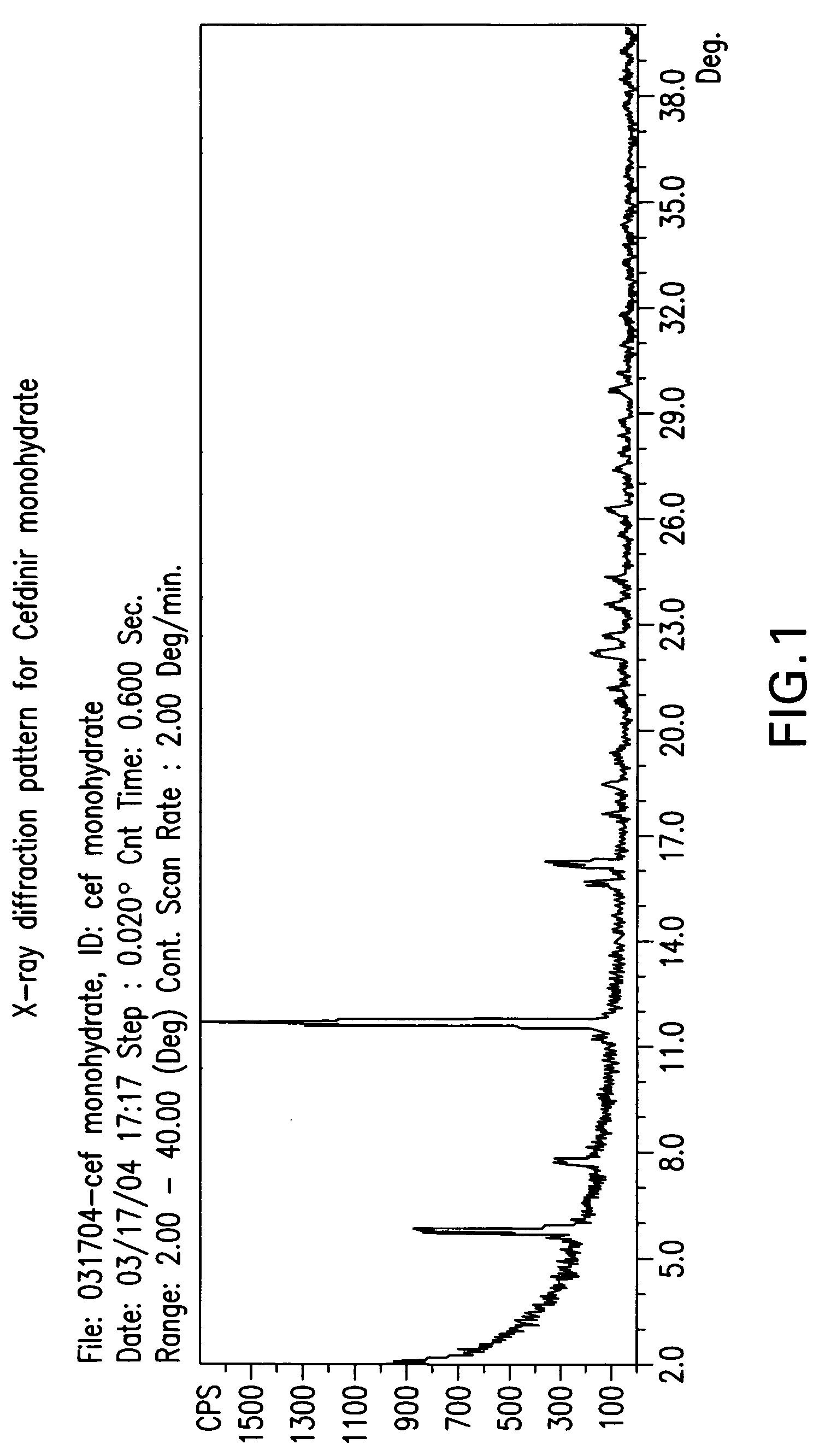
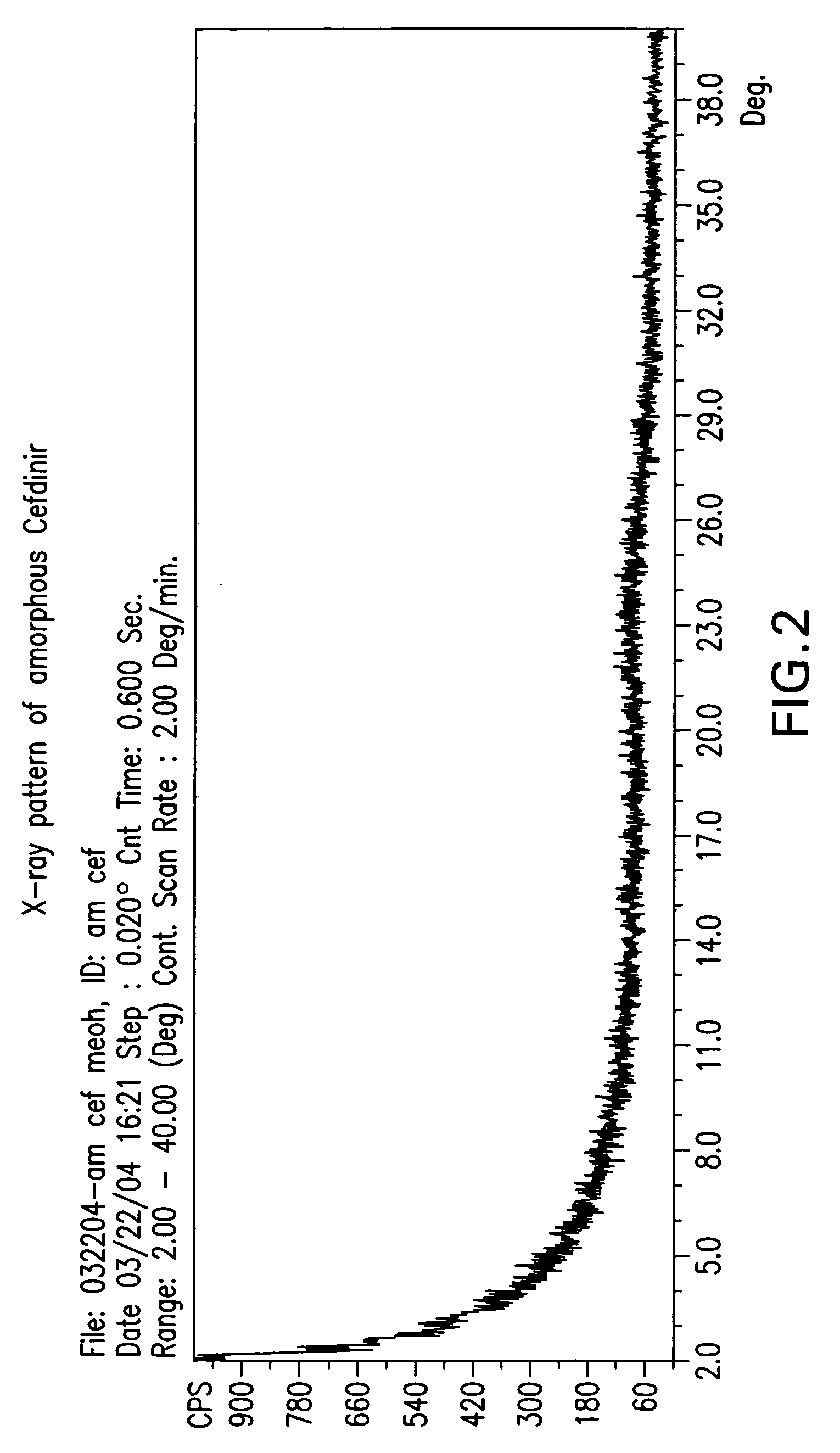
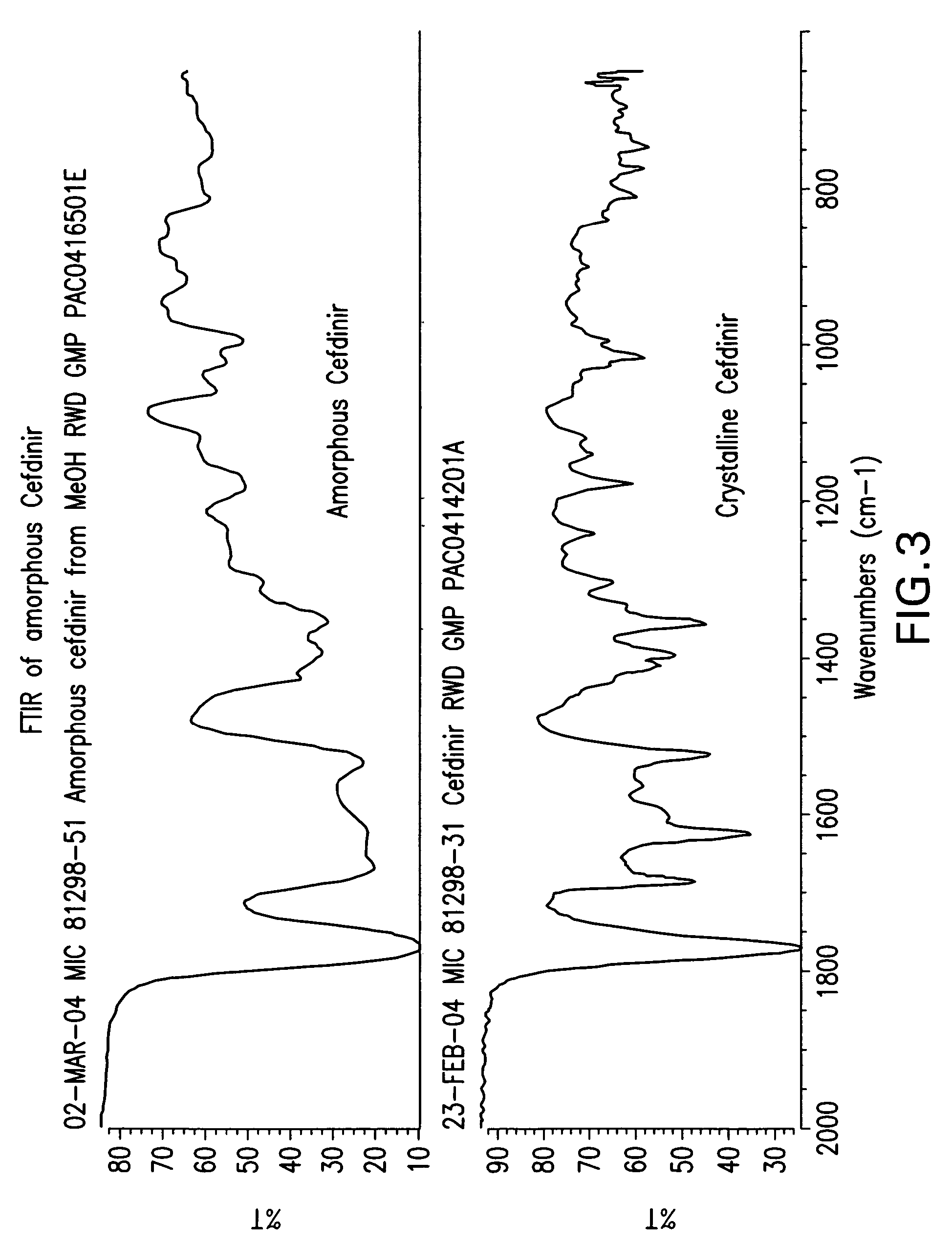
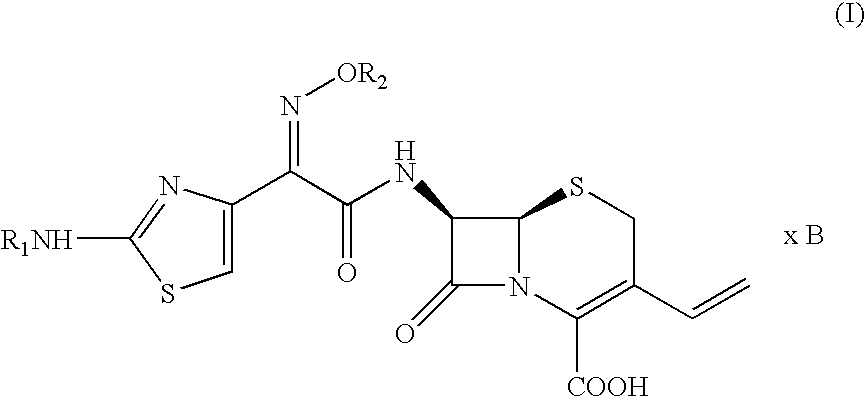
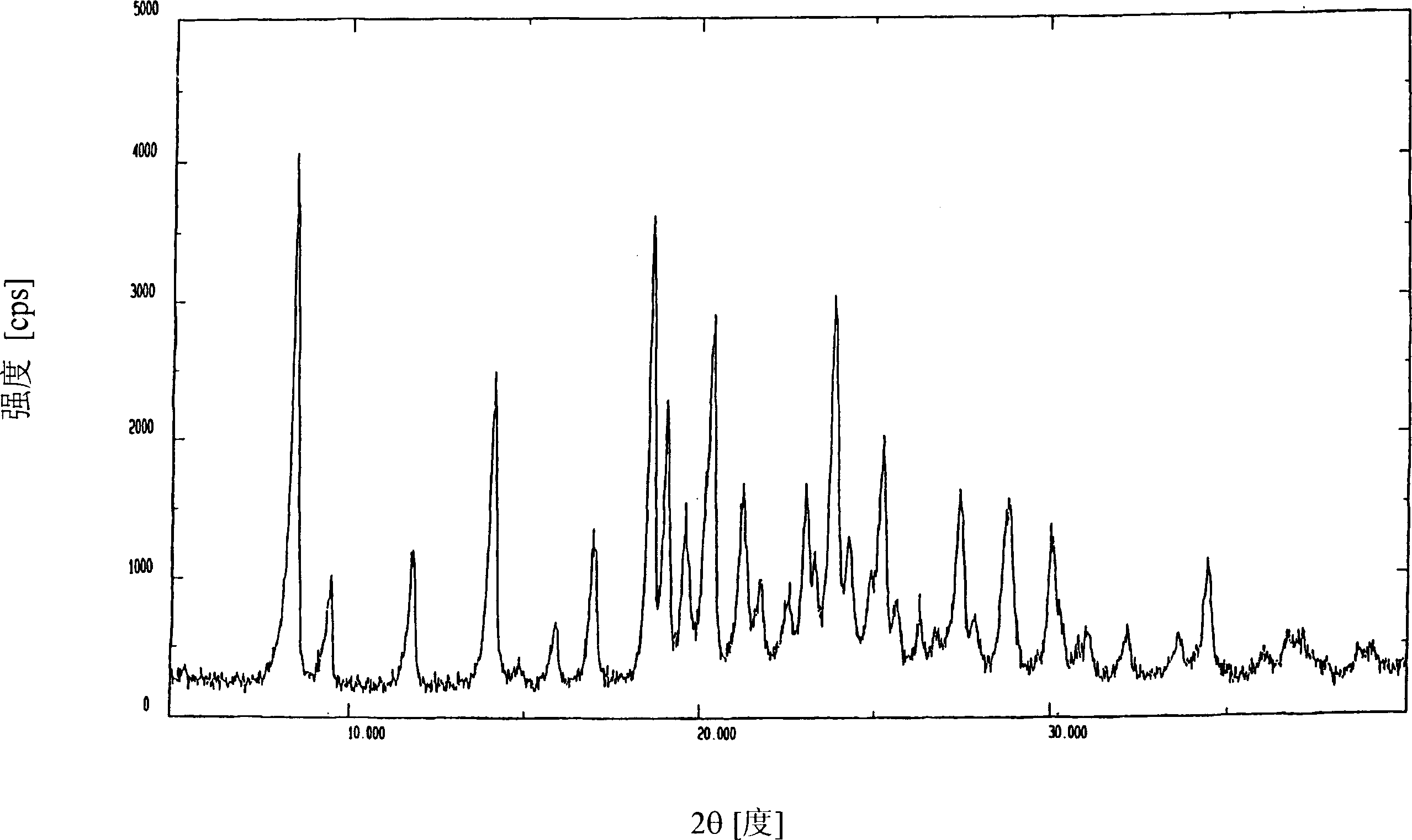
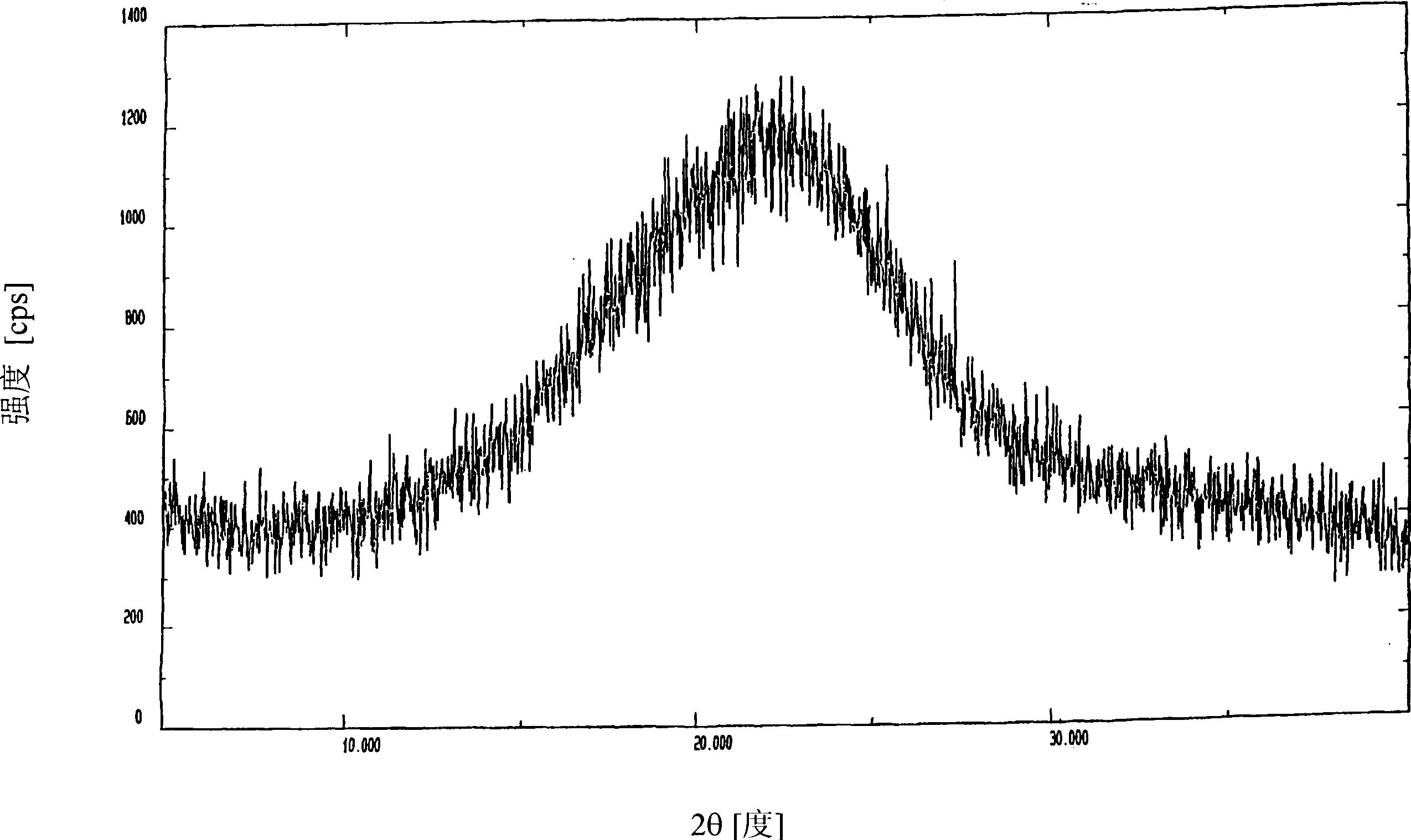
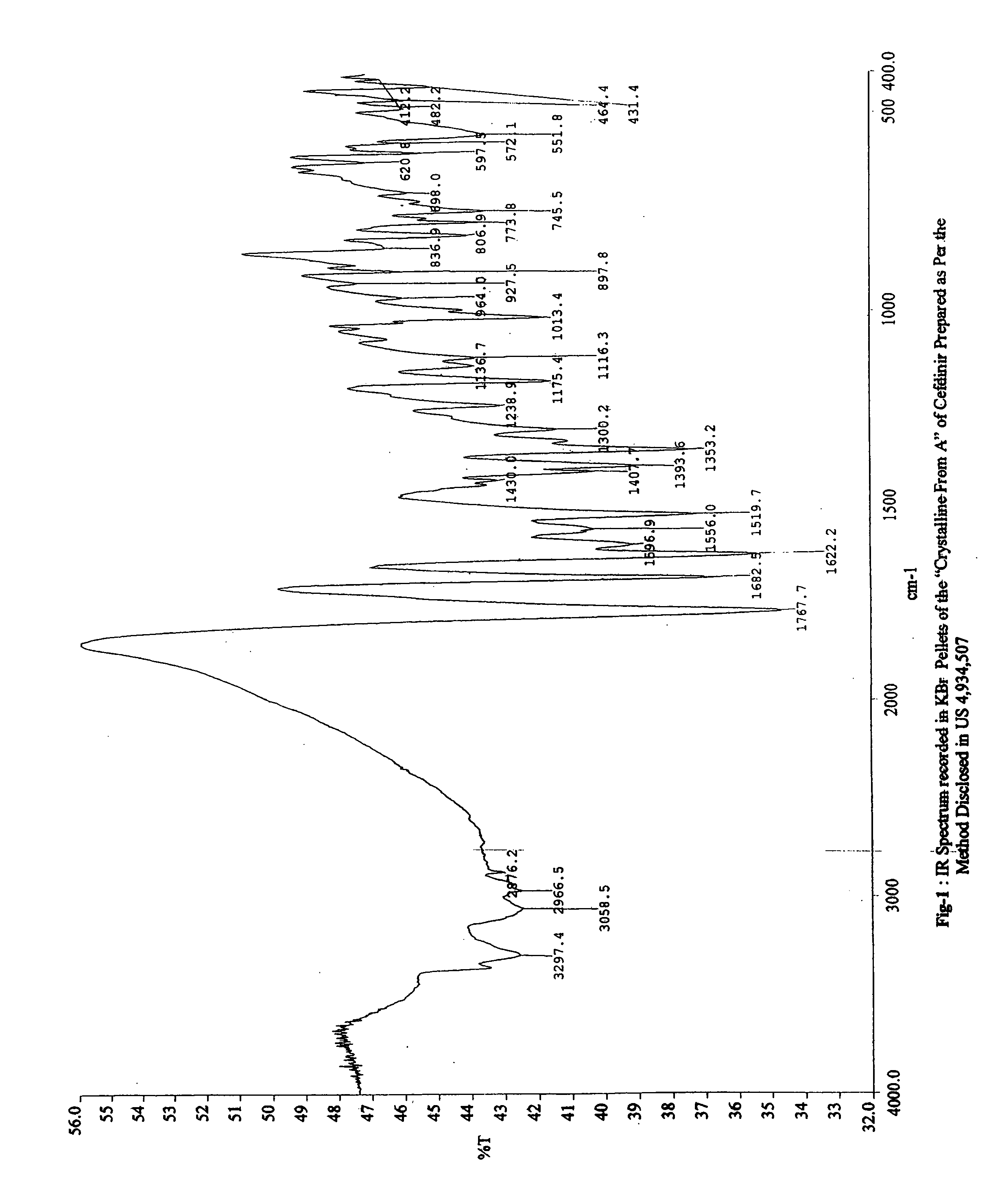
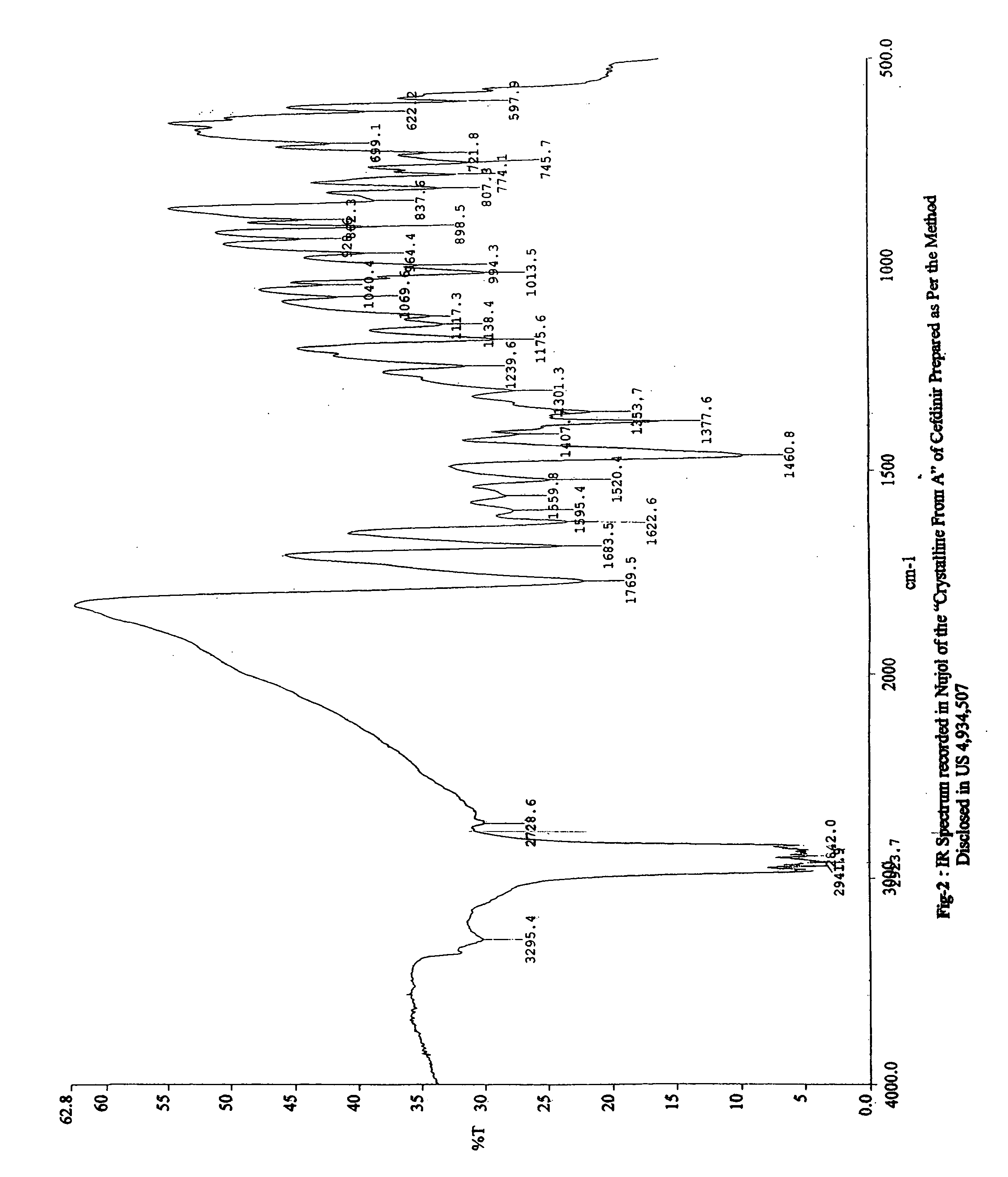
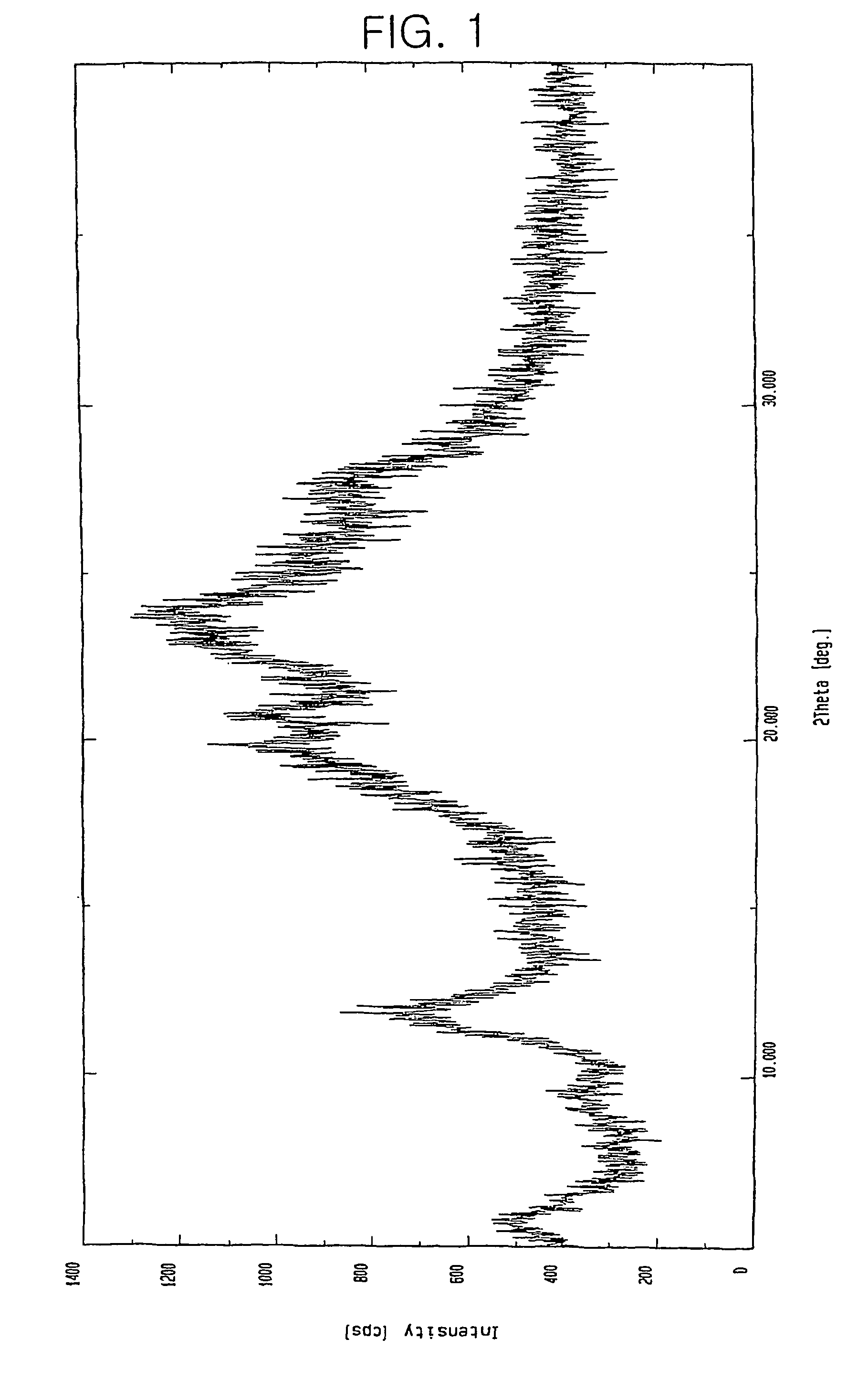
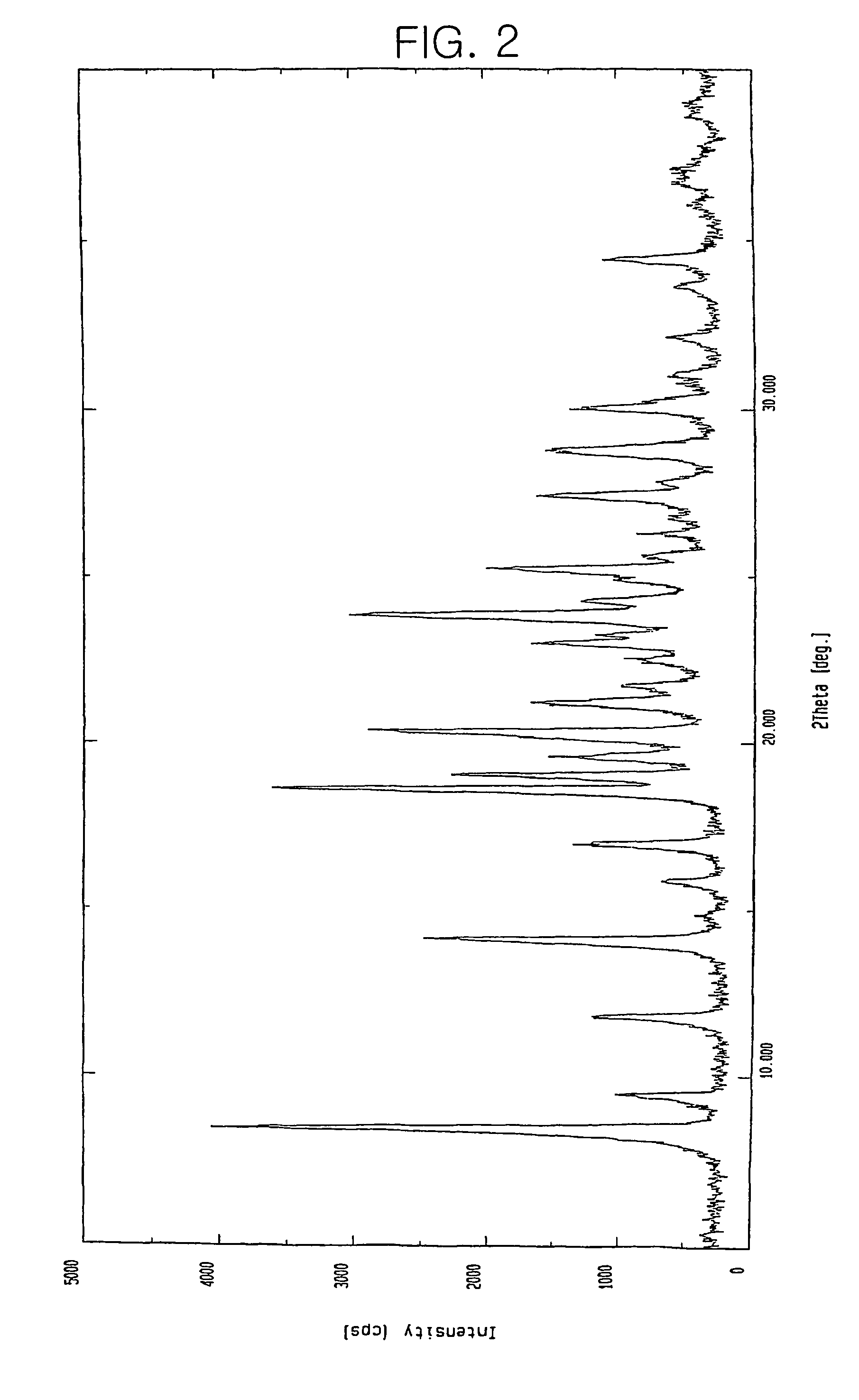
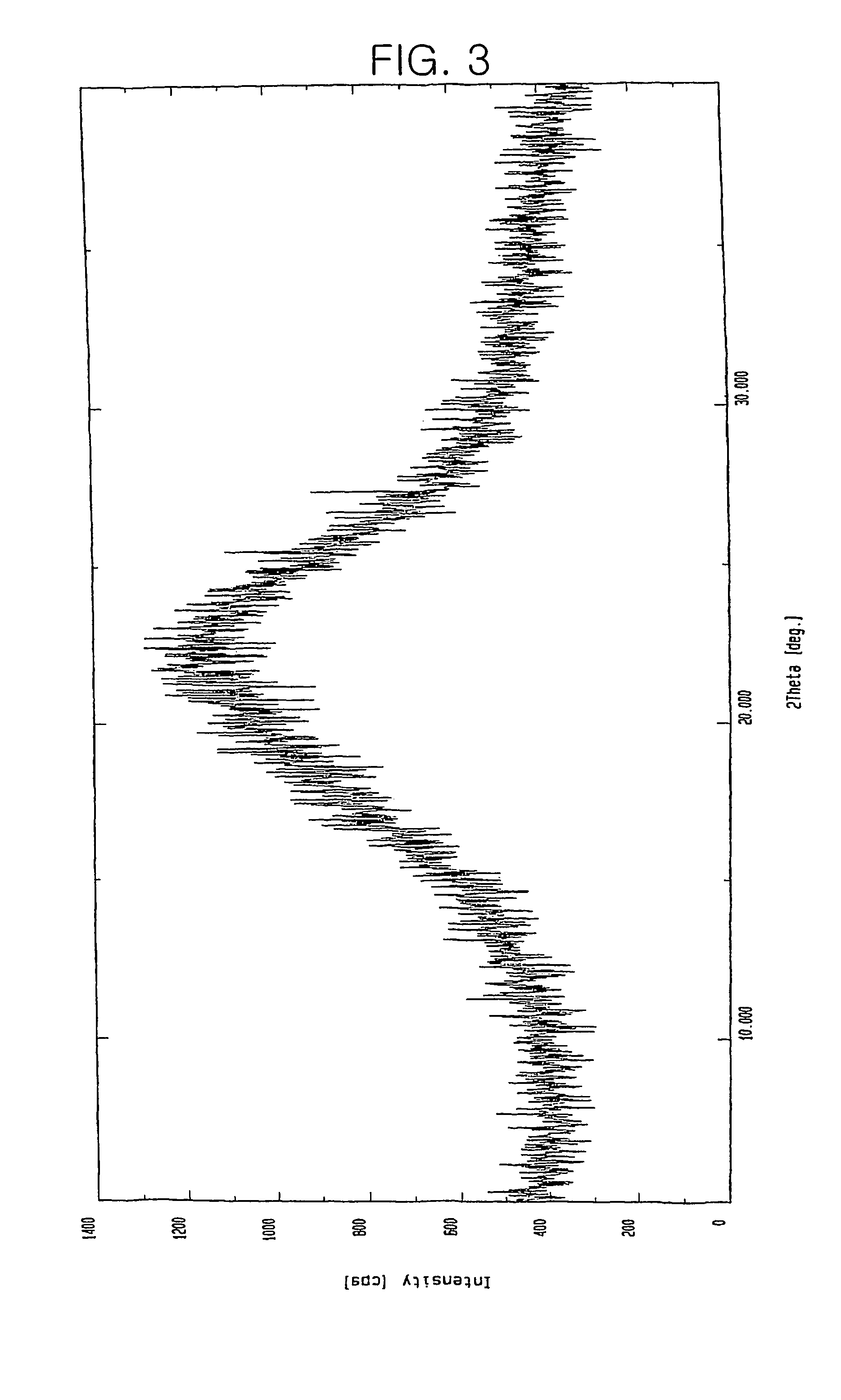
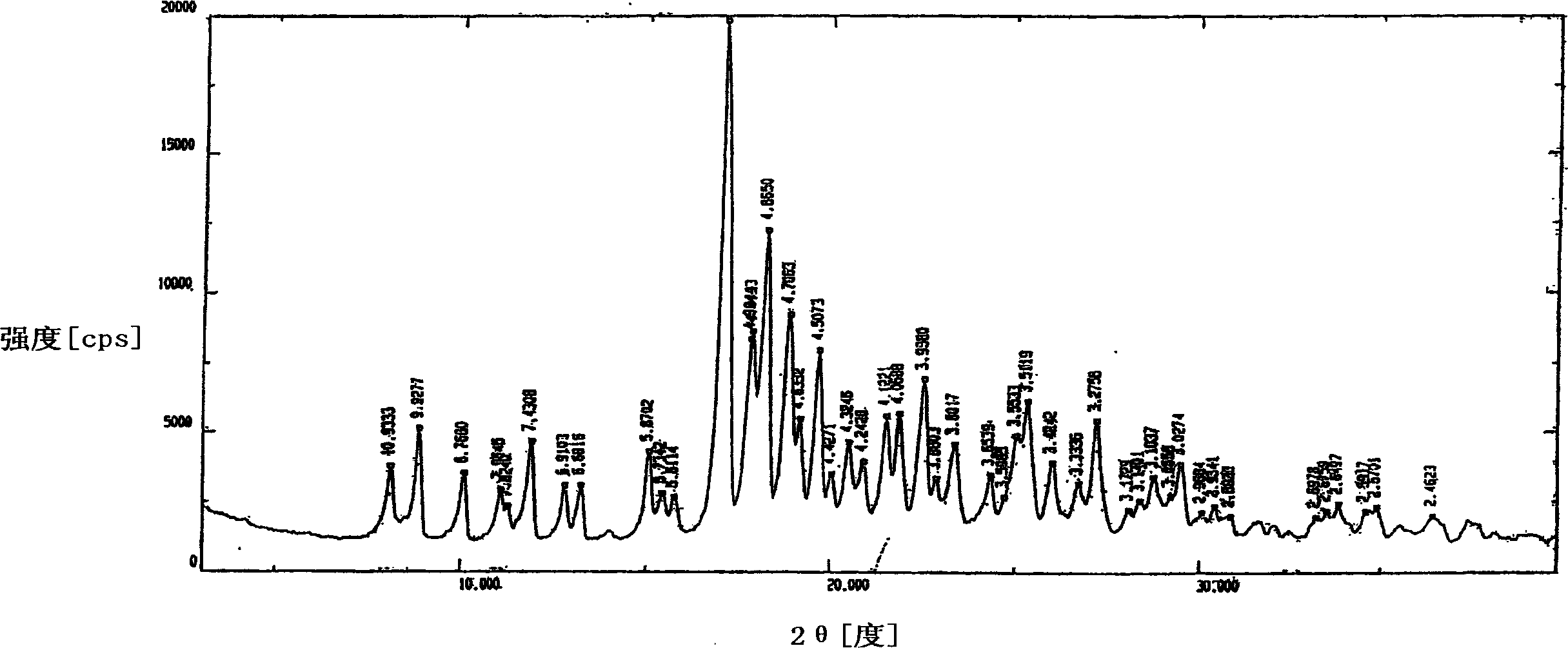
