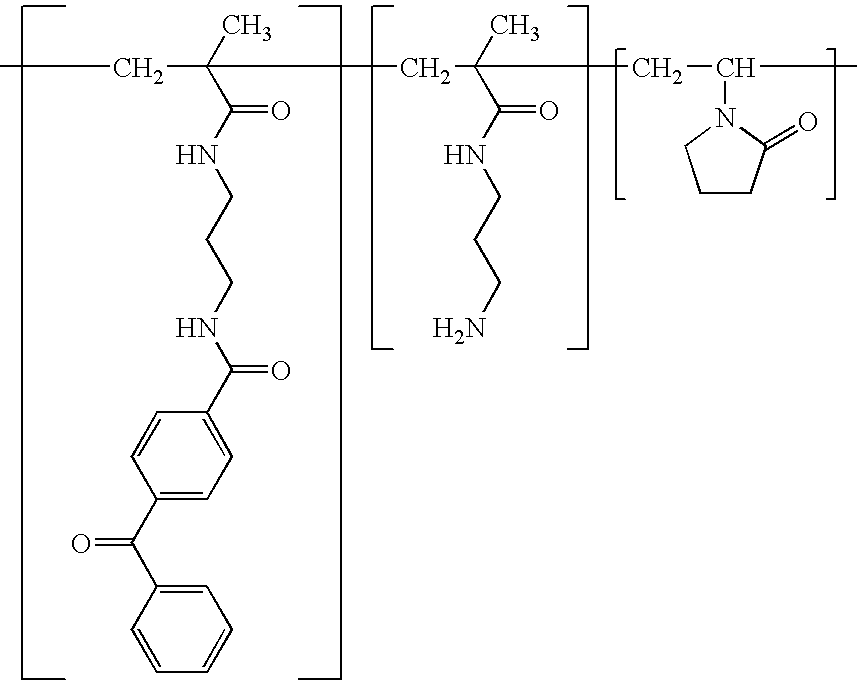
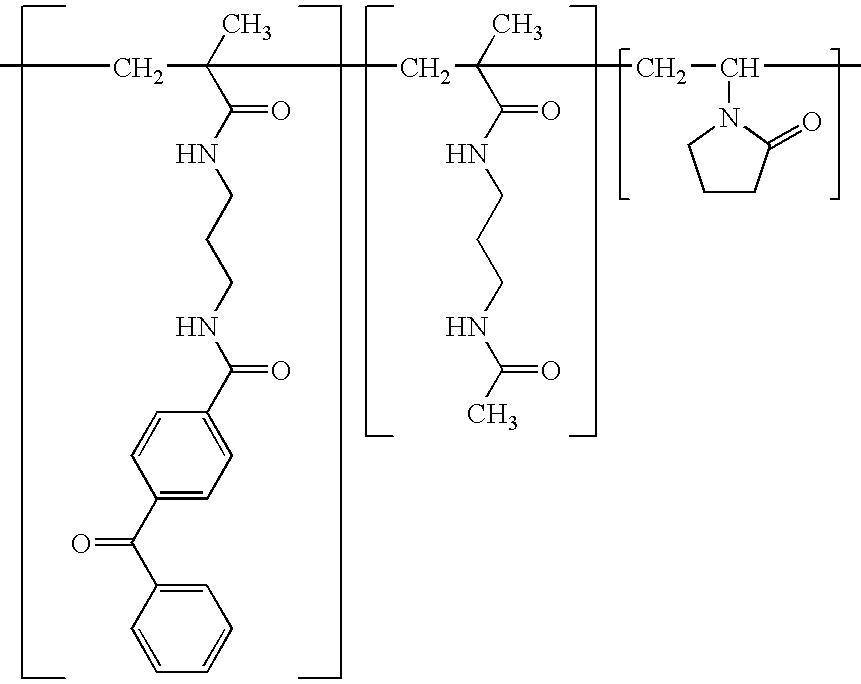
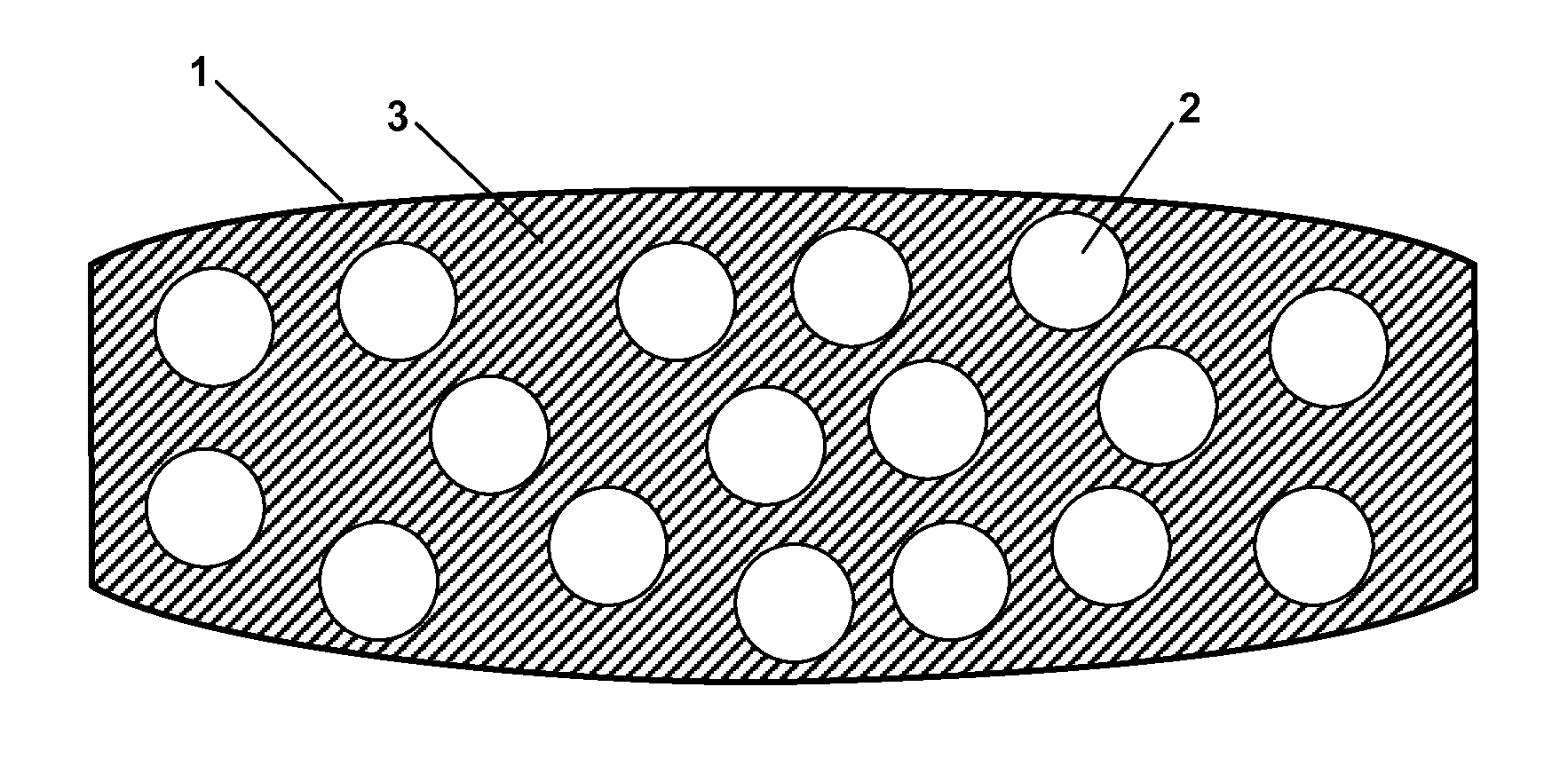
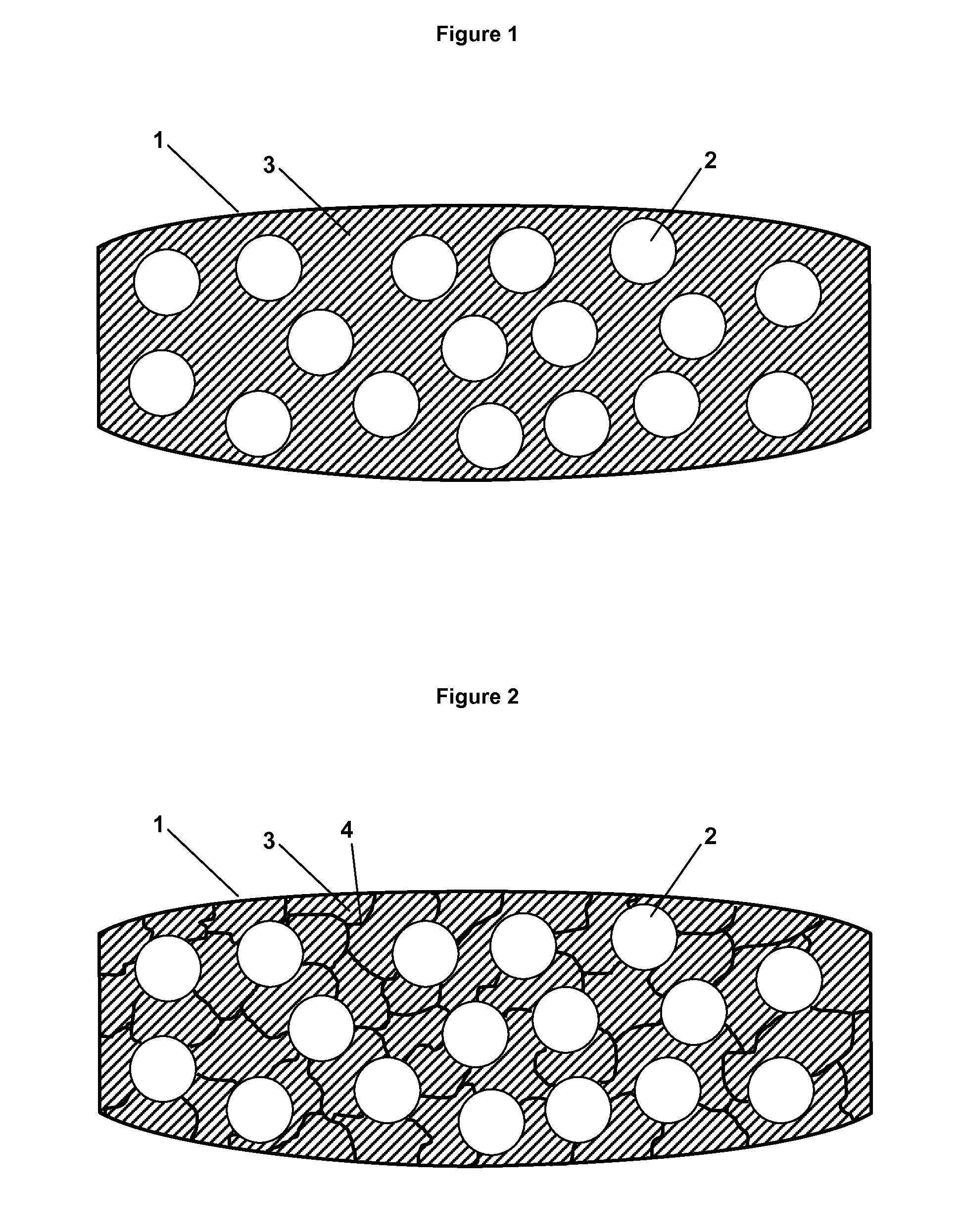
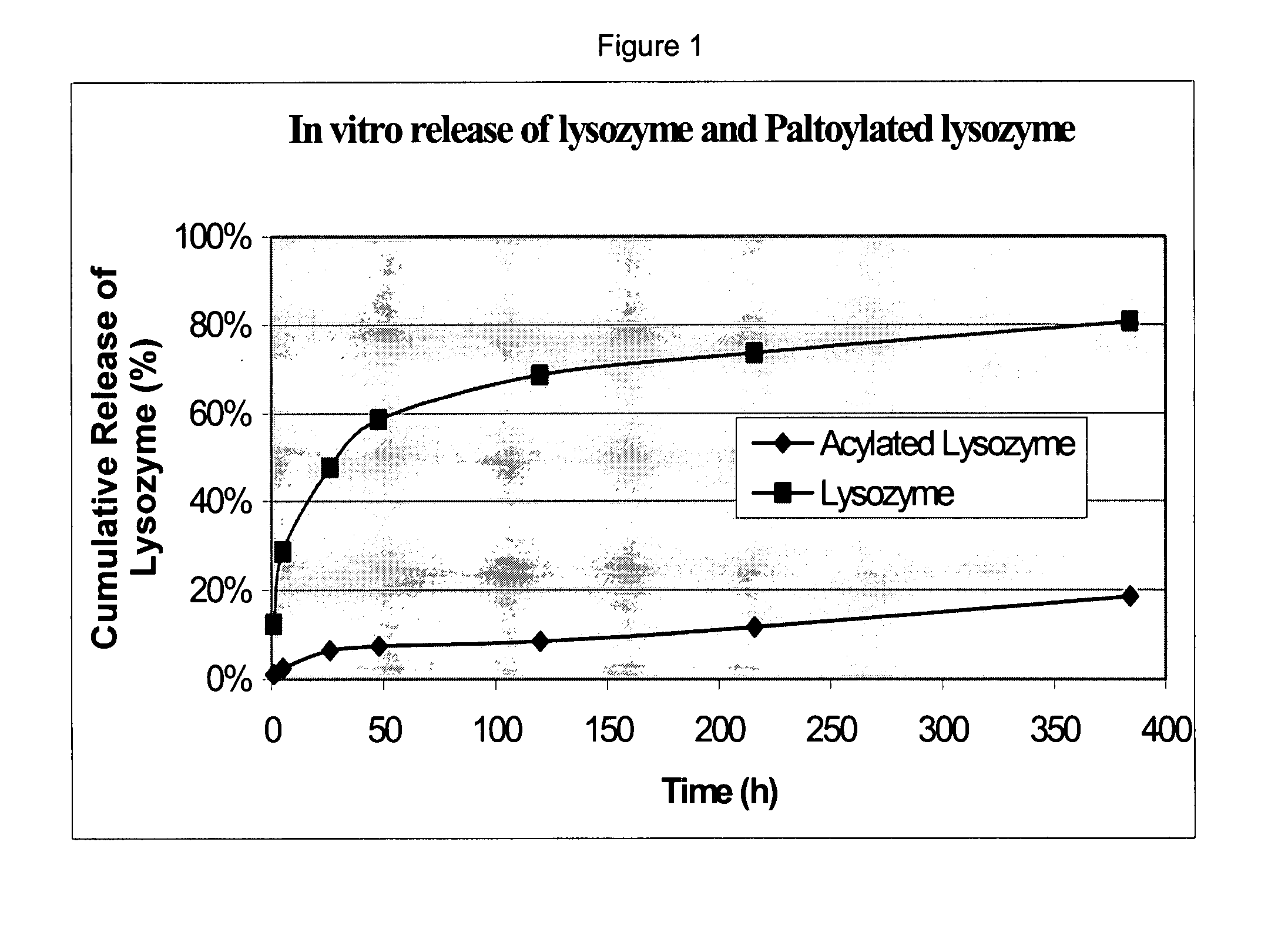
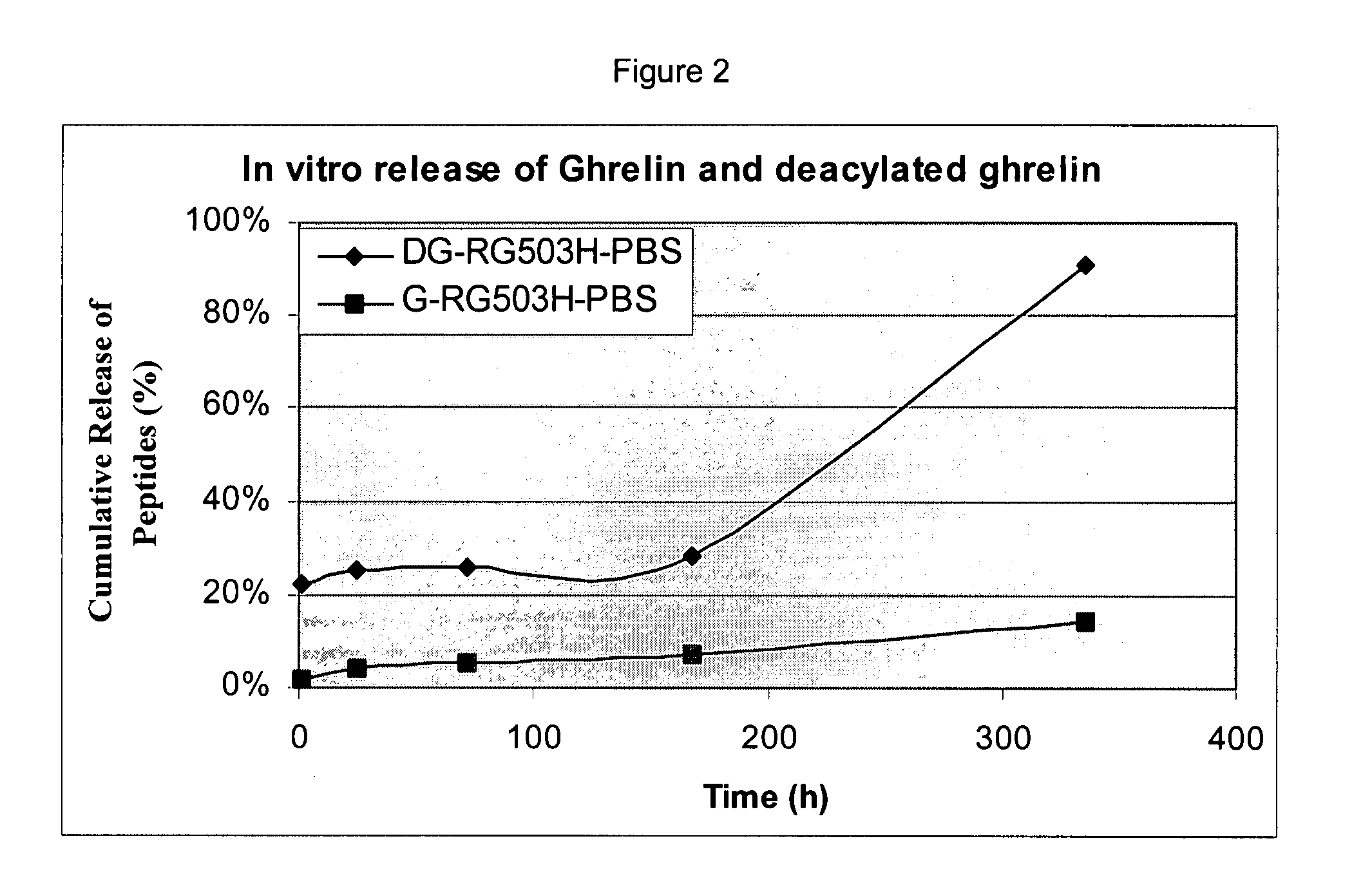
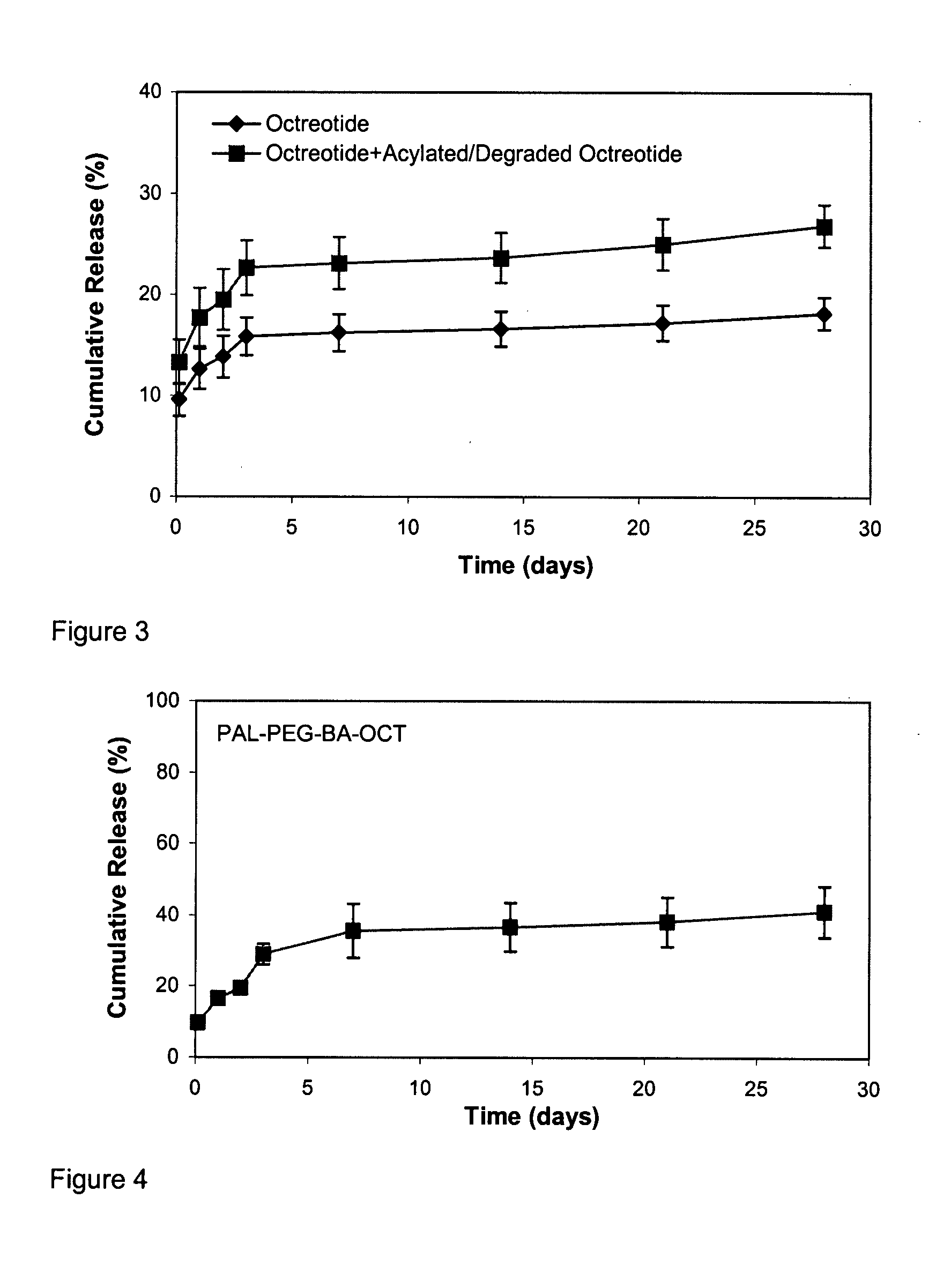
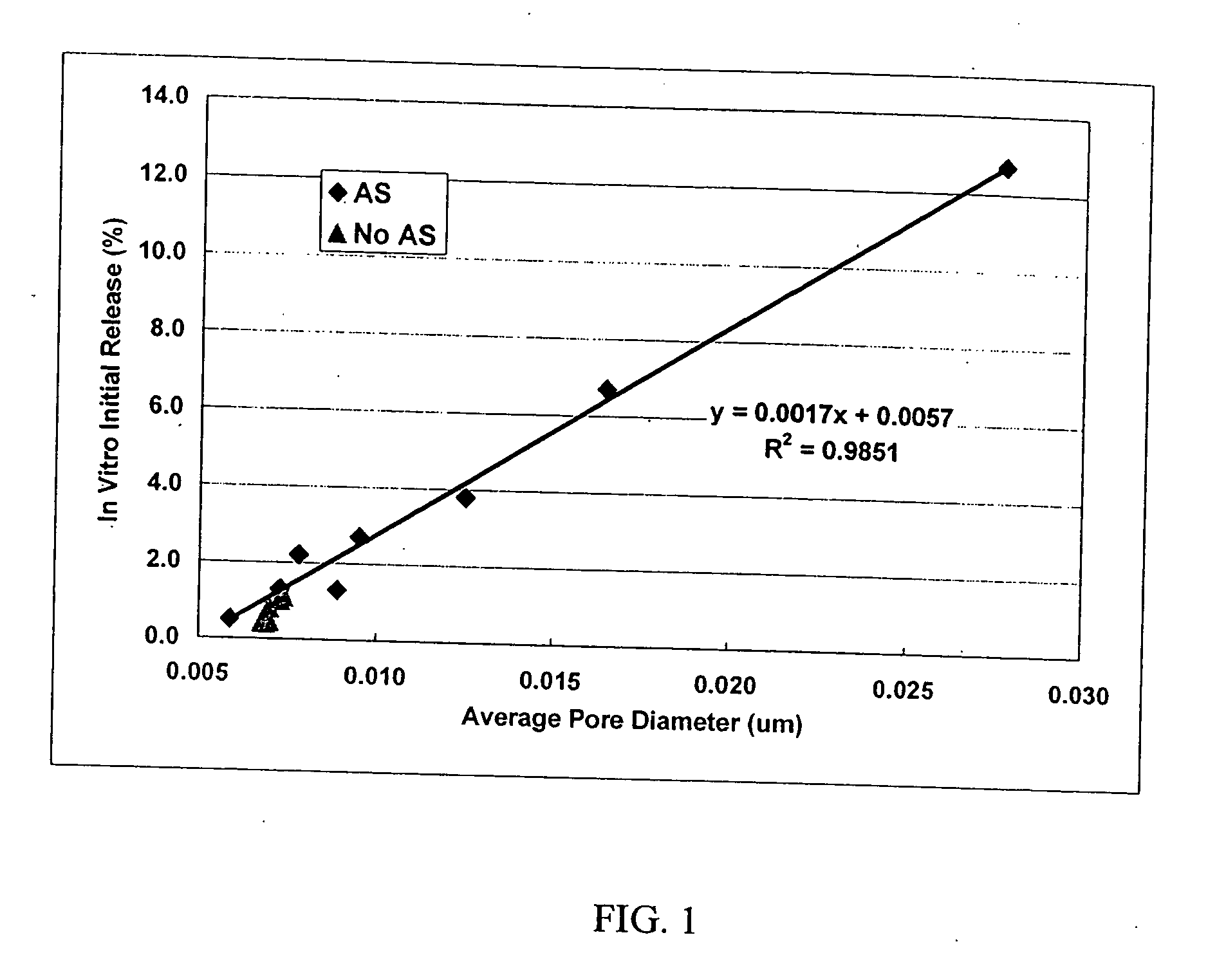
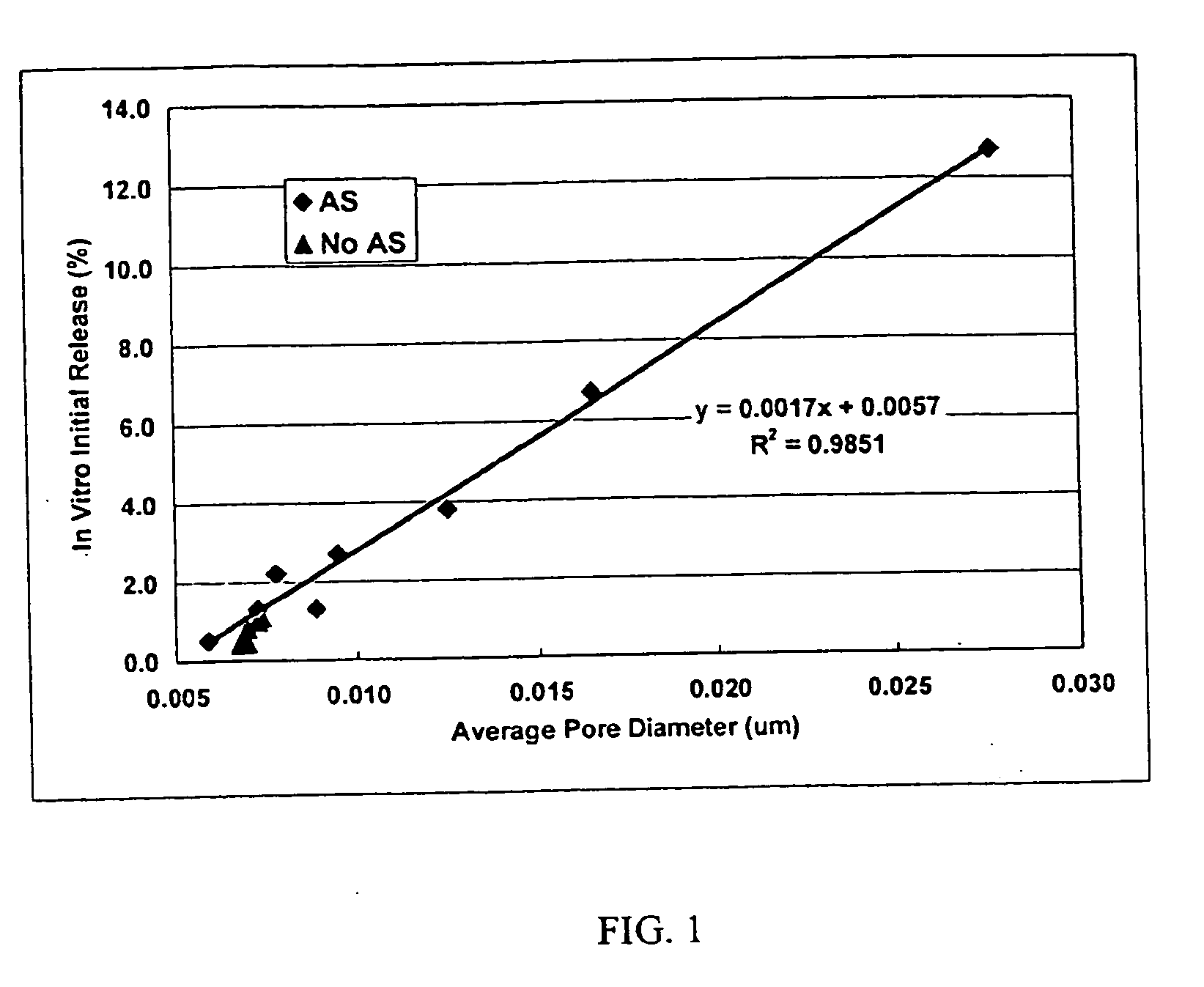
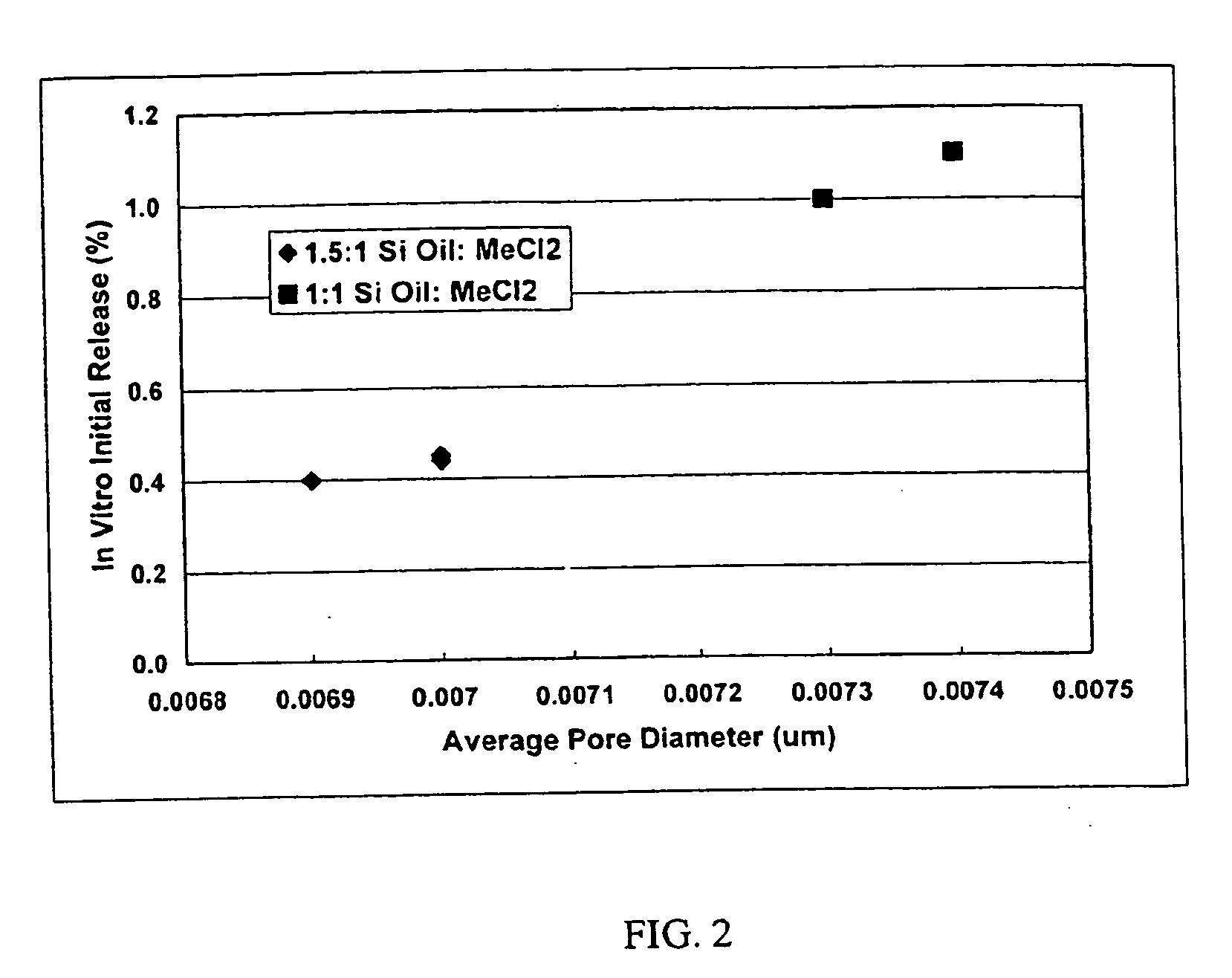
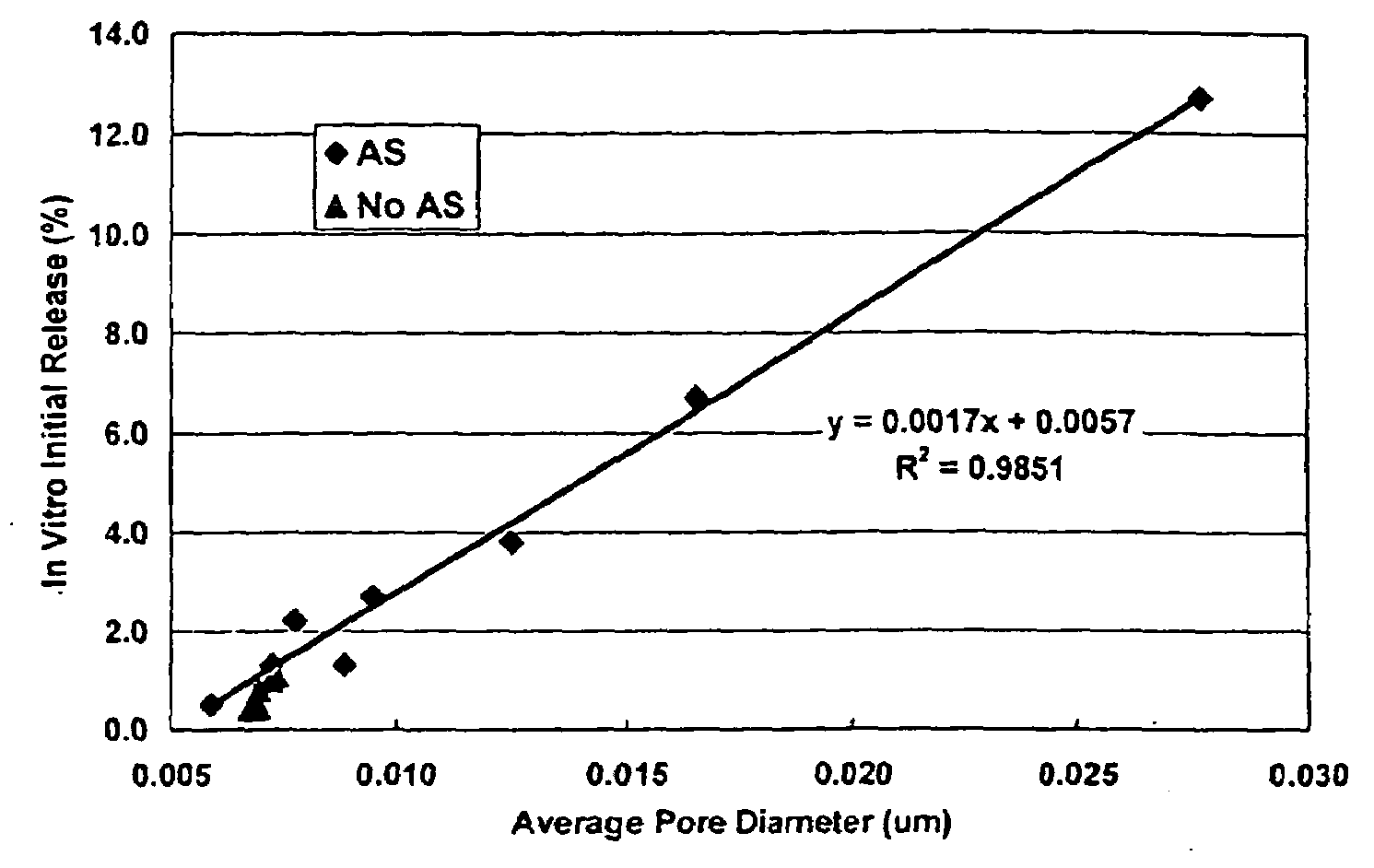
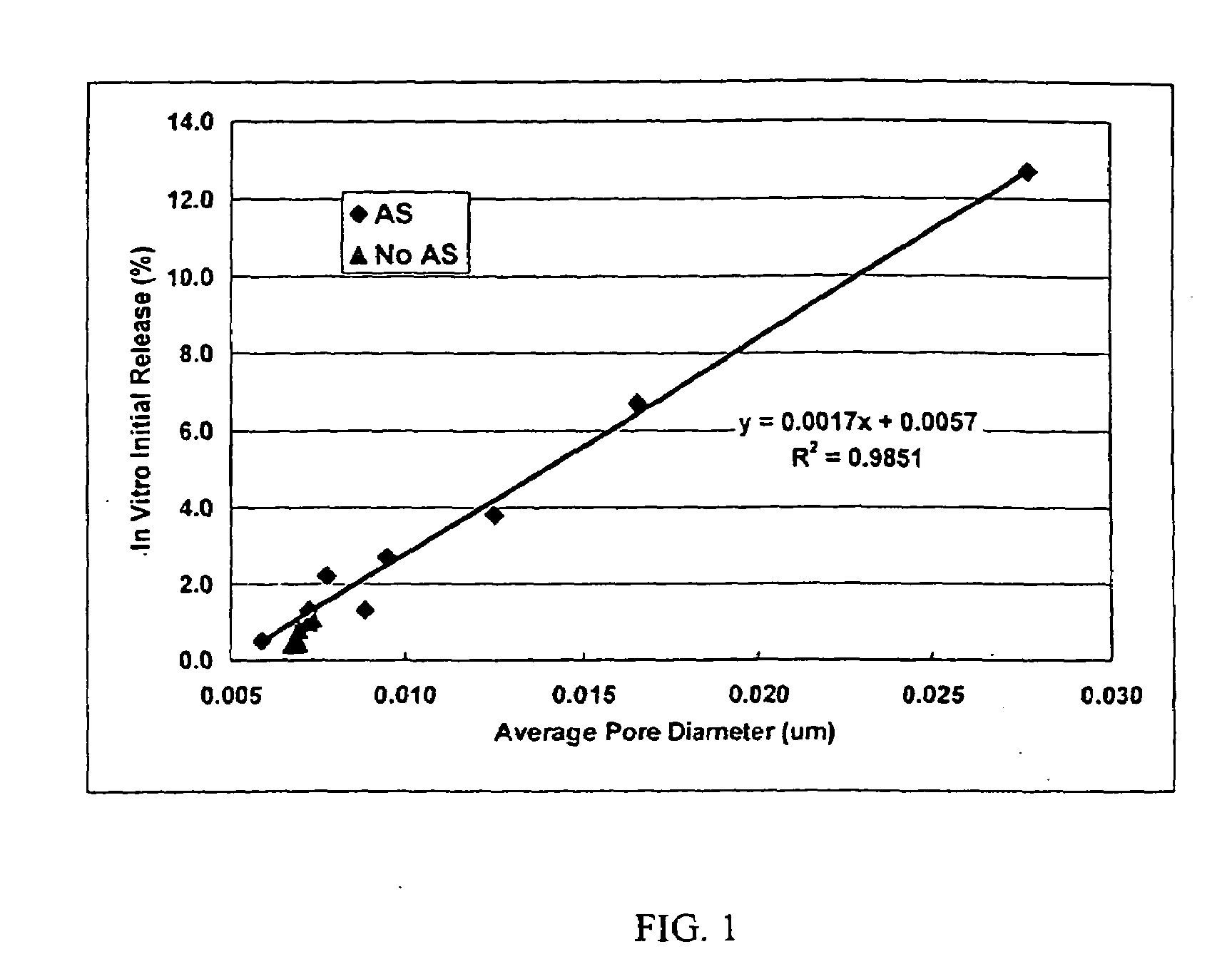
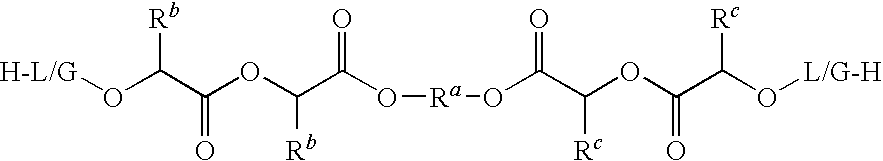Patents
Literature
87results about How to "Improved release profile" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Composition and method for preparing biocompatible surfaces
ActiveUS20050244453A1Reduced throughput timeEfficient and cost-effectiveBiocideOrganic active ingredientsPre irradiationActive agent
The invention provides methods and compositions for providing biocompatible surfaces to medical articles. In particular the invention provides biocompatible coatings with heparin activity. In some aspects, the biocompatible coatings of the invention are able to release a bioactive agent. The coatings can be formed using biostable or biodegradable polymeric material and photoreactive groups. The invention also provides methods for improving the quality of bioactive agent-containing coatings by performing pre-irradiation of biocompatible coating compositions.
