Stable polymer micelle medicine carrging system
A technology of mixing polymers and micelles, which is applied in the fields of cardiovascular system diseases, antipyretics, antineoplastic drugs, etc., can solve the problem of low drug loading, and achieve the effect of large drug loading and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The synthesis of embodiment 1 amphiphilic block copolymer mPEG-PDLLA
[0038] Weigh 16g of methyl polyethylene glycol and 24g of lactide, place in a closed reactor, add 50mg of stannous octoate toluene solution (2mL), raise the temperature to 120-140°C under nitrogen flow to melt the solid and remove the toluene , raising the temperature to 150-180° C. for 6 hours. After cooling, a white solid crude product was obtained. After the crude product was dissolved in 1ml of dichloromethane, it was added into 100ml of diethyl ether under stirring, filtered, washed with diethyl ether three times, and the product was vacuum-dried for 24 hours.
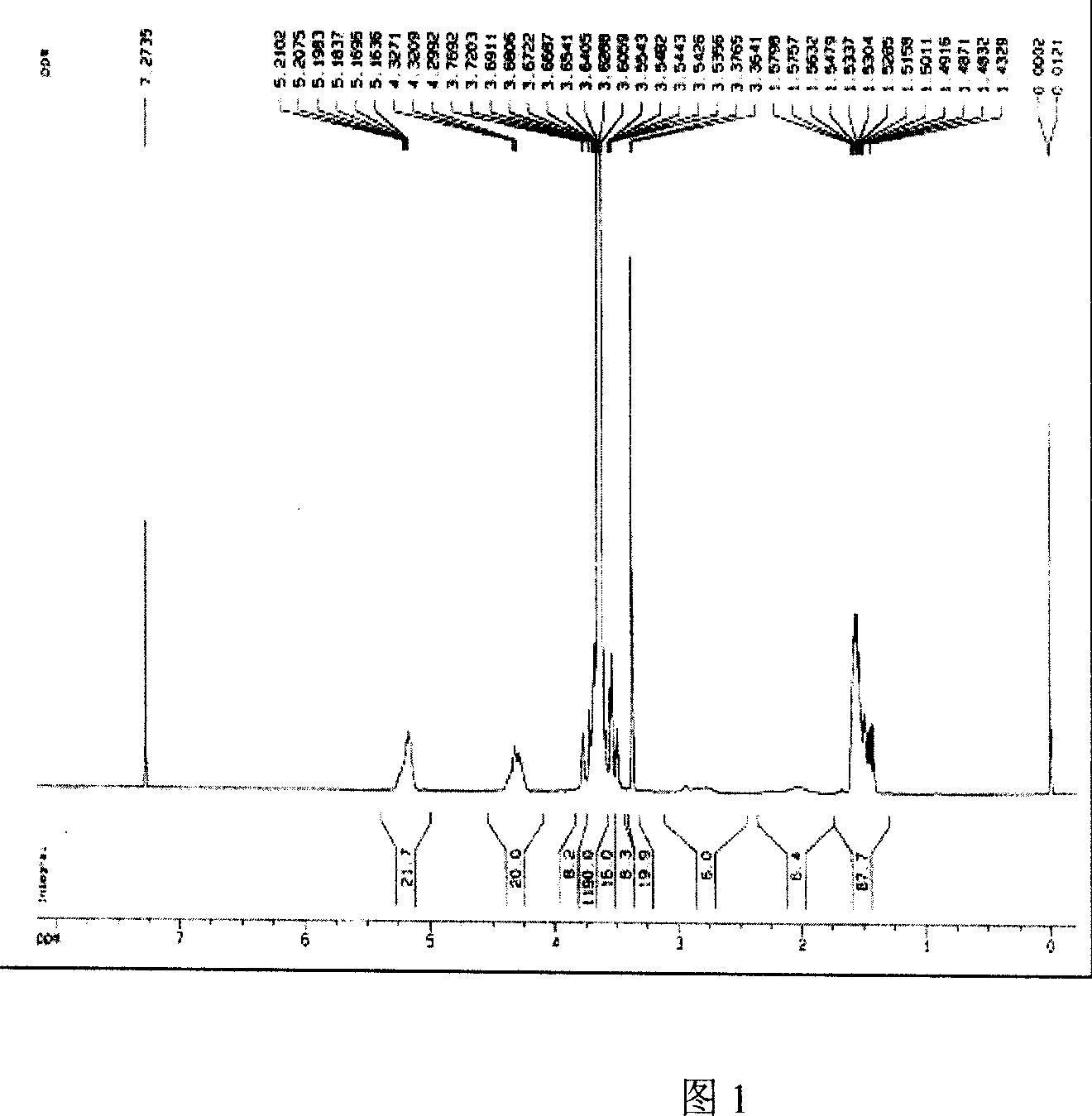
[0039] The NMR (D-chloroform as solvent) spectrum of the product confirmed the mass ratio and molecular weight of methylpolyethylene glycol and polylactic acid in the polymer with the peak area ratio of 5.2ppm (PLA) and 3.6ppm (PEG) (Figure 1).
