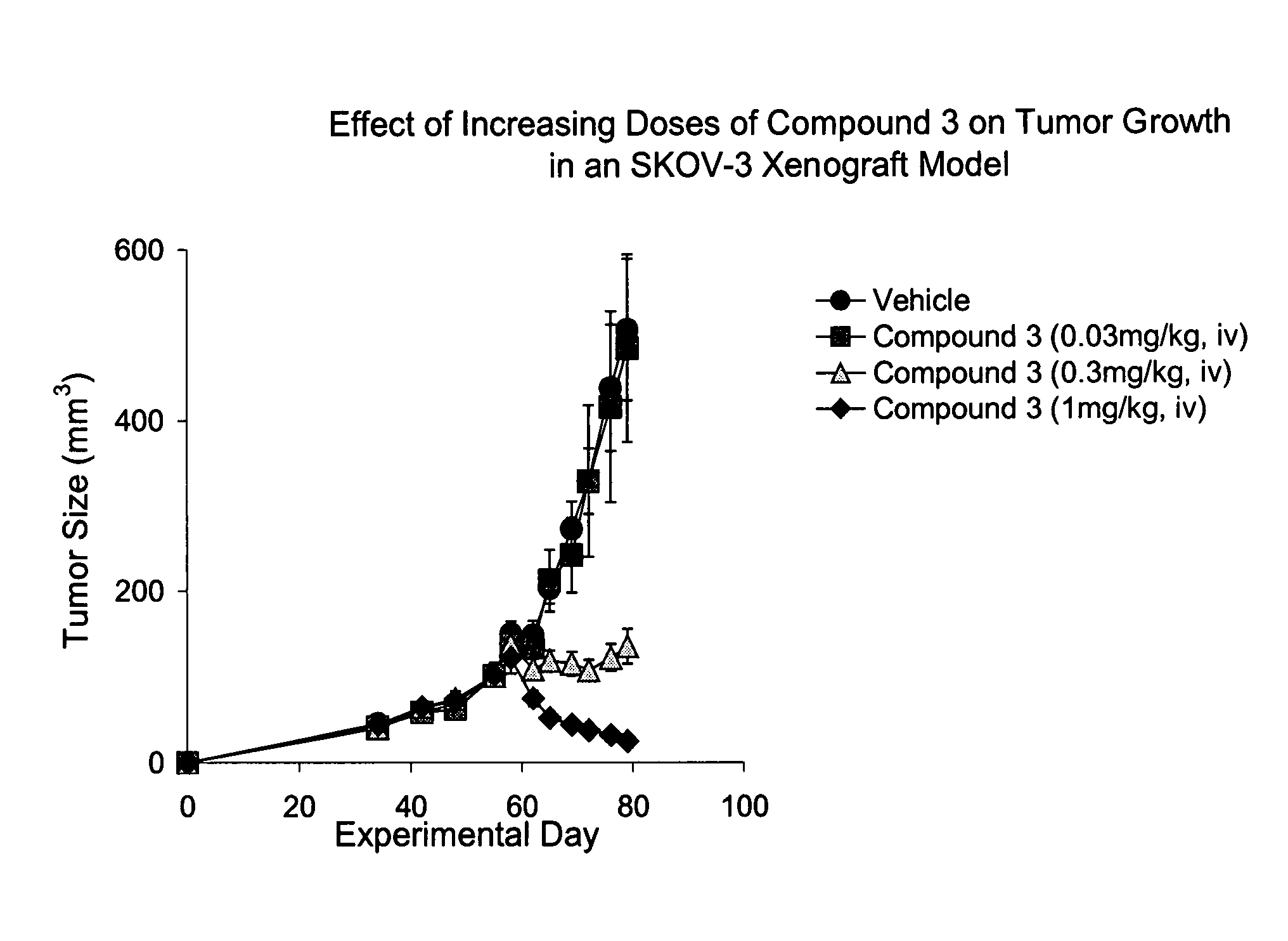
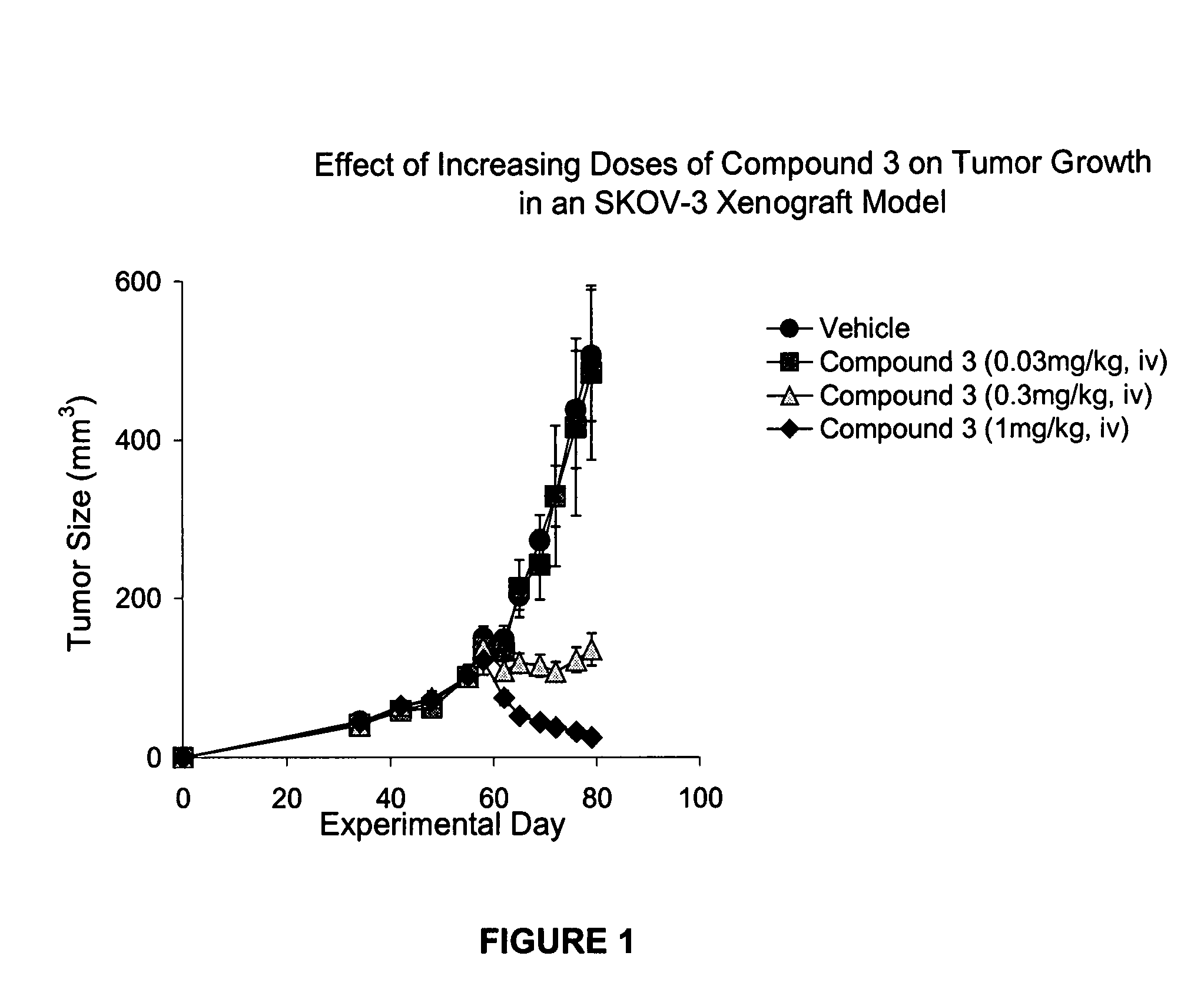
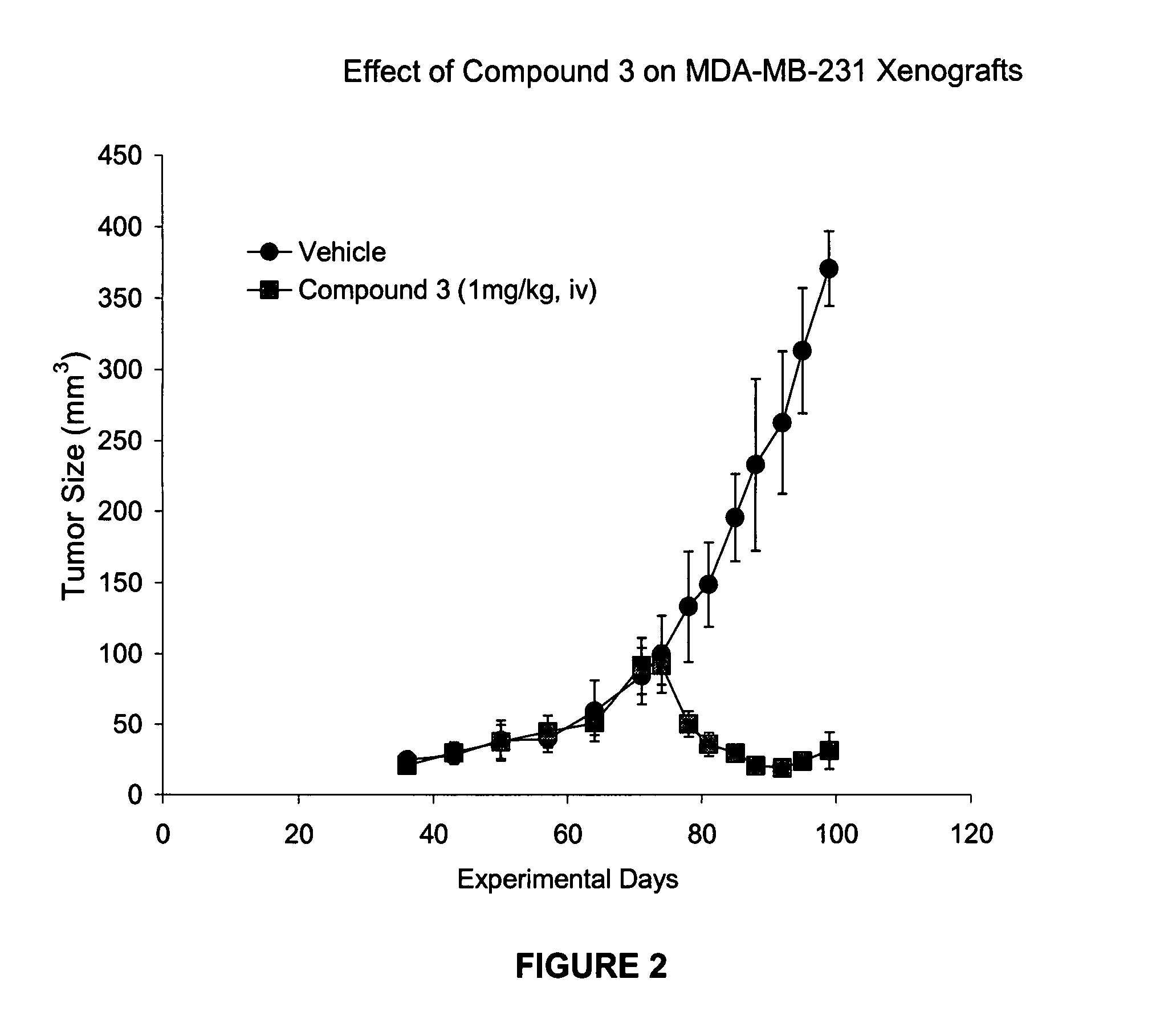
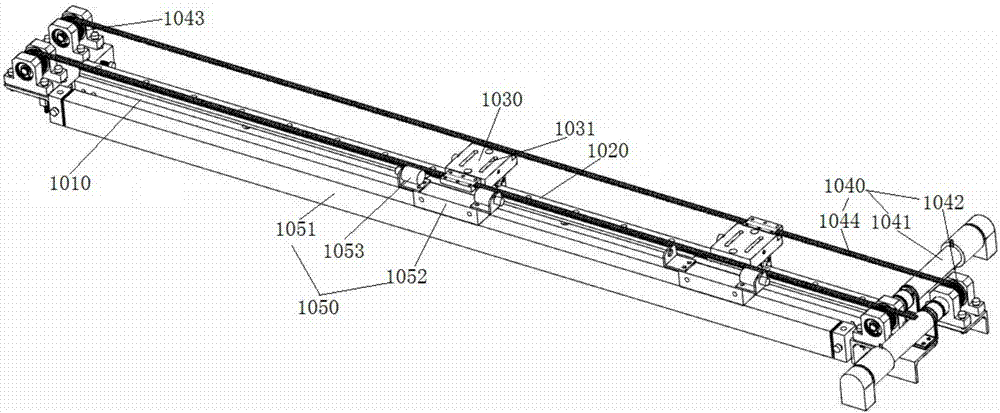
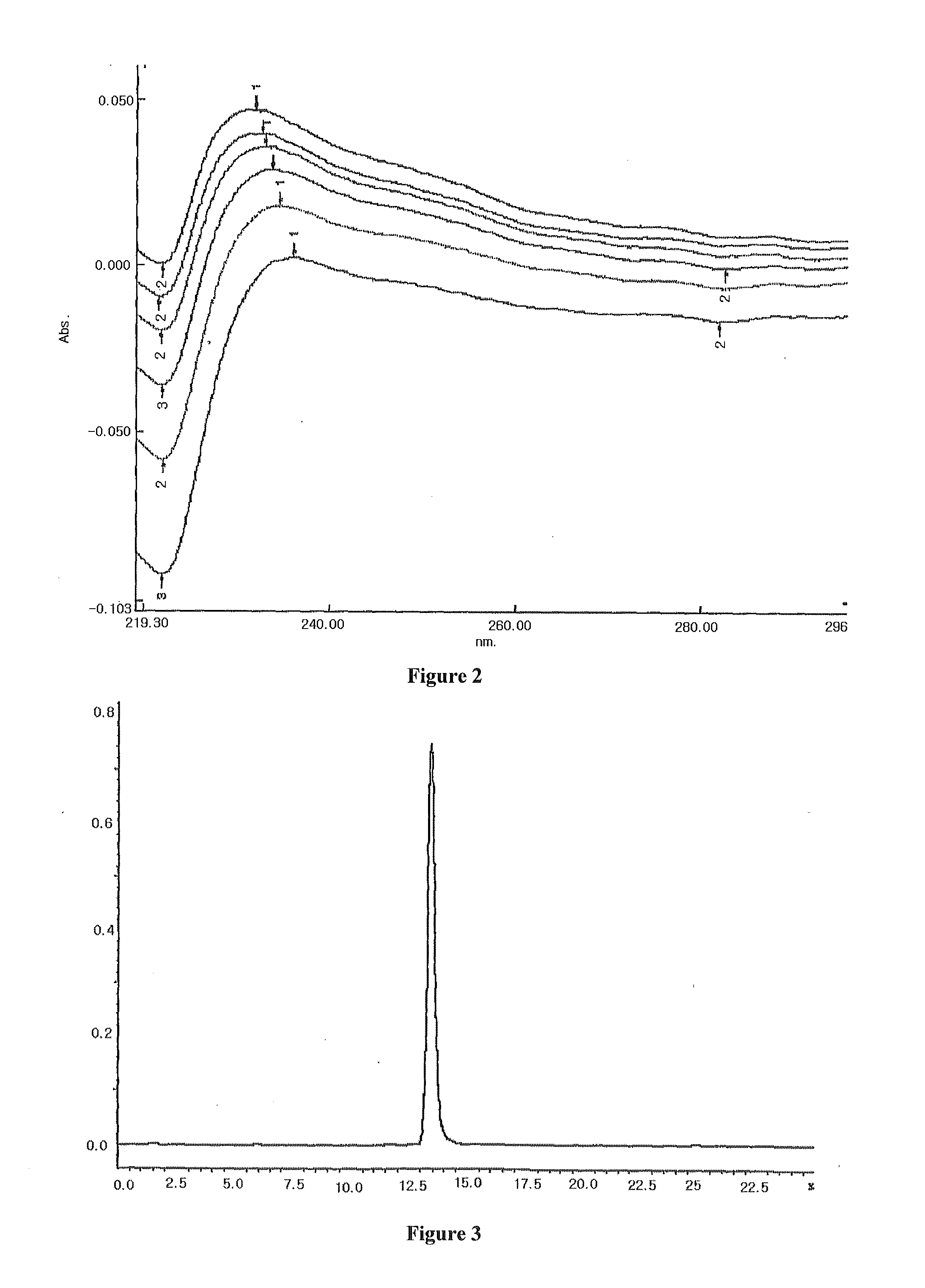
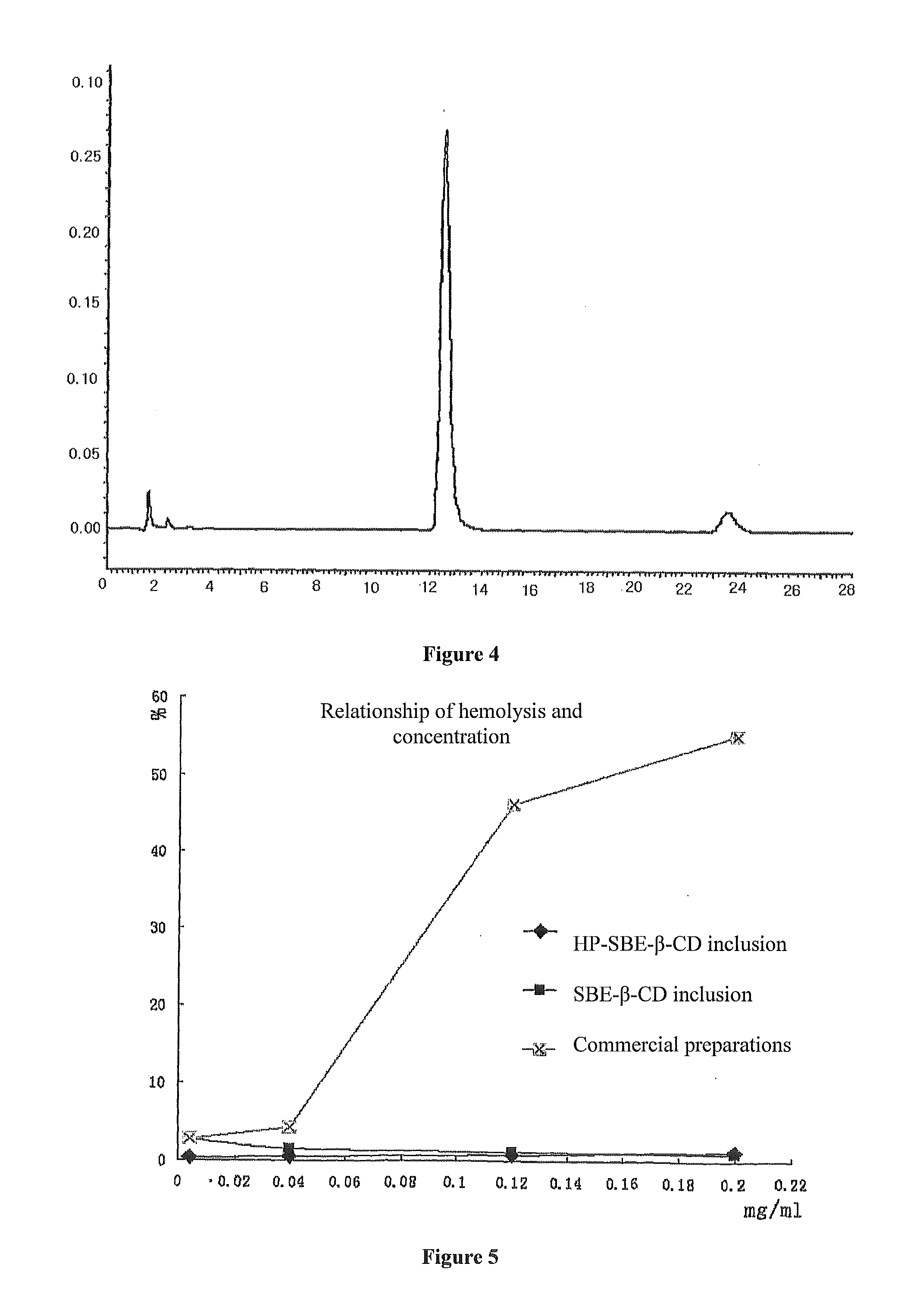
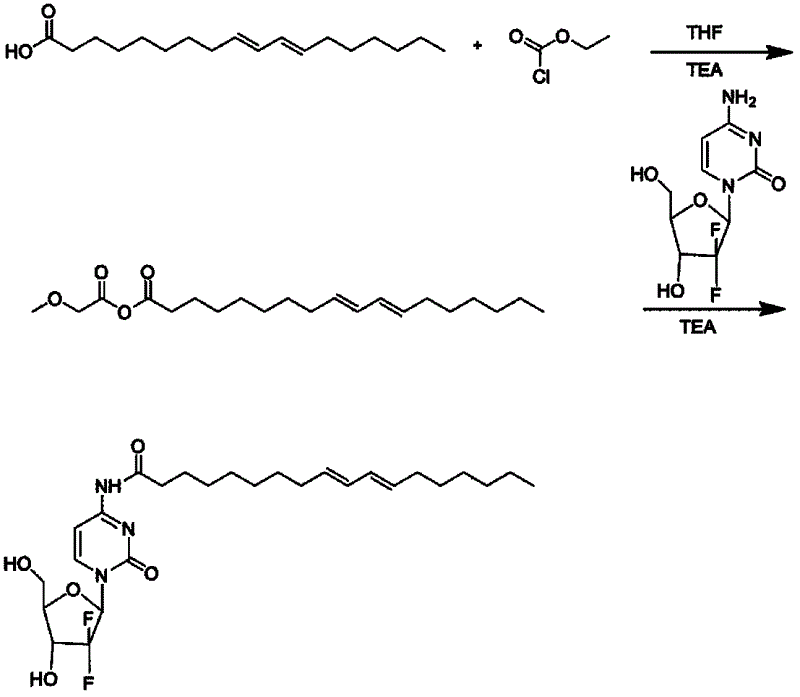
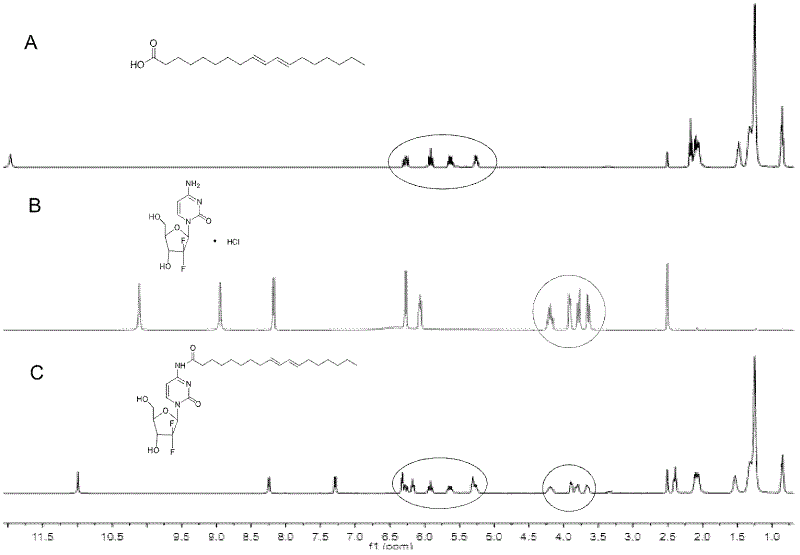
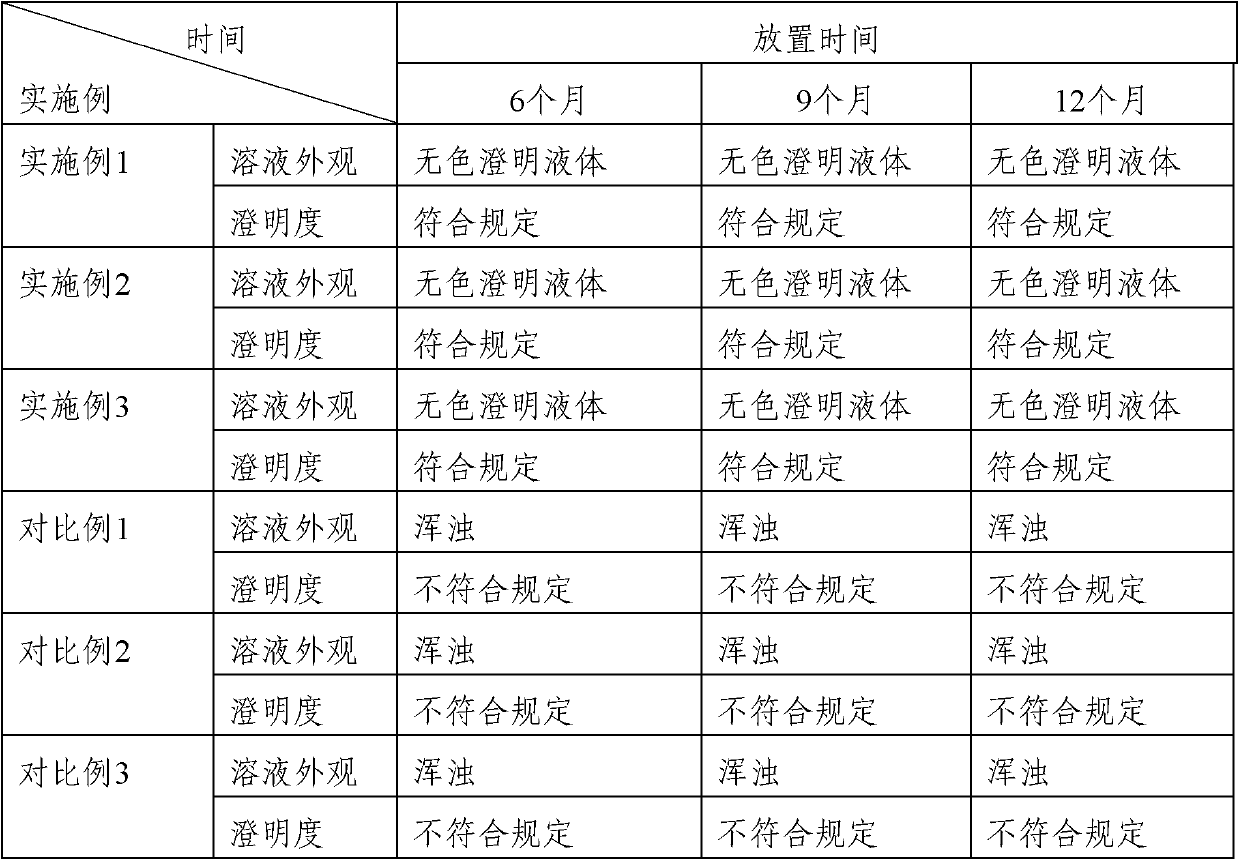
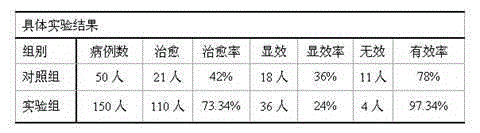
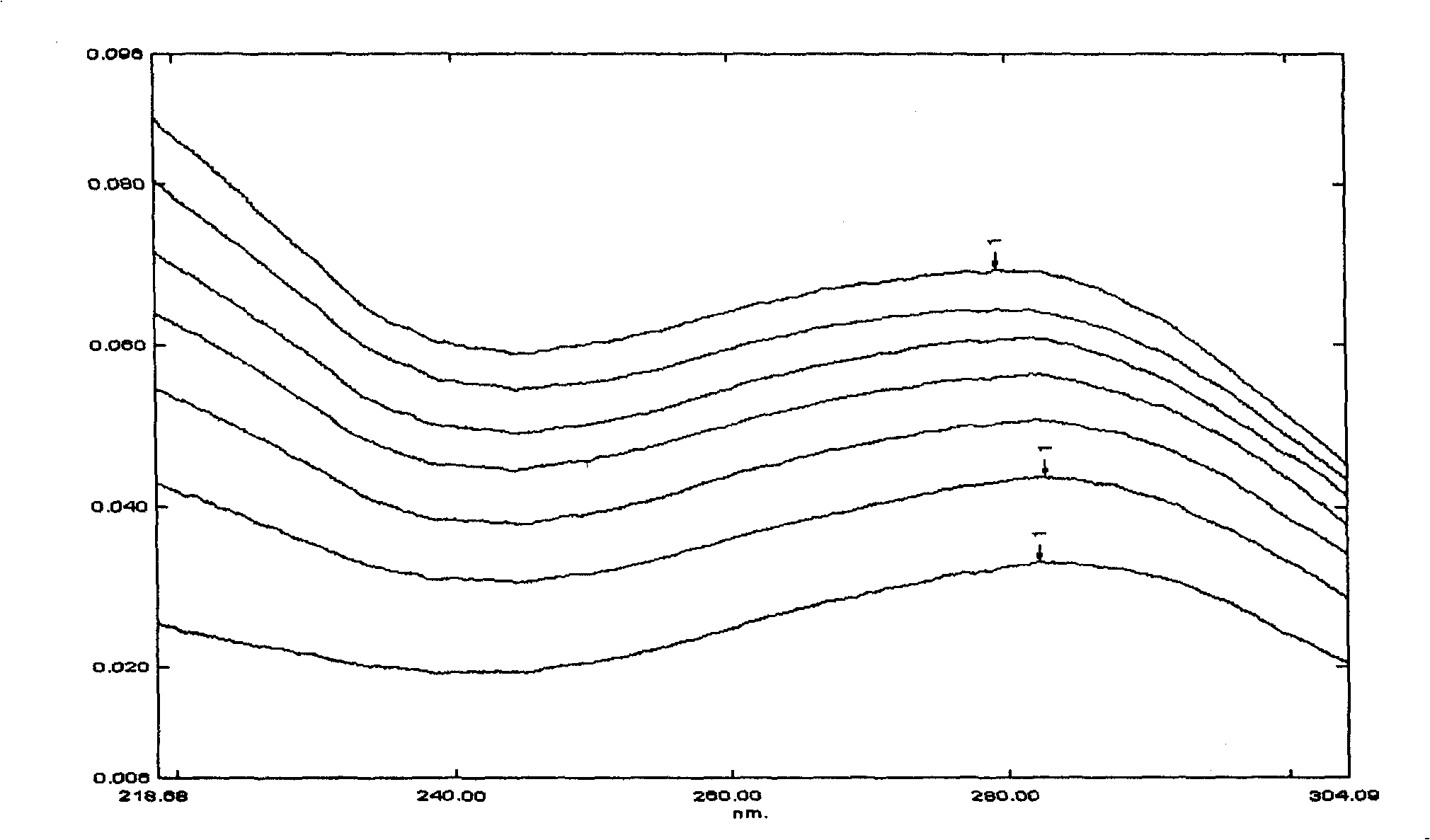
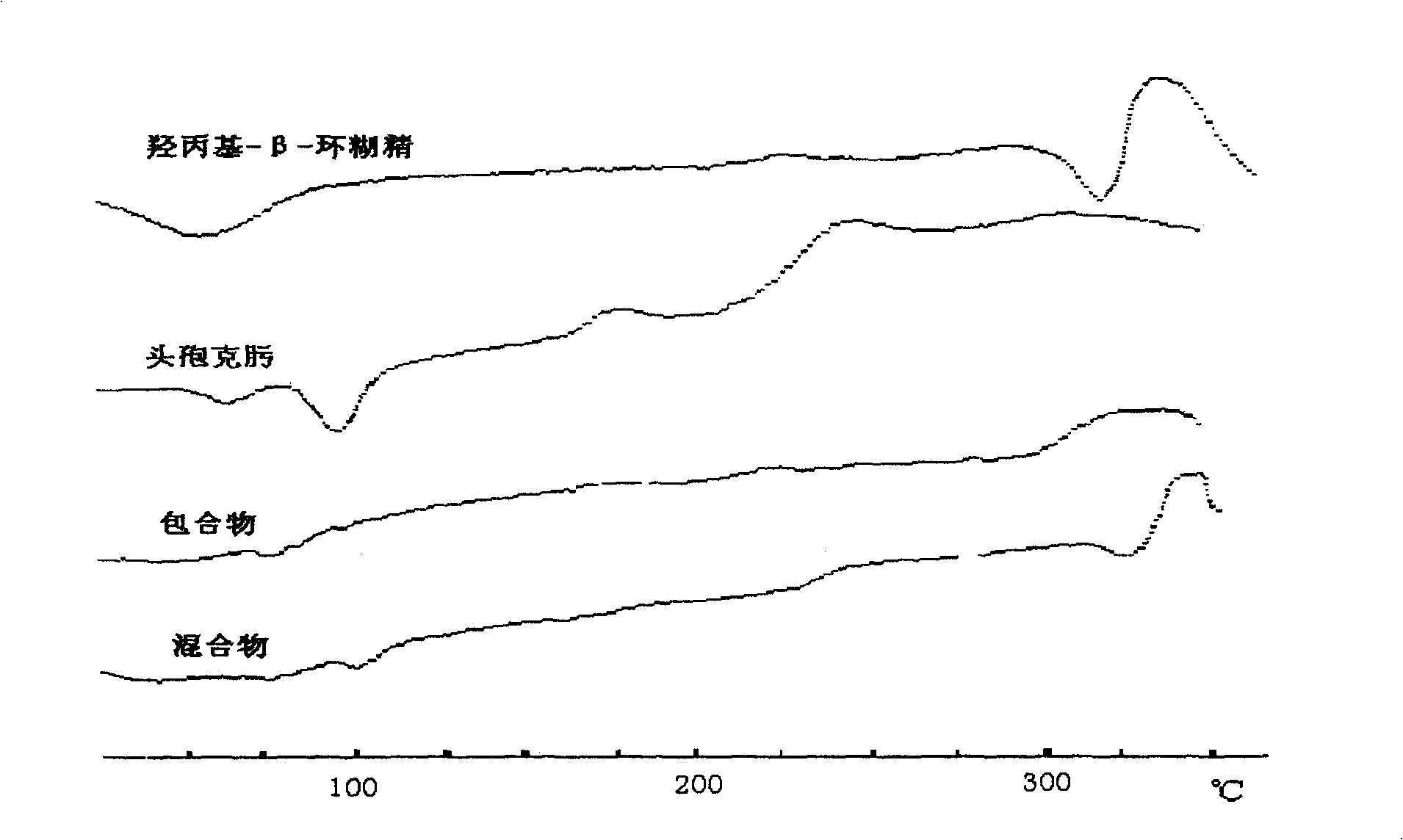
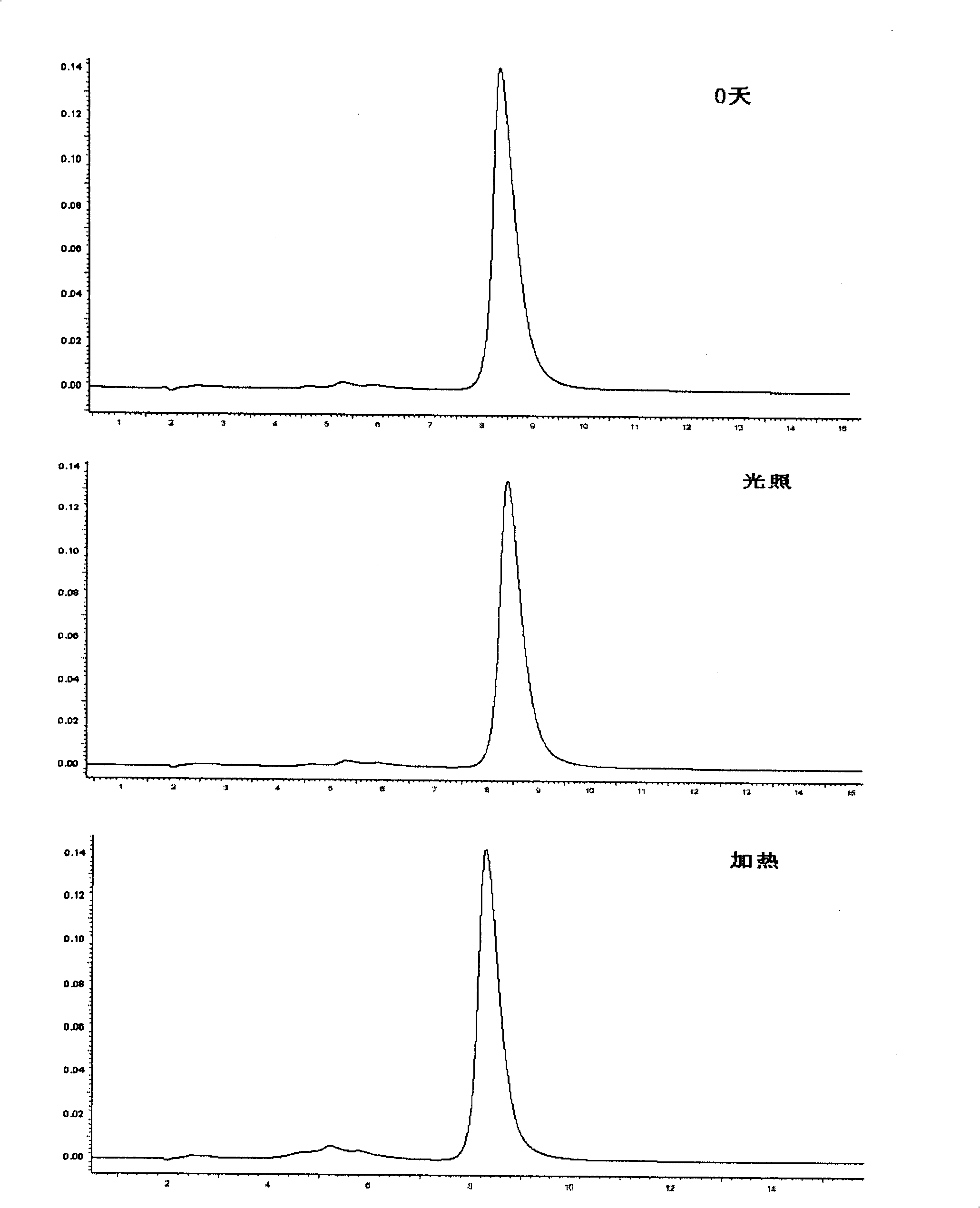
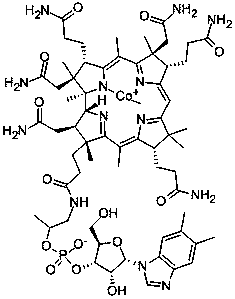
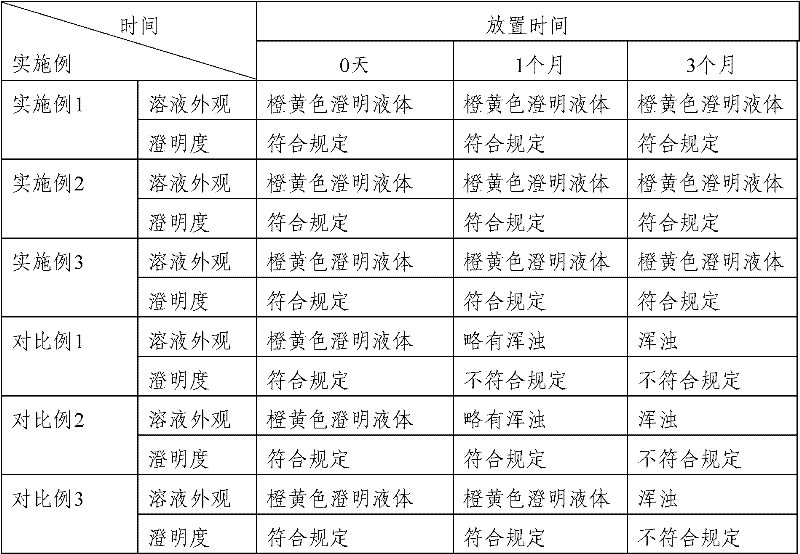
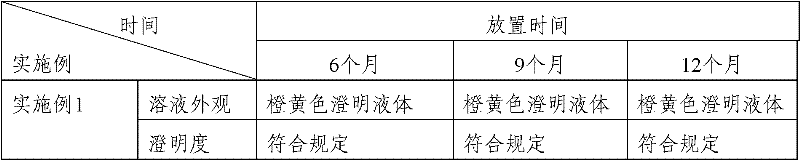
Patents
Literature
77results about How to "Suitable for clinical use" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
IAP bir domain binding compounds
InactiveUS20080069812A1Improve effectivenessPharmaceutically acceptable stabilityPeptide/protein ingredientsAntipyreticEnantiomerTautomer
