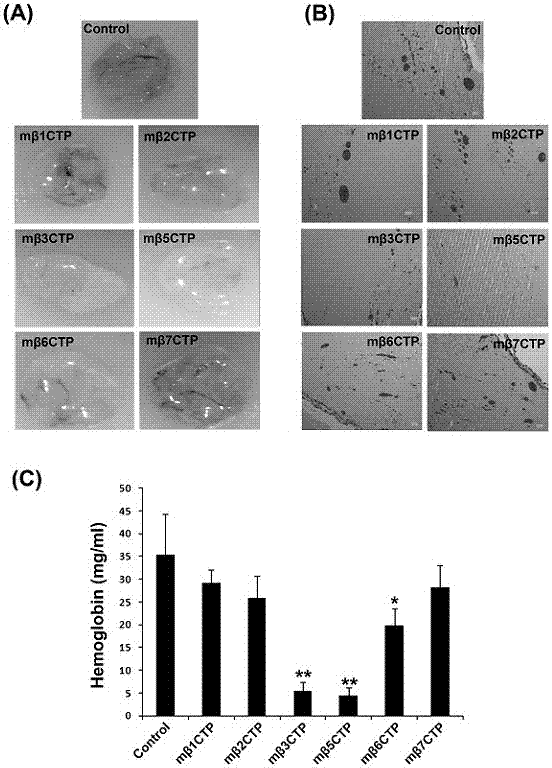
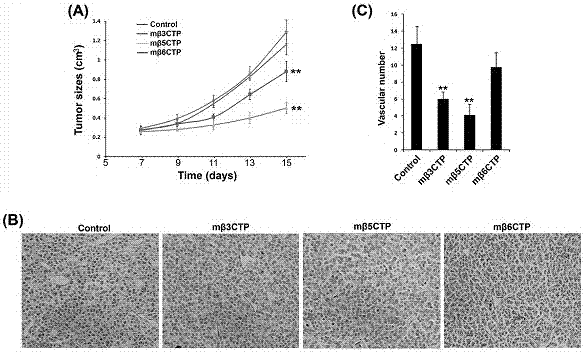
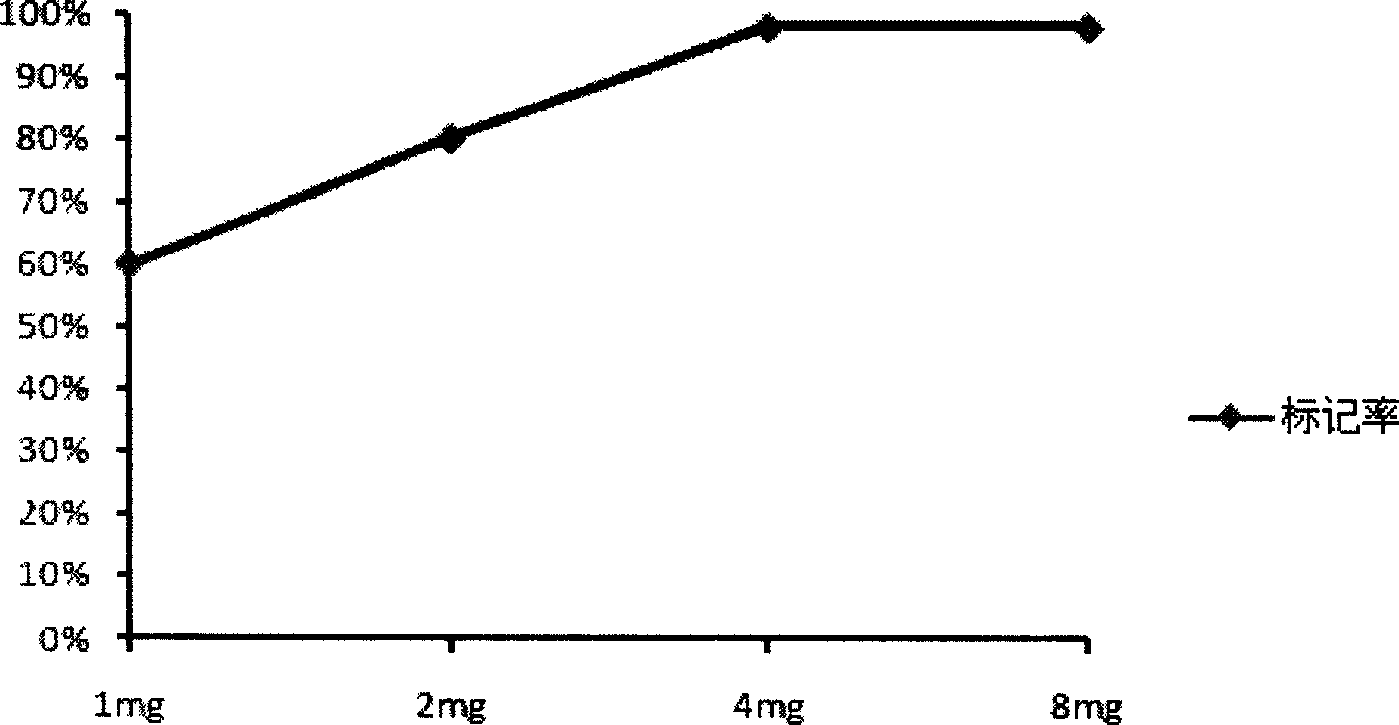
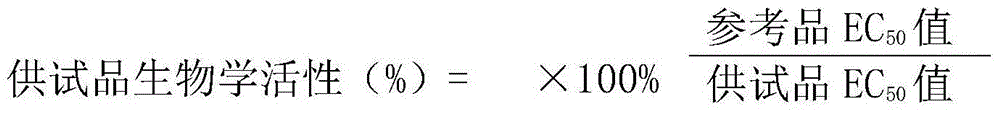Patents
Literature
103 results about "HUVEC Cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
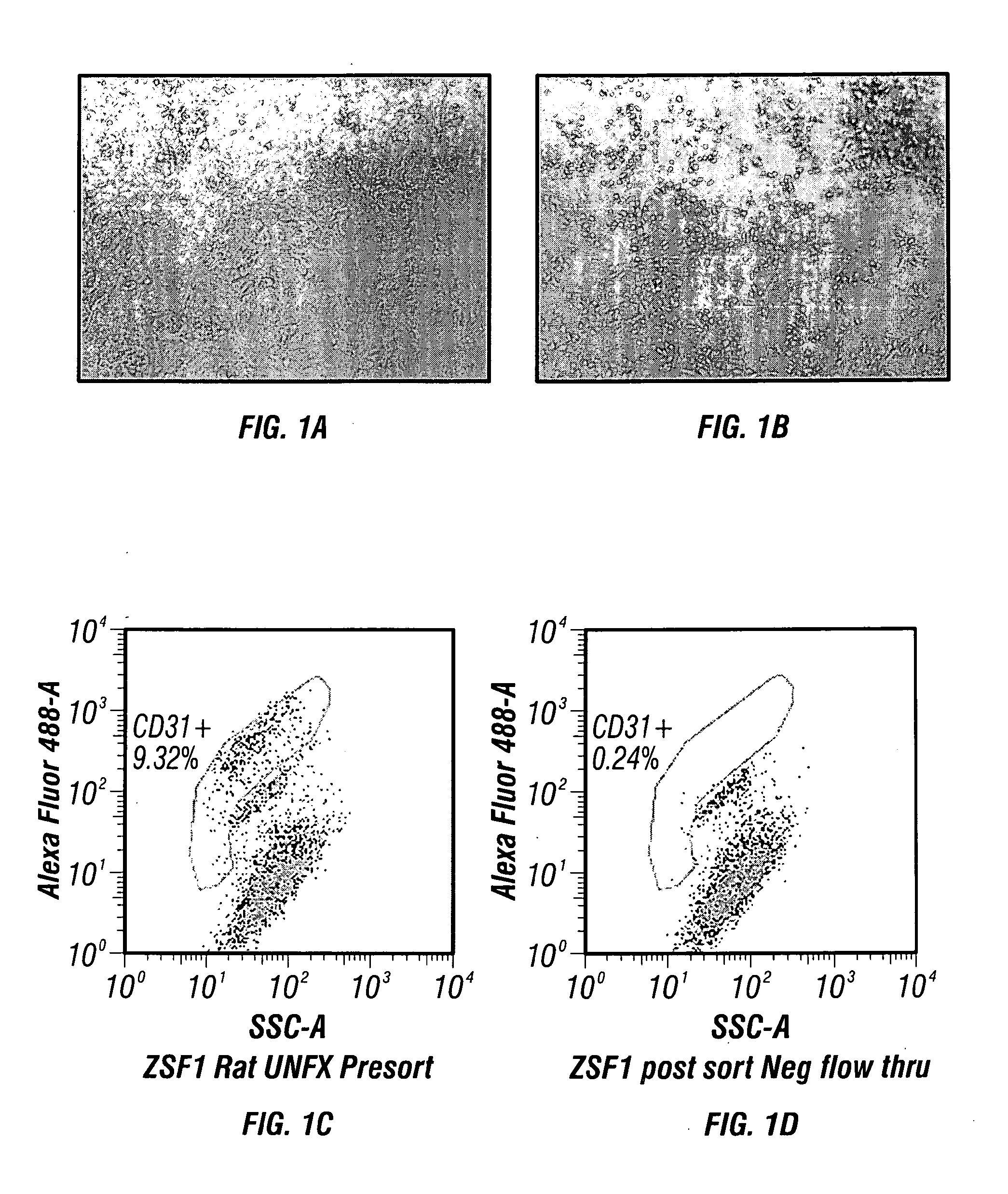
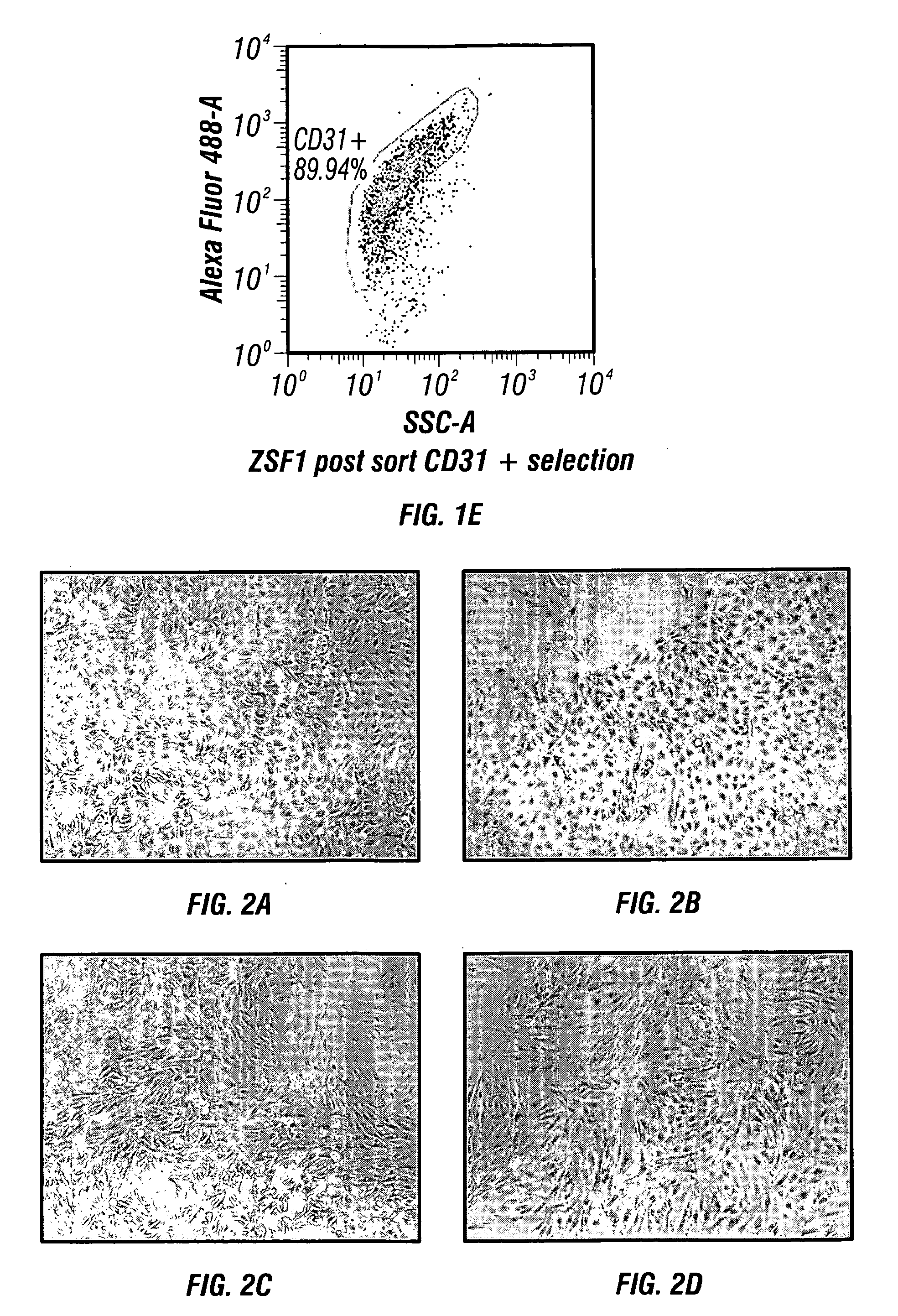
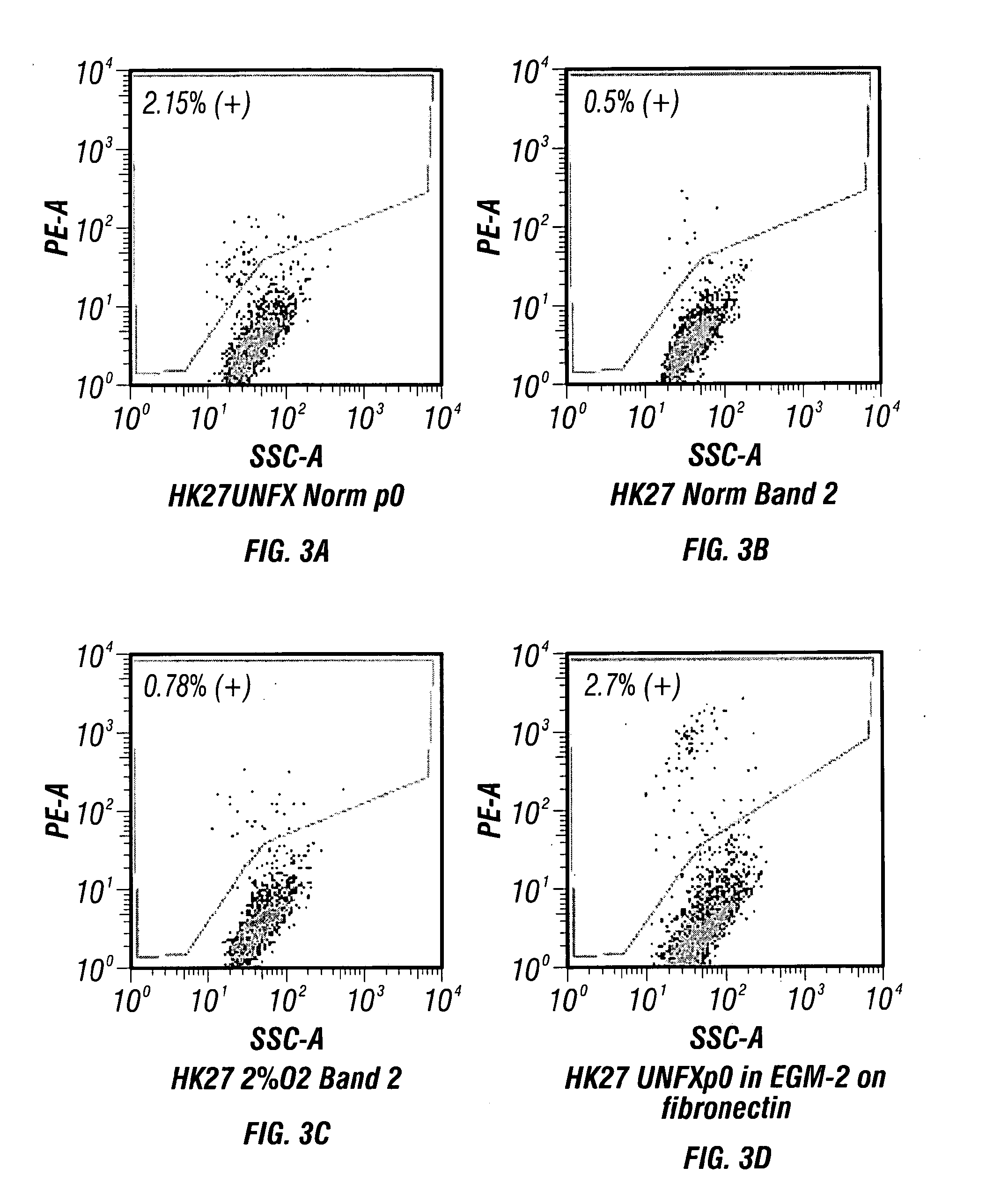
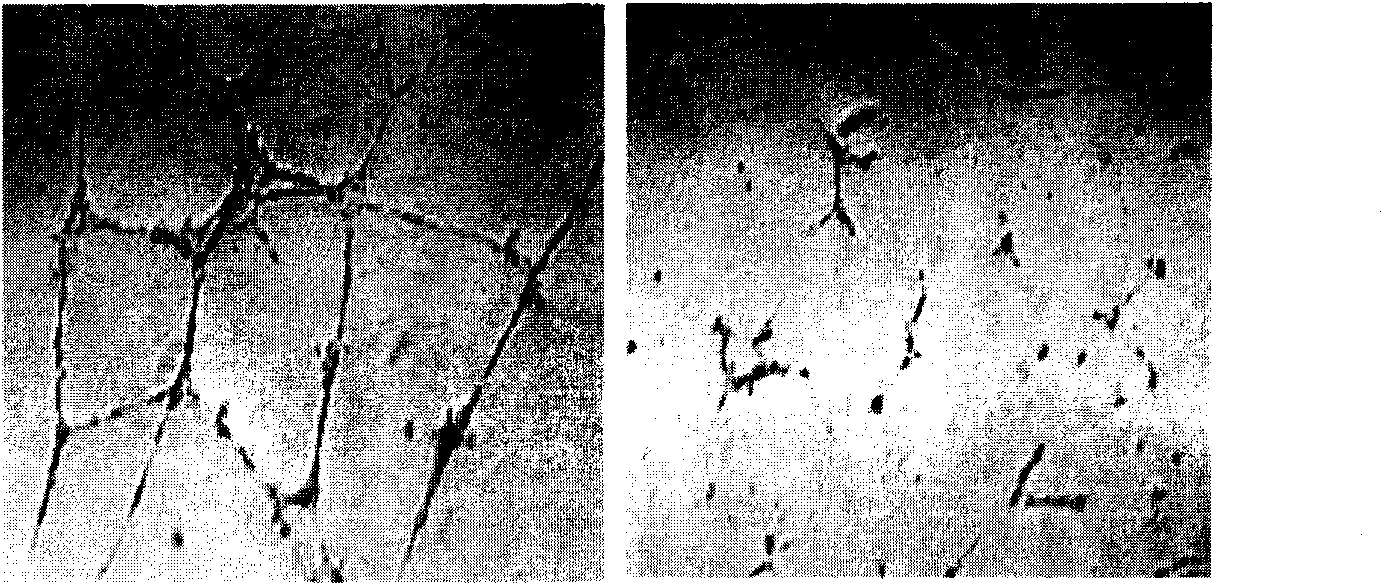
Microscopic image of the keratin cytoskeleton of a HUVEC cell. Human umbilical vein endothelial cells (HUVECs) are cells derived from the endothelium of veins from the umbilical cord. They are used as a laboratory model system for the study of the function and pathology of endothelial cells (e.g., angiogenesis).
