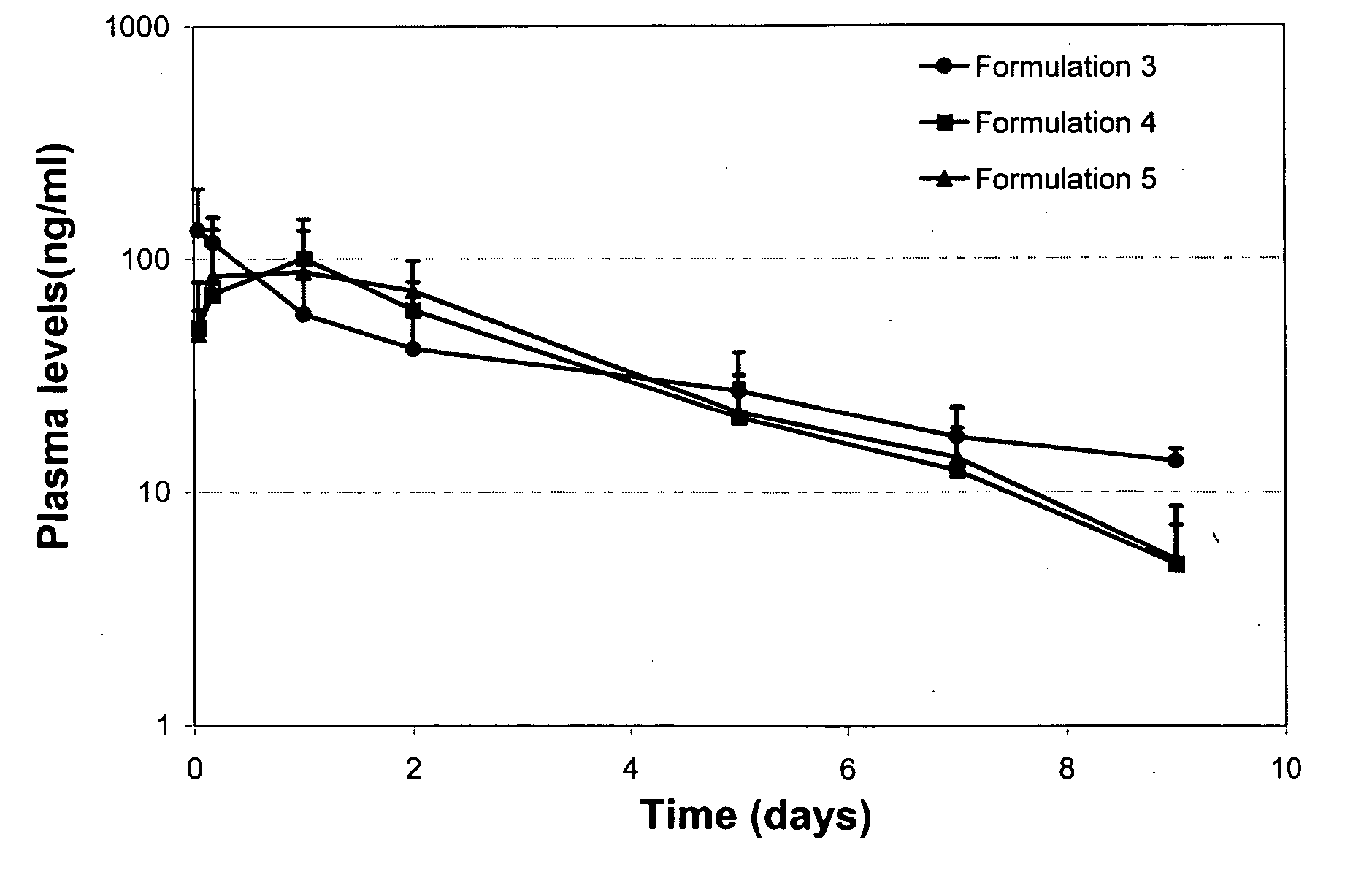
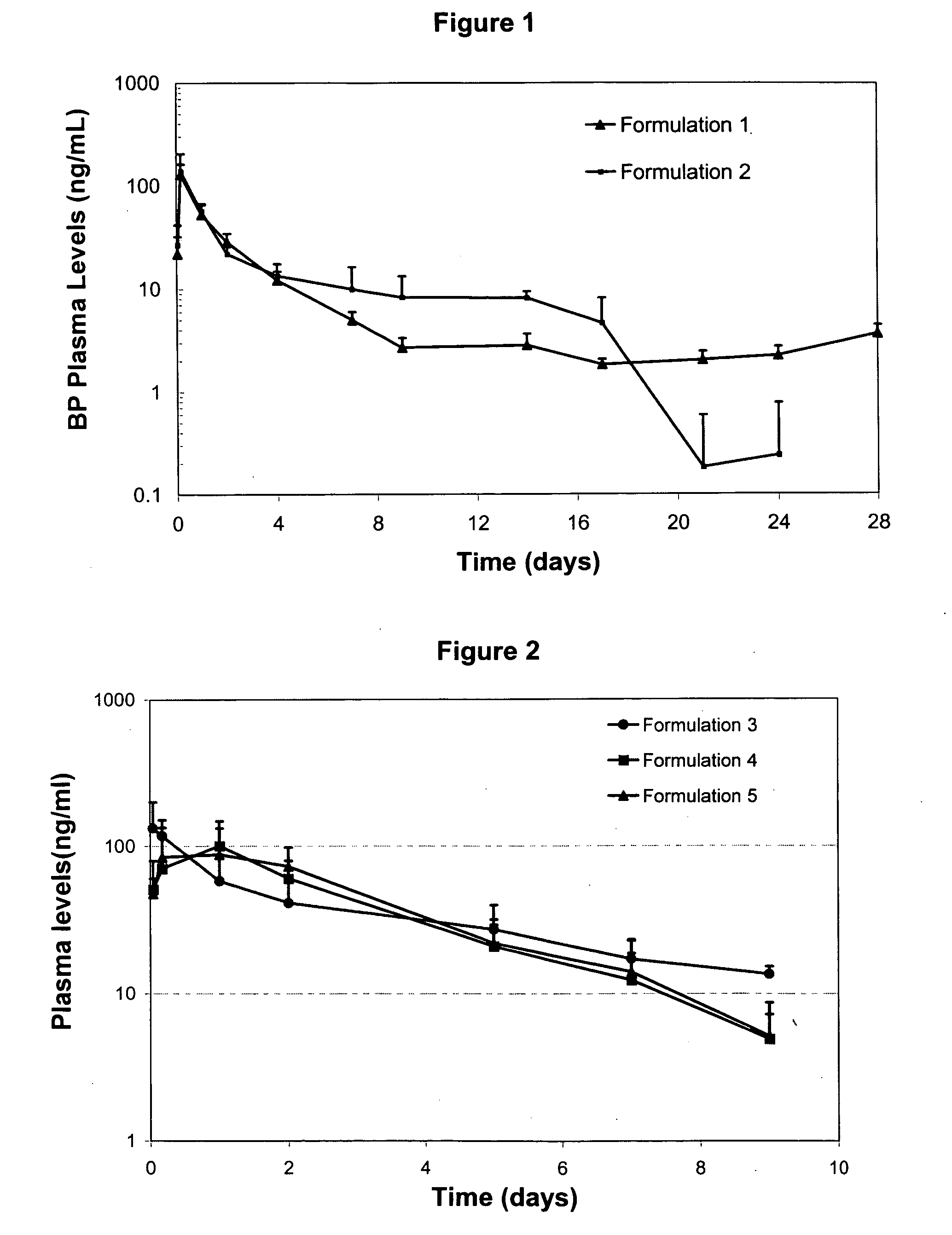
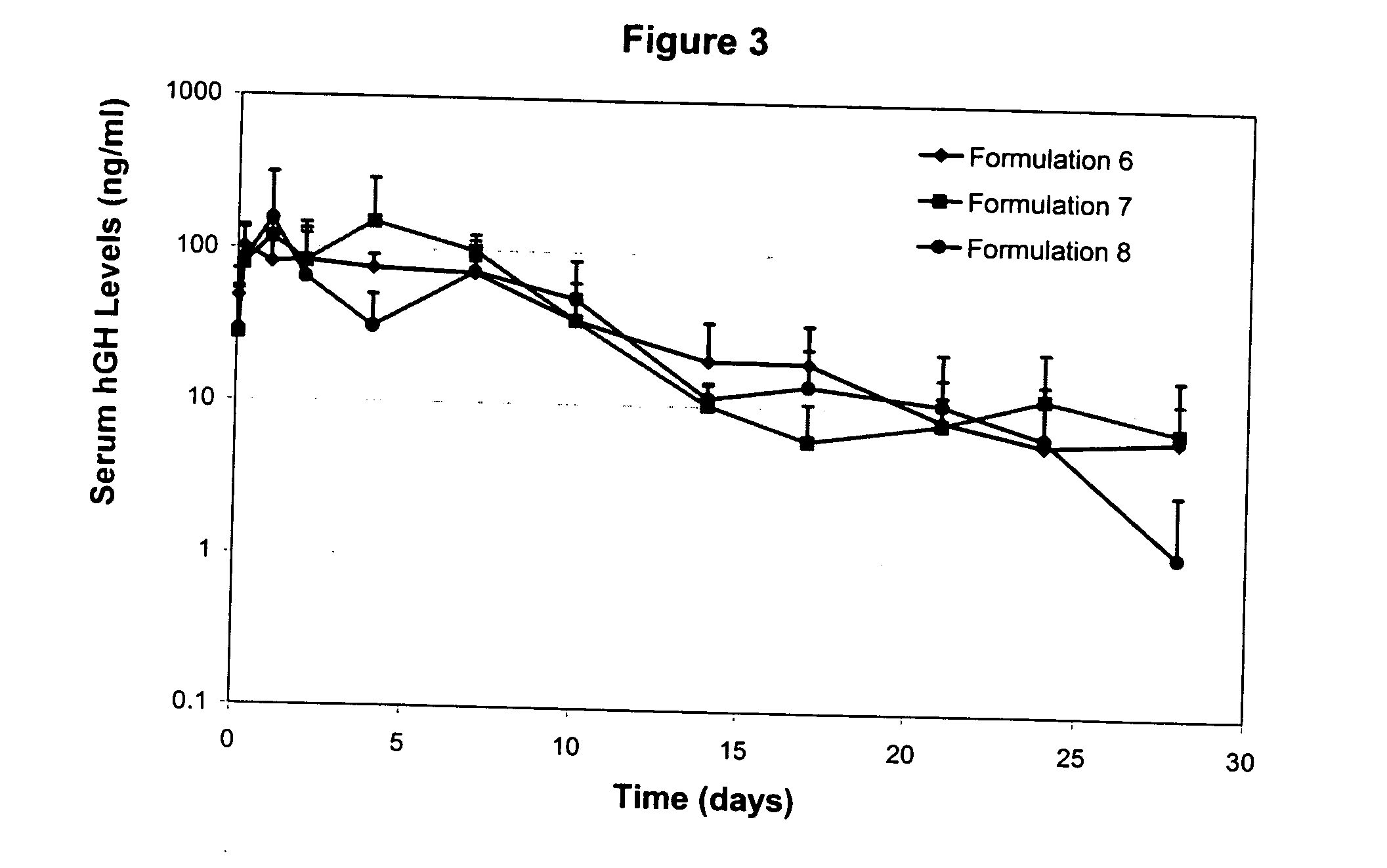
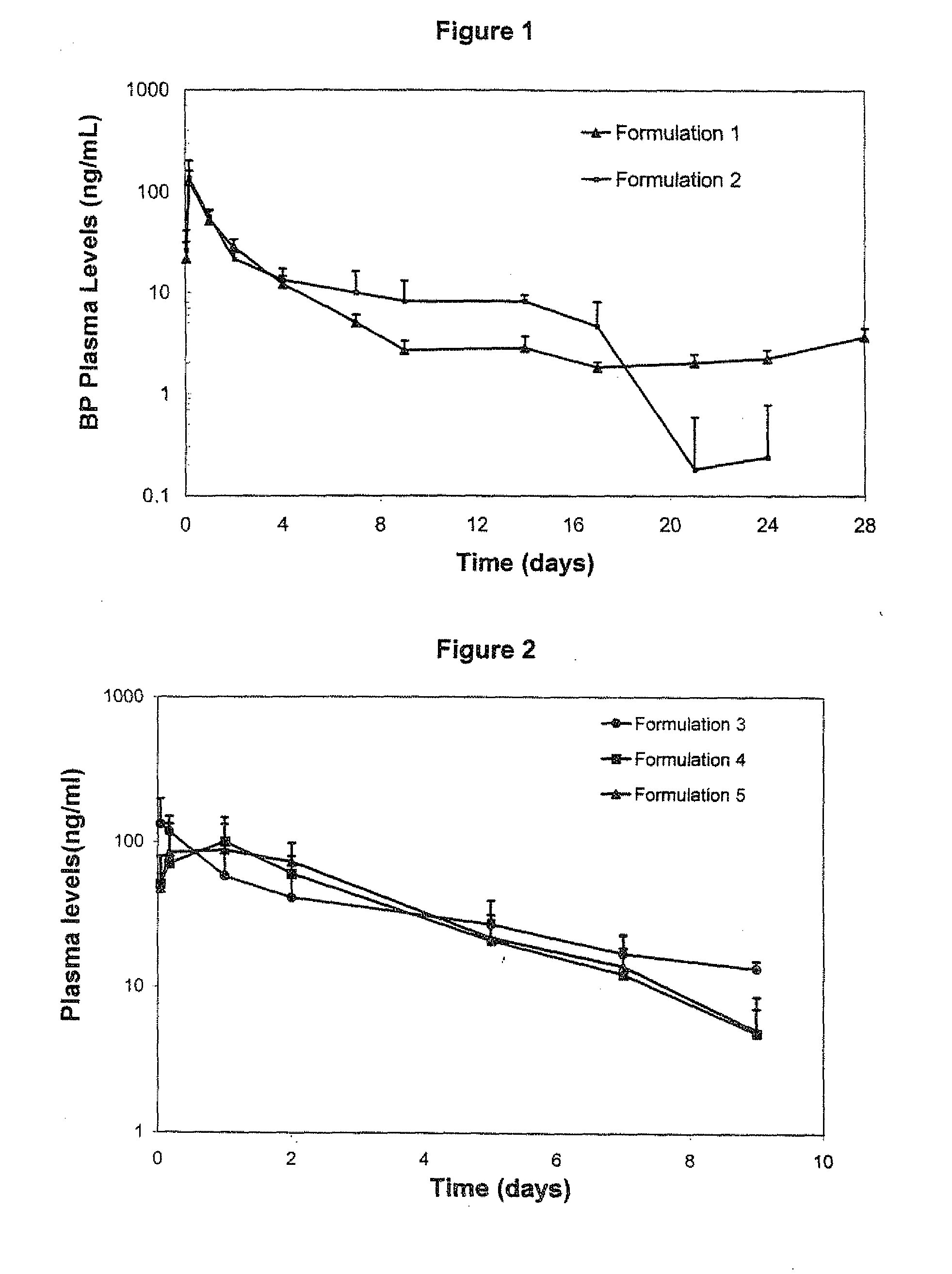
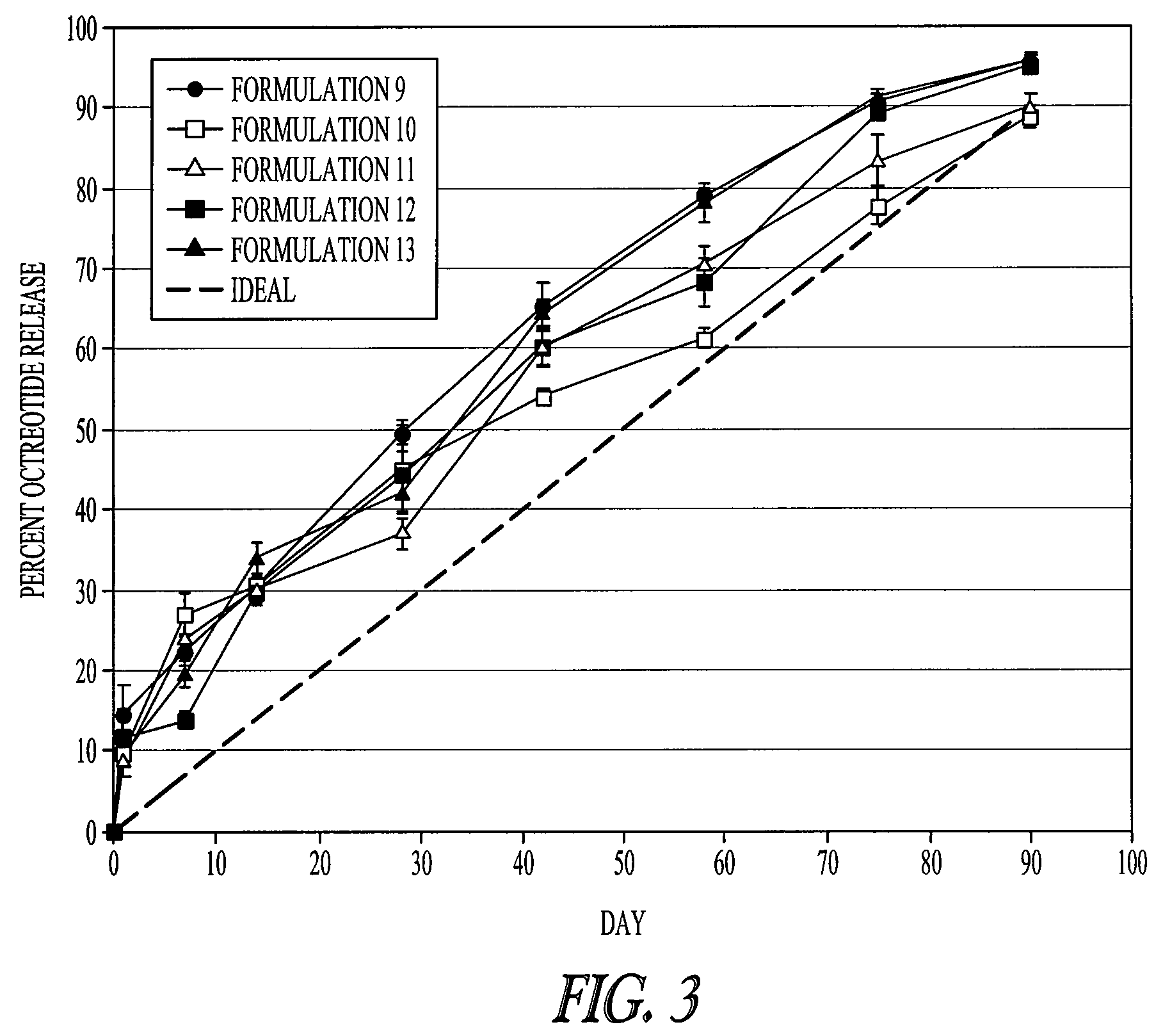
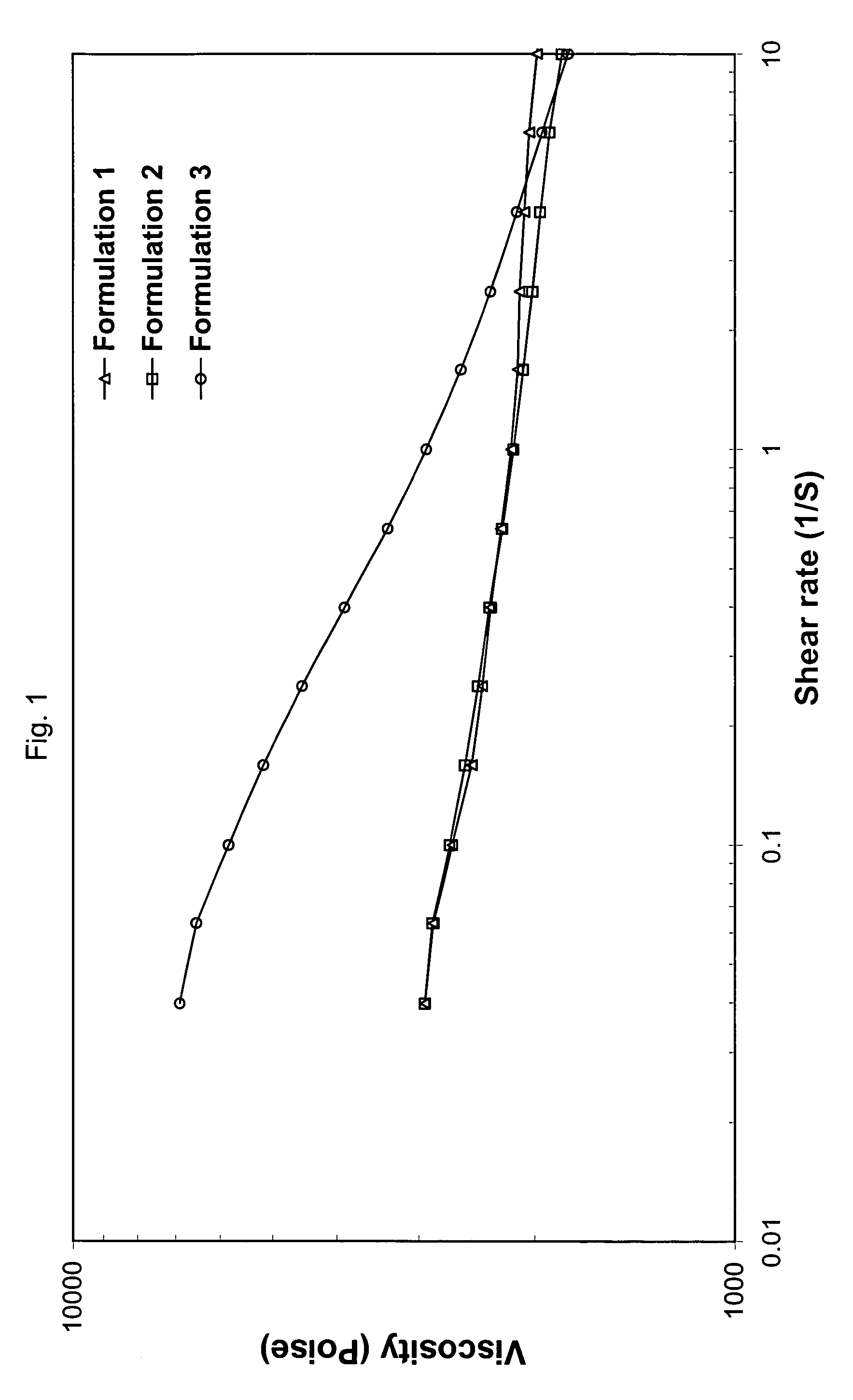
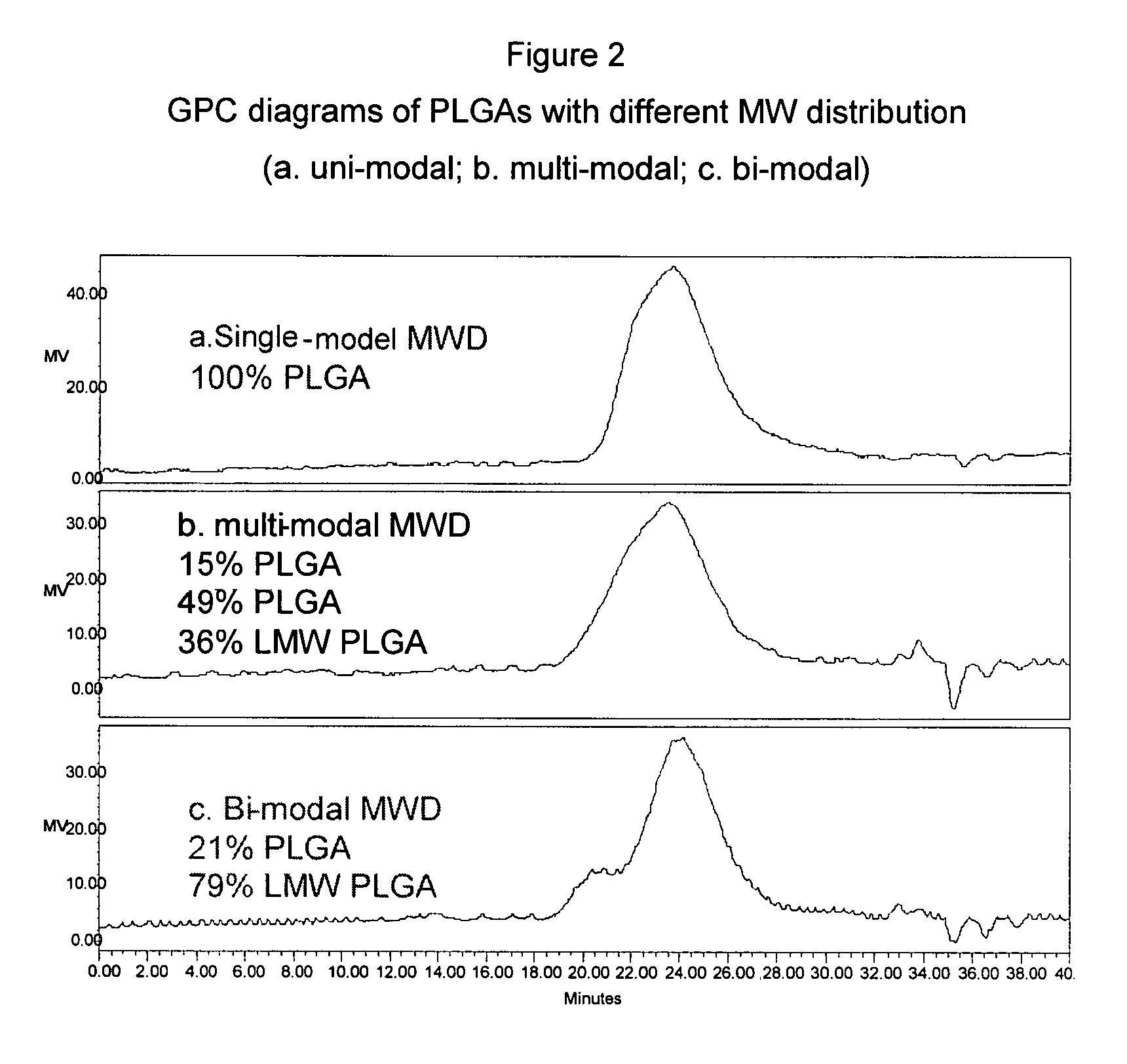
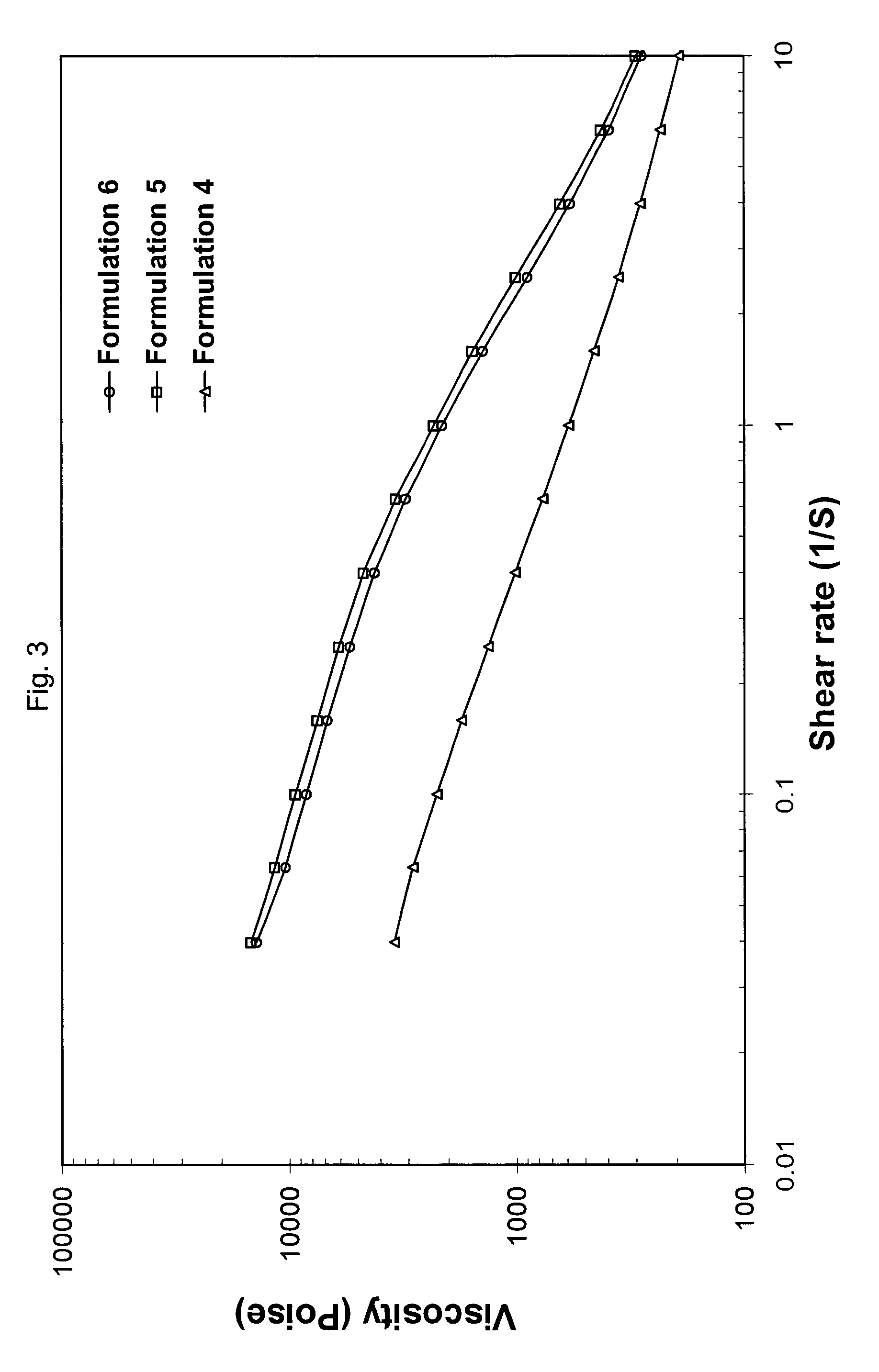
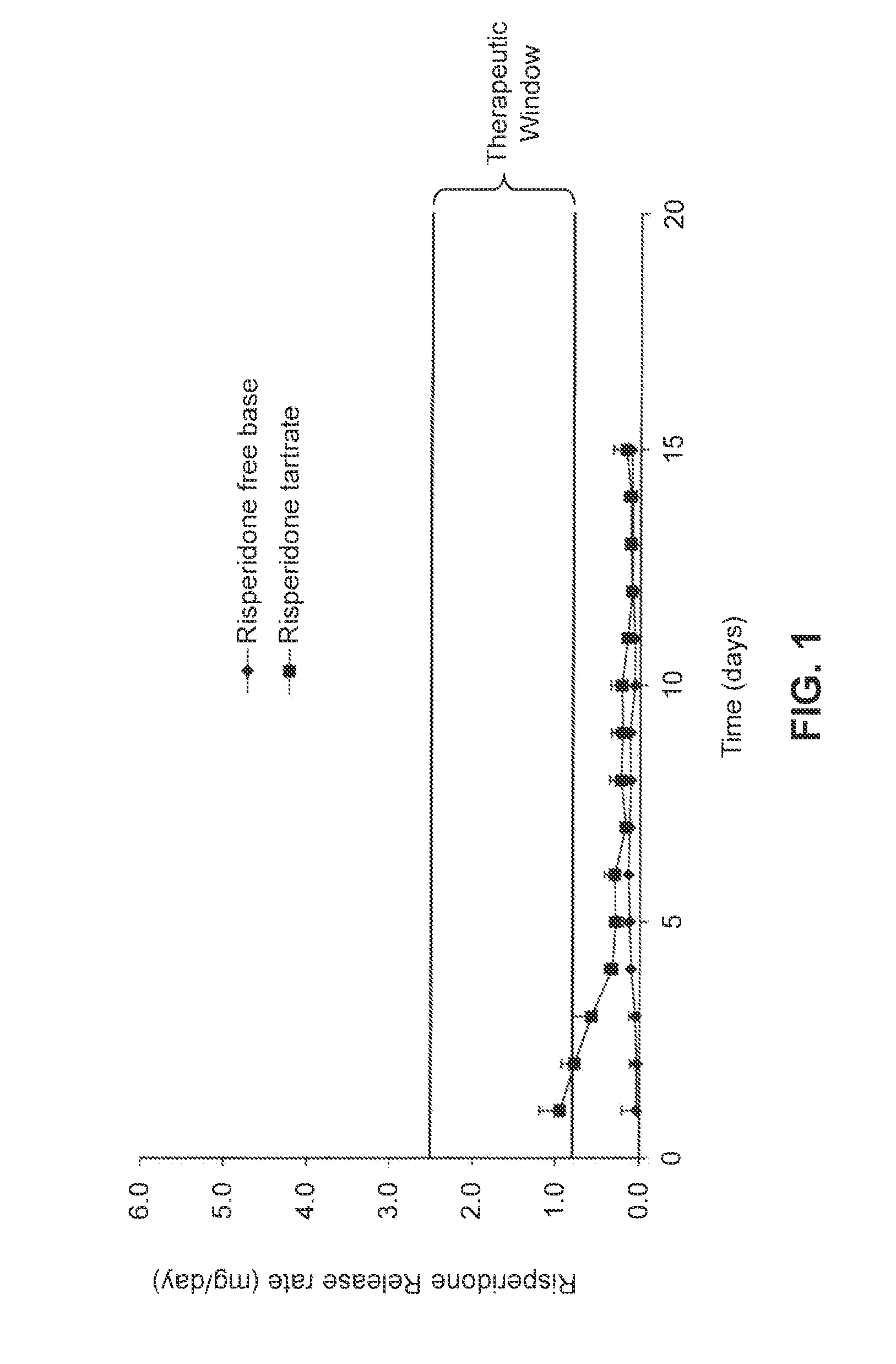
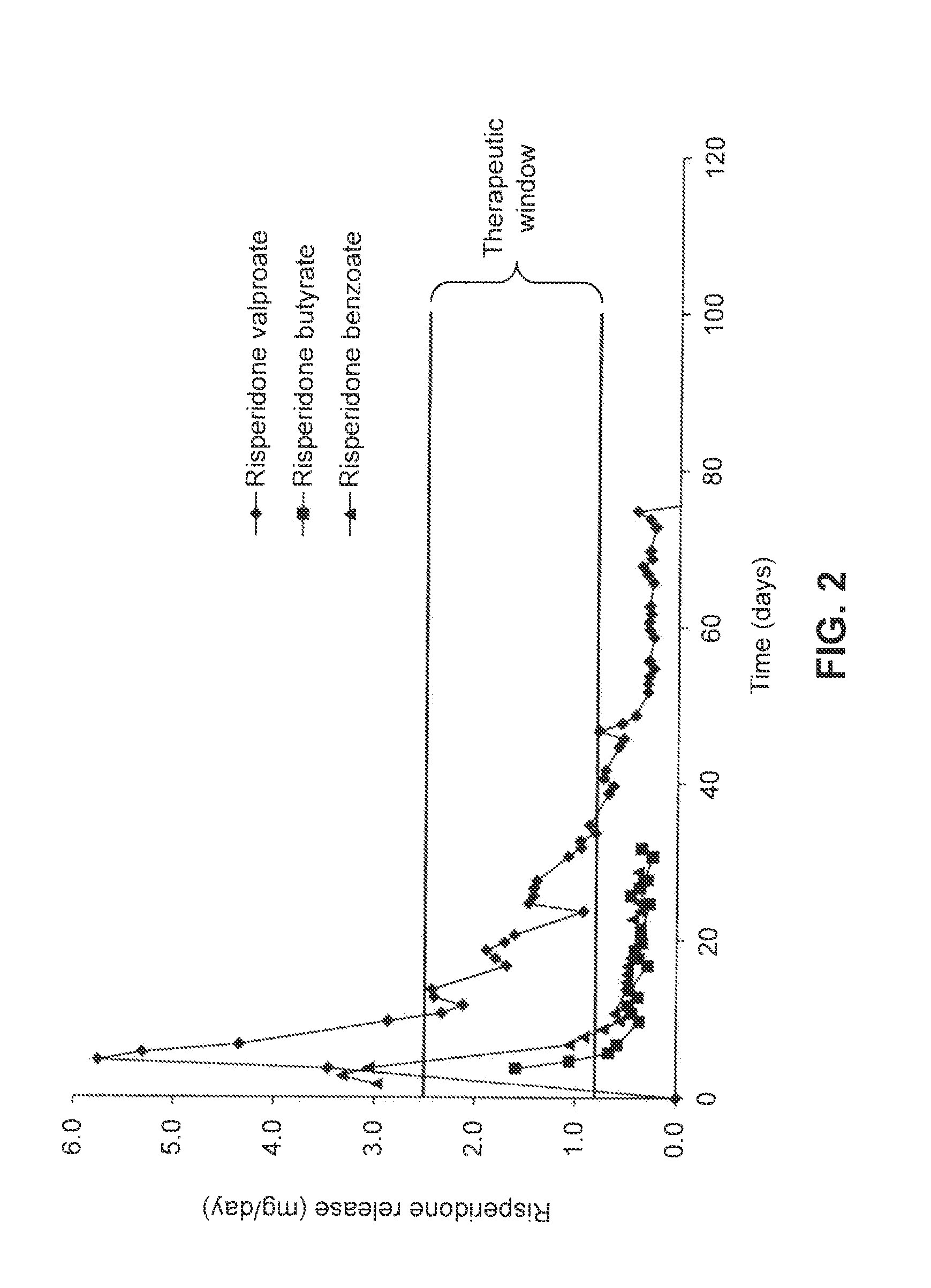
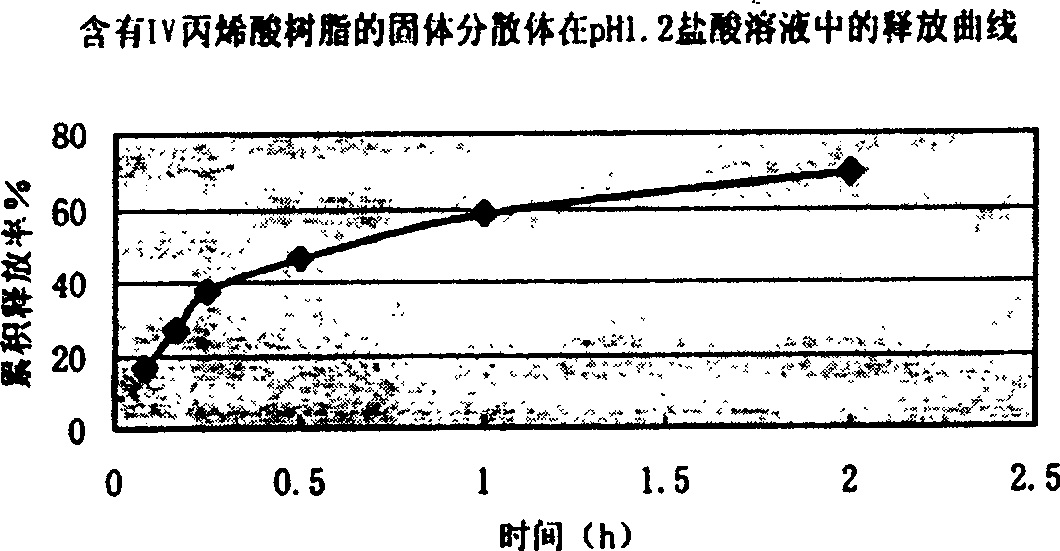
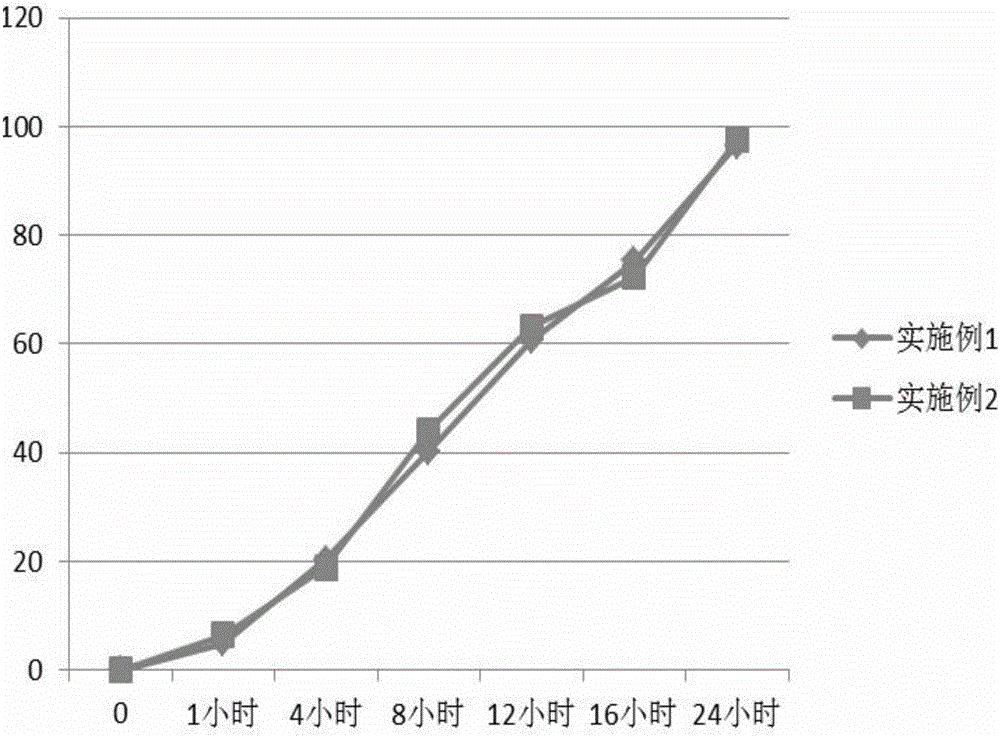
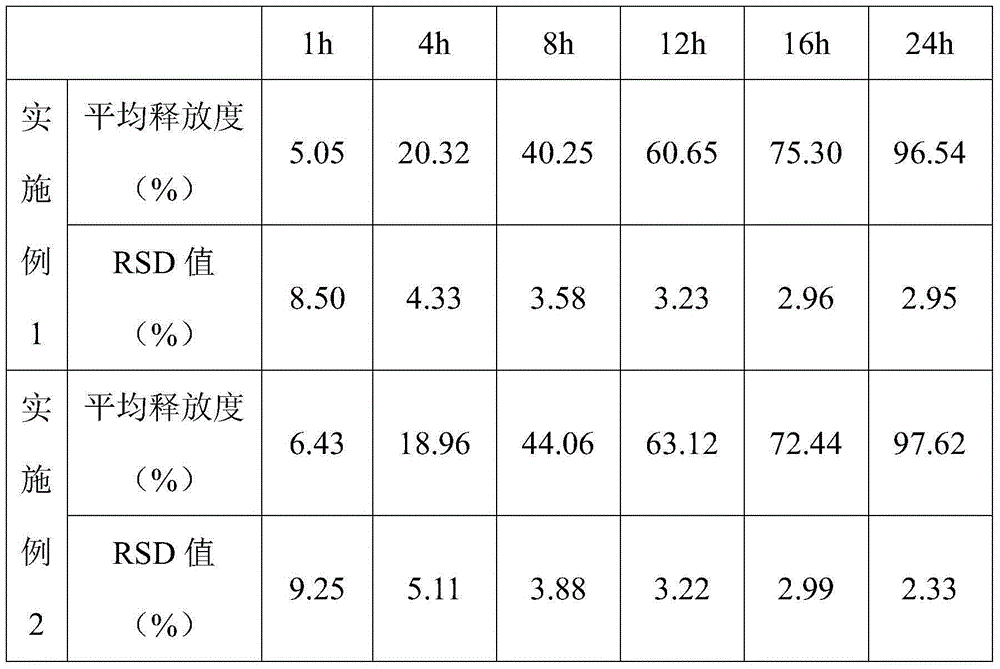
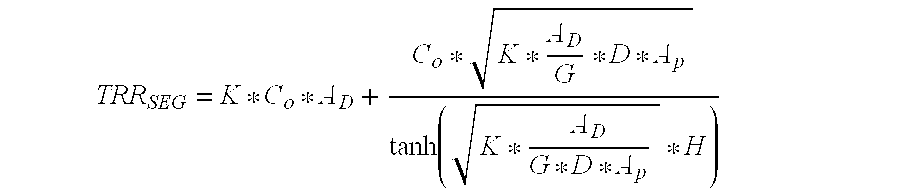Patents
Literature
66results about How to "Constant release rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Excipients in drug delivery vehicles
InactiveUS20050106214A1Effective distributionLow loading ratePowder deliveryPeptide/protein ingredientsAntioxidantBioerodible polymers
Injectable depot gel compositions and kits that provide an excipient for modulating a release rate and stabilizing beneficial agents are provided. Methods of administering and preparing such systems are also provided. The gel compositions comprise biodegradable, bioerodible polymers and water-immiscible solvents in amounts effective to plasticize the polymers and form gels with the polymers. Suitable excipients include pH modifiers, reducing agents, and antioxidants.