Disclosed is an isomer, enantiomer, diastereoisomer or tautomer of a compound represented by Formula I or II or a salt thereof, in which R1, R2, R3, R100, R200, R300, A, A1, BG, Q and Q1 are substituents described herein. Also disclosed is the use of compounds of Formula I and II to treat proliferative disorders such as cancer.
Owner:PHARMASCIENCE INC
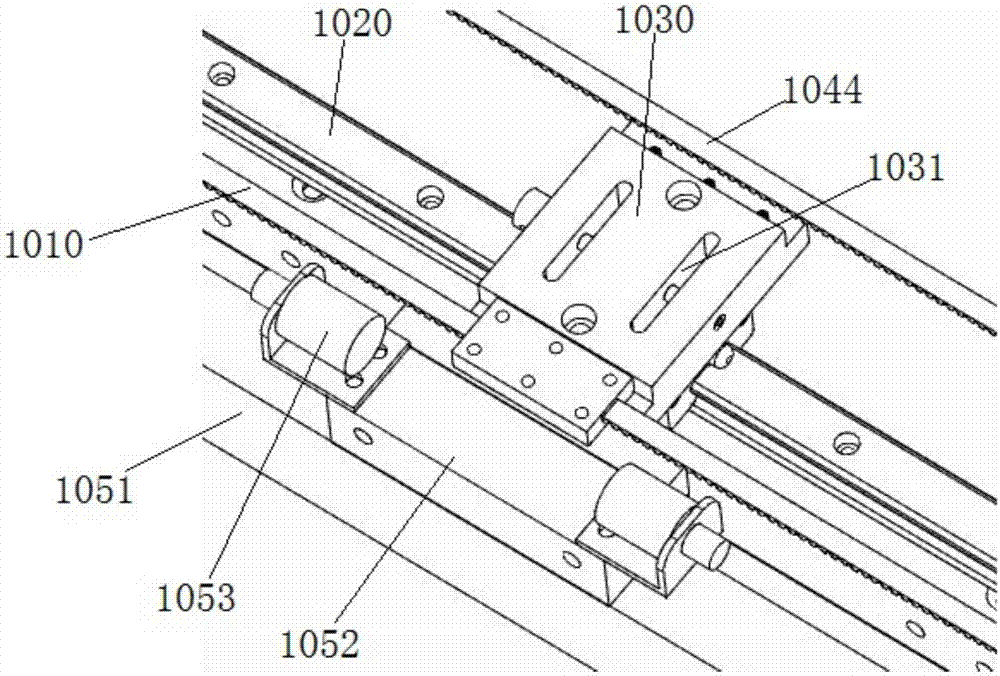
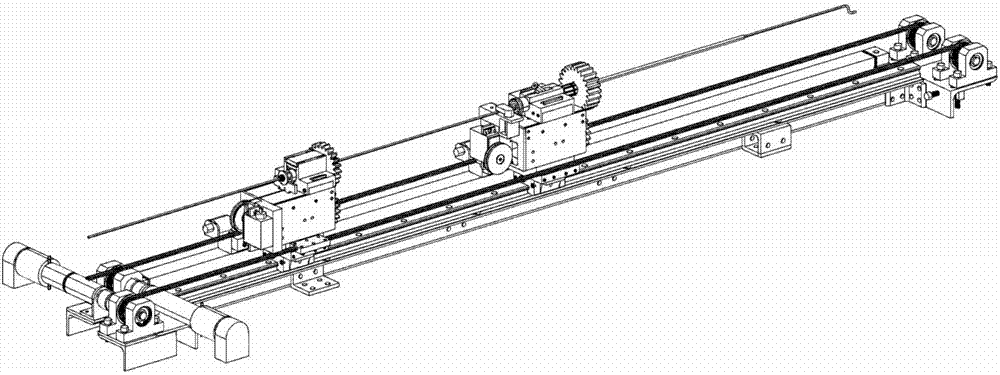
Interventional surgical robot subordinate end and mobile platform and control method of robot subordinate end
ActiveCN107374738AMeet the requirements for collaborative operationHigh transmission precisionDiagnosticsSurgical robotsSurgical robotThree vessels
The invention discloses an interventional surgical robot subordinate end and a mobile platform and a control method of the robot subordinate end and belongs to the technical field of minimally invasive blood vessel interventional operations. The mobile platform comprises a platform main beam, and platform connection blocks are arranged on the platform main beam through a linear guide rail pair D; the platform connection blocks are driven by a platform driving mechanism and used for installing a catheter controller or a guide wire controller. The