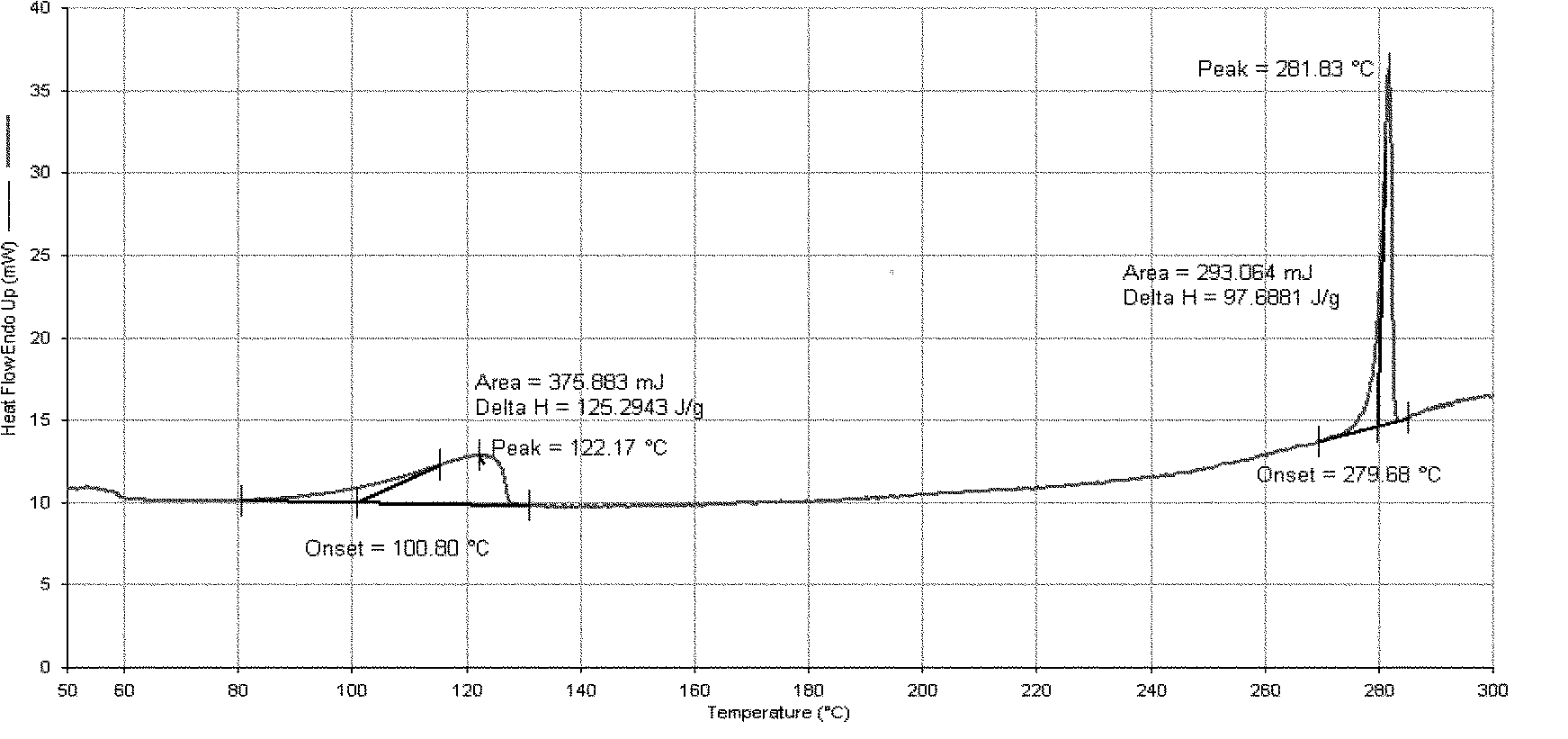
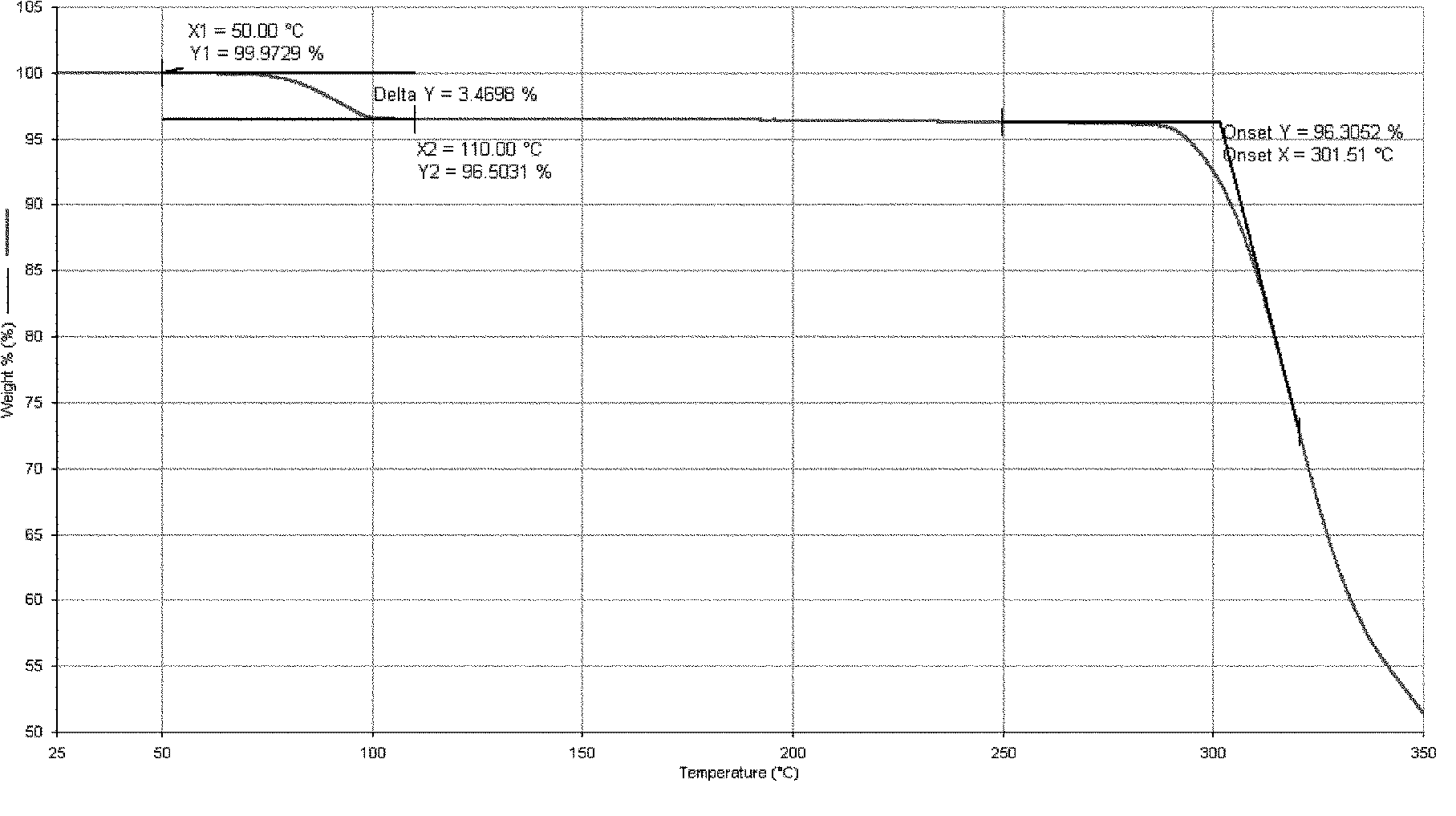
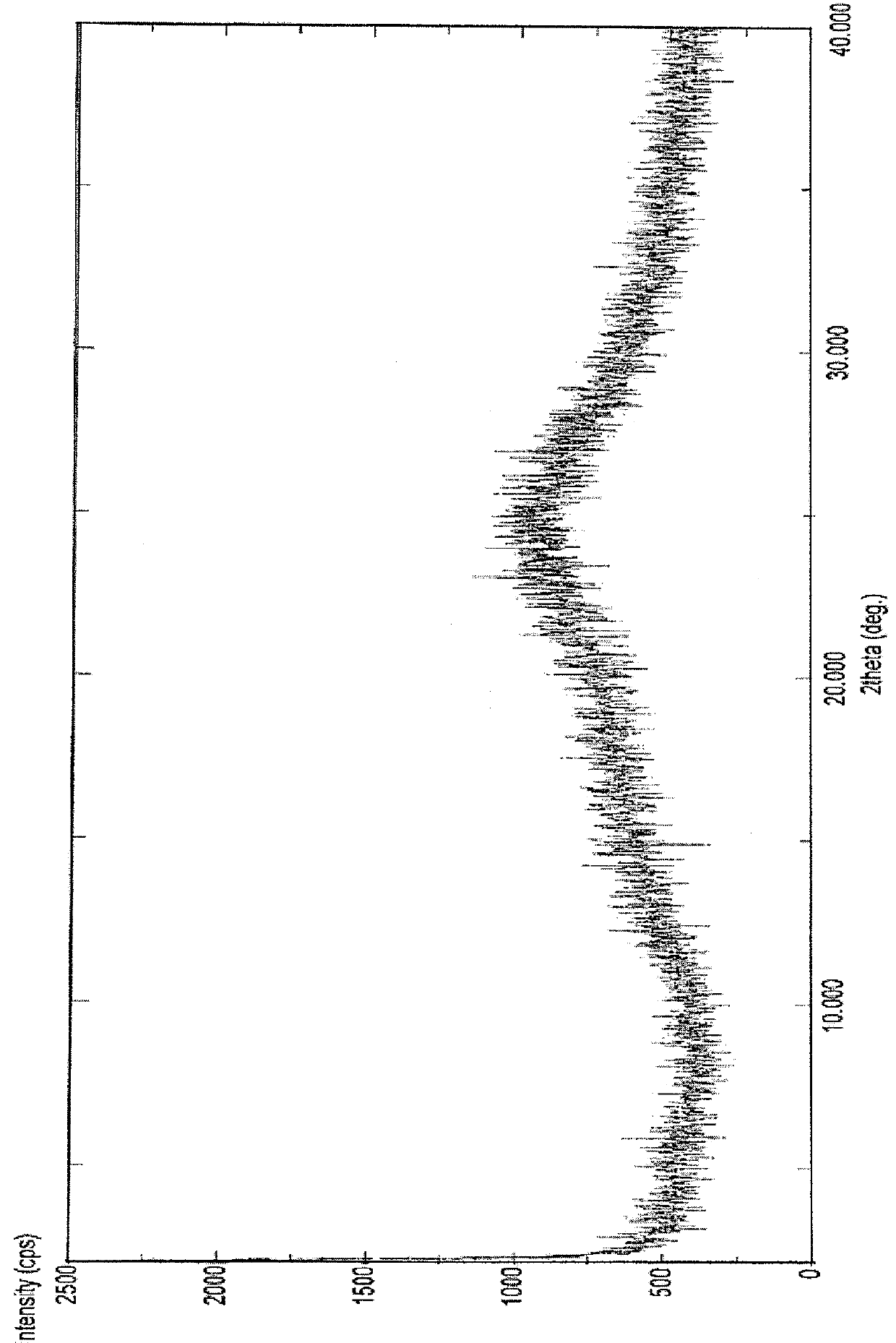
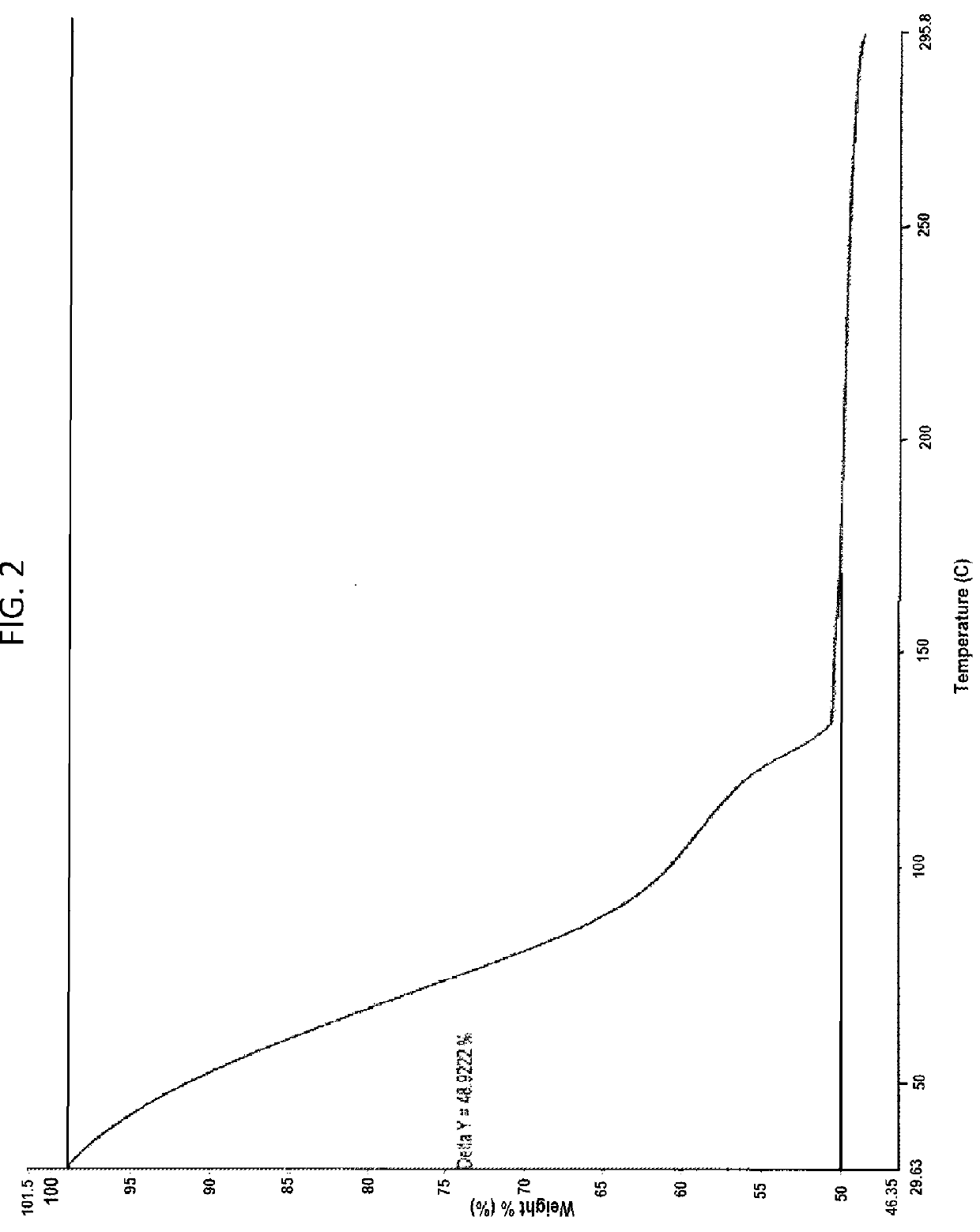
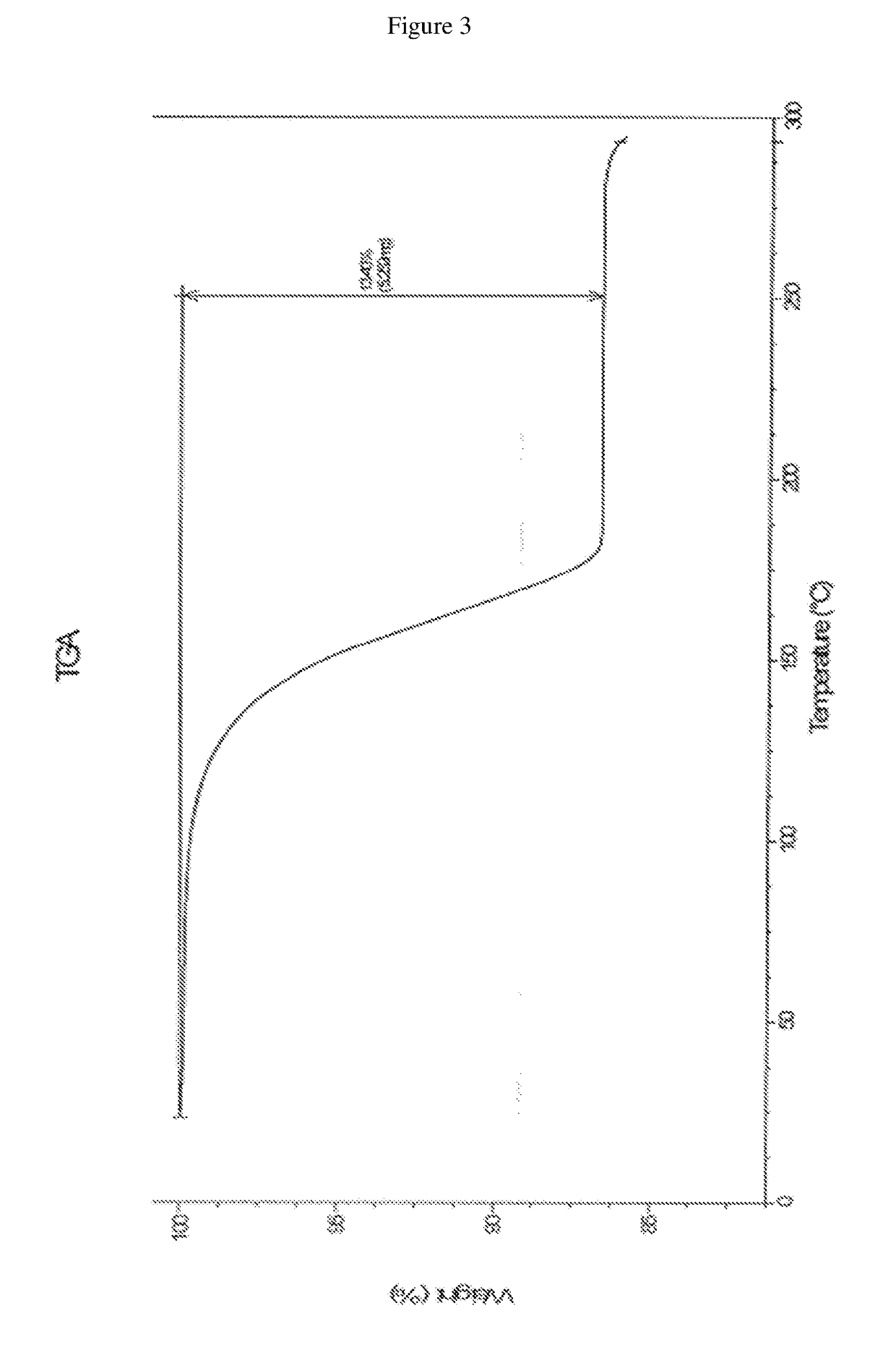
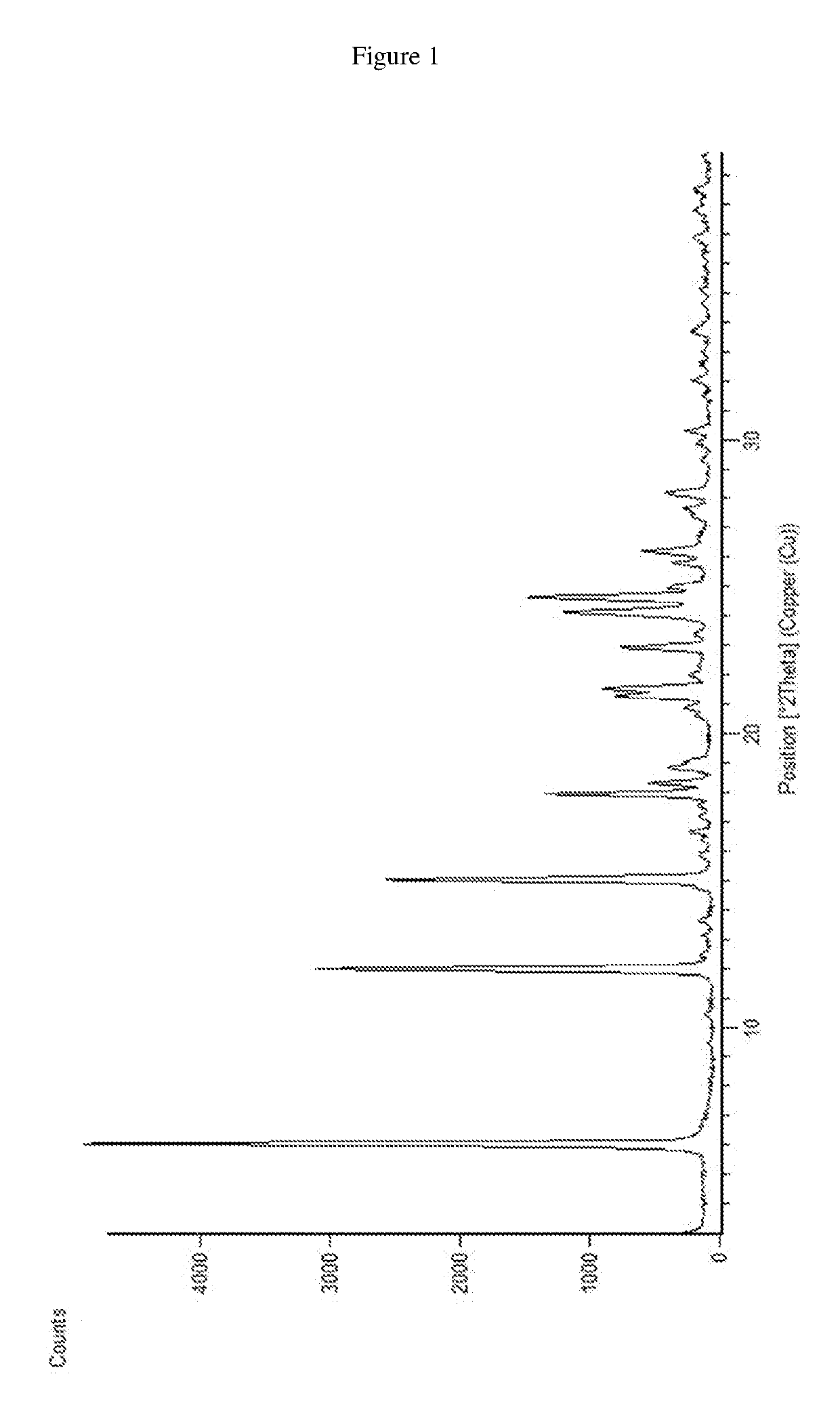
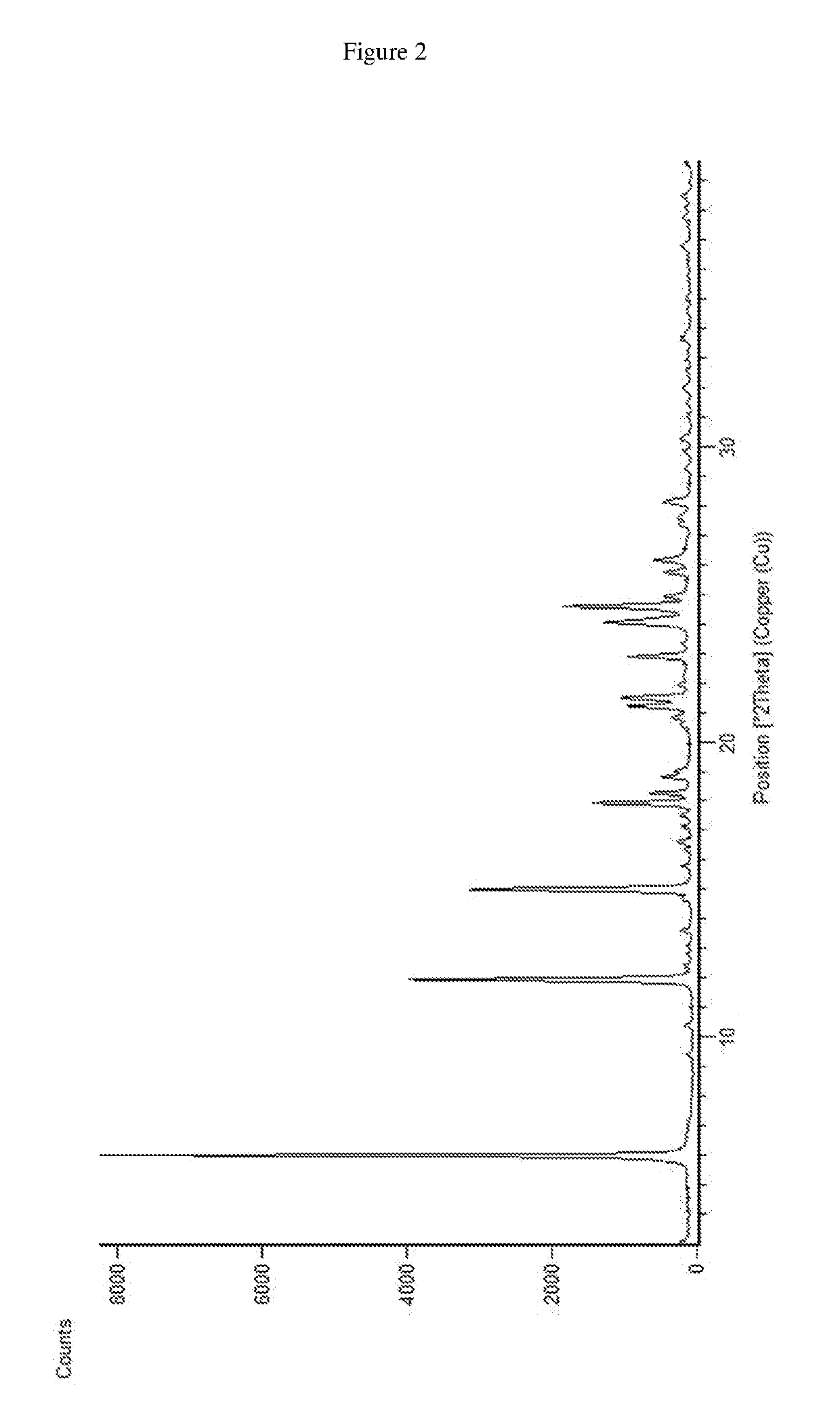
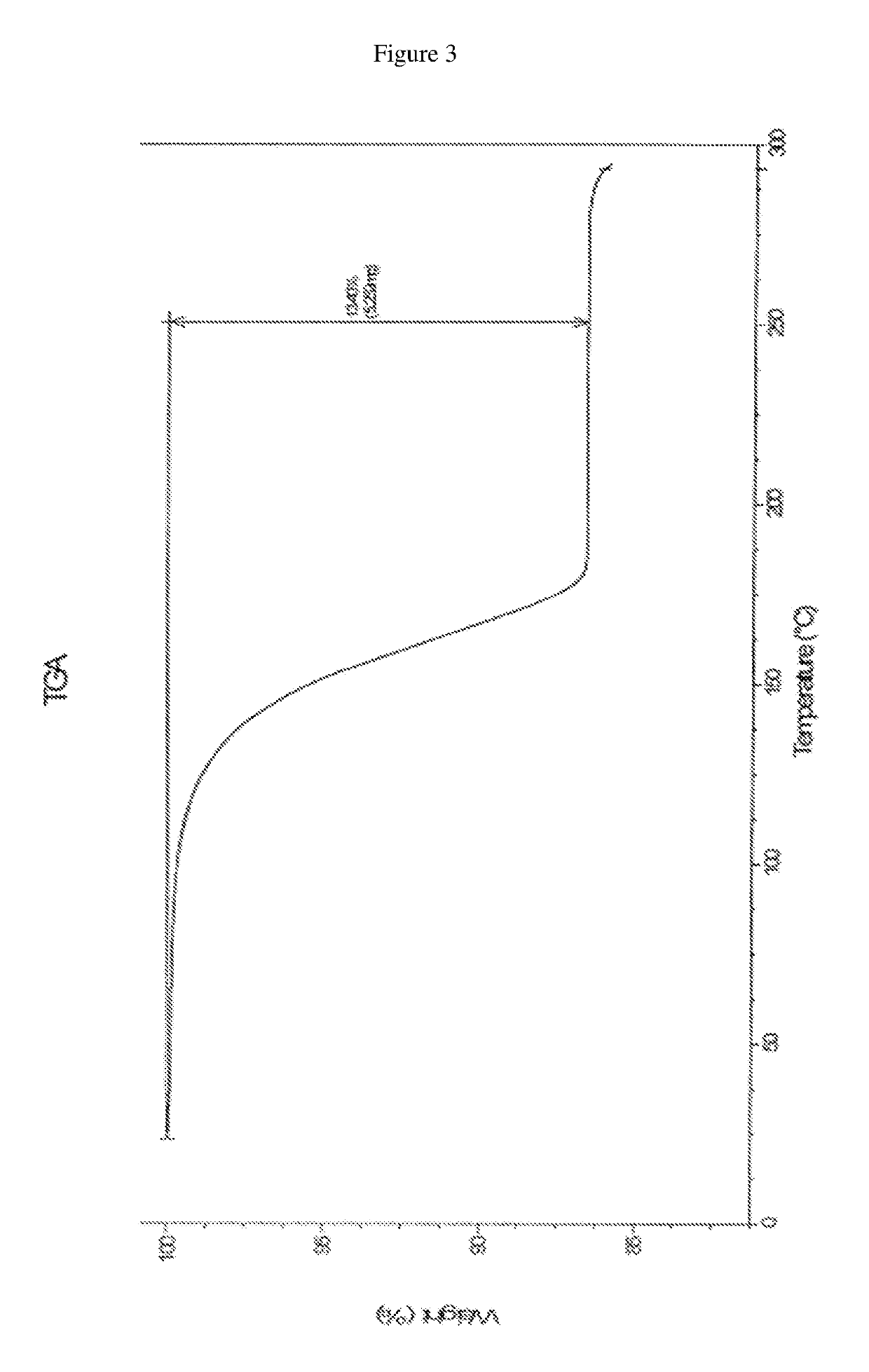
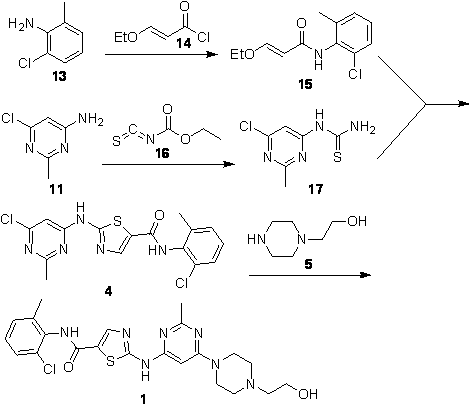
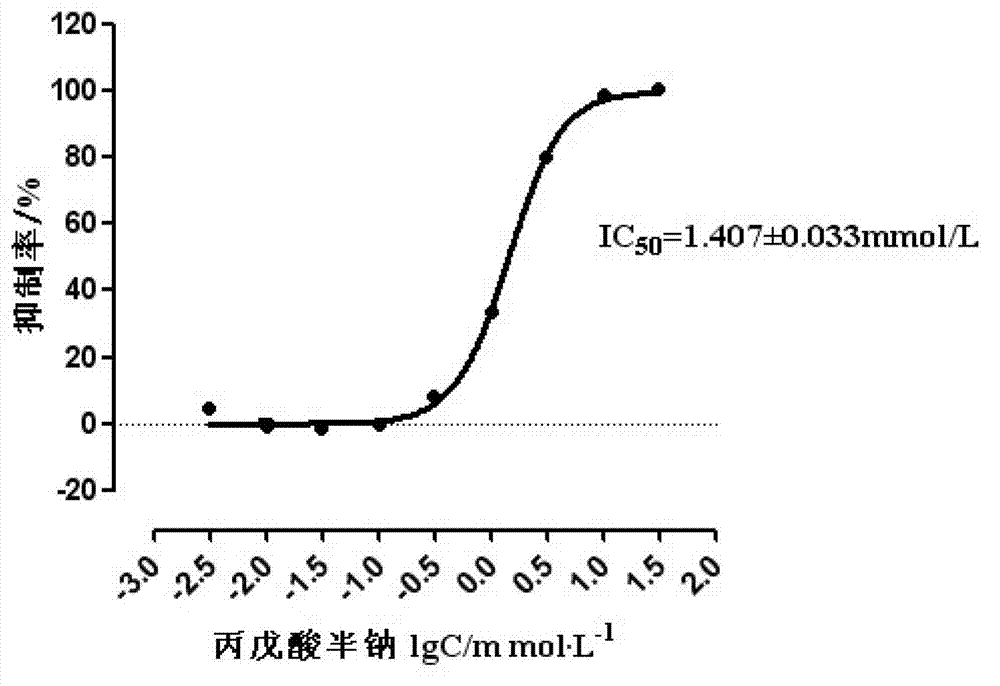
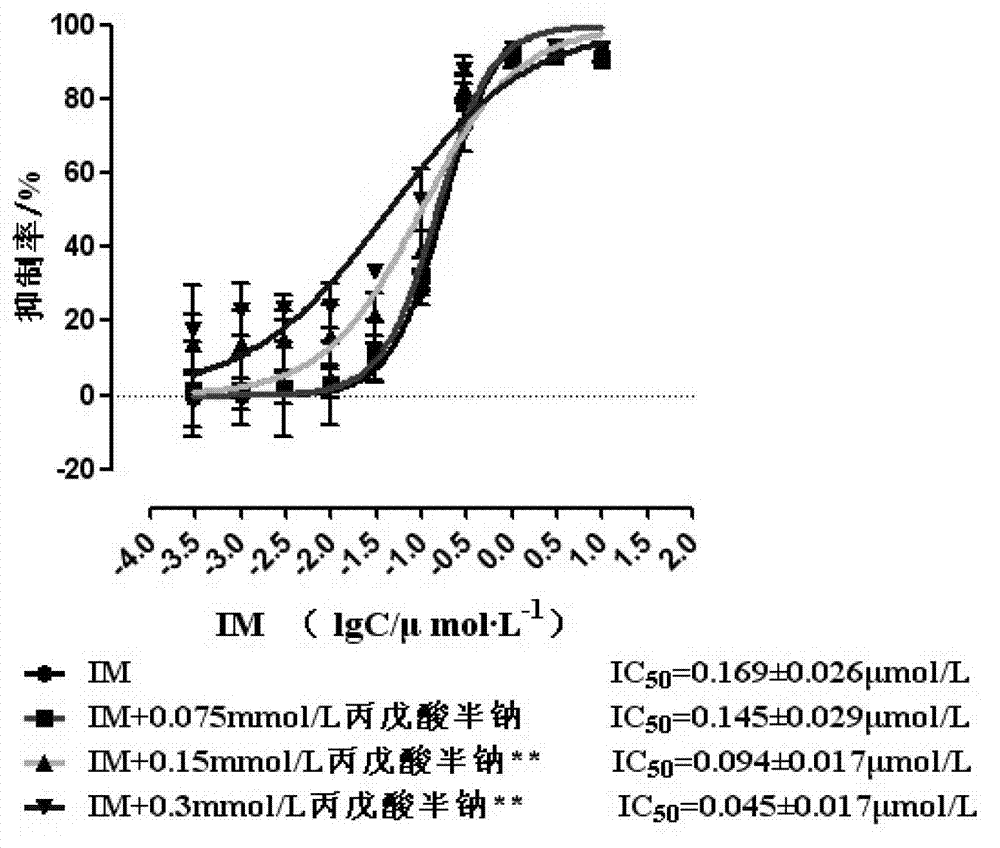
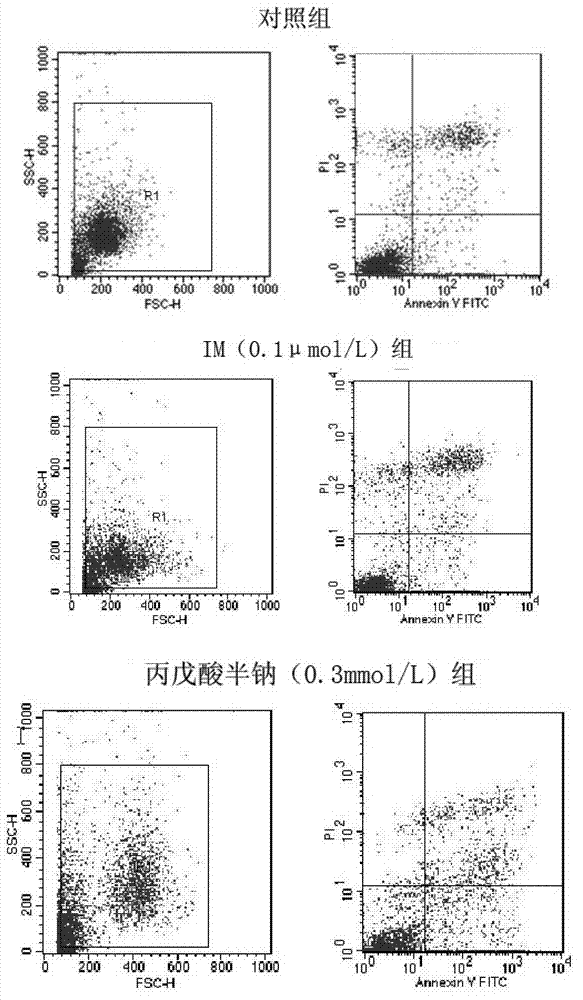
Patents
Literature
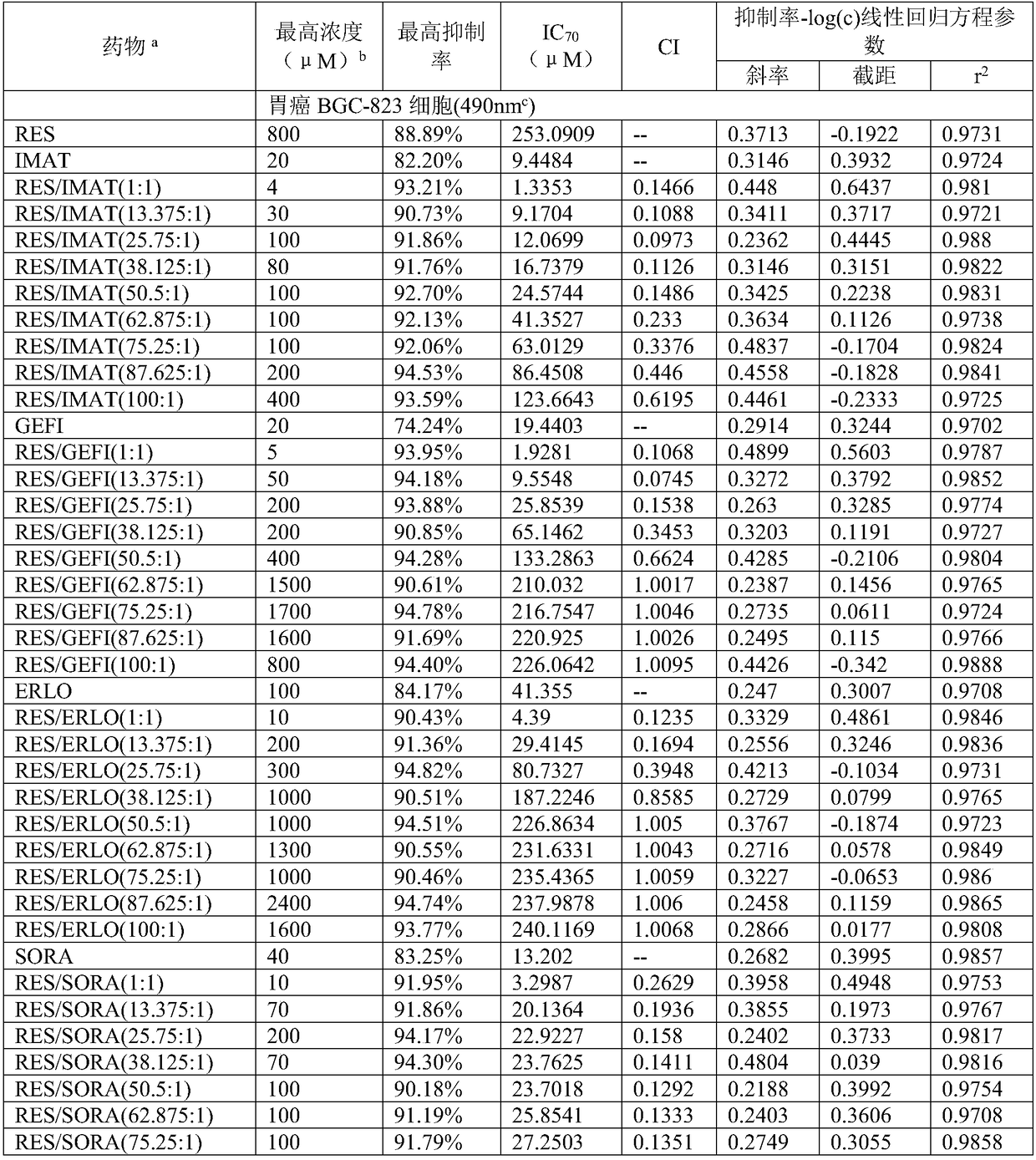
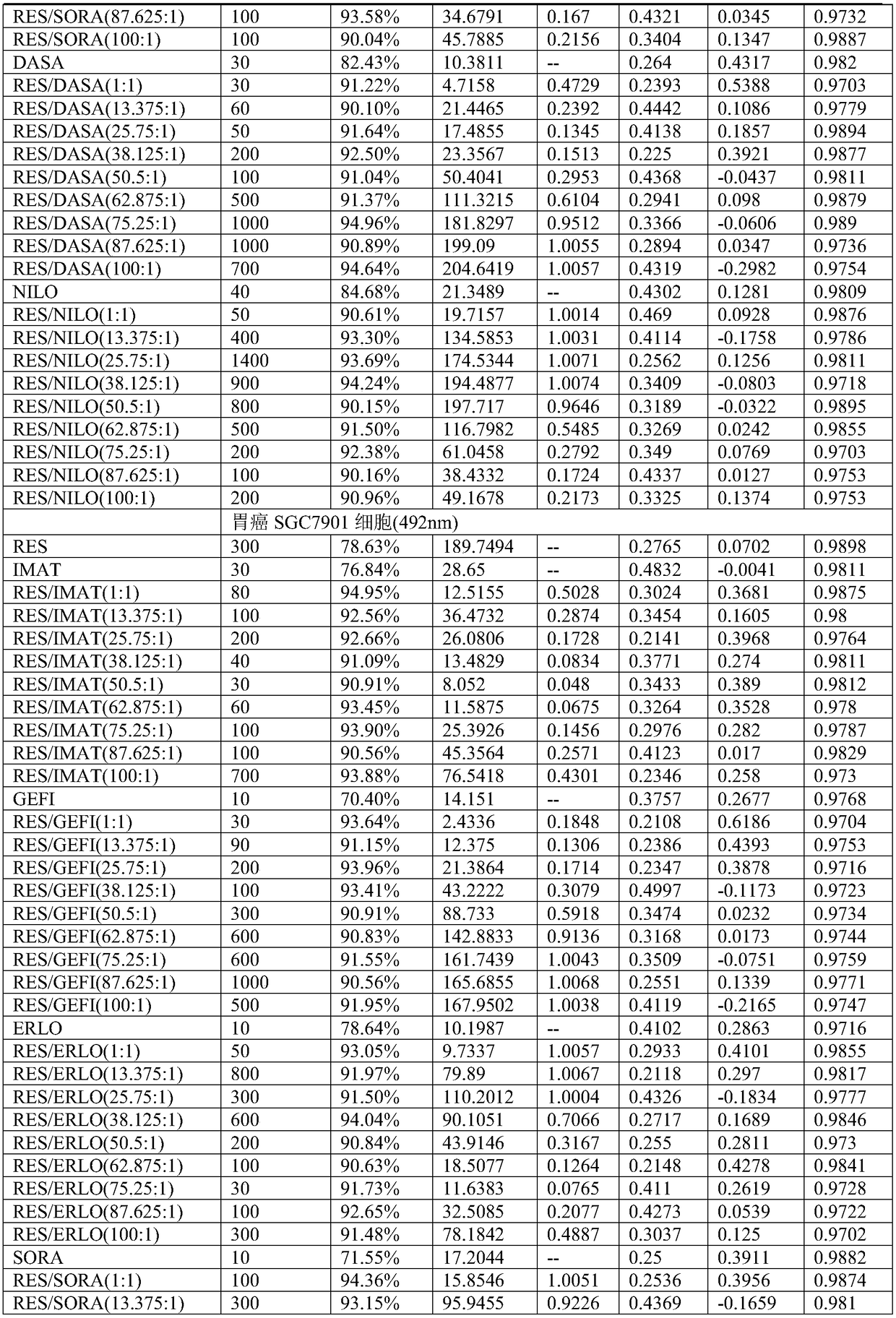
196 results about "Dasatinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat certain types of cancer (chronic myeloid leukemia-CML, acute lymphoblastic leukemia-ALL).
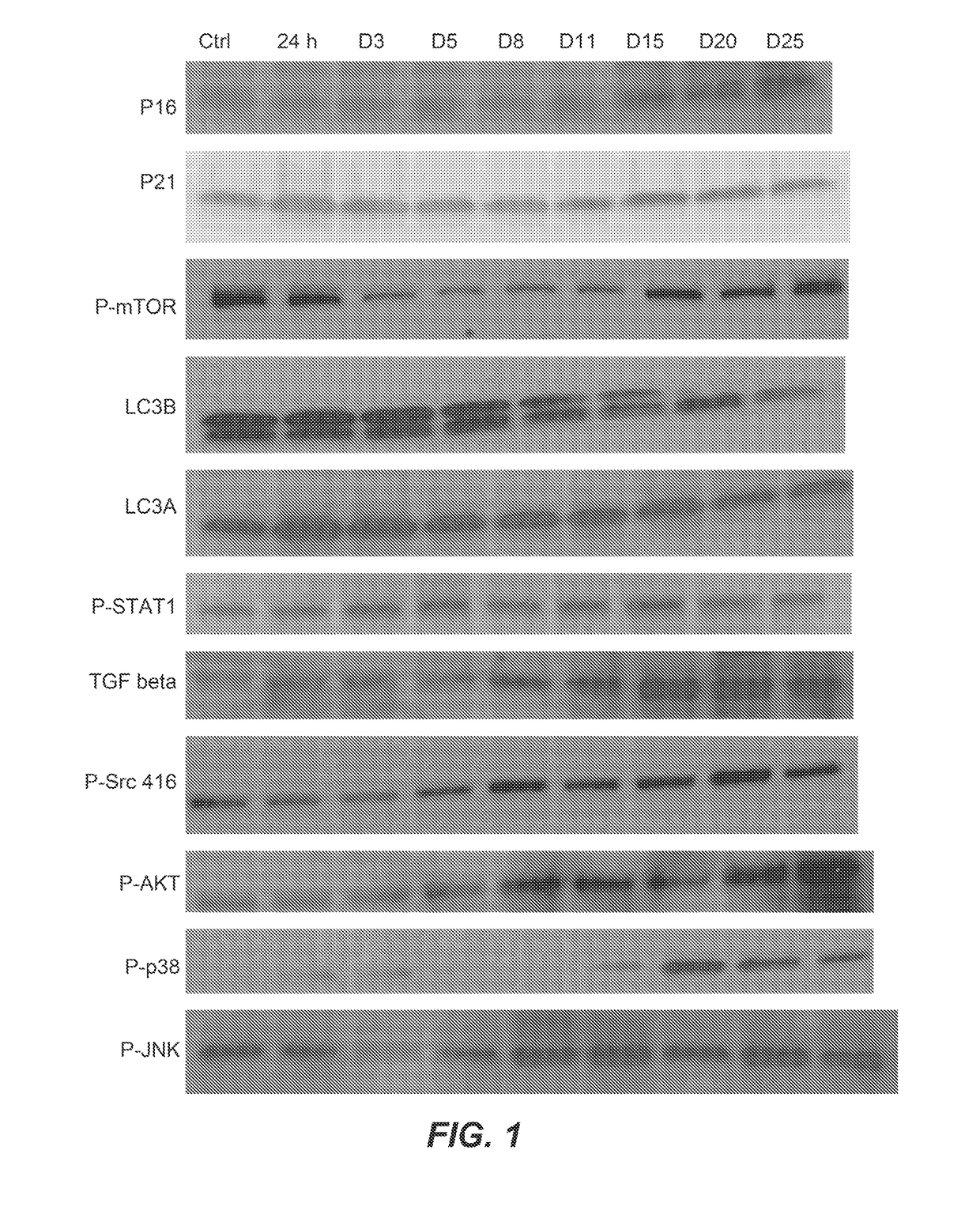
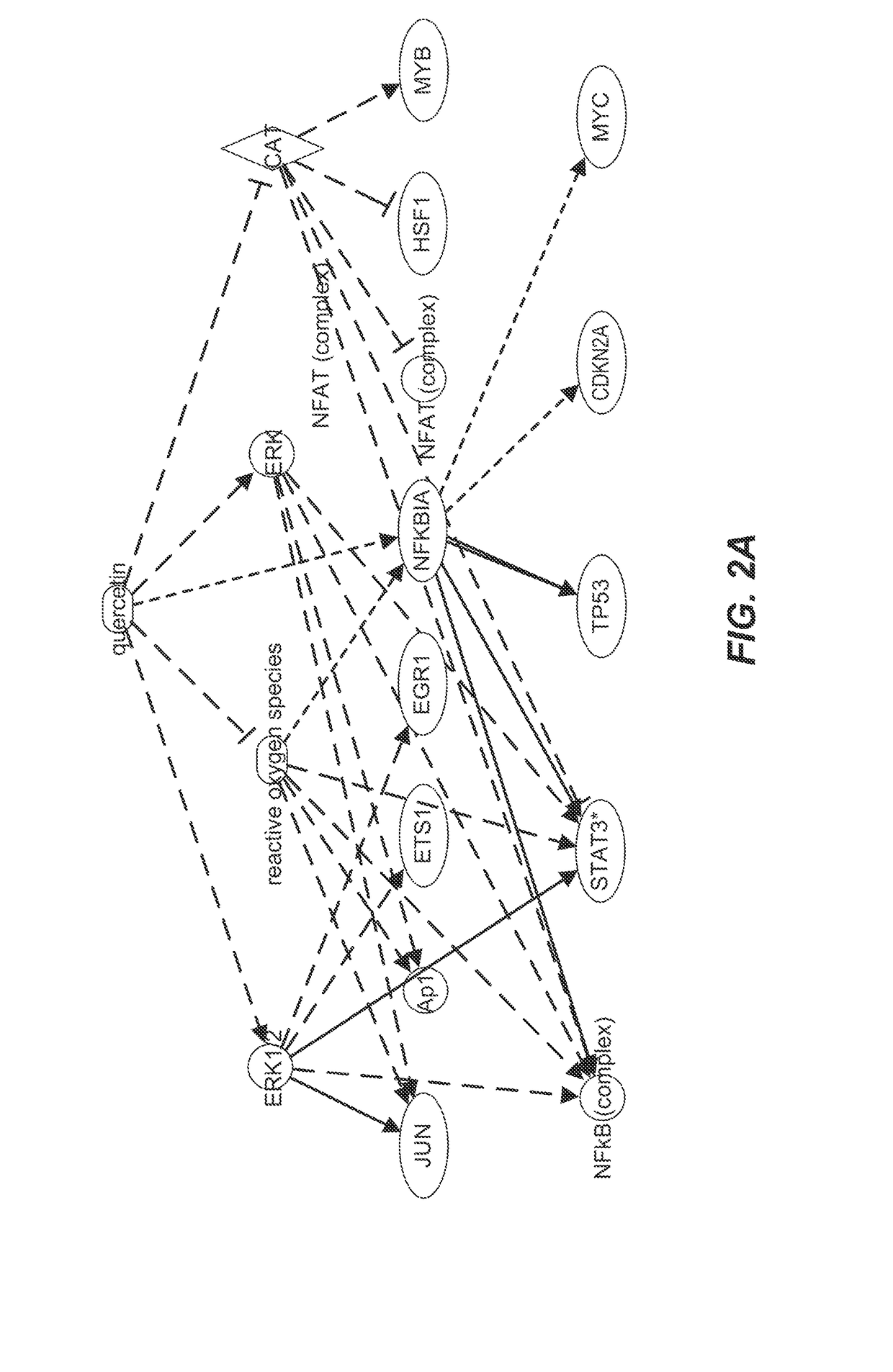
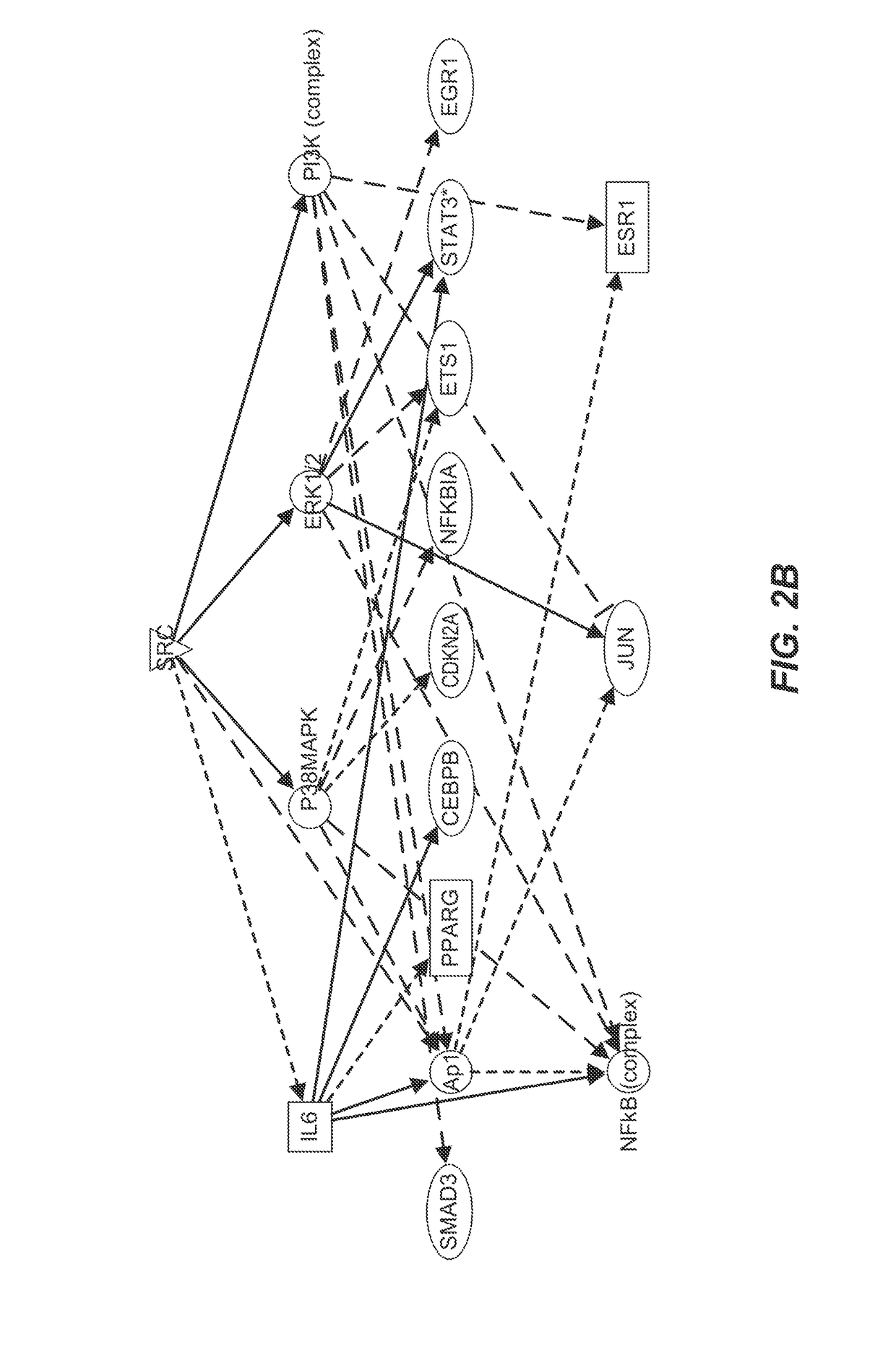
Killing senescent cells and treating senescence-associated conditions using a src inhibitor and a flavonoid
InactiveUS20170216286A1Cause deathReduce the possibilityBiocideCarbohydrate active ingredientsSrc inhibitorDasatinib
Provided herein are methods and uses for treatment or prophylaxis of a senescent cell associated disease or disorder by administering a senolytic combination comprising dasatinib and quercetin or an analog thereof to a subject in need thereof. In certain embodiments, the senescent cell associated disease or disorder is a cardiovascular disease or disorder, inflammatory disease or disorder, a pulmonary disease or disorder, a neurological disease or disorder, or a metabolic disease or disorder.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
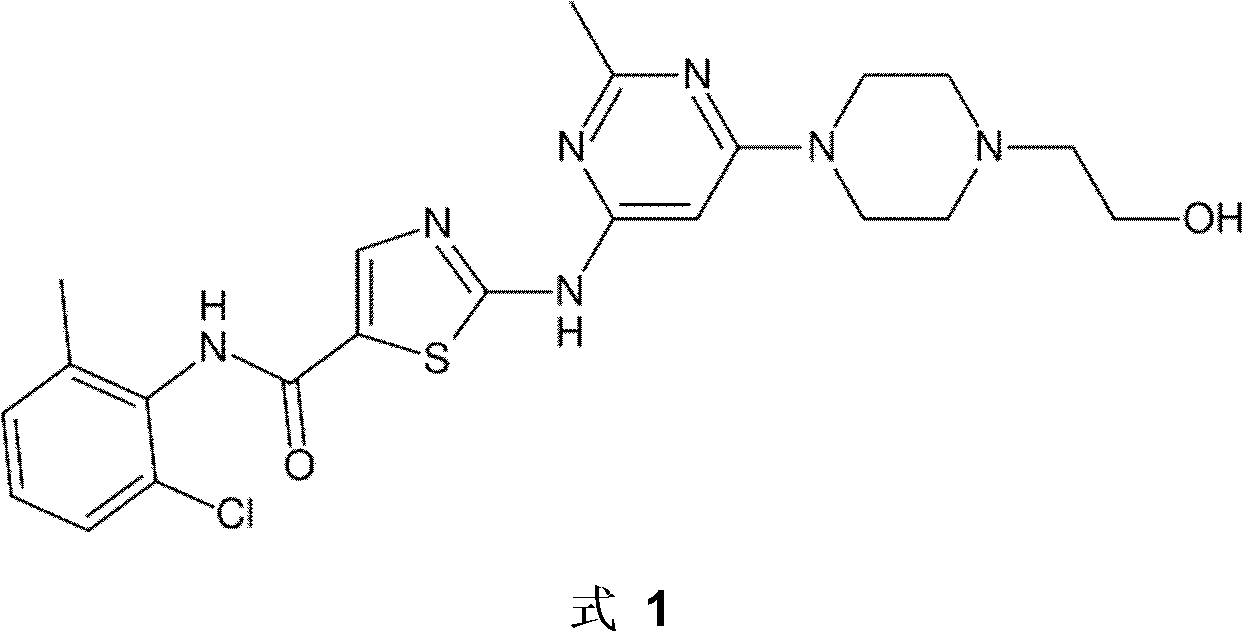
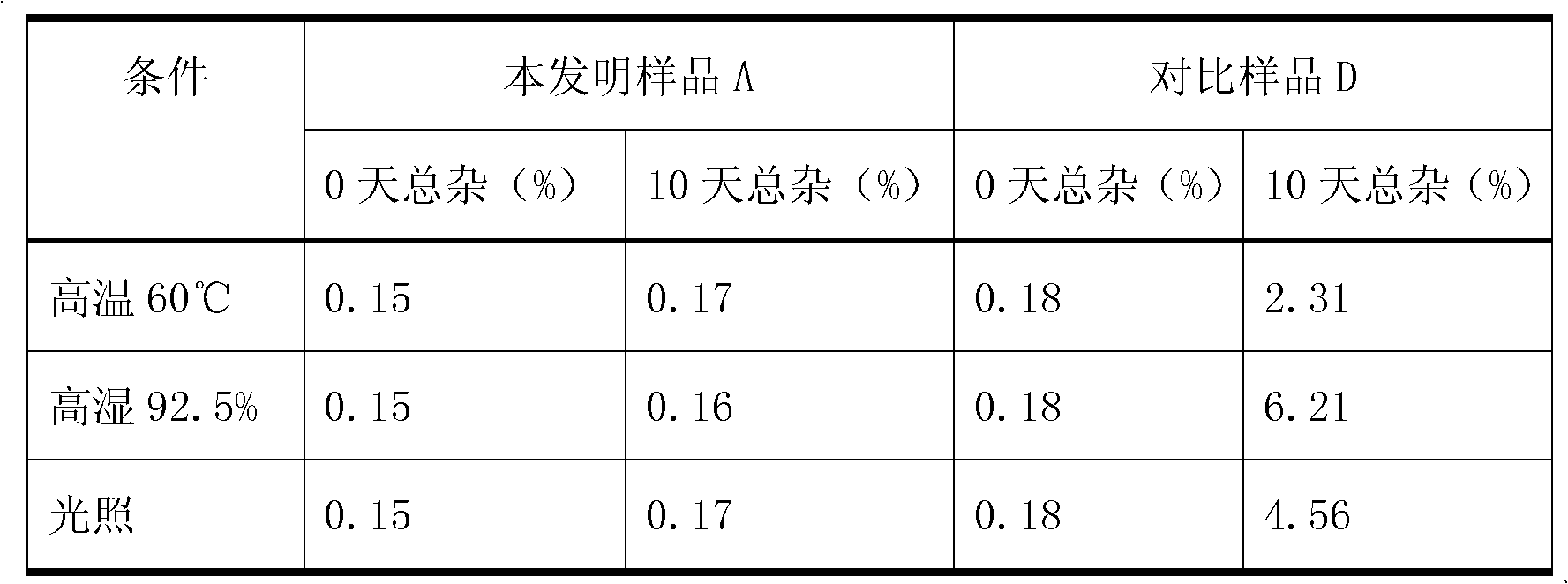
Dasatinib polymorphic substance as well as preparation method and medicinal composition thereof
ActiveCN102086195ASimple processHigh purityOrganic active ingredientsNervous disorderDasatinibIndustrial scale