Owner:DURECT CORP
Excipients In Drug Delivery Vehicles
InactiveUS20120177697A1Effective distributionLow loading ratePowder deliveryPeptide/protein ingredientsAntioxidantBioerodible polymers
Owner:DURECT CORP
Sustained release system for reservoir treatment and monitoring
ActiveUS20180298277A1Reduce releaseMinimize effect of usingSurveyConstructionsCompound (substance)Polymer
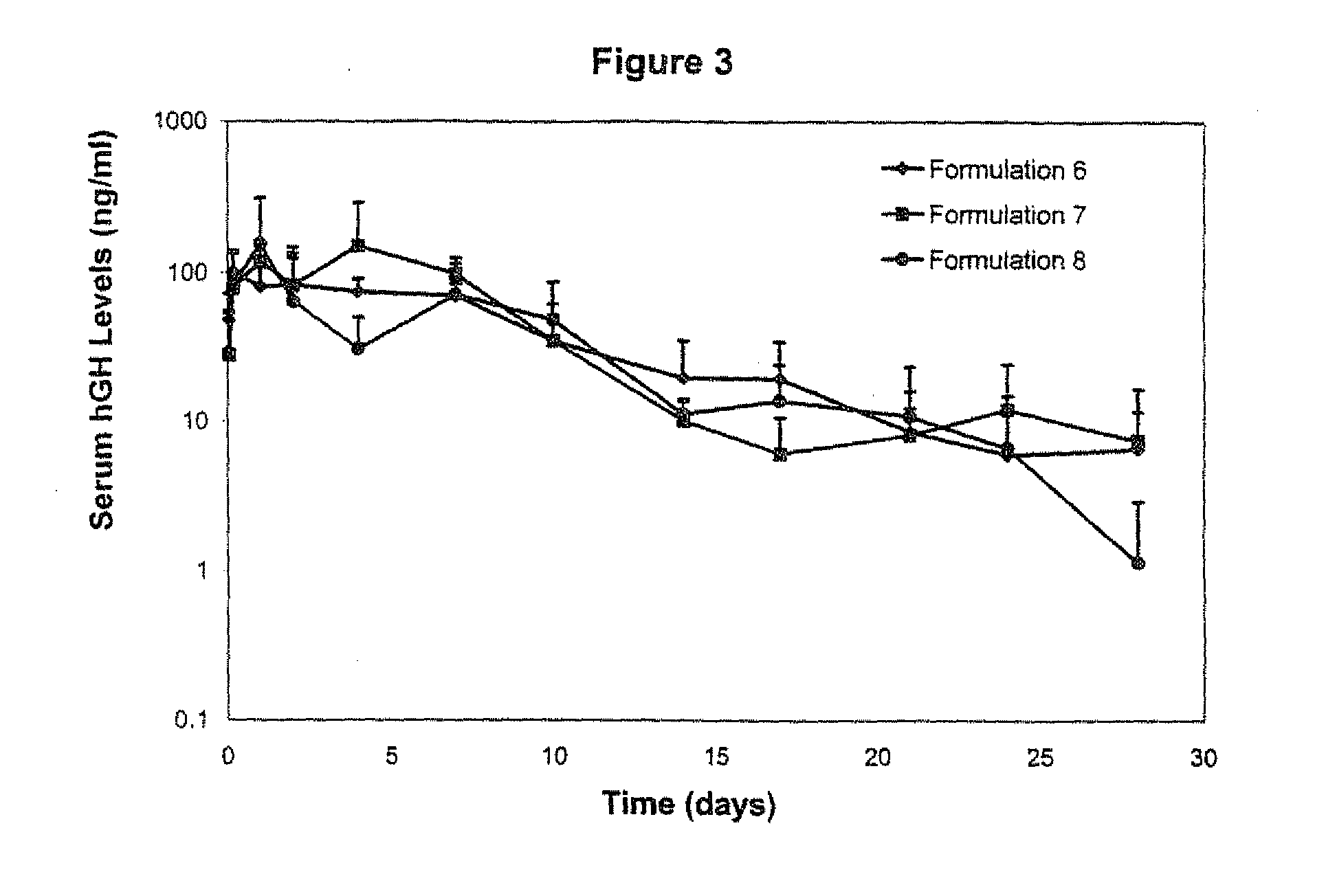
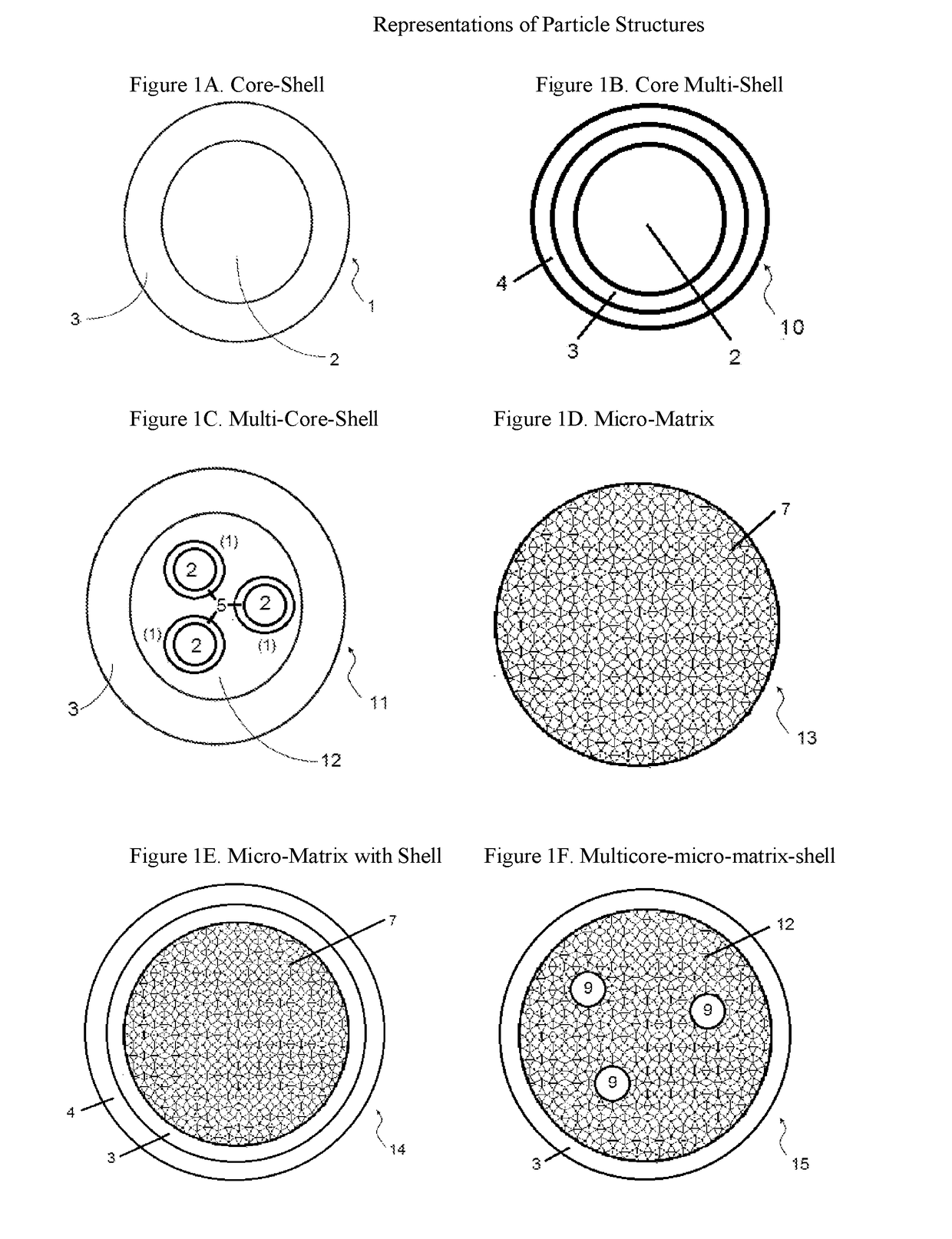
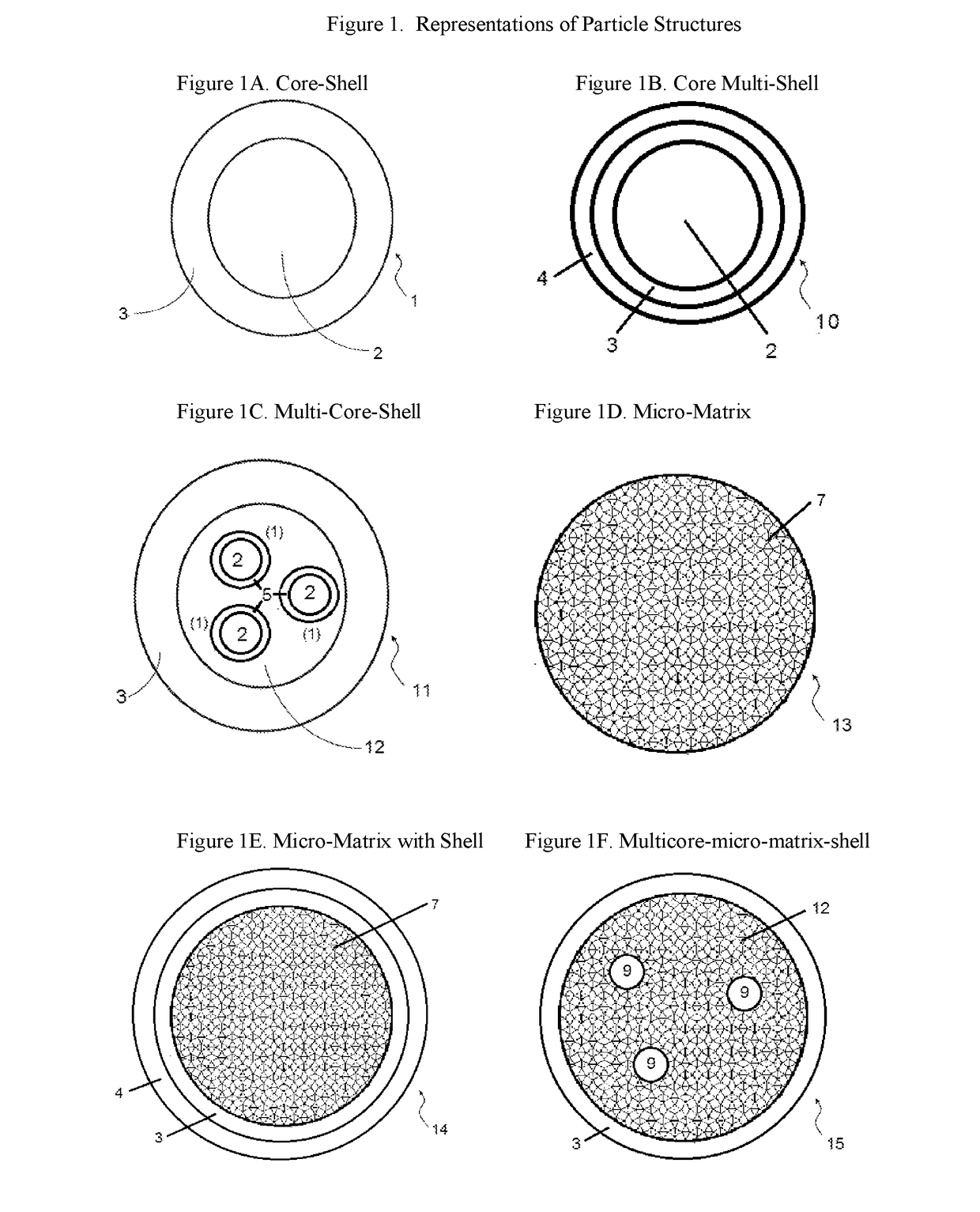
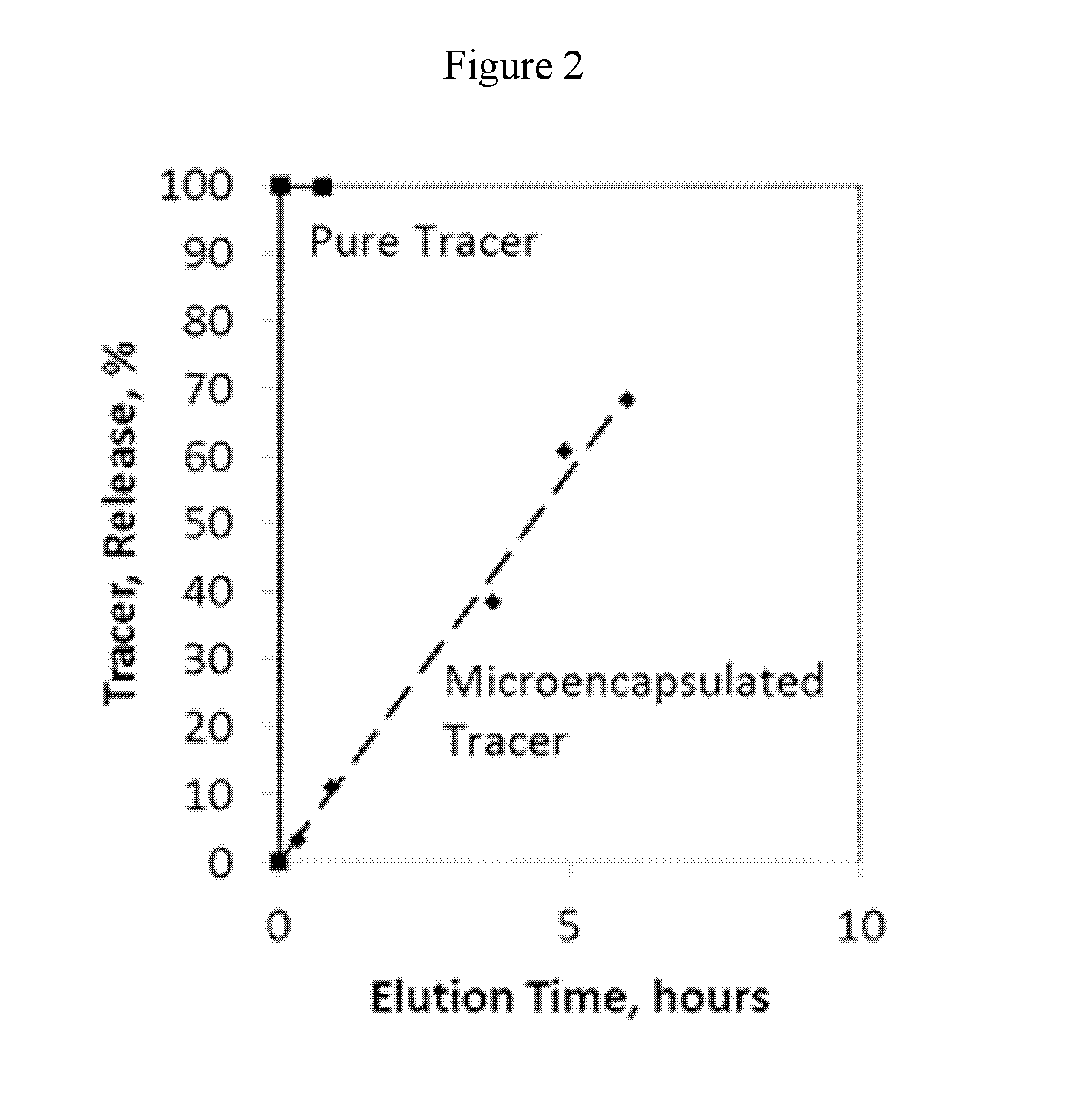
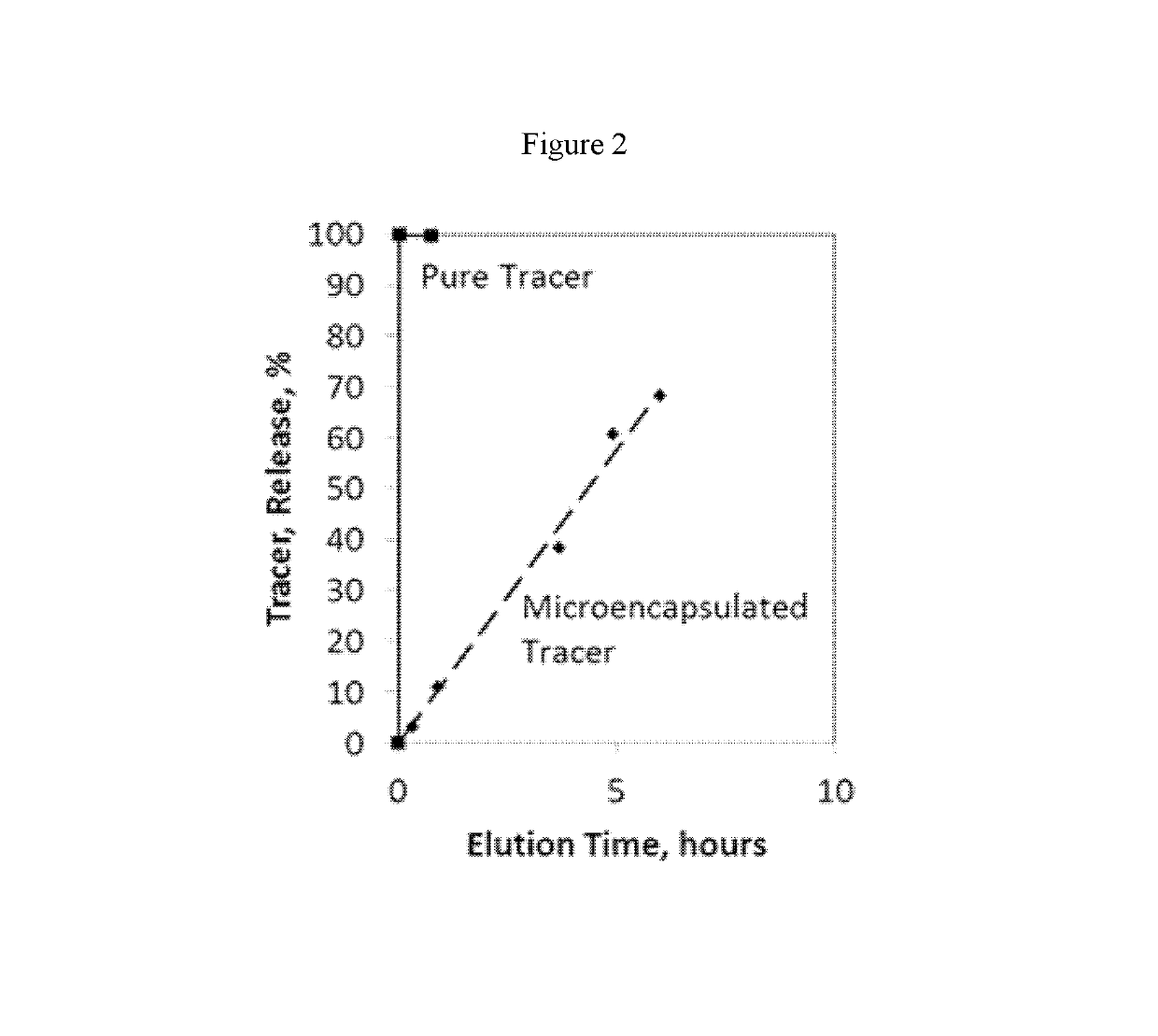
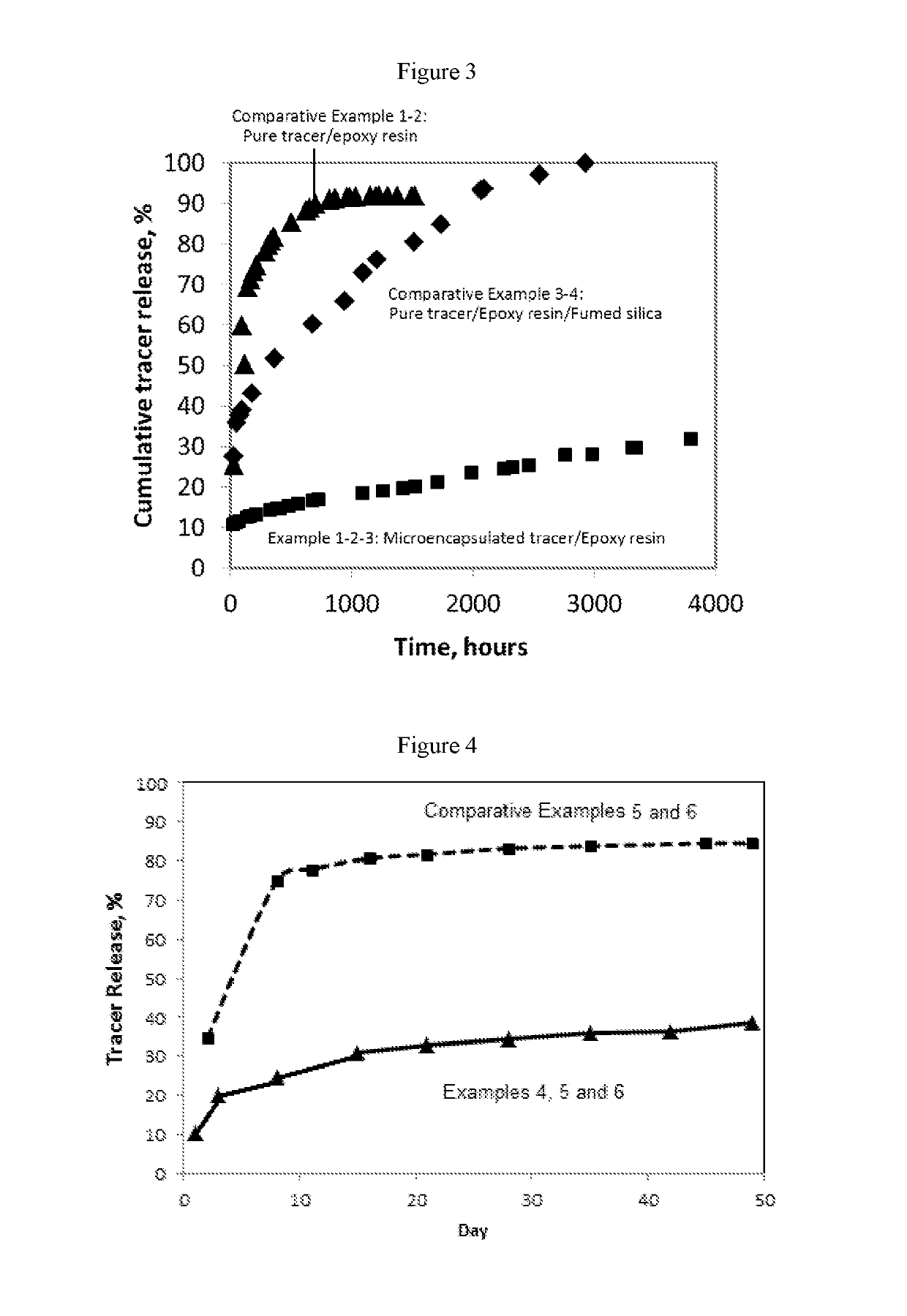
Compositions containing a mixture of microcapsules and a bulk polymer, where the microcapsules have an oil field chemical contained within the microcapsules are described. The microcapsules can be present in a variety of configurations. The microcapsules contain a core or a micro-matrix containing the oil field chemicals. The core or micro-matrix can be surrounded by one or more polymeric shells, where each shell contains at least one polymer that affects the release of the oil field chemical from the composition. The compositions provide for the sustained release of an oil field chemical into fluid in an oil field reservoir over long periods of time. Methods of making the compositions and articles containing the compositions are described. Methods of tracing the movement of fluid in a hydrocarbon reservoir using the compositions, and methods of providing for the sustained release of oil field chemicals are also described.
