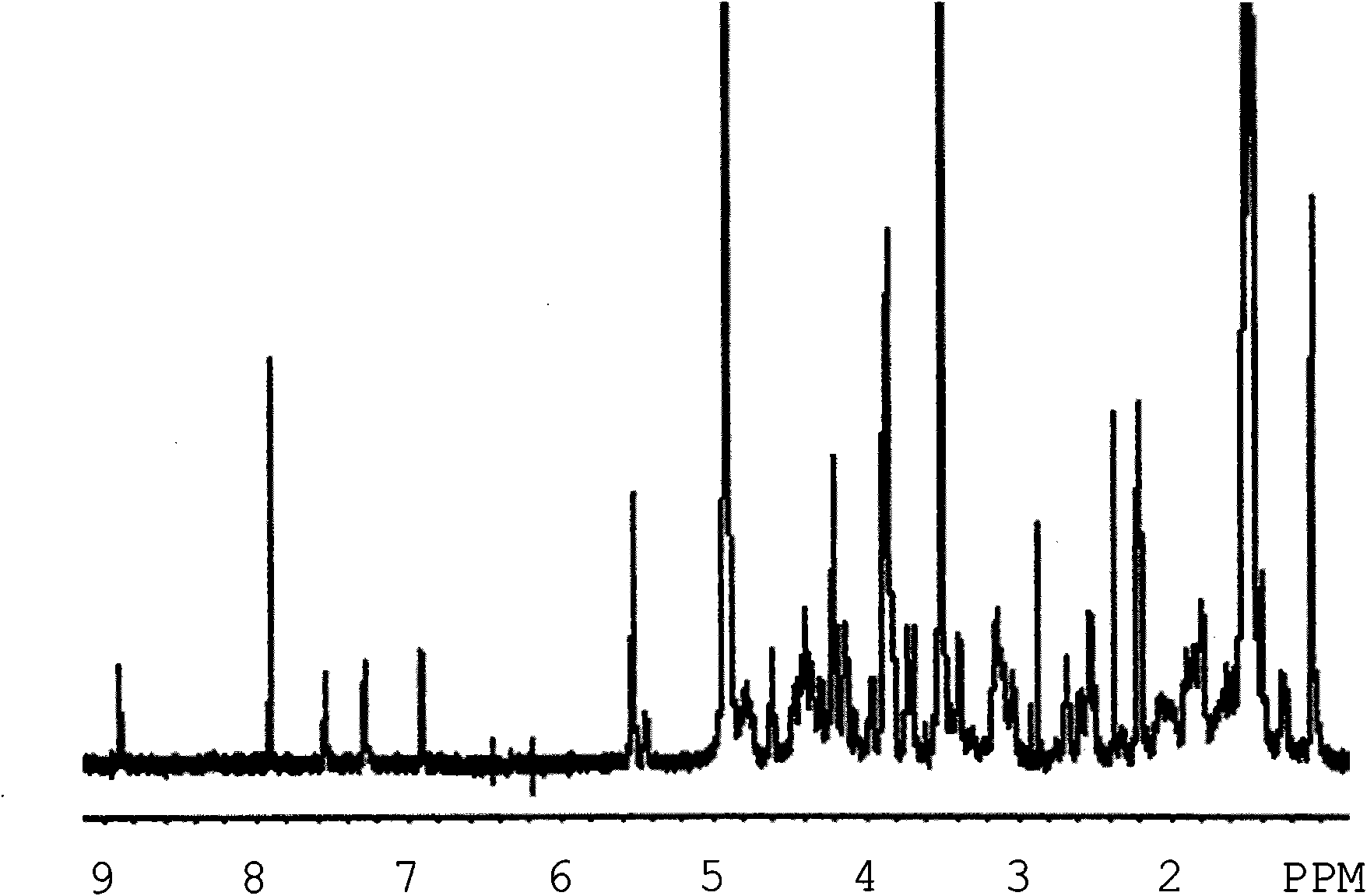
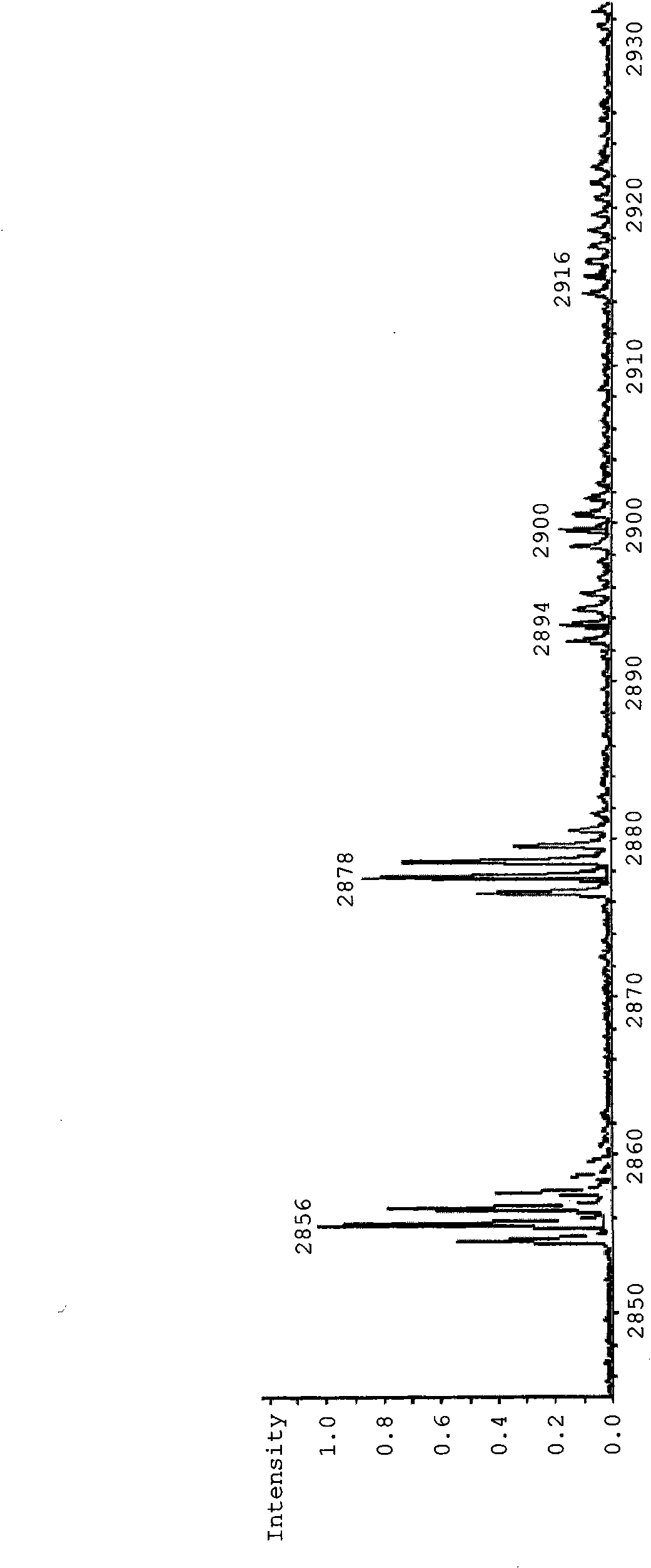
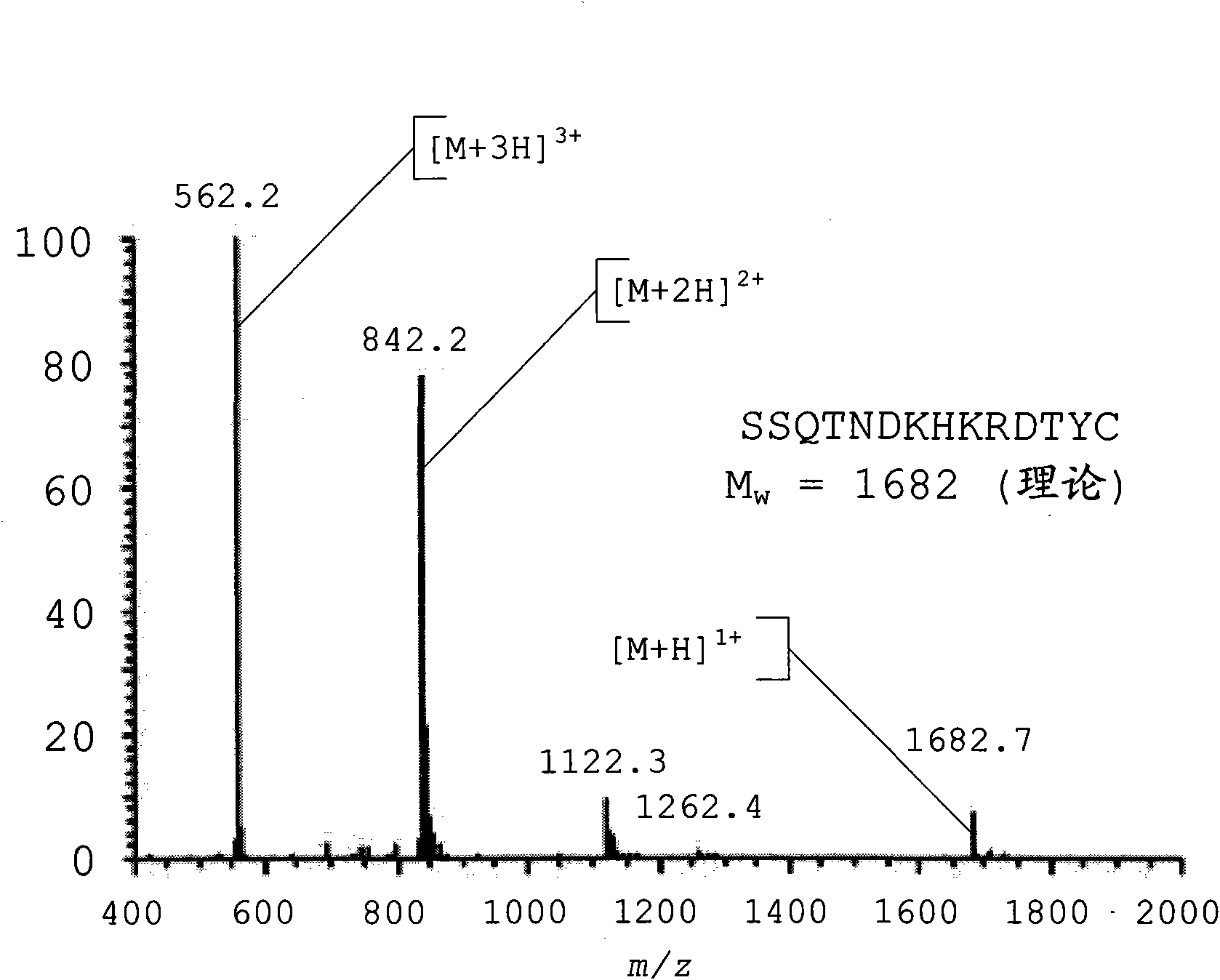
Peptide-lipid constructs and their use in diagnostic and therapeutic applications
A constructive, reactive technique for peptide-lipid constructs for diagnostic and therapeutic use, the field of construction of methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0228] In general, the present invention provides a structure (L-S-) i F(-S-L) j The peptide-lipid structure of which:
[0229] F is a peptide;
[0230] S is a spacer covalently linking F to L;
[0231] L is a lipid selected from the group consisting of diacyl-glycerides and dialkyl-glycerides, including glycerophospholipids;
[0232] i and j are each 0 or 1;
[0233] As well as the use of these peptide-lipid constructs in diagnostic and therapeutic applications.
[0234] When i is 0 and j is 1, the peptide-lipid construct has the structure:
[0235] F-S-L
[0236] When i is 1 and j is 0, the peptide-lipid construct has the structure:
[0237] L-S-F
[0238] When S is linked to F through the amino terminus of the peptide, this configuration is represented by the structure or substructure L-S-F.
[0239] When S is linked to F through the carboxyl terminus of the peptide, this configuration is represented by the structure or substructure F-S-L.
[0240] When S is linke...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com