The invention discloses a dasatinib polymorphic substance. In addition, the invention also discloses a preparation method and a medicinal composition of the dasatinib polymorphic substance. The dasatinib polymorphic substance provided by the invention has the advantages of good physicochemical property and good stability, is more suitable for industrial scale preparation, and the like.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Process for preparation of amorphous form of dasatinib
A stable amorphous form of dasatinib of Formula (I) wherein amorphous dasatinib after exposure to a relative humidity of 75% at 40 ° C. or 60% at 25 ° C. for a period of at least three months doesn't change to crystalline form and a process for the preparation of the amorphous form of dasatinib of Formula (I).
Owner:CADILA HEALTHCARE LTD
Eutectic complex composed of resveratrol and protein kinase inhibitor, and composition comprising eutectic complex
InactiveCN110283052AHydroxy compound active ingredientsOrganic chemistry methodsLenvatinibPTK Inhibitors
The invention provides a eutectic complex composed of resveratrol and a protein kinase inhibitor, and a composition comprising the eutectic complex. The eutectic complex is characterized in that the protein kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib, sunitinib, sorafenib, dasatinib, lapatinib, nilotinib, pazopanib, afatinib, crizotinib, axitinib, regorafenib, ibrutinib, lenvatinib, palbociclib, osimertinib and alectinib or pharmaceutically acceptable salt thereof. The eutectic complex provided by the invention can produce synergistic effects of inhibiting histamine release.
Owner:黄泳华
Treatment of Restenosis and Stenosis with Dasatinib
A method for treating or inhibiting artery obstructive disease, such as restenosis after angioplasty and stenting procedures and stenosis after coronary artery bypass surgery, in a subject by administering to the subject a therapeutically effective amount of dasatinib or a derivative thereof Also provided are drug-eluting medical devices, including stents, having a therapeutically effective amount of dasatinib.