Owner:JOHNSON MATTHEY PLC
Semi-enclosed gel delivery device
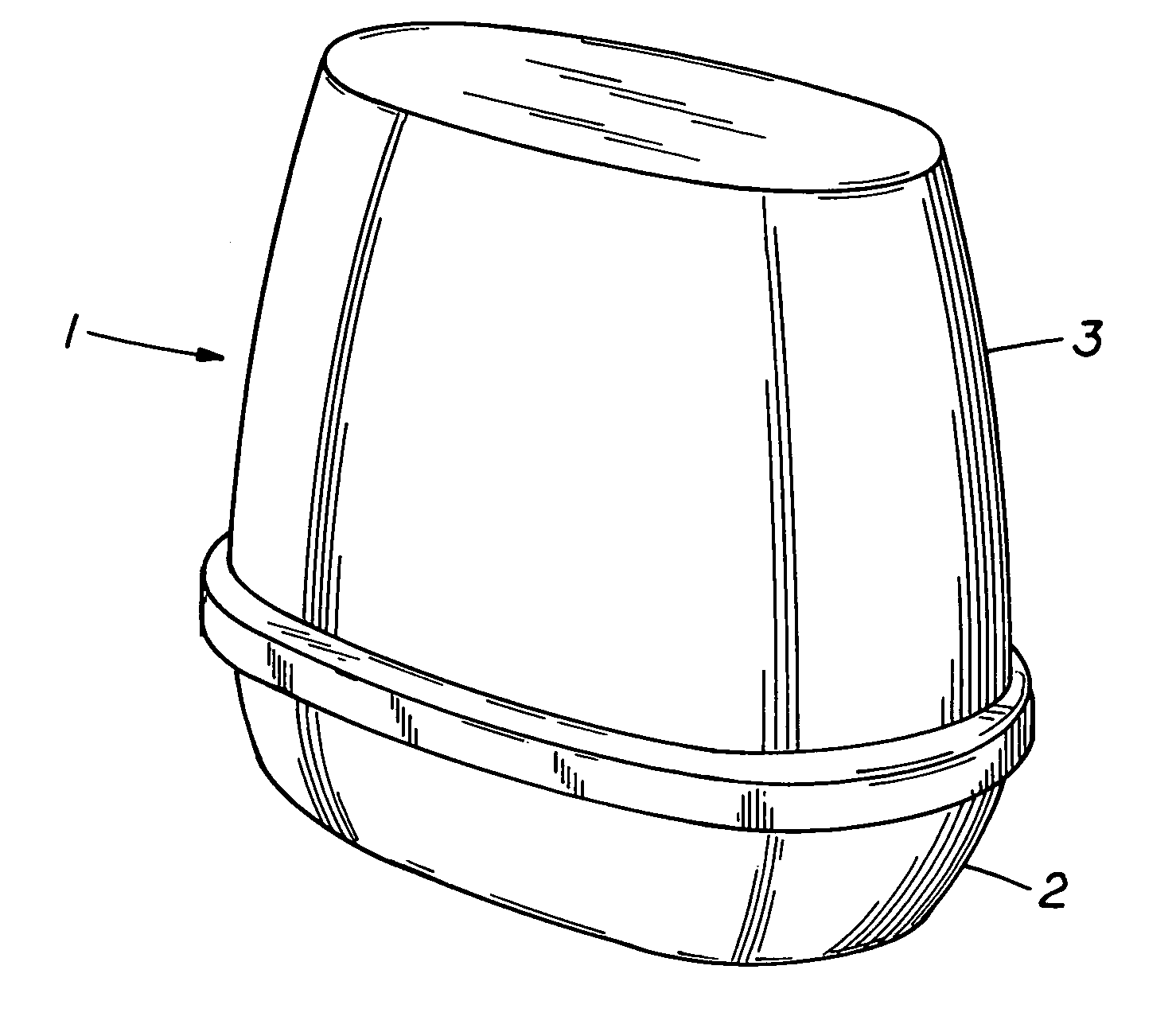
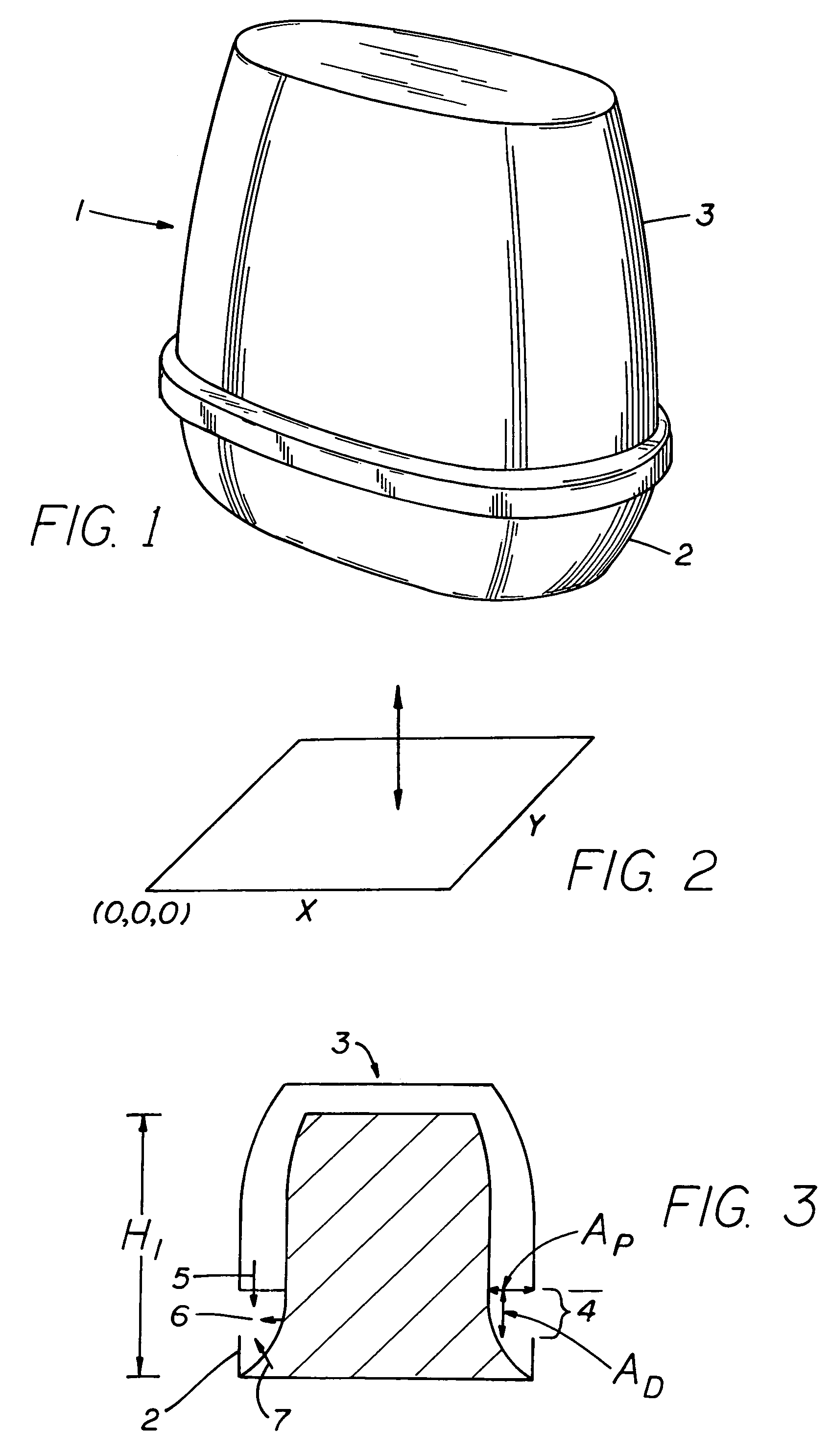
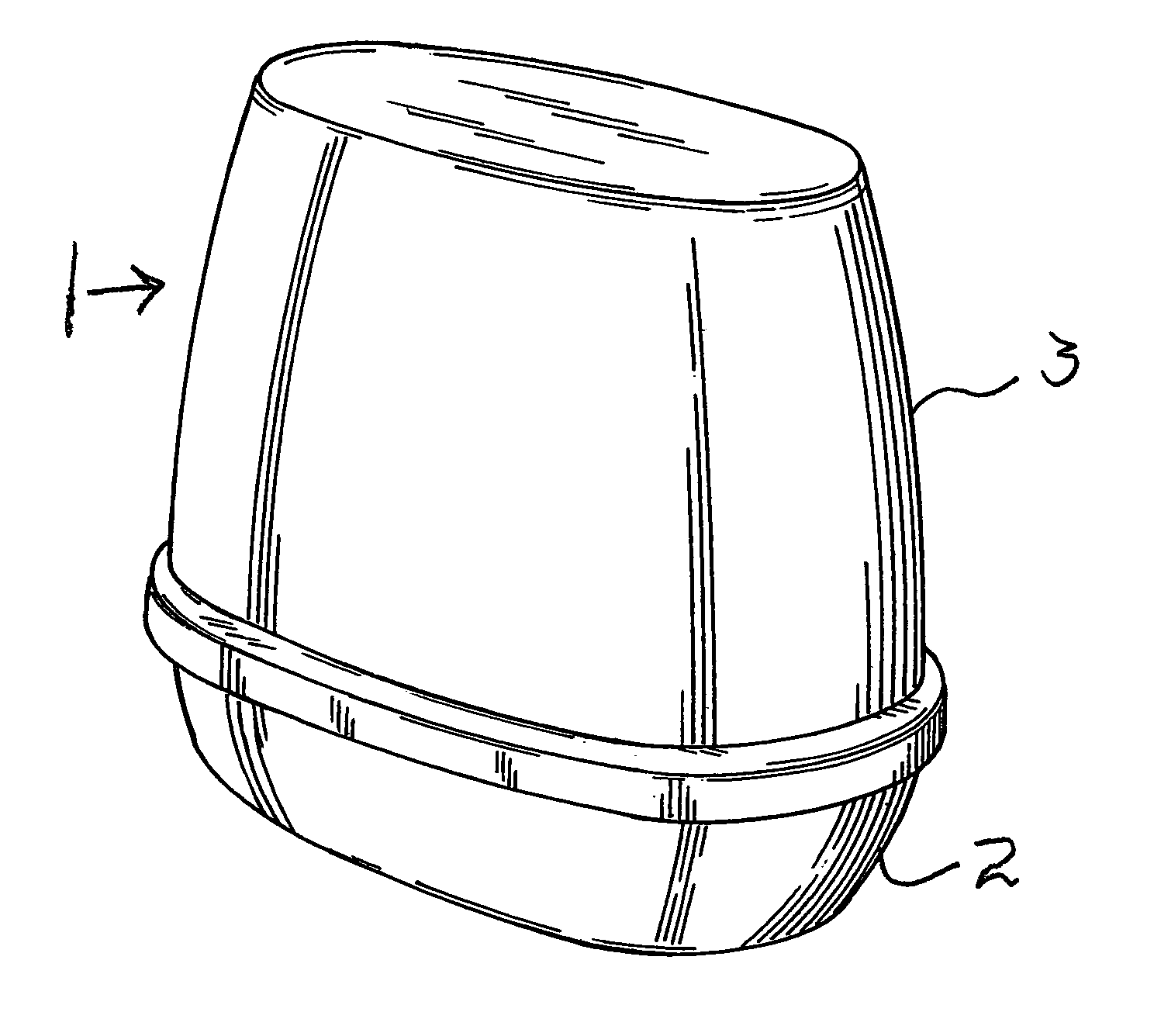
ActiveUS6994270B2Reduce deliveryConstant release rateTobacco devicesGaseous substancesEngineeringAtmosphere
A dispenser of actives having a linear release rate may be achieved by providing a volatile containing gel system wherein the gel system is proportioned in specified dimensional ratios, so that the sum of the rate of volatile release from directly exposed areas of the surface of the gel system and the rate of volatile release from areas of the surface of the gel system which are not in direct exposure to the atmosphere remains essentially constant through out the life of the dispensing device.
Owner:SC JOHNSON & SON INC
Open gel delivery device
InactiveUS7278589B2Reduce deliveryConstant release rateTobacco devicesGaseous substancesBiomedical engineeringConstant rate
An open gel system for delivery of an active volatile possesses dimensions in the x, y, and z directions such as to release actives at an essentially constant rate from initiation of volatilization until completion of volatilization.
Owner:SC JOHNSON & SON INC
Controlled release copolymer formulation with improved release kinetics
InactiveUS8877225B2Constant of releaseImproved release profileBiocideOrganic active ingredientsOligomerControl release
Owner:TOLMAR THERAPEUTICS
Injectable multimodal polymer depot compositions and uses thereof
InactiveUS8501215B2Effective distributionLow loading ratePowder deliveryAerosol deliverySolventCompatibilization
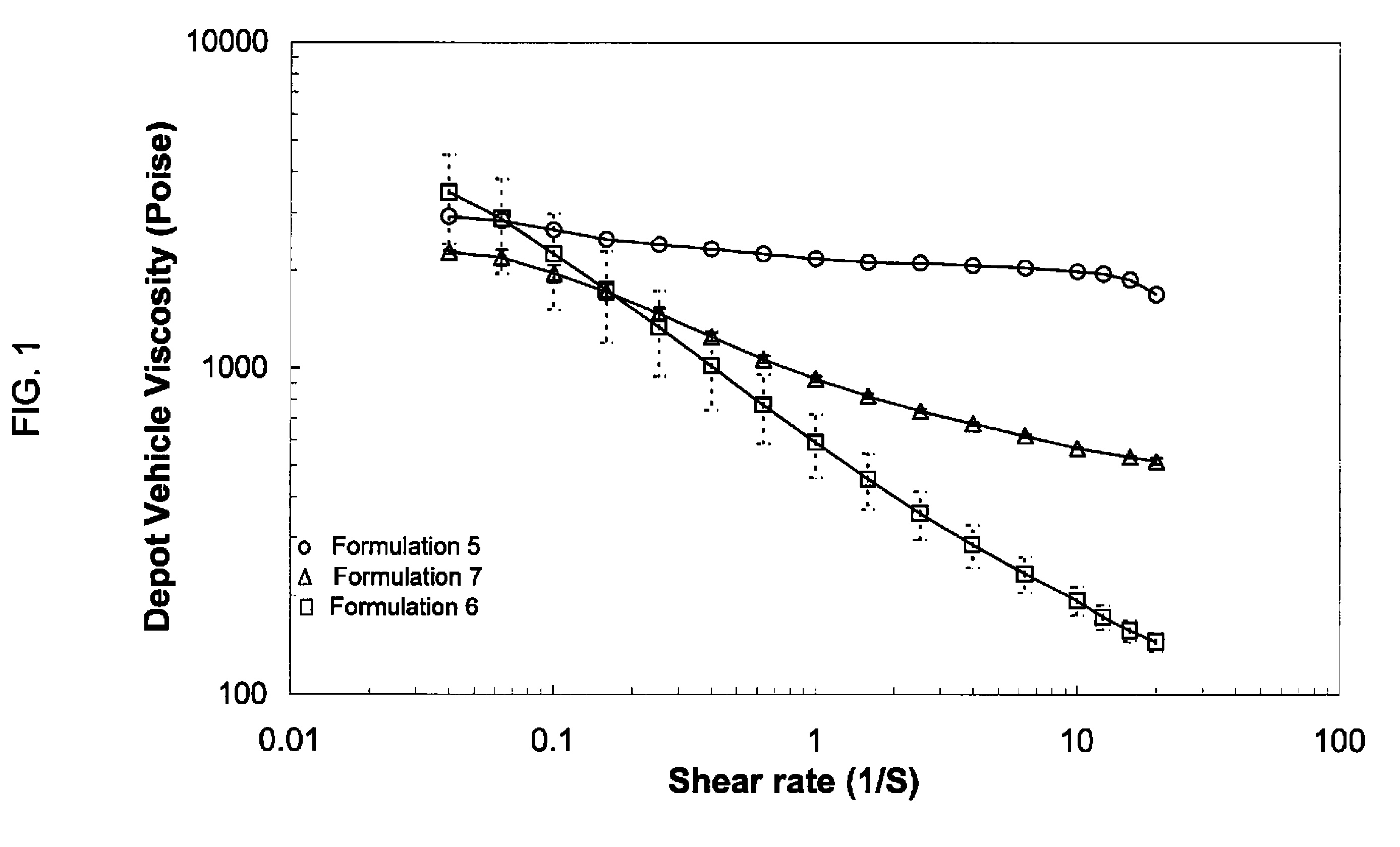
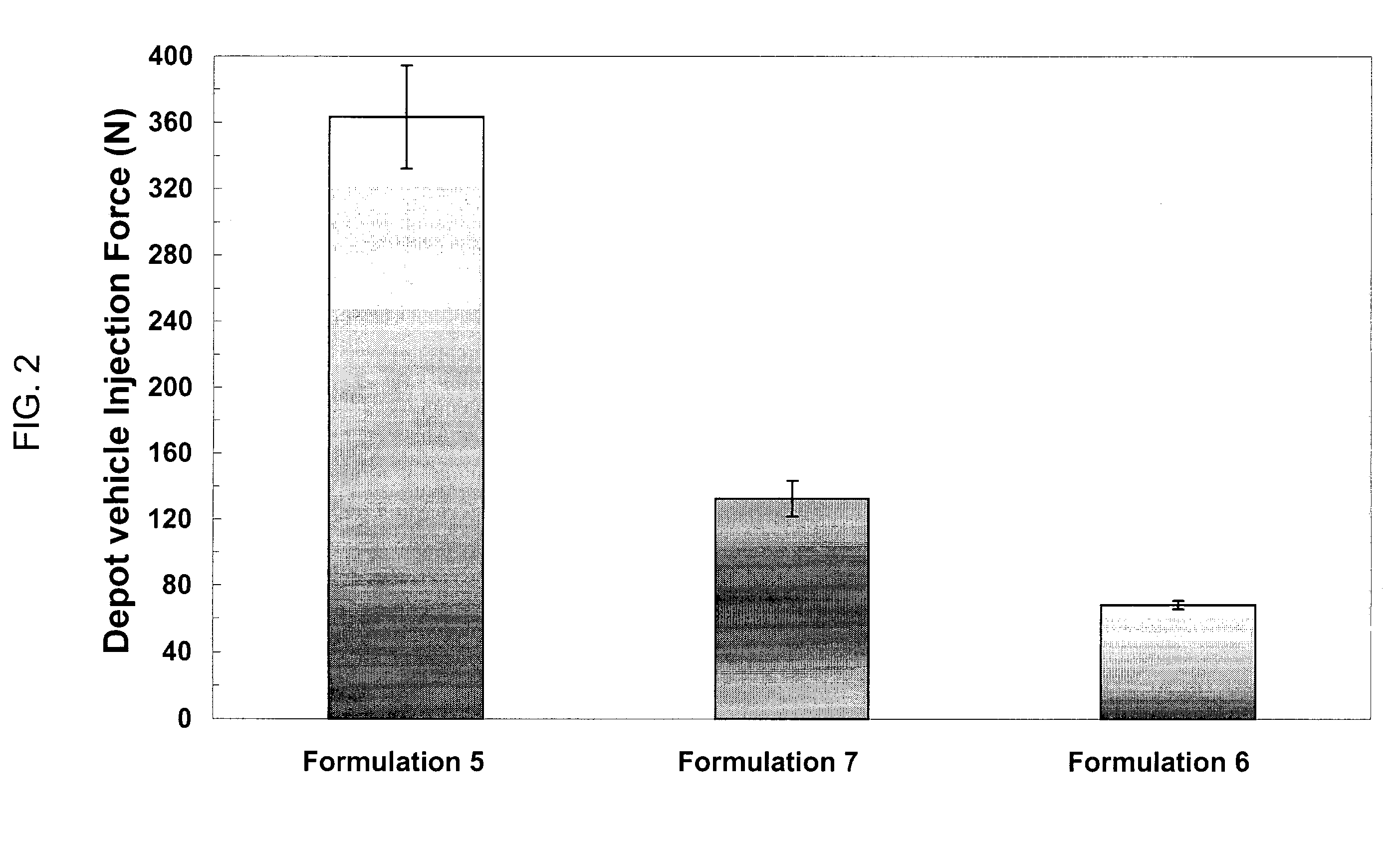
Injectable depot compositions are provided that include a polymer matrix having a plurality of bioerodible, biocompatible polymers wherein each polymer of the plurality of polymers has a specified average molecular weight, and the polymer matrix has a broad molecular weight distribution of the plurality of polymers; a solvent having a miscibility in water of less than or equal to 7 wt % at 25° C., in an amount effective to plasticize the polymer and form a gel therewith; and a beneficial agent. The compositions have substantially improved shear thinning behavior and reduced injection force, rendering the compositions readily implanted beneath a patient's body surface by injection.
