Sphingolipid-polyalkylamine-oligonucleotide compounds
一种聚烷基胺、寡核苷酸的技术,应用在基因治疗、药物组合、基因工程等方向,能够解决使用受限细胞毒性阳离子脂质等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
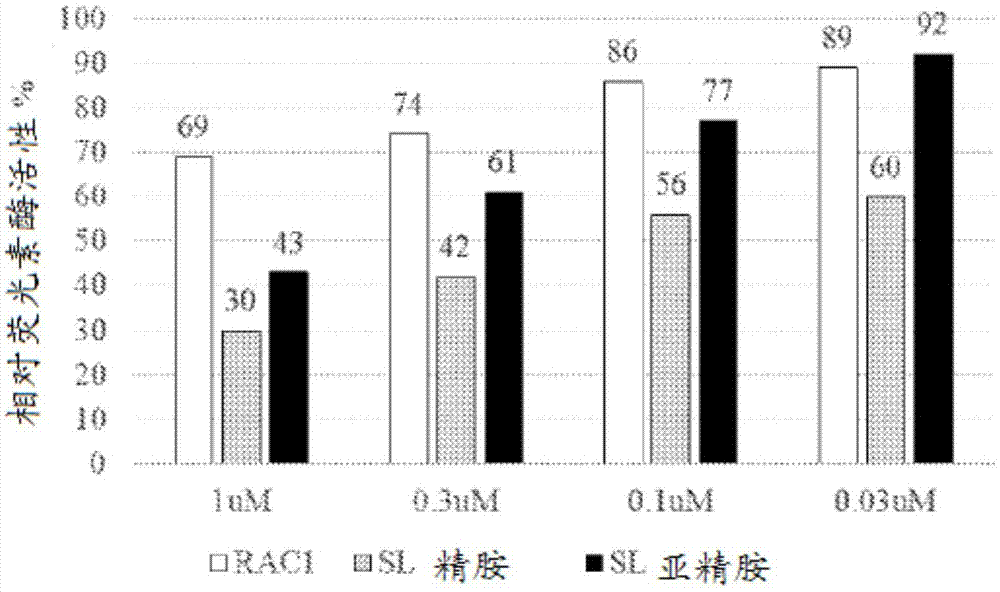
Image
Examples
Embodiment 1
[0310] The selection and generation of the sense strand and the antisense strand of embodiment 1.dsRNA
[0311] 18-mer and 19-mer sequences of potential dsNAs are generated using a proprietary algorithm and the known sequence of the target gene. The antisense strand sequence generated by this method is completely or substantially complementary to the target mRNA sequence segment. In some embodiments, the antisense sequence is fully complementary to the corresponding segment of mRNA sequence. In general, double-stranded nucleic acid molecules with specific sequences selected for in vitro testing are specific for both human and a second species such as rat, mouse non-human primate or rabbit genes.
[0312] Exemplary compounds target Rac1 (Homosapiensras-related C3 botulinum toxin substrate 1 (rho family, small GTP-binding protein Rac1) (RAC1), transcript variant Rac1, mRNA) gi|156071503|ref|NM_006908.4|( SEQ ID NO: 1); PLK1 (Homosapienspolo-like kinase 1) gi|34147632|ref|NM_...
Embodiment 2
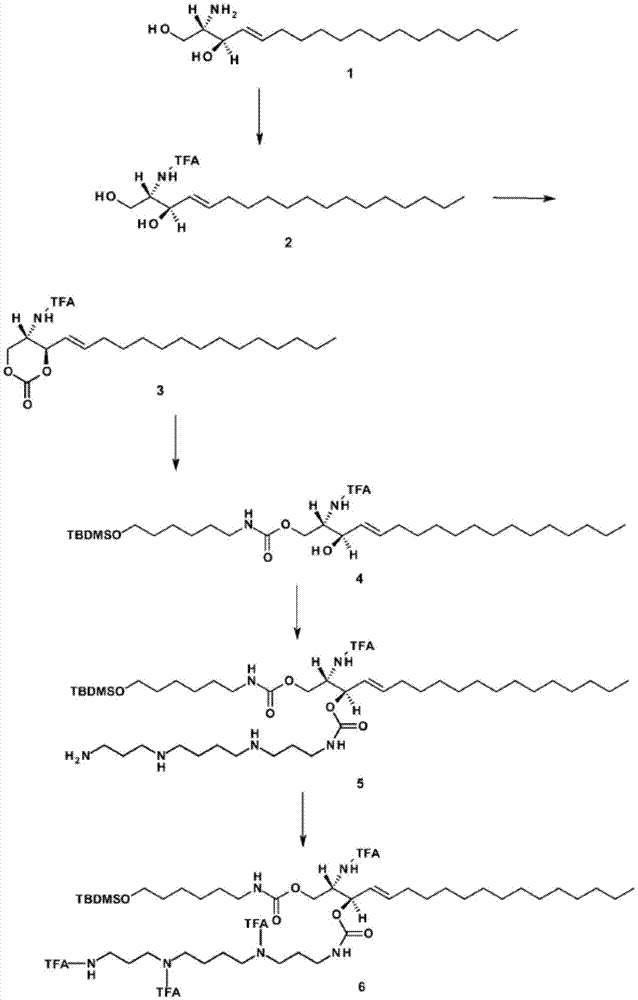
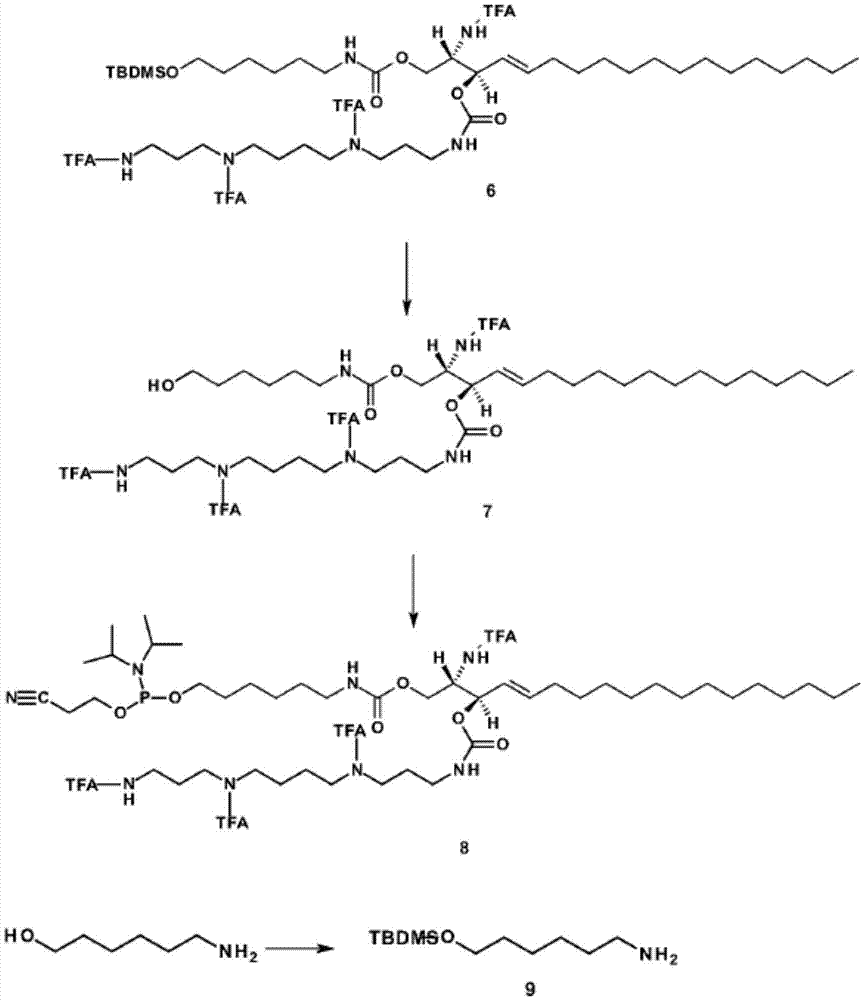
[0357] Embodiment 2: the synthesis of sphingolipid-spermine / spermidine phosphoramidite
[0358] figure 1 A scheme for the synthesis of sphingolipid-spermine / spermidine-phosphoramidites is shown. Abbreviations: TFA: trifluoroacetoxy; TBDMS: tert-butyldimethylsilyl; CC-column chromatography; MeOH-methanol; THF-tetrahydrofuran; DSC-Di(succinate)carbonate; DMAP-4-di methylaminopyridine; DCM-4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran; TBAF-tetra-n-butylammonium fluoride.
