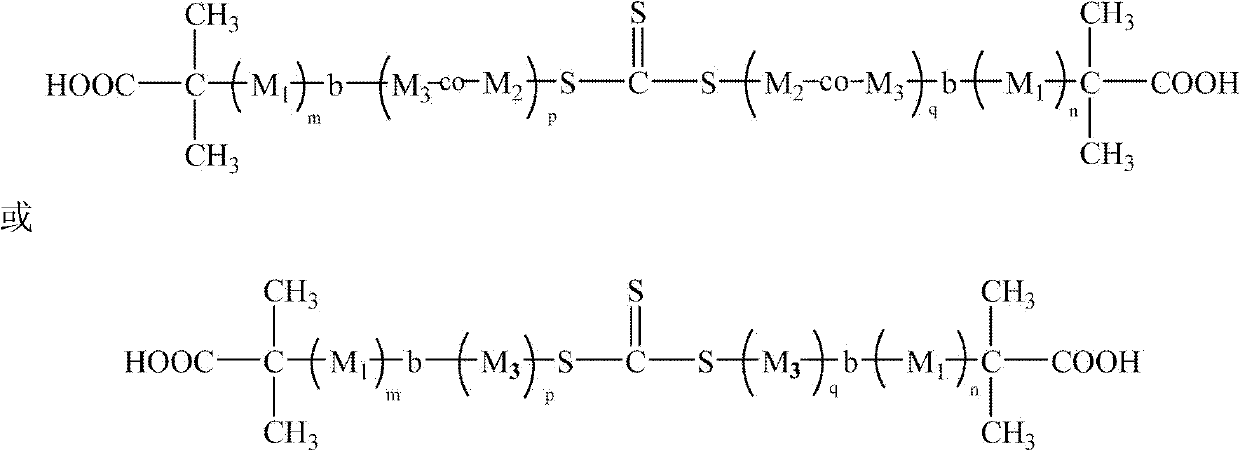Patents
Literature
990results about How to "Good physical and chemical stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
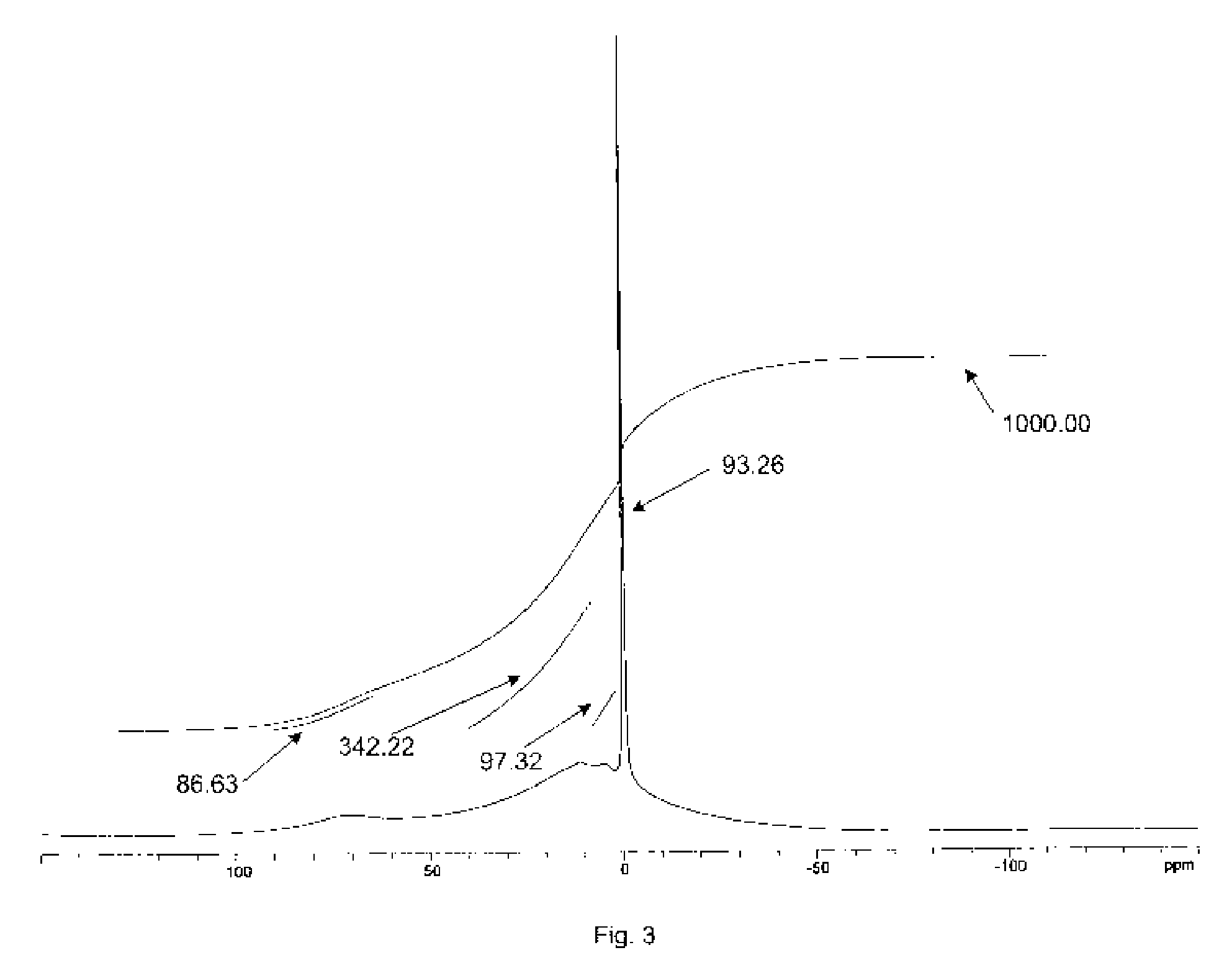
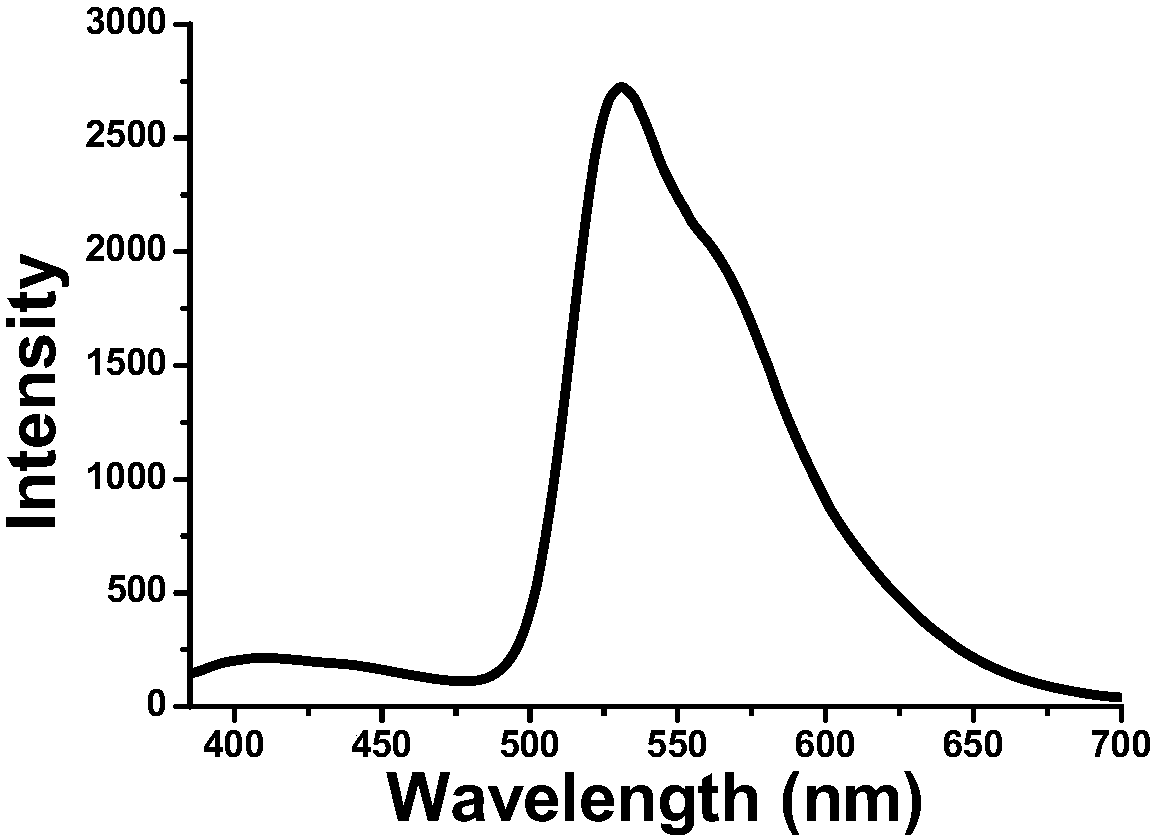
Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide
InactiveUS20110064811A1Improve solubilityGood physical and chemical stabilityBiocidePowder deliveryFormamideOxoquinolines
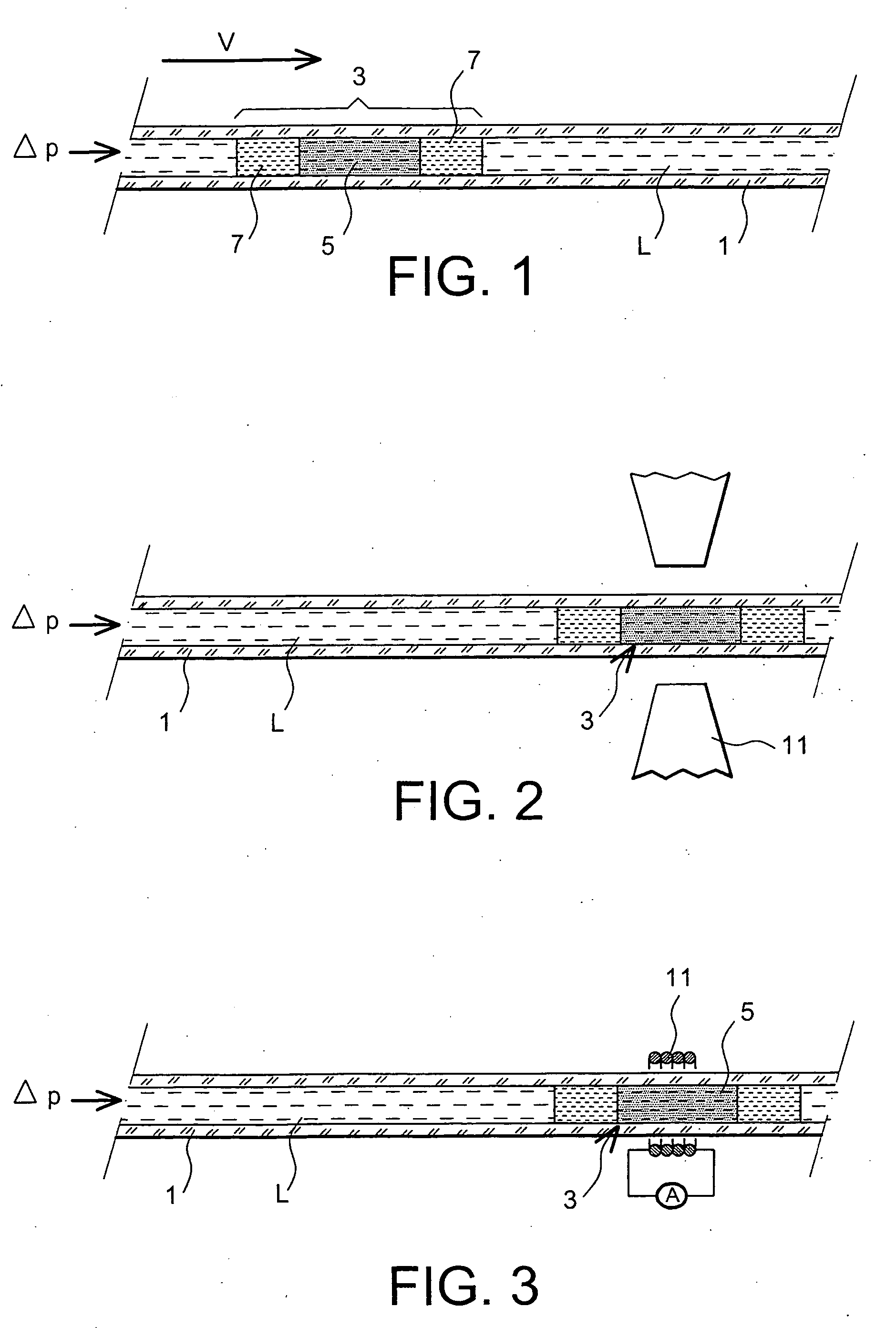
The present invention relates to solid state forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide (Compound 1), pharmaceutical compositions thereof and methods therewith.
Owner:VERTEX PHARMA INC
Solid lipid particles, particles of bioactive agents and methods for the manufacture and use thereof
InactiveUS6207178B1Suppresses decrease in specific surface areaImprove bioavailabilityBiocideCosmetic preparationsLipid formationLipid particle
The present invention is in the area of administration forms and delivery systems for drugs, vaccines and other biologically active agents. More specifically the invention is related to the preparation of suspensions of colloidal solid lipid particles (SLPs) of predominantly anisometrical shape with the lipid matrix being in a stable polymorphic modification and of suspensions of micron and submicron particles of bioactive agents (PBAs); as well as to the use of such suspensions or the lyophilizates thereof as delivery systems primarily for the parenteral administration of preferably poorly water-soluble bioactive substances, particularly drugs, and to their use in cosmetic, food and agricultural products. SLPs and PBAs are prepared by the following emulsification process: (1) A solid lipid or bioactive agent or a mixture of solid lipids or bioactive agents is melted. (2) Stabilizers are added either to the lipid or bioactive agent and to the aqueous phase or to the aqueous phase only depending on their physicochemical characteristics. Stabilizers may also be added or exchanged after homogenization. (3) Drugs or other bioactive substances to be incorporated into the SLPs may be melted together with the lipids if the physicochemical characteristics of the substance permit or may be dissolved, solubilized or dispersed in the lipid melt before homogenization. (4) The aqueous phase is heated to the temperature of the melt before mixing and may contain for example stabilizers, isotonicity agents, buffering substances, cryoprotectants and / or preservatives. (5) The molten lipid compounds and the bioactive agents are emulsified in an aqueous phase preferably by high-pressure homogenization.