Owner:DURECT CORP
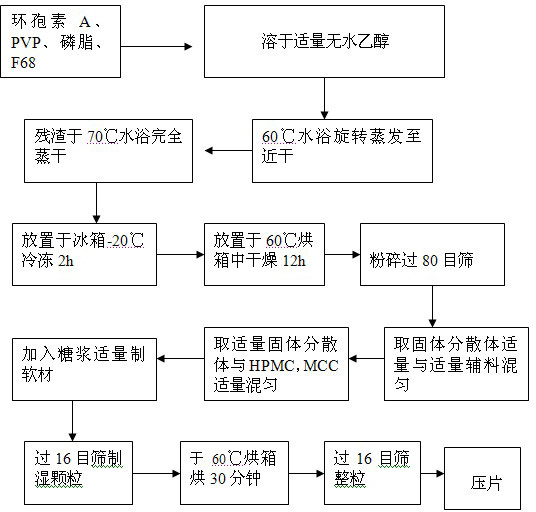
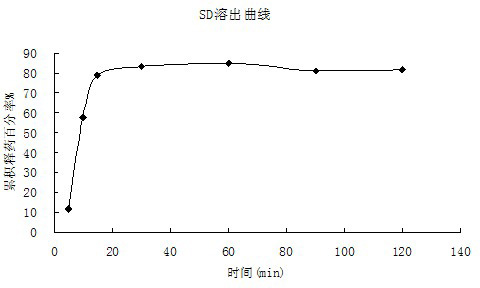
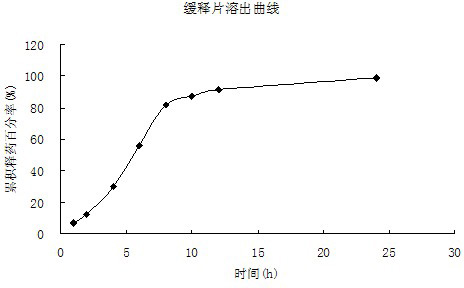
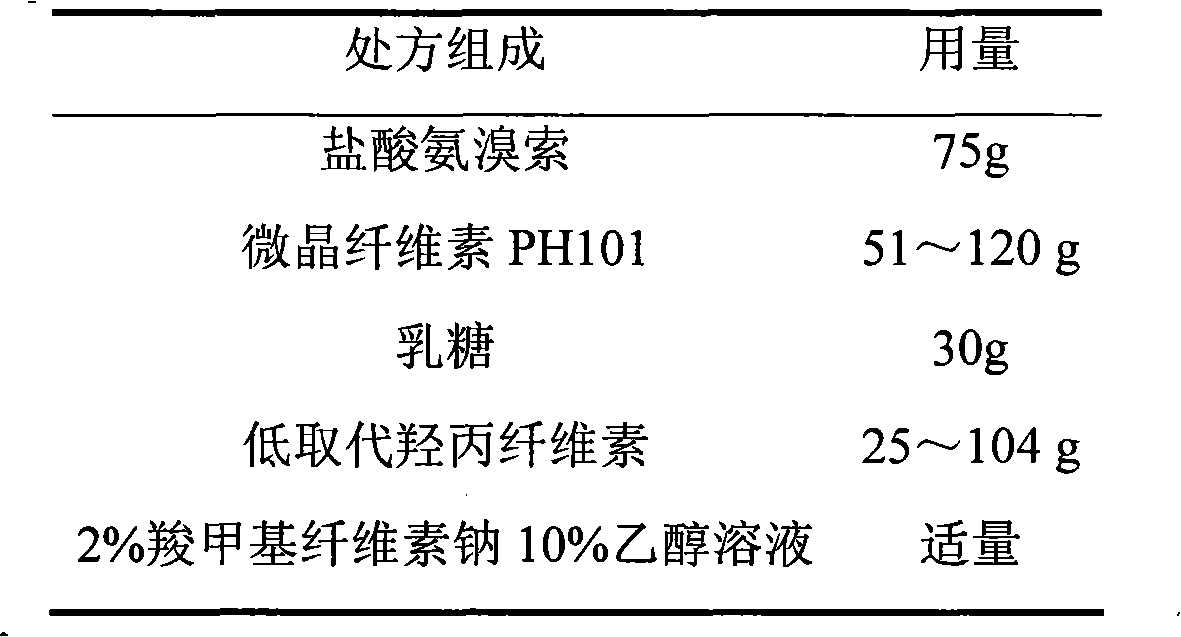
Oral ciclosporin A sustained-release agent and preparation method thereof
ActiveCN102166201AStable drug releaseImprove bioavailabilityPharmaceutical delivery mechanismCyclic peptide ingredientsCiclosporinSolubility
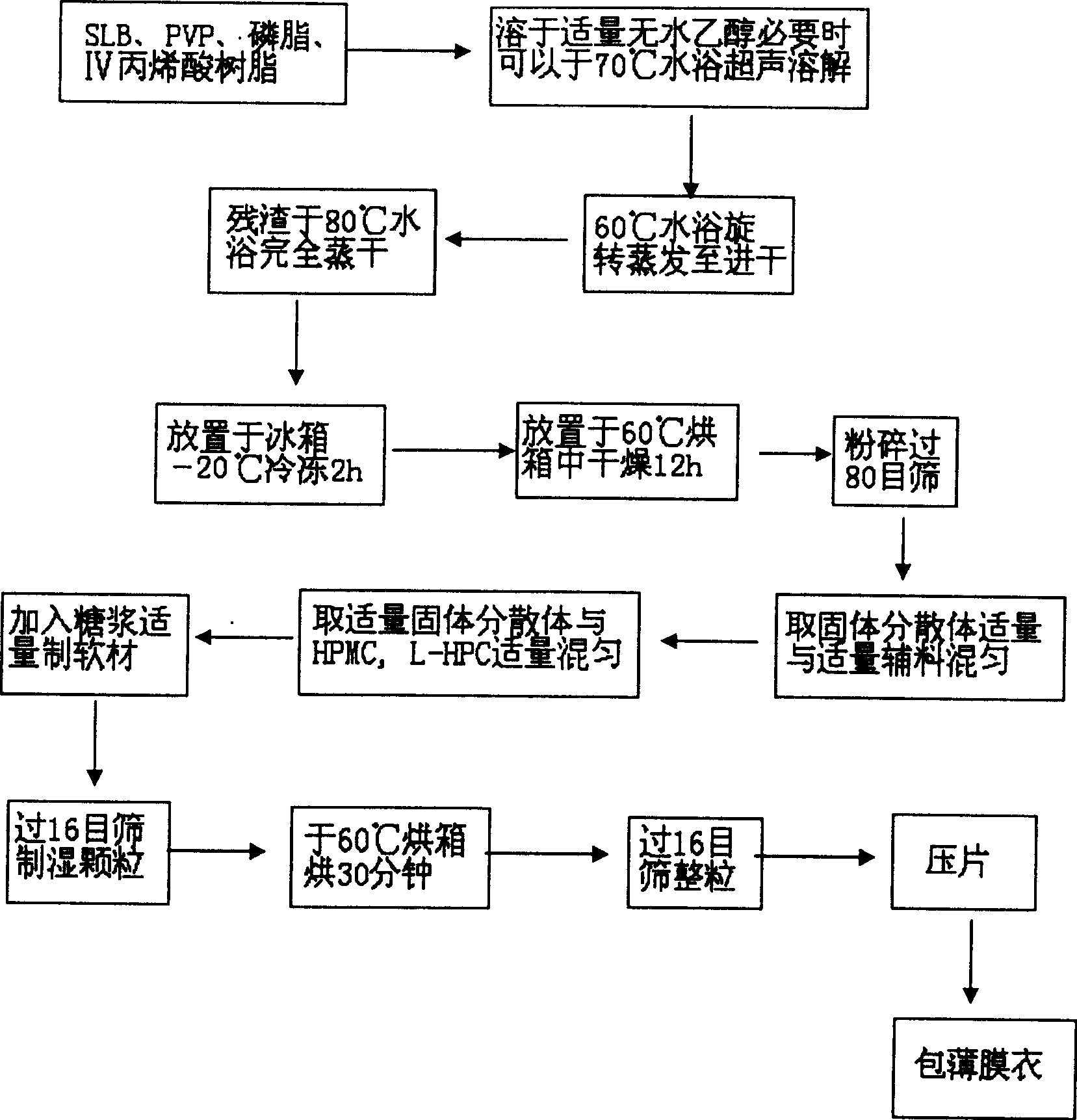
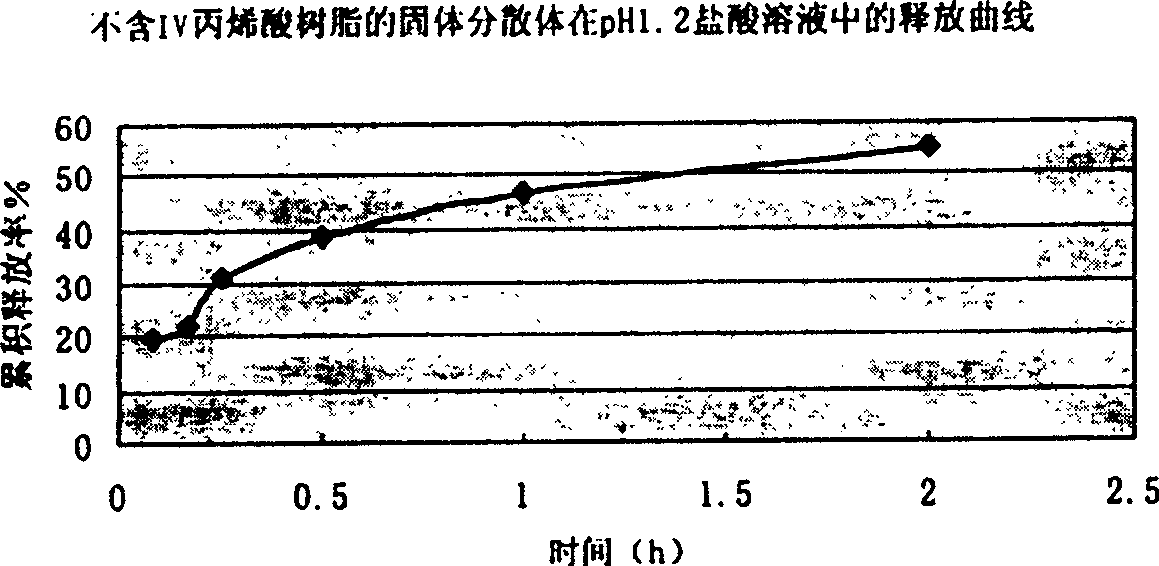
The invention provides an oral ciclosporin A sustained-release agent. The sustained-release agent comprises the following components in part by mass: 1 part of ciclosporin A, 2.5 to 7.5 parts of polyvidone-K30, 0.15 to 4.82 parts of hydroxypropyl methyl cellulose 4000cPa.s, 0.1 to 3.94 parts of microcrystalline cellulose, 0 to 2.57 parts of hydroxypropyl methyl cellulose 15000cPa.s and 0.1 to 1.0part of phospholipid. By combining a solid dispersing technology and a sustained-release hydrophilic gel matrix technology and based on the 'double release' principle of fast release first and slow release second, the method prepares the insoluble medicament of ciclosporin A sustained-release agent; and the method has the obvious advantages that the solubility of ciclosporin A is improved, and the oral agent can quickly play the effect first and then smoothly and slowly release drug. The ciclosporin A sustained-release tablets reduce the lowered blood concentration peak-valley phenomenon and lower the drug-taking frequency. The invention also discloses a method for preparing the oral ciclosporin A sustained-release agent.
Owner:JIANGSU UNIV +1
Acipimox film-controlled slow-release pellet capsule
ActiveCN103211785AAccelerated agingReduce permeabilityOrganic active ingredientsMetabolism disorderMedicineLactose
The invention relates to an acipimox film-controlled slow-release pellet capsule. A slow-release film of the acipimox film-controlled slow-release pellet utilizes Eurdragit NE 30D as a film-formation material. A pellet core of the acipimox film-controlled slow-release pellet contains low-substituted hydroxypropyl cellulose having high expansibility, and also contains pharmaceutically acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 10 to 40wt% of the low-substituted hydroxypropyl cellulose. The slow-release film comprises the Eurdragit NE 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit NE 30D to triethyl citrate to talcum powder is 30: 2: 4 and coating weight gain is in a range of 20 to 39%. The acipimox film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxypropyl cellulose having high water expansibility and thus after absorbing water, the acipimox film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the acipimox film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept prior to expiration date.
Owner:北京天衡药物研究院有限公司
Isosorbide mononitrate sustained-release pellet, and isosorbide mononitrate quick-release and sustained-release pellet capsule adopting it
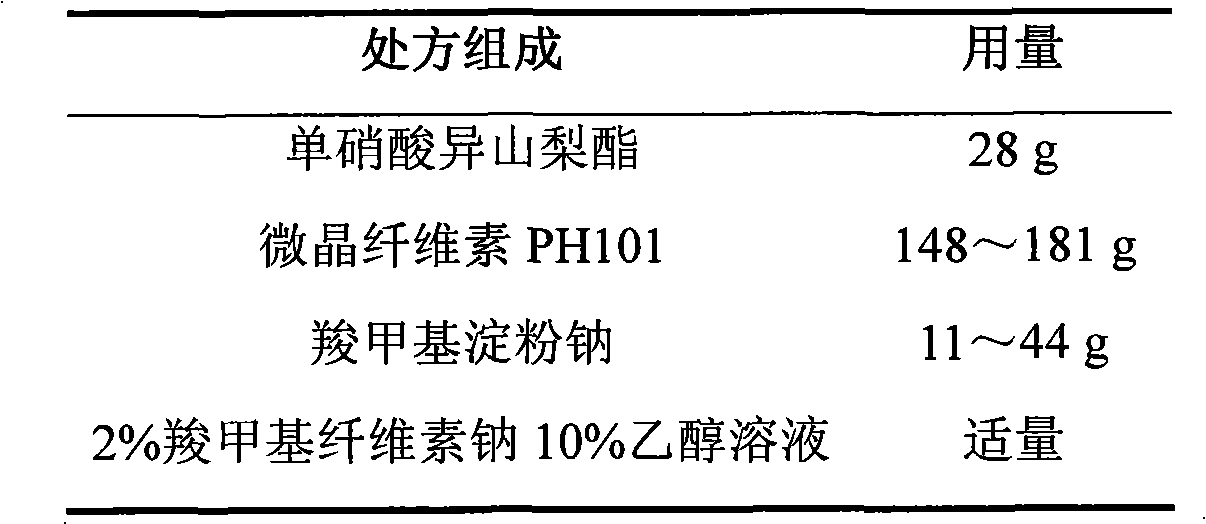
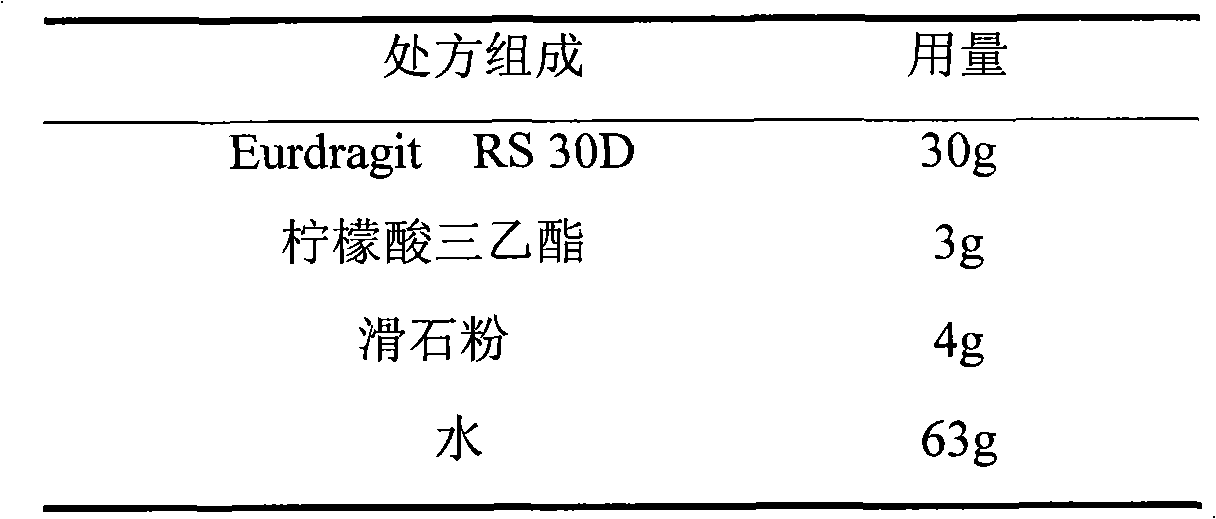
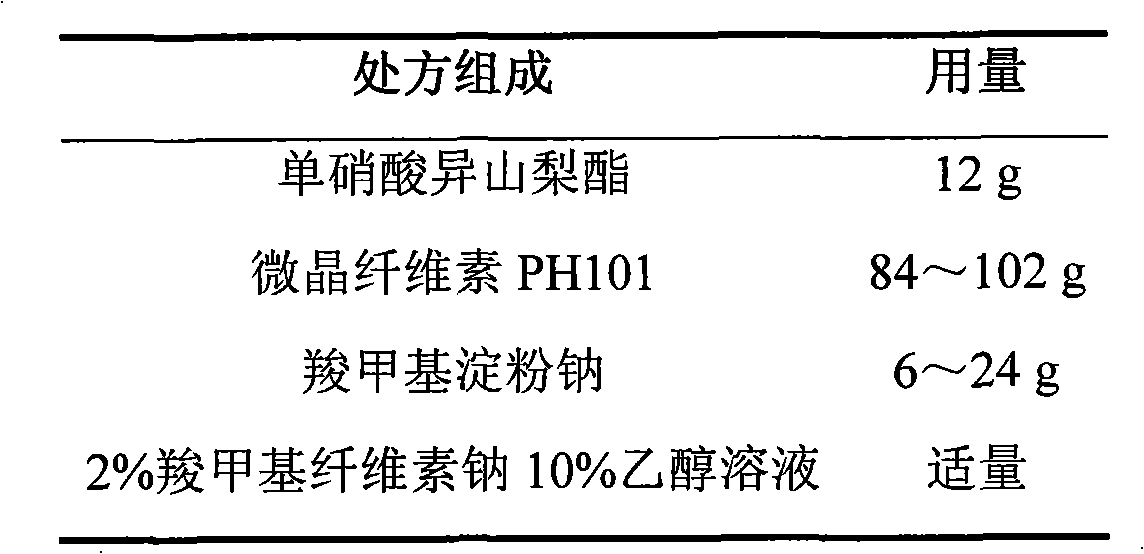
ActiveCN103211768AAccelerated agingReduce permeabilityPharmaceutical non-active ingredientsGranular deliverySustained release pelletsMedicine
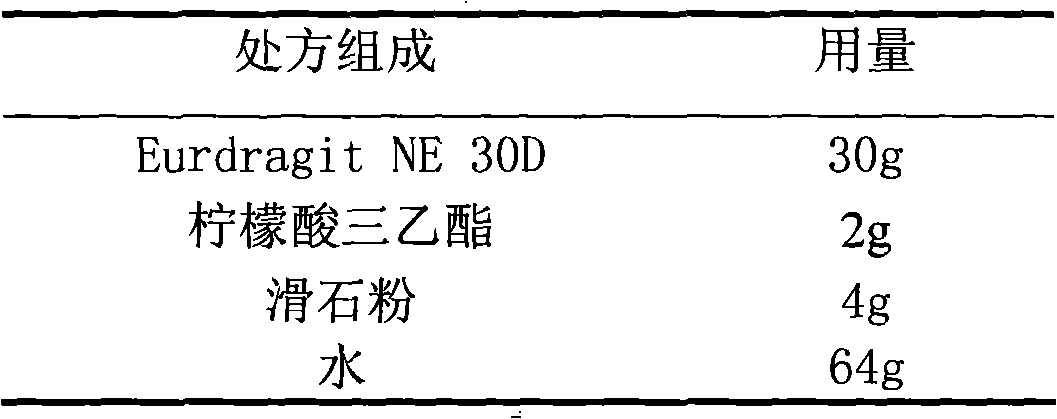
The invention relates to an isosorbide mononitrate sustained-release pellet, and an isosorbide mononitrate quick-release and sustained-release pellet capsule adopting it. The sustained-release film of the sustained-release pellet adopts Eurdragit RS 30D as a film forming material, the core of the sustained-release pellet contains high-expansibility sodium carboxymethyl starch and a pharmaceutically-acceptable excipient commonly used for sustained-release pellets, and optimally, the excipient is microcrystalline cellulose, wherein the weight percentage of sodium carboxymethyl starch in the core of the sustained-release pellet is 5-20%. The sustained-release film of the sustained-release pellet includes the Eurdragit RS 30D, a plasticizer triethyl citrate and an anti-adherent talcum powder, the optimal ratio of the Eurdragit RS 30D to triethyl citrate to the talcum powder is 30:3:4, and the optimal coating weight gain is 19-38%. The core will obviously expand after absorbing water because of the containment of sodium carboxymethyl starch highly expanding after contacting with water, so the sustained-release film is stretched, the thickness of the film is thinned, the apertures of water-pervious micro-pores are increased, the permeability is good, and the permeability decrease caused by film ageing is compensated, thereby the middle and later stage release speed is basically constant, the last stage residue is small, and a stable release performance is always maintained prior to the expiration date.