interventional surgical robot subordinate end comprises the catheter controller, the guide wire controller and the mobile platform; the catheter controller and the guide wire controller are mounted on the two platform connection blocks respectively. According to the interventional surgical robot subordinate end, the catheter controller and the guide wire controller are installed on the mobile platform, and the problem is solved that it is difficult for an existing robot to complete co-operating of a catheter and a guide wire.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
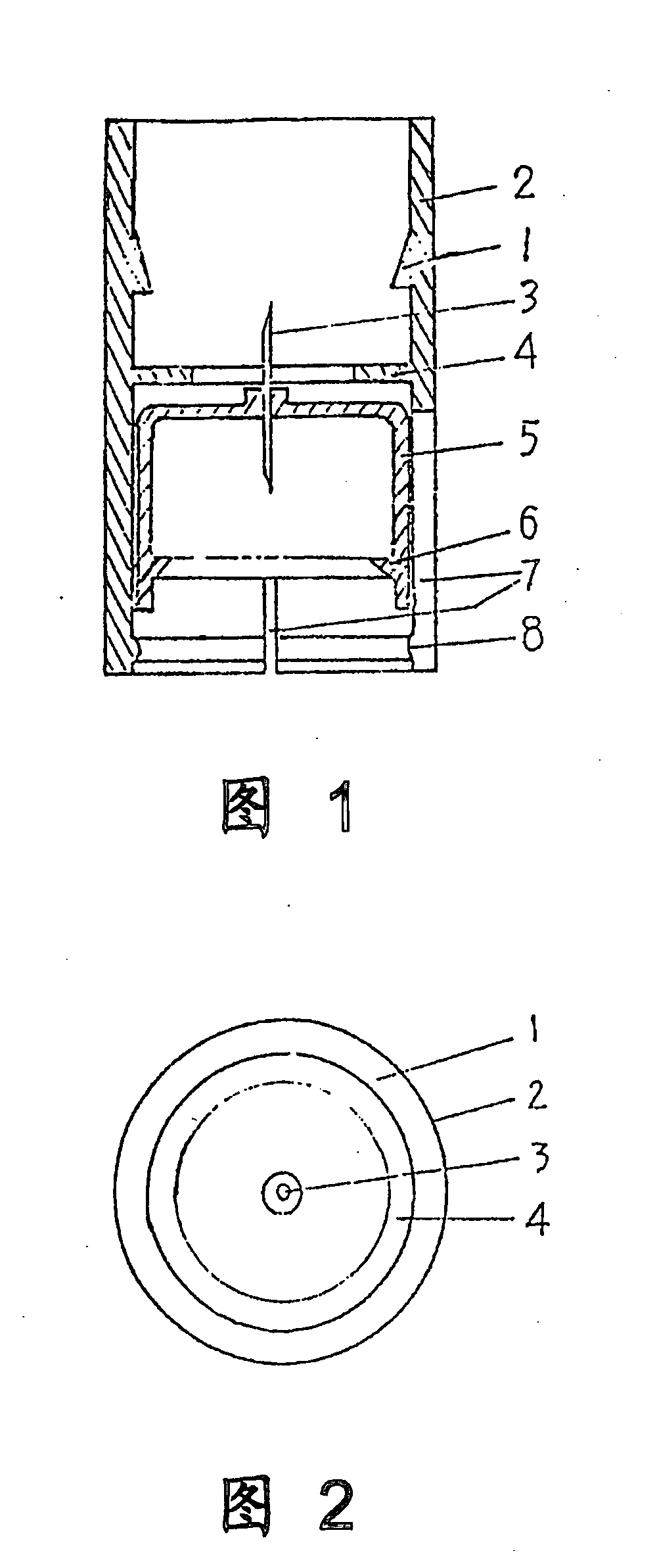
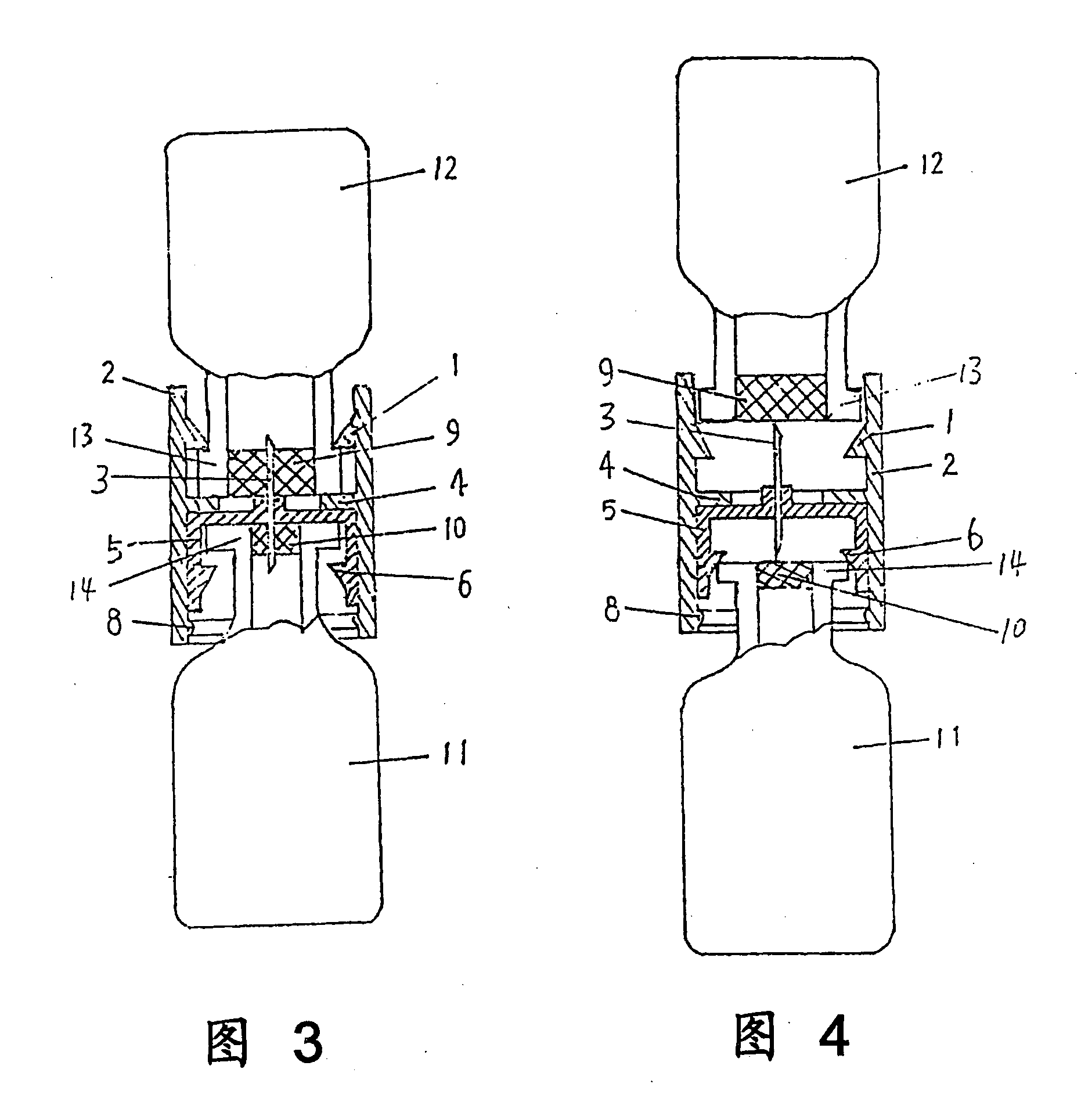
Drug Mixing and Delivery Device
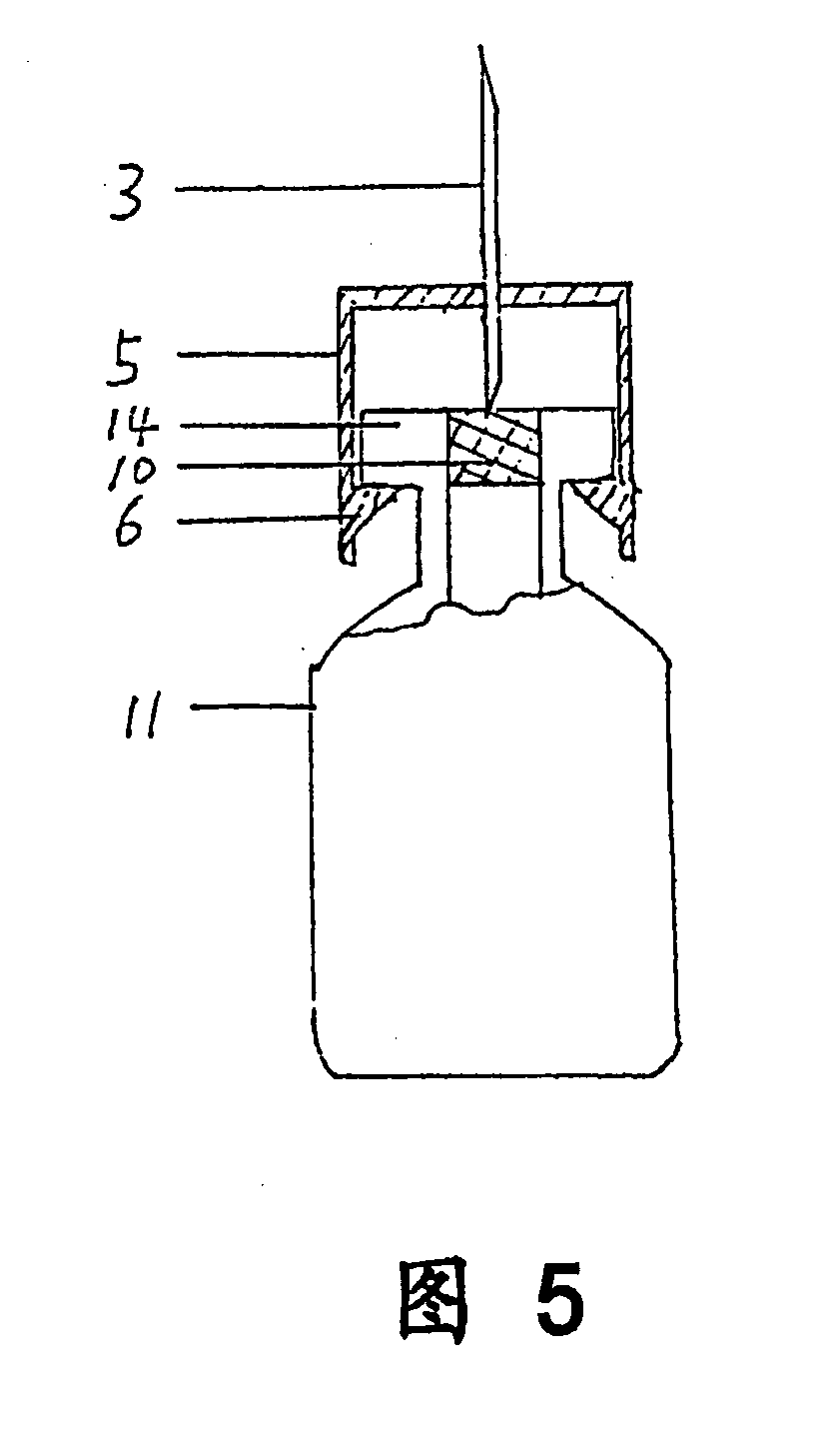
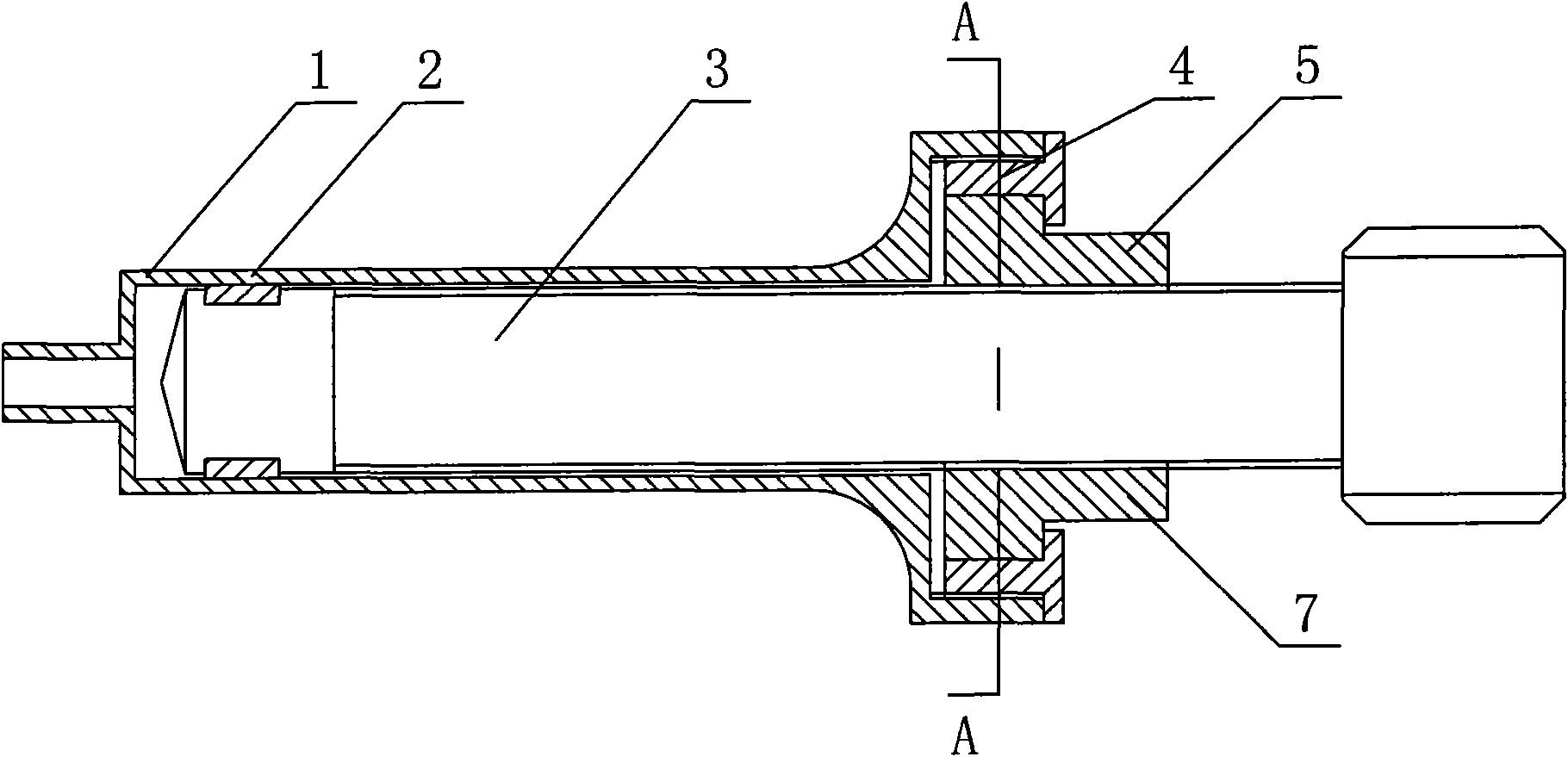
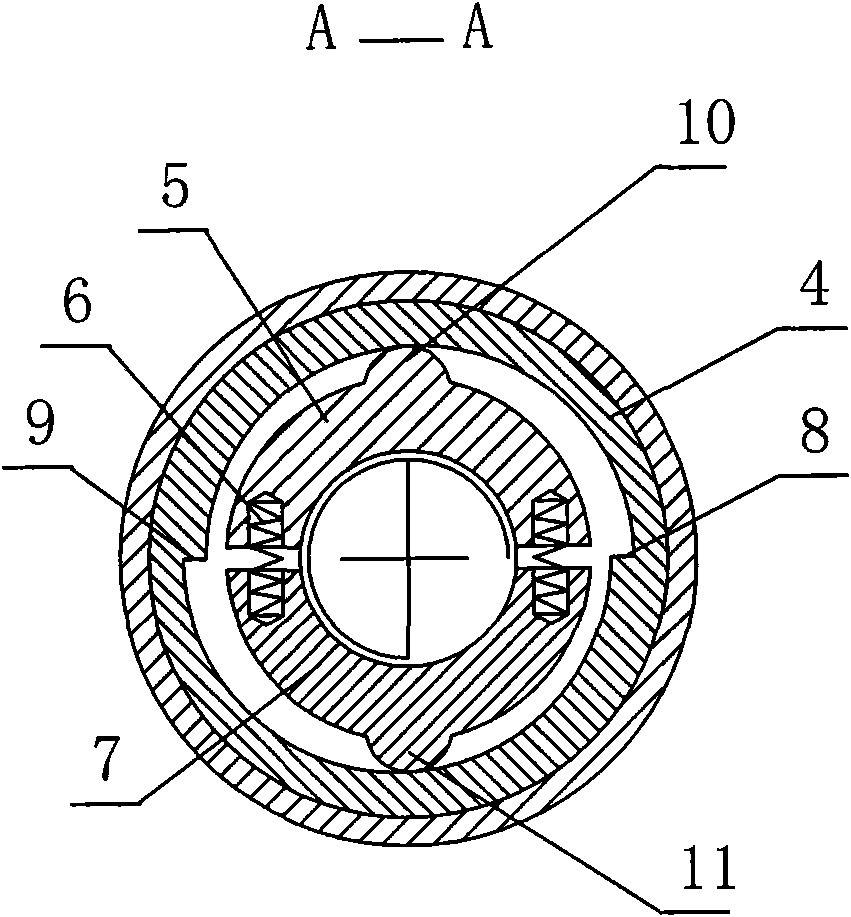
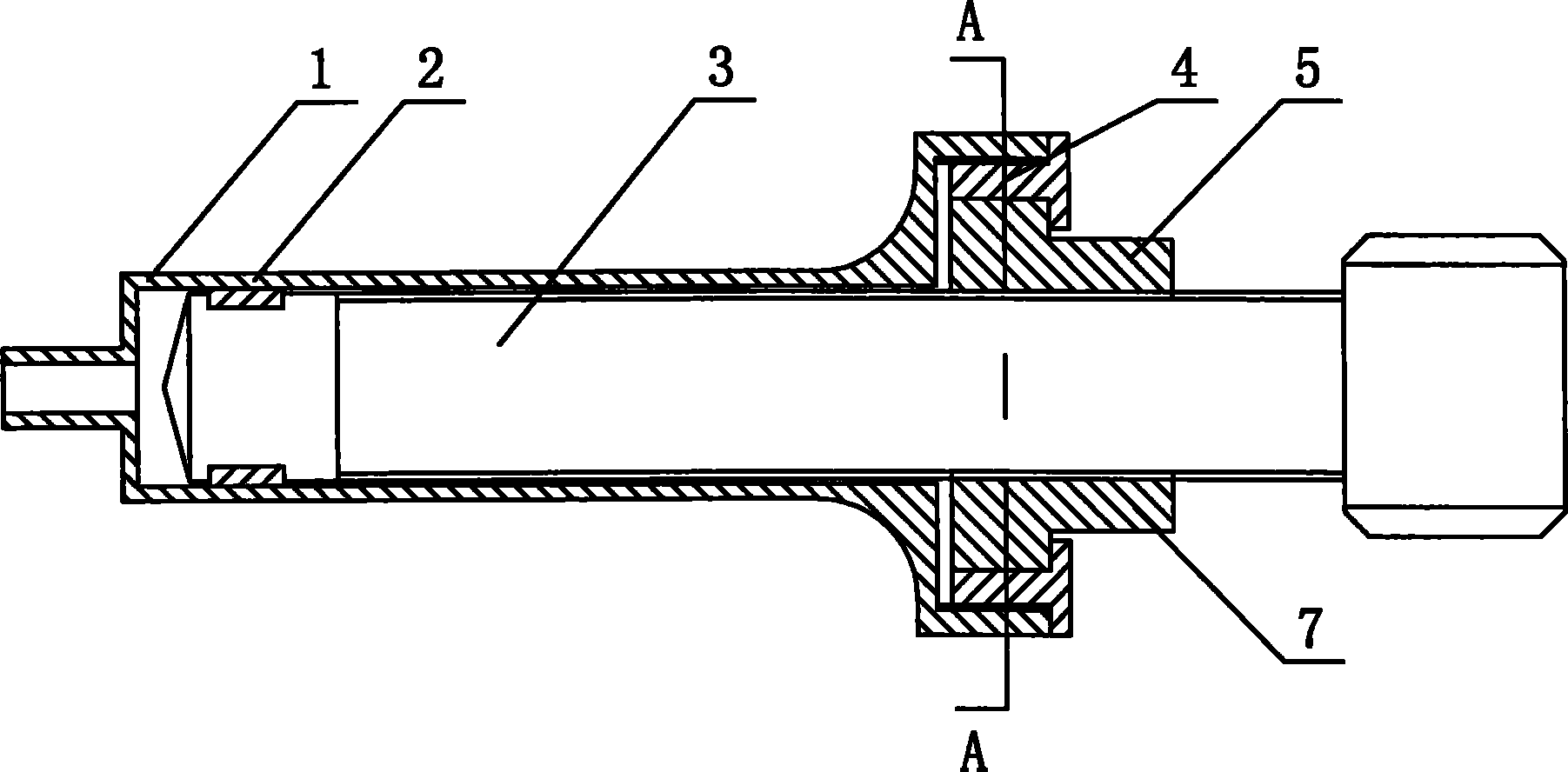
InactiveUS20080300536A1Easy to operateSuitable for clinical useInfusion syringesPharmaceutical containersOuter CannulaBottle
A medicine mixer for applying drug comprises a menstruum vial (12), an outer cannula (2) and a solute vial (11) (powdered drug ampoule) which are in one. Retaining ring (1), chuck ring (4) and convex ring (8) are disposed respectively at up portion, middle portion and nether portion of the inner wall of the outer cannula (2). An inner cannula (5) with a ducting needle (3) is disposed between the chuck ring (4) and the convex ring (8). In use the lower end of the outer cannula (2) is inserted to the opening (14) of the solute vial (11), and the opening (13) of the menstruum vial (12) is inserted into the retaining ring (1) of the outer cannula (2), so that rubber plugs (10,9) are pierced successively by the ducting needle (3) to connect two vials and thus mix drug. Then the outer cannula (2) is unfixed and the drug is applied to an infusion bottle (15). A medicine mixer for applying drug which can be repositioned automatically and a medicine mixer for applying drug which can delivery drug to many ampoules are also provided. The structure of the device is simple and cost is low. It is suitable to be combined with commercial ampoules. It is used conveniently and simply, and applied broadly.