Vascularized human skin equivalent
InactiveUS20070207125A1Accelerated rate of vascularizationImprove clinical utilityBiocideEpidermal cells/skin cellsSkin equivalentIn vivo
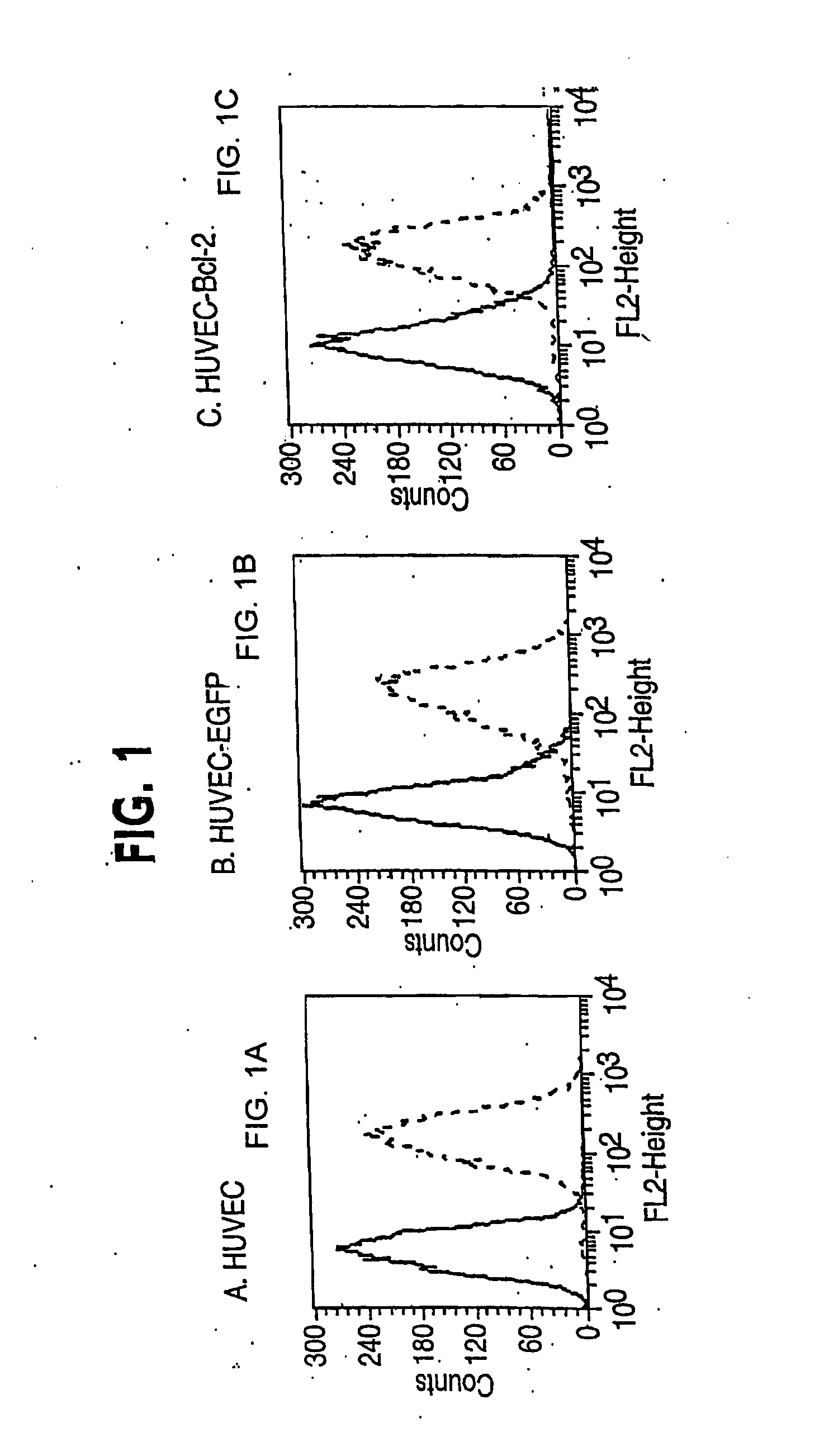
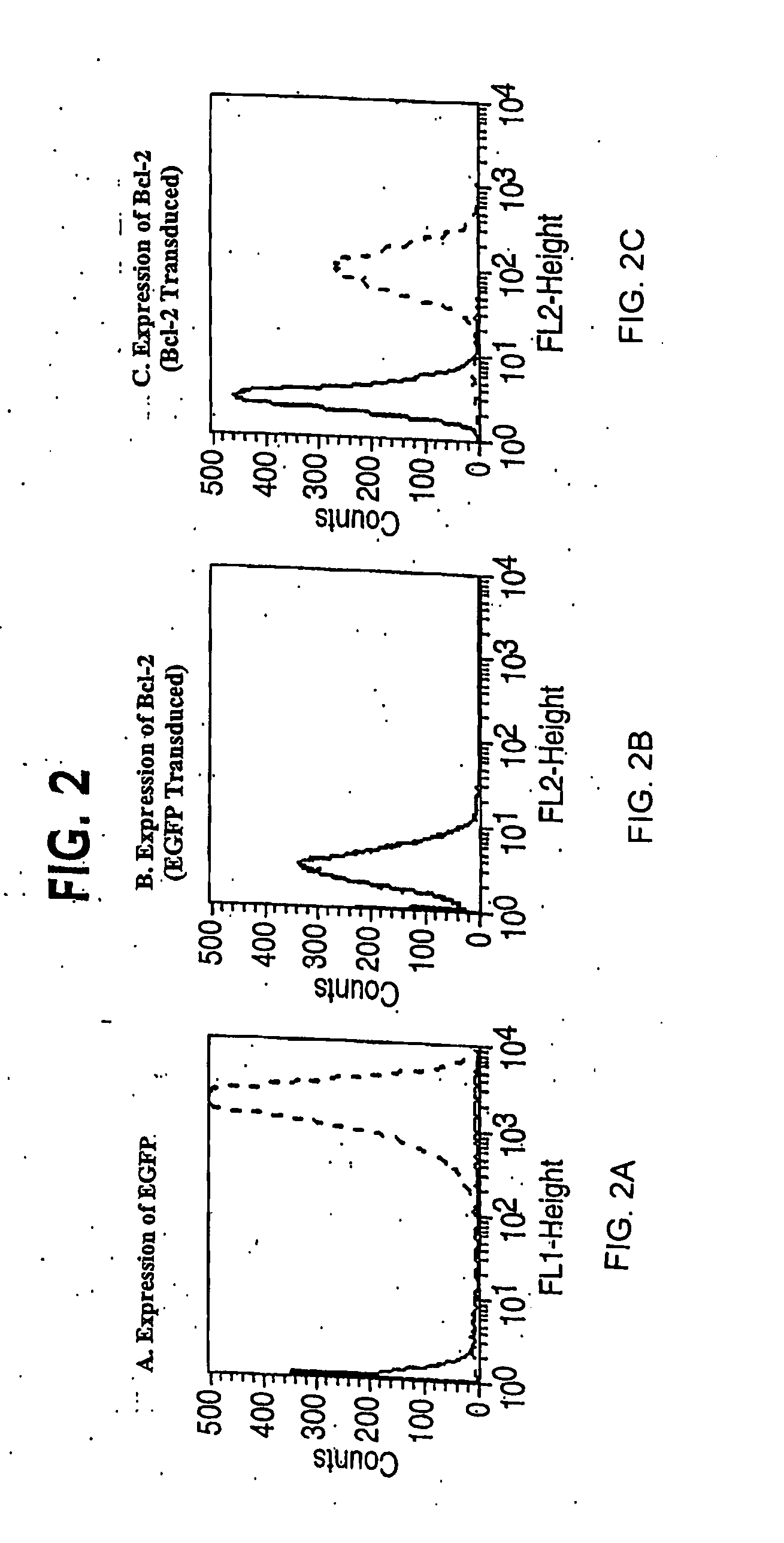
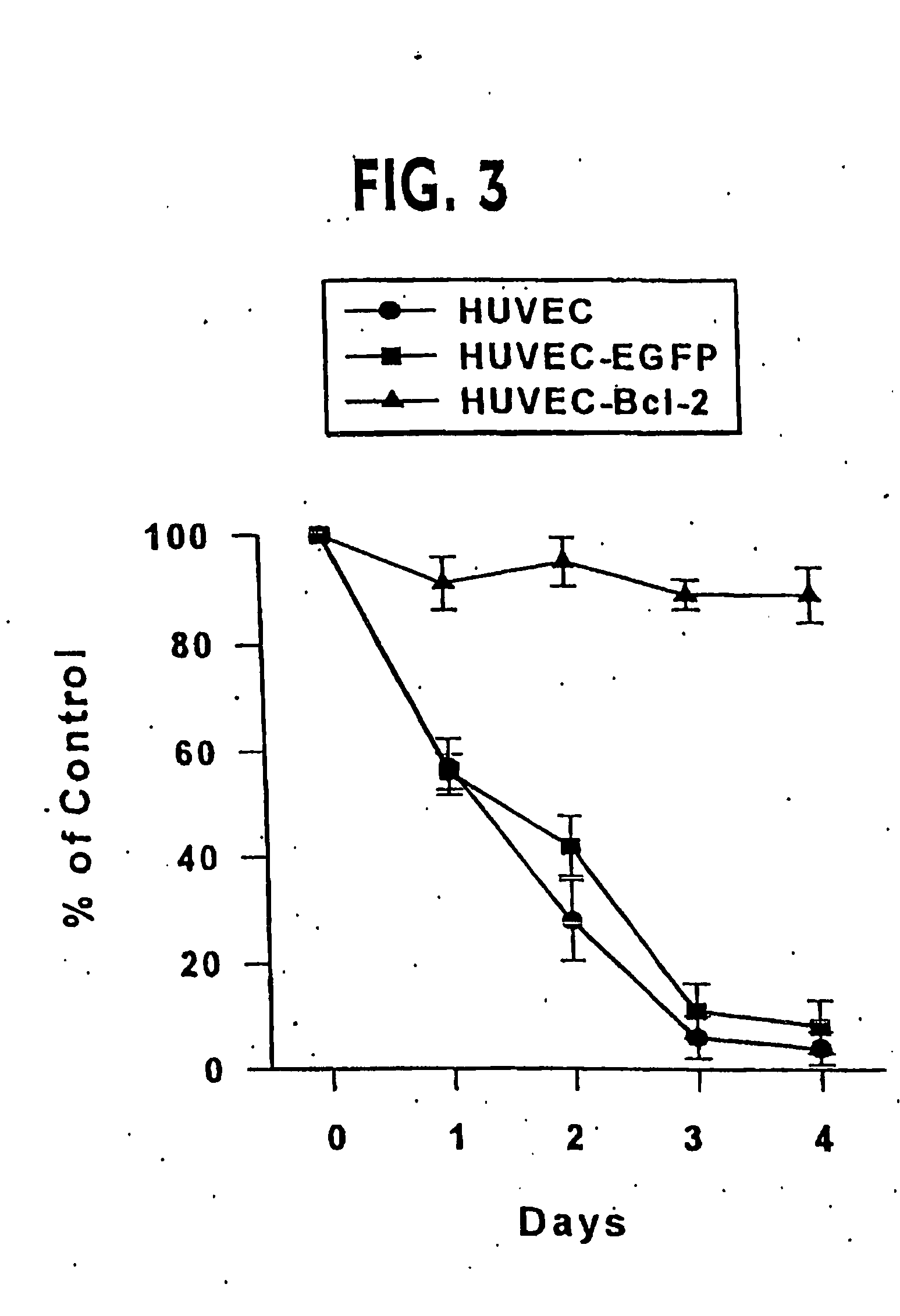
Clinical performance of currently available human skin equivalents is limited by failure to develop perfusion. To address this problem we have developed a method of endothelial cell transplantation that promotes vascularization of human skin equivalents in vivo. Living skin equivalents were constructed by sequentially seeding the apical and basal surfaces of acellular dermis with cultured human keratinocytes and Bcl-2 transduced HUVEC or umbilical cord cells sequentially. After orthotopic implantation of grafts comprising cultured human keratinocytes and Bcl-2 transduced HUVEC cells onto mice, the grafts displayed both a differentiated human epidermis and perfusion through the HUVEC-lined microvessels. These vessels, which showed evidence of progressive maturation, accelerated the rate of graft vascularization. Successful transplantation of such vascularized human skin equivalents should enhance clinical utility, especially in recipients with impaired angiogenesis.
Owner:YALE UNIV
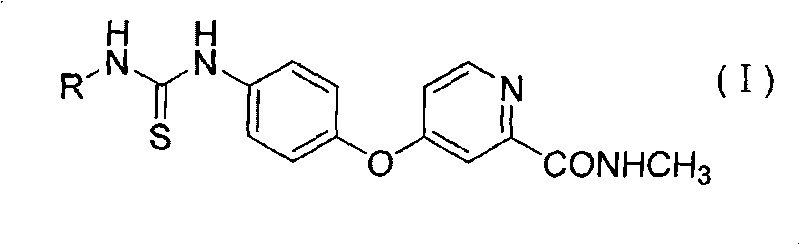
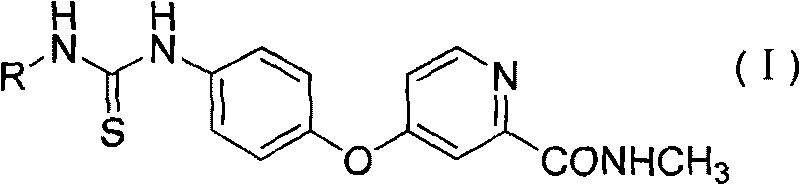
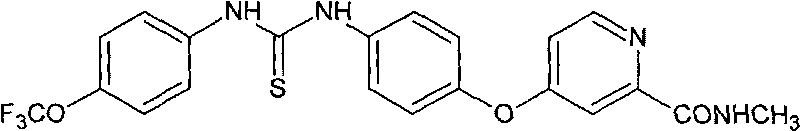
Aryl thiourea and preparation method and application thereof
The invention discloses aryl thiourea and a preparation method and applications thereof. The aryl thiourea is a compound shown in the general formula I. The new compound-aryl substituted thiourea shows very high activity of inhibiting the growth of tumor cells and especially has significant effect of inhibiting the growth of HUVEC cells with high expression of VEGFR, wherein IC 50 is more than 70% in 10mu g / ml. The invention also provides a preparation method of the compound.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Organoids comprising isolated renal cells and use thereof
InactiveUS20160101133A1BiocidePharmaceutical delivery mechanismBlood Vessel EndotheliumErythroid cell
Described herein are organoids comprising admixtures of selected bioactive primary renal cells and a bioactive cell population, e.g., an endothelial cell populations, e.g. HUVEC cells, and methods of treating a subject in need thereof with such organoids. Further, the isolated renal cells, which may include tubular and erythropoietin {EPO}-producing kidney cell populations, and / or the endothelial cell populations may be of autologous, syngeneic, allogeneic or xenogeneic origin, or any combination thereof. Further provided are methods of treating a subject in need with the organoids.
Method for testing vascellum esoderma inhibin bioactivity
InactiveCN101256139AWith characteristicsRepetitiveMicrobiological testing/measurementColor/spectral properties measurementsAngiogenesis InhibitionVascular endothelium
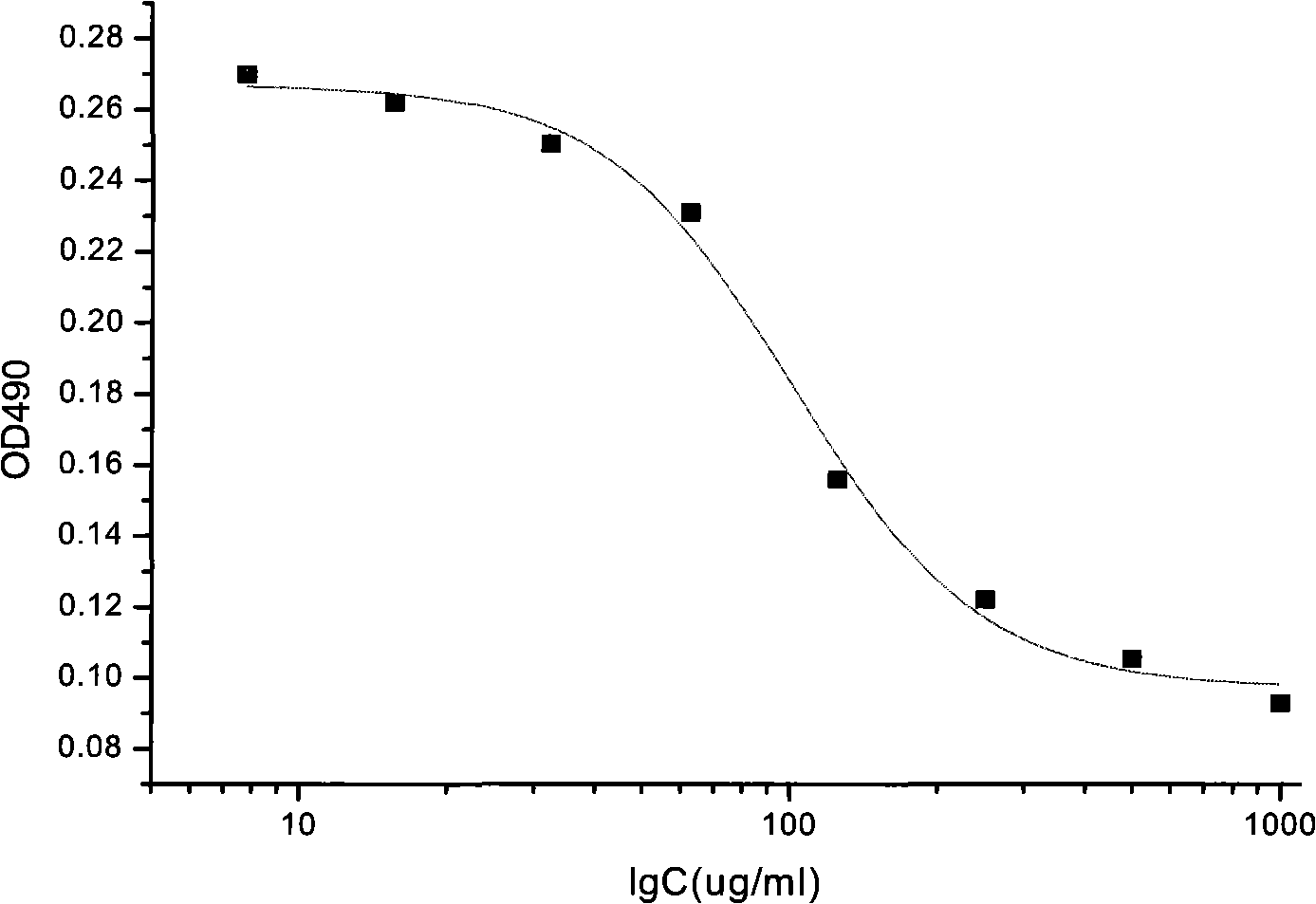
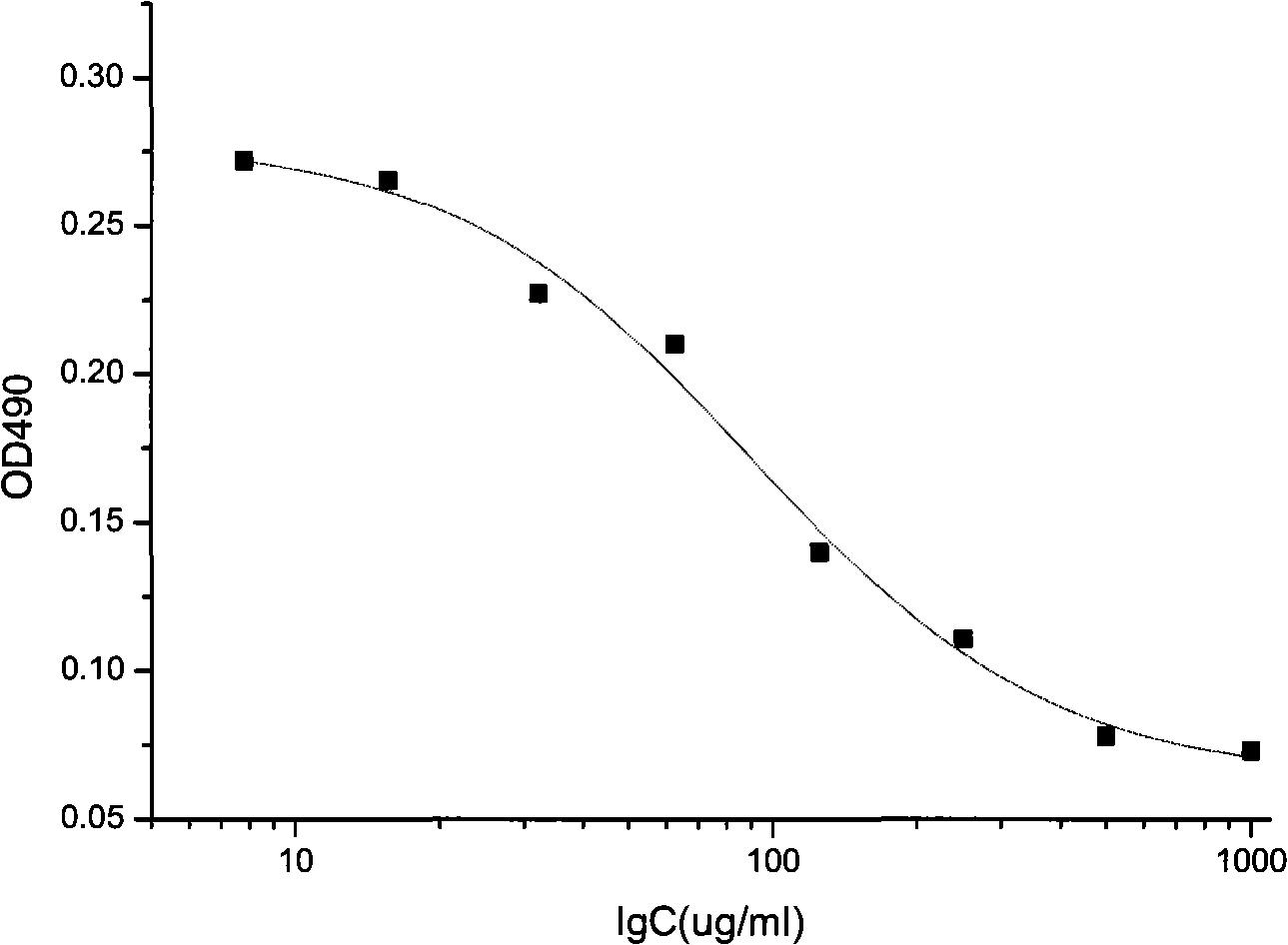
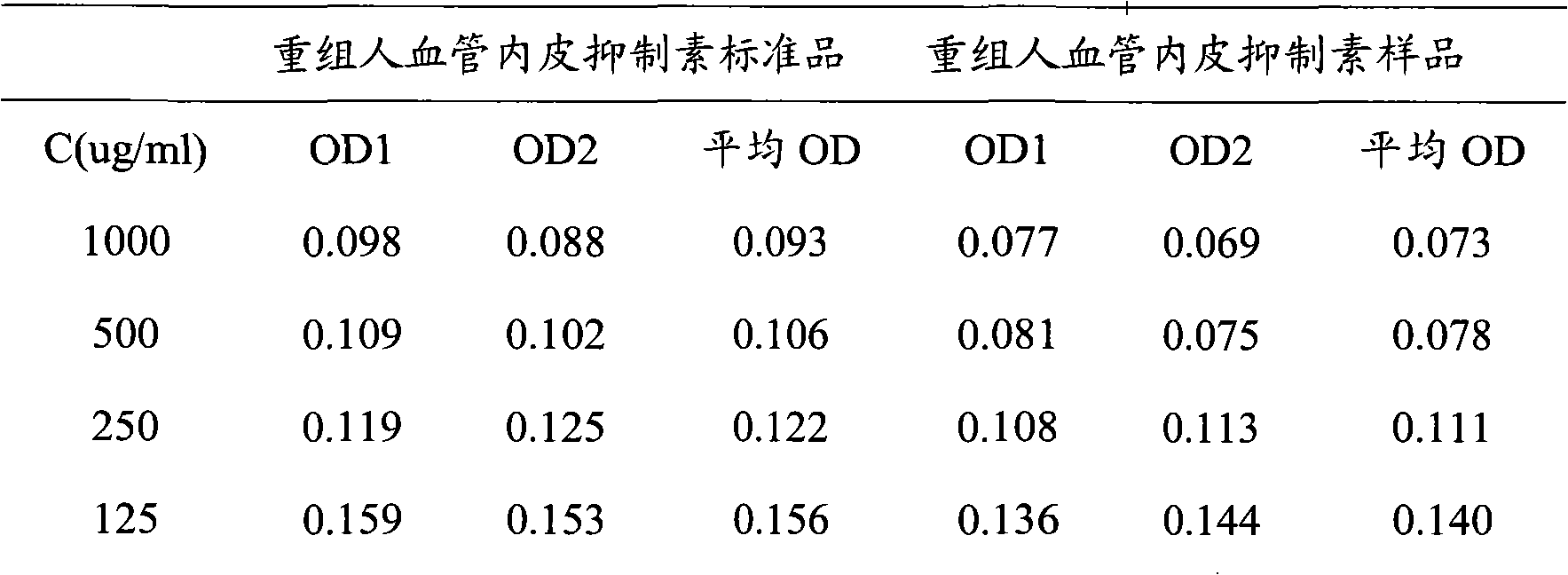
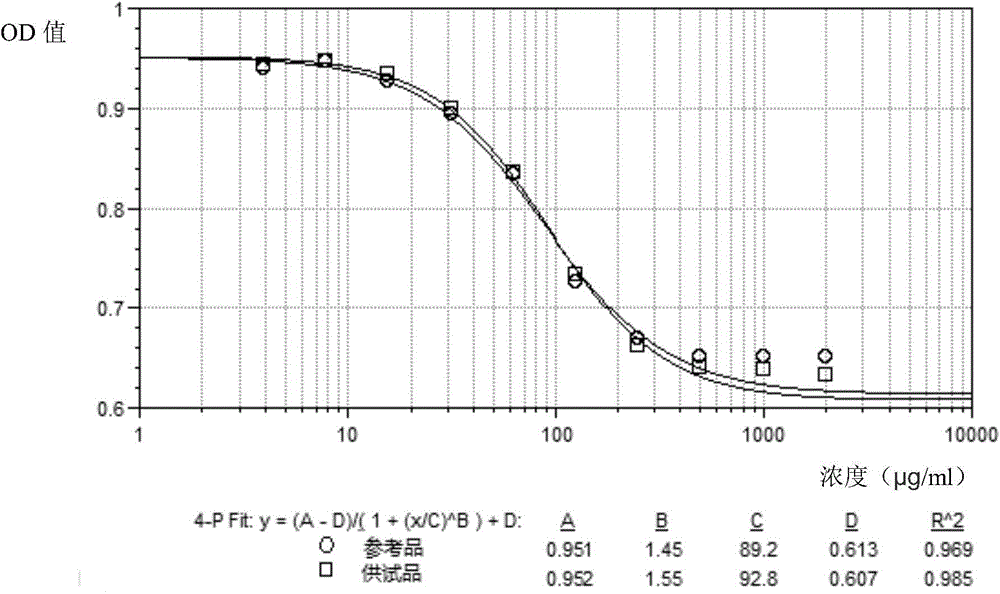
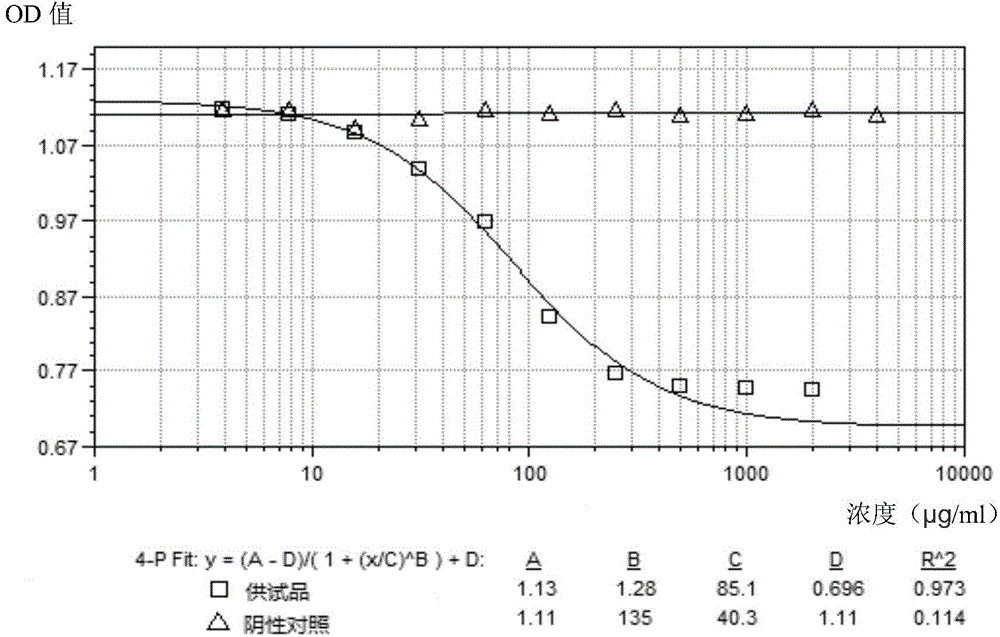
The invention discloses a method for testing vascular endothelial inhibitor biological activity, the method utilizes vascular endothelial inhibitor standard and sample with the same concentration gradient after dilution in multiple proportions to inhibit HUVEC cell proliferation, and detects the proliferate inhibition activity of vascular endothelial inhibitor to HUVEC cells with trace enzyme reaction colorimetry, and then analyzes biological activity of angiogenesis inhibition of vascular endothelial inhibitor. The method uses the vascular endothelial inhibitor standard to correct testing errors of vascular endothelial inhibitor samples in different batches, which causes the cell proliferation inhibition activity testing results of vascular endothelial inhibitor have specificity and repeatability, and overcomes the defects that existed in prior vascular endothelial inhibitor activity testing method that the repeatability is bad, the method can not be unified, and the method can not meet the needs of large scale production and multiple-batch quality control.