Owner:SURMODICS INC
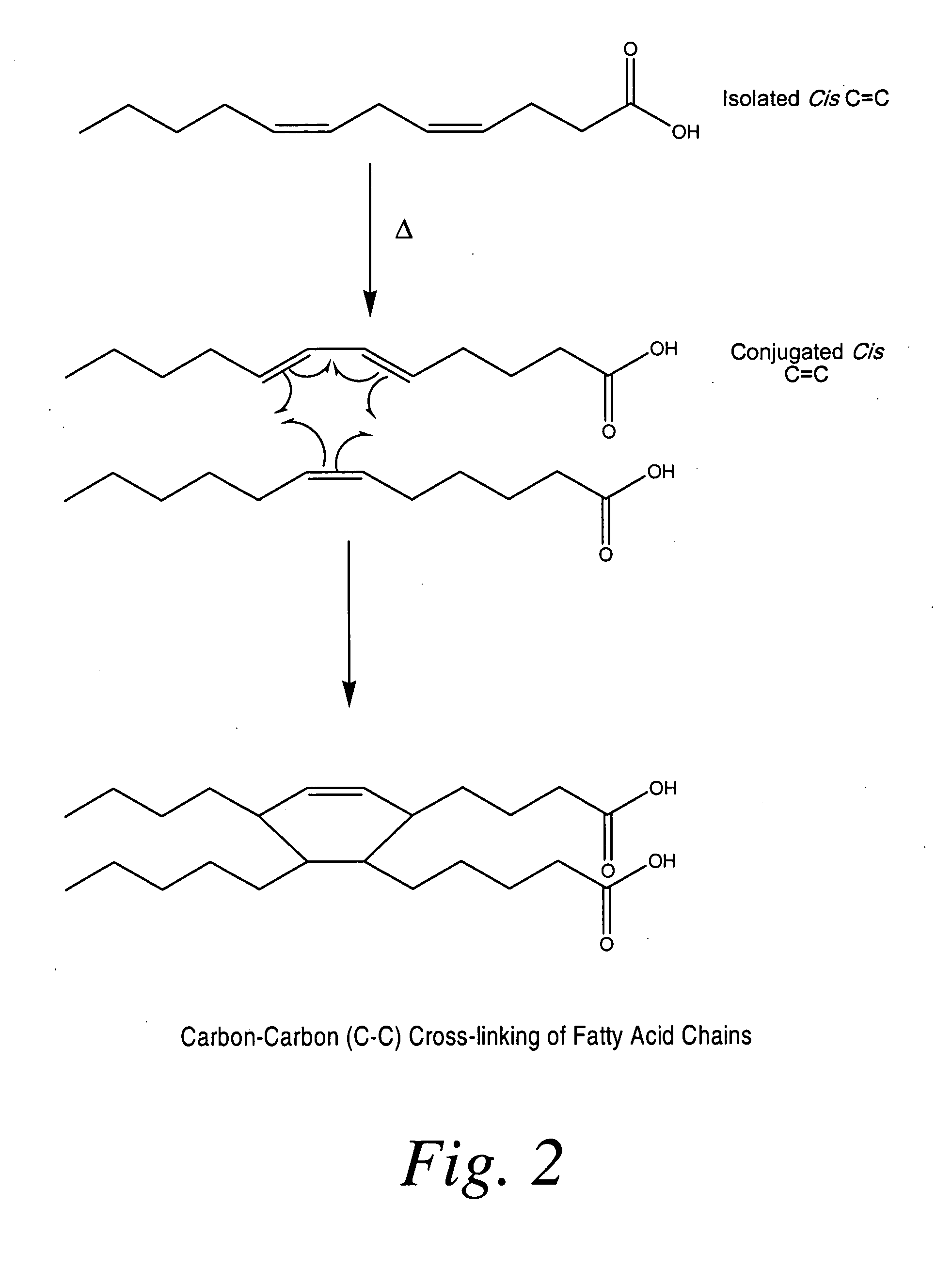
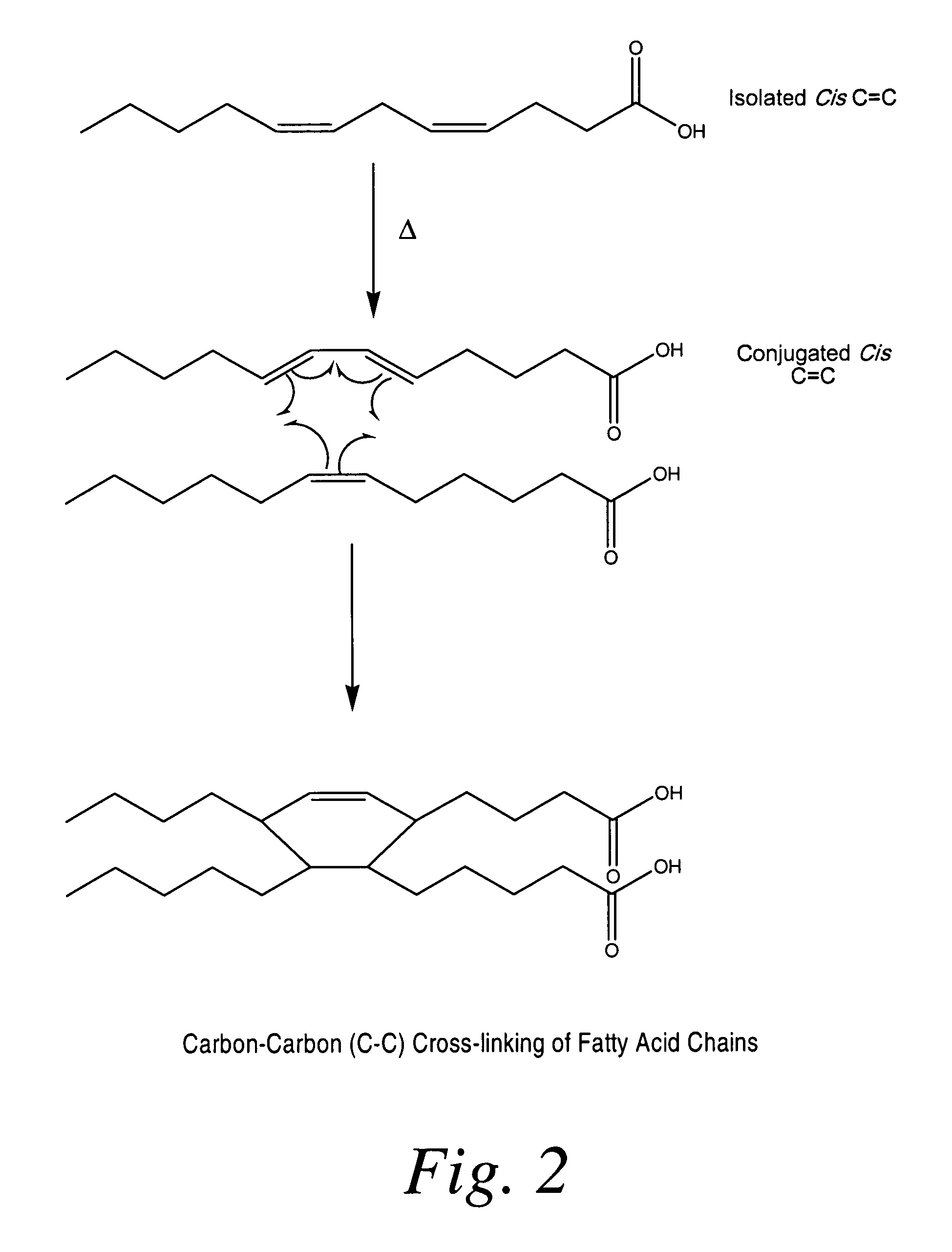
Cross-linked fatty acid-based biomaterials
InactiveUS20090208552A1Reduced oxidizable reactive sitesPrevent oxidationOrganic active ingredientsPeptide/protein ingredientsCross-linkMedicine
Fatty acid-based, pre-cure-derived biomaterials, methods of making the biomaterials, and methods of using them as drug delivery carriers are described. The fatty acid-derived biomaterials can be utilized alone or in combination with a medical device for the release and local delivery of one or more therapeutic agents. Methods of forming and tailoring the properties of said biomaterials and methods of using said biomaterials for treating injury in a mammal are also provided.
Owner:ATRIUM MEDICAL
Tamper-resistant tablet providing immediate drug release
InactiveUS20130028970A1High weight ratioImproved release profileOrganic active ingredientsBiocideParticulatesImmediate release
The invention relates to a tamper-resistant tablet comprising(i) a matrix material in an amount of more than one third of the total weight of the tablet; and(ii) a plurality of coated particulates in an amount of less than two thirds of the total weight of the tablet; wherein said particulates comprise a pharmacologically active compound and a physiologically acceptable polymer, preferably a polyalkylene oxide; and form a discontinuous phase within the matrix material;which preferably provides under in vitro conditions immediate release of the pharmacologically active compound in accordance with Ph. Eur.;and method of using said tablet to treat pain and other conditions.
Owner:GRUNENTHAL GMBH
Polymer-based sustained release device
ActiveUS20050271702A1Improve bioavailabilityMinimizes loss of activityPowder deliveryPeptide/protein ingredientsMedicineSugar
This invention relates to compositions for the sustained release of biologically active polypeptides, and methods of forming and using said compositions, for the sustained release of biologically active polypeptides. The sustained release compositions of this invention comprise a biocompatible polymer having dispersed therein, a biologically active polypeptide and a sugar.
Owner:ALKERMES PHARMA IRELAND LTD
Composition and method for preparing biocompatible surfaces
InactiveUS20060216324A1Reduced activityChallenge can be overcomeImpression capsSurgeryPre irradiationBiocompatible coating
The invention provides methods and compositions for providing biocompatible surfaces to medical articles. In particular the invention provides biocompatible coatings with heparin activity. In some aspects, the biocompatible coatings of the invention are able to release a bioactive agent. The coatings can be formed using biostable or biodegradable polymeric material and photoreactive groups. The invention also provides methods for improving the quality of bioactive agent-containing coatings by performing pre-irradiation of biocompatible coating compositions.
Owner:STUCKE SEAN M +3
Tamper-resistant tablet providing immediate drug release
InactiveUS20130028972A1Increase relative weight ratioHigh weight ratioBiocideOrganic active ingredientsOxideImmediate release
The invention relates to a tamper-resistant tablet comprising(i) a matrix material in an amount of more than one third of the total weight of the tablet; and(ii) a plurality of particulates in an amount of less than two thirds of the total weight of the tablet; wherein said particulates comprise a pharmacologically active compound and a polyalkylene oxide; and form a discontinuous phase within the matrix material;and method of using said tablet to treat pain and other conditions.
Owner:GRUNENTHAL GMBH
Microencapsulation and sustained release of biologically active polypeptides
ActiveUS7164005B2Avoid a lot of problemsIncrease acceptancePowder deliveryOrganic active ingredientsMedicineSugar
This invention relates to compositions for the sustained release of biologically active polypeptides, and methods of forming and using said compositions, for the sustained release of biologically active polypeptides. The sustained release compositions of this invention comprise a biocompatible polymer having dispersed therein, a biologically active polypeptide, a sugar and a salting-out salt.
Owner:ALKERMES PHARMA IRELAND LTD
Pharmaceutical compositions for sustained release delivery of peptides
ActiveUS20080020016A1Reduce undesired initial burst releaseImproved profileAntibacterial agentsPeptide/protein ingredientsOrganic solventMedicine
The present invention provides methods of forming a solid, biodegradable implant in-situ in a body by administering a liquid pharmaceutical composition comprising an effective amount of a biocompatible, water-insoluble, biodegradable polymer and an effective amount of a therapeutic peptide covalently modified with one or more lipophilic or amphiphilic moieties, which are dissolved or dispersed in a biocompatible, water-soluble organic solvent. This invention also provides related compositions and methods.
Owner:FORESEE PHARMA CO LTD
Polymer-based sustained release device
InactiveUS20060110423A1Improve bioavailabilityMinimizes loss of activityPeptide/protein ingredientsMicrocapsulesMedicineSugar
This invention relates to compositions for the sustained release of biologically active polypeptides, and methods of forming and using said compositions, for the sustained release of biologically active polypeptides. The sustained release compositions of this invention comprise a biocompatible polymer having dispersed therein, a biologically active polypeptide and a sugar.
