Killing senescent cells and treating senescence-associated conditions using a src inhibitor and a flavonoid
a technology of senescent cells and flavonoid, which is applied in the field of treatment and prophylaxis of senescent cellassociated diseases and disorders, can solve the problems that the presence of senescent cells may have deleterious effects on millions of patients worldwid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
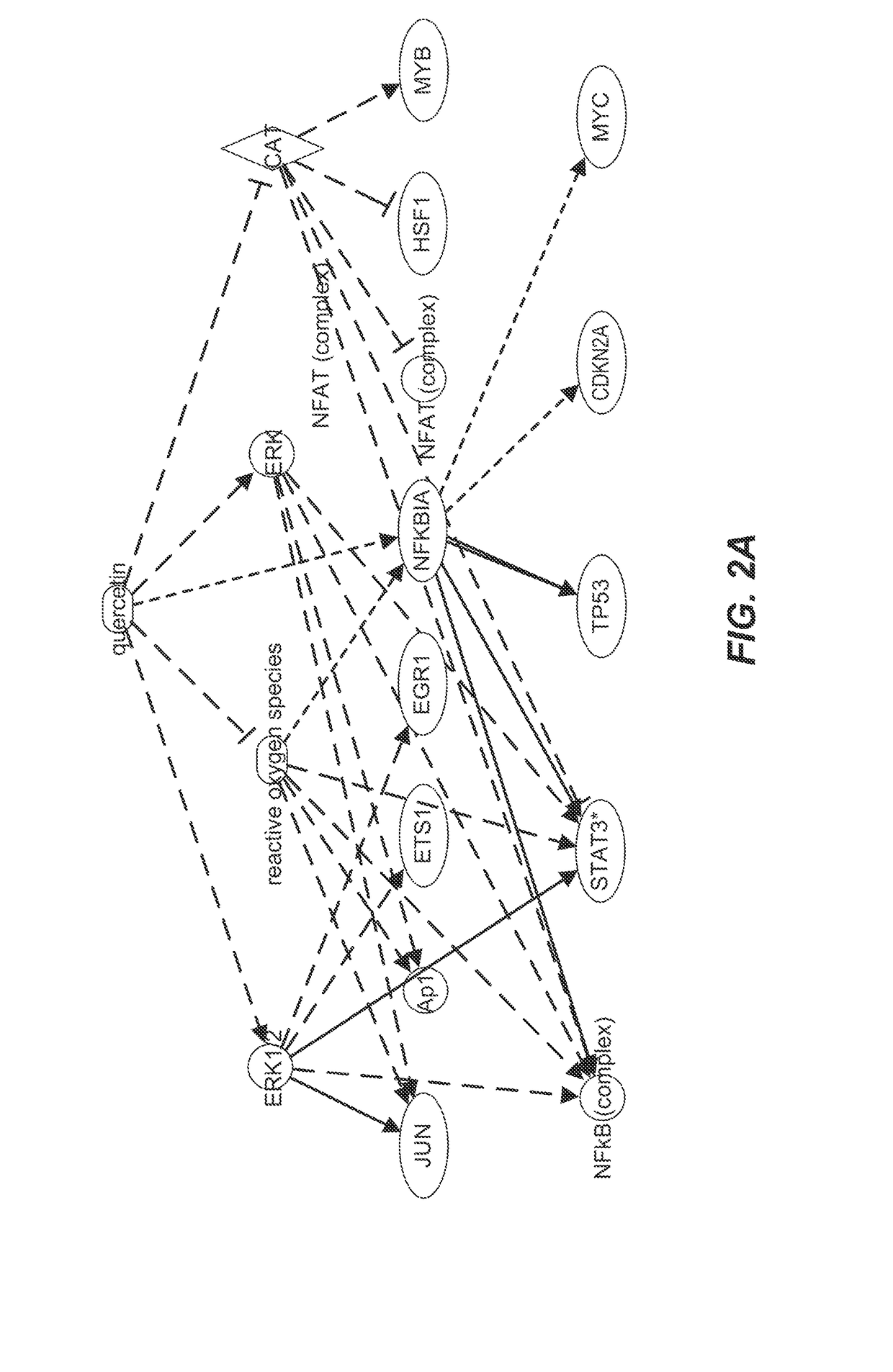
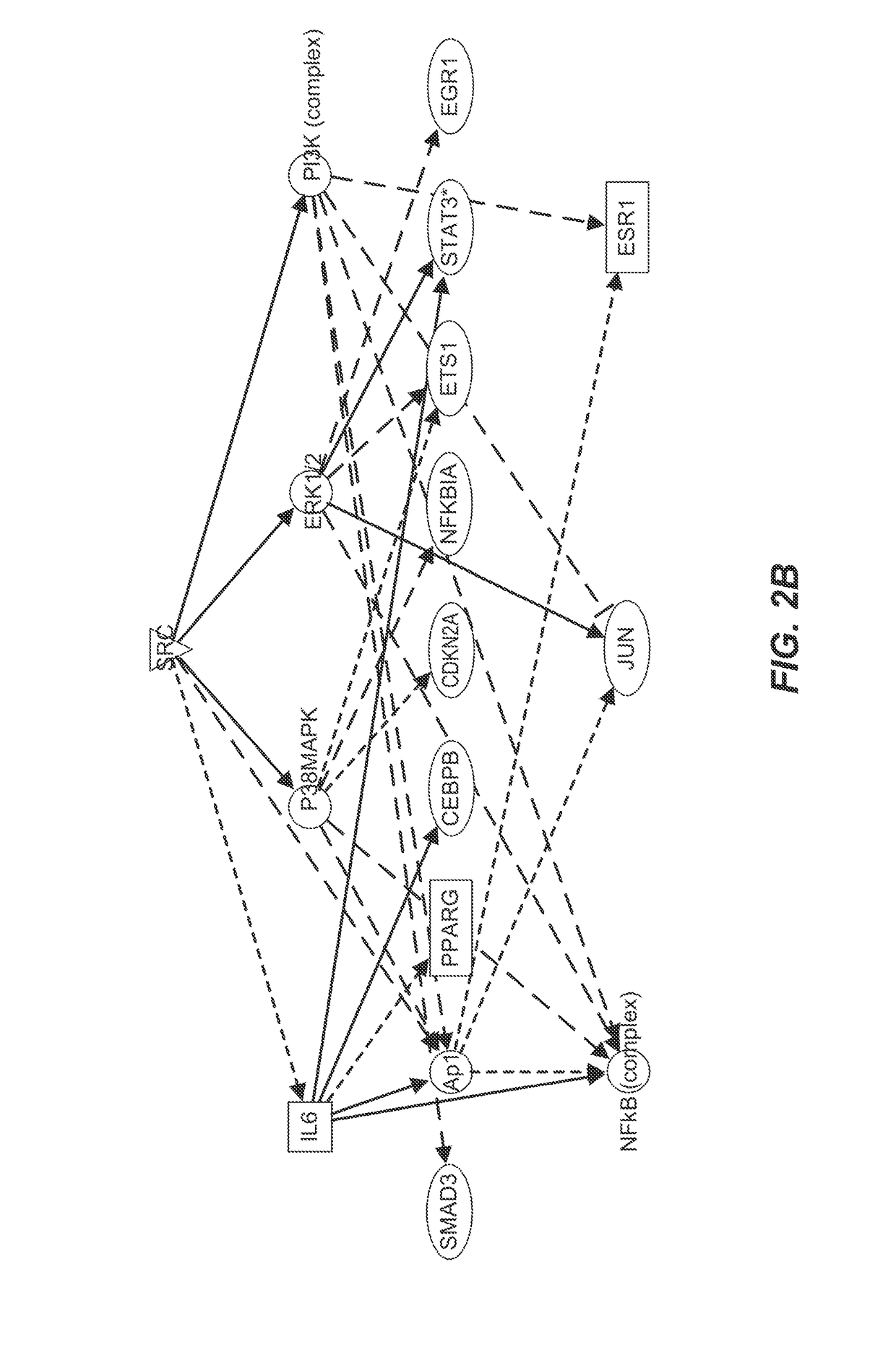
Identification of Senescence Associated Pathways
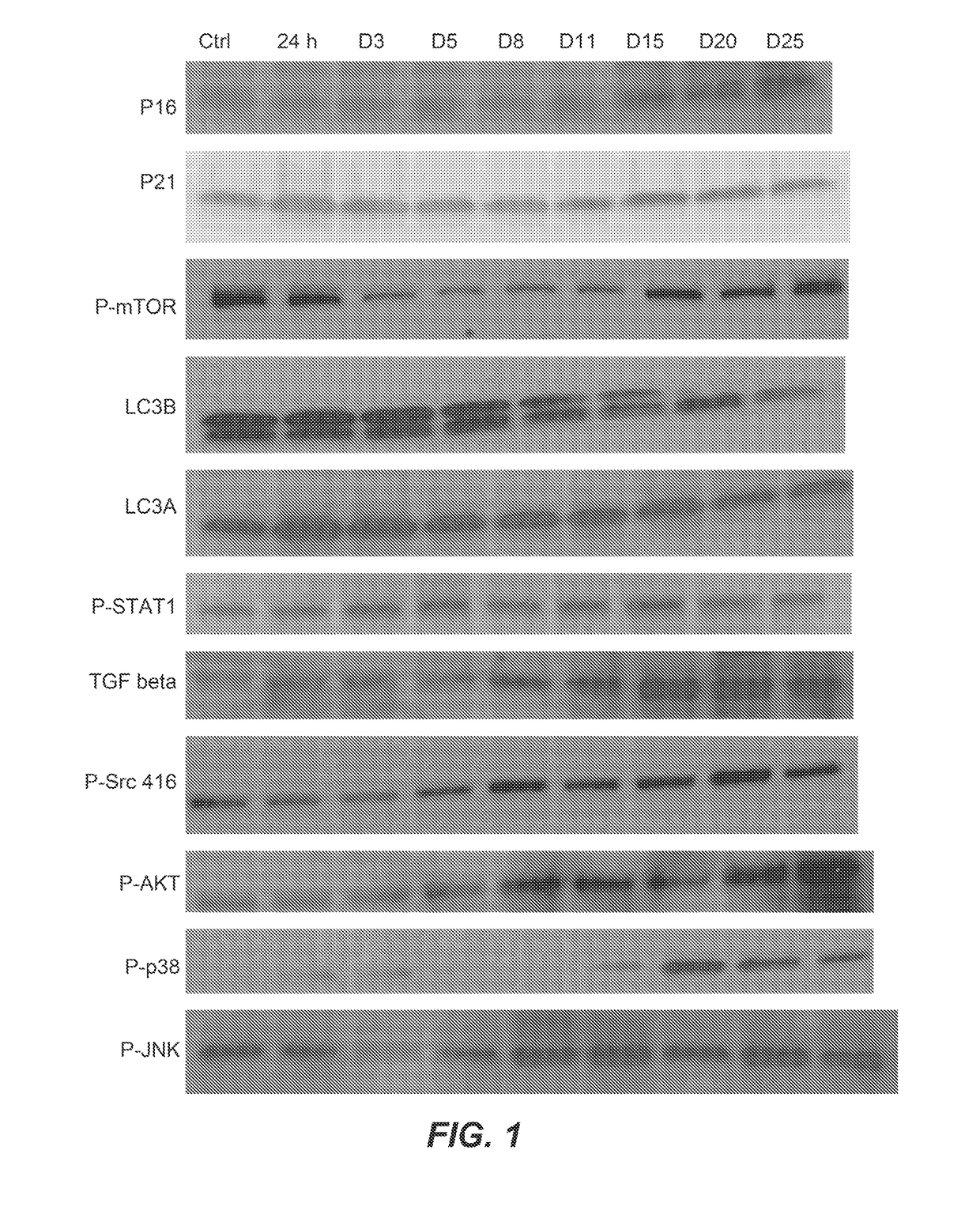
[0264]Proteomic analyses by nano LC MS / MS were performed on lysates on human abdominal subcutaneous preadipocytes that were senescent or non-senescent. Preadipocytes, one of the most abundant cell types in humans susceptible to senescence, were extracted from fat tissues of nine different healthy kidney transplant donors by collagenase digestion. Prior consent from the donors was obtained. Senescence was induced by 10 Gy radiation or by serial subculturing. Bioinformatics methods were used to identify pathways that were susceptible to existing drugs and that could mediate cell death.
