Synthetic method of nitrogen mustard-glycerol phosphatidyl choline compound
A technology of glycerol phosphatidylcholine and synthesis method, which is applied in the field of drug synthesis, can solve the problems of low reaction efficiency, high reaction temperature, and long time, and achieve the effects of solvent saving, short reaction time, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
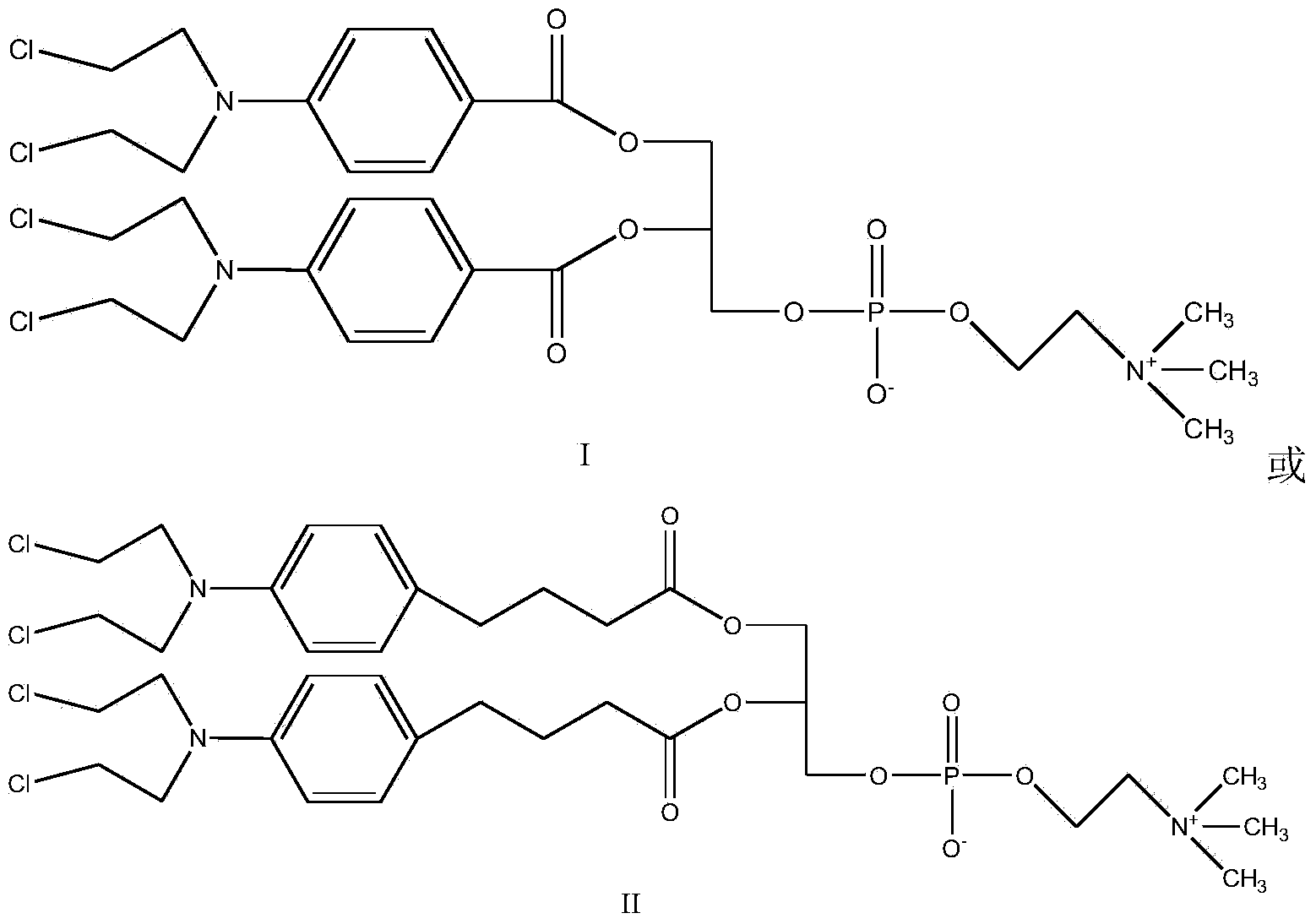
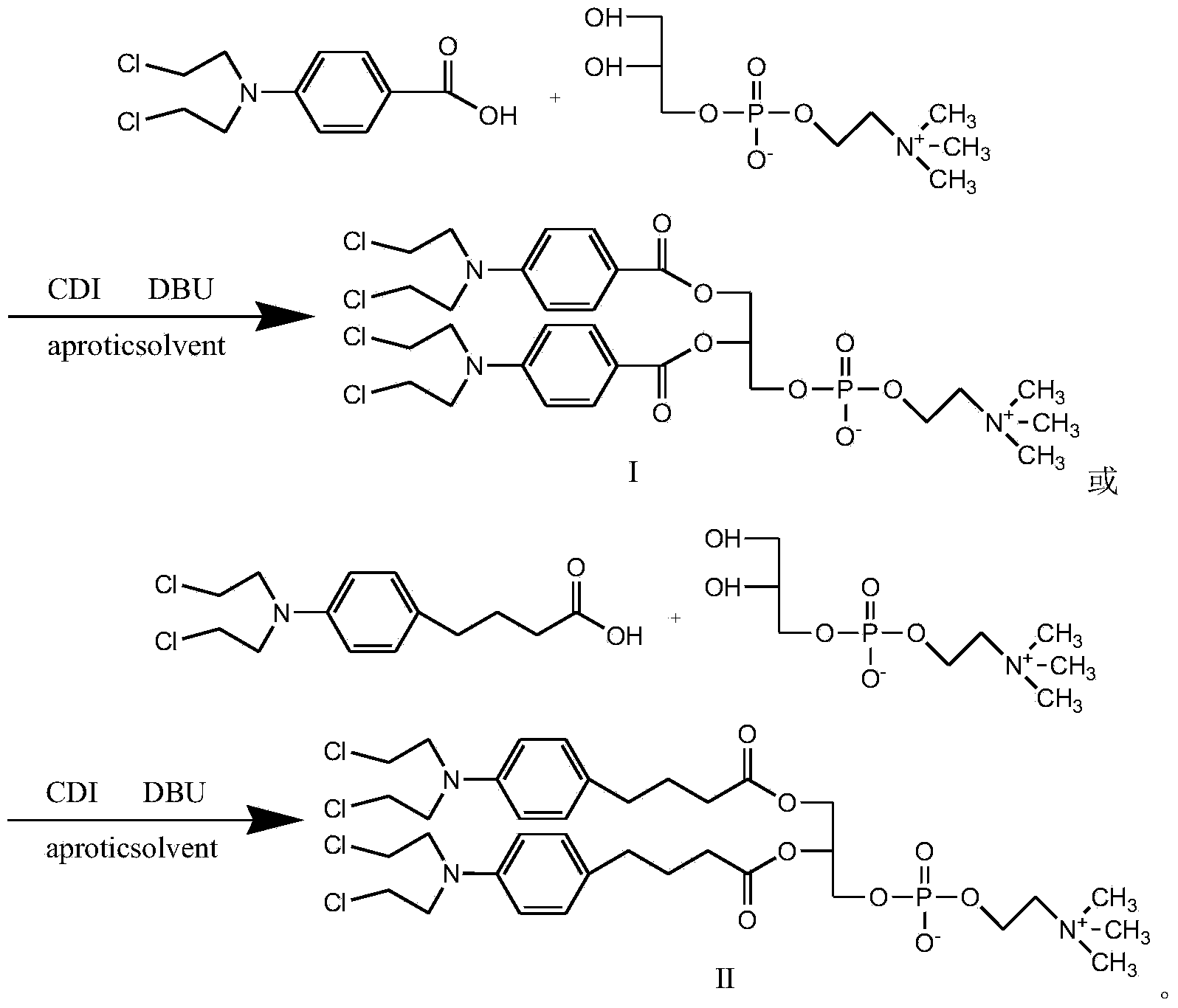
[0024] Synthesis of Compound I
[0025]
[0026] In round bottom flask, add benzoic acid mustard (1mol), CDI (0.5mol), dichloromethane is made solvent, room temperature reaction 0.5 hour, after TLC (thin layer chromatography) shows that reaction is finished, adds GPC (0.2 mol) in the system. mol) and DBU (0.8mol), reacted for 1 hour, TLC showed that after the reaction was completed, a white or yellowish product was obtained by precipitation and column separation, with a yield of 90%.
Embodiment 2