Owner:PHARMACIA AB
Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide
ActiveUS8410274B2Good physical and chemical stabilityPromote dissolutionBiocideSenses disorderFormamideOxoquinolines
Owner:VERTEX PHARMA INC
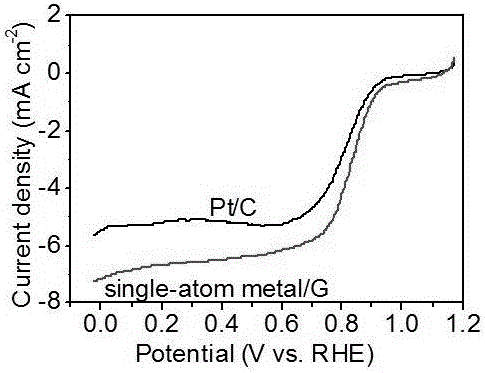
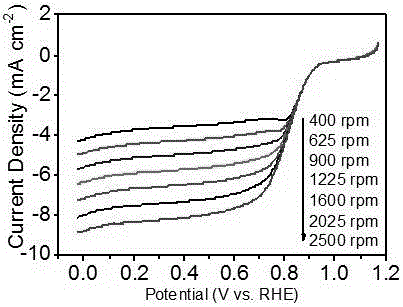
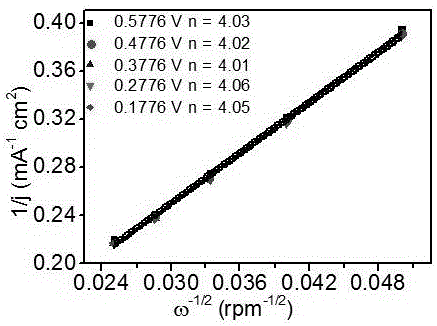
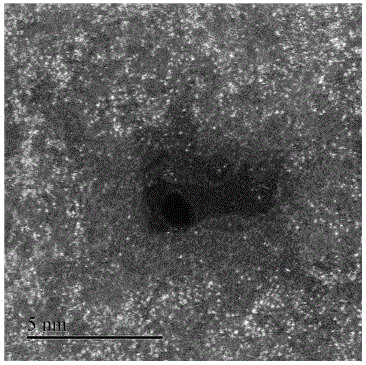
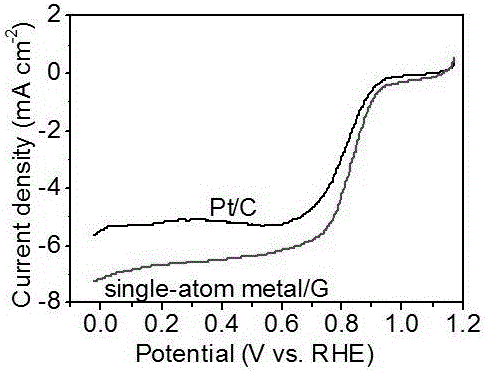
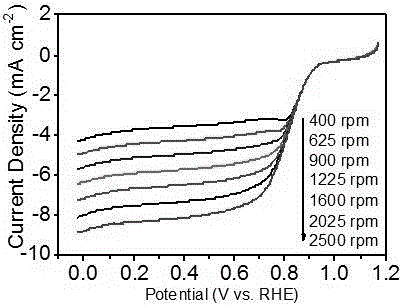
Monodisperse metal atom/graphene composite catalyst and preparation method and application thereof
ActiveCN106694007ALow costSimple stepsCell electrodesCatalyst activation/preparationCvd grapheneChemistry
The invention discloses a monodisperse metal atom / graphene composite catalyst and a preparation method and application thereof. The catalyst is formed by loading monodisperse metal atoms on / in graphene, wherein the content of the monodisperse metal atoms is 0.0001wt%-5.0wt%; the content of heteroatom doped graphene is 95wt%-99.9999wt%; and the content of heteroatom in graphene is 0wt%-90wt%. The monodisperse metal atom / graphene composite catalyst is obtained through an electrochemical method, and the preparation method is simple, easy to operate and control, low in cost, high in efficiency, good in quality, high in security and favorable for industrial production. The composite catalyst has the characteristics of high activity and good stability when being applied to electro-catalytic oxygen reduction, and has better comprehensive performance and good application prospects relative to commercial 20wt% of Pt / C.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
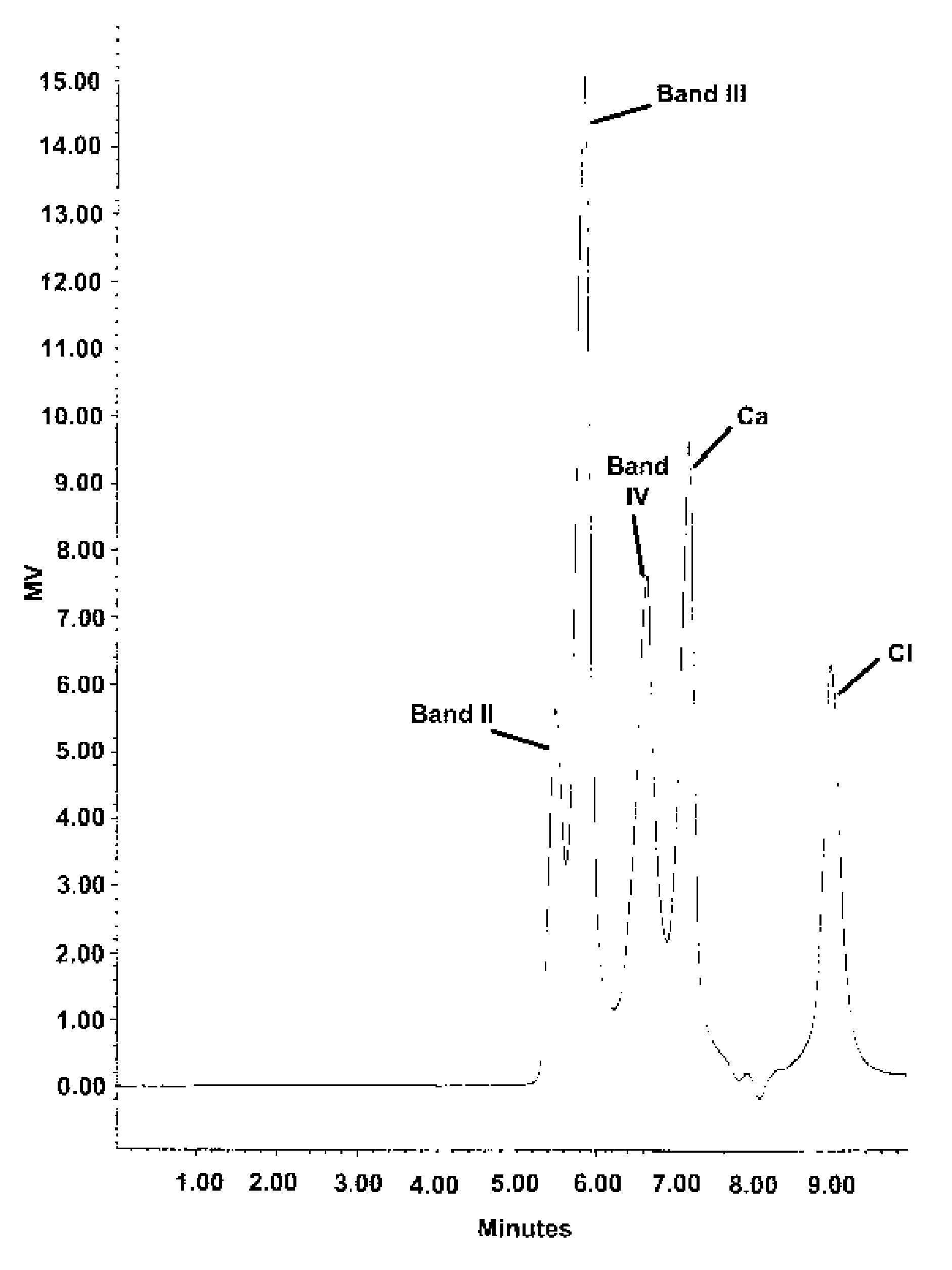
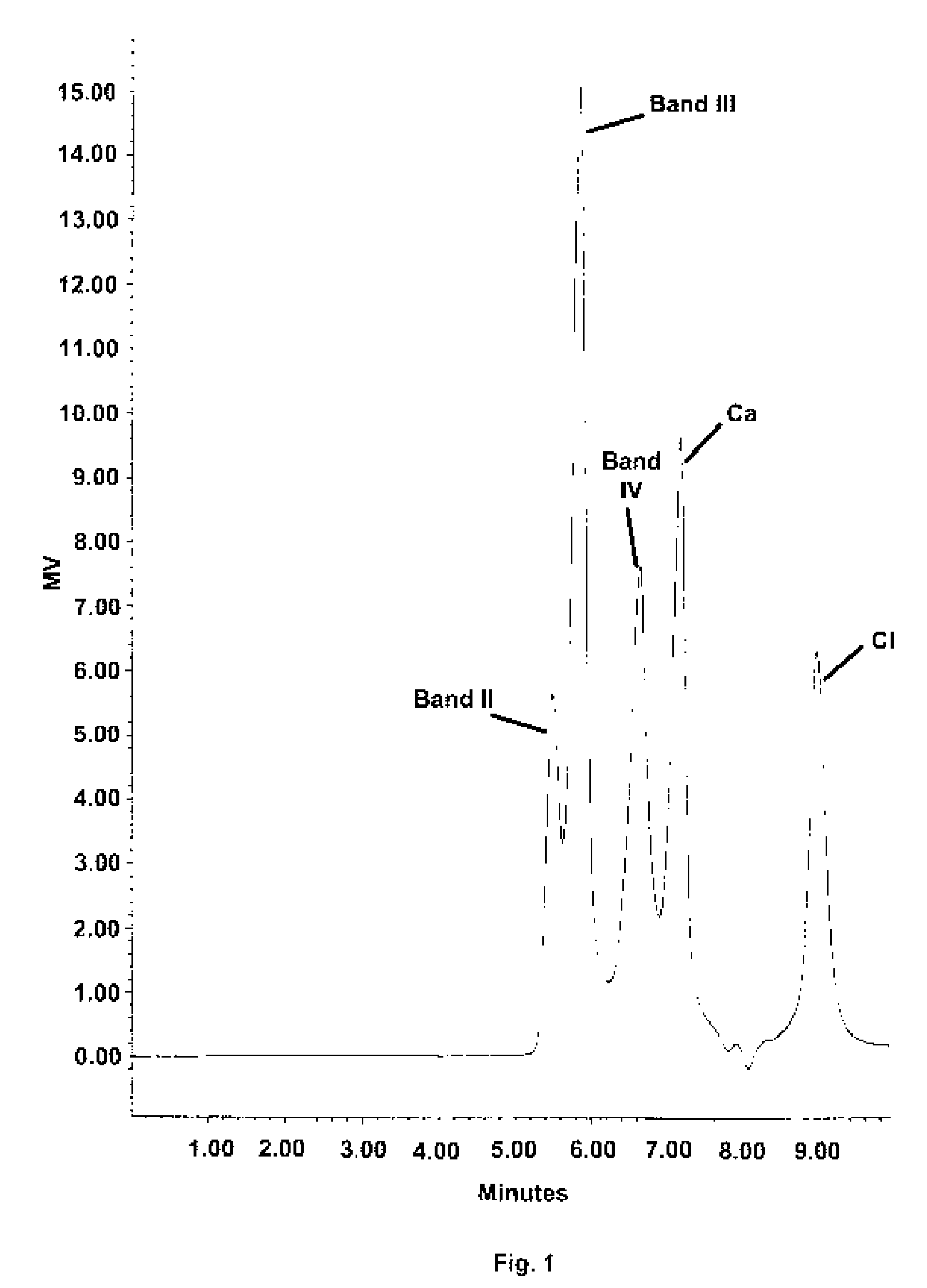
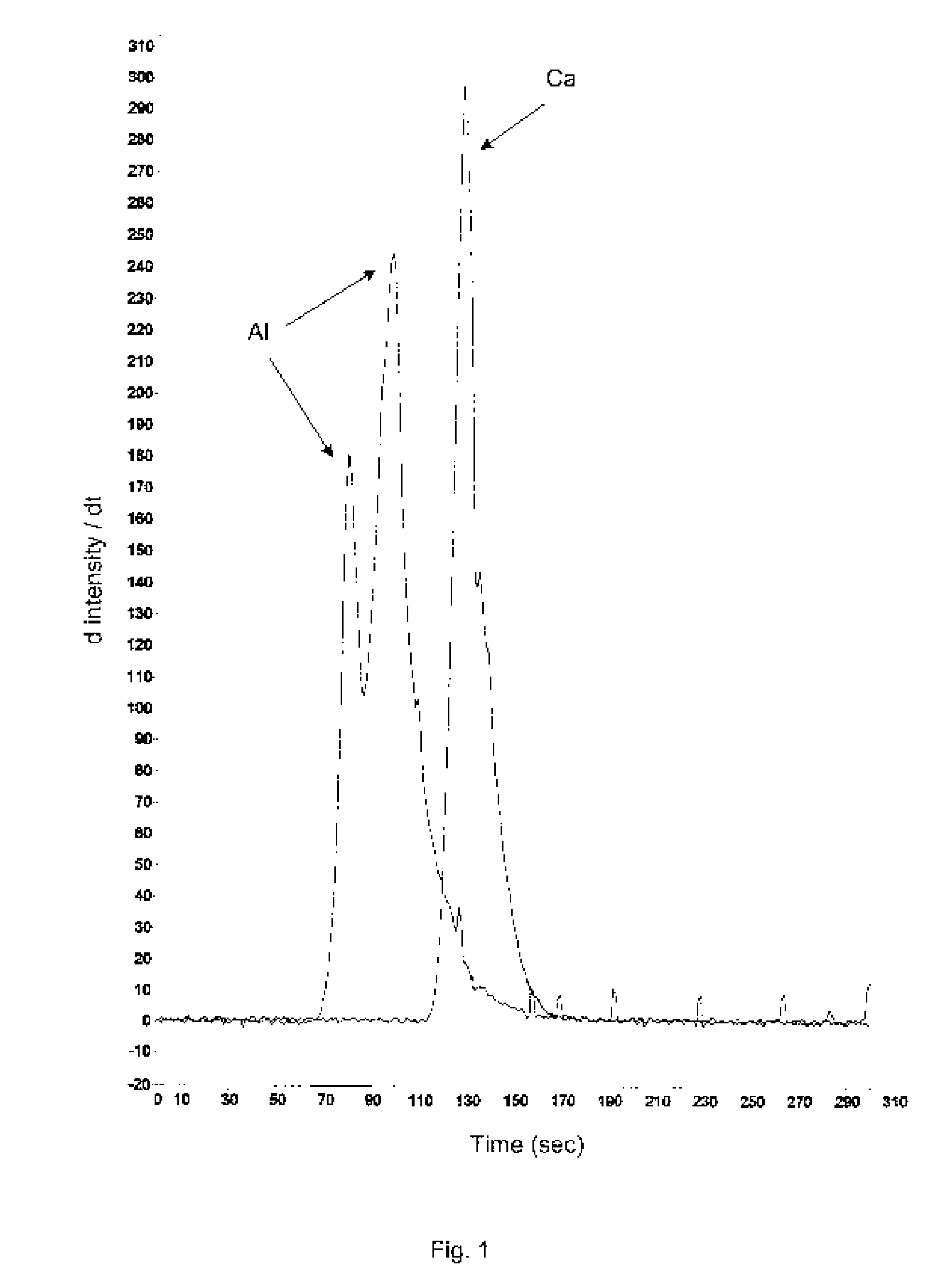
Stable buffered aluminum compositions having high HPLC bands iii and iv containing calcium/strontium
InactiveUS20070196303A1Efficiently dryEnhance efficacyCosmetic preparationsToilet preparationsNitrite concentrationAluminium
A novel aluminum antiperspirant active composition of enhanced efficacy is provided. The composition is activated by both heat and acid and characterized by having high HPLC Band III and high HPLC Band IV, prepared by the addition of an acid such as AlCl3 to (1) the aluminum calcium and / or strontium betaine solutions, where the calcium and / or strontium is derived from a soluble salt such as calcium and / or strontium chloride or nitrate and (2) the aluminum calcium and / or strontium glycine and / or betaine solutions, where calcium and / or strontium is from strongly alkaline calcium / strontium oxide or hydroxide. The novel aluminum compositions derived according to the invention have HPLC Band I of less than 2%; a Band III of at least 20%; a Band III / II ratio of at least about 0.7; and a Band IV of at least 20%.