Owner:WRIGHT STEVEN G +8
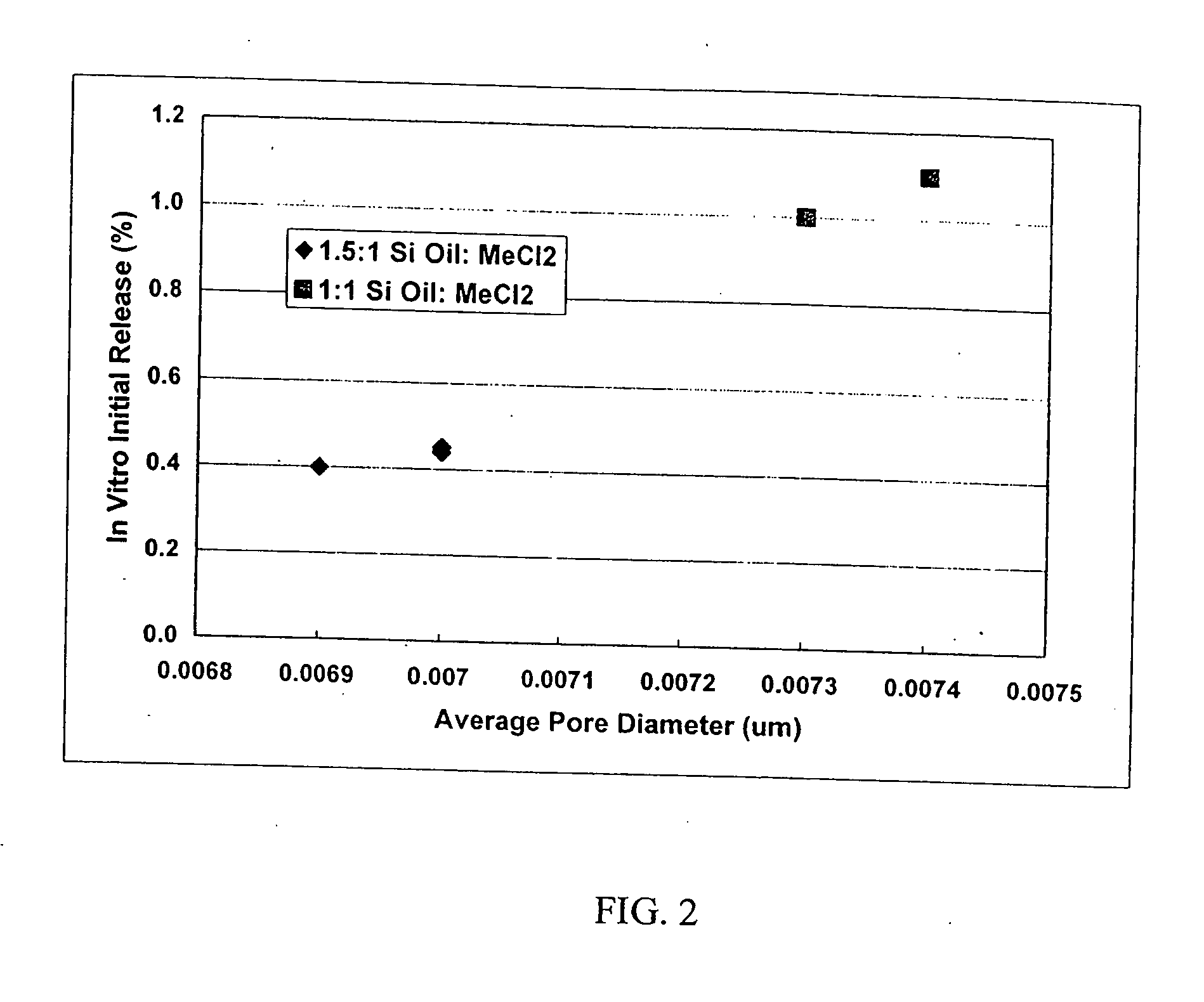
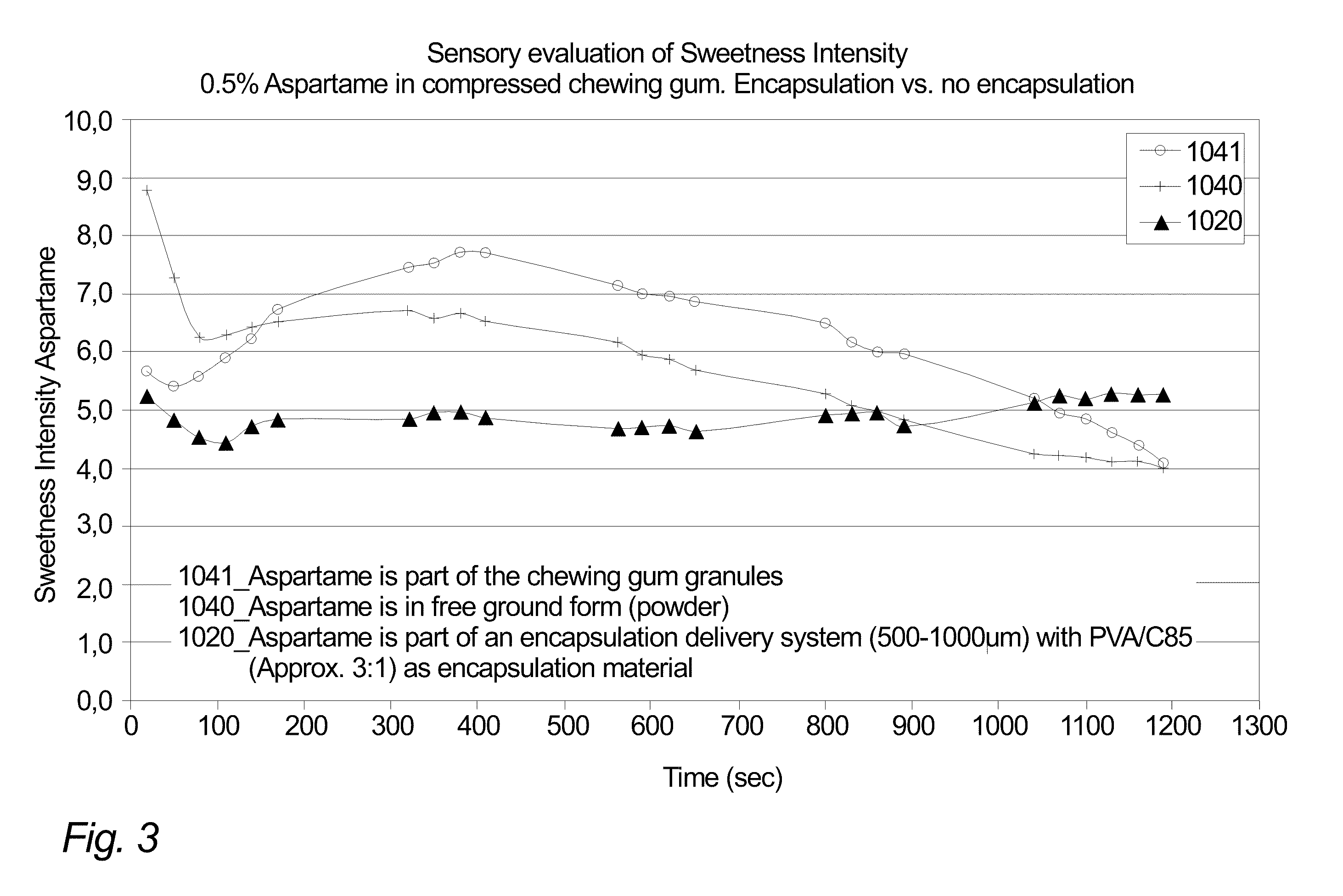
Compressed Chewing Gum Comprising An Encapsulation Delivery System Comprising Natural Resin
InactiveUS20100104689A1Avoid interactionWithout compromising texture propertyContainers for annular articlesChewing gumNatural resinAdditive ingredient
A compressible chewing gum composition includes i) chewing gum granules containing gum base and ii) particles of one or more encapsulation delivery systems including at least one encapsulation material and at least one active ingredient being encapsulated by the encapsulation material, wherein the encapsulation material includes at least one natural resin. The compressible chewing gum composition, which has been provided, is suitable for compression into compressed chewing gum tablets. Such compressed chewing gum tablets thus include a compression of a quantity of the compressible chewing gum composition and thus include chewing gum granules containing gum base and particles of one or more encapsulation delivery systems including an active ingredient encapsulated by an encapsulation material including natural resin.
Owner:GUMLINK AS
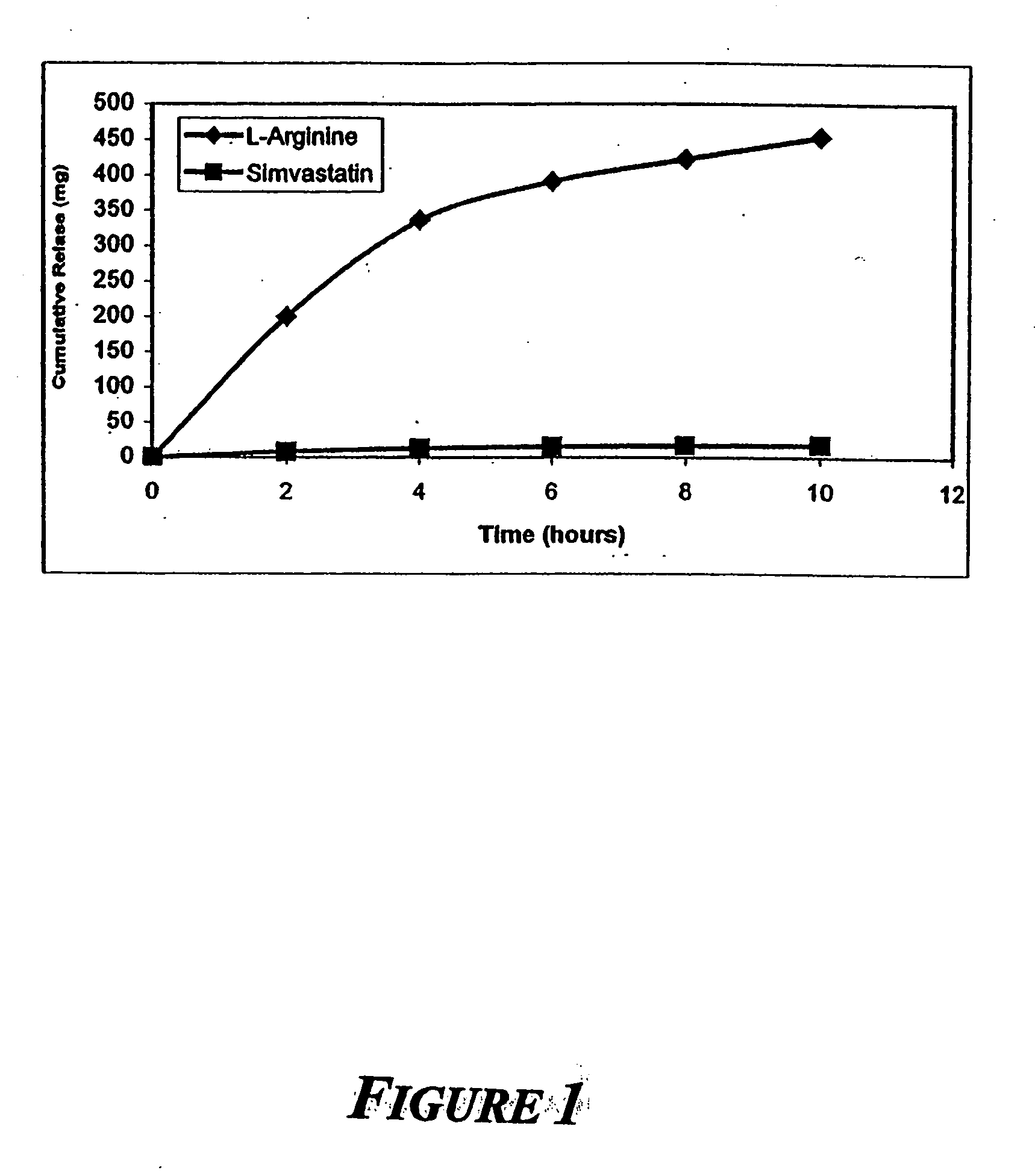
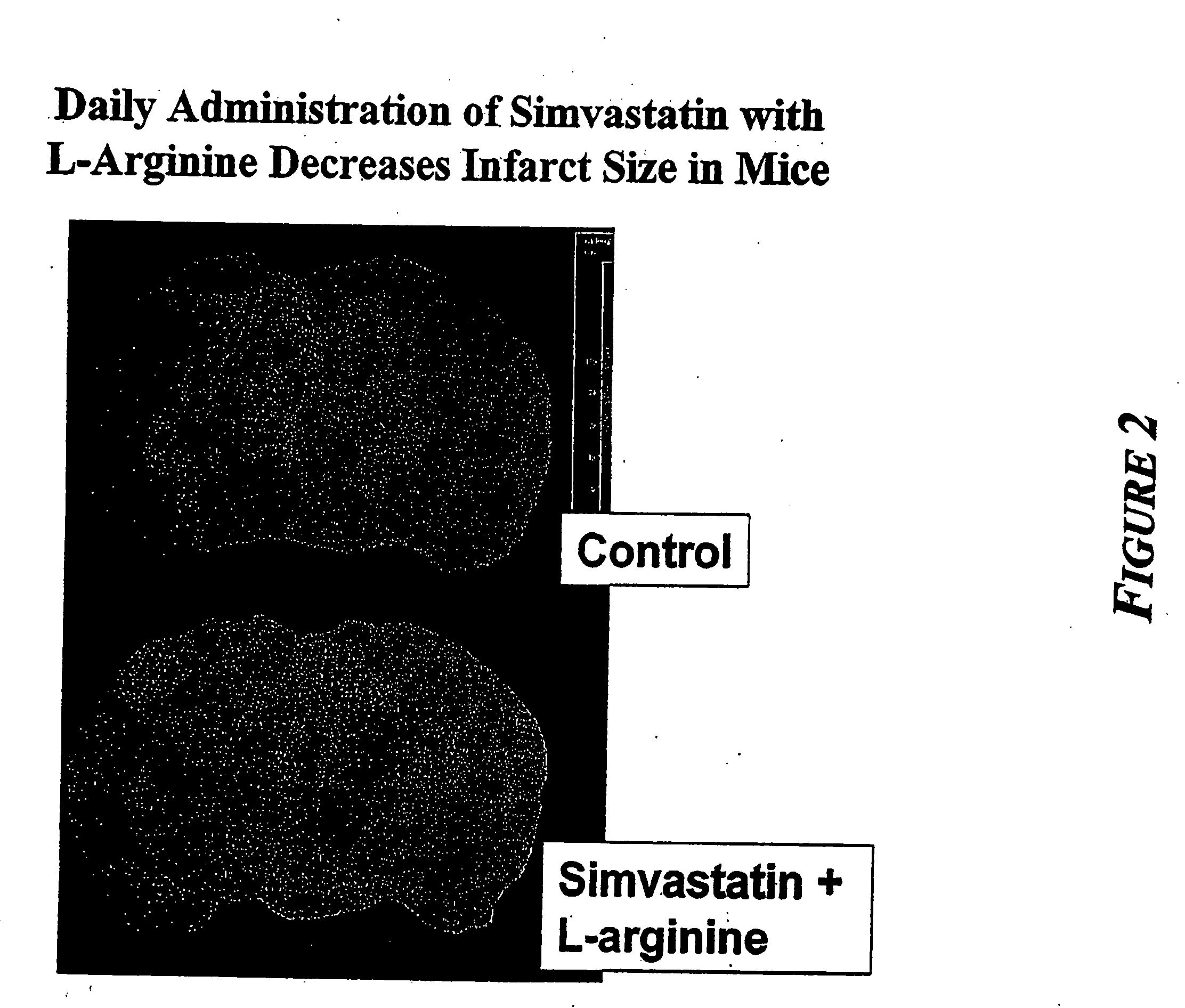
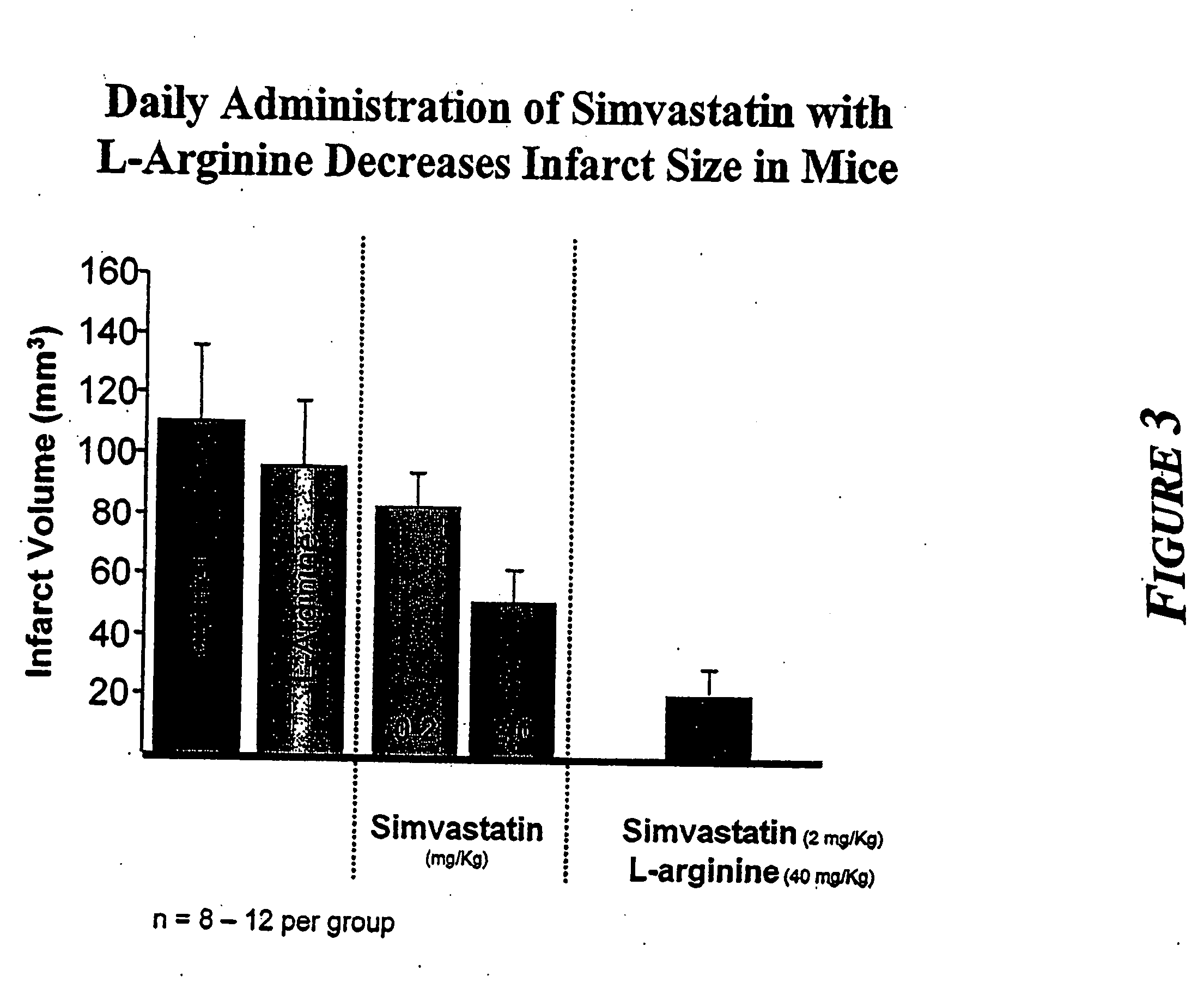
Sustained release L-arginine formulations and methods of manufacture and use
InactiveUS20050287210A1Easy to compressReduce cholesterolOrganic active ingredientsBiocideHMG-CoA reductaseArginine
The present invention provides methods and formulations for the treatment and prevention of cerebrovascular and cardiovascular diseases and disorders. The present invention is based, at least in part, on the discovery that administering to a subject a formulation comprising an agonist of endothelial nitric oxide synthase (eNOS), such as an HMG-CoA reductase inhibitor, and a formulation comprising a precursor of NO, such as L-arginine, may be used to treat or prevent cerebrovascular and / or cardiovascular diseases or disorders.
Owner:PALMETTO PHARMA
Polymer-based sustained release device
InactiveUS20080125348A1Improve bioavailabilityMinimizes loss of activityPeptide/protein ingredientsMetabolism disorderMedicineSugar
Owner:ALKERMES INC
Cross-linked fatty acid-based biomaterials
InactiveUS9000040B2Reduced oxidizable reactive sitesPrevent oxidationBiocideAntipyreticCross-linkMedicine
Fatty acid-based, pre-cure-derived biomaterials, methods of making the biomaterials, and methods of using them as drug delivery carriers are described. The fatty acid-derived biomaterials can be utilized alone or in combination with a medical device for the release and local delivery of one or more therapeutic agents. Methods of forming and tailoring the properties of said biomaterials and methods of using said biomaterials for treating injury in a mammal are also provided.
