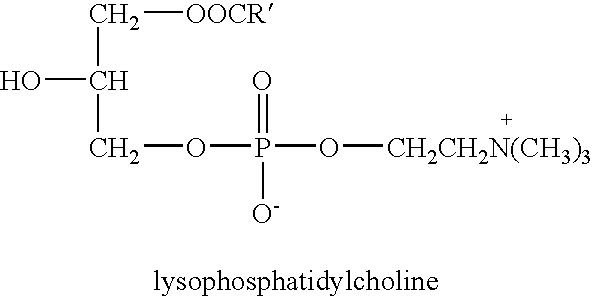Patents
Literature
64 results about "Glycerophospholipids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
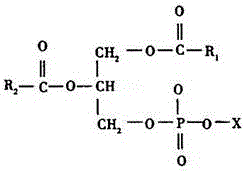
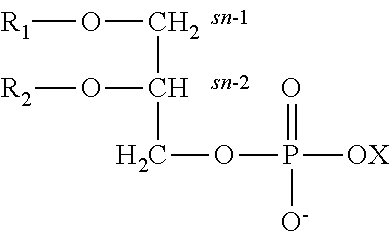
Derivatives of phosphatidic acid in which the hydrophobic regions are composed of two fatty acids and a polar alcohol is joined to the C-3 position of glycerol through a phosphodiester bond. They are named according to their polar head groups, such as phosphatidylcholine and phosphatidylethanolamine.
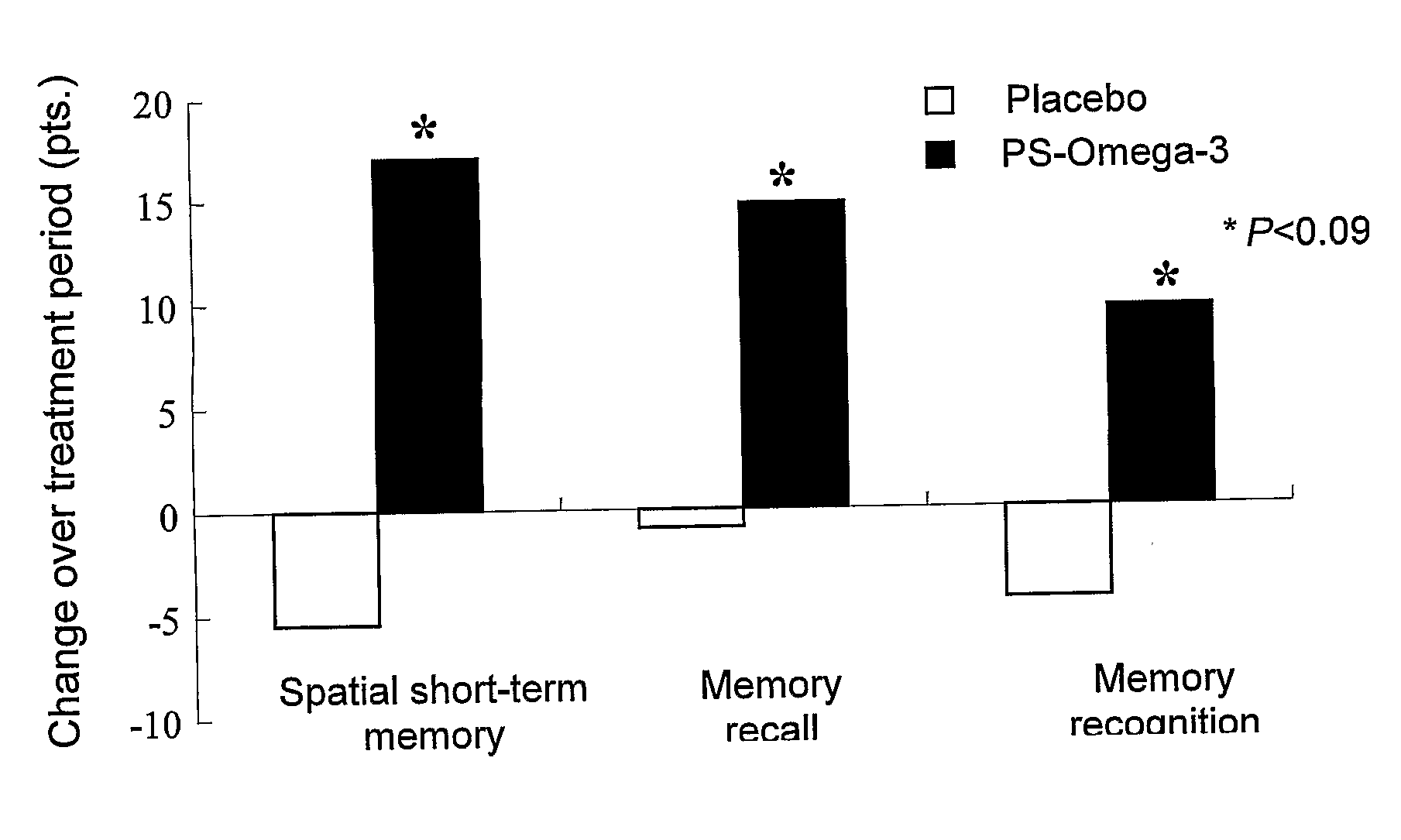
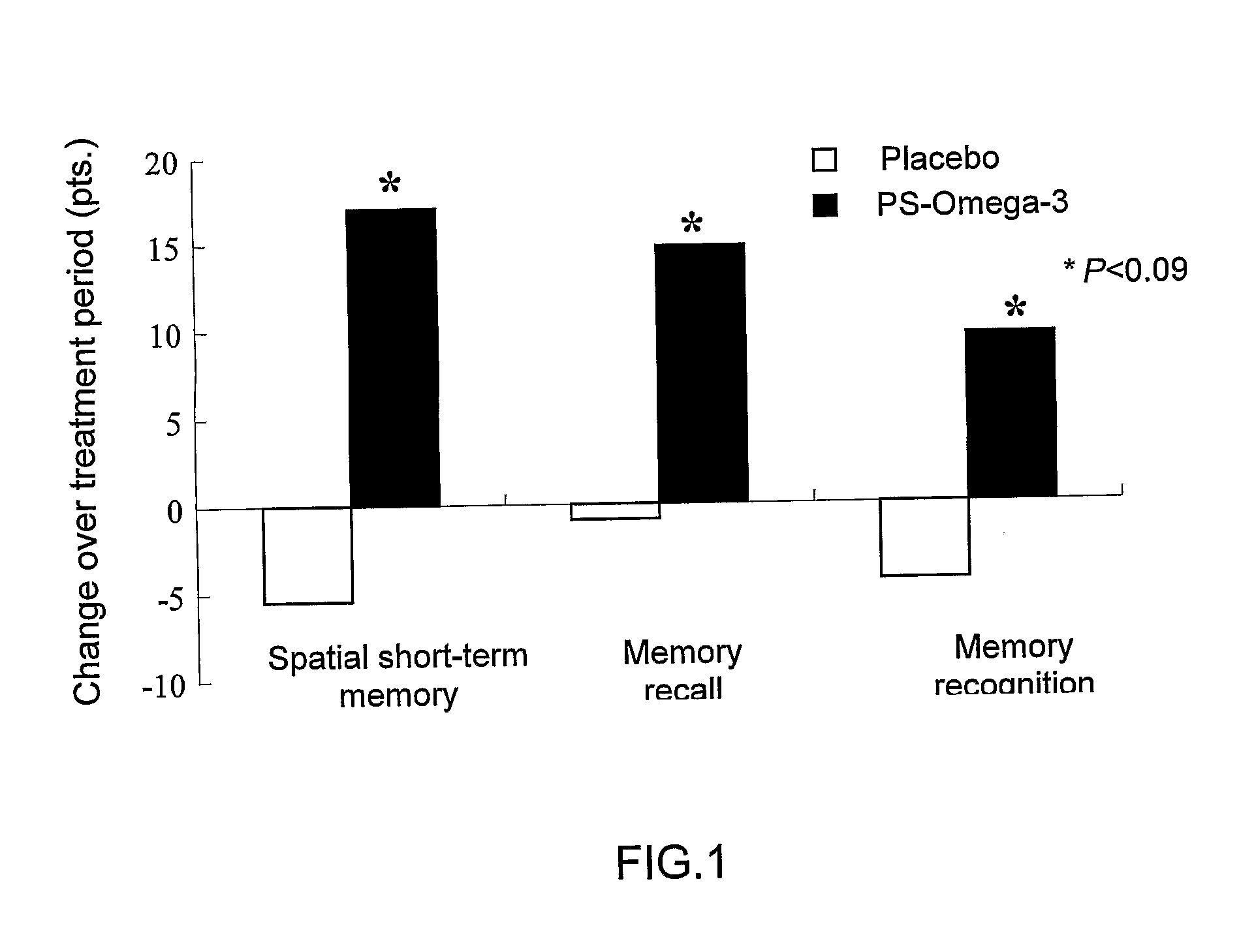
Glycerophospholipids for the improvement of cognitive functions
ActiveUS20090074857A1Improve cognitive functionEnhanced and cheapBiocideNervous disorderDiseaseErogorgiaene
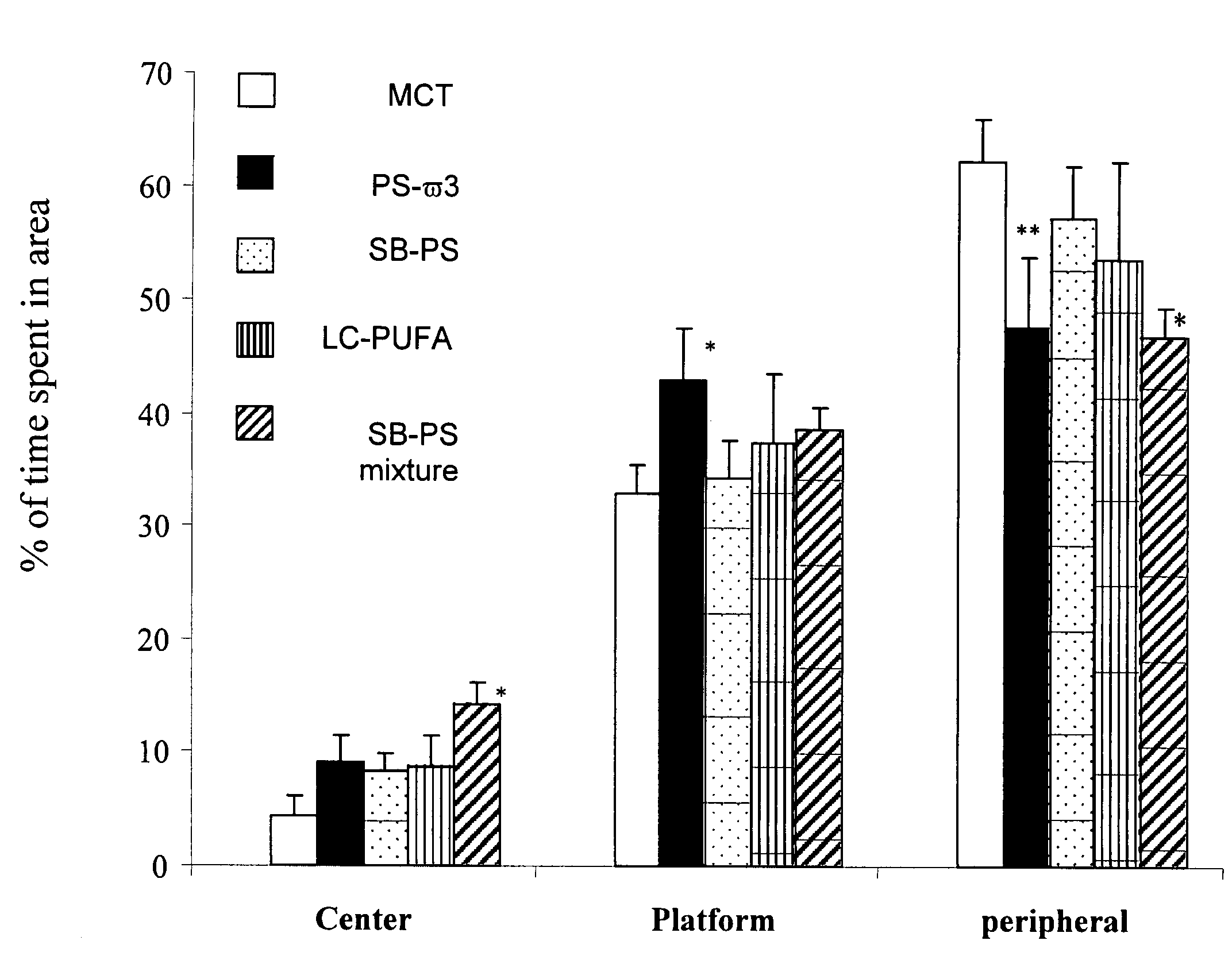
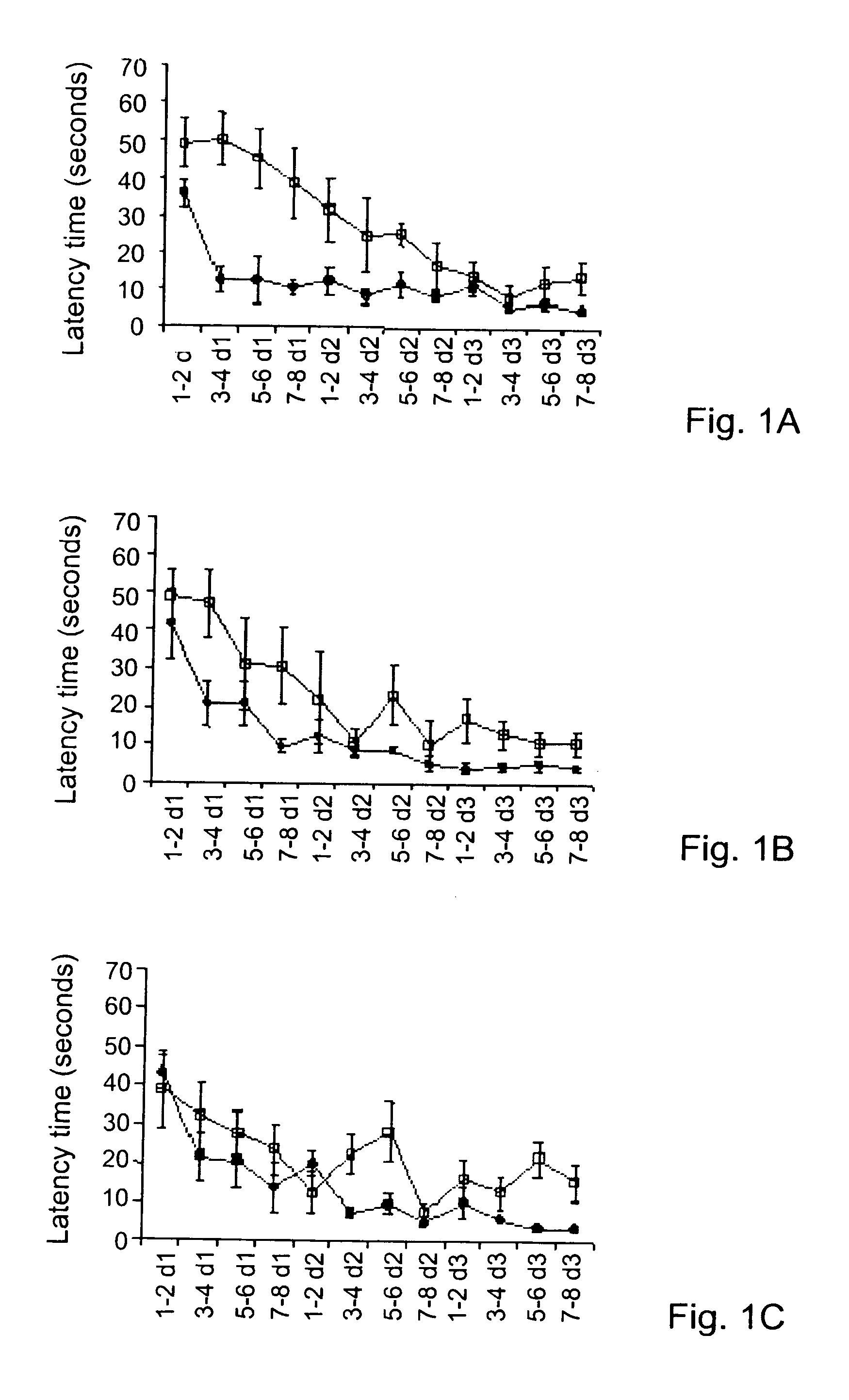
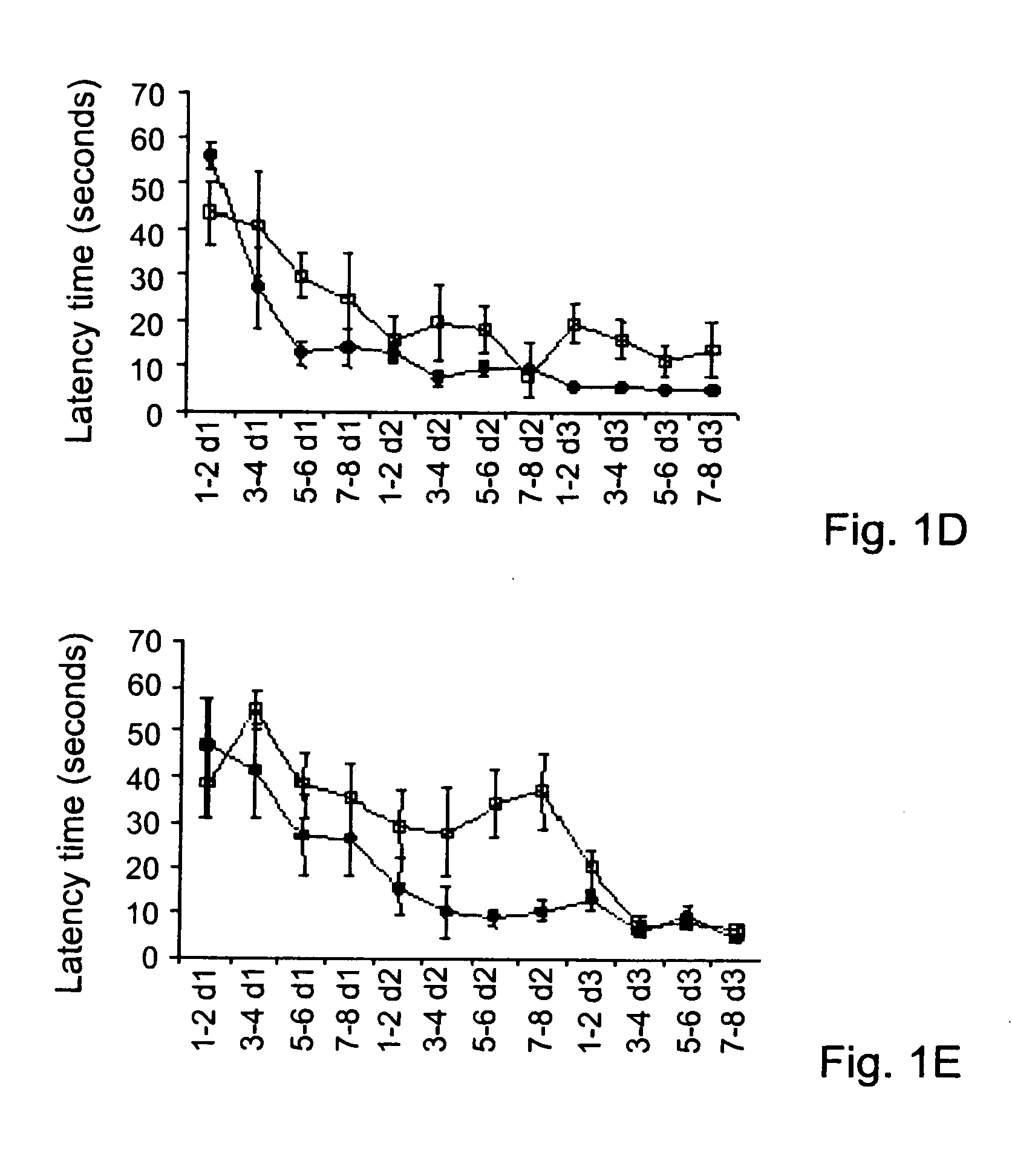
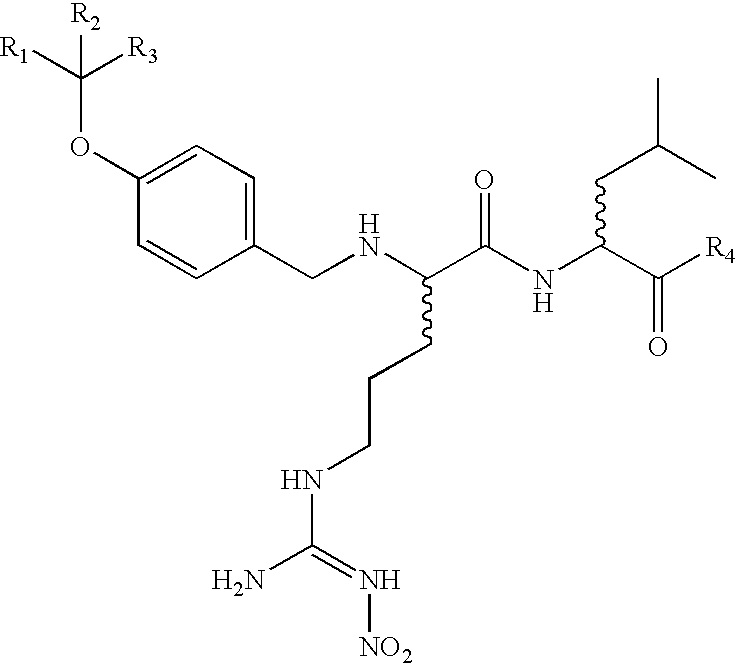
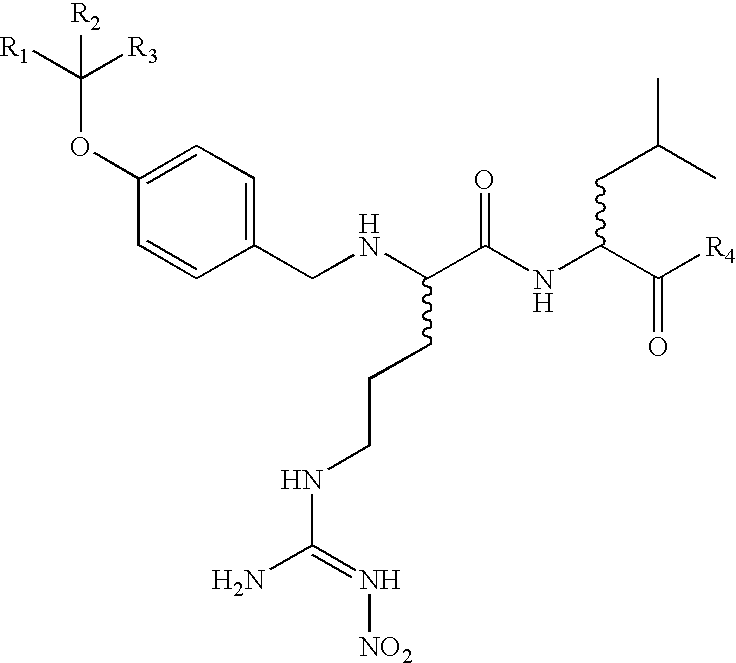
Disclosed herein are alternative, enhanced, and cheaper methods of improving cognitive functions in a subject using a lipid composition conjugated with omega-3 and omega-6 fatty acids, with specific amounts and specific conjugation patterns of LA, linolenic acid (alpha-linolenic acid, gamma-linolenic acid) DHA and eicosapentaenoyl (EPA), e.g. utilizing different sources of lipids.Disclosed herein is a lipid preparation, said preparation comprising a phosphatidylserine moiety, and poly-unsaturated fatty acid (PUFA) acyl groups, particularly long-chain poly-unsaturated fatty acid (LC-PUFA) acyl groups such as omega-3 and / or omega-6 acyl groups, wherein said PUFA is covalently bound to said glycerophospholipid. Said lipid preparations are particularly useful in the treatment of mental and cognitive disorders, e.g. ADHD (attention deficit hyperactivity disorder) and Alzheimer's disease.The disclosed preparations present improved bioactivity, and are useful in the treatment of various cognitive and mental conditions and disorders, as well as for maintenance of normal functions of brain-related systems and processes.