Owner:SUMMIT RES LAB
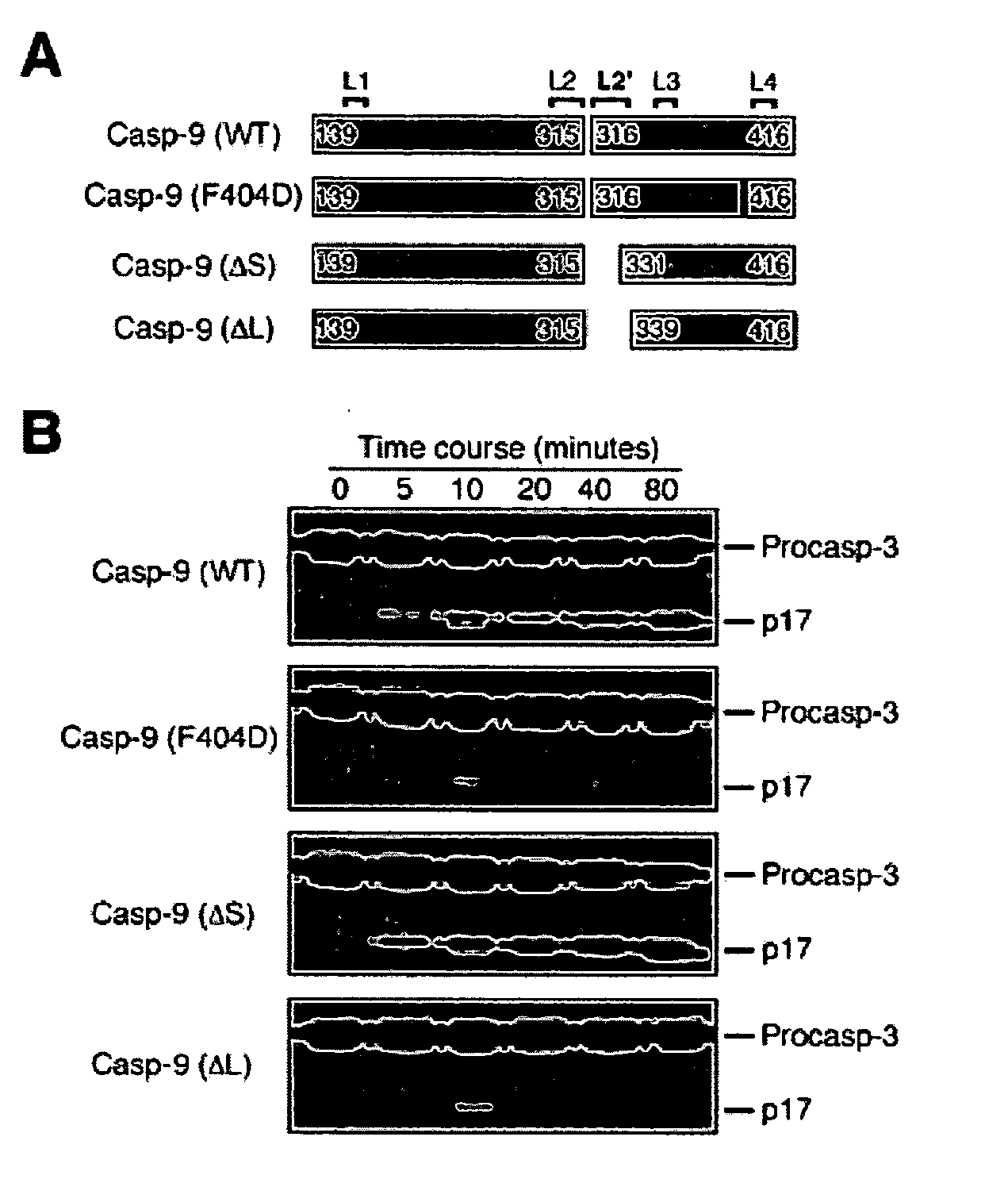
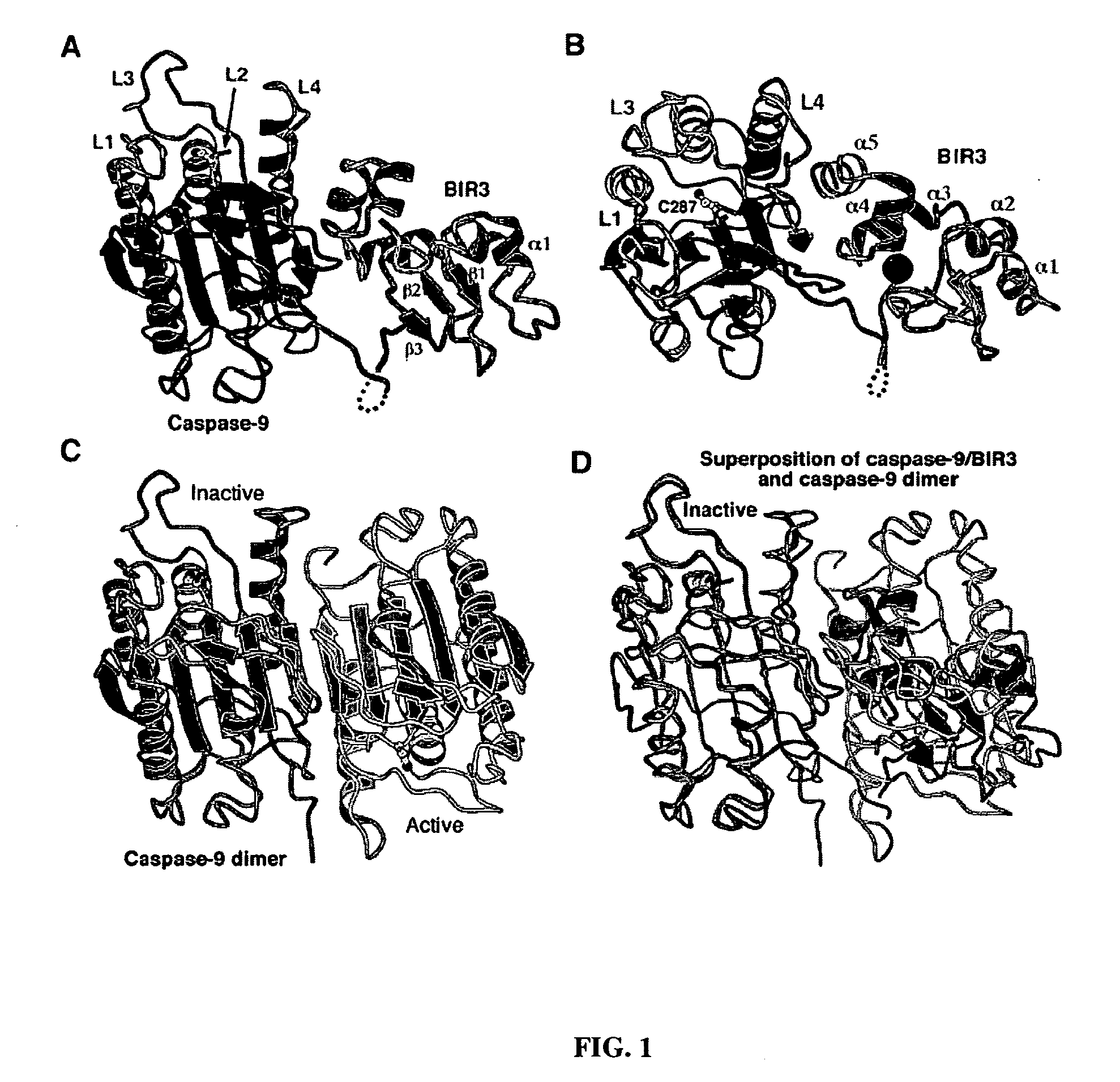
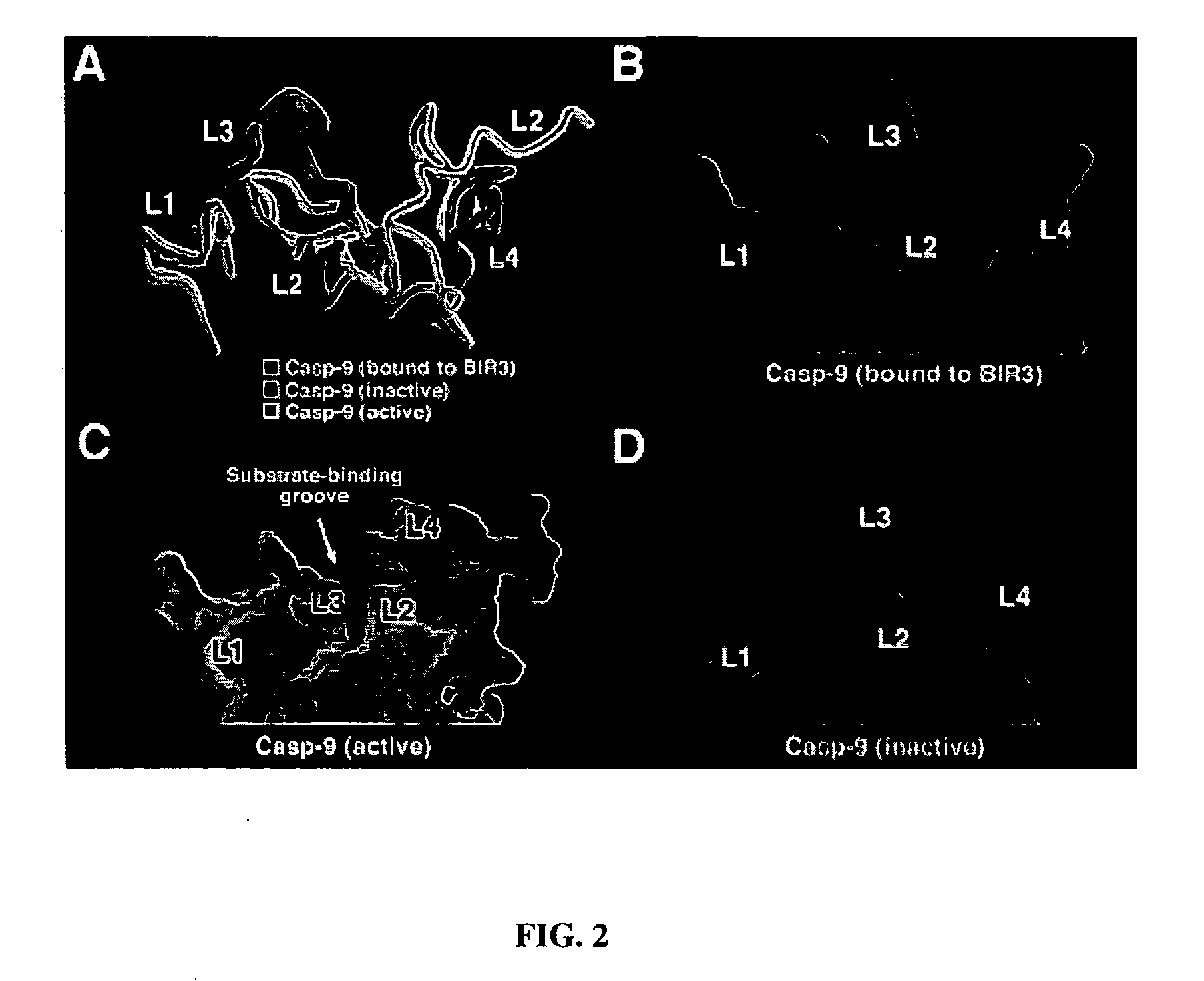
Caspase-9 : BIR domain of XIAP complexes and methods of use
InactiveUS20040180828A1Avoid possibilityIncreased apoptosisPowder deliveryElectrotherapyApoptosisCaspase-9
The present invention provides polypeptides and specific binding agents that modify the activity of an initiator caspase involved in apoptosis, caspase-9. The polypeptides include the third baculoviral IAP repeat (BIR3) of an IAP and form a heterodimer complex with caspase-9. Nucleic acid molecules including expression vectors encoding the polypeptides and variants thereof as well as variants of caspase-9 are provided. Such polypeptide and nucleic acid molecules may be used for modifying apoptosis.