Owner:ATRIUM MEDICAL
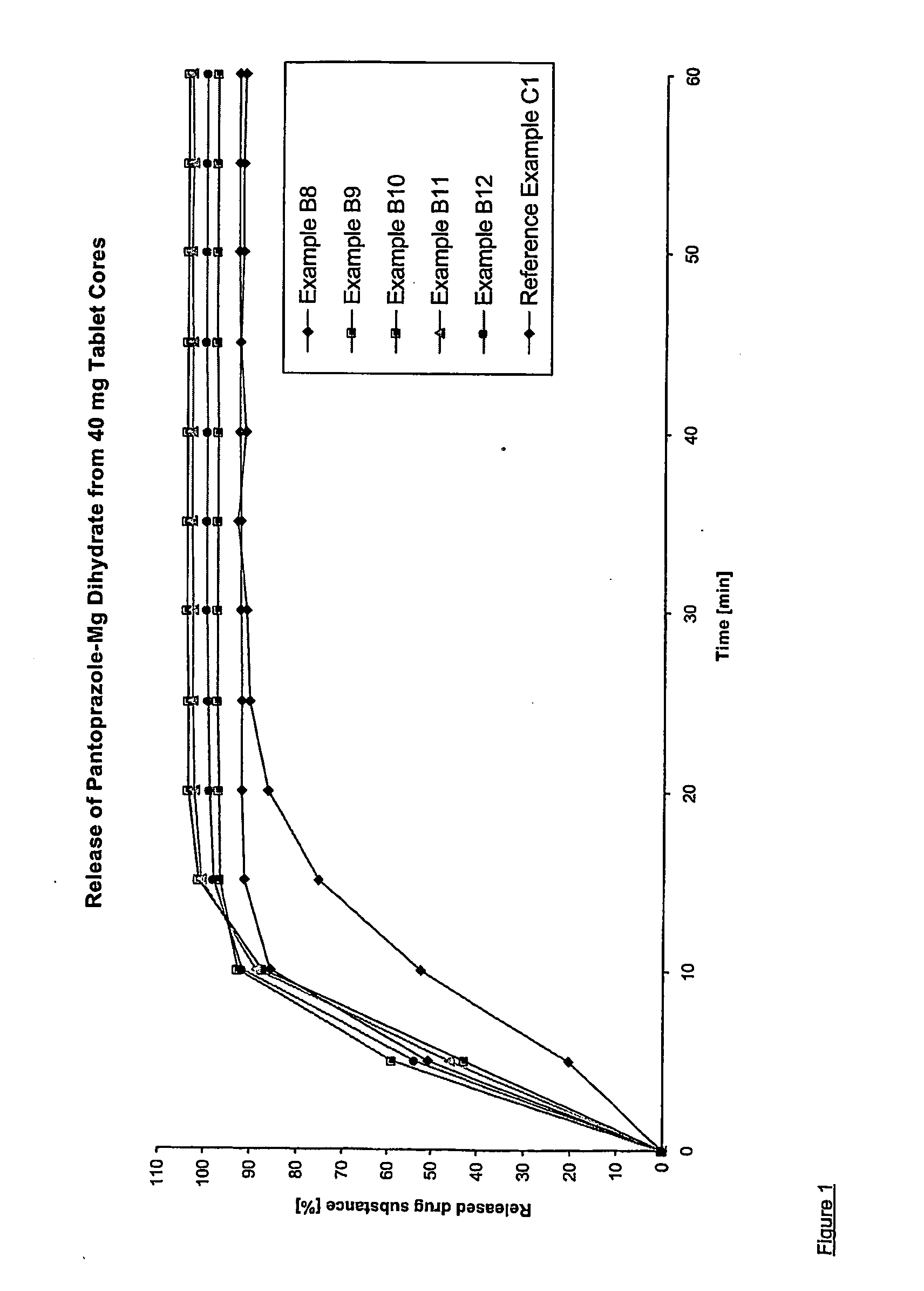
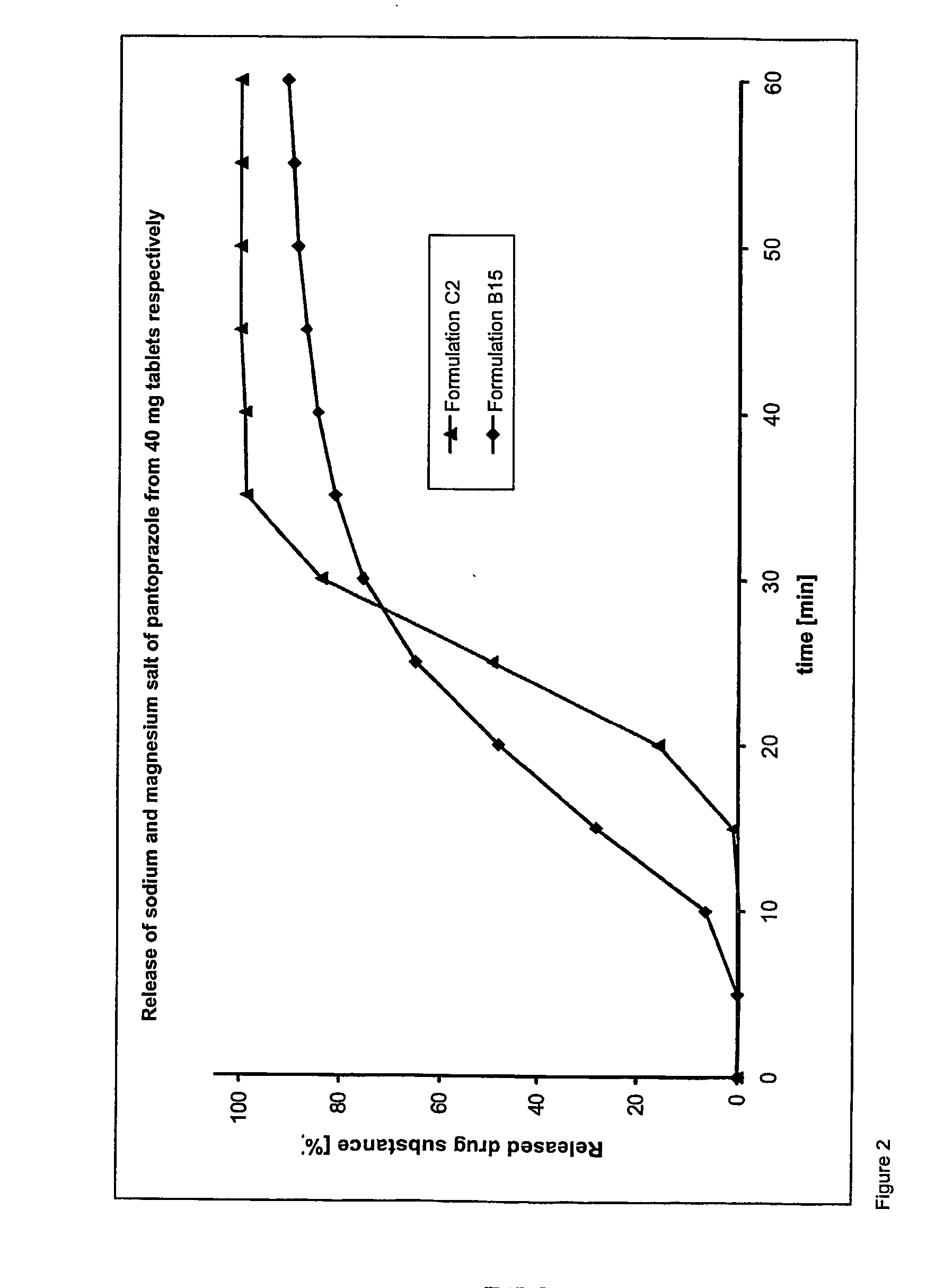
Dosage form containing pantoprazole as active ingredient
InactiveUS20060240100A1Without great technical complexityEnhance the imageBiocideDigestive systemOral medicationMagnesium salt
Owner:NYCOMED GMBH
Polymer-based sustained release device
InactiveUS20080125351A1Improve bioavailabilityMinimizes loss of activityOrganic active ingredientsPeptide/protein ingredientsMedicineSugar
This invention relates to compositions for the sustained release of biologically active polypeptides, and methods of forming and using said compositions, for the sustained release of biologically active polypeptides. The sustained release compositions of this invention comprise a biocompatible polymer having dispersed therein, a biologically active polypeptide and a sugar.
Owner:ALKERMES INC
Polymer-based sustained release device
ActiveUS20080125349A1Improve bioavailabilityMinimizes loss of activityPeptide/protein ingredientsMicrocapsulesMedicineSugar
This invention relates to compositions for the sustained release of biologically active polypeptides, and methods of forming and using said compositions, for the sustained release of biologically active polypeptides. The sustained release compositions of this invention comprise a biocompatible polymer having dispersed therein, a biologically active polypeptide and a sugar.
Owner:ALKERMES PHARMA IRELAND LTD
Non-polymeric compositions for controlled drug delivery
InactiveUS8586103B2Maintain physical stabilityEnhanced chemical and physical stabilityAntibacterial agentsBiocidePharmaceutical SubstancesCompatibilization
The present invention provides a novel liquid composition suitable for in-situ formation of a depot system to deliver a bioactive substance in a controlled manner. The composition of the present invention comprises: (a) a hydrophobic non-polymeric carrier material; (b) a water miscible biocompatible organic solvent that dissolves the hydrophobic non-polymeric material; (c) an ionic complex that is formed between an amphiphilic molecule and a bioactive substance having a net charge at neutral pH in water. The present invention also provides a method of manufacturing and use of the composition thereof.
Owner:FORESEE PHARMA CO LTD
Liquid composition for air freshener systems
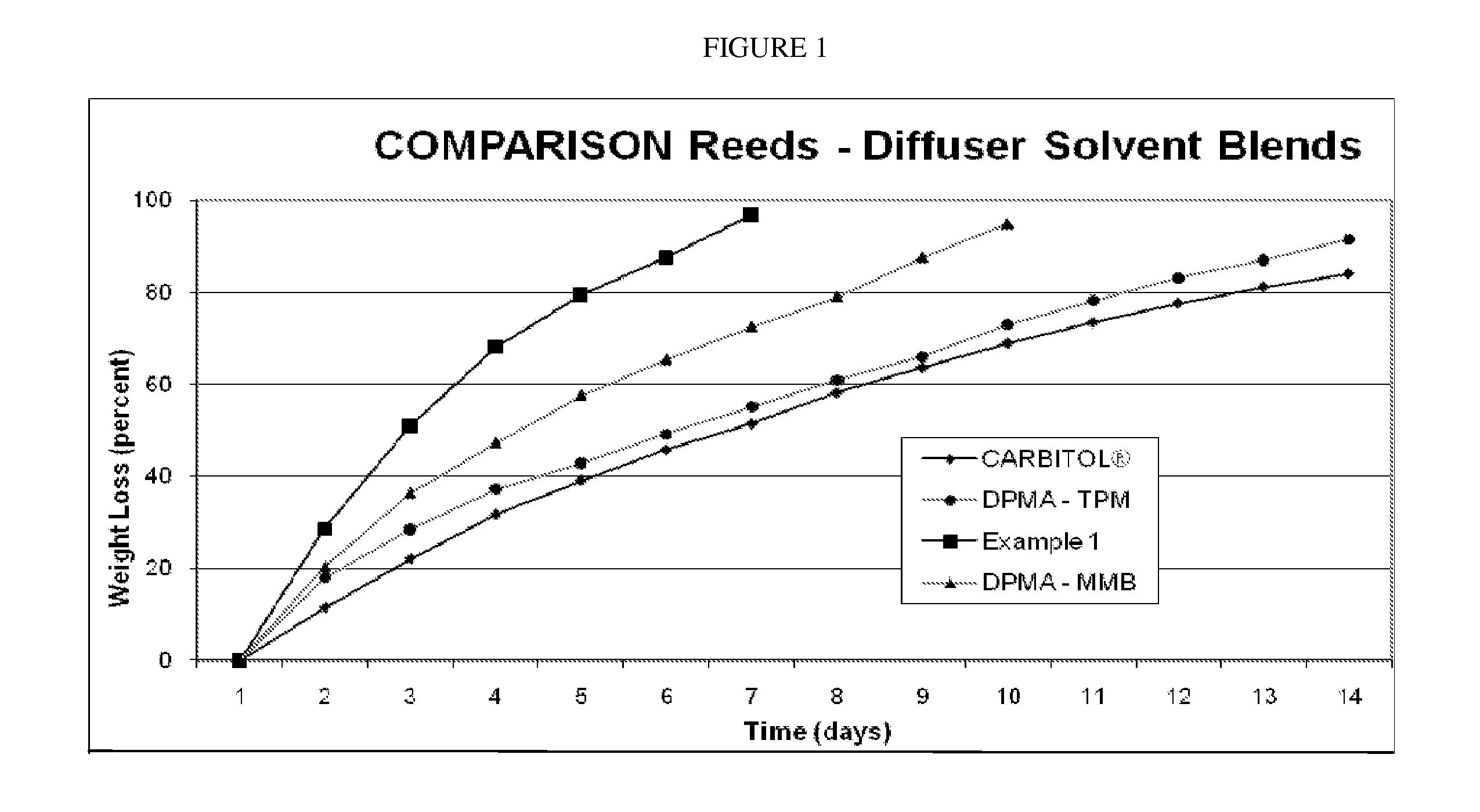
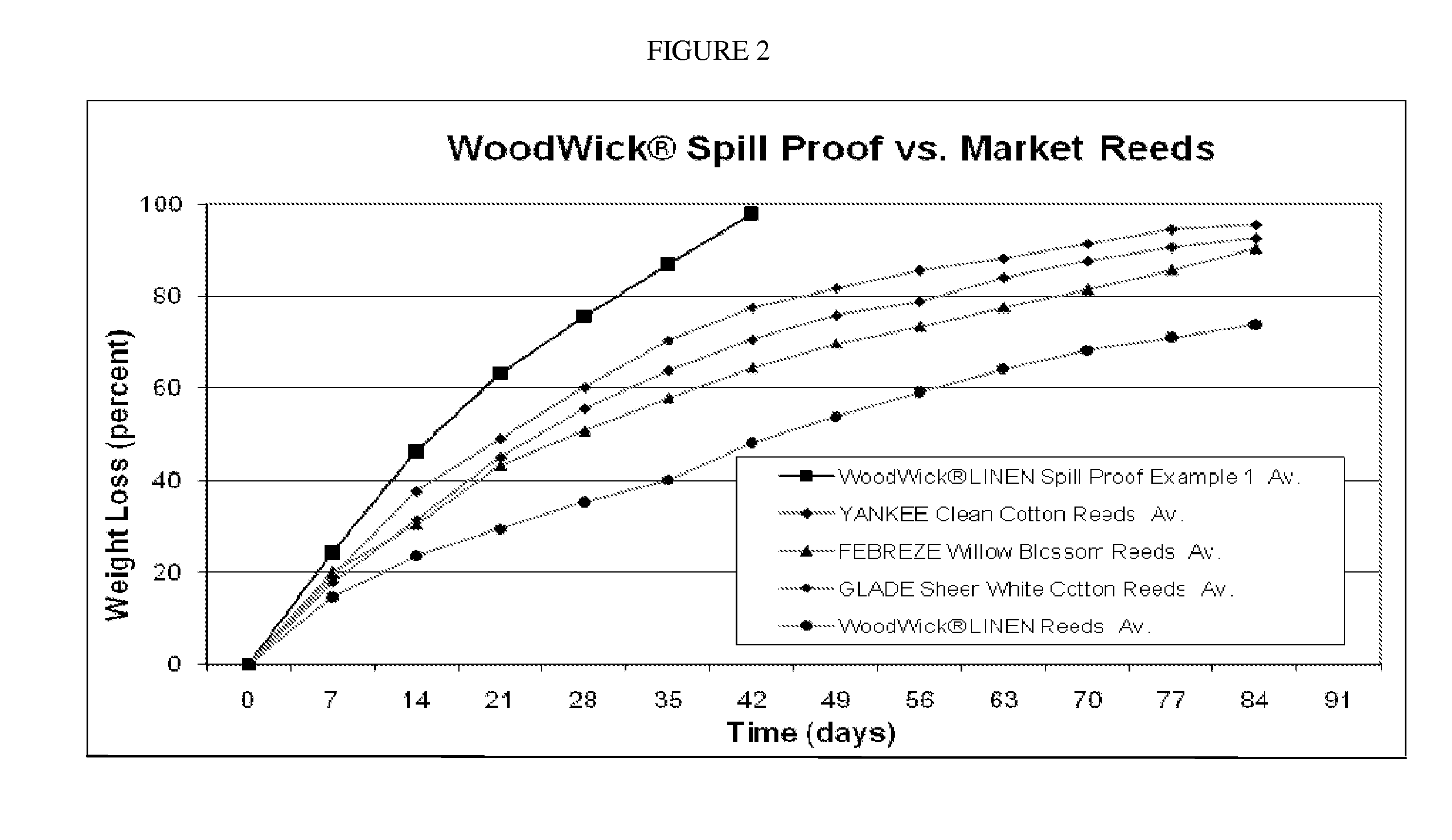
InactiveUS20110095097A1Increase evaporation rateFacilitate stabilization and solubilizationTobacco devicesGaseous substancesSolventLiquid composition
Embodiments relate to liquid air freshener systems that allow fragrances to evaporate through use of a wicking element where fluid moves by capillary action towards the emanating surface, as well as the fragrance stabilizing compositions used therein. Cyclomethicone-based liquid compositions, preferably clear, provide an improved rate of evaporation of perfume materials from the air freshener device emanating surface and allow the use of fragrance materials with a wide range of characteristics. Embodiments utilize cyclomethicone in combination with other solvents specifically to increase solubility, enhance performance, maintain consistent fragrance character and maximize the range of aromatic materials that can be used.
Owner:BELMAY
Polymer-based sustained release device
InactiveUS20080119569A1Improve bioavailabilityMinimizes loss of activityOrganic active ingredientsBiocideMedicineSugar
This invention relates to compositions for the sustained release of biologically active polypeptides, and methods of forming and using said compositions, for the sustained release of biologically active polypeptides. The sustained release compositions of this invention comprise a biocompatible polymer having dispersed therein, a biologically active polypeptide and a sugar.