Owner:H LEE MOFFITT CANCER CENT +1
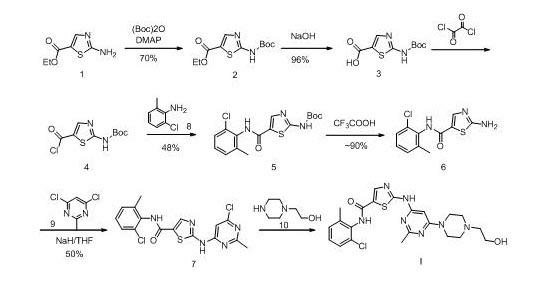
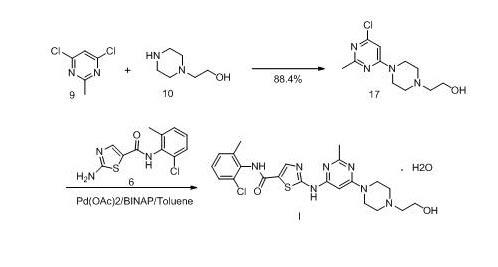
New method for synthesizing dasatinib
InactiveCN102010407AMild reaction conditionsEasy to operateOrganic chemistryBiochemical engineeringDasatinib
The invention discloses a new method for synthesizing dasatinib. The method comprises the following steps: a compound 38 and a compound 8 are subjected to amidation reaction to obtain a compound 7; and the compound 7 reacts with a compound 10 under alkaline condition to obtain dasatinib I. The method provided by the invention has the following advantages: the method is mild in reaction conditions, simple to operate, convenient for purification, low in production cost, environmental-friendly and suitable for industrial production.
Owner:SHANGHAI SYNCORES TECH INC
Dasatinib dispersoid, preparation method thereof and application thereof in tablets
ActiveCN102836159APromote dissolutionPromote absorptionOrganic active ingredientsPharmaceutical non-active ingredientsTherapeutic effectBULK ACTIVE INGREDIENT
The invention belongs to the field of medicinal preparations and in particular relates to a Dasatinib dispersoid, a preparation method thereof and an application thereof in preparation of Dasatinib dispersible tablets. Dasatinib solid dispersoid is prepared by using Dasatinib as an active ingredient and is prepared into tablets of different specifications. The Dasatinib solid dispersible tablets overcome the defects of incomplete release of the medicine and low bioavailability caused by that the dispersible common oral tablets are easy to adhere in the medicine releasing process, so that the bioavailability of the Dasatinib medicine can be further improved and the therapeutic effect of the medicine can be effectively exerted. The preparation process is simple, convenient and practical.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Polymorphs of Dasatinib, preparation methods and pharmaceutical compositions thereof
