Loxoprofen sodium matrix sustained-release tablet
A technology of loxoprofen sodium and skeleton sustained-release material, which is applied in the directions of non-active ingredient medical preparations, pill delivery, pharmaceutical formulations, etc., can solve the problems of increased adverse reactions, rapid initial release, and unfavorable blood drug concentrations, etc. Achieve the effect of increasing drug diffusion resistance, constant release rate and good control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Comparative Example 1: Loxoprofen Sodium Common Single-Layer Sustained Release Tablet
[0079] 1. Prescription composition:
[0080]
[0081] Prescription composition 1000 tablets
[0082]
[0083] Loxoprofen Sodium 90g
[0084] Hydroxypropyl Methyl Cellulose K4M 95g
[0085] Hydroxypropyl Methyl Cellulose K100 60g
[0086] Microcrystalline Cellulose 30g
[0087] Lactose 60g
[0088] Stearic acid 15g
[0089] Micronized silica gel 20g
[0090] 8% povidone K30 ethanol solution appropriate amount
[0092]
[0093] Second, the preparation process:
[0094] 1. Take the prescription amount of loxoprofen sodium, hypromellose K4M, hypromellose K100, microcrystalline cellulose, lactose, micropowder silica gel and stearic acid and mix evenly;
[0095] 2. 8% povidone K30 ethanol solution is used to make soft materials, and 24 m...
Embodiment 1
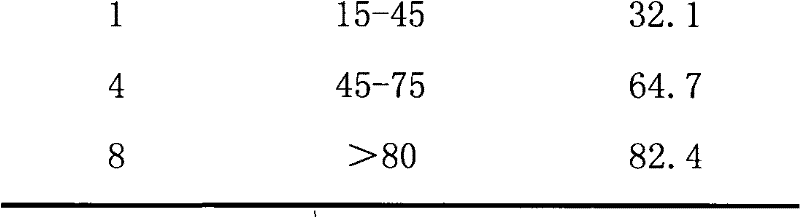
[0102] Comparative example 1 release test result
[0103]
[0104]
[0105] * n=6, the average value of 6 samples
[0106] The result shows, because the dissolubility of loxoprofen sodium is bigger, according to the loxoprofen sodium common matrix slow-release tablet of single-layer structure prepared by traditional process, has used a larger amount of matrix slow-release material, the result releases better in the early stage, but The release value at the end point 8h is on the low side, although it is still within the limit, the residue is serious (about 15-20%), and the ideal release effect has not been achieved.
Embodiment 2
[0107] Comparative Example 2: Loxoprofen Sodium Common Single-Layer Sustained Release Tablet
[0108] 1. Prescription composition:
[0109]
[0110] Prescription composition 1000 tablets
[0111]
[0112] Loxoprofen Sodium 90g
[0113] Hydroxypropyl Methyl Cellulose K4M 90g
[0114] Hydroxypropyl Methyl Cellulose K100 50g
[0115] Microcrystalline Cellulose 30g
[0116] Lactose 60g
[0117] Stearic acid 15g
[0118] Micronized silica gel 20g
[0119] 8% povidone K30 ethanol solution appropriate amount
[0121]
[0122] Two, preparation technology: with comparative example 1.
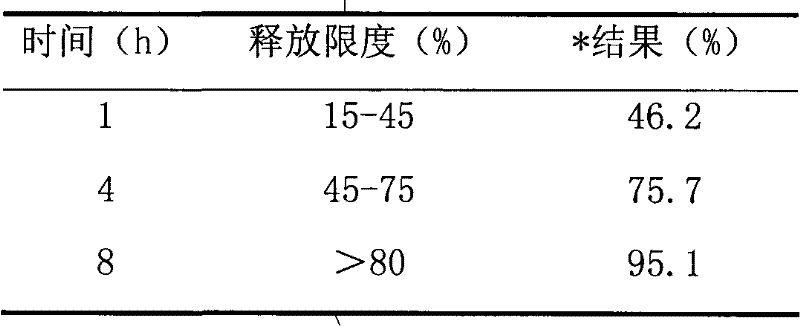
[0123] Three, release measurement: method is with comparative example 1, and result is as shown in the table below:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com