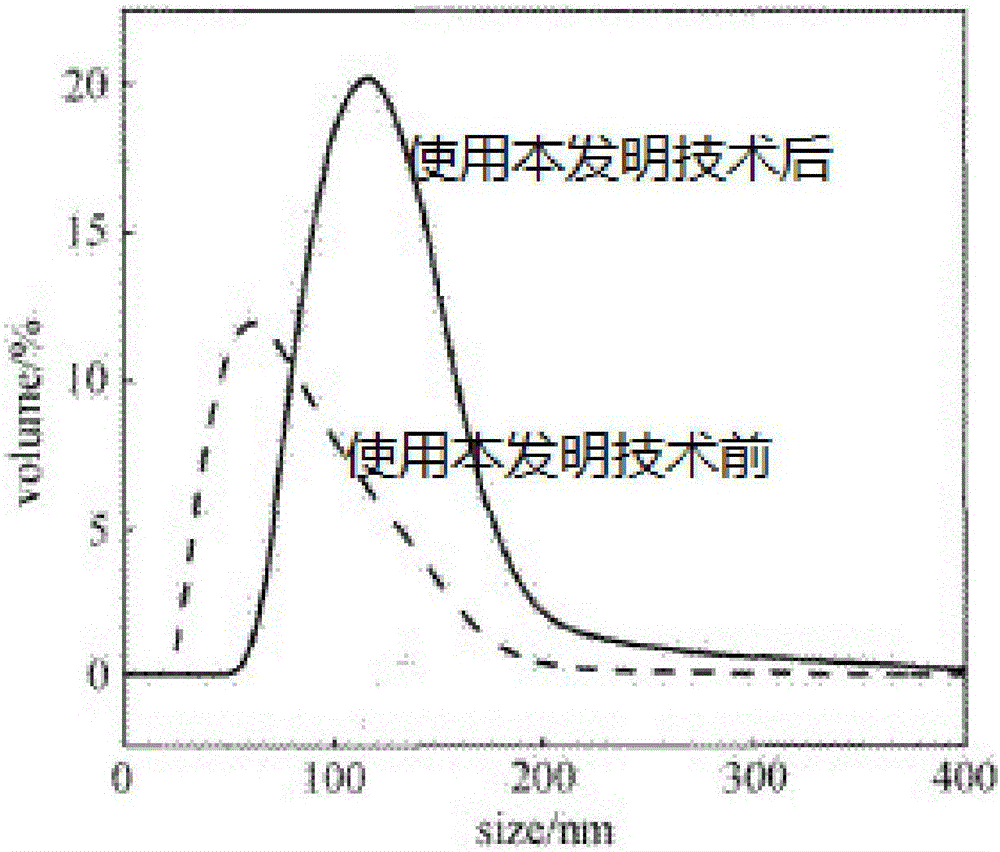
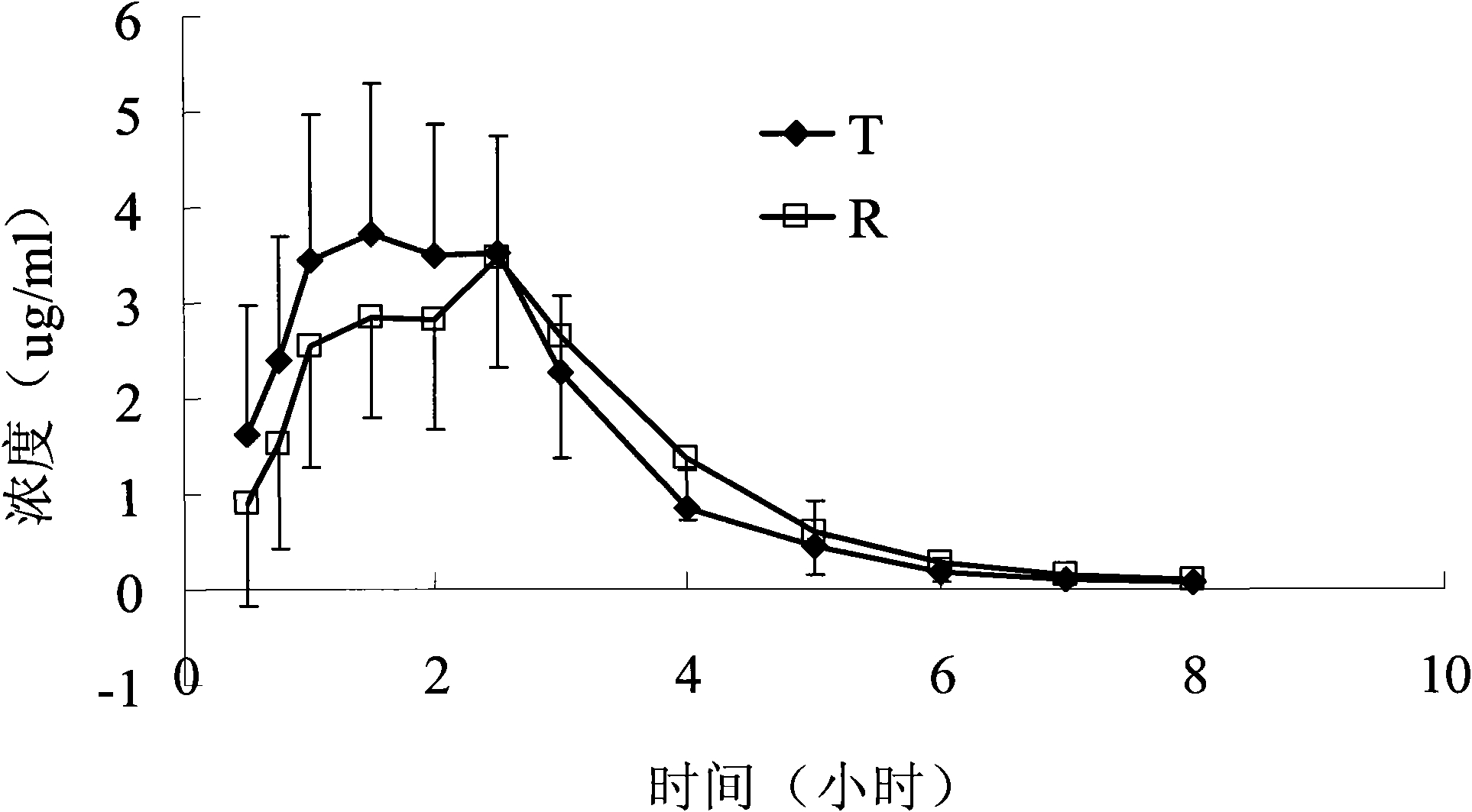
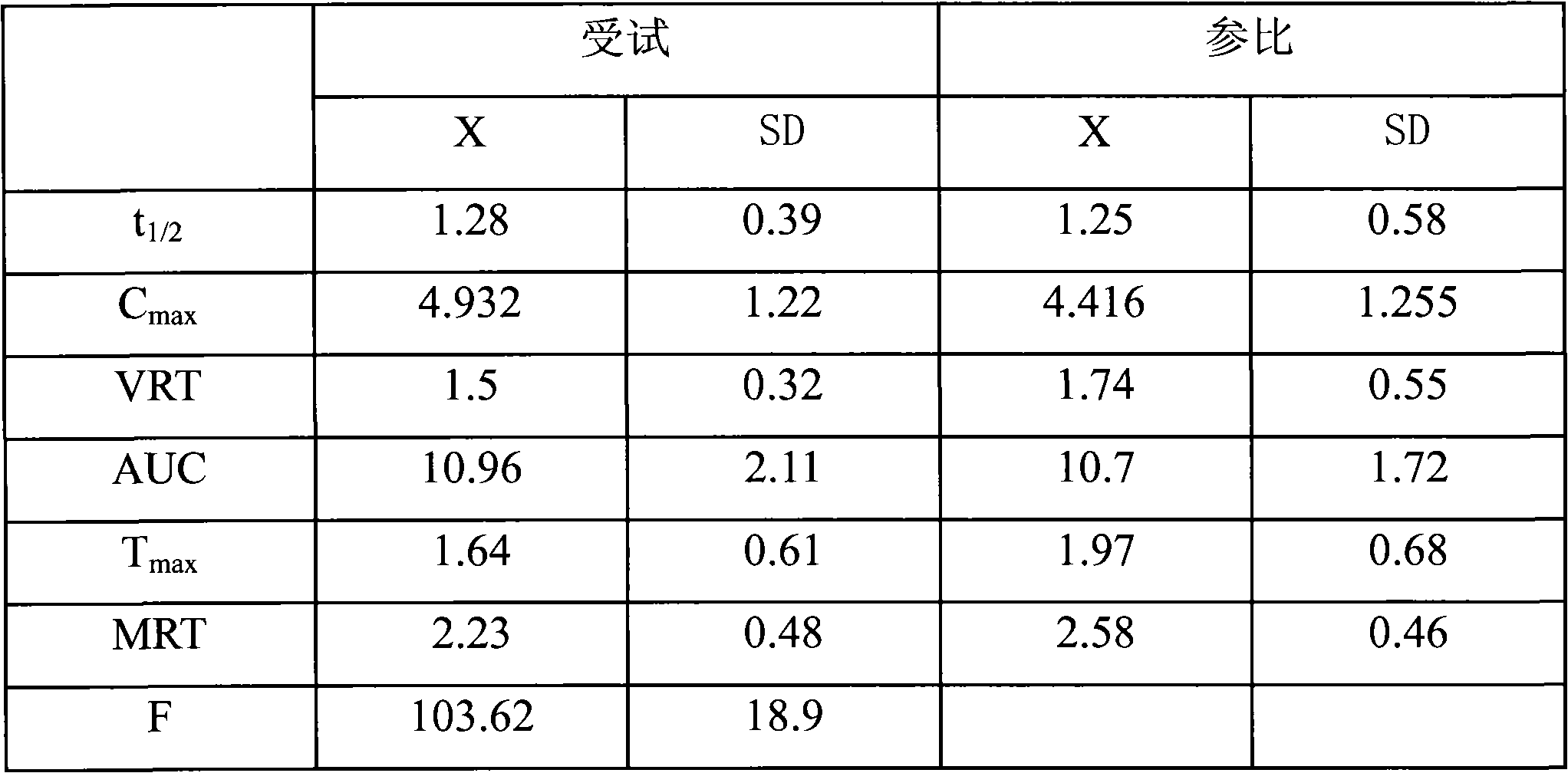
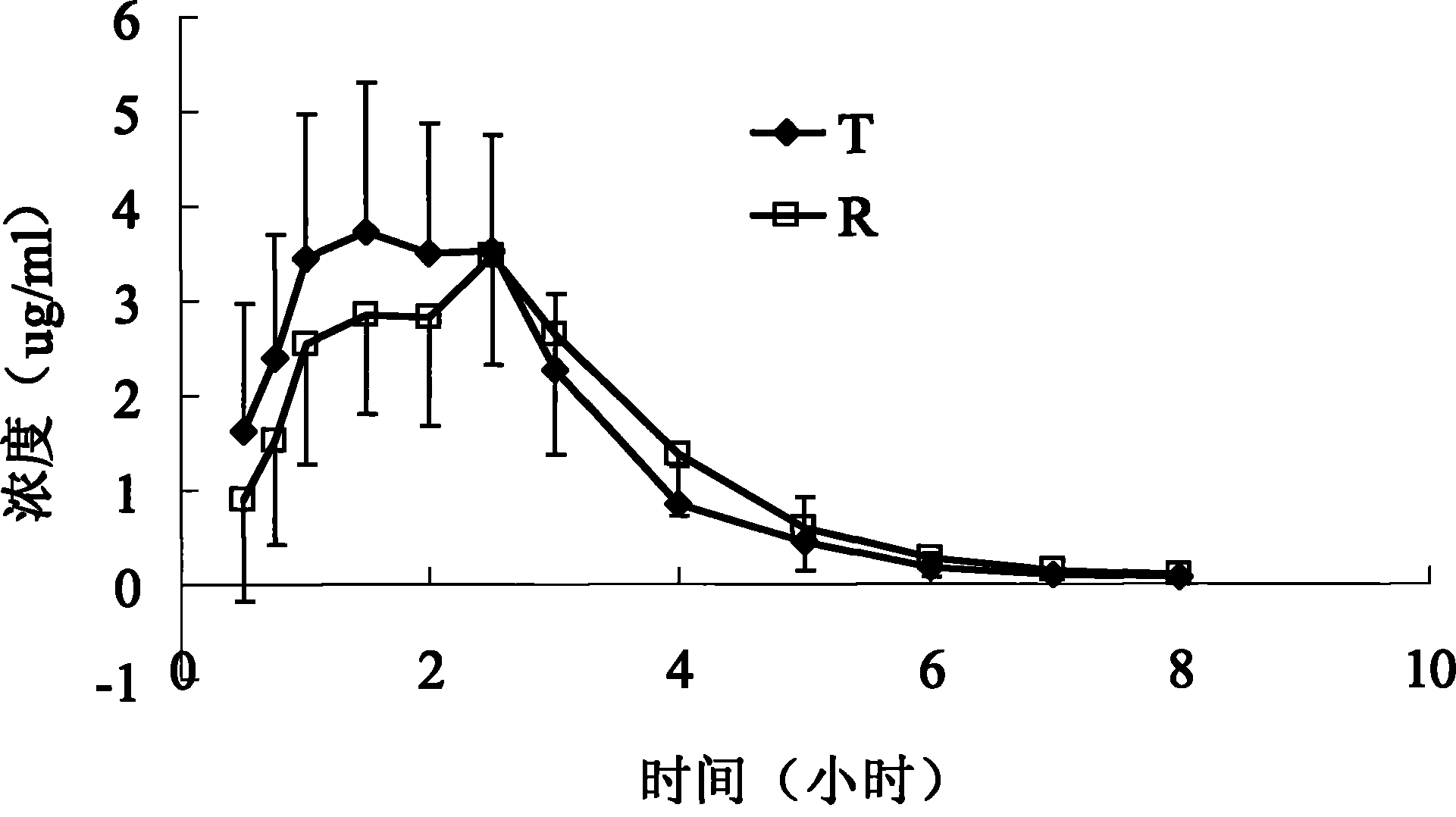
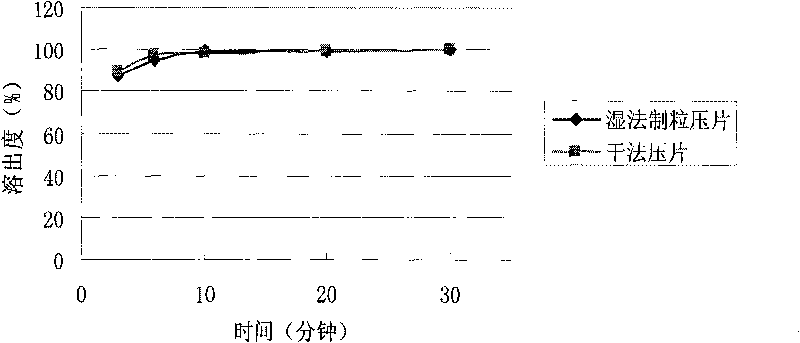
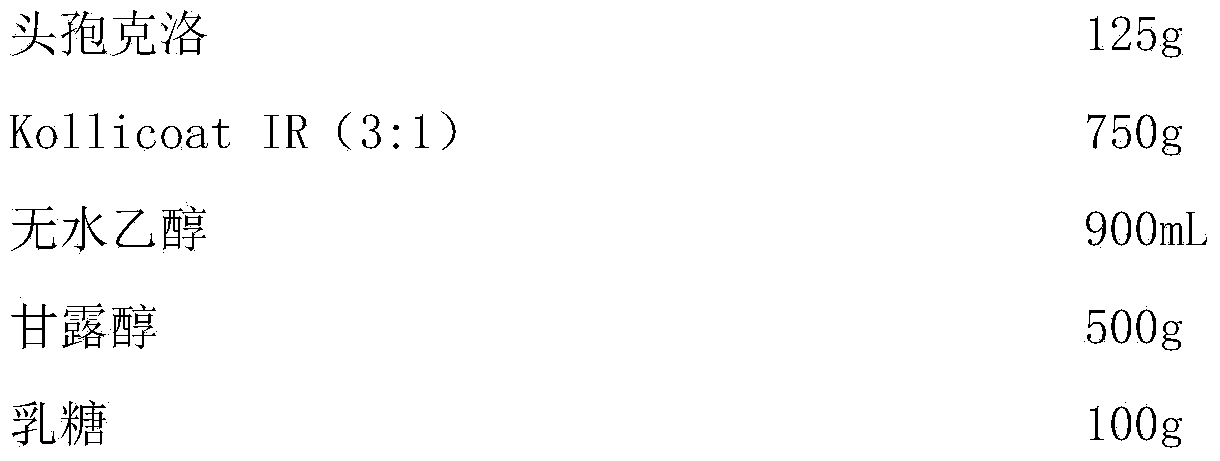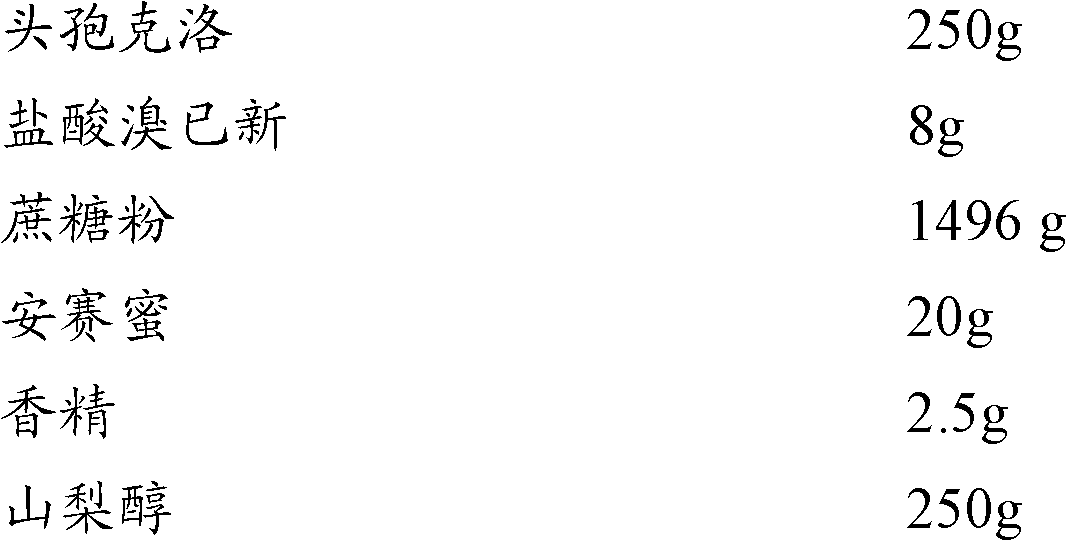Patents
Literature
132 results about "Cefaclor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