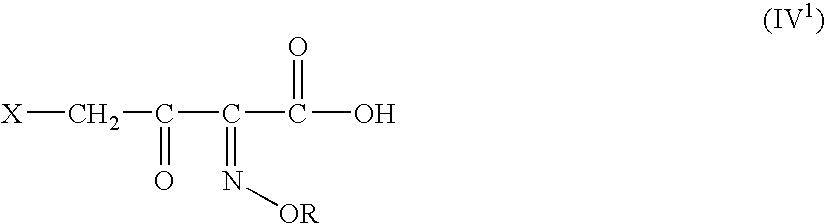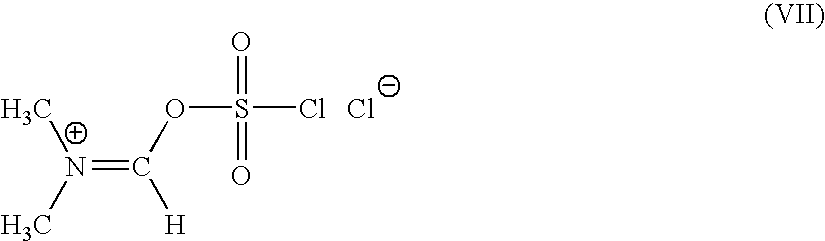Patents
Literature
150 results about "Cefdinir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
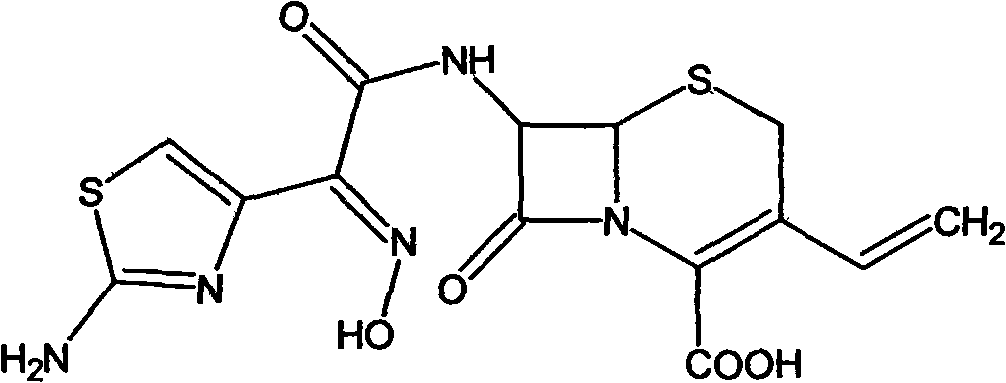
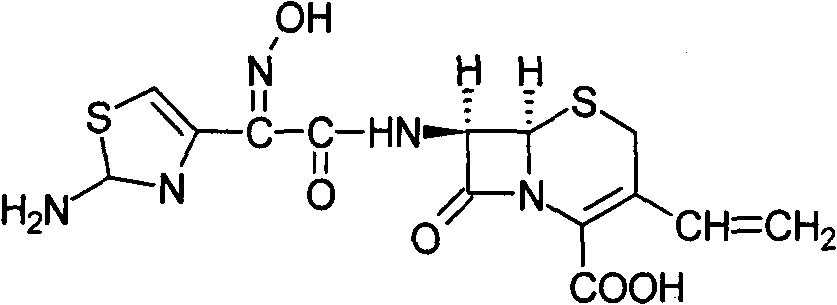
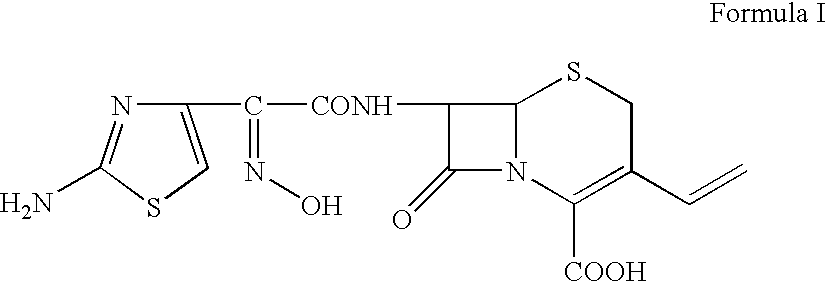
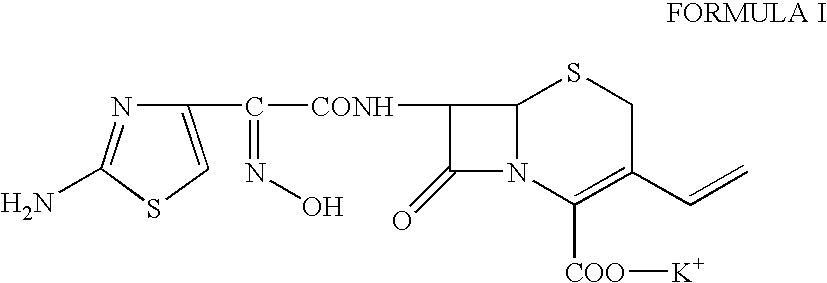
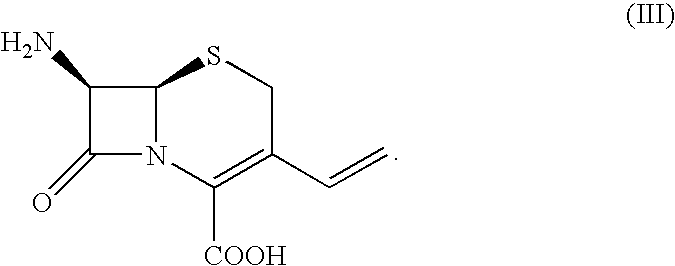
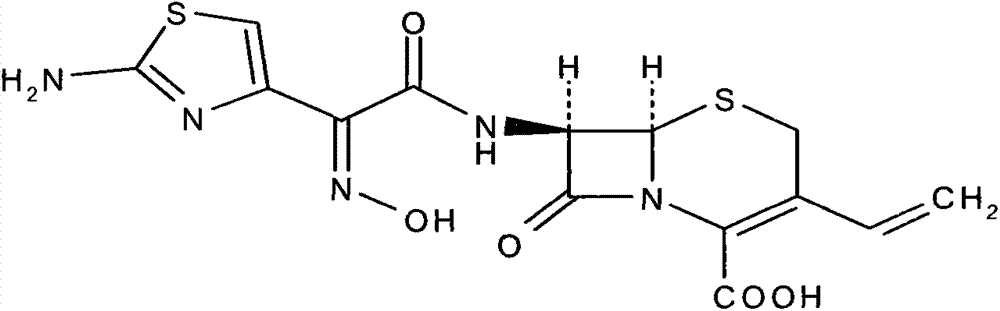
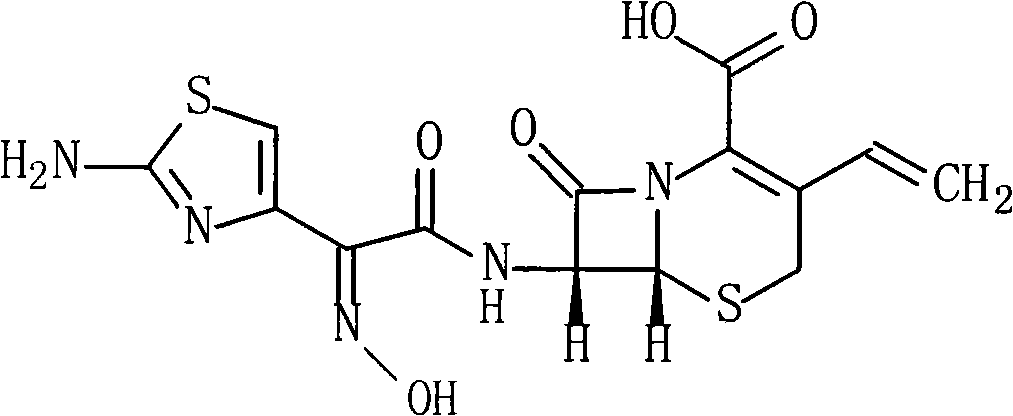
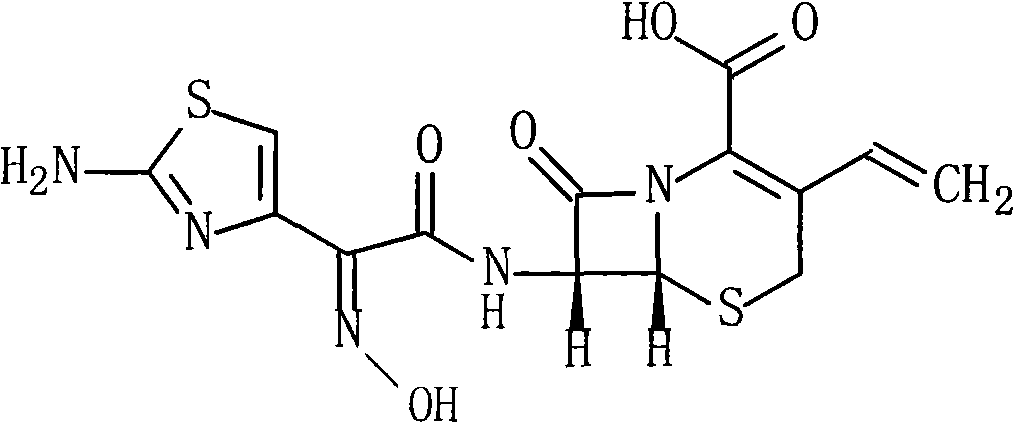
Cefdinir is used to treat a wide variety of bacterial infections.