Owner:北京天衡药物研究院有限公司
Device and method for sustained release of antipsychotic medications
A drug delivery device comprising a non-erodible, non-porous housing member defining a reservoir is provided. The reservoir is loaded with a dry formulation of a selected salt of a neuroleptic agent. The housing member has one or more porous partitions, where the pores of the partitions are sufficiently small to retain the insoluble powder particles within the reservoir yet large enough to allow diffusion of the active agent once the device is hydrated. A therapeutic dose of the drug is released from the device at a constant rate over a period of approximately 2-6 months.
Owner:DELPOR
Water-retention and fertilizer-retention air ventilation nutritional soil
InactiveCN106748317AMeet the law of growthEasy maintenanceMagnesium fertilisersAnimal corpse fertilisersPhosphateHeat stability
The invention discloses water-retention and fertilizer-retention air ventilation nutritional soil. The water-retention and fertilizer-retention air ventilation nutritional soil is prepared from the following raw materials in parts by weight: 20 to 40 parts of garden soil, 15 to 25 parts of leaf mold, 5 to 18 parts of rice chaff ash, 2 to 9 parts of ammonium chloride, 1 to 6 parts of ground phosphate rock, 2 to 8 parts of potassium nitrate, 4 to 10 parts of fish bone meal, 10 to 20 parts of germinated brown rice, 2 to 8 parts of castor meal, 3 to 6 parts of oil residues, 4 to 10 parts of earthworm excretion, 0.01 to 0.06 part of diaminoferric xanthohumate, 1 to 2 parts of sepiolite powder, 2 to 6 parts of grass carbon, 2 to 4 parts of diatomite, 0.5 to 1.5 parts of shell powder, 10 to 20 parts of alfalfa, and 5 to 15 parts of tobacco stem and clay compound. The water-retention and fertilizer-retention air ventilation nutritional soil has the advantages that the degrading rate and nutrient releasing rate can be regulated, the utilization rate of fertilizer is high, nitrogen, phosphor and potassium necessary to the growth of plants can be provided, the releasing rate meets the growth rule of the plants, the slow-release effect is obvious, and the heat stability is good.
Owner:蚌埠市凯婷农民种植专业合作社
Injectable depot compositions and uses thereof
InactiveUS8252303B2Effective distributionBeneficial agent loading rateOrganic active ingredientsPowder deliveryAromatic alcoholAromatic ketones
Injectable depot compositions are provided that include a bioerodible, biocompatible polymer, a solvent having a miscibility in water of less than or equal to 7 wt. % at 25° C., in an amount effective to plasticize the polymer and form a gel therewith, a thixotropic agent, and a beneficial agent. The solvent comprises an aromatic alcohol, an ester of an aromatic acid, an aromatic ketone, or mixtures thereof. The compositions have substantially improved the shear thinning behavior and reduced injection force, rendering the compositions readily implanted beneath a patient's body surface by injection.
Owner:DURECT CORP
Sustained-release microsphere of nomegestrol acetate of analogs thereof and preparation method and application thereof
InactiveCN101612111ALow first-day burst drug releaseImprove securityPowder deliveryOrganic active ingredientsPolymer scienceMicrosphere
The invention discloses a sustained-release microsphere to achieve the aims of contraception and long-term treatment of endometriosis uterine, which comprises the following components: (1) nomegestrol acetate or analogs thereof, and (2) a biodegradable polymer, wherein the weight ratio of an ester-terminated polymer to a carboxyl-terminated polymer is between 1:0.01-1:1; the ester-terminated polymer is selected from polylactide-glycolide, polylactide, poly-beta-hydroxybutyrate, poly ortho ester, polycaprolactone, poly-phosphate, polyanhydride, or poly-cyanoacrylate; the carboxyl-terminated polymer is selected from polyglycolic acid and polylactic acid-glycolic acid; and the weight of the nomegestrol acetate or the analogs thereof accounts for 5 to 80 percent of the total weight of the microsphere. The invention also discloses a preparation method and application of the sustained-release microsphere.
Owner:SHANGHAI INST OF PHARMA IND +1
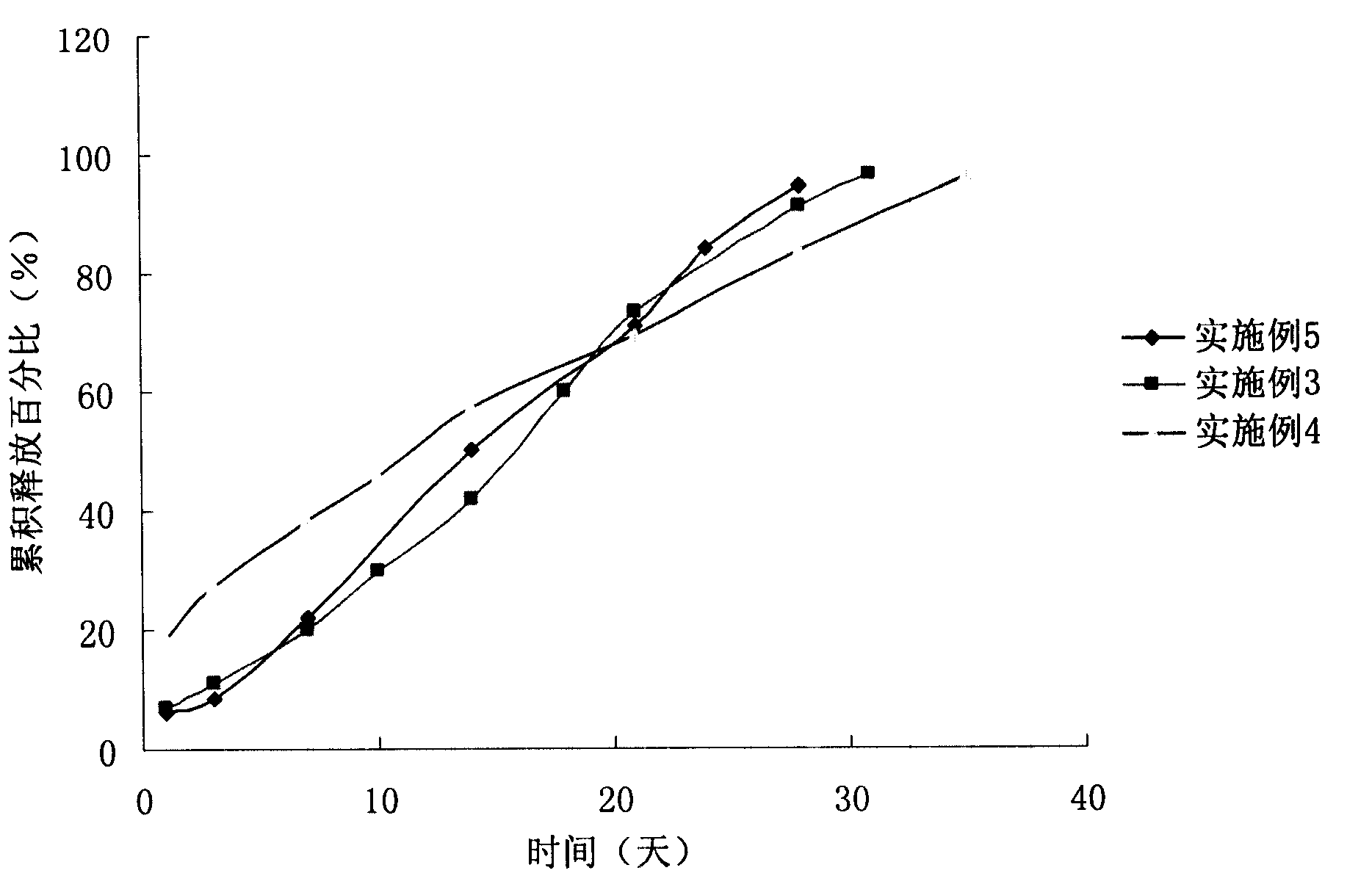
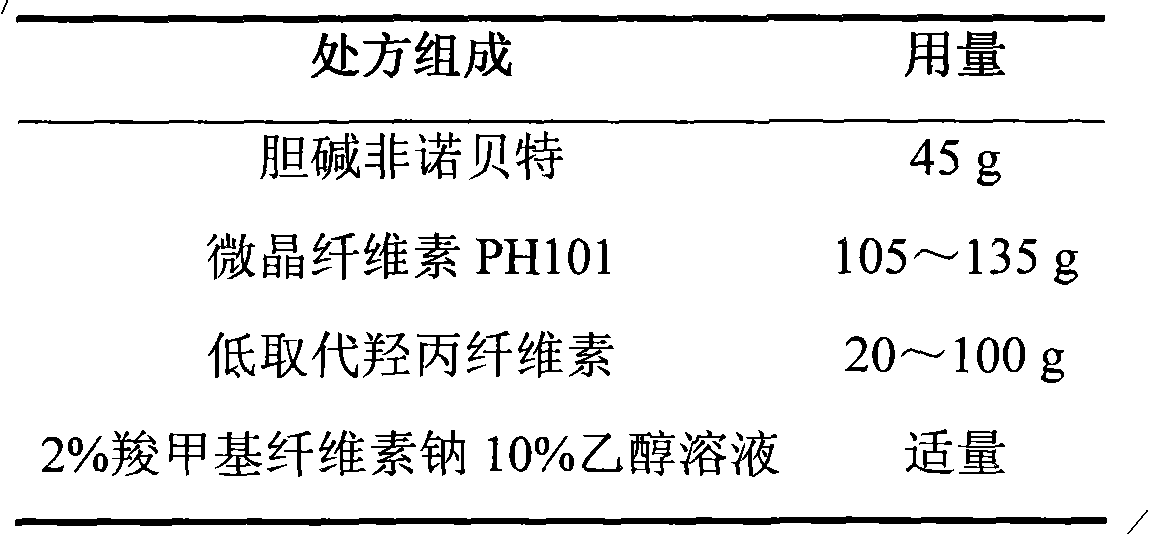
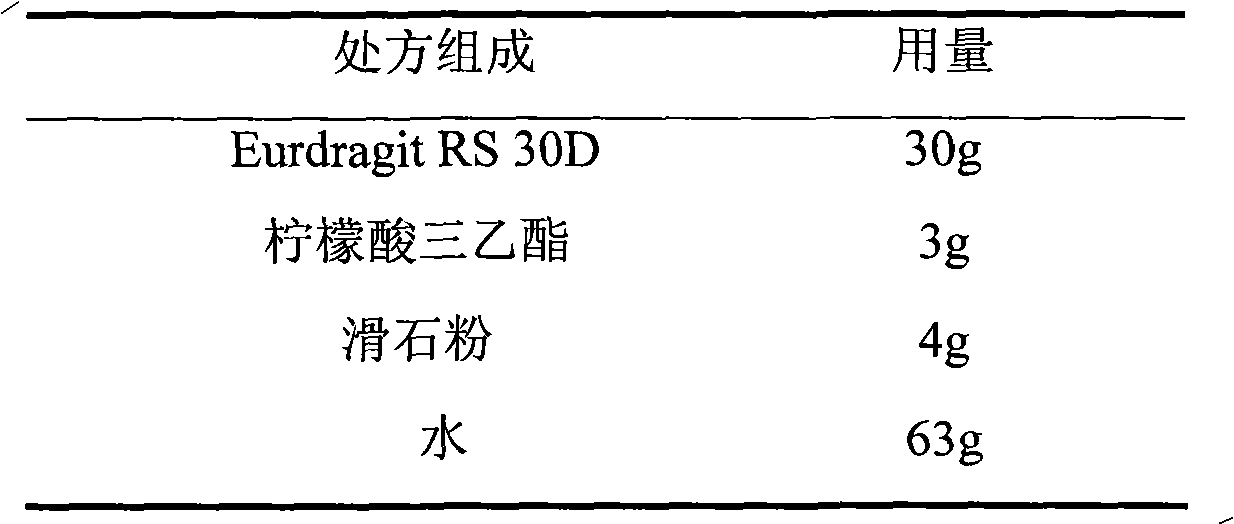
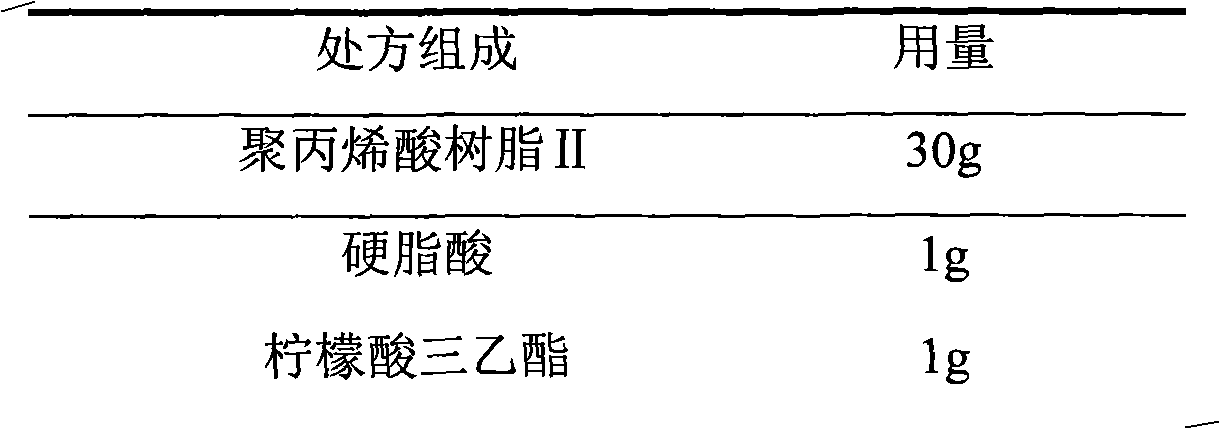
Choline fenofibrate film-controlled enteric slow-release pellet capsule
ActiveCN103211786AAccelerated agingReduce permeabilityOrganic active ingredientsMetabolism disorderSustained release pelletsMedicine
The invention relates to a choline fenofibrate film-controlled enteric slow-release pellet capsule. A slow-release film of the choline fenofibrate film-controlled enteric slow-release pellet utilizes Eurdragit RS 30D as a film-formation material. A pellet core of the choline fenofibrate film-controlled enteric slow-release pellet contains low-substituted hydroxypropyl cellulose having high expansibility, and also contains a pharmaceutically acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 10 to 40wt% of the low-substituted hydroxypropyl cellulose. The slow-release film comprises Eurdragit RS 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of urdragit RS 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 20 to 38%. The choline fenofibrate film-controlled enteric slow-release pellet comprises the pellet core containing low-substituted hydroxypropyl cellulose having high water expansibility and thus after absorbing water, the choline fenofibrate film-controlled enteric slow-release pellet expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the choline fenofibrate film-controlled enteric slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
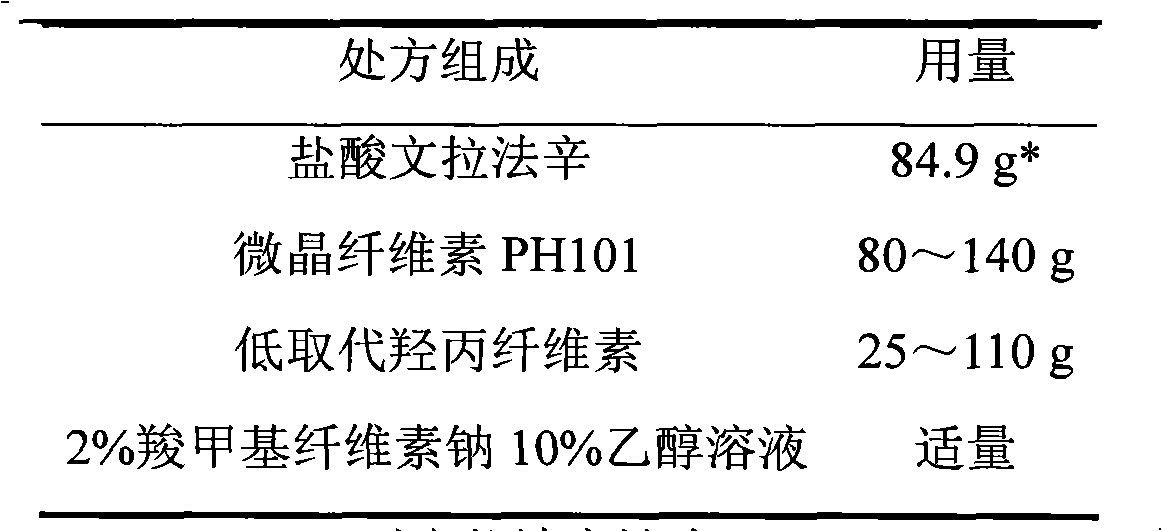
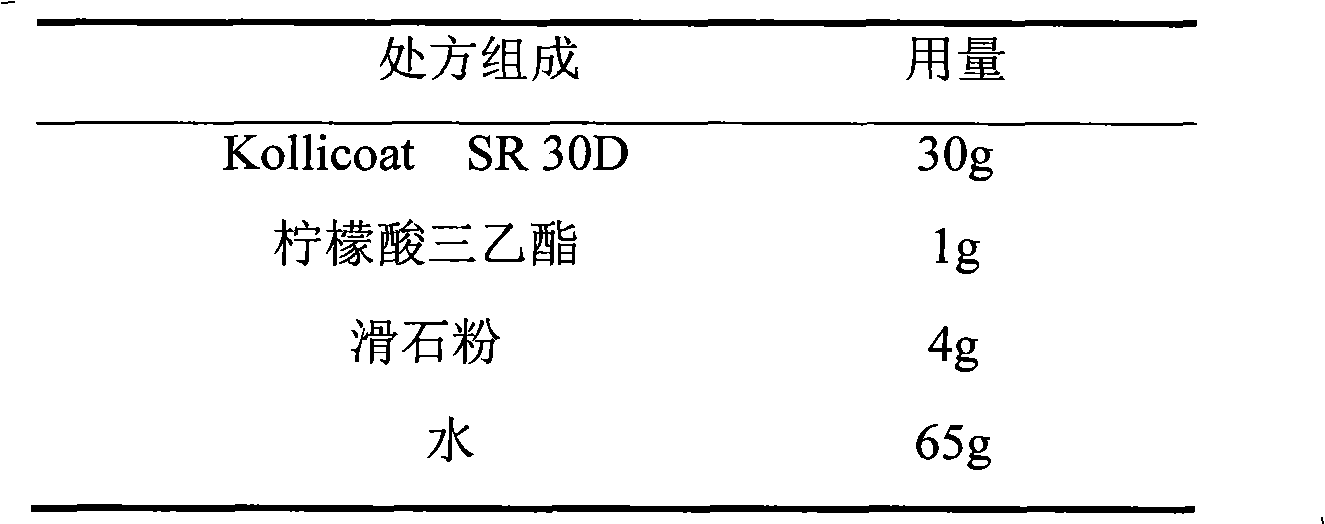
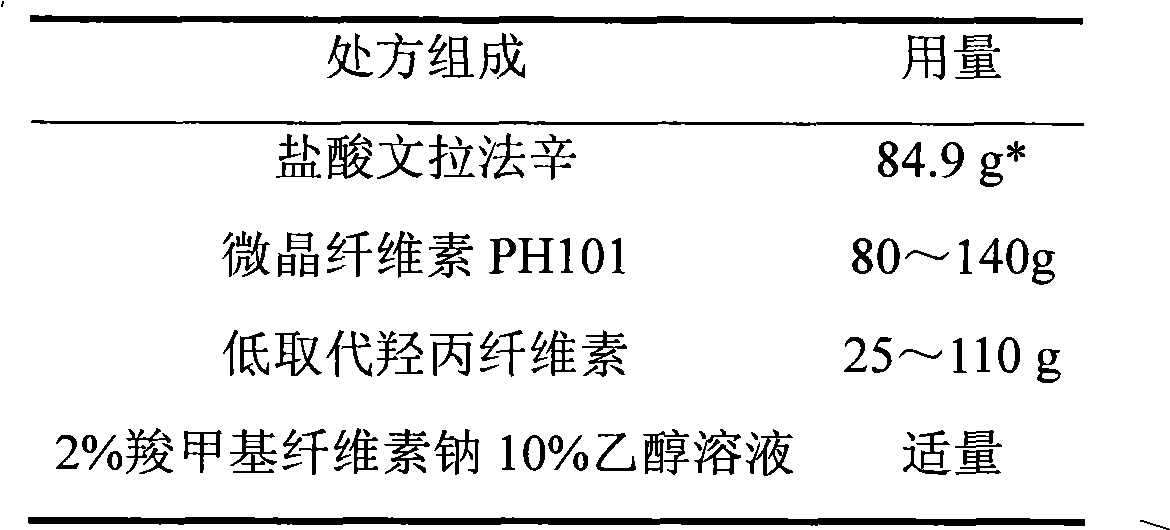
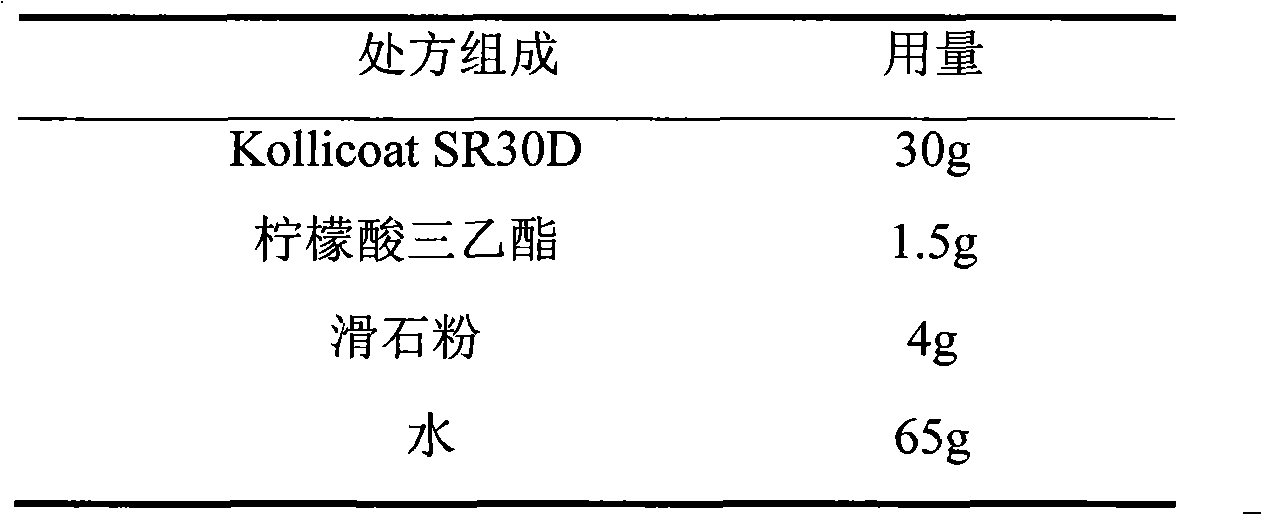
Venlafaxine hydrochloride film-controlled slow-release pellet capsule
ActiveCN103211791AAccelerated agingReduce permeabilityOrganic active ingredientsNervous disorderSustained release pelletsLow-substituted hydroxypropylcellulose
The invention relates to a venlafaxine hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the venlafaxine hydrochloride film-controlled slow-release pellet utilizes Kollicoat SR30D as a film-formation material. A pellet core of the venlafaxine hydrochloride film-controlled slow-release pellet contains low-substituted hydroxyprepyl cellulose having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 10 to 40wt% of low-substituted hydroxyprepyl cellulose. The slow-release film comprises Kollicoat SR30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Kollicoat SR30D to triethyl citrate to talcum powder is 30: 1: 4 and a film weight increasing ratio is in a range of 21 to 39%. The venlafaxine hydrochloride film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxyprepyl cellulose having high water expansibility and thus after absorbing water, the venlafaxine hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the venlafaxine hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
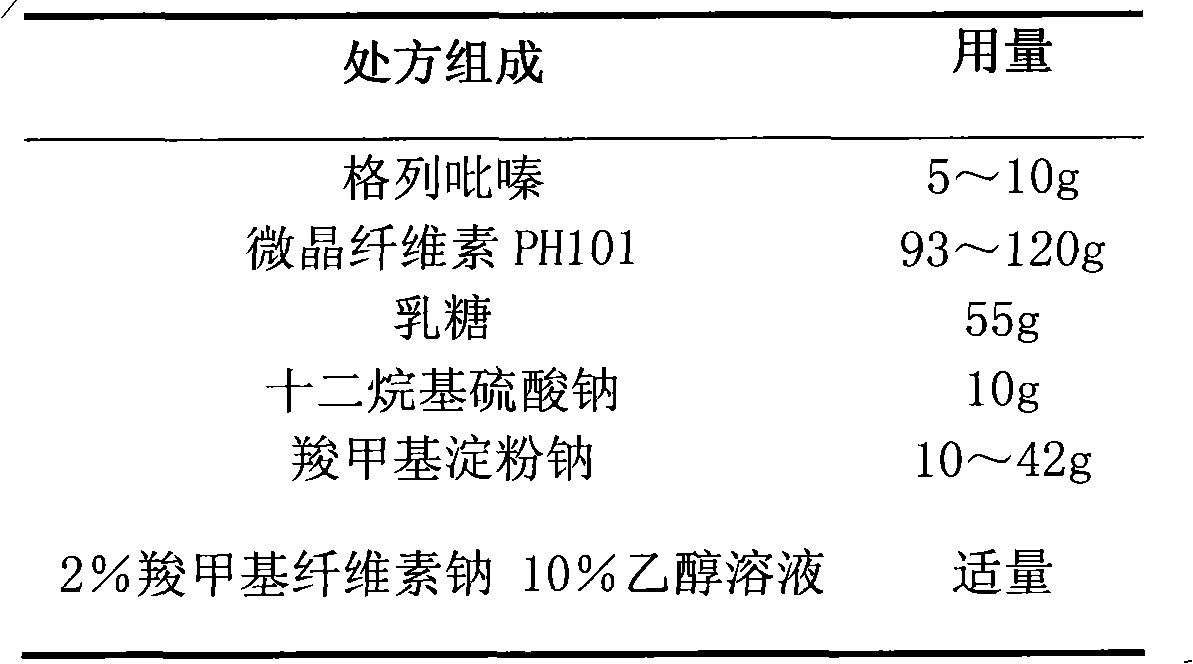
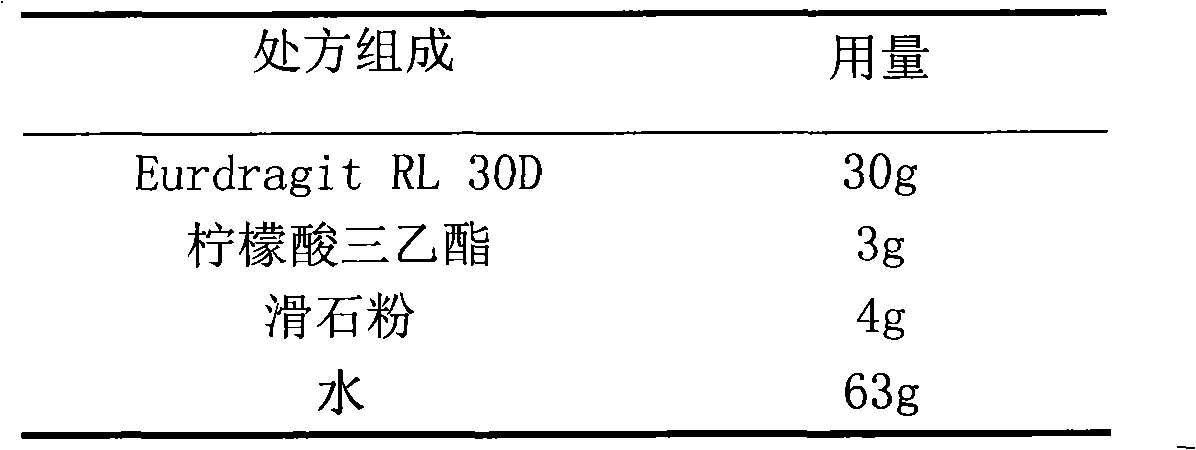
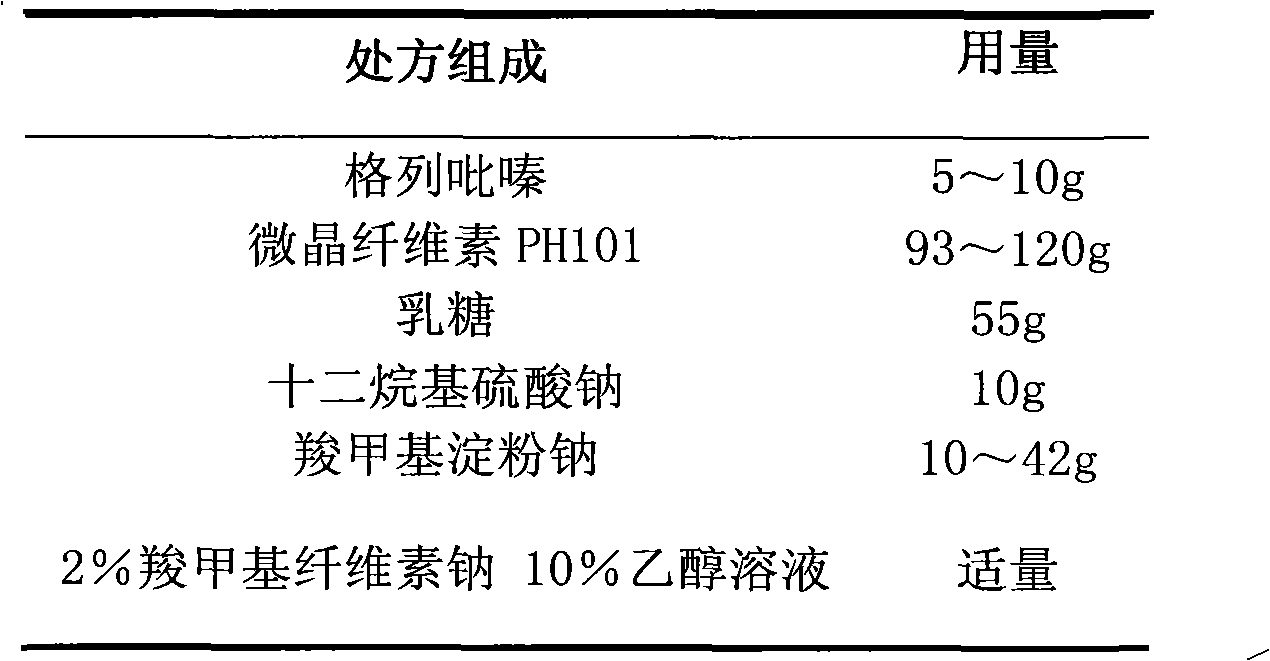
Glipizide film-controlled slow-release pellet capsule
ActiveCN103211787AAccelerated agingReduce permeabilityMetabolism disorderSulfonylurea active ingredientsSustained release pelletsMedicine