Owner:THE TRUSTEES FOR PRINCETON UNIV
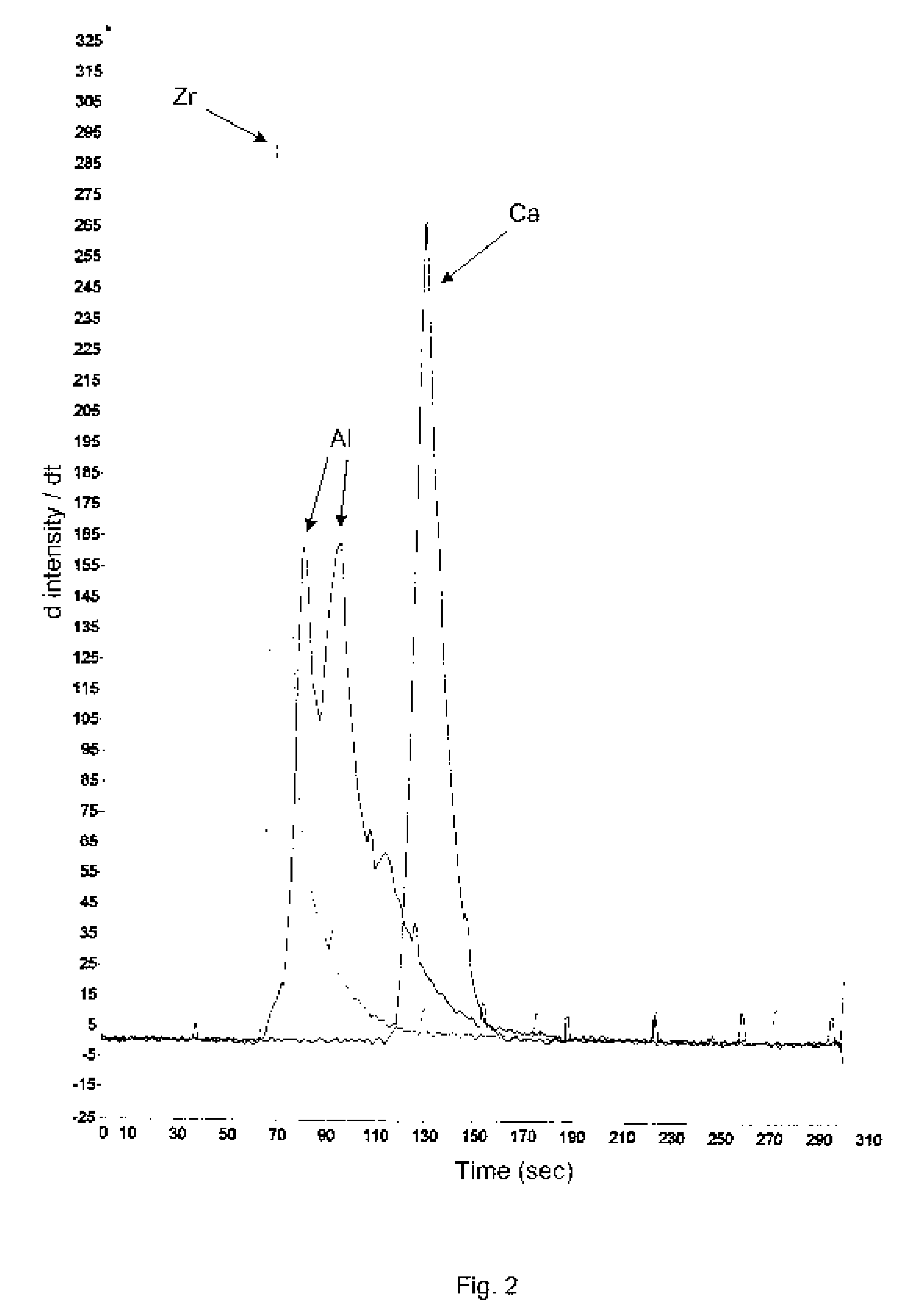
Betaine with Calcium and/or Strontium Antiperspirants
InactiveUS20070020211A1Good curative effectImprove skinCosmetic preparationsToilet preparationsAntiperspirantsBetaine
Aluminum and aluminum-zirconium antiperspirant compositions comprising basic aluminum chlorides that have a particular molecular size distribution defined by having an SEC-HPLC Band III / II ratio of at least 0.5, having SEC-HPLC Band III plus Band II area of at least 70% of the total area and having SEC-HPLC Band I content no more than 5% and containing betaine (trimethylglycine), calcium and / or strontium are disclosed. Also disclosed are the methods of making these compositions and the use thereof in consumer acceptable antiperspirant vehicles such as aerosols, gels, roll-on, sticks and soft solids.
Owner:SUMMIT RES LAB
Method for moving a fluid of interest in a capillary tube and fluidic microsystem
InactiveUS20040241693A1Low viscosityGood physical and chemical stabilityBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteCapillary channel
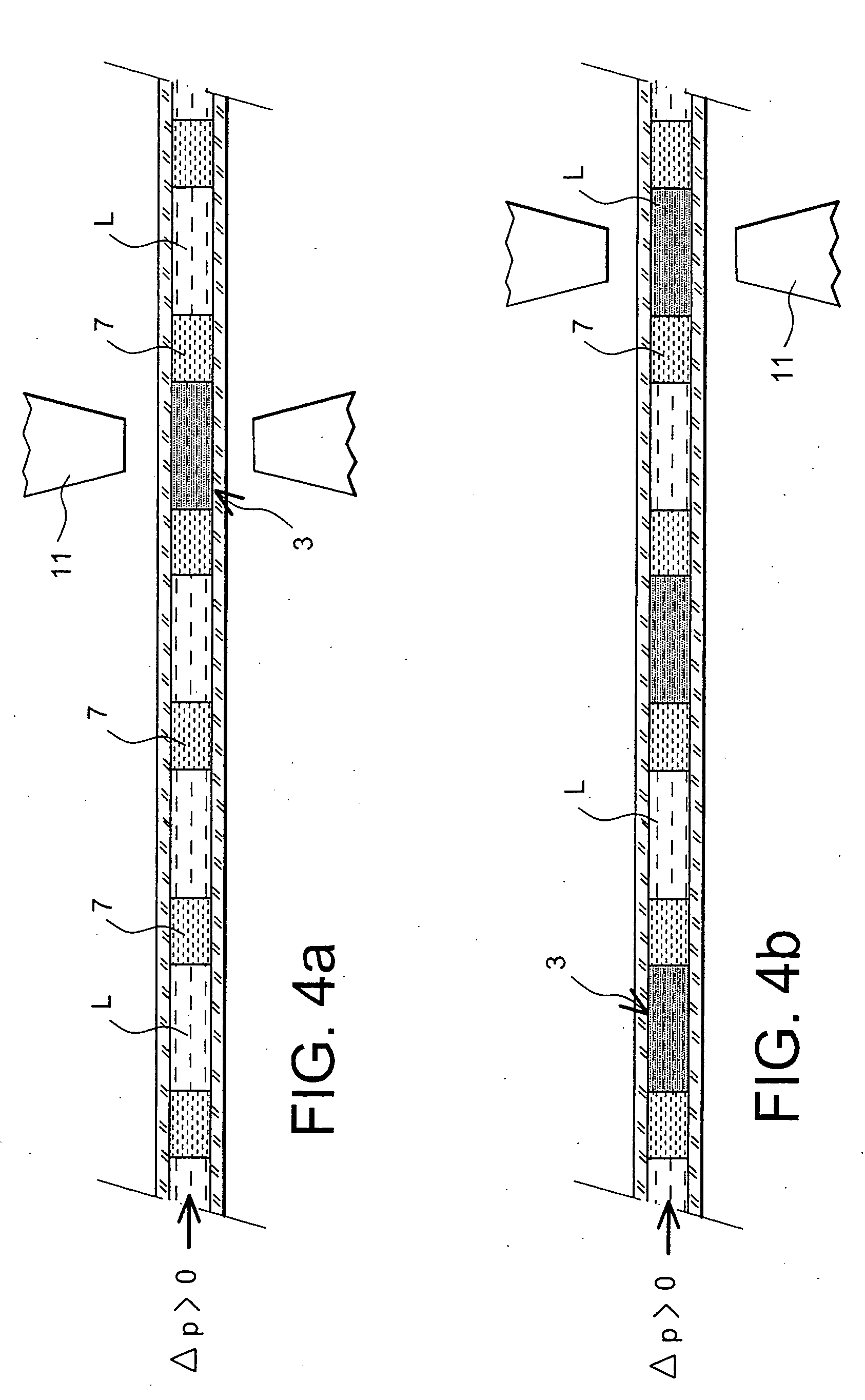
The present invention relates to a method for the displacement of an analyte fluid within a capillary microchannel and to a microfluidic system. In particular, it relates to the field of microfluidics, and especially to microfluidic systems. The method comprises steps which consist in introducing at least one ferrofluid train (3) into the said capillary channel (1), the said ferrofluid train (3) comprising a slug of ferrofluid (5) and, placed against at least one of the two ends of the slug of ferrofluid and in contact with it, a slug of liquid (7) immiscible with both the ferrofluid and the analyte fluid; in introducing the said analyte fluid (9) into the said capillary channel, in proximity to the ferrofluid train and on the side having the slug of liquid (7) immiscible with both the ferrofluid and the analyte fluid; and in controlling the analyte fluid displacement within the said capillary channel by the action of a magnetic field on the ferrofluid train, which field is generated by a magnet system placed on the outside of the said capillary channel.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Storage stable powder compositions of interleukin-4 receptor
InactiveUS6896906B2Good chemical stabilityImprove physical stabilityPowder deliveryPeptide/protein ingredientsWhite blood cellPhysical chemistry
The present invention provides storage stable dry powder compositions of IL-4R. The powder compositions demonstrate superior chemical and physical stability over their solution counterparts, particularly upon storage under varying conditions of temperature and humidity. Moreover, the powders, as prepared, possess good aerosol properties, which are maintained upon storage.
Owner:NOVARTIS FARMA
Composite phase-change energy storage material for microcapsule and preparation method thereof
InactiveCN101824307ANo leaksPlay the role of self-control temperature controlHeat-exchange elementsMicroballoon preparationCrack resistanceSolvent
The invention discloses a composite phase-change energy storage material for microcapsules and a preparation method thereof. The coating of the microcapsule is made of silicon dioxide, and the core of the microcapsule is made of a phase-change energy storage material, wherein the phase-change energy storage material is a paraffin organic solid-liquid phase-change energy storage material. 0.2 to 0.5 wt.% of dispersed emulsifier, 52.5 to 62.5 wt.% of solvent water, 18.75 to 31.5 wt.% of phase-change energy storage material and 15.5 to 18.75 wt.% of inorganic silica source are matched and put into a reactor for stirring for 5 to 8 hours; the mixture is uniformly dispersed and emulsified at the temperature 3 to 8 DEG C higher than that for solid-liquid phase change; hydrochloric acid aqueous solution catalyst with the pH value of 0.93 to 4.07 or sodium hydroxide aqueous solution catalyst with the pH value of 8.0 to 12.0 is added into the emulsion; the reacting solution is naturally cooled to room temperature and precipitation solution is obtained; the precipitate is washed with the combination of water and petroleum ether, wherein the mass percent of the petroleum ether is 30 wt.%; then the precipitate is washed with deionized water and is filtered, and the product is naturally aired. The invention improves the technology of phase-change energy storage and conservation, and has the function of automatic temperature regulation, favorable physical and chemical stability, crack resistance, flame retardancy, wear resistance and high thermal conductivity.
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing monodisperse metal atom/graphene composite material employing electrochemical dissolved graphite
ActiveCN106654300ALow costSimple stepsCell electrodesMetal/metal-oxides/metal-hydroxide catalystsGraphiteElectrochemistry
The invention discloses a method for preparing a monodisperse metal atom / graphene composite material employing electrochemical dissolved graphite. The method comprises the steps of (1) preparing an electrode from a graphite-based material; (2) electrolyzing the prepared electrode in an electrolytic cell, carrying out solid-liquid separation and recycling an electrolyte; (3) further stripping the solid obtained by separation and carrying out solid-liquid separation to obtain a crude monodisperse metal atom / graphene composite material; (4) separating and purifying the crude monodisperse metal atom / graphene composite material; and (5) carrying out thermal treatment on the composite material obtained in the step (4) under inert atmosphere protection and cooling and drying the material to obtain a monodisperse metal atom / graphene composite catalyst. The preparation method is simple in process steps, high in efficiency and low in energy consumption, and massive production can be achieved.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
Ceramic wall flow filter manufacture
InactiveUS20060272306A1Good chemical stabilityImprove physical stabilityDispersed particle filtrationLoose filtering material filtersHoneycombPorous ceramics
Porous ceramic wall flow filter bodies of unitary or segmented construction wherein the honeycomb channels are alternately plugged with plugging cements incorporating low expansion refractory fillers and permanent inorganic bonding agents, the latter imparting improved plug integrity and plug bonding to the porous ceramic honeycomb channel walls.
Owner:CORNING INC
Dispersible pharmaceutical compositions
ActiveUS7842791B2Good dispersionEfficacious level of antibacterialAntibacterial agentsOrganic active ingredientsDiseaseMicrocrystalline wax
A pharmaceutical composition is provided comprising a vehicle that comprises (a) an amphipathic oil that is water dispersible and ethanol insoluble, (b) microcrystalline wax, and (c) a pharmaceutically acceptable non-aqueous carrier; and having an antibacterial substance in an antibacterially effective amount stably dispersed in the vehicle. The composition is suitable for administration by intramammary infusion to a milk producing animal for treatment and / or prevention of mastitis or other diseases of the udder, as well as for otic administration for treatment and / or prevention of an ear infection.