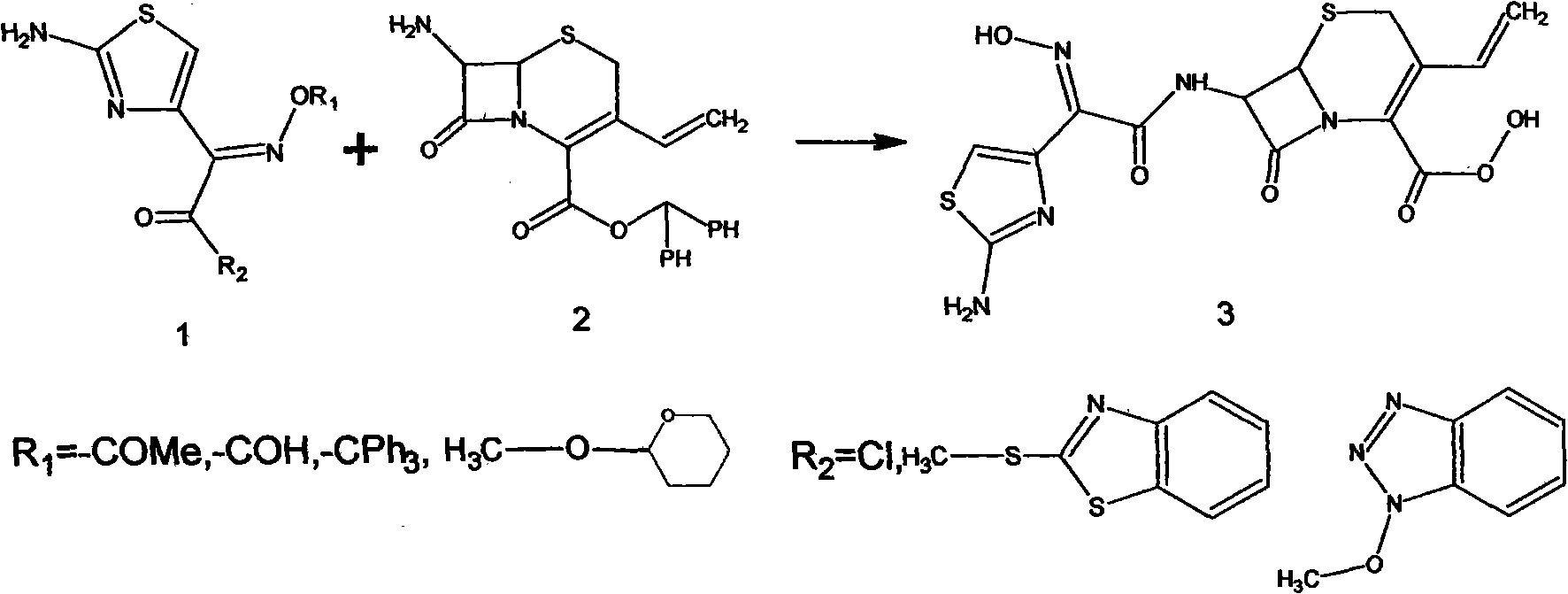
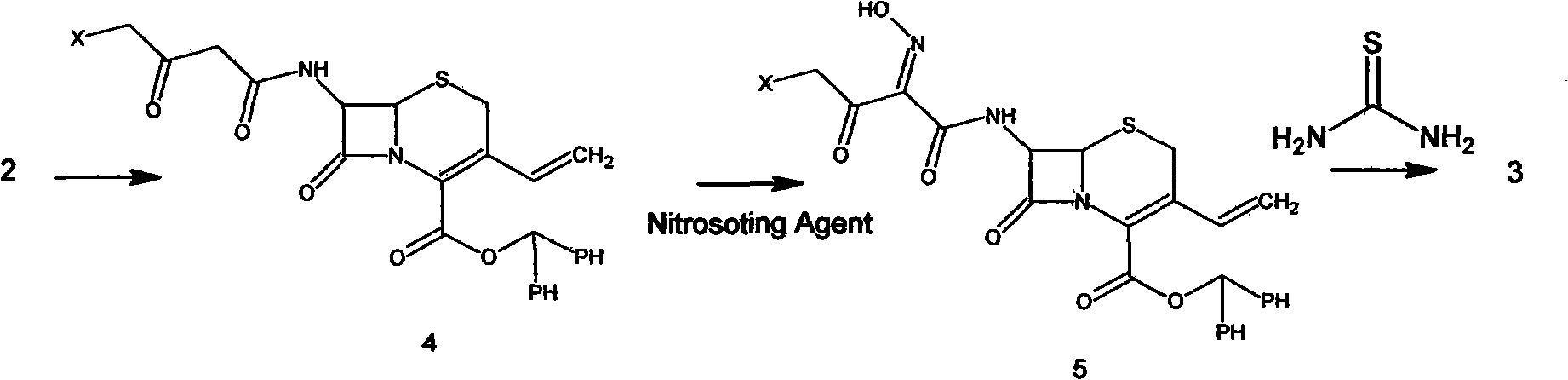
Process for preparation of cefdinir
InactiveUS6093814ALow priceManufacturing timeAntibacterial agentsOrganic active ingredientsP-Toluenesulfonic acidCefdinir
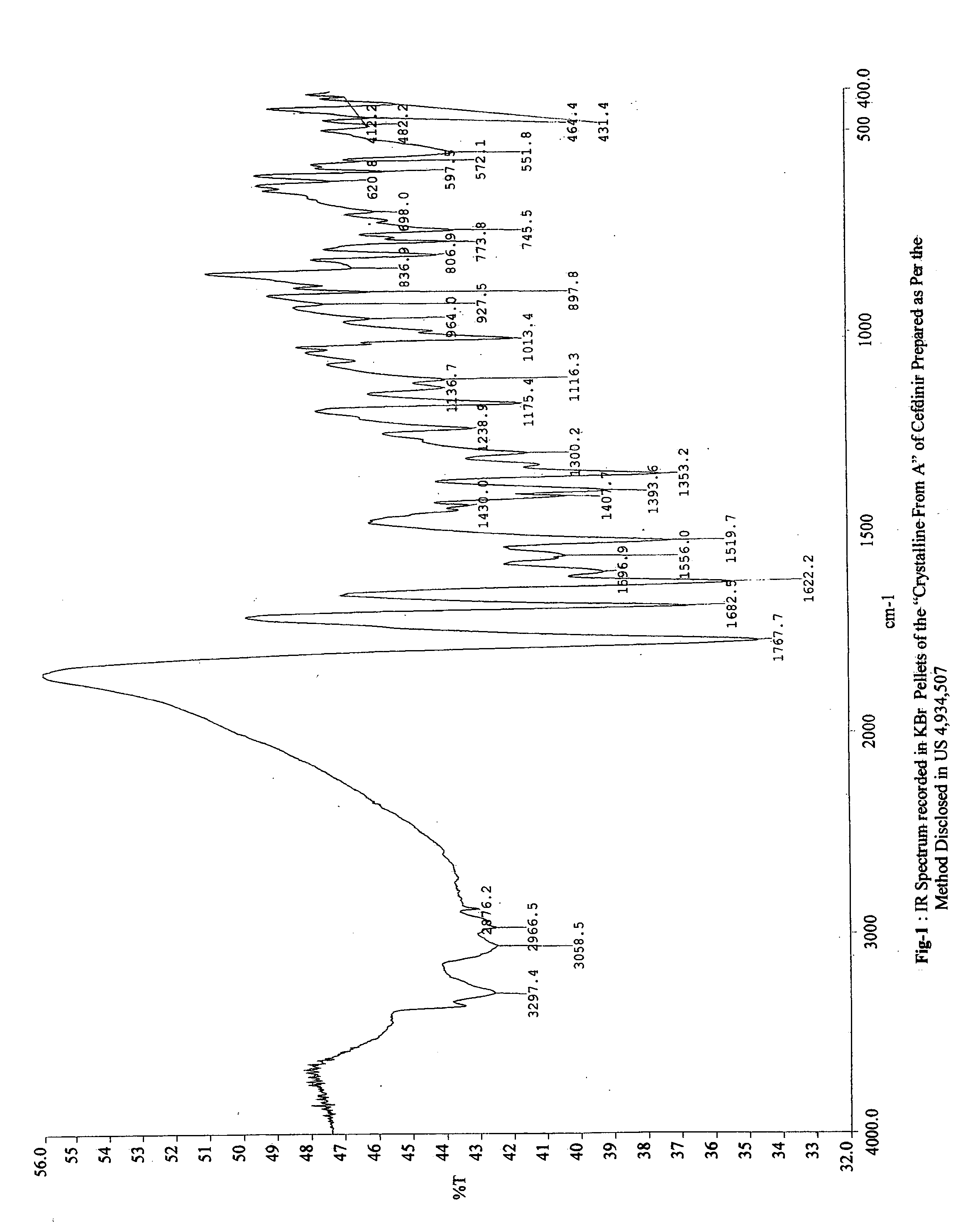
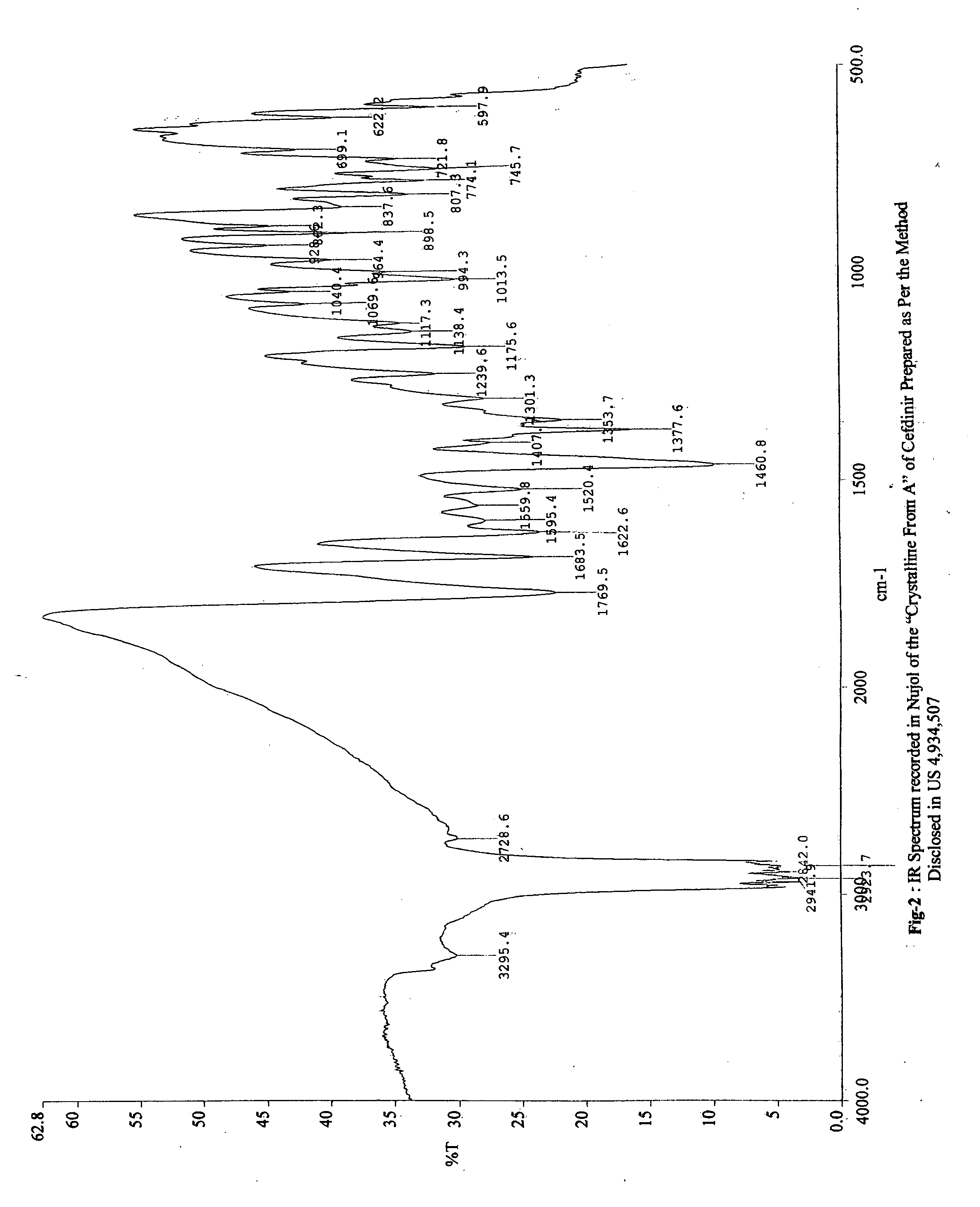
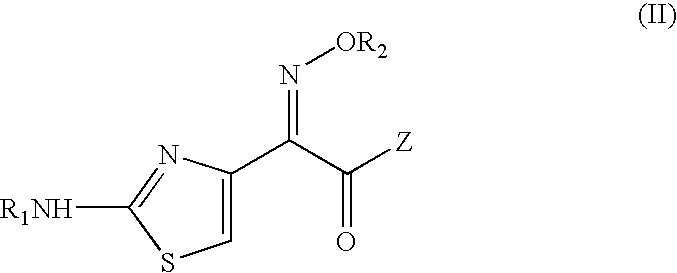
PCT No. PCT / KR96 / 00250 Sec. 371 Date May 18, 1998 Sec. 102(e) Date May 18, 1998 PCT Filed Dec. 26, 1996 PCT Pub. No. WO97 / 24358 PCT Pub. Date Jul. 10, 1997The present invention relates to a novel crystalline cefdinir intermediate having formula (II) which can be used very usefully for preparing a cephalosporin antibiotics, cefdinir, in which Ph represents phenyl, p-TsOH represents p-toluenesulfonic acid, and DMAC represents N,N-dimethylacetamide, to a process for preparation thereof and to a process for preparing cefdinir using the compound of formula (II). According to the present invention, cefdinir can be prepared in an excellent color and purity and with a good yield.
Owner:HANMI SCI CO LTD
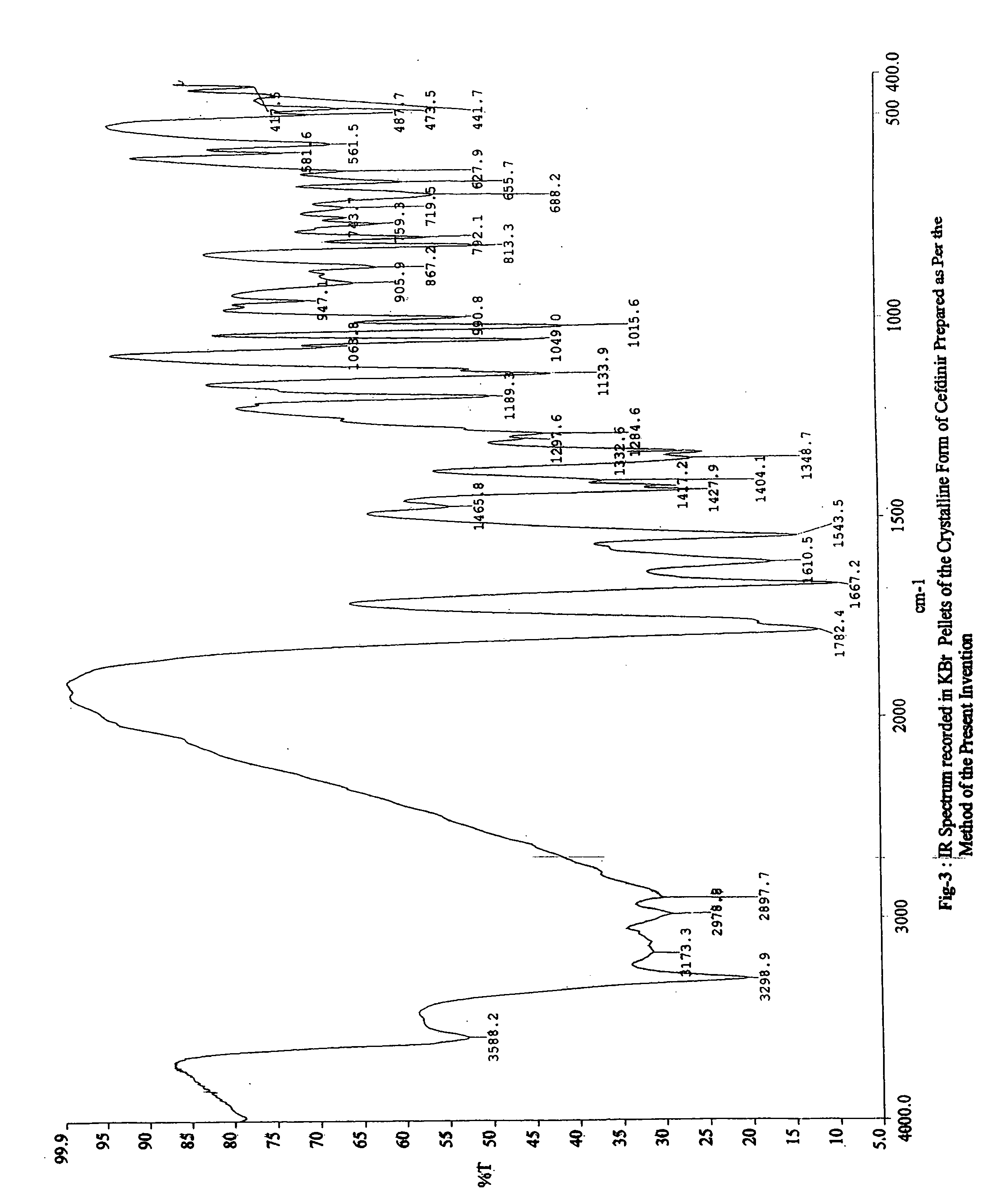
Stable bioavailable crystalline form or cefdinir and a process for the preparation thereof
InactiveUS20050245738A1Reduce usageHighly bioavailableOrganic active ingredientsOrganic chemistryAntibiotic YCefdinir
The present invention relates to a stable and bioavailable crystalline form of a third generation cephalosporin antibiotic, cefdinir and a process for the preparation thereof. The present invention also relates to a pharmaceutical composition containing the novel crystalline cefdinir, useful in the treatment of maladies such as bacterial infections.