Owner:SHANDONG SIMCERE BIO PHARMA CO LTD
Centipede toxin anti-tumour active polypeptide
InactiveCN101899095AHas antitumor activityGrowth inhibitionPeptide/protein ingredientsPeptidesReversed-Phase Liquid ChromatographyAmino acid composition
The invention discloses polypeptide separated from centipede toxin, and determination on anti-tumour activity thereof is carried out. Ultrafilteration and reversed-phase chromatography separation methods are adopted to separate and purify one polypeptide composed of twelve amino acids from centipede toxin, the sequence thereof detected by applying edman degradation method is Phe-Thr-Gly-Gly-Asp-Glu-Ser-Arg-Ile-Gln-Glu-Gly, and the molecular weight thereof measured by applying mass-spectrometric method is 1296.05Da. The anticancer cell viability of the polypeptide is determined by applying MTT method. The polypeptide in the invention has anti-tumour activity, has inhibition action on propagation and growth of human umbilical vein endothelial cell, is in dose-response relationship and can be applied to treatment and auxiliary treatment of various cancers.
Owner:CHINA PHARM UNIV
Tricyclic derivatives or pharmaceutically acceptable salts thereof, their preparations and pharmaceutical compositions containing them
The present invention relates to tricyclic derivatives or pharmaceutically acceptable salts thereof, their preparations and pharmaceutical compositions containing them. More precisely, the present invention relates to tricyclic derivatives as colchicine derivatives, pharmaceutically acceptable salts thereof, their preparations and pharmaceutical compositions containing them. Tricyclic derivatives of the present invention show very powerful cytotoxici ty to cancer cell lines but were much less toxic than colchicine or taxol, confirmed through animal toxicity test. Tricyclic derivatives of the inventi on also decrease the volume and weight of a tumor and have a strong angiogenesi s inhibiting activity in HUVEC cells. Thus, tricyclic derivatives of the prese nt invention can effectively be used as an anticancer agent, anti-proliferation agent and an angiogenesis inhibitor.
Owner:JEIL PHARM CO LTD
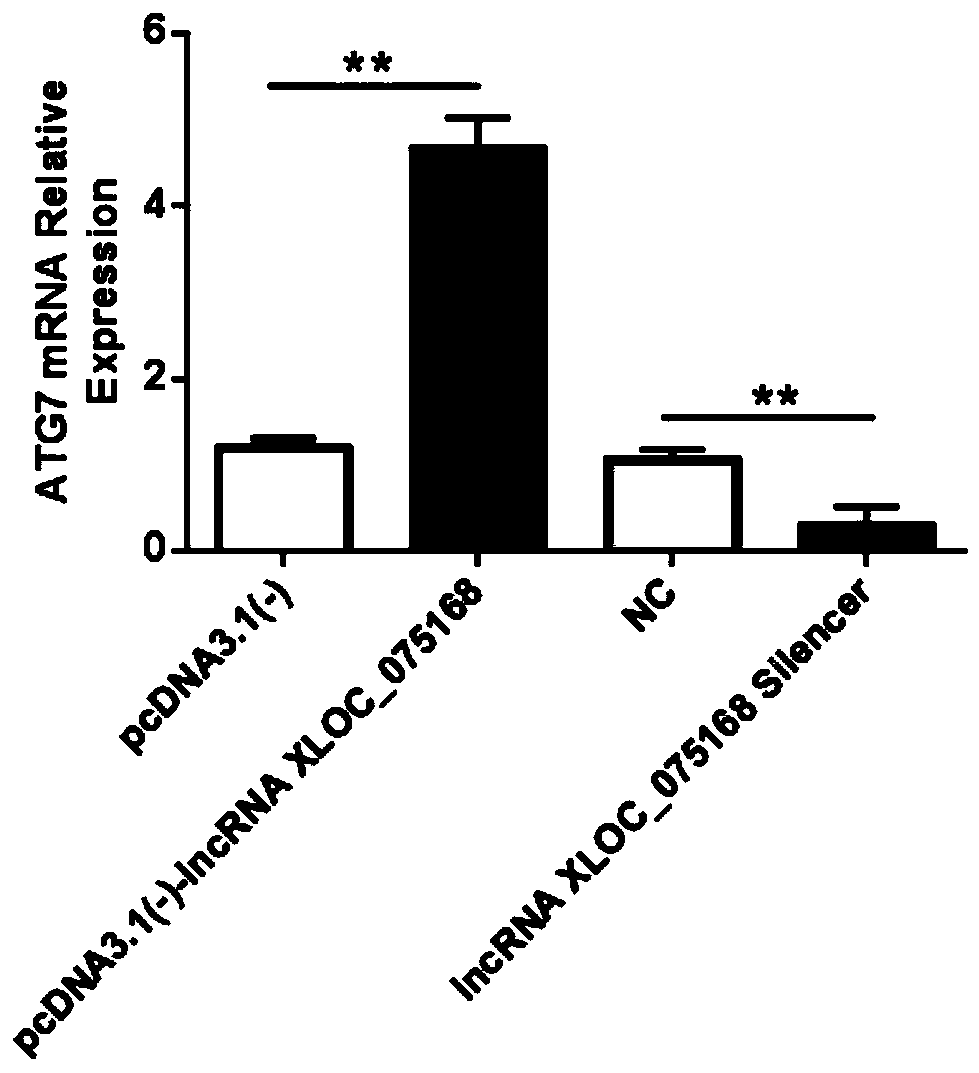
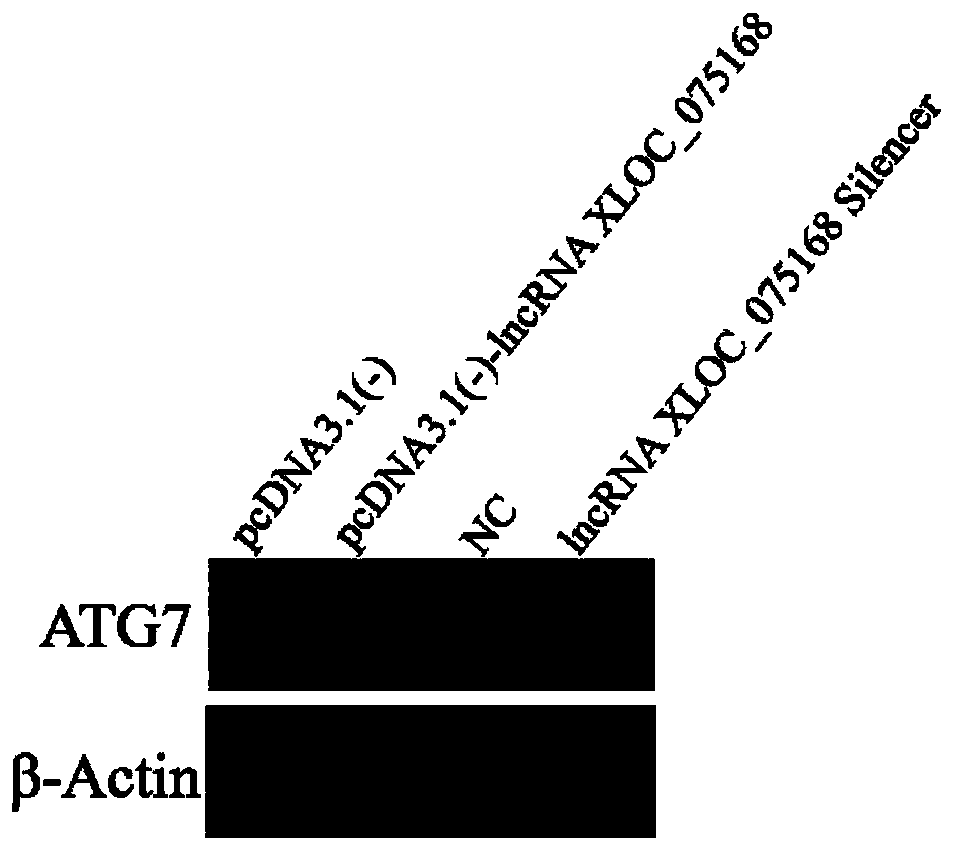
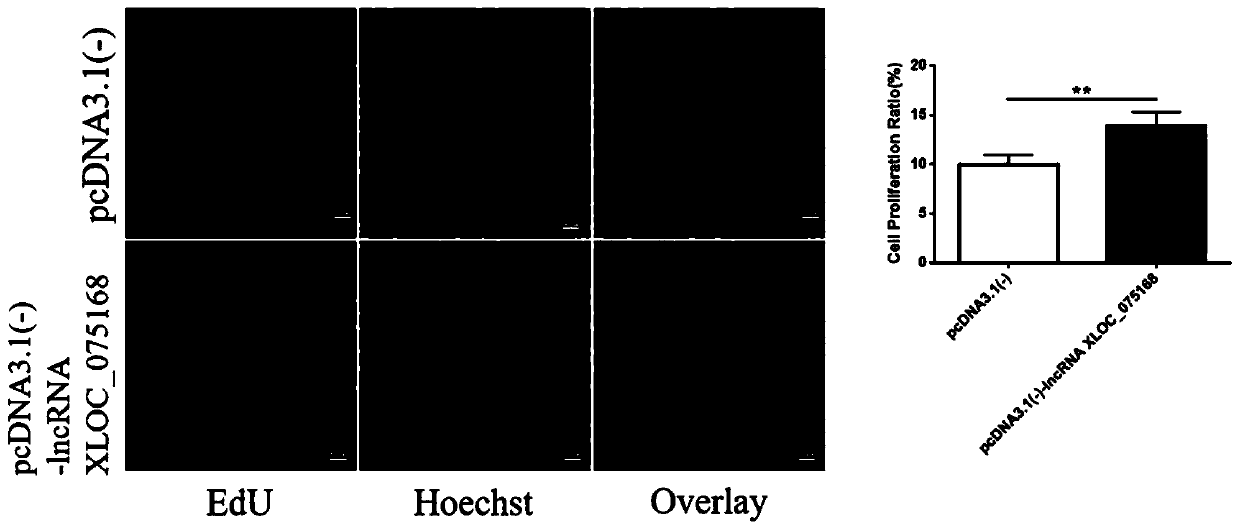
Application of LncRNA XLOC_075168 to preparation of medicines for promoting angiogenesis
ActiveCN110384800APromote proliferationPrevent proliferationOrganic active ingredientsCardiovascular disorderApoptosisAngiogenesis growth factor
The invention discloses an application of LncRNA XLOC_075168 to preparation of medicines for promoting angiogenesis. Overexpressing and silencing of lncRNA XLOC_075168 confirm that the lncRNA XLOC_075168 can influence the expression of an ATG7 gene and protein in human umbilical vein endothelial cells, also confirm that the lncRNA XLOC_075168 participates in promoting the proliferation of the human umbilical vein endothelial cells, restraining the apoptosis of the human umbilical vein endothelial cells and promoting the canaliculization of the human umbilical vein endothelial cells, and further illustrate that the lncRNA XLOC_075168 can possibly participate in promoting the angiogenesis after miocardial infarction. Therefore, the lncRNA XLOC_075168 or a reagent for promoting expression ofthe lncRNA XLOC_075168 can be used for preparing medicines for promoting angiogenesis and can particularly be used for preparing medicines for promoting angiogenesis after miocardial infarction.
Owner:GUANGDONG LAB ANIMALS MONITORING INST +1
Biological activity detection method for VEGF targeted therapy drugs
InactiveCN105628910AAccurate evaluationEffective evaluationBiological testingDrug biological activityTargeted therapy
The present invention provides a biological activity detection method for VEGF targeted therapy drugs. The biological activity detection method comprises: taking HUVEC cells being in a logarithmic growth phase, adjusting to achieve an appropriate cell density, plating, and culturing to make cells be adhered on the plate; carrying out series gradient dilution on a reference substance and a sample, sequentially adding to a culture plate with paved cells, and continuously incubating for a certain time; and carrying out a coloration reaction on the cell culture plate by using a detection system for detecting the amount of living cells or dead cells, and obtaining the biological activity of the sample according to the coloration reaction results. According to the present invention, the requirements on the specificity, the accuracy, the precision (including repeatability, day difference, personnel operating error, and the like) and other verifications can be met, and the method can be effectively applied for screening and quality control of VEGF targeted therapy drugs.
Owner:SUNSHINE GUOJIAN PHARMA (SHANGHAI) CO LTD
Application of cycloicaritin in preparation of anti-tumor composition
InactiveCN103860542AGrowth inhibitionInhibit migrationOrganic active ingredientsAntineoplastic agentsApoptosisMitogen-activated protein
The invention discloses an application of cycloicaritin in preparation of an anti-tumor composition. The cycloicaritin disclosed by the invention can effectively inhibit growth and pipe formation of HUVEC (Human Umbilical Vein Endothelial Cells) and cause apoptosis to inhibit migration of the HUVEC, and has a remarkable inhibiting effect on highly activated MAPK (Mitogen-Activated Protein Kinase) pathway and AKT pathway. Through the above mechanism, the composition can effective prevent tumors, and particularly can effectively inhibit growth of liver cancer, breast cancer, lung cancer, cervical cancer and colon cancer.
Owner:贾晓斌
Decapeptide inhibiting angiogenesis and use thereof
InactiveCN101597324AEnhanced inhibitory effectSenses disorderPeptide/protein ingredientsDiseaseVascular endothelium
The invention relates to a decapeptide inhibiting angiogenesis and use thereof, belonging to the technical field of biological medicine. The decapeptide inhibiting angiogenesis provided by the invention is deprived from human Homo sapien with an amino acid sequence of YNWNSFGLRF and the molecular weight of 1303.4, and is named as Kp-10. The invention further provides use of the decapeptide inhibiting angiogenesis for preparing medicines for inhibition of angiogenesis, in particular relevant use of Kp-10 for preparing medicines for inhibition of angiogenesis dependent diseases such as cancer, rheumatoid arthritis, psoriasis, diabetes syndrome, and hemangioma. A series of experimental results in VEGF (Vascular Endothelial Growth Factor) induction shows that Kp-10 has obvious inhibition function in the proliferation experiment of human umbilical vein endothelial cells (HUVEC), transfer experiment, micro-tube forming experiment, angiogenesis experiment of chick embryo allantois and mouse cornea experiment, and the decapetide can be used for preparing medicines for inhibiting angiogenesis.
Owner:EAST CHINA NORMAL UNIV
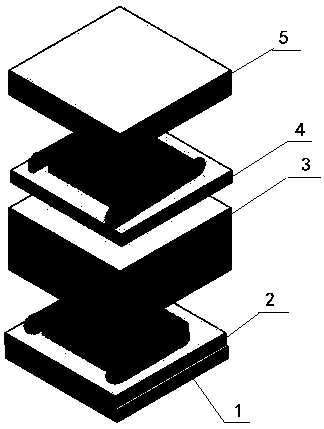
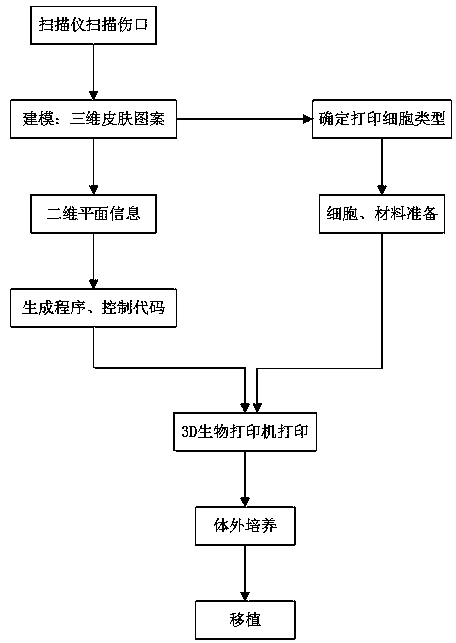
Tissue engineering skin with layered structure and preparation method of tissue engineering skin
InactiveCN108452381AIncreased delivery distribution rateImprove survivabilityAdditive manufacturing apparatusProsthesisFiberHuman body
The invention discloses tissue engineering skin with a layered structure and a preparation method of the tissue engineering skin, and relates to the technical field of tissue engineering skin. The tissue engineering skin with the layered structure comprises a collagen layer, a collagen layer embedded in human skin fibroblast, a first multilayer collagen layer, a collagen layer embedded in keratinocyte and a second multilayer collagen layer. According to the tissue engineering skin disclosed by the invention, a manner that channels are innovatively generated in tissues by a temporary material such as gelatin is adopted, and human umbilical vein endothelial cells are adhered to the inner parts of the channels, so that the formed blood vessels are better fit with human body blood vessels; thetransporting and distributing rate of the formed blood vessels is increased; cells have higher viability and high multiplication rate; the printed skin with the layered structure is adopted, so thatthe interaction between cells of the formed skin structure and the interaction of the skin tissues and epimatrix are more stable, and regeneration of the skin tissue is facilitated.
Owner:TAIYUAN UNIV OF TECH
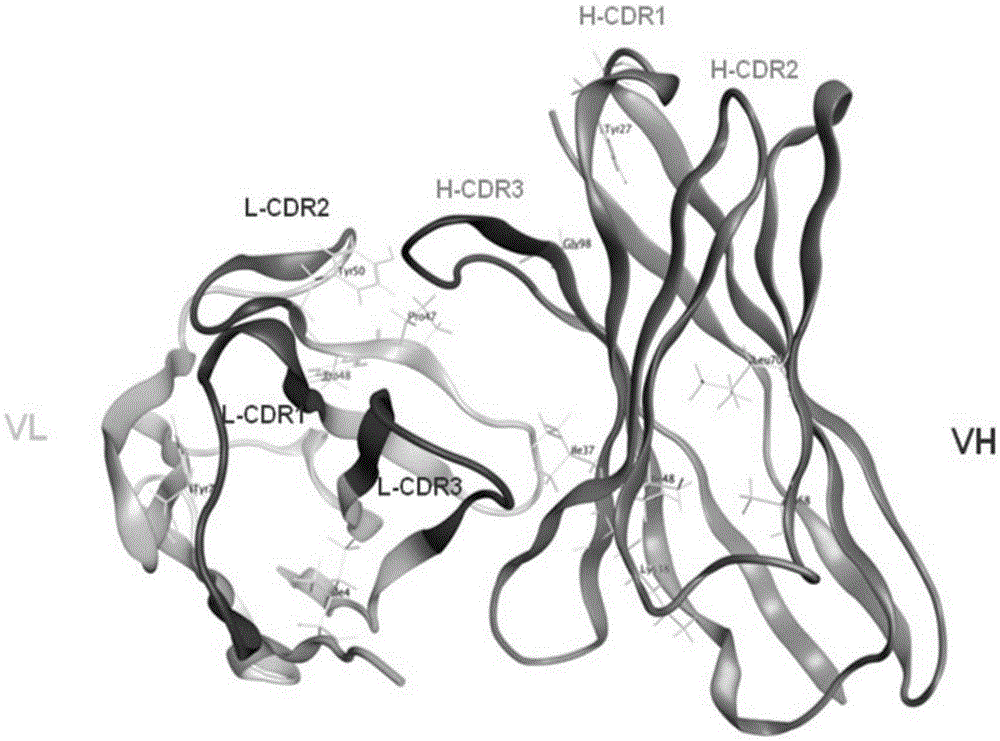
Anti-human Delta-like4 humanized antibody and preparation and application thereof
ActiveCN105384819APreserve affinityRetain biological activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenDisease
The present invention provides anti-human DLL4 (delta-like 4) humanized monoclonal antibody H3L2 and a preparation method and application thereof. A CDR-grafting-based FR region important base reversible mutant humanized antibody of a murine monoclonal antibody is designed by use of bioinformatics software, the heavy chain variable region of the anti-human DLL4 humanized antibody has an amino acid sequence shown as SEQ IDNO: 3, the light chain variable region of the anti-human DLL4 humanized antibody has an amino acid sequence shown as SEQ IDNO: 4; and the humanized antibody H3L2 is capable of high affinity binding to human DLL4 antigen (KD value of 2.26* 10<-12>M), can specifically target HUVEC cell surface DLL4 antigen, blocks DLL4 inhibition effect on HUVEC cell proliferation, and retains the biological activity of the parent murine monoclonal antibody. An antibody-based treatment method for diagnosis and treatment of diseases characterized by DLL4 overexpression is provided.