ActiveUS8884013B2Excellent physical and chemical propertyImprove stabilityOrganic active ingredientsSenses disorderDasatinibIndustrial scale
Polymorph I of dasatinib monohydrate and Polymorph II of dasatinib, their preparation methods and pharmaceutical compositions containing the same are provided. These polymorphs have better physicochemical properties, are more stable and are more suitable for industrial scale production.
Owner:YAN RONG
Dasatinib liposome preparation, and preparation method thereof
ActiveCN107260680AAchieve slow and controlled releaseImprove lipophilicityOrganic active ingredientsInorganic non-active ingredientsDasatinibBiocompatibility Testing
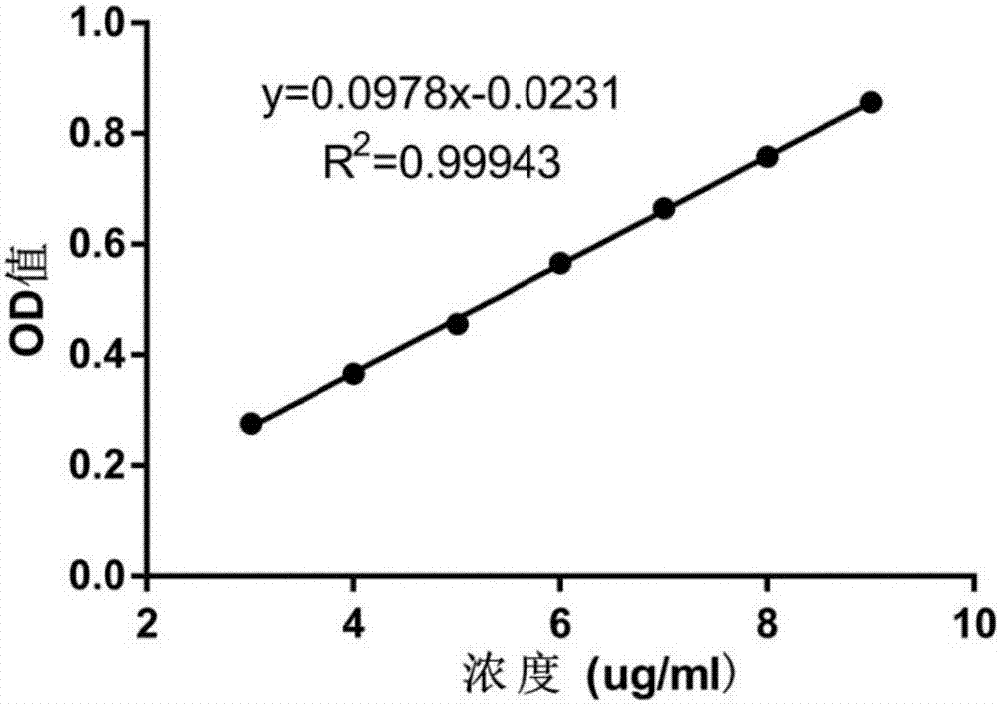
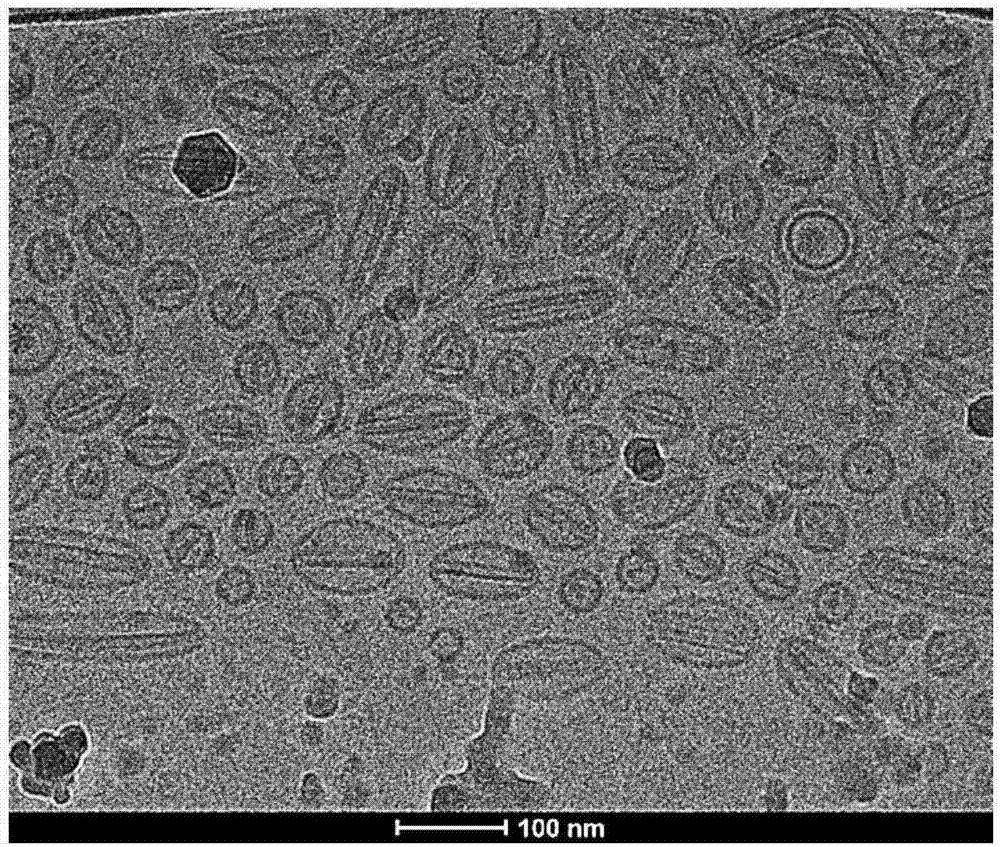
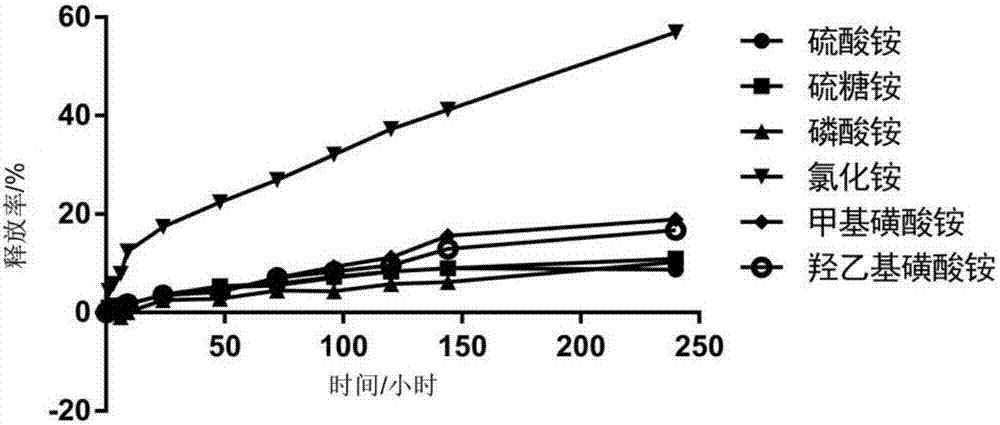
The invention relates to a dasatinib liposome preparation, and a preparation method thereof. The dasatinib liposome preparation is high in biocompatibility; target modification can be carried out; sustained and controlled release of dasatinib can be realized; and it is beneficial for maintenance of relatively high plasma drug concentration in a long term, improvement of drug distribution, and increasing of drug bioavailability. The dasatinib liposome preparation possesses excellent lipophilic performance, is capable of passing through phospholipid bilayer in a molecular form and entering into inner water phase; pH value of liposome inner water phase is relatively low, the dasatinib moleculars are capable of bonding with hydrogen ions so as to form dasatinib ions, and combination with anions in an ammonium salt solution and forming of insoluble salts are realized, diffusion of dasatinib in the inner water phase into an outer water phase is inhibited, dasatinib is coated by the liposome inner water phase steadily, encapsulation efficiency and storage stability are improved, and excellent in-vitro slow release effect is achieved.