Embodiment 2
[0040] Embodiment 2 Preparation and particle size determination of etoposide mixed polymer micelles
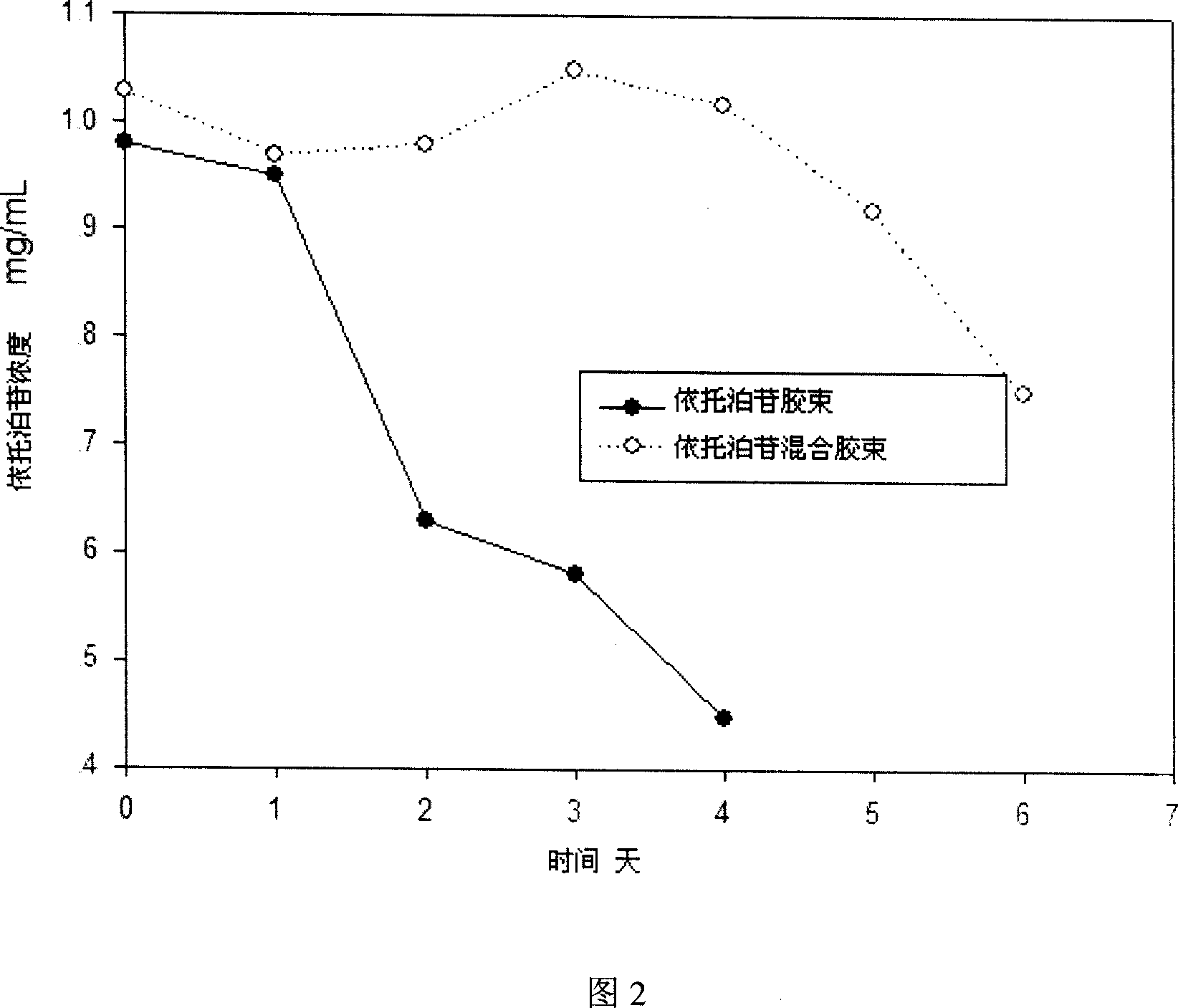
[0041] Weigh 1 g of etoposide, 2 g of mPEG-PDLLA, and 100 mg of lecithin into a flask, add 200 ml of acetonitrile, and stir until dissolved. Slowly evaporate acetonitrile under vacuum at 60°C (rotary evaporator). After no obvious acetonitrile evaporates, increase the temperature and vacuum to continue removing acetonitrile to obtain a colorless gel. Add water for injection at 60°C to 150ml, and hydrate to obtain a mixed polymer micelle solution with light blue opalescence. The product is sterilized by filtration with a 0.22 mm microporous membrane and then freeze-dried to obtain etoposide mixed polymer micelles (etoposide, mPEG-PDLLA and lecithin, 30 mg etoposide per bottle).
[0042] Weigh 400mg of etoposide and 1gmPEG-PDLLA respectively into a flask, add 200ml of acetonitrile, and stir until dissolved. Slowly evaporate acetonitrile under vacuum at 60°C (rotary evaporator)....
