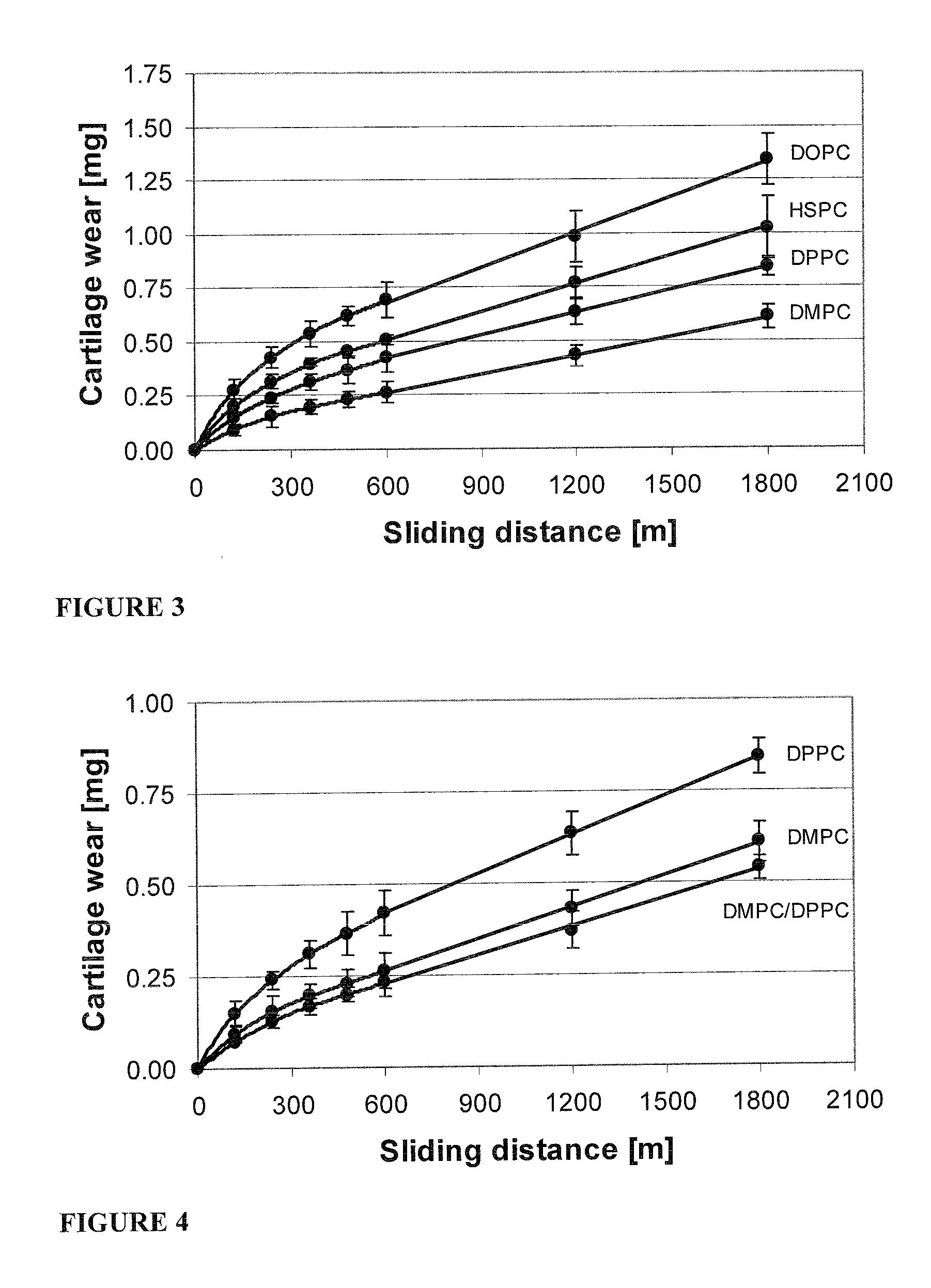
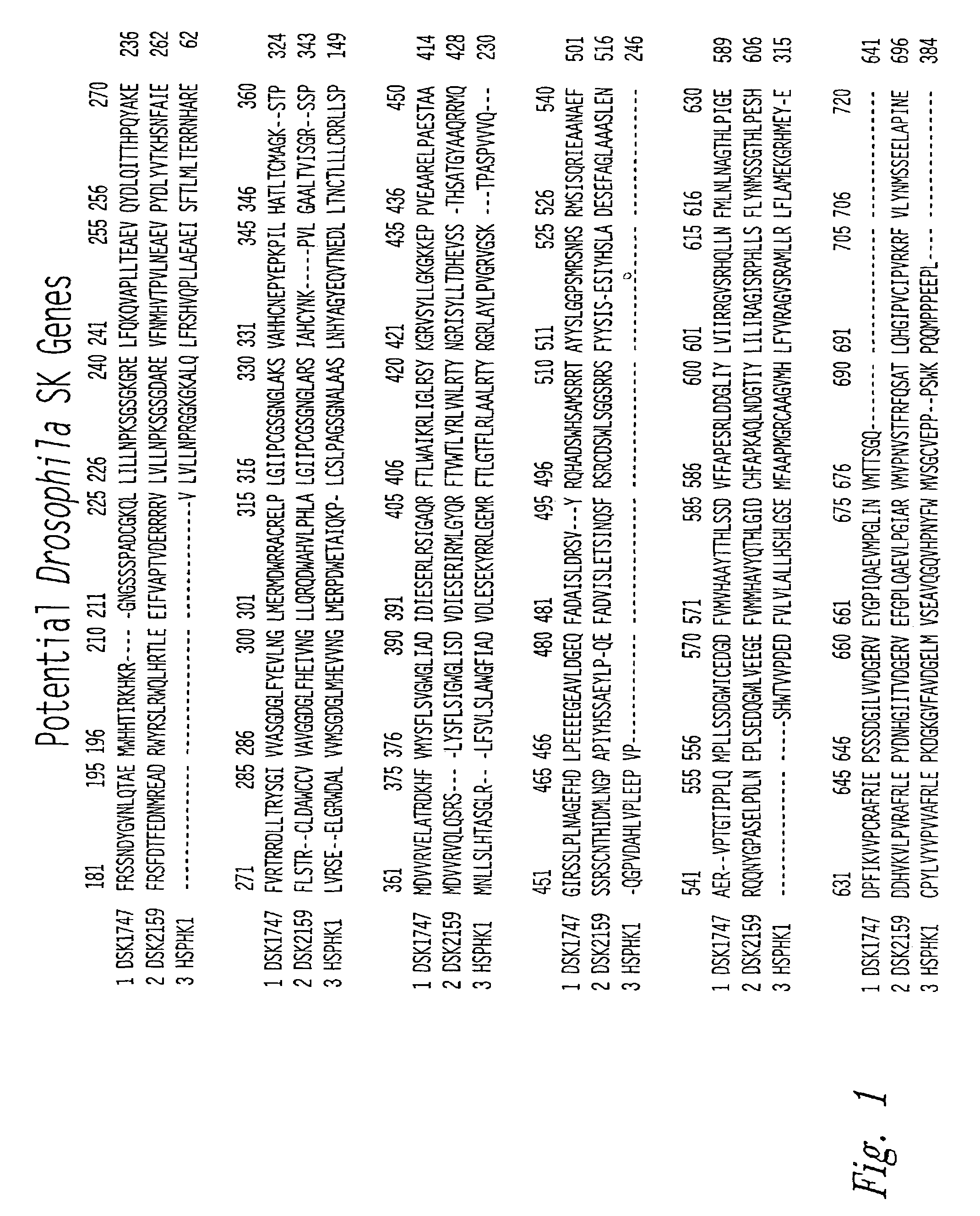
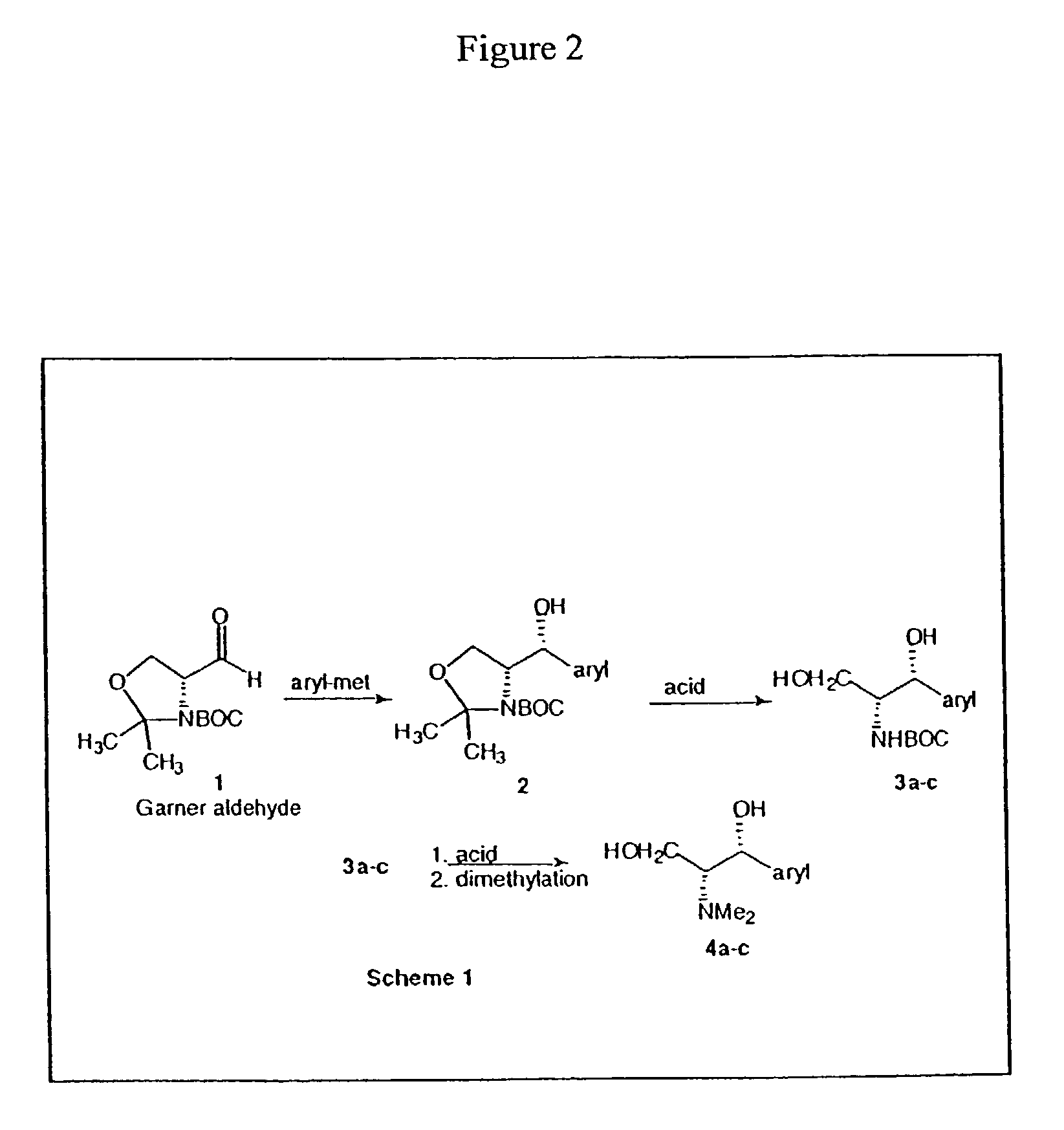
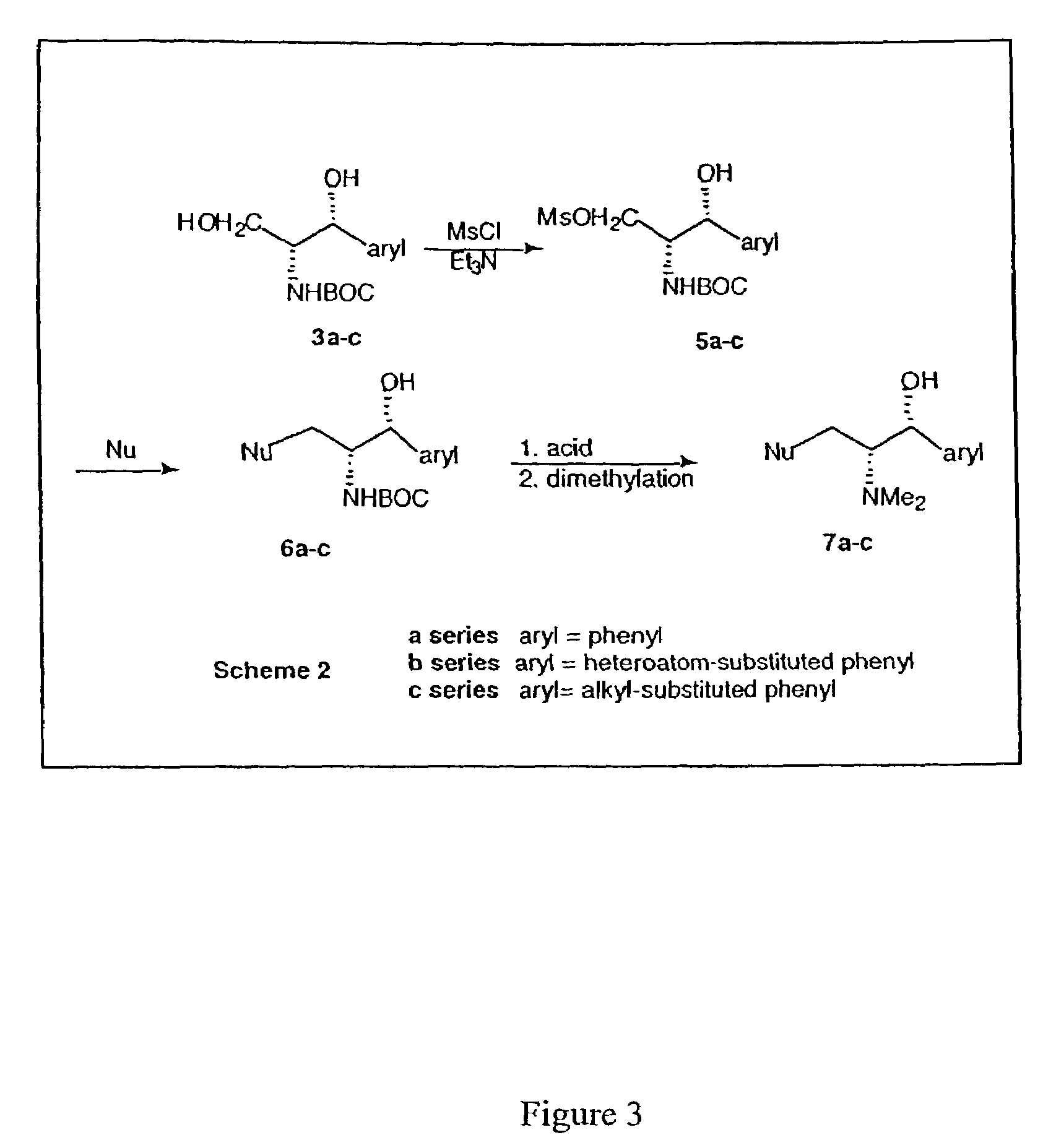
Patents
Literature
168 results about "Sphingolipid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
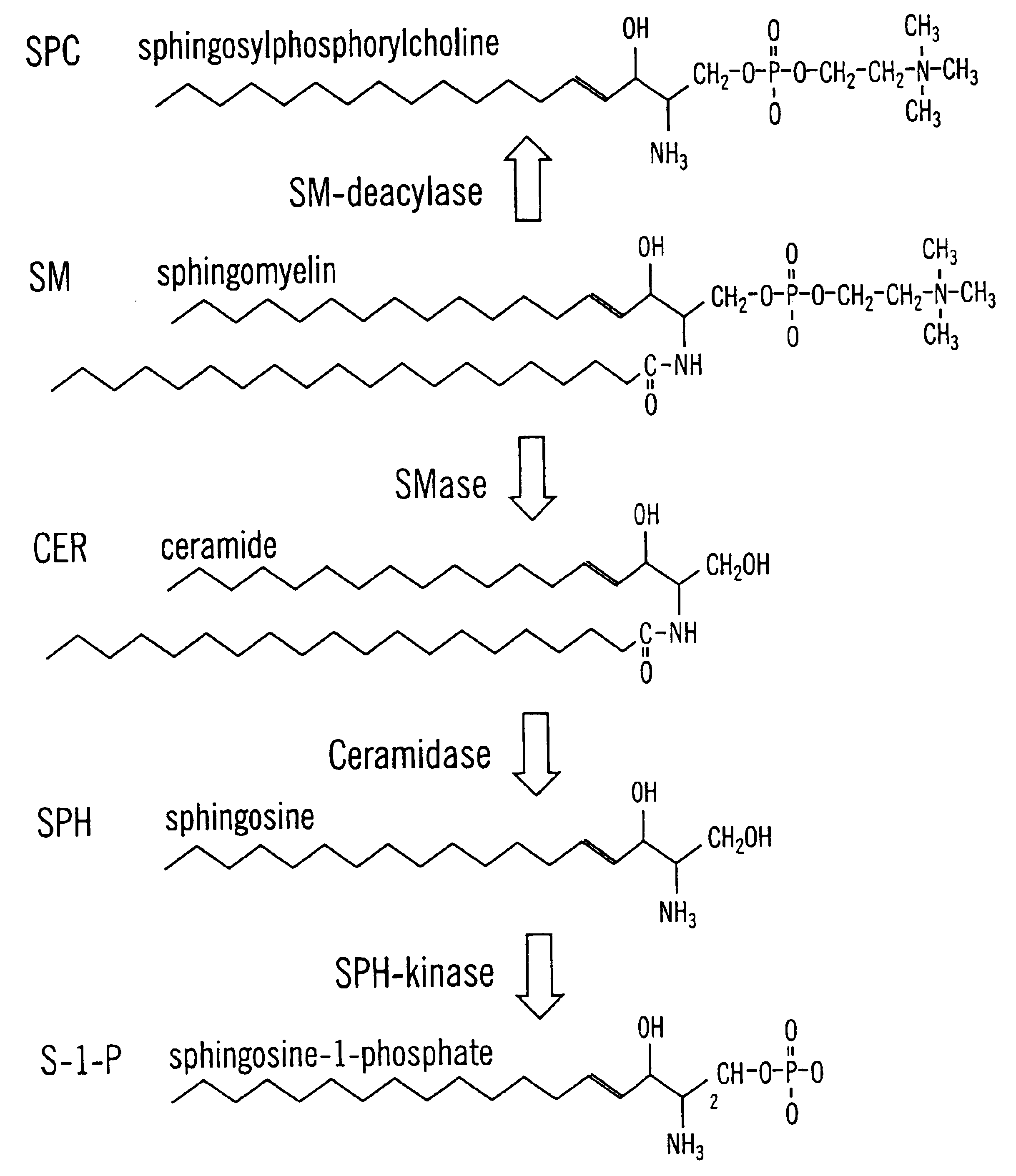
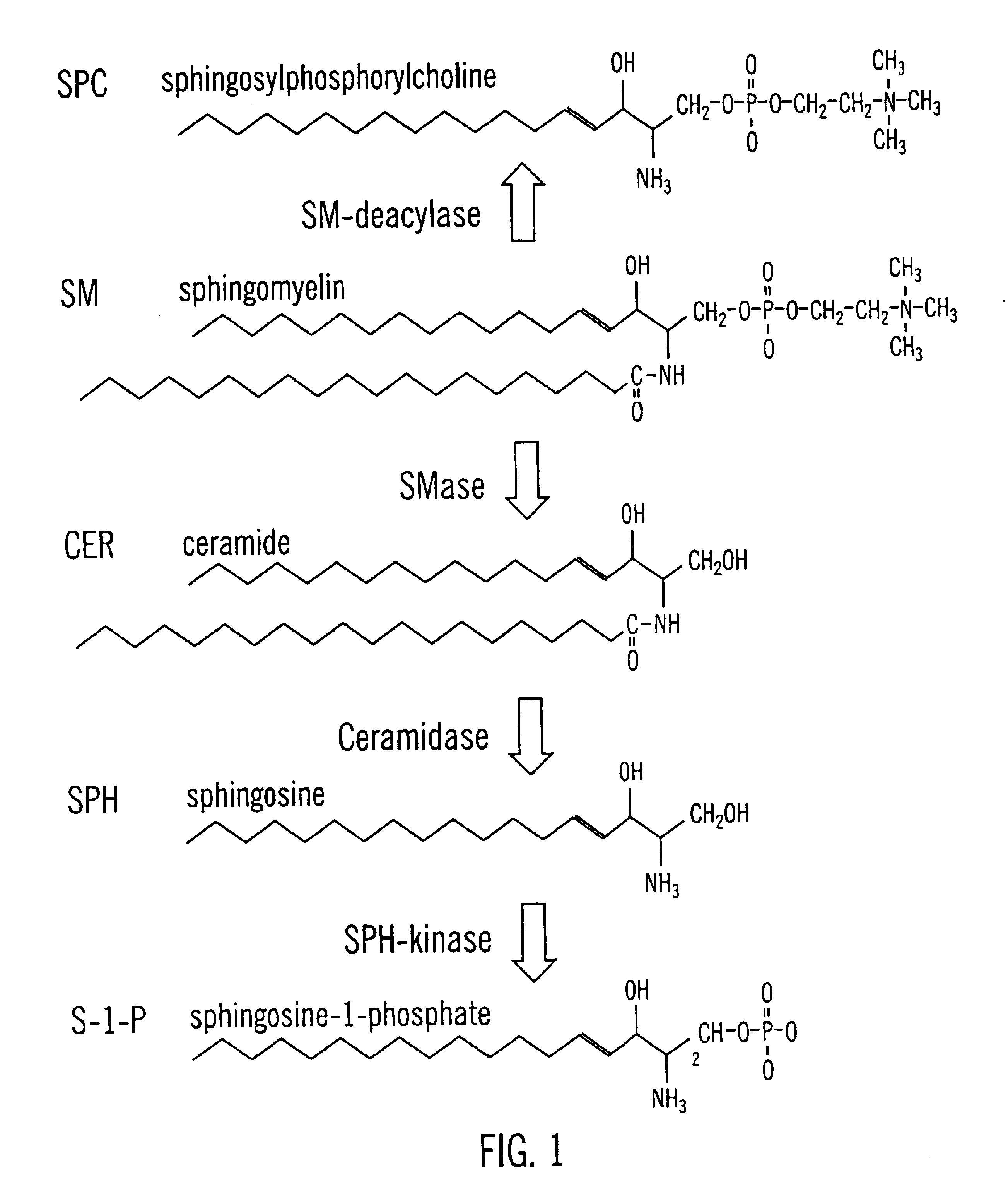
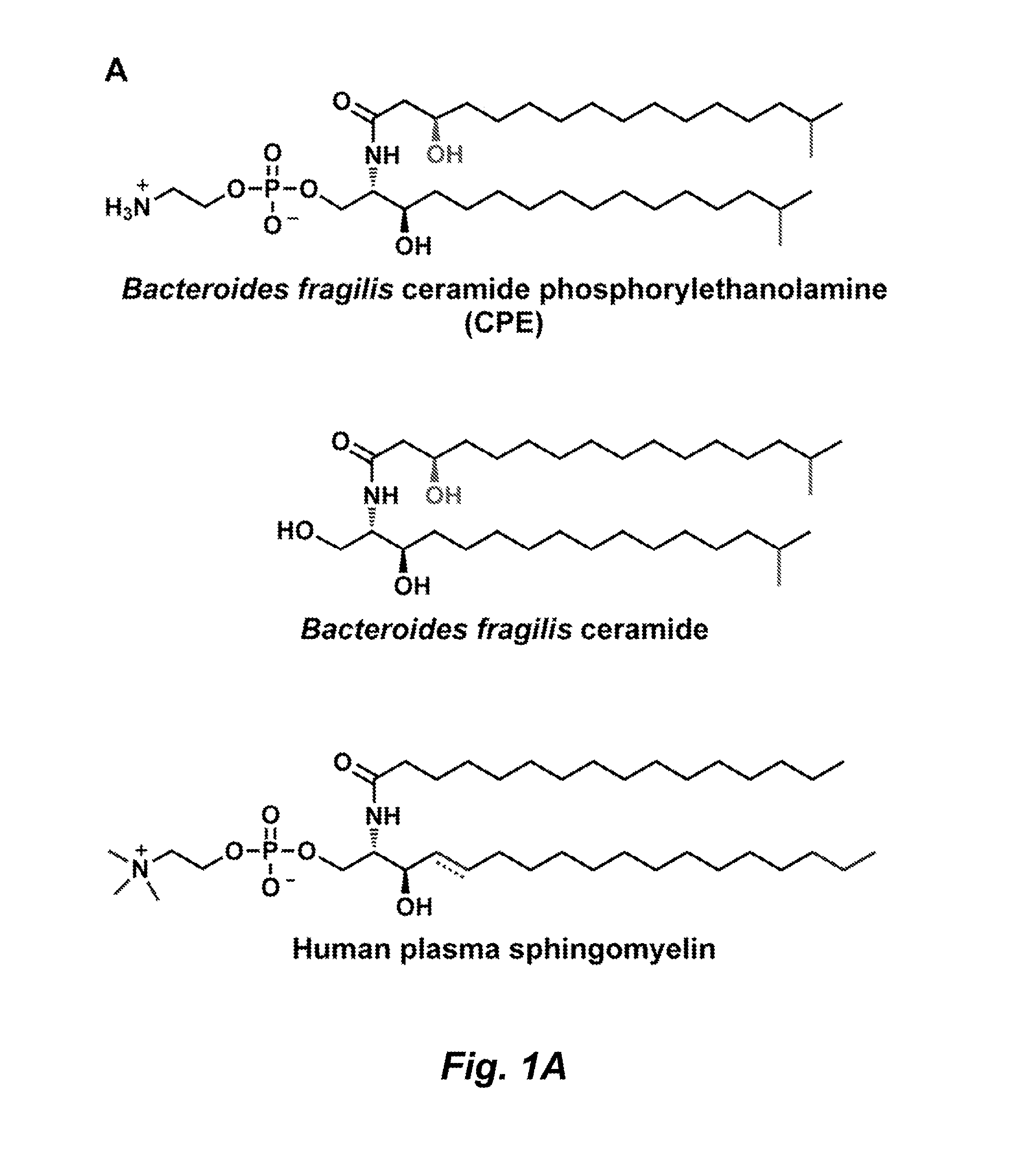
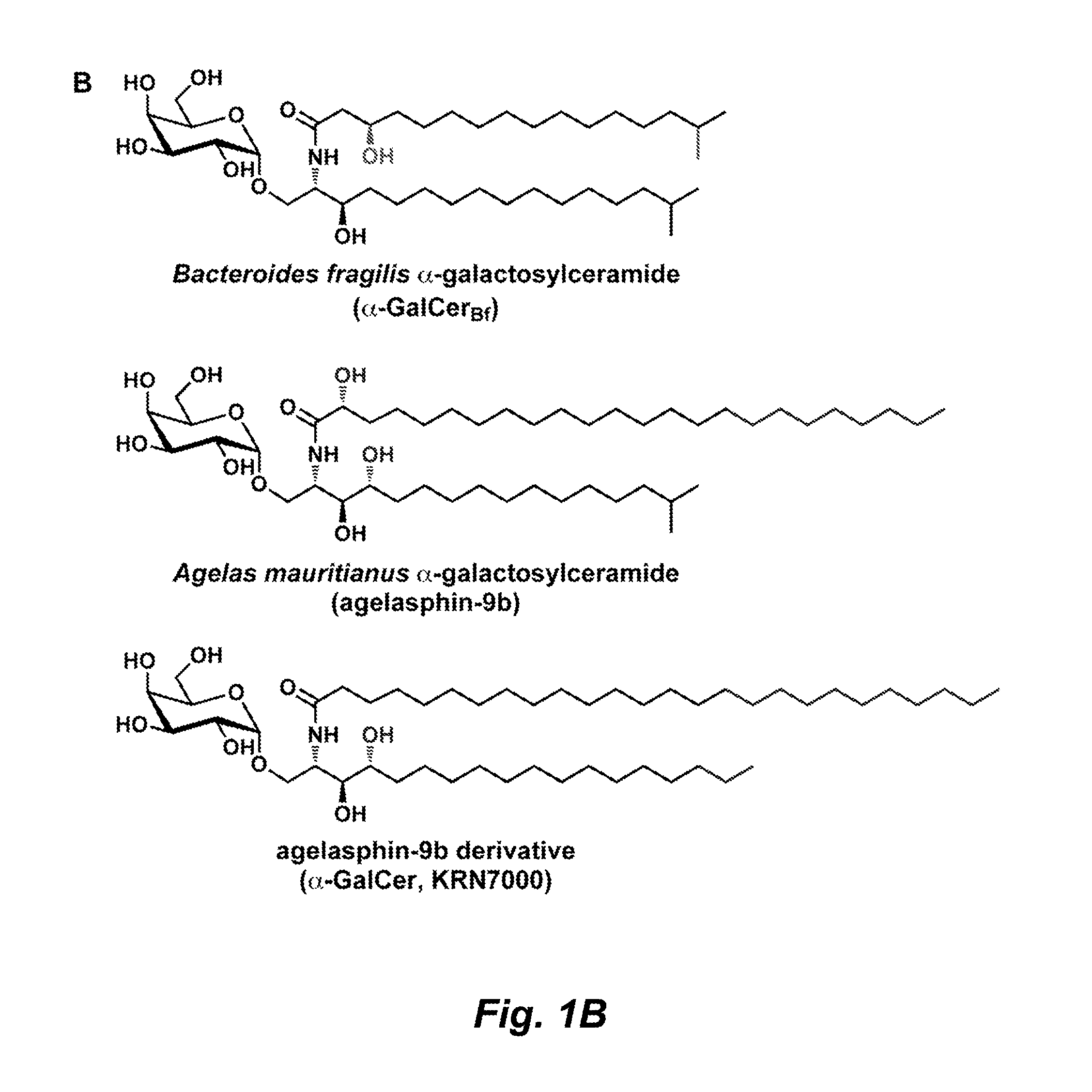
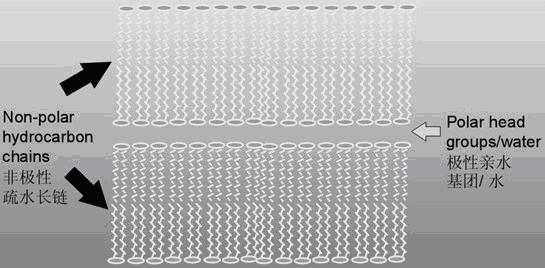
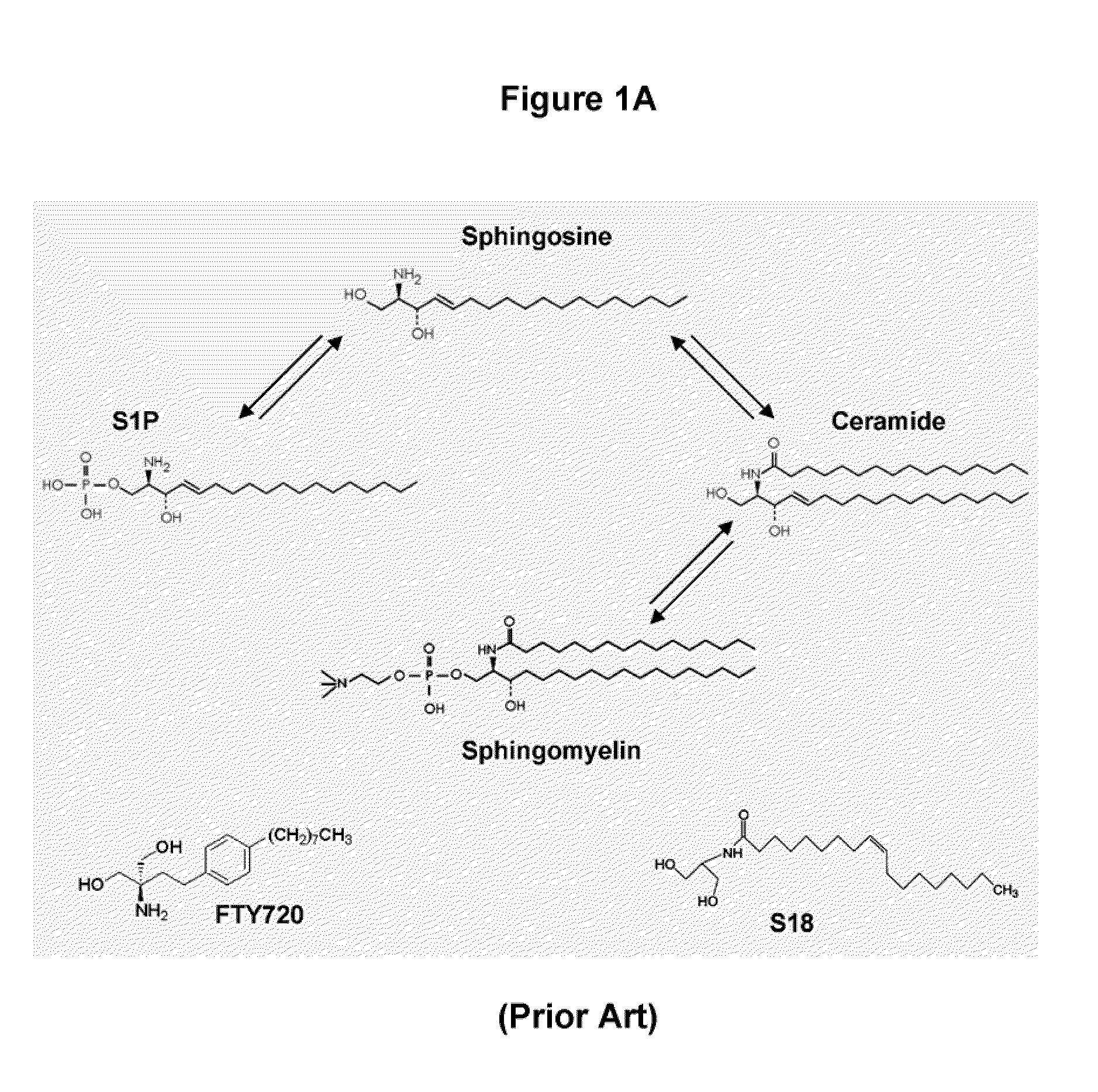
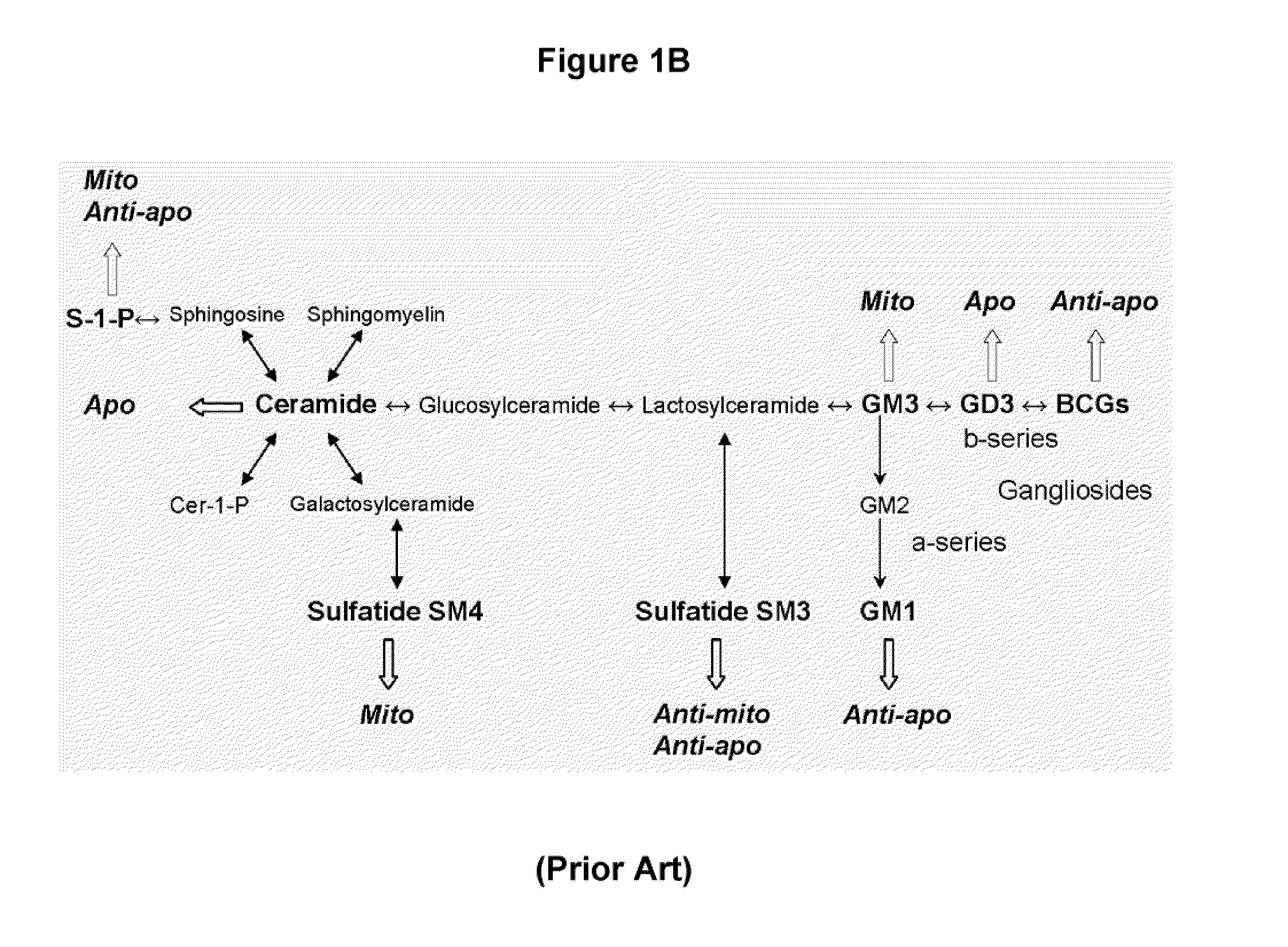
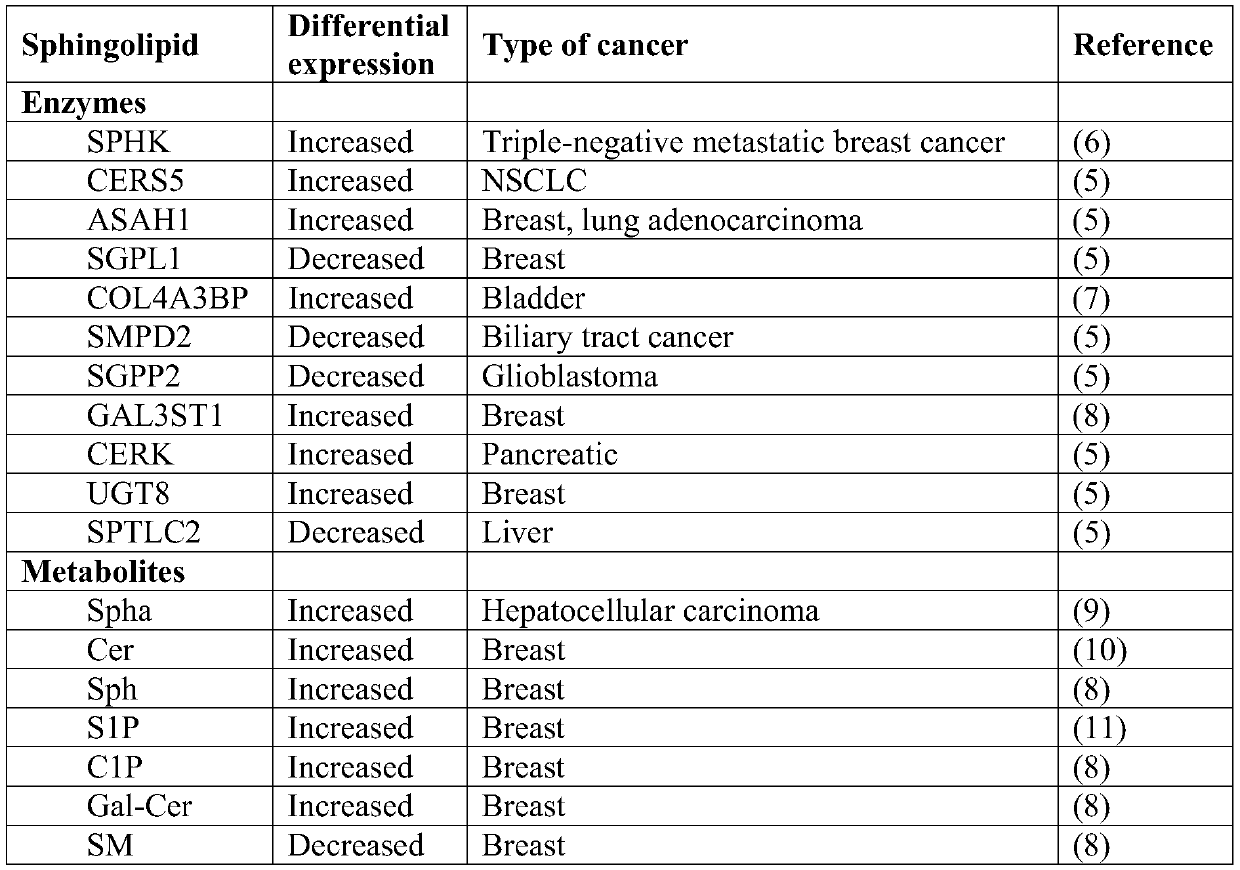
Sphingolipids are a class of lipids containing a backbone of sphingoid bases, a set of aliphatic amino alcohols that includes sphingosine. They were discovered in brain extracts in the 1870s and were named after the mythological sphinx because of their enigmatic nature. These compounds play important roles in signal transduction and cell recognition. Sphingolipidoses, or disorders of sphingolipid metabolism, have particular impact on neural tissue. A sphingolipid with an R group consisting of a hydrogen atom only is a ceramide. Other common R groups include phosphocholine, yielding a sphingomyelin, and various sugar monomers or dimers, yielding cerebrosides and globosides, respectively. Cerebrosides and globosides are collectively known as glycosphingolipids.