Owner:SHANGHAI JIAO TONG UNIV
Novel anti-cancer medicaments using NGR(NO2) as targeting carrier, preparation thereof and use thereof
ActiveCN101948507AInhibition of anti-tumor effectImprove targetingTripeptide ingredientsPeptidesTumor targetCytarabine
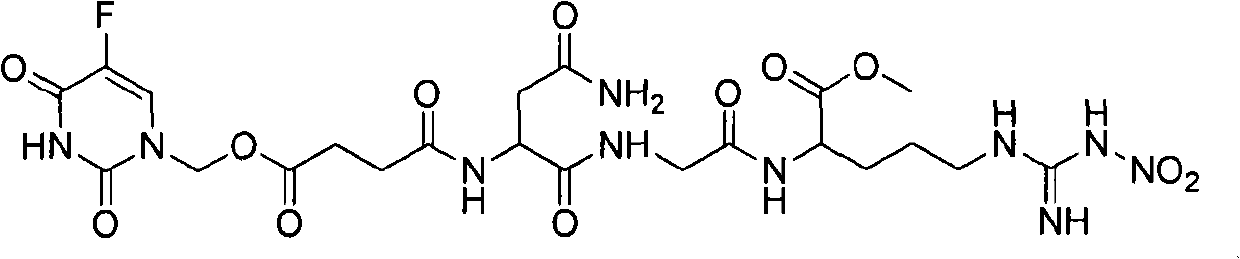
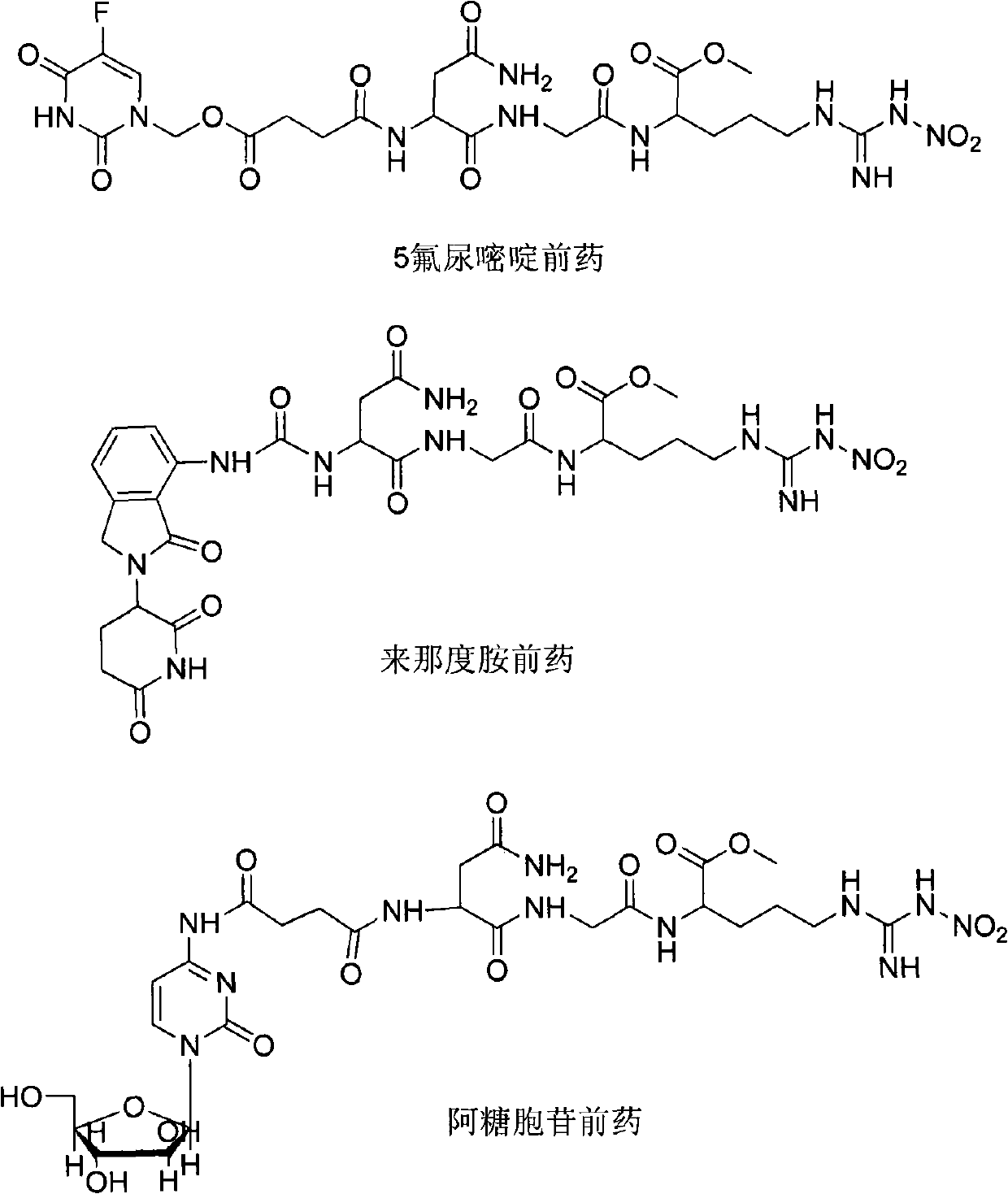
The invention provides novel anti-cancer precursor medicaments using NGR(NO2) as a targeting carrier. The novel anti-cancer precursor medicaments are prepared by designing and synthesizing a 5-fluorouracil precursor medicament, a lenalidomide precursor medicament, a cytarabine precursor medicament, an epirubicin precursor medicament and a dasatinib precursor medicament. According to the initial research on the anti-tumor activity of the 5-fluorouracil precursor medicament, the 5-fluorouracil precursor medicament can inhibit the invasion and metastasis of tumor cells and the growth of solid tumors. The 5-fluorouracil precursor medicament is modified in both effectiveness and preparation compared with the 5-fluorouracil serving as a parent medicament and is widely applicable. Concretely, the invention mainly relates to three aspects: (1) design and antiangiogenic effect of a novel tumor-targeted tripeptide NGR(NO2); (2) preparation of anti-cancer precursor medicaments by coupling the tumor-targeted tripeptide NGR(NO2) and 5 anti-cancer medicaments through covalent bonds; and (3) antitumor and antiangiogenic medical use of the novel 5-fluorouracil precursor medicament.
Owner:廖年生 +1
Dasatinib tablet
ActiveCN102429880AMeet the requirements of industrial mass productionThe quality meets the requirementsOrganic active ingredientsPill deliveryDasatinibSaturation solubility
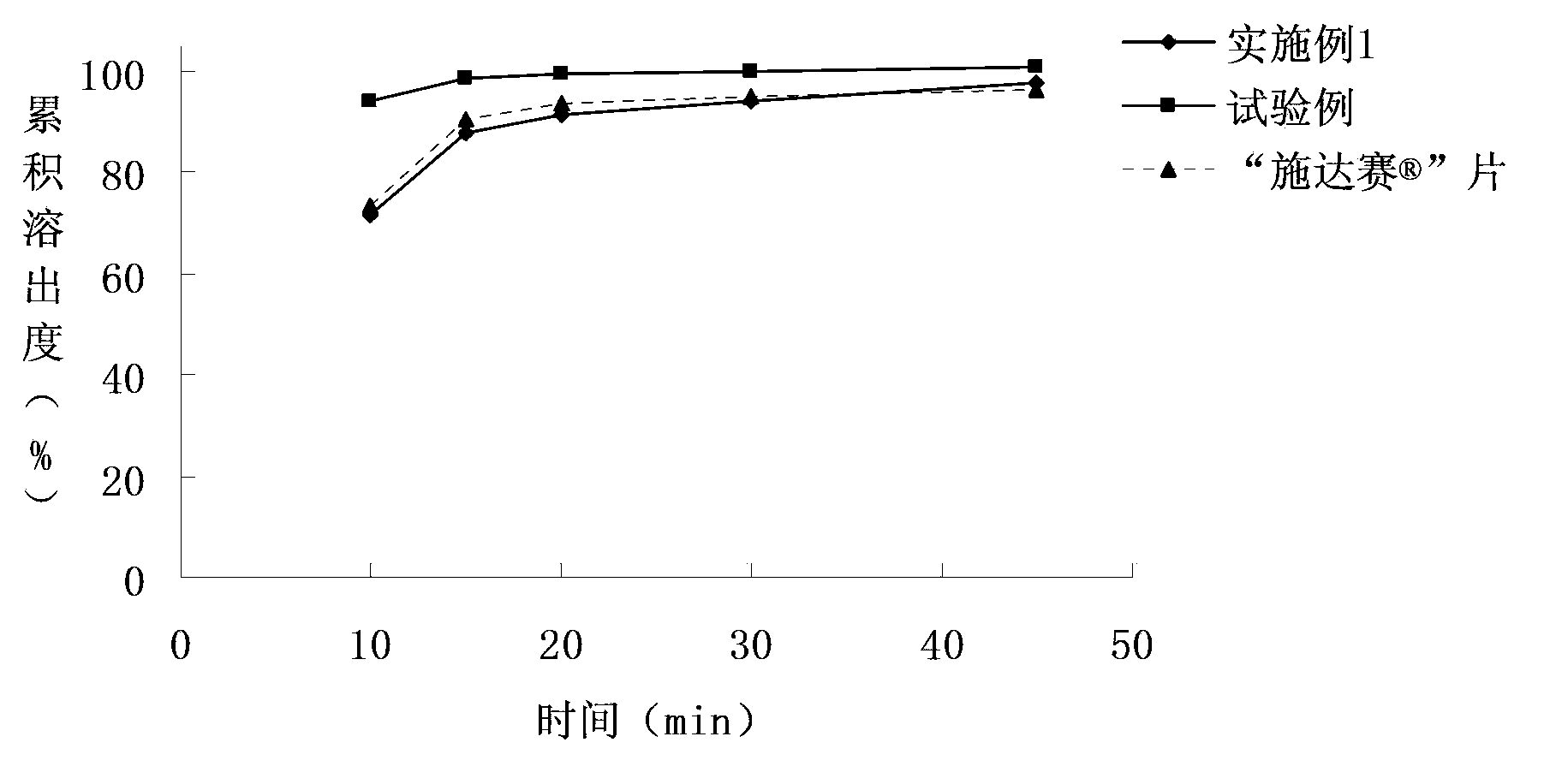
The invention discloses a dasatinib tablet, which comprises dasatinib and pharmaceutically-acceptable medicinal auxiliary materials, and is characterized in that: the dasatinib is an anhydride of which the particle size D(0.1) is equal to 3.0-10 mum, D(0.5) is equal to 15-60 mum and D(0.9) is equal to 100-150 mum. A qualified dasatinib tablet is prepared by taking the dasatinib anhydride as a raw material, so that the problems of over-high dissolution rate of a tablet prepared from the anhydride, untoward reactions caused by initial burst release of a medicament, reduction in the acting time, easy occurrence of unqualified dissolution rate and the like, which are caused by relatively high water saturation solubility of the dasatinib anhydride, are solved, and the industrial batch production requirements of low cost, simple process and qualification on a dasatinib raw material and a preparation can be met.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
Solid state forms of dasatinib and processes for their preparation
The present invention provides crystalline form of (R)-propylene glycol solvate of dasatinib and crystalline form of (S)-propylene glycol solvate of dasatinib.
Owner:DR REDDYS LAB LTD
Solid state forms of dasatinib and processes for their preparation
The present invention provides crystalline form of (R)-propylene glycol solvate of dasatinib and crystalline form of (S)-propylene glycol solvate of dasatinib.
Owner:DR REDDYS LAB LTD
Salts of dasatinib in amorphous form
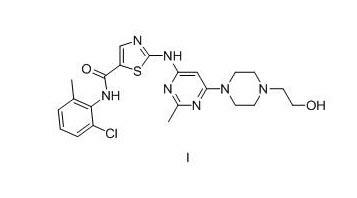
InactiveUS9884857B2Improved profileReduce complexityOrganic active ingredientsOrganic chemistryGlutaric acidDasatinib
The present invention primarily relates to salts of Dasatinib, wherein the salts are in amorphous form. The salts described herein preferably comprise a cation of a compound of formula 1and an anion of a second compound selected from the group consisting of glutaric acid, nicotinic acid and saccharin. The invention is further related to pharmaceutical compositions comprising such a salt. Furthermore, the invention relates to processes for preparing said salts. The invention also relates to several aspects of using said salt or pharmaceutical composition to treat a disease.
Owner:BASF SE
Application of compound containing tyrosine kinase inhibitor and resveratrol in preparing antitumor drugs
InactiveCN108939080AGood synergyHydroxy compound active ingredientsAntineoplastic agentsTyrosine-kinase inhibitorDasatinib
The invention provides an application of a compound containing tyrosine kinase inhibitor and resveratrol in preparing antitumor drugs. The application is characterized in that the tyrosine kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib hydrochloride tablets, sorafenib, dasatinib tablets and tasigna or pharmaceutically acceptable salts or solvates or pharmaceutically acceptable salt solvents thereof; the tumor is selected from one of gastric cancer, liver cancer, lung cancer, kidney cancer, cervical cancer, pancreatic cancer, breast cancer, esophagus cancer, nasopharynxcancer and ovarian cancer; the mole ratio of resveratrol to tyrosine kinase inhibitor is (1-100):1.