Owner:LUPIN LTD
Cephalosporin suspension granule and preparation method thereof
ActiveCN101816635AGranularityAvoid damageAntibacterial agentsOrganic active ingredientsFluidized bed dryingAdditive ingredient
The invention discloses a cephalosporin suspension granule of which the formula composition comprises drug active ingredients, excipient, suspending agent, disintegrating agent, flavoring agent, coloring agent, stabilizing agent, adhesive and spice. The granule takes cefixime, cefprozil, cefaclor or cefdinir as the main drug ingredient, disintegrating agent and suspending agent with a certain quantity are added into the formula to ensure that the effective ingredients are dissolved out, and the solution has favorable suspending effect. Meanwhile, the invention also discloses a preparation method of the cephalosporin suspension granule, which adopts the wet granulation method to prepare granules and adopts the fluidized bed drying method to dry. The invention adopts the fluidized bed drying technology, so that compared with the traditional oven drying, the invention has the advantages of short drying time, small influence on active matter quality and the like, not only ensures product quality but also improves production efficiency. The granule prepared by the formula of the invention has solid granularity and lowers damage to the granule by fluidized drying.
Owner:广东恒健制药有限公司
Preparation method of cefdinir
ActiveCN101565427AEasy to recycleReduce pollutionAntibacterial agentsOrganic chemistryOrganic baseCarboxylic acid
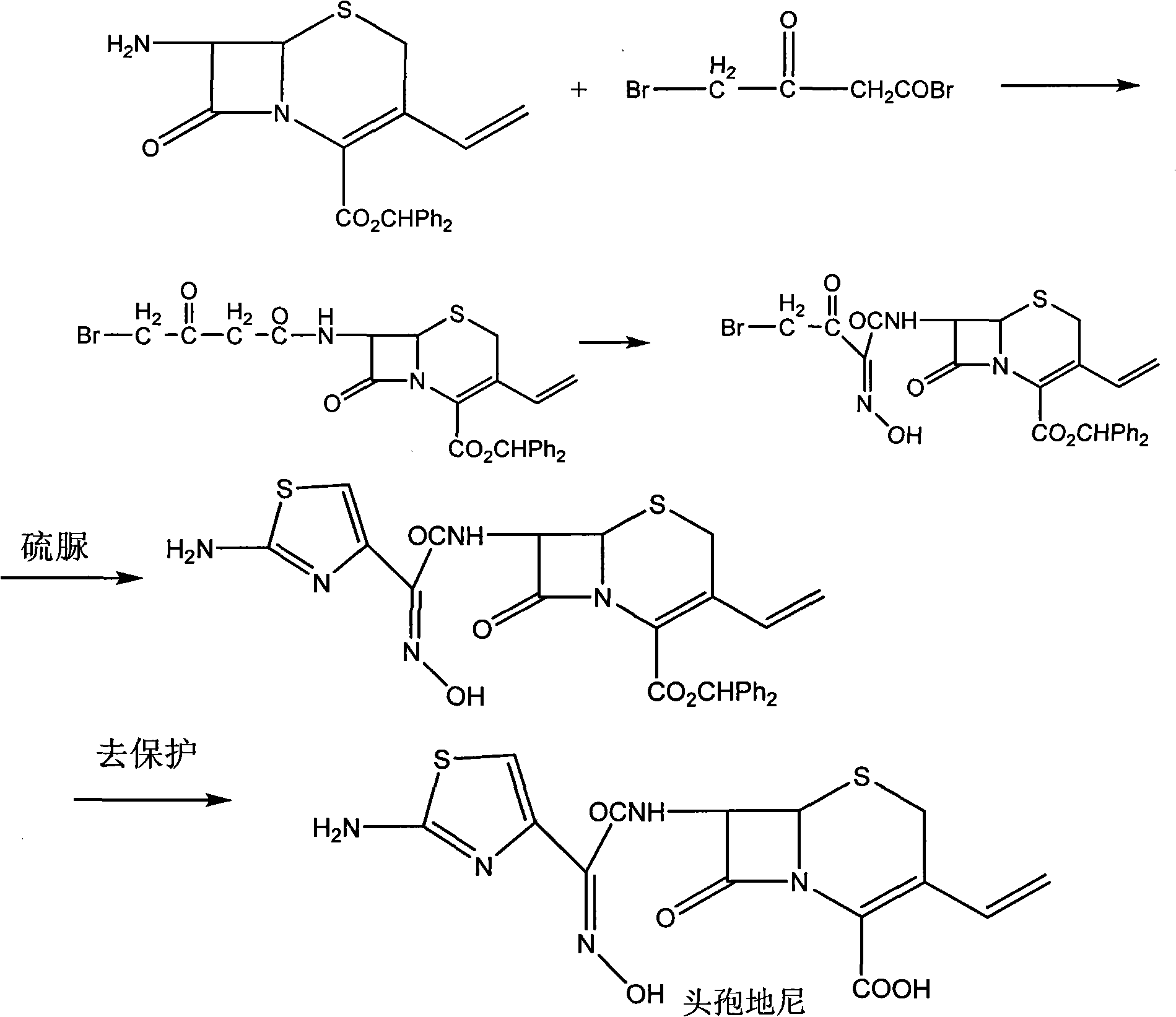
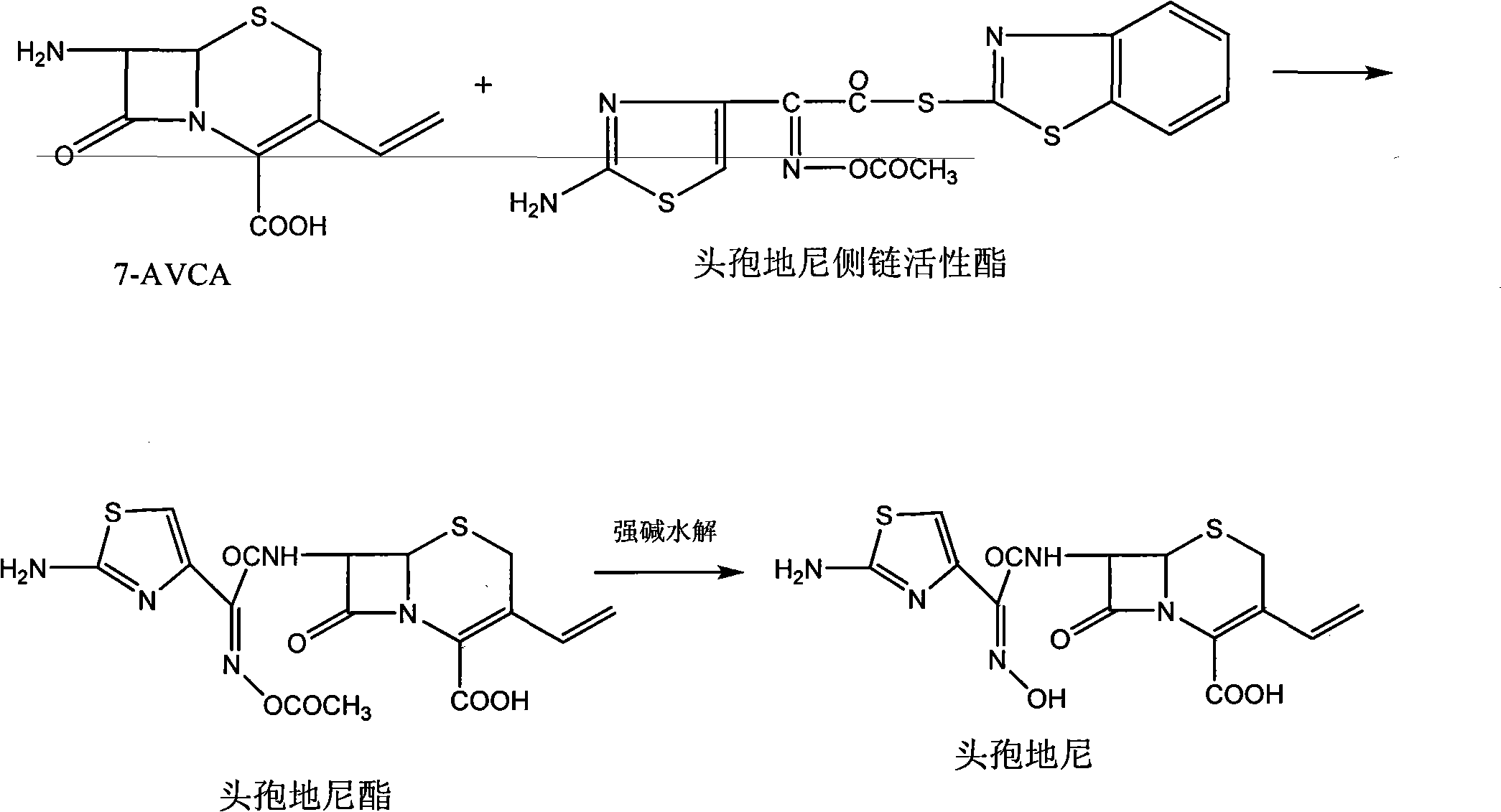
The invention relates to a preparation method of cefdinir, comprising the following steps: reacting 7-amino-3-vinyl-8-oxy-5-thia-1- nitric heterocyclic dicyclo[4.2.0]octyl-2-en-2-carboxylic acid with (Z)-2-(2-aminothiazole-4-yl)-2-acetoxy imino thioacetic acid (S-2-benzothiazole)ester in the presence of organic base at low temperature; extracting, adjusting p H value, preparing the intermediate of the cefdinir, removing the ester-group protective group of the intermediate of the cefdinir to obtain the cefdinir. The preparation method uses the low-temperature reaction technique, capable of increasing the reaction yield and reducing the impurities generated by the high temperature reaction. The hydrolysis and crystallization process is very easily controlled. The used alcohols, ketones or esters solvent is easily recovered, thus the production cost and the three-wastes drain are reduced, therefore the pollution to the environment is reduced. The preparation method of cefdinir is suitable for large-scale production.
Owner:ZHEJIANG ANGLIKANG PHARMA
Novel intermediates for synthesis of cephalosporins and process for preparation of such intermediates
InactiveUS20060135761A1Easily hydrolysableSulfuric acid esters preparationBulk chemical productionCefmenoximeAntibiotic Y
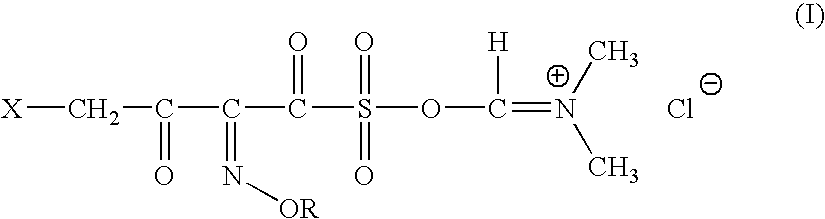
A novel 4-halo-2-oxyimino-3-oxo butyric acid-N,N-dimethyl formiminium chloride chlorosulfate of formula (I) useful in the preparation of cephalosporin antibiotics wherein X is chlorine or bromine; R is hydrogen, C1-4 alkyl group, an easily removable hydroxyl protective group, —CH2COOR5, or —C(CH3)2COOR5, wherein R5 is hydrogen or an easily hydrolysable ester group. The compound of formula (I) is prepared by reacting 4-halo-2-oxyimino-3-oxobutyric acid of formula (IV1), wherein X, R and R5 are as defined above, with N,N-dimethylformiminium chloride chlorosulphate of formula (VII) in an organic solvent at a temperature ranging from −30° C. to −15° C. The cephalosporins that may be prepared from the intermediate include cefdinir, cefditoren pivoxil, cefepime, cefetamet pivoxil, cefixime, cefmenoxime, cefodizime, cefoselis, cefotaxime, cefpirome, cefpodoxime proxetil, cefquinome, ceftazidime, cefteram pivoxil, ceftiofur, ceftizoxime, ceftriaxone and cefuzonam.
Owner:LUPIN LTD
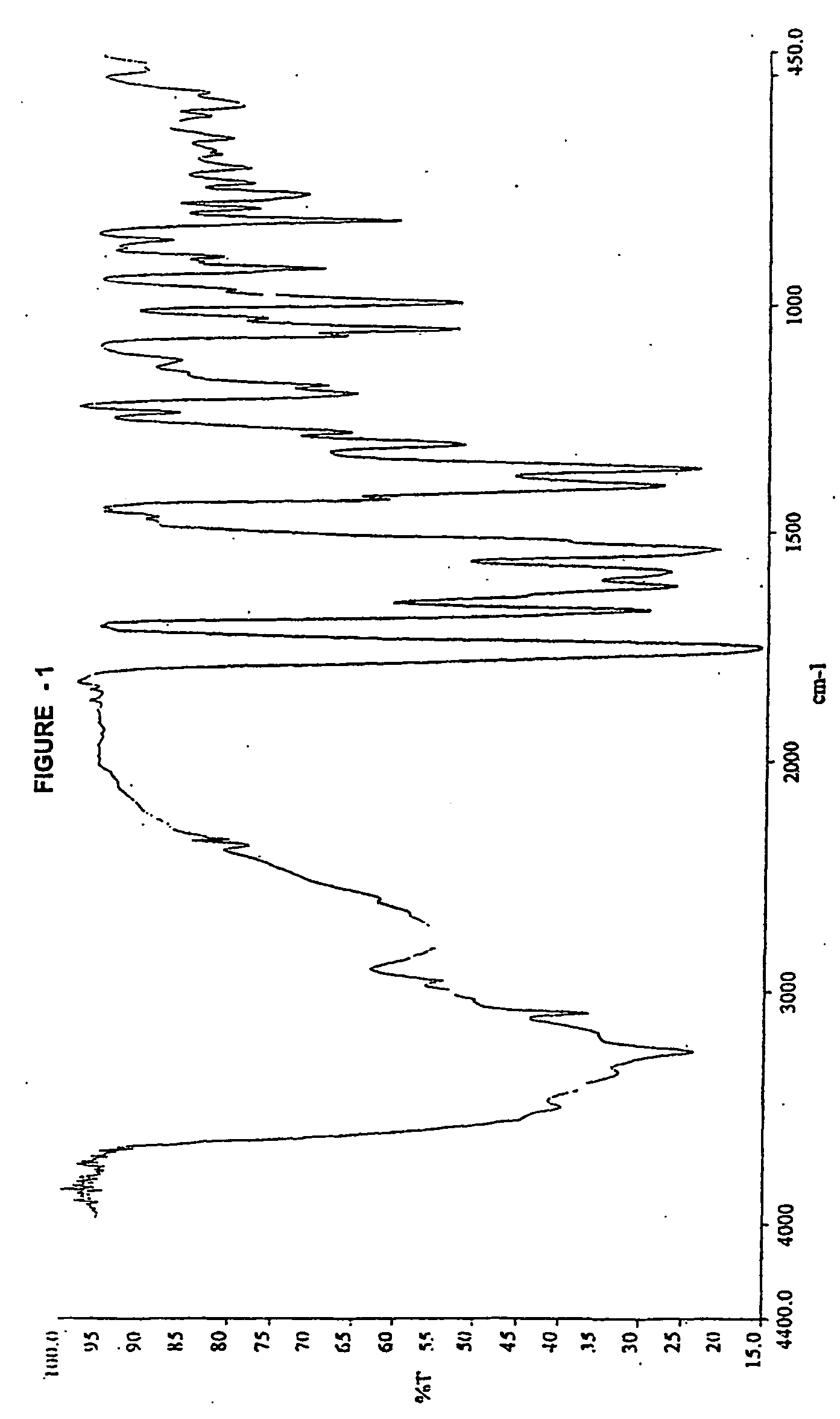
Novel amorphous hydrate of a cephalosporin antibiotic
InactiveUS20060094703A1Improve bioavailabilityUseful for developmentOrganic active ingredientsOrganic chemistryOrganic solventCarboxylic acid
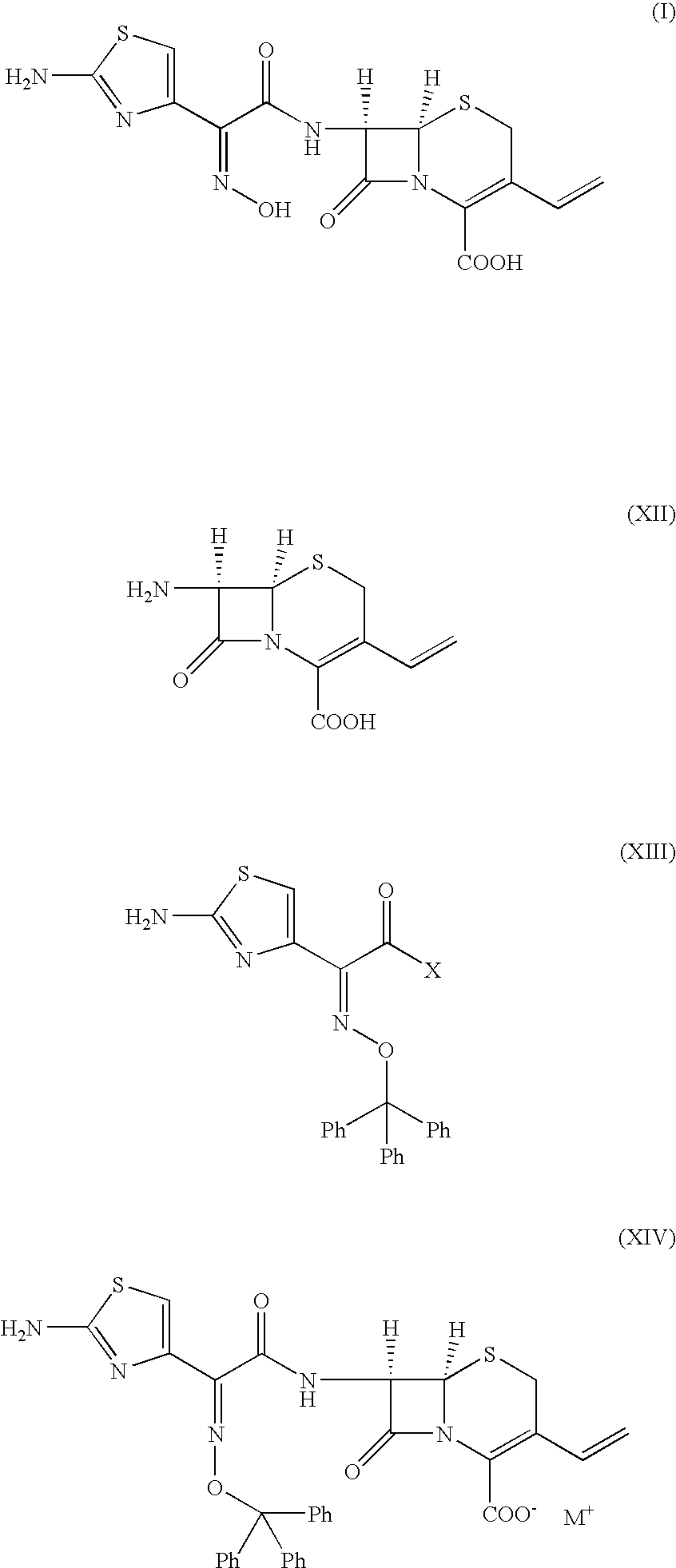
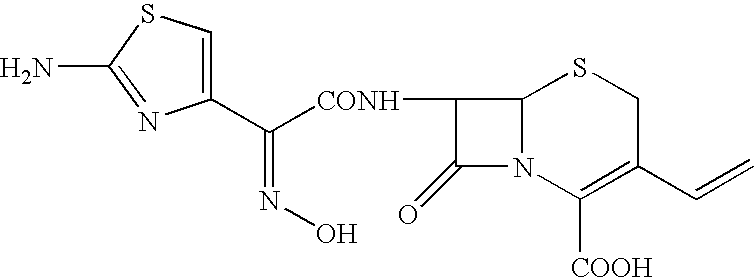
A process for the preparation of cefdinir of the formula (I) the said process comprising the steps of: i) condensing 7-amino-3-cephem-4-carboxylic acid of the formula (XII) wherein R1 is as defined above with compound of the formula (XIII) in the presence of a tertiary amine and an organic solvent, followed by treatment with a base to produce a salt of compound formula (XIV), wherein M+ is a counter ion and ii) hydrolyzing the compound of the formula (XIV) using an acid in the presence of a solvent to produce cefdinir of formula (I).