Owner:WANG XINMING +2
Kit for detecting helicobacter pylori drug-resistant gene polymorphism by multiple fluorescent PCR melting curve method
ActiveCN111850154AShort detection timeImprove efficiencyMicrobiological testing/measurementMicroorganism based processesMultiplexLysis
The invention discloses a kit for detecting helicobacter pylori drug resistance gene polymorphism by a multiplex fluorescence PCR melting curve method. The helicobacter pylori drug resistance gene comprises the following three genes: a 23S rRNA gene, a 16S rRNA gene and a gyr A gene, the kit comprises a nucleic acid extraction reagent and a nucleic acid amplification reagent, the nucleic acid extraction reagent comprises superparamagnetic silicon oxide nano magnetic beads, a lysis solution, a washing solution and an eluent; the nucleic acid amplification reagent comprises a primer pair and a probe which respectively correspond to the helicobacter pylori drug resistance gene 23S rRNA gene, the helicobacter pylori 16S rRNA gene, the gyr A gene and an internal standard gene human housekeepinggene beta-globin. According to the invention, three drug-resistant sites and one internal standard gene can be simultaneously detected in a single tube, so that the detection flux of a sample is improved; meanwhile, interference between multiple pairs of primer probes in a detection system is improved, and the sensitivity and specificity of reagent detection are effectively improved.
Owner:SHANG OUTDO BIOTECH CO LTD
Reagent kit for detecting high-risk human mammilla papillomavirus, as well as preparation and application thereof
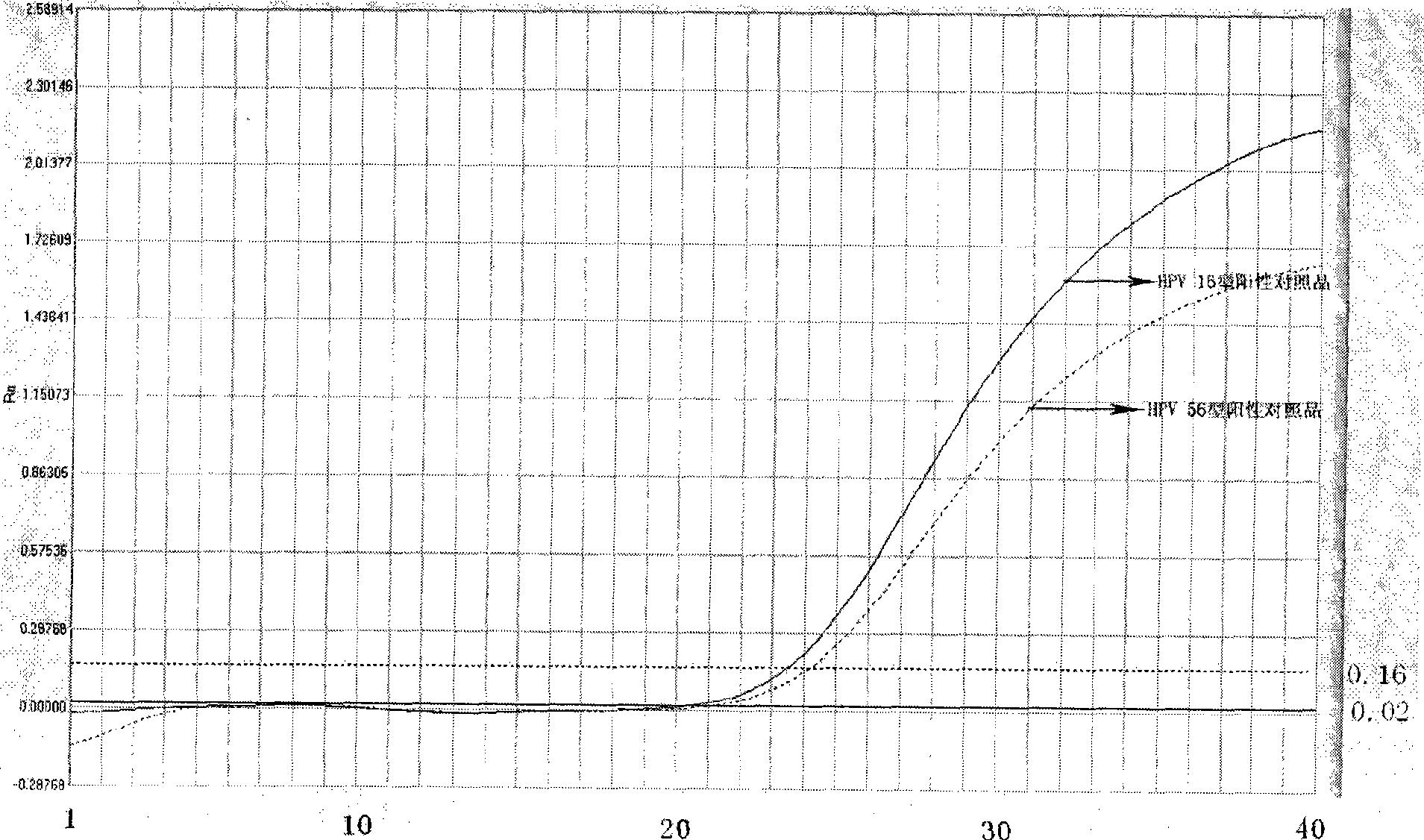
ActiveCN101503741AAvoid False Positive ResultsIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesFluorescent pcrBiology
The invention relates to a kit for auxiliary diagnosis of cervical carcinoma and high-grade cervical intraepithelial lesion, and discloses a kit for detecting high-risk HPV. The kit for detecting the high-risk HPV comprises a high-risk HPV nucleic acid fluorescent PCR detection mixture and Taq enzyme. The invention also discloses a method for preparing the kit for detecting the high-risk HPV and a method for using the same. The kit can be used for detecting the high-risk HPV and overcomes the defects of high false positive rate, low specificity, high cost and the like of typing detection of the high-risk HPV in the prior art.
Owner:SHANGHAI ZJ BIO TECH
Dasatinib pharmaceutical composition and preparation method thereof
InactiveCN102048736AGuaranteed stabilityImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsDasatinibHydroxymethyl cellulose
The invention relates to a dasatinib pharmaceutical composition which comprises dasatinib and pharmaceutical auxiliary materials, wherein the auxiliary materials comprise pregelatinized starch and bonding agent. The dasatinib pharmaceutical composition comprises the following concrete components in percentage by weight: 1-20% of dasatinib, 20-35% of pregelatinized starch and 60-79% of bonding agent, wherein the bonding agent is selected from one or mixture of microcrystalline cellulose, hydroxypropyl cellulose sodium, hydroxymethyl cellulose sodium and hydroxypropyl cellulose. The preparation method of the dasatinib pharmaceutical composition comprises the following steps: weighing the dasatinib, pregelatinized starch and bonding agent according to component proportions; sieving the dasatinib by a sieve with 100 meshes, sieving the pregelatinized starch and bonding agent by a sieve with 80 meshes, and uniformly mixing the dasatinib and the sieved auxiliary materials to obtain powder; and directly pressing the powder to obtain the dasatinib pharmaceutical composition. Because the pregelatinized starch is adopted, the product quality and product stability are greatly improved. The dasatinib pharmaceutical composition is a new preparation which has the advantages of better liquidity, higher stability and low hygroscopicity and is suitable for clinical pharmaceutical applications.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Prodrug of antitumor medicament as well as preparation method and application thereof
InactiveCN103861116AImprove anti-tumor efficacyLow toxicityOrganic active ingredientsOrganic chemistryMicrosphereDocetaxel
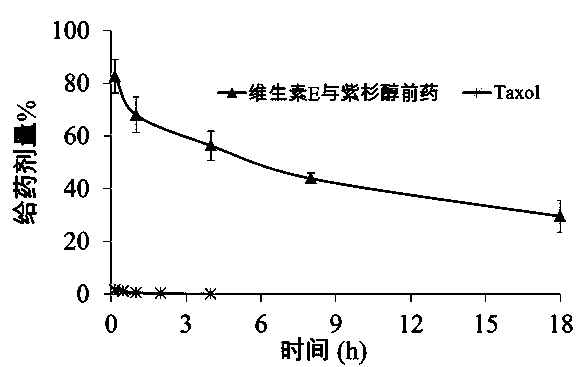
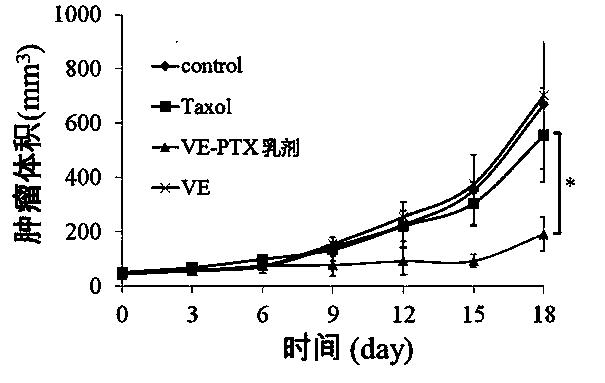
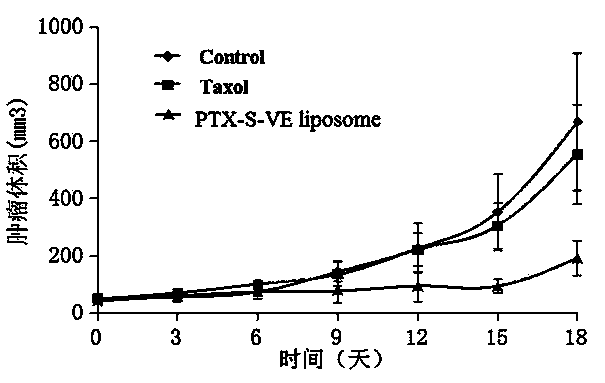
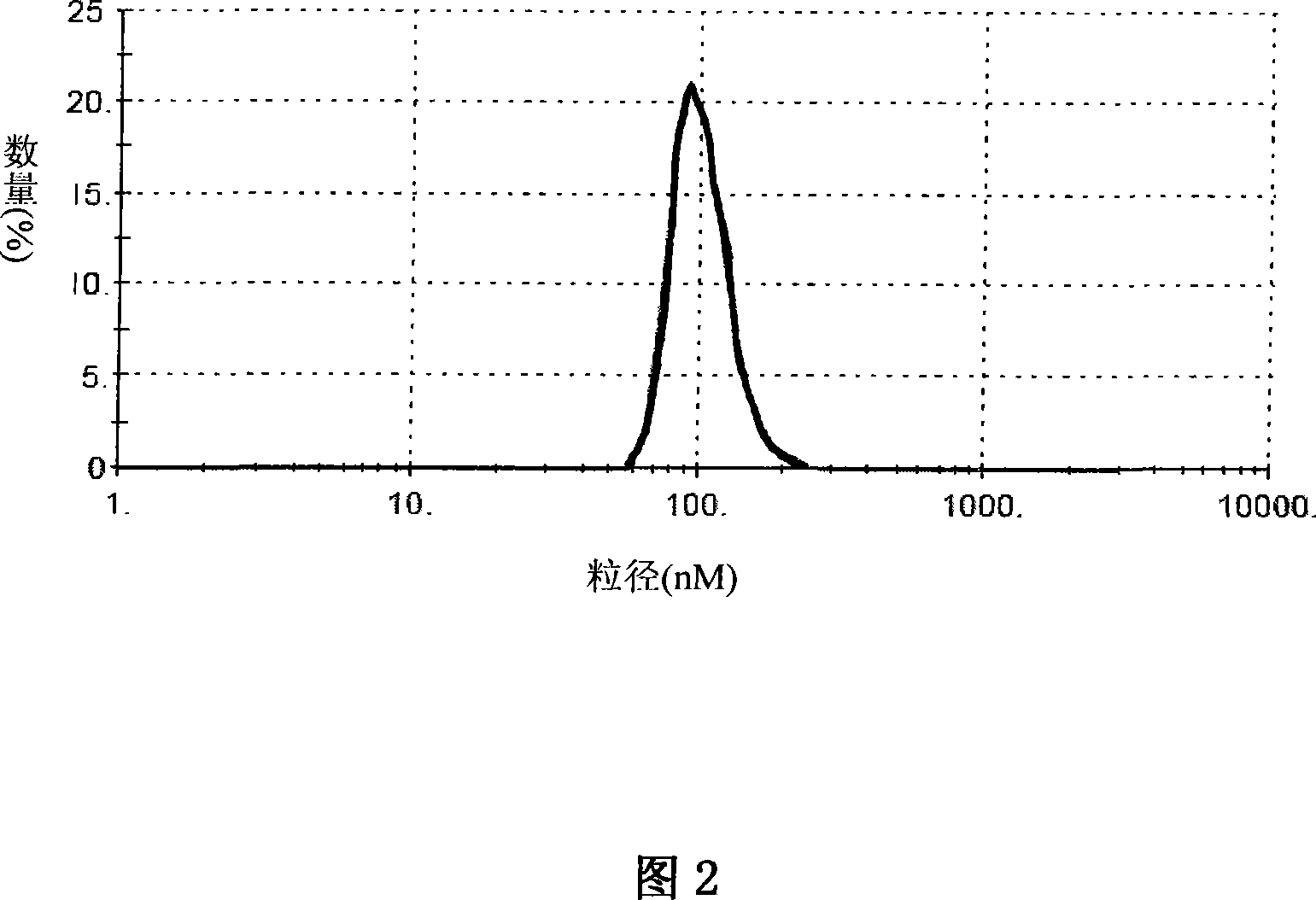
The invention belongs to the technical field of medicines and relates to a prodrug of an antitumor medicament as well as a preparation method and application thereof. The prodrug is prepared by connecting vitamin E and the antitumor medicament by virtue of a chemical bond. The chemical bond is one of a sulfide bond, an ester bond, an interval disulfide bond and a hydrazone bond. The antitumor medicament is one of paclitaxel and docetaxel. The prodrug of the antitumor medicament and a pharmaceutically acceptable carrier can be prepared into a clinically acceptable dosage form for injection or oral administration, including lipidosome, an emulsion, a lipid microsphere and a PLGA (Poly(Lactic-co-Glycolic Acid) nanoparticle or microsphere. According to the prodrug and the preparation, the activity of the antitumor medicament can be obviously improved, the toxicity is reduced and the development of the preparation is facilitated.
Owner:沈阳药大制剂新技术有限公司
Cationic elaioplast and its adenovirus composition, its preparing method and use
ActiveCN101045037AImprove stabilityUnique formulaGenetic material ingredientsPharmaceutical non-active ingredientsCholesterolElaioplast
A cationic liposome is proportionally prepared from DOPE, DOTMA and cholesterol and can be used to encapsulate the object (adenovirus carrier) to obtain a recombinant human endostatin adenovirus-cationic liposome composition carrying the active antineoplastic genes for suppressing the growth of tumor. Its preparing process is also disclosed.
Owner:SICHUAN UNIV
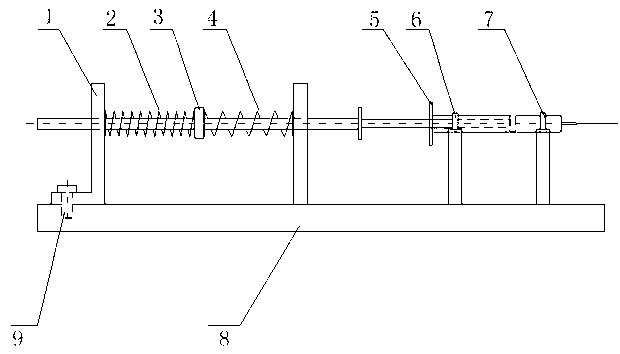
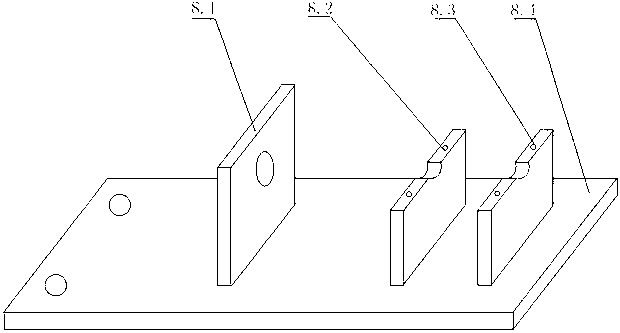
Syringe-microinjection-type infusion device based on shape memory alloy driven and method thereof
The invention discloses a syringe-microinjection-type infusion device based on shape memory alloy driven and a method thereof. A baffle, a first fixing plate and a second fixing plate are arranged on a base plate. Positioning holes are formed on a support plate and the baffle. Semi-cycle fixing grooves are formed on the first fixing plate and the second fixing plate. A stopping block is arranged in the middle of a push rod. Two sides of the stopping block are provided with a shape memory alloy spring and a reset spring. The end, with the reset spring mounted on, of the push rod penetrates the positioning hole on the baffle and contacts with a disposable syringe. The disposable syringe is placed in the semi-cycle fixing grooves of the first fixing plate and the second fixing plate, and is fixed on the first fixing plate and the second fixing plate through a first clamp and a second clamp. The end, with the shape memory alloy spring mounted on, of the push rod penetrates the positioning hole on the support plate which is fixed on the base plate through a bolt. The syringe-microinjection-type infusion device has the advantages that structure is simple, no noise is produced, size is small and the like, and is adaptable to micro-drug injection and the like.