Owner:ALKERMES INC
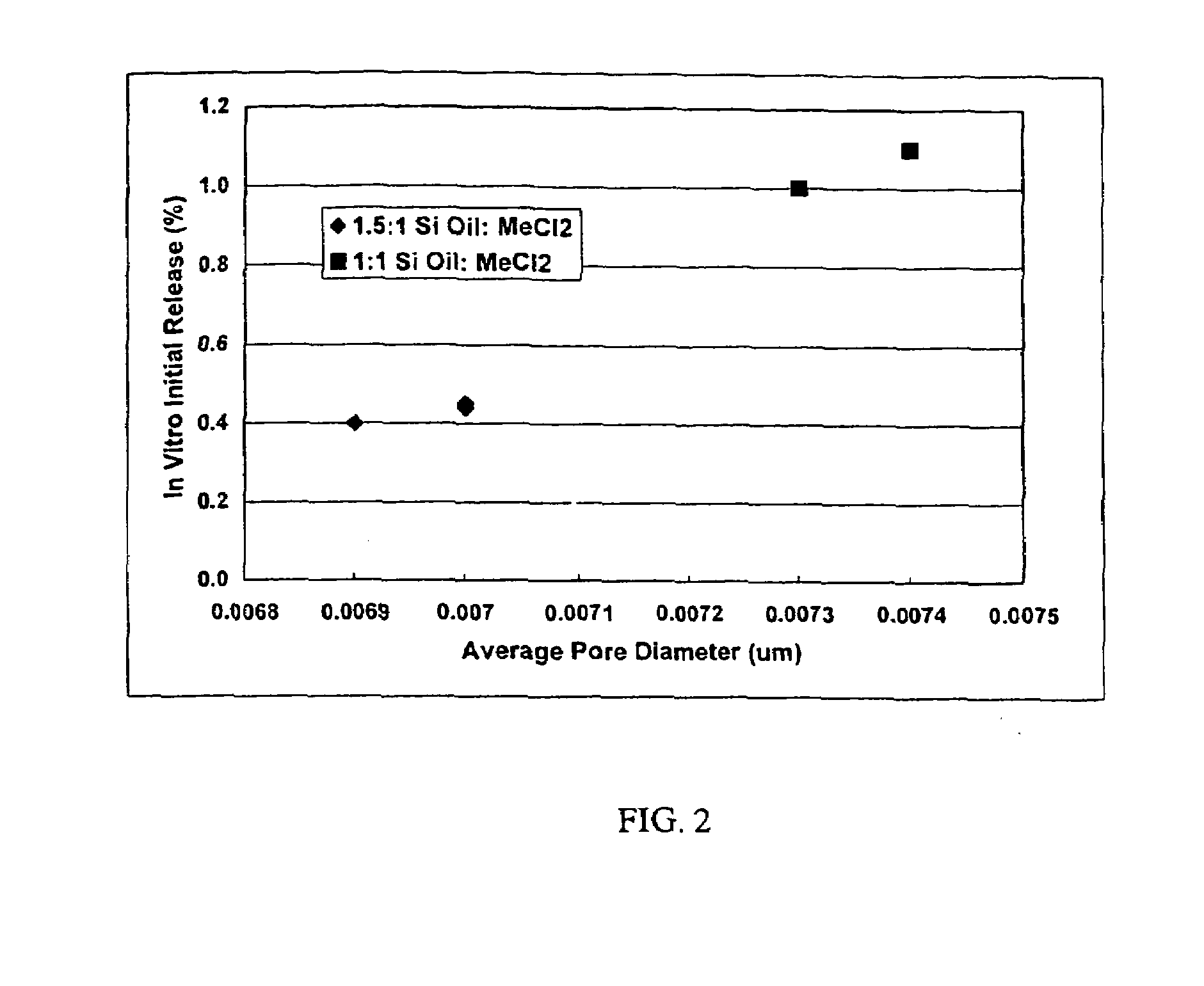
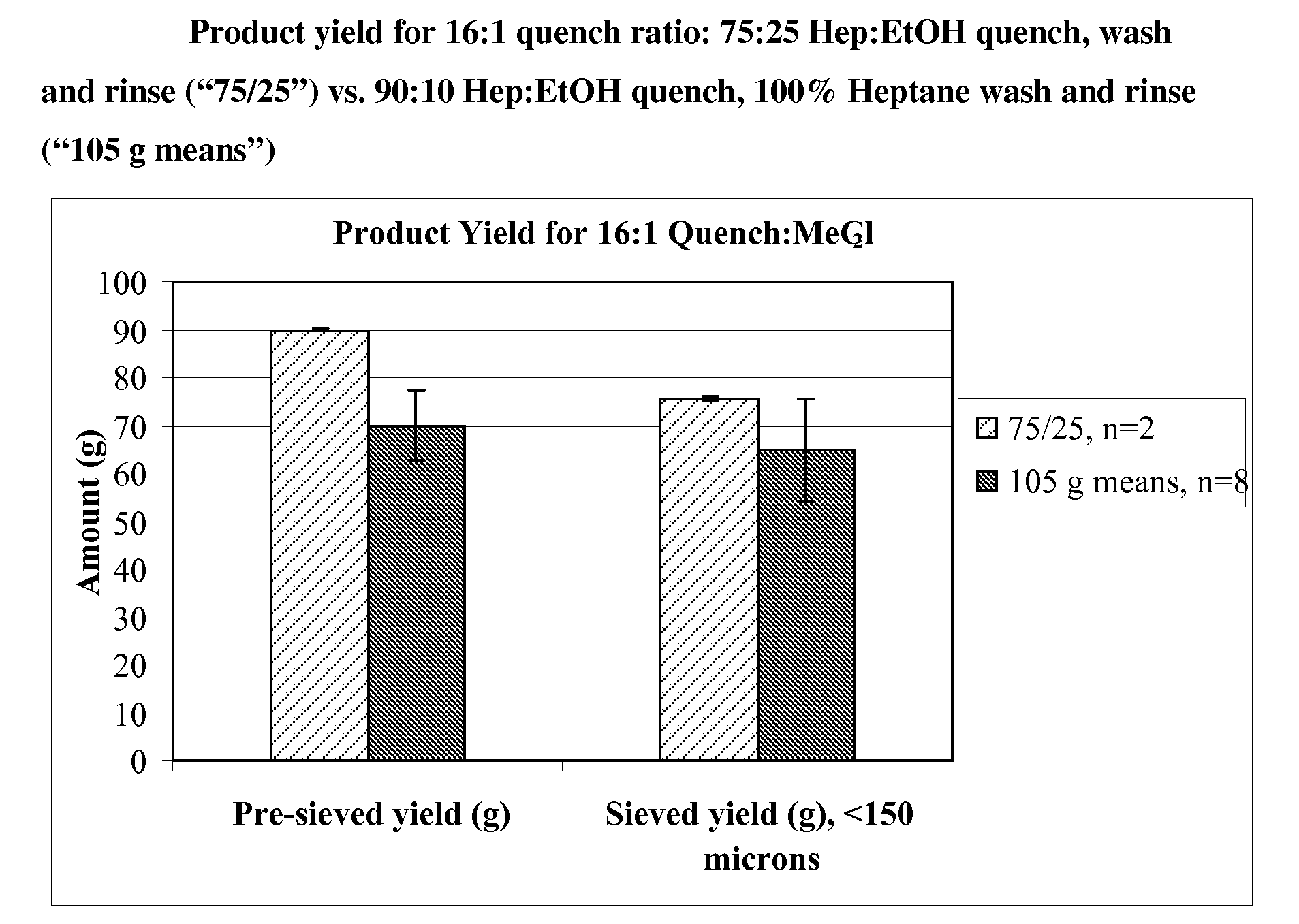
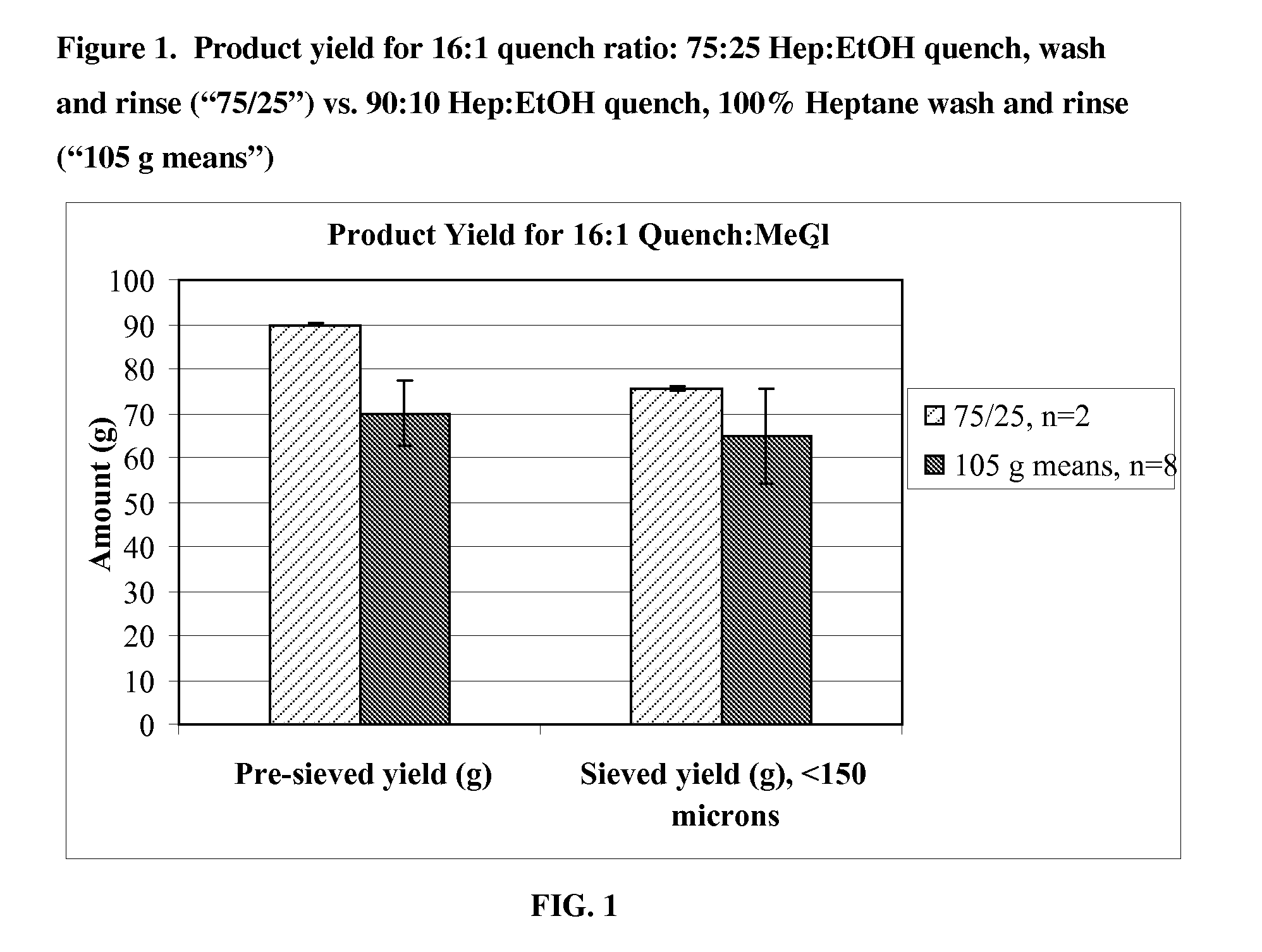
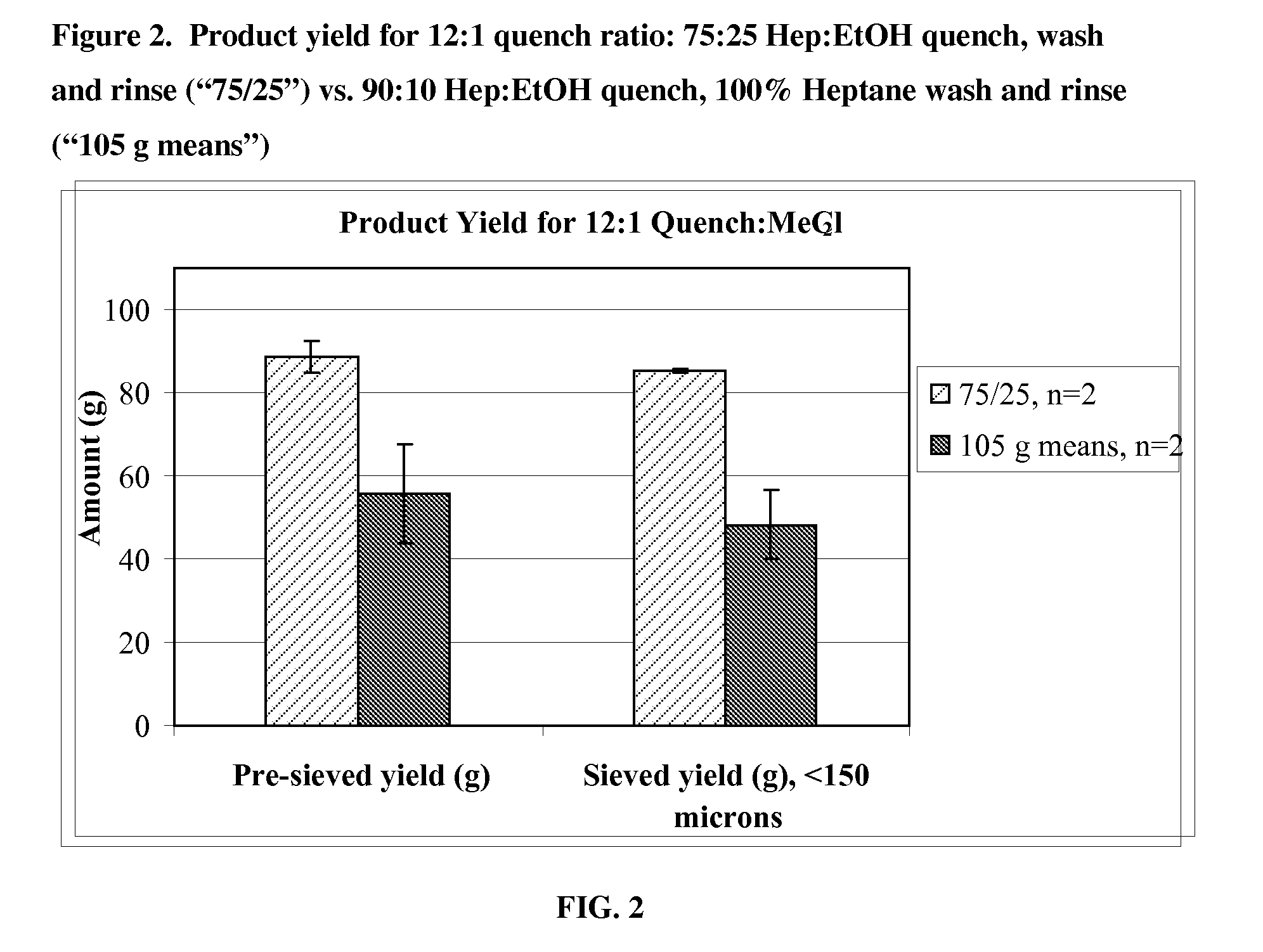
Quench liquids and washing systems for production of microparticles
InactiveUS20080317865A1Reduce amount and concentrationImprove bioavailabilityPowder deliverySaccharide peptide ingredientsActive agentWater soluble
The present invention provides coacervation methods forming compositions for the sustained release water soluble active agents, including biologically active polypeptides. The invention further relates to the discovery of improved non-aqueous quench liquids and washing systems, which enable a reduction in the amount and concentration of hardening agents such as heptane used to produce microparticles, while providing acceptable product yields and residual solvent levels.
