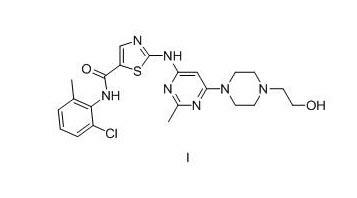
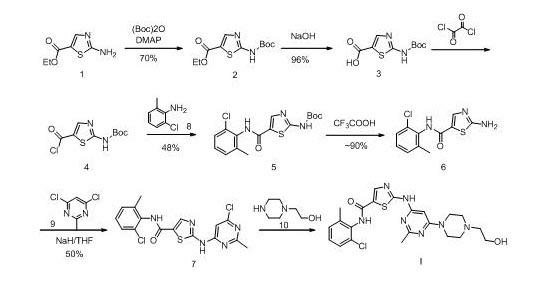
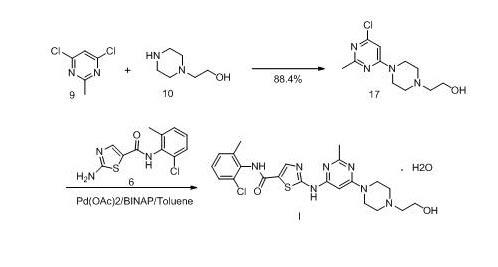
New method for synthesizing dasatinib
A technology of dasatinib and a new method, which is applied in the field of synthesizing dasatinib, can solve the problems of high production cost, harsh reaction conditions, and unfriendly environment, and achieve the effects of low production cost, mild reaction conditions, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Preparation of N-(2-chloro-6-methylphenyl)-2-[[6-chloro-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide (Compound 7)
[0063] 13.2 g of compound 2-(6-chloro-2-methylpyrimidinyl-4-amino)thiazole-5-carboxylic acid and 100 ml of thionyl chloride were stirred and mixed at room temperature, 2 ml of DMF was added, and then heated under reflux for 4 hours Complete the reaction. Concentrate under reduced pressure to dryness, add 50ml of dichloromethane, add 6.9g of 2-chloro-6-methylaniline dropwise after cooling to 0°C, stir for 5 minutes after the addition, and then add dropwise 15ml of triethylamine. After dripping, the temperature was naturally raised to room temperature and stirred overnight, and a large amount of solid was precipitated. The mixture was concentrated to dryness, 1N sodium hydroxide was added and stirred for 15 minutes, filtered, the filter cake was washed twice with 100 ml of water and washed with 100 ml of methanol to obtain 12.5 g of light yellow solid ...
Embodiment 2
[0065] Preparation of N-(2-chloro-6-methylphenyl)-2-[[6-chloro-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide (Compound 7)
[0066] 13.2 g of compound 2-(6-chloro-2-methylpyrimidinyl-4-amino)thiazole-5-carboxylic acid and 150 ml of toluene were stirred and mixed at room temperature, 20 ml of thionyl chloride were added, and then 1.0 ml of DMF , The temperature was raised to 80°C and reacted overnight. Concentrate under reduced pressure to dryness, then add 150 ml of toluene, and then remove toluene under reduced pressure to obtain a solid. Add 50ml of dichloromethane, after cooling to 0°C, add dropwise 6.9g of 2-chloro-6-methylaniline, stir for 15 minutes after the addition, and then add dropwise 15ml of triethylamine. Warm to room temperature and stir overnight. The mixture is concentrated to dryness under reduced pressure, then 1N sodium hydroxide is added and stirred for 15 minutes, filtered, and the filter cake is washed twice with 100 ml of water and washed with 100 m...
Embodiment 3
[0068] Preparation of N-(2-chloro-6-methylphenyl)-2-[[6-chloro-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide (Compound 7)
[0069] 13.2 g of compound 2-(6-chloro-2-methylpyrimidinyl-4-amino)thiazole-5-carboxylic acid and 130 ml of dichloromethane were stirred and mixed at room temperature, 0.5 ml of DMF was added, and then cooled to 0°C. 28.0 ml of a dichloromethane solution of 2mol / L oxalyl chloride was added dropwise, after the addition, the temperature was raised to room temperature, and the reaction was continued to be stirred for 2.5 hours to complete the reaction. The reaction mixture was concentrated to dryness as much as possible, and then 150 ml of toluene was added, and concentrated under reduced pressure to obtain a solid. Add 50ml of dichloromethane, stir and mix. After cooling to 0°C, add 6.9g of 2-chloro-6-methylaniline dropwise, stir for 15 minutes after the addition, and then dropwise add 15ml of triethylamine. After dripping, warm to room temperature and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com