Cephalosporin suspension granule and preparation method thereof
A technology for suspending granules and cephalosporins, which is applied in the directions of antibacterial drugs, pharmaceutical formulations, and medical preparations of inactive ingredients, etc., can solve problems such as difficulty in guaranteeing the dosage of drugs, poor solution suspension effect, and poor quality stability, etc. To achieve the effect of good medication compliance, good suspension effect and stable quality in children
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] A method for preparing cephalosporin suspension particles, the preparation steps are as follows: first, the polyvinylpyrrolidone mixed solution is prepared in advance: the polyvinylpyrrolidone is dissolved in water to prepare a polyvinylpyrrolidone aqueous solution, and the polyvinylpyrrolidone accounts for polyethylene The weight percent of the pyrrolidone aqueous solution is 3-20%; tartrazine (or sunset yellow, carmine) and edetate disodium (EDTA-2Na) are added to the above solution, stirred and dissolved to prepare a polyvinylpyrrolidone mixed solution for use.
[0018] Then, weigh cefixime, sucrose, low-substituted hydroxypropyl cellulose, aspartame, and strawberry essence according to the prescription amount, grind sucrose and cefixime into 120-mesh fine powder and mix with low-substituted hydroxypropyl cellulose, The auxiliary materials such as spathin are mixed evenly, and the polyvinylpyrrolidone mixed solution prepared above is added, and after being stirred eve...
Embodiment 2
[0022] A kind of cephalosporin suspension granule, its prescription composition comprises cefixime, sucrose powder, low-substituted hydroxypropyl cellulose, aspartame, disodium edetate, strawberry essence, tartrazine, polyvinylpyrrolidone (PVPK 30 ). The percentage by weight of the cephalosporin suspension particles in each component is: 3% cefixime; 65% sucrose powder; 30% low-substituted hydroxypropyl cellulose; 0.5% aspartame; 0.02% edetate disodium ; Strawberry essence powder 1%; Yellow pigment 0.05%; 20% polyvinylpyrrolidone (PVPK 30 ) solution in an appropriate amount.
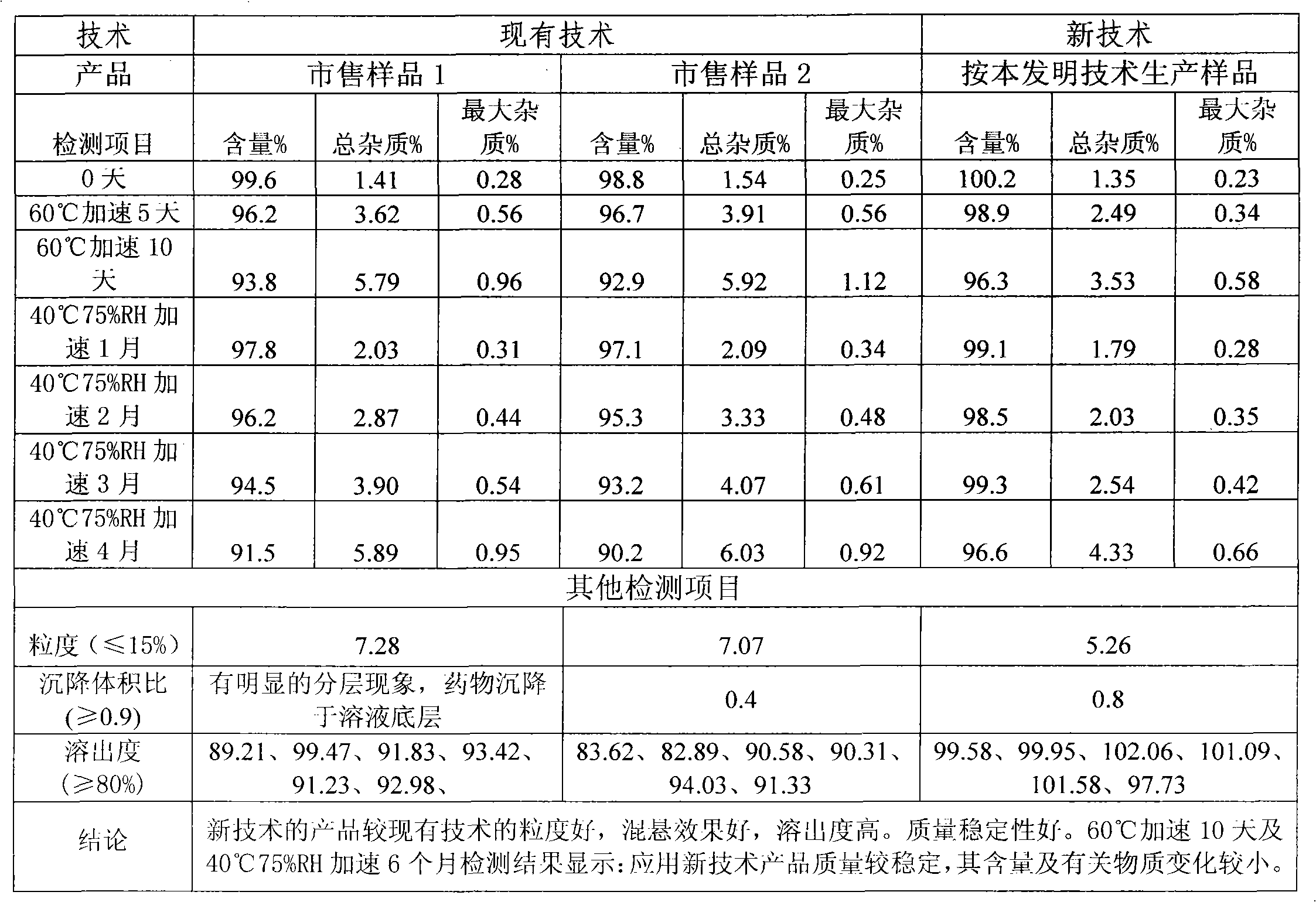
[0023] The cephalosporin suspension granule sample produced by the technology described in Example 1 of the present invention and the commercially available sample of the prior art are carried out to conduct a comparative study of the quality of the above prescription, and the results are shown in Table 1:
[0024] Table 1 The quality test results of cefixime granules produced by different manufacturer...
Embodiment 3
[0027] The cephalosporin suspension granules of present embodiment 2, its prescription composition comprises cefprozil, mannitol, carboxymethyl starch sodium, stevioside, disodium edetate, strawberry essence, yellow pigment, polyvinylpyrrolidone (PVPK 30 ). The percentage by weight of the cephalosporin suspension particles in each component is: 10% cefprozil; 50% sucrose; 20% mannitol; 15% sodium starch glycolate; 1% stevioside; 0.02% edetate disodium; Orange flavor powder 1%; yellow pigment 0.5%; 15% polyvinylpyrrolidone (PVPK 30 ) in an appropriate amount of aqueous solution.
[0028] The sample that this prescription is produced by the technology described in embodiment 1 of the present invention and the commercially available sample of prior art are carried out the comparative study of quality situation, and the result is: the particle size of present embodiment product is better than prior art, and suspension effect is good, High dissolution rate. Good quality and stab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com