Owner:ZHEJIANG UNIV
Traditional Chinese medicine composition with sleep improving function
InactiveCN105596702AExtensive resourcesReduce manufacturing costOrganic active ingredientsNervous disorderSide effectGamma-Aminobutyric acid
The invention belongs to the technical field of traditional Chinese medicine, relates to a traditional Chinese medicine composition with a sleep improving function, and further relates to a preparation with the composition as the main component, and a preparation method of the preparation. The traditional Chinese medicine composition is prepared from 50-300 parts of American ginseng, 500-800 parts of fried spina date seeds, 75-200 parts of fructus schizandrae, 200-600 parts of lucid ganoderma, 250-450 parts of lily, 250-450 parts of lotus seeds and 25-50 parts of gamma-aminobutyric acid. The traditional Chinese medicine composition is prepared through the method including the steps of weighing lucid ganoderma, fried spina date seeds, lily, lotus seeds and fructus schizandrae, and adding water for decocting 2-3 times, wherein 8-12 times of water is added each time, soaking is conducted for 1-2 hours before each time of decocting, decocting is conducted for 1-2 hours, obtained decoctions are filtered and then combined and condensed, and American ginseng powder and gamma-aminobutyric acid are added after the decoctions are dried. The prepared traditional Chinese medicine composition is mainly prepared from traditional Chinese medicine, raw materials are wide in resource, low in price and easy to obtain, and the composition is low in production cost, free of dependence after long-term use, free of body accumulation, free of medicine resistance, safe, free of toxic and side effects and definite in sleep improving function.
Owner:LIAONING TAIYANG PHARMA TECH DEV
Ornithine aspartate injection and preparation method thereof
ActiveCN107441038AAvoid decomposition catalysisReduce hydrolysisOrganic active ingredientsNervous disorderIonDecomposition
The invention relates to the field of drug preparations and particularly relates to an ornithine aspartate injection and a preparation method thereof. Aiming at the phenomenon that impurities such as ornithamide, arginine, dimerized ornithine and the like exceed standard caused by instability of the existing ornithine aspartate injection in the storage and usage processes, the prescription adopted by the invention is as follows: the ornithine aspartate injection is prepared from pre-purified ornithine aspartate, a metal ion complexing agent, a pH buffering agent and injection water, wherein the pH buffering agent is used for maintaining stable pH of the injection; the metal ion complexing agent is capable of avoiding the decomposition and catalysis actions of metal ions on the main drugs. The preparation method of the ornithine aspartate injection comprises two steps of pre-purifying ornithine aspartate and preparing drug liquid, wherein the step of pre-purifying adopts a recrystallization manner; the drug liquid preparation process adopts two sterilization manners of sterilizing for 15 minutes by using high-pressure steam at 121 DEG C and sterilizing by using a micropore filtration membrane with the pore size of 0.22 micron. The ornithine aspartate injection is stable in physical and chemical properties and relatively low in content of impurities, and can be stored for a relatively long time.
Owner:JINAN KANGHE MEDICAL TECH
Method for processing Abelmoschus manihot
ActiveCN103591772AGuaranteed efficacyEasy to storeDrying using combination processesPlant ingredientsMaterials scienceWater content
The invention discloses a method for processing Abelmoschus manihot. The method includes the steps that sorting is carried out; the Abelmoschus manihot is placed into a tray; the temperature is lowered gradually till the temperature is lower than the eutectic point temperature of the Abelmoschus manihot, and the Abelmoschus manihot is frozen for 6-10h at the temperature of -35DEG C to -40DEG C; vacuuming is carried out when the temperature reaches -40DEG C, and heating is carried out when the vacuum degree reaches 200-260Pa; the first step of heating is carried out, specially, the temperature is raised to -25DEG C from -40DEG C in 2h and kept for 1h when reaching -25DEG C, the temperature is raised to -10DEG C from -25DEG C in 2h and kept for 1h when reaching -10DEG C, the temperature is raised to 5DEG C from -10DEG C in 2h and kept for 1h when reaching 5DEG C, the temperature is raised to 20DEG C from 5DEG C in 2h and kept for 1h when reaching 20DEG C, the temperature is raised to 35DEG C from 20DEG C in 2h and kept for 1h when reaching 35DEG C, and the temperature is raised to 40DEG C from 35DEG C in 2h and kept for 1h when reaching 40DEG C; the second step of heating is carried out, specially, the temperature is kept ranging from 35DEG C to 40DEG C, and processed Abelmoschus manihot is discharged when the water content is less than 3%. An Abelmoschus manihot medicinal material prepared through the method has essential characteristics of fresh medicine, can keep medicinal properties of fresh traditional Chinese medicine for a long time, and is convenient to store and transport and suitable for industrial production and clinical use, and pharmaceutical effects can be guaranteed.
Owner:西藏求本生物科技发展有限公司
Implantable cyanoacrylate medical adhesive and application thereof
PendingCN113827765AImprove securityGood flexibilitySurgical adhesivesTissue regenerationCyanoacrylatePlasticizer
The invention relates to an implantable cyanoacrylate medical adhesive and an application thereof. The medical adhesive comprises the following components in parts by mass: 60 to 80 parts of cyanoacrylate, 10 to 30 parts of a plasticizer, 0.3 to 5 parts of a polymerization inhibitor, 0 to 5 parts of a flexibilizer and 0 to 5 parts of a stabilizer. The addition amounts of the flexibilizer and the stabilizer are not 0 at the same time. Compared with the prior art, the medical adhesive has the controllable curing speed, low heat release during bonding, high bonding strength, capability of being injected into veins to cure and block blood vessels and relieve varicosity, soft cured film and capability of reducing foreign body sensation.
Owner:ENOVE PRECISION PLASTICS CATHETER
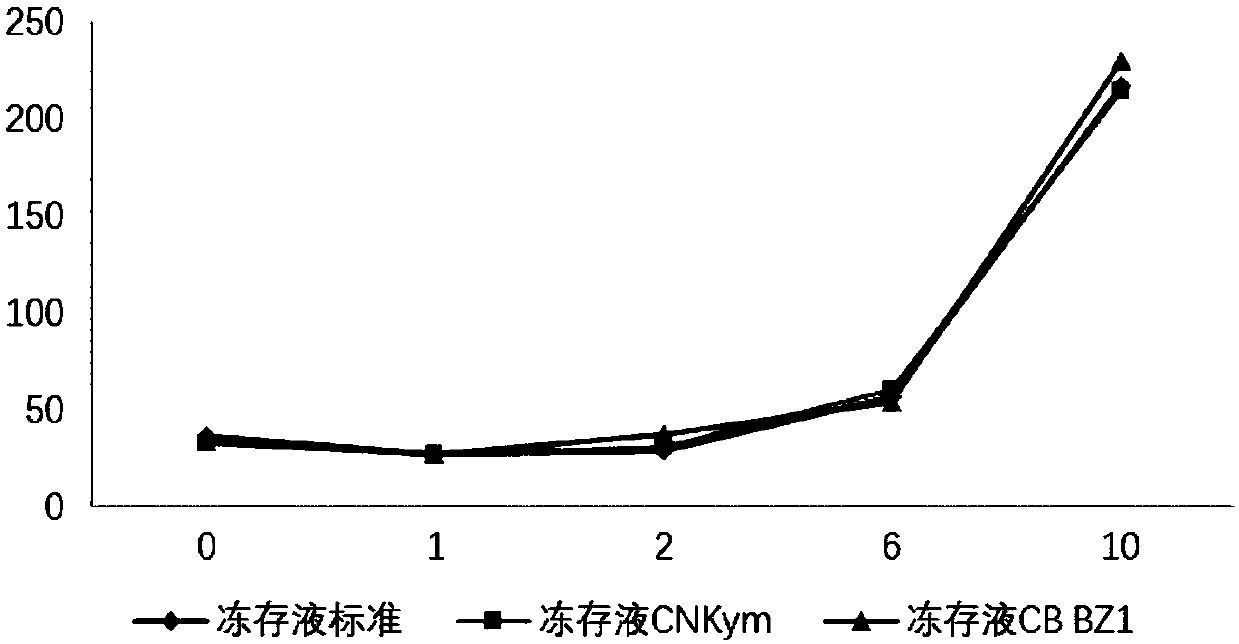
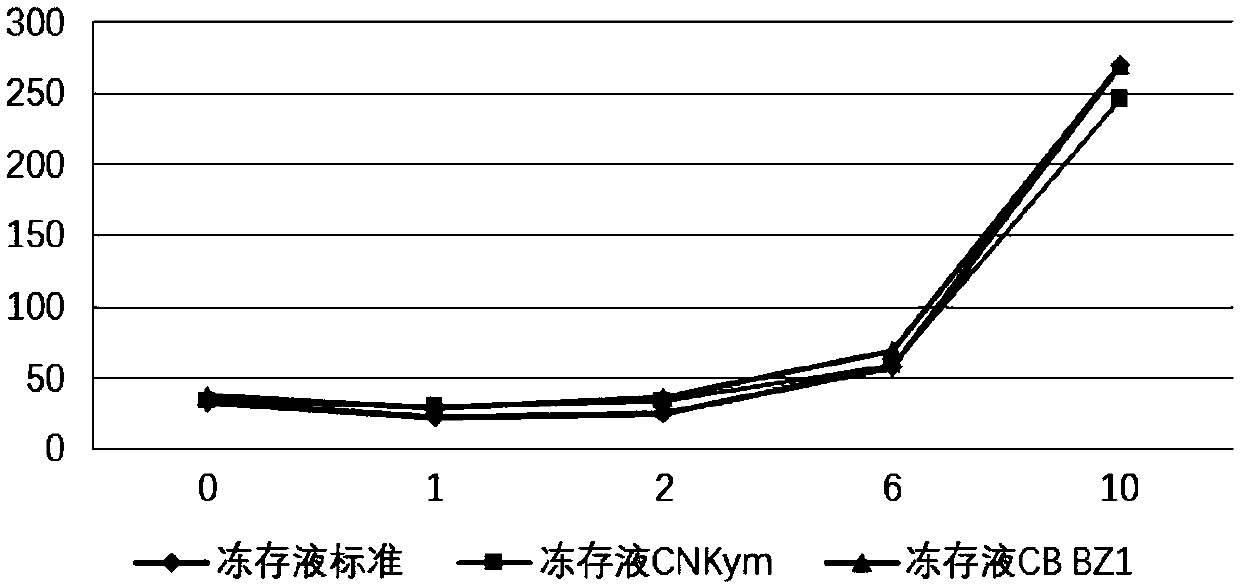
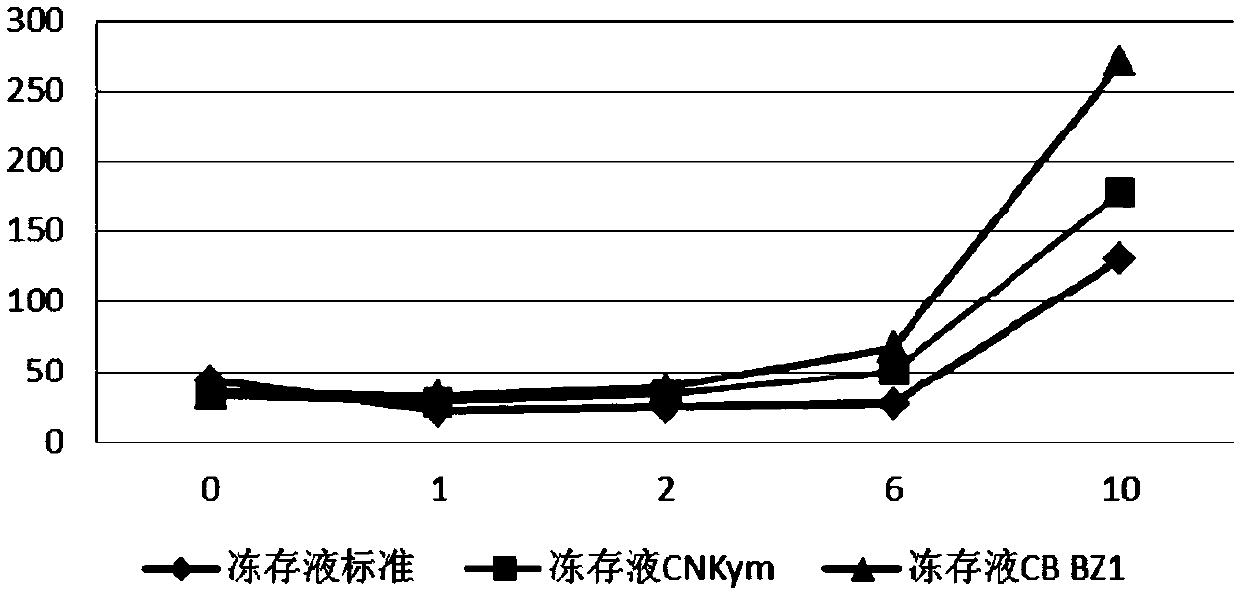
Immune effector cell cryoprotectant and application thereof
ActiveCN110959606AHigh activityIncreased proliferationDead animal preservationSodium acetateHuman albumin
The invention relates to an immune effector cell cryoprotectant and application thereof. Specifically, the invention provides a cell cryoprotectant which comprises human albumin, dimethyl sulfoxide, dextran, glucose, sodium chloride, sodium gluconate, sodium acetate, potassium chloride, magnesium chloride and water. The invention also provides application of the cell cryoprotectant in preparationof immune effector cell suspension. The cell cryoprotectant has the advantages of improving the viability and the proliferation level of immune effector cells, and has excellent application prospects.
Owner:SHANGHAI CELL THERAPY GRP CO LTD
Pharmaceutical composition comprising cyclodextrin paclitaxel inclusion and preparation method thereof
ActiveUS20100041625A1Reduce animal body weightImprove anti-tumor activityBiocideOrganic active ingredientsExcipientBeta-Cyclodextrins
A pharmaceutical composition comprising cyclodextrin / paclitaxel inclusion, which consists of paclitaxel, cyclodextrin and a pharmaceutically acceptable excipient, wherein the mass ratio of the paclitaxel to cyclodextrin is 1:10-150, the said cyclodextrin is hydroxylpropyl-sulfobutyl-7-β-cyclodextrin, or sulfobutylether-7-β-cyclodextrin, or their mixture; the stability constant of the cyclodextrin / paclitaxel inclusion is Ka=5396M−1−1412M−1. The preparation method of the pharmaceutical composition is as follow: (a) A solution of cyclodextrin is added dropwise to a solution of paclitaxel in ethanol. (b) The resulting mixture is filtered through microporous membrane of 0.2-0.4 μm after being dissolved. (c) Ethanol is removed under reduced pressure to give a liquid inclusion which has the ethanol level of less than 2%, or alternatively water is also removed under reduced pressure, the resulting product is dried giving a solid inclusion.
Owner:SUN XIAODONG +1
Preparation method and application of connected conjugated linoleic acid and gemcitabine prodrug
ActiveCN102617679AImprove anti-tumor efficacyLow toxicityOrganic active ingredientsSugar derivativesEthyl chloroformateNitrogen gas
The invention relates to a preparation method and application of a connected conjugated linoleic acid and gemcitabine prodrug. The conjugated linoleic acid is connected with the gemcitabine via an amide bond. The preparation method comprises the following steps that: under the protection of nitrogen, the conjugated linoleic acid is dissolved in a solvent, and an activator is added at a low temperature of -15 DEG C under stirring to activate the conjugated linoleic acid to form a highly reactive mixed acid anhydride; the gemcitabine is dissolved in a solvent to be added dropwise to the mixed acid anhydride, and the reaction is undergone under stirring at room temperature; the solvent refers to one or a mixed solution of triethylamine, tetrahydrofuran, N,N-dimethyl formamide, and the activator is ethyl chloroformate; the reaction time is 2-72 hours, and the feed molar ratio of the gemcitabine to the conjugated linoleic acid is 1:(0.5-5); and the molar ratio of the activator to the conjugated linoleic acid is 1:(0.5-5). The object of the invention is to increase the anti-tumor efficacy of the gemcitabine.