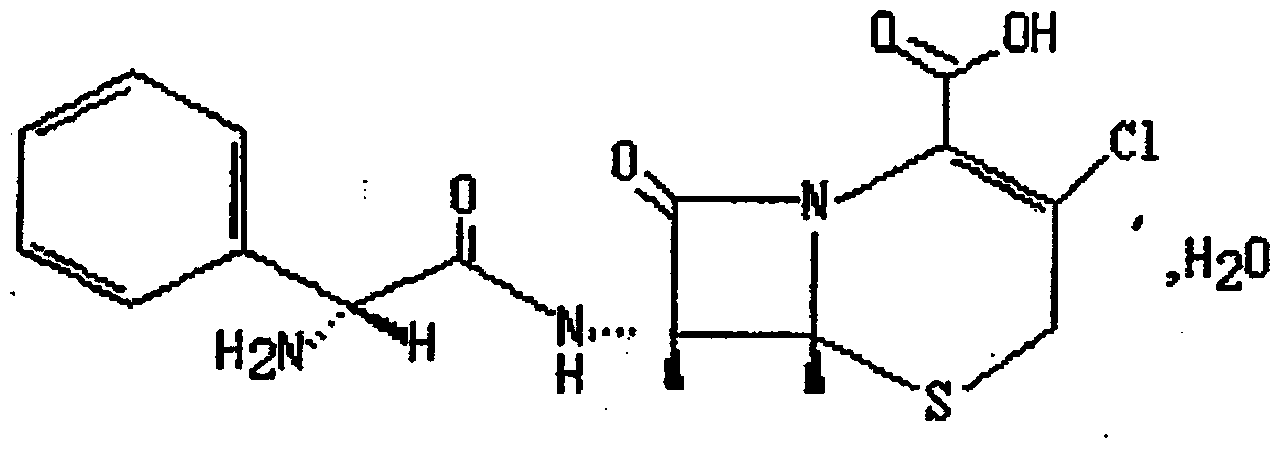
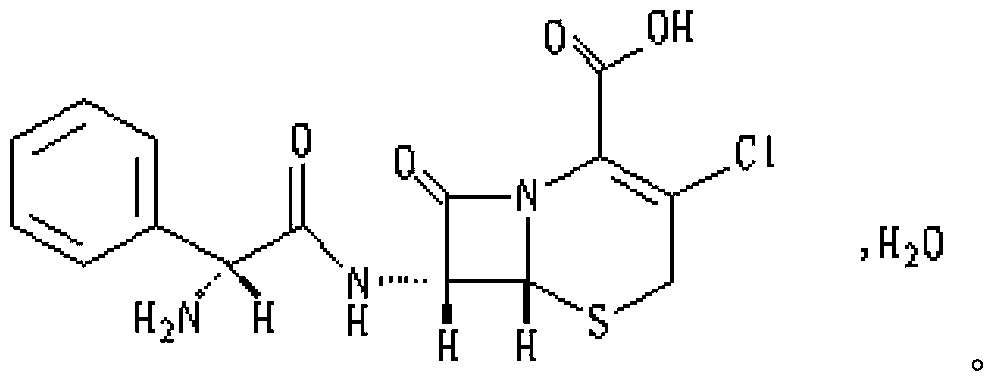
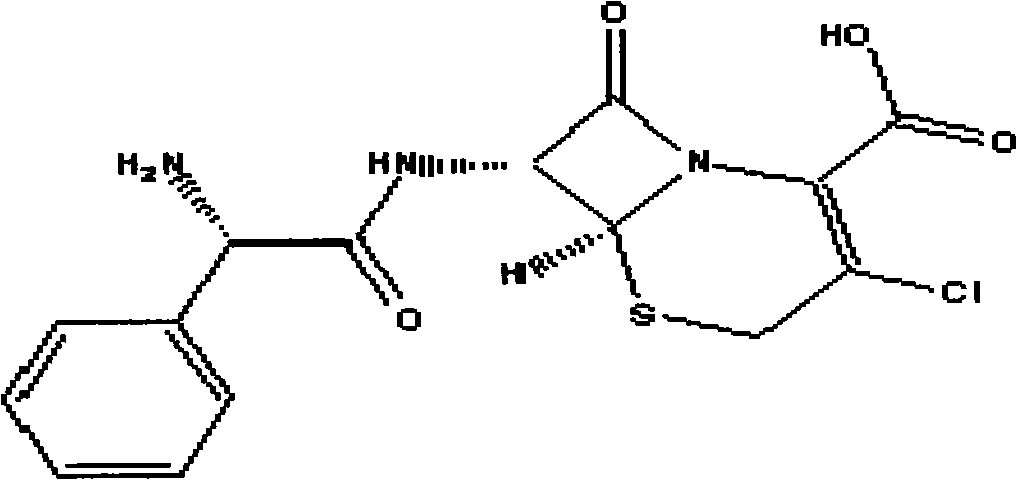
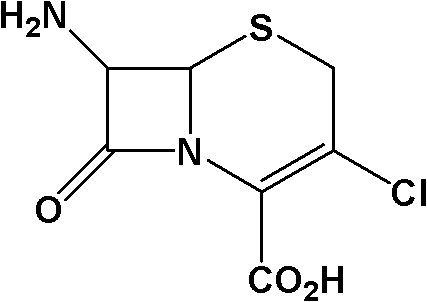
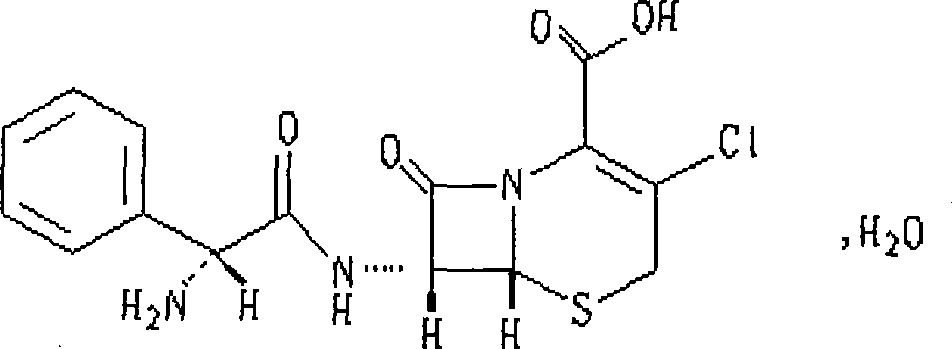
This medication is a cephalosporin-type antibiotic used to treat a wide variety of bacterial infections (e.g., middle ear, skin, urine and respiratory tract infections).