Owner:CHINA PHARM UNIV
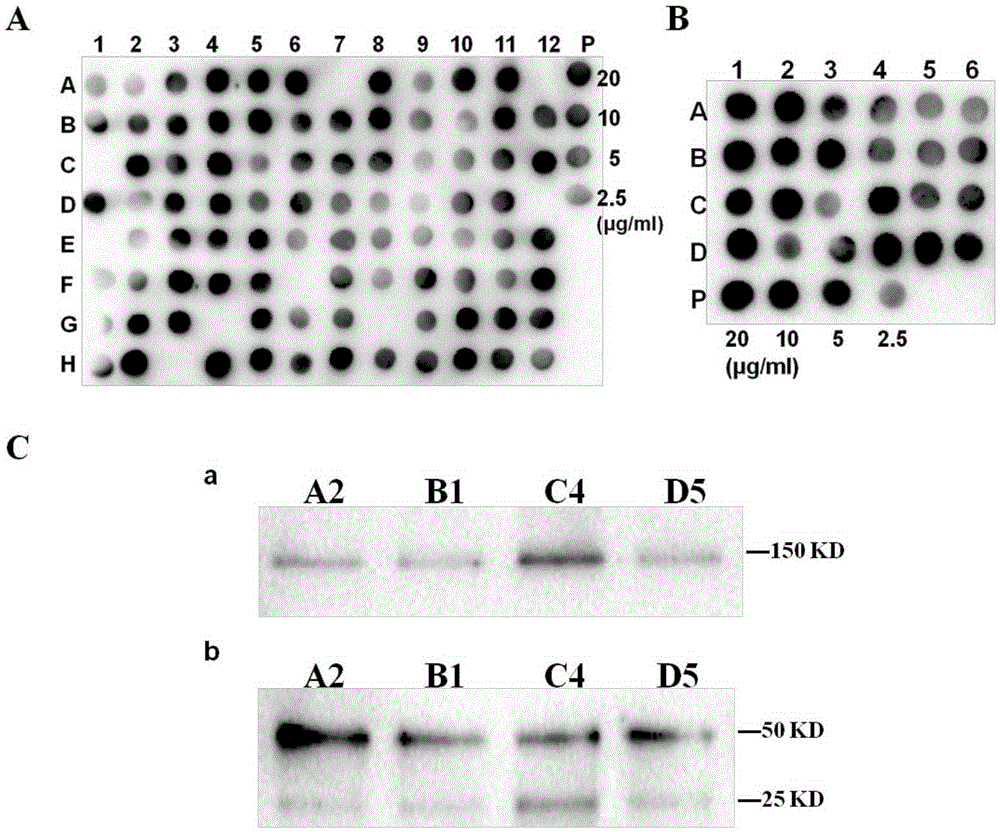
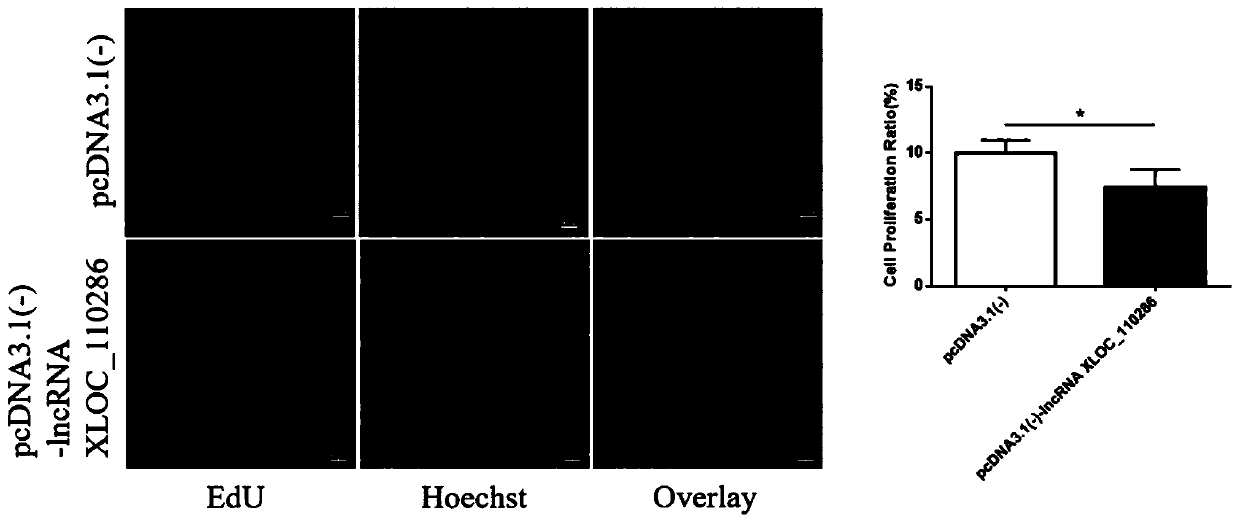
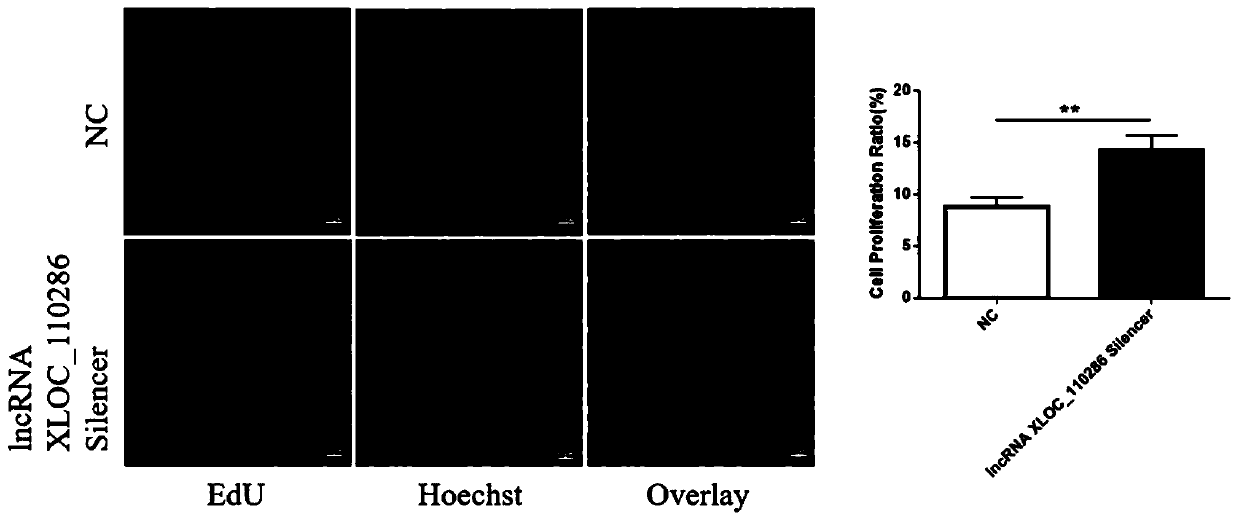
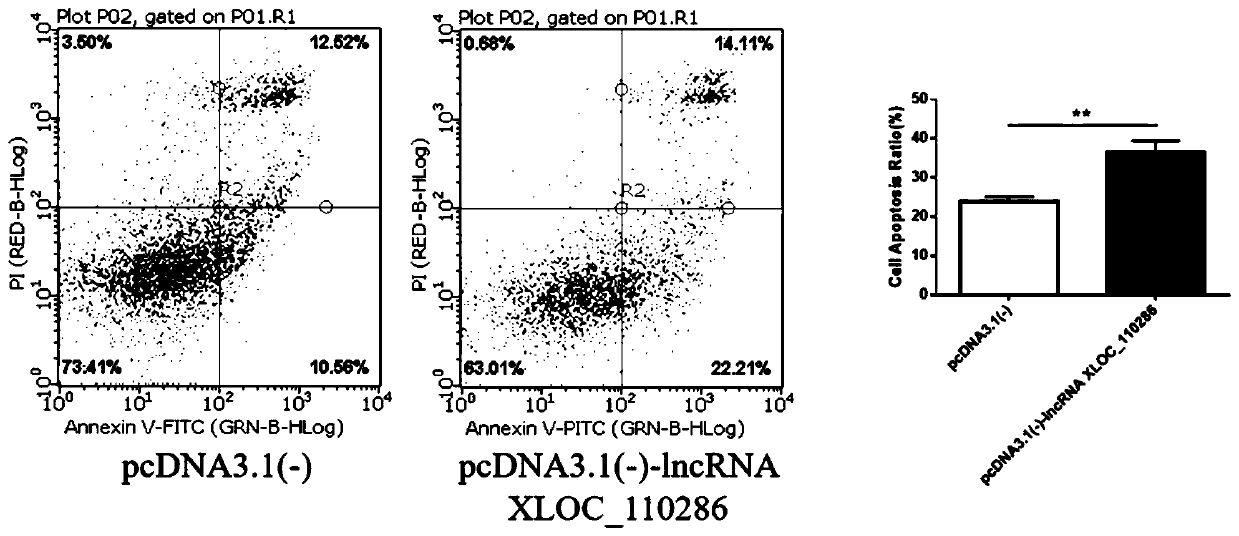
Application of inhibitor of LncRNA XLOC_110286 in preparation of medicine for promoting angiogenesis
ActiveCN110403954AReduce proliferation rateHigh proliferation rateOrganic active ingredientsCardiovascular disorderApoptosisAngiogenesis growth factor
The invention discloses application of an inhibitor of LncRNA XLOC_110286 in preparation of medicine for promoting angiogenesis. It is confirmed that the LncRNA XLOC_110286 is involved in inhabitationof proliferation of human umbilical vein endothelial cells through overexpression and silence of the LncRNA XLOC_110286, apoptosis of the human umbilical vein endothelial cells are promoted, migration of the human umbilical vein endothelial cells is inhibited, formation of human umbilical vein endothelial cell canaliculi is inhabited, and it is further stated that the LncRNA XLOC_110286 may has the inhibiting effect on angiogenesis after infarct. Thus, the inhibitor of the LncRNA XLOC_110286 can be used for preparation of the medicines for promoting angiogenesis. Specifically, the inhibitor of the LncRNA XLOC_110286 can be used for preparation of medicine for promoting angiogenesis after myocardial infarction.
Owner:GUANGDONG LAB ANIMALS MONITORING INST +1
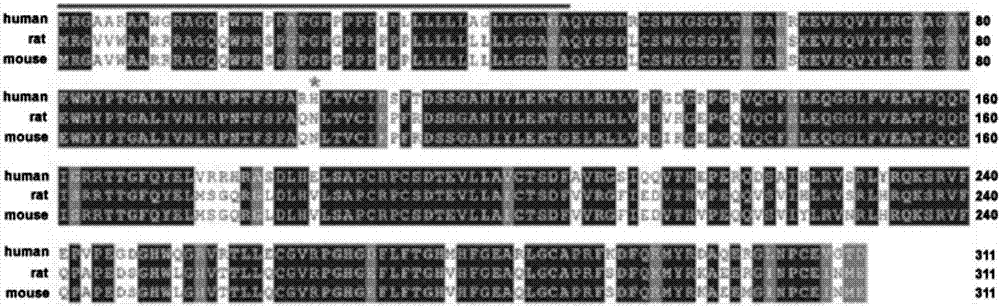
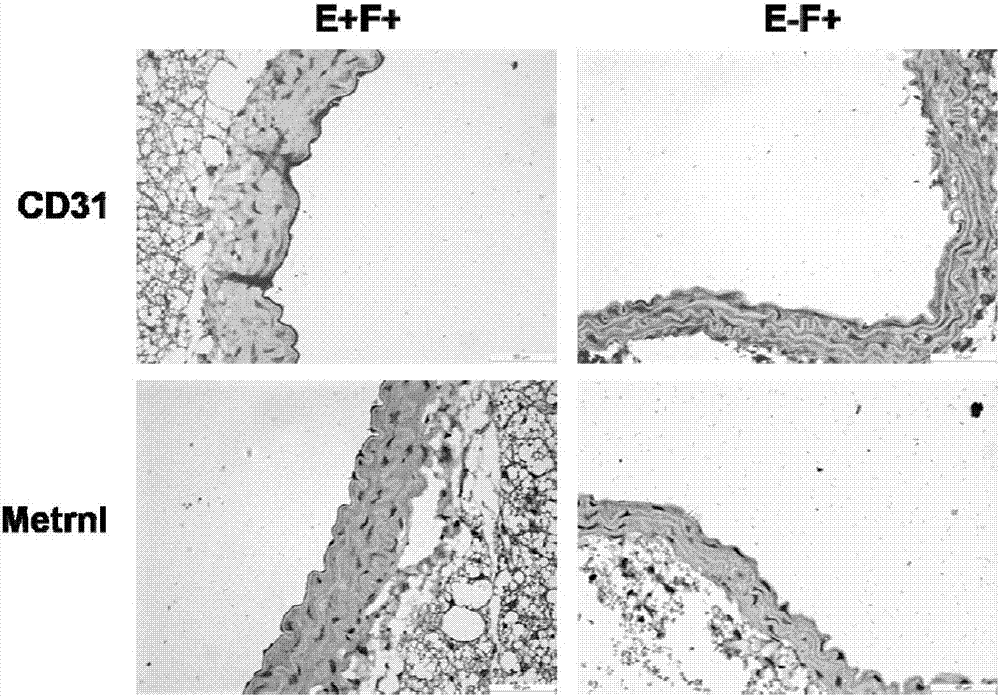
Application of Metrnl protein or gene in treatment of endothelial function impairment
ActiveCN107088223APromote proliferationPeptide/protein ingredientsMetabolism disorderDiseaseVascular endothelium
The invention relates to application of Metrnl protein or gene in treatment of endothelial function impairment. On one hand, based on a Metrnl endothelial specific knockout mouse model, it is proved that due to lacking of Metrnl in blood vessel endothelia, an endothelium-dependent relaxation function is lowered, while Metrnl has the function of dilating blood vessels; on the other hand, based on a human umbilical vein endothelial cell line and Metrnl endothelial specific knockout mouse primary endothelial cells, it is proved that the cell proliferation is lowered after knockout of blood vessel endothelia Metrnl, showing that Metrnl has the effect of promoting endothelial cell proliferation. Therefore, the Metrnl protein or gene and a synergist of the Metrnl protein or gene can be used for preparing reagents for treating endothelium-dependent blood vessel relaxation dysfunction or regulating endothelial cell proliferation, and are applied to development of drugs of diseases related to endothelial function impariment.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Myxobacteria strain and antitumor activity metabolite thereof
The invention relates to a myxobacteria strain and an antitumor activity metabolite thereof. The myxobacteria strain is the myxococcus STXZ72, Myxococcus sp.STXZ72, and the preservation number of the strain is CCTCC NO:M2015353. The invention further comprises a separation and purification method of the antitumor activity metabolite of myxobacteria. The metabolite of Myxococcus sp.STXZ72 is prepared by utilizing the separation and purification method. Antitumor activity determination proves that the myxobacteria strain is capable of effectively inhibiting the activity of a variety of tumor cells and also has very low toxicity to normal human umbilical vein endothelial cells.
Owner:湖南晴天生物科技有限公司
Euphausia superba oxidation resisting oligopeptide and preparation method thereof
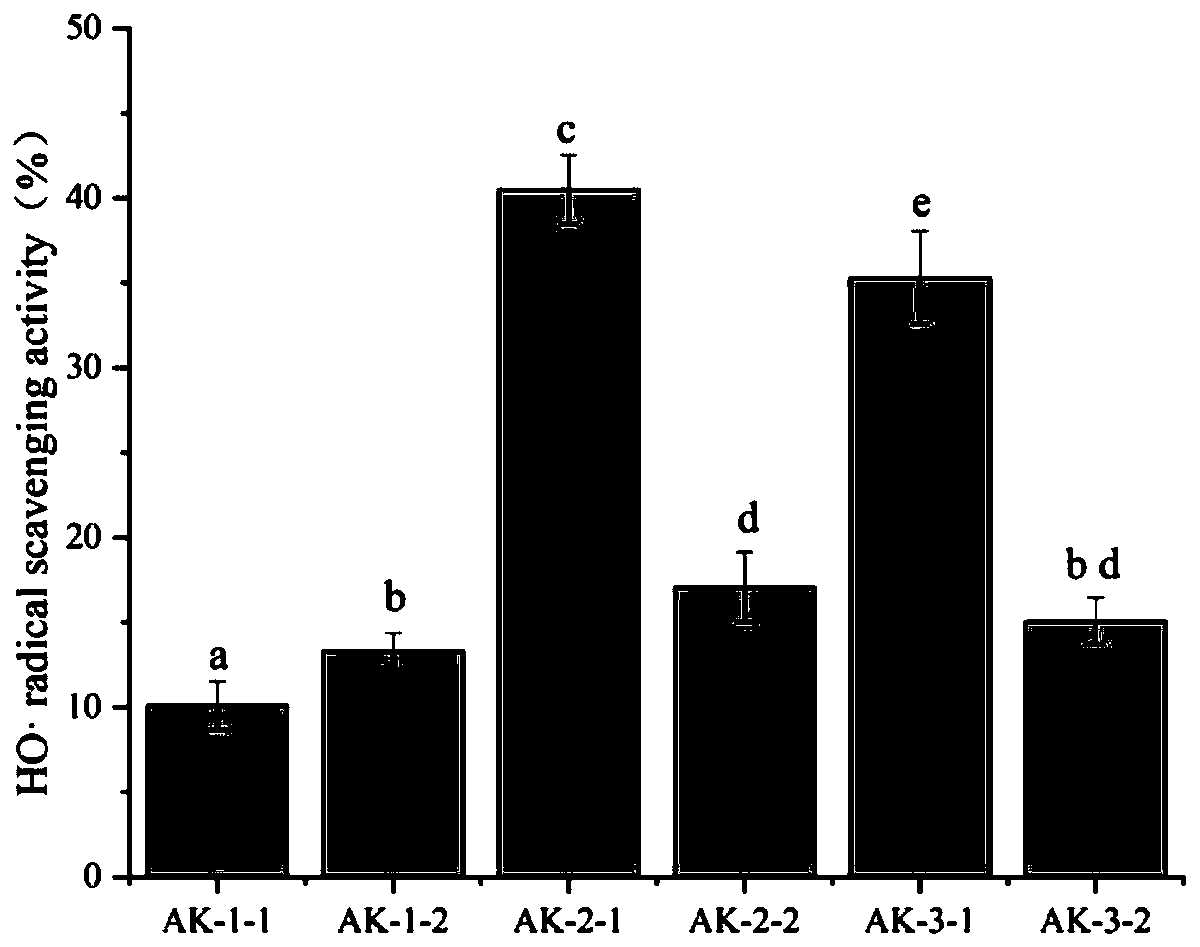
ActiveCN110563808AImprove cleanlinessIncrease vitalityPeptide/protein ingredientsAntinoxious agentsDPPHSuperoxide
The invention provides a preparation method of euphausia superba oxidation resisting oligopeptide. Euphausia superba is used as a raw material, through defatting, enzymolysis, ultrafiltration, cationexchange resin chromatography, gel column chromatography and antiphase high performance liquid chromatogram separation and purification, the oxidation resisting oligopeptide is obtained. The preparedhigh activity oxidation resisting peptide disclosed by the invention can have favorable elimination effects on DPPH free radicals, hydroxyl free radicals and superoxide anion free radicals, can significantly increase the vitality of SOD and GSH-Px in HUVEC cells, and can reduce the contents of intracellular NO and MDA.