Owner:ZOETIS SERVICE LLC
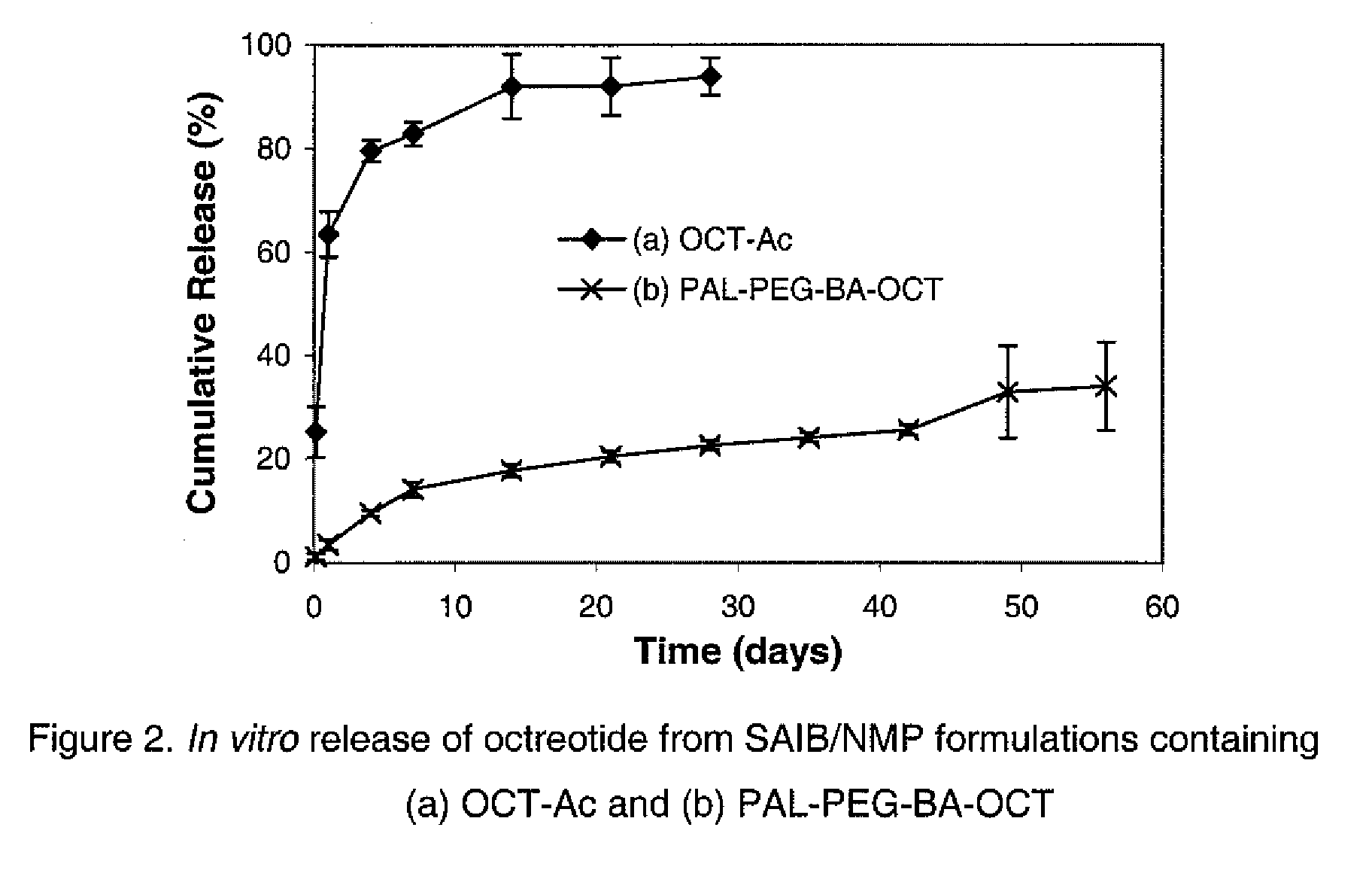
Composition for Sustained Release Delivery of Proteins or Peptides
InactiveUS20090202481A1Improve compatibilityImproved release profilePeptide/protein ingredientsMetabolism disorderCrystallographyOrganic solvent
The present invention provides a novel liquid composition suitable for in-situ formation of a depot system to deliver a protein or peptide in a controlled manner. The composition of the present invention comprises: (a) a hydrophobic non-polymeric carrier material; (b) a water miscible biocompatible organic solvent that dissolves the hydrophobic non-polymeric material; (c) a protein or peptide covalently conjugated with one or more formulation performance-enhancing compounds. The present invention also provides a method of manufacturing and use of the composition thereof.
Owner:FORESEE PHARMA CO LTD
Cell culture compositions and methods for polypeptide production
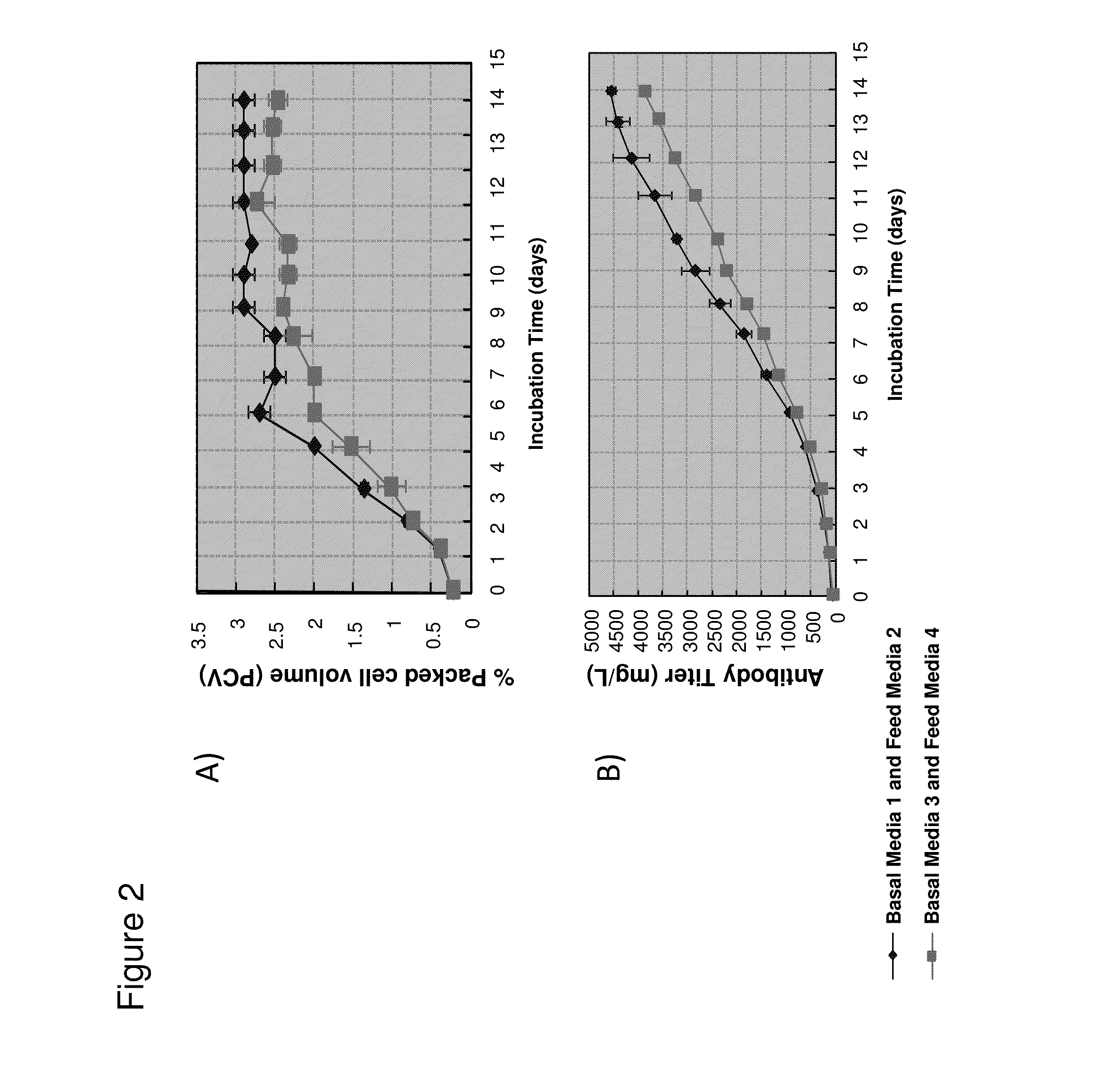
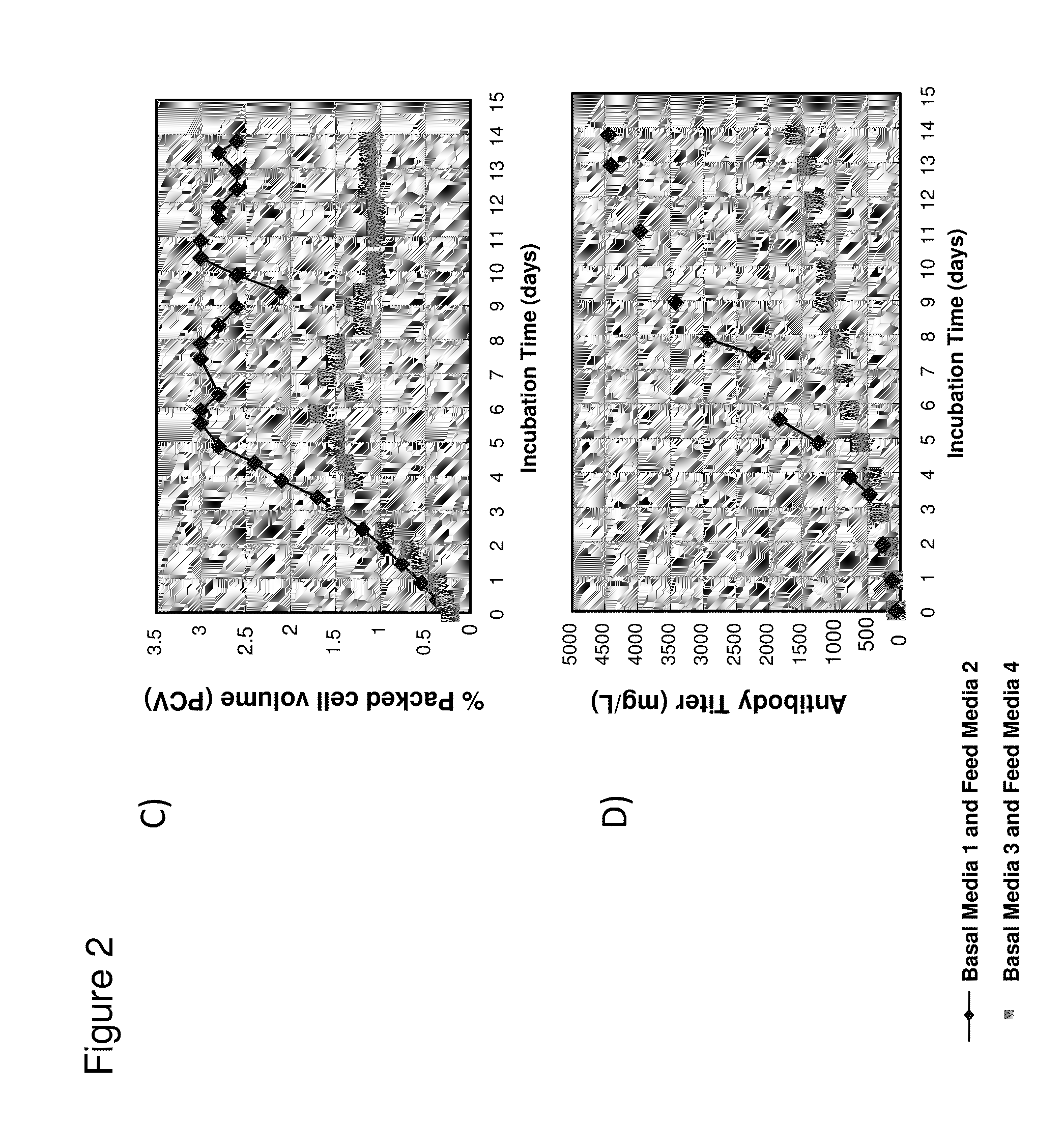
PendingUS20130281355A1Improve stabilityReduce oxidationPeptide/protein ingredientsCulture processCell culture mediaAntibody
Owner:F HOFFMANN LA ROCHE & CO AG
Stable glucagon formulations for the treatment of hypoglycemia
InactiveUS20120046225A1Promote uniform deliveryProvide for shelf stabilityPeptide/protein ingredientsMetabolism disorderGlucagon preparationCuticle
The delivery of biopharmaceutical and other therapeutic agents parenterally to an animal via a minimally invasive, low pain administration is provided. The agents are delivered to the patient via, e.g., the epidermal, dermal, or subcutaneous layer of the skin in a concentrated form of injectable glucagon that is dissolved in a pharmaceutically acceptable carrier.
Owner:XERIS PHARMA +1
Electrode-active anion-deficient non-stoichiometric lithium iron phosphate, method for preparing the same, and electrochemical device using the same
ActiveUS20100183924A1Good physical and chemical stabilityHigh capacityElectrode manufacturing processesActive material electrodesCompound (substance)Reaction conditions
The invention provides an anion-deficient non-stoichiometric lithium iron phosphate as an electrode-active material, which is represented by the formula Li1−xFe(PO4)1−y, wherein 0<x≦0.15 and 0<y≦0.05. The invention provides a method for preparing said Li1−xFe(PO4)1−y, which comprises preparing a precursor of lithium iron phosphate; mixing said precursor with water under reaction conditions of 200˜700° C. and 180˜550 bar to produce lithium iron phosphate; and calcining, or granulating and calcining the resultant compound. The invention also provides electrochemical devices employing said Li1−xFe(PO4)1−y, as an electrode-active material.
Owner:HANWHA CHEMICAL CORPORATION
Stabilized liquid protein formulations in pharmaceutical containers
ActiveUS20070092487A1Reduce surface tensionArea minimizationSmall article dispensingPeptide/protein ingredientsMedicineMethionine biosynthesis
A container comprising a closure means coated by an inert fluorinated material and containing a liquid pharmaceutical composition. In particular, the container comprises a closure means coated by TEFLON and contains a HSA-free Interferon-β formulation having the following composition: 30 to 100 μg / ml of interferon-β, an isotonicity agent, 0.1 to 2 mg / ml of Poloxamer 188, at least 0.12 mg / ml of L-Methionine and a buffer solution capable of maintaining the pH of the liquid formulation at a value between 3.0 and 4.0.
Owner:ARES TRADING SA
Gelatin-chitosan composite food packaging film and preparation method thereof
The invention relates to a gelatin-chitosan composite food packaging film. The gelatin-chitosan composite food packaging film is prepared by the following steps of: dissolving 0.5 to 6 grams of gelatin into 0.5 to 10 milliliters of distilled water to stir uniformly at the temperature of between 25 and 60 DEG C; dissolving 0.05 to 0.4 gram of chitosan into 10 to 40 milliliters of acetum at the concentration of 0.5 to 4 percent to stir uniformly at the temperature of between 20 and 30 DEG C; adding the prepared gelatin solution and the chitosan solution in a volume ratio of the gelatin solution to the chitosan solution of 1:9-6:4 to heat and stir for 5 to 25 minutes at the temperature of between 50 and 70 DEG C; adding 0.5 to 2.5 grams of glycerin and 0.05 to 0.5 gram of sorbitol sequentially into the mixture to continue to heat and stir for 5 to 25 minutes; ultrasonically oscillating the obtained solution for 15 to 60 minutes and then performing vacuum degassing for 0.5 to 2 hours; pouring the solution into a mould to form a film; drying the film and dip-coating the film with 0.5 to 5 percent edible oil solution; and then drying and cooling the film to obtain the gelatin-chitosan composite food packaging film.