Compositions and methods for the treatment and prevention of cardiovascular diseases and disorders, and for identifying agents therapeutic therefor
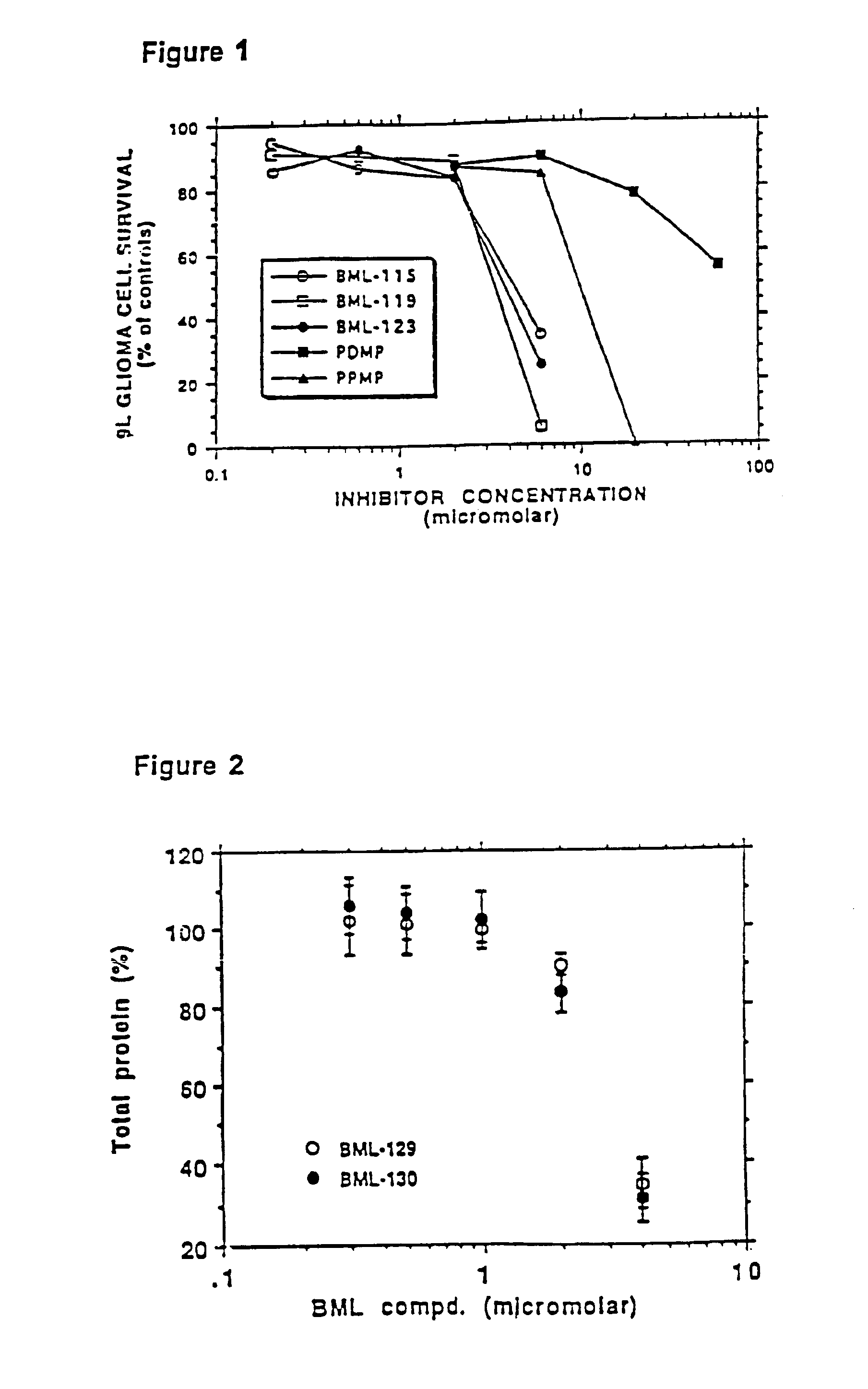
InactiveUS6858383B2High serum cholesterol levelDecreased blood flowOrganic active ingredientsHydrolasesMetaboliteSphingolipid metabolism
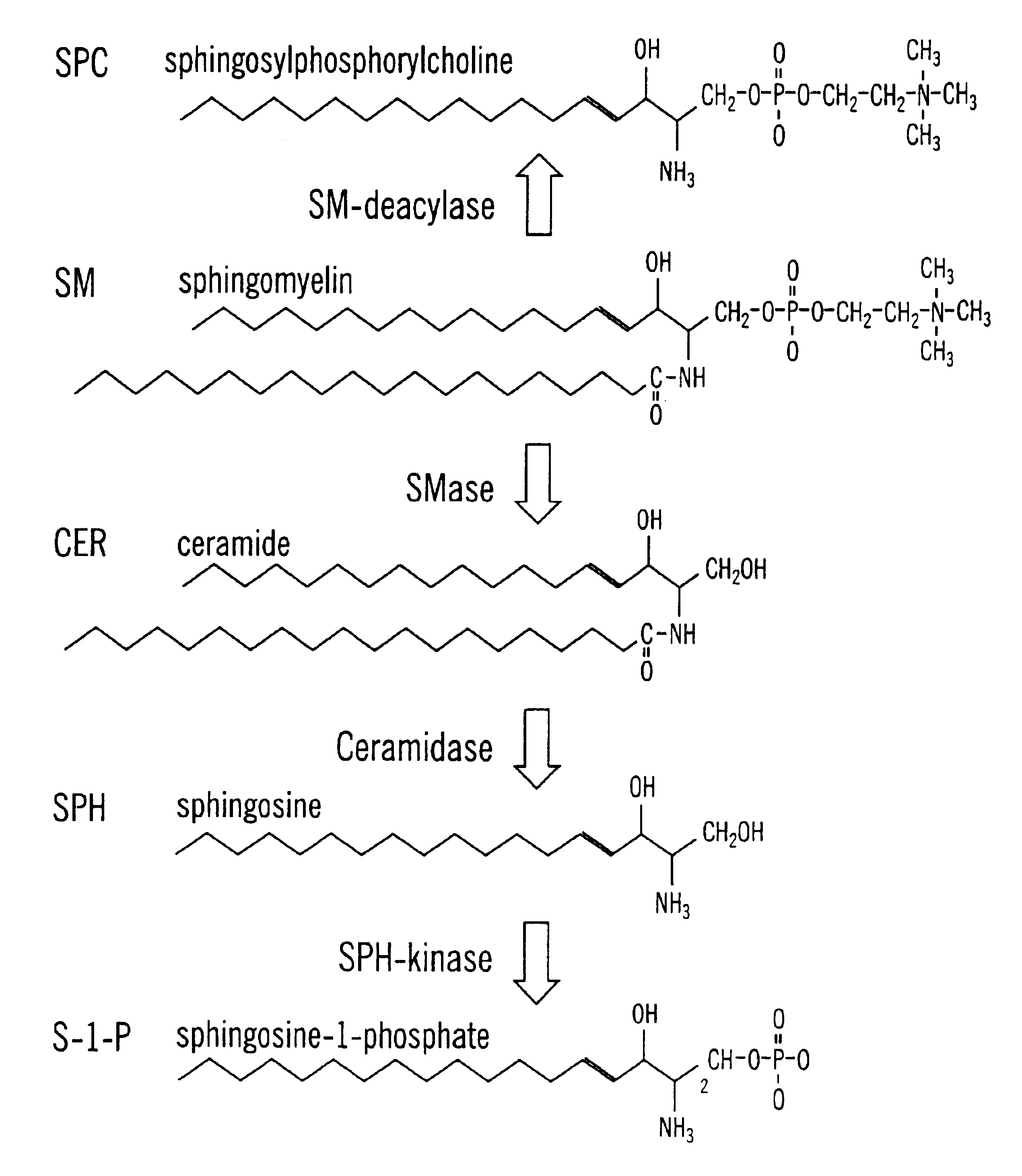
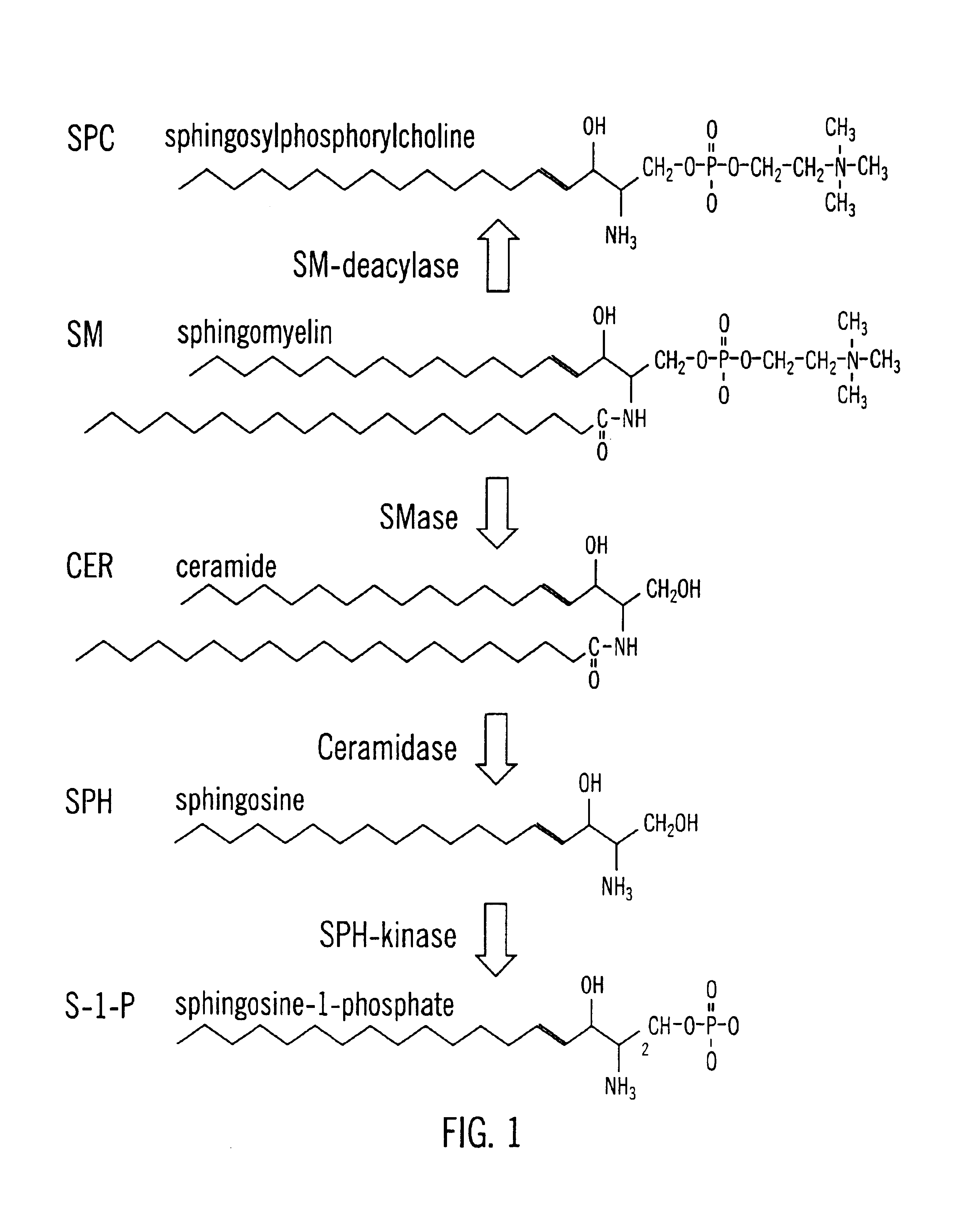
Methods and compositions are disclosed that are useful for the prevention and / or treatment of cardiovascular and cardiac diseases and disorders, or damage resulting from surgical or medical procedures that may cause ischemic or ischemic / reperfusion damage in humans; and cardiovascular trauma. The beneficial effects of the compositions and methods are achieved through the use of pharmaceutical compositions that include agents that interfere with the production and / or biological activities of sphingolipids and their metabolites, particularly sphingosine (SPH) and sphingosine-1-phosphate (S-1-P). Also disclosed are methods for identifying and isolating therapeutic agents.