Owner:ENZYMOTEC
Formulation for spray-drying large porous particles
InactiveUS7279182B2Reduce and eliminate needEasy to preparePowder deliveryBiocidePrillVolumetric Mass Density
Particles having a tap density less than about 0.4 g / cm3 are formed by spray drying from a colloidal solution including a carboxylic acid or salt thereof, a phospholipid, a divalent salt and a solvent such as an aqueous-organic solvent. The colloidal solution can also include a therapeutic, prophylactic or diagnostic agent. Preferred carboxylic acids include at least two carboxyl groups. Preferred phospholipids include phosphatidylcholines, phosphatidylethanolamines, phosphatidylglycerols, phophstidylserines, phosphatidylinositols and combinations thereof. The particles are suitable for pulmonary delivery.
Owner:CIVITAS THERAPEUTICS
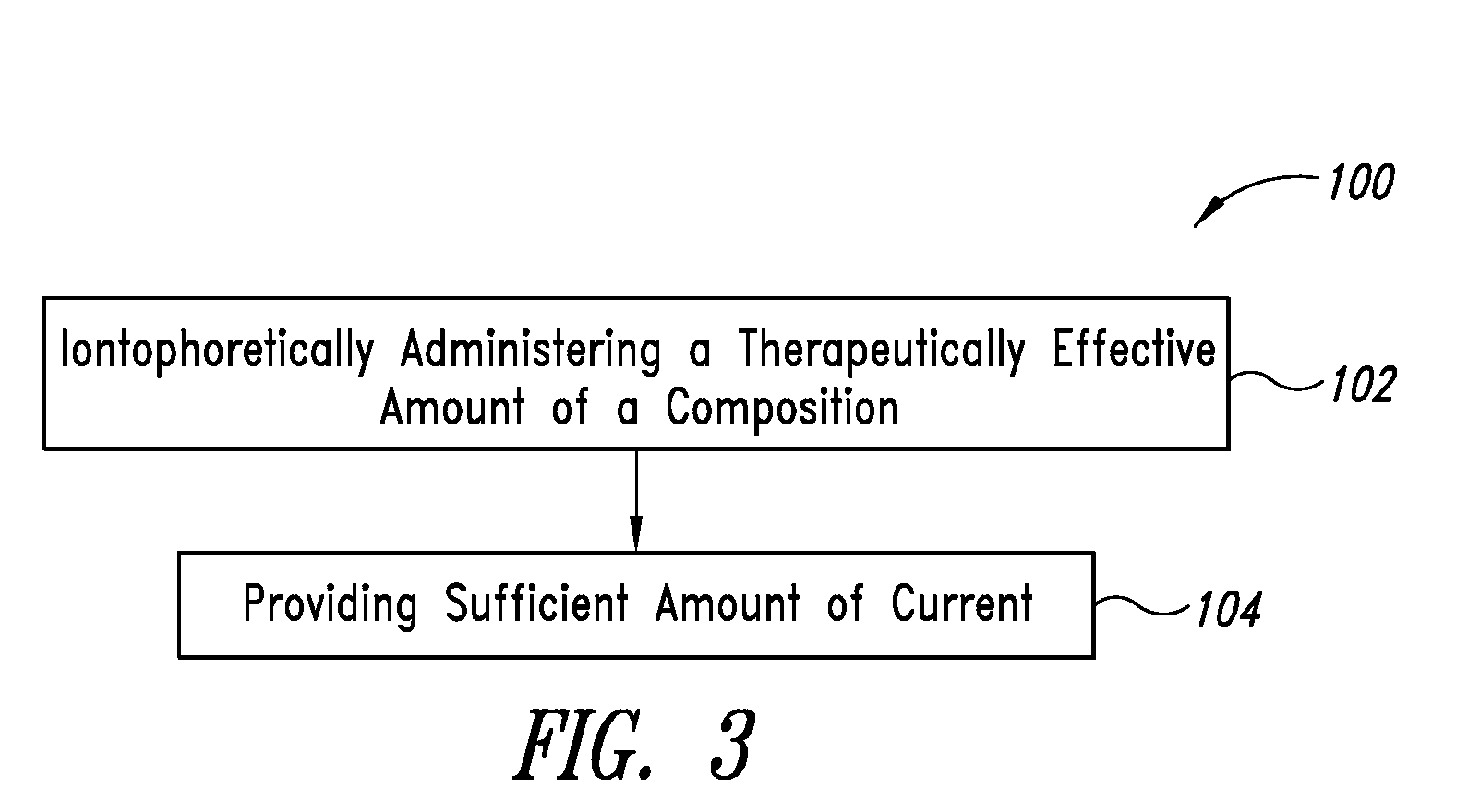
System, devices, and methods for iontophoretic delivery of compositions including antioxidants encapsulated in liposomes
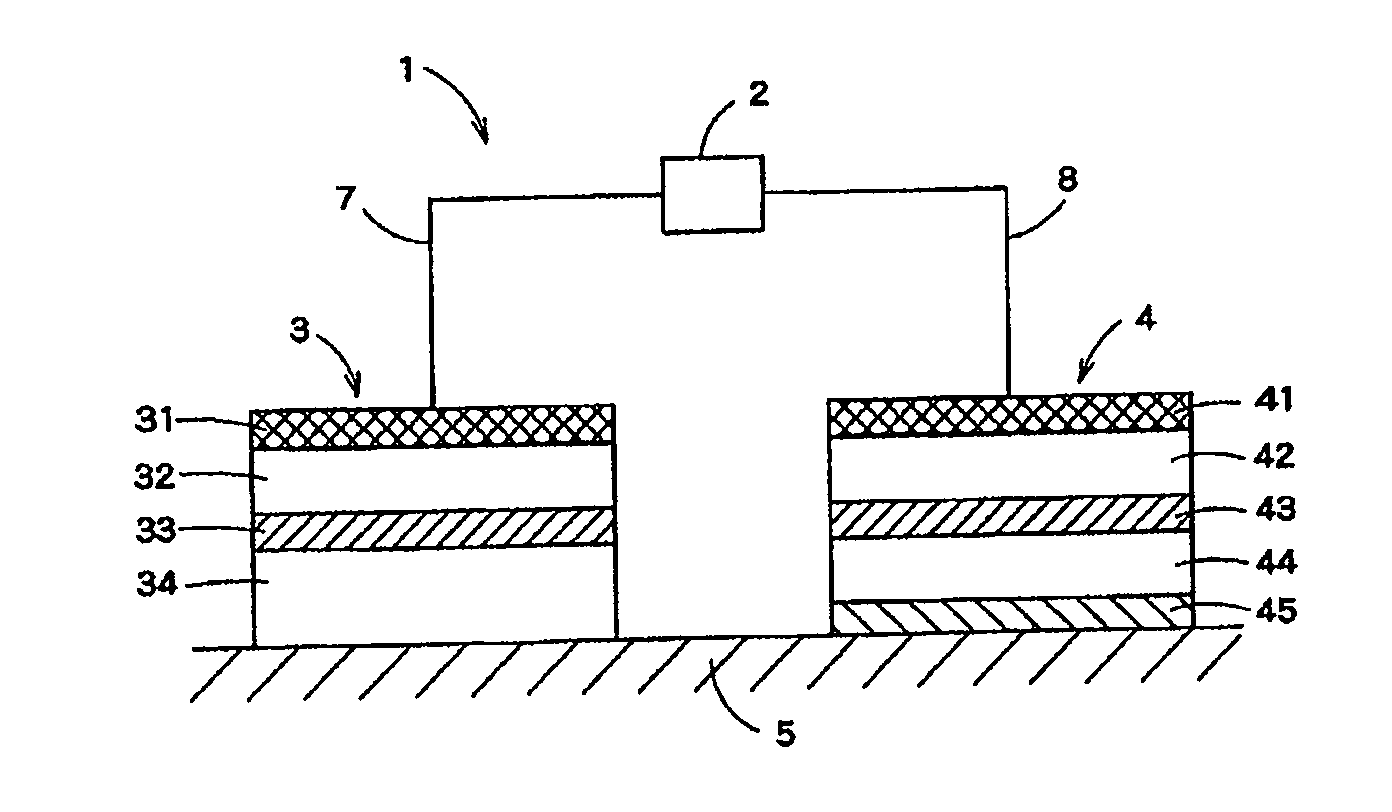
Systems, devices, and methods for delivering one or more active ingredients to intradermal tissues, deep regions of pores, and intradermal tissues in the vicinity of the pores. In some embodiments, a composition is provided including a plurality of liposomes including a cationic lipid, and an amphiphilic glycerophospholipid having a saturated fatty acid moiety and an unsaturated fatty acid moiety, and one or more antioxidants and / or antioxidant enzymes.
Owner:KOGURE KENTARO +2
Stable polymer micelle medicine carrging system
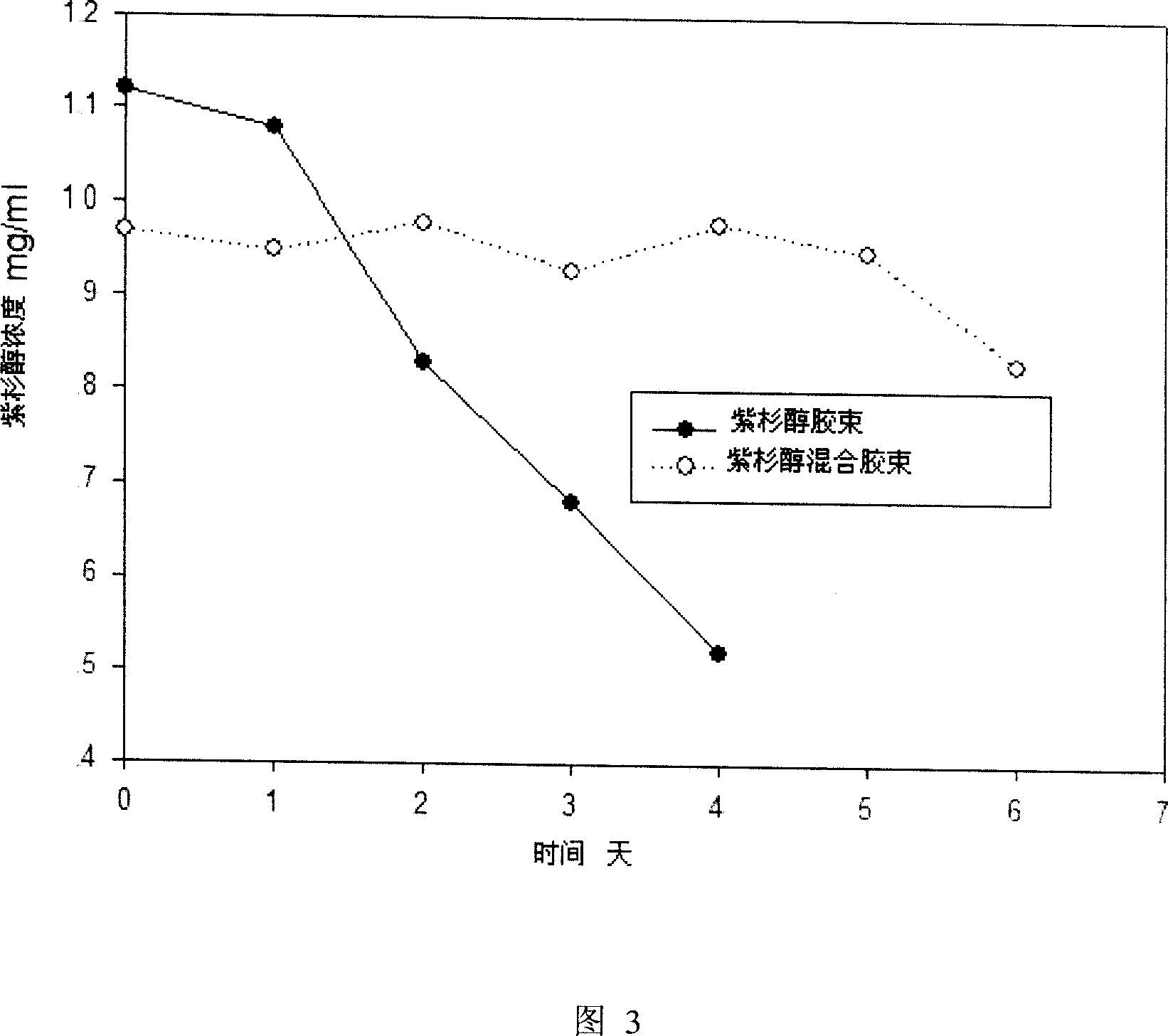
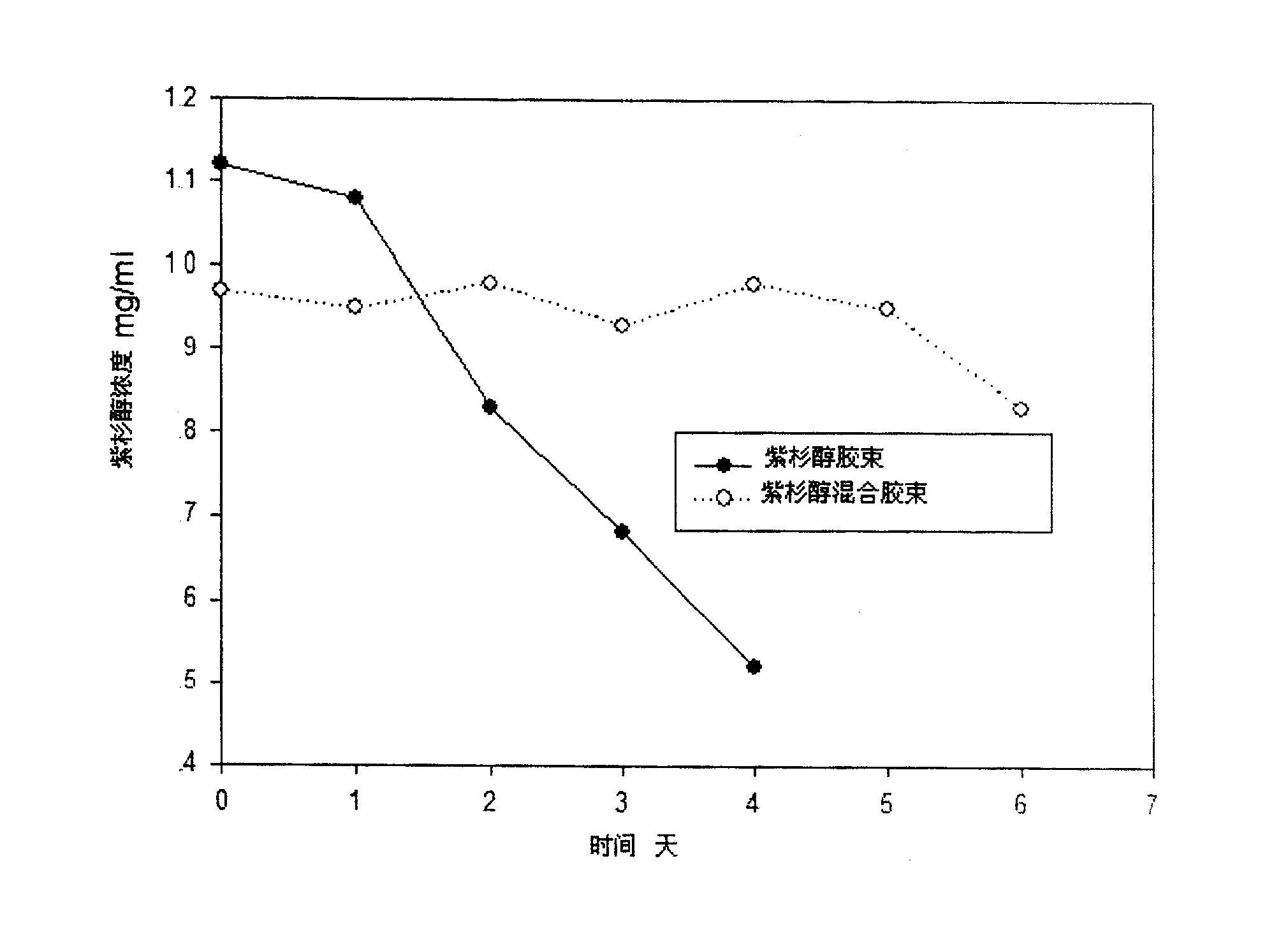
InactiveCN100998870AHigh drug loadingImprove stabilityAntipyreticMetabolism disorderDrug carrierCarrier system
A stable polymer micella carrier system for medicines is composed of an amphipathic block copolymer including diblock copolymer and triblock copolymer and a phosphatide chosen from glyceryl phosphatide, sphingomyelin, and their derivatives.