Owner:FUZHOU UNIV
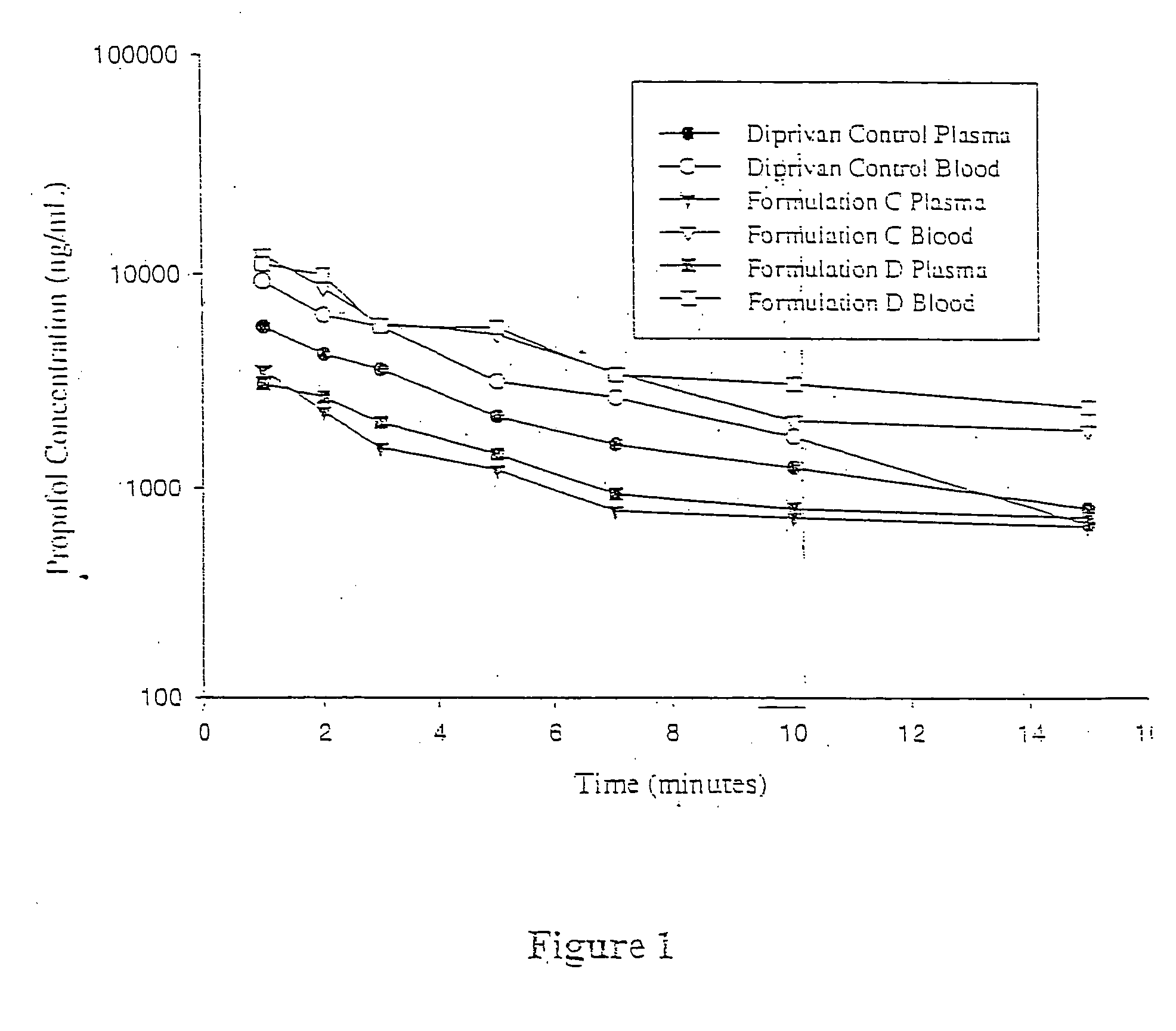
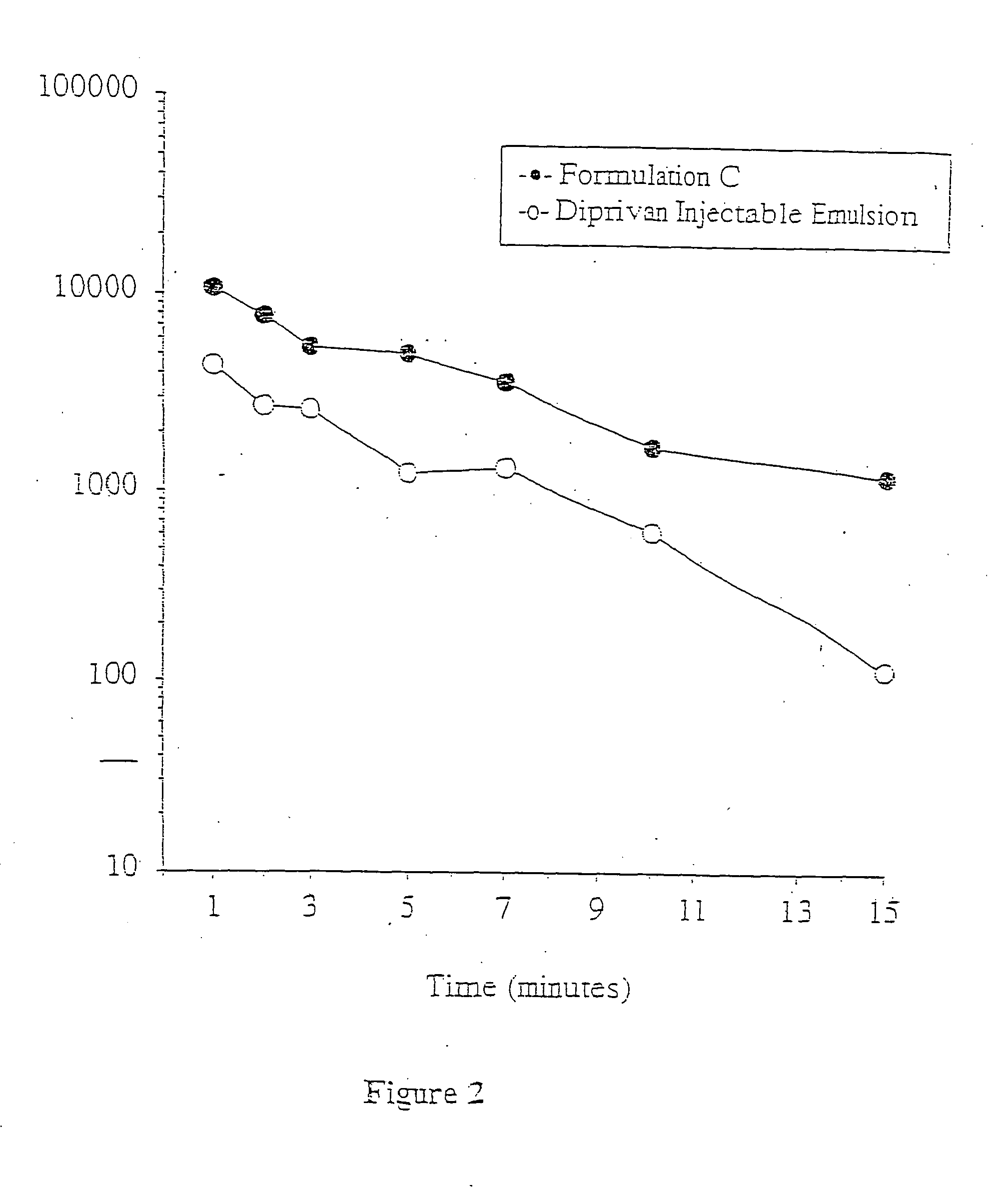
Aqueous 2,6-diisopropylphenol pharmaceutical compositions
InactiveUS20050027019A1Good chemical stabilityImprove physical stabilityBiocideHydroxy compound active ingredientsAqueous solutionExcipient
The present invention relates to aqueous pharmaceutical compositions comprising 2,6-diisopropylphenol (propofol). A composition of the present invention can comprise propofol and two or more excipients as an aqueous mixture. The propofol containing compositions are preferably sterile and are parenterally administered to any animal, including humans. The compositions are also chemically and physically stable over a wide range of environmental conditions.
Owner:JANSSEN BIOTECH INC
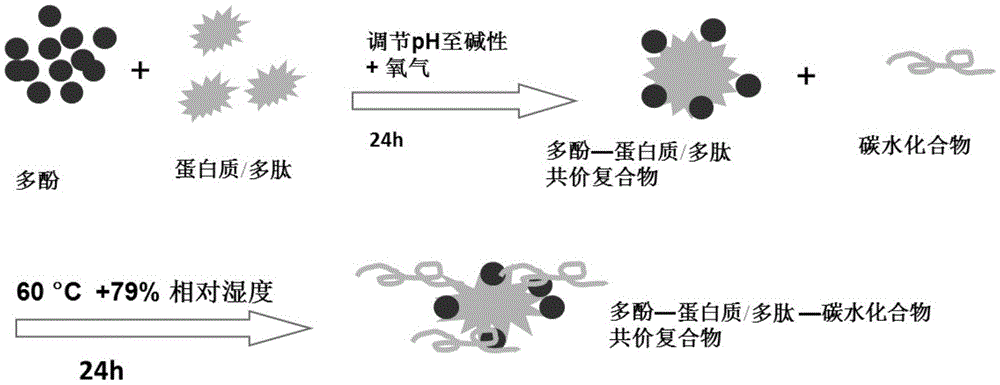
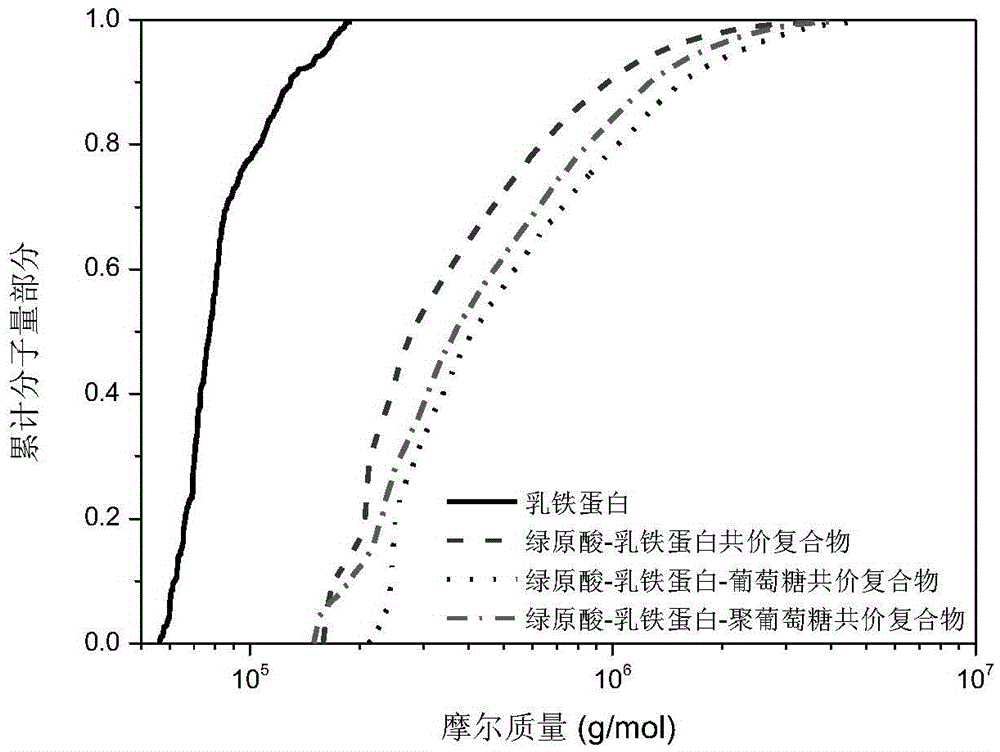
Preparation method and application of polyphenol-protein/polypeptide-carbohydrate covalent complexes
ActiveCN105639651AImprove performanceGood physical and chemical stabilitySugar food ingredientsNatural extract food ingredientsMaillard reactionBeta-Carotene
The invention discloses a preparation method and application of polyphenol-protein / polypeptide-carbohydrate covalent complexes, and belongs to the technical field of food ingredients and food processing. The method comprises the steps that polyphenol, protein / polypeptide are used as raw materials, an alkaline method is used for inducing a reaction, and polyphenol-protein / polypeptide covalent complexes are prepared; then, a carbohydrate and the prepared polyphenol-protein / polypeptide covalent complexes are used as raw materials, and a maillard reaction is utilized for preparing the polyphenol-protein / polypeptide-carbohydrate covalent complexes. According to the preparation method, the natural raw materials are adopted, no any organic reagent is adopted, the polyphenol-protein / polypeptide-carbohydrate covalent complexes with the good performance can be obtained through two steps, the preparation method is simple, efficient and safe, and production is easy. The polyphenol-protein / polypeptide-carbohydrate covalent complexes have the high oxidation resistance and emulsibility, and the beta-carotene emulsion stabilized by means of the polyphenol-protein / polypeptide-carbohydrate covalent complexes has the good physical and chemical stability.
Owner:CHINA AGRI UNIV
Topical compositions comprising 5-alpha reductase inhibitors
InactiveUS20100048598A1Enhanced topical deliveryShorten the progressBiocideAerosol delivery5 Alpha-Reductase InhibitorChemical composition
The present invention relates to topical compositions comprising 5α-reductase inhibitors. The present invention also includes processes for preparation of such topical compositions and methods of using them.
Owner:DR REDDYS LAB LTD +1
Amphiphilic triblock copolymer, preparation method thereof, and polyethersulfone hollow fiber membrane blend-modified by using amphiphilic triblock copolymer
InactiveCN102432782AGood blood compatibilityGood physical and chemical stabilitySemi-permeable membranesHollow filament manufactureDecompositionAcrylonitrile
The invention discloses an amphiphilic triblock copolymer with a structural general formula represented as the following. In the formula, when M1 is vinylpyrrolidone and M2 is acrylic acid, M3 is styrene or acrylonitrile or methyl methacrylate; or when M1 is vinylpyrrolidone and M2 is a chemical bond, M3 is styrene or acrylonitrile. m, n, p, q, are all lager than 1. A number-average molecular weight of the copolymer is 30000 to 100000, a glass-transition temperature of the copolymer is 90-180 DEG C, and a decomposition temperature of the copolymer is 180-430 DEG C. The invention also discloses a preparation method of the copolymer, and a polyethersulfone hollow fiber membrane blend-modified by using the amphiphilic triblock copolymer. The amphiphilic triblock copolymer provided by the invention is insoluble in water. When the amphiphilic triblock copolymer is blended with polyethersulfone and is prepared into a polyethersulfone hollow fiber membrane, the amphiphilic triblock copolymeris hard to precipitate. Therefore, the polyethersulfone hollow fiber membrane is provided with permanent hydrophilicity, protein pollution resistance and excellent blood compatibility. The polyethersulfone hollow fiber membrane can be used in the field of blood purification. The preparation method provided by the invention is simple, and is easy to operate. With the method, industrialization is easy to realize.