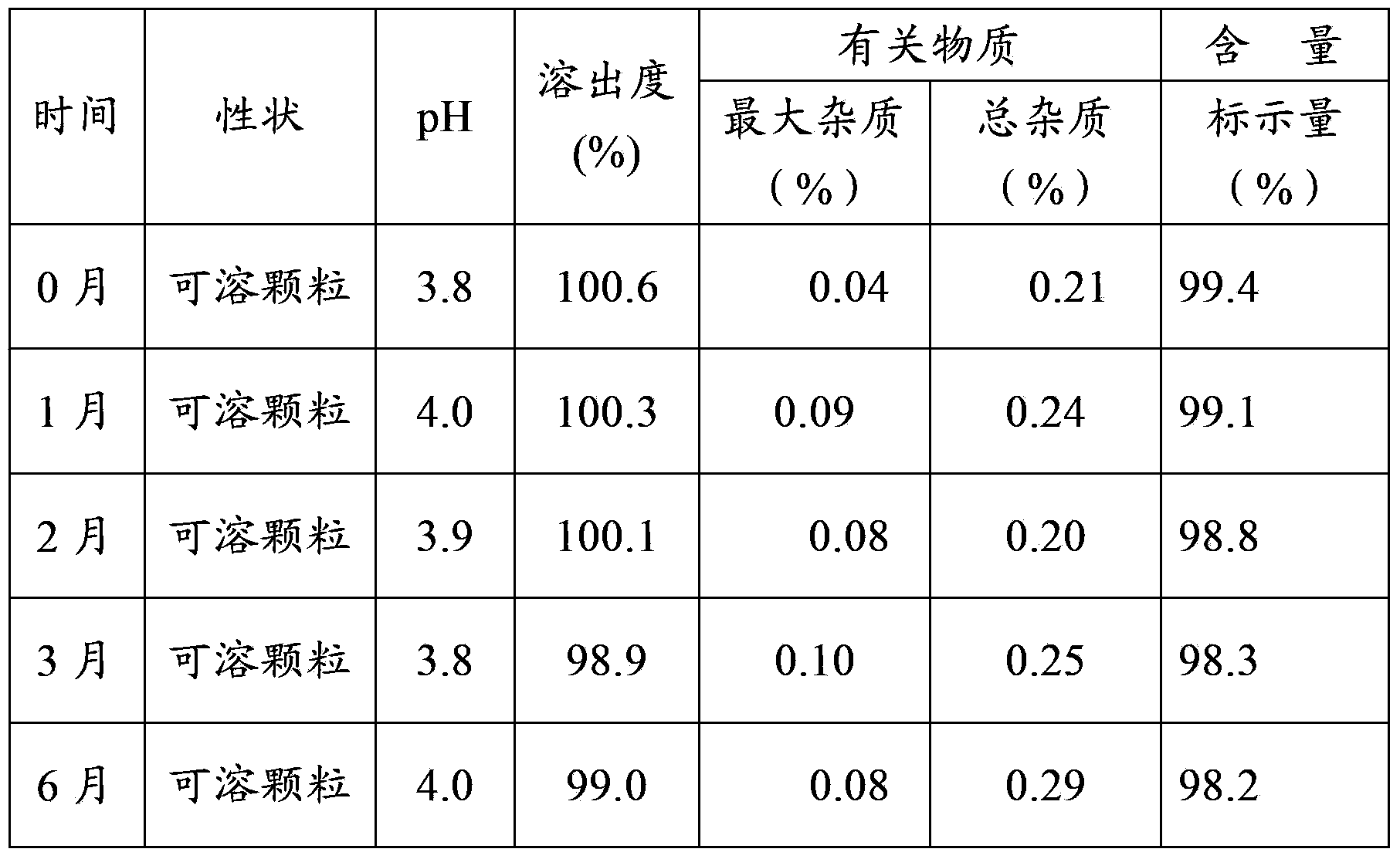
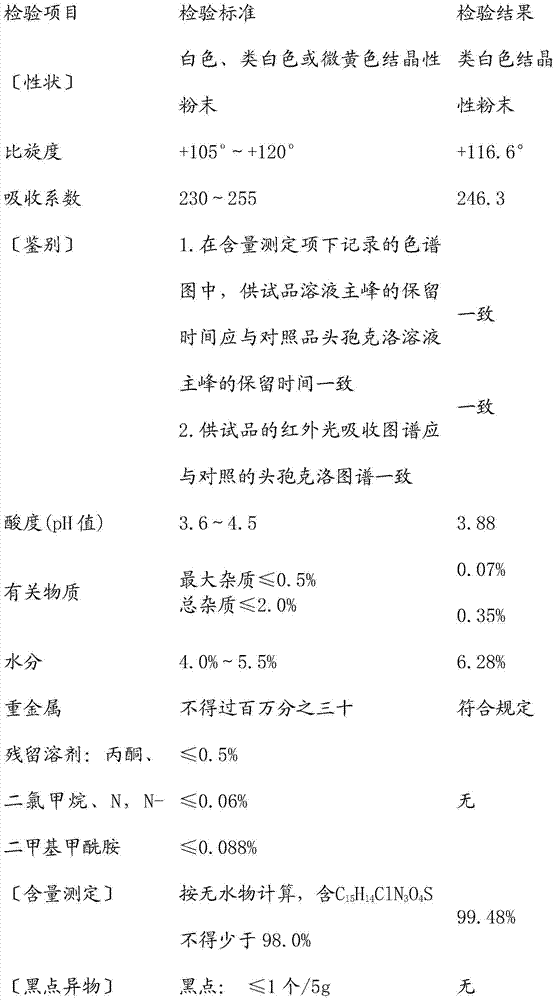
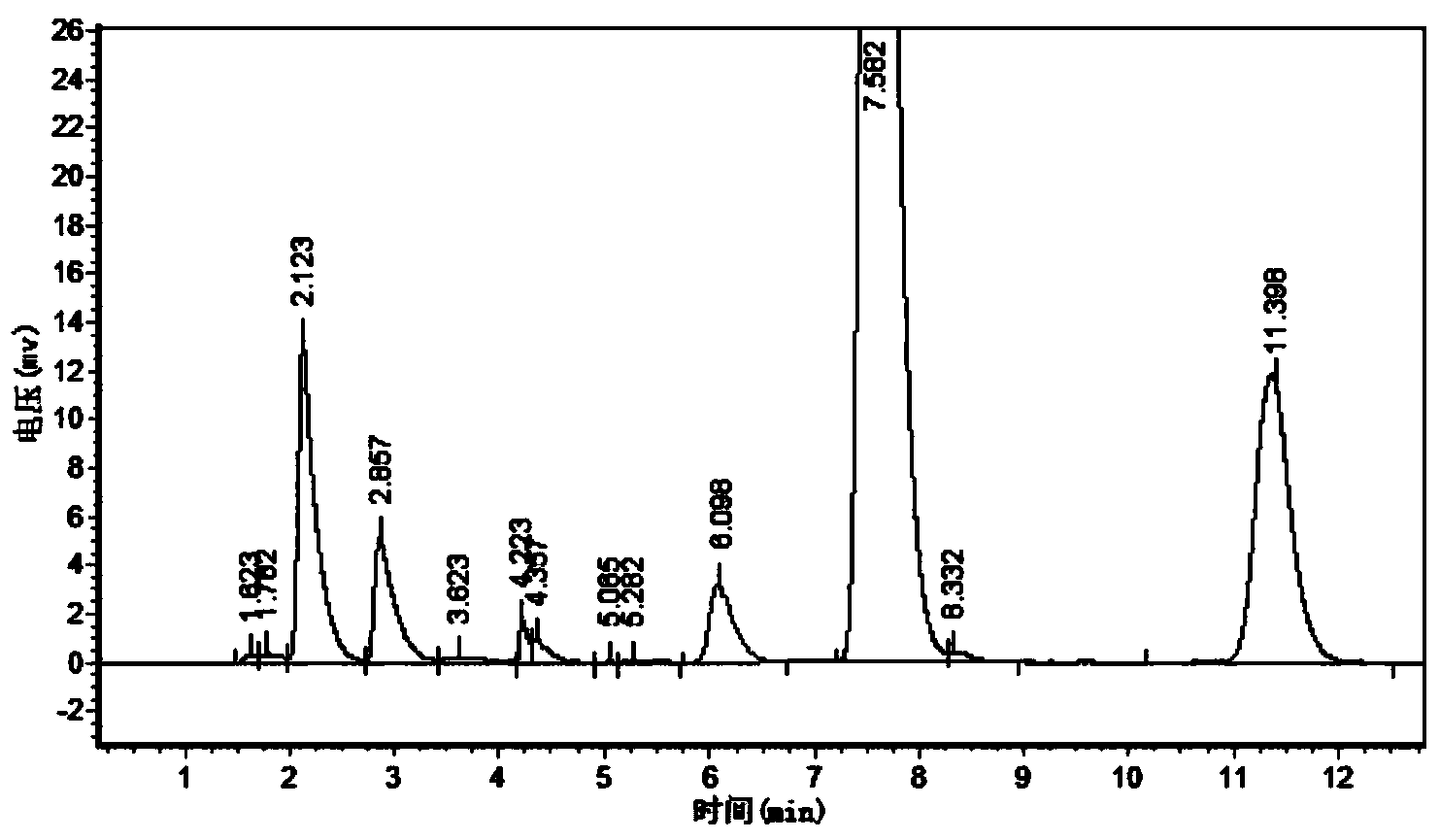
Cephalosporin suspension granule and preparation method thereof
ActiveCN101816635AGranularityAvoid damageAntibacterial agentsOrganic active ingredientsFluidized bed dryingAdditive ingredient
The invention discloses a cephalosporin suspension granule of which the formula composition comprises drug active ingredients, excipient, suspending agent, disintegrating agent, flavoring agent, coloring agent, stabilizing agent, adhesive and spice. The granule takes cefixime, cefprozil, cefaclor or cefdinir as the main drug ingredient, disintegrating agent and suspending agent with a certain quantity are added into the formula to ensure that the effective ingredients are dissolved out, and the solution has favorable suspending effect. Meanwhile, the invention also discloses a preparation method of the cephalosporin suspension granule, which adopts the wet granulation method to prepare granules and adopts the fluidized bed drying method to dry. The invention adopts the fluidized bed drying technology, so that compared with the traditional oven drying, the invention has the advantages of short drying time, small influence on active matter quality and the like, not only ensures product quality but also improves production efficiency. The granule prepared by the formula of the invention has solid granularity and lowers damage to the granule by fluidized drying.