The invention relates to a glipizide film-controlled slow-release pellet capsule. A slow-release film of the glipizide film-controlled slow-release pellet utilizes Eurdragit RL 30D as a film-formation material. A pellet core of the glipizide film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose, lactose and sodium dodecyl sulfate, and the pellet core comprises 5 to 20wt% of sodium carboxymethyl starch. The slow-release film comprises Eurdragit RL 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RL 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 17 to 36%. The glipizide film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the glipizide film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the glipizide film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:内蒙古天衡医院管理有限公司
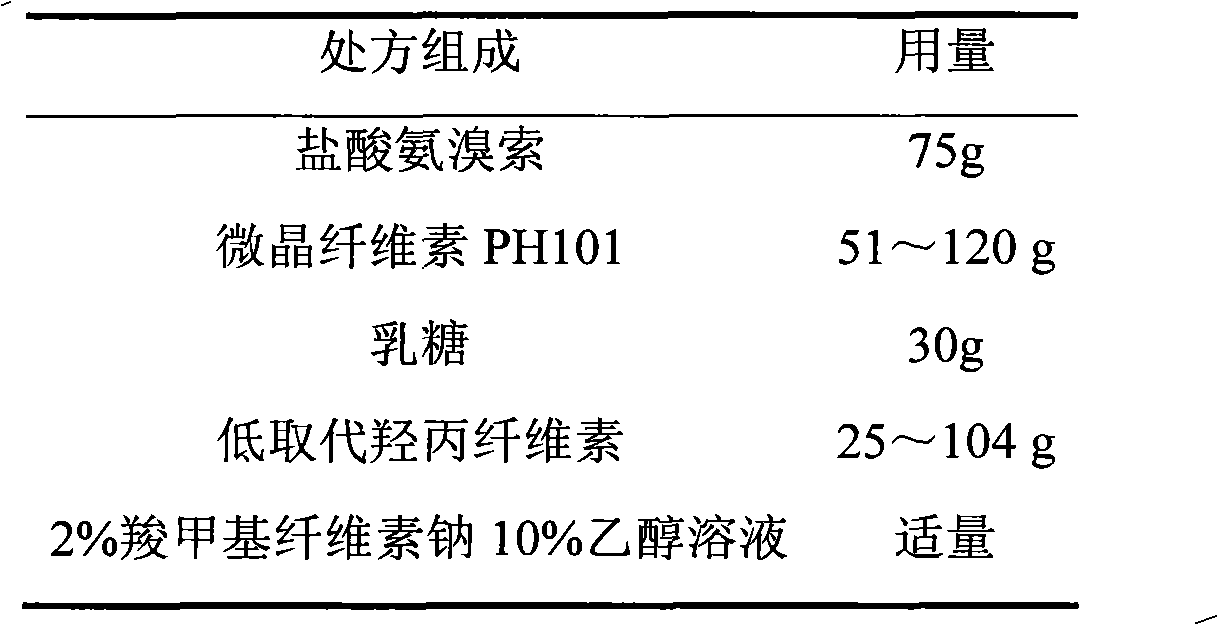
Ambroxol hydrochloride film-controlled slow-release pellet capsule
ActiveCN103211789AAccelerated agingReduce permeabilityOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsLactose
The invention relates to an ambroxol hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the ambroxol hydrochloride film-controlled slow-release pellet utilizes Kollicoat SR 30D as a film-formation material. A pellet core of the ambroxol hydrochloride film-controlled slow-release pellet contains low-substituted hydroxyprepyl cellulose having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 10 to 40wt% of low-substituted hydroxyprepyl cellulose. The slow-release film comprises Kollicoat SR 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Kollicoat SR 30D to triethyl citrate to talcum powder is 30: 1.5: 4 and a film weight increasing ratio is in a range of 20 to 36%. The ambroxol hydrochloride film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxyprepyl cellulose having high water expansibility and thus after absorbing water, the ambroxol hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the ambroxol hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
Sustained pheromone releaser
InactiveUS8828374B2Increase release rateConstant release rateBiocideAnimal repellantsPaleontologyPolymer
Provided is a sustained releaser of a sex pheromone having a high release rate, especially preferably of a sex pheromone of an aliphatic acetate compound having 10 to 16 carbon atoms, wherein the release of the sex pheromone is controlled so that it can be released at a constant rate over a control period of a pest insect. More specifically, provided is a sustained pheromone releaser comprising a polymer container and having, enclosed therein, a mixture having a melting point of 15 to 35° C. and being obtained by mixing a sex pheromone substance and a compound having a melting point of 10 to 40° C.
Owner:SHIN ETSU CHEM CO LTD
Enteric Sustained-Release Tablet Comprising Paroxetine
InactiveUS20080292696A1Minimize interactionConstant release rateBiocideAnimal repellantsSustained Release TabletDrug release
The present invention relates to an enteric, sustained-release tablet comprising paroxetine or a hydrates or anhydrides of a pharmaceutically acceptable salt thereof as active substance, more particularly to a tablet prepared by coating a sustained-release tablet core containing paroxetine with an enteric polymer, wherein the interaction between the tablet core and the enteric coating layer is minimized to enable constant drug release without regard to the residence time of the tablet in the stomach.
Owner:GL PHARMTECH
Controlled release transdermal patch for preventing and treating cardiovascular and cerebrovascular system diseases and preparation method thereof
InactiveCN101810596AGood film formingExcellent water vapor permeabilityOrganic active ingredientsOil/fats/waxes non-active ingredientsTransdermal patchComposite film
The invention relates to a controlled release transdermal patch for preventing and treating cardiovascular and cerebrovascular system diseases, which consists of a protectively layer, a medicine release layer, a medicine storage layer and a back lining layer through being sequentially overlapped, wherein the protectively layer is siliconized anti-sticking paper or a polyethylene film or a siliconized polyester film, the back lining layer is an aluminum foil, an aluminum foil and polyethylene composite film or a polyester composite film, the medicine releasing layer is prepared from 3 to 8 percent of cyclovimbuxine D, 60 to 70 percent of pressure sensitive adhesive substrates, 5 to 10 percent of composite transdermal penetrating agents, 20 to 30 percent of plasticizing agents and 1 to 3 percent of cross-linking agents, the medicine storage layer consists of 8 to 15 percent of the cyclovimbuxine D, 50 to 60 percent of the pressure sensitive adhesive substrates, 1 to 5 percent of composite transdermal penetrating agents, 20 to 30 percent of plasticizing agents and 1 to 3 percent of cross-linking agents. The patch of the invention is prepared by an organic solvent dispersing method, the preparation process is simple, the prepared patch has the exact curative effect, stable quality, high safety and convenient use, and is applicable to the long-time prevention and treatment of the cardiovascular and cerebrovascular diseases.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Loxoprofen sodium matrix sustained-release tablet
ActiveCN102525989AIncreased diffusion resistanceReduced Diffusion ResistanceOrganic active ingredientsAntipyreticSustained Release TabletLoxoprofen
The invention relates to a novel matrix sustained-release tablet containing loxoprofen sodium, in particular to a locoprofen sodium matrix sustained-release tablet with a regulation layer, which is capable of stably releasing for 8 hours, good in releasing effect, simple in process and low in cost.
Owner:北京天衡药物研究院有限公司
Posaconazole double-layer osmotic pump controlled release tablet and preparation method thereof
InactiveCN103948558AReduced responseDelay drug resistanceOrganic active ingredientsAntimycoticsIn vivoPharmaceutical formulation
The invention belongs to the field of medicinal preparation and specifically provides a posaconazole double-layer osmotic pump controlled release tablet and a preparation method thereof. The tablet comprises a tablet core and a semipermeable controlled release film. The tablet core comprises a drug containing layer and a boosting layer. The drug containing layer is located inside the controlled release film which is provided with a drug releasing hole. The boosting layer is located inside the controlled release film at a place far away from the drug releasing hole. One end of the water-permeable controlled release film is provided with one or more drug releasing holes. The release curve of the preparation drug shows a zero-order release pattern, and thus the problem of drug resistance caused by long-term use of posaconazole dosage forms on the market is solved. Besides, the problems that the preparation technology is complex, the preparation cost is high, and the drug is unsuitable for industrialized mass production due to the adoption of a hot-melt extrusion technology are solved. Simultaneously, the in vivo-in vitro correlation of the drug is better ensured and the clinical monitoring of plasma concentration is better facilitated.
Owner:JINAN KANGHE MEDICAL TECH
Sustained pheromone releaser
InactiveUS20060093638A1Increase release rateConstant release rateBiocideAnimal repellantsPaleontologyCompound (substance)
Provided is a sustained releaser of a sex pheromone having a high release rate, especially preferably of a sex pheromone of an aliphatic acetate compound having 10 to 16 carbon atoms, wherein the release of the sex pheromone is controlled so that it can be released at a constant rate over a control period of a pest insect. More specifically, provided is a sustained pheromone releaser comprising a polymer container and having, enclosed therein, a mixture having a melting point of 15 to 35° C. and being obtained by mixing a sex pheromone substance and a compound having a melting point of 10 to 40° C.