Owner:ALKERMES INC
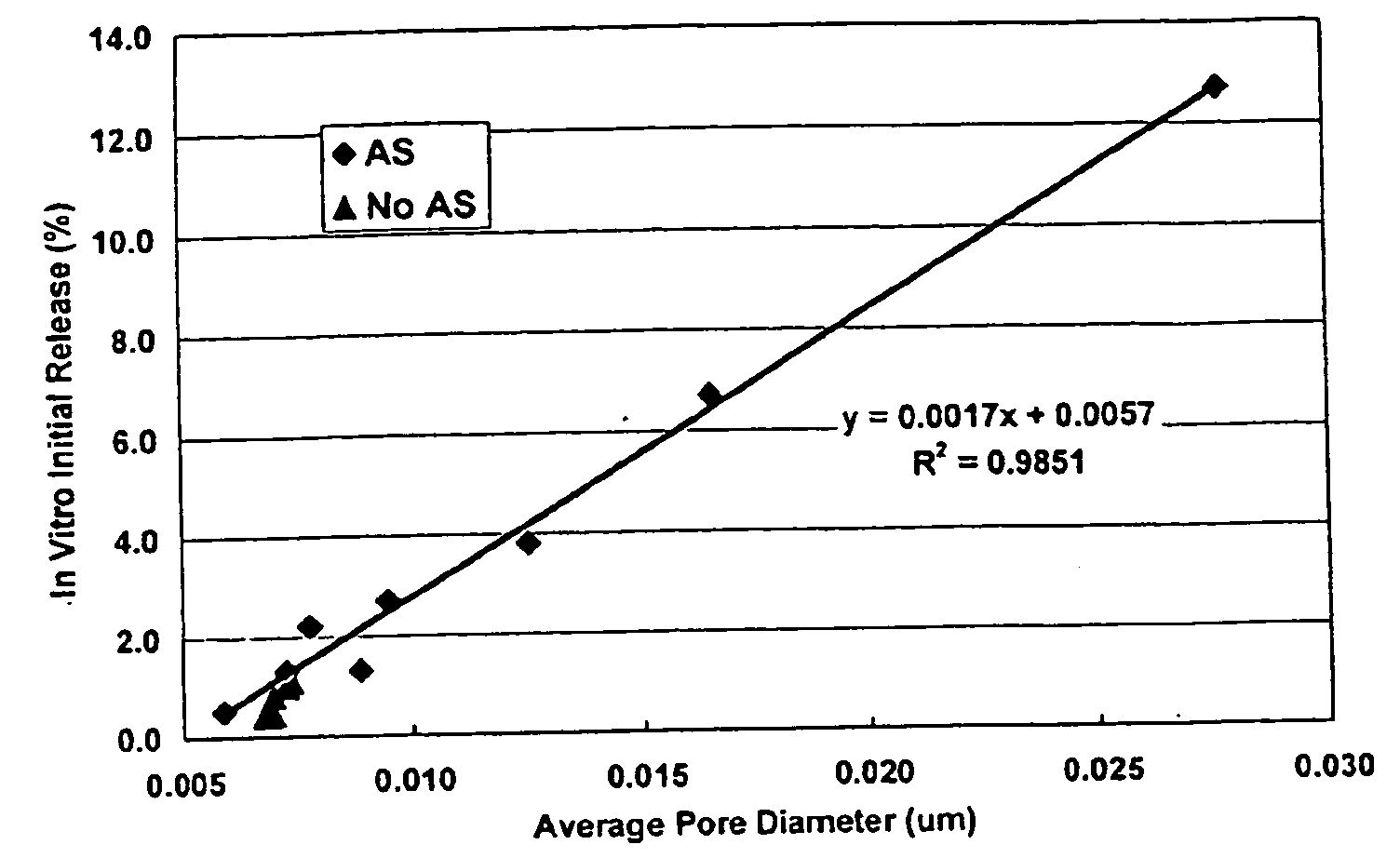
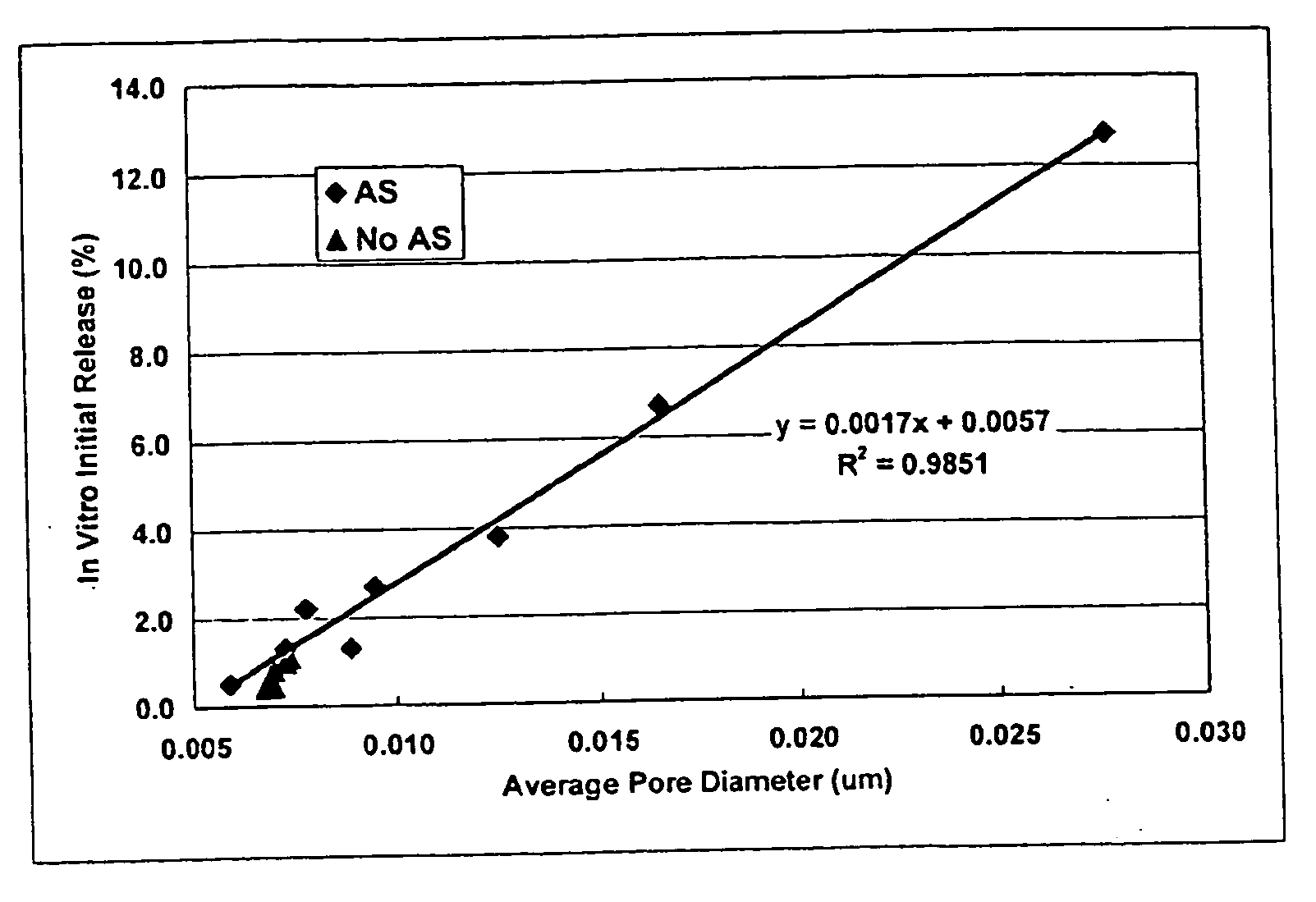
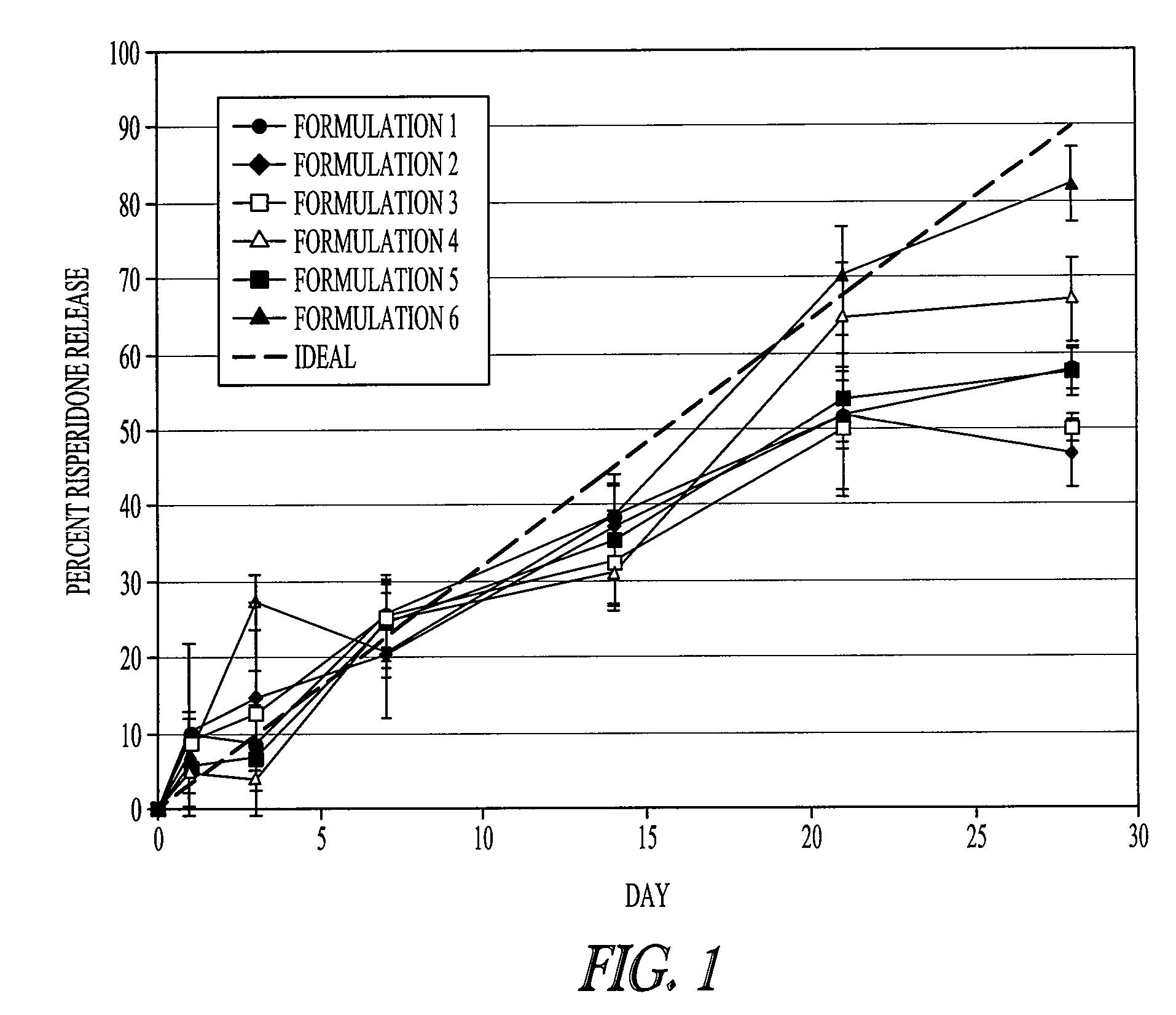
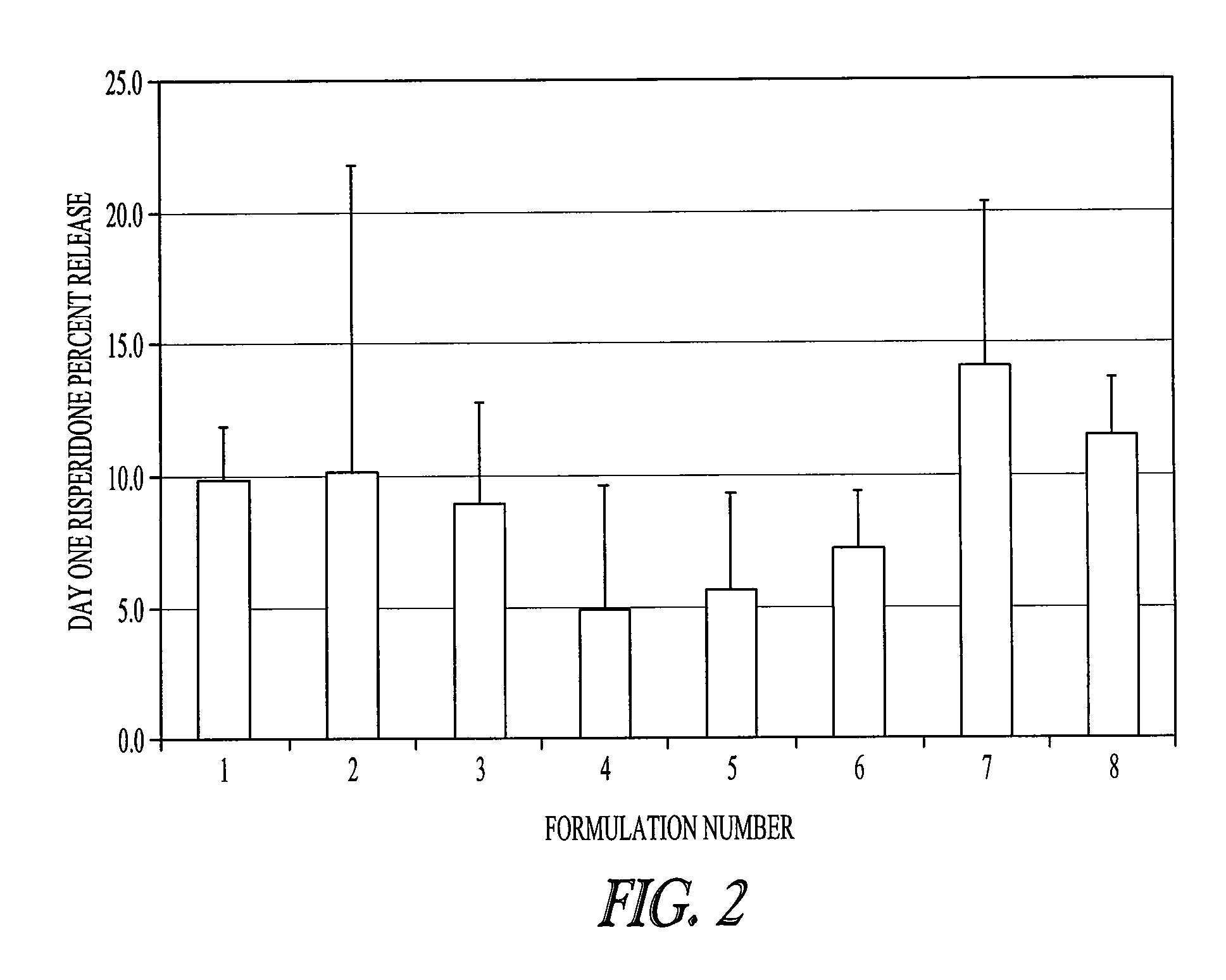
Method of modifying the release profile of sustained release compositions
InactiveUS20080057131A1Improve bioavailabilityDampen potentialOrganic active ingredientsPeptide/protein ingredientsIn vivoPharmacology
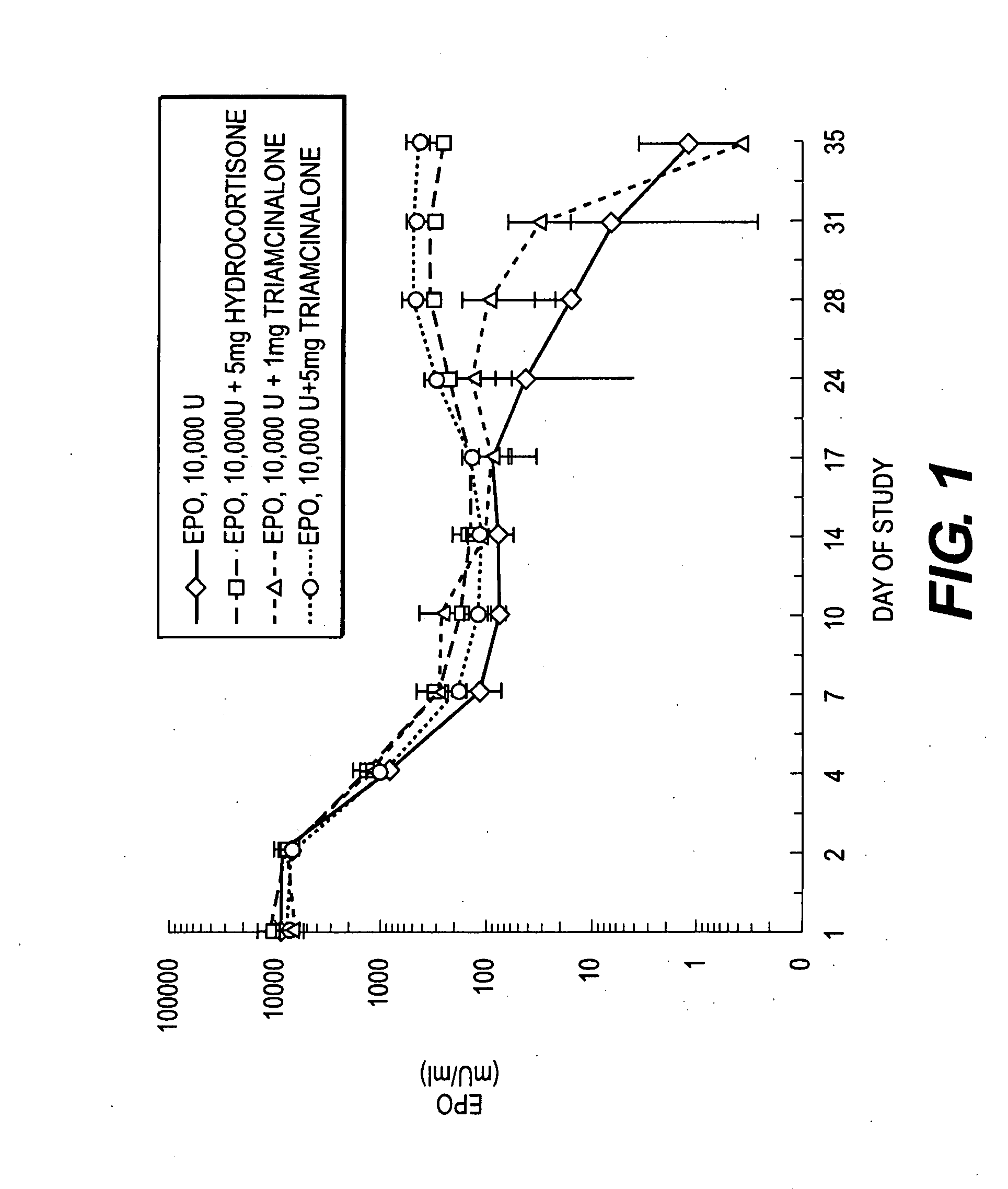
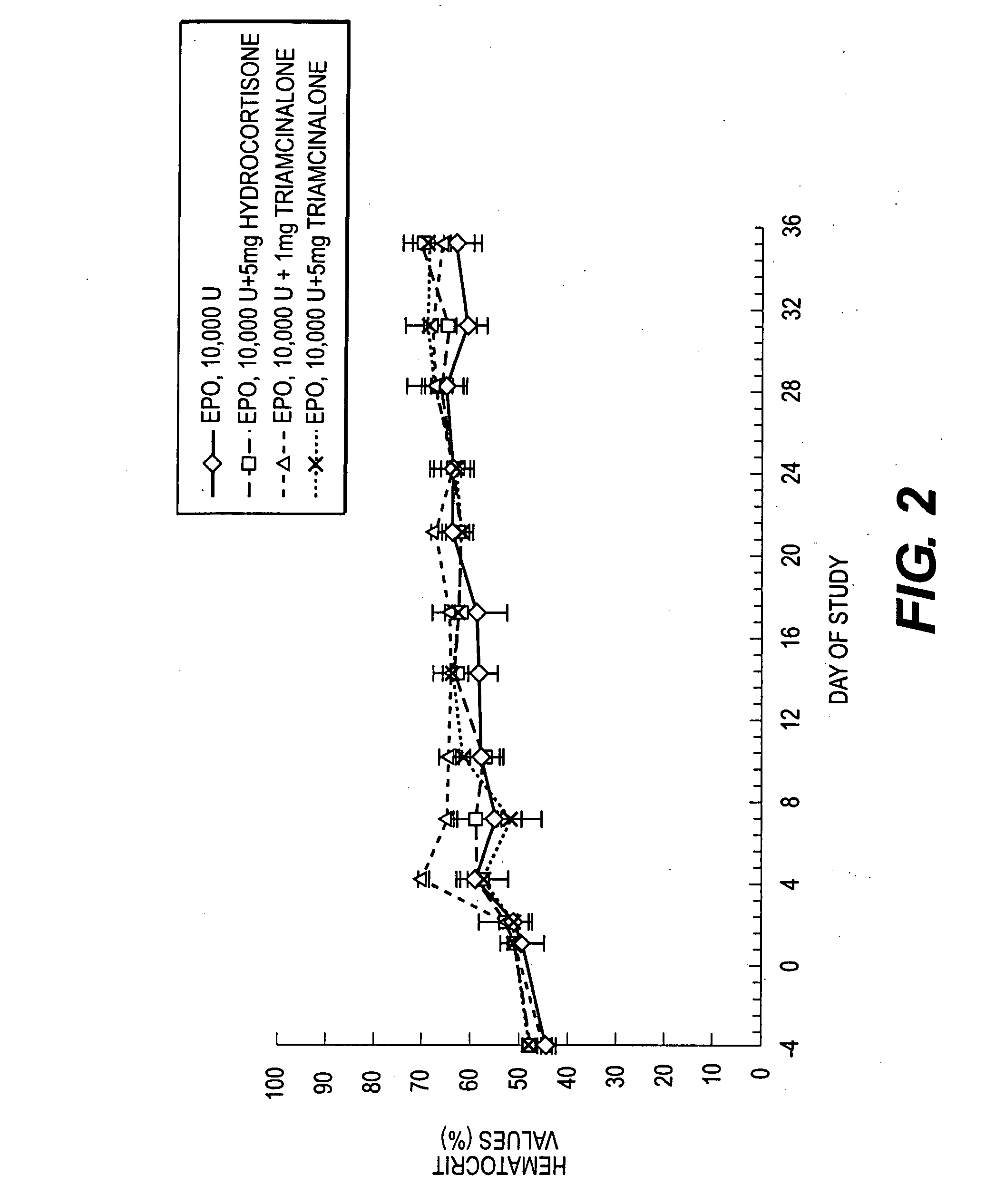
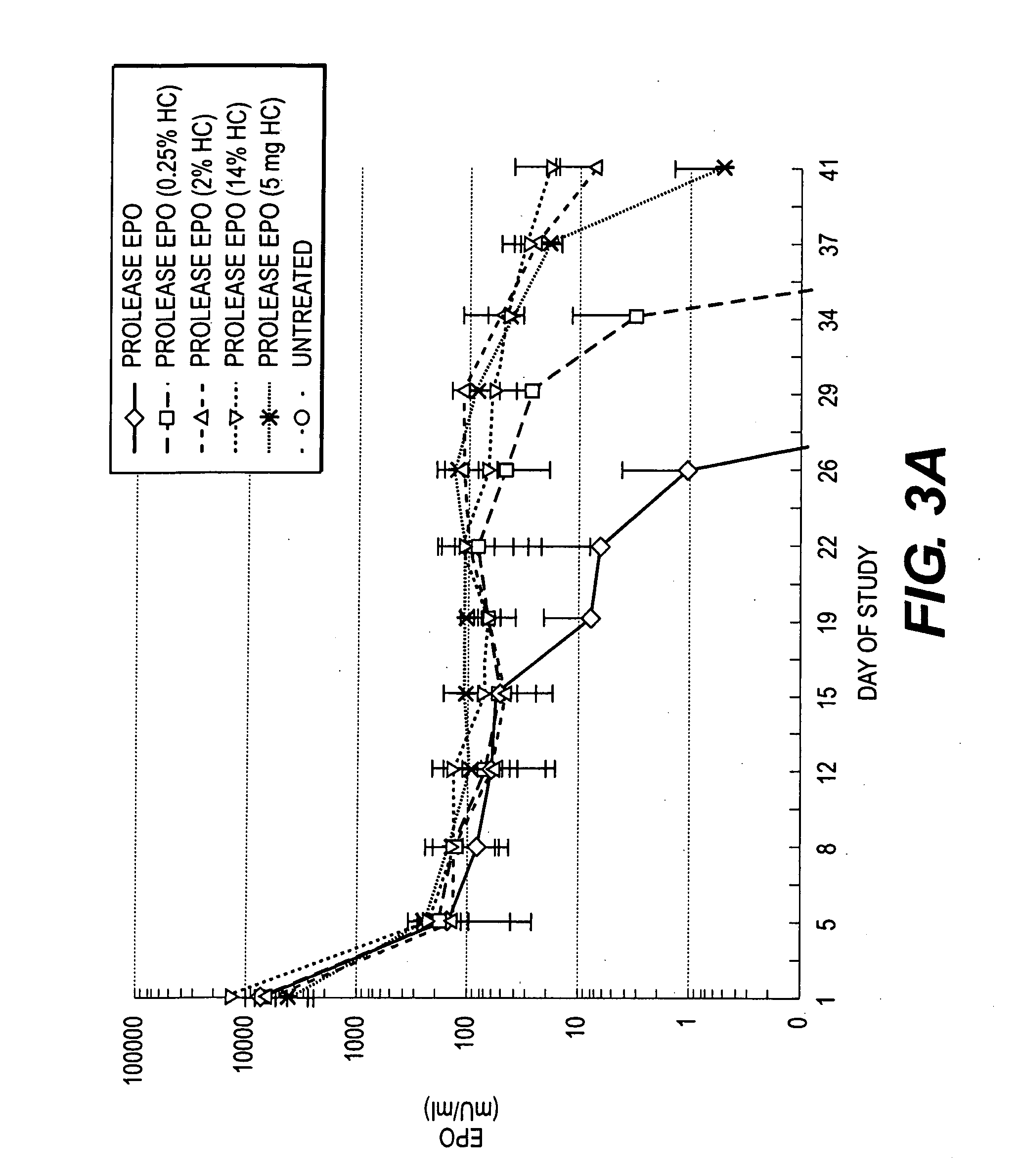
The present invention relates to a method for the sustained release in vivo of a biologically active labile agent comprising administering to a subject in need of treatment an effective amount of a sustained release composition comprising a biocompatible polymer having the biologically active labile agent incorporated therein, and a corticosteroid wherein the labile is released for a period of at least about two weeks. It is understood that the corticosteroid is present in an amount sufficient to modify the release profile of the biologically active labile agent from the sustained release composition. Pharmaceutical compositions suitable for use in the method of the invention are also disclosed.