Owner:APOLLO ENDOSURGERY INC
Amino ceramide-like compounds and therapeutic methods of use
InactiveUS6916802B2Lower Level RequirementsSolve low usageAntibacterial agentsBiocideDiseaseGlycosphingolipid
Owner:GENZYME CORP +1
Compositions and methods for the treatment and prevention of cardiovascular diseases and disorders, and for identifying agents therapeutic therefor
InactiveUS6881546B2High levelOrganic active ingredientsPeptide/protein ingredientsMetaboliteSphingolipid metabolism
Methods and compositions are disclosed that are useful for the prevention and / or treatment of cardiovascular and cardiac diseases and disorders, or damage resulting from surgical or medical procedures that may cause ischemic or ischemic / reperfusion damage in humans; and cardiovascular trauma. The beneficial effects of the compositions and methods are achieved through the use of pharmaceutical compositions that include agents that interfere with the production and / or biological activities of sphingolipids and their metabolites, particularly sphingosine (SPH) and sphingosine-1-phosphate (S-1-P). Also disclosed are methods for identifying and isolating therapeutic agents.
Owner:APOLLO ENDOSURGERY INC
Ophthalmic devices for delivery of hydrophobic comfort agents
ActiveUS20100140114A1Easy to understandPackage sterilisationPharmaceutical delivery mechanismLipid formationMonoglyceride
The invention relates to a soft hydrogel contact lens, especially a silicone hydrogel contact lens, which has a capability of delivering a hydrophobic comfort agent into the eye of a wearer. The hydrophobic comfort agent includes without limitation a monoglyceride, a diglyceride, a triglyceride, a glycolipid, a glyceroglycolipid, a sphingolipid, a sphingo-glycolipid, a phospholipid, a fatty acid, a fatty alcohol, a hydrocarbon having a C12-C28 chain in length, a mineral oil, a silicone oil, or a mixture thereof. It can be released from the soft hydrogel contact lens into the eye of a wearer when being worn so as to strengthen and stabilize the tear film lipid layer and alleviate the dryness of the eye.
Owner:ALCON INC
Amino ceramide-like compounds and therapeutic methods of use
InactiveUS7148251B2Lower Level RequirementsImprove concentrationBiocideOrganic chemistryDiseaseGlycosphingolipid
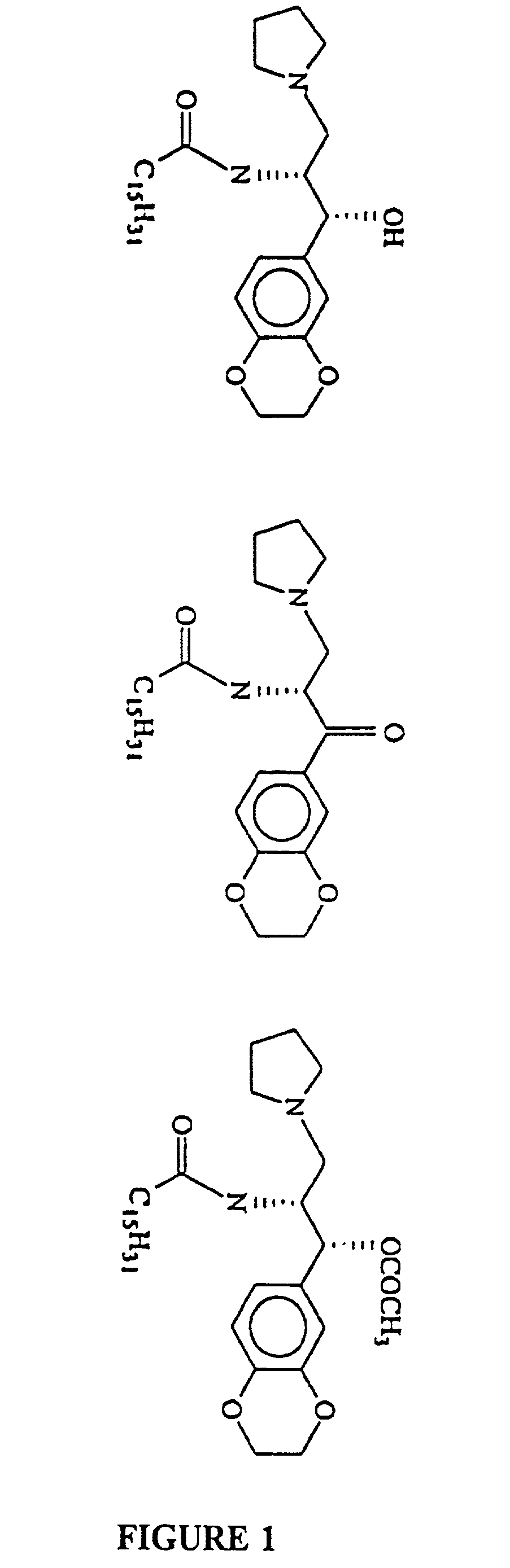
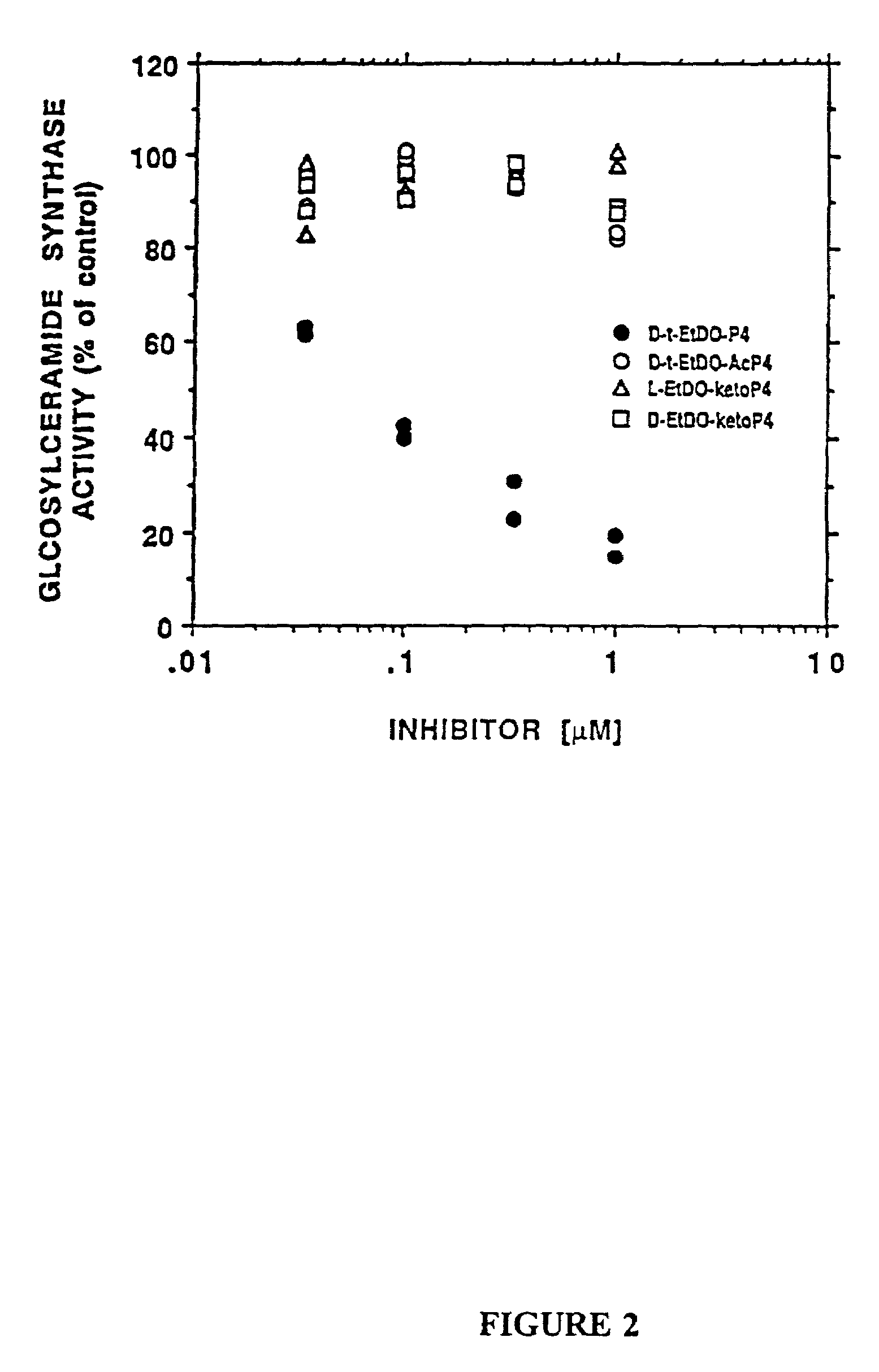
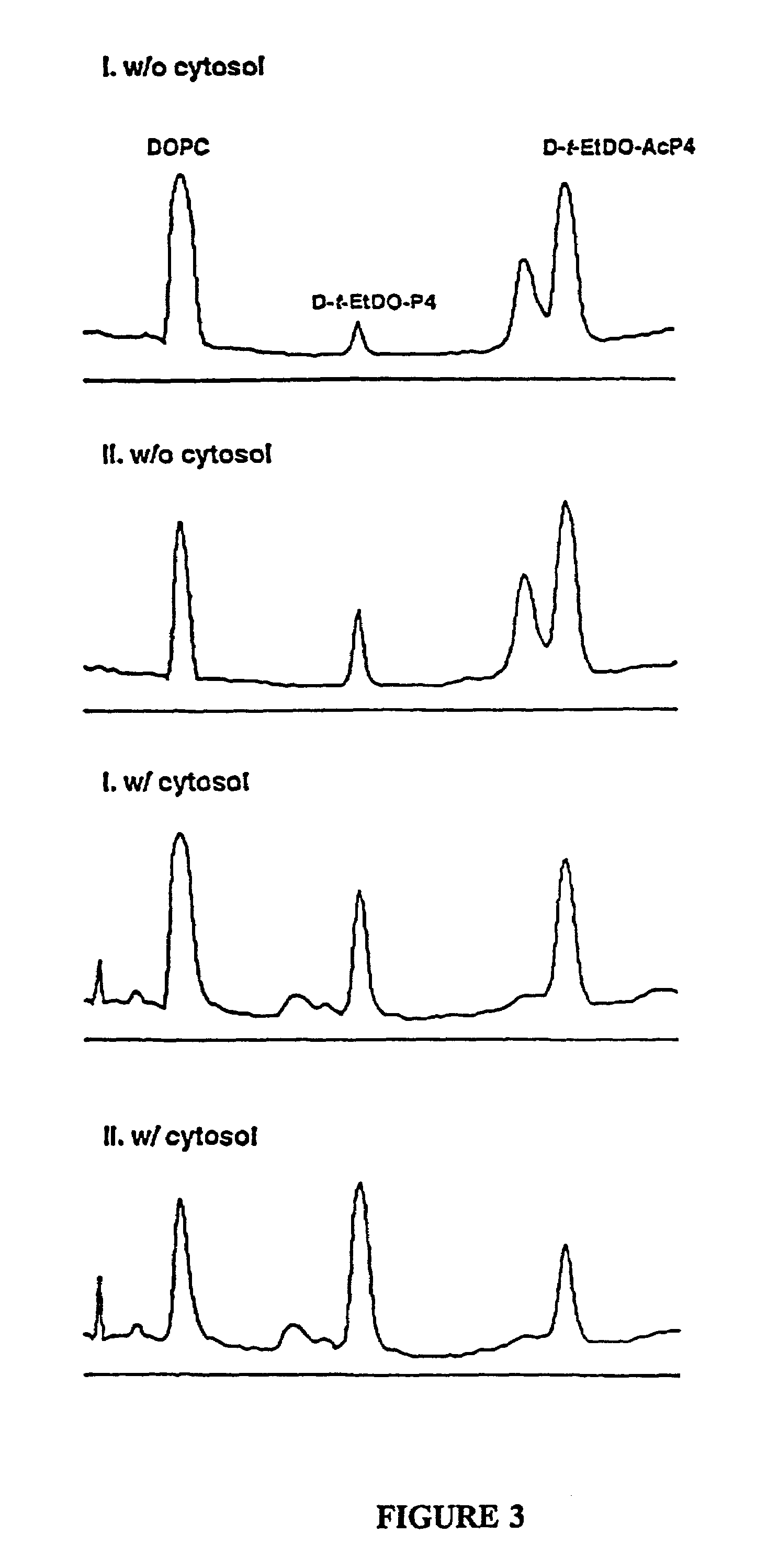
Novel prodrugs of amino ceramide-like compounds are provided which inhibit glucosyl ceramide (GlcCer) formation by inhibiting the enzyme GlcCer synthase, thereby lowering the level of glycosphingolipids. The compounds of the present invention have improved GlcCer synthase inhibition activity and are therefore highly useful in therapeutic methods for treating various conditions and diseases associated with altered glycosphingolipid levels.
Owner:RGT UNIV OF MICHIGAN
Amino ceramide-like compounds and therapeutic methods of use
Novel amino ceramide-like compounds (1) are provided which inhibit glucosyl ceramide (GlcCer) formation by inhibiting the enzyme GlcCer synthase, thereby lowering the level of glycosphingolipids. The compounds of the present invention have improved GlcCer synthase inhibition activity and are therefore highly useful in therapeutic methods for treating various conditions and diseases associated with altered glycosphingolipid levels.
Owner:RGT UNIV OF MICHIGAN
Plants with Increased Yield
InactiveUS20120227134A1MicroorganismsVector-based foreign material introductionUbiquitin-Protein LigasesSerine hydroxymethyltransferase
A method for producing a plant with increased yield as compared to a corresponding wild type plant whereby the method comprises at least the following step: increasing or generating in a plant or a part thereof one or more activities of a polypeptide selected from the group consisting of 2-oxoglutarate-dependent dioxygenase, 3-ketoacyl-CoA thiolase, 3′-phosphoadenosine 5′-phosphate phosphatase, 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase, 5OS chloroplast ribosomal protein L21, 57972199. R01.1-protein, 60952769. R01.1-protein, 60S ribosomal protein, ABC transporter family protein, AP2 domain-containing transcription factor, argonaute protein, AT1 G29250.1-protein, AT1 G53885-protein, AT2G35300-protein, AT3G04620-protein, AT4G01870-protein, AT5G42380-protein, AT5G47440-protein, CDS5394-protein, CDS5401_TRUNCATED-protein, cold response protein, cullin, Cytochrome P450, delta-8 sphingolipid desaturase, galactinol synthase, glutathione-S-transferase, GTPase, haspin-related protein, heat shock protein, heat shock transcription factor, histone H2B, jasmonate-zim-domain protein, mitochondrial asparaginyl-tRNA synthetase, Oligosaccharyltransferase, OS02G44730-protein, Oxygen-evolving enhancer protein, peptidyl-prolyl cis-trans isomerase, peptidyl-prolyl cis-trans isomerase family protein, plastid lipid-associated protein, Polypyrimidine tract binding protein, PRLI-interacting factor, protein kinase, protein kinase family protein, rubisco subunit binding-protein beta subunit, serine acetyltransferase, serine hydroxymethyltransferase, small heat shock protein, S-ribosylhomocysteinase, sugar transporter, Thioredoxin H-type, ubiquitin-conjugating enzyme, ubiquitin-protein ligase, universal stress protein family protein, and Vacuolar protein.