Owner:广东恒健制药有限公司
Penicillin G acylase immobilized with a crosslinked mixture of gelled gelatin and amino polymer
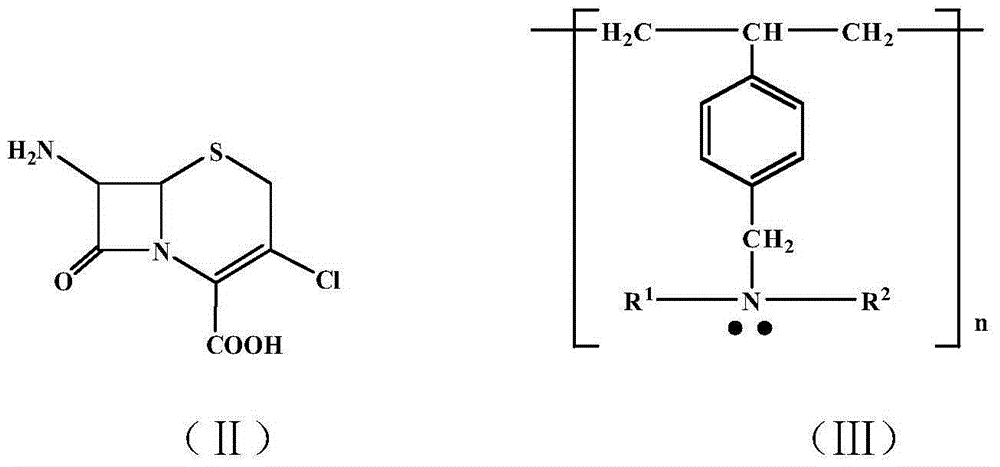
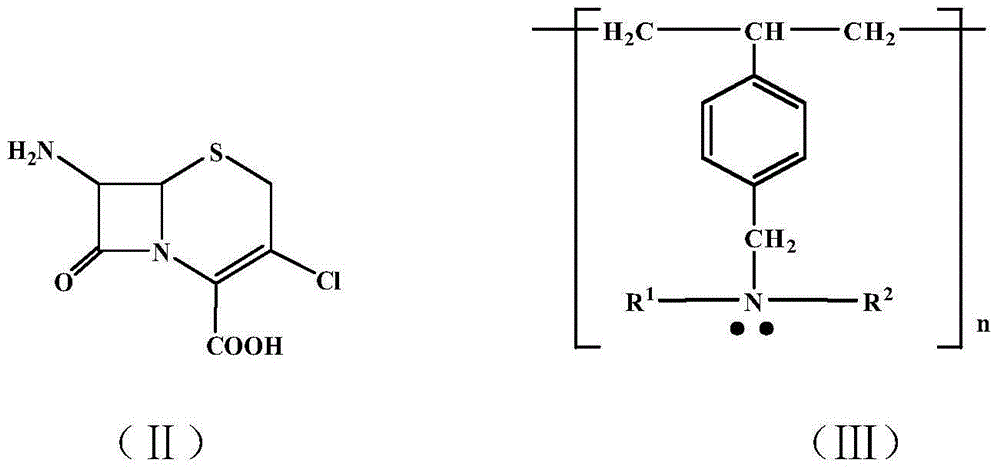
PCT No. PCT / EP96 / 03253 Sec. 371 Date Jan. 15, 1998 Sec. 102(e) Date Jan. 15, 1998 PCT Filed Jul. 16, 1996 PCT Pub. No. WO97 / 04086 PCT Pub. Date Feb. 6, 1997Penicillin G acylase is immobilized by covalent bonding to a crosslinked mixture of a gelled gelling agent such as gelatin and a polymer containing free amino groups such as alginate amine, chitosan or polyethylene imine. The immobilized penicillin G acylase provides a higher synthesis / hydrolysis ratio as compared to immobilizing with other carriers when producing beta -lactam derivatives by a condensing reaction of an amino beta -lactam with an acylating agent. The acylating agent may be a derivative of D-phenylglycine, a derivative of D-p-hydroxyphenylglycine or a derivative of D-2,5-dihydro-phenylglycine. Examples of beta -lactam derivatives that can be produced are amoxycillin, ampicillin, cephaclor, cephadroxil, cephprozil, cephalexin and cephradine.
Owner:GIST BROCADES NV
Pharmaceutical composition for controlled release of a beta-lactam antibiotic
ActiveUS20050031685A1Maintenance characteristicAvoid disadvantagesBiocideDrug compositionsHydrophilic polymersWater dispersible
An improved stable pharmaceutical composition for controlled release of an active ingredient comprises a betalactam antibiotic such as cephalexin, cefaclor or their pharmaceutically acceptable hydrates, salts or esters as active ingredient, a calcium salt and a mixture of hydrophilic polymers selected from the group consisting of at least one sodium alginate and one xanthan gum and with or without hydroxypropyl methylcellulose, said composition optionally containing probenecid. The composition may also contain one or more of a water soluble and / or water dispersible diluent, wherein the quantities of the hydrophilic polymers and water soluble and / or water dispersible diluents are such that the therapeutically effective active ingredient is released at a rate suitable for once or twice daily administration of the pharmaceutical composition.
Owner:LUPIN LTD
Slow release preparation of cefaclor
InactiveCN101002747AImprove securityImprove effectivenessAntibacterial agentsOrganic active ingredientsCurative effectSustained-Release Preparations
Owner:刘凤鸣
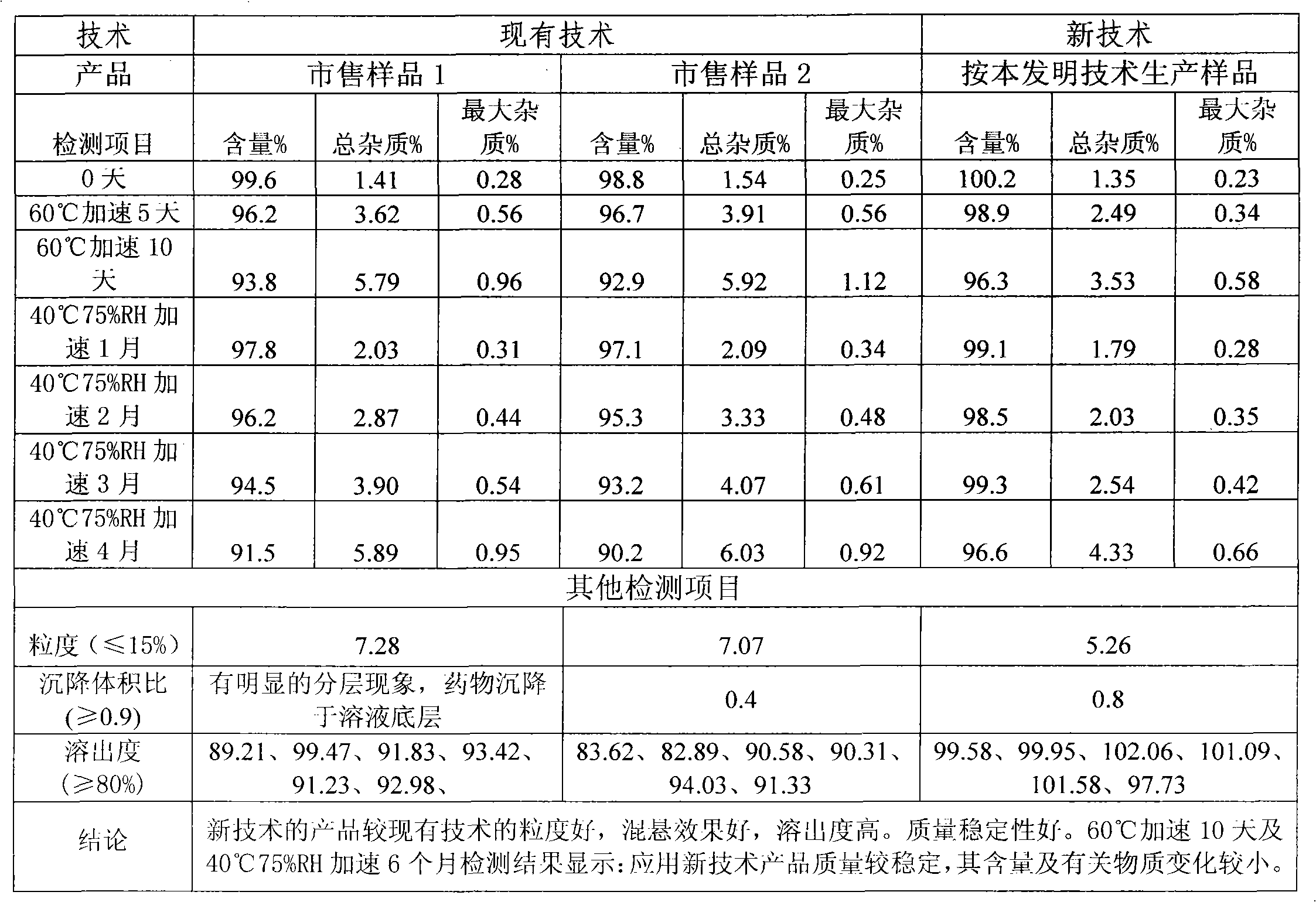
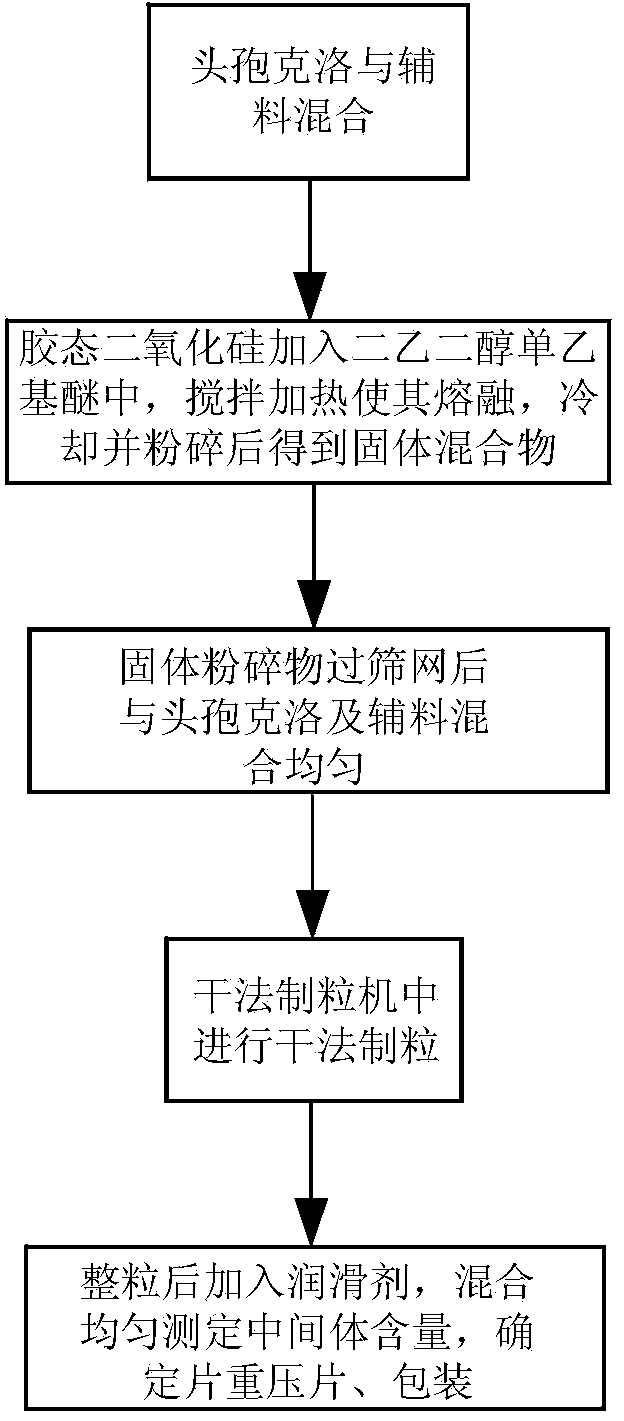
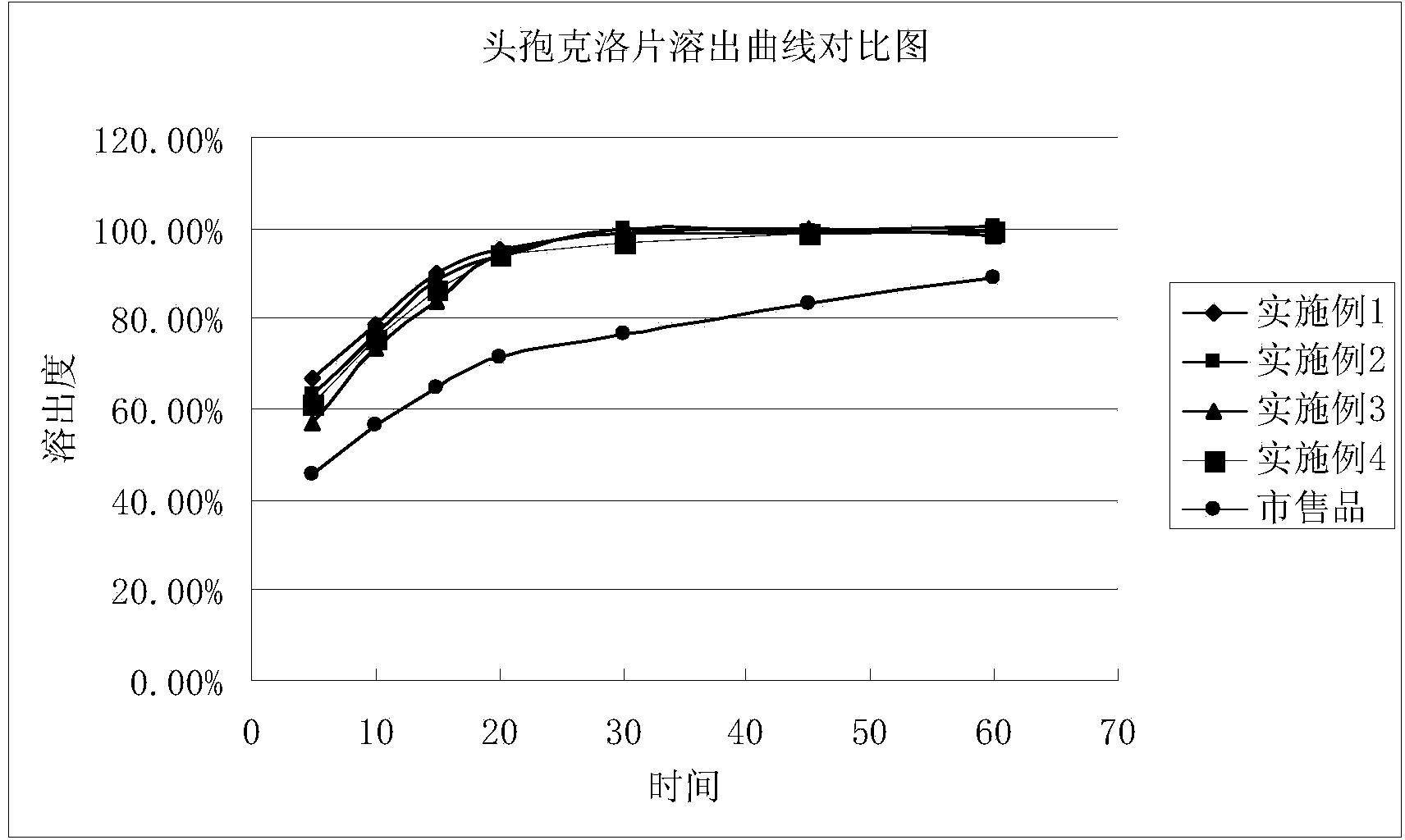
Stable cefaclor tablet composition and preparation method thereof
ActiveCN103623412AImprove solubilityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsDiethylene glycol monoethyl etherBioavailability
The invention discloses a cefaclor tablet pharmaceutical tablet composition which comprises cefaclor, lactose, microcrystalline cellulose, crosslinked povidone, diethylene glycol monoethyl ether, colloidal silicon dioxide and glyceryl behenate or sodium stearyl fumarate. The preparation method comprises the following steps: evenly mixing the cefaclor with the pharmaceutical auxiliary materials; adding the colloidal silicon dioxide into the diethylene glycol monoethyl ether, heating while stirring for melting, and cooling to obtain a solid mixture; screening, and evenly mixing the cefaclor auxiliary materials; granulating by a dry process; and after finishing the granules, adding the lubricant, evenly mixing, measuring the intermediate content, determining the tablet weight, tabletting and packaging. The invention provides a legal and reasonable cefaclor table composition and a preparation method thereof, thereby obtaining the cefaclor tablet which has the advantages of stabler and more controllable quality, higher bioavailability and simpler technique.