Owner:ORCHID CHEM & PHARM LTD
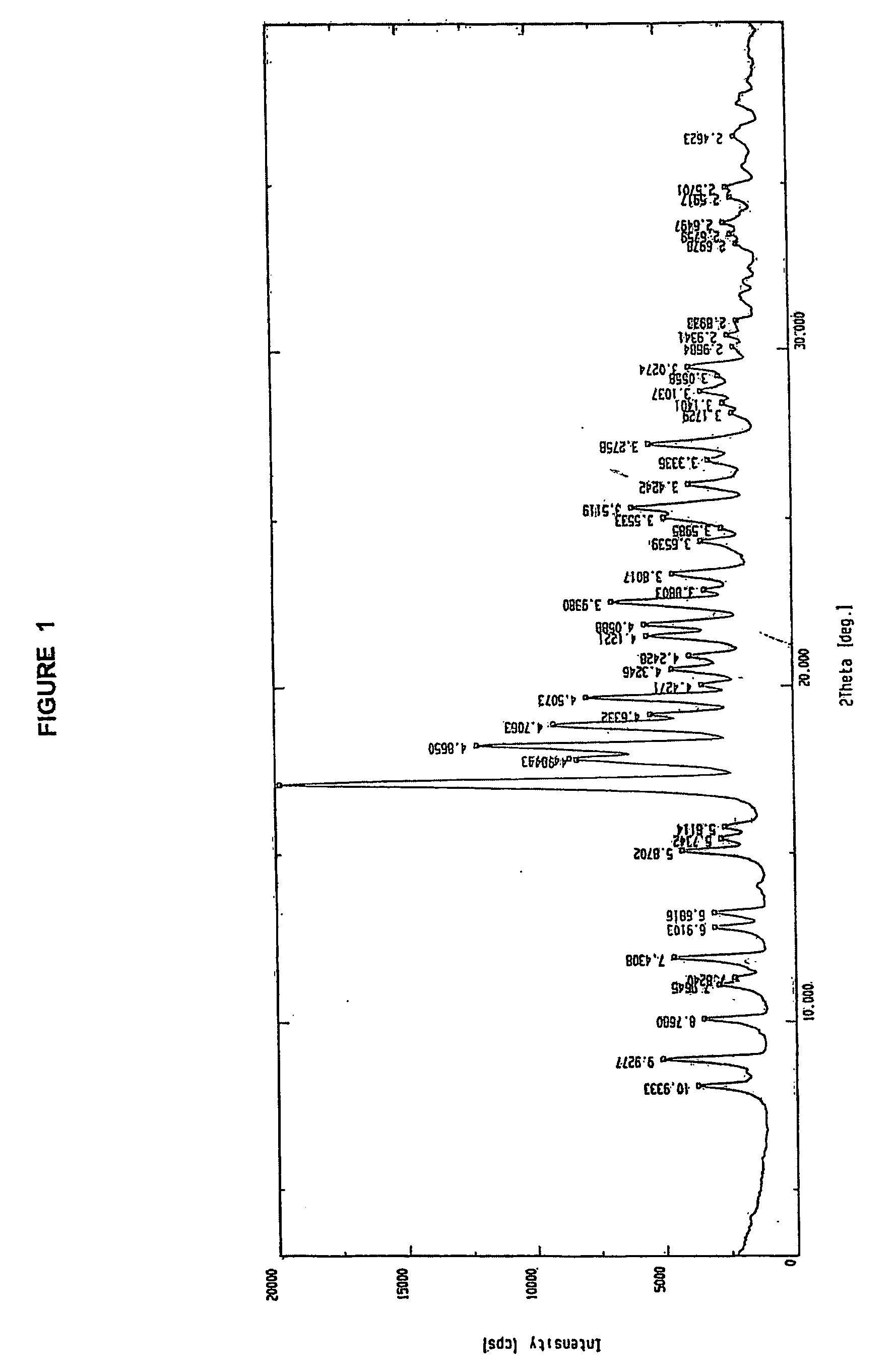
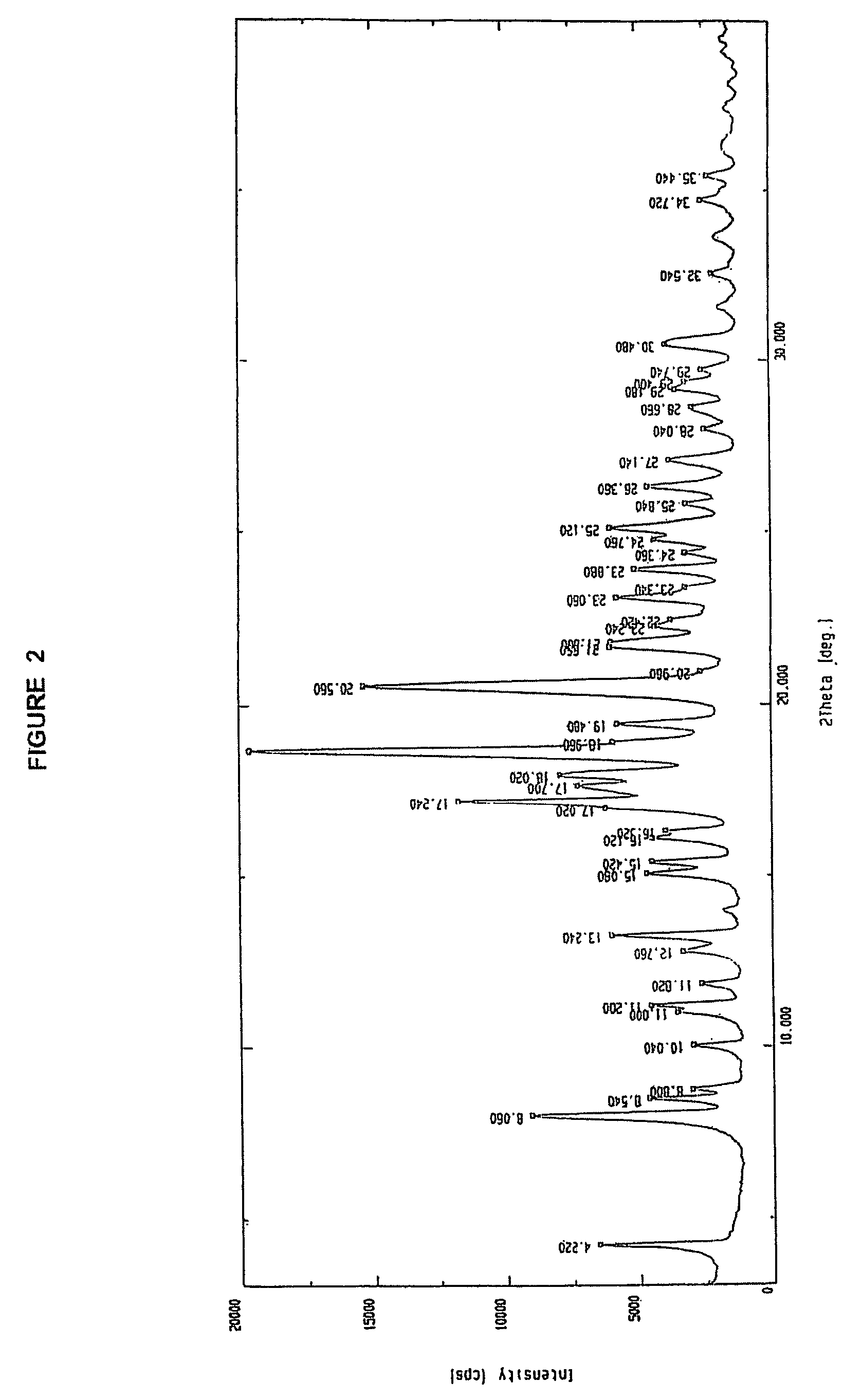
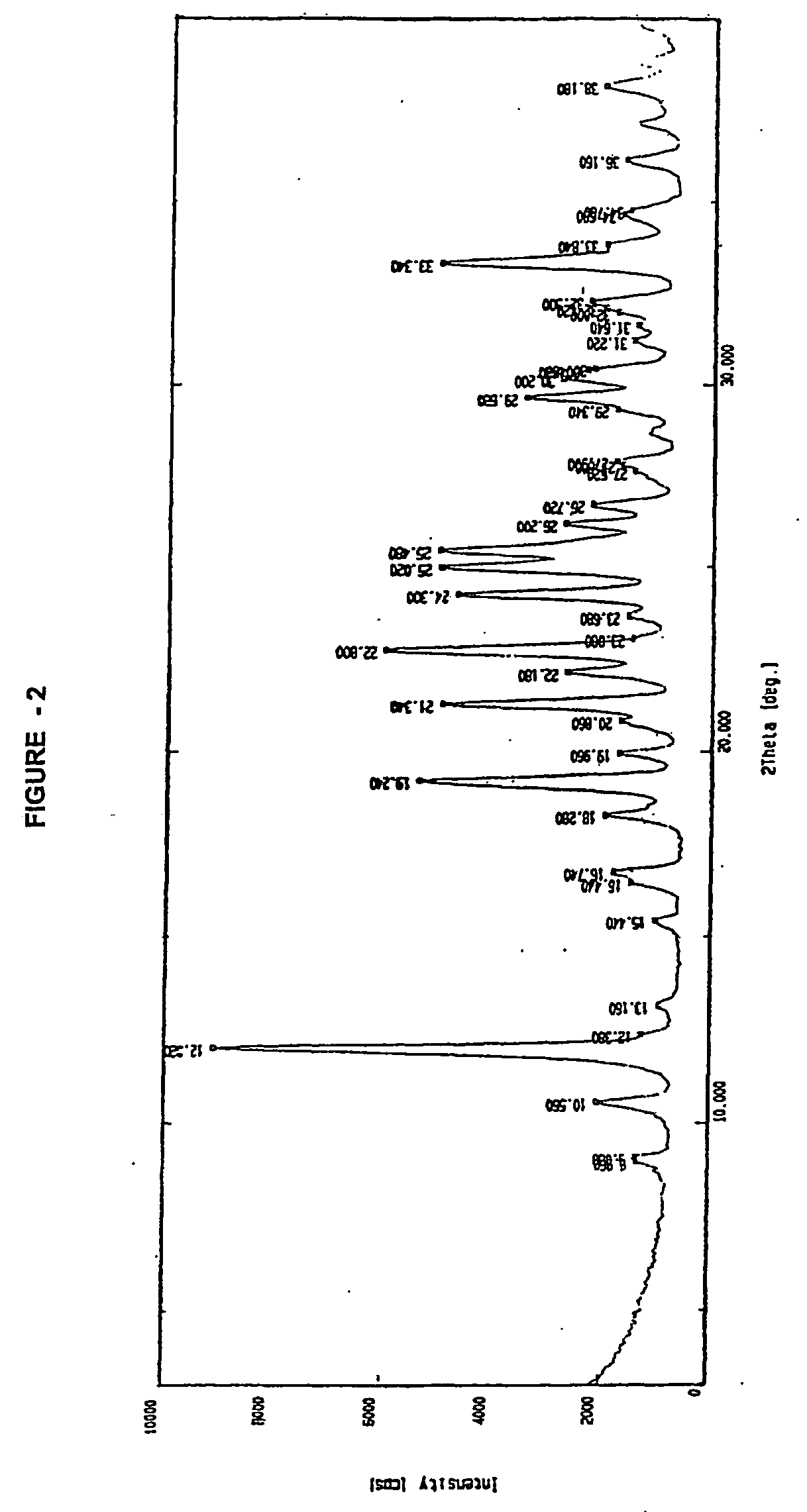
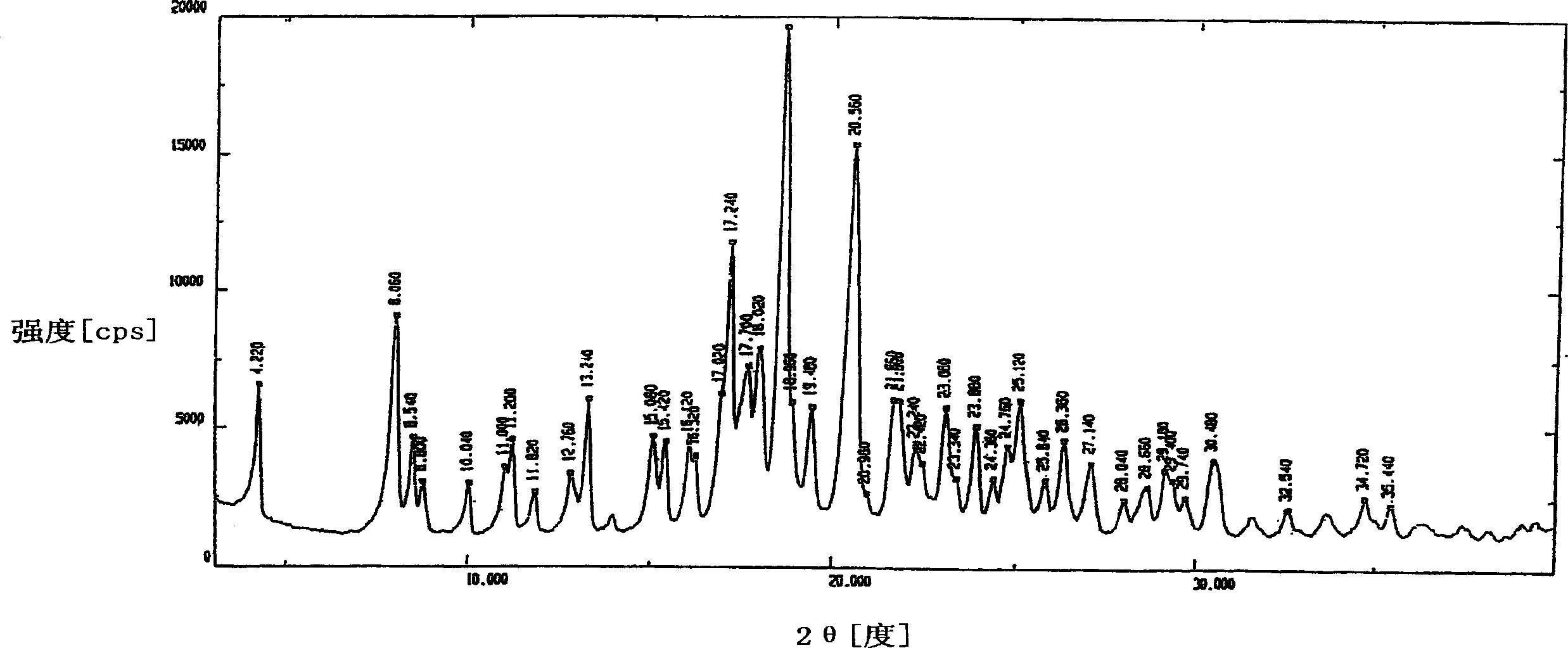
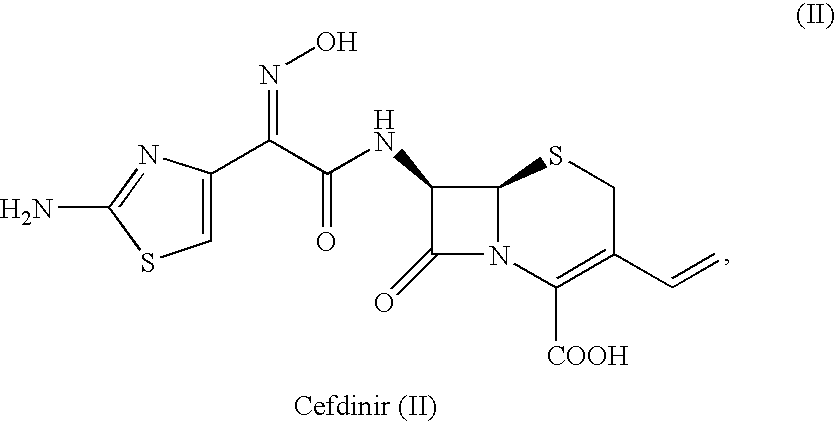
Novel polymorph of cefdinir
InactiveUS20050215781A1Improve stabilityUseful for developmentOrganic chemistryCefdinirMedicinal chemistry
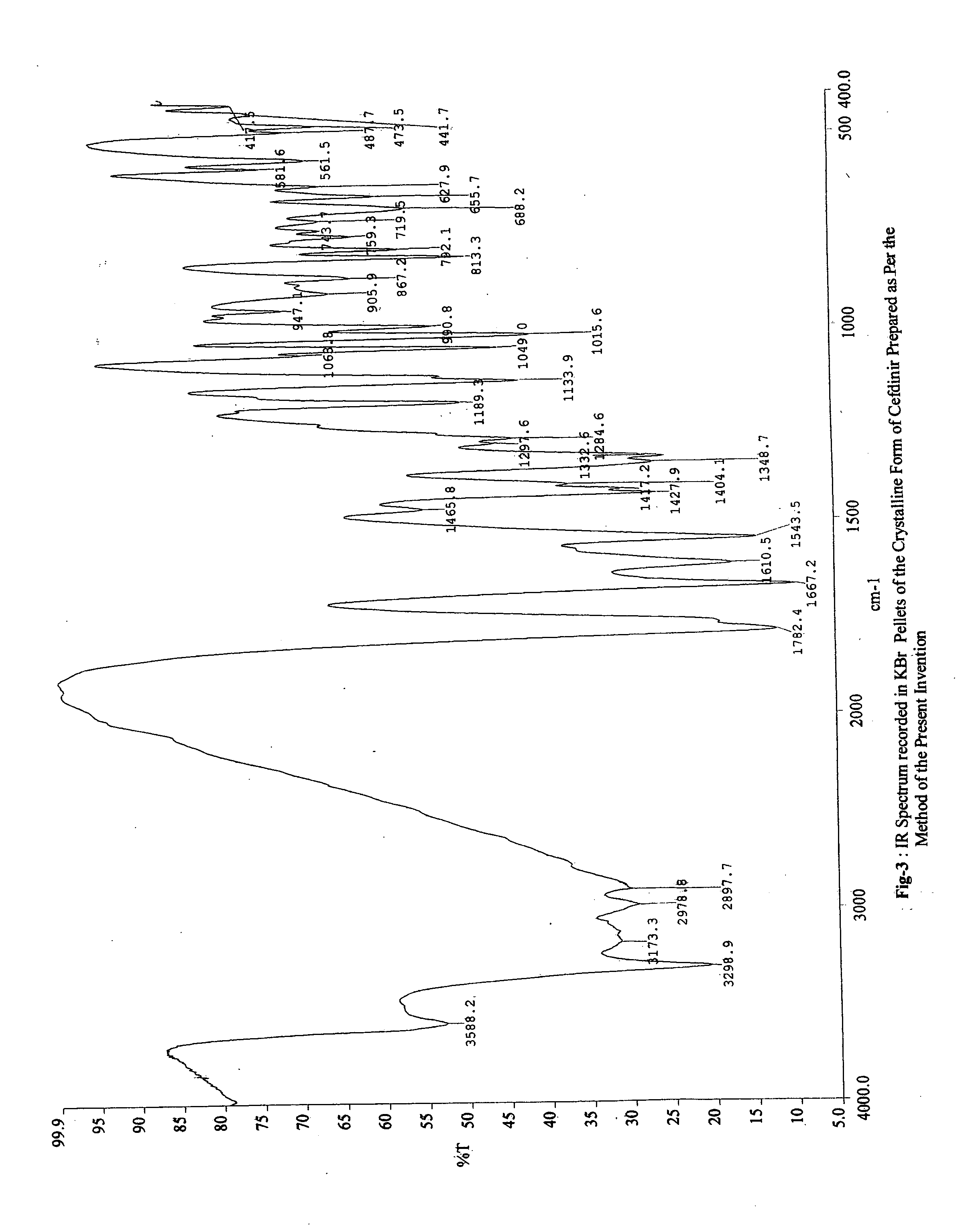
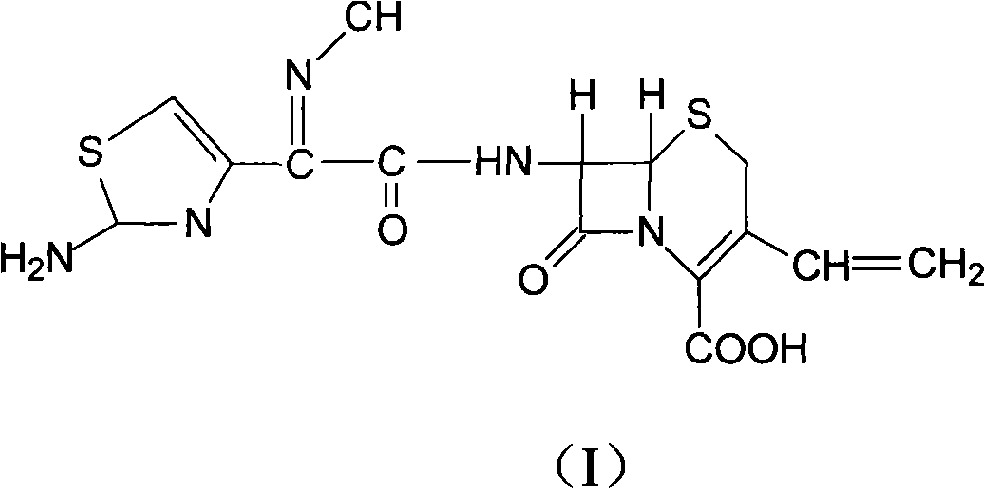
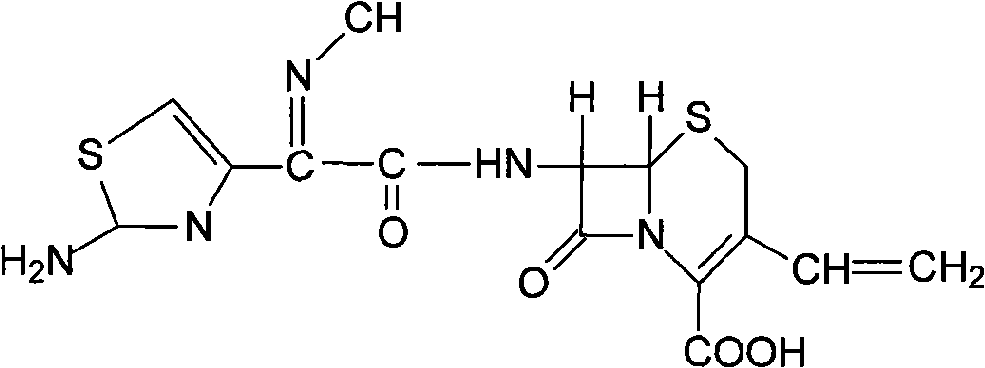
The present invention relates to novel polymorph of cefdinir represented by formula (I). More particularly, the present invention relates to novel crystalline form (Crystal D) of cefdinir. The present invention also provides a process for the preparation of novel crystalline form (Crystal D) of cefdinir.