Owner:涂家生 +1
Glycerophospholipids for the improvement of cognitive functions
ActiveUS7935365B2Improve cognitive functionReduce dosageBiocideNervous disorderAcyl groupMammalian brain
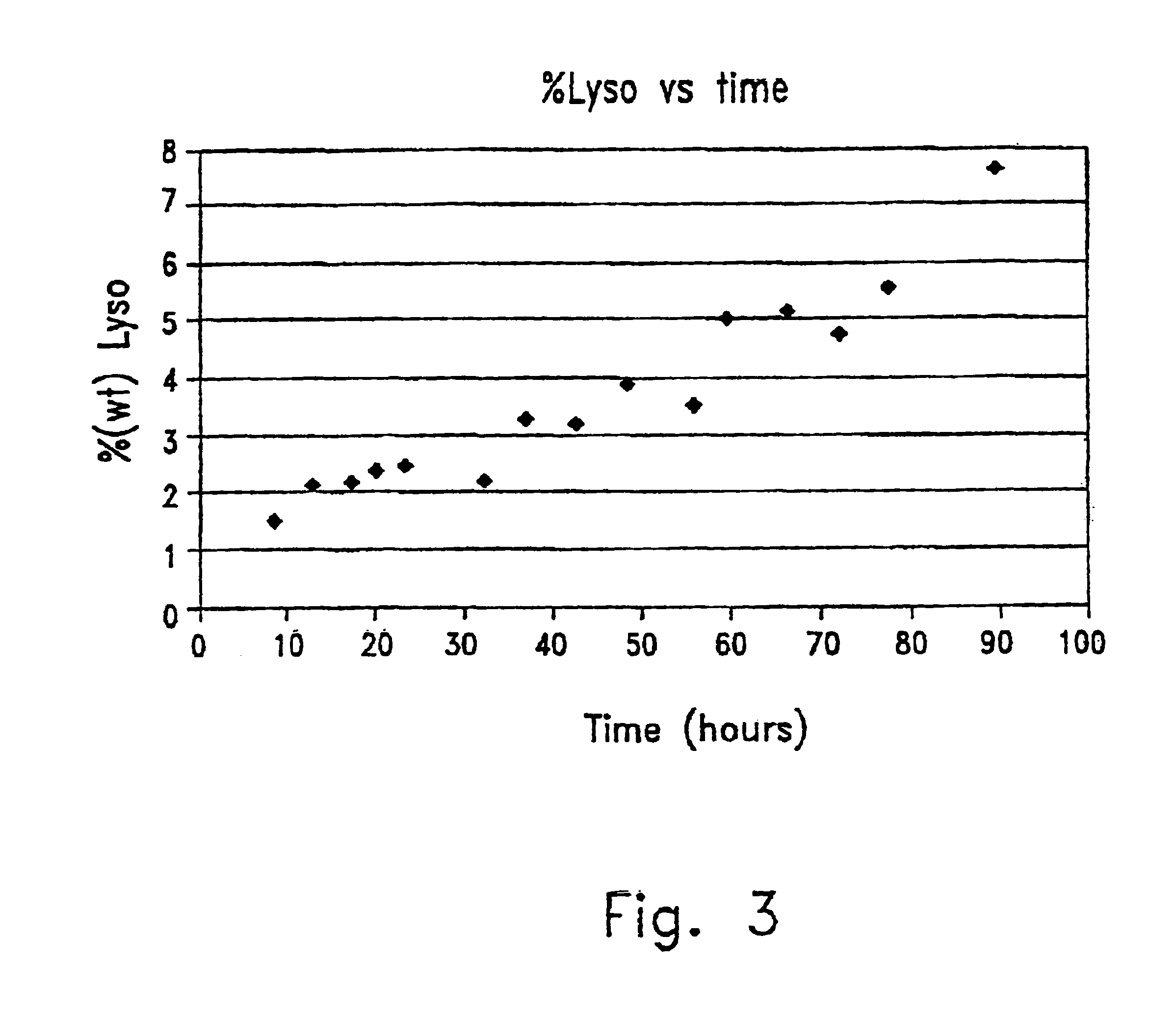
This invention provides a preparation comprising serine glycerophospholipids which comprise a mixture of serine glycerophospholipids comprising eicosapentaenoic acid (EPA) and serine glycerophospholipids comprising docosahexaenoic acid (DHA), wherein each such serine glycerophospholipid comprising EPA and each such serine glycerophospholipid comprising DHA has the formula (I):wherein R″ is serine; wherein one of R or R′ is acyl EPA or acyl DHA and the other of R or R′ is hydrogen or an acyl group; wherein the combined amount of EPA and DHA present in such mixture of serine glycerophospholipids constitutes 10-50% by weight of the total fatty acids content of the serine glycerophospholipids in said preparation; and wherein the mixture is not identical to naturally occurring human or mammalian brain PS.
Owner:ENZYMOTEC
Compositions for use in darkening the skin
The present invention features glycerophospholipids having a single fatty acid moiety and the use thereof in darkening the skin.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Polar lipid mixtures, their preparation and uses
InactiveUS20110294757A1Milk preparationOrganic active ingredientsPhosphatidyl inositolPhosphatidylethanolamine
Disclosed herein are polar lipid mixtures, comprising glycerophospholipids such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS) and phosphatidyl-inositol (PI), and sphingolipids such as sphyngomyelin (SM). Most importantly, the ratio of phospholipids in said mixture is comparable to that of HMF, and is represented by SM>PC>PE>PS>PI or SM=PC>PE>PS>PI. Processes for the preparation of said mixtures and uses thereof are also described herein.
Owner:ENZYMOTEC
Lipids containing omega-3 and omega-6 fatty acids
InactiveUS20080085319A1Enhance memoryReduce stressBiocideEdible oils/fats ingredientsLipid formationAcyl group
A lipid preparation including a glycerophospholipid or salt, conjugate and derivatives thereof, particularly phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidyl-inositol (PI), phosphatidylglycerol (PG) and phosphatidic acid (PA), and poly-unsaturated fatty acid (PUFA) acyl groups, particularly long-chain poly-unsaturated fatty acid (LC-PUFA) acyl groups such as omega-3 and / or omega-6 acyl groups, wherein said PUFA is covalently bound to said glycerophospholipid. The preparation possesses an improved bioactivity, and is useful in the treatment of various cognitive and mental conditions and disorders and for maintenance of normal functions of brain-related systems and processes.
Owner:ENZYMOTEC
Process for the production of phospholipids
ActiveUS7034168B2High yieldHigh purityMicroorganism based processesPhosphatide foodstuff compositionsPhospholipaseCarboxylic acid
A new enzymatic process for preparing 1,2-diacylated phospholipids using an enzyme preparation possessing phospholipase activity towards acylation at the sn-1 and sn-2 sites in a microaqueous reaction system. More particularly, the 1,2-diacyl-phospholipids produced according to the esterification / transesterification process are obtainable in high yield and purity and carry identical desired carboxylic acid, preferably fatty acid, acyl groups at the sn-1 and sn-2 positions. The process involves esterification / transesterification (acylation) of a glycerophospholipid, preferably glycerophosphoryl choline (GPC) with a desired carboxylic acid, preferably fatty acid, or their derivatives in the presence of the above mentioned appropriate enzyme preparation. The process of the invention further relates to a process for the production of 1-acyl-2-lyso-glycerophospholipid, preferably 2-lyso-PC by reacting glycerophospholipid, preferably glycerophosphoryl choline (GPC) with a desired carboxylic acid, preferably fatty acid, or their derivatives in the presence of a sn-1 specific phospholipase (PLA1 or PLA1,2) and a solvent, in a microaqueous medium.