Owner:ALKERMES CONTROLLED THERAPAUTICS INC
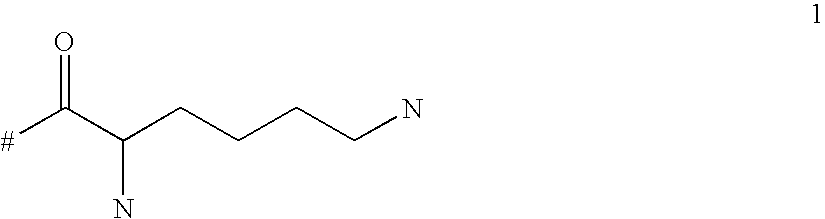
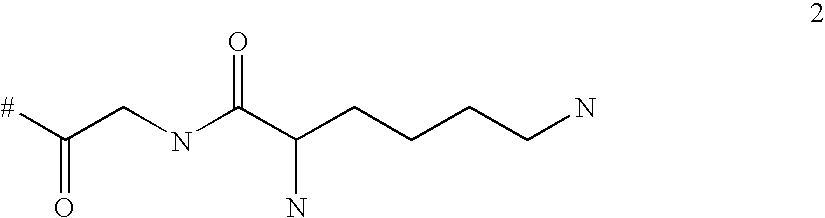
Targeted polylysine dendrimer therapeutic agent
ActiveUS20100292148A1Improve performanceImproved release profileNervous disorderDipeptide ingredientsChemistryMacromolecule
The present invention relates generally to branched macromolecules bearing functional moieties. In particular, the invention relates to dendrimers, derived from lysine or lysine analogues, bearing a plurality of functional moieties. The invention further relates to the use of such macromolecules, particularly in therapeutic applications, and compositions comprising them.
Owner:STARPHARMA PTY LTD
Controlled release copolymer formulation with improved release kinetics
InactiveUS20090325879A1Slight solubilityConstant release rateBiocideOrganic active ingredientsOligomerActive agent
The present invention provides a constant release copolymer composition adapted for use in a controlled release formulation for a bioactive agent, such as a formulation adapted for implantation within a patient's body tissues as a depot to release the agent over a period of time, wherein the copolymer provides a substantially constant rate of release of the bioactive agent over the time period for which the depot persists in the body tissues. The copolymer includes a PLG copolymer and a PLG oligomer of about 5-10 kDa average molecular weight, which can lack free carboxylic acid groups. When the PLG copolymer is a low burst copolymer, the constant release copolymer composition is a low burst, constant release copolymer composition adapted for implantation in the body tissues of a mammal, wherein a substantially constant rate of release of the bioactive agent is achieved.
Owner:TOLMAR THERAPEUTICS
Prepn process of slow release parathyroid hormone microballoon
InactiveCN1739795AHigh drug loadingImprove stabilityPeptide/protein ingredientsGranular deliveryFreeze-dryingMicrosphere
The slow release parathyroid hormone microballoon contains parathyroid hormone 2-20 wt%, high molecular weight polysaccharide 2-20 wt%, small molecular weight saccharide 0-10 wt%, and slow release polymer 75-95 wt%. The preparation process includes the following steps: A. dissolving parathyroid hormone in water solution of high molecular weight polysaccharide to form inner water phase; B. adding the inner water phase into the dichloromethane solution of polylactic acid-hydroxy acetic acid copolymer to form initial W / O emulsion; C. emulsifying the initial W / O emulsion into compound W / O / W emulsion via saturating outer water phase of PVA-NaCl aqua with dichloromethane and adding the initial W / O emulsion; D. adding compound W / O / W emulsion to NaCl aqua via stirring to solidify microballoon; and E. water washing the solidified microballoon and freeze drying. The present invention has improved release curve of parathyroid hormone microballoon, high stability of parathyroid hormone and increased medicine carrying amount of the microballoon.
Owner:SHANGHAI JIAO TONG UNIV
Pharmaceutical compositions for sustained release delivery of peptides
ActiveUS8206735B2Reduce undesired initial burst releaseImproved profileAntibacterial agentsPeptide/protein ingredientsBiocompatibilityPharmaceutical Substances
The present invention provides methods of forming a solid, biodegradable implant in-situ in a body by administering a liquid pharmaceutical composition comprising an effective amount of a biocompatible, water-insoluble, biodegradable polymer and an effective amount of a therapeutic peptide covalently modified with one or more lipophilic or amphiphilic moieties, which are dissolved or dispersed in a biocompatible, water-soluble organic solvent. This invention also provides related compositions and methods.
Owner:FORESEE PHARMA CO LTD
Kit for the treatment of onychomycosis by nitric oxide
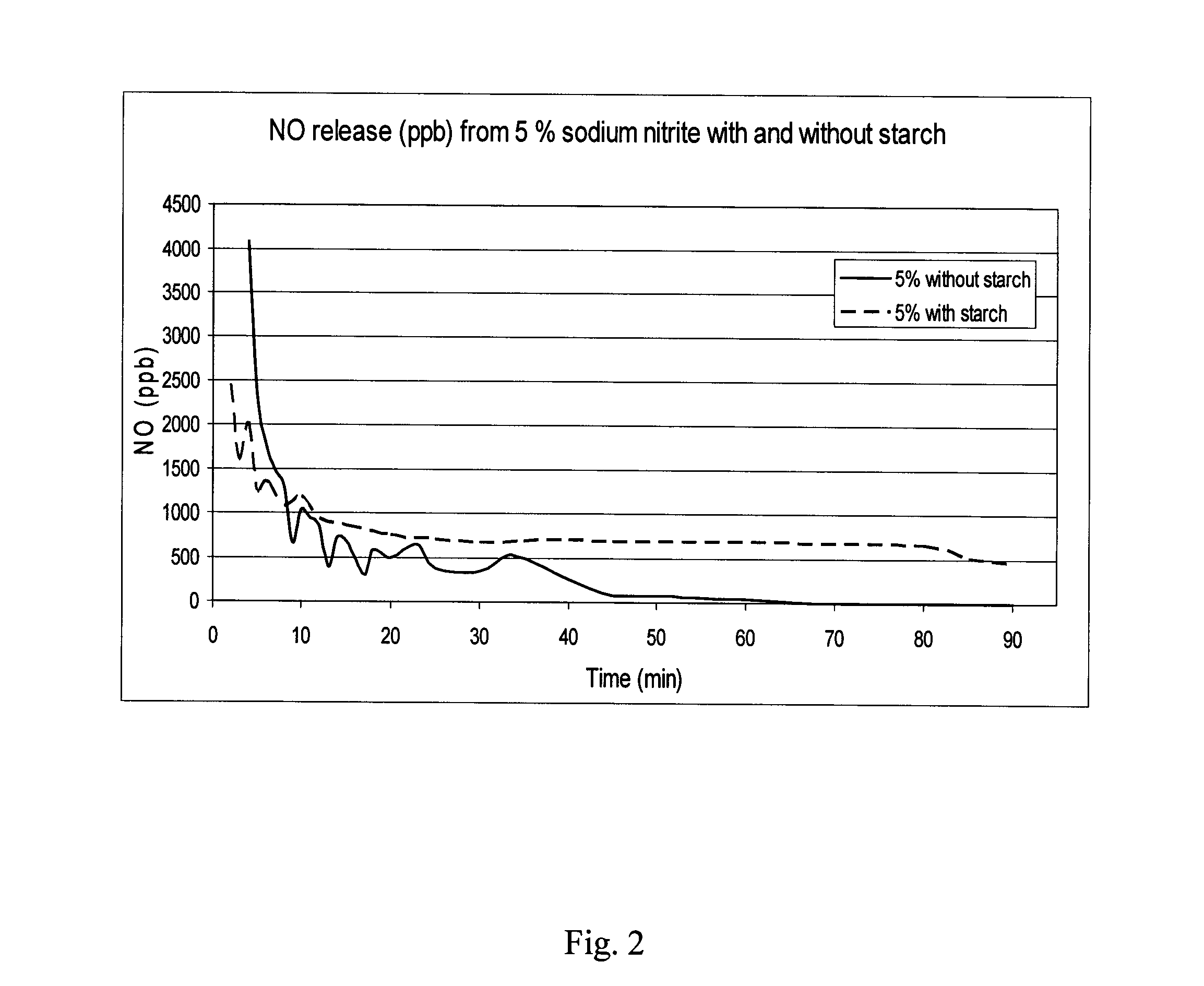
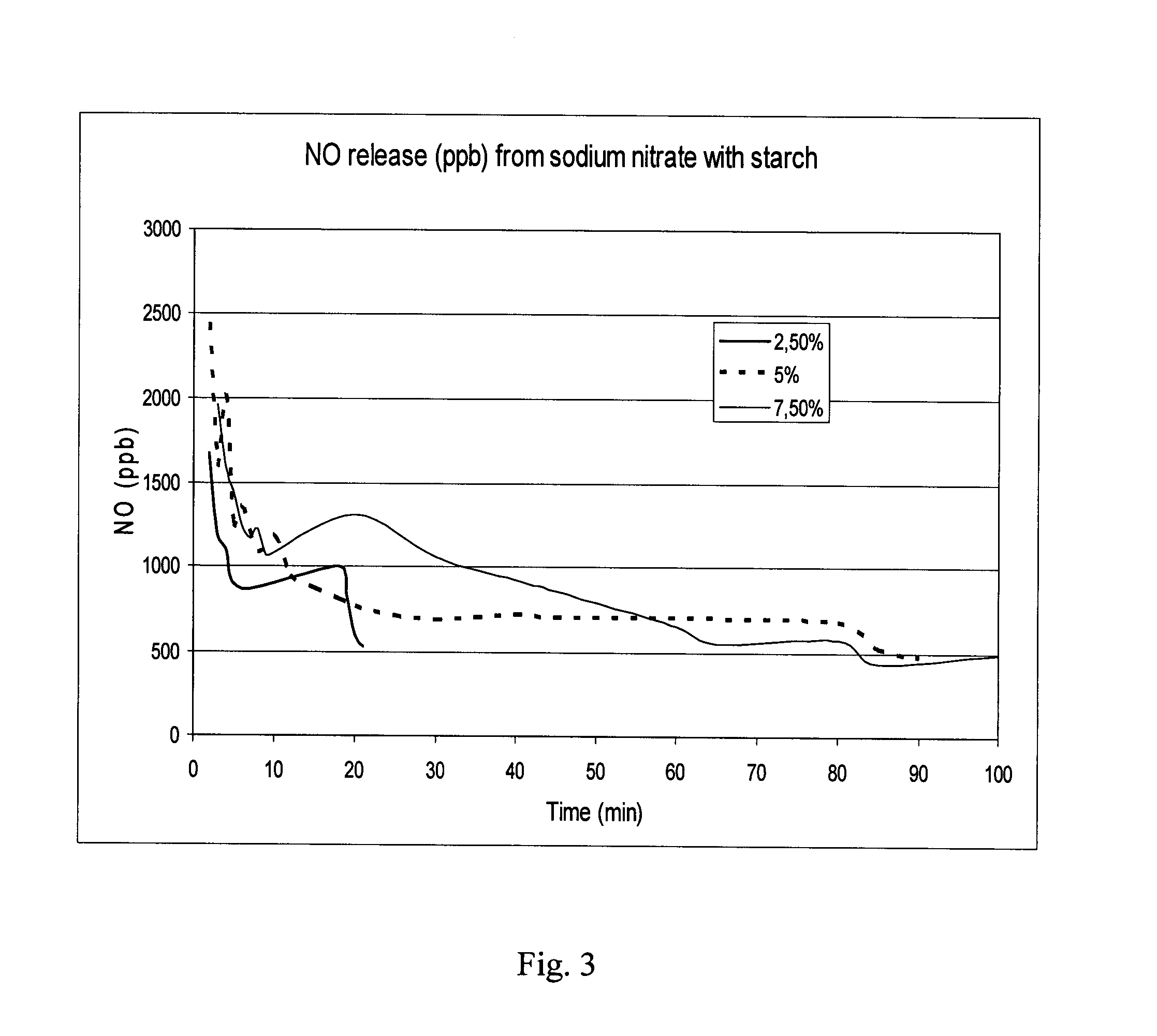
InactiveUS20130089629A1Reduce and eliminate onychomycosisFast and efficientBiocideSalicyclic acid active ingredientsNitriteNitric oxide
A kit for the treatment of onychomycosis by nitric oxide, including: a) a pre-treatment part comprising a pharmaceutically acceptable acidifying agent in an amount sufficient to loosen up the superficial outer nail plate layer, b) a treatment part comprising a pharmaceutically acceptable nitrite and at least one polysaccharide or a NO eluting polymer in one container and ascorbic acid in another container in amounts sufficient to produce nitric oxide in an amount that reduces and / or eliminates the onychomycosis upon being mixed and c) at least two devices suitable to apply a) and b) at the treatment site and upon treatment secure that the treatment site is substantially sealed.
Owner:TOPICAL PHARMA
Controlled release copolymer formulation with improved release kinetics
InactiveUS8877225B2Constant of releaseImproved release profileBiocideOrganic active ingredientsOligomerControl release
Owner:TOLMAR THERAPEUTICS
Sustained-release compositions
InactiveUS20050084537A1Improved release profilePowder deliveryPeptide/protein ingredientsMedicineMicroparticle
Microparticles comprise a therapeutic agent dispersed within a polymer matrix, the matrix comprising a first polymer of hyaluronic acid and a second polymer of either a non-ionic polymer, a polymeric gum or a combination thereof. The microparticles may be formulated for nasal or pulmonary delivery.
Owner:QUADRANT DRUG DELIVERY
Glp-1 pharmaceutical compositions
InactiveUS20100137204A1Raise the pHDecreasing initial burstPeptide/protein ingredientsMetabolism disorderGlucagon-like peptide-1Peptide
Owner:IPSEN PHARMA SAS
Cosmetic skincare applications employing mineral-derived tubules for controlled release
InactiveUS20070202061A1Improve health and appearance of skinGood lookingCosmetic preparationsHair cosmeticsActive agentGlycerol
Compositions and methods pertaining to the topical treatment of skin are disclosed. The present invention relates to topical compositions for regulating the condition of skin, especially for regulating visible and / or tactile discontinuities in skin associated with skin aging, environmental affects and the like. One embodiment of the present invention relates to improving skin with compositions containing one or more non-volatile, slowly absorbed, liquid or semi-liquid organic substances (active agents) via controlled release of active agents associated with mineral-derived tubules such as halloysite nanotubules. For example, the active agents, in the form of organic substances may include vitamin compounds and glycerin, among others.
Owner:NATURALNANO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com