Owner:SHIN ETSU CHEM IND CO LTD
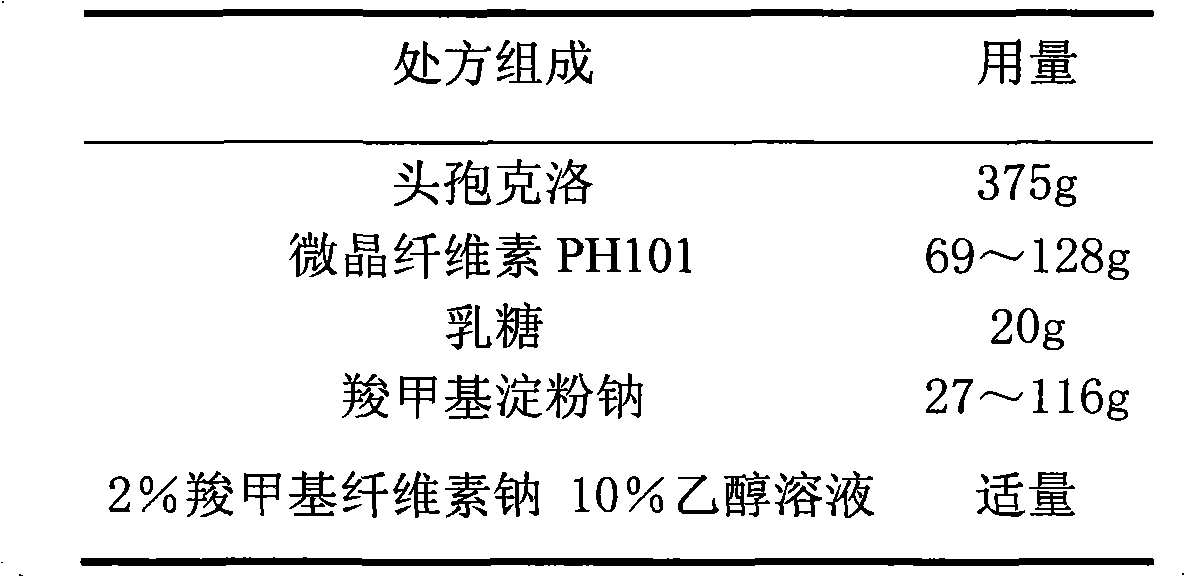
Cefaclor film-controlled slow-release pellet capsule
ActiveCN103211795AAccelerated agingReduce permeabilityAntibacterial agentsOrganic active ingredientsMedicineLactose
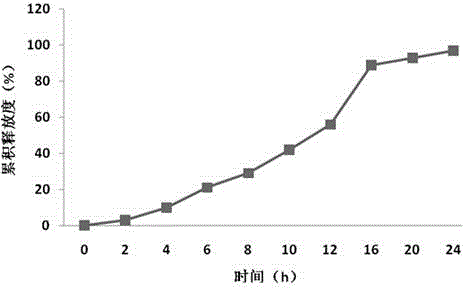
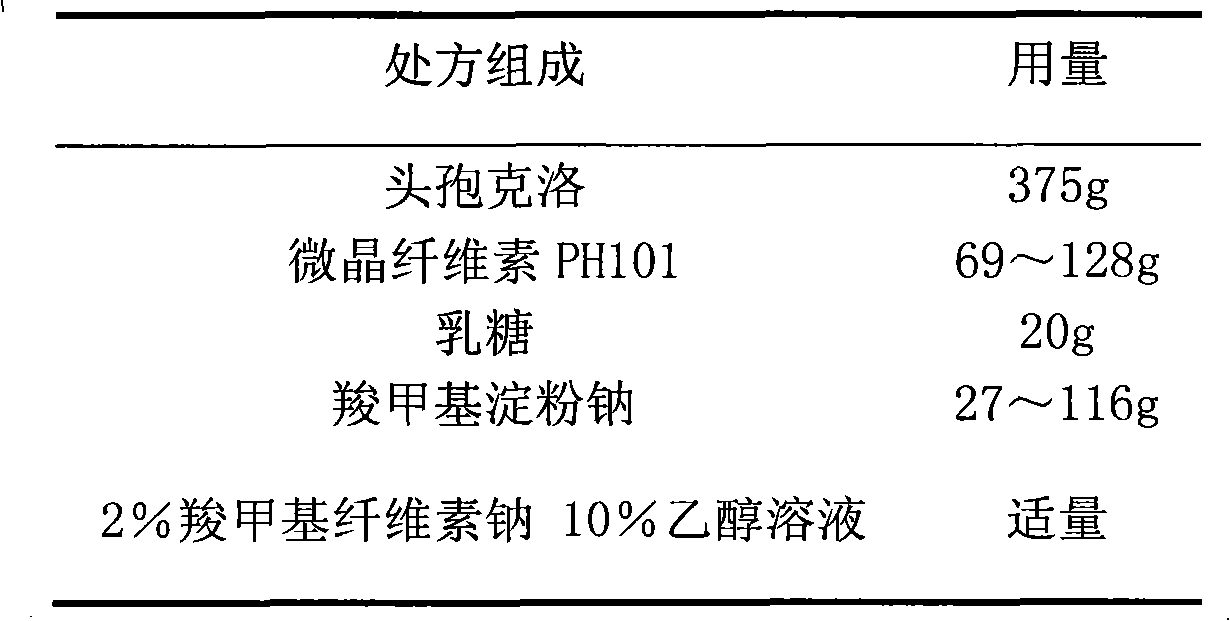
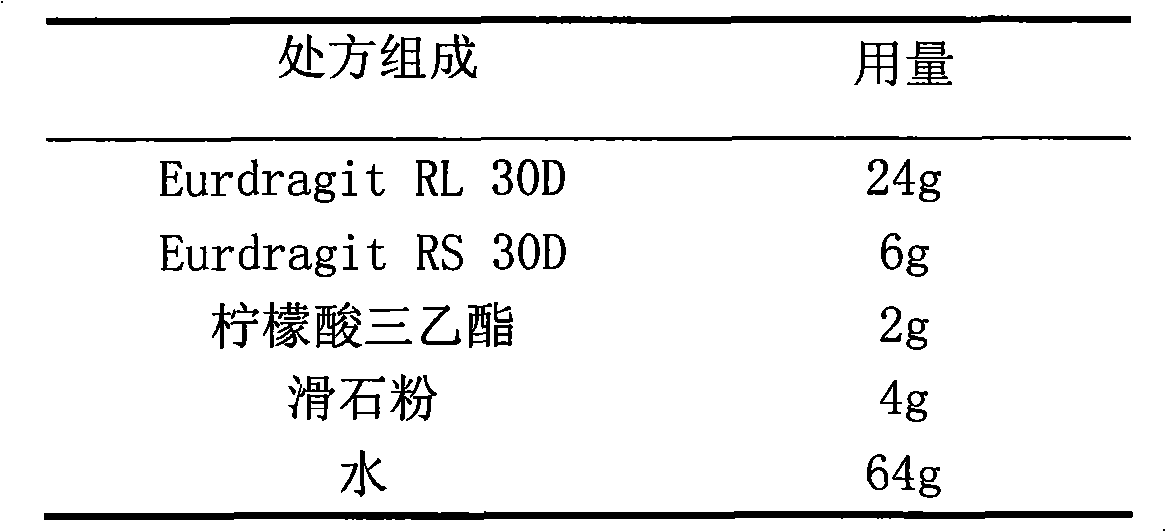
The invention relates to a cefaclor film-controlled slow-release pellet capsule. A slow-release film of the cefaclor film-controlled slow-release pellet utilizes a mixture of aqueous dispersion Eurdragit RL 30D and Eurdragit RS 30D as a film-formation material, wherein a weight ratio of Eurdragit RL 30D to Eurdragit RS 30D in the mixture is 4: 1. A pellet core of the cefaclor film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 5 to 20wt% of the sodium carboxymethyl starch. The slow-release film comprises the mixture of Eurdragit RL 30D and Eurdragit RS 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RL 30D to Eurdragit RS 30D to triethyl citrate to talcum powder is 24: 6: 2: 4 and a film weight increasing ratio is in a range of 23 to 40%. The cefaclor film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the cefaclor film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the cefaclor film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
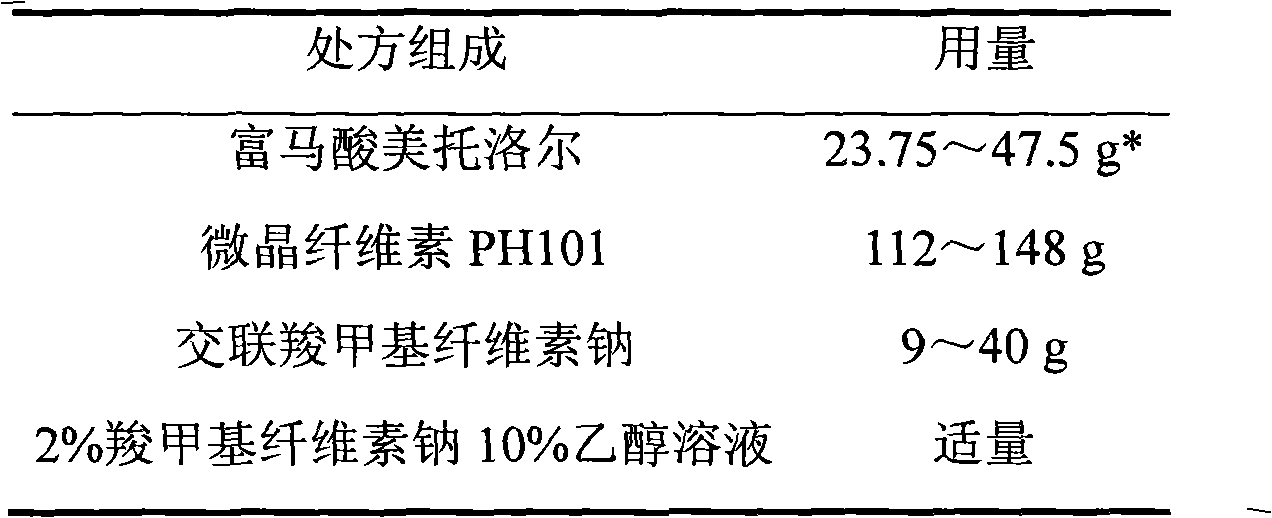
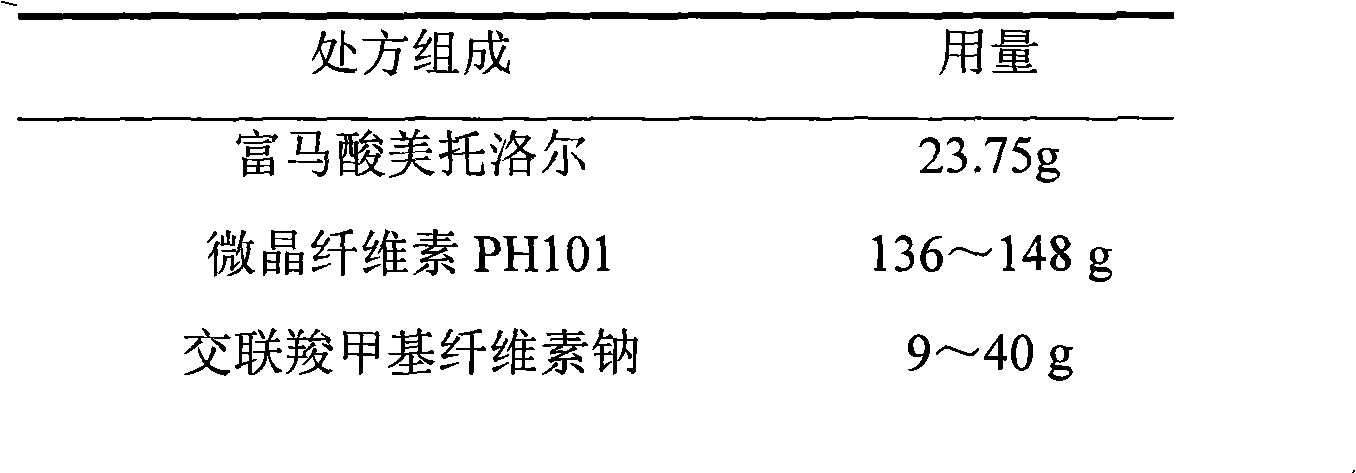
Metoprolol fumarate film-controlled slow-release pellet capsule
ActiveCN103211792AAccelerated agingReduce permeabilityOrganic active ingredientsNervous disorderMetoprolol FumarateMedicine
The invention relates to a metoprolol fumarate film-controlled slow-release pellet capsule. A slow-release film of the metoprolol fumarate film-controlled slow-release pellet utilizes Eurdragit RS 30D as a film-formation material. A pellet core of the metoprolol fumarate film-controlled slow-release pellet contains croscarmellose sodium having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 5 to 20wt% of croscarmellose sodium. The slow-release film comprises Eurdragit RS 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RS 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 18 to 33%. The metoprolol fumarate film-controlled slow-release pellet comprises the pellet core containing croscarmellose sodium having high water expansibility and thus after absorbing water, the metoprolol fumarate film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the metoprolol fumarate film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
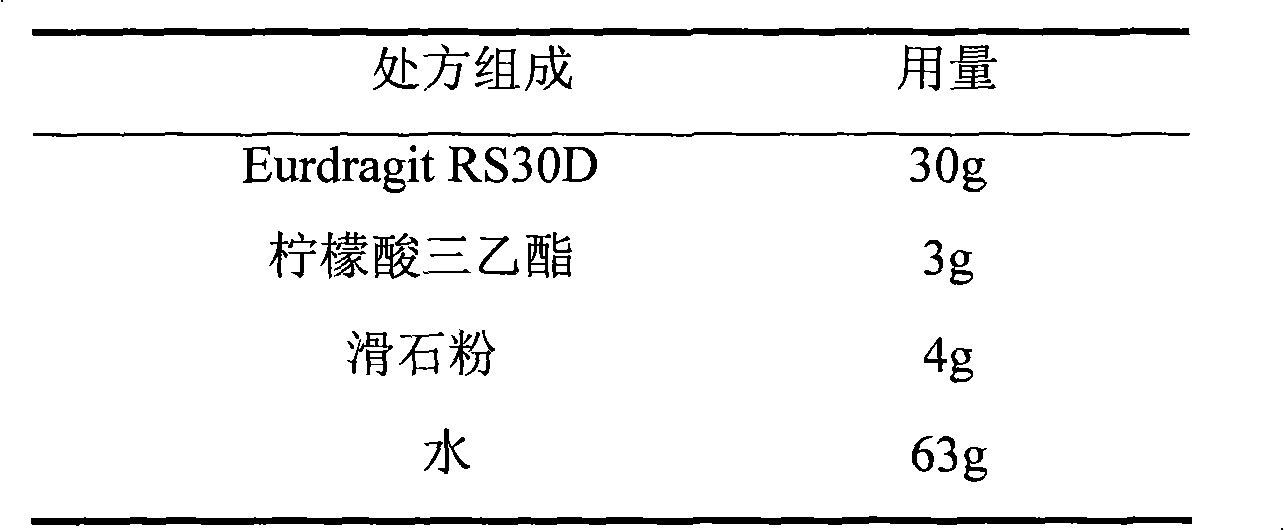
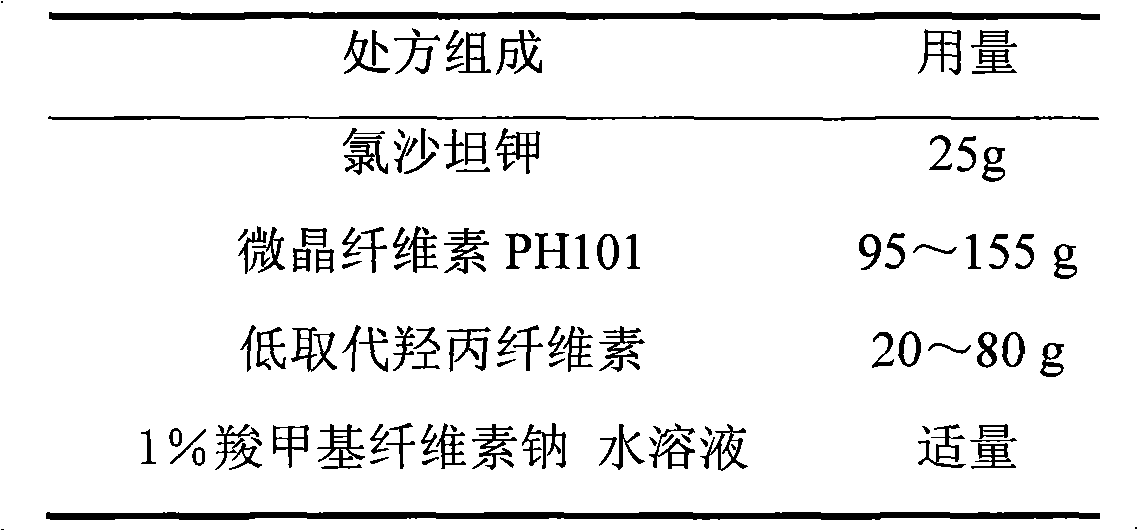
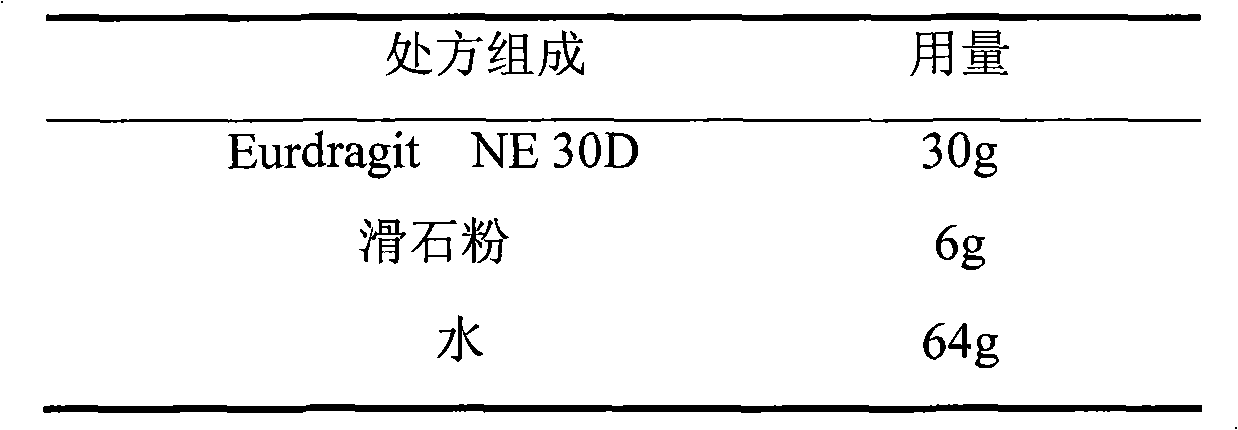
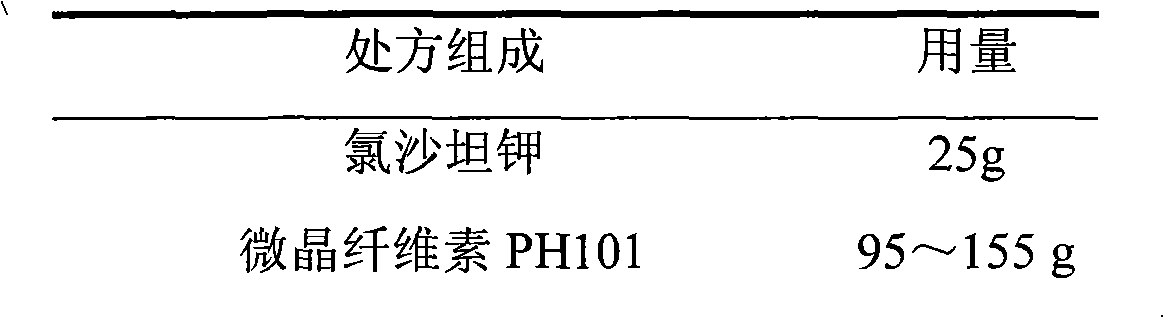
Losartan potassium membrane controlled-release pellet capsule
InactiveCN103211798AReduce permeabilityImprove permeabilityOrganic active ingredientsPharmaceutical delivery mechanismControlled releaseMedicine
The invention relates to a losartan potassium membrane controlled-release pellet capsule. The controlled-release film of the pellet adopts Eurdragit NE 30D as a film forming material, the core of the controlled-release pellet contains high-expansibility low-substituted hydroxypropylcellulose and a pharmaceutically-acceptable excipient commonly used for controlled-release pellets, and optimally, the excipient is microcrystalline cellulose, wherein the weight percentage of the low-substituted hydroxypropylcellulose in the core of the controlled-release pellet is 10-40%. The controlled-release film of the controlled-release pellet includes the Eurdragit NE 30D and an anti-adherent talcum powder, the optimal ratio of the Eurdragit NE 30D to the talcum powder is 30:6, and the optimal coating weight gain is 19-36%. The core will obviously expand after absorbing water because of the containment of the low-substituted hydroxypropylcellulose highly expanding after contacting with water, so the controlled-release film is stretched, the thickness of the film is thinned, the apertures of water-pervious micro-pores are increased, the permeability is good, and the permeability decrease caused by film ageing is compensated, thereby the middle and later stage release speed is basically constant, the last stage residue is small, and a stable release performance is always maintained prior to the expiration date.
Owner:内蒙古天衡医院管理有限公司
Sustained release system for reservoir treatment and monitoring
Compositions containing a mixture of microcapsules and a bulk polymer, where the microcapsules have an oil field chemical contained within the microcapsules are provided. The microcapsules can be present in a variety of configurations. The microcapsules contain a core or a micro-matrix containing the oil field chemicals. The core or micro-matrix can be surrounded by one or more polymeric shells, where each shell contains at least one polymer that affects the release of the oil field chemical from the composition. The compositions provide for the sustained release of an oil field chemical into fluid in an oil field reservoir over long periods of time. Methods of making the compositions and articles containing the compositions are also provided. Further provided are methods of tracing the movement of fluid in a hydrocarbon reservoir using the compositions, and methods of providing for the sustained release of oil field chemicals.
Owner:TRACERCO LTD
High-efficient oral silibinin sustained-release preparation and preparation method thereof
ActiveCN101164537BImprove solubilityRapid drug releaseOrganic active ingredientsDigestive systemMedicineMethyl cellulose
The present invention relates to a high-effective oral silibinin (SLB) slow-released preparation. Its composition includes (by mass component portion) 1 portion of silibinin, 1.5-2.5 portions of polyvidone-K30, 0.23-0.58 portion of hydroxypropyl methyl cellulose 4000cPa.S, 0.46-1.38 portions of low-substituted hydroxypropyl cellulose. Said invention adopts the combination of solid dispersion technique and slow-released hydrophilic gel skeleton technique to raise the dissolubility of silibinin.
Owner:JIANGSU UNIV
Method for preparing indapamide sustained-release preparation
ActiveCN105012275ASolve the low rate of drug useSolve solubilityOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsAcrylic resin
The invention discloses a method for preparing an indapamide sustained-release preparation. The preparation method includes the following steps: premixing microcrystalline cellulose, lactose, indapamide and starch, making acrylic resin, triethyl citrate and talcum powder into a suspension using water, spraying most suspension into the premixed material in an atomizing manner at a high mixing speed and a high cut-off velocity, reducing the mixing speed and adding the remaining suspension, and stirring continuously at a low speed to prepare sustained-release pellets; in combination with pharmaceutically acceptable excipients, carrying out tablet coating or filling hollow capsules to prepare the sustained-release preparation. The method provided by the invention not only solves the defects that the drug applying rate is low, active substances are liable to crystal transformation, and the stability is low, but also solves the problems that the production safety is low and environment pollution is caused due to the use of a large amount of organic solvents when high-viscosity sustained-release framework materials are prepared into granules. The production process of the invention is simple, safe and environment-friendly, and avoids loss of active ingredient substances; the prepared drug can keep a constant release speed after being taken, and can continuously and stably lower the blood pressure.
Owner:广东安诺药业股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com