Owner:黄泳华
A new preparation method for Dasatinib N-6 crystal form
ActiveCN102643275AAvoid the disadvantages of cumbersome operation and prone to crystal transformationLow costOrganic chemistryDasatinibCrystallization
Owner:JIANGSU SIMCERE PHARMA
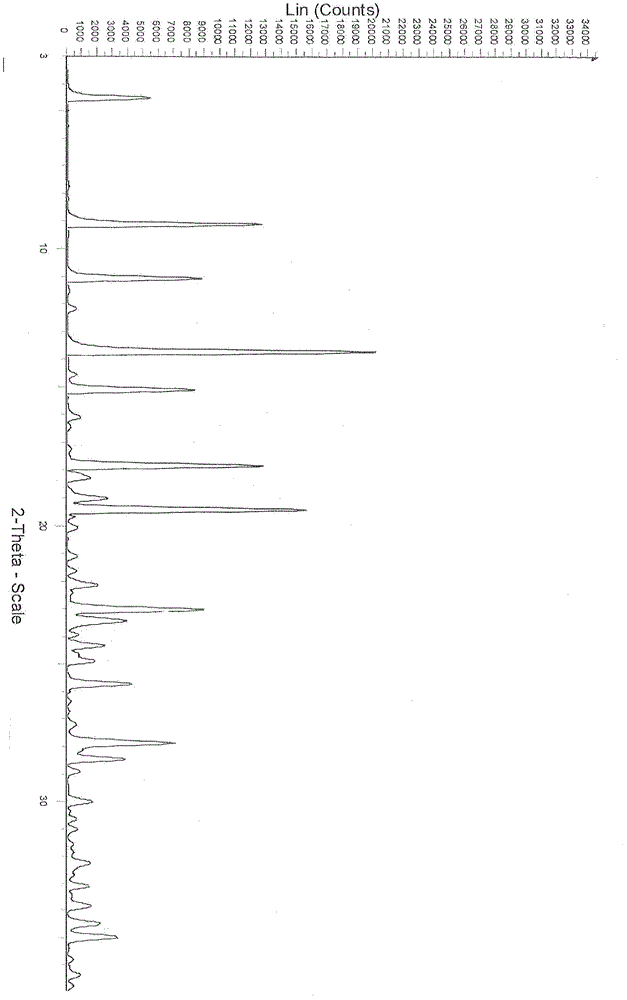
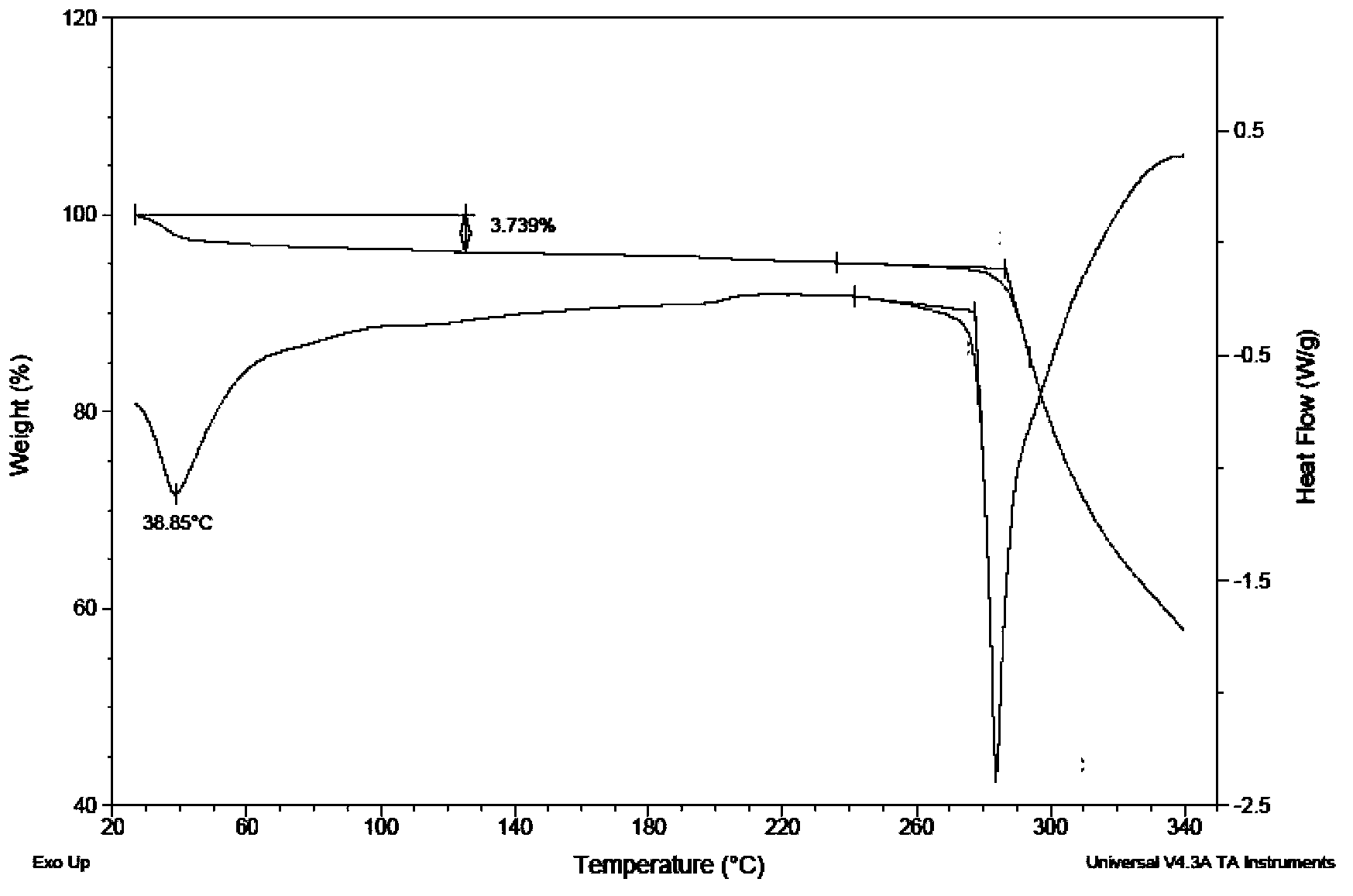
New crystal of Dasatinib monohydrate and preparation method thereof
ActiveCN103059013AQuality improvementHigh dissolution rateOrganic active ingredientsOrganic chemistryHigh humidityDasatinib
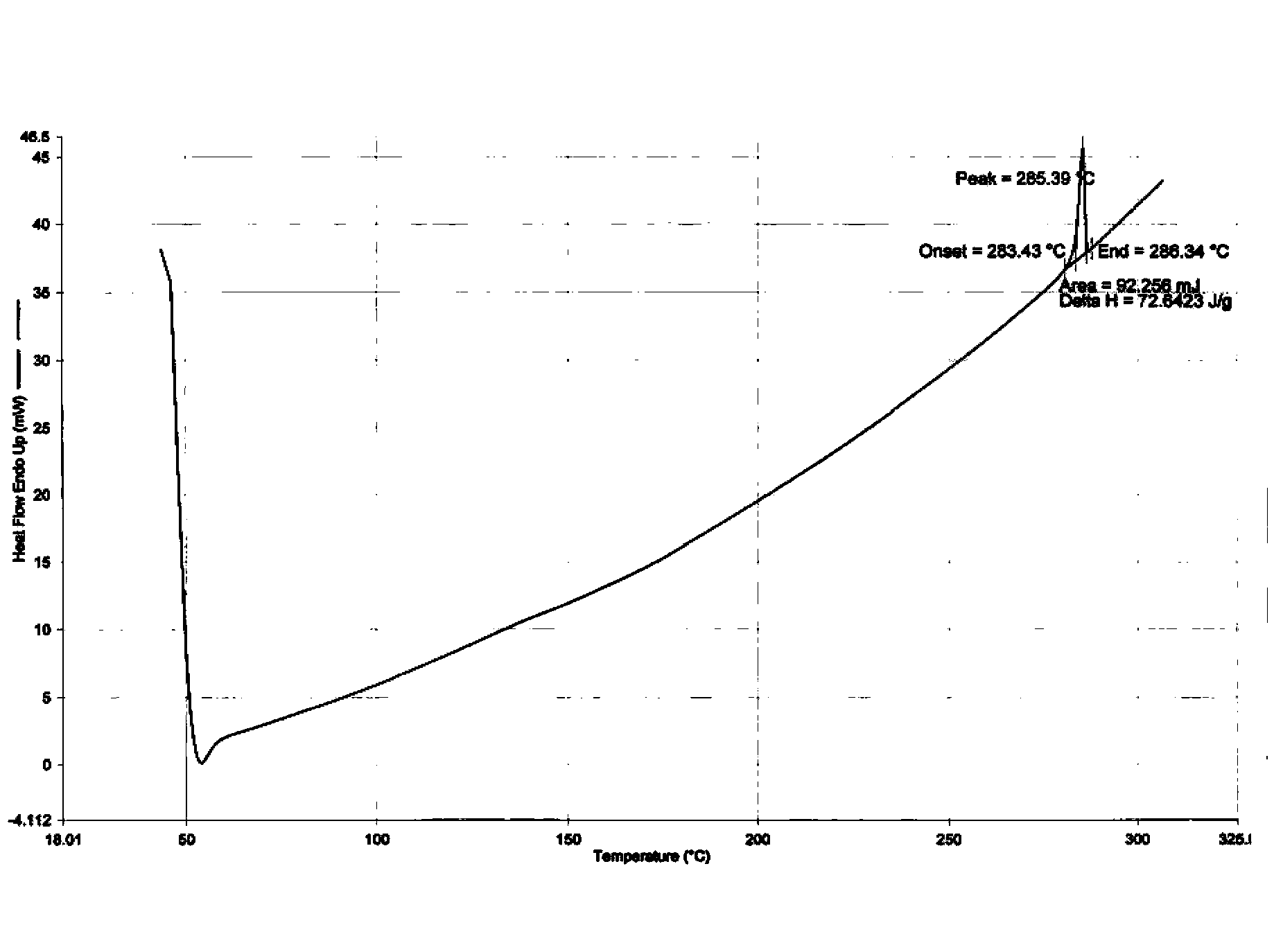
The invention relates to the technical field of medicines, in particular to a crystal III of Dasatinib monohydrate, its preparation method and pharmaceutical composition. The crystal III of Dasatinib monohydrate provided in the invention is very stable under the conditions of illumination, high temperature, high humidity and accelerated testing. The pharmaceutical composition of crystal III of Dasatinib monohydrate has good dissolubility, and can be stable under high temperature conditions. In addition, compared with prior art, the preparation method provided in the invention has the advantages of simple operation and controllable quality.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Dasatinib pharmaceutical composition and preparation method thereof
InactiveCN102048736AGuaranteed stabilityImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsDasatinibHydroxymethyl cellulose
The invention relates to a dasatinib pharmaceutical composition which comprises dasatinib and pharmaceutical auxiliary materials, wherein the auxiliary materials comprise pregelatinized starch and bonding agent. The dasatinib pharmaceutical composition comprises the following concrete components in percentage by weight: 1-20% of dasatinib, 20-35% of pregelatinized starch and 60-79% of bonding agent, wherein the bonding agent is selected from one or mixture of microcrystalline cellulose, hydroxypropyl cellulose sodium, hydroxymethyl cellulose sodium and hydroxypropyl cellulose. The preparation method of the dasatinib pharmaceutical composition comprises the following steps: weighing the dasatinib, pregelatinized starch and bonding agent according to component proportions; sieving the dasatinib by a sieve with 100 meshes, sieving the pregelatinized starch and bonding agent by a sieve with 80 meshes, and uniformly mixing the dasatinib and the sieved auxiliary materials to obtain powder; and directly pressing the powder to obtain the dasatinib pharmaceutical composition. Because the pregelatinized starch is adopted, the product quality and product stability are greatly improved. The dasatinib pharmaceutical composition is a new preparation which has the advantages of better liquidity, higher stability and low hygroscopicity and is suitable for clinical pharmaceutical applications.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Medicine composition containing tyrosine kinase restraining agent
InactiveCN101081207APharmaceutical delivery mechanismPharmaceutical non-active ingredientsDocetaxelWhole body
The anticancer medicine composition containing tyrosine kinase inhibitor is slow released injection and slow released implant. The effective anticancer components include tyrosine kinase inhibitor selected from Erbitux, Iressa, Tarceva, Sunitinib, Trastuzumab, etc, and / or composition selected from Docetaxel, deacetyl taxol, taxol, etc. The slow releasing supplementary material is selected from p(LAEG-EOP), p(DAPG-EOP), etc. The released injection and slow released implant may be injected or set in tumor for slow releasing to maintain effective medicine concentration for over 50 days, and has obviously lowered systemic reaction on the medicine and capacity of enhancing the chemotherapeutic and radiotherapeutic effect.
Owner:JINAN KANGQUAN PHARMA TECH
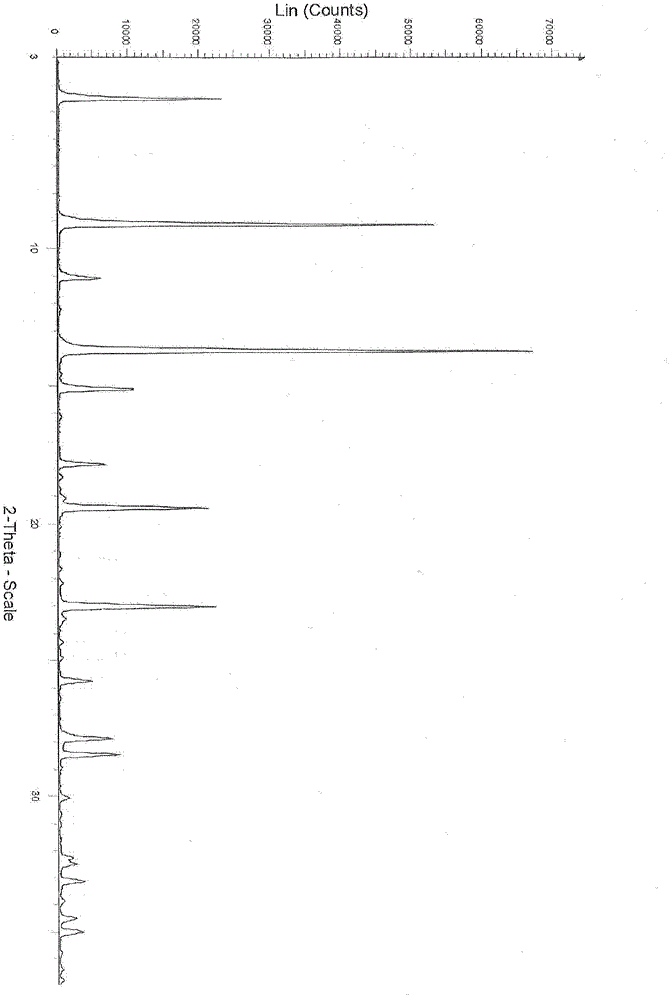
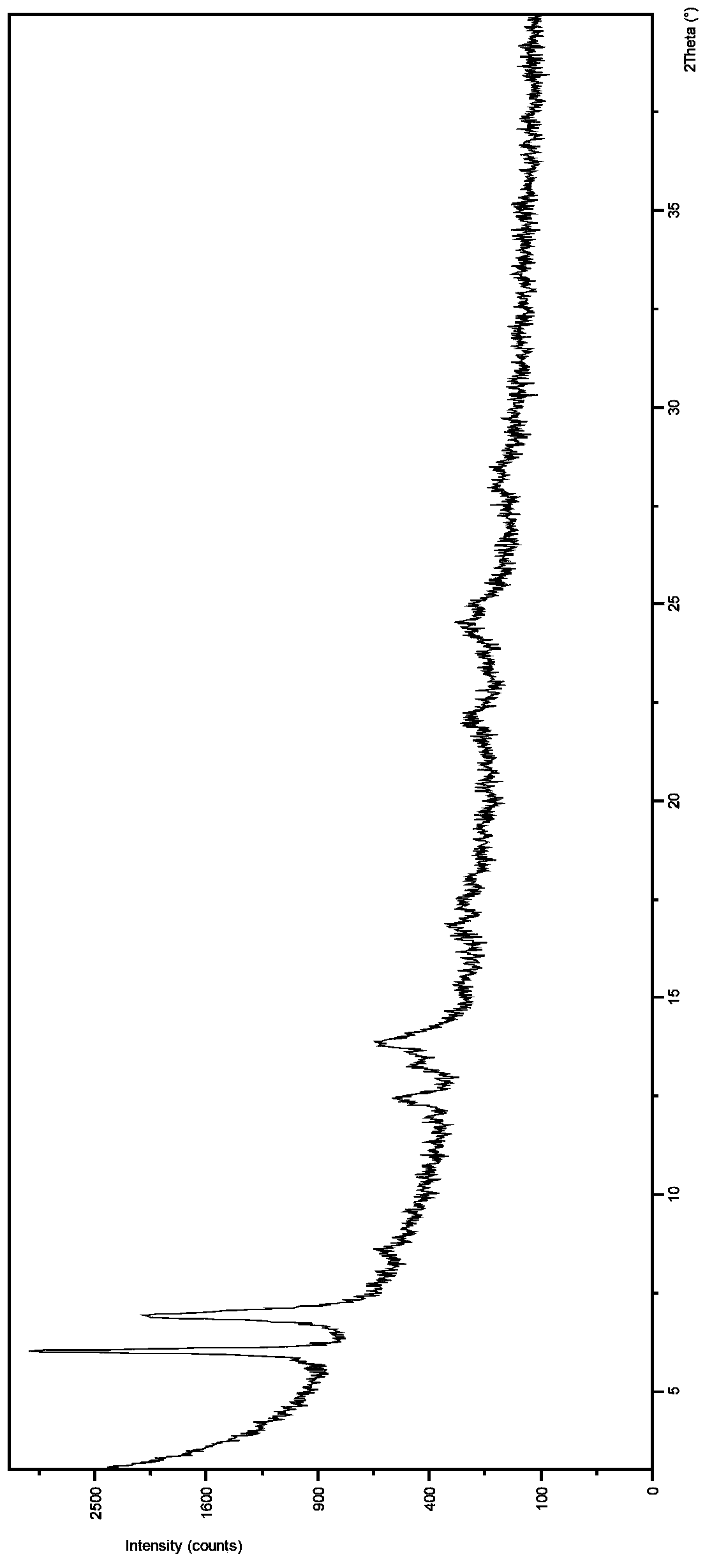
New Dasatinib crystal form and preparation method thereof
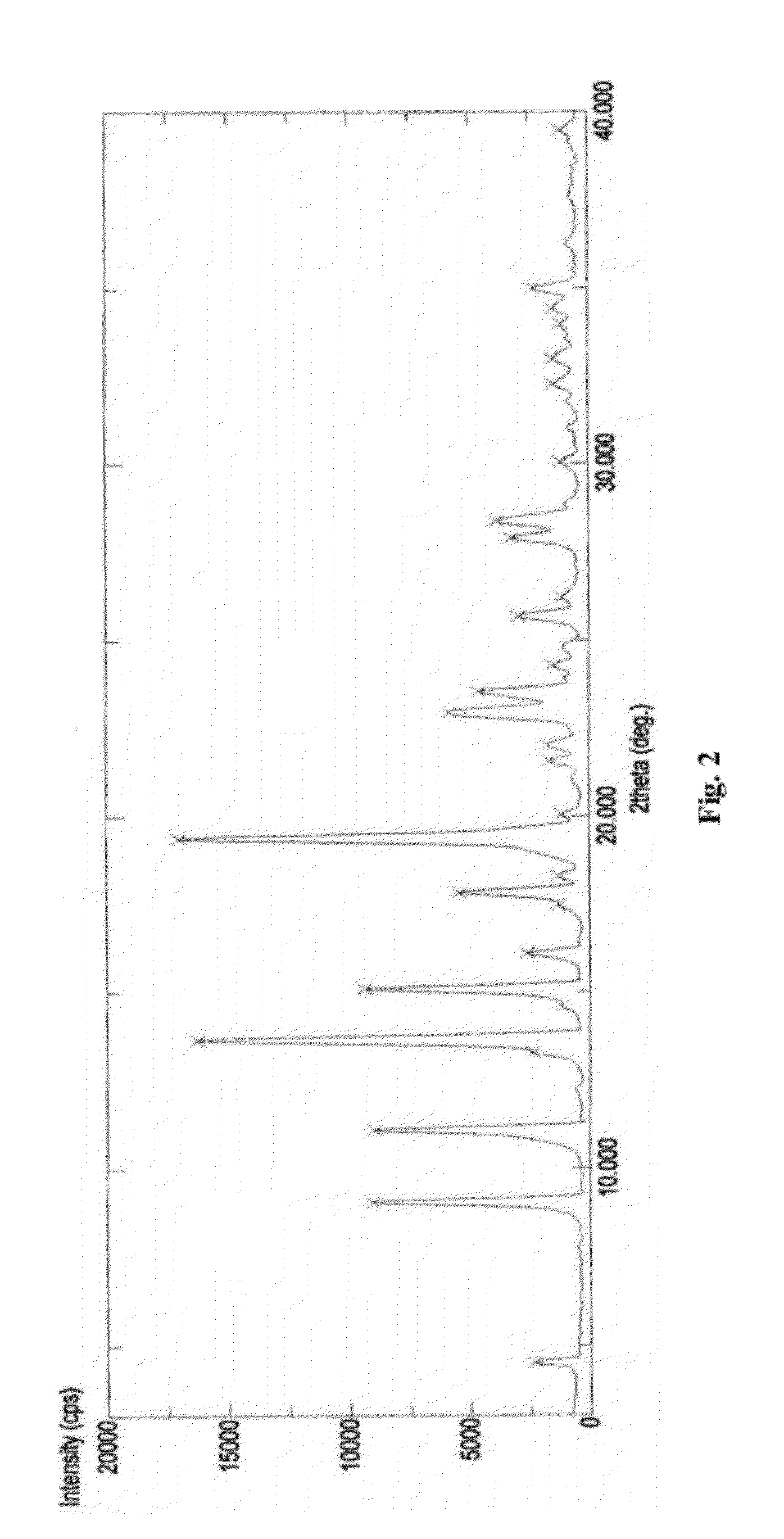