[0265]Senescence-associated β-galactosidase (SA-β gal) activity was used to assess the percentage of senescent cells present in the irradiated cell cultures. To be considered a senescent culture, 75% or more of the cells needed to demonstrate SA-β gal activity. Both whole cell lysates and cellular supernatants were collected. Proteins were separated on 1D S...
example 2
Selective Killing of Senescent Fibroblasts
[0268]Senescence of human primary lung fibroblasts (IMR90) (IMR-90 (ATCC® CCL-186™, Mannassas, Va.) was induced by irradiation. IMR90 cells in culture were subjected to 10 Gy radiation (Day −7). Four days after irradiation (Day −3), the culture media was changed. Three days after the media change (Day 0), the cells were exposed to media containing quercetin. Non-irradiated IMR90 cells were included as controls. Non-irradiated and irradiated IMR90 cells were exposed to quercetin at concentrations of 5, 10, 15, and 45 μM. Percent survival was determined four days after exposure to the drugs (Day 4). The number of viable cells was determined by using ATPLITE (Perkin-Elmer, Waltham, Mass.). The results are presented in FIG. 3.
[0269]In a separate experiment, IMR90 cells were irradiated as described above to induce senescence. Approximately 20 days after irradiation, the senescent cells and proliferating IMR90 cells were exposed to enzastaurin at ...
example 3
Selective Killing of Senescent Endothelial Cells
[0271]Human umbilical endothelial cells (HUVEC) (Lonza Group, Basel, Switzerland) were induced to senescence by exposure to 10 Gy radiation. Twenty days after irradiation, markers of cellular senescence (SA-β Gal) and growth arrest, determined by incorporation of BRdU, were evident. Non-senescent HUVEC cells used as control were plated at low density in culture media so that the cells were proliferating when exposed to the test drug. Senescent HUVEC cells and non-senescent, proliferating HUVEC cells were treated for 48 hours with quercetin, dasatinib, and enzastaurin as follows: (1) quercetin alone at 3.75, 7.5 and 15 μM; (2) dasatinib alone at 25, 50, 100, and 200 nM; (3) enzastaurin alone at concentrations of 0.25 μM, 0.5 μM, 1 μM, and 2 μM; (4) dasatinib at 100 nM plus quercetin at 7.5 μM; (5) with dasatinib at 50 nM plus quercetin at 7.5 μM; (6) dasatinib at 100 nM plus enzastaurin at 1.0 μM; (7) dasatinib at 100 nM plus enzastauri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com