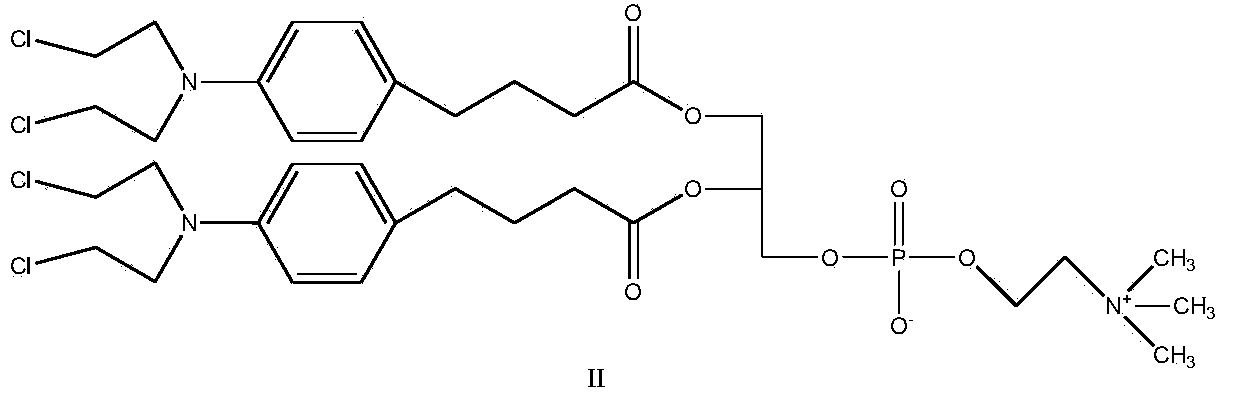
[0028] Synthesis of Compound II
[0029]
[0030] Add chlorambucil (1mol), CDI (1.3mol) and THF as solvent in the round bottom flask, react at room temperature for 1 hour, after TLC shows that the reaction is complete, add GPC (0.2mol) and DBU (1.1mol) to the system ), reacted for 4 hours, TLC showed that after the reaction was completed, a white or off-yellow product was obtained by precipitation and column separation, and the yield was 80%.
Embodiment 3
[0032] Synthesis of Compound I
[0033] In the round bottom flask, add benzoic acid mustard (1mol), CDI (1mol), and ethylene dichloride is used as solvent, react at room temperature for 3 hours, after TLC shows that the reaction is complete, add GPC (0.5mol) and DBU (1mol) in the system ), reacted for 3 hours, TLC showed that after the completion of the reaction, a white or off-yellow product was obtained by precipitation and column separation, and the yield was 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com