Owner:ENZYMOTEC
Lipids containing omega-3 and omega-6 fatty acids
InactiveUS20080085320A1Enhance memoryReduce stressOrganic active ingredientsBiocideLipid formationAcyl group
Owner:ENZYMOTEC
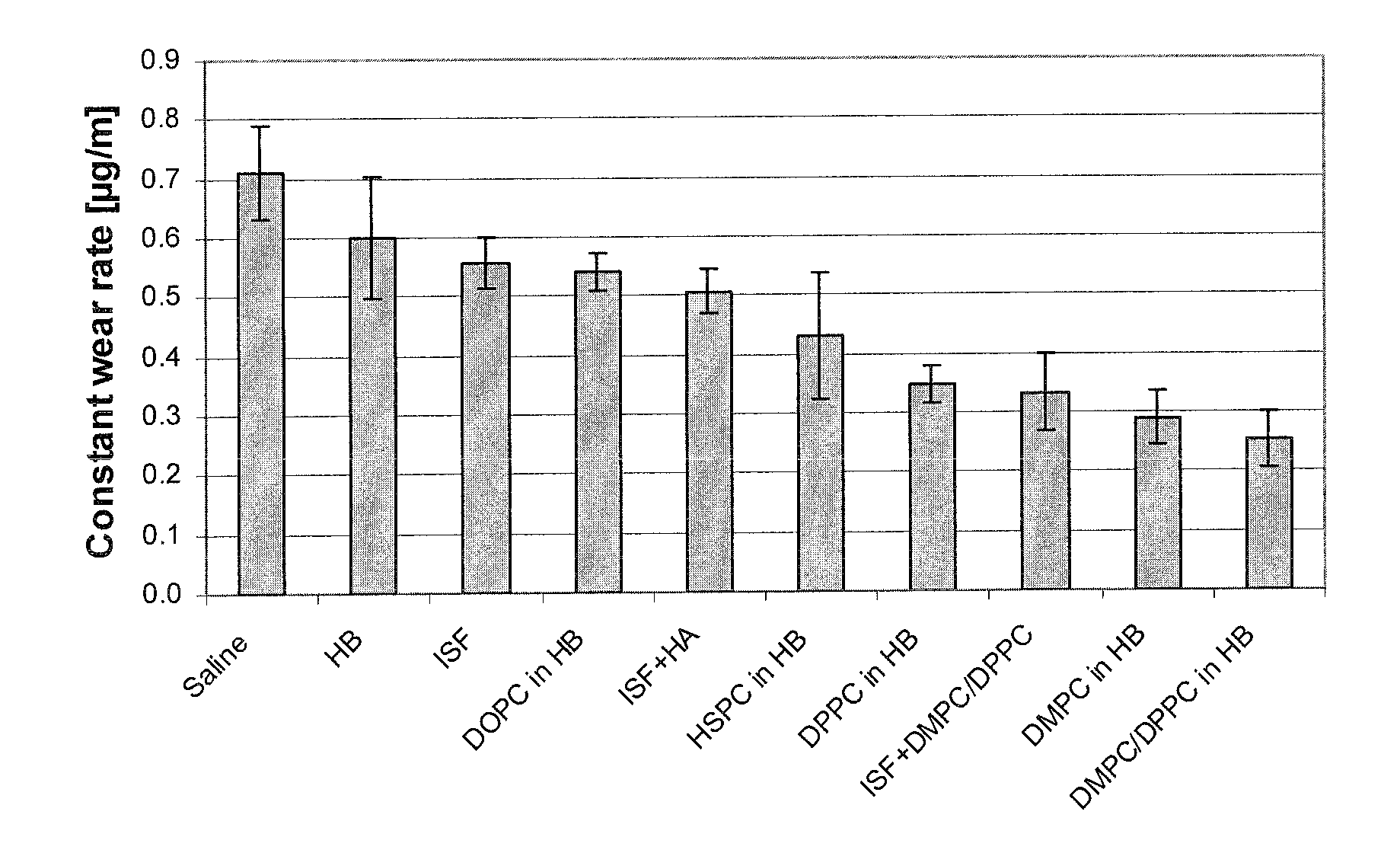
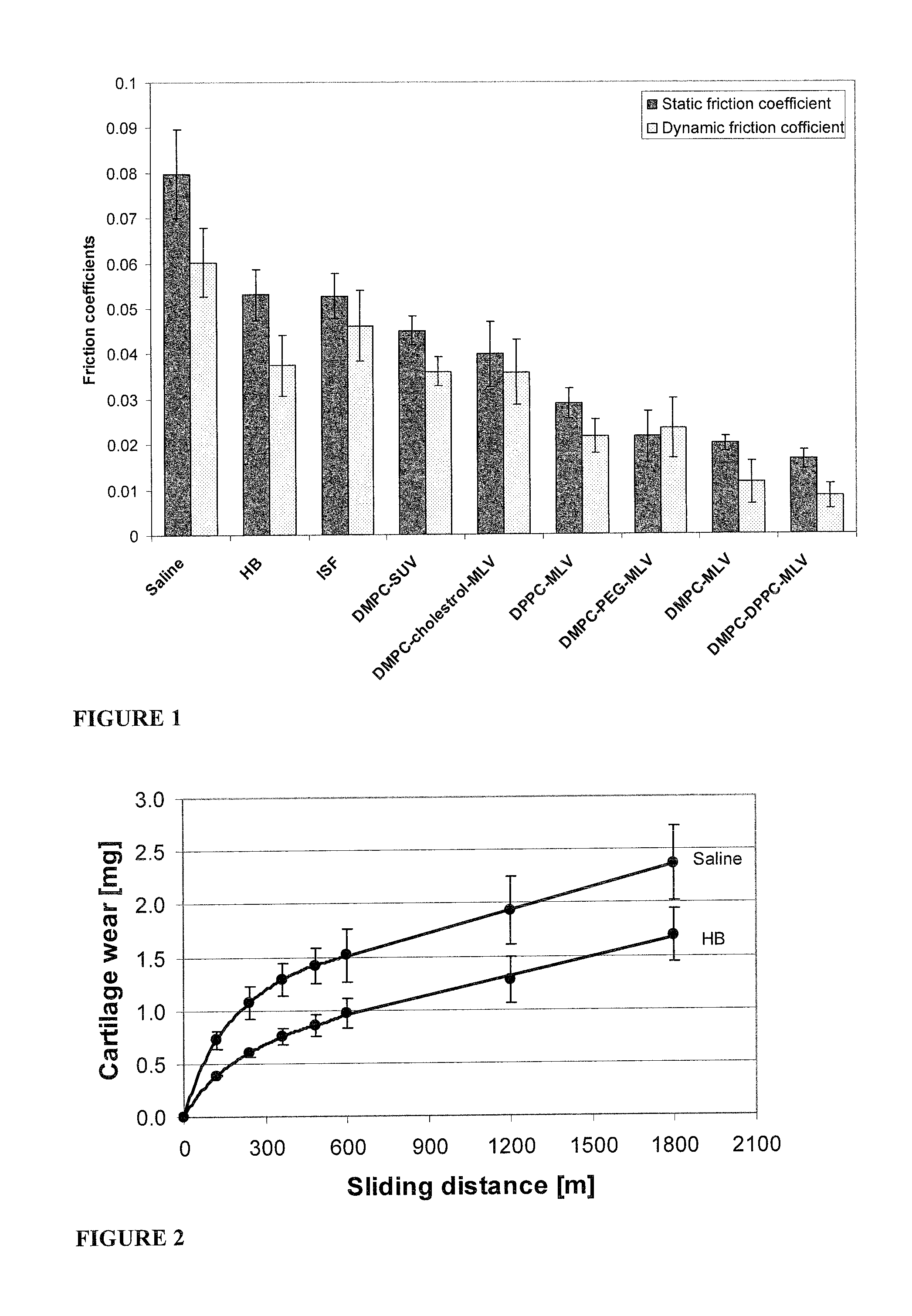
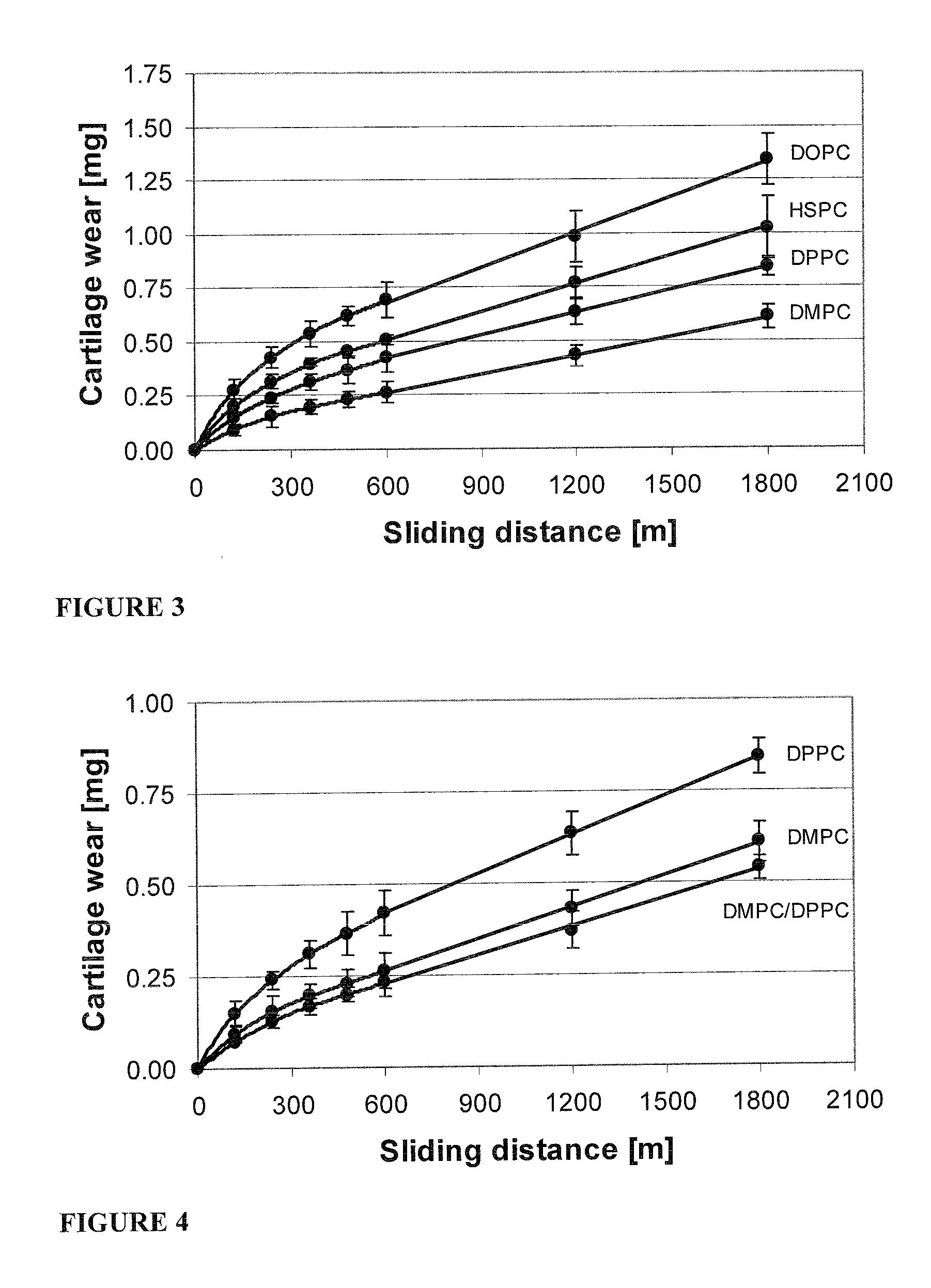
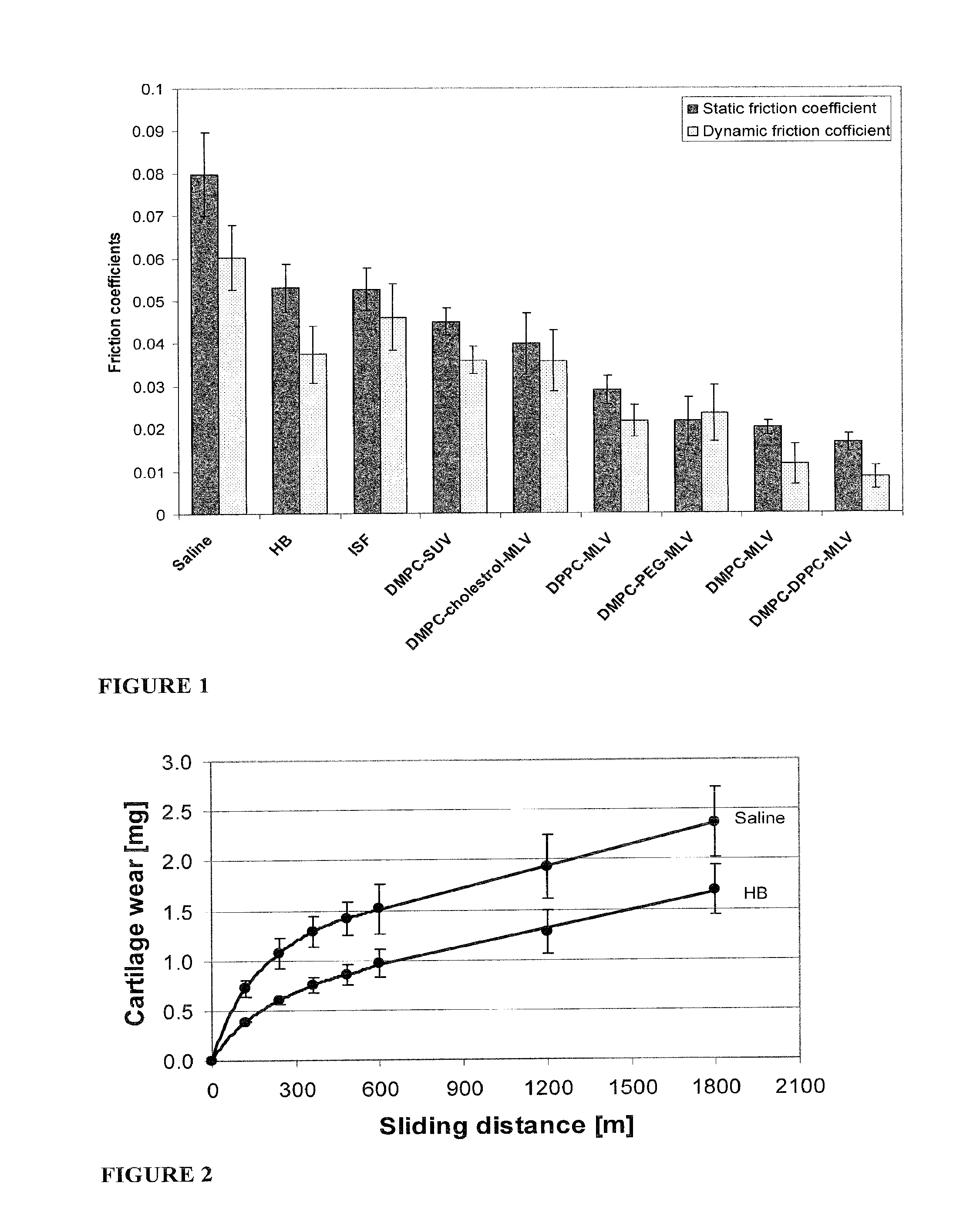
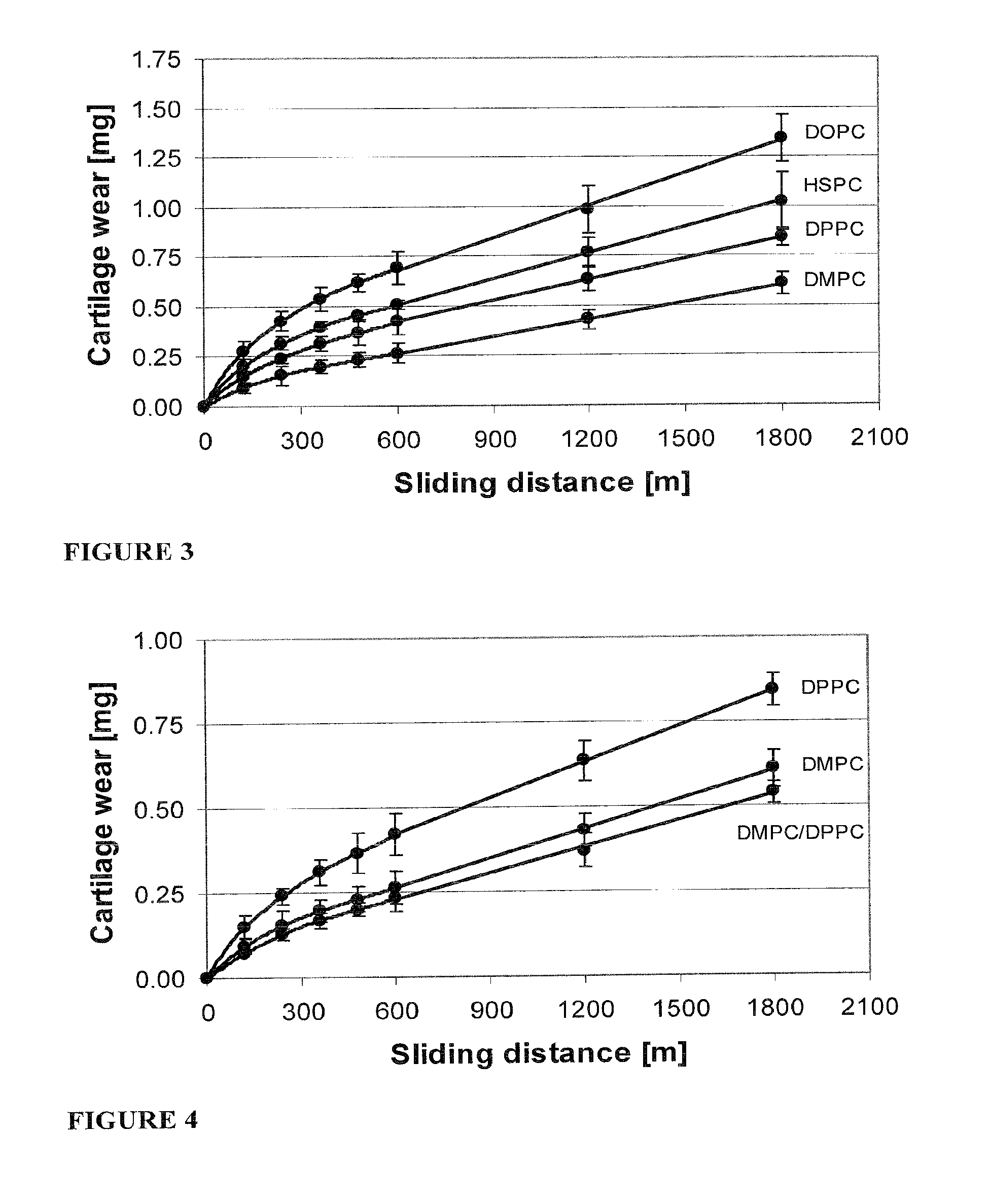
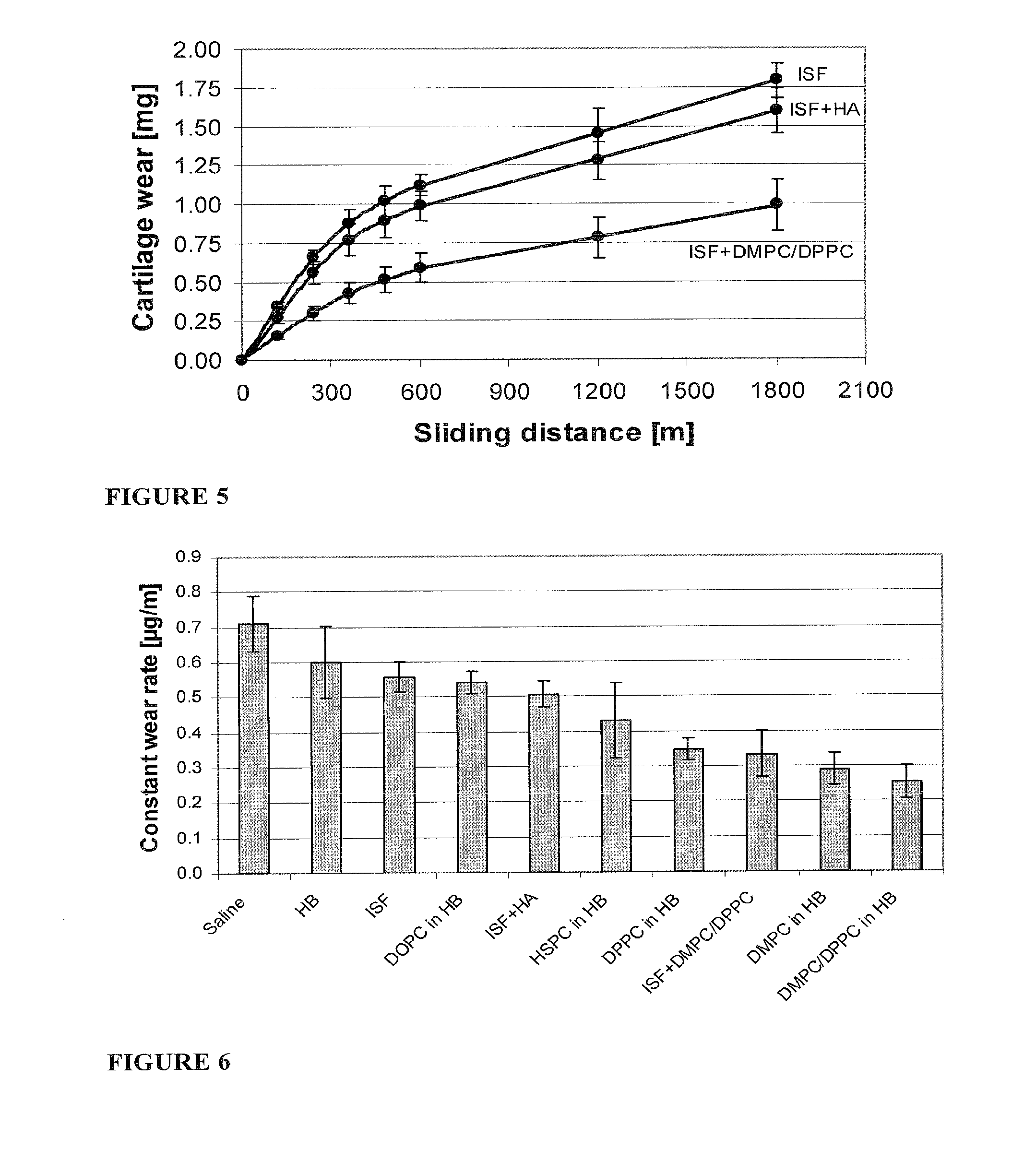
Methods for joint lubrication and cartilage wear prevention making use of glycerophospholipids
ActiveUS8895054B2Improve and restore joint mobilityReduce wearOrganic active ingredientsAntipyreticLiposomeLubrication
The present invention concerns methods of joint lubrication and / or prevention of cartilage wear making use of liposomes having membranes with at least one phospholipid (PL) of the group consisting of a glycerophospholipid (GPL) having two, being the same or different, C12-C16 hydrocarbon chain and a sphingolipid (SPL) having a C12-C15 hydrocarbon chain, the one or more membranes having a phase transition temperature in which solid ordered (SO) to liquid disordered (LD) phase transition occurs, the phase transition temperature being within a temperature of about 20° C. to about 39° C. for lubrication of joints.
Owner:TECHNION RES & DEV FOUND LTD +2
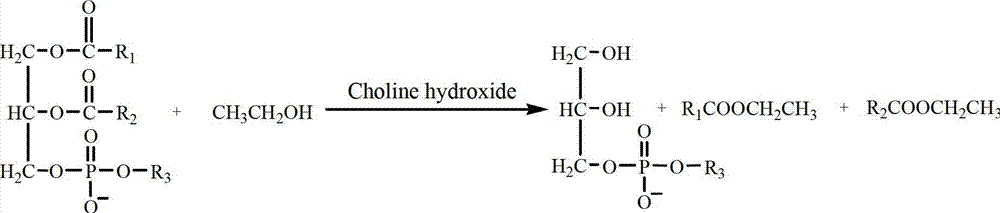
Method for preparing natural L-alpha-glycerol phosphatidylcholine
InactiveCN102964377AHigh purityGuaranteed stabilityPhosphorus organic compoundsFood additiveGlycerol
The invention discloses a method for preparing natural L-alpha-glyceryl phosphatide dipalmitoyl phosphatidyl choline. The method comprises the following steps of: dissolving natural lecithin having the known content of phosphatidylcholine into methyl tertiary butyl ether; filtering out undissolved substances and regulating the temperature to 5-55 DEG C; adding anhydrous low carbon alcohol and hydroxide choline into mixing liquid in a molar rate of anhydrous low carbon alcohol to phosphatidylcholine of (3:1)-(600:1)_to stir and react for 0.25-4 hours; pouring out supernate; dissolving oily precipitates with absolute ethyl alcohol and precipitating the oily precipitates by methyl tertiary butyl ether; removing the supernate after the precipitation is totally finished; repeatedly dissolving the precipitates by the absolute ethyl alcohol and precipitating the precipitates by the methyl tertiary butyl ether for 2-3 times; vacuumizing and drying obtained oily products. By the method, transesterification between the phosphatidylcholine and the anhydrous low carbon alcohol is carried out by using hydroxide choline as a catalyst and the methyl tertiary butyl ether as a solvent so as to obtain glycerol phosphatidylcholine (GPC) or natural lecithin deacylation substances using the GPC as a main part; and moreover, the method is simple in process, mild and safe in reaction conditions, and can guarantee the safety of the obtained products used as functional food addictives or medical health-care products.
Owner:NORTHWEST UNIV(CN)
Glycerophospholipids containing omega-3 and omega-6 fatty acids
InactiveCN1897955AGuaranteed functionImprove and treat ADHDOrganic active ingredientsSenses disorderGlycerolOmega-6 fatty acid
Disclosed is a lipid preparation comprising a glycerophospholipid or salt, conjugate and derivatives thereof, particularly phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidyl-inositol (PI), phosphatidylglycerol (PG) and phosphatidic acid (PA), and poly-unsaturated fatty acid (PUFA) acyl groups, particularly long-chain poly-unsaturated fatty acid (LC-PUFA) acyl groups such as omega-3 and / or omega-6 acyl groups, wherein said PUFA is covalently bound to said glycerophospholipid. The disclosed preparations possess an improved bioactivity, and are useful in the treatment of various cognitive and mental conditions and disorders and for maintenance of normal functions of brain-related systems and processes, for example ADHD.