Owner:ORCHID CHEM & PHARM LTD
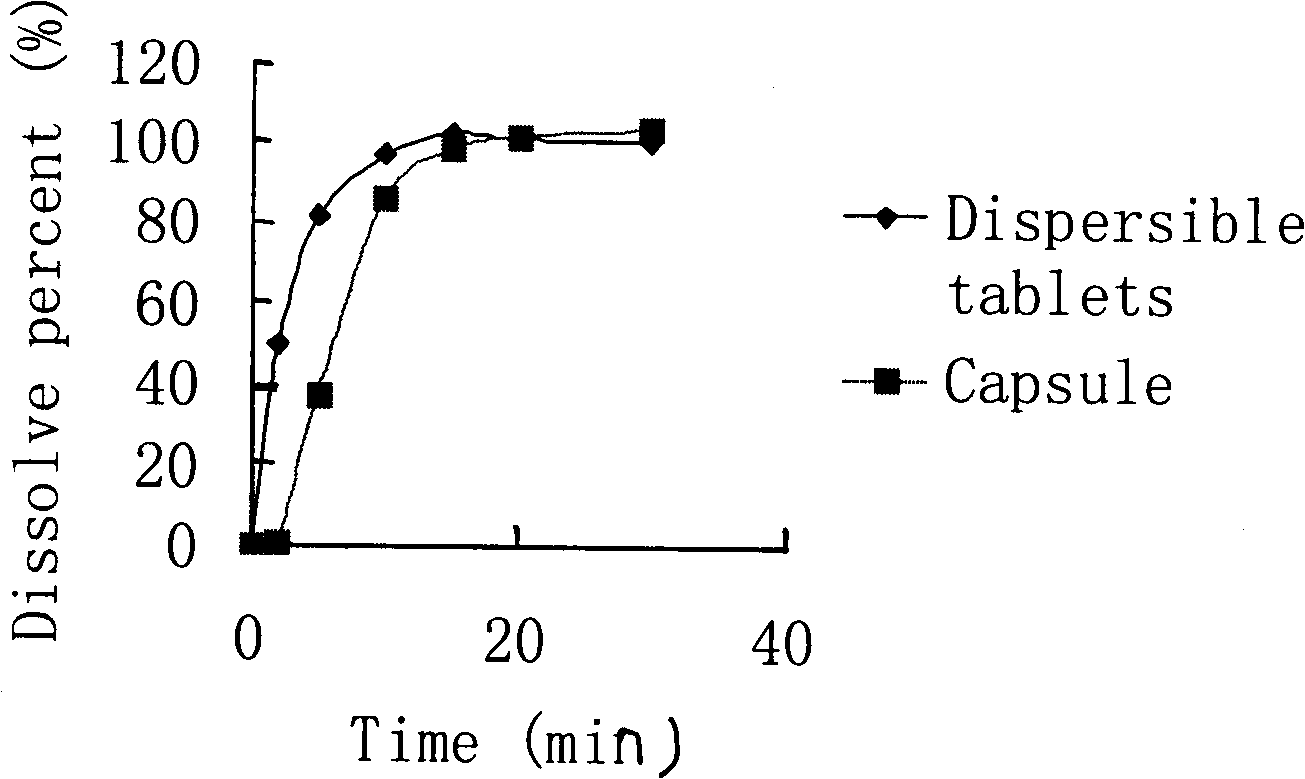
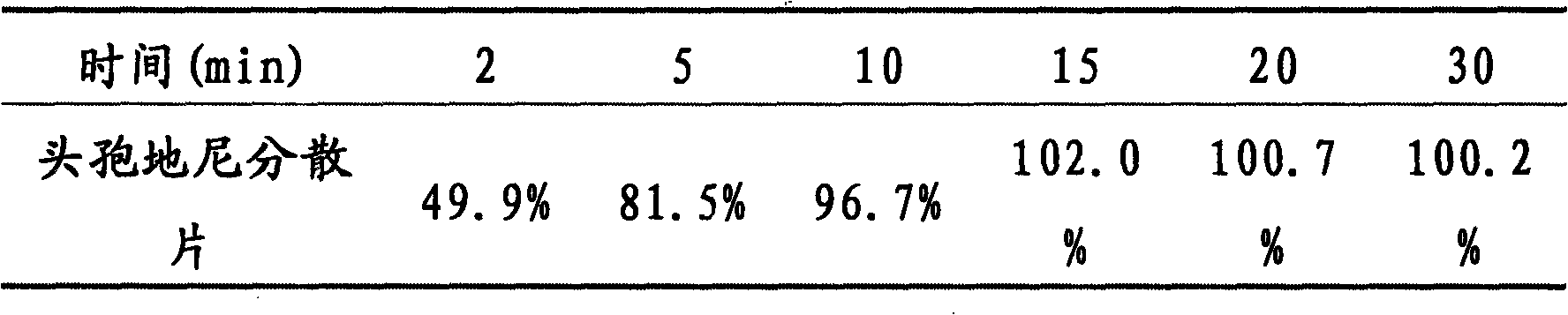
Cefdinir dispersible tablet and preparation method thereof
InactiveCN101352424AImprove solubilityLarge distribution areaAntibacterial agentsOrganic active ingredientsCefdinirPharmaceutic Adjuvant
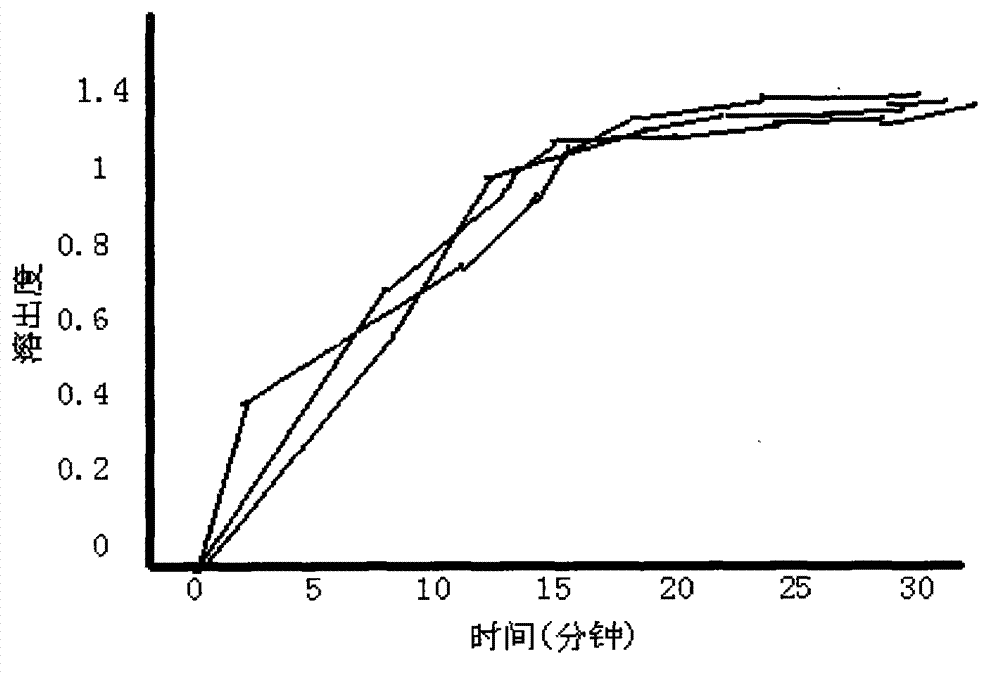
The invention provides a cefdinir dispersible tablet and a preparation method thereof, and the cefdinir dispersible tablet contains cefdinir with effective dose and pharmaceutic adjuvant which includes disintegrant and disintegrant-promoting aerosol; in every 100 parts of cefdinir, the dosage of the disintegrant is 2-60 parts, and the dosage of the disintegrant-promoting aerosol is 0.1-45 parts; after being taken orally, the cefdinir dispersible tablet of the invention can quickly disintegrate, and the cefdinir which is the active ingredient of the dispersible tablet has the accumulating dissolution rate of over 102.0% within 15 minutes. Compared with other medicines, the accumulating dissolution rate of cefdinir capsule is 98.3%. The cefdinir dispersible tablet are evenly dispersed into fine particles, so as to have remarkable disintegration and leachability capacity compared with the compressed tablets, realize the rapid absorption of medicine, the function of rapid onset, and improve the bioavailability of human body.
Owner:TIANJIN CENT PHARM CO LTD
Process for the preparation of cefdinir
InactiveUS20060040915A1High yieldHigh purityOrganic active ingredientsOrganic chemistryCefdinirOrganic chemistry
Owner:RANBAXY LAB LTD
Crystalline cefdinir potassium dihydrate
InactiveUS20050080255A1Simple and efficient processEasy to prepareAntibacterial agentsOrganic active ingredientsPotassiumCefdinir
Owner:SEKISUI CHEM CO LTD +1
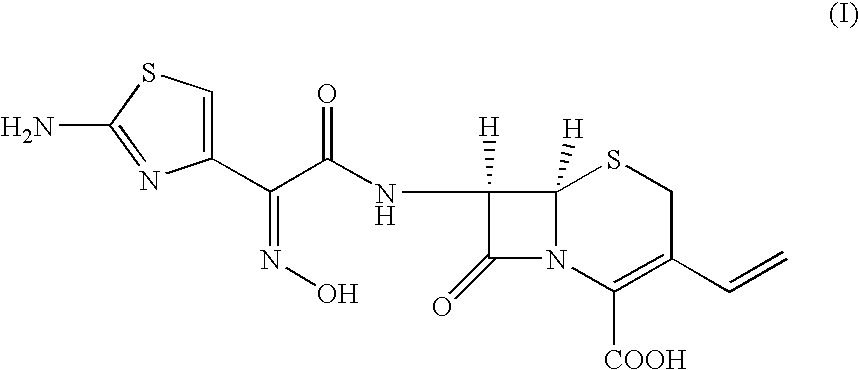
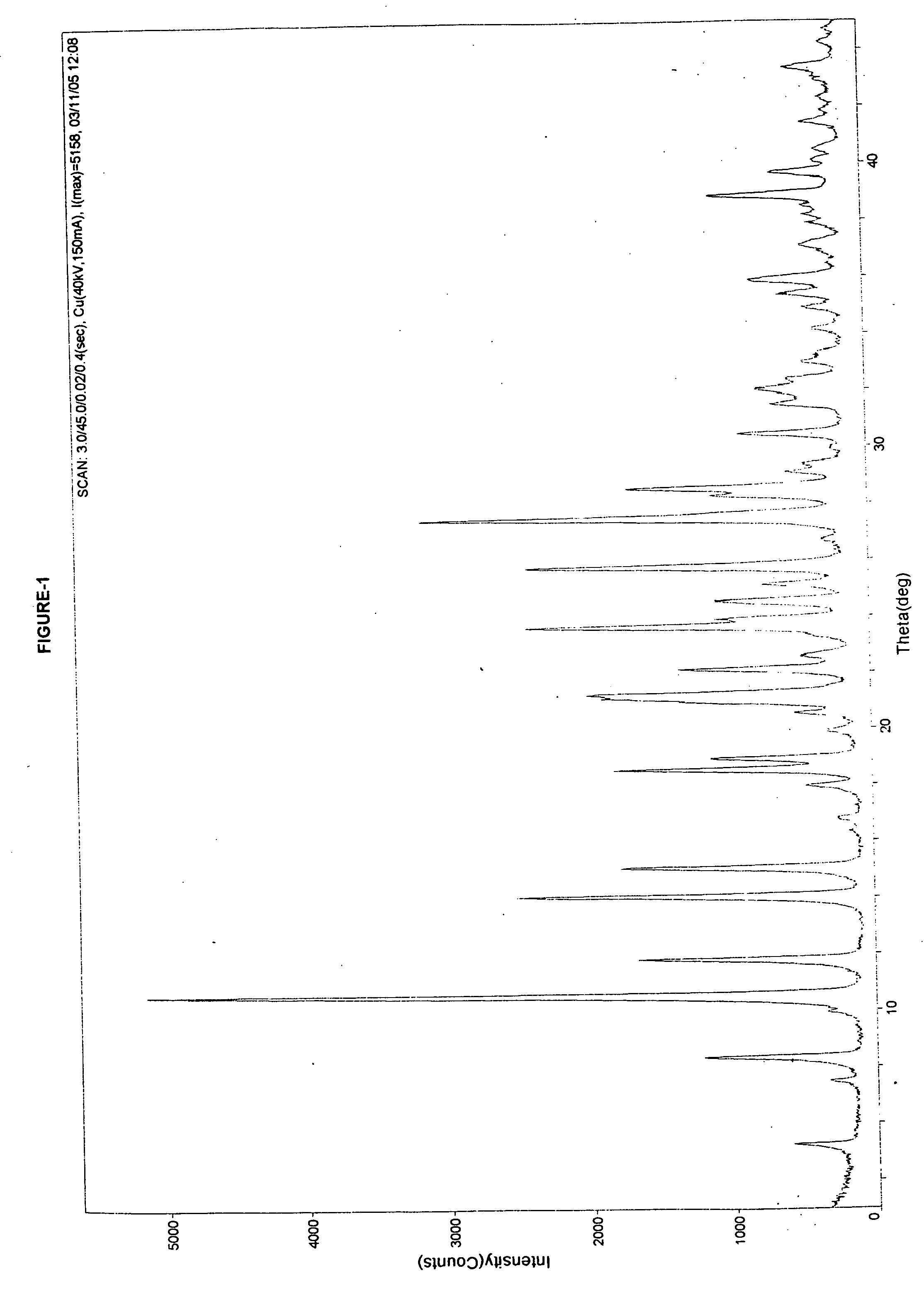
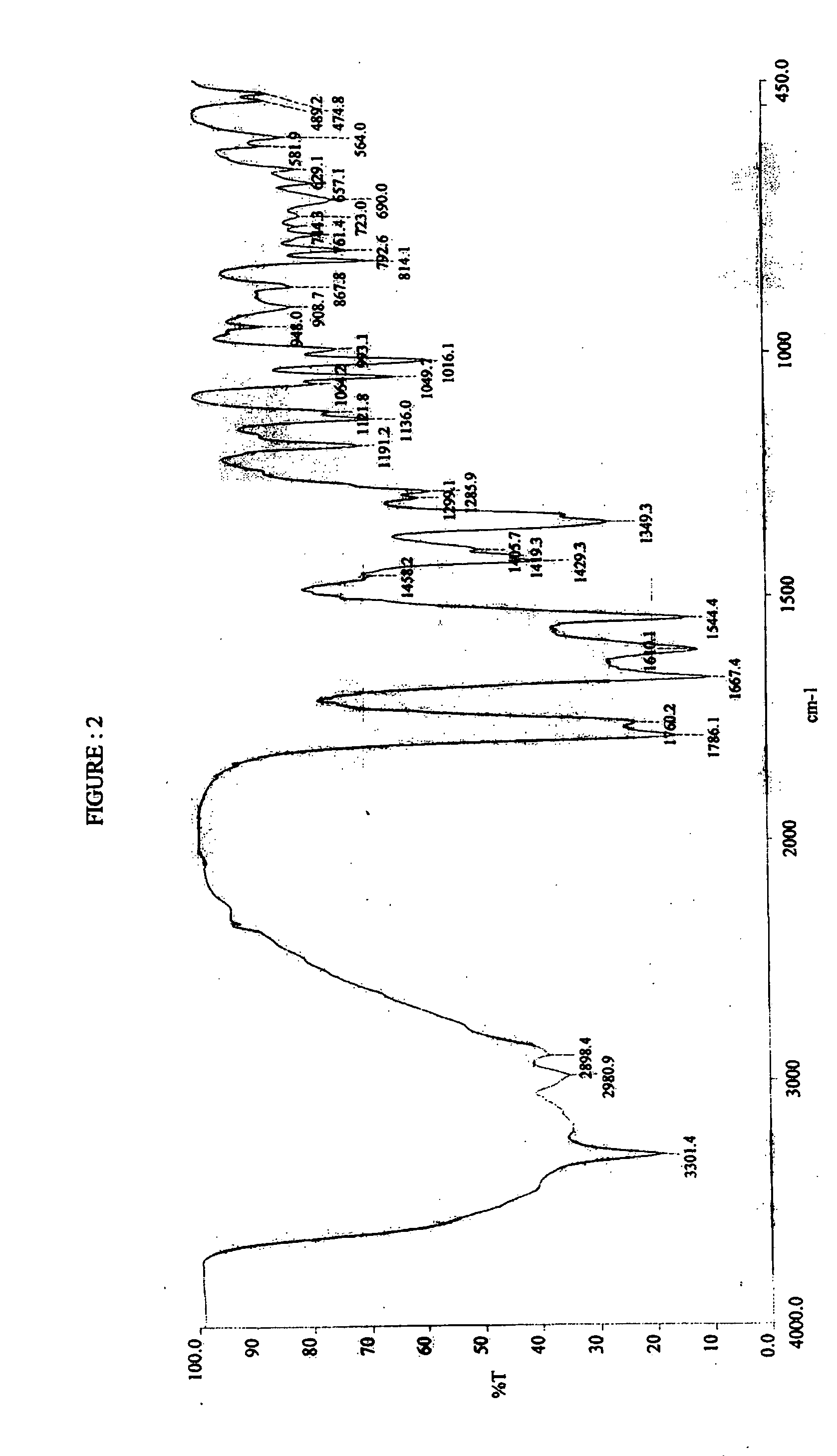
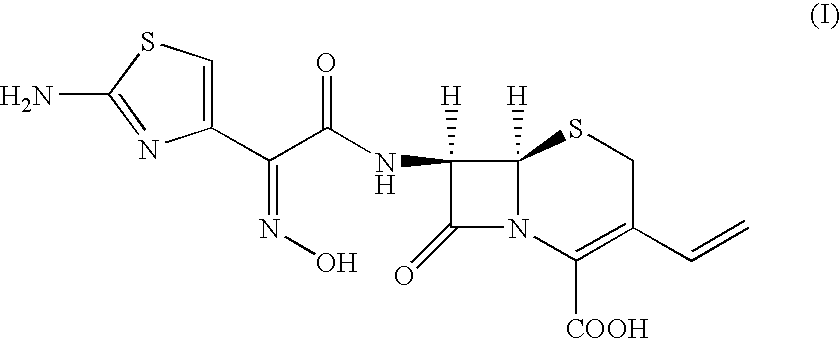
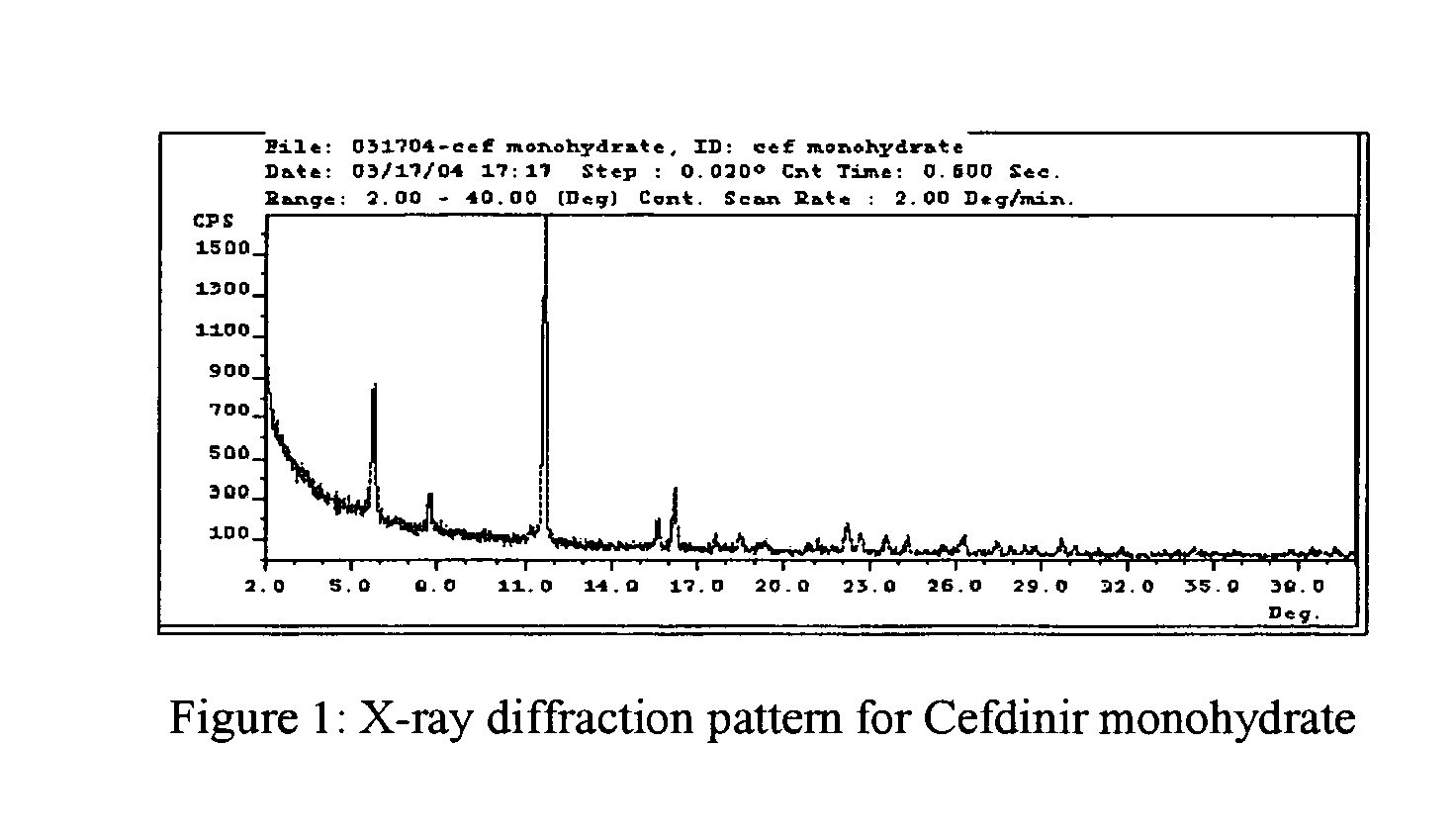
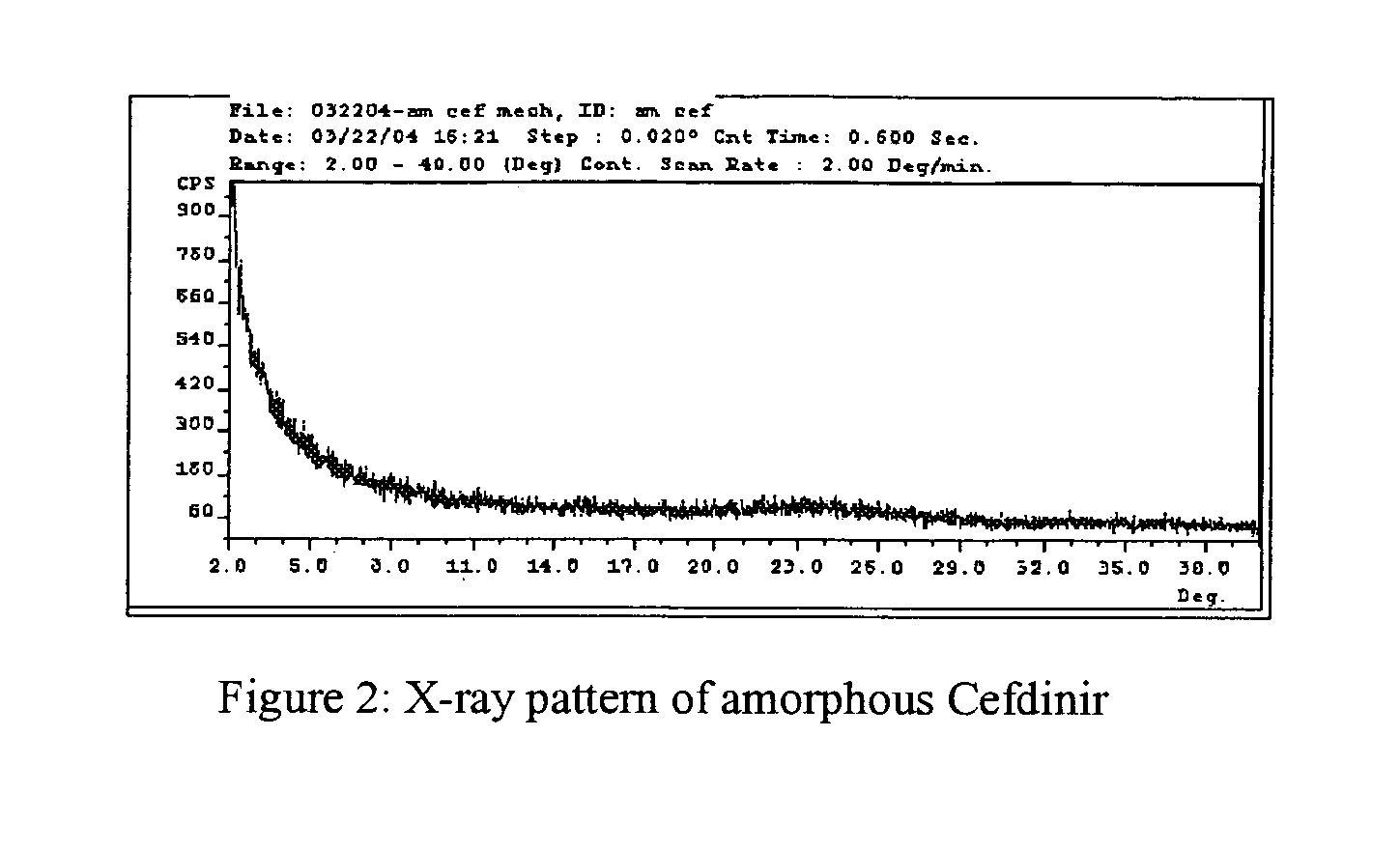
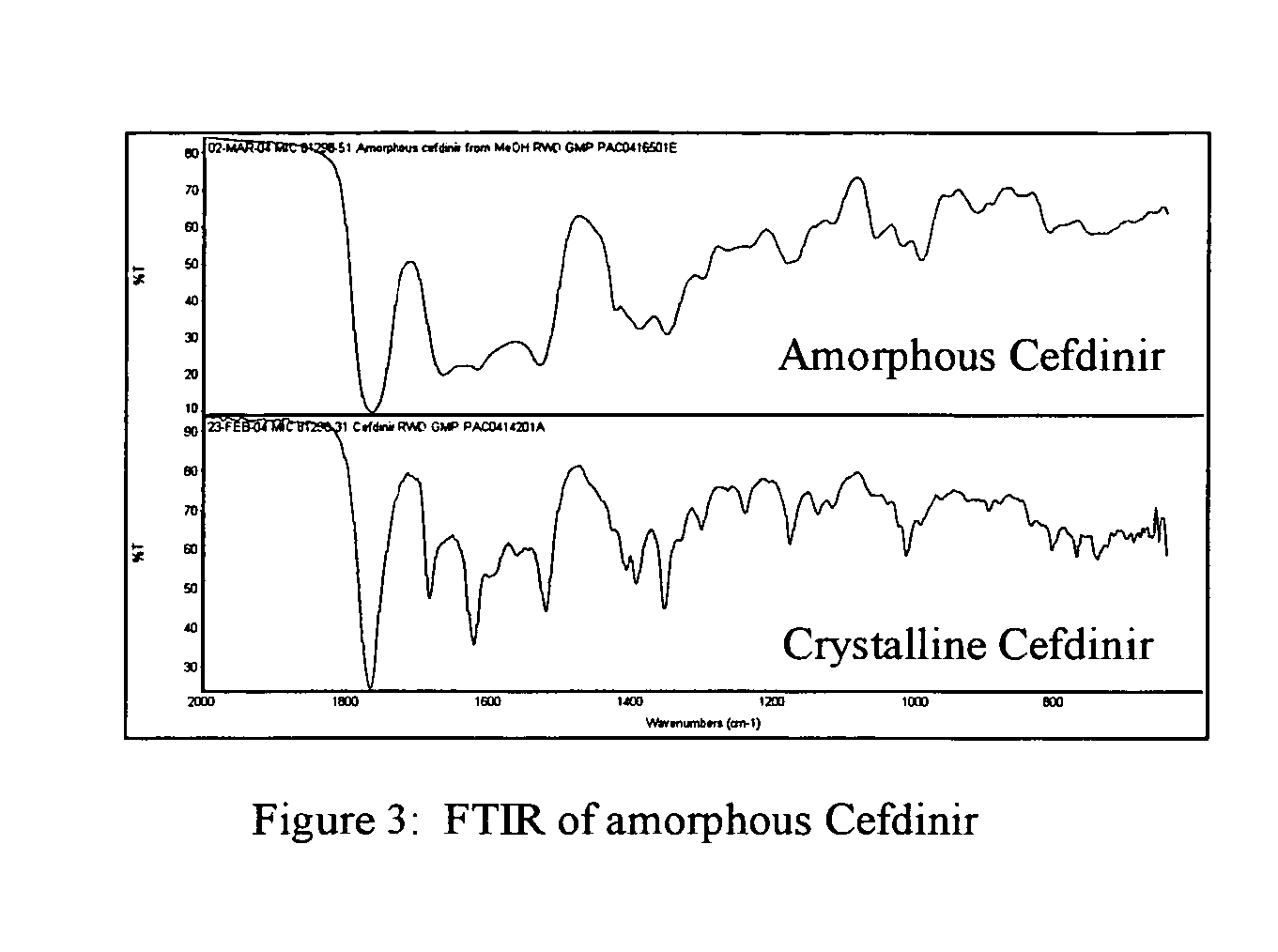
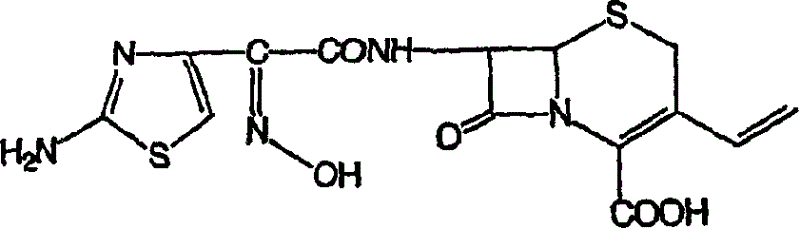
Stable amorphous Cefdinir
The present invention relates to stable amorphous 7-[2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamide]-3-vinyl-3-cephem-4-carboxylic acid (syn isomer), methods for its preparation, and pharmaceutical compositions comprising stable amorphous 7-[2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamide]-3-vinyl-3-cephem-4-carboxylic acid (syn isomer).