Owner:SHANGHAI NEW ASIATIC PHARMA MINHANG
Cefaclor capsule and preparation method thereof
InactiveCN102793687AConvenient sourceReduce typesAntibacterial agentsOrganic active ingredientsMagnesium stearateSilicon dioxide
The present invention relates to a cefaclor capsule and a preparation method thereof. The cefaclor capsule is composed by the following components by weight: 100 parts of cefaclor, 5-100 parts of pregelatinized starch, 1-5 parts of silica and 0.5-2.5 parts of magnesium stearate. The preparation method comprises: (1) sieving the cefaclor after pulverization; (2) mixing pregelatinized starch and silica with the sieved cefaclor powder uniformly; (3) adding magnesium stearate and mixing uniformly; and (4) making the mixed powder into capsules, and packing the qualified capsules into resulting products after detection. The sources of raw and auxiliary materials selected by the present invention are convenient. The auxiliary materials are few in types, are all commonly used auxiliary materials for preparations, and are low in prices. The production process is simple, and the operation is convenient and fast. The preparation process is simple, easy in operation, low in cost, and high in yield, and is suitable for large-scale industrial production.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
Compound cefaclor suspension and preparation method thereof
InactiveCN101912368AGood dissolution effectPromote dissolutionAntibacterial agentsOrganic active ingredientsBiotechnologyCellulose
The invention discloses a compound cefaclor suspension and a preparation method thereof, belonging to the field of pharmaceutical preparations. The compound cefaclor suspension comprises the following components in percentage by weight: 4-50 percent of cefaclor, 0.14-1.75 percent of bromhexine hydrochloride, 0.5-6 percent of macromolecule suspending agent, 0.1-1.8 percent of sweetener, 38.45-95.256 percent of cane sugar, 0.003-1 percent of sunset yellow and 0.001-1 percent of flavoring orange essence. The preparation method comprises the following steps of: after uniformly mixing the macromolecule suspending agent sodium carboxymethylcellulose with the cane sugar, adding an ethanol-water solution containing the sunset yellow and the sweetener saccharin sodium; and after preparing a soft material, granulating and drying, uniformly mixing with the cefaclor, the bromhexine hydrochloride and the flavoring orange essence to obtain the compound cefaclor suspension. The compound cefaclor suspension can be used for treating respiratory tract mild-to-severe infection caused by sensitive bacteria, tonsillitis, chronic bronchitis acute exacerbation, pneumonia, nasosinusitis, and the like.
Owner:UNIV OF SHANGHAI FOR SCI & TECH +1
Preparation method of ceftibuten
The invention provides a preparation method of ceftibuten. The preparation method comprises the steps of adding cefaclor nucleus, methyltetrahydrofuran and magnesium powder into a reactor, and reacting until the magnesium powder disappears; then, adding a proper quantity of distilled water, stirring, standing for layering, separating out an organic layer, and drying by using anhydrous magnesium sulfate; after filtering, adding D301 week basic ion exchange resin and 2-(2-carbobenzoxy-aminothiazole-4-yl)-5-carbobenzoxy-2-pentenoic acid, and reacting at a certain temperature for a certain time; after ending the reaction, filtering to remove the week basic ion exchange resin; and then, hydrolyzing to obtain a target product, namely ceftibuten. The method is a novel preparation method of ceftibuten, is high in product yield and purity as well as simple and convenient in operation, is a green and clean production process and is suitable for industrial production on a certain scale; and the cefaclor nucleus is a main product in the company, and a sustainable development technology of the company is to prepare ceftibuten serving as a mainly developed variety in the Tenth Five-year Plan period from the product of the company.
Owner:山东昌邑四方医药化工有限公司
Compound cefaclor dispersible tablet
InactiveCN1666743AEasy to take and carryHigh degree of industrialization of productionAntibacterial agentsOrganic active ingredientsDiluentDissolution
The invention relates to a new drug from of cefaclor belonging to heavy oral cephalosporin antibacterial drugs, comprising following compositions in weight fractions: cefaclor is 250 deals in non water cefaclor; bromhexine hydrochloride is 8.77 deals in bromhexinum; filling agent is 0-140 deals selected from lactose, white dextrine, starch or / and compressible starch; diluent is 30-140 deals selected from microcrystalline cellulose; disintegrating adminicle is 20-150 deals selected from L-HPC or / and crospovidone; lubricating agent is 7-15 deals selected from micropowder silica gel or / and magnesium. The compound cefaclor dispersible tablet provided can disintegrate quickly in the body, improve its dissolution, promote absorption, and have high bioavailability.
Owner:黄本东
Medicinal composition containing cefaclor particles, and preparation method and application thereof
ActiveCN103349646AAvoid breakingShorten production timeAntibacterial agentsOrganic active ingredientsFormularyAqueous solubility
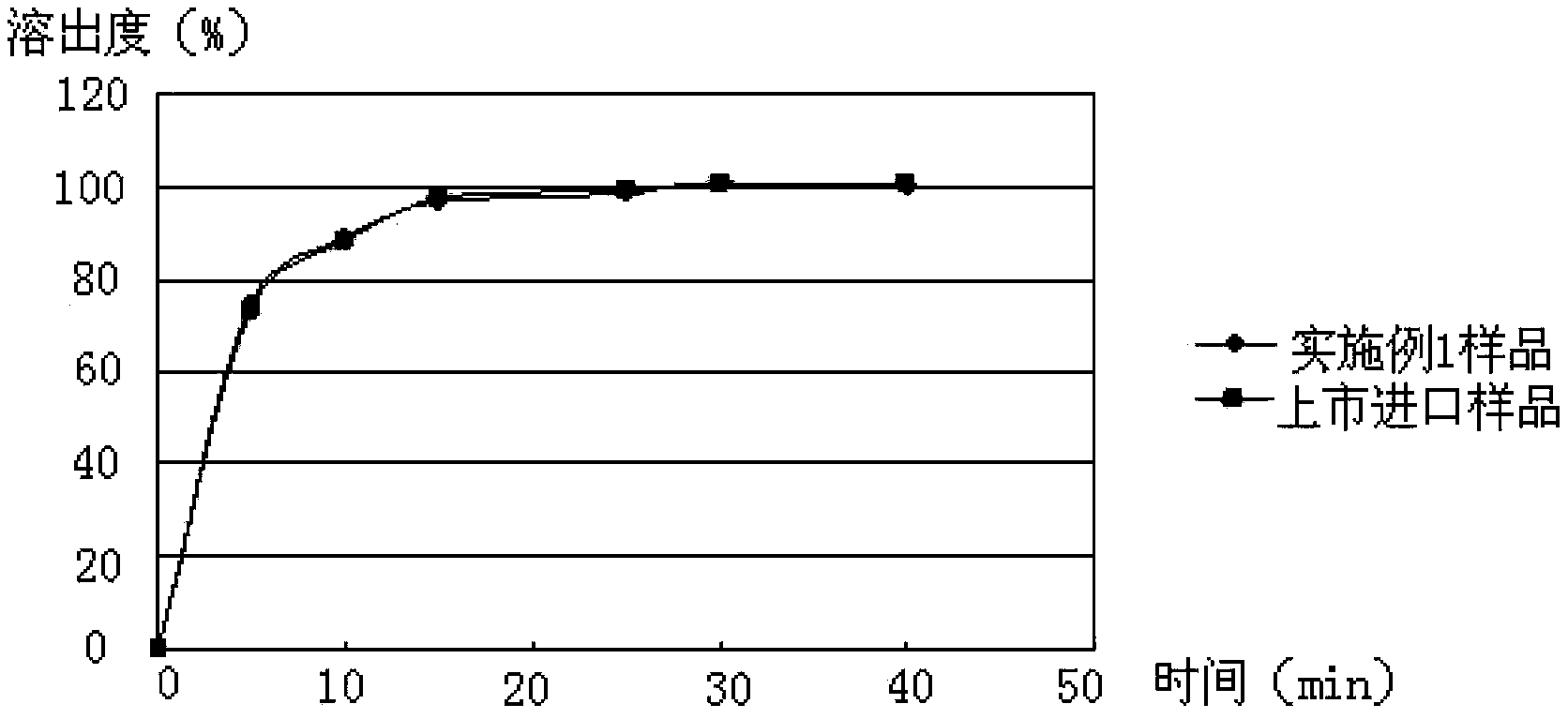
The invention provides a medicinal composition containing cefaclor particles, and a preparation method and an application thereof. The preparation method which adopts a fluidized bed preparation technology to prepare the medicinal composition comprises the following steps: burdening materials preprocessed by a 80 mesh sieve according to a specific formula, placing the obtained mixture in a fluidized bed granulator, and carrying out intake mixing; and rising the intake temperature, spraying an adhesive solution, and mixing and drying under a continuous intake condition to obtain cefaclor particles. The preparation method of the cefaclor particles, which is a one step granulation method, substantially shortens the production time; the whole production process is carried out in the closed environment and accords with medicinal GMP standard requirements; and the prepared cefaclor particles are porous particles having round shapes, have the characteristics of good water solubility, uniform granularity, good fluidity, uniform distribution of a cefaclor raw material in the cefaclor particles, and very good stability and in-vitro dissolution curve, and can be used for treating clinic indications.