Owner:SICHUAN UNIV
Extracting and purifying method for natural capsanthin pigment
InactiveCN1392201ALarge adsorption capacityShorten the production cycleNatural dyesOrganic solventPigment
The present invention relates to the extracting and purifying method of capsanthin pigment with high color number, low spicy degree and no bad taste from ripe chili fruit or peel. The extracting and purifying process includes organic solvent extraction, depression concentration of extractive liquid and separation and purification of the concentrate with macroporous adsorption resin. Measurement in ultraviolet spectrophotometric method shows that the capsanthin pigment of the present invention has color number up to 100 (E449nm) and no spicy taste.
Owner:SUN YAT SEN UNIV
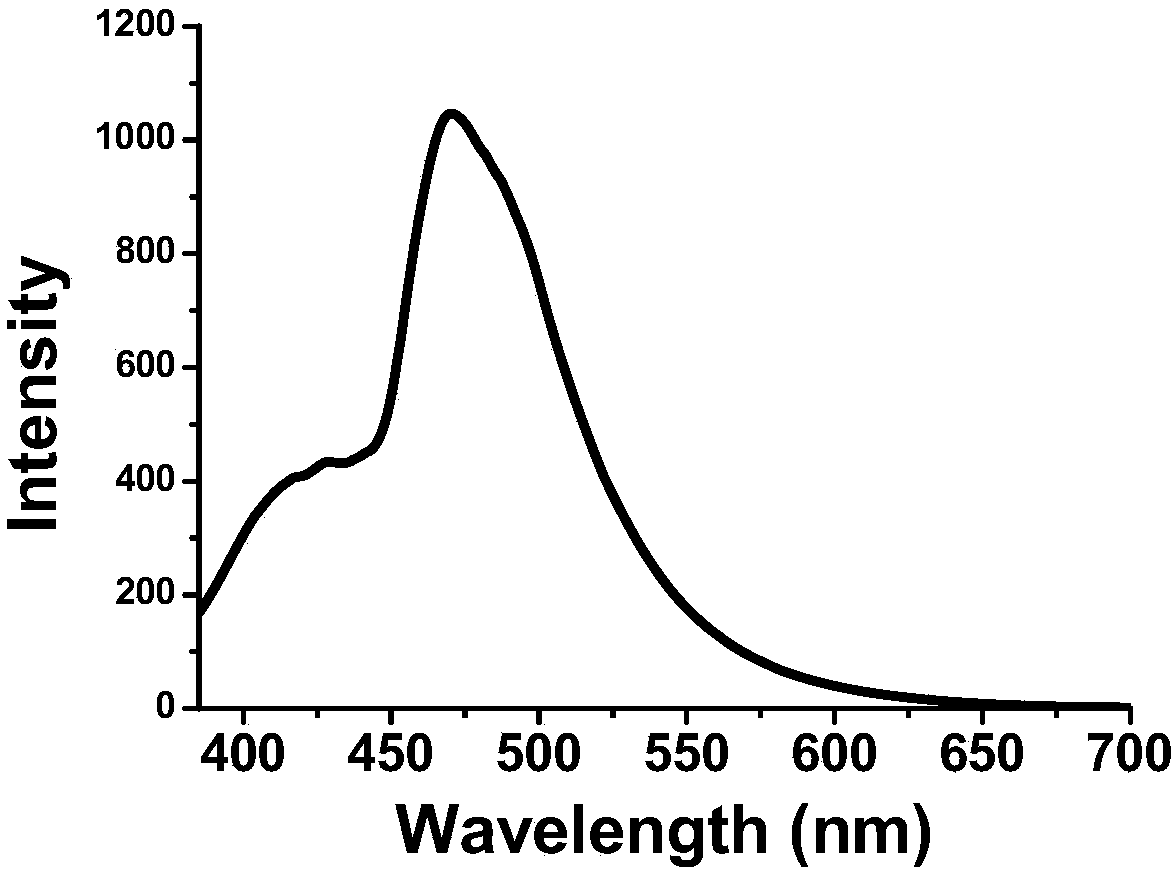
Preparation method of transition metal doped carbon fluorescent quantum dots
The invention relates to a preparation method of transition metal doped carbon fluorescent quantum dots. According to the method, a metal chelator and transition metal salt are dissolved in an organicphase and water which are incompatible with each other, then, a heating reaction is conducted, concentration and purification are performed after the reaction, and transition metal doped carbon fluorescent quantum dots with different solubility are prepared. The method is convenient to operate, doping of carbon fluorescent quantum dots with metal ions can be realized without harsh reaction conditions or large instruments. The obtained carbon dots have multiple different characteristics according to different types of doping metal ions and different solvents. The prepared carbon dots have great application value in preparation of bio-labeling sensing and medical imaging, photoelectric and light-emitting devices and the like due to these characteristics.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Citric acid loofah sponge preparation method and application
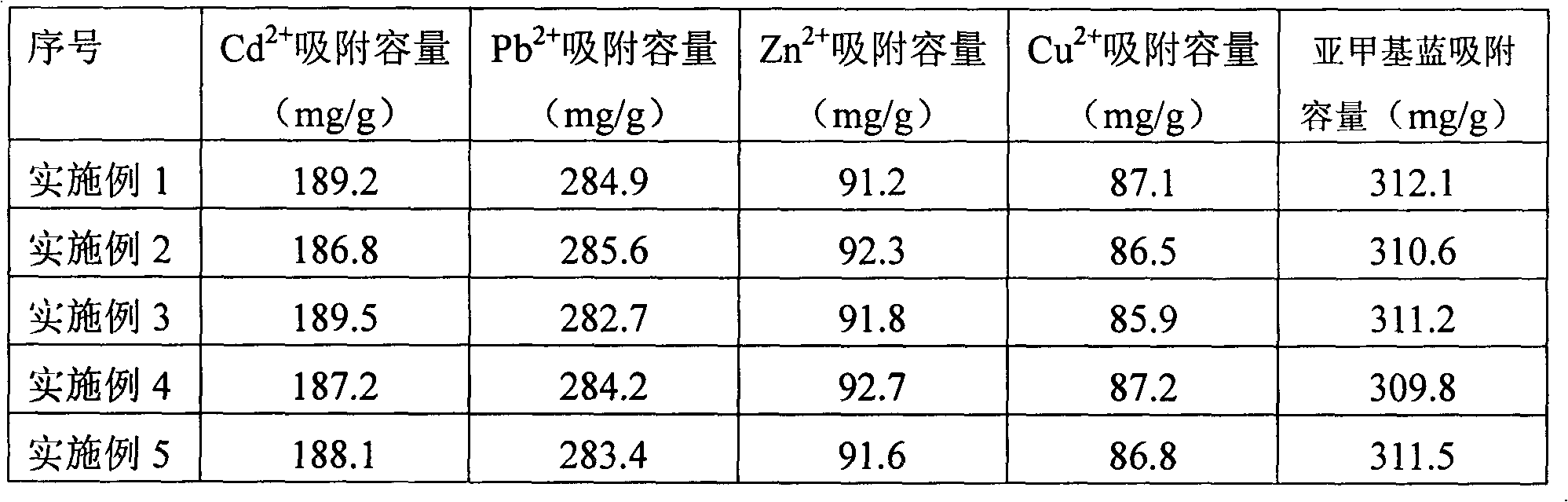
InactiveCN102430393AImprove adsorption efficiencyGood physical and chemical stabilityOther chemical processesAlkali metal oxides/hydroxidesFiltrationOrganic dye
The invention discloses a citric acid loofah sponge preparation method and an application technology. The preparation method provided by the invention is characterized by comprising the following steps of: adding 18-25 wt% of citric acid, 18-25 wt% of saponified loofah sponge and 50-60 wt% of water into a plugged Erlenmeyer flask, putting on a plug, carrying out refluxing at the temperature of 60-70 DEG C for 2-3h with stirring, heating up to the temperature of 110-120 DEG C, reacting for 2-3h, cooling, washing by using deionized water, carrying out pumping filtration until a filtrate is neutral, washing by using a few ethanol, and drying in a baking oven of 75 DEG C to obtain the citric acid loofah sponge. The citric acid loofah sponge has strong adsorption capability, can directly used for the adsorption and elution of various metal ions and organic dyes in a water body, is characterized by high adsorption efficiency, fast adsorption speed, good physical and chemical stability and excellent mechanical stability, and can be used within a wide soda acid range. Simultaneously, the citric acid loofah sponge has regeneration capability and is a natural green adsorbent.
Owner:UNIV OF JINAN
Dispersible pharmaceutical composition for treatment of mastitis and otic disorders
InactiveUS20050009931A1Improve resuspendabilityImproved physical stabilityBiocideOrganic non-active ingredientsDrugDisease
A method is provided for treatment and / or prevention of an infective condition in a fluid-containing organ having a natural exterior orifice, such as the udder of a milk-producing animal or an ear of a subject. The invention also relates to a dispersible pharmaceutical composition suitable for infusion into the organ according to the method of the invention, and to a process for preparing such a composition.
Owner:BRITTEN NANCY JEAN +4
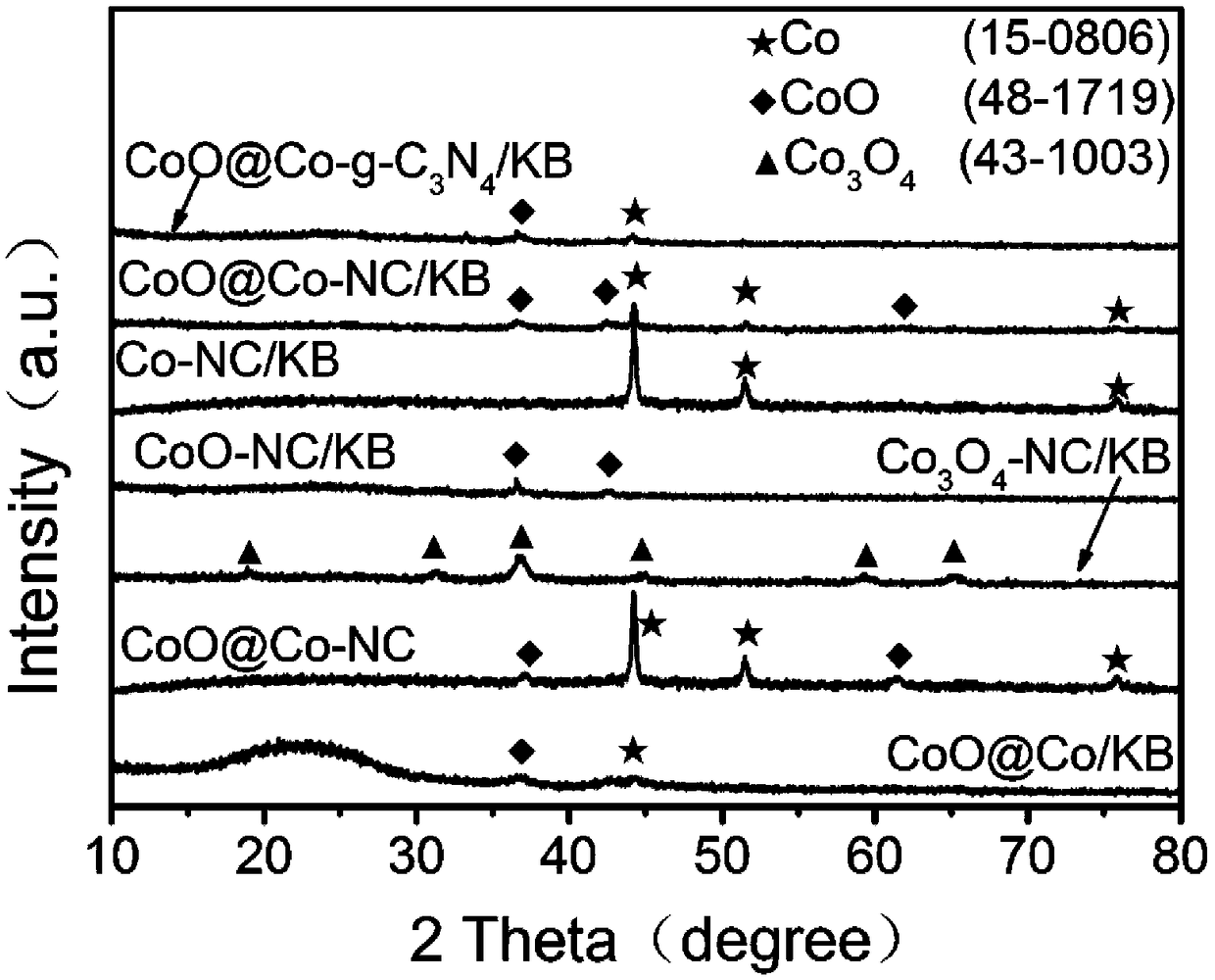
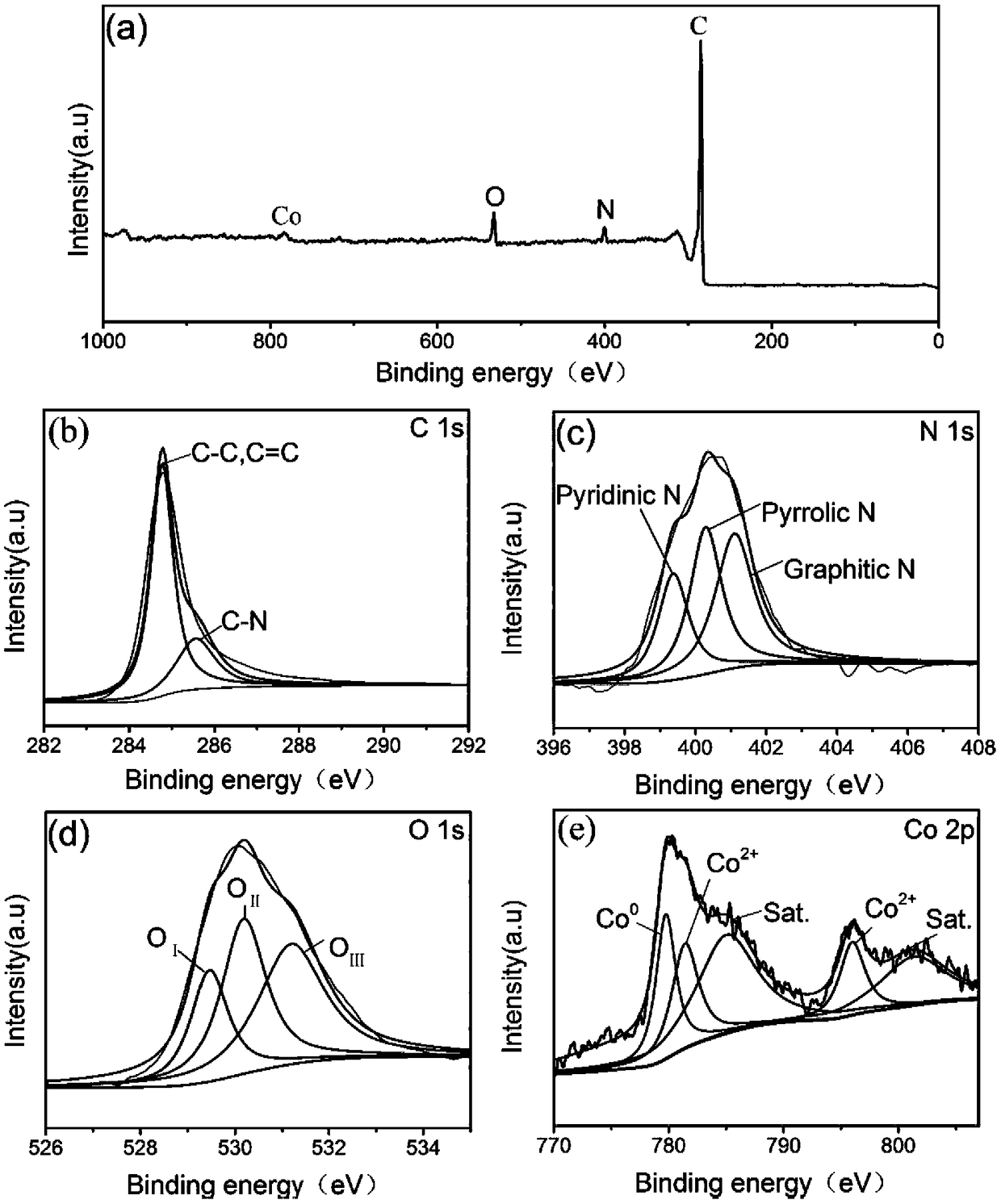
High-performance oxygen evolution CoO@Co-NC/C composite catalyst as well as preparation method and application thereof
ActiveCN108855184AImprove stabilityImprove catalytic performancePhysical/chemical process catalystsElectrodesChemistryOrganic acid
The invention discloses a high-performance oxygen evolution CoO@Co-NC / C composite catalyst as well as preparation method and application thereof. The composite catalyst is prepared by loading Co-coated CoO nano particles and nitrogen doped carbon on a carbon material; the preparation method comprises the following steps: mixing a nitrogen-containing organic small molecule compound, the carbon material and organic acid cobalt salt through a liquid phase, and performing evaporation and drying to obtain mixed powder; putting the mixed powder in a protection atmosphere, and performing two-stage roasting treatment to obtain the CoO@Co-NC / C composite catalyst. The preparation method is simple, low in cost and favorable for industrial production; the prepared CoO@Co-NC / C composite catalyst is applied to water decomposition or the storage and conversion system of renewable resources such as metal-air secondary batteries, and has the characteristics of high activity and high stability; comparedwith a RuO2 commercial catalyst, the CoO@Co-NC / C composite catalyst has better comprehensive performance and shows a good application prospect.