Owner:德州蓝力生物技术有限公司
Cyclopentanopolyhydrophenanthrene skeleton compound capable of regulating and controlling blood coagulation factor VIII level to play anti-tumor role and application thereof
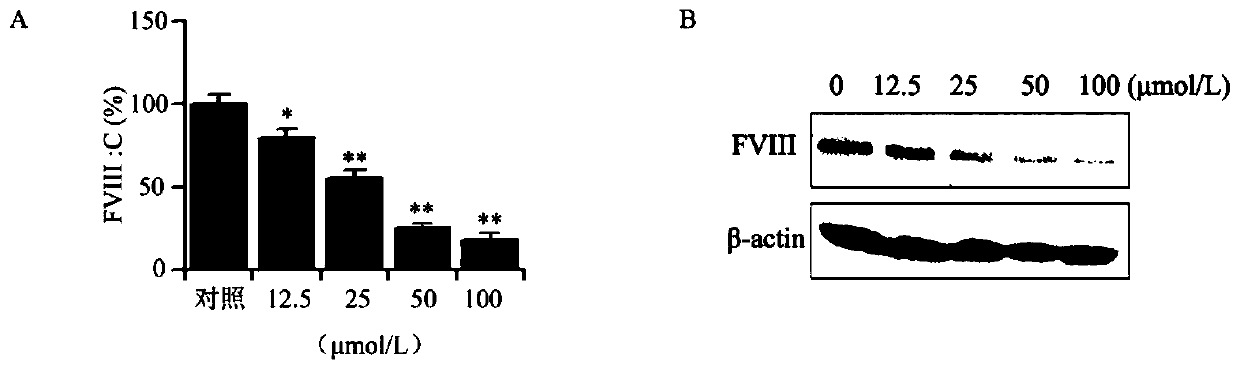
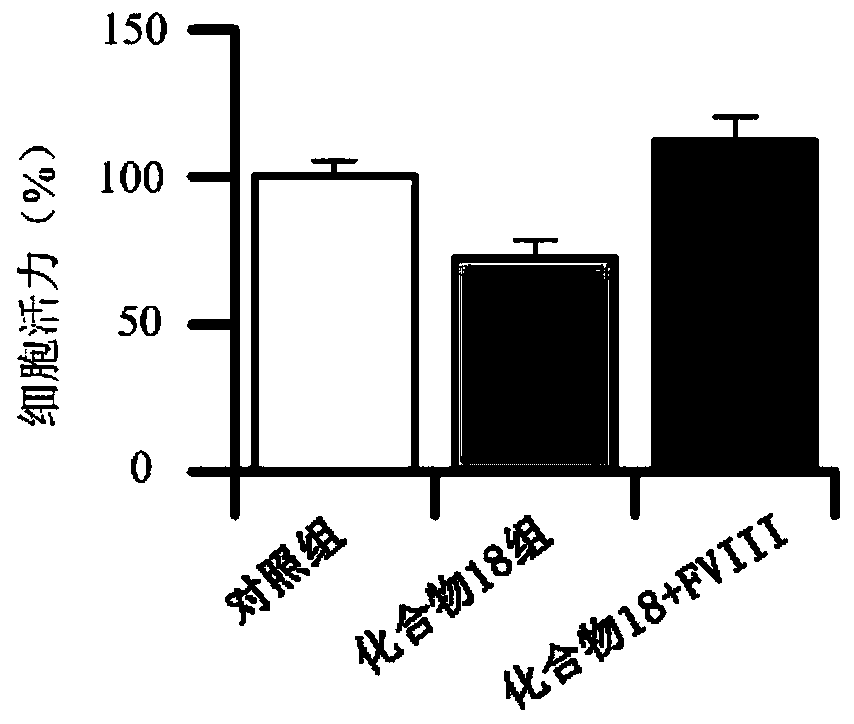
ActiveCN110028546APrevent proliferationInhibition of adhesionSteroidsAntineoplastic agentsBlood coagulation factor VIIIFactor ii
The invention provides a cyclopentanopolyhydrophenanthrene skeleton compound capable of regulating and controlling the blood coagulation factor VIII level to play an anti-tumor role, and application of the cyclopentanopolyhydrophenanthrene skeleton compound to preparation of drugs for treating blood coagulation factor VIII-mediated tumors, in particular to drugs for treating a blood coagulation factor VIII-mediated tumor which is blood coagulation factor VIII-mediated liver cancer. Experiments prove that the cyclopentanopolyhydrophenanthrene skeleton compound can inhibit expression and secretion of HUVEC endothelial cells FVIII, inhibit HUVEC endothelial cell FVIII mediated hepatoma carcinoma cell proliferation, and inhibit adhesion of tumor cells and platelets mediated by FVIII secreted by HUVEC cells, and in-vivo experiments prove that the cyclopentanopolyhydrophenanthrene skeleton compound can inhibit lung metastasis of liver cancer by inhibiting the level of FVIII.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
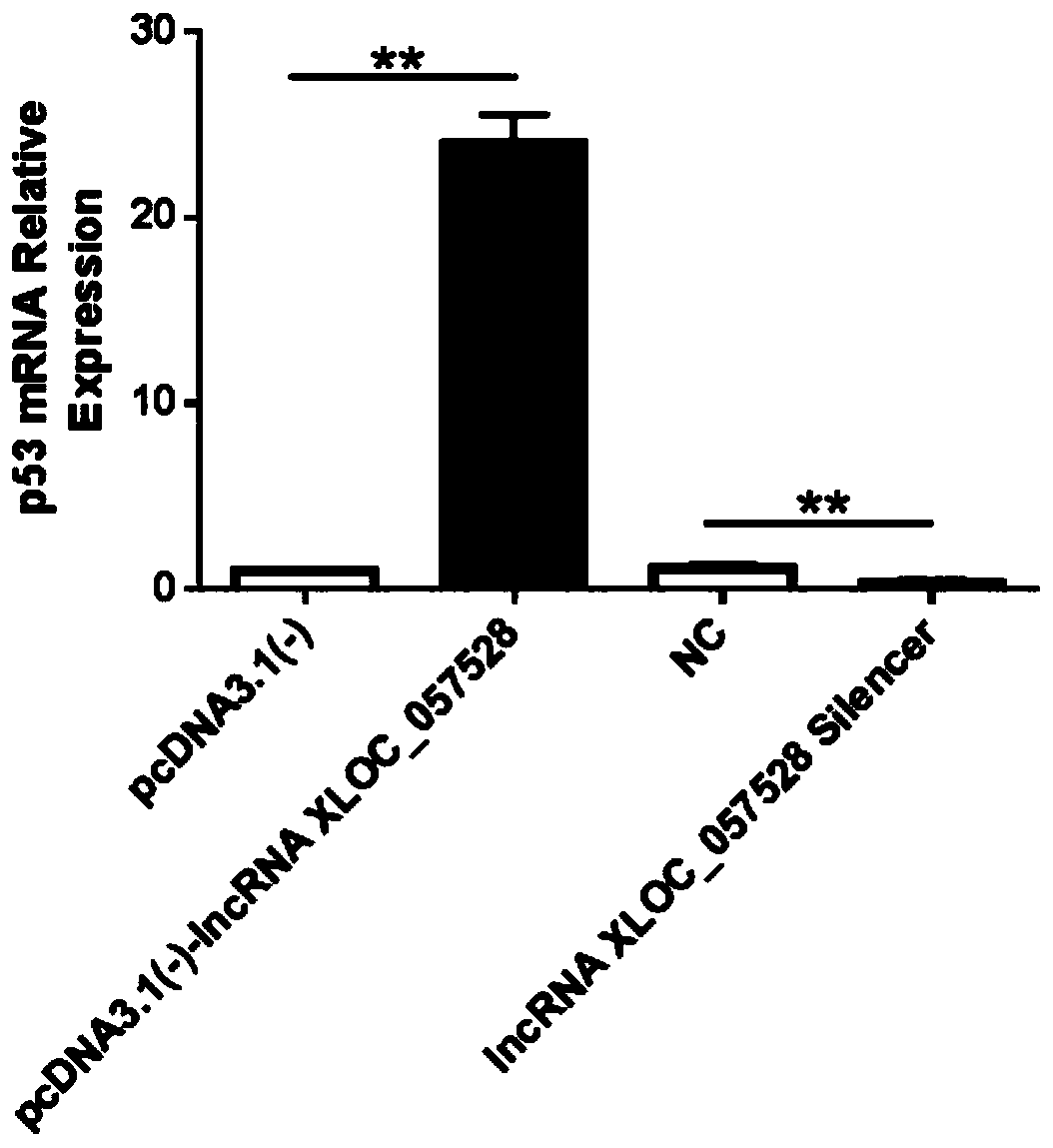
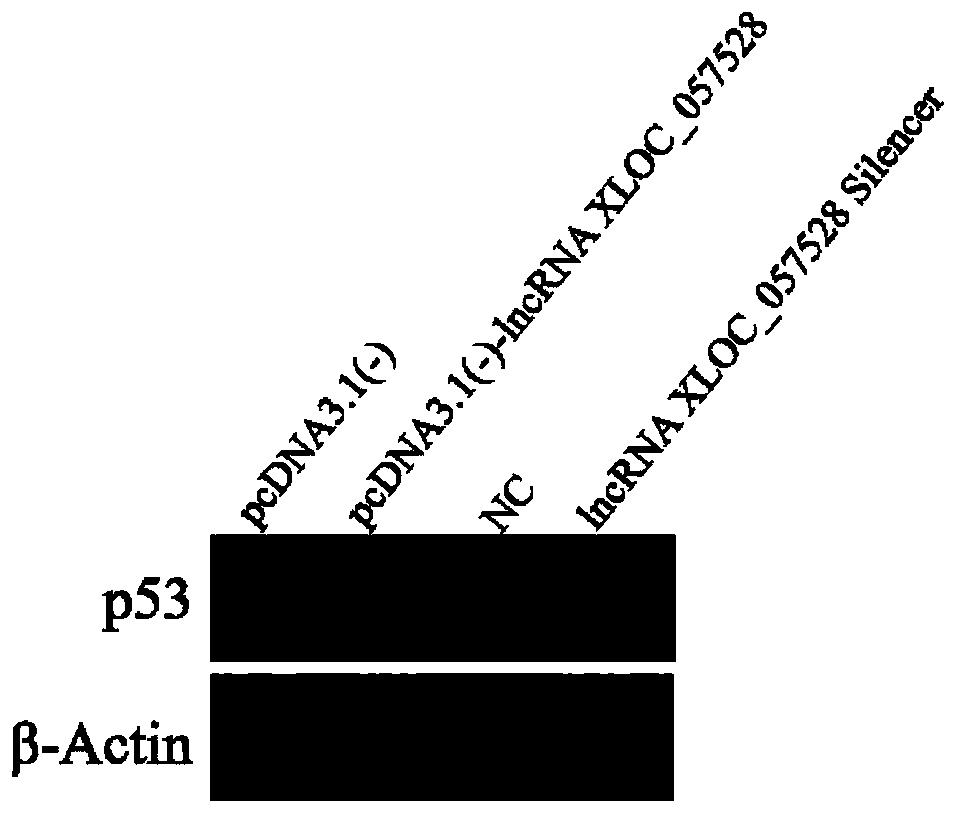
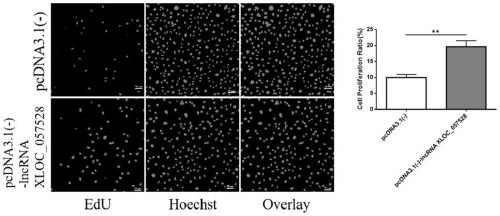
Application of LncRNA XLOC_057528 inhibitor to preparation of medicines for promoting blood vessel regeneration
ActiveCN110484614AReduce expressionHigh proliferation rateOrganic active ingredientsMicrobiological testing/measurementVeinMedicine
The invention discloses an application of an LncRNA XLOC_057528 inhibitor to preparation of medicines for promoting blood vessel regeneration. Overexpression and silence of lncRNA XLOC_057528 confirmthat lncRNA XLOC_057528 can influence expression of a p53 gene and protein to human umbilical vein endothelial cells, and besides, confirm that the lncRNA XLOC_057528 participates in promotion of proliferation of the human umbilical vein endothelial cells, restraining of apoptosis of the human umbilical vein endothelial cells, and restraining of canaliculization of the human umbilical vein endothelial cells, and further confirm that the lncRNA XLOC_057528 can participate in restraining of blood vessel regeneration after miocardial infarction. Therefore, the lncRNA XLOC_057528 inhibitor can beused for preparing the medicines for promoting blood vessel regeneration, and particularly the lncRNA XLOC_057528 inhibitor can be used for preparing the medicines for promoting blood vessel regeneration after miocardial infarction.
Owner:GUANGDONG LAB ANIMALS MONITORING INST +1
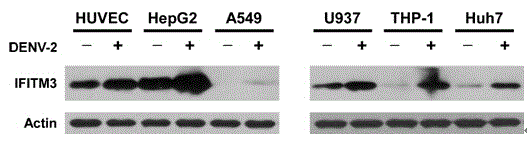
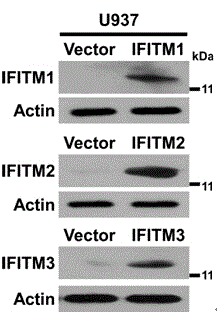
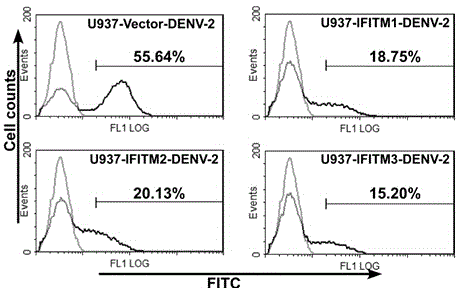
Application of IFITM3 (interferon induced transmembrane protein 3) packaging exosome to preparation of dengue virus infection prevention medicine
InactiveCN104587447APeptide/protein ingredientsGenetic material ingredientsZINC FINGER ANTIVIRAL PROTEINInterferon alpha
The invention discloses application of IFITM3 (interferon induced transmembrane protein 3) packaging exosome to preparation of a dengue virus infection prevention medicine. A preparation method of the exosome comprises the following steps: collecting the supernatant of an HUVEC (Human Umbilical Vein Endothelial Cell) culture medium with high-expressed IFITM3, and performing sucrose density gradient ultracentrifugation on the supernatant to prepare the exosome. The action mechanism is that the exosome with high-expressed IFITM3 can strongly inhibit dengue virus from adsorbing and penetrating host cells. The invention builds a new strategy for resisting dengue virus with the IFITM3 packaging exosome. Whether the antiviral protein packaging exosome can replace interferon to be directly applied to research and development of antiviral medicines opens up a new vision and new application of humanized antiviral protein in the field of antiviral prevention and treatment, thus providing new ideas and directions for development of novel antiviral medicines.
Owner:SUN YAT SEN UNIV
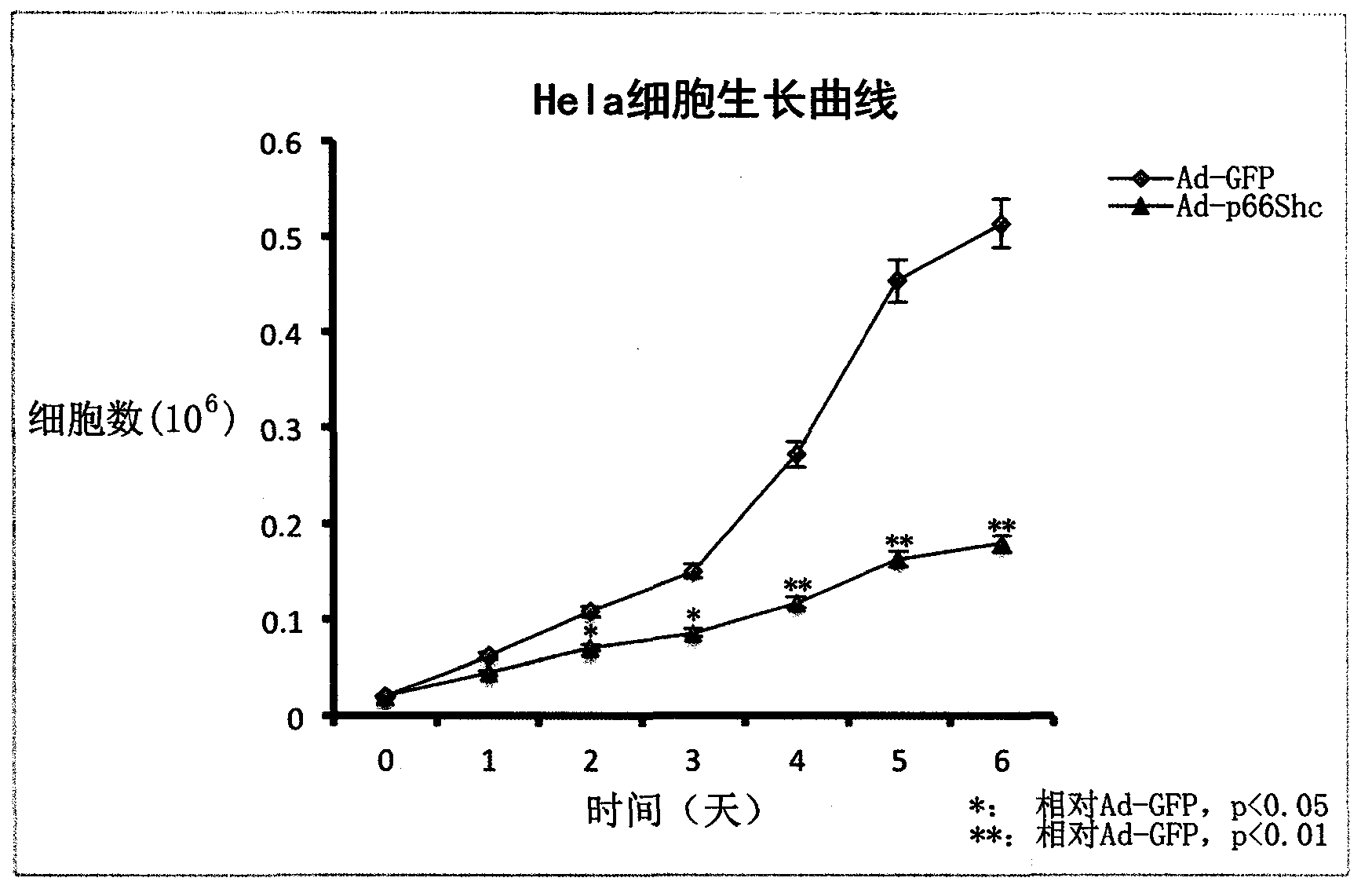
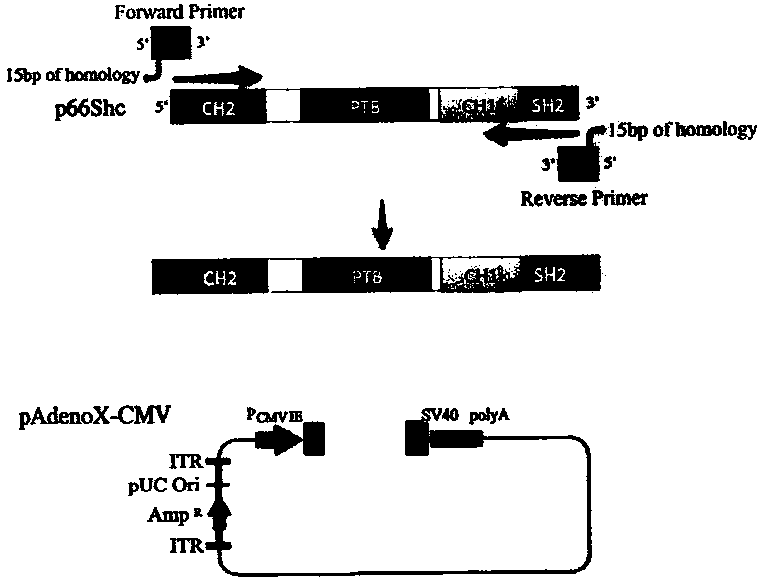
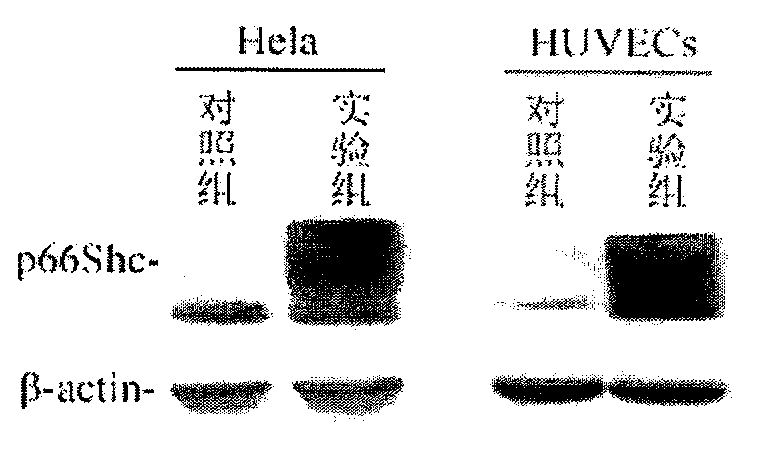
p66shc recombinant adenovirus vector as well as construction and application thereof
InactiveCN104073517AGrowth inhibitionGenetic material ingredientsFermentationHUVEC CellsGene engineering
The invention provides a p66Shc recombinant adenovirus vector. Homologous recombination in vitro of a human p66Shc fragment and a pAdeno X-CMV vector is realized by use of the gene engineering technology, and then competent bacteria are used for transformation to screen out correct recombinant adenoviruses; next, the recombinant adenoviruses are linearized and then used for transfecting HEK293A cells, and therefore, the recombinant adenoviruses are packaged and amplified largely in the HEK293A cells. After Hela cells are infected with the p66Shc recombinant adenovirus vectors, the growth and proliferation of the cells can be obviously inhibited; if human umbilical vein endothelial cells (HUVEC) are in infected with the p66Shc recombinant adenovirus vectors, no obvious growth inhibition effect is caused. The p66Shc recombinant adenovirus vector is expected to achieve a special growth inhibition effect on tumor cells.
