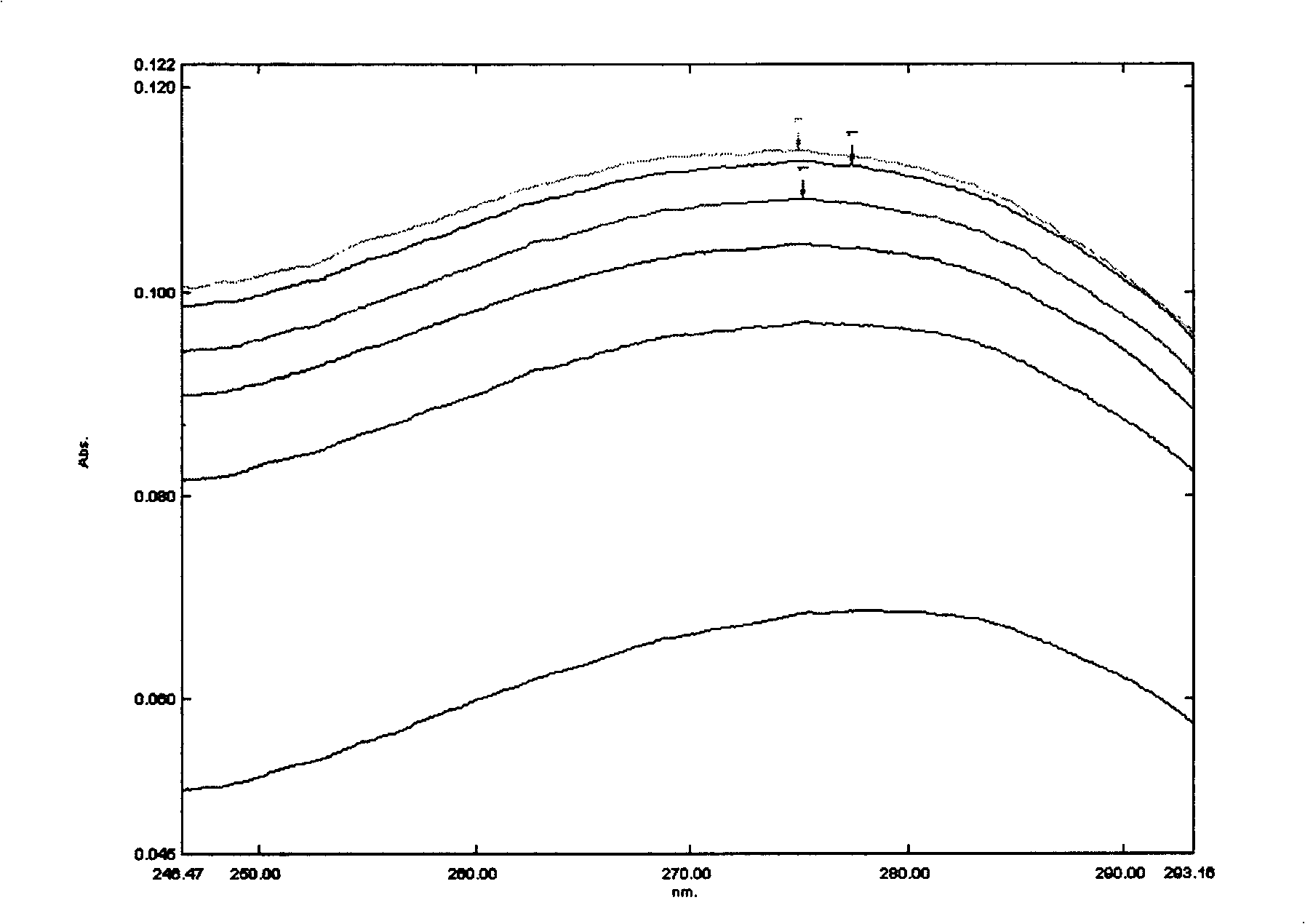
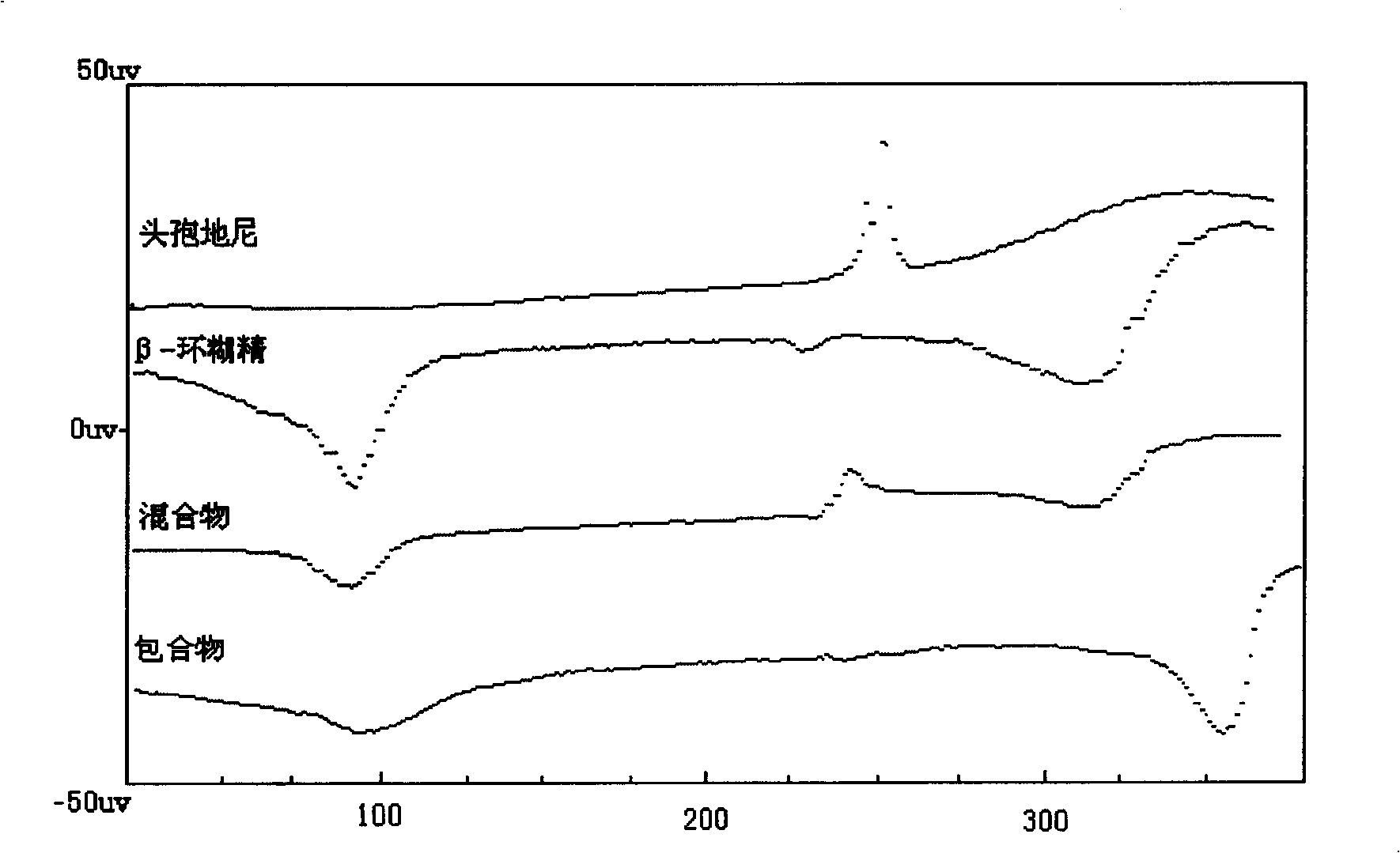
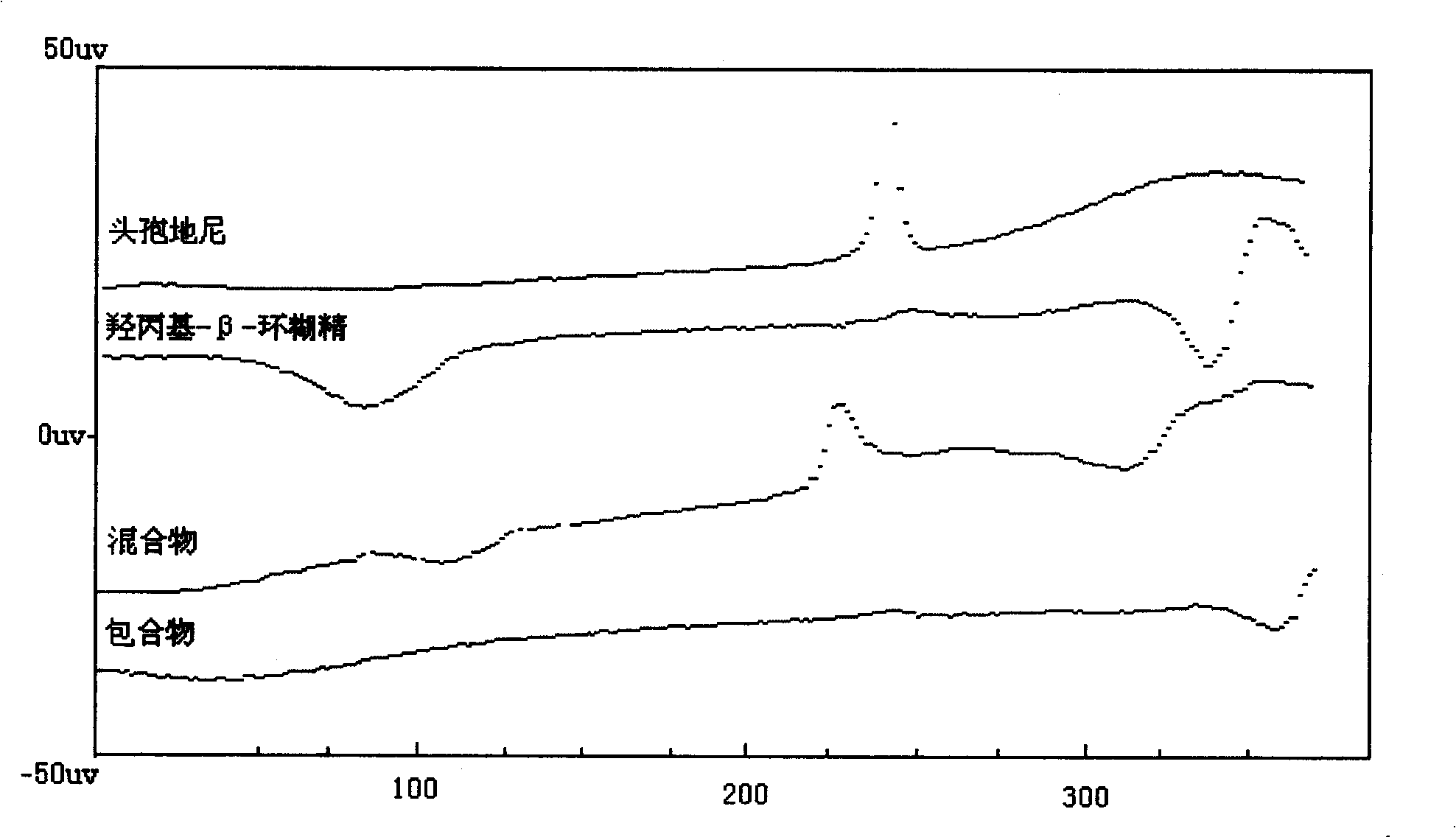
Medicinal composition containing cefdinir cyclodextrin inclusion compound and preparation thereof
A technology of cyclodextrin inclusion complex and cefdinir, which is applied in the directions of medical preparations containing active ingredients, medical preparations without active ingredients, nano-drugs, etc., to achieve easy dissolution, high water solubility, and little hemolytic side effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Mix 28g of β-cyclodextrin with 30ml of pure water, heat, add 10g of cefdinir at 50°C, mix well, add dilute NaHCO drop by drop 3 (0.1M) solution, stirred for 3 hours, neutralized by adding an equal volume of dilute hydrochloric acid (0.1M) dropwise, then cooled at 5°C for 24 hours; removed water under reduced pressure at a temperature not higher than 50°C, and dried to obtain a solid clathrate.
Embodiment 2
[0076] Basically the same as Example 1, but with 100 g of β-cyclodextrin mixed with 100 ml of pure water.
Embodiment 3
[0078] Basically the same as Example 1, but with 1000 g of β-cyclodextrin mixed with 1000 ml of pure water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com