Owner:ENZYMOTEC
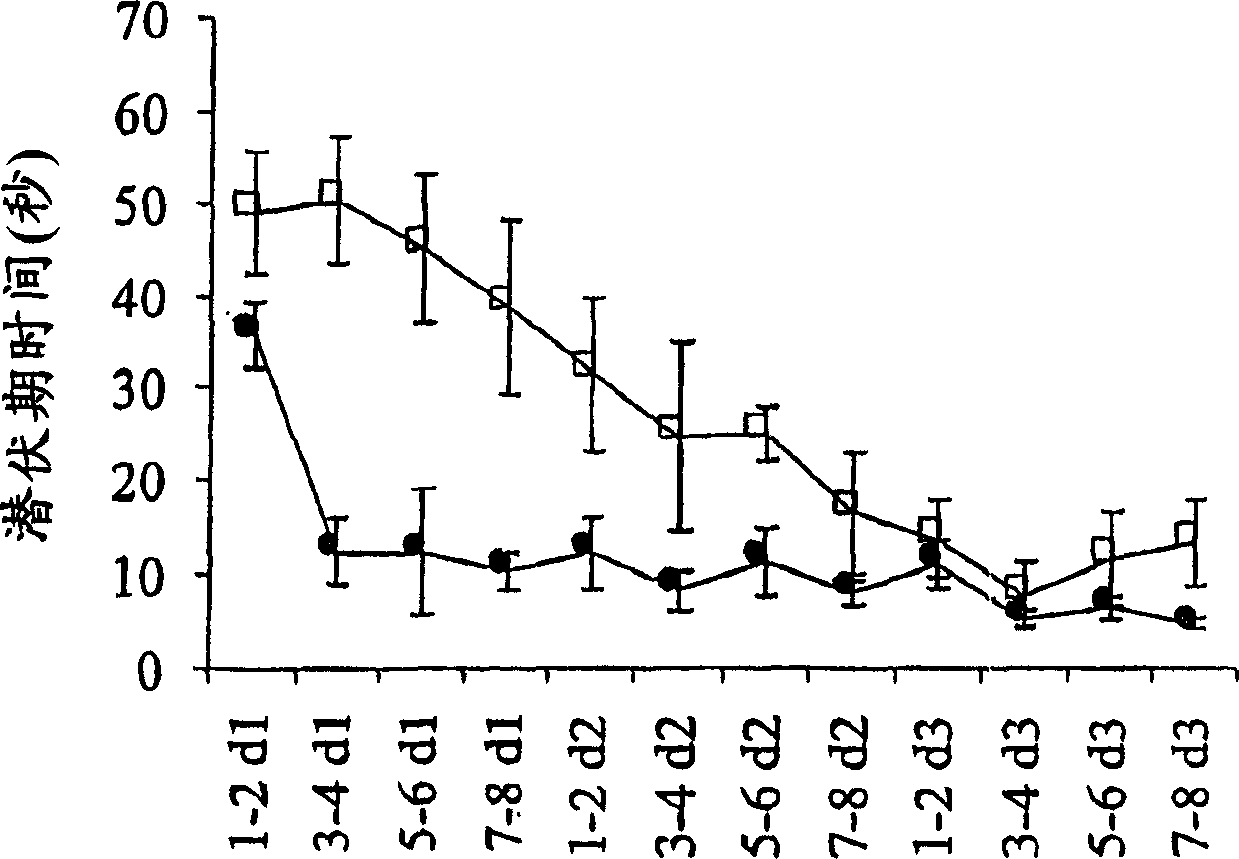
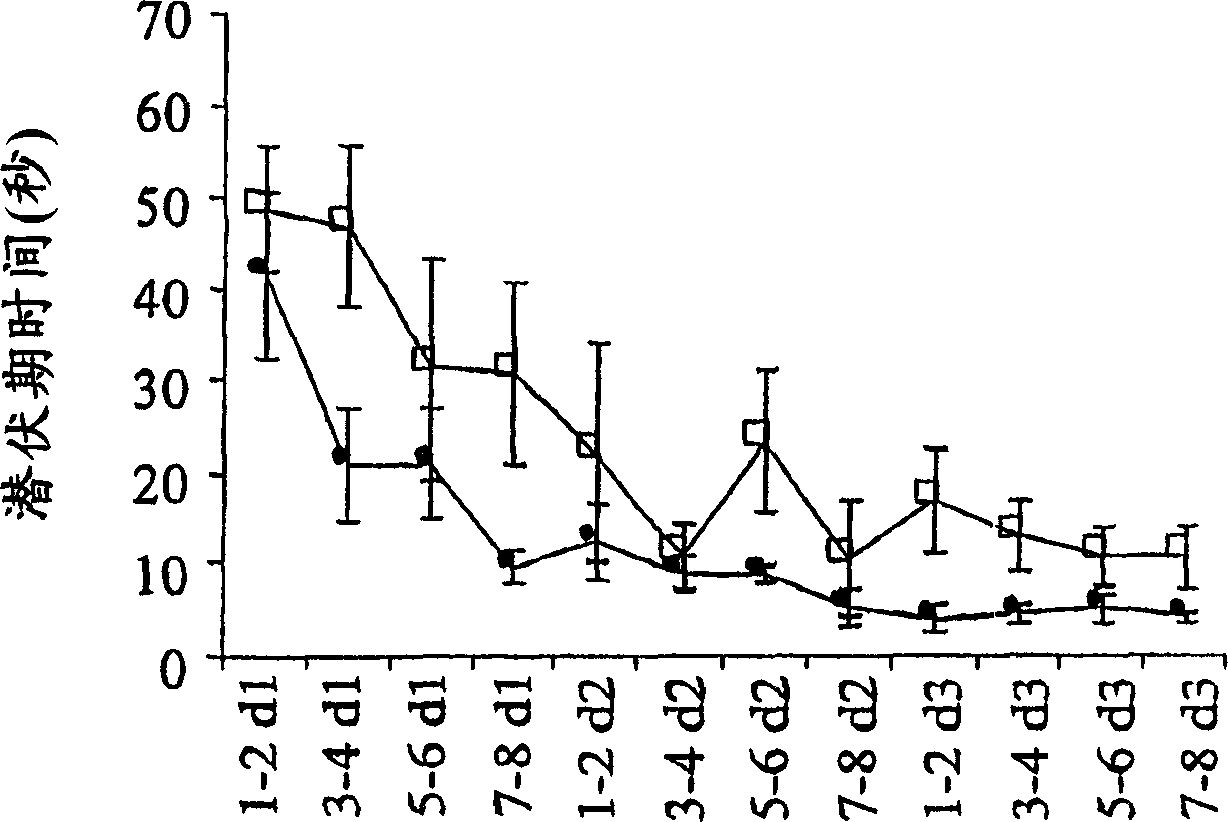
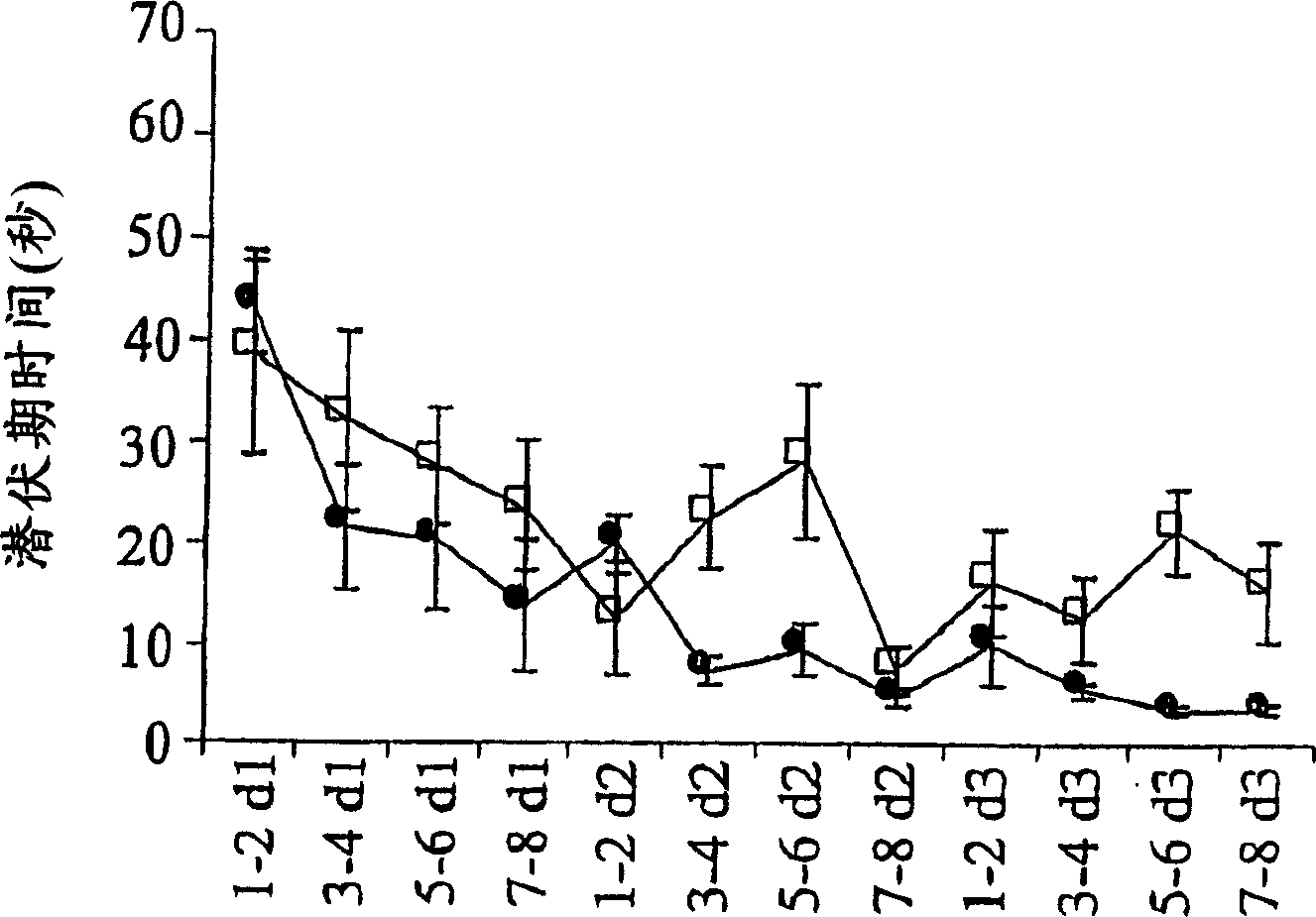
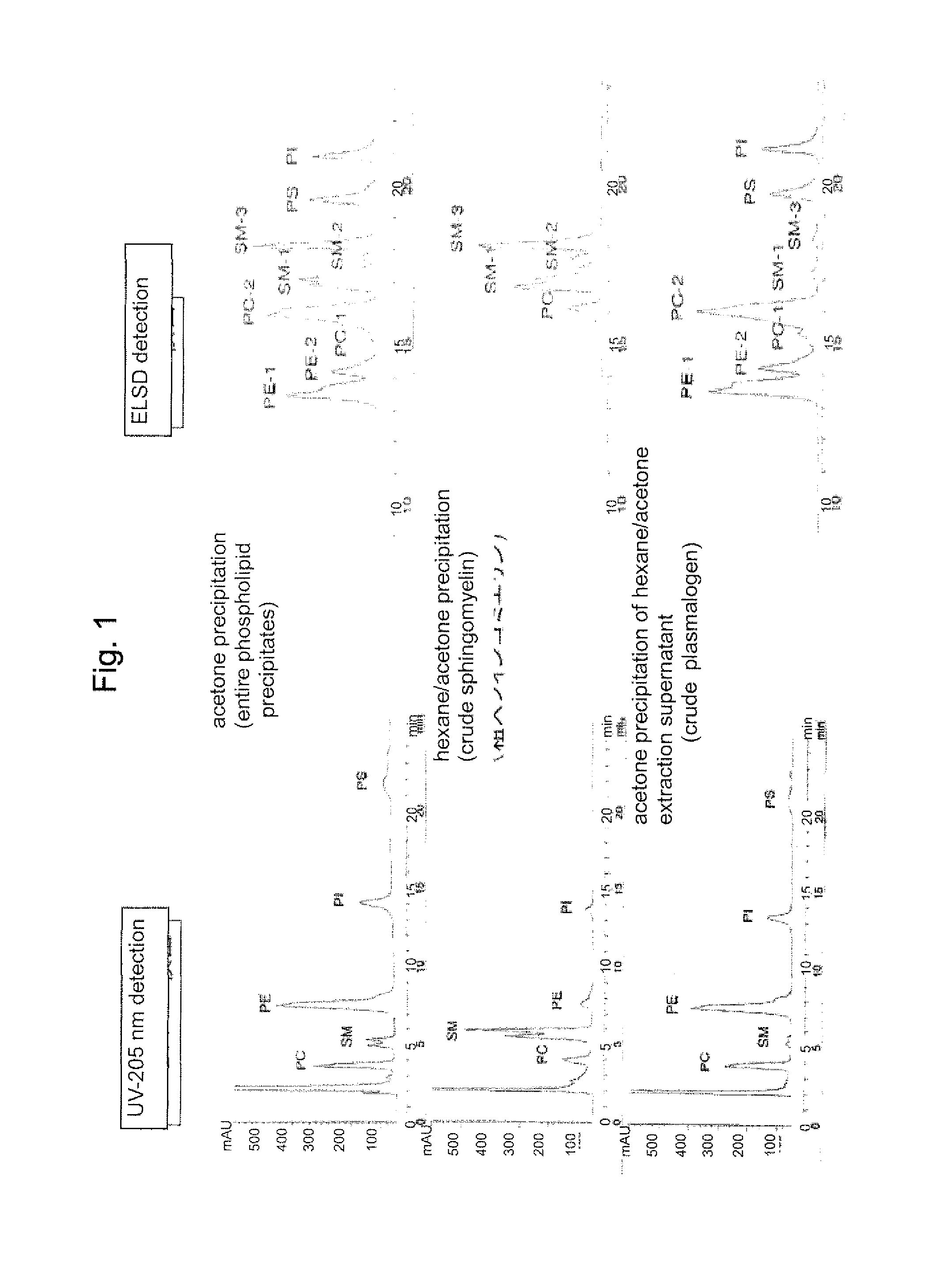
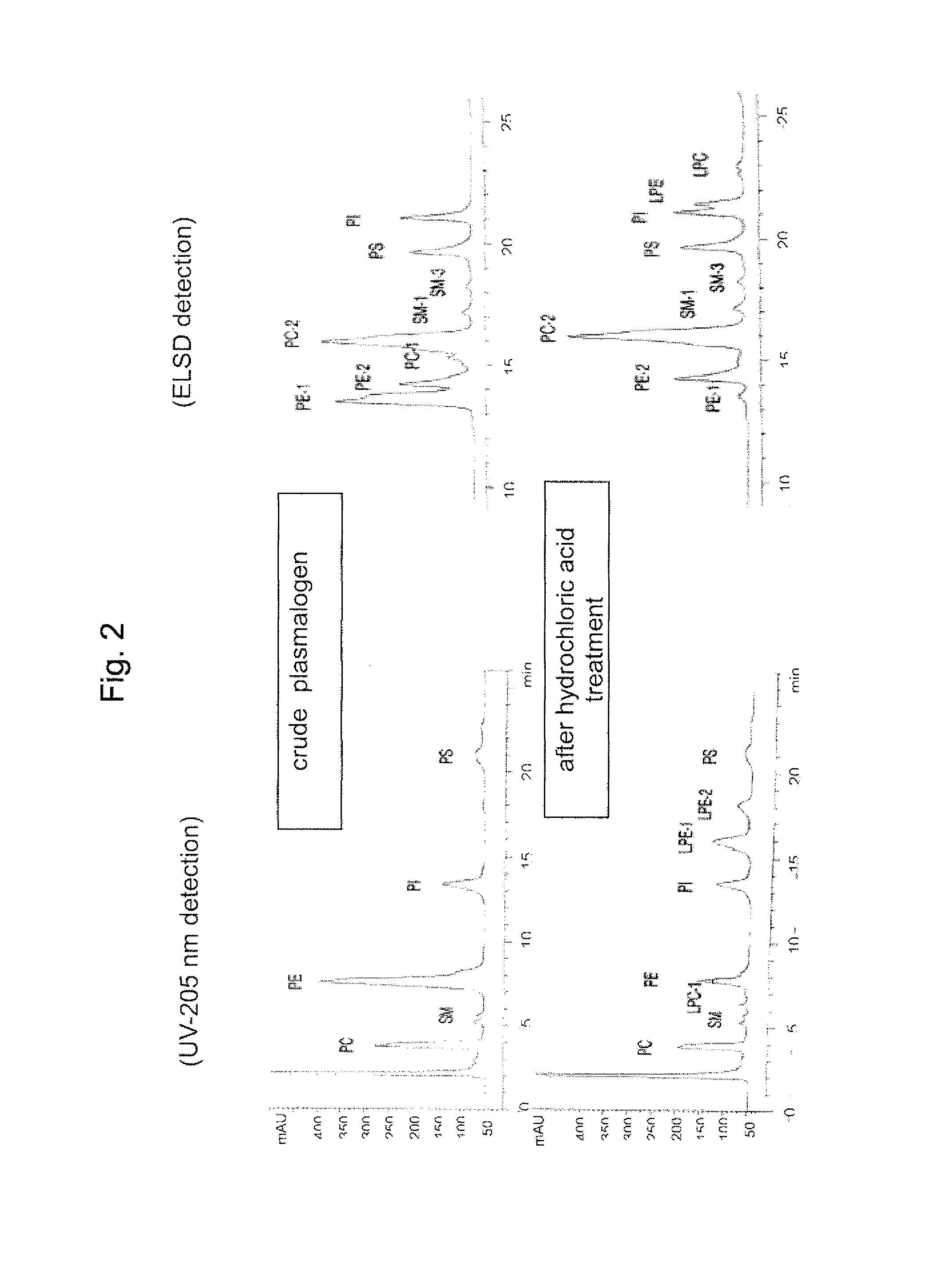
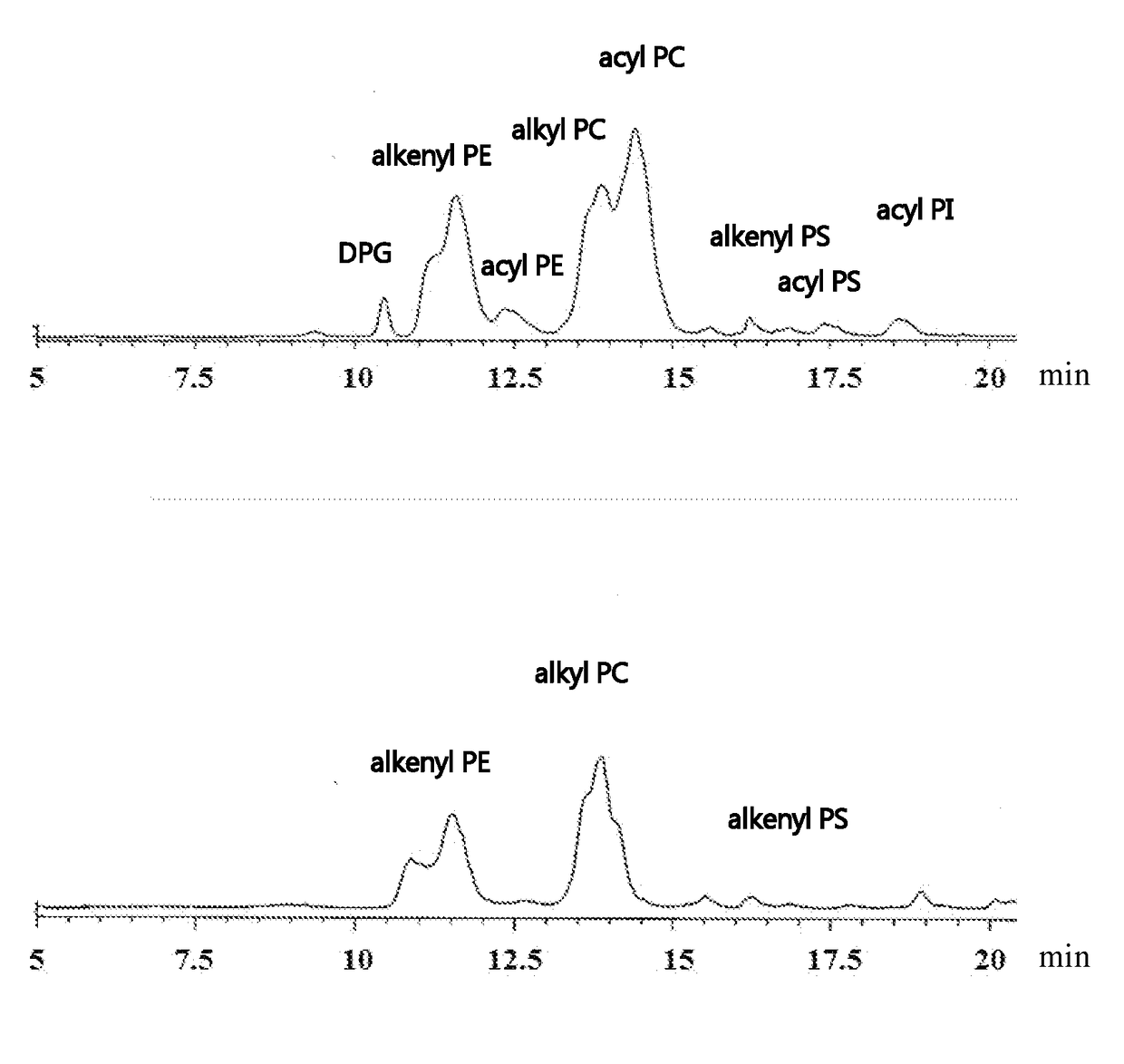
Process for producing sphingomyelin and plasmalogen-form glycerophospholipid
ActiveUS20100029966A1Simple procedureHigh yieldFatty-oils/fats productionKetone solventsAliphatic hydrocarbon
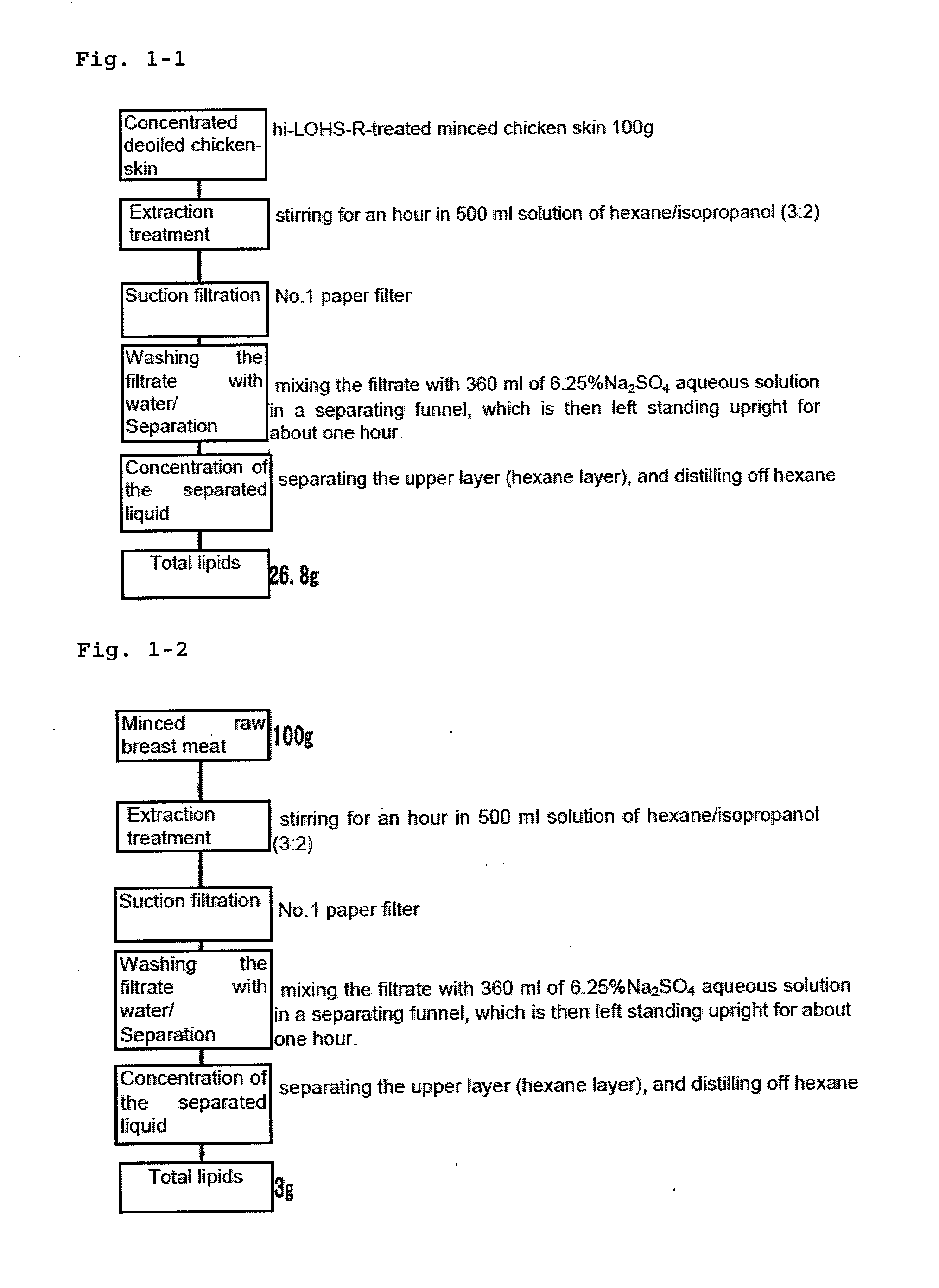
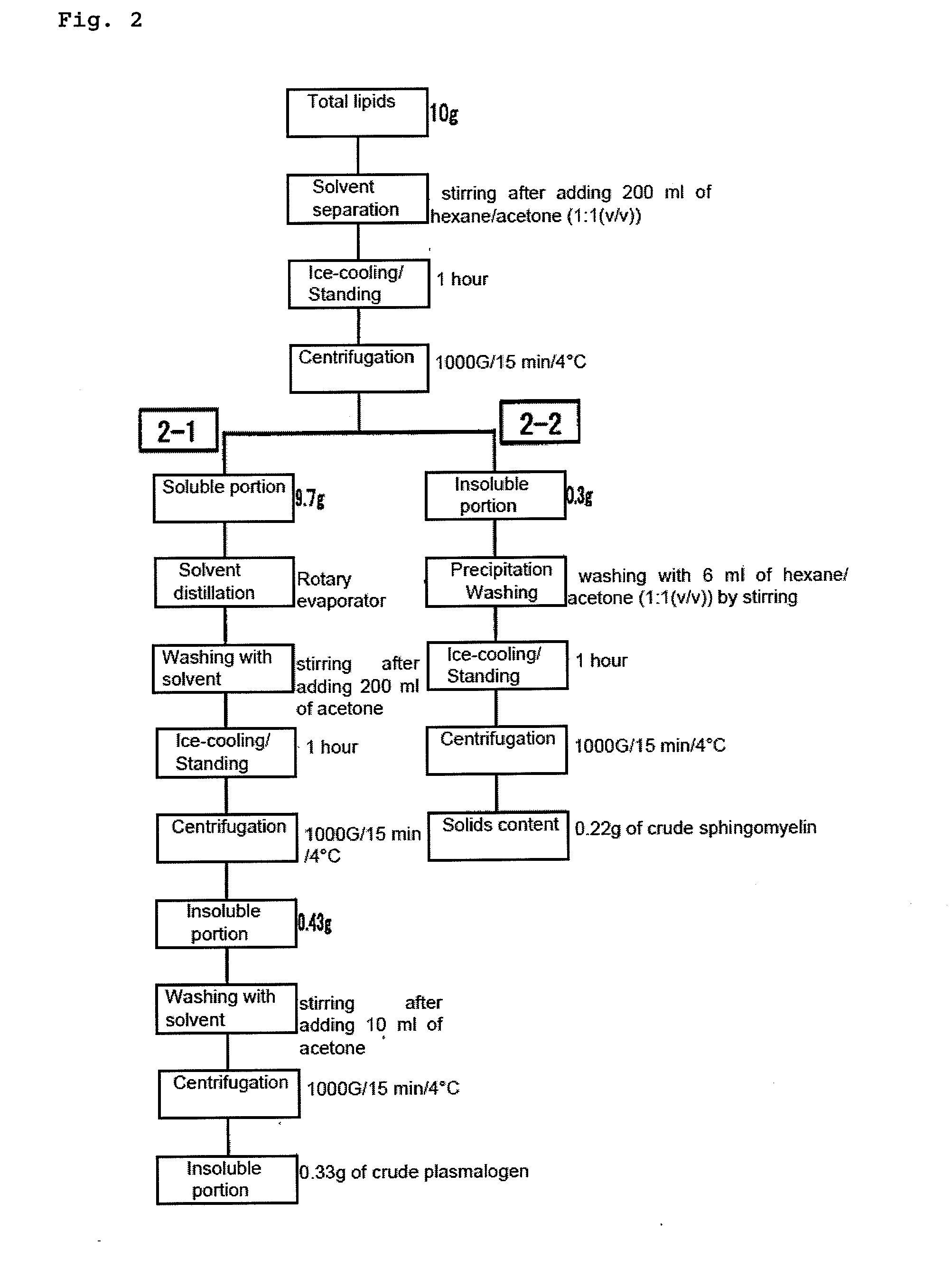
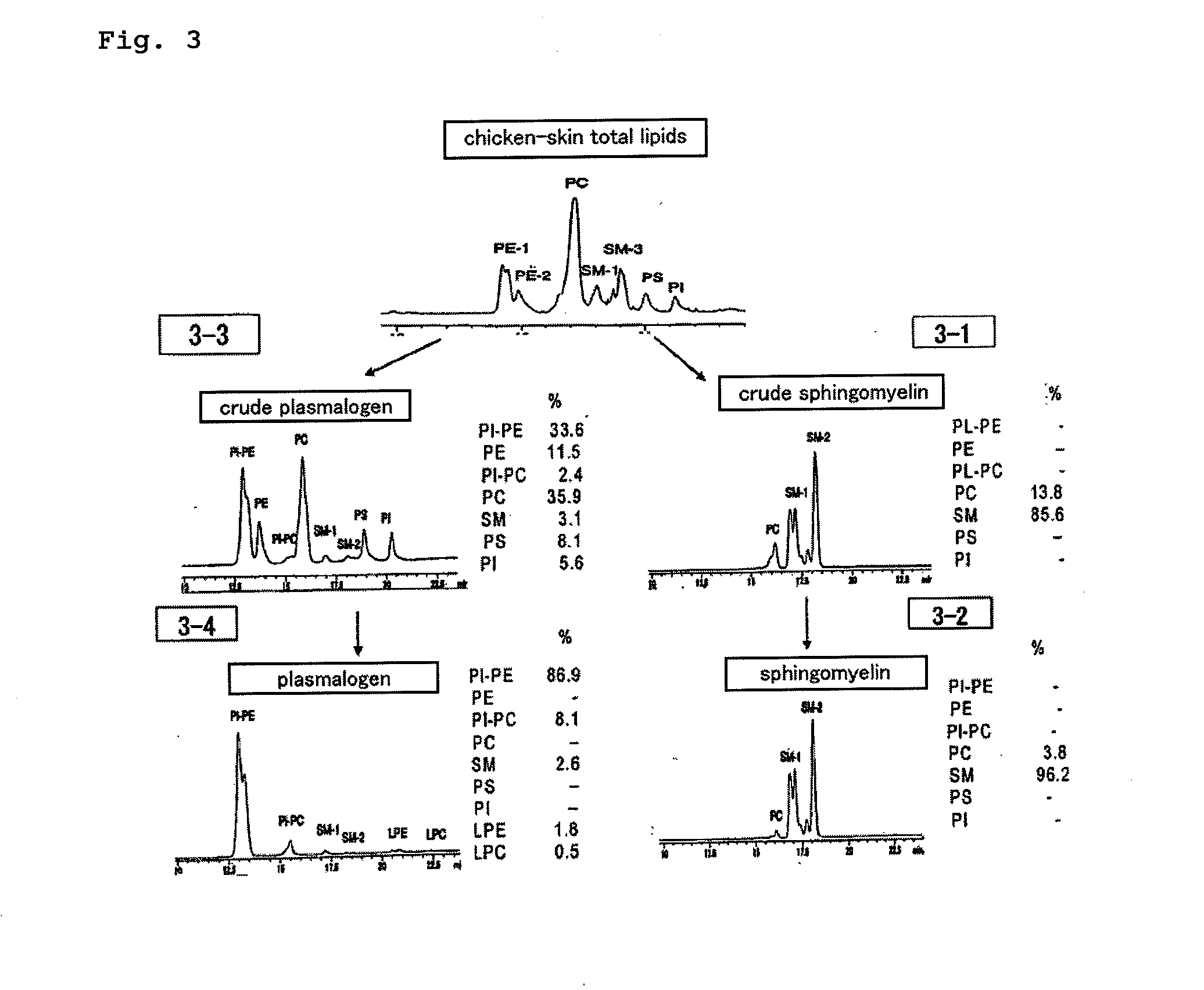
Disclosed is a process for producing sphingomyelin and plasmalogen-form glycerophospholipid, which comprises the step (A) of extracting a total lipids from a chicken skin powder and drying the extract, the step (B) of subjecting the dried total lipids obtained in said step (A), to extraction treatment with a solvent mixture of an aliphatic hydrocarbon solvent and a water-soluble ketone solvent to separate an insoluble portion composed mainly of sphingomyelin and a soluble portion, the step (C) subjecting the insoluble portion composed mainly of sphingomyelin, obtained in said step (B), to extraction treatment with a solvent mixture of water and a water-soluble ketone solvent to remove a non-lipid component contained in the soluble portion, and the step (D) of drying the soluble portion obtained in said step (B), and subjecting the thus-obtained dried product to extraction treatment with a water-soluble ketone solvent to separate and recover an insoluble portion composed mainly of plasmalogen-form giycerophospholiuld.According to the above production process, high-purity sphingomyelin, in particular, human-form sphingomyelin and plasmalogen-form sphingomyelin can be produced from chicken skin at high yields with simple procedures.
Owner:MARUDAI FOOD +2
Peptide-lipid constructs and their use in diagnostic and therapeutic applications
Peptide-lipid constructs of the structure L-S—F are disclosed, where F is a peptide, S is a spacer covalently linking F to L via an oligomer of ethylene glycol, and L is a diacyl- or dialkyl-glycerolipid (including glycerophospholipids). The spacer ideally has 6 to 14 ethylene glycol repeats, corresponding to PEG with a molecular weight of approximately 250 to 600. Also disclosed is a method of detecting reactive antibodies in serum by contacting serum with cells modified to incorporate a peptide-lipid construct, where the peptide is an epitope of the antibody, and determining the degree of aggluthination of the cells.
Owner:KODE BIOTECH
Peptide-lipid constructs and their use in diagnostic and therapeutic applications
Peptide-lipid constructs of the structure L-S-F are disclosed, where F is a peptide, S is a spacer covalently linking F to L via an oligomer of ethylene glycol, and L is a diacyl- or dialkyl-glycerolipid (including glycerophospholipids). The spacer ideally has 6 to 14 ethylene glycol repeats, corresponding to PEG with a molecular weight of approximately 250 to 600. Also disclosed is a method of detecting reactive antibodies in serum by contacting serum with cells modified to incorporate a peptide-lipid construct, where the peptide is an epitope of the antibody, and determining the degree of agglutination of the cells.