Owner:BEIJING HOSPITAL
Anti-vegf antibody, and pharmaceutical composition for preventing, diagnosing or treating cancer or angiogenesis-related diseases, containing same
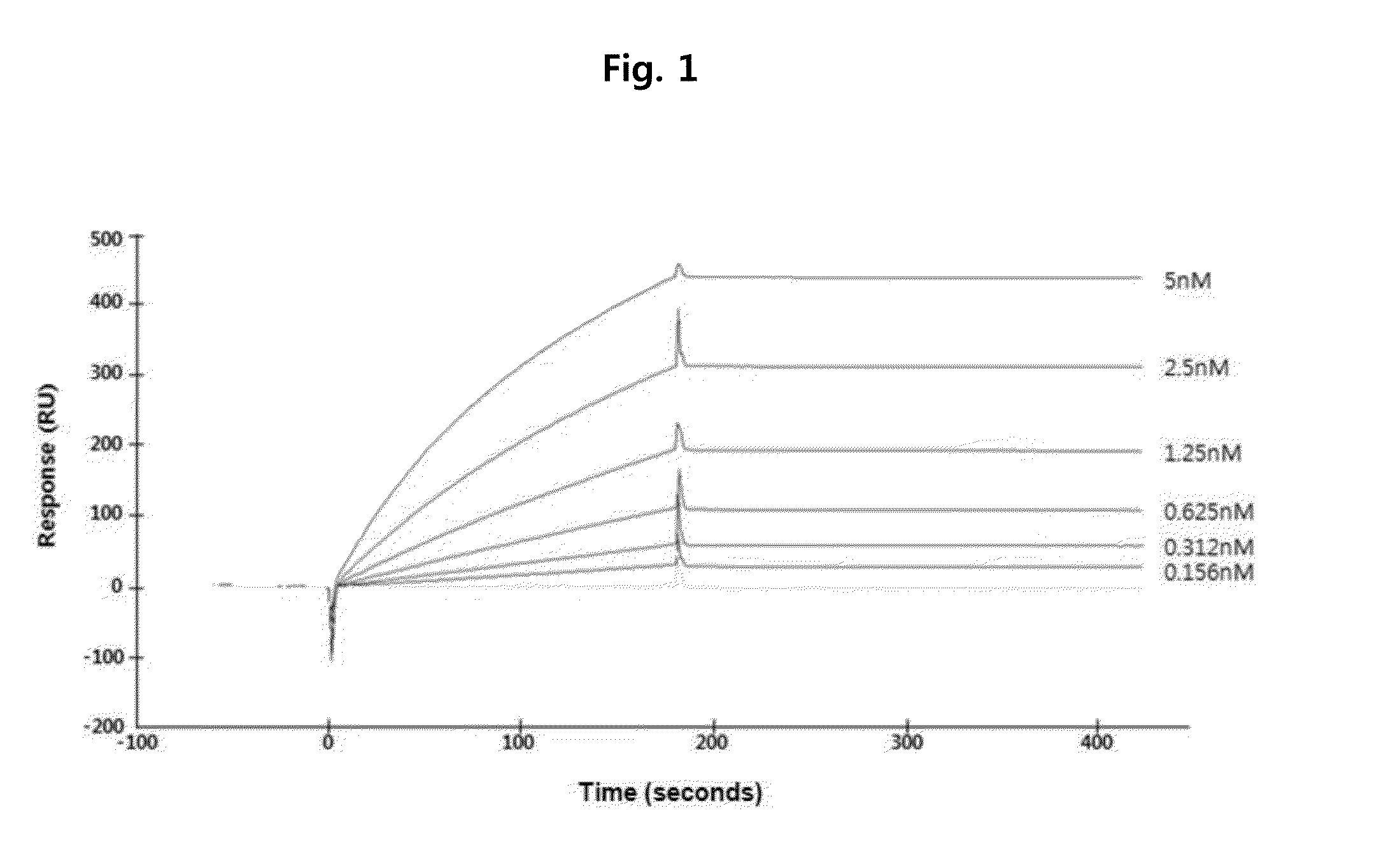
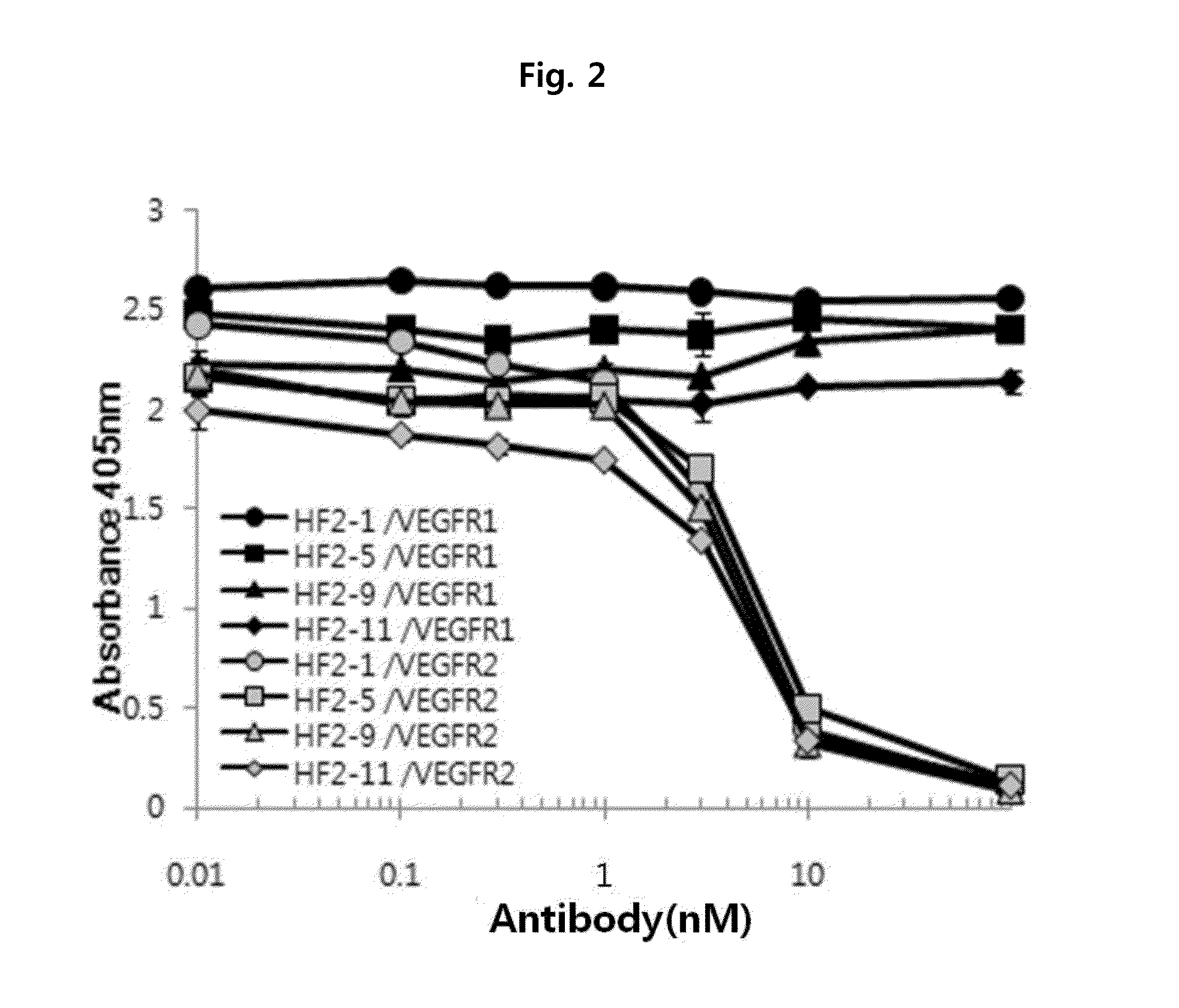
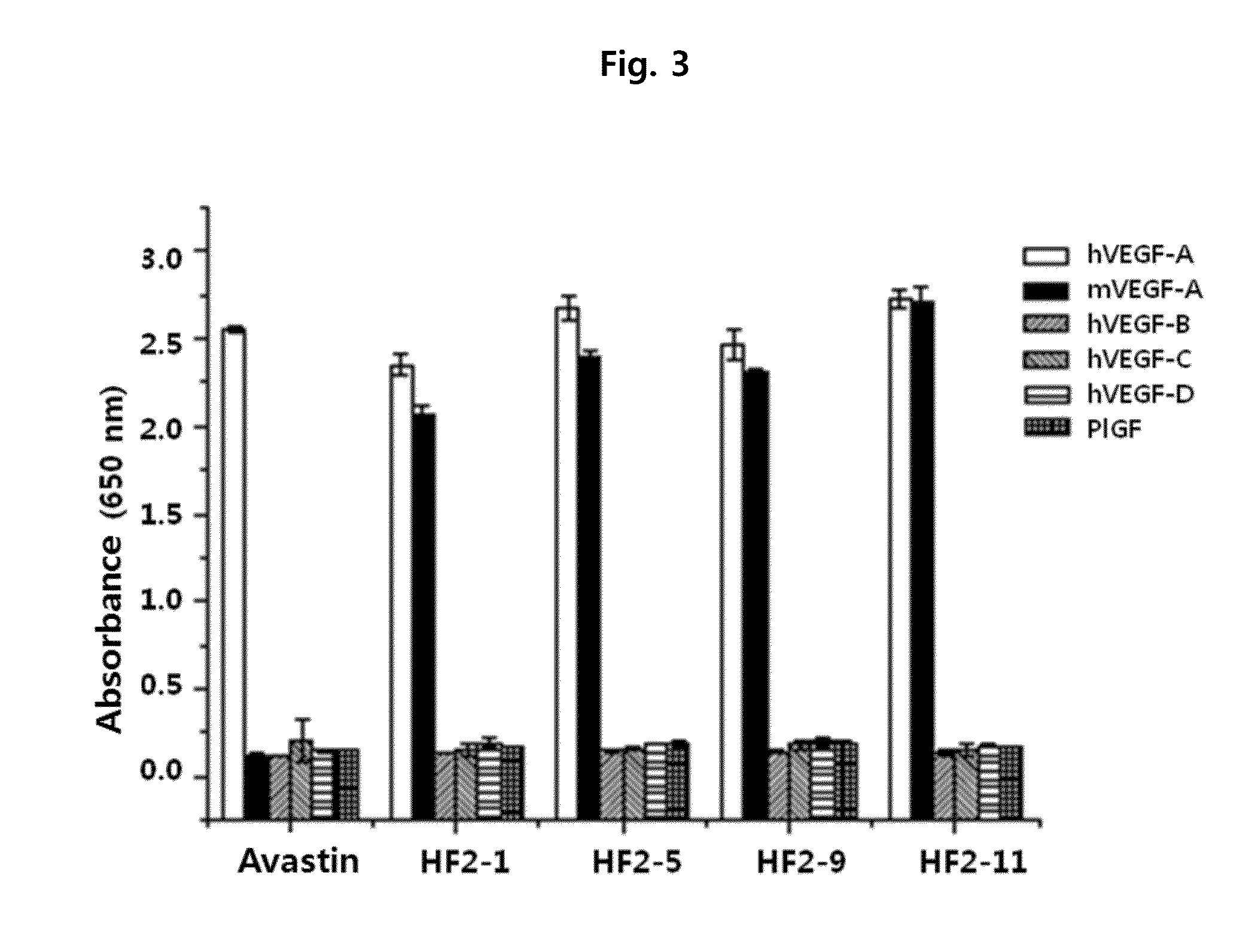
ActiveUS20160122426A1Remarkable binding propertySuppresses permeabilityAnimal cellsSenses disorderAnti vegf antibodyAngiogenesis growth factor
A novel anti-vascular endothelial growth factor (VEGF) antibody having a strong binding affinity for VEGF and capable of inhibiting in vivo tumor growth and a composition for the treatment of cancer, containing the same. The antibody shows a remarkable binding property to human and mouse VEGF, suppresses the proliferation and permeability of a human umbilical vein endothelial cell (HUVEC) and inhibits tumor growth, and thus can be useful as an antibody for the treatment of cancer.
Owner:DONG A SOCIO HLDG CO LTD +1
Application of integrin intracellular peptide sequences to inhibiting neovascularization
ActiveCN107253986AGrowth inhibitionCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsVaccinationConserved sequence
The invention relates to a series of peptide compounds containing integrin conserved sequences and having a function of inhibiting in vitro and vivo neovascularization. The functional peptide compounds have amino acid sequences shown as the first item of claims. The micromolecular peptide compounds can be coupled to cell-penetrating peptides to enter cells and then be combined with common signaling protein molecules to inhibit neovascularization and to achieve the effect of inhibiting in vivo solid tumor growth of tested mice. The peptide compounds belong to the field of biomedicine. The functional micromolecular peptide compounds and the effect protein molecules of the functional micromolecular peptide compounds can inhibit in vitro human umbilical vein endothelial cells from forming vascular structures, inhibit vascularization in subcutaneous vaccination matrix gels of nude mice and inhibit growth of subcutaneous vaccination solid tumors of the nude mice. The invention not only provides a number of leading compounds capable of inhibiting neovascularization, but also discloses a new neovascularization regulating mechanism, thereby providing new target sites for developing a new generation of neovascularization inhibiting and antineoplastic drugs.
Owner:SHANGHAI UNIV
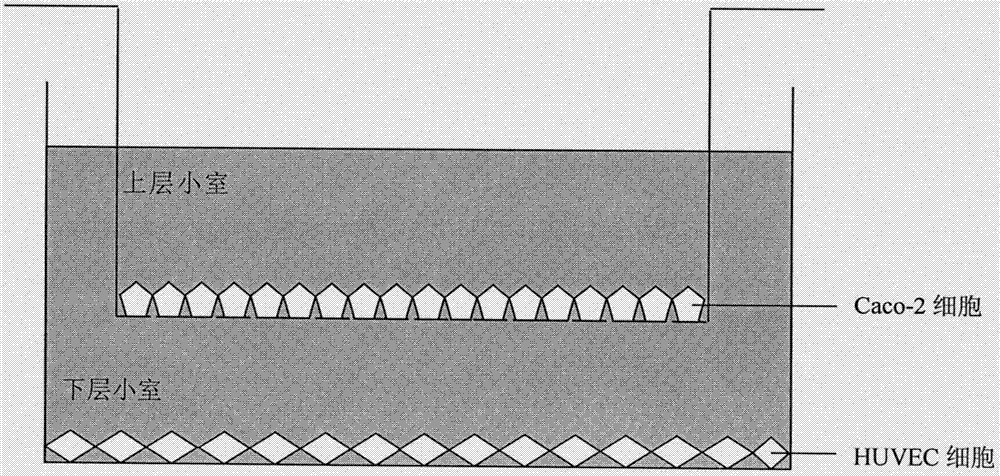
Method for constructing Caco-2/HUVEC cell co-culture system
InactiveCN107083364AComprehensive reflection of bioavailabilityTrue reflection of bioavailabilityCulture processArtificial cell constructsHUVEC CellsBioavailability
The invention discloses a method for constructing a Caco-2 and HUVEC cell co-culture system. The method comprises inoculating an upper small cell of a Transwell plate with 25 to 40 passages of Caco-2 cells in a logarithmic phase, carrying out culture until the cells are completely differentiated, inoculating a lower small cell of the Transwell plate with 2 to 6 passages of HUVEC cells in a logarithmic phase, carrying out culture until 80-90% of the lower small cell is filled with the cells, inserting the completely differentiated Caco-2 cell Transwell upper small cell into the cultured HUVEC cell Transwell lower small cell, fine adjusting a DMEM medium and carrying out non-contact co-culture at 37 DEG C in 5% of CO2. The Caco-2 / HUVEC co-culture system realizes simultaneous research of absorption, metabolism and physiological activity of drugs, simulates the pharmacological action of the drug in the circulatory system after absorption and metabolism in the intestinal tract and provides a cell model for showing the bioavailability of the drug.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Tumor vascular targeting peptide GX1
InactiveCN101531706AGood radiochemical purityGood specific activityIn-vivo radioactive preparationsPeptide preparation methodsTumor vesselDrug biological activity
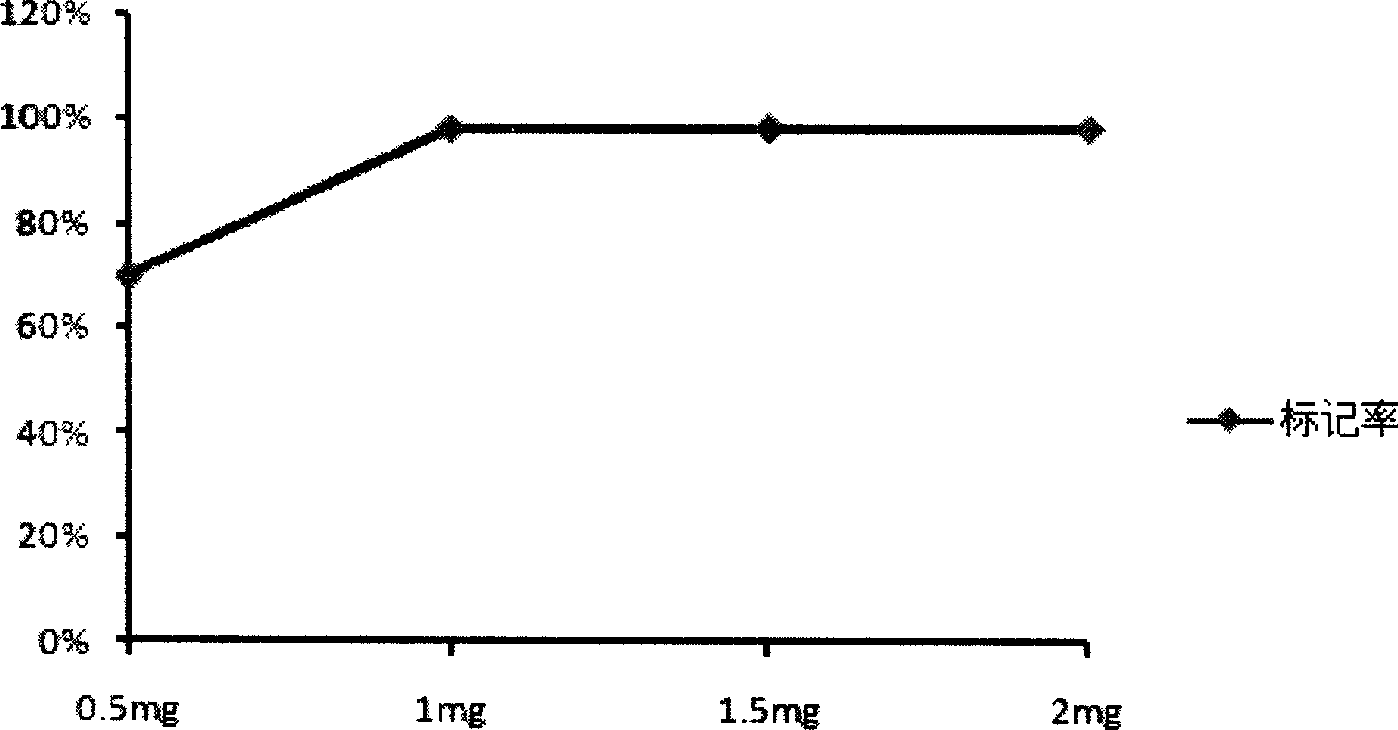
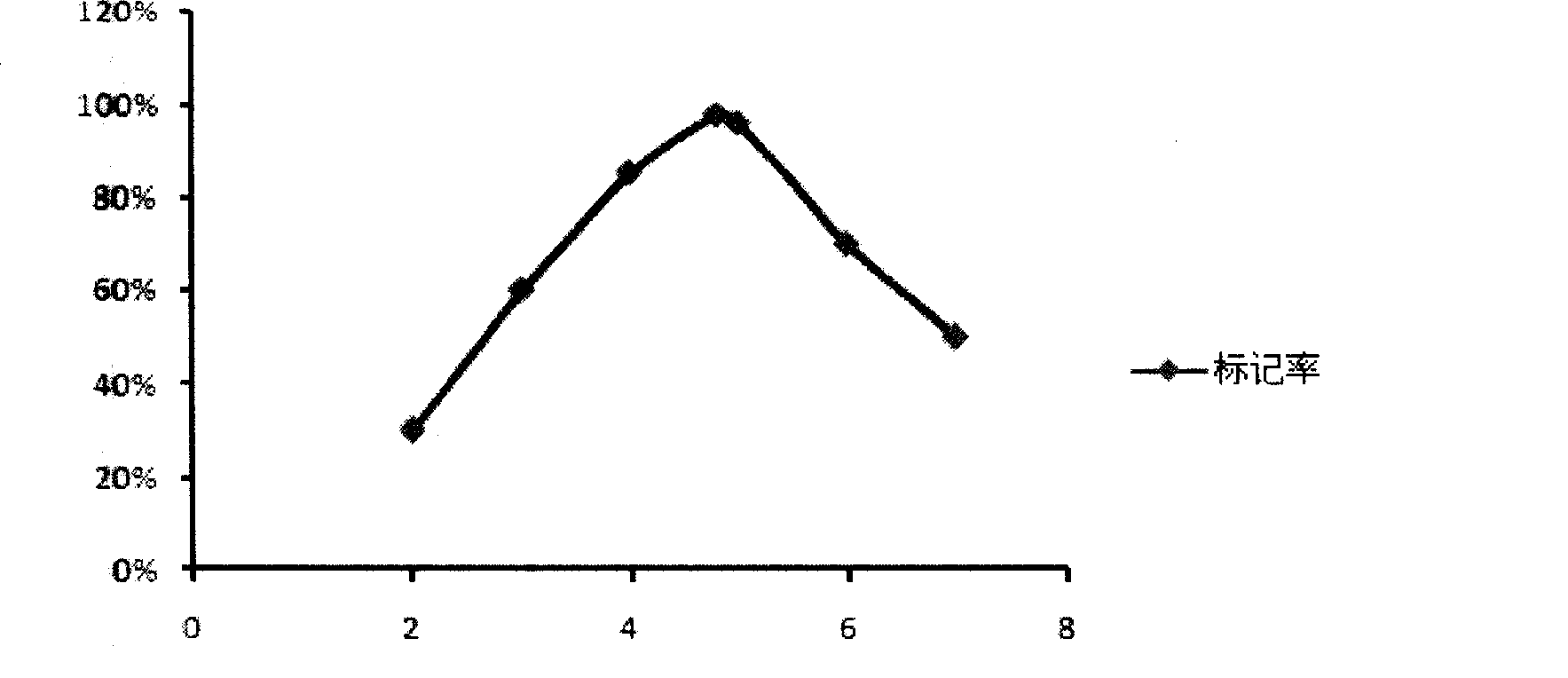
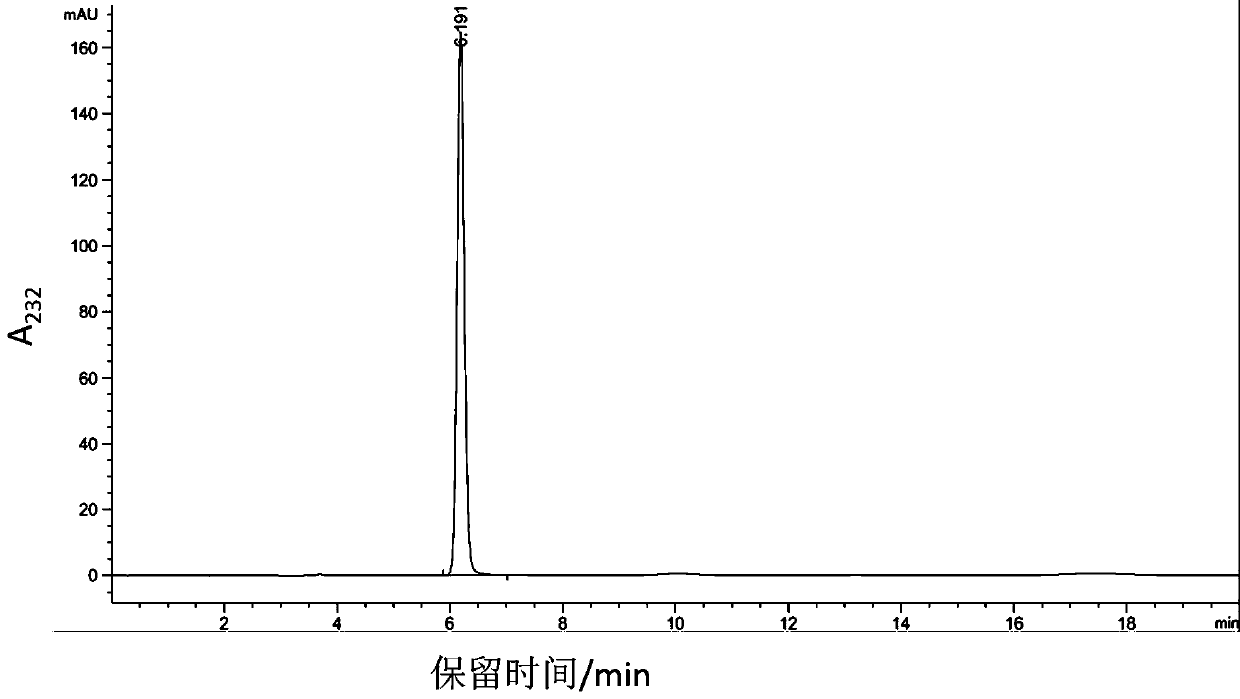
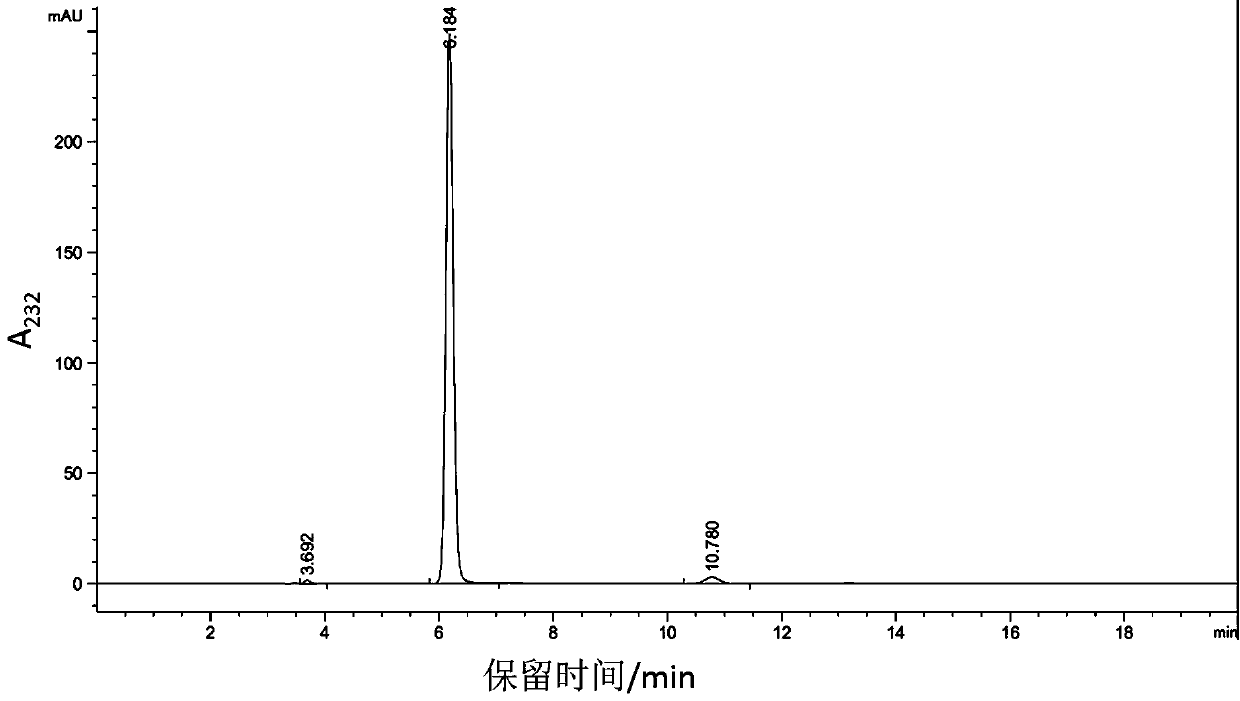