Owner:BASF PLANT SCI GMBH
Glycosphingolipids for use in modulating immune responses
ActiveUS20140377291A1Modulate immune responseEnhance immune responseAntibacterial agentsBiocideAutoimmune diseaseSphingolipid metabolism
Provided herein are sphingolipid compounds that are useful for activating natural killer T cells. Also provided are methods for treating or preventing a disease or disorder that is treatable by activating the immune system by stimulating natural killer T cells. The compounds are therefore useful for treating or reducing the likelihood of occurrence of an immune diseases and disorders, such as autoimmune diseases or disorders. The compounds may also be used for treating or reducing the likelihood of occurrence of a microbial infection or for treating or reducing the likelihood of occurrence of a cancer in a subject by administering the sphingolipid compounds described herein.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
Amino ceramide-like compounds and therapeutic methods of use
InactiveUS7335681B2Lower Level RequirementsImprove concentrationAntibacterial agentsBiocideDiseaseGlycosphingolipid
Owner:RGT UNIV OF MICHIGAN
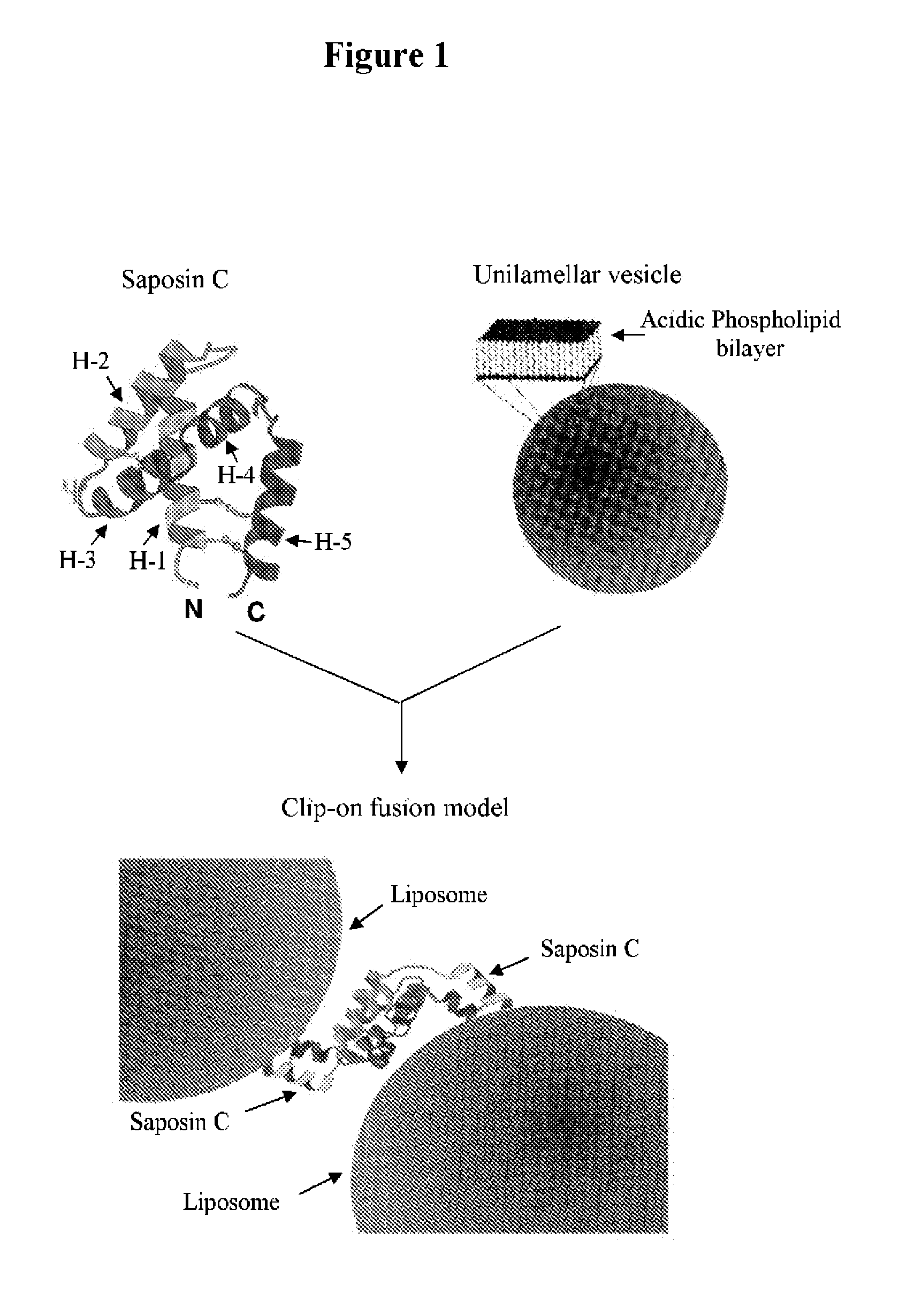
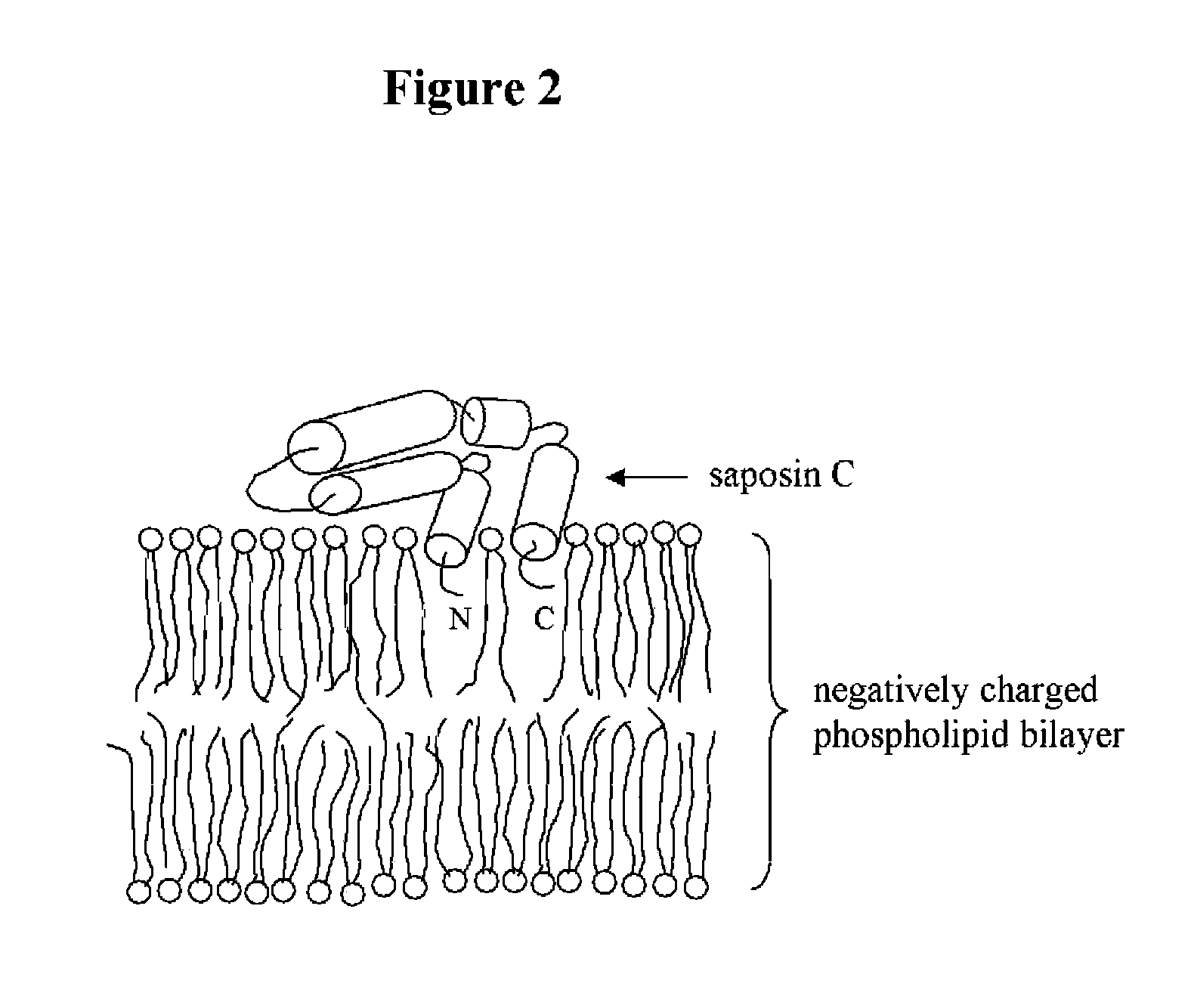
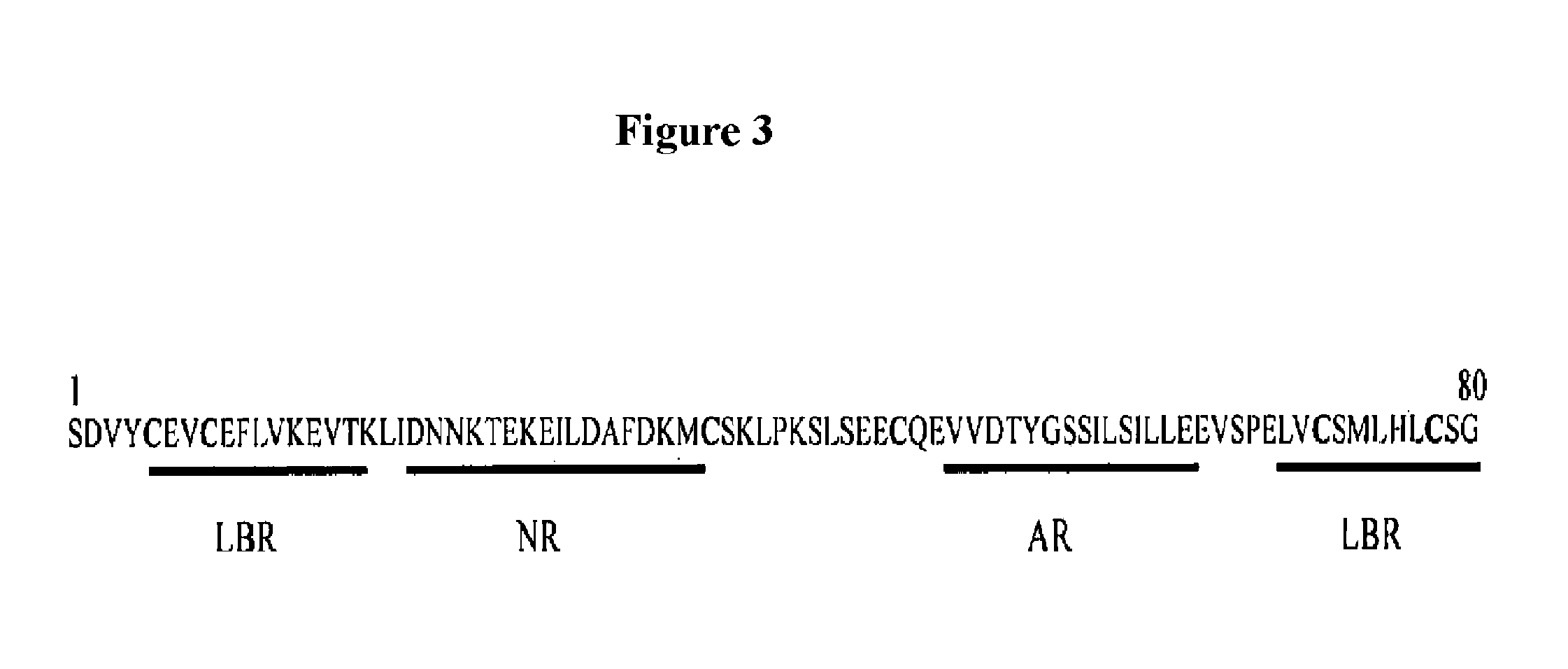
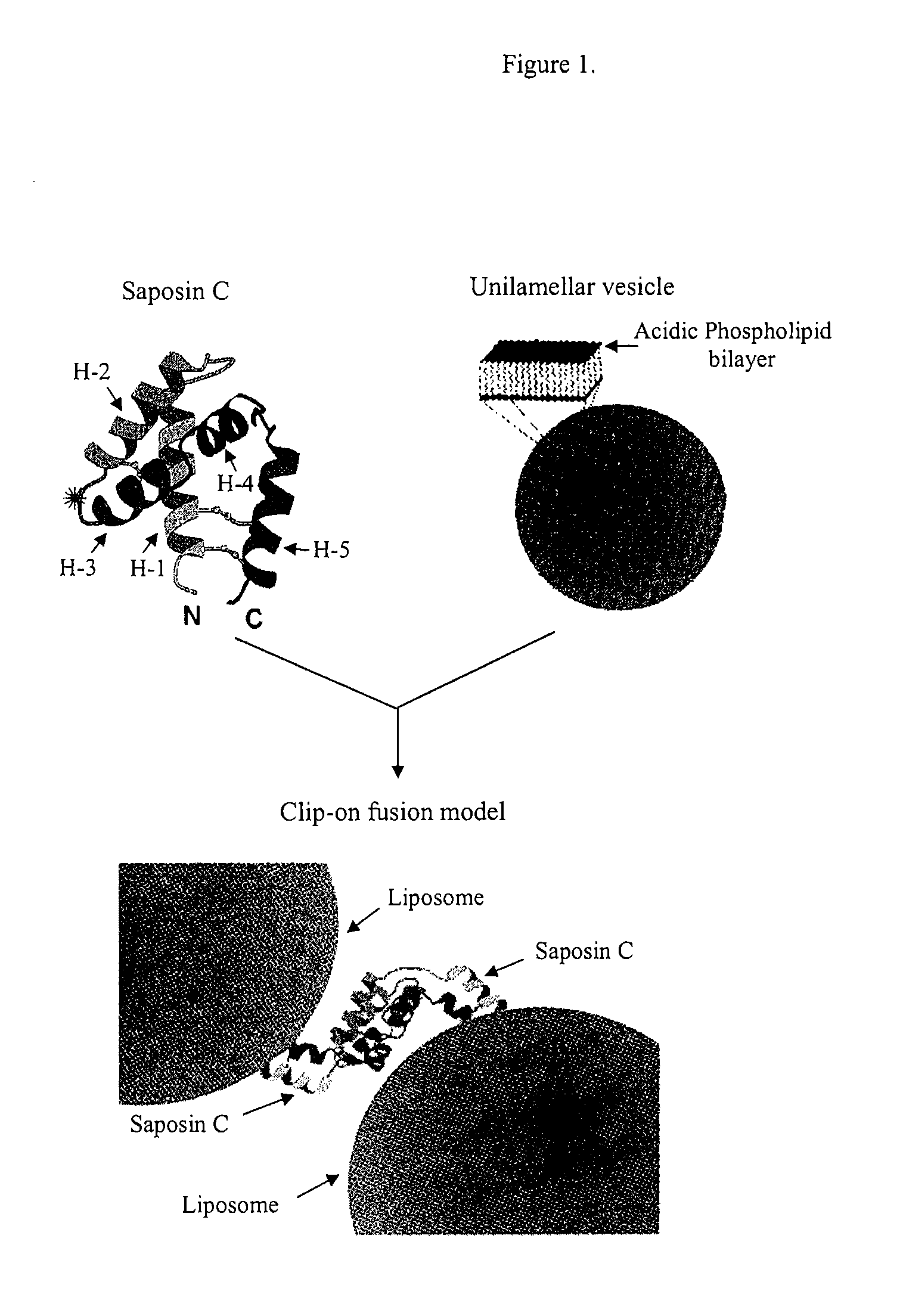
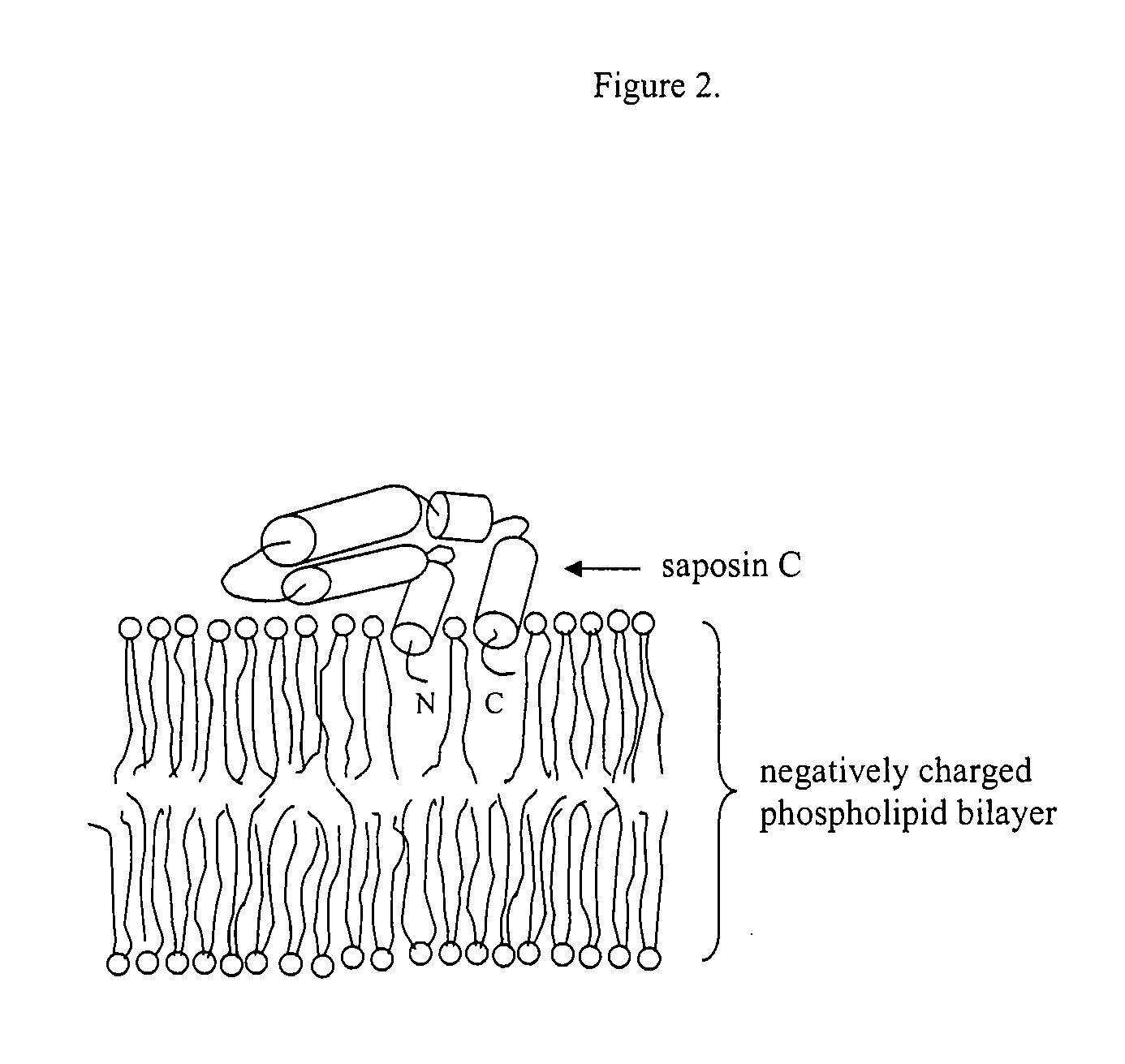
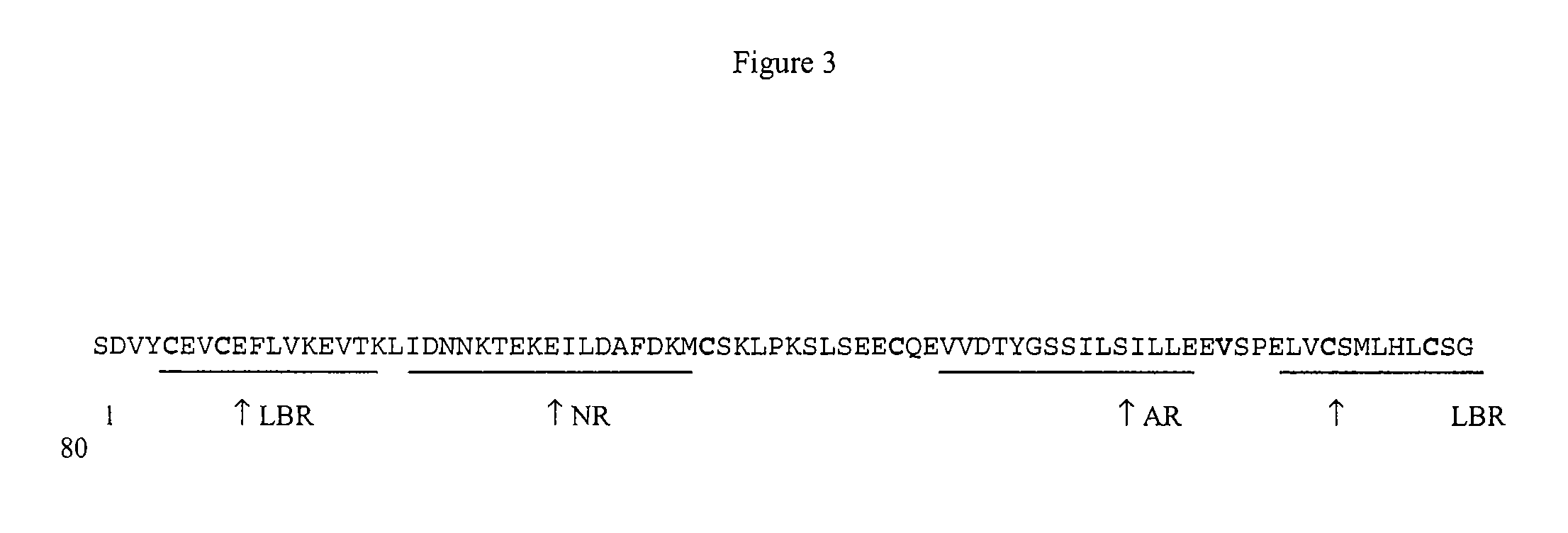
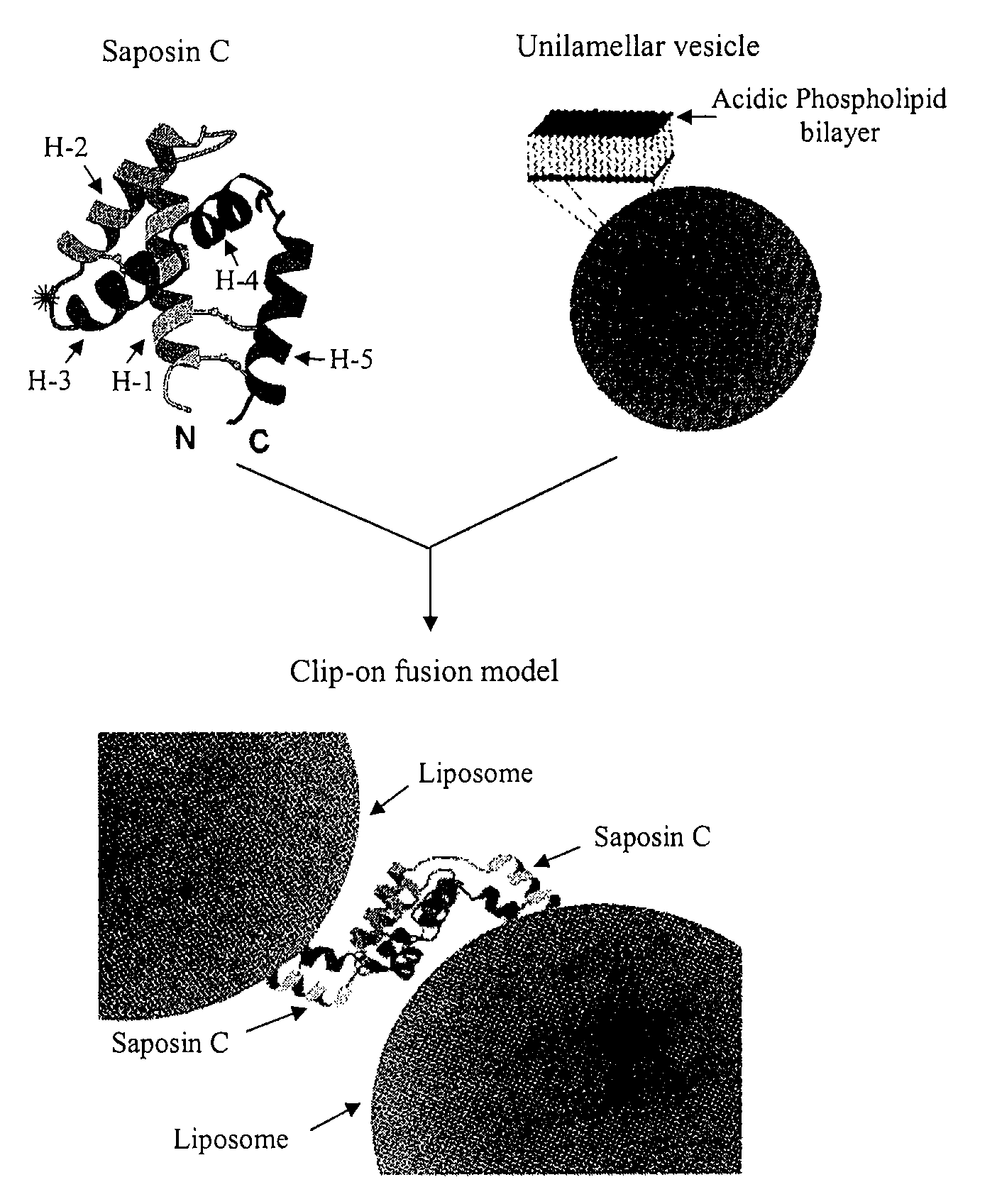
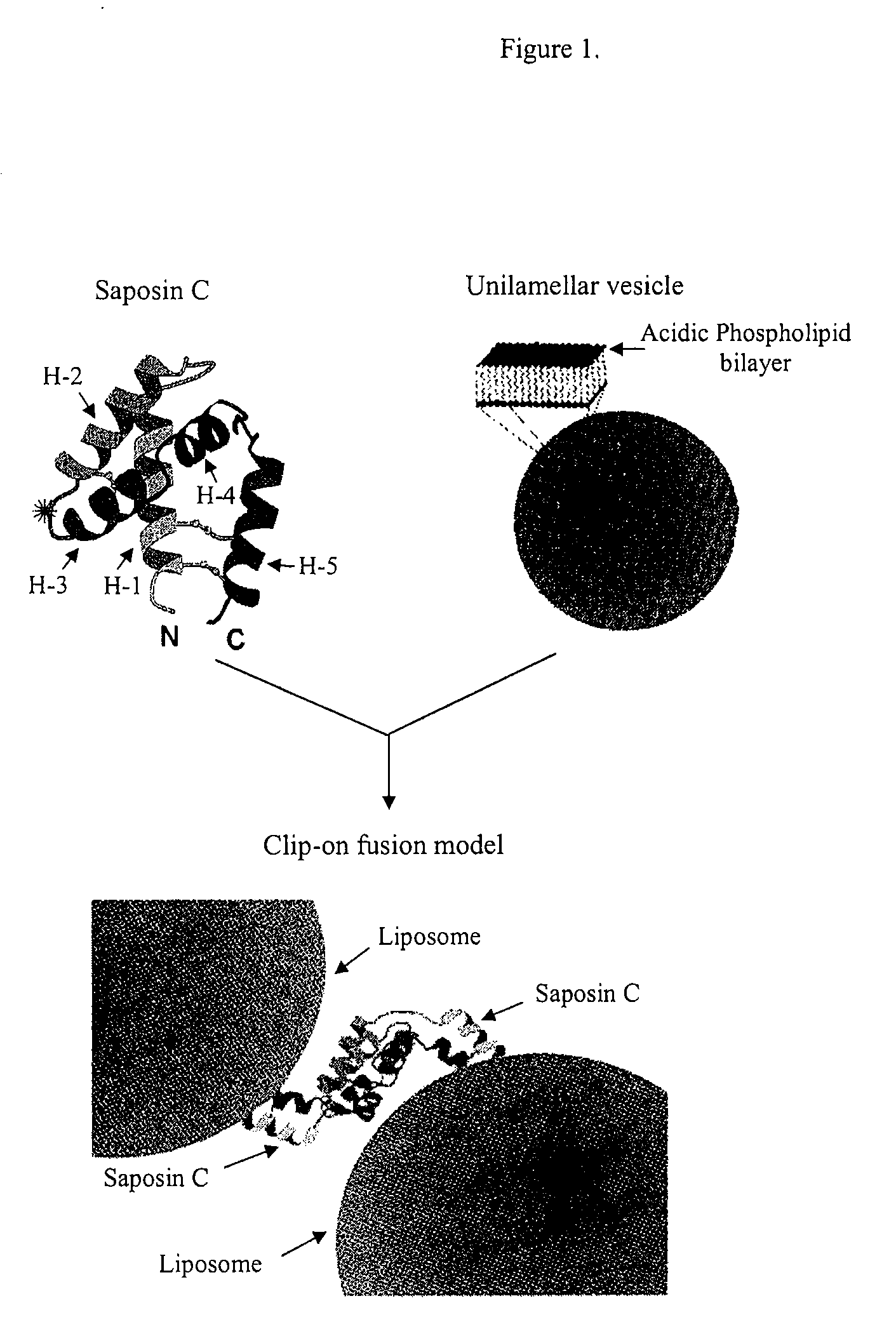
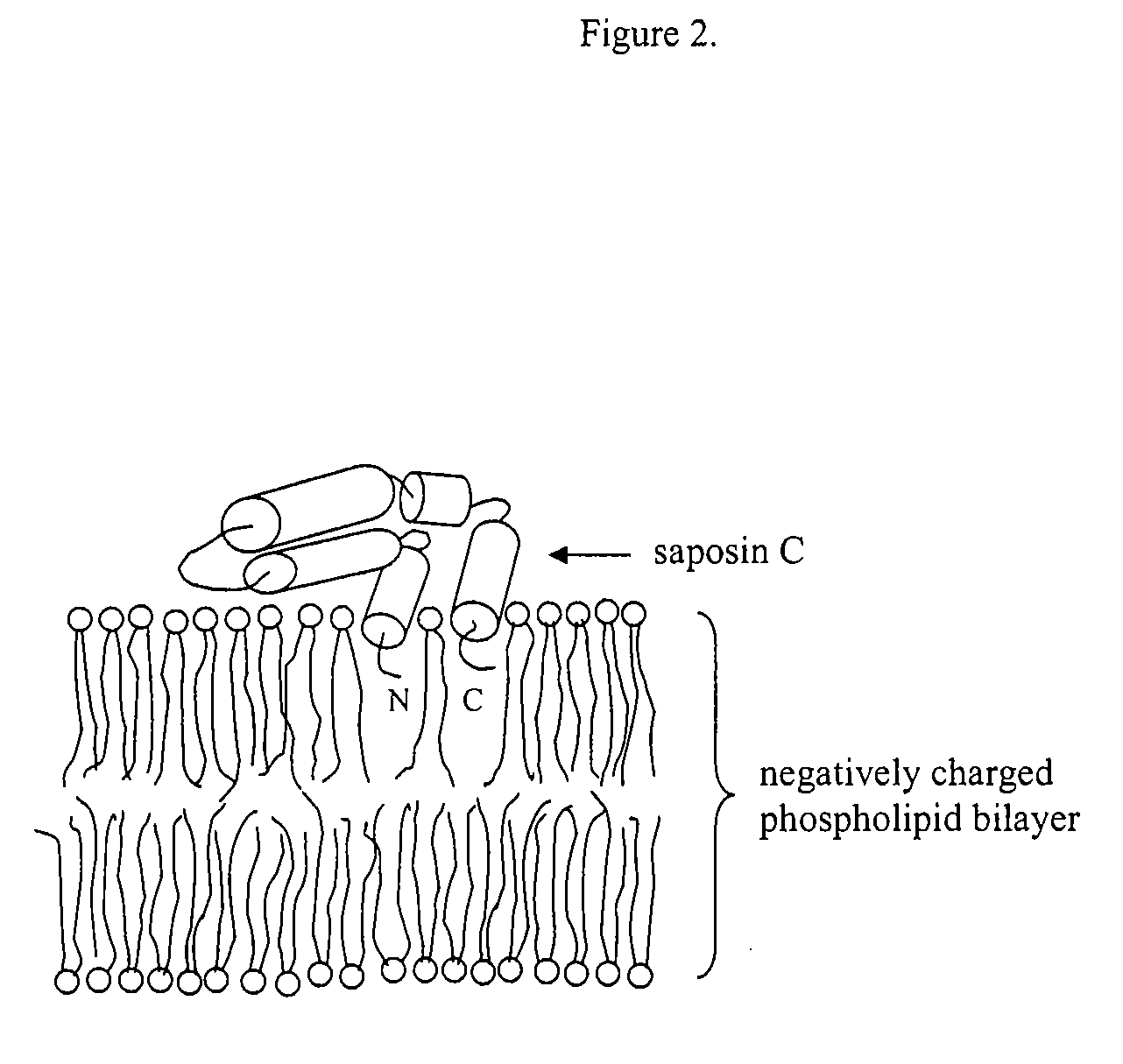
Fusogenic properties of saposin c and related proteins and peptides for application to transmembrane drug delivery systems
InactiveUS20120020878A1Reduce deliveryUltrasonic/sonic/infrasonic diagnosticsPeptide/protein ingredientsLipid formationActive agent
The present invention comprises a method for delivering pharmaceutical and / or imaging agents within and / or through the dermal, mucosal and other cellular membranes, and across the blood-brain barrier, utilizing a fusogenic protein. The fusogenic protein is associated with a phospholipid membrane, such as a liposome. The liposome may include dioleoylphosphatidylserine, a negatively charged long-chain lipid. Alternatively, the liposome is comprised of a mixture of negatively charged long-chain lipids, neutral long-chain lipids, and neutral short-chain lipids. Preferred fusogenic proteins include saposin C and other proteins, polypeptides and peptide analogs derived from saposin C. The active agent contained within the liposome may comprise biomolecules and / or organic molecules. This technology can be used for both cosmetic and medicinal applications in which the objective is delivery of the active agent within and / or beneath biological membranes or across the blood-brain barrier and neuronal membranes.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Polar lipid mixtures, their preparation and uses
InactiveUS20110294757A1Milk preparationOrganic active ingredientsPhosphatidyl inositolPhosphatidylethanolamine
Disclosed herein are polar lipid mixtures, comprising glycerophospholipids such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS) and phosphatidyl-inositol (PI), and sphingolipids such as sphyngomyelin (SM). Most importantly, the ratio of phospholipids in said mixture is comparable to that of HMF, and is represented by SM>PC>PE>PS>PI or SM=PC>PE>PS>PI. Processes for the preparation of said mixtures and uses thereof are also described herein.
Owner:ENZYMOTEC
Medical skin care agent and preparation method thereof
InactiveCN102626374AHas broad-spectrum antibacterial propertiesAchieve antibacterial and anti-inflammatory effectsCosmetic preparationsToilet preparationsPhospholipidAnti inflammation
The invention discloses a medical skin care agent and a preparation method thereof, wherein, the medical skin care agent provided in the invention mainly comprises chitosan or derivatives thereof, a gelling agent and preservative. Specifically, the gelling agent is advantaged in that: ceramide, a mixture of sphingolipids and phospholipids, ceramide analogue and the like are used to form a layered-structure gel phase structure which is similar to skin lipoid structure and capable of enhancing the lipoid structure of the skin itself, thereby realizing the function of repairing skin barrier; due to the adding of the chitosan or the derivatives thereof in the medical skin care agent, the medical skin care agent is provided with broad-spectrum anti-microbial property and can effectively repair common skin inflammation of redness, swelling, peeling, itching and the like for dry, damaged and sensitive skin, thereby realizing anti-microbial function and anti-inflammation function in medical skin care.