Owner:ABBOTT LAB INC
Stable amorphous cefdinir
The present invention relates to stable amorphous 7-[2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamide]-3-vinyl-3-cephem-4-carboxylic acid (syn isomer), methods for its preparation, and pharmaceutical compositions comprising stable amorphous 7-[2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamide]-3-vinyl-3-cephem-4-carboxylic acid (syn isomer).
Owner:ABBOTT LAB INC
Cefdinir capsule and preparation method thereof
ActiveCN102058561AGood curative effectEnhanced in vitro releaseAntibacterial agentsOrganic active ingredientsMedicineCurative effect
The invention relates to a Cefdinir capsule and a preparation method thereof. Each Cefdinir capsule contains equivalently 50-100mg of Cefdinir and also comprises a filling agent, a disintegrating agent, a solubilizer, a lubricating agent and a coating agent. The method of mixing and screening raw materials and hydrophilic excipients, granulating by adopting a material dry method and then coating with a moistureproof film coat is adopted in the invention, thus the drug in vitro release degree is improved, the activity of the Cefdinir is avoided from reducing in a high-moisture environment and the related substances of the Cefdinir are avoided from increasing, and the treatment effect, the stability and the safety of the drug are improved.
Owner:JIANGSU YABANG QIANGSHENG PHARMA
Intermediate cefdinir salts
InactiveUS20060111566A1High antibacterial activityHigh activityOrganic chemistryBulk chemical productionCefdinirOrganic chemistry
Disclosed are salts of the general formula (I) wherein R1, R2 and as defined in the description and a process for the preparation thereof. These salts are useful intermediates for the preparation of cefdinir.
Owner:ANTIBIOTICOS SPA
Cefdinir oral disintegration tablet, and its prepn. method
InactiveCN1723905AEasy to takeAntibacterial agentsOrganic active ingredientsOrally disintegrating tabletCefdinir
An oral disintegrating tablet of Toubaodini is prepared from Toubaodini, filler, disintegration agent, flouring agent, sweeten and appropriate. It features that it can be easily dissolved in saliva.
Owner:张志生
Effervescent cefdinir prepn and its prepn process
InactiveCN1706389ASlow onsetQuick effectAntibacterial agentsOrganic active ingredientsCefdinirOrganoleptic
The present invention relates to cefdinir as one kind of cephalosporin, and is especially effervescent cefdinir preparation and its preparation process. The present invention features that the effervescent cefdinir preparation consists of cefdinir 15-25 wt%; disintegrating agent including acid 13-23 wt% and alkali 25-35 wt%; and other components including stuffing 14-24 wt%, adhesive 1-5 wt%, moistener 1-5 wt%, lubricant 5-9 wt%, sweetener 6-10 wt% and aromatic 8-16 wt%. The effervescent cefdinir preparation has the advantages of easy taking, fast absorption in body, good taste, etc.
Owner:JINAN PINGZHI MEDICINE SCI TECH
Crystalline acid salts of cefdinir and process for preparing cefdinir using same
High purity cefdinir is prepared in a high yield by a process comprising the steps of: treating a cefdinir intermediate with a formic acid-sulfuric acid mixture or a formic acid-methanesulfonic acid mixture to obtain a crystalline salt of cefdinir and reacting the crystalline salt with a base in a solvent.
Owner:HANMI SCI CO LTD
Stable bioavailable crystalline form of cefdinir and a process for the preparation thereof
InactiveUS20060149056A1Simple, convenient, non-hazardous, environment friendly and cost-effectiveStable and bioavail ableOrganic active ingredientsOrganic chemistryAntibiotic YCefdinir
The present invention relates to a stable and bioavailable crystalline form of a third generation cephalosporin antibiotic, cefdinir and a process for the preparation thereof. The present invention also relates to a pharmaceutical composition containing the novel crystalline cefdinir, useful in the treatment of maladies such as bacterial infections.
Owner:LUPIN LTD
Process for preparing cefdinir
InactiveUS7105659B2Prevent overconsumptionMinimizes exothermicityOrganic active ingredientsOrganic chemistryTriphenylphosphineSolvent
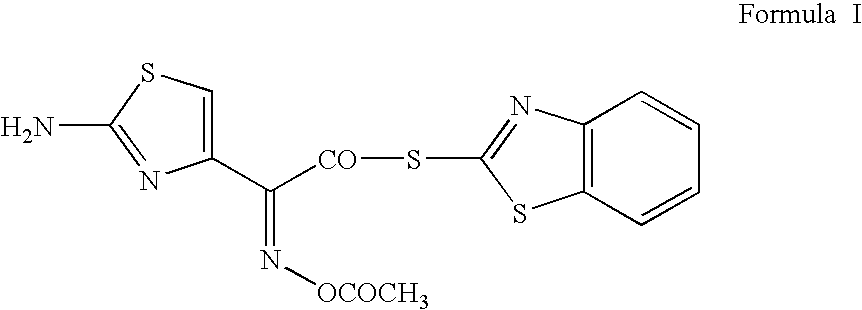
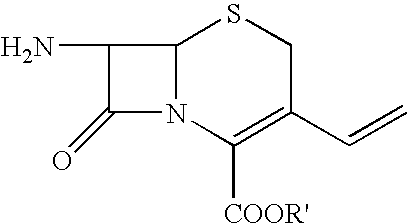
The present invention relates to a novel process for the preparation of a cefdinirby reacting O-acetyl thioester of Formula Iwithin the presence of a base in suitable solvent wherein R′ represents H or any carboxyl protecting group, and then converting to the cefdinir by the removal of protecting groups. This invention also relates to making the cefdinir using a novel process to prepare the O-acetyl thioester intermediate (Formula I) by condensing (Z)-2-(2-amino-4-thiazolyl)-2-acetyloxyiminoacetic acid with bis(benzothiazol-2-yl)disulphide in the presence of triphenylphosphine and a base in a suitable solvent.
Owner:AUROBINDO PHARMA LTD
Medicinal composition containing cefdinir cyclodextrin inclusion compound and preparation thereof
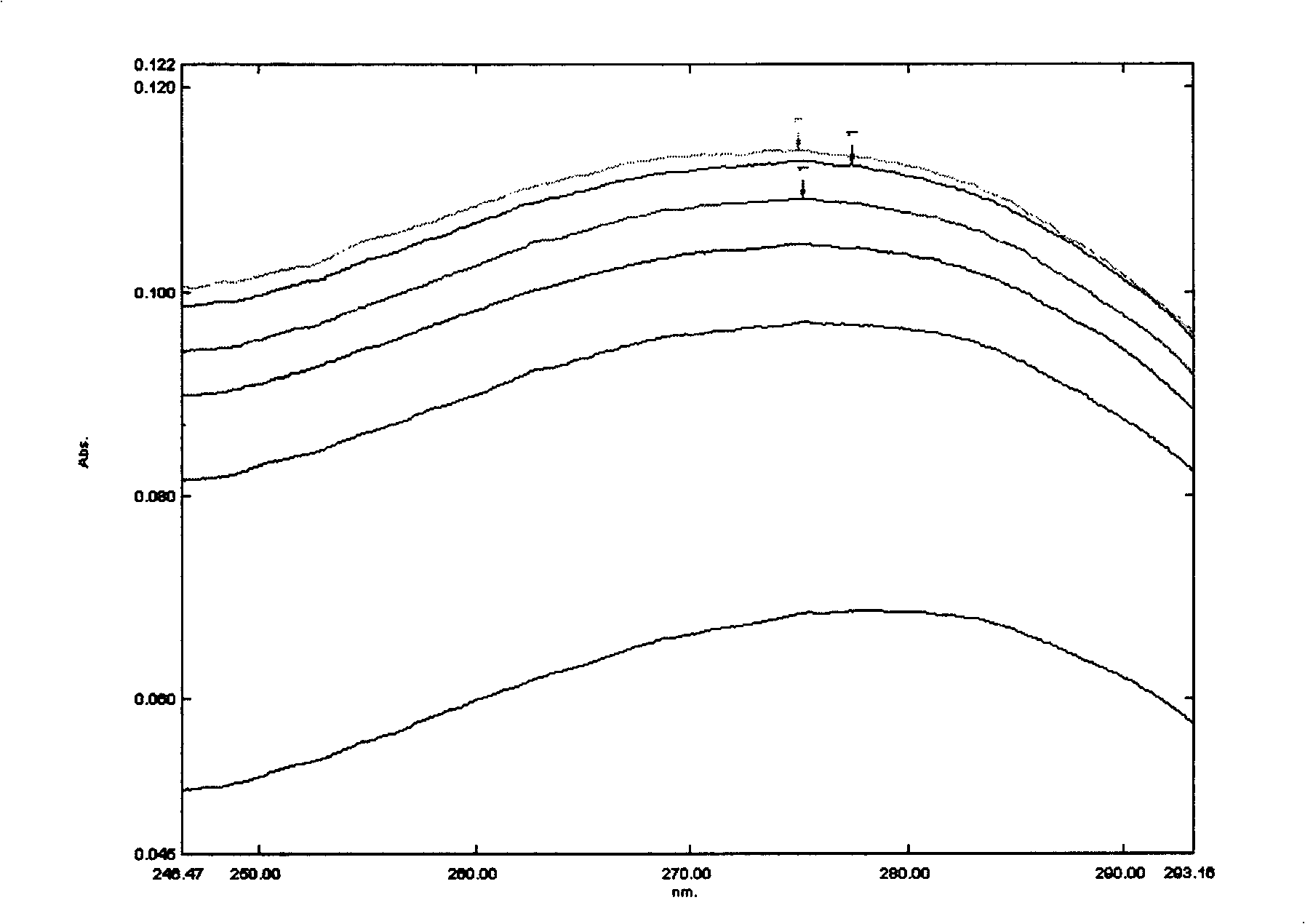
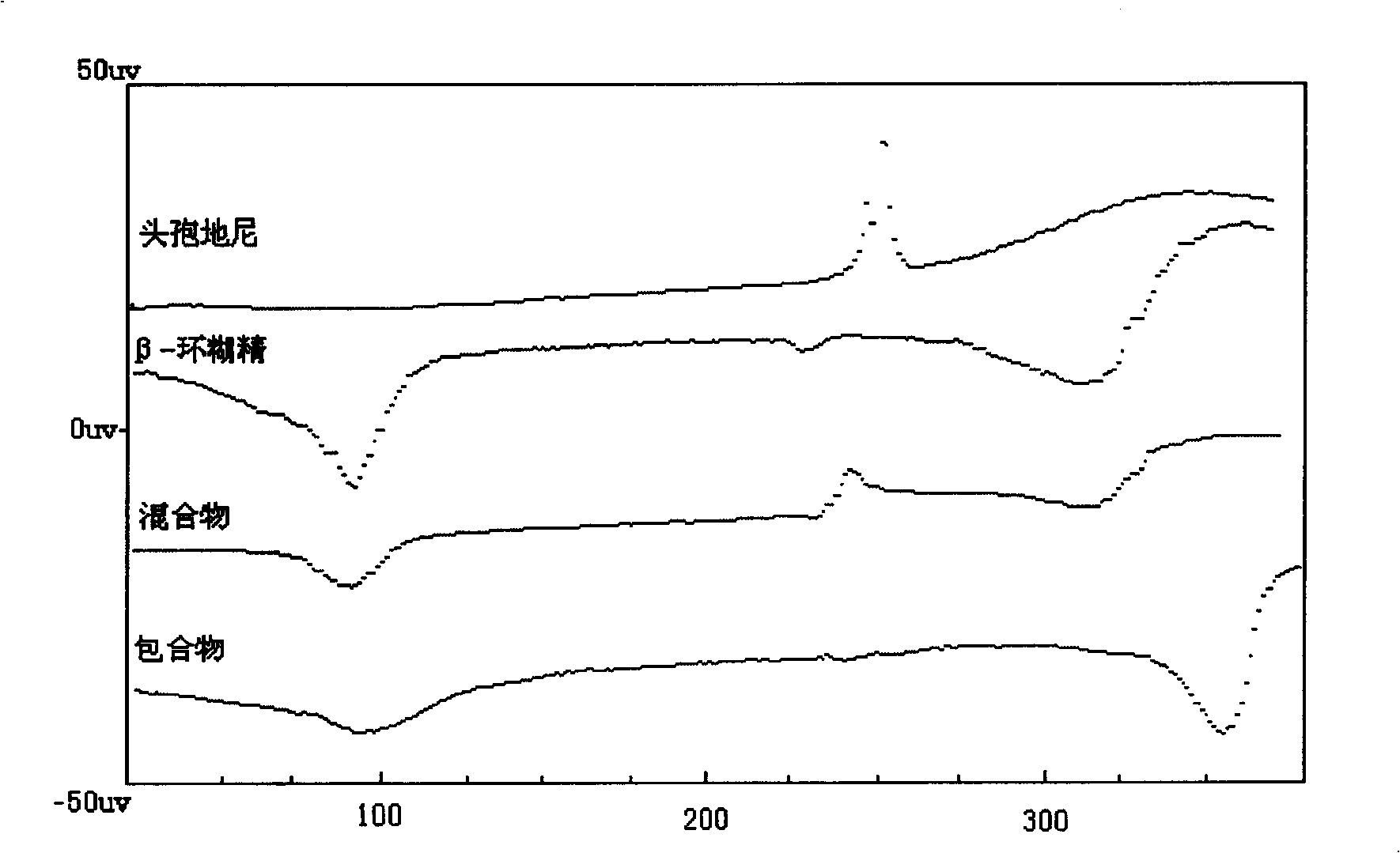
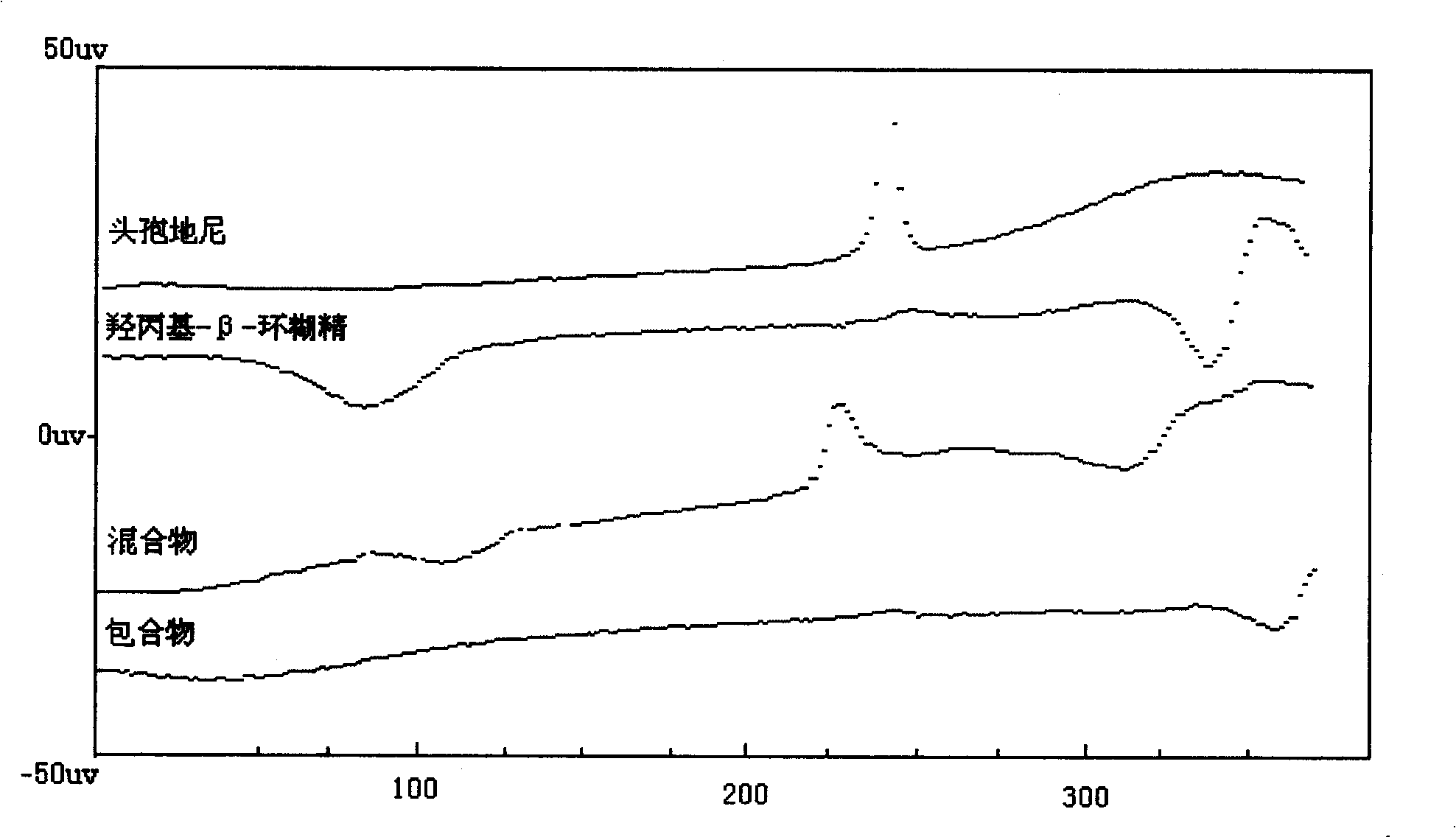
InactiveCN101264085AGood water solubilityImprove solubilityAntibacterial agentsOrganic active ingredientsSolubilityCefdinir
The invention relates to a medicine composition comprising the cefdinir cyclodextrin inclusion compound. The basic composition comprises the cefdinir and the pharmacy-acceptable cyclodextrin; the cyclodextrin is chosen from one or a plurality of Beta-cyclodextrin, sulfobutyl- Beta -cyclodextrin, hydroxypropyl- Beta-cyclodextrin or hydroxypropyl-sulfobutyl- Beta -cyclodextrin. The invention has an advantage of increasing the solubility, stability and activity of the cefdinir. The invention also provides the preparation method of the medicine composition.