Owner:PEKING UNIV
Traditional Chinese medicine preparation for treating insomnia
InactiveCN101653573ASedative and hypnoticReduce adverse reactionsHeavy metal active ingredientsNervous disorderCinnabarOyster
The invention discloses a traditional Chinese medicine preparation for treating insomnia, which is made of traditional Chinese medicine raw materials based on parts by weight: 8-12 parts of grass leafsweelflag rhizome, 10-14 parts of lotus plumule, 43-47 parts of lily, 18-22 parts of spina date seed, 8-12 parts of citrus chachiensis hortorum, 10-14 parts of poria cocos, 28-32 parts of light wheat, 18-22 parts of raw fossilizid, 18-22 parts of raw oyster shell, 18-22 parts of dried longan, 3-7 parts of backed licorice and 1-3 parts of cinnabar. The traditional Chinese medicine preparation hasthe functions of sedation and hypnosis, and reliable effect in treating insomnia with less untoward effect, thus being suitable for clinical use.
Owner:北京国奥健康科技研究院有限公司
Betulinic acid derivative, preparation method and application thereof
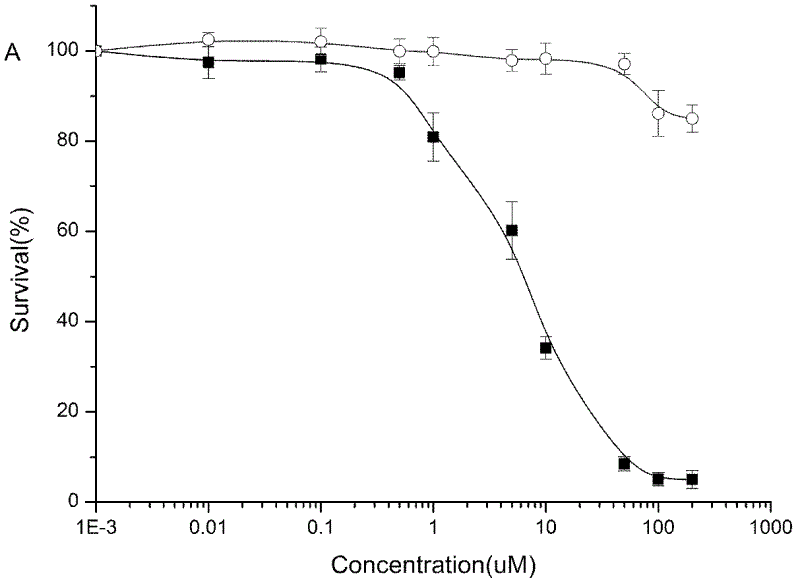
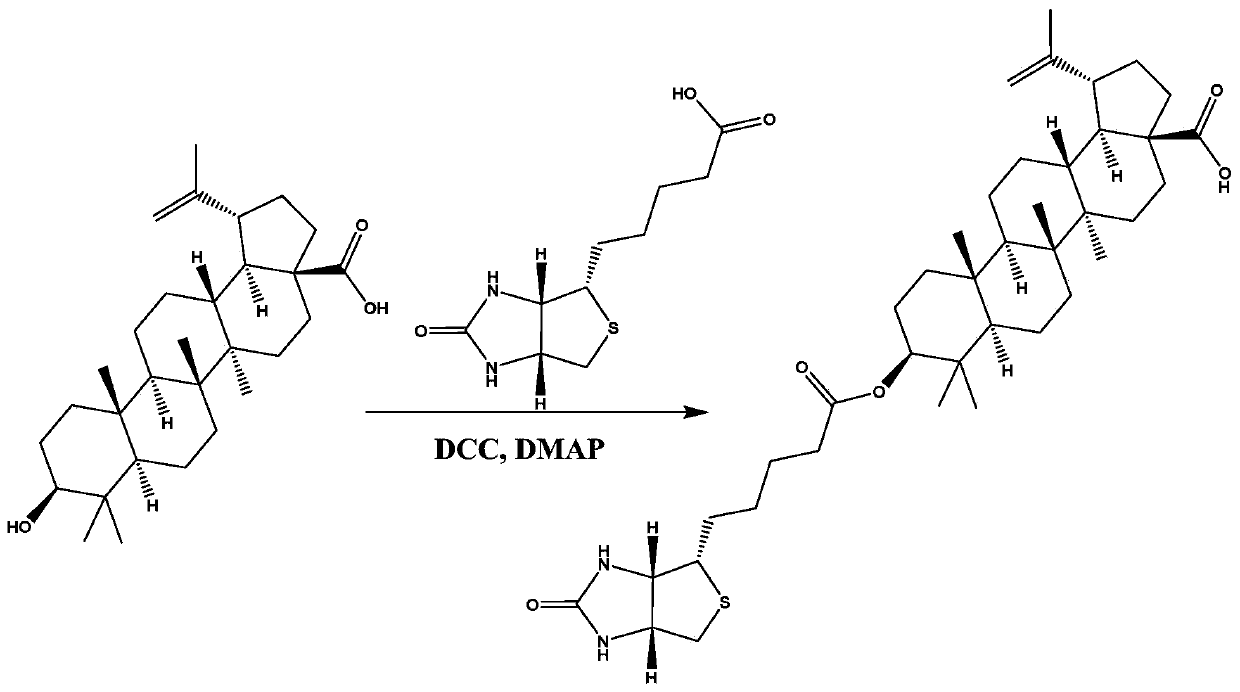
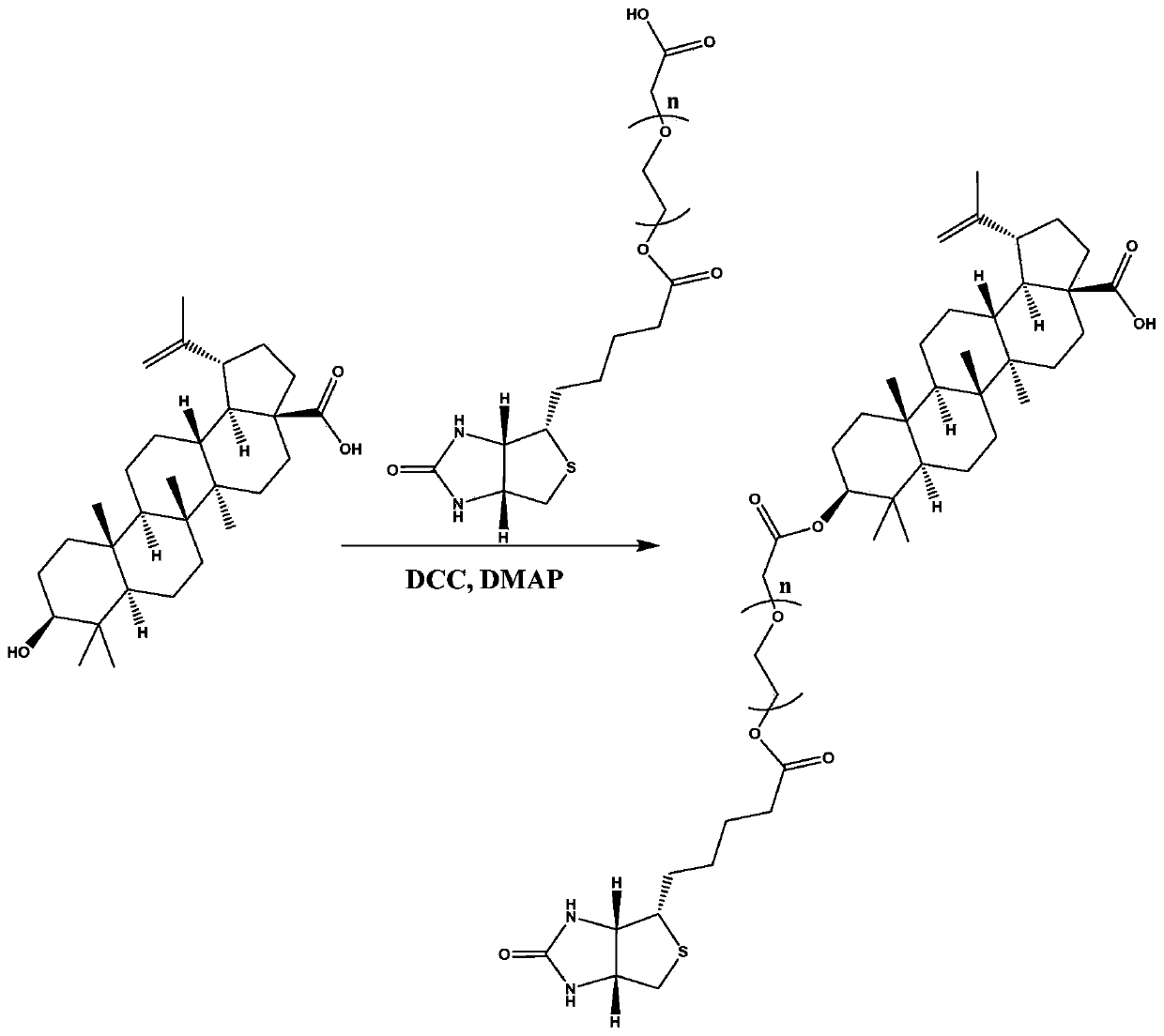
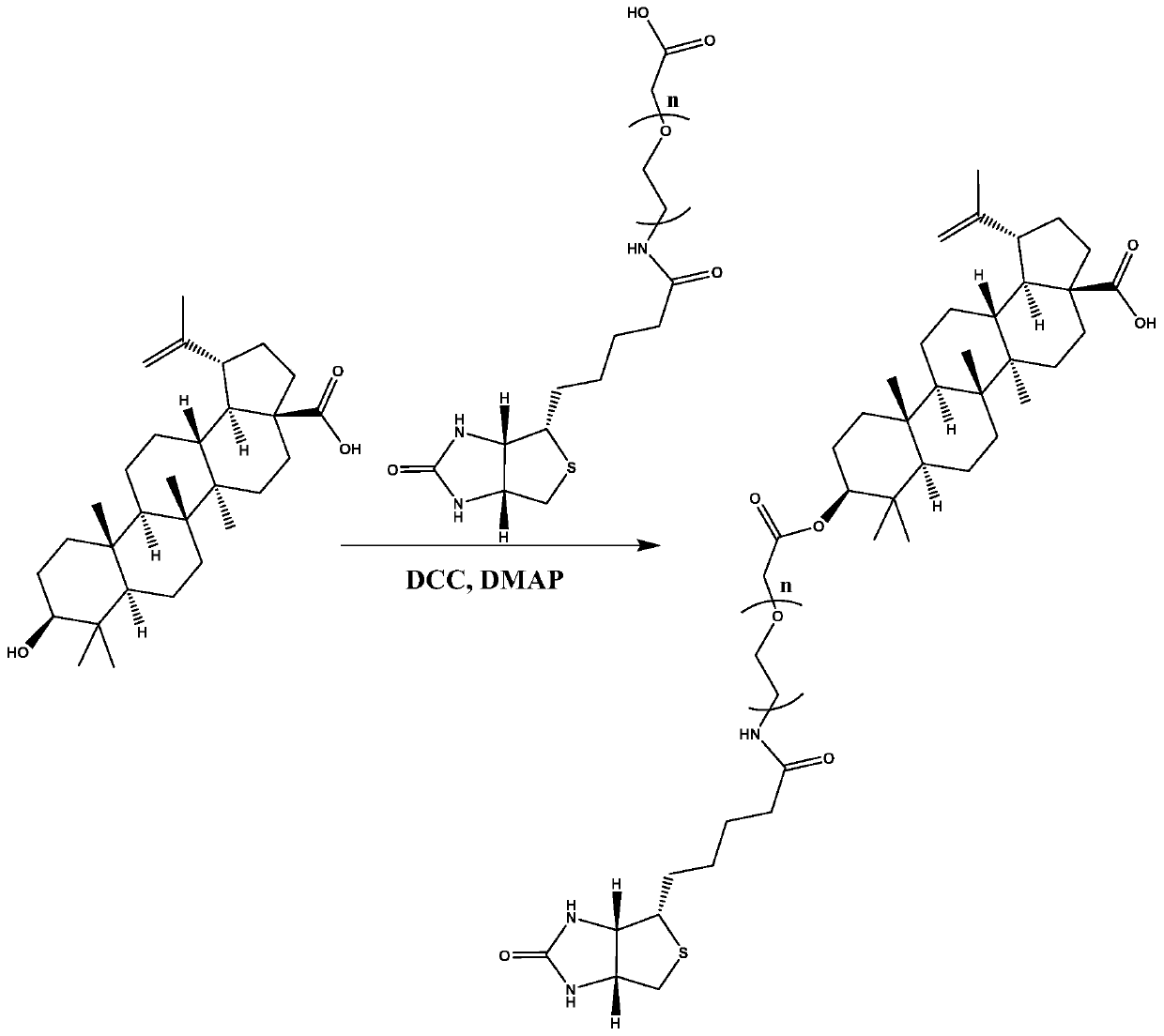
ActiveCN111333692AHigh selectivityGood water solubilityOrganic active ingredientsMetabolism disorderCarboxylic acidPharmaceutical medicine
The invention belongs to the technical field of biological medicines, and discloses a betulinic acid derivative, a preparation method and application thereof. The preparation method comprises the following steps: preparing biotin esterification coupled oligomerization ethylene glycol carboxylic acid or biotin amide coupled oligomerization ethylene glycol carboxylic acid; dissolving betulinic acidin a solvent, and carrying out a stirring reaction at 0 DEG C for 1-6 hours under the action of a dehydrating agent and a catalyst; adding biotin or the biotin esterification coupled oligomerization ethylene glycol carboxylic acid or biotin amide coupled oligomerization ethylene glycol carboxylic acid according to a betulinic acid molar ratio of 1:1-3, heating to room temperature from an ice bath,and stirring in a dark place overnight; and concentrating the filtrate, re-crystallizing with ice diethyl ether or isopropanol, carrying out chromatography or preparative liquid phase purification, and freeze-drying to obtain the betulinic acid derivative. The betulinic acid derivative, the pharmaceutically acceptable salt and the isotope marker thereof can be applied to preparation of anti-cancer drugs and drugs for treating obesity or non-alcoholic fatty liver disease.
Owner:湖南省中医药研究院
Fast-retractable type pressurizing injector
The invention provides a fast-retractable type pressurizing injector. A spiral push rod is arranged in an injection tube, an inner cam sleeve is embedded at the back end of the injection tube, a left lug boss and a right lug boss are symmetrically arranged on the inner wall of the inner cam sleeve, an upper opening screw nut and a lower opening screw nut are arranged on the spiral push rod and are respectively arranged in the inner cam sleeve, the upper ends of the outer edges of the middle parts of the upper opening screw nut and the lower opening screw nut are respectively provided with an upper convex baffle and a lower convex baffle 11, the upper convex baffle and the lower convex baffle are respectively and correspondingly butted against the left lug boss and the right lug boss, both ends of the upper opening screw nut and the lower opening screw nut are respectively in butt joint, and springs are respectively arranged at the joints of both ends of the upper opening screw nut and the lower opening screw nut. The invention has the advantages of scientific and reasonable design, original effect, good practical effect and better development prospects, and is suitable for clinic application.
Owner:SHENYANG LIGONG UNIV
Set of genes used for bladder cancer detection and application thereof
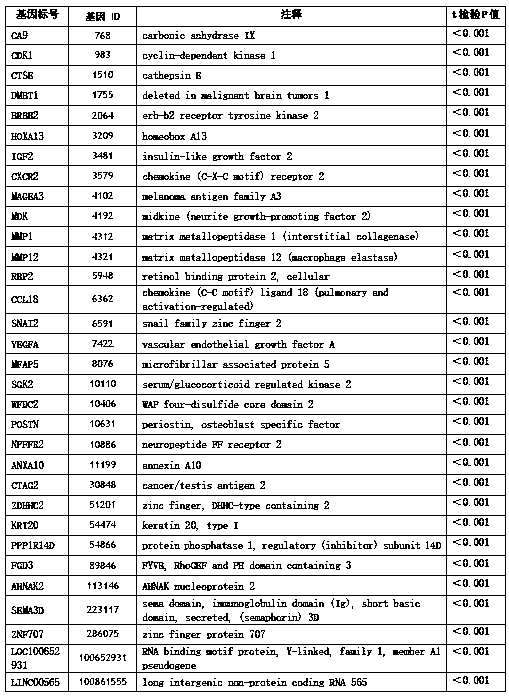
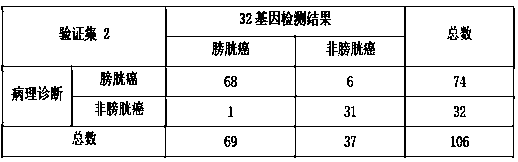
ActiveCN108866194AHigh yieldHigh purityMicrobiological testing/measurementBiostatisticsBladder cancerCCL18
The invention discloses a set of genes used for bladder cancer detection and application. The set of genes used for bladder cancer detection comprise the following 32 genes: a CA9 gene, a CDK1 gene, aCTSE gene, a DMBT1 gene, an ERBB2 gene, an HOXA13 gene, an IGF2 gene, a CXCR2 gene, an MAGEA3 gene, an MDK gene, an MMP1 gene, an MMP12 gene, a RBP2 gene, a CCL18 gene, an SNAI2 gene, a VEGFA gene, an MFAP5 gene, an SGK2 gene, a WFDC2 gene, a POSTN gene, an NPFFR2 gene, an ANXA10 gene, a CTAG2 gene, a ZDHHC2 gene, a KRT20 gene, a PPP1R14D gene, an FGD3 gene, an AHNAK2 gene, an SEMA3D gene, a ZNF707 gene, a LOC100652931 gene and an LINC00565 gene. High accuracy and objective result interpretation by adopting a detection kit of the set of genes for bladder cancer detection are proven clinically; meanwhile, as non-invasive detection, compared with existing cystoscopy, the detection kit has the advantage that the compliance of a patient is greatly improved, and the detection kit has importantclinical significance to early detection and postoperation monitoring of bladder cancer.