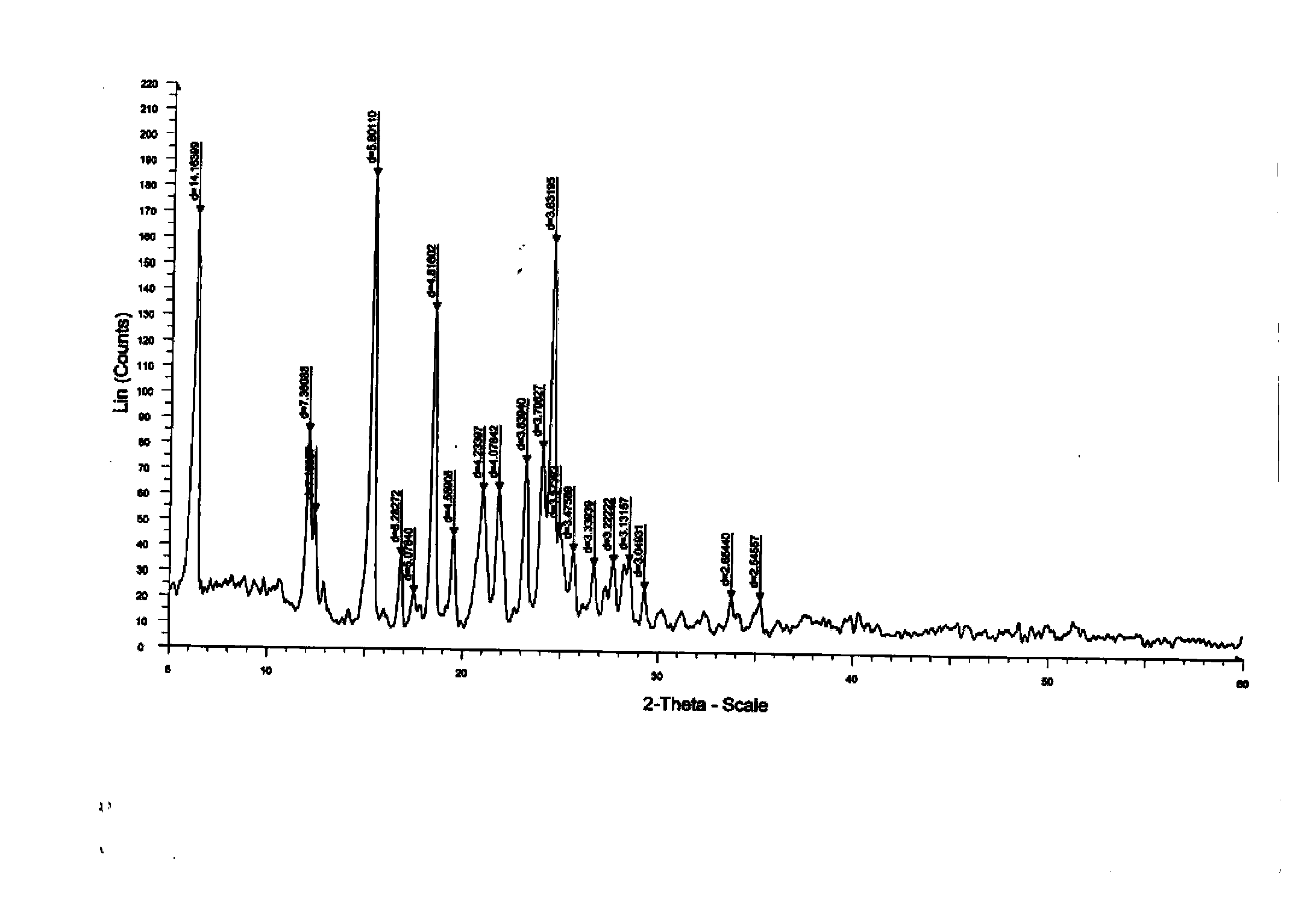
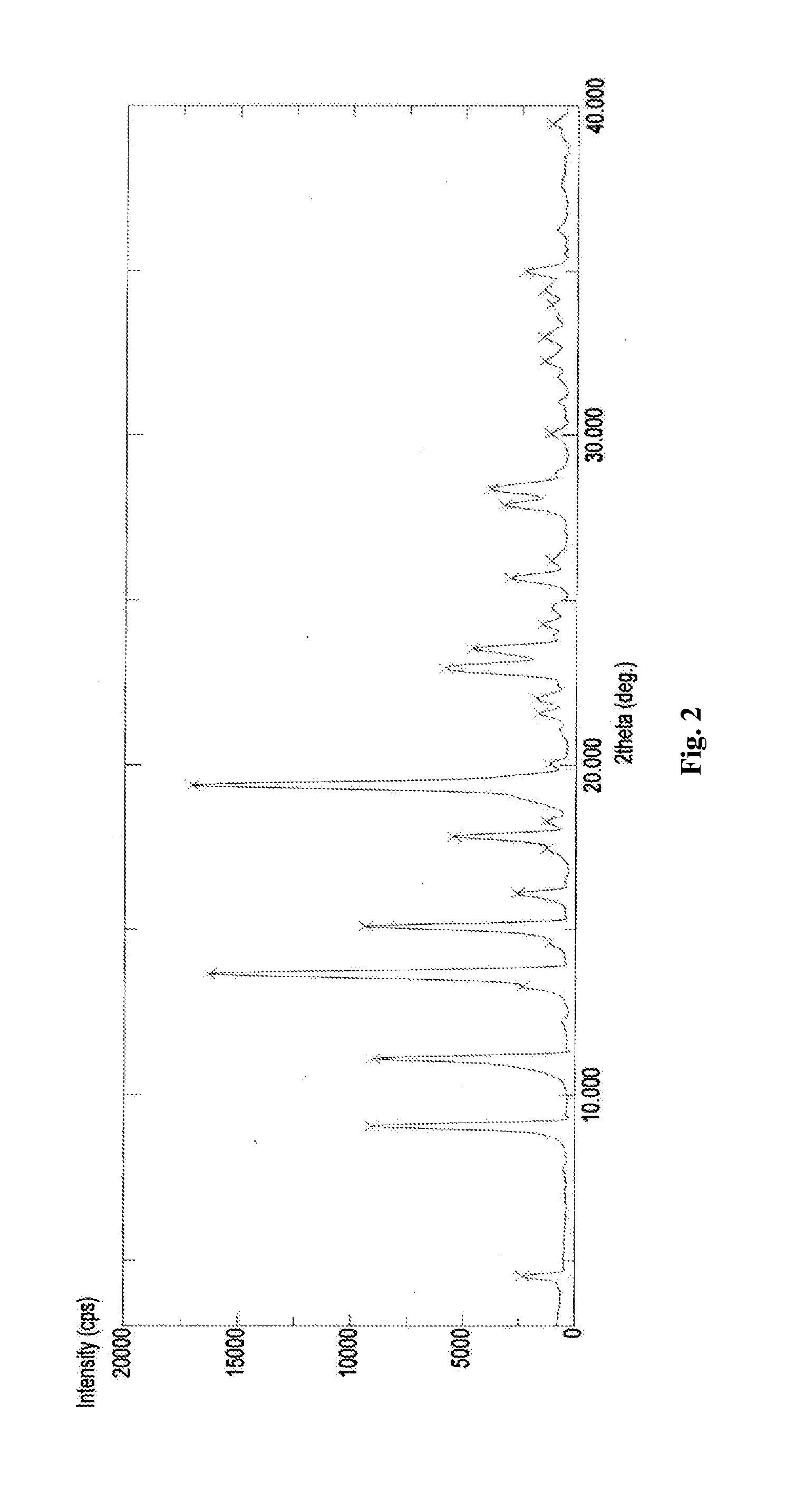
The invention relates to a new Dasatinib crystal form and a preparation method thereof. The new Dasatinib crystal form is named as a crystal form V and has characteristic peaks at 6.02 degrees, 6.91 degrees, 8.55 degrees, 12.44 degrees, 13.30 degrees, 13.84 degrees, 16.83 degrees, 24.41 degrees and 24.96 degrees (2theta) in an X ray powder diffraction spectrum obtained by means of Cu-Ka radiation detection. The invention also provides a method for preparing the new Dasatinib crystal form V. The method is simple, convenient and good in reproducibility, and the obtained new Dasatinib crystal form V is high in purity, good in stability and applicable to industrial production.
Owner:SHANGHAI SYNCORES TECH INC
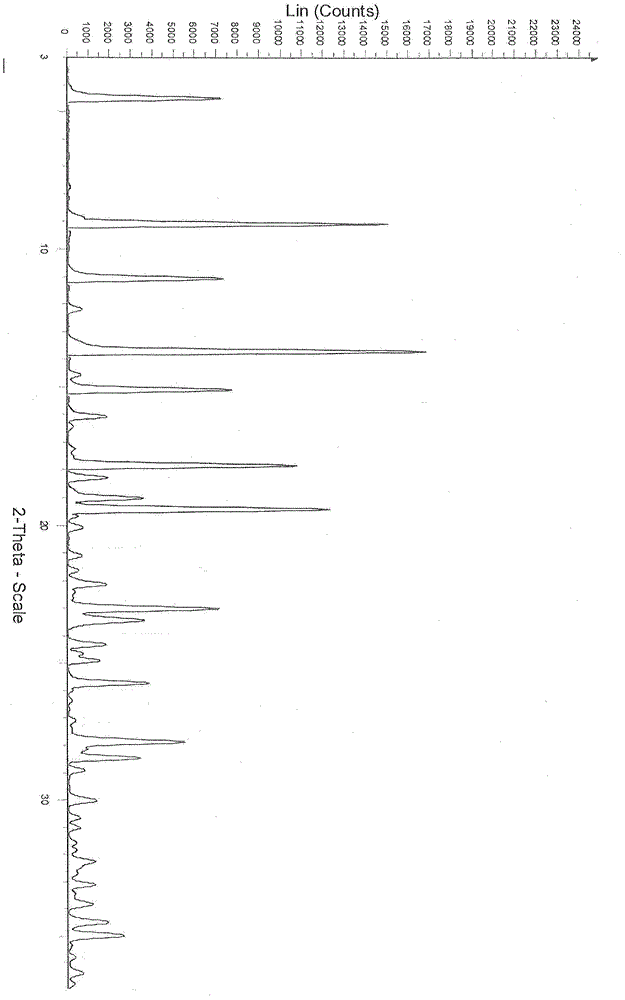
Dasatinib polycrystalline form medicament and preparation method thereof
ActiveCN103664929AImprove liquidityGood compressibilityOrganic active ingredientsOrganic chemistryMedicineDasatinib
The invention provides a new dasatinib polycrystalline form medicament and a preparation method thereof. In a powder diffraction spectrum of a dasatinib polycrystalline form matter III provided by the invention, diffraction peaks exist at 2 theta of 6.2 plus or minus 0.2, 12.0 plus or minus 0.2, 15.3 plus or minus 0.2 and 18.4 plus or minus 0.2. The crystal form prepared by the preparation method provided by the invention has good chemical stability and high purity. The preparation method has the advantages of mild process conditions, simplicity in operation, stable and controllable quality and high yield, and is suitable for industrial production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Medicinal dasatinib composition and preparation method thereof
ActiveCN103845332AOrganic active ingredientsPharmaceutical non-active ingredientsMethyl celluloseDasatinib
The invention relates to a medicinal dasatinib composition and preparation method thereof. The composition comprises an effective amount of dasatinib and medicinal auxiliary materials, wherein dasatinib is an anhydride, and the medicinal materials comprise 3-15 percent by weight of retardant; the retardant is a hydrophilic gel material, and is selected from hydroxypropylmethyl cellose, methyl cellulose, carbopol or alginate. The invention also provides a preparation method of the medicinal dasatinib composition. According to the medicinal dasatinib composition, the microenvironment of the solid preparation can be improved due to the retardant, so that the preparation stability can be improved on one hand, and on the other hand, the dissolution velocity of the oral preparation can be controlled. The medicinal dasatinib composition is simple in process and low in cost, and does not have pollution.
Owner:QILU PHARMA HAINAN
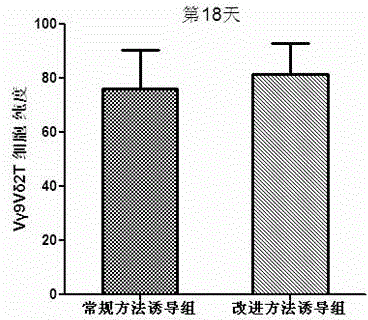
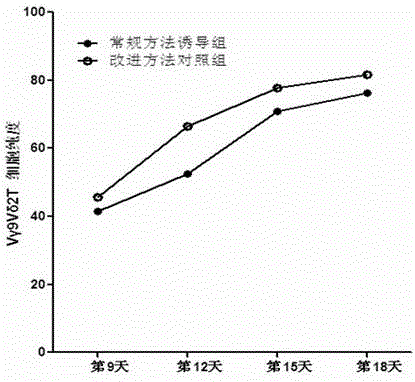
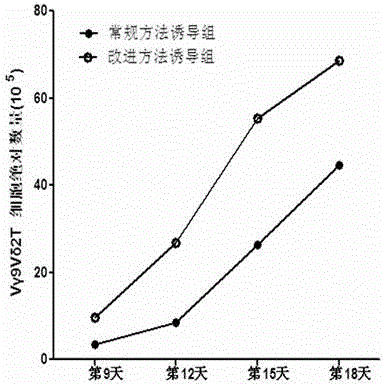
Culture method for increasing amplification efficiency and activity of Vgamma9Vdelta2T cells
InactiveCN103555666AImprove induction efficiencyHigh activityBlood/immune system cellsCell activityTyrosine-kinase inhibitor
The present invention provides a culture method for increasing amplification efficiency and activity of Vgamma9Vdelta2T cells, wherein IL-2 and zoledronic acid are combined with a tyrosine kinase inhibitor dasatinib to carry out in vitro Vgamma9Vdelta2T cell induction production to obtain the Vgamma9Vdelta2T cells with high induction efficiency and high activity. According to the present invention, the method has characteristics of simple and easy performing induction culture process, short period, low cost, high induction efficiency and good repeatability, the cells obtained through induction has enhanced activity, the tumor immunotherapy effect of the current Vgamma9Vdelta2T cells is expected to be increased with the number and the function of the induction-cultured cells, and good application potential is provided.