Owner:CENT SOUTH UNIV
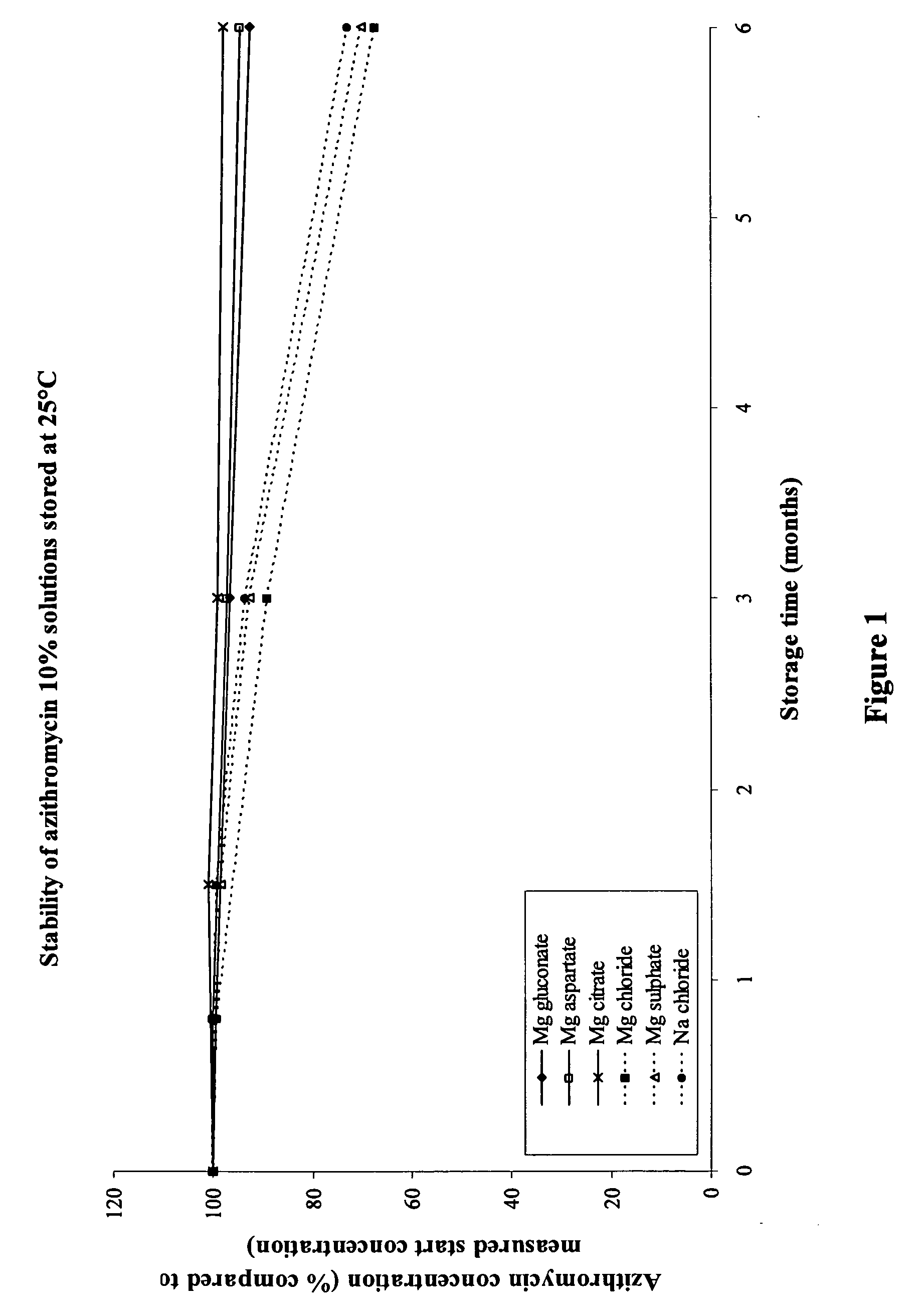
Macrolide compositions having improved taste and stability
InactiveUS20090232744A1Improve stabilityBad tasteBiocideDispersion deliverySodium acetateSodium lactate
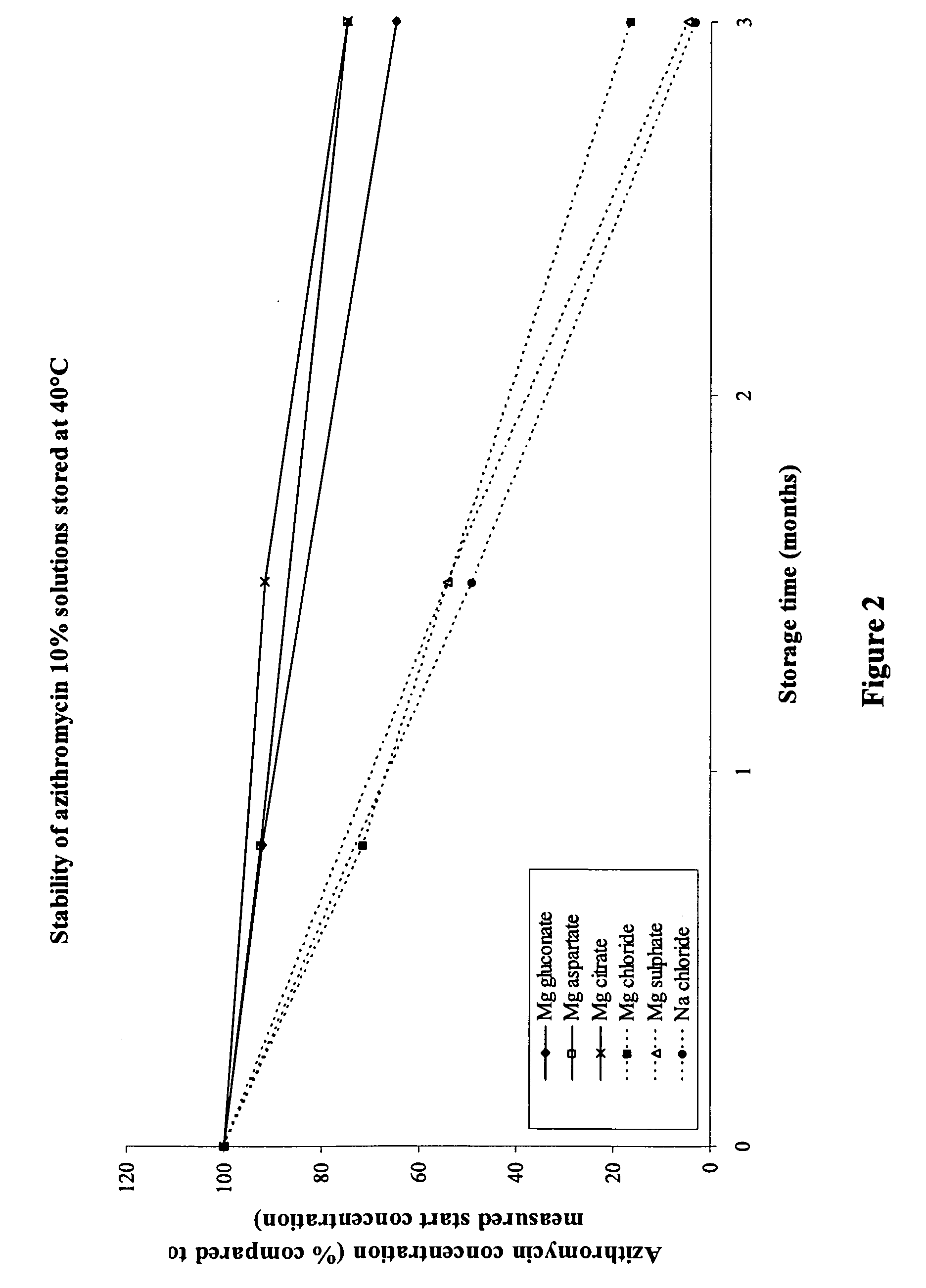
The invention provides an aqueous pharmaceutical composition for administration as an aerosol to the respiratory tract, nose or oropharyngeal region comprising (i) a macrolide having a poor taste and poor chemical stability in aqueous solution; (ii) at least one salt selected from the group consisting of sodium gluconate, sodium aspartate, sodium acetate, sodium lactate, sodium succinate, sodium maleate, magnesium gluconate, magnesium aspartate, magnesium citrate, magnesium acetate, magnesium lactate, magnesium succinate, and magnesium maleate; or mixtures thereof and (iii) a taste-masking agent different from said salt; wherein (a) the concentration of said macrolide in the composition is in the range of about 0.25 wt.-% to about 15 wt.-%; (b) the molar ratio of said macrolide:said salt is in the range from about 1:0.5 to about 1:100; (c) the pH of the composition is in the range of about 3 to 9; and (d) the osmolality of the composition is in the range of about 150 mOsmol / kg to about 1500 mOsmol / kg. The invention further provides a method of generating an aerosol, preferably by means of a nebuliser, which uses such an aqueous pharmaceutical composition. The macrolide may be used alone or in combination with other drugs. The composition is suitable to treat inflammatory disorders and / or infections of the respiratory tract. It has an improved taste and stability.
Owner:PARI PHARMA GMBH
Pulmonary pharmaceutical formulations
ActiveUS8614255B2Reliable deliveryGood physical and chemical stabilityBiocidePowder deliveryDiseaseDiagnostic agent
The present invention provides improved pharmaceutical formulations for pulmonary delivery having improved chemical and physical stability of the therapeutic, prophylactic or diagnostic agent as compared to formulations known in the art. The improved pharmaceutical formulations of the invention for administration to the respiratory system of a patient for the treatment of a variety of disease conditions comprise a mass of biocompatible particles comprising an active agent, and a hydrogenated starch hydrosylate (HSH). The improvement over the prior art comprises the presence of HSH in the pharmaceutical formulation. The invention further relates to a method of treating diseases comprising administering the pharmaceutical formulations of the present invention to the respiratory system of a patient in need of treatment.
Owner:CIVITAS THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/a113b4d7-c198-49c0-a9ea-68b5e5fb4c63/US20110064811A1-20110317-D00000.png)
![Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/a113b4d7-c198-49c0-a9ea-68b5e5fb4c63/US20110064811A1-20110317-D00001.png)
![Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Solid forms of N-[2,4-BIS(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/a113b4d7-c198-49c0-a9ea-68b5e5fb4c63/US20110064811A1-20110317-D00002.png)
![Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/dcede4dd-2f3e-4f55-87e4-8901c2ca1068/US08410274-20130402-D00001.png)
![Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/dcede4dd-2f3e-4f55-87e4-8901c2ca1068/US08410274-20130402-D00002.png)
![Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide](https://images-eureka.patsnap.com/patent_img/dcede4dd-2f3e-4f55-87e4-8901c2ca1068/US08410274-20130402-D00003.png)