Owner:佛山拜澳生物科技有限公司
Pharmaceutical formulations employing short-chain sphingolipids and their use
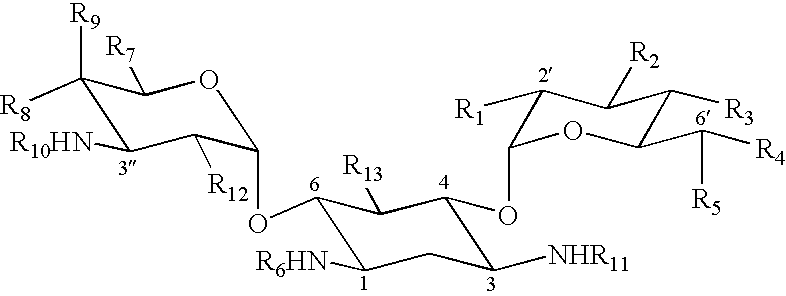
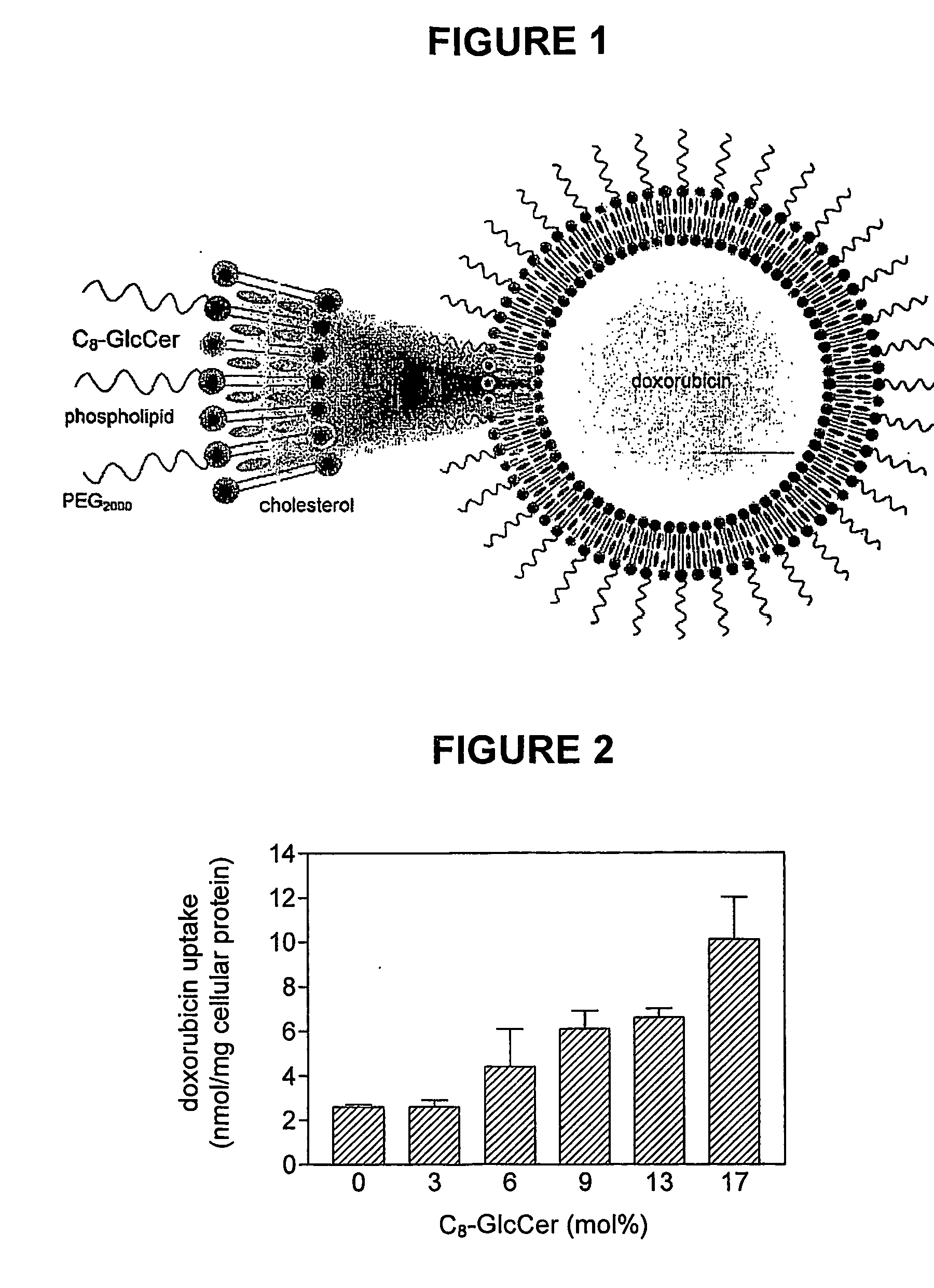
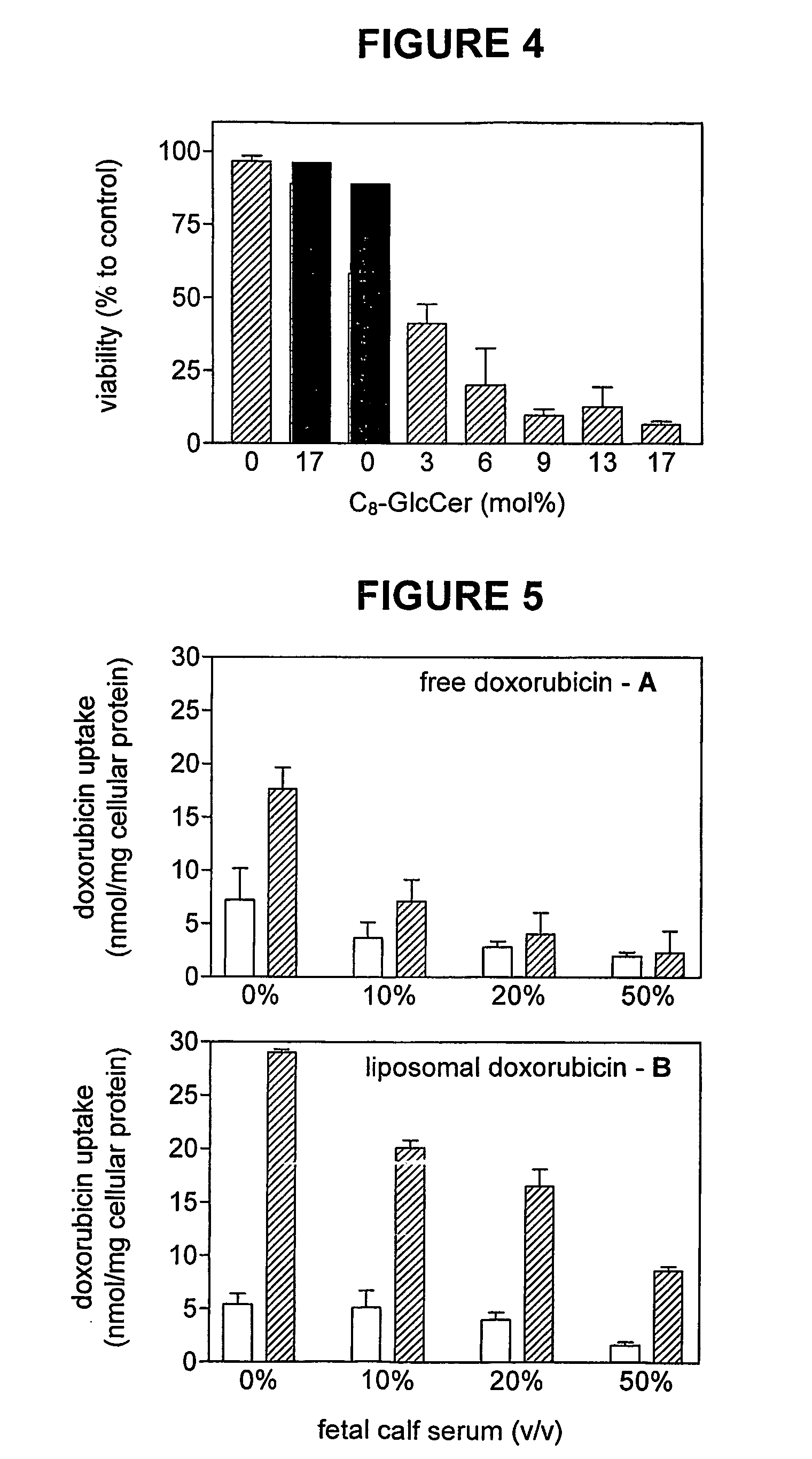
This invention pertains to pharmaceutical formulations which comprise (i) a drug (e.g., an amphiphilic drug) (e.g., an anthracycline) (e.g., doxorubicin) and (ii) a short-chain sphingolipid (e.g., a short-chain glycosphingolipid or a short-chain sphingomyelin) (e.g., N-octanoyl-glucosylceramide, referred to as C8-GlcCer) (e.g., N-hexanoyl-sphingomyelin, referred to herein as C6-SM), and which provide improved drug delivery and efficacy. The short-chain sphingolipidis selected from compounds of the following formula: wherein: R1 is independently: an O-linked saccharide group; or an O-linked polyhydric alcohol group; or: R1 is independently: an O-linked (optionally N-(C1-4alkyl)-substituted amino)-C1-6alkyl-phosphate group; or an O-linked (polyhydric alcohol-substituted)-C1-6alkyl-phosphate group; R2 is independently C3-9alkyl, and is independently unsubstituted or substituted; R3 is independently C7-19alkyl, and is independently unsubstituted or substituted; R4 is independently —H, —OH, or —O—C1-4alkyl; RN is independently —H or C1-4alkyl; the bond marked with an alpha (α) is independently a single bond or a double bond; if the bond marked with an alpha (α) is a double bond, then R5 is —H; if the bond marked with an alpha (α) is a single bond, then R5 is —H or —OH; the carbon atom marked (*) is independently in an R-configuration or an S-configuration; the carbon atom marked (**) is independently in an R-configuration or an S-configuration; and pharmaceutically acceptable salts, solvates, esters, ethers, chemically protected forms thereof. In one embodiment, the pharmaceutical formulation is a liposomal pharmaceutical formulation prepared using a mixture of lipids comprising, at least, vesicle-forming lipids (e.g., phospholipids) (e.g., phosphatidylcholines) (e.g., fully hydrogenated soy phosphatidylcholine (HSPC)) (e.g., dipalmitoyl-phosphatidylcholine (DPPC)) and said short-chain sphingolipid, and optionally cholesterol and optionally a vesicle-forming lipid which is derivatized with a polymer chain (e.g., a phosphatidylethanolamine (PE) which is derivatized with polyethyleneglycol (PEG)) (e.g., N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (MPEG2000-DSPE). The present invention also pertains to methods for the preparation and use of such formulations.
Owner:NETHERLANDS CANCER INST
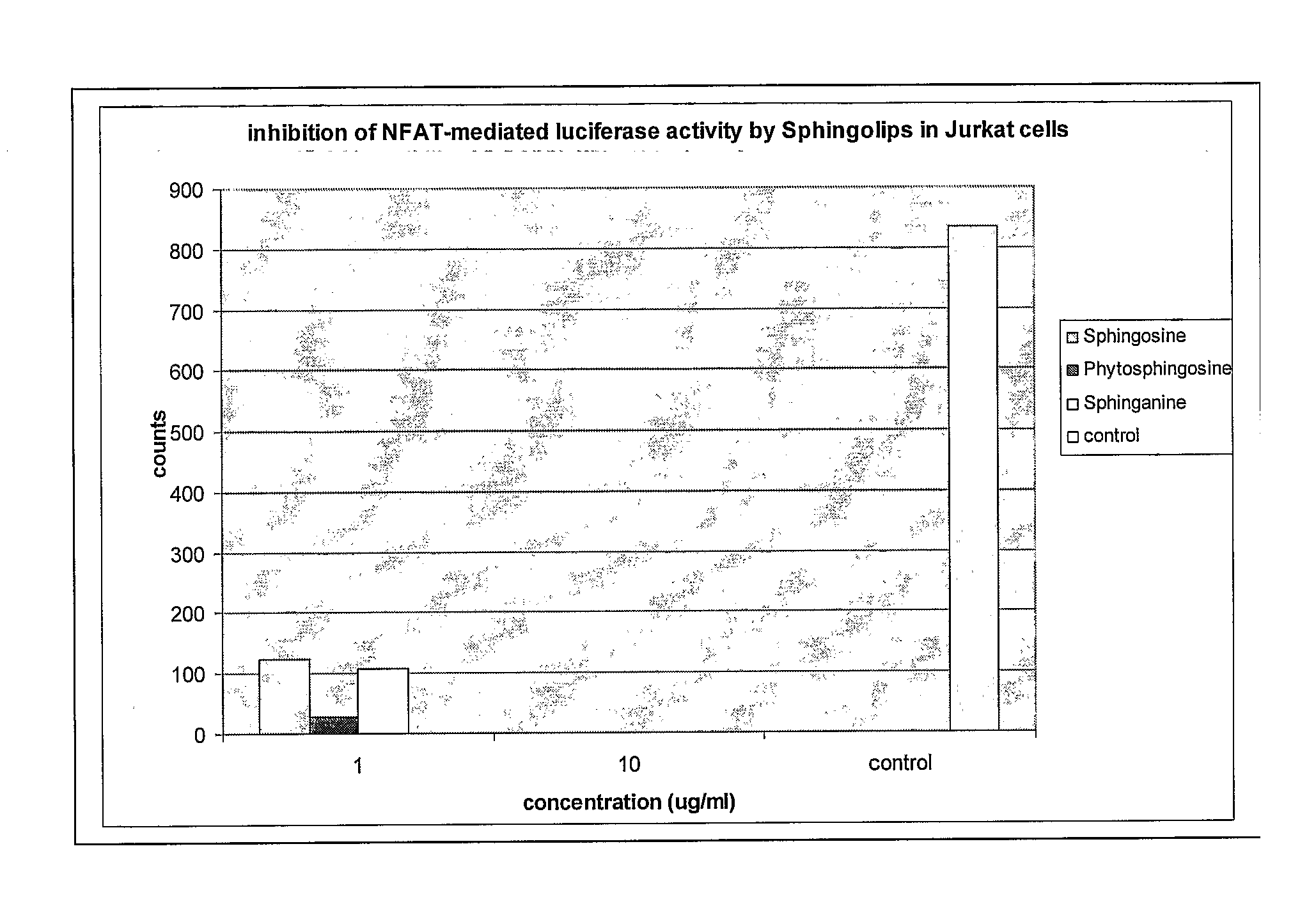
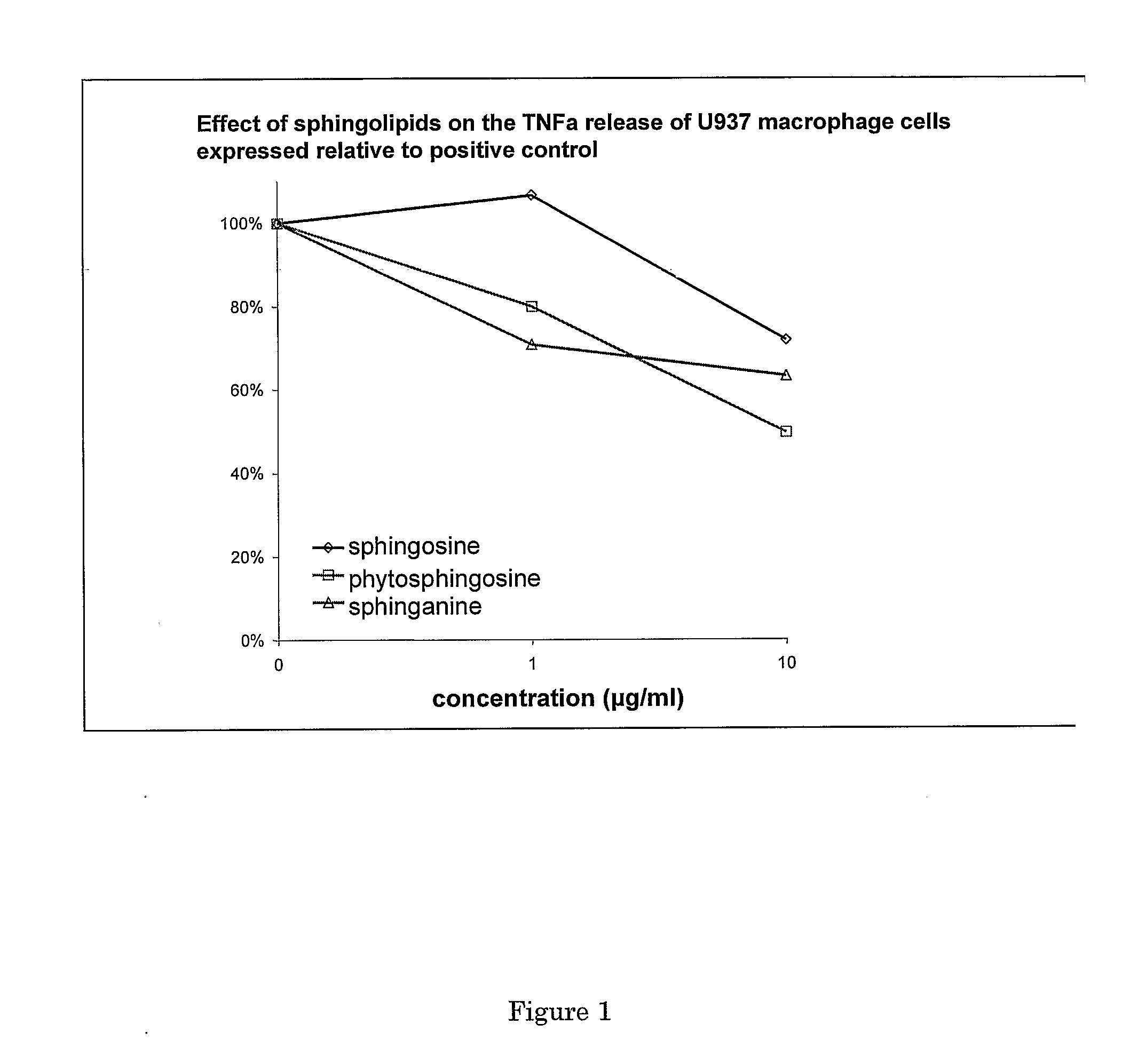
Sphingolipids for improvement of the composition of the intestinal flora
InactiveUS20060134182A1Efficient killingDifficult to controlAntibacterial agentsBiocideLysosphingomyelinGut flora
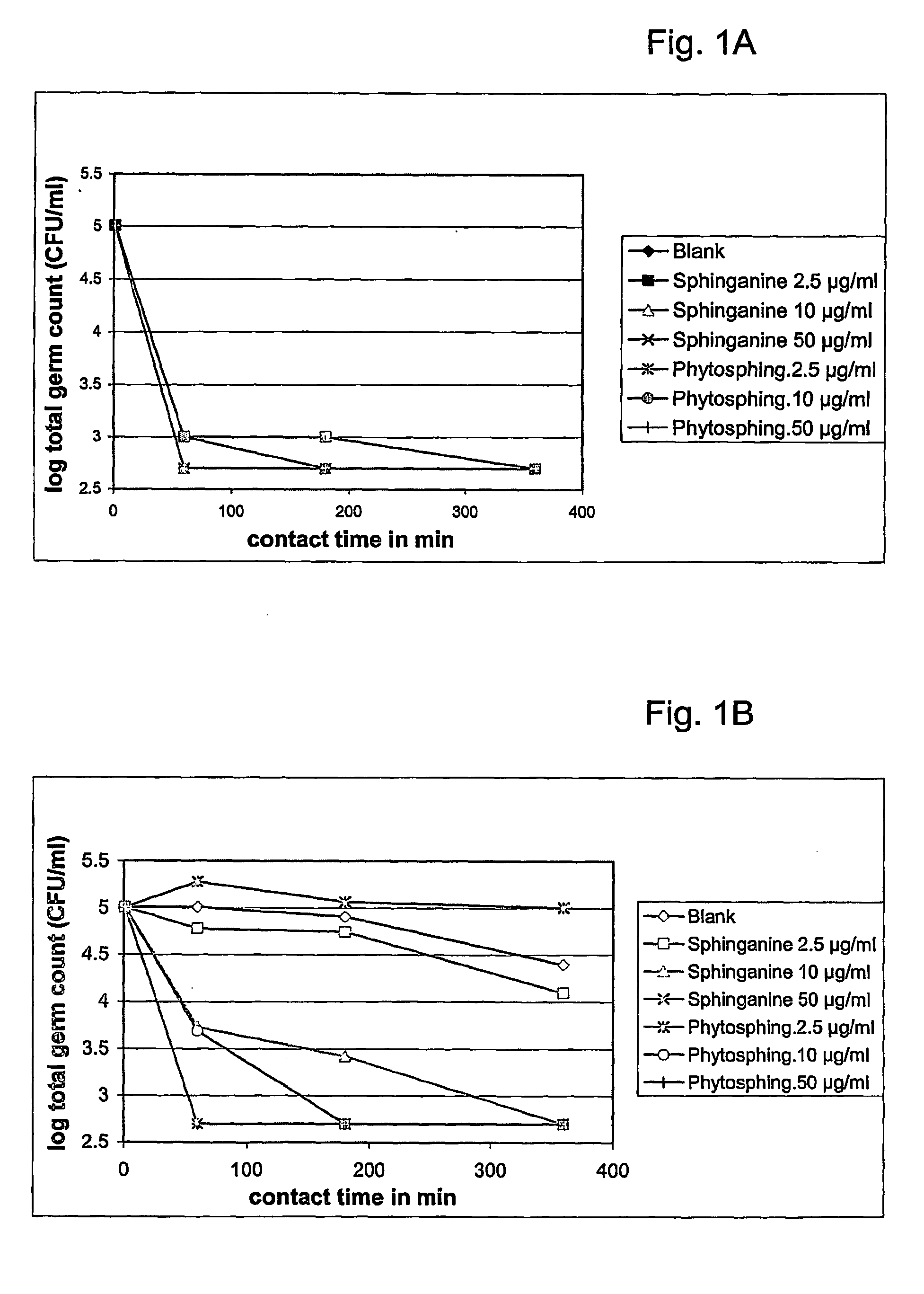
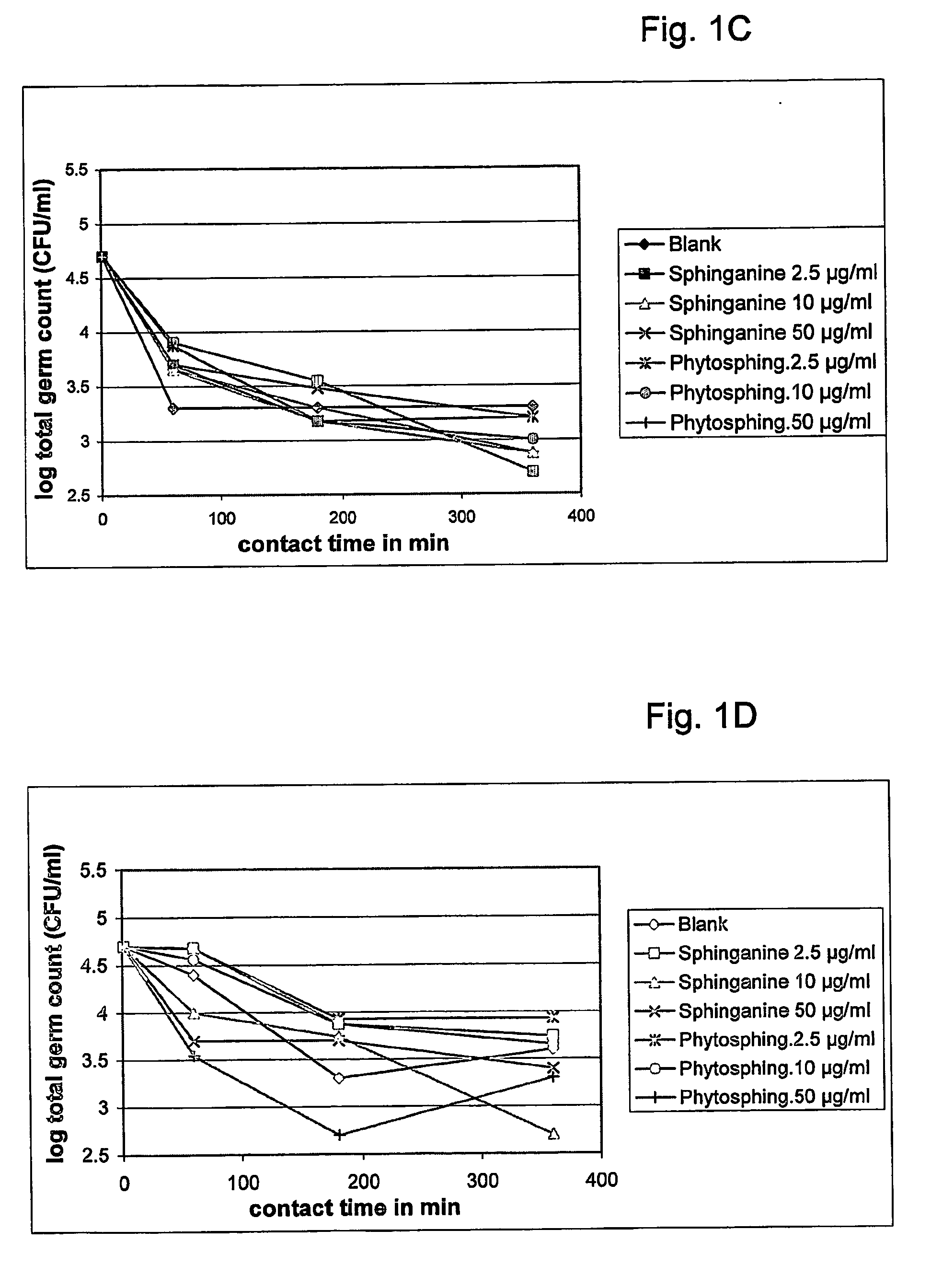
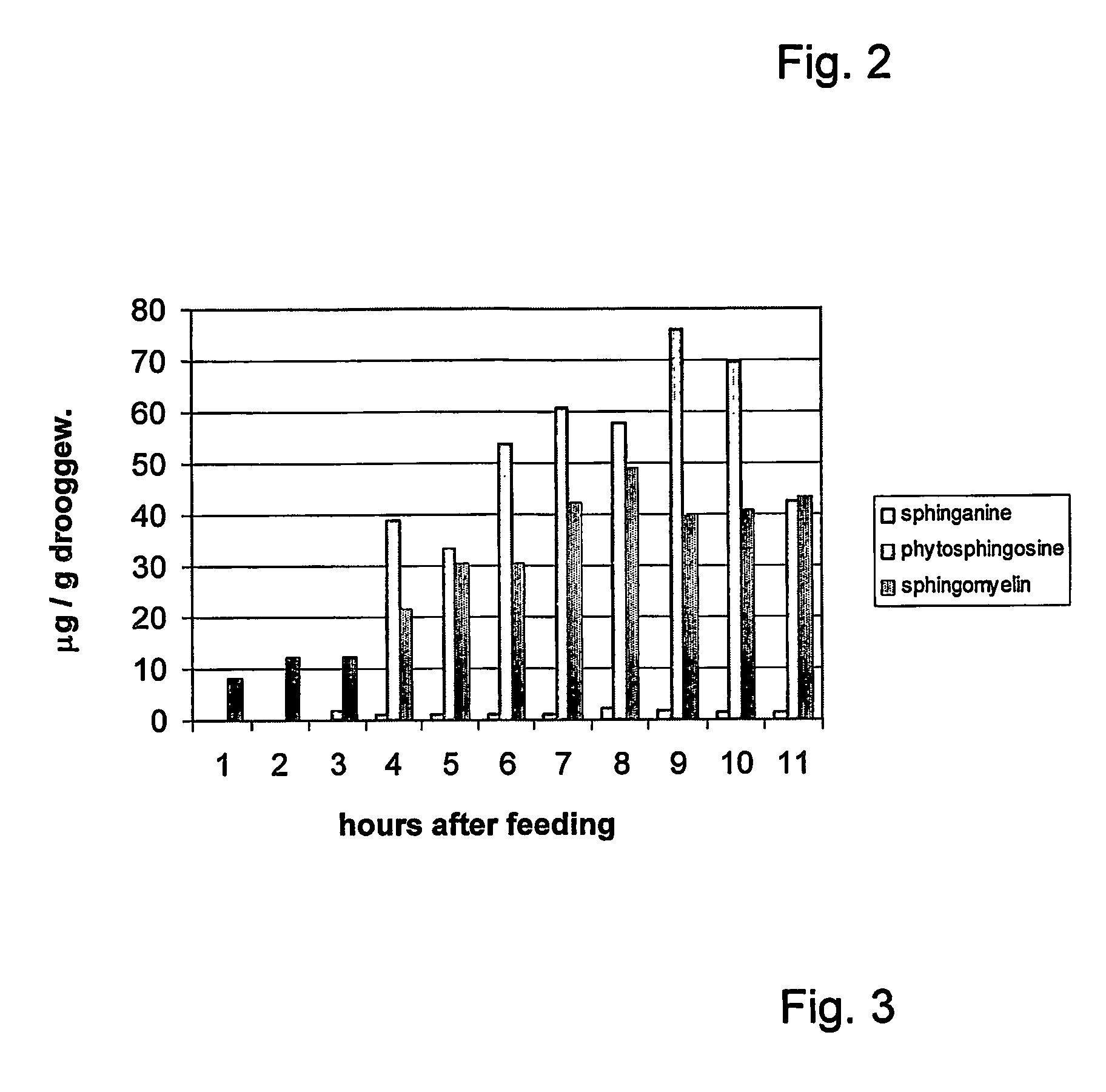
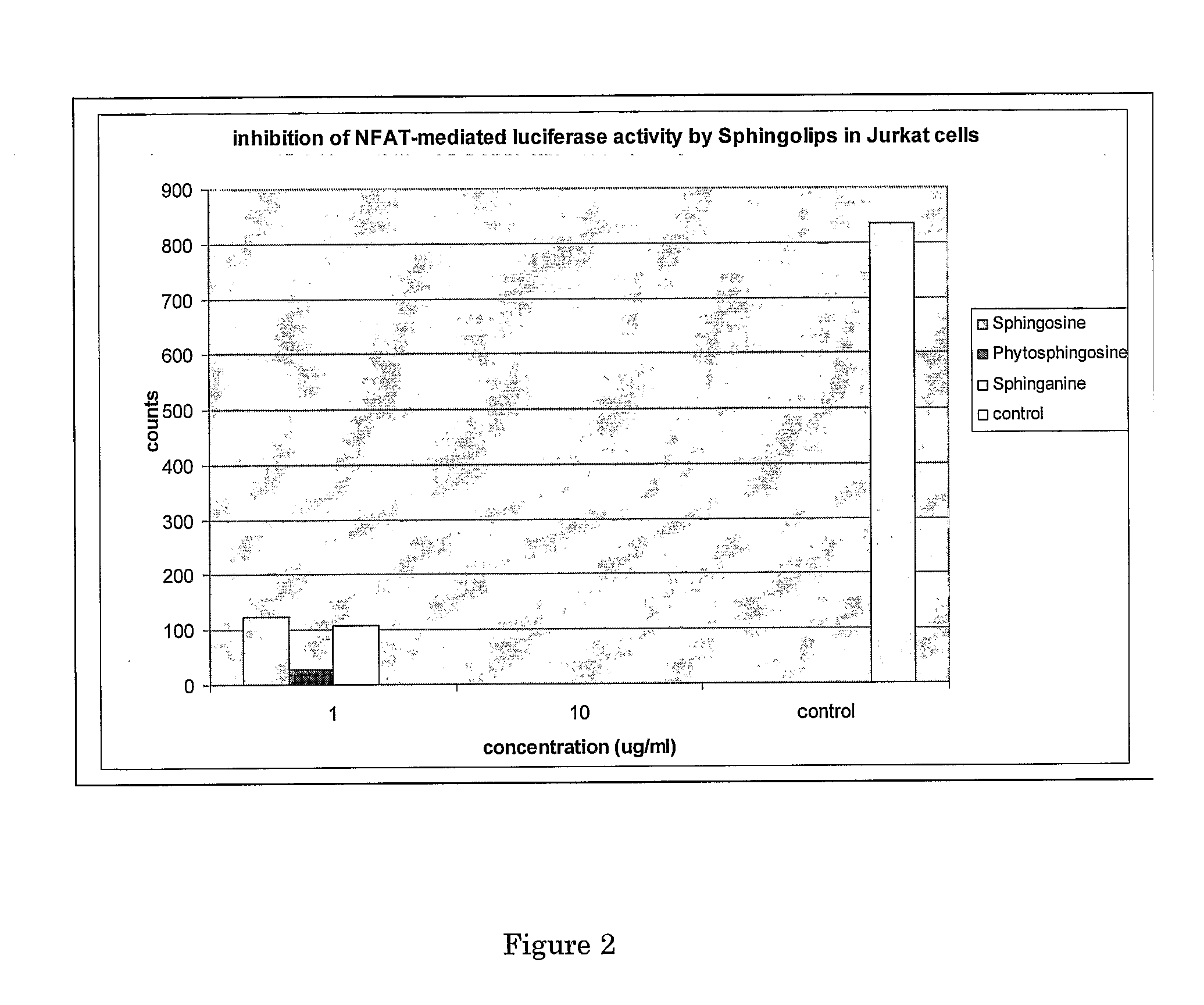
The present invention relates to a method for improving the composition of the intestinal flora, to a food comprising a sphingolipid for use in such a method, to methods for the preparation of such a food and to the use of sphingolipids for the preparation of a medicine for improving the composition of the intestinal flora. More in particular, the present invention relates to a method and food in which a sphingolipid chosen from the group consisting of phytosphingosine, sphingosine, lysosphingomyelin and sphinganine, or a precursor, a derivative, or suitable salt thereof is used.
Owner:NEDERLANDSE ORG VOOR TOEGEPAST-NATUURWETENSCHAPPELIJK ONDERZOEK (TNO)
Fusogenic properties of saposin C and related proteins and peptides for application to transmembrane drug delivery systems
ActiveUS9271932B2Reduce deliveryPeptide/protein ingredientsMetabolism disorderLipid formationActive agent
The present invention comprises a method for delivering pharmaceutical and / or imaging agents within and / or through the dermal, mucosal and other cellular membranes, and across the blood-brain barrier, utilizing a fusogenic protein. The fusogenic protein is associated with a phospholipid membrane, such as a liposome. The liposome may include dioleoylphosphatidylserine, a negatively charged long-chain lipid. Alternatively, the liposome is comprised of a mixture of negatively charged long-chain lipids, neutral long-chain lipids, and neutral short-chain lipids. Preferred fusogenic proteins include saposin C and other proteins, polypeptides and peptide analogs derived from saposin C. The active agent contained within the liposome may comprise biomolecules and / or organic molecules. This technology can be used for both cosmetic and medicinal applications in which the objective is delivery of the active agent within and / or beneath biological membranes or across the blood-brain barrier and neuronal membranes.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Use Of Sphingolipids For Prevention And Treatment Of Atherosclerosis