Owner:KODE BIOTECH
Methods for joint lubrication and cartilage wear prevention making use of glycerophospholipids
InactiveUS20150037404A1Improve and restore joint mobilityReduce wearBiocideAntipyreticMean diameterMedicine
A method for lubricating a joint of a mammal by administering into a cavity of the joint a composition of liposomes that are multilamellar vesicles (MLV) dispersed in a fluid medium, the liposomes having a mean diameter of between about 0.8 μm to about 10 μm and including membranes of at least one glycerophospholipid (GPL) having two C12-C16 hydrocarbon chains which are the same or different. These membranes have a phase transition temperature in which solid ordered (SO) to liquid disordered (LD) phase transition occurs, the phase transition temperature being at a temperature of about 20° C. to about 39° C. and being lower than the temperature of the joint.
Owner:TECHNION RES & DEV FOUND LTD +2
Systems, devices, and methods for iontophoretic delivery of compositions including liposome-encapsulated insulin
InactiveUS20090022784A1Lower blood sugar levelsImprove the quality of lifeElectrotherapyPeptide/protein ingredientsMedicineBULK ACTIVE INGREDIENT
Systems, devices, and methods for delivering one or more active ingredients to intradermal tissues, deep regions of pores, and intradermal tissues in the vicinity of pores. In some embodiments, a composition is provided including a plurality of liposomes including a cationic lipid, and an amphiphilic glycerophospholipid having a saturated fatty acid moiety and an unsaturated fatty acid moiety, and at least one insulin, insulin analog, or insulin derivative.
Owner:TITI ELLEBEAU INC +1
Degradable hydrate accelerant and preparation method and application thereof
ActiveCN104692383ANo pollution in the processRealize industrial greeningCarbon compoundsGlycerolAlginic acid
The invention relates to the technical fields of production and utilization of gas hydrates, in particular to a degradable hydrate accelerant and a preparation method and an application thereof. The degradable hydrate accelerant is prepared from the following raw materials by mass concentration: 12%-18% of glyceryl phosphatide, 20%-27% of alginic acid and the balance of deionized water. The degradable hydrate accelerant provided by the invention is degradable, environment-friendly, free of pollution, low in cost, good in economical performance, safe to use and wide in application range; greening of industry is really realized; the hydrate generation and acceleration effects are good; the hydrate generation pressure can be reduced by 40%-60% within the range of 1-15 DEG C; and the generation time is shortened by 70%-85%.