Owner:ZHEJIANG UNIV
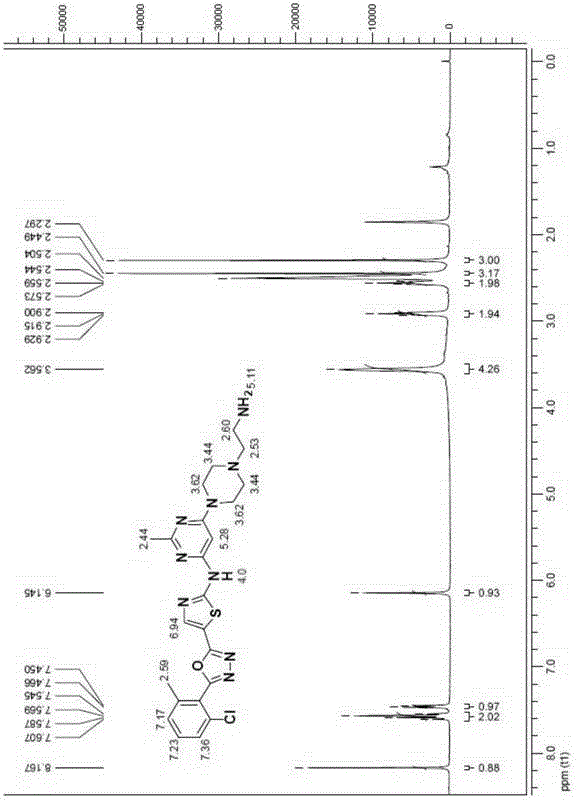
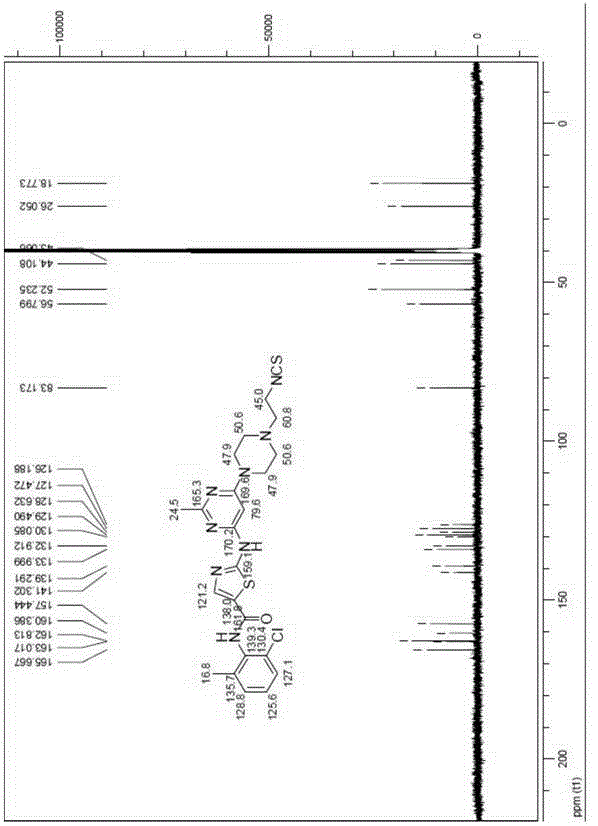
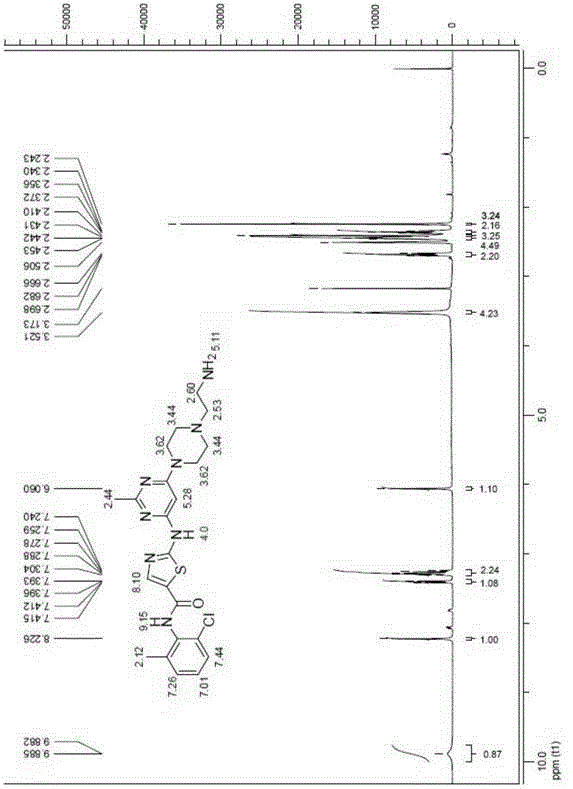
Thiazole heterocyclic compounds, and preparation method and application thereof
InactiveCN106279143APrevent proliferationInhibition of proliferative abilityOrganic active ingredientsOrganic chemistryThiazolePositive control
Owner:TIANJIN INT JOINT ACADEMY OF BIOTECH & MEDICINE
Polymorphs of dasatinib, preparation methods and pharmaceutical compositions thereof
ActiveUS20120309968A1Excellent physicalGood chemical propertiesOrganic active ingredientsSenses disorderDasatinibIndustrial scale
Polymorph I of dasatinib monohydrate and Polymorph II of dasatinib, their preparation methods and pharmaceutical compositions containing the same are provided. These polymorphs have better physicochemical properties, are more stable and are more suitable for industrial scale production.
Owner:YAN RONG
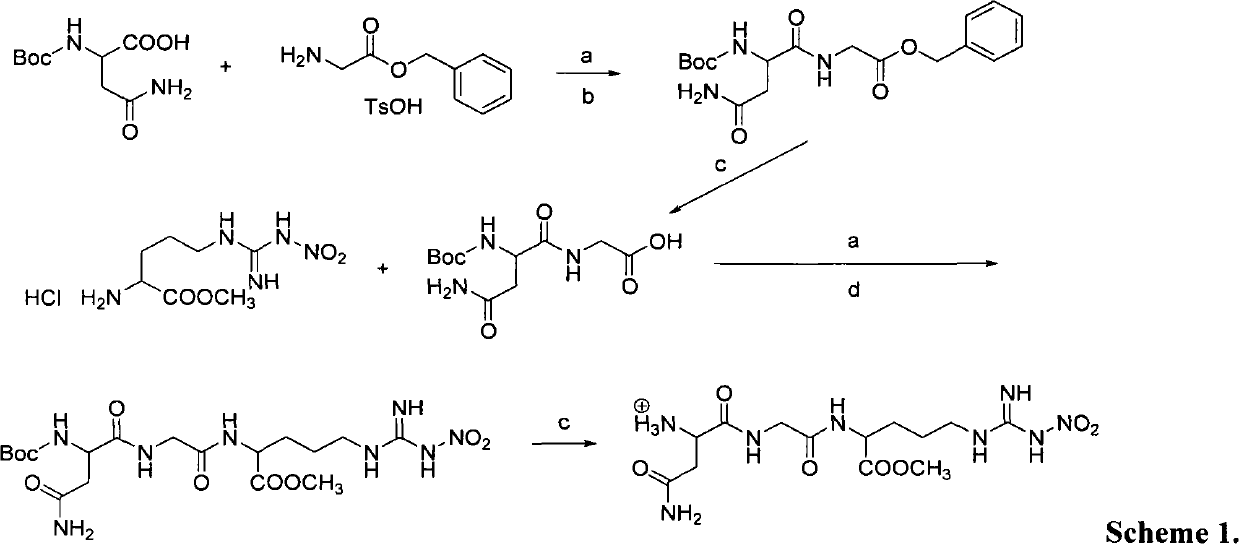
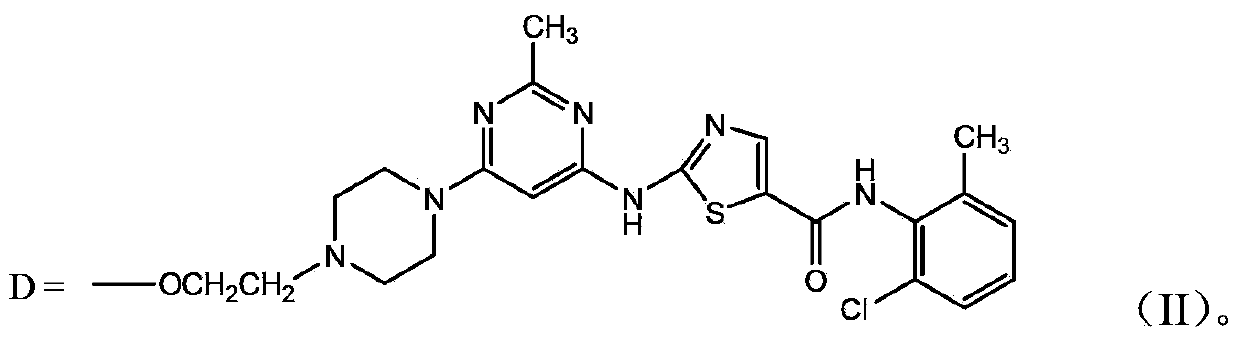
Polyethylene glycol-amino acid oligopeptide-dasatinib conjugate and pharmaceutical composition thereof
ActiveCN103965458AImprove clinical outcomesHigh load rateOrganic active ingredientsPharmaceutical non-active ingredientsSide effectTreatment effect
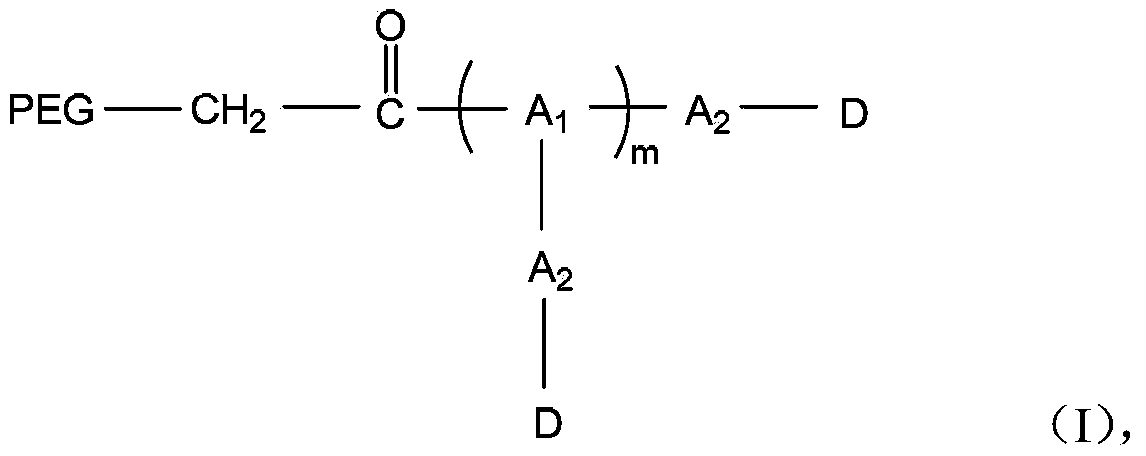
The invention discloses a polyethylene glycol-amino acid oligopeptide-dasatinib conjugate represented by a general formula I and a pharmaceutical composition containing the conjugate. In the conjugate, PEG represents polyethylene glycols residues, A1 and A2 represent same or different amino acid residues, m is an integer from 2 to 12, and D is a dasatinib residue connected with A2. In the conjugate, each polyethylene glycol terminal group is connected with multiple dasatinib residues via amino acid oligopeptides, so that the medicine loading rate is substantially increased, the medicine hydrophilia is increased, medicine absorption is improved, the action time is prolonged, and therefore the treatment effect is increased or the toxic and side effects are reduced.
Owner:JENKEM TECH CO LTD TIANJIN
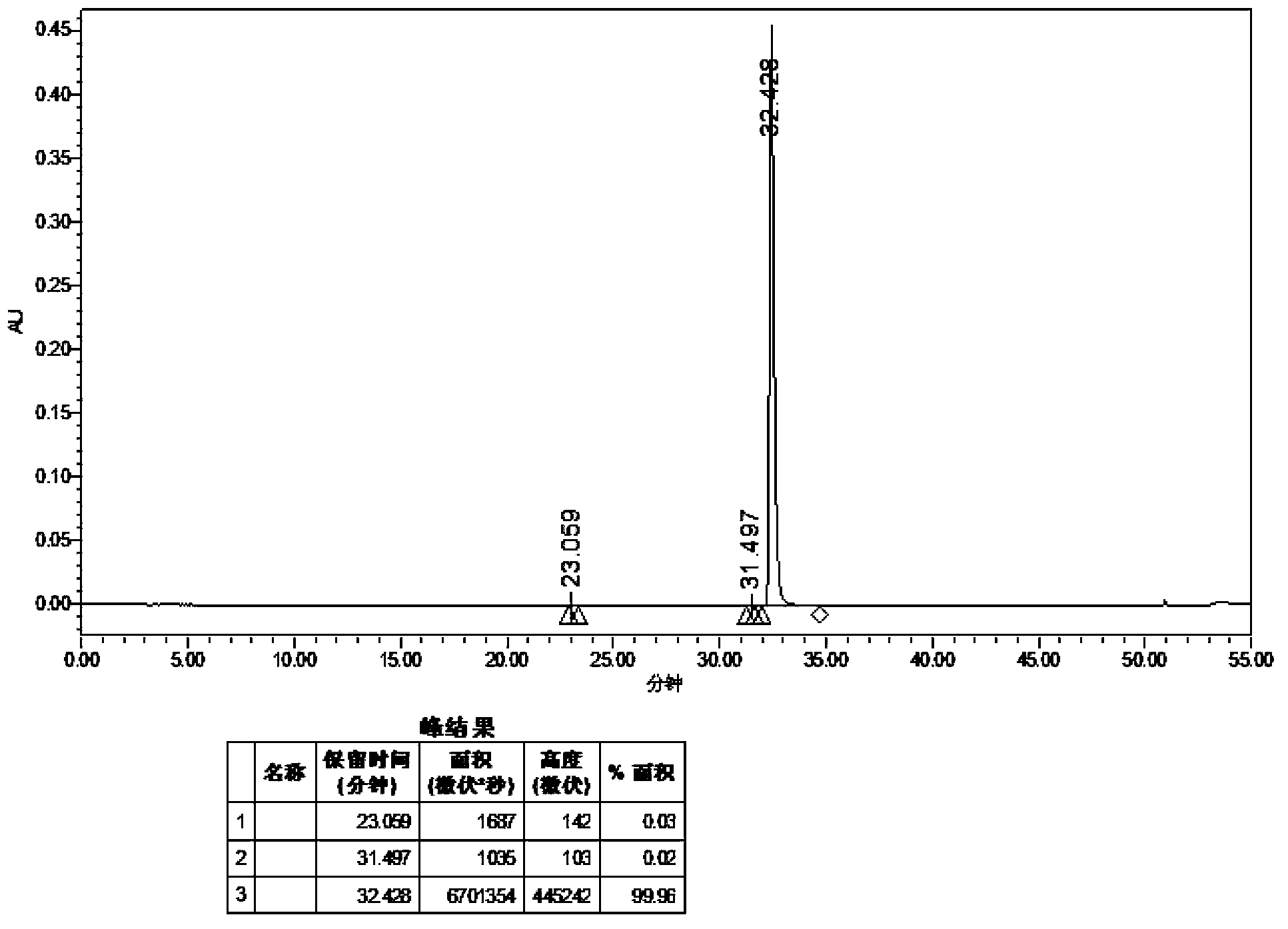
Preparation method of high-purity dasatinib and by-product of dasatinib
InactiveCN102838595AReasonable controlHigh purityOrganic chemistryBiochemical engineeringChemical compound
The invention relates to a preparation method of high-purity dasatinib and a by-product of the dasatinib and belongs to the technical field of medicines. An optimized preparation method of the dasatinib is disclosed, and the prepared dasatinib is high in product purity and low in compound 6 content. The invention further discloses the by-product of the dasatinib and a synthetic method. The by-product which is difficult to separate is generated in a synthetic process of the dasatinib, and the by-product is determined to be a compound 6 through systemization and identification. The compound has important significant on quality control of the dasatinib. A structural formula of the compound 6 is shown in the specification.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Medicine composition of tyrosine kinase inhibitor and histone deacetylase inhibitor
InactiveCN103083671ASignificant synergyDelayed drug resistanceOrganic active ingredientsAntineoplastic agentsTreatment effectHydroxamic acid
The invention discloses a medicine composition of a tyrosine kinase inhibitor and a histone deacetylase inhibitor. The prepared medicine composition comprises a tyrosine kinase inhibitor and a histone deacetylase inhibitor according to a mass ratio of (0.5:1)-(1:4). The tyrosine kinase inhibitor is selected from imatinib, dasatinib, nilotinib, gefitinib and the like; and the histone deacetylase inhibitor is selected from short-chain fatty acid such as butyric acids and valproic acids, hydroxamic acids such as trichostatin A, cyclic tetrapeptides and benzamides. Compared with the tyrosine kinase inhibitor of treating tumors by one target, the medicine composition has the advantages of strong treatment effect, high medicine tolerance, and small clinical medicine dosage, and is applied clinically by using a medicine preparation form.
Owner:毛幼桦 +1
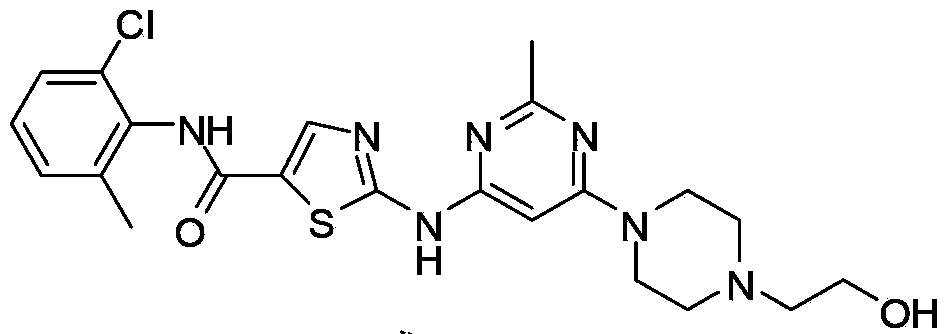
Preparation and refining methods of dasatinib
The invention relates to the field of pharmaceutical chemistry, in particular to the synthesis field of dasatinib and novel preparation and refining methods of the dasatinib. The preparation method of the dasatinib comprises the step of allowing an intermediate N-(2-chlorine-6-methoxyphenyl)-2[(6-chlorine-2-methyl-4-pyrimidyl) amino]-5-thiazole formamide to perform homogeneous nucleophilic substitution reaction with 1-(2-ethoxyl) piperazine in a high-polar solvent. The preparation method solves the problems that n-butyl alcohol is adopted as a solvent in the traditional synthesis method, the cost is high, the reaction time is long, the reaction temperature is high, and the requirements on a reaction vessel are high. The invention provides a simple and feasible refining method, which improves the purity of the dasatinib effectively.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com