InactiveUS20080085939A1Anti-inflammatory effectAvoid complicationsBiocideOrganic chemistryPhospholipidAtheroma
The present invention is in the field of prevention and treatment of atherosclerosis and atherosclerosis-related clinical conditions. In particular, the present invention relates to the use of sphingolipids, more preferably phytosphingosine, sphingosine, sphinganine, ceramide, cerebroside lyso-sphingomyelin and / or sphingomyelin for prevention and treatment of inflammatory processes associated with atherosclerosis, psoriasis, colitis or auto-immune diseases in a subject.
Owner:NIEUWENHUIZEN WILLEM FERDINAND +2
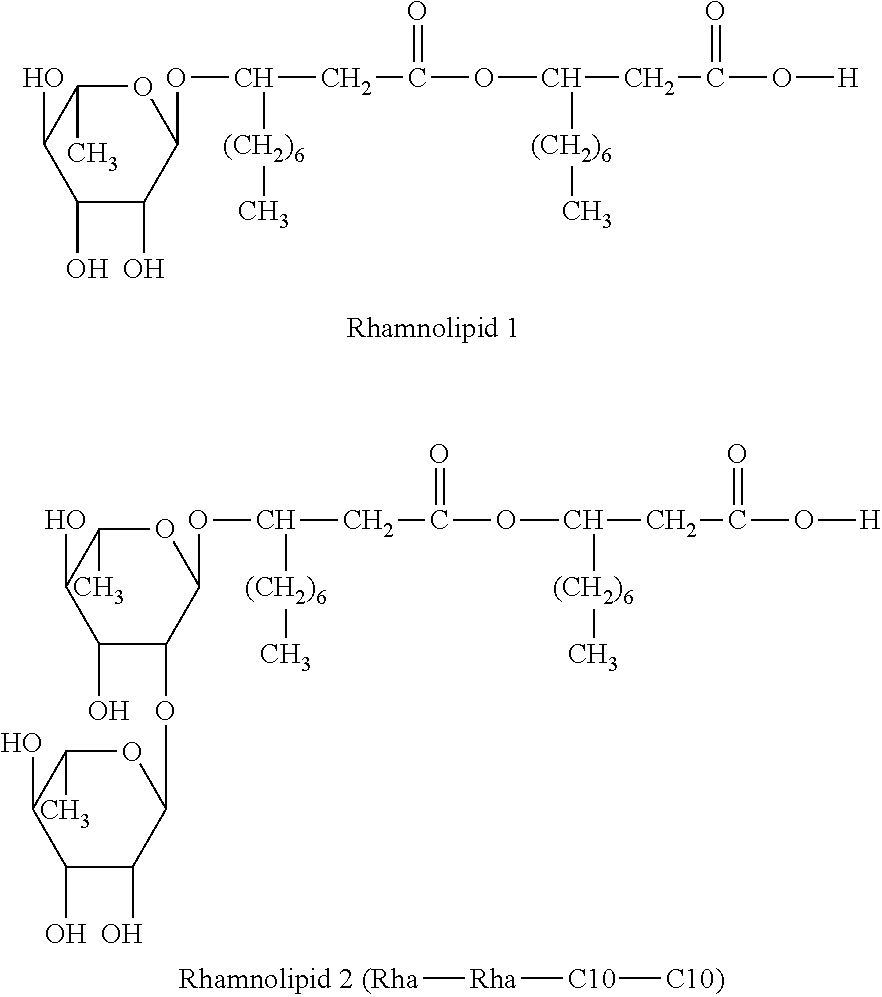
Method for treating rhinitis and sinusitis by rhamnolipids
The present invention is directed to methods for treating rhinitis or sinusitis in a subject. In one embodiment, the method comprises the steps of: identifying a subject in need thereof, and administering intranasally to the subject a formulation comprising an only active ingredient of an effective amount of rhamnolipid. In another embodiment, the method comprises the steps of: identifying a subject in need thereof, and administering intranasally to the subject a first active ingredient of an effective amount of a rhamnolipid and a second active ingredient of an effective amount of a corticosteroid, an antihistamine, a leukotriene antagonist, cromylin, an antibiotic, a sphingolipid, or a decongestant.
Owner:LEIGHTON ANTON
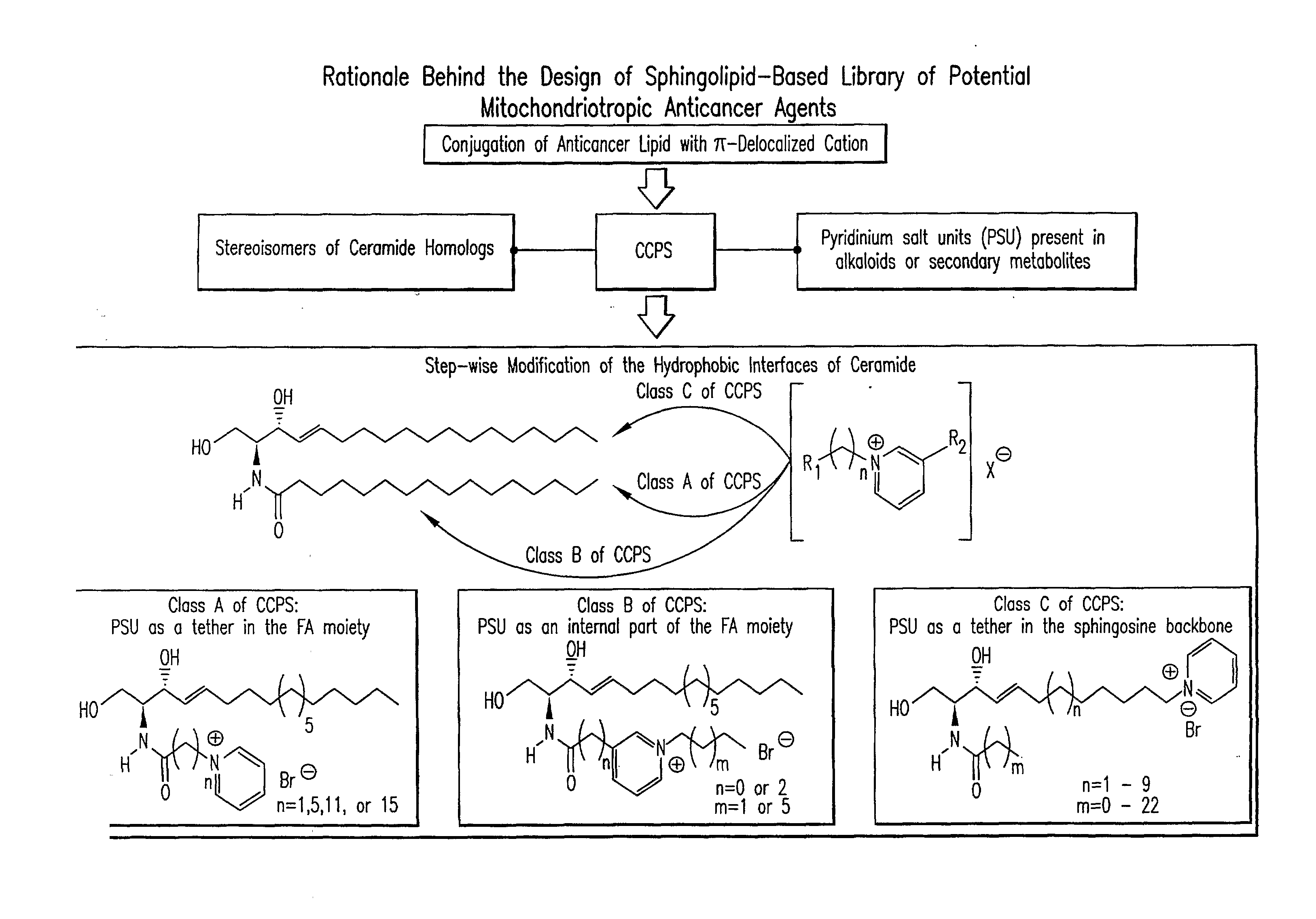
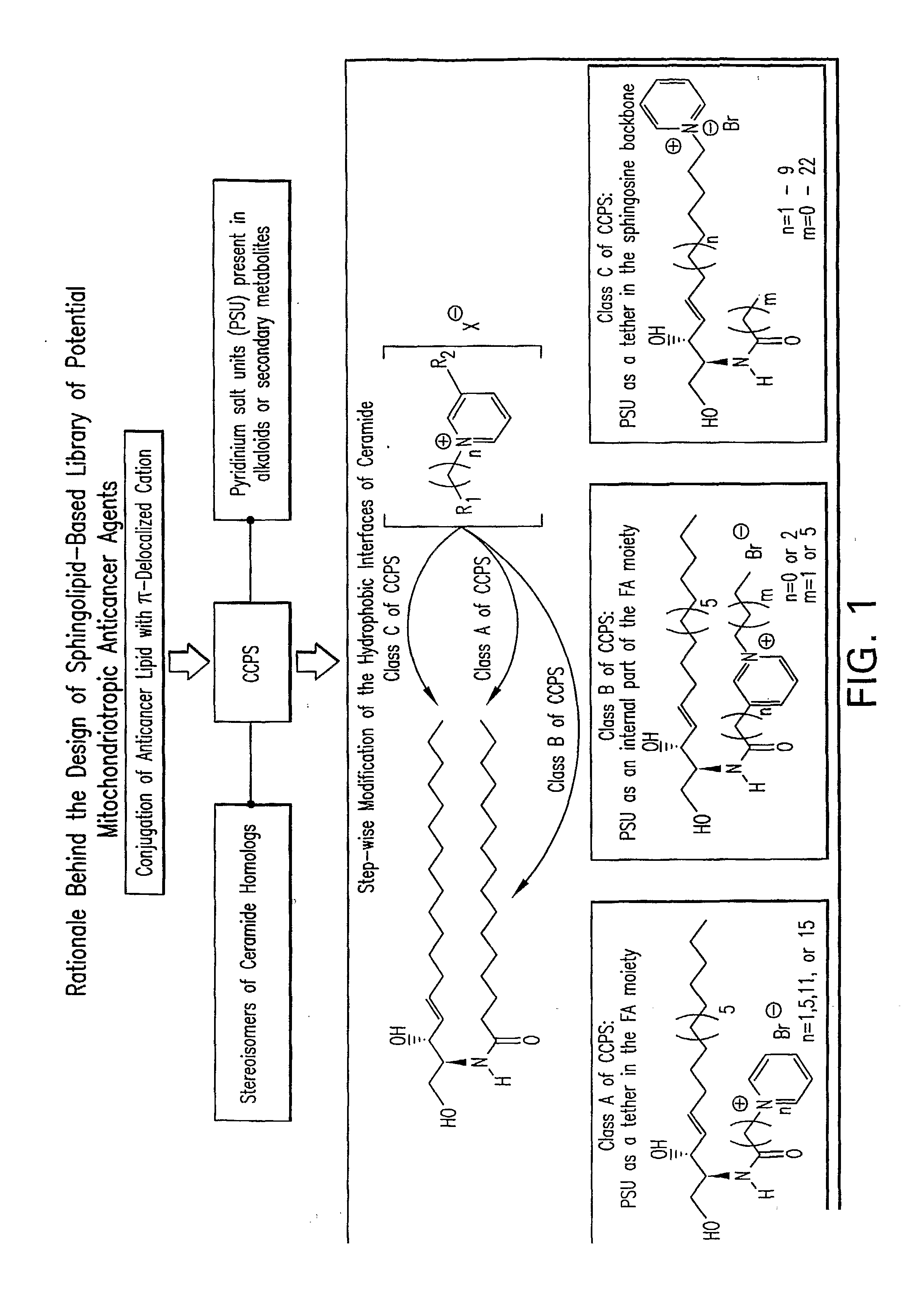
Cationic Ceramides, And Analogs Thereof, And Their Use For Preventing Or Treating Cancer
InactiveUS20110071099A1Reduce cell proliferationReduced viabilityBiocideOrganic chemistryDiseasePyridinium
The present invention relates to cationic ceramides, their dihydro-analogs and aromatic analogs and their derivatives, comprising a pyridinium group. Also provided are methods for making cationic ceramides comprising a pyridinium group, and their use for treating or preventing diseases associated with cell overproliferation and sphingolipid signal transduction, such as cancer, inflammation, and stenosis. The compounds are also useful as mitochondritropic agents that are localized to mitochondria carrying with them chemical cargoes, such as drugs, or signaling molecules, such as fluorophores for probing organelle structure and functions.
Owner:MUSC FOUND FOR RES DEV
Cosmetic composition promoting oxygen transport into the skin
InactiveUS20070098662A1Good curative effectImprove stabilityCosmetic preparationsToilet preparationsTransport systemStratum corneum
A cosmetic composition containing from about 1% to about 50% by weight of membrane-forming sphingolipids and / or galactolipids and about 5% to about 50% by weight of a fluorocarbon or fluorocarbon mixture charged with oxygen. Surprisingly, it has been shown that the use of membrane-forming sphingolipids and / or galactolipids as transport vesicles or for the formation of transport vesicles for a fluorocarbon or fluorocarbon mixture charged with oxygen results in excellent transport of oxygen through the stratum corneum of the skin and the epidermis in a manner which is far superior to known transport systems.
Owner:ROVI COSMETICS INT
Methods for joint lubrication and cartilage wear prevention making use of glycerophospholipids
ActiveUS8895054B2Improve and restore joint mobilityReduce wearOrganic active ingredientsAntipyreticLiposomeLubrication
The present invention concerns methods of joint lubrication and / or prevention of cartilage wear making use of liposomes having membranes with at least one phospholipid (PL) of the group consisting of a glycerophospholipid (GPL) having two, being the same or different, C12-C16 hydrocarbon chain and a sphingolipid (SPL) having a C12-C15 hydrocarbon chain, the one or more membranes having a phase transition temperature in which solid ordered (SO) to liquid disordered (LD) phase transition occurs, the phase transition temperature being within a temperature of about 20° C. to about 39° C. for lubrication of joints.
Owner:TECHNION RES & DEV FOUND LTD +2
Use of nutritional compositions with phospholipid, sphingolipid and cholesterol.
InactiveUS20090186803A1Increased adipose tissue massMany formatsNervous disorderPeptide/protein ingredientsEnvironmental healthCholesterol
A nutritional composition comprising phospholipids, sphingolipids and cholesterol for the prevention of obesity and / or diabetes is provided.