Owner:泉州职业技术大学
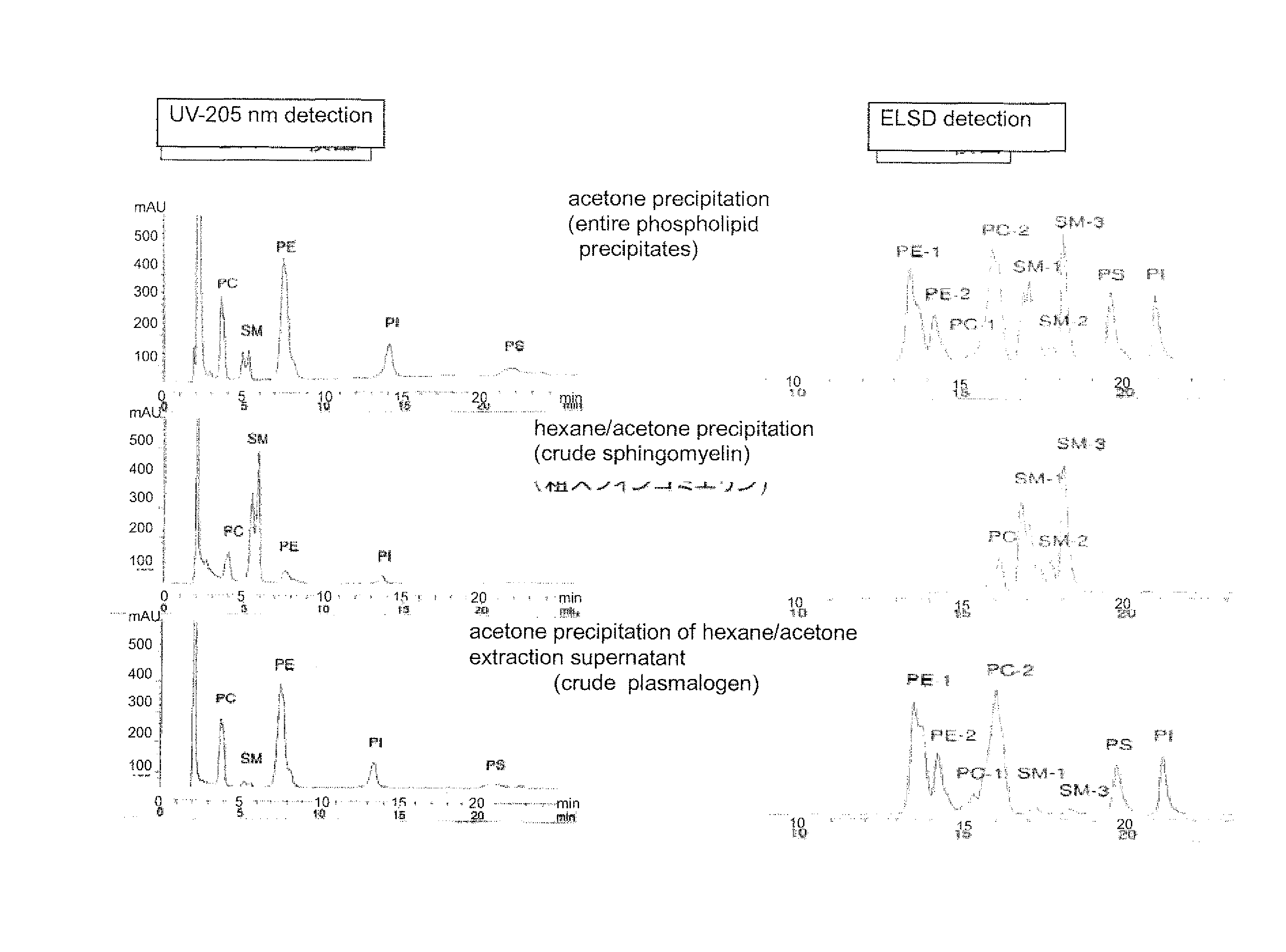
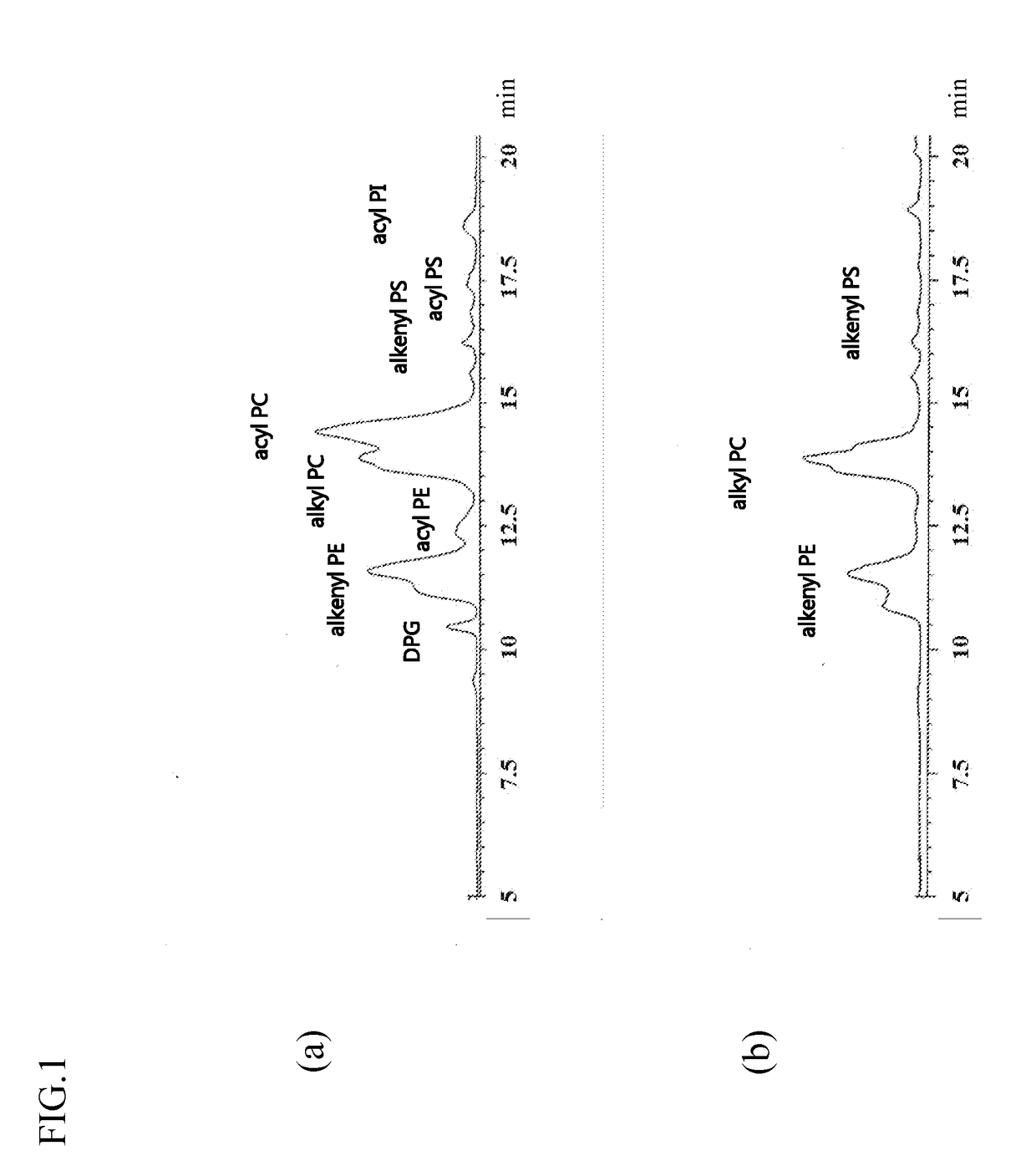
Method for production of highly pure phospholipid, and highly pure sphingomyelin and plasmalogen-type glycerophospholipid produced by the method
ActiveUS20110160471A1Simple procedureHigh yieldFatty oils/acids recovery from wasteFatty acid chemical modificationKetone solventsWater soluble
This invention provides a process for producing a high-purity sphingomyelin and a high-purity plasmalogen-form glycerophospholipid from a biological material by simple procedures at high yields. The process comprises the steps of: (A) subjecting dried total lipids extracted from a biological material to extraction treatment with a specific mixture solution to separate an insoluble portion composed mainly of sphingomyelin and a soluble portion; (B) subjecting the insoluble portion, obtained in said Step (A), to washing treatment with a specific mixture solution to obtain crude sphingomyelin; (C) subjecting the soluble portion, obtained in said Step (A), to washing treatment with a water-soluble ketone solvent to obtain crude plasmalogen-form glycerophospholipid; (D) causing an enzyme to act on the crude sphingomyelin, obtained in said Step (B), to obtain sphingomyelin having a purity of 90% or more; and (E) causing an enzyme to act on the crude plasmalogen-form glycerophospholipid, obtained in said Step (C), to obtain plasmalogen-form glycerophospholipid having a purity of 40% or more.
Owner:MARUDAI FOOD +1
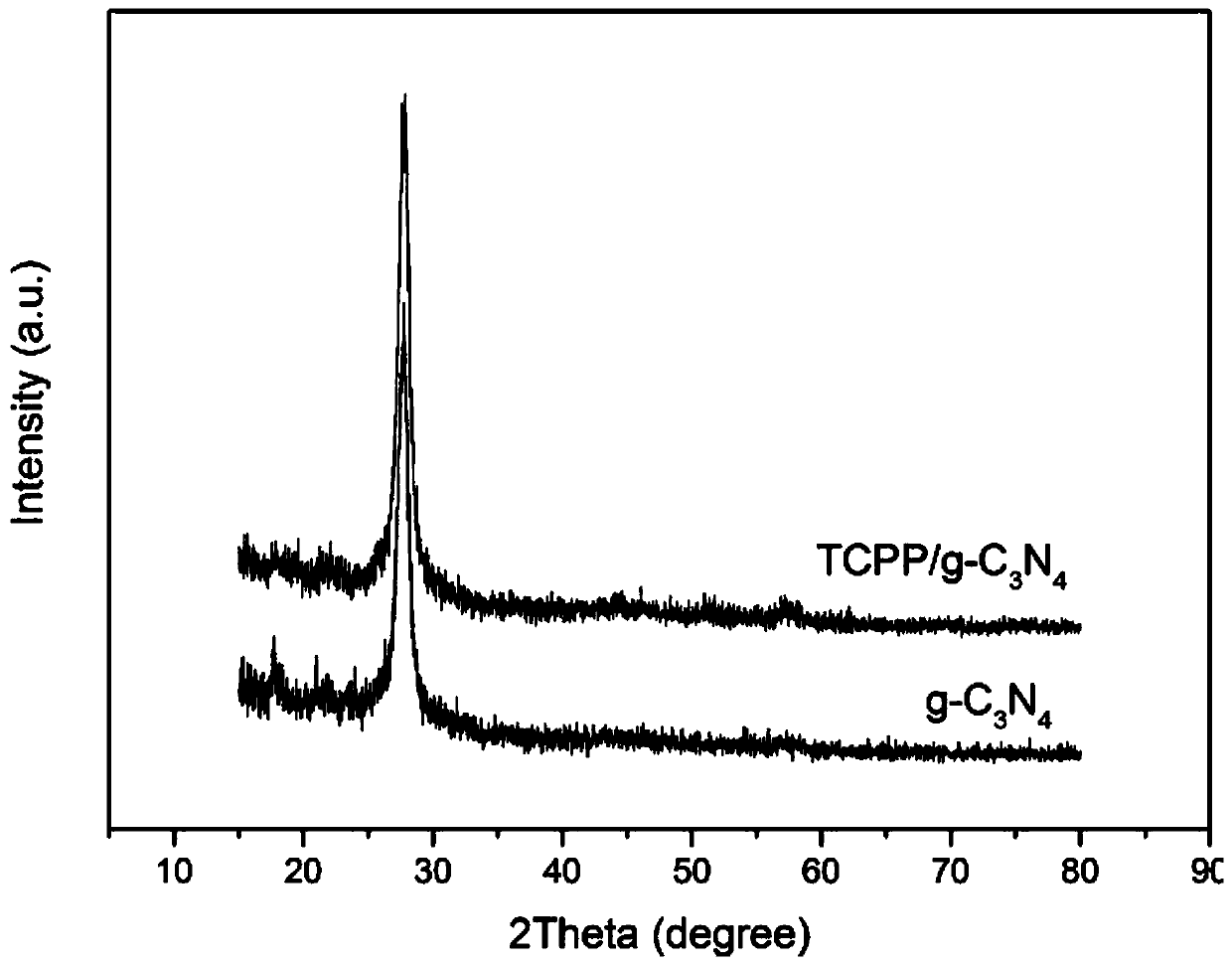
Preparation method of porphyrin/carbon nitride layer-by-layer composite structure photocatalytic nano-composite material
InactiveCN110961150AIncrease migration ratePromote migrationOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationFiberCarbon fibers
The invention discloses a preparation method of a porphyrin / carbon nitride layer-by-layer composite structure photocatalytic nano-composite material. The preparation method comprises the following steps: taking and dissolving melamine and L-alpha glycerylphosphorylcholine in deionized water, carrying out a hydrothermal reaction in a hydrothermal kettle, centrifuging the obtained solution, and washing and drying the obtained reaction product to obtain a solid; adding KOH and a carbon fiber into the solid, performing mixing, putting the obtained mixture into a crucible, putting the crucible intothe resonant cavity of a microwave oven, and performing vacuum heating to obtain a heated product; cooling the heated product to room temperature, and washing, drying and grinding the cooled heated product to obtain a carbon nitride powder; dissolving the carbon nitride powder into anhydrous ethanol to obtain a solution A; adding TCPP into anhydrous ethanol to prepare a solution B; adding the solution B into the solution A to obtain a mixed solution C; and continuously stirring the mixed solution C until the solvent is completely volatilized, and performing drying to obtain the porphyrin / carbon nitride layer-by-layer composite structure photocatalytic nano-composite material. The specific surface area of carbon nitride can be increased, the charge transmission and separation efficiency ofcarbon nitride is improved, and absorption and utilization of carbon nitride on visible light are improved.
Owner:PINGDINGSHAN UNIVERSITY
Glycerophospholipids for the improvement of cognitive functions
ActiveUS20110144063A1Improve cognitive functionBiocideNervous disorderAlpha-Linolenic acidGamma-Linolenic acid
The invention described herein provides a preparation comprising a non-mammalian derived mixture of serine glycerophospholipid conjugates with a specific content and specific conjugation patterns of LA, linolenic acid (alpha-linolenic acid, gamma-linolenic acid) DHA and EPA which depend on utilizing different sources of lipids, and uses of such preparations.
Owner:ENZYMOTEC
Methods for analysis of isomeric lipids
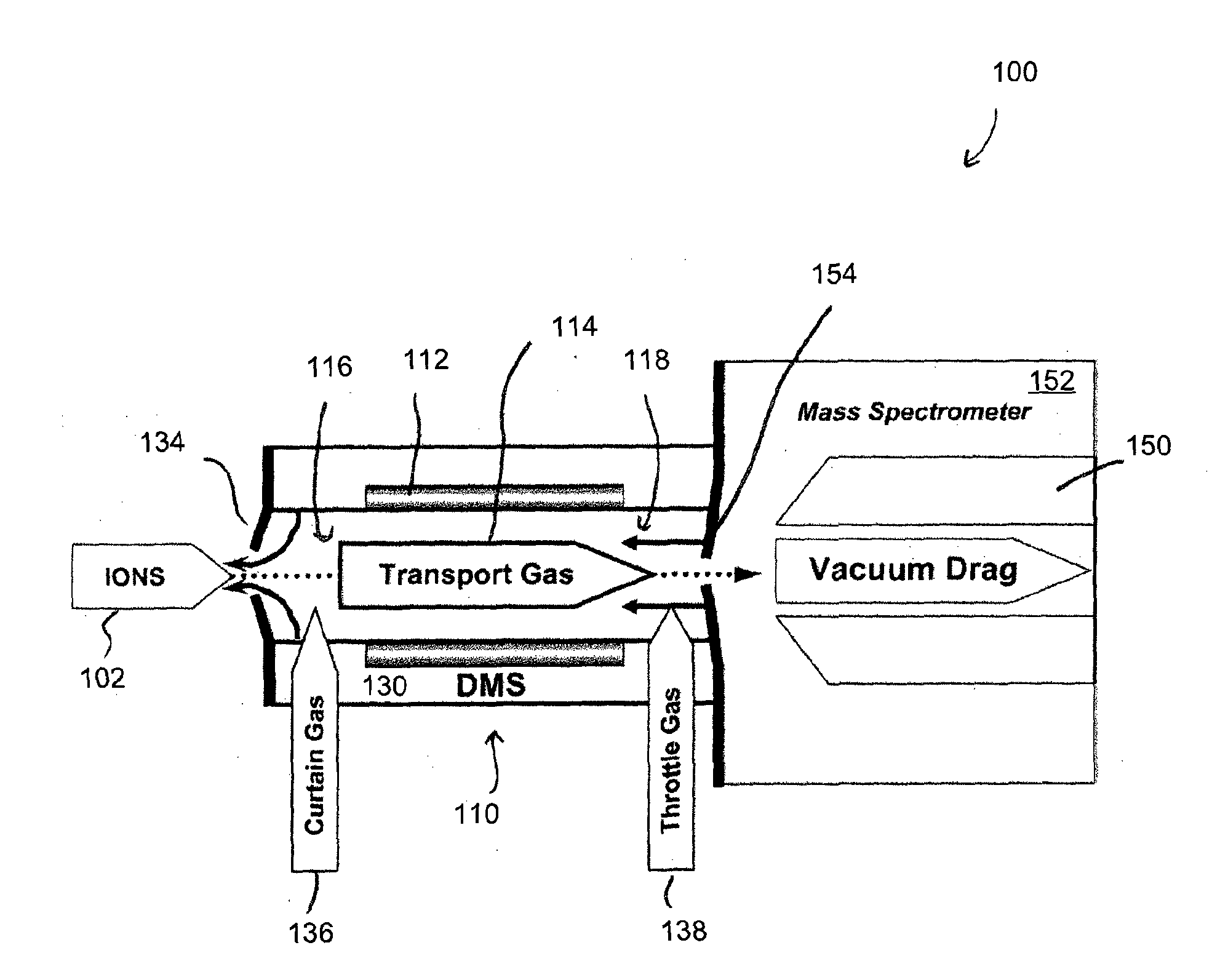
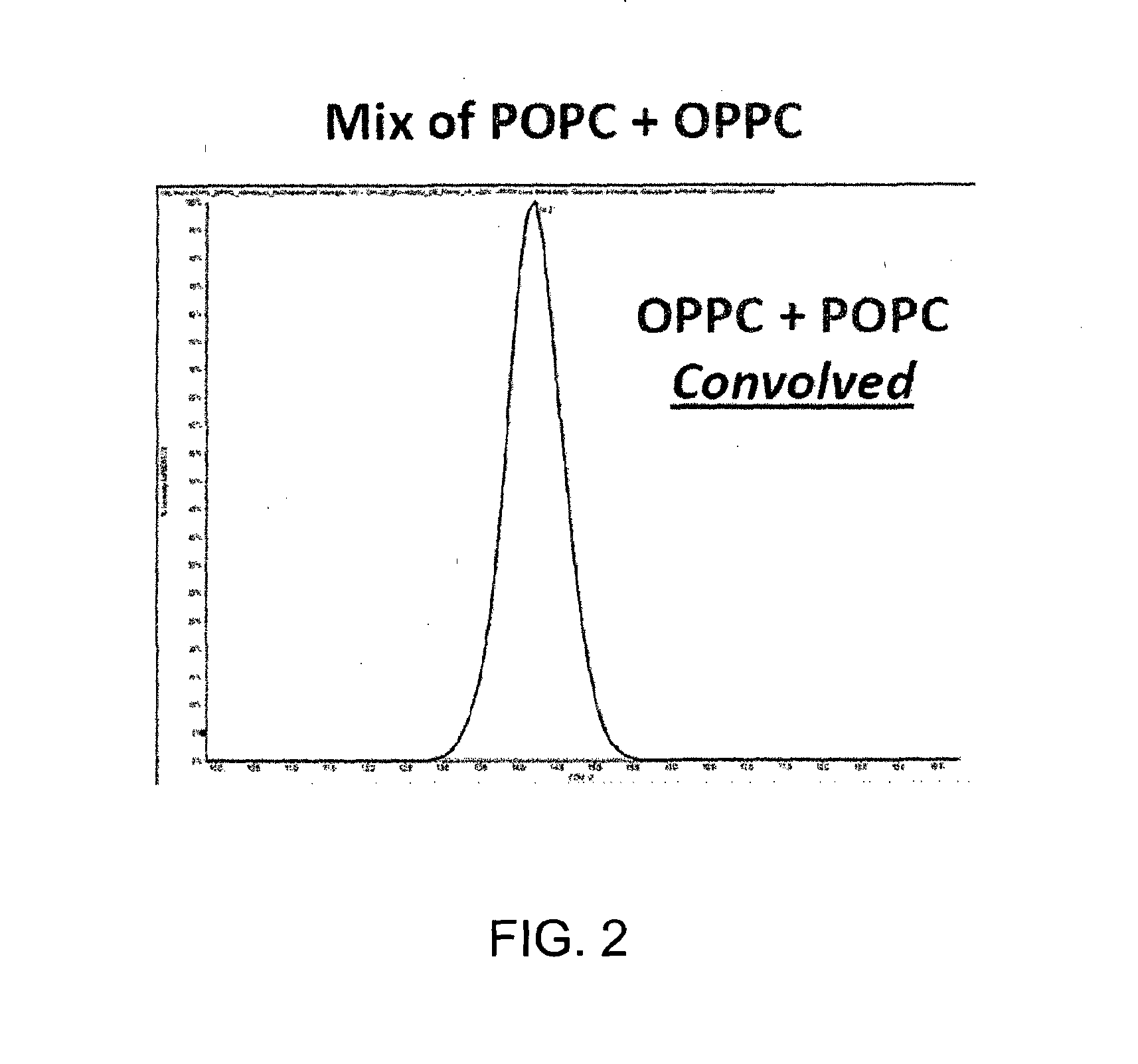
ActiveUS20150316507A1Allow separationEasy to separateSamples introduction/extractionMaterial analysis by electric/magnetic meansLipid formationPolyketide
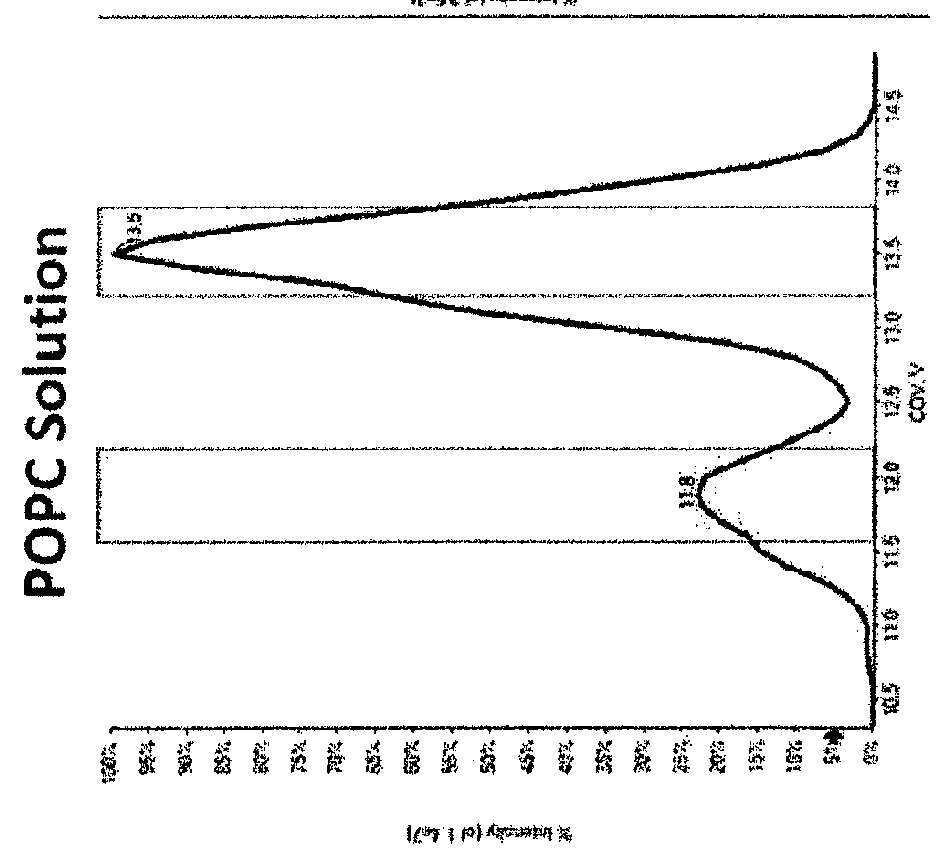
A method for analyzing a sample that contains a plurality of lipid isomers is described that involves forming one or more lipid metal ion adducts and transporting the one or more lipid metal ion adducts through a Mix of POPC+OPPC differential mobility spectrometer to cause separation of the one or more lipid metal ion adducts from each other. The lipid isomers can be chosen, for example, from fatty acids, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, polyketides, sterol lipids, and prenol lipids. Particular examples include phosphatidylcholine regioisomers such as 1-palmatoyl-2-oleoyl-sn-phosphatidylcholine (POPC) and 1-oleoyl-2-palmatoyl-sn-phosphatidylcholine and triacylglycerols containg palmetic and oleic acid groups. The metal chosen can include a cationization reagent that contains sodium, potassium, silver or lithium.