Owner:HAIKOU PHARMA FACTORY +1
Separation and purification method for cefaclor by enzymatic synthesis
ActiveCN103571907AResolve separabilitySolution concentrationOrganic chemistryFermentationPurification methodsPhenylglycine methyl ester
The invention discloses a separation and purification method for cefaclor by enzymatic synthesis. The separation and purification method comprises the following steps: dissolving 7-ACCA by ammonia water in a water phase, thereafter adding immobilized enzyme; keeping the temperature within 10-35 DEG C; dripping phenylglycine methyl ester hydrochloride or phenylglycine methyl ester mesylate; continuously maintaining the pH value of the reaction within 5.5-7.5; continuously making the generated cefaclor be precipitated from the system with occurrence of enzyme digestion; continuously separating the immobilized enzyme M-1 from the cefaclor by a filtering separation method in an enzymatic liquid; circulating the mother liquid back to a reactor until the 7-ACCA has complete reaction; purifying the obtained wet cefaclor product, and then obtaining the cefaclor. According to the separation and purification method disclosed by the invention, during precipitation of the cefaclor, the cefaclor is separated from the enzymatic reaction liquid, so that the problems of separation and reaction concentration of the enzymic catalytic reaction are solved.
Owner:苏州盛达药业有限公司
Penicillin G acylase mutant, and coding gene and application thereof
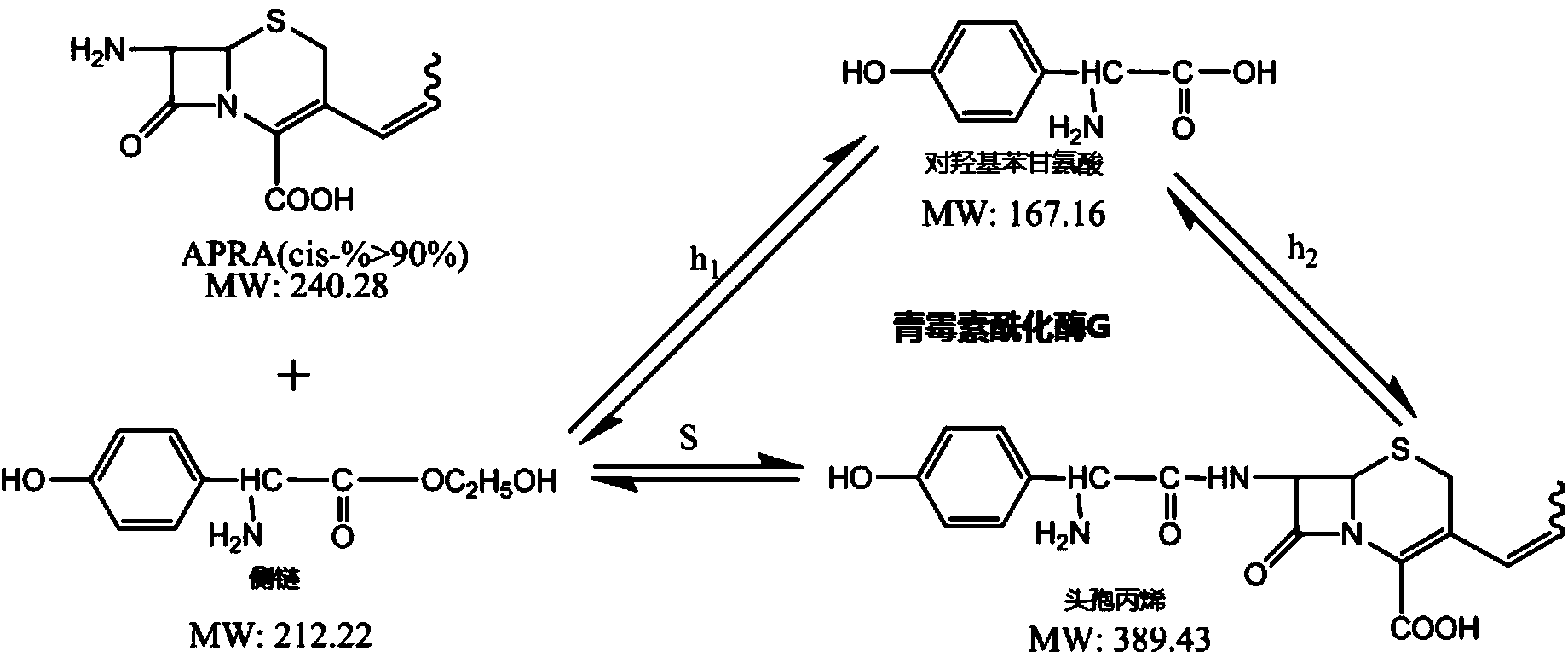
The invention discloses a penicillin G acylase mutant, and coding gene and application thereof; the amino acid sequence of the penicillin G acylase mutant is displayed by SE Q ID NO.1; the nucleotide sequence of the coding gene is presented by the SEQ ID NO.2; the invention also provides the application of the penicillin G acylase mutant in synthesis of cephalo-type antibiotic. Compared with wild type, the novel penicillin G acylase mutant has higher activity in synthesis of the cephalo-type antibiotic like cephalosporin propylene, cofactor or cephalosporin amoxicillin, and has highly improved enzyme vitality when catalyzing 7-APRA and side chain 2-hydroxy ethyl to react with hydroxy benzenes glycine ester so as to synthesis the cephalosporin propylene; specific vitality is improved from 1.5U / mg to 35U / mg, and synthesis hydrolysis vitality ratio is not reduced, and the synthesis hydrolysis vitality ratio can reach 1.8.
Owner:ZHEJIANG APELOA TOSPO PHARMA +1
Sustained release tablets containing cefaclor active component and preparation method thereof
The invention discloses a sustained-release preparation containing the active ingredient of cefaclor, which consists of the cefaclor, acrylics II, slow release stroma of hydroxypropyl methylcellulose, cementing agent, loading agent and other supplementary materials; wherein, the proportion by weight among the cefaclor, the slow release stroma and the acrylics II is 1:0.1-1:0.01-0.1. After being taken, the cefaclor sustained-release tablets can be slowly and continuously released according to the requirements to maintain effective blood level, thus achieving the action of controlled release. The sustained-release preparation has the characteristics of lasting action, low toxic and side effect, few times for taking medicine, and utilizing the minimum dose to achieve the best healing efficacy; furthermore, blood concentration is stable, the incidence rate of untoward effect is reduced, and the sustained-release preparation is more applicable to the use of the patients.
Owner:TIANJIN CENT PHARM CO LTD
Cefaclor capsule and preparation method thereof
ActiveCN103446075APromote precipitationImprove stabilityAntibacterial agentsOrganic active ingredientsAlcoholPolyethylene glycol
The invention discloses a cefaclor capsule and a preparation method thereof. The preparation is obtained by mixing a cefaclor inclusion complex and pharmaceutically acceptable auxiliary materials. The preparation method of the cefaclor inclusion complex comprises the following steps: dissolving polyvinyl alcohol-polyethylene glycol grafted copolymer (Kollicoat IR) in ethyl alcohol, adding cefaclor, carrying out reduced-pressure drying for removing ethyl alcohol while stirring, and filing into capsules, thus obtaining the cefaclor capsules. The cefaclor capsule is good in stability, high in dissolve-out speed, simple in production technique, and suitable for industrial production.
Owner:回音必集团浙江齐齐制药有限公司
Compound cefaclor preparation and preparation method
InactiveCN102114019AGood water solubilityGreat tasteAntibacterial agentsOrganic active ingredientsSolubilitySuspending Agents
The invention relates to a preparation of a medicine and a preparation method, in particular to a compound cefaclor preparation and a preparation method, and belongs to the technical field of pharmacy. The preparation is characterized by being prepared by adding a proper amount of pharmaceutical excipients into 25 to 35 weight parts of medicine, namely cefaclor and 0.5 to 1.5 weight parts of bromhexine hydrochloride, wherein the cefaclor is calculated based on anhydrous cefaclor, and the bromhexine hydrochloride is calculated based on bromhexine; and the excipients consist of a suspending agent, a flocculating agent, a flavoring agent and a flow aid. The invention provides a formula capable of improving the dissolubility and suspension stability of medicines and the mouthfeel of finished products, and a preparation technology.
Owner:汤明昌 +1
Cefaclor submicro-emulsion solid preparation and novel application thereof
InactiveCN101711742AImprove stabilityImprove solubilityAntibacterial agentsOrganic active ingredientsEmulsionBioavailability
The invention aims at providing a cefaclor submicro-emulsion solid preparation and a novel application thereof, in particular to a solid preparation of cefaclor processed by micro-emulsification and a novel application thereof. The invention solves the problems of poor stability and low bioavailability of the on-sale cefaclor solid preparation at present very well, can also be used for preparing a medicine for treating bacterial peritonitis and obtains a satisfactory technical effect.
Owner:HAINAN MEIDA PHARMA
Novel beta-lactam antibiotic synthetase production method
ActiveCN103695405AIncrease enzyme activityImprove stabilityHydrolasesVector-based foreign material introductionNucleotideAntibiotic Y
The present invention discloses a method for producing novel lactam antibiotics by using specific engineered bacteria transformant BL21(DE3) / PET28-ASPGA. According to the method, a penicillin acylase amino acid sequence represented by SEQIDNO:1 is adopted as basis, a mutation site is introduced, reverse design is performed to obtain a nucleotide sequence, optimization is performed, complete gene synthesis and recombinant vector construction are sequentially performed to construct a recombinant strain, and the constructed recombinant strain is treated through steps of fermentation, cell disruption, separation, purification, decoloration and immobilization to finally obtain the novel lactam antibiotic immobilized enzyme finished product capable of being directly used for industrial production. According to the present invention, the immobilized enzyme prepared by using the method has characteristics of high enzyme activity, good stability and excellent repeated use effect, and can be provided for catalyzing synthesis of a variety of lactam antibiotics such as amoxicillin, cephalexin and cefaclor.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Cefadole oral liquor antibacterial composition
InactiveCN1742734AImprove antibacterial propertiesImprove the bactericidal effectAntibacterial agentsHeterocyclic compound active ingredientsSulbactam PivoxilVeterinary medicine
The present invention discloses an oral antimicrobial medicine composite composed of cefaclor and sulbactam pivoxil. The weight ratio range of cefaclor and sulbactam pivoxil in said oral antimicrobial medicine composite is 1:1 to 15:1, and the optimized weight ratio range is 1:1 to 6:1.