Owner:NV NUTRICIA
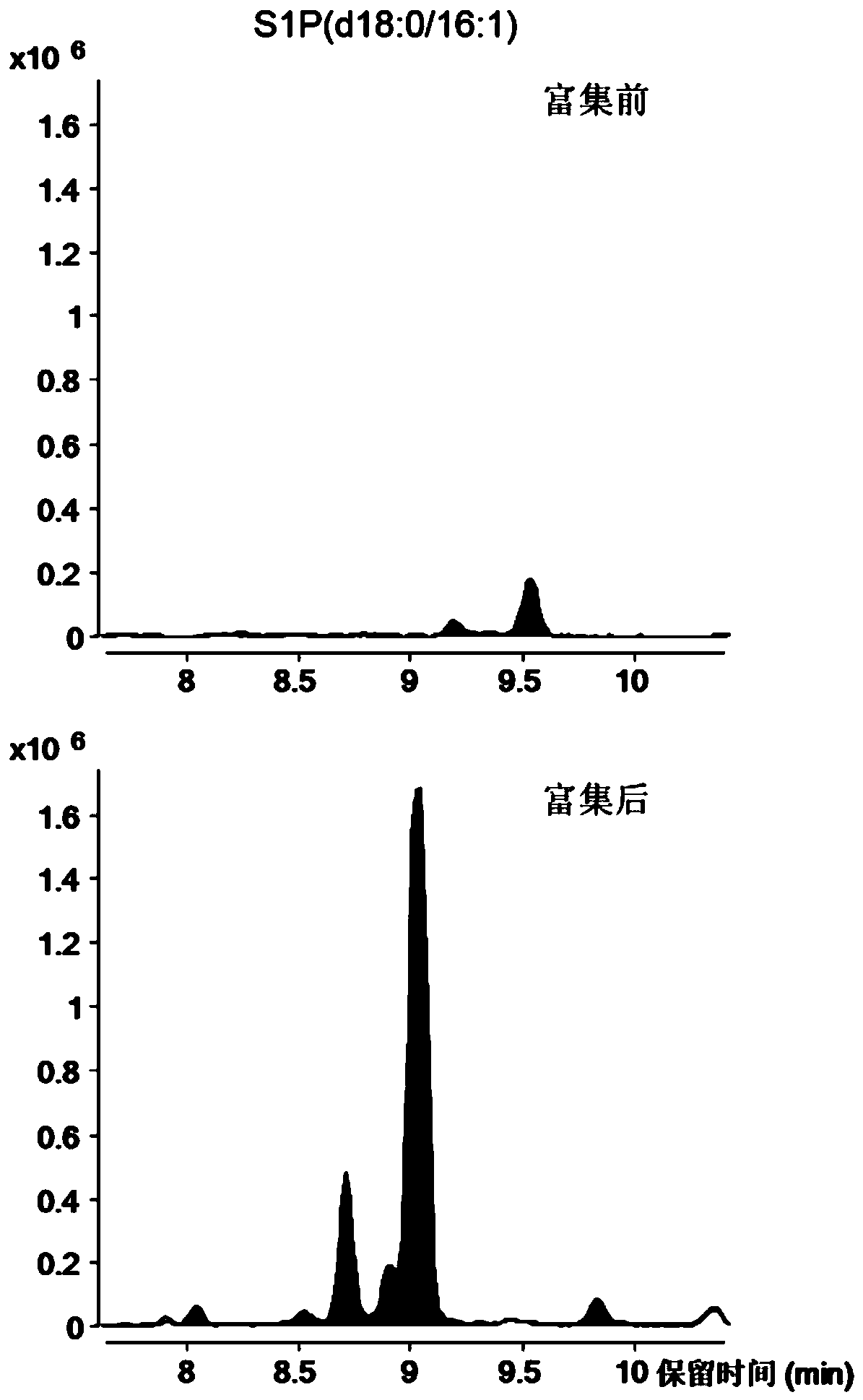
Large-scale blood plasma sphingolipid profile analysis method based on liquid chromatography-mass spectrometry combination
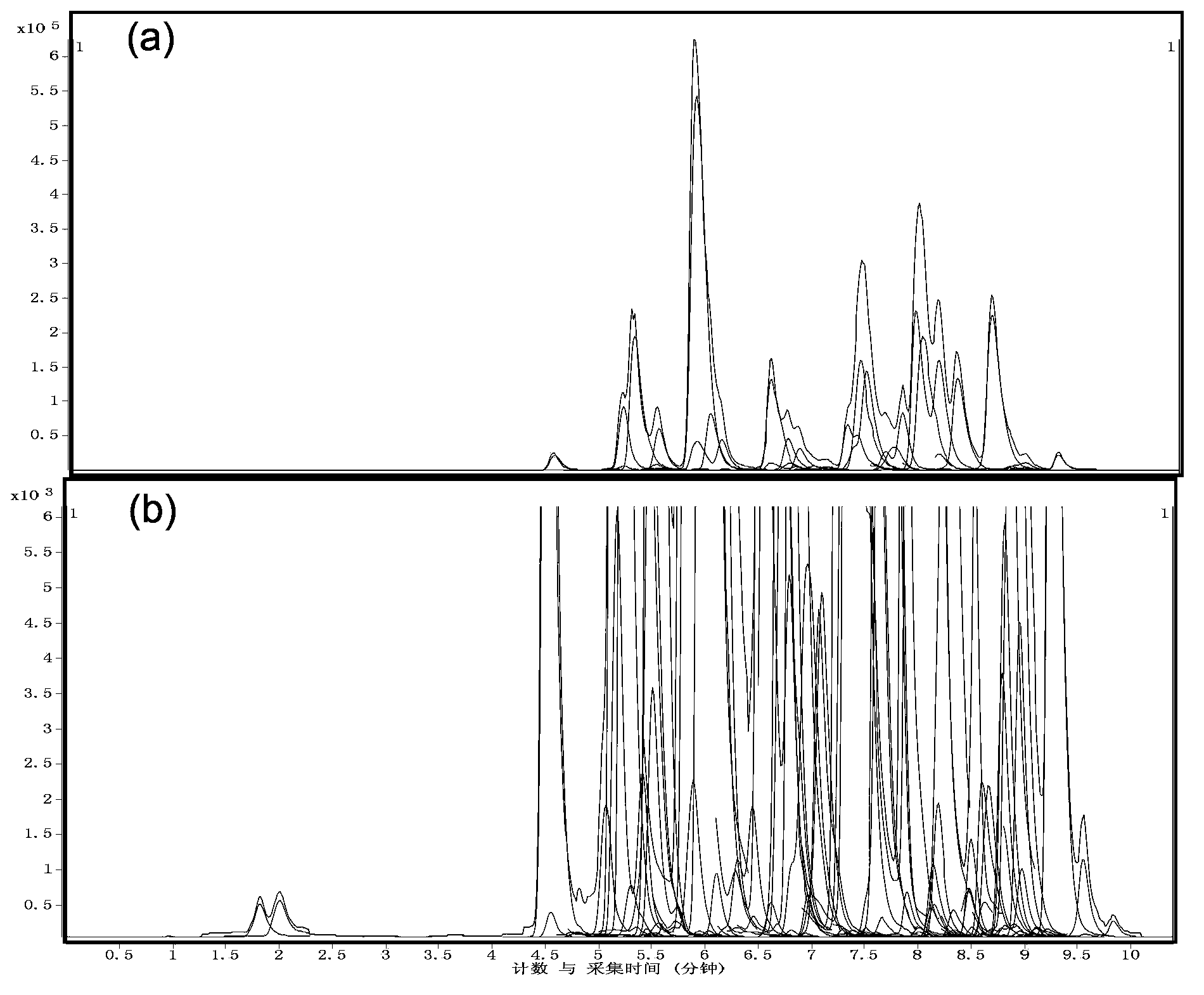
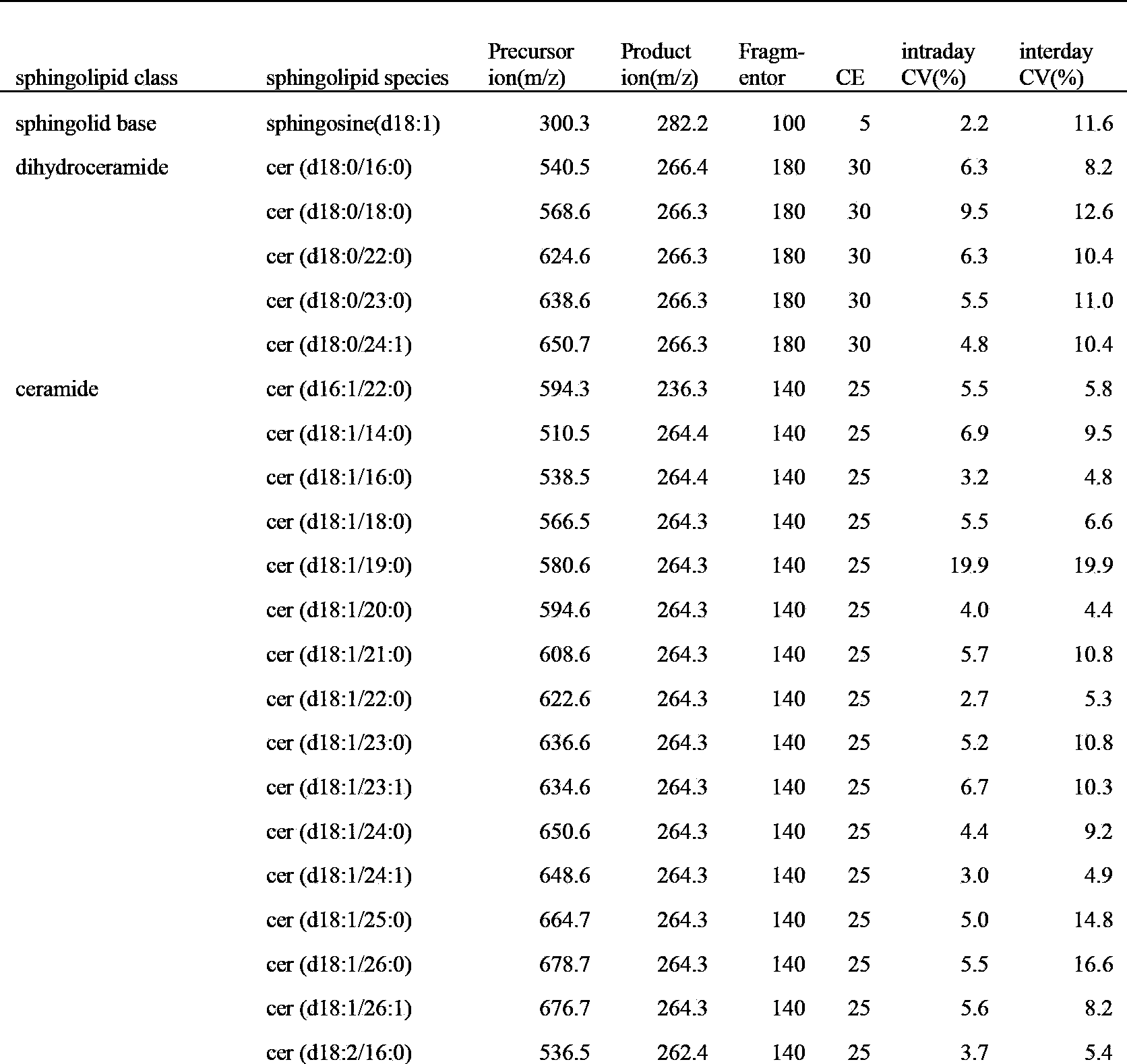
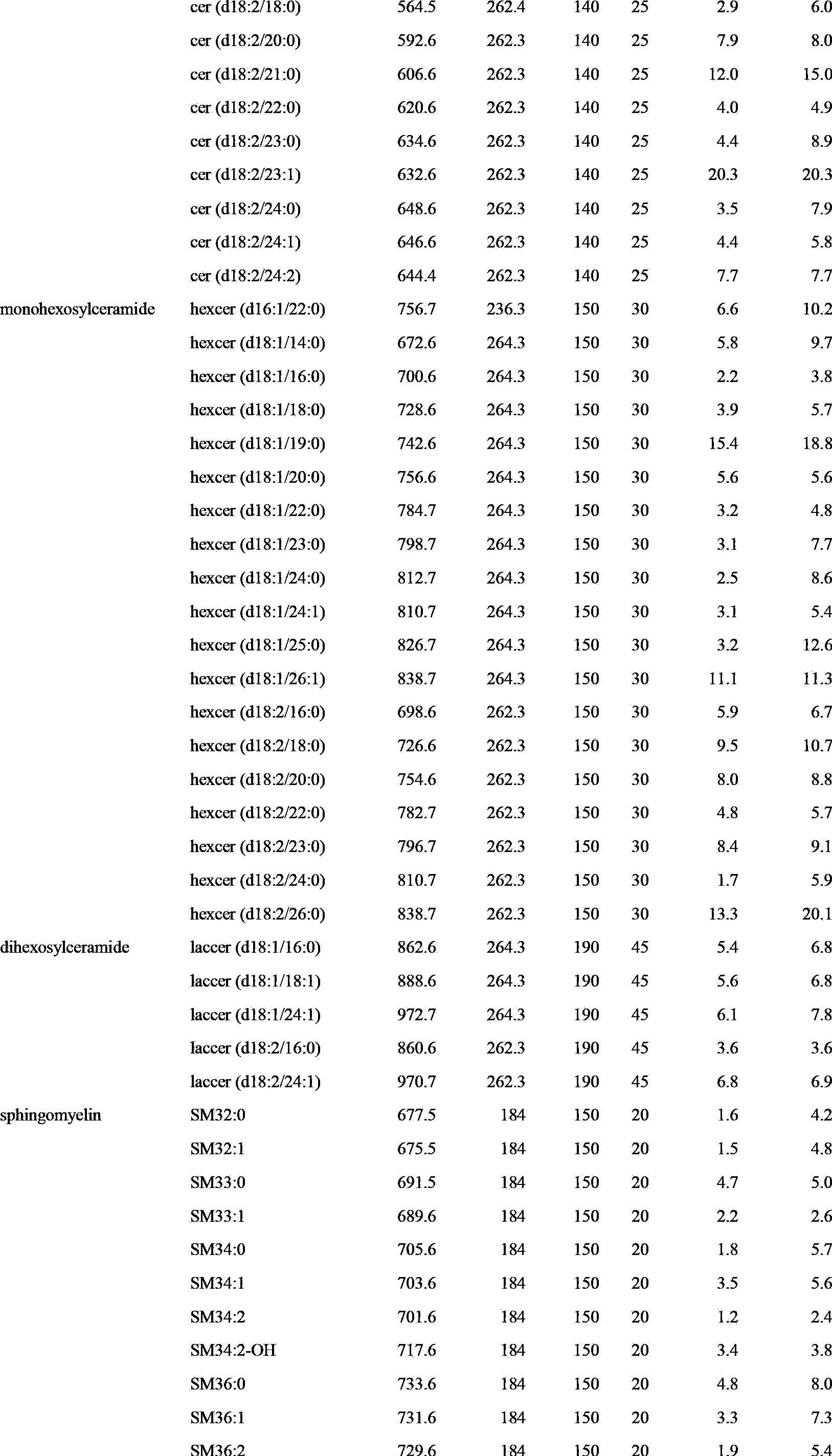
ActiveCN103884782ALow toxicityGood repeatabilityComponent separationPhospholipidMass spectrometry imaging
The invention discloses a large-scale blood plasma sphingolipid profile analysis method based on liquid chromatography-mass spectrometry combination, which is characterized in that sphingolipid in blood plasma is extracted by double-phase extraction with methyl tertiary butyl ether / methanol / water (MTBE / MeOH / H2O) combined with mild basic hydrolysis. Then rapid semi-quantitative analysis is realized within 15 min by combination of ultra-high performance liquid chromatography-electro-spray ionization-mass spectrometry. In the invention, the MTBE / MeOH / H2O extraction system is simple, rapid, and easy to operate, has less protein interference in the extract, and has good repeatability; interference of high-abundance phospholipid and glyceride in the lipid group is eliminated by hydrolysis; ion inhibition is reduced; therefore, the purpose of detecting low-abundance sphingolipid with high sensitivity is achieved. With the high resolution separation capability of subsequent ultra-high performance liquid chromatography (UHPLC) and the specificity of MRM, effective distinguishing of isomers is realized, and the introduction of complex isotope correction during quantification is avoided, which makes the method more rapid and accurate.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
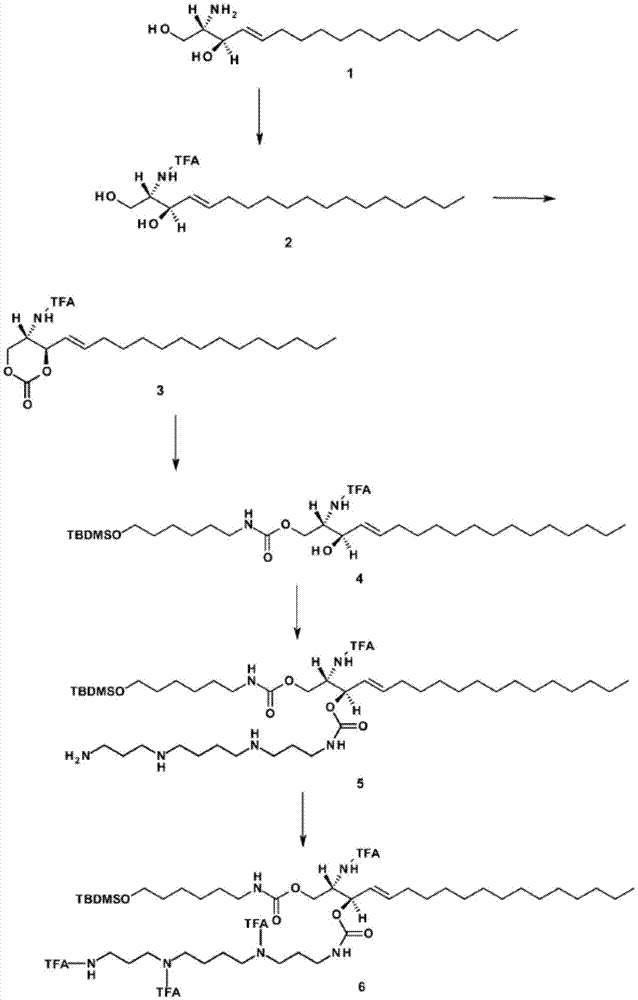
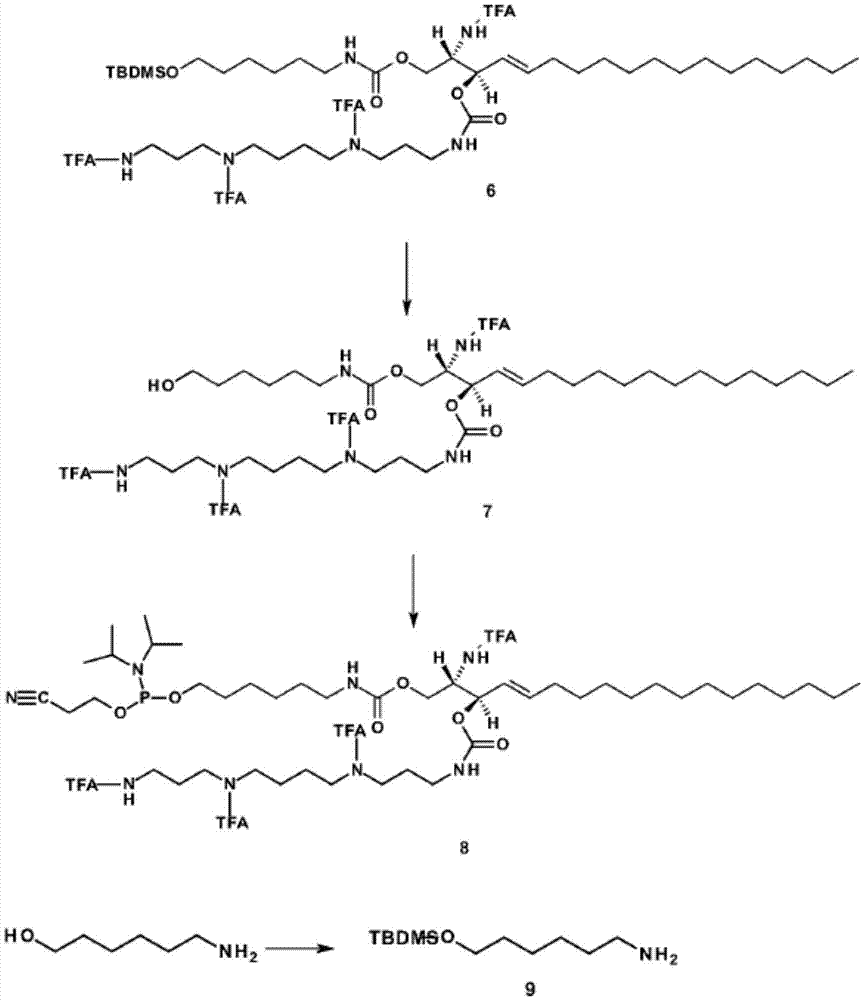
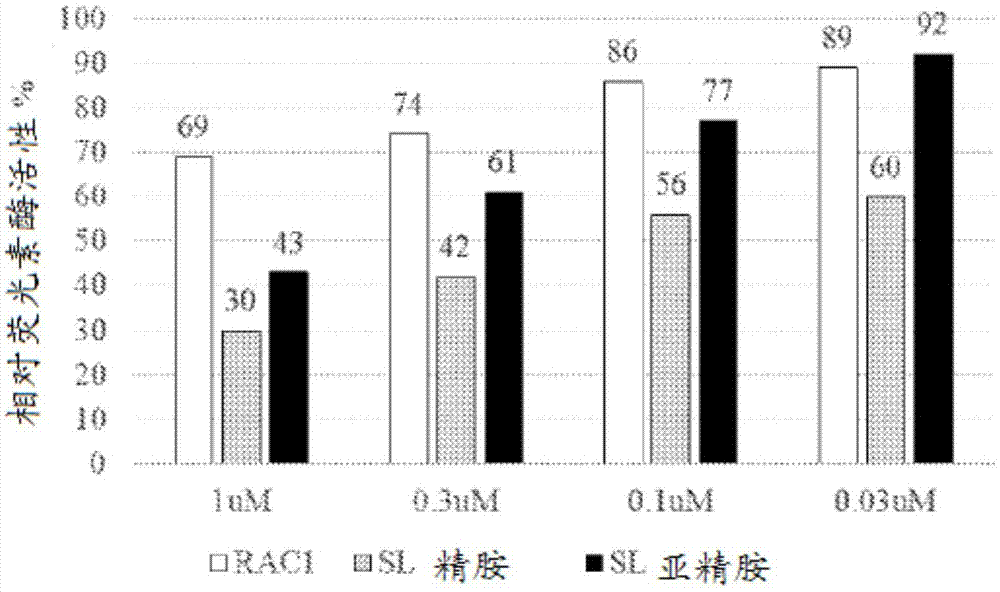
Sphingolipid-polyalkylamine-oligonucleotide compounds
Owner:QBI ENTERPRISES +1
Oligodendrocyte precursor cell composition and methods of use
The present invention provides a cell culture enriched for sphingolipid enhances neural stem cells (SENSe), particularly oligodendrocyte precursor cells (ODPCs), that do not form teratomas after transplanted in vivo. Methods for producing and use of the invention ODPCs or the cell culture enriched with these ODPCs for stem cell therapy are also provided. The invention method comprises culturing a stem cell culture with a cell culture medium comprising a ceramide compound and a S1P receptor agonist in sequence, overlapping intervals or concurrent manners. The present invention further provides a cellular or gene therapy using a composition comprising a ceramide compound in conjunction with a S1P1 agonist to proliferate or differentiate endogeneous neural stem cells to ODPCs and further to oligodendrocytes.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
Compositions and methods to protect cells by blocking entry of pathogen proteins
InactiveUS20100093601A1Avoid infectionEfficient responseBiocidePeptide/protein ingredientsADAMTS ProteinsPhosphoric acid
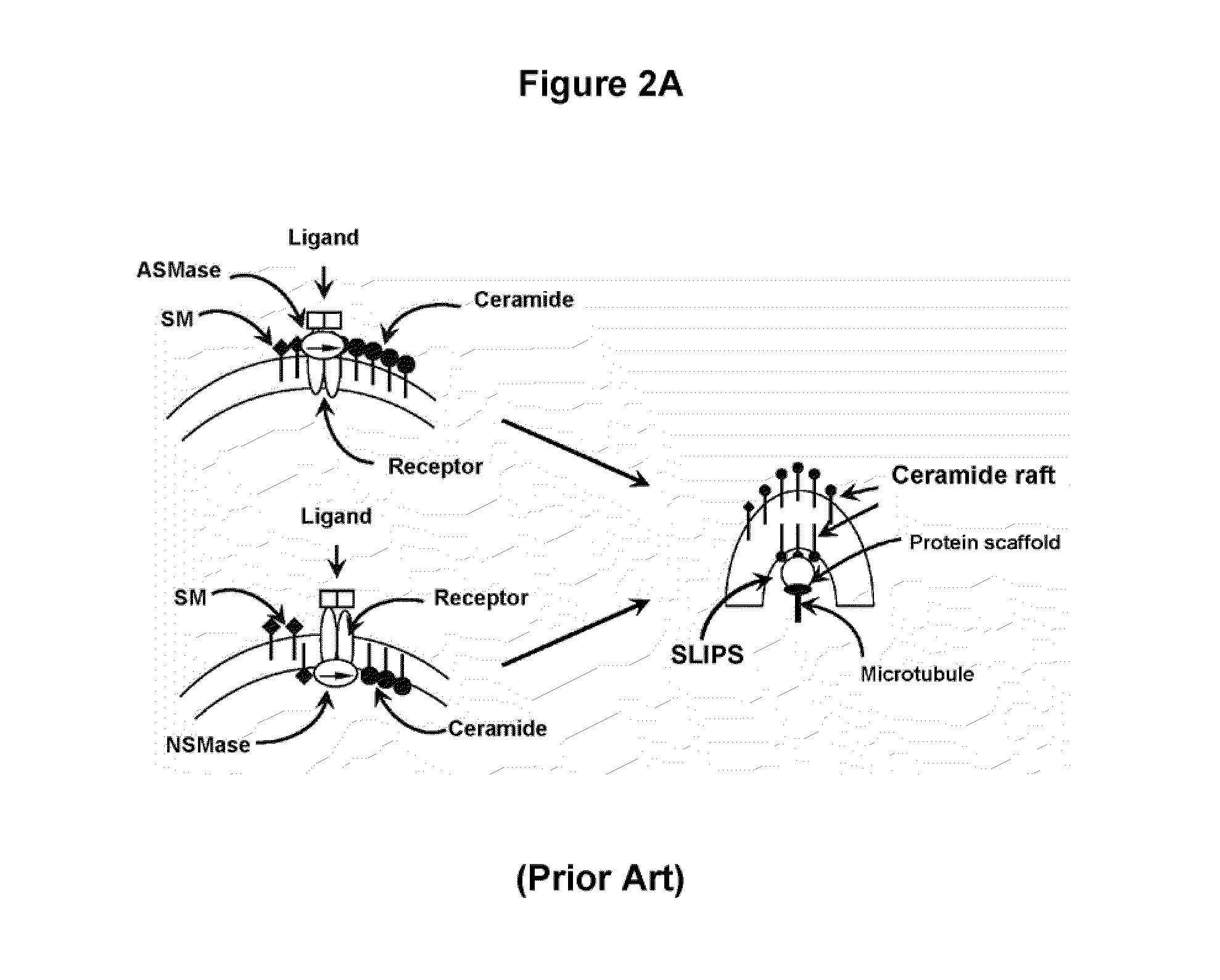
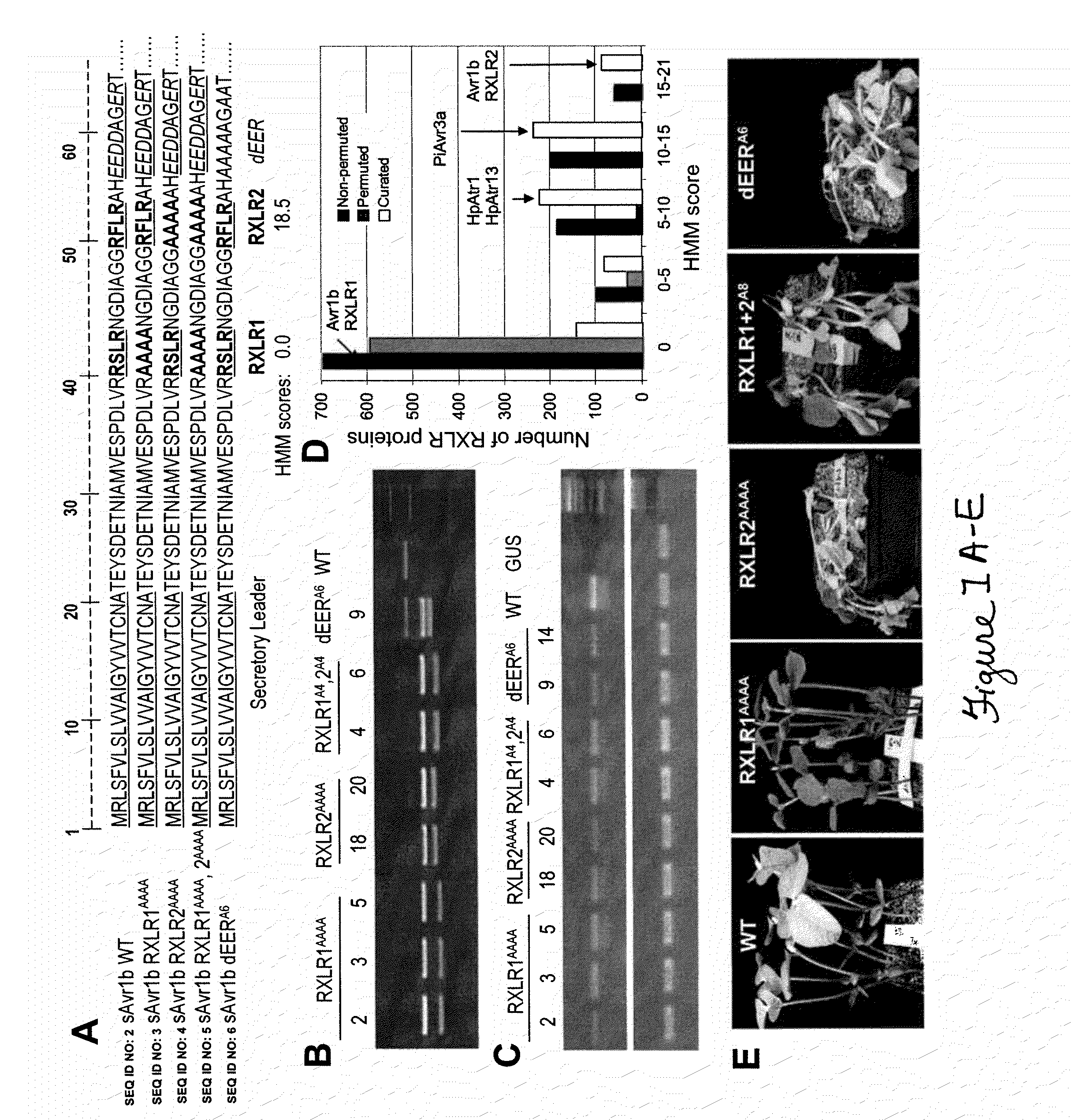
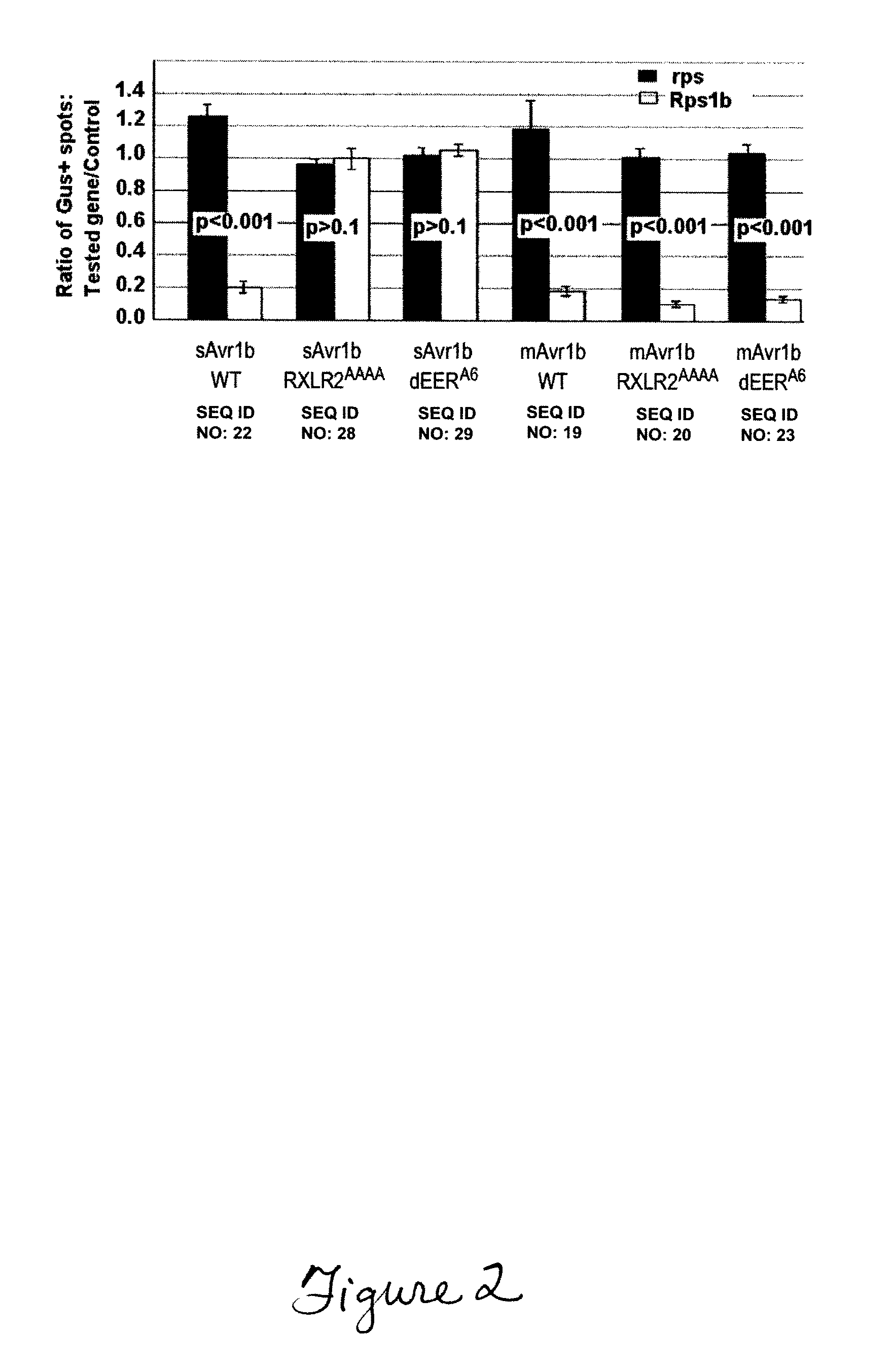
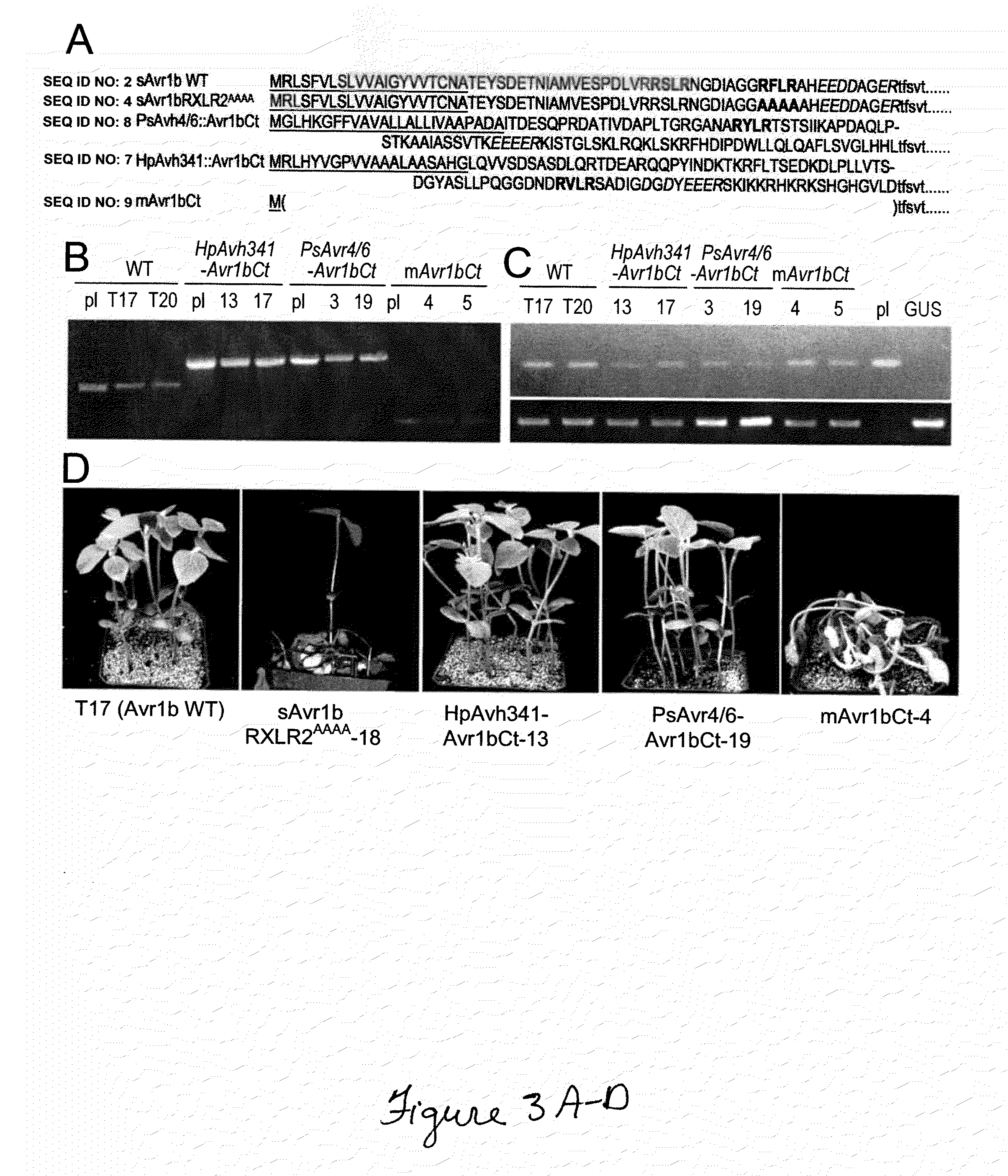
Pathogenic effector proteins which include one or more RxLR, dEER, Pexel or analogous motifs are blocked from entry into plant or animal cells by binding one or more of the motifs with a blocking compound which prevents binding of phosphoinositides or other polar lipids to the motifs which is a prerequisite for translocation of the pathogenic effector proteins into the plant or animal cell. The blocking compounds can take a variety of forms including synthetic peptides or the hydrophilic head-groups of phosphoinositides, phosphatidic acids, phospholipids, or sphingolipids. Suitable blocking compounds can be identified by assays demonstrating binding to RxLR, dEER, Pexel or analogous motifs. In addition, pathogenic effector proteins can be identified by analyzing whether they contain structural RxLR motifs using hidden markov modeling.
Owner:VIRGINIA TECH INTPROP INC
Compositions and methods for the modulation of sphingolipid metabolism and/or signaling
Compositions, methods and kits for diagnosing and treating cancer and muscular disorders are provided. Therapeutic compositions may comprise agents that modulate sphingolipid metabolism and / or signaling pathways. Such compositions may be administered to a mammal afflicted with cancer. Diagnostic methods and kits may employ an agent suitable for detecting alterations in endogenous genes involved in sphingolipid metabolism. Such methods and kits may be used to detect the presence of a cancer or to evaluate the prognosis of a known disease. SPL polypeptides, polynucleotides and antibodies are also provided.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Ophthalmic devices for delivery of hydrophobic comfort agents