[0359] Details of the synthetic steps are provided below:
[0360] Compound 2
[0361] To a solution of D-erythro-sphingosine (1 g of compound 1) in MeOH (16 mL) was added ethyl trifluoroacetate (0.6 mL) and triethylamine (0.93 mL). The mixture was stirred for 16 hours, then the solvent was evaporated to dryness under reduced pressure to afford crude compound 2. The crude product was purified by CC (silica, DCM / MeOH as eluent).
[0362] Compound 3
[0363] Compound 2 (1 g) was disso...
Embodiment 3
[0376] Example 3: Synthesis of chimeric oligonucleotides
[0377] Incorporation of sphingolipid-polyalkylamine phosphoramidites into oligonucleotides during synthesis by conjugation to oligonucleotides, in particular into oligonucleotides that can be used to generate antisense oligonucleotide compounds or double-stranded RNA nucleic acid molecules Antisense and / or sense strand, including siRNA, siNA, anti-miR and miRNA.
[0378] For large-scale synthesis (20 μmol), sphingolipid-polyalkylamine phosphoramidites (300 mg) were dissolved in acetonitrile (1.65 ml, 0.15M). The sphingolipid-polyalkylamine coupling was performed twice (coupling time 10 min for each coupling step).
[0379] Cleavage and deprotection of siRNA-sphingolipid-polyalkylamine
[0380] For oligonucleotide strands (e.g., siRNA strands) bound to resin (344 mg), add NH in a sealed tube 4 OH (33% in water): Methylamine (33% in EtOH) (v / v; total 3.44ml) and incubated in a hot zone at 65°C for 3.5 hours. After ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com