Owner:HANGZHOU CANHELP GENOMICS TECH CO LTD
Freeze-drying composition containing cerebroprotein hydrolysates and preparation method of freeze-drying composition
InactiveCN102166200ASuitable for clinical usePowder deliveryNervous disorderFreeze dryMANNITOL/SORBITOL
The invention provides a freeze-drying composition containing cerebroprotein hydrolysates and a preparation method of the freeze-drying composition. The freeze-drying composition containing the cerebroprotein hydrolysates consists of cerebroprotein hydrolysates, mannitol, sodium hydroxide and reduced type glutathione. The freeze-drying composition containing cerebroprotein hydrolysates can overcome the defects in the conventional cerebroprotein injection that the stability is poor and polypeptide matters are easily subject to environmental influence and generate polymerization, and are more suitable for clinical application.
Owner:罗诚
Spleen and stomach strengthening preparation for treating stomachache and preparation method of preparation
InactiveCN105125789ASignificant effectAppropriate compatibilityDigestive systemPlant ingredientsCrocosmiaCinnamomum burmannii
The invention discloses a spleen and stomach strengthening preparation for treating stomachache, wherein the preparation is mainly prepared from the following raw materials in parts by mass: 9-13 parts of coptis chinensis, 7-15 parts of rhizome of largeleaf Japanese ginseng, 9-16 parts of beet root, 6-14 parts of betel nut, 10-15 parts of bigfruit phymatopsis herb, 8-16 parts of creepingthymeleaf sandwort herb, 6-13 parts of girald daphne bark, 7-12 parts of ground common crocosmia, 9-14 parts of dentiferous dendropanax root and stem, 10-13 parts of burmann cinnamon bark, 8-15 parts of chinarue, 11-14 parts of girald acanthopanax bark, 9-15 parts of pilea cadierei and 7-13 parts of blueflower cicerbita root. The spleen and stomach strengthening preparation for treating stomachache disclosed by the invention has functions of warming stomach for dispelling cold, promoting qi circulation and relieving pain, promoting digestion and relieving dyspepsia, soothing liver and relieving depression, and removing dampness-heat, and the preparation is significant in curative effect on stomachache.
Owner:霍传生
Medicinal composition containing cefixime cyclodextrin inclusion compound and preparation thereof
InactiveCN101264086AGood water solubilityImprove solubilityAntibacterial agentsOrganic active ingredientsSolubilityHigh activity
The invention provides a medicine composition comprising the cefixime cyclodextrin inclusion compound. The basic composition comprises the cefixime and the pharmacy-acceptable cyclodextrin; the cyclodextrin is chosen from one or a plurality of Beta -cyclodextrin, sulfobutyl- Beta -cyclodextrin, hydroxypropyl- Beta -cyclodextrin or hydroxypropyl-sulfobutyl- Beta -cyclodextrin. The invention has the advantages of increasing the solubility and stability of the medicine, and higher activity. The invention also provides the preparation method of the medicine composition.
Owner:NANJING NORMAL UNIVERSITY +1
Manufacturing process of leonurus
The invention discloses a manufacturing process of leonurus. The process comprises the following steps of: selecting leonurus, washing and cutting and then draining off till the water content is 78-82%; placing the drained leonurus in a tray, wherein the weight in each tray is 2-4kg / m<2> and thickness is 2-3cm; gradually cooling for 2.5-3.2 hours and freezing at -30 to -37 DEG C; vacuumizing till the absolute pressure of the system is 70-100Pa, starting heating to 60-70 DEG C, and drying for 15-18 hours; and maintaining the temperature at 30-40 DEG C, and discharging till the water content is below 3%. The leonurus decoction piece obtained by the manufacturing process disclosed by the invention not only has the essential characteristics of fresh medicines to store the drug properties of fresh traditional Chinese medicines for a long time and ensure the effect, but also is convenient to store and convey and suitable for industrial production and clinical use. The effective components are further effectively prevented from being decomposed in the storage process, and the leonurus decoction piece has good use value and wide market prospect.
Owner:西藏求本生物科技发展有限公司
Pharmaceutical composition containing butyphthalide as well as application of pharmaceutical composition in preparing medicines for treating cerebrovascular diseases
ActiveCN107595874AGood treatment effectReduce the effective doseOrganic active ingredientsNervous disorderTherapeutic effectEffective treatment
The invention discloses a pharmaceutical composition, which consists of butyphthalide and mecobalamine. The pharmaceutical composition, when used for treating cerebrovascular diseases and dementia, can achieve a synergistic effect, wherein with the adoption of the mecobalamine, the therapeutic effect of the butyphthalide can be obviously enhanced, so that the effective dosage of the butyphthalideis reduced and a using dose is reduced; and the pharmaceutical composition, when used for a long time, can reduce the occurrence of adverse reactions. Therefore, the pharmaceutical composition can take a more effective on treating the cerebrovascular diseases and dementia, and a therapeutic effect can be achieved at a relatively low dose, so that the pharmaceutical composition is suitable for clinical application.
Owner:SHIJIAZHUANG PHARMA GRP NBP PHARMA CO LTD
Medicinal composition containing carbazochrome sodium sulfonate compound and preparation method thereof
ActiveCN102210656ASuitable for clinical usePowder deliveryOrganic active ingredientsSolubilityCarbazochrome Sodium Sulfonate
The invention provides a medicinal composition containing carbazochrome sodium sulfonate compound and a preparation method thereof. The medicinal composition containing carbazochrome sodium sulfonate compound consists of carbazochrome sodium sulfonate, mannitol, dextran and sodium hydroxide. The medicinal composition containing carbazochrome sodium sulfonate compound can solves the problems of low stability, low solubility and the like of the conventional carbazochrome sodium sulfonate powder injection and is more suitable for use in clinic.
Owner:福建康成医药有限公司
Preparation method of curcumin-containing medical dressing based on silk fibroin
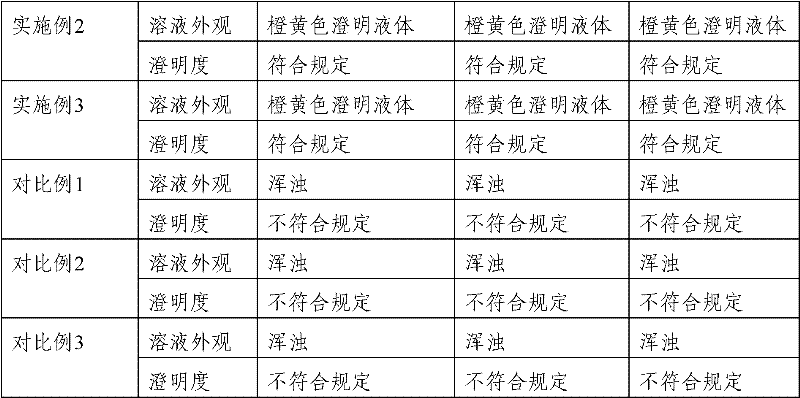
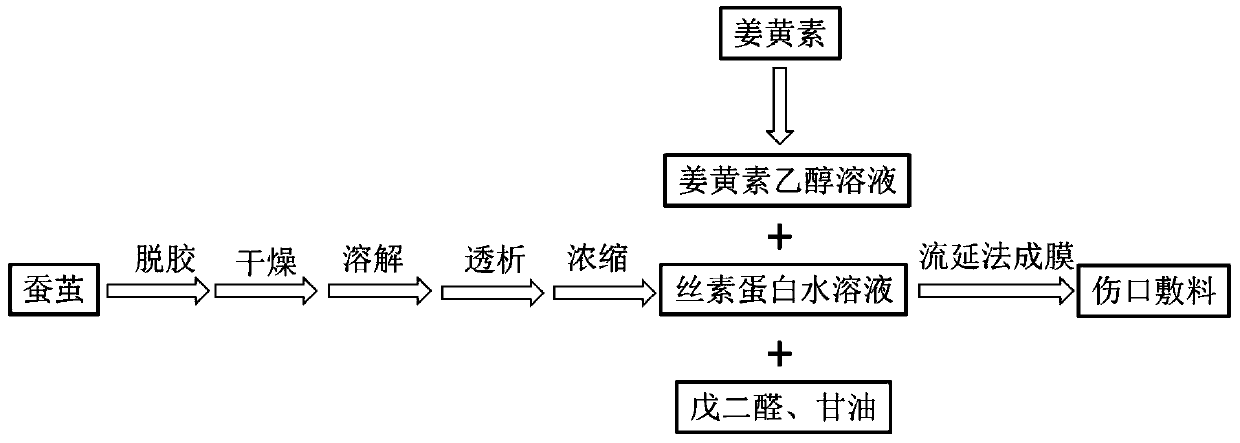
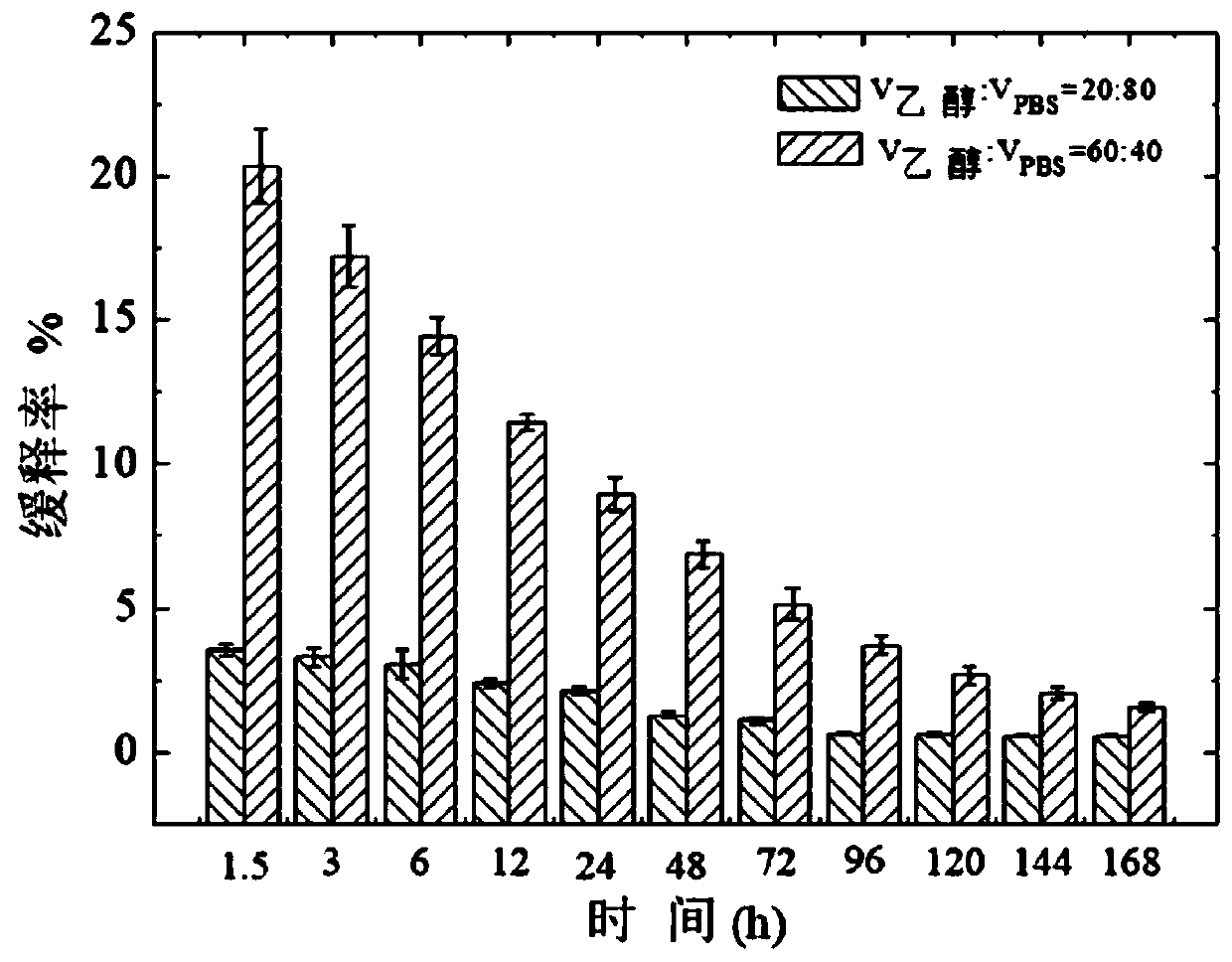
PendingCN110448715AFacilitated releaseControl slow release rateAbsorbent padsBandagesPorosityMoisture permeability
The invention provides a preparation method of a curcumin-containing medical dressing based on silk fibroin. According to the invention, silk cocoon is degummed, dried, dissolved, dialyzed, and concentrated to produce a silk fibroin aqueous solution, the silk fibroin aqueous solution is then mixed with glutaraldehyde, glycerin, and a curcumin ethanol solution to prepare the curcumin-containing medical dressing based on silk fibroin. The dressing prepared by the method effectively avoids the defect of easy penetration of bacteria in high porosity materials, and the prepared dressing has good air permeability and moisture permeability, which can provide the suitable environment for a wound area; the slow-release rate of curcumin can be adjusted by changing a slow-release medium; the producthas good antibacterial properties, the antibacterial rate against Staphylococcus aureus is higher than 70% within 12 hours, and is used for wound repair and is more conducive to cell proliferation andadhesion than a pure silk fibroin film.
Owner:SOUTHWEST UNIV
Compound fluconazole gel for pets and preparation process thereof
InactiveCN103083349AImprove uniformityImprove breathabilityHydroxy compound active ingredientsPharmaceutical delivery mechanismIrritationCurative effect
The invention belongs to the technological field of veterinary medicines. Specifically, the invention mainly relates to a prescription of a compound fluconazole gel treating mixed infection of bacteria, fungi and mites in pets and a preparation process thereof. The invention provides an externally applied gel preparation with good breathability and transdermal absorption, small irritation as well as good curative effects, i.e. the compound fluconazole gel. The compound fluconazole gel prepared according to the prescription and process provided in the invention overcomes the disadvantages of high oil content and poor breathability in existing veterinary compound fluconazole ointments, fills the blank of the veterinary drug market, and has the characteristics of good curative effects and good breathability.
Owner:QINGDAO VLAND BIOTECH INC
Fluorescent quantitative PCR detection kit for chlamydia trachomatis
InactiveCN105018573AStrong specificityShort detection timeMicrobiological testing/measurementFluoProbesA-DNA
The invention provides a fluorescent quantitative PCR detection kit for chlamydia trachomatis in clinic samples, used for assisted diagnosis of chlamydia trachomatis. The kit comprises a PCR solution, a DNA polymerase solution, positive quality control, weakly positive quality control, negative quality control, positive quantitative reference, lysate and protease K, wherein the PCR solution contains forward and reverse primers and the fluorescence probe are specific primers and probe designed for the specific sequence of chlamydia trachomatis and are capable of amplifying a target DNA sequence specifically so as to conveniently and quickly detect chlamydia trachomatis infection in clinic samples. The kit has the characteristics of high specificity and high sensitivity.
Owner:兰州安康伯乐生物技术有限公司
Traditional Chinese medicine composition for treating hyperthyroidism due to hyperactivity of heart-liver fire and preparation method thereof
InactiveCN104352987ALittle side effectsDefinite curative effectConiferophyta medical ingredientsEndocrine system disorderAdemetionineTreatment effect
The invention discloses a traditional Chinese medicine composition for treating hyperthyroidism due to hyperactivity of heart-liver fire and a preparation method thereof and belongs to the field of Chinese medicine. The traditional Chinese medicine composition for treating the hyperthyroidism due to hyperactivity of heart-liver fire is prepared from the following medicinal raw materials: selfheal, uncaria, semen cassiae, pinellia ternate, semen raphani, barbary wolfberry fruit, bidens tripartite, holly leaves, cortex lycii radicis, salviae miltiorrhizae, radix curcumae, platycladi seed and the like. The traditional Chinese medicine composition for treating the hyperthyroidism due to hyperactivity of heart-liver fire has the advantages that all the medicines are combined and jointly realize the effects of removing heat from the liver and purging intense heat, reducing phlegm and activating blood as well as eliminating goiter and eliminating stagnation, and the treatment effect on the hyperthyroidism due to hyperactivity of heart-liver fire is definite.
Owner:崔合芳
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com