ActiveUS10155349B2Easy to understandPackage sterilisationPharmaceutical delivery mechanismLipid formationMonoglyceride
The invention relates to a soft hydrogel contact lens, especially a silicone hydrogel contact lens, which has a capability of delivering a hydrophobic comfort agent into the eye of a wearer. The hydrophobic comfort agent includes without limitation a monoglyceride, a diglyceride, a triglyceride, a glycolipid, a glyceroglycolipid, a sphingolipid, a sphingo-glycolipid, a phospholipid, a fatty acid, a fatty alcohol, a hydrocarbon having a C12-C28 chain in length, a mineral oil, a silicone oil, or a mixture thereof. It can be released from the soft hydrogel contact lens into the eye of a wearer when being worn so as to strengthen and stabilize the tear film lipid layer and alleviate the dryness of the eye.
Owner:ALCON INC
Fusogenic properties of saposin c and related proteins and peptides for application to tansmembrane drug delivery systems
ActiveUS20090142267A1Reduce deliveryPeptide/protein ingredientsMetabolism disorderLipid formationActive agent
The present invention comprises a method for delivering pharmaceutical and / or imaging agents within and / or through the dermal, mucosal and other cellular membranes, and across the blood-brain barrier, utilizing a fusogenic protein. The fusogenic protein is associated with a phospholipid membrane, such as a liposome. The liposome may include dioleoylphosphatidylserine, a negatively charged long-chain lipid. Alternatively, the liposome is comprised of a mixture of negatively charged long-chain lipids, neutral long-chain lipids, and neutral short-chain lipids. Preferred fusogenic proteins include saposin C and other proteins, polypeptides and peptide analogs derived from saposin C. The active agent contained within the liposome may comprise biomolecules and / or organic molecules. This technology can be used for both cosmetic and medicinal applications in which the objective is delivery of the active agent within and / or beneath biological membranes or across the blood-brain barrier and neuronal membranes.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Isolation, identification and application of pollen-specific promoter
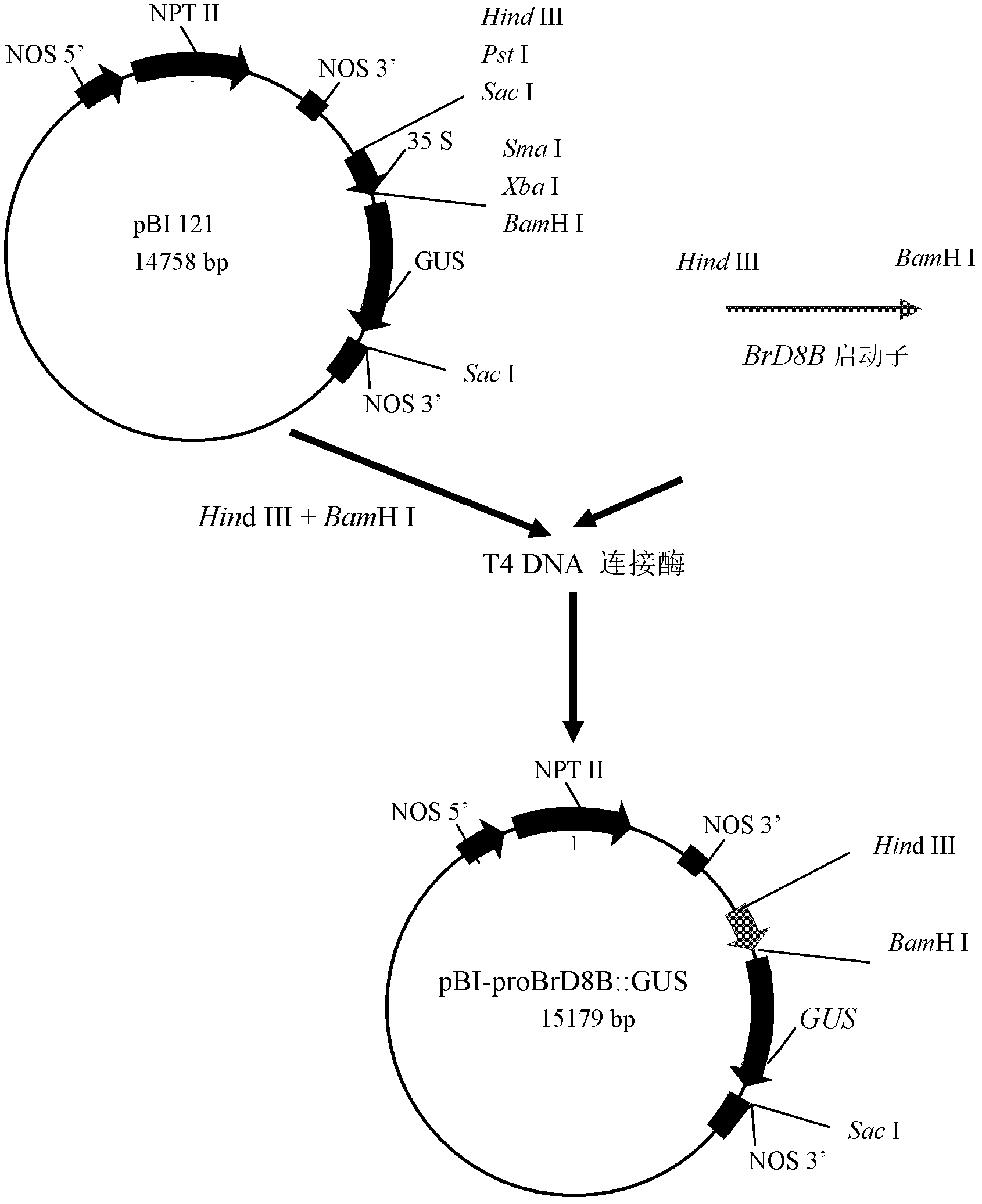
InactiveCN102286486AVector-based foreign material introductionAngiosperms/flowering plantsBrassica rapaSphingolipid desaturase
The invention provides a pollen-specific promoter and application thereof. The promoter is derived from Brassica rapa and is an upstream sequence of a Delta8 sphingolipid desaturase gene. The promoter provided by the invention is a stable pollen-specific promoter having high specificity. The promoter can be used to promote the expression of various genes in pollen, and can be used for cultivatingmale sterile crop varieties and cultivating landscaping plants and the like with pollen eliminated or greatly reduced.
Owner:INST OF GENETICS & DEVELOPMENTAL BIOLOGY CHINESE ACAD OF SCI
Method for specific separation and enrichment of acid sphingolipid and glycosphingolipid in human serum
ActiveCN110068638AImprove throughputHigh sensitivityComponent separationPhosphorylationSeparation technology
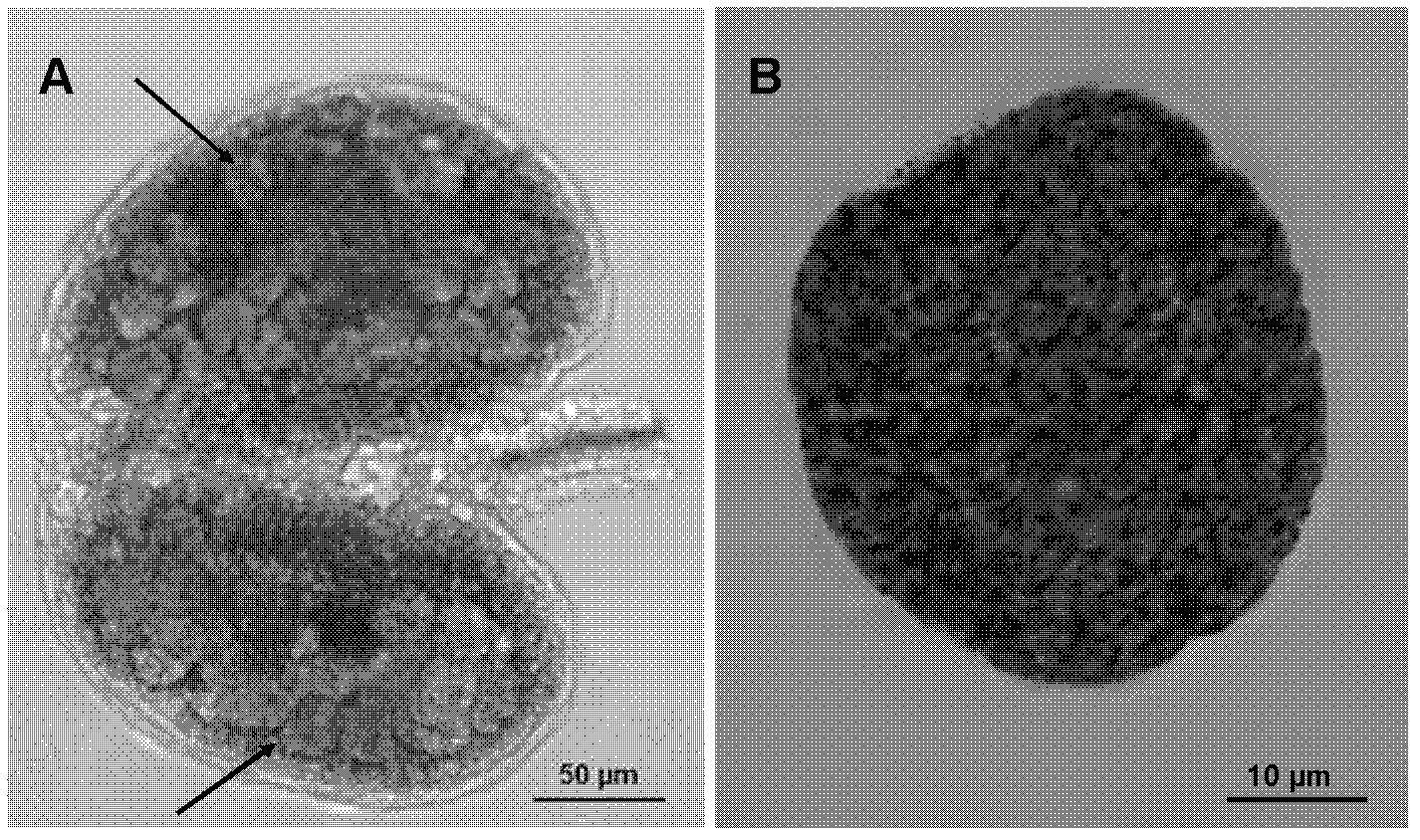
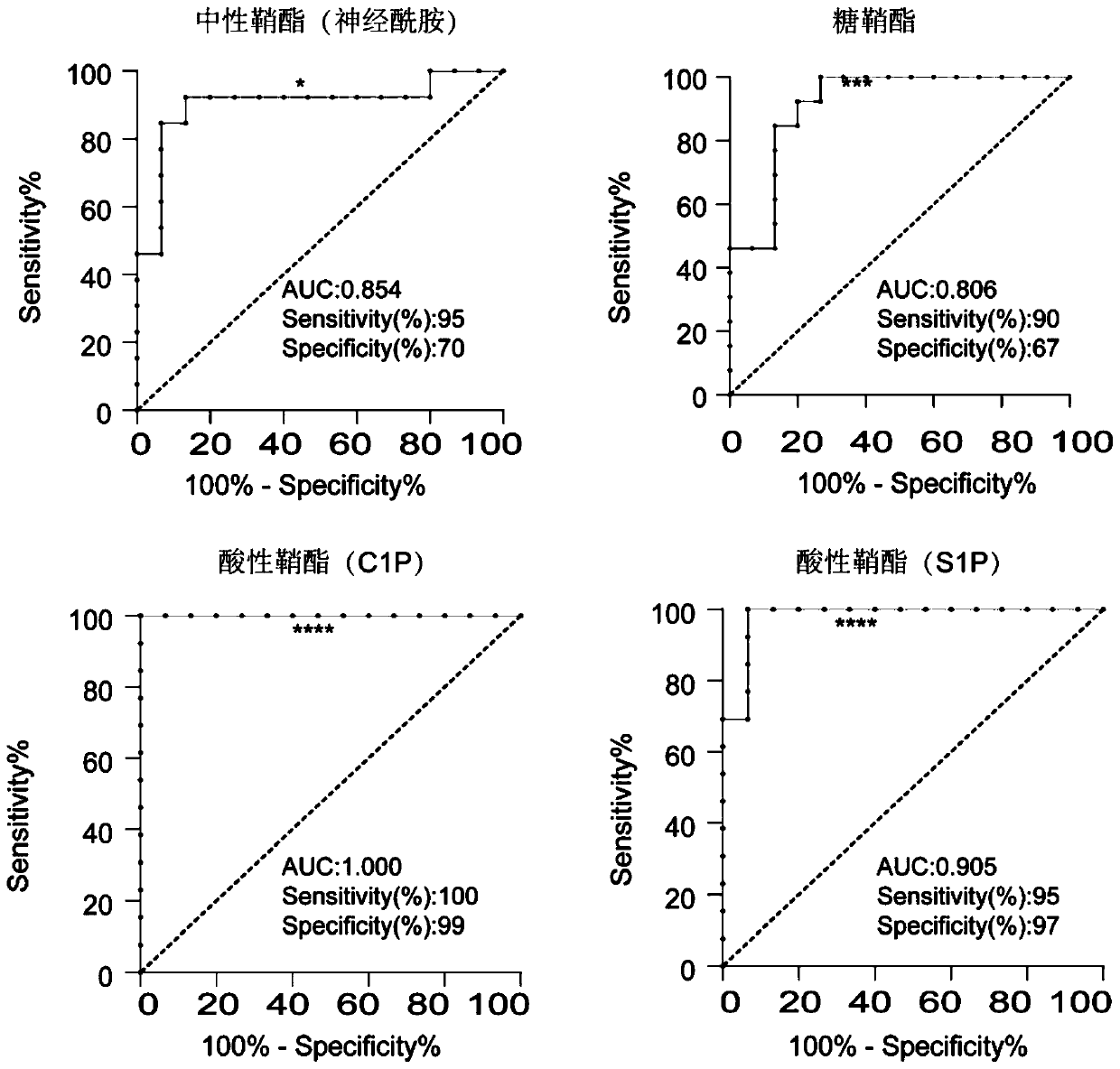
The invention discloses a method for specific separation and enrichment of acid sphingolipid and glycosphingolipid in human serum. The method realizes a sample preparation step of complex sphingolipidomics in serum through a titanium dioxide column separation technology, and comprises separation of neutral sphingolipid and the acid sphingolipid, and separation of the acid sphingolipid and the glycosphingolipid. The method provided by the invention can separate the neutral sphingolipid, the glycosphingolipid and the acid sphingolipid (such as phosphorylated sphingolipid and sulfatide), and consequently, realizes removal of nature determination and quantification to other minute amount even trace amount of sphingolipid (the glycosphingolipid and the phosphorylated sphingolipid) by high abundance neutral sphingolipid in mass spectrum. According to a titanium dioxide enrichment method in the method provided by the invention, eight biomarkers are detected from a clinical specimen, whereinthe biomarkers comprise ceramide in the neutral sphingolipid, S1P and C1p in the acid sphingolipid and the glycosphingolipid. And the eight biomarkers can distinguish Early and middle and advanced stage patients well.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com