Owner:DH TECH DEVMENT PTE
Glycerophospholipids containing omega-3 and omega-6 fatty acids
InactiveCN103381172AFacilitate cognitionFunction increaseOrganic active ingredientsSenses disorderGlycerolFatty acids.polyunsaturated
Disclosed is a lipid preparation comprising a glycerophospholipid or salt, conjugate and derivatives thereof, particularly phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylglycerol (PG) and phosphatidic acid (PA), and poly-unsaturated fatty acid (PUFA) acyl groups, particularly long-chain poly-unsaturated fatty acid (LC-PUFA) acyl groups such as omega-3 and / or omega-6 acyl groups, wherein said PUFA is covalently bound to said glycerophospholipid. The disclosed preparations possess an improved bioactivity, and are useful in the treatment of various cognitive and mental conditions and disorders and for maintenance of normal functions of brain-related systems and processes, for example ADHD.
Owner:ENZYMOTEC
Methods for analysis of isomeric lipids
ActiveUS9360455B2Allow separationEasy to separateSamples introduction/extractionMaterial analysis by electric/magnetic meansLipid formationPolyketide
A method for analyzing a sample that contains a plurality of lipid isomers is described that involves forming one or more lipid metal ion adducts and transporting the one or more lipid metal ion adducts through a differential mobility spectrometer to cause separation of the one or more lipid metal ion adducts from each other. The lipid isomers can be chosen, for example, from fatty acids, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, polyketides, sterol lipids, and prenol lipids. Particular examples include phosphatidylcholine regioisomers such as 1-palmatoyl-2-oleoyl-sn-phosphatidylcholine (POPC) and 1-oleoyl-2-palmatoyl-sn-phosphatidylcholine and triacylglycerols containing palmetic and oleic acid groups. The metal chosen can include a cationization reagent that contains sodium, potassium, silver or lithium.
Owner:DH TECH DEVMENT PTE
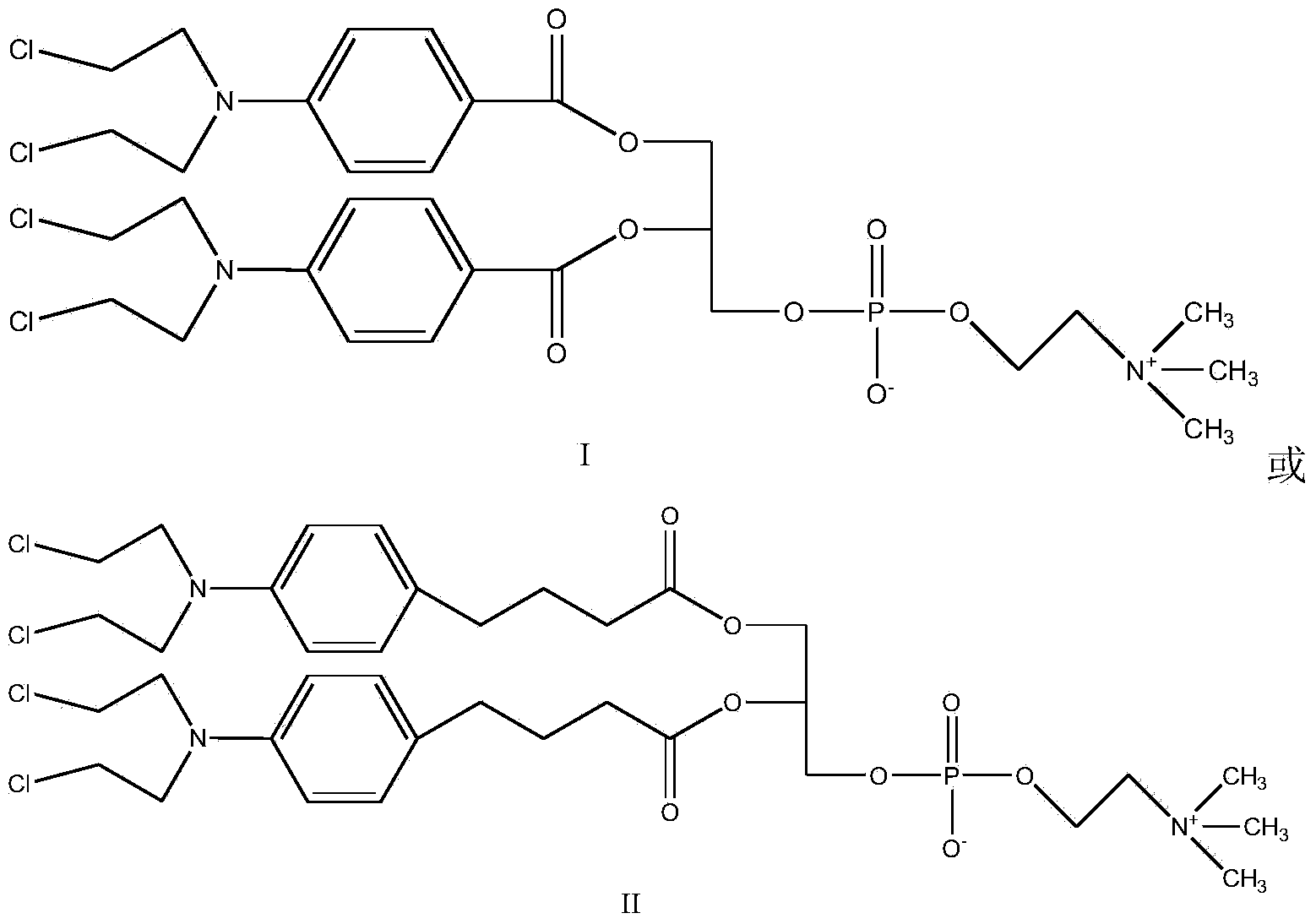
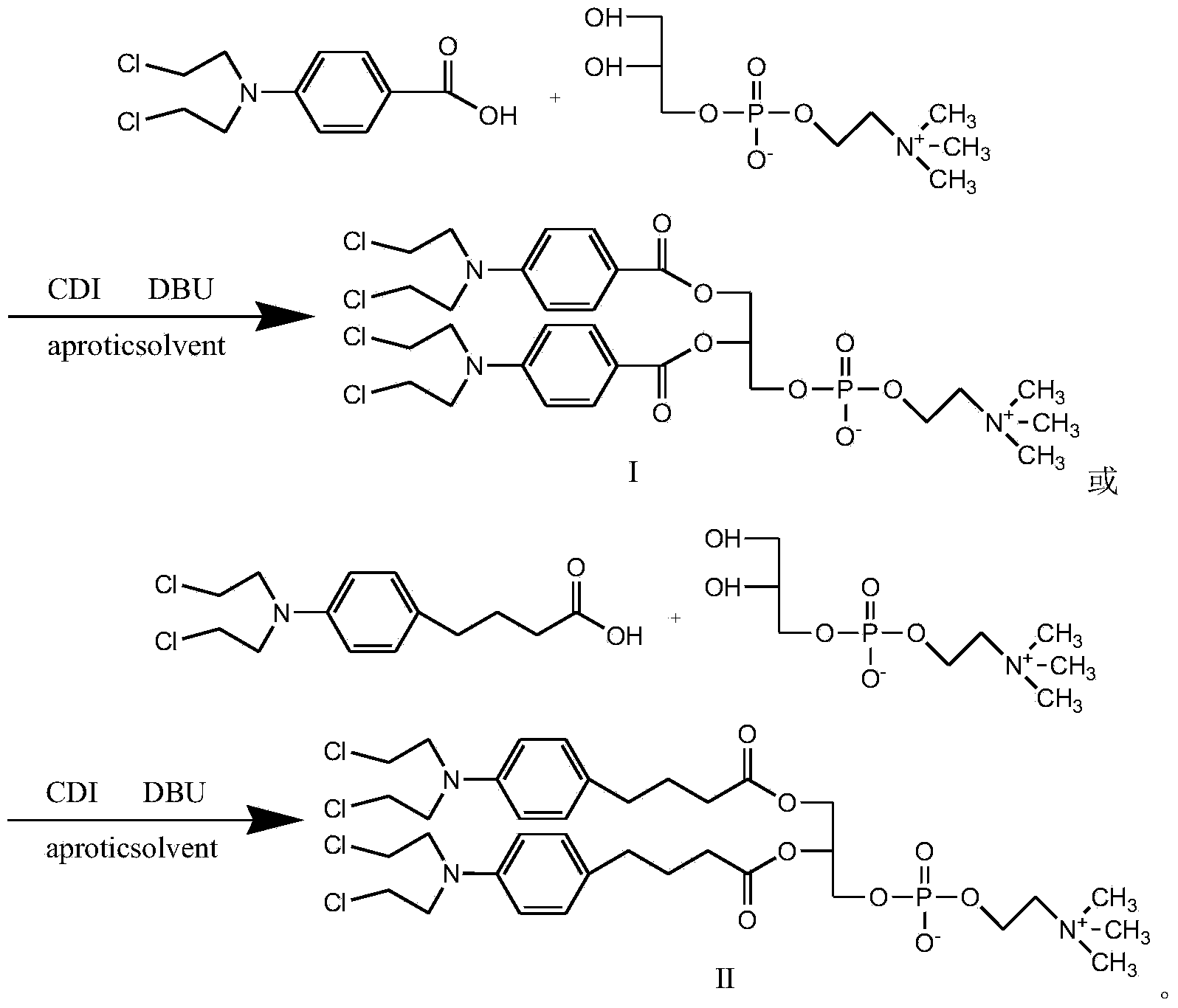
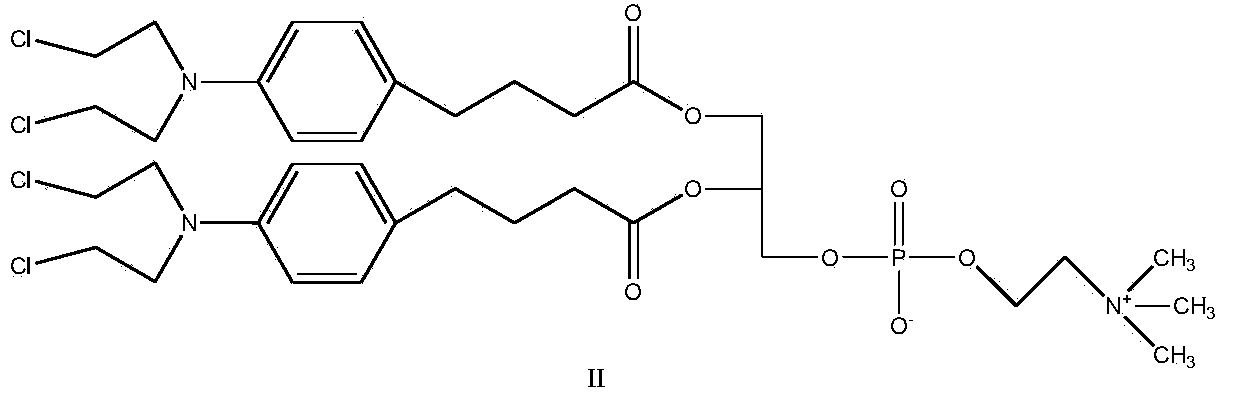
Synthetic method of nitrogen mustard-glycerol phosphatidyl choline compound
InactiveCN104017019AMild reaction conditionsShort reaction timePhosphatide foodstuff compositionsAntineoplastic agentsGlycerolRoom temperature
The invention discloses a synthetic method of a nitrogen mustard-glycerol phosphatidyl choline compound. The synthetic method comprises the following step of reacting at room temperature by taking a nitrogen mustard-type compound and glycerol phosphatidyl choline as raw materials, taking N, N'-carbonyldimidazole and 1, 8-diazabicyclo C11-7-alkene and taking an aprotic solvent as a reaction solvent, thereby preparing the nitrogen mustard-glycerol phosphatidyl choline compound. The method disclosed by the invention is simple to operate, is mild in reaction condition, and is high in yield.
Owner:SOUTHEAST UNIV
Method for producing ether phospholipids
InactiveUS20180127680A1Easy to processHigh purityFatty substance recovery/refiningGroup 5/15 element organic compoundsAlcoholOrganic solvent
[PROBLEM TO BE SOLVED] To provide a method for producing ether phospholipids of high purity by an easy process. [SOLUTION] This production method comprises: (A) a step of extracting total lipids from organisms with non-polar organic solvent and branched alcohol mixture; and (B) a step of reacting the total lipids obtained by the step (A) with phospholipase A1 to decompose mixed diacyl-glycerophospholipids. This makes it possible to produce plasmalogen-type glycerophospholipids or ether phospholipids of high purity by an easy process.
Owner:INST OF RHEOLOGICAL FUNCTION OF FOOD
Method for masking bitterness of composition containing collagen peptide
InactiveUS20170028032A1Reduce bitternessImprove skinDispersion deliveryPeptide/protein ingredientsPeptideChemistry
An object of the present invention is to provide a technique for masking the bitterness of collagen peptide containing compositions.By incorporating glycerophospholipids comprising neutral glycerophospholipids and acidic glycerophospholipids in specified proportions or incorporating sphingoglycolipids in a specified proportion relative to collagen peptides, there can be provided methods for masking the bitterness of collagen peptide containing compositions.
Owner:SUNTORY HLDG LTD
Use of a phospholipase A2 for the preparation of pharmaceutical and/or cosmetic compositions for the local and/or systematic treatment and/or prevention of diseases and/or processes caused by intra- and extracellular pathogens expressing membrane phospholipids
The invention relates to the use of phospholipases, v.gr. sPLA2, as an active agent in the production of pharmaceutical and / or cosmetic compositions for the treatment of diseases caused by micro-organisms, the envelope or membrane of which comprises phospholipids, particularly glycerophospholipids. In particular, said invention relates to the use of sPLA2 from Crotalus durissus terrificus venom as a biocide component for the preparation of compositions for the treatment of viral infections, particularly acquired immunodeficiency states VIH-1 and VIH-2, bacterial and parasitic infections, such as infections caused by plasmodium and leishmania-type parasites.
Owner:COSTA LUIS A +1
Stable polymer micelle medicine carrging system
InactiveCN100589843CHigh drug loadingImprove stabilityMetabolism disorderAntipyreticDrug carrierCarrier system
A stable polymer micella carrier system for medicines is composed of an amphipathic block copolymer including diblock copolymer and triblock copolymer and a phosphatide chosen from glyceryl phosphatide, sphingomyelin, and their derivatives.
Owner:涂家生 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com