The invention belongs to the technical field of biomedicine, and in particular provides tumor vascular targeting peptide GX1. The invention utilizes a radioactive nuclide <99>Tc<m>O4 <-> to successfully mark GX1 by a direct method. Quality identification proves that <99>Tc<m>-GX1 has good radiochemical purity, specific activity and in vivo / vitro stability. In vitro microcosmic receptor autoradiography shows that the <99>Tc<m>-GX1 can combine with human umbilical vein endothelial cells and has good biological activity. In vitro microcosmic autoradiography, receptor radioligand conjoint analysis and competitive inhibition analysis confirm that the combination of the <99>Tc<m>-GX1 and tumor vascular endothelial cells has good specificity and affinity. ECT imaging and biological distribution prove that the <99>Tc<m>-GX1 has good targeting property to in vivo tumor tissue and specific distribution characteristicto tumor tissue. The <99>Tc<m>-GX1 has certain theoretical significance and great potential application value in diagnosis of gastric cancer, study on vascular targeting therapy, and other aspects.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method for large-scale preparation of high-purity unsaturated hyaluronic acid disaccharide
ActiveCN110982862AHigh purityLess impuritiesSugar derivativesDisaccharidesOrganic solventHyaluronidase
The invention discloses a method for large-scale preparation of high-purity unsaturated hyaluronic acid disaccharide. The method adopts two-step enzymolysis and two-step purification, and specificallyincludes the steps of: firstly, carrying out enzymolysis on macromolecular hyaluronic acid or salt thereof by hyaluronidase, purifying the obtained product with a low organic solvent multiple to obtain low-molecular-weight hyaluronic acid or salt thereof with high purity, further performing thorough enzymolysis on the low-molecular-weight hyaluronic acid or salt thereof, and purifying the obtained product with a high organic solvent multiple to obtain the high-purity unsaturated hyaluronic acid disaccharide. The method has simple technological process operation, mild conditions, high production efficiency, low energy consumption, and is suitable for large-scale industrial production. The obtained product has high purity and low cytotoxicity, has the characteristic of promoting the proliferation of human umbilical vein endothelial cells and human corneal epithelial cells, can be used as a standard substance for content and purity detection of hyaluronic acid and related products thereof, and also has wide application prospect in the field of medicines.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
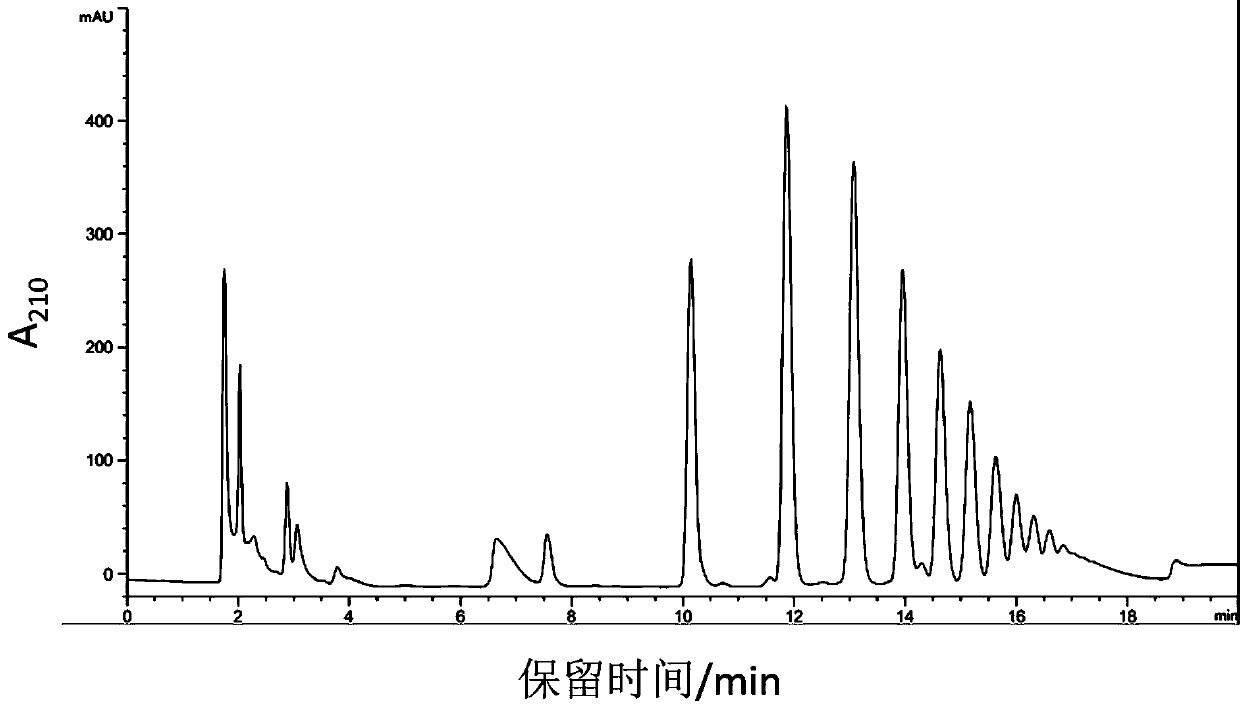
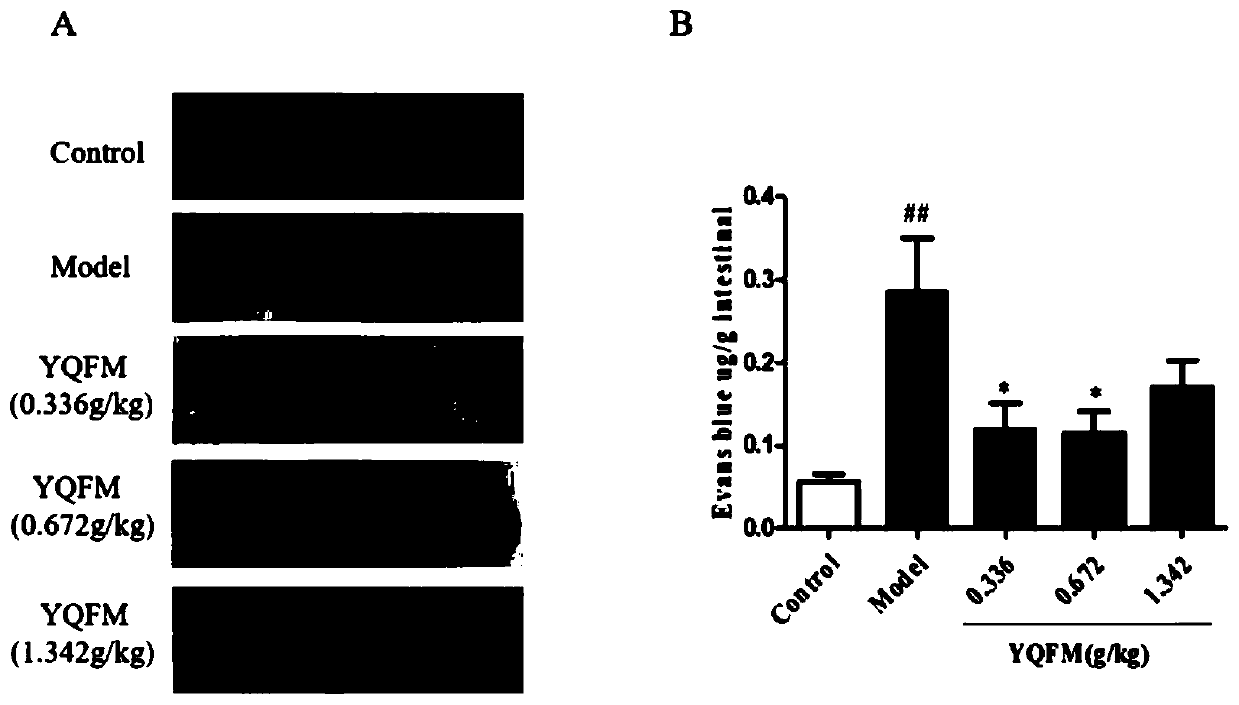
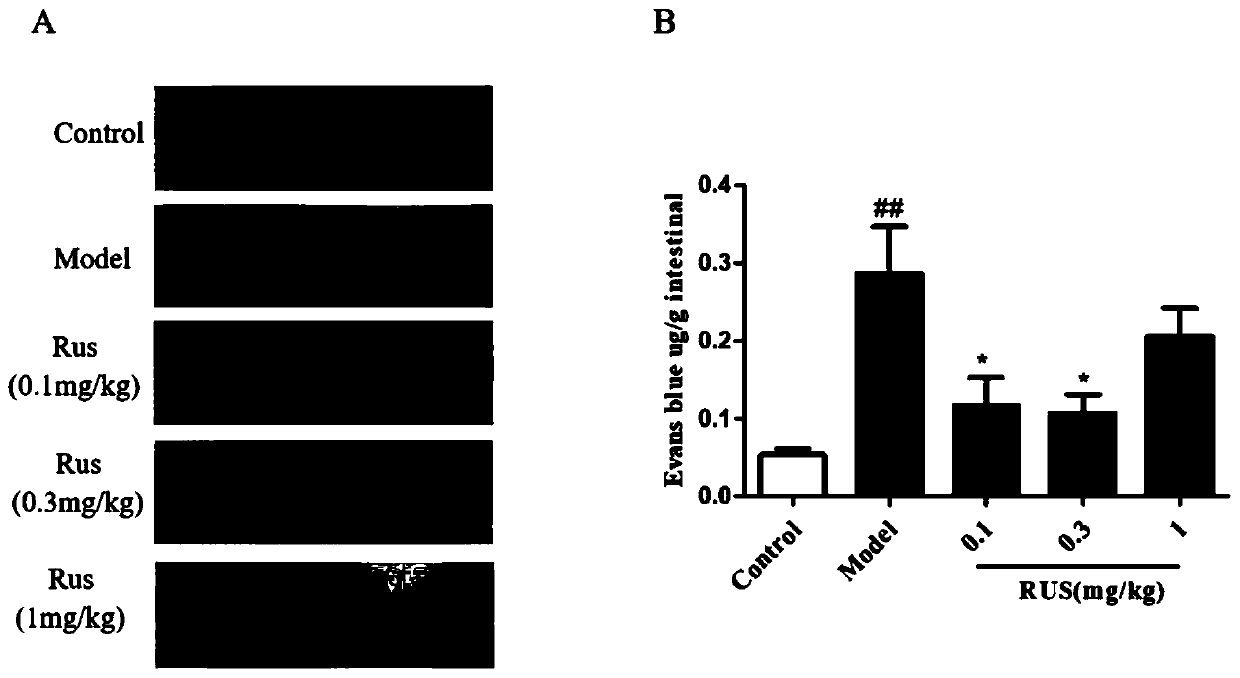
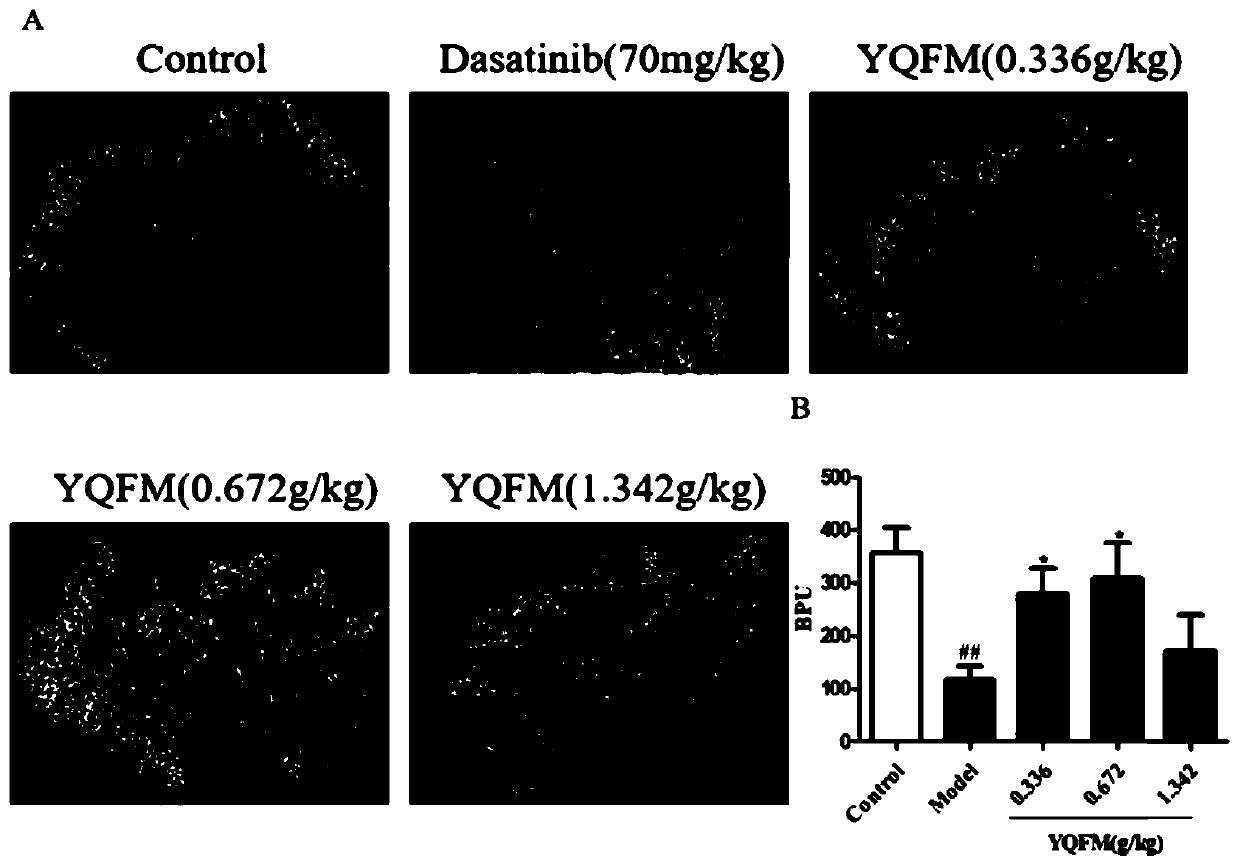
Application of qi-tonifying and pulse-restoring (freeze-drying) or ruscogenin for injection in prevention of pharmaceutical organ hemorrhage
InactiveCN110882258AOrganic active ingredientsPharmaceutical delivery mechanismDasatinibPharmaceutical drug
The invention discloses a new application of qi-tonifying and pulse-restoring (freeze-drying) or ruscogenin for injection in prevention of pharmaceutical organ hemorrhage. A dasatinib-induced mouse gastrointestinal hemorrhage model is used for verifying the improvement effect of qi-tonifying and pulse-restoring (freeze-drying) or ruscogenin for injection on dasatinib-induced mouse gastrointestinalhemorrhage. In addition, a dasatinib damaged HUVEC cell model is also used for verifying the improvement effect of the qi-tonifying and pulse-restoring (freeze-drying) or luscogenin for injection onthe cell permeability.
Owner:CHINA PHARM UNIV
Mytilus crassitesta oligopeptide and use thereof
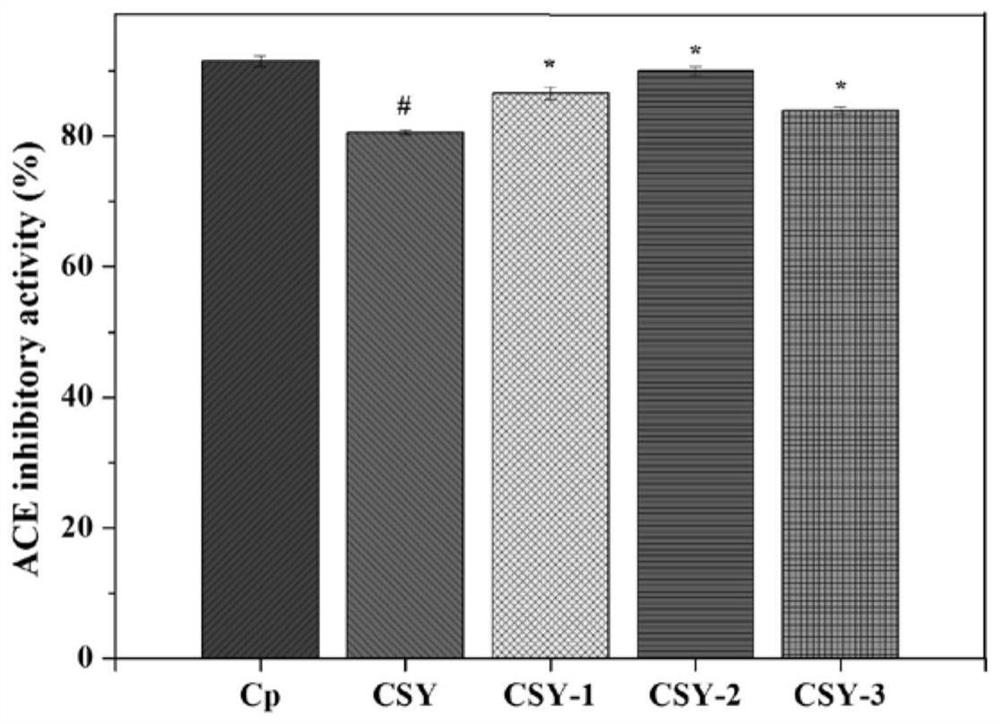
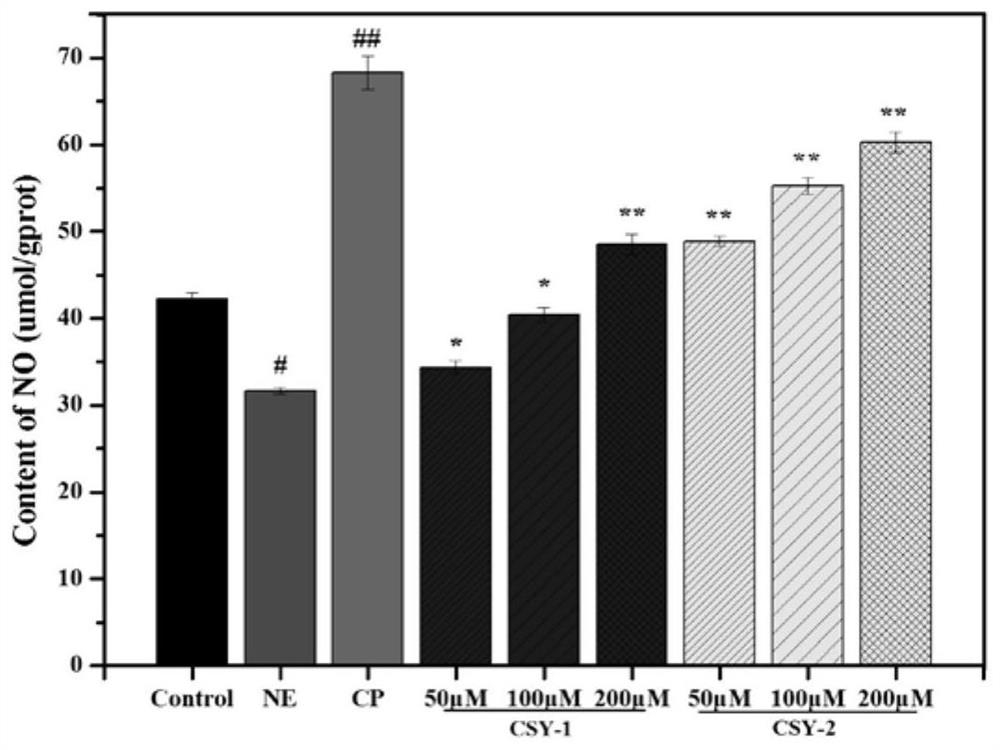
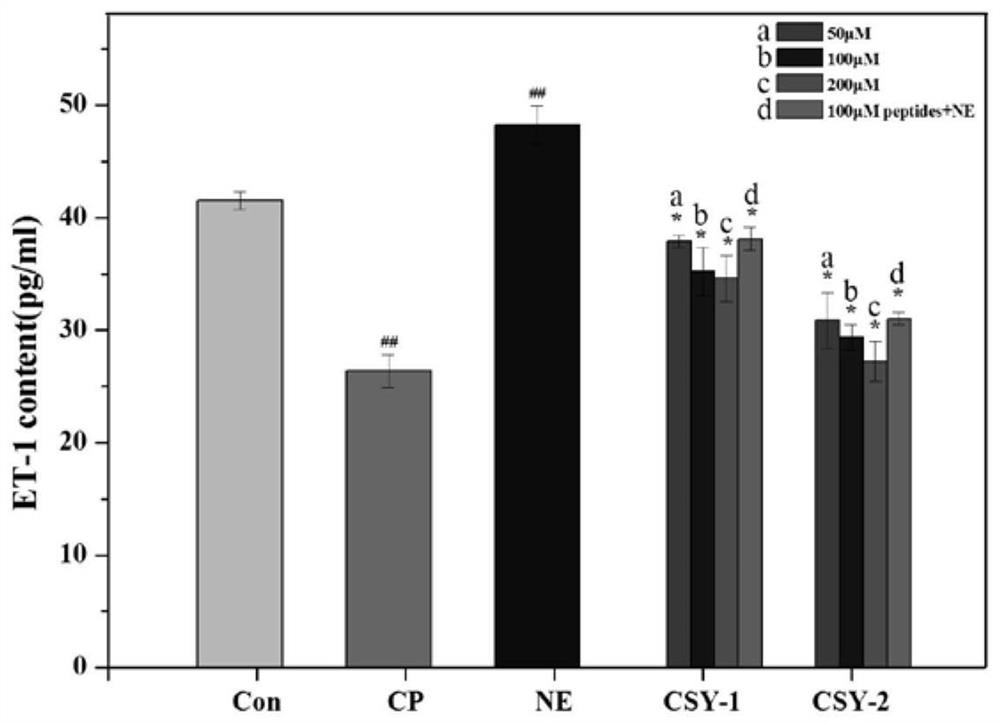
InactiveCN112457387AHigh ACE inhibitory activityNon-cytotoxicPeptide/protein ingredientsAnimals/human peptidesCytotoxicityOligopeptide
The invention provides a mytilus crassitesta oligopeptide and use thereof and belongs to the technical field of bioactive peptides. An amino acid sequence of the mytilus crassitesta oligopeptide is Leu-Ala-Ala-Ser-His or Tyr-Glu-Leu-His-Asp. The mytilus crassitesta oligopeptide provided by the invention has relatively high ACE inhibiting activity and is free of cytotoxicity, the content of an endogenous dilatation factor NO in blood vessel endothelial cells (HUVEC cells) can be increased, the content of an endogenous contraction factor ET-1 can be decreased, namely, the pressure release actionis exerted through affecting functions of the blood vessel endothelial cells. The mytilus crassitesta oligopeptide provided by the invention can be applied to preparation of health-care foods and / ormedicines with a blood pressure regulating action.