Owner:NANJING NORMAL UNIVERSITY +1
New preparation method of Cefdinir
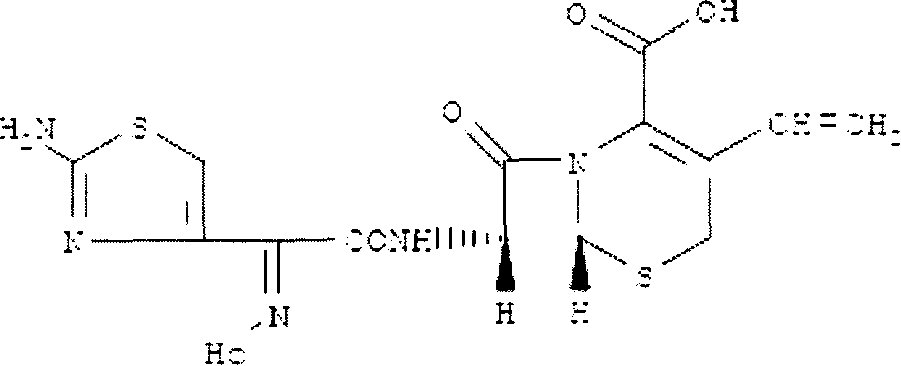
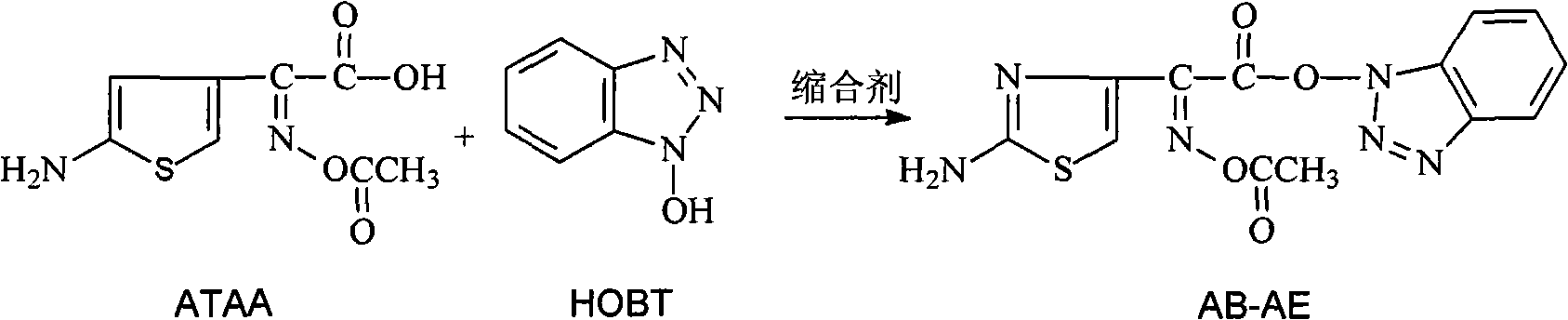
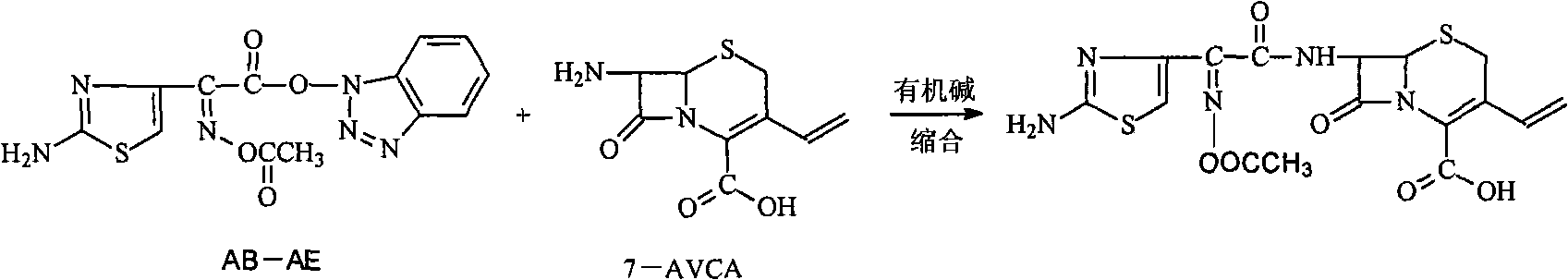
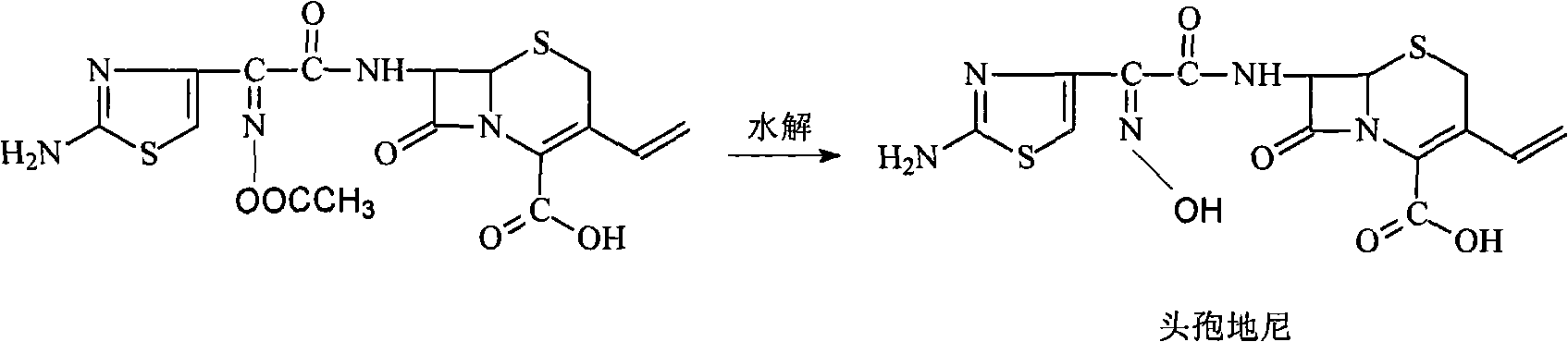
The invention provides a new preparation method of Cefdinir, in particular to a method for preparing Cefdinir from an intermediate of a new active ester. The preparation method comprises the following steps: using (Z)-2-(2-aminothiazole-4-yl)-2-acetoxyiminoacetic acid (ATAA) and 1-hydroxybenzotrizole (HOBT) to be subject to dehydrolysis condensation to generate a new active ester, i.e. 1-[(Z)-2-(2-amino-4-thiazyl)-2-(acetoxyimino) acetoxy] benzotrizole (AB-AE), then using the AB-AE and 7-amino-vinyl-3-cephalosporin-4-carboxylic acid (7-AVCA) as raw materials to be subject to the condensation reaction, and then hydrolyzing to prepare the Cefdinir. The invention has the advantages that the AB-AE has good stability and is convenient to store, the yield of the synthesized Cefdinir is more than 90%, and the process is more applicable to the industrial production.
Owner:ZHEJIANG YONGNING PHARMA
Method for preparing cefdinir
The invention discloses a method for preparing cefdinir. The method comprises the following steps of: generating a cefdinir ester intermediate by 7-amino-3-vinyl-8-oxo-5-thia-1-azabicyclo [4.2.0] octy-2-alkene-2-carboxylic acid (7-AVCA) and cefdinir active ester (acetoxy imide) in the presence of organic base, and hydrolyzing the cefdinir ester intermediate by using immobilized carboxylic ester hydrolase to generate the cefdinir. In the method for preparing the cefdinir, the reaction condition is mild, an ultralow temperature reaction is avoided, process steps are reduced, and organic solvent is saved; and due to the adoption of enzyme hydrolysis, the reaction is easy to control, and yield is high, so the method is suitable for industrial production.
Owner:GUANGDONG LIGUO PHARMACY
Cefdinir compound and preparation method thereof
InactiveCN101550147ASolve the shortcomings of low purityQuality improvementAntibacterial agentsOrganic chemistryOrganic solventSolid acid
The invention relates to a cefdinir compound and a preparation method thereof. In the method, the crude product of cefdinir prepared by the prior art is processed by the following steps to obtain a relatively pure cefdinir compound: aqueous alkali is added into the crude product of the cefdinir and full reaction is conducted till a clear solution is obtained, and then a cefdinir solution is obtained, and the cefdinir solution is absorbed with added active carbon and filtered; or the crude product of cefdinir is dissolved in an organic solvent and reacts with the added aqueous alkali to obtain a cefdinir solution, and the cefdinir solution is absorbed with added active carbon and filtered; and then the two filter liquors are added with acid or solid acid salt respectively; and crystal is separated out, filtered, washed and dried to obtain a cefdinir refined product.
Owner:HAINAN MEIDA PHARMA
Crystalline acid salts of cefdinir and process for preparing cefdinir using same
High purity cefdinir is prepared in a high yield by a process comprising the steps of: treating a crystalline salt of a cefdinir intermediate with a formic acid-sulfuric acid mixture or a formic acid-methanesulfonic acid mixture to obtain a crystalline salt of cefdinir and reacting the crystalline salt with a base in a solvent.
Owner:HANMI SCI CO LTD
Process for prepn. of cefdinir
Owner:RANBAXY LAB LTD
Cefdinir dispersible tablet and preparation method thereof
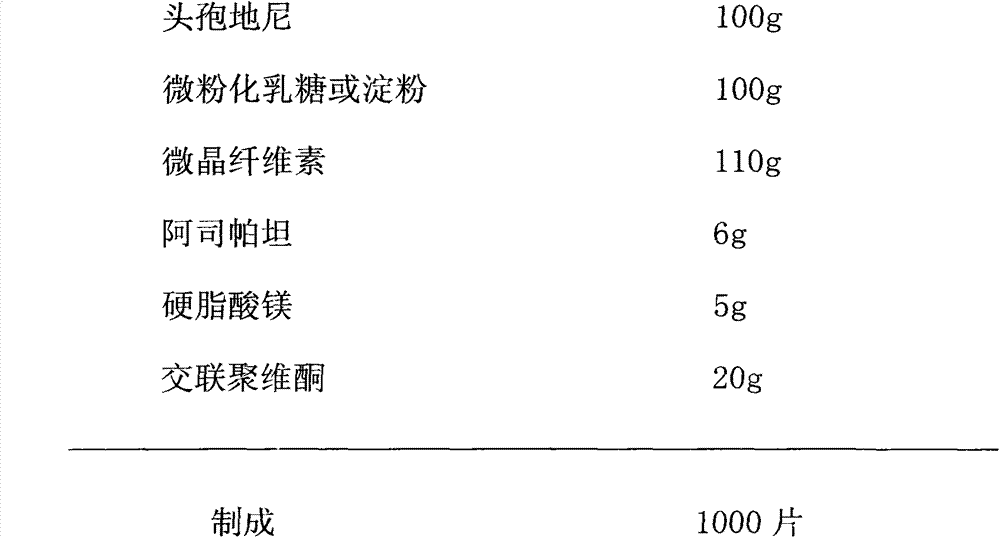
ActiveCN103110595AWell mixedImprove dispersion uniformityAntibacterial agentsOrganic active ingredientsMagnesium stearateLactose
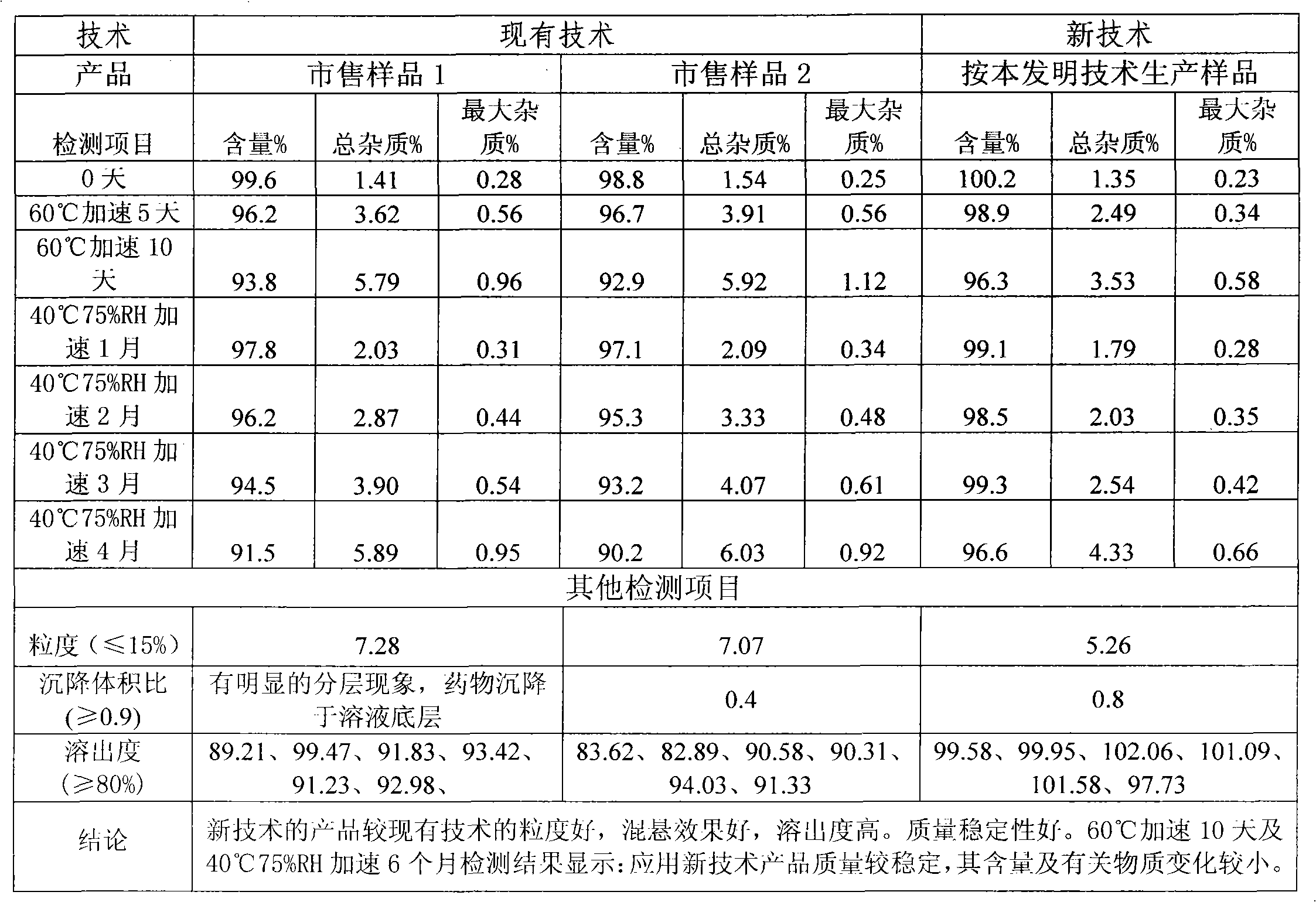
The invention relates to a preparation method of a medicinal preparation taking cefdinir as a main ingredient, and in particular relates to the preparation method of a cefdinir dispersible tablet. The method comprises the following steps: taking the cefdinir in a therapeutic dose as a main raw material; micronizing the cefdinir to form the powder with the particle diameter of 60 to 150 mu m; adding assistant medicinal materials in proper quantity into the cefdinir to be directly pelletized by using a dry method; and directly pressing a pellet into the tablet, wherein the assistant medicinal materials comprise micronized milk sugar or starch, microcrystalline cellulose, aspartame and cross-linking povidone. The cefdinir dispersible tablet comprises the following ingredients in parts by weight: 100 parts of the cefdinir, 30 to 100 parts of the starch, 80 to 200 parts of the microcrystalline cellulose, 5 to 15 parts of the aspartame, 8 to 20 parts of the cross-linking povidone and 20 to 50 parts of magnesium stearate. The method provided by the invention has the advantages that the operation is simple; and the prepared cefdinir dispersible tablet is stable in quality, rapid in dissolution rate and high in bioavailability.
Owner:GUANGDONG BOZHOU PHARMA
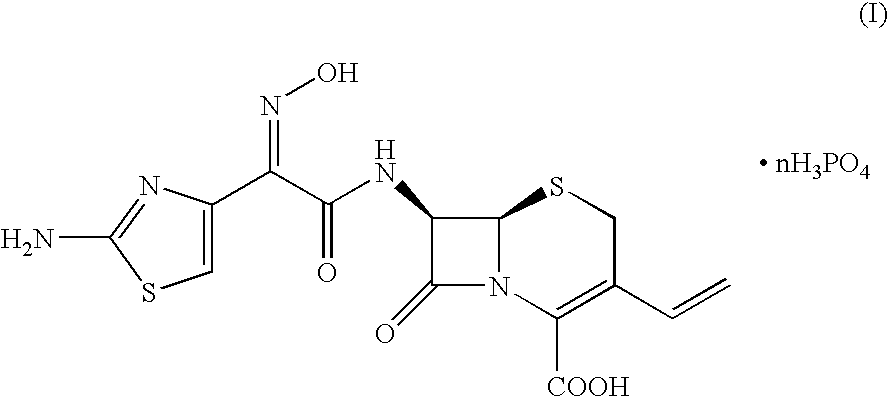
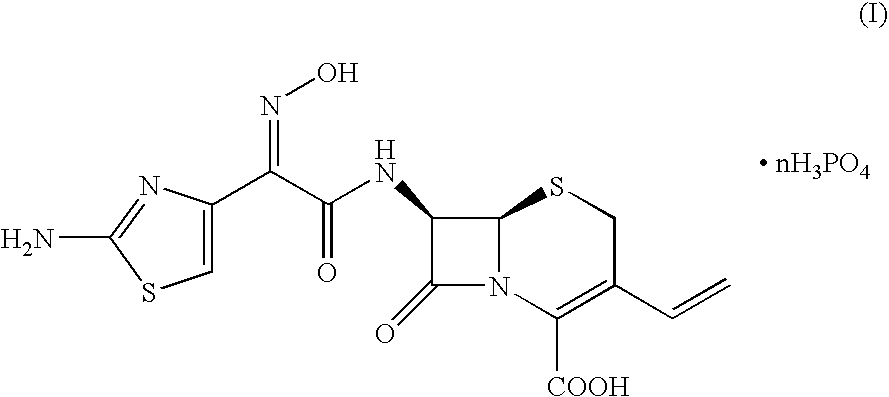
Crystalline cefdinir salts
Cefdinir crystalline salts of formula (I),in which n ranges from 1 to 3, the preparation and use thereof for the preparation and purification of cefdinir are herein disclosed. The salts of formula (I) can be obtained from cefdinir intermediates or crude cefdinir by treatment with phosphoric acid.
Owner:ANTIBIOTICOS SPA
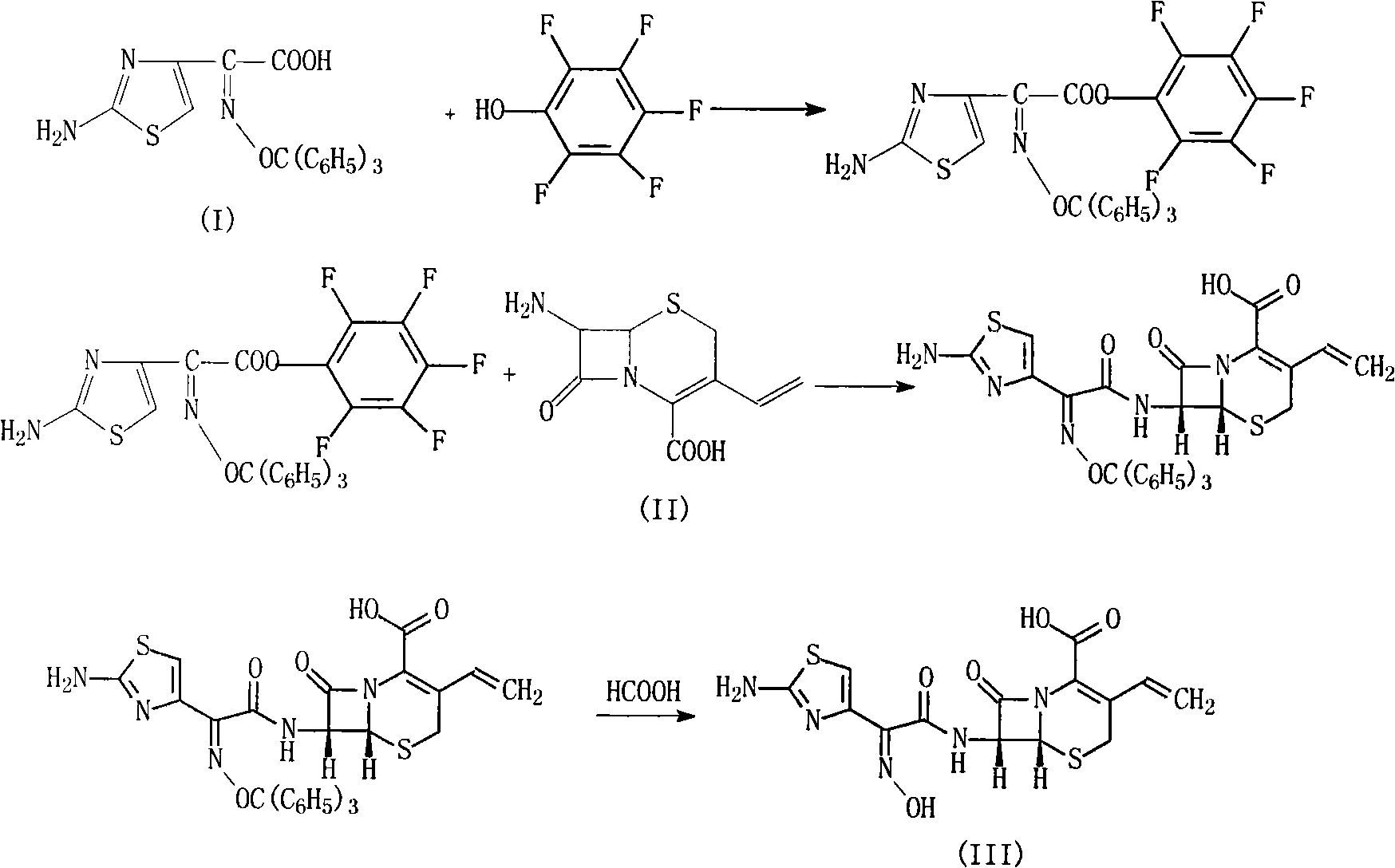
Cefdinir compound and new preparation method thereof
The invention discloses a cefdinir compound and a new preparation method thereof. The method prepares the cefdinir by performing reaction on (Z)-2-(2-aminothiazole-4-group)-2-triphenylmethyl iminoacetic acid serving as an initial raw material, pentafluorophenol serving as an activating group and 7-AVCA.
Owner:HAINAN MEIDA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com