Embodiment 3
[0044] The preparation of embodiment 3 paclitaxel mixed polymer micelles
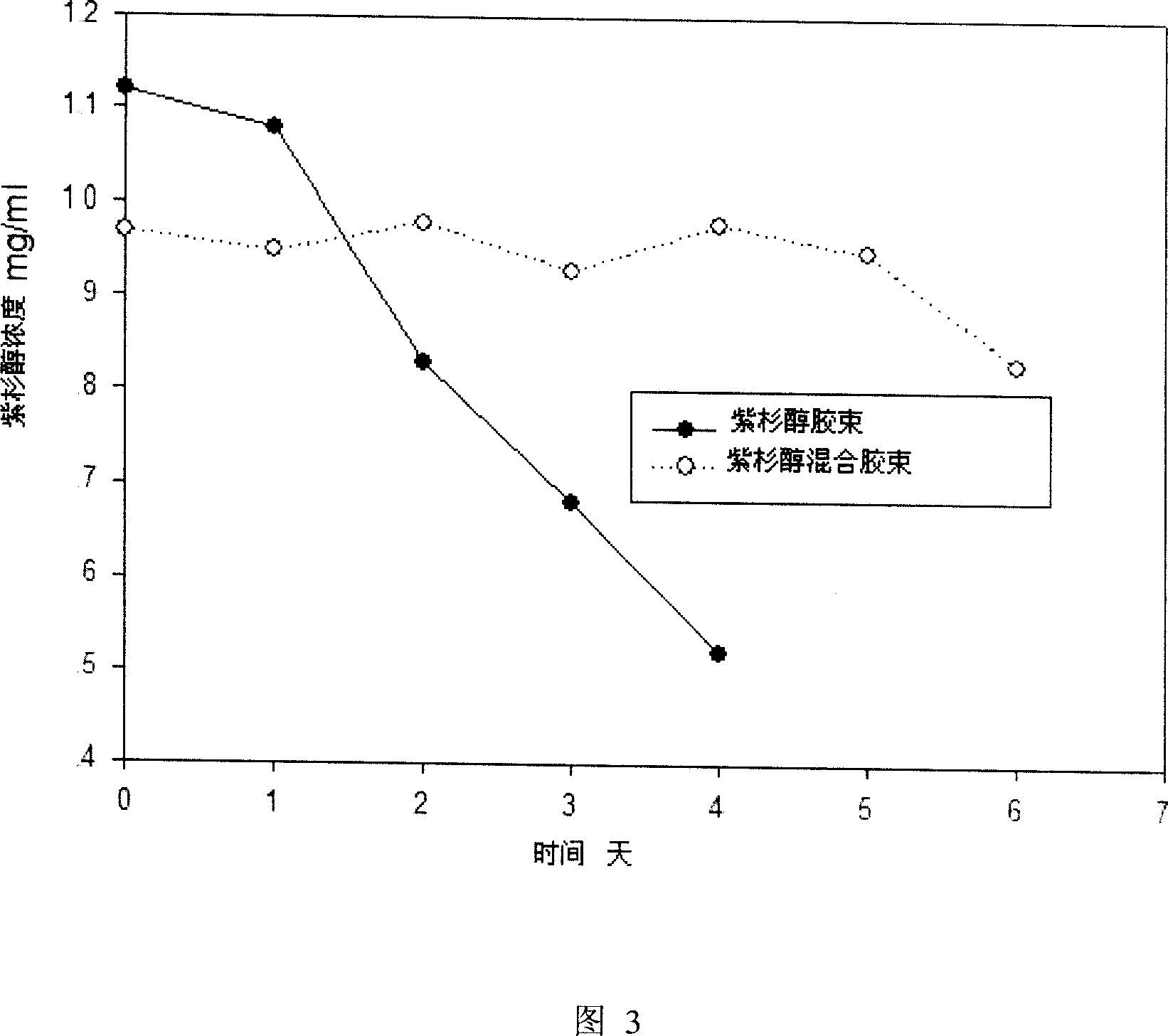
[0045] Weigh 300mg of paclitaxel, 1g of mPEG-PDLLA and 100mg of lecithin into a flask, add 150ml of acetonitrile, and stir until dissolved. Slowly evaporate acetonitrile under vacuum at 60°C (rotary evaporator). After no obvious acetonitrile evaporates, increase the temperature and vacuum to continue removing acetonitrile to obtain a colorless gel. Add water for injection at 60°C to 150ml, hydrate to obtain a mixture with light blue opalescence, and homogenize with a high-speed homogenizer at 12000rpm for 30 seconds to obtain a mixed polymer micelle solution. The product is sterilized by filtration with a 0.22mm microporous membrane and then freeze-dried to obtain paclitaxel mixed polymer micelles (paclitaxel, mPEG-PDLLA and lecithin, 30mg paclitaxel per bottle).
[0046] Weigh 300mg of paclitaxel and 1gmPEG-PDLLA respectively into a flask, add 150ml of acetonitrile, and stir until dissolved. Slowly e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com