Owner:舟山市和朴海洋生物科技有限公司
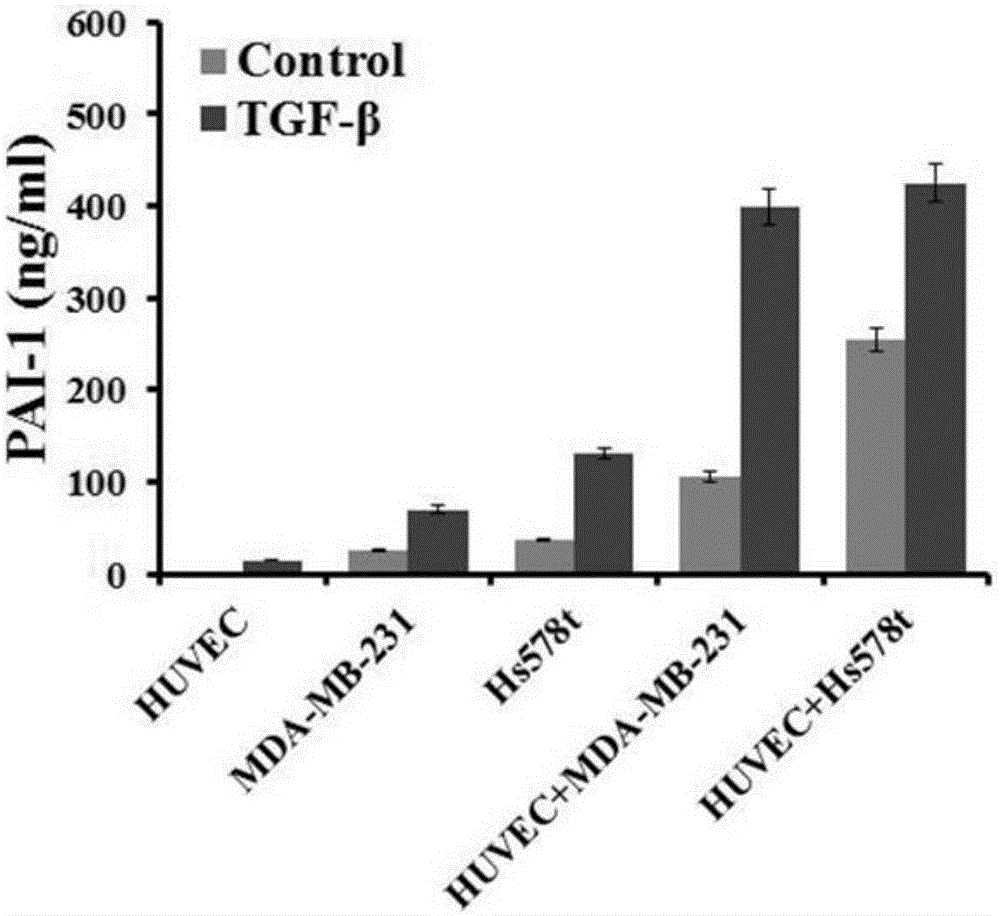
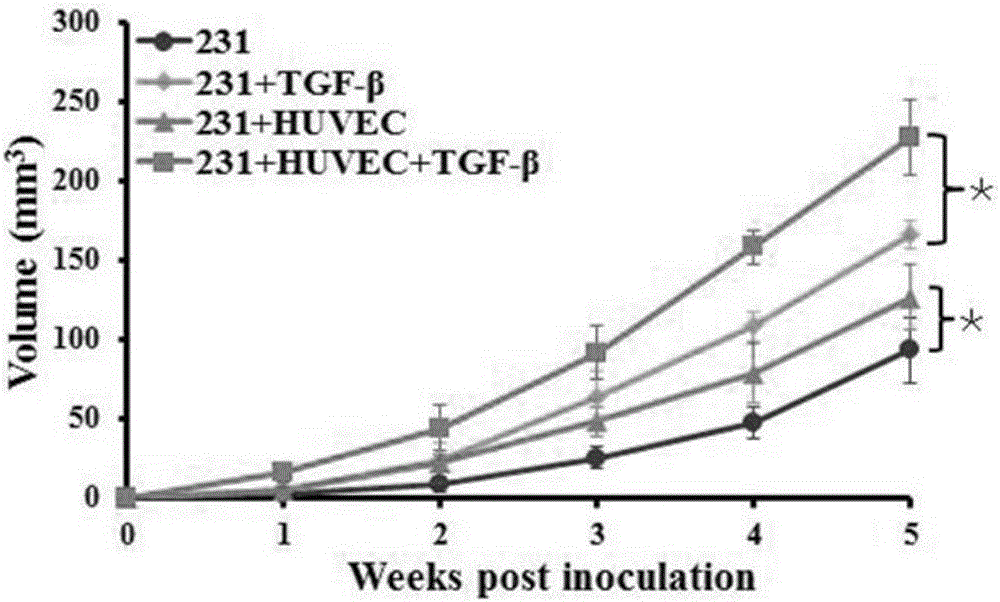
Method for detecting influence of triple negative breast cancer cell on endothelial cell secretion function in epithelial-mesenchymal transition process
ActiveCN106701886AEasy transferPromote secretionCompound screeningApoptosis detectionMda mb 231Factor ii
The invention relates to a method for detecting the influence of a triple negative breast cancer cell on an endothelial cell secretion function in the epithelial-mesenchymal transition process. A triple negative breast cancer cell line MDA-MB-231 and a human umbilical vein endothelial cell HUVEC are selected, and experimental groups including an MDA-MB-231 and HUVEC co-culture group and a control group, namely a MDA-MB-231 individual culture group are set; after the experimental groups and the control group are applied to 10ng / ml TGF-beta stimulation for 36 hours, a TMT quantitative proteome expression profile is applied to analyze differential expressions of secretory proteins in cellular supernatants in the two groups, and secretion results of SERPINE (PAI-1) factors in the co-culture group and the individual culture group are compared. The triple negative breast cancer cell line of the triple negative breast cancer cell in the epithelial-mesenchymal transition process can promote secretion of chemotactic factors CCL5 of endothelial cells and accordingly promotes transfer of the triple negative breast cancer cell.
Owner:管晓翔 +2
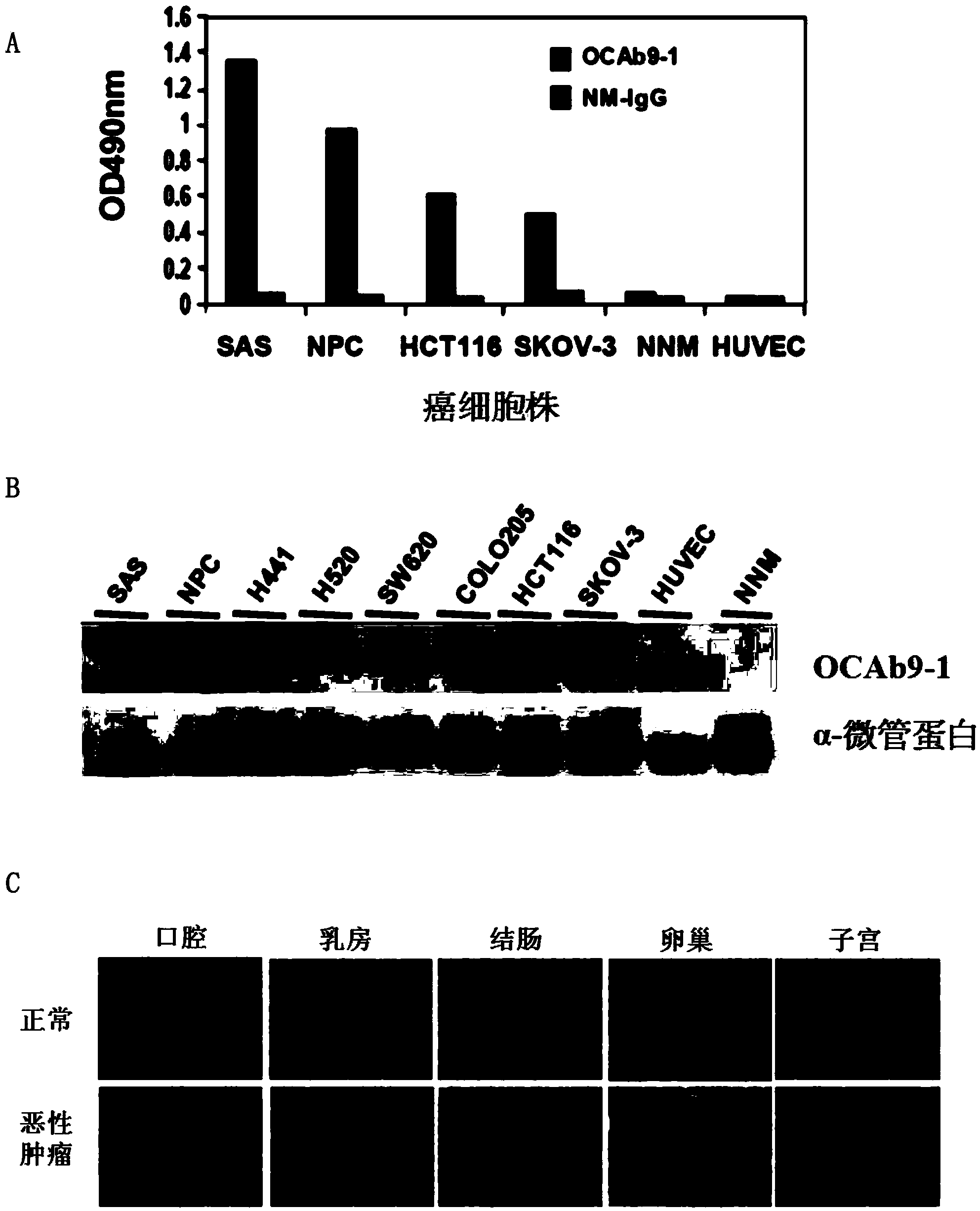
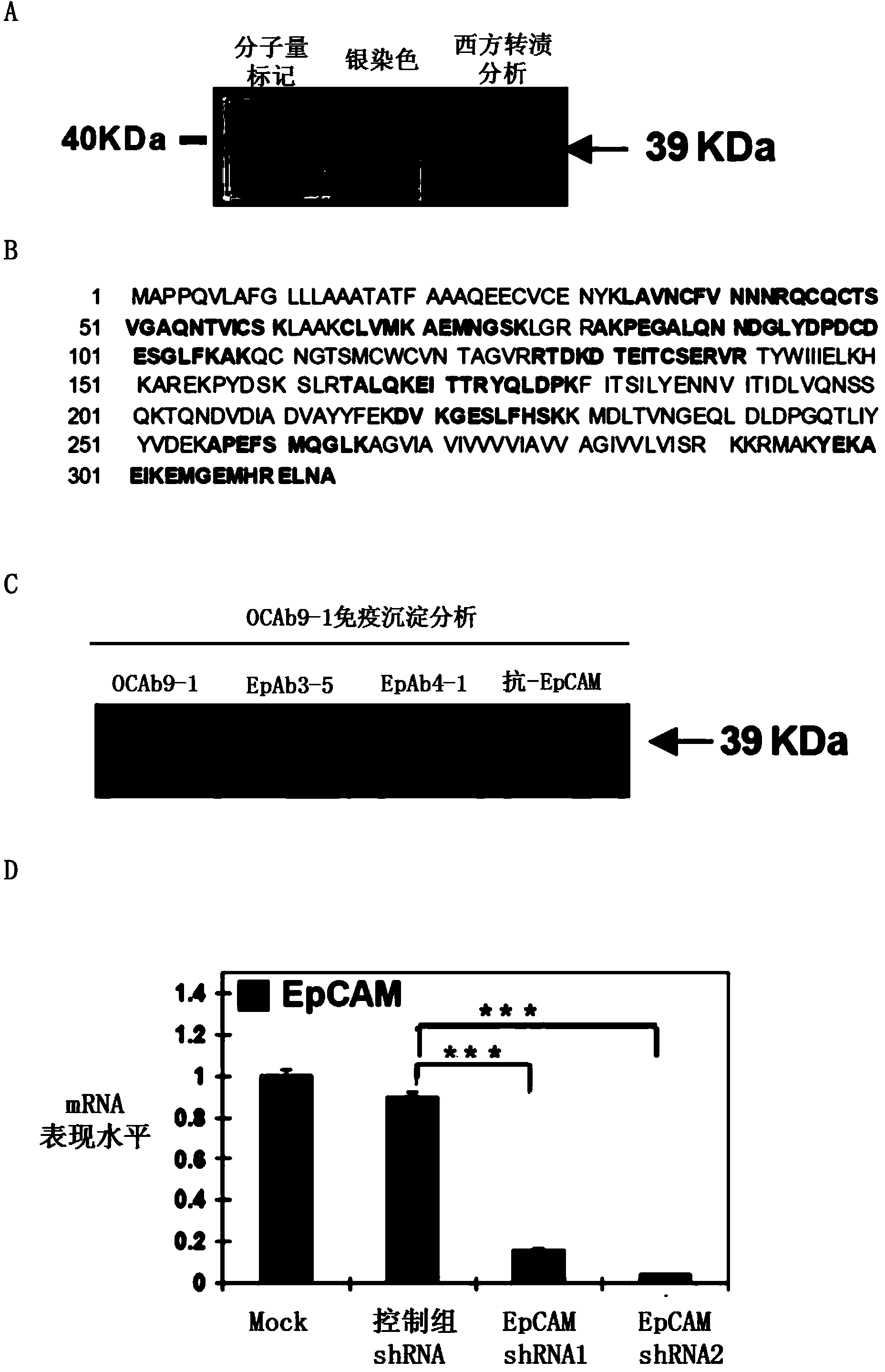
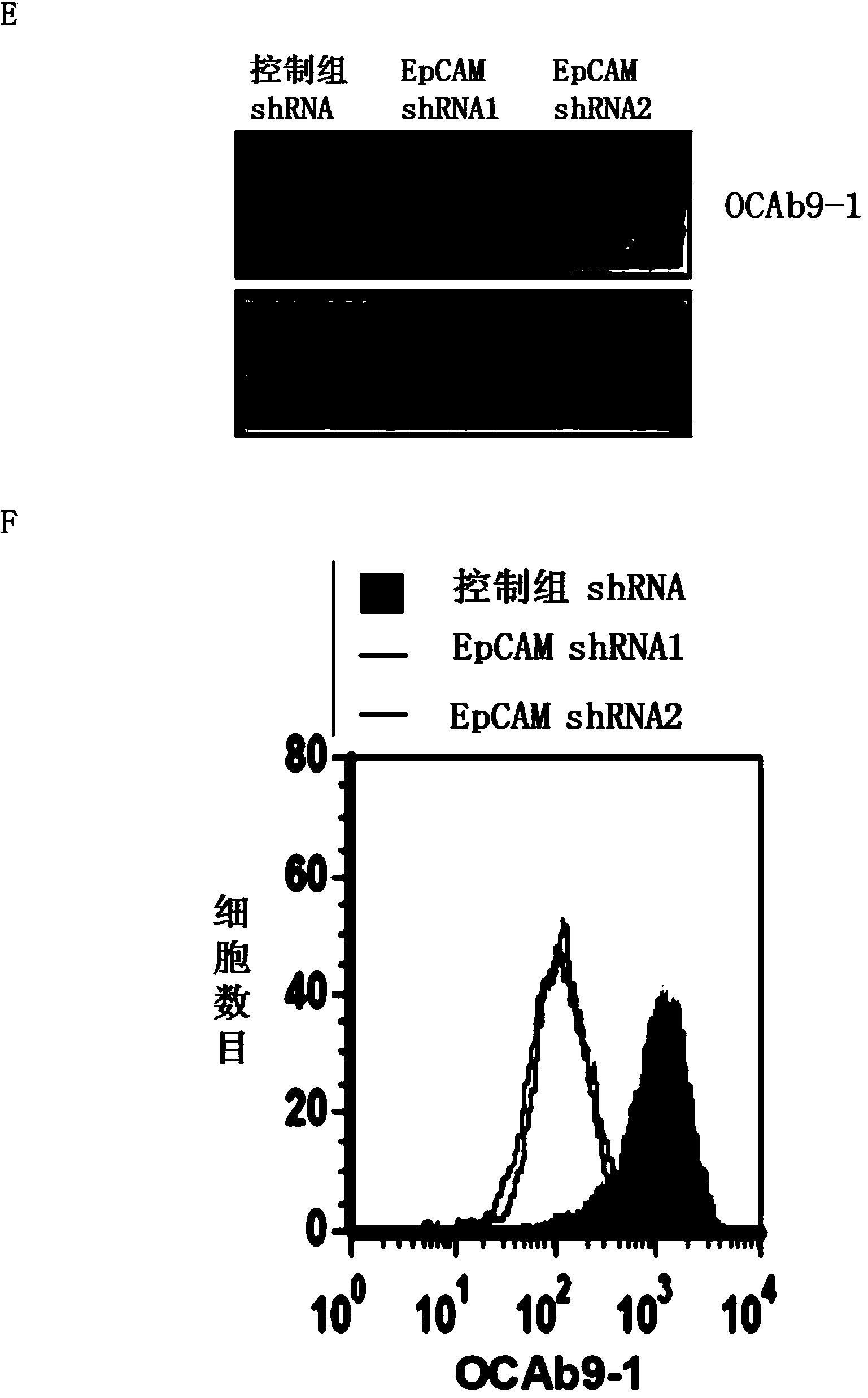
Anti-epithelial cell adhesion molecule (EpCAM) antibodies and methods of use thereof
ActiveCN104168916AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCancer cellAntigen Binding Fragment
An isolated monoclonal antibody or an antigen-binding fragment thereof is disclosed. The antibody or the antigen-binding fragment is characterized by: (a) having a specific binding affinity to epithelial cell adhesion molecule (EpCAM) comprising the amino acid sequence of SEQ ID NO: 1; (b) having a specific binding affinity to cancer cells expressing EpCAM, said cancer cells being selected from the group consisting of oral cancer cells, nasopharyngeal cancer cells (NPC), colorectal cancer cells, and ovarian cancer cells; and(c) having no binding affinity to human umbilical vein endothelial cell (HUVEC) and normal nasal mucosal epithelia (NNM). Also disclosed is an isolated monoclonal antibody or an antigen-binding fragment thereof that has a specific binding affinity to an epitope within the sequence of KPEGALQNNDGLYDPDCDE (SEQ ID NO: 63) located within the EGF-like domain II of epithelial cell adhesion molecule (EpCAM). Methods of using the same are also disclosed.
Owner:ACAD SINIC
Research on antitumor mechanism of Combretastatin A-4 derivative
InactiveCN106727629AInhibit migrationInhibit phosphorylationOrganic active ingredientsAntineoplastic agentsVascular endotheliumFocal adhesion
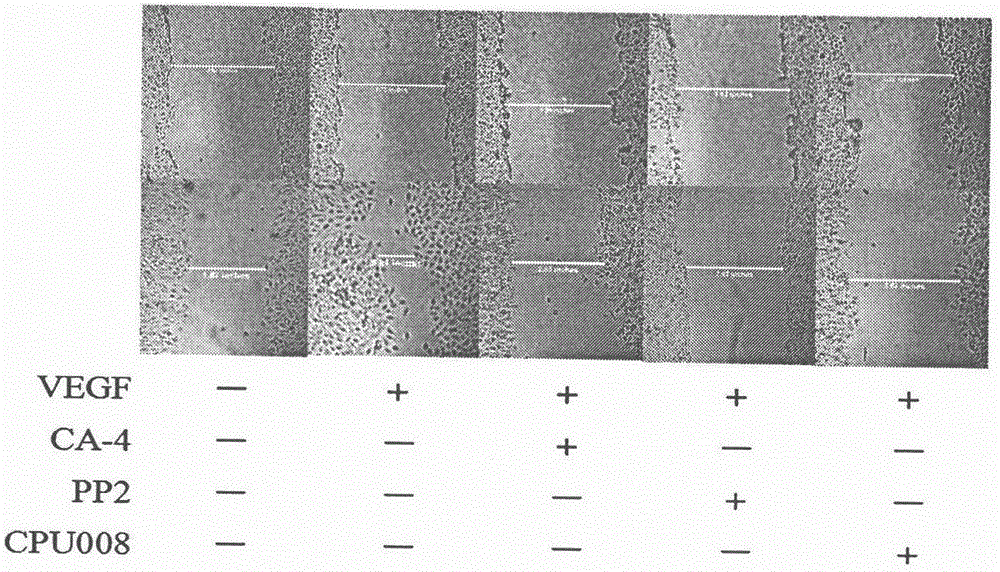
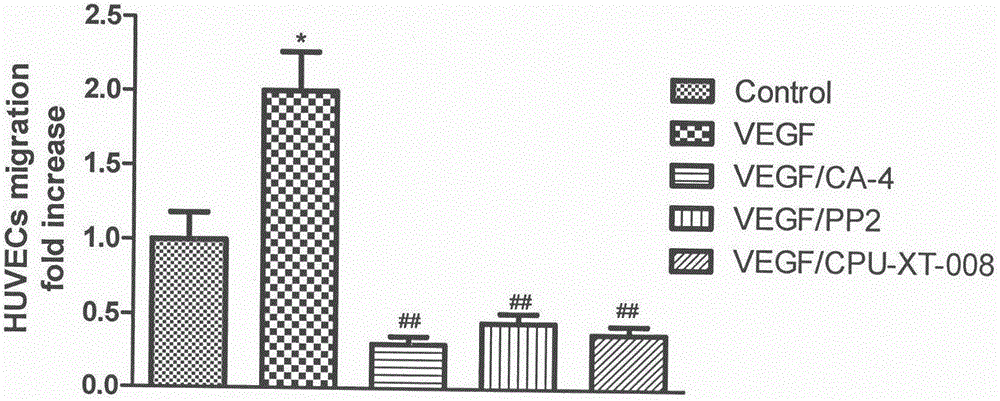
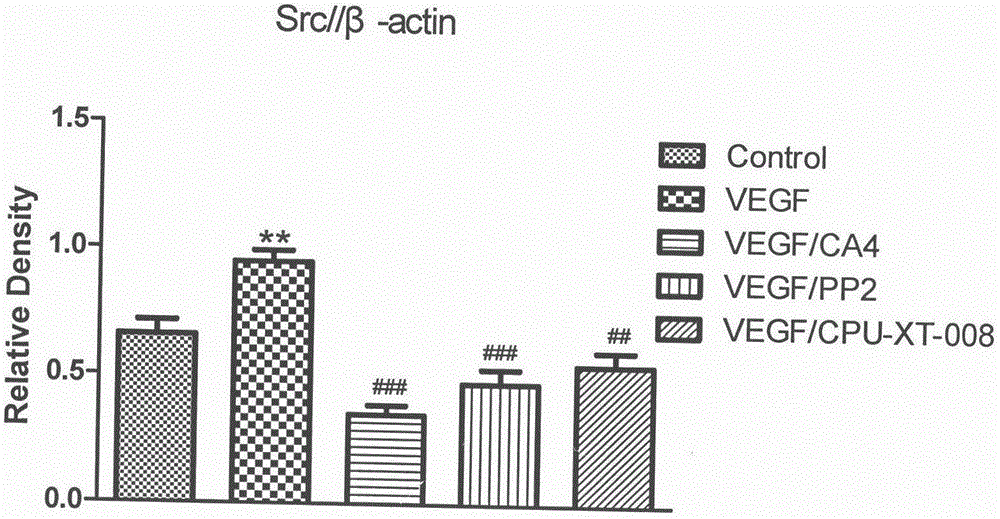
The invention discloses and relates to the field of biomedicine, in particular to inhibition of Src-FAK (Src focal adhesion kinase) signal pathways by Combretastatin A-4 derivative CPU-TX-008 to inhibit VEGF-induced (vascular endothelial growth factor induced) migration of vascular endothelial cells. As a CA-4 (Combretastatin A-4) derivative, CPU-TX-008 is acquired by replacing hydroxy of CA-4 structure with more water-soluble glucosamine groups. In-vitro cell experiments show that the derivative plays a role in inhibiting the migration of HUVECs (human umbilical vein endothelial cells); it is discovered through the research on the influence of related migrated proteins Src and FAK that CPU-TX-008 inhibits cell migration by inhibiting the phosphorylation of Src and FAK proteins in HUVECs, finally inhibiting the growth of tumor vessels; by adding chemical groups to CA-4 to prepare tumor vessel inhibitory drugs, new clinical antitumor paths are provided.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com