Owner:HAINAN JINXING PHARMA
Green method of enzymatic synthesis of cefaclor
InactiveCN106222230AHigh selectivityImprove catalytic performanceImmobilised enzymesHydrolasesChemical synthesisEnzymatic synthesis
The invention relates to a green method of enzymatic synthesis of cefaclor. The method includes the steps of: (S1) adding a parent nucleus 7-ACCA into a buffer liquid; (S2) under the pH of 5-8, adding a D-p-hydroxyphenylglycinate derivative or a salt thereof and / or D-p-hydroxyphenylglycine amide, and immobilized cefaclor synthesis enzyme, and performing a reaction for 1-3 h at 5-30 DEG C under the pH value of 6.2-7.8; after a certain reaction time, adding a seed crystal to perform crystallization, and when the reaction is finished, separating a reaction liquid and the immobilized cefaclor synthesis enzyme to obtain a cefaclor coarse product; and (S3) acid-hydrolyzing and dissolve-clarifying the coarse product, filtering and re-crystallizing the product to prepare the cefaclor. The enzymatic synthesis method, compared with a conventional chemical synthesis method, is simple in operation, is low in cost, reduces synthetic period, improves production efficiency and total yield, has strong controllability and satisfies industrial production.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
Cefaclor composition particles and preparation method thereof
ActiveCN102525949AImprove antibacterial propertiesNot very stableAntibacterial agentsOrganic active ingredientsSolubilityDisease
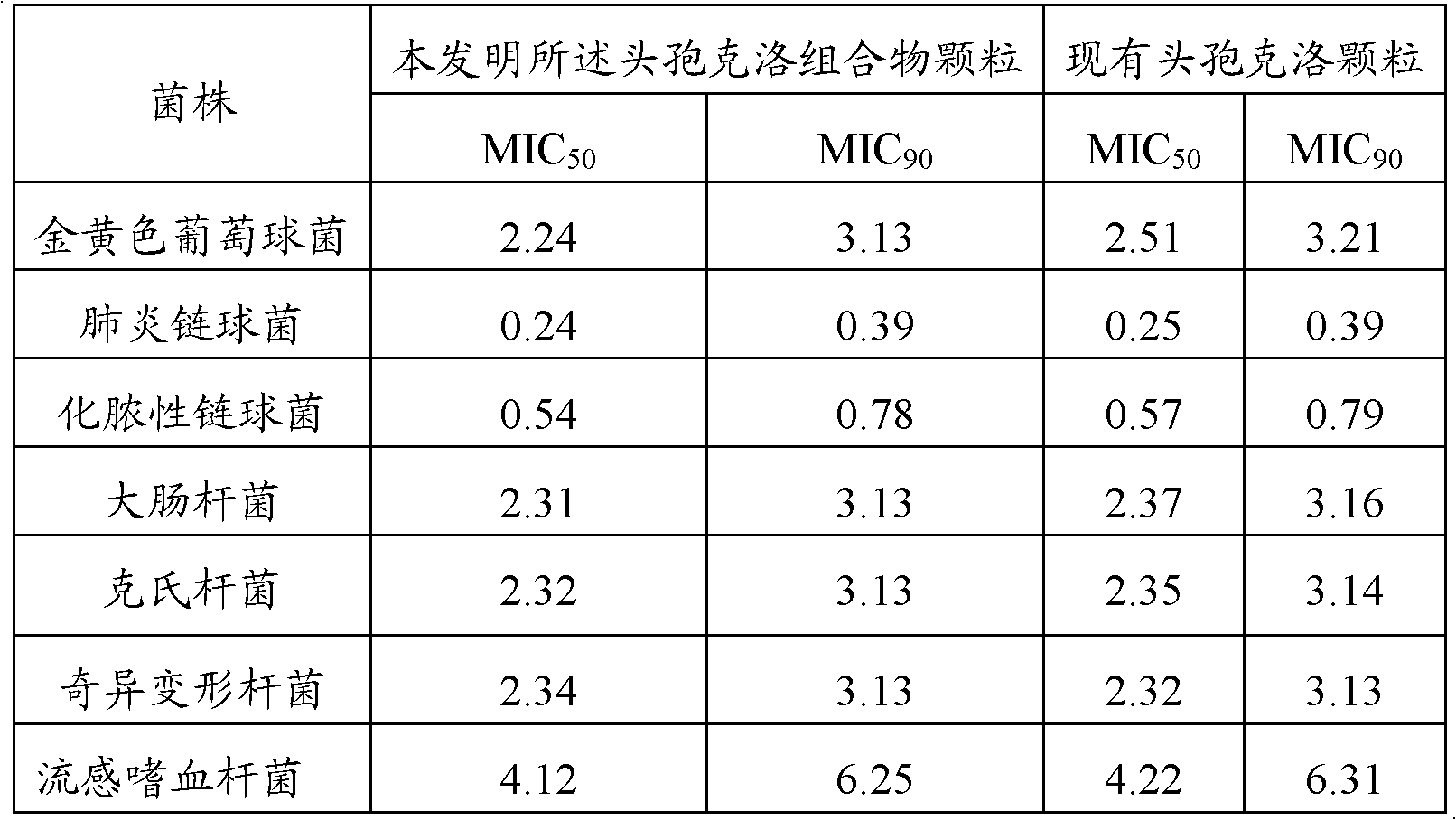
The invention relates to the technical field of medicine, and discloses cefaclor composition particles and a preparation method thereof. The cefaclor composition particles disclosed by the invention are prepared from the following raw materials in parts by weight: 250 parts of cefaclor, 8 parts of bromhexine hydrochloride, 1,490 parts of sucrose, 20 parts of acesulfame, 2.5 parts of essence and 250 parts of sorbitol. The cefaclor composition particles disclosed by the invention comprise sorbitol which can be taken as a medicament dispersing agent for enhancing the medicament solubility and can be used for enhancing the antibacterial action of cefaclor. As proved by a test, the cefaclor composition particles disclosed by the invention have enhanced antibacterial action and high stability and can be widely applied to treatment of bacterial infection diseases such as respiratory tract infection, bone and joint infection, pelvic infection, abdominal infection and the like.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
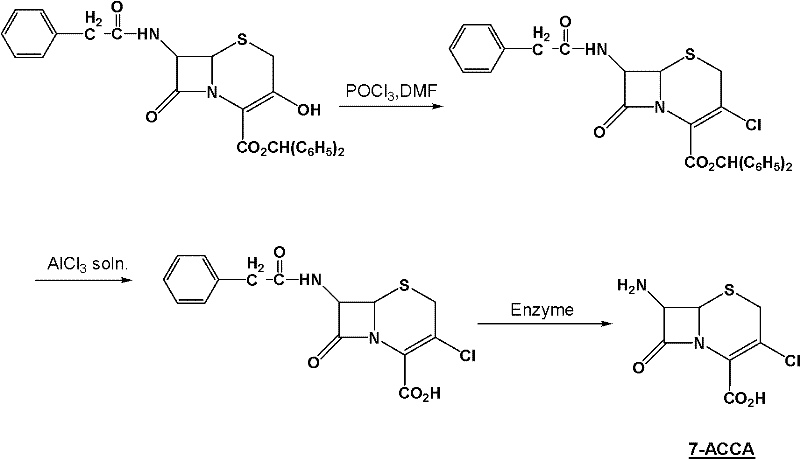
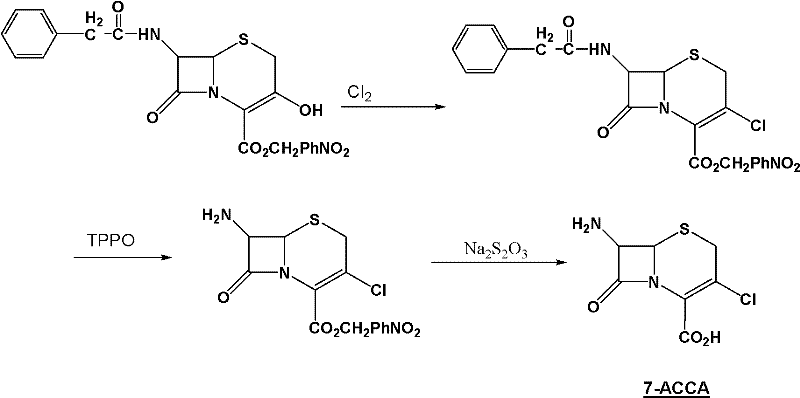
Method for preparing 7-ACCA
The invention relates to an important intermediate 7-ACCA of cephalosporin cefaclor, comprising the following steps of: 1) preparation of 3-amino-2-ethoxyformyl anesthesin; 2) preparation of 2-ethoxybenzimidazole-4-carboxylic acid ethyl ester; 3) preparation of candesartan cyclic compound. According to the invention, the benzimidazole ring is constructed, tetraehtyl orthocarbonate is avoided, andintramolecular dehydration is accomplished; and alkylation is accomplished in the final stage and cyanobromobiphenyl is introduced to minimize the consumption of cyanobromobiphenyl as well as the cost of the candesartan cyclic compound.
Owner:ZHANGJIAGANG XINYI CHEM
Cefaclor medicinal composition and acute lung injury protection effect thereof
InactiveCN106083980AHas a preventive effectNovel structureOrganic active ingredientsSteroidsNatural productALI - Acute lung injury
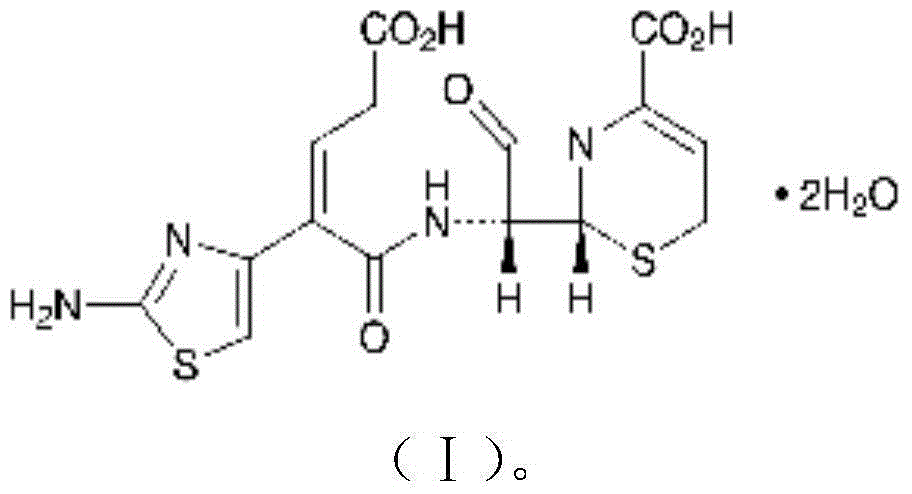
The invention discloses a cefaclor medicinal composition and an acute lung injury protection effect thereof. The cefaclor medicinal composition contains cefaclor and a natural product compound (I) having a novel structure and separated from dry rhizome of Grassleaf Sweelflag Rhizome, and cefaclor and the natural product have lung injury prevention and treatment effects in the individual action process; and cefaclor and the natural product have further improved lung injury prevention and treatment effects when being combined, so cefaclor and the natural product can be used to develop lung injury prevention and treatment medicines. Compared with medicines in the prior art, the medicinal composition has protruding characteristics and substantial progresses.
Owner:赵吉永
Cefaclor sustained-release tablets and preparation method thereof
InactiveCN102028667ARelease stabilityIdeal plasma concentrationAntibacterial agentsOrganic active ingredientsSustained Release TabletCarboxymethyl starch
The invention discloses cefaclor sustained-release tablets, which are characterized by being prepared from the following raw materials in part by weight: 75 parts of cefaclor 3, 20 to 40 parts of mannitol, 15 to 30 parts of sodium carboxymethyl starch, 15 to 30 parts of hydroxypropyl methyl cellulose E5, 15 to 30 parts of hydroxypropyl methyl cellulose K100M, 5 to 10 parts of polyvinylpyrrolidone K90 and 3 to 6 parts of magnesium stearate. The cefaclor sustained-release tablets are a dispersible sustained release preparation taking the hydroxypropyl methyl cellulose (HPMC) as a water-soluble skeleton, after the cefaclor sustained-release tablets are administered, medicaments can be slowly released at an unconstant speed according to the requirement, the release is stable, the ideal blood concentration is provided, an obvious peak-valley phenomenon is absent, the times for taking medicines is reduced, and the cefaclor sustained-release tablets have durable medicament effects and obvious curative effects.
Owner:山东淄博新达制药有限公司
Cefaclor preparation and preparation method thereof
ActiveCN105769873AHigh reactivityGood choiceAntibacterial agentsOrganic active ingredientsChemical synthesisPotassium
The invention discloses a cefaclor preparation and a preparation method thereof. The preparation method comprises: preparing cefaclor crystal, pretreating a main material and auxiliary materials, weighing, mixing, forming, and packaging; wherein the preparation of the cefaclor crystal includes subjecting 7-ACCA and potassium (R)-[(3-ethoxy-1-methyl-3-oxoprop-1-enyl)amino]phenylacetate to silylation, acylation, condensation, acid hydrolysis, extraction and cleaning, decoloring, and crystallization; the preparation comprises the cefaclor crystal as the main material and auxiliary materials, the cefaclor crystal is < / =33 degrees in angle of repose, 0.50-0.60 g / m in bulk density, 0.70-0.80 g / ml in compactness and 40-60 Mum in D10 of particle size distribution, 120-140 Mum in D50 and 210-230 Mum in D90. The conversion rate of cefaclor in chemical synthesis is increased, reaction conditions are simplified, the crystal form and particle size distribution of the cefaclor crystal are improved, and the quality of finished cefaclor crystal is improved.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Sustained-release composition of cefaclor
ActiveCN101897678AReduce releaseAntibacterial agentsOrganic active ingredientsMedicineWater insoluble
The invention relates to the technical field of drug release, in particular to a novel sustained-release composition of cefaclor. The sustained-release composition of cefaclor comprises 60-90% of cefaclor, 5-25% of water-soluble adhesives, 0.5-5% of water-insoluble retardants, 2-10% of porogenic agents and 2-15% of cellulose derivatives with water viscosity being more than 500mmPa.s by total weight of the composition. The sustained-release composition of cefaclor can be completely released within 4-8h, has sustained-release effect in bodies and is applicable to administration twice a day.
Owner:ZHEJIANG ANGLIKANG PHARMA
Method for preparing cefaclor dispersible tablets by dry method direct tablet compressing and cefaclor dispersible tablets prepared by same
InactiveCN101711748ADisintegrates quicklyImprove stabilityAntibacterial agentsOrganic active ingredientsRoom temperaturePharmaceutical formulation
The invention relates to the field of medicinal preparations, in particular to a method for preparing cefaclor dispersible tablets by dry method direct tablet compressing and cefaclor dispersible tablets prepared by the same. The method comprises the following steps of: firstly, respectively sieving cefaclor and various auxiliary materials by a sieve of 80 meshes for later use; then respectively drying the auxiliary materials and cooling to room temperature; and finally, mixing the dried and cooled auxiliary materials with the cefaclor and then directly pressing tablets. The method has less production working procedures, simple equipment, short period and smaller medicament loss. The cefaclor dispersible tablets prepared by the dry method direct tablet compressing have quick disintegration and high stability.
Owner:ANHUI ANKE BIOTECHNOLOGY (GRP) CO LTD
Method for recovery of cefaclor
InactiveCN1830982AThe reduction ratio is not highReduce lossesOrganic chemistryRecovery methodN dimethylformamide
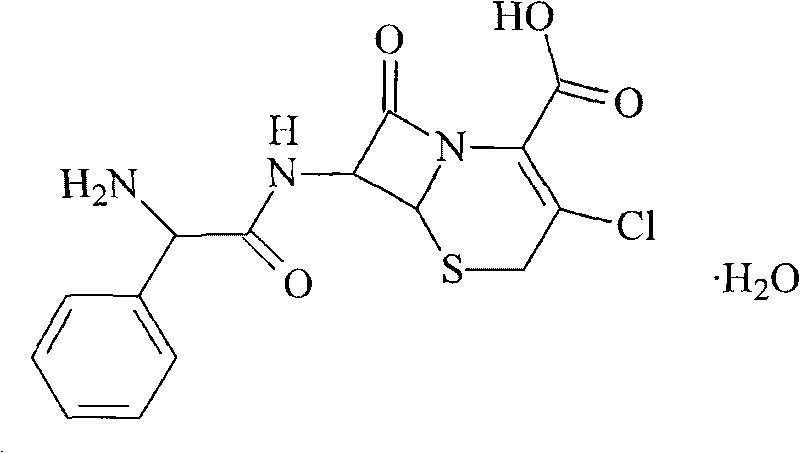
The invention discloses a recovering technique of reclaiming the cefaclor from the water liquor. Firstly, the cefaclor in the water liquor is transformed into the cefaclor composite; secondly, the cefaclor composite is transformed into the cefaclor N, N-dimethylformamide composite or the cefaclor N, N-dimethyl acetamide composite, finally, the cefaclor N, N- dimethylformamide or the cefaclor N, N-dimethyl acetamide composite is transformed into the cefaclor hydrate to reclaim the cefaclor. Comparing the invention with the existing technique, we can perorate that the invention can reclaim the height ratio of the cefaclor from the cefaclor equeous solution and is easy to operating.
Owner:苏州盛达药业有限公司
Method for extracting cefaclor from cefaclor naphthalenol complexes
InactiveCN101314605AHigh purityLow impurity contentOrganic chemistryOrganic solventPurification methods
The invention provides a method for purifying cefaclor from a cefaclor naphthol compound. The method comprises the steps as follows: (1) rinsing the cefaclor naphthol compound, extracting the impurities in water phase by an organic solvent, and collecting the water phrase; (2) adjusting the pH value of the water phrase solution to 1-2 and adsorbing by macroporous absorbent resin; and (3) eluting cefaclor with low-carbon organic solvent-water mixed solution as a mobile phase and collecting the eluate. By adopting the purification method, high-purity cefaclor can be prepared from the cefaclor naphthol compound, and has low impurity content, thus meeting the 'pharmacopeia' standard. The prepared cefaclor can be directly taken as the commercial drug material.
Owner:EAST CHINA UNIV OF SCI & TECH
Cefaclor orally disintegrating tablet and preparation method thereof
ActiveCN101444513AImprove bioavailabilityImprove biostabilityAntibacterial agentsOrganic active ingredientsPharmacyAdditive ingredient
The invention discloses a Cefaclor orally disintegrating tablet and a preparation method thereof. The tablet comprises Cefaclor with pharmaceutically effective dose, pharmaceutically acceptable coating material, diluent, disintegrant, glidant, flavouring and lubricant. Ingredients, such as the coating material, the diluent, the disintegrant and the like, and contents are screened, and the preparation technology is improved by numerous studies by the applicant of the invention, thereby obtaining a prepared product with faster effect and more sufficient absorption. The tablet can be disintegrated or dissolved in more than ten seconds in an oral cavity by swallowing without drinking water. The tablet is more suitable for the elderly and patients with solid-swallowing difficulty, and provides great convenience for patients which are busy in work or can not take water conveniently.
Owner:金鸿药业股份有限公司
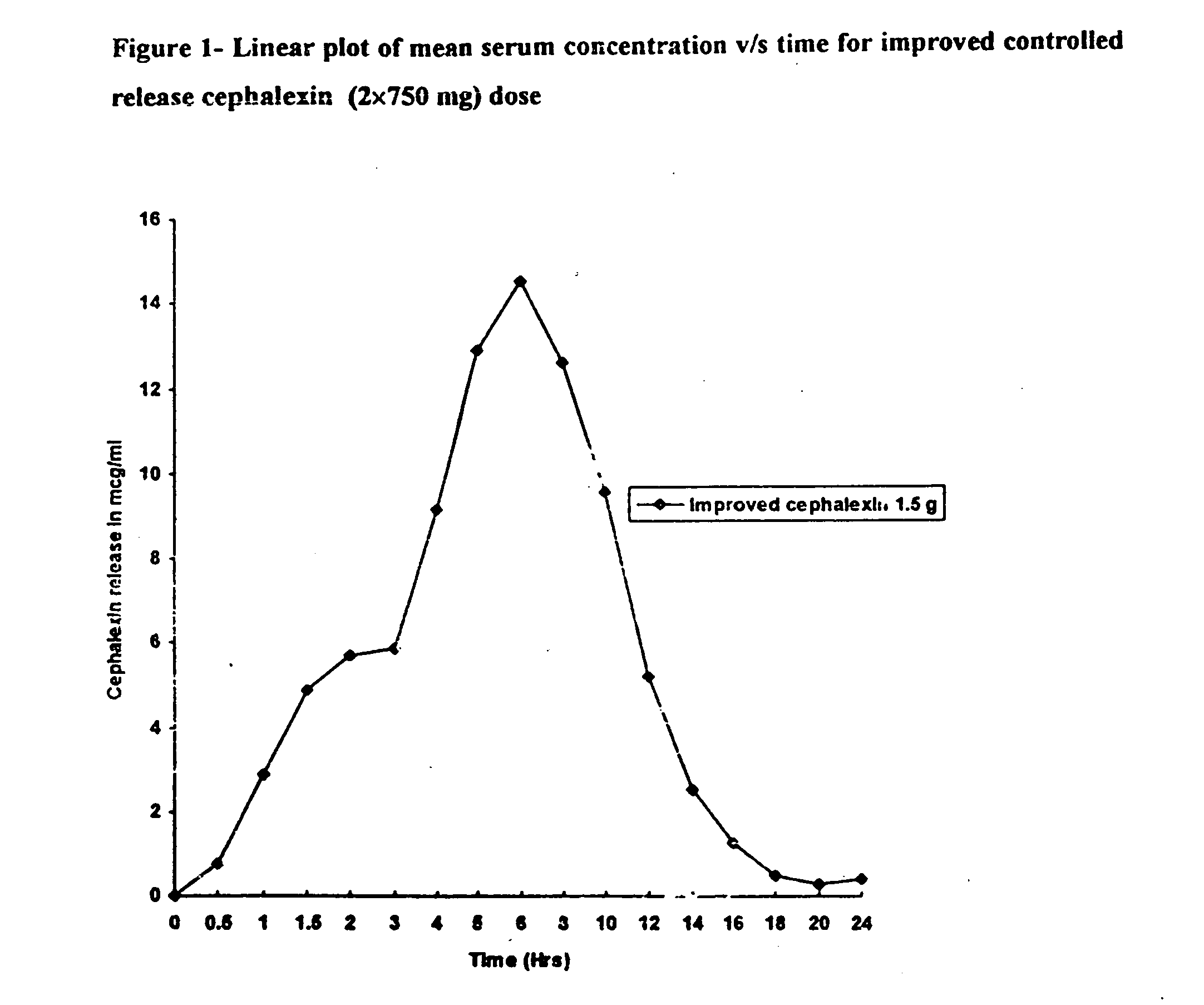
Extended release matrix tablets
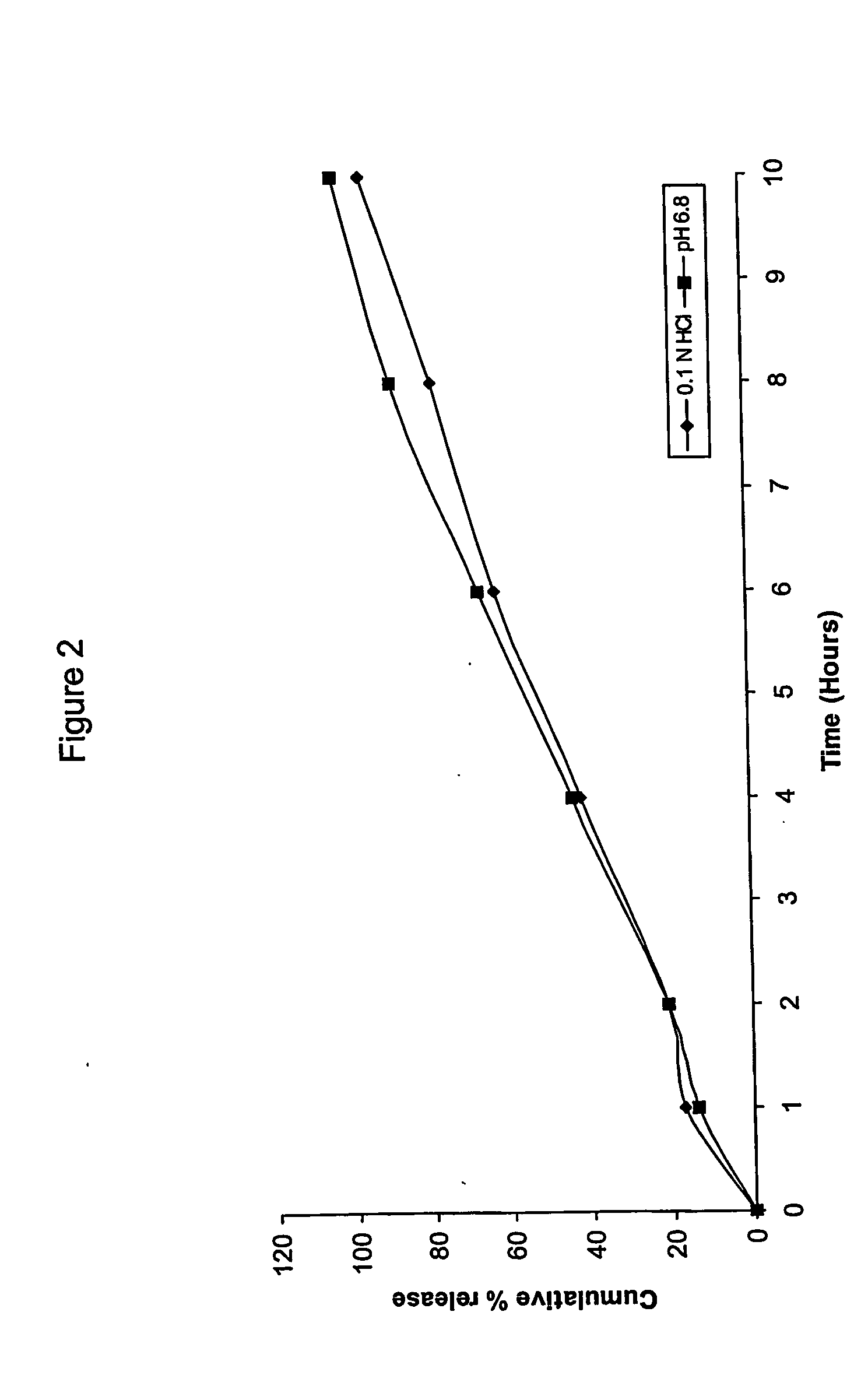
The present invention relates to extended release matrix tablets for oral administration that include a cationic polymer, a water-swellable polymer, and an alginic acid derivative to cause the release rate of the active ingredient of the tablets to be independent of pH and gastric residence time. The active pharmaceutical ingredient may be one or more of antibiotics, sympathomimetics, sympatholytic agents, cholinergic agents, antimuscarinics, gastro-intestinal drugs, gentio-urinary smooth muscle relaxants, cardiac drugs, anticonvulsants, tranquilizers and sedatives, and in particular may be an antibiotic, such as cefaclor, or may be a sympatholytic agent, such as carvedilol.
Owner:RANBAXY LAB LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com