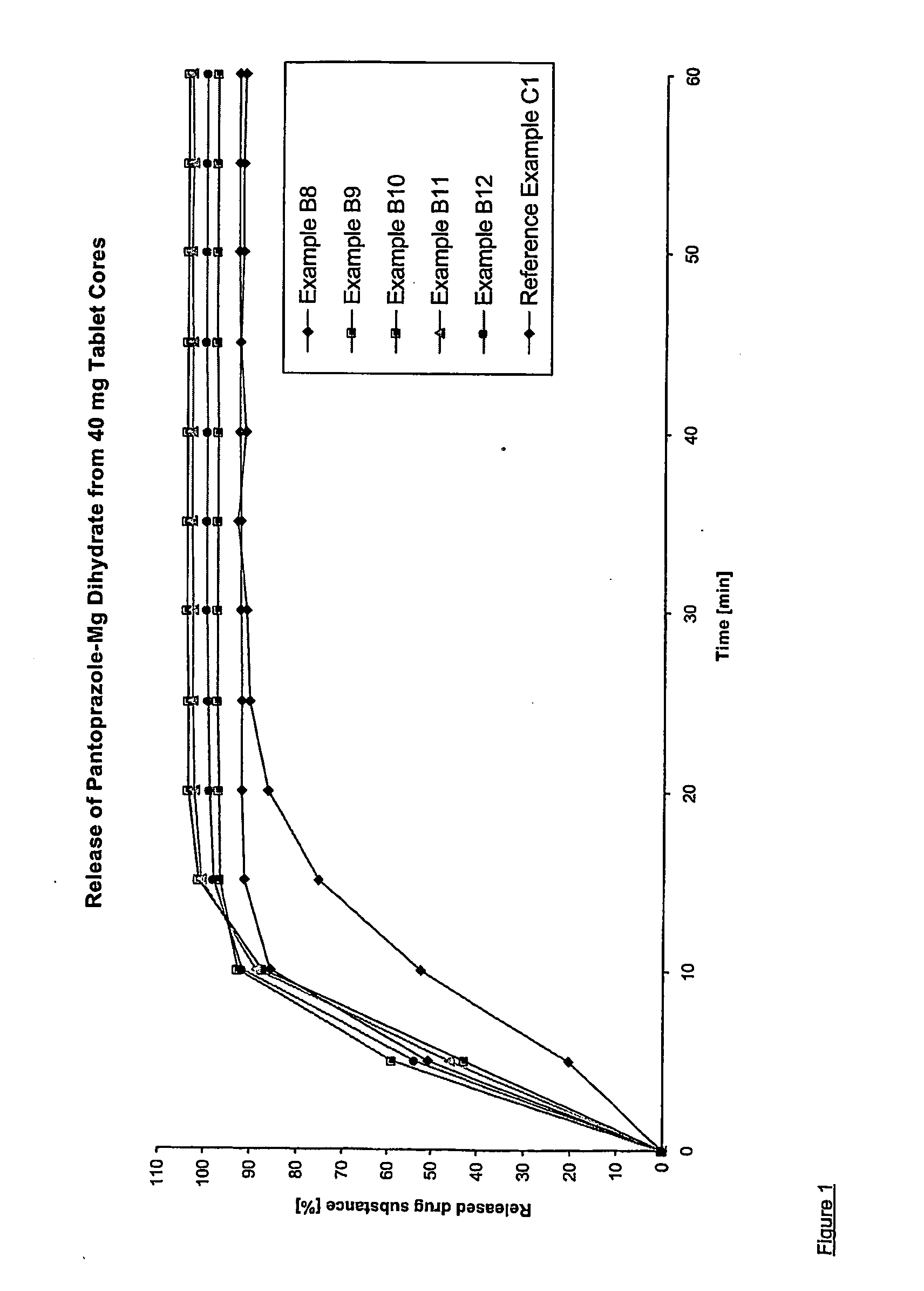
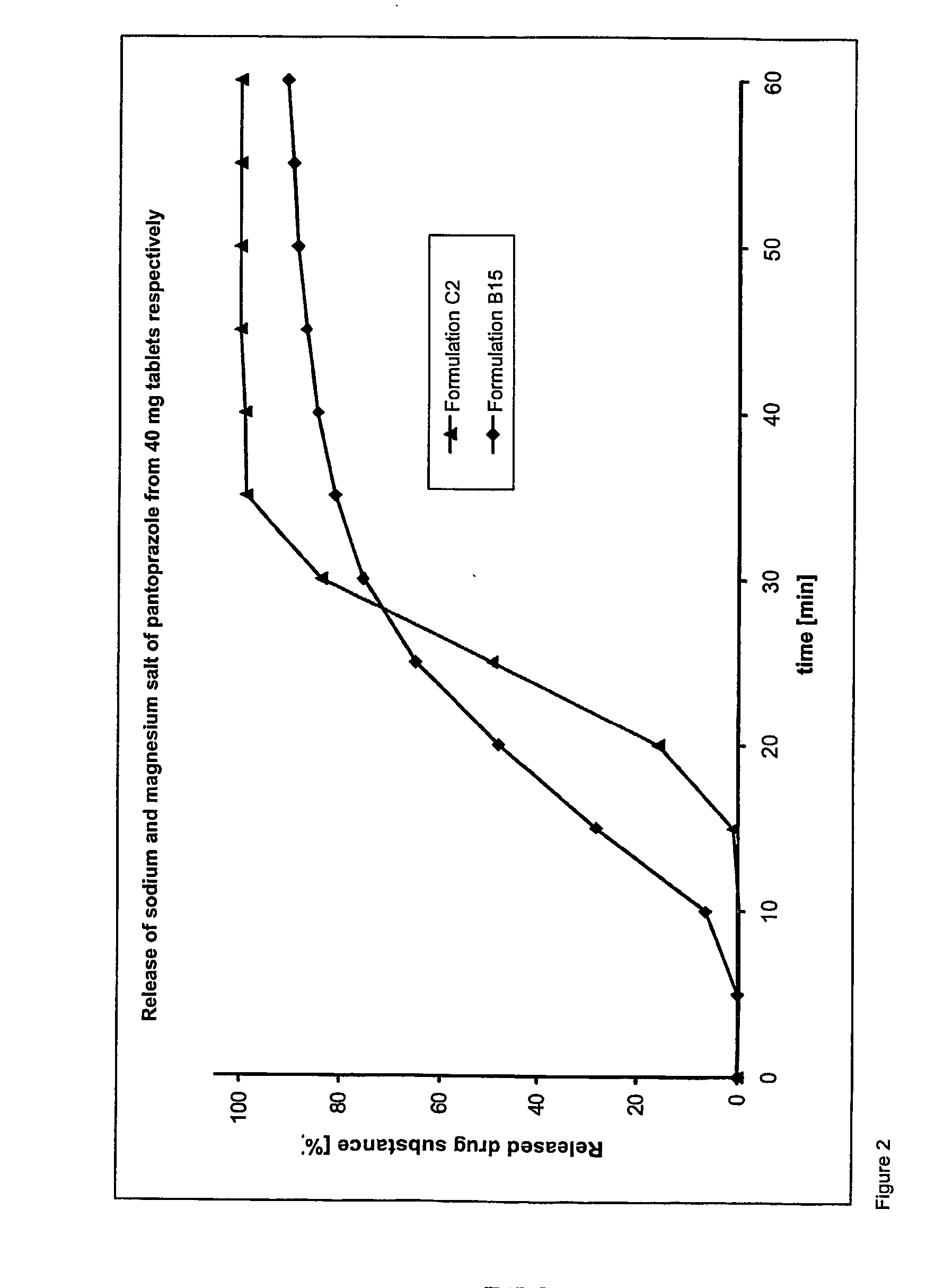
Dosage form containing pantoprazole as active ingredient
a technology of active ingredients and dosage forms, applied in the field of pharmaceutical technology, can solve the problems of affecting the effect of adsorption, and requiring a complex process to produce the peroral administration form of acid-labile active compounds,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
A. Synthesis of Magnesium bis[5-[difluoromethoxy]-2-[[3,4-dimethoxy-2-pyridinyl]-methyl]sulfinyl]1H-benzimidazolide]dihydrate
[0069] 3.85 kg (8.9 mol) of pantoprazole Na sesquihydrate [sodium [5[-difluoromethoxy]-2-[[3,4-dimethoxy-2-pyridinyl]methyl]sulfinyl]-1H-benzimidazolide]sesquihydrate] are dissolved at 20-25° C. in 38.5 l of purified water in a stirring vessel. A solution of 1.0 kg (4.90 mol) of magnesium dichloride hexahydrate in 8 l of purified water is added with stirring at 20-30° C. in the course of 3 to 4 h. After stirring for a further 18 h, the precipitated solid is centrifuged, washed with 23 l of purified water, stirred at 20-30° C. for 1 to 2 h in 35 l of purified water, centrifuged again and washed again with 30-50 l of purified water. The solid product is dried at 50° C. in vacuo (30-50 mbar) until a residual water content of <4.8% is achieved. The product is then ground.
[0070] The title compound is obtained as a white to beige powder, which is employed directly...
example b.1
Pellets Made by Wurster Coating (Nonpareilles):
[0074] I. Active Pellets:
a.) Sucrose starter pellets (0.425-0.5 mm)500.0gb.) Sodium carbonate30.0gc.) Pantoprazole-Mg dihydrate300.0gd.) Polyvinylpyrrolidone K 2535.0g
a. is sprayed with an aqueous dispersion of b., c. and d. in a fluidised bed process (Wurster equipment) or other suitable equipments (e.g. coating pan).
[0075] II. Intermediate Layer (Subcoating):
e.) Hydroxypropylmethylcellulose120.0gf.) Titanium dioxide2.0gg.) LB Iron oxide yellow0.2gh.) Propylene glycol24.0g
e. is dissolved in water (A). f. and g. are suspended in water using a high shear mixer (B). A and B are combined and after addition of h. the resulting suspension is sieved through a suitable sieve. The suspension is sprayed onto 500 g of the active pellets obtained under I using a fluidised bed process (Wurster) or other suitable processes (e.g. coating pan).
[0076] III. Coating with a Layer which is Resistant to Gastric Juice (Enteric Coating):
i.) Eudragit...
example b.2
Pellets Made by Wurster Coating (Nonpareilles):
[0078] I. Active Pellets:
a.) Cellulose pellets (0.6-0.7 mm)1000.0gb.) Sodium carbonate75.0gc.) Pantoprazole-Mg dihydrate650.0gd.) Polyvinylpyrrolidone K 2580.0g
a. is sprayed with an aqueous dispersion of b., c. and d. in a fluidised bed process (Wurster equipment) or other suitable equipments (e.g. coating pan).
[0079] II. Intermediate Layer (Subcoating):
e.) Hydroxypropylmethylcellulose250.0gf.) Titanium dioxide5.0gg.) LB Iron oxide yellow0.45g
e. is dissolved in water (A). f. and g. are suspended in water using a high shear mixer (B). A and B are combined and the resulting suspension is sieved through a suitable sieve. The suspension is sprayed onto 1000 g of the active pellets obtained under I using a fluidised bed process (Wurster) or other suitable processes (e.g. coating pan).
[0080] III. Coating with a Layer which is Resistant to Gastric Juice (Enteric Coating):
h.) Eudragit ® L 30 D365.0gi.) Triethyl